Abstract
Background and Aims
A composite endoscopic‐histologic remission is increasingly explored as an important endpoint in ulcerative colitis (UC). We investigated combined endoscopic‐histologic remission for predicting clinical outcomes at 12 months compared with endoscopic remission alone using the high definition virtual chromoendoscopy (VCE) Paddington International virtual ChromoendoScopy ScOre (PICaSSO) and histology scores.
Methods
Ulcerative colitis patients, prospectively enrolled from 11 international centres, underwent VCE with targeted biopsies and followed up for 12 months. Endoscopic activity was assessed by Mayo Endoscopic Score (MES), Ulcerative Colitis Endoscopic Index Severity (UCEIS) followed by VCE‐PICaSSO. Robarts Histopathological Index|Robarts Histological index≤3 without neutrophils in mucosa, and Nancy Histological index (NHI)≤ 1 were used to define histologic remission. Combined endoscopic‐histologic remission was compared with endoscopic remission alone by Cox proportional hazards model and by two‐ and three‐proportion analysis using pre‐specified clinical outcomes.
Results
307 patients were recruited and 302 analysed. There was no difference in survival without specified clinical outcomes between PICaSSO defined endoscopic remission alone and endoscopic plus histologic remission in the rectum (HR 0.42, 95%CI 0.16‐1.11 and HR 1.03, 95%CI 0.42‐2.52 for Robarts Histological index and NHI respectively) at 12 months. There was however a significant survival advantage without specified clinical outcome events for UCEIS combined with histology compared with UCEIS alone (HR 0.30, 95%CI 0.12‐0.75, p = 0.02) at 12 months (but not combined with NHI). For MES there was no advantage for predicting specified clinical outcomes at 12 months for endoscopy alone versus endoscopy plus histology, but there were differences in two and three proportion analysis at 6 months.
Conclusion
Endoscopic remission by VCE‐PICaSSO alone was similar to combined endoscopic and histologic remission for predicting specified clinical outcomes at 12 months. Larger studies with specific therapeutic interventions are required to further confirm the findings.
Keywords: endoscopic remission, histological remission, mucosal healing, ulcerative colitis, virtual electronic chromoendoscopy ‐ PICaSSO – clinical outcomes – prediction
INTRODUCTION
Endoscopic remission is an established treatment goal in ulcerative colitis (UC) since it is associated with improved clinical outcomes with fewer complications such as hospitalization and colectomy. 1 However, several studies have shown the importance of looking beyond white light defined endoscopic remission, as up to 40% of patients may still have persistent histologic inflammatory activity. 2 , 3 Histological normalisation or remission is being recognized as a “deeper “target for UC as some studies have associated it with better outcomes with lower rates of clinical relapse, corticosteroid use and acute severe colitis requiring hospitalization over a median of 6 years follow up. 4 , 5 However, as neither endoscopy or histology predicts outcomes accurately, the definition of mucosal healing (MH) has been evolving towards a composite endpoint of endo‐histology MH (alternatively designated endo‐histology mucosal improvement/remission) encompassing both endoscopic remission/improvement and histologic remission (neutrophils infiltration <5% of crypts, no crypts destruction, no erosions or ulcerations). 6 Such an endpoint has been reported from the ustekinumab trial in UC. 7 However, after achieving endoscopic remission {Mayo Endoscopic Score (MES) = 0}, incremental benefit of histologic remission is doubtful. 8
The discrepancy between endoscopy and histology may be explained by the current endoscopic scores used to assess grade of inflammation, that do not incorporate a definition of endoscopic healing {including MES = 1 as endoscopic improvement} and of previous generation of standard definition (SD) white light endoscopy (WLE) on which current scores are based. 9 , 10 , 11 , 12
Key summary.
Summarize the established knowledge on this subject
Recently we have developed and validated the first virtual electronic chromoendoscopy (virtual chromoendoscopy (VCE)) score Paddington International virtual ChromoendoScopy ScOre (PICaSSO) that can accurately define endoscopic remission and accurately predict histological remission in patients with ulcerative colitis (UC).
However, the magnitude of benefit in treating UC patients by achieving the more rigorous combination of endoscopic and histologic remission is still controversial.
What are the significant and/or new findings of this study?
Endoscopic remission defined by VCE PICaSSO scoring system alone predicts specified clinical outcomes of interest such as hospitalization, colectomy, change in medical therapies with no incremental advantage in combining it with histology remission at follow‐up over 12 months.
The VCE score PICaSSO could serve as a sole assessment method reflecting both endoscopic and histologic remission in patients with UC.
The advent of Virtual electronic ChromoEndoscopy (VCE) potentially overcomes these limitations of WLE and hence endoscopy now getting closer to histology by providing details of mucosal and vascular architecture. 9 Recently we have developed, validated and reproduced the first VCE score the Paddington international virtual ChromoendoScopy ScOre (PICaSSO) and shown that it strongly correlates with five most commonly used histological indices predicting histologic remission. 11 , 13 , 14 , 15 This new score addresses the need for a definition of endoscopic findings of MH not just as absence of inflammatory lesions and ulcers but it accurately reflects mucosal and vascular healing changes and correlates with histologic scores better than UC Endoscopic Index of Severity (Ulcerative Colitis Endoscopic Index Severity (UCEIS)) and MES 11 , 15 , 16
While several studies suggest that histologic remission predicts clinical outcomes better than endoscopic remission, this requires optimised prospective studies. 17 , 18 , 19 , 20 , 21 , 22 A recent meta‐analysis by Gupta 19 involving UC patients in endoscopic remission showed that persistent histologic activity is associated with higher rates of relapse. However, the magnitude of benefit in treating UC patients by achieving the more rigorous combination of endoscopic and histologic remission is still controversial. 3 , 22 , 23 A recent retrospective study involving 269 patients with UC in endoscopic remission showed that histologic remission had no additional impact on time to relapse. 8 A systematic review by Yonn 24 reported that UC patients achieving endoscopic and histologic remission had a favourable clinical outcome with substantially lower risk of clinical relapse compared with patients in only clinical remission. In a recent sub‐analysis of endpoints of the UNIFI study, 6 the combined endoscopic ‐ histologic endpoint was superior to endoscopy or histology endpoints individually in predicting inflammatory activity after maintenance with ustekinumab in moderate‐severe UC. 6 However, the endoscopic endpoint used is endoscopic improvement rather than remission.
By utilizing data from our prospective multicentre international study 15 we aimed to investigate the performance of the combination of endoscopic and histologic remission for predicting specified clinical outcomes over 6 and 12 months in comparison with endoscopic remission alone by using the new VCE ‐PICaSSO along with several endoscopic and histological scores in patient with UC.
PATIENTS AND METHODS
The study was approved by Research Ethics Committee (17/WM/0223) for the UK centres and all international sites obtained local research ethics committee approvals in their respective regions and countries. All patients provided written informed consent to take part to the study.
Patient cohort
The prospective study was performed in 11 international centres between September 2016 and November 2019. A cohort of IBD patients (age ≥18 and ≤ 80 years),who were enrolled in the international multicentre real‐life study of VCE score PICaSSO with an established diagnosis of UC for ≥1 year, was analysed. The characteristics of this cohort and methodology has been reported in details recently. 15 The patients underwent colonoscopy for assessment of activity or surveillance of UC. With regards to clinical activity of disease at baseline at the time of recruitment, for the first 20 patients, each participating site included quiescent, mild, moderate, and severe inflammatory activity based on the clinical partial Mayo score 25 (0–1 = quiescent, 2–4 = mild, 5–6 = moderate, 7–9 = severe). Subsequently, to assess the ability of PICaSSO to predict HR, sites were asked to recruit (n = 20) patients with mainly mild/quiescent disease (clinical partial Mayo score 0– 4). Details of recruitment protocol are published elsewhere. 15
Exclusion criteria were inability to provide consent, patients with unclassified colitis, Crohn's colitis, ischaemic colitis or infectious colitis, presence of serious co‐morbidities, toxic megacolon, pregnancy or breast feeding, contraindication to biopsies and Boston bowel preparation score <2 in the rectum or sigmoid colon.
Study objectives and outcomes
The primary objective was to determine the rates of specified clinical outcomes by considering a composite of endoscopic with histologic remission versus endoscopic or histological remission alone at 12 months. The definition of specified clinical outcomes was clarified in the designated paragraph below.
The outcome measure was the incremental clinical benefit of combined endoscopic ‐histologic MH/remission assessed by specific histological scores (Robarts Histological index (RHI)) ≤3 with no neutrophils in epithelium or lamina propria 26 or Nancy Histological index (NHI) ≤1 27 among patients with endoscopic remission defined by specific endoscopic scores [MES = 0, 25 UCEIS ≤1, 28 , 29 PICaSSO ≤3 15 ] compared with each measure alone.
Endoscopy assessment
Endoscopic assessment was performed by designated endoscopists (MI, PB, JF, MG, BH, ML, APB, LP, TR, GT, RB) experienced in IBD and optical diagnosis and well trained in VCE (i‐Scan imaging). At the time of the colonoscopy, data collected included demographic details, duration of disease, extent of colitis and current/previous medication history. These data were recorded on case report forms before being transferred to REDCap (The Vanderbilt University, Nashville, Tennessee, USA). Patients were followed‐up over 12 months and protocol specified clinical outcomes (hospitalization, colectomy, initiation or change of steroids or change of other therapies such as immunosuppressants or biologics) were collected at 6 and 12 months.
Examinations at all centres were performed using HD Pentax (Tokyo, Japan) 7010 processor and three (iScan1, iScan2 and iScan3) modes by simply switching in real time the button of the handpiece of the endoscope. The standardized settings used have been reported recently. 15 Endoscopic activity was assessed first with HD‐WLE using MES, 25 UCEIS 28 followed by VCE and PICaSSO score. 15 Details of PICaSSO score are shown in Table S1.
All the endoscopies were video recorded and included the target site of biopsies which corresponded to the area scored by the endoscopists. 11 expert endoscopists scored high quality videos independently; the intra‐class correlation coefficient (ICC) for the MES, UCEIS total and PICaSSO total score have been previously reported in our recent publication including methodology. 15
Histological assessment and definition of endo‐histologic mucosal healing:
During endoscopy protocol defined two targeted biopsies were taken from the worst or the most representative area of endoscopic healing or active inflammation assessed by expert endoscopists in the rectum and sigmoid colon. For each biopsy the histological activity was scored by using RHI and NHI and the worst was used for analysis 30 (Figure 1). The definitions of endoscopic‐histological MH/remission based on selected endoscopic and histological scores are summarized in Table 1.
FIGURE 1.
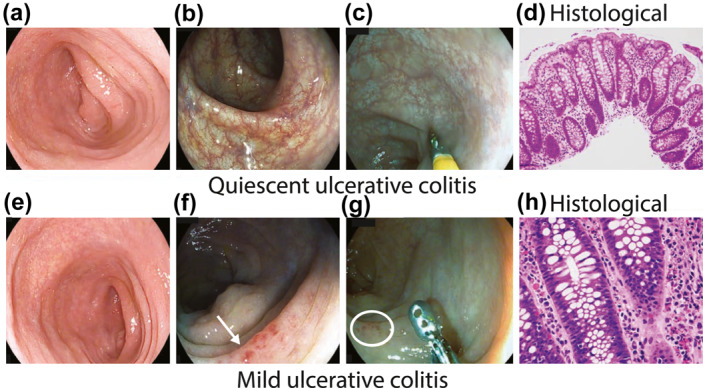
(a–c) quiescent ulcerative colitis (UC) assessed by HD‐white light endoscopy (a); i‐scan modes 2 (b); i‐scan modes 3 (c); histology showing minimal architectural distortion of crypts and no active inflammatory infiltrate in lamina propria (d); (e, f) mild UC assessed by HD‐white light endoscopy (e); i‐scan modes 2 showing vessels with dilatation (arrow) (f); i‐scan modes 3 showing micro erosions (circle) (g); histology showing architectural distortion of crypts and focal active inflammatory infiltrate in lamina propria (h)
TABLE 1.
Definition of composite endoscopic and histological remission determined by selected endoscopic and histological scores
| Endoscopic/Histological scores | Endoscopic remission | Histological remission | Endo‐histological remission |
|---|---|---|---|
| MES/RHI | MES 0 | RHI ≤3 | MES 0‐RHI ≤ 3 |
| MES/NHI | MES 0 | NHI ≤1 | MES 0‐NHI ≤ 1 |
| UCEIS/RHI | UCEIS ≤1 | RHI ≤3 | UCEIS ≤1 ‐RHI ≤3 |
| UCEIS/NHI | UCEIS ≤1 | NHI ≤1 | UCEIS ≤ 1‐NHI ≤ 1 |
| PICaSSO/RHI | PICaSSO ≤3 | RHI ≤3 | PICaSSO ≤ 3‐RHI ≤3 |
| PICaSSO/NHI | PICaSSO ≤3 | NHI ≤1 | PICaSSO ≤ 3‐NHI ≤ 1 |
Abbreviations: MES, Mayo endoscopic score; NHI, Nancy histological index; PICaSSO, Paddington international virtual ChromoendoScopy ScOre; RHI, Robarts Histological index; UCEIS, ulcerative colitis endoscopic index severity.
All biopsies were fixed in 10% formalin and then processed at institutional pathology laboratories in routine embedding and staining protocol. The haematoxylin‐eosin stained slides of the biopsies were digitized using high‐speed slide scanners by participating centres to allow central reading. Five pathologists (DZ‐UK, MV‐Germany, VV‐Italy, GDH‐Belgium and GX‐USA/Canada), who were blinded to patients' clinical features and endoscopic activity performed histological analysis. Each slide was scored using RHI and NHI. The inter‐rater agreement between the pathologists was formally assessed and it was almost perfect, as reflected by ICC (ICCs): RHI 0.77 (95% CI 0.69–0.85), NHI 0.85 (95% CI 0.79–0.90). Any discrepancy was discussed among the pathologists before determining the final diagnosis.
The rectal and sigmoid biopsies were used for Cox proportional hazards model and Z‐test for comparison of two and three proportions (data of sigmoid biopsies provided in the Supporting Information).
Specified clinical outcomes:
Prospectively specified clinical adverse outcomes at 6 and 12 months follow‐up was defined as (i) hospitalization as a result of UC relapse (ii) colectomy, (iii) initiation or changes in medical therapy including steroids, immunomodulators and biologics due to UC relapse. All the clinical outcome events were recorded through telephone calls and clinical records assessment at 6 and 12 months after colonoscopy. Such outcome measures have been used in studies such as REACT 31 and CALM extension 32 studies in Crohn's disease and in observational follow up studies in UC. 4
Statistical analysis
Statistical analysis was predominantly done with a statistical software R [R Core Team (2019), https://www.R‐project.org/]. In order to compare the rates of specified clinical outcomes (hospitalization, colectomy, initiation or changes in medical therapy due to UC relapse) predicted by the score cut‐offs we used two‐ and 3‐sample two‐sided test for equality of proportions. The corresponding binomial 95% confidence intervals were computed. We used Cox proportional hazards model within an R‐package survival 33 to create survival curves for patients in endoscopic remission without histologic remission and both endoscopic and histologic remission according to different endoscopic and histological scores. Each pair of curves was compared using likelihood ratio test with significance level at 0.05. The corresponding hazard ratios were reported. To assess the inter‐rater agreement of the histological scourings, we used one‐way ICC coefficient by means of R package irr (http://cran.r‐project.org/package=irr). According to Landis and Koch benchmarks, 37 ICC of <0.2, 0.2–0.4, >0.4–≤0.6, >0.6–0.8, and >0.8 was considered ‘poor’, ‘fair’, ‘moderate’, ‘good’, ‘substantial’, and ‘almost perfect’, respectively. To assess the role of disease extent for predicting clinical outcome 2‐sample two‐sided test for equality of proportions was used and the difference was not significant for p > 0.05. Multiple univariate regressions (for each endoscopic and histology score) as well bivariate regression for endo‐histologic scores were further performed to look at independent predictors of specified clinical adverse outcomes at 12 months.
All results were exported from REDCap to STATA Version 14 [StataCorp]. Mean ± SD and median ± interquartile range were determined on continuous variables. In the original study 11 we estimated whether we have adequate sample size to detect a difference in outcomes at 6 and 12 months follow up. We accepted a relapse rate of 10% for Mayo score 0 based on the results of Barreiro‐de Acosta et al 34 and we assumed 6.4% for PICaSSO score cut‐off that best predicted HR. 13 For this study, to demonstrate a 5% margin between endoscopic remission and endo‐histologic remission in predicting specified clinical outcome events one would need 270 patients.
RESULTS
Patient demographics:
The study prospectively recruited 307 patients with UC from 11 centres in the international PICaSSO study. The final analysis consisted of 302 patients as five patients were excluded due to missing data (Table 2). A total of 289 and 270 patients completed 6 and 12 months of follow‐up after colonoscopy. 15 Overall 32 patients withdrew at month 12 because of noncompliance with follow‐up contact with investigator team.
TABLE 2.
Baseline patient demographics of the study cohort
| Characteristics | Patients (n = 307) |
|---|---|
| Age (y) mean ± sd | 48.4 ± 14.8 |
| Gender male n (%) | 182 (59.3%) |
| Disease duration (y) mean ± sd | 15.0 ± 10.8 |
| Extension of disease n (%) | |
| Left‐sided colitis | 130 (42.3%) |
| Extensive or pan colitis | 172 (56.0%) |
| Missing data a | 5 (1.6%) |
| Therapy at time of colonoscopy n (%) | |
| No treatment | 14 (4.6%) |
| 5‐ASA | 234 (76.2%) |
| Corticosteroids | 74 (24.1%) |
| Immunomodulators | 68 (22.1%) |
| Biologics | 118 (38.4%) |
| Endoscopic activity | |
| Mayo endoscopic score n (%) | |
| Mayo 0 | 168 (54.7%) |
| Mayo 1 | 47 (15.3%) |
| Mayo 2 | 56 (18.2%) |
| Mayo 3 | 31 (10.1%) |
| Missing data a | 5 (1.6%) |
| UCEIS rectum n (%) | |
| Remission (≤1) | 209 (68.1%) |
| Mild (2–4) | 62 (20.2%) |
| Moderate (5–7) | 33 (10.7%) |
| Severe (>7) | 1 (0.3%) |
| Missing data a | 2 (0.6%) |
| UCEIS sigmoid n (%) | |
| Remission (≤1) | 219 (71.3%) |
| Mild (2–4) | 62 (20.2%) |
| Moderate (5–7) | 21 (6.8%) |
| Severe (>7) | 3 (1.0%) |
| Missing data a | 2 (0.6%) |
| PICaSSO Score rectum n (%) | |
| Remission (≤3) | 220 (71.7%) |
| Active (>3) | 85 (27.7%) |
| Missing data a | 2 (0.6%) |
| PICaSSO Score sigmoid n (%) | |
| Remission (≤3) | 229 (74.6%) |
| Active (>3) | 76 (24.8%) |
| Missing data a | 2 (0.6%) |
Missing data: These patients were not included in the overall analysis (302 patients analysed) due to solid stool present which preclude endoscopy assessment. 15 patients (4.9%) received non‐steroidal anti‐inflammatory drugs.
The median age was 48 (range 19–77) years and 182 (59.3%) were men. The mean duration of disease was 15.0 (SD 10.8) years. 172 (56.0%) had extensive (E3) colitis, while 130 (42.3%) had left‐side (E2) colitis and none of the patients had Montreal E1 disease. At the time of endoscopic assessment, 234 patients (76.2%) were on 5‐ASA, 74 (24.1%) on corticosteroids, 68 (22.1%) on immunomodulators, 118 (38.4%) on biologics and 14 (4.6%) were not on any UC treatment. None were on any topical therapies.
In the rectum, 168 (54.7%), 209 (68.1%) and 220 (71.7%) patients were in endoscopic remission by MES 0, UCEIS ≤1 and PICaSSO ≤3 respectively. In the rectum, 207 (67.4%) and 181 (59%) of patients were in histologic remission by RHI ≤3 and NHI ≤1 respectively (Tables 2 and 3).
TABLE 3.
Summarizes the definition of endoscopic, histologic and combined endoscopic and histologic remission based on the scores used
| Endoscopic score/Histological score | ER | ER no HR | HR no ER | HR and ER |
|---|---|---|---|---|
| MES 0/RHI≤ 3 | 168 (54.7%) | 6 (3.6%) | 51 (24.6%) | 156 (50.8%) |
| MES 0/NHI ≤1 | 168 (54.7%) | 28 (16.6%) | 42 (23.2%) | 139 (45.3%) |
| UCEIS ≤1/RHI ≤3 | 209 (68.1%) | 52 (24.8%) | 10 (4.8%) | 186 (60.6%) |
| UCEIS ≤1/NHI ≤1 | 209 (68.1%) | 41 (19.6%) | 17 (9.4%) | 163 (53.1%) |
| PICaSSO ≤3/RHI ≤3 | 220 (71.7%) | 9 (4.09%) | 9 (4.3%) | 198 (64.5%) |
| PICaSSO ≤3/NHI ≤1 | 220 (71.7%) | 41 (18.6%) | 6 (3.3%) | 175 (57.0%) |
Note: It provides the rates of patients who have both histological and endoscopic (HR and ER), endoscopic remission alone with persistent histologic activity (ER no HR) and vice versa (HR no ER).
Abbreviations: ER, endoscopic remission; MES, Mayo endoscopic score; NHI, Nancy histological index; PICaSSO, Paddington international virtual ChromoendoScopy ScOre; RHI, Robarts Histological index; UCEIS, ulcerative colitis endoscopic index severity.
Of the 270 patients who completed 12 months of clinical follow‐up after colonoscopy the proportion of patients with endoscopic, histologic and combined endoscopic and histologic remission in the rectum at the start of follow up are provided in Table S2. The equivalent sigmoid data for endoscopic, histologic remission (ER, HR) and combined endoscopic and histologic remission of 270 patients who completed 12 months clinical follow up are shown in the Table S3. 289 patients completed 6 months follow‐up.
Combined endoscopic and histologic remission in predicting specified clinical outcomes (Figure 2)
FIGURE 2.
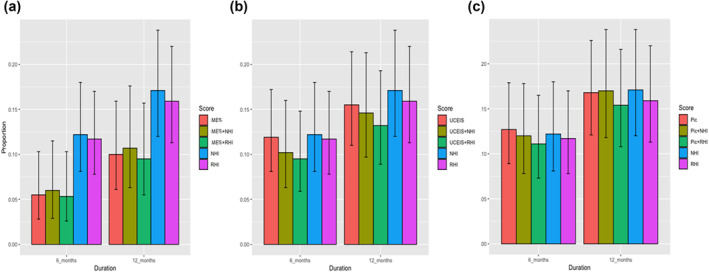
Bar graphs showing proportions of patients in endoscopic remission, combined endoscopic and histologic remission, and histologic remission assessed by using Mayo Endoscopic Score 0 (a); Ulcerative Colitis Endoscopic Index Severity ≤1 (b); Paddington International virtual ChromoendoScopy ScOre ≤3 (c) in rectum
MES: The three‐proportion analysis (endoscopic remission vs. endoscopic and histologic remission vs. histologic remission) showed a significant difference in predicting specified clinical outcome between MES 0 Versus MES 0 + RHI ≤3 Versus RHI ≤3 in rectum at 6 months (p = 0.04), whereas it was not significant at 12 months (p = ns). For NHI, there was a significant difference between MES 0 Versus MES 0 + NHI≤1 Versus NHI≤1 in the rectum at 6 months (p = 0.04), whereas it was not significantly different at 12 months (p = ns).
The pairwise comparisons of MES 0 Versus MES 0+ RHI≤3 and MES 0+ RHI≤3 Versus RHI ≤3 in predicting specified clinical outcomes was not significant in the rectum at 6 and 12 months (p = ns). With regards to NHI ≤1, the pairwise comparison of combination of MES 0 + NHI ≤1 was superior to NHI ≤1 alone at 6 months in the rectum (p = 0.01), but not at 12 months.
UCEIS: In three‐proportion analysis, no significant difference was observed between UCEIS ≤1 Versus UCEIS ≤1 + RHI ≤3 Versus RHI ≤3 in the rectum at 6 and 12 months (p = 0.ns) in predicting specified clinical outcomes. With regards to the NHI, the comparison of three proportions between UCEIS ≤1 Versus UCEIS ≤1 + NHI ≤1 Versus NHI ≤1 was significant in the rectum at 6 months (p = 0.04) but not at 12 months in predicting specified clinical outcomes.
The pairwise comparison of the combined endo‐histologic remission defined as UCEIS + RHI≤ 3 was superior to RHI ≤3 in the rectum (p = 0.01) at 6 months in predicting specified clinical outcomes. However, at 12 months, the differences between UCEIS ≤1 Versus UCEIS ≤1 + RHI≤ 3 or UCEIS≤1 + RHI≤ 3 Versus RHI ≤3 were not significant in the rectum (p = ns). There was no significant difference between UCEIS + NHI≤1 Versus UCEIS ≤1 or versus NHI ≤1 at 6 months or 12 months in the rectum in predicting specified clinical outcomes.
PICaSSO: In three‐proportion analysis, no significant difference was found between the composite PICaSSO ≤3 + RHI≤3 Versus PICaSSO ≤3 Versus RHI ≤3 in the rectum at six or 12 months in predicting specified clinical outcomes (p = ns). Similarly, for NHI, the comparison of PICaSSO ≤3 +NHI ≤1 Versus PICaSSO ≤3 Versus NHI ≤1 was not significant in the rectum either at 6 months or at 12 months in predicting specified clinical outcomes.
The pairwise comparisons of the combined endo‐histologic remission, PICaSSO ≤3 + RHI<3 Versus PICaSSO ≤3 and PICaSSO ≤3 + RHI<3 Versus RHI ≤3 were not significantly different in predicting specified clinical outcomes in the rectum at 6 and 12 months (p = ns). The same was true for PICaSSO ≤3 +NHI ≤1 Versus PICaSSO ≤3 and PICaSSO ≤3 +NHI ≤1 Versus NHI ≤1 at 6 and 12 months in the rectum (Figure 2). The equivalent data for sigmoid colon are shown in Figure S1.
A further analysis showed that there was no significant difference in terms of adverse clinical outcomes at 6 and 12 months between all patients in combined endo‐histologic remission assessed by selected endoscopic (MES, UCEIS, PICaSSo) and histological scores (RHI, NHI), those with extensive/pancolitis and those with left‐sided colitis p > 0.05.
Multivariate analysis of endoscopy, endoscopy + histology for the specified clinical outcome
Multiple univariate regressions (for each endoscopic and histology score) as well bivariate ones for endo‐histologic scores were further performed for predicting specified clinical outcomes. All endoscopic and histological scores were significant for predicting specified clinical outcome (Table 4). With regards to bivariate analysis all endoscopic scores were significant whilst histological scores were not significant (Table 5).
TABLE 4.
Multiple univariate regressions (for each endoscopic and histology score in rectum and sigmoid colon) to predict specified clinical outcomes at 12 months
| Regression variable | OR, 95% CI | p‐value |
|---|---|---|
| MES | 2.879 (2.177, 3.88) | <10−3 |
| PICaSSO total score rectum | 1.231 (1.159, 1.314) | <10−3 |
| PICaSSO total score sigmoid | 1.266 (1.178, 1.370) | <10−3 |
| UCEIS rectum | 1.672 (1.435, 1.973) | <10−3 |
| UCEIS sigmoid | 1.809 (1.523, 2.183) | <10−3 |
| NHI rectum | 1.829 (1.501, 2.252) | <10−3 |
| NHI sigmoid | 1.900 (1.544, 2.364) | <10−3 |
| RHI rectum | 1.265 (1.169, 1.374) | <10−3 |
| RHI sigmoid | 1.281 (1.181, 1.396) | <10−3 |
Abbreviations: CI, confidence interval; MES, Mayo endoscopic score; NHI, Nancy histological index; OR, odds ratio; PICaSSO, Paddington international virtual ChromoendoScopy ScOre; RHI, Robarts Histological Index, UCEIS, ulcerative colitis endoscopic index of severity.
TABLE 5.
Bivariate regression analysis for endo‐histologic scores using MES, UCEIS and PICaSSO combined with RHI and NHI in prediction of specified clinical outcomes
| Regression variables | OR, 95% CI | p‐value |
|---|---|---|
| MES | 2.558 (1.739, 3.840) | <10−3 |
| NHI, rectum | 1.114 (0.832, 1.486) | 0.464 |
| MES | 2.502 (1.742, 3.656) | <10−3 |
| RHI, rectum | 1.058 (0.951, 1.177) | 0.301 |
| MES | 2.394 (1.703, 3.427) | <10−3 |
| NHI, sigmoid | 1.263 (0.967, 1.653) | 0.086 |
| PICaSSO total rectum | 1.186 (1.082, 1.308) | <10−3 |
| NHI¥, rectum | 1.179 (0.857, 1.612) | 0.303 |
| PICaSSO total rectum | 1.189 (1.088, 1.307) | <10−3 |
| RHI, rectum | 1.065 (0.943, 1.200) | 0.305 |
| PICaSSO total sigmoid | 1.178 (1.073, 1.301) | 0.001 |
| NHI, sigmoid | 1.371 (1.028, 1.825) | 0.030 |
| PICaSSO total sigmoid | 1.180 (1.076, 1.301) | 0.001 |
| RHI, sigmoid | 1.130 (1.014, 1.260) | 0.026 |
| UCEIS, rectum | 1.482 (1.202, 1.854) | <10−3 |
| NHI, rectum | 1.252 (0.938, 1.665) | 0.123 |
| UCEIS rectum | 1.504 (1.217, 1.884) | <10−3 |
| RHI, rectum | 1.081 (0.963, 1.212) | 0.181 |
| UCEIS sigmoid | 1.595 (1.275, 2.027) | <10−3 |
| NHI sigmoid | 1.261 (0.942, 1.683) | 0.116 |
| UCEIS sigmoid | 1.585 (1.268, 2.013) | <10−3 |
| RHI sigmoid | 1.098 (0.982, 1.228) | 0.098 |
Abbreviations: CI, confidence interval; MES, Mayo endoscopic score; NHI, Nancy histological index; OR, odds ratio; PICaSSO, Paddington international virtual ChromoendoScopy ScOre; RHI, Robarts Histological Index, UCEIS, ulcerative colitis endoscopic index of severity.
Analysis of survival without any events specified as clinical outcomes for combination of endoscopic and histologic remission: survival curves at 12 months follow‐up
Cox proportional hazard models were plotted to compare probability of survival without specified clinical outcome events in those with combined endo‐histologic remission Versus those with endoscopic remission without histologic remission.
For rectal assessments, there was no significant survival advantage for specified clinical outcome events for either MES 0 + RHI ≤3 Versus MES 0 + RHI >3 (HR 0.42, 95% CI 0.09, 1.9; p = 0.3) or MES 0 + NHI ≤1 Versus MES + NHI >1 (HR 1.4, 95% CI 0.31, 6.28; p = 0.6) at 12 months.
There was however a significant survival advantage without specified clinical outcome events for UCEIS ≤1 + RHI ≤3 Versus UCEIS ≤1 + RHI >3 in the rectum (HR 0.30, 95% CI 0.12, 0.75, p = 0.02) at 12 months. While there was no significant survival advantage for UCEIS ≤1 + NHI ≤1 Versus UCEIS ≤1 + NHI >1 at 12 months in the rectum (HR 0.79, 95% CI 0.34, 1.87; p = 0.6).
With regards to PICaSSO, there was no difference in survival without specified clinical outcomes between PICaSSO ≤3 + RHI ≤3 Versus PICaSSO ≤3 + RHI >3 in the rectum (HR 0.42, 95% CI 0.16, 1.11; p = 0.1) at 12 months. Similarly, there was no significant survival advantage for PICaSSO ≤3 + NHI≤1 Versus PICaSSO ≤3 + NHI >1 in the rectum (HR 1.03, 95% CI 0.42, 2.52; p = 0.9) at 12 months (Figure 3)
FIGURE 3.
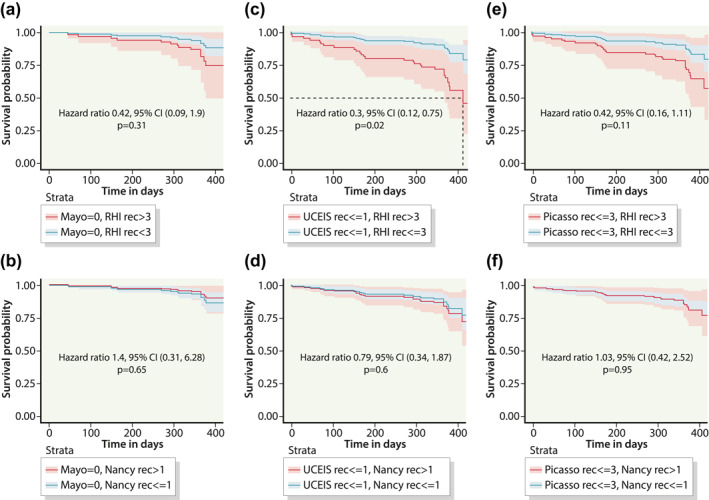
Cox proportional hazard curves in predicting likelihood of survival without specified outcome events at 12 months in the rectum (a) Mayo Endoscopic Score (MES) 0 + Robarts Histological index (RHI) ≤3 (blue) versus MES 0 + RHI ≥3 (red); (b) MES 0 + Nancy Histological index (NHI) ≤1 (blue) versus MES 0 + NHI ≥1 (red); (c) Ulcerative Colitis Endoscopic Index Severity (UCEIS) ≤1 + RHI ≤3 (blue) versus UCEIS ≤1+ RHI ≥3 (red); (d) UCEIS ≤1 + NHI ≤1 (blue) versus UCEIS ≤1 + NHI ≥1 (red); (e) Paddington International virtual ChromoendoScopy ScOre (PICaSSO) ≤3 + RHI ≤3 (blue) versus PICaSSO ≤3 + RHI ≥3 (red); (f) PICaSSO ≤3 + NHI ≤1 (blue) versus PICaSSO ≤3 + NHI ≥1 (red)
Further Cox proportional hazard model survival analysis of the combination of endoscopic and histologic remission Versus endoscopic remission without histologic remission at 12 months in the sigmoid colon are presented in the Figure S2.
In summary, pairwise, 3‐proportion analysis and Cox proportional hazard model survival analysis demonstrated that VCE PICaSSO endoscopic score alone predicted specified clinical outcomes as well as a combination of endoscopic and histologic remission over a 12 months follow‐up.
DISCUSSION
The combination of histologic and endoscopic improvement has been proposed to be a more ‘complete’ measure of colonic MH as it includes complementary information from both endoscopy and histology assessments blended into a single outcome measure. This is already being used in clinical trials as an exploratory outcome. 6
The current treatment targets by International Organization for the study of Inflammatory Bowel Disease consensus have recently been revised. 35 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) II retains clinical and endoscopic remission as main targets in UC treatment. 35 The discrepancy between endoscopy and histology reported in several studies 11 have led to hot debate regarding the target of possible ‘deep remission’ associated with better clinical outcomes than endoscopic remission alone. Precise and objective disease related measures is becoming crucial in drug development and clinical management. 16
In our study, endoscopic remission defined by recently developed PICaSSO VCE score accurately predicts specified clinical outcomes at 12 months; when combining histologic assessment defined by either RHI or NHI to PICaSSO this does not improve the accuracy of the prediction. Thus, PICaSSO score alone could be sufficient to characterize deep MH (endoscopic and histological remission).
Survival analysis at 12 months using the Cox proportional hazards model did not show any difference in survival without specified clinical outcomes between composite PICaSSO and histologic remission and endoscopic remission alone determined by using PICaSSO (Figure 3). This may be the consequence of very strong correlation between PICaSSO score and several histology scores reported recently including RHI and NHI. 15 Accordingly, for the PICaSSO endoscopic score, histologic remission does not add incremental advantage in predicting outcomes and therefore combination of endoscopic and histologic remission does not demonstrate an advantage. Therefore, a single measure of endoscopic remission defined by PICaSSO ≤3 may be sufficient for prediction of clinically relevant outcomes.
Conversely, for endoscopic remission assessed with MES by using HD endoscopes and defined by the more rigorous MES = 0 in combination with histologic remission defined by either RHI or NHI, there was statistically significant difference in predicting specified clinical outcomes at 6 months but not at 12‐month follow‐up (Figure 2). Ulcerative Colitis Endoscopic Index Severity results were different in some aspects and depended on whether UCEIS remission was combined with NHI or RHI defined histologic remission in pairwise comparison or in Cox proportional hazard model survival analysis.
While a meta‐analysis has suggested composite endpoints of endo‐histologic improvement (alternatively designated as endoscopic‐histologic MH) as a preferable endpoint which correlated with substantially lower risk of clinical relapse, 24 there was substantial limitations due to heterogeneity from the different studies published with different definition of endoscopic and histologic remission and different use of scores, outcome measures and duration of follow up. 18 , 23 , 36 , 37 Apart from choosing a realistic endpoint aligned with current therapeutic efficacy, the data for combining endoscopy and histology has to use optimum endoscopy definitions and technology and especially evaluate incremental benefit of histology over endoscopic remission and not just endoscopic improvement.
An expert panel of gastroenterologists and pathologists have recently developed standardisation and recommendations to address the heterogeneity in biopsy acquisition, measurement tools, item definitions for histologic activity, and thresholds for classifying histologic response and remission. 38 In addition a recent ECCO position paper, in the attempt to harmonize the approach to UC histopathology, has proposed that randomized control trials use of the RHI or NHI. 20 Hence we have not included in this current study other histological score such as Villanacci 39 and the Extent Chronicity, Activity, Plus score, 40 which has been investigated in our previous study. 15
However a recent large study has reported that for patients already in endoscopic remission, histologic remission did not provide additional outcomes benefits. 8
Our large prospective multicentre study does not support that more rigorous endpoint such as the composite endo‐histology improvement/MH shows overall advantages in predicting specified clinical outcomes after 12 months in patients with UC, especially when the PICaSSO is used to define endoscopic remission (not improvement). This was valid even when only MES was combined with histology scores at 12 months. However, our study deliberately recruited more quiescent patients at baseline, unlike studies which had all actively inflamed UC at baseline and hence caution is required in interpreting our results. It is noteworthy that all the endoscopic assessments were done by experts using HD endoscopy as well as histology slides were examined by experts. 15
Our results were in contrast with the data published in connection with ustekinumab in UC trial (UNIFI) where achievement of histo‐endoscopic MH after induction therapy was associated with lower disease activity at the end of maintenance therapy than either histologic or endoscopic improvement alone and better outcomes. 6 However, the endoscopic criteria was improvement MES = 0/1/and not complete remission as we have considered in our study. However, unlike UNIFI 6 our study had variable baseline drugs and a large proportion of quiescent patients.
Several studies suggested that there might be patchiness in the distribution of inflammatory infiltrate especially in quiescent/mild UC 41 , 42 , 43 , 44 and the outcomes of partial MH is still unexplored. In this study we specifically targeted the biopsies where endoscopic assessment was done. The variability and discrepancy between endoscopy and histology may be also influenced by the different endoscopic scoring system used as these may not include a clear and validated definition of MH and not as well aligned with histology. 42 , 43 , 44 In addition, this variability is exaggerated by the different performance of endoscopists and pathologists and their training. 45 The reason that endoscopic remission defined by VCE score PICaSSO may be accurate in prediction of MH is likely due to comprehensive definition of mucosal and vascular healing and targeted biopsies in the study and its interobserver agreement.
Recently we have reported that correlation between the PICaSSO endoscopic score and histologic scores RHI and NHI were superior to correlations with MES and UCEIS; PICaSSO score of ≤3 was associated with histological remission with a high degree of accuracy. 15 These observations confirm that the endoscopic scores of inflammatory activity used in UC (MES and UCEIS) that were developed with previous generation of endoscopes may have limitations.
This current study has several strengths. Firstly, this study used endoscopic assessment with new generation VCE scopes which getting closer to histology and it allows an accurate evaluation of both mucosal and vascular patterns. As reported by us recently, PICaSSO correlated very strongly with histological activity and better than MES and UCEIS. 15 In addition, endoscopic and histologic remission were assessed by experts with almost perfect kappa agreement. Indeed, the one‐way ICC between raters was 0.88 (95% CI 0.83‐0.92) for the overall PICaSSO score, as compared with MES 0.82 (95% CI 0.74‐0.88) and UCEIS total score 0.84 (95% CI 0.77‐0.87). 15
Targeted biopsies were taken in the same areas scored by the endoscopists and the histological specimens were evaluated by experienced histopathologists in blinded fashion. The involvement of several endoscopist and pathologists improved generalizability of our results and kappa agreement was excellent as reported recently in a multicentre international study. 15 Targeted biopsies using VCE can be easily used during endoscopy as we described recently and may ensure better correlation with histology which was a key finding of our recent report. 15 Furthermore, our definition for histologic improvement (NHI ≤1 and RHI ≤3 no neutrophils) is in accordance with a recently published European Crohn's and Colitis Association (ECCO) position paper on UC histopathology in which the absence of intraepithelial neutrophils, erosions and ulceration are required for histological remission. 20
With the STRIDE II recommendations, 35 histological remission is being considered as an important adjunctive measurement of ‘deep healing’, but our study shows that advanced endoscopic scores such as VCE PICaSSO or even HD endoscopic assessment were fairly accurate in predicting specified clinical outcomes. The specific clinical outcomes used in the PICaSSO study has been widely used in studies relating to baseline endoscopic and histologic scores both in UC 4 and in Crohn's disease 28 and indeed discrepancy between endoscopy and histology are based on such studies 46
Nevertheless, our study has several limitations that need to be considered. First the involvement of endoscopists who were experienced in optical diagnosis. Therefore, it can be argued that the same levels of performance for endoscopy and histology cannot be reproduced amongst non‐experts not accustomed to VCE and standardized histological scores in a real‐life setting. We have previously shown that the level of performance can be reproduced even in non‐expert endoscopists and trainees by using a short training module in PICaSSO similar to that used in this study and also using Narrow Banding Imaging. 14 We did not follow‐up patients using patient reported outcomes, but used events indicative of specified clinical outcomes, such as hospitalisation, surgery or changes in therapy due to UC relapse. Patients were on different therapies at baseline as it has been the case with studies that showed large discrepancies between endoscopic remission and histological remission. 15 Recent studies have reported comparative differences between biologics using endoscopy improvement plus histologic remission endpoints. 47 Furthermore, we defined endoscopic remission and histological remission based on the assessment of the sigmoid colon and rectum only. We did not examine response to specific therapies by follow‐up endoscopies or biopsies and acknowledge the importance of change in neutrophilic infiltration as a predictor of long‐term response to biologics. 48 Changes in medications were confirmed by the investigator but not standard protocol was established to analyse the cause.
Recently Kaneshiro 49 et al. investigated the impact of the disease extension on the risk of clinical outcome. Importantly pancolonic assessment was strongly associated with relapse prediction in patients in clinical remission and the combination of pancolonic and histological evaluations represented the highest predictive value for the prognosis of UC patients. However, when we look at our patients in endo‐histologic remission who had left sided colitis or extensive/pancolitis and compared their specified clinical outcomes rates at 12 months we did not find significant difference. This is consistent with previous results that histologic and endoscopic findings in the left colon on colonoscopy had excellent accuracy for detecting pancolonic histologic remission, histologic normalization, endoscopic improvement, and endoscopic remission. 50
Two biopsies each were taken from rectum and sigmoid colon as defined in the protocol as rectosigmoid involvement indicates the worst affected disease location in UC.
In conclusion, endoscopic remission defined by VCE PICaSSO score predicts specified clinical outcomes as well as histological remission defined by RHI or NHI and combination of endoscopic and histologic assessment did not appear to improve the prediction in this study. If confirmed in further large‐scale studies, endoscopic remission (as opposed to improvement) assessed using PICaSSO score may be adequate as a single accurate outcome measure to predict clinical outcomes of interest; however for this prospective studies involving cohorts that are more homogenous at baseline is required.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Marietta Iacucci: Study conception and design, Data acquisition, Analysis and interpretation of data, Draughting of the manuscript, Critical revision of manuscript for important intellectual content.
Olga M Nardone: Study conception and design, Data acquisition, draughting of the manuscript, analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Alina Bazarova: Analysis and interpretation of data, Critical revision of manuscript for important intellectual content, Statistical analysis.
Pradeep Bhandari: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Rosanna Cannatelli: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Marco Daperno: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Jose Ferraz: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Martin Goetz: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Xianyong Gui: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Bu Hayee: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Gert De Hertogh: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Mark Lazarev: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Ji Li: Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Adolfo Parra‐Blanco: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Luca Pastorelli: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Remo Panaccione: Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Vincenzo Occhipinti: Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Timo Rath: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Samuel CL Smith: Data acquisition, Analysis and interpretation of data, Draughting of the manuscript, Critical revision of manuscript for important intellectual content.
Uday N Shivaji: Data acquisition, Critical revision of manuscript for important intellectual content.
Gian Eugenio Tontini: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Michael Vieth: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Vincenzo Villanacci: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Davide Zardo: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Raf Bisschops: Data acquisition, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Ralf Kiesslich: Study conception and design, Analysis and interpretation of data, Critical revision of manuscript for important intellectual content.
Subrata Ghosh: Study conception and design, Analysis and interpretation of data, Draughting of the manuscript, Critical revision of manuscript for important intellectual content.
Supporting information
Supporting Information S1
Figure S1
Figure S2
ACKNOWLEDGENT
MI is part‐funded by the NIHR Birmingham Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Nardone OM, Bazarova A, Bhandari P, Cannatelli R, Daperno M, Ferraz J, et al. PICaSSO virtual electronic chromendoscopy accurately reflects combined endoscopic and histological assessment for prediction of clinical outcomes in ulcerative colitis. United European Gastroenterol J. 2022;10(2):147–59. 10.1002/ueg2.12185
[Correction added on 02 March 2022, after first online publication: Title has been revised]
Olga Maria Nardone and Alina Bazarova contribute equally.
DATA AVAILABILITY STATEMENT
Data are available on request.
REFERENCES
- 1. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long‐term outcomes of patients with ulcerative colitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–55. [DOI] [PubMed] [Google Scholar]
- 2. Bessissow T, Lemmens B, Ferrante M, Bisschops R, Van Steen K, Geboes K, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107(11):1684–92. [DOI] [PubMed] [Google Scholar]
- 3. Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta‐analysis. Am J Gastroenterol. 2016;111 (12):1692–701. [DOI] [PubMed] [Google Scholar]
- 4. Bryant RV, Burger DC, Delo J, Walsh AJ, Thomas S, von Herbay A, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow‐up. Gut. 2016;65(3):408–14. [DOI] [PubMed] [Google Scholar]
- 5. Cushing KC, Tan W, Alpers DH, Deshpande V, Ananthakrishnan AN. Complete histologic normalisation is associated with reduced risk of relapse among patients with ulcerative colitis in complete endoscopic remission. Aliment Pharmacol Ther. 2020;51(3):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li K, Marano C, Zhang H, Yang F, Sandborn WJ, Sands BE, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterol. 2020;159(6):2052–64. [DOI] [PubMed] [Google Scholar]
- 7. Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. [DOI] [PubMed] [Google Scholar]
- 8. Narula N, Aruljothy A, Alshahrani AA, Fadida M, Al‐Saedi M, Marshall JK, et al. Histologic remission does not offer additional benefit for ulcerative colitis patients in endoscopic remission. Aliment Pharmacol Ther. 2020;52(11‐12):1676–82. [DOI] [PubMed] [Google Scholar]
- 9. Nardone OM, Cannatelli R, Zardo D, Ghosh S, Iacucci M. Can advanced endoscopic techniques for assessment of mucosal inflammation and healing approximate histology in inflammatory bowel disease? Therap Adv Gastroenterol. 2019;12:1756284819863015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iacucci M, Furfaro F, Matsumoto T, Uraoka T, Smith S, Ghosh S, et al. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: new technology, new era. Gut. 2019;68(3):562–72. [DOI] [PubMed] [Google Scholar]
- 11. Iacucci M, Cannatelli R, Gui X, Zardo D, Bazarova A, Gkoutos GV, et al. Assessment of endoscopic healing by using advanced technologies reflects histological healing in ulcerative colitis. J Crohns Colitis. 2020;14(9):1282–9. [DOI] [PubMed] [Google Scholar]
- 12. Iacucci M, Kiesslich R, Gui X, Panaccione R, Heatherington J, Akinola O, et al. Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy. 2017;49(6):553–9. [DOI] [PubMed] [Google Scholar]
- 13. Iacucci M, Daperno M, Lazarev M, Arsenascu R, Tontini GE, Akinola O, et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: the PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest Endosc. 2017;86(6):1118–27. [DOI] [PubMed] [Google Scholar]
- 14. Trivedi PJ, Kiesslich R, Hodson J, Bhala N, Boulton RA, Cooney R, et al. The Paddington International Virtual Chromoendoscopy Score in ulcerative colitis exhibits very good inter‐rater agreement after computerized module training: a multicenter study across academic and community practice (with video). Gastrointest Endosc. 2018;88(1):95–106. [DOI] [PubMed] [Google Scholar]
- 15. Iacucci M, Smith SCL, Bazarova A, Shivaji UN, Bhandari P, Cannatelli R, et al. An international multicenter real‐life prospective study of electronic chromoendoscopy score PICaSSO in ulcerative colitis. Gastroenterol. 2021;160(5):1558–69. [DOI] [PubMed] [Google Scholar]
- 16. Meserve J, Singh S. Pathologist, meet Picasso! Virtual chromoendoscopy for detecting histologic remission in ulcerative colitis. Gastroenterol. 2021;160(5):1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jangi S, Yoon H, Dulai PS, Valasek M, Boland BS, Jairath V, et al. Predictors and outcomes of histological remission in ulcerative colitis treated to endoscopic healing. Aliment Pharmacol Ther. 2020;52(6):1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lobaton T, Bessissow T, Ruiz‐Cerulla A, De Hertogh G, Bisschops R, Guardiola J, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: a prospective multicenter study. United European Gastroenterol J. 2018;6(5):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta A, Yu A, Peyrin‐Biroulet L, Ananthakrishnan AN. Treat to target: the role of histologic healing in inflammatory bowel diseases, A systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2020;19(9):1800–13. [DOI] [PubMed] [Google Scholar]
- 20. Magro F, Doherty G, Peyrin‐Biroulet L, Svrcek M, Borralho P, Walsh A, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020;14(11):1503–11. [DOI] [PubMed] [Google Scholar]
- 21. Narang V, Kaur R, Garg B, Mahajan R, Midha V, Sood N, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Int Res. 2018;16(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christensen B, Hanauer SB, Erlich J, Kassim O, Gibson PR, Turner JR, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15(10):1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calafat M, Lobaton T, Hernandez‐Gallego A, Manosa M, Torres P, Canete F, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig Liver Dis. 2017;49(12):1327–31. [DOI] [PubMed] [Google Scholar]
- 24. Yoon H, Jangi S, Dulai PS, Boland BS, Prokop LJ, Jairath V, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta‐analysis. Gastroenterol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317 (26):1625–9. [DOI] [PubMed] [Google Scholar]
- 26. Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, et al. Development and validation of a histological index for UC. Gut. 2017;66(1):50–8. [DOI] [PubMed] [Google Scholar]
- 27. Marchal‐Bressenot A, Salleron J, Boulagnon‐Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and validation of the Nancy histological index for UC. Gut. 2017;66(1):43–9. [DOI] [PubMed] [Google Scholar]
- 28. Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61(4):535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh A, Kormilitzin A, Hinds C, Sexton V, Brain O, Keshav S, et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis‐a prospective analysis. J Crohns Colitis. 2019;13(4):424–30. [DOI] [PubMed] [Google Scholar]
- 30. Pai RK, Khanna R, D'Haens GR, Sandborn WJ, Jeyarajah J, Feagan BG, et al. Definitions of response and remission for the Robarts histopathology index. Gut. 2019;68(11):2101–2. [DOI] [PubMed] [Google Scholar]
- 31. Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386(10006):1825–34. [DOI] [PubMed] [Google Scholar]
- 32. Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D'Haens GR, et al. Deep remission at 1 Year prevents progression of early crohn's disease. Gastroenterol. 2020;159(1):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. New York: Springer; 2000:ISBN 0‐387‐98784‐3. [Google Scholar]
- 34. Barreiro‐de Acosta M, Vallejo N, de la Iglesia D, Uribarri L, Baston I, Ferreiro‐Iglesias R, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. Journal of Crohn's & colitis. 2016;10(1):13–19. [DOI] [PubMed] [Google Scholar]
- 35. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterol; 2020;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 36. Pai RK, Hartman DJ, Rivers CR, Regueiro M, Schwartz M, Binion DG, et al. Complete resolution of mucosal neutrophils associates with improved long‐term clinical outcomes of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(11):2510–17. [DOI] [PubMed] [Google Scholar]
- 37. Battat R, Vande Casteele N, Pai RK, Wang Z, Zou G, McDonald JWD, et al. Evaluating the optimum number of biopsies to assess histological inflammation in ulcerative colitis: a retrospective cohort study. Aliment Pharmacol Ther. 2020;52(10):1574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma C, Sedano R, Almradi A, Casteele NV, Parker CE, Guizzetti L, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterol. 2021;160(7):2291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villanacci V, Antonelli E, Lanzarotto F, Bozzola A, Cadei M, Bassotti G. Usefulness of different pathological scores to assess healing of the mucosa in inflammatory bowel diseases: a real life study. Sci Rep. 2017;7(1):6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iacucci M, Fort Gasia M, Hassan C, Panaccione R, Kaplan GG, Ghosh S, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy. 2015;47(8):726–34. [DOI] [PubMed] [Google Scholar]
- 41. Christensen B, Hanauer SB, Gibson PR, Turner JR, Hart J, Rubin DT. Segmental histological normalisation occurs in ulcerative colitis but does not improve clinical outcomes. J Crohns Colitis. 2020;14(10):1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim B, Barnett JL, Kleer CG, Appelman HD. Endoscopic and histological patchiness in treated ulcerative colitis. Am J Gastroenterol. 1999;94(11):3258–62. [DOI] [PubMed] [Google Scholar]
- 43. Harpaz N, Ballentine S, Colombel JF, Sands BE, E Sands HM. Microscopic heterogeneity in ulcerative colitis: implications for microscopic measurement of disease activity. Gut. 2020;69(2):401–2. [DOI] [PubMed] [Google Scholar]
- 44. Bernstein CN, Shanahan F, Anton PA, Weinstein WM. Patchiness of mucosal inflammation in treated ulcerative colitis: a prospective study. Gastrointest Endosc. 1995;42(3):232–7. [DOI] [PubMed] [Google Scholar]
- 45. Bossuyt P, Bisschops R, Vermeire S, De Hertogh G. Variability in the distribution of histological disease activity in the colon of patients with ulcerative colitis. J Crohns Colitis. 2021;15(4):603–8. [DOI] [PubMed] [Google Scholar]
- 46. Travis SP, Higgins PD, Orchard T, Van Der Woude CJ, Panaccione R, Bitton A, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34(2):113–24. [DOI] [PubMed] [Google Scholar]
- 47. Peyrin‐Biroulet L, Loftus EV Jr., Colombel JF, Danese S, Rogers R, Bornstein JD, et al. Histologic outcomes with Vedolizumab versus adalimumab in ulcerative colitis: results from an efficacy and safety study of Vedolizumab intravenous compared to adalimumab subcutaneous in participants with ulcerative colitis (VARSITY). Gastroenterol. 2021;161(4):1156–67. [DOI] [PubMed] [Google Scholar]
- 48. Narula N, Wong ECL, Colombel JF, Riddell R, Marshall JK, Reinisch W, et al. Early change in epithelial neutrophilic infiltrate predicts long‐term response to biologics in ulcerative colitis. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):00716‐3. [DOI] [PubMed] [Google Scholar]
- 49. Kaneshiro M, Takenaka K, Suzuki K, Fujii T, Hibiya S, Kawamoto A, et al. Pancolonic endoscopic and histologic evaluation for relapse prediction in patients with ulcerative colitis in clinical remission. Aliment Pharmacol Ther. 2021;53(8):900–7. [DOI] [PubMed] [Google Scholar]
- 50. Jangi S, Holmer AK, Dulai PS, Boland B, Valasek M, Jairath V, et al. Spatial evolution of histologic and endoscopic healing in the left and right colon in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):00108‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Figure S1
Figure S2
Data Availability Statement
Data are available on request.


