Abstract
As a momentous energetic group, a nitro group widely exists in high-energy-density materials (HEDMs), such as trinitrotoluene (TNT), 1,3,5-triamino-2,4,6-trinitrobenzene (TATB), cyclo-1,3,5-trimethylene-2,4,6-trinitramine (RDX), etc. The nitro group has a significant effect on improving the oxygen balance and detonation performances of energetic materials (EMs). Moreover, the nitro group is a strong electron-withdrawing group, and it can increase the acidity of the acidic hydrogen-containing nitrogen-rich energetic compounds to facilitate the construction of energetic ionic salts. Thus, it is possible to design nitro-nitrogen-rich energetic compounds with adjustable properties. In this paper, the nitration methods of azoles, including imidazole, pyrazole, triazole, tetrazole, and oxadiazole, as well as azines, including pyrazine, pyridazine, triazine, and tetrazine, have been concluded. Furthermore, the prospect of the future development of nitrogen-rich heterocyclic energetic compounds has been stated, so as to provide references for researchers who are engaged in the synthesis of EMs.
Keywords: nitro group, nitration, nitrogen rich heterocycle, energetic materials
1. Introduction
Nitrogen-rich compounds have attracted widespread attention in energetic materials (EMs) because of their advantages of outstanding density, excellent positive enthalpy of formation, remarkable detonation performance, and high thermal stability [1,2,3]. They can be used in explosives, propellants, gas generators, and smokeless pyrotechnic fuels [4,5]. In order to meet the increasing performance requirements, new nitrogen-rich EMs are being developed with upgradable density, better detonation performance, lower impact and friction sensitivity, and higher thermal stability.
One of the most popular strategies for the design of promising new EMs is the incorporation of both fuel and oxidizer moieties into one molecule. [6,7] The nitro group is a pivotal explosive group, which exists in trinitrotoluene (TNT), 1,3,5-triamino-2,4,6-trinitrobenzene (TATB), cyclo-1,3,5-trimethylene-2,4,6-trinitramine (RDX), cyclo-1,3,5-7-tetramethylene-2,4,6,8-tetranitr-amine (HMX), and hexanitrohexaazaisowurtzitane (CL-20), etc. Nitro groups in the molecular structure can heighten the oxygen balance, increase the density, and significantly enhance the detonation performance of EMs [8,9]. Moreover, the nitro group is a strong electron-withdrawing group, and it can increase the acidity of hydrogen-containing nitrogen-rich energetic compounds and is conducive to the construction of energetic ionic salts [10]. Therefore, this paper reviews the nitrification methods for nitrogen-rich heterocyclic energetic compounds so as to give some suggestions for this special reaction.
The main skeletons of nitrogen-rich heterocyclic energetic compounds are azole rings (imidazole, pyrazole, triazole, tetrazole, oxadiazole) and azine rings (pyrazine, pyridazine, triazine, tetrazine) [11,12]. Different nitration systems can be used to nitrate specific compounds on the basis of the structural characteristics of compounds [13]. According to the diversity of the nitration positions on these frameworks, this paper classifies the nitration of H on heterocyclic C, the nitration of -NH2 on heterocyclic C, the nitration of H on heterocyclic N, and the nitration of -NH2 on heterocyclic N.
2. Nitrification of Azoles Nitrogen-Rich Heterocyclic Energetic Compounds
2.1. Imidazoles
2.1.1. Nitrification of H on Imidazole Ring C
4,4′,5,5′-tetranitro-2,2′-benzimidazole (TNBI) is an important precursor for the synthesis of EMs. It has been synthesized by two nitrification systems.
-
(1)
NaNO3/H2SO4 system
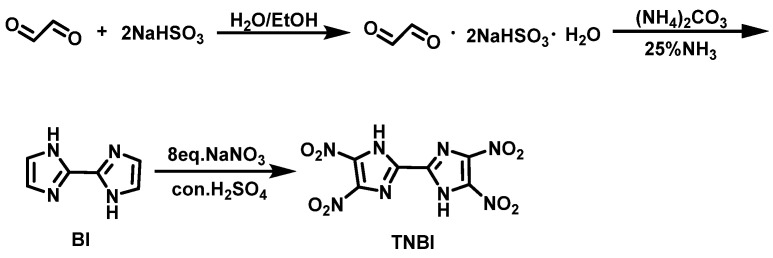
Klapötke et al. [14] synthesized 2,2′-biimidazole (BI) from glyoxal and sodium bisulfite by cyclization, suspended NaNO3 and urea in concentrated H2SO4 (96–98%) at 0 °C, and added BI in small batches. The suspension was stirred for 1 h at ambient temperature, and then heated to 85–90 °C for 16 h. Thereafter, the suspension was poured onto crushed ice, filtered, and washed with ice water to obtain TNBI·2H2O, with a yield of 51%. The synthesis route is shown in Scheme 1.
Scheme 1.
Synthesis of 4,4′,5,5′-tetranitro-2,2′-biimidazole [14].
-
(2)
80% HNO3/H2SO4 system
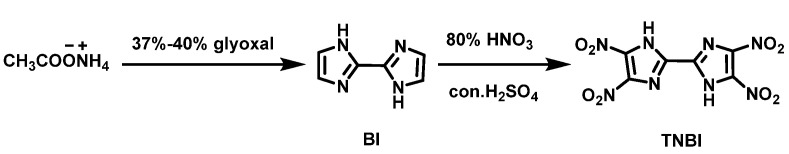
Li et al. [15] synthesized BI using ammonium acetate (CH3COONH4) and glyoxal as raw materials. Then, BI was added to 95–98% concentrated H2SO4 at 20–25 °C. After that, the mixed solution of 80% HNO3 and 95–98% H2SO4 was added dropwise as the mixture was heated to 45 °C. Four hours later, the reaction solution was poured onto crushed ice, filtered, washed with cold water, and dried to obtain TNBI. The synthesis route is shown in Scheme 2. The yield was 51.7%. Compared with the traditional operation process [14], this strategy saves the reaction time and lowers the reaction temperature via a gentle and stable reaction process, thus, the danger of the reaction is reduced.
Scheme 2.
Synthesis of 4,4′,5,5′-tetranitro-2,2′-biimidazole [15].
2.1.2. Nitration of -NH2 on Imidazole Ring C/N
The H atom of NH2 can be nitrified to obtain different nitration products using the HNO3/H2SO4 system.
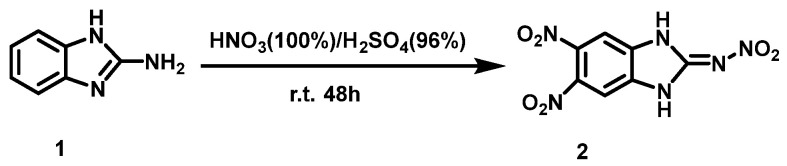
Thomas et al. [16] added 2-aminobenzimidazole (1) to the solution of 100% HNO3 and concentrated H2SO4 (96–98%) while stirring at 0 °C. After stirring for 48 h at 25 °C, the mixture was poured onto crushed ice, then filtered and washed with 20% HNO3 and a small amount of water. The light yellow solid, 2-nitroammonium-5,6-dinitrobenz imidazole (2), was obtained with a yield of 41%. The synthesis route is shown in Scheme 3.
Scheme 3.
Synthesis of 2-nitrimino-5,6-dinitrobenzimidazole [16].
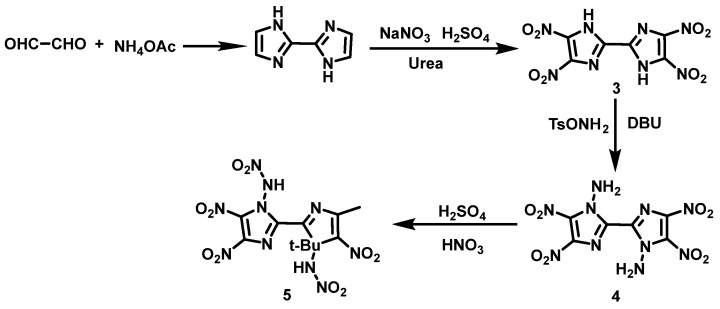
Another example is nitration of -NH2 on imidazole ring N. Yin et al. [17] synthesized 4,4′,5,5′-tetranitro-1H,1′H-(2,2′-benzimidazole)-1,1′-diamine (4) from glyoxal and ammonium acetate by using condensation, nitration, and N-amination reactions. Compound 4 was then slowly added to the HNO3/H2SO4 solution at −10 °C and stirred for 90 min. The solution was subsequently poured onto crushed ice, stirred for about 10–15 min, filtered, and washed with ice-cooled water, ethanol, and ether to obtain N,N′-dinitroamino-4,4′,5,5′-tetranitro-bisimidazole (5). The synthesis route is shown in Scheme 4. In order to prevent the N-N bond from being broken, the nitration reaction should be performed in a mixed acid at a temperature of −15 to −10 °C because the N-amino group is highly reactive. It has been found that the nitrification ability of HNO3/H2SO4 is stronger than that of HNO3, so HNO3/H2SO4 with an appropriate ratio is always selected for nitrating NH2 to NHNO2. The reaction condition is mostly mild and the operation is simple.
Scheme 4.
Synthesis of N,N′-dinitramino-4,4′,5,5′-tetranitro-bisimidazole [17].
2.1.3. Nitrification of H on Imidazole Ring N
-
(1)
HNO3/Ac2O system
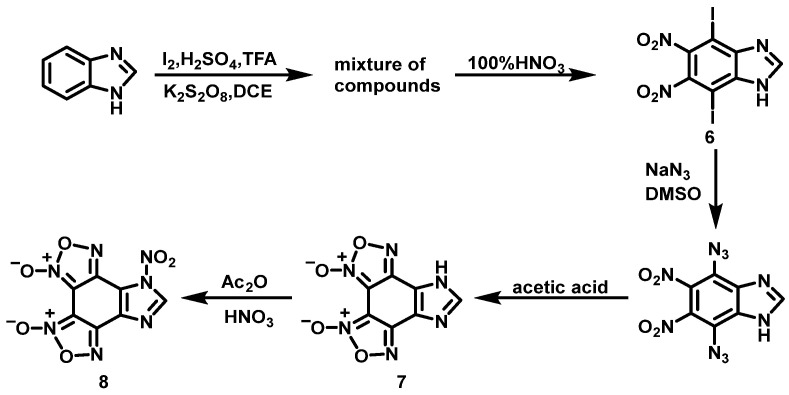
Chand et al. [18] synthesized compound 7 using benzimidazole as a raw material through iodination, nitration, substitution, and ring formation reactions (Scheme 5). Then, 100% HNO3 was added dropwise to acetic anhydride (Ac2O) at −5 °C, and the resulting mixture was stirred for 0.5 h. Compound 7 was then added to the reaction solution in portions and stirred for 2 h. Afterwards, the reaction mixture was poured onto crushed ice. Compound 8 was obtained by filtration with a yield of 67%. Compared with the nitrifying agent HNO3/H2SO4, the nitrifying ability of HNO3/Ac2O mixed solution is weaker, but Ac2O can effectively decrease the oxidizing property of HNO3 and avoid the formation of by-products of compound 8.
Scheme 5.
Synthesis of 7-nitro-7H-imidazo[4′,5,5,6]benzo[1,2,3,4]bis([1,2,5]oxadiazole)-3,4-dioxide [18].
2.2. Nitrification of Pyrazoles
2.2.1. Nitrification of H on Pyrazole Ring C
-
(1)
Bismuth nitrate/montmorillonite
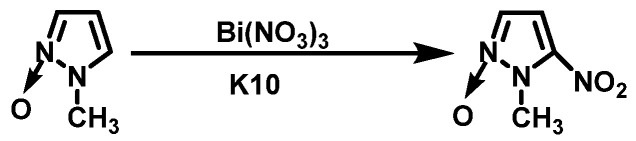
P. Ravi et al. [19] added 1-methylpyrazole-2-oxide and montmorillonite (K-10) to a suspension of bismuth nitrate (Bi(NO3)3) in tetrahydrofuran (THF) and stirred for 2.5 h. The solvent was then evaporated under reduced pressure using a vacuum pump for 5 min. The mixture was repeatedly washed with dichloromethane (CH2Cl2) and concentrated to give the crude K-10. The pure product was isolated by column chromatography with a yield of 98%. The synthesis route is shown in Scheme 6. The ranking of nitration capacities of some nitrates is as follows: Bi(NO3)3 > AgNO3 > KNO3 > NaNO3 > NH4NO3 > Pb(NO3)2 > Ba(NO3)2. In the reaction, Bi(NO3)3, which is impregnated on K-10, has a fast nitration rate and high yield and is easy to separate from the product by filtration. The methyl group in the pyrazole ring contributes to the nitration process, and the nitration rate will increase with the number of methyl groups.
Scheme 6.
Synthesis of 1-methyl-5-nitropyrazole- 2-oxide (K-10) [19].
-
(2)
HNO3/H2SO4 system
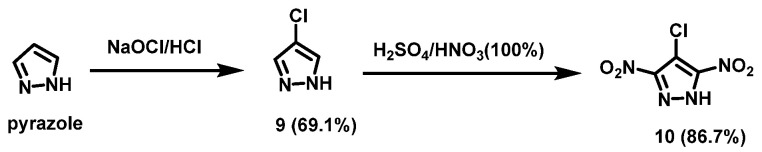
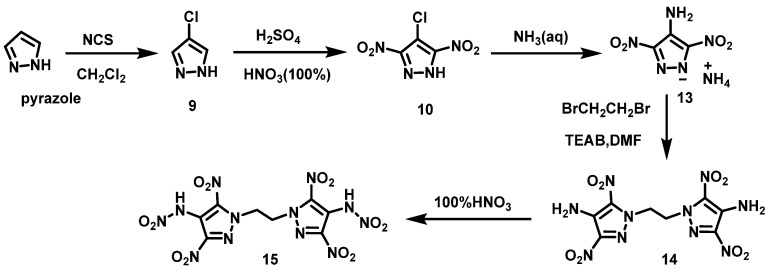
The HNO3/H2SO4 system usually has a strong nitration effect. Fischer et al. [20] synthesized 4-chloropyrazole (9) using pyrazole as a raw material via chlorination reaction. Compound 9 was then dissolved in the concentrated H2SO4, and 100% HNO3 was slowly added below 40 °C. Then, the mixture was heated to 100 °C and stirred for 5 h under reflux. After cooling to room temperature, the final solution was poured onto crushed ice water and extracted with acetoacetic acid. The organic layers were washed with water, dried over magnesium sulfate, and dried under nitrogen to give 4-chloro-3,5-dinitro-1H-pyrazole (10) in a yield of 86.7%. The synthesis route is shown in Scheme 7. The reaction is simple, no further purification is required, and the reaction yield is high.
Scheme 7.
Synthesis of 4-chloro-3,5-dinitropyrazole [20].
-
(3)
HNO3/P2O5 system
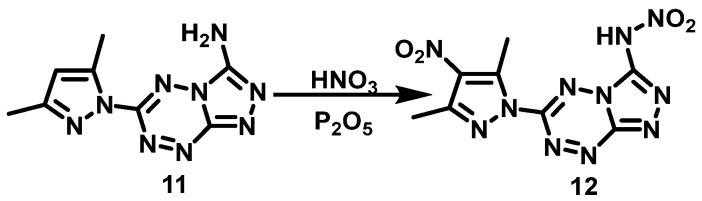
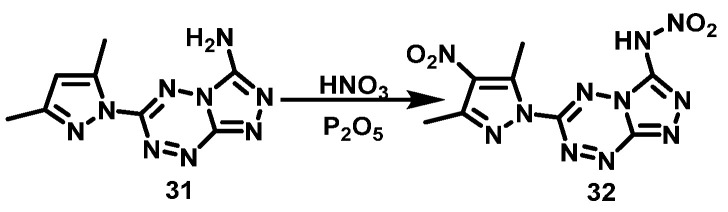
Wang et al. [21] dissolved P2O5 in fuming HNO3 and then added (6-(3,5-dimethyl-1H- pyrazole-1-yl)-1,2,4-triazole[4,3-b]-1,2,4,5-tetrazine-3-amino (11) at 0 °C. The reaction mixture was stirred for 10 h at room temperature. Then, the mixture was subsequently poured onto crushed ice, extracted with ethyl acetate, and purified by column chromatography to obtain N-(6-(3,5-dimethyl- 4-nitro-1H-pyrazole-1-yl)-1,2,4-triazolo[4,3-b]-1,2,4,5-tetrazin-3-yl)nitramide (12) with a yield of 58%. In this reaction, the NH2 in 1,2,4-triazol ring is also nitrated to NHNO2. The synthesis route is shown in Scheme 8. The nitrification system used in this reaction is HNO3/P2O5. P2O5 is not only a dehydrating agent, but also a nitrification promoter. This nitration system is not only suitable for aromatics but also for amines. Even amines that are difficult to nitrate can get satisfactory results sometimes.
Scheme 8.
Synthesis of N-(6-(3,5-dimethyl-4-nitro-1H-pyrazol-1-yl) -1,2,4-triazolo[4,3-b]-1,2,4,5-tetrazine-3-yl)nitramide [21].
2.2.2. Nitrification of -NH on Pyrazole Ring C
-
(1)
100% HNO3 system
HNO3 is a strong nitration agent, it can be used to nitrate NH2 to NHNO2. Yin et al. [22] synthesized 1,1′-(ethane-1,2-diyl)bis(3,5-dinitro-)1H-pyrazole-4-amine) (14) using pyrazole as raw material through halogenation, nitration, neutralization, and alkylation reaction. Afterwards, compound 14 was added to 100% HNO3 in portions below 10 °C. The reaction was held for 10 min at 5 °C, and HNO3 was removed by blowing in air. The residue was dried under vacuum to give N,N′-[1,1′-(ethane-1,2-diyl)bis(3,5-dinitro-1H-pyrazole-4,1-diyl)]dinitramide (15). The synthesis route is shown in Scheme 9. This process uses 100% HNO3 as the nitration system. For the nitration of different azole rings, different concentrations of HNO3 are required.
Scheme 9.
Synthesis of N,N′-[1,1′-(ethane-1,2-diyl)bis(3,5-dinitro-1H-pyrazole-4,1-diyl)]dinitramide [22].
-
(2)
70% HNO3/H2SO4 system
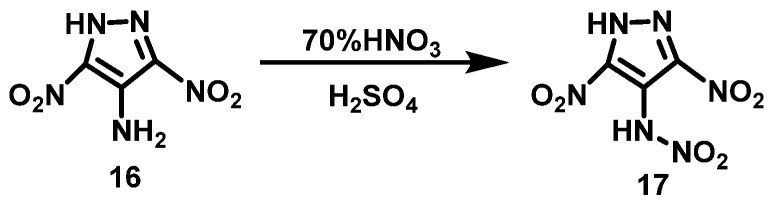
Zhang et al. [23] added 4-amino-3,5-dinitropyrazole (16) to a mixture of HNO3 (70%) and concentrated H2SO4 in a volume ratio of 1:1 at 0 °C. The mixture was stirred for 2 h and then slowly warmed to room temperature. After stirring for another 4 h, the reaction mixture was poured into ice water and extracted with ether to obtain 4-nitroamino-3,5-dinitropyrazole (17). The synthesis route is shown in Scheme 10.
Scheme 10.
Synthesis of 4-nitramino-3,5-dinitropyrazole [23].
-
(3)
HNO3/Ac2O system
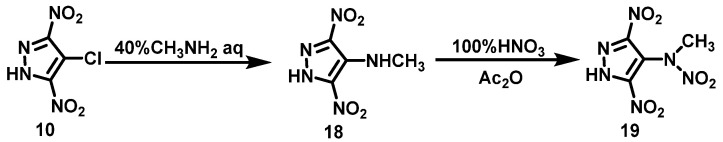
He et al. [23] synthesized 4-methylamino-3,5-dinitropyrazole (18) using 4-chloro-3,5-dinitro pyrazole (10) as a raw material through nucleophilic substitution reaction. Firstly, 100% HNO3 was slowly added to a cooled solution of compound 18 in acetic acid, after which Ac2O was added, and the mixture was stirred for 1.5 h at room temperature. 4-(N-methylnitramino)-3,5-dinitropyrazole (19) was finally obtained by removing excess acid under vacuum with a yield of 95%. The synthesis route is shown in Scheme 11. This reaction uses HNO3/Ac2O as a nitration system, which is simple to operate, has no by-products, and the yield is as high as 95%.
Scheme 11.
Synthesis of 4-(N-methylnitramino)-3,5-dinitropyrazole [24].
2.2.3. Nitrification of H on Pyrazole Ring N
-
(1)
HNO3/Ac2O system
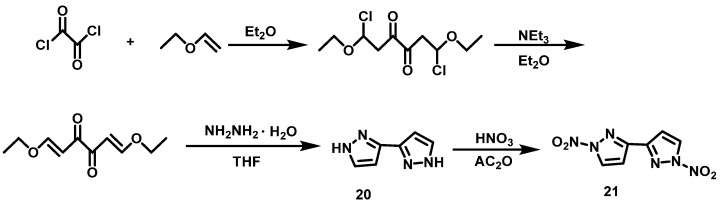
Tang et al. [25] synthesized 1H,1′H-3,3′-bispyrazole(20) using oxalyl chloride and ethoxyethylene as raw materials through addition, elimination, and cyclization reactions. Firstly, 100% HNO3 was added dropwise to AC2O at 0 °C, then compound 20 was slowly added to the mixed acid. The reaction mixture was warmed to room temperature, and stirred for 6 h. The reaction mixture was subsequently poured into ice water, filtered, and washed with trifluoroacetic acid (TFAA) to obtain 1,1′-dinitro-1H, 1′H-3,3′-bispyrazole (21), with a yield of 71%. The synthesis route is shown in Scheme 12.
Scheme 12.
Synthesis of 1,1′-dinitro-1H,1H’-3,3′-bipyrazole [25].
-
(2)
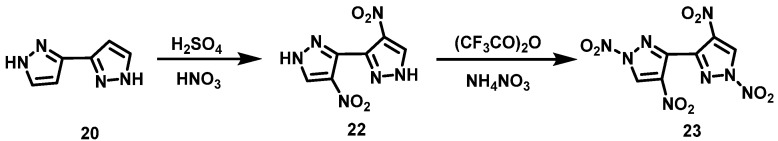
NH4NO3/TFAA system
Kumar et al. [26] prepared 4,4′-dinitro-1H,1′H-3,3′-bipyrazole (22) by nitrating bipyrazole (20) with nitric–sulfur mixed acid. Then, NH4NO3 was added to the suspension of compound 5 in TFAA ((CF3CO)2O) in batches at 0–5 °C. The reaction mixture was stirred for 5 h at room temperature, filtered, and washed with water to obtain 1,1′,4,4′-tetranitro-1H,1′H-3,3′-dipyrazole (23). The synthesis route is shown in Scheme 13. NH4NO3/TFAA are used as nitration reagents to make reaction conditions mild, and post-treatment of waste acid is not needed at the end of the reaction.
Scheme 13.
Synthesis of 4,4′,5,5′-tetranitro-2H,2′H-3,3′-bipyrazole [26].
2.2.4. Nitrification of -NH2 Connected with the Pyrazole Ring N
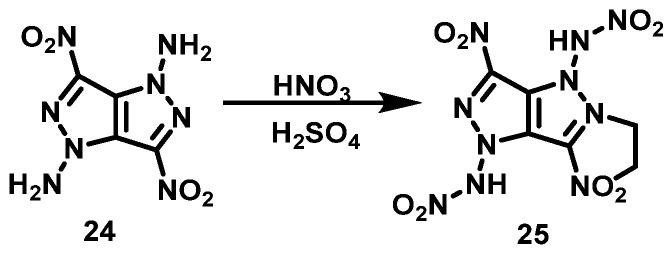
A fuming HNO3/con. H2SO4 system can be used to nitrate -NH2 to NHNO2, which is connected with N in a pyrazole ring.
Yin et al. [27] cooled a concentrated H2SO4 suspension of 3,6-dinitropyrazole[4,3-c] pyrazole-1,4-diamine (24) to −15 °C in an ice-salt bath, then fuming HNO3 was added dropwise to the mixture. After the mixture was stirred for 2 h at −15 °C, N,N′-(3,6-dinitropyrazole[4,3-c] pyrazole-1,4-diyl)dinitramine (25) was obtained by filtering the reaction solution and washing with TFAA. The synthesis route is shown in Scheme 14.
Scheme 14.
Synthesis of N,N′-(3,6-dinitropyrazole [4,3-c] pyrazole-1,4-diyl) dinitramine [27].
2.3. Triazoles
2.3.1. Nitrification of H on 1,2,4-Triazole Ring C
-
(1)
70% HNO3 system
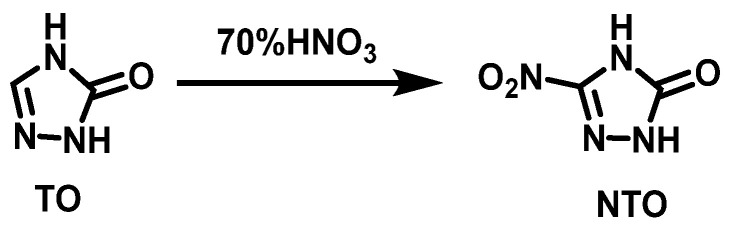
3-nitro-1,2,4-triazol-5-one (NTO) is a kind of insensitive high-energy explosive with excellent comprehensive performance [28,29]. The more mature synthesis method of NTO is by nitration of TO(1,2,4-triazol-5-one), which is synthesized from semicarbazide hydrochloride and formic acid using condensation and cyclization reaction. The synthesis route is shown in Scheme 15. The nitrating agent is 70% or 98% HNO3. Huang et al. [30] added TO to 70% HNO3 in batches at 60–65 °C, reacted for 1 h, then cooled to 3 °C in an ice-water bath, filtered, and collected the HNO3 filtrate, which was recycled in the next batch of reactions. The filter cake was rinsed with water and followed by vacuum filtration. Finally, NTO was obtained via recrystallization from water with a yield of 75.4% and a purity of 99.94%. Compared with the use of 98% HNO3 as the nitrating agent, this nitration method has a simpler process, safer operation, and HNO3 filtrate can be reused, so the cost of raw materials can be lowered.
Scheme 15.
Synthesis of 3-nitro-1,2,4-triazol-5-one [30].
-
(2)
HNO3/Ac2O system
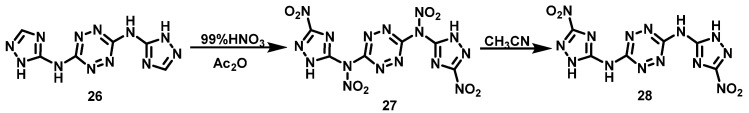
Aizikovich et al. [31] added 99% HNO3 to Ac2O at 0 °C while stirring for 30 min. The solid N3,N6-bis(1H-1,2,4-triazol-5-yl)-1,2,4,5-tetrazine-3,6-diamine (26) was slowly added to the mixture, after which, the solution was stirred for 2 h under anhydrous conditions. Then, the reaction solution was warmed to room temperature, filtered and washed quickly with TFAA, and immediately redissolved in hot CH3CN. The obtained CH3CN solution was heated for 30 min at 65 °C, then cooled to room temperature. Pure N3,N6-bis(3-nitro-1H-1,2,4-triazole-5-yl)-1,2,4,5-tetrazine-3,6-diamine (28) was filtrated and washed with CH3CN to give a yield of 31%. The synthesis route is shown in Scheme 16. Although the yield of the reaction is low, the operation is simple with short reaction time, and the product does not need further purification.
Scheme 16.
Synthesis of N3,N6-Bis(3-nitro-1H-1,2,4-triazol-5-yl)-1,2,4,5-tetrazine-3,6-diamine [31].
2.3.2. Nitration of -NH2 on 1,2,4-Triazole Ring C
-
(1)
HNO3/concentrated H2SO4 system
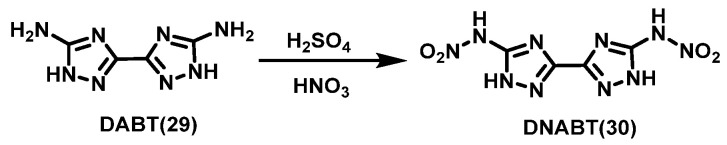
From the synthetic method by Astachov et al. [32], Dippold et al. [33] synthesized 3,3′-diamino-5,5′-bis (1H-1,2,4-triazole) (DABT,29) via condensation reaction using oxalic acid and aminoguanidine bicarbonate as raw materials. After that, HNO3 was slowly added to a concentrated H2SO4 solution of compound 29 at 0 °C. The mixture was warmed to room temperature and stirred for 1 h. The clear solution was then poured onto ice and the precipitate was collected by filtration. A yellow crystalline solid of 3,3′-dinitramine-5,5′-bis (1H-1,2,4-triazole) (DNABT, 30) was obtained by recrystallization from boiling water with a yield of 77%. The synthesis route is shown in Scheme 17.
Scheme 17.
Synthesis of 3,3′-dinitrimino-5,5′-bis(1H-1,2,4-triazole) [33].
-
(2)
HNO3/P2O5 system
Wang et al. [21] dissolved P2O5 in fuming HNO3, then (6-(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4- triazole[4,3-b]-1,2,4,5-tetrazine-3-amino (31) was added to the mixture at 0 °C and stirred at room temperature for 10 h. Then, the mixture was poured onto crushed ice and extracted with ethyl acetate, after which, N-(6-(3,5-dimethyl-4-nitro-1H-pyrazol-1-yl)-1,2,4-triazole[4,3-b]-1,2,4,5-tetrazin-3-yl) nitramine (32) was purified by column chromatography with a yield of 58%. The synthesis route is shown in Scheme 18. The nitrating reagents used in this reaction are relatively mild, but the post-treatment is relatively complicated.
Scheme 18.
Synthesis of N-(6-(3,5-dimethyl-4-nitro-1H-pyrazol-1-yl) -1,2,4-triazolo[4,3-b]-1,2,4,5-tetrazin-3-yl)nitramide [21].
2.3.3. Nitrification of H on 1,2,4-Triazole Ring N
-
(1)
HNO3/Ac2O system
Yin et al. [34] dissolved TFAA and 3-nitro-1,2,4-triazole in chloroform at 0~5 °C, and fuming HNO3 was added dropwise while maintaining the temperature. After the dropwise addition was completed, the reaction was warmed and stirred for 1 h at room temperature. The reaction solution was then poured onto crushed ice, and 1,3-dinitro-1H-1,2,4-triazole (33) was obtained by extraction with chloroform with a yield of 53%. The synthesis route is shown in Scheme 19.
Scheme 19.
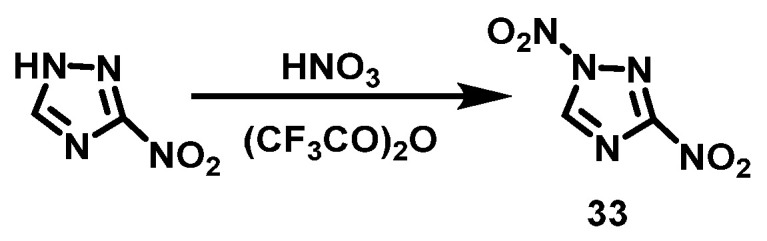
Synthesis of 1,3-dinitro-1H-1,2,4-triazole [34].
2.3.4. Nitrification of -NH2 on 1,2,4-Triazole Ring N
-
(1)
HNO3 system
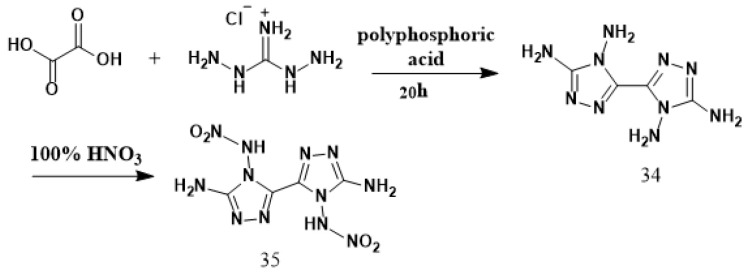
Klapçtke et al. [35] synthesized 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazole (34) using oxalic acid and diaminoguanidine hydrochloride as raw materials via a condensation reaction under the action of polyphosphoric acid. HNO3 was cooled to −10 °C and compound 1 was slowly added while the temperature was kept below 0 °C. After that, the mixture was stirred for 90 min at −5 °C. Then, the solution was poured onto crushed ice, stirred for 10–15 min, filtered, and 5,5′-diamino-4,4′-dinitroammonium-3,3′-di-(1,2,4-triazole) (35) was obtained by washing with cold water, ethanol, and ether, respectively, with a yield of 69%. The synthesis route is shown in Scheme 20. The synthetic method is simple in operation, and the obtained product does not require further purification.
Scheme 20.
Synthesis of 5,5′-diamino-4,4′-dinitramino-3,3′-bi-1,2,4-triazole [35].
2.3.5. Nitration of -NH2 Connected with 1,2,3-Triazole Ring C
Zhang et al. [36] added 4-amino-5-nitro-1,2,3-2H-triazole (36) to a mixture of HNO3 (70%) and concentrated H2SO4 in batches and stirred for 2 h at 0 °C. After stirring for another 2 h at room temperature, the reaction mixture was poured into ice water. 4-nitramine-5-nitro-1,2,3-2H-triazole (37) was obtained by ether extraction, and the yield was 76%. The synthesis route is shown in Scheme 21.
Scheme 21.
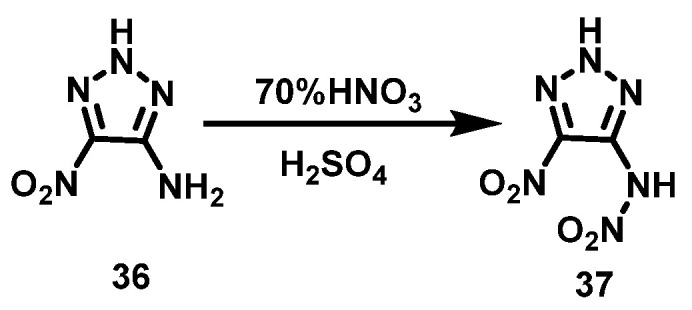
Synthesis of 4-nitramino-5-nitro-1,2,3-2H-triazole [36].
2.3.6. Nitrification of H on 1,2,3-Triazole Ring N
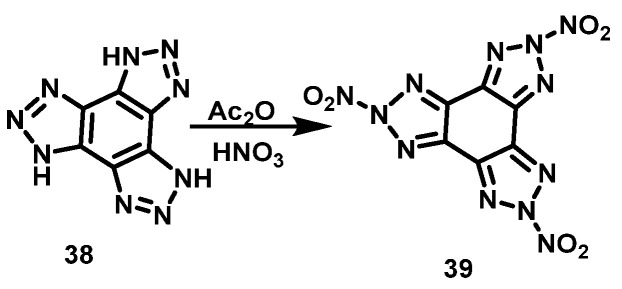
Thottempudi et al. [37] added concentrated HNO3 to Ac2O dropwise at −5 °C, the mixture was stirred for 30 min, and then stirred for another 45 min at room temperature. Subsequently, the mixture was cooled to −5 °C, and tris(triazolo) benzene (38) was added in portions, stirred for about 15 min, and stirred overnight at room temperature. The mixture was then poured onto ice, filtered, and washed with water to obtain the final product of trinitrotris(triazolo)benzene (39) with a yield of 53%. The synthesis route is shown in Scheme 22.
Scheme 22.
Synthesis of 2,5,8-trinitrotris(triazolo)benzene [37].
2.4. Tetrazoles
2.4.1. Nitrification of -NH2 on Tetrazole C
-
(1)
NO2BF4 system
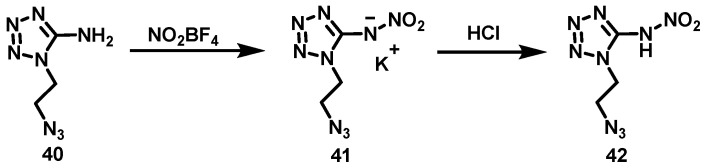
Stierstorfer et al. [38] dissolved 1-(2-azidoethyl)-5-aminotetrazole (40) in MeCN in an ice-water bath, and added nitronium tetrafluoroborate (NO2BF4) to the solution. The solution was then stirred for 30 min at 0 °C and for another 1 h at 25 °C. The solvent was evaporated and KOH ethanol solution was added. The precipitated KBF4 was removed by filtration and another equivalent of KOH in ethanol was added. The solvent was evaporated and cold water was added. The insoluble material was removed by filtration and the water was evaporated. Potassium 1-(2-azidoethyl)-5-nitroaminotetrazole (41) was finally obtained by recrystallization from hot ethanol with a yield of 33%. After that, an equimolar amount of dilute (1N) HCl was added to compound 41. Then, the solvent was evaporated and acetone was added to the residue. The solution was filtered to remove KCl. After acetone was evaporated, the crude product was recrystallized from a small amount of methanol to yield colorless 42 (90%). The synthesis route is shown in Scheme 23. The reaction conditions are mild and the temperature is easy to control. However, HBF4 must be removed after the reaction, the yield of compound 41 is low, and the purification is difficult.
Scheme 23.
Synthesis of 1-(2-azidoethyl)-5-nitriminotetrazole [38].
-
(2)
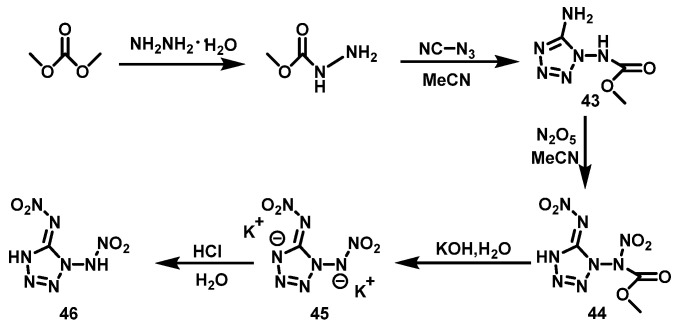
N2O5 system
Fischer et al. [39] synthesized 1-methoxycarbonyl-1,5-diaminotetrazole (43) using dimethyl carbonate via nucleophilic substitution and a ring formation reaction. After that, compound 43 was suspended in anhydrous acetonitrile at 0 °C, a solution of N2O5 in acetonitrile (MeCN) was added, and the mixture was stirred for 1 h. KOH aqueous solution was then added dropwise, the aqueous phase was separated, and the water was evaporated under high vacuum. The residue was stirred in methanol for several hours. The reaction solution was filtered, washed with methanol, and dried. The obtained solid was dissolved in 2M HCl, and 1,5-bis(nitroamino)tetrazole (46) was obtained by extraction with ethyl acetate in a yield of 50%. The synthesis route is shown in Scheme 24. The reaction has less heat generation and the temperature is easy to control. Moreover, the product separation is simple with no waste acid treatment.
Scheme 24.
Synthesis of 1,5-di(nitramino)tetrazole [39].
-
(3)
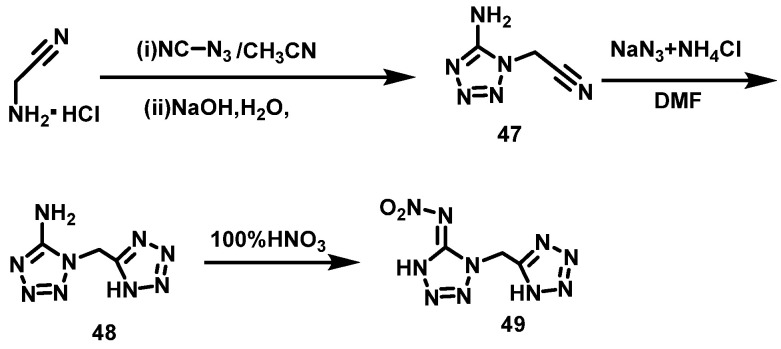
100% HNO3 system
Kumar et al. [40] synthesized amino 1-((1H-tetrazol-5-yl)methyl)-1H-tetrazole-5-amino (48) using aminoacetonitrile hydrochloride and cyanide azide (47) as raw materials through two-step ring formation reaction. Then, compound 2 was slowly added to 100% HNO3 at 0–2 °C. After stirring for 12 h at room temperature, the mixture was poured into cold water. Thus, N-(1-((1H-tetrazol-5-yl)methyl)-1H-tetrazole-5 (4H)-alkylene)nitramine (49) was obtained by extraction with ethyl acetate in a yield of 65%. The synthesis route is shown in Scheme 25.
Scheme 25.
Synthesis of N-(1-((1H-tetrazol-5-yl)methyl)-1H-tetrazol-5(4H)-ylidene)nitramide [40].
2.4.2. Nitrification of -NH2 Connected with Tetrazole Ring N
-
(1)
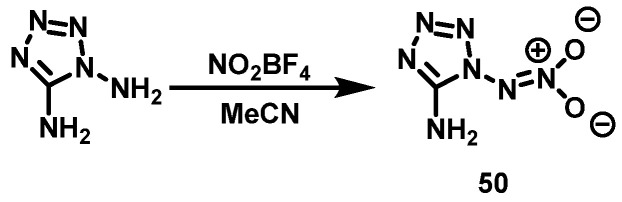
NO2BF4 system
Klapçtke et al. [41] dissolved 1,5-diaminotetrazole in anhydrous acetonitrile at 0 °C and added NO2BF4 to the solution with stirring. The reaction mixture was then stirred overnight at room temperature. A pale yellow solid was obtained by evaporating acetonitrile under vacuum. The solid was re-dissolved in a small amount of ethanol, and then a mixed solution of KOH and ethanol was added to precipitate potassium tetrafluoroborate. Followed by filtration, the filtrate was evaporated and 5-amino-1-nitroaminotetrazole (HDATNO2, 50) was obtained with a yield of 59%. The synthesis route is shown in Scheme 26. The nitrating agent, NO2BF4, is environmentally friendly and does not require waste acid treatment. The reaction conditions are mild and the temperature is easy to control. There are fewer by-products in the reaction and the selectivity of the reaction is high, but NO2BF4 is expensive and has high costs.
Scheme 26.
Synthesis of 5-amino-1-nitriminotetrazole [41].
-
(2)
N2O5 system
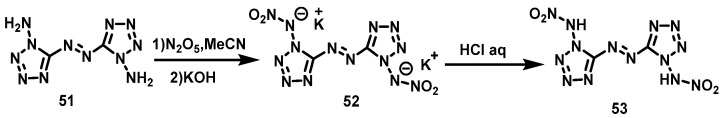
Fischer et al. [42] suspended 1,1′-diamino-5,5′-azobitetrazole (51) in dry acetonitrile at 0 °C and added a solution of N2O5 in cold acetonitrile dropwise. The KOH solution was added dropwise until 1,1′-diamino-5,5′-azobitetrazole was dissolved, and then the red crystal of 1,1′-dinitroammonium-5,5′-azobitetrazole dipotassium salt (52) was obtained via filtration. The yield was 62%. Subsequently, the salt 4 was dissolved in 2M HCl, and a colorless single crystal of 1,1′-dinitroammonium-5,5′-azotetrazole (53) was obtained via extraction with ethyl acetate. The synthesis route is shown in Scheme 27. This reaction uses N2O5 as a nitration system, which has higher nitration selectivity, less side reactions, superior yield, and lower equipment requirements, thereby reducing the cost of the entire process.
Scheme 27.
Synthesis of 1,1′-dinitramino-5,5′-azobitetrazole [42].
2.5. Oxadiazoles
2.5.1. Nitration of -NH2 on 1,2,4-Oxadiazole Ring C
-
(1)
100% HNO3 system
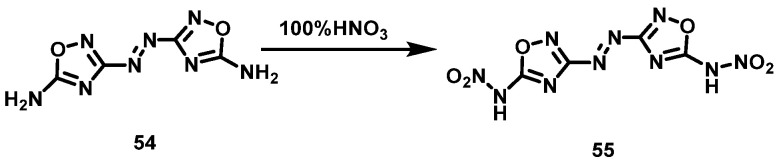
Tang et al. [43] slowly added 5,5′-diamino-3,3′-azo-1,2,4-oxadiazole (54) to 100% HNO3 at −5 °C and then slowly raised the temperature to room temperature. The mixture was stirred overnight. 5,5′-dinitroammonium-3,3′-azo-1,2,4-oxadiazole (55) was obtained via filtration and washed with TFAA, with a yield of 75%. The synthesis route is shown in Scheme 28. The reaction yield is high and the obtained product is pure without further purification.
Scheme 28.
Synthesis of 5,5′-dinitramino-3,3′-azo-1,2,4-oxadiazole [43].
-
(2)
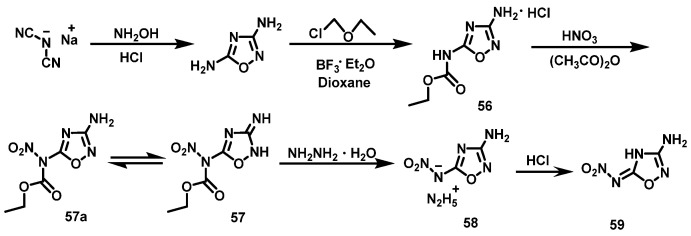
HNO3/Ac2O system
Tang et al. [44] synthesized 3-amino-5-N-ethoxychloroamido-1,2,4-oxadiazole hydrochloride (56) using the sodium salt of malononitrile and hydroxylamine as raw materials (Scheme 29) through a ring formation reaction and substitution reaction. HNO3 (100%) was slowly added to the acetic anhydride at 0 °C. Compound 56 was then added to the mixture and stirred for 1 h. Afterwards, the reaction solution was poured into ice water. The pure product of 3-amino-5-N-nitro-ethoxyformamido-1,2,4-oxadiazole (57) was obtained via filtration and washing with cold water. The yield was 73%. Subsequently, compound 57 was treated with a solution of hydrazine in acetonitrile to obtain 3-amino-5-nitroamino-1,2,4-oxadiazole hydrazine salt (58). 3-amino-5- nitroamino-1,2,4-oxadiazole monohydrate (59) was obtained via acidification with concentrated hydrochloric acid.
Scheme 29.
Synthesis of 3-amino-5-nitramino-1,2,4-oxadiazole monohydrate [44].
2.5.2. Nitrification of -NH2 on 1,3,4-Oxadiazole Ring C
-
(1)
100% HNO3 system
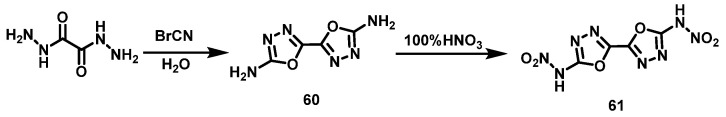
Tobias et al. [45] synthesized intermediate 2,2′-diamino-5,5′-bis(1-3,4-oxadiazole) (60) using oxalodiazide and BrCN as raw materials. Compound 60 was added to 100% HNO3 in portions at 0 °C, and the mixture was stirred for 12 h at room temperature. 2,2′-dinitroamino-5,5-bi(1-3,4-oxadiazole) (61) was obtained by filtering and washing with water, methanol, and ether, respectively, with a yield of 84%. The synthesis route is shown in Scheme 30. In this reaction, 100% HNO3 is used as a nitrating agent, and the yield is greater than 80% with a high purity of the nitrated product.
Scheme 30.
Synthesis of 2,2′-dinitramino-5,5-bi(1-oxa-3,4-diazole) [45].
2.5.3. Nitration of -NH2 Connected with 1,2,5-Oxadiazole(Furazan) Ring C
-
(1)
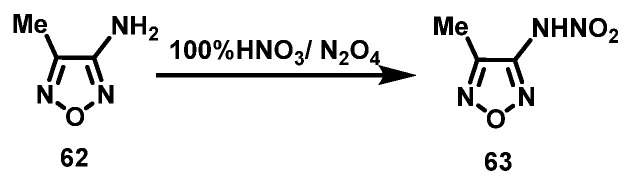
N2O4/100% HNO3 system
Sheremetev et al. [46] added 3-amino-4-methylfurazan (62) to a mixture of 100% HNO3 and 2% N2O4 at −5 °C, and stirred for 1 h. Then, the reaction mixture was heated to 20 °C and poured onto ice. 3-nitroamino-4-methylfurazan (63) was obtained via extraction with diethyl ether, washing with cold water, and drying with MgSO4, with a yield of 40–45%. The synthesis route is shown in Scheme 31.
Scheme 31.
Synthesis of 3-nitroamino-4 -methylfurazan with N2O4/100% HNO3 system [46].
-
(2)
NO2BF4 system
Sheremetev et al. [46] also added 3-amino-4-methylfurazan (62) to a CH2Cl2 suspension of NO2BF4. The mixture was cooled to 0 °C, stirred for 1 h, and then the temperature was slowly raised to 5 °C. Subsequently, the reaction mixture was poured onto ice, and the resulting emulsion was extracted with diethyl ether to obtain 3-nitroamino-4-methylfurazan (63) in a 63% yield. The synthesis route is shown in Scheme 32.
Scheme 32.
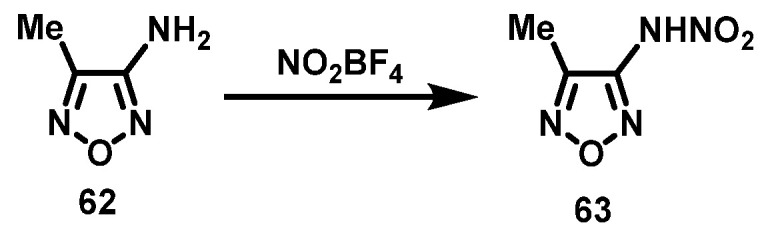
Synthesis of 3-nitroamino-4 –methylfurazan with NO2BF4 system [46].
-
(3)
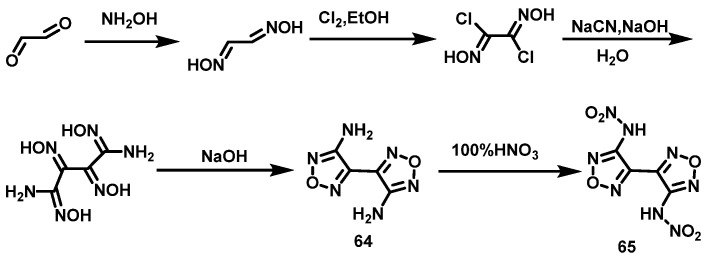
100% HNO3 system
Fischer et al. [47] synthesized 3,3′-diamino-4,4′-bifurazan (64) using glyoxal and hydroxylamine by nucleophilic addition, chlorination, substitution, nucleophilic addition, and cyclization reactions. Then, compound 64 was slowly added to 100% HNO3 at −5 °C to 0 °C, and stirred for 45 min. The suspension was poured onto ice and 3,3′-dinitroamino-4,4′-furazan (65) was obtained by filtering and washing with ice water, with a yield of 80%. The synthesis route is shown in Scheme 33. The yield can be increased to more than 90% by using an organic solvent (such as ethyl acetate) to extract filtrate.
Scheme 33.
Synthesis of 3,3′-dinitramino-4,4′-bifurazane [47].
-
(4)
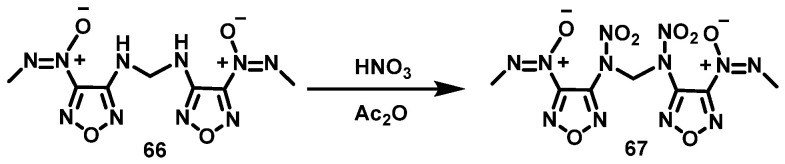
HNO3/Ac2O system
Zhang et al. [48] added compound (66) in batches to a mixture of acetic anhydride and 100% HNO3 at 0 °C. The reaction mixture was stirred for 6 h at room temperature and then poured into ice water. N,N′-dinitro-N,N′-bis [3-(methyl-azo-nitrogen oxide) furazan-4-yl] methylene diamine (67) was obtained by filtering and washing the precipitate with ethanol in a yield of 66%. The synthesis route is shown in Scheme 34. In this nitration system, the acid anhydride can effectively reduce the oxidizing property of HNO3, preventing the generation of by-products. The reaction has simple operation procedures and a high yield.
Scheme 34.
Synthesis of N,N′-dinitro-N,N′-bis[3-(methyl-NNO-azoxy)furazan-4-yl]methylenediamine [48].
-
(5)
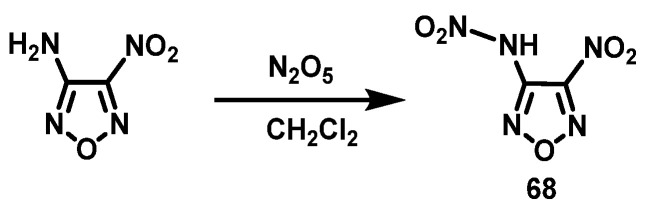
N2O5 system
Klapötke et al. [49] cooled dichloromethane (CH2Cl2) to −20 °C and added N2O5, while keeping the temperature at −20 °C. After it was completely dissolved, 3-amino-4-nitrofurazan was slowly added at −20 °C. The solution was slowly warmed up to 0–5 °C and stirred for 3 h. The solvent was removed under a constant nitrogen stream until most of the solvent was removed and 3-nitramino-4-nitrofurazan (68) was obtained in a yield of 66%. The synthesis route is shown in Scheme 35.
Scheme 35.
Synthesis of 3-nitramino-4-nitrofurazan [49].
3. Nitrification of Azines as Nitrogen-Rich Heterocyclic Energetic Compounds
3.1. Pyridazines
Nitrification of H on Pyridazine Ring C
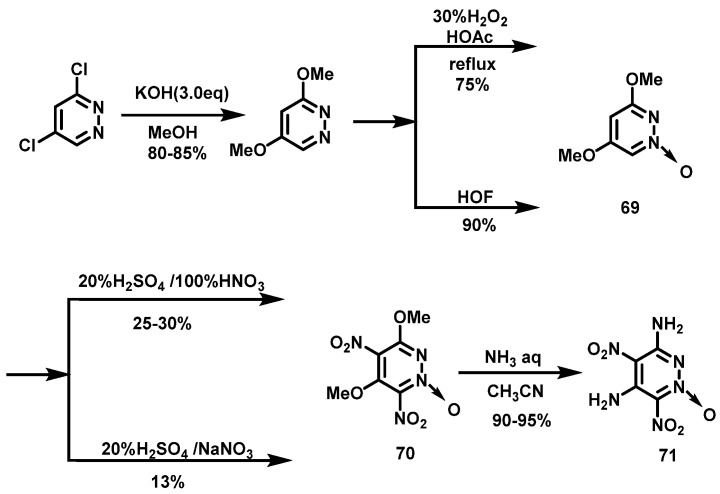
Gospodinov et al. [50] prepared the compound 3,5-dimethoxypyridazine-1-oxide (69) using dichloropyridazine as a raw material by substitution reaction and oxidation reaction, and then used two nitration systems to obtain compound 70. The synthesis route is shown in Scheme 36.
Scheme 36.
Synthesis of 3,6-diamino-4,6-dinitropyridazine-1-oxide [50].
-
(1)
20% H2SO4/100% HNO3 system
Compound 69 was dissolved in 20% fuming H2SO4 at 5 °C, after which, NaNO3 was added in batches, the reaction mixture was stirred for 1 h, and was then slowly warmed up to room temperature. After that, the reaction mixture was stirred overnight at 60 °C and then poured onto crushed ice. The resulting suspension was stirred until the ice dissolved and the resulting precipitate was filtered. The crude product was dissolved in conc. H2SO4, stirred for 3 h at 60 °C, and then poured on ice and the precipitate was filtered. 3,5-dimethoxy-4,6-dinitropyridazine-1-oxide (70) was obtained after washing with ice water several times in a yield of 13%.
-
(2)
20% H2SO4/100% NaNO3 system
Compound 69 was dissolved in 20–25% fuming H2SO4 at 10 °C, and 100% HNO3 was added dropwise below 8 °C. The reaction mixture was first stirred at 0 °C for 1.5 h, then at room temperature for 2 h, and finally stirred at 45–50 °C for 20 h. After cooling, the reaction was poured onto crushed ice. The resulting suspension was stirred for 2 h and the obtained yellowish precipitate was filtered off and washed with water. The crude product was dissolved in conc. H2SO4 and stirred at 60 °C for 2 h. The mixture was poured onto crushed ice, filtered, and washed with ice water to obtain compound 70 in a yield of 28%. Finally, 3,6-diamino-4,6-dinitropyridazine-1-oxide (71) was synthesized by reacting compound 70 with concentrated ammonia in acetonitrile solution.
3.2. Pyrazines
Nitrification of H on Pyrazine Ring C
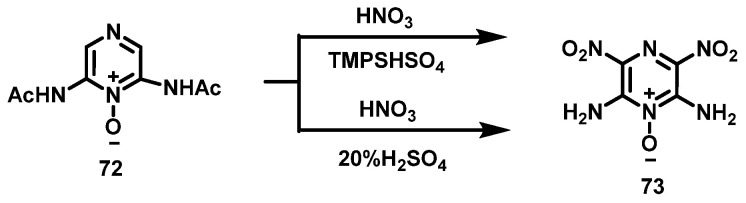
The density of 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105, 73) is 1.92 g·cm−3 with the detonation velocity of 8516 m·s−1 and the detonation pressure of 35.9 GPa. It is a new explosive with excellent energy and safety performance. In the process of its preparation, there is a typical nitration reaction of H on pyrazine C, and the preparation method of LLM-105 has been improving. In 2014, Zhou et al. [51] used two nitration systems to nitrate the intermediate 2,6-diacetamidopyrazine-1-oxide (72) to obtain LLM-105. The synthesis route is shown in Scheme 37.
Scheme 37.
Synthesis of 2,6-diamino-3,5-dinitropyrazine-1-oxide [51].
-
(1)
20% H2SO4/100% HNO3 system
Compound 72 was added into 20% fuming H2SO4 when the temperature was lower than 25 °C, the temperature of the mixture was controlled to be lower than 10 °C at the end of feeding, and then fuming HNO3 was slowly added into the mixture. Afterwards, the system was controlled to react for 1 h within 10–15 °C and then heated to room temperature for 2 h. Finally, the mixture was poured onto crushed ice, filtered, washed with water, and dried to obtain the bright yellow LLM-105 solid with the yield of 72%.
-
(2)
100% HNO3/TMPSHSO4 system
N,N,N-trimethyl-N-propanesulfonate-ammonium bisulfate (TMPSHSO4), as an ionic liquid, was dissolved in fuming HNO3, cooled to about 0 °C, and then compound 72 was slowly added. The reaction solution was stirred for 0.5 h; then heated to 25 °C, reacting for 1 h; and heated to 75 °C, reacting for 4 h. After the reaction was completed, the mixture was cooled to room temperature, diluted with distilled water, filtered and washed with water three times, and dried to obtain yellow LLM-105, with a yield of 68.4%. Compared with nitration with mixed acid, HNO3/acid ionic liquid has the advantages of simpler post-treatment and less waste acid discharge.
-
(3)
H2SO4/95% HNO3 system
Liu et al. [52] synthesized 2,6-dipyramidopyrazine (74) using 2,4,6-trinitrochlorobenzene and 2,6-diaminopyrazine by condensation reaction. Next, 95% fuming HNO3 and concentrated H2SO4 were stirred for 0.5 h at 0 °C. After that, compound 74 was slowly added, and then the temperature was gradually raised to 50 °C for 3 h. Subsequently, the reaction solution was cooled, poured into ice water, filtered and washed with acetone, and dried in a vacuum to obtain 2,6-dipyramido-3,5-dinitro- pyrazine (BPNP, 75). The yield was 66%. The synthesis route is shown in Scheme 38.
Scheme 38.
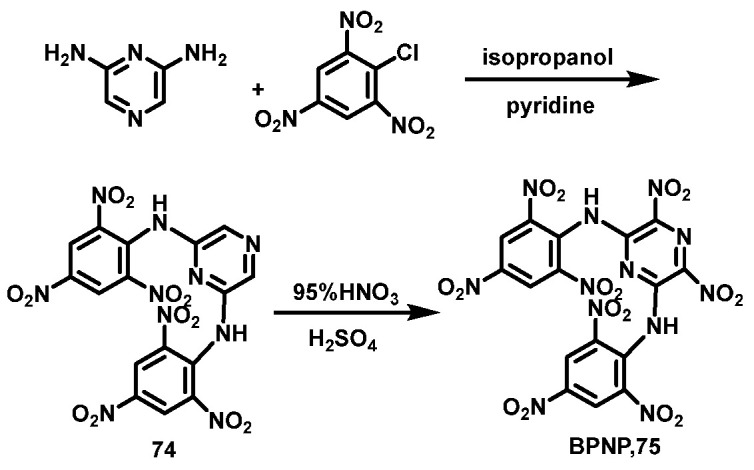
Synthesis of BPNP [52].
3.3. Nitrification of -NH2 Connected with Triazine Ring C
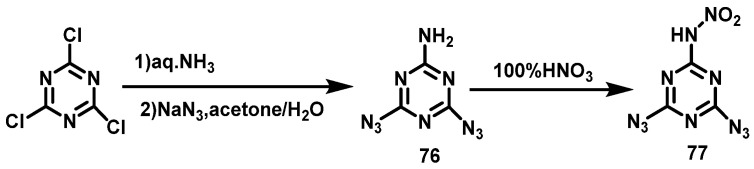
Huang et al. [53] synthesized 6-amino-2,4-diazio[1,3,5]triazine (76) using cyanuric acid chloride with aqueous NH3 at 0 °C and then sodium azide in acetone/H2O(1:1). After that, compound (76) was added to HNO3 (100%) in batches below 5 °C, after which the reaction mixture was stirred for 30 min at 0 °C, slowly warmed up to room temperature, and stirred for another 3 h. Afterwards, the reaction mixture was poured onto ice, filtered, washed with cold water, and dried in air to obtain 2-nitroamino-4,6-diazo[1,3,5]triazine (77) in a yield of 54%. The synthesis route is shown in Scheme 39.
Scheme 39.
Synthesis of 2-Nitroamino-4,6-diazido[1,3,5]triazine [53].
3.4. Nitrification of -NH2 on Tetrazine Ring C
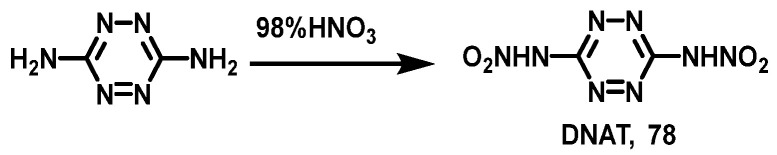
Zhang et al. [54] added 3,6-diamino-1,2,4,5-tetrazine to fuming HNO3 at 0 °C, then gradually stirred for 1 h, filtered, and dried. 3,6-dinitroamino-1,2,4,5-tetrazine (DNAT, 78) was obtained by recrystallization from ethyl acetate with 85.0% yield. The synthesis route is shown in Scheme 40.
Scheme 40.
Synthesis of 3,6-dinitroamine-1,2,4,5-tetrazine [54].
4. Conclusions
The characteristics of the used nitration agents are listed in Table 1.
Table 1.
Characteristics of different nitrification systems.
| Nitrification System | Characteristic |
|---|---|
| HNO3 | Cheap. To nitrate different azole rings, different concentrations of HNO3 are required. |
| HNO3/H2SO4 | The nitration ability is stronger than that of HNO3, and it is often used for the nitration of a variety of azole compounds. According to the structural characteristics of different azole rings and the difficulty of the aromatic electrophilic substitution reaction, a suitable ratio of HNO3/H2SO4 should be selected for nitration. The reaction conditions are mostly mild, the required temperature range is generally from 20 °C to 100 °C, the reaction time is about 1 h to 2.5 h, and the yield can reach about 80% or higher. For some azole substrates with relatively inert reaction activity (such as the existence of strong electron-withdrawing group), the reaction rate is generally slow and the reaction time needs to be extended to 10–48 h. |
| HNO3/Ac2O | Compared with the nitrating reagent HNO3/H2SO4, the HNO3/Ac2O mixture has a weaker nitrification ability, but the acid anhydride can effectively reduce the oxidation of HNO3. |
| NO2BF4 | An environmentally friendly nitrating reagent without waste acid treatment. The reaction condition is relatively mild, and the temperature is easy to control. It is non-oxidizing and has high reaction selectivity. It can be used for the aromatic electrophilic nitration reaction of various azole rings, acetonitrile is often used as the solvent, the reaction time is generally 10 h, and the yield is 30–50%. |
| N2O5 | N2O5 is a green nitrification reagent that can be used for the nitration of sensitive compounds. Compared with nitration reagents such as HNO3, HNO3/H2SO4, HNO3/acid anhydride, it has the following advantages: no need for waste acid treatment, the reaction has less heat release and the temperature is easy to control, the post-treatment is simple as only the solvent needs to be evaporated, the reaction is usually carried out with CH2Cl2 as solvent, the temperature is generally from 20 °C to 100 °C, and the yield is about 90%. |
| HNO3/P2O5 | In this system, P2O5 is not only a dehydrating agent, but also a nitrification accelerator. The nitration system is suitable for the nitration of aromatics as well as amines. |
| Nitrate | Under catalysis, some nitrates can also act as nitrating reagents, which are mainly used in the synthesis of some nitropyrazole compounds. Their nitrating capacities are as follows: Bi(NO3)3 > AgNO3 > KNO3 > NaNO3 > NH4NO3 > Pb(NO3)2 > Ba(NO3)2. |
The nitration methods of nitrogen-rich heterocyclic EMs of azoles (imidazole, pyrazole, triazole, tetrazole, oxadiazole) and azines (pyrazine, pyridazine, triazine, tetrazine) were reviewed. Most of the above nitro-containing nitrogen-rich heterocyclic EMs have higher density, outstanding enthalpy of formation, and excellent oxygen balance. Generally speaking, nitrogen-rich compounds with high density and enthalpy of formation always have high detonation performance. Although there are many nitration methods of nitrogen-rich heterocyclic EMs reported in the literature, there are still some problems that need to be explored and studied. The authors believe that the following aspects of research should be noted in the future:
Nitramino-containing nitrogen-rich EMs have higher sensitivity, and these materials can be used as primary explosives. From the viewpoint of chemical reactivity, the nitro group attached to the heterocyclic carbon adjacent to the nitrogen on the heterocyclic ring is unstable to hydrolysis. The existence of adjacent C-amino groups will improve the stability of the ring, and the lone pair of electrons on the amino nitrogen provides electrons to the ring system, thus, the sensitivity of the nitro group to hydrolysis can be significantly reduced. In addition, the presence of amino and nitro groups will boost the formation enthalpy, oxygen balance, density, and stability of the compound, thereby enhancing the detonation and safety of the compound [6,20,55]. Therefore, when designing the molecular structure of EMs, it should be designed as far as possible with compounds where nitro and amino groups cross, such as 3,6-diamino-4,6-dinitropyridazine-1-oxide (Scheme 36) and 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) (Scheme 37);
Applying organic synthesis technologies, such as ultrasound and microwave, to the nitration process of nitrogen-rich heterocyclic energetic compounds to shorten the time and improve the overall yield is imperative;
In view of the traditional methods for synthesizing nitro-containing heterocyclic energetic compounds, including a series of problems brought about by the application of oleum sulfuric acid and fuming nitric acid, it is necessary to continue research so as to discover new nitrification methods to adapt to new needs, especially paying more attention to the development of some low-toxic, cheap, efficient, and environmentally friendly nitrification strategies to adapt to the implementation of sustainable development strategies and the practical application of green chemistry.
Author Contributions
Conceptualization, X.W. and Y.L.; investigation, W.Z. and F.S.; writing—original draft preparation, Y.L. and W.Z.; writing—review, X.W. and Y.L.; editing, F.S.; Y.L. and W.Z. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang C., Chen X., Bai Y., Guo Z.Q., Song J.R., Ma H.X. 6-((2H-tetrazol-5-yl)-amino)-1,2,4,5-tetrazin-3(2H)-one: High-nitrogen insensitive energetic compound stabilized by π-stacking and hydrogen-bonding interaction. Chin. J. Energetic Mater. 2020;28:182–189. [Google Scholar]
- 2.Peng F., Yao Y., Liu H., Ma Y. Crystalline LiN5 predicted from first-principles as a possible high-energy material. J. Phys. Chem. Lett. 2015;6:2363–2366. doi: 10.1021/acs.jpclett.5b00995. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y., Lin Q., Wang P., Lu M. Syntheses, crystal structures and properties of a series of 3D metal-inorganic frameworks containing pentazolate anion. Chem. Asian J. 2018;13:2786–2790. doi: 10.1002/asia.201800476. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C., Sun C., Hu B., Yu C., Lu M. Synthesis and characterization of the pentazolate anion cyclo-N5-in(N5)6(H3O)3(NH4)4Cl. Science. 2017;355:374–376. doi: 10.1126/science.aah3840. [DOI] [PubMed] [Google Scholar]
- 5.Chavez D.E., Parrish D.A., Mitchell L. Energetic Trinitro- and Fluorodinitroethyl Ethers of 1,2,4,5-Tetrazines. Angew. Chem. Int. Ed. 2016;55:8666–8669. doi: 10.1002/anie.201604115. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Liu Y.J., Song S.W., Yang Z.J., Qi X.J., Wang K.C., Liu Y., Zhang Q.H., Tian Y. Accelerating the discovery of insensitive high-energy-density materials by a materials genome approach. Nat. Commun. 2018;9:2444. doi: 10.1038/s41467-018-04897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas M.K., Carles M.S. Nitrogen-Rich Tetrazolium Azotetrazolate Salts: A New Family of Insensitive Energetic Materials. Chem. Mater. 2008;20:1750–1763. [Google Scholar]
- 8.Wang Y., Ye J., Yang N., Ma H.X., Zhang Y.Z., Guo Z.Q. Strong intermolecular interaction induced methylene-bridged asymmetric heterocyclic explosives. CrystEngComm. 2021;23:7635–7642. doi: 10.1039/D1CE01083B. [DOI] [Google Scholar]
- 9.Fu W., Zhao B., Zhang M., Li C., Gao H., Zhang J., Zhou Z. 3,4-Dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine (HANTP) and its salts: Primary and secondary explosives. J. Mater. Chem. A. 2017;5:5044–5054. doi: 10.1039/C6TA08376E. [DOI] [Google Scholar]
- 10.Huang S., Tian J., Qi X., Wang K., Zhan Q. Synthesis of gem-dinitromethylated and fluorodinitromethylated derivatives of 5,5′-dinitro-bis-1,2,4-triazole as promising high-energy-density materials. Chem. Eur. J. 2017;23:12787–12794. doi: 10.1002/chem.201702451. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y., Wang Q., Shen C., Lin Q., Wang P., Lu M. A series of energetic metal pen-tazolate hydrates. Nature. 2017;549:78–81. doi: 10.1038/nature23662. [DOI] [PubMed] [Google Scholar]
- 12.Yin P., Zhang Q., Shreeve J.M. Dancing with energetic nitro-gen atoms: Versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials. Acc. Chem. Res. 2016;49:14–16. doi: 10.1021/acs.accounts.5b00477. [DOI] [PubMed] [Google Scholar]
- 13.Dippold A.A., Klapötke T.M. Synthesis and Characterization of 5-(1,2,4-Triazol-3-yl)tetrazoles with Various Energetic Functionalities. Chem. An. Asian J. 2013;8:1463–1471. doi: 10.1002/asia.201300063. [DOI] [PubMed] [Google Scholar]
- 14.Klapötke T.M., Preimesser A., Stierstorfer J. Energetic derivatives of 4,4’,5,5’-tetranitro-2,2′-bisimidazole(TNBI) Z. Anorg. Allg. Chem. 2012;638:1278–1286. doi: 10.1002/zaac.201200188. [DOI] [Google Scholar]
- 15.Li Y.N., Shu Y.J., Zhang S.Y., Wang B.Z., Zhai L.J. Synthesis and Thermal Properties of 4,4′,5,5′-Tetranitro-2,2′-biimidazole and Its Energetic Ion Salts. Chin. J. Energ. Mater. 2017;25:298–303. [Google Scholar]
- 16.Klapötke T.M., Preimesser A., Stierstorfer J. Energetic Derivatives of 2-Nitrimino-5,6-dinitrobenzimidazole. Propell. Explos. Pyrot. 2015;40:60–66. doi: 10.1002/prep.201400045. [DOI] [Google Scholar]
- 17.Yin P., He C., Shreeve J.M. Fully C/N-Polynitro-Functionalized 2,2′-Biimidazole Derivatives as Nitrogen- and Oxygen-Rich Energetic Salts. Chem. Eur. J. 2016;22:2108–2113. doi: 10.1002/chem.201504407. [DOI] [PubMed] [Google Scholar]
- 18.Chand D., He C., Mitchell L.A., Parrish D.A., Shreeve J.M. Electrophilic iodination: A gateway to high iodine compounds and energetic materials. Dalton Trans. 2016;45:13827–13833. doi: 10.1039/C6DT02438F. [DOI] [PubMed] [Google Scholar]
- 19.Ravi P., Tewari S.P. Facile and environmentally friendly synthesis of nitropyrazoles using montmorillonite K-10 impregnated with bismuth nitrate. Catal. Commun. 2012;19:37–41. doi: 10.1016/j.catcom.2011.12.016. [DOI] [Google Scholar]
- 20.Fischer D., Gottfried J.L., Klapötke T.M., Karaghiosoff K., Stierstorfer J., Witkowski T.G. Synthesis and Investigation of Advanced Energetic Materials Based on Bispyrazolylmethanes. Angew. Chem. Int. Ed. 2016;55:16132–16135. doi: 10.1002/anie.201609267. [DOI] [PubMed] [Google Scholar]
- 21.Wang G.L., Lu T., Fan G.J., Li C., Yin H. The Chemistry and Properties of Energetic Materials Bearing [1,2,4]Triazolo[4,3-b][1,2,4,5]tetrazine Fused Rings. Chem. Asian J. 2018;13:3718–3722. doi: 10.1002/asia.201801145. [DOI] [PubMed] [Google Scholar]
- 22.Yin P., Zhang J.H., Parrish D.A., Shreeve J.M. Energetic N,N′-Ethylene-Bridged Bis(nitropyrazoles): Diversified Functionalities and Properties. Chem. Eur. J. 2014;20:16529–16536. doi: 10.1002/chem.201404991. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y.Q., Parrish D.A., Shreeve J.M. 4-Nitramino-3,5-dinitropyrazole Based Energetic Salts. Chem. Eur. J. 2012;18:987–994. doi: 10.1002/chem.201102773. [DOI] [PubMed] [Google Scholar]
- 24.He C.L., Zhang J.H., Parrish D.A., Shreeve J.M. 4-Chloro-3,5-dinitropyrazole: A precursor for promising insensitive energetic compounds. Mater. Chem. A. 2013;1:2863–2868. doi: 10.1039/c2ta01359b. [DOI] [Google Scholar]
- 25.Tang Y.X., Kumar D., Shreeve J.M. Balancing Excellent Performance and High Thermal Stability in a Dinitropyrazole Fused 1,2,3,4-Tetrazine. J. Am. Chem. Soc. 2017;139:13684–13687. doi: 10.1021/jacs.7b08789. [DOI] [PubMed] [Google Scholar]
- 26.Kumar D., Tang Y.X., He C.L., Imler G.H., Parrish D., Shreeve J.M. Multipurpose Energetic Materials by Shuffling Nitro Groups on a 3,3′-Bipyrazole Moiety. Chem. Eur. J. 2018;24:17220–17224. doi: 10.1002/chem.201804418. [DOI] [PubMed] [Google Scholar]
- 27.Yin P., Zhang J.H., Mitchell L.A., Parrish D.A., Shreeve J.M. 3,6-Dinitropyrazolo[4,3-c]pyrazole-Based Multipurpose Energetic Materials through Versatile N-Functionalization Strategies. Angew. Chem. Int. Ed. 2016;55:12895–12897. doi: 10.1002/anie.201606894. [DOI] [PubMed] [Google Scholar]
- 28.Ma H.X., Xiao H.M., Song J.R., Ju X.H., Zhu W., Yu K.B. Molecular structure of 4-amino-1,2,4- triazol-5-one and a density-functional theoretical investigation of its dimers and crystal band structure. Chem. Phys. 2008;344:79–89. doi: 10.1016/j.chemphys.2007.11.020. [DOI] [Google Scholar]
- 29.Chang P., Zhou C., Wang B.Z., Huang X.P., Zhu Y. Safety Analysis of Two NTO Synthesis Processes. J. Chem. Eng. Chin. Univ. 2018;32:1223–1227. (In Chinese) [Google Scholar]
- 30.Huang X.P., Chang P., Wang B.Z., Li P.R., Wang M.C., Fan X.Z., Feng H.L. Recycling use of waste acids in preparation of 3-nitro-1,2,4-triazol-5-one (NTO) Chin. J. Energ. Mater. 2013;21:363–366. (In Chinese) [Google Scholar]
- 31.Aizikovich A., Shlomovich A., Cohen A. The nitration pattern of energetic 3,6-diamino-1,2,4,5-tetrazine derivatives containing azole functional groups. Dalton Trans. 2015;44:13939–13946. doi: 10.1039/C5DT01641J. [DOI] [PubMed] [Google Scholar]
- 32.Astachov A.M., Revenko V.A., Buka E.S. Comparative characteristics of two isomeric explosives: 4-nitro-5-nitrimino-1H-1,2,4-triazole and 3-nirro-5-nitrimino-1,4H-1,2,4-triazole; Proceedings of the Seminar on New Trends in Research of Energetic Materials; Pardubice, Czech Republic. 20 April 2004. [Google Scholar]
- 33.Dippold A.A., Klapötke T.M. Nitrogen-Rich Bis-1,2,4-triazoles-A Comparative Study of Structural and Energetic Properties. Chem. Eur. J. 2012;18:16742–16753. doi: 10.1002/chem.201202483. [DOI] [PubMed] [Google Scholar]
- 34.Yin P., Shreeve J.M. From N-Nitro to N-Nitroamino: Preparation of High-Performance Energetic Materials by Introducing Nitrogen-Containing Ions. Angew. Chem. Int. Ed. 2015;54:14513–14517. doi: 10.1002/anie.201507456. [DOI] [PubMed] [Google Scholar]
- 35.Klapötke T.M., Leroux M., Schmid P.C., Stierstorfer J. Energetic Materials Based on 5,5′-Diamino-4,4′-dinitramino-3,3′-bi-1,2,4-triazole. Chem. Asian J. 2016;11:844–851. doi: 10.1002/asia.201500701. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y.Q., Parrish D.A., Shreeve J.M. Derivatives of 5-nitro-1,2,3-2H-triazole-high performance energetic materials. J. Mater. Chem. A. 2013;1:585–593. doi: 10.1039/C2TA00136E. [DOI] [Google Scholar]
- 37.Thottempudi V., Forohor F., Parrish D.A., Shreeve J.M. Tris(triazolo)benzene and Its Derivatives: High-Density Energetic Materials. Angew. Chem. Int. Ed. 2012;51:9881–9885. doi: 10.1002/anie.201205134. [DOI] [PubMed] [Google Scholar]
- 38.Stierstorfer J., Tarantik K.R., Klapötke T.M. New Energetic Materials: Functionalized 1-Ethyl-5-aminotetrazoles and 1-Ethyl-5-nitriminotetrazoles. Chem. Eur. J. 2009;15:5775–5792. doi: 10.1002/chem.200802203. [DOI] [PubMed] [Google Scholar]
- 39.Fischer D., Klapötke T.M., Stierstorfer J. 1,5-Di(nitramino)tetrazole: High Sensitivity and Superior Explosive Performance. Angew. Chem. Int. Ed. 2015;54:10299–10302. doi: 10.1002/anie.201502919. [DOI] [PubMed] [Google Scholar]
- 40.Kumar D., Imler G.H., Parrish D.A., Shreeve J.M. Aminoacetonitrile as precursor for nitrogen rich stable and insensitive asymmetric N-methylene-C linked tetrazole-based energetic compounds. Mater. Chem. A. 2017;5:16767–16775. doi: 10.1039/C7TA05394K. [DOI] [Google Scholar]
- 41.Klapötke T.M., Martin F.A., Stierstorfer J. N-Bound Primary Nitramines Based on 1,5-Diaminotetrazole. Chem. Eur. J. 2012;18:1487–1501. doi: 10.1002/chem.201102142. [DOI] [PubMed] [Google Scholar]
- 42.Fischer D., Klapötke T.M., Stierstorfer J., Szimhardt N. 1,1′-Nitramino-5,5′-bitetrazoles. Chem. Eur. J. 2016;22:4966–4970. doi: 10.1002/chem.201600177. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y.X., Gao H.X., Mitchell L.A., Parrish D.A., Shreeve J.M. Syntheses and Promising Properties of Dense Energetic 5,5′-Dinitramino-3,3′-azo-1,2,4-oxadiazole and Its Salts. Angew. Chem. Int. Ed. 2016;55:3200–3203. doi: 10.1002/anie.201600068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y.X., Gao H.X., Mitchell L.A., Parrish D.A., Shreeve J.M. Enhancing Energetic Properties and Sensitivity by Incorporating Amino and Nitramino Groups into a 1,2,4-Oxadiazole Building Block. Angew. Chem. Int. Ed. 2016;55:1147–1150. doi: 10.1002/anie.201509985. [DOI] [PubMed] [Google Scholar]
- 45.Hermann T.S., Karaghiosoff K., Klapötke T.M., Stierstorfer J. Synthesis and Characterization of 2,2′-Dinitramino-5,5′-bi(1-oxa-3,4-diazole) and Derivatives as Economic and Highly Dense Energetic Materials. Chem. Eur. J. 2017;23:12087–12091. doi: 10.1002/chem.201702191. [DOI] [PubMed] [Google Scholar]
- 46.Sheremetev A.B., Aleksandrova N.S. Reactions of 3-amino-4-methylfurazan with nitrating agents. Russ. Chem. Bull. Int. Ed. 2005;54:1665–1669. doi: 10.1007/s11172-006-0027-3. [DOI] [Google Scholar]
- 47.Fischer D., Klapötke T.M., Reymann M., Stierstorfer J. Dense Energetic Nitraminofurazanes. Chem. Eur. J. 2014;20:6401–6411. doi: 10.1002/chem.201400362. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J.H., Dharavath S., Mitchell L.A., Parrish D.A., Shreeve J.M. Bridged bisnitramide-substituted furazan-based energetic materials. J. Mater. Chem. A. 2016;4:16961–16967. doi: 10.1039/C6TA08055C. [DOI] [Google Scholar]
- 49.Klapötke T.M., Schmid P.C., Stierstorfer J. Crystal Structures of Furazanes. Crystals. 2015;5:418–432. doi: 10.3390/cryst5040418. [DOI] [Google Scholar]
- 50.Gospodinov I., Klapötke T.M., Stierstorfer J. Energetic Functionalization of the Pyridazine Scaffold: Synthesis and Characterization of 3,5-Diamino-4,6- dinitropyridazine-1-Oxide. Eur. J. Org. Chem. 2018;8:1004–1010. doi: 10.1002/ejoc.201800068. [DOI] [Google Scholar]
- 51.Zhou X.L., Liu Z.L., Cheng J., Zhao X. Study on nitration reaction of 2,6-diacetamidopyrazine-1-oxide. Explos. Mater. 2014;43:16–21. (In Chinese) [Google Scholar]
- 52.Liu J.D., Shen C., Wang P.C., Lu M. Synthesis and Properties of 2,6-Bis(picryIamino)-3,5-dinitroPyrazine. Chin. J. Energetic Mater. 2017;25:486–492. [Google Scholar]
- 53.Huang Y.G., Zhang Y.Q., Shreeve J.M. Nitrogen-Rich Salts Based on Energetic Nitroaminodiazido [1,3,5]triazine and Guanazine. Chem. Eur. J. 2011;17:1538–1546. doi: 10.1002/chem.201002363. [DOI] [PubMed] [Google Scholar]
- 54.Zhang T.H., Du J., Li Z.M., Lin X., Wang L., Yang L., Zhang T.L. Alkali metal salts of 3,6-dinitramino-1,2,4,5-tetrazine: Promising nitrogen-rich energetic materials. Cryst. Eng. Comm. 2019;21:765–772. doi: 10.1039/C8CE01827H. [DOI] [Google Scholar]
- 55.Tang Y.X., He C.L., Imler G.H., Parrish D.A., Shreeve J.M. A C–C bonded 5,6-fused bicyclic energetic molecule: Exploring an advanced energetic compound with improved performance. Chem. Commun. 2018;54:10566–10569. doi: 10.1039/C8CC05987J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.