Abstract
The medical term xerostomia refers to the subjective sensation of oral dryness. The etiology seems to be multifactorial with the most frequently reported causes being the use of xerostomic medications, neck and head radiation, and systematic diseases (such as Sjögren’s syndrome). Xerostomia is associated with an increased incidence of dental caries, oral fungal infections, and difficulties in speaking and chewing/swallowing, which ultimately affect the oral health-related quality of life. The development of successful management schemes is regarded as a highly challenging project due to the complexity of saliva. This is why, in spite of the fact that there are therapeutic options aiming to improve salivary function, most management approaches are alleviation-oriented. In any case, polymers are an integral part of the various formulations used in every current treatment approach, especially in the saliva substitutes, due to their function as thickening and lubricating agents or, in the case of mucoadhesive polymers, their ability to prolong the treatment effect. In this context, the present review aims to scrutinize the literature and presents an overview of the role of various polymers (or copolymers) on either already commercially available formulations or novel drug delivery systems currently under research and development.
Keywords: xerostomia, artificial saliva, salivary substitutes, salivary stimulants, advanced polymers, mucoadhesive
1. Introduction
Xerostomia (or dry mouth) is the medical term used to describe the subjective sensation of oral dryness, which commonly exists as a consequence of reduced salivary flow (hyposalivation) [1,2,3]. However, despite its connection to salivation, studies have shown that in various cases, patients with xerostomia appear to have normal salivary flow [4,5,6]. Hence, the term “symptomatic” xerostomia (or else “pseudo” xerostomia) is nowadays used to refer to oral dryness despite the salivary gland function [7,8,9]. In general, patients with xerostomia suffer symptoms that significantly affect their health as well as social and emotional aspects of their life. Currently, the diagnosis and therapeutic approaches of this condition vary, while it is difficult to achieve favorable results, since the etiology seems to be multifactorial. The majority of the management options aim to relieve oral discomfort by keeping mouth moisture at an acceptable level. In most of these therapeutic scenarios, polymers are an integral part of the different formulations used at every current treatment approach (as discussed in detail hereunder) due to their pivotal role as thickening and lubricating agents, while mucoadhesive polymers (i.e., polymers, synthetic or natural, which are capable of attaching to mucosal surfaces) are frequently used for prolonging the treatment effect. In this vein, this review sets the foundations for the identification of the polymer’s exact task on the xerostomia’s treatment. Characteristically, specific examples of commercially available products with a polymeric base and innovative drug delivery systems currently under research are provided in order to establish the vital role of polymers at the development of various management schemes, whether in commerce or in the research field. Furthermore, the specific properties that polymers attribute to the products are revealed.
2. Approach of the Review
The aim of this review was to scrutinize the literature regarding xerostomia and salivary gland hypofunction and, specifically, the underlying causes, the impact on quality of life, and the recommended management approaches. Significant attention was paid especially to the polymers’ role in the various therapeutic approaches. A literature search was conducted during September 2021 to January 2022, utilizing the electronic databases of MEDLINE/PubMed, SCOPUS, and Google scholar, for original research articles in the English language, already published, or at “in press” status in peer-reviewed literature. The terms “xerostomia”, “saliva substitute”, “artificial saliva”, “salivary stimulation”, “cholinergic agents”, “pilocarpine”, “cevimeline”, “xerostomia drug formulations”, and “dry mouth drug formulations” were used. Related links until 2021, as well as articles referenced in the initially retrieved papers, have also been taken into consideration and were included if pertinent. After a careful analysis of the output of these searches, 132 articles fitted our criteria, as referenced below, and were included in this review.
In regard to the management of xerostomia, various management approaches of xerostomia, concerning either already commercially available formulations or delivery systems currently under research and development, have been taken into consideration. Additionally, the crucial role that polymers play in the development of the various management approaches in this research is indisputable, being referred to in 68% of the 63 studies comprised in the section for disease management.
3. Saliva Production in Humans
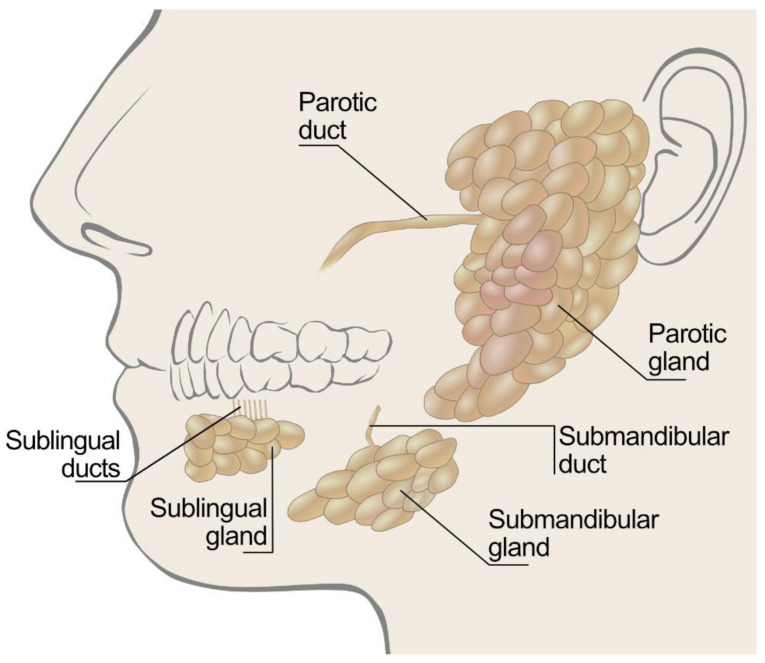
Saliva is a very complex fluid that is mainly composed of water combined with electrolytes, minerals, buffers, growth factors, enzymes, cytokines, proteins, and immunoglobulins [10,11,12,13]. In humans, it is produced from the major and minor salivary glands of the mouth, as depicted in Figure 1.
Figure 1.
Illustration of salivary glands types and position.
Ninety percent of the average daily salivary secretion (≈1–1.5 L) is produced by the major salivary glands (this is the parotid, submandibular, and sublingual glands), while the minor salivary glands spontaneously produce the remaining 10% of the total salivary secretions [12,14]. The minor glands are important, as their ducts open onto most areas of the oral mucosa except for the area covering the dorsum of the tongue, the anterior part of the hard palate, and the gingiva. They can be grouped into lingual, labial, buccal, palatine, and glossopalatine [1]. The secretions from the salivary glands are innervated by both the parasympathetic and sympathetic nervous systems [15,16]. Specifically, when parasympathetic innervations dominate, the secretions are more watery, whereas the sympathetic system produces a more viscous flow, since the secretions contain more proteins from acinar cells [17]. Therefore, a sensation of dryness may occur, for example, during episodes of acute anxiety or stress, which cause changes in salivary composition owing to predominant sympathetic stimulation during such periods [18]. In general, there is great variability in the salivary flow rate for each individual. The average unstimulated whole salivary flow rate is 0.3 to 0.4 mL/min during waking hours, with an unstimulated flow rate below 0.1 mL/min indicating hyposalivation [19,20,21,22].
4. Diagnosis of Xerostomia
Xerostomia is often referred as hyposalivation [4]; however, these two terms do not correspond to identical conditions and should not be used interchangeably. Hyposalivation refers solely to the objective observation of reduced salivary flow due to external or internal influences, while xerostomia encompasses the subjective sensation of oral dryness [23]. The clinical method most often employed for the diagnosis of salivary dysfunction is the sialometry test, in which hyposalivation is considered to appear when salivary flow rates are under 0.1 mL/min at rest (UWS) or 0.7 mL/min under stimulation (SWS) [24].
A systematic approach is used to distinguish patients with symptoms of xerostomia, using measurable salivary gland hypofunction. The diagnosis of xerostomia requires a thorough medical history, which includes a detailed description of the symptoms (patients with xerostomia often complain of a dry and sticky sensation in the mouth, which makes it considerable difficulty to swallow and speak, while a decrease in taste sensation might also be presented) and of the medication used [25,26,27]. Several scientifically validated questionnaires have been designed specifically to evaluate a possible salivary glandular dysfunction and xerostomia [5], among which the questionnaire developed by Fox et al. [28,29,30] is the most frequently used to take the medical history of patients. Salivary hypofunction could also be diagnosed using four additional clinical measures: dryness of the lips, dryness of the buccal mucosa, absence of saliva produced by gland palpation, and total decayed–missing–filled teeth (DMFT) [31].
5. Causes of Xerostomia
Depending on their nature, the causes of xerostomia can be classified as systemic or local [32]. Based on the duration of the symptoms, the condition is qualified as persistent or periodic. Systemic causes of xerostomia include endocrinological (e.g., diabetes mellitus, autoimmune thyroid diseases), autoimmune (e.g., Sjögren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus), infectious (e.g., hepatitis C virus), and granulomatous (e.g., tuberculosis and sarcoidosis) diseases. Local factors that are recognized as responsible for xerostomia are multiple medications (polypharmacy), radical radiotherapy for treatment of head and neck malignancies, and lifestyle factors, such as alcohol, tobacco, and caffeine consumption [33,34,35]. Interestingly, a correlation between xerostomia and the recent coronavirus disease-19 (COVID-19) has been reported. Specifically, according to the results of the study conducted by Fantozzi et al., 45.9% of patients with confirmed SARS-CoV-2 infection suffered xerostomia, with a significant majority (76.5%) of them mentioning that it was their first-time experiencing xerostomia in their lifetime [36].
5.1. Local Factors
Polypharmacy (the prescription of multiple medicines) is regarded as the most common cause of xerostomia, which in some way explains the aforementioned association between the elderly population and the prevalence of dry mouth, since chronic diseases and multi-morbidities of geriatric patients result in widespread polymedication [37,38]. From 2005 to 2011, in the United States, more than one-third of older adults used ≥5 prescription medications concurrently [39], and more than 75% of people over the age of 65 took at least one medication prescription that may affect salivary function [40]. It is estimated that more than 400 medications favor the occurrence of xerostomia and affect the salivary gland function [41]. Although the exact mechanisms by which some drugs cause xerostomia are still unknown, the common offenders of xerostomia include antiparkinsonian medications, antipsychotic agents, antidepressant medications, diuretic agents; opioids; cytotoxic agents, and antihypertensive medications [42,43,44]. While drug-induced xerostomia is generally reversible, the conditions for which these medications are prescribed are frequently chronic. A detailed list of agents that cause drug-related xerostomia is presented in Table 1.
Table 1.
| Category | Drug Substance |
|---|---|
| Antidepressant agents and antipsychotic agents | citalopram, fluoxetine, paroxetine, sertraline, venlafaxine, amitriptyline, imipramine, reboxetine, bupropion hydrochloride, clozapine, chlorpromazine, haloperidol, olanzapine |
| Anticholinergic agents | dicyclomine, mepenzolate |
| Antihypertensive agents | captopril, clonidine, methyldopa, prazosin |
| Antiparkinsonian agents | biperiden, selegiline |
| Diuretic agents | spirnolactone, chlorothiazide, furosemide, hydrochlorothiazide |
| Opioids | morphine, codeine, methadone, pethidine |
| Immunostimulants | interferon-alpha |
Radiotherapy is one of the prominent integral components in the multidisciplinary management of head and neck cancer, yet it produces considerable acute and long-term side effects. One of the most frequent complications of conventional radiotherapy is xerostomia, since the major salivary glands are usually included in the radiation portals [46,47,48,49]. Specifically, it is assumed that the radiation exposure harms the blood vessels or nerves supplying these glands and not the salivary glands themselves [50]. However, there is a consensus that xerostomia is sufficiently limited by keeping the mean dose to the total parotid volume below 26 Gy [51].
5.2. Systematic Diseases
Sjögren’s syndrome is an autoimmune disease characterized by the inflammation of the exocrine glands, mainly of the lacrimal and salivary glands [52,53]. The two categories of the disease are the primary form, which is characterized by the independent occurrence of the disease, and the secondary form, which is associated with other autoimmune diseases, such as rheumatoid arthritis, scleroderma, and systemic lupus erythematosus. Some common oral manifestations of Sjögren’s syndrome are mainly xerostomia and hyposalivation, autoimmune sialadenitis, and dental caries [54]. The xerostomia that is associated with primary and secondary Sjögren’s syndrome has been attributed to the progressive lymphocytic infiltration that gradually destroys the secretory acini of the major and minor salivary glands.
Diabetes mellitus is a chronic multi-systemic metabolic disease characterized by hyperglycemia due to either a deficiency of insulin secretion or resistance to the action of insulin or both [55,56]. The oral manifestations and complications related to diabetes mellitus include, among others, xerostomia, tooth decay, gingivitis, periodontal disease, oral candidiasis, burning mouth, and altered taste [57,58]. Researchers have identified a bidirectional adverse relationship between diabetes and oral diseases [59]. Diabetic patients suffer from xerostomia and salivary gland hypofunction, which may be related to polydipsia and polyuria, autonomic neuropathies, and alterations in the basement membranes of salivary glands.
All the aforementioned xerostomia’s reasons are summarized in Figure 2.
Figure 2.
The classification of xerostomia’s causes as systemic or local.
6. Effects of Xerostomia
Although xerostomia can affect a person at any age, it appears to be most prevalent in postmenopausal women and the elderly population [60]. In a study of over 5000 individuals, Johansson et al. [61] examined the prevalence, the progression, the yearly incidence of xerostomia, and its effect on 50- to 80-year-old people. In all age groups, xerostomia was significantly more prevalent in women than in men; the prevalence increased with age and was more frequent during night-time. Xerostomia is associated with an increased incidence of dental caries, oral fungal infections (e.g., candidiasis), halitosis or burning mouth, and periodontal disease. Furthermore, clinical effects include dysphagia, dysgeusia, and difficulty in speaking, chewing, and swallowing, which ultimately affect oral health-related quality of life (OHRQOL) [50,62,63,64,65,66]. Additionally, looking at the literature, a correlation between decreased salivary flow rate and low nutritional assessment score is suggested, as determined by body mass index (BMI), mid-arm circumference, triceps skinfold thickness, and serum albumin level [67,68].
7. Management of Xerostomia
The establishment of the correct diagnosis is considered as the most crucial step in the management of patients with xerostomia, since it encompasses the distinguishment of patients with subjective complaints from those presenting salivary gland hypofunction as well [4]. Once a diagnosis is established and an underlying etiology is identified, a stepwise management approach can be implemented, aiming to institute preventive measures, alleviate symptoms, treat oral manifestations, and improve salivary function.
7.1. Preventive Approaches
In a first step, preventive measures must be followed by every patient who suffers from xerostomia in order to prevent the development of oral infections associated to the disorder. Specifically, diligent oral hygiene and regular dental care—with examinations very 4–6 months—are essential [69]. It is also important to inform patients about the role of dietary sugars and refined carbohydrates in the development of caries, so their intake is minimized or discouraged [1,70,71]. Furthermore, a topical application of fluorides (e.g., fluoridated toothpaste, daily fluoridated mouth washes, and application of fluoridated gel) is also beneficial for the management of hyposalivation-induced caries, especially in cases of patients whose xerostomia has resulted from radiation therapy to the head and neck [72,73,74,75].
7.2. Symptomatic Relief: Salivary Substitutes
The symptomatic relief or control of oral dryness includes hydration (frequent sipping of water), discontinuation or reduction in xerogenic medications, and elimination of common dry mouth offenders, such as tobacco and alcohol [76]. Moreover, artificial salivary substitutes (i.e., commercial products containing specific ingredients, whose properties resemble those of the natural saliva) are frequently used as symptomatic treatments for patients with decreased salivary flow rate [77,78]. In fact, they act as oral lubricants that maintain the lubrication of the mucosa and, hence, relieve the sensation of dryness, without stimulating the salivary flow. However, it should be pointed out that saliva substitutes’ action present limited duration and, therefore, a frequent re-application is required, which creates issues around patient adherence and increases the cost of therapy.
An ideal salivary substitute should resemble the properties of human saliva and, simultaneously, provide a pleasant taste aiming to a convenient self-administration and increased patient compliance [79,80]. The development of artificial saliva requires in-depth understanding of both biological and rheological (e.g., viscosity and film-forming wettability) properties of human saliva, which is composed of a mixture of macromolecules. Specifically, human saliva is regarded as a non-Newtonian fluid, because of the salivary glycoproteins’ presence. This characterization means, in essence, that saliva’s viscosity varies, depending on the shear rate [81]. Thus, the efficacy of artificial saliva as a lubricant is partially dependent on its viscosity and how this changes with shear rates [82]. Since different shear rates may be present in the oral cavity—from 60 to 160 s−1 during processes such as swallowing and speaking—the high importance of the aforementioned phenomenon is apparently highlighted [83]. So, salivary substitutes are expected to have a viscoelastic pattern similar to normal human saliva in order to provide similar viscosity and film-forming properties. Taking into consideration that the principal aim of saliva substitutes is to ensure the lubrication of oral tissues, it is obvious that apart from the viscosity, lubrication is considered as another important factor for the clinical acceptance of saliva substitutes. Concerning the biological properties of artificial saliva, substances of natural origin, including enzymes such as lysozyme and peroxidase or proteins such as lactoferrin and mucin, may be utilized in order to provide high biocompatibility [84].
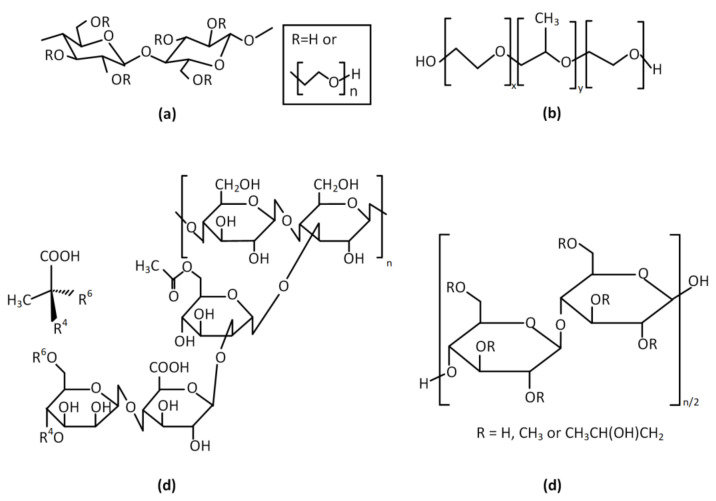
Different dosage forms are available in the market such as cleansers, gels, sprays, and lozenges. The majority of the commercial salivary substitutes are commonly based on animal mucin or on polymeric thickening and moisturizing agents, such as cellulose-based polymers (e.g., carboxymethylcellulose (CMC), hydroxyethylcellulose (HEC), hydroxypropylmethylcellulose (HPMC) [85,86]) and water-soluble polymers, such as xanthan gum and carbomer [87]. The referred polymers are integrated in saliva substitutes, since they provide certain properties at formulations and, hence, fulfill the significant aforementioned standards. Specifically, CMC—the most commonly used polymer in the saliva substitutes’ development, even though it is not a natural lubricant—has been proved as a decent clinical choice for the basis of a saliva substitute, improving a formulation’s viscoelastic properties. It was shown that the wetting properties of CMC-containing saliva substitutes on human enamel were significantly better than those of human whole saliva and comparable with those on human oral mucosa [88]. Moreover, a prospective cross-over study in patients with xerostomia comparing four different polymers used in saliva substitutes showed that the majority of patients preferred the CMC-based product due to its palatability and easy handling. Moreover, saliva substitutes containing other cellulose derivatives (i.e., sodium carboxymethyl cellulose (SCMC), methyl cellulose (MC), and HPMC) have been prepared. The examined polymers provide to final formulations some physical properties resembling those of human saliva, rendering them high-quality standard formulations [89]. As for xanthan, it is an anionic biopolymer with repeated chains of cellulose monosaccharides and oligosaccharides, which is utilized in the pharmaceutical industry due to its tunable thickening, stabilizing, suspending, and emulsifying properties. Characteristically, the most noticeable xanthan’s property is its very high low-shear viscosity coupled with its strongly shear-thinning character. Relatively low viscosity at high shear means that it is easy to mix, pour, and swallow, but its high viscosity at low shear gives decent suspension and coating properties and lends stability to colloidal suspensions. These features could explain why xanthan gum-containing saliva substitutes have presented synergistic effects on the elastic and rheologic properties of human whole saliva [90]. The structures of the most commonly used polymers at saliva substitutes are presented in Figure 3.
Figure 3.
Structures of commonly used polymers for artificial saliva: (a) hydroxy ethylcellulose (HEC); (b) poloxamer or Pluronic® (PEO-PPG-PEO); (c) xanthan gum; (d) hydroxypropyl methylcellulose (HPMC).
Each formulation differs from another in respect to the base substance, the chemical composition, and the viscosity. Studies have shown that the viscosity of mucin-based saliva substitutes resembles natural saliva more closely than formulations based on CMC or polyethylene oxide; notwithstanding, there is no evidence regarding whether one formulation is superior to another [91]. Patients should select different products based on the severity of xerostomia, their daily routine and, even, the time of the day [92]. Characteristically, in severe xerostomia, a gel-like salivary substitute should be used overnight, whereas a more liquid substitute is recommended as more appropriate during the day.
In any case, polymers play a crucial role in the saliva substitutes’ development and, specifically, in the exact property of mimicking the techno-functionalities of real human saliva. This is confirmed by the fact that a polymeric base substitute is trusted to be used in numerous commercially available salivary substitutes. Table 2 summarizes different commercial saliva substitutes and highlights the polymers that are utilized by these formulations.
Table 2.
Commercially available saliva substitutes mentioned in published studies.
| Dosage Forms | Brand Name | Polymers Used | Product Composition | Characteristics of the Formulation: Advantages or Disadvantages | Manufacturer | Ref. |
|---|---|---|---|---|---|---|
| Oral Sprays | Aldiamed® | CMC | Water, propylene glycol, xylitol, glycerol, microcrystalline cellulose, panthenol, CMC, sodium, sodium benzoate, lactoferrin, disodium EDTA, lysozyme, hydrochloride, aroma, Aloe Barbadensis | Significant improvement of xerostomia and increased life quality. Diminished use frequency, as compared to the other respective saliva substitutes, which may be associated to the improved results on mouth dryness. | Certmedica International | [93] |
| Artisial® | Sodium CMC | Sodium CMC, sorbitol, calcium chloride dihydrate, magnesium chloride, dipotassium phosphate, monopotassium phosphate, potassium chloride, sodium chloride | Only minimal enamel mineral loss was observed in relevant published studies. | Jouveinal Laboratoires | [94] | |
| Aqwet® | CMC | Water, CMC, sorbitol, potassium chloride, sodium chloride, magnesium chloride, calcium chloride | Improved wetting ability as compared to similar commercially available saliva substitutes; comparable properties with human saliva. | Cipla Ltd. (Mumbai, India) | [95] | |
| Biotene® | Xanthan gum | Water, glycerin, xylitol, PEG-60, hydrogenated castor oil, VP/NA copolymer, sodium benzoate, Xanthan gum, methylparaben, propylparaben sodium saccharin, cetylpyridinium chloride, limonene | Effective in reducing mouth dryness, taste alteration, and chewing difficulties. Not well-tolerated and limited acceptance from patients. | GlaxoSmithKline | [96] | |
| EMOFLUOR® | HEC | Water, glycerin, sorbitol, maltitol, ammonium phosphate, HEC, ammonium fluoride, methylparaben, sodium saccharin, sodium chloride, potassium chloride, propylparaben | Erosion-protective potential, which may be connected to the product’s film-forming properties. | Dr. Wild&Co AG | [93] | |
| Entertainer® | CMC | Water, CMC, aloe vera, glycerin, dibasic sodium phosphate, potassium chloride | High popularity among performers and voice clinicians; has gained increased interest as possible laryngeal lubricants due to quick throat comfort and vocal quality improvement. However, it has a relatively short-term effect. | KLI Corporation (Carmel, IN, USA) | [97] | |
| Glandosane® | Sodium CMC | Potassium chloride, sodium chloride, magnesium chloride, Magnesii chloridum, calcium chloride, potassium monohydrogen phosphate, sodium CMC, sorbitol | Preferred by patients due to the good taste and the easy handling. However, it has revealed a high demineralizing potential in several in vitro studies. | Helvepharm | [98] | |
| Oasis® | Copovidone | Cetylpyridinium chloride, copovidone, flavor, methylparaben, PEG-60 hydrogenated castor oil, propylparaben, sodium benzoate, sodium saccharin, water, xanthan gum, xylitol | Significantly reduced enamel loss as compared to a positive control. | Oasis Consumer Healthcare | [99] | |
| Stoppers 4® | HEC | Water, glycerin, xylitol, HEC, lysozyme, lactoferrin, glucose oxidase, spearmint (natural), sodium benzoate | Increased enamel loss as compared to a positive control. | Jocott Brands Inc. (Van Nuys, CA, USA) | [93] | |
| Oral Solutions | Act® | Poloxamer | Provides immediate but not long-lasting effect. | Sanofi | [40] | |
| Orazyme | Poloxamer and Sodium CMC |
Gluconate, aloe Barbadensis, sodium CMC, poloxamer, water | Similarly with the abovementioned oral solution, it fails to provide long-lasting effect. | Dr. Fresh | [100] | |
| Xeros® | HEC | HEC, betaine, xylitol, sodium fluoride, water, allantoin | Decreases the patients’ discomfort during night but presents more significant effects in patients whose residual secretory potential was severely compromised. | Dentaid | [101] | |
| Gels | Biotene oralbalance | HEC | Lactoperoxidase, lysozyme, glucose oxidase, lactoferrin, hydrogenated starch hydrolysate, xylitol, HEC, glyceryl polymethacrylate beta-D-glucose, aloe vera, potassium thiocyanate | Significant improvement in dryness, swallowing, and taste. Low retention time, which may be attributed to the relatively low viscosity. | GlaxoSmithKline | [87,102,103] |
| OralSeven | HEC | Hydrogenated starch hydrosylate, glycerin, water, xylitol, glyceryl acrylate, acrylic acide copolymer, HEC, aloe barbadenisis, lactoperoxidase, dextrose monohydrate, glucose oxidase, lactoferrin, lysozyme, potassium thiocyanate, cellulose gum | Considerable problems with the application and the handling of the gel were referred by patients. |
Oral7 International | [3] | |
| Lozenges | Salese | Ethylcellulose and xanthan gum | Ethyl cellulose, xanthan gum, xylitol, sodium bicarbonate, eucalyptus oil, wintergreen oil, glycerol, zinc gluconate, thymol, calcium sulfate, potassium phosphate dibasic | Significantly low erosive potential on enamel, probably due to formulation’s high pH. However, the efficacy and patients’ acceptance of higher pH products are not yet known. | Nuvora Inc. (Santa Clara, CA, USA) | [56,104] |
| SalivaSure® | CMC | Xylitol, malic acid, dibasic calcium phosphate, CMC, sodium citrate dihydrate, stearic acid, citric acid, magnesium stearate, silica colloidal | Xylitol contained in the formulation reduces plaque formation and minimizes dental caries. Furthermore, no interaction with prescription medications has been reported, and the formulation is regarded as safe for people with diabetes. Main drawback is the short-lasting relief on contact. | Scandinavian Formulas Inc. (Sellersville, PA, USA) | [102,103] |
7.3. Salivary Stimulation
One point to be highlighted here is that salivary substitutes are utilized when salivary glands are completely damaged. In case there is residual functional salivary tissue, one of the alternatives for xerostomia and hyposalivation is the use of salivary stimulants [100,105]. Generally, salivary stimulation can be divided into acid-, pharmaceutically-, and mechanically- driven [77].
Acid-driven stimulation of salivary secretion is generated by the acidification of the oral cavity, with malic and citric acid being the most commonly preferred sialogogues acid [106]. Mechanical salivary stimulation, on the other hand, includes the utilization of chewing gums—usually sugar-free and artificially sweetened with aspartame and sorbitol—acupuncture, and electrostimulation [107,108,109,110]. In xerostomia, sugarless chewing gums are used to stimulate the major salivary glands, aiming to increase the saliva secretion through mechanical stimulation and decrease the oral mucosal friction [107,111,112]. The stimulation of the saliva secretion also increases the plaque pH, reducing the risk of caries formation [113]. A gum composition includes a water-soluble bulk portion and a water-insoluble gum base, consisting of several ingredients such as fillers, elastomers, emulsifiers, sweeteners, flavoring, and texture-regulating agents. The gum base can be natural or synthetic, composed mostly of elastomers, which are polymers providing elasticity and flexibility to the gum formulation [113]. Table 3 presents the polymers used in chewing gums’ formulations, while Table 4 refers to some commercially available chewing gums for xerostomia and their presented characteristics.
Table 3.
Polymers used in chewing gums’ formulations.
Table 4.
Commercially available chewing gums used in xerostomia.
| Product Name | Characteristics | Manufacturer | Ref. |
|---|---|---|---|
| Freedent WhiteTM | As a low-tack chewing gum, it provides a better tolerance in patients with dental prostheses as compared to the normal-tack chewing gums. Nevertheless, several adverse effects (i.e., irritation of mouth, nausea etc.) have been reported. | Wrigley company | [108,115] |
| V6 chewing gum | Acceptable consistency and no reports of mouth irritation. | Gadbury | [116,120] |
| Dentirol chewing gum | Satisfying taste and acceptable consistency. Alleviates the symptoms without increasing the saliva flow rate. | Continental Candy Company, Denmark | [117] |
| Xerostom Chewable Relief Capsules® | Improves speech, swallowing; decreases subjective xerostomia. | Biocosmetics laboratories, Spain | [121] |
| Biotene chewing gum | Xylitol contained in the formulation reduces plaque formation and minimizes dental caries; improved results when combined with the respective oral solution and mouth paste. | GlaxoSmithKline | [122] |
As far as the pharmaceutical approach is concerned, the administration of cholinergic agents, such as the parasympathomimetics and muscarinic agonists pilocarpine and cevimeline, in order to stimulate residual glandular function is widely implemented [123,124,125]. However, pharmaceutical stimulation may result in systemic adverse effects and, consequently, limited patient acceptance. Specifically, the use of orally administrated pilocarpine is contraindicated in patients suffering from gastric ulcer and uncontrolled asthma, while the risk of cardiovascular effects associated to the systemic administration is also a matter worth taking into consideration [126,127]. Cevimeline is a salivary gland stimulant with a stronger affinity for M3 muscarinic receptors. Since it has no effect on M2 receptors, it is expected to present fewer adverse effects when compared to pilocarpine [69,128]. However, clinical trials revealed similar adverse side effects to between cevimeline and pilocarpine.
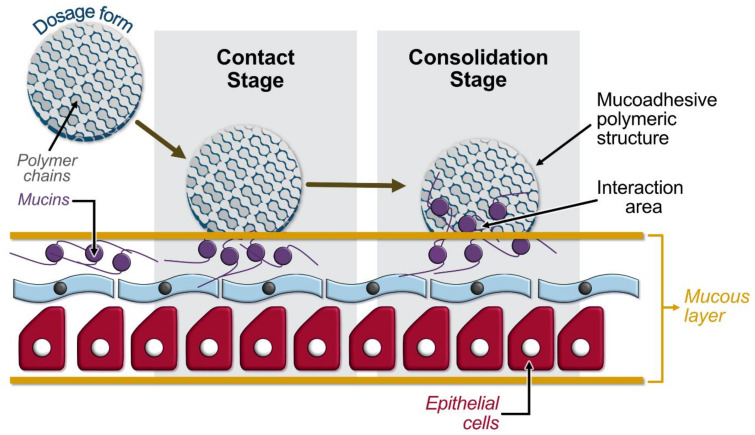
Although the systemic administration of the cholinergic agents has been characterized by success, because of the increased risk of side effects, there is an urgent need to design novel and effective dosage forms presenting adhesive and sustained release properties for on-site demand to the intraoral surface. As a result, recent studies focusing on xerostomia’s treatment have turned their interest to mucoadhesive polymers and mucoadhesive dosage forms. Briefly, mucoadhesion is commonly defined as the adhesion between two materials, one of which, at least, is a mucus membrane. It can be affected by a number of factors, including hydrophilicity, molecular weight, cross-linking, swelling, pH, and the concentration of the active polymer. Mucoadhesive polymers have numerous hydrophilic groups, such as hydroxyl, carboxyl, amide, and sulfate. These groups attach to mucus or the cell membrane by various interactions such as hydrogen bonding and hydrophobic or electrostatic interactions [129]. The mechanism by which mucoadhesion takes place comprises two stages: the contact (wetting) stage, which is characterized by the initiation of interaction between the mucoadhesive polymeric and the mucous membrane, followed by the consolidation stage that involves interpenetration or entanglement of the polymeric and mucin chains [130]. Figure 4 presents an illustration of the aforementioned mechanism. Oral mucoadhesive drug delivery systems have received a great deal of attention for their potential to optimize localized drug delivery, since the oral mucosa is easily accessible and highly vascularized by a relative fast blood flow, allowing a direct access to the systemic circulation, bypassing the liver first-pass effect with consequent high bioavailability and acceptability by the patient [131]. Moreover, the oral mucosa is less susceptible to damage or irritation potentially related to drugs or excipients used, since it is characterized by a rapid cellular turnover (5–6 days).
Figure 4.
Illustration of the two-stage (contact and consolidation stage) mucoadhesion model.
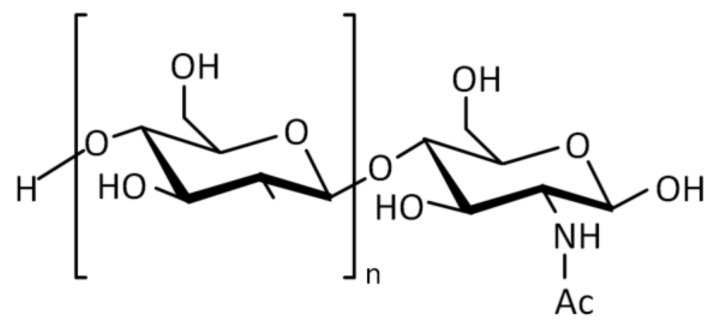
Looking at the literature, recently published studies provide a clear indication of the promising properties of chitosan as a mucoadhesive polymeric material for the preparation of novel xerostomia-treating formulations. Chitosan (Figure 5) is a cationic polysaccharide derived from chitin by partially deacetylating its acetamido groups with strong alkaline solutions [132]. Over the last two decades, it has been used for various biomedical and drug delivery applications due to its low toxicity, good biocompatibility, and mucoadhesive properties [133]. Chitosan has been reported to show excellent mucoadhesion on buccal mucosa, which makes it a promising candidate for the development of formulations aiming at the treatment of xerostomia.
Figure 5.
Structure of chitosan.
In the case of xerostomia, Laffluer et al. [134] investigated the synthesis of novel preactivated chitosan conjugates and the development of a buccal adhesive semisolid dosage form comprising pilocarpine for patients with xerostomia. Specifically, unmodified chitosan was covalently linked to sulfhydryl possessing mercaptonicotinic acid (MNA) via the formation of amide bond. As for the safety profile, according to the obtained results from the carried out cell viability assay, no cytotoxicity was presented. Furthermore, mucoadhesion was improved in the presence of preactivated chitosan, and pilocarpine showed a controlled drug release in the presence of chitosan–MNA–MNA. The aforementioned observations, which might be attributed to the polymeric stability, render preactivated chitosan–MNA–MNA as a promising solution for the treatment of xerostomia.
Liposomes are nano-sized spherical vesicles composed of a lipid bilayer membrane that are able to encapsulate water-soluble molecules in their aqueous core. They have gained increased interest as carriers of active pharmaceutical ingredients, since they can present high biocompatibility and provide sustained drug release [135]. Furthermore, liposomes have been investigated in dental tissue regeneration, providing promising results [136]. The long-term stability of liposomes can be immensely improved by coating them with various polymers. In this context, Adamczak et al. [137] prepared different types of liposomes coated with five different types of polymers (i.e., low-methoxylated pectin (LM-pectin), high-methoxylated pectin (HM-pectin), alginate, chitosan, and hydrophobically modified ethyl hydroxyethyl cellulose (HM-EHEC)) and studied their efficacy on relieving dry mouth symptoms. Coating the liposomes with polymers significantly improved the water sorption capacity of the formulations in all cases. It is worth mentioning that the highest water sorption capacity along with a high mucoadhesion to the mucus-producing cells appeared in the case of the chitosan coated liposomes, demonstrating that these formulations could be another possible selection for relieving dry mouth symptoms. In a similar context, the research team of Tsibouklis et al. [40] published a review highlighting the need for novel hydrogel formulations with an affinity for buccal cells aiming at the management of xerostomia. Once again, various mucoadhesive polymers, such as chitosan, play the major role at the design of these hydrogels.
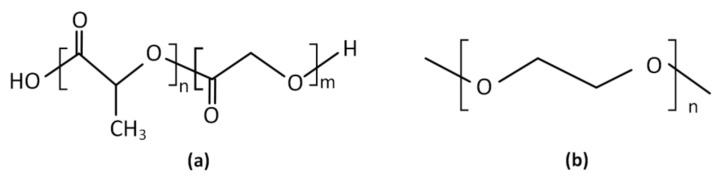
Finally, another promising drug delivery system utilizing advanced polymeric materials for the treatment of xerostomia was that of Muthumariappan et al. [138], who developed a localized formulation consisting of pilocarpine-loaded poly(lactic-co-glycolic acid) (PLGA)/poly(ethylene glycol) (PEG) nanofiber mats via electrospinning. The selection of these polymers, whose structures are presented at Figure 6, was made according to their favorable biodegradability and biocompatibility. Results showed that within the first 24 h of the application, pilocarpine-loaded nanofiber mats had a higher saliva secretion compared to the conventional systemic pilocarpine.
Figure 6.
Structures of: (a) poly(lactic-co-glycolic acid) (PLGA); (b) poly(ethylene glycol) (PEG).
8. Summary and Conclusions
Oral dryness is a complex condition expressed as a physiological deficiency with or without perceived dysfunction. Xerostomia is most commonly presented in patients treated with certain medications, those subjected to head and neck radiotherapy, or in individuals with specific systemic diseases, such as Sjögren’s syndrome. Even though it mostly affects geriatric patients, xerostomia can also be observed in young individuals. The development of a successful treatment approach requires the establishment of the correct diagnosis, which encompasses the distinguishment of patients with subjective complaints from those presenting salivary gland hypofunction, and, subsequently, the identification of the underlying etiology. However, the complex nature and functions of saliva pose challenges that needs to be surpassed during the development of the management approach. Remedies for patients with hyposalivation and xerostomia are mainly directed at the relief of symptoms and the prevention of oral complications. In any case, based on the detailed literature survey conducted above, it is an indisputable conclusion that advanced polymeric materials play a vital role in the development of the various management approaches of xerostomia, concerning either already commercially available formulations or drug delivery systems currently under research and development.
Abbreviations
| BMI | Body mass index |
| CMC | Carboxymethyl cellulose |
| DMFT | Decayed missing filled teeth |
| HEC | Hydroxyethyl cellulose |
| HM-EHEC | Hydrophobically modified ethyl hydroxyethyl cellulose |
| HPMC | Hydroxypropylmethyl cellulose |
| LM-pectin | Low-methoxylated pectin |
| MC | Methyl cellulose |
| MNA | Mercaptonicotinic acid |
| OHRQOL | Oral health-related quality of life |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene) oxide |
| PLGA | Poly(lactic-co-glycolic acid) |
| PPG | Poly(propylene glycol) |
| SCMC | Sodium carboxymethyl cellulose |
| SWS | Stimulated whole saliva |
| UWS | Unstimulated whole saliva |
Author Contributions
Conceptualization, A.K.P., L.T., A.N.A. and P.B.; methodology, A.K. and A.-E.M.; resources, P.B.; writing—original draft preparation, A.K., K.N.K. and A.-E.M.; writing—review and editing, A.K., A.K.P., L.T., A.N.A. and P.B.; visualization, K.N.K. and P.B.; supervision, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE-INNOVATE (project code: T2EDK-00842, XeroPharmPaste).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cassolato S.F., Turnbull R.S. Xerostomia: Clinical aspects and treatment. Gerodontology. 2003;20:64–77. doi: 10.1111/j.1741-2358.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 2.Ship J.A., Pillemer S.R., Baum B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002;50:535–543. doi: 10.1046/j.1532-5415.2002.50123.x. [DOI] [PubMed] [Google Scholar]
- 3.Dawes C. How much saliva is enough for avoidance of xerostomia? Caries Res. 2004;38:236–240. doi: 10.1159/000077760. [DOI] [PubMed] [Google Scholar]
- 4.Hopcraft M.S., Tan C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010;55:238–244. doi: 10.1111/j.1834-7819.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- 5.Van der Putten G.J., Brand H.S., Schols J.M., de Baat C. The diagnostic suitability of a xerostomia questionnaire and the association between xerostomia, hyposalivation and medication use in a group of nursing home residents. Clin. Oral Investig. 2011;15:185–192. doi: 10.1007/s00784-010-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox P.C., van der Ven P.F., Sonies B.C., Weiffenbach J.M., Baum B.J. Xerostomia: Evaluation of a symptom with increasing significance. J. Am. Dent. Assoc. 1985;110:519–525. doi: 10.14219/jada.archive.1985.0384. [DOI] [PubMed] [Google Scholar]
- 7.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J. Dent. Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 8.Guggenheimer J., Moore P.A. Xerostomia: Etiology, recognition and treatment. J. Am. Dent. Assoc. 2003;134:61–69. doi: 10.14219/jada.archive.2003.0018. [DOI] [PubMed] [Google Scholar]
- 9.Tanasiewicz M., Hildebrandt T., Obersztyn I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016;25:199–206. doi: 10.17219/acem/29375. [DOI] [PubMed] [Google Scholar]
- 10.Hanning S.M., Yu T., Jones D.S., Andrews G.P., Kieser J.A., Medlicott N.J. Lecithin-based emulsions for potential use as saliva substitutes in patients with xerostomia—Viscoelastic properties. Int. J. Pharm. 2013;456:560–568. doi: 10.1016/j.ijpharm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 12.Neyraud E., Palicki O., Schwartz C., Nicklaus S., Feron G. Variability of human saliva composition: Possible relationships with fat perception and liking. Arch. Oral Biol. 2012;57:556–566. doi: 10.1016/j.archoralbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Amerongen A.V., Veerman E.C. Saliva—The defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 14.Dawes C., Wood C.M. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch. Oral Biol. 1973;18:337–342. doi: 10.1016/0003-9969(73)90156-8. [DOI] [PubMed] [Google Scholar]
- 15.Proctor G.B., Carpenter G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. Basic Clin. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Rao R.S., Akula R., Satyanarayana T.S.V., Indugu V. Recent Advances of Pacemakers in Treatment of Xerostomia: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019;9:311–315. doi: 10.4103/jispcd.JISPCD_389_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culp D.J., Graham L.A., Latchney L.R., Hand A.R. Rat sublingual gland as a model to study glandular mucous cell secretion. Am. J. Physiol. 1991;260:C1233–C1244. doi: 10.1152/ajpcell.1991.260.6.C1233. [DOI] [PubMed] [Google Scholar]
- 18.Scully C., Felix D.H. Oral medicine—Update for the dental practitioner: Dry mouth and disorders of salivation. Br. Dent. J. 2005;199:423–427. doi: 10.1038/sj.bdj.4812740. [DOI] [PubMed] [Google Scholar]
- 19.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008;139:18s–24s. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 20.Sreebny L.M., Valdini A. Xerostomia: A Neglected Symptom. Arch. Intern. Med. 1987;147:1333–1337. doi: 10.1001/archinte.1987.00370070145022. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen A.M., Bardow A., Jensen S.B., Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 22.Sreebny L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000;50:140–161. doi: 10.1111/j.1875-595X.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 23.Thomson W.M., Chalmers J.M., Spencer A.J., Williams S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health. 1999;16:12–17. [PubMed] [Google Scholar]
- 24.López-Pintor R.M., Casañas E., González-Serrano J., Serrano J., Ramírez L., de Arriba L., Hernández G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016;2016:4372852. doi: 10.1155/2016/4372852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visvanathan V., Nix P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010;64:404–407. doi: 10.1111/j.1742-1241.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 26.Valdez I.H., Fox P.C. Diagnosis and management of salivary dysfunction. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1993;4:271–277. doi: 10.1177/10454411930040030301. [DOI] [PubMed] [Google Scholar]
- 27.Villa A., Connell C.L., Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014;11:45–51. doi: 10.2147/TCRM.S76282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox P.C., Busch K.A., Baum B.J. Subjective reports of xerostomia and objective measures of salivary gland performance. J. Am. Dent. Assoc. 1987;115:581–584. doi: 10.1016/S0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 29.Ship J.A., Fox P.C., Baum B.J. How Much Saliva is Enough? J. Am. Dent. Assoc. 1991;122:63–69. doi: 10.14219/jada.archive.1991.0098. [DOI] [PubMed] [Google Scholar]
- 30.Pai S., Ghezzi E.M., Ship J.A. Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001;91:311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 31.Navazesh M., Christensen C., Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J. Dent. Res. 1992;71:1363–1369. doi: 10.1177/00220345920710070301. [DOI] [PubMed] [Google Scholar]
- 32.Bustillos H., Indorf A., Alwan L., Thompson J., Jung L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Supportive Care Cancer. 2022;30:1681–1687. doi: 10.1007/s00520-021-06535-9. [DOI] [PubMed] [Google Scholar]
- 33.Napeñas J.J., Brennan M.T., Fox P.C. Diagnosis and treatment of xerostomia (dry mouth) Odontology. 2009;97:76–83. doi: 10.1007/s10266-008-0099-7. [DOI] [PubMed] [Google Scholar]
- 34.Gil-Montoya J.A., Guardia-López I., González-Moles M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology. 2008;25:3–9. doi: 10.1111/j.1741-2358.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 35.Epstein J.B., Jensen S.B. Management of Hyposalivation and Xerostomia: Criteria for Treatment Strategies. Compend. Contin. Educ. Dent. 2015;36:600–603. [PubMed] [Google Scholar]
- 36.Fantozzi P.J., Pampena E., di Vanna D., Pellegrino E., Corbi D., Mammucari S., Alessi F., Pampena R., Bertazzoni G., Minisola S., et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am. J. Otolaryngol. 2020;41:102721. doi: 10.1016/j.amjoto.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nederfors T., Isaksson R., Mörnstad H., Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—Relation to age, sex and pharmacotherapy. Community Dent. Oral Epidemiol. 1997;25:211–216. doi: 10.1111/j.1600-0528.1997.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Eijk J., van Campen J.P.C.M., van der Jagt H., Beijnen J.H., Tulner L.R. Prevalence of Xerostomia and Its Relationship with Underlying Diseases, Medication, and Nutrition: A Descriptive Observational Study. J. Am. Geriatr. Soc. 2013;61:1836–1837. doi: 10.1111/jgs.12469. [DOI] [PubMed] [Google Scholar]
- 39.Marcott S., Dewan K., Kwan M., Baik F., Lee Y.-J., Sirjani D. Where Dysphagia Begins: Polypharmacy and Xerostomia. Fed Pract. 2020;37:234–241. [PMC free article] [PubMed] [Google Scholar]
- 40.Tsibouklis J., Middleton A.M., Patel N., Pratten J. Toward mucoadhesive hydrogel formulations for the management of xerostomia: The physicochemical, biological, and pharmacological considerations. J. Biomed. Mater. Res. 2013;101:3327–3338. doi: 10.1002/jbm.a.34626. [DOI] [PubMed] [Google Scholar]
- 41.Peker I., Alkurt M.T., Usalan G. Clinical evaluation of medications on oral and dental health. Int. Dent. J. 2008;58:218–222. doi: 10.1111/j.1875-595X.2008.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 42.Scully C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003;9:165–176. doi: 10.1034/j.1601-0825.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- 43.Tan E.C.K., Lexomboon D., Sandborgh-Englund G., Haasum Y., Johnell K. Medications That Cause Dry Mouth As an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018;66:76–84. doi: 10.1111/jgs.15151. [DOI] [PubMed] [Google Scholar]
- 44.Ship J.A. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002;8:77–89. doi: 10.1034/j.1601-0825.2002.2o837.x. [DOI] [PubMed] [Google Scholar]
- 45.Arany S., Kopycka-Kedzierawski D.T., Caprio T.V., Watson G.E. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;132:662–670. doi: 10.1016/j.oooo.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner M.D. Hyposalivation and Xerostomia: Etiology, Complications, and Medical Management. Dent. Clin. N. Am. 2016;60:435–443. doi: 10.1016/j.cden.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Dirix P., Nuyts S., van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer. 2006;107:2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 48.Messmer M.B., Thomsen A., Kirste S., Becker G., Momm F. Xerostomia after radiotherapy in the head & neck area: Long-term observations. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011;98:48–50. doi: 10.1016/j.radonc.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Wu V.W.C., Leung K.Y. A Review on the Assessment of Radiation Induced Salivary Gland Damage After Radiotherapy. Front. Oncol. 2019;9:1090. doi: 10.3389/fonc.2019.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logemann J.A., Smith C.H., Pauloski B.R., Rademaker A.W., Lazarus C.L., Colangelo L.A., Mittal B., MacCracken E., Gaziano J., Stachowiak L., et al. Effects of xerostomia on perception and performance of swallow function. Head Neck. 2001;23:317–321. doi: 10.1002/hed.1037. [DOI] [PubMed] [Google Scholar]
- 51.Tribius S., Sommer J., Prosch C., Bajrovic A., Muenscher A., Blessmann M., Kruell A., Petersen C., Todorovic M., Tennstedt P. Xerostomia after radiotherapy. Strahlenther. Und Onkol. 2013;189:216–222. doi: 10.1007/s00066-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 52.Mignogna M.D., Fedele S., Russo L.L., Muzio L.L., Wolff A. Sjögren’s syndrome: The diagnostic potential of early oral manifestations preceding hyposalivation/xerostomia. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2005;34:1–6. doi: 10.1111/j.1600-0714.2004.00264.x. [DOI] [PubMed] [Google Scholar]
- 53.Stankeviciene I., Puriene A., Mieliauskaite D., Stangvaltaite-Mouhat L., Aleksejuniene J. Detection of xerostomia, Sicca, and Sjogren’s syndromes in a national sample of adults. BMC Oral Health. 2021;21:552. doi: 10.1186/s12903-021-01917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Reijden W.A., Vissink A., Veerman E.C.I., Amerongen A.V.N. Treatment of oral dryness related complaints (xerostomia) in Sjögren’s syndrome. Ann. Rheum. Dis. 1999;58:465–474. doi: 10.1136/ard.58.8.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abadi P.A., Koopaie M., Montazeri R. Comparison of salivary nitric oxide and oral health in diabetic patients with and without xerostomia. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:11–15. doi: 10.1016/j.dsx.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Al-Maskari A.Y., Al-Maskari M.Y., Al-Sudairy S. Oral Manifestations and Complications of Diabetes Mellitus: A review. Sultan Qaboos Univ. Med. J. 2011;11:179–186. [PMC free article] [PubMed] [Google Scholar]
- 57.Sandberg G.E., Sundberg H.E., Fjellstrom C.A., Wikblad K.F. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res. Clin. Pract. 2000;50:27–34. doi: 10.1016/S0168-8227(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 58.Guggenheimer J., Moore P.A., Rossie K., Myers D., Mongelluzzo M.B., Block H.M., Weyant R., Orchard T. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: I. Prevalence and characteristics of non-candidal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000;89:563–569. doi: 10.1067/moe.2000.104476. [DOI] [PubMed] [Google Scholar]
- 59.Valerio M.A., Kanjirath P.P., Klausner C.P., Peters M.C. A qualitative examination of patient awareness and understanding of type 2 diabetes and oral health care needs. Diabetes Res. Clin. Pract. 2011;93:159–165. doi: 10.1016/j.diabres.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Åstrøm A.N., Lie S.A., Ekback G., Gülcan F., Ordell S. Self-reported dry mouth among ageing people: A longitudinal, cross-national study. Eur. J. Oral Sci. 2019;127:130–138. doi: 10.1111/eos.12601. [DOI] [PubMed] [Google Scholar]
- 61.Johansson A.-K., Johansson A., Unell L., Ekbäck G., Ordell S., Carlsson G.E. Self-reported dry mouth in 50- to 80-year-old Swedes: Longitudinal and cross-sectional population studies. J. Oral Rehabil. 2020;47:246–254. doi: 10.1111/joor.12878. [DOI] [PubMed] [Google Scholar]
- 62.Loesche W.J., Bromberg J., Terpenning M.S., Bretz W.A., Dominguez B.L., Grossman N.S., Langmore S.E. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J. Am. Geriatr. Soc. 1995;43:401–407. doi: 10.1111/j.1532-5415.1995.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 63.Fife R.S., Chase W.F., Dore R.K., Wiesenhutter C.W., Lockhart P.B., Tindall E., Suen J.Y. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: A randomized trial. Arch. Intern Med. 2002;162:1293–1300. doi: 10.1001/archinte.162.11.1293. [DOI] [PubMed] [Google Scholar]
- 64.Dodds M.W.J., Johnson D.A., Yeh C.-K. Health benefits of saliva: A review. J. Dent. 2005;33:223–233. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Nadig S.D., Ashwathappa D.T., Manjunath M., Krishna S., Annaji A.G., Shivaprakash P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral Maxillofac. Pathol. JOMFP. 2017;21:316. doi: 10.4103/jomfp.JOMFP_231_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koshimune S., Awano S., Gohara K., Kurihara E., Ansai T., Takehara T. Low salivary flow and volatile sulfur compounds in mouth air. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;96:38–41. doi: 10.1016/S1079-2104(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 67.Dormenval V., Budtz-Jørgensen E., Mojon P., Bruyère A., Rapin C.H. Associations between malnutrition, poor general health and oral dryness in hospitalized elderly patients. Age Ageing. 1998;27:123–128. doi: 10.1093/ageing/27.2.123. [DOI] [PubMed] [Google Scholar]
- 68.Iwasaki M., Borgnakke W.S., Yoshihara A., Ito K., Ogawa H., Nohno K., Sato M., Minagawa K., Ansai T., Miyazaki H. Hyposalivation and 10-year all-cause mortality in an elderly Japanese population. Gerodontology. 2018;35:87–94. doi: 10.1111/ger.12319. [DOI] [PubMed] [Google Scholar]
- 69.Atkinson J.C., Grisius M., Massey W. Salivary hypofunction and xerostomia: Diagnosis and treatment. Dent. Clin. N. Am. 2005;49:309–326. doi: 10.1016/j.cden.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Alhejoury H., Mogharbel L., Al-Qadhi M., Shamlan S., Alturki A., Babatin W., Alaishan R.M., Pullishery F. Artificial saliva for therapeutic management of xerostomia: A narrative review. J. Pharm. Bioallied Sci. 2021;13:903–907. doi: 10.4103/jpbs.jpbs_236_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newbrun E. Current treatment modalities of oral problems of patients with Sjögren’s syndrome: Caries prevention. Adv. Dent. Res. 1996;10:29–34. doi: 10.1177/08959374960100010401. [DOI] [PubMed] [Google Scholar]
- 72.Jansma J., Vissink A., Spijkervet F.K., Roodenburg J.L., Panders A.K., Vermey A., Szabó B.G., Gravenmade E.J. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer. 1992;70:2171–2180. doi: 10.1002/1097-0142(19921015)70:8<2171::AID-CNCR2820700827>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 73.Barbe A.G. Medication-Induced Xerostomia and Hyposalivation in the Elderly: Culprits, Complications, and Management. Drugs Aging. 2018;35:877–885. doi: 10.1007/s40266-018-0588-5. [DOI] [PubMed] [Google Scholar]
- 74.Zero D.T., Brennan M.T., Daniels T.E., Papas A., Stewart C., Pinto A., Al-Hashimi I., Navazesh M., Rhodus N., Sciubba J., et al. Clinical practice guidelines for oral management of Sjögren disease: Dental caries prevention. J. Am. Dent. Assoc. 2016;147:295–305. doi: 10.1016/j.adaj.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Taylor S.E. Efficacy and economic evaluation of pilocarpine in treating radiation-induced xerostomia. Expert Opin. Pharmacother. 2003;4:1489–1497. doi: 10.1517/14656566.4.9.1489. [DOI] [PubMed] [Google Scholar]
- 76.Mese H., Matsuo R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007;34:711–723. doi: 10.1111/j.1365-2842.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 77.Hu J., Andablo-Reyes E., Mighell A., Pavitt S., Sarkar A. Dry mouth diagnosis and saliva substitutes-A review from a textural perspective. J. Texture Stud. 2021;52:141–156. doi: 10.1111/jtxs.12575. [DOI] [PubMed] [Google Scholar]
- 78.Lam-Ubol A., Matangkasombut O., Trachootham D., Tarapan S., Sattabanasuk V., Talungchit S., Paemuang W., Phonyiam T., Chokchaitam O., Mungkung O.O. Efficacy of gel-based artificial saliva on Candida colonization and saliva properties in xerostomic post-radiotherapy head and neck cancer patients: A randomized controlled trial. Clin. Oral Investig. 2021;25:1815–1827. doi: 10.1007/s00784-020-03484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonda P.L.F., Farinone M., Pattarino F., Bonda A.F. Artificial saliva substitutes evaluation: The role of some chemical-physical properties. Glob. J. Res. Anal. 2018;7:67–70. [Google Scholar]
- 80.Salum F.G., Medella-Junior F.A.C., Figueiredo M.A.Z., Cherubini K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology. 2018;35:305–316. doi: 10.1111/ger.12353. [DOI] [PubMed] [Google Scholar]
- 81.Park M.-S., Chung J.-W., Kim Y.-K., Chung S.-C., Kho H.-S. Viscosity and wettability of animal mucin solutions and human saliva. Oral Dis. 2007;13:181–186. doi: 10.1111/j.1601-0825.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 82.Davis S.S. The rheological properties of saliva. Rheol. Acta. 1971;10:28–35. doi: 10.1007/BF01972473. [DOI] [Google Scholar]
- 83.Łysik D., Niemirowicz-Laskowska K., Bucki R., Tokajuk G., Mystkowska J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019;20:3199. doi: 10.3390/ijms20133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jornet P.L., Hernandez L., García F.G., Molero F.G., López E.P.-F., Tvarijonaviciute A. A Clinical Study on the Efficacy and Tolerability of a New Topical Gel and Toothpaste in Patients with Xerostomia: A Randomized Controlled Trial. J. Clin. Med. 2021;10:5641. doi: 10.3390/jcm10235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Epstein J.B., Emerton S., Le N.D., Stevenson-Moore P. A double-blind crossover trial of Oral Balance gel and Biotene toothpaste versus placebo in patients with xerostomia following radiation therapy. Oral Oncol. 1999;35:132–137. doi: 10.1016/S1368-8375(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 86.Shahdad S.A., Taylor C., Barclay S.C., Steen I.N., Preshaw P.M. A double-blind, crossover study of Biotène Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia. Eur. J. Cancer Care. 2005;14:319–326. doi: 10.1111/j.1365-2354.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 87.Wolff A., Fox P.C., Porter S., Konttinen Y.T. Established and novel approaches for the management of hyposalivation and xerostomia. Curr. Pharm. Des. 2012;18:5515–5521. doi: 10.2174/138161212803307509. [DOI] [PubMed] [Google Scholar]
- 88.Vissink A., de Jong H.P., Busscher H.J., Arends J., Gravenmade E.J. Wetting Properties of Human Saliva and Saliva Substitutes. J. Dent. Res. 1986;65:1121–1124. doi: 10.1177/00220345860650090301. [DOI] [PubMed] [Google Scholar]
- 89.Amal A.S.S., Hussain S., Jalaluddin M.A. Preparation of Artificial Saliva Formulation; Proceedings of the ICB Pharma II “Current Breakthrough in Pharmacy Materials and Analyses”; Indonesia. 2015. [(accessed on 26 January 2022)]. Available online: https://publikasiilmiah.ums.ac.id/xmlui/handle/11617/6203. [Google Scholar]
- 90.Jellema A.P., Langendijk H., Bergenhenegouwen L., van der Reijden W., Leemans R., Smeele L., Slotman B.J. The efficacy of Xialine® in patients with xerostomia resulting from radiotherapy for head and neck cancer: A pilot-study. Radiother. Oncol. 2001;59:157–160. doi: 10.1016/S0167-8140(01)00336-X. [DOI] [PubMed] [Google Scholar]
- 91.Hahnel S., Behr M., Handel G., Bürgers R. Saliva substitutes for the treatment of radiation-induced xerostomia—A review. Supportive Care Cancer. 2009;17:1331–1343. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 92.Momm F., Volegova-Neher N.J., Schulte-Mönting J., Guttenberger R. Different Saliva Substitutes for Treatment of Xerostomia Following Radiotherapy. Strahlenther. Onkol. 2005;181:231–236. doi: 10.1007/s00066-005-1333-7. [DOI] [PubMed] [Google Scholar]
- 93.Aykut-Yetkiner A., Wiegand A., Attin T. The effect of saliva substitutes on enamel erosion in vitro. J. Dent. 2014;42:720–725. doi: 10.1016/j.jdent.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 94.Kielbassa A.M., Shohadai S.P., Schulte-Mönting J. Effect of saliva substitutes on mineral content of demineralized and sound dental enamel. Supportive Care Cancer. 2001;9:40–47. doi: 10.1007/s005200000148. [DOI] [PubMed] [Google Scholar]
- 95.Jaiswal N., Patil P.G., Gangurde A., Parkhedkar R.D. Wettability of 3 different artificial saliva substitutes on heat-polymerized acrylic resin. J. Prosthet. Dent. 2019;121:517–522. doi: 10.1016/j.prosdent.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 96.Salom M., Hachulla E., Bertolus C., Deschaumes C., Simoneau G., Mouly S. Efficacy and safety of a new oral saliva equivalent in the management of xerostomia: A national, multicenter, randomized study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015;119:301–309. doi: 10.1016/j.oooo.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Roy N., Tanner K., Gray S.D., Blomgren M., Fisher K.V. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure (PTP) J. Voice. 2003;17:331–342. doi: 10.1067/S0892-1997(03)00078-X. [DOI] [PubMed] [Google Scholar]
- 98.Smith G., Smith A.J., Shaw L., Shaw M.J. Artificial saliva substitutes and mineral dissolution. J. Oral Rehabil. 2001;28:728–731. doi: 10.1046/j.1365-2842.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 99.Shirodaria S., Kilbourn T., Richardson M. Subjective assessment of a new moisturizing mouth spray for the symptomatic relief of dry mouth. J. Clin. Dent. 2006;17:45–51. [PubMed] [Google Scholar]
- 100.Malallah O.S., Garcia C.M.A., Proctor G.B., Forbes B., Royall P.G. Buccal drug delivery technologies for patient-centred treatment of radiation-induced xerostomia (dry mouth) Int. J. Pharm. 2018;541:157–166. doi: 10.1016/j.ijpharm.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Skrinjar I., Boras V.V., Bakale I., Rogulj A.A., Brailo V., Juras D.V., Alajbeg I., Vrdoljak D.V. Comparison between three different saliva substitutes in patients with hyposalivation. Clin. Oral Investig. 2015;19:753–757. doi: 10.1007/s00784-015-1405-8. [DOI] [PubMed] [Google Scholar]
- 102.Fehder W.P. Nursing care & management of pathological oral conditions among women and children, MCN. Am. J. Matern. Child Nurs. 2008;33:38–44. doi: 10.1097/01.NMC.0000305656.86495.e2. [DOI] [PubMed] [Google Scholar]
- 103.News and Innovations. J. Pain Palliat. Care Pharmacother. 2006;20:83–104. doi: 10.1080/J354v20n02_15. [DOI] [Google Scholar]
- 104.Delgado A., Ribeiro A.P.D., Aslam M., Olafsson V.G., Pereira P.N. Erosive assessment of dry mouth lozenges and tablets on enamel and dentin. J. Dent. 2021;105:103496. doi: 10.1016/j.jdent.2020.103496. [DOI] [PubMed] [Google Scholar]
- 105.Femiano F., Rullo R., di Spirito F., Lanza A., Festa V.M., Cirillo N. A comparison of salivary substitutes versus a natural sialogogue (citric acid) in patients complaining of dry mouth as an adverse drug reaction: A clinical, randomized controlled study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;112:e15–e20. doi: 10.1016/j.tripleo.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 106.Gómez-Moreno G., Guardia J., Aguilar-Salvatierra A., Cabrera-Ayala M., Maté-Sánchez de-Val J.-E., Calvo-Guirado J.L. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med. Oral Patol. Oral Y Cir. Bucal. 2013;18:e49–e55. doi: 10.4317/medoral.18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ozen N., Sayilan A.A., Mut D., Sayilan S., Avcioglu Z., Kulakac N., Ecder T., Akyolcu N. The effect of chewing gum on dry mouth, interdialytic weight gain, and intradialytic symptoms: A prospective, randomized controlled trial, Hemodialysis international. Int. Symp. Home Hemodial. 2021;25:94–103. doi: 10.1111/hdi.12878. [DOI] [PubMed] [Google Scholar]
- 108.Bots C.P., Brand H.S., Veerman E.C., Korevaar J.C., Valentijn-Benz M., Bezemer P.D., Valentijn R.M., Vos P.F., Bijlsma J.A., Wee P.M.T., et al. Chewing gum and a saliva substitute alleviate thirst and xerostomia in patients on haemodialysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2005;20:578–584. doi: 10.1093/ndt/gfh675. [DOI] [PubMed] [Google Scholar]
- 109.Tulek A., Mulic A., Hogset M., Utheim T.P., Sehic A. Therapeutic Strategies for Dry Mouth Management with Emphasis on Electrostimulation as a Treatment Option. Int. J. Dent. 2021;2021:6043488. doi: 10.1155/2021/6043488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaae J.K., Stenfeldt L., Eriksen J.G. Xerostomia after Radiotherapy for Oral and Oropharyngeal Cancer: Increasing Salivary Flow with Tasteless Sugar-free Chewing Gum. Front. Oncol. 2016;6:111. doi: 10.3389/fonc.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bossola M. Xerostomia in patients on chronic hemodialysis: An update. Semin. Dial. 2019;32:467–474. doi: 10.1111/sdi.12821. [DOI] [PubMed] [Google Scholar]
- 112.Olsson H., Spak C.J., Axell T. The effect of a chewing gum on salivary secretion, oral mucosal friction, and the feeling of dry mouth in xerostomic patients. Acta Odontol. Scand. 1991;49:273–279. doi: 10.3109/00016359109005919. [DOI] [PubMed] [Google Scholar]
- 113.Maggi L., Segale L., Conti S., Machiste E.O., Salini A., Conte U. Preparation and evaluation of release characteristics of 3TabGum, a novel chewing device. Eur. J. Pharm. Sci. 2005;24:487–493. doi: 10.1016/j.ejps.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 114.Fathima S.R., Viswanath V., Malleswari M., Panitha M., Reddy N.P., Reddy R., Sreedevi N.S. Medicated chewing gums—An Overview. Int. J. Pharm. Anal. Res. 2019;8:138–144. [Google Scholar]
- 115.Davies A.N. A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat. Med. 2000;14:197–203. doi: 10.1191/026921600672294077. [DOI] [PubMed] [Google Scholar]
- 116.Björnström M., Axéll T., Birkhed D. Comparison between saliva stimulants and saliva substitutes in patients with symptoms related to dry mouth. A Multi-Centre Study. Swed. Dent. J. 1990;14:153–161. [PubMed] [Google Scholar]
- 117.Risheim H., Arneberg P. Salivary stimulation by chewing gum and lozenges in rheumatic patients with xerostomia. Scand. J. Dent. Res. 1993;101:40–43. doi: 10.1111/j.1600-0722.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 118.Khatun S., Sutradhar B.K. Medicated chewing gum: An unconventional drug delivery system. Int. Curr. Pharm. J. 2012;1:86–91. doi: 10.3329/icpj.v1i4.10064. [DOI] [Google Scholar]
- 119.Aslani A., Rostami F. Medicated chewing gum, a novel drug delivery system. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015;20:403–411. [PMC free article] [PubMed] [Google Scholar]
- 120.Aagaard A., Godiksen S., Teglers P.T., Schiødt M., Glenert U. Comparison between new saliva stimulants in patients with dry mouth: A placebo-controlled double-blind crossover study. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 1992;21:376–380. doi: 10.1111/j.1600-0714.1992.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 121.Lapiedra R.C., Gomez G.E., Sanchez B.P., Pereda A.A., Turner M.D. The Effect of a Combination Saliva Substitute for the Management of Xerostomia and Hyposalivation. J. Maxillofac. Oral Surg. 2015;14:653–658. doi: 10.1007/s12663-015-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warde P., Kroll B., O’Sullivan B., Aslanidis J., Tew-George E., Waldron J., Maxymiw W., Liu F.F., Payne D., Cummings B. A phase II study of Biotene in the treatment of postradiation xerostomia in patients with head and neck cancer. Supportive Care Cancer. 2000;8:203–208. doi: 10.1007/s005200050286. [DOI] [PubMed] [Google Scholar]
- 123.Riley P., Glenny A.M., Hua F., Worthington H.V. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst. Rev. 2017;7:1–150. doi: 10.1002/14651858.CD012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opin. Drug Metab. Toxicol. 2008;4:1333–1340. doi: 10.1517/17425255.4.10.1333. [DOI] [PubMed] [Google Scholar]
- 125.Navazesh M. Ship, II, Xerostomia: Diagnosis and treatment. Am. J. Otolaryngol. 1983;4:283–292. doi: 10.1016/S0196-0709(83)80072-6. [DOI] [PubMed] [Google Scholar]
- 126.Bernardi R., Perin C., Becker F.L., Ramos G.Z., Gheno G.Z., Lopes L.R., Pires M., Barros H.M. Effect of pilocarpine mouthwash on salivary flow. Braz. J. Med. Biol. Res. = Rev. Bras. De Pesqui. Med. E Biol. 2002;35:105–110. doi: 10.1590/S0100-879X2002000100015. [DOI] [PubMed] [Google Scholar]
- 127.Braga M.A., Tarzia O., Bergamaschi C.C., Santos F.A., Andrade E.D., Groppo F.C. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int. J. Dent. Hyg. 2009;7:126–130. doi: 10.1111/j.1601-5037.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 128.Chambers M.S., Posner M., Jones C.U., Biel M.A., Hodge K.M., Vitti R., Armstrong I., Yen C., Weber R.S. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1102–1109. doi: 10.1016/j.ijrobp.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 129.Shaikh R., Singh T.R.R., Garland M.J., Woolfson A.D., Donnelly R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011;3:89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smart J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 131.Harris D., Robinson J.R. Drug Delivery via the Mucous Membranes of the Oral Cavity. J. Pharm. Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 132.Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 133.Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004;30:985–993. doi: 10.1081/DDC-200037245. [DOI] [PubMed] [Google Scholar]
- 134.Laffleur F., Röttges S. Buccal adhesive chitosan conjugate comprising pilocarpine for xerostomia. Int. J. Biol. Macromol. 2019;135:1043–1051. doi: 10.1016/j.ijbiomac.2019.05.219. [DOI] [PubMed] [Google Scholar]
- 135.Chong J.R., Le D.L., Sato H., Sou K. Nanocapsule pH Regulator: Sustained Continuous Alkali Release from Thermosensitive Liposomes Reduces Acid Erosion. ACS Appl. Mater. Interfaces. 2020;12:21463–21469. doi: 10.1021/acsami.0c03814. [DOI] [PubMed] [Google Scholar]
- 136.Shafiei M., Ansari M.N.M., Razak S.I.A., Khan M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers. 2021;13:2529. doi: 10.3390/polym13152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adamczak M.I., Martinsen Ø.G., Smistad G., Hiorth M. Polymer coated mucoadhesive liposomes intended for the management of xerostomia. Int. J. Pharm. 2017;527:72–78. doi: 10.1016/j.ijpharm.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 138.Muthumariappan S., Ng W.C., Adine C., Ng K.K., Davoodi P., Wang C.H., Ferreira J.N. Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. Int. J. Mol. Sci. 2019;20:541. doi: 10.3390/ijms20030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.