Abstract
Excessive or insufficient maternal nutrition can influence fetal development and the susceptibility of offspring to adult disease. As eating a fructose-rich diet is becoming more common, the effects of maternal fructose intake on offspring health is of increasing relevance. The gut is required to process fructose, and a high-fructose diet can alter the gut microbiome, resulting in gut dysbiosis and metabolic disorders. Current evidence from animal models has revealed that maternal fructose consumption causes various components of metabolic syndrome in adult offspring, while little is known about how gut microbiome is implicated in fructose-induced developmental programming and the consequential risks for developing chronic disease in offspring. This review will first summarize the current evidence supporting the link between fructose and developmental programming of adult diseases. This will be followed by presenting how gut microbiota links to common mechanisms underlying fructose-induced developmental programming. We also provide an overview of the reprogramming effects of gut microbiota-targeted therapy on fructose-induced developmental programming and how this approach may prevent adult-onset disease. Using gut microbiota-targeted therapy to prevent maternal fructose diet-induced developmental programming, we have the potential to mitigate the global burden of fructose-related disorders.
Keywords: fructose, hypertension, gut microbiota, developmental origins of health and disease (DOHaD), short chain fatty acid, probiotics, prebiotics, metabolic syndrome
1. Introduction
The developmental origins of health and disease (DOHaD) hypothesis supports that in utero adverse events prime the risk for developing chronic disease later in life [1]. Maternal nutrition plays a crucial role in fetal growth and development. Inappropriate intakes of certain nutrients have been linked to many adult diseases of developmental origins [2]. The consumption of fructose has risen in the last half-century and is thought to play a potential role in the epidemic of hypertension, obesity, diabetes, kidney disease, etc. [3]. Emerging evidence shows increased fructose intake in pregnancy can cause fetal programming and result in a variety of adverse outcomes in offspring, such as hypertension, obesity, diabetes, cardiovascular disease, and non-alcoholic fatty liver disease [4,5].
The major organ for fructose metabolism is the liver, while excessive consumption of fructose can be spilled over to the gut microbiota [6]. Intestinal fructose absorption is limited due to selective absorption [6]. Unabsorbed fructose is converted by the gut microbiome into hydrogen, short-chain fatty acids (SCFAs), methane and carbon dioxide [7]. Accordingly, a high-fructose diet can alter gut microbiome, leading to gut dysbiosis and microbial metabolite disorder. A growing body of evidence supports that maternal diet impacts gut microbiota establishment in offspring [8]. Maternal high-fructose diet has been shown to increase risk for developing adult disease, which is associated with alterations of gut microbiota and its metabolites [9,10,11]. However, there remains gaps in our knowledge about whether early life interventions may target gut microbiota, resulting in protection against adult diseases programmed by maternal fructose exposure.
This review will focus specifically on gut microbiome implicated in the developmental origins of adult disease programmed by maternal fructose exposure. The molecular and mechanistic pathways mediating fetal programming will be a special focus, and their interconnections with gut microbiome will be addressed. Further, the potential of the reprogramming approach targeting gut microbiota to protect progeny against maternal high-fructose diet-induced adult disease will be discussed. A schematic diagram summarizing the adverse impact of a maternal fructose diet on adult offspring and the proposed mechanisms by which gut microbiota may implicate in adult diseases of developmental origins is depicted in Figure 1.
Figure 1.
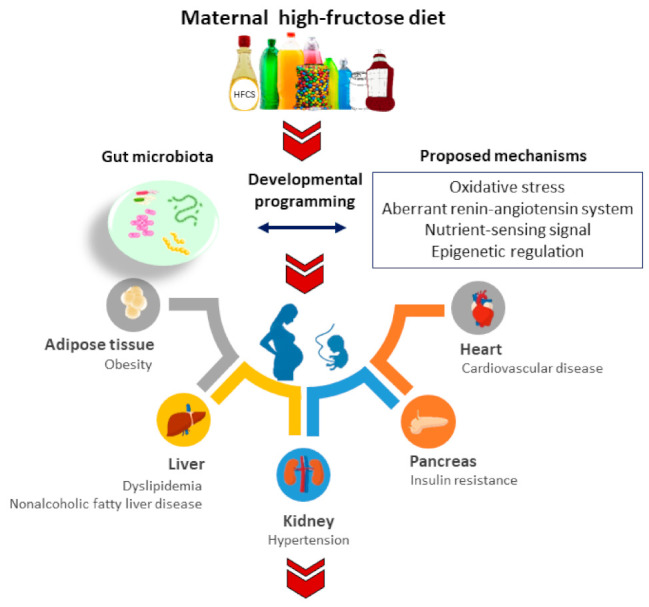
Schematic diagram summarizing the proposed mechanisms linking gut microbiota to maternal fructose-induced developmental programming in different organ systems resulting in various adult diseases in later life.
We searched the PubMed/MEDLINE databases for studies published in English between January 1980 to December 2021. Search terms were as follows: “fructose”, “gut microbiota”, “short chain fatty acid”, “blood pressure”, “metabolic syndrome”, “hypertension”, “obesity”, “diabetes”, “fatty liver”, “cardiovascular disease”, “kidney disease”, “developmental programming”, “DOHaD”, “mother”, “maternal”, “pregnancy”, “gestation”, “offspring”, “progeny”, and “prenatal”. Additional studies were then selected and assessed based on appropriate references in eligible papers.
2. Maternal Fructose Diet Programs Adult Diseases
Fructose is a structural isomer of glucose and galactose. Although fructose occurs naturally in fruits and vegetables, current fructose consumption is mostly derived from refined sugars and processed foods made up of high-fructose corn syrup (HFCS). Fructose is absorbed in the gut through facilitated glucose transporters 5 (Glut 5) and Glut 2 [6] and almost entirely metabolized in the liver [12]. Today, epidemiological and animal studies have revealed that excessive fructose consumption is a risk factor in the development of obesity and several metabolic disturbances [3]. A systematic meta-analysis recruiting 15 human studies demonstrated that fructose consumption was positively associated with elevated systolic blood pressure (BP), increased fasting blood sugar, and elevated triglycerides [13]. Another meta-analysis study demonstrated sugar-sweetened beverages that contain fructose were associated with a risk of developing hypertension [14].
Notably, both negative and positive effects of fructose consumption have been reported in human studies [15,16,17,18,19]. These controversies arise largely because all sources of fructose are not the same, and not all people respond to fructose in the same way [15,16]. Fructose in fruit tends to be safer because of the additional nutrients and antioxidants in fruit, whereas fructose in high fructose corn syrup (HFCS) and refined sugar is mostly harmful [17,18,19]. Additionally, the response to fructose in young healthy people is much lower than in older obese persons. Therefore, most pooling epidemiological studies that contain various participants as well as fructose from different sources carries great risk for diluting any real findings. Since human studies have not yet established the direct cause-and-effect relationship between excessive fructose consumption and adverse offspring outcomes, it stands to reason that the use of animal models is essential to investigate maternal fructose-induced developmental programming for further translational research.
So far, no information exists regarding the impact of excessive fructose consumption during gestation and/or lactation on offspring health in humans. Given this limitation, much of what is known about the implication of maternal fructose intake on offspring health is only based on experimental animal models.
Recent evidence has emerged from animal models that maternal fructose diet causes various components of metabolic syndrome in their offspring [20,21], including obesity [22], hypertension [22,23,24], dyslipidemia [25], and insulin resistance [22,26]. Additionally, maternal high-fructose consumption is associated with impaired spatial learning and memory [27], and early onset retinopathy [28]. Nevertheless, the role of gut microbiome in the developmental origins of adult disease programmed by maternal fructose exposure remains largely unknown.
3. Fructose and Gut Microbiota
Major functions of the gut microbiota include the maintenance of the structural integrity of the gut, host nutrient metabolism, regulation of immune homeostasis, xenobiotic and drug metabolism, and fermentation of non-digestible substrates [29]. It is becoming increasingly obvious that a loss of balance in gut microbiota, termed dysbiosis, is implicated in numerous human diseases. Gut microbiota-derived metabolites are key molecular mediators between the microbiota and the host [30]. Certain metabolites, notably bile acids, SCFAs, branched-chain amino acids, tryptophan derivatives, and trimethylamine N-oxide (TMAO), have been connected to the pathogenesis of metabolic disorders [30]. Emerging data have demonstrated an association between the fructose and gut microbiota dysbiosis in metabolic syndrome-related disorders [31,32]. Although prior research reports that the gut microbiota compositions differ between healthy subjects and patients with metabolic syndrome [33], the causality is still insufficiently demonstrated.
3.1. How Fructose Alters Gut Microbiota and Their Metabolites
The tight junctions form a selectively permeable barrier defending the host by avoiding the entry of intestinal microbes and their products. Chronic consumption of fructose is accompanied with a loss of intestinal tight junction proteins, resulting in elevated translocation of endotoxin [34]. A high-fructose diet fed to mice has been shown to result in the development of hyperglycemia, adiposity, dyslipidemia, endotoxemia, and glucose intolerance, which coincided with lost gut microbial diversity in these mice [35]. A high-fructose diet also altered gut microbiota compositions, characterized by a lower abundance of Bacteroidetes and a markedly increased proportion of Proteobacteria. Another study demonstrated that a high-fructose diet induced steatosis with dyslipidemia and was associated with decreased beneficial microbes Bifidobacterium and Lactobacillus [36].
Additionally, several lines of evidence indicate fructose is able to mediate microbiota-derived metabolites. First, SCFA levels in plasma from rats fed with a high-fructose diet were reduced [37]. Another line of evidence comes from metabonomic analysis. The metabolic profiling from high-fructose- and salt-fed rats showed the increase of TMAO in urine was associated with metabonomic progression axes, progressing from normal to insulin resistance and hypertension status [38]. This final status of hypertension is an observation regarding mice fed a HFCS-moderate fat diet and displayed anxio-depressive behavior coinciding with altered gut microbiota compositions and tryptophan metabolites [39]. Altogether, these studies indicate that fructose is able to induce gut microbiota dysbiosis in three ways: it disrupts the gut barrier triggering endotoxemia and inflammation; it alters gut microbial profile and diversity; and it influences key microbial metabolites.
3.2. The Impact of Maternal Fructose Diet on Gut Microbiome
Much of the work investigating the actions of fructose on gut microbiota has directly studied the fructose-fed animals, yet relatively little data exist on its programming effect on the offspring’s gut microbiota. A summary of animal studies demonstrating the association between gut microbiome, maternal high-fructose intake, and subsequent development of diseases in adult offspring is provided in Table 1 [9,10,40,41,42]. The current review is merely narrowed to a fructose diet starting in the pregnancy and/or lactation period. As shown in Table 1, rats were the dominant animal species being used. The major adverse outcome is hypertension [9,40,41,42].
Table 1.
Maternal high-fructose diet-induced adult disease of developmental origins related to gut microbiota dysbiosis in animal models.
| Animal Models | Species/ Gender |
Programming Mechanisms Related to Gut Microbiota | Adverse Offspring Outcomes | References |
|---|---|---|---|---|
| Maternal 60% fructose diet | SD rat/M | Decreased SCFA receptor GPR41 and GPR43 expression | Hypertension | [9] |
| Maternal 10% fructose water | Wistar rat/F | Reduced genera Lactobacillus and Bacteroides | Adiposity, dyslipidemia, and insulin resistance | [10] |
| Maternal 60% fructose diet | SD rat/M | Reduced genus Akkermansia abundance; Increased plasma TMA level | Hypertension | [40] |
| Maternal plus post-weaning 60% fructose diet | SD rat/M | Decreased abundance of genera Lactobacillus, Leuconostoc, and Turicibacter | Hypertension | [41] |
| Maternal 60% fructose diet and minocycline administration | SD rat/M | Reduced α-diversity, Decreased genera abundance of Lactobacillus, Ruminococcus, and Odoribacter; Increased abundance of Akkermansia; Increased SCFA receptor expression | Hypertension | [42] |
SCFA, Short-chain fatty acid. GPR, G protein-coupled receptor. TMA, Trimethylamine.
Adding 10% fructose to the drinking water of pregnant rats significantly altered the maternal microbiome [10]. Notably, there was a significant reduction in Lactobacillus and Bacteroides; both are commonly known as beneficial microbes. Their female offspring developed adiposity, dyslipidemia, and insulin resistance at 8 weeks of age. These findings were associated with a reduction in the expression of tight junction proteins in the offspring; however, the offspring microbiome was not assessed in this study. Similarly, a maternal high-fructose diet also alters the microbiome in rat offspring. Maternal high-fructose diet-induced hypertension in adult male offspring is related to decreased genus Akkermansia abundance [40]. Another study showed adult offspring born to dams that received a 60% fructose diet during pregnancy and lactation displayed an increase in the Firmicutes to Bacteroidetes ratio [41], a microbial marker of hypertension [42]. A follow-up study identified that maternal plus post-weaning high-fructose diet programs caused hypertension and coincided with a decreased abundance of genera Lactobacillus, Leuconostoc and Turicibacter [41].
Moreover, a maternal high-fructose diet not only alters microbiota compositions but also their metabolites in adult offspring. An association has been found between maternal high-fructose-induced programmed hypertension and gut microbial metabolites, trimethylamine (TMA), and acetate [40]. TMA is a microbiota-derived precursor of TMAO. Like TMAO, TMA is emerging as a cardiovascular risk marker [43,44]. The major SCFAs produced are acetate, propionate, and butyrate. Evidence shows that SCFAs regulate BP via interacting with SCFA receptors, including G protein-coupled receptor 41 (GPR41), GPR43, and olfactory receptor 78 (Olfr78) [45]. Feeding mother rats with a 60% fructose diet causes elevation of BP in adult offspring, relevant to an increase in plasma acetate level and a decrease in renal GPR41 and GPR43 expression [9]. Considering acetate is a ligand for Olfr78 to induce vasoconstriction and GPR41 exhibits vasodilatory action [46], these findings suggest that SCFAs and their receptors may be involved in maternal high-fructose diet programs leading to hypertension in their offspring.
Abnormalities in early-life gut microbiota were related to a number of adverse offspring outcomes, including obesity [47,48], insulin resistance [47,49], dyslipidemia [49], nonalcoholic fatty liver disease [48], and cardiovascular disease (CVD) [49]. All of these diseases are connected with fructose-induced developmental programming. In the current review, limited information is available about the use of gut microbiota-targeted therapies to study other adult diseases programmed by maternal fructose consumption, such as obesity, liver steatosis, dyslipidemia, insulin resistance, and cardiovascular disease.
Using Lactobacillus as a probiotic intervention, previous studies demonstrated it slowed progression of liver steatosis [50] and type II diabetes [51] in fructose-fed rats and mice. Additionally, maternal Lactiplantibacillus plantarum WJL treatment prevented adult offspring against CVD [49]. Another study revealed that maternal oligofructose therapy attenuated hepatic steatosis and insulin resistance induced in adult offspring born to dams received high-sucrose/-fat diets [52]. At this point, these studies evaluating the effect of gut microbiota-targeted therapies have only examined the established disease model or developmental models programmed by other insults. There will be a growing need to examine their reprogramming effects in adult diseases related to maternal fructose-induced developmental programming.
Furthermore, prior research demonstrated that excess maternal fructose consumption that caused adverse fetal outcomes was related to increased placental uric acid levels, while treatment of mother mice with the xanthine oxidase inhibitor allopurinol reduced placental uric acid levels and improved fetal weights and serum triglycerides [53]. Given that uric acid is a key mediator in high-fructose intake-related disorders [3] and intestinal microbes like Lactobacillus and Pseudomonas are known to participate in the metabolism of uric acid [54], probiotics Lactobacilli with uric acid-lowering effects targeting the gut microbiota may be a potential therapy for fructose-induced programming in further research.
3.3. How Gut Microbiota Links to Common Mechanisms Underlying Fructose-Induced Developmental Programming
In addition to gut microbiota dysbiosis, a number of mechanisms are proposed to be involved in fructose-induced developmental programming and the resulting adult disease. These molecular mechanisms include oxidative stress, aberrant renin–angiotensin system (RAS), nutrient sensing signals, epigenetic regulation, arachidonic acid metabolism pathway, etc. [4,5,14,55,56]. Some aforementioned mechanisms are interrelated to gut microbiota. We will discuss each of these mechanisms in turn.
3.3.1. Oxidative Stress
The fetus is extremely sensitive to oxidative damage during development because of its low antioxidant capacity [57]. A maternal high-fructose diet has been shown to induce various features of metabolic syndrome in adult offspring. Among them, dyslipidemia [58], insulin resistance [26], and hypertension [55,56] have been related to oxidative stress. Oxidative stress can reduce nitric oxide (NO) production by enhancing asymmetric dimethylarginine production (ADMA, an endogenous inhibitor of NO synthase) [59]. Increased plasma ADMA levels and decreased NO bioavailability have been reported in maternal fructose diet-induced programmed hypertension [23].
Conversely, antioxidants can reduce oxidative stress and prevent adult disease of developmental origins [60]. Melatonin is an antioxidant. Its use in pregnancy and lactation has shown to have beneficial effects on hypertension programmed by maternal high-fructose consumption [23]. Because fructose-induced developmental programming induces various features of metabolic syndrome targeting different organs, there is still a lack of reliable data on which organ-specific redox-sensitive signals are responsible for fructose-triggered programming processes.
Fructose-induced developmental programming, apart from oxidative stress, has been linked to gut microbiota. Gut microbial communities have been shown to trigger redox signaling and maintain host–microbiota homeostasis [61]. When an imbalance in the redox state occurs, inflammatory responses may mediate collateral tissue damage and end organ dysfunction [61]. Therefore, oxidative stress seems to work together with gut microbiota behind fructose-induced developmental programming. Attention will be needed to be paid to understanding how gut microbiota interrelates with oxidative stress to trigger organ-dependent programming processes, and whether interventions targeting gut microbiota in pregnancy may also reduce oxidative stress to prevent adult progeny against adult disease of developmental origins.
3.3.2. Aberrant RAS
The RAS is closely connected with adult disease of developmental origins [62]. The RAS is composed of different angiotensin peptides with diverse biological actions mediated by distinct receptors [63]. In general, activation of the classical axis of ACE/angiotensin II (ANG II)/ANG II type 1 receptor (AT1R) triggers vasoconstriction, oxidative stress, and inflammation. Maternal high-fructose diet-induced hypertension is relevant to the aberrant activation of RAS, represented by increases in (pro)renin receptor, angiotensinogen, and angiotensin-converting enzyme (ACE) in the kidneys (minocycline). In contrast, the non-classical RAS, composed mainly by the ACE2/angiotensin-(1-7) (Ang-(1-7))/Mas receptor (MasR)/ANG II type 2 receptor (AT2R), can counterbalance the adverse effects of ANG II. In the high-fructose diet plus 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure model, 3,3-dimethyl-1-butanol (DMB) therapy protected against hypertension coinciding with decreased AT1R and increased AT2R protein abundance [11]. Emerging evidence suggests a bidirectional interaction between the gut microbiome and RAS; gut microbiota-derived metabolites can modulate the gut RAS, while alterations in RAS shape microbiota composition and metabolic activity [64]. Considering maternal fructose consumption altered gut microbiota and the RAS concurrently, more work is required to explore the interaction between gut microbiome and the RAS implicating the pathogenesis of fructose-induced developmental programming.
3.3.3. Nutrient-Sensing Signals
During fetal development, nutrient-sensing signals regulate fetal metabolism in response to maternal nutritional status [65]. Accordingly, disturbed nutrient-sensing signals in pregnancy have a distinctive role in the pathogenesis of adult disease of developmental origins [66]. A number of signals, including silent information regulator transcript (SIRT), AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptors (PPARs), and PPARγ coactivator-1α (PGC-1α), are related to developmental programming [67]. SIRT1 and AMPK can mediate deacetylation and phosphorylation of PGC-1α, respectively. The downstream signaling effect of PGC-1α is PPARγ, which governs the expression of specific sets of PPAR target genes involved in hypertension of developmental origins [68]. In a maternal and post-weaning high-fructose diet rat model, renal mRNA expression of AMPK, PGC-1α, and PPARs were shown to decrease [41]. On the contrary, resveratrol, an AMPK activator, can mediate these nutrient-sensing signals to activate PPAR target genes and thereby protect offspring against metabolic syndrome-related programmed processes [41]. Additionally, maternal insulin therapy, shown to prevent the elevation of BP in adult offspring born to fructose-fed dams, was associated with enhanced phosphorylated AMPKα2 protein levels [41]. These observations suggest a potential connection between nutrient-sensing signals and gut microbiota underlying fructose-induced developmental programming.
3.3.4. Epigenetic Regulation
The epigenetic modification of genes has emerged as a key mechanism for developmental programming [69]. These modifications include DNA methylation, histone modification, and noncoding RNAs, all of which control gene activation or silencing [70]. Our previous work recognized significant alterations of renal transcriptome in 1-day-old male offspring exposed to maternal high-fructose intake by using next-generation RNA sequencing (NGS) analysis [55]. In total, 2706 differential expressed genes (DEGs) (1214 up- and 1492 down-regulated genes) were identified. Among them, Cyp2c23, Hpgds, Ptgds and Ptges belonging to arachidonic acid metabolism were involved in maternal high-fructose diet-induced hypertension [56]. Moreover, a number of genes regulating fructose metabolism, fatty acid metabolism, glycolysis/gluconeogenesis, and insulin signaling appear to be regulated by a maternal high-fructose diet in different organs at 1 day of age in our follow-up study [20]. Notably, a maternal high-fructose diet induces differential alterations of gene expression in the brain, kidney, heart, and urinary bladder in progeny. Our NGS results suggest that epigenetic regulation may be involved in the developmental programming of various adult diseases in an organ-specific manner. Additionally, maternal fructose exposure altered the miR-206 expression level in offspring liver that increased promoter methylation at Lxra gene [70,71]. Gut microbiota and their metabolites have shown the ability of epigenetic programming of multiple host tissues [72]. The SCFAs can form acetyl-CoA, the substrate for histone acetyltransferase (HAT) enzymes. Further, butyrate is a known histone deacetylase (HDAC) inhibitor. Both scenarios could affect histone modification. Thus, maternal fructose diet-related gut dysbiosis induces epigenetic programming of offspring genes, and corresponding epigenetic mechanisms together with associated gut microbes await further elucidation.
4. Reprogramming Strategies Targeted on Gut Microbiota
Concerning our advanced understanding of developmental programming, it turns out that a therapeutic approach can be shifted from adulthood to early life, even before disease occurs. This is termed as reprogramming [73]. In view of the fact that research into gut microbiota has uncovered a number of interesting contributions to health and disease, researchers are turning their attention to focus on gut microbiota as a potential target for therapeutics [74,75,76]. We propose a diagram to illustrate the potential gut microbiota-targeted therapies as a reprogramming strategy for the prevention of maternal fructose diet-induced adult disease, which is depicted in Figure 2.
Figure 2.
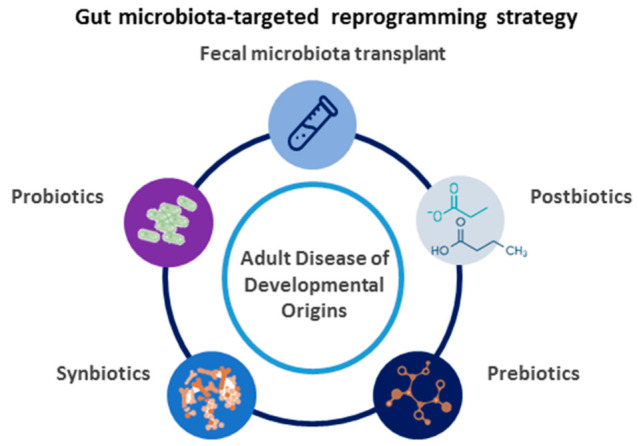
Schematic diagram of the potential gut microbiota-targeted therapies used for adult disease of developmental origins.
4.1. Gut Microbiota-Targeted Therapy
The gut microbiome can be targeted with a variety of modalities, including probiotics (i.e., beneficial microbes), prebiotics (i.e., nutrition or food for those beneficial microbes), synbiotics (i.e., mixture of probiotics and prebiotics), postbiotics (i.e., substances produced through the metabolism of the gut microbes), and fecal microbiota transplant.
As reviewed elsewhere [75,76,77,78], gut microbiota-targeted therapy has shown benefits against a broad range of diseases. The widespread clinical use of gut microbiota-targeted treatments are probiotics and prebiotics. Though results from human studies reported beneficial effects of probiotics during pregnancy on maternal outcomes, little is known about the effectiveness in protecting offspring against DOHaD-related disorders. Due to ethical considerations in humans, experiments to target gut microbiota for the prevention of adult disease of developmental origins have provided emerging evidence in animal models. Nevertheless, targeting gut microbiota and derived metabolites remain a flourishing area of research with potential reprogramming interventions for DOHaD-related disorders.
4.2. The Use of Gut Microbiota-Targeted Therapy as a Reprogramming Strategy
Here, we summarize studies documenting reprogramming strategies in animal models of fructose-induced developmental programming, focusing on interventions aimed at the gut microbiota. Notably, gut microbiota-targeted therapy will only be restricted to key periods during early development. A review of the literature exposes that currently there are only a few reports on the reprogramming effects of gut microbiota-targeted therapy related to fructose-induced developmental programming. As illustrated in Table 2, rats have dominated in experiments for studying programmed hypertension. Reported reprogramming interventions consist of probiotics, prebiotics, and postbiotics. Though fecal microbiota transplant has also been shown to rescue metabolic syndrome-related phenotypes in a high-fructose diet rat model [79], its reprogramming effect on offspring born to fructose-fed dam has yet to be studied.
Table 2.
Gut microbiota-targeted therapies used as a reprogramming strategy for maternal fructose diet-induced adult disease.
| Gut Microbiota-Targeted Therapy | Animal Models | Species/ Gender |
Reprogramming Effects | Ref. |
|---|---|---|---|---|
| Lactobacillus casei (2 × 10⁸ CFU/day) by oral gavage from pregnancy through lactation | Maternal 60% fructose diet | SD rat/M | Prevented hypertension |
[40] |
| Long chain inulin (5% w/w) in drinking water from pregnancy through lactation | Maternal 60% fructose diet | SD rat/M | Prevented hypertension | [40] |
| Magnesium acetate (200 mmol/L) in drinking water from pregnancy through lactation | Maternal 60% fructose diet | SD rat/M | Prevented hypertension | [41] |
| DMB (1%, v/v in drinking water) from pregnancy through lactation | Maternal 60% diet | SD rat/M | Prevented hypertension | [41] |
| DMB (1%, v/v in drinking water) from pregnancy through lactation | Maternal 60% fructose diet and TCDD exposure | SD rat/M | Prevented hypertension | [42] |
SD, Sprague–Dawley rat; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; DMB, 3,3-maternal dimethyl-1-butanol.
Though several probiotic bacteria are associated with health benefits [78,79,80], there is currently no information to suggest their roles on the fructose-induced developmental programming. Only one study documented that Lactobacillus casei supplementation during gestation and lactation protects adult male rat offspring against hypertension induced by a maternal high-fructose diet [40]. The beneficial effects of Lactobacillus casei supplementation against maternal fructose diet-induced hypertension is related to a decrease in plasma acetate level and renal Olfr78 expression. Considering acetate can induce hypertension by activation of Olf78, SCFA and their receptors are involved in the protective benefits of maternal probiotics treatment. In the same study, long-chain inulin was investigated in regards to their reprogramming effects [40]. Although several types of prebiotics provide various health benefits [76,77], only inulin has been examined in the maternal fructose diet model. Supplementation with inulin during pregnancy and lactation protected maternal high-fructose diet-induced programmed hypertension and was associated with an increased plasma propionate level and renal GPR43 expression [40]. As propionate can activate GPR43 to elicit vasodilatation [45,46], the protective effects of inulin are attributed to gut microbiota-derived metabolite SCFAs. Of note, probiotic and prebiotic therapy show different mechanisms on modulation of gut microbiota despite them exhibiting similar BP-lowering effects [40]. As there are many prebiotic-like components, such as flavonoids, polyphenols, and vitamins, in functional foods, in moving towards their widespread clinical use, there will be a growing need to better understand their reprogramming effects on fructose-induced developmental programming. For example, resveratrol therapy has emerged as a reprogramming agent to protect against several adult diseases of developmental origin [80]. Somewhat surprisingly, there is no research evaluating its protective effects on maternal fructose diet-induced adverse offspring outcomes; such studies are warranted.
The SCFAs have been used as postbiotics as they are fermentation products of polysaccharides by gut microbiota [64]. So far, there is only one report showing that maternal high-fructose diet-induced hypertension can be protected by acetate supplementation [41]. Its protective effects are associated with decreased plasma TMA levels and TMA-to-TMAO ratios, and increased expression of SCFA receptors. As previously mentioned, gut microbiota-dependent TMA and TMAO formation is related to cardiovascular risk. Maternal TMAO administration has been reported to program hypertension in adult male progeny [81]. Conversely, TMA inhibition shows reprogramming effects in fructose-exposed offspring. A structural analogue of choline, 3,3-dimethyl-1-butanol (DMB), showed non-lethal inhibition of TMA and host TMAO formation [82]. Until now, two studies have shown that DMB treatment during pregnancy and lactation protect adult offspring against hypertension programmed by a maternal high-fructose diet [41] or high-fructose diet plus TCDD exposure [42]. The protective effects of DMB therapy are associated with alterations of gut microbes involved in TMA–TMAO metabolism, including the phyla Firmicutes and Proteobacteria, Enterobacteriaceae and Deferribacteraceae, and genus Holdemania families.
5. Conclusions and Future Perspectives
Prior research has indicated that maternal fructose exposure and early life imbalance of gut microbiome might cause adverse offspring outcomes in later life. This review has aimed to highlight the value of gut microbiota-targeted therapy; if applied early, it may protect adult disease of developmental origins programmed by maternal fructose consumption. We fully understand that the presented mechanisms in the current review might not cover the whole picture of the programming effects of fructose related to alterations of gut microbiome. A thorough examination of the relationships between fructose-induced developmental programming and mechanisms behind gut microbiota dysbiosis is worthy of further study. Moreover, what is missing from the literature is a greater understanding of whether maternal or offspring gut microbiota affects developmental programming, due to the inability to longitudinally analyze gut microbiome dynamics at different developmental stages from all reported studies. More attention needs to be paid to analyze how gut microbiome interacts with fructose at the early developmental stage to help explore the causal relationship and elucidate the underlying programmed processes.
Studies to date have documented some evidence regarding how early life gut microbiota-targeted therapies reprogram unexpected fructose-induced programming processes and protect disease in later life; however, almost all studies were focused on hypertension. Given that fructose can program many organs, thus resulting in different phenotypes in adult offspring, there is a pressing need for more studies in their organ-specific reprogramming effects; it is also necessary to evaluate other gut microbiota-related interventions.
Regardless of recent advances in developing potential reprogramming strategies targeting gut microbiota for adult disease of developmental origins, none of them have been translated in human trials. The literature is missing a greater understanding of whether the current uses of probiotics or prebiotic-rich food in pregnant women may also alter gut microbiota and derived metabolites to prevent their children from chronic diseases in adulthood.
In summary, gut microbiota is an important pathophysiological link in early life fructose exposure and developmental programming of adult disease. After a better understanding of fructose-induced developmental programming and this remarkable growth in gut microbiota-target therapy, we expect that translating animal results into optimal clinical practice is a valuable strategy that could reduce the global pandemic of fructose-related disorders.
Author Contributions
C.-N.H. contributed to data interpretation, concept generation, methodology, drafting of the manuscript, and approval of the article; H.-R.Y. contributed to drafting of the manuscript, critical revision of the manuscript, and approval of the article; J.Y.H.C. contributed to drafting of the manuscript, critical revision of the manuscript, and approval of the article; K.L.H.W. contributed to critical revision of the manuscript, and approval of the article; W.-C.L. contributed to critical revision of the manuscript and approval of the article; Y.-L.T. contributed to methodology, concept generation, drafting of the manuscript, critical revision of the manuscript, and approval of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan, grant MOST 110-2314-B182A-029, and Chang Gung Memorial Hospital, Kaohsiung, Taiwan, grants CMRPG8J0251, CMRPG8J0252, and CMRPG8J0253.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barker D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 2.Hsu C.-N., Tain Y.-L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients. 2019;11:894. doi: 10.3390/nu11040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson R.J., Segal M.S., Sautin Y., Nakagawa T., Feig D.I., Kang D.-H., Gersch M.S., Benner S., Sánchez-Lozada L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 4.Thompson M.D., De Bosch B.J. Maternal Fructose Diet-Induced Developmental Programming. Nutrients. 2021;13:3278. doi: 10.3390/nu13093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee W.-C., Wu K.L., Leu S., Tain Y.-L. Translational insights on developmental origins of metabolic syndrome: Focus on fructose consumption. Biomed. J. 2018;41:96–101. doi: 10.1016/j.bj.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G., Liu W., Tesz G.J., Birnbaum M.J., Rabinowitz J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018;27:351–361.e3. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson P.R., Newnham E., Barrett J.S., Shepherd S.J., Muir J.G. Review article: Fructose malabsorption and the bigger picture. Aliment. Pharmacol. Ther. 2006;25:349–363. doi: 10.1111/j.1365-2036.2006.03186.x. [DOI] [PubMed] [Google Scholar]
- 8.Al Rubaye H., Adamson C.C., Jadavji N.M. The role of maternal diet on offspring gut microbiota development: A review. J. Neurosci. Res. 2021;99:284–293. doi: 10.1002/jnr.24605. [DOI] [PubMed] [Google Scholar]
- 9.Hsu C.-N., Lin Y.-J., Hou C.-Y., Tain Y.-L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients. 2018;10:1229. doi: 10.3390/nu10091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astbury S., Song A., Zhou M., Nielsen B., Hoedl A., Willing B.P., Symonds M.E., Bell R.C. High Fructose Intake During Pregnancy in Rats Influences the Maternal Microbiome and Gut Development in the Offspring. Front. Genet. 2018;9:203. doi: 10.3389/fgene.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu C.-N., Chan J.Y.H., Yu H.-R., Lee W.-C., Wu K.L.H., Chang-Chien G.-P., Lin S., Hou C.-Y., Tain Y.-L. Targeting on Gut Microbiota-Derived Metabolite Trimethylamine to Protect Adult Male Rat Offspring against Hypertension Programmed by Combined Maternal High-Fructose Intake and Dioxin Exposure. Int. J. Mol. Sci. 2020;21:5488. doi: 10.3390/ijms21155488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S., Jang C., Liu J., Uehara K., Gilbert M., Izzo L., Zeng X., Trefely S., Fernandez S., Carrer A., et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelishadi R., Mansourian M., Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrients. 2014;30:503–510. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Jayalath V.H., de Souza R.J., Ha V., Mirrahimi A., Blanco-Mejia S., Di Buono M., Jenkins A.L., Leiter L.A., Wolever T.M.S., Beyene J., et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015;102:914–921. doi: 10.3945/ajcn.115.107243. [DOI] [PubMed] [Google Scholar]
- 15.Khan T.A., Sievenpiper J.L. Controversies about sugars: Results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Zeitschrift für Ernährungswissenschaft. 2016;55:25–43. doi: 10.1007/s00394-016-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik V.S., Hu F.B. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015;66:1615–1624. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semnani-Azad Z., Khan T.A., Blanco Mejia S., de Souza R.J., Leiter L.A., Kendall C.W.C., Hanley A.J., Sievenpiper J.L. Association of Major Food Sources of Fructose-Containing Sugars With Incident Metabolic Syndrome: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020;3:e209993. doi: 10.1001/jamanetworkopen.2020.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanhope K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016;53:52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan T.A., Tayyiba M., Agarwal A., Mejia S.B., de Souza R.J., Wolever T.M.S., Leiter L.A., Kendall C.W.C., Jenkins D.J.A., Sievenpiper J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars With the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019;94:2399–2414. doi: 10.1016/j.mayocp.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Chao Y.-M., Tain Y.-L., Leu S., Wu K.L.H., Lee W.-C., Chan J.Y.H. Developmental programming of the metabolic syndrome: Next-generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao [Acta Physiol. Sin.] 2016;68:557–567. [PubMed] [Google Scholar]
- 21.Koo S., Kim M., Cho H.M., Kim I. Maternal high-fructose intake during pregnancy and lactation induces metabolic syndrome in adult offspring. Nutr. Res. Pract. 2021;15:160–172. doi: 10.4162/nrp.2021.15.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad A.F., Dickerson J., Kechichian T.B., Yin H., Gamble P., Salazar A., Patrikeev I., Motamedi M., Saade G.R., Costantine M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016;215:378.e1–378.e6. doi: 10.1016/j.ajog.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Tain Y.-L., Leu S., Wu K.L.H., Lee W.-C., Chan J.Y.H. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014;57:80–89. doi: 10.1111/jpi.12145. [DOI] [PubMed] [Google Scholar]
- 24.Seong H.Y., Cho H.M., Kim M., Kim I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin-Angiotensin-Aldosterone System. Hypertension. 2019;74:518–525. doi: 10.1161/HYPERTENSIONAHA.119.12941. [DOI] [PubMed] [Google Scholar]
- 25.Smith E.V.L., Dyson R.M., Berry M.J., Gray C. Fructose Consumption During Pregnancy Influences Milk Lipid Composition and Offspring Lipid Profiles in Guinea Pigs. Front. Endocrinol. 2020;11:550. doi: 10.3389/fendo.2020.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez L., Otero P., Panadero M.I., Rodrigo S., Álvarez-Millán J.J., Bocos C. Maternal Fructose Intake Induces Insulin Resistance and Oxidative Stress in Male, but Not Female, Offspring. J. Nutr. Metab. 2015;2015:1–8. doi: 10.1155/2015/158091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K.L., Wu C.-W., Tain Y.-L., Huang L.-T., Chao Y.-M., Hung C.-Y., Wu J.-C., Chen S.-R., Tsai P.-C., Chan J.Y. Environmental stimulation rescues maternal high fructose intake-impaired learning and memory in female offspring: Its correlation with redistribution of histone deacetylase 4. Neurobiol. Learn. Mem. 2016;130:105–117. doi: 10.1016/j.nlm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Huang H.-M., Wu C.-W., Chen I.-C., Lee Y.-C., Huang Y.-S., Hung C.-Y., Wu K.L.-H. Maternal high-fructose diet induced early-onset retinopathy via the suppression of synaptic plasticity mediated by mitochondrial dysfunction. Am. J. Physiol. Metab. 2021;320:E1173–E1182. doi: 10.1152/ajpendo.00001.2021. [DOI] [PubMed] [Google Scholar]
- 29.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 30.Agus A., Clément K., Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rienzi S.C., Britton R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. 2020;11:616–629. doi: 10.1093/advances/nmz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambertz J., Weiskirchen S., Landert S., Weiskirchen R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front. Immunol. 2017;8:1159. doi: 10.3389/fimmu.2017.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabke K., Hendrick G., Devkota S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spruss A., Bergheim I. Dietary fructose and intestinal barrier: Potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2009;20:657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Do M.H., Lee E., Oh M.-J., Kim Y., Park H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients. 2018;10:761. doi: 10.3390/nu10060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jegatheesan P., Beutheu S., Ventura G., Sarfati G., Nubret E., Kapel N., Waligora-Dupriet A.-J., Bergheim I., Cynober L., De-Bandt J.-P. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin. Nutr. 2016;35:175–182. doi: 10.1016/j.clnu.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Wang C., Chen Y., Tain Y.-L., Chang S.K., Huang L., Hsieh C., Hou C. Fast quantification of short-chain fatty acids in rat plasma by gas chromatography. J. Food Sci. 2020;85:1932–1938. doi: 10.1111/1750-3841.15172. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Zheng L., Wang L., Wang S., Wang Y., Han Z. Effects of high fructose and salt feeding on systematic metabonome probed via1H NMR spectroscopy. Org. Magn. Reson. 2015;53:295–303. doi: 10.1002/mrc.4198. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborti A., Graham C., Chehade S., Vashi B., Umfress A., Kurup P., Vickers B., Chen H.A., Telange R., Berryhill T., et al. High Fructose Corn Syrup-Moderate Fat Diet Potentiates Anxio-Depressive Behavior and Alters Ventral Striatal Neuronal Signaling. Front. Neurosci. 2021;15:669410. doi: 10.3389/fnins.2021.669410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu C.N., Chang-Chien G.P., Lin S., Hou C.Y., Tain Y.L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019;63:e1900073. doi: 10.1002/mnfr.201900073. [DOI] [PubMed] [Google Scholar]
- 41.Tain Y.-L., Lee W.-C., Wu K.L.H., Leu S., Chan J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018;62:e1800066. doi: 10.1002/mnfr.201800066. [DOI] [PubMed] [Google Scholar]
- 42.Hsu C.-N., Chan J.Y.H., Wu K.L.H., Yu H.-R., Lee W.-C., Hou C.-Y., Tain Y.-L. Altered Gut Microbiota and Its Metabolites in Hypertension of Developmental Origins: Exploring Differences between Fructose and Antibiotics Exposure. Int. J. Mol. Sci. 2021;22:2674. doi: 10.3390/ijms22052674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T., Richards E.M., Pepine C.J., Raizada M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018;14:442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fennema D., Phillips I.R., Shephard E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016;44:1839–1850. doi: 10.1124/dmd.116.070615. Erratum in Drug Metab. Dispos. 2016, 44, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pluznick J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Physiol. 2013;305:F439–F444. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017;19:25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M., Zhang Y., Miller D., Rehman N.O., Cheng X., Yeo J.-Y., Joe B., Hill J.W. Microbial Reconstitution Reverses Early Female Puberty Induced by Maternal High-fat Diet During Lactation. Endocrinology. 2020;161:bqz041. doi: 10.1210/endocr/bqz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wankhade U., Zhong Y., Kang P., Alfaro M., Chintapalli S.V., Thakali K.M., Shankar K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE. 2017;12:e0175675. doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guimarães K.S.D.L., Braga V.D.A., de Noronha S.I.S.R., da Costa W.K.A., Makki K., Cruz J.D.C., Brandão L.R., Junior D.A.C., Meugnier E., Leulier F., et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020;11:8939–8950. doi: 10.1039/D0FO01718C. [DOI] [PubMed] [Google Scholar]
- 50.Wagnerberger S., Spruss A., Kanuri G., Stahl C., Schröder M., Vetter W., Bischoff S.C., Bergheim I. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: A mouse model. J. Nutr. Biochem. 2013;24:531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Yadav H., Jain S., Sinha P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrients. 2007;23:62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Paul H.A., Collins K.H., Nicolucci A.C., Urbanski S.J., Hart D.A., Vogel H.J., Reimer R.A. Maternal prebiotic supplementation reduces fatty liver development in offspring through altered microbial and metabolomic profiles in rats. FASEB J. 2019;33:5153–5167. doi: 10.1096/fj.201801551R. [DOI] [PubMed] [Google Scholar]
- 53.Asghar Z.A., Thompson A., Chi M., Cusumano A., Scheaffer S., Al-Hammadi N., Saben J.L., Moley K.H. Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Sci. Rep. 2016;6:25091. doi: 10.1038/srep25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., Chen Y., Zhong H., Chen F., Regenstein J., Hu X., Cai L., Feng F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2021:1–11. doi: 10.1080/10408398.2021.1874287. [DOI] [PubMed] [Google Scholar]
- 55.Tain Y.-L., Wu K.L., Lee W.-C., Leu S., Chan J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015;26:642–650. doi: 10.1016/j.jnutbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Tain Y.-L., Lee W.-C., Wu K.L., Leu S., Chan J.Y. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016;38:86–92. doi: 10.1016/j.jnutbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Thompson L.P., Al-Hasan Y. Impact of Oxidative Stress in Fetal Programming. J. Pregnancy. 2012;2012:1–8. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ching R.H.H., Yeung L.O.Y., Tse I.M.Y., Sit W.-H., Li E.T.S. Supplementation of Bitter Melon to Rats Fed a High-Fructose Diet During Gestation and Lactation Ameliorates Fructose-Induced Dyslipidemia and Hepatic Oxidative Stress in Male Offspring. J. Nutr. 2011;141:1664–1672. doi: 10.3945/jn.111.142299. [DOI] [PubMed] [Google Scholar]
- 59.Huang L.-T., Hsieh C.-S., Chang K.-A., Tain Y.-L. Roles of Nitric Oxide and Asymmetric Dimethylarginine in Pregnancy and Fetal Programming. Int. J. Mol. Sci. 2012;13:14606–14622. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu C.-N., Tain Y.-L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants. 2020;9:1034. doi: 10.3390/antiox9111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell E.L., Colgan S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019;16:106–120. doi: 10.1038/s41575-018-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu C.-N., Tain Y.-L. Targeting the Renin–Angiotensin–Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021;22:2298. doi: 10.3390/ijms22052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Te Riet L., van Esch J.H., Roks A.J., van den Meiracker A.H., Danser A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 64.Jaworska K., Koper M., Ufnal M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol. Liver Physiol. 2021;321:G355–G366. doi: 10.1152/ajpgi.00099.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018;39:489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansson T., Powell T. Role of Placental Nutrient Sensing in Developmental Programming. Clin. Obstet. Gynecol. 2013;56:591–601. doi: 10.1097/GRF.0b013e3182993a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Efeyan A., Comb W.C., Sabatini D.M. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tain Y.-L., Hsu C.-N., Chan J.Y.H. PPARs Link Early Life Nutritional Insults to Later Programmed Hypertension and Metabolic Syndrome. Int. J. Mol. Sci. 2015;17:20. doi: 10.3390/ijms17010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goyal D., Limesand S.W., Goyal R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019;242:T105–T119. doi: 10.1530/JOE-19-0009. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigo S., Fauste E., de la Cuesta M., Rodríguez L., Álvarez-Millán J.J., Panadero M.I., Otero P., Bocos C. Maternal fructose induces gender-dependent changes in both LXRα promoter methylation and cholesterol metabolism in progeny. J. Nutr. Biochem. 2018;61:163–172. doi: 10.1016/j.jnutbio.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Yamazaki M., Munetsuna E., Yamada H., Ando Y., Mizuno G., Fujii R., Nouchi Y., Kageyama I., Teshigawara A., Ishikawa H., et al. Maternal fructose consumption down-regulates Lxra expression via miR-206-mediated regulation. J. Nutr. Biochem. 2020;82:108386. doi: 10.1016/j.jnutbio.2020.108386. [DOI] [PubMed] [Google Scholar]
- 72.Krautkramer K., Kreznar J.H., Romano K.A., Vivas E.I., Barrett-Wilt G.A., Rabaglia M.E., Keller M.P., Attie A.D., Rey F.E., Denu J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell. 2016;64:982–992. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tain Y.-L., Joles J.A. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int. J. Mol. Sci. 2015;17:23. doi: 10.3390/ijms17010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu C.-N., Hou C.-Y., Hsu W.-H., Tain Y.-L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients. 2021;13:2290. doi: 10.3390/nu13072290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S.-K., Guevarra R.B., Kim Y.-T., Kwon J., Kim H., Cho J.H., Kim H.B., Lee J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 76.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 77.Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peluzio M.D.C.G., Martinez J.A., Milagro F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021;108:11–26. doi: 10.1016/j.tifs.2020.12.004. [DOI] [Google Scholar]
- 79.Di Luccia B., Crescenzo R., Mazzoli A., Cigliano L., Venditti P., Walser J.-C., Widmer A., Baccigalupi L., Ricca E., Iossa S. Rescue of Fructose-Induced Metabolic Syndrome by Antibiotics or Faecal Transplantation in a Rat Model of Obesity. PLoS ONE. 2015;10:e0134893. doi: 10.1371/journal.pone.0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu C.-N., Hou C.-Y., Tain Y.-L. Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021;22:4210. doi: 10.3390/ijms22084210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu C.-N., Hou C.-Y., Chang-Chien G.-P., Lin S., Chan J.Y., Lee C.-T., Tain Y.-L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N-oxide. J. Nutr. Biochem. 2021;93:108630. doi: 10.1016/j.jnutbio.2021.108630. [DOI] [PubMed] [Google Scholar]
- 82.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., Gu X., Huang Y., Zamanian-Daryoush M., Culley M.K., et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.


