Background:
Understanding the effect of lifestyle and genetic risk on the lifetime risk of coronary heart disease (CHD) is important to improving public health initiatives. Our objective was to quantify remaining lifetime risk and years free of CHD according to polygenic risk and the American Heart Association’s Life’s Simple 7 (LS7) guidelines in a population-based cohort study.
Methods:
Our analysis included data from participants of the ARIC (Atherosclerosis Risk in Communities) study: 8372 White and 2314 Black participants; 45 years of age and older; and free of CHD at baseline examination. A polygenic risk score (PRS) comprised more than 6 million genetic variants was categorized into low (<20th percentile), intermediate, and high (>80th percentile). An overall LS7 score was calculated at baseline and categorized into “poor,” “intermediate,” and “ideal” cardiovascular health. Lifetime risk and CHD-free years were computed according to polygenic risk and LS7 categories.
Results:
The overall remaining lifetime risk was 27%, ranging from 16.6% in individuals with an ideal LS7 score to 43.1% for individuals with a poor LS7 score. The association of PRS with lifetime risk differed according to ancestry. In White participants, remaining lifetime risk ranged from 19.8% to 39.3% according to increasing PRS categories. Individuals with a high PRS and poor LS7 had a remaining lifetime risk of 67.1% and 15.9 fewer CHD-free years than did those with intermediate polygenic risk and LS7 scores. In the high-PRS group, ideal LS7 was associated with 20.2 more CHD-free years compared with poor LS7. In Black participants, remaining lifetime risk ranged from 19.1% to 28.6% according to increasing PRS category. Similar lifetime risk estimates were observed for individuals of poor LS7 regardless of PRS category. In the high-PRS group, an ideal LS7 score was associated with only 4.5 more CHD-free years compared with a poor LS7 score.
Conclusions:
Ideal adherence to LS7 recommendations was associated with lower lifetime risk of CHD for all individuals, especially in those with high genetic susceptibility. In Black participants, adherence to LS7 guidelines contributed to lifetime risk of CHD more so than current PRSs. Improved PRSs are needed to properly evaluate genetic susceptibility for CHD in diverse populations.
Keywords: atherosclerosis, cohort studies, coronary disease, genetic predisposition to disease, lifestyle, public health, risk factors
Clinical Perspective.
What Is New?
To date, no study has reported the lifetime risk of coronary heart disease (CHD), or the years free of CHD, according to polygenic risk and adherence to the American Heart Association’s Life’s Simple 7 (LS7) recommendations in both White and Black participants.
Participants with high polygenic risk may offset lifetime risk for CHD by up to 50% through managing their health according to LS7 recommendations, depending on ancestry.
Individuals with high polygenic risk scores and ideal LS7 scores had 4.5 to 20 more CHD-free years than individuals with high polygenic risk scores but low LS7 scores, depending on ancestry.
What Are the Clinical Implications?
Appropriate management of lifestyle and clinical risk factors of CHD play larger roles in overall lifetime risk of CHD than presently available genetic information.
Improved polygenic risk scores are needed to expand the utility of them to ancestries beyond European.
Communicating the effects of LS7 and polygenic risk on CHD in terms of absolute risk may have important implications for education, policy, and environmental changes, which can benefit not only high-risk individuals, but the whole population.
Reducing the global burden of coronary heart disease (CHD) remains a crucial public health focus. Improving prevention and intervention strategies for CHD requires summarizing the effects of both environmental and genetic causes. With the emergence of large genome-wide association studies (GWAS), the effects of millions of genetic variants on CHD have been described and translated into clinically relevant terms using polygenic risk scores (PRSs).1–5 To date, GWAS for CHD have primarily included individuals of European ancestry. As such, PRSs are less effective at predicting disease in individuals from other ancestry groups, with particularly poor prediction in those of African ancestry.6,7 In individuals of European ancestry, a high PRS can confer a risk of CHD comparable to monogenic mutations in familial hypercholesteremia.8,9 Furthermore, PRSs are independent of common risk factors including age, providing opportunity for risk ascertainment early in life10,11 which is vital to the reduction of CHD and has proven effective in individuals with familial hypercholesteremia.12–15
Adherence to a healthy lifestyle is a key preventive approach to CHD and optimizing lifestyle choices through education, policies, and environmental change remain important public health initiatives. The effect of a healthy lifestyle on clinical risk factors for CHD has been well described, but the interplay between polygenic risk and present lifestyle guidelines is still a topic of interest. While a PRS is often considered an immutable trait, there is evidence that a healthy lifestyle can offset high polygenic risk.16–19 In 2010, the American Heart Association created Life’s Simple 7 (LS7) to define ideal cardiovascular health according to 7 risk factors that are modifiable through lifestyle changes.20 To our knowledge, no studies have examined whether adherence to the LS7 guidelines can offset polygenic risk.
Most studies that have examined the interplay between lifestyle and polygenic risk have communicated their results in terms of relative risk during a limited follow-up period.16–18 Lifetime risk is the preferred method of risk communication for many patients.21 While traditional 10-year estimates are more commonly used, a low or intermediate 10-year risk can often disguise a high lifetime risk, particularly at younger ages.22–25 This limits their utility in describing the potential burden of CHD and may delay important lifestyle and clinical intervention. Hindy et al reported a strong gradient in lifetime risk of CHD according to polygenic risk in individuals of European ancestry.10 This gradient was still present within clinical risk stratum defined by the pooled cohort equations, suggesting polygenic risk plays a key role in lifetime risk of CHD and complements current clinical risk assessment strategies.
Our primary objective was to quantify differences in the lifetime risk of CHD in White and Black individuals according to polygenic risk and adherence to LS7 guidelines. We also examined these differences in terms of years lived free of CHD, an intuitive measure of absolute lifetime risk.
Methods
Genotype and phenotype data supporting the findings from this study are available through the Database of Genotypes and Phenotypes (accession no. phs000090.v7.p1).
Study Population
The ARIC (Atherosclerosis Risk in Communities) study is a population-based, prospective cohort study of cardiovascular disease and associated risk factors sponsored by the National Institute of Health’s National Heart, Lung, and Blood Institute. ARIC included 15 792 individuals, predominantly White and Black participants, 45 to 64 years of age at baseline (1987–1989), chosen by probability sampling from 4 US communities. Cohort members completed 3 more recent triennial follow-up examinations: a fifth examination between 2011 and 2013, a sixth examination between 2016 and 2017, and a seventh examination between 2018 and 2019. Community surveillance and annual telephone interviews identify study outcomes in the interim. The ARIC study has been described in detail previously.26 The ARIC study has been approved by the institutional review board at all participating institutions. All participants provided written informed consent.
Of the 15 792 ARIC participants, 11 478 participants reported being White (defined by the NIH as European, Middle Eastern, or North African ancestry) and 4266 participants reported being Black (or of African ancestry) and were included in these analyses. Participants who reported other ancestry groups, declined to answer, or reported other were not included in these analyses (n=48). Of these, 12 219 participants provided genetic data: 9345 White and 2874 Black participants. The primary analysis was restricted to 8372 White and 2314 Black ARIC participants with appropriate values for principal components for genetic ancestry who were free of CHD at the baseline examination and had LS7 data available.
Outcome
CHD events were ascertained through cohort follow-up interviews, as well as through the routine community-wide surveillance, from 1987 through 2017. Medical records and death certificates were used, when available, as source documentation for CHD events. Incident CHD included hospitalized myocardial infarction (MI), fatal CHD, or a cardiac revascularization procedure.27 Hospitalized myocardial infarctions were classified by trained ARIC personnel based on combinations of cardiac pain, cardiac biomarkers, and/or ECG patterns. Fatal CHD was defined as the absence of a lethal process of known nonatherosclerotic or noncardiac atherosclerotic causes, presence of chest pain within 72 hours of death and/or ever having had chronic ischemic heart disease such as coronary insufficiency or angina pectoris. Final classification of CHD was adjudicated by trained personnel.
Polygenic Risk Score
Participants were genotyped using the Affymetrix 6.0 array (Affymetrix Inc, Santa Clara, CA). Genotyped variants were used to impute to the TOPMed (version R2) reference panel. Haplotype phasing and imputation was performed using the Michigan Imputation Server28 (available at https://imputationserver.sph.umich.edu).
A PRS for CHD, on the basis of more than 6 million genetic variants, was developed using the LDPred algorithm on individuals of European ancestry in UK Biobank by Khera et al.8 On the basis of the publicly available weights from this published score, a PRS was created by multiplying the risk allele dosage with the weights. After restricting to single nucleotide polymorphisms with an imputation quality r2 >0.3 in ARIC, 6 483 355 single nucleotide polymorphisms were included in an additive weighted genetic risk score calculated by summing the weighted dosages for each individual. A residual PRS was then created after adjusting for the first 11 principal components for ancestry. Individuals were further categorized into low (<20th percentile), intermediate (20th–80th percentile), and high (>80th percentile) genetic risk categories according to their self-reported race. To maximize the statistical precision and analyze the effects of low and high polygenic risk, the intermediate genetic risk category was used as the reference.
LS7 and Baseline Covariates
At baseline, questionnaires assessing information on demographic characteristics, health behaviors, medical history and medication use were administered. Family history of CHD was considered if an individual reported maternal or paternal history of heart attack. If the incident occurred before 60 years of age for women and 55 years of age for men, then it was considered premature CHD. Physical assessments including weight, height and blood pressure were performed by trained staff members. Fasting blood samples were also obtained and blood glucose and cholesterol were measured using standard laboratory techniques.
LS7 was previously calculated in ARIC.29,30 In brief, the 7 cardiovascular health factors include smoking status, body weight, total cholesterol, blood glucose, physical activity, and diet. Each cardiovascular health factor was categorized into 3 groups (ideal, intermediate, poor). Smoking was considered “ideal” if individuals never smoked or quit >1 year ago, intermediate if they quit within the past year, and “poor” if they currently smoked. Body weight was classified according to an individual’s body mass index. Body mass index was calculated as weight in kilograms divided by height in square meters and classified into 3 categories: <25 kg/m2 (ideal), ≥25 to <30 kg/m2 (intermediate), and ≥30 kg/m2 (poor). Serum total cholesterol was separated into 3 categories: <200 mg/dL and untreated (ideal), 200 to 239 mg/dL or treated (intermediate), and ≥240 mg/dL (poor). Ideal fasting blood glucose was considered <100 mg/dL and was not treated, 100 to 125 mg/dL or treated to goal range was considered intermediate, and ≥126 mg/dL was considered poor. Ideal blood pressure was systolic blood pressure <120 mm Hg and diastolic blood pressure <80 mm Hg without medication; intermediate was systolic blood pressure of 120 to 139 mm Hg or diastolic blood pressure 80 to 89 mm Hg or on blood pressure–lowering medication and treated to appropriate low/intermediate range; and systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg was considered poor. Physical activity was considered ideal in individuals who participated in ≥150 min/week of moderate or vigorous physical activity, intermediate in those who participated in 1 to 149 min/week of moderate or vigorous intensity, and poor in individuals who did not participate in any moderate or vigorous physical activity. Last, a healthy diet was evaluated on the basis of 5 components: if an individual consumed ≥4.5 cups/day of fruits and vegetables, ≥2 3.5-oz servings/week of fish, 3 1-oz equivalent servings/day of fiber-rich whole grains, and restricted their diet to <1500 mg/day of sodium and ≤450 kcal/week of sugar-sweetened beverages, they were considered to meet all 5 criteria for an ideal healthy diet. Individuals who met the criteria for a healthy diet for at least 4 of the 5 areas were also considered to have an ideal diet. Those who met the criteria for 2 to 3 components were considered to have an intermediate diet, and those who met the criteria for one or none of the components were considered to have a poor diet.
As done previously,29,31 each of the 7 cardiovascular health factors was assigned a value of 0 if poor, 1, if intermediate and 2, if ideal. The values for the factors were then summated to create a total score that reflects the overall lifestyle and clinical management of risk factors an individual maintains. An overall LS7 score of 0 to 4 was considered poor, 5 to 9 was intermediate, and 10 to 14 was ideal. Intermediate LS7 score was used as the reference.
Statistical Analysis
Baseline characteristics of the study population were described according to PRS and LS7 categories. Median and interquartile range were reported for continuous variables. Frequency and percentages were calculated for categorical variables. Differences between PRS and LS7 groups were evaluated using Kruskal–Wallis tests for continuous variables and Pearson chi-square for categorical variables. To determine whether each characteristic increased ordinally with increasing PRS and LS7 score category, Jonckheere–Terpstra test for ordinal categorical or continuous variables and Cochran-Armitage tests for binary variables were used.
Remaining lifetime risk of CHD for polygenic risk and LS7 score were computed using a nonparametric survival model accounting for the competing risk of death.32 Cumulative incidence functions (CIF) were calculated from study entry age in 1987 to 1989 to age of incident CHD, death, or to censor date because of participant withdrawal or end of follow-up (2017). Under the competing risk framework and using age-related outcome definitions, these CIF translate directly into absolute, or lifetime, risk estimates.33 Remaining lifetime risk of CHD was calculated for polygenic risk and LS7 for the total population starting at the earliest entry age.
Lifetime risk of CHD for the joint association of PRS and LS7 score category was computed for the total population, accounting for different starting ages. Irwin’s restricted mean survival time were used to calculate the years free of CHD and overall survival time. Restricted mean survival time used the area under the survival curve up to 95 years given that few participants achieved this age during the follow-up period. Adjusted CIF for CHD were also calculated using Fine and Gray subdistribution regression models. All models adjusted for family history of CHD, sex, and study center.
Remaining lifetime risk and Fine and Gray subdistribution hazard regression analyses were also used to evaluate the effect of individual components of LS7, family history of CHD, and sex. All analyses were performed in the total population and stratified according to self-reported race. All P values are 2-sided and significant at P<0.05. Analyses were performed using R (version 3.6; etm package) and SAS (version 9.4).
Results
Baseline Characteristics
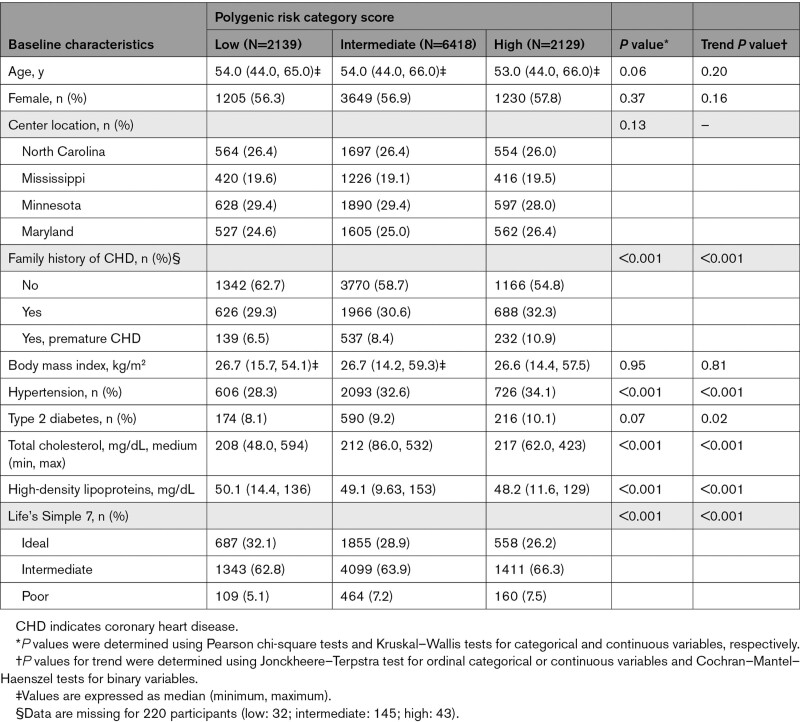
Participants were a median age of 54 years on entry into the cohort and were followed for a median 26.4 years (interquartile range, 16.5–29.1). At baseline, participants with a high PRS had a higher prevalence of hypertension, type 2 diabetes, higher total cholesterol, and lower high-density lipoprotein cholesterol as compared with participants with an intermediate or low PRS (P<0.001; Table 1). Participants were most commonly assigned poor LS7 scores because of poor physical activity (82.9%), poor blood pressure (70.3%), high body mass index (68.2%), and poor total cholesterol (61.3%; Table S1).
Table 1.
Baseline Characteristics of All Participants. by Polygenic Risk Score
Incidence and Lifetime Risk of CHD
During a total of 239 942 person-years of follow-up, 1725 White and 427 Black participants had a CHD event (incidence rate, 9.2 [95% CI, 8.7–9.6]) per 1000 person-years and 8.3 [95% CI, 7.5–9.1] per 1000 person-years, respectively). CHD events occurred more frequently in the high-PRS group for both White and Black participants. Overall remaining lifetime risk was 27.8% in the total population, 28.8% for White participants and 24.4% for Black participants. Men had higher lifetime risk estimates than women across both race groups. In the total population, men had a remaining lifetime risk of 38.2% and women had a lifetime risk of 19.8%.
Lifetime Risk Estimate and Years Free of CHD by LS7 Score
The lifetime risk of CHD ranged from 16.6% in individuals with an ideal LS7 score to 43.1% individuals with a poor LS7 score. Both races had similar lifetime risk of CHD according to LS7 category (Figure 1). Without adjustment for other risk factors or polygenic risk, poor blood sugar, elevated blood pressure, and total cholesterol were the components of LS7 with the highest observed lifetime risk of CHD. After controlling for polygenic risk and all cardiovascular health factors, the highest risk of CHD was observed with poor blood sugar. Ideal body mass index, cholesterol, and physical activity had the strongest significant inverse associations with CHD risk (Tables S2 and S3).
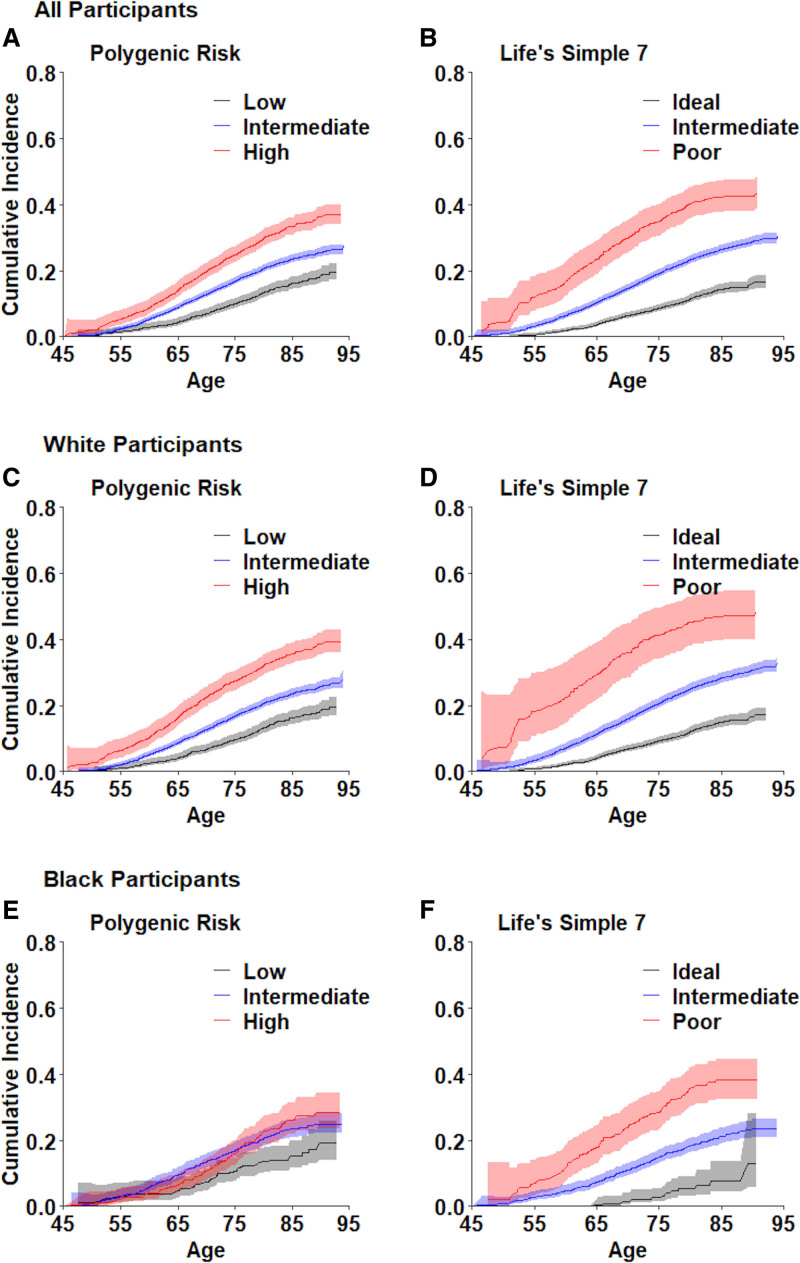
Figure 1.
Cumulative incidence of coronary heart disease according to polygenic risk and Life’s Simple 7 score.
A, C, and E, Cumulative incidence function (CIF) and 95% CI bands stratified by PRS category and self-reported race. In all participants, a low, intermediate, or high PRS was associated with an overall CIF of 19.5% (17.1%–22.1%), 27.3% (25.1%–29.6%), and 36.9 (34.0%–40.0%), respectively. In White participants, a low, intermediate, or high PRS was associated an overall CIF of 19.6% (16.9%–22.6%), 28.0% (25.2%–31.1%), and 39.5% (36.0%–43.1%), respectively. In Black participants, a low, intermediate, or high PRS was associated with an overall CIF 19.1% (14.0%–25.8%), 24.9% (22.1%–28.0%), and 28.6% (23.5%–34.4%), respectively. B, D, and F, CIF and 95% CI bands stratified by LS7 category and self-reported race. In all participants, a poor, intermediate, or ideal LS7 score was associated with an overall CIF of 16.6% (14.7%–18.7%), 30.4 (28.5–32.5), and 43.1% (38.5%–48.0%), respectively. In White participants a poor, intermediate, or ideal LS7 score was associated with an overall CIF of 17.1% (15.1%–19.2%), 32.6% (30.3%–35.0%), and 48.0% (40.9%–55.8%), respectively. In Black participants a poor, intermediate, or ideal LS7 score was associated with an overall CIF of 12.8% (5.6%–28.0%), 23.5% (20.9%–26.4%), and 38.2% (32.5%–44.6%), respectively
After stratification, the remaining lifetime risk for White participants ranged from 17.1% to 48.0% according to increasing LS7 score. A poor LS7 score was associated with an absolute difference in lifetime risk of 15.5% compared with intermediate LS7 score, and 30.9% compared with an ideal LS7 score (Figure 1). For White individuals with poor LS7 scores, this translated into 5.9 fewer years free of CHD than individuals with intermediate LS7 scores, and 15.6 fewer CHD-free years than individuals with ideal LS7 scores (Table S4). Similarly, remaining lifetime risk for Black participants ranged from 12.8% to 38.2% according to LS7 score, with an absolute difference of 14.7% between poor and intermediate LS7 scores and 25.4% between a poor and ideal LS7 score. In Black individuals with a poor LS7 score, this translated into 5.2 fewer years free of CHD than individuals with an intermediate LS7 score and 11.6 fewer years free of CHD than individuals with an ideal LS7 score.
Lifetime Risk and Years Free of CHD by PRS
Polygenic risk significantly predicted incident CHD for all participants, but the magnitude of association differed according to race. For White individuals, remaining lifetime risk ranged from 19.6% to 39.5% according to increasing PRS, with an absolute difference of 11.4% between a high and intermediate PRS and 19.9% between a high and low PRS (Figure 1). While Black participants had a lifetime risk ranging from 19.1% to 28.6% according to increasing PRS, the absolute difference between PRS categories was smaller in Black than White participants, as Black individuals had an absolute difference of 3.7% between high and intermediate PRS and 9.5% between high and low PRS. This translated into 5.4 fewer years free of CHD for White participants and only 2.1 fewer years free of CHD for Black participants with a high PRS as compared with a low PRS (Table S4).
Lifetime Risk and Years Free of CHD by LS7 and PRS
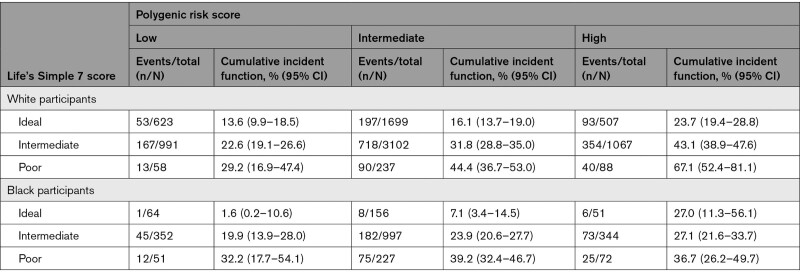
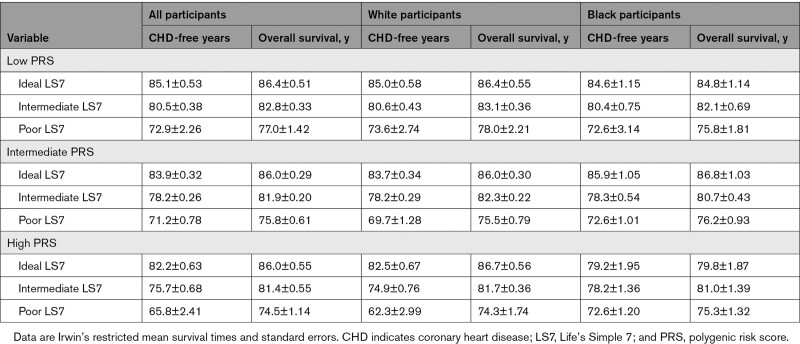
Overall, individuals with a high PRS and poor LS7 score had a higher lifetime risk than either high PRS or poor LS7 alone (54.3% [42.0%–67.5%]). However, the joint association of LS7 and PRS differed according to self-reported race. In White participants, a clear increase in lifetime risk of CHD was observed with each increase in PRS and LS7 score category (Figure S1). Participants with both a high PRS and a poor LS7 score had 35.3% greater risk than participants with intermediate PRS and intermediate LS7 and 53.5% greater risk than participants with a low PRS and an ideal LS7 score (Table 2). Furthermore, for those in the high-PRS group, remaining lifetime risk of CHD ranged from 23.7% to 67.1% according to increasing LS7 score. Individuals with both a high PRS and a poor LS7 score had 15.9 fewer years free of CHD than those with an intermediate PRS and an intermediate LS7 score (Figure 2; Table 3). Within Black participants, similar lifetime risk estimates were observed for individuals of poor LS7 score regardless of PRS category. Participants with both a high PRS and poor LS7 score had higher lifetime risk than participants with an intermediate PRS and LS7 score (absolute difference, 12.7%; Table 2). In the high-PRS group, an ideal LS7 score was associated with 1.0 more year free of CHD and a poor LS7 score was associated with 6.6 fewer CHD-free years as compared with an intermediate LS7 score (Figure 2; Table 3). There was no evidence of significant multiplicative interactions between PRS and LS7 for either race group.
Table 2.
Remaining Lifetime Risk of Coronary Heart Disease According to Polygenic Risk and Life’s Simple 7 Score
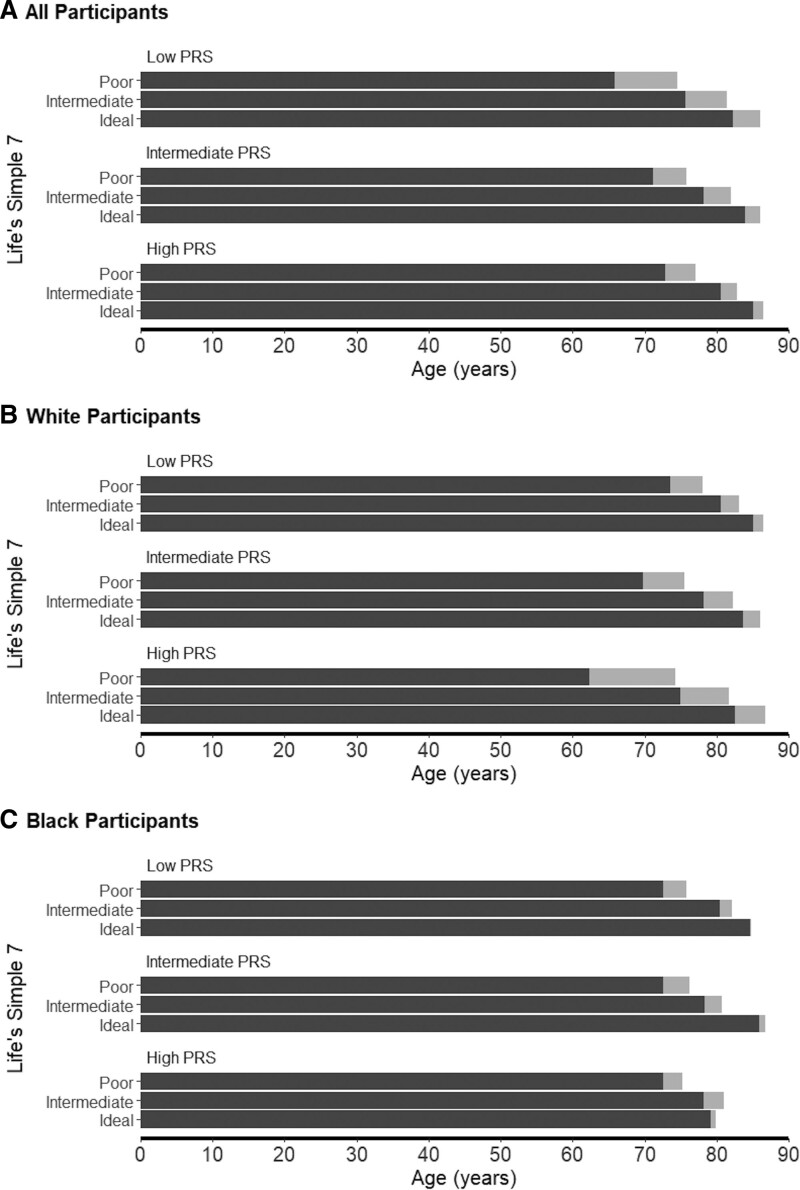
Figure 2.
Years free of coronary heart disease and overall survival according to polygenic risk and lifestyle.
Each bar represents Irwin’s mean restricted survival time for incident coronary heart disease or years free of coronary heart disease, and overall survival for participants according to PRS and Life’s Simple 7 score categories. A, Data for all participants. B, Data for White participants. C, Data for Black participants. Dark gray bars indicate CHD-free years; light gray bars indicate overall survival. PRS indicates polygenic risk score.
Table 3.
CHD-Free Years According to Polygenic Risk and LS7
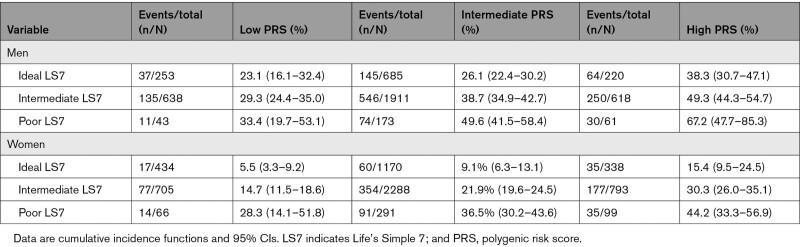
Lifetime Risk by PRS and LS7 According to Sex
In all participants, men had higher lifetime risk across all PRS and LS7 score categories (Table S5). Within the high PRS category, men in the poor LS7 score group had nearly twice the lifetime risk of those in the ideal group, while women in the poor group had nearly 3× the risk of those in the ideal group (Table 4). In the high-PRS group, women with a poor LS7 had 7.7 fewer years free of CHD compared with the intermediate group and 14.4 fewer years free of CHD than ideal group. Men had 12.2 fewer years free of CHD compared with the intermediate group and 17.9 fewer years free of CHD compared with the ideal group (Figure S2).
Table 4.
Lifetime Risk of Coronary Heart Disease According to Polygenic Risk, LS7, and Sex of Participants
Lifetime Risk Estimates for CHD According to PRS, LS7, and Family History of CHD
The remaining lifetime risk for individuals with family history of premature CHD (N=908) was 32.0% (95% CI, 27.9%–36.4%; Table S6). In White participants, the lifetime risk of CHD did not meaningfully differ by presence or absence of family history of premature CHD within PRS categories (Table S7). Individuals who had a high PRS and no family history of CHD had a higher lifetime risk of CHD than those with intermediate or low PRS regardless of their family history. In Black participants, the highest lifetime risk of CHD was observed in individuals with a high PRS and a family history of premature CHD. (Table S7). Individuals with family history of premature CHD had a clear increase in lifetime risk of CHD according to increasing PRS. However, clear differences in lifetime risk were not observed across PRS groups for individuals according to family history of CHD.
Discussion
Approximately 1 in 3 individuals with high polygenic risk and 1 in 2 individuals with a poor LS7 score, which is reflective of a poor lifestyle and clinical risk factors, will experience a CHD event in their lifetime. Participants with high polygenic risk may offset their lifetime risk for CHD by up to 50% through adherence to the LS7 recommendations. Notable differences were observed in the joint association of PRS and LS7 according to self-reported race. White participants with a high PRS and poor LS7 score had a remaining lifetime risk of 67.1% and nearly 16 fewer years free of CHD than those with intermediate PRS and LS7 scores. While polygenic risk was associated with high lifetime risk of CHD in Black participants, the association was attenuated. As such, lifetime risk estimates were observed for individuals of poor LS7 regardless of PRS category, suggesting the benefits of a healthy lifestyle play a much stronger role in this population than current polygenic risk scores, which were established based largely on previous studies in people of European ancestry.
Overall lifetime risk estimates for CHD (to 95 years of age) were previously reported as 48% for men and 32% for women.34 Similar estimates for atherosclerotic cardiovascular disease at an index age of 50 years were approximately 50% for men and 39% for women.35 Our estimates of 38.2% for men and 19.8% for women are lower, most likely attributable to our more restricted outcome definition. Previous studies used broader definitions of CHD by including angina pectoris or used a composite definition of atherosclerotic cardiovascular disease, which includes additional endpoints such as stroke. We restricted our outcome to hospitalized myocardial infarction, cardiac revascularization procedures and fatal CHD in accordance with the outcomes analyzed in the GWAS used in the creation of the PRS. Recent lifetime risk estimates for CHD were reported according to polygenic risk deciles and ranged from 16.3% to 47.7%.10
Our finding that high lifetime risk conferred by high PRS can be offset by healthy lifestyle echoes the findings of previous studies that used more limited genetic risk scores and did not account for the competing risk of death.16,17 We used a PRS composed of more than 6 million genetic variants that accounted for the effects of linkage disequilibrium, which has been shown to improve the accuracy of genetic risk scores.8,36 As such, the resulting risk strata based on PRS may more accurately stratify individuals according to their underlying genetic risk of CHD. Similarly, our study evaluates risk across the lifespan while accounting for the competing risk of non-CHD death, improving the accuracy of our estimates. Without such an adjustment, the risk of CHD may be overestimated.34
Furthermore, using lifetime risk and years free of disease further highlights the particular importance of establishing healthy habits in a high-risk population. Lifetime risk for individuals with a high PRS and a poor lifestyle was >50% (>67% in men; 44% in women). On average, individuals with a high polygenic risk who maintained a poor lifestyle experienced a CHD event 20 years earlier than those with a same genetic risk profile but adhered to an ideal lifestyle. While the relative impact of lifestyle on lifetime CHD risk was greater for women than men, men with a high PRS and a healthy lifestyle experienced nearly 2 more decades of CHD-free years. The American College of Cardiology and American Heart Association guidelines for the treatment and prevention of atherosclerotic cardiovascular disease stress the importance of lifetime risk estimates to guide individuals younger than 40 years of age.37 Beyond 40 years of age, the American College of Cardiology/American Heart Association guidelines lean heavily on 10-year risk estimates derived from the pooled cohort equations. However, in a middle-aged cohort, we note large absolute effects of a healthy lifestyle. While polygenic risk provides important information for early intervention, communicating lifetime risks may also encourage lifestyle changes later in life, particularly in those who have low 10-year risk, but high lifetime risk. Lifestyle counseling, along with policies and environmental changes, have proven effective at reducing cardiovascular risk.38,39 Smoking cessation or improved aerobic activity can also effectively reduce vascular aging.40
Last, family history of premature CHD is a well-established surrogate variable for genetic risk, and is considered a risk enhancing factor, which is considered in shared decision making about the initiation of statin therapy among individuals at low and intermediate risk in the 2019 American College of Cardiology/American Heart Association guidelines.37 In the past decade, increasingly well-powered GWAS of CHD and advances in method development have fueled the creation of more effective PRSs.4,36 Previous work in ARIC suggested a high PRS did not predict incident CHD as well as family history.41 However, this study significantly truncated follow-up time, starting at ARIC visit 4, which may have underestimated predictive accuracy of this PRS, as described by Hindy et al.10 Using the complete ARIC follow-up time, we found lifetime risk of CHD predicted by polygenic risk versus family history of CHD was similar. As PRSs continue to improve, it is likely that PRSs will become markedly more effective at capturing genetic risk than family history. Results from the present study and other recent studies suggest that we may have already reached this inflection point for individuals of European ancestry.10
Some limitations of this study must be acknowledged. First, the current PRS8 was developed using a primarily European population. While our analysis emphasizes the importance of a healthy lifestyle in all populations, the effect of the PRS on lifetime risk in Black participants was attenuated. This resonates with current literature, which thoroughly documents the attenuated association of European-derived PRS among individuals of African ancestry.6,42 Thus, our study highlights the need for diverse GWAS to create polygenic risk scores for individuals of all ancestral backgrounds to improve appropriate risk assignments based on PRS. If the gap in the effectiveness of PRSs in different ancestry groups is not addressed, PRSs may exacerbate health disparities.43
Also, although lifestyle is a mutable variable, LS7 scoring was only available at baseline. Individuals who met criteria for a poor LS7 score may have changed course over time. Similarly, the starting age varied across our study sample. As such, the number of years that an individual may have adhered to the LS7 guidelines is not reflected in their lifetime risk estimates. Further research is needed to determine how improving lifestyle offsets the lifetime risk for CHD conferred by PRS. Similarly, we acknowledge that LS7 guidelines consist of lifestyle and clinical risk factors, and some of the clinical risk factors have a heritable, genetic component and are not strictly modifiable. However, after controlling for genetic information, summarized through PRSs, we still note large differences between ideal and poor LS7 categories, suggesting modifiable portions of these risk factors still play an important role in the lifetime risk of CHD.
Last, our sample size provided adequate power for our main analyses but resulted in a low number of cases for some combinations of PRS and LS7 strata. Similarly, with our current sample size, we were unable to examine extreme thresholds for high PRS. Large, diverse study populations are needed to evaluate the effects of defining high polygenic risk at different thresholds to determine the best risk threshold for future risk stratification.
Both high polygenic risk and poor lifestyle confer a high lifetime risk of CHD. Lifestyle had a larger impact on the lifetime risk of CHD than genetic information. Managing one’s cardiovascular health according to LS7 guidelines is associated with lower lifetime risk of CHD for all individuals, suggesting that individuals with high genetic susceptibility for CHD may experience a lifetime risk of CHD comparable to those with an intermediate polygenic risk and intermediate lifestyle. Communicating the effects of polygenic risk on CHD in terms of absolute risk may have important implications for education, policy, and environmental changes, which not only benefit high-risk individuals, but the whole population. Further research is needed to determine how much lifestyle improvements offset the lifetime risk for CHD conferred by PRS. Additional consideration should be given to using polygenic risk as a risk-enhancing factor in current treatment guidelines and in current lifetime risk estimates for CHD.
Article Information
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
This study was supported by the National Heart, Lung, and Blood Institute (grant No. R01 HL146860). Paul S. de Vries and Allison Bebo were also supported by American Heart Association (grant no. 18CDA34110116). The ARIC (Atherosclerosis Risk in Communities) study has been funded in whole or part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract Nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I, HHSN268200625226C, R01HL087641, R01HL059367, and R01HL086694); and National Human Genome Research Institute (contract U01HG004402). Infrastructure was supported in part by grant No. UL1RR025005, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research.
Disclosures
None.
Supplemental Material
Figures S1–S2
Tables S1–S7
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ARIC
- atherosclerosis risk in communities
- CHD
- coronary heart disease
- GWAS
- genome-wide association study
- LS7
- life’s simple 7
- PRS
- polygenic risk score
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.053730.
For Sources of Funding and Disclosures, see page 817.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Symen Ligthart, Email: s.ligthart@erasmusmc.nl.
Michael R. Brown, Email: michael.r.brown@uth.tmc.edu.
Adam S. Heath, Email: adam.s.heath@uth.tmc.edu.
Allison Bebo, Email: allison.bebo@uth.tmc.edu.
Kellan E. Ashley, Email: keashley@umc.edu.
Eric Boerwinkle, Email: eric.boerwinkle@uth.tmc.edu.
Alanna C. Morrison, Email: alanna.c.morrison@uth.tmc.edu.
Aaron R. Folsom, Email: folso001@umn.edu.
David Aguilar, Email: david.aguilar@uth.tmc.edu.
References
- 1.Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World J Cardiol. 2016; 8:1–23. doi: 10.4330/wjc.v8.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vries PS, Kavousi M, Ligthart S, Uitterlinden AG, Hofman A, Franco OH, Dehghan A. Incremental predictive value of 152 single nucleotide polymorphisms in the 10-year risk prediction of incident coronary heart disease: the Rotterdam Study. Int J Epidemiol. 2015; 44:682–688. doi: 10.1093/ije/dyv070 [DOI] [PubMed] [Google Scholar]
- 3.Howe LJ, Dudbridge F, Schmidt AF, Finan C, Denaxas S, Asselbergs FW, Hingorani AD, Patel RS. Polygenic risk scores for coronary artery disease and subsequent event risk amongst established cases. Hum Mol Genet. 2020; 29:1388–1395. doi: 10.1093/hmg/ddaa052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. ; UK Biobank CardioMetabolic Consortium CHD Working Group. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018; 72:1883–1893. doi: 10.1016/j.jacc.2018.07.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison AC, Bare LA, Chambless LE, Ellis SG, Malloy M, Kane JP, Pankow JS, Devlin JJ, Willerson JT, Boerwinkle E. Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2007; 166:28–35. doi: 10.1093/aje/kwm060 [DOI] [PubMed] [Google Scholar]
- 6.Dikilitas O, Schaid DJ, Kosel ML, Carroll RJ, Chute CG, Denny JA, Fedotov A, Feng Q, Hakonarson H, Jarvik GP, et al. Predictive utility of polygenic risk scores for coronary heart disease in three major racial and ethnic groups. Am J Hum Genet. 2020; 106:707–716. doi: 10.1016/j.ajhg.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahed AC, Aragam KG, Hindy G, Chen YI, Chaudhary K, Dobbyn A, Krumholz HM, Sheu WHH, Rich SS, Rotter JI, et al. Transethnic transferability of a genome-wide polygenic score for coronary artery disease. Circ Genom Precis Med. 2021; 14:e003092. doi: 10.1161/CIRCGEN.120.003092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018; 50:1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera AV, Chaffin M, Zekavat SM, Collins RL, Roselli C, Natarajan P, Lichtman JH, D’Onofrio G, Mattera J, Dreyer R, et al. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation. 2019; 139:1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindy G, Aragam KG, Ng K, Chaffin M, Lotta LA, Baras A, Drake I, Orho-Melander M, Melander O, Kathiresan S, et al. ; Regeneron Genetics Center. Genome-wide polygenic score, clinical risk factors, and long-term trajectories of coronary artery disease. Arterioscler Thromb Vasc Biol. 2020; 40:2738–2746. doi: 10.1161/ATVBAHA.120.314856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mars N, Koskela JT, Ripatti P, Kiiskinen TTJ, Havulinna AS, Lindbohm JV, Ahola-Olli A, Kurki M, Karjalainen J, Palta P, et al. ; FinnGen. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020; 26:549–557. doi: 10.1038/s41591-020-0800-0 [DOI] [PubMed] [Google Scholar]
- 12.Chung RJ, Touloumtzis C, Gooding H. Staying young at heart: cardiovascular disease prevention in adolescents and young adults. Curr Treat Options Cardiovasc Med. 2015; 17:61. doi: 10.1007/s11936-015-0414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg D. The rationale for initiating treatment of hypercholesterolemia in young adulthood. Curr Atheroscler Rep. 2013; 15:296. doi: 10.1007/s11883-012-0296-2 [DOI] [PubMed] [Google Scholar]
- 14.Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Borén J, et al. ; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015; 36:2425–2437. doi: 10.1093/eurheartj/ehv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Büller HR, Sijbrands EJ, Kastelein JJ. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004; 292:331–337. doi: 10.1001/jama.292.3.331 [DOI] [PubMed] [Google Scholar]
- 16.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016; 375:2349–2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol. 2018; 3:693–702. doi: 10.1001/jamacardio.2018.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Chen X, Han J, Jiang W, Natarajan P, Zhao H. Interactions between enhanced polygenic risk scores and lifestyle for cardiovascular disease, diabetes, and lipid levels. Circ Genom Precis Med. 2021; 14:e003128. doi: 10.1161/CIRCGEN.120.003128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ligthart S, Hasbani N, Ahmadizar F, Herpt T, Leening M, Uitterlinden A, Sijbrands E, Morrison A, Boerwinkle E, Pankow J, et al. Genetic susceptibility, obesity, and lifetime risk of type 2 diabetes: the ARIC study and Rotterdam Study. Diabetic Medicine. 2021; 38::e14639. doi: 10.1111/dme.14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). 2011; 12:255–257. doi: 10.2459/JCM.0b013e328343e986 [DOI] [PubMed] [Google Scholar]
- 21.Fortin JM, Hirota LK, Bond BE, O’Connor AM, Col NF. Identifying patient preferences for communicating risk estimates: a descriptive pilot study. BMC Med Inform Decis Mak. 2001; 1:2. doi: 10.1186/1472-6947-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 23.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009; 119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM. Short-term versus long-term risk for coronary artery disease: implications for lipid guidelines. Curr Opin Lipidol. 2006; 17:619–625. doi: 10.1097/MOL.0b013e3280108740 [DOI] [PubMed] [Google Scholar]
- 25.Persell SD, Zei C, Cameron KA, Zielinski M, Lloyd-Jones DM. Potential use of 10-year and lifetime coronary risk information for preventive cardiology prescribing decisions: a primary care physician survey. Arch Intern Med. 2010; 170:470–477. doi: 10.1001/archinternmed.2009.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989; 129:687–702 [PubMed] [Google Scholar]
- 27.Rosamond WD, Folsom AR, Chambless LE, Wang CH. Coronary heart disease trends in four United States communities. The Atherosclerosis Risk in Communities (ARIC) study 1987-1996. Int J Epidemiol. 2001; 30:S17–22. doi: 10.1093/ije/30.suppl_1.s17 [DOI] [PubMed] [Google Scholar]
- 28.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016; 48:1284–1287. doi: 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folsom AR, Olson NC, Lutsey PL, Roetker NS, Cushman M. American Heart Association’s Life’s Simple 7 and incidence of venous thromboembolism. Am J Hematol. 2015; 90:E92. doi: 10.1002/ajh.23950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57:1690–1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson NC, Cushman M, Judd SE, McClure LA, Lakoski SG, Folsom AR, Safford MM, Zakai NA. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2015; 4:e001494. doi: 10.1161/JAHA.114.001494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putter H, Spitoni C. Non-parametric estimation of transition probabilities in non-Markov multi-state models: the landmark Aalen-Johansen estimator. Stat Methods Med Res. 2018; 27:2081–2092. doi: 10.1177/0962280216674497 [DOI] [PubMed] [Google Scholar]
- 33.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012; 308:1795–1801. doi: 10.1001/jama.2012.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999; 353:89–92. doi: 10.1016/S0140-6736(98)10279-9 [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006; 113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 36.Vilhjálmsson BJ, Yang J, Finucane HK, Gusev A, Lindström S, Ripke S, Genovese G, Loh PR, Bhatia G, Do R, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium, Discovery, Biology, and Risk of Inherited Variants in Breast Cancer (DRIVE) study. Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am J Hum Genet. 2015; 97:576–592. doi: 10.1016/j.ajhg.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stonerock GL, Blumenthal JA. Role of counseling to promote adherence in healthy lifestyle medicine: strategies to improve exercise adherence and enhance physical activity. Prog Cardiovasc Dis. 2017; 59:455–462. doi: 10.1016/j.pcad.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siren R, Eriksson JG, Vanhanen H. Observed changes in cardiovascular risk factors among high-risk middle-aged men who received lifestyle counselling: a 5-year follow-up. Scand J Prim Health Care. 2016; 34:336–342. doi: 10.1080/02813432.2016.1248649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000; 102:1351–1357. doi: 10.1161/01.cir.102.12.1351 [DOI] [PubMed] [Google Scholar]
- 41.Mosley JD, Gupta DK, Tan J, Yao J, Wells QS, Shaffer CM, Kundu S, Robinson-Cohen C, Psaty BM, Rich SS, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020; 323:627–635. doi: 10.1001/jama.2019.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, Agler C, Love SM, Okello S, Petty LE, et al. Importance of genetic studies of cardiometabolic disease in diverse populations. Circ Res. 2020; 126:1816–1840. doi: 10.1161/CIRCRESAHA.120.315893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019; 51:584–591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]