Graphical abstract
Keywords: Ulcerative colitis, Proctocolectomy, Pouch, Pouchitis
Highlights
-
•
Approximately 20% of patients with acute pouchitis will develop chronic pouch inflammation.
-
•
There are no randomized controlled trials to guide management.
-
•
Biologic therapies have efficacy rates of 45–65% for chronic pouch inflammation.
1. Introduction
The staged total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA) is the gold standard surgical treatment for ulcerative colitis (UC) complicated by treatment refractory disease or dysplasia. Acute pouchitis is the most common post-IPAA inflammatory condition of the pouch with reported cumulative incidence rates of 50% (Barnes et al., 2020; Ferrante et al., 2008; Kayal et al., 2019). Symptoms of acute pouchitis include increased stool frequency, urgency, pelvic pain, and hematochezia. Antibiotics are the mainstay of treatment and induce remission in 80% of patients, however approximately 20% will progress to chronic pouch inflammation, often requiring continuous antibiotic use or escalation to biologic or small molecule therapy (Fazio et al., 1995; Shen, 2012).
Chronic pouch inflammation is categorized as antibiotic dependent, antibiotic refractory or Crohn's disease like. Chronic antibiotic dependent pouchitis (CADP) involves persistent ( 4 weeks) or recurrent symptoms of pouchitis ( 4 episodes per year) that are responsive to long-term, continuous antibiotic use (Zezos and Saibil, 2015; Quinn and Raffals, 2020). In contrast, chronic antibiotic refractory pouchitis (CARP) involves persistent or recurrent symptoms of pouchitis that are unresponsive to a 4-week course of antibiotics and require escalation of therapy (Zezos and Saibil, 2015; Quinn and Raffals, 2020; Shen et al., 2012; Tulchinsky et al., 2003). Crohn's disease like pouch inflammation (CDLPI) presents similarly to traditional CD with severe inflammation of the pouch body and pre-pouch ileum, strictures of the pre-pouch ileum or proximal small bowel, and/or fistulae involving the perineum or proximal small bowel (Barnes et al., 2019). Refractory cuffitis involves inflammation of the residual rectal mucosa and may also be a feature of CDLPI (Wu et al., 2013).
The etiology of chronic pouch inflammation remains largely unknown, however is theorized to be multifactorial and involve a complex interaction between the pouch mucosal immune system and microbiome in genetically susceptible patients, not dissimilar to the etiopathogenesis of inflammatory bowel disease (IBD) (Dalal et al., 2018; Yanai et al., 2015). The pouch undergoes a pro-inflammatory transcriptional shift after ostomy closure consistent with colonic metaplasia, changes in the extracellular matrix and enhanced immune activation (Huang et al., 2017; Luukkonen et al., 1988; Hinata et al., 2012). In this context, chronic pouch inflammation is triggered by microbial dysbiosis secondary to fecal stasis in patients with genetic predisposition (Tyler et al., 2013; Reshef et al., 2015; Turpin et al., 2019). Risk factors for acute and chronic pouchitis include genetic polymorphisms of interleukin-1-receptor antagonist and NOD2/CARD 15, history of extensive UC, extra-intestinal manifestations such as primary sclerosing cholangitis (PSC) and the presence of perinuclear anti-neutrophil cytoplasmic antibodies (pANCA). (Aisenberg et al., 2004; Meier et al., 2005; Schmidt et al., 1998; Fleshner et al., 2001).
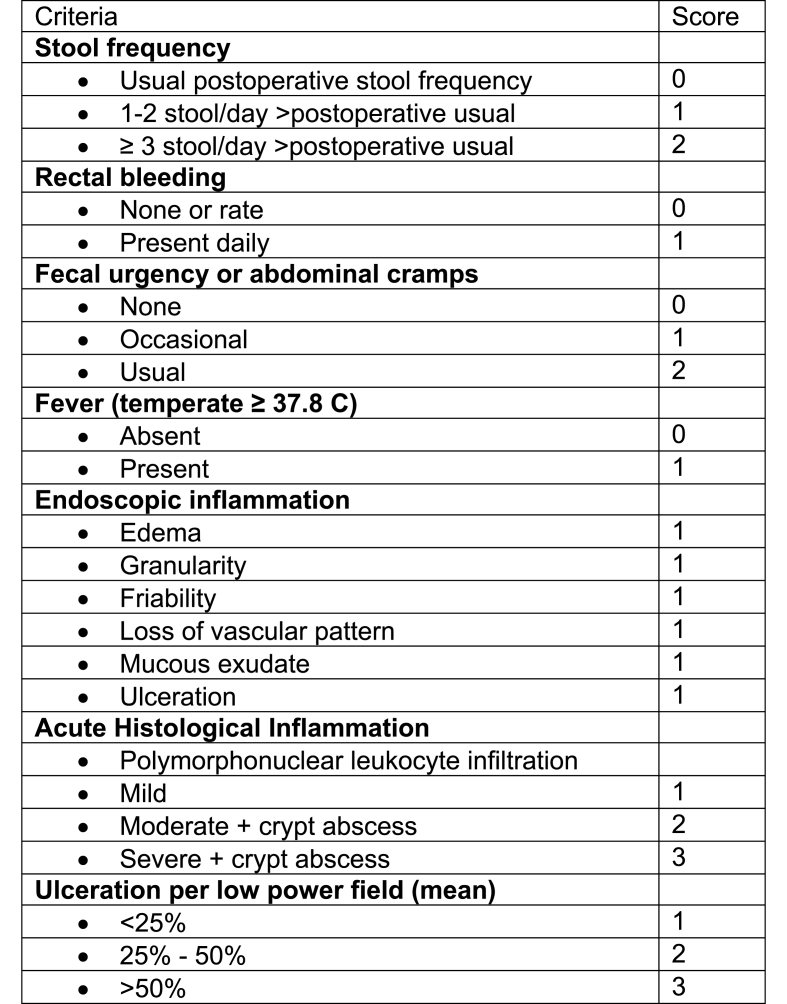
There are no currently approved medications for acute or chronic pouchitis, and drug development has partly been limited by the lack of a fully validated instrument that measures pouchitis disease activity or response to therapy. The Pouchitis Disease Activity Index (PDAI) involves a composite of clinical, endoscopic and histologic scores to define pouchitis and is the most commonly used pouchitis classification measure (Fig. 1), yet it is limited by reliability and the lack of established treatment targets (Sandborn et al., 1994). Furthermore, there are no large, randomized controlled trials and evidence for currently used therapies is limited. As a result, the treatment of chronic pouch inflammation may be challenging. In this review, we aim to discuss the medical management of chronic pouch inflammation and its supporting evidence.
Fig. 1.
Pouchitis disease activity index.
2. Antibiotics
In patients with CADP, maintenance antibiotic therapy is used to mitigate symptoms and prevent recurrent episodes of pouchitis. Ciprofloxacin and/or metronidazole at the lowest effective dose are the most commonly prescribed antibiotics in CADP, however the supporting evidence is limited. In a cohort study of 44 patients with CADP who received combination ciprofloxacin and metronidazole for 28 days, 36 (82%) achieved clinical and endoscopic remission after antibiotic therapy (Mimura et al., 2002). Median PDAI and IBD questionnaire (IBDQ) scores improved significantly even in the eight patients who did not achieve remission at 28 days (Mimura et al., 2002). The risks of chronic ciprofloxacin and metronidazole therapy include tendinitis/tendon rupture and peripheral neuropathy, respectively.
In addition to these adverse effects, long-term maintenance therapy with ciprofloxacin and metronidazole CADP may promote resistance. In an observational study of 39 patients with CADP and continuous antibiotic use for at least one year, 78% had evidence of antibiotic resistance on stool coliform testing (Segal et al., 2018). In a prospective study of 49 patients, 27 of whom had chronic pouch inflammation, antibiotics reduced virulent disease associated bacteria such as Clostridium perfringens, Ruminocooccus gnavus, Klebsiella penumoniae, in addition to beneficial bacteria such as Faecalibacterium prausnitzii (Dubinsky et al., 2020). Furthermore, antibiotic therapy resulted in multiple ciprofloxacin-resistance mutations in drug target genes and confirmed drug resistance. Reassuringly, microbiome composition recovered after cessation of antibiotic therapy in these patients, with resurgence of baseline pre-therapy bacteria. Alternating antibiotic regimens at the lowest effective dose may decrease the risk of antibiotic resistance, however there is insufficient data to recommend this practice in CADP.
Rifaximin is a non-absorbable alternative to ciprofloxacin and metronidazole for CADP in patients who have developed resistance or adverse effects. In an open-label study of 51 patients with CADP who received a two-week course of ciprofloxacin or metronidazole for induction of remission followed by rifaximin for maintenance, 33 (65%) were in clinical and endoscopic remission at 3 months as determined by the modified Pouchitis Disease Activity Index (mPDAI) (Shen et al., 2008). Of these patients, the majority (23, 70%) received 200 mg/day of rifaximin during the maintenance period, and the remainder required rifaximin dose escalation to a maximum of 1200 mg/day. A high percentage (58%) of responsive patients remained on maintenance therapy at one year, suggesting rifaximin is effective at sustained, long-term remission.
Alternative antibiotics to consider in patients with CADP refractory or intolerant to those discussed above include tinidazole, amoxicillin-clavulanate, or vancomycin, particularly in patients with Primary Sclerosing Cholangitis, though data is limited (Shen et al., 2007, 2022; Ardalan and Sparrow, 2019). Antibiotics have been used as adjunct induction therapy in patients with an inflammatory phenotype of CDLPI, however outcomes are unknown (Shen et al., 2022; Jarchin et al., 2019).
3. Probiotics
Probiotics may be used as maintenance therapy and secondary prophylaxis in patients with CADP, however data is limited. In a randomized controlled trial of 40 patients with CADP in clinical and endoscopic remission, only 15% of patients randomized to daily VSL#3 had disease relapse within nine months, compared to 100% of patients randomized to placebo (Gionchetti et al., 2000). In a subsequent randomized controlled trial of 36 patients with CADP in whom remission was induced via four weeks of combination ciprofloxacin and metronidazole, 85% randomized to daily VSL#3 maintained remission at 12 months compared to 6% randomized to placebo (p < 0.0001) (Mimura et al., 2004). However, real-world data has not replicated these positive results. In an observational study of 31 patients with CADP prescribed VSL#3, 81% discontinued the therapy at 8 months due to symptom relapse or adverse effects such as bloating and flatulence (Shen et al., 2005). There is no data to support the use of probiotics in patients with CARP.
4. Mesalamine
There is no data regarding mesalamine in CADP and the evidence to support their use in CARP is limited to a single case series (Shen et al., 2007). In a study of 16 patients with CARP comparing combination ciprofloxacin and tinidazole to mesalamine (oral 4 g/day, enema 8 g/day, or suppository 1 g/day formulation), there was a significant reduction in total PDAI scores in the mesalamine group and the rates of clinical remission and response were 50% and 50%, respectively. This small case series included both oral and topical mesalamine formulations and did not delineate which formulation was superior. As a result, there is no data to support the choice of oral vs topical mesalamine formulation for patients with CARP.
Importantly, cuffitis and pouchitis often co-exist with overlapping symptoms (Hashimoto et al., 2014). Mesalamine suppositories are considered first line therapy for patients with cuffitis, however data is limited to a single study. In an open label trial of 14 patients with cuffitis treated with mesalamine suppositories (500 mg twice a day), there was significant improvement in symptomatic, endoscopic, and histologic endpoints with 92% of patients with hematochezia noting improvement (Shen et al., 2004). There are no other therapies that have been investigated for the management of cuffitis.
5. Corticosteroids
Budesonide is an oral synthetic steroid that provides luminal control of inflammation with minimal systemic absorption and may be used as induction therapy in CARP. There are few studies that support the use of budesonide in CARP, and they are limited by small sample sizes. In a case series of 13 patients with CARP, 60% reported a favorable clinical response with budesonide (Chopra et al., 2006). In a subsequent prospective study of 20 patients with CARP treated with budesonide 9 mg/day for eight weeks, 75% achieved remission and the median total PDAI scores significantly decreased from 14 to 3 (p < 0.001) (Gionchetti et al., 2007). There is no data to guide subsequent therapy in patients who respond to budesonide after eight weeks, however consideration should be given to maintenance at the lowest effective dose or escalation to biologic.
There is limited data to support the use of beclomethasone in CARP. In an open-label study of 10 patients with CARP treated with oral beclomethasone 10 mg per day for eight weeks, 80% of patients achieved clinical remission and the median number of bowel movements decreased significantly from 10 to 6, p < 0.001 (Gionchetti et al., 2014). There are no studies that have evaluated the efficacy of other oral (ie prednisone) or topical steroids in CARP.
6. Biologics
Biologics such as anti-tumor necrosis factor (TNF), anti-integrin and anti-interleukin agents should be considered in patients with CARP or CDLPI. Multiple case reports and retrospective studies have demonstrated the efficacy of biologics in CARP and CDLPI, however there is insufficient evidence to recommend one biologic agent over the other. In a meta-analysis of 311 patients with CARP treated with biologics, the pooled rate of clinical remission was 65.7%, 31%, 47.4% with infliximab, adalimumab, and vedolizumab, respectively (Chandan et al., 2021). Selection of a biologic should take into account previous pre-colectomy biologic class exposure, as patients exposed to anti-TNF therapy pre- and post-IPAA may be less likely to experience clinical remission and more likely to have pouch failure (Kayal et al., 2020).
6.1. Anti-tumor necrosis factor (TNF) therapy – infliximab and adalimumab
In a retrospective, multicenter study of 33 patients with CARP treated with infliximab, 21% of patients achieved clinical remission at week eight, 33% at week 26, and 27% at week 52 (Barreiro-de Acosta et al., 2012a). In a separate analysis of only the patients who continued therapy beyond week eight, 44% achieved clinical remission at week 26 and 56% at week 52 (Barreiro-de Acosta et al., 2012a). In a subsequent retrospective study of eight patients with CARP previously treated with infliximab, rescue adalimumab therapy achieved clinical remission in 13% at week eight, 13% at week 26, and 25% at week 52 (Barreiro-de Acosta et al., 2012b).
There is only one randomized, placebo-controlled trial that evaluated the efficacy of adalimumab in CARP (Kjær et al., 2019). The primary outcome was reduction in PDAI 2 at any time and the secondary outcomes were clinical remission, endoscopic and histologic response, and quality of life. In the small number of patients (n = 13) included, six received adalimumab and seven received placebo. There was no significant difference in the primary or secondary outcomes among the two groups however no conclusions can be drawn from this highly underpowered study.
Two small, single center open-label studies have investigated adalimumab for the management of CDLPI. In the first, 17 patients with inflammatory (n = 10), fibrostenotic (n = 2) or fistulizing (n = 5) CDLPI treated with adalimumab were included (Shen et al., 2009). At week four, seven (41.2%) patients reported clinical remission and six (35.3%) reported clinical response. There was a significant improvement in the mean PDAI endoscopic sub-score at week 4 from a score of 3 pre-treatment to a score of 0 post-treatment, p < 0.001. However, three (17.7%) patients ultimately required pouch excision. In the second study, 48 patients with CDLPI were treated with adalimumab. Among these, 24 (50%) patients achieved clinical remission, 10 (21%) achieved partial remission, and 14 (29%) had no response at eight weeks (Li et al., 2012). At the end of follow-up, 13 (27%) patients achieved mucosal remission and nine (19%) required pouch excision.
In a meta-analysis of 313 patients treated with infliximab or adalimumab for CARP or CDLPI, the overall rates of short-term and long-term clinical remission were 50% and 52%, respectively (Huguet et al., 2018). In further analysis, rates of remission after induction and after maintenance were 10% and 37% in patients with CARP and 64% and 57% in patients with CDLPI, respectively, suggesting anti-TNF agents may be more efficacious for CDLDPI than CARP. There was no significant difference in remission rates among patients treated with infliximab or adalimumab.
Thiopurine use in chronic pouch inflammation as monotherapy or combination therapy with anti-TNF agents has not been comprehensively studied. In a retrospective review of 22 patients with CDLPI who were treated with azathiopurine/6-mercaptopurine and infliximab, six (29%) patients with fistulizing disease had no clinical response and required a permanent ileostomy (Haveran et al., 2011). In the subset group of eight patients with stricturing disease or inflammation limited to the pouch body, all were treated successfully with azathiopurine/6-mercaptopurine (7/8) or infliximab (1/8).
6.2. Anti-interleukin therapy - ustekinumab
Ustekinumab has demonstrated efficacy in CARP and CDLPI with improvement in endoscopic and clinical outcomes. In a retrospective, multicenter study of 56 patients (47 with CDLPI and 9 with CARP), 83% demonstrated clinical response six months after induction with ustekinumab, although none were in clinical remission (Weaver et al., 2019). Among the responders, 60% were able to completely stop antibiotics within the initial six months of therapy (Weaver et al., 2019). Higher mean body mass index at induction (26.3 vs 23.7, p = 0.033) and male sex (83% vs 30%, p = 0.014) were predictors of non-response in patients with CDLPI. In a subsequent, retrospective single-center study of 24 patients with CARP treated with ustekinumab, 12 (50%) patients had a clinical response with a decrease in the median number of bowel movements per 24 h from 8 to 6, p = 0.002 (Ollech et al., 2019). In the subset of patients who had pouchoscopy data available after ustekinumab treatment, the median endoscopic PDAI score decreased by only one point, from 5 to 4, yet met statistical significance (p = 0.016).
Ustekinumab dose escalation and optimization may be necessary to achieve remission in patients with chronic pouch inflammation. In a recent retrospective study of 46 patients (six with CARP, four with cuffitis, and 36 with CDLPI), 80.4% of patients had clinical response 8–16 weeks after ustekinumab induction. (Dalal et al., 2021). Dose intensification was required in 50% of patients after a median of 223 days due to breakthrough symptoms before eight weeks. After dose intensification, 70% of patients had endoscopic improvement in pouch inflammation.
6.3. Anti-integrin therapy - vedolizumab
Multiple small, retrospective observational studies have reported on the efficacy on vedolizumab in chronic pouch inflammation. In a retrospective, multicenter study of 20 patients with CADP and CARP treated with vedolizumab, 64% achieved clinical remission and there was no difference in efficacy among anti-TNF naïve and anti-TNF experienced patients (Bär et al., 2018). In a subsequent retrospective, multicenter study of 83 patients (54 with CDLPI and 29 with CARP) treated with vedolizumab, 71.1% of patients had a clinical response and 19.3% achieved clinical remission (Gregory et al., 2019). Separate analysis of only the CARP patients was unavailable, however there was no difference in efficacy in patients with CDLPI or CARP. Patients who developed symptoms of pouch inflammation within one year of IPAA were less likely to have clinical response to vedolizumab (Gregory et al., 2019). In a case series of 15 patients with CARP treated with vedolizumab, clinical remission was achieved in 60% of patients, and significantly more patients continued vedolizumab compared to anti-TNF therapy due to less adverse effects (Verstockt et al., 2019).
The first randomized, double-blind, placebo-controlled multicenter study of vedolizumab for the management of CARP was recently completed. In this study of 102 patients, 51 were randomized to vedolizumab and 51 to placebo (Efficacy and safety of in, 2790). Clinical remission rates at weeks 14 and 34 in the vedolizumab vs placebo group were 31.4% vs 9.8% (p = 0.013), and 35.2% vs 17.6% (p = 0.043), respectively. Analysis of secondary outcomes is forthcoming.
7. Small molecules
The data regarding tofacitinib in chronic pouch inflammation is limited and conflicting. In a case report of a 20-year-old woman with CARP refractory to anti-TNF therapy, tofacitinib resulted in clinical and endoscopic remission within six months (Okano et al., 2020). In a separate case report of a 68-year-old man with CDLPI, tofacitinib similarly resulted in clinical and endoscopic remission within six months (Bauer et al., 2020). In a single center, retrospective study of 426 patients who underwent TPC with IPAA, seven (1.6%) were treated with tofacitinib for chronic pouchitis. Only one (14.2%) patient had clinical improvement and four (57.1%) discontinued tofacitinib due to lack of improvement, worsening of symptoms, or severe anemia (Akiyama et al., 2020). There is an ongoing study to evaluate the effectiveness of tofacitinib in patients with CARP (Melmed, 2020). Additional data is needed before tofacitinib can be routinely recommended for the management of chronic pouch inflammation.
8. Tacrolimus
Topical tacrolimus administered via enema has demonstrated efficacy and safety in CARP. In an open-label study of 10 patients with CARP treated with once daily tacrolimus enema for eight weeks, 70% of patients achieved clinical remission (Uchino et al., 2013). The mean PDAI score decreased significantly from 15.9 to 7.8 after eight weeks of therapy (p < 0.01). No serious adverse events were reported.
9. Fecal microbiota transplant
Bacterial dysbiosis has been implicated in the pathogenesis of chronic pouch inflammation, and there is evidence that increased proportions of certain microbial groups correlate with increased clinical pouchitis disease activity and inflammatory markers (Tyler et al., 2013; Reshef et al., 2015; Turpin et al., 2019; Dubinsky et al., 2020). Given this, manipulation of the pouch microbiome via fecal microbiota transplantation (FMT) has been theorized to be a promising therapeutic approach for patients with chronic pouch inflammation. Unfortunately, studies thus far using donor stool obtained from healthy patients with intact colons have not shown FMT to be efficacious for CARP and donor microbiota engraftment has been poor (Kayal ML et al., 2020).
In a pilot study of eight patients with CARP treated with FMT via nasogastric infusion, no patient achieved clinical remission (Landy et al., 2015). Two patients had a reduction of composite PDAI score ≥3 at four weeks post-FMT however both still had a composite PDAI score ≥7. No major adverse events following FMT were observed (Landy et al., 2015). In a subsequent pilot study of 19 patients with CARP treated with FMT via pouchoscopy, bowel movement frequency significantly decreased from 9.25 movements per day pre-FMT to 7.25 post-FMT, p = 0.03 (Selvig et al., 2020). Despite this, there was no significant change in PDAI and seven patients required additional therapy during follow-up.
In a recent double-blind, randomized controlled trial comparing FMT with placebo in 26 patients with CARP, nine patients in the FMT group and eight patients in the placebo group relapsed during follow-up (p = 0.18) (Karjalainen et al., 2021). Furthermore, five patients in the FMT group relapsed within the first four weeks following FMT, while no patient in the placebo group relapsed during this time period. Additional prospective studies are needed to investigate the benefit of FMT in CARP, with particular consideration to protocol issues such as use of fresh or frozen stool from pooled or non-pooled multi-donors, frequency and interval of FMT, and concomitant use of antibiotics and probiotics.
10. Hyperbaric oxygen therapy
Hyperbaric oxygen therapy for the management of CARP has been investigated in a single case series. In this retrospective case series of 46 patients with CARP treated with repeated sessions of hyperbaric oxygen therapy (range 10–60), there was a significant reduction in the mean PDAI symptom sub-score (3.19–1.19, p < 0.05) and endoscopic PDAI sub-scores in the afferent limb (2.31 tot 0.85, p = 0.006), pouch body (2.34–1.29, p < 0.001), and cuff (1.93–0.63, p < 0.001) (Hasan et al., 2021). Transient adverse effects included ear barotrauma and hyperbaric myopic changes.
11. Conclusion
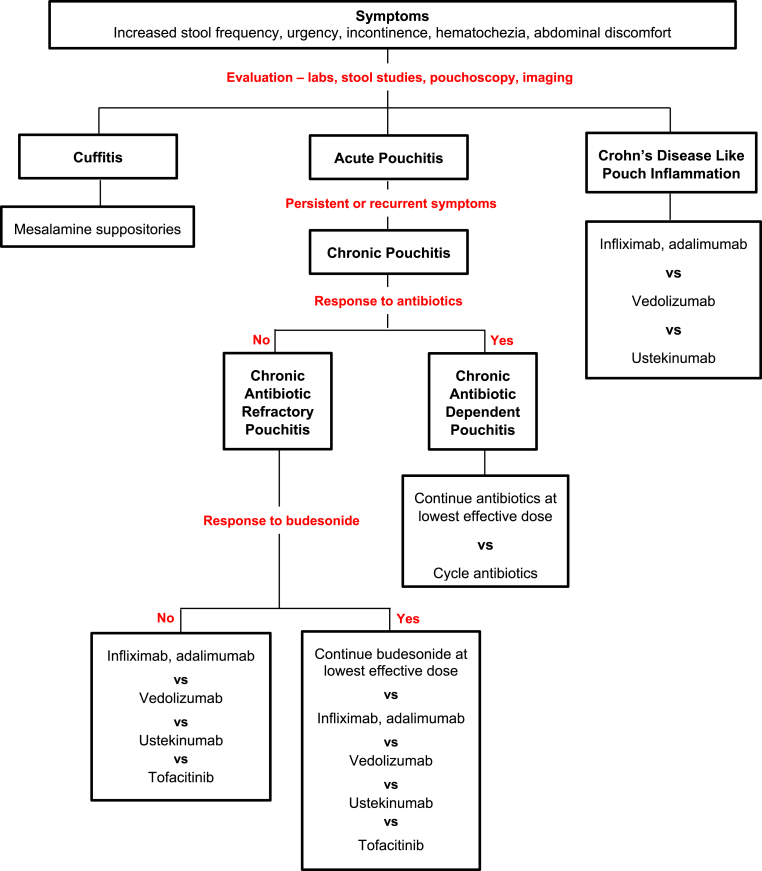
Approximately 20% of patients with acute pouchitis will develop chronic pouch inflammation and require continuous antibiotic use or escalation to immunomodulatory therapy (Fazio et al., 1995; Shen, 2012). Appropriate assessment and treatment is imperative for patients with chronic pouch inflammation to decrease symptoms, improve quality of life, and mitigate the risk of pouch failure. A summary of the available data discussed in this review is presented in Table 1 and a recommended approach is offered in Fig. 2 with the consideration that choice of therapy should be tailored to each patient and prior medication exposure. Additional studies are needed to develop evidenced based treatment algorithms for patients with chronic pouch inflammation.
Table 1.
Summary of available evidence.
| Therapy | Chronic Antibiotic Dependent Pouchitis | Chronic Antibiotic Refractory Pouchitis | Crohn's Disease Like Pouch Inflammation |
|---|---|---|---|
| Antibiotics | + | – | + |
| Probiotics | ++ | – | – |
| Mesalamine | – | + | – |
| Corticosteroids | – | + | – |
| Adalimumab, Infliximab | – | ++ | + |
| Ustekinumab | – | + | + |
| Vedolizumab | + | ++ | + |
| Tofacitinib | – | + | – |
| Tacrolimus | – | + | – |
| Fecal Microbiota Transplant | – | ++ | – |
+ Indicates supportive evidence is based on cohort studies, ++ indicates supportive evidence is based on randomized controlled trials.
- Indicates no supportive evidence.
Fig. 2.
Approach to symptoms in patients after total proctocolectomy with ileal pouch anal anastomosis.
CRediT authorship contribution statement
Maia Kayal: acquisition and review of data, drafting of the manuscript. Marla C. Dubinsky: critical revision of the manuscript for important intellectual content.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MCD has served as a consultant for Abbvie, Arena Pharmaceuticals, Boehringer Ingelheim International, Bristol-Myers Squibb, Celgene, Eli Lilly, F. Hoffman-La Roche, Genentech, Gilead, Janssen, Pfizer, Prometheus, Takeda, UCB and has research grants from Pfizer, Abbvie, Janssen, and Prometheus.
Marla C. Dubinsky reports a relationship with AbbVie Inc that includes: consulting or advisory and funding grants. Marla C. Dubinsky reports a relationship with Arena Pharmaceuticals Inc that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Boehringer Ingelheim Corp USA that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Bristol Myers Squibb Co that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Celgene SL that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Eli Lilly and Company that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with F Hoffmann-La Roche AG that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Genentech Inc that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Gilead Sciences Inc that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with Janssen Pharmaceuticals Inc that includes: consulting or advisory and funding grants. Marla C. Dubinsky reports a relationship with Pfizer that includes: consulting or advisory and funding grants. Marla C. Dubinsky reports a relationship with Prometheus Inc that includes: consulting or advisory and funding grants. Marla C. Dubinsky reports a relationship with Takeda Pharmaceutical Co Ltd that includes: consulting or advisory. Marla C. Dubinsky reports a relationship with UCB Inc that includes: consulting or advisory.
References
- Aisenberg J., Legnani P.E., Nilubol N., et al. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am. J. Gastroenterol. 2004;99:432–441. doi: 10.1111/j.1572-0241.2004.04107.x. 2004/04/02. [DOI] [PubMed] [Google Scholar]
- Akiyama S., Traboulsi C., Rai V., et al. S3202 treatment of chronic pouchitis with tofacitinib: real world experience from a tertiary center. Off. J. Am. Coll. Gastroenterol. ACG. 2020;115:S1679. doi: 10.14309/01.ajg.0000714856.39315.e7. [DOI] [Google Scholar]
- Ardalan Z.S., Sparrow M.P. A personalized approach to managing patients with an ileal pouch-anal anastomosis. Front. Med. 2019;6:337. doi: 10.3389/fmed.2019.00337. 2020/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär F., Kühbacher T., Dietrich N.A., et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment. Pharmacol. Ther. 2018;47:581–587. doi: 10.1111/apt.14479. 2017/12/22. [DOI] [PubMed] [Google Scholar]
- Barnes E.L., Kochar B., Jessup H.R., et al. The incidence and definition of crohn's disease of the pouch: a systematic review and meta-analysis. Inflamm. Bowel Dis. 2019;25:1474–1480. doi: 10.1093/ibd/izz005. 2019/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E.L., Herfarth H.H., Kappelman M.D., et al. Incidence, risk factors, and outcomes of pouchitis and pouch-related complications in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.035. 2020/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro-de Acosta M., García-Bosch O., Souto R., et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm. Bowel Dis. 2012;18:812–817. doi: 10.1002/ibd.21821. 2011/08/10. [DOI] [PubMed] [Google Scholar]
- Barreiro-de Acosta M., García-Bosch O., Gordillo J., et al. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur. J. Gastroenterol. Hepatol. 2012;24:756–758. doi: 10.1097/MEG.0b013e3283525a7b. 2012/03/08. [DOI] [PubMed] [Google Scholar]
- Bauer C.M., Barnes E.L., Herfarth H.H. Tofacitinib in the treatment of crohn's-like disease of the pouch. Am. J. Gastroenterol. 2020;115:2116–2117. doi: 10.14309/ajg.0000000000000801. 2020/08/03. [DOI] [PubMed] [Google Scholar]
- Chandan S., Mohan B.P., Kumar A., et al. Safety and efficacy of biological therapy in chronic antibiotic refractory pouchitis: a systematic review with meta-analysis. J. Clin. Gastroenterol. 2021;55:481–491. doi: 10.1097/mcg.0000000000001550. 2021/05/29. [DOI] [PubMed] [Google Scholar]
- Chopra A., Pardi D.S., Loftus E.V., Jr., et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice. Inflamm. Bowel Dis. 2006;12:29–32. doi: 10.1097/01.mib.0000192323.82426.83. 2005/12/24. [DOI] [PubMed] [Google Scholar]
- Dalal R.L., Shen B., Schwartz D.A. Management of pouchitis and other common complications of the pouch. Inflamm. Bowel Dis. 2018;24:989–996. doi: 10.1093/ibd/izy020. 2018/04/25. [DOI] [PubMed] [Google Scholar]
- Dalal R.S., Gupta S., Goodrick H., et al. Outcomes of standard and intensified dosing of ustekinumab for chronic pouch disorders. Inflamm. Bowel Dis. 2021 doi: 10.1093/ibd/izab156. 2021/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky V., Reshef L., Bar N., et al. Predominantly antibiotic-resistant intestinal microbiome persists in patients with pouchitis who respond to antibiotic therapy. Gastroenterology. 2020;158:610–624. doi: 10.1053/j.gastro.2019.10.001. e613. 2019/10/13. [DOI] [PubMed] [Google Scholar]
- Efficacy and Safety of Intravenous Vedolizumab for Treatment of Chronic Pouchitis: Results of the Phase 4 EARNEST Trial. https://clinicaltrials.gov/ct2/show/results/NCT02790138
- Fazio V.W., Ziv Y., Church J.M., et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann. Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. 1995/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M., Declerck S., De Hertogh G., et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm. Bowel Dis. 2008;14:20–28. doi: 10.1002/ibd.20278. 2007/11/02. [DOI] [PubMed] [Google Scholar]
- Fleshner P.R., Vasiliauskas E.A., Kam L.Y., et al. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. doi: 10.1136/gut.49.5.671. 2001/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Venturi A., et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. 2000/08/10. [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Poggioli G., et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment. Pharmacol. Ther. 2007;25:1231–1236. doi: 10.1111/j.1365-2036.2007.03306.x. 2007/04/25. [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Calabrese C., Calafiore A., et al. Oral beclomethasone dipropionate in chronic refractory pouchitis. J. Crohns. Colitis. 2014;8:649–653. doi: 10.1016/j.crohns.2013.12.001. 2014/01/08. [DOI] [PubMed] [Google Scholar]
- Gregory M., Weaver K.N., Hoversten P., et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm. Bowel Dis. 2019;25:1569–1576. doi: 10.1093/ibd/izz030. 2019/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan B., Yim Y., Ur Rashid M., et al. Hyperbaric oxygen therapy in chronic inflammatory conditions of the pouch. Inflamm. Bowel Dis. 2021;27:965–970. doi: 10.1093/ibd/izaa245. 2020/09/19. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Itabashi M., Ogawa S., et al. Treatment strategy for preventing pouchitis as a postoperative complication of ulcerative colitis: the significance of the management of cuffitis. Surg. Today. 2014;44:1730–1734. doi: 10.1007/s00595-014-0974-5. 2014/07/16. [DOI] [PubMed] [Google Scholar]
- Haveran L.A., Sehgal R., Poritz L.S., et al. Infliximab and/or azathioprine in the treatment of Crohn's disease-like complications after IPAA. Dis. Colon Rectum. 2011;54:15–20. doi: 10.1007/DCR.0b013e3181fc9f04. 2010/12/17. [DOI] [PubMed] [Google Scholar]
- Hinata M., Kohyama A., Ogawa H., et al. A shift from colon- to ileum-predominant bacteria in ileal-pouch feces following total proctocolectomy. Dig. Dis. Sci. 2012;57:2965–2974. doi: 10.1007/s10620-012-2165-9. 2012/04/28. [DOI] [PubMed] [Google Scholar]
- Huang Y., Dalal S., Antonopoulos D., et al. Early transcriptomic changes in the ileal pouch provide insight into the molecular pathogenesis of pouchitis and ulcerative colitis. Inflamm. Bowel Dis. 2017;23:366–378. doi: 10.1097/mib.0000000000001027. 2017/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet M., Pereira B., Goutte M., et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and crohn's disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm. Bowel Dis. 2018;24:261–268. doi: 10.1093/ibd/izx049. 2018/01/24. [DOI] [PubMed] [Google Scholar]
- Jarchin L., Spencer E.A., Khaitov S., et al. De novo crohn's disease of the pouch in children undergoing ileal pouch-anal anastomosis for ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 2019;69:455–460. doi: 10.1097/mpg.0000000000002406. 2019/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karjalainen E.K., Renkonen-Sinisalo L., Satokari R., et al. Fecal microbiota transplantation in chronic pouchitis: a randomized, parallel, double-blinded clinical trial. Inflamm. Bowel Dis. 2021 doi: 10.1093/ibd/izab001. 2021/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal M., Plietz M., Rizvi A., et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm. Bowel Dis. 2019 doi: 10.1093/ibd/izz227. 2019/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal M., Lambin T., Plietz M., et al. Recycling of precolectomy anti-tumor necrosis factor Agents in chronic pouch inflammation is associated with treatment failure. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.07.008. 2020/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal Ml T., Pinotti R., Dubinsky M., Grinspan A. A systematic review of fecal microbiota transplant for the management of pouchitis. Crohn’s and Colitis. 2020;360:2. doi: 10.1093/crocol/otaa034. May 12 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjær M.D., Qvist N., Nordgaard-Lassen I., et al. Adalimumab in the treatment of chronic pouchitis. A randomized double-blind, placebo-controlled trial. Scand. J. Gastroenterol. 2019;54:188–193. doi: 10.1080/00365521.2019.1569718. 2019/02/12. [DOI] [PubMed] [Google Scholar]
- Landy J., Walker A.W., Li J.V., et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci. Rep. 2015;5:12955. doi: 10.1038/srep12955. 2015/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Lopez R., Queener E., et al. Adalimumab therapy in Crohn's disease of the ileal pouch. Inflamm. Bowel Dis. 2012;18:2232–2239. doi: 10.1002/ibd.22933. 2012/03/13. [DOI] [PubMed] [Google Scholar]
- Luukkonen P., Valtonen V., Sivonen A., et al. Fecal bacteriology and reservoir ileitis in patients operated on for ulcerative colitis. Dis. Colon Rectum. 1988;31:864–867. doi: 10.1007/bf02554850. 1988/11/01. [DOI] [PubMed] [Google Scholar]
- Meier C.B., Hegazi R.A., Aisenberg J., et al. Innate immune receptor genetic polymorphisms in pouchitis: is CARD15 a susceptibility factor? Inflamm. Bowel Dis. 2005;11:965–971. doi: 10.1097/01.mib.0000186407.25694.cf. 2005/10/22. [DOI] [PubMed] [Google Scholar]
- Melmed G. 2020. Tofacitinib for Treatment of Chronic Pouchitis.https://clinicaltrials.gov/ct2/show/NCT04580277 [Google Scholar]
- Mimura T., Rizzello F., Helwig U., et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment. Pharmacol. Ther. 2002;16:909–917. doi: 10.1046/j.1365-2036.2002.01203.x. 2002/04/23. [DOI] [PubMed] [Google Scholar]
- Mimura T., Rizzello F., Helwig U., et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. 2003/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano S., Yoshimura N., Sako M., et al. A case of refractory chronic pouchitis successfully treated with tofacitinib. Clin. J. Gastroenterol. 2020;13:560–563. doi: 10.1007/s12328-020-01108-5. 2020/03/05. [DOI] [PubMed] [Google Scholar]
- Ollech J.E., Rubin D.T., Glick L., et al. Ustekinumab is effective for the treatment of chronic antibiotic-refractory pouchitis. Dig. Dis. Sci. 2019;64:3596–3601. doi: 10.1007/s10620-019-05697-1. 2019/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K.P., Raffals L.E. An update on the medical management of inflammatory pouch complications. Am. J. Gastroenterol. 2020;115:1439–1450. doi: 10.14309/ajg.0000000000000666. 2020/05/27. [DOI] [PubMed] [Google Scholar]
- Reshef L., Kovacs A., Ofer A., et al. Pouch inflammation is associated with a decrease in specific bacterial taxa. Gastroenterology. 2015;149:718–727. doi: 10.1053/j.gastro.2015.05.041. 2015/06/01. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Tremaine W.J., Batts K.P., et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin. Proc. 1994;69:409–415. doi: 10.1016/s0025-6196(12)61634-6. 1994/05/01. [DOI] [PubMed] [Google Scholar]
- Schmidt C.M., Lazenby A.J., Hendrickson R.J., et al. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann. Surg. 1998;227:654–662. doi: 10.1097/00000658-199805000-00006. ; discussion 663-655. 1998/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J.P., Poo S.X., McLaughlin S.D., et al. Long-term follow-up of the use of maintenance antibiotic therapy for chronic antibiotic-dependent pouchitis. Frontline Gastroenterol. 2018;9:154–158. doi: 10.1136/flgastro-2017-100913. 2018/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvig D., Piceno Y., Terdiman J., et al. Fecal microbiota transplantation in pouchitis: clinical, endoscopic, histologic, and microbiota results from a pilot study. Dig. Dis. Sci. 2020;65:1099–1106. doi: 10.1007/s10620-019-05715-2. 2019/07/16. [DOI] [PubMed] [Google Scholar]
- Shen B. Acute and chronic pouchitis--pathogenesis, diagnosis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2012;9:323–333. doi: 10.1038/nrgastro.2012.58. 2012/04/18. [DOI] [PubMed] [Google Scholar]
- Shen B., Lashner B.A., Bennett A.E., et al. Treatment of rectal cuff inflammation (cuffitis) in patients with ulcerative colitis following restorative proctocolectomy and ileal pouch-anal anastomosis. Am. J. Gastroenterol. 2004;99:1527–1531. doi: 10.1111/j.1572-0241.2004.30518.x. 2004/08/17. [DOI] [PubMed] [Google Scholar]
- Shen B., Brzezinski A., Fazio V.W., et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment. Pharmacol. Ther. 2005;22:721–728. doi: 10.1111/j.1365-2036.2005.02642.x. 2005/10/04. [DOI] [PubMed] [Google Scholar]
- Shen B., Fazio V.W., Remzi F.H., et al. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis. Colon Rectum. 2007;50:498–508. doi: 10.1007/s10350-006-0828-3. 2007/02/07. [DOI] [PubMed] [Google Scholar]
- Shen B., Remzi F.H., Lopez A.R., et al. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 2008;8 doi: 10.1186/1471-230x-8-26. 26. 2008/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Remzi F.H., Lavery I.C., et al. Administration of adalimumab in the treatment of Crohn's disease of the ileal pouch. Aliment. Pharmacol. Ther. 2009;29:519–526. doi: 10.1111/j.1365-2036.2008.03920.x. 2009/02/03. [DOI] [PubMed] [Google Scholar]
- Shen B., Yu C., Lian L., et al. Prediction of late-onset pouch failure in patients with restorative proctocolectomy with a nomogram. J. Crohns. Colitis. 2012;6:198–206. doi: 10.1016/j.crohns.2011.08.006. 2012/02/14. [DOI] [PubMed] [Google Scholar]
- Shen B., Kochhar G.S., Rubin D.T., et al. Treatment of pouchitis, Crohn's disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol. Hepatol. 2022;7:69–95. doi: 10.1016/s2468-1253(21)00214-4. 2021/11/15. [DOI] [PubMed] [Google Scholar]
- Tulchinsky H., Hawley P.R., Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann. Surg. 2003;238:229–234. doi: 10.1097/01.sla.0000082121.84763.4c. 2003/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin W., Kelly O., Borowski K., et al. Mucosa-associated microbiota in ileoanal pouches may contribute to clinical symptoms, particularly stool frequency, independent of endoscopic disease activity. Clin. Transl. Gastroenterol. 2019;10:1–7. doi: 10.14309/ctg.0000000000000038. 2019/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler A.D., Knox N., Kabakchiev B., et al. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066934. 2013/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M., Ikeuchi H., Matsuoka H., et al. Topical tacrolimus therapy for antibiotic-refractory pouchitis. Dis. Colon Rectum. 2013;56:1166–1173. doi: 10.1097/DCR.0b013e31829ebd83. 2013/09/12. [DOI] [PubMed] [Google Scholar]
- Verstockt B., Claeys C., De Hertogh G., et al. Outcome of biological therapies in chronic antibiotic-refractory pouchitis: a retrospective single-centre experience. United Eur. Gastroenterol. J. 2019;7:1215–1225. doi: 10.1177/2050640619871797. 2019/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K.N., Gregory M., Syal G., et al. Ustekinumab is effective for the treatment of crohn's disease of the pouch in a multicenter cohort. Inflamm. Bowel Dis. 2019;25:767–774. doi: 10.1093/ibd/izy302. 2018/10/09. [DOI] [PubMed] [Google Scholar]
- Wu B., Lian L., Li Y., et al. Clinical course of cuffitis in ulcerative colitis patients with restorative proctocolectomy and ileal pouch-anal anastomoses. Inflamm. Bowel Dis. 2013;19:404–410. doi: 10.1097/MIB.0b013e31828100ed. 2013/01/19. [DOI] [PubMed] [Google Scholar]
- Yanai H., Ben-Shachar S., Baram L., et al. Gene expression alterations in ulcerative colitis patients after restorative proctocolectomy extend to the small bowel proximal to the pouch. Gut. 2015;64:756–764. doi: 10.1136/gutjnl-2014-307387. 2014/07/02. [DOI] [PubMed] [Google Scholar]
- Zezos P., Saibil F. Inflammatory pouch disease: the spectrum of pouchitis. World J. Gastroenterol. 2015;21:8739–8752. doi: 10.3748/wjg.v21.i29.8739. 2015/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]