Abstract
Anaplasmosis, caused by intracellular gram-negative bacteria Anaplasma marginale is one of the most frequently reported tick-borne disease (TBDs) in tropical and sub-tropical countries, including Pakistan. In the present study, a total of 428 cattle blood samples were collected to examine the prevalence and phylogenetic origin of A. marginale in two important livestock regions of Punjab Province in Pakistan, i.e. Lodhran and Dera Ghazi Khan Districts. In addition, association between occurrence of A. marginale in cattle blood and selected epidemiological factors has been also investigated. The presence of A. marginale genetic material was confirmed in 9% of the tested blood samples taken from cattle in Lodhran and in 17% from Dera Ghazi Khan. Prevalence of A. marginale was significantly higher in cattle from Dera Ghazi Khan. All the cattle breeds from both districts were equally susceptible to A. marginale infection. We reported higher prevalence of A. marginale in cattle living indoors or with other dairy animals in Dera Ghazi Khan district. However, no such relationship was observed in the Lodhran district. Sequencing of the msp1b gene shows 96–99% similarity of A. marginale in the study area to those reported from other parts of Pakistan, South Africa, and Israel. We recommend that large scale tick and tick-borne disease control strategies must be implemented in both districts.
Keywords: Molecular characterization, Cattle, Punjab, Pakistan
1. Introduction
Majority of Pakistani population resides in rural areas. Rearing and trading livestock is a major source of income and employment in these societies (Irshad et al., 2010). Majority of livestock owners are small farm holders and they are heavily contributing in meeting the dietary requirements of their countrymen as well as in national economy (Ashraf, 2021). 15 indigenous cattle breeds of zebu (one-humped) have been reported from Pakistan that includes local breeds like Sahiwal and Cholistani (known to be tick and heat resistant) and exotic breeds like Holstein-Friesian and Jersey (that are known for high milk production) (Saeed, 2016). Livestock and dairy industry face a lot of challenges in Pakistan including unawareness of livestock owners regarding feed, tick management artificial insemination, finance constrains and limited available health facilities (Arif, et al., 2011, Ramzan et al., 2018). Parasitism is a crucial problem causing health problems in livestock farms. Ticks are one of the most important ectoparasites in this region. This is favored by the climate in Pakistan, which provides optimal conditions for the development and reproduction of ticks. A large variety of ticks (belonging to genera Hyalomma, Rhiphicephalus and Ixodes) has been reported from Pakistan that infests variety of domestic, wild animals as well as humans (Ashraf, 2021); (Durrani and Shakoori, 2009).
Anaplasma marginale is an intra-erythrocyte gram-negative bacterium that causes bovine anaplasmosis (Ashraf, 2013). Ticks of Dermacentor; Ixodes, Amblyomma and Rhipicephalus genera act as important vector of A. marginale in Pakistan (Kocan et al., 2004, Hairgrove, 2015). The most characteristic symptoms of anaplasmosis are anemia, anorexia, pyrexia, depression, and jaundice in large ruminants. Infection with A. marginale can also result in reduced milk production, can lead to abortion in pregnant animals and, in extreme cases can kill animal (Camus et al., 2010, Kumar, 2015).
Prevalence of A. marginale is known to vary with sampling sites, vector species, breeds and breeding system as well as the climatic conditions (Bursakov and Kovalchuk, 2019). In the past a few studies have been conducted in various parts of Pakistan showing the prevalence of A. marginale in cattle ranging from 6 to 41% (Hussain, 2017, Farooqi et al., 2017, Turi et al., 2018, Ashraf et al., 2021). These reports are mainly from Punjab and Khyber Pakhtonkhwa (KPK) provinces and most of the remaining part of Pakistan is still unexplored for tick-borne diseases (TBDs) (Parveen et al., 2021).
Lodhran and Dera Ghazi Khan are among those districts in Punjab where local population is heavily dependent on livestock for their earnings, but the TBDs are not explored in detail in these areas. Most local farmers consider all ticks as one species and is mostly unaware of their specific health effects to various animal species they are rearing. To the best of our knowledge, there are no previous reports, from both the districts, regarding molecular detection of A. marginale in cattle breeds. Hence, the present study was designed to document the PCR based prevalence of A. marginale in cattle breeds enrolled from Lodhran and Dera Ghazi Khan, with a note on epidemiology and phylogenetic origin of this bacterium with the hypothesis that bacterium prevalence would be higher in Dera Ghazi Khan district due to its poor socio economic and hygiene conditions.
2. Materials and methods
2.1. Study area
Two districts (Dera Ghazi Khan and Lohdran) in South Punjab (Pakistan) were included during present investigation (Fig. 2). Lohdran is located at 29° 32′ 34″ N, 71 37′ 48″ E. Lodhran is located on the northern side of river Satluj. On its north are the districts of Multan, Khanewal and Vehari while Bahawalpur is on its southern side. On the east lie the districts of Vehari and Bahawalpur; while district Multan lies on the western side. The climate of the district is hot and dry in summer and cold in winter. The maximum and minimum temperature ranges between 42 °C and 28 °C in summer. During winter, the temperature fluctuates between 21Cand 5C. The entire district is smooth plain. District Dera Ghazi Khan is located at 30° 1′ 59″ N, 70° 38′ 24″ E. The Dera Ghazi Khan is located in a strip between the river Indus and the Koh-Suleman range of mountains separating it from the Baluchistan Province. It is surrounded by Dera Ismail Khan on the North and Rajanpur on its South. Indus river flows on the East across which lie the districts of Muzafargarh and Layyah. Loralai and Dera Bugti districts of Baluchistan Province lies on the West separated by the Koh-Suleman range of mountains. The overall climate of this district is dry with little rainfall. The winter is mild, lasting from November till January with average temperature 4 °C but summer is extremely hot and extended, starting in May, and lasting till September with average temperature around 42 °C. The different geographic location of these districts was one of the key reasons that they were investigated for the presence of A. marginale in cattle.
Fig. 2.
Map of Pakistan with highlighted Punjab Province and Lodhran and Dera Ghazi Khan districts. Map was generated in the Datawrapper 1.25 web tool (Datawrapper GmbH, https://app.datawrapper.de/) and modified in GIMP 2.10 software (GIMP Development Team, https://www.gimp.org/).
2.2. Sample collection
Ethical Research Committee of the Institute of Pure and Applied Biology at Bahauddin Zakariya University Multan (Pakistan) approved all the experimental procedures and protocols applied in this study via letter number IP&ABB/Ethics/2019-17. In total 428 cattle blood samples were collected between June and October 2019 (District Lodhran, n = 218 and Dera Ghazi Khan, n = 210) from cattle breeds: Cholistani, Sahiwal, Crossbreed, Dajli, Holstein Friesian, Australian Ayrshire and Jersey. Solvin’s formula was used to estimate sample size that were randomly collected during present study. Solvin’s formula was computed as;
Whereas: n = no. of samples, N = total population, e = margin of error.
Only apparently healthy animals were included in this study. Body of each animal was carefully inspected to detect the presence of tick(s) on them. A questionnaire was filled on sampling site with the help of cattle owners used to assess the epidemiological situation on farms (sex, age, number of animals in herd, presence, or absence of other dairy animals in herds, presence or absence of dogs, history of disease, animals’ living environment, water supply to farm and ticks feed on animals).
2.3. DNA extraction
Blood sample (3–5 ml) was collected through jugular vein puncture. Genomic DNA from the collected blood was extracting following the protocol of Saeed et al. (Malik, 2018). Briefly, blood samples were suspended in 500 μL of lysis buffer (20 mM Tris-HCl, 1 mM EDTA, 30 mM DTT, 0.5% SDS) with 0.4 mg/mL Proteinase K (Fermentas, USA) and incubated at 55 °C overnight. Subsequently, samples were heated at 95 °C for 10 min and equal volume of phenol: chloroform: isoamylalcohol (25:24:1 v/v/v), was added to the lysate, vortexed for 30 s and centrifuged at 12,000g for 10 min. The aqueous phase was transferred to new Eppendorf tube and equal volumes of ice cold isopropanol were added. The DNA was pelleted by centrifugation at 12,000g for 15 min and washed with 70% ethanol and dried at 65 °C for 5 min. The DNA was finally re-suspended in 50 μL sterile double distilled water and stored at −20 °C.
2.4. PCR amplification
Primer sequence R 5ʹCTG CTT GGG AGA ATG CACCT 3ʹ and F 5ʹGCT CTA GCA GGT TAT GCG TC 3ʹ were applied to generate 265 bp fragment from A. marginale’s msp1b (major surface protein–1b) gene following Ashraf et al. (Ashraf, 2021) A reaction mixture (50 µL) that contained 65 mM KCl, 13 mM Tris–HCl (pH 8.3), 1U of DNA polymerase (Vivantas, USA), 2 mM MgCl2, 250 µM of dNTP, 0.5 µM of primers and 5 µL of template DNA was prepared for PCR. Thermal profile of PCR included 5 min of denaturation at 94 °C followed by 25 repeated cycles of DNA denaturation for 50 s at 95 °C, primer annealing for 50 s at 56 °C and primer extension for 50 s at 72 ⁰C. A final primer extension for 5 min was carried out at 72 °C (Tay et al., 2014). A. marginale positive sample (previously isolated from equine and confirmed by DNA sequencing: GenBank accession number MK792344) and parasite negative samples (no extraction from the cattle blood samples) were run as controls (positive and negative respectively) during each reaction for quality control.
2.5. DNA sequencing followed by phylogenetic analysis
To confirm that amplified DNA is of A. marginale, 4 amplified PCR amplicons were sent to a commercial lab (First base Sequencing, Malaysia) for DNA sequencing by using the same primer sequences as used during PCR. The obtained DNA sequences were compare using the Basic Local Alignment Search Tool (BLAST) with the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and similar sequences were obtained from the GenBank database (https:// www.ncbi.nlm.nih.gov/), to perform multiple alignments among the sequences using MUSCLE.
MEGA version X was used to perform the phylogenetic analysis, with a bootstrap analysis with 1000 replicates. For msp1b evolutionary history was inferred using the Maximum Likelihood method with Kimura 2-parameter model (Kimura, 1980). The tree was scaled based and the length of branches and evolutionary distances was expressed in the same units. Datasets of sequences obtained from GenBank and those generated in this study were trimmed to have similar length of all sequences.
2.6. Statistical analysis
Minitab 17.0 software (Minitab Inc., USA) was used to analyze data. Association of A. marginale infection with studied epidemiological factors was evaluated through Fisher’s exact test. Comparison of A. marginale prevalence between cattle breeds was done by one way ANOVA while Chi square test was used to compare A. marginale prevalence in cattle from two districts Dera Ghazi Khan and Lohdran. Significance level was set at p < 0.05.
3. Results
3.1. Prevalence of A. Marginale
In present study, a fragment specific for msp1b gene of A. marginale (265 bp) was amplified by PCR in 20/218 (9%) cattle blood samples from District Lodhran and in 36/210 (17%) blood samples from District Dera Ghazi Khan (Table 1). A significantly higher A. marginale prevalence was observed in cattle from Dera Ghazi Khan (p = 0.01). 3 cattle breeds, i.e. Cholistani, Sahiwal and cross breed from Lodhran District were equally susceptible to A. marginale infection (p = 0.60). A similarly relationship was observed in 6 other cattle breeds (Cholistani, Sahiwal and cross breed, Jersy, Dajali, Holstein Friesian, Australian Ayshire) from Dera Ghazi Khan (p = 0.20) (Table 1).
Table 1.
Comparison of A. marginale prevalence in blood samples of various cattle breeds collected from two Lodhran and Dera Ghazi Khan districts.
| Breed | Districts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lohdran |
Dera Ghazi Khan |
|||||||
| Number of tested blood samples | A. marginale positive samples | A. marginale negative samples | p-value | Number of tested blood samples | A. marginale positive samples | A. marginale negative samples | p-value | |
| Cholistani | 87 | 8 (9%) | 79 (91%) | 0.60 | – | – | – | – |
| Sahiwal | 122 | 12 (10%) | 110 (90%) | 51 | 13 (25%) | 38 (75%) | 0.20 | |
| Crossbreed | 9 | 0 (0%) | 9 (100%) | 19 | 5 (26%) | 14 (74%) | ||
| Dajli | – | – | – | – | 57 | 6 (11%) | 51 (89%) | |
| Holstein Friesian | – | – | – | – | 37 | 5 (14%) | 32 (86%) | |
| Australian Ayrshire | – | – | – | – | 35 | 6 (17%) | 29 (83%) | |
| Jersey | – | – | – | – | 11 | 1 (9%) | 10 (91%) | |
| Total | 218 | 20 (9%) | 198 (90%) | 210 | 3 (17%) | 174 (83%) | ||
3.2. DNA sequencing and phylogenetic analysis
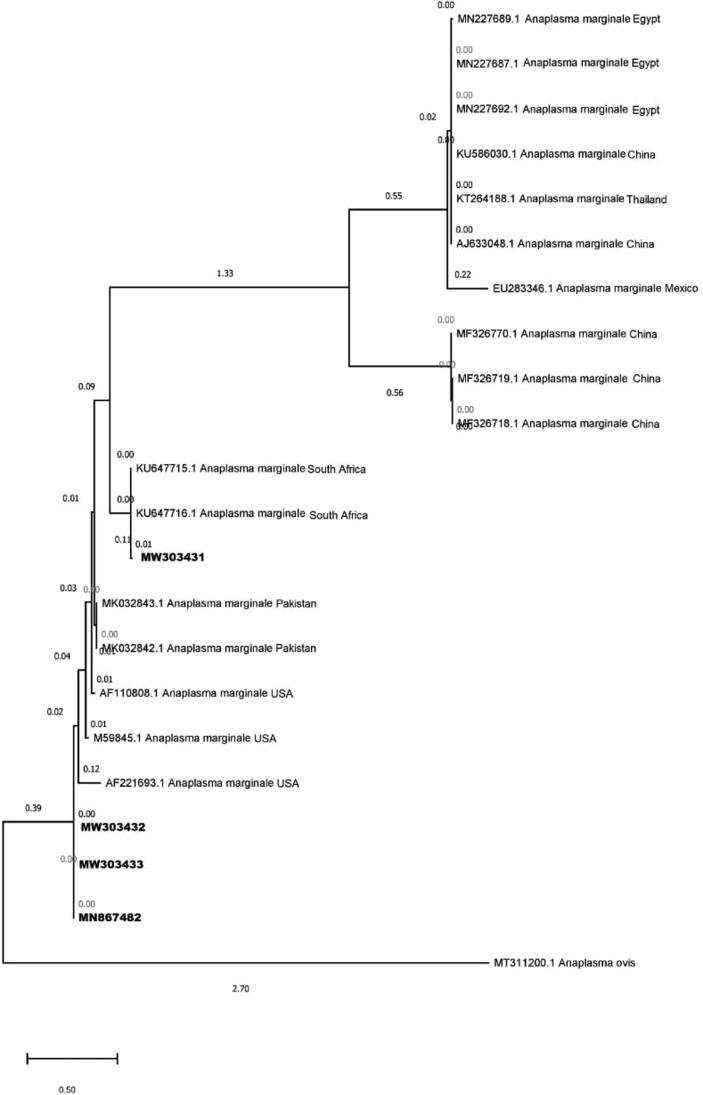
Six amplified PCR products of A. marginale were confirmed by BLAST analysis and four were deposited to the GenBank (Accession numbers MN867482 and MW303431-33). BLAST analysis showed that amplified PCR products of msp1b gene in present study were 96–99% similar to msp1b gene sequence of A. marginale from Pakistan, South Africa and USA (Fig. 1).
Fig. 1.
Phylogenetic tree of A. marginale based on partial msp1bgen sequences from isolates taken from cattle blood samples in Pakistan and cattle worldwide, available in GenBank. Four new sequences of A. marginale obtained in the present study are represented in bold. Scale bar represents 0.2 substitutions per nucleotide position.
3.3. Epidemiological factors analysis
Indoor living environment (p = 0.05), living with other dairy animals (p = 0.03) and absence of dogs at dairy farms (p = 0.04) were found to be associated with A. marginale infection in cattle from Dera Ghazi Khan District. On the other hand no such relation was observed in Lodhran district (p > 0.05) (Table 2).
Table 2.
Association of A. marginale prevalence and epidemiological parameters from Lodhran and Dera Gahzi Khan districts. Based on age, 5 years old animals were considered young while older than 5 years were mature.
| Parameters | Districts |
||||||
|---|---|---|---|---|---|---|---|
| Lohdran |
Dera Ghazi Khan |
||||||
| A. marginale positive samples | A. marginale negative samples | p-value | A. marginale positive samples | A. marginale negative samples | p-value | ||
| Gender | Male | 2 (8%) | 23 (92%) | 1.00 | 18 (24%) | 58 (76%) | 0.08 |
| Female | 18 (9%) | 175 (91%) | 18 (13%) | 116 (87%) | |||
| Age | >5 year | 12 (10%) | 106 (90%) | 0.6 | 25 (18%) | 112 (82%) | 0.7 |
| <5 year | 08 (9%) | 92 (92%) | 11 (15%) | 62 (85%) | |||
| Health status | Healthy | 20 (10%) | 189 (91%) | 1.00 | – | – | – |
| Fever | 0 (0%) | 09 (100 %) | – | – | – | ||
| Dogs presence | Present | 11 (9%) | 118 (90%) | 0.8 | 21 (14%) | 131 (86%) | 0.04 |
| Absent | 9 (10%) | 80 (90%) | 15 (26%) | 43 (74%) | |||
| Presence of feeding ticks | Present | 5 (10%) | 46 (90%) | 0.8 | 36 (18%) | 161(82%) | 0.1 |
| Absent | 15 (9%) | 152 (91%) | 0 (0%) | 13 (100%) | |||
| Living environment | Outdoor | – | – | – | 22 (14%) | 134 (86%) | 0.05 |
| Indoor | – | – | – | 14 (26%) | 40 (74%) | ||
| Presence of other dairy animals | Present | – | – | – | 28 (15%) | 158 (85%) | 0.03 |
| Absent | – | – | – | 8 (33%) | 16 (67%) | ||
| Water supply | Pump | – | – | – | 16 (19%) | 69 (81%) | 0.7 |
| Pool | – | – | – | 20(16%) | 105(84%) | ||
4. Discussion
Tick-borne diseases are one of the most important factors limiting the dairy industry and cause huge economic losses (Ashraf, 2013, Malik, 2018). This relationship is also observed in Pakistan, from where there are numerous reports of ticks collected from animals, including livestock. This is favored by the climate. Pakistan has sub-tropical climatic conditions and due to high air temperature and humidity, ticks and TBDs are common in this area (Ashraf, 2013).
In present study we confirmed the occurrence of genetic material of A. marginale in 9% and 17% of cattle blood samples collected from Lodhran and Dera Ghazi Khan Districts respectively (Table 1). Our results are in line with those of Ashraf et al. (Ashraf, 2021) who had reported 8.6% infection rate of A. marginale in cattle from Layyah District in Pakistan. There are few other studies regarding prevalence of A. marginale in cattle from Pakistan. Farooqi et al. (Farooqi, 2018) had documented 18.3% prevalence of A. marginale in bovine samples collected from three distinct zones of Khyber Pakhtunkhwa (KPK) province. In the other study conducted in KPK, 41.6% cattle from District Lakki Marwat and Peshawar were reported to be infected with A. marginale (Turi et al., 2018). Hussain et al. (Hussain, 2017) had reported that 6.1% of apparently healthy cattle (Cholistan breed) from District Bahawalpur in Punjab were infected with A. marginale. Bovine anaplasmosis has been reported from various countries across the globe including Turkey (2.3%) (Aktas et al., 2011); Tunisia (3.7–25.4%) (Belkahia, 2015) and Brazil (5.4%) (Barbosa da Silva, 2014). These variations in prevalence of A. marginale reported from different areas of the world are probably due to variation in tick control and management programs in these regions, suitability of the climatic conditions for tick growth, different farm management techniques and animal husbandry practices that are used in those regions (Belkahia, 2015, Yukari and Umur, 2002).
To report the genetic diversity of A. marginale in studied cattle populations, phylogenetic analysis based on msp1b gene of parasite was conducted. The obtained sequences (MW303432, MW303433 and MN867482) were like sequences previously reported from Pakistan from District Layyah (MK032843-1 and MK032842-1), and USA (AF110808.1, M59845.1 and AF221693.1)). The MW30343.1 presented a higher similarly with the South Africa (KU647715, and KU647715) sequences (Fig. 1). The obtained sequences didn’t group with sequences from Egypt (MN227689.1, MN227687.1) China (KU586030.1, MF326718.1, MF326719.1, AJ533048.1, MF326770.1) Thailand (KT264188.1) and Mexico (EU283346.1) showing a complex diversity worldwide.
Specific epidemiological factors can influence the host–parasite associations including climate conditions, behavioral traits, host sociality, population density, diet, habitat, age, sex, host immunocompetence, supplementary feeding and animal translocations (Hussain et al., 2021). During the present investigation, we have also recorded a number of epidemiological factors explaining the characters of cattle as well those of the farms where they were kept in order to report their association with the prevalence of A. marginale. In present study the prevalence of A. marginale did not differ statistically between cattle breeds from Lohdran and Dera Ghazi Khan Districts (Table 2). This observation is contradictory to Ashraf et al. (Ashraf et al., 2021) who had documented highest prevalence of A. marginale in exotic Holstein Friesian than in local Sahiwal breed. Similarly, Khan et al. (Khan, 2019) and Tay et al. (Tay et al., 2014) had also confirmed higher Anaplasma spp. prevalence in Holstein Friesian breed than in local cattle breeds. They assumed that Holstein Friesian breed has long and thick hairs compared to local breeds that make them a preferred host for ticks to infest. There are two potential reasons for this difference in parasite prevalence in cattle breeds from two studies; first, local breeds are more resistant against rickettsial infection (Ashraf, 2021) and the second reason is different number of samples that were examined in two studies. The number of samples tested by Ashraf et al. (Ashraf et al., 2021) were almost double than the sample we have included in present study. This difference can clearly affect the breed specific prevalence ratios of A. marginale.
In our study, we have reported that absence of the dogs at the dairy farms and cattle living indoor are important factor associated with A. marginale infection. We also reported that presence of other dairy animals on farms with cattle is significantly associated with the A. marginale infection in cattle from District Dera Ghazi Khan (Table 2). Our results are also in agreement with those of Ashraf et al. (Ashraf, 2013) who had confirmed that A. marginale prevalence was higher in herds that do not had dogs and they had proposed that A. marginale infection was probably transmitted from infected to healthy by physical contact. Although, no association was found between the presences of dogs at dairy with the prevalence of A. marginale during present study but it is also an established facts that rearing dogs at the livestock farms is a common practice in Pakistan, especially in rural areas. It has been reported that dogs in Pakistan (Abid, 2021) are infested with Rhipicephalus spp. and these ticks are also known to infest cattle in Pakistan (Ashraf, 2021, Parveen et al., 2021). There is a possibility that physical contact of dogs with cattle can result in tick transfer from one host to other resulting in spread of tick borne diseases among them.
During the second half of the 20th century, many dairy farmers moved away from pasture-based housing systems and housed their dairy cows indoors year-round. This shift in housing system decreased the exposure of the cattle to the various parasites and their vectors. In addition, development and use of pharmaceutical parasiticides decreased the likelihood of heavy parasite infestations and reduced related production losses (Sorge et al., 2015). At dairy farms where modern scientific procedures are applied, the dairy cattle shows greater economic importance as compared to farms where old traditional methods of animal rearing are in practise, encouraging the farmers to adopt appropriate hygiene practices during milking and reproduction of the herd (Simioni et al., 2013). Most of the farms in district Dera Ghazi Khan are poorly managed and old traditional techniques for animal rearing and management are still in practice that leads to poor hygiene and increases the risk of tick infestation and prevalence of TBDs which is event from the fact that the prevalence of A. marginale was higher in cattle of Dera Ghazi Khan district as compared to Lodhran. Swai et al. (Swai, 2005) and Atif et al. (Atif et al., 2012) had also documented higher infection rate of A. marginale in farms that were managed by old traditional techniques than the farms where modern scientific techniques were applied.
5. Conclusions
In conclusion, we are reporting that prevalence of A. marginale was higher in cattle from Dera Ghazi Khan than in cattle from Lodhran. No specific cattle breed was found susceptible to A. marginale infection. We observed that poor farm management practices are responsible for increasing prevalence of TBDs in studied regions. As we did not include any cattle suffering from anaplasmosis in this study, so none of the enrolled animals showed the symptoms of anaplasmosis but still A. marginale was detected in their blood. Studies similar to the present one are necessary for the prophylactic detection of this bacterium in cattle, so that they must be diagnosed and treated before the onset of acute form of disease preventing the economic losses.
Author contributions
F.I., Z.Z. and M.F. had designed and supervised this study. S.N.A.Z., S.N. and M.M.A. had collected blood and epidemiological data from cattle. A.P., I.A.E., M.A. and M.M.A. had extracted DNA from the blood samples and performed PCR. A.K. had purified PCR products and analyzed DNA sequences. M.A. A.A. A.S. and S.O. had constructed phylogenetic tree and analyzed the data. All authors reviewed the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors thank the researchers supporting project for their funding this work number (RSP-2021/232) at King Saud University, Riyadh, Saudi Arabia.
Ethics approval
This study was revised and approved by the Ethical Research Committee of Institute of Pure and Applied Biology at Bahauddin Zakariya University Multan (Pakistan) according to the ethical principles of human and animal research. This study was carried out in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments, PLoS Bio 8(6), e1000412,2010) guidelines.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abid K., et al. Molecular detection and prevalence of Theileria ovis and Anaplasma marginale in sheep blood samples collected from district Layyah in Punjab Pakistan. Trop. Anim. Health Prod. 2021;53(4):439. doi: 10.1007/s11250-021-02870-5. [DOI] [PubMed] [Google Scholar]
- Aktas M., Altay K., Dumanli N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick Borne Dis. 2011;2:62–65. doi: 10.1016/j.ttbdis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Arif, M. et al., 2013. Prospects and limitations of dairying in Gujranwala district (Punjab-Pakistan). In: Proceedings of International Workshop on Dairy Sciences. Park. Nov 21-23, 2011, Agriculture University Peshawar. Pak. J. Anim. Plant Sci. 23, 34–37.
- Ashraf Q.U.A., et al. A report on the high prevalence of Anaplasma sp. in buffaloes from two provinces in Pakistan. Ticks Tick Borne Dis. 2013;4:395–398. doi: 10.1016/j.ttbdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Ashraf S., et al. A report on molecular detection and phylogenetic evaluation of Anaplasma marginale in ticks and blood samples collected from cattle in District Layyah in Punjab (Pakistan) Curr. Microbiol. 2021;7:278–281. doi: 10.1007/s00284-020-02256-0. [DOI] [PubMed] [Google Scholar]
- Ashraf S., Parveen A., Asif M., Alanazi A.D., Alouffi A., Muhammad Awais M., Khan A., Aktas M., Ozubek S., Iqbal F. First report regarding molecular epidemiology and novel variant identification of Anaplasma centrale in cattle from Pakistan. Saudi J. Biol. Sci. 2021;28(11):6488–6494. doi: 10.1016/j.sjbs.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif F.A., Khan M.S., Iqbal H.J., Ali Z., Ullah S. Prevalence of cattle tick infestation in three districts of the Punjab, Pakistan. Pak. J. Sci. 2012;64:49–53. [Google Scholar]
- Barbosa da Silva J., et al. Molecular and serological prevalence of Anaplasma marginale in water buffaloes in northern Brazil. Ticks Tick Borne Dis. 2014;5:100–104. doi: 10.1016/j.ttbdis.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Belkahia H., et al. First molecular survey and novel genetic variants’ identification of Anaplasma marginale, A. centrale and A. bovis in cattle from Tunisia. Inf. Gen. Evol. 2015;34:361–371. doi: 10.1016/j.meegid.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Bursakov S.A., Kovalchuk S.N. Co-infection with tick-borne disease agents in cattle in Russia. Ticks Tick Borne Dis. 2019;10(3):709–713. doi: 10.1016/j.ttbdis.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Camus, E., Uilenberg, G. Anaplasmosis. In Infectious and Parasitic Diseases of Livestock. Bacterial Diseases, Fungal Diseases, Parasitic Diseases. Lefevre, P.C., Blancou, J., Chermette, R., Uilenberg, G. (Eds.), Lavoisier, 1247–1263 (Paris, 2010).
- Durrani A.Z., Shakoori A.R. Study on ecological growth conditions of cattle Hyalomma ticks in Punjab, Pakistan. Iran. J. Parasitol. 2009;4:19–25. [Google Scholar]
- Farooqi S.H., et al. Molecular epidemiology of bovine anaplasmosis in Khyber Pakhtunkhwa, Pakistan. Trop. Animal Health Prod. 2018;50:1591–1598. doi: 10.1007/s11250-018-1599-2. [DOI] [PubMed] [Google Scholar]
- Farooqi S.H., Ijaz M., Saleem M.H., Rashid M.I., Oneeb M., Khan A., Aqib A.I., Mahmood S. Distribution of ixodid tick species and associated risk factors in temporal zones of Khyber Pakhtunkhwa Province, Pakistan. Pak. J. Zool. 2017 doi: 10.17582/journal.pjz/2017.49.6.2011.2017. [DOI] [Google Scholar]
- Hairgrove T., et al. Molecular and serological in-herd prevalence of Anaplasma marginale infection in Texas cattle. Prev. Vet. Med. 2015;119:1–9. doi: 10.1016/j.prevetmed.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Hussain M.F., et al. Molecular detection of Anaplasma in apparently healthy Cholistan breed of cattle from the Bahawalpur district, Pakistan. Trop. Biomed. 2017;34:37–44. [PubMed] [Google Scholar]
- Hussain S., Hussain A., Ho J., Li J., George D., Rehman A., Zeb J., Sparagano O. An epidemiological survey regarding ticks and tick-borne diseases among livestock owners in Punjab, Pakistan: a one health context. Pathogen. 2021;10:361. doi: 10.3390/pathogens10030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad N., Qayyum M., Hussain M., Khan M.Q. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 2010;30:178–180. [Google Scholar]
- Khan N.U., et al. Prevalence and risk factors analysis associated with anaplasmosis in symptomatic cattle under field conditions in southern Khyber Pakhtoonkhwa, Pakistan. Pu. Appl. Biol. 2019;8:2119–2127. [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kocan K.M., De La Fuente J., Blouin E.F., Garcia-Garcia J.C. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host–pathogen adaptations of a tick-borne rickettsia. Parasitology. 2004;129(S1):S285–S300. doi: 10.1017/s0031182003004700. [DOI] [PubMed] [Google Scholar]
- Kumar T., et al. Emerging status of anaplasmosis in cattle in Hisar. Vet. World. 2015;8:768–771. doi: 10.14202/vetworld.2015.768-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M.I., et al. Molecular detection of Ehrlichia canisin dogs from three districts in Punjab (Pakistan) Vet. Med. Sci. 2018;4:126–132. doi: 10.1002/vms3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen, A., Ashraf, S., Khan, A., Asif, M., Iqbal, F., 2021. Epidemiology of tick-borne diseases. In: Kumar, S., Bayugar, R.C., Sharma, A.K., Miranda, E.M., Chaubey, A.K. (Eds.), The Entomological Guide to Rhipicephalus. Nova Science Publishers, New York.
- Ramzan M., Unsar N.U., Syed H.M.B., Ghulam M., Alamgir A.K. Knowledge, attitude and practices of herdsmen about ticks and tick-borne diseases in district Multan. Pak. Entomol. 2018;40:13–18. [Google Scholar]
- Saeed Z., et al. Molecular prevalence and hematology of tropical theileriosis in Cholistani cattle from nomadic herds of the Cholistan desert, Pakistan. Kaf. Uni. Vet. Fak. Derg. 2016;22:281–286. [Google Scholar]
- Simioni F.S., Baretta C.R.D.M., Stefani L.M., Lopes L.S., Tizziani T. Qualidade do leite proveniente de propriedades com diferentes níveis de especialização. Semina: Ciênc. Agr. 2013;34(4):1901–1912. [Google Scholar]
- Sorge U.S., Moon R.D., Stromberg B.E., Schroth S.L., Michels L., Wolff L.J., Kelton D.F., Heins B.J. Parasites and parasite management practices of organic and conventional dairy herds in Minnesota. J. Dairy Sci. 2015;98(5):3143–3151. doi: 10.3168/jds.2014-9031. [DOI] [PubMed] [Google Scholar]
- Swai E.S., et al. Seroprevalence estimation and risk factors for A. marginale on smallholder dairy farms in Tanzania. Trop. Animal Health Prod. 2005;37:599–610. doi: 10.1007/s11250-005-4307-y. [DOI] [PubMed] [Google Scholar]
- Tay S.T., Koh F.X., Kho K.L., Ong B.L. Molecular survey and sequence analysis of Anaplasma spp. in cattle and ticks in a Malaysian farm. Trop. Biomed. 2014;31:769–776. [PubMed] [Google Scholar]
- Turi, A.T., Rahman, A., Ali, I., 2018. Comparative analysis of indirect ELISA and real time PCR for the detection of Anaplasma marginale in buffalo, cattle and sheep in district Peshawar and Lakki Marwat, Pakistan. South Asi. J. Life Sci. 6 (2018), 1–6.
- Yukari B.A., Umur S. The prevalence of tick species (Ixodoidea) in cattle, sheep and goats in the Burdur region, Turkey. Turk. J. Vet. Anim. Sci. 2002;26:1260–1270. [Google Scholar]