Abstract
Background
Vitamin C deprivation can lead to fatigue, dyspnea, oedema and chest pain, which are also symptoms of heart failure (HF). In animal studies vitamin C has improved contractility and mechanical efficiency of the heart. Compared with healthy people, patients with HF have lower vitamin C levels, which are not explained by differences in dietary intake levels, and more severe HF seems to be associated with lower plasma vitamin C levels. This meta-analysis looks at the effect of vitamin C on left ventricular ejection fraction (LVEF).
Methods
We searched for trials reporting the effects of vitamin C on LVEF. We assessed the quality of the trials, and pooled selected trials using the inverse variance, fixed effect options. We used meta-regression to examine the association between the effect of vitamin C on LVEF level and the baseline LVEF level.
Results
We identified 15 trials, three of which were excluded from our meta-analysis. In six cardiac trials with 246 patients, vitamin C increased LVEF on average by 12.0% (95% CI 8.1–15.9%; P < 0.001). In six non-cardiac trials including 177 participants, vitamin C increased LVEF on average by 5.3% (95% CI 2.0–8.5%; P = 0.001). In meta-regression analysis we found that the effect of vitamin C was larger in trials with the lowest baseline LVEF levels with P = 0.001 for the test of slope. The meta-regression line crossed the null effect level at a baseline LVEF level close to 70%, with progressively greater benefit from vitamin C with lower LVEF levels. Some of the included trials had methodological limitations. In a sensitivity analysis including only the four most methodologically sound cardiac trials, the effect of vitamin C was not substantially changed.
Conclusions
In this meta-analysis, vitamin C increased LVEF in both cardiac and non-cardiac patients, with a strong negative association between the size of the vitamin C effect and the baseline LVEF. Further research on vitamin C and HF should be carried out, particularly in patients who have low LVEF together with low vitamin C intake or low plasma levels. Different dosages and different routes of administration should be compared.
Keywords: antioxidant, coronary artery bypass graft surgery (CABG), heart failure, left ventricular function, oxidative stress, percutaneous coronary intervention (PCI), randomized trials, systematic review
Introduction
In the 18th century, James Lind described extreme intolerance for exercise as a characteristic of scorbutic patients (1). In addition, other signs compatible with heart failure (HF) such as shortness of breath, lethargy, and swelling of legs were described as symptoms of vitamin C deficiency in the major monographs on scurvy (1, 2). Autopsies of patients who died of scurvy revealed cardiac hypertrophy and congestion of the lungs (2). Experimental vitamin C deprivation in healthy volunteers led to fatigue, dyspnea, oedema, chest pain and reduced autonomic reflexes (3–8).
Case reports of patients with severe vitamin C deficiency have reported fatigue, dyspnea, cardiac enlargement, oedema and orthostatic hypotension, which often disappeared quite rapidly after vitamin C administration (9–15). A few animal models found that vitamin C can improve contractility and mechanical efficiency of the heart, including effects on left ventricular ejection fraction (LVEF) (16–21). Given the overlap of the symptoms of vitamin C deficiency with the symptoms of HF, and the findings from the animal studies, it seems appropriate to investigate whether administration of vitamin C may be beneficial for some HF patients.
Vitamin C exerts a multitude of biochemical effects influencing several cardiovascular processes relevant for HF. It participates in the synthesis of norepinephrine, carnitine, nitric oxide, and in the terminal amidation of dozens of neuropeptides such as vasopressin (22–25). Vitamin C hydroxylates specific proline residues in hypoxia-inducible factor-I, which regulates hundreds of genes, and it also participates in the demethylation of DNA and histones and thereby influences the epigenome (23–26).
In randomized trials, vitamin C has affected the cardiovascular system in various ways. Meta-analyses have indicated that in some contexts vitamin C may reduce blood pressure and risk of atrial fibrillation (27, 28). It has also improved endothelial functions and baroreflex sensitivity (29–36). In patients with ischemia/reperfusion injury, vitamin C may reduce reperfusion damage with amelioration of myocardial stunning (37). Through these types of mechanisms, vitamin C may impact HF.
The goal of this meta-analysis was to analyze the findings of trials that have reported on the effect of vitamin C on LVEF as a measure of the mechanical function of the heart.
Results
Description of the Included Trials
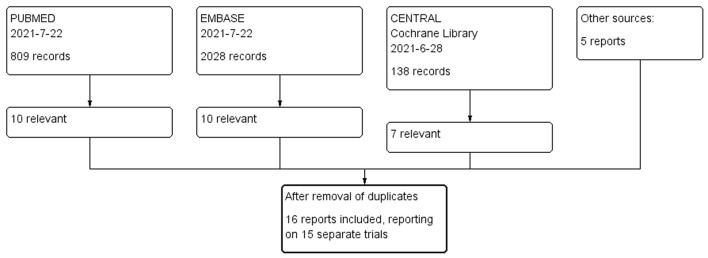
We identified 16 publications that reported 15 separate trials (Figure 1, Table 1, Supplementary Table S1). Seven of them were parallel group trials and five were cross-over trials (Table 1). Three further trials were before-after trials: LVEF was first measured at baseline and then a second LVEF measurement was carried out following vitamin C administration of 1 month (49) or 6 months (38). The Basili et al. trial was published in two reports (43, 44) and the Fernhall et al. trial in three reports (45–47). Sabri et al. (49) and Scalzo et al. (52) reported two separate trials in one paper. The total number of patients in the 15 trials was 469, with 246 participants in six cardiac trials, and 223 participants in nine non-cardiac trials, six of which were included in the meta-analysis.
Figure 1.
Flow diagram of the searches, which identified 16 publications reporting 15 trials on the effect of vitamin C on LVEF (38–53). The search terms are listed in Supplementary File 1.
Table 1.
Characteristics of included trials.
| Trial [ref] | N (vit C/ Control) | Age (y, mean) | Country | Context | LVEF at baselinea | Vit C route | Vit C dose (g/day) | Vit C days |
|---|---|---|---|---|---|---|---|---|
| Cardiac trials | ||||||||
| Guan et al. (39) | 10/11 | 65 | Japan | PCI | 50% | iv | 6 | 1 |
| Oktar et al. (40) | 12/12 | 56 | Turkey | CABG | 61.5% | iv | 4 | 1 |
| Ho (41) | 37b | 69 | Taiwan | HF | 35% | po | 4 | 28 |
| Basili et al. (43) Pignatelli et al. (44) |
28/28 | 67 | Italy | PCI | 53% | iv | 1 | 1 |
| Safaei et al. (51) | 29/29 | 57 | Iran | CABG | 49% | iv | 2 | 1 |
| Emadi et al. (53) | 25/25 | 62 | Iran | CABG | 56% | iv | 10 | 1 |
| Non-cardiac trials | ||||||||
| Jensen et al. (38) | 9c | 52 | Denmark | Iron-loaded adults | 56% | po | 0.2 | 180 |
| Glavas et al. (42) | 8b | 37 | Croatia | Diving | 66% | po | 1 | 1 |
| Fernhall et al. (45) Fahs et al. (46) Fernhall et al. (47) |
34/35 | 28 | USA | Fire fighters | 59% | po | 2 | 1 |
| Gao et al. (48) | 8d | 26 | USA | Hyperoxia | 59% | iv | 3 | 1 |
| Sabri et al. (49) | 19c | 13 | Iran | Healthy | 66% | po | 0.25 | 30 |
| Sabri et al. (49) | 18c | 11 | Iran | T1D | 66% | po | 0.25 | 30 |
| Sabri et al. (50) | 20/20 | 13 | Iran | T1D | 60% | po | 0.25 | 180 |
| Scalzo et al. (52) | 21b | 45 | USA | Exercise; Healthy | 66% | iv | 7.5 | 1 |
| Scalzo et al. (52) | 31b | 46 | USA | Exercise; T2D | 64% | iv | 7.5 | 1 |
Baseline LVEF is the mean of vitamin C and control groups.
Cross-over; random order.
Before-after study.
Cross-over; first was the control test and thereafter the vitamin C test.
CABG, coronary artery bypass graft surgery; iv, intravenous; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; po, per oral; T1D, type 1 diabetes; T2D, type 2 diabetes.
In the six cardiac trials, the mean age ranged from 56 to 69 years, and the baseline LVEF ranged from 35 to 62%. Five of the cardiac trials investigated the effect of a single intravenous dose of vitamin C before or during cardiac surgery with the dose ranging from 1 to 10 g (39, 40, 43, 51, 53). A trial with HF patients used a cross-over design to investigate the effect of 4-week oral administration of vitamin C at 4 g/day compared with placebo (41).
The nine non-cardiac LVEF trials form a heterogeneous group of studies. The mean age of participants ranged from 11 to 52 years. The baseline LVEF was rather high in all these trials, ranging from 56 to 66%. In children with diabetes, Sabri et al. administered 0.25 g/day of vitamin C orally for 6 months (50). In two cross-over trials published in one trial report, Scalzo et al. administered 7.5 g of vitamin C intravenously before an exercise test for type 2 diabetes (T2D) patients and matched healthy participants (52). In a cross-over trial, Gao et al. studied the effect of 3 g/day intravenous vitamin C on cardiac effects of hyperoxia (48). In one before-after trial, Jensen et al. administered 0.2 g/day of vitamin C orally for 12 months to adults who suffered from transfusion-induced iron overload (38), and in two before-after trials, Sabri et al. gave vitamin C to healthy participants and to type 1 diabetes mellitus (T1D) patients (49).
The risk of bias assessment for the 15 included trials is shown in Figure 2. Eleven trials were randomized. Oktar et al. did not describe the method of allocation in their CABG trial (40). In three self-controlled trials, a baseline LVEF measurement was taken, followed by a period of vitamin C treatment, and then a second LVEF measurement was taken (38, 49). The reported baseline variables for the treatment groups in the parallel-group trials were balanced (Supplementary Table S1). Eight trials used an explicit placebo (41–43, 45, 50, 52, 53), whereas four trials reported “no treatment” in the control groups (39, 40, 48, 51). The trials by Gao et al. (48), Jensen et al. (38), Oktar et al. (40), Safaei et al. (51), and Sabri et al. (49) had the most methodological concerns (Figure 2). The Jensen et al. trial (38) and the both Sabri et al. (49) trials were excluded from our meta-analysis as they did not have an explicit control group or control treatment period.
Figure 2.
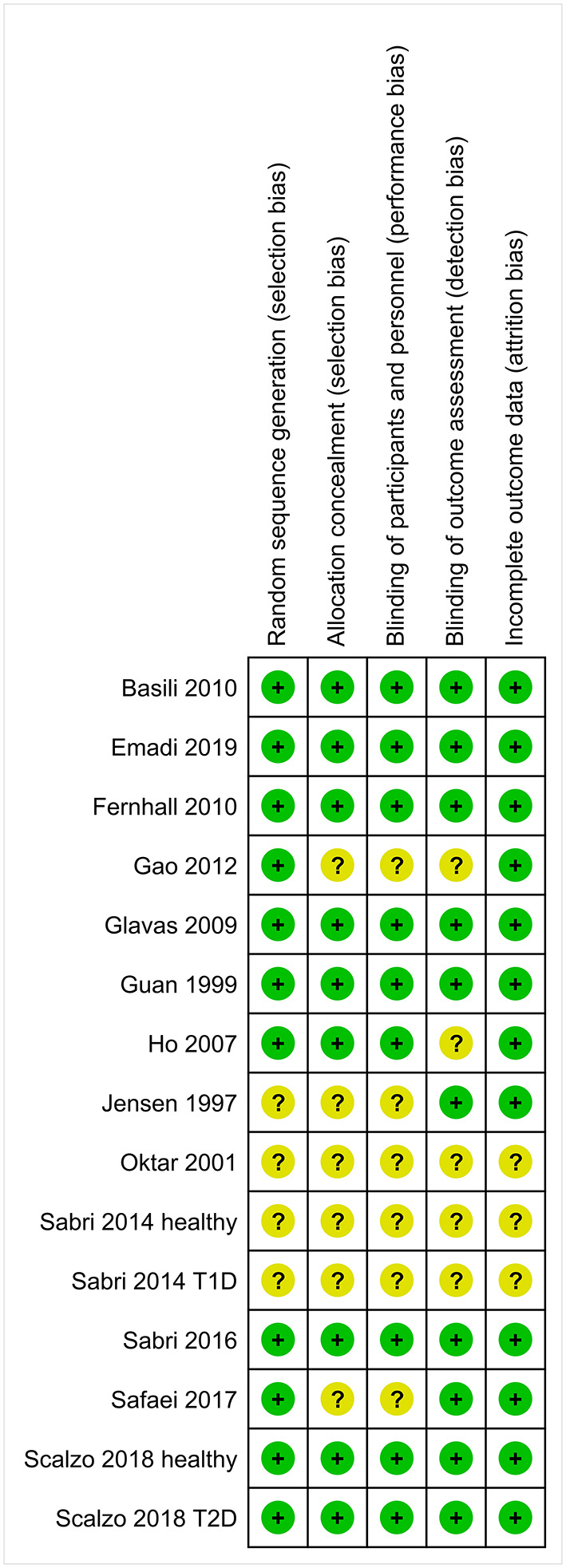
Risk of bias summary of the trials that reported on the effect of vitamin C on LVEF. Review authors' judgments are shown for each risk of bias item for each included trial. A green plus sign (+) indicates that there is no substantial concern for bias in the particular quality item. A question mark (?) indicates that conclusions are unable to be drawn regarding potential bias. The reference numbers to the trials are shown in Table 1. Justifications for the quality assessments are described in Supplementary Table S1.
In 11 trials LVEF was measured by transthoracic echocardiography (40–50, 52, 53). Biplane Simpson's rule was used to calculate LVEF in 3 trials (41, 43, 48), 2-D guided M-mode measurement of the cross-sectional axis of the LV at the papillary muscle tip level in 1 trial (42) and in the other 7 trials this was not specified (40, 45–47, 49, 50, 52, 53). Jensen et al. (38) estimated LVEF by resting multigated acquisition (MUGA) scans, Oktar et al. (40) by both MUGA scan and echocardiography and Guan et al. by left ventriculography (39). Safaei et al. did not describe the method of measurement of LVEF in their CABG trial (51).
Results of the Included Trials
Vitamin C and LVEF
For our quantitative study, we excluded three before-after trials (38, 49), restricting the meta-analysis to seven parallel group trials and five cross-over trials – 12 trials in total.
We calculated the difference in LVEF changes between the parallel vitamin C and control groups, and between the vitamin C and control periods in the cross-over trials. We used the relative scale which has been shown to be superior in the analysis of continuous outcomes (54–56). See Figure 3 for an illustration of the calculation for the effect of vitamin C on LVEF. A similar approach was previously used in the analysis of changes in FEV1 levels in β2-agonist trials (54). We analyzed the findings of cardiac and non-cardiac trials separately (Figure 4, Table 1).
Figure 3.
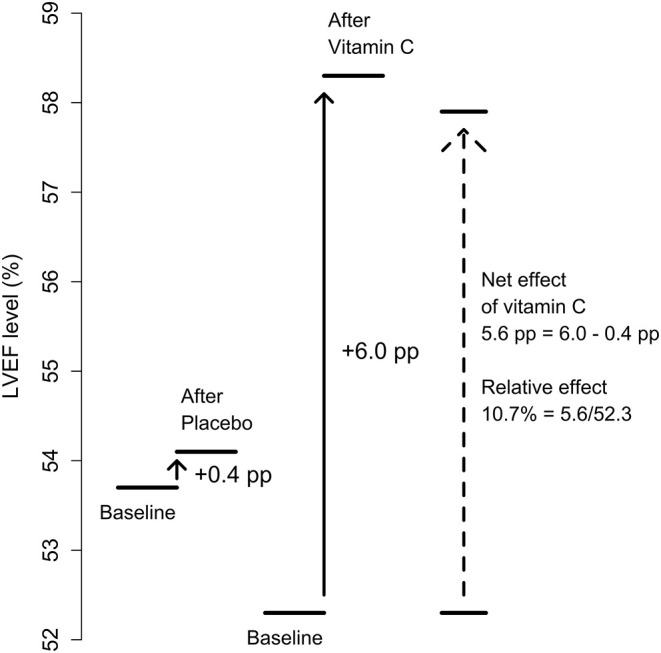
Calculation of the effect of vitamin C on LVEF. The calculation is illustrated with figures from the Basili et al. trial during PCI (percutaneous coronary intervention) (43). In the placebo group, LVEF was increased from a baseline level of 53.7 to 54.1% after the intervention, while in the vitamin C group from 52.3 to 58.3%. These correspond to 0.4 and 6.0 percentage point (pp) increases in LVEF, respectively. Thus, subtracting the placebo group change from the vitamin C group change gives the net effect of vitamin C as 5.6 pp. On the relative scale this corresponds to a 10.7% (= 5.6/52.3) increase in LVEF due to vitamin C from the baseline level.
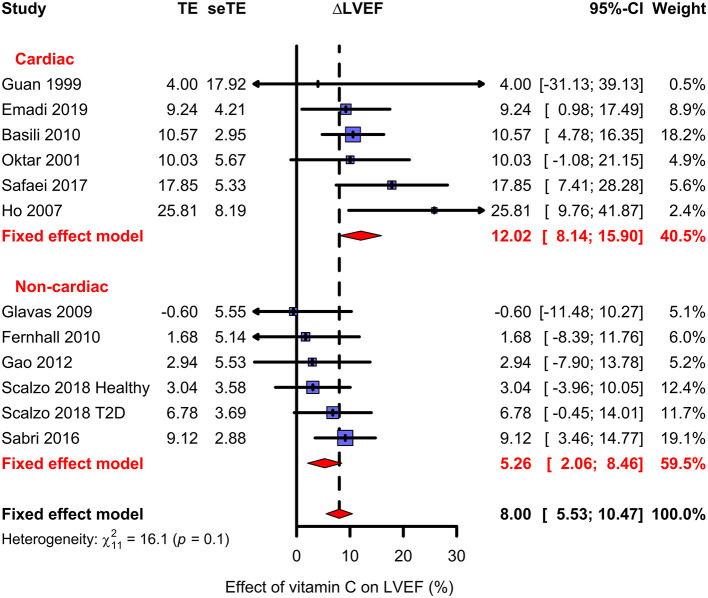
Figure 4.
Effect of vitamin C on LVEF. The upper subgroup shows the cardiac trials and the lower subgroup shows the non-cardiac trials. The effect of vitamin C is shown as the percentage difference between the vitamin C and placebo groups from the baseline LVEF; see Figure 3. The horizontal lines indicate the 95% CI for the vitamin C effect and the blue squares in the middle of the horizontal lines indicate the point estimate of the effect in the particular trial. The size of the blue square reflects the weight of the trial in the meta-analysis. The red diamond shape indicates the pooled effect and 95% CI for the two subgroups and for all 12 trials. See Supplementary Files 1, 2 for the description of the trials and the calculations.
In six cardiac trials with 246 patients, vitamin C increased LVEF on average by 12.0% (95% CI 8.1–15.9%; P = 10−9) (Figure 4). In four trials, the benefit was statistically significant. There was no evidence of heterogeneity between the six cardiac trials (P = 0.4). There are methodological concerns with the Oktar and Safaei trials (Figure 2). Nevertheless, if these two trials are excluded in a sensitivity analysis, the estimate of vitamin C effect changes only slightly to 11.3% (95% CI 6.7–15.8%; P = 10−6). The methodologically sound Basili trial has the greatest weight in the cardiac meta-analysis, with an estimate of effect close to the pooled effect in the cardiac group (Figure 4). Thus, there is no indication that methodologically inferior trials exaggerate the estimate of effect.
In the meta-analysis of six non-cardiac trials including 177 participants, vitamin C increased LVEF on average by 5.3% (95% CI 2.0–8.5%; P = 0.0013) (Figure 4). A statistically significant benefit was found in two of the individual trials. There was no evidence of heterogeneity (P = 0.5).
In explorative meta-regression analyses, we investigated a few relevant variables over the 12 trials as follows. There was no evidence of a difference in estimates between four trials with oral administration and eight trials with intravenous administration (P = 0.8). In meta-regression, the dose of vitamin C was not associated with the size of the effect (P = 0.7). For example, Basili (43) administered 1 g and Emadi (53) administered 10 g on one single day, but the reported effects were quite similar (Figure 4). See Supplementary File 1 for the analyses.
In the 12 trials included in our meta-analysis, baseline LVEF varied from 35 to 66%, but only the Ho trial with HF patients (41) had a baseline LVEF below 48%. The relationship between the effect of vitamin C and the baseline LVEF is plotted in Figure 5 with the cardiac trials indicated by filled circles and the non-cardiac trials by open circles. The evidence for modification of the vitamin C effect by the baseline LVEF is very strong (P = 0.0008). There is no evidence of residual heterogeneity around the regression line, which indicates that the meta-regression fully captures the findings of the 12 trials. The slope for all 12 trials crosses the null-effect line at a baseline LVEF of close to 70%. Given that the Ho trial (41) had by far the lowest baseline LVEF level of 35%, and is thereby particularly influential in defining the slope, it was removed in a sensitivity analysis, however, the slope did not change substantially and remained significant (P = 0.011). It is worth noting that most of the non-cardiac trials had participants with high baseline LVEF levels, which explains their rather low pooled estimate of effect in Figure 4.
Figure 5.
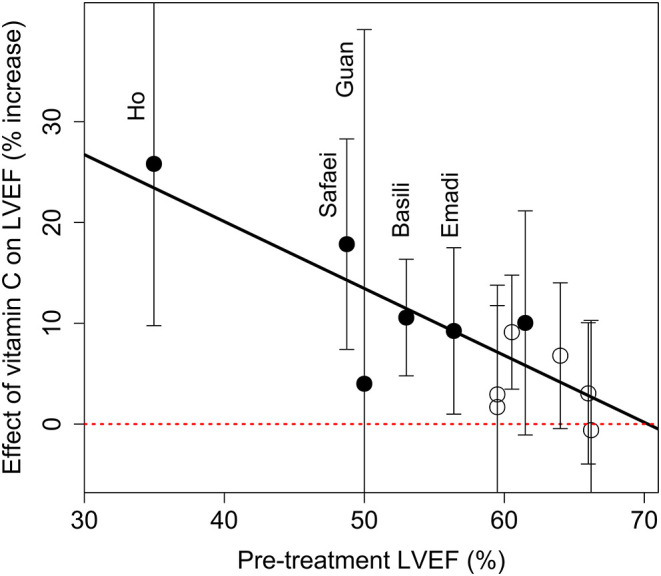
Effect of vitamin C on LVEF by the baseline LVEF. The effect of vitamin C is shown as the percentage differences between the vitamin C and control groups from the baseline LVEF. The vertical lines indicate the 95% CI for the vitamin C effect in each trial. The cardiac trials are indicated by filled circles, and the non-cardiac trials by open circles. The red horizontal dotted line indicates the null effect. The diagonal line shows the meta-regression line with P = 0.0008 for the test that the slope is null. There are no indications of residual heterogeneity over the regression line, P = 0.9. For the calculations, see Supplementary File 1.
Three before-after trials were excluded from our meta-analysis. The Jensen trial (38) was a study of adult patients with transfusional iron overload. During 12-month iron chelation therapy with desferrioxamine, the LVEF level of the patients significantly decreased. Thereafter, 0.2 g/day of vitamin C was administered in order to increase the efficacy of iron chelation. After 6 months of vitamin C administration, there was a mean increase in LVEF of 7.0% (P < 0.01) in 9 patients compared with a LVEF of about 56% before vitamin C; see Supplementary File 2 for the calculation. Because of the continuous gradual decline in LVEF before vitamin C administration, the net effect of vitamin C would be even greater, if the period before vitamin C was adjusted for. In two pediatric trials with T1D patients and healthy controls, Sabri et al. (49) gave 0.25 g/day vitamin C for 1 month. No increase in LVEF was observed from the baseline LVEF of 66% (49). Given the baseline LVEF levels of these three trials, these findings are consistent with the predictions of our meta-regression in Figure 5.
Vitamin C and Other Measures of Cardiac Function in the Included Trials
Five of the included publications also reported other outcomes relevant to the topic of this study.
Basili et al. reported that after the percutaneous coronary intervention (PCI), the Thrombolysis in Myocardial Infarction Myocardial Perfusion Grade (TMPG, a categoric coronary flow grading system) was normal in 79% (22/28) of the participants in the vitamin C group, compared with just 39% (11/28) in the placebo group (P = 0.01) (43). In addition, after the PCI, the corrected Thrombolysis in Myocardial Infarction frame count (cTFC; a quantitative index for the assessment of myocardial perfusion) was lower, indicating better reperfusion in the vitamin C group (median change −41%) compared with the placebo group (median change −23%; P < 0.0001).
Ho found that, compared with the placebo group, the 4-week vitamin C administration increased the distance covered in a 6-min walk test by 26% in the HF patients (P < 0.001) and the Minnesota Living with HF questionnaire score by 53% (P = 0.01) (41).
Scalzo et al. found that the effect of vitamin C was not significant on left ventricular circumferential and longitudinal strain, which are measures of systolic function, whereas there was a marginally significant effect of vitamin C on the decrease in the post-peak exercise measurement of circumferential strain (P = 0.052) (52). In both the healthy and the T2D patients, vitamin C infusion improved diastolic function, estimated by lower values of lateral and septal E:E' (P < 0.05 for both) indicating enhanced relaxation. In the mitral valve deceleration time, there was interaction between the vitamin C intervention and the participant group (healthy vs. T2D) (P = 0.018), such that vitamin C decreased mitral valve deceleration time in participants with T2D.
Gao et al. studied the same participants before and after hyperoxia. Systolic myocardial velocity was higher during hyperoxia with a vitamin C infusion compared with the control day on which no vitamin C infusion was given (P = 0.001) (48). Vitamin C also led to a higher coronary blood velocity during hyperoxia compared with the control day (P = 0.005), and a decrease in coronary vascular resistance (P = 0.04).
Guan et al. reported that in patients with acute myocardial infarction undergoing direct PCI, intravenous vitamin C did not affect the cardiac index, measured by thermodilution technique at 3–4 weeks after the onset (39).
Discussion
Textbooks often describe the effect that vitamin C has on wound healing, explaining the effect through the role of vitamin C in collagen metabolism. However, this is a simplistic view of the physiological functions of the vitamin. Biochemistry of vitamin C is complex, extending from several cofactor roles in diverse parts of metabolism to non-specific antioxidant effects, and further to wide-ranging epigenetic effects (22–26). The numerous biochemical effects translate to diverse changes at the clinical level. Vitamin C deficiency is associated with many symptoms characteristic of HF (1–15).
Compared with healthy people, patients with HF have lower vitamin C levels, which are not explained by differences in dietary intake levels (57–59). More severe HF seems to be associated with lower plasma vitamin C levels (57, 58). The apparent depletion of vitamin C in HF may be explained by increased metabolic consumption due to the oxidative stress associated with HF (60). In the early literature, vitamin C was suggested for treatment of HF (61–64), yet interest in the topic waned. It seems probable that the early findings were ignored because of wide-spread bias against vitamin C having effects other than treating and preventing scurvy (65).
Decreased plasma vitamin C levels have also been reported after cardiac surgery in parallel with increases in the oxidized forms of the vitamin, i.e., dehydroascorbate and ascorbate free radical (66–72). Thus, if vitamin C has an effect on cardiac function it is plausible that the decreased plasma vitamin C levels may contribute to the postoperative compromise in myocardial function after cardiac surgery.
In this systematic review, six cardiac trials were included. One of them was a 28-day oral vitamin C supplementation trial in patients with HF, whereas the others were 1-day intravenous vitamin C trials in patients who had undergone cardiac surgery or PCI. In the cardiac trials, vitamin C increased the LVEF levels on average by 12%. We also pooled six non-cardiac trials with diverse clinical contexts. In these trials vitamin C increased the LVEF levels on average by 5%. The statistically highly significant effect of vitamin C in both the cardiac and non-cardiac trials provides strong evidence that in some contexts vitamin C can influence the mechanical functions of the heart. Three further before-after trials were consistent with our meta-regression analysis.
We did not demonstrate a dose response on the size of the effect. There was also no difference between oral and intravenous administration, which suggests that intravenous administration is not necessarily needed, despite the fact that this was the mode used in most studies (Table 1). However, the included trials were small and examined mostly ambulant patients, or patients undergoing elective cardiac surgery, and not patients with more severe disease with greater oxidative stress. Therefore, our comparison of oral and intravenous administration, while indicating that in some contexts oral vitamin C is effective, is not definitive and should not be generalized widely.
We found a strong negative association between the size of the vitamin C effect and baseline LVEF (Figure 4). However, only a single trial, which investigated HF patients (41), had baseline LVEF levels below 48% and that trial has the greatest weight in the linear regression. Nevertheless, the association remained significant even when the HF trial was removed. This modification of the vitamin C effect by baseline LVEF should be examined in further trials. Previously, the severity of disease was found to modify the effect of vitamin C on intubation time (73), the effect of vitamin C on FEV1 decline as a result of exercise tests (74), and the effect of vitamin C on the duration of COVID-19 in outpatients (65, 75).
A few of the included trials reported effects of vitamin C on measures of cardiac physiology other than LVEF and found that vitamin C increased cardiac perfusion after PCI (43, 44), improved diastolic function in an exercise test (52), and increased systolic myocardial velocity during hyperoxia (48). In addition to the included trials, a few other trials have reported that vitamin C had an effect on the mechanical function of the heart (76–79).
Two cohort studies examined the effects of dietary vitamin C intake in HF patients who had average LVEF levels of 34% (80, 81). In both studies, higher dietary vitamin C intake was associated with a lower rate of cardiac events during follow-up. In addition, higher vitamin C intake was associated with a better health related quality of life (81). Residual confounding is a potential concern in cohort studies. However, in the randomized cross-over trial with HF patients included in our analysis, Ho found significant benefit from vitamin C administration on health-related quality of life and on the 6-min walk test (41). One of the trials included in our meta-analysis reported both a significant increase in LVEF and a significant decrease in ICU stay in the vitamin C participants (53), and another reported a significant increase in LVEF and a significant decrease in intubation time (51). It is plausible that the effect of vitamin C on the mechanical function of the heart is one of the explanations for the benefits seen in some ICU patients (65, 73, 82–84).
No effect of vitamin C on cardiovascular events was found in the Physicians' Health Study II (PHS-II) (85), in which 14,641 participants received 0.5 g/day of vitamin C for 8 years. However, the PHS-II trial recruited physicians, a group of extremely health-literate professionals who are not representative of the average population in terms of health-related lifestyles. Thus, the participants in the PHS-II trial were very different from the participants in the trials we included in our meta-analysis (Table 1) and so the PHS-II trial should not be considered a relevant comparison for the current meta-analysis.
It is plausible that increased intake of vitamin C may have no beneficial effect for well-nourished healthy people, but higher doses may have effects for people under heavy physiological stress, for example during cardiac surgery, or for people with HF. The possible effects of vitamin C for patients under heavy physiological stress is supported by controlled trials which found vitamin C to be beneficial for ICU patients (65, 73, 82–84), patients undergoing cardiac operations (28), patients with exercise-induced bronchoconstriction (74, 86), and patients with short-term respiratory symptoms induced by heavy physical activity (87–89).
One potential concern with meta-analyses is publication bias. Most of the included trials did not mention the findings on vitamin C and LVEF in their abstracts (39, 43, 44, 46–50, 52), and the Ho study was published only as a monograph (41). This indicates that the specific findings on LVEF were not the primary reason for publication. Furthermore, it is highly unlikely that publication bias could generate the association shown in Figure 5.
The method of allocation and the level of blinding during controlled trials are important issues. There was no risk of bias associated with these issues for most of the included trials (Figure 2). Nevertheless, even when the analysis was restricted to the four cardiac trials for which there were no methodological concerns, the evidence indicating a benefit from vitamin C was very strong.
In conclusion, in this meta-analysis, vitamin C increased LVEF in both cardiac and non-cardiac patients, with a strong negative association between the size of the vitamin C effect and the baseline LVEF. Further research on vitamin C and HF should be carried out. The most informative approach would be to investigate patients who have low LVEF together with low vitamin C intake or plasma levels, and to compare different dosages and different routes of administration. If an effect of vitamin C is demonstrated in such a setting, it would be reasonable thereafter to examine patients with higher LVEF levels or higher plasma vitamin C levels to determine the relationship between baseline characteristics and the effects of vitamin C.
Methods
We searched for controlled trials that reported LVEF levels in parallel vitamin C and control groups, in cross-over trials (separate vitamin C and control periods for the same participants), and before-after studies. We included trials in which the administration of vitamin C was the only difference between the study groups or periods. We did not limit our search to randomized trials and did not require placebo control. We included all doses, all routes of administration and all durations of vitamin C administration.
We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials with the search phrases described in Supplementary File 1. Two authors (HH and AM) independently screened the titles and abstracts and identified potentially relevant trials. Discrepancies between reviewers were resolved by discussion. We also perused the reference lists of the selected trials and relevant reviews. We identified 16 publications reporting on 15 distinct trials that satisfied our selection criteria (38–53) (Figure 1, Table 1). One author (HH) extracted study characteristics and outcomes from the trial reports and entered the data in Supplementary Tables S1, S2 and in a spreadsheet, which are available in Supplementary Files 1, 2. All authors checked the data entered against the original trial reports and all authors assessed the methodological quality of the trials (Figure 2, Supplementary Table S1). We contacted several authors to ask for details of their trials, but only three authors responded; see Supplementary Table S1.
The primary outcome in this analysis was the change in LVEF after the initiation of vitamin C administration. For most trials, we used variance analysis to calculate the P-value for the interaction between time and vitamin C treatment; see Supplementary File 1 for the formula to calculate the F-test from the reported mean and standard deviation (SD) values of LVEF, and Supplementary File 2 for the calculations. Thereafter we used the P-values to calculate comparable standard error (SE) values for the differences between the vitamin C and control observations. For the Jensen trial, we measured the LVEF changes from the 6-month time point of their figure 4A (38); see Supplementary File 2.
As secondary outcomes, we collected other measures of cardiac function from the included trial reports. The findings for the secondary outcomes are described in the Results section, but we did not construct forest plots for any of them because of their heterogeneity and low number.
In our analysis of the changes in LVEF, we used the relative scale, which has been shown to be superior in the analysis of continuous outcomes, because it adjusts for baseline variations and frequently leads to less heterogeneity (54–56). An illustration of the measurement of the vitamin C effect on LVEF is shown in Figure 3 by using the Basili trial (43) as an example. Our analysis of the changes in LVEF is closely analogous to a previous analysis of changes in FEV1 (54). In both cases the original observations are expressed as a percentage change, and the relative change caused by the treatment is calculated as a percentage effect of the percentages (Figure 3). We pooled the included trials with the metagen function of the R package meta (90–92), using the inverse variance, fixed effect options. For the meta-regression of the vitamin C effect on baseline LVEF, we used the metareg function of the meta package.
Although the Cochran Q test has been criticized (93), in the absence of a suitable alternative we used it to assess statistical heterogeneity among the trials in the meta-analysis, but we did not calculate the I2 statistic. Our calculations are described in Supplementary Files 1, 2.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author Contributions
HH planned the study, extracted the data and entered the data into a spreadsheet, assessed the quality of the included trials, carried out the statistical analysis, and wrote the draft manuscript. EC and AM checked that the entered data were consistent with the published data, independently assessed the quality of the included trials, and participated in the critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.789729/full#supplementary-material
Detailed descriptions of the included trials and statistical calculations.
Spreadsheet with the calculations of the results.
References
- 1.Lind J. The diagnostics, or signs (Chap. II). In: A Treatise of the Scurvy in Three Parts. Edinburgh: Kincaid A, Donaldson A. (1753). 10.1017/CBO9781107256644 Available online at: https://archive.org/details/b30507054/page/n6/mode/2up (accessed January 8, 2022). [DOI] [Google Scholar]
- 2.Hess AF. Pathology; Symptomatology and diagnosis. In: Scurvy: Past and Present. Philadelphia, PA, USA: Lippincott. (1920). p. 81–110, 176–224. Available online at: https://chla.library.cornell.edu; https://archive.org/details/b29823778/page/n4/mode/2up [Google Scholar]
- 3.Crandon JH, Lund CC, Dill DB. Experimental human scurvy. N Engl J Med. (1940) 223:353–69. 10.1056/NEJM194009052231001 [DOI] [Google Scholar]
- 4.Vitamin C. Subcommittee. Vitamin C requirement of human adults; experimental study of vitamin-C deprivation in man. Lancet. (1948) 251:853–58. 10.1016/S0140-6736(48)90572-8 [DOI] [PubMed] [Google Scholar]
- 5.Hodges RE, Baker EM, Hood J, Sauberlich HE, March SC. Experimental scurvy in man. Am J Clin Nutr. (1969) 22:535–48. 10.1093/ajcn/22.5.535 [DOI] [PubMed] [Google Scholar]
- 6.Hodges RE, Hood J, Canham JE, Sauberlich HE, Baker EM. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. (1971) 24:432–43. 10.1093/ajcn/24.4.432 [DOI] [PubMed] [Google Scholar]
- 7.Abboud FM, Hood J, Hodges RE, Mayer HE. Autonomic reflexes and vascular reactivity in experimental scurvy in man. J Clin Invest. (1970) 49:298–307. 10.1172/JCI106239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. (1996) 93:3704–9. 10.1073/pnas.93.8.3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh D, Chan W. Cardiomegaly and generalized oedema due to vitamin C deficiency. Singapore Med J. (1974) 15:60–3. Available online at: http://smj.sma.org.sg/1501/1501smj11.pdf [PubMed] [Google Scholar]
- 10.Meisel JL, McDowell RK. Case 39-1995: a 72-year-old man with exertional dyspnea, fatigue, and extensive ecchymoses and purpuric lesions. N Engl J Med. (1995) 333:1695–702. 10.1056/NEJM199512213332508 [DOI] [PubMed] [Google Scholar]
- 11.Kupari M, Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest. (2012) 142:225–7. 10.1378/chest.11-1857 [DOI] [PubMed] [Google Scholar]
- 12.Zipursky JS, Alhashemi A, Juurlink D. A rare presentation of an ancient disease: scurvy presenting as orthostatic hypotension. BMJ Case Rep. (2014) 2014:bcr2013201982. 10.1136/bcr-2013-201982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas F, Ha LD, Sterns R, von Doenhoff L. Reversible right heart failure in scurvy: rediscovery of an old observation. Circ Heart Fail. (2016) 9:e003497. 10.1161/CIRCHEARTFAILURE.116.003497 [DOI] [PubMed] [Google Scholar]
- 14.Bennett SE, Schmitt WP, Stanford FC, Baron JM. Case 22-2018: a 64-year-old man with progressive leg weakness, recurrent falls, and anemia. N Engl J Med. (2018) 379:282–9. 10.1056/NEJMcpc1802826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gayen SK, Abdelrahman AA, Preston IR, Petit RD, Hill NS. Vitamin C deficiency-induced pulmonary arterial hypertension. Chest. (2020) 157:e21–3. 10.1016/j.chest.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 16.Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic Biol Med. (2004) 37:1419–29. 10.1016/j.freeradbiomed.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Bae S, Kim Y, Cho CH, Kim SJ, Kim YJ, et al. Vitamin C prevents stress-induced damage on the heart caused by the death of cardiomyocytes, through down-regulation of the excessive production of catecholamine, TNF-α, and ROS production in Gulo(-/-)Vit C-Insufficient mice. Free Radic Biol Med. (2013) 65:573–83. 10.1016/j.freeradbiomed.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 18.Tsai MS, Huang CH, Tsai CY, Chen HW, Cheng HJ, Hsu CY, et al. Combination of intravenous ascorbic acid administration and hypothermia after resuscitation improves myocardial function and survival in a ventricular fibrillation cardiac arrest model in the rat. Acad Emerg Med. (2014) 21:257–65. 10.1111/acem.12335 [DOI] [PubMed] [Google Scholar]
- 19.El-Shitany NA, El-Desoky K. Protective effects of carvedilol and vitamin C against azithromycin-induced cardiotoxicity in rats via decreasing ROS, IL1-β, and TNF-α production and inhibiting NF-κB and caspase-3 expression. Oxid Med Cell Longev. (2016) 2016:1874762. 10.1155/2016/1874762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akolkar G, da Silva Dias D, Ayyappan P, Bagchi AK, Jassal DS, Salemi VMC, et al. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. (2017) 313:H795–809. 10.1152/ajpheart.00253.2017 [DOI] [PubMed] [Google Scholar]
- 21.Cheng C, Li H, Liang L, Jin T, Zhang G, Bradley JL, et al. Effects of ω-3 PUFA and ascorbic acid combination on post-resuscitation myocardial function. Biomed Pharmacother. (2021) 133:110970. 10.1016/j.biopha.2020.110970 [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA. Ascorbic acid: what do we really NO? J Appl Physiol. (2011) 111:1542–3. 10.1152/japplphysiol.01187.2011 [DOI] [PubMed] [Google Scholar]
- 23.Padayatty SJ, Levine M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. (2016) 22:463–93. 10.1111/odi.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. (2009) 157:1097–110. 10.1111/j.1476-5381.2009.00282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. (2013) 19:2068–83. 10.1089/ars.2013.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young JI, Züchner S, Wang G. Regulation of the epigenome by vitamin C. Annu Rev Nutr. (2015) 35:545–64. 10.1146/annurev-nutr-071714-034228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juraschek SP, Guallar E, Appel LJ, Miller ER. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. (2012) 95:1079–88. 10.3945/ajcn.111.027995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemilä H, Suonsyrjä T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2017) 17:49. 10.1186/s12872-017-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. (1998) 97:363–8. 10.1161/01.CIR.97.4.363 [DOI] [PubMed] [Google Scholar]
- 30.Ellis GR, Anderson RA, Chirkov YY, Morris-Thurgood J, Jackson SK, Lewis MJ, et al. Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J Cardiovasc Pharmacol. (2001) 37:564–70. 10.1097/00005344-200105000-00008 [DOI] [PubMed] [Google Scholar]
- 31.Erbs S, Gielen S, Linke A, Möbius-Winkler S, Adams V, Baither Y, et al. Improvement of peripheral endothelial dysfunction by acute vitamin C application: different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J. (2003) 146:280–5. 10.1016/S0002-8703(03)00184-4 [DOI] [PubMed] [Google Scholar]
- 32.Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. (2012) 235:9–20. 10.1016/j.atherosclerosis.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 33.Nightingale AK, Blackman DJ, Field R, Glover NJ, Pegge N, Mumford C, et al. Role of nitric oxide and oxidative stress in baroreceptor dysfunction in patients with chronic heart failure. Clin Sci (Lond). (2003) 104:529–35. 10.1042/CS20020334 [DOI] [PubMed] [Google Scholar]
- 34.Piccirillo G, Nocco M, Moisè A, Lionetti M, Naso C, di Carlo S, et al. Influence of vitamin C on baroreflex sensitivity in chronic heart failure [dose correction to grams in 2003;42:e5]. Hypertension. (2003) 41:1240–5. 10.1161/01.HYP.0000073581.74107.22 [DOI] [PubMed] [Google Scholar]
- 35.Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol. (2004) 286:H2113–7. 10.1152/ajpheart.01054.2003 [DOI] [PubMed] [Google Scholar]
- 36.Bruno RM, Daghini E, Ghiadoni L, Sudano I, Rugani I, Varanini M, et al. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. Am J Clin Nutr. (2012) 96:302–8. 10.3945/ajcn.112.035022 [DOI] [PubMed] [Google Scholar]
- 37.Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. (2018) 22:70. 10.1186/s13054-018-1996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen PD, Olsen N, Bagger JP, Jensen FT, Christensen T, Ellegaard J. Cardiac function during iron chelation therapy in adult non-thalassaemic patients with transfusional iron overload. Eur J Haematol. (1997) 59:221–30. 10.1111/j.1600-0609.1997.tb00981.x [DOI] [PubMed] [Google Scholar]
- 39.Guan W, Osanai T, Kamada T, Ishizaka H, Hanada H, Okumura K. Time course of free radical production after primary coronary angioplasty for acute myocardial infarction and the effect of vitamin C. Jpn Circ J. (1999) 63:924–8. 10.1253/jcj.63.924 [DOI] [PubMed] [Google Scholar]
- 40.Oktar GL, Sinci V, Kalaycioglu S, Soncul H, Gökgöz L, Halit V, et al. Biochemical and hemodynamic effects of ascorbic acid and alpha-tocopherol in coronary artery surgery. Scand J Clin Lab Invest. (2001) 61:621–9. 10.1080/003655101753267982 [DOI] [PubMed] [Google Scholar]
- 41.Ho CC. Effects of antioxidant on cardiovascular performance, exercise capacity, and functional status in patients with chronic heart failure (PhD thesis). Case Western Reserve University, Cleveland, Ohio: (2007). Available online at: https://rave.ohiolink.edu/etdc/view?acc_num=case1157980994 [Google Scholar]
- 42.Glavas D, Bakovic B, Obad A, Palada I, Breskovic T, Valic Z, et al. Effects of tetrahydrobiopterin on venous bubble grade and acute diving-induced changes in cardiovascular function. Clin Physiol Funct Imaging. (2009) 29:100–7. 10.1111/j.1475-097X.2008.00845.x [DOI] [PubMed] [Google Scholar]
- 43.Basili S, Tanzilli G, Mangieri E, Raparelli V, Di Santo S, Pignatelli P, et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovasc Interv. (2010) 3:221–9. 10.1016/j.jcin.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 44.Pignatelli P, Tanzilli G, Carnevale R, Di Santo S, Loffredo L, Celestini A, et al. Ascorbic acid infusion blunts CD40L upregulation in patients undergoing coronary stent. Cardiovasc Ther. (2011) 29:385–94. 10.1111/j.1755-5922.2010.00168.x [DOI] [PubMed] [Google Scholar]
- 45.Fernhall B, Fahs C, Agiovlasitis S, Ranadive S, Yan H, Rossow L, et al. Effect of vitamin C on cardiac performance following live firefighting [Abstract]. Circulation. (2010) 122:A11915. Available online at: https://www.ahajournals.org/doi/10.1161/circ.122.suppl_21.A11015 [Google Scholar]
- 46.Fahs CA, Yan H, Ranadive S, Rossow LM, Agiovlasitis S, Echols G, et al. Acute effects of firefighting on arterial stiffness and blood flow. Vasc Med. (2011) 16:113–8. 10.1177/1358863X11404940 [DOI] [PubMed] [Google Scholar]
- 47.Fernhall B, Fahs CA, Horn G, Rowland T, Smith D. Acute effects of firefighting on cardiac performance. Eur J Appl Physiol. (2012) 112:735–41. 10.1007/s00421-011-2033-x [DOI] [PubMed] [Google Scholar]
- 48.Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. (2012) 112:483–92. 10.1007/s00421-011-1997-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabri MR, Tavana EN, Ahmadi A, Hashemipour M. The effect of vitamin C on endothelial function of children with type 1 diabetes: an experimental study. Int J Prev Med. (2014) 5:999–1004. [PMC free article] [PubMed] [Google Scholar]
- 50.Sabri M, Ghaffari G, Hashemipour M, Mostofizadeh N, Koushki AM. Effect of long-term vitamin C intake on vascular endothelial function in diabetic children and adolescents: a pilot study. J Res Med Sci. (2016) 21:119. 10.4103/1735-1995.193510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safaei N, Babaei H, Azarfarin R, Jodati AR, Yaghoubi A, Sheikhalizadeh MA. Comparative effect of grape seed extract (Vitis vinifera) and ascorbic acid in oxidative stress induced by on-pump coronary artery bypass surgery. Ann Card Anaesth. (2017) 20:45–51. 10.4103/0971-9784.197834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scalzo RL, Bauer TA, Harrall K, Moreau K, Ozemek C, Herlache L, et al. Acute vitamin C improves cardiac function, not exercise capacity, in adults with type 2 diabetes. Diabetol Metab Syndr. (2018) 10:7. 10.1186/s13098-018-0306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emadi N, Nemati MH, Ghorbani M, Allahyari E. The effect of high-dose vitamin C on biochemical markers of myocardial injury in coronary artery bypass surgery. Braz J Cardiovasc Surg. (2019) 34:517–24. 10.21470/1678-9741-2018-0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemilä H, Friedrich JO. Many continuous variables should be analyzed using the relative scale: a case study of β[[sb]]2[[/s]]-agonists for preventing exercise-induced bronchoconstriction. Syst Rev. (2019) 8:282. 10.1186/s13643-019-1183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol. (2011) 64:556–64. 10.1016/j.jclinepi.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 56.Hemilä H. Duration of the common cold and similar continuous outcomes should be analyzed on the relative scale: a case study of two zinc lozenge trials. BMC Med Res Methodol. (2017) 17:82. 10.1186/s12874-017-0356-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. (1998) 31:1352–6. 10.1016/S0735-1097(98)00101-6 [DOI] [PubMed] [Google Scholar]
- 58.de Lorgeril M, Salen P, Accominotti M, Cadau M, Steghens JP, Boucher F, et al. Dietary and blood antioxidants in patients with chronic heart failure: insights into the potential importance of selenium in heart failure. Eur J Heart Fail. (2001) 3:661–9. 10.1016/S1388-9842(01)00179-9 [DOI] [PubMed] [Google Scholar]
- 59.Polidori MC, Praticó D, Savino K, Rokach J, Stahl W, Mecocci P. Increased F[[sb]]2[[/s]] isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. (2004) 10:334–8. 10.1016/j.cardfail.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 60.Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature. J Am Coll Cardiol. (2017) 70:212–29. 10.1016/j.jacc.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbasy MA. The diuretic action of vitamin C. Biochem J. (1937) 31:339–42. 10.1042/bj0310339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans W. Vitamin C in heart failure. Lancet. (1938) 231:308–9. 10.1016/S0140-6736(00)62412-1 [DOI] [Google Scholar]
- 63.Editorial . Ascorbic acid as a diuretic. Lancet. (1944) 244:6310. 10.1016/S0140-6736(00)42799-6 [DOI] [Google Scholar]
- 64.Shaffer CF. The diuretic effect of ascorbic acid. JAMA. (1944) 124:700–1. 10.1001/jama.1944.0285011002400625996397 [DOI] [Google Scholar]
- 65.Hemilä H, Chalker E. Bias against vitamin C in mainstream medicine: examples from trials of vitamin C for infections. Life. (2022) 12:62. 10.3390/life12010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietri S, Séguin JR, d'Arbigny PD, Culcasi M. Ascorbyl free radical: a noninvasive marker of oxidative stress in human open-heart surgery. Free Radic Biol Med. (1994) 16:523–8. 10.1016/0891-5849(94)90131-7 [DOI] [PubMed] [Google Scholar]
- 67.Sharma MK, Buettner GR, Spencer KT, Kerber RE. Ascorbyl free radical as a real-time marker of free radical generation in briefly ischemic and reperfused hearts: an electron paramagnetic resonance study [correction: 1994;75:601]. Circ Res. (1994) 74:650–8. 10.1161/01.RES.74.4.650 [DOI] [PubMed] [Google Scholar]
- 68.Ballmer PE, Reinhart WH, Jordan P, Buhler E, Moser UK, Gey KF. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations [correction: 1995;110:1972]. J Thorac Cardiovasc Surg. (1994) 108:311–20. 10.1016/S0022-5223(94)70013-3 [DOI] [PubMed] [Google Scholar]
- 69.Haramaki N, Stewart DB, Aggarwal S, Ikeda H, Reznick AZ, Packer L. Networking antioxidants in the isolated rat heart are selectively depleted by ischemia-reperfusion. Free Radic Biol Med. (1998) 25:329–39. 10.1016/S0891-5849(98)00066-5 [DOI] [PubMed] [Google Scholar]
- 70.Lahet JJ, Courderot-Masuyer C, Lenfant F, Tatou E, Vergely C, David M, et al. The influence of extracorporeal circulation on the susceptibility of erythrocytes to oxidative stress. Free Radic Res. (2004) 38:683–9. 10.1080/10715760410001702512 [DOI] [PubMed] [Google Scholar]
- 71.Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, et al. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. (2005) 38:1323–32. 10.1016/j.freeradbiomed.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 72.Rodemeister S, Duquesne M, Adolph M, Nohr D, Biesalski HK, Unertl K. Massive and long-lasting decrease in vitamin C plasma levels as a consequence of extracorporeal circulation. Nutrition. (2014) 30:673–8. 10.1016/j.nut.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 73.Hemilä H, Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care. (2020) 8:15. 10.1186/s40560-020-0432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemilä H. Vitamin C may alleviate exercise-induced bronchoconstriction: a meta-analysis. BMJ Open. (2013) 3:e002416. 10.1136/bmjopen-2012-002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hemilä H, Carr A, Chalker E. Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: reanalysis of the COVID A to Z randomized clinical trial. Front Immunol. (2021) 12:674681. 10.3389/fimmu.2021.674681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozemek C, Hildreth KL, Groves DW, Moreau KL. Acute ascorbic acid infusion increases left ventricular diastolic function in postmenopausal women. Maturitas. (2016) 92:154–61. 10.1016/j.maturitas.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mak S, Overgaard CB, Newton GE. Effect of vitamin C and L-NMMA on the inotropic response to dobutamine in patients with heart failure. Am J Physiol Heart Circ Physiol. (2005) 289:H2424–8. 10.1152/ajpheart.00453.2005 [DOI] [PubMed] [Google Scholar]
- 78.Shinke T, Shite J, Takaoka H, Hata K, Inoue N, Yoshikawa R, et al. Vitamin C restores the contractile response to dobutamine and improves myocardial efficiency in patients with heart failure after anterior myocardial infarction. Am Heart J. (2007) 154:645.e1-e8. 10.1016/j.ahj.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 79.Schmitt M, Nightingale AK, Ellis GR, Morris-Thurgood J, Broadley A, Cockcroft JR, et al. Vitamin C acutely improves ejection duration in chronic heart failure (CHF) patients in a heart rate independent fashion [abstract]. Eur J Heart Failure. (2000) 2:96. 10.1016/S1388-9842(00)80341-4 [DOI] [Google Scholar]
- 80.Song EK, Kang SM. Vitamin C deficiency, high-sensitivity C-reactive protein, and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. (2018) 33:6–12. 10.1097/JCN.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 81.Wu JR, Song EK, Moser DK, Lennie TA. Dietary vitamin C deficiency is associated with health-related quality of life and cardiac event-free survival in adults with heart failure. J Cardiovasc Nurs. (2019) 34:29–35. 10.1097/JCN.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. (2019) 11:708. 10.3390/nu11040708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. (2019) 322:1261–70. 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemilä H, Chalker E. Reanalysis of the effect of vitamin C on mortality in the CITRIS-ALI trial: important findings dismissed in the trial report. Front Med. (2020) 7:590853. 10.3389/fmed.2020.590853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. (2008) 300:2123–33. 10.1001/jama.2008.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hemilä H. The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: a review and statistical analysis. Allergy Asthma Clin Immunol. (2014) 10:58. 10.1186/1710-1492-10-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemilä H. Vitamin C and common cold incidence: a review of studies with subjects under heavy physical stress. Int J Sports Med. (1996) 17:379–83. 10.1055/s-2007-972864 [DOI] [PubMed] [Google Scholar]
- 88.Hemilä H. Vitamin C supplementation and respiratory infections: a systematic review. Mil Med. (2004) 169:920–5. 10.7205/MILMED.169.11.920 [DOI] [PubMed] [Google Scholar]
- 89.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. (2013) 2013:CD000980. 10.1002/14651858.CD000980.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.R Core Team . R Project for Statistical Computing. Available online at: https://www.r-project.org (accessed January 8, 2022).
- 91.Schwarzer G, Carpenter JR, Rucker G. Meta-analysis with R. London: Springer; (2015). 10.1007/978-3-319-21416-0 [DOI] [Google Scholar]
- 92.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoaglin DC. Misunderstandings about Q and Cochran's Q test in meta-analysis. Stat Med. (2016) 35:485–95. 10.1002/sim.6632 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed descriptions of the included trials and statistical calculations.
Spreadsheet with the calculations of the results.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.