Abstract
Despite numerous advances in all areas of cardiovascular care, cardiovascular disease (CVD) is the leading cause of death in the United States (US). There is compelling evidence that interventions to improve diet are effective in cardiovascular disease prevention. This clinical practice statement emphasizes the importance of evidence-based dietary patterns in the prevention of atherosclerotic cardiovascular disease (ASCVD), and ASCVD risk factors, including hyperlipidemia, hypertension, diabetes, and obesity. A diet consisting predominantly of fruits, vegetables, legumes, nuts, seeds, plant protein and fatty fish is optimal for the prevention of ASCVD. Consuming more of these foods, while reducing consumption of foods with saturated fat, dietary cholesterol, salt, refined grain, and ultra-processed food intake are the common components of a healthful dietary pattern. Dietary recommendations for special populations including pediatrics, older persons, and nutrition and social determinants of health for ASCVD prevention are discussed.
Keywords: Nutrition, Cardiovascular disease prevention, Cardiovascular disease, Clinical trials, Social determinants of health
Acronyms: ACC, American college of cardiology; AHA, American heart association; ASCVD, atherosclerotic cardiovascular disease; CER, continuous energy restriction; CHD, coronary heart disease; CVD, cardiovascular disease; DASH, dietary approaches to stop hypertension; DHA, docosahexaenoic acid; EPA, eicosapentanoic acid; EVOO, extra virgin olive oil; HDL-C, high density lipoprotein cholesterol; HR, hazards ratio; IER, intermittent energy restriction; LD, low density lipoprotein cholesterol; MUFA, monounsaturated fatty acids; NHANES, national health and nutrition examination survey; OMT, optimal medical therapy; PUFA, polyunsaturated fatty acids; PURE, prospective urban rural epidemiology; RCT, randomized controlled trial; SFA, saturated fatty acids; TER, timed energy restriction; T2D, type 2 diabetes; US, United States; VD, vegetarian diet
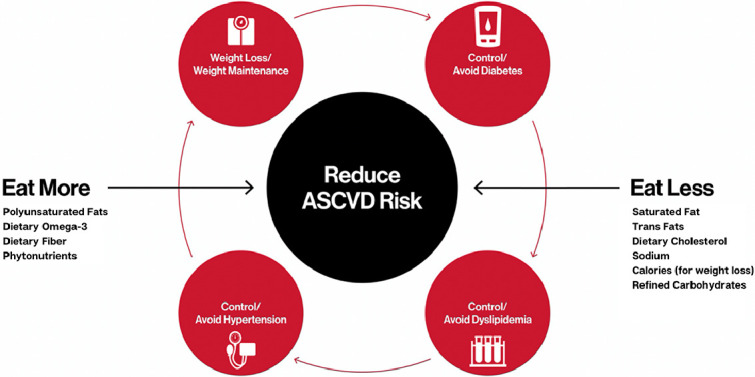
Central Figure
LEGEND: ASCVD= atherosclerotic cardiovascular disease
1. Background
Cardiovascular disease (CVD) is the leading cause of death in the United States (US), accounting for 868,662 deaths in 2018, with substantial health care costs, reaching 363.4 billion in 2016–2017 [1]. Concerningly, over the past decade, there has been plateau in the rate of decline in CV mortality [1]. Despite numerous advances in all areas of cardiovascular care, heart disease remained the leading cause of death, even during the global viral SARS-CoV-2 pandemic [2]. Accordingly, recent gains in control of risk factors such as smoking and lipids have been outpaced by discouraging trends in obesity, diabetes and hypertension. Estimates for US adults with obesity (body mass index ≥ 30 kg/m2) increased from 30.5% in 1999–2000 to 42.4% in 2017–2018 [3]. Type 2 diabetes prevalence increased over this time period as well, reaching 26 million (9.8%) US adults diagnosed, 9.4 million (3.7%) undiagnosed, and 91.8 million (37.6%) classified as having prediabetes in 2013–2016. Additionally, hypertension has increased from 31.8% in 1999–2000, peaking at 53.8% in 2013–2014, with a subsequent decline to 43.7% in 2017–2018 [4]. Underlying these trends are stark disparities in CVD incidence, mortality, and many of its risk factors, where those of minority race/ethnicity and those with disadvantaged socioeconomic status are disproportionately burdened [1,3,5].
Adverse lifestyle, including poor quality diet, are established risk factors for CVD, and collectively contribute substantially to the cumulative morbidity and life years lost from CVD [6]. Based on the 2013–2016 National health and nutrition examination survey (NHANES) data, less than 10% of US adults meet the USDA dietary guidelines for daily consumption of whole grains (≥3 servings per day), whole fruits (≥2 cups per day) and non-starchy vegetables (≥2.5 cups per day) [1]. Unfortunately, the current food environment stacks the deck against patients making and sustaining improvements in diet quality, and various social and economic factors may strongly affect the ability of individual patients to effectively make such lifestyle changes. Nevertheless, there is compelling evidence that interventions to improve diet quality and other lifestyle strategies are effective in cardiovascular prevention. For example, the Dietary Approaches to Stop Hypertension (DASH) -Sodium randomized feeding trial demonstrated the efficacy of dose-response reduction in dietary sodium intake in the context of an overall healthy diet for lowering blood pressure in adults with and without baseline hypertension [7]. Meanwhile, poorer quality diets such as the Southern dietary pattern – which is high in saturated fats, refined carbohydrates and sodium, and low in potassium - underlies more prevalent hypertension in Black adults compared to White adults and also associated with coronary heart disease (CHD) [8].
The Diabetes Prevention Program trial randomized participants with prediabetes to an intensive physical activity and diet weight loss program vs. no intervention; lifestyle improvement reduced progression to type 2 diabetes by 58% over 2.8 years follow-up [9]. Further, a randomized trial among high risk Spanish older adults tested the effects of Mediterranean vs. low-fat control diet advice and observed a 30% reduction in atherosclerotic cardiovascular disease (ASCVD) risk [10]. Mechanisms for individual diet and lifestyle factors, or their collective influence, on ASCVD risk are complex, including but not limited to improvements in lipids, blood pressure, carbohydrate metabolism, body weight and composition, inflammation, oxidative stress, and endothelial function. Although it is often difficult to disentangle the precise downstream mechanisms by which behaviors and environmental exposures impact the development and progression of ASCVD, this does not preclude the need for clinical recommendations and guidelines for adopting optimal lifestyle factors for lifelong ASCVD prevention [11].
The purpose of this clinical practice statement is to provide an overview of the nutritional approaches to ASCVD prevention. This paper includes specific approaches targeting individual risk factors, consideration for distinct approaches in children, and addresses potential patient barriers to adherence related to cultural differences and social determinants of health.
2. Primary and Secondary Prevention of ASCVD Using Dietary Approaches
At a population level, 90% of the global CV risk is attributable to nine modifiable factors: abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, low consumption of fruits, vegetables, and alcohol, and regular physical activity) [9]. Thus, a critical approach to prevent ASCVD is maintenance of a healthy lifestyle throughout the life course [12]. The INTERHEART study identified low fruit/vegetable consumption as one of the nine major modifiable factors associated with myocardial infarction (MI) [13]. Similarly, the Prospective Urban Rural Epidemiology (PURE) study observed that adults with higher consumption of fruits, vegetables, and legumes had a lower risk of all-cause mortality [14].
In 2019, the American college of cardiology (ACC) and American heart association (AHA) Guideline on the primary prevention of CVD, as well the 2021 European society of cardiology guidelines on cardiovascular disease prevention in clinical practice, emphasized the consumption of a plant predominant diet with a class I recommendation [15,16]. Specifically, the guidelines also advised minimizing processed meats, refined carbohydrates and sweetened beverages, reducing the amount of dietary cholesterol and sodium, replacing saturated fat with monounsaturated and polyunsaturated fats, and avoiding trans fats [16]. The ACC/AHA recommendations can be met with a Mediterranean, DASH, healthy vegetarian, and exclusively plant-based diets, as discussed further below [17,18]. Furthermore, the 2021 Scientific Statement from the AHA on dietary guidance to improve cardiovascular health was consistent with previous recommendations emphasizing the importance of a plant predominant dietary pattern, encouraging healthy sources of protein and added a recommendation to choose minimally processed foods instead of ultra-processed foods [19]. For both primary and secondary prevention, similar dietary patterns are recommended for improving cardiovascular health.
2.1. Mediterranean Diet
The Mediterranean diet is inspired by the traditional eating habits of countries such as Spain, Italy, and Southern Greece that border the Mediterranean Sea. It emphasizes leafy green vegetables, fruits, nuts, legumes, whole grains, extra virgin olive oil (EVOO), fish and seafood, with only moderate consumption of poultry, eggs, and dairy, and limited intake of red meats and sweets. It has demonstrated efficacy for both primary and secondary CVD prevention, with a reduced risk of both cardiovascular and all-cause mortality [10,20–22].
The Seven Countries Study was among the first to suggest cardiometabolic benefits of the Mediterranean diet. The study recruited men aged 40–59 years from Yugoslavia, Italy, Greece, Finland, Netherlands, Japan, and United States, and found countries with a Mediterranean diet style-pattern reported a lower incidence of CHD and the highest survival rates compared with non-Mediterranean regions [23]. This was bolstered by another large cohort study of 22,043 adults in Greece who completed a validated, food frequency questionnaire. Over an average 44 months of follow-up, a greater adherence to a traditional Mediterranean diet (per 2-point increment in Mediterranean diet score) was associated with a 25% reduction in all-cause mortality [Hazards Ratio (HR) 0.75 (0.64–0.87)], 33% reduction in CHD death [HR 0.67 (0.47–0.94)], and also a 24% reduction in cancer death [HR 0.76 (0.59–0.98)] [20]. In a secondary prevention cohort of 15,482 patients with stable CHD followed for an average of 3.7 years, higher adherence to a Mediterranean-style diet (per 1 unit increase in score >12) was independently associated with a 5% reduction in cardiovascular events in a multivariable-adjusted analysis [HR 0.95 (0.91–0.98)] [22].
In addition to the aforementioned observational studies, there are also randomized controlled trial (RCT) data supporting the favorable benefits of the Mediterranean diet for CVD prevention. The Lyon Diet Heart Study, was an RCT among a secondary prevention population that evaluated whether the Mediterranean diet reduced the rates of recurrence after a first MI compared to a “prudent diet” [21]. Over a 4 year period, post-MI patients who were randomized to the Mediterranean diet experienced a 72% reduction in recurrent non-fatal MI and a 56% decline in mortality compared to the control diet [21].
The Prevención con Dieta Mediterránea (PREDIMED) study was a primary prevention RCT that enrolled 7447 participants at high cardiovascular risk, but with no CVD at enrollment [10]. Participants were randomized to one of three diets, a Mediterranean diet enriched with EVOO, a Mediterranean diet enriched with nuts, or a low fat diet and were followed for 4.8 years. Compared to the low-fat diet, both Mediterranean diet patterns were associated with a reduced risk of composite cardiovascular endpoint (MI, stroke, or CV death), with HR 0.69 (0.53–0.91) for the Mediterranean diet+EVOO and HR 0.72 (0.54–0.95) for Mediterranean diet+nuts, predominantly driven by the reduction in stroke [10].
2.2. The DASH Diet
The DASH diet consists of fruits, vegetables, lean protein, low-fat dairy, and limits intake of sodium and saturated fats. It was original proposed in the 1990’s by the National Institutes of Health as an intervention to treat hypertension, and was subsequently shown to reduce blood pressure among patients with and without hypertension. (See section on Hypertension below for the evidence relating to the DASH diet and its role on blood pressure).
In observational data, the DASH dietary pattern has also been associated with decreased incidence of diabetes, CVD, and heart failure [24,25]. In a recent RCT of 92 patients with non-obstructive coronary artery disease identified by computed tomography angiography, participants randomized to DASH diet plus optimal medical therapy (OMT) experienced greater reduction in non-calcified plaque and less atherosclerosis progression than those randomized to OMT alone [26].
2.3. Vegetarian and Plant‐Based Diets
A healthy vegetarian diet also emphasizes intake of vegetables, fruits, whole grains, legumes, nuts, and seeds, but substitutes meat, seafood, and poultry with soy products or other plant-based protein sources. Some vegetarian diets include dairy and/or eggs, while an exclusively plant-based diet (PBD) eliminates all animal-based products. A meta-analysis of 8 observational studies including 131,869 adults concluded that a vegetarian diet was associated with a 30% reduction in ischemic heart disease mortality [RR 0.70 (0.55–0.89)] compared to non-vegetarian diet [27]. However, not all vegetarian diets are necessarily beneficial and depend on what foods are chosen to replace traditional protein sources (meat, poultry, and seafood).
In a large meta-analysis of 209,298 people eating PBD, Satija et al compared diets made up of healthier foods (fruits, vegetables, nuts, legumes, whole grains, and vegetable oils) versus less healthy foods (juices, sugar-sweetened beverages, refined grains, potatoes, and sweets) [28]. Higher adherence to a more healthful PBD was associated with a 25% reduction in incident CHD [HR 0.75 (0.68–0.83)]; conversely, PBD consisting more of the unhealthy foods was associated with a 32% increased CHD risk [HR 1.32 (1.20–1.46)] [28]. In a cross-over RCT, a low-fat PBD lead to a greater calorie deficit and subsequently improved body weight, lipids, and insulin sensitivity more than a Mediterranean diet, although Mediterranean had slightly greater blood pressure reduction [29].
2.4. Low Carbohydrate and Ketogenic Diets
Low carbohydrate diets are defined as having net dietary carbohydrates contribute ∼10–25% of total energy intake, whereas very low carbohydrate, ketogenic diets restrict net carbohydrate consumption to ≤10% of energy intake and may also limit protein consumption because amino acids stimulate insulin secretion and gluconeogenesis [30]. Within these macronutrient constraints, there are a wide variety of possible dietary patterns such as different relative contributions of plant versus animal products, non-starchy vegetables, nuts, low carbohydrate fruit, and degree of processing. Such dietary patterns are advocated for both potential weight loss and improved diabetes control versus higher carbohydrate dietary patterns [31]. Nevertheless, longitudinal cohort data indicate that the healthfulness of relatively lower carbohydrate intake with long-term CVD risk depended on the plant vs animal sources of non-carbohydrate calories. In other words, diets generally higher in vegetable sources of protein and fat were related to lower CVD risk while diets higher in animal sources were associated with higher risk [[32], [33], [34], [35]]. Furthermore, some studies raise concerns about the safety of an animal-based ketogenic diet [30,36] including occasional marked increases in LDL-C [30,37]. In addition, lower carbohydrate dietary patterns may limit the consumption of healthful foods, such as fruits, unrefined whole grains, and legumes [30,36,38]. While a high-fiber plant-predominant low carbohydrate dietary pattern with a low saturated fat content may hypothetically be of benefit, further investigation is needed. Thus, we do not suggest encouraging patients to adopt a high saturated fat, animal-based ketogenic diet-pattern for primary prevention of CVD.
2.5. Intermittent Energy Restriction
Intermittent energy restriction (IER), includes intermittent fasting (i.e. fasting for 16–48 h) and time restricted eating (TRE) (i.e. restricting feeding to specific windows of time each day, such as for 8–10 or fewer hours) [39]. IER has been advocated as a weight loss/maintenance strategy and to improve cardiometabolic health. These feeding strategies may be useful for the primary prevention of CVD. However, while supported by promising animal data and preclinical human data, IER has largely shown no to minimal benefit above continuous energy restriction (CER) for weight loss and for cardiometabolic health in humans, although poor compliance in long-term trials undermines quality and interpretation of prior studies [[39], [40], [41], [42]].
In an analysis of eleven randomized studies comparing intermittent fasting to CER, while weight loss was seen in both groups, no difference in weight loss and minimal to no differences in a variety of cardiometabolic parameters were found between the groups [39]. In an RCT of TRE vs CER in 116 individuals, no difference in weight loss or cardiometabolic parameters were found [41]. However, in a smaller RCT with only 8 men, a 6-hour TRE period ending by 3pm versus a 12 h feeding period was associated with improved cardiometabolic health without a difference in weight loss [43].
While IER may be helpful for modest weight loss, IER does not appear to have clear incremental benefit above CER for either weight loss or for cardiometabolic health. Furthermore, we are not aware of IER studies with hard cardiovascular outcomes.
2.6. Challenges in Nutritional Studies for CVD Prevention
Although RCTs provide the best evidence base to shape guidelines, RCTs are difficult to do for dietary interventions as participants are not blinded, may not adhere to their assigned diet, and require long term follow-up for hard ASCVD outcomes. Most of the RCTs on dietary interventions have been short-term studies with surrogate endpoints such as blood pressure, lipids, and weight change, with a few exceptions as noted above for the Mediterranean diet. Thus, data from observational studies with long-term follow-up for ASCVD outcomes play an important role in informing health promotion and prevention recommendations. Nevertheless, a methodological challenges of observational studies include valid ascertainment of habitual long-term diet and confounding from social and other behavioral/lifestyle determinants of CVD, potentially biasing diet/outcome associations; [[44], [45], [46]] thus, recommendations should be based on the accumulation of evidence across a number of research domains, incorporating their consistency, quality, validity, and certainty of individual studies and the body of evidence overall.
3. Addressing ASCVD Risk Factors: Nutritional Approaches
3.1. Hyperlipidemia
The intake of major macronutrient groupings on serum lipid and lipoprotein levels have been substantially investigated in controlled, metabolic feeding trials in inpatients and in diet assignment trials in free-living settings [47,48]. Macronutrients are typically grouped as: (1) carbohydrate; (2) saturated fatty acids (SFAs); (3) cis monounsaturated fatty acids (MUFAs), rich in oleic acid; (4) cis polyunsaturated fatty acids (PUFAs), with high omega 6 linoleic acid and often accompanying omega 3 alpha-linolenic acid; (5) protein; (6) marine source omega 3s, rich in eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA). In addition to the macronutrients, a sizable body of randomized trial evidence evaluating the relationship of other food components, including dietary cholesterol, dietary fibers, phytosterols/stanols, and polyphenol subclasses (e.g. isoflavones), and lipids/lipoproteins exists.
Consistent evidence across numerous meta-analyses allow for pairwise macronutrient comparisons and the general conclusions [48] that: (1) SFA sources, predominantly feeding tropical oils like coconut oil, eggs, meats, and dairy fats, increase total, and LDL-C, relative to all other macronutrient classes; and (2) carbohydrates have relatively neutral effects on LDL-C, though effect modification by concomitant intakes of viscous fiber have relevant effects in decreasing LDL-C. MUFAs exhibit similar, or slightly greater, total and LDL-C lowering effects than carbohydrate. Linoleic-acid-rich PUFAs exhibit the largest reductions in total and LDL-C, with the greatest reduction in total and LDL-C resulting when these replace SFA sources. A regression analysis of 69 metabolic ward trials demonstrated a positive linear relationship between predicted and observed apoB based on saturated fat intake, a linear relationship between SFA intake apoB, and a consistent effect on serum lipids and lipoproteins across the entire range of SFA intakes observed [49]. Both MUFA and PUFA modestly lower triglycerides when replacing SFA, and to a greater extent when replacing carbohydrate. Thus, the net apoB lowering when carbohydrates replace SFA is modest, relative to more significant decreases observed when SFA is replaced by MUFA and PUFA.
Marine source omega 3 fatty acids found in fatty fish exhibit small effects on serum lipids when consumed at typical nutritional doses [50,51]. Dietary cholesterol's effect on LDL-C is smaller in magnitude relative to changes induced by fatty acid composition and are likely non-linear [52]. While the most robust and repeatable evidence of diet's effects on lipoproteins relate to the influence of macronutrient, other components of the food matrix, as well as their supplemental forms, can influence LDL-C. Phytosterols/stanols (∼2 g/day) can lower LDL-C significantly [53,54]. Modest effects of viscous soluble fibers on total and LDL-C have been consistently reported with larger effects reported for more viscous fiber types (e.g. konjac root; psyllium) [[55], [56], [57]]. Dietary polyphenols may lower LDL-C as well, though the impact of this heterogeneous class of chemicals has not been repeatedly investigated in controlled studies broadly; a body of evidence exists for soy, both enriched versus depleted for isoflavones, and soy protein replacement studies (most commonly replacing dairy/milk proteins), point towards modest, and heterogeneous, LDL-C lowering effects [[57], [58], [59]].
3.2. Hypertension
Despite pathophysiologic and therapeutic advances, hypertension remains a leading modifiable risk factor for CVD mortality and disability adjusted life-years in the US and worldwide [60]. In the US, the estimated lifetime risk of developing hypertension after age 45 is 84–93% [61]. This trajectory, however, may not be immutable, as dietary modification is important or beneficial to both prevent and treat hypertension [61]. In a case series from the mid-20th century, Dr. Walter Kempner showed that hypertension could be improved with dietary change, specifically when consuming a low sodium plant-based diet comprised largely of fruits and whole grains [62]. Subsequently, in 1986, a small RCT found that a vegetarian diet lowered blood pressure significantly more than an omnivorous diet and in 1988, Dr. Frank Sacks and colleagues showed that vegetarians tended to have lower blood pressure compared to the general population [63,64]. As such, the DASH diet was created to have the blood pressure lowering benefits of a vegetarian diet, yet contain enough animal products to make them palatable to non-vegetarian [65].
The DASH diet is described above (see Dietary Approaches). Relative to a traditional American “western diet”, the DASH diet includes more vegetables, fruits, legumes, nuts, fiber, fish, and low-fat dairy, and less processed and red meat, saturated fat, and sweets. In a pivotal 8-week RCT comparing a Western diet to a DASH diet, the DASH diet lowered systolic and diastolic blood pressure, by 5.5 mmHg and 3.0 mmHg, more, respectively, than did the Western diet. In study participants with hypertension, the impact of the DASH diet was greater, as systolic and diastolic blood pressure fell by 11.4 mmHg and 5.5 mmHg, respectively [66]. The blood pressure lowering effect of the DASH dietary pattern was further supported by an umbrella review of systematic reviews and meta-analyses of controlled trials, where a DASH dietary pattern led to lower blood pressure [24].
Dietary sodium also appears to impact blood pressure [67]. Over the course of human evolution, naturally occurring sodium was the only dietary source, leading to an intake of ∼0.5 gm/d [68,69]. Currently however, the mean intake in the US is around 3.6 gm/d and the global average is ∼3.6gm/d to 4gm/d, with some countries nearing 10 gm/d [70]. In 2017, a systematic analysis of the Global Burden of Disease study noted that 3 million deaths were attributed to excess sodium intake annually [71]. In a recent Mendelian Randomization analysis, dietary sodium was causally associated with salt-sensitive hypertension [72]. Furthermore, observational studies, meta-analyses, and systematic reviews have shown a direct association between dietary sodium intake and blood pressure [[73], [74], [75], [76]]. Given this concern regarding dietary sodium and blood pressure, the impact of differing amounts of sodium (low, intermediate, and high) within a DASH dietary pattern was studied in a RCT. The lowest sodium DASH dietary group achieved the greatest reductions in blood pressure, supporting lowering sodium intake to achieve lower blood pressure [77]. In 2021, the U.S. Food and Drug Administration released guidance for the food industry regarding salt content, encouraging voluntary salt reduction goals over the next 2.5 years, with the goal to improve the health of the nation [78].
In further support of a DASH dietary pattern, large prospective cohort studies reinforce the beneficial impact of fruit and vegetable consumption on blood pressure, while also demonstrating an association between greater red and processed meat consumption and higher blood pressure [[79], [80], [81], [82], [83]]. In a recent meta-analysis of 36 randomized controlled trails and seven crossover studies, a plant based dietary pattern significantly lowered systolic and diastolic blood pressure [84]. This beneficial association of fruit and vegetable consumption on blood pressure may be mediated, in part, by their anti-inflammatory and antioxidant effects, vasodilation, and changes in the renin-angiotensin and sympathetic nervous systems [85]. In addition, dietary nitrate load appears to play a role. In a recent analysis of 53,150 participants with 23-year follow up in the Danish Diet, Cancer, and Health Study it was noted that the highest quintile of vegetable nitrate intake was associated with lower systolic and diastolic blood pressure. In addition, a moderate vegetable nitrate of ∼60 mg/day (one cup of green leafy vegetables) was associated with 15% lower risk of CVD [86]. In a recent open-label large cluster RCT that enrolled 20,995 individuals over 60 with prior history of stroke, salt substitute high in potassium, compared to regular salt was shown to significantly lower the rates of stroke, major cardiovascular events, and death at a median follow up duration of 4.7 years [87]. Based on the totality of evidence and in alignment with the ACC, the AHA and the International Society of Hypertension, the American society for preventive cardiology (ASPC) recommends a low sodium DASH dietary pattern, rich in fruits and vegetables, for the prevention and treatment of hypertension [88,89].
3.3. Type 2 Diabetes and Obesity
The prevalence of obesity, defined as body mass index ≥ 30 kg/m2 has escalated in recent decades, reaching 42.4% of US adults [90]. Globally, the incidence of diabetes increased from 11.3 million in 1990 to 22.9 million in 2017 [91], and annual US healthcare expenditures for 34 million Americans with the disease exceed $325 billion. Both obesity and type 2 diabetes (T2D) are risk factors in the development and progression of CVD. Thus, the integration of effective and sustainable dietary recommendations for prevention and treatment of obesity and diabetes is a top public health priority.
The importance of diet for prevention of both obesity and T2D is well-demonstrated. Weight gain in adulthood is primarily due to gains in fat mass, and accumulates gradually peaking after midlife about 1 lb/year [92]. Modest increases in body weight, often imperceptible in the short-term, have been associated with a number of dietary changes in longitudinal observational studies, including increased intakes of potato chips, fries, refined grains, processed meats, unprocessed red meats, and sugar-sweetened beverages [[92], [93], [94]]. While diets high in calorically dense ultra-processed food can cause excess energy intake and weight gain [95]. it is important to differentiate healthful “smartly processed” foods such as fortified plant milks, grains, and plant protein based meat and egg substitutes, which can add nutrition value in a well-planned dietary pattern. “Smartly processed” foods are those low in saturated fat, refined carbohydrates and dietary cholesterol, consistent with our guideline recommendations for reducing ASCVD risk. Increasing intake of yogurt, fruits (not fruit juices), whole grains, legumes, fish, and nuts have been associated with less weight gain and lower risk of developing obesity. Other foods including cheese, milk, and artificially sweetened beverages may not be related to gradual long-term weight change. The macronutrient composition of the diet may also play a role in long-term weight gain, although the role of total fat, carbohydrates, and protein per se independent of food sources or overall diet quality, is less clear. For example, long-term modest increases in calories from fat from animal sources, but not vegetable sources, has been associated with weight gain [96,97]. Increasing trans fat and saturated fat at the expense of calories from carbohydrates is also related to long-term gradual weight gain [96,97].
In addition to weight gain prevention, diet plays a critical role in weight loss for treatment of overweight and obesity. Experimental evidence supports long-term weight loss (≥12 months) is achievable with interventions that emphasize a variety of diets and eating patterns, with or without explicit guidance for calorie restriction [[98], [99], [100]].
Globally, 24.7% of deaths and 34.9% of disability-adjusted life years due to diabetes are estimated to be attributable to poor diet [90]. Epidemiologic studies and short-term RCTs of T2D-related intermediate traits collectively suggest a number of dietary factors and eating patterns are related to lower T2D risk [101]. For example, using the Alternative Healthy Eating Index, an overall diet quality score reflecting the USDA dietary guideline recommendations, is associated with lower T2D risk in general cohort populations [102], as well as among high risk women with a history of gestational diabetes mellitus [103]. Other dietary patterns that similarly emphasize vegetables, fruits, whole grains, legumes, nuts, and lean meats, while discouraging sugar-sweetened beverages and red and processed meats, are also consistently associated with lower T2D risk in observational cohorts [104].
The PREDIMED trial randomized participants to a Mediterranean dietary pattern or low-fat control group for primary prevention of CVD in high-risk older adults in Spain [105]. In a subgroup analysis of n = 418 participants without T2D at baseline, those randomized to the Mediterranean diets vs. the low-fat diet had >50% reduction in T2D risk over ∼4 years follow-up. Accumulating evidence also supports low-carbohydrate plant-based diet [34], and other food and nutrient patterns for T2D prevention [106]. The Diabetes Prevention Program is a landmark randomized trial that observed a 58% reduction in T2D risk among participants receiving an intensive lifestyle program, compared with control group receiving usual care [9]. As a result of its demonstrated effectiveness, this curriculum has been implemented nationally and world-wide, adapted to several languages, and is even covered by some healthcare insurances for T2D prevention.
Despite advances in understanding and appreciation for the role of diet in obesity and diabetes, trends in prevalence continue to escalate. We therefore emphasize the need to maintain dedicated research efforts to identify optimal dietary interventions and effective strategies for their implementation
4. Vitamin Supplementation
Dietary supplement use is widespread in the general population, and patients are frequently interested in the potential for dietary supplements (e.g. vitamins, minerals, herbs, other bioactives) to modify CVD risk [[107], [108], [109]]. The broad variety of dietary supplements, lack of a structured clinical research program to study their safety profiles or efficacy for clinical endpoints in well-powered trials, as well as the lack of a broader regulatory system to ensure product quality and limit contamination [110], have led nearly all professional societies, guideline panels and clinicians to not recommend specific dietary supplements for general prevention/treatment of CVD. Practitioners should be prepared to have patient-centered conversations with regard to dietary supplement use, and be able to communicate the uncertainty in clinical effects of dietary supplements with regard to both disease prevention and risks of supplements. Practitioners may benefit from utilizing available educational resources, such as the Supplement Fact Sheets from the NIH's Office of Dietary Supplements [111], as well as information on supplements provided by the National Center for Complementary and Integrative Health [112]. Referral to practitioners with subject matter expertise and allotted time for patient counseling (e.g. registered dietitians; outpatient pharmacists) may be considered for patients who are interested in additional discussion about dietary supplements, or are suspected of dietary inadequacy that may require supplementation.
While enthusiasm for many dietary supplements relies on proposed mechanisms of action with limited clinical data, some supplements have been the subject of significant investigation and are the subject of common queries by patients. A large body of RCT and prospective cohort evidence demonstrates that multivitamin-multimineral supplementation does not confer CVD benefits in the general population [[113], [114], [115]]. Some supplements (e.g. phytosterols/stanols; viscous fibers) have a significant body of evidence to support risk factor lowering (e.g. LDL-C lowering) [[116], [117], [118], [119]]; however, their utilization in clinical practice remains limited, likely due to unclear net benefits, cost and practicality of continued, long-term use. While enthusiasm for some botanical/herbal supplements abounds (e.g. green tea; turmeric), it should be noted that formulations are not standardized, vary substantially in bioactives across batches and products, can interact with medications, and can contribute to acute liver injury [120,121].
Many patients assume dietary supplements, which can be purchased without a prescription, are benign and harmless. However, some supplements have been associated with increased cardiovascular risk such as antioxidant mixtures and niacin [122]. Calcium supplements, particularly in higher doses and when taken without vitamin D, have also been associated with increased risk of vascular events in some (but not all) studies; [115,[123], [124], [125]] whereas no such risk has been seen with calcium intake from food sources. Nevertheless, patients are frequently not counseled on potential risks of dietary supplements. Potential risks and benefits of supplement use should be discussed as part of clinician-patient risk discussion.
5. Special Considerations in Nutritional Approaches in Pediatric Populations for ASCVD Prevention
Primary prevention of ASCVD should begin as early as possible. Although adverse (what about other vascular disease which appears earlier-ED) cardiovascular outcomes are typically not seen until middle age, ASCVD risk factors can occur during childhood and adolescence [[126], [127], [128], [129], [130]]. Identifying modifiable early-life risk factors (e.g. weight, lipid levels, blood pressure, dietary patterns) is necessary to address the high morbidity and mortality from cardiovascular events [131].
Evidence suggests that childhood risk factors can lead to clinical manifestations of CVD [118]. Familial hypercholesterolemia, which causes high LDL levels in children, increases the risk of midlife coronary events and reduces life expectancy [131]. Similarly, the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study found strong associations between atherosclerosis and non-HDL-C and hyperglycemia in young individuals [132].
Of the risk factors identified, weight correlates most strongly from childhood to adulthood [131]. According to the Centers for Disease Control and Prevention (CDC), the prevalence of obesity among children and adolescents is 18.5%, equating to about 13.7 million youth. The Bogalusa Heart Study, which followed >2000 children, found that most participants with a BMI in the 95th to 99th percentile and all those with a BMI >99th percentile developed obesity later in life [133]. Children and adolescents with elevated blood pressure and cholesterol levels were more likely to carry these risk factors into adulthood, especially if weight was a risk factor [133].
ASCVD prevention in pediatric populations requires compassionate, effective, and individualized approaches to address modifiable risk factors. The recommendations outlined in Table 1 provide practical guidance related to clinical assessment and healthcare delivery in pediatric populations. Recommendations for macronutrients in children by age are outlined in Table 2.
Table 1.
Dietary recommendations in pediatric populations.
| Dietary Recommendations in Pediatric Populations |
|---|
| Focus on clinical indicators of ASCVD risk (e.g. lipids, blood pressure, glucose) rather than weight. Discussing or prescribing weight loss in pediatric populations may increase the risk of disordered eating and eating disorders [164]. Providers should focus on clinical indicators that provide a more comprehensive assessment of cardiometabolic risk. |
| Focus on nutrient-dense foods and beverages as recommended in the 2020–2025 Dietary Guidelines for Americans. Consistent evidence suggests that nutrition-dense dietary patterns can decrease future CVD risk [131]. Dietary recommendations should emphasize fruits, vegetables, whole grains, and varied protein foods (e.g. legumes, nuts, seeds, seafood) [165]. Acceptable macronutrient composition for age for children are shown in Table 2. |
| Emphasize small changes in eating behaviors to promote nutrient-dense dietary patterns. Providers should work collaboratively with pediatric patients to establish nutrition-related SMART goals that are focused, feasible, and measurable [166]. Remember to acknowledge and celebrate all forms of progress. |
| Tailor nutrition recommendations based on cultural and personal food preferences and support children's ability to self-regulate intake. Clinicians should be aware of cultural dynamics and food practices when working with pediatric patients and their families [167]. Encourage caregivers to foster positive eating environments and introduce nutrient-dense foods but trust children to determine how much and whether to eat these foods [168]. |
| Provide support for navigating food choices at care centers, schools, and afterschool programs. School meals and child care programs can provide a significant proportion of children's daily nutritional needs and are an important resource for low-income families [169]. Clinicians should encourage consumption of nutrient-dense options (e.g. whole fruits, fruit cups, steamed or roasted vegetables, salads) when available in school and child care settings. |
| Involve pediatric patients in discussions about their health status and care plans [170]. Providers should consider patients’ age, ability to comprehend information, and food choice autonomy during clinical visits. Use accessible language and emphasize positive, weight-neutral outcomes of health behavior changes (e.g. increased energy, improved learning ability). |
Table 2.
Acceptable macronutrient distribution ranges for children aged 2–18 years.
| Macronutrient | 2 to 3 years | 4 to 8 years | 9 to 13 years | 14 to 18 years |
|---|---|---|---|---|
| Carbohydrate (% kcal) | 45–65% | 45–65% | 45–65% | 45–65% |
| Fat (% kcal) | 30–40% | 25–35% | 25–35% | 25–35% |
| Protein (% kcal) | 5–20% | 10–30% | 10–30% | 10–30% |
*Based on the 2020–2025 Dietary Guidelines for Americans.
6. Special Considerations in Nutritional Approaches in Older Adult Populations for ASCVD Prevention
While all nutrition recommendations for ASCVD prevention should ideally be individualized, special considerations for older adult populations are worth highlighting. Evidence from experimental studies indicate similar or greater effects of medical nutrition therapy targeting risk factors (i.e. blood pressure, blood lipids) in the older adult population (>65 year olds). Implementation of specific cardioprotective dietary changes should consider and accommodate the unique metabolic and practical aspects of nutrition that arise as individuals age, as well as the increasing prevalence of comorbidities. For example, older adults are at increased risk of protein-energy malnutrition, as well as micronutrient deficiencies (e.g. Vitamin B12). As individuals age, changes in oral and dental health, as well as neurological function, can influence mastication and oral control, swallowing and taste perception that may impair the ability to readily and safely implement ASCVD prevention recommendations. Where appropriate, individualized recommendations to include fortified and/or medical foods and supplements, as well as to modify food texture, form and gustatory/olfactory profiles may be required to facilitate the intake of cardioprotective dietary patterns [134].
7. Nutrition and Social Determinants of Health for ASCVD Prevention
Despite advances in ASCVD treatment and prevention, demographic and socioeconomic disparities persist. Black Americans are more likely to die from ASCVD than any other racial/ethnic group [135]. The prevalence of heart disease is highest among some Native American populations and Black adults have the highest hypertension rates, although awareness and treatment among Black women has recently declined [135,136].
It is important to acknowledge that race/ethnicity are social constructs and not reflective of genetic or biological differences in health status. However, race/ethnicity are potential indicators of structural racism and adverse social, economic, and environmental conditions (i.e. social determinants of health) that lead to health inequities [137]. The social determinants of health affecting ASCVD outcomes include inadequate housing, exposure to adversity (e.g. violence, safety concerns), suboptimal health literacy, and food insecurity (lack of access to sufficient, affordable and nutritious food) [138]. Therefore, addressing social determinants of health is critical to primary and secondary ASCVD prevention.
Nutrient-dense dietary patterns emphasizing intake of vegetables, fruits, legumes, nuts, whole grains, and fatty fish are recommended to decrease ASCVD risk factors (Table 3) [138]. The REGARDS (REasons for Geographic and Racial Differences in Stroke) trial linked the Southern dietary pattern higher in refined grains, fried foods, and sweetened beverages with elevated ASCVD risk.[8] An emerging area of research is the causal relationship between the gut microbiota, increased consumption of sodium and cooked meats, and hypertension and ASCVD among Black adults [139].
Table 3.
Recommendations for practice in all patients.
| Recommendations for practice in all patients |
|---|
| Screen all patients for food insecurity and refer to food assistance programs and other community food resources. The Hunger Vital Sign is a two-question screening tool to assess food insecurity in clinical settings [171]. [Routine screening for food insecurity can be integrated into the Electronic Medical Record as part of standard patient intake procedures [171,172]. Patients identified as food insecure should be referred to food resources such as the Supplemental Nutrition Assistance Program (formerly known as food stamps), food pantries, and home-delivered meals. Tailor dietary recommendations to reflect cultural food habits and budgetary considerations. Cardioprotective dietary patterns can incorporate foods from diverse cultures and food choices . Clinicians should be aware of cultural dynamics, patient food preferences, and access to resources when providing nutritional recommendations. |
| Utilize literacy-level appropriate, culturally-relevant educational materials. Clinicians should refer to existing resources from health-focused organizations and seek patient feedback in developing culturally-relevant nutrition education materials. (Table 4) |
However, notable disparities in dietary behaviors exist among US racial/ethnic groups. Black and Mexican American adults have a higher prevalence of poor heart healthy diet scores as compared with White adults [135]. Culturally relevant nutrition resources should be provided when appropriate (Table 4). Fueled by inequities in food access and socioeconomic resources, racial/ethnic disparities in food insecurity have persisted for decades and been further exacerbated by the COVID-19 pandemic [140,141]. Overall, living in food deserts is associated with increased cardiovascular risk factors and preclinical ASCVD, mainly driven by social determinants of health such as income and access to nutritious food [142].
Table 4.
Examples of culturally relevant nutrition resources for ASCVD prevention.
| Organization | Resource(s) | Resource Link |
|---|---|---|
| Association of Black Cardiologists | Cooking for your Heart and Soul (heart-healthy plant-based recipes) | https://abcardio.org/wp-content/uploads/2020/06/ABC_Cookbook.pdf |
| Doras Table | Easy and Delicious Mexican Vegan Recipes | https://dorastable.com |
| Oldways | Traditional food pyramids (African, Latin American, Asian) and recipes | Food pyramids: https://oldwayspt.org/traditional-diets Recipes: https://oldwayspt.org/recipes |
8. Practical Tips/Implementations for Clinicians and Patients
While diet-related risk continues to be one of the leading causes of CVD, there is an urgent and an unmet need for nutrition related education at all levels of medical education and clinical practice [143,144]. Most physicians receive minimal or no training in evidence based clinical nutrition and this frequently results in physicians not actively engaging in educating their patients. This has direct and profound effect on disease prevention and clinical outcomes [144]. In a survey of 930 cardiovascular specialists, 90% admitted to receiving minimal or no training in nutrition and 95% believed that it is their personal responsibility to teach nutrition to their patients. However, only 20% of cardiologists admitted to eating ≥ 5 servings of fruits and vegetables a day [145].
Most of the current CVD-related conferences in the US offer minimal nutrition related continuing medical education (CME) programming [146]. In an effort fill this gap, in 2018, the AHA has issued a Scientific Advisory of Medical Nutrition, Training, and Competencies to Advance Guidelines-Based Counselling by Physicians [147]. In a recent article tilted, A Clinicians Guide to Healthy Eating for Cardiovascular Disease Prevention, the authors outline practical tips to take evidence based clinical nutrition to day-to-day practice of preventive cardiology [148]. In addition, the two-part series on Trending Cardiovascular Nutrition Controversies is a useful resource for cardiovascular trainees and practitioners [149,150].
Currently, there is an increasing trend and demand to include formal culinary training programs at US medical schools [151]. Recently in 2020, the American college of lifestyle medicine has published the first, comprehensive, open-source culinary medicine curriculum for health professional training programs: a global reach [152]. Over the course of the past 10–15 years, the percentage of Americans cooking at home has gradually increased [153]. However, according to the 2021 AHA statistics, the number of adults meeting the Healthy Diet Index for ages 12–19 is 0.0% and for age >19 is 0.3% [154]. This trend provides an opportunity to empower the general population with the information needed to eat healthy, in accordance with the USDA and ACC/AHA guidelines [155]. The American journal of preventive cardiology recently published a practical and implementable outline of 10 dietary strategies for cardiovascular risk reduction [156]. The American society for preventive cardiology (ASPC) supports these recommendations.
9. Referral to Registered Dietitians
Implementation of a cardioprotective dietary pattern and associated reductions in ASCVD risk factors can be facilitated by nutritional counseling; a substantial body of evidence supports counseling by Registered Dietitians (RDs) as effectively improving diet quality, facilitating intentional weight loss, improving blood glucose, HbA1c, blood lipids and blood pressure. However, insurance and Medicare coverage of dietetic consultations remains variable and often with a limited scope; Medicare Part B coverage for medical nutrition therapy extends only to type 2 diabetes and renal conditions. Providers should be encouraged to refer patients to RDs for medical nutrition interventions when appropriate, and advocate for expanded coverage of medical nutrition therapy. Existing proposed legislation aims to broadly expand medical nutrition therapy coverage for various ASCVD risk factors including obesity, prediabetes, hypertension, dyslipidemia and other cardiovascular diseases, and stands to broadly expand access to preventive nutrition services [19,[157], [158], [159], [160], [161], [162]].
10. Precision/Gaps/Future research
While the current evidence for diet's relationship to ASCVD risk is actionable, important questions remain. Firstly, areas of uncertainty should be resolved with rigorous and valid research approaches. Relative to the traditional medical paradigm, diet represents a complex exposure, and advancing nutrition science faces both inherent and pragmatic limitations to conducting rigorous double-blind placebo-controlled trials. It is imperative to develop and validate novel analytic tools and study designs to accommodate the complexities of relating habitual intakes of foods/nutrients with long-term CVD outcomes.
Also paramount is the funding and investment in domiciled facilities that allow for tightly controlled experimental manipulation of specific dietary exposures for significant durations [163]. Such efforts would have a substantial impact on nutrition science, advancing our understanding of diet, metabolism, and their effects on traditional and emerging risk factors (e.g. novel metabolites; imaging modalities). Controlled feeding experiments also facilitate the discovery of objective biomarkers of diet, which may be subsequently leveraged in free-living populations to monitor adherence and complement self-reported diet in large-scale observational studies. Precision nutrition initiatives, which seek to tailor diet for populations or individuals to optimize impact, also readily benefit from such controlled research environments.
As actionable evidence for dietary interventions accumulates, better strategies for dissemination are needed. Currently, interventions to modify diet in free-living populations are difficult to achieve, significantly impairing the feasibility of long-term dietary modification trials. Scaling up of implementation research is urgently needed to identify barriers of long-term adherence and strategies to foster sustainable changes in diet.
Finally, descriptive evidence highlighting disparities in prevalence and incidence of ASCVD and its risk factors must be urgently addressed. Improving nutrition should remain a priority across all populations. Thus, it is imperative that advances in nutrition science and implementation are made in the context of reducing, not exacerbating, disparities. This may include, but is not limited to, ensuring equity in the access to food product development and dietary counseling informed by ‘precision’ nutrition approaches, and removing systemic barriers to adopting meaningful dietary changes.
Clinical Recommendations
-
1
Primary and Secondary Prevention of ASCVD: A diet consisting predominantly of fruits, vegetables, legumes, nuts, seeds, plant protein, and fatty fish is optimal for the prevention of ASCVD.
-
2
Hyperlipidemia: Replacing saturated fat with polyunsaturated and monounsaturated fat, reducing dietary cholesterol intake, and increasing intake of fiber rich foods, can all lead to a reduction in LDL-C and apoB.
-
3
Hypertension: Eating a low sodium DASH dietary pattern, rich in dietary potassium from fruits and vegetables, is recommended for the prevention and treatment of hypertension.
-
4
Type 2 Diabetes: Preventing weight gain and obesity is pivotal for diabetes prevention. Thus, effective strategies for weight loss and management are likely beneficial for mitigating diabetes progression.
-
5
Obesity: The importance of diet for prevention of weight gain and obesity is well demonstrated, with poor nutritional habits playing a role in gradual midlife weight gain.
-
6
Vitamin Supplementation: Vitamin supplementation is not routinely recommended for the prevention of ASCVD; supplementation should be individualized and recommended in those where it is necessary to meet nutrient requirements or as otherwise medically indicated.
7. Children: Primary prevention of ASCVD should begin as early as possible, emphasizing small changes in eating behaviors to promote nutrient-dense dietary patterns to establish nutrition-related goals that are focused, feasible, and measurable.
-
7
8. Older Adults:: Nutrition therapy can have substantial benefits for individuals across the life span, including older adults with existing disease burden. Dietary recommendations to reduce cardiovascular risk should be counseled, tailored to the unique nutrient requirements and challenges associated with achieving adequate nutrition encountered by older adults.
9. Social Determinants of Health: Healthcare providers should evaluate patients for inequities in food access and socioeconomic resources, while providing culturally relevant nutrition resources when appropriate.
10. Multidisciplinary approach: Medical nutrition therapy, in collaboration with registered dietitians, results in greater improvements in cardiovascular disease risk factors and referral should be encouraged. Greater advocacy by allied health providers is necessary to ensure adequate coverage and reimbursement for the provision of nutrition counseling services.
11. Conclusion
Although often there is debate in popular media about which dietary pattern is best, the nutrition science is clear regarding which dietary patterns reduce risk for CVD: A diet consisting predominantly of fruits, vegetables, legumes, nuts, seeds, plant protein and fatty fish is optimal for the prevention of ASCVD (Central Figure). Consuming more of these foods, while reducing consumption of foods with saturated fat, dietary cholesterol, salt, refined grains, and ultra-processed food intake are the common components of a healthful dietary pattern.
While there is clear evidence that diet impacts ASCVD risk and risk factors, adherence to a healthy dietary pattern remains difficult to achieve. As a result, the incidence of CVD continues to increase in our population, increasingly impacting the younger population. Understanding the effects of different dietary approaches on cardiovascular health will help us partner with our patients on choosing a nutritional strategy that should be part of every patient's lifestyle prescription. Consideration of barriers to obtaining healthy foods, including food insecurity should be a component of any cardiovascular risk assessment. Cultural competence related to food is essential as we care for diverse patient populations. In addition, providing education on both clinical nutrition and culinary training to all healthcare professionals involved in ASCVD prevention must be a priority within the medical community.
Disclosures
RJO receives research grants from the Purjes and Greenbaum Foundations. Consulted for Bright Plate.
Funding
None
References
- 1.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad F.B., Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325:1829–1830. doi: 10.1001/jama.2021.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020:1–8. [PubMed] [Google Scholar]
- 4.Muntner P., Hardy S.T., Fine L.J., et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y.J., Kanaya A.M., Araneta M.R.G., et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Metabolic Risk Factors for Chronic Diseases C Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks F.M., Svetkey L.P., Vollmer W.M., et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 8.Shikany J.M., Safford M.M., Newby P.K., Durant R.W., Brown T.M., Judd S.E. Southern dietary pattern is associated with hazard of acute coronary heart disease in the reasons for geographic and racial differences in stroke (REGARDS) study. Circulation. 2015;132:804–814. doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estruch R., Ros E., Salas-Salvado J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 11.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;74:e177. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micha R., Shulkin M.L., Peñalvo J.L., et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the nutrition and chronic diseases expert group (NutriCoDE) PLoS One. 2017;12 doi: 10.1371/journal.pone.0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusuf S., Hawken S., Ounpuu S., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. (London, England) [DOI] [PubMed] [Google Scholar]
- 14.Miller V., Mente A., Dehghan M., et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037–2049. doi: 10.1016/S0140-6736(17)32253-5. (London, England) [DOI] [PubMed] [Google Scholar]
- 15.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 16.Arnett Donna K., Blumenthal Roger S., Albert Michelle A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer N.M., Pallazola V.A., Xun H., Cainzos-Achirica M., Michos E.D. The evolution of the heart-healthy diet for vascular health: a walk through time. Vasc Med. 2020;25:184–193. doi: 10.1177/1358863X19901287. [DOI] [PubMed] [Google Scholar]
- 18.Ferraro R.A., Fischer N.M., Xun H., Michos E.D. Nutrition and physical activity recommendations from the United States and European cardiovascular guidelines: a comparative review. Curr Opin Cardiol. 2020;35:508–516. doi: 10.1097/HCO.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein A.H., Appel L.J., Vadiveloo M., et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American heart association. Circulation. 2021 doi: 10.1161/CIR.0000000000001031. CIR0000000000001031. [DOI] [PubMed] [Google Scholar]
- 20.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 21.de Lorgeril M., Salen P., Martin J.L., Monjaud I., Delaye J., Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 22.Stewart R.A., Wallentin L., Benatar J., et al. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37:1993–2001. doi: 10.1093/eurheartj/ehw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menotti A., Puddu P.E. How the seven countries study contributed to the definition and development of the Mediterranean diet concept: a 50-year journey. Nutr Metab Cardiovasc Dis. 2015;25:245–252. doi: 10.1016/j.numecd.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Chiavaroli L., Viguiliouk E., Nishi S.K., et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019:11. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos C.L., Wood A., Burke G.L., Bahrami H., Bertoni AG. Dietary approaches to stop hypertension diet concordance and incident heart failure: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2019;56:819–826. doi: 10.1016/j.amepre.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henzel J., Kepka C., Kruk M., et al. High-risk coronary plaque regression after intensive lifestyle intervention in nonobstructive coronary disease: a randomized study. JACC Cardiovasc Imaging. 2021;14:1192–1202. doi: 10.1016/j.jcmg.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Jabri A., Kumar A., Verghese E., et al. Meta-analysis of effect of vegetarian diet on ischemic heart disease and all-cause mortality. Am J Prev Cardiol. 2021 doi: 10.1016/j.ajpc.2021.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satija A., Bhupathiraju S.N., Spiegelman D., et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70:411–422. doi: 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard N.D., Alwarith J., Rembert E., et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. J Am Coll Nutr. 2021:1–13. doi: 10.1080/07315724.2020.1869625. [DOI] [PubMed] [Google Scholar]
- 30.Kirkpatrick C.F., Bolick J.P., Kris-Etherton P.M., et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the national lipid association nutrition and lifestyle task force. J Clin Lipidol. 2019;13:689–711. doi: 10.1016/j.jacl.2019.08.003. e1. [DOI] [PubMed] [Google Scholar]
- 31.Hall K.D., Chung S.T. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2018;21:308–312. doi: 10.1097/MCO.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 32.Li S., Flint A., Pai J.K., et al. Low carbohydrate diet from plant or animal sources and mortality among myocardial infarction survivors. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halton T.L., Willett W.C., Liu S., et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 34.de Koning L., Fung T.T., Liao X., et al. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:844–850. doi: 10.3945/ajcn.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung T.T., van Dam R.M., Hankinson S.E., Stampfer M., Willett W.C., Hu F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi S., Ostfeld R.J., McMacken M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern Med. 2019;179:1163–1164. doi: 10.1001/jamainternmed.2019.2633. [DOI] [PubMed] [Google Scholar]
- 37.O'Neill B., Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. 2020;292:119–126. doi: 10.1016/j.atherosclerosis.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Joshi S., Ostfeld R.J., McMacken M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2019.2633. [DOI] [PubMed] [Google Scholar]
- 39.Rynders C.A., Thomas E.A., Zaman A., Pan Z., Catenacci V.A., Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11 doi: 10.3390/nu11102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 41.Lowe D.A., Wu N., Rohdin-Bibby L., et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patikorn C., Roubal K., Veettil S.K., et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221. doi: 10.1016/j.cmet.2018.04.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ioannidis J.P.A. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320:969–970. doi: 10.1001/jama.2018.11025. [DOI] [PubMed] [Google Scholar]
- 45.Cainzos-Achirica M., Bilal U., Al Rifai M., et al. Communication issues in nutritional observational research. Prev Med. 2018;115:76–82. doi: 10.1016/j.ypmed.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Cainzos-Achirica M., Bilal U., Kapoor K., et al. Methodological issues in nutritional epidemiology research—sorting through the confusion. Curr Cardiovasc Risk Rep. 2018;12:4. [Google Scholar]
- 47.Clarke R., Frost C., Collins R., Appleby P., Peto R. Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies. BMJ. 1997;314:112–117. doi: 10.1136/bmj.314.7074.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mensink R. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva: World Health Organisation, 2016.
- 49.Mensink R.P., World Health O . World Health Organization; Geneva: 2016. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. [Google Scholar]
- 50.Mozaffarian D., Rimm E.B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. J Am Med Assoc. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 51.Balk E.M., Adams G.P., Langberg V., et al. Omega-3 fatty acids and cardiovascular disease: an updated systematic review. Evid Rep Technol Assess (Full Rep) 2016:1–1252. doi: 10.23970/AHRQEPCERTA223. [DOI] [PubMed] [Google Scholar]
- 52.Vincent M.J., Allen B., Palacios O.M., Haber L.T., Maki KC. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am J Clin Nutr. 2019;109:7–16. doi: 10.1093/ajcn/nqy273. [DOI] [PubMed] [Google Scholar]
- 53.Amir Shaghaghi M., Abumweis S.S., Jones P.J. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113:1494–1503. doi: 10.1016/j.jand.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Ras R.T., Geleijnse J.M., Trautwein EA. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vuksan V., Jenkins A.L., Rogovik A.L., Fairgrieve C.D., Jovanovski E., Leiter LA. Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br J Nutr. 2011;106:1349–1352. doi: 10.1017/S0007114511001711. [DOI] [PubMed] [Google Scholar]
- 56.Brown L., Rosner B., Willett W.W., Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson T.A., Maki K.C., Orringer C.E., et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9:S1–122. doi: 10.1016/j.jacl.2015.09.002. e1. [DOI] [PubMed] [Google Scholar]
- 58.Blanco Mejia S., Messina M., Li S.S., et al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr. 2019;149:968–981. doi: 10.1093/jn/nxz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taku K., Umegaki K., Sato Y., Taki Y., Endoh K., Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007;85:1148–1156. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 60.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carson A.P., Howard G., Burke G.L., Shea S., Levitan E.B., Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klemmer P., Grim C.E., Luft F.C. Who and what drove Walter Kempner? Hypertension. 2014;64:684–688. doi: 10.1161/HYPERTENSIONAHA.114.03946. [DOI] [PubMed] [Google Scholar]
- 63.Margetts B.M., Beilin L.J., Vandongen R., Armstrong BK. Vegetarian diet in mild hypertension: a randomised controlled trial. Br Med J (Clin Res Ed) 1986;293:1468–1471. doi: 10.1136/bmj.293.6560.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sacks F.M., Kass EH. Low blood pressure in vegetarians: effects of specific foods and nutrients. Am J Clin Nutr. 1988;48:795–800. doi: 10.1093/ajcn/48.3.795. [DOI] [PubMed] [Google Scholar]
- 65.Sacks F.M., Obarzanek E., Windhauser M.M., et al. Rationale and design of the dietary approaches to stop hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–118. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- 66.Appel L.J., Moore T.J., Obarzanek E., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 67.Grillo A., Salvi L., Coruzzi P., Salvi P., Parati G. Sodium intake and hypertension. Nutrients. 2019:11. doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batuman V. Salt and hypertension: why is there still a debate? Kidney Int Suppl. 2013;3:316–320. doi: 10.1038/kisup.2013.66. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson F.L. An evolutionary perspective on salt, hypertension, and human genetic variability. Hypertension. 1991;17:I129–I132. doi: 10.1161/01.hyp.17.1_suppl.i129. [DOI] [PubMed] [Google Scholar]
- 70.Cook N.R., He F.J., MacGregor G.A., Graudal N. Sodium and health-concordance and controversy. BMJ. 2020;369:m2440. doi: 10.1136/bmj.m2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Health effects of dietary risks in 195 countries 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi J.R. Genetically, dietary sodium intake is causally associated with salt-sensitive hypertension risk in a community-based cohort study: a Mendelian randomization approach. Eur Heart J. 2020:41. doi: 10.1007/s11906-020-01050-4. [DOI] [PubMed] [Google Scholar]
- 73.Intersalt: an international study of electrolyte excretion and blood pressure Results for 24 h urinary sodium and potassium excretion. Intersalt cooperative research group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graudal N.A., Hubeck-Graudal T., Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochr Datab Syst Rev. 2017;4 doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He F.J., Li J., Macgregor G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 76.He F.J., Pombo-Rodrigues S., MacGregor G.A. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juraschek S.P., Miller E.R., Weaver C.M., Appel L.J. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70:2841–2848. doi: 10.1016/j.jacc.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guidance for industry: voluntary sodium reduction goals. In: Nutrition FCfFSaA, editor, 2021, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals.
- 79.Alexander S., Ostfeld R.J., Allen K., Williams K.A. A plant-based diet and hypertension. J Geriatr Cardiol. 2017;14:327–330. doi: 10.11909/j.issn.1671-5411.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borgi L., Curhan G.C., Willett W.C., Hu F.B., Satija A., Forman J.P. Long-term intake of animal flesh and risk of developing hypertension in three prospective cohort studies. J Hypertens. 2015;33:2231–2238. doi: 10.1097/HJH.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du H., Li L., Bennett D., et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374:1332–1343. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lajous M., Bijon A., Fagherazzi G., Rossignol E., Boutron-Ruault M.C., Clavel-Chapelon F. Processed and unprocessed red meat consumption and hypertension in women. Am J Clin Nutr. 2014;100:948–952. doi: 10.3945/ajcn.113.080598. [DOI] [PubMed] [Google Scholar]
- 83.Steffen L.M., Kroenke C.H., Yu X., et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the coronary artery risk development in young adults (CARDIA) study. Am J Clin Nutr. 2005;82:1169–1177. doi: 10.1093/ajcn/82.6.1169. quiz 1363-4. [DOI] [PubMed] [Google Scholar]
- 84.Gibbs J., Gaskin E., Ji C., Miller M., Cappuccio F. The effect of plant-based diets on blood pressure: a systematic review and meta-analysis of controlled clinical trials. J Hypertens. 2021;39:e322. doi: 10.1097/HJH.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 85.Lin P.H., Allen J.D., Li Y.J., Yu M., Lien L.F., Svetkey L.P. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab. 2012;2012 doi: 10.1155/2012/472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bondonno C.P., Dalgaard F., Blekkenhorst L.C., et al. Vegetable nitrate intake, blood pressure and incident cardiovascular disease: Danish diet, cancer, and health study. Eur J Epidemiol. 2021 doi: 10.1007/s10654-021-00747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neal B., Wu Y., Feng X., et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067–1077. doi: 10.1056/NEJMoa2105675. [DOI] [PubMed] [Google Scholar]
- 88.Unger T., Borghi C., Charchar F., et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 89.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 90.Ogden C.L., Fryar C.D., Martin C.B., et al. Trends in obesity prevalence by race and Hispanic origin-1999–2000 to 2017–2018. JAMA. 2020;324:1208–1210. doi: 10.1001/jama.2020.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin X., Xu Y., Pan X., et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malik V.S., Pan A., Willett W.C., Hu F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlesinger S., Neuenschwander M., Schwedhelm C., et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10:205–218. doi: 10.1093/advances/nmy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hall K.D., Ayuketah A., Brychta R., et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019;30:67–77. doi: 10.1016/j.cmet.2019.05.008. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Li Y., Tobias D.K., et al. Changes in types of dietary fats influence long-term weight change in US women and men. J Nutr. 2018;148:1821–1829. doi: 10.1093/jn/nxy183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hosseini-Esfahani F., Koochakpoor G., Mirmiran P., Ebrahimof S., Azizi F. The association of dietary macronutrients with anthropometric changes, using iso-energetic substitution models: Tehran lipid and glucose study. Nutr Metab. 2019;16:83. doi: 10.1186/s12986-019-0411-2. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tobias D.K., Chen M., Manson J.E., Ludwig D.S., Willett W., Hu F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968–979. doi: 10.1016/S2213-8587(15)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardner C.D., Trepanowski J.F., Del Gobbo L.C., et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319:667–679. doi: 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salas-Salvado J., Diaz-Lopez A., Ruiz-Canela M., et al. Effect of a lifestyle intervention program with energy-restricted mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42:777–788. doi: 10.2337/dc18-0836. [DOI] [PubMed] [Google Scholar]
- 101.Marventano S., Vetrani C., Vitale M., Godos J., Riccardi G., Grosso G. Whole grain intake and glycaemic control in healthy subjects: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9 doi: 10.3390/nu9070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morze J.D.A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2020;120:1998–2031. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 103.Tobias D.K., Hu F.B., Chavarro J., Rosner B., Mozaffarian D., Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;172:1566–1572. doi: 10.1001/archinternmed.2012.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jannasch F., Kroger J., Schulze M.B. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147:1174–1182. doi: 10.3945/jn.116.242552. [DOI] [PubMed] [Google Scholar]
- 105.Estruch R., Ros E., Salas-Salvado J., et al. Retraction and republication: primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. N Engl J Med 2018;378:2441-2442. [DOI] [PubMed] [Google Scholar]
- 106.Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Radimer K., Bindewald B., Hughes J., Ervin B., Swanson C., Picciano M.F. Dietary supplement use by US adults: data from the national health and nutrition examination survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 108.Li S., Petraskova T., Stys N., Blue M., Stys T., Stys A. Dietary supplement use in patients with cardiovascular disease. S D Med. 2020;73:72–76. [PubMed] [Google Scholar]
- 109.Qato D.M., Wilder J., Schumm L.P., Gillet V., Alexander G.C. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473–482. doi: 10.1001/jamainternmed.2015.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bailey R.L. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit Rev Food Sci Nutr. 2020;60:298–309. doi: 10.1080/10408398.2018.1524364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dietary supplement fact sheets [Internet]. Available from: https://ods.od.nih.gov/factsheets/list-all/ [accessed April 27], 2021.
- 112.Resources for health care providers | NCCIH [Internet]. Available from: https://www.nccih.nih.gov/health/providers [accessed April 28], 2021.
- 113.Jenkins D.J.A., Spence J.D., Giovannucci E.L., et al. Supplemental vitamins and minerals for cvd prevention and treatment. J Am Coll Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 114.Kim J., Choi J., Kwon S.Y., et al. Association of multivitamin and mineral supplementation and risk of cardiovascular disease: a systematic review and meta-analysis. Circ Cardiovas Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.004224. [DOI] [PubMed] [Google Scholar]
- 115.Khan S.U., Khan M.U., Riaz H., et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190–198. doi: 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ras R.T., Geleijnse J.M., Trautwein E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amir Shaghaghi M., Abumweis S.S., Jones P.J.H. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113:1494–1503. doi: 10.1016/j.jand.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 118.Brum J., Ramsey D., McRorie J., Bauer B., Kopecky SL. Meta-analysis of usefulness of psyllium fiber as adjuvant antilipid therapy to enhance cholesterol lowering efficacy of statins. Am J Cardiol. 2018;122:1169–1174. doi: 10.1016/j.amjcard.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 119.Ho H.V.T., Jovanovski E., Zurbau A., et al. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr. 2017;105:1239–1247. doi: 10.3945/ajcn.116.142158. [DOI] [PubMed] [Google Scholar]
- 120.Tachjian A., Maria V., Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Navarro V.J., Khan I., Björnsson E., Seeff L.B., Serrano J., Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jenkins D.J.A., Spence J.D., Giovannucci E.L., et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 123.Michos E.D., Cainzos-Achirica M., Heravi A.S., Appel LJ. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC focus seminar. J Am Coll Cardiol. 2021;77:437–449. doi: 10.1016/j.jacc.2020.09.617. [DOI] [PubMed] [Google Scholar]
- 124.Yang C., Shi X., Xia H., et al. The evidence and controversy between dietary calcium intake and calcium supplementation and the risk of cardiovascular disease: a systematic review and meta-analysis of cohort studies and randomized controlled trials. J Am Coll Nutr. 2020;39:352–370. doi: 10.1080/07315724.2019.1649219. [DOI] [PubMed] [Google Scholar]
- 125.Bolland M.J., Avenell A., Baron J.A., et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Enos W.F., Holmes R.H., Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 127.McNamara J.J., Molot M.A., Stremple J.F., Cutting R.T. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216:1185–1187. [PubMed] [Google Scholar]
- 128.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:I19–I32. [PubMed] [Google Scholar]
- 129.Tanaka K., Masuda J., Imamura T., et al. A nation-wide study of atherosclerosis in infants, children and young adults in Japan. Atherosclerosis. 1988;72:143–156. doi: 10.1016/0021-9150(88)90075-5. [DOI] [PubMed] [Google Scholar]
- 130.Imakita M., Yutani C., Strong J.P., et al. Second nation-wide study of atherosclerosis in infants, children and young adults in Japan. Atherosclerosis. 2001;155:487–497. doi: 10.1016/s0021-9150(00)00595-5. [DOI] [PubMed] [Google Scholar]
- 131.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strong J.P., Malcom G.T., McMahan C.A., et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the pathobiological determinants of atherosclerosis in youth study. JAMA. 1999;281:727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 133.Freedman D.S., Mei Z., Srinivasan S.R., Berenson G.S., Dietz W.H. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa heart study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. e2. [DOI] [PubMed] [Google Scholar]
- 134.Ship J.A., Duffy V., Jones J.A., Langmore S. Geriatric oral health and its impact on eating. J Am Geriatr Soc. 1996;44:456–464. doi: 10.1111/j.1532-5415.1996.tb06419.x. [DOI] [PubMed] [Google Scholar]
- 135.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 136.Villarroel M.A., Blackwell D.L., Jen A. National Center for Health Statistics; 2019. Tables of summary health statistics for U.S. adults: 2018 national health interview survey. [Google Scholar]
- 137.National Academies of Sciences E, Medicine a. Washington, DC: The National Academies Press, 2017.
- 138.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferdinand K.C., Graham R.M. Disparities in hypertension and cardiovascular disease in African Americans: Is the answer in the gut microbiota? Int J Cardiol. 2018;271:340–342. doi: 10.1016/j.ijcard.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 140.Odoms-Young A., Bruce M.A. Examining the Impact of structural racism on food insecurity: implications for addressing racial/ethnic disparities. Fam Community Health. 2018;41(Suppl 2) doi: 10.1097/FCH.0000000000000183. Suppl, Food Insecurity and Obesity:S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolfson J.A., Leung C.W. Food insecurity and COVID-19: disparities in early effects for US adults. Nutrients. 2020;12:1648. doi: 10.3390/nu12061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kelli H.M., Hammadah M., Ahmed H., et al. Association between living in food deserts and cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reddy K.R., Freeman A.M., Esselstyn C.B. An urgent need to incorporate evidence-based nutrition and lifestyle medicine into medical training. Am J Lifestyle Med. 2019;13:40–41. doi: 10.1177/1559827618781764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aggarwal M., Devries S., Freeman A.M., et al. The deficit of nutrition education of physicians. Am J Med. 2018;131:339–345. doi: 10.1016/j.amjmed.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 145.Devries S., Agatston A., Aggarwal M., et al. A deficiency of nutrition education and practice in cardiology. Am J Med. 2017;130:1298–1305. doi: 10.1016/j.amjmed.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 146.Johnston E., Mathews T., Aspry K., Aggarwal M., Gianos E. Strategies to fill the gaps in nutrition education for health professionals through continuing medical education. Curr Atheroscler Rep. 2019;21:13. doi: 10.1007/s11883-019-0775-9. [DOI] [PubMed] [Google Scholar]
- 147.Aspry K.E., Horn L.V., Carson J.A.S., et al. Medical nutrition education, training, and competencies to advance guideline-based diet counseling by physicians: a science advisory from the American heart association. Circulation. 2018;137:e821–e841. doi: 10.1161/CIR.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 148.Pallazola V.A., Davis D.M., Whelton S.P., et al. A clinician's guide to healthy eating for cardiovascular disease prevention. Mayo Clin Proc Innov Qual Outcomes. 2019;3:251–267. doi: 10.1016/j.mayocpiqo.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Freeman A.M., Morris P.B., Barnard N., et al. Trending cardiovascular nutrition controversies. J Am Coll Cardiol. 2017;69:1172–1187. doi: 10.1016/j.jacc.2016.10.086. [DOI] [PubMed] [Google Scholar]
- 150.Freeman A.M., Morris P.B., Aspry K., et al. A clinician's guide for trending cardiovascular nutrition controversies: part II. J Am Coll Cardiol. 2018;72:553–568. doi: 10.1016/j.jacc.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 151.La Puma J. What is culinary medicine and what does it do? Popul Health Manag. 2016;19:1–3. doi: 10.1089/pop.2015.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hauser M.E., Nordgren J.R., Adam M., et al. The first, comprehensive, open-source culinary medicine curriculum for health professional training programs: a global reach. Am J Lifestyle Med. 2020;14:369–373. doi: 10.1177/1559827620916699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taillie LS. Who's cooking? Trends in US home food preparation by gender, education, and race/ethnicity from 2003 to 2016. Nutr J. 2018;17:41. doi: 10.1186/s12937-018-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 155.U.S. Department of Agriculture and U.S. Department of Health and Human Services . USDA; 2020. Dietary guidelines for Americans. Dietary guidelines for Americans -tEDAaDg. [Google Scholar]
- 156.Sikand G., Severson T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am J Prev Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ross L.J., Barnes K.A., Ball L.E., et al. Effectiveness of dietetic consultation for lowering blood lipid levels in the management of cardiovascular disease risk: a systematic review and meta-analysis of randomised controlled trials. Nutr Diet. 2019;76:199–210. doi: 10.1111/1747-0080.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mitchell L.J., Ball L.E., Ross L.J., Barnes K.A., Williams L.T. Effectiveness of dietetic consultations in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2017;117:1941–1962. doi: 10.1016/j.jand.2017.06.364. [DOI] [PubMed] [Google Scholar]
- 159.Sikand G., Cole R.E., Handu D., et al. Clinical and cost benefits of medical nutrition therapy by registered dietitian nutritionists for management of dyslipidemia: a systematic review and meta-analysis. J Clin Lipidol. 2018;12:1113–1122. doi: 10.1016/j.jacl.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 160.Riegel G.R., Ribeiro P.A.B., Rodrigues M.P., Zuchinali P., Moreira L.B. Efficacy of nutritional recommendations given by registered dietitians compared to other healthcare providers in reducing arterial blood pressure: systematic review and meta-analysis. Clin Nutr. 2018;37:522–531. doi: 10.1016/j.clnu.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 161.Moller G., Andersen H.K., Snorgaard O. A systematic review and meta-analysis of nutrition therapy compared with dietary advice in patients with type 2 diabetes. Am J Clin Nutr. 2017;106:1394–1400. doi: 10.3945/ajcn.116.139626. [DOI] [PubMed] [Google Scholar]
- 162.Congress.gov . Library of Congress; Washington, D.C.: 2020. Medical nutrition therapy act of 2020. [Google Scholar]
- 163.Hall K.D. Challenges of human nutrition research. Science. 2020;367:1298–1300. doi: 10.1126/science.aba3807. [DOI] [PubMed] [Google Scholar]
- 164.Golden N.H., Schneider M., Wood C. Preventing obesity and eating disorders in adolescents. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1649. [DOI] [PubMed] [Google Scholar]
- 165.United States Department of Agriculture, United States Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. 9th ed., United States Department of Agriculture, December 2020.
- 166.Bailey R.R. Goal setting and action planning for health behavior change. Am J Lifestyle Med. 2017;13:615–618. doi: 10.1177/1559827617729634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tervalon M., Murray-García J. Cultural humility versus cultural competence: a critical distinction in defining physician training outcomes in multicultural education. J Health Care Poor Underserved. 1998;9:117–125. doi: 10.1353/hpu.2010.0233. [DOI] [PubMed] [Google Scholar]
- 168.Satter E.M. The feeding relationship. J Am Diet Assoc. 1986;86:352–356. [PubMed] [Google Scholar]
- 169.Fox M.K., Gearan E., Cabili C. et al. School Nutrition and meal cost study, final report volume 4: student participation, satisfaction, plate waste, and dietary intakes. 2019, https://www.mathematica.org/publications/school-nutrition-and-meal-cost-study-final-report-volume-4-student-participation-satisfaction-plate.
- 170.Katz A.L., Webb S.A. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1485. [DOI] [PubMed] [Google Scholar]
- 171.Hager E.R., Quigg A.M., Black M.M., et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26–e32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- 172.Ashbrook A., Essel K., Montez K., Bennett-Tejes D. Screen and intervene: a toolkit for pediatricians to address food insecurity. 2021.


