Abstract
The mussel-watch concept was firstly proposed in 1975, which was later adopted by several international monitoring programs worldwide. However, for the very first time, a field experiment with caged mussels was performed in three reservoirs in Bulgaria to follow the harmful effects of sub-chronic pollution (30 days) of metals, trace, and macro-elements, as well as some organic toxicants, such as polybrominated diphenyl ethers and chlorinated paraffins. Therefore, we studied the biometric indices, histochemical lesions in the gills, biochemical changes in the digestive glands (antioxidant defense enzymes, such as catalase, glutathione reductase, and glutathione peroxidase; metabolic enzymes, such as lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase, and the neurotransmitter cholinesterase), in addition to the DNA damage in the Chinese pond mussel, Sinanodonta woodiana (Lea, 1834) in Kardzhali, Studen Kladenets and Zhrebchevo reservoirs in Bulgaria. Significant correlation trends between the pollution levels, which we reported before, and the biomarker responses were established in the current paper. Overall, we found that both tested organs were susceptible to pollution-induced oxidative stress. The different alterations in the selected biomarkers in the caged mussels compared to the reference group were linked to the different kinds and levels of water pollution in the reservoirs, and also to the simultaneously conducted bioaccumulation studies.
Keywords: Water, Pollution, Transplants, Caged mussels, Biomarkers
Highlights
-
•
The effects of water pollution in caged mussels from three large dam reservoirs in Bulgaria were assessed.
-
•
A cocktail of different inorganic and organic toxicants was measured both in waters and mussels for the first time.
-
•
Different biomarker responses (cellular to individual) were also followed in gills and digestive glands of the transplants.
-
•
Correlation trends between the pollution levels and the applied biological tools were established.
Water; Pollution; Transplants; Caged mussels; Biomarkers.
1. Introduction
Pollution with metals and toxic elements has been a worldwide phenomenon for decades and affects various aquatic environments [1, 2, 3, 4, 5, 6, 7]. Metals and metalloids, such as arsenic, aluminum, and mercury are considered to be among the most dangerous pollutants, because of their persistence in the environment, nonbiodegradability, toxicity character, bioaccumulation, and even biomagnification [8].
According to Müller et al. and Zhang et al., polybrominated diphenyl ethers (PBDEs) are a group of chemicals that have been marketed since the 1960s [9, 10]. They have been commonly applied as flame retardants in various commercial products, such as electronic equipment, furniture, plastic materials, polyurethane foams, textile fabrics, etc. PBDEs belong to a class of hydrophobic, poorly degradable organic pollutants, which are persistent and, are prone to adsorb onto particulate matter, bioaccumulate in the fatty tissues and biomagnify through the food web [11]. Accordingly, the production and application of technical penta-, octa- and deca-BDE mixtures has been prohibited in the EU [12] and partially restricted in America. Novak et al. stated that despite the bans, PBDEs will continue to be discharged in nature from the available large depots of PBDE-containing products for many years to come, and hence, will remain an environmental issue in the foreseeable future [13].
Among the high production volume commercial organics, chlorinated paraffins (CPs) are of growing environmental concern. As explained by Feo et al. they have been commonly used as metal flame retardants, fat liquors of leather, plasticizers and working fluids [14]. According to Zhou et al., the synthesized products are conventionally classified as short-chain (SCCPs, C10–13), medium-chain (MCCPs, C14–17) or long-chain CPs (LCCPs, C ≥ 18), and hazard evaluation and chemical regulation of CPs have focused on the chain length categories, which has thus taken most CP formulations into account [15]. In particular, SCCPs have been under global regulation as persistent organic pollutants (POPs) since 2018 [16], and MCCPs and LCCPs have been on the EU priority list of chemicals, considering their impact on the environment [17].
Bivalve mollusks, especially mussels, are considered to be good bioindicator organisms of water pollution. First of all, it is so because of their broad distribution, sedentary life mode and high abundance, and secondly, because of their filtering feeding mode, ability to accumulate high concentrations of substances in their organs, and high tolerance to abiotic changes [18]. Mussels also attract the attention regarding the assessment of human health risks associated with water deterioration [19]. In addition, bivalves have been used as bioindicators of pollutants throughout the United States and also around the globe [20, 21]. Until today, mussels have been widely applied in many regional environmental monitoring programs, such as the U.S. Mussel Watch Project, Assessment and Control of Pollution in the Mediterranean region (MEDPOL), and OSPAR's Coordinated Environmental Monitoring Program (CEMP) [22]. Also, resident and transplanted mussels have been used to assess patterns in the process of bioaccumulation in the Californian waters for over four decades, including some of the earliest performed work, using the “Mussel Watch” approach [23]. Thus, in the “Mussel Watch” monitoring programs, resident and/or caged mussels can be used as bioindicators of chemical pollutants [24]. Recently, Avio et al., Catarino et al., d'Ericco et al., Manfra et al., and Railo et al. used caged mussels in specific areas to investigate pollution effects related to different anthropogenic activity, such as plastic pollution, wastewater discharge, wreck removal, gas production and dredging activities [25, 26, 27, 28, 29]. Furthermore, according to Schøyen et al., mussel caging is particularly valuable when the indigenous mussels are absent in the study sites [30].
According to Turja et al. and Larsson et al., responses to environmental stress are usually measured using multiple biomarkers of different biological functions and from different stages of biological organization [31, 32]. Thus, an integrated biomarker approach employs a set of biochemical, histological, genotoxic, and physiological features [31, 32], which represent the kind, level, and state of alterations that need to be studied thoroughly to understand the extent of the damage, which is a vital aspect of ecotoxicological research [33].
The results of the current field experiment are part of a pilot study оn bioaccumulation of selected priority substances in water, as well as transplanted moss and mussels. The hypothesis, which was tested in the present work was that the long-term pollution waters of three large reservoirs in Bulgaria would alter selected biomarkers in caged mussels after a 30 days’ exposure period. Hence, we aimed to develop an integrated methodology for the assessment of metals, trace and macro-elements, polybrominated diphenyl ethers, and short-chain chlorinated paraffins using transplants in Kardzhali, Student Kladenets and Zhrebchevo reservoirs, located in Bulgaria, which have been subjected to anthropogenic stress for several decades. In addition, we aimed to apply a multi-biomarker approach on the Chinese pond mussel, Sinanodonta woodiana (Lea, 1834), which included biometric measurements, histochemical, biochemical, and genotoxic assays to study the possible link between pollutant levels and biological parameters. To our knowledge, this is the very first study, which was carried out on transplanted mussels with the selected reservoirs, bivalve species, pollutants, and biological effect tools.
2. Materials and methods
2.1. Test organism
According to Klimova et al., while mollusks of the genus Mytilus are considered the most sensitive test organisms in the biomonitoring of marine ecosystems, a model similar in characteristics has not yet been proposed for freshwater ecosystems [34]. In this regard, the identification of a freshwater bivalve bioindicator with suitable parameters as the Mytilus species will play a crucial role for future biomonitoring and ecotoxicological studies of inland waters [35]. There are several studies [36, 37, 38], which suggest that zebra mussel, Dreissena polymorpha (Pallas, 1771) could be a substitute for marine bivalves, but its relatively smaller size could be a problem in terms of multiple analyses.
In the present study, we selected as a test organism the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (the Eastern Asiatic freshwater clam or swan-mussel). Overall, the reasons we chose this particular bivalve were because it is a natural biofilter and has a wide distribution, but also a relatively big size, which is essential in terms of performing several different analyses [39, 40]. However, in terms of its quick range expansion, we also carefully selected a test organism, which occurs naturally, in both the study and reference sites. The Chinese pond mussel is a species, with a native range that expands into East Asia from the Russian Far East (Amur Basin) to Indo-China, Malaysia, and Taiwan [41, 42]. According to Demayo et al., Soroka et al., and Lopes-Lima et al., this mussel has spread broadly across many areas, e.g., Costa Rica, Europe, Hispaniola, Indonesia, Philippines, and the USA [43, 44, 45]. Therefore, the Chinese pond mussel can be classified as an invasive species in Europe and its presence could have adverse effects on other indigenous Unionid species in the same habitat. In Europe, the Chinese pond mussel was firstly seen in Romania in 1979. Currently, the Chinese pond mussel is known to exist in 16 European countries, including Bulgaria and Hungary, and also in the study sites of the presently reported research [46, 47]. Contrary to the European countries and the USA, the populations of the Chinese pond mussel are also perceived as an important protein source to local communities, which has a great economic value in Indonesia [48]. Moreover, this mussel is also a traditionally edible species in its native range, including China as explained by Chen et al. [49].
2.2. Study sites
Three large dam reservoirs in Bulgaria were selected as study sites – Kardzhali (41.638475 N, 25.304432 E), Studen Kladenets (41.622244 N, 25.441933 E), and Zhrebchevo (42.585571 N, 25.885592 E) reservoirs. They have been subjected to long-term anthropogenic stress, which also differs in its type and level (Figure 1).
Figure 1.
Map of South-Eastern Europe and the localities of the study sites: Kardzhali (K), Studen Kladenets (SK) and Zhrebchevo (Z) reservoirs, and reference site in Plovdiv (P) in Bulgaria. Geocoordinates of the localities: K – 41.638475 N, 25.304432 E; SK – 41.622244 N, 25.441933 E; Z – 42.585571 N, 25.885592 E; P – 42.164785 N, 24.756515 E.
Kardzhali is an artificial water reservoir, formed by damming the waters of the Arda River in the Rhodope Mountains. It is among the first reservoirs in Bulgaria where fish were reared in net cages and currently there are 7 net cage farms [50]. In addition, Kardzhali Reservoir has also been intensively used for power production and cage farming for over 30 years, with one of the largest sturgeon farms in Europe [51]. Arda River is a large Bulgarian river, passing through other Balkan countries, such as Greece and Turkey, which subsequently flows into the Aegean Sea. There used to be anthropogenic sources of metal pollution along its valley on the territory of Bulgaria; the lead-zinc processing plant and ore mines are now closed, but they have caused permanent water deterioration [52]. However, there is relatively scarce recent data on the pollution levels of this dam reservoir and their effects on different bioindicator species [53].
Studen Kladenets Reservoir is the third largest dam in Bulgaria and it has been significantly impacted by the activity of the former lead-zinc ore processing plant “Kardzhali” and the very few published and old data were on metal accumulation and its effects on some freshwater fish species [54, 55]. Furthermore, until now, according to Bachvarov and Velcheva [56], in the area of Studen Kladenets Reservoir, studies have been mainly conducted related to the metal content in different fish organs. There is almost no data about the impact of anthropogenic pollution on the various biomarkers in bioindicators, such as fish or mussels [57, 58].
Zhrebchevo Reservoir is located along Tundzha River (in the drainage area of Maritsa River, Aegean Sea basin) and it is mainly polluted due to the intense agricultural activity in the region, but there is also some data on metal pollution [59, 60]. The reservoir is used for power generation, irrigation, aquaculture, recreation, and sport fishing [61]. In addition, the changes of zooplankton structure in Zhrebchevo Reservoir as a result of water pollution and hydro-technical constructions were described as early as 1981 by Naidenow [62].
2.3. Field experiment
Mussels were hand-collected from one of the fish ponds (dig-out) of the Institute of Fisheries and Aquaculture (42.143611 N, 24.816111 E) in Plovdiv, Bulgaria (reference site) where they exist naturally a few days before field deployment. The mussels were selected from one size class (weight: 154 ± 5.5 g; shell length: 11 ± 3.5 cm). The Institute of Fisheries and Aquaculture rears fish under strict and controlled conditions, and there is no known or published data on anthropogenic pressure in the area (www.ira-plovdiv.bg). Plovdiv is situated in south-central Bulgaria, standing on the two banks of the Maritsa River. Plovdiv was аlso the cultural capital of Europe in 2019 and it is the second-largest city after Sofia. Even though the city is rapidly growing, the Institute of Fisheries and Aquaculture is far from the city center, located in an uninhabited area surrounded by fields, which creates perfect conditions for fish farming.
No mussels from elsewhere were transplanted in the reference site and no mussels from other locations were used as transplants in the three studied reservoirs.
The mussels were transported to the laboratory at Plovdiv University in Bulgaria on the same day. They were placed in a 100 L water tank filled with dechlorinated water, mixed with water from the reference site (50:50) and fitted with air pumps. Conductivity, dissolved oxygen, pH and temperature were measured to be relatively constant, thus we verified that they did not influence the tested biomarkers. Shortly after that, the mussels (n = 30) were transported to the reservoirs in clean plastic containers filled with oxygenated water and put randomly in stainless-steel cages (30 × 15 × 10 cm) at a depth of 2 m in each of the studied reservoirs (n = 10 in each reservoir) [63]. The cages' mesh size was 25 mm allowing water circulation and preventing mussels from falling out of the cages as explained by Kazour and Amara [64]. The mussels were not artificially fed. The cages were collected after 30 days of exposure. No set of animals was sampled at time 0, only at day 30. There were funding limitations and therefore, we used to control from the reference site for comparison. In addition, we thought that at time 0 we would not find any disturbances in the studied biomarkers, which normally take a longer time to manifest. The mussels’ total soft tissues were cut out at the selected study sites for further biomarker analyses according to the adapted for mussels EMERGE protocol of Rosseland et al. [65], but the sex was not determined for the current study as we were not interested in the sex-specific differences in regards to the toxicological responses in the present study. The transplant experiment was performed once in June–July 2019.
2.4. Laboratory methodology
2.4.1. Bioaccumulation analyses
Concentrations of 17 elements, which included metals, trace and macro-elements (Al, As, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Mg, Mn, Na, Ni, P, Pb, Zn), as well as organic toxicants, such as PBDEs (PBDE 28, PBDE 47, PBDE 99, PBDE 100, PBDE 153, PBDE 154) and SCCPs were determined simultaneously in surface water samples and the same set of transplanted mussel samples (whole mussel soft tissues) at day 30. The methods of ICP-AES and ICP-MS in addition to the method of gas chromatography-mass spectrometry (GC-MS) (Thermo Scientific, USA) were applied for the bioaccumulation analyses [63, 66]. Metal pollution index (MPI) was assessed to compare the total content of metals and trace elements, excluding the macro-elements for the mussels from the reference and sampling sites (Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Zn). The MPI formula is the following [67, 68] [Eq. (1)]:
| MPI = (C1 × C2 × C3 × …Cn)1/n, | (1) |
where Cn is the mean concentration of the element n in the analyzed tissue (mg kg−1 wet weight).
The results on bioaccumulation and bioaccumulation factors in the transplanted mussels from Kardzhali, Studen Kladenets, and Zhrebchevo reservoirs were previously calculated and reported as well [63, 66]. Therefore, the main focus of the present research was the biomarker responses of the Chinese pond mussel associated with the toxicant levels in the waters, which are presented below.
2.4.2. Biometric measurements
The biometric calculations were carried out as described by Gasmi et al. [69]. Thus, the whole weight (ww) of each mussel was measured and reported in g with an analytical scale (Kern, Germany) after cleaning the shell from epibionts and other debris. The mussels were then thoroughly opened to separate the flesh from the shell with a stainless scalpel. The intervalvular liquid was drained, and thus the soft tissues were dried on absorbent paper and wet-weighed. The shells were measured with calipers in mm to calculate the studied indices following Marques [70]; Kagley et al. [71] and Galvao et al. [72] [Eqs. (2), (3), (4), (5), and (6)]:
| CI total = soft tissue ww/total ww x 100 | (2) |
| CI 2 = soft tissue ww/shell weight ww x 100 | (3) |
| CI 3 (state index) = soft tissue ww/shell length x 100 | (4) |
| CI 4 (shell component index) = shell ww/(shell ww + meat ww) x 100 | (5) |
| CI 5 (condition factor) = soft tissue ww/shell length3 x 100 | (6) |
2.4.3. Histochemical technique
The histochemical analysis was carried out with a cryostat (Leica, CM 1520, Germany) to cut the samples. Multiple mussel gill sections (6 μm) of each specimen were prepared according to a standard PAS methodology [73] and observed with a light microscope (Leica DM 2000 LED, Germany), attached with a camera in a blinded manner. The gill histochemical lesions of all the specimens, including the reference group, were appraised individually and semi-quantitatively by using the grading system of Bernet, which was adopted for this study [74]. The positive PAS reaction was presented in purple-magenta staining. The evaluation of the histochemical changes was performed and presented as an average value. Each grade represents specific histochemical characteristics and was classified as follows: (0) – negative reaction of the histochemical staining; (1) – very weak positive reaction of the histochemical staining; (2) – weak positive reaction of the histochemical staining; (3) – moderate positive reaction of the histochemical staining; (4) – strong positive reaction of the histochemical staining.
2.4.4. Biochemical analyses
All the chemicals, which were used in the biochemical assays were of analytical grade. They were purchased from Sigma Aldrich-Merck (Germany). The biochemical analyses were measured at 25 °C with a spectrophotometer (Beckman Coulter, DU 800, USA).
The digestive glands were first thawed fast on ice and then, manually homogenized, using a Potter Elvehjem homogenizer fitted with a Teflon pestle (Thomas Scientific, USA) in chilled phosphate buffer (pH 7.4, 50 mM, 300 mM NaCl). The homogenates were centrifuged at 4 °C for 15 min at 9000 rpm in a cooling centrifuge (MPW 351 R, Poland). The supernatant fractions were aliquoted, transferred in new Eppendorf tubes, and stored at ‒80 °C for further analyses of both, antioxidant and metabolic enzymes.
The catalase (CAT EC 1.11.1.6) activity was measured by the decrease in absorbance at 240 nm by H2O2 decomposition according to Aebi and Beutler [75, 76].
The glutathione reductase activity (GR, E.C. 1.8.1.7) was determined by monitoring the glutathione-dependent oxidation of NADPH at 340 nm [15].
The glutathione peroxidase (GPx, E.C. 1.11.1.9) was measured using the method described by Wendel [77].
The cholinesterase (ChE, E.C. 3.1.1.8) activity was determined by the decrease in absorbance at 405 nm according to Burtis and Ashwood [78].
The lactate dehydrogenase (LDH, E.C. 1.1.1.27) activity was measured by measuring the amount of pyruvate consumed due to NADH oxidation at 340 nm (backward reaction) according to Vassault [79].
The alanine aminotransferase (ALAT, E.C. 2.6.1.2) and aspartate aminotransferase (ASAT, E.C. 2.6.1.1) activities were determined by the method of Reitman and Frankel [80] as described by Bergmeyer et al., using commercially available kits (Merck, Germany) [81].
The total protein levels were detected by the method described by Bradford [82] with Coomassie Brilliant Blue G-250 using bovine serum albumin as standard. The absorbance was set at 595 nm and expressed as milligram protein per milliliter homogenate.
All the assays were performed in triplicates and the enzyme activities were presented in international units per milligram of protein (U/mg protein).
2.4.5. Comet assay
Hemolymph was collected from the adductor muscle of each mussel, and the samples were centrifuged for 10 min at 1000 rpm (MPW 351 R, Poland) before the comet assay analysis. The alkaline version was conducted, following the procedure of Singh et al. [83], which we slightly modified. The preparation of the agarose layers on the microscope slides was performed according to Kolarević et al. [84]. Nucleoids forming comet-like shapes were observed at a magnification of × 400 after staining with SYBR Green I (1: 10.000 dilution) with a Leica DM1000 LED epifluorescence microscope, equipped with an I3 filter (Leica, Germany) and a camera. Fifty nucleoids per individual were scored, using the Comet Assay IV Computer Software (Perceptive Instruments, UK). The DNA damage was evaluated by the comet parameter Tail intensity (TI%), which reflects the percentage of DNA in the comet tail.
2.5. Integrated biomarker response (IBR) calculation
Integrative indices are widely used to summarizing biomarker responses and simplifying their interpretation in biomonitoring programs [28, 29]. Beliaeff and Burgeot [85] created the integrated biomarker response (IBR) especially for biomonitoring purposes with mussels. The basis of the calculation is described here briefly. For each biomarker:
-
(1)
Calculation of mean and standard deviation for each sampling site.
-
(2)
Standardisation of data for each sampling site [Eq. (7)]:
| Y = (X – m)/s | (7) |
where Y = the standardized value of the biomarker; X = mean value of a biomarker from each sampling site, m = mean of the biomarker calculated for all the sampling sites, and s = standard deviation of m.
-
(3)
Then we computation of Z value [Eq. (8)],
| Z = –Y or Z = Y | (8) |
in the case of a biological effect corresponding, respectively, to inhibition or activation.
-
(4)
Finally, the score (S) was computed as adding the absolute value of minimum value (min) for all sampling sites for each biomarker to Z value [Eq. (9)]:
| S = Z + |min| | (9) |
where S ≥ 0.
All the biomarkers were computed this way. Calculation of star plot areas by multiplication of the obtained value of each biomarker (Si), with the value of the next biomarker, arranged as a set, dividing each calculation by 2 and summing up all values [86]. The corresponding IBR value is [Eq. (10)]:
| IBR = (((S1 × S2)/2) + ((S2 × S3)/2) + … ((Sn-1 × Sn)/2) +((Sn × S1)/2))) | (10) |
2.6. Data processing
Past 3.03 [87] and GraphPad Prism 7 for Windows (USA) were used for the statistical evaluation of the obtained data. The normality of data was tested by the Shapiro-Wilk test. The homogeneity of variances was tested with Levene's test. The results were analyzed for the significance of differences among the mussels from the reference and the exposed groups by one-way analysis of variance (ANOVA), followed by Tukey's test (means comparison). In addition, Spearman's non-parametric correlation test was used to check for significant relationships between the MPI and biomarker responses of mussels from the reference and sampling sites. Principal component analysis (PCA) was also used to assess the differences in mussels from the studied sampling sites in Bulgaria, based on the biometric measurements; PAS-reaction in the gills; CAT, GPx, GR, ChE, ASAT, ALAT, LDH activities in the digestive glands, and DNA damage in the hemocytes. The significance was set at p < 0.05. The results from all the performed biomarker analyses were presented as mean ± standard deviation (SD).
3. Results and discussion
3.1. Bioaccumulation analyses
Оverall, in the present study we could link all the observed changes in the studied biomarkers with the results on bioaccumulation of different elements and organic pollutants in the caged mussels, which were determined simultaneously and published separately in our previous manuscripts [63, 66] (Table 1). Our results showed that Studen Kladenets was highly polluted compared to the other two, which were also impacted, but to a lesser degree. Оne probable reason for the pollution of the reservoir is that it is the final precipitator of the various emitters along Arda River, including the existing alloy factory and four sites for discharge from terminated mine sections.
Table 1.
Concentrations (mean mg L−1 in water and mg kg−1 in mussels and their relative standard deviation (RSD%)) of studied elements and organic pollutants in water and mussel samples from different sampling sites in Bulgaria.
| Reference site | Sampling sites |
|||
|---|---|---|---|---|
| Zhrebchevo | Kardzhali | Studen Kladenets | ||
| Water | ||||
| Macro elements (mg L−1) | ||||
| Ca | n.a. | n.a. | n.a. | n.a. |
| K | n.a. | n.a. | n.a. | n.a. |
| Mg | 10.7 (3.3%) | 12.1 (2.6%) | 2.6 (5.9%) | 3.6 (6.4%) |
| Na | 14.9 (2.6%) | 11.1 (2.7%) | 4.8 (3.6%) | 7.6 (2.1%) |
| P |
0.13 (3.5%) |
<0.01 |
0.11 (7.6%) |
0.04 (8.2%) |
| Trace elements (μg L−1) | ||||
| Al | 130 (3.5%) | 50 (11.4%) | 110 (7.6%) | 30 (13.3%) |
| As | 1.7 (8.2%) | <1 | 1.2 (9.4%) | 5.2 (4.9%) |
| Cd | <0.1 | <0.1 | <0.1 | 0.32 (7.9%) |
| Co | 0.28 (5.1%) | <0.01 | <0.01 | 0.26 (5.4%) |
| Cr | 0.38 (5.3%) | 0.13 (4.2%) | 0.18 (4.1%) | 0.06 (6.9%) |
| Cu | 6.4 (4.2%) | 0.3 (7.3%) | 1.7 (6.2%) | 1.8 (5.7%) |
| Fe | 0.27 (9.8%) | <0.01 | <0.01 | <0.01 |
| Hg | <0.05 | <0.05 | <0.05 | <0.05 |
| Mn | 0.049 (4.3%) | 0.005 (5.3%) | 0.008 (5.2%) | 0.039 (3.3%) |
| Ni | 1.0 (3.1%) | 0.4 (2.5%) | 0.4 (7.5%) | 0.6 (3.4%) |
| Pb | 2.1 (4.5%) | 0.3 (5.9%) | 0.6 (5.0%) | 17.7 (4.6%) |
| Zn |
<1 |
<1 |
<1 |
19.9 (4.8%) |
| Organic compounds (μg L−1) | ||||
| BDE 28 | <0.004 | <0.004 | 0.023 (26.0%) | 0.032 (28.1%) |
| BDE 47 | 0.005 (0.1%) | 0.005 (0.1%) | 0.012 (0.1%) | 0.005 (0.1%) |
| BDE 99 | 0.012 (0.1%) | <0.004 | 0.017 (0.1%) | 0.018 (0.1%) |
| BDE 100 | 0.009 (0.1%) | <0.004 | <0.004 | <0.004 |
| BDE 153 | 0.014 (0.1%) | 0.014 (0.1%) | 0.012 (0.1%) | 0.018 (0.1%) |
| BDE 154 | <0.004 | <0.004 | 0.010 (0.1%) | <0.004 |
| SCCPs |
0.58 (20.7%) |
3.9 (20.5%) |
0.86 (19.8%) |
1.2 (20.0%) |
| Mussels | ||||
| Macro elements (mg kg−1) | ||||
| Ca | 89 (3.1%) | 196 (2.3%) | 145 (2.1%) | 185 (2.4%) |
| K | 211 (4.2%) | 201 (4.7%) | 229 (4.0%) | 234 (5.5%) |
| Mg | 268 (3.8%) | 659 (2.6%) | 454 (2.8%) | 450 (3.1%) |
| Na | 331 (3.6%) | 514 (3.0%) | 780 (3.0%) | 436 (3.1%) |
| P |
0.43 (6.4%) |
1.20 (5.7%) |
0.93 (6.0%) |
1.07 (5.1%) |
| Trace elements (mg kg−1) | ||||
| Al | 14.4 (11.1%) | 36.9 (6.9%) | 36.6 (6.2%) | 35.7 (5.7%) |
| As | 0.37 (6.4%) | 1.15 (8.8%) | 0.80 (7.5%) | 0.63 (9.3%) |
| Cd | 0.09 (10%) | 0.19 (13%) | 0.17 (6.0%) | 0.38 (5.6%) |
| Co | 0.14 (6.0%) | 0.27 (7.4%) | 0.20 (7.1%) | 0.21 (5.8%) |
| Cr | 0.15 (3.4%) | 0.19 (8.6%) | 0.22 (3.2%) | 0.14 (11.3%) |
| Cu | 5.32 (4.9%) | 25.35 (4.3%) | 63.03 (3.4%) | 31.43 (3.9%) |
| Fe | 170 (6.4%) | 294 (3.4%) | 212 (3.4%) | 196 (5.0%) |
| Hg | 0.004 (16%) | 0.009 (12%) | 0.007 (12%) | 0.009 (11%) |
| Mn | 1.34 (7.5%) | 1.88 (5.0%) | 2.54 (4.0%) | 1.98 (5.6%) |
| Ni | 0.14 (5.0%) | 0.4 (2.5%) | 0.37 (6.5%) | 0.6 (3.4%) |
| Pb | 1.6 (6.8%) | 1.9 (3.6%) | 1.7 (5.7%) | 4.0 (7.9%) |
| Zn |
55.1 (3.3%) |
15.70 (3.8%) |
11.47 (3.6%) |
32.59 (4.7%) |
| Organic compounds (mg kg−1) | ||||
| BDE 28 | 0.005 (0.1%) | <0.003 | <0.003 | 0.005 (0.1%) |
| BDE 47 | 0.005 (0.1%) | 0.005 (0.1%) | 0.012 (0.1%) | 0.005 (0.1%) |
| BDE 99 | 0.013 (0.1%) | <0.003 | 0.015 (0.1%) | 0.010 (0.1%) |
| BDE 100 | 0.007 (0.1%) | <0.003 | <0.003 | <0.003 |
| BDE 153 | 0.014 (0.1%) | <0.003 | 0.016 (0.1%) | 0.014 (0.1%) |
| BDE 154 | <0.003 | <0.003 | <0.003 | 0.010 (0.1%) |
| SCCPs | 7.4 (29.7%) | 0.22 (31.8%) | 0.56 (30.4%) | 6.1 (29.5%) |
BDE: brominate diphenyl ethers; SCCPs: short-chain chlorinated paraffins.
The MPI values of mussels from the reference site, Zhrebchevo, Kardzhali, and Studen Kladenets, and reservoirs were 0.95; 1.58; 1.65, and 1.72, respectively. According to the MPI values of mussels, the amount of bioaccumulated metals was the lowest in the case of the reference site, and the highest was in the mussels from Studen Kladenets, respectively. At the same time, the mean concentrations of BDEs were highest in the mussels from Studen Kladanets too, and those of SCCPs were the highest in the mussels from Zhrebchevo (Table 1).
3.2. Biomarkers
No lethal outcome was observed throughout the exposures in any of the tested reservoirs.
3.2.1. Biometric indices
The results on calculated biological measurements are presented in Table 2. After one month of caging, we observed deviations in the biometric values of reference and transplanted mussels. There were no significant differences among CI, CI 2, CI 4 of mussels from the different sampling sites (CI: ANOVA, F = 0.6378, p = 0.6062; CI 2: ANOVA, F = 2.583, p = 0.1063; CI 4: ANOVA, F = 2.713, p = 0.0959; CI 5 ANOVA, F = 1.074, p = 0.1764) (Table 2). At the same time, the CI 3 of mussels from Studen Kladanets Reservoir was significantly lower compared to the reference and the other sampling sites (ANOVA, F = 7.656, p < 0.01) (Table 2). We consider that such results can indicate that the individuals exposed to pollution put their energy in detoxification processes, instead of other processes associated with metabolism, growth, etc. On one hand, some authors [88, 89, 90] explained that this period (1 month) is potentially too short to register changes in the biometric indices, which was not the scenario in our case. On the other hand, our results are in line with Lacroix et al. [90] that the period was long enough for histochemical, biochemical, and genotoxic alterations in the transplanted mussels.
Table 2.
Average results (± standard deviation) of biometric measurements; PAS-reaction in the gills; oxidative stress related enzymes' activities (catalase – CAT, glutathione peroxidase – GPx, and glutathione reductase – GR) and metabolic related enzymes’ activities (cholinesterase – ChE, aspartate aminotransferase – ASAT, alanine aminotransferase – ALAT, and lactate dehydrogenase – LDH) in the digestive glands (U/mg protein); and DNA damage (percentage of DNA in the tail of the comet (tail intensity – TI%)) in the hemocytes; and the integrated biomarker response (IBR) values of mussels from different sampling sites in Bulgaria.
| Reference site | Sampling sites |
|||
|---|---|---|---|---|
| Zhrebchevo | Kardzhali | Studen Kladenets | ||
| Biometric indices | ||||
| CI total | 25.57 ± 1.50a | 22.78 ± 2.04a | 25.57 ± 2.10a | 24.29 ± 3.31a |
| CI 2 | 62.10 ± 10.99a | 39.49 ± 1.57a | 51.17 ± 8.28a | 51.95 ± 6.84a |
| CI 3 | 419.63 ± 24.72a | 330.43 ± 13.15a | 329.13 ± 58.60a | 255.94 ± 38.17b |
| CI 4 | 61.97 ± 4.15a | 71.70 ± 0.81a | 66.35 ± 3.73a | 65.94 ± 2.99a |
| CI 5 |
0.0030 ± 0.0003a |
0.0027 ± 0.0001a |
0.0025 ± 0.0003a |
0.0024 ± 0.0004a |
| Histochemical alterations in gills | ||||
| Intensity of PAS-reaction |
++ |
+ |
+/- |
+/- |
| Oxidative stress related enzymes in digestive gland | ||||
| CAT | 25.79 ± 2.99a,c | 17.74 ± 2.52a | 34.23 ± 7.59c | 104.75 ± 5.06b |
| GPx | 0.68 ± 0.19a | 0.74 ± 0.18a | 0.93 ± 0.23a,b | 1.27 ± 0.29b |
| GR |
0.31 ± 0.11a |
0.49 ± 0.09a |
0.86 ± 0.28b |
1.10 ± 0.25b |
| Metabolic related enzymes in digestive gland | ||||
| ChE | 31.82 ± 3.73a | 30.39 ± 2.46b | 5.98 ± 2.76d | 14.52 ± 3.18c |
| ASAT | 84.35 ± 2.97a | 72.19 ± 3.00b | 24.98 ± 2.54d | 65.18 ± 2.79c |
| ALAT | 47.07 ± 3.64a | 46.12 ± 2.70a | 34.41 ± 1.72b | 35.32 ± 2.85b |
| LDH |
147.28 ± 16.22a |
52.28 ± 6.70b |
140.81 ± 8.76a,c |
125.43 ± 9.16c |
| DNA damage in haemocytes | ||||
| Tail inensity, TI% |
5.54 ± 0.92a |
13.23 ± 1.31b |
21.84 ± 2.60c |
25.79 ± 1.58d |
| Integrated biomarker response (IBR) | ||||
| IBR | 0 | 0.50 | 7.51 | 4.38 |
a,b,c The values with different letters in the same row are significantly different (Tukey's test, p < 0.05).
CI total = weight of soft tissue (g)/total weight (g) x 100. CI 2 = weight of soft tissue (g)/shell weight (g) x 100. CI 3 (state index) = weight of soft tissue (g)/shell length (cm) x 100. CI 4 (shell component index) = shell weight (g)/(shell weight (g) + meat weight (g)) x 100. CI 5 (condition factor) = weight of soft tissue (g)/shell lengthˆ3 (mm) x 100.
There are various approaches in the scientific literature [91, 92, 93, 94, 95] for calculating the status indices in bivalve mollusks; they can be based on the ratio of weight and length of the shell, with some formulas, using wet weight and others - dry weight, respectively. Furthermore, some authors showed that the fluctuations in the index values can be affected by water pollution [96, 97], which we also confirmed in our study. We agree with Galvao et al. [72] according to whom the studied indices are not limited only to aquaculture, but are also commonly applied as biological tools in studies on environmental pollution, as well as to assess the relationship between toxic substances and mussels’ health.
3.2.2. Histochemical observations
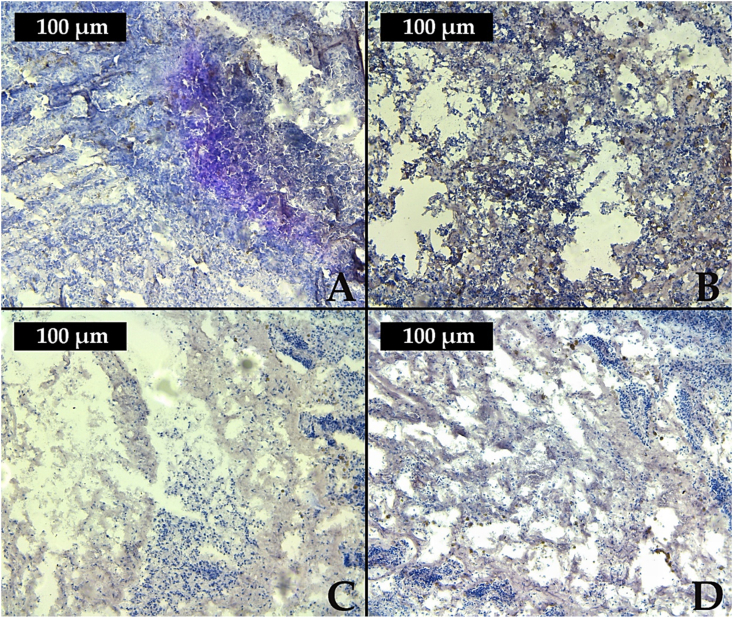
The results on histochemical analyses showed a moderately positive PAS reaction in the gills of the reference group. In contrast, in the case of tested reservoirs, we observed a general tendency towards reducing the amount of glycogen in the mussels' gills. Furthermore, in the mussels' gills from Kardzhali and Student Kladenets reservoirs, a weak positive reaction of the histochemical staining was revealed, which was also expressed in discrete pink-violet colors. Regarding the mussels from Zhrebchevo Reservoir, we found a moderately positive PAS reaction with similar intensity to the reference group (Table 2). This in turn also showed a similar level of glycogen accumulation in the mussels’ gill cells (Figure 2).
Figure 2.
Intensity of PAS-reaction in the gills of mussels: A – reference site, B – Zhrebchevo; C – Kardzhali; D – Studen Kladenets reservoirs.
Based on the obtained results, we can summarize that in the gills of the tested individuals from Kardzhali and Studen Kladenets reservoirs there was a stronger decrease in the amount of glycogen compared to Zhrebchevo Reservoir. This is a possible indication of chemical stress in the mussels' organisms as a result of the polluted waters with metals, toxic elements, and organic compounds [63, 66]. The different degrees of intensity of PAS reaction in the caged mussels (Table 2) also showed a different degree of glycogen depletion in the mussels’ cells. This could be explained by varying stages of glycolysis processes, associated with depletion of energy reserves, as a result of induced chemical stress.
Our opinion is in line with Au [98] that the use of histological and histochemical biomarkers to determine the effects of environmental pollution is perceived as an extremely important approach, as they specifically reflect the health status of organisms. Moreover, we agree with Pathan et al. [99] who indicated that histochemical studies help to demonstrate the localization of proteins, lipids, and glycogen at the cellular level, with the main advantage of applying such methods, being in the analysis of biological phenomena in “individual cells”.
Overall, a similar result to ours was observed by Bakry et al. [100] who explained that a reduction in the glycogen content in tissues leads to an increase in the glucose level, thus providing the organism enough energy to detoxify, breakdown and excrete toxicants. The authors reported a decrease in the levels of glycogen and an increase in the levels of glucose in hemolymph from the freshwater snail Bulinus truncates (Audouin, 1827) exposed to pesticides. We also confirmed the findings of Ansaldo et al. [101] of the potential use of glycogen level as a biomarker of chemical stress in caged mussels for the aim of environmental monitoring and risk assessment in natural water bodies or under laboratory conditions. We agree that under conditions of oxidative stress, caused by water pollution the body responds by including defense mechanisms, such as increasing the antioxidant defense mechanisms. However, in the case of excessive free radical formation, antioxidant mechanisms can be completely compromised. Our opinion is in line with Bickler and Buck [102] that another important response to oxidative stress in the regulation of the glycolysis process, which is an easily available energy resource. Hence, the main function of body fat is associated with lipid, glycogen, and protein storage [103]. In addition, we confirmed that the biotransformation of toxic compounds requires the use of energetic reserves, which are needed for other vital processes as explained by Köhler et al. [104] and Ansaldo et al. [101]. Furthermore, our findings are in line with Triebskorn and Köhler [105] who reported depletion of lipids and glycogen in the hepatopancreas of the mollusk Deroceras reticulatum (O. F. Müller, 1774), with Köhler et al. [104] who found a decrease in the number of lipids in the hepatopancreas of the isopod Porcellio scaber (Latreille, 1804), and also with Ansaldo et al. [101] who observed that the exposure to toxicants resulted in glycogen depletion in the gastropod Biomphalaria glabrata (Say, 1818). Lastly, we confirmed the results of Da Silva Souza et al. [106] that the caged mussels from Kardzhali, Studen Kladenets, and Zhrebchevo reservoirs most likely consumed their energy reserves in an attempt to reestablish the organism integrity, however, we did not observe lethal outcome as the reported authors did.
3.2.3. Biochemical responses
Data of all examined enzyme activities were normally distributed (Shapiro-Wilk test, p > 0.05) and their variances were homogeneous (Levene's test, p > 0.05).
3.2.4. Oxidative stress-related enzymes
Oxidative stress can take place in mussels under a series of environmental adverse conditions, including water pollution, which we showed in our study. In general, the biomarkers of oxidative stress can be split into two main groups as explained by Uchendu [107]: biomarkers for free radicals in the biological systems and antioxidant defense factors. The biomarkers used in the present study belong to the group of antioxidant defense factors. The analyses revealed changes in the enzymatic activity of the following antioxidant enzymes - catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) in the digestive glands of caged mussels from the tested reservoirs (Table 2).
Overall, the analyses showed that generally there were no significant differences between the oxidative stress-related enzymatic activities in mussels from Zhrebchevo Reservoir and those in mussels from the reference site. However, oxidative stress-related enzymatic activities in mussels from Studen Kladenets were significantly higher than those from Zhrebchevo Reservoir and the reference site, respectively. We consider that these phenomena indicated that Studen Kladenets Reservoir was the most polluted water basin according to the changes in enzymatic activities, and thus Zhrebchevo Reservoir was the least polluted water body (the biochemical values did not differ significantly from the reference site). Our bioaccumulation analyses also confirmed these results [63, 66].
The CAT activity in mussels from Studen Kladenets and Kardzhali reservoirs was significantly higher compared to the reference group (ANOVA, F = 324.3, p < 0.001) (Table 2). At the same time, there were no significant differences between the oxidative stress-related enzymatic activities in mussels from Zhrebchevo Reservoir and mussels from the reference site (p > 0.05) (Table 2).
We agree with Regoli et al. [108] that when organisms are under stress conditions, antioxidant enzymes, including the CAT activity could be increased to eliminate the excessively produced reactive oxygen species (ROS), more precisely the higher rates of H2O2 production and prevent further cellular damage. Moreover, our findings are in agreement with Duarte et al. [109], Freitas et al. [110], and Velez et al. [111] who assessed the impact of metals on the CAT activity in different mussel species. These results endorsed the capacity of mussel's cells to detoxify superoxide (O2−), which is the precursor of most of the other ROS through the antioxidant enzymatic system.
The activity of GPx in mussels from Studen Kladenets Reservoir was significantly higher compared to the reference site and Zhrebchevo Reservoir (ANOVA, F = 7.066, p < 0.01) (Table 2).
The GPx catalyzes the degradation of hydrogen peroxide (H2O2), which originates from the dismutation of superoxide radical by the superoxide dismutase (SOD) activity. As explained by Lesser [112] it also catalyzes the degradation of lipid hydroperoxides in alcohols. Our results for the elevated levels of GPx are in line with Orbea and Cajaraville [113] who found an increased GPx activity in invertebrate species from highly polluted sites compared to the respective reference sites, and also with Cong et al. who reported an increased GPx activity in bivalves exposed to metals under laboratory conditions [114].
The GR activity in the mussels from Studen Kladenets and Kardzhali reservoirs was significantly higher than that of the reference and Zhrebchevo sites (ANOVA, F = 15.680, p < 0.001) (Table 2). Our findings are coherent with Lacroix et al. [90] whose data suggested a higher GSH production, which could subsequently enhance ROS scavenging and phase-II biotransformation. Furthermore, we agree with Ferreira et al. who found in their study on transplanted oysters from an estuary in Brazil that the increased GR activity could be explained with a possible increase in the cellular GSH supply [115].
As explained by Regoli and Guiliani, and Ferreira et al., GR is an NADPH-dependent enzyme, involved in the reduction of oxidized glutathione (GSSG) to GSH, previously needed by antioxidant and biotransformation enzymes (e.g., GPx and GST) for ROS and chemical detoxification [115, 116].
We agree that the biochemical changes observed in the caged mussels are expected to be energetically costly and therefore, lead to increased energy consumption as shown by Sokolova et al. [117]. We also consider that the energy needed for the normal function of the body will be put into detoxification processes and our results on the biometric measurements and histochemical analyses confirmed this suggestion. Similarly to Lacroix et al. [90], we could hypothesize that there was probably activation of aerobic metabolism in the caged mussels to fulfill the energy demand, associated with an increase in the ROS generation and lipid peroxidation due to water pollution. Moreover, ROS could be generated directly by toxicants, but also indirectly by increased aerobic metabolism to sustain energetic costs of metabolic responses to pollutants, since the electron transport chain is a major site of ROS production [116]. We consider that there was perhaps activation of aerobic metabolism in the digestive glands of transplanted mussels to fulfill the energy demand associated with water pollution and a ROS metabolism activation, leading to an increased ROS generation and probably lipid peroxidation. Our results correspond with those of Murphy and Sokolova et al. [117, 118].
Regarding metal and organic (PAHs, PCBs) pollution of waters, it is well documented that it promotes the generation of reactive oxygen species (ROS) [115, 119, 120]. In addition, according to Wang et al. [121], it can trigger defense mechanisms or cause oxidative cellular damage to macromolecules, such as lipid peroxidation. In the present study we were interested in the response of transplanted mussels to oxidative stress, which includes enzymes, such as CAT, GPx, and GR. Due to funding limitations, the activity of other key enzymes, such as GST, SOD, and lipid peroxidation was not measured. Hence, further studies in this particular area are highly recommended, which will also provide information if the protective defense mechanisms in transplanted mussels were impaired due to exceeded ROS generation, resulting in lipid peroxidation. This is in agreement with Lacroix et al. who proved that oxidative pressure subsequently leads to oxidative damage (lipid peroxidation) in cases when the antioxidant mechanisms are not sufficient to maintain a balance between the generation and neutralization of ROS [90].
3.2.5. Metabolic related enzymes
According to Dong et al. [122], metabolism is the basic process of material circulation and energy exchange in a living entity. Metabolic enzymes perform different cellular functions vital for survival and homeostasis, including proteolysis and digestion, cellular respiration, energy storage, transcription, and response to the environment. These life-sustaining pathways are essential for the growth and maintenance of cellular integrity. Metabolic enzymes encompass a wide range of different classes, including carboxylases, dehydrogenases, lipoxygenases, oxidoreductases, kinases, lyases, transferases, and more.
Generally, the metabolic-related enzymes’ activities from the sampling sites were significantly lower than those of the reference site, which indicated disturbances in the metabolism linked with polluted waters.
The activity of LDH in caged mussels from Zhrebchevo and Studen Kladenets reservoirs was significantly lower than that of the mussels from the reference site (ANOVA, F = 81.69, p < 0.001). The levels of LDH in the specimens from Kardzhali Reservoir were similar to the reference site (Table 2).
LDH plays a key role in the cellular metabolic activity under the influence of oxidative stress, especially after exposure to metals [123]. LDH as a metabolic enzyme is also involved in cellular respiration and the production of the high-energy compound adenosine triphosphate (ATP) from glucose. Furthermore, LDH is responsible for the regeneration of nicotinamide adenine dinucleotide (NAD+) necessary in the metabolism of glucose and for the subsequent production of ATP from NADH for the continuation of glycolysis. Therefore, the LDH activity is a measure of the anaerobic capacity and status of the cell as explained in detail by Gagnon and Holdway [124].
The activity of ASAT differed significantly among the sampling sites (ANOVA, F = 412.80, p < 0.001). At the same time, the levels of ASAT in mussels from the tested reservoirs were lower than the reference site. In addition, the activity of ALAT in mussels from Studen Kladenets and Kardzhali reservoirs was significantly lower than that from the reference site (ANOVA, F = 29.20, p < 0.001) (Table 2).
ASAT and ALAT are sensitive criteria for hepatotoxicity and can be assessed in a shorter time [125, 126]. According to Narvia and Rantamaki [127], transaminases are among the crucial enzymes in amino acid metabolism, which in aquatic organisms is known to be affected by exposure to organic pollutants. In addition, ASAT and ALAT not only function as link enzymes between the protein and carbohydrate metabolism but also serve as indicators of the altered physiological condition under chemical stress (Table 2).
From the obtained results we could consider that the decrease in the enzymatic activities is a result of tissue lesions in the particular organ associated with degenerative processes. Therefore, we agree with Almeida et al. [128] who consider that changes in the LDH, ASAT, and ALAT activity can be used as biomarkers for tissue damage and further determination and confirmation of the presence of histological lesions. Thus, we suggest that such analyses on the digestive gland of transplanted mussels in Kardzhali, Studen Kladenets, and Zhrebchevo reservoirs should be also assessed in the research to come.
From the conducted analyses on the changes in the ChE activity, we found that there were significant differences among the sampling sites (ANOVA, F = 83.57, p < 0.001) (Table 2) with the lowest levels measured in mussels from Kardzhali and Studen Kladenets reservoirs compared to the reference site (Table 2).
The ChE activity has traditionally been applied as a specific indicator of exposure to some pesticides [96, 97], initially for organophosphorus and carbamate insecticides, but it also responds to a large variety of chemicals, such as metals and toxic elements, hydrocarbons, detergents, etc. Thus, it has been also suggested as an indicator of general health [129]. We agree with Bocquené et al. [130, 131] and Galgani and Bocquené [132] that the inhibition of ChE activity can be used as an indicator of stress under the influence of environmental toxicants. In addition, our results confirmed that in bivalves the neurotoxic impacts of different in nature pollutants were proven by the decrease of ChE activity due to its high affinity for many neurotoxic compounds, including metal(loid)s and organic chemicals [133, 134, 135, 136].
The action of metals, toxic elements, and organic pollutants, which are contained in the waters of Kardzhali, Student Kladenets and Zhrebchevo reservoirs led to disturbances in the biochemical processes that occurred in the caged mussels. In recent years, the measurement of enzymatic activity in various organs of bivalves has been used as a reliable biomarker to identify the negative effects of a polluted environment. Our findings are in line with Sevgiler et al. [137] who identified changes in the enzymatic levels as one of the most sensitive biomarkers for the negative effects of different anthropogenic toxicants. Furthermore, we agree with Rogers et al. [138] that the damage to vital organs or tissues in bivalves is ecologically relevant because it reflects the negative impact on important physiological functions, such as toxicant transformations and digestion, detoxification, growth, energy substrate storage, ionic regulation, and respiration. Thus, we consider that it is crucial to determine the harmful effects by considering the alterations in the activity of complex enzymes and to study the mechanisms of their action, as well as to try to imply different ways to reduce possible adverse effects.
3.2.6. DNA damage
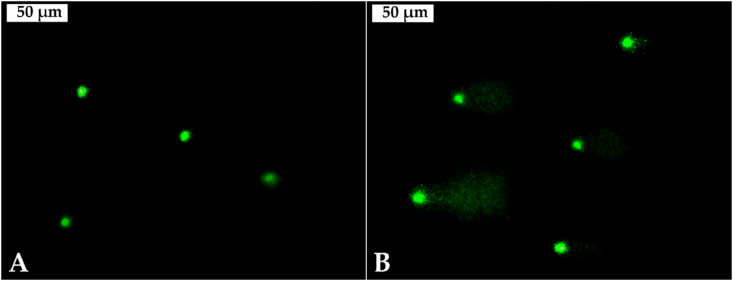
There were significant differences among the DNA tail intensity percentage in the hemocytes of mussels from the different sampling sites (ANOVA, F = 277.1, p < 0.001) (Table 2). Furthermore, a significant increase of DNA migration in the hemocytes’ nucleoids of caged mussels was observed in the three investigated reservoirs and the individuals from Studen Kladenets Reservoir showed the most intensive migration (Figure 3).
Figure 3.
Comet cells from the mussels' haemocytes: A – reference site; B – Studen Kladenets Reservoir (magnification 400 ×).
In more recent ecotoxicological studies the DNA damage is mostly evaluated by the use of DNA comets [139, 140, 141]. Furthermore, the individual cell's DNA damage is detected by the use of comet assay (single-cell gel electrophoresis) for quick evaluation of genotoxicity in aquatic organisms [142, 143, 144] caused by pollution. As a result, we agree that the method can be used for early detection of exposure to genotoxic agents like primary DNA damage (single or double-strand breaks, alkali labile sites, and crosslinks) [140, 141, 145].
We proved that the monitoring of genotoxicity in freshwater, indigenous and caged mussels, using the comet assay of hemocytes is a rather sensitive method as stated by other authors [146, 147, 148]. Our study also confirmed that the Chinese pond mussel is a good bioindicator species for in situ genotoxicity assessment. The latter has been demonstrated already through the method of comet assay in native populations from polluted waters [149, 150, 151]. The same is also valid for transplanted mussels [148].
The significant differences among the TI% in the hemocytes of caged mussels from the different sampling sites in our study confirmed the high sensitivity of the tested freshwater mussel in detecting genotoxicity induced by waterborne pollutants, such as metals, toxic elements, and organic compounds.
Our results revealed elevated levels of DNA damage in the mussels’ hemocytes from all the studied reservoirs. This confirms the genotoxic effect, caused by the polluted waters [63, 66]. DNA damage detected in the mussels sampled from Studen Kladenets Reservoir was the highest (Table 2, Figure 3) and can be attributed to the former lead-zinc ore processing plant “Kardzhali”. This corresponds to the results for bioaccumulation of metals and toxic elements, as well as organic priority substances: six polybrominated diphenyl ethers (PBDEs) congeners and short-chain chlorinated paraffins (SCCPs) in caged mussels from the sampling sites [63]. The highest values for Cd, Pb, Zn, as well as for PBDEs and SCCPs, were measured in the mussels from Studen Kladenets Reservoir. Cd, Pb, and Zn caused genotoxic responses – DNA and/or chromosomal fragmentations, and DNA strand breakage in aquatic freshwater organisms as previously described [151, 152, 153]. Moreover, metals cause DNA damage in living cells because they form strong covalent bonds with DNA [154]. This results in the formation of DNA adducts, which prevent replication. The genotoxic effect of Cd is indirect and is due to the oxidative stress and inactivation of several DNA repair enzymes [153]. The genotoxic effects of Pb in vitro and in vivo are widely known, but the biochemical and molecular mechanisms of Pb impact are still unclear. Inhibition of DNA repair or production of free radicals was reported as indirect mechanism of Pb genotoxicity [155]. It is also known that the brominated compounds cause double-strand breaks, as well as DNA damage mediated through reactive oxygen species (ROS) [156]. The comet assay revealed that all the assessed PBDEs exerted genotoxic effects in HepG2 cells according to Pereira et al. [157], and BDE-209 induced DNA damages in in vivo exposed freshwater bivalve zebra mussel, Dreissena polymorpha under laboratory conditions [158]. According to Wang et al. [121], the metabolism of SCCPs can cause the generation of ROS. The deduction of oxidative stress destroys cellular components, causing DNA damage and genetic mutations, which may underlie the toxic effect mechanisms of SCCPs [121]. In our study, oxidative stress-related enzymatic activities in mussels from Studen Kladenets Reservoir were significantly higher than those from Zhrebchevo Reservoir and the reference site. The SCCPs (6.12 μg kg−1) in the mussels from Studen Kladenets Reservoir were 27 times higher than those measured in the Zhrebchevo Reservoir and 10 times higher than those in the Kardzhali Reservoir [63]. Therefore, we consider that the highest level of DNA damage in Studen Kladenets Reservoir can be attributed to pollutants, such as metals and toxic elements, PBDEs, and SCCPs accumulated in the soft tissues of caged mussels.
The genotoxicity detected at the other two reservoirs could be attributed mainly to the measured metals concentrations, with the highest levels for Cr, Cu, Ni in the mussels from Kardzhali Reservoir, and for Al, As, Co, Fe, Mn in the mussels from Zhrebchevo Reservoir [63]. A similar finding was observed by Khan et al. (Khan et al., 2019) who reported that the higher concentrations of metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn) in the soft tissues of freshwater mussels showed significantly higher DNA damage from metal polluted waters as compared to the reference site.
The observed DNA damage in transplants from all three reservoirs can also be further explained by the effect of synergism between the different toxicants. The various combinations of pesticides and metals act synergistically and result in a more severe toxic effect compared to a single molecule alone [159]. The effect of synergism can also explain the statistically significant DNA damage in the mussels from Zhrebchevo Reservoir, mainly polluted due to intense agricultural activity, as well as with the presence of metal pollution reported before [59, 60].
The DNA damage due to genotoxic pollution was proved by the comet assay technique on hemocytes in transplants in other studies during the last decade [84, 148, 149]. The highest values of TI% (28.8 ± 1.6 for Unio sp. and 21.9 ± 1.5 for S. woodiana) in specimens collected from different sites under pollution pressure in Danube River [84] were close to those for Studen Kladenets Reservoir (25.79 ± 1.58) and Kardzhali Reservoir (21.84 ± 2.60) in our study. Slightly lower levels of DNA damage of the other polluted sites along the Danube River were obtained in the study of Marić et al. [148] – the highest level of TI% recorded in hemocytes of Chinese pond mussel specimens was 18.42 ± 0.72, while the lowest was 10.95 ± 0.58.
Our results confirmed the comet assay as a sensitive biomarker for the detection of DNA damage in bivalves from polluted waters. Based on the obtained results, we can summarize that the significantly higher levels of DNA damage measured in the hemocytes of caged mussels from Studen Kladenets, Kardzhali, and Zhrebchevo reservoirs compared to the reference site revealed the genotoxic potential of the waters of the three reservoirs, which we associated with the presence of metals, toxic elements and organic toxicants, such as PBDEs and SCCPs.
3.2.7. Relationships between pollutant levels and biomarker responses
The results of correlation analysis, which was performed to study the possible relationships between the MPI and the different biomarker responses, such as biometric indices, enzymatic responses, and tail intensity (DNA damage), were summarized in Table 3.
Table 3.
Correlation coefficients between metal pollution index (MPI) and biomarker responses of mussels from different sampling sites in Bulgaria, significant at p < 0.05 (N = 20).
| Biometric indices/biomarker responses | Spearman's rang correlation coefficient |
|---|---|
| CI total | n.s. |
| CI 2 | n.s. |
| CI 3 | -0.824 |
| CI 4 | n.s. |
| CI 5 | -0.592 |
| CAT | 0.745 |
| GPx | 0.718 |
| GR | 0.904 |
| ChE | -0.710 |
| ASAT | -0.760 |
| ALAT | -0.772 |
| LDH | n.s. |
| Tail intensity | 0.953 |
n.s.: Non-significant.
CI total = weight of soft tissue (g)/total weight (g) x 100, CI 2 = weight of soft tissue (g)/shell weight (g) x 100, CI 3 (state index) = weight of soft tissue (g)/shell length (cm) x 100, CI 4 (shell component index) = shell weight (g)/(shell weight (g) + meat weight (g)) x 100, CI 5 (condition factor) = weight of soft tissue (g)/shell lengthˆ3 (mm) x 100.
ASAT – aspartate aminotransferase, ALAT – alanine aminotransferase, CAT – catalase, ChE – cholinesterase, GPx – glutathione peroxidase, GR – glutathione reductase, and LDH – lactate dehydrogenase in the digestive glands of caged mussels (U/mg protein).
Tail intensity (TI%) – average results of DNA damage (percentage of DNA in the tail of the comet) in the haemocytes of caged mussels.
In the case of biometric indices, only the state index (CI 3) and the condition factor (CI 5) showed a significant (p < 0.05) negative correlation with the MPI of caged mussels. According to these results, with the increasing values of MPI, the state index (CI 3) and condition factor (CI 5) decreased significantly. Therefore, the mussels with higher concentrations of metals had lower biometric indices.
Between the MPI and the oxidative stress-related enzymatic activities (CAT, GPx, GR) there were significant (p < 0.05) positive correlations. At the same time, the metabolic-related enzyme activities (except for LDH) showed a significant decrease (p < 0.05) with the increasing values of MPI. These results indicated that the mussels with higher values of MPI increased the activities of oxidative stress-related enzymes. On the other hand, the metabolic-related enzymes (ASAT, ALAT, ChE) showed a significant negative correlation with MPI. This means that mussels with higher values of MPI had lower activities of metabolic-related enzymes.
Furthermore, it is also important to highlight that between the MPI and TI% a significant (p < 0.05) positive correlation (Table 3) was established. This result confirmed that the mussels with higher MPI had notable DNA damage.
The IBR values also showed that overall, the pollution status based on the investigated biomarkers was the lowest in the case of reference site (Table 2). Zhrebchevo Reservoir had a relatively low IBR value, which indicates a lower pollution status of this sampling site. At the same time, Kardzhali, and Studen Kladanets reservoirs were more impacted, which supports the presently reported results.
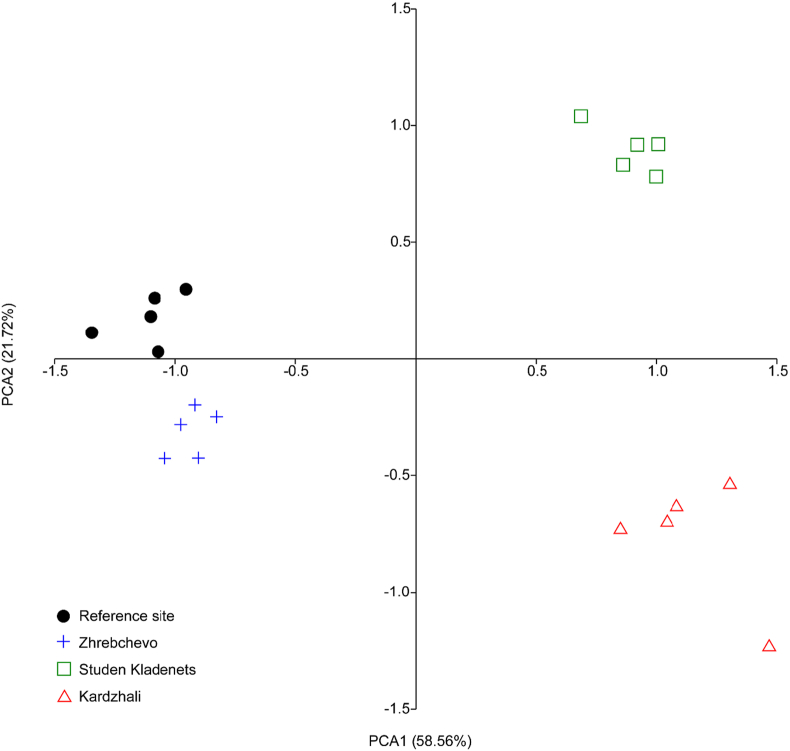
The PCA showed clear separations among all investigated biomarkers of mussels from the different sampling sites in Bulgaria (Figure 4). The first component (PCA 1) contributed 58.56% of the total variance, while the second (PCA 2) contributed 21.72% of the total variance (Figure 4). Based on the biomarker pattern, the groups were completely separated from the other groups, which supported the results of the regression analyses.
Figure 4.
Principal component analysis of biometric measurements; PAS-reaction in the gills; oxidative stress related enzymes' activities (catalase – CAT, glutathione peroxidase – GPx, and glutathione reductase – GR) and metabolic related enzymes' activities (cholinesterase – ChE, aspartate aminotransferase – ASAT, alanine aminotransferase – ALAT, and lactate dehydrogenase – LDH) in the digestive glands (U/mg protein); and DNA damage (percentage of DNA in the tail of the comet (tail intensity – TI%)) in the hemocytes of mussels from different sampling sites in Bulgaria.
In a similar way, Ferreira et al. [115] studied the possible relationships between metal levels and biochemical biomarkers in oysters (Crassostrea gasar Deshayes, 1830). Ferreira et al. [119] also investigated the relationship between organic toxicants (polycyclic aromatic hydrocarbons, PAHs, polychlorinated biphenyls, PCBs, and linear alkylbenzenes, LABs) and molecular, and biochemical markers in transplanted oysters. Moreover, our results are in agreement with Capolupo et al. [160] who followed the correlation trends between CAT activity, DNA damage, and caffeine concentrations in the Mediterranean mussel (Mytillus galloprovincialis Lamark, 1819), which were dose-dependent.
4. Conclusions
In summary, we can conclude that the mussel caging is a relatively novel method in Bulgaria. The waters of Studen Kladenets and Kardzhali reservoirs overall changed different biomarker responses in the caged mussels more significantly compared to the waters of Zhrebchevo Reservoir due to a different anthropogenic load. The results from this pilot study are essential for improving the assessment and monitoring programs of water pollution effects. In addition, these data suggest that the approach with transplanted mussels should be applied regularly in ecotoxicological studies in Bulgaria and respectively on the Balkans, along with classical bioaccumulation analyses of water/sediments and biota in polluted reservoirs. We recommend that a battery of biomarkers, which include biometric measurements, histochemical, biochemical, and genotoxic assays are also integrated because it is essential to use easily applicable and representative biological tools for determining the toxicant impact on bivalves in freshwater ecosystems. Lastly, we recommend that further investigation is performed to study in detail the combined exposure to inorganic and organic pollutants, and their effects on the Chinese pond mussel, which may reveal antagonistic or synergistic interactions.
Declarations
Author contribution statement
Elenka Georgieva: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
László Antal & Ifeanyi Emmanuel Uzochukwu: Analyzed and interpreted the data; Wrote the paper.
Stela Stoyanova & Vesela Yancheva: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Desislava Aranudova, Iliana Velcheva, Ilia Iliev, Tonka Vasileva, Veselin Bivolarski, Vesela Mitkovska, Tsenka Chassovnikarova & Borislava Todorova: Performed the experiments.
Krisztián Nyeste: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Live water, air and health with transplants - LIFE (FP19-BF-013), NPD – Plovdiv University “Paisii Hilendarski”, the National Research, Development and Innovation Fund of Hungary (TKP2021-NKTA-32) and the New National Excellence Program of the Ministry of Human Capacities (ÚNKP-21-4).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We sincerely thank Assoc. Prof. Gana Gecheva for fulfilling a wish to work on caged mussels despite all challenges in the field.
References
- 1.Chapman P. Heavy metal—music, notscience. Environ. Sci. Technol. 2007;41:6. [PubMed] [Google Scholar]
- 2.Czédli H., Csedreki L., Szíki Á.G., Jolánkai G., Pataki B., Hancz C., Antal L., Nagy S.A. Investigation of the bioaccumulation of Copper in fish. Fresenius Environ. Bull. 2014;23:1547–1552. [Google Scholar]
- 3.Ji Z., Zhang H., Zhang Y., Chen T., Long Z., Li M., Pei Y. Distribution, ecological risk and source identification of heavy metals in sediments from the Baiyangdian Lake, Northern China. Chemosphere. 2019:237. doi: 10.1016/j.chemosphere.2019.124425. [DOI] [PubMed] [Google Scholar]
- 4.Maghrebi M., Karbassi A., Lak R., Noori R., Sadrinasab M. Temporal metal concentration in coastal sediment at the north region of Persian Gulf. Mar. Pollut. Bull. 2018;135:880–888. doi: 10.1016/j.marpolbul.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Salam M.A., Paul S.C., Shaari F.I., Rak A.E., Ahmad R.B., Kadir W.R. Geostatistical distribution and contamination status of heavy metals in the sediment of Perak River, Malaysia. Hydrology. 2019;6 [Google Scholar]
- 6.Smal H., Ligęza S., Wójcikowska-Kapusta A., Baran S., Urban D., Obroslak R., Pawłowski A. Spatial distribution and risk assessment of heavy metals in bottom sediments of two small dam reservoirs (south-east Poland) Arch. Environ. Protect. 2015;41:67–80. [Google Scholar]
- 7.Somlyai I., Berta C., Nagy S.A., Dévai G., Ács É., Szabó L.J., Nagy J., Grigorszky I. Heterogeneity and anthropogenic impacts on a small lowland stream. Water (Switzerland) 2019:11. [Google Scholar]
- 8.Kostka A., Leśniak A. Spatial and geochemical aspects of heavy metal distribution in lacustrine sediments, using the example of Lake Wigry (Poland) Chemosphere. 2020:240. doi: 10.1016/j.chemosphere.2019.124879. [DOI] [PubMed] [Google Scholar]
- 9.Müller M.H.B., Polder A., Brynildsrud O.B., Lie E., Løken K.B., Manyilizu W.B., Mdegela R.H., Mokiti F., Murtadha M., Nonga H.E., Skaare J.U., Lyche J.L. Brominated flame retardants (BFRs) in breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Int. 2016;89–90:38–47. doi: 10.1016/j.envint.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M., Guo W., Wei J., Shi J., Zhang J., Ge H., Tao H., Liu X., Hu Q., Cai Z. Determination of newly synthesized dihydroxylated polybrominated diphenyl ethers in sea fish by gas chromatography-tandem mass spectrometry. Chemosphere. 2020;240 doi: 10.1016/j.chemosphere.2019.124878. [DOI] [PubMed] [Google Scholar]
- 11.Frederiksen M., Vorkamp K., Thomsen M., Knudsen L.E. Human internal and external exposure to PBDEs - a review of levels and sources. Int. J. Hyg Environ. Health. 2009;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Cox P., Efthymiou P. Directive 2003/11/EC of the European parliament and of the council of February 6 2003 amending for the 24th time Council Directive 76/669/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether), off. J. Eur. Union. 2003;42:45–46. [Google Scholar]
- 13.Novak P., Zuliani T., Milačič R., Ščančar J. Development of an analytical method for the determination of polybrominated diphenyl ethers in mussels and fish by gas chromatography—inductively coupled plasma mass spectrometry. J. Chromatogr., A. 2017;1524:179–187. doi: 10.1016/j.chroma.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 14.Feo M.L., Eljarrat E., Barceló D. Occurrence, fate and analysis of polychlorinated n-alkanes in the environment. TrAC Trends Anal. Chem. (Ref. Ed.) 2009;28:778–791. [Google Scholar]
- 15.Zhou J., Kang H.M., Lee Y.H., Jeong C.B., Park J.C., Lee J.S. Adverse effects of a synthetic pyrethroid insecticide cypermethrin on life parameters and antioxidant responses in the marine copepods Paracyclopina nana and Tigriopus japonicus. Chemosphere. 2019;217:383–392. doi: 10.1016/j.chemosphere.2018.10.217. [DOI] [PubMed] [Google Scholar]
- 16.Environment U. United Nations Environment Programme; 2018. UN Environment Annual Report 2017.https://www.unep.org/resources/un-environment-annual-report-2017 [Google Scholar]
- 17.European Commission, Commission Staff Working Document . 2020. Chemicals Strategy for Sustainability towards a Toxic-free Environment, Brussels.https://ec.europa.eu/environment/pdf/chemicals/2020/10/SWD_on_Endocrines_disruptors.pdf [Google Scholar]
- 18.Olenycz M., Sokotowski A., Niewińska A., Wołowicz M., Namies¨nik J., Hummel H., Jansen J. Comparison of PCBs and PAHs levels in European coastal waters using mussels from the Mytilus edulis complex as biomonitors. Oceanologia. 2015;57:196–211. [Google Scholar]
- 19.Van Cauwenberghe L., Janssen C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Melwani A.R., Gregorio D., Jin Y., Stephenson M., Ichikawa G., Siegel E., Crane D., Lauenstein G., Davis J.A. Mussel watch update: long-term trends in selected contaminants from coastal California, 1977-2010. Mar. Pollut. Bull. 2014;81:291–302. doi: 10.1016/j.marpolbul.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Sárkány-Kiss A., Herczeg I., Palombi B., Grigorszky I., Antal L., Bácsi I., Mozsár A., Kalmár A.F., Nagy S.A. Toxicity tests of chlorinated hydrocarbons on the river mussel, Unio crassus (Bivalvia, Unionidae), North. West. J. Zool. 2012;8:358–361. http://biozoojournals.3x.ro/nwjz/index.html [Google Scholar]
- 22.Beyer J., Green N.W., Brooks S., Allan I.J., Ruus A., Gomes T., Bråte I.L.N., Schøyen M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: a review. Mar. Environ. Res. 2017;130:338–365. doi: 10.1016/j.marenvres.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Butler P.A. Organochlorine residues in estuarine molluscs, 1965-1972 - national pesticide monitoring program. Pesticides Monit. J. 1973;6:238–262. [PubMed] [Google Scholar]
- 24.Glad M., Bihari N., Jakšić Ž., Fafanđel M. Comparison between resident and caged mussels: polycyclic aromatic hydrocarbon accumulation and biological response. Mar. Environ. Res. 2017;129:195–206. doi: 10.1016/j.marenvres.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Avio C.G., Cardelli L.R., Gorbi S., Pellegrini D., Regoli F. Microplastics pollution after the removal of the Costa Concordia wreck: first evidences from a biomonitoring case study. Environ. Pollut. 2017;227:207–214. doi: 10.1016/j.envpol.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 26.Catarino A.I., Macchia V., Sanderson W.G., Thompson R.C., Henry T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018;237:675–684. doi: 10.1016/j.envpol.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 27.Railo S., Talvitie J., Setälä O., Koistinen A., Lehtiniemi M. Application of an enzyme digestion method reveals microlitter in Mytilus trossulus at a wastewater discharge area. Mar. Pollut. Bull. 2018;130:206–214. doi: 10.1016/j.marpolbul.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 28.d’Errico G., Nardi A., Benedetti M., Mezzelani M., Fattorini D., Di Carlo M., Pittura L., Giuliani M.E., Macchia S., Vitiello V., Sartori D., Scuderi A., Morroni L., Chiaretti G., Gorbi S., Pellegrini D., Regoli F. Application of a multidisciplinary weight of evidence approach as a tool for monitoring the ecological risk of dredging activities. Front. Mar. Sci. 2021;8:765256. [Google Scholar]
- 29.Manfra L., Maggi C., D’Errico G., Rotini A., Catalano B., Maltese S., Moltedo G., Romanelli G., Sesta G., Granato G., Lanera P., Amici M., Martuccio G., Onorati F., Di Mento R., Berducci M.T., Chiaretti G., Faraponova O., Regoli F., Tornambè A. A weight of evidence (Woe) approach to assess environmental hazard of marine sediments from adriatic offshore platform area. Water (Switzerland) 2021;13:1691. [Google Scholar]
- 30.Schøyen M., Allan I.J., Ruus A., Håvardstun J., Hjermann D., Beyer J. Comparison of caged and native blue mussels (Mytilus edulis spp.) for environmental monitoring of PAH, PCB and trace metals. Mar. Environ. Res. 2017;130:221–232. doi: 10.1016/j.marenvres.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Turja R., Lehtonen K.K., Meierjohann A., Brozinski J.M., Vahtera E., Soirinsuo A., Sokolov A., Snoeijs P., Budzinski H., Devier M.H., Peluhet L., Pääkkönen J.P., Viitasalo M., Kronberg L. The mussel caging approach in assessing biological effects of wastewater treatment plant discharges in the Gulf of Finland (Baltic Sea) Mar. Pollut. Bull. 2015;97:135–149. doi: 10.1016/j.marpolbul.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Larsson J., Smolarz K., Świeżak J., Turower M., Czerniawska N., Grahn M. Multi biomarker analysis of pollution effect on resident populations of blue mussels from the Baltic Sea. Aquat. Toxicol. 2018;198:240–256. doi: 10.1016/j.aquatox.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Swaleh S.B., Banday U.Z., Al Asadi M., Usmani N. Biochemical profile and gene expression of Clarias gariepinus as a signature of heavy metal stress. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114693. [DOI] [PubMed] [Google Scholar]
- 34.Klimova Y.S., Chuiko G.M., Pesnya D.S., Ivanova E.S. Biomarkers of oxidative stress in freshwater bivalve mollusks (Review) Inl. Water Biol. 2020;13:674–683. [Google Scholar]
- 35.Binelli A., Della Torre C., Magni S., Parolini M. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environ. Pollut. 2015;196:386–403. doi: 10.1016/j.envpol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Binelli A., Cogni D., Parolini M., Provini A. Multi-biomarker approach to investigate the state of contamination of the R. Lambro/R. Po confluence (Italy) by zebra mussel (Dreissena polymorpha) Chemosphere. 2010;79:518–528. doi: 10.1016/j.chemosphere.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 37.Faria M., Ochoa V., Blázquez M., Juan M.F.S., Lazzara R., Lacorte S., Soares A.M.V.M., Barata C. Separating natural from anthropogenic causes of impairment in Zebra mussel (Dreissena polymorpha) populations living across a pollution gradient. Aquat. Toxicol. 2014;152:82–95. doi: 10.1016/j.aquatox.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Evariste L., David E., Cloutier P.L., Brousseau P., Auffret M., Desrosiers M., Groleau P.E., Fournier M., Betoulle S. Field biomonitoring using the zebra mussel Dreissena polymorpha and the quagga mussel Dreissena bugensis following immunotoxic reponses. Is there a need to separate the two species? Environ. Pollut. 2018;238:706–716. doi: 10.1016/j.envpol.2018.03.098. [DOI] [PubMed] [Google Scholar]
- 39.Kim B.H., Lee J.H., Hwang S.J. Inter- and intra-specific differences in filtering activities between two unionids, Anodonta woodiana and Unio douglasiae, in ambient eutrophic lake waters. Ecol. Eng. 2011;37:1957–1967. [Google Scholar]
- 40.Douda K., Vrtílek M., Slavík O., Reichard M. The role of host specificity in explaining the invasion success of the freshwater mussel Anodonta woodiana in Europe. Biol. Invasions. 2012;14:127–137. [Google Scholar]
- 41.Watters G.T. A synthesis and review of the expanding range of the asian freshwater mussel Anodonta woodiana (Lea, 1834)(Bivalvia, Unionidae). Síntesis y revisión del rango de expansión del mejillón asiático de agua dulce Anodonta woodiana (Lea, 1834)(Bivalvia, Unionidae) Veliger. 1997;40:152–156. [Google Scholar]
- 42.Popa O.P., Bartáková V., Bryja J., Reichard M., Popa L.O. Characterization of nine microsatellite markers and development of multiplex PCRs for the Chinese huge mussel Anodonta (Sinanodonta) woodiana Lea, 1834 (Mollusca, Bivalvia) Biochem. Systemat. Ecol. 2015;60:234–237. [Google Scholar]
- 43.Demayo C.G., Cabacaba K.M.C., Torres M.A.J. Shell shapes of the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) from Lawis stream in Iligan city and lake Lanao in Mindanao, Philippines. Adv. Environ. Biol. 2012;6:1468–1473. [Google Scholar]
- 44.Soroka M., Urbańska M., Andrzejewski W. Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia): origin of the Polish population and GenBank data. J. Limnol. 2014;73 [Google Scholar]
- 45.Lopes-Lima M., Burlakova L.E., Karatayev A.Y., Mehler K., Seddon M., Sousa R. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia. 2018;810 [Google Scholar]
- 46.Labecka A.M., Domagala J. Continuous reproduction of Sinanodonta woodiana (Lea, 1824) females: an invasive mussel species in a female-biased population. Hydrobiologia. 2018;810:57–76. [Google Scholar]
- 47.Packet J., Van den Neucker T., Sablon R. 2009. Distribution of the Chinese Pond Mussel, Sinanodonta Woodiana (Lea, 1834) in Flanders (Belgium): Ready for the Invasion?, Brussels, Belgium Res. [Google Scholar]
- 48.Bolotov I.N., Bespalaya Y.V., Gofarov M.Y., Kondakov A.V., Konopleva E.S., Vikhrev I.V. Spreading of the Chinese pond mussel, Sinanodonta woodiana, across Wallacea: one or more lineages invade tropical islands and Europe. Biochem. Systemat. Ecol. 2016;67:58–64. [Google Scholar]
- 49.Chen X., Liu H., Su Y., Yang J. Morphological development and growth of the freshwater mussel Anodonta woodiana from early juvenile to adult. Invertebr. Reprod. Dev. 2015;59:131–140. [Google Scholar]
- 50.Iliev I., Hadjinikolova L. Seasonal and vertical dynamics of the water temperature and oxygen content in Kardzhali reservoir, Bulgaria. Agric. Sci. Technol. 2013;5:212–215. [Google Scholar]
- 51.Dochin K., Iliev I. Functional classifi cation of phytoplankton in Kardzhali reservoir (Southeast Bulgaria) Bulg. J. Agric. Sci. 2019;25:385–395. [Google Scholar]
- 52.Velcheva I.G. Zinc content in the organs and tissues of freshwater fish from the Kardjali and studen Kladenets dam lakes in Bulgaria. Turk. J. Zool. 2006;30:1–7. [Google Scholar]
- 53.Zhelev Z.M., Popgeorgiev G.S., Mehterov N.H. Haematological parameters of Pelophylax ridibundus(Amphibia: Ranidae) from the region of the Lead and Zinc Plant “Kardzhali” (South Bulgaria) and their use in the environmental quality assessment. Acta Zool. Bulg. 2015;67:271–282. http://www.bg-ibr.org [Google Scholar]
- 54.Velcheva I. Investigation on content of Càdmium (Cd) in different origins of chub (Leuciscus cephalus L./Pisces, Famille Cyprinide) from Arda river valley. Biology (Basel) 1995;6:86–89. [Google Scholar]
- 55.Velcheva I. Research on the content of cadmium in different organs of perch (Perca fluviatilis L.) (Pisces, Percidae) from the artificial lakes “Kardjali” and “studen Kladenetz” on river Arda. Acta Zool. Bulg. 1997:43–48. [Google Scholar]
- 56.Bachvarov G., Velcheva I. Etude du contenu de plomb (Pb), zinc (Zn), cadmium (Cd) dans Leuciscus cephalus L. (Pisces, Famille Cyprinidae) du bassin de la riviere Arda (Bulgarie du Sud) Traveaux Sci. l’Univ. Plovdiv. Biol. 1995;6:71–75. [Google Scholar]
- 57.Arnaudova D., Arnaudov A., Tomova E. Selected hematological indices of freshwater fish from Studen Kladenetsh Reservoir. Bulg. J. Agric. Sci. 2008;14:244–250. [Google Scholar]
- 58.Velcheva I., Tomova E., Arnaudova D., Arnaudov A. Morphological investigation on gills and liver of fresh water fish from Dam Lake" Studen Kladenets. Bulg. J. Agric. Sci. 2010;16:364–368. [Google Scholar]
- 59.Kalchev R., Beshkova M., Botev I., Kalcheva H., Kozuharov D., Trichkova T. Effect of Dreissena polymorpha (Bivalvia: Dreissenidae) on physicochemical characteristics of Zhrebchevo reservoir (Central Bulgaria) Compt. Rendus l’Acad ´emie Bulg. Des Sci. 2013;66:1571–1578. [Google Scholar]
- 60.Zhelyazkov G., Georgiev D., Dospatliev L., Staykov Y. Determination of heavy metals in roach (Rutilus rutilus) and bleak (Alburnus alburnus) in Zhrebchevo Dam Lake. Ecol. Balk. 2014;5:15–20. [Google Scholar]
- 61.Ognjanova-Rumenova N.G., Rashkova L.E., Botev I.S., Trichkova T.A. Diversity of benthic diatoms in relation to environmental factors in two Bulgarian reservoirs. Phytol. Balc. 2013;19:307–322. [Google Scholar]
- 62.Naidenow W. Changes of zooplankton structure in the Zhrebchevo Reservoir as a result of pollution and hydrotechnical devices. Hydrobiology. 1981;15:22–43. [Google Scholar]
- 63.Gecheva G., Yancheva V., Velcheva I., Georgieva E., Stoyanova S., Arnaudova D., Stefanova V., Georgieva D., Genina V., Todorova B., Mollov I. Integrated monitoring with moss-bag and mussel transplants in reservoirs. Water (Switzerland) 2020;12 [Google Scholar]
- 64.Kazour M., Amara R. Is blue mussel caging an efficient method for monitoring environmental microplastics pollution? Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.135649. [DOI] [PubMed] [Google Scholar]
- 65.Rosseland B.O., Massabuau J.C., Grimalt J., Hofer R., Lackner R., Raddum G., Rognerud S., Vives I. Norwegian Institute for Water Research (NIVA); Oslo, Norway: 2003. Fish Ecotoxicology: European mountain lake Ecosystems Regionalisation, Diagnostic and Socio-Economic Evaluation (EMERGE) [Google Scholar]
- 66.Gecheva G., Mollov I., Yahubyan G., Gozmanova M., Apostolova E., Nikolova M., Dimitrova-Dyulgerova I., Radoukova T., Vasileva T. Can biomarkers respond upon freshwater pollution?—a moss-bag approach. Biology (Basel) 2021;10:1–10. doi: 10.3390/biology10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ju Y.R., Chen C.W., Chen C.F., Chuang X.Y., Di Dong C. Assessment of heavy metals in aquaculture fishes collected from southwest coast of Taiwan and human consumption risk. Int. Biodeterior. Biodegrad. 2017;124:314–325. [Google Scholar]
- 68.Nyeste K., Dobrocsi P., Czeglédi I., Czédli H., Harangi S., Baranyai E., Simon E., Nagy S.A., Antal L. Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): Juveniles are useful bioindicators of recent pollution. Ecol. Indicat. 2019;101:1–10. [Google Scholar]
- 69.Gasmi S., Bernard I., Pouvreau S., Maurer D., Schaal G., Ganthy F., Cominassi L., Allain G., Sautour B., David V. Spatial patterns in the condition index of the wild Pacific oyster Crassostrea gigas in a macrotidal coastal ecosystem: influence of tidal processes and beyond. J. Sea Res. 2017;119:28–36. [Google Scholar]
- 70.Marques H.L.A. In: Criaçao Comer. Mexlhoes, Nobel. Marques H.L.A., editor. Sao Paulo; 1998. Aspectos relativos a produçao; pp. 77–81. [Google Scholar]
- 71.Kagley A.N., Snider R.G., Krishnakumar P.K., Casillas E. Assessment of seasonal variability of cytochemical responses to contaminant exposure in the blue mussel Mytilus edulis (complex) Arch. Environ. Contam. Toxicol. 2003;44:43–52. doi: 10.1007/s00244-002-1303-3. [DOI] [PubMed] [Google Scholar]
- 72.Galvao P., Longo R., Torres J.P.M., Malm O. Estimating the potential production of the brown mussel Perna perna (Linnaeus, 1758) reared in three tropical bays by different methods of condition indices. J. Mar. Biol. 2015 [Google Scholar]
- 73.McManus J.F.A. Histological and histochemical uses of periodic acid. Stain Technol. 1948;23:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- 74.Bernet D., Schmidt H., Meier W., Burkhardt-Holm P., Wahli T. Histopathology in fish: proposal for a protocol to assess aquatic pollution. J. Fish. Dis. 1999;22:25–34. [Google Scholar]
- 75.Beutler E. Grune & Stratton Inc.; Orlando, FL: 1984. Red Cell Metabolism: A Manual of Biochemical Methods. [Google Scholar]
- 76.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 77.Wendel A. Academic Press Inc; New York, NY: 1980. Enzymatic Basis of Detoxication. [Google Scholar]
- 78.Burtis C.A., Ashwood E.R. Amer Assn for Clinical Chemistry; 1994. Tietz Textbook of Clinical Chemistry. [Google Scholar]
- 79.Vassault A. In: Methods Enzym. Anal. Bergmeyer H.U., editor. Academic Press; New York: 1983. Lactate dehydrogenase, UV-method with pyruvate and NADH; pp. 118–126. [Google Scholar]
- 80.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. http://ajcp.oxfordjournals.org/ [DOI] [PubMed] [Google Scholar]
- 81.Bergmeyer H.U., Hørder M., Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase. J. Clin. Chem. Clin. Biochem. Zeitschrift Fur Klin. Chemie Und Klin. Biochem. 1986;24:497–510. [PubMed] [Google Scholar]
- 82.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 83.Singh N.P., Mccoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 84.Kolarević S., Kračun-Kolarević M., Kostić J., Slobodnik J., Liška I., Gačić Z., Paunović M., Knežević-Vukčević J., Vuković-Gačić B. Assessment of the genotoxic potential along the Danube River by application of the comet assay on haemocytes of freshwater mussels: the Joint Danube Survey 3. Sci. Total Environ. 2016;540:377–385. doi: 10.1016/j.scitotenv.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 85.Beliaeff B., Burgeot T. Integrated biomarker response: a useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002;21:1316–1322. [PubMed] [Google Scholar]
- 86.Damiens G., Gnassia-Barelli M., Loquès F., Roméo M., Salbert V. Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere. 2007;66:574–583. doi: 10.1016/j.chemosphere.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 87.Hammer Ø., Harper D.A.T., Ryan P.D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 88.Cappello T., Maisano M., D’Agata A., Natalotto A., Mauceri A., Fasulo S. Effects of environmental pollution in caged mussels (Mytilus galloprovincialis) Mar. Environ. Res. 2013;91:52–60. doi: 10.1016/j.marenvres.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Turja R., Soirinsuo A., Budzinski H., Devier M.H., Lehtonen K.K. Biomarker responses and accumulation of hazardous substances in mussels (Mytilus trossulus) transplanted along a pollution gradient close to an oil terminal in the Gulf of Finland (Baltic Sea) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013;157:80–92. doi: 10.1016/j.cbpc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Lacroix C., Richard G., Seguineau C., Guyomarch J., Moraga D., Auffret M. Active and passive biomonitoring suggest metabolic adaptation in blue mussels (Mytilus spp.) chronically exposed to a moderate contamination in Brest harbor (France) Aquat. Toxicol. 2015;162:126–137. doi: 10.1016/j.aquatox.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Lucas’ A., Beningerl P.G. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture. 1985;44:187–200. [Google Scholar]
- 92.Davenport J., Chen X. A comparison of methods for the assessment of condition in the mussel (Mytilus edulis L.) Moll. Stud. 1987;53:293–297. http://mollus.oxfordjournals.org/ [Google Scholar]
- 93.Crosby M.P., Gale L.D. A review and evaluation of bivalve condition index methodologies with a suggested standard method. J. Shellfish Res. 1990;9:233–237. [Google Scholar]
- 94.Lagade V.M., Taware S.S., Muley D.V. Seasonal variation in the biochemical constituents, percentage edibility and condition index of the estuarine clam, Soletellina diphos (Linnaeus, 1771)(Mollusca: Bivalvia: veneroida: Psammobiidae) Int. J. Zool. Res. 2015;11:127–139. [Google Scholar]
- 95.Ingle R.M. A comparative study of oyster condition. Science (80) 1949;109:593. doi: 10.1126/science.109.2841.593. [DOI] [PubMed] [Google Scholar]
- 96.Almeida C., Pereira C., Gomes T., Bebianno M.J., Cravo A. DNA damage as a biomarker of genotoxic contamination in Mytilus galloprovincialis from the south coast of Portugal. J. Environ. Monit. 2011;13:2559–2567. doi: 10.1039/c1em10190k. [DOI] [PubMed] [Google Scholar]
- 97.Rouane-Hacene O., Boutiba Z., Belhaouari B., Guibbolini-Sabatier M.E., Francour P., Risso-De Faverney C. Seasonal assessment of biological indices, bioaccumulation and bioavailability of heavy metals in mussels Mytilus galloprovincialis from Algerian west coast, applied to environmental monitoring. Oceanologia. 2015;57:362–374. doi: 10.1007/s11356-017-8946-0. [DOI] [PubMed] [Google Scholar]
- 98.Au D.W.T. The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Mar. Pollut. Bull. 2004;48:817–834. doi: 10.1016/j.marpolbul.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 99.Pathan T.S., Thete P.B., Shinde S.E., Sonawane D.L., Khillare Y.K. Histochemical changes in the liver of freshwater fish, Rasbora daniconius, exposed to paper mill effluent. Emir. J. Food Agric. 2009;21:71–78. http://cfa.uaeu.ac.ae/ejfa.shtml [Google Scholar]
- 100.Bakry F.A., Ismail S.M., Abd El-Atti M.S. Glyphosate herbicide induces genotoxic effect and physiological disturbances in Bulinus truncatus snails. Pestic. Biochem. Physiol. 2015;123:24–30. doi: 10.1016/j.pestbp.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Ansaldo M., Nahabedian D.E., Holmes-Brown E., Agote M., Ansay C.V., Guerrero N.R.V., Wider E.A. Potential use of glycogen level as biomarker of chemical stress in Biomphalaria glabrata. Toxicology. 2006;224:119–127. doi: 10.1016/j.tox.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 102.Bickler P.E., Buck L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- 103.Silvia Fontanetti C., Tiritan B., Izabel Camargo-Mathias M. Mineralized bodies in the fat body of Rhinocricus padbergi (Diplopoda) Braz. J. Morphol. Sci. 2006;23:487–493. [Google Scholar]
- 104.Köhler H.-R., Hüttenrauch K., Berkus M., Graff S., Alberti G. Cellular hepatopancreatic reactions in Porcellio scaber (Isopoda) as biomarkers for the evaluation of heavy metal toxicity in soils. Appl. Soil Ecol. 1996;3:1–15. [Google Scholar]
- 105.Triebskorn R., Köhler H.R. The impact of heavy metals on the grey garden slug, Deroceras reticulatum (Müller): metal storage, cellular effects and semiquantitative evaluation of metal toxicity. Environ. Pollut. 1996;93:321–343. doi: 10.1016/s0269-7491(96)00048-6. [DOI] [PubMed] [Google Scholar]
- 106.Da Silva Souza T., De Franceschi De Angelis D., Fontanetti C.S. Histological and histochemical analysis of the fat body of Rhinocricus padbergi (Diplopoda) exposed to contaminated industrial soil. Water Air Soil Pollut. 2011;221:235–244. [Google Scholar]
- 107.Uchendu C., Ambali S.F., Ayo J.O. The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: a review. Afr. J. Agric. Res. 2012;7:2720–2728. [Google Scholar]
- 108.Regoli F., Pellegrini D., Cicero A.M., Nigro M., Benedetti M., Gorbi S., Fattorini D., D’Errico G., Di Carlo M., Nardi A., Gaion A., Scuderi A., Giuliani S., Romanelli G., Berto D., Trabucco B., Guidi P., Bernardeschi M., Scarcelli V., Frenzilli G. A multidisciplinary weight of evidence approach for environmental risk assessment at the Costa Concordia wreck: integrative indices from Mussel Watch. Mar. Environ. Res. 2014;96:92–104. doi: 10.1016/j.marenvres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 109.Duarte C.A., Giarratano E., Amin O.A., Comoglio L.I. Heavy metal concentrations and biomarkers of oxidative stress in native mussels (Mytilus edulis chilensis) from Beagle Channel coast (Tierra del Fuego, Argentina) Mar. Pollut. Bull. 2011;62:1895–1904. doi: 10.1016/j.marpolbul.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 110.Freitas R., Martins R., Campino B., Figueira E., Soares A.M.V.M., Montaudouin X. Trematode communities in cockles (Cerastoderma edule) of the Ria de Aveiro (Portugal): influence of inorganic contamination. Mar. Pollut. Bull. 2014;82:117–126. doi: 10.1016/j.marpolbul.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 111.Velez C., Figueira E., Soares A., Freitas R. Spatial distribution and bioaccumulation patterns in three clam populations from a low contaminated ecosystem. Estuar. Coast Shelf Sci. 2015;155:114–125. [Google Scholar]
- 112.Lesser M.P. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 113.Orbea A., Cajaraville M.P. Peroxisome proliferation and antioxidant enzymes in transplanted mussels of four Basque estuaries with different levels of polycyclic aromatic hydrocarbon and polychlorinated biphenyl pollution. Environ. Toxicol. Chem. 2006;25:1616–1626. doi: 10.1897/04-520r2.1. [DOI] [PubMed] [Google Scholar]
- 114.Cong M., Wu H., Liu X., Zhao J., Wang X., Lv J., Hou L. Effects of heavy metals on the expression of a zinc-inducible metallothionein-III gene and antioxidant enzyme activities in Crassostrea gigas. Ecotoxicology. 2012;21:1928–1936. doi: 10.1007/s10646-012-0926-z. [DOI] [PubMed] [Google Scholar]
- 115.Ferreira C.P., Lima D., Paiva R., Vilke J.M., Mattos J.J., Almeida E.A., Grott S.C., Alves T.C., Corrêa J.N., Jorge M.B., Uczay M., Vogel C.I.G., Gomes C.H.A.M., Bainy A.C.D., Lüchmann K.H. Metal bioaccumulation, oxidative stress and antioxidant responses in oysters Crassostrea gasar transplanted to an estuary in southern Brazil. Sci. Total Environ. 2019;685:332–344. doi: 10.1016/j.scitotenv.2019.05.384. [DOI] [PubMed] [Google Scholar]
- 116.Regoli F., Giuliani M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014;93:106–117. doi: 10.1016/j.marenvres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Sokolova I.M., Frederich M., Bagwe R., Lannig G., Sukhotin A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012;79:1–15. doi: 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 118.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferreira C.P., Lima D., Souza P., Piazza T.B., Zacchi F.L., Mattos J.J., Jorge M.B., Almeida E.A., Bianchini A., Taniguchi S., Sasaki S.T., Montone R.C., Bícego M.C., Bainy A.C.D., Lüchmann K.H. Short-term spatiotemporal biomarker changes in oysters transplanted to an anthropized estuary in Southern Brazil. Sci. Total Environ. 2020;709 doi: 10.1016/j.scitotenv.2019.136042. [DOI] [PubMed] [Google Scholar]
- 120.Ferreira C.P., Piazza T.B., Souza P., Lima D., Mattos J.J., Saldaña-Serrano M., Piazza R.S., Jorge M.B., Bianchini A., Taniguchi S., Sasaki S.T., Montone R.C., Bícego M.C., Bainy A.C.D., Lüchmann K.H. Integrated biomarker responses in oysters Crassostrea gasar as an approach for assessing aquatic pollution of a Brazilian estuary. Mar. Environ. Res. 2021;165 doi: 10.1016/j.marenvres.2021.105252. [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Zhu J., Xue Z., Jin X., Jin Y., Fu Z. The environmental distribution and toxicity of short-chain chlorinated paraffins and underlying mechanisms: implications for further toxicological investigation. Sci. Total Environ. 2019;695:133834. doi: 10.1016/j.scitotenv.2019.133834. undefined. [DOI] [PubMed] [Google Scholar]
- 122.Dong B.-W., Qin G.-M., Luo Y., Mao J.-S. Metabolic enzymes: key modulators of functionality in cancer stem-like cells. Oncotarget. 2017;8:14251–14267. doi: 10.18632/oncotarget.14041. www.impactjournals.com/oncotarget [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das P.C., Ayyappan S., Das B.K., Jena J.K. Nitrite toxicity in Indian major carps: sublethal effect on selected enzymes in fingerlings of Catla catla, Labeo rohita and Cirrhinus mrigala. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;138:3–10. doi: 10.1016/j.cca.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Gagnon M.M., Holdway D.A. Metabolic enzyme activities in fish gills asbiomarkers of exposure to petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 1999;44:92–99. doi: 10.1006/eesa.1999.1804. http://www.idealibrary.comon [DOI] [PubMed] [Google Scholar]
- 125.Williams A.L.B., Hoofnagle J.H. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 126.Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ (Can. Med. Assoc. J.) 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narvia M., Rantamaki P. Aminotransferases in the bivalve mollusc Mytilus edulis L. and short term effects of crude oil in brackish water. Biomarkers. 1997;2:253–258. doi: 10.1080/135475097231634. [DOI] [PubMed] [Google Scholar]
- 128.Almeida J.A., Diniz Y.S., Marques S.F.G., Faine L.A., Ribas B.O., Burneiko R.C., Novelli E.L.B. The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ. Int. 2002;27:673–679. doi: 10.1016/s0160-4120(01)00127-1. www.elsevier.com/locate/envint [DOI] [PubMed] [Google Scholar]
- 129.Lastumäki A., Turja R., Brenner M., Vanninen P., Niemikoski H., Butrimavičienė L., Stankevičiūtė M., Lehtonen K.K. Biological effects of dumped chemical weapons in the Baltic Sea: a multi-biomarker study using caged mussels at the Bornholm main dumping site. Mar. Environ. Res. 2020;161 doi: 10.1016/j.marenvres.2020.105036. [DOI] [PubMed] [Google Scholar]
- 130.Bocquené G., Galgani F., Truquet P. Characterization and assay conditions for use of AChE activity from several marine species in pollution monitoring. Mar. Environ. Res. 1990;30:75–89. [Google Scholar]
- 131.Bocquené G., Bellanger C., Cadiou Y., Galgani F. Joint action of combinations of pollutants on the acetylcholinesterase activity of several marine species. Ecotoxicology. 1995;4:266–279. doi: 10.1007/BF00116345. [DOI] [PubMed] [Google Scholar]
- 132.Galgani F., Bocquené G. In: Use Biomarkers Environ. Qual. Assess. Lagadic L., editor. Elsevier Science Publisher; Amsterdam: 2000. Molecular biomarkers of exposure of marine organisms to organophosphorus pesticides and carbamates; pp. 113–137. [Google Scholar]
- 133.Maisano M., Cappello T., Natalotto A., Vitale V., Parrino V., Giannetto A., Oliva S., Mancini G., Cappello S., Mauceri A., Fasulo S. Effects of petrochemical contamination on caged marine mussels using a multi-biomarker approach: histological changes, neurotoxicity and hypoxic stress. Mar. Environ. Res. 2017;128:114–123. doi: 10.1016/j.marenvres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 134.Freitas R., Coppola F., De Marchi L., Codella V., Pretti C., Chiellini F., Morelli A., Polese G., Soares A.M.V.M., Figueira E. The influence of Arsenic on the toxicity of carbon nanoparticles in bivalves. J. Hazard Mater. 2018;358:484–493. doi: 10.1016/j.jhazmat.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 135.Coppola F., Tavares D.S., Henriques B., Monteiro R., Trindade T., Soares A.M.V.M., Figueira E., Polese G., Pereira E., Freitas R. Remediation of arsenic from contaminated seawater using manganese spinel ferrite nanoparticles: ecotoxicological evaluation in Mytilus galloprovincialis. Environ. Res. 2019;175:200–212. doi: 10.1016/j.envres.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 136.Pirone G., Coppola F., Pretti C., Soares A.M.V.M., Solé M., Freitas R. The effect of temperature on Triclosan and Lead exposed mussels. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019;232:42–50. doi: 10.1016/j.cbpb.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 137.Sevgiler Y., Karaytug S., Karayakar F. Antioxidative effects of N-acetylcysteine, lipoic acid, taurine, and curcumin in the muscle of Cyprinus carpio L. exposed to cadmium. Arh. Hig. Rada. Toksikol. 2011;62:1–9. doi: 10.2478/10004-1254-62-2011-2082. [DOI] [PubMed] [Google Scholar]
- 138.Rogers J.J., Henley W.F., Weberg A.G., Jones J.W., Gregory Cope W. Assessment of growth, survival, and organ tissues of caged mussels (Bivalvia: unionidae) in a river-scape influenced by coal mining in the southeastern USA. Sci. Total Environ. 2018;645:1273–1286. doi: 10.1016/j.scitotenv.2018.07.142. [DOI] [PubMed] [Google Scholar]
- 139.Jha A.N. Ecotoxicological applications and significance of the comet assay. Mutagenesis. 2008;23:207–221. doi: 10.1093/mutage/gen014. [DOI] [PubMed] [Google Scholar]
- 140.Collins A.R. The comet assay: a heavenly method! Mutagenesis. 2015;30:1–4. doi: 10.1093/mutage/geu079. [DOI] [PubMed] [Google Scholar]
- 141.de Lapuente J., Lourenço J., Mendo S.A., Borràs M., Martins M.G., Costa P.M., Pacheco M. The Comet Assay and its applications in the field of ecotoxicology: a mature tool that continues to expand its perspectives. Front. Genet. 2015;6:180. doi: 10.3389/fgene.2015.00180. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dixon D.R., Pruski A.M., Dixon L.R.J., Jha A.N. Marine invertebrate eco-genotoxicology: a methodological overview. Mutagenesis. 2002;17:495–507. doi: 10.1093/mutage/17.6.495. https://academic.oup.com/mutage/article/17/6/495/1274864 [DOI] [PubMed] [Google Scholar]
- 143.Frenzilli G., Nigro M., Lyons B.P. The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat. Res. Rev. Mutat. Res. 2009;681:80–92. doi: 10.1016/j.mrrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 144.Bolognesi C., Cirillo S. Genotoxicity biomarkers in aquatic bioindicators. Curr. Zool. 2014;60:273–284. [Google Scholar]
- 145.Glei M., Schneider T., Schlörmann W. Comet assay: an essential tool in toxicological research. Arch. Toxicol. 2016;90:2315–2336. doi: 10.1007/s00204-016-1767-y. [DOI] [PubMed] [Google Scholar]
- 146.öran G., Klobučar I.V., Pavlica M., Erben R., Papeš D. Application of the micronucleus and comet assays to mussel Dreissena polymorpha haemocytes for genotoxicity monitoring of freshwater environments. Aquat. Toxicol. 2003;64:15–23. doi: 10.1016/s0166-445x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 147.Vuković-Gačić B., Kolarević S., Sunjog K., Tomović J., Knežević-Vukčević J., Paunović M., Gačić Z. Comparative study of the genotoxic response of freshwater mussels Unio tumidus and Unio pictorum to environmental stress. Hydrobiologia. 2014;735:221–231. [Google Scholar]
- 148.Marić J.J., Kračun-Kolarević M., Kolarević S., Sunjog K., Kostić-Vuković J., Deutschmann B., Hollert H., Tenji D., Paunović M., Vuković-Gačić B. Selection of assay, organism, and approach in biomonitoring significantly affects the evaluation of genotoxic potential in aquatic environments. Environ. Sci. Pollut. Res. 2020;27:33903–33915. doi: 10.1007/s11356-020-09597-0. [DOI] [PubMed] [Google Scholar]
- 149.Kolarević S., Knezević-Vukćević J., Paunović M., Tomović J., Gaćić Z., Vuković-Gaćić B. The anthropogenic impact on water quality of the River Danube in Serbia: microbiological analysis and genotoxicity monitoring. Arch. Biol. Sci. 2011;63:1209–1217. [Google Scholar]
- 150.Kolarević S., Knežević-Vukčević J., Paunović M., Kračun M., Vasiljević B., Tomović J., Vuković-Gačić B., Gačić Z. Monitoring of DNA damage in haemocytes of freshwater mussel Sinanodonta woodiana sampled from the velika morava river in Serbia with the comet assay. Chemosphere. 2013;93:243–251. doi: 10.1016/j.chemosphere.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 151.Vincent-Hubert F., Arini A., Gourlay-Francé C. Early genotoxic effects in gill cells and haemocytes of Dreissena polymorpha exposed to cadmium, B[a]P and a combination of B[a]P and Cd. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;723:26–35. doi: 10.1016/j.mrgentox.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 152.Ali D., Alarifi S., Kumar S., Ahamed M., Siddiqui M.A. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat. Toxicol. 2012;124–125:83–90. doi: 10.1016/j.aquatox.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 153.Mahaye N., Thwala M., Cowan D.A., Musee N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: a review. Mutat. Res. Rev. Mutat. Res. 2017;773:134–160. doi: 10.1016/j.mrrev.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 154.Anastassopoulou J. Metal-DNA interactions. J. Mol. Struct. 2003;651–653:19–26. www.elsevier.com/locate/molstruc [Google Scholar]
- 155.García-Lestón Julia J., Méndez J., Pásaro E., Laffon B. Genotoxic effects of lead: an updated review. Environ. Int. 2010;36:623–636. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 156.Ji K., Choi K., Giesy J.P., Musarrat J., Takeda S. Genotoxicity of several polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDEs, and their mechanisms of toxicity. Environ. Sci. Technol. 2011;45:5003–5008. doi: 10.1021/es104344e. [DOI] [PubMed] [Google Scholar]
- 157.Pereira L.C., de Souza A.O., Meireles G., Franco-Bernardes M.F., Tasso M.J., Bruno V., Dorta D.J., de Oliveira D.P. Comparative study of genotoxicity induced by six different PBDEs. Basic Clin. Pharmacol. Toxicol. 2016;119:396–404. doi: 10.1111/bcpt.12595. [DOI] [PubMed] [Google Scholar]
- 158.Riva C., Binelli A., Cogni D., Provini A. Evaluation of DNA damage induced by decabromodiphenyl ether (BDE-209) in hemocytes of Dreissena polymorpha using the comet and micronucleus assays. Environ. Mol. Mutagen. 2007;48:735–743. doi: 10.1002/em.20353. [DOI] [PubMed] [Google Scholar]
- 159.Singh N., Gupta V.K., Kumar A., Sharma B. Synergistic effects of heavy metals and pesticides in living systems. Front. Chem. 2017;5:70. doi: 10.3389/fchem.2017.00070. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Capolupo M., Valbonesi P., Kiwan A., Buratti S., Franzellitti S., Fabbri E. Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus galloprovincialis. Sci. Total Environ. 2016;563–564:538–548. doi: 10.1016/j.scitotenv.2016.04.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.