Abstract
As the worldwide prevalence of diabetes and obesity continues to rise, so does the risk of debilitating cardiovascular complications. Given the significant association between diabetes and cardiovascular risk, the actions of glucose-lowering therapies within the cardiovascular system must be clearly defined. Incretin hormones, including GLP-1 (glucagon-like peptide 1) and GIP (glucose-dependent insulinotropic polypeptide), are gut hormones secreted in response to nutrient intake that maintain glycemic control by regulating insulin and glucagon release. GLP-1 receptor agonists (GLP-1Ras) and dipeptidyl peptidase 4 inhibitors (DPP-4is) represent two drug classes used for the treatment of type 2 diabetes mellitus (T2DM) that improve glucose regulation through stimulating the actions of gut-derived incretin hormones or inhibiting their degradation, respectively. Despite both classes acting to potentiate the incretin response, the potential cardioprotective benefits afforded by GLP-1Ras have not been recapitulated in cardiovascular outcome trials (CVOTs) evaluating DPP-4is. This review provides insights through discussion of clinical and preclinical studies to illuminate the physiological mechanisms that may underlie and reconcile observations from GLP-1Ra and DPP-4i CVOTs. Furthermore, critical knowledge gaps and areas for further investigation will be emphasized to guide future studies and, ultimately, facilitate improved clinical management of cardiovascular disease in T2DM.
Introduction
The gastrointestinal tract coordinates nutrient intake and utilization by peripheral tissues, and unraveling the integrative physiological network connecting these functions has revealed several drug targets. Glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are hormones released in response to nutrient intake that potentiate glucose-stimulated insulin secretion (1). Their hormonal action is limited to the postprandial period because of their rapid inactivation by dipeptidyl peptidase 4 (DPP-4), a serine protease that cleaves two N-terminal amino acids, and subsequent renal elimination. Two classes of drugs that potentiate the effects of gut-derived hormones have been developed for type 2 diabetes mellitus (T2DM): GLP-1 receptor agonists (GLP-1Ras), which are peptides based on human or nonmammalian structures, and DPP-4 inhibitors (DPP-4is), which stabilize endogenous GLP-1 and other substrates, including GIP.
While glucose-lowering therapies have demonstrated efficacy in reducing microvascular events in patients with T2DM, preventing macrovascular complications has proven more difficult. The number of pharmacological tools available to endocrinologists has increased in recent years, with significant advancements in effective glucose-lowering drugs. Since the introduction of these incretin-based therapies to the market, DPP-4is have been widely adopted to manage glucose levels with few side effects, and the use of GLP-1Ras is steadily increasing (2). In agreement with the 2008 guidance of the U.S. Food and Drug Administration, cardiovascular outcome trials (CVOTs) have been performed to evaluate the cardiovascular safety of these agents. Most use the 3-point major adverse cardiovascular end point (MACE), comprised of cardiovascular mortality, nonfatal myocardial infarction (MI), and nonfatal stroke. Here, we review the results of incretin therapy CVOTs and provide molecular insights into potential mechanisms using smaller clinical studies and translationally relevant studies in animal and cellular models.
GLP-1Ras and Cardiovascular Outcomes
The first completed GLP-1Ra CVOT, the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA), demonstrated that lixisenatide treatment of subjects with T2DM was noninferior to placebo for 3-point MACE, with similar observations reported in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Conversely, 3-point MACE results from CVOTs on the longer-acting GLP-1Ras liraglutide (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER]), semaglutide (Trial to Evaluate Cardiovascular and Other Long-Term Outcomes with Semaglutide in Subjects with Type 2 Diabetes [SUSTAIN-6]), albiglutide (Harmony Outcomes), and dulaglutide (Researching Cardiovascular Events with a Weekly Incretin in Diabetes [REWIND]) were quite positive (Fig. 1A). GLP-1Ra CVOTs have also demonstrated inconsistencies regarding the secondary outcome of hospitalization for heart failure (HF), whereby the LEADER and Harmony Outcomes CVOTs reported trends toward reduced hospitalization rates, whereas the SUSTAIN-6 and REWIND CVOTs reported no differences, as reviewed in Gopal et al. (3).
Figure 1.
Summary of CVOTs for GLP-1Ra and DPP-4i. A: Six large-scale randomized CVOTs evaluating cardiovascular safety/efficacy of different GLP-1Ras in T2DM patients with established CVD : EXSCEL, LEADER, SUSTAIN-6, ELIXA, Harmony Outcomes, and REWIND. A dagger indicates a composite of cardiovascular death (CD) or hospitalization for heart failure (HHF). An asterisk indicates hospitalization for HF or an urgent visit. B: Five large-scale randomized CVOTs evaluating cardiovascular safety/efficacy of different DPP-4i in T2DM patients with established CVD: EXAMINE, SAVOR-TIMI 53, TECOS, CARMELINA, and CAROLINA. Of note, the patients included in these 5 CVOTs already received standard care with cardiovascular protection (i.e., statins and antiplatelet agents) and T2DM management. PCO, primary composite outcome; stroke, nonfatal stroke. The numbers provided represent the first occurrence of the primary composite end point if data were available and thus may not match reported PCOs that include subjects who reached multiple end points.
Potential Mechanisms Underlying GLP-1Ra–Mediated Cardioprotection
GLP-1Ras and Atherosclerosis
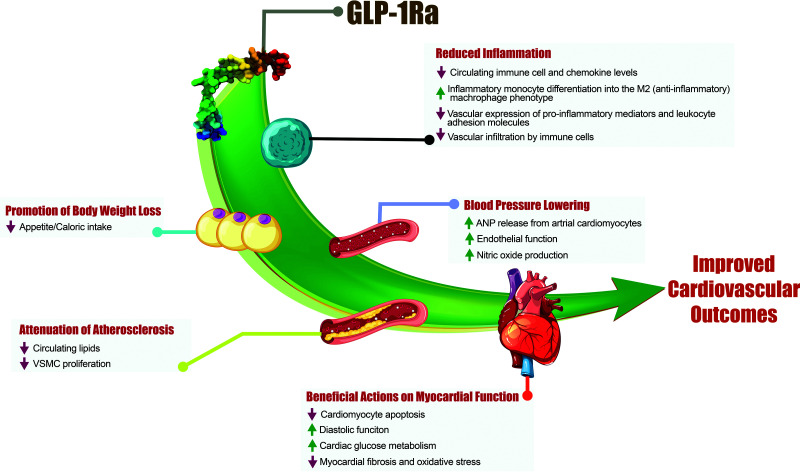
Post hoc analysis of the LEADER trial demonstrated that patients with a history of MI, stroke, or established disease demonstrate more significant cardiovascular benefit using liraglutide than subjects with elevated risk factors alone (4). Patients enrolled in these CVOTs receive standard-of-care measures, including optimal lipid lowering and antihypertensives; therefore, together with the timeline required for protection, GLP-1Ras are likely antiatherogenic (5). Consistent with this, preclinical mouse studies have described the reduced progression of atherosclerosis with liraglutide and semaglutide through reduced residual inflammation in the atherosclerotic plaques of Western diet (WD)-fed Ldlr−/− and Apoe−/− mice (6). Dulaglutide treatment reduced the atherosclerotic plaque area and aortic arch macrophage infiltration in Apoe−/− mice with streptozotocin (STZ)-induced experimental type 1 diabetes (7). This benefit was also associated with reduced aortic expression of markers of inflammation and increased plaque stability. Interestingly, the antiatherogenic effect of dulaglutide was more pronounced when administered to younger diabetic mice (at 10–18 weeks old rather than 18–26 weeks old). As assessed in the brachiocephalic artery by iMAP intravascular ultrasound, plaque stability was increased with lixisenatide in Watanabe heritable hyperlipidemic rabbits (8). Moreover, lixisenatide decreased circulating lymphocytes and interleukin-6 levels in Apoe−/−:Irs2+/− mice, and plaque macrophages displayed increased arginase and decreased inducible nitric oxide (NO) synthase expression, indicating an anti-inflammatory (M2) phenotype (9). However, establishing reductions in atherogenesis or plaque composition in patients with T2DM has proven more challenging. Treatment of subjects with exenatide once weekly for up to 18 months improved glycemic control but did not significantly alter the volume of carotid plaque (MRI) or the lipid-rich necrotic core or calcification (10). Exploratory analysis of the LEADER trial evaluating factors contributing to the time to first MACE identified several factors, including HbA1c, body weight, systolic blood pressure (BP), and LDL cholesterol (11). Nevertheless, preclinical studies demonstrated that reduced lesion progression with GLP-1Ras was independent of changes in body weight or total cholesterol (6). Additionally, the collective effect size on atherogenic LDL cholesterol in the MACE trials is modest (12). Postprandial triacylglycerol-rich lipoproteins are reduced by GLP-1Ra (13), and treatment with liraglutide in a prospective 18-month real-world study did reduce carotid intermedial thickness, which was associated with reduced plasma triacylglycerols (14). However, the contribution of reduced triacylglycerol-rich lipoproteins to reduced atherogenesis is currently unclear.
GLP-1Ras and Hypertension
GLP-1Ras frequently produce antihypertensive effects in murine hypertension models and in T2DM MACE trials, which may contribute to their cardioprotective properties (11), independent of glucose lowering and weight loss (15). Liraglutide-mediated reductions in systolic and diastolic BP in angiotensin II–infused C57BL/6J mice were due to atrial natriuretic peptide (ANP) release from atrial cardiomyocytes, which relaxes the vasculature (16). Intriguingly, liraglutide failed to lower systolic and diastolic BP in angiotensin II–infused ANP-deficient mice. Nonetheless, the clinical relevance of the proposed GLP-1r–ANP axis in reducing human BP is questionable, as liraglutide increased plasma ANP levels in subjects with T2DM in some studies (17) but not in others (18). The enhancement of the vasodilatory response may also explain the antihypertensive effects of GLP-1Ras through direct GLP-1R activation in the vasculature. Endothelium-dependent vasodilation in response to acetylcholine was improved in preconstricted aortic rings isolated from WD-fed Apoe−/− mice treated with liraglutide versus the control, which was abolished by cotreatment with the GLP-1R antagonist exendin(9-39) (19). Conversely, intracoronary infusion of GLP-1(7-36) did not augment coronary flow in open-chest, anesthetized dogs and failed to induce vasodilation in preconstricted coronary artery rings (20). GLP-1Ras may also reduce BP by decreasing vascular inflammation. In C57BL/6J mice infused with angiotensin II for 1 week, liraglutide treatment attenuated aortic wall infiltration by LY6G−:LY6C+ monocytes (where LY6G is lymphocyte antigen 6 complex, locus G) and LY6G+:LY6C+ neutrophils while decreasing systolic BP (21). Furthermore, these effects were associated with decreased aortic levels of proinflammatory mediators (nuclear factor κB [NF-κB] and tumor necrosis factor-α) and leukocyte adhesion molecules (vascular cell adhesion molecule 1 and intercellular adhesion molecule 1). Interestingly, these beneficial effects were abolished in mice with endothelium-specific but not myeloid-specific GLP-1R deficiencies.
While these rodent studies have improved our potential understanding of antihypertensive mechanisms, as already mentioned, GLP-1Ra-mediated reductions in BP are not as potent in humans, and this is also observed in larger animal species (e.g., pigs and dogs), as reviewed in Ussher and Drucker (22). Therefore, BP reductions are unlikely to be a major contributor to the cardioprotection reported in GLP-1Ra CVOTs. Further emphasizing this point, albiglutide had negligible actions on BP lowering despite significant improvements in cardiovascular outcomes in Harmony Outcomes, whereas exenatide significantly lowered BP, albeit mildly, but did not improve cardiovascular outcomes in EXSCEL.
GLP-1Ras and MI
In patients undergoing percutaneous coronary intervention, a randomized, placebo-controlled study involving a 6-h exenatide infusion prior to reperfusion onset reported an improved myocardial salvage index (23). This translated into a reduced infarct size if infusion occurred <132 min from the time of first medical contact to balloon treatment, and benefits were observed in subjects with and without diabetes. However, as mentioned previously, the EXSCEL CVOT did not observe decreases in cardiovascular outcomes or fatal/nonfatal MI events (24).
GLP-1 and GLP-1Ras decrease infarct size in mice, rats, rabbits, and pigs following temporary ligation of the left anterior descending (LAD) or circumflex coronary arteries (22). However, a caveat to these studies is that nearly all were performed in healthy young animals, with surprisingly few studies performed in animals with experimental obesity and/or T2DM. Likewise, similar to issues relating to studying antihypertensive actions described previously, the effect of GLP-1Ras on infarct size is not as reproducible in larger animal models, with several studies in pigs reporting no benefit, though this could also be partially due to their limited collateral circulation. It is possible that decreased inflammation is responsible for increased vascular function/coronary blood flow and attenuated infarct size (25). GLP-1Ras may also decrease infarct size during ischemia/reperfusion by inhibiting cardiomyocyte apoptosis (26). Furthermore, albiglutide-mediated reductions in infarct size are associated with increased myocardial glucose oxidation, which may improve cardiac efficiency, as carbohydrates are a more oxygen-efficient fuel (27).
GLP-1Ras and HF
While the LEADER and Harmony Outcomes trials demonstrated trends toward reduced hospitalization rates for HF, all other GLP-1Ra CVOTs have been neutral for this end point. Furthermore, two randomized placebo-controlled trials suggested no clear benefit and potential adverse effects for liraglutide in HF subjects with reduced ejection fraction (HFrEF). The Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) trial included subjects with HFrEF who were recently hospitalized for acute HF and treated with either liraglutide or a placebo for 6 months (28). Although the FIGHT trial reported no changes in HF-related outcomes or cardiac function, a mild but nonsignificant signal for harm was observed for liraglutide, which appeared greater in subjects with coexistent T2DM. Similarly, the Effect of Liraglutide on Left Ventricular Function in Stable Chronic Heart Failure Patients (LIVE) trial demonstrated that treatment with liraglutide for 24 weeks did not affect cardiac function in subjects with stable HFrEF with or without T2DM (29). However, a higher prevalence of serious adverse cardiac events, including atrial fibrillation and aggravation of ischemic heart disease, was observed with liraglutide, raising concerns about the safety of GLP-1Ras in individuals with HFrEF.
Conversely, preclinical studies examining the impact of liraglutide and other GLP-1Ras in HFrEF have been largely positive. Indeed, a 7-day pretreatment with liraglutide prior to MI induction via permanent LAD coronary artery ligation increased survival and ameliorated adverse left ventricular (LV) remodeling in both nondiabetic and diabetic mice (26). Moreover, in obese mice fed a WD for 20 weeks, liraglutide treatment during the final week improved LV ejection fraction in a 5′AMP activated protein kinase (AMPK)-dependent manner, as improvement was not observed in mice concurrently treated with the AMPK inhibitor compound C (30). In Ossabaw swine fed a WD for 6 months and subjected to MI using an ameroid constrictor placed around the LAD coronary artery, liraglutide treatment for 4 weeks did not reduce infarct size but did improve cardiac efficiency (31). This effect was attributed to β1-adrenoceptor downregulation, which would decrease myocardial O2 demands. In contrast but drawing similar parallels to observations in the FIGHT and LIVE trials, liraglutide treatment for 42 days exacerbated cardiac hypertrophy and fibrosis in nondiabetic J2N-k hamsters, which develop a spontaneous dilated cardiomyopathy (32).
HF with preserved ejection fraction (HFpEF) is more prevalent in patients with diabetes than the general population, and early diastolic dysfunction (a form of diabetic cardiomyopathy) is often undiagnosed because of a lack of routine cardiovascular screening in the early stages of T2DM (3). Currently, no clinical studies have investigated the impact of GLP-1Ras in subjects with HFpEF. However, liraglutide treatment for 6 months improved diastolic function, indicated by an increased peak early diastolic tissue velocity (e′) and decreased LV end diastolic volume in the Magnetic Resonance Assessment of Victoza Efficacy in the Regression of Cardiovascular Dysfunction in Type 2 Diabetes Mellitus (MAGNA VICTORIA) study (29). In addition, 6 months of liraglutide treatment improved diastolic function in subjects with T2DM, indicated by an increased e′/peak late diastolic tissue velocity (a′) ratio and a decreased peak early diastolic flow velocity (E)/e′ ratio (33). These observations have been recapitulated in preclinical studies, as liraglutide treatment improved global longitudinal strain in aged WD-fed, angiotensin II-induced female mice, indicating reduced diastolic dysfunction (34). Treatment alleviated fibrosis and proinflammatory gene expression and decreased capillary density, all of which promote diabetic cardiomyopathy (3). Furthermore, liraglutide-mediated improvements in diastolic function (increased E/peak atrial flow velocity [A] and decreased E/e′) in WD-fed mice administered low-dose STZ to induce T2DM were associated with increased myocardial glucose oxidation rates (35). As direct impairments in myocardial glucose oxidation precipitate diastolic dysfunction (3), liraglutide’s ability to stimulate glucose oxidation may explain how GLP-1Ras alleviate diabetic cardiomyopathy. GLP-1Ras may also mitigate diastolic dysfunction in T2DM by decreasing oxidative stress. Exenatide administration for 4 weeks increased the myocardial expression of antioxidant enzymes (manganese-dependent superoxide dismutase and catalase) in mice fed a WD for 24 weeks, increasing the E/A ratio (36). Liraglutide therapy for 4 weeks also increased myocardial catalase activity in Sprague-Dawley rats with WD/low-dose STZ-induced T2DM, although diastolic function was not assessed (37).
Reconciling Preclinical Mechanisms of Action With Observations From GLP-1Ra CVOTs
It is not surprising that most GLP-1Ra CVOTs have yielded positive findings based on the available preclinical data and associated mechanisms (Fig. 2). ELIXA’s neutral outcomes may have been due to lixisenatide’s shorter half-life and the higher-risk population studied (subjects had acute coronary events within 180 days of screening). Improvements in vascular function and decreases in oxidative stress as well as circulating lipids may contribute to the antiatherosclerotic properties of GLP-1Ras, possibly explaining the decreased cardiovascular events, including MI, in subjects with T2DM. A highly contested aspect of GLP-1Ra–induced cardioprotection that requires further interrogation involves GLP-1R expression in the cardiovascular system, which is often impacted by the species and tools (e.g., antibodies) used. In rodents, ventricular cardiomyocytes do not express the canonical GLP-1R, while recent studies of human heart extracts demonstrated GLP-1R expression in all four chambers (16,38). However, efforts to determine whether the GLP-1R is meaningfully expressed in vascular smooth muscle cells, endothelial cells, and immune cells have been plagued by inconsistencies. GLP-1Ra–induced heart rate elevations may contribute to the potential worsened outcomes observed for subjects with HFrEF in the FIGHT and LIVE trials. It should be noted that the average increase in heart rate in the LIVE trial (∼7 bpm) is greater than those observed in most GLP-1Ra CVOTs (0.4 to 3.0 bpm).
Figure 2.
Potential mechanisms that may contribute to the cardiovascular benefit afforded by GLP-1Ra. GLP-1Ra-mediated cardioprotection likely results from multiple contributing factors, including a reduction in inflammatory processes and body weight, improvements in vascular function that decrease BP, attenuation of atherosclerosis, and cardiomyocyte-independent actions that improve myocardial function (see the text for references). VSMC, vascular smooth muscle cells.
DPP-4is and Cardiovascular Outcomes
The DPP-4i trials have been extensively reviewed elsewhere (22) and are summarized in Fig. 1B. Although their designs were not entirely consistent, the five CVOTs yielded similar results: DPP-4is (alogliptin, saxagliptin, sitagliptin, and linagliptin) were noninferior to placebo, demonstrating their cardiovascular safety when added to standard care (39–42). The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) demonstrated both cardiovascular and renal safety of linagliptin versus placebo (43). In addition to disappointing results for obvious cardiovascular benefit, the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial revealed an increased risk of hospitalization for HF (40), which was highest among patients with elevated baseline brain natriuretic peptide levels, prior HF, or impaired renal function at study entry (44). However, the increased HF hospitalization risk was not associated with an increased risk of cardiovascular death (40). Interestingly, these results were not replicated in the Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) and CARMELINA, as their HF hospitalization rates did not differ among patients with T2DM, even between those with a history of HF or chronic kidney disease, and were independent of LV ejection fraction (41). In a secondary analysis of TECOS, cardiovascular and all-cause deaths after HF hospitalization were also similar between the sitagliptin and control groups (45). Thus, the increase in HF hospitalization observed in the SAVOR-TIMI 53 trial may be related to its design or to properties of the saxagliptin molecule itself, which may directly impair cardiomyocyte function (46).
Reconciling the Preclinical Cardioprotection of DPP-4is With Observations of Neutrality in CVOTs
Modest improvements in classic cardiovascular risk factors, including HbA1c, BP, fasting, and postprandial blood lipids, have been observed with DPP-4is, albeit much lower in magnitude than those observed with GLP-1Ras (12), consistent with the sustained elevation in physiological GLP-1. Here, we discuss aspects of DPP-4 biology that may contribute to the MACE differences observed between DPP-4is and GLP-1Ras.
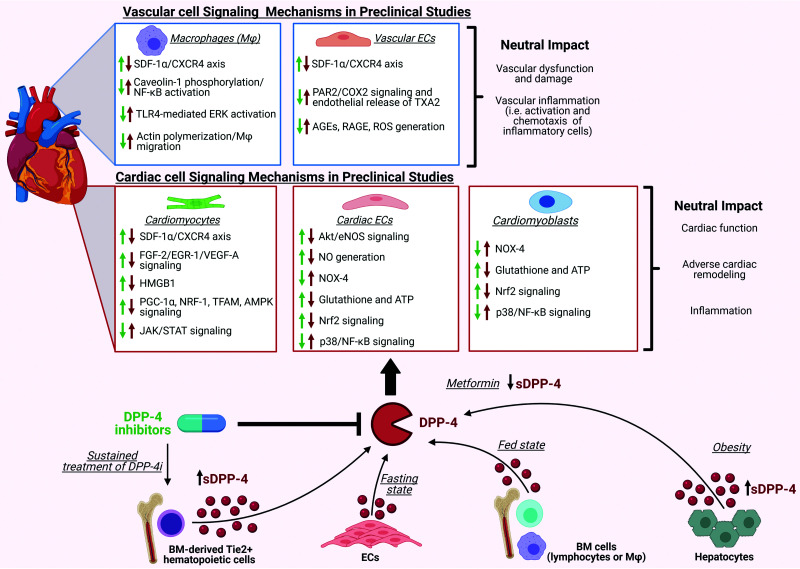
DPP-4 exists as both a membrane-bound isoform and a soluble form shed from the membrane to circulate in most bodily fluids (sDPP-4), which lacks the intracellular tail and transmembrane domain but maintains enzymatic activity (47). Plasma sDPP-4 level increases are positively associated with coronary artery disease (48,49), endothelial dysfunction in patients with T2DM (50), and diabetic nephropathy (51).
Adding to the complexity of its association with disease progression, sDPP-4 originates from various sources depending on the metabolic state. For example, major sDPP-4 sources in healthy rodents are endothelial cells and hematopoietic cells, including bone marrow cells, lymphocytes, and macrophages (52). DPP-4 is stored in secretory granules by cytotoxic lymphocyte populations, and, upon stimulation, these vesicles rapidly translocate to the cell surface in a Ca2+-dependent manner to release proteolytically active sDPP-4 (53). Conversely, the increased sDPP-4 in obesity appears to originate from hepatocytes (54,55), as soluble factors released from dysfunctional adipocytes promote hepatic hypoxia-inducible factor 1α expression, increasing hepatic DPP-4 shedding (56). Furthermore, in obese mice, hepatocyte-derived sDPP-4 induces inflammation in macrophages by directly interacting with surface caveolin 1, increasing its phosphorylation and dissociation from complexes with Toll-interacting protein and interleukin-1 receptor-associated kinase 1, which activates NF-κB (54,57). Collectively, these data suggest that obesity-mediated increases in hepatic sDPP-4 activate inflammatory programs in several cell types; however, whether targeting hepatocyte DPP-4 has merit in preventing metabolic disease has not yet been explored clinically.
Surprisingly, sustained DPP-4 inhibition is associated with elevated circulating sDPP-4 levels in mice (55,58). This increase originates predominantly from bone marrow-derived tunica intima endothelial kinase receptor tyrosine kinase-positive hematopoietic cells (58). The upregulation of hematopoiesis-derived DPP-4 did not affect tissue or systemic inflammation, dissociating changes in DPP-4 activity from plasma sDPP-4 and inflammatory marker levels (55,58). These data parallel other studies in which DPP-4i treatment abrogates the inflammatory effects of sDPP-4 (59,60). Contrary to findings in mice, continuous DPP-4i treatment did not increase circulating sDPP-4 levels in humans with established cardiovascular disease (CVD) and T2DM (58). Therefore, although some pools of sDPP-4 may contribute to the subclinical inflammation observed in metabolic disease, evidence suggests that, in human subjects with diabetes, DPP-4is have an overall neutral effect on pathways regulating inflammation (Fig. 3).
Figure 3.
Potential mechanisms underlying the neutral cardiovascular actions of DPP-4 and DPP-4i. In healthy animals, several pathways within the cardiovascular system have been identified that mediate the effects of DPP-4is; however, the majority have not translated to benefits in subjects with T2DM. Inhibition of DPP-4 in preclinical studies facilitates the homing of CXCR4+ progenitor stem cells at sites of myocardial damage, prevents cardiomyocyte apoptosis, and improves postischemic recovery of cardiac contractility through increasing SDF-1α levels. In addition, DPP-4i can activate Akt/endothelial NO synthase (eNOS) signaling along with NO generation by the endothelium, enhancing FGF-2/EGR-1/VEGF-A signaling, inhibiting HMGB1 inactivation, promoting signaling pathways related to PGC-1α/NRF-1/TFAM/AMPK, downregulating the JAK/STAT signaling pathway, reducing the expression of NOX-4, and restoring intracellular levels of antioxidant glutathione as well as ATP. Furthermore, DPP-4i can reduce p38/NF-κB signaling while inducing Nrf2 signaling. Additionally, DPP-4i can inhibit PAR2/NF-κB signaling cascades, TLR4-mediated extracellular signal–regulated kinase activation, and the expression levels of advanced glycation end products (AGEs) as well as their receptor, RAGE. DPP-4i can also cause macrophage activation and chemotaxis. Despite the identification of many signaling pathways in cell and preclinical models, in MACE trials neutral outcomes are observed. Adding to the complexity of signaling, sDPP-4 may originate from various sources, such as endothelial cells, bone marrow cells, or hepatocytes, depending on the metabolic states, and activate the complementary pathways. Additionally, sustained treatment of DPP-4i also increases sDPP-4 shedding from bone marrow-derived Tie2+ hematopoietic cells; conversely, metformin decreases sDPP-4 levels. The green arrows represent the effects of DPP-4i, whereas the red arrows represent the effects of sDPP-4 on the cardiovascular system. Generated with BioRender (biorender.com; publication license FF233Q5E1I).
Progenitor Cell Homing
Another example where mechanisms for improved cardiovascular function with DPP-4is identified in young mouse models failed to translate was potentiation of C-X-C motif chemokine ligand 12 (CXCL12) signaling to facilitate the homing of C-X-C motif chemokine receptor 4+ progenitor stem cells to sites of myocardial damage, preventing cardiomyocyte apoptosis (61). Genetic elimination and pharmacological inhibition of DPP-4 (62) or potentiation of CXCL12 improves postischemic recovery of cardiac contractility in the hearts of healthy young adult mice (63). However, cardioprotection with DPP-4is was not reproduced in aged, obese, diabetic mice (63,64). Aspects of this model have been tested clinically in the Sitagliptin Plus Granulocyte Colony-Stimulating Factor in Patients Suffering From Acute Myocardial Infarction (SITAGRAMI) trial, which combined sitagliptin with colony-stimulating factor 3–mediated stem cell mobilization. Long-term follow-up data showed no improvement in cardiac function or the clinical outcomes of patients with acute MI receiving the combined therapy (65). It is unclear if this mechanism is disrupted due to characterized progenitor cell dysfunction induced by diabetes or whether it can be influenced by the increase in sDPP-4 observed with DPP-4i.
DPP-4is and Metformin
Although large clinical CVOTs failed to demonstrate obvious cardioprotective benefits for DPP-4is, a recent meta-analysis of three trials demonstrated that individuals receiving baseline metformin treatment had improved cardiovascular outcomes with DPP-4is compared with metformin nonusers, most notably for MI, stroke, cardiovascular mortality, and hospitalization for unstable angina (66). Similarly, another meta-analysis indicated that metformin-DPP-4i combination therapy markedly reduced nonfatal cardiovascular events and CVD mortality compared with metformin plus sulfonylurea (67). The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus with Coronary Artery Disease (SPREAD-DIMCAD) indicated that metformin substantially reduced MACE compared with glipizide, despite similar HbA1c levels (68), suggesting that metformin’s effects are independent of its glucose-lowering activity. Metformin may potentiate DPP-4i cardioprotection by regulating the incretin pathway. Metformin stimulates intestinal GLP-1 production and promotes GLP-1R as well as GIP receptor expression in islet β-cells via peroxisome proliferator-activated receptor-α, which may increase incretin sensitivity (69). It also lowers circulating sDPP-4 levels (58). Collectively, these mechanisms may enhance the cardiovascular benefits when combined with DPP-4is. However, at this time we cannot discount the contribution of DPP-4-independent mechanisms.
GLP-1Ras Versus DPP-4is
It remains unclear why DPP-4is, despite stabilizing endogenous GLP-1 levels, have not yielded the positive cardiovascular outcomes seen with GLP-1Ras. The amplified cardiovascular risk in metabolic disease involves a complex interplay between inflammatory, lipid-regulatory, and metabolic factors (70). GLP-1Ras produce clear improvements in glycemia, lipid levels, and metabolism in obesity, whereas DPP-4is impart only modest or limited improvements (12). Moreover, while GLP-1 is the primary DPP-4–regulated substrate responsible for the glucose-lowering actions of DPP-4is, DPP-4 substrates influence multiple facets of the cardiovascular system (47). This adds a unique layer of complexity in extrapolating how DPP-4is and GLP-1Ras differentially influence cardiac function in human studies, especially since both native and DPP-4–cleaved peptides can affect the cardiovascular system. For example, the DPP-4–cleaved GLP-1 peptide GLP-1(9-36), which was originally thought to be inactive, may have direct vascular effects that improve cardiac function (22, 71). However, in swine, GLP-1(9-36) had no impact on cardiac function relative to GLP-1(7-36) (72), and both the Harmony Outcomes and REWIND trials were associated with improvements in cardiovascular outcomes despite albiglutide and dulaglutide being highly DPP-4 resistant. These points argue against GLP1(9-36) being critically involved in GLP-1–mediated cardioprotection. Additionally, reduced sensitivity of endogenous signaling pathways to several DPP-4 substrates, including GLP-1, has been described (73, 74). While we have provided the currently known key details from preclinical studies that indicate potential mechanisms explaining GLP-1Ra-induced cardioprotection in T2DM and accounting for discrepancies between preclinical and clinical studies involving DPP-4is, the field is ripe for growth. Studies in aged and diseased mice did not demonstrate cardiovascular efficacy for DPP-4is. Therefore, it is imperative to use preclinical models that reproduce the multifaceted features of diabetes-related CVD (e.g., structural issues, fibrosis, inflammation, and dyslipidemia) more accurately. Metabolic benefit (HbA1c and obesity) with semaglutide has been determined to closely associate with circulating concentrations of the drug (75). Therefore, despite complex signaling mechanisms, plasma exposure of GLP-1Ra may predict efficacy to prevent MACE and explain the discrepancy between DPP-4i and the range of benefits observed in GLP-1Ra trials.
The Evolving Field of Cardiovascular Endocrinology
The completion of numerous T2DM CVOTs, including those investigating DPP-4is and GLP-1Ras, has sparked excitement in the rapidly evolving field of cardiovascular endocrinology. Nonetheless, many questions remain unanswered. For instance, the CVOTs completed to date do not indicate whether one agent is more efficacious than another regarding cardiovascular outcomes. Recent guidelines developed by the American Diabetes Association, Diabetes Canada, and the European Association for the Study of Diabetes indicate that individuals with T2DM at high cardiovascular risk should be preferentially prescribed liraglutide and other therapies with demonstrated cardiovascular benefits (2,76). Additionally, ageing, quality of life (i.e., prevention of hypoglycemia), and adherence must be considered.
Current evidence strongly supports that GLP-1R activation is cardioprotective, whereas DPP-4is are not despite increasing physiological GLP-1 action; thus, these two incretin-based therapies cannot be considered equivalent despite a shared glucose-lowering mechanism of action. It will be imperative for the field to continue defining the mechanisms responsible for GLP-1R–induced cardioprotection in people with T2DM and to better understand the cardiac biology of other DPP-4 substrates. A limitation with many of the mechanisms described here is that most were identified in small rodents, where it is easy to genetically manipulate the mechanistic target to confirm its involvement. However, these cardioprotective effects are often more robust in small rodents, such as that observed for GLP-1Ra-mediated BP reductions, which are often milder in humans and dissociated from the actual impact on cardiovascular outcomes (e.g., EXSCEL). Hence, future studies will need to determine whether such mechanisms translate to larger animal models, as this may also pave the way for the development of new therapies that specifically target T2DM-related CVD.
Another important aspect to consider is that increased survival of acute cardiac events has led to an increased prevalence of HF. Cardiac function end points for HF are not actively included in MACE outcomes in most CVOTs, and HFpEF and HFrEF subjects are often conglomerated, given their shared therapeutic regimen. This is highly relevant, given that ∼40% of patients with HF have HFpEF, and patients with T2DM are overrepresented in this cohort (3). This may explain some of the inconsistencies in studies investigating the effects of GLP-1Ras in HF. Therefore, careful studies evaluating glucose-lowering therapies used in early-stage T2DM to determine their impact on diastolic dysfunction, which goes unrecognized until more overt cardiac dysfunction develops, are required. Additionally, as therapies like sodium–glucose cotransporter 2 inhibitors have established merit for treating HFpEF in the presence or absence of diabetes (76), more integration and collaboration between endocrinologists and cardiologists is required to address these questions and progress toward a more personalized CVD management approach for patients with T2DM.
Article Information
Acknowledgments. We thank Seyed Amirhossein Tabatabaei Dakhili (University of Alberta, Edmonton, Alberta, Canada) for his artistic contributions to Figs. 1 and 2.
Funding. This research was funded by Canadian Institutes of Health Research project grants to E.E.M. and J.R.U. E.E.M. is the recipient of a Diabetes Canada New Investigator Award. J.R.U. is a Tier 2 Canada Research Chair (Pharmacotherapy of Energy Metabolism in Obesity). A.A.G. is a Canadian Institutes of Health Research Vanier Scholar.
Duality of Interest. The Mulvihill lab receives funding from the Merck & Co. Investigator Studies Program for preclinical studies unrelated to this work. No other potential conflicts of interest relevant to this article were reported.
References
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Canada Clinical Practice Guidelines Expert Committee, Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020;44:575–591 [DOI] [PubMed] [Google Scholar]
- 3. Gopal K, Chahade JJ, Kim R, Ussher JR. The impact of antidiabetic therapies on diastolic dysfunction and diabetic cardiomyopathy. Front Physiol 2020;11:603247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verma S, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation 2018;138:2884–2894 [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Holst AG, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017;376:891–892 [DOI] [PubMed] [Google Scholar]
- 6. Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci 2018;3:844–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanada J, Obata A, Obata Y, et al. Dulaglutide exerts beneficial anti atherosclerotic effects in ApoE knockout mice with diabetes: the earlier, the better. Sci Rep 2021;11:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudo M, Li Y, Hiro T, et al. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2017;265:283–291 [DOI] [PubMed] [Google Scholar]
- 9. Vinué Á, Navarro J, Herrero-Cervera A, et al. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia 2017;60:1801–1812 [DOI] [PubMed] [Google Scholar]
- 10. Koska J, Migrino RQ, Chan KC, Cooper-Cox K, Reaven PD. The effect of exenatide once weekly on carotid atherosclerosis in individuals with type 2 diabetes: an 18-month randomized placebo-controlled study. Diabetes Care 2021;44:1385–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buse JB, Bain SC, Mann JFE, et al.; LEADER Trial Investigators . Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care 2020;43:1546–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136:849–870 [DOI] [PubMed] [Google Scholar]
- 13. Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012;32:1513–1519 [DOI] [PubMed] [Google Scholar]
- 14. Rizzo M, Rizvi AA, Patti AM, et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol 2016;15:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications 2014;28:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013;19:567–575 [DOI] [PubMed] [Google Scholar]
- 17. Li CJ, Yu Q, Yu P, et al. Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 2014;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015;38:132–139 [DOI] [PubMed] [Google Scholar]
- 19. Gaspari T, Liu H, Welungoda I, et al. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diab Vasc Dis Res 2011;8:117–124 [DOI] [PubMed] [Google Scholar]
- 20. Moberly SP, Berwick ZC, Kohr M, Svendsen M, Mather KJ, Tune JD. Intracoronary glucagon-like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Exp Biol Med (Maywood) 2012;237:334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helmstädter J, Frenis K, Filippou K, et al. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler Thromb Vasc Biol 2020;40:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res 2014;114:1788–1803 [DOI] [PubMed] [Google Scholar]
- 23. Lønborg J, Kelbæk H, Vejlstrup N, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv 2012;5:288–295 [DOI] [PubMed] [Google Scholar]
- 24. Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009;58:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bao W, Aravindhan K, Alsaid H, et al. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One 2011;6:e23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Margulies KB, Hernandez AF, Redfield MM, et al.; NHLBI Heart Failure Clinical Research Network . Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bizino MB, Jazet IM, Westenberg JJM, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 2019;18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noyan-Ashraf MH, Shikatani EA, Schuiki I, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 2013;127:74–85 [DOI] [PubMed] [Google Scholar]
- 31. Sassoon DJ, Tune JD, Mather KJ, et al. Glucagon-like peptide 1 receptor activation augments cardiac output and improves cardiac efficiency in obese swine after myocardial infarction. Diabetes 2017;66:2230–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiraki A, Oyama JI, Nishikido T, Node K. GLP-1 analog liraglutide-induced cardiac dysfunction due to energetic starvation in heart failure with non-diabetic dilated cardiomyopathy. Cardiovasc Diabetol 2019;18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saponaro F, Sonaglioni A, Rossi A, et al. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment. Diabetes Res Clin Pract 2016;118:21–28 [DOI] [PubMed] [Google Scholar]
- 34. Withaar C, Meems LMG, Markousis-Mavrogenis G, et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res 2021;117:2108–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almutairi M, Gopal K, Greenwell AA, et al. The GLP-1 receptor agonist liraglutide increases myocardial glucose oxidation rates via indirect mechanisms and mitigates experimental diabetic cardiomyopathy. Can J Cardiol 2021;37:140–150 [DOI] [PubMed] [Google Scholar]
- 36. Ding W, Chang WG, Guo XC, et al. Exenatide protects against cardiac dysfunction by attenuating oxidative stress in the diabetic mouse heart. Front Endocrinol (Lausanne) 2019;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hussein AM, Eid EA, Taha M, Elshazli RM, Bedir RF, Lashin LS. Comparative study of the effects of GLP1 analog and SGLT2 inhibitor against diabetic cardiomyopathy in type 2 diabetic rats: possible underlying mechanisms. Biomedicines 2020;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baggio LL, Yusta B, Mulvihill EE, et al. GLP-1 receptor expression within the human heart. Endocrinology 2018;159:1570–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 40. Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 41. Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 42. Rosenstock J, Kahn SE, Johansen OE, et al.; CAROLINA Investigators . Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 2019;322:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenstock J, Perkovic V, Johansen OE, et al.; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scirica BM, Braunwald E, Raz I, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2015;132:e198. [DOI] [PubMed] [Google Scholar]
- 45. McGuire DK, Van de Werf F, Armstrong PW, et al.; Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) Study Group . Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:126–135 [DOI] [PubMed] [Google Scholar]
- 46. Koyani CN, Trummer C, Shrestha N, et al. Saxagliptin but not sitagliptin inhibits CaMKII and PKC via DPP9 inhibition in cardiomyocytes. Front Physiol 2018;9:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 2014;35:992–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang G, Li Y, Cui L, et al. Increased plasma dipeptidyl peptidase-4 activities in patients with coronary artery disease. PLoS One 2016;11:e0163027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng TP, Liu YH, Yang LX, Qin SH, Liu HB. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of subclinical atherosclerosis in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Atherosclerosis 2015;242:580–588 [DOI] [PubMed] [Google Scholar]
- 50. Barchetta I, Ciccarelli G, Barone E, et al. Greater circulating DPP4 activity is associated with impaired flow-mediated dilatation in adults with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2019;29:1087–1094 [DOI] [PubMed] [Google Scholar]
- 51. Cho EH, Kim SW. Soluble dipeptidyl peptidase-4 levels are associated with decreased renal function in patients with type 2 diabetes mellitus. Diabetes Metab J 2019;43:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nargis T, Chakrabarti P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life 2018;70:112–119 [DOI] [PubMed] [Google Scholar]
- 53. Lettau M, Dietz M, Vollmers S, et al. Degranulation of human cytotoxic lymphocytes is a major source of proteolytically active soluble CD26/DPP4. Cell Mol Life Sci 2020;77:751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghorpade DS, Ozcan L, Zheng Z, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 2018;555:673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varin EM, Mulvihill EE, Beaudry JL, et al. Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic DPP4 inhibition. Cell Metab 2019;29:320–334 [DOI] [PubMed] [Google Scholar]
- 56. Lee YS, Riopel M, Cabrales P, Bandyopadhyay GK. Hepatocyte-specific HIF-1α ablation improves obesity-induced glucose intolerance by reducing first-pass GLP-1 degradation. Sci Adv 2019;5:eaaw4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ohnuma K, Yamochi T, Uchiyama M, et al. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol 2005;25:7743–7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baggio LL, Varin EM, Koehler JA, et al. Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat Commun 2020;11:3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ikeda T, Kumagai E, Iwata S, Yamakawa A. Soluble CD26/dipeptidyl peptidase IV enhances the transcription of IL-6 and TNF-α in THP-1 cells and monocytes. PLoS One 2013;8:e66520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee DS, Lee ES, Alam MM, et al. Soluble DPP-4 up-regulates toll-like receptors and augments inflammatory reactions, which are ameliorated by vildagliptin or mannose-6-phosphate. Metabolism 2016;65:89–101 [DOI] [PubMed] [Google Scholar]
- 61. Kubota A, Takano H, Wang H, et al. DPP-4 inhibition has beneficial effects on the heart after myocardial infarction. J Mol Cell Cardiol 2016;91:72–80 [DOI] [PubMed] [Google Scholar]
- 62. Sauvé M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010;59:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Batchu SN, Thieme K, Zadeh FH, et al. The dipeptidyl peptidase 4 substrate CXCL12 has opposing cardiac effects in young mice and aged diabetic mice mediated by Ca2+ flux and phosphoinositide 3-kinase γ. Diabetes 2018;67:2443–2455 [DOI] [PubMed] [Google Scholar]
- 64. Mulvihill EE, Varin EM, Ussher JR, et al. Inhibition of dipeptidyl peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes 2016;65:742–754 [DOI] [PubMed] [Google Scholar]
- 65. Gross L, Theiss HD, Grabmaier U, et al. Combined therapy with sitagliptin plus granulocyte-colony stimulating factor in patients with acute myocardial infarction-Long-term results of the SITAGRAMI trial. Int J Cardiol 2016;215:441–445 [DOI] [PubMed] [Google Scholar]
- 66. Crowley MJ, Williams JW Jr, Kosinski AS, D’Alessio DA, Buse JB. Metformin use may moderate the effect of DPP-4 inhibitors on cardiovascular outcomes. Diabetes Care 2017;40:1787–1789 [DOI] [PubMed] [Google Scholar]
- 67. Wang F, He Y, Zhang R, Zeng Q, Zhao X. Combination therapy of metformin plus dipeptidyl peptidase-4 inhibitor versus metformin plus sulfonylurea and their association with a decreased risk of cardiovascular disease in type 2 diabetes mellitus patients. Medicine (Baltimore) 2017;96:e7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hong J, Zhang Y, Lai S, et al.; SPREAD-DIMCAD Investigators . Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology 2011;152:4610–4619 [DOI] [PubMed] [Google Scholar]
- 70. Dugani SB, Moorthy MV, Li C, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol 2021;6:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 72. Goodwill AG, Tune JD, Noblet JN, et al. Glucagon-like peptide-1 (7-36) but not (9-36) augments cardiac output during myocardial ischemia via a Frank-Starling mechanism. Basic Res Cardiol 2014;109:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Varin EM, Hanson AA, Beaudry JL, et al. Hematopoietic cell- versus enterocyte-derived dipeptidyl peptidase-4 differentially regulates triglyceride excursion in mice. JCI Insight 2020;5:e140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab 2017;26:278. [DOI] [PubMed] [Google Scholar]
- 75. Overgaard RV, Hertz CL, Ingwersen SH, Navarria A, Drucker DJ. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes. Cell Rep Med 2021;2:100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Anker SD, Butler J, Filippatos G, et al.; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461 [DOI] [PubMed] [Google Scholar]