Abstract
Melanomas arising in the mucous membranes are a rare and aggressive subtype. New treatment approaches are needed, yet accumulating sufficient evidence to improve patient outcomes is difficult. Clinical and pathological correlates between human and canine mucosal melanomas (MM) are substantial, and the relatively greater incidence of spontaneous naturally occurring MM in dogs represents a promising opportunity for predictive modeling. The genomic landscapes of human and canine MM appear highly diverse and generally lack recurring hotspot mutations associated with cutaneous melanomas. Although much remains to be determined, evidence indicates that Ras/MAPK and/or PI3K/Akt/mTOR signaling pathway activations are common in both species and may represent targets for therapeutic intervention. Sapanisertib, an mTORC1/2 inhibitor, was selected from a PI3K/mTOR inhibitor library to collaborate with MEK inhibition; the latter preclinical efficacy was demonstrated previously for canine MM. Combined inhibition of MEK and mTORC1/2, using trametinib and sapanisertib, produced apoptosis and cell cycle alteration, synergistically reducing cell survival in canine MM cell lines with varying basal signaling activation levels. Compared to individual inhibitors, a staggered sapanisertib dose, coupled with daily trametinib, was optimal for limiting primary MM xenograft growth in mice, and tumor dissemination in a metastasis model, while minimizing hematologic and renal side effects. Inhibitors downmodulated respective signaling targets and the combination additionally suppressed pathway reciprocal crosstalk. The combination did not significantly change plasma sapanisertib pharmacokinetics, however trametinib area under the curve was increased in the presence of sapanisertib. Targeting Ras/MAPK and PI3K/Akt/mTOR signal transduction pathways appear rational therapies for canine and human MM.
Keywords: trametinib, sapanisertib, BRAF and NRAS wild type, dog, melanoma therapy, mass spectrometry
Introduction
While the majority of melanomas occur in the skin, mucosal melanomas (MM) constitute a rare and aggressive form comprising approximately 1–2% of melanomas (1, 2). Unlike cutaneous melanoma, for which ultraviolet (UV) exposure is a significant risk factor, circumstances surrounding melanoma development in the mucosae are more occult. Since MM is rare and few patients get included in clinical trials, progress against this form of melanoma remains challenging (3). Poor therapeutic responsiveness also contributes to the challenges and new treatments are critically needed.
Mouse models analogous to those used for preclinical development in cutaneous melanoma are not widely available to advance therapies for MM (4). We and others have credentialed spontaneous, naturally occurring canine MM as a preclinical model of human MM; one that represents the entire cancer spectrum from benign disease to metastatic spread, and can be utilized to study the disease progression as well as for developing novel treatments for both dogs and humans (5, 6). Both human and canine MM share similar growth pattern, disease histogenesis, and poor therapeutic responsiveness. Furthermore, dogs with spontaneous melanoma are immune‐competent and phylogenetically more similar to humans than are rodents. With shorter natural life span and relatively brief disease course compared to humans, it is feasible to clinically study veterinary patient cohorts amenable to frequent monitoring and sampling.
Recently, studies assessing the genomic landscape in human and canine MM are revealing highly diverse mutation patterns (7–9). Since MM arises from sun-shielded areas, the UV-induced mutation signatures noted in many cutaneous melanomas do not characterize MM. In contrast to human cutaneous melanoma, both human and canine MM exhibited low single nucleotide variation rate (6, 10). The genomic studies of MM thus far have not consistently revealed recurrent driver mutation candidates. The limited overlapping gene mutations among studies appear to complicate identifying a recurring target for drug development. Despite the paucity of recurrent functional mutations discovered in MM, both human and canine MM appear to exhibit aberrant activities in Ras/ERK and PI3K/Akt/mTOR signaling pathways with some frequency (8, 9, 11). Thus, targeting these pathways may be an attractive treatment alternative.
In examining naturally occurring MM in dogs as a means to identify a targeted therapeutic approach potentially applicable for MM in both dogs and humans, we previously combined trametinib (NDC 0078–0666), targeting MEK, with dactolisib, a dual PI3k/mTOR inhibitor (11). Although this combination synergistically reduced survival and growth of canine MM cells in vitro and in a xenograft model, the latter PI3K/mTOR kinase inhibitor appears to have a fairly narrow safety profile and poor tolerability (12, 13). Therefore, an alternative inhibitor to target PI3K/Akt/mTOR signal transduction was sought to pair with trametinib for treating MM. Trametinib is an FDA-approved MEK inhibitor used as a single agent or in combination with dabrafenib, a BRAF inhibitor, for the treatment of BRAF-V600 mutated melanomas, metastatic non-small cell lung cancer, and thyroid cancer in people (14–16). Phase III trial results indicated that tumor cells developed survival mechanisms independent of Ras/ERK signaling pathways when treated with the BRAF/MEK inhibitors. Continuing trials include coupling trametinib with immunotherapy, including anti-PD-1 and anti-CTLA-4, or with a second targeted therapy, such as GSK2141795, an AKT inhibitor (17). Combined trametinib and immunotherapy resulted in some toxicities, while the efficacy/toxicity of trametinib plus GSK2141795 remains unclear. It is therefore rational to continue evaluating drug combinations as well as dose and treatment schedules, particularly for patients with aggressive forms of melanoma (14).
Overlapping downstream feedback influences between the PI3K/Akt/mTOR and Ras/MAPK signaling pathways is known in a variety of human cancers, and AKT activation in the face of BRAF and MEK inhibition can be a source of drug resistance in melanoma (18). Therefore, our goal was to identify a well-tolerated and therapeutically effective inhibitor combination to couple with trametinib for MM therapy. Cognizant of the recurring activation of one or both RAS/ERK and PI3K/Akt/mTOR signal transduction pathways in MM (8, 9), we screened a PI3K/Akt/mTOR pathway inhibitor library to evaluate whether MM cells were more susceptible to a specific category of PI3K pathway target inhibition. This was pursued in combination with trametinib as a strategy to circumvent possible resistance due to signaling crosstalk for MM (19, 20). Based upon screening and on initial testing, we chose sapanisertib, a dual mTORC1/2 inhibitor (https://pubchem.ncbi.nlm.nih.gov/compound/Sapanisertib), to combine with trametinib to further examine the efficacy of tandem parallel signaling pathway inhibition using NRAS-mutated or triple wild-type (nonmutated NRAS, BRAF, and NF1) MM models.
Materials and Methods
Melanoma specimens and cell lines
All canine MM cell lines, kindly provided by Dr. Michael Kent (University of California, Davis) [UCDK9M1 (M1), UCDK9M2 (M2), UCDK9M3 (M3), UCDK9M5 (M5)], and Drs. Jared Fowles and Dan Gustafson (Colorado State University, Fort Collins) (Jones), were maintained as described (11). Human cutaneous melanoma cell lines, WM3011, WM2032, WM853–2 and WM858 were purchased from Rockland Immunochemicals (Limerick, PA) and cultured according to the vendor’s guidance. Basal activation levels of selected MAPK and Akt/mTOR signaling mediators are shown for all cell lines (Supplemental Figure S1A).
Inhibitor library screening and drug inhibition studies
For PI3K/mTOR inhibitor library screening, M1 and M5 canine MM cells were plated in 96-well plates. Inhibitors were added at 625 and 160 nM concentration, with and without 10 nM trametinib for 72h (HY-L015, MedChemExpress, Monmouth Junction, NJ). For growth inhibition, canine MM cells and human cutaneous melanoma cells were plated and inhibitors, or DMSO-only control were added once to wells 24h after plating cells. For dose responses, inhibitors were dosed from 10 μM to 0.125 nM (serial 1:4 dilutions); for combination treatment trametinib (https://chemtechbio.com/products/gsk1120212), and either sapanisertib (https://www.medchemexpress.com/INK-128.html) or Torin 2 (https://pubchem.ncbi.nlm.nih.gov/compound/Torin-2; https://www.medchemexpress.com/Torin-2.html), were combined at a 1:1 molar ratio. Viability was determined by MTS assay (Promega, Madison, WI), 72h after the addition of drugs. Each experiment was repeated at least twice.
Animal studies
All animal experiments using 6‐8‐week‐old female nu/nu athymic nude mice (Charles River Laboratories, Frederick, MD) were carried out under review and approval by NCI animal care and use programs in compliance with current US Public Health Service Guide for the Care and Use of Laboratory Animals (8th edition, 2015). For localized tumor growth, 1×106 M1 or M5 cells were injected subcutaneously into the flanks of mice. Mice with established tumors (75–150 mm3) were randomized into groups. Mice were treated with trametinib, sapanisertib or Torin 2, or with either of the latter two combined with trametinib, at multiple indicated schedule and doses (indicated in figures). The vehicle, 0.5% hydroxypropyl methylcellulose/0.2% Tween, was used for all inhibitors and the drug suspension was administered via oral gavage. Melanoma tumor dimensions were measured two to three times a week using a caliper, and tumor volumes were calculated using π/6 × dimension 1 × dimension 2 × dimension 3. Body weights were recorded at least twice a week to gauge tolerability of treatments. Approximately 4h after final gavage in a series, tumors and tissues were either snap frozen or fixed in 10% neutral‐buffered formalin. The latter specimens were routinely prepared for histology (HistoServ, Inc., Gaithersburg, MD). Mice were electively taken off study when tumors approached 2000 mm3, or loss of ≥20% body weight occurred.
Evidence of drug target modulation was examined in vivo in two separate cohorts of mice. The first cohort of two tumor-bearing mice per treatment had blood plasma and subcutaneous tumor collected and processed for drug concentration determination (supplemental methods), at 2, 4, 8, 12, 24, and 48 hours following a single dose of individual inhibitor, or drug combination, at the indicated concentrations. In a second study, five tumor-bearing mice per group were dosed daily for three days and tumors tissues were collected and analyzed by western blot and immunohistochemistry to evaluate target inhibition efficacy (supplemental methods).
To evaluate efficacy to interrupt tumor metastases, 5×105 cells of an M1-derived metastatic subline were administered intravenously in a tail vein injection metastasis study and mice were treated with either single agent, combination treatments, or control vehicle (10 mice/treatment group) for up to 28 days, beginning on day 6 following cell injection. Intrathoracic soft-tissue organs were removed at necropsy to assess propensity for metastasis in this model using automated machine learning image analysis to map and quantify lesions in tissue sections (supplemental methods).
In order to assess cumulative adverse acute organ toxicity, multiple organs were collected, fixed and processed for histopathology from non-tumor bearing mice treated for 21 days (n=5 per group). Immediately prior to removal from study, mice evaluated for lesions associated with repeat drug dosing were bled. Whole blood was collected in EDTA (Fisher Scientific, Hanover Park, IL) and complete blood cell counts were obtained (Laboratory Animal Sciences, Leidos Biomedical Research, Frederick, MD).
Pharmacokinetics
Trametinib and sapanisertib were quantified in mouse plasma in validated liquid chromatographic-tandem mass spectrometric (LC-MS/MS) assays. Sapanisertib was detected by multiple reaction monitoring (MRM) of the mass transition m/z 310.2→268.1 over a calibrated range of 1–1000 ng/mL. Trametinib was detected by MRM m/z 616.0→491.6 over a calibrated range of 0.25 – 500 ng/mL. (Also supplemental methods).
Statistics
Statistical significance was determined by Wilcoxon signed-rank or t-tests. Assessment of synergy for the inhibitor combinations was performed using the Chou‐Talalay algorithm (CompuSyn, ComboSyn Inc., Paramus, NJ). Plasma pharmacokinetics (PK) were calculated using noncompartmental methods for destructive sampling using a validated Phoenix WinNonlin® v8.1 method (Certara, Cary, NC), with error around the plasma concentration area under the time curve (AUC) calculated to the last observed timepoint (AUCLAST) using Bailer’s Method. A t-test was used to determine statistical significance between two groups (p<0.05) following error calculations of mean estimated values.
Results
PI3K/AKT/mTOR inhibitor library screening
Previously, we showed that the MEK inhibitor, trametinib, was effective in inhibiting canine MM cell growth in vitro at low inhibitory concentration (IC50~10nM) (11). Although dactolisib, a dual PI3k/mTOR inhibitor, exhibited dose dependent cytotoxicity in the same series of experiments, further examination of canine MM sensitivity to PI3K pathway inhibition was investigated with a PI3K/AKT/mTOR inhibitor library. The 156-inhibitor-compound library targeted various nodes within the PI3K pathway, including AKT, AMPK, GSK3, PDK-1, PTEN, PI3K, and mTOR. Canine MM cell lines M1 and M5 were treated with two concentrations of inhibitors, 625 and 160 nM, with or without the presence of 10 nM trametinib. Cell survival indicated greatest sensitivities to subgroups of both mTOR and PI3K/mTOR dual inhibitors (Supplemental Figure S2). This pattern of diminished cell survival was similar, but enhanced, for the various PI3K library compounds with the presence of trametinib, indicating potential advantage of combination treatment. Since we sought an alternative to known adverse effects associated with the use of some PI3K/mTOR dual inhibitors in human clinical trials (21, 22), we selected mTORC1/2 inhibitors sapanisertib (INK-128/TAK228) and Torin 2 for further examination of their efficacy, based upon this screen. Sapanisertib is currently in active human clinical trials for various advanced or refractory solid tumors (https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/sapanisertib?redirect=true) while there are no evident trials of Torin 2.
The efficacy and toxicity of Torin 2
Our analyses modeling MM indicated that the mTORC1/2 inhibitor Torin 2 was highly effective in cytotoxicity tests and target inhibition in vitro, either as a single agent or in combination with trametinib (Supplemental Figure S3A,B). In an attempt to define a maximum tolerated dose however, canine MM xenograft bearing mice lost weight during oral administration of Torin 2 at therapeutic levels sufficient to inhibit tumor growth (40, 60, and 80 mg/Kg) (Supplemental Figure S3C). Although treated mice appeared capable of recovering body weight loss upon skipping some doses (e.g., 40 mg/Kg), the Torin 2 dose range for efficacy and safety was narrow. As a consequence, sapanisertib, a second mTORC1/2 inhibitor, was examined.
Synergistic effects of combined trametinib and sapanisertib in vitro
The combination of sapanisertib and trametinib was tested on a panel of canine MM cell lines with various degrees of basal PI3K/mTOR and Ras/ERK pathway activities (Supplemental Figure S1). Canine MM, and for comparison human cutaneous melanoma cell lines harboring various mutations, were treated with trametinib and sapanisertib individually or in combination at 1:1 molar ratios. All cells tested were sensitive to sapanisertib with IC50 between 10 and 100 nM (Figure 1A). Cells were also sensitive to trametinib regardless of Ras and NF1 mutation status. We noted that >100 nM trametinib did not appear to provide further cytotoxicity, likely indicating saturation of the MEK binding site at ~100 nM.
Figure 1.
Cytotoxicity and target inhibition of trametinib and sapanisertib individually, and as a combination. A) Reduced cell survival occurred in canine MM cells (M1, M5, M2, M3, and Jones) and human cutaneous melanoma cells (WM3311, WM2032, WM853–2 and WM858) shown for comparison, treated with escalated dose concentrations of trametinib and sapanisertib individually or in combination at 1:1 molar ratio. The treatments were added to wells on day 0 and incubated for 72 hours (h), at which time cell titers were determined by MTS assay. The results are presented as ratio of OD490,treated/OD490,DMSO control. Each data point represents mean of 3–6 wells and error bars indicate replicate standard deviation. Each treatment was repeated at least twice; representative results are shown. B) Western blots to examine target inhibition in M1, M5 and Jones canine MM cell lines treated with trametinib (10 nM) and sapanisertib (40 nM) individually or as a combination for 4 and 24h. Cell lysates were prepared from treated cells and anti-p-AKT, p-S6 and p-ERK were used to gauge the activation status of PI3K/mTOR and Ras/ERK pathways. C) Chou-Talalay computation of combination indices (CI) for treated cells are shown for 50% and 90% affected fraction (Fa) values at 72h post-exposure. Indication of synergistic two-drug effect was interpreted at <1.0 Fa. D) Apoptosis and cell cycle alteration may contribute to the decreased cell titers of treated cells. Western blots were used to detect the level of cyclin D1, cyclin B1, Bim, Bcl-xl, Mcl-1 and p-Bad in cells treated with trametinib and/or sapanisertib.
Both trametinib and sapanisertib inhibited their respective Ras/MAPK and PI3K/Akt/mTOR signal transduction targets when used individually or in combination (Figure 1B). We also observed apparent reciprocal activation of ERK1/2 when AKT activity was diminished in some cell lines treated with sapanisertib alone (Figure 1B). Under combined-drug exposure, the reciprocal activation of the two pathways was suppressed and the effect on canine melanoma appeared synergistic (Figure 1C). An analogous target inhibition pattern was also observed in similarly exposed human cutaneous melanoma cells (Figure 1C, and Supplemental Figure S1B), although reciprocal cross talk among the Ras/MAPK and PI3K/Akt/mTOR signal transduction pathways was not as evident in human cutaneous melanoma under the experimental conditions examined.
Mechanistically, trametinib treatment reduced the expression of cyclins D1 and B1 in canine MM, while sapanisertib was effective in reducing cyclin B1 (Figure 1D). The two-drug combination reduced the cyclins further, suggesting inhibition of G2/M cell cycle progression. In addition, both drugs altered the balance of Bcl2 family protein expression by increasing the levels of pro-apoptotic family member Bim, while reducing the levels of pro-survival family members, Bcl-xl and Mcl1 (Figure 1D). Sapanisertib also inhibited phosphorylation of Bad, a pro-apoptotic protein whose phosphorylation reduces its function. Despite the changes in cell cycle and pro- and anti-apoptotic protein expressions, the annexin V-labeling showed a trend toward increased apoptosis in trametinib, and to a greater degree combination-treatments, relative to sapanisertib-treated (and sham control) cultures (Supplemental Figure S4). Taken together, a blend of cell cycle inhibition as well as apoptosis likely contribute to reduced cell titers in single and combination-treated canine MM cells in vitro.
Dose range finding and safety in mice
We next tested whether the influence on survival observed in cells in vitro could be translated in tumor-bearing mice. A preliminary study in mice with established canine M1 or M5 MM xenografts was initiated in three treated cohorts using daily 5.0 mg/Kg trametinib or 3.0 mg/Kg sapanisertib, as well as the two-drugs combined. The initial daily 3.0 mg/Kg sapanisertib dose was not well tolerated, as reflected in body weight loss and the fact that four mice had to be removed from study; therefore, dose and frequency of administration were reduced in the cohort three days after initiation of the treatment (Supplemental Figure S5A). This sapanisertib intermittent-dose modification permitted some recovery in body weights, while maintaining tumor growth inhibition (Supplemental Figure S5B). This finding inferred maintenance of tumor growth inhibition with improved tolerability when staggering an intermittent dose frequency for sapanisertib.
To refine a more optimal treatment schedule for sapanisertib, while evaluating the nature of dose-related systemic toxicity, we tested tolerability of a lower 2.0 mg/Kg dose, given daily or every other day (EOD). In this study, mice receiving daily 2.0 mg/Kg oral doses of sapanisertib experienced body weight loss (11–14% in 4 of 5 mice) after two weeks on treatment (Supplemental Figure S6). These mice had to be removed from study by 16 days due to poor body condition, with one exception. However, the same sapanisertib dose given alone EOD, or EOD in combination with daily trametinib, was well tolerated. White blood cell counts from blood samples collected prior to elective euthanasia revealed lymphopenia in daily sapanisertib-treated mice (Supplemental Figure S6). Other treated mice differed, exhibiting some variable degree of neutrophilia in the absence of lymphopenia. Histological examination of brain, intestines, stomach, lung, heart, liver, spleen, kidney and bone marrow revealed compound-related changes primarily in the liver, spleen, kidney and bone marrow in mice receiving sapanisertib alone or in combination (Table 1). Consistent with most prominent weight losses befalling mice receiving sapanisertib daily were renal tubular degeneration and necrosis, as well as bone marrow hypocellularity (Supplemental Figure S7). These mice also had degenerative hepatocellular cytoplasmic vacuolation (Table 1). Reduction of splenic cellularity overall was most pronounced in mice treated daily with sapanisertib. When sapanisertib was administered EOD, or EOD in combination with daily trametinib, renal tubular changes were limited to minimal necrosis of isolated cells accompanied by tubular epithelial regeneration, which was not considered an adverse event (Table 1, Supplemental Figure S7). When sapanisertib exposure was limited to EOD, with or without trametinib, adverse impact on the bone marrow, spleen, liver and kidney was minimized, while moderate lymphoid and hematopoietic hyperplastic responses, not considered detrimental events, were observed in spleens in the combination treatment group (Table 1).
Table 1.
Lesions and findings in mice treated with sapanisertib and/or trametinib for up to 21 days.
| sapanisertib 2mg/kg daily |
sapanisertib 2mg/kg every other day |
sapanisertib 2mg/kg every + trametinib 5mg/kg daily |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no lesion | lesion/feature severity* |
no lesion | lesion/feature severity* |
no lesion | lesion/feature severity* |
||||||||
| + | ++ | +++ | + | ++ | +++ | + | ++ | +++ | |||||
| Bone marrow | Hypocellularity | 3† | 2 | 5 | 4 | 1 | |||||||
| Renal tubules | Zonal cortical degeneration/necrosis | 1 | 1 | 3 | 5 | 5 | |||||||
| Scattered isolated necrosis and regeneration | 5 | 1 | 4 | 1 | 4 | ||||||||
| Spleen | Red and white pulp hypocellularity | 1 | 2 | 2 | 5 | 5 | |||||||
| Lymphocytolysis | 5 | 2 | 3 | 5 | |||||||||
| Lymphoid and hematopoietic hyperplasia | 2 | 3** | 2 | 3 | 5 | ||||||||
| Liver | Hepatocelluar vacuolation | 2 | 1 | 1 | 1 | 5 | 5 | ||||||
severity: + minimal, ++ moderate, +++ severe
numbers of animals with described feature / severity; n = 5 per treatment; group blank = 0
+ evidence of cell turnover
Vehicle and trametinib treated mice had no significant lesions. Minimal splenic hyperplasia including welldeveloped marginal zones and hematopoiesis was uniformly present in these mice.
Efficacy and target inhibition in vivo
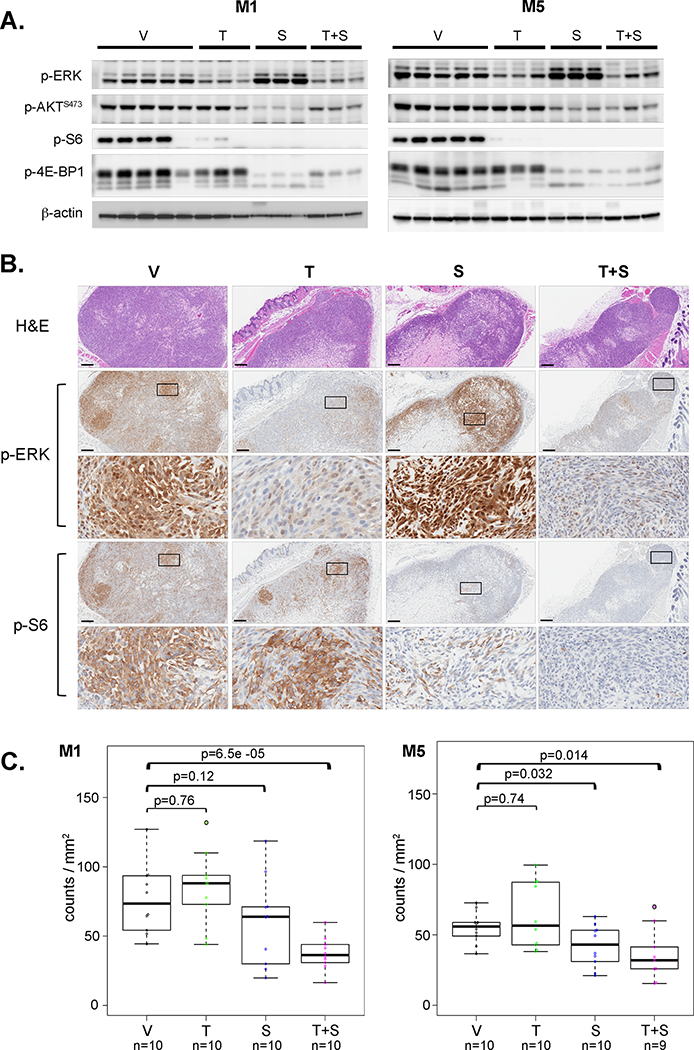
Evidence of pathway inhibition in vivo was first tested in the context of our initial dose range finding experiments. Mice with established tumor grafts were treated orally with sapanisertib at 2.0 mg/Kg and/or trametinib at 5.0 mg/Kg for three consecutive days. Respective targets were inhibited in tumors with individual drug treatments (Figure 2A). In trametinib-treated tumors, p-ERK was less compared to vehicle-only treated mice. Compared to controls, sapanisertib reduced p-AKT as well as downstream effectors p-S6 and p-4EBP-1 levels (Figure 2A). Diminution of Akt/mTOR signal activation due to sapanisertib-only treatment was accompanied by an apparent increased p-ERK level in all tumors, indicating reciprocal activation of alternative pathways, as noted in vitro. Further, p-ERK and p-S6 IHC confirmed both the suppression of two signaling pathway activations as well as the reciprocal activation of p-ERK, in tumor sections when the respective single-agent treatments were provided (Figure 2B). Similarly, tumors from mice receiving drug combination had reduced activities in both PI3K/Akt/mTOR and Ras/ERK pathways, including ERK, AKT, S6 and 4EBP-1, as demonstrated by western blots and IHC. Combination-inhibitor treatment of mice appeared to further diminish pathway activation in tumors while also negating the reciprocal signaling activation cross-talk that occurred with single agent treatment (Figure 2A,B). Tumor proliferation was evaluated ex vivo as counts of anti-p-HH3 immunolabeled cells per tumor area. Despite evidence of target inhibition, fewer anti-p-HH3 immunopositive cells compared to controls were only observed in tumors from both M1 and M5 when the two-drugs were administered together (Figure 2C), indicating that proliferation was more reliably diminished during initial treatment stage (three days) by the simultaneous inhibition of both pathways with repeated doses.
Figure 2.
Trametinib and sapanisertib target inhibition in vivo. Nude mice bearing subcutaneous M1 or M5 canine MM xenografts were treated daily with 5.0 mg/Kg trametinib and/or 2.0 mg/Kg sapanisertib to investigate target inhibition in vivo. A) Tumors were harvested and lysed after three daily doses of inhibitors trametinib (T), sapanisertib (S), the two-drug combination (T+S), or vehicle only control (V) for western blots of p-ERK, p-AKT, p-S6 and p-4E-BP1. B) Fixed tumor tissues were sectioned for H&E staining, and anti-p-ERK and p-S6 IHC was performed to independently assess affirmation of western blot results in the same mouse experiment as in A). Photomicrographs from M1 canine MM xenografts of treated mice, displayed at low and high magnification for IHC (scale bar=200 µm), are representative of five mice per group. Area of higher magnification location is shown as box in respective low magnification image. C) To assess the early responses to inhibitor action, anti-p-HH3 IHC was performed to investigate the proliferative activity of MM cells after three daily doses of inhibitor treatment. Two regions of interest were randomly selected for each tumor, five tumor tissues per treatment group. Anti-p-HH3 immunopositive cells were counted and the results were presented as positive cell number per tumor area. Median counts, solid line, with 1st and 3rd quartile ranges indicated in box plots. Wilcoxon signed-rank tests were used to compare the proliferation activities between treatment groups; p-values are indicated.
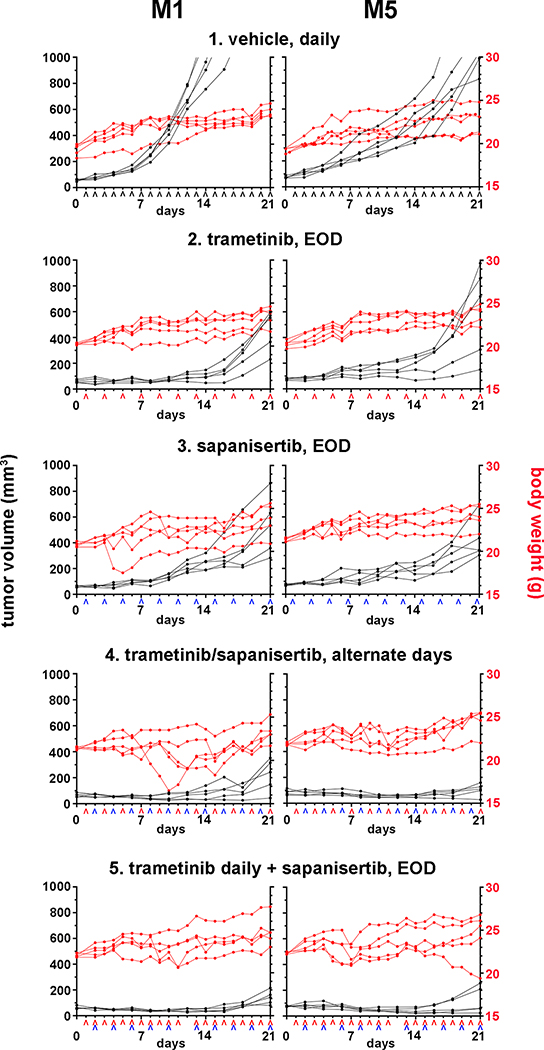
Since tolerability was improved with dose reduction and intermittent administration, the therapeutic potential of sapanisertib and trametinib was evaluated further in the interest of moderating adverse events, using the canine M1 and M5 MM xenograft models. Oral administration of either 5.0 mg/Kg trametinib or 2.0 mg/Kg sapanisertib EOD for 3 weeks, as single agents, was tolerated with some varying body weight fluctuation (Figure 3). Tumor growth was inhibited compared to vehicle-treated controls initially, but progression became evident over time. For the combination treatment, two different drug schedules were employed in separate cohorts of mice. Since trametinib was well tolerated when given daily (Supplemental Figure S5), we tested a dose schedule combination including 5.0 mg/Kg trametinib every day in conjunction with 2.0 mg/Kg sapanisertib EOD. This combination induced a nearly stable disease course as assessed by monitoring body weight and tumor size (Figure 3). In the second dose schedule employing the same formulations, trametinib was alternated in single daily doses with sapanisertib, so that drug exposure for each agent was every other day. This combination exhibited variable and reversible body weight fluctuation. ANOVA analysis indicated tumor-size effect differences for each treatment condition from day 4 to up to 21 days was significantly different than control conditions (p<0.03) (Figure 3). Therapeutically, this combination was interpreted to suppress tumor growth in both M1 and M5 models compared to single agent treatments. Hence, combining trametinib with sapanisertib, in staggered dose schedules for the latter inhibitor, improved tolerability and maintained commensurate improved treatment effects in preclinical modeling compared to treatment with either drug alone.
Figure 3.
Pre-clinical therapeutic efficacy and tolerability in a staggered repeat-dose treatment regimen. Nude mice bearing M1 or M5 canine MM subcutaneous xenografts were randomly divided into five treatment groups, 1) vehicle, daily, 2) trametinib, every other day (EOD), 3) sapanisertib, every other day, 4) trametinib and sapanisertib on alternate days, and 5) trametinib daily plus sapanisertib every other day, (n=5 mice per group). Trametinib (^ red caret) was given at 5.0 mg/Kg and sapanisertib (^ blue caret) at 2.0 mg/Kg; the treatment schedule is labeled along the x-axis. The body weights and tumor volumes were followed through the 21-day treatment duration.
We next tested capacity of the treatments to affect experimental metastasis. In this series, trametinib was given daily and sapanisertib was administered three times a week, in an interest to further explore a balance between efficacy and tolerability. This combination treatment was compared to cohorts provided either sapanisertib three times per week or trametinib daily, as single agent treatments, as well as compared to vehicle-only treated mice. At the end of the four-week treatment, trametinib-only treated mice had significantly less metastatic tumor burdens in the thorax compared to control vehicle-treated mice (p<0.0001), while sapanisertib provided alone three times a week was not as effective as trametinib in abating metastasis (Figure 4). Moreover, the two-drug combination further significantly minimized intrathoracic metastatic tumor burden compared to the trametinib-only treatment group (p<0.0001). Our results signified that the combination treatment inhibited some elements of the melanoma metastatic cascade most efficiently, in addition to reducing tumor growth.
Figure 4.
Combination trametinib and sapanisertib impeded tumor metastases in a MM metastatic model. A metastatic subline of M1 was injected hematogenously into nude mice via tail vein. Four treatment groups included vehicle control, daily (V); sapanisertib, 3 times a week (S), trametinib, daily (T), and combination (T+S); n=10 mice per group. Treatments started approximately one week after tumor cell injection and extended four weeks. Trametinib was given at 5.0 mg/Kg and sapanisertib 2.0 mg/Kg. The intra-thoracic tumor burden was evaluated using H&E stained sections of thoracic tissues using quantitative machine learning pattern classifier image analyses. A) Representative illustrations of H&E-stained tissue photomicrographs of a vehicle control and a combination treated mouse, and the corresponding morphometric pseudocolor markup of the identical images segmented as tumor (red) and lung plus other non-neoplastic thoracic tissues (green) are shown (bars=1 mm). B) Ratios of tumor to total intra-thoracic tissue, the latter representing tumor plus non-neoplastic tissue areas, by treatment group. Five step sections of thoracic tissues, approximately 400 μm apart, were evaluated for each animal (sections per treatment = 45 – 50). Each point represents analysis of individual tissue sections. Comparisons of the tumor burdens between groups displayed as box plots depicting median counts, solid line, with 1st and 3rd quartile ranges indicated. Wilcoxon signed-rank tests were used to compare the experimental metastatic areas between treatment groups. p-values are indicated.
Based upon evidence of diminished primary and metastatic melanoma progression in these repeat-dose efficacy studies, we conducted a further evaluation of respective target modulation in xenografts in coordination with blood plasma inhibitor concentration kinetics. This was accomplished in cohorts of two tumor-bearing mice exposed to a single administration of individual or two-drug-combination. The PK results are shown in Figure 5A,B for trametinib and sapanisertib, respectively. There was a significant increase in trametinib plasma AUC in the presence of sapanisertib relative to trametinib alone (Figure 5A). By contrast, there was no significant change in sapanisertib PK based on the presence of trametinib in mice, although the T1/2 was approximately 50% (6.21h), suggesting more rapid clearance in the presence of trametinib, compared to sapanisertib alone (Figure 5B); however this influence on the AUC was not significant. A nadir of pathway activation was evident in tumors from these mice at 4h or 12h post oral dosing (Figure 5C). p-S6 was not detected in combination treated mice at 4h and 12h, while Akt and ERK activation were also diminished compared to other conditions and timepoints.
Figure 5.
Single administration plasma pharmacokinetic profiles of trametinib (5.0 mg/Kg) and sapanisertib (2.0 mg/Kg), or combination, were associated with respective target inhibition in tumor xenografts. Plasma samples and tumor tissues were collected at 2, 4, 6, 8, 12, 24 and 48 hours after administration of inhibitors, two mice per treatment group per time point. The plasma pharmacokinetics of trametinib alone (black tracing) or in combination with sapanisertib (purple tracing) A), or of sapanisertib alone (red tracing) or with trametinib (purple tracing) B), were determined using mass spectrometry. Maximum concentration (Cmax), time of ½ clearance (T1/2), and the plasma concentration over time (AUCLAST) are indicated for individual inhibitors when administered alone and in combination (single/combination). † (p<0.001). C) Target inhibition in tumor tissues by western blot. Selected representative tumor samples are shown to demonstrate the activity changes of ERK, AKT and S6 over the course of 48 hours post single oral treatment with trametinib (T), sapanisertib (S) and the combination (T+S).
Discussion
Activation of one or both of Ras/MEK/ERK and PI3K/Akt/mTOR signal transduction pathways appear to be recurring mechanisms for tumorigenesis and growth in both canine and human MM (23). Combined targeting of the two signaling pathways was considered to be an attractive strategy for treating MM when a precisely characterized targetable mutation was undefined. Combined therapeutic targeting has been beneficially applied in clinical cancer treatments, including melanoma (24). Inhibiting multiple nodes in an activated signaling pathway has attained better clinical outcome than single drug therapy; prolonged response rate and duration were achieved (25). In circumstances with multiple signal transduction pathways capable of reciprocal cross interaction (20, 26), a tandem (horizontal) targeting approach in a parallel manner was considered to be an effective strategy to inhibit pathways needed for cancer cell survival simultaneously.
In this study, the combination of trametinib and sapanisertib was effective in inhibiting canine MM growth in vitro, despite various levels of basal signal transduction pathway activities. Basal levels of signaling activation don’t necessarily predict responsiveness to combined targeting, as all canine MM tested showed similar degree of sensitivity towards MEK and mTORC1/2 inhibition in this study, and previously to PI3K/mTOR inhibition (11). This would appear to implicate broad potential application of such combination. In addition, optimal minimization of localized canine MM xenograft growth and metastasis was linked to PK conditions providing therapeutically-effective (and tolerated) concentrations of combined trametinib and sapanisertib, which also resulted in dynamic down modulation of targeted signaling pathway activations in primary tumors of treated mice. Mechanisms of the observed putative plasma interactions between trametinib and sapanisertib when administered together, from PK studies, are unknown and should be substantiated further. Despite noteworthy boundaries in extrapolating between mouse and human studies, there were some parallels between sapanisertib in mice compared to a phase I study (27). For example, the Cmax, Tmax and AUC in mice were generally comparable (or lower), despite the relative differences in per kilogram exposures (example, 20 mg human dose) (27). The concentrations of trametinib measured in mice receiving 5 mg/Kg plus 2 mg/Kg sapanisertib were on average higher than the concentrations in patients receiving the clinically approved dose of 2 mg trametinib, (AUC = 644 vs 248 hr*ng/mL; Cmax = 34.8 vs 8.03 ng/mL); however, some patients at the high end of the exposure range experienced concentrations that approximated levels in mice (28). We advise interpreting these data in the context of species differences that may include varied drug binding, metabolism, distribution, and elimination, as well as the fact the sample sizes are small in both mouse and human PK studies.
Our results also indicated that the combination of trametinib and sapanisertib, compared to the individual inhibitors, more effectively diminished in vitro survival of a panel of human cutaneous melanoma cell lines that harbored various mutations, including BRAF-V600. This also suggested that combined targeting of the two pathways could be a constructive strategy more broadly, because of possible convergence on regulatory signaling pathway master nodes, in the face of diverse genomic events in melanoma.
Selection of sapanisertib was influenced by experience with dactolisib and Torin 2. Dactolisib yielded undesirable side effects during clinical trials and was subsequently abandoned for further clinical development (12, 13). Torin 2 exhibited detrimental side effects at therapeutic levels in mice bearing MM; the fact that Torin 2 targets additional PIKK kinases in an analogous fashion to dactolisib (29), may be a factor in limitations encountered in the current study. Sapanisertib is an ATP-competitive mTOR selective inhibitor with >100 fold selectivity for PI3K (https://adisinsight.springer.com/drugs/800030541; https://drugs.ncats.io/substance/JGH0DF1U03#activemoieties). Clinical trials of sapanisertib are demonstrating that sapanisertib can be tolerated, although its efficacy is under further investigation (27, 30). Dose limiting toxicities have occurred in trial patients receiving sapanisertib, including evidence of renal insufficiency and infrequent blood cytopenias (27, 31). In our acute mouse study, body weight loss and deteriorating condition associated with lymphopenia, bone marrow depletion, and renal tubular necrosis, primarily attributed to sapanisertib, may indicate some overlap with clinical trial adverse events, although further evidence is needed. Reducing the frequency of sapanisertib administration moderated the adverse lesions while continuing to provide synergistic benefits in combination with trametinib, inducing nearly stable disease and significantly limiting metastasis in the model.
In addition to tolerability, development of drug resistance remains a concern for small molecule inhibitors (32, 33). For example, resistance to targeting one signal transduction pathway can originate from re-activation of targeted pathways due to disabled negative feedback loops (34), de novo mutagenesis (35), changes in transcriptional plasticity (36), and activation of other signaling nodes or pathways (37). In particular, direct or indirect PI3K/AKT signaling pathway activation can be a chief cause of resistance to BRAF- and/or MEK-targeted therapy (19). Likewise, inhibition of PI3K/mTOR pathway is known to elevate ERK activities as a consequence (20). Patients treated with high doses of RAD001 (everolimus, a rapalog), in contrast to lower doses used, exhibited p-S6 inhibition, which was accompanied by elevated p-ERK (38). Although such reciprocal signaling cross talk may not be universal (39), p-ERK level increases were observed both in vitro and in vivo for some canine MM cell lines treated with sapanisertib. This implicated a mechanism MM may utilize to bypass inhibition of mTOR signal transduction; however, the reciprocal ERK activity increase elicited by sapanisertib in canine MM was suppressed by the addition of trametinib.
Target inhibition and downmodulation of signal transduction nodes was accompanied by cell cycle arrest and cell death responses in these MM and other studies (40, 41). Trametinib exposure was associated with pro-apoptotic Bim and reduced pro-survival Bcl-xl and Mcl-1 expression, similar to other reports (42, 43). Sapanisertib had no effect on Bim expression but did reduce Bad phosphorylation, thereby moderating its degradation, which logically would sustain its pro-apoptotic function. Trametinib influence on Mcl-1 depletion has been shown previously in lung adenocarcinoma cells (44). Sapanisertib had a role in apoptosis of myeloid leukemia cells when combined with a Bcl2/xl antagonist, implicating a collaborative relationship between sapanisertib and Bcl-xl reduction (45). In this current study, trametinib reduced Bcl-xl levels, which may indirectly contribute to the cytotoxicity observed, and thereby contribute to synergistic cytotoxicity (46).
Although much about MM remains to be determined, investigating naturally occurring MM in dogs continues to be reinforced as a useful model for the human disease. As MM is rare in humans, its greater relative frequency in the US companion dog population (47), poses advantages for drug development with potential to benefit both dogs and humans with MM (4). Evaluation of this as well as additional drug combinations to combat naturally occurring melanoma in dogs provides a number of potential advantages as a human MM model. Among these, canine clinical trials can take advantage of the short natural life span and the course of disease of dogs, relative greater frequency of MM occurrence in dogs, and the fact clinical trials can proceed in treatment-naïve dogs with spontaneous melanomas without having to first fail a standard of care (48). In the context of further clinical development, experience obtained here in the minimization of side effects sustained, in particular due to sapanisertib, may require continued refinement depending on patient characteristics, be they dogs or humans with MM. The therapeutic development pathway is anticipated to be advanced more rapidly by piloting combined therapy in canine melanoma as a means to benefit both dogs and humans stricken by MM.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland. TJP is a molecular pathology fellow in the NIH Comparative Biomedical Scientist Training Program, an NCI-administered Graduate Partnership Program in partnership with Purdue University. HAA is currently at Covance, Inc., Chantilly, VA.
Footnotes
Conflict of interest: none
References
- 1.Goldemberg DC, Thuler LCS, de Melo AC, An Update on Mucosal Melanoma: Future Directions. Acta dermatovenerologica Croatica : ADC 27, 11–15 (2019). [PubMed] [Google Scholar]
- 2.Yde SS, Sjoegren P, Heje M, Stolle LB, Mucosal Melanoma: a Literature Review. Current oncology reports 20, 28 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Tacastacas JD et al. , Update on primary mucosal melanoma. J Am Acad Dermatol 71, 366–375 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Hernandez B et al. , Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson RM et al. , Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment Cell Melanoma Res 27, 37–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prouteau A, Andre C, Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong K et al. , Cross-species genomic landscape comparison of human mucosal melanoma with canine oral and equine melanoma. Nature communications 10, 353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendricks WPD et al. , Somatic inactivating PTPRJ mutations and dysregulated pathways identified in canine malignant melanoma by integrated comparative genomic analysis. PLoS genetics 14, e1007589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward NK et al. , Whole-genome landscapes of major melanoma subtypes. Nature 545, 175–180 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Furney SJ et al. , Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol 230, 261–269 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Wei BR et al. , Synergistic targeted inhibition of MEK and dual PI3K/mTOR diminishes viability and inhibits tumor growth of canine melanoma underscoring its utility as a preclinical model for human mucosal melanoma. Pigment Cell Melanoma Res 29, 643–655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlo MI et al. , A Phase Ib Study of BEZ235, a Dual Inhibitor of Phosphatidylinositol 3-Kinase (PI3K) and Mammalian Target of Rapamycin (mTOR), in Patients With Advanced Renal Cell Carcinoma. The oncologist 21, 787–788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei XX et al. , A Phase I Study of Abiraterone Acetate Combined with BEZ235, a Dual PI3K/mTOR Inhibitor, in Metastatic Castration Resistant Prostate Cancer. The oncologist 22, 503–e543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C et al. , Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. The New England journal of medicine 10.1056/NEJMoa1904059 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Alvarez JGB, Otterson GA, Agents to treat BRAF-mutant lung cancer. Drugs in context 8, 212566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljubas J, Ovesen T, Rusan M, A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer. Cancers 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffner B, Benchich K, Trametinib: A Targeted Therapy in Metastatic Melanoma. Journal of the advanced practitioner in oncology 9, 741–745 (2018). [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza MC, Er EE, Blenis J, The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36, 320–328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakadia S et al. , Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. OncoTargets and therapy 11, 7095–7107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozengurt E, Soares HP, Sinnet-Smith J, Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Molecular cancer therapeutics 13, 2477–2488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X et al. , Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PloS one 13, e0192464–e0192464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J et al. , Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 18, 26–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asati V, Mahapatra DK, Bharti SK, PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. European journal of medicinal chemistry 109, 314–341 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T et al. , The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 18, 2316–2325 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Long GV et al. , Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet (London, England) 386, 444–451 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Jokinen E, Koivunen JP, MEK and PI3K inhibition in solid tumors: rationale and evidence to date. Therapeutic advances in medical oncology 7, 170–180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KN et al. , Phase I study of the investigational oral mTORC1/2 inhibitor sapanisertib (TAK-228): tolerability and food effects of a milled formulation in patients with advanced solid tumours. ESMO open 3, e000291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonowens C et al. , Concomitant oral and intravenous pharmacokinetics of trametinib, a MEK inhibitor, in subjects with solid tumours. British Journal of Clinical Pharmacology 78, 524–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q et al. , Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer research 73, 2574–2586 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghobrial IM et al. , TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: A phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenstrom’s macroglobulinemia. American journal of hematology 91, 400–405 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Al-Kali A et al. , A phase II study (NCI9775) of sapanisertib (MLN0128/TAK-228) in relapsed and/or refractory acute lymphoblastic leukemia (ALL): Interim analysis. 37, e18506–e18506 (2019). [Google Scholar]
- 32.Long GV et al. , Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. The New England journal of medicine 371, 1877–1888 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Larkin J et al. , Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. The New England journal of medicine 371, 1867–1876 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Wagle N et al. , MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer discovery 4, 61–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y et al. , V211D mutation in MEK1 causes resistance to MEK inhibitors in colon cancer. Cancer discovery 10.1158/2159-8290.cd-19-0356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emmons MF et al. , HDAC8 Regulates a Stress Response Pathway in Melanoma to Mediate Escape from BRAF Inhibitor Therapy. Cancer research 79, 2947–2961 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winder M, Virós A, “Mechanisms of Drug Resistance in Melanoma” in Mechanisms of Drug Resistance in Cancer Therapy, Mandalà M, Romano E, Eds. (Springer International Publishing, Cham, 2018), 10.1007/164_2017_17, pp. 91–108. [DOI] [Google Scholar]
- 38.Carracedo A et al. , Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation 118, 3065–3074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C et al. , The preclinical evaluation of the dual mTORC1/2 inhibitor INK-128 as a potential anti-colorectal cancer agent. Cancer biology & therapy 16, 34–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posch C et al. , Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 110, 4015–4020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadwick ML et al. , Combined mTOR and MEK inhibition is an effective therapy in a novel mouse model for angiosarcoma. Oncotarget 9, 24750–24765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Zhu A, Gu X, Xie G, Inhibition of MEK suppresses hepatocellular carcinoma growth through independent MYC and BIM regulation. Cellular oncology (Dordrecht) 42, 369–380 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Petigny-Lechartier C et al. , The mTORC1/2 Inhibitor AZD8055 Strengthens the Efficiency of the MEK Inhibitor Trametinib to Reduce the Mcl-1/[Bim and Puma] ratio and to Sensitize Ovarian Carcinoma Cells to ABT-737. Molecular cancer therapeutics 16, 102–115 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Tada M et al. , MCL1 inhibition enhances the therapeutic effect of MEK inhibitors in KRAS-mutant lung adenocarcinoma cells. Lung cancer (Amsterdam, Netherlands) 133, 88–95 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Rahmani M et al. , Co-administration of the mTORC1/TORC2 inhibitor INK128 and the Bcl-2/Bcl-xL antagonist ABT-737 kills human myeloid leukemia cells through Mcl-1 down-regulation and AKT inactivation. Haematologica 100, 1553–1563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jokinen E, Koivunen JP, Bcl-xl and Mcl-1 are the major determinants of the apoptotic response to dual PI3K and MEK blockage. International journal of oncology 47, 1103–1110 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Bosenberg M, Arnheiter H, Kelsh R, Melanoma in mankind’s best friend. Pigment Cell Melanoma Res 27, 1 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Gordon I, Paoloni M, Mazcko C, Khanna C, The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med 6, e1000161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.