Abstract
OBJECTIVE
We examined lifestyle factors with midlife weight change according to history of gestational diabetes mellitus (GDM) in a large longitudinal female cohort.
RESEARCH DESIGN AND METHODS
In the Nurses’ Health Study II, we categorized changes in lifestyle within 4-year periods and estimated their associations with concurrent changes in body weight (kilograms) among parous women after age 40 years by GDM history status (N = 54,062; 5.3% with a history of GDM) for the following: diet quality (Alternate Healthy Eating Index [AHEI]), leisure-time physical activity (PA), alcohol consumption, and smoking status.
RESULTS
Over a median follow-up of 13 years, average 4-year weight gain was 1.10 and 1.33 kg for women with and without prior GDM, respectively. Women with improved diet quality had favorable 4-year weight change, particularly those with a history of GDM (AHEI change [95% CI] from low to high −2.97 kg [−4.34, −1.60] vs. −1.19 kg [−1.41, −0.96] for GDM vs. non-GDM, respectively; P heterogeneity = 0.04). Increasing PA was associated with weight maintenance for GDM women only (PA increase [95% CI] from low to high 0.26 kg [−0.25, 0.77] vs. 0.90 kg [0.80, 1.01] for GDM vs. non-GDM, respectively; P heterogeneity = 0.02). For both GDM and non-GDM women, weight change did not differ significantly with change in alcohol consumption, while women who quit smoking had significant weight gain (4.38 kg for GDM and 3.85 kg for non-GDM).
CONCLUSIONS
Improvements in diet quality and PA were related to less weight gain in midlife among parous women, and the benefit of such improvements on weight management was particularly pronounced among women with a history of GDM.
Introduction
Despite extensive public health efforts, prevalence of obesity in the U.S. remains high and continues to increase at an alarming rate; more than one in three U.S. adults has obesity (1), with nearly one in two projected to have obesity by 2030 (2). Obesity is a risk factor for chronic disease and mortality, including cardiovascular disease (CVD) (3), type 2 diabetes (4), and at least 13 types of cancer (5). Midlife weight gain typically occurs in often imperceptible increments (∼0.5 kg per year), and without proper management, excess body weight accumulates, with health consequences (6).
Gestational diabetes mellitus (GDM) is a common pregnancy complication, affecting ∼8% of pregnancies in the U.S. (7). Obesity is a strong risk factor for GDM (8). National data in 2011–2014 reported 34.4% of reproductive-aged women had a BMI of ≥30 kg/m2, raising concerns of an upward trend in GDM incidence (9). Furthermore, compared with the general population, women with a history of GDM are at substantially higher risk of obesity-related chronic diseases after pregnancy, including a three- to seven-fold higher risk for type 2 diabetes and a one- to three-fold higher risk for CVD events (10,11). These associations are at least in part due to greater post-pregnancy weight retention or weight gain (8,11). Consequently, women with a history of GDM are a particularly important group for whom to identify effective and sustainable interventions for long-term weight management.
Maintaining a healthy lifestyle, including high-quality diet emphasizing fruits and vegetables, whole grains, healthy fats, and low sodium or added sugar (12,13), sufficient volume of physical activity (14), no smoking (15), and moderate alcohol consumption (16), is pivotal for long-term maintenance of a healthy body weight. Even modest and achievable improvements in lifestyle factors are associated with favorable weight change in midlife in the general population (6). While several randomized clinical trials (RCTs) have demonstrated the efficacy of lifestyle interventions on weight change shortly after the GDM index pregnancy (17,18), evidence focusing on long-term weight management among women with a history of GDM is generally lacking. In particular, compared with the general population, whether realistic lifestyle improvements are similarly effective for women with a history of GDM with regard to midlife weight management is unknown. Therefore, we prospectively examined the associations of lifestyle changes after age 40 years with concurrent weight change in a longitudinal cohort of U.S. women, according to their history of GDM.
Research Design and Methods
Study Population
The Nurses’ Health Study II (NHS II) is a large prospective cohort of U.S. women. Details of the NHS II have been described elsewhere (19). Briefly, 116,429 female registered nurses aged 24 to 42 years enrolled in the study in 1989. Participants return questionnaires every 2 to 4 years to collect information on demographics, health-related characteristics, lifestyle factors, and disease outcomes (follow-up rate >90%). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required, with participants’ consent implied by the return of the questionnaires.
Assessment of GDM
On the questionnaire in 1989, participants were asked “have you had any of the following physician-diagnosed conditions,” with GDM listed as one of the options. Study participants continued to report incident pregnancies and pregnancy complications since the last follow-up cycle, including GDM, from 1991 to 2001, after which a majority of participants had passed reproductive age. A previous validation study in a subset demonstrated a high rate of concordance (94%) between self-reported GDM and GDM diagnosis confirmed via medical records (20). High surveillance of GDM in this cohort was previously reported in a separate study (21).
Lifestyle and Body Weight Ascertainment
The modifiable lifestyle factors of interest in the current analysis were diet quality, physical activity, alcohol consumption, and cigarette smoking. Diet was assessed every 4 years by a validated 130-item food frequency questionnaire (FFQ) inquiring how often, on average, a participant had consumed specified amounts of commonly consumed foods during the preceding year (22). We derived participants’ Alternate Healthy Eating Index (AHEI) score (range 0–110), which estimates the adherence to a healthy dietary pattern, with higher score indicating greater adherence (13). Participants’ physical activity was assessed every 2 to 4 years by reporting the average time per week spent in various moderate- or vigorous-intensity leisure-time activities in the preceding year (23). Based on the intensity and duration of each activity, weekly expenditure in metabolic equivalents (MET-h per week) was calculated and summed over all activities for total physical activity. Alcohol consumption in the past year was assessed on the FFQ. Total alcohol consumption (servings per day) was calculated by summing the intakes from beer (regular and light), wine (red and white), and liquor in servings per day. Current smoking status (current, past, or never) and quantity (cigarettes per day) were queried biennially. In addition, average daily sleep duration was assessed in 2001 and 2009 (<5, 5, 6, 7, 8, 9, or 10+ h).
Participants self-reported their height (feet and inches) at enrollment and body weight (pounds) at enrollment and biennially thereafter. A previous validation study in a subset of participants indicated high correlation between self-reported versus staff-measured body weights on average (spearman correlation 0.97; weight difference 3.3 lb) (24).
Statistical Analysis
For the current investigation, analyses were restricted to women who reported at least one pregnancy lasting ≥6 months at baseline enrollment or during follow-up through 2001 (n = 94,995). Baseline was defined as the date of return of the first FFQ after reaching age 40 years. At baseline, we excluded women who had missing date of birth (n = 271) or a diagnosis of cancer (n = 1,728), CVD (n = 478), or type 2 diabetes (n = 153). Participants were subsequently followed until the first of the following events: diagnosis of cancer, death, age 65 years, return of the last available questionnaire, or end of the study follow-up period (June 2017). We censored participants’ follow-up time at diagnosis of cancer or age 65 years, given the potential influence on body weight. For a given participant, data collected from each follow-up cycle were skipped if she was currently pregnant (n = 3,114), missing body weight or physical activity information, or had missing or implausible FFQ (n = 35,189) (25). Change in body weight every 4 years was calculated by subtracting the more recent weight measure from the earlier weight measure and was then converted to kilograms, with positive value indicating weight gain. We similarly calculated 4-year changes for each lifestyle factor (Supplementary Table 1). For analysis, we categorized AHEI scores at each cycle in tertiles (T1 = low, T2 = medium, and T3 = high), and 4-year change in AHEI between two adjacent cycles was categorized into the following nine groups: stay low, low to medium, low to high, stay medium, medium to low, medium to high, stay high, high to medium, and high to low. Physical activity was dichotomized to <7.5 or ≥7.5 MET-h/week, with the cut point based on the current physical activity guidelines for U.S. adults (7.5 MET-h/week is equivalent to 150 min/week of moderate-intensity physical activity) (26), and 4-year change was then categorized as stay low, increase from low to high, stay high, and decrease from high to low. Change in alcohol consumption was similarly defined as nondrinker, recent starter, quitter, stable drinker, drinker with increasing consumption, and drinker with decreasing consumption. Change in smoking status was classified as the following: never smoker, recent starter, past smoker, restarter, recent quitter, and continued smoker.
Our primary analysis was to calculate the mean weight changes in body weight (kilograms) according to the magnitude of concurrent changes in the following lifestyle factors, stratified by history of GDM: diet quality (AHEI), physical activity, alcohol consumption, and smoking. We defined history of GDM as ever having GDM before age 40 years. For each lifestyle factor, we used multivariable marginal models with generalized estimating equations to calculate the least squares mean of 4-year weight change and 95% CI within each category of lifestyle change, using an autorecessive variance-covariance matrix to account for repeated within-person measures; the least squares mean was calculated as the marginal mean with adjusted covariates set as mean for continuous covariate and prevalence for categorical covariate (27,28). Models were adjusted for follow-up period, race, marital status, family history of diabetes, age, BMI, oral contraceptive use, menopausal and hormonal replacement therapy status, sleep duration, and concurrent changes in the other lifestyle factors. All the covariates, except race, marital status, and family history of diabetes, were updated at each questionnaire cycle. Because the definitions of change accounted for cycle-specific baseline status, we did not additionally adjust for lifestyle at baseline. For each lifestyle factor, we tested for effect modification by GDM history by including the cross-product terms (e.g., categorical changes in AHEI ∗ history of GDM), with the main effects included in the model; significance of heterogeneity by GDM was assessed using the generalized score statistics for the cross-product terms (28). For lifestyle factors that showed significant heterogeneity by GDM status on weight change, we further examined the joint associations of these factors by modeling the cross-product terms reflecting the joint changes with weight change, stratified by GDM.
We performed sensitivity analyses, including modeling changes in diet and physical activity by quintiles with additional adjustment for baseline level, using unstructured variance-covariance matrix in the models, excluding women with nonsingleton births (n = 1,596), excluding women who developed GDM at or after age 40 years (n = 25), further censoring at incident CVD events (n = 246 with nonfatal myocardial infarction or stroke), and restricting analyses to baseline BMI ≥25 kg/m2 (n = 30,180 for non-GDM and n = 2,067 for GDM). All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). All statistical tests were two sided, with P values <0.05 considered statistically significant.
Data and Resource Availability
Further information, including the procedures to obtain and access data from the NHS II, is described at https://www.nurseshealthstudy.org/researchers (contact e-mail: nhsaccess@channing.harvard.edu).
Results
Study Population Characteristics at Baseline
In total, 54,062 women were eligible for inclusion in our analysis, 2,888 (5.3%) of whom reported a history of GDM (Table 1). Average age and weight at baseline were 43.2 years and 75.1 kg, respectively, for GDM women and 43.4 years and 69.6 kg, respectively, for non-GDM women. Compared with women without a history of GDM, women with a history of GDM at baseline were more likely to have a higher BMI and family history of diabetes. Women with a history of GDM reported less physical activity and modestly lower alcohol consumption; baseline AHEI scores and smoking status were similar between the two groups (Table 1).
Table 1.
Baseline characteristics of participants from the NHS II by history of GDM
| No history of GDM (n = 51,174; 94.7%) | History of GDM† (n = 2,888; 5.3%) | |
|---|---|---|
| Mean (SD) age, years | 43.4 (3.9) | 43.2 (3.8) |
| Mean (SD) weight, kg | 69.6 (15.9) | 75.1 (19.3) |
| Mean (SD) BMI, kg/m2 | 25.6 (5.6) | 27.9 (6.8) |
| White | 50,272 (98.2) | 2,824 (97.8) |
| Married | 49,682 (97.1) | 2,752 (95.3) |
| Family history of diabetes | 18,107 (35.4) | 1,482 (51.3) |
| Parity | ||
| 0 | 243 (0.5) | 17 (0.6) |
| 1 | 8,771 (17.1) | 393 (13.6) |
| 2 | 24,924 (48.7) | 1,308 (45.3) |
| 3 | 12,796 (25.0) | 810 (28.0) |
| 4+ | 4,440 (8.7) | 360 (12.5) |
| Oral contraceptive use | ||
| Current user | 3,113 (6.1) | 151 (5.2) |
| Past user | 41,632 (81.4) | 2,338 (81.0) |
| Never user | 6,429 (12.6) | 399 (13.8) |
| Combined menopausal and HRT use status | ||
| Premenopausal never HRT user | 44,556 (87.1) | 2,538 (87.9) |
| Postmenopausal never HRT user | 829 (1.6) | 41 (1.4) |
| Postmenopausal current HRT user | 1,347 (2.6) | 90 (3.1) |
| Postmenopausal past HRT user | 2,898 (5.7) | 128 (4.4) |
| Missing | 1,544 (3.0) | 91 (3.2) |
| Total daily sleep, h‡ | ||
| ≤6 | 13,699 (28.5) | 819 (29.9) |
| 7–8 | 31,979 (66.5) | 1,770 (64.7) |
| >8 | 2,408 (5.0) | 149 (5.4) |
| Mean (SD) total energy intake, kcal | 1,809 (549) | 1876 (573) |
| Mean (SD) AHEI score | 51.2 (11.9) | 51.2 (11.8) |
| Median physical activity (IQR), MET-h/week§ | 13.1 (5.7, 25.9) | 11.3 (4.9, 22.2) |
| Mean (SD) alcohol use, servings/day | 0.3 (0.6) | 0.2 (0.5) |
| Smoking status | ||
| Never | 33,875 (66.2) | 1,951 (67.6) |
| Past | 12,693 (24.8) | 700 (24.2) |
| Current | 4,606 (9.0) | 237 (8.2) |
Data are n (%) unless stated otherwise. Baseline was defined as first follow-up cycle when a woman reached age 40 years.
HRT, hormone replacement therapy; IQR, interquartile range.
History of ever reporting a pregnancy complicated by GDM.
Duration of sleep was assessed in 2001 and 2009 questionnaires only. Numbers are presented based on sleep data collected in the 2001 questionnaires.
Metabolic equivalents from leisure-time physical activities of moderate or vigorous intensity were summed.
Changes in Lifestyle and Weight Over the Follow-up Period
On average, participants had increased AHEI scores, physical activity, alcohol consumption, and smoking cessation over the mean 13.4 years of follow-up (Supplementary Fig. 1). These trends differed by GDM status for alcohol, with women who had prior GDM reporting less increase in alcohol consumption compared with non-GDM counterparts. The average changes in AHEI, physical activity, and alcohol consumption within each 4-year period were similar between GDM and non-GDM women (results not shown). During this period, mean 4-year weight change across all periods was a gain of 1.10 kg (SD 7.55) and 1.33 kg (SD 6.31) for women with and without a history of GDM, respectively (Supplementary Table 2).
Diet Quality and Weight Change
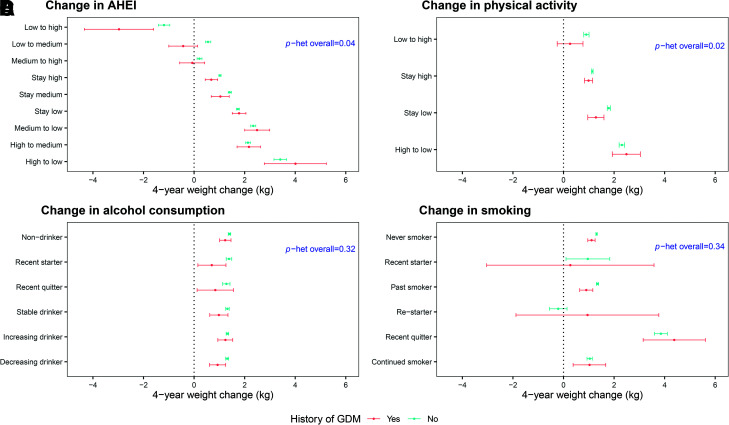
Across categories of AHEI change, an increasing AHEI was associated with favorable weight change, with a greater magnitude among women with a history of GDM than among women without a history of GDM (P heterogeneity = 0.04) (Fig. 1A and Supplementary Table 2). For example, improving AHEI from low to high was associated with the highest weight loss (−2.97 kg for GDM and −1.19 kg for non-GDM), while decreasing AHEI from high to low was correlated with the highest weight gain (4.01 kg for GDM and 3.41 kg for non-GDM) (Fig. 1A and Supplementary Table 2).
Figure 1.
Associations between 4-year change in lifestyle in mid-life and weight change, including AHEI (A), physical activity (B), alcohol consumption (C), and smoking status (D), stratified by history of GDM. Least squares (LS) means of 4-year weight change were modeled in multivariable marginal models with generalized estimating equations adjusting for follow-up period, race (White or non-White), marital status (ever married or other), family history of diabetes (yes or no), age (years), BMI (underweight <18.5, normal 18.5–24.9, overweight 25–29.9, or obese ≥30.0 kg/m2), oral contraceptive use (current, past, or never), menopausal and hormone replacement therapy (HRT) use status (premenopausal never HRT use, postmenopausal never HRT use, postmenopausal current HRT use, postmenopausal past HRT use, or missing), sleep duration (≤6, 7–8, or >8 h), and concurrent changes in the other lifestyle factors (continuous change in AHEI score including alcohol, continuous change in physical activity [MET-h/week], and categorical change in smoking status) depending on the model. LS means were calculated as marginal means with adjusted covariates set as mean for continuous covariates and prevalence for categorical covariates. P heterogeneity (het) was calculated by the generalized score statistics for the interaction term between each lifestyle change and history of GDM.
Physical Activity and Weight Change
Women increasing their physical activity had less weight gain than women decreasing physical activity, and this magnitude was stronger for women with a history of GDM (P heterogeneity = 0.02) (Fig. 1B and Supplementary Table 2). Specifically, increasing volume of physical activity from low to high was associated with maintaining stable body weight only for women with a history of GDM (0.26 kg [95% CI −0.25, 0.77] vs. 0.90 kg [95% CI 0.80, 1.01] for GDM vs. non-GDM, respectively) (Fig. 1B and Supplementary Table 2).
Alcohol Consumption and Weight Change
Patterns of weight change across changes in alcohol consumption were similar for women with and without prior GDM (P heterogeneity = 0.32) (Fig. 1C and Supplementary Table 2). Overall, we observed weight change did not differ widely by category of change in alcohol consumption, ranging between 0.70 and 1.24 kg and 1.28 and 1.40 kg for women with and without a history of GDM, respectively (Fig. 1C and Supplementary Table 2).
Smoking Status and Weight Change
The associations between change in smoking status and weight change were similar between women with and without prior GDM (P heterogeneity = 0.34) (Fig. 1D and Supplementary Table 2). Women quitting smoking within the past 4 years had the highest weight gain (4.38 kg [95% CI 3.15, 5.60] for GDM and 3.85 kg [95% CI 3.60, 4.11)] for non-GDM), while similar degrees of weight gain were observed in the other change category of cigarette smoking, overall (Fig. 1D and Supplementary Table 2).
Joint Associations of Diet and Physical Activity With Weight Change
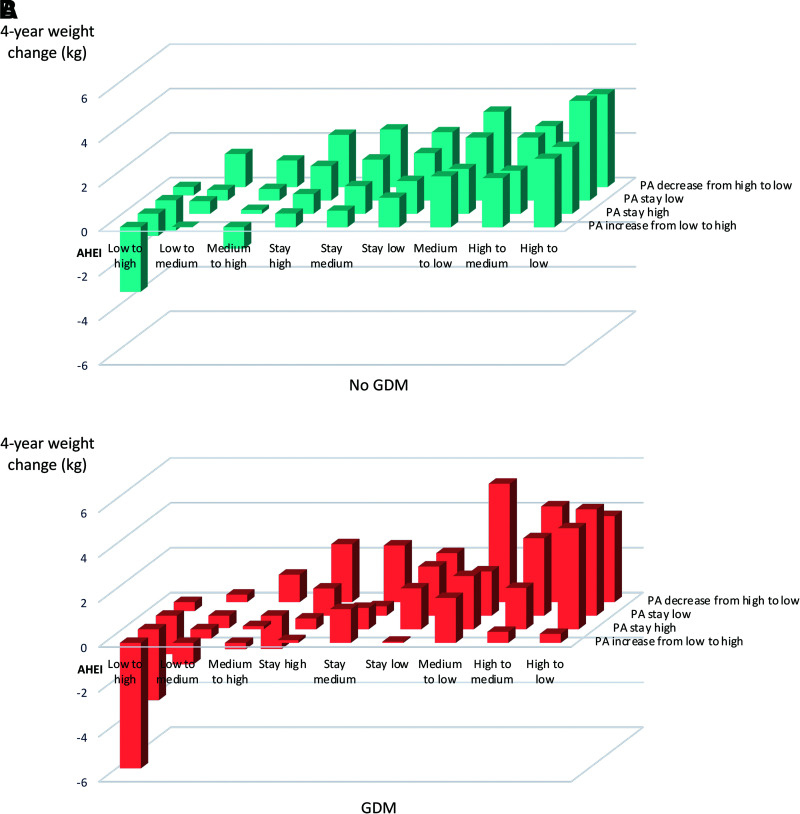
Overall, for both GDM and non-GDM women, the effect size of 4-year weight change related to improving diet and physical activity in the same 4-year period was more favorable compared with improving either diet or physical activity alone, and the joint role of these two factors with regard to weight change appeared to be more than additive (Fig. 2 and Supplementary Table 3). While the overall joint associations did not statistically differ by GDM status (P heterogeneity = 0.28), the effect sizes related to the joint improvement were suggestively greater among GDM women (e.g., increased AHEI from low to high and increased physical activity −5.59 kg vs. −2.90 kg for GDM vs. non-GDM) (Fig. 2 and Supplementary Table 3).
Figure 2.
Joint associations of 4-year change in diet (AHEI) and physical activity (PA) with weight change, stratified by history of GDM: no (A) and yes (B). Least squares (LS) means of 4-year weight change were modeled in multivariable marginal models with generalized estimating equations adjusting for follow-up period, race (White or non-White), marital status (ever married or other), family history of diabetes (yes or no), age (years), BMI (underweight <18.5, normal 18.5–24.9, overweight 25–29.9, or obese ≥30.0 kg/m2), oral contraceptive use (current, past, or never), menopausal and hormone replacement therapy (HRT) use status (premenopausal never HRT use, postmenopausal never HRT use, postmenopausal current HRT use, postmenopausal past HRT use, or missing), sleep duration (≤6, 7–8, or >8 h), and categorical change in smoking status. LS means were calculated as marginal means with adjusted covariates set as mean for continuous covariates and prevalence for categorical covariates. P heterogeneity was calculated by the generalized score statistics for the interaction term between categorical joint variables of AHEI and PA and history of GDM. Overall P heterogeneity by history of GDM = 0.28.
Additional sensitivity analyses were overall consistent with the main findings. Among women with baseline BMI ≥25 kg/m2 particularly, while the magnitudes of weight change related to improving diet, physical activity, or diet and physical activity simultaneously were greater compared with the primary analysis for both GDM and non-GDM women, the magnitudes remained consistently higher among GDM versus non-GDM women with adjustment for continuous BMI (Supplementary Tables 4 and 5; other results not shown).
Conclusions
In this longitudinal study of 54,062 women followed for >13 years from age 40 years, we observed that women who improved their diet quality and physical activity had more favorable long-term weight change, and these associations were stronger for women with a history of GDM. Importantly, for both GDM and non-GDM women, the improvements in both diet quality and physical activity, which were moderate and attainable, were associated with clinically meaningful prevention of weight gain and/or weight loss.
Our findings in the NHS II are consistent with those in previous cohort studies. Women in our study experienced gradual weight gain, with an average of 1.3 kg over each 4-year period, which was similar to the 4-year weight change reported in other U.S. female cohort studies (1.0 kg with average baseline age 52 years and 0.8 kg with average baseline age 54 years) (6,29). Numerous studies, including both RCTs and observational evidence, support the benefits of healthy diet and habitual physical activity for short-term and long-term weight gain prevention (6,30). Observational studies have suggested smoking cessation causes transient short-term weight gain despite its long-term health benefits (15), and heavy alcohol drinking is related to weight gain (16). The consistent results between our study in non-GDM women and the overall literature evidence support the validity of our overall findings.
We observed clinically meaningful associations between long-term improvement in diet quality and long-term weight gain, particularly among women with a history of GDM. Tobias et al. (25) previously examined long-term weight change with several dietary patterns in this subset of women with a history of GDM (n = 3,397 with 20 years of follow-up). Change in AHEI was similarly associated with a meaningful reduction in body weight gain (−1.24 kg [95% CI −1.42, −1.06] per SD increase in AHEI). This updated analysis of AHEI reaches similar conclusions among women with a history of GDM, and it additionally presents complementary findings for parous women without a history of GDM.
To our knowledge, this is the first study assessing change in physical activity and long-term weight change among women with a history of GDM. Our results suggest increasing the amount of physical activity is associated with less weight gain among women with a history of GDM. Our observations on weight gain across the four change groups for non-GDM women were in line with previous studies suggesting that a relatively high level of physical activity (60 min/day of moderate intensity) was required for body weight maintenance (29), especially for adults with overweight or obesity (60–90 min/day of moderate intensity) (31). In this study, volume of physical activity at baseline was on average higher in the non-GDM group compared with the GDM group. Because we modeled physical activity as a dichotomous variable according to the national guidelines, there were fewer women in the non-GDM group who could have crossed the threshold over the follow-up. However, critically, our results among GDM women suggested that, despite having a higher mean body weight at baseline compared with non-GDM women, women with a history of GDM achieved weight maintenance with increasing physical activity from ∼5.0 MET-h/week at baseline to 15.0 MET-h/week (equivalent to an additional 30 min/day of moderate-intensity physical activity) over a 4-year period, on average. This highlights the importance of physical activity in addressing weight management among women with a history of GDM. Future intervention studies are warranted to validate this observational evidence and determine the optimally achievable level of physical activity for long-term weight maintenance for women with a history of GDM.
Our results also highlight the potential joint role of diet and physical activity with regard to long-term weight gain prevention for GDM women. The Diabetes Prevention Program (DPP) previously conducted an RCT in the U.S. and examined the effects of intensive lifestyle (ILS) intervention and metformin therapy compared with placebo, respectively, on progression to type 2 diabetes in a subset of middle-aged high-risk parous women with and without a history of GDM (32). The ILS intervention had goals of weight reduction and maintenance through healthy eating and physical activity (at least 150 min/week of moderate intensity). After 3 years of follow-up, the study observed a lower adherence to the intervention and a smaller amount of weight loss (−1.6 kg vs. −4.0 kg) comparing GDM vs. non-GDM women assigned to the ILS intervention. Nevertheless, the ILS intervention was more effective than metformin in reducing type 2 diabetes risk for both GDM and non-GDM women (32). Compared with the DPP trial, we used data from a large observational cohort and corroborated the effectiveness of attainable sustained improvements in diet and physical activity with regard to long-term weight management for women in midlife. Taken together, our results support that simultaneously emphasizing diet quality and physical activity should continue to be advocated as an effective strategy to prevent long-term weight gain and delay type 2 diabetes progression, particularly among women with a history of GDM.
For both GDM and non-GDM women, we did not observe significant differences in weight change related to change in alcohol consumption, possibly because of the overall low levels of intake and modest changes during follow-up. The relationship between alcohol consumption and weight change has been controversial, except with heavy drinking, which is linked with weight gain (16). For both GDM and non-GDM women, the observed patterns of weight change with smoking cessation were consistent with the literature on postcessation weight gain (15). Our analysis of smoking may be underpowered, because changes in status were less common in our study population.
Multiple factors likely influence the drivers of long-term gradual weight change, namely energy intake and energy expenditure (22,33). A healthy lifestyle, including healthy diet (22), sufficient volume of physical activity (29), nonheavy alcohol drinking (16), and nonsmoking (15), potentially affects several mechanisms related to energy intake and expenditure.
We observed greater benefit for increases in diet quality and physical activity on long-term weight change among high-risk GDM women compared with their non-GDM counterparts. These differences may be due to differences in underlying physiological profiles between the two groups, particularly factors related to metabolism. Pregnancy is hypothesized by some to be a cardiometabolic stress test (34), whereby subclinical metabolic impairments may be uncovered. A GDM pregnancy may inflict permanent metabolic alterations, with molecular changes persisting beyond the pregnancy (35). Other studies have presented evidence on differential influences of lifestyle on obesity and obesity-related diseases by underlying risk profiles or subclinical status, with greater benefits conferred with healthy lifestyle for groups of higher risk (36–38); therefore, it is plausible that GDM women might be particularly sensitive to lifestyle changes. Differences in barriers and sustainability of long-term adherence to lifestyle interventions, however, should be investigated for their optimal implementation.
This study was conducted among a female cohort consisting of health professionals. Women included in the study are likely to be more aware of their health and pursue a healthier lifestyle compared with the general population, particularly those with lower socioeconomic status. Because most U.S. adults do not meet dietary recommendations or physical activity guidelines (39,40), levels of adherence to a healthy diet and the volume of physical activity observed in this cohort might not be applicable to other populations. However, given the magnitude of the favorable weight changes associated with increased diet quality and volume of physical activity observed in this health professional cohort, the study suggests a potentially greater public health benefit and larger economic savings in the long run in preventing obesity-related health consequences by encouraging women to adopt a healthier lifestyle.
Strengths of this study include the large cohort size, allowing statistical power to investigate associations across several degrees of within-person changes in multiple lifestyle factors, the long-term follow-up period with repeated measures of lifestyle and body weight, and the adjustment for concurrent changes in other lifestyle and health-related factors that might confound the associations of interest. Of note, the observational nature of this cohort therefore reflects realistic and real-world attainable sustained lifestyle changes. This study has some potential limitations. First, body weight and diet were self-reported, and therefore, measurement error may have resulted in underestimation of diet and body weight changes. We also cannot exclude the possibility of systematic measurement error of under- or overreporting of certain lifestyle factors in relation to weight change, because these were ascertained during the same time periods. Total physical activity that includes leisure-time, transportation, and household activities is important for caloric balance; however, the NHS II assessed only leisure-time physical activity and therefore may have underestimated the potential role of physical activity in weight change. We also cannot rule out the possibility of confounding by other determinants of weight change that could be correlated with lifestyle. Our analyses on change in smoking and the joint associations of lifestyle were likely underpowered, particularly in some strata where data were sparse. Finally, our results are generalizable to a predominantly White population with characteristics similar to those of our analysis sample. Studies in diverse populations (e.g., lower socioeconomic status or race/ethnicity) are needed to confirm the generalizability of our findings to populations and subgroups with different magnitudes and patterns of lifestyle changes. Studies of contemporary birth cohorts are warranted to examine the impact of more recent trends in lifestyle behaviors on midlife weight gain.
In conclusion, improvements in diet quality and physical activity are promising strategies for mitigating long-term weight gain for women in midlife, particularly among high-risk women with a history of GDM. Barriers of lifestyle improvements and strategies for implementation of long-term sustainability should be further evaluated among GDM women in future studies.
Article Information
Acknowledgments. The authors acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School (Boston, MA), as the home of the NHS I and II. The NHS II thanks the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Funding. The NHS II is supported by National Institutes of Health (NIH) grant U01 CA176726. J.Y. was supported by the PhD program in Population Health Sciences at Harvard University. C.Z. is supported by the NIH intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. J.E.C. received grants from NIH (U01HL145386 and P30 DK046200). J.E.M. received grants from NIH. D.K.T. received grants from the American Diabetes Association.
The funding sources had no role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.Y. and D.K.T. designed the study. J.Y. performed the analyses, designed the figures, and drafted the first version of the manuscript. C.Z., J.W.R.-E., M.W., W.W.F., and D.K.T. provided critical feedback on the analyses. M.W. provided statistical expertise. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript. J.Y. and D.K.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form during the virtual American Heart Association EPI|Lifestyle Scientific Sessions, 20–21 May 2021.
Footnotes
J.Y. is currently affiliated with Bia-Echo Asia Centre for Reproductive Longevity & Equality (ACRLE) and Department of Obstetrics and Gynaecology, National University of Singapore, Singapore.
This article contains supplementary material online at https://doi.org/10.2337/figshare.17003881.
References
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450 [DOI] [PubMed] [Google Scholar]
- 3. Colpani V, Baena CP, Jaspers L, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol 2018;33:831–845 [DOI] [PubMed] [Google Scholar]
- 4. Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab 2011;96:1654–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 2011;13:71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tobias DK. Prediction and prevention of type 2 diabetes in women with a history of GDM. Curr Diab Rep 2018;18:78. [DOI] [PubMed] [Google Scholar]
- 8. Bao W, Yeung E, Tobias DK, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia 2015;58:1212–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief 2015;1–8 [PubMed] [Google Scholar]
- 10. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 11. Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 2017;177:1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM. Predictors of weight gain in the Pound of Prevention study. Int J Obes Relat Metab Disord 2000;24:395–403 [DOI] [PubMed] [Google Scholar]
- 13. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hruby A, Manson JE, Qi L, et al. Determinants and consequences of obesity. Am J Public Health 2016;106:1656–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systematic review and meta-analysis of prospective cohort studies. Obes Rev 2015;16:883–901 [DOI] [PubMed] [Google Scholar]
- 16. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep 2015;4:122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goveia P, Cañon-Montañez W, Santos DP, et al. Lifestyle intervention for the prevention of diabetes in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2018;9:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, Wang L, Zhang S, et al. One-year weight losses in the Tianjin Gestational Diabetes Mellitus Prevention Programme: a randomized clinical trial. Diabetes Obes Metab 2018;20:1246–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnard ME, Poole EM, Curhan GC, et al. Association of analgesic use with risk of ovarian cancer in the Nurses’ Health Studies. JAMA Oncol 2018;4:1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomon CG, Willett WC, Rich-Edwards J, et al. Variability in diagnostic evaluation and criteria for gestational diabetes. Diabetes Care 1996;19:12–16 [DOI] [PubMed] [Google Scholar]
- 21. Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 22. Willett W. Nutritional Epidemiology. Oxford, U.K., Oxford University Press, 2012 [Google Scholar]
- 23. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999 [DOI] [PubMed] [Google Scholar]
- 24. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 25. Tobias DK, Zhang C, Chavarro J, et al. Healthful dietary patterns and long-term weight change among women with a history of gestational diabetes mellitus. Int J Obes 2016;40:1748–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U.S. Department of Health and Human Services Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Accessed 4 August 2021. Available from https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
- 27. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ, John Wiley & Sons, 2012 [Google Scholar]
- 28. Littell RC, Freund RJ, Spector PC. SAS System for Linear Models. Cary, NC, SAS Institute Inc., 1991 [Google Scholar]
- 29. Lee IM, Djoussé L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA 2010;303:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown T, Avenell A, Edmunds LD, et al. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev 2009;10:627–638 [DOI] [PubMed] [Google Scholar]
- 31. Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev 2003;4:101–114 [DOI] [PubMed] [Google Scholar]
- 32. Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filozof C, Fernández Pinilla MC, Fernández-Cruz A. Smoking cessation and weight gain. Obes Rev 2004;5:95–103 [DOI] [PubMed] [Google Scholar]
- 34. Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ 2017;358:j3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018;19:3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 2016;375:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang T, Heianza Y, Sun D, et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohan D, Mente A, Dehghan M, et al.; PURE, ONTARGET, TRANSCEND, and ORIGIN investigators . Associations of fish consumption with risk of cardiovascular disease and mortality among individuals with or without vascular disease from 58 countries [published correction appears in JAMA Intern Med 2021;181:727]. JAMA Intern Med 2021;181:631–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med 2014;174:1587–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh R, Pattisapu A, Emery MS. US physical activity guidelines: current state, impact and future directions. Trends Cardiovasc Med 2020;30:407–412 [DOI] [PubMed] [Google Scholar]