Abstract
This cross-sectional study determined income disparities in age-adjusted prevalence and trends of 10-year high absolute cardiovascular disease (CVD) risk, metabolic syndrome, hypertension, diabetes, obesity, chronic kidney disease (CKD), leisure-time physical activity (LTPA), and current tobacco smoking within racial/ethnic groups in the US. National Health and Nutrition Examination Survey 2001–2016 data of 40–79-year-old people were analyzed. Survey periods were grouped as 2001–2006, 2007–2012, and 2013–2016. Race/ethnicity was grouped as non-Hispanic whites, non-Hispanic blacks, and other races/ethnicities. Three equal-sized strata (low-, middle-, and high income) were made from the family income-to-poverty ratio. Of the 25,777 participants (mean age: 55.6 years, 48% males), a majority of the studied prevalence was higher in most survey years among non-Hispanic blacks compared to non-Hispanic whites. Most studied prevalence was also higher among low-income people than middle-/high-income people. Within racial/ethnic groups, the prevalence also differed by income for high CVD risk, metabolic syndrome, hypertension, diabetes, obesity, CKD, LTPA, and tobacco smoking (P < 0.05) in most survey periods. After stratifying by race/ethnicity, the prevalence of many conditions remained disproportionately higher among low- and middle-income people, compared to those with high income during most survey periods in all racial/ethnic groups. These results reveal income in addition to race/ethnicity to be an important correlate of cardiovascular health and underscore the need to consider each when controlling for risk factors.
Introduction
Cardiovascular disease (CVD) is a leading cause of death and disability globally and in the US [1–3]. Regularly monitoring the prevalence and trends of CVD risk factors is thus essential to reduce these adverse consequences. Hypertension, abnormal cholesterol levels, diabetes, obesity, chronic kidney disease (CKD), physical inactivity, and smoking are some major modifiable risk factors for CVD [2, 4, 5]. Clustering of some of these conditions also constitutes metabolic syndrome or Syndrome X, a common term used to predict higher CVD risk [6]. In addition, 10-year absolute CVD risk, estimated based on age and other coexisting CVD risk factors measurements, is used to predict future CVD events [7]. Overall, within the past several decades, the prevalence of risk factors for CVD has remained mostly static or declined within the US [8, 9]. However, CVD risk remains substantially higher among non-Hispanic blacks compared to non-Hispanic whites [9, 10]. Take hypertension as an example: the age-adjusted prevalence of hypertension is about 1.5 times higher among non-Hispanic blacks compared to their non-Hispanic white counterparts [11]. Other studies have estimated that the prevalence of the metabolic syndrome is higher among non-Hispanic whites compared to non-Hispanic blacks, specifically among younger people and males [12, 13]. In addition to the race, the disparities have been reported by socioeconomic status, including income. For instance, Odutayo et al. have observed that the prevalence of high absolute CVD risk and other risk factors is substantially higher among low-income adults compared to high-income adults [14]. Similar results have been observed among adolescents [15].
Socioeconomic status may differ by race/ethnicity, which may contribute to a difference in CVD prevalence within racial/ethnic groups. Earlier studies have reported the racial/ethnic and income disparities associated with CVD risk, metabolic syndrome, and other major CVD risk factors; however, to our knowledge, none of the previous studies report income disparities associated with prevalence and trends of these risk factors within the strata of races/ethnicities. This study attempts to fill the gaps in the literature by investigating whether income disparities in CVD risk factors’ prevalence exist within racial/ethnic groups in a nationally representative sample of middle-aged and older adults in the US.
Methods
Data source
This cross-sectional study analyzed National Health and Nutrition Examination Survey (NHANES) data from 2001–2016 survey years [16]. This survey primarily aims to obtain a nationally representative sample of the non-institutionalized US population. Details of NHANES are available elsewhere [16]. The datasets are publicly available. The survey protocols were approved by the Ethics Review Board of the National Center for Health Statistics [17]. We used pooled cohort risk equations to calculate CVD risk. Because this method is recommended for people 40–79 years of age, we limited our analysis to this age group [7].
Study variables
Participants reported their race/ethnicity. Because stratifying income by race/ethnicity brings a lower sample size, especially the racial/ethnic groups other than non-Hispanic whites, we merged races/ethnicities other than non-Hispanic whites and non-Hispanic blacks (i.e., Mexican–Americans, Asians, and other Hispanics, including multiraces) as “other races/ethnicities” and divided the 2001–2016 survey years into three periods: 2001–2006, 2007–2012, and 2013–2016 to increase the sample size for all racial/ethnic group.
The family income-to-poverty ratio is the ratio of a family’s income and poverty threshold based on the number of family members. Thus, a higher ratio indicates a higher socioeconomic status [18, 19]. We used this variable to stratify the entire study population into three equal-sized groups: low-, middle-, and high income.
Per the pooled cohort risk equations, among people without CVD events’ history (i.e., no history of myocardial infarction, coronary heart disease, stroke, or heart failure), we obtained the 10-year predicted CVD risk and considered a ≥ 20% 10-year CVD risk as high CVD risk [7].
Based on the criteria described by the joint scientific statement [13, 20], metabolic syndrome was defined as having at least three of the following five conditions: abdominal obesity (i.e., ≥102 cm for men and ≥88 cm for women); high serum triglycerides (i.e., ≥150 mg/dL); low high-density lipoprotein (HDL) concentration (i.e., <40 mg/dL for men and <50 mg/dL for women); raised systolic/diastolic blood pressure (i.e., ≥135/85 mmHg); and raised fasting glucose level (i.e., ≥100 mg/dL). In addition, people who were taking any drug to control blood lipids, blood pressure, and blood glucose were considered to have high triglyceride/low HDL, raised blood pressure, and raised blood glucose, respectively [13].
Among other major CVD risk factors, we reported hypertension, diabetes, obesity, CKD, physical inactivity, and smoking status. Hypertension was defined as having a systolic/diastolic blood pressure ≥130/80 mmHg or if the participants reported that they were taking a blood pressure-lowering drug [21]. A person was considered to have diabetes if the glycohemoglobin was ≥6.5%, previous diagnosis of diabetes by a physician, or if the person was taking an antidiabetic drug [14]. The “weight in kilograms” was divided by “height in meters squared” to calculate body mass index (BMI) and a BMI of ≥30 kg/m2 was considered obese [22]. We used the CKD epidemiology (CKD-EPI) equation to estimate the glomerular filtration rate (GFR). A person was categorized as CKD if the albumin–creatinine ratio was ≥30 mg/g or the GFR was <60 ml/min per 1.73 m2 [23]. Serum creatinine was also calibrated for the required survey years [17]. Participants’ reports of the usual amount of time spent doing aerobic moderate and vigorous recreational physical activity (PA) in a week were used to estimate aerobic leisure-time PA (LTPA). Minutes spent performing vigorous aerobic PA were multiplied by 2 and added to moderate PA to obtain the aerobic LTPA. A person was considered as no aerobic LTPA or physically inactive if the aerobic LTPA was “0” min/week. As the LTPA data was available for 2007–2016 survey years, we only included those survey years. Persons reported their current tobacco smoking status [18, 24]. To describe sample characteristics, we also used age, gender (i.e., male or female), and education level.
Statistical analysis
First, the study sample was stratified by survey years. Mean and standard errors (SE) were reported for continuous variables and weighted percentages (%) and unweighted frequencies (n) were reported for categorical variables. To compare, continuous variables were tested with analysis of variance and categorical variables (including the trends over the sampling years) were tested with chi-square tests. Then, the age-adjusted prevalence (with 95% confidence interval (CI)) of individuals with high 10-year absolute CVD risk, metabolic syndrome, and other risk factors were reported. To report the age-adjusted estimates, the population distribution from the 2015 population was used [25]. This age distribution was used to report estimates for most recent years. The provided sample weights were also used to calculate the estimates. To handle missing data, we used pairwise deletion (i.e., available case analysis) [26]. Due to a low proportion of missing data (<10%) for most variables, the overall bias using that approach will be low. Stata 14.0 (Statistical software, College Station, TX, USA) was used to analyze data [27].
Results
From the 2001–2016 survey years, a total of 25,777 respondents between 40 and 79 years of age were included in the analysis (mean age: 55.6 years, SE: 0.1, Table 1). Over time, the mean age of the respondents was roughly constant, with averages of 54.8 (SE: 0.1), 55.6 (SE: 0.2), and 56.5 (SE: 0.2) years in 2001–2006 (n = 8388), 2007–2012 (n = 10536), and 2013–2016 (n = 6853), respectively. More than two-thirds of the respondents were non-Hispanic whites in each survey period. The proportion of participants with a bachelor or above education level increased over time. When assessing the overall prevalence of conditions that comprise metabolic syndrome, we found that 62.3%, 41.2%, 29.5%, 49.5%, and 56.3% of respondents had abdominal obesity, high triglyceride, low HDL, raised blood pressure, and raised glucose level, respectively.
Table 1.
Background characteristics of the study participants (N = 25,777)a.
| Variable | Overall (n = 25,777) | 2001–2006 (n = 8388) | 2007–2012 (n = 10536) | 2013–2016 (n = 6853) | P values |
|---|---|---|---|---|---|
| Age, y, mean (SE) | 55.6 (0.1) | 54.8 (0.2) | 55.6 (0.2) | 56.5 (0.2) | <0.001 |
| Male | 48.0 (12677/25777) | 48.1 (4228/8388) | 48.0 (5172/10536) | 47.9 (3277/6853) | 0.94 |
| Race/ethnicity | |||||
| NHW | 72.6 (11401/25777) | 76.5 (4389/8388) | 71.9 (4537/10536) | 68.6 (2475/6853) | 0.019 |
| NHB | 10.7 (5738/25777) | 10.4 (1828/8388) | 10.9 (2422/10536) | 10.9 (1488/6853) | |
| Other | 16.7 (8638/25777) | 13.1 (2171/8388) | 17.1 (3577/10536) | 20.5 (2890/6853) | |
| Family income-to-poverty ratio | |||||
| Low | 20.3 (7792/25538) | 18.3 (2237/7848) | 21.4 (3387/9498) | 21.4 (2168/6192) | 0.30 |
| Middle | 31.9 (7884/25538) | 33.1 (2747/7848) | 31.1 (3061/9498) | 31.6 (2076/6192) | |
| High | 47.7 (7862/25538) | 48.6 (2864/7848) | 47.5 (3050/9498) | 47.0 (1948/6192) | |
| College education | 29.2 (5573/25737) | 26.4 (1664/8366) | 29.2 (2218/10522) | 32.8 (1691/6849) | 0.008 |
| High CVD risk | 8.9 (2988/22622) | 9.0 (1050/7279) | 8.5 (1193/9249) | 9.4 (745/6094) | 0.033 |
| Metabolic synd. | 43.5 (4995/10800) | 41.6 (1570/3465) | 43.6 (2077/4451) | 45.7 (1348/2884) | 0.12 |
| Hypertension | 59.4 (15856/24511) | 60.5 (5182/7842) | 58.6 (6471/10082) | 59.2 (4203/6587) | 0.33 |
| High cholesterol | 51.5 (12803/24357) | 48.2 (3885/7862) | 51.8 (5287/9959) | 55.3 (3631/6536) | <0.001 |
| Diabetes | 10.9 (3896/25207) | 8.9 (1072/8226) | 11.3 (1668/10320) | 12.8 (1156/6661) | <0.001 |
| Obesity | 38.5 (9696/24361) | 36.1 (2862/7740) | 38.2 (4079/10066) | 42.1 (2755/6555) | <0.001 |
| CKD | 16.0 (4935/24611) | 16.1 (1615/7903) | 15.1 (2017/10119) | 17.2 (1303/6589) | 0.064 |
| No aerobic LTPA | 50.8 (10019/17366) | – | 51.8 (6249/10523) | 49.5 (3770/6843) | 0.30 |
| Smoker | 20.2 (5360/25750) | 22.2 (1855/8374) | 19.5 (2149/10526) | 18.8 (1356/6850) | 0.009 |
| Metabolic syndrome conditions | |||||
| Abdominal obesity | 62.3 (14710/23516) | 60.0 (4654/7603) | 62.3 (6079/9650) | 65.4 (3977/6263) | 0.004 |
| High triglyceride | 41.2 (10148/23978) | 38.2 (3045/7732) | 43.0 (4317/9805) | 42.5 (2786/6441) | <0.001 |
| Low HDL | 29.5 (7222/23488) | 28.4 (2170/7588) | 30.8 (3059/9575) | 29.2 (1993/6325) | 0.088 |
| Raised BP | 49.5 (13676/24493) | 49.4 (4419/7834) | 48.8 (5613/10076) | 50.6 (3644/6583) | 0.43 |
| Raised glucose | 56.3 (7005/11616) | 49.3 (1999/3717) | 59.1 (3026/4796) | 61.0 (1980/3103) | <0.001 |
BP blood pressure, CKD chronic kidney disease, CVD cardiovascular disease risk, HDL high-density lipoprotein, LTPA leisure-time physical activity, NHB non-Hispanic black, NHW non-Hispanic white, SE standard error.
Number and column percentage unless otherwise specified. Numbers may not add up to total because of missing values.
Races/ethnicities other than non-Hispanic whites and non-Hispanic blacks as ‘other races/ethnicities’ (i.e., Mexican–Americans, Asians, and other Hispanics, including multiraces).
The family income-to-poverty ratio is the ratio of a family’s income and poverty threshold based on the number of family members. The population was stratified into three equal-sized groups: low-, middle-, and high income.
High CVD risk was defined as a ≥ 20% 10-year CVD risk among people without CVD events history (i.e., no history of myocardial infarction, coronary heart disease, stroke, or heart failure), using the pooled cohort risk equations.
Metabolic syndrome was defined as having at least three of the following five conditions: abdominal obesity (i.e., ≥102 cm for men and ≥88 cm for women); high serum triglycerides (i.e., ≥150 mg/dL); low high-density lipoprotein (HDL) concentration (i.e., <40 mg/dL for men and <50 mg/dL for women); raised systolic/diastolic blood pressure (i.e., ≥135/85 mmHg); and raised fasting glucose level (i.e., ≥100 mg/dL). Then, people who were taking any drug to control blood lipids, blood pressure, and blood glucose were considered to have high triglyceride/low HDL, raised blood pressure, and raised blood glucose, respectively.
Hypertension was defined as a systolic/diastolic blood pressure ≥130/80 mmHg or taking a blood pressure-lowering drug.
Diabetes was defined as the glycohemoglobin ≥6.5%, previous diagnosis of diabetes, or if the person was on antidiabetic drugs.
Obesity was defined as a body mass index of ≥30 kg/m2.
The chronic kidney was considered as albumin–creatinine ratio ≥30 mg/g or glomerular filtration rate <60 ml/min per 1.73 m2.
Minutes spent performing vigorous physical activity (PA) were multiplied by 2 and added to moderate PA to obtain the LTPA. A person was considered as no LTPA or physically inactive if the LTPA was “0” min/week. The LTPA data were available for 2007–2016 survey years.
Figure 1 shows the prevalence and trends of high 10-year absolute CVD risk, metabolic syndrome, and other major risk factors by race/ethnicity. Among all races/ethnicities, the prevalence of high CVD risk and metabolic syndrome did not change significantly over time (P > 0.05). For instance, among non-Hispanic whites, the prevalence of high CVD risk was 9.0% (95% CI: 8.3–9.7%), 8.4% (95% CI: 7.6–9.2%), and 8.6% (95% CI: 7.4–10.0%) in 2001–2006, 2007–2012, and 2013–2016 survey periods, respectively; this was also the lowest prevalence among all races/ethnicities across the periods. Except for high CVD risk and metabolic syndrome, the prevalence of all other characteristics differed significantly by race/ethnicity in all survey years. For tobacco smoking, non-Hispanic blacks had the highest prevalence among all races/ethnicities. The prevalence of obesity increased among all races/ethnicities and non-Hispanic blacks had the highest prevalence across the survey years.
Fig. 1. Prevalence and trends of high cardiovascular disease risk, metabolic syndrome, hypertension, and other cardiovascular risk factors by race/ethnicity, 2001–2016 (N = 25,777).
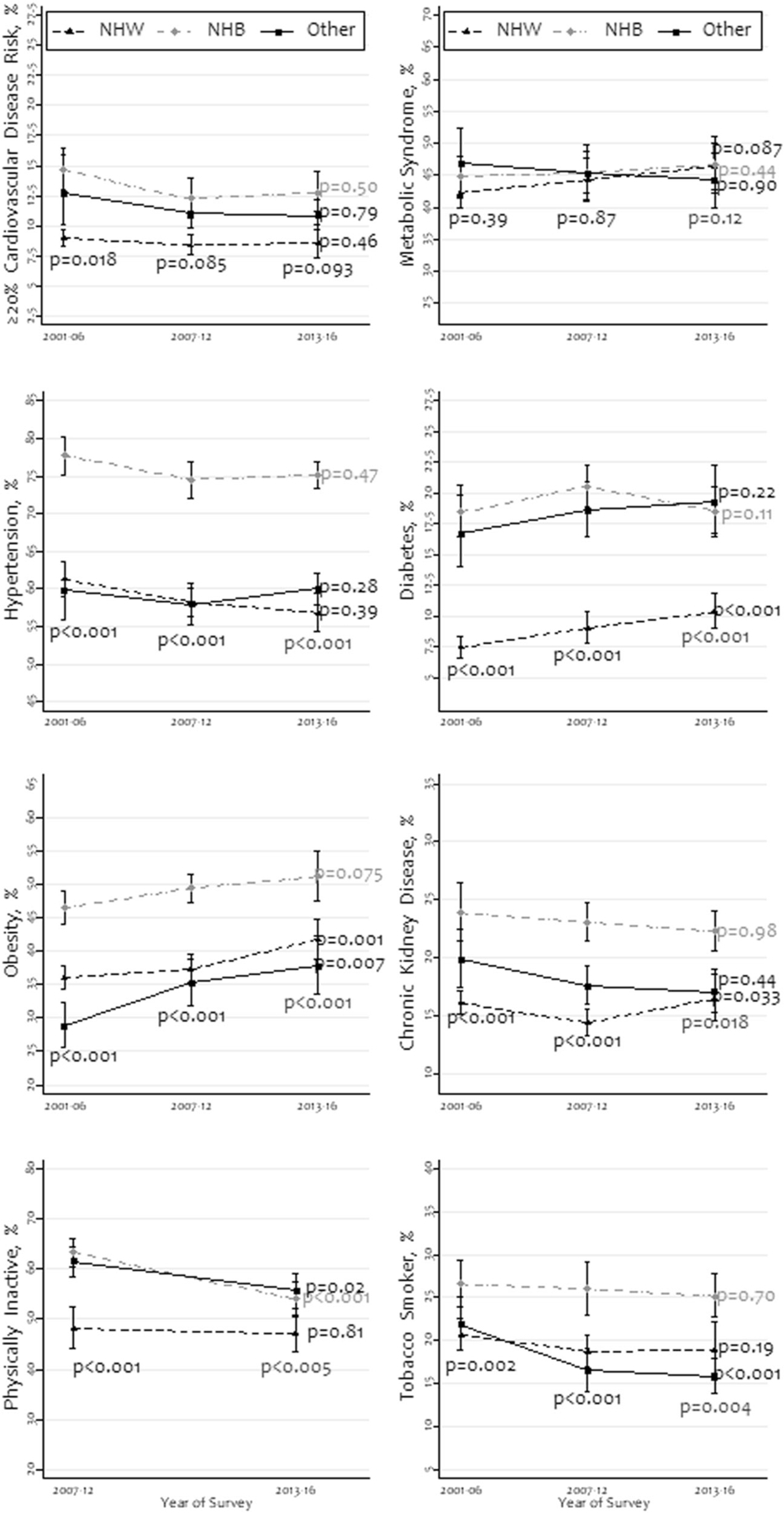
NHW non-Hispanic whites, NHB non-Hispanic blacks; all other races/ethnicities were grouped as “other”. The P values were obtained using chi-square tests.
Figure 2 summarizes the prevalence and trends by income level. The overall prevalence of high CVD risk, metabolic syndrome, hypertension, diabetes, CKD, no LTPA, and tobacco smoking was substantially higher among people with low income in most survey periods. Across the survey periods, the overall prevalence of high CVD risk, metabolic syndrome, hypertension, and CKD did not change significantly among all income groups (P > 0.05). The prevalence of tobacco smoking and no aerobic LTPA did not show such a consistent pattern by income across survey periods. The overall prevalence of diabetes and obesity increased across the survey period in all income groups (P < 0.05).
Fig. 2. Prevalence and trends of high cardiovascular disease risk, metabolic syndrome, hypertension, and other cardiovascular risk factors by family income-to-poverty ratio, 2001–2016 (N = 25,777).
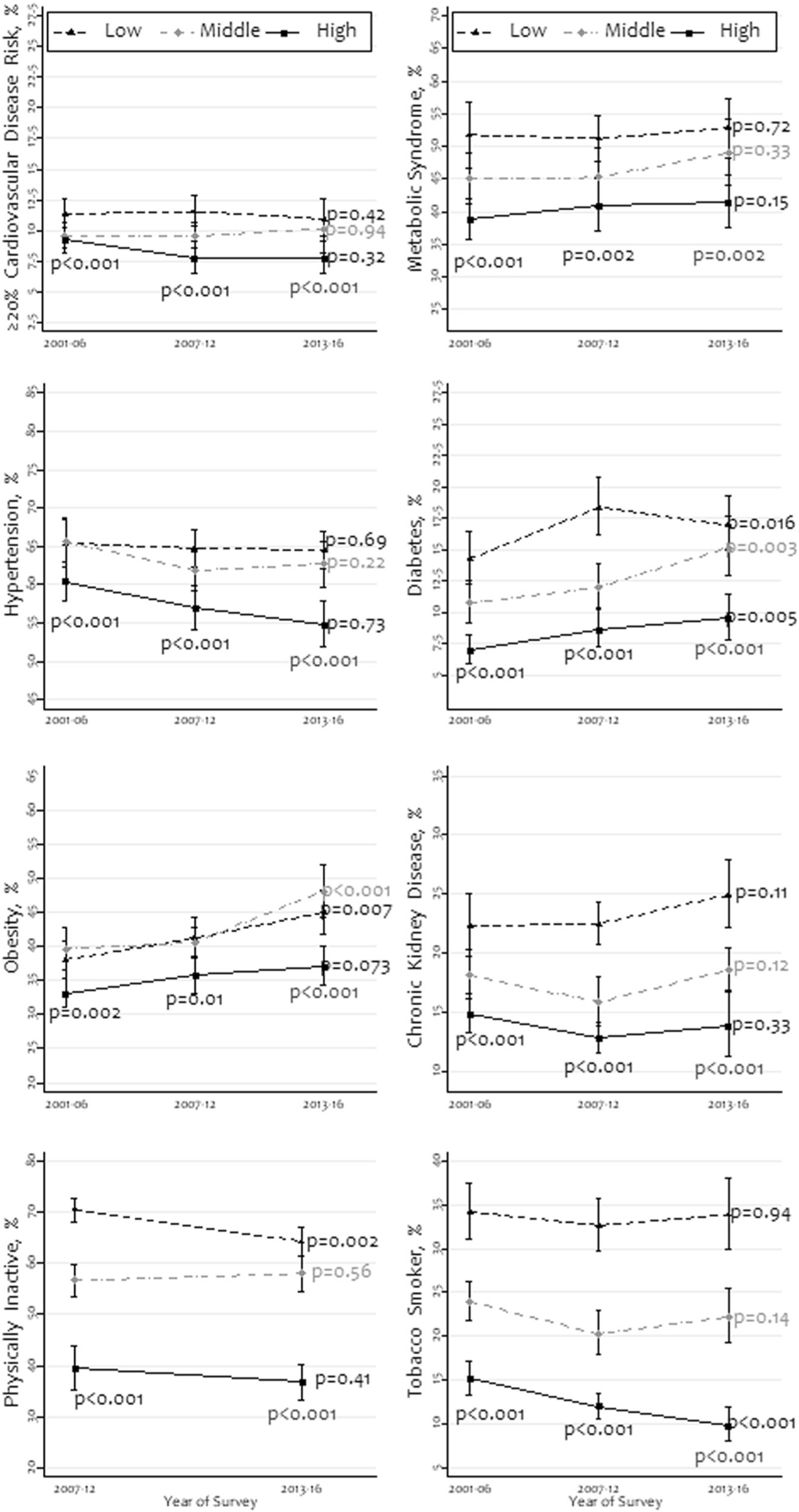
This was stratified as three equal-sized groups: low, middle, and high. Races other than non-Hispanic whites and non-Hispanic blacks were merged as “other races/ethnicities”. The P values were obtained using chi-square tests.
In Fig. 3, we illustrate the differences in prevalence and trends of high CVD risk, metabolic syndrome, hypertension, and diabetes according to income level within each racial/ethnic group. Among all races/ethnicities, high-income people had an overall lower prevalence of high CVD risk in the latest survey period compared to low- and middle-income people. For instance, in 2013–2016, within people of other races/ethnicities, the prevalence for high CVD risk was 11.9% (95% CI: 9.7–14.5%), 11.4% (95% CI: 9.2–14.0%), and 8.8% (95% CI: 6.1–12.6%) among people with low-, middle- and high income, respectively. The prevalence of metabolic syndrome was higher among low-income people than high-income people in all racial/ethnic groups during all survey periods. The prevalence of hypertension did not show any consistent pattern across racial/ethnic groups; however, it was lower among high-income people among non-Hispanic whites and non-Hispanic blacks. The prevalence of diabetes was also lower among high-income people in all racial/ethnic groups. The change in prevalence was statistically non-significant (P > 0.05) for most characteristics across income groups within all races/ethnicities.
Fig. 3. Prevalence and trends of high cardiovascular disease risk, metabolic syndrome hypertension, and diabetes by family income-to-poverty ratio within racial/ethnic groups, 2001–2016 (N = 25,777).
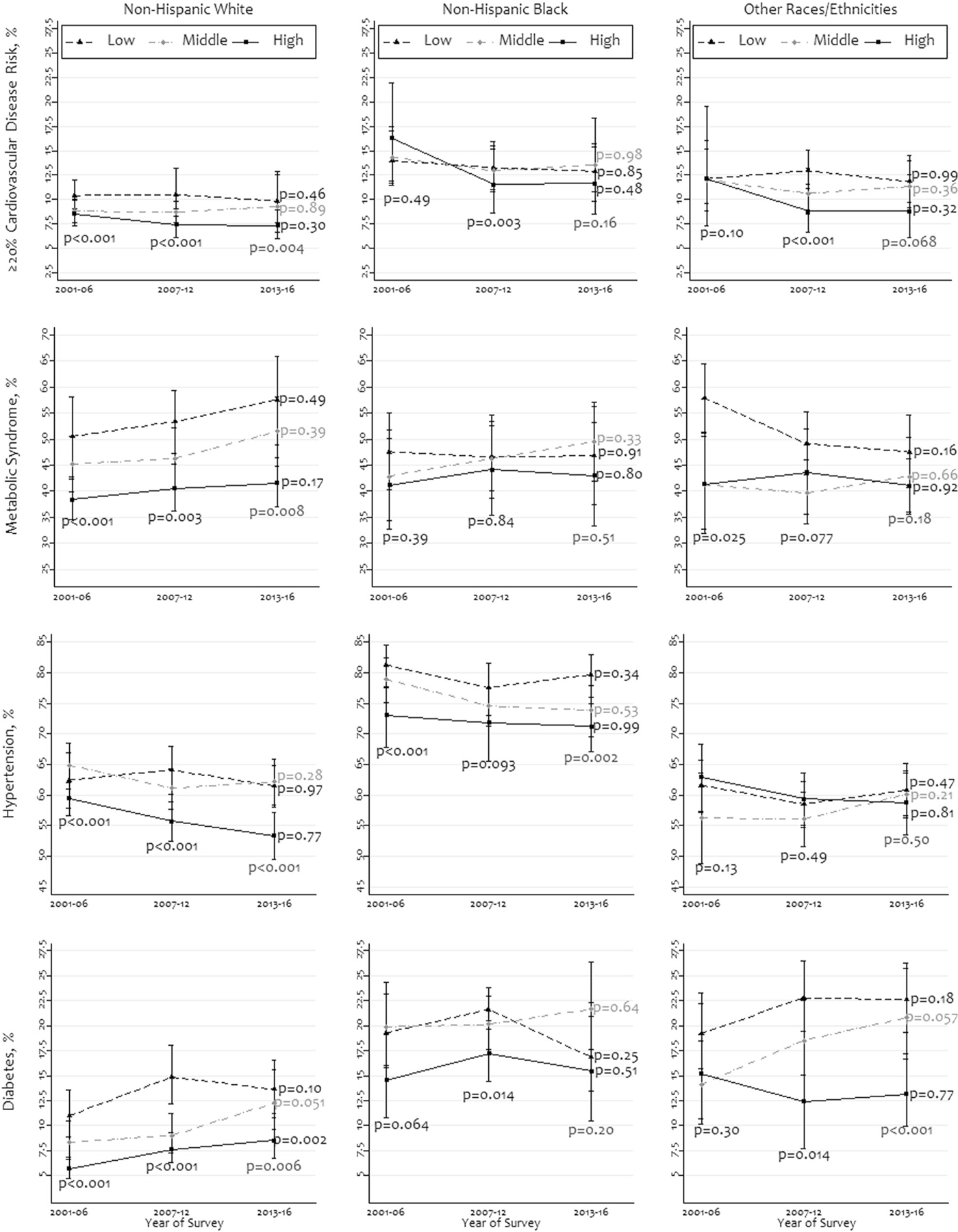
The ratio was stratified as three equal-sized groups: low, middle, and high. Races other than non-Hispanic whites and non-Hispanic blacks were merged as “other races/ethnicities”. The P values were obtained using chi-square tests.
As shown in Fig. 4, the overall prevalence of all four studied characteristics was higher among people with low- and middle income than those with high income in most racial/ethnic groups. The prevalence change did not show any consistent pattern for most characteristics during the survey periods.
Fig. 4. Prevalence and trends of obesity, chronic kidney disease, no physical activity, and tobacco smoking by family income-to-poverty ratio within racial/ethnic groups, 2001–2016 (N = 25,777).
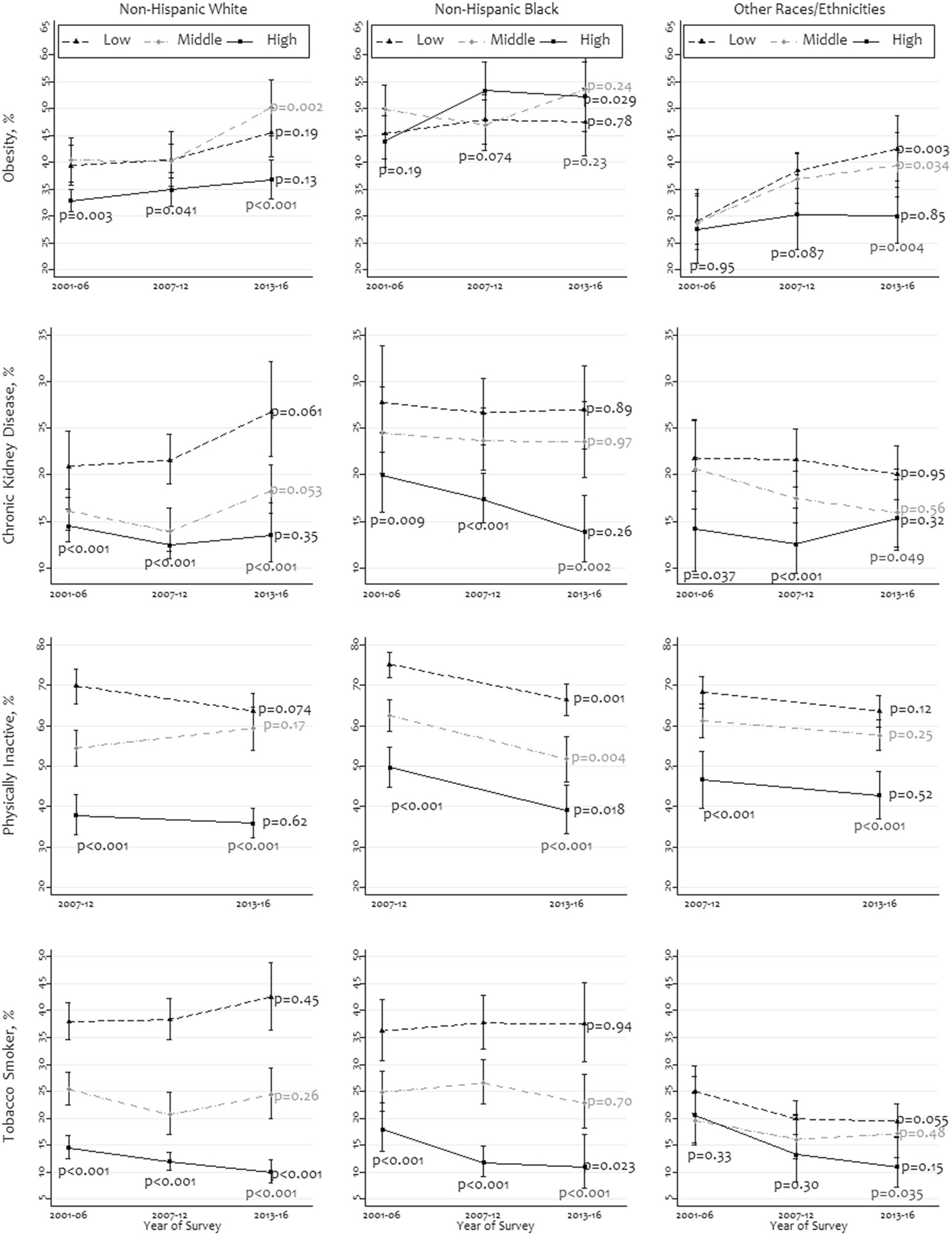
The ratio was stratified as three equal-sized groups: low, middle, and high. Races other than non-Hispanic whites and non-Hispanic blacks were merged as “other races/ethnicities“. The P values were obtained using chi-square tests.
Discussion
This study described income disparities in high absolute CVD risk, metabolic syndrome, hypertension, and other major CVD risk factors by race/ethnicity among middle-aged and older people. The overall prevalence of many CVD risk factors was higher among non-Hispanic blacks and low-/middle-income people compared to non-Hispanic whites and high-income people. Within the racial/ethnic groups, low- or middle-income people had a higher prevalence of most characteristics than high-income people during most periods.
This study supports previous research that found an overall higher prevalence of many CVD risk factors among non-Hispanic blacks and low-income people [8, 9, 14, 28]. These populations also have higher hospitalization rates due to acute myocardial infarction compared to non-Hispanic whites or high-income people [14, 29]. Even among people who receive treatment, there may be racial/ethnic and income disparities in CVD control. For instance, although non-Hispanic blacks are more likely to receive anti-hypertensive treatment, they have a higher prevalence of uncontrolled hypertension [11, 18]. We have also observed differences in the proportion of people with high income within racial/ethnic groups (Supplementary Table 1). This may also contribute to the differences by races/ethnicities.
To reduce the burden of CVD and the adverse consequences resulting from it, it is important to develop prevention strategies that target people who have a higher likelihood of developing CVD. As many of the observed characteristics were higher among low-income people of all races/ethnicities, it is important to design health education programs for them. Although the differences in the prevalence of high CVD risk and metabolic syndrome were insignificant according to race/ethnicity, prevalence differed by income, indicating a substantial association of income instead of race/ethnicity in prevalence for these two variables. However, most other characteristics differed by race/ethnicity as well as income. Studies have also reported differences in education, insurance, dietary habits, and health awareness according to race/ethnicity and income [14, 28, 30]. The two reported behavioral characteristics (i.e., no aerobic LTPA and tobacco smoking) in this study were also significantly more prevalent among low-income people compared to other income groups. These differences were observed overall and within racial/ethnic groups. The prevention strategies are similar for high CVD risk, metabolic syndrome, hypertension, diabetes, obesity, and CKD; increasing LTPA and smoking cessation are two of them [21, 24]. Reducing the racial/ethnic and income disparities in the CVD burden should help address life expectancy disparities as well [31]. Furthermore, increased age is a known risk factor for CVD and a growing concern as the US population ages in the upcoming decades [13]. Reducing burden by adopting healthy behaviors from a young age is thus essential [21, 28].
The study findings also indicate that the recent decline in the burden of many CVD risk factors has not been achieved across all income groups within all races/ethnicities [14, 28]. It is important to halt the rising prevalence of risk factors that disproportionately afflict non-Hispanic blacks and low-income people. Previous studies have reported that the prevalence of obesity and diabetes has increased during the past several decades [12, 13, 32]. The absolute increment in prevalence by insignificant (i.e., P value > 0.05) or small (e.g., 1–2%) percentage points in each survey year may look trivial but the population-wide increase may be several millions. Moreover, the prevalence increment for these characteristics may obviate the benefits of prevalence reduction for characteristics like tobacco smoking or no LTPA.
Our study has several notable strengths. First, we have analyzed a nationally representative survey’s data and reported the prevalence and trends after adjusting for the age and sample weights. Second, NHANES uses standardized validated methods. To our knowledge, this is the first study to report these disparities by income strata among racial/ethnic groups.
Study limitations
The limitations of the present study also merit discussion. First, due to a lower number of participants from racial/ethnic groups other than non-Hispanic whites and non-Hispanic blacks, grouping all of them together may conceal the different prevalence estimates among them. In addition, because the diagnoses were made based on a single day’s measure, there may be some overestimation of prevalence. Also, using different cutoffs (e.g., intermediate CVD risk or other hypertension definitions) may result in different estimates. Lastly, the associations we observe may not be causal due to the cross-sectional nature of the dataset.
Conclusion
In addition to race/ethnicity, this study highlights the importance of family income in the burden of CVD risk factors. Considering the observed income disparities in the prevalence of these studied characteristics among all racial/ethnic groups, it is essential to design prevention strategies focused on the people with the higher burden.
Supplementary Material
Summary table.
What is known about topic
The prevalence of some cardiovascular risk factors can differ by race/ethnicity and income.
What this study adds
Within racial/ethnic groups, low- or middle-income people had a higher prevalence of most cardiovascular risk factors than high-income people during most periods.
This study indicates that income disparities in the burden of cardiovascular risk factors also exist within racial/ethnic groups.
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41371-021-00513-8.
Compliance with ethical standards
Conflict of interest The authors declare no competing interests.
References
- 1.Heron M. National Vital Statistics Reports National Center for Health Statistics. Annual Hyattsville, MD. 2018. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_06.pdf. Accessed 5 Dec 2018. [Google Scholar]
- 2.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD. DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2016;2017:1260–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lottenberg SA, Glezer A, Turatti LA. Metabolic syndrome: identifying the risk factors. J de Pediatr 2007;83:204–8. [DOI] [PubMed] [Google Scholar]
- 5.Cassells HB, Haffner SM. The metabolic syndrome: risk factors and management. J Cardiovascular Nurs 2006;21:306–13. [DOI] [PubMed] [Google Scholar]
- 6.Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem 2007;44:232–63. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES. Trends in Predicted 10-year risk of coronary heart disease and cardiovascular disease among U.S. adults from 1999 to 2010. J Am Coll Cardiol 2013;61:2249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J Am Heart Assoc 2018;7:e008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pool LR, Ning H, Lloyd‐Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US Adults from 1999–2012. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Kibria GM. Racial/ethnic disparities in prevalence, treatment and control of hypertension among US adults following application of the 2017 American College of Cardiology/American Heart Association guideline. Prevent Med Rep 2019;14:100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. J Am Med Assoc 2015;313:1973. [DOI] [PubMed] [Google Scholar]
- 13.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing Chronic Disease 2017;14. 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odutayo A, Gill P, Shepherd S, Akingbade A, Hopewell S, Tennankore K, et al. Income disparities in absolute cardiovascular risk and cardiovascular risk factors in the United States, 1999–2014. JAMA Cardiol 2017;2:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SL, Zhang Z, Wiltz JL, Loustalot F, Ritchey MD, Goodman AB, et al. Hypertension among youths—United States, 2001–2016. MMWR Morbidity Mortal Wkly Rep 2018;67: 758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital-Health Stat 2013;1:1–37. [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. National health and nutrition examination survey 2020. https://wwwn.cdc.gov/nchs/nhanes/default.aspx. Accessed 26 Feb 2020.
- 18.Ostchega Y, Zhang G, Hughes JP, Nwankwo T. Factors associated with hypertension control in US adults using 2017 ACC/AHA guidelines: National Health and Nutrition Examination Survey 1999–2016. Am J Hypertens 2018;31:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health & Human Services. Poverty Guidelines 2019. https://aspe.hhs.gov/poverty-guidelines. Accessed 16 Feb 2019.
- 20.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–5. [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, Casey DEJ, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017:HYP.0000000000000065. [Google Scholar]
- 22.Khosla T, Lowe CR. Indices of obesity derived from body weight and height. Br J Prev Soc Med 1967;21:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services. 2008 Physical activity guidelines for Americans. US; 2008. https://health.gov/paguidelines/pdf/paguide.pdf. Accessed 30 Jun 2018.
- 25.United States Census Bureau. Population distribution 2019. https://www.census.gov/data/datasets/2017/demo/popest/nation-detail.html. Accessed 26 Feb 2020.
- 26.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol 2013;64:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stata Corporation, College Station, Texas USA. StataCorp. 2017 2017. https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/. Accessed 8 May 2017.
- 28.Jackson SL, Yang EC, Zhang Z. Income disparities and cardio-vascular risk factors among adolescents. Pediatrics 2018;142: e20181089 10.1542/peds.2018-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spatz ES, Beckman AL, Wang Y, Desai NR, Krumholz HM. Geographic variation in trends and disparities in acute myocardial infarction hospitalization and mortality by income levels, 1999–2013. JAMA Cardiol 2016;1:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell CN, Thorpe RJ, Bowie JV, LaVeist TA. Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol 2018;28:147–52. [DOI] [PubMed] [Google Scholar]
- 31.Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, et al. The association between income and life expectancy in the United States, 2001–2014. J Am Med Assoc 2016;315: 1750–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. J Am Med Assoc 2015;314:1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


