Abstract
Background
Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome are rare, severe cutaneous adverse reactions usually triggered by medications. In addition to tertiary‐level supportive care, various systemic therapies have been used including glucocorticoids, intravenous immunoglobulins (IVIGs), cyclosporin, N‐acetylcysteine, thalidomide, infliximab, etanercept, and plasmapheresis. There is an unmet need to understand the efficacy of these interventions.
Objectives
To assess the effects of systemic therapies (medicines delivered orally, intramuscularly, or intravenously) for the treatment of SJS, TEN, and SJS/TEN overlap syndrome.
Search methods
We searched the following databases up to March 2021: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, and Embase. We also searched five clinical trial registers, the reference lists of all included studies and of key review articles, and a number of drug manufacturer websites. We searched for errata or retractions of included studies.
Selection criteria
We included only randomised controlled trials (RCTs) and prospective observational comparative studies of participants of any age with a clinical diagnosis of SJS, TEN, or SJS/TEN overlap syndrome. We included all systemic therapies studied to date and permitted comparisons between each therapy, as well as between therapy and placebo.
Data collection and analysis
We used standard methodological procedures as specified by Cochrane. Our primary outcomes were SJS/TEN‐specific mortality and adverse effects leading to discontinuation of SJS/TEN therapy. Secondary outcomes included time to complete re‐epithelialisation, intensive care unit length of stay, total hospital length of stay, illness sequelae, and other adverse effects attributed to systemic therapy. We rated the certainty of the evidence for each outcome using GRADE.
Main results
We included nine studies with a total of 308 participants (131 males and 155 females) from seven countries. We included two studies in the quantitative meta‐analysis.
We included three RCTs and six prospective, controlled observational studies. Sample sizes ranged from 10 to 91. Most studies did not report study duration or time to follow‐up. Two studies reported a mean SCORe of Toxic Epidermal Necrosis (SCORTEN) of 3 and 1.9. Seven studies did not report SCORTEN, although four of these studies reported average or ranges of body surface area (BSA) (means ranging from 44% to 51%). Two studies were set in burns units, two in dermatology wards, one in an intensive care unit, one in a paediatric ward, and three in unspecified inpatient units. Seven studies reported a mean age, which ranged from 29 to 56 years. Two studies included paediatric participants (23 children).
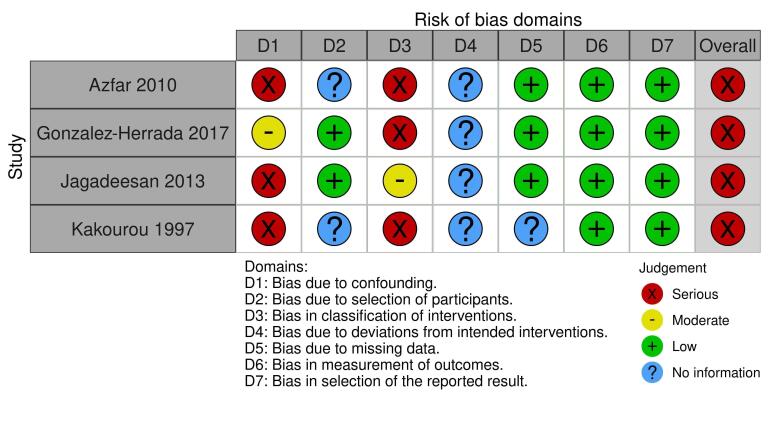
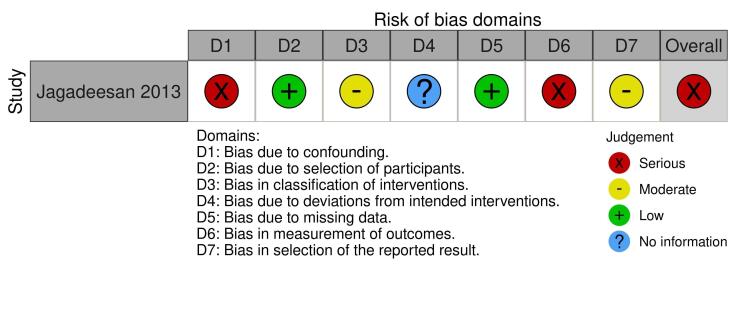
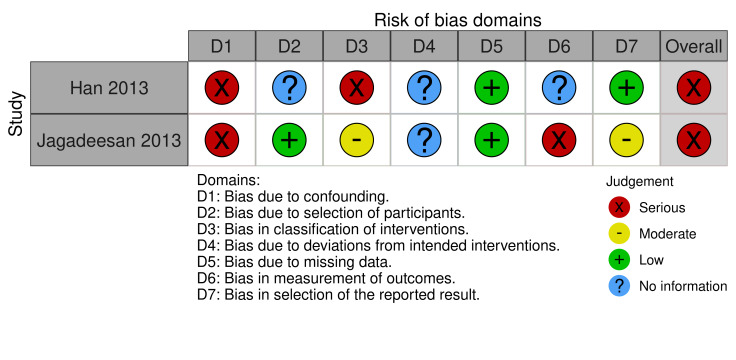
We assessed the results from one of three RCTs as low risk of bias in all domains, one as high, and one as some concerns. We judged the results from all six prospective observational comparative studies to be at a high risk of bias. We downgraded the certainty of the evidence because of serious risk of bias concerns and for imprecision due to small numbers of participants.
The interventions assessed included systemic corticosteroids, tumour necrosis factor‐alpha (TNF‐alpha) inhibitors, cyclosporin, thalidomide, N‐acetylcysteine, IVIG, and supportive care. No data were available for the main comparisons of interest as specified in the review protocol: etanercept versus cyclosporin, etanercept versus IVIG, IVIG versus supportive care, IVIG versus cyclosporin, and cyclosporin versus corticosteroids.
Corticosteroids versus no corticosteroids
It is uncertain if there is any difference between corticosteroids (methylprednisolone 4 mg/kg/day for two more days after fever had subsided and no new lesions had developed) and no corticosteroids on disease‐specific mortality (risk ratio (RR) 2.55, 95% confidence interval (CI) 0.72 to 9.03; 2 studies; 56 participants; very low‐certainty evidence). Time to complete re‐epithelialisation, length of hospital stay, and adverse effects leading to discontinuation of therapy were not reported.
IVIG versus no IVIG
It is uncertain if there is any difference between IVIG (0.2 to 0.5 g/kg cumulative dose over three days) and no IVIG in risk of disease‐specific mortality (RR 0.33, 95% CI 0.04 to 2.91); time to complete re‐epithelialisation (mean difference (MD) −2.93 days, 95% CI −4.4 to −1.46); or length of hospital stay (MD −2.00 days, 95% CI −5.81 to 1.81). All results in this comparison were based on one study with 36 participants, and very low‐certainty evidence. Adverse effects leading to discontinuation of therapy were not reported.
Etanercept (TNF‐alpha inhibitor) versus corticosteroids
Etanercept (25 mg (50 mg if weight > 65 kg) twice weekly "until skin lesions healed") may reduce disease‐specific mortality compared to corticosteroids (intravenous prednisolone 1 to 1.5 mg/kg/day "until skin lesions healed") (RR 0.51, 95% CI 0.16 to 1.63; 1 study; 91 participants; low‐certainty evidence); however, the CIs were consistent with possible benefit and possible harm. Serious adverse events, such as sepsis and respiratory failure, were reported in 5 of 48 participants with etanercept and 9 of 43 participants with corticosteroids, but it was not clear if they led to discontinuation of therapy. Time to complete re‐epithelialisation and length of hospital stay were not reported.
Cyclosporin versus IVIG
It is uncertain if there is any difference between cyclosporin (3 mg/kg/day or intravenous 1 mg/kg/day until complete re‐epithelialisation, then tapered off (10 mg/day reduction every 48 hours)) and IVIG (continuous infusion 0.75 g/kg/day for 4 days (total dose 3 g/kg) in participants with normal renal function) in risk of disease‐specific mortality (RR 0.13, 95% CI 0.02 to 0.98, 1 study; 22 participants; very low‐certainty evidence). Time to complete re‐epithelialisation, length of hospital stay, and adverse effects leading to discontinuation of therapy were not reported.
No studies measured intensive care unit length of stay.
Authors' conclusions
When compared to corticosteroids, etanercept may result in mortality reduction. For the following comparisons, the certainty of the evidence for disease‐specific mortality is very low: corticosteroids versus no corticosteroids, IVIG versus no IVIG and cyclosporin versus IVIG. There is a need for more multicentric studies, focused on the most important clinical comparisons, to provide reliable answers about the best treatments for SJS/TEN.
Plain language summary
Which treatments that affect the whole body work best to treat severe skin reactions (Stevens‐Johnson syndrome and toxic epidermal necrolysis)?
Key messages
Treating severe skin reactions with etanercept (a medicine that acts to reduce a specific part of the immune system), rather than steroids (which broadly reduces the immune system), may result in a lower number of deaths from Stevens‐Johnson syndrome and toxic epidermal necrolysis.
We are uncertain about the effectiveness of other treatments, such as steroids and cyclosporin (medicines that act on the immune system) or immunoglobulins (naturally produced antibodies). We need more studies set across multiple sites that compare the effectiveness and safety of the most important treatments.
What causes severe skin reactions?
Certain medications may trigger severe skin reactions known as Stevens‐Johnson syndrome (SJS), a more severe condition known as toxic epidermal necrolysis (TEN), or an overlap syndrome (SJS/TEN). Large skin blisters form, which leave painful sores. The affected skin eventually peels off (necrosis). Cases are rare, but can be fatal if they lead to infections or problems affecting other organs.
Severe skin reactions are medical emergencies, and require treatment in a hospital, often in an intensive care or a burns unit. Treatments include supportive care, such as fluids and nutrition, wound care, and painkilling medications.
What did we want to find out?
In addition to supportive care, treatments taken by mouth or injected include medicines that act on the immune system (e.g. etanercept) and intravenous immunoglobulins (antibodies).
We wanted to find out how well these treatments work to treat severe skin reactions.
What did we do?
We searched for studies of all currently used treatments taken by mouth or injected to treat SJS, TEN, or SJS/TEN overlap. We were interested in studies that compared one of these treatments against another, or against supportive treatment alone to determine whether one worked better than the other.
What did we find?
We found 9 studies in 308 people (adults and a few children), which took place in India, Europe, China, and Taiwan. We found three randomised controlled trials (RCTs) (a type of study where participants are randomly assigned to one of two or more treatment groups). The other six studies observed the effectiveness of a treatment compared to another, without randomly assigning participants to a treatment (observational study). Most patients in the studies (where reported) had a moderate severity of disease, with 44% to 51% of their body surface area affected by rash. Studies were set in burn units, intensive care units and inpatient hospital wards.
Main results
The evidence comparing no steroids to steroids came from only 2 observational studies in 56 people; we are uncertain about this result due to our lack of confidence in the evidence: on average, out of 1000 people given steroids, 232 people are at risk of dying, compared with 91 out of every 1000 not given steroids. The number of days to full skin healing and length of hospital stay were not reported.
The evidence comparing immunoglobulins to no immunoglobulins came from 1 observational study in 36 people; we are uncertain about these results due to our lack of confidence in the evidence: on average, for every 1000 people given immunoglobulins, 55 people are at risk of dying, compared with 167 of every 1000 people not given immunoglobulins. The skin of people given immunoglobulins healed almost three days faster, and people spent two days less in hospital.
Compared with steroids, etanercept may reduce the number of people who die: on average, for every 1000 people given etanercept, 83 people would die from complications of their severe skin reaction (usually infection), compared with 163 out of every 1000 people given steroids (evidence from 1 RCT in 91 participants). Unwanted side effects (such as breathing problems or severe infections) occurred in both etanercept and steroids study groups, but it was not clear if they caused participants to stop treatment. Other studies did not report on unwanted effects leading to discontinuation of treatment. The number of days to full skin healing and length of hospital stay were not reported.
The evidence comparing cyclosporin with immunoglobulins was from 1 observational study in 22 people; we are uncertain about this result due to our lack of confidence in the evidence: on average, for every 1000 people given cyclosporin, 65 people are at risk of dying, compared with 500 out of every 1000 people given immunoglobulins. The number of days to full skin healing and length of hospital stay were not reported.
No studies measured the length of stay in intensive care units.
What are the limitations of the evidence?
We are not confident in our results because they came from few studies with small numbers of participants. In most studies, the way in which the studies were conducted could have influenced the findings of the study.
How up‐to‐date is this evidence?
The evidence is current to March 2021.
Summary of findings
Summary of findings 1. Corticosteroids compared to no corticosteroids.
| Summary of findings: | ||||||
| Corticosteroids compared to no corticosteroids for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: corticosteroids and supportive care Comparison: supportive care1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supportive care | Risk with corticosteroids | |||||
|
Disease‐specific mortality ‐ SJS and TEN (time to follow‐up not reported) |
91 per 1000 | 232 per 1000 (65 to 821) |
RR 2.55 (0.72 to 9.03) |
56 (2 observational studies) | ⨁◯◯◯ VERY LOW a,b | Effect estimates calculated from 1 study (Azfar 2010). A second study did not report any events (Kakourou 1997). |
| Time to complete re‐epithelialisation – not reported | Not reported | ‐ | ‐ | ‐ | ‐ | |
| Intensive care unit (ICU) length of stay ‐ not reported | Not reported | ‐ | ‐ | ‐ | ‐ | |
| Total hospital length of stay – not reported | Not reported | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects leading to discontinuation of SJS/TEN therapy – not reported | Not reported | ‐ | ‐ | ‐ | ‐ | |
|
1 Supportive care process in 1 study was not described (Azfar 2010). In the second study this was described in detail and includes topical saline compresses and sprays, petroleum jelly, bathing, topical lidocaine gel to the oral mucosa and topical antibiotics and artificial tears to the eyes (Kakourou 1997). *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of selection bias and lack of control of any confounding variables. bDowngraded two levels for imprecision, as results only based on small studies, and the confidence interval includes serious harms and an important benefit.
Summary of findings 2. Intravenous immunoglobulin (IVIG) compared to no IVIG.
| Summary of findings: | ||||||
| Intravenous immunoglobulin (IVIG) compared to no IVIG for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: IVIG and supportive care1 Comparison: supportive care1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supportive care | Risk with IVIG | |||||
|
SJS/TEN‐specific mortality (time to follow‐up not reported) |
167 per 1000 | 55 per 1000 (6 to 386) | RR 0.33 (0.04 to 2.91) | 36 (1 observational study) | ⨁◯◯◯ VERY LOW a, b | An additional observational study, Saraogi 2016, that included a comparison of IVIG with steroids versus steroids alone did not provide useable data for meta‐analysis, but reported that “SCOR‐TEN (Score of Toxic Epidermal Necrosis) analysis showed that treatment with steroids led to an increased mortality rate, whereas treatment with IVIg decreased it". |
|
Time to complete re‐epithelialisation (time to follow‐up not reported) |
The mean time to complete re‐epithelialisation was 10.93 days. | MD 2.93 days lower (4.4 lower to 1.46 lower) | ‐ | 36 (1 observational study) | ⨁◯◯◯ VERY LOW a, c | An additional observational study, Saraogi 2016, included a comparison of IVIG with steroids versus steroids alone did not provide useable data for meta‐analysis, but reported that “The average time for complete re‐epithelialization and hospitalization was lowest in patients given IVIg and highest in those given steroids”. |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ |
‐ | ‐ | ‐ | ‐ |
|
Total hospital length of stay (time to follow‐up not reported) |
The mean total hospital length of stay was 15.33 days. | MD 2.00 days lower (5.81 lower to 1.81 higher) | ‐ | 36 (1 observational study) | ⨁◯◯◯ VERY LOW a, c | An additional observational study, Saraogi 2016, that included a comparison of IVIG with steroids versus steroids alone did not provide useable data for meta‐analysis, but reported that “The average time for complete re‐epithelialization and hospitalization was lowest in patients given IVIg and highest in those given steroids”. |
| Adverse effects leading to discontinuation of SJS/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
1 Jagadeesan 2013 compared IVIG plus corticosteroids to supportive care plus corticosteroids. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of selection bias, as the studies were not randomised and prespecified confounders were not properly addressed, and performance bias. bDowngraded two levels due to imprecision, as the confidence interval includes harms and important benefits, and results were from one small study. cDowngraded one level due to imprecision, as results were from one small study.
Summary of findings 3. Etanercept compared to no etanercept.
| Summary of findings: | ||||||
| Etanercept compared to no etanercept for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: etanercept and supportive care Comparison: supportive care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supportive care | Risk with etanercept | |||||
| Disease‐specific mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies met the criteria for evaluation of etanercept to supportive care. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJT/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 4. Cyclosporin compared to no cyclosporin.
| Summary of findings: | ||||||
| Cyclosporin compared to no cyclosporin for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: cyclosporin and supportive care Comparison: supportive care alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supportive care | Risk with cyclosporin | |||||
| Disease‐specific mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Unpublished prospective data for 22 participants were obtained from 1 cohort study, Gonzalez‐Herrada 2017, for the comparison of cyclosporin versus other treatments (IVIG n = 4, corticosteroids n = 1, or no specified treatment n = 1). 4 participants died (1 on cyclosporin and 3 on IVIG) (RR 0.13, 95% CI 0.02 to 0.98; Analysis 4.1; Fisher's exact test P = 0.046). There were 43% fewer deaths with cyclosporin compared to other treatments (95% CI 2.0% to 85.5% fewer). Retrospective data as reported in the published paper are not included here. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJT/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
4.1. Analysis.

Comparison 4: Cyclosporin versus IVIG, Outcome 1: Disease‐specific mortality
Summary of findings 5. Intravenous immunoglobulin (IVIG) compared to corticosteroids.
| Summary of findings: | ||||||
| Intravenous immunoglobulin (IVIG) compared to corticosteroids for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: IVIG Comparison: corticosteroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with corticosteroids | Risk with IVIG | |||||
| Disease‐specific mortality | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies comparing IVIG to corticosteroids. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJT/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 6. Etanercept compared to corticosteroids.
| Summary of findings: | ||||||
| Etanercept compared to corticosteroids for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital, serving as a regional referral centre for SJS/TEN cases Intervention: etanercept and supportive care Comparison: corticosteroids and supportive care (specifics of supportive care not identified by study) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with corticosteroids | Risk with etanercept | |||||
|
Disease‐specific mortality (time to follow‐up not reported) |
163 per 1000 | 83 per 1000 (26 to 265) |
RR 0.51 (0.16 to 1.63) |
91 (1 RCT) | ⨁⨁◯◯ LOW a | ‐ |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJS/TEN therapy ‐ not reported (see comments) | ‐ | ‐ | ‐ | ‐ | ‐ | 1 study reported that 5/48 participants in the etanercept group had serious adverse events (sepsis, respiratory failure, and bipolar disorder) and 9/43 in the corticosteroids group (sepsis, respiratory failure, upper gastrointestinal haemorrhage, stridor and vocal cord palsy) (Wang 2018). It is unclear from the trial report if any of these adverse events led to discontinuation of treatment or if they were attributed to systemic therapy. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to imprecision, as the confidence interval is wide and includes benefits and harms, and results were from one small study.
Summary of findings 7. Cyclosporin compared to corticosteroids.
| Summary of findings: | ||||||
| Cyclosporin compared to corticosteroids for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: cyclosporin Comparison: corticosteroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with corticosteroids | Risk with cyclosporin | |||||
| Disease‐specific mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Unpublished prospective data for 22 participants were obtained from 1 cohort study, Gonzalez‐Herrada 2017, for the comparison of cyclosporin versus other treatments (IVIG n = 4, corticosteroids n = 1, or no specified treatment n = 1). 4 participants died (1 on cyclosporin and 3 on IVIG) (RR 0.13, 95% CI 0.02 to 0.98; Analysis 4.1; Fisher's exact test P = 0.046). There were 43% fewer deaths with cyclosporin compared to other treatments (95% CI 2.0% to 85.5% fewer). See Table 9 for additional details. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJT/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 8. Etanercept compared to intravenous immunoglobulin (IVIG).
| Summary of findings: | ||||||
| Etanercept compared to intravenous immunoglobulin (IVIG) for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: no studies found Intervention: etanercept Comparison: IVIG | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with IVIG | Risk with etanercept | |||||
| Disease‐specific mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies for the comparison of etanercept to IVIG. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJS/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 9. Cyclosporin compared to intravenous immunoglobulin (IVIG).
| Summary of findings: | ||||||
| Cyclosporin compared to intravenous immunoglobulin (IVIG) for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: inpatient hospital wards Intervention: cyclosporin Comparison: IVIG | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other treatments | Risk with cyclosporin | |||||
|
Disease‐specific mortality (time to follow‐up not reported) |
500 per 1000 | 65 per 1000 (10 to 468) |
RR 0.13 (0.02 to 0.98) |
22
(1 observational study) |
⨁◯◯◯ VERY LOW a, b | Unpublished prospective data for 22 participants were obtained from 1 cohort study, Gonzalez‐Herrada 2017, for the comparison of cyclosporin versus other treatments (IVIG n = 4, corticosteroids n = 1, or no specified treatment n = 1). 4 participants died (1 on cyclosporin and 3 on IVIG) (RR 0.13, 95% CI 0.02 to 0.98; Analysis 4.1; Fisher's exact test P = 0.046). There were 43% fewer deaths with cyclosporin compared to other treatments (95% CI 2.0% to 85.5% fewer). Retrospective data as reported in the published paper are not included here. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Complete re‐epithelialisation was reported only for participants treated with cyclosporin but not for other interventions. No comparison between therapies was possible. |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJT/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of bias from potential confounding effects and selection bias. bDowngraded one level due to imprecision, as these results were from one small study.
Summary of findings 10. Etanercept compared to cyclosporin.
| Summary of findings: | ||||||
| Etanercept compared to cyclosporin for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome | ||||||
|
Patient or population: Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome Setting: no studies found Intervention: etanercept Comparison: cyclosporin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with cyclosporin | Risk with etanercept | |||||
| Disease‐specific mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies for the comparison of etanercept to cyclosporin. |
| Time to complete re‐epithelialisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intensive care unit (ICU) length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Total hospital length of stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse effects leading to discontinuation of SJS/TEN therapy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
A glossary of technical terms is provided in Table 11.
1. Glossary.
| Term | Definition |
| Apoptosis | programmed cell death |
| Cutaneous | skin |
| Epidermal | top‐most layer of the skin |
| Exogenous | external to the body |
| Fas‐mediated apoptosis | Fas‐ligand belongs to a group of proteins known as tumour necrosis factor (TNF) transmembrane proteins, which are molecules present on the surfaces of skin cells. These proteins bind (connect) with receptors, which causes the skin cells to apoptose (die). |
| IVIG | intravenous immunoglobulin; a medical therapy that consists of concentrated antibodies extracted from the blood of healthy donors |
| Mucous membrane | skin that lines internal body cavities such as the oral cavity and the vagina |
| Necrosis | tissue death |
| NF‐kB | Nuclear factor kappa‐light chain enhancer of activated B cells (NF‐kB) is a protein that influences DNA transcription, thereby regulating cellular responses to various stimuli. |
| Pathogenesis | the mechanism (process) of a disease |
| Re‐epithelialisation | the process of skin re‐growing its outermost layer (epidermis) |
Description of the condition
Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome are rare severe skin reactions most commonly triggered by medications. These three entities represent a spectrum of disease, with SJS the least and TEN the most severe, and the severity of SJS/TEN overlap syndrome in between. This spectrum of disease will be collectively referred to as SJS/TEN. The annual incidence of SJS and TEN in the general population is estimated to be 1 to 6 and 0.4 to 1.2 per million people, respectively (Yang 2016). The condition is a potentially fatal dermatological emergency, with mortality between 1% to 5% for SJS, and 25% to 40% for TEN (Patel 2013).
Over 200 drugs have been associated with SJS/TEN, most frequently antibiotics, allopurinol, non‐steroidal anti‐inflammatory drugs, and anticonvulsants. The risk of SJS/TEN is greatest within weeks of the start of therapy (Roujeau 1995). Risk factors include immunocompromised status, concomitant radiotherapy with anticonvulsant use, and a slow acetylator genotype (associated with slow drug metabolism) (Dietrich 1995). Certain human leucocyte antigen (HLA) alleles are associated with the development of SJS/TEN, including HLA‐B*15:02 in Asians and East Indians taking carbamazepine; HLA‐B*15:02 in Han Chinese taking carbamazepine, lamotrigine, or phenytoin; HLA‐B*58‐01 in Han Chinese taking allopurinol; and HLA‐A*31‐01 in Europeans taking carbamazepine (Cheung 2013; Chung 2004; Hsu 2016; Hung 2005; McCormack 2011). Screening programmes in Asia have resulted from these discoveries; however, despite the knowledge of these risk factors, the pathogenesis of SJS/TEN is not entirely understood.
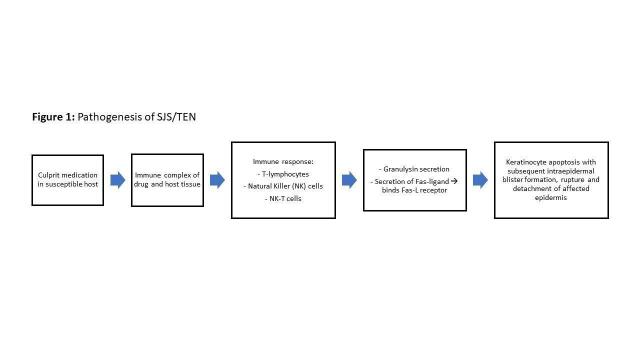
It is hypothesised that SJS/TEN may be due to an immune response to an antigenic complex between the culprit drug and host tissue HLAs in predisposed individuals, whereby T lymphocytes, natural killer cells, and natural killer T cells secrete granulysin and Fas‐ligand. This immune response induces apoptosis upon binding to the Fas‐ligand death receptor on keratinocytes (Figure 1) (Nickoloff 2008).
1.
Proposed pathogenesis of SJS/TEN.
This spectrum of disease is characterised by widespread epidermal necrosis, which leads to separation of the epidermis from the underlying dermis. This separation causes erythema and erosion of both cutaneous and mucous membrane skin (< 10% body surface area for SJS, 10% to 30% for SJS/TEN overlap, and > 30% for TEN) (Bastuji‐Garin 1993). Acute effects of epidermal necrosis include abnormalities in fluid and electrolyte balance, temperature regulation, and protection from infection. Significant secondary complications can be acute (sepsis, respiratory distress, hypothermia, fluid loss, electrolytic abnormalities) or chronic (ocular symblepharon, entropion, blindness, chronic pain, and genital scarring with associated urethral stenosis and phimosis) (Revuz 1987), and include mental health sequelae such as depression and anxiety (Hoffman 2021).
Description of the intervention
Limited evidence is available to guide the treatment of SJS/TEN, thus there is wide variability in dosage, duration, and treatment regimen, with no international standard dosing for systemic therapies. In addition to tertiary‐level supportive care, various systemic therapies have been used, including glucocorticoids, intravenous immunoglobulin (IVIG), cyclosporin (calcineurin inhibitor), N‐acetylcysteine, thalidomide (immunomodulator), infliximab or etanercept (tumour necrosis factor‐alpha (TNF‐alpha) inhibitors), and plasmapheresis. Through various mechanisms (see How the intervention might work), these systemic therapies potentially halt the progression and lessen the severity of SJS/TEN. Supportive care measures include wound care; eye, mouth, and genital skin care; nutrition; fluid replacement; and care provided at a tertiary care centre. This review examines systemic medical interventions only.
Few randomised controlled trials (RCTs) have examined systemic therapy for SJS/TEN. A study that compared thalidomide versus supportive care in patients with TEN was stopped early owing to higher‐than‐predicted mortality (10 of 12 participants in the thalidomide group versus 3 of 10 in the placebo group) (Wolkenstein 1998). This is the only study to be included in a prior Cochrane Review of systemic therapies specifically for TEN, which was published in 2002 (Majumdar 2002). The authors of that review concluded that there was no reliable evidence to support treatment decisions for TEN. More recently, an RCT of 96 participants with SJS/TEN reported less‐than‐predicted mortality for patients treated with etanercept (8.3% observed versus 17.7% predicted deaths) based on severity of illness score or SCORe of Toxic Epidermal Necrolysis (SCORTEN) criteria; this mortality was less than that predicted for patients treated with corticosteroids (16.3%), and neither result was statistically significant (Wang 2018).
Retrospective cohorts from the EuroSCAR and RegiSCAR trials provide robust data on systemic therapies for SJS/TEN, with close to 1000 participants from these studies combined. The studies have examined survival benefit in patients treated with a variety of regimens including corticosteroids, supportive care, IVIG, and cyclosporin (Campione 2003; Faye 2005; Prins 2003; Sekula 2013; Stella 2001; Trent 2008; Tristani‐Firouzi 2002; Viard 1998). Other case series and small cohorts and a phase 2 non‐randomised trial assessed the effect of cyclosporin in halting the progression of SJS/TEN (Arevalo 2000; Gonzalez‐Herrada 2017; Jarrett 1997; Kirchhof 2014; Poizeau 2018; Rai 2008; Reese 2011; Robak 2001; Sullivan 1996; Zaki 1995).
Tumour necrosis factor inhibitors (anti‐TNF agents) are the newest agents under study for use in SJS/TEN. Several reports have shown that infliximab given as a single infusion of 5 mg/kg halts skin sloughing and induces rapid re‐epithelialisation (no erosions or active lesions) of denuded skin (Patmanidis 2012; Scott‐Lang 2014; Wojtkiewicz 2008; Zarate‐Correa 2013). A few case series have described similar results with a single 50 mg subcutaneous injection of etanercept (Famularo 2007; Gubinelli 2009; Paradisi 2014). The true benefit of anti‐TNF agents in SJS/TEN is difficult to ascertain because published studies on this topic are few.
In addition to mortality, time to complete re‐epithelialisation is an important and validated endpoint. Several studies have also attempted to evaluate re‐epithelialisation when treated with supportive care, corticosteroids, IVIG, cyclosporin, or etanercept (Famularo 2007; Lalosevic 2015; Napolitano 2013; Paradisi 2014; Singh 2013; Valeyrie‐Allanore 2010; Wang 2018).
How the intervention might work
The interventions described above involve several potential mechanisms. As the SJS/TEN disease spectrum is believed to be an immune response to an exogenous agent, initial studies investigated the use of steroids to reduce this response (Yamane 2016). Dysregulation of Fas‐mediated apoptosis has also been implicated in SJS/TEN, and IVIG is thought to act through autoantibodies against Fas (Romanelli 2008). The ultimate target of immunomodulating or suppressive therapies is to reduce the action of activated T‐lymphocytes and cytokines whilst reducing granulysin at the cellular level to arrest cytotoxicity and apoptosis of the skin and mucosal surfaces. This is the case of TNF‐alpha inhibitors such as etanercept or infliximab (Chave 2005), or thalidomide (Klausner 1996), although thalidomide is a weak inhibitor of TNF alpha. TNF‐alpha inhibitors have also been shown to have an effect on increasing the Treg population to downregulate T‐cells and reduce granulysin, which is the ultimate effector of SJS‐TEN (Wang 2018). Cyclosporin is supposed to act through inhibiting interleukin‐15 (IL‐15) and IL‐17, which are the main drivers of TNF‐alpha (Su 2017). Cyclophosphamide leads as well to cell apoptosis through DNA alkylation and T‐cell inhibition. Other mechanisms of action include the removal of pathogenic particles from blood (like plasmapheresis), as investigated by Yamane 2016, or haemoperfusion (Hall 1992), enhanced bioregeneration of the skin tissues through accelerated re‐epithelialisation with granulocyte colony‐stimulating factor (de Sica‐Chapman 2010), and downregulation of NF‐kB (cyclosporin and N‐acetylcysteine), as studied by Kohanim 2016 and Hasan 2020.
Why it is important to do this review
Given the rarity of this disease, evidence of treatment efficacy is limited, and most has been derived from retrospective, uncontrolled studies including few participants. Most patients thus continue to be treated according to institutional experience. In a practice survey of 147 North American centres treating patients with SJS/TEN (130 burn centres and 17 academic dermatology centres), only 54% of physicians reported that they followed treatment guidelines or an institutional standard of care for SJS/TEN, and only a minority of these physicians used professionally published guidelines (Dodiuk‐Gad 2015). IVIG was the first choice at more than 80% of sites, followed by systemic corticosteroids, cyclosporin, anti‐TNF medications, and supportive care alone (provided at 14% of centres). This pattern of practice is markedly different from published expert opinions (iSCAR meeting 2013, as reported by Dodiuk‐Gad 2015, and a multidisciplinary expert group meeting in 2017, as reported by White 2018).
A recent meta‐analysis of 96 studies including 3248 participants showed survival benefit for cyclosporin and glucocorticoids but not for supportive care alone, IVIG, plasmapheresis, thalidomide, cyclophosphamide, anti‐TNF agents, haemoperfusion, or granulocyte colony‐stimulating factor (Zimmermann 2017). This is the only meta‐analysis to comprehensively evaluate treatments for SJS/TEN. Proposed strengths of our own review will include use of Cochrane methods, which involve rigorous quality assessment of included studies and inclusion of only prospective studies (cohort and prospective patient registry studies) to ensure the highest quality of included data.
The topic of this review was covered in part by the Cochrane Review titled 'Interventions for toxic epidermal necrolysis' (Majumdar 2002).
Objectives
To assess the effects of all systemic therapies (medicines delivered orally, intramuscularly, or intravenously) for the treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and prospective comparative studies only, due to a lack of validated tools for assessing risk of bias in uncontrolled studies. We defined 'prospective' as studies that collected data from the point of patient inclusion in the study, and 'comparative' as when studies had a control group or a second arm to compare with. We excluded cross‐over trials due to the inability to get an adequate wash‐out period for patients with this condition. There were no restrictions on language or publication status (published, unpublished, in press, or in progress).
Types of participants
We included participants of any age with a clinical diagnosis of SJS, TEN, or SJS/TEN overlap syndrome. Given the rarity of SJS/TEN and the limited number of studies to date, we also included studies in which participants with SJS/TEN represented a subset of the overall study population, but only included data for the SJS/TEN patients specifically. We classified SJS/TEN as per published criteria (Bastuji‐Garin 1993), but included all studies reporting a clinical diagnosis of SJS/TEN.
Types of interventions
We included all systemic therapies studied to date, including corticosteroids, IVIG, cyclosporin, N‐acetylcysteine, thalidomide, infliximab, plasmapheresis, and etanercept. We included comparisons between each of the therapies outlined when data were available (28 possible comparisons). In addition, we included comparisons of some therapies versus placebo (supportive care alone versus the intervention with supportive care) when such data were available.
Types of outcome measures
We considered the following primary and secondary outcome measures, where data were available.
Primary outcomes
SJS/TEN‐specific mortality: mortality within one month of onset of SJS/TEN that is not clearly attributed to another cause
Adverse effects leading to discontinuation of SJS/TEN therapy: events that occur within one month following administration of therapy that are listed as potential adverse effects in the product monograph and lead to discontinuation of therapy
Secondary outcomes
Time to complete re‐epithelialisation: number of days to full skin healing
Intensive care unit (ICU) length of stay: time during which participant is admitted to ICU ward, as reported when available
Total hospital length of stay: time during which participant is admitted to hospital, as reported when available
Illness sequelae (chronic mucocutaneous morbidity): sequelae that clinically makes sense as possible outcomes of SJS/TEN including cutaneous (scarring, dyspigmentation, loss of nails), ocular (cicatricial conjunctivitis, corneal perforation/ ulceration/epithelial defects, entropion/ectropion, chronic dry eye, symblepharon, blindness), gastrointestinal (ulceration, perforation, strictures), genitourinary (vaginal stenosis, phimosis, urethral strictures), and respiratory events (bronchiolitis, bronchiectasis, obstructive lung disease), and chronic pain
Other adverse effects attributed to systemic therapy: events that occur within one month following administration of therapy that are listed as potential adverse effects in the product monograph and do not lead to discontinuation of therapy
We included these outcomes because literature reviews and clinical experience indicate that they are important considerations for patients with SJS/TEN. Besides reducing mortality, the purpose of treating SJS/TEN is to increase the speed of skin healing to minimise the potential for illness sequelae. Length of stay in hospital, including the ICU, is an important determinant of healthcare costs. Furthermore, minimising time spent in hospital reduces the risk of hospital‐acquired illness among these patients. We collected data on these measures at any and all outcome time points.
Prespecified confounders and co‐interventions for non‐randomised studies
Confounders identified a priori include disease duration (first day of symptoms to initiation of treatment), disease severity (as determined by SCORTEN; Bastuji‐Garin 2000), use of diagnostic criteria (as published in Table 12; Bastuji‐Garin 1993), baseline comorbidities, age distribution, duration of follow‐up, and the use of co‐interventions (i.e. supportive care, other systemic medical treatments). These variables may confound the relationship between SJS/TEN treatment and disease‐specific mortality, as they are related to these variables and, when not adjusted for, may impact the measure of treatment effect. For example, if treatment X is used only for patients who have better disease prognosis (present to hospital early in their disease, have less severe disease, are younger, have fewer baseline comorbidities), the effect of treatment X on reducing mortality may be overestimated. Similarly, studies that do not use diagnostic criteria may include other diseases that are less severe than SJS/TEN (such as erythema multiforme), which may lead to the overestimation of treatment effects. Duration of follow‐up is also important, as studies with shorter follow‐up (i.e. less than one month) may underestimate SJS/TEN mortality, which again would lead to overestimation of the treatment effect. We included non‐randomised studies in the analysis that did not adjust for these prespecified confounders, and assessed the possible impact of these confounders using the Risk of Bias In Non‐randomised Studies – of Interventions (ROBINS‐I) tool (Sterne 2016), as detailed below.
2. Diagnostic criteria for Stevens‐Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) as proposed by Bastuji and colleagues (1993).
| Classification | Types of lesions* | Distribution | Percentage of body surface area detached/detachable |
| Bullous erythema multiforme | Typical or atypical raised targets | Acral | < 10 |
| SJS | Spots ± flat atypical targets | Generalised | < 10 |
| Overlap SJS/TEN | Spots ± flat atypical targets | Generalised | ≥ 10 to 30 |
| TEN with spots | Spots ± flat atypical targets | Generalised | ≥ 30 |
| TEN without spots | No spots or targets | Generalised | ≥ 10 |
*Typical targets: lesions < 3 cm with well‐defined borders and regular round shape with 3 separate zones of colour; atypical targets: flat or palpable lesions with 2 zones of colour and poorly defined borders.
Search methods for identification of studies
We aimed to identify all relevant RCTs and prospective observational comparative studies regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist (Liz Doney) searched the following databases up to 10 March 2021 using strategies based on the draft strategy for MEDLINE in our published protocol (Langley 2018):
the Cochrane Skin Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2021, Issue 3, in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4.
Trial registers
One of two review authors (AL or BO) searched the following trial registers using the search terms: Stevens‐Johnson syndrome, toxic epidermal necrolysis, SJS, and Lyell’s syndrome or Lyell’s disease, up to 21 May 2020.
ISRCTN register (www.isrctn.com)
ClinicalTrials.gov (www.clinicaltrials.gov)
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
EU Clinical Trials Register (www.clinicaltrialsregister.eu)
Searching other resources
Reference lists
One of two review authors (AL or BO) checked the reference lists of all included studies and key review articles for additional references to relevant trials.
Relevant organisations
One of three review authors (AL, BO, or FS) searched up to March 2021 for clinical trials records on the following drug manufacturers’ websites using the search terms ‘Steven Johnson syndrome’ and ‘toxic epidermal necrolysis’:
Intravenous immunoglobulins: Grifols (www.grifols.com);
Cyclosporin: Novartis (www.novartisclinicaltrials.com);
N‐acetylcysteine: Pfizer (www.pfizer.com/science/clinical-trials);
Thalidomide: Bristol Myers‐Squibb (www.bms.com/researchers-and-partners/clinical-trials-and-research.html);
Infliximab: Janssen (www.globaltrialfinder.janssen.com);
Etanercept: Amgen (www.amgen.com/science/clinical-trials).
Errata or retractions
One of two review authors (AL or BO) searched for errata or retractions of the included studies up to 16 February 2021 using MEDLINE and the Retraction Watch database.
Adverse effects
We did not perform a separate search for adverse effects of interventions used for the treatment of SJS, TEN, and SJS/TEN overlap syndrome. We considered adverse effects described in the included studies only.
Data collection and analysis
Selection of studies
All search results were merged into Covidence reference management software and duplicates removed (Covidence).
Two review authors (AL, AS, EM, BO, RP, or AJ) independently reviewed and selected abstracts based on relevancy to the research question. Any discrepancies were resolved by discussion or by consulting a third review author (AS, EM, BO, RP, or AJ) if required. The full texts of selected abstracts were obtained and stored in Covidence. Two review authors (AL, EM, BO, RP, or AJ) reviewed the full texts to determine if they met the inclusion criteria, with any discrepancies resolved by discussion or with input from a third review author (AS, BO, RP, or AJ) if required. When selected abstracts meeting the inclusion criteria did not have associated full‐text publications, we contacted the study authors to obtain full data. When full data were not available but abstracts still met the inclusion criteria, data from the abstracts were extracted and managed as outlined below. We created a PRISMA flow diagram (Figure 2) to outline study selection, and a Characteristics of excluded studies highlighting the reasons for exclusion of the excluded studies (Eden 2011). We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review.
2.
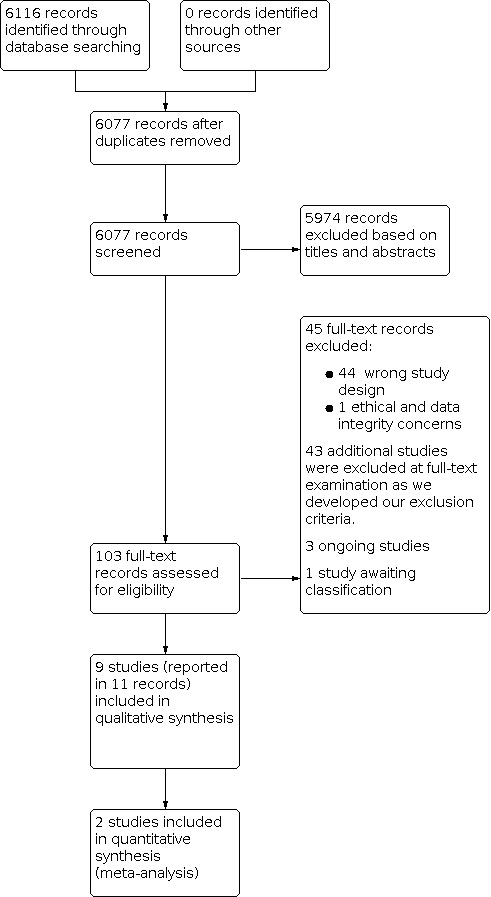
Study flow diagram.
Data extraction and management
We extracted data for each included study into Characteristics of included studies tables. Two review authors (AL, EM, BO, RP, or AJ) independently extracted the following study characteristics from reports of the included studies following the form in Table 13.
3. Data collection.
| Study information | Methods | Participants | Interventions | Outcomes |
| Citation | Study design | Number randomised | Description of intervention | Mean time to initial skin healing |
| Date of study | Total duration of study | Mean age, range | Description of comparison | Mean time to full skin healing |
| Funding | Details of any run‐in period | Sex | Concomitant medications | Mean hospital length of stay |
| Notable declarations of interest of study authors | Number of study centres | Ethnicity | Excluded medications | Mean intensive care unit (ICU) length of stay |
| Study locations | Inclusion criteria | All‐cause mortality | ||
| Study setting | Exclusion criteria | Disease‐specific mortality | ||
| Diagnostic criteria | SMR | |||
| Disease severity | Adverse effects of treatment | |||
| Disease duration | Illness sequelae (chronic mucocutaneous morbidity) | |||
| Mean day of illness at which treatment was initiated |
Abbreviations: SMR: standardised mortality ratio, a ratio of the observed number of deaths to the number of deaths expected for a standard population of known age and sex distribution.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, study dates.
Participants: number of participants, mean age, age range, sex, ethnicity, disease duration, severity of condition, diagnostic criteria, baseline comorbidities, inclusion criteria, exclusion criteria.
Interventions: interventions, comparisons, concomitant medications, supportive care measures, excluded medications.
Outcomes: primary and secondary outcomes specified above (number of events and number of participants per treatment group for dichotomous outcomes, and means and standard deviations and number of participants per treatment group for continuous outcomes. Both adjusted and unadjusted measures of treatment effect were collected, as well as time points for data collection).
Notes: funding for trial, notable declarations of interest of trial authors.
Any disagreements were resolved by consensus or by involving a third review author if required (JPP). One review author (AL or BO) transferred extracted data into the Review Manager 5 (Review Manager 2020) and RevMan Web (RevMan Web 2020). We compared each study against the PRISMA checklist for inclusion of information reported in the study protocol, when this was available.
Assessment of risk of bias in included studies
Four review authors (AL, BO, AJ, and FS) independently assessed risk of bias for each study's outcome result included in the summary of findings tables. We evaluated bias in RCTs using Cochrane’s RoB 2 tool (22 August 2019 version) for effect of assignment to the intervention (Higgins 2021b; Sterne 2019). We assessed risk of bias as low, high, or some concerns, for the domains described below.
Domain 1: Risk of bias arising from the randomisation process
Domain 2: Risk of bias due to deviations from the intended interventions
Domain 3: Missing outcome data
Domain 4: Risk of bias in measurement of the outcome
Domain 5: Risk of bias in selection of the reported result
We then determined an overall risk of bias for each result. If any domain was graded as high, the overall risk of bias for the study was considered to be high. We planned that if we identified cluster‐RCTs we would use Cochrane’s RoB 2 tool adding a domain specific for cluster‐RCTs (Eldridge 2020), using the signalling questions in combination with guidance on cluster‐RCTs in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c).
We assessed each outcome result from non‐randomised studies for bias using the ROBINS‐I tool as developed by members of the Cochrane Non‐Randomized Studies for Interventions Methods Group (Sterne 2016). We evaluated the effect of assignment to the intervention. We assessed risk of bias as 'low', 'moderate', 'serious', or 'critical' for the domains described below, with an additional option of 'no information'. We then determined an overall risk of bias for each result. If any domain was graded as critical, the overall risk of bias was considered to be critical.
Bias due to confounding
Bias in selection of participants into the study
Bias in classification of interventions
Bias due to deviations from intended interventions
Bias due to missing data
Bias in measurement of outcomes
Bias in selection of the reported result
We used the templates for the Cochrane RoB 2 and ROBINS‐I tools to assess risk of bias, available at www.riskofbias.info. Any disagreements were resolved through consensus with the Cochrane Skin Group. We used the robvis tool to create risk of bias summary figures (McGuinness 2020).
Measures of treatment effect
We collected effect estimates from each study. When researchers provided both adjusted and unadjusted measures of treatment effect, we would collect both. 'Adjusted measures' refers to those produced from multi‐variate analyses that adjust for the confounding effects of covariates. Although adjusted data were preferred for the analysis, the collection of unadjusted data would have allowed us to perform a sensitivity analysis for inclusion of these data in the results.
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). This was completed using RevMan Web (RevMan Web 2020).
We analysed continuous data as mean differences (MDs) or standardised mean differences (SMDs), depending on whether the same scale was used to measure a given outcome, and 95% CIs. We entered data presented as a scale with a consistent direction of effect across studies.
If in future updates different scales are used to measure the same conceptual outcome (e.g. disability), we will calculate SMDs instead with corresponding 95% CIs. We plan to convert SMDs back to MDs on a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical amongst‐person standard deviation (e.g. standard deviation of the control group at baseline from the most representative trial), as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b).
We intended to analyse time‐to‐event data as hazard ratios and rate data using Poisson methods (Lawless 1986); however, insufficient data precluded this analysis.
When pooling results, for dichotomous outcomes we planned to calculate the absolute risk difference using the risk difference statistic in Review Manager 5 (Review Manager 2020) or RevMan Web (RevMan Web 2020) and to express the result as a percentage. We also planned to calculate the relative per cent change for dichotomous data as 'Risk ratio − 1', and express this as a percentage, if possible.
Unit of analysis issues
The unit of analysis in each study was the individual participant, and we sought to obtain participant‐level data for all included studies. We planned to conduct a meta‐analysis only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense based on heterogeneity assessment. We would analyse studies by grouping them according to study design, and provide a global estimate in the context of analysis of each study design. If data from RCT versus non‐RCT studies were sufficiently similar with minimal heterogeneity, we would cautiously consider a pooled meta‐analysis.
We included cluster‐RCTs by accounting for within‐cluster participant correlation in the analysis (Higgins 2021a). We excluded cross‐over trials due to concerns with carryover effects. Within‐participant (split‐body) RCTs are not relevant to this topic, which pertains to systemic therapies only, and were therefore excluded.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only, or when data were not available for all participants). When this was not possible, and missing data were thought to introduce serious bias, we would explore the impact of including such studies in the overall assessment of results by performing a sensitivity analysis. We would clearly describe any assumptions and imputations used to handle missing data and would explore the effect of imputation by performing sensitivity analyses.
For dichotomous outcomes, we calculated event rates using the number of participants randomised in the group as the denominator.
For continuous outcomes, we calculated the mean difference based on the number of participants analysed at that time point. If the number of participants analysed was not presented for each time point, we would use the number of randomised participants in each group at baseline.
Where possible, we would compute missing standard deviations from other statistics such as standard errors, CIs, or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). If standard deviations could not be calculated, we would impute them (e.g. from other studies in the meta‐analysis).
Assessment of heterogeneity
In the case of sufficient included studies, we would perform meta‐regression to investigate potential sources of heterogeneity. We would assess clinical and methodological diversity in terms of participants, interventions, outcomes, and study characteristics for the included studies to determine whether a meta‐analysis was appropriate. We would do this using data from the data extraction tables. We would assess statistical heterogeneity by visually inspecting the forest plot for obvious differences in results between studies, and by performing I² and Chi² statistical tests.
As recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), we would interpret the I² value as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
As noted in the Cochrane Handbook, we would keep in mind that the importance of I² depends on (1) the magnitude and direction of effects, and (2) the strength of evidence for heterogeneity. We would interpret the Chi² test with P ≤ 0.10 as indicating evidence of statistical heterogeneity. Had we identified substantial heterogeneity, we would have reported this and investigated possible causes by following the recommendations provided in Section 10.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022).
Assessment of reporting biases
In the case of sufficient included studies, we would perform tests to detect publication bias. We would create and examine a funnel plot to explore possible small‐study biases. In interpreting funnel plots, we would examine different possible reasons for funnel plot asymmetry as outlined in Section 13.3 of the Cochrane Handbook, and relate this to the review results. If we were able to pool more than 10 trials, we would undertake formal statistical tests to investigate funnel plot asymmetry, following the recommendations provided in Section 13.3 of the Cochrane Handbook (Page 2022).
To assess outcome reporting bias, we checked trial protocols against the published reports. When a protocol was not available, we would request access from study authors. If this was not obtainable, we would list the study as 'reporting bias cannot be ruled out'. For studies published after 1 July 2005, we would screen the WHO ICTRP (apps.who.int/trialsearch/) for the a priori trial protocol. We would evaluate whether selective reporting of outcomes was present.
Data synthesis
In the case of sufficient included studies, we would perform meta‐analysis, and only when this was meaningful (i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense). We would analyse studies by grouping them according to study design, providing a global estimate in the context of the analysis of each study design. We did not pool together different measures of effect (e.g. odds ratio (OR) and RR).
If meta‐analysis was possible, we would employ a random‐effects model using Review Manager 5 (Review Manager 2020) or RevMan Web (RevMan Web 2020). Where meta‐analysis was not possible, we summarised results narratively including data from non‐comparative studies.
When results were estimated for individual studies with low numbers of events (< 10 in total), or when the total sample size was less than 30 participants and a risk ratio was used, we would report the proportion of events in each group together with a P value from Fisher’s exact test.
In studies appraised using ROBINS‐I, we would exclude studies from pooled or narrative analysis if the ROBINS‐I assessment was critical.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis by category of disease severity (SCORTEN ≥ 3), body surface area (≥ 30%), advanced age (≥ 75 years), and co‐interventions if adequate data for meta‐analysis were available.
Sensitivity analysis
If adequate data were available for meta‐analysis, we would conduct sensitivity analyses to assess the robustness of data analysis, specifically to test the impact of the following.
Treatment effect estimates that were unadjusted (because we believed this would help support our decision to exclude studies that did not adjust for important prespecified confounding variables, by showing that the results may change when this confounding is not accounted for).
Missing data that required assumptions or imputations, or both.
Studies with brief (less than one month) follow‐up.
Quality assessment of the included studies (removing studies that were at high risk of bias or serious risk of bias).
Summary of findings and assessment of the certainty of the evidence
Based on our protocol, the most important comparisons of interventions that we sought to prepare the summary of findings tables were as follows.
Etanercept versus cyclosporin
Etanercept versus IVIG
IVIG versus cyclosporin
Cyclosporin versus corticosteroids
However, of these comparisons, data were only available for IVIG versus cyclosporin. We therefore prepared summary of findings tables for the following comparisons.
Corticosteroids versus no corticosteroids
IVIG versus no IVIG
Etanercept versus corticosteroids
Cyclosporin versus IVIG
We included the following prespecified outcomes in each summary of findings table, where reported.
Primary outcomes
Disease‐specific mortality
Adverse events leading to discontinuation of therapy
Secondary outcomes
Time to complete re‐epithelialisation
ICU length of stay
Total hospital length of stay
For each summary of findings table, five review authors (AL, BO, RP, AJ, and JPP) independently assessed the quality of the evidence using the five GRADE considerations (study limitations/risk of bias based on the assessments from the risk of bias tools, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence (GRADEpro GDT). If only one study contributed to a comparison, the quality assessment was limited to data from the single study. We assessed the certainty of the evidence as high, moderate, low, or very low for each outcome. Evidence from RCTs is automatically assessed as high quality, with the certainty of the evidence downgraded for any of the factors listed above by one level (serious concerns) or two levels (very serious concerns). Evidence from observational studies also starts at high quality, whenever the ROBINS‐I tool is used. However, in all cases the evidence was downgraded two levels due to the inherent risk of bias associated with the lack of randomisation. However, we did consider the following criteria for upgrading the certainty of evidence, if appropriate: large effect, dose‐response gradient, and plausible confounding effect. We used the methods and recommendations described in Sections 8.5 and 8.7 and Chapters 14 and 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022; Dijkers 2013; Higgins 2021a; Schunemann 2022a; Schunemann 2022b). We justified all decisions to down‐ or upgrade the certainty of evidence using footnotes, and provided comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
Our search (see Search methods for identification of studies) retrieved 6116 records. We removed 39 duplicates, and screened the remaining 6077 records. We excluded 5974 records based on titles and abstracts, and obtained the full texts of the remaining 103 records. We excluded 45 studies based on full‐text review (see Characteristics of excluded studies). We excluded another 43 studies after full‐text examination as our exclusion criteria developed (we restricted the review to only RCTs and prospective comparative studies, and excluded cross‐over trials). These studies can be viewed in a citation list at file repository https://osf.io/gth7c/?view_only=e0316ead836a436894a2e7c41346682a. We identified three ongoing studies (see Characteristics of ongoing studies), and one completed but unpublished study (see Characteristics of studies awaiting classification). We included nine studies reported in 11 references in the qualitative synthesis. We included two studies in the quantitative synthesis (meta‐analysis). For a further description of our screening process, see the study flow diagram (Figure 2).
Included studies
Design
We included three RCTs, Paquet 2014; Wang 2018; Wolkenstein 1998, and six prospective, controlled observational studies, Azfar 2010; Gonzalez‐Herrada 2017; Han 2017; Jagadeesan 2013; Kakourou 1997; Saraogi 2016, in the review.
Sample sizes
The nine studies included a total of 308 participants (range: 10 participants in Paquet 2014 to 91 participants in Wang 2018). There were 22 included participants in Gonzalez‐Herrada 2017; these data represent only the prospective data and were obtained directly from the study authors.
Settings
Burn units in tertiary care hospitals were explicitly reported as the setting for Gonzalez‐Herrada 2017 (Madrid, Spain) and Paquet 2014 (Brussels, Belgium). An inpatient dermatology ward at a tertiary care centre in India served as the setting for Jagadeesan 2013 and Azfar 2010; an inpatient referral centre for the largest medical system in Taiwan for Wang 2018; an intensive care unit in a hospital in Xi'an, China for Han 2017; an inpatient paediatrics ward in Athens, Greece for Kakourou 1997; and a single‐centre inpatient hospital ward for Saraogi 2016. Wolkenstein 1998 was set at nine hospitals across France.
Participants
Across the nine included studies where demographic data were available, there were a total of 131 males and 155 females (sex distribution not available for Gonzalez‐Herrada 2017). Two studies included paediatric participants, with a total of 23 children represented (Han 2017; Kakourou 1997). The mean age of the participants in the adult trials (where available) ranged from 29 to 56 years.
More specifically, participants in Azfar 2010 had a mean age of 29.07 years, a sex distribution of 16 males versus 24 females, and a distribution of 29 SJS cases compared to 11 cases of TEN; body surface area (BSA) was not reported. The Han 2017 study included both adults (n = 21) and children (n = 7), with 15 males and 13 females and a mean age of 25. The distribution of SJS/TEN or BSA was not reported. In Jagadeesan 2013, participants had a mean age of 37 years, a sex distribution of 16 males versus 20 females, and a mean baseline BSA of 51.16%; all participants were reported to have TEN. Kakourou 1997 included only paediatric participants, with 10 males and 6 females with a mean age ranging between 6 and 6.6 years; the distribution of SJS/TEN or BSA was not reported. Participants in Paquet 2014 had a mean age of 49 years, and 8 of 10 participants were female; mean BSA at baseline was 44.5%, with all participants diagnosed with TEN. In Saraogi 2016, reported baseline characteristics of the study population were 56% aged 20 to 39 years and 51% male, with 21 participants categorised as having SJS, 11 as SJS/TEN overlap, and 11 as TEN; BSA was not reported. The Wang 2018 study reported a mean age of 56.09 years, and a predominance of females: 51 females and 40 males, with the majority of cases (61.5%) classified as having less than 10% BSA affected; the distribution of SJS/TEN was not reported. Finally, participants in Wolkenstein 1998 had an age range of 23 to 81 years, a sex distribution of 10 males and 12 females, and a range of BSA at baseline of 10% to 90%; a distinction between SJS/TEN overlap and TEN was not reported.
Demographics of the participants from the prospective data, which were obtained directly from the authors of Gonzalez‐Herrada 2017, were not available. SCORTEN for participants was reported in Jagadeesan 2013 (mean: 3, range: 2 to 3) and Wang 2018 (mean: 1.90).
Interventions
Our main comparisons of interest, as specified in the review protocol, included: etanercept versus cyclosporin, etanercept versus IVIg, IVIg versus supportive care, IVIG versus cyclosporin, and cyclosporin versus corticosteroids. However, we identified no studies that directly made these comparisons. Some studies included more than one comparison, whilst others compared an intervention to supportive care alone. Most studies did not specify the exact supportive care measures provided. Only two studies reported on hospital follow‐up after discharge (Jagadeesan 2013; Wang 2018). We included the following comparisons in this review.
Corticosteroids versus no corticosteroids
IVIG versus no IVIG
Etanercept versus corticosteroids
Cyclosporin versus IVIG
N‐acetylcysteine and infliximab versus infliximab alone
Thalidomide versus placebo
Plasmapheresis versus other treatments
The interventions of interest are listed below along with their corresponding studies. Please note that studies may be repeated between groups due to multiple comparisons.
Cyclosporin
In Gonzalez‐Herrada 2017 (n = 22), cyclosporin was compared to other treatments including IVIG (n = 4), systemic corticosteroids (n = 1), or no specified treatment (n = 1). Cyclosporin was dosed at oral 3 mg/kg/day or intravenous (IV) 1 mg/kg/day until complete re‐epithelialisation, then tapered off (10 mg/day reduction every 48 hours). IVIG in the Gonzalez‐Herrada 2017 study was infused continuously at a dose of 0.75 g/kg/day for four days (total dose = 3 g/kg) in participants with normal renal function (a lower dose was used in participants with renal insufficiency). Finally, a systemic corticosteroid dose of prednisone‐equivalent dose ranging from 37.5 to 100 mg for 9 to 12 days was used. We identified no additional studies with a cyclosporin comparison arm.
Corticosteroids
Five studies involved comparisons of corticosteroids. In Azfar 2010 (n = 40), corticosteroids were compared to supportive care; however, details on dose, duration, and the specifics of supportive care measures were not reported. Jagadeesan 2013 (n = 36) compared IVIG plus corticosteroids to supportive care plus corticosteroids. IVIG in this study was dosed at 0.2 to 0.5 g/kg cumulative dose divided over three days, whilst IV dexamethasone 0.1 to 0.3 mg/kg/day was rapidly tapered within one to two weeks according to response. Participants in the Jagadeesan 2013 study were scheduled for a six‐month visit after hospital discharge for follow‐up. Saraogi 2016 (n = 43) also considered both corticosteroids and IVIG, comparing IVIG versus IVIG plus corticosteroids versus supportive care alone; dosages and duration were not available. Kakourou 1997 (n = 16) compared corticosteroids to supportive care alone, with the dose of methylprednisolone described as a bolus infusion of 4 mg/kg/day for two more days after fever had subsided and no new lesions had developed. Details on supportive care measures for Kakourou 1997 included topical saline compresses and sprays, petroleum jelly, bathing, topical lidocaine gel to the oral mucosa, and topical antibiotics and artificial tears to the eyes. Finally, Wang 2018 (n = 91) compared etanercept to corticosteroids. Etanercept was dosed at 25 mg (50 mg if weight > 65 kg) subcutaneously twice weekly "until skin lesions healed", whilst IV prednisolone 1 to 1.5 mg/kg/day was given "until skin lesions healed". Participants in the Wang 2018 study were followed until three weeks post‐hospital discharge.
Etanercept
Wang 2018 (n = 91) evaluated a comparison including etanercept for treatment of SJS/TEN (see details above under 'Corticosteroids').
IVIG
Two studies, Jagadeesan 2013 and Saraogi 2016, evaluated IVIG as a comparator (see details above under 'Corticosteroids').
Other interventions
We identified three studies matching our inclusion criteria but not our prespecified systemic interventions of interest. No summary of findings tables were constructed for these comparisons, but the interventions are described here. In Han 2017 (n = 28), plasmapheresis was compared to non‐plasmapheresis treatments including IVIG or corticosteroids, or both. Plasmapheresis was dosed as a one‐time dose of 1000 mL of Ringer‐Locke and 2000 to 3000 mL of plasma at a rate of 1000 mL an hour. No details on the dosages of IVIG or corticosteroids were provided.
In Paquet 2014 (n = 10), infliximab combined with IV N‐acetylcysteine was compared to IV N‐acetylcysteine alone. Infliximab was given at 5 mg/kg intravenously over a two‐hour period. N‐acetylcysteine was diluted in 5% glucose solution and given intravenously over a 20‐hour period (150 mg/kg in 250 mL of solution during the first hour, followed by 150 mg/kg in 500 mL during a 4‐hour period, and finally 150 mg/kg in 1000 mL for 15 hours).
Finally, in Wolkenstein 1998 (n = 22), thalidomide (200 mg by mouth twice a day for 5 days) was compared to placebo given at the same frequency and duration. Participants were followed for up to seven days after treatment.
Outcomes
The two primary outcomes of this review were disease‐specific mortality and adverse events leading to discontinuation. No disease‐specific mortality data were reported for Han 2017, and it was not possible to extract the supplied mortality data in Saraogi 2016 for analysis; however, all other studies reported this outcome. Also, no studies reported on adverse effects leading to discontinuation of therapy.
Reporting on secondary outcomes was inconsistent, with only two studies providing data on time to complete re‐epithelialisation (Jagadeesan 2013; Wang 2018). Two studies reported total hospital length of stay (Han 2017; Jagadeesan 2013). Jagadeesan 2013 reported on illness sequelae. Jagadeesan 2013 and Wang 2018 reported on other adverse events.
Two studies reported participant follow‐up after hospital discharge, one at three weeks, Wang 2018, and the other at six months (Jagadeesan 2013). Timing of outcome assessment was not reported.
No studies measured ICU length of stay.
Funding sources
Four studies reported funding sources, which included a research grant from the Instituto de Salud Carlos III–Ministerio de Economía, Industria y Competitividad (Gonzalez‐Herrada 2017), the Scientific Fund for the Young Talent of Shaanxi Province (Han 2017), a grant from Fonds d'Investissement de la Recherche Scientifique of the University Hospital of Liege (Paquet 2014), and grants from the National Science Council of Taiwan and Ministry of Health and Welfare of Taiwan (Wang 2018). In one study (Paquet 2014), free samples of the study drug (infliximab) were provided by industry.
Excluded studies
We excluded 45 studies (see Characteristics of excluded studies). We excluded 44 of these due to wrong study design. We excluded one prospective study comparing IVIG to supportive care (Firoz 2012). The Journal of the American Academy of Dermatology published an erratum stating that institutional review board approval was not obtained as stated in the paper (Firoz 2012). An expression of concern was also published that reported of a significant discrepancy between the number of patients recorded in the hospital database as having SJS/TEN and those reported in the paper. Furthermore, the original paper states that all diagnoses were biopsy‐confirmed, but only 75% of patients are recorded in the hospital database as having a confirmatory biopsy (Firoz 2012). Based on this information, we decided to exclude this study.
We also excluded 43 other studies after full‐text examination as our exclusion criteria developed (we restricted the review to only RCTs and prospective comparative studies, and excluded cross‐over trials). These studies can be viewed in a citation list at file repository https://osf.io/gth7c/?view_only=e0316ead836a436894a2e7c41346682a.
Ongoing studies
We identified a further three ongoing studies (see Characteristics of ongoing studies). Two RCTs are comparing granulocyte colony‐stimulating factor with placebo (NCT02739295), and cyclosporin versus etanercept versus supportive care (NCT02987257). One prospective cohort study is evaluating cyclosporin, IVIG, etanercept, and steroids (NCT03585946).
Studies awaiting classification
One study comparing glucocorticoid alone to glucocorticoid plus IVIG is finished but not yet published (ChiCTR‐TRC‐13003550), so is awaiting classification (see Characteristics of studies awaiting classification).
Risk of bias in included studies
Randomised controlled trials
We assessed three RCTs, Paquet 2014; Wang 2018; Wolkenstein 1998, for risk of bias using Cochrane’s RoB 2 tool (Higgins 2021b; Sterne 2019). See the risk of bias summary (Figure 3) and the risk of bias in each study for each domain for the outcome of mortality (Figure 4). Please also see the interactive RoB 2 tables for each analysis (Table 15; Table 16; Table 17). Detailed assessment notes and responses to the signal questions are available here.
3.
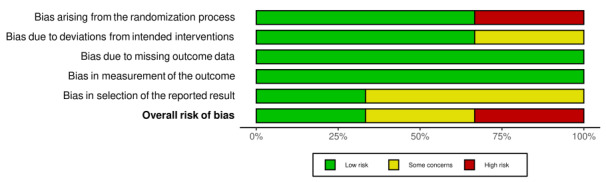
RoB 2 summary.
4.
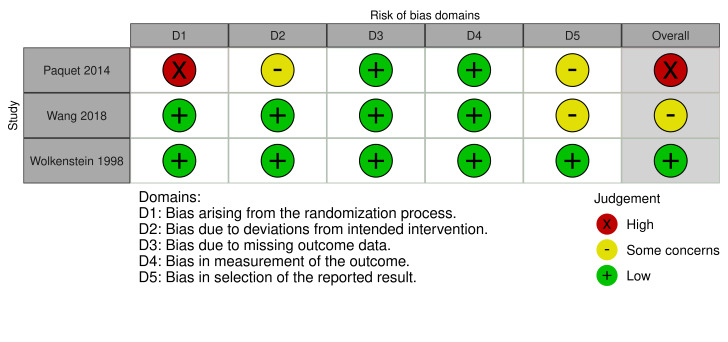
Risk of bias judgements for RCTs using the RoB 2 tool. Outcome: SJS/TEN‐specific mortality. Follow‐up: end of study.
Risk of bias for analysis 3.1 Disease‐specific mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Wang 2018 | Low risk of bias | ‐allocation sequence was random and concealed: “a random number was generated by the clinical trial center... after which the investigator was given the random number and initiated the appropriate treatment” ‐no baseline differences between intervention groups to suggest problem with randomization process |
Low risk of bias | Participants could not be blinded. Quote: "Because the injection methods are quite different for etanercept and corticosteroids, this was not a blinded clinical trial." Carers do not seem to have been blinded. Quote: "investigator was given the random number and initiated the appropriate treatment" Nothing to suggest deviations from the intended intervention because of the trial context. All participants analysed according to the group they were assigned to. | Low risk of bias | ‐Full data reported for all participants | Low risk of bias | ‐The outcomes were collected from this study are felt to be objective measures, and measurement unlikely to have differed between groups despite a lack of blinding of outcome assessors | Some concerns | ‐Mortality was not specified as an outcome in the trial registry record | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
Risk of bias for analysis 5.1 Disease‐specific mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Paquet 2014 | High risk of bias | ‐Seems to be alternate allocation rather than truly random. Quote: "The patients were randomized alternatively in one of two groups", "successive randomized TEN patients" ‐Illness auxiliary scores at admission are similar. No other baseline characteristics are described per group. | Some concerns | No mention of blinding of carers. Quote: "Each drug was administered intravenously (IV). NAC diluted in 5% glucose solution was administered at decreasing doses over a 20‐h period [...] In addition, IV infliximab was administered at a 5 mg/kg dosage as a single IV shot over a 2‐h period." No evidence to suggest any deviations from the intended intervetion because of the trial context. All participants analysed according to the group to which they were randomised and all participants received allocated intervention. | Low risk of bias | ‐Data are available for all participants for the outcome of disease‐specific mortality. | Low risk of bias | ‐Outcome is mortality so it would be measured the same way regardless of intervention group. ‐Mortality is unlikely to have been influenced if outcome assessors were aware of group allocation. | Low risk of bias | ‐No pre‐specified analysis plan was reported ; however, the only outcome used from this study was mortality rate which is reasonable to assume was an important outcome identified a priori as it is the most significant outcome for SJS‐TEN | High risk of bias | The study is judged to be at high risk of bias in at least one domain for this result. |
Risk of bias for analysis 6.1 Disease‐specific mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Wolkenstein 1998 | Low risk of bias | Quote: "The randomisation was done in blocks of six patients stratified according to two categories of study centres—dermatological centres or burns and intensive‐care units. Two lists (one for each category of centres) were generated from tables of random numbers. These numbers were assigned to the capsule boxes. Local investigators telephoned a private randomisation service and were given an identification number that matched numbers on capsule boxes distributed in the centres. This schedule was prepared by Laboratoires Laphal; the investigators were unaware of the allocation. A set of sealed envelopes containing the code were supplied to each centre In an emergency, the code could be broken and the investigator was required to write, sign, and date an explanation." | Low risk of bias | Quote: "The placebo and the thalidomide capsules were identical in appearance, and the investigators and patients did not know which capsules were given." All participants were analysed according to the group to which they were assigned. | Low risk of bias | ‐Although study was terminated early (due to a high death rate) prior to assessment of the study’s primary endpoint (extent of epidermal detachment at days 0, 5, 7, this outcome was not one of our pre‐specified outcomes ‐The only outcome available from this study that was included as one of our pre‐specified outcomes was mortality, and this data was available for all participants |
Low risk of bias | ‐Methods for measurement of the outcome (mortality) were appropriate – this is a highly objective measure, and measurement could not have differed between groups ‐Outcome assessors were not aware of the intervention received by study groups (blinded) |
Low risk of bias | ‐No pre‐specified analysis plan was reported as finalized before unblinded data were available for analysis; however, the only outcome used from this study was mortality rate which is reasonable to assume was an important outcome identified a priori as it is the most significant outcome for SJS‐TEN | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
For disease‐specific mortality, risk of bias was low in all domains and overall for Wolkenstein 1998. However, we assessed Paquet 2014 as at high risk of bias related to the randomisation process, and some concerns due to deviations from the intended interventions and selection of the reported result, therefore the risk of bias overall for this outcome was high. We assessed Wang 2018 as some concerns related to the selection of the reported result; overall the risk of bias was some concerns.
Non‐randomised trials
Prospective observational controlled studies
We assessed five prospective observational controlled studies, Azfar 2010; Gonzalez‐Herrada 2017; Han 2017; Jagadeesan 2013; Kakourou 1997, for risk of bias using the ROBINS‐I tool as developed by members of the Cochrane Non‐Randomized Studies for Interventions Methods Group (Sterne 2016). For individual assessments of each study, see Table 14 and Figure 5 for the outcome disease‐specific mortality; Table 15 and Figure 6 for days to full skin healing; and Table 16 and Figure 7 for hospital length of stay. We assessed all outcome results as overall serious risk bias. The major concerns were confounding not having been addressed and bias in the classification of interventions. Saraogi 2016 did not contribute to any of the outcomes, so we were not able to assess risk of bias using ROBINS‐I.
4. Summary of risk of bias assessments for non‐randomised studies ‐ outcome: disease‐specific mortality.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Azfar 2010 | Serious | No information | Serious | No information | Low | Low | Low |
Serious |
| Rationale for judgement | Missing information on most confounders ‐ unclear if comparable between groups | No information provided ‐ cannot determine from study text. | Intervention status was likely determined in such a way that could have been affected by knowledge of the outcome. | Unable to assess ‐ no details provided in study text | Data available for all reported outcomes for all participants. | Death was the only outcome measure of interest in this study. | Death was the only outcome measure of interest in this study. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
| Gonzalez‐Herrada 2017 | Moderate | Low | Serious | No information | Low | Low | Low | Serious |
| Rationale for judgement | Most confounding variables accounted for, apart from duration of follow‐up. | All consecutive patients over the age of 14 who fulfilled diagnostic criteria during the study time period were included in the study. | No information provided on how intervention assignment took place within each burn unit. | Unable to assess ‐ no details provided in text | Study authors provided mortality data on all prospectively recruited patients. | Death was the only outcome measure of interest in this study. | Death was the only outcome measure of interest in this study. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
| Jagadeesan 2013 | Serious | Low | Moderate | No information | Low | Low | Low | Serious |
| Rationale for judgement | Missing information on some confounders ‐ unclear if comparable between study groups | Consecutively diagnosed patients during the study period were included in the study. | Little information provided on how intervention assignment took place other than alternate allocation. | Unable to assess ‐ no details provided in text | Data were available for all participants. | The outcome was disease‐specific mortality, which is an objective measure. | This study assessed various outcomes of interest; this risk of bias analysis relates to disease‐specific mortality. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
| Kakourou 1997 | Serious | No information | Serious | No information | No information | Low | Low | Serious |
| Rationale for judgement | Missing information on most confounders ‐ unclear if comparable between study groups | No information provided ‐ cannot determine from text. | Very few details provided on intervention groups and how group assignment took place. | Unable to assess ‐ no details provided in text | Unclear from information provided in text | Disease‐specific mortality is the only outcome of interest in this study. | Disease‐specific mortality is the only outcome of interest in this study. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
5.
Risk of bias judgements for observational studies using the ROBINS‐I tool. Outcome: SJS/TEN‐specific mortality. Follow‐up: end of study.
5. Summary of risk of bias assessments for non‐randomised studies ‐ outcome: days to full skin healing.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Jagadeesan 2013 | Serious | Low | Moderate | No information | Low | Serious | Moderate | Serious |
| Rationale for judgement | Missing information on some confounders ‐ unclear if comparable between study groups | Consecutively diagnosed patients during the study period were included in the study. | Little information provided on how intervention assignment took place, other than alternate allocation. | Unable to assess ‐ no details provided in text | Data were available for all participants. | Concerns due to non‐blinding: evaluations and outcome measurement could have been influenced by knowledge of treatment allocation | No prespecified analysis plan was reported as finalised before unblinded data were available for analysis. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
6.
Risk of bias judgements for observational studies using the ROBINS‐I tool. Outcome: time to complete re‐epithelialisation. Follow‐up: end of study.
6. Summary of risk of bias assessments for non‐randomised studies ‐ outcome: hospital length of stay.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Han 2017 | Serious | No information | Serious | No information | Low | No information | Low | Serious |
| Rationale for judgement | Missing information on most confounders ‐ unclear if comparable between study groups | No information provided ‐ cannot determine from article. | Very few details provided on intervention groups and how group assignment took place: “divided into two groups on the basis of whether plasma exchange was performed after admission”. | Unable to assess ‐ no details provided in study text | Data reported on hospital length of stay for each participant in supplementary table. | Information about outcome assessors and whether they were blinded was not provided. | Individual patient data and outcomes provided in supplementary material. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
| Jagadeesan 2013 | Serious | Low | Moderate | No information | Low | Serious | Moderate | Serious |
| Rationale for judgement | Missing information on some confounders ‐ unclear if comparable between study groups | Consecutively diagnosed patients during the study period were included in the study. | Little information provided on how intervention assignment took place, other than alternate allocation. | Unable to assess ‐ no details provided in text | Data were available for all participants. | Concerns due to non‐blinding: evaluations and outcome measurement could have been influenced by knowledge of treatment allocation | No prespecified analysis plan was reported as finalised before unblinded data were available for analysis. | We assessed the study as at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. |
7.
Risk of bias judgements for observational studies using the ROBINS‐I tool. Outcome: total hospital length of stay. Follow‐up: end of study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
There were available data for only seven comparisons, details of which are described below.
Corticosteroids versus no corticosteroids
IVIG versus no IVIG
Etanercept versus corticosteroids
Cyclosporin versus IVIG
N‐acetylcysteine and infliximab versus infliximab alone
Thalidomide versus placebo
Plasmapheresis versus other treatments
It was not possible to pool studies for any of the comparisons, which precluded subgroup or sensitivity analyses.
Corticosteroids versus no corticosteroids
The evidence is very uncertain regarding the effect of corticosteroids on disease‐specific mortality compared to supportive care. One prospective study with 16 participants reported zero events (Kakourou 1997). Evidence from a second prospective study with 40 participants resulted in a risk ratio (RR) of 2.55 (95% confidence interval (CI) 0.72 to 9.03; very low‐certainty evidence; Analysis 1.1; Fisher's exact test P = 0.25) (Azfar 2010). There were 141 more deaths out of a thousand with corticosteroids compared to supportive care (95% CI 26 fewer deaths to 730 more). In Azfar 2010, data for mortality were available separately for SJS (RR 3.83, 95% CI 0.67 to 21.88) and TEN (RR 1.71, 95% CI 0.26 to 11.47) respectively, but a formal subgroup analysis was not possible due to the small numbers of participants. See Analysis 1.1.
1.1. Analysis.

Comparison 1: Corticosteroids versus no corticosteroids, Outcome 1: Disease‐specific mortality
Data were not available for ICU length of stay, total hospital length of stay, time to complete re‐epithelialisation, withdrawal due to adverse events, or other adverse events.
See Table 1.
IVIG versus no IVIG
The evidence is very uncertain regarding the effect of IVIG (plus corticosteroids) compared to no IVIG (corticosteroids alone) on disease‐specific mortality (RR 0.33, 95% CI 0.04 to 2.91; 1 observational study; 36 participants; very low‐certainty evidence; Analysis 2.1; Fisher's exact test P = 0.60). There were 11.2% fewer deaths with IVIG plus corticosteroids compared to corticosteroids alone (95% CI 16% fewer to 31.8% more). There was a 67% lower risk of mortality with IVIG plus corticosteroids compared to corticosteroids alone (Jagadeesan 2013).
2.1. Analysis.

Comparison 2: IVIG versus no IVIG, Outcome 1: Disease‐specific mortality
The evidence is very uncertain regarding the effect of IVIG plus corticosteroids compared to corticosteroids alone for the following outcomes: time to complete re‐epithelialisation (days to full skin healing, mean difference (MD) −2.93, 95% CI −4.40 to −1.46; 1 observational study; 36 participants; very low‐certainty evidence; Analysis 2.2) and total hospital length of stay (days, MD −2.00, 95% CI −5.81 to 1.81; 1 observational study; 36 participants; very low‐certainty evidence; Analysis 2.3) (Jagadeesan 2013). This study qualitatively reports the outcome illness sequelae, but does not state in which treatment group the complications occurred. This study also qualitatively reports other adverse events, but does not state in which treatment group the complications occurred. The authors note that bacterial infections was the most common complication encountered overall.
2.2. Analysis.

Comparison 2: IVIG versus no IVIG, Outcome 2: Days to full skin healing
2.3. Analysis.

Comparison 2: IVIG versus no IVIG, Outcome 3: Hospital length of stay
Data were not available for ICU length of stay or withdrawal due to adverse events.
Saraogi 2016 had three participants on IVIG plus corticosteroids and 10 participants on intravenous corticosteroids. This study also included participants on IVIG alone and supportive care alone. Although this study provided mortality data, it was not possible to extract the data for this analysis.
See Table 2.
Etanercept versus no etanercept
No trials were identified for this comparison. See Table 3.
Cyclosporin versus no cyclosporin
No trials were identified for this comparison. See Table 4.
IVIG versus corticosteroids
No trials were identified for this comparison. See Table 5.
Etanercept versus corticosteroids
Etanercept may reduce disease‐specific mortality compared to corticosteroids (RR 0.51, 95% CI 0.16 to 1.63; 1 RCT; 91 participants; low‐certainty evidence; Analysis 3.1). There were 8% fewer deaths with etanercept compared to corticosteroids (95% CI 13.7% fewer to 10.3% more). There was a 49% lower risk of mortality with etanercept compared to corticosteroids (Wang 2018).
3.1. Analysis.

Comparison 3: Etanercept versus corticosteroids, Outcome 1: Disease‐specific mortality
We assessed the overall risk of bias for this result as some concerns due to concerns about bias in selection of the reported result, as mortality was not specified as an outcome in the trial registry record.
Wang 2018 reported that 5 of 48 participants in the etanercept group experienced serious adverse events (sepsis, respiratory failure, and bipolar disorder), and 9 of 43 participants in the corticosteroids group (sepsis, respiratory failure, upper gastrointestinal haemorrhage, stridor and vocal cord palsy). It is unclear from the trial report if any of these adverse events led to discontinuation of treatment. The authors also report that in participants with 10% or greater BSA detachment, the time to complete skin healing was significantly shorter in the etanercept‐treated group compared to the corticosteroid‐treated group (P = 0.010, by Kaplan‐Meier analysis), with the median time to skin healing 14 and 19 days, respectively.
Data were not available for ICU length of stay, time to complete re‐epithelialisation, or total hospital length of stay.
See Table 6.
Cyclosporin versus corticosteroids
No trials were identified for this comparison. See Table 7.
Etanercept versus IVIG
No trials were identified for this comparison. See Table 8.
Cyclosporin versus IVIG
One cohort study with 22 prospective patients provided data for this comparison on the following prespecified outcome: disease‐specific mortality (RR 0.13, 95% CI 0.02 to 0.98; Analysis 4.1; Fisher's exact test P = 0.046) (Gonzalez‐Herrada 2017). There were 43% fewer deaths with cyclosporin compared to other treatments (95% CI 2.0% to 85.5% fewer).
Data were not available for ICU length of stay, total hospital length of stay, time to complete re‐epithelialisation, withdrawal due to adverse events, or other adverse events. We assessed risk of bias as some concerns due to potential confounding effects and selection bias.
See Table 9.
Etanercept versus cyclosporin
No trials were identified for this comparison. See Table 10.
N‐acetylcysteine and infliximab versus infliximab alone
One small RCT with 10 participants provided data for this comparison on the following prespecified outcome: disease‐specific mortality (RR 2.00, 95% CI 0.26 to 15.62; Analysis 5.1; Fisher's exact test P = 1.00) (Paquet 2014). Characterisation of participant deaths included events up to 10 days after initiation of therapy. Otherwise, time to follow‐up was not reported. The overall risk of bias for this result was high due to concerns about bias arising from the randomisation process.
5.1. Analysis.

Comparison 5: N‐acetylcysteine and infliximab versus infliximab alone, Outcome 1: Disease‐specific mortality
Data were not available for ICU length of stay, total hospital length of stay, time to complete re‐epithelialisation, withdrawal due to adverse events, or other adverse events.
Thalidomide versus placebo
One RCT provided data for this comparison on the following prespecified outcome: disease‐specific mortality (RR 2.78, 95% CI 1.04 to 7.40; Analysis 6.1; Fisher's exact test P = 0.027) (Wolkenstein 1998). Participants were followed for up to seven days after treatment or death if it occurred sooner. The overall risk of bias for this result was low.
6.1. Analysis.

Comparison 6: Thalidomide versus placebo, Outcome 1: Disease‐specific mortality
Data were not available for ICU length of stay, total hospital length of stay, time to complete re‐epithelialisation, withdrawal due to adverse events, or other adverse events.
Plasmapheresis versus other treatments
One prospective study with 28 participants provided data for this comparison on the following prespecified outcome: total hospital length of stay (days, MD −7.37, 95% CI −16.09 to 1.35; Analysis 7.1) (Han 2017). Participants were followed from admission to hospital discharge.
7.1. Analysis.

Comparison 7: Plasmapheresis versus other treatments, Outcome 1: Hospital length of stay
Data were not available for disease‐specific mortality, ICU length of stay, time to complete re‐epithelialisation, withdrawal due to adverse events, or other adverse events.
Discussion
Summary of main results
We included nine studies with a total of 308 participants, including adults and children, from seven countries. We included two of these studies in the quantitative meta‐analysis.
We included three RCTs and six prospective, controlled observational studies. Only one of the randomised trials was considered as at low risk of bias overall (Wolkenstein 1998). Of the other two, one was judged at high risk of bias overall due to bias arising from the randomisation process and some concerns due to deviations from the intended interventions and selection of the reported result (Paquet 2014). For the other RCT, we made a risk of bias assessment of some concerns due to selection of the reported result (Wang 2018). All risk of bias assessments for the RCTs related to the outcome disease‐specific mortality. For the five observational studies included in the analysis, all outcome results (i.e. disease‐specific mortality, days to full skin healing, and length of hospital stay) were considered at overall serious risk bias; the major concerns were not addressing confounding and bias in the classification of interventions.
We found very low‐certainty evidence for the outcome of mortality in the following comparisons: corticosteroids versus no corticosteroids (two observational studies, 56 participants, Table 1); IVIG versus no IVIG (one observational study, 36 participants, Table 2); and cyclosporin versus IVIG (one observational study, 22 participants, Table 9). Hence, we are uncertain of the effect of these treatment comparisons.
For the comparison of IVIG versus no IVIG, we found only very low‐certainty evidence for the secondary outcomes reduction in time to complete re‐epithelialisation and reduction in total hospital length of stay (one observational study, 36 participants, Table 2), therefore we are uncertain of their effects. These outcomes were not measured for the comparisons of corticosteroids versus no corticosteroids and cyclosporin versus IVIG.
Based on one study with 91 participants, etanercept may decrease mortality when compared to corticosteroids; however, the 95% CI was wide and included null (low‐certainty evidence). Serious adverse events, such as sepsis and respiratory failure, were reported in both groups, but it was not clear if they led to discontinuation of treatment (Table 6). No other key treatment comparison measured adverse effects leading to discontinuation of SJS/TEN therapy. Time to complete re‐epithelialisation and length of hospital stay were not reported.
We did not find any studies addressing our most important comparisons: cyclosporin versus corticosteroids (Table 7), etanercept versus IVIG (Table 8), cyclosporin versus IVIG (Table 9), or etanercept versus cyclosporin (Table 10).
No studies measured ICU length of stay.
Overall completeness and applicability of evidence
The nine studies included in this review were conducted in a diverse variety of settings, including India, Taiwan, Europe, and China. No studies based in other countries met the inclusion criteria. Study locations included inpatient dermatology wards, an ICU, and burns units, which represent the typical setting where such patients would be treated. We found no studies of our prespecified comparisons between systemic therapies of interest including cyclosporin, etanercept, and IVIG, nor for cyclosporin versus corticosteroids. Comparisons between the above systemic agents versus corticosteroids or supportive care were more commonly represented in our included studies; however, the way in which data were presented and analysed made comparisons unfeasible. The included studies were not able to fully address the objective of this review to assess the effects of all systemic therapies for the treatment of SJS, TEN, and SJS/TEN overlap syndrome. None of the interventions was very well assessed: corticosteroids versus supportive care was the only comparison assessed by more than one study.
Only two studies included paediatric participants, and we were unable to perform subgroup analyses of mortality between paediatric and adult participants in this review. The participants in four studies had a mean age under 40 years, and the other two studies that reported participant mean age had means of 49 and 56 years. A skew towards adult age is expected, as this represents an increasing use of drugs associated with SJS‐TEN in the population overall. Separate data were not provided for elderly adults.
Seven studies reported extractable mortality data but with very limited data on other outcomes or adverse events. Only two studies reported on adverse events, and no studies reported our primary outcome of adverse events leading to discontinuation of SJS/TEN therapy. None of the studies reported on length of stay in ICU or illness sequelae, although one study reported overall illness sequelae for all participants. Three studies reported on time to re‐epithelialisation, and two on length of stay in hospital.
Reporting of the key prognostic characteristics of the included participants was, in general, poor. Specifically, SCORTEN was not consistently reported between studies, which has implications for predicting mortality. For example, a SCORTEN of 0 to 1 is associated with a predicted mortality rate of 3.2%, whereas scores of ≥ 5 are associated with a predicted mortality rate of 90% (Bastuji‐Garin 2000). Other reporting of severity, such as BSA affected, was only available for three studies, with means ranging from 44% to 51%. One study simply reported that 61.5% of the participants had more than 10% of BSA. Reporting of age or sex was done at baseline for most studies, but not always addressed in the analysis. Only two studies reported baseline comorbidities.
Due to the paucity of data, it was not possible to perform any of the following planned subgroup analyses: disease severity (SCORTEN ≥ 3), BSA (≥ 30%), advanced age (≥ 75 years), and co‐interventions.
We identified three ongoing randomised trials, and one completed study awaiting assessment.
Quality of the evidence
We did not find any studies addressing the main comparisons of interest. Of the comparisons for which data were available, the studies were generally small and at high risk of bias. Most of the evidence was from observational studies, and there were concerns regarding selection bias and lack of controlling for confounders. Data were generally sparse, and in most cases, mortality was the only outcome properly reported. We assessed the evidence for all outcome results, apart from one, to be of very low certainty, mainly due to imprecision (small studies and wide confidence intervals). Hence, we are uncertain about the results for these comparisons. The comparison of etanercept versus corticosteroids (Table 6) was based on one RCT and was judged to be low certainty of evidence for mortality. We downgraded the evidence twice due to imprecision, firstly for being based on a small study with few participants, and secondly for having a wide confidence interval that included important benefits and increased risk of harms. Despite the study being unblinded, we decided not to downgrade further, as we considered it unlikely to influence an objective measure such as mortality. We did not downgrade any results for heterogeneity, indirectness, or publication bias.
Potential biases in the review process
During the process of the review, we made several decisions that could affect the results. First, we decided to further restrict our inclusion criteria to only RCTs and prospective, comparative studies. This decision limited the number of participants that could be included from the Gonzalez‐Herrada 2017 study. They conducted a retrospective‐prospective study, taking advantage of the two units that covered most patients from Madrid. Each of these centres/units had its own protocol for managing patients with SJS/TEN. Although this could be considered a natural experiment closer to an RCT, our decision to only consider prospective data limited the number of participants that we were able to include from this study significantly. Second, we originally planned to limit the inclusion of non‐randomised studies to those that adjusted for all our prespecified confounders. This decision would have excluded all the currently included observational studies, thus we decided it could be more informative to flag the risk of bias and include the studies instead.
We did not find any studies addressing our main comparisons of interest. We therefore created empty summary of findings tables for them, and added four other comparisons as summary of findings based on the availability of data.
Agreements and disagreements with other studies or reviews
Due to the sparsity of prospective studies on SJS/TEN, we have looked for different strategies to provide an evidence base for clinicians.
A previous Cochrane Review focused on TEN identified only one RCT, which found a higher mortality on TEN with thalidomide compared to placebo (Majumdar 2002). This RCT is also included in our review.
A review from Australia focused on studies performed in burns centres (Mahar 2014). They identified 20 studies, but focused on describing the different management strategies without evaluating different approaches.
Law 2015 looked for experimental and observational studies assessing the role of corticosteroids on the management of SJS/TEN. They included six observational, retrospective studies. Only one of them showed a positive effect of corticosteroids on mortality.
Barron 2015 addressed the effect of IVIG in 13 studies, 8 with a control group, although most of them retrospective. The focus of the study was to establish whether IVIG improved standardised mortality rate. They claimed the meta‐regression showed a decrease of mortality with an increase of dose.
Zimmermann 2017 did an exhaustive literature search of SJS/TEN studies up to 2012 evaluating mortality. They found only one RCT (the same one as Majumdar 2002) and 157 non‐randomised studies, of which 96 were included in their analysis. They identified corticosteroids and cyclosporin as the most promising systemic immunomodulating therapies for SJS/TEN.
A systematic review that focused on treatment of SJS/TEN in the paediatric population summarised treatment outcomes with biologics (infliximab or etanercept) in case series in 12 patients from 10 identified studies (Sachdeva 2021). The review did not provide an effect estimator for treatment with biologics.
Tsai 2021 performed a systematic review and network meta‐analysis focused on mortality rates and SCORTEN‐based standardised mortality ratio including 67 studies and 2079 participants. They included 10 different treatments, none of which was found to be superior to supportive care. As in our review, thalidomide was associated with a higher mortality rate. When using SCORTEN‐based standardised mortality ratio, the combination of corticosteroids and IVIG was the only treatment to show statistically significant survival benefits. It should be noted that the Tsai 2021 authors had an unequal allocation of the groups in their network meta‐analysis, which they did not adjust for in their results or conclusions.
All reviews agreed on the need for prospective RCTs to provide reliable answers to the effects of interventions.
Authors' conclusions
Implications for practice.
We did not find any studies addressing our most important comparisons: etanercept versus cyclosporin, etanercept versus intravenous immunoglobulin (IVIG), cyclosporin versus corticosteroids, or IVIG versus cyclosporin.
Compared to corticosteroids, etanercept may result in a reduction in mortality; however, the 95% CIs are consistent with possible benefit and possible harm (low‐certainty evidence). Although serious adverse events, such as sepsis and respiratory failure, were reported in both treatment groups, it was not clear if they led to discontinuation of therapy. Only one other study reported on adverse events, finding that bacterial infections were the most common complication encountered overall. The study also qualitatively reported other adverse events, but did not confirm in which treatment group the complications had occurred.
The evidence is very uncertain (very low‐certainty evidence) for the outcome of mortality for the comparisons corticosteroids versus no corticosteroids, IVIG versus no IVIG, and cyclosporin versus IVIG.
The following outcomes were reported for the comparison IVIG versus no IVIG, but very low‐certainty evidence means we are uncertain about the results:
time to complete re‐epithelialisation;
length of hospital stay.
This comparison did not report adverse effects leading to discontinuation of Stevens‐Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) therapy.
The following outcomes were not reported by the comparisons corticosteroids versus no corticosteroids and cyclosporin versus IVIG:
time to complete re‐epithelialisation;
length of hospital stay;
adverse effects leading to discontinuation of SJS/TEN therapy.
The following outcomes were not reported by the comparison etanercept versus corticosteroids:
time to complete re‐epithelialisation;
length of hospital stay.
None of the included studies measured intensive care unit (ICU) length of stay.
The treatment of SJS/TEN varies widely across institutions, and mortality remains high. Etanercept, a tumour necrosis factor‐alpha inhibitor, has emerged as a potential treatment option for people with SJS/TEN. However, the availability and cost may limit its use in resource‐poor settings.
Implications for research.
Limitations to this review include the high risk of bias, selection bias, lack of controlling for confounders in the various studies as well as wide confidence intervals. There is a need for randomised studies, with more participants to reach optimal information size. The optimal information size will depend on SCORe of Toxic Epidermal Necrosis (SCORTEN) scores; for example, in people with scores ≥ 5, to detect a 10% reduction in mortality with 80% power and alpha 0.05, 398 participants would be needed. For SCORTEN scores 0 to 1, 2872 participants would be needed to detect a 50% reduction in mortality, with 80% power and alpha 0.05.
Given the rarity of SJS/TEN, this may be difficult to achieve with single‐centre studies. Larger collaborative, multicentric studies are needed to provide more definitive evidences for the main comparisons considered in this review. In particular, studies are needed comparing cyclosporin, etanercept, IVIG, and corticosteroids to one another, including their effect on mortality, adverse effects, length of time in an ICU, and time to re‐epithelialisation. These outcomes were inconsistently reported in the studies included in this review. There is one ongoing study evaluating cyclosporin versus etanercept versus best supportive care that is adequately powered. Trials for the other comparisons will help to provide a complete view of all treatment options.
Additionally, severity of disease is a critical factor in the prediction of mortality in SJS/TEN. For improved comparison, consistent reporting of validated disease severity scores, such as SCORTEN, are needed. Implementing Core Outcome Sets, with validated scoring systems such as SCORTEN, so that subgroup analyses can be made, is critical for future studies of SJS/TEN. Reporting results for age groups where the risk‐benefit ratio might change (i.e. ≥ 75 years) will improve the applicability of the findings.
Finally, clinicians must rely on medical history, clinical morphology, and histopathology, as there are no validated biomarkers to aid in the diagnosis or prognostication of SJS/TEN. It is important to also report baseline comorbidities of the patients included. An adequate description of supportive care and other co‐interventions is necessary to improve the interpretability of the results in different settings.
Further research on the association of human leukocyte antigens, cytochrome P450 isoforms, and inflammatory markers associated with SJS/TEN is warranted.
History
Protocol first published: Issue 9, 2018
Risk of bias
Acknowledgements
Acknowledgements from the authors
The authors would like to acknowledge Dr Prof Francisco J de Abajo Iglesias for providing the data for the prospective patients included in their study (Gonzalez‐Herrada 2017), and Dr Gabriel Aedo Inostroza for facilitating access to a hard‐to‐reach article.
The authors would also like to thank Robert Boyle and Marialena Trivella, who assisted with risk of bias assessments.
Editorial and peer‐reviewer contributions
Cochrane Skin supported the authors in the development of this review.
The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Robert Boyle and Robert Dellavalle, Cochrane Skin Joint Co‐ordinating Editors
Managing Editor and Assistant Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Laura Prescott and Helen Scott; also Emma Axon (methods guidance) and Information Specialist Liz Doney (search methods)
Cochrane Skin is grateful to the following people:
Copy Editor (copy‐editing and production): Lisa Winer
Peer reviewers* (provided comments and recommendations): Carlo Pincelli (clinical/content review), Doaa Wael (consumer review), Tess Moore, Methods Support Unit (methods review), Ben Carter (statistical review)
*Peer reviewers provided peer‐review comments on this article, but were not otherwise involved in the editorial process or decision making for this article.
Appendices
Appendix 1. Cochrane Skin Specialised Register (CRS Web)
1. (Steven* Johnson Syndrome*):ti,ab,kw AND INREGISTER 2. MESH DESCRIPTOR Stevens‐Johnson Syndrome AND INREGISTER 3. (toxic and epidermal and necrolys*):ti,ab,kw AND INREGISTER 4. (lyell* and syndrome*):ti,ab,kw AND INREGISTER 5. (lyell* and disease*):ti,ab,kw AND INREGISTER 6. #1 OR #2 OR #3 OR #4 OR #5
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Stevens‐Johnson Syndrome] this term only #2 Steven* Johnson Syndrome*:ti,ab,kw #3 sjs:ti,ab,kw #4 (toxic next epidermal next necrolys*):ti,ab,kw #5 (lyell* next syndrome*):ti,ab,kw #6 (lyell* next disease*):ti,ab,kw #7 {OR #1‐#6} #8 cyclosporine:ti,ab,kw #9 MeSH descriptor: [Cyclosporine] explode all trees #10 (steroid* or corticosteroid*):ti,ab,kw #11 MeSH descriptor: [Adrenal Cortex Hormones] this term only #12 corticoid*:ti,ab,kw #13 dexamethasone:ti,ab,kw #14 MeSH descriptor: [Dexamethasone] this term only #15 prednisolone:ti,ab,kw #16 MeSH descriptor: [Prednisolone] this term only #17 methylprednisolone:ti,ab,kw #18 MeSH descriptor: [Methylprednisolone] this term only #19 MeSH descriptor: [Immunoglobulins] this term only #20 (immunoglobulin* or IVIG):ti,ab,kw #21 enbrel:ti,ab,kw #22 etanercept:ti,ab,kw #23 MeSH descriptor: [Etanercept] explode all trees #24 MeSH descriptor: [Tumor Necrosis Factor‐alpha] this term only #25 anti‐tumo?r necrosis factor*:ti,ab,kw #26 anti‐tnf:ti,ab,kw #27 TNF‐alpha inhibitor*:ti,ab,kw #28 anti‐interleukin*:ti,ab,kw #29 infliximab:ti,ab,kw #30 MeSH descriptor: [Infliximab] this term only #31 remicade:ti,ab,kw #32 Plateletpheres*:ti,ab,kw #33 MeSH descriptor: [Plateletpheresis] this term only #34 (platelet and rich and pheres*):ti,ab,kw #35 plasmapheres*:ti,ab,kw #36 MeSH descriptor: [Plasmapheresis] this term only #37 Thalidomid*:ti,ab,kw #38 MeSH descriptor: [Thalidomide] this term only #39 Acetylcystein*:ti,ab,kw #40 MeSH descriptor: [Acetylcysteine] this term only #41 N‐acetylcystein*:ti,ab,kw #42 nac:ti,ab,kw #43 systemic immunomodulating therap*:ti,ab,kw #44 MeSH descriptor: [Glucocorticoids] this term only #45 (glucocorticosteroid* or glucocorticoid*):ti,ab,kw #46 cyclophosphamide:ti,ab,kw #47 MeSH descriptor: [Cyclophosphamide] this term only #48 granulocyte stimulating factor*:ti,ab,kw #49 hemoperfusion:ti,ab,kw #50 MeSH descriptor: [Hemoperfusion] this term only #51 {OR #8‐#50} #52 #7 and #51
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Stevens‐Johnson Syndrome/ 2. Steven$ Johnson Syndrome$.ti,ab,kw. 3. sjs.ti,ab,kw. 4. (toxic and epidermal and necrolys$).ti,ab,kw. 5. (Lyell$ and (syndrome$ or disease$)).ti,ab,kw. 6. or/1‐5 7. cyclosporine.mp. or CYCLOSPORINE/ 8. STEROIDS/ 9. (steroid$ or corticosteroid$).mp. 10. Adrenal Cortex Hormones/ 11. corticoid$.mp. 12. dexamethasone.mp. or DEXAMETHASONE/ 13. prednisolone.mp. or PREDNISOLONE/ 14. METHYLPREDNISOLONE/ or methylprednisolone.mp. 15. Immunoglobulins/ 16. (immunoglobulin$ or IVIG).mp. 17. enbrel.mp. 18. etanercept.mp. or ETANERCEPT/ 19. Tumor Necrosis Factor‐alpha/ 20. anti‐tumo?r necrosis factor$.mp. 21. anti‐tnf.mp. 22. TNF‐alpha inhibitor$.mp. 23. anti‐interleukin$.mp. 24. infliximab.mp. or INFLIXIMAB/ 25. remicade.mp. 26. exp PLATELETPHERESIS/ 27. Plateletpheres$.mp. 28. (platelet and rich and pheres$).mp. 29. PLASMAPHERESIS/ 30. plasmapheres$.mp. 31. THALIDOMIDE/ 32. Thalidomid$.mp. 33. ACETYLCYSTEINE/ 34. Acetylcystein$.mp. 35. N?acetylcystein$.mp. 36. NAC.mp. 37. systemic immunomodulating therap$.mp. 38. Glucocorticoids/ 39. (glucocorticosteroid$ or glucocorticoid$).mp. 40. cyclophosphamide.mp. or CYCLOPHOSPHAMIDE/ 41. granulocyte stimulating factor$.mp. 42. hemoperfusion.mp. or HEMOPERFUSION/ 43. or/7‐42 44. 6 and 43 45. exp animals/ not humans.sh. 46. 44 not 45 47. exp Stevens‐Johnson Syndrome/ 48. Steven$ Johnson Syndrome$.ti,ab. 49. sjs.ti,ab. 50. (toxic and epidermal and necrolys$).ti,ab. 51. (Lyell$ and (syndrome$ or disease$)).ti,ab. 52. or/47‐51 53. cyclosporine.ti,ab,kw. or CYCLOSPORINE/ 54. STEROIDS/ 55. (steroid$ or corticosteroid$).ti,ab,kw. 56. Adrenal Cortex Hormones/ 57. corticoid$.ti,ab,kw. 58. dexamethasone.ti,ab,kw. or DEXAMETHASONE/ 59. prednisolone.ti,ab,kw. or PREDNISOLONE/ 60. METHYLPREDNISOLONE/ or methylprednisolone.ti,ab,kw. 61. Immunoglobulins/ 62. (immunoglobulin$ or IVIG).ti,ab,kw. 63. enbrel.ti,ab,kw. 64. etanercept.ti,ab,kw. or ETANERCEPT/ 65. Tumor Necrosis Factor‐alpha/ 66. anti‐tumo?r necrosis factor$.ti,ab,kw. 67. anti‐tnf.ti,ab,kw. 68. TNF‐alpha inhibitor$.ti,ab,kw. 69. anti‐interleukin$.ti,ab,kw. 70. infliximab.ti,ab,kw. or INFLIXIMAB/ 71. remicade.ti,ab,kw. 72. exp PLATELETPHERESIS/ 73. Plateletpheres$.ti,ab,kw. 74. (platelet and rich and pheres$).ti,ab,kw. 75. PLASMAPHERESIS/ 76. plasmapheres$.ti,ab,kw. 77. THALIDOMIDE/ 78. Thalidomid$.ti,ab,kw. 79. ACETYLCYSTEINE/ 80. Acetylcystein$.ti,ab,kw. 81. N?acetylcystein$.ti,ab,kw. 82. NAC.ti,ab,kw. 83. systemic immunomodulating therap$.ti,ab,kw. 84. Glucocorticoids/ 85. (glucocorticosteroid$ or glucocorticoid$).ti,ab,kw. 86. cyclophosphamide.ti,ab,kw. or CYCLOPHOSPHAMIDE/ 87. granulocyte stimulating factor$.ti,ab,kw. 88. hemoperfusion.ti,ab,kw. or HEMOPERFUSION/ 89. or/53‐88 90. 52 and 89 91. exp animals/ not humans.sh. 92. 90 not 91
Note, there is no restriction by study design as we aim to identify RCTs, cohort studies, and those studies using prospective data from patient registries.
Appendix 4. Embase (Ovid) search strategy
1. exp Stevens Johnson syndrome/ 2. Steven$ Johnson Syndrome$.ti,ab. 3. sjs.ti,ab. 4. (toxic and epidermal and necrolys$).ti,ab. 5. exp toxic epidermal necrolysis/ 6. (Lyell$ and (syndrome$ or disease$)).ti,ab. 7. or/1‐6 8. exp cyclosporine/ 9. cyclosporine.ti,ab,kw. 10. steroid/ 11. (steroid$ or corticosteroid$).ti,ab,kw. 12. adrenal cortex hormone$.ti,ab,kw. 13. corticosteroid/ 14. corticoid$.ti,ab,kw. 15. dexamethasone/ 16. dexamethasone.ti,ab,kw. 17. prednisolone/ 18. prednisolone.ti,ab,kw. 19. methylprednisolone/ 20. methylprednisolone.ti,ab,kw. 21. immunoglobulin/ 22. (immunoglobulin$ or IVIG).ti,ab,kw. 23. enbrel.ti,ab,kw. 24. etanercept/ 25. etanercept.ti,ab,kw. 26. tumor necrosis factor/ 27. anti‐tumo?r necrosis factor$.ti,ab,kw. 28. anti‐tnf.ti,ab,kw. 29. tumor necrosis factor inhibitor/ 30. TNF‐alpha inhibitor$.ti,ab,kw. 31. anti‐interleukin$.ti,ab,kw. 32. infliximab/ 33. infliximab.ti,ab,kw. 34. remicade.ti,ab,kw. 35. thrombocytopheresis/ 36. Plateletpheres$.ti,ab,kw. 37. (platelet and rich and pheres$).ti,ab,kw. 38. plasmapheresis/ 39. plasmapheres$.ti,ab,kw. 40. thalidomide/ 41. Thalidomid$.ti,ab,kw. 42. acetylcysteine/ 43. acetylcystein$.ti,ab,kw. 44. N?acetylcystein$.ti,ab,kw. 45. nac.ti,ab,kw. 46. systemic immunomodulating therap$.ti,ab,kw. 47. glucocorticoid/ 48. (glucocorticosteroid$ or glucocorticoid$).ti,ab,kw. 49. cyclophosphamide.ti,ab,kw. 50. cyclophosphamide/ 51. granulocyte stimulating factor$.ti,ab,kw. 52. hemoperfusion/ 53. hemoperfusion.ti,ab,kw. 54. or/8‐53 55. 7 and 54 56. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 57. human/ or normal human/ 58. 56 and 57 59. 56 not 58 60. 55 not 59
Data and analyses
Comparison 1. Corticosteroids versus no corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Disease‐specific mortality | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.72, 9.03] |
| 1.1.1 SJS | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [0.67, 21.88] |
| 1.1.2 TEN | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.26, 11.47] |
Comparison 2. IVIG versus no IVIG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Disease‐specific mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Days to full skin healing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Hospital length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Etanercept versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Disease‐specific mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Cyclosporin versus IVIG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Disease‐specific mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. N‐acetylcysteine and infliximab versus infliximab alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Disease‐specific mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Thalidomide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Disease‐specific mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Plasmapheresis versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Hospital length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azfar 2010.
| Study characteristics | ||
| Methods |
Study Design: prospective observational comparative study Study Duration: not reported Number of Participants: 40 Study Dates: December 2006 to December 2009 |
|
| Participants |
Setting: patients treated for SJS/TEN at an inpatient dermatology ward of a tertiary care hospital, Jinnah Hospital, Lahore, India Inclusion Criteria: clinical diagnosis of SJS‐TEN, "40 patients of both sexes and all ages, clinically diagnosed as having SJS or TEN, were enrolled. Their detailed history was taken and physical examination was performed". No further details are provided. Exclusion Criteria: none reported Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Data not presented by groups:
Data not reported:
|
|
| Interventions |
Intervention: Corticosteroids (details on dose and duration not provided) (n = 29) Comparator: Supportive care (details not provided) (n = 11) |
|
| Outcomes | Disease‐specific mortality | |
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
Gonzalez‐Herrada 2017.
| Study characteristics | ||
| Methods |
Study Design: prospective controlled study Study Duration: not reported Number of Participants: 22 Study Dates: 2011 to 2015 (prospective data only)* |
|
| Participants |
Setting: 2 inpatient burn units in the community of Madrid, Spain Inclusion Criteria: SJS/TEN over the age of 14; cases were validated by clinical data, photographs, and histology by an expert committee to establish the final diagnosis Exclusion Criteria: not reported Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Data not presented by groups:
Data not reported:
|
|
| Interventions |
Intervention Cyclosporin (oral dosing at 3 mg/kg/day or intravenous 1 mg/kg/day until complete re‐epithelialisation, then taper off (10 mg/day reduction every 48 hours)) (n = 16) Comparator IVIG (continuous infusion at a dose of 0.75 g/kg/day for 4 days (total dose = 3 g/kg) in participants with normal renal function. A lower dose was used in participants with renal insufficiency) (n = 4) Supportive care (n = 1) Systemic corticosteroids (prednisone‐equivalent daily doses ranging from 37.5 to 100 mg for 9 to 12 days) (n = 1) |
|
| Outcomes | All‐cause mortality Expected death rate using SCORTEN Time to stabilisation of BSA involvement Time to re‐epithelialisation start Time to complete re‐epithelialisation |
|
| Funding source | Research grant from the Instituto de Salud Carlos III–Ministerio de Economía, Industria y Competitividad (#PI12/02267), cofunded by FEDER. The Spanish Agency of Medicinal Products and Medical Devices supports the management of the registry and database. | |
| Declarations of interest | None declared. | |
| Notes | *The published cohort of patients included both retrospectively and prospectively recruited patients. Published data represented the entire cohort rather than each arm separately, therefore the study authors kindly provided the data related to the prospective arm of the study for us to use in the quantitative synthesis of this review. Only the data for the prospective arm (n = 22), which were collected from 2011 to 2015, are reported here. | |
Han 2017.
| Study characteristics | ||
| Methods |
Study Design: prospective comparative study Study Duration: not reported Number of Participants: 28 Study Dates: February 2009 to August 2016 |
|
| Participants |
Setting: intensive care unit at a hospital in Xi'an, China Inclusion Criteria: children and adults with TEN or SJS/TEN overlap Exclusion Criteria: not reported Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Intervention
Comparator
Data not reported by group:
|
|
| Interventions |
Intervention: Plasmapheresis (one‐time dose of 1000 mL of Ringer‐Locke and 2000 to 3000 mL of plasma at a rate of 1000 mL an hour) (n = 13) Comparator: Non‐plasmapheresis (details not provided) (n = 15) |
|
| Outcomes | Hospital length of stay (days) | |
| Funding source | This work was supported by the Scientific Fund for the Young Talent of Shaanxi Province (2015KJXX‐06). | |
| Declarations of interest | None declared. | |
| Notes | ||
Jagadeesan 2013.
| Study characteristics | ||
| Methods |
Study Design: prospective observational comparative study Study Duration: all participants were scheduled for follow‐up visits for 6 months Number of Participants: 36 Study Dates: February 2008 to January 2012 |
|
| Participants |
Setting: inpatient dermatology ward at a single tertiary care hospital in India Inclusion Criteria: diagnosis of TEN based on WHO causality definition of certain/probable drug rash, Bastuji‐Garin 1993 criteria, Tzanck smear, complete blood investigations, urine microscopy and culture, chest x‐ray, Veneral Disease Research Laboratory test, ELISA for HIV, antinuclear antibody, blood cultures, pus cultures, and in doubtful cases, skin biopsy Exclusion Criteria: contraindications to the use of IVIG or corticosteroids Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Overall study group:
Intervention:
Comparator:
|
|
| Interventions |
Intervention: Low‐dose IVIG (GAMMA I.V., Bharat Serumes and Vaccines Limited) 0.2 to 0.5 g/kg cumulative dose divided over 3 days, and dexamethasone 0.1 to 0.3 mg/kg/d intravenous rapidly tapered within 1 to 2 weeks according to response (n = 18) Comparator: Dexamethasone 0.1 to 0.3 mg/kg/d intravenous rapidly tapered within 1 to 2 weeks according to response (n = 18) Supportive Care Measures (for all): "Patients in both groups were given intensive supportive treatment along with prompt and meticulous ophthalmic care and care of oral and genital mucosa" |
|
| Outcomes | Disease‐specific mortality Adverse events leading to discontinuation of therapy Other adverse events Mean days to full skin healing Mean length of hospital stay, days Illness sequelae (not reported by study arm) |
|
| Funding source | None reported. | |
| Declarations of interest | None declared. | |
| Notes | ||
Kakourou 1997.
| Study characteristics | ||
| Methods |
Study Design: prospective comparative study Study Duration: not reported Number of Participants: 16 Study Dates: 1989 to 1994 |
|
| Participants |
Setting: inpatient paediatric unit in Athens, Greece Inclusion Criteria: children admitted to the department within 3 days from onset of rash who had a diagnosis of EMM based on the following criteria:
Exclusion Criteria: not reported Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Intervention:
Comparator:
Data not reported:
|
|
| Interventions |
Intervention: Corticosteroids (bolus infusion of methylprednisolone (4 mg/kg/day) for 2 more days after the fever had subsided and no new lesions had developed) (n = 10) Comparator: Supportive care only (n = 6) |
|
| Outcomes | Mortality | |
| Funding source | None reported. | |
| Declarations of interest | None declared. | |
| Notes | ||
Paquet 2014.
| Study characteristics | ||
| Methods |
Study Design: open‐label randomised controlled trial Study Duration: not reported: "assessments were performed 48h after treatment initiation"; the authors did not comment on follow‐up/assessments past 48 h, but characterisation of participant deaths included events up to 10 days after initiation of therapy Number of Participants Randomised: 10 Study Dates: not reported |
|
| Participants |
Setting: inpatient burn units of 2 tertiary care hospitals, University Hospital of Liege and Military Hospital of Brussels in Belgium Inclusion Criteria: diagnosis of TEN based on Bastuji‐Garin 1993 criteria and skin biopsy Exclusion Criteria: none reported Baseline Data: Overall:
Intervention:
Comparator:
Data not reported:
|
|
| Interventions |
Intervention: N‐acetylcysteine (Lysomucil, Zambone) diluted in 5% glucose solution intravenous over 20‐hour period (150 mg/kg in 250 mL of solution during the first hour, followed by 150 mg/kg in 500 mL during a 4‐hour period, and finally 150 mg/kg in 1000 mL for 15 h) and infliximab (Remicade, MSD) 5 mg/kg intravenous over 2‐hour period (n = 5) Comparator: N‐acetylcysteine (Lysomucil, Zambone) diluted in 5% glucose solution intravenous over 20‐hour period (150 mg/kg in 250 mL of solution during the first hour, followed by 150 mg/kg in 500 mL during a 4‐hour period, and finally 150 mg/kg in 1000 mL for 15 h) (n = 5) Supportive Care Measures (for all): "Each patient was placed on fluidized beds and benefited from supportive and antiseptic care measures including daily baths" |
|
| Outcomes | Disease‐specific mortality | |
| Funding source | Grant from Fonds d'Investissement de la Recherche Scientifique of the University Hospital of Liege MSD pharmaceutical company provided free samples of infliximab. |
|
| Declarations of interest | The authors declared no conflicts of interest directly relevant to the content of the trial. | |
| Notes | ||
Saraogi 2016.
| Study characteristics | ||
| Methods |
Study Design: prospective observational study Study Duration: not reported Number of Participants: 43 Study Dates: 16 months (dates not reported) |
|
| Participants |
Setting: inpatient unit not otherwise specified Inclusion Criteria: not reported Exclusion Criteria: not reported Baseline Data: Disease classification, number of participants with SJS: SJS‐TEN overlap: TEN
Data not reported:
|
|
| Interventions |
Intervention Intravenous corticosteroids (dose/duration not reported) (n = 10) IVIG (dose/duration not reported) (n = 5) Combination of intravenous corticosteroids and IVIG (dose/duration not reported) (n = 3) Comparator Supportive care (n = 25) |
|
| Outcomes | Arrest of disease progression Time to complete re‐epithelialisation Mortality |
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | We contacted the study authors 3 times via e‐mail to request further data; however, no response was received. | |
Wang 2018.
| Study characteristics | ||
| Methods |
Study Design: open‐label randomised controlled trial Study Duration: participants were followed for an additional 3 weeks after hospital discharge Number of Participants Randomised: 91 Study Dates: 2009 to 2015 |
|
| Participants |
Setting: the study was undertaken at Chuang Gung Memorial Hospital, the largest medical health system in Taiwan, which receives SJS‐TEN referral cases from other hospitals in northern Taiwan. Inclusion Criteria: patients over 4 years of age diagnosed with "probable" or "definite" SJS‐TEN using the Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) phenotypic criteria, and for most patients histopathological analysis including direct immunofluorescence and blister granulysin levels. All patients were also "assessed and diagnosed by at least two experienced dermatologists". Exclusion Criteria: (a) pregnant or breastfeeding women; (b) patients with a previous allergy to any TNF‐α biological product; (c) patients with active or latent tuberculosis confirmed by chest x‐ray; (d) patients with severe, active infection and septicaemia; (e) carriers of active hepatitis B or C; (f) suspected carriers of HIV with a CD4+ T cell count below 200; and (g) patients with poor compliance or safety concerns, as judged by an investigator Baseline Data: Overall study group:
Intervention:
Comparator:
Data not reported:
|
|
| Interventions |
Intervention: Etanercept 25 mg (50 mg if weight > 65 kg) subcutaneous twice weekly "until skin lesions healed" (n = 48) Comparator: Prednisolone 1 to 1.5 mg/kg/day intravenous "until skin lesions healed" (n = 43) Supportive Care Measures (for all): No details provided. |
|
| Outcomes | Disease‐specific mortality Other adverse events Days to full skin healing, median (mean and SD not provided) |
|
| Funding source | This work was supported by grants from the National Science Council of Taiwan (MOST101‐2320‐B‐010‐072‐MY3, MOST101‐2321‐B‐010‐027, MOST101‐2628‐B‐182‐001‐MY3, MOST101‐2321‐B‐182‐008, MOST102‐2314‐B‐010‐014‐MY3, MOST102‐2321‐B‐182‐006, and MOST103‐2321‐B‐182‐001); the CGMH (BMRPG‐290011, OMRPG‐2C0011, OMRPG‐2C0021, CMRPG‐290051‐3, CMRPG‐3D0351‐2, CMRPG‐3D0361‐2, CORPG3F0041‐2, and CLRPG‐2E0051); and the Ministry of Health and Welfare of Taiwan (DOHW103‐TDU‐B‐212‐113003, DOHW104‐TDU‐B‐212‐113003, and DOHW105‐TDU‐B‐212‐113003). | |
| Declarations of interest | The authors declare that they have no conflicts of interest. | |
| Notes | ||
Wolkenstein 1998.
| Study characteristics | ||
| Methods |
Study Design: double‐blind randomised controlled trial Study Duration: participants were followed for up to 7 days after treatment (or death if it occurred sooner) Number of Participants Randomised: 22 Study Dates: May 1995 to September 1996 |
|
| Participants |
Setting: patients with TEN treated at 9 centres in France "representing recruitment of about half the cases of TEN in France" Inclusion Criteria: patients over 18 years of age with epidermal detachment of greater than 10% BSA, with disease evolution of less than 4 days after the first mucocutaneous symptoms at presentation, predicted survival greater than 48 h, consistent skin biopsy (showing full‐thickness detachment of epidermis), exclusion of staphylococcal scalded‐skin syndrome, and review of clinical photographs Exclusion Criteria: patients with skin detachment > 90% BSA at presentation, a lack of progression of skin detachment during the previous 48 h, and if they had already received "therapies that have been claimed to influence TEN evolution (systemic corticosteroids, plasmapheresis, cyclosporin, cyclophosphamide) or other experimental drugs targeted at TNF‐ (such as oxpentifylline or monoclonal antibodies to TNF‐)". Women of child‐bearing age required a negative serum pregnancy test before inclusion. Baseline Data: Intervention:
Comparator:
Data not reported:
|
|
| Interventions |
Intervention: Thalidomide 200 mg by mouth twice a day x 5 days (n = 12) Comparator: Placebo tablets of same frequency and duration (n = 10) Supportive Care Measures (for all): No details provided. |
|
| Outcomes | Disease‐specific mortality | |
| Funding source | None reported. | |
| Declarations of interest | None declared. | |
| Notes | ||
BSA: Body surface area; ELISA: enzyme‐linked immunoassay; EMM: erythema multiforme major; IVIG: Intravenous immune globulin; SCORTEN: Severity of illness SCORe of Toxic Epidermal Necrosis; SD: standard deviation; SJS: Stevens‐Johnson Syndrome; TEN: toxic epidermal necrolysis; TNF‐α: anti–tumor necrosis alpha; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Auyeung 2018 | Wrong study design |
| Campione 2003 | Wrong study design |
| Choudhury 2008 | Wrong study design |
| Danby 2007 | Wrong study design |
| De Juan 2002 | Wrong study design |
| Del 1996 | Wrong study design |
| Dequidt 2019 | Wrong study design |
| Dhar 1996 | Wrong study design |
| Didona 2015 | Wrong study design |
| Eliades 2020 | Wrong study design |
| Feldmeyer 2011 | Wrong study design |
| Firoz 2012 | The Journal of the American Academy of Dermatology published an erratum stating that institutional review board approval was not obtained as stated in the paper. An expression of concern informed that the institution reported a significant discrepancy between the number of patients recorded in the hospital database as having SJS/TEN and those reported in the paper. Furthermore, the original paper states that all diagnoses were biopsy‐confirmed, but only 75% of patients are recorded in the hospital database as having a confirmatory biopsy. Based on this information, we decided to exclude the study. |
| Gomez‐Flores 2020 | Wrong study design |
| Halebian 1986 | Wrong study design |
| Hertl 1993 | Wrong study design |
| Hirahara 2013 | Wrong study design |
| Journet 1980 | Wrong study design |
| Kaushik 2019 | Wrong study design |
| Khapii 1987 | Wrong study design |
| Kim 2002 | Wrong study design |
| Krajewski 2019 | Wrong study design |
| Lalevee 2018 | Wrong study design |
| Lalosevic 2015 | Wrong study design |
| Mockenhaupt 2000 | Wrong study design |
| Morgado‐Carrasco 2019 | Wrong study design |
| Mori 2019 | Wrong study design |
| NCT02037347 | Wrong study design |
| Nixon 1985 | Wrong study design |
| Noe 2018 | Wrong study design |
| Paquet 2006 | Wrong study design |
| Patterson 1992 | Wrong study design |
| Pham 2020 | Wrong study design |
| Pisani 1981 | Wrong study design |
| Revuz 1978 | Wrong study design |
| Sasidharanpillai 2015 | Wrong study design |
| Shah 2019 | Wrong study design |
| Stella 2001 | Wrong study design |
| Sudusinghe 2018 | Wrong study design |
| Tao 2013 | Wrong study design |
| Trautmann 1998 | Wrong study design |
| Viard 1998 | Wrong study design |
| Wolkenstein 2002 | Wrong study design |
| Yeung 2005 | Wrong study design |
| Zajicek 2012 | Wrong study design |
| Zhang 2017 | Wrong study design |
SJS/TEN: Stevens‐Johnson syndrome/toxic epidermal necrolysis
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR‐TRC‐13003550.
| Methods |
Study Type: interventional study Study Design: randomised parallel controlled trial |
| Participants |
Inclusion Criteria
Exclusion Criteria
Age minimum: 18 Age maximum: 65 Gender: both |
| Interventions | Glucocorticoid group: IVIG 400 mg/kg/d, for 5 days after the withdrawal Glucocorticoid plus immunoglobulin group: IVIG 400 mg/kg/d for 5 days, then IVIG 400 mg/kg/d, for 5 days after the withdrawal |
| Outcomes |
Primary Outcome(s)
Secondary Outcome(s)
|
| Notes | We contacted the authors three times via e‐mail (twice in English on 20 May 2020 and 23 February 2021 and once in Chinese on 5 March 2021), but received no response. |
CT: computed tomography IVIG: intravenous immunoglobulin SCORTEN: SCORe of Toxic Epidermal Necrosis
Characteristics of ongoing studies [ordered by study ID]
NCT02739295.
| Study name | G‐CSF in the Treatment of Toxic Epidermal Necrolysis (NeupoNET) |
| Methods |
Allocation: randomised Intervention Model: parallel assignment |
| Participants | 10 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Experimental: G‐CSF An intravenous dose of 5 μg/kg of G‐CSF (Neupogen) will be administered daily, from admission (day 0) to day 4. Drug: recombinant granulocyte colony‐stimulating factor Other Name: Neupogen (Amgen) Placebo Comparator: placebo An intravenous dose of 5 mL of sodium chloride 0.9% will be administered daily, from admission (day 0) to day 4. |
| Outcomes |
Primary Outcome Measures Time for complete cutaneous healing, considered as healing of 90% of the body surface area Changes in immunohistologic typing (MAC 387, CD15, CD68, CD45Ro, fact XIIIa) Neutrophilic count
Secondary Outcome Measures WBC count WBC formula
|
| Starting date | July 2016 |
| Contact information | Anne‐Françoise Rousseau, MD, PhD; afrousseau@chu.ulg.ac.be ClinicalTrials.gov ID: NCT02739295 |
| Notes | Estimated Study Completion Date: July 2022 |
NCT02987257.
| Study name | NATIENS: Optimal Management and Mechanisms of SJS/TEN (NATIENS) |
| Methods |
Allocation: randomised Intervention Model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | 267 participants 18 years of age and older Inclusion Criteria
|
| Interventions | Subjects will be allocated 1:1:1 to cyclosporine plus best supportive care, etanercept plus best supportive care or best supportive care alone. Placebo Comparator: Harmonised supportive care with placebo cyclosporin days 0 to 14 or placebo etanercept at days 0 and 3 Active Comparator: Cyclosporin 5 mg/kg twice a day on days 0 to 14 with harmonized supportive care Active Comparator: Etanercept 50 mg subcutaneous day 0 and day 3 with harmonized supportive care |
| Outcomes |
Primary Outcome Measures Patients will be assessed by 2 independent raters (burn surgeons, dermatologists, wound care experts) to determine the day of full re‐epithelialisation. For disagreements on the day of re‐epithelialisation, the case with supporting photographs will be referred to an independent adjudication committee comprised of a minimum of 3 experts (a burn surgeon, dermatologist, wound care expert). In the instance of death will be the maximum period of re‐epithelialisation (21 days + 1).
Secondary Outcome Measures Progression will be considered significant if there are any new blistering lesions or any new detached or detachable skin. Number of participants with mortality at 30 days, 3 months, and 1 year Number of participants with actual mortality versus expected mortality for each SCORTEN risk Time to cessation of acute ocular involvement acutely, then extent of ocular involvement at follow‐up will be assessed by 2 independent ophthalmology experts. Will be tracked by photography Incidence of nosocomial infections Duration of time in hospital Adverse events due to assigned treatment arm
Other Outcome Measures Featured exploratory secondary outcome measuring difference overtime within an individual as well as differences between treatment arms at various time measurements. C0 and C2 levels will be measured in all patients with analysis after unblinding in those randomised to cyclosporin (n = 89). Relationship between levels and adverse drug events and treatment outcome will be measured.
|
| Starting date | April 2021 |
| Contact information | Contact: Elizabeth J Phillips; elizabeth.j.phillips@vumc.org ClinicalTrials.gov ID: NCT02987257 |
| Notes | Estimated Study Completion Date: July 2027 |
NCT03585946.
| Study name | Outcomes in Stevens Johnsons syndrome and toxic epidermal necrolysis |
| Methods |
Observational Model: cohort Time Perspective: prospective |
| Participants | 750 participants 18 years of age and older Sampling Method: non‐probability sample Study Population Adults hospitalised with a diagnosis of Stevens Johnson syndrome/toxic epidermal necrolysis confirmed by examination of a dermatologist and/or skin biopsy plus described appropriate clinical findings (epidermal necrosis plus 2 involved mucosal surfaces) presenting within 1 week of disease onset Criteria Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Cyclosporin Intravenous Immunoglobulin Etanercept Steroids Drug: site‐specific standard‐of‐care comparison Patient outcomes will be assessed and compared based on the medication they receive, which will be assigned based on the standard of care at each enrolling site. |
| Outcomes |
Primary Outcome Measures Per cent of deaths in each group Days until no new lesions arise from time of initiation of therapy Days until skin has completely healed Time from hospital admission to discharge
|
| Starting date | 1 September 2018 |
| Contact information | Daniela Kroshinsky, Associate Professor of Dermatology, Massachusetts General Hospital |
| Notes | Estimated Study Completion Date: 31 August 2021 |
C0: conventionally measured trough level C2: cyclosporin levels at 2 hours postdose G‐CSF: granulocyte colony‐stimulating factor IL‐15: interleukin‐15 SJS/TEN: Stevens‐Johnson syndrome/toxic epidermal necrolysis WBC: white blood cell
Differences between protocol and review
Since the publication of the review protocol, we have made the following changes to the methodology of the review, with justifications.
Our initial search strategy was to include any prospective studies, including randomised controlled trials (RCTs) and observational prospective studies, including single‐arm trials. However, we opted to further restrict our study selection to only RCTs and prospective comparative studies at the analysis stage due to a lack of validated tools for assessing the risk of bias in uncontrolled studies. Our final list of included studies therefore included only RCTs and prospective observational comparative studies. We also added cross‐over trials as an exclusion criterion given the inability to obtain an adequate washout period for patients with this condition.
In the protocol, we documented that non‐randomised studies would be included in the analysis only when researchers adjusted for the following prespecified confounders: disease duration, disease severity, use of diagnostic criteria, baseline comorbidities, age distribution, duration of follow‐up, and the use of co‐interventions. However, this approach would have markedly limited the number of studies available to include in the analysis, therefore we instead included non‐randomised studies that did not adjust for prespecified confounders in our analyses and assessed the possible impact of these confounders using the ROBINS‐I tool (Sterne 2016).
Based on our protocol, the most important comparisons of interventions that we sought to prepare summary of findings tables for were as follows.
Etanercept versus cyclosporin
Etanercept versus intravenous immunoglobulin (IVIG)
IVIG versus cyclosporin
Cyclosporin versus corticosteroids
However, data for these comparisons were not available, therefore we included the following comparisons as the main summary of findings tables in the review.
Cyclosporin versus other treatments
Corticosteroids versus supportive care
Etanercept versus corticosteroids
IVIG versus supportive care
We initially planned to use the original Cochrane risk of bias tool. However, as we were including assessments of observational evidence, it became clear that the Cochrane RoB 2 tool was more appropriate to assess in pair with the ROBINS‐I tool. In order to assess these points we made the following changes.
We specified that we were interested in the effect of assignment in the Assessment of risk of bias in included studies section.
We stated that we would assess risk of bias for each of the outcomes of the summary of findings table.
We added further detail on how we would deal with cluster studies.
We indicated that we would apply the guidance in the Cochrane Handbook for Systematic Reviews of Interventions to assess whether to downgrade for study limitations.
We clarified that we planned to perform sensitivity analysis by removing studies at serious risk of bias.
We made explicit that we would exclude studies from pooled or narrative analysis if the ROBINS‐I assessment was critical, as recommended in the Cochrane Handbook.
Contributions of authors
AL and BO were the contact people with the editorial base. JB came up with the concept of the review. JB and BW determined the primary and secondary outcomes in the Methods. JB and BW performed the preliminary literature search to draft the Background and rationale for the review. BO co‐ordinated contributions from the co‐authors and wrote the final draft of the review. AL, AS, EM, BO, RP, or AJ screened papers against the eligibility criteria. AL and BO obtained data on ongoing and unpublished studies. AL, JPP, BO, AJ, and RP appraised the quality of papers. AL, FS, BO, AJ, and RP extracted data for the review and sought additional information about papers. AL, JPP, BO, AJ, and RP entered data into Revman 5 or RevMan Web. AL, JPP, BO, AJ, and RP analysed and interpreted data. AL, JPP, BO, AJ, and RP worked on the Methods section. AL and BO drafted the clinical sections of the Background and responded to clinical comments of the referees. JPP responded to the methodological comments of the referees. TR provided statistical advice and approved final manuscript. AJ wrote and addressed the response to comments.
All authors approved the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Audrey Jacobsen reports award of American Academy of Dermatology 2019 Cochrane Scholarship; support for travel to Cochrane Colloquium, personal payment.
Bayanne Olabi reports support for travel to meetings from Celgene; personal payment.
Annie Langley: declared that they have no conflict of interest.
Jennifer Beecker: declared that they have no conflict of interest.
Eric Mutter: declared that they have no conflict of interest.
Amanda Shelley: declared that they have no conflict of interest.
Brandon Worley reports a prior National Institute of Allergy and Infectious Diseases (NIAID) grant for the development of an experimental therapeutics clinical trial for SJS/TEN; payment to institution. However, transfer of responsibility completed to Vanderbilt and no direct affiliation at this time with the continuation of the work. This work was separate from the present publication, which summarises what is currently known about available and widely used treatments.
Timothy Ramsay: declared that they have no conflict of interest.
Arturo Saavedra: declared that they have no conflict of interest.
Roses Parker: declared that they have no conflict of interest.
Fiona Stewart: declared that they have no conflict of interest.
Jordi Pardo Pardo: declared that they have no conflict of interest.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
New
References
References to studies included in this review
Azfar 2010 {published data only}
- Azfar NA, Zia MA, Malik LM, Khan AR, Jahangir M.Role of systemic steroids in the outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. Journal of Pakistan Association of Dermatologists 2010;20(3):158-62. [Google Scholar]
Gonzalez‐Herrada 2017 {published and unpublished data}
- González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al.Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. Journal of Investigative Dermatology 2017;137(10):2092-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Han 2017 {published data only}
Jagadeesan 2013 {published data only}
- Jagadeesan S, Sobhanakumari K, Sadanandan SM, Ravindran S, Divakaran MV, Skaria L, et al.Low dose intravenous immunoglobulins and steroids in toxic epidermal necrolysis: a prospective comparative open-labelled study of 36 cases. Indian Journal of Dermatology, Venereology and Leprology 2013;79(4):506-11. [DOI] [PubMed] [Google Scholar]
Kakourou 1997 {published data only}
- Kakourou T, Klontza D, Soteropoulou F, Kattamis C.Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. European Journal of Pediatrics 1997;156(2):90-3. [DOI] [PubMed] [Google Scholar]
Paquet 2014 {published data only}
- Paquet P, Jennes S, Rousseau AF, Libon F, Delvenne P, Piérard GE.Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. A proof-of-concept study. Burns 2014;40(8):1707-12. [DOI: 10.1016/j.burns.2014.01.027] [DOI] [PubMed] [Google Scholar]
Saraogi 2016 {published data only (unpublished sought but not used)}
- Agrawal P, Mahajan S, Khopkar U.Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective study of epidemiology and clinical course. Allergy: European Journal of Allergy and Clinical Immunology 2013;68(Suppl 97):140. [Google Scholar]
- Saraogi P, Mahajan S, Khopkar U.Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective study of epidemiology and clinical course. British Journal of Dermatology 2016;175(Suppl 1):46. [Google Scholar]
Wang 2018 {published data only}
- Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al.Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. Journal of Clinical Investigation 2018;128(3):985-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolkenstein 1998 {published data only}
- Wolkenstein P, Latarget J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al.Randomized double-blind comparison of thalidomide versus placebo in toxic epidermal necrolysis: overmortality in treated group. Journal of Dermatological Science 1998;16(Suppl 1):S44. [DOI: 10.1016/S0923-1811(98)83259-3] [DOI] [Google Scholar]
- Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al.Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352(9140):1586-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Auyeung 2018 {published data only}
- Auyeung J, Lee M.Successful treatment of Stevens-Johnson Syndrome with cyclosporine and corticosteroid. Canadian Journal of Hospital Pharmacy 2018;71(4):272-5. [PMC free article] [PubMed] [Google Scholar]
Campione 2003 {published data only}
- Campione E, Marulli GC, Carrozzo AM, Chimenti MS, Costanzo A, Bianchi L.High-dose intravenous immunoglobulin for severe drug reactions: efficacy in toxic epidermal necrolysis. Acta Dermato-Venereologica 2003;83(6):430-2. [DOI] [PubMed] [Google Scholar]
Choudhury 2008 {published data only}
- Choudhury CD, Agarwal V.Toxic epidermal necrolysis managed with immunoglobulin. Medical Journal, Armed Forces India 2008;64(3):272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danby 2007 {published data only}
- Danby FW.Ciclosporin use in toxic epidermal necrolysis. British Journal of Dermatology 2007;156(2):390-1. [DOI] [PubMed] [Google Scholar]
De Juan 2002 {published data only}
- De Juan Martin F, Bouthelier Moreno M, Marin Bravo Ma C, Melendo Gimeno J.Toxic epidermal necrolysis treated with intravenous immunoglobulin. Anales Espanoles de Pediatria 2002;56(4):370-1. [PubMed] [Google Scholar]
Del 1996 {published data only}
- Del Pozo J, De Lucas R, Suarez-Marrero MC, Fernandez-Diaz ML, Casado M, Garcia-Torres V.Toxic epidermal necrolysis: treatment with cyclophosphamide. Journal of Dermatological Treatment 1996;7(2):127. [Google Scholar]
Dequidt 2019 {published data only}
- Dequidt L, Darrigade AS, Camus T, Taieb A, Milpied B.Intravenous immunoglobulins for delayed wound healing associated with toxic epidermal necrolysis. European Journal of Dermatology: EJD 2019;29(2):229-31. [DOI] [PubMed] [Google Scholar]
Dhar 1996 {published data only}
- Dhar S.Systemic corticosteroids in toxic epidermal necrolysis. Indian Journal of Dermatology, Venereology, and Leprology 1996;62(4):270-1. [PubMed] [Google Scholar]
Didona 2015 {published data only}
- Didona B, Paradisi A, Didona D, Abeni D.Etanercept for toxic epidermal necrolysis: a confirm on efficacy and safety. Journal of Investigative Dermatology 2015;1:S72. [Google Scholar]
Eliades 2020 {published data only}
- Eliades P, Fonseca M, Harp J.Use of etanercept in a series of pediatric patients with Stevens-Johnson Syndrome-Toxic Epidermal Necrolysis spectrum disease. JAMA Dermatology 2020;29:29. [DOI] [PubMed] [Google Scholar]
Feldmeyer 2011 {published data only}
- Feldmeyer L, Kerdel FA, French LE.Use of intravenous immunoglobulin in toxic epidermal necrolysis. Archives of Dermatology 2011;147(12):1440-1. [DOI] [PubMed] [Google Scholar]
Firoz 2012 {published data only}
- Editors.Erratum. Journal of the American Academy of Dermatology 2013;69(6):1048. [DOI] [PubMed] [Google Scholar]
- Editors.Expression of Concern. Journal of the American Academy of Dermatology 2014;71(3):583. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Firoz BF, Henning JS, Zarzabal LA, Pollock BH.Toxic epidermal necrolysis: five years of treatment experience from a burn unit. Journal of the American Academy of Dermatology 2012;67(4):630-5. [DOI] [PubMed] [Google Scholar]
Gomez‐Flores 2020 {published data only}
- Gomez-Flores M, Villarreal-Villarreal CD, Welsh O, Cardenas-de la Garza JA, Sanchez-Meza E, Ancer-Arellano J, et al.Lower than expected mortality with dexamethasone in toxic epidermal necrolysis: 30-year experience of a Northern Mexico reference hospital. International Journal of Dermatology 2020;59(1):e4-5. [DOI] [PubMed] [Google Scholar]
Halebian 1986 {published data only}
- Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT.Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Annals of Surgery 1986;204(5):503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hertl 1993 {published data only}
- Hertl M, Bohlen H, Merk HF.Efficacy of cyclophosphamide in toxic epidermal necrolysis. Journal of the American Academy of Dermatology 1993;28(3):511. [DOI] [PubMed] [Google Scholar]
Hirahara 2013 {published data only}
- Hirahara K, Kano Y, Sato Y, Horie C, Okazaki A, Ishida T, et al.Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. Journal of the American Academy of Dermatology 2013;69(3):496-8. [DOI] [PubMed] [Google Scholar]
Journet 1980 {published data only}
- Journet M.Treatment of polymorph erythema and Stevens-Johnson syndrome. Union Medicale du Canada 1980;109(10):1494-5. [PubMed] [Google Scholar]
Kaushik 2019 {published data only}
- Kaushik A, Kumaran MS, Vinay K, Mehta S, Singh P.Cyclosporine-induced toxic leukoencephalopathy in toxic epidermal necrolysis. International Journal of Dermatology 2019;58(2):e36-8. [DOI] [PubMed] [Google Scholar]
Khapii 1987 {published data only}
- Khapii KK, Kulikova LP, Kil'diushevskii AV.Plasmapheresis in the combined therapy of Lyell's syndrome. Anesteziologiia I Reanimatologiia 1987;2:61-2. [PubMed] [Google Scholar]
Kim 2002 {published data only}
- Kim KJ, Jee MS, Han MH, Choi JH, Sung KJ, Moon KC, et al.The effect of high-dose intravenous immunoglobulin for the treatment of toxic epidermal necrolysis. Korean Journal of Dermatology 2002;40(7):766-71. [Google Scholar]
Krajewski 2019 {published data only}
- Krajewski A, Mazurek MJ, Mlynska-Krajewska E, Piorun K, Knakiewicz M, Markowska M.Toxic Epidermal Necrolysis therapy with TPE and IVIG-10 years of experience of the Burns Treatment Center. Journal of Burn Care & Research 2019;40(5):652-7. [DOI] [PubMed] [Google Scholar]
Lalevee 2018 {published data only}
- Lalevee S, Audureau E, Riou A, Colin A, Anquetin M, Barau C, et al.Cytokine profile in 133 patients with severe cutaneous adverse reactions. Clinical and Translational Allergy 2018;8(Suppl 3):P01. [Google Scholar]
Lalosevic 2015 {published data only}
- Lalosevic J, Nikolic M, Gajic-Veljic M, Skiljevic D, Medenica L.Stevens-Johnson syndrome and toxic epidermal necrolysis: a 20-year single-center experience. International Journal of Dermatology 2015;54(8):978-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mockenhaupt 2000 {published data only}
- Mockenhaupt M, Schlingmann J, Schopf E.Immunomodulating therapy in Stevens-Johnson syndrome and toxic epidermal necrolysis. H+G Zeitschrift fur Hautkrankheiten 2000;75(7-8):467. [Google Scholar]
Morgado‐Carrasco 2019 {published data only}
- Morgado-Carrasco D, Fusta-Novell X, Iranzo P.FR-ciclosporin as a first-line treatment in epidermal necrolysis. Actas Dermo-Sifiliograficas 2019;110(7):601-3. [DOI] [PubMed] [Google Scholar]
Mori 2019 {published data only}
- Mori F, Liccioli G, Parronchi P, Barni S, Capone M, Sarti L, et al.Aetiopathogenesis of severe cutaneous adverse reactions (SCARS) in children: a 9-year experience in a tertiary-care paediatric hospital setting. Allergy: European Journal of Allergy and Clinical Immunology 2019;74(Suppl 106):30. [DOI] [PubMed] [Google Scholar]
NCT02037347 {published data only}
- NCT02037347.Study to evaluate the use of palifermin to treat toxic epidermal necrolysis. clinicaltrials.gov/ct2/show/NCT02037347 (first received 15 January 2014).
Nixon 1985 {published data only}
- Nixon PGF, Freeman LJ.Asymptomatic hyperuricaemia and allopurinol induced toxic epidermal necrolysis. British Medical Journal 1985;291(6493):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noe 2018 {published data only}
- Noe MH, Mostaghimi A, Rosenbach M, Shinkai K, Micheletti RG.Selective use of cyclosporine for Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis may exclude patients with poor prognostic factors. Journal of Investigative Dermatology 2018;138(9):2068-72. [DOI] [PubMed] [Google Scholar]
Paquet 2006 {published data only}
- Paquet P, Kaveri S, Jacob E, Pirson J, Quatresooz P, Pierard GE.Skin immunoglobulin deposition following intravenous immunoglobulin therapy in toxic epidermal necrolysis. Experimental Dermatology 2006;15(5):381-6. [DOI] [PubMed] [Google Scholar]
Patterson 1992 {published data only}
- Patterson R, Grammer LC, Greenberger PA, Lawrence ID, Zeiss CR, Detjen PF, et al.Stevens-Johnson syndrome (SJS): effectiveness of corticosteroids in management and recurrent SJS. Allergy and Asthma Proceedings 1992;13(2):89-95. [DOI] [PubMed] [Google Scholar]
Pham 2020 {published data only}
- Pham CH, Gillenwater TJ, Garner WL.Re: Combination therapy for Toxic Epidermal Necrolysis: It is time for anti-TNFa biologics comparison. Burns 2020;46(1):245-6. [DOI] [PubMed] [Google Scholar]
Pisani 1981 {published data only}
- Pisani M, Ruocco V.Beneficial effect of hyperbaric oxygen on toxic epidermal necrolysis and pemphigus vulgaris. Annali Italiani di Dermatologia Clinica e Sperirentale 1981;35(4):581-4. [Google Scholar]
Revuz 1978 {published data only}
- Revuz J, Laroche L, Touraine R.The treatment of acute epidermal necrolysis (Lyell's syndrome). The contribution of two recent techniques [Traitement de la nécrolyse épidermique aiguë (Syndrome de Lyell). Apport de deux techniques récentes]. La Nouvelle Presse Medicale 1978;7(47):4273-6. [PubMed] [Google Scholar]
Sasidharanpillai 2015 {published data only}
- Sasidharanpillai S, Riyaz N, Khader A, Rajan U, Binitha MP, Sureshan DN.Severe cutaneous adverse drug reactions: a clinicoepidemiological study. Indian Journal of Dermatology 2015;60(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2019 {published data only}
- Shah R, Chen ST, Kroshinsky D.Use of cyclosporine for the treatment of Steven Johnson Syndrome/Toxic Epidermal Necrolysis. Journal of the American Academy of Dermatology 2019;85(2):512-513. [DOI] [PubMed] [Google Scholar]
Stella 2001 {published data only}
- Stella M, Cassano P, Bollero D, Clemente A, Giorio G.Toxic epidermal necrolysis treated with intravenous high-dose immunoglobulins: our experience. Dermatology 2001;203(1):45-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sudusinghe 2018 {published data only}
- Sudusinghe H, Pirabakaran S, Nanayakkara K, Seneviratne J, Samaraweera S.Patterned behaviour and response to current treatments among children with Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum: a prospective study. Australian Journal of Dermatology 2018;59(Suppl 1):16-7. [Google Scholar]
Tao 2013 {published data only}
- Tao Y, Wang Y, Ye L.Clinical efficacy of hemoperfusion in 10 children with severe drug eruption. Pediatric Nephrology 2013;28(8):1674. [Google Scholar]
Trautmann 1998 {published data only}
- Trautmann A, Klein CE, Kampgen E, Brocker EB.Severe bullous drug reactions treated successfully with cyclophosphamide. British Journal of Dermatology 1998;139(6):1127-8. [DOI] [PubMed] [Google Scholar]
Viard 1998 {published data only}
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al.Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282(5388):490-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wolkenstein 2002 {published data only}
- Wolkenstein P.Thalidomide and emergent conditions. Annales de Dermatologie et de Venereologie 2002;129:WK0718. [Google Scholar]
Yeung 2005 {published data only}
- Yeung CK, Lam LK, Chan HH.The timing of intravenous immunoglobulin therapy in Stevens-Johnson syndrome and toxic epidermal necrolysis. Clinical and Experimental Dermatology: Viewpoints in Dermatology 2005;30(5):600-2. [DOI] [PubMed] [Google Scholar]
Zajicek 2012 {published data only}
- Zajicek R, Pintar D, Broz L, Suca H, Konigova R.Toxic epidermal necrolysis and Stevens-Johnson syndrome at the Prague Burn Centre 1998-2008. Journal of the European Academy of Dermatology and Venereology 2012;26(5):639-43. [DOI] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang F, Zhou J.Toxic epidermal necrolysis: 13 years of experience in the management at a Department of Dermatology in China. Cutaneous and Ocular Toxicology 2017;36(1):19-24. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐TRC‐13003550 {published data only}
- ChiCTR-TRC-13003550.Use of glucocorticoid alone or plus intravenous immunoglobulin(IVIG) for the treatment of severe drug eruption. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-13003550 (first received 3 September 2013).
References to ongoing studies
NCT02739295 {published data only}
- NCT02739295.G-CSF in the treatment of toxic epidermal necrolysis. clinicaltrials.gov/ct2/show/NCT02739295 (first received 15 April 2016).
NCT02987257 {published data only}
- NCT02987257.NATIENS: Optimal management and mechanisms of SJS/TEN (NATIENS). clinicaltrials.gov/show/NCT02987257 (first received 8 December 2016).
NCT03585946 {published data only}
- NCT03585946.Outcomes in Stevens Johnsons Syndrome and Toxic Epidermal Necrolysis. clinicaltrials.gov/show/NCT03585946 (first received 13 July 2018).
Additional references
Arevalo 2000
- Arévalo JM, Lorente JA, González-Herrada C, Jiménez- Reyes J.Treatment of toxic epidermal necrolysis with cyclosporin A. Journal of Trauma 2000;48(3):473-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barron 2015
- Barron SJ, Del Vecchio MT, Aronoff SC.Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational studies. International Journal of Dermatology 2015;54(1):108-15. [DOI] [PubMed] [Google Scholar]
Bastuji‐Garin 1993
- Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC.Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Archives of Dermatology 1993;129(1):92-6. [PubMed] [Google Scholar]
Bastuji‐Garin 2000
- Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P.SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. Journal of Investigative Dermatology 2000;115(2):149-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campione 2003
- Campione E, Marulli GC, Carrozzo AM, Chimenti MS, Costanzo A, Bianchi L.High-dose intravenous immunoglobulin for severe drug reactions: efficacy in toxic epidermal necrolysis. Acta Dermato-Venereologica 2003;83(6):430-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chave 2005
- Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE.Toxic epidermal necrolysis: current evidence, practical management and future directions. British Journal of Dermatology 2005;153(2):241-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheung 2013
- Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, Kwan P.HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia 2013;54(7):1307-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2004
- Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al.Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428(6982):486. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence.Version accessed 5 June 2018. Melbourne, Australia: Veritas Health Innovation, 2018. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG.Chapter 10. Analyzing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
de Sica‐Chapman 2010
- Sica-Chapman A, Williams G, Soni N, Bunker CB.Granulocyte colony-stimulating factor in toxic epidermal necrolysis (TEN) and Chelsea & Westminster TEN management protocol [corrected]. British Journal of Dermatology 2010;162(4):860-5. [DOI] [PubMed] [Google Scholar]
Dietrich 1995
- Dietrich A, Kawakubo Y, Rzany B, Mockenhaupt M, Simon JC, Schöpf E.Low N-acetylating capacity in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Experimental Dermatology 1995;4(5):313-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dijkers 2013
- Dijkers M.Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development. ktdrr.org/products/update/v1n5/dijkers_grade_ktupdatev1n5.pdf (accessed prior to 5 June 2018).
Dodiuk‐Gad 2015
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, Cartotto R, Fish J, Shear NH.Treatment of toxic epidermal necrolysis in North America. Journal of the American Academy of Dermatology 2015;73(5):876-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eden 2011
- Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist. In: Eden J, Levit L, Berg A, et al, editors(s). Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
Eldridge 2020
- Eldridge S, Campbell MK, Campbell MJ, Drahota AK, Giraudeau B, Reeves BC, et al.Revised Cochrane risk of bias tool for randomized trials (RoB 2). Additional considerations for cluster-randomized trials (RoB 2 CRT). www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials (accessed 20 January 2021).
Famularo 2007
- Famularo G, DiDona B, Canzona F, Girardelli CR, Cruciani G.Etanercept for toxic epidermal necrolysis. Annals of Pharmacotherapy 2007;41(6):1083-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Faye 2005
- Faye O, Roujeau JC.Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IV Ig): clinical experience to date. Drugs 2005;65(15):2085-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 5 June 2018. Available at gradepro.org.
Gubinelli 2009
- Gubinelli E, Canzona F, Tonanzi T, Raskovic D, Didona B.Toxic epidermal necrolysis successfully treated with etanercept. Journal of Dermatology 2009;36(3):150-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hall 1992
- Hall AG, Tilby MJ.Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Reviews 1992;6(3):163-73. [DOI] [PubMed] [Google Scholar]
Hasan 2020
- Hasan MJ, Rabbani R.Intravenous N-acetylcysteine in severe cutaneous drug reaction treatment: a case series. SAGE Open Medical Case Reports 2020;8:2050313X2093470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from handbook.cochrane.org.
Higgins 2021b
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021c
- Higgins JPT, Eldridge S, Li T.Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hoffman 2021
- Hoffman M, Chansky PB, Bashyam AR, Boettler MA, Challa N, Dominguez A, et al.Long-term physical and psychological outcomes of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. JAMA Dermatology 2021 May 5 [Epub ahead of print]. [DOI: 10.1001/jamadermatol.2021.1136] [PMID: ] [DOI] [PMC free article] [PubMed]
Hsu 2016
- Hsu DY, Brieva J, Silverberg NB, Silverberg JI.Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. Journal of Investigative Dermatology 2016;136(7):1387-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hung 2005
- Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al.HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proceedings of the National Academy of Sciences of the United States of America 2005;102(11):4134-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarrett 1997
- Jarrett P, Rademaker M, Havill J, Pullon H.Toxic epidermal necrolysis treated with cyclosporin and granulocyte colony stimulating factor. Clinical and Experimental Dermatology 1997;22(3):146-7. [PMID: ] [PubMed] [Google Scholar]
Kirchhof 2014
- Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP.Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. Journal of the American Academy of Dermatology 2014;71(5):941-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klausner 1996
- Klausner JD, Freedman VH, Kaplan G .Thalidomide as an anti-TNF-α inhibitor: implications for clinical use. Clinical Immunology and Immunopathology 1996;81:219-23. [DOI] [PubMed] [Google Scholar]
Kohanim 2016
- Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, et al.Stevens-Johnson syndrome/toxic epidermal necrolysis – a comprehensive review and guide to therapy. I. Systemic disease. Ocular Surface 2016;14(1):2-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lalosevic 2015
- Lalosevic J, Nikolic M, Gajic-Veljic M, Skiljevic D, Medenica L.Stevens-Johnson syndrome and toxic epidermal necrolysis: a 20-year single-center experience. International Journal of Dermatology 2015;54(8):978-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Law 2015
- Law EH, Leung M.Corticosteroids in Stevens-Johnson Syndrome/toxic epidermal necrolysis: current evidence and implications for future research. Annals of Pharmacotherapy 2015;49(3):335-42. [DOI] [PubMed] [Google Scholar]
Lawless 1986
- Lawless JF.Regression methods for Poisson process data. Journal of the American Statistical Association 1986;82(399):808-15. [DOI: 10.2307/2288790] [DOI] [Google Scholar]
Mahar 2014
- Mahar PD, Wasiak J, Hii B, Cleland H, Watters DA, Gin D, et al.A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns 2014;40(7):1245-54. [DOI] [PubMed] [Google Scholar]
McCormack 2011
- McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al.HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. New England Journal of Medicine 2011;364(12):1134-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuinness 2020
- McGuinness LA, Higgins JPT.Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2020;12(1):55-61. [DOI: 10.1002/jrsm.1411] [DOI] [PubMed] [Google Scholar]
Napolitano 2013
- Napolitano M, Giampetruzzi AR, Didona D, Papi M, Didona B.Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus successfully treated with a single dose of etanercept: report of three cases. Journal of the American Academy of Dermatology 2013;69(6):E303-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nickoloff 2008
- Nickoloff BJ.Saving the skin from drug-induced detachment. Nature Medicine 2008;14(12):1311-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JPT, Sterne JAC.Chapter 13. Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Paradisi 2014
- Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B.Etanercept therapy for toxic epidermal necrolysis. Journal of the American Academy of Dermatology 2014;71(2):278-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patel 2013
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C.A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian Journal of Dermatology, Venereology and Leprology 2013;79(3):389-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patmanidis 2012
- Patmanidis K, Sidiras A, Dolianitis K, Simelidis D, Solomonidis C, Gaitanis G, et al.Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Reports in Dermatological Medicine 2012;2012:915314. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poizeau 2018
- Poizeau F, Gaudin O, Le Cleach L, Duong TA, Hua C, Hotz C, et al.Ciclosporine for epidermal necrolysis: absence of beneficial effect in a retrospective cohort of 174 patients - exposed/unexposed and propensity-score matched analyses. Journal of Investigative Dermatology 2018;138(6):1293-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Prins 2003
- Prins C, Kerdel FA, Padilla RS, Hunziker T, Chimenti S, Viard I, et al.Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: multicenter retrospective analysis of 48 consecutive cases. Archives of Dermatology 2003;139(1):26-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rai 2008
- Rai R, Srinivas CR.Suprapharmacologic doses of intravenous dexamethasone followed by cyclosporine in the treatment of toxic epidermal necrolysis. Indian Journal of Dermatology, Venereology and Leprology 2008;74(3):263-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reese 2011
- Reese D, Henning JS, Rockers K, Ladd D, Gilson R.Cyclosporine for SJS/TEN: a case series and review of the literature. Cutis 2011;87(1):24-9. [PMID: ] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web).Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Revuz 1987
- Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, et al.Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Archives of Dermatology 1987;123(9):1160-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Robak 2001
- Robak E, Robak T, Góra-Tybor J, Chojnowski K, Strzelecka B, Waszczykowska E, et al.Toxic epidermal necrolysis in a patient with severe aplastic anemia treated with cyclosporin A and G-CSF. Journal of Medicine 2001;32(1-2):31-9. [PMID: ] [PubMed] [Google Scholar]
Romanelli 2008
- Romanelli P, Schlam E, Green JB, Trent JT, Ricotti C, Elgart GW, et al.Immunohistochemical evaluation of toxic epidermal necrolysis treated with human intravenous immunoglobulin. Giornale Italiano di Dermatologia e Venereologia 2008;143(4):229-33. [PMID: ] [PubMed] [Google Scholar]
Roujeau 1995
- Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al.Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. New England Journal of Medicine 1995;333(24):1600-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sachdeva 2021
- Sachdeva M, Mufti A, Kim P, Maliyar K, Sibbald C.Biologic treatment in pediatric Stevens-Johnson syndrome/toxic epidermal necrolysis: a systematic review. Journal of the American Academy of Dermatology 2021;85(4):1011-3. [DOI] [PubMed] [Google Scholar]
Schunemann 2022a
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH.Chapter 14. Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schunemann 2022b
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al.Chapter 15. Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Scott‐Lang 2014
- Scott-Lang V, Tidman M, McKay D.Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatric Dermatology 2014;31(4):532-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sekula 2013
- Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al.Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Journal of Investigative Dermatology 2013;133(5):1197-204. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2013
- Singh GK, Chatterjee M, Verma R.Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian Journal of Dermatology, Venereology and Leprology 2013;79(5):686-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stella 2001
- Stella M, Cassano P, Bollero D, Clemente A, Giorio G.Toxic epidermal necrolysis treated with intravenous high dose immunoglobulins: our experience. Dermatology 2001;203(1):45-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al.ROBINS-1: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Su 2017
- Su S-C, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen C-B, et al.Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal necrolysis. Journal of Investigative Dermatology 2017;137(5):1065-73. [DOI] [PubMed] [Google Scholar]
Sullivan 1996
- Sullivan JR, Watson A.Lamotrigine-induced toxic epidermal necrolysis treated with intravenous cyclosporin: a discussion of pathogenesis and immunosuppressive management. Australian Journal of Dermatology 1996;37(4):208-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Trent 2008
- Trent JT, Kirsner RS, Romanelli P, Kerdel FA.Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: the University of Miami experience. Archives of Dermatology 2008;139(1):39-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tristani‐Firouzi 2002
- Tristani-Firouzi P, Petersen MJ, Saffle JR, Morris SE, Zone JJ.Treatment of toxic epidermal necrolysis with intravenous immunoglobulin in children. Journal of the American Academy of Dermatology 2002;47(4):548-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsai 2021
- Tsai TY, Huang IH, Chao YC, Li H, Hsieh TS, Wang HH, et al.Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. Journal of the American Academy of Dermatology 2021;84(2):390-7. [DOI] [PubMed] [Google Scholar]
Valeyrie‐Allanore 2010
- Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al.Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. British Journal of Dermatology 2010;163(4):847-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Viard 1998
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al.Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282(5388):490-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
White 2018
- White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al.SJS/TEN 2017: Building multidisciplinary networks to drive science and translation. Journal of Allergy and Clinical Immunology Practice 2018;6(1):38-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wojtkiewicz 2008
- Wojtkiewicz A, Wysocki M, Fortuna J, Chrupek M, Matczuk M, Koltan A, et al.Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Dermato-Venereologica 2008;88(4):420-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamane 2016
- Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y, Nakamura K, Kambara T, et al.Retrospective analysis of Stevens–Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients. Allergy International 2016;65(1):74-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2016
- Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al.Incidence of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: a nationwide population-based study using national health insurance database in Korea. PLOS ONE 2016;11(11):e0165933. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaki 1995
- Zaki I, Patel S, Reed R, Dalziel KL.Toxic epidermal necrolysis associated with severe hypocalcaemia, and treated with cyclosporin. British Journal of Dermatology 1995;133(2):337-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zarate‐Correa 2013
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, Serrano-Reyes C.Toxic epidermal necrolysis successfully treated with infliximab. Journal of Investigational Allergology & Clinical Immunology 2013;23(1):61-3. [PMID: ] [PubMed] [Google Scholar]
Zimmermann 2017
- Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al.Systemic immunomodulating therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: a systematic review and meta-analysis. JAMA Dermatology 2017;153(6):514-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Langley 2018
- Langley A, Worley B, Pardo Pardo J, Beecker J, Ramsay T, Saavedra A, et al.Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013130. [DOI: 10.1002/14651858.CD013130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Majumdar 2002
- Majumdar S, Mockenhaupt M, Roujeau JC, Townshend AP.Interventions for toxic epidermal necrolysis. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD001435. [DOI: 10.1002/14651858.CD001435] [DOI] [PMC free article] [PubMed] [Google Scholar]