Significance
The authors propose that odors are consciously perceived or not, depending on whether the olfactory cortex succeeds in activating the endopiriform nucleus—a structure that, in turn, is capable of activating multiple downstream brain areas. The authors further propose that the cellular mechanisms of endopiriform nucleus activation are an attenuated form of cellular events that occur during epileptic seizure initiation. If correct, the authors’ hypothesis could help explain the mechanisms of action of certain general anesthetics.
Keywords: consciousness, endopiriform nucleus, kindling, synchronization, claustrum
Abstract
Odors of similar intensity may be perceived or not by human subjects. Perceived odors correlate with brain magnetic fields, delayed some hundreds of milliseconds, that are not present for unperceived ones. How might this occur? The endopiriform nucleus is an excitable structure, considered part of the claustrum, that is interconnected with the primary olfactory (piriform) cortex. A procedure called kindling allows the endopiriform nucleus to generate epileptiform activities in vitro that are delayed ∼100 ms after a stimulus, suggesting a mechanism for delayed activity. Using a detailed computational model of the piriform cortex, consistent with an in vitro experiment, we show that the addition of neurons with endopiriform properties could allow similar stimuli to generate either brief responses or prolonged ones, depending on parameters such as a persistent Na+ conductance. Brief responses putatively correlate with lack of conscious perception, and prolonged responses correlate with the presence thereof.
Crick and Koch (1) suggested that the claustrum might play a role in consciousness, given its widespread reciprocal connectivity with multiple cortical regions. By way of caution, however, some data indicate that connectivity between claustral principal neurons may be limited (2), raising questions as to how consciousness might be sustained. Nevertheless, endopiriform principal neurons (multipolar cells) are reciprocally interconnected (3), and this nucleus is considered to be part of the claustrum, at least in primates (4). As well, the endopiriform nucleus is interconnected with the primary olfactory cortex or piriform cortex. These data led to a hypothesis that the endopiriform nucleus might play a role in initiating olfactory conscious perception.
Two pieces of experimental evidence support the hypothesis: 1) the existence of delayed synchronized neuronal bursting in the endopiriform nucleus in vitro after a procedure called kindling (for example, bathing tissue in low external [Mg2+]) that renders the tissue prone to epileptiform bursting and 2) the existence of delayed MEG (magnetoencephalographic) signals in human subjects who consciously perceive an odor.
With respect to piece 1 above, Hoffmann and Haberly (5–8), following on earlier in vivo observations of epileptic properties of the endopiriform nucleus (9), observed that a kindled piriform/endopiriform brain slice could generate a synchronized burst of activity following a localized stimulus, with delays of tens of milliseconds or more, and this would occur despite the presence of synaptic inhibition. [This latter phenomenon had been observed previously in other epilepsy models (10).]
With respect to piece 2 above, in accord with the notion that a given sensory stimulus may be perceived or not [and perception perhaps involving an “ignition” process (11)], it has been noted that odor stimuli, of comparable intensity, evoke different MEG signals; the MEG distributions over the brain depend on whether the odor was perceived or not, with odor perception evoking more widespread and delayed activation over timescales in the hundreds of milliseconds (12).
We, therefore, asked whether the above observations might be mechanistically linked in the following way; there is cellular machinery within the endopiriform nucleus—enhanced by the kindling process but present in a less intense form under normal conditions—that could generate, or not generate, sustained signals capable of widespread communication, the latter being presumed to lead to perception.
Results
In beginning to address this question, of particular note are the intrinsic burst properties of at least some multipolar neurons, the principal cell type of the endopiriform nucleus (13, 14), with evidence that persistent sodium conductance contributes to depolarizing events in these cells (14); the presence of persistent sodium conductances, gNa(P), in other cortical neurons (15); and the control of gNa(P) by neuromodulators known to be important in controlling brain states (16–18). To investigate this question, we used a detailed computational model of piriform cortex, designed to account for cell assemblies arising with odor stimulation (19); to this model, we added a subnetwork of endopiriform multipolar neurons, cells with intrinsic burst properties that interconnect with each other as well as with piriform pyramidal neurons (“L2pyr” and “L3pyr” for layers 2 and 3 pyramids, respectively) (Materials and Methods and Fig. 1). Our database was 133 network and single-cell simulations in addition to the simulations of ref. 19.
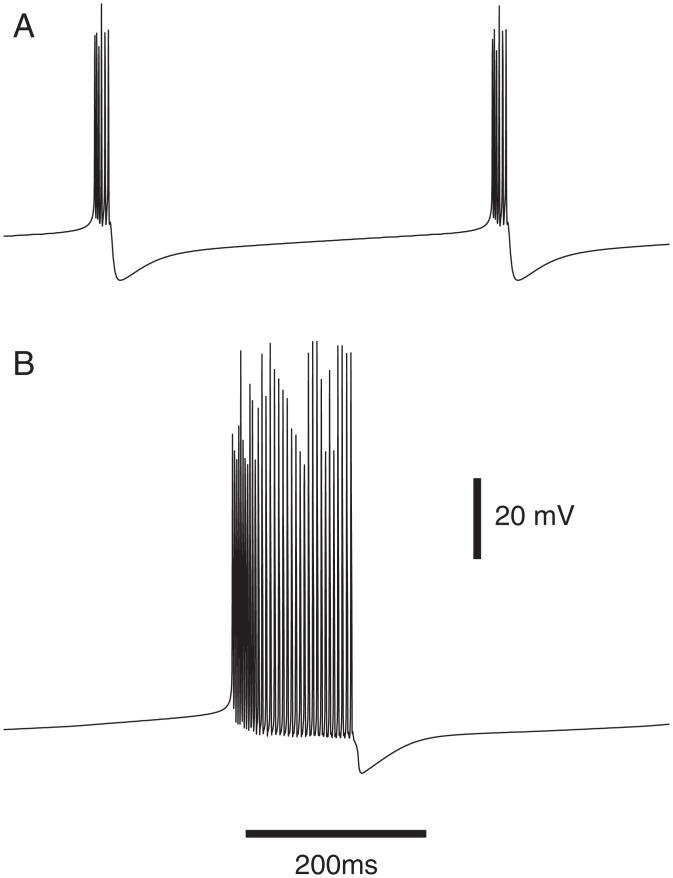
Fig. 1.
Single-model multipolar neuron behavior (soma voltage) with small and large gNa(P): (A) 0.5 nA injected into the soma and density of persistent gNa = 0.005 × the density of the transient gNa; (B) 0.35 nA injected into the soma and density of persistent gNa = 0.05 × the density of the transient gNa. Injected currents were constant and lasted 2.5 s.
Because the purpose of this paper is to present a mechanistic hypothesis, we did not attempt an exhaustive search of the parameter space, but rather, we present specific examples that illustrate the relevant physiological principles and that indicate a path toward future experiments—the same approach as in the modeling portion of ref. 20.
We first confirmed (Fig. 2) that the network model could replicate the activity patterns—delayed bursts after local stimulation—observed by Hoffman and Haberly in vitro (5–8). This type of collective behavior, a consequence of cell-intrinsic properties and recurrent synaptic excitation, follows established principles concerning the spread of firing among principal neurons during states of altered synaptic inhibition (10, 20). Of particular importance is that each principal neuron (in this case, deep multipolar neurons) connects to more than one other and that enough principal neurons are initially excited that multiple stages of population firing growth can occur. In the simulation of Fig. 2, each multipolar neuron receives synaptic input from five others. These physiological principles are reviewed in ref. 21.
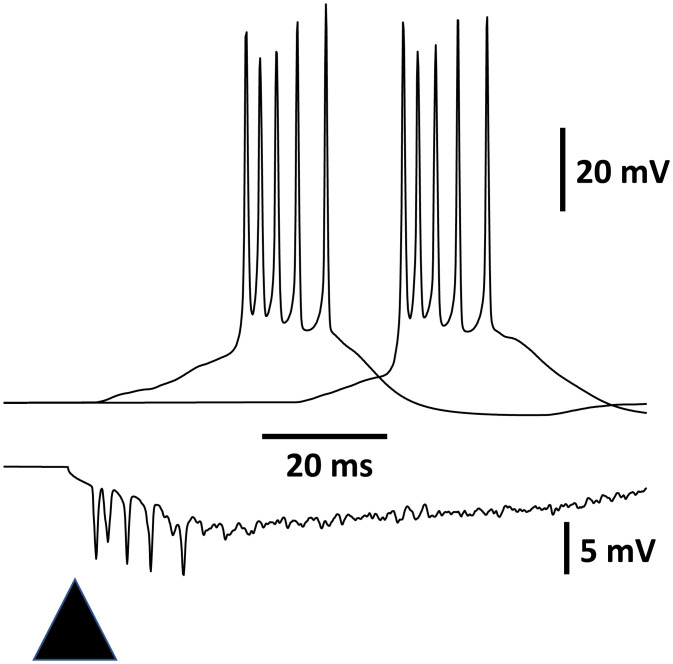
Fig. 2.
Delayed bursting in the model endopiriform nucleus. Traces in Upper are somatic potentials of two model multipolar neurons (of 500); the trace in Lower is the inverted average of multipolar somata, approximating a field. The triangle marks a 5-ms 0.6-nA current pulse to 50 of the multipolar neurons, which were randomly chosen. The density of gNa(P) was 0.001 × the density of fast transient gNa. Compare with figure 2 of ref. 7, which illustrates activity in a kindled endopiriform nucleus in vitro.
We next examined conditions under which the piriform plus endopirform network model would generate a firing pattern, in which piriform and endopiriform neurons both participate together or instead, a pattern occurs mostly confined to piriform pyramidal neurons. The first such pattern would putatively correspond to a perceived olfactory response, while the second type of pattern would putatively correspond to a registered but nonperceived olfactory response. Three main classes of parameters were varied: the mean frequency of afferent (lateral olfactory tract [LOT]) spikes, the connectivity within the endopiriform and to/from the piriform, and the intrinsic properties of multipolar neurons (varied by setting different densities of persistent gNa).
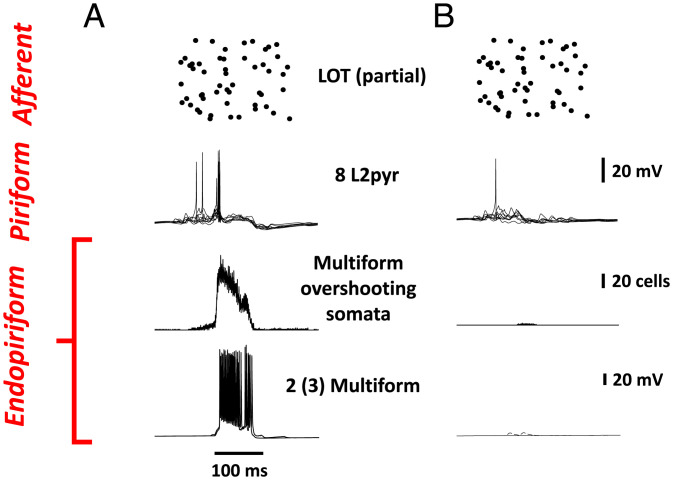
Fig. 3A illustrates a situation where sufficient (but sparse) LOT activation combined with sufficient multipolar excitability [in this case, from gNa(P)] leads to sustained collective bursting within the endopirform nucleus and also, time-limited reactivation of pyramidal cells within the piriform cortex. Such intense activation could readily spread not only from the endopiriform/claustrum but also, to other connected regions, such as the amygdala, the orbitofrontal cortex, and the lateral entorhinal cortex. In contrast (Fig. 3B), limited endopiriform activation can occur if the multipolar neurons are not “bursty” enough (in our model, because of limited persistent gNa—recalling, as noted above, that this conductance is subject to neuromodulation). SI Appendix, Fig. 1 illustrates model behavior, where network parameters are as in Fig. 3 but using two intermediate values of persistent gNa density in the mulitpolar neurons. Fig. 3 and SI Appendix, Fig. 1 suggest that a threshold density of persistent gNa is required for endopiriform collective bursting. This notion could possibly be tested with the application of drugs that block currents contributing to bursting, including persistent gNa but also, Ca2+ channel subtypes.
Fig. 3.
Sustained model endopiriform bursting, following afferent stimulation, when persistent gNa in multipolar neurons is large enough. (A) The rasters in Upper show firing times of 100 (of 500) afferent axons, representing the LOT. Note the delayed firing of L2pyrs due to the sustained activity seen in the multipolar neurons (Lower). The density of gNa(P) was 0.5 × the density of fast transient gNa. (SI Appendix, Fig. 1 shows smaller values of this parameter.) The mean interval between LOT spikes was 400 ms. (B) A comparable simulation with gNa(P) 0.05 × the density of fast transient gNa. The afferent stimulation was exactly as in A. Note the much reduced multipolar firing and the synaptic potentials in multipolar neurons. A similar simulation with a mean interval between LOT spikes of 500 ms is shown in Fig. 4; in that case, both L2pyr and multipolar activities are present but limited.
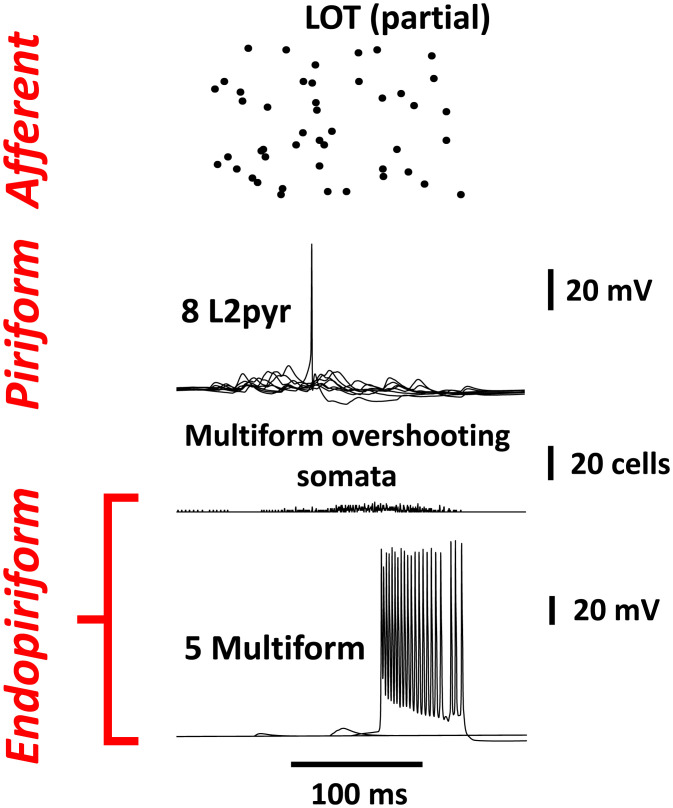
Another example of afferent stimulation evoking limited or virtually no endopiriform activation is illustrated in Fig. 4. Thus, limited activation can also occur if LOT inputs are below threshold frequencies. Other structural parameters limiting endopiriform activation are the densities and strengths of synaptic connections from the piriform cortex or within the endopiriform itself. SI Appendix, Figs. 2 and 3 show examples of the effects of altering within-endopiriform structural parameters. These data indicate that—other things being equal—the strength of recurrent synaptic excitation within the endopiriform is critical for collective bursting, provided that connectivity is above the percolation limit (one multiform cell exciting, on average, more than one other). SI Appendix, Fig. 4 shows further examples of the effects of LOT mean frequency (other parameters remaining fixed); SI Appendix, Fig. 4 also indicates critical behavior in the model endopiriform network as another parameter is varied.
Fig. 4.
An example of the much reduced delayed activity, with large persistent gNa but less afferent stimulation (as compared with the stimulation used in Fig. 3). The mean interval between LOT spikes was 500 ms, compared with 400 ms in Fig. 3. In the present case, only 17 (of 500) multipolar neurons generate bursts. Initial L2pyr activity is reduced, and intense delayed firing does not occur. Such a pattern is proposed to correspond to an odor that is detectable but does not lead to conscious perception. SI Appendix, Fig. 4 shows further examples of varying the LOT mean interval.
Discussion
Our hypothesis then is that activation of the endopiriform nucleus, with its recurrent connections and intrinsic membrane conductances that are subject to neuromodulation, could be a determining element for 1) the odor response leading to widespread brain activation and hence, conscious awareness or 2) an odor response remaining relatively confined but still able to influence behavior. Experimental recordings of endopiriform activity during in vivo odor stimulation, particularly with combined intracellular/extracellular techniques, will help determine the validity of this hypothesis (22).
It is interesting that the anesthetic agents propofol (23) and isoflurane (24) block gNa(P), suggesting that persistent neuronal activity as described here could be a contributing mechanism for general anesthesia. It is also striking that cellular mechanisms apparently permissive for consciousness—persistent synchronized activity depending on recurrent synaptic excitation—are also mechanisms central to epilepsy, a condition that in many patients (depending on seizure type), renders them unconscious.
While we have here concentrated attention on gNa(P) as a relevant membrane conductance in endopiriform neurons following experimental data of Tseng and Haberly (14) and also, recognizing related data in CA1 pyramidal neurons (25), we are nevertheless well aware of contributions made by Ca2+ conductances to dendritic electrogenesis, intrinsic bursting, and plateau generation (26, 27) (among many other references). Indeed, Tseng and Haberly (14) themselves discuss gCa in multipolar neurons, and we have incorporated various types of gCa into our own models of neocortical pyramidal cells (28). Again, this is a matter requiring further experimental study.
Materials and Methods
The model used here is built upon a previous detailed model of a piece of piriform cortex designed to study cell assemblies (19); an additional collection of deep multipolar principal cells was then added, intended to represent a portion of the endopiriform nucleus. The multipolar cells were interconnected with each other and with some of the piriform cells. We first briefly describe the piriform portion, recapitulating portions of the methods section of ref. 19. Further details for this portion are in ref. 19, following the principles of ref. 28.
The piriform part of the network model contained 2,700 multicompartment (∼100 compartments each), multiconductance neurons, with 500 afferent axons representing LOT. There were 1,000 L2pyrs; 500 L3pyrs; 500 semilunar cells; and 200 superficial feed-forward interneurons of two types, one sort to correspond to horizontal cells (100) and another sort for neurogliaform cells (100). There were 500 deep feedback interneurons: fast-spiking “basket” cells (200); neurogliaform (200); and low–threshold spiking, dendrite-contacting interneurons (100). The network model contained synaptic conductances mediated by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors (excitatory) and gamma-aminobutyric acid (GABA)A and GABAB receptors (inhibitory), with AMPA and GABAA receptor-mediated interactions predominating.
Full details are embedded in the Fortran code, which is available at ModelDB (accession no. 267280).
Model(s) of Multipolar Neurons.
To model a multipolar neuron, we started with the compartmental structure of a model basket cell (28); then, we increased the dendritic lengths twofold and doubled dendritic compartment surface areas to allow for the contribution of spines. The densities of some of the ionic conductances (in milli-Siemens per centimeter squared) were gCaL 0.2 to 2.5 in different simulations (this is a high-threshold, noninactivating Ca2+ conductance), soma gKC 10 (a fast Ca2+ and voltage-dependent K+ conductance), gK(AHP) 0.12 (the slow after hyperpolarization), and gKA 1 and 2 (transient K+ conductance); gNaP (persistent; i.e., noninactivating gNa) varied and was scaled relative to the fast transient Na+ conductance. Further details on the properties of model multipolar neurons are in the SI Appendix.
A somatic bias current of −0.25 nA was typically used to suppress spontaneous firing. Specific details are in the code (integrate_multipolar.f). Examples of firing patterns for single multipolar model neurons are shown in Fig. 1.
Connectivity between Multipolar Neurons and with the Original Piriform Model.
There were 500 model multipolar cells. Connectivity for these cells and indeed, all synaptic connectivities in the network model were random. A multipolar neuron received synaptic input from 5 multipolar cells in the simulations illustrated (except as noted), with a range of 1 to 25 in other runs. It received input from five L2pyrs, five L3pyrs, and 15 deep basket cells. Fifteen multipolar neurons connected to each L2pyr, 15 connected to each L3pyr, and 10 connected to each deep basket cell. Excitatory synaptic conductances were predominantly AMPA receptor mediated, and inhibitory synaptic conductances were predominantly GABAA receptor mediated, with specific conductance values specified in the code.
Code was written in Fortran. It uses the mpi parallel environment. Differential equations were integrated with a second-order Taylor series method with a time step of 2 µs.
The program ran on an IBM Power GPU compute node residing in the IBM Cognitive Computing Cluster and running Linux. Top-level program names include piriformENDO.f and multipolar.f. A simulation of 1.1 s of network activity (piriform plus multipolar interconnected regions) ran for ∼2.9 h.
Supplementary Material
Acknowledgments
R.D.T. was supported by IBM Exploratory Research Councils.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120093119/-/DCSupplemental.
Data Availability
Computer programs have been deposited in ModelDB (Yale) (accession no. 267280).
References
- 1.Crick F. C., Koch C., What is the function of the claustrum? Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1271–1279 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J., Matney C. J., Roth R. H., Brown S. P., Synaptic organization of the neuronal circuits of the claustrum. J. Neurosci. 36, 773–784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behan M., Haberly L. B., Intrinsic and efferent connections of the endopiriform nucleus in rat. J. Comp. Neurol. 408, 532–548 (1999). [PubMed] [Google Scholar]
- 4.Smith J. B., et al. , The relationship between the claustrum and endopiriform nucleus: A perspective towards consensus on cross-species homology. J. Comp. Neurol. 527, 476–499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman W. H., Haberly L. B., Bursting induces persistent all-or-none EPSPs by an NMDA-dependent process in piriform cortex. J. Neurosci. 9, 206–215 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman W. H., Haberly L. B., Bursting-induced epileptiform EPSPs in slices of piriform cortex are generated by deep cells. J. Neurosci. 11, 2021–2031 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman W. H., Haberly L. B., Role of synaptic excitation in the generation of bursting-induced epileptiform potentials in the endopiriform nucleus and piriform cortex. J. Neurophysiol. 70, 2550–2561 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Hoffman W. H., Haberly L. B., Kindling-induced epileptiform potentials in piriform cortex slices originate in the underlying endopiriform nucleus. J. Neurophysiol. 76, 1430–1438 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Piredda S., Gale K., A crucial epileptogenic site in the deep prepiriform cortex. Nature 317, 623–625 (1985). [DOI] [PubMed] [Google Scholar]
- 10.Traub R. D., Dingledine R., Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. J. Neurophysiol. 64, 1009–1018 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Olcese U., Oude Lohuis M. N., Pennartz C. M. A., Sensory processing across conscious and nonconscious brain states: From single neurons to distributed networks for inferential representation. Front. Syst. Neurosci. 12, 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walla P., et al. , Evidence of conscious and subconscious olfactory information processing during word encoding: A magnetoencephalographic (MEG) study. Brain Res. Cogn. Brain Res. 14, 309–316 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Tseng G. F., Haberly L. B., Deep neurons in piriform cortex. I. Morphology and synaptically evoked responses including a unique high-amplitude paired shock facilitation. J. Neurophysiol. 62, 369–385 (1989a). [DOI] [PubMed] [Google Scholar]
- 14.Tseng G. F., Haberly L. B., Deep neurons in piriform cortex. II. Membrane properties that underlie unusual synaptic responses. J. Neurophysiol. 62, 386–400 (1989b). [DOI] [PubMed] [Google Scholar]
- 15.Mittmann T., Alzheimer C., Muscarinic inhibition of persistent Na+ current in rat neocortical pyramidal neurons. J. Neurophysiol. 79, 1579–1582 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Carlier E., et al. , Metabotropic glutamate receptor subtype 1 regulates sodium currents in rat neocortical pyramidal neurons. J. Physiol. 577, 141–154 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr D. B., Cooper D. C., Ulrich S. L., Spruston N., Surmeier D. J., Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J. Neurosci. 22, 6846–6855 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorelova N., Seamans J. K., Cell-attached single-channel recordings in intact prefrontal cortex pyramidal neurons reveal compartmentalized D1/D5 receptor modulation of the persistent sodium current. Front. Neural Circuits 9, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traub R. D., Tu Y., Whittington M. A., Cell assembly formation and structure in a piriform cortex model. Rev. Neurosci. 33, 111–132 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Traub R. D., Wong R. K. S., Cellular mechanism of neuronal synchronization in epilepsy. Science 216, 745–747 (1982). [DOI] [PubMed] [Google Scholar]
- 21.Traub R. D., Miles R., Neuronal Networks of the Hippocampus (Cambridge University Press, 1991). [Google Scholar]
- 22.Ferreyra-Moyano H., Cinelli A. R., Molina J. C., Barragán E., Current generators and properties of late components evoked in rat olfactory cortex. Brain Res. Bull. 20, 433–446 (1988). [DOI] [PubMed] [Google Scholar]
- 23.Martella G., et al. , Inhibition of persistent sodium current fraction and voltage-gated L-type calcium current by propofol in cortical neurons: Implications for its antiepileptic activity. Epilepsia 46, 624–635 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Zhao W., et al. , Isoflurane modulates hippocampal Cornu Ammonis pyramidal neuron excitability by inhibition of both transient and persistent sodium currents in mice. Anesthesiology 131, 94–104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azouz R., Jensen M. S., Yaari Y., Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J. Physiol. 492, 211–223 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong R. K., Prince D. A., Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 159, 385–390 (1978). [DOI] [PubMed] [Google Scholar]
- 27.Schwindt P., Crill W., Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. J. Neurophysiol. 81, 1341–1354 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Traub R. D., et al. , Single-column thalamocortical network model exhibiting gamma oscillations, sleep spindles, and epileptogenic bursts. J. Neurophysiol. 93, 2194–2232 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Computer programs have been deposited in ModelDB (Yale) (accession no. 267280).