Abstract
With climate warming, drought becomes a vital challenge for agriculture. Extended drought periods affect plant–pathogen interactions. We demonstrate an interplay in tomato between drought and infection with tomato yellow leaf curl virus (TYLCV). Infected plants became more tolerant to drought, showing plant readiness to water scarcity by reducing metabolic activity in leaves and increasing it in roots. Reallocation of osmolytes, such as carbohydrates and amino acids, from shoots to roots suggested a role of roots in protecting infected tomatoes against drought. To avoid an acute response possibly lethal for the host organism, TYLCV down‐regulated the drought‐induced activation of stress response proteins and metabolites. Simultaneously, TYLCV promoted the stabilization of osmoprotectants' patterns and water balance parameters, resulting in the development of buffering conditions in infected plants subjected to prolonged stress. Drought‐dependent decline of TYLCV amounts was correlated with HSFA1‐controlled activation of autophagy, mostly in the roots. The tomato response to combined drought and TYLCV infection points to a mutual interaction between the plant host and its viral pathogen.
Keywords: begomovirus, drought, osmoprotective metabolites, plant–virus interaction
Tomato yellow leaf curl virus infection is able to extend the survival of tomato plants subjected to drought, mainly by promoting the stabilization of stress markers and by reallocating important osmoprotective metabolites from shoots to roots.

1. INTRODUCTION
Environmental stresses affect agricultural production worldwide, leading to yield reductions of many crops. Drought and heat are the most serious abiotic stresses, especially in countries with hot climates. Drought, together with heat, usually stimulates plant pathogens such as viruses, bacteria, fungi, and insects. Interactions between the plant environment and pathogens modulate the plant defence responses (Prasch & Sonnewald, 2013), either weakening or enhancing them (Atkinson & Urwin, 2012).
An increasing research body indicates that plant viruses modulate host responses to changes in their environment such as wounding, elevated salinity, high temperature, and atmospheric CO2. These changes are accompanied by alterations in the virus biology such as titre, virulence, and transmission efficiency (Bergès et al., 2020; van Munster et al., 2017).
Abiotic stresses may affect the life cycle of viruses as well as the interactions between host susceptibility factors and viruses. Conversely, viruses can influence the plant response to abiotic stresses. For example, turnip mosaic virus (TuMV)‐infected plants display an enhanced expression of defence genes, which is abolished in those plants exposed to abiotic stresses. Deactivation of defence responses leads to a higher susceptibility of plants to virus (Prasch & Sonnewald, 2013). Abiotic stress sensing through the Ca2+ signalling pathway has been shown for potato virus A (PVA). PVA gene expression can be induced by exposing PVA‐infected Nicotiana benthamiana plants to saline, osmotic, or wounding stresses (Suntio & Makinen, 2012). The titre of barley yellow dwarf virus (BYDV) increases by more than 30% in BYDV‐infected wheat growing under elevated CO2 or elevated temperature (Trębicki et al., 2015).
Viruses are parasites multiplying in host living cells, using host resources to support their own reproduction, thereby harming the host. Nonetheless, many viral infections can be beneficial for the host. Rising number of publications have shown that virus infections can alleviate the plant from the negative effect of some abiotic stresses (González et al., 2021). Four different RNA viruses, brome mosaic virus (BMV), cucumber mosaic virus (CMV), tobacco mosaic virus (TMV), and tobacco rattle virus (TRV), have been shown to improve the infected plant's tolerance to drought (Xu et al., 2008). In addition, CMV‐infected beet plants exhibit improved tolerance to freezing. Metabolite profiling showed that virus infection improves plant tolerance to abiotic stress by increasing their levels of osmoprotectants and antioxidants. Another example of a plant beneficial trade‐off from infection with potato virus X (PVX) and plum pox virus (PPV) was described for N. benthamiana and Arabidopsis thaliana grown under drought conditions. Virus infection enhanced plant tolerance to drought by increasing salicylic acid amounts in an abscisic acid (ABA)‐independent manner (Aguilar et al., 2017). TRV has been shown to change the stress response of A. thaliana to low temperature (Fernandez‐Calvino et al., 2014). PVX increases adaptation to environmental oxidation in N. benthamiana (Shabala et al., 2011).
Tomato yellow leaf curl virus (TYLCV) is a begomovirus transmitted in nature by the whitefly Bemisia tabaci. It has a circular c.2800 nucleotide single‐stranded DNA genome encapsidated in a 25 × 30 nm geminate particle. TYLCV causes a disease affecting tomato crops in tropical and semitropical countries, which may result in crop total loss (Czosnek, 2021). The exposure of TYLCV‐infected tomatoes to heat enhances viral multiplication. On the other hand, preinfection of tomato plants with TYLCV using viruliferous whiteflies results in an increased tolerance to heat in the laboratory and in the field under hot Middle Eastern summers (Anfoka et al., 2016). The dual effect of viral and heat stresses on tomato plants was investigated by following the patterns of the key chaperones/heat shock proteins (HSP70, HSP90), the prevailing heat shock transcription factor HSFA2, and HSFA2‐dependent genes (Hsp17, Apx1, Apx2), induced by high temperatures. When TYLCV‐infected leaves are subjected to heat, the increase in the amounts of HSFA2 and HSFA2‐dependent genes is less pronounced versus uninfected plants. It was reported recently that tomato and N. benthamiana agroinoculated with TYLCV present enhanced tolerance to drought; the C4 protein of TYLCV has been found to be involved in conferring drought tolerance through an ABA‐independent manner in a still unknown mechanism (Corrales‐Gutierrez et al., 2020).
In the current study, we studied the role of osmolyte amino acids and carbohydrates, stress proteins and transcription factors, and autophagy in tomato. Our results deepen our understanding of the molecular mechanism involved in drought tolerance induced by whitefly‐mediated TYLCV infection. The results showed that TYLCV was able to extend the survival of tomato plants subjected to drought, mainly by promoting a stabilization of stress marker patterns and by a reallocation of important osmoprotective metabolites from shoots to roots.
2. RESULTS
2.1. When exposed to drought, TYLCV‐infected tomatoes are more resilient than uninfected plants
TYLCV‐susceptible plants (cv. Ikram) were grown in a 2‐L pot, in standard soil (three seedlings in one pot). Then the plants were divided into four groups: healthy (uninfected) and watered (HW), uninfected and subjected to drought (HD), TYLCV‐infected and watered (VW), and TYLCV‐infected subjected to drought (VD). Each group contained 15 seedlings in the repeated experimental growths. VW and VD plants were infected with TYLCV using viruliferous whiteflies. At 5 days postinfection (dpi), VD tomatoes were exposed to drought together with the HD group. To prevent early plant death and provide long‐term growth in conditions of water deficit until 25 days of drought application (25 dd), 50 ml of water was added once in 4 days to drought‐exposed plants. Even such a minor addition of water was started only after 14 dd. Watered and drought‐treated plants were regularly photographed, from the first appearance of drought symptoms until the noninoculated plants showed wilted shoot tips or entirely collapsed. Plant growth and development of drought symptoms in virus‐infected and uninfected tomatoes were compared during prolonged drought treatment (shown from 10 dd until 25 dd throughout the manuscript). Virus infection essentially diminished the growth rate of TYLCV‐susceptible tomatoes. VW plants were smaller than HW tomatoes after 15–25 days of growth (Figure 1a). HD plants ceased to grow very quickly; drought symptoms appeared clearly after 10 dd, while after 25 dd HD plants were completely wilted and collapsed. Cessation of VD growth was delayed in comparison with HD tomatoes, and even after prolonged exposure to drought (20–25 dd) virus‐infected plants did not show those dramatic features (Figure 1a). The comparative growth of HW versus HD and VW versus VD showed that on exposure to prolonged water withholding, the TYLCV‐infected plants were more resilient than the noninfected plants.
FIGURE 1.
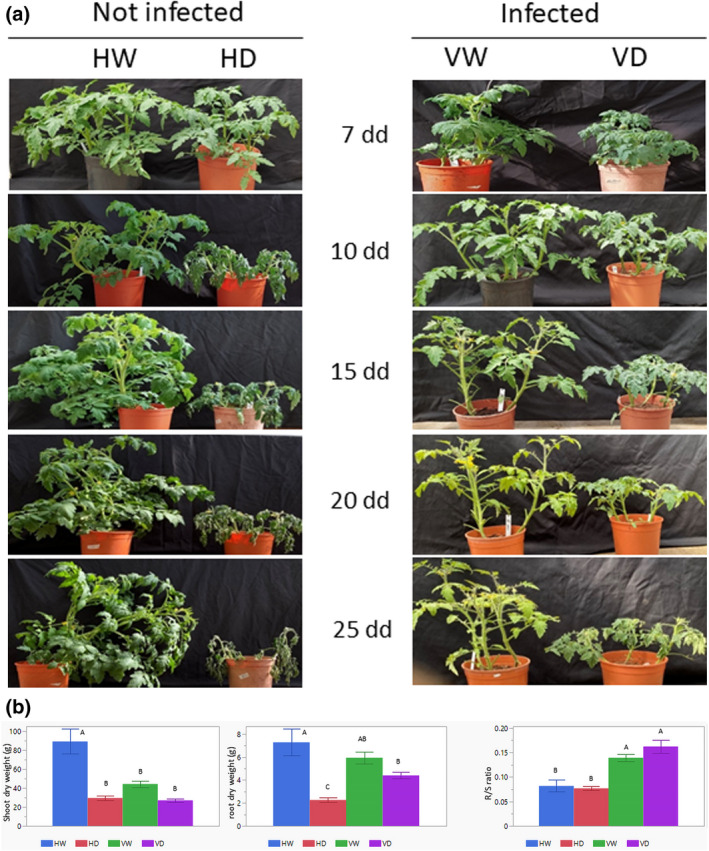
TYLCV infection improves the tolerance of virus‐susceptible tomato to drought. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were infected by viruliferous whiteflies 5 days before drought application. Three independent biological replicates were performed with similar results. (a) Appearance of drought symptoms and plant collapse were monitored 7, 10, 15, 20, and 25 days after the onset of water withdrawal (days of drought application, dd), in addition to 5 days after TYLCV infection (12, 15, 20, 25, and 30 days postinoculation). (b) Dry weights in grams (g) of shoots (left) and roots (middle) of HW, HD, VW, and VD tomatoes, harvested at the end of experimental growth. Mean ± SD values with different letters are significant (p < 0.05, Tukey HSD test). (Right) Root to shoot ratio (R/S) measured for dry weights of tomato roots and shoots. Mean ± SD of R/S ratio, values with different letters are significantly different (p < 0.05, Tukey HSD test)
The reduction in shoot dry weight was approximately 65%–70% in HD versus HW (Figure 1b). The biomass of VW was about half that of HW because we used a TYLCV‐susceptible line, but VD weight loss was only 35% that of VW. An even more convincing difference was obtained by comparing root dry weights. The root weight of uninfected plants decreased by 60%–70%, while that of infected plants decreased by no more than 25%–30%. Under irrigation, TYLCV infection caused a lesser biomass reduction of roots than of shoots, and a significantly lower biomass loss under drought (Figure 1b). The root to shoot ratio (R/S) of TYLCV‐infected plants was higher than of healthy tomatoes during irrigated growth (Figure 1c, HW vs. VW). Under drought growth, the R/S ratio of VD increased, while that of HD remained unchanged. Interestingly, the R/S ratio of VD slightly exceeded that of VW (Figure 1b). TYLCV infection led to significantly lower biomass losses under drought conditions.
2.2. Comparison of water balance relations in uninfected and TYLCV‐infected plants grown under drought conditions
Using the whole‐plant diagnostic system for functional phenotyping of a large number of plants, coined iCORE (https://plantscience.agri.huji.ac.il/icore‐center; details in Halperin et al., 2017), we defined several parameters that pointed to physiological differences between HW, HD, VW, and VD plants. The principal alteration was found for daily transpiration rates: noninfected plants HW showed the highest transpiration rates, while transpiration of VW tomatoes was lower almost from the first days of water withholding (Figure 2a). Under drought, the transpiration of HD decreased much more quickly than that of VD. If HD transpiration started to decrease after 6–7 dd, VD transpiration decreased only after 9–10 dd; the rate of transpiration decline of HD was higher than that of VD plants.
FIGURE 2.
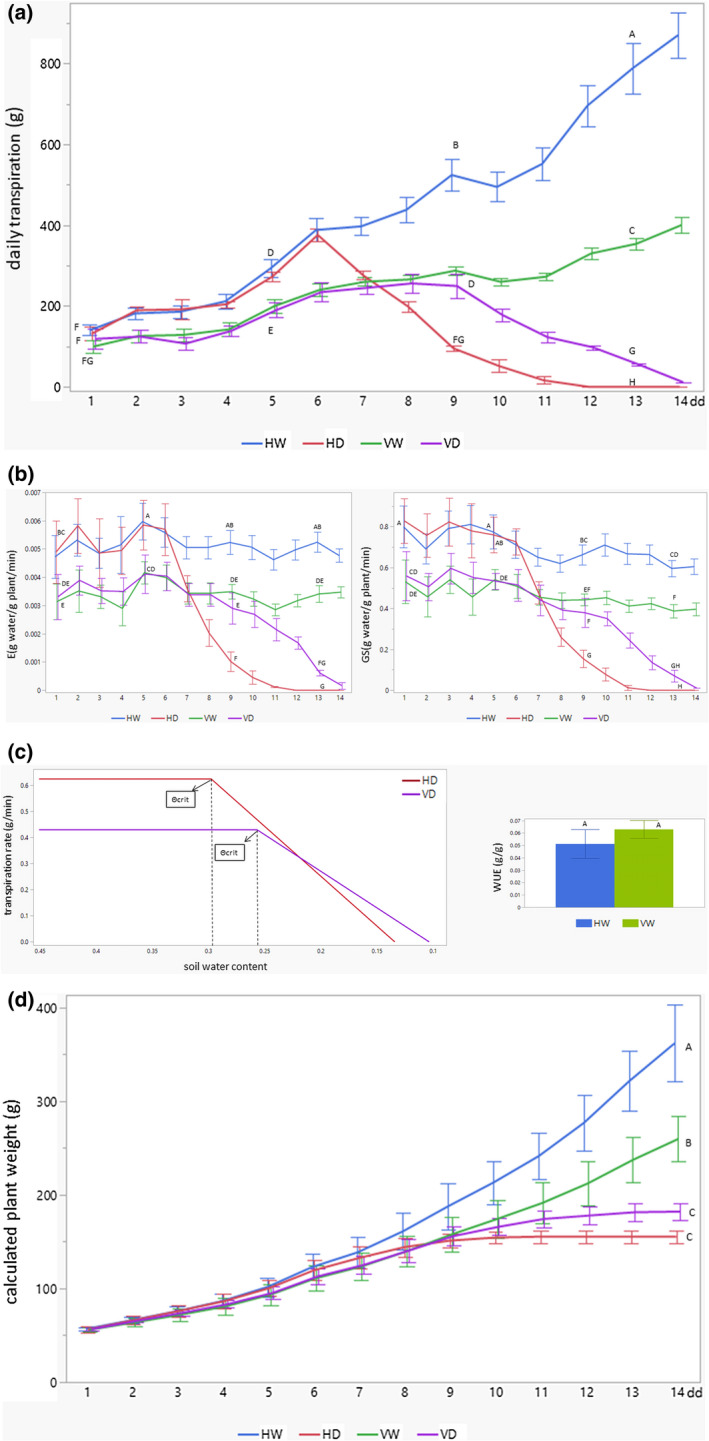
TYLCV infection influences water balance in plants exposed to drought stress. Several physiological parameters were constantly fixated for HW, HD, VW, and VD tomatoes grown in an iCore greenhouse during 14 days of drought application (dd). HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. (a) Efficiency of daily transpiration. Daily transpiration was calculated according to follow equation: difference of plant mass (g) between plant weight, fixated in morning and evening. Mean ± SD continuous daily whole‐plant transpiration during the entire experimental period, values with different letters are significantly different (Tukey HSD, p < 0.05, Tukey post hoc was done only at certain time points: 1, 5, 9, and 13 dd). (b) Normalized transpiration rate (E) and canopy stomatal conductance (GS). Mean ± SD of midday transpiration rate normalized to calculated plant weight (E) and whole‐canopy stomatal conductance (GS) during the entire experimental period between 11:00 and 13:00; values with different letters are significantly different (Tukey HSD, p < 0.05, Tukey post hoc was done only at certain time points: 1, 5, 9, and 13 dd). (c) Physiological drought point (Θcrit) and water use efficiency (WUE). Θcrit was identified as midday transpiration rate versus the soil water content (SWCcrit) of tomatoes grown in water‐withholding conditions (HD and VD). WUE is identified as the ratio between the gain of daily weight and the daily transpiration. Mean ± SD of WUE calculated automatically by SPAC‐analytics software; values with same letter are not significantly different (p < 0.05, Student's t test). (d) Calculated plant weight (CPW). CPW was determined as the sum of the initial plant weight (fixated before start of iCore growth) with the cumulative transpiration multiplied by WUE. Mean ± SD of CPW for the entire experimental period; values with different letters are significantly different (Tukey HSD, p < 0.05, Tukey post hoc was done only for 14 dd)
The lower transpiration rate of VW tomatoes was related not only to its smaller size (Figure 1), but also to a lesser transpiration rate per unit of leaf area (E) (Figure 2b). E levels of VW were approximately 30%–40% lower than those of HW during the entire growth period. Under drought, E levels of VD were also lower than those of HD, but only until 6–7 dd; afterwards, the situation inverted, and E VD was higher than E HD. If E levels of HD plants dropped after 8 dd, VD plants were maintained at high levels until 11 dd. Even more interesting, E of virus‐infected plants essentially did not differ under full watering or under severe drought until 9–10 dd (Figure 2b). Whole‐canopy stomatal conductance (GS) and E levels were measured at the same time, at midday (Halperin et al., 2017). GS levels in HD were higher than in VD during the first week of drought (Figure 2b); after 8 dd, they started to drop quickly, while in VD, GS patterns were maintained at high levels during the additional time and only at 14 dd were undetectable. These results defined the time (at about 7 dd; Figure 2a,b) when drought started to be crucial. TYLCV provides plants with an advantage in terms of drought stress resistance, as evaluated through the improved stability of GS levels.
The other parameter of stressed plants checked in the iCore system was the physiological drought point (Θcrit), which reflects the midday transpiration rate versus soil water content. Θcrit is the point when the soil water content begins to limit the transpiration rate. A Θcrit of 0.258 (25.8% of soil water content, SWC) characterized VD, lower than the Θcrit of 0.3 for HD (Figure 2c), indicating a critical delay of SWC in the infected plants, probably caused by the reduction of transpiration related to infection. The slope representing the decrease of the midday transpiration rate (Figure 2c) was less in VD (2.29 g/min) than in HW (3.73 g/min), which reflected the higher survival of TYLCV‐infected tomatoes even after the critical drought point had been reached. Furthermore, the water use efficiency (WUE), expressed as the amount of biomass per unit of water taken by a plant, was enhanced by 22% (but not statistically significant) in infected VW versus noninfected HW plants (Figure 2c). The gained fresh weight (calculated plant weight, CPW) of experimental plants was estimated during growth. In normal irrigated conditions, HW gained more fresh weight compared to VW plants. In drought conditions, CPW of VD exceeded that of HD (Figure 2d). HD CPW reached a plateau at 10 dd, while VD did so at 13 dd, once again pointing to the improved survival of virus‐infected tomatoes under drought stress.
2.3. Differential changes in patterns of heat shock proteins and heat shock transcription factors in uninfected and infected tomatoes exposed to drought
Various stresses induce protein denaturation, which requires the recruitment of heat shock protein (HSP)/chaperone to cope with potential damage. Therefore, several chaperones and their heat shock transcription factors (HSFs) were studied on drought and virus infection, and their patterns followed for up to 25 dd in HW, HD, VW, and VD plants (Figure 3a). The abundance of HSP70, HSP90, and BiP (HSP70 isoform located in the endoplasmic reticulum lumen) decreased in leaf and root samples of uninfected plants subjected to drought: HD versus HW. The most significant decline in HSPs was observed at the late stages of drought treatment in leaf samples of HD, coinciding with the weakening of total protein patterns. TYLCV infection resulted in the stabilization of chaperone amounts in leaves and roots of VD versus HD (Figure 3a). The densitometry measurements of immunodetected protein bands demonstrated the sharp decline of chaperone abundance in leaf samples because of drought stress (Figure S3a). In root samples, a dramatic decrease was detected only after prolonged stress treatment. In both tissues, stabilization of chaperone abundance was obvious in virus‐infected plants. The relative stabilization of protein, especially HSPs, in infected plants indicated that TYLCV infection caused proteostasis in response to drought stress.
FIGURE 3.
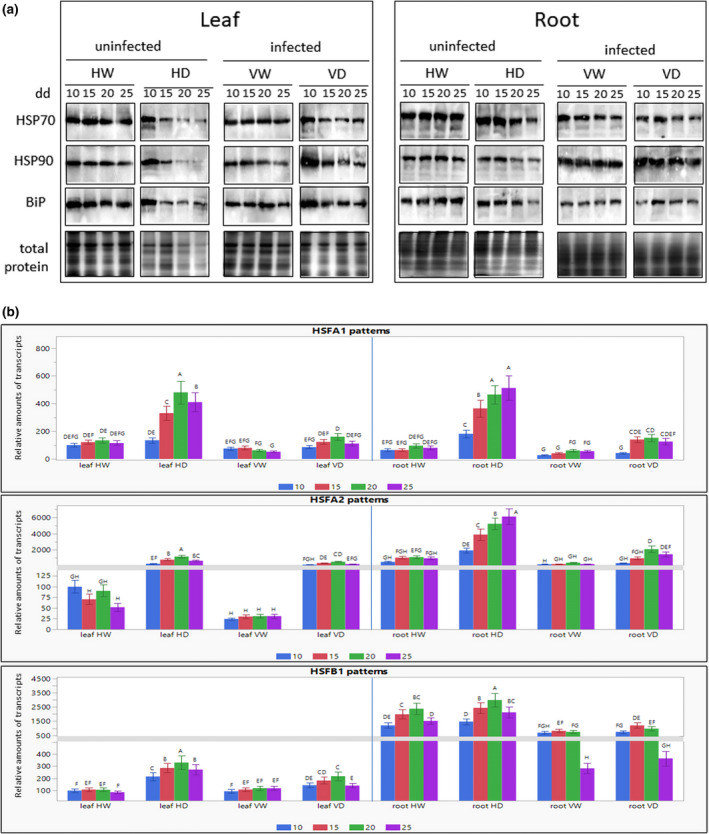
Differential effects of drought on heat shock protein (HSP) patterns and heat shock transcription factor (HSF) transcripts in leaf and root tissues of not infected and of TYLCV‐infected tomato. Four groups of plantlets HW, HD, VW, and VD were used for leaf and root samples, collected after 10, 15, 20, and 25 days of drought application (dd). HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. (a) HSP patterns were appraised by western blot analysis. The same amounts (100 mg fresh weight) of chopped leaves and roots from three different plants were taken for each sample. Plant tissues were drill‐homogenized in 500 µl of SDS‐PAGE buffer. The same volumes (30 µl) of extracted proteins were subjected to SDS‐PAGE. Total proteins were extracted and subjected to SDS‐PAGE. The gels stained with Coomassie Brilliant blue were used as protein loading controls (total protein). Immunodetection was performed with antibodies raised against HSP70, HSP90, and BiP. The photographs show the relevant regions of the gel. Three independent biological repeats were used with similar results. (b) HSF transcription patterns were appraised by reverse transcription quantitative PCR analysis. The expression level of each gene in leaf and root samples is calculated in relation to HW leaves after 10 dd (taken as 100). The results were normalized using the β‐actin gene as an internal marker. Bars represent the average and standard deviation of the relative expression from three independent biological repeats, which gave similar results. Different uppercase letters (A–H) above the bars denote significant differences (Tukey HSD, p < 0.05), and were done separately for leaf and root
The role of HSFs in leaves and roots of TYLCV‐infected tomatoes subjected to drought was studied using as an example the transcription factor HSFA1, a master regulator of heat shock and other abiotic stresses (Liu et al., 2011). The transcription pattern of HsfA1a in plants watered over 25 days remained largely unchanged. In contrast, water withholding was associated with a c.2.5‐fold increase in the amount of HsfA1a transcript in leaves and roots as early as after 15 dd, reaching a c.4‐fold induction by 25 dd (Figure 3b). TYLCV infection suppressed the drought‐activated transcription of HsfA1a, especially in roots, where it was down‐regulated by 2‐ to 3‐fold, compared to a 1.5‐ to 1.8‐fold down‐regulation in leaves. Moreover, the amount of HsfA1a transcript was much lower in irrigated infected (HW) than in uninfected plants (VW) (Figure 3b). In addition to HSFA1, two other transcription factors, HSFA2 and HSFB1, control the plant response to prolonged stresses. We have shown previously that TYLCV infection modifies their transcription patterns (Anfoka et al., 2016). Here, while the amounts of HsfA1 transcripts were similar in leaves and roots of HW plants, those of HsfA2 and HsfB1 were much higher in the roots than in the leaves (Figure 3b). Under drought, the amounts of HsfA2 and HsfB1 transcripts in the leaves of HD plants increased with time. In the roots of HD plants after 25 dd, the amount of HsfA2 transcript was about 6‐fold that in roots of HW plants. HsfB1 transcription was less induced by drought. These results demonstrate the TYLCV‐dependent mitigation of transcription of all three HSFs analysed, especially in roots.
2.4. Osmoprotective amino acids accumulate in response to drought in the background of TYLCV infection
Several amino acids have been shown to accumulate in plants subjected to drought (Krasensky & Jonak, 2012; Obata et al., 2015). Among those, proline is the best known, as its amount increases in many plant species exposed to drought (Fàbregas & Fernie, 2019; Pires et al., 2016; Todaka et al., 2017). At 25 dd, proline amounts increased about 80‐fold in leaves and about 10‐fold in roots of healthy plants (Figure 4 and Figure S1). TYLCV infection resulted in a reduction of proline amounts by half in leaves of watered plants, but in its doubling in the roots. In plants subjected to drought, infection reduced the amount of proline by about half in leaves and roots (Figure 4 and Figure S1).
FIGURE 4.
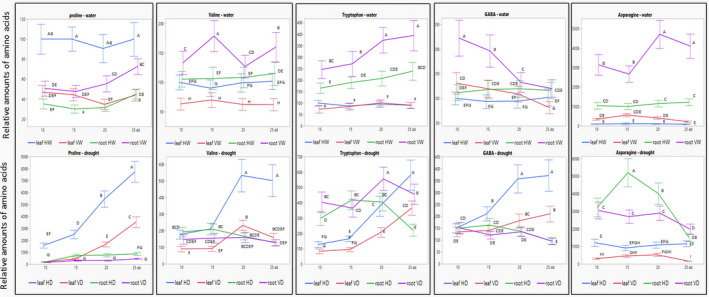
Amino acid patterns in leaves and roots in not infected (H) and virus‐infected (V) tomato, watered and subjected to drought: proline, valine, tryptophan, γ‐aminobutyric acid (GABA), and asparagine. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were preinfected by viruliferous whiteflies 5 days before drought application. Leaf and root samples were collected after 10, 15, 20, and 25 days of drought application (dd). The concentration of each amino acid in leaf HW on the first day of sampling (10 dd) was considered to be 100; the concentration of each amino acid was calculated relative to this value. Bars represent the standard deviation of the relative amino acid levels from three independent biological repeats. Different uppercase letters (A–I) above the bars denote significant differences (Tukey HSD, p < 0.05, which was done for results obtained from watered and drought‐treated plants separately)
γ‐aminobutyric acid (GABA) is another well‐known stress‐responsive amino acid (Urano et al., 2009). GABA quantities increased in tomato leaves after 15 dd, and to a lesser extent in tomato roots (Figure 4 and Figure S1). Interestingly, TYLCV infection caused an increase in root GABA content at the early stages of drought treatment. Basal levels of GABA in infected plants were higher than drought‐induced GABA amounts in uninfected plants (Figure S1). On stress, the valine patterns resembled those of GABA; in contrast, those of asparagine and tryptophan were different from GABA (Figure 4 and Figure S1). Amino acids levels were higher in roots, especially in virus‐infected tomatoes, compared to leaves. Those high levels were weakly or not induced by water withholding (Figure S1). The amounts of asparagine, leucine, isoleucine, and, particularly, glutamine were higher in roots than in leaves (Figure S1). Drought‐dependent induction of aspartic acid and glutamate was surprisingly minor, contradicting previous reports (Batista‐Silva et al., 2019). The amounts of glutamate decreased in leaves and increased in roots of virus‐infected tomatoes; such redistribution was not detected for aspartic acid, leucine, and isoleucine (Figure S1).
Most selected amino acids were induced in leaves of tomatoes subjected to drought, while TYLCV caused a decrease of their levels before and after application of drought (Figure 4 and Figure S1). However, in roots of watered plants, levels of valine, tryptophan, GABA, asparagine, and glutamate were higher in infected (VW) than in noninfected plants (HW). Water withholding levelled these differences, and amino acid amounts were comparable in HD and VD roots. We suggest that enhanced amounts of key osmoprotective amino acids in TYLCV‐infected tomatoes prime plants to cope with drought stress.
2.5. Virus infection induces major changes in the pattern of carbohydrates
High responsiveness of sugars to water deficit has been shown in various plants (reviewed by Fàbregas & Fernie, 2019). In our experiments, the amounts of glucose, fructose, and sucrose increased in leaves and roots of plants subjected to drought, sucrose being the highest induced sugar (Figure 5). Galactose and arabinose presented similar patterns (data not shown). TYLCV mitigated the sugar increase in leaves of plants subjected to drought, but not in roots. Virus infection led to a redistribution of sugars from leaves to roots. In virus‐infected leaves, sugar levels decreased dramatically compared to uninfected plants, while in roots, sugar amounts greatly increased. Moreover, drought mainly induced sugar levels in leaves of uninfected plants and in roots of infected plants (Figure 5 and Figure S2). The sucrose increase in VD plants was less pronounced than in HD plants, which was an exception.
FIGURE 5.
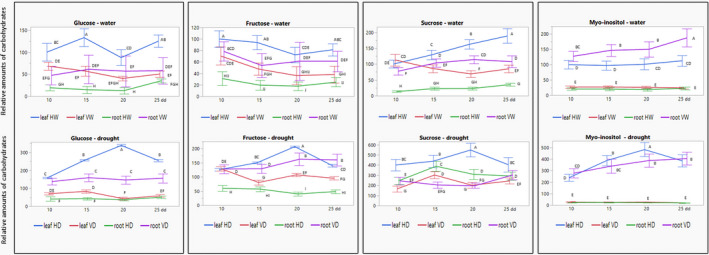
Glucose, fructose, sucrose, and myo‐inositol in tomatoes not infected (H) and infected (V), watered and subjected to drought: reallocation from leaves to roots on infection. Leaf and root samples were collected after 10, 15, 20, and 25 days of drought application (dd). HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. The amount of each carbohydrate in leaves of HW was considered to be 100 on the first day of sampling (10 dd); the amounts of each carbohydrate were calculated relative to this value. Bars represent the standard deviation of the carbohydrate levels from three independent biological repeats. Different uppercase letters (A–I) above the bars denote significant differences (Tukey HSD, p < 0.05, which was done for results obtained from watered and drought‐treated plants separately)
Drought‐stressed plants accumulate cyclitols, such as myo‐inositol, in the cytosol. Myo‐inositol has been described as an osmoprotective carbohydrate in the drought stress tolerance of pepper plants (Yildizli et al., 2008). In our plants, the basal levels of myo‐inositol were much higher in leaves of uninfected than infected plants, and much higher in roots of infected than noninfected plants. Virus infection led to a large increase of myo‐inositol levels in roots, which surpassed those in the roots of noninfected tomatoes (Figure 5 and Figure S2). Myo‐inositol is the finest example of TYLCV‐induced redistribution of carbohydrates, from leaf to root tissues. Altogether, based on these results, we propose that TYLCV led to the redistribution of carbohydrate osmolytes from shoots to roots, making the roots the organ protecting the plant against drought stress.
2.6. Drought influences TYLCV levels in tomatoes
We investigated not only the influence of virus infection on plants subjected to drought, but also the accumulation of TYLCV in these plants. Figure 6a shows the relative TYLCV DNA amounts in the leaves and roots of watered plants (W) and of plants subjected to drought (D) after 10, 15, 20, and 25 dd, corresponding to 15, 20, 25, and 30 dpi. We considered the amount of virus in watered plants at the early stage of infection (15 dpi) as 100. Tomatoes exposed to prolonged drought contained reduced amounts of viral DNA. The reduction was more pronounced in roots (2.5‐ to 3‐fold) than in leaves (about 1.5‐fold) during almost the entire period, except for the late stages (25 dd), when the rates of reduction were comparable (1.5‐ to 1.7‐fold). Western blot immunodetection of the TYLCV coat protein (CP) confirmed the decrease of viral amounts in leaves and especially in roots, and of plants subjected to drought (Figure 6b). Results of densitometry measurements (Figure S3b) confirmed the difference in TYLCV DNA levels obtained by quantitative PCR (qPCR) (Figure 6a). The decline of TYLCV amounts in roots could be explained by the absence of active viral replication in roots (Ber et al., 1990).
FIGURE 6.
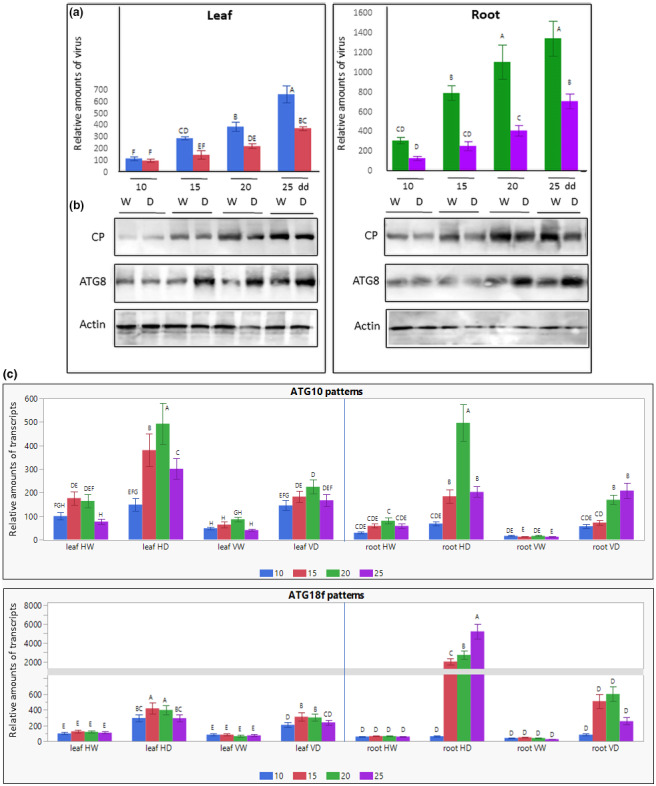
Changes in TYLCV amounts, viral coat protein (CP) and autophagy ATG8 patterns, and transcription profiles of autophagy genes ATG10 and ATG18f in tomato plants subjected to drought. Tomatoes were inoculated with TYLCV using viruliferous whiteflies. Five days after the beginning of virus infection, half of the plants were exposed to drought, while the second half was irrigated. Leaves and roots were sampled after 10, 15, 20, and 25 days of drought application (dd). HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. (a) TYLCV DNA levels in leaves and roots estimated by quantitative PCR. The amounts of viral DNA in infected leaves at 10 dd was considered to be 100; the amounts of DNA in all other samples were calculated relative to this value. The results were normalized using the tomato β‐actin gene as an internal marker. Bars represent the average and standard deviation of the relative expression from five independent biological repeats. Uppercase letters (A–F) above the bars denote significant differences (Tukey HSD, p < 0.05). (b) Western blot analyses of TYLCV CP and cellular ATG8 in total protein extracts of leaves and roots of TYLCV‐infected tomatoes. Actin was used as loading control. The photographs show the relevant region of the gel. Results represent three independent biological repeats. (c) The expression levels of ATG10 and ATG18f in leaf and root samples were measured using reverse transciption quantitative PCR and calculated in relation to HW leaves after 10 dd (taken as 100). The results were normalized using the β‐actin gene as an internal marker. Bars represent the average and standard deviation of the relative expression from three independent biological repeats. Different uppercase letters (A–H) above the bars denote significant differences (Tukey HSD, p < 0.05) and were done separately for leaf and root
A general deterioration of protein patterns was observed for root and mainly for leaf tissues of healthy plants, while TYLCV infection stabilized the patterns of total proteins and selected HSPs (Figure 3a). Hence, the tangible decrease of CP abundance in VD versus VW (Figure 6b) could not be explained only by the decline of total protein amounts. We have shown previously that the six TYLCV proteins are targeted by the autophagy degradation machinery (Gorovits et al., 2016). The decrease in virus amounts in tomato tissues could also be due to activation of autophagy by drought. As shown previously, drought is able to induce tomato autophagy by activating ATG genes such as ATG10 and ATG18f (Wang et al., 2015). An increase in the amount of ATG10 and ATG18f transcripts was observed in leaves and roots of plants subjected to drought (Figure 6c). Transcription activation in the roots exceeded that in the leaves because the basal amounts of ATG10 and ATG18f transcripts in roots were lower than in leaves. Virus infection was associated with a decrease in the amounts of ATG10 and ATG18f transcripts found in uninfected plants (HD vs. VD).
The activation of ATG10 and ATG18f transcription in root tissues of infected plants continued during the plant growth. The patterns of the key autophagy marker ATG8 (Haxim et al., 2018) were estimated in the same plant samples. Some increase of ATG8 levels was detected, particularly in roots of tomatoes subjected to drought (Figure 6b and Figure S3b). Therefore, we suggest that viral CP was degraded by autophagy more efficiently in roots than in leaves.
3. DISCUSSION
Drought is one of the main crucial environmental stresses that inhibits production of crops, including tomatoes. The decline of water content interferes with numerous plant processes and results in reduction of plant growth. In the current experimental system, tomatoes exposed to water withholding showed a dramatic growth reduction after 10 dd. Infection of tomatoes by TYLCV triggered a critical preservation of plants from such effects; after 25 dd, VD plants continued to survive, while uninfected tomatoes were completely wilted and collapsed (Figure 1a).
Harsh environmental stresses, especially deficit of water, influence changes in plant biomass and its distribution between root and shoot (R/S ratio); the increase of root biomass allocation is an adaptive response to drought (Wilson, 1988). Measuring dry weights of experimental tomatoes identified significant losses in shoots and roots of HD in comparison with HW, while VD tissues lost much less biomass in comparison with VW (Figure 1b). Furthermore, TYLCV infection caused an enhancement of the R/S ratio in normally watered and drought‐grown plants (Figure 1c). It could be proposed that virus presence led to increased plant survival under drought stress and a relative root biomass allocation in TYLCV‐infected tomatoes played an important role in this adaptation to drought.
The comparison of physiological water balance parameters measured in healthy and TYLCV‐infected tomatoes revealed significant differences in response to drought stress (Figure 2). The reduced transpiration in TYLCV‐infected tomatoes led to a slower use of the available water in the soil and might promote better survival under severe drought conditions (Figure 2a). It is important to note that E levels of VW were decreased in comparison with HW and withholding of water did not really affect them during a prolonged period of stress (Figure 2b), which pointed to some specific state of the infected plants, contributing physiological stabilization while exposed to extra abiotic stresses. The improved stability of the other parameters, such as GS (Figure 2b), Θcrit and SWCcrit, and WUE (Figure 2c), was a characteristic of TYLCV‐infected plants. The comparison of CPW patterns is of particular interest (Figure 2d). The clear CPW HW advantage over VW was inverted under stress, when CPW VD exceeded that of HD. Altogether, the parameters studied point to an enhanced stabilization of water balance in TYLCV‐infected plants grown under drought stress, allowing their adaptation to severe environmental conditions.
The increase in R/S ratio in response to drought was associated with a higher proportion of soluble sugars in roots than in leaves due to an increased efficiency of transport of sucrose and other carbohydrates from leaves to roots. Carbohydrate partitioning is a key determinant of plant growth and osmoprotection (Lemoine et al., 2013). The synthesis of carbohydrates such as glucose, fructose, sucrose, and myo‐inositol was induced by drought in tomato leaves and roots (Figure 5). Because TYLCV has been shown to suppress the activation of several cellular stress responses (reviewed by Gorovits et al., 2019), it was expected that TYLCV infection would mitigate the induction of carbohydrates. However, the virus‐induced down‐regulation occurred in leaves but not in roots, where induction was extremely high, with the exception of sucrose (Figure 5). Moreover, virus infection resulted in the reallocation of sugars from leaves to roots. Highly sensitive, rapid, and early induction of sugars by water stress ensures the carbohydrate supply from source to sink plant tissues, protecting plants from stress (reviewed by Fàbregas & Fernie, 2019). Our results indicated that TYLCV provided carbohydrate buffering, protecting infected plants from the effects of drought, predominantly in the roots.
In response to withholding water, plants accumulate not only carbohydrate metabolites, but also amino acids (Krasensky & Jonak, 2012). The patterns of several osmoprotective amino acids shown previously to be involved in tomato stress response to abiotic stresses (Gorovits et al., 2020) were notable in plants subjected to drought. Indeed, drought strongly induced several selected amino acids (Figure 4 and Figure S1). TYLCV infection resulted in the down‐regulation of the induction observed in leaves (to a minor degree in roots) of drought‐subjected tomatoes. In particular, the redistribution of stress‐protective amino acids from leaves to roots in TYLCV‐infected plants is worth highlighting.
In tomato roots, TYLCV infection caused not only an increase in the amounts of several important osmoprotective amino acids (compare HW and VW levels in Figure 4 and Figure S1), but also led to the development of some homeostasis, when severe drought stress could not induce the accumulation of these amino acids and their levels remained unchangeable during prolonged growth under water deficit. Levels of valine, asparagine, glutamine, and glutamate were not induced in roots by drought throughout the growth of VD plants but increased in HD tomatoes. The amounts of GABA, leucine, and isoleucine showed minor induction not earlier than 20 dd in VD, while the amounts of these amino acids were induced by drought in HD roots (Figure 4 and Figure S1).
The prominent synthesis of osmolytes remodels the metabolism and the plant defences, playing a central role in the response to simultaneous stresses (Hahn et al., 2011; Woodrow et al., 2017). In addition to the major allocation of carbohydrate osmolytes from shoots to roots, the enhanced levels of amino acids in the roots of unstressed plants could contribute to the tolerance to drought, enhanced in TYLCV‐infected tomatoes.
In the case of simultaneous abiotic and biotic stresses, we took advantage of pleiotropic HSPs and HSFs to perform a comparative plant response. Tomato HSPs develop heterodimers with HSFA1. HSFA1 regulates the function of HSFA2 and HSFB1, and consequently the transcription of HSPs (Hahn et al., 2011). The amounts of HSPs/HSFs may constitute markers for the cell capacity to maintain a stable proteostasis in stressed plants. Even more, overexpression of HSPs or HSFs leads to enhanced plant resistance to several environmental stresses (Nishizawa‐Yokoi et al., 2011). We found that drought caused a very mild induction of HSPs in leaf and root tissues (Figure 3a). TYLCV infection may serve as buffering drought stress. Indeed, HSPs are considered as powerful buffers against environmental stresses (Carey et al., 2006). The multifunctional transcription factor HSFA1a is known to be responsive to drought treatment in tomato leaves (Wang et al., 2015). Withholding water induced the transcription not only of HsfA1, but also HsfA2 and HsfB1 in leaf and root tissues (Figure 3b). TYLCV infection reduced the transcriptional levels of all three HSFs, not only in stressed but also in control plants. Furthermore, virus infection affected the drought‐induced activation of HsfA1, but not that of HsfA2 and HsfB1. The ability of TYLCV to mitigate the stress response of tomato plants has been demonstrated with heat shock (Anfoka et al., 2016). Alleviation of cell death, caused by the other factors, has been shown in TYLCV‐infected plants (Moshe et al., 2016); thus, mitigation of drought‐induced Hsfs transcription is in line with a series of analogous examples.
The crucial role of tomato HsfA1a in tolerance to drought has been shown to occur through the activation of ATG genes and the induction of autophagy (Wang et al., 2015). Among the many autophagy genes, ATG10 and ATG18f are HSFA1‐dependent. Assays such as electrophoretic mobility shift, chromatin immunoprecipitation, and qPCR have shown that HSFA1a binds to the promoter of ATG10 and ATG18f. Silencing of ATG10 and ATG18f down‐regulates the HsfA1a‐dependent tomato tolerance to drought and inhibites autophagosome formation in plants overexpressing HsfA1a. In the current study, we showed that the induction of HsfA1a, caused by drought stress, was followed by an increase in the amounts of ATG10 and ATG18f transcripts, especially pronounced in roots (Figure 6c). Autophagy is a powerful degradative mechanism against geminiviruses in plants (Haxim et al., 2018), and the TYLCV six proteins are prone to autophagy degradation in host cells (Gorovits et al., 2016). The bulk of TYLCV replication occurs in the nucleus of leaf phloem‐associated cells, while viral replication in the roots is minor in comparison; the detection of large amounts of TYLCV in the roots is probably due to long‐distance movement (Ber et al., 1990; Hipper et al., 2013). In infected tomatoes subjected to drought, a decrease in TYLCV DNA amount and CP abundance was detected in shoots, but especially in roots (Figure 6a,b). We suggest that drought activates autophagy, which in turn degrades TYLCV CP (Figure 6c) and, consequently, virions.
The present study showed that the interaction between abiotic drought and biotic virus stresses is mutual. In drought‐exposed infected plants, the amounts of virus decreased. Tomatoes infected by viruliferous whiteflies, as happens in the field, became more tolerant to drought. We suggest that the increased tolerance originates from the reallocation of various key metabolites from shoots to roots. In conditions of prolonged withholding of water, shoots started to be less metabolically active and reduced their nutrient uptake. The behaviour of roots was the opposite: the uptake of metabolites was activated to protect the whole plants from stress. In many plants, drought causes a critical decline of growth metabolism in leaves (e.g., lower concentrations of sugars and amino acids), while the levels of the same metabolites increase in roots (Gargallo‐Garriga et al., 2014). Such metabolite redistribution is a major requirement for buffering the effects of drought. To protect plants against heat stress, TYLCV uses a strategy of down‐regulating the overactivated heat stress response genes. Viral proteins are able to capture HSFA2 in response to heat shock, preventing its translocation into nuclei (Anfoka et al., 2016). TYLCV infection not only down‐regulated the drought‐induced activation of stress response proteins and metabolites to avoid an acute response possibly lethal for the host organism, it also induced a stabilization and redistribution of metabolites to the roots to prepare the plant protection capacities against drought stress.
The current study provides one more compelling example of how the interplay between a plant virus and its host can evolve from pathogenic to mutualistic interaction under stress conditions. Following the recently published research describing TuMV evolution in its Arabidopsis host under well‐watered or dry environments (González et al., 2021), we suggest that virus transition from parasitism to mutualism is not plant‐specific, but is a general feature of plant–virus–environment interactions.
4. EXPERIMENTAL PROCEDURES
4.1. Drought treatment
Tomato seedlings 3 weeks after sowing (Solanum lycopersicum ‘Ikram’; Syngenta) were transferred into 2‐L pots (three seedlings in each pot) with standard soil and irrigated with tap water for a week, then water was withheld. No additional minerals or fertilizers were used. The temperature range of the growth rooms for uninfected and virus‐infected tomatoes was 20°C (night) to 26°C (day). To prevent early plant death while still providing long‐term stress, after 14 dd, 50 ml of water was added to each pot once in 4 days. The drought‐treated plants were photographed every 3 days from the onset of drought symptoms until the noninoculated plants showed wilted shoot tips or entirely collapsed.
At the end of the experiment, dry weights of shoots and roots (g) were measured as followed: shoots were removed from the plants and were dried in a hot air oven at 60°C until no further reduction in weight was measured; roots were washed thoroughly to remove the soil, then dried similarly to shoots.
4.2. Measurements of quantitative physiological traits
The experimental growth was performed in a commercial‐like greenhouse located at the Faculty of Agriculture, Food and Environment in Rehovot, Israel, called iCore throughout this manuscript (https://plantscience.agri.huji.ac.il/icore‐center). The temperature (25°C) and humidity (30%–80%) in the greenhouse were controlled by the Plantarray meteorological station (Plant‐Ditech Ltd, Israel); plants grew under natural light. The functional Plantarray system was used to monitor plant behaviour during 14‐day periods by controlling the quantity of irrigation. Each Plantarray unit had a personalized controller collecting the data of each plant separately grown in a 2‐L pot. The data were analysed by SPACanalytics (Plant‐Ditech), monitored online by web‐based software. Quantitative physiological traits of the plants were determined according to Halperin et al. (2017), and equations were implemented in the SPAC‐analytics software: daily transpiration (g); E, normalized transpiration (g water/g plant/min); GS, whole canopy stomatal conductance (g water/g plant/min); transpiration rate (g/min) versus soil water content; and WUE, water use efficiency (g/g). CPW (calculated plant weight [g]) was determined as the sum of the initial plant weight (fixated before start of iCore growth) with the cumulative transpiration multiplied by the WUE, which than was identified as the ratio between the gain of daily weight and the daily transpiration.
4.3. Whitefly‐mediated inoculation of TYLCV
TYLCV infection started by placing the plants (usually 15 seedlings per treatment) in a net house swarming with viruliferous whiteflies. In the first 1–2 days of the experiment, there were about 20 whiteflies per plant. TYLCV infection occurred over 5 days. The whitefly population was eliminated by a treatment with pesticide against insects, Spirotetramat (Movento, Bayer Inc.). Whitefly‐free tomatoes were transferred to a clean greenhouse, where drought experiments were performed.
4.4. Determination of TYLCV DNA levels in infected tomatoes
A mixture of finely chopped leaves and roots from three plants (50 mg tissues per sample) were prepared every day during the drought treatment. For clarity, the results of only four time points, 10, 15, 20, and 25 dd, are shown. DNA was extracted using the Wizard genomic DNA purification Kit (Promega). DNA was dissolved in 100 µl rehydration buffer and its quality was assessed by agarose gel electrophoresis. The DNA concentrations were measured using a NanoDrop spectrophotometer (ThermoFisher Scientific). The amounts of TYLCV were measured by qPCR using TYLCV CP‐specific primers in the presence of SYBR Green I (Takara) in a Corbett Research Rotor‐Gene 6000 cycler (Corbett Robotics Pty Ltd). The accompanying software (Rotor Gene Q‐Series Software v. 2.0) was used for qPCR data normalization and quantification. The ∆∆C t method was applied for relative quantifications. Viral DNA amounts were measured in two technical replicates for each of three biologically independent experiments. The reaction was as follows: 30 s at 94°C, followed by 40 cycles consisting of 10 s at 94°C, 30 s at 59°C, and 20 s at 72°C. The primers used to amplify a 200 bp fragment of TYLCV CP (V1) were sense 5′‐GAAGCGACCAGGCGATATAA‐3′ and complementary‐sense 5′‐GGAACATCAGGGCTTCGATA‐3′. A 60‐bp fragment of the tomato β‐actin gene served as an internal reference; it was amplified using the primer pair sense 5′‐TGGAGGATCCATCCTTGCATCAC‐3′ and complementary‐sense 5′‐TCGCCCTTTGAAATCCACATCTGC‐3′. Standard curves were generated using 10‐fold dilutions of a CP gene‐carrying plasmid of known size and concentration.
4.5. Transcription patterns in experimental tomatoes
A mixture of chopped leaves from three plants (100 mg per sample) was prepared every 5 days during prolonged drought treatments. A mixture of chopped roots was similarly prepared. The results of 10, 15, 20, and 25 dd are shown only. RNA was prepared from plant tissues using the Tri‐reagent method (Sigma‐Aldrich); cDNA was used for qPCR in triplicate for each cDNA sample. cDNA was subjected to qPCR in the presence of SYBR Green I (Takara), using a Corbett Research Rotor‐Gene 6000 cycler. The reaction was as follows: 30 s at 94°C, followed by 40 cycles consisting of 10 s at 94°C, 30 s at 59°C, and 20 s at 72°C. For tomato HsfA2 (XR742804), a 66‐bp fragment was amplified using the sense (5′‐ACCTTGTGGATCAGCTTGGTTTCC‐3′) and complementary‐sense (5′‐AATAGTGGAGGAGGCCAGAGGAAC‐3′) primers. For tomato HsfB1(CAA39034), a 74‐bp fragment was amplified using the sense (5′‐GGTCAGGCGAAGAAACAATGC‐3′) and complementary‐sense (5′‐TCATATCGGGTGCAACCTTCACG‐3′) primers. For tomato HsfA1 (Sl08g005170), a 113‐bp fragment was amplified using the sense (5′‐TAGCTGAAGGCAGCAAGAAA‐3′) and complementary‐sense (5′‐CTGCCTCATTTATCCCAGGT‐3′) primers. For tomato ATG10 (Sl09g047840), a 147‐bp fragment was amplified by using sense (5′‐GGAGAACCCTTGGCAATAGA‐3′) and complementary‐sense (5′‐TAGTCCCACATGGATGCAAT‐3′) primers. For tomato ATG18f (Sl12g005230), a 125 bp was amplified by using sense (5′‐CCGAAGCAGAACTCCAAAT‐3′) and complementary‐sense (5′‐AACCTCAGCCTCTCCACGAC‐3′) primers. β‐actin gene served as an internal reference.
4.6. Amino acid quantification
Tomato root and leaf tissues (50 mg/sample) were used for each replicate. Three independent experiments were performed. For each time/concentration point, analyses were done in triplicate. Amino acids were quantified according to the procedure described previously (Zwighaft et al., 2015). The concentrations based on standard curves were calculated using TargetLynx (Waters). The amino acid amount in leaves of plants regularly watered at the first sampling time (10 dd) was considered as 100; leaf and root amino acids levels in all plants used throughout the experiments were estimated relative to this value.
4.7. Analysis of carbohydrates
Each sample consisted of a mix of leaf or root pieces from three seedlings (total 10 mg, dry weight). One millilitre of water/methanol solution (1:1) was added to each sample followed by the addition of 50 µg of mannitol as an internal standard. After shaking for 24 h (150 rpm), the samples were centrifuged at 10,000 × g for 10 min. One hundred microlitre aliquots of the extract were transferred to a gas chromatography (GC) vial and evaporated on a steam of nitrogen at 50°C to dryness. The solid residue was converted to trimethyl silyl ethers for the GC‐mass spectrometry (GC‐MS) analysis. Sugar amounts in leaves of plants watered at the first sampling time (10 dd) were considered as 100. Levels of leaf and root carbohydrates in the plants used throughout the experiments were estimated relative to this value.
4.8. Protein immunodetection
Exactly 100 mg fresh weight of leaves and roots was frozen, minced, and drill‐homogenized in 500 µl of standard SDS‐PAGE loading buffer supplemented with 2% sodium dodecyl sulphate (SDS). Samples were boiled for 10 min and centrifuged for 10 min at 10,000 × g; 30 µl of each supernatant was subjected to SDS‐PAGE. Western blotting and immunodetections were performed as described before (Gorovits et al., 2013). Antibodies against the following proteins were purchased from Agrisera: HSP70 (AS08371‐100), HSP90 (AS08346), BiP (AS09481), and ATG8 (AS142769). Antibodies were prepared against TYLCV CP (Hadar Biotech) expressed in Escherichia coli (Gorovits et al., 2013), and anti‐actin polyclonal antibodies were purchased from Abcam. Incubation with primary antibodies was followed by exposure to secondary goat peroxidase‐coupled antibodies (Agrisera) and by ECL detection (Amersham). Each immunodetection was repeated at least three times for each set of plants and tissues. Blots were documented using an Image Quant LAS500 imager (GE Healthcare Life Sciences). Only the relevant parts of the blots are shown in the figures.
The signals were scanned and quantified using ImageJ software (https://imagej.nih.gov/ij/features.html).
4.9. Statistical analysis
Three independent biological replicates were used in each experiment. The levels of amino acids, sugars, viral DNA, and plant transcripts were determined using three technical replicates. Comparisons were performed between control and drought‐treated plants by a univariate analysis of variance (ANOVA) with repeated measures. Significant differences were tested with either Tukey's HSD post hoc (p < 0.05) or Student's t test (p < 0.05) using the statistical analysis software package JMP Pro v. 10.0 (SAS Inc., https://www.jmp.com/ensues/software/data‐analysis‐software.html). Error bars represent standard deviation obtained using JMP graph builder.
AUTHOR CONTRIBUTIONS
R.G., R.M., and M.S. designed the experiments. R.M., M.S., and R.G. planned and performed the experiments. D.S., G.A., M.S., H.C., and R.G. analysed the data. H.C. and R.G. drafted the manuscript. G.A. and D.S. revised the manuscript. All authors read and agreed to the published version of the manuscript.
Supporting information
FIGURE S1 Profiles of osmoprotective amino acids: proline, valine, tryptophan, aspartic acid, γ‐aminobutyric acid, asparagine, glutamine, glutamate, isoleucine, and leucine in not infected and TYLCV‐infected tomato leaves and roots. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were pre‐infected by viruliferous whiteflies 5 days before drought application. Leaves and roots were sampled 10, 15, 20, and 25 days of drought application (dd) after the beginning of water withholding. The levels of each amino acid in leaf HW the first day of sampling (10 dd) was considered to be 100; the concentration of each amino acid was calculated relative to this value. Bars represent the standard deviation of the relative amino acid levels from three independent biological repeats. Different uppercase letters (A–K) above the bars denote significant differences (Tukey HSD, p < 0.05)
FIGURE S2 Profiles of osmoprotective carbohydrates: glucose, fructose, sucrose, and myo‐inositol in uninfected and TYLCV‐infected tomato leaves and roots. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were preinfected by viruliferous whiteflies 5 days before drought application. Leaves and roots were sampled 10, 15, 20, and 25 days of drought application (dd) after the beginning of water withholding. The levels of each carbohydrate in leaf HW the first day of sampling (10 dd) was considered to be 100; the concentration of each sugar was calculated relative to this value. Bars represent the standard deviation of the relative carbohydrate levels from three independent biological repeats. Different uppercase letters (A–J) above the bars denote significant differences (Tukey HSD, p < 0.05)
FIGURE S3 Densitometric analysis of protein samples. Using ImageJ software (https://imagej.nih.gov/ij/index.html), the intensity of immunodetected proteins was measured. (a) HSP abundance in leaf and root. The intensity of immunodetected HSP’s bands was measured and normalized to the respective total protein bands. The obtained measured protein intensity in the leaf sample collected from uninfected tomatoes at 10 days of drought application (dd) was considered to be 1; the measurements of the other intensities were calculated relative to this value. Different uppercase letters (A–F) above the bars denote significant differences (Tukey HSD, p < 0.05). (b) TYLCV CP and cellular ATG8 abundances in leaf and root. The intensity of immunodetected CP and ATG8 bands was measured and normalized to the respective actin bands. The obtained measured protein intensity in the leaf sample collected from TYLCV‐infected tomatoes at 10 dd was considered to be 1; the measurements of the other intensities were calculated relative to this value. Different uppercase letters (A–I) above the bars denote significant differences (Tukey HSD, p < 0.05)
ACKNOWLEDGEMENTS
This research was supported financially by a grant from the United States Agency for International Development, Middle East Research and Cooperation (MERC) program, grant M34‐013. R.M. is the recipient of a Postdoctoral Fellowship from the Planning and Budgeting Committee (PBC) of the Council for Higher Education of Israel (2020‐2022).
Mishra, R. , Shteinberg, M. , Shkolnik, D. , Anfoka, G. , Czosnek, H. & Gorovits, R. (2022) Interplay between abiotic (drought) and biotic (virus) stresses in tomato plants. Molecular Plant Pathology, 23, 475–488. 10.1111/mpp.13172
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aguilar, E. , Cutrona, C. , del Toro, F.J. , Vallarino, J.G. , Osorio, S. , Pérez‐Bueno, M.L. et al. (2017) Virulence determines beneficial trade‐offs in the response of virus‐infected plants to drought via induction of salicylic acid. Plant, Cell & Environment, 40, 2909–2930. [DOI] [PubMed] [Google Scholar]
- Anfoka, G. , Moshe, A. , Fridman, L. , Amrani, L. , Rotem, O.R. , Kolot, M. et al. (2016) Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Scientific Reports, 6, 19715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, N.J. & Urwin, P.E. (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. Journal of Experimental Botany, 63, 3523–3543. [DOI] [PubMed] [Google Scholar]
- Batista‐Silva, W. , Heinemann, B. , Rugen, N. , Nunes‐Nesi, A. , Araújo, W.L. , Braun, H.‐P. et al. (2019) The role of amino acid metabolism during abiotic stress release. Plant, Cell & Environment, 42, 1630–1644. [DOI] [PubMed] [Google Scholar]
- Ber, R. , Navot, N. , Zamir, D. , Antignus, Y. , Cohen, S. & Czosnek, H. (1990) Infection of tomato by the tomato yellow leaf curl virus: susceptibility to infection, symptom development and accumulation of viral DNA. Archives of Virology, 112, 169–180. [DOI] [PubMed] [Google Scholar]
- Bergès, S.E. , Vasseur, F. , Bediée, A. , Rolland, G. , Masclef, D. , Dauzat, M. et al. (2020) Natural variation of Arabidopsis thaliana responses to Cauliflower mosaic virus infection upon water deficit. PLoS Pathogens, 16, e1008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, C.C. , Gorman, K.F. & Rutherford, S. (2006) Modularity and intrinsic evolvability of Hsp90‐buffered change. PLoS One, 1, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales‐Gutierrez, M. , Medina‐Puche, L. , Yu, Y. , Wang, L. , Ding, X. , Luna, A.P. et al. (2020) The C4 protein from the geminivirus Tomato yellow leaf curl virus confers drought tolerance in Arabidopsis through an ABA‐independent mechanism. Plant Biotechnology Journal, 18, 1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnek, H. (2021) Tomato yellow leaf curl viruses (Geminiviridae). In Bamford, D. & Zuckerman, M. (Eds.), Encyclopedia of virology (Fourth Edition). Elsevier. [Google Scholar]
- Fàbregas, N. & Fernie, A.R. (2019) The metabolic response to drought. Journal of Experimental Botany, 70, 1077–1085. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Calvino, L. , Osorio, S. , Hernandez, M.L. , Hamada, I.B. , del Toro, F.J. , Donaire, L. et al. (2014) Virus induced alterations in primary metabolism modulate susceptibility to Tobacco rattle virus in Arabidopsis. Plant Physiology, 166, 1821–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo‐Garriga, A. , Sardans, J. , Pérez‐Trujillo, M. , Rivas‐Ubach, A. , Oravec, M. , Vecerova, K. et al. (2014) Opposite metabolic responses of shoots and roots to drought. Scientific Reports, 4, 6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González, R. , Butković, A. , Escaray, F.J. , Martínez‐Latorre, J. , Melero, Í. , Pérez‐Parets, E. et al. (2021) Plant virus evolution under strong drought conditions results in a transition from parasitism to mutualism. Proceedings of the National Academy of Sciences of the United States of America, 118, e2020990118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovits, R. , Fridman, L. , Kolot, M. , Rotem, O.R. , Ghanim, M. , Shriki, O.Z. et al. (2016) Tomato yellow leaf curl virus confronts host degradation by sheltering in small/midsized protein aggregates. Virus Research, 213, 304–313. [DOI] [PubMed] [Google Scholar]
- Gorovits, R. , Moshe, A. , Kolot, M. , Sobol, I. & Czosnek, H. (2013) Progressive aggregation of Tomato yellow leaf curl virus coat protein in systemically infected tomato plants, susceptible and resistant to the virus. Virus Research, 171, 33–43. [DOI] [PubMed] [Google Scholar]
- Gorovits, R. , Sobol, I. , Akama, K. , Hefetz, B. & Czosnek, H. (2020) Pharmaceuticals in treated wastewater induce a stress response in tomato plants. Scientific Reports, 10, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovits, R. , Sobol, I. , Altaleb, M. , Czosnek, H. & Anfoka, G. (2019) Taking advantage of a pathogen: understanding how a virus alleviates plant stress response. Phytopathology Research, 1, 20. [Google Scholar]
- Hahn, A. , Bublak, D. , Schleiff, E. & Scharf, K.D. (2011) Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. The Plant Cell, 23, 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin, O. , Gebremedhin, A. , Wallach, R. & Moshelion, M. (2017) High‐throughput physiological phenotyping and screening system for the characterization of plant–environment interactions. The Plant Journal, 89, 839–850. [DOI] [PubMed] [Google Scholar]
- Haxim, Y. , Ismayil, A. , Jia, Q.I. , Wang, Y. , Zheng, X. , Chen, T. et al. (2018) Autophagy functions as an antiviral mechanism against geminiviruses in plants, eLife, 6, 23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipper, C. , Brault, V. , Ziegler‐Grff, V. & Revers, F. (2013) Viral and cellular factors involved in phloem transport of plant viruses. Frontiers in Plant Science, 4, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky, J. & Jonak, C. (2012) Drought, salt, and temperature stress induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany, 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine, R. , Camera, S.L. , Atanassova, R. , Dédaldéchamp, F. , Allario, T. , Pourtau, N. et al. (2013) Source‐to‐sink transport of sugar and regulation by environmental factors. Frontiers in Plant Science, 4, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.C. , Liao, H.T. & Charng, Y.Y. (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis . Plant, Cell & Environment, 34, 738–751. [DOI] [PubMed] [Google Scholar]
- Moshe, A. , Gorovits, R. , Liu, Y. & Czosnek, H. (2016) Tomato plant cell death induced by inhibition of HSP90 is alleviated by Tomato yellow leaf curl virus infection. Molecular Plant Pathology, 17, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Munster, M. , Yvon, M. , Vile, D. , Dader, B. , Fereres, A. & Blanc, S. (2017) Water deficit enhances the transmission of plant viruses by insect vectors. PLoS One, 12, e0174398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa‐Yokoi, A. , Nosaka, R. , Hayashi, H. , Tainaka, H. , Maruta, T. , Tamoi, M. et al. (2011) HsfA1d and HasfA1e involved in the transcriptional regulation of HsfA2 function as key regulators for the Hsf signaling network in response to environmental stress. Plant Cell Physiology, 52, 933–945. [DOI] [PubMed] [Google Scholar]
- Obata, T. , Witt, S. , Lisec, J. , Palacios‐Rojas, N. , Florez‐Sarasa, I. , Araus, J.L. et al. (2015) Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiology, 169, 2665–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, M.V. , Pereira, J.A.A. , Medeiros, D.B. , Daloso, D.M. , Pham, P.A. et al. (2016) The influence of alternative pathways of respiration that utilize branched‐chain amino acids following water shortage in Arabidopsis . Plant, Cell & Environment, 39, 1304–1319. [DOI] [PubMed] [Google Scholar]
- Prasch, C.M. & Sonnewald, U. (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiology, 162, 1849–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala, S. , Baekgaard, L. , Shabala, L. , Fuglsang, A. , Babourina, O. , Palmgren, M.G. et al. (2011) Plasma membrane Ca2+ transporters mediate virus‐induced acquired resistance to oxidative stress: calcium efflux and oxidative stress tolerance. Plant, Cell & Environment, 34, 406–417. [DOI] [PubMed] [Google Scholar]
- Suntio, T. & Makinen, K. (2012) Abiotic stress responses promote Potato virus A infection in Nicotiana benthamiana . Molecular Plant Pathology, 13, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka, D. , Zhao, Y. , Yoshida, T. , Kudo, M. , Kidokoro, S. , Mizoi, J. et al. (2017) Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown stress conditions. The Plant Journal, 90, 61–78. [DOI] [PubMed] [Google Scholar]
- Trębicki, P. , Nancarrow, N. , Cole, E. , Bosque‐Pérez, N.A. , Constable, F.E. , Freeman, A.J. et al. (2015) Virus disease in wheat predicted to increase with changing climate. Global Change Biology, 21, 3511–3519. [DOI] [PubMed] [Google Scholar]
- Urano, K. , Maruyama, K. , Ogata, Y. , Morishita, Y. , Takeda, M. , Sakurai, N. et al. (2009) Characterization of the ABA‐regulated global responses to dehydration in Arabidopsis by metabolomics. The Plant Journal, 57, 1065–1078. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Cai, S. , Yin, L. , Shi, K. , Xia, X. , Zhou, Y. et al. (2015) Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy, 11, 2033–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J.B. (1988) A review of evidence on the control of shoot: root ratio, in relation to models. Annals of Botany, 61, 433–449. [Google Scholar]
- Woodrow, P. , Ciarmiello, L.F. , Annunziata, M.G. , Pacifico, S. , Iannuzzi, F. , Mirto, A. et al. (2017) Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiologia Plantarum, 159, 290–312. [DOI] [PubMed] [Google Scholar]
- Xu, P. , Chen, F. , Mannas, J.P. , Feldman, T. , Sumner, L.W. & Roossinck, M.J. (2008) Virus infection improves drought tolerance. New Phytologist, 180, 911–921. [DOI] [PubMed] [Google Scholar]
- Yildizli, A. , Çevik, S. & Ünyayar, S. (2008) Effects of exogenous myo‐inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiologiae Plantarum, 122, 35–42. [Google Scholar]
- Zwighaft, Z. , Aviram, R. , Shalev, M. , Rousso‐Noori, L. , Kraut‐Cohen, Ju. , Golik, M. , et al. (2015) Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metabolism, 22, 874–885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Profiles of osmoprotective amino acids: proline, valine, tryptophan, aspartic acid, γ‐aminobutyric acid, asparagine, glutamine, glutamate, isoleucine, and leucine in not infected and TYLCV‐infected tomato leaves and roots. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were pre‐infected by viruliferous whiteflies 5 days before drought application. Leaves and roots were sampled 10, 15, 20, and 25 days of drought application (dd) after the beginning of water withholding. The levels of each amino acid in leaf HW the first day of sampling (10 dd) was considered to be 100; the concentration of each amino acid was calculated relative to this value. Bars represent the standard deviation of the relative amino acid levels from three independent biological repeats. Different uppercase letters (A–K) above the bars denote significant differences (Tukey HSD, p < 0.05)
FIGURE S2 Profiles of osmoprotective carbohydrates: glucose, fructose, sucrose, and myo‐inositol in uninfected and TYLCV‐infected tomato leaves and roots. Four groups of plantlets were used: HW, not infected (healthy) and watered; HD, not infected and subjected to drought; VW, infected and watered; VD, infected and subjected to drought. Tomatoes were preinfected by viruliferous whiteflies 5 days before drought application. Leaves and roots were sampled 10, 15, 20, and 25 days of drought application (dd) after the beginning of water withholding. The levels of each carbohydrate in leaf HW the first day of sampling (10 dd) was considered to be 100; the concentration of each sugar was calculated relative to this value. Bars represent the standard deviation of the relative carbohydrate levels from three independent biological repeats. Different uppercase letters (A–J) above the bars denote significant differences (Tukey HSD, p < 0.05)
FIGURE S3 Densitometric analysis of protein samples. Using ImageJ software (https://imagej.nih.gov/ij/index.html), the intensity of immunodetected proteins was measured. (a) HSP abundance in leaf and root. The intensity of immunodetected HSP’s bands was measured and normalized to the respective total protein bands. The obtained measured protein intensity in the leaf sample collected from uninfected tomatoes at 10 days of drought application (dd) was considered to be 1; the measurements of the other intensities were calculated relative to this value. Different uppercase letters (A–F) above the bars denote significant differences (Tukey HSD, p < 0.05). (b) TYLCV CP and cellular ATG8 abundances in leaf and root. The intensity of immunodetected CP and ATG8 bands was measured and normalized to the respective actin bands. The obtained measured protein intensity in the leaf sample collected from TYLCV‐infected tomatoes at 10 dd was considered to be 1; the measurements of the other intensities were calculated relative to this value. Different uppercase letters (A–I) above the bars denote significant differences (Tukey HSD, p < 0.05)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


