Abstract
Metabolic syndrome (MetS), as a health-threatening factor, consists of various symptoms including insulin resistance, high blood sugar, hypertension, dyslipidemia, inflammation, and abdominal obesity that raise the risk of diabetes mellitus and cardiovascular disease. Cardiovascular diseases are important causes of mortality among the world population. Recently, there has been a growing interest in using phytomedicine and natural compounds in the prevention and treatment of various diseases. The data was gathered by searching various standard electronic databases (Google Scholar, Scopus, Web of Science, and PubMed) for English articles with no time limitations. All in vivo, in vitro, and clinical studies were included. Elettaria cardamomum (cardamom) is a rich source of phenolic compounds, volatile oils, and fixed oils. Cardamom and its pharmacologically effective substances have shown broad-spectrum activities including antihypertensive, anti-oxidant, lipid-modifying, anti-inflammatory, anti-atherosclerotic, anti-thrombotic, hepatoprotective, hypocholesterolemic, anti-obesity, and antidiabetic effects. This review aims to highlight the therapeutic effects of cardamom on MetS and its components including diabetes, hyperlipidemia, obesity, and high blood pressure as well as the underlying mechanisms in the management of MetS. Finally, it can be stated that cardamom has beneficial effects on the treatment of MetS and its complications.
Key Words: Anti-inflammatory agents, Anti-oxidants, Elettaria cardamomum, Hypoglycemic agents, Hypolipidemic agents, Metabolic syndrome
Introduction
Metabolic syndrome (MetS), as a metabolic disorder, includes a set of major symptoms such as impaired glucose level, dyslipidemia, central obesity, overweight, insulin resistance, and high blood pressure that lead to cardiovascular diseases (CVD), cancer, diabetes mellitus type 2 (DMT2), short lifespan, and low-quality life. MetS has increased concerns about public health problems throughout the world population (1). It is mentioned as a leading public health problem and the cause of morbidity and mortality via high risks for increasing diabetes mellitus and CVD (2-4). Pathophysiology of MetS -induced complications is possibly due to an imbalance in calorie and energy intake associated with alterations in genetic and lifestyle. In addition, it may be affected by the type of food and gut microorganisms (5). Gut microorganisms are sensitive to the changes in nutrition in the intestine and they can also directly participate in the process of absorption (6).
The prevalence of the MetS is not the same in different countries such as the USA (34%), India (25.6%), Kuwait (24.8%), and Australia (22.1%) (7-10). MetS is a threat to human health that requires vital prevention and treatment; thus, effective strategies must be applied to decrease the burden of this disease (11). Because of the side effects and inefficiency of drugs, herbal medicines have been considered for their potential impact on improving and maintaining human health. Medicinal plants and their active constituents such as grapes, saffron, rosemary, garlic, and rutin have long been used to treat various disorders (3, 12-15). Generally, the purpose of spices using in food is to increase the flavor and to take advantage of their medicinal properties (16). Many of the spices belong to herbal families. Therefore, the demand for these herbs has increased because of their beneficial effects on various disorders. Herbal medicines such as Vitis vinifera (15), Silybum marianum L. (17), Nigella sativa (18, 19), Allium sativum (12), Persea americana (20), Solanum melongena (21), and Berberis vulgaris (22) can ameliorate MetS.
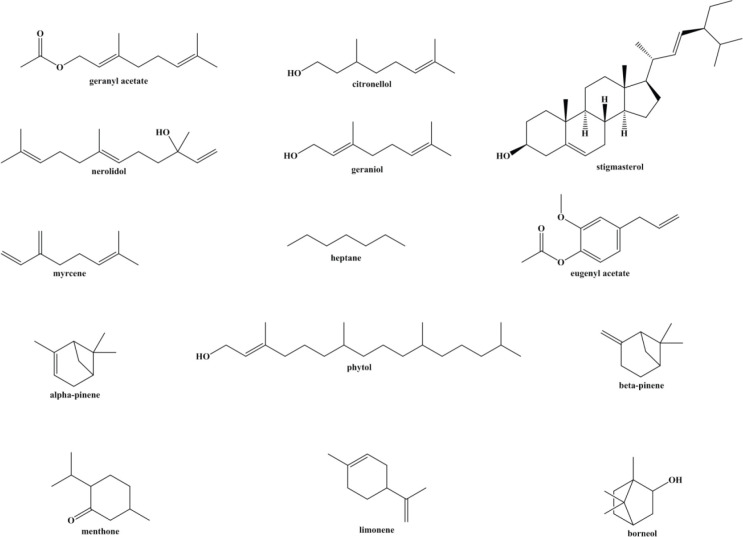
Elettaria cardamomum also known as cardamon, belongs to the Zingiberaceae family. It is native to the Indonesia and Indian subcontinent, Pakistan, Burma, Bangladesh, tropical and subtropical Asia. It is perennial, herbaceous monocots with 4-5 m in height. Cardamom flowers are the whitish lip located at the tip of the corolla tube (23). Phytochemical investigations illustrated that E. cardamomum contains terpinene, stigmasterol, geranyl acetate, geraniol, β-pinene, citronellol, borneol, bisabolene, eugenyl acetate, phytol, β-sitostenone, nerolidol, linalol, α-pinene, menthone, cineol, limonene, subinene, heptane, myrcene, and α-terpineol (24, 25) (Figure 1). The small seeds of cardamom have a triangular pod in cross-section that is covered by a thin papery black shell (26). Several studies have reported the beneficial effects of cardamom on Gram-positive and Gram-negative bacteria (27, 28), teeth and gums health (29), lung disorders (30), and gastrointestinal disorders (31, 32). The therapeutic impacts of cardamom are due to its pharmacological effects including anti-oxidant, antimutagenic (33), antibacterial, anti-inflammatory (34), antidiabetic (35), cardioprotective (36), hepatoprotective, and chemoprotective properties (37).
Figure 1.
Active ingredients of cardamom
This review summarizes the potential efficacy of cardamom and its active constituent in MetS and its related complications.
Methods
PubMed, Web of Science, Google Scholar, and Scopus databases were used to find articles from 1995 to 2021. The search terms used were “cardamom”, “Elettaria cardamomum”, “hypertension”, “diabetes”, “hyperglycemia”, “antihyperglycemic”, “antidiabetic”, “blood glucose”, “dyslipidemia”, “hyperlipidemia”, “hypercholesterolemia”, “hyper-triglyceridemia”, “atherosclerosis”, “obesity”, “appetite”, “anti-obesity”, “weight loss”, and “metabolic syndrome”. Our team collected all published in vivo, in vitro articles, and clinical studies investigating the ameliorative effects of cardamom and its effective constituents on MetS.
Effect of E. cardamomum on dyslipidemia
Dyslipidemia, which is linked to a changed lipoprotein spectrum and modified lipoproteins, is one of the main risk factors in MetS (38). Increased triglyceride (TG)-rich lipoproteins (TRLs), reduced high-density lipoprotein (HDL) and augmented small low-density lipoprotein (LDL) particles are the three major components of dyslipidemia associated with MetS (39). Dyslipidemia plays an important role in developing atherosclerotic CVD associated with MetS. The association between low-density lipoprotein cholesterol (LDL-C) levels and the initiation and development of arterial plaques, for example, is well known, and LDL-lowering therapy has been shown in several clinical trials to substantially reduce the frequency of cardiovascular events. Furthermore, epidemiologic studies have shown that high-density lipoprotein cholesterol (HDL-C) levels and coronary artery disease have a clear inverse relationship (40). Some studies have also stated that the C-reactive protein (CRP) level is a marker for dyslipidemia, diabetes, and MetS (41, 42).
Cardamom and its active ingredients have been demonstrated to modify blood total cholesterol (TC), TG, LDL, and HDL in several investigations.
Clinical trials
A clinical trial was carried out on obese or overweight pre-diabetic women taking 3 grams of cardamom for 2 months. The obtained results disclosed that mean TC (from 192.6 to 183.7 mg/dl) and LDL-C (from 118.1 to 110.5 mg/dl) significantly reduced. It also showed a protective effect on HDL-C (from 44.1 to 42.7 mg/dl) amount in pre-diabetic subjects (43). Another study reported that cardamom supplementation (3 g, 10 weeks) could significantly decrease TG (from 158.4 to 125.8 mg/dl) in T2DM patients in comparison with the placebo group (44). A study aimed to assess the cardamom (3 g/day, 8 weeks) effects on inflammation and oxidative stress in hyperlipidemic pre-diabetic women. In comparison to the placebo group, cardamom supplementation significantly reduced serum hs-CRP (from 5.2 to 5.06 mg/dl), hs-CRP/IL-6 ratio (from 775.04 to 623.5), and MDA (from 8.7 to 7.3 μM) level. Cardamom has been shown to control certain inflammatory and oxidative stress parameters in pre-diabetic people. As a result, it may help these patients avoid complications related to inflammation and oxidative stress (45).
In vivo studies
Pre-clinical research reported that the administration of cardamom powder suspension in 2% gum acacia (1 g/kg/10 ml, oral, for 12 days) decreased the levels of TC and TG, and increased HDL levels in rats with dexamethasone-induced hepatic steatosis (46). In an animal study, cardamom oil (3 g/kg, equivalent to 50 g/kg cardamom, 8 weeks, PO) is effective in restoring lipid homeostasis in hypercholesterolemia rats. The substantial reduction in atherogenicity index following dietary intervention with cardamom powder and oil suggests that cardamom may have a cardioprotective effect (47).
In vitro studies
An in vitro study indicated that 1,8-cineole, a constituent of cardamom, exhibited anti-oxidant properties in lipoprotein metabolism and reduced lipid accumulation in THP-1 cells (48).
Cardamom may exhibit its anti-hyperlipidemic effects through the enhanced rate of cholesterol degradative processes or lipoprotein lipase activity, as well as the efficient reduction in lipid absorption from the intestine (Table 1).
Table 1.
Therapeutic effects of cardamom and its active constituents on dyslipidemia
| Compounds | Dose | Study design | Results | Ref |
|---|---|---|---|---|
| Cardamom | 3g/day, 2 months | Clinical trial, pre-diabetic women | ↓ TC ↓ LDL-C ↓ Insulin sensitivity |
(43) |
| Cardamom | 3g/day, 10 weeks | Clinical trial, T2DM patients | ↓ HbA1C ↓ insulin ↓ HOMA-IR ↓ TG ↑ SIRT1 |
(44) |
| Cardamom | 3g/day, 8 weeks | Clinical trial, hyperlipidemic, overweight, and obese pre-diabetic women | ↓ hs-CRP ↓ hs-CRP/IL-6 ↓ MDA |
(45) |
| Cardamom suspension of 2% gum acacia | 1 g/kg/10 mL, p.o. | In vivo, albino rats | ↓ Hepatomegaly ↓ Dyslipidemia ↓ Hyperglycemia |
(46) |
| Cardamom oil | 3 g/kg, 8 weeks, p.o. | In vivo, Wistar rats | ↓ TC ↓ LDL-C ↓ TG |
(47) |
| 1,8-cineole | 10 μg/ml | In vitro | ↓ HDL ↓ Lipid accumulation |
(48) |
TC: total cholesterol, TG: triglyceride, LDL-C: Low-density lipoproteins-cholesterol, T2DM: type 2 diabetes mellitus, HbA1C: hemoglobin A1c, HOMA-IR: homeostasis model assessment index, SIRT1: sirtuin-1, hs-CRP: high-sensitivity C-reactive protein, MDA: Malondialdehyde, HDL: high-density lipoprotein
Effect of E. cardamomum on obesity
Obesity is a medical condition in which extra body fat has stored to the point that it can be harmful to one’s health. It is characterized by BMI, which is a metric for measuring body fatness (49, 50). Obesity might result in several diseases, including DMT2 (51), cardiovascular disease (52), hypertension (53), as well as respiratory disease (54). Oxidative stress and inflammation have important roles in metabolic disorders linked to obesity (55, 56). According to previous reports, obese patients have lower levels of anti-oxidant markers (superoxide dismutase (SOD), glutathione (GSH) in their blood (55, 57, 58).
Moreover, several parts of the hypothalamus are implicated in the development and maintenance of obesity through several pathways. As one of these important metabolic regulators, sirtuins (SIRTs) are a well-conserved family of class III deacetylases (59). In mammals, seven members of the SIRT family (SIRT1-7) have been identified. SIRT1 is expressed in different organs including the pancreas, liver, adipose tissue, muscle, heart, as well as important metabolic centers of the brain such as the ventromedial hypothalamus (VMH), dorsomedial nucleus, and paraventricular nucleus of the hypothalamus (PVN) (60). SIRT1 regulates body weight by controlling metabolic processes such as food intake, adiposity, energy expenditure, thermogenesis of brown adipose tissue, and browning of white adipose tissue (61, 62). A high-fat diet and obesity result in downregulation of sirtuins especially SIRT1 expression in humans (63). SIRT1 regulates systemic homeostasis by preventing Forkhead box protein O1 (FoxO1) acetylation. In the insulin signaling pathway, FoxO1 is a downstream transcription factor and its activation or overexpression in the hypothalamus decreases insulin anorexigenic property (64), increases adiposity, and results in weight gain (65).
The anti-obesity effects of cardamom and its active constituents have been stated in numerous studies (Table 2).
Table 2.
Therapeutic effects of cardamom and its active constituents on obesity
| Compounds | Dose | Study design | Results | Ref |
|---|---|---|---|---|
| Cardamom | two 500 mg capsules, three times a day, 3 months | Clinical trial, overweight or obese patients with non-alcoholic fatty liver disease | ↑ SIRT1 ↓ hs-CRP ↓ TNF-α ↓ IL-6 ↓ ALT ↓ Degree of fatty liver |
(66) |
| Cardamom | 3 g/day , 16 weeks | Clinical trial, obese women with polycystic ovary syndrome | ↓ Anthropometric indices ↑ Glycemic indices ↓ Expression level of Carnitine palmitoyltransferase 1A, leptin receptor, and lamin A/C ↑ PPAR-γ |
(67) |
| Cardamom | 3g/day, 8 weeks | In vivo, Male Wistar rats | ↓ Glucose intolerance ↓ abdominal fat deposition ↓ Dyslipidemia ↑ Antioxidant enzymes ↓ ALT, AST and ALP ↓ Fibrosis in liver |
(16) |
SIRT1: sirtuin-1, hs-CRP: high-sensitivity C-reactive protein, TNF-α: Tumor necrosis factor-alpha, ↓ IL-6: Interleukine-6, ALT: alanine aminotransferase, PPAR-γ: peroxisome proliferator-activated receptor gamma, AST: aspartate transaminase, ALP: alkaline phosphatase
Clinical trials
An investigation suggested that the administration of cardamom (3 g/day, 3 months) caused an increase in SIRT1 (from 1.2 to 1.3 ng/ml) in the non-alcoholic fatty liver patient (66). Another clinical trial explained that administration of green cardamom (3 g/day, 16 weeks) controlled the expression of some diabetes and obesity genes including fat mass and obesity-associated (FTO), carnitine palmitoyltransferase 1A (CPT1A), leptin receptor (LEPR), lamin A/C, and peroxisome proliferator-activated receptor gamma (PPAR-γ) in women with polycystic ovary syndrome (67).
In vivo studies
It was also found that administration of cardamom powder (1% of powder chow diet w/w, 8 weeks) prevented obesity in high-fat diet-induced obese rats (16).
Effect of E. cardamomum on hypertension
Hypertension is a metabolic risk factor for CVD. It raises the possibility of numerous CVDs, such as peripheral vascular disease, heart failure, coronary artery disease, and stroke (18).
Several lines of evidence have revealed that cardamom and its active constituents show hypotensive and cardiovascular protective properties.
Clinical trials
A study showed that treatment with cardamom (3 g/day, for 12 weeks) caused a significant decrease in systolic blood pressure (SBP) (from 154.2 to 134.8 mmHg) and diastolic blood pressure (DBP) (from 91.8 to 79.6 mmHg) in individuals with primary hypertension of stage 1. Also, cardamom ameliorated fibrinolysis and improved anti-oxidant status after 3 months (68). In contrast, it has been reported that the administration of cardamom (3 g/day, 8 weeks) did not significantly reduce SBP (from 120.7 to 115.5 mmHg) and DBP (from 78.1 to 77.5 mmHg) in obese pre-diabetic women which might be because of a short intervention period and a small sample size (43).
In vivo studies
An in vivo study showed that cardamom crude extract (3-100 mg/kg) reduced blood pressure by a combination of cholinomimetic and calcium channel blocking mechanisms in rats (69).
It has been reported that α-terpineol (25, 50, or 100 mg/kg/day, a week, PO) was able to attenuate mean arterial pressure in hypertensive rats. The authors suggested that α-terpineol can decrease arterial pressure, Probably by decreasing vascular resistance and repairing enzymatic anti-oxidants in these animals (70). These findings reinforce the results of another research that demonstrated that α-terpineol (1, 5, 10, 20 and 30 mg/kg, IV) could induce hypotension and vasorelaxation mediated, partly by the endothelium, probably through releasing NO and activating the NO–cGMP pathway (71). The IV administration of 1,8-cineole (0.3–10 mg/kg) to rats reduced blood pressure, most likely due to active vascular stimulation rather than a reduction of sympathetic tone. These results propose that 1,8-cineole helps to mediate the hypotensive effects of essential oils from certain aromatic plants that are commonly used to treat hypertension (72).
In vitro studies
The vasodilatory effect of cardamom (3-10 mg/ml) has been shown against contraction induced by phenylephrine (1 µM) and potassium (80 mM) in the isolated rat aorta (69). An in vitro study on rabbit aortic endothelial cell line revealed that α-terpineol (10−6, 10−5, and 10−4 mol/l for 15 min) increased nitric oxide (NO) levels via NO–cGMP pathway activation (71).
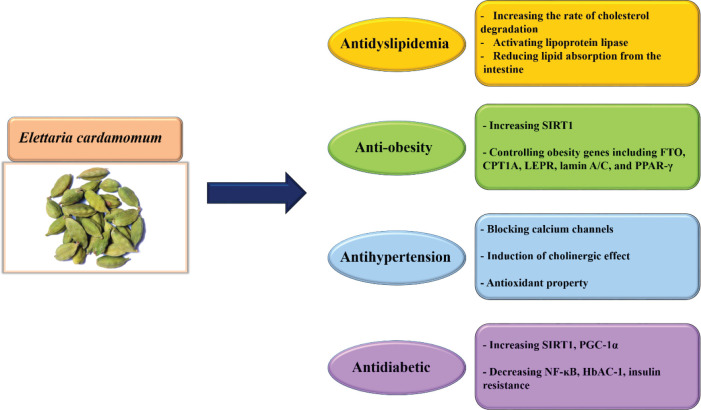
As a result, different mechanisms such as calcium channel blocking, induction of NOS and endothelial NO, cholinergic effect, and anti-oxidant property are included in the cardioprotective and hypotensive effects of cardamom and its active ingredients (Figure 2). Based on these studies, cardamom is a high potential cardiovascular protective agent (Table 3).
Figure 2.
Schematic effect of cardamom and its active component on metabolic syndrome
Table 3.
Therapeutic effects of cardamom and its active constituents on hypertension
| Compounds | Dose | Study design | Results | Ref |
|---|---|---|---|---|
| Cardamom | 3g/day, 12 weeks | Clinical trial, individuals with stage 1 hypertension | ↓ Systolic, diastolic and mean blood pressure ↑ Fibrinolytic activity ↑ Antioxidant status |
(68) |
| Cardamom | 3g/day, 2 months | Clinical trial, pre-diabetic women | ↓ TC ↓ LDL-C ↓ Insulin sensitivity |
(43) |
| Cardamom | 3-100 mg/kg | In vivo, rats | ↓ Arterial blood pressure ↓ Phenylephrine (1 microM)-induced contractions |
(69) |
| α-terpineol | 25, 50, or 100 mg/kg/day, 1 week, p.o. | In vivo, rats | ↓ Mean arterial pressure ↓ Vascular resistance ↑ Antioxidant status |
(70) |
| α-terpineol | 1, 5, 10, 20 and 30 mg/kg, i.v. | In vivo, Wistar rats | ↓ Blood pressure ↑ Vasorelaxation |
(71) |
| 1,8-cineole | 0.3-10 mg/kg, i.v. | In vivo, rats | ↓ Mean arterial pressure ↓ Heart rate |
(72) |
TC: total cholesterol, LDL-C: Low-density lipoproteins-cholesterol
Effect of E. cardamomum on hyperglycemia
Hyperglycemia is one of the risk factors of metabolic syndrome. Hyperglycemia can induce vascular inflammation (73), microvascular damage (74), and atherosclerosis (75). It also impairs the immune status by stimulating cell adhesion molecules and inflammatory cytokines besides inhibiting the function of leukocytes (76).
Cardamom can ameliorate high blood glucose, insulin resistance, and glucose metabolic disorders. E. cardamomum and its active constituents can control insulin secretion, insulin resistance through increasing the amount of SIRT1, PPAR-γ coactivator-1 alpha (PGC-1α), and attenuating the function of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) as well as controlling glucose metabolism by inhibiting α-glucosidase and α-amylase.
Relevant studies regarding the effect of cardamom on diabetes, insulin resistance, and glucose metabolism will be discussed below.
Clinical trials
A clinical trial demonstrated that administration of cardamom (3 g/day, 3 months) to a non-alcoholic fatty liver patient caused an increase in SIRT1 (from 1.2 to 1.3 ng/ml) (66). SIRT1 is responsible to regulate insulin secretion, insulin resistance, lipid/glucose/energy metabolism, inflammatory process, CVD, and kidney diseases (77). Moreover, SIRT1 can upregulate PGC-1α that inhibits NF-κB activation. It also impacts obesity, hepatic glucose production, insulin sensitivity (78), inhibits oxidative stress, and inflammation in pancreatic β-cells (79). On the other hand, NF-κB activation in adipose tissue macrophage of liver and muscle adipose tissue can contribute to the development of insulin resistance in these tissues (80). The administration of cardamom (3 g/day, 10 weeks) declined serum hemoglobin-A1C (HbA1C) (from 8.19 to 7.71 %), homeostatic model assessment-insulin resistance (HOMA-IR) (from 5.01 to 3.80), insulin (from 12.8 to 10.7 μIU/dl), TG levels (from 158.4 to 125.8 mg/dl), and elevated SIRT1 level (from 8.73 to 11.10 ng/dl) in overweight/obese T2DM patients (44). It was also observed that cardamom (3 g, 2 months) could increase insulin sensitivity (from 0.30 to 0.31 QUICKI) in pre-diabetic subjects (43).
In vivo studies
It has been shown that oral administration of cardamom suspension (1 g/kg/10 ml of 2% gum acacia, 6 days before dexamethasone and 6 days during dexamethasone administration (to induce hyperglycemia, dyslipidemia, and hepatic steatosis)) reduced hepatomegaly and hyperglycemia in Albino rats (46). Nanoliposome of 1,8-cineole rich extract of cardamom (550 mg/kg, 35 days) could manage T2DM and hypercholesterolemia by decreasing fasting blood sugar and controlling serum lipid profile (81). It was demonstrated that cardamom powder (1% of powder chow diet w/w, 8 weeks) could significantly reduce glucose intolerance, oxidative stress, and inflammation in the liver of obese rats fed with a high carbohydrate high-fat diet (16).
Moreover, it was reported that supplementation of E. cardamomum extract (100, 200, and 400 mg/kg) for 8 weeks in diabetic rats reduced insulin resistance in animals’ brains by increasing glutamate receptor expression (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor 1 (GluR1) subunit and N-methyl-D-aspartate receptor (NMDA) receptor subunits NR1, NR2A, NR2B) (82).
In vitro studies
It has been reported that supercritical carbon dioxide extract of cardamom increased both the liver insulin sensitivity and glucose uptake in the gut. The results of this study showed that this extract of spices is a safe alternative to metformin and blood glucose regulator-34m (BGR-34) in the treatment of T2DM and could be tested in clinical trials (83). The hydrolysis of starch by pancreatic α-amylase and the uptake of glucose by intestinal α-glucosidases generate a quick spike in blood glucose levels, which causes hyperglycemia in T2DM patients (84). Therefore, an effective strategy to manage DM is inhibiting intestinal α-glucosidase and α-amylase. An in vitro study on aqueous and methanolic cardamom extracts showed that these extracts have α-glucosidase and α-amylase inhibitory effects and can be used in managing diabetes (35).
It can be suggested that cardamom and its active ingredients disclose their therapeutic or preventive properties against diabetes via several mechanisms including decreasing glucose level, insulin resistance, increasing insulin level, glucose uptake, anti-oxidant effects, and the number of β-cells in the pancreas (Table 4) (Figure 2). However, there are not enough clinical studies on the effectiveness of cardamom in diabetes. Therefore, more human studies should be performed on the preventive or curative effect of this herb on diabetes.
Table 4.
Therapeutic effects of cardamom and its active constituents on diabetes
| Compounds | Dose | Study design | Results | Ref |
|---|---|---|---|---|
| Cardamom | 3g/day, 10 weeks | Clinical trial, T2DM patients | ↓ HbA1C ↓ insulin ↓ HOMA-IR ↓ TG ↑ SIRT1 |
(44) |
| Supercritical carbon dioxide extract of cardamom seeds | 550 mg/kg, 35 days, p.o. | In vivo, Wistar albino rats | ↓ Fasting blood sugar | (81) |
| Supercritical carbon dioxide extract of cardamom seeds | in vitro | ↑ Insulin sensitivity ↑Glucose uptake in the gut |
(83) | |
| Aqueous and methanol cardamom extracts | 1 mg/mL | in vitro | ↓ a-glucosidase activity ↓ a-amylase activity |
(35) |
HbA1C: hemoglobin A1c, HOMA-IR: homeostasis model assessment index, TG: triglyceride, SIRT1: sirtuin-1
Conclusion
This manuscript reviewed the chief aspects of MetS and protective mechanisms of E. cardamomum and its active constituents in ameliorating and attenuating the components of MetS including dyslipidemia (by increasing the rate of cholesterol degradation, activating lipoprotein lipase, reducing lipid absorption from the intestine), obesity (through increasing the amount of SIRT1 and controlling some genes involved in obesity), hypertension (via blocking calcium channels, induction of cholinergic effects and anti-oxidant property), and high blood glucose (by enhancing the amount of SIRT1, PGC-1α, reducing NF-κB function and inhibiting α-glucosidase and α-amylase ) that have been reported in clinical trials, in vivo, and in vitro documents. Although there are growing numbers of animal studies demonstrating positive effects of cardamom in MetS, the number of studies linked to cardamom protective properties in humans is still limited. This review article also suggests that cardamom may be implicated as a preventative or therapeutic drug against MetS after being examined in different randomized clinical trials.
Authors’ Contributions
HH and GK Study conception and design; RY Acquisition of data; RY Wrote the manuscript in consultation with MR and MGR; HH Critical revision.
Conflicts of Interest
The authors express no conflict of interest.
Acknowledgment
The authors are thankful to Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Mollazadeh H, Hosseinzadeh H. Cinnamon effects on metabolic syndrome: a review based on its mechanisms. Iran J Basic Med Sci. 2016;19:1258–1270. doi: 10.22038/ijbms.2016.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tousian Shandiz H, Razavi BM, Hosseinzadeh H. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother Res. 2017;31:1173–1182. doi: 10.1002/ptr.5862. [DOI] [PubMed] [Google Scholar]
- 3.Hassani FV, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 4.Baxter AJ, Coyne T, McClintock C. Dietary patterns and metabolic syndrome-a review of epidemiologic evidence. Asia Pac J Clin Nutr. 2006;15:134–142. [PubMed] [Google Scholar]
- 5.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:1–8. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. 2012;8:613–616. doi: 10.6026/97320630008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Rashdan I, Al Nesef Y. Prevalence of overweight, obesity, and metabolic syndrome among adult Kuwaitis: results from community-based national survey. Angiology. 2010;61:42–48. doi: 10.1177/0003319709333226. [DOI] [PubMed] [Google Scholar]
- 9.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index; United States, 2003-2006. Natl Health Stat Report . 2009:1–7. [PubMed] [Google Scholar]
- 10.Janus ED, Laatikainen T, Dunbar JA, Kilkkinen A, Bunker SJ, Philpot B, et al. Overweight, obesity and metabolic syndrome in rural southeastern Australia. Med J Aust. 2007;187:147–152. doi: 10.5694/j.1326-5377.2007.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 11.Magkos F, Yannakoulia M, Chan JL, Mantzoros CS. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu Rev Nutr. 2009;29:223–256. doi: 10.1146/annurev-nutr-080508-141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 14.Razavi BM, Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97:1679–1685. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- 15.Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 16.Rahman MM, Alam MN, Ulla A, Sumi FA, Subhan N, Khan T, et al. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017;16:1–12. doi: 10.1186/s12944-017-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahabzadeh M, Amiri N, Karimi G. Effects of silymarin on metabolic syndrome: a review. J Sci Food Agric. 2018;98:4816–4823. doi: 10.1002/jsfa.9115. [DOI] [PubMed] [Google Scholar]
- 18.Razavi B, Hosseinzadeh H. A review of the effects of Nigella sativa L and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 19.Fadishei M, Ghasemzadeh Rahbardar M, Imenshahidi M, Mohajeri A, Razavi BM, Hosseinzadeh H. Effects of Nigella sativa oil and thymoquinone against bisphenol A-induced metabolic disorder in rats. Phytother Res. 2021;35:2005–2024. doi: 10.1002/ptr.6944. [DOI] [PubMed] [Google Scholar]
- 20.Tabeshpour J, Razavi BM, Hosseinzadeh H. Effects of avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytother Res. 2017;31:819–837. doi: 10.1002/ptr.5805. [DOI] [PubMed] [Google Scholar]
- 21.Yarmohammadi F, Ghasemzadeh Rahbardar M, Hosseinzadeh H. Effect of eggplant (Solanum melongena) on the metabolic syndrome: A review. Iran J Basic Med Sci. 2021;24:420–427. doi: 10.22038/ijbms.2021.50276.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci. 2017;20:557–568. doi: 10.22038/IJBMS.2017.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravindran P, Madhusoodanan K. Cardamom: the genus Elettaria. CRC Press; 2002. [Google Scholar]
- 24.Gopalakrishnan M, Narayanan C, Grenz M. Nonsaponifiable lipid constituents of Cardamom. J Agric Food Chem. 1990;38:2133–2136. [Google Scholar]
- 25.Duke JA. Handbook of phytochemical constituent grass, herbs and other economic plants. CRC press LLC. 1992: 239–240. [Google Scholar]
- 26.Anwar F, Abbas A, Alkharfy KM. Cardamom (Elettaria cardamomum Maton) Oils. In: Preedy VR, editor. Essential oils in food preservation, flavor and safety. Academic Press; 2016. pp. 295–301. [Google Scholar]
- 27.Alam A, Rehman NU, Ansari MN, Palla AH. Effects of essential oils of Elettaria cardamomum grown in India and Guatemala on gram-negative bacteria and gastrointestinal disorders. Molecules. 2021;26:2546. doi: 10.3390/molecules26092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik P, Goyal P, Chauhan A, Chauhan G. In vitro evaluation of antibacterial potential of dry fruitextracts of Elettaria cardamomum Maton (Chhoti Elaichi) Iran J Pharm Sci. 2010;9:287–292. [PMC free article] [PubMed] [Google Scholar]
- 29.Jesylne P, Soundarajan S, Murthykumar K, Meenakshi M. The role of cardamom oil in oral health: A short review. Res J Pharm Technol. 2016;9:272–274. [Google Scholar]
- 30.Kumari S, Dutta A. Protective effect of Elettaria cardamomum (L ) Maton against Pan masala induced damage in lung of male Swiss mice. Asian Pac J Trop Med. 2013;6:525–531. doi: 10.1016/S1995-7645(13)60090-5. [DOI] [PubMed] [Google Scholar]
- 31.Al-Zuhair H, El-Sayeh B, Ameen H, Al-Shoora H. Pharmacological studies of cardamom oil in animals. Pharmacol Res. 1996;34:79–82. doi: 10.1006/phrs.1996.0067. [DOI] [PubMed] [Google Scholar]
- 32.Jamal A, Javed K, Aslam M, Jafri M. Gastroprotective effect of cardamom, Elettaria cardamomum Maton. fruits in rats. J Ethnopharmacol. 2006;103:149–153. doi: 10.1016/j.jep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Saeed A, Sultana B, Anwar F, Mushtaq M, Alkharfy KM, Gilani A-H. Antioxidant and antimutagenic potential of seeds and pods of green cardamom (Elettaria cardamomum) Int J Pharmacol. 2014;10:461–469. [Google Scholar]
- 34.Souissi M, Azelmat J, Chaieb K, Grenier D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe. 2020;61:102089. doi: 10.1016/j.anaerobe.2019.102089. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed AS, Ahmed QU, Saxena AK, Jamal P. Evaluation of in vitro antidiabetic and anti-oxidant characterizations of Elettaria cardamomum (L ) Maton (Zingiberaceae) Piper cubeba L (Piperaceae), and Plumeria rubra L (Apocynaceae) Pak J Pharm Sci. 2017;30:113–126. [PubMed] [Google Scholar]
- 36.Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S, et al. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. 2015;16:27457–27469. doi: 10.3390/ijms161126040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elguindy NM, Yacout GA, El Azab EF, Maghraby HK. Chemoprotective effect of Elettaria cardamomum against chemically induced hepatocellular carcinoma in rats by inhibiting NF-κB, oxidative stress, and activity of ornithine decarboxylase. S Afr J Bot. 2016;105:251–258. [Google Scholar]
- 38.Blaton VH, Korita I, Bulo A. How is metabolic syndrome related to dyslipidemia? Biochem Med. 2008;18:14–24. [Google Scholar]
- 39.Ruotolo G, Howard BV. Dyslipidemia of the metabolic syndrome. Curr Cardiol Rep. 2002;4:494–500. doi: 10.1007/s11886-002-0113-6. [DOI] [PubMed] [Google Scholar]
- 40.Cefalu WT. Diabetic dyslipidemia and the metabolic syndrome. Diabetes Metab Syndr. 2008;2:208–222. [Google Scholar]
- 41.Jeong H, Baek S-Y, Kim SW, Park E-J, Lee J, Kim H, et al. C reactive protein level as a marker for dyslipidaemia, diabetes and metabolic syndrome: results from the Korea National Health and Nutrition Examination Survey. BMJ open. 2019;9:e029861. doi: 10.1136/bmjopen-2019-029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Engelsen C, Koekkoek PS, Gorter KJ, van den Donk M, Salomé PL, Rutten GE. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: a cross-sectional analysis. Cardiovasc Diabetol. 2012;11:1–7. doi: 10.1186/1475-2840-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fatemeh Y, Siassi F, Rahimi A, Koohdani F, Doostan F, Qorbani M, et al. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: a randomized controlled trial. J Diabetes Metab Disord. 2017;16:1–9. doi: 10.1186/s40200-017-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghasi M, Koohdani F, Qorbani M, Nasli-Esfahani E, Ghazi-Zahedi S, Khoshamal H, et al. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric. 2019;99:3933–3940. doi: 10.1002/jsfa.9617. [DOI] [PubMed] [Google Scholar]
- 45.Kazemi S, Yaghooblou F, Siassi F, Rahimi Foroushani A, Ghavipour M, Koohdani F, et al. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: A randomized double-blind clinical trial. J Sci Food Agric. 2017;97:5296–5301. doi: 10.1002/jsfa.8414. [DOI] [PubMed] [Google Scholar]
- 46.Bhat GN, Nayak N, Vinodraj K, Chandralekha N, Mathai P, Cherian J. Comparison of the efficacy of cardamom (Elettaria cardamomum) with pioglitazone on dexamethasone-induced hepatic steatosis, dyslipidemia, and hyperglycemia in albino rats. J Adv Pharm Technol Res. 2015;6:136–140. doi: 10.4103/2231-4040.157981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagashree S, Archana KK, Srinivas P, Srinivasan K, Sowbhagya HB. Anti-hypercholesterolemic influence of the spice cardamom (Elettaria cardamomum) in experimental rats. J Sci Food Agric. 2017;97:3204–3210. doi: 10.1002/jsfa.8165. [DOI] [PubMed] [Google Scholar]
- 48.Cho K-H. 1, 8-cineole protected human lipoproteins from modification by oxidation and glycation and exhibited serum lipid-lowering and anti-inflammatory activity in zebrafish. BMB Rep. 2012;45:565–570. doi: 10.5483/bmbrep.2012.45.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Razavi BM, Abazari AR, Rameshrad M, Hosseinzadeh H. Carnosic acid prevented olanzapine-induced metabolic disorders through AMPK activation. Mol Biol Rep. 2020;47:7583–7592. doi: 10.1007/s11033-020-05825-5. [DOI] [PubMed] [Google Scholar]
- 50.Malekzadeh S, Heidari MR, Razavi BM, Rameshrad M, Hosseinzadeh H. Effect of safranal, a constituent of saffron, on olanzapine (an atypical antipsychotic) induced metabolic disorders in rat. Iran J Basic Med Sci. 2019;22:1476–1482. doi: 10.22038/IJBMS.2019.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118:1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 53.Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12:2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xanthopoulos M, Tapia IE. Obesity and common respiratory diseases in children. Paediatr Respir Rev. 2017;23:68–71. doi: 10.1016/j.prrv.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Malti N, Merzouk H, Merzouk S, Loukidi B, Karaouzene N, Malti A, et al. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–416. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 57.Madhikarmi NL, Singh PP, Khatun T. Lipid peroxidation and anti-oxidant status in male and female patients with type 1 and type 2 diabetes mellitus. Med Phoenix. 2016;1:10–14. [Google Scholar]
- 58.Molnár D, Decsi T, Koletzko B. Reduced anti-oxidant status in obese children with multimetabolic syndrome. Int J Obes. 2004;28:1197–1202. doi: 10.1038/sj.ijo.0802719. [DOI] [PubMed] [Google Scholar]
- 59.Quiñones M, Martínez-Grobas E, Fernø J, Pérez-Lois R, Seoane LM, Al Massadi O. Hypothalamic actions of SIRT1 and SIRT6 on energy balance. Int J Mol Sci. 2021;22:1430. doi: 10.3390/ijms22031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al Massadi O, Quiñones M, Lear P, Dieguez C, Nogueiras R. The brain: A new organ for the metabolic actions of SIRT1. Horm Metab Res. 2013;45:960–966. doi: 10.1055/s-0033-1351322. [DOI] [PubMed] [Google Scholar]
- 61.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Velásquez DA, Martínez G, Romero A, Vázquez MJ, Boit KD, Dopeso-Reyes IG, et al. The central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes. 2011;60:1177–1185. doi: 10.2337/db10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.dos Santos Costa C, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20:633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 64.Kim M-S, Pak YK, Jang P-G, Namkoong C, Choi Y-S, Won J-C, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 65.Susanti VY, Sasaki T, Yokota-Hashimoto H, Matsui S, Lee YS, Kikuchi O, et al. Sirt1 rescues the obesity induced by insulin-resistant constitutively-nuclear FoxO1 in POMC neurons of male mice. Obesity. 2014;22:2115–2119. doi: 10.1002/oby.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, et al. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: a double-blind randomized placebo-controlled clinical trial. Nutr Metab. 2018;15:1–12. doi: 10.1186/s12986-018-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheshmeh S, Elahi N, Ghayyem M, Mosayebi E, Moradi S, Pasdar Y, et al. Effects of green cardamom supplementation on obesity and diabetes gene expression among obese women with polycystic ovary syndrome; A double blind randomized controlled trial. 2021;Research Square [Google Scholar]
- 68.Verma S, Jain V, Katewa S. Blood pressure lowering, fibrinolysis enhancing and anti-oxidant activities of cardamom (Elettaria cardamomum) Indian J Biochem Biophys. 2009;46:503–506. [PubMed] [Google Scholar]
- 69.Gilani AH, Jabeen Q, Khan A-u, Shah AJ. Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. J Ethnopharmacol. 2008;115:463–472. doi: 10.1016/j.jep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Sabino CKB, Ferreira-Filho ES, Mendes MB, da Silva-Filho JC, Ponte MPTR, Moura LHP, et al. Cardiovascular effects induced by α-terpineol in hypertensive rats. Flavour Fragr J. 2013;28:333–339. [Google Scholar]
- 71.Ribeiro TP, Porto DL, Menezes CP, Antunes AA, Silva DF, De Sousa DP, et al. Unravelling the cardiovascular effects induced by α-terpineol: A role for the nitric oxide–cGMP pathway. Clin Exp Pharmacol Physiol. 2010;37:811–816. doi: 10.1111/j.1440-1681.2010.05383.x. [DOI] [PubMed] [Google Scholar]
- 72.Lahlou S, Figueiredo AF, Magalhães PJC, Leal-Cardoso JH. Cardiovascular effects of 1, 8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol. 2002;80:1125–1131. doi: 10.1139/y02-142. [DOI] [PubMed] [Google Scholar]
- 73.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Du X, Edelstein D, Obici S, Higham N, Zou M-H, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. The Journal of clinical investigation. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clinical cornerstone. 2007;8:S30–S42. doi: 10.1016/s1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- 76.Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocrine Practice. 2006;12:22–26. doi: 10.4158/EP.12.S3.22. [DOI] [PubMed] [Google Scholar]
- 77.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2011;90:276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 78.Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013;37:315–325. doi: 10.4093/dmj.2013.37.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets. 2013;14:622–636. doi: 10.2174/1389450111314060002. [DOI] [PubMed] [Google Scholar]
- 80.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paul K, Bhattacharjee P, Chatterjee N, Pal TK. Nanoliposomes of supercritical carbon dioxide extract of small cardamom seeds redresses type 2 diabetes and hypercholesterolemia. Recent Pat Biotechnol. 2019;13:284–303. doi: 10.2174/1872208313666190404101336. [DOI] [PubMed] [Google Scholar]
- 82.Gomaa AA, Makboul RM, El-Mokhtar MA, Abdel-Rahman EA, Ahmed IA, Nicola MA. Terpenoid-rich Elettaria cardamomum extract prevents Alzheimer-like alterations induced in diabetic rats via inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Cytokine. 2019;113:405–416. doi: 10.1016/j.cyto.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 83.Paul K, Chakraborty S, Mallick P, Bhattacharjee P, Pal TK, Chatterjee N, et al. Supercritical carbon dioxide extracts of small cardamom and yellow mustard seeds have fasting hypoglycaemic effects: diabetic rat, predictive iHOMA2 models and molecular docking study. Br J Nutr. 2021;125:377–388. doi: 10.1017/S000711452000286X. [DOI] [PubMed] [Google Scholar]
- 84.Mohamed EAH, Siddiqui MJA, Ang LF, Sadikun A, Chan SH, Tan SC, et al. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement Altern Med. 2012;12:1–7. doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]