Abstract
Decorin is a stromal-derived prototype member of the small leucine-rich proteoglycan gene family. In addition to its functions as a regulator of collagen fibrillogenesis and TGF-β activity soluble decorin acts as a pan-receptor tyrosine kinase (RTK) inhibitor. Decorin binds to various RTKs including EGFR HER2 HGFR/Met VEGFR2 TLR and IGFR. Although the molecular mechanism for the action of decorin on these receptors is not entirely elucidated overall decorin evokes transient activation of these receptors with suppression of downstream signaling cascades culminating in growth inhibition followed by their physical downregulation via caveosomal internalization and degradation. In the case of Met decorin leads to decreased β-catenin signaling pathway and growth suppression. As most of these RTKs are responsible for providing a growth advantage to cancer cells the result of decorin treatment is oncosuppression. Another decorin-driven mechanism to restrict cancer growth and dissemination is by impeding angiogenesis via vascular endothelial growth factor receptor 2 (VEGFR2) and the concurrent activation of protracted endothelial cell autophagy. In this review we will dissect the multiple roles of decorin in cancer biology and its potential use as a next-generation protein-based adjuvant therapy to combat cancer.
Keywords: angiogenesis, autophagy, small leucine-rich proteoglycans, receptor tyrosine kinase
INTRODUCTION
Decorin (DCN), is a well-characterized small leucine-rich proteoglycan (SLRP) and serves as the archetype for this group of proteoglycans (1, 2). SLRPs are an 18-member gene family, forming a distinct subgroup of proteoglycans that is a microcosm of the multifunctional nature of extracellular matrix (ECM) proteins (2–5). SLRPs, aptly named after their identifiable leucine-rich structural motif repeats, contain three canonical classes, I-III, and two noncanonical classes, IV-V. These classifications are defined through parameters such as homologies at the genomic and protein levels. The distribution of SLRP-encoding genes is spread across seven chromosomes with some in gene clusters, indicating there is functional redundancy among the SLRPs. The evolutionary conservation of SLRP function underscores the critical function of these proteins in the ECM and overall organismal homeostasis (3, 6). Decorin is one of these highly conserved SLRPs, and is present across species. In mammals, the proteoglycan consists of a central domain of 10 leucine-rich repeats, a single glycosaminoglycan chain (GAG), and a 42 kDa conserved protein core. Originally categorized as a collagen-binding protein, decorin, a class I SLRP, was initially characterized as a critical structural factor in collagen fibrillogenesis and tissue integrity. The myriad of interactions decorin has with its ligands (7) primarily involves its protein core, but the single GAG chain, existing as either chondroitin or dermatan sulfate, also plays an essential role in tissue homeostasis (8).
A BRIEF HISTORY OF DECORIN
In the latter half of the 1980s, a heavy emphasis was put on proteoglycan research, as understanding the associations and interactions between single gene products lied at the epicenter of advancing the field of biochemistry. Naturally, connective tissue became a locus of particular interest due to its layout as a complex multicellular system with different components working in tandem to sustain critical function in both maintaining shape and resisting physical stressors (9, 10). The characterization of proteoglycans became a focal point in the study of connective tissue, with decorin identified as a chondroitin-dermatan sulfate proteoglycan. Decorin is indeed heavily involved in collagen fibrillogenesis and along with other dermatan sulfate-rich proteoglycans, was shown to associate with tendon collagen at the d band in the gap region (11). Thus the eponym of decorin was aptly proposed for its ability to “decorate” collagen fibrils (12). With further investigation, it was determined that decorin possesses a much broader range of function, with the discovery that the protein core inhibits, rather than aids, collagen fibrillogenesis by binding to type I collagen (13) and maintaining collagen fibril structure, fiber realignment, and mechanical properties of various tissues (14–24) that regulate homeostasis. Moreover, the realization that decorin harbored a single GAG chain at its N-terminus was “surprise” at that time as proteoglycans were believed to have a higher amount of carbohydrates related to the protein core. After its initial cloning in 1986 from human fibroblasts (25), the decorin gene was fully sequenced in both humans and mice (26, 27), and its promoter region was also partially characterized (28, 29). Notably, the transcriptional regulation of DCN is quite complex and is induced by quiescence and repressed by tumor necrosis factor α (TNFα) (30), and is also transcriptionally repressed by FOXD1 (31) and MEIS1 (32). Moreover, decorin is involved in controlling cell proliferation, adhesion, and migration (33, 34). To fully understand the in vivo functions of decorin, we generated Dcn−/− mice and discovered that the lack of decorin caused lax and fragile skin, telltale of dermal thinning (35), consistent with its ascribed roles in collagen fibrillogenesis. As these mice are viable and fertile, they have been used in many studies and in various pathological processes, in both experimental and congenital settings.
Adding to its already versatile interactions, decorin potently binds TGF-β (36), effectively sequestering the cytokine, attenuating its function, and blocking cell proliferation (37–40). It was this discovery that propelled decorin into the forefront of proteoglycan research, particularly in cancer as malignant progression requires constitutive cell proliferation, making decorin a promising target for oncogenic therapeutics. As study of the proteoglycan continued, it was revealed that the breadth and power of its biological function primarily lies in its functional interactions with multiple cell surface receptors tyrosine kinases, effectively ascribing ECM remodeling as a cardinal role of decorin (41–43). Because cancerous tissues require constant remodeling of the extracellular matrix, this groundbreaking discovery has wholly reshaped our understanding of the physiological role of decorin and its significance in the tumor microenvironment, especially in terms of its potential as an oncosuppressive agent.
A CURRENT VIEW OF DECORIN
Today, decorin is understood as a far more complex unit of the ECM. Genetic ablation of this SLRP leads to a wide range of debilitating conditions that range from structurally compromised skin and tendon to impaired metabolism and obesity, abnormal angiogenesis, myocardial infarction, and fibrosis, demonstrating its multifunctional nature via direct and indirect interactions with a multitude of signaling complexes (18, 35, 44–48). Early in its discovery, decorin was characterized as having an inhibitory role in cancer proliferation and metastasis in tumor cell lines (49, 50). Dcn−/− mice have been studied in depth, and, in addition to the skin fragility phenotype, they show a strong trend toward spontaneous tumor development (51–56) and metastatic spread (57). Notably, decorin levels are markedly reduced in several solid malignancies including prostate, breast, colon, renal, and esophageal carcinomas (58–66). Decorin primary function can be attributed to cell cycle regulation via p21-induced G1 cell cycle arrest. Upon ectopic decorin expression, upregulation of p21 allows for nuclear translocation in cells with de novo decorin expression subsequently inhibiting cell cycle machinery. Recently, our studies have shown that decorin can induce autophagy in endothelial cells and mitophagy in breast cancer cells, independently of nutrient conditions (67–75). We should also note that decorin has been linked to the pathophysiology of epidermolysis bullosa (76–78), a disease due to deficiency of collagen VII (79–81), and to other congenital diseases primarily those involving ocular abnormalities (82–87).
THE STRUCTURE AND FUNCTION OF DECORIN
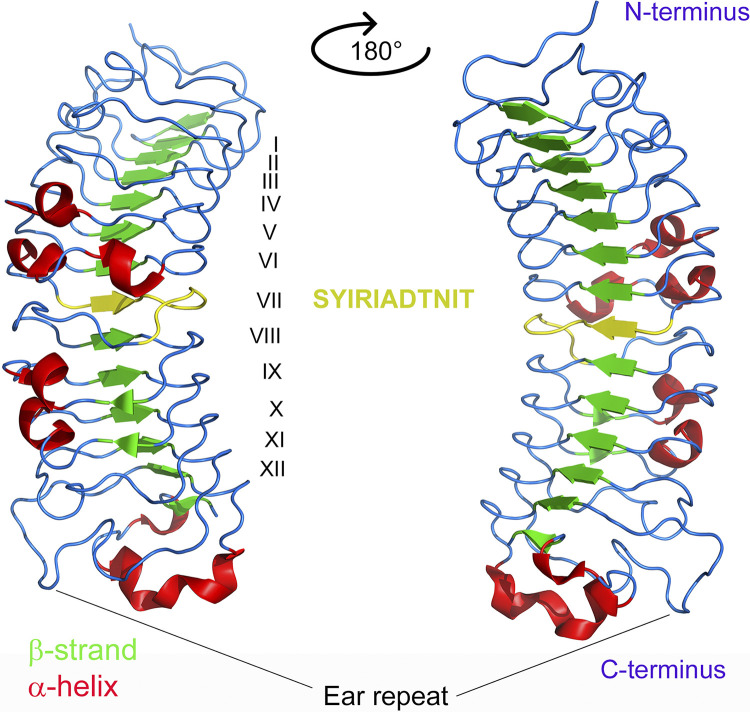
Decorin is horseshoe-shaped, in which its 14 curved β-strands, located on the inner concave surface contains protein sequences for recognizing most of the known decorin-binding partners (7, 88). In contrast, the outer convex surface of decorin contains multiple α-helices (Fig. 1) (70). The leucine rich repeats (LRR) architecture of the decorin solenoid provides a plastic interface that encodes biological information necessary for coordinating a myriad of protein-protein interactions, the hallmark of decorin multiplicity of functions. To understand how decorin interacts, it is useful to think of the decorin structure as an amalgam of two parts. The central domain is composed of the characteristic 12 LRRs forming short β-strands in a parallel conformation, and the N-terminal attachment of the GAG chain, of either dermatan or chondroitin sulfate (Fig. 1). The LRR protein core forms the interface for binding receptor tyrosine kinases, most notably vascular endothelial growth factor receptor 2 (VEGFR2). Specifically, LRRV/VI aid in the binding of decorin to VEGFR2 (89), whereas LRRXII is utilized for decorin binding to CCN2/CTGF and for suppressing its biological activity (90). Perhaps, the most established sequence (SYIRIADTNIT) is located in LRRVII and contains the area with a high affinity for collagen type I (91), the most classic and well-known binding partner of decorin (92). The C-terminal includes a structure known as the “ear” repeat and participates in protein folding (Fig. 1). This structure has been investigated extensively and its functionality has been determined via truncation of the decorin C-terminal, which causes protein misfolding and endoplasmic reticulum stress. These issues subsequently cause disease manifestation, including congenital stromal corneal dystrophy (82–85). The GAG chain remains crucial for decorin-ligand interactions. The chondroitin/dermatan sulfate (CS/DS) chain attached to decorin performs many functions related to wound healing (93), keratinocyte function (94), and collagen assembly in adipose and skeletal muscle tissues (95). In addition, the chain appears to increase the affinity of decorin to collagen, with its absence often phenotypically emerging as increased skin fragility (35). Because decorin appears in both a monomeric and dimeric form, it is likely that the dimeric complex would sterically hinder most of the core region, thus making binding to other substrates, especially cell surface receptors, quite difficult or impossible. Thus, although decorin forms a dimer in physiological solutions (96), its biologically active form is that of a monomer (97).
Figure 1.
Three-dimensional (3-D) structure of decorin visualized as a cartoon ribbon diagram rendered with PyMOL (PDB accession number:1XKU). Monomeric bovine decorin is depicted where secondary structures are color coded: vertical arrows designate β-strands and are shaded in green, whereas coiled ribbons indicate α-helices and shaded red. The leucine-rich repeats are numbered in Roman numerals I-XII. Decorin contains a central domain composed of 14 β-strands, 12 of which are leucine-rich repeats. This domain mainly participates in interaction with RTKs, collagen, and growth factors. The type I collagen-binding sequence, SYIRIADTNIT, located in LRRVII is shaded in yellow. Other noteworthy areas include the C-terminal LRR Cys capping motif, known as the ear repeat, which is involved in protein folding, and the single GAG chain which is located between the N-terminus and the leucine-rich region. See text for additional information. GAG, glycosaminoglycan chain; RTK, receptor tyrosine kinase.
ROLE OF DECORIN IN ANTITUMORIGENIVC SIGNALING
Decorin has been considered a “guardian from the matrix” because of its antitumorigenic activity, which manifests itself by inhibiting several RTKs and their downstream signaling cascades that originate from the ECM (98). In general, by blocking these RTK-mediated pathways, decorin ultimately interferes with the continued growth and survival of the tumor by inhibiting key processes such as metastasis and angiogenesis (72, 99) These two processes are integral in determining the fate of a tumor in terms of remaining silent or becoming malignant. In angiogenesis, new blood vessels are formed from preexisting blood vessels, which enhances the survival of tumor cells, the so-called angiogenic switch (100). This is because a greater amount of nutrients and oxygen pertinent to cancer growth can reach tumorigenic tissue and feed metabolic processes (101). During metastatic dissemination, tumors gain migratory abilities and spread to distal areas in relation to the primary site, which makes it a beneficiary of angiogenesis. After binding to RTKs, decorin mitigates both tumor metastasis and angiogenesis (102–107). The latter process is performed in a way similar to other proteoglycan-derived bioactive molecules such perlecan/endorepellin (108–110) or collagen XVIII/endostatin (111–113). Apart from RTKs, decorin can also bind other growth factor receptors like TGF-βR and Toll-like receptors, TLR2 and TLR4 to stimulate the anti-inflammatory response, which similarly curbs cancer lethality (7, 114). In the next section, we provide a succinct summary of the functional involvement of several RTKs interacting with the decorin protein core.
EGFR Signaling
Decorin binds with several RTKs on the cell surface with high affinity. Epidermal growth factor receptor (EGFR) was the first RTK discovered as a binding partner of decorin (41–43). In A431 squamous carcinoma cells, decorin binding to EGFR induces dimerization, internalization, and the subsequent degradation of EGFR via caveolar-mediated endocytosis (41, 42, 115). Moreover, soluble decorin elevates cytosolic Ca2+ in squamous carcinoma cells overexpressing EGFR (116). After binding to the receptor, decorin evokes sustained downregulation of EGFR and an overall attenuation of the EGFR signaling cascade (117), a mechanism for controlling tumor growth in vivo. Activation of this EGFR signaling through PI3 kinase (PI3K) and RAS is crucial for sustained tumor growth and proliferation (Fig. 2A). By suppressing oncogenic signaling, decorin is believed to restrict tumor growth, survival, and metastatic potential. On the other hand, through EGFR signaling, decorin can simultaneously activate an antioncogenic pathway that leads to cell cycle arrest. Decorin induces rapid transautophosphorylation of EGFR and concurrent activation of mitogen-activated protein (MAP) kinase for a protracted induction of endogenous p21 (118, 119), a potent inhibitor of cyclin-dependent kinases, and induction of caspase-3, which ultimately results in cell cycle arrest (42). In line with these findings, the antiangiogenic effect of decorin is also reported to signal via EGFR in breast carcinoma cells (120). Decorin evokes the rapid secretion of thrombospondin-1 (TSP-1), a potent antiangiogenic effector via inhibition of the RhoA/ROCK1 complex (Fig. 2A) (120). The importance of decorin in EGFR signaling is further emphasized when osteosarcoma cells that constitutively produce decorin were shown to be resistant to decorin-induced growth arrest through the sustained expression and activation of EGFR signaling (121). Decorin has recently been established as a suppressor of invasion and tumor growth in inflammatory breast cancer by inhibiting EGFR/Erk signaling. In addition, its overexpression leads to decreased migration and invasion of the tumor both in vitro and in mouse xenograft models (122). Collectively, through EGFR, decorin suppresses oncogenic signaling and activates oncosuppressive functions in tumor cells.
Figure 2.
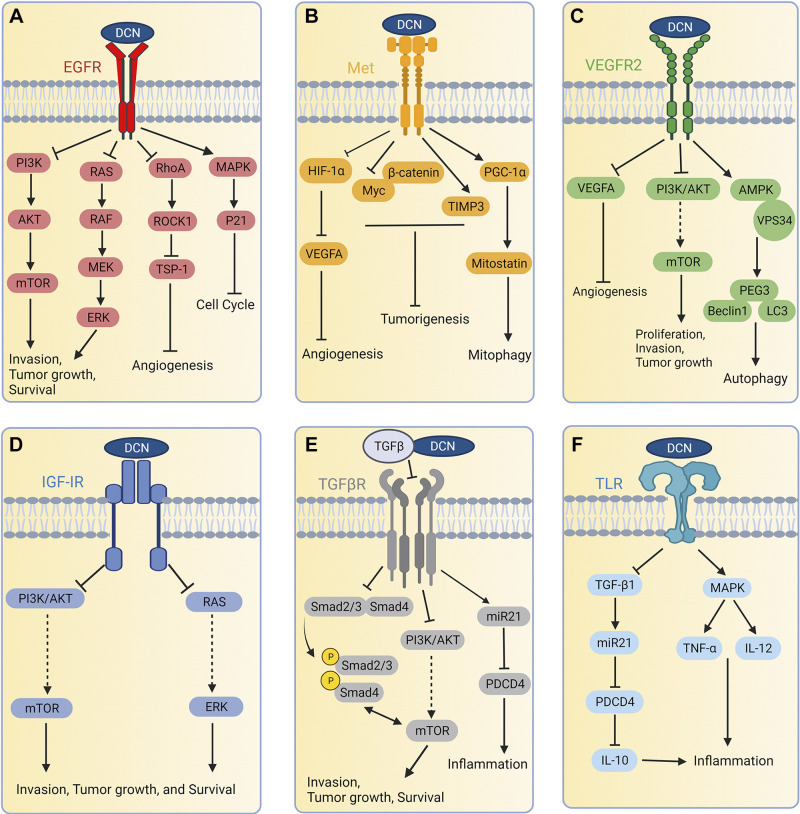
Interaction of decorin with different receptor tyrosine kinase (RTKs) and other receptors (A–F). As it pertains to the TGF-β receptor, decorin binds the ligand, TGF-β, and inhibits its downstream signaling. After binding with variety of receptors or ligand, decorin inhibits several oncogenic biochemical pathways and activates some oncosuppressive genes to restrict the growth and proliferation of the tumor. AMPK, AMP-activated protein kinase; EGFR, epidermal growth factor receptor; HIF-1α, hypoxia-inducible factor 1α; IGF-IR, insulin-like growth factor receptor 1; IL, interleukin; mTOR, mammalian target of rapamycin; Met, mesenchymal-epithelial transition factor also known as hepatocyte growth factor receptor (HGFR); miR21, microRNA 21; MAPK, mitogen-activated protein kinase; Myc, myelocytomatosis proto-oncogene transcription factor; Peg3, paternally expressed 3; PGC-1α, peroxisome proliferator-activated receptor γ, coactivator 1α; PI3K, PI3 kinase; Rho, RAS homolog family member A; ROCK1, Rho-associated coiled-coil kinase 1; TSP-1, thrombospondin-1; TIMP3, tissue inhibitor metalloprotease 3; TLR, Toll-like receptor; TGF-β1, transforming growth factor β isoform 1; TNFα, tumor necrosis factor α; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2; VPS34, vacuolar protein sorting 34PDCD4, programmed cell death protein 4. Created with Biorender.com.
MET Signaling
To investigate the possibility that the antioncogenic effects of decorin could integrate with RTKs other than EGFR and related ErbB receptors, we utilized an antibody array system to assess tyrosine phosphorylation of 42 RTKs (123). We discovered that the addition of soluble decorin affected phosphorylation of the hepatocyte growth factor (HGF) receptor Met in a serum-independent manner that resembled its effects on EGFR. Decorin can directly bind to the Met receptor, a proven mediator of malignant transformation, invasive growth, and metastasis (123–125). Decorin binds to the extracellular domain of Met that leads to receptor downregulation through a combination of increased ectodomain shedding and internalization (126). Notably, decorin evokes a marked proteasome-dependent degradation of the transcription factor β-catenin and downregulates the protein expression of both β-catenin and Myc (Fig. 2B) (123, 127). In tumor xenograft models, decorin downregulates Met with concurrent suppression of β-catenin, which is mechanistically implicated in mediating HGF- and Met-dependent cell invasion, and Myc, a key oncogenic factor for tumor progression (127). Not limited to this, decorin was also shown to suppress the expression of two proangiogenic genes, hypoxia-inducible factor (HIF)-1α, and vascular endothelial growth factor A (VEGFA) in breast carcinoma cells and inhibits VEGFA-mediated angiogenesis (128). In line with this, decorin reduces the expression and activity of matrix metalloprotease (MMP)-9 and MMP-2, two proangiogenic proteases, and evokes the expression of potent angiostatic agents like TIMP3 (120). Decorin antagonizes the angiogenic network by inhibiting proangiogenic factors and activating angiostatic agents via Met, which reduces tumorigenicity. In addition, in triple-negative and luminal breast carcinoma cells, decorin triggered mitochondrial depolarization followed by augmented mitophagy downstream of Met. Mechanistically, decorin mobilizes PGC-1α for the cytosolic accumulation of mitostatin to evoke mitophagy (71). Thus, by increasing mitostatin levels and evoking the autophagic catabolism of mitochondria, decorin suppresses VEGFA ultimately leading to tumor angiostasis.
VEGFR2 Signaling
In recent years, decorin has been established as a novel VEGFR2 antagonist in endothelial cells as well as in human trophoblasts (67, 89, 129). Presumably, these biological interactions affect in vivo neovascularization in several organs including the cornea (130). Decorin directly binds the ectodomain of VEGFR2 in a region that partially overlaps with its endogenous agonist, VEGFA, and, as such, inhibits VEGFA-mediated angiogenesis. By interacting with VEGFR2, decorin induces AMPK to initiate a signaling cascade that activates vacuolar protein sorting 34 (Vps34) and inhibits mTOR for excessive autophagy. Due to the protracted nature of decorin-evoked autophagy in endothelial cells, decorin transcriptionally activates paternally expressed gene 3 (Peg3). Peg3 is critical for sustaining the decorin-evoked autophagy response as it is necessary and sufficient for driving the expression and accumulation of Beclin-1 and LC3 (131), two key proteins required for successful autophagy (Fig. 2C) (67, 131). Moreover, Peg3 has been conclusively implicated in mediating autophagic flux downstream of decorin/VEGFR2 interactions (67), in part by transcriptionally promoting TFEB expression (129). Loss of Peg3 or Beclin 1 significantly abrogates decorin-evoked autophagy. Decorin-VEGFR2 binding also inhibits the Akt phosphorylation axis that ultimately blocks oncogenic signaling via mTOR pathway (132). Recently, a connection that unifies the proautophagic properties of decorin with the well-established antiangiogenic functions has been uncovered. Decorin clears intracellular VEGFA by mobilizing this potent proangiogenic growth factor into LC3-positive autophagosomes in a Peg3-dependent manner (133). Moreover, VEGFA is sensitive to autophagic flux in vivo as application of chloroquine prevented a starvation-induced reduction of VEGFA in cardiac and aortic tissues (133). Thus, decorin-induced VEGFR2 signaling attenuates tumor progression by blocking angiogenesis or by inhibiting oncogenic signaling through autophagy.
IGF-IR Signaling
Activation of signaling cascades through insulin-like growth factor receptor I (IGF-IR) is involved in the development of many carcinomas. In some experimental models, it has been established that activation of IGF-IR is directly linked to tumor progression and epithelial-mesenchymal transition (EMT) (134, 135). Notably, previous studies have shown that in invasive bladder cancer IGF-IR expression is generally upregulated, whereas Dcn mRNA expression is downregulated (136–138). In addition, it has been shown that decorin binds IGF-IR and inhibits IGF-I-induced migration and invasion of bladder cancer through the inhibition of downstream signaling cascades (137). Decorin severely mitigates IGF-I-stimulated activation of Akt and ERK1/2, two key pathways for tumor development and progression (Fig. 2D). Therefore, decorin binding with IGF-IR inhibits the oncogenic signaling through Akt and ERK, whereas loss of decorin indirectly induces IGF-IR activity and signaling, thereby promoting enhanced cellular motility, invasion, and tumor progression.
TGF-β Signaling
Limited reports are available concerning the role of decorin in transforming growth factor β (TGF-β) signaling. In 2002, decorin was shown to disrupt the TGF-β/Smad signaling pathway in human mesangial cells where decorin induced the phosphorylation of different Smad proteins (139). This initial discovery was supported by subsequent reports whereby decorin has been genetically ablated. This was sufficient to allow for rampant Erk and Smad signaling favoring the development of hepatic (140, 141) and renal fibrosis (142, 143). Another way in which decorin affects TGF-β is through interaction with LDL receptor-related protein 1 (LRP-1) (144), which is a large endocytic receptor involved in lipoprotein metabolism, catabolism of proteinases, coagulation (145), and cancer cell migration (146). Notably, the internal LRRVI is responsible for decorin binding to LRP-1 and subsequent TGF-β-evoked signaling (147). Very recently, decorin has also been reported as an antagonist of TGF-β in astrocytes of the optic nerve (148). In this report also, decorin deficiency has been shown to increase the expression and synthesis of TGF-βs. Importantly, treatment with decorin reduced TGF-β expression in murine astrocytes. In addition, this report claims Smad-independent TGF-β signaling where decorin exerts its suppressive effect over TGF-β expression via pAKT/AKT signaling (Fig. 2E) (148).
TLR Signaling
Beyond the interactions with growth factors and cytokines to control cell growth and proliferation, decorin also mediates inflammatory responses by acting as an endogenous ligand for TLR2/4 (Toll-like receptor) (149, 150). Decorin binds to TLR2 and TLR4 on macrophages with high affinity and, in turn, causes rapid activation of p38, MAPK, and NF-κB pathways, all of which are involved in proinflammatory responses (150, 151). Decorin binding to the TLRs prevents transcriptional repression of programmed cell death protein 4 (PDCD4) by decreasing TGF-β1 activity; this leads to an increase of oncogenic miR-21, a posttranscriptional repressor of PDCD4 (Fig. 2F) (150, 152). Subsequently, increased PDCD4 decreased the release of IL-10, an anti-inflammatory cytokine, thereby making the overall cytokine environment more proinflammatory. In addition, through these Toll-like receptors, decorin enhances the synthesis of the proinflammatory cytokines TNFα and IL-12 (Fig. 2F). Thus by stimulating proinflammatory molecules and reducing the abundance of anti-inflammatory ones, decorin shifts the immune response to a more proinflammatory state that is associated with reduced tumor growth. It is important to note that inflammation in cancer remains a highly debated topic, in which the current outlook diverges for the effects of acute versus chronic inflammation on tumorigenesis.
CONSEQUENCES OF DECORIN INTERACTION WITH VARIOUS RECEPTORS
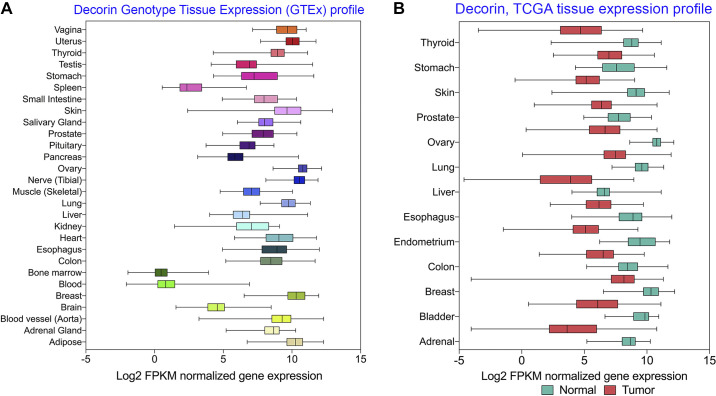
To curb the lethality of tumorigenesis and malignant transformation, the interaction of SLRPs with different cell surface receptors has become an emerging field of study in the fight against cancer. After binding with different receptors, decorin modulates key processes vital for tumor growth, invasion, and progression such as autophagy, mitophagy, cell cycle arrest, inflammation, and angiogenesis. By attenuating several oncogenic processes, promoting oncosuppressive functions, and/or inducing inflammation and autophagy, decorin acts as a soluble master tumor repressor to determine whether a tumor remains silent or becomes malignant (62, 63, 153–156). Because decorin is ubiquitously expressed in all tissue types, its presence and potential use as a therapeutic agent is relevant to all types of carcinomas (Fig. 3A). Interestingly, in patients with cancer, the expression of decorin is downregulated in most tumorigenic tissues (Fig. 3B). It is believed this facilitates tumor metastases due to reduced decorin expression from the homeostatic level. Therefore, abundant expression of decorin or treatment with decorin may lead to an “organized” ECM presenting itself as a physical barrier against tumor cell metastasis.
Figure 3.
Ubiquitous expression of decorin and downregulation in various malignant tissues. A: from data generated in GTEx tissue expression via the Xena database provided by the University of California in San Diego, decorin is expressed at measurable levels ubiquitously in different tissue types, further expounding its pertinent function in maintaining wild type, and healthy tissue function. Expression is measured via fragments/Kb of transcripts/million mapped reads (FPKM). B: from a cohort of 19,131 patients with cancer aggregate in the TCGA Target GTEx database published by UCSD, normal and tumor tissue RNA-Seq signaling was measured in a variety of tissue types ranging from esophageal to ovarian. These data illustrate a significant downregulation (P values ranging from 1.74 × 10−8 to 2.63 × 10−206) of decorin in tumorigenic tissue. These findings strongly indicate that higher decorin concentrations are beneficial in preventing primary tumor growth and proliferation. Along with its ubiquitous expression in mammalian tissue, decorin is a nondiscriminant antitumorigenic agent.
DECORIN IN CANCER STUDIES
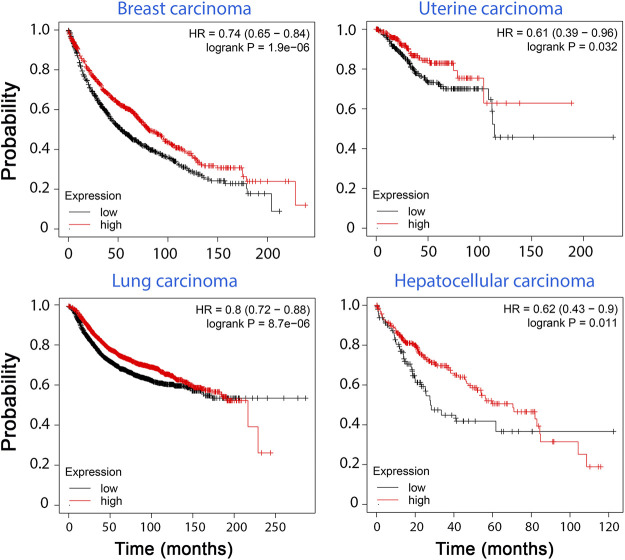
Although clinical studies involving decorin date to the 1990s, knowledge of this proteoglycan was rather limited and was thought to strictly involve collage fibrillogenesis for tissue integrity. Therefore, in these initial studies, the focus was more on tissue and wound healing (157, 158). In comparison, after experimental findings suggested that decorin evokes autophagy (159), there has been remarkable interest in evaluating decorin in clinical studies involving a wide spectrum of malignancies. Its effectiveness in animal models has inspired numerous groups to use decorin for prognosis and intervention. Since the 2000s, there have been numerable noteworthy studies concerning decorin and various forms of cancer. For instance, in 2003, Troup et al. analyzed 140 invasive breast carcinomas without axillary node involvement that were treated with adjuvant endocrine therapy (160). In their study, an increase in tumor size was associated with a statistically significant (P = 0.0496) reduction in decorin levels (160). Over the years, this proteoglycan has only snowballed in attention. Recently, Kawaguchi et al. set out to examine the relationship between decorin levels, exercising, and hepatocellular carcinoma (HCC) (161). In their study, 65 patients with a history of HCC that were treated with embolization were enrolled. The study population was divided into two groups, high decorin, and low decorin, after performing enzyme-linked immunosorbent assays. Increased serum decorin levels correlated with an increase in 6-min walking distance with overall survival being significantly higher in the high decorin group (P = 0.0353) (161). Glioblastoma multiforme (GBM) is devastating cancer with a poor prognosis due to being very invasive and the current chemotherapy regimens generally failing to fight it effectively. Therefore, the 5-year survival rate from this cancer is as low as 5.1% in recent studies (162). A 2021 study by Jia et al. (163) focused on the effects of decorin on GBM. In their multifaceted study, they obtained tumors from 42 patients with GBM and analyzed decorin expression via qRT-PCR. After dividing their patients into two groups, high and low expression, they have observed that the high DCN expression group had an overall higher survival rate (P = 0.0159). Although this is an impressive finding by itself, the group took it a step further by utilizing patient-derived xenograft models. As such, some of the tumors were implanted into nude mice and their metastatic behaviors were observed. Remarkably, the tumors with high decorin expression had markedly less invasive cells when compared with those possessing low decorin expression (163). Like other proteins and proteoglycans in the human body, decorin is differentially expressed across different tissues. The abundance of decorin also varies among different types of cancer. Using the recently developed Xena platform (164) and data from resources like The Cancer Genome Atlas (TCGA), we analyzed decorin expression profiles across different cancers and their normal counterparts (Fig. 3B). According to this database, decorin expression is suppressed in a variety of primary tumors indicative of the pertinent oncosuppressive role decorin plays (164). In addition, in breast, uterine, liver, and lung carcinomas, survival is markedly lower in patients with lower decorin expression levels as measured by the Kaplan–Meier cancer survival estimator database (Fig. 4). Following these studies, the reasonable way forward would be to utilize decorin isolates directly in a clinical trial. Unfortunately, at the time of writing, there are no studies utilizing this proteoglycan in such a way.
Figure 4.
Kaplan–Meier database for cancer prognosis shows the survival probability of patients with cancer in retrospective studies with high and low decorin levels. We used KM plotter (175, 176) from Gene Expression Omnibus (GEO) and European Genome-Phenome Archive (EGA) repositories. There is a clear correlation between low decorin expression and lower survival rates for patients suffering from breast, uterine, lung, and hepatocellular carcinomas, which are some of the most common metastatic malignancies.
CONCLUSIONS
The extracellular matrix has emerged as a novel locus for cancer therapeutics, especially considering the interactions between proteoglycans and cell surface growth factor receptors. Tumor tissue differs greatly in terms of extracellular matrix composition and RTK density when compared with healthy tissue. Indeed, the emergence of the matrisome as a bioinformatics ensemble of extracellular matrix-associated proteins (165, 166) needs to be considered as tumors can show unique features and variants of the matrisome (167, 168). Decorin acts as a vital SLRP that helps reprogram constitutive metabolic activity tailored toward cell growth, proliferation, and migration. Decorin is an endogenous matrix-centric pan-RTK inhibitor that possesses hierarchical binding for various RTKs expressed by a “target-rich” environment such as tumor cells. This property might function to integrate the activity of decorin across multiple RTKs with differential binding kinetics for sustained and proficient cross talk for optimal tumorigenic suppression. In this manner, decorin acts as a soluble cell cycle arrest agent against metastasis by inhibiting the activities of known oncogenic genes such as mTOR, ERK, β-catenin, and Myc while simultaneously upregulating genes such as p21, which serve oncosuppressive roles. Clinically, decorin was found to be ubiquitously expressed in most bodily tissues. Utilizing cancer databases that measure gene expression in normal and tumor tissues, decorin was found to be significantly downregulated in most solid tumors. Results of the clinical investigations and in vivo animal studies strongly suggest that decorin might be used in the near future as an adjuvant “protein therapeutic” for solid tumors where RTKs play a pivotal role. Decorin could be delivered as either fully glycanated proteoglycan or as a protein core, the size of which is similar to that of antibodies routinely used in the clinics. It could also be delivered as bioactive fragments harboring the internal leucine-rich repeats where all the bindings occur. Notably, it has been recently generated a fusion protein of decorin harboring a CAR peptide that targets inflammatory and angiogenic vasculature (169). This CAR-DCN is a multifunctional biotherapeutic that inhibits numerous growth factor signaling pathways involved in fibrosis (170, 171). It has been safely administered to mice to block fibrosis and the formation of abdominal aortic aneurysms (172) as well as to attenuate the pathology of murine muscular dystrophy (173). Finally, an analogous decorin-based fusion protein (DCN-tCRK) systemically delivered to Col7−/− mice, an animal model of recessive dystrophic epidermolysis bullosa, results in suppression of TGF-β signaling in the skin with an overall improvement of survival (174). Thus, we believe that the strategy of “monitoring from the matrix” with SLRPs like decorin provides a new paradigm that could be exploited as an additional therapeutic tool in the fight against cancer.
GRANTS
The original research was supported, in part, by the National Institutes of Health Grants RO1 CA39481 and RO1 CA245311 (to R.V.I.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTION
C.X. and R.V.I. conceived and designed research; C.X. and R.V.I. analyzed data; C.X., D.K.M., M.U., T.N., and R.V.I. interpreted results of experiments; C.X., D.K.M., M.U., T.N., and R.V.I. prepared figures; C.X., D.K.M., M.U., T.N., and R.V.I. drafted manuscript; C.X., D.K.M., M.U., T.N., and R.V.I. edited and revised manuscript; C.X., D.K.M., and R.V.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all past and present members of the laboratory and apologize for not referencing many valuable contributions to the fields because of space limitation.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Dr. Liliana Schaefer served as Guest Editor of this collection.
REFERENCES
- 1.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67: 609–652, 1998. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 2.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274: 18843–18846, 1999. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 277: 3864–3875, 2010. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iozzo RV, Karamanos N. Proteoglycans in health and disease: emerging concepts and future directions. FEBS J 277: 3863, 2010. doi: 10.1111/j.1742-4658.2010.07796.x. [DOI] [PubMed] [Google Scholar]
- 6.Hua R, Jiang JX. Small leucine-rich proteoglycans in physiological and biomechanical function of bone. Matrix Biol Plus 11: 100063, 2021. doi: 10.1016/j.mbplus.2021.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV. Decorin interacting network: a comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol 55: 7–21, 2016. doi: 10.1016/j.matbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidler DG. The galactosaminoglycan-containing decorin and its impact on diseases. Curr Opin Struct Biol 22: 578–582, 2012. doi: 10.1016/j.sbi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainio A, Järveläinen H. Extracellular matrix-cell interactions: focus on therapeutic applications. Cell Signal 66: 109487, 2020. doi: 10.1016/j.cellsig.2019.109487. [DOI] [PubMed] [Google Scholar]
- 11.Scott JE, Orford CR. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J 197: 213–216, 1981. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol 4: 229–255, 1988. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- 13.Vogel KG, Paulsson M, Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223: 587–597, 1984. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häkkinen L, Strassburger S, Kähäri VM, Scott PG, Eichstetter I, Lozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest 80: 1869–1880, 2000. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 15.Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationship in a transgenic age mouse model using multiple regression models. Ann Biomed Eng 32: 924–931, 2004. doi: 10.1023/B:ABME.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98: 1436–1449, 2006. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem 284: 8888–8897, 2009. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 280: 2120–2137, 2013. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng 126: 252–257, 2004. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- 20.Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV, Soslowsky LJ, Birk DE. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol 64: 81–93, 2017. doi: 10.1016/j.matbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chery DR, Han B, Zhou Y, Wang C, Adams SM, Chandrasekaran P, Kwok B, Heo SJ, Enomoto-Iwamoto M, Lu XL, Kong D, Iozzo RV, Birk DE, Mauck RL, Han L. Decorin regulates cartilage pericellular matrix micromechanobiology. Matrix Biol 96: 1–17, 2021. doi: 10.1016/j.matbio.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han B, Li Q, Wang C, Patel P, Adams SM, Doyran B, Nia HT, Oftadeh R, Zhou S, Li CY, Liu XS, Lu XL, Enomoto-Iwamoto M, Qin L, Mauck RL, Iozzo RV, Birk DE, Han L. Decorin regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix. ACS Nano 13: 11320–11333, 2019. doi: 10.1021/acsnano.9b04477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Han B, Wang C, Tong W, Wei Y, Tseng WJ, Han LH, Liu XS, Enomoto-Iwamoto M, Mauck RL, Qin L, Iozzo RV, Birk DE, Han L. Mediation of cartilage matrix degeneration and fibrillation by decorin in post-traumatic osteoarthritis. Arthritis Rheumatol 72: 1266–1277, 2020. doi: 10.1002/art.41254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Buyank F, Sinha NR, Grant DG, Sinha PR, Iozzo RV, Chaurasia SS, Mohan RR. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp Eye Res 216: 108933, 2022. doi: 10.1016/j.exer.2022.108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci USA 83: 7683–7687, 1986. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5' untranslated region, and mapping of the gene to chromosome 12q23. Genomics 15: 146–160, 1993. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- 27.Scholzen T, Solursh M, Suzuki S, Reiter R, Morgan JL, Buchberg AM, Siracusa LD, Iozzo RV. The murine decorin. Complete cDNA cloning, genomic organization, chromosomal assignment, and expression during organogenesis and tissue differentiation. J Biol Chem 269: 28270–28281, 1994. [PubMed] [Google Scholar]
- 28.Santra M, Danielson KG, Iozzo RV. Structural and functional characterization of the human decorin gene promoter. A homopurine-homopyrimidine S1 nuclease-sensitive region is involved in transcriptional control. J Biol Chem 269: 579–587, 1994. [PubMed] [Google Scholar]
- 29.Mauviel A, Korang K, Santra M, Tewari D, Uitto J, Iozzo RV. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J Biol Chem 271: 24824–24829, 1996. doi: 10.1074/jbc.271.40.24824. [DOI] [PubMed] [Google Scholar]
- 30.Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-α. J Biol Chem 270: 11692–11700, 1995. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- 31.Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 141: 17–27, 2014. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanOpstall C, Perike S, Brechka H, Gillard M, Lamperis S, Zhu B, Brown R, Bhanvadia R, Vander Griend DJ. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. eLife 9: e53600, 2020. doi: 10.7554/eLife.53600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferdous F, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res A 93: 419–428, 2010. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J 19: 249–255, 2002. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 35.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136: 729–743, 1997. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J 302: 527–534, 1994. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature 336: 244–246, 1988. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346: 281–284, 1990. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 39.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Yu, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 360: 361–364, 1992. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 40.Ferdous Z, Wei VM, Iozzo R, Höök M, Grande-Allen KJ. Decorin-transforming growth factor-β interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem 282: 35887–35898, 2007. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 41.Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 274: 4489–4492, 1999. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 42.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest 101: 406–412, 1998. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem 277: 35671–35681, 2002. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- 44.Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr, Dalton N, Jones Y, Reed CC, Iozzo RV, McCulloch AD. A role for decorin in the remodeling of myocardial infarction. Matrix Biol 24: 313–324, 2005. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Schönherr E, Sunderkötter C, Schaefer L, Thanos S, Grässel S, Oldberg Å, Iozzo RV, Young MF, Kresse H. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res 41: 499–508, 2004. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- 46.Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem 287: 33926–33933, 2012. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaefer L, Macakova K, Raslik I, Micegova M, Gröne H-J, Schönherr E, Robenek H, Echtermeyer FG, Grässel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol 160: 1181–1191, 2002. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang ZX, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS One 7: e45559, 2012. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci USA 92: 7016–7020, 1995. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest 100: 149–157, 1997. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA 96: 3092–3097, 1999. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem 281: 26408–26418, 2006. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 53.Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 29: 1435–1440, 2008. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 33: 326–330, 2012. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horváth Z, Kovalszky I, Fullár A, Kiss K, Schaff Z, Iozzo RV, Baghy K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol 35: 194–205, 2014. doi: 10.1016/j.matbio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bi X, Xia X, Fan D, Mu T, Zhang Q, Iozzo RV, Yang W. Oncogenic activin C interacts with decorin in colorectal cancer in vivo and in vitro. Mol Carcinog 55: 1786–1795, 2016. doi: 10.1002/mc.22427. [DOI] [PubMed] [Google Scholar]
- 57.Mao L, Yang J, Yue J, Chen Y, Zhou H, Fan D, Zhang Q, Buraschi S, Iozzo RV, Bi X. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol 95: 1–14, 2021. doi: 10.1016/j.matbio.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henke A, Grace OC, Ashley GR, Stewart GD, Riddick ACP, Yeun H, O'Donnell M, Anderson RA, Thomson AA. Stromal expression of decorin, semaphorin6D, SPARC, Sprouty 1 and Tsukushi in developing prostate and decreased levels of decorin in prostate cancer. PLoS One 7: e4251, 2012. doi: 10.1371/journal.pone.0042516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards IJ. Proteoglycans in prostate cancer. Nat Rev Urol 9: 196–206, 2012. doi: 10.1038/nrurol.2012.19. [DOI] [PubMed] [Google Scholar]
- 60.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 15: 1013–1031, 2011. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theocharis AD, Skandalis SS, Neill T, Multhaupt HA, Hubo M, Frey H, Gopal S, Gomes A, Afratis N, Lim HC, Couchman JR, Filmus J, Sanderson RD, Schaefer L, Iozzo RV, Karamanos NK. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim Biophys Acta 1855: 276–300, 2015. doi: 10.1016/j.bbcan.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nyman MC, Sainio AO, Pennanen MM, Lund RJ, Vuorikoski S, Sundström JT, Järveläinen HT. Decorin in human colon cancer: localization in vivo and effect on cancer cell behavior in vitro. J Histochem Cytochem 63: 710–720, 2015. doi: 10.1369/0022155415590830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nyman MC, Jokilammi AB, Böstrom PC, Kurki SH, Sainio AO, Grenman SE, Orte KJ, Hietanen SH, Elenius K, Järveläinen HT. Decorin expression in human vulva carcinoma: oncosuppressive effect of decorin cDNA transduction on carcinoma cells. J Histochem Cytochem 67: 511–522, 2019. doi: 10.1369/0022155419845373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y, Xia Q, Rao Q, Shi S, Shi Q, Ma H, Lu Z, Chen H, Zhou X. DCN deficiency promotes renal cell carcinoma growth and metastasis through downregulation of P21 and E-cadherin. Tumour Biol 37: 5171–5183, 2016. doi: 10.1007/s13277-015-4160-1. [DOI] [PubMed] [Google Scholar]
- 65.Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J 284: 10–26, 2017. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji C, Liu H, Xiang M, Liu J, Yue F, Wang W, Chu X. Deregulation of decorin and FHL1 are associated with esophageal squamous cell carcinoma progression and poor prognosis. Int J Clin Exp Med 8: 20965–20970, 2015. [PMC free article] [PubMed] [Google Scholar]
- 67.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci USA 110: E2582–E2591, 2013. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neill T, Schaefer L, Iozzo RV. Instructive roles of extracellular matrix on autophagy. Am J Pathol 184: 2146–2153, 2014. doi: 10.1016/j.ajpath.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neill T, Schaefer L, Iozzo RV. Decoding the matrix: instructive roles of proteoglycan receptors. Biochemistry 54: 4583–4598, 2015. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neill T, Schaefer L, Iozzo RV. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev 97: 174–185, 2016. doi: 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neill T, Torres A, Buraschi S, Owens RT, Hoek JB, Baffa R, Iozzo RV. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin. J Biol Chem 289: 4952–4968, 2014. doi: 10.1074/jbc.M113.512566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gubbiotti MA, Buraschi S, Kapoor A, Iozzo RV. Proteoglycan signaling in tumor angiogenesis and endothelial cell autophagy. Semin Cancer Biol 62: 1–8, 2020. doi: 10.1016/j.semcancer.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neill T, Buraschi S, Kapoor A, Iozzo RV. Proteoglycan-driven autophagy: a nutrient-independent mechanism to control intracellular catabolism. J Histochem Cytochem 68: 733–746, 2020. doi: 10.1369/0022155420937370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neill T, Kapoor A, Xie C, Buraschi S, Iozzo RV. A functional outside-in signaling network of proteoglycans and matrix molecules regulating autophagy. Matrix Biol 100–101: 118–149, 2021. doi: 10.1016/j.matbio.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen CG, Iozzo RV. Extracellular matrix guidance of autophagy: a mechanism regulating cancer growth. Open Biol 12: 210304, 2022. doi: 10.1098/rsob.210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odorisio T, Di Salvio M, Orecchia A, Di ZG, Piccinni E, Cianfarani F, Travaglione A, Uva P, Bellei B, Conti A, Zambruno G, Castiglia D. Monozygotic twins discordant for recessive dystrophic epidermolysis bullosa phenotype highlight the role of TGF-β signalling in modifying disease severity. Hum Mol Genet 23: 3907–3922, 2014. doi: 10.1093/hmg/ddu102. [DOI] [PubMed] [Google Scholar]
- 77.Cianfarani F, De Domenico E, Nyström A, Mastroeni S, Abeni D, Baldini E, Ulisse S, Uva P, Bruckner-Tuderman L, Zambruno G, Castiglia D, Odorisio T. Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa. Matrix Biol 81: 3–16, 2019. doi: 10.1016/j.matbio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Uitto J, Has C, Vahidnezhad H, Youssefian L, Bruckner-Tuderman L. Molecular pathology of the basement membrane zone in heritable blistering diseases: the paradigm of epidermolysis bullosa. Matrix Biol 57–58: 76–85, 2017. doi: 10.1016/j.matbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Ryynänen M, Ryynänen J, Sollberg S, Iozzo RV, Knowlton RG, Uitto J. Genetic linkage of Type VII collagen (COL7A1) to dominant dystrophic epidermolysis bullosa in families with abnormal anchoring fibrils. J Clin Invest 89: 974–980, 1992. doi: 10.1172/JCI115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudnicka L, Varga J, Christiano AM, Iozzo RV, Jimenez SA, Uitto J. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. Regulation by transforming growth factor-β. J Clin Invest 93: 1709–1715, 1994. doi: 10.1172/JCI117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nyström A, Bornert O, Kühl T. Cell therapy for basement membrane-linked diseases. Matrix Biol 57–58: 124–139, 2017. doi: 10.1016/j.matbio.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Bredrup C, Knappskog PM, Majewski J, Rødahl E, Boman H. Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Invest Ophthalmol Vis Sci 46: 420–426, 2005. doi: 10.1167/iovs.04-0804. [DOI] [PubMed] [Google Scholar]
- 83.Bredrup C, Stang E, Bruland O, Palka BP, Young RD, Haavik J, Knappskog PM, Rødahl E. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Invest Ophthalmol Vis Sci 51: 5578–5582, 2010. doi: 10.1167/iovs.09-4933. [DOI] [PubMed] [Google Scholar]
- 84.Chen S, Sun M, Meng X, Iozzo RV, Kao WWY, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy. C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol 179: 2409–2419, 2011. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen S, Sun M, Iozzo RV, Kao WW, Birk DE. Intracellularly-retained decorin lacking the C-terminal ear repeat causes ER stress: a cell-based etiological mechanism for congenital stromal corneal dystrophy. Am J Pathol 183: 247–256, 2013. doi: 10.1016/j.ajpath.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mellgren AEC, Bruland O, Vedeler A, Saraste J, Schönheit J, Bredrup C, Knappskog PM, Rødahl E. Development of congenital stromal corneal dystrophy is dependent on export and extracellular deposition of truncated decorin. Invest Ophthalmol Vis Sci 56: 2909–2915, 2015. doi: 10.1167/iovs.14-16014. [DOI] [PubMed] [Google Scholar]
- 87.Schneider M, Pawlak R, Weber GR, Dillinger AE, Kuespert S, Iozzo RV, Quigley HA, Ohlmann A, Tamm ER, Fuchshofer R. A novel ocular function for decorin in the aqueous humor outflow. Matrix Biol 97: 1–19, 2021. doi: 10.1016/j.matbio.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J. Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc Natl Acad Sci USA 101: 15633–15638, 2004. doi: 10.1073/pnas.0402976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol 25: 1431–1443, 2011. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vial C, Gutiérrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem 286: 24242–24252, 2011. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalamajski S, Aspberg A, Oldberg Å. The decorin sequence SYIRIADTNIT binds collagen type I. J Biol Chem 282: 16062–16067, 2007. doi: 10.1074/jbc.M700073200. [DOI] [PubMed] [Google Scholar]
- 92.Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. Decorin binds near the C terminus of type I collagen. J Biol Chem 275: 21801–21804, 2000. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 93.Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J 274: 4246–4255, 2007. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 94.Nikolovska K, Renke JK, Jungmann O, Grobe K, Iozzo RV, Zamfir AD, Seidler DG. A decorin-deficient matrix affects skin chondroitin/dermatan sulfate levels and keratinocyte function. Matrix Biol 35: 91–102, 2014. doi: 10.1016/j.matbio.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daquinag AC, Gao Z, Fussell C, Sun K, Kolonin MG. Glycosaminoglycan modification of decorin depends on MMP14 activity and regulates collagen assembly. Cells 9: 2646, 2020. doi: 10.3390/cells9122646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scott PG, Grossmann JG, Dodd CM, Sheehan JK, Bishop PN. Light and X-ray scattering show decorin to be a dimer in solution. J Biol Chem 278: 18353–18359, 2003. doi: 10.1074/jbc.M211936200. [DOI] [PubMed] [Google Scholar]
- 97.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J Biol Chem 279: 6606–6612, 2004. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 98.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol 181: 380–387, 2012. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mongiat M, Buraschi S, Andreuzzi E, Neill T, Iozzo RV. Extracellular matrix: the gatekeeper of tumor angiogenesis. Biochem Soc Trans 47: 1543–1555, 2019. doi: 10.1042/BST20190653. [DOI] [PubMed] [Google Scholar]
- 100.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 438: 967–974, 2005. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 101.Folkman J, Shing Y. Angiogenesis. J Biol Chem 267: 10931–10934, 1992. [PubMed] [Google Scholar]
- 102.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene 21: 3688–3695, 2002. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 103.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene 24: 1104–1110, 2005. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 104.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. An antimetastatic role for decorin in breast cancer. Am J Pathol 173: 844–855, 2008. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21: 4765–4777, 2002. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 106.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage E H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 14: 443–452, 2006. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 107.Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol 43: 15–26, 2015. doi: 10.1016/j.matbio.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zoeller JJ, Whitelock JM, Iozzo RV. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol 28: 284–291, 2009. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goyal A, Pal N, Concannon M, Paul M, Doran M, Poluzzi C, Sekiguchi K, Whitelock JM, Neill T, Iozzo RV. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): a dual receptor antagonism. J Biol Chem 286: 25947–25962, 2011. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goyal A, Poluzzi C, Willis CD, Smythies J, Shellard A, Neill T, Iozzo RV. Endorepellin affects angiogenesis by antagonizing diverse vascular endothelial growth factor receptor 2 (VEGFR2)-evoked signaling pathways: transcriptional repression of hypoxia-inducible factor 1α and VEGFA and concurrent inhibition of nuclear factor of activated T cell 1 (NFAT1) activation. J Biol Chem 287: 43543–43556, 2012. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J 19: 716–728, 2005. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 112.Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T. Collagen XVIII in tissue homeostasis and dysregulation-lessons learned from model organisms and human patients. Matrix Biol 57–58: 55–75, 2017. doi: 10.1016/j.matbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev 97: 156–173, 2016. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diehl V, Huber LS, Trebicka J, Wygrecka M, Iozzo RV, Schaefer L. The role of decorin and biglycan signaling in tumorigenesis. Front Oncol 11: 801801, 2021. doi: 10.3389/fonc.2021.801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu J-X, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem 280: 32468–32479, 2005. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 116.Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 cells. J Biol Chem 273: 3121–3124, 1998. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- 117.Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem 275: 32879–32887, 2000. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 118.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with upregulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem 271: 18961–18965, 1996. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Wang Y, Du Z, Wang Q, Wu M, Wang X, Wang L, Cao L, Hamid AS, Zhang G. Recombinant human decorin suppresses liver HepG2 carcinoma cells by p21 upregulation. Onco Targets Ther 5: 143–152, 2012. doi: 10.2147/OTT.S32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neill T, Jones HR, Crane-Smith Z, Owens RT, Schaefer L, Iozzo RV. Decorin induces rapid secretion of thrombospondin-1 in basal breast carcinoma cells via inhibition of Ras homolog gene family, member A/Rho-associated coiled-coil containing protein kinase 1. FEBS J 280: 2353–2368, 2013. doi: 10.1111/febs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zafiropoulos A, Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Decorin-induced growth inhibition is overcome through protracted expression and activation of epidermal growth factor receptors in osteosarcoma cells. Mol Cancer Res 6: 785–794, 2008. doi: 10.1158/1541-7786.MCR-07-0165. [DOI] [PubMed] [Google Scholar]
- 122.Hu X, Villodre ES, Larson R, Rahal OM, Wang X, Gong Y, Song J, Krishnamurthy S, Ueno NT, Tripathy D, Woodward WA, Debeb BG. Decorin-mediated suppression of tumorigenesis, invasion, and metastasis in inflammatory breast cancer. Commun Biol 4: 72, 2021. doi: 10.1038/s42003-020-01590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 185: 743–754, 2009. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Danilkovitch-Miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest 109: 863–867, 2002. doi: 10.1172/JCI15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. MET, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925, 2003. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 126.Goldoni S, Iozzo RV. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer 123: 2473–2479, 2008. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 127.Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and down-regulates β-catenin and Myc levels. J Biol Chem 285: 42075–42085, 2010. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3. J Biol Chem 287: 5492–5506, 2012. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neill T, Sharpe C, Owens RT, Iozzo RV. Decorin-evoked paternally expressed gene 3 (PEG3) is an upstream regulator of the transcription factor EB (TFEB) in endothelial cell autophagy. J Biol Chem 292: 16211–16220, 2017. doi: 10.1074/jbc.M116.769950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Balne PK, Gupta S, Zhang J, Bristow D, Faubion M, Heil SD, Sinha PR, Green SL, Iozzo RV, Mohan RR. The functional role of decorin in corneal neovascularization in vivo. Exp Eye Res 207: 108610, 2021. doi: 10.1016/j.exer.2021.108610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torres A, Gubbiotti MA, Iozzo RV. Decorin-inducible Peg3 evokes Beclin 1-mediated autophagy and Thrombospondin 1-mediated angiostasis. J Biol Chem 292: 5055–5069, 2017. doi: 10.1074/jbc.M116.753632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV. Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol 34: 46–54, 2014. doi: 10.1016/j.matbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Neill T, Chen CG, Buraschi S, Iozzo RV. Catabolic degradation of endothelial VEGFA via autophagy. J Biol Chem 295: 6064–6079, 2020. doi: 10.1074/jbc.RA120.012593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Doerr ME, Jones JI. The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J Biol Chem 271: 2443–2447, 1996. doi: 10.1074/jbc.271.5.2443. [DOI] [PubMed] [Google Scholar]
- 135.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-κB and snail. Mol Cell Biol 27: 3165–3175, 2007. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Metalli D, Lovat F, Tripodi F, Genua M, Xu S-Q, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, Iozzo RV, Morrione A. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol 176: 2997–3006, 2010. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC, Gomella LG, Owens RT, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem 286: 34712–34721, 2011. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dyrskjøt L, Kruhøffer M, Thykjaer T, Marcussen N, Jensen JL, Møller K, Ørntoft TF. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res 64: 4040–4048, 2004. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 139.Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. Decorin suppresses transforming growth factor-β-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J 362: 643–649, 2002. doi: 10.1042/bj3620643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baghy K, Dezsó K, László V, Fullár A, Péterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest 91: 439–451, 2011. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baghy K, Iozzo RV, Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem 60: 262–268, 2012. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol 60, Suppl 4: 5–13, 2009. [PMC free article] [PubMed] [Google Scholar]
- 143.Nastase MV, Iozzo RV, Schaefer L. Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology. Biochim Biophys Acta 1840: 2460–2470, 2014. doi: 10.1016/j.bbagen.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1. J Biol Chem 282: 18842–18850, 2007. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 145.Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost 1: 1663–1670, 2003. doi: 10.1046/j.1538-7836.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 146.Appert-Collin A, Bennasroune A, Jeannesson P, Terryn C, Fuhrmann G, Morjani H, Dedieu S. Role of LRP-1 in cancer cell migration in 3-dimensional collagen matrix. Cell Adh Migr 11: 316–326, 2017. doi: 10.1080/19336918.2016.1215788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cabello-Verrugio C, Santander C, Cofre C, Acuña MJ, Melo F, Brandan E. The internal region leucine-rich repeat 6 of decorin interacts with low density lipoprotein receptor-related protein-1, modulates transforming growth factor (TGF)-β-dependent signaling, and inhibits TGF-β-dependent fibrotic response in skeletal muscles. J Biol Chem 287: 6773–6787, 2012. doi: 10.1074/jbc.M111.312488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schneider M, Dillinger AE, Ohlmann A, Iozzo RV, Fuchshofer R. Decorin-An antagonist of TGF-β in astrocytes of the optic nerve. Int J Mol Sci 22: 7660, 2021. doi: 10.3390/ijms22147660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Frey T, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J 280: 2165–2179, 2013. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci Signal 4: ra75, 2011. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023, 2017. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27: 2128–2136, 2008. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 153.Köninger J, Giese NA, di Mola FF, Berberat P, Giese T, Esposito I, Bachem MG, Büchler MW, Friess H. Overexpressed decorin in pancreatic cancer: potential tumor growth inhibition and attenuation of chemotherapeutic action. Clin Cancer Res 10: 4776–4783, 2004. doi: 10.1158/1078-0432.CCR-1190-03. [DOI] [PubMed] [Google Scholar]
- 154.Boström P, Sainio A, Kakko T, Savontaus M, Söderström M, Järveläinen H. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochem Cell Biol 139: 161–171, 2013. doi: 10.1007/s00418-012-1026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Appunni S, Anand V, Khandelwal M, Gupta N, Rubens M, Sharma A. Small leucine rich proteoglycans (decorin, biglycan and lumican) in cancer. Clin Chim Acta 491: 1–7, 2019. doi: 10.1016/j.cca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 156.Sainio AO, Järveläinen HT. Decorin-mediated oncosuppression - a potential future adjuvant therapy for human epithelial cancers. Br J Pharmacol 176: 5–15, 2019. doi: 10.1111/bph.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hunzelmann N, Anders S, Fierlbeck G, Hein R, Herrmann K, Albrecht M, Bell S, Muche R, Wehner-Caroli J, Gaus W, Krieg T. Double-blind, placebo-controlled study of intralesional interferon gamma for the treatment of localized scleroderma. J Am Acad Dermatol 36: 433–435, 1997. doi: 10.1016/s0190-9622(97)80221-6. [DOI] [PubMed] [Google Scholar]
- 158.Schönherr E, Lügering N, Stoll R, Domschke W, Kresse H. Differences in decorin and biglycan expression in patients with gastric ulcer healing. Scand J Gastroenterol 32: 785–790, 1997. doi: 10.3109/00365529708996535. [DOI] [PubMed] [Google Scholar]
- 159.Neill T, Torres A, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy 9: 1626–1628, 2013. doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- 160.Troup S, Njue C, Kliewer EV, Parisien M, Roskelley C, Chakravarti S, Roughley PJ, Murphy LC, Watson PH. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res 9: 207–214, 2003. [PubMed] [Google Scholar]
- 161.Kawaguchi T, Yoshio S, Sakamoto Y, Hashida R, Koya S, Hirota K, Nakano D, Yamamura S, Niizeki T, Matsuse H, Torimura T. Impact of decorin on the physical function and prognosis of patients with hepatocellular carcinoma. J Clin Med 9: 936, 2020. doi: 10.3390/jcm9040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol 20, Suppl 4: iv1–iv86, 2018. [Erratum in Neuro Oncol 23: 508–522, 2018]. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jia Y, Feng Q, Tang B, Luo X, Yang Q, Yang H, Li Q. Decorin suppresses invasion and EMT phenotype of glioma by Inducing autophagy via c-Met/Akt/mTOR axis. Front Oncol 11: 659353, 2021. doi: 10.3389/fonc.2021.659353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 38: 675–678, 2020. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 11: M111.014647, 2012. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the "omics" era. Matrix Biol 49: 10–24, 2016. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Izzi V, Lakkala J, Devarajan R, Kääriäinen A, Koivunen J, Heljasvaara R, Pihlajaniemi T. Pan-cancer analysis of the expression and regulation of matrisome genes across 32 tumor types. Matrix Biol Plus 1: 100004, 2019. doi: 10.1016/j.mbplus.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Izzi V, Davis MN, Naba A. Pan-cancer analysis of the genomic alterations and mutations of the matrisome. Cancers (Basel) 12: 2046, 2020. doi: 10.3390/cancers12082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Järvinen TAH, Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci USA 107: 21671–21676, 2010. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Järvinen TAH, Ruoslahti E. Generation of a multi-functional, target organ-specific, anti-fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin. Br J Pharmacol 176: 16–25, 2019. doi: 10.1111/bph.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Järvinen TAH, Pemmari T. Systemically administered, target-specific, multi-functional therapeutic recombinant proteins in regenerative medicine. Nanomaterials (Basel) 10: 226, 2020. doi: 10.3390/nano10020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shen Y, Russo V, Zeglinski MR, Sellers SL, Wu Z, Oram C, Santacruz S, Merkulova Y, Turner C, Tauh K, Zhao H, Bozin T, Bohunek L, Zeng H, Seidman MA, Bleackley RC, McManus BM, Ruoslahti E, Järvinen TAH, Granville DJ. Recombinant decorin fusion protein attenuates murine abdominal aortic aneurysm formation and rupture. Sci Rep 7: 15857, 2017. doi: 10.1038/s41598-017-16194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Iqbal A, May U, Prince SN, Järvinen TAH, Heydemann A. Systemically administered homing peptide targets dystrophic lesions and delivers transforming growth factor-β (TGFβ) inhibitor to attenuate murine muscular dystrophy pathology. Pharmaceutics 13: 1506, 2021. doi: 10.3390/pharmaceutics13091506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pemmari T, Ivanova L, May U, Lingasamy P, Tobi A, Pasternack A, Prince S, Ritvos O, Makkapati S, Teesalu T, Cairo MS, Järvinen TAH, Liao Y. Exposed CendR domain in homing peptide yields skin-targeted therapeutic in epidermolysis bullosa. Mol Ther 28: 1833–1845, 2020. doi: 10.1016/j.ymthe.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 123: 725–731, 2010. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 176.Mihály Z, Kormos M, Lánczky A, Dank M, Budczies J, Szász MA, Györffy B. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res Treat 140: 219–232, 2013. doi: 10.1007/s10549-013-2622-y. [DOI] [PubMed] [Google Scholar]