Abstract
One of the primary functions of the intestinal epithelium is to transport fluid and electrolytes to and from the luminal contents. Under normal circumstances, absorptive and secretory processes are tightly regulated such that absorption predominates, thereby enabling conservation of the large volumes of water that pass through the intestine each day. However, in conditions of secretory diarrhea, this balance becomes dysregulated, so that fluid secretion, driven primarily by Cl− secretion, overwhelms absorptive capacity, leading to increased loss of water in the stool. Secretory diarrheas are common and include those induced by pathogenic bacteria and viruses, allergens, and disruptions to bile acid homeostasis, or as a side effect of many drugs. Here, we review the cellular and molecular mechanisms by which Cl− and fluid secretion in the intestine are regulated, how these mechanisms become dysregulated in conditions of secretory diarrhea, currently available and emerging therapeutic approaches, and how new strategies to exploit intestinal secretory mechanisms are successfully being used in the treatment of constipation.
Keywords: chloride secretion, diarrhea, epithelial transport
INTRODUCTION
The gastrointestinal system deals with large volumes of fluid daily in the process of digesting and absorbing nutrients. Including around 1 L of fluid that is ingested in the form of beverages or as part of food, the intestinal fluid load averages ∼9 L/day. The majority of the fluid load derives from digestive secretions from the salivary glands, stomach, pancreas, and biliary system, and the intestine itself also secretes fluid to maintain appropriate fluidity of the luminal contents and lubricate their passage along the length of the gut. In health, the vast majority of the fluid load is absorbed by the gut epithelium, driven either by the uptake of solutes derived from nutrients (such as glucose, amino acids, and fatty acids) or by the reabsorption of electrolytes. This typically leaves only 100–200 mL/day to be lost to the stool. Both the small and the large intestines, moreover, have significant reserve capacity for fluid absorption. Thus, under normal circumstances, the secretory function of the gut can be stimulated severalfold, and/or the absorptive function inhibited, before fluid losses to the stool increase. This is a key homeostatic mechanism to avoid dehydration.
The ability of the intestine to absorb large volumes of fluid is underpinned by its substantial surface area. However, this also represents a liability in that if a potent prosecretory stimulus is present, an increase in secretion of fluid, driven by the active secretion of Cl− ions, can ensue and overwhelm the anatomical reserve for fluid absorption (even in the setting of normal levels of fluid absorption). This represents the underlying pathogenesis of secretory diarrheal illnesses. The classical example is cholera, where a bacterial toxin irreversibly stimulates active Cl− secretion resulting in watery diarrhea, and in which diarrheal volumes of several liters per day are common. Like many other secretory diarrheal illnesses, moreover, fluid losses in cholera are exacerbated by the fact that cholera toxin also inhibits the ability of the epithelium to absorb NaCl, and because there is also activation of the enteric nervous system with release of additional Cl− secretagogues. Secretory diarrhea should be contrasted with osmotic diarrheal illnesses, with the latter resulting from a failure to absorb a specific solute from the gut (typically from the diet, and other than an electrolyte) that then retains fluid in the lumen osmotically (e.g., lactose intolerance). The distinction between secretory and osmotic diarrhea may be useful clinically (e.g., measurement of the stool osmotic gap can reveal an osmotic pathogenesis of diarrheal illness).
Despite additional mechanisms that may amplify disease, secretory diarrhea is driven fundamentally by an increase in Cl− secretion. Because of this fact, the Cl− secretory mechanism has long been considered as an attractive target to counter disease and prevent dehydration, which may be life-threatening especially in vulnerable individuals. Classically, antidiarrheal medications (such as loperamide) acted predominantly to slow propulsive motility, but such drugs are not wholly effective, may exert side effects, and often lose efficacy over time and/or cause rebound constipation. On the other hand, a focus on Cl− secretion as a therapeutic target is attractive because disease control might be accomplished luminally, without a need for a systemically active agent. Furthermore, inhibition of Cl− secretion has the potential to be effective even if the precise etiology of the diarrheal illness has not yet been established, which is often the case in developing countries where diarrheal disease outbreaks are commonplace. Recent studies have in fact identified a number of small molecules and natural products that may act as potent inhibitors of various aspects of the Cl− secretory mechanism. Considering the other side of the coin, moreover, the ability to therapeutically activate Cl− secretion is increasingly recognized as an effective strategy in many forms of constipation.
The goal of this review, therefore, is to describe the molecular mechanisms of Cl−-driven fluid secretion in the gut, and their involvement in secretory diarrheal diseases of varying etiologies. Building on this knowledge, we will discuss mechanisms of action for new and emerging inhibitors of Cl− secretion that are likely to become important additions to our armamentarium for treating diarrheal diseases. Finally, we will address the therapeutic strategy of inducing limited “diarrhea” iatrogenically to treat serious constipation.
MOLECULAR MECHANISMS OF CHLORIDE-DRIVEN FLUID SECRETION
The molecular pathway underlying Cl− secretion, the primary driving force for fluid secretion in the intestine, has been quite well elucidated (Fig. 1). Studies in cultured epithelial cells and intact intestinal tissues have revealed that the process occurs primarily via crypt enterocytes and is driven by the concerted activity of several transport proteins arranged in a polarized fashion. Polarization refers to a fundamental characteristic of epithelial cells whereby specific transport (and other) proteins are differentially localized to their apical and basolateral membranes, enabling vectorial electrolyte transport to occur. Recent years have seen significant advances in our understanding of how these proteins are regulated at the molecular level.
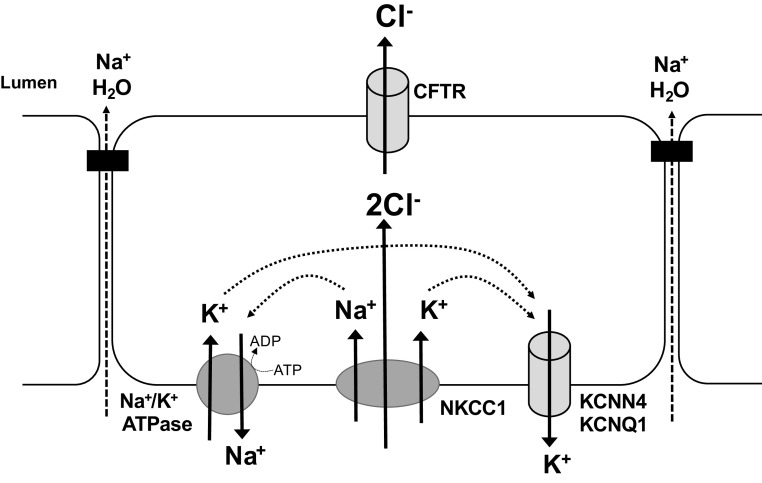
Figure 1.
Intestinal epithelial Cl− secretory mechanism. Chloride secretion is the primary driving force for water secretion across the intestinal epithelium. The energy for this process is derived from the activity of Na+-K+-ATPase pumps in the basolateral membrane. This transporter exudes 3 Na+ from the cell in exchange for 2 K+ into the cell with each molecule of ATP consumed. K+ is recycled across the basolateral membrane through the cAMP and Ca2+-regulated K+ channels, KCNQ1 and KCNN4, respectively. Through the activity of the Na+-K+-ATPase pump, intracellular Na+ is maintained at low levels creating a gradient for its entry via the Na+-K+-2Cl− cotransporter, NKCC1. Since Na+ and K+ entering the cell via this transporter are recycled across the basolateral membrane, a net increase in chloride concentration inside the cell results, with the result that when the apical Cl− channel, cystic fibrosis transmembrane conductance regulator (CFTR), opens (or other Cl− channels, not shown), there is an electrochemical gradient for chloride secretion into the lumen. Cations, most notably Na+, follow passively through the paracellular pathway, with the net accumulation of NaCl in the lumen creating an osmotic gradient that drives water secretion.
Na-K-ATPase
Fluid secretion is driven by osmotic gradients established by active transmembrane electrolyte transport, with the maintenance of such gradients requiring considerable amounts of cellular energy. This energy is provided by the activity of Na+-K+-ATPase pumps located in the basolateral membrane. With each cycle of activity, these pumps extrude three Na+ ions from the cell in exchange for two K+ ions moving into the cell. Movement of these ions occurs against their electrochemical gradients and therefore energy is required in the form of ATP hydrolysis. Thus, the primary function of Na+-K+-ATPase pumps is to maintain intracellular Na+ concentrations at low levels, ensuring that a gradient exists for its uptake via secondary active transport pathways that in turn can drive the uptake of additional solutes (Cl−, in the case of transepithelial Cl− secretion).
The Na+-K+-ATPase pump consists of three subunits α, β, and FXYD, of which multiple isoforms exist (1). The α subunit is the catalytically active component and is responsible for hydrolysis of ATP and the transport of Na+ and K+. In its resting state, the binding pocket of the α subunit is open to the cytosol and has a higher affinity for Na+ than K+. Upon phosphorylation, the protein changes conformation and opens to the extracellular side of the membrane. In this conformation, the pump has a higher affinity for K+, resulting in the release of Na+ into the extracellular fluid and binding of K+. When K+ binds, the pump becomes dephosphorylated and returns its original conformation with the release of K+ inside the cell and is then ready for another cycle of activity. Four isoforms of the α subunit are known to exist, with the α1 isoform being ubiquitously expressed and responsible for Na+/K+ exchange in the intestine (2). The Na-K-ATPase β subunit is a regulatory glycoprotein that is thought to function as a chaperone that ensures appropriate folding as well as insertion of the α subunit into the basolateral membrane. Some studies suggest that β subunits are also important in maintaining cellular polarity and intercellular contact between neighboring cells (3). Three different isoforms of the β subunit are known to exist, with the β1 isoform predominating in the intestine (2). The third component of Na+-K+-ATPase oligomers are the FXYD family of proteins, of which there are currently seven known members (4, 5). FXYD proteins are expressed in a tissue-specific manner with different isoforms conferring different properties on the pump, most notably its affinity for Na+ and K+.
NKCC1
The low intracellular levels of Na+ generated by Na+-K+-ATPase pump activity create an electrochemical gradient favoring Na+ uptake into the cell. The primary pathway by which this occurs in secretory intestinal epithelial cells is through the Na+-K+-2Cl− cotransporter (NKCC1; Slc12a2). This cotransporter, which is also located on the basolateral membrane, imports Na+ along with K+ and 2 Cl− ions and since its activity does not result in the net transfer of charge, it is termed an electroneutral transporter. NKCC1 is a single 130-kDa protein that spans the basolateral membrane 12 times (6). The cytosolic NH2-terminal domain is important for the binding of regulatory kinases, whereas the COOH-terminal domain directs trafficking of the protein to the basolateral membrane (7). The transmembrane core of the protein contains the ion binding sites and glycosylation of this domain is necessary for transporter activity (8).
K+ Channels
For Cl− secretion to occur, the cations that are cotransported with Na+ and Cl− ions into epithelial cells by NKCC1 must subsequently be extruded to maintain the driving force for Cl− to exit across the apical membrane, along with a lumen-positive electrochemical gradient. Na+ exits via the ATPase pump, while K+ entering the cell, via either NKCC1 or the Na+-K+-ATPase, exits through channels in the basolateral membrane. Two types of K+ channel are established as being particularly important in the Cl− secretory pathway; KCNQ1 and KCNN4 (SK4). KCNQ1 is a protein that spans the membrane six times and forms a tetramer to create a voltage-gated low-conductance K+ channel, the gating of which is modulated by regulatory subunits, known as KCNEs (9, 10). Association of KCNQ1 with the KCNE1 subunit creates a slowly activating, voltage-dependent channel, whereas association with KCNE3 converts the channel into a voltage-independent and constitutively open form that is sensitive to elevations in intracellular cAMP (11, 12). On the other hand, KCNN4 is a K+ channel that is regulated by intracellular Ca2+. Similar to KCNQ1, the channel is a tetramer, with each of its subunits having six transmembrane domains (13). KCNN4 channels have a calmodulin binding domain in their COOH-terminal that confers their sensitivity to intracellular Ca2+ levels.
Cl− Channels
The concerted activity of basolateral Na+-K+-ATPase pumps, NKCC1 and K+ channels results in accumulation of Cl− within the cell, thereby creating an intracellular to extracellular electrochemical gradient. Chloride channels are present on the apical membrane and, when open, they allow Cl− to flow from the cell into the lumen. The primary route for Cl− exit in the intestinal epithelium is through the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is a member of the ATP-binding cassette (ABC) transporter family and consists of a single polypeptide chain with two transmembrane domains (TMDs; each of which spans the membrane 6 times), two nucleotide binding domains (NBDs), and a regulatory (R) domain (14, 15). Binding of ATP to the NBDs along with phosphorylation of the R domain is necessary for channel opening. Although cAMP-dependent protein kinase A (PKA) is recognized as the primary regulator of CFTR phosphorylation and activity, several other kinases, such as cGMP-dependent protein kinase G (PKG) and PKC, and phosphatases, also contribute to modulating phosphorylation and gating of the channel (16–18). Other Cl− channels are also present in the intestinal epithelium, including the calcium-activated Cl− channel (CaCC), TMEM16A, otherwise known as anoctamin 1 (19). However, although studies in neonatal mice suggest that TMEM16A may be an important pathway for Cl− secretion in this species (20), studies of human intestine indicate the channel plays only a minor role (21).
Regulation of Chloride-Driven Fluid Secretion
Normally, fluid secretion into the intestinal lumen is required for appropriate hydration of the mucosa and maintenance of barrier function. However, the rate at which this process occurs varies depending upon a myriad of factors, including hormones, neurotransmitters, immune cell mediators, and dietary components, as well as microbes and their metabolites. At any given time, the integrated actions of these extracellular factors on intracellular regulatory pathways govern the extent of fluid secretion into the lumen.
Extracellular Regulation of Chloride Secretion
The lamina propria underlying the epithelium is densely packed with cells that are important in regulation of Cl− secretion. For example, cells of the mucosal immune system (MIS) are present, including mast cells, eosinophils, neutrophils, macrophages, monocytes, and lymphocytes, each of which produces a potent cocktail of mediators and cytokines that are key to regulating the rate of Cl− secretion into the lumen. Some mediators, such as histamine and adenosine, are preformed and stored in granules to be released upon stimulation, while others, such as prostaglandins, leukotrienes, and other lipid-derived substances, are synthesized de novo. Neurons of the enteric nervous system (ENS) are also present within the mucosa and, when activated, release their neurotransmitters in close proximity to the epithelium to either promote or inhibit secretion (22, 23). Such nerves can be activated by signals arising from within the intestinal wall, from the central nervous system (CNS), or by intrinsic reflex arcs initiated by luminal factors, such as the presence of nutrients or distension of the mucosal wall (24). It is also important to note that cells of the MIS and ENS do not act independently of one another in regulation of Cl− secretion but rather, each system has the capacity to recruit the other so that they exert their actions in an integrated fashion (25). Another cell type present within the mucosa that is likely to have an important role in regulating intestinal secretion are the myofibroblasts, which exist as a sheath underlying the epithelial basement membrane. In response to inflammatory cytokines, such as IL-1β, these cells release prostaglandins that act in a paracrine fashion to enhance epithelial secretory function (26).
In addition to signals arising from cells within the mucosa, the rate of intestinal fluid and electrolyte secretion is also determined by factors present within the lumen. Perhaps the most predominant of these are the bacteria that are present in the microbiota and the panel of metabolites they produce. It has long been known that the presence of pathogenic bacteria in the lumen can cause diarrhea (see Toxigenic Diarrhea for more details), but it is now becoming increasingly apparent that even under normal circumstances, the luminal microbiota is critical to setting the basal transport tone of the colonic epithelium. Evidence for this comes from studies showing that mucosal tissues from germ-free mice display altered Cl− secretory function (27, 28), and that various bacterial species within the microbiome can regulate the expression and activity of epithelial transport proteins, including CFTR (29, 30).
Precisely, how the microbiota regulates epithelial transport function is still poorly understood but such actions are mediated, at least in part, by the metabolites that the members of the microbiota produce. Some of these metabolites are derived from endogenous precursors, while others are derived from the diet. For example, short-chain fatty acids (SCFAs), produced by bacterial metabolism of dietary fiber, have been shown to regulate Cl− secretion by a number of mechanisms, including altered expression/activity of transport proteins, altered production of neuroimmune mediators, or modulation of the receptors and signaling pathways involved (31–37). On the other hand, bile acids are produced endogenously and are released from the liver into the intestine in response to food ingestion. In the small intestine, bile acids are important for fat digestion and absorption, with most of them then being reabsorbed in the terminal ileum and recycled to the liver by the enterohepatic circulation (EHC). However, with each cycle of the EHC, a small quantity of bile acids enters the colon where they are extensively metabolized by the microbiota to secondary bile acids (38, 39). When present at pathophysiologically high concentrations, bile acids stimulate Cl− and fluid secretion, leading to the onset of diarrhea (see Bile Acid Diarrhea). However, at more physiologically relevant concentrations, bile acids exert antisecretory actions, an effect we have proposed to promote normal colonic absorptive function (40). Such antisecretory actions of bile acids appear to be mediated by both cell surface and nuclear bile acid receptors. For example, activation of the nuclear receptor, farnesoid X receptor (FXR), downregulates CFTR expression and Na+-K+-ATPase activity to chronically dampen epithelial secretory capacity (41), whereas activation of the cell surface bile acid receptor, TGR5, can lead to the release of more rapidly acting antisecretory hormones and mediators (42–44).
In addition to bacteria and their metabolites, there are a number of other factors that contribute to dynamic regulation of epithelial transport function. For example, O2 supply to the mucosa determines the extent to which NKCC1 and CFTR are expressed (45, 46). This sensory mechanism is triggered by oxygen-sensing prolyl hydroxylases in the epithelium and likely serves to coordinate energy-expending transport processes with changes in splanchnic blood flow during and between digestive periods (47). Meanwhile, during digestion, the physical presence of a food bolus within the lumen can rapidly upregulate fluid and electrolyte secretion by activating enteroendocrine cells and neuronally mediated reflex arcs in the mucosa (48–50).
Intracellular Regulation of Cl− Secretion
Epithelial cells primarily sense changes in their extracellular environment through receptors expressed either at the cell surface or within the cytosol (Table 1). When activated, for example by neuroimmune agonists, growth factors, bile acids, or SCFAs, as described previously, these receptors engage a highly integrated network of signaling pathways that regulate all aspects of epithelial function, including fluid and electrolyte transport. The receptors primarily responsible for promoting Cl− secretion are G protein-coupled receptors (GPCRs), in particular Gq- and GsPCRs, expressed on the cell surface. These receptors are primarily activated in response to neurotransmitters and immune cell mediators and are predominantly expressed on the basolateral side of the epithelium, although some receptors are preferentially localized to the apical domain (81, 82), while others can be expressed both apically and basolaterally but be linked to differing downstream signaling pathways (83, 84). Such differential localization of GPCRs confers the epithelium with the property of “sidedness,” enabling it to respond appropriately to signals arising from either the serosal or mucosal environment. GqPCRs mediate their prosecretory actions through inositol trisphosphate-induced elevations in intracellular Ca2+, while GsPCRs stimulate adenylate cyclase activity, leading to the accumulation of cAMP. In turn, these second messengers bring about rapid alterations in transport protein activity, either through phosphorylation-induced changes in activity or altered trafficking to and from the cell membrane (85–90). Neuroimmune agonists that act via GsPCRs are typified by the neurotransmitter vasoactive intestinal polypeptide (VIP). Binding of VIP to its receptor on intestinal epithelial cells leads to accumulation of cAMP and activation of PKA. PKA then phosphorylates the regulatory domain of CFTR to induce channel opening and Cl− efflux (Fig. 2). Agents such as the bacterial toxin, STa, or the endogenous peptide, guanylin, elevate levels of another important cyclic nucleotide, cGMP, in intestinal epithelial cells to induce CFTR-mediated intestinal Cl− secretion (61, 91, 92). In this case, responses are mediated by protein kinase G (PKG), which appears to induce CFTR activation through pathways that overlap with those of cAMP (16, 93).
Table 1.
Positive and negative regulation of intestinal epithelial Cl− secretion
| Receptor Type | Signaling Pathway | Endogenous Agonists | References |
|---|---|---|---|
| Prosecretory | |||
| GqPCR | Increase [Ca2+]i | ACh, histamine, bradykinin, 5-HT, ATP | (51–55) |
| GsPCR | Increase cAMP | VIP, 5-HT, adenosine, prostaglandin E2, PACAP | (56–60) |
| Guanylate cyclase C | Increase cGMP | Guanylin | (61) |
| IL-1 receptor | Upregulation of transport protein expression | IL-1β | (62) |
| Antisecretory | |||
| Muscarinic M3 receptor | Generation of IP4 | ACh | (63) |
| Muscarinic M3 receptor | Activation of MAPKs | ACh | (64, 65) |
| GiPCR | Reduce cAMP | Somatostatin, Neuropeptide Y, SCFAs | (35, 37, 66–68) |
| Receptor tyrosine kinases | PI-3 kinase activation | EGF, insulin | (69, 70) |
| Steroid hormone receptors | Repression of transport protein expression; direct interaction with CFTR | Estrogen, progesterone, bile acids | (41, 71, 72) |
| Various cytokine receptors (e.g., TNF receptor superfamily, class II cytokine receptors) | Repression of transport protein expression; inhibition of cAMP generation | IFN-γ, TNF, TGF-β, | (73–80) |
5-HT, 5-hydroxytryptamine; ACh, acetylcholine; ATP, adenosine 5′-triphosphate; GPCR, G protein-coupled receptor; IFN, interferon; IL-1, interleukin 1; PACAP, pituitary adenylate cyclase activating protein; SCFA, short chain fatty acid; TGF, transforming growth factor; TNF, tumor necrosis factor; VIP, vasoactive intestinal polypeptide.
Figure 2.
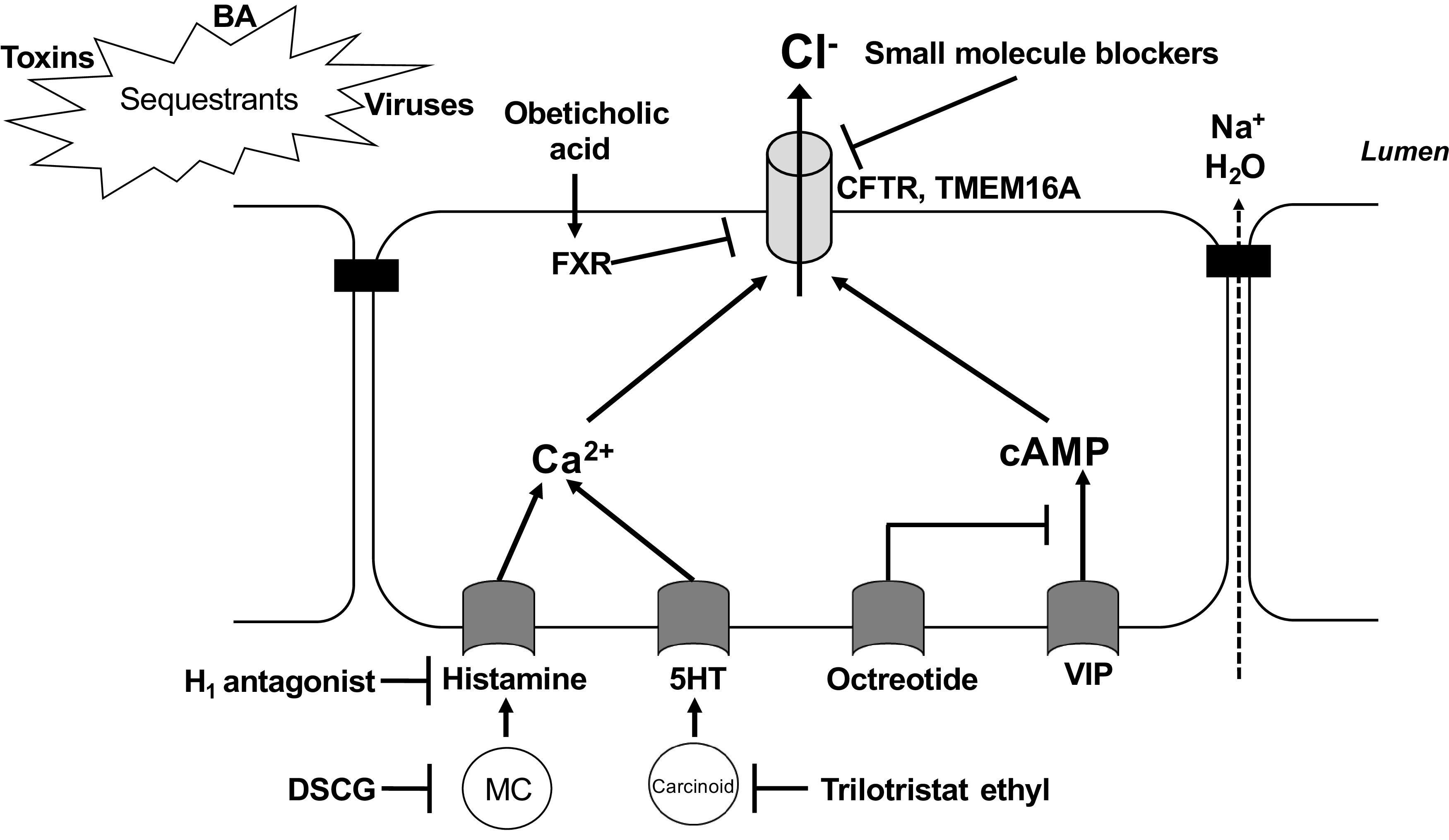
Modulation of intestinal secretory mechanisms as a modality to treat diarrhea. Selected agents discussed in the text are illustrated. In allergic diarrhea, histamine released from mast cells (MC) may evoke calcium-dependent Cl− secretion, which can be blocked by the mast cell stabilizer disodium cromoglycate (DSCG) or antagonists of the histamine H1 receptor. Excessive release of serotonin (5HT) in patients suffering from carcinoid syndrome also evokes calcium-dependent Cl− secretion; production of 5HT can be inhibited by trilotristat ethyl. Release of excess amounts of vasoactive intestinal polypeptide (VIP) in the setting of VIPoma triggers profound cAMP-dependent Cl− secretion; this action (and release of VIP from the tumor) can be antagonized by somatostatin analogs such as octreotide. Small molecule inhibitors of CFTR or TMEM16A can block Cl− secretion directly, whereas the farnesoid X receptor (FXR) agonist, obeticholic acid, reduces CFTR expression levels. Finally, various resins can sequester prosecretory agents such as bile acids (BA), viruses, and toxins in the intestinal lumen, preventing their interaction with the epithelium. For further details, see text.
The best studied GqPCR agonist in the regulation of Cl− secretion is probably acetylcholine (ACh), which acts at Gq-coupled M3 receptors expressed on the basolateral side of intestinal epithelia. Elevations in intracellular Ca2+ subsequent to activation of M3 receptors brings about activation of basolateral KCNN4 channels, both through direct interactions of the channel with Ca2+ or indirectly through binding of the Ca2+ effector protein, calmodulin (94). Efflux of K+ through KCNN4 hyperpolarizes the cell, creating an electronegative driving force for anion secretion into the lumen through apical Cl− channels, most notably CFTR. Concomitant with elevations in intracellular Ca2+, GqPCRs also induce activation of protein kinase C, which can either promote or suppress CFTR opening depending on which of the nine PKC motifs in the CFTR regulatory domain are phosphorylated (95, 96). At the same time that elevations in cAMP and Ca2+ alter the gating of K+ and Cl− channels, they also regulate the trafficking of these channels, and other transporters involved in Cl− secretion, to and from the cell surface. Altered endo- and exocytosis of transport proteins can occur within seconds of agonist stimulation and is mediated by complex signaling cascades involving multiple protein kinases and intramolecular docking molecules (90, 97–99).
At any given time, epithelial cells in the intestine are exposed to a cocktail of neuroimmune mediators acting through both Ca2+ and cAMP-dependent signaling pathways. It is perhaps not surprising then that significant interactions between these pathways occur in the regulation of Cl− secretion. Indeed, our studies have shown that cAMP- and Ca2+-dependent signaling pathways do not act independently of one another but, when activated simultaneously, they interact synergistically to induce more powerful and prolonged secretory responses. This is due to the fact that in the presence of both types of agonist, both basolateral K+ channels and apical Cl− channels are simultaneously activated, thereby removing the rate limiting steps for both types of secretory pathway. This, along with the capacity for cross talk between the two pathways in the generation of Ca2+ and cAMP (100), provides the molecular mechanisms that enable intestinal fluid secretion to be rapidly and finely tuned to match immediate requirements. Such rapid onset responses allow the intestine to quickly adapt to changes within the intestinal environment, but they can also be followed by more long-term, genomically mediated alterations in transport function.
Another class of receptors important in regulating intestinal fluid and electrolyte secretion is the receptor tyrosine kinases, the most studied of which with respect to intestinal Cl− secretion is the epidermal growth factor receptor (EGFR). Binding of EGF or TGF-α to EGFR rapidly dampens the ability of the epithelium to respond to Ca2+-, but not to cAMP-dependent agonists. The pathway mediating this effect involves phosphatidylinositol 3-kinase (PI3-K), leading to inhibition of basolateral K+ channels (101, 102). The physiological relevance of EGFR-induced downregulation of epithelial fluid and electrolyte secretion is unclear, but such observations should be considered in the context of other studies which have shown that EGF also promotes intestinal Na+ absorption (103). Thus, through its capacity to both inhibit secretion and to promote absorption, increases in mucosal levels of EGFR ligands are likely to conserve against water loss in the intestine. Importantly, EGFR does not only mediate responses to its own ligand, but it can also be recruited by GPCR-activating agonists. Transactivation of the EGFR in this way occurs by Src-dependent signaling mechanisms and differentially modulates responses to cAMP and Ca2+-dependent secretagogues. Activation of EGFR by Ca2+-dependent agonists leads to downstream activation of ERK and p38 MAPKs, which in turn act to limit secretory responses to such agonists (64, 65, 104). In contrast, EGFR recruitment by cAMP-dependent agonists leads to activation of PI3-K and appears to be necessary for the full expression of secretory responses mediated by this pathway (105).
Nuclear receptors (NR) also have important roles in regulating intestinal fluid and electrolyte secretion. In their unstimulated state, these receptors are usually found in the cytosol, but are activated by lipid-soluble ligands that can cross the cell membrane. Upon ligand binding, NRs translocate to the nucleus and bind to promoter elements within responsive genes, resulting in either up- or downregulation of target gene expression. To date, several NRs have been shown to chronically downregulate epithelial secretory function, including peroxisome proliferator-activated receptor (PPARγ), FXR, and the progesterone receptor (41, 71, 106). However, in addition to regulating Cl− secretion through genomically mediated mechanisms, steroid hormones also have the capacity to exert more rapid, nongenomic, antisecretory effects. For example, treatment of female, but not male, rat colonic tissues with estrogen leads, within minutes, to inhibition of both Ca2+- and cAMP-dependent secretory responses (107–109). Such rapid antisecretory actions of estrogen appear to be mediated by a membrane-associated receptor that is coupled to PKCδ and inhibition of basolateral K+ KCNQ1 channels (110). Estrogen is not the only steroid hormone reported to rapidly attenuate colonic epithelial secretory function; this is also a property of aldosterone, which appears to exert its inhibitory effects on KCNN4 K+ channels (111).
SECRETORY DIARRHEAS
In this section, recent advances in our understanding of various forms of secretory diarrheal disease will be discussed, as a prelude to covering treatments that are in use or under development for the control of these conditions.
Toxigenic Diarrhea
As alluded to aforementioned, secretory diarrhea has classically been exemplified by cholera, where disease is induced by cholera toxin, a multimeric toxin that evokes irreversible increases in cAMP within intestinal epithelial cells. Cholera toxin is made up of five identical B (for binding) subunits that, as their name suggests, are responsible for binding the toxin complex to the apical pole of epithelial cells, whereupon the holotoxin is translocated into the cell cytosol. The internalized toxin undergoes retrograde vesicular trafficking to the endoplasmic reticulum, where proteolytic cleavage releases the A (active) subunit into the cell cytosol. The A subunit mediates ADP-ribosylation of adenylyl cyclase, irreversibly activating the protein and triggering profound increases in cAMP (112). These, in turn, trigger ongoing Cl− secretion by the mechanisms discussed earlier and thus profound watery diarrhea. The disease is likewise exacerbated by the fact that cAMP also inhibits electroneutral NaCl absorption across the intestinal epithelium [via inhibitory effects on Na+/H+ exchanger 3 (NHE3)] although not Na+-coupled nutrient absorption (113). The fact that the latter absorptive process is spared is exploited by oral rehydration therapies.
Additional clinically relevant pathogens elaborate enterotoxins to induce diarrheal symptoms. For example, some strains of enterotoxigenic Escherichia coli (ETEC) produce the so-called heat-labile toxin (LT) that is structurally related to cholera toxin, and produces diarrhea via similar mechanisms, albeit with a milder phenotype (114). More characteristically, ETEC also elaborates a heat-stable toxin, STa, that is a ligand for the apically localized membrane guanylyl cyclase C (GC-C) (115). Toxin binding results in production of cGMP, which triggers active Cl− secretion via cGMP-dependent protein kinase G phosphorylation and opening of CFTR, as well as cross-activation of PKA (116, 117). STa is a compact peptide that not only is heat-stable, but also relatively resistant to proteolytic cleavage in the gut lumen, doubtlessly contributing to its potency in vivo. It is a major causative agent of traveler’s diarrhea but also causes numerous deaths in developing countries, and as such has recently been tested as a candidate for an ETEC vaccine (115). A related toxin, EAST1 (produced by certain strains of enteroaggregative E. coli) also binds to GC-C and has emerged as a causative agent in sporadic diarrheal diseases in both children and domesticated farm species (118). Moreover, the existence of STa and related toxins, which can be considered “super-agonists” of GC-C, led to the discovery of guanylin and related peptides that are endogenous GC-C ligands (119) and believed to be responsible for fine-tuning fluid and electrolyte homeostasis between the gut and kidneys.
Finally, Clostridium difficile is an opportunistic pathogen that frequently overgrows in the gut when the microbiota is disturbed, secondary (for example) to the use of broad-spectrum antibiotics, particularly in hospitalized and/or vulnerable patients, such as the elderly. Clostridium difficile causes a spectrum of disease from mild diarrhea to life-threatening colitis, and is the most common cause of antibiotic-associated diarrhea. Because the pathogen is difficult to eradicate and symptoms may recur, it is seen as a major emerging health hazard. Symptoms are predominantly ascribed to the production of two toxins, TcdA and TcdB, which both contain a glucosyltransferase domain that is capable of glucosylating Rho (and Ras) G proteins. The toxins thereby disturb a variety of Rho-dependent functions in intestinal epithelial cells, including maintenance of cytoskeletal integrity, resulting in severely diminished epithelial barrier function and ultimately an uncontrolled inflammatory response in the lamina propria (120, 121). Diarrhea likely results secondarily from the effects of mediators produced by infiltrating inflammatory cells, as well as via “leak-flux” mechanisms dependent on barrier dysfunction (122).
Bile Acid Diarrhea
If it is necessary to resect the terminal ileum, the bile acids used during digestion of lipids cannot be fully reabsorbed, and spill over into the colon where they are effective Cl− secretagogues when present in high concentrations. Interruption of the enterohepatic circulation will also increase the rate of hepatic bile acid synthesis, further exacerbating the problem. Bile acid diarrhea (BAD) may also result when production or release of the homeostatic factor produced by ileal enterocytes, fibroblast growth factor 19 (FGF19) is reduced, releasing an inhibitory influence that normally keeps bile acid synthesis in check when concentrations of these molecules reaching the ileum are adequate. This has been described as a mechanism underlying diarrhea in a subset of patients with irritable bowel syndrome (IBS), now more accurately classified as suffering from bile acid diarrhea (123–125). Bile acids have also been implicated in diarrhea that arises as a complication postbariatric surgery (126). Finally, disorders of bile acid absorption secondary to reduced expression and/or mutations in ileal bile acid transporters will also lead to hypersecretion in the colon (127).
The ability of bile acids to cause Cl− secretion in the colon has largely been attributed to an increase in cytosolic calcium levels (128–130). However, unexpectedly, recent studies have indicated that this secretory response, rather than being mediated by CaCCs, instead is largely dependent on activation of CFTR. Thus, chenodeoxycholic acid evoked Cl− secretion in cell lines and enteroid-derived monolayers, as well as a fluid secretory response in colonic loops, that was substantially reduced by an experimental CFTR inhibitor. The fluid secretory response was similarly reduced in mice lacking CFTR expression (131). On the other hand, bile acids likely are capable also of stimulating the opening of CaCCs such as TMEM16A, as evidenced by the fact that their effects on cholangiocytes were suppressed by knockdown of these channels (132). In these latter studies, likely also applicable to the intestinal epithelium, the secretory effects of bile acids were also dependent on their ability to evoke extracellular release of ATP, activation of purinergic receptors, and calcium mobilization secondary to the production of inositol trisphosphate.
Rotaviral Diarrhea
Rotavirus is an important and often deadly diarrheal pathogen in the pediatric population. There has been significant recent progress toward understanding the basis of its diarrheal symptoms. Particularly, infection with the virus results in production of nonstructural protein 4 (NSP4) that is capable of triggering calcium-dependent Cl− secretion via its ability to activate TMEM16A (20, 133). The propensity of rotavirus to activate Cl− secretion across the intestinal epithelium apparently is also amplified by the ability of the virus to trigger waves of calcium signaling that emanate from directly infected cells, mediated by paracrine release of ADP and activation of P2Y1 receptors, as well as the release of serotonin from enteroendocrine cells (134). Importantly, interruption of such paracrine signaling lessened fluid secretion in vitro and diarrheal symptoms in a neonatal mouse model of rotaviral diarrhea, suggesting possible adjunctive therapeutic strategies.
VIPoma
A particularly challenging form of diarrhea is that observed in patients with (typically) pancreatic neuroendocrine tumors that secrete excessive quantities of VIP (135). Although VIPomas (also known as Verner–Morrison syndrome) are rare, they are almost invariably accompanied by profuse watery diarrhea and hypokalemia that can be life-threatening. This outcome is presumably secondary to uncontrolled release of high levels of VIP, a highly potent Cl− secretagogue that triggers CFTR activation via cAMP-dependent mechanisms (56). Where possible, resection of the offending tumor is curative, but such patients often must be managed medically, at least to stabilize them before surgery. Diarrheal symptoms in VIPoma are rarely responsive to standard antidiarrheal therapies (136) but typically are controlled by somatostatin analogues, whose efficacy may be attributed not only to direct Gi-mediated downregulation of cAMP production and CFTR activity in the epithelium, but also reductions in secretion of VIP from the offending tumor (137).
Drug-Induced Diarrhea
Diarrheal symptoms may result inadvertently from a variety of different drug regimens. The case of diarrhea associated with the use of broad-spectrum antibiotics was addressed earlier. In many other situations, underlying mechanisms are obscure, but two additional specific scenarios are worthy of mention here due to specific links to dysregulated Cl− secretion and because diarrheal symptoms may be dose limiting, compromising treatment efficacy. For example, use of the cyclin-dependent kinase inhibitor flavopiridol, evaluated for the therapy of a variety of refractory solid tumors and hematological malignancies (138), was accompanied by diarrhea of sufficient severity as to require treatment cessation in many patients, apparently related to interindividual differences in the ability to glucuronidate the agent (139). Clinically relevant concentrations of flavopiridol were shown to enhance the Cl− secretory effect of calcium-dependent agonists such as carbachol and bile acids, without themselves inducing Cl− secretion independently (140). These findings may suggest strategies to mitigate dose-limiting diarrhea in patients receiving flavopiridol or related agents. Indeed, the bile acid-binding resin, cholestyramine, was effective in reducing diarrheal symptoms in some patients (141).
In a similar vein, small molecule tyrosine kinase inhibitors that target the EGF receptor (EGFR-TKI) have emerged as potent first-line treatments for nonsmall cell lung cancers and are being evaluated for their efficacy in other tumors. Like the experience with flavopiridol, their efficacy is often limited by their propensity to induce diarrhea. Based on the earlier discussion of the role of EGFR in limiting calcium-dependent Cl− secretion, it was reasonable to speculate that the ability of EGFR-TKI to reduce receptor function could contribute to undesirable diarrheal side-effects. Indeed, several groups have now demonstrated that various such agents, or others that target EGFR, potentiate calcium-dependent Cl− secretion in vitro, with their efficacy in this regard correlated with their propensity to evoke diarrhea in the clinical setting (142–144). Ultimately, this understanding may allow the more effective use of drugs in this class in patients suffering from various classes of malignancies.
TARGETING CHLORIDE SECRETION FOR TREATMENT OF DIARRHEA
There are several therapeutic approaches currently available to treat secretory diarrhea, with options ranging from FDA-approved drugs to centuries-old, but often clinically unproven, traditional remedies (some examples, along with their sites of action, are shown in Fig. 2). These agents exert antidiarrheal actions by very diverse mechanisms, including interference with neuroimmune signaling in the mucosa, sequestration of luminal prosecretory stimuli, inhibition of intestinal transit, downregulation of epithelial second messenger production, or through altering the microbiota and its metabolites.
In many cases, diarrhea occurs due to the actions of prosecretory mediators released from cells in the mucosa. For example, mast cells and eosinophils are key players in allergic diarrhea, mastocytosis, mast cell activation syndrome, and eosinophilic enteritis, with diarrhea in these conditions being largely due to induction of epithelial Cl− secretion by histamine or other cell-specific mediators. Thus, drugs that prevent mast cell and eosinophil degranulation (e.g., disodium cromoglycate) or which block the actions of histamine at H1 receptors can be effective in alleviating diarrhea under such circumstances (145–148). As mentioned in the previous section, another approach to inhibiting the actions of prosecretory mediators involves the use of drugs that block the production of intracellular second messengers. Somatostatin analogs, such as octreotide, act at the Gi-coupled SSTR1 in intestinal epithelial cells to inhibit cAMP production and are commonly used in the treatment of diarrhea associated with neuroendocrine tumors, such as VIPomas and carcinoid syndrome (149, 150). Diarrhea can also be prevented by drugs that inhibit the production of prosecretory mediators from the epithelium. For example, a 5-hydroxytryptophan hydroxylase inhibitor, tilotristat ethyl, was recently approved by the Food and Drug Administration (FDA) for use in treating diarrhea associated with carcinoid syndrome. This drug exerts its antidiarrheal actions by preventing production of 5-HT, an important prosecretory and prokinetic messenger in the intestine (151).
Another strategy to treat secretory diarrhea involves the use of drugs that slow intestinal transit, thereby increasing the time available for water absorption to occur. Loperamide is such an antidiarrheal drug that has its effects by activating opioid receptors in the myenteric plexus to inhibit smooth muscle contraction and peristalsis. It has also been reported to exert at least some of its antidiarrheal actions by inhibition of epithelial Cl− secretion through an opioid-receptor-independent pathway that reduces basolateral K+ channel activity (152, 153). Although most useful for treating diarrhea associated with dysregulated intestinal motility, loperamide is also commonly used to reduce symptoms of acute infectious diarrheas. However, although it is among the top selling antidiarrheals worldwide, there are a number of limitations to the therapeutic use of loperamide. First, in the setting of upregulated fluid and electrolyte secretion, it relies on absorptive processes remaining functional. Second, it has tendency to cause rebound constipation or ileus and is contraindicated for use in young children (154). Third, it is also contraindicated in the setting of diarrhea caused by invasive or toxigenic bacteria since in such situations, slower intestinal transit can facilitate bacterial colonization of the epithelium and subsequent systemic infection. Loperamide can also have significant interactions with other drugs, particularly those that inhibit cytochrome P450 or p-glycoprotein as these enzymes are important in determining the extent of loperamide metabolism or uptake into the CNS, respectively (155). An alternative drug for targeting opioid pathways in the treatment of diarrhea is racecadotril. In contrast to being an opioid receptor agonist, racecadotril (or acetorphan) acts by inhibiting neutral endopeptidase (NEP), an enzyme that degrades endogenous enkephalins and prevents intracellular production of cAMP (156). Racecadotril has been shown to be as effective as loperamide in preventing acute diarrhea in animal models and humans but since its active metabolite, thiorphan, does not alter intestinal motility or cross the blood brain barrier, it does not have similar side effects (155, 157–159). Racecadotril is approved for use in the treatment of acute diarrhea in several European and Asian countries but is currently not available in the United States.
Another approach to the treatment of secretory diarrhea involves the use of sequestrants that bind pathogens, toxins, and other prosecretory agents in the lumen. For example, diosmectite is a widely used aluminum-based clay that is available over the counter as an oral preparation. In the intestinal lumen, diosmectite avidly absorbs water and binds bacteria, viruses, and toxins so that they are unable to interact with the epithelium. Diosmectite has been shown to prevent acute diarrhea both in adults and children with minimal side effects (160–162). Another type of luminally acting sequestrant frequently used in the treatment of diarrhea is anionic resins known as bile acid sequestrants (BAS). Cholestyramine was the first of these drugs on the market and, by virtue of its ability to drive hepatic bile acid synthesis from cholesterol, was originally developed to treat hypercholesterolemia. However, for a considerable time BAS have also been used to treat patients with bile acid diarrhea (124, 163). Nevertheless, although clearly of use in treating certain types of secretory diarrhea, a significant drawback to the use of sequestrants is the high likelihood of their interactions with other orally administered drugs.
Although the treatments described earlier can have clear benefits in treating various types of secretory diarrhea, there is still a lack of clinically available therapeutic strategies that directly target the epithelial transport pathways involved in Cl− secretion. Such drugs, acting at the most fundamental level of transport protein expression and activity would be expected to have enhanced efficacy, safety, and specificity over currently available treatments. Much research has focused on this area over the past two decades and a number of strategies are currently in development. One promising avenue of research involves the use of probiotics, various strains of which have been shown to regulate the expression of intestinal transport proteins, either by downregulating those involved in fluid secretion or upregulating those involved in absorption (30, 164–166). Indeed, clinical trials suggest that probiotics can be of benefit in treatment of acute diarrhea in adults and children (167–169) although other well-designed trials have reported negative findings (169, 170).
An important mechanism by which the microbiota communicates with the host is through the generation of bioactive metabolites in the colonic lumen, Such metabolites, which include secondary bile acids and SCFAs, also present another promising avenue for the development of new antidiarrheal drugs. Studies from the Keely group have shown that activation of FXR downregulates the expression of CFTR in colonic epithelial cells, inhibits cholera toxin-induced fluid accumulation, and prevents the onset of allergic diarrhea in mice (41). Such antisecretory actions, along with their well-established role in downregulating hepatic bile acid synthesis, suggests that FXR agonists should be particularly useful in the treatment of patients with BAD, and indeed, a recent Phase II trial of obeticholic acid supports this idea (171, 172). Whether FXR agonists might also be useful in treating other intestinal disorders remains to be determined but studies in mouse models support their development for treatment of inflammatory diarrhea (173).
Similar to secondary bile acids, SCFAs are also produced in the colonic lumen by bacterial metabolism of dietary fiber and have the capacity to regulate epithelial transport. For example, butyrate has been shown to inhibit Cl− secretion and promote Na+ absorption across colonic epithelial cells through mechanisms that involve direct blockade of the CFTR channel, downregulation of NKCC1, upregulation of NHE3, and reductions in adenylyl cyclase expression (174–178). Such actions may contribute to the perhaps paradoxical antidiarrheal effects of dietary fiber (179–181) and suggest that drugs that act specifically at receptors for SCFAs [e.g., free fatty acid (FFA)2 and FFA3] might be of benefit in such conditions (37, 66).
Traditional medicines have been used around the world for centuries to treat both acute and chronic diarrhea (182, 183). For example, Dragon’s blood, an extract from the bark of the South American tree, Croton lechleri, is effective in treating acute diarrhea (184), while extracts of Sclerocarya birrea are used for a similar purpose in South Africa (185). In China, Coptis and Berberis plant species are just two of many plant extracts used for their antidiarrheal properties (186). Thus, although often not properly validated by clinical trials, there is a long history for the use of herbal remedies in the treatment of diarrhea. The availability of such treatments gives patients an alternative, “drug-free,” and inexpensive approach to treating their diarrhea but there are also several limitations. For example, a lack of standardization in extracts from different sources, as well as poor understanding of their chemical makeup, their mechanisms of action, and their interactions with other medications are all important factors to consider before traditional herbal remedies can be clinically approved for routine use as antidiarrheals.
Small molecules that specifically act on the transport proteins that constitute the Cl− secretory pathway continue to be the holy grail for researchers searching for more effective and specific approaches to treat secretory diarrhea. Agents that target apical Cl− channels are the most attractive candidates and one approach to identifying such drug candidates is through isolating and characterizing the antisecretory components of traditional herbal medicines. For example, Dragon’s blood contains crofelemer, a molecule that has been approved by the FDA for treatment of HIV-associated diarrhea (187). Crofelemer also inhibits Cl− secretion in vitro, prevents secretory diarrhea in vivo, and shortens the duration of traveler’s diarrhea (188). Crofelemer has been shown to inhibit Cl− conductance through both CFTR and CaCCs (187, 189, 190). However, given the lack of evidence for CaCCs as being important in intestinal Cl− secretion in humans, it is unclear how much their inhibition by crofelemer contributes to its antidiarrheal activity (21). Other traditional remedies have also yielded new antidiarrheal drug candidates that target Cl− channels. For example, stilbenes (e.g., resveratrol), catechins (e.g., epigallocatechin-3-gallate), and terpenes, (e.g., oridonin), are all found in traditional antidiarrheal remedies and, similar to crofelemer, can inhibit both CFTR and CaCCs (191–193). Other molecules found in traditional medicines target different components of the Cl− secretory pathway. For example, Berberis vulgaris is particularly rich in berberine, an alkaloid that has been shown to alleviate infectious diarrhea and IBS-D in humans (186, 194). Studies in cultured epithelial cells suggest the antidiarrheal effects of berberine are due, at least in part, to inhibition of basolateral K+ channels (195). There are many other examples of plant-derived molecules that can directly regulate epithelial transport protein activity or expression but, to date, none have been approved by the FDA for treatment of diarrhea.
An alternative strategy to identifying new small molecule antidiarrheals involves the use of high-throughput screening methods. For example, Verkman and coworkers have used such approaches to screen chemical libraries to identify new blockers of CFTR and CaCCs. Although these drugs show specificity and efficacy in vitro and in animal models of diarrhea, none, as yet, have reportedly progressed into clinical trials (196, 197).
Promoting “Diarrhea” to Treat Constipation
Constipation is a common human malady. Whether idiopathic or seen as the predominant symptom in a patient suffering from IBS (IBS-C), constipation has classically been considered to result from alterations in bowel motility that result in excessive dehydration of the stool and/or an inability to propel it efficiently in an aboral direction, and has typically been treated symptomatically with the use of bulk-forming or stimulant laxatives. However, in recent years, the idea that Cl− secretion might be triggered in a controlled fashion to offset constipation has come to the fore with the availability of new agents (Fig. 3). For example, lubiprostone is a prostanoid that was considered initially to be a specific agonist of ClC Cl− channels and was shown to activate Cl− secretion in intestinal epithelial cell lines and animal models (198, 199). It is now believed to have a broader mechanism of action, including triggering CFTR-dependent Cl− and bicarbonate secretion, bolstering epithelial barrier function secondary to induced expression of claudin-1, and reduction of visceral pain (perhaps secondary to its barrier protective effects) (199–204). In any event, it has consistently been shown to be more effective than placebo in relieving chronic idiopathic constipation (CIC) and IBS-C in clinical trials (205).
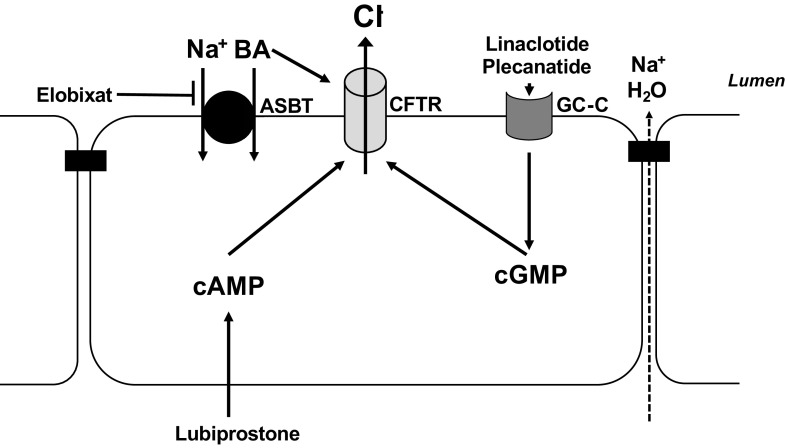
Figure 3.
Stimulation of intestinal secretory mechanisms as an emerging modality to treat constipation. Lubiprostone elicits activation of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (and perhaps other channels) via an increase in cAMP. Linaclotide and plecanatide also activate CFTR and thus Cl− secretion, but by binding to apical guanylyl cyclase-C (GC-C) receptors and thus triggering an increase in cGMP. Elobixat is an inhibitor of the ileal apical Na+-coupled bile acid transporter, ASBT. In the presence of this agent, bile acids (BA) accumulate luminally and stimulate Cl− secretion, in part via CFTR. For further details, see text.
Linaclotide is a peptide analog of STa, and activates Cl− secretion secondary to its ability to trigger the activity of apical GC-C and cGMP-dependent activation of CFTR. Like lubiprostone, it has proven safe and effective for the treatment of CIC and IBS-C, with the main side effect being (perhaps not surprisingly) diarrhea (206). In addition to its direct effect on gut secretory function, linaclotide was revealed to have an unexpected beneficial effect on visceral pain in human patients and in animal models, apparently secondary to extracellular release of cGMP from the epithelium that acts, in turn, to inhibit colonic nociceptors (207). Plecanatide has been developed and approved more recently as an additional GC-C agonist, based on the structure of uroguanylin, that is active predominantly in the proximal small intestine (208).
Finally, the ability of bile acids to trigger colonic Cl− secretion has been exploited for the treatment of constipation (209). Indeed, a subset of patients with CIC exhibit reduced fecal concentrations of bile acids, presumably reflecting a deficit in the colonic lumen (210). Elobixibat, an inhibitor of the ileal Na+-dependent bile acid transporter (ASBT) (Fig. 3), improved bowel function by accelerating colonic transit and loosening stool consistency in a small trial in patients with functional constipation (211). Drugs of this class may be particularly important for the treatment of CIC in patients with demonstrated reductions in fecal bile acids, but may ultimately prove useful for the treatment of constipation more broadly (209).
CONCLUSIONS
Secretory diarrheal diseases arise from a broad array of mechanisms, but rest centrally on activation of Cl− secretory mechanisms across epithelial cells lining the small intestine and colon. Recent years have seen the rapid expansion of our understanding not only of the transport proteins that comprise the Cl− secretory mechanism, but also the ways that these are regulated (both positively and negatively) by a panoply of intra- and extracellular influences. Knowledge in this area, in turn, is leading to the development of a wide array of drugs that could be used to arrest secretory diarrheal fluid losses, as well as an understanding of the mechanisms of action of traditional medicines that have been used for millennia to treat diarrhea. Conversely, intestinal secretory mechanisms are increasingly exploited to increase stool fluidity and thus passage in constipation of various etiologies.
Diarrheal diseases, particularly those caused by infectious agents acquired from contaminated food and water, remain major causes of morbidity and mortality worldwide, especially in developing countries and in settings where normal infrastructure is compromised (such as following natural disasters). Repeated bouts of diarrhea, especially in infancy, are associated with failure to thrive and long-lasting cognitive side-effects that may further amplify the toll of these disease states in developing countries most vulnerable to such compromises. The promise, therefore, of small molecule inhibitors that target Cl− secretory pathways and can arrest diarrhea arising from multiple etiologies is tantalizing and perhaps in reach given advances in our knowledge of how Cl− secretion occurs and is regulated, particularly if such agents can be provided cheaply.
GRANTS
Studies from the authors’ laboratories have been supported by grants from the National Institutes of Health (DK28305, to K. E. Barrett), Science Foundation Ireland (to S. J. Keely), and an unrestricted grant from the Estratest Settlement Fund (to K. E. Barrett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.K. and K.E.B. prepared figures; S.J.K. and K.E.B. drafted manuscript; S.J.K. and K.E.B. edited and revised manuscript; S.J.K. and K.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the late Alan Hofmann, who inspired both authors to study the multifaceted roles of bile acids in the gastrointestinal tract, including triggering of diarrhea when present in excess.
REFERENCES
- 1.Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol 8: 371, 2017. doi: 10.3389/fphys.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller PJ, Verity K. Colonic sodium-potassium adenosine triphosphate subunit gene expression: ontogeny and regulation by adrenocortical steroids. Endocrinology 127: 32–38, 1990. doi: 10.1210/endo-127-1-32. [DOI] [PubMed] [Google Scholar]
- 3.Vagin O, Dada LA, Tokhtaeva E, Sachs G. The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am J Physiol Cell Physiol 302: C1271–C1281, 2012. doi: 10.1152/ajpcell.00456.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr 37: 387–392, 2005. doi: 10.1007/s10863-005-9476-x. [DOI] [PubMed] [Google Scholar]
- 5.Garty H, Karlish SJ. Role of FXYD proteins in ion transport. Annu Rev Physiol 68: 431–459, 2006. doi: 10.1146/annurev.physiol.68.040104.131852. [DOI] [PubMed] [Google Scholar]
- 6.Chew TA, Zhang J, Feng L. High-resolution views and transport mechanisms of the NKCC1 and KCC transporters. J Mol Biol 433: 167056, 2021. doi: 10.1016/j.jmb.2021.167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmosino M, Gimenez I, Caplan M, Forbush B. Exon loss accounts for differential sorting of Na-K-Cl cotransporters in polarized epithelial cells. Mol Biol Cell 19: 4341–4351, 2008. doi: 10.1091/mbc.e08-05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+-K+-2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl− transport. Am J Physiol Renal Physiol 290: F1094–F1102, 2006. doi: 10.1152/ajprenal.00071.2005. [DOI] [PubMed] [Google Scholar]
- 9.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch 442: 896–902, 2001. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 10.Abbott GW. Regulation of human cardiac potassium channels by full-length KCNE3 and KCNE4. Sci Rep 6: 38412, 2016. doi: 10.1038/srep38412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barro-Soria R, Ramentol R, Liin SI, Perez ME, Kass RS, Larsson HP. KCNE1 and KCNE3 modulate KCNQ1 channels by affecting different gating transitions. Proc Natl Acad Sci USA 114: E7367–E7376, 2017. doi: 10.1073/pnas.1710335114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 13.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 14.Callebaut I, Chong PA, Forman-Kay JD. CFTR structure. J Cyst Fibros 17: S5–S8, 2018. doi: 10.1016/j.jcf.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Cant N, Pollock N, Ford RC. CFTR structure and cystic fibrosis. Int J Biochem Cell Biol 52: 15–25, 2014. doi: 10.1016/j.biocel.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Tien XY, Brasitus TA, Kaetzel MA, Dedman JR, Nelson DJ. Activation of the cystic fibrosis transmembrane conductance regulator by cGMP in the human colonic cancer cell line, Caco-2. J Biol Chem 269: 51–54, 1994. doi: 10.1016/S0021-9258(17)42310-6. [DOI] [PubMed] [Google Scholar]
- 17.French PJ, Bijman J, Edixhoven M, Vaandrager AB, Scholte BJ, Lohmann SM, Nairn AC, de Jonge HR. Isotype-specific activation of cystic fibrosis transmembrane conductance regulator-chloride channels by cGMP-dependent protein kinase II. J Biol Chem 270: 26626–26631, 1995. doi: 10.1074/jbc.270.44.26626. [DOI] [PubMed] [Google Scholar]
- 18.Poroca DR, Amer N, Li A, Hanrahan JW, Chappe VM. Changes in the R-region interactions depend on phosphorylation and contribute to PKA and PKC regulation of the cystic fibrosis transmembrane conductance regulator chloride channel. FASEB Bioadv 2: 33–48, 2020. doi: 10.1096/fba.2019-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem 284: 28698–28703, 2009. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ousingsawat J, Mirza M, Tian Y, Roussa E, Schreiber R, Cook DI, Kunzelmann K. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflugers Arch 461: 579–589, 2011. doi: 10.1007/s00424-011-0947-0. [DOI] [PubMed] [Google Scholar]
- 21.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem 286: 2365–2374, 2011. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci 915: 77–80, 2000. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 23.Wood JD. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr Opin Gastroenterol 26: 102–108, 2010. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- 24.Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci 133: 55–63, 2007. doi: 10.1016/j.autneu.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127: 635–657, 2004. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol Cell Physiol 277: C271–C279, 1999. doi: 10.1152/ajpcell.1999.277.2.C271. [DOI] [PubMed] [Google Scholar]
- 27.Lomasney KW, Cryan JF, Hyland NP. Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. Am J Physiol Gastrointest Liver Physiol 307: G241–G247, 2014. doi: 10.1152/ajpgi.00401.2013. [DOI] [PubMed] [Google Scholar]
- 28.Lomasney KW, Hyland NP. The application of Ussing chambers for determining the impact of microbes and probiotics on intestinal ion transport. Can J Physiol Pharmacol 91: 663–670, 2013. doi: 10.1139/cjpp-2013-0027. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, Klegeris A, Vallance BA, Gibson DL. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol 301: G39–G49, 2011. doi: 10.1152/ajpgi.00509.2010. [DOI] [PubMed] [Google Scholar]
- 30.Urdaci MC, Lefevre M, Lafforgue G, Cartier C, Rodriguez B, Fioramonti J. Antidiarrheal Action of Bacillus subtilis CU1 CNCM I-2745 and Lactobacillus plantarum CNCM I-4547 in Mice. Front Microbiol 9: 1537, 2018. doi: 10.3389/fmicb.2018.01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yajima T. Chemical specificity of short-chain fatty acid-induced electrogenic secretory response in the rat colonic mucosa. Comp Biochem Physiol A Comp Physiol 93: 851–856, 1989. doi: 10.1016/0300-9629(89)90511-2. [DOI] [PubMed] [Google Scholar]
- 32.Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol 313: G80–G87, 2017. doi: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno S, Gerbig S, Schulz S, Spengler B, Diener M, Bader S. Epithelial propionyl- and butyrylcholine as novel regulators of colonic ion transport. Br J Pharmacol 173: 2766–2779, 2016. doi: 10.1111/bph.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagher PC, Egnor RW, Taglietta-Kohlbrecher A, Charney AN. Short-chain fatty acids inhibit cAMP-mediated chloride secretion in rat colon. Am J Physiol Cell Physiol 271: C1853–C1860, 1996. doi: 10.1152/ajpcell.1996.271.6.C1853. [DOI] [PubMed] [Google Scholar]
- 36.Vidyasagar S, Barmeyer C, Geibel J, Binder HJ, Rajendran VM. Role of short-chain fatty acids in colonic HCO3 secretion. Am J Physiol Gastrointest Liver Physiol 288: G1217–G1226, 2005. doi: 10.1152/ajpgi.00415.2004. [DOI] [PubMed] [Google Scholar]
- 37.Tough IR, Forbes S, Cox HM. Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol Motil 30: e13454, 2018. doi: 10.1111/nmo.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med 56: 54–65, 2017. doi: 10.1016/j.mam.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome 9: 140, 2021. doi: 10.1186/s40168-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating N, Keely SJ. Bile acids in regulation of intestinal physiology. Curr Gastroenterol Rep 11: 375–382, 2009. doi: 10.1007/s11894-009-0057-8. [DOI] [PubMed] [Google Scholar]
- 41.Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, Donowitz M, Fallon PG, Keely SJ. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut 63: 808–817, 2014. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]
- 42.Tough IR, Schwartz TW, Cox HM. Synthetic G protein-coupled bile acid receptor agonists and bile acids act via basolateral receptors in ileal and colonic mucosa. Neurogastroenterology and Motil 32: e13943, 2020. doi: 10.1111/nmo.13943. [DOI] [PubMed] [Google Scholar]
- 43.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil 25: 708–711, 2013. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- 44.Duboc H, Tolstanova G, Yuan PQ, Wu V, Kaji I, Biraud M, Akiba Y, Kaunitz J, Million M, Tache Y, Larauche M. Reduction of epithelial secretion in male rat distal colonic mucosa by bile acid receptor TGR5 agonist, INT-777: role of submucosal neurons. Neurogastroenterol Motil 28: 1663–1676, 2016. doi: 10.1111/nmo.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibla JC, Khoury J, Kong T, Robinson A, Colgan SP. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol 291: C282–C289, 2006. doi: 10.1152/ajpcell.00564.2005. [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Kuhlicke J, Jackel K, Eltzschig HK, Singh A, Sjoblom M, Riederer B, Weinhold C, Seidler U, Colgan SP, Karhausen J. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J 23: 204–213, 2009. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward JB, Keely SJ, Keely SJ. Oxygen in the regulation of intestinal epithelial transport. J Physiol 592: 2473–2489, 2014. doi: 10.1113/jphysiol.2013.270249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 263: G91–G96, 1992. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- 49.Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10: 729–740, 2013. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 50.Schulzke JD, Pfaffenbach S, Fromm A, Epple HJ, Troeger H, Fromm M. Prostaglandin I(2) sensory input into the enteric nervous system during distension-induced colonic chloride secretion in rat colon. Acta physiologica (Oxford, UK) 199: 305–316, 2010. doi: 10.1111/j.1748-1716.2010.02096.x. [DOI] [PubMed] [Google Scholar]
- 51.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baird AW, Skelly MM, O'Donoghue DP, Barrett KE, Keely SJ. Bradykinin regulates human colonic ion transport in vitro. Br J Pharmacol 155: 558–566, 2008. doi: 10.1038/bjp.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wasserman SI, Barrett KE, Huott PA, Beuerlein G, Kagnoff MF, Dharmsathaphorn K. Immune-related intestinal Cl− secretion. I. Effect of histamine on the T84 cell line. Am J Physiol Cell Physiol 254: C53–C62, 1988. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]
- 54.Siriwardena A, Kellum JM Jr.. A 5-HT2 receptor mediates serotonin-induced electrolyte transport in rat left colon. J Surg Res 55: 323–329, 1993. doi: 10.1006/jsre.1993.1149. [DOI] [PubMed] [Google Scholar]
- 55.Dho S, Stewart K, Foskett JK. Purinergic receptor activation of Cl− secretion in T84 cells. Am J Physiol Cell Physiol 262: C67–C74, 1992. doi: 10.1152/ajpcell.1992.262.1.C67. [DOI] [PubMed] [Google Scholar]
- 56.Dharmsathaphorn K, Mandel KG, Masui H, McRoberts JA. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl− cotransport system. J Clin Invest 75: 462–471, 1985. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albuquerque FC Jr, Smith EH, Kellum JM. 5-HT induces cAMP production in crypt colonocytes at a 5-HT4 receptor. J Surg Res 77: 137–140, 1998. doi: 10.1006/jsre.1998.5361. [DOI] [PubMed] [Google Scholar]
- 58.Hosoda Y, Karaki S, Shimoda Y, Kuwahara A. Substance P-evoked Cl− secretion in guinea pig distal colonic epithelia: interaction with PGE2. Am J Physiol Gastrointest Liver Physiol 283: G347–G356, 2002. doi: 10.1152/ajpgi.00504.2001. [DOI] [PubMed] [Google Scholar]
- 59.Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol Cell Physiol 256: C197–C203, 1989. doi: 10.1152/ajpcell.1989.256.1.C197. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen TD, Heintz GG, Cohn JA. Pituitary adenylate cyclase-activating polypeptide stimulates secretion in T84 cells. Gastroenterology 103: 539–544, 1992. doi: 10.1016/0016-5085(92)90844-o. [DOI] [PubMed] [Google Scholar]
- 61.Forte LR, Eber SL, Turner JT, Freeman RH, Fok KF, Currie MG. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest 91: 2423–2428, 1993. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cafferata EG, González-Guerrico AM, Giordano L, Pivetta OH, Santa-Coloma TA. Interleukin-1beta regulates CFTR expression in human intestinal T84 cells. Biochim Biophys Acta 1500: 241–248, 2000. doi: 10.1016/s0925-4439(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 63.Vajanaphanich M, Schultz C, Rudolf MT, Wasserman M, Enyedi P, Craxton A, Shears SB, Tsien RY, Barrett KE, Traynor-Kaplan A. Long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4. Nature 371: 711–714, 1994. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- 64.Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J Biol Chem 273: 27111–27117, 1998. doi: 10.1074/jbc.273.42.27111. [DOI] [PubMed] [Google Scholar]
- 65.Keely SJ, Barrett KE. p38 mitogen-activated protein kinase inhibits calcium-dependent chloride secretion in T84 colonic epithelial cells. Am J Physiol Cell Physiol 284: C339–C348, 2003. doi: 10.1152/ajpcell.00144.2002. [DOI] [PubMed] [Google Scholar]
- 66.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest 125: 908–917, 2015. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol 135: 1505–1512, 2002. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 111: 325–333, 1996. doi: 10.1053/gast.1996.v111.pm8690197. [DOI] [PubMed] [Google Scholar]
- 69.Uribe JM, Keely SJ, Traynor-Kaplan AE, Barrett KE. Phosphatidylinositol 3-kinase mediates the inhibitory effect of epidermal growth factor on calcium-dependent chloride secretion. J Biol Chem 271: 26588–26595, 1996. doi: 10.1074/jbc.271.43.26588. [DOI] [PubMed] [Google Scholar]
- 70.Chang N, Uribe JM, Keely SJ, Calandrella S, Barrett KE. Insulin and IGF-I inhibit calcium-dependent chloride secretion by T84 human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 281: G129–G137, 2001. doi: 10.1152/ajpgi.2001.281.1.G129. [DOI] [PubMed] [Google Scholar]
- 71.Mayol JM, Arbeo-Escolar A, Alarma-Estrany P, Adame-Navarrete Y, Fernandez-Represa JA. Progesterone inhibits chloride transport in human intestinal epithelial cells. World J Surg 26: 652–656, 2002. doi: 10.1007/s00268-001-0284-0. [DOI] [PubMed] [Google Scholar]
- 72.Singh AK, Schultz BD, Katzenellenbogen JA, Price EM, Bridges RJ, Bradbury NA. Estrogen inhibition of cystic fibrosis transmembrane conductance regulator-mediated chloride secretion. J Pharmacol Exp Ther 295: 195–204, 2000. [PubMed] [Google Scholar]
- 73.Besançon F, Przewlocki G, Baró I, Hongre AS, Escande D, Edelman A. Interferon-gamma downregulates CFTR gene expression in epithelial cells. Am J Physiol Cell Physiol 267: C1398–C1404, 1994. doi: 10.1152/ajpcell.1994.267.5.C1398. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura H, Yoshimura K, Bajocchi G, Trapnell BC, Pavirani A, Crystal RG. Tumor necrosis factor modulation of expression of the cystic fibrosis transmembrane conductance regulator gene. FEBS Lett 314: 366–370, 1992. doi: 10.1016/0014-5793(92)81507-i. [DOI] [PubMed] [Google Scholar]
- 75.Bertelsen LS, Eckmann L, Barrett KE. Prolonged interferon-gamma exposure decreases ion transport, NKCC1, and Na+-K+-ATPase expression in human intestinal xenografts in vivo. Am J Physiol Gastrointest Liver Physiol 286: G157–G165, 2004. doi: 10.1152/ajpgi.00227.2003. [DOI] [PubMed] [Google Scholar]
- 76.Howe KL, Wang A, Hunter MM, Stanton BA, McKay DM. TGFβ down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp Cell Res 298: 473–484, 2004. doi: 10.1016/j.yexcr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 77.Colgan SP, Parkos CA, Matthews JB, D'Andrea L, Awtrey CS, Lichtman AH, Delp-Archer C, Madara JL. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol Cell Physiol 267: C402–C410, 1994. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 78.Zünd G, Madara JL, Dzus AL, Awtrey CS, Colgan SP. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem 271: 7460–7464, 1996. doi: 10.1074/jbc.271.13.7460. [DOI] [PubMed] [Google Scholar]
- 79.Kolachala V, Asamoah V, Wang L, Srinivasan S, Merlin D, Sitaraman SV. Interferon-gamma down-regulates adenosine 2b receptor-mediated signaling and short circuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. J Biol Chem 280: 4048–4057, 2005. doi: 10.1074/jbc.M409577200. [DOI] [PubMed] [Google Scholar]
- 80.Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology 120: 1393–1403, 2001. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- 81.Rossi O, Karczewski J, Stolte EH, Brummer RJ, van Nieuwenhoven MA, Meijerink M, van Neerven JR, van Ijzendoorn SC, van Baarlen P, Wells JM. Vectorial secretion of interleukin-8 mediates autocrine signalling in intestinal epithelial cells via apically located CXCR1. BMC Res Notes 6: 431, 2013. doi: 10.1186/1756-0500-6-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69: 2826–2832, 2009. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270: 2387–2394, 1995. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 84.Smitham JE, Barrett KE. Differential effects of apical and basolateral uridine triphosphate on intestinal epithelial chloride secretion. Am J Physiol Cell Physiol 280: C1431–C1439, 2001. doi: 10.1152/ajpcell.2001.280.6.C1431. [DOI] [PubMed] [Google Scholar]
- 85.Kansen M, Bajnath RB, Groot JA, de Jonge HR, Scholte B, Hoogeveen AT, Bijman J. Regulation of chloride channels in the human colon carcinoma cell line HT29.cl19A. Pflugers Arch 422: 539–545, 1993. doi: 10.1007/BF00373999. [DOI] [PubMed] [Google Scholar]
- 86.Tabcharani JA, Boucher A, Eng JW, Hanrahan JW. Regulation of an inwardly rectifying K channel in the T84 epithelial cell line by calcium, nucleotides and kinases. J Membr Biol 142: 255–266, 1994. doi: 10.1007/BF00234947. [DOI] [PubMed] [Google Scholar]
- 87.Krolczyk AJ, Bear CE, Lai PF, Schimmer BP. Effects of mutations in cAMP-dependent protein kinase on chloride efflux in Caco-2 human colonic carcinoma cells. J Cell Physiol 162: 64–73, 1995. doi: 10.1002/jcp.1041620109. [DOI] [PubMed] [Google Scholar]
- 88.Chalfoun AT, Kreydiyyeh SI. Involvement of the cytoskeleton in the effect of PGE2 on ion transport in the rat distal colon. Prostaglandins Other Lipid Mediat 85: 58–64, 2008. doi: 10.1016/j.prostaglandins.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 13: 1072–1082, 2012. doi: 10.1111/j.1600-0854.2012.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582: 507–524, 2007. doi: 10.1113/jphysiol.2007.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charney AN, Egnor RW, Alexander-Chacko JT, Zaharia V, Mann EA, Giannella RA. Effect of E. coli heat-stable enterotoxin on colonic transport in guanylyl cyclase C receptor-deficient mice. Am J Physiol Gastrointest Liver Physiol 280: G216–G221, 2001. doi: 10.1152/ajpgi.2001.280.2.G216. [DOI] [PubMed] [Google Scholar]
- 92.Vaandrager AB, Bot AG, De Jonge HR. Guanosine 3',5'-cyclic monophosphate-dependent protein kinase II mediates heat-stable enterotoxin-provoked chloride secretion in rat intestine. Gastroenterology 112: 437–443, 1997. doi: 10.1053/gast.1997.v112.pm9024297. [DOI] [PubMed] [Google Scholar]
- 93.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3- secretion. J Physiol 505: 411–423, 1997. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morales P, Garneau L, Klein H, Lavoie MF, Parent L, Sauvé R. Contribution of the KCa3.1 channel-calmodulin interactions to the regulation of the KCa3.1 gating process. J Gen Physiol 142: 37–60, 2013. doi: 10.1085/jgp.201210933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chappe V, Hinkson DA, Howell LD, Evagelidis A, Liao J, Chang XB, Riordan JR, Hanrahan JW. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 101: 390–395, 2004. doi: 10.1073/pnas.0303411101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seavilleklein G, Amer N, Evagelidis A, Chappe F, Irvine T, Hanrahan JW, Chappe V. PKC phosphorylation modulates PKA-dependent binding of the R domain to other domains of CFTR. Am J Physiol Cell Physiol 295: C1366–C1375, 2008. doi: 10.1152/ajpcell.00034.2008. [DOI] [PubMed] [Google Scholar]
- 97.Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB. Activated PKC{δ} and PKC{ε} inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl− cotransporter NKCC1. J Biol Chem 285: 34072–34085, 2010. doi: 10.1074/jbc.M110.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amacher JF, Zhao R, Spaller MR, Madden DR. Chemically modified peptide scaffolds target the CFTR-associated ligand PDZ domain. PLoS One 9: e103650, 2014. doi: 10.1371/journal.pone.0103650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lobo MJ, Amaral MD, Zaccolo M, Farinha CM. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J Cell Sci 129: 2599–2612, 2016. doi: 10.1242/jcs.185629. [DOI] [PubMed] [Google Scholar]
- 100.Vajanaphanich M, Schultz C, Tsien RY, Traynor-Kaplan AE, Pandol SJ, Barrett KE. Cross-talk between calcium and cAMP-dependent intracellular signaling pathways. Implications for synergistic secretion in T84 colonic epithelial cells and rat pancreatic acinar cells. J Clin Invest 96: 386–393, 1995. doi: 10.1172/JCI118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uribe JM, Gelbmann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhibits Ca(2+)-dependent Cl- transport in T84 human colonic epithelial cells. Am J Physiol Cell Physiol 271: C914–C922, 1996. doi: 10.1152/ajpcell.1996.271.3.C914. [DOI] [PubMed] [Google Scholar]
- 102.Barrett KE, Smitham J, Traynor-Kaplan A, Uribe JM. Inhibition of Ca2+-dependent Cl− secretion in T84 cells: membrane target(s) of inhibition is agonist specific. Am J Physiol Cell Physiol 274: C958–C965, 1998. doi: 10.1152/ajpcell.1998.274.4.C958. [DOI] [PubMed] [Google Scholar]
- 103.Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem 271: 9919–9927, 1996. doi: 10.1074/jbc.271.17.9919. [DOI] [PubMed] [Google Scholar]
- 104.Keely SJ, Calandrella SO, Barrett KE. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular Ca2+, PYK-2, and p60(src). J Biol Chem 275: 12619–12625, 2000. doi: 10.1074/jbc.275.17.12619. [DOI] [PubMed] [Google Scholar]
- 105.Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem 279: 6271–6279, 2004. doi: 10.1074/jbc.M311612200. [DOI] [PubMed] [Google Scholar]
- 106.Bajwa PJ, Lee JW, Straus DS, Lytle C. Activation of PPARγ by rosiglitazone attenuates intestinal Cl- secretion. Am J Physiol Gastrointest Liver Physiol 297: G82–G89, 2009. doi: 10.1152/ajpgi.90640.2008. [DOI] [PubMed] [Google Scholar]
- 107.Alzamora R, O’Mahony F, Bustos V, Rapetti-Mauss R, Urbach V, Cid LP, Sepúlveda FV, Harvey BJ. Sexual dimorphism and oestrogen regulation of KCNE3 expression modulates the functional properties of KCNQ1 K(+) channels. J Physiol 589: 5091–5107, 2011. doi: 10.1113/jphysiol.2011.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Condliffe SB, Doolan CM, Harvey BJ. 17β-Oestradiol acutely regulates Cl− secretion in rat distal colonic epithelium. J Physiol 530: 47–54, 2001. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Mahony F, Alzamora R, Betts V, LaPaix F, Carter D, Irnaten M, Harvey BJ. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17β-estradiol in rat distal colonic crypts. J Biol Chem 282: 24563–24573, 2007. doi: 10.1074/jbc.M611682200. [DOI] [PubMed] [Google Scholar]
- 110.O'Mahony F, Thomas W, Harvey BJ. Novel female sex-dependent actions of oestrogen in the intestine. J Physiol 587: 5039–5044, 2009. doi: 10.1113/jphysiol.2009.177972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowley KA, Linley JE, Robins GG, Kopanati S, Hunter M, Sandle GI. Role of protein kinase C in aldosterone-induced non-genomic inhibition of basolateral potassium channels in human colonic crypts. J Steroid Biochem Mol Biol 104: 45–52, 2007. doi: 10.1016/j.jsbmb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Muanprasat C, Chatsudthipong V. Cholera: pathophysiology and emerging therapeutic targets. Future Med Chem 5: 781–798, 2013. doi: 10.4155/fmc.13.42. [DOI] [PubMed] [Google Scholar]
- 113.Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev 56: 622–647, 1992. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiglmeier PR, Rosch P, Berkner H. Cure and curse: E. coli heat-stable enterotoxin and its receptor guanylyl cyclase C. Toxins (Basel) 2: 2213–2229, 2010. doi: 10.3390/toxins2092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J 13: 1065–1072, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005. doi: 10.1152/ajpcell.00544.2004. [DOI] [PubMed] [Google Scholar]
- 118.Ménard LP, Dubreuil JD. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST1): a new toxin with an old twist. Crit Rev Microbiol 28: 43–60, 2002. doi: 10.1080/1040-840291046687. [DOI] [PubMed] [Google Scholar]
- 119.Forte LR Jr. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104: 137–162, 2004. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 120.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers 2: 16020, 2016. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aktories K, Schwan C, Jank T. Clostridium difficile toxin biology. Annu Rev Microbiol 71: 281–307, 2017. doi: 10.1146/annurev-micro-090816-093458. [DOI] [PubMed] [Google Scholar]
- 122.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 123.Weaver MJ, McHenry SA, Sayuk GS, Gyawali CP, Davidson NO. Bile acid diarrhea and NAFLD: shared pathways for distinct phenotypes. Hepatol Commun 4: 493–503, 2020. doi: 10.1002/hep4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 9: 332–339, 2015. doi: 10.5009/gnl14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Intestinal absorption of bile acids in health and disease. Compr Physiol 10: 21–56, 2019. doi: 10.1002/cphy.c190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borbély YM, Osterwalder A, Kröll D, Nett PC, Inglin RA. Diarrhea after bariatric procedures: diagnosis and therapy. World J Gastroenterol 23: 4689–4700, 2017. doi: 10.3748/wjg.v23.i26.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vijayvargiya P, Camilleri M. Update on bile acid malabsorption: finally ready for prime time? Curr Gastroenterol Rep 20: 10, 2018. doi: 10.1007/s11894-018-0615-z. [DOI] [PubMed] [Google Scholar]
- 128.Dharmsathaphorn K, Huott PA, Vongkovit P, Beuerlein G, Pandol SJ, Ammon HV. Cl− secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J Clin Invest 84: 945–953, 1989. doi: 10.1172/JCI114257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moschetta A, Portincasa P, Debellis L, Petruzzelli M, Montelli R, Calamita G, Gustavsson P, Palasciano G. Basolateral Ca2+-dependent K+-channels play a key role in Cl− secretion induced by taurodeoxycholate from colon mucosa. Bio Cell 95: 115–122, 2003. doi: 10.1016/S0248-4900(03)00011-X. [DOI] [PubMed] [Google Scholar]
- 130.Devor DC, Sekar MC, Frizzell RA, Duffey ME. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84). J Clin Invest 92: 2173–2181, 1993. doi: 10.1172/JCI116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duan T, Cil O, Tse CM, Sarker R, Lin R, Donowitz M, Verkman AS. Inhibition of CFTR-mediated intestinal chloride secretion as potential therapy for bile acid diarrhea. FASEB J 33: 10924–10934, 2019. doi: 10.1096/fj.201901166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Q, Dutta A, Kresge C, Bugde A, Feranchak AP. Bile acids stimulate cholangiocyte fluid secretion by activation of transmembrane member 16A Cl(−) channels. Hepatology 68: 187–199, 2018. doi: 10.1002/hep.29804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morris AP, Estes MK. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol 281: G303–G310, 2001. doi: 10.1152/ajpgi.2001.281.2.G303. [DOI] [PubMed] [Google Scholar]
- 134.Chang-Graham AL, Perry JL, Engevik MA, Engevik KA, Scribano FJ, Gebert JT, Danhof HA, Nelson JC, Kellen JS, Strtak AC, Sastri NP, Estes MK, Britton RA, Versalovic J, Hyser JM. Rotavirus induces intercellular calcium waves through ADP signaling. Science 370: eabc3621, 2020. doi: 10.1126/science.abc3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Unwin RJ, Calam J, Peart WS, Dimaline R, Lightman SL. Vipoma and watery diarrhea. N Engl J Med 307: 377–378, 1982. doi: 10.1056/NEJM198208053070617. [DOI] [PubMed] [Google Scholar]
- 136.Ito T, Jensen RT. Perspectives on the current pharmacotherapeutic strategies for management of functional neuroendocrine tumor syndromes. Expert Opin Pharmacother 22: 685–693, 2021. doi: 10.1080/14656566.2020.1845651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O'Dorisio TM, Mekhjian HS, Gaginella TS. Medical therapy of VIPomas. Endocrinol Metab Clin North Am 18: 545–556, 1989. doi: 10.1016/S0889-8529(18)30381-5. [DOI] [PubMed] [Google Scholar]
- 138.Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs 17: 313–320, 1999. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 139.Innocenti F, Stadler WM, Iyer L, Ramírez J, Vokes EE, Ratain MJ. Flavopiridol metabolism in cancer patients is associated with the occurrence of diarrhea. Clin Cancer Res 6: 3400–3405, 2000. [PubMed] [Google Scholar]
- 140.Kahn ME, Senderowicz A, Sausville EA, Barrett KE. Possible mechanisms of diarrheal side effects associated with the use of a novel chemotherapeutic agent, flavopiridol. Clin Cancer Res 7: 343–349, 2001. [PubMed] [Google Scholar]
- 141.Thomas JP, Tutsch KD, Cleary JF, Bailey HH, Arzoomanian R, Alberti D, Simon K, Feierabend C, Binger K, Marnocha R, Dresen A, Wilding G. Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol 50: 465–472, 2002. doi: 10.1007/s00280-002-0527-2. [DOI] [PubMed] [Google Scholar]
- 142.Kim Y, Quach A, Das S, Barrett KE. Potentiation of calcium-activated chloride secretion and barrier dysfunction may underlie EGF receptor tyrosine kinase inhibitor-induced diarrhea. Physiol Rep 8: e14490, 2020. doi: 10.14814/phy2.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duan T, Cil O, Thiagarajah JR, Verkman AS. Intestinal epithelial potassium channels and CFTR chloride channels activated in ErbB tyrosine kinase inhibitor diarrhea. JCI Insight 4: e126444, 2019. doi: 10.1172/jci.insight.126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moisan A, Michielin F, Jacob W, Kronenberg S, Wilson S, Avignon B, Gérard R, Benmansour F, McIntyre C, Meneses-Lorente G, Hasmann M, Schneeweiss A, Weisser M, Adessi C. Mechanistic investigations of diarrhea toxicity induced by Anti-HER2/3 combination therapy. Mol Cancer Ther 17: 1464–1474, 2018. doi: 10.1158/1535-7163.MCT-17-1268. [DOI] [PubMed] [Google Scholar]
- 145.Edwards AM. Oral sodium cromoglycate: its use in the management of food allergy. Clin Exp Allergy 25, Suppl 1: 31–33, 1995. doi: 10.1111/j.1365-2222.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 146.Soderholm JD. Mast cells and mastocytosis. Dig Dis 27, Suppl 1: 129–136, 2009. doi: 10.1182/blood-2007-11-078097. [DOI] [PubMed] [Google Scholar]
- 147.Pineton de Chambrun G, Dufour G, Tassy B, Riviere B, Bouta N, Bismuth M, Panaro F, Funakoshi N, Ramos J, Valats JC, Blanc P. Diagnosis, natural history and treatment of eosinophilic enteritis: a review. Curr Gastroenterol Rep 20: 37, 2018. doi: 10.1007/s11894-018-0645-6. [DOI] [PubMed] [Google Scholar]
- 148.Frieri M. Mast cell activation syndrome. Clin Rev Allergy Immunol 54: 353–365, 2018. doi: 10.1007/s12016-015-8487-6. [DOI] [PubMed] [Google Scholar]
- 149.Peeters M, Van den Brande J, Francque S. Diarrhea and the rationale to use Sandostatin. Acta Gastroenterol Belg 73: 25–36, 2010. [PubMed] [Google Scholar]
- 150.Farthing MJ. The role of somatostatin analogues in the treatment of refractory diarrhoea. Digestion 57, Suppl 1: 107–113, 1996. doi: 10.1159/000201412. [DOI] [PubMed] [Google Scholar]
- 151.Lamarca A, Barriuso J, McNamara MG, Hubner RA, Valle JW. Telotristat ethyl: a new option for the management of carcinoid syndrome. Expert Opin Pharmacother 17: 2487–2498, 2016. doi: 10.1080/14656566.2016.1254191. [DOI] [PubMed] [Google Scholar]
- 152.Epple HJ, Fromm M, Riecken EO, Schulzke JD. Antisecretory effect of loperamide in colon epithelial cells by inhibition of basolateral K+ conductance. Scand J Gastroenterol 36: 731–737, 2001. doi: 10.1080/003655201300191996. [DOI] [PubMed] [Google Scholar]
- 153.Hughes S, Higgs NB, Turnberg LA. Antidiarrhoeal activity of loperamide: studies of its influence on ion transport across rabbit ileal mucosa in vitro. Gut 23: 974–979, 1982. doi: 10.1136/gut.23.11.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li ST, Grossman DC, Cummings P. Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS Med 4: e98, 2007. doi: 10.1371/journal.pmed.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fischbach W, Andresen V, Eberlin M, Mueck T, Layer P. A comprehensive comparison of the efficacy and tolerability of racecadotril with other treatments of acute diarrhea in adults. Front Med (Lausanne) 3: 44, 2016. doi: 10.3389/fmed.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eberlin M, Mück T, Michel MC. A comprehensive review of the pharmacodynamics, pharmacokinetics, and clinical effects of the neutral endopeptidase inhibitor racecadotril. Front Pharmacol 3: 93, 2012. doi: 10.3389/fphar.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schwartz JC. Racecadotril: a new approach to the treatment of diarrhoea. Int J Antimicrob Agents 14: 75–79, 2000. doi: 10.1016/s0924-8579(99)00151-x. [DOI] [PubMed] [Google Scholar]
- 158.Gordon M, Akobeng A. Racecadotril for acute diarrhoea in children: systematic review and meta-analyses. Arch Dis Child 101: 234–240, 2016. doi: 10.1136/archdischild-2015-309676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eberlin M, Chen M, Mueck T, Däbritz J. Racecadotril in the treatment of acute diarrhea in children: a systematic, comprehensive review and meta-analysis of randomized controlled trials. BMC Pediatr 18: 124, 2018. doi: 10.1186/s12887-018-1095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Szajewska H, Dziechciarz P, Mrukowicz J. Meta-analysis: Smectite in the treatment of acute infectious diarrhoea in children. Aliment Pharmacol Ther 23: 217–227, 2006. doi: 10.1111/j.1365-2036.2006.02760.x. [DOI] [PubMed] [Google Scholar]
- 161.Das RR, Sankar J, Naik SS. Efficacy and safety of diosmectite in acute childhood diarrhoea: a meta-analysis. Arch Dis Child 100: 704–712, 2015. doi: 10.1136/archdischild-2014-307632. [DOI] [PubMed] [Google Scholar]
- 162.Khediri F, Mrad AI, Azzouz M, Doughi H, Najjar T, Mathiex-Fortunet H, Garnier P, Cortot A. Efficacy of diosmectite (smecta) in the treatment of acute watery diarrhoea in adults: a multicentre, randomized, double-blind, placebo-controlled, parallel group study. Gastroenterol Res Pract 2011: 783196, 2011. doi: 10.1155/2011/783196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol 3: 349–357, 2010. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307: C1084–C1092, 2014. doi: 10.1152/ajpcell.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 166.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52: 988–997, 2003. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sudha MR, Bhonagiri S, Kumar MA. Efficacy of Bacillus clausii strain UBBC-07 in the treatment of patients suffering from acute diarrhoea. Benef Microbes 4: 211–216, 2013. doi: 10.3920/BM2012.0034. [DOI] [PubMed] [Google Scholar]
- 168.Ianiro G, Rizzatti G, Plomer M, Lopetuso L, Scaldaferri F, Franceschi F, Cammarota G, Gasbarrini A. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients 10: 1074, 2018. doi: 10.3390/nu10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guarino A, Guandalini S, Lo Vecchio A. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 49, Suppl 1: S37–S45, 2015. doi: 10.1097/MCG.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 170.Freedman SB, Williamson-Urquhart S, Farion KJ, Gouin S, Willan AR, Poonai N, Hurley K, Sherman PM, Finkelstein Y, Lee BE, Pang XL, Chui L, Schnadower D, Xie J, Gorelick M, Schuh S; PERC PROGUT Trial Group. Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med 379: 2015–2026, 2018. doi: 10.1056/NEJMoa1802597. [DOI] [PubMed] [Google Scholar]
- 171.Keely SJ, Walters JR. The farnesoid X receptor: good for bad. Cell Mol Gastroenterol Hepatol 2: 725–732, 2016. doi: 10.1016/j.jcmgh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther 41: 54–64, 2015. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 173.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60: 463–472, 2011. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 174.Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl- secretory capacity by butyrate. J Clin Invest 101: 2072–2079, 1998. doi: 10.1172/JCI1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687–G693, 2001. doi: 10.1152/ajpgi.2001.280.4.G687. [DOI] [PubMed] [Google Scholar]
- 176.Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci 44: 1924–1930, 1999. doi: 10.1023/a:1018871412748. [DOI] [PubMed] [Google Scholar]
- 177.Linsdell P. Direct block of the cystic fibrosis transmembrane conductance regulator Cl(−) channel by butyrate and phenylbutyrate. Eur J Pharmacol 411: 255–260, 2001. doi: 10.1016/s0014-2999(00)00928-6. [DOI] [PubMed] [Google Scholar]
- 178.Resta-Lenert S, Truong F, Barrett KE, Eckmann L. Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281: C1837–C1849, 2001. doi: 10.1152/ajpcell.2001.281.6.C1837. [DOI] [PubMed] [Google Scholar]
- 179.Correa-Matos NJ, Donovan SM, Isaacson RE, Gaskins HR, White BA, Tappenden KA. Fermentable fiber reduces recovery time and improves intestinal function in piglets following Salmonella typhimurium infection. J Nutr 133: 1845–1852, 2003. doi: 10.1093/jn/133.6.1845. [DOI] [PubMed] [Google Scholar]
- 180.Generoso SV, Lages PC, Correia M. Fiber, prebiotics, and diarrhea: what, why, when and how. Curr Opin Clin Nutr Metab Care 19: 388–393, 2016. doi: 10.1097/MCO.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 181.Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol 108: 718–727, 2013. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 182.Rawat P, Singh PK, Kumar V. Evidence based traditional anti-diarrheal medicinal plants and their phytocompounds. Biomed Pharmacother 96: 1453–1464, 2017. doi: 10.1016/j.biopha.2017.11.147. [DOI] [PubMed] [Google Scholar]
- 183.Semenya SS, Maroyi A. Medicinal plants used by the Bapedi traditional healers to treat diarrhoea in the Limpopo Province, South Africa. J Ethnopharmacol 144: 395–401, 2012. doi: 10.1016/j.jep.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 184.Gurgel LA, Silva RM, Santos FA, Martins DT, Mattos PO, Rao VS. Studies on the antidiarrhoeal effect of dragon's blood from Croton urucurana. Phytother Res 15: 319–322, 2001. doi: 10.1002/ptr.728. [DOI] [PubMed] [Google Scholar]
- 185.Ojewole JA, Mawoza T, Chiwororo WD, Owira PM. Sclerocarya birrea (A. Rich) Hochst. ['Marula'] (Anacardiaceae): a review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phytother Res 24: 633–639, 2010. doi: 10.1002/ptr.3080. [DOI] [PubMed] [Google Scholar]
- 186.Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract—a review of actions and therapeutic implications. Am J Chin Med 42: 1053–1070, 2014. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 187.Castro JG, Chin-Beckford N. Crofelemer for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on anti-retroviral therapy. Expert Rev Clin Pharmacol 8: 683–690, 2015. doi: 10.1586/17512433.2015.1082424. [DOI] [PubMed] [Google Scholar]
- 188.Cottreau J, Tucker A, Crutchley R, Garey KW. Crofelemer for the treatment of secretory diarrhea. Expert Rev Gastroenterol Hepatol 6: 17–23, 2012. doi: 10.1586/egh.11.87. [DOI] [PubMed] [Google Scholar]
- 189.Gabriel SE, Davenport SE, Steagall RJ, Vimal V, Carlson T, Rozhon EJ. A novel plant-derived inhibitor of cAMP-mediated fluid and chloride secretion. Am J Physiol Gastrointest Liver Physiol 276: G58–G63, 1999. doi: 10.1152/ajpgi.1999.276.1.G58. [DOI] [PubMed] [Google Scholar]
- 190.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol 77: 69–78, 2010. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J 24: 4178–4186, 2010. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Das S, Jayaratne R, Barrett KE. The role of ion transporters in the pathophysiology of infectious diarrhea. Cell Mol Gastroenterol Hepatol 6: 33–45, 2018. doi: 10.1016/j.jcmgh.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Luan J, Zhang Y, Yang S, Wang X, Yu B, Yang H. Oridonin: a small molecule inhibitor of cystic fibrosis transmembrane conductance regulator (CFTR) isolated from traditional Chinese medicine. Fitoterapia 100: 88–94, 2015. doi: 10.1016/j.fitote.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 194.Rabbani GH. Mechanism and treatment of diarrhoea due to Vibrio cholerae and Escherichia coli: roles of drugs and prostaglandins. Dan Med Bull 43: 173–185, 1996. [PubMed] [Google Scholar]
- 195.Taylor CT, Winter DC, Skelly MM, O'Donoghue DP, O'Sullivan GC, Harvey BJ, Baird AW. Berberine inhibits ion transport in human colonic epithelia. Eur J Pharmacol 368: 111–118, 1999. doi: 10.1016/S0014-2999(99)00023-0. [DOI] [PubMed] [Google Scholar]
- 196.Thiagarajah JR, Donowitz M, Verkman AS. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol 12: 446–457, 2015. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thiagarajah JR, Ko EA, Tradtrantip L, Donowitz M, Verkman AS. Discovery and development of antisecretory drugs for treating diarrheal diseases. Clin Gastroenterol Hepatol 12: 204–209, 2014. doi: 10.1016/j.cgh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 199.Du C, Liu J, Wan H, Dong H, Zhao X. Functional role of basolateral ClC-2 channels in the regulation of duodenal anion secretion in mice. Dig Dis Sci 64: 2527–2537, 2019. doi: 10.1007/s10620-019-05578-7. [DOI] [PubMed] [Google Scholar]
- 200.Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology 137: 976–985, 2009. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 201.Norimatsu Y, Moran AR, MacDonald KD. Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP(4)). Biochem Biophys Res Commun 426: 374–379, 2012. doi: 10.1016/j.bbrc.2012.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nishii N, Oshima T, Li M, Eda H, Nakamura K, Tamura A, Ogawa T, Yamasaki T, Kondo T, Kono T, Tozawa K, Tomita T, Fukui H, Miwa H. Lubiprostone induces claudin-1 and protects intestinal barrier function. Pharmacology 105: 102–108, 2020. doi: 10.1159/000503054. [DOI] [PubMed] [Google Scholar]
- 203.Zong Y, Zhu S, Zhang S, Zheng G, Wiley JW, Hong S. Chronic stress and intestinal permeability: Lubiprostone regulates glucocorticoid receptor-mediated changes in colon epithelial tight junction proteins, barrier function, and visceral pain in the rodent and human. Neurogastroenterol Motil 31: e13477, 2019. doi: 10.1111/nmo.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kato T, Honda Y, Kurita Y, Iwasaki A, Sato T, Kessoku T, Uchiyama S, Ogawa Y, Ohkubo H, Higurashi T, Yamanaka T, Usuda H, Wada K, Nakajima A. Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: a prospective randomized pilot study in healthy volunteers. PLoS One 12: e0175626, 2017. doi: 10.1371/journal.pone.0175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li F, Fu T, Tong WD, Liu BH, Li CX, Gao Y, Wu JS, Wang XF, Zhang AP. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc 91: 456–468, 2016. doi: 10.1016/j.mayocp.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 206.Bassotti G, Usai-Satta P, Bellini M. Linaclotide for the treatment of chronic constipation. Expert Opin Pharmacother 19: 1261–1266, 2018. doi: 10.1080/14656566.2018.1494728. [DOI] [PubMed] [Google Scholar]
- 207.Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, Cohen MB, MacDougall JE, Lavins BJ, Kurtz CB, Silos-Santiago I, Johnston JM, Currie MG, Blackshaw LA, Brierley SM. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology 145: P1334–P1346.e11, 2013. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 208.Love BL. Plecanatide for treatment of chronic constipation and irritable bowel syndrome. Am J Med 132: 572–575, 2019. doi: 10.1016/j.amjmed.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 209.Chedid V, Vijayvargiya P, Camilleri M. Elobixibat for the treatment of constipation. Expert Rev Gastroenterol Hepatol 12: 951–960, 2018. doi: 10.1080/17474124.2018.1522248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol 16: 522–527, 2018. doi: 10.1016/j.cgh.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 106: 2154–2164, 2011. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]