Abstract
Nicotinamide adenine dinucleotide (NAD) acts as a cofactor in several oxidation-reduction (redox) reactions and is a substrate for a number of nonredox enzymes. NAD is fundamental to a variety of cellular processes including energy metabolism, cell signaling, and epigenetics. NAD homeostasis appears to be of paramount importance to health span and longevity, and its dysregulation is associated with multiple diseases. NAD metabolism is dynamic and maintained by synthesis and degradation. The enzyme CD38, one of the main NAD-consuming enzymes, is a key component of NAD homeostasis. The majority of CD38 is localized in the plasma membrane with its catalytic domain facing the extracellular environment, likely for the purpose of controlling systemic levels of NAD. Several cell types express CD38, but its expression predominates on endothelial cells and immune cells capable of infiltrating organs and tissues. Here we review potential roles of CD38 in health and disease and postulate ways in which CD38 dysregulation causes changes in NAD homeostasis and contributes to the pathophysiology of multiple conditions. Indeed, in animal models the development of infectious diseases, autoimmune disorders, fibrosis, metabolic diseases, and age-associated diseases including cancer, heart disease, and neurodegeneration are associated with altered CD38 enzymatic activity. Many of these conditions are modified in CD38-deficient mice or by blocking CD38 NADase activity. In diseases in which CD38 appears to play a role, CD38-dependent NAD decline is often a common denominator of pathophysiology. Thus, understanding dysregulation of NAD homeostasis by CD38 may open new avenues for the treatment of human diseases.
Keywords: CD38, diseases, NAD metabolism
INTRODUCTION
A series of studies by Warburg and others between the 1920s and the 1950s led to the discovery that nicotinamide adenine dinucleotide (NAD) is an essential cofactor in oxidation-reduction (redox) reactions (1). These discoveries shed light on reactions that drive the transfer of reducing equivalents from energy-rich substrates such as glucose, fatty acids, and amino acids to the mitochondrial electron transport system (ETS), resulting in the synthesis of ATP (2). The NAD+-to-NADH ratio (NAD+/NADH) is considered a readout of the cell’s redox state (3). The phosphorylated form of NADH (NADPH) is also a critical substrate for enzymes charged with scavenging reactive oxygen species (ROS) and preventing oxidative damage to cells (4, 5).
In addition to these “classical” functions, NAD plays a crucial role in cell signaling as a substrate for protein-modifying enzymes (e.g., ADP-ribosylation and deacetylation) and the formation of putative second messengers such as cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (6). This review critically discusses the mounting literature demonstrating that NAD dysregulation is consequential to cellular homeostasis. In fact, dysregulation of NAD metabolism is currently reported in preclinical animal models of age-related disease and increasingly identified in human diseases (7). NAD homeostasis relies on several enzymes including those associated with NAD degrading and synthetic pathways. This review pays particular attention to the role of the NAD-catabolizing enzyme CD38, which plays critical roles in the pathogenesis of diseases related to infection, inflammation, fibrosis, metabolism, and aging (Fig. 1) (7–9).
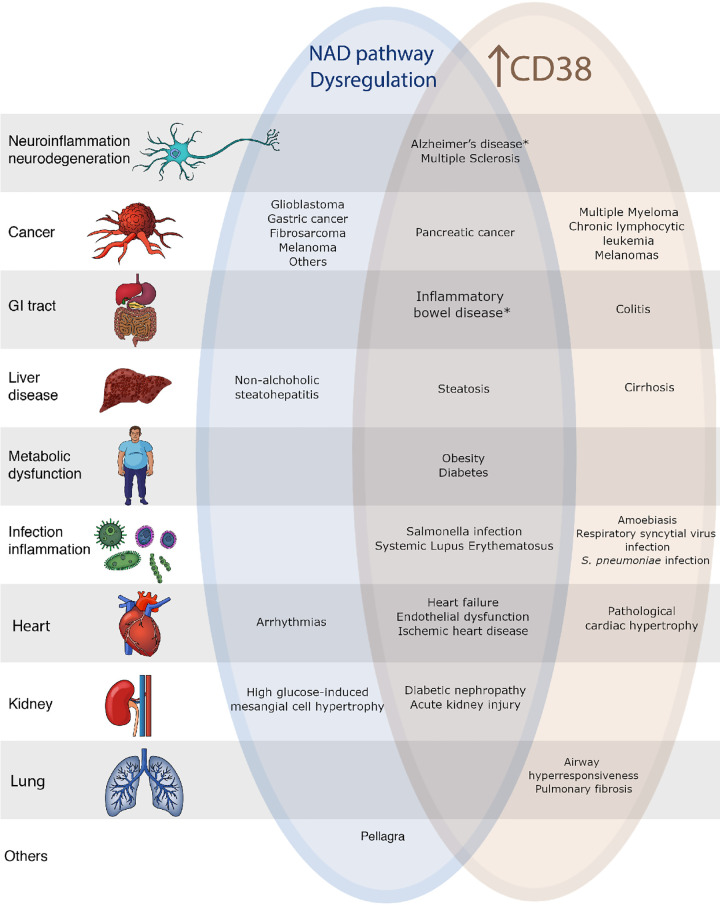
Figure 1.
Diseases associated with CD38 and/or nicotinamide adenine dinucleotide (NAD) dysfunction. Asterisks indicate diseases in which CD38 and NAD metabolism play a role in pathophysiology but the link between CD38 and NAD dysregulation in these diseases is yet to be established. GI, gastrointestinal.
NAD METABOLISM OVERVIEW: SYNTHESIS AND DEGRADATION
Cellular NAD levels are dynamically controlled by the balance between its synthesis and degradation (6). As a redox carrier, NAD is interconverted between its oxidized form (NAD+) and its reduced form (NADH) by dehydrogenases or oxidoreductases that catalyze hydride transfer. In redox reactions, the ratio between the oxidized and reduced forms (NAD+/NADH) is changed but no NAD is consumed and the total NAD+/NADH pool is maintained. However, NAD-degrading enzymes such as CD38, ARTCs (ADP-ribosyltransferase C2 and C3 toxin-like), sirtuins (SIRTs), and poly(ADP-ribose) polymerases (PARPs) break the glycosidic bond between the nicotinamide ring and the ribose of the dinucleotide. This generates free nicotinamide and an ADP-ribosyl moiety and contributes to a decrease in the levels of NAD (6). The activity of these enzymes is reflected by high turnover of NAD in some tissues (10). NAD half-life varies across tissues from 15 min to 15 h in mice, averaging 2–4 h in most tissues (10). The dynamic turnover of NAD in tissues highlights the previously underappreciated metabolic fluxes of NAD in vivo. Why NAD turnover is especially rapid is not entirely understood (11). The total NAD pool is recycled in hours and requires a significant expenditure of energy to maintain cellular levels (Table 1). Conservative estimates predict that the mouse recycles its NAD pool nearly three times a day to maintain its steady-state level (Table 1).
Table 1.
Energy cost of NAD salvage pathway synthesis
| Tissue | NAD, nmol/mg* | Tissue Weight, g | NAD, µmol | NAD Half-Life, h | NAD Turnover, µmol/h | ATP |
% Daily Caloric Requirements | ||
|---|---|---|---|---|---|---|---|---|---|
| µmol/h | µmol in 24 h | kcal in 24 h | |||||||
| Liver | 4.77 | 1.4 | 6.9 | 2.1 | 1.6 | 9.8 | 235.7 | 0.0017 | 0.021 |
| Heart | 4.12 | 0.2 | 0.8 | 2.6 | 0.2 | 1.0 | 22.8 | 0.0002 | 0.002 |
| Spleen | 0.38 | 0.1 | 0.0 | 0.4 | 0.0 | 0.2 | 5.5 | 0.0000 | 0.000 |
| Intestine | 0.84 | 0.9 | 0.8 | 0.3 | 1.3 | 7.8 | 187.6 | 0.0014 | 0.016 |
| Skeletal muscle | 2.26 | 19.7 | 44.5 | 14.6 | 1.5 | 9.1 | 219.6 | 0.0016 | 0.019 |
| Fat | 4.34 | 3.6 | 15.6 | 2.3 | 3.4 | 20.4 | 488.6 | 0.0036 | 0.043 |
| Brain | 2.36 | 0.4 | 1.0 | 3.9 | 0.1 | 0.8 | 18.3 | 0.0001 | 0.002 |
| Lung | 1.75 | 0.2 | 0.3 | 2.4 | 0.1 | 0.3 | 8.2 | 0.0001 | 0.001 |
| Kidneys | 7.78 | 0.3 | 2.6 | 2.6 | 0.5 | 3.0 | 72.8 | 0.0005 | 0.006 |
| Total | 26.9 | 72.5 | 8.74 | 52.5 | 1,259.0 | 0.0092 | 0.110 | ||
Nicotinamide adenine dinucleotide (NAD) levels are derived from 3-mo-old C57BL/6 male mice (n = 4). Tissue weights are provided by the Jackson Laboratory database. NAD half-life in tissues is determined according to Liu et al. (10). Daily caloric requirement of young C57BL/6 male mice is 8.33 kcal/day based on the Jackson Laboratory database. *Calculations are based on an expenditure of 6 ATP molecules per recycled nicotinamide (NAM) [i.e., 2 ATP equivalents in the ribose-5-phosphate isomerase reaction, 2 ATP in the reaction catalyzed by nicotinamide phosphoribosyltransferase (NAMPT), and 2 ATP equivalents in the reaction catalyzed by nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT)].
NAD can be synthesized by multiple pathways (Fig. 2). De novo NAD synthesis requires the essential amino acid tryptophan and proceeds through the kynurenine pathway (KP) mainly in the liver (10, 12). A portion of de novo-synthesized NAD is likely metabolized in the liver to nicotinamide (NAM) and then distributed organism-wide. It is speculated that NAM released from the liver or NAM formed as a product of NAD breakdown in cells is used to generate NAD in other tissues through a second pathway called the NAD salvage pathway (10). The first step of the salvage pathway is catalyzed by the rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT), which synthesizes nicotinamide mononucleotide (NMN) from NAM and a phospho-ribosyl group from 5-phospho-α-d-ribosyl 1-diphosphate (PRPP), a metabolite that originates from the pentose-phosphate pathway (13). NMN is a substrate for NMN-adenylyltransferases (NMNATs 1–3), which catalyze the conversion of NMN and ATP to NAD and pyrophosphate. Interestingly, the synthesis of NAD from NAM is energetically costly (Table 1), particularly given the requirement of 2–4 molecules of ATP per molecule of NMN (14, 15). Based on 24-h NAD turnover, one would expect a mouse to use ∼1.25 mmol of ATP each day to recycle its NAD pool via the salvage pathway (Table 1). This amount would correspond to ∼0.7 g of ATP per day, ∼1–3% of the weight of a young mouse, to maintain steady-state levels of NAD. Although NAD turnover has not been determined in humans, data in rodents suggest that the energy requirement for recycling this cofactor is quite significant.
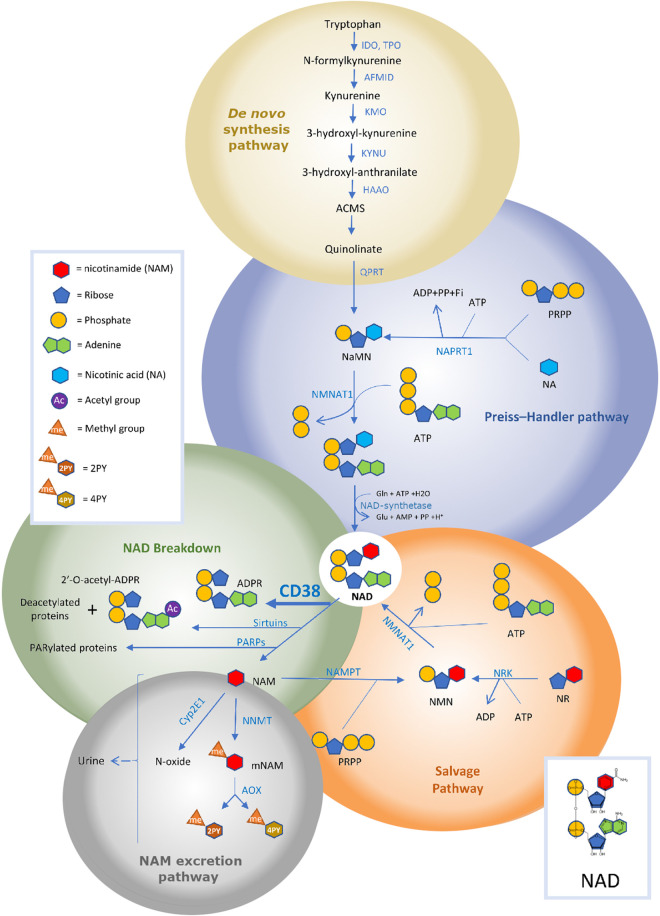
Figure 2.
Nicotinamide adenine dinucleotide (NAD) pathways: synthesis, degradation, and excretion. Overview of the pathways related to NAD metabolism. 2PY, N-methyl-2-pyridone-5-carboxamide; 4PY, N-methyl-4-pyridone-3-carboxamide; ACMS, 2-amino-3-carboxymuconic acid semialdehyde; ADPR, ADP-ribose; AFMID, arylformamidase; AOX, aldehyde oxidase; Cyp2E1, cytochrome P-450 2E1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HAAO, 3-hydroxyanthranilate 3,4-dioxygenase; IDO, indoleamine 2,3-dioxygenase; KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; M-NAM, methyl nicotinamide; NA, nicotinic acid; NaAD, nicotinic acid adenine dinucleotide; NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; NaMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NaPRT1, nicotinic acid phosphoribosyltransferase 1; NMN, nicotinamide mononucleotide; NMNAT1, nicotinamide/nicotinic acid mononucleotide adenylyltransferase 1; NNMT, nicotinamide N-methyltransferase; NR, nicotinamide riboside; PARP, poly(ADP-ribose) polymerase; PRPP, phosphoribosyl pyrophosphate; QPRT, quinolinate phosphoribosyl transferase; SARM1, sterile α and TIR motif-containing protein 1; TPO, tryptophan 2,3-dioxygenase. Figure modified from Chini et al. (319) with permission from Cell Metabolism.
NAD is alternatively generated from nicotinic acid (NA), a dietary form of vitamin B3, through a third pathway called the Preiss–Handler pathway. Here the enzyme nicotinate phosphoribosyltransferase 1 (NAPRT1) catalyzes the reaction between NA and PRPP using energy derived from ATP and produces nicotinic acid mononucleotide (NaMN), ADP, and inorganic pyrophosphate (PPi) (16, 17). The next steps involve the incorporation of AMP (from ATP) by NMNATs and an amidation in the pyridine ring of NA by the NAD-synthetase, which uses l-glutamine or ammonia as a nitrogen source (18) to generate NAD. Interestingly, changes in the intestinal microbiota can affect NAD production via the Preiss–Handler pathway (19). In the gut, metabolism of NA by the intestinal microbial nicotinamidase PncA generates NAM (19). As expected, germfree mice are more vulnerable to the inhibition of the salvage pathway, indicating that gut microbiota indeed contribute to NAD production through the Preiss–Handler pathway. Furthermore, gut microbiota potentiates the NAD-boosting effect of NAM supplementation by providing deaminated intermediates for NAD synthesis. Gut microbiota also participates in conversion of NR to NAM, NA, and nicotinic acid riboside (NAR) in mice (19). Taken together, gut microbiota contributes to the energy balance of the organism by maintaining NAD levels within a healthy range mainly by diversifying the array of NAD precursors for NAD synthesis available to the host.
NMN, another NAD precursor, can be generated intracellularly from NR via a phosphorylation reaction catalyzed by nicotinamide riboside kinase 1 (NRK1) (20–22). A reduced form of NR (NRH) can also serve as a potent NAD precursor (23, 24). NRH is phosphorylated to NMNH by adenosine kinase and results in enhanced NAD boosting (23–25). The interplay between these multiple pathways for NAD synthesis has not been completely elucidated.
NAD CONSUMERS: PARPs, SIRTs, SARMs
The common feature of enzymes that degrade NAD is that each breaks the glycosidic bond between the NAM ring and the ribose of the dinucleotide. This section highlights ADP-ribosyltransferases (PARPs), NAD-dependent protein deacylases (SIRTs), and the sterile α and toll/IL-1 receptor motif containing 1 enzyme (SARM1).
ADP-Ribose Polymerases
ADP-ribosyltransferases such as PARPs [poly(ADP-ribose) polymerases] and tankyrases catalyze the transfer of ADP-ribose from NAD to a number of amino acids in substrate proteins (i.e., arginine, aspartate, glutamate, cysteine, serine, lysine, or tyrosine residues) and release NAM (26, 27). This posttranslational modification can then be recognized by other proteins that possess specific interaction domains such as PIN, PEPPAR, PBZ, BRCT, MDPAR, WWE, OB-fold, and/or FHA. A well-known example of poly ADP-ribosylation (PAR) is the PARylation of proteins by ribosyltransferases that sense single- or double-strand DNA breaks (26). The resulting PAR chains recruit DNA repair machinery. ADP-ribosylation additionally controls many other important cellular processes such as transcription (28, 29), cell cycle progression (30), proteasome regulation (31), and metabolism (32). Among the ADP-ribosyltransferases, PARP1 is the most abundant and significantly impacts NAD levels when overactivated (33–36). Another example of ADP-ribosylation is the recently described ADP-ribosylation of DNA on thymidine, which is performed by DNA ADP-ribosyltransferase (DarT) and is induced by bacterial toxins (e.g., DarT-DarG-toxin antitoxin system) requiring NAD consumption (37).
NAD-Dependent Protein Deacetylases
Largely known as NAD-dependent protein deacetylases, sirtuins (SIRTs) transfer the acetyl group of an acetylated substrate protein to the ADPR moiety of NAD, forming 2′-O-acetyl-ADPR, NAM, and deacetylated proteins (38). Some sirtuins such as SIRT4–7 display lower deacetylase activity (39–42). Apart from protein deacetylation activity, sirtuins also perform other reactions such as defatty-acylation by SIRT1–3 and -6 (43–46), deacylation by SIRT4 (47), and desuccinylation, demalonylation, and deglutarylation by SIRT5 (48–50) in an NAD-dependent manner. NAD levels regulate these activities as well as biological processes related to these enzymes (51, 52). Having low binding affinity to NAD+ (27), sirtuins are more likely to be influenced by changes in NAD homeostasis as a consequence of the activity of other NAD-dependent enzymes.
Sterile α and Toll/IL-1 Receptor Motif-Containing 1
Sterile α and Toll/interleukin-1 receptor motif-containing 1 (SARM1) has been identified in neurons as a new member of the NADase enzyme family (53, 54). It was shown to degrade NAD and generate ADPR, cADPR, and NAM as products (53). SARM1 may also generate the second messenger NAADP via the base-exchange reaction (55). In neurons, SARM1-dependent NAD depletion plays a key role in the axonal degeneration pathway in vitro and in vincristine-induced traumatic injury models in vivo (53). SARM1 is an important NADase of the nervous system, yet it is still not known whether the enzyme contributes to NAD depletion in other tissues.
NAD CONSUMERS: CD38
CD38 was first observed on thymocytes and T lymphocytes (56) and is widely reported on immune, endothelial, and smooth muscle cells (9, 57–64). CD38 is upregulated in a cell-dependent manner by several stimuli in the presence of 1) proinflammatory or secreted senescence factors (9, 64–66) or in response to a bacterial infection (63, 67); 2) retinoic acid (68); or 3) gonadal steroids (60, 69). CD38 is stimulated in a cell-specific manner by lipopolysaccharide (LPS), tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and interferon-γ (IFN-γ) (9, 66, 70, 71). Among the NAD-degrading enzymes, CD38 is the main regulator of NAD levels in mouse tissues (51, 52, 72–74).
CD38 exhibits three main functions using NAD as a substrate. ADP-ribosyl cyclase activity produces cADPR and NAM, which represents <1% of the enzymatic activity of CD38. cADPR is a putative second messenger that induces calcium release from the endoplasmic reticulum by binding to ryanodine receptors (75). CD38 also catalyzes a rare base-exchange reaction that substitutes the NAM group with an NA moiety, which produces NAADP and free NAM (76, 77). NAADP is a putative second messenger that mediates Ca2+ signaling acting on two-pore channel receptor (TPC) in acid endolysosomes (78) and is generated by CD38-dependent base-exchange reaction in vitro (76, 77, 79) with uncertain physiological relevance in vivo (7, 80, 81). Because the base-exchange reaction in vitro occurs in excess of NA and low pH (82), it may have some importance in specific cellular compartments such as the endolysosomal system (83). NAADP is also shown to mediate calcium release from the endoplasmic reticulum through its interaction with the NAADP binding protein HN1L/JPT2 (hematological and neurological expressed 1-like protein/Jupiter microtubule associated homolog 2), which forms complex with ryanodine receptor 1 (84–86). Upon T-cell receptor (TCR)/CD3 complex stimulation, the dual NADPH oxidases DUOX1 and DUOX2 produce NAADP from NAADPH, eliciting calcium signaling that ultimately leads to T-cell activation (87). CD38 does not participate in NAADP formation in this context. Rather, CD38 appears to coordinate NAADP degradation in T cells, perhaps acting on the desensitization of the NAADP-dependent signaling (88).
Most significantly, CD38 NAD glycohydrolase activity produces ADPR and NAM and represents >90% of enzyme activity (89). ADPR acts on an intracellular domain of transient receptor melastatin 2 (TRPM2) channels, thus eliciting Ca2+ influx across the plasma membrane (90). CD38 can also degrade NMN, producing NAM and, presumably, phosphoribose (52, 91), regulating NMN levels in vivo (64).
Although CD38 can be found in the cytoplasm and in the membranes of intracellular organelles, the vast majority of CD38 activity is in the plasma membrane, facing outside the cell (7, 92). That CD38 regulates NAD homeostasis inside the cell while its catalytic site faces the extracellular space has been known as the CD38 “topological paradox.” One explanation for this paradox is that the roles of extracellular and intracellular CD38 differ. For instance, whereas intracellular CD38 targets NAD for degradation, extracellular-facing CD38 additionally degrades the NAD precursor NMN, limiting the production of NAD intracellularly (51, 52, 64, 91). In addition to its function in NAD catabolism, CD38 is also required in purinergic signaling pathways, where it interacts with extracellular nucleotidases and modulates the production of the immunosuppressive molecule adenosine (93). Neither α-NAD derivatives, NR and NRH, nor reduced forms of NAD can be efficiently hydrolyzed by CD38 (24, 52, 94). CD38 also produces NAM as a product of intracellular NAD breakdown and as a product of NMN extracellular breakdown. Considering the importance of CD38 as the main NAD-consuming enzyme in the body (74), it may also be responsible for local NAM accumulation (36, 95) and subsequent inhibition of NAD-utilizing enzymes, but this possibility remains to be explored.
CD38 interferes with the activity of other NAD-dependent enzymes such as sirtuins. For example, CD38 inhibits sirtuins both by reducing NAD levels and by generating NAM, a well-characterized sirtuin inhibitor (36, 51, 96). Inhibition of CD38 by 78c, a specific and potent CD38 inhibitor, promotes protein deacetylation (51). Genetic deletion or inhibition of CD38 also protects mice from conditions that impair sirtuin activity such as a high-fat diet (97), dysregulation of glucose and lipid homeostasis in obesity (72), age-associated mitochondrial dysfunction (52), and d-galactose-induced myocardial cell senescence (98). Thus, the interplay between CD38 and sirtuins is an important component of the pathophysiology of diseases associated with NAD decline (Fig. 3).
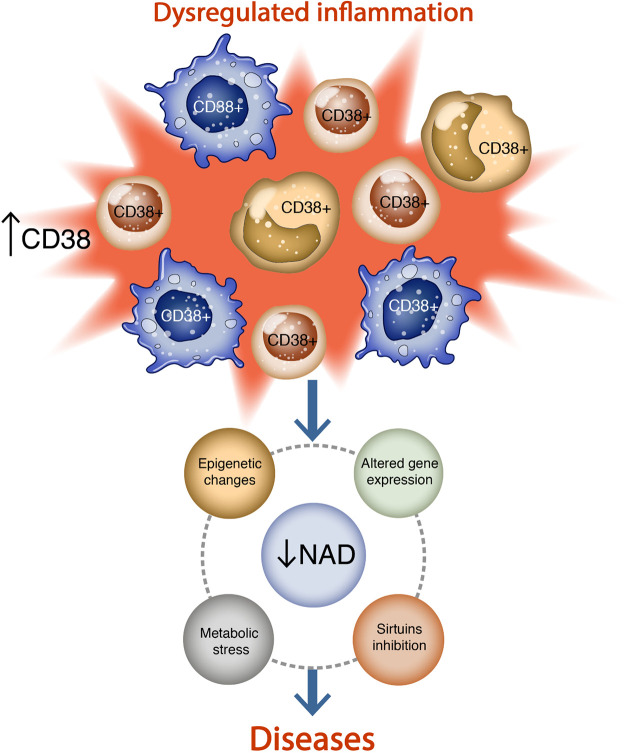
Figure 3.
Interplay between CD38, nicotinamide adenine dinucleotide (NAD) decline, and inflammation. Inflammatory signals induce the recruitment of CD38+ cells to a specific site, where increased CD38 activity leads to NAD decline, inhibition of sirtuins and epigenetic changes, altered gene expression, and metabolic stress.
CD38 AND INFECTIOUS DISEASE
Bacterial and Protozoan Infections
CD38+ immune cells are emerging as important pathogen responders. The reliance of bacteria on uptake of NAD precursors for NAD synthesis provides some insight into how CD38 might disrupt the metabolic demands of bacteria (99, 100). The presence of CD38 on immune cells is likely to be an evolutionary strategy for limiting availability of NAD precursors to bacteria and affecting infection control (7). Evidence in genetically altered mice suggests a role for CD38 in the innate immune response against pathogens. CD38-knockout mice have increased susceptibility to direct intratracheal administration of Streptococcus pneumoniae and intravenous administration of Listeria monocytogenes (101, 102). The recruitment of neutrophils to the site of infection is impaired in CD38-knockout mice, and neutrophil chemotaxis to the bacterial antigen fMLP is dependent on CD38/cADPR/Ca2+ signaling (101), but some controversy surrounds the contribution of ADPR versus cADPR in neutrophil recruitment (103). Macrophages derived from CD38-knockout mice are less efficient at engulfing Listeria than those from wild-type (WT) mice, but both are equally adept at killing bacteria (102) or limiting their growth (63). Interestingly, NAD decline, but not cADPR/Ca2+ signaling, induces cellular cytoskeleton modifications and protects macrophages from excessive bacterial internalization (63). Overall, the bacteriostatic effects of CD38 rely on modulation of immune cell migration/phagocytosis and on scarcity of NAD precursors, which may result in metabolic collapse in these microorganisms (7, 99). The effect of CD38 could be especially important for some bacteria such as Haemophilus influenzae that support metabolism by obtaining intact NAD or other precursors such as NMN from the surrounding environment (99, 100).
CD38 also protects against protozoan infections such as hepatic amoebiasis (i.e., Entamoeba histolytica) by potentiating CD38+ neutrophil migration to amoebic liver abscesses (ALAs) (104). In CD38-knockout mice, there is a delay in neutrophil migration compared with wild-type mice.
Viral Infection
The role of CD38 in viral infections is not well understood but is especially important given the emergence of novel viruses worldwide. Cell-specific increases in CD38 levels are found in patients infected with Epstein–Barr virus, cytomegalovirus (CMV), and human immunodeficiency virus (HIV) (105–107), but what role CD38 plays in mitigating infection remains unclear. One possibility is that CD38 modifies cytoskeletal dynamics required for immune cell migration by controlling either NAD levels or cADPR-dependent Ca2+ signaling (63, 101). Another alternative, which is observed in respiratory syncytial virus (RSV) infection, is CD38-mediated induction of innate immune response genes (e.g., IFN-β, IFN-λ1/IL-29, RANTES, MxA, ISG15) in a cADPR-dependent manner (108).
NAD dysregulation and decline occur in SARS-CoV-2 models of coronavirus infections as a consequence of NAD consumption by PARPs (109). Furthermore, accumulation of senescent cells is observed in a mouse model of coronavirus. In vitro, senescent cells exposed to SARS-CoV-2-derived spike protein produce a hyperinflammatory response (110). Aged coronavirus-infected mice administered senolytic drugs show a reduction in senescence, inflammation, and mortality (110). Given the clear association between the phenomenon of inflammaging, senescence, and CD38, as well as the impact of CD38 on degradation of NAD and the NAD precursor NMN (64, 65), future studies should focus on CD38 as a druggable target in viral illnesses (111, 112). Administration of NAD precursors for the purpose of boosting NAD levels in the presence of viral infections shows promise preclinically and in human clinical trials. For example, NAD boosting attenuates neuronal cell death and increases brain weight in mice infected with Zika virus (113), and administration of a combination of glutathione and NAD precursors in patients with SARS-CoV-2 infection results in improved recovery (114).
In summary, CD38 participates in the innate immune response against bacterial, protozoan, and viral infections. Future studies on the role of CD38 in infection should explore how aging influences the immune response and whether the apparent resiliency observed in aging CD38-knockout mice extends to infection in humans.
CD38 IN AUTOIMMUNITY AND FIBROSIS
Autoimmune Disease
CD38 plays a critical role in inflammation, migration, and immunometabolism, but equally important is the resolution of the inflammatory response, if left unchecked, leads to loss of self-tolerance (115), tissue infiltration of lymphocytes, and circulation of autoantibodies (116). Mounting evidence suggests that CD38 acts as a double-edged sword in the formation of autoimmunity. Depending upon context, CD38 can either promote or protect against an autoimmune response (117–121). Here, the role of CD38 in four autoimmune disorders is discussed briefly and outstanding questions highlighted.
Systemic lupus erythematosus.
Systemic lupus erythematosus (SLE) involves formation of autoantibodies against nuclear and cytoplasmic antigens (122, 123), resulting in tissue damage in multiple organs including skin and kidneys (124). Initial observations of dysregulated NAD metabolism in SLE show decreased ADP-ribosylation in patient-derived peripheral blood lymphocytes (125). Individuals with SLE have CD8+ T cells with high CD38 expression (CD8+CD38high) (117, 121, 126, 127) and accompanying NAD decline, Sirt1 inactivation, acetylation of the methyl-transferase EZH2, and subsequent suppression of cytotoxicity-related transcription factors (128). The result is a lower T-cell cytotoxic response observed in SLE, which predisposes patients to infection. Pharmacological inhibition of EZH2 restores cytotoxic capacity of CD8+CD38high T cells in SLE. What triggers increased CD38 expression in CD8+ T cells in this condition remains unknown.
Multiple sclerosis.
Multiple sclerosis (MS) is a chronic autoimmune degenerative disease of the central nervous system (CNS) characterized by inflammation, demyelination, and destruction of the blood-brain barrier (129). The pathogenesis of MS involves activation of microglia and macrophages, which when reversed significantly decreases disease severity (129). In a cuprizone (CPZ)-induced demyelination mouse model, CD38 is upregulated in both astrocytes and microglia (130). CD38 inhibition in this model attenuates glial activation and demyelination by restoring NAD levels. Furthermore, in a myelin-oligodendrocyte-glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) mouse model, CD38 is involved in T-cell activation (131), which is attenuated in CD38-knockout mice. Further studies are required to evaluate whether CD38 expression on inflammatory cells also disrupts NAD homeostasis in EAE.
Interestingly, a Western diet potentiates demyelination in the CNS of mice, which is improved by genetic ablation or pharmacological CD38 inhibition (132). Furthermore, CD38 levels increase in mouse spinal cord after chronic high-fat diet exposure, after focal toxin-mediated demyelinating injury, and in reactive astrocytes in active MS lesions (132). CD38-catalytically inactive mice are protected from high-fat-induced NAD depletion, oligodendrocyte loss, oxidative damage, and astrogliosis. Likewise, the CD38 inhibitor 78c increases NAD and attenuates neuroinflammatory changes in astrocytes treated with saturated fats in vitro (132). Taken together, a high-fat diet impairs oligodendrocyte survival and differentiation by a CD38-mediated mechanism and underscores the potential therapeutic value of CD38 inhibitors in myelin regeneration (132).
Inflammatory bowel disease.
Inflammatory bowel disease (IBD) is an idiopathic chronic and progressive inflammatory condition of the gastrointestinal tract mucosa that includes Crohn’s disease and ulcerative colitis (133). The chronic mucosal inflammation and tissue damage characteristic of the disease predispose IBD patients to the development of colorectal cancer, and the risks increase with duration, extent, and severity of inflammation (134). CD38 expression is increased in macrophages from intestinal tissues of individuals with IBD (135). Additionally, IBD shows systemic chronic inflammation with increased activation of CD38+ T lymphocytes in the blood compared with healthy individuals (136, 137). Dysregulation of NAD metabolism partially explains the pathogenesis of IBD, which includes upregulation of the NAD consumers CD38, PARP9, PARP14, and SIRT1; the NAD synthesis enzyme NAMPT; and the NAD excretion enzyme nicotinamide N-methyltransferase (NNMT) (135).
The direct link between CD38 and intestinal inflammation is demonstrated in CD38-knockout mice in a dextran sulfate sodium (DSS)-induced colitis model (119). Wild-type mice exposed to DSS display dense CD38+ cells in the mucosa, including resident T cells, granulocytes, and inflammatory monocytes, and exhibit weight loss, shortening of the colon, and alterations in the morphology of the epithelium, crypts, and submucosa. Conversely, CD38-knockout mice displayed only mild disease during DSS treatment (119). CD38 depletion is likely to be protective in DSS-treated mice because of impaired migration of immune cells to mucosal tissue. It is proposed that ADPR produced by CD38 breakdown of NAD acts on TRPM2, a plasma membrane Ca2+-permeable cation channel (119). Curiously, DSS-induced ulcerative colitis is also suppressed in TRPM2-knockout mice (138). It remains to be established whether CD38-induced cell migration, CD38-dependent secretion of proinflammatory cytokines, and/or CD38 NADase activity contribute to the role of CD38 in intestinal inflammation.
Rheumatoid arthritis.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease more frequently observed in females and the elderly and associated with progressive disability and premature death. RA mainly targets the lining of synovial joints, includes extra-articular involvement, and results in inflammatory arthritis (139, 140).
Antibody-producing plasma cells and their B cell precursors contribute considerably to the pathogenesis of RA by synthesizing autoantibodies that either bind to tissue antigens or form immune complexes within tissues (9). CD38 is highly expressed in plasma cells compared with other immune cell populations in synovial biopsies and peripheral blood mononuclear cells (PBMCs) (121). In fact, pathogenic autoantibodies such as anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor (RF), synthesized by infiltrating plasma cells, are considered biomarkers of rheumatoid synovitis and serve to gauge the severity of RA and disease activity (141). Importantly, B cells, T cells, and macrophages, all of which express CD38, infiltrate the joint tissue (synovium), producing various proinflammatory cytokines that facilitate inflammation and ultimately destroy surrounding tissue (142).
CD38 expression is higher in synovial tissues of individuals with RA compared with other inflammatory conditions like ankylosing spondylitis (AS) and osteoarthritis (OA) (143). In the peripheral blood of RA patients, the proportions of CD38+ cells and CD38+ CD56+ cells [which represents CD38+ natural killer (NK) cells] are significantly higher, and the level of CD38+ cells correlates with the level of autoantibodies (143). CD38+ NK cells release proinflammatory cytokines IFN-γ and TNF-α, which contribute to RA progression (144). Conversely, suppression of CD38 in cultured patient-derived synovial fibroblasts results in decreased IL-1α and IL-1β secretion (143).
Several drugs targeting CD38 show promise in the treatment and management of RA. Daratumumab, a CD38-targeting monoclonal antibody approved for the treatment of multiple myeloma (MM), reduces autoantibody levels (145) and depletes autoreactive plasma cells (145). Additionally, the anti-CD38 monoclonal antibody TAK-079 prevents arthritis by decreasing NK cells, B cells, and T cells in primates (146). Likewise, cyanidin-3-O-glucoside, a competitive inhibitor of CD38 cyclase activity, ameliorates RA synovial fibroblast proliferation, IL-6 and IFN-γ levels, and the percentage of CD38+ NK cells in rats (144).
Future studies will seek to understand the implications of dysregulated NAD homeostasis in autoimmune conditions like RA and the ways in which targeting CD38 may reverse NAD-related metabolic imbalance (147).
Fibrosis
Differentiation of quiescent progenitor cells into activated myofibroblasts and their persistence is a common feature to all forms of fibrosis. However, what triggers the process remains obscure. Mounting evidence suggests that dysregulation of the NAD metabolome may underlie processes leading to fibrosis and, importantly, that CD38 may be a promising therapeutic target.
Systemic sclerosis.
Systemic sclerosis (SSc) is a chronic systemic orphan disease associated with high mortality (148). A hallmark of SSc is synchronous fibrosis in multiple tissues including skin, lung, heart, and muscle that leads to permanent and irreversible organ dysfunction with no effective treatment (8). One hypothesis is that dysregulation of NAD metabolism resulting from the interplay of immune cells and fibroblasts plays a key role in SSc pathogenesis. Indeed, skin biopsies of SSc patients that show signatures of fibrosis have increased expression of key NAD-depleting enzymes including CD38 and NNMT (8). Conversely, genetic ablation or pharmacological inhibition of CD38 in mice increases NAD levels in skin and lung and substantially attenuates bleomycin-induced fibrosis (8).
Pulmonary fibrosis.
Inflammatory processes are pathognomonic for common disorders of the lung [e.g., asthma, chronic obstructive pulmonary disease (COPD)]. Therefore, the link between CD38 and inflammatory diseases of the lung may shed light on early events contributing to fibrosis. Asthma pathophysiology involves a series of events including increased airway smooth muscle contractility, mucus hypersecretion, impaired lung elasticity, and disruption of epithelial integrity, which lead to airway narrowing and impact lung function (149). The influx of inflammatory cells contributes to airway hyperresponsiveness (AHR), which is the main characteristic of asthma and defined as an exacerbated response to nonspecific stimuli present in the environment. Studies in CD38-knockout mice using different models of AHR such as inhaled methacholine, TNF-α, IL-13, or ovalbumin (OVA) challenge reveal a dual role for CD38 in airway smooth muscle (ASM) reactivity via cADPR-dependent Ca2+ release from sarcoplasmic reticulum and modulation of inflammation (150–153). There is a paucity of data elucidating the role of CD38 as an NAD consumer in this context.
Pulmonary fibrosis occurs in the presence of unresolved inflammation and dysregulated tissue repair and results from an array of injurious stimuli including infection, toxicant exposure, adverse effects of drugs, and autoimmune response. Fibrosis is irreversible and is associated with a poor prognosis (154). Interestingly, CD38+ cells are found in the peripheral blood and in the bronchoalveolar space of individuals with pulmonary fibrosis (155, 156), although the contribution of CD38 remains unexplored. One possibility is that CD38 is involved in the epithelial-to-mesenchymal transition (EMT) (157). EMT is a phenotypic transition of epithelial cells necessary for normal tissue repair (158) but can be induced by stress and lead to loss of apical/basal polarization, loss of cell-cell adhesion, and acquisition of a fibroblast-like phenotype (158). Interestingly, it is reported that postinfluenza viral lung fibrosis observed in old mice is mediated by a subset of CD8+ cells (159). Additionally, CD8+ cells are shown to be enriched with CD38 in some studies (128, 160, 161). Thus, it is possible that CD38+CD8+ cells may contribute to lung fibrosis observed after viral infections such as influenza or coronavirus.
Renal fibrosis.
CD38 plays an important role in kidney physiology, namely renal vasoconstriction responsible for regulating both renal blood flow and glomerular filtration (162, 163). Renal vasoconstriction is controlled hormonally by angiotensin II (ANG II), endothelin-1 (ET-1), and norepinephrine (NE) (164), which in turn are modulated by CD38 expressed on preglomerular resistance arterioles (162). CD38-knockout mice display attenuated hemodynamic response to administration of ANG II, ET-1, and NE, which impact renal microcirculation functioning by reducing basal renal blood flow and urine excretion (162). Furthermore, thromboxane prostanoid (TP)-induced vasoconstriction is also mediated by CD38 (163). Additionally, CD38 is important for differentiation and function of podocytes, which are epithelial cells of the glomerulus that act as a glomerular filtration barrier (165, 166). Although the mechanisms are unknown, CD38 deficiency leads to podocyte EMT, resulting in enhanced glomerular injury and sclerosis (166).
CD38 deficiency significantly elevates levels of renal NAD (74, 167). The kidney is among the organs with the highest mitochondrial abundance (168). Therefore, regulation of NAD is critical for maintaining renal homeostasis. Reduced NAD+-to-NADH ratios (NAD+/NADH) are reported in numerous renal diseases including diabetic nephropathy (169) and acute kidney injury (AKI) (170–172) and in high-glucose-induced mesangial cell hypertrophy (173). By inhibiting CD38 or boosting NAD to increase NAD+/NADH and reduce mitochondrial stress, an improvement of renal conditions is observed. For example, inhibiting CD38 with apigenin restores NAD+/NADH and SIRT3 function in renal cells and improves renal injury in a model of diabetic nephropathy (174). In an LPS-induced AKI mouse model, inhibition of CD38 with quercetin ameliorates LPS-induced AKI and improves kidney function and inflammation by inhibiting LPS-induced M1 polarization and activation of NF-κB signaling in kidney macrophages (172). Supplementation with NR (175) and NMN promotes NAD boosting and protects against age-associated susceptibility to AKI (170). Similarly, NAM supplementation is associated with reduced AKI in cardiac surgery patients (171). These studies demonstrate that modulation of CD38 and NAD levels in kidney disease may provide therapeutic approaches for the prevention of inflammatory conditions of the kidney that predispose the kidney to fibrosis and altered function.
Cirrhosis.
Fibrosis is a critical step in the progression of most chronic liver diseases and a precursor of cirrhosis (176). Liver cirrhosis arises from a variety of conditions including alcoholism, chronic hepatitis virus infection, nonalcoholic fatty liver disease (NAFLD), exposure to drugs and toxins, and inherited diseases among others (177, 178). Despite many etiologies of cirrhosis, some common pathological findings include degeneration and necrosis of hepatocytes, appearance of regenerative nodules, and fibrotic tissue (176).
The specific role of CD38 in the development of liver cirrhosis is still unclear. An association between CD38 and cirrhosis is shown in a thioacetamide‐induced rat model of liver cirrhosis. Cirrhotic rat livers demonstrate increased CD38 expression and cyclase activity resulting in higher cADPR levels in liver microsomes (179). These findings raise the possibility that increased CD38 activity and cADPR could be involved in the pathogenesis of cirrhosis. Immunohistochemical studies in liver biopsies from patients with chronic liver disease show an increased number of CD38+ hepatic stellate cells (HSCs), which are cells known to contribute to hepatic fibrosis (180). Furthermore, there is a positive correlation between the number of CD38+ HSCs and the METAVIR score, which is based on the intensity of necroinflammatory activity, interface hepatitis, and lobulitis. These findings highlight CD38+ HSCs as a potential biomarker of fibrosis in chronic liver diseases (180). Moreover, markers of inflammation and senescence are present in the pathophysiology of chronic liver diseases that progress to cirrhosis (181–186). CD38 plays a role in both inflammation and senescence. Age-related NAD decline and increased inflammation are partially mediated by senescence-induced accumulation of CD38+ inflammatory cells in tissues (64, 65). This may offer new insight into a role for CD38 in cirrhosis.
METABOLIC DISEASES
Obesity and Metabolic Syndrome
Obesity is a disease of epidemic proportions and represents a major public health problem worldwide (187). Besides being a risk factor for increased morbidity and mortality, obesity is a feature of metabolic syndrome, which is a cluster of conditions that increase risk of cardiovascular disease (CVD), type 2 diabetes, and stroke (188). CD38 plays a key role as a regulator of the obesity phenotype (97, 189), and inhibition of CD38 has the potential to ameliorate obesity and metabolic syndrome. In mice, CD38 deficiency results in a higher metabolic rate and resistance to high-fat diet-induced obesity (97). In humans, several lines of evidence positively associate CD38 with metabolic phenotypes including CD38 methylation and adiposity and linkage between CD38 and cholecystokinin A receptor genes and lipid levels (190–192). Previous studies also demonstrate a relationship between obesity and NAD decline in multiple metabolic tissues including liver, pancreas, and adipose (193, 194). Most notably, inhibition of CD38 ameliorates high-fat diet-induced hepatic steatosis and protects against obesity and metabolic syndrome by increasing availability of NAD (72, 194, 195). Taken together, elevation of NAD levels by genetic ablation of CD38 affords protection from the diet-induced insulin resistance, accumulation of fat, and metabolic inflexibility observed in wild-type mice (195), which is likely to translate to metabolic syndrome observed in humans.
In addition to its role as the primary NADase in mammalian tissues, CD38 regulates SIRT enzymes by regulating NAD availability (189). CD38-knockout mice show increased NAD levels compared with wild type and are protected against high-fat diet (HFD)-induced obesity, metabolic syndrome, glucose intolerance, and liver steatosis through a SIRT1-dependent mechanism. CD38-knockout mice also have higher energy expenditures compared with wild-type mice, with increased basal and activity-induced metabolic rates (97). The mechanism underlying these changes is mediated at least in part by NAD-dependent activation of SIRT1-peroxisome proliferator-activated receptor-γ coactivator α (PGC1α) axis and downstream effects on energy metabolism (97). Furthermore, SIRT1 regulates mitochondrial biogenesis and response to stress through the deacetylation of PGC-1α (196). Consistent with these findings, inhibition of CD38 by the small-molecule inhibitor 78c in mice ameliorates age-related glucose intolerance and insulin resistance, suggesting CD38 as a possible pharmacological target for aging-related metabolic dysfunction (51). A possible mechanism for amelioration of obesity through inhibition of CD38 is impaired adipogenesis and lipogenesis through the activation of a SIRT1-peroxisome proliferator-activated receptor γ (PPARγ) or a SIRT1-sterol regulatory element binding protein 1 (SREBP1) signaling pathway, respectively (197). Expression of adipogenic genes including PPARγ, fatty acid-binding protein 4 (FABP4), and CCAAT/enhancer-binding protein α (C/EBPα) is attenuated in CD38-knockout mice. In addition, in vitro expression of CD38 is increased during adipocyte differentiation in mouse embryonic fibroblasts (MEFs). CD38-deficient MEFs show increased expression of SIRT1 and downregulation of SREBP1‐mediated FASN (fatty acid synthase) expression, suggesting that CD38 may also influence lipogenesis. Altogether, these results suggest an important link between CD38 deficiency and the development of obesity through activation of Sirt1 signaling (197).
NAD-dependent SIRT3, which is localized in mitochondria, also plays a CD38-dependent role in obesity and other features of metabolic syndrome (197). To illustrate, CD38-knockout mice demonstrate improved glucose tolerance profiles compared with wild-type mice, but this observation is reversed in CD38/SIRT3 double-knockout mice (52). Furthermore, CD38-knockout mice exhibit increased oxygen consumption coupled to ATP synthesis compared with wild-type mice, a phenomenon that is also reversed in double-knockout mice. Taken together, these observations suggest that ablation of SIRT3 in CD38-knockout mice abrogates the protective effect of the CD38 inhibition in HFD-induced obesity, suggesting a role for NAD-dependent enzymes in obesity (52).
Alterations in white adipose tissue (WAT), in particular, may play a part in the pathogenesis of obesity and metabolic diseases (198). Low-grade chronic inflammation is characteristic of obesity and accompanied by macrophage infiltration in WAT (199, 200). Moreover, the observed macrophage burden and proinflammatory secretome of adipose tissue are correlated with obesity-associated metabolic derangements (201). Macrophage-induced inflammation and insulin resistance in obesity are attenuated by quercetin, a CD38 inhibitor (202), which highlights the importance of CD38+ immune cells in the pathogenesis of metabolic diseases.
Curiously, CD38 is implicated in browning of white fat and the development of brown fat in mice. CD38 downregulation occurs during cold-induced thermogenesis and results in increased NAD+ and NADP(H) levels in brown fat (203). Thus, the role of CD38 in obesity and energy expenditure is linked to thermogenesis in brown fat through SIRT1-dependent mechanisms including the inactivation of the NAD/SIRT1/caveolin-1 axis (204, 205).
Taken together, CD38 inhibition resulting in increased NAD availability to SIRT1 and 3 may have therapeutic potential in the treatment of obesity-related metabolic syndrome.
Nonalcoholic Fatty Liver Disease
A common disease closely associated with metabolic syndrome is nonalcoholic fatty liver disease (NAFLD). NAFLD may lead to nonalcoholic steatohepatitis (NASH), the most common form of chronic liver disease characterized by inflammation and hepatocellular damage. Progression to fibrosis, the first stage of liver scarring, occurs in ∼32–37% of individuals with NASH and may increase risk of hepatocellular carcinoma (119, 206, 207). Comorbidities that accompany NAFLD including cardiovascular diseases, type 2 diabetes, and dyslipidemia often contribute to the morbidity and mortality of this disease (208).
SIRT1 and 3 appear to be important targets of NASH pathophysiology. Overexpression of SIRTs not only protects the liver from steatosis and progression to NASH but also can reverse effects of this disease (209–214). In a methionine and choline diet-deficient mouse model of NASH (209), sirtuins are downregulated, resulting in increased expression of lipogenic genes such as fatty acid synthase (215). Increased gene expression is observed as well in mice fed a HFD, which results in decreased NAD levels, reduced SIRT3 activity in liver, hyperacetylation of liver proteins, and reduced activity of mitochondrial complexes III and IV (213).
A role for CD38 in the pathophysiology of NASH has also been proposed. In a HFD model, CD38-knockout mice show significantly lower liver fat infiltration in comparison to wild-type control (97). Additionally, CD38 inhibition in a mouse model of HFD-induced obesity treated with the flavonoid apigenin demonstrates decreased lipid accumulation in liver through increased lipid oxidation, NAD boosting, and SIRT1 activation (72).
Taken together, these studies show a role for sirtuins and CD38 in metabolic syndrome and NAFLD and suggests that therapeutic interventions targeting SIRT1 activators or CD38 inhibitors or supplementation with NAD synthesis precursors may be beneficial for management of metabolic diseases (216–218). For example, NMN administration improves glucose metabolism in obese mice and muscle insulin sensitivity in prediabetic women (216, 217), whereas NR supplementation in mice activates both SIRT1 and SIRT3 and ameliorates metabolic dysfunction by improving mitochondrial function (219). In patients with mitochondrial myopathy, administration of niacin improves NAD metabolism and muscle function and decreases liver fat infiltration (220). Furthermore, NAD precursors, alone or in combination with other metabolic activators, have the potential to reverse obesity, enhance exercise performance, and ameliorate NAFLD (221–224).
AGING AND AGE-RELATED DISEASES
Inflammaging and NAD Decline
Tissue NAD levels decline with age and in progeroid syndromes (52, 225–228) despite NAD synthesis being relatively maintained (229). Boosting NAD levels in vivo is protective against some age-associated disorders in animal models (36, 230–233). One of the causes of NAD decline during aging is increase of NAD breakdown in the presence of increased CD38 expression and activity on immune cells, thus linking inflammaging with tissue NAD decline (51, 52, 64, 65). Other sources of NAD decline include increased DNA damage requiring PARP1 activation and decreased NAMPT levels leading to diminished NAD synthesis through the salvage pathway (216, 225, 226, 234–237).
Although age-related NAD decline seems to be multifactorial, CD38 appears to have a significant contribution in this process (51, 52, 64–66). Among the NAD-degrading enzymes, CD38 expression is upregulated with chronological aging in mice, whereas PARP1 and SIRT1 are downregulated (52). In addition, no changes are observed in the expression of NAD-synthesizing enzymes in multiple tissues in mice including liver, white adipose tissue (WAT), spleen, and skeletal muscle (52). Interestingly, CD38-knockout mice demonstrate a delayed age-related NAD decline with age compared with wild-type animals (52). This observation indicates that CD38 plays a major role in NAD dysregulation during aging. Moreover, CD38-dependent age-related NAD decline occurs as a consequence of SIRT3-mediated mitochondrial dysfunction. SIRT3, a key modulator of mitochondrial metabolism, undergoes an age-related decrease in activity, causing increased acetylation of mitochondrial proteins and impaired mitochondrial function independent of mitochondrial biogenesis (52, 238). Aged CD38-knockout mice display more robust mitochondrial function including increased oxygen consumption rates, higher mitochondrial membrane potential, and a higher NAD+/NADH compared with wild-type aged mice (52). Administration of the highly potent and specific CD38 inhibitor 78c restores NAD levels and ameliorates metabolic dysfunction in aged mice, thus improving glucose tolerance, muscle function, exercise capacity, and cardiac function (51). 78c-induced NAD boosting also increases sirtuin and PARP activity; prevents the accumulation of telomere-associated foci (TAFs), a marker of DNA damage associated with aging; and promotes the activation of longevity pathways in tissues of aged mice (51). Taken together, CD38 is a critical regulator of NAD levels during the aging process in rodents.
Whereas CD38 plays a major role in aging-related systemic NAD decline, other mechanisms such as the downregulation of the NAD salvage pathway likely contribute to the NAD decline observed in specific tissues during aging. NAMPT is found inside the cells (iNAMPT) as well as in the extracellular environment (eNAMPT). Plasma eNampt released from adipocytes increases NAD levels in remote tissues such as the hypothalamus by increasing the capacity of the NAD salvage pathway. Increased NAD levels in the hypothalamus regulate SIRT1 activity and neural activation in response to fasting (239). The circulating levels of eNAMPT-containing extracellular vesicles (EVs) decrease with age both in mice and in humans (235). In addition, overexpression of NAMPT in adipose tissue increases NAD biosynthesis in the hypothalamus, hippocampus, pancreas, and retina. Exposure to circulating eNAMPT-containing vesicles results in increased physical activity and sleep quality, delayed aging, and extended life span (235). Conversely, NAMPT decline is correlated with decreased Sirt1 expression/activity in retinal pigment epithelium cells from old mice and in skeletal muscle of aged rats (236, 240). Although the age-associated decline in NAMPT expression is tissue specific, this decrease may also have a systemic impact.
In instances where there is an increase in NAD breakdown by CD38, there will be an excess of NAM. NNMT catalyzes the N-methylation of NAM using S-adenosylmethionine (SAM) as a methyl donor, resulting in the generation of methyl-NAM and S-adenosyl-homocysteine (SAH) (Ref. 95, Fig. 1). Thus, increased NNMT expression has been associated with decreased cellular methylation capacity, reduced histone methylation, and alterations to the epigenetic landscape (95, 241). Also, by limiting the availability of NAM to the NAD salvage pathway, NNMT overexpression is associated with reduced NAD levels in specific contexts such as in high-fat diet-induced fatty liver disease (242) and obesity (243), thereby impacting NAD levels both in liver and in WAT. Accordingly, NNMT inhibitors are shown to increase NAD levels in adipocytes (244). NNMT expression increases in skeletal muscle of old mice, whereas NNMT inhibition enhances muscle regeneration in aging by rescuing muscle stem cell (muSC) function (245). Downregulation of Sirt1 activity as a result of NAD decline is a possible mechanism by which NNMT induces muSC activation in aged muscle tissue. Since NNMT is mostly associated with specific tissues such as liver and fat, it is unlikely that NNMT upregulation contributes to systemic NAD decline in aging. As a NAM generator, CD38 may upregulate NNMT activity in tissues such as fat and liver, thus contributing to a methylation sink, NAD decline, and metabolic dysfunction in specific organs. Whereas modulation of both NAMPT and NNMT expression seems to be context specific, the age-related increase in CD38 expression/activity appears to have the greatest impact on NAD levels.
CD38 also appears to play a wider role in aging by disrupting telomere integrity. In patient-derived cells from individuals with congenital telomere shortening, CD38 expression is higher, resulting in lower NAD levels and reduced PARP and SIRT1 activity (246). However, modification of systemic NAD metabolism by CD38 also occurs, at least partially, by way of circulating CD38-expressing immune cells that infiltrate tissues under inflammatory conditions. Indeed, inflammation is among the major risk factors that predispose organisms to age-associated diseases. During aging, the accumulation of senescent cells (SCs) creates an environment rich in proinflammatory signals, leading to “inflammaging” (247). SCs are metabolically active cells that lose their replicative capacity by entering an irreversible quiescent state and are considered both a cause and a consequence of inflammaging (248). SCs secrete several factors collectively known as the senescence-associated secretory phenotype (SASP), which in turn serves as a source of chemotactic factors for immune cell recruitment. SCs and SASP factors upregulate CD38 in bone marrow-derived macrophages and endothelial cells in culture as well as in vivo (64–66). Increased accumulation of CD38+ immune cell clusters is observed in WAT and liver during aging (64, 65). Furthermore, CD38-knockout mice receiving wild-type donor bone marrow cells accumulate CD38+ cells in tissues, indicating that these cells infiltrate from the circulation (64). In tissues where high levels of immune cells typically reside, such as spleen and intestine, NAD levels decline in CD38-knockout mice transplanted with bone marrow from WT mice. LPS treatment in this model leads to a decline in NAD levels as well (64). Furthermore, clearance of p16ink4a-positive cells from aged INK-ATTAC mice decreases CD38 expression and promotes recovery of NAD levels in WAT and liver (64). Blocking SASP production also leads to restored tissue NAD levels (64, 65). Moreover, the senescence-inducing agent doxorubicin reduces NAD levels in WT mice but has no effect in mice expressing a catalytically inactive form of CD38, thereby linking NAD decline, senescence, and CD38 activity. Taken together, these studies support a role for senescent cells and their SASP factors in the accumulation of CD38+ cells and age-related NAD decline (11, 64).
Cardiovascular Disease
Cardiovascular disease is a leading cause of death among the aged and by 2030 will result in 40% of all deaths in individuals aged 65 yr and older. An abundance of mitochondria in cardiac muscle ensures high levels of NAD, which is required to sustain the metabolic demands of the heart (249). Thus, the NAD-consuming CD38, which is present on nonparenchymal cells of the heart, is emerging as a target of pathogenesis as well as a druggable target in heart disease.
Heart failure.
Cardiac muscle requires high energetic demands because of continuous pumping of oxygenated blood throughout the circulation. Under physiological conditions, the heart relies almost entirely on the oxidation of short-chain fatty acids (250). However, during the development of heart failure, there is a shift from fatty acid oxidation to the utilization of other substrates such as carbohydrates and ketone bodies (251, 252). This transition leads to a decline in NAD+/NADH, which increases susceptibility to stress (252). Preclinical studies report that supplementation with NAD precursors such as NMN and NR prevents decline of NAD+/NADH and adverse cardiac remodeling in a mouse model of heart failure induced by pressure overload through transaortic constriction (253).
CD38 also plays a crucial role in the signaling pathway that leads to pathological cardiac hypertrophy in numerous models of heart failure. For example, in a mouse model of β-adrenergic stimulation-induced heart failure, genetic ablation of CD38 prevents myocardial hypertrophy, interstitial fibrosis, and decreased fractional shortening/ejection fraction in a cADPR-dependent manner (254). CD38-dependent cardiac remodeling is also studied in both two-kidney, one-clip and angiotensin II continuous infusion heart failure models (255, 256). In the latter, the proposed mechanism is CD38 upregulation, inhibition of mitochondrial SIRT3 (by NAD depletion), mitochondrial Ca2+ accumulation and dysfunction, and, consequently, cardiac hypertrophy. In this model, CD38-knockout mice are protected from cardiac hypertrophy (256).
One of the hallmarks of heart failure is the dysregulation of Ca2+ homeostasis in failing cardiomyocytes. A crucial feature is the decrease in expression and activity of sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), the most critical Ca2+-ATPase located in the sarcoplasmic reticulum of myocytes, which replenishes the intrasarcoplasmic Ca2+ pool to maintain a continuously efficient Ca2+ release (and therefore, contraction) in every beat. Decrease in SERCA2a activity and expression leads to a drop in reticular Ca2+ concentration, which causes insufficient contractility and impairs excitation-contraction coupling mechanisms (257). In this context, CD38-knockout mice have a higher SERCA2a-to-phospholamban (PLB) ratio than wild-type mice. Thus, what results is enhanced SERCA2a function with PLB inhibition (258). In addition, CD38-knockout male, but not female, mice demonstrate improved contractility, better contraction and relaxation velocities, and upregulated expression of SERCA2a, Na+/Ca2+ exchanger (NCX), ryanodine receptor (RyR), and myosin heavy chain α isoform (α-MHC) (259).
Expression of the α-MHC isoform results in higher contraction velocity and attachment time with actin and improves contraction. These changes are associated with increased testosterone levels in CD38-knockout mice compared with wild-type mice, which is prevented by treatment with an androgen receptor antagonist (259). With age, the α-MHC-to-β-MHC ratio drops, leading to cardiac contraction impairment. This change is enhanced when heart failure is added to the equation. Therefore, upregulation of α-MHC by CD38 inhibition could lead to improved cardiovascular performance. Indeed, the CD38 inhibitor 78c significantly improves ejection fraction, fractional shortening, and other echocardiographic parameters related to cardiac strain compared with control aged mice (51). Interestingly, recent studies also indicate a role for NAD metabolism in heart failure with preserved ejection fraction (HFpEF) (260, 261). However, to date, the role of CD38 and NAD turnover has not been explored in this condition.
Myocardial infarction.
Ischemic heart disease is another cardiovascular disease of paramount importance due to its high prevalence. In an animal model of postischemic heart disease, CD38-dependent NAD and NADP depletion leads to endothelial dysfunction. In this study, CD38-knockout postischemic hearts show recovered endothelial function compared with wild-type hearts. In another in vitro model of ischemia-reperfusion of isolated heart, genetic ablation of CD38 results in postischemic protection by enhancing glutathione levels, improving contractile performance, reducing infarction size, and decreasing enzymatic release from cardiomyocytes compared with wild-type mice (262). After reperfusion, wild-type hearts present severe endothelial dysfunction due to uncoupling of endothelial nitric oxide synthase (eNOS), whereas CD38-knockout hearts display near-complete recovery of NOS-dependent coronary flow. Cardiac endothelial cells express high levels of CD38, whereas resident fibroblasts and actual cardiomyocytes express little CD38 (61). Predictably, endothelial cells present high CD38 NADase activity compared with other cell types. When subjected to hypoxia/ischemia, endothelial cells significantly increase expression and activity of CD38, leading to depletion of both NAD and NADP levels in heart and endothelial dysfunction (61). Another feature of the ischemic process in the heart is the metabolic shift toward glycolysis, leading ultimately to NAD depletion, a decline in ATP production, inactivation of ATPases, Ca2+ overload, and inevitable cell death (263). In this context, several studies report that boosting NAD levels by supplementation of NAD precursors increases mouse survival in a SIRT1-dependent manner (264). This evidence suggests an essential role of CD38 and NAD metabolism in mediating ischemia-reperfusion injury (Fig. 4).
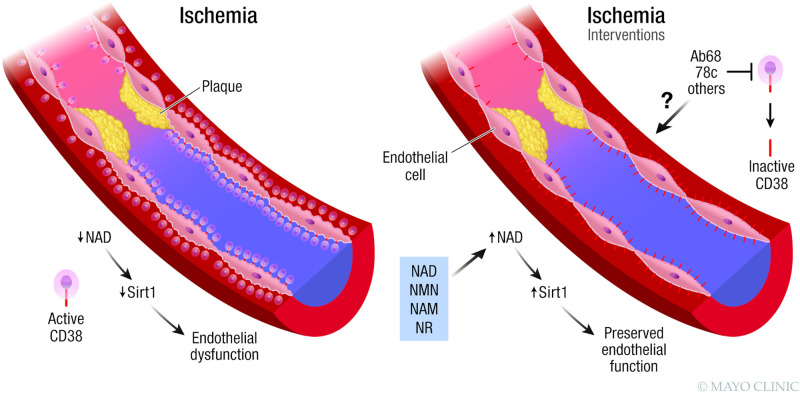
Figure 4.
Ischemia and CD38. During ischemia there is increased expression of CD38 on endothelial cells in the vasculature, resulting in nicotinamide adenine dinucleotide (NAD) decline, downregulation of Sirt1, and endothelial dysfunction. NAD boosting by supplementation with NAD precursors has a protective effect on endothelial function. Although not yet extensively explored, CD38 inhibition could be a target to treat ischemia-induced endothelial dysfunction. NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Vascular dysfunction.
Inflammation-associated metabolic diseases impair vascular function (265). Chronic inflammation can lead to vascular senescence and dysfunction. As discussed above, CD38 leads to NAD depletion related to metabolic syndromes and aging-related diseases (52, 64, 65). Accordingly, CD38 deficiency or NAD supplementation alleviates angiotensin II-induced vascular remodeling and dysfunction by increasing NAD levels in vascular smooth muscle cells (VSMCs) (266). Increased NAD levels mitigate the degree of fibrosis in the vascular wall and, consequently, the media thickness, thereby preserving physiological pressure levels. This protection is also accomplished by decreasing the secretion of senescence-inducing factors, which are secreted from VSMCs and not bone marrow-derived cells (266). CD38 is also involved in vascular remodeling and dysfunction associated with diabetes (267). Under this pathological condition, CD38 upregulation leads to mitochondrial damage, VSMC remodeling, vascular wall fibrosis, and endothelium-independent dysfunction, which is tied to the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome and prevented by CD38 inhibitors (267).
Neurodegeneration
The relationship between pellagra pathophysiology and its effect on the nervous system (NS), when first described, revealed a link between NAD metabolism and proper NS functioning (268, 269). Functional pellagra is likely to cause premature aging and is a risk factor for neurodegeneration. Healthy aged brains demonstrate a decline of NAD+/NADH compared with the brains of younger subjects, and this decline has a potential impact on mitochondrial function (228). Furthermore, the hippocampus of 10- to 12-mo old mice shows a decline in NAD levels and the NAD rate-limiting enzyme NAMPT compared with 1-mo-old mice (270). Considering that CD38 expression and activity increase in multiple tissues and organs with age (52) and are detected in the CNS (130, 271–273), CD38 is proposed to have a role in age-associated neurodegenerative diseases like Alzheimer’s disease (AD) and multiple sclerosis (MS).
AD is the most prevalent form of dementia (274) and is characterized by accumulation of β-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs) (275). CD38 promotes activation of microglia, the resident immune cells of the CNS. Furthermore, CD38 knockdown or 8-Br-cADPR treatment results in reduced secretion of proinflammatory cytokines and nitric oxide (NO) production in LPS-stimulated murine microglial BV2 cells (276, 277). In one study, knockout of CD38 in AD‐prone mice (APP.PS.CD38-KO) results in decreased microglia/macrophage accumulation (278), reduced activity of α-, β-, and γ-secretases, less amyloidogenic Aβ peptide formation, and improved spatial learning (278). However, the role of CD38 in AD and other neurodegenerative diseases has not been well established. In fact, no functional or physiological data are presented in the studies described above (278).
Although a direct association with AD pathology remains to be determined, CD38 appears to play an important role in the nervous system. For instance, CD38 improves neuronal survival after cerebral ischemia by facilitating mitochondrial transfer from astrocytes to neurons, neurons to astrocytes, and astrocytes to astrocytes (272, 279). Mitochondria transfer is proposed to release extracellular mitochondrial particles mediated by CD38/cADPR signaling. The introduction of Alexander disease-associated point mutations into the GFAP (glial fibrillary acidic protein) gene of astrocytes results in impaired mitochondrial transfer from astrocytes and decreased CD38 expression (279). It is not yet known how mutations in the GFAP gene affect CD38 expression. Moreover, changes in NAD levels are not shown to affect mitochondrial transfer among neuronal cells. Thus, CD38 participation in mitochondrial transfer deserves further investigation, particularly because of its potential neuroprotective function. Interestingly, transcriptome-wide association data identify CD38 as a possible gene associated with susceptibility to Parkinson’s disease (280). However, akin to AD, the role of CD38 in this condition is not well explored.
Cancer and Carcinogenesis
Cancer is the quintessential disease of aging, occurring on a backdrop of inflammation, failed DNA repair, senescence, and impaired immune surveillance (281). Because of high metabolic demand and reliance on NAD-dependent signaling, cancer cell growth is tied to NAD metabolism (282, 283). In fact, lowering levels of NAD/NADP by NAMPT inhibition is a promising anticancer strategy in both NAPRT-depleted and CD38-overexpressing tumors (283–285). In addition, NAMPT inhibition sensitizes cancer cells to oxidative stress and chemotherapeutics (283, 285). Interestingly, CD38 is shown to participate in both tumor progression and tumor suppression, depending on tumor type.
CD38 appears to play an important role in multiple myeloma (MM), the second most common hematological malignancy (286, 287). MM cells strongly express CD38, which is used as a marker for myeloma cell immunophenotyping (288, 289). A possible role for CD38 in MM appears to be related to the transfer of mitochondria from bone marrow stromal cells to MM cells via tunneling nanotubes, a process that supports oxidative phosphorylation in MM cells and contributes to cancer cell survival (290). It is still not clear, however, how CD38 facilitates this process. In astrocytes, CD38/cADPR/Ca2+ signaling is proposed to induce release of extracellular mitochondrial particles, promoting mitochondria transfer to adjacent neurons (272). Whether the CD38/cADPR/Ca2+ signaling axis is also part of tunneling nanotube formation in MM remains to be established. Therapeutically, anti-CD38 monoclonal antibodies such as isatuximab and daratumumab are being investigated and used as therapies in patients with MM and other hematological malignancies (291, 292). The process by which anti-CD38 antibodies exert their anticancer effects is not completely understood and comprises several different mechanisms including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP) (291, 293–296).
Another hematological malignancy in which CD38 plays an important role is chronic lymphocytic leukemia (CLL) (297, 298). CD38 positivity in B-CLL cells is a prognostic factor, indicating decreased patient survival (299). Overexpression of CD38 increases aggressiveness of CLL, and CD38 inhibition by the flavonoid kuromanin results in downstream disruption of calcium signaling necessary for CLL chemotaxis, adhesion, and homing (300). Furthermore, migration and homing of CLL lymphocytes requires interaction between CD38 and its endothelial cell coreceptor CD31, which favors localization of neoplastic cells to growth-permissive sites (301). In addition to the CD38/CD31 axis, ADPR and NAADP signaling appear to mediate CD38-dependent effects of CLL migration via activation of the Ras family GTPase Rap1 through regulation of the Ca2+-sensitive Rap1 guanine-nucleotide exchange factor RasGRP2 (302). Not surprisingly, the anti-CD38 MAb daratumumab has antitumor effects in a partially humanized CLL xenograft model (303). Furthermore, anti-CD38 targeting agents modulate the tumor microenvironment by inducing apoptosis of B regulatory cell-like CLL cells and Treg cells, which have immunosuppressive roles. CD38 inhibition also increases activated cytotoxic CD8+ T cells, which target CLL cells (304). Thus, CD38 likely regulates the CLL microenvironment by modulating migration and proliferation of CLL cells, in addition to having immunomodulatory effects on B and T cells.
An alternative CD38-mediated mechanism of tumorigenesis relies on the interplay of adenosine (ADO)-generating ectonucleotidases expressed on tumor cells, stromal cells, and/or tumor-infiltrating immune cells (305). ADO is a signaling molecule that suppresses multiple immune subsets such as cytotoxic T cells via activation of purinergic receptors (306, 307). ADPR generated by CD38 can be used as a substrate by CD203a to generate AMP, a substrate for the primary adenosine-producing ectoenzyme CD73, which then produces ADO. ADO-producing ectoenzymes are expressed on multiple cell types within the tumor microenvironment or specifically on tumor cells (305). For instance, CD38 expressed on melanoma cells plays a prominent role in adenosinergic signaling leading to suppression of T-cell proliferation (308). Kuromanin-mediated inhibition of CD38 activity on melanocytes partially restores T-cell proliferation in coculture experiments. The immunosuppressive quality of the adenosinergic pathway highlights CD38 as a promising target for cancer immunotherapy (93, 305).
Cancer cells elude or modify the immune system by several mechanisms. One mechanism involves increased expression of cell surface immune checkpoint proteins such as the programmed cell death ligand 1 (PD-L1), which binds to the programmed cell death 1 (PD1) receptor present on the surface of T cells (309). PD1/PD-L1 binding triggers T-cell suppression and prevents an antitumor cytotoxic response. By blocking PD1/PD-L1 binding, the immune system targets cancer cells that express high levels of PD-L1. Increased CD38 expression on tumors and the subsequent inhibition of tumor-infiltrating lymphocytes via purinergic signaling is the main mechanism for reversing PD1/PD-L1 blockade in preclinical models of lung cancer (93, 310). Accordingly, coinhibition of CD38 and PD1/PD-L1 improves antitumor immune response (93, 310).
Another CD38-mediated protumor immune response is the expansion of myeloid-derived suppressor cells (MDSCs), which are an immature cell population with immunosuppressive functions. Cancer-related inflammation alters myelopoiesis and stimulates generation of MDSCs, which in turn are recruited to tumor tissues in response to chemokines and act as a barrier to antitumor immunity (311). In a murine esophageal cancer model, administration of a CD38 antibody inhibits the expansion and survival of MDSCs (312). Moreover, CD38+ MDSCs are found in peripheral blood of advanced-stage cancer patients (312). Cytokines such as IFN-γ, TNF-α, IGFBP3, CXCL16, and IL-6 induce CD38 expression in MDSCs, leading to expansion and maintenance of undifferentiated cells with greater immunosuppressive capacity and higher tumor-promoting activity (312). This mechanism is proposed to be mediated by CD38 induction of inducible nitric oxide synthase (iNOS), presumably through binding of CD38 to surface receptors. Another possibility is that the CD38 glycohydrolase activity modulates NAD-dependent signaling and alters gene expression to favor MDSC expansion. Taken together, CD38 is an important immune checkpoint for carcinogenesis with promising therapeutic potential.
CD38 may facilitate interaction between a tumor and its microenvironment including cancer-associated fibroblasts and tumor-associated blood vessels by a mechanism yet to be elucidated. Tumor outgrowth induced by subcutaneous injection of melanoma cells is reduced in CD38-knockout mice compared with wild-type mice and is partially explained by fewer cancer-associated fibroblasts and reduced density of tumor-associated blood vessels in CD38-knockout mice (313). In an opposing scenario, CD38 expression is negatively correlated with disease progression in prostate cancer, in which low CD38 mRNA is prognostic for tumor recurrence and metastasis (314, 315). CD38 is epigenetically regulated in prostate cancer. Suppression of CD38 by methylation may increase the availability of extracellular NAD in prostate cancer and lead to disease progression (314). Higher CD38 expression results in lower NAD and leads to cell cycle arrest. The presence of CD38 also reduces glycolytic and mitochondrial metabolism, inhibits fatty acid metabolism, activates AMPK, and promotes a nonproliferative phenotype (316).
Similarly, the presence of CD38+ cells in hepatocarcinomas improves clinical outcome in cancer patients. Infiltration of CD38+ leukocytes in human hepatocarcinomas is correlated with better patient survival (317). Also, the presence of CD38+ M1 macrophages is associated with a positive prognosis after surgery (318). Although there is no direct evidence, CD38 may contribute to an antitumor immune response. Furthermore, CD38 expression sensitizes pancreatic cancer cells to NAMPT-mediated metabolic collapse (284). In pancreatic cancer cells, CD38 overexpression reduces NAD levels and inhibits cell growth both in vitro and in a xenograft mouse model. In addition, CD38 knockdown in the pancreatic cancer cell line PaTu8988t results in increased NAD levels and resistance to NAMPT inhibition (284). Therefore, CD38-dependent NAD decline has possible antitumor properties. Akin to what is observed in an infection, CD38 plays a complex role in cancer biology that is highly cell and context dependent. These opposing effects of CD38 on cancer cells, inflammatory cells, and cancer stromal cells, which can be pro- or antitumor, can be explained by the multifunctionality of the ectoenzyme, which seems to be tissue specific and/or depend on interactions between the tumor and its microenvironment. Collectively, these findings highlight the importance of understanding the role of CD38 in different types of cancer, in immune cells, and in the tumor microenvironment.
CONCLUSIONS AND FUTURE PERSPECTIVES
NAD is a cofactor of paramount importance for an array of cellular processes related to mitochondrial function and metabolism, redox reactions, signaling, cell division, inflammation, and DNA repair. It is not surprising, therefore, that dysregulation of NAD is associated with multiple diseases. Since CD38 is the main NADase in mammalian tissues, its contribution to pathological processes has been explored in multiple disease models, and it is becoming evident that CD38 is a potential pharmacological target for several conditions. Although there may be links between disease and NAD decline, the role of CD38 in the pathogenesis of many of these diseases is still emerging. Studies presented in this review are largely based on animal models. Future studies are needed to explore the possibility of targeting the CD38-NAD axis for treatment of human diseases.
GRANTS
This work was supported by the Helen Diller Family Foundation, the Glenn Foundation for Medical Research via the Paul F. Glenn Laboratories for the Biology of Aging, Calico Life Sciences LLC, National Institute on Aging Grants AG-26094 and AG58812, and National Cancer Institute Grant CA233790 to E.N.C.
DISCLOSURES
E.N.C holds a patent on CD38 inhibitors licensed by Elysium Health. E.N.C. consults for Calico, Mitobridge, and Cytokinetics. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.D.Z., T.R.P., S.K., K.S.K., C.C.S.C., and E.N.C. conceived and designed research; J.D.Z. and E.N.C. analyzed data; J.D.Z. and E.N.C. interpreted results of experiments; J.D.Z. and G.A. prepared figures; J.D.Z., K.A.H., G.A., T.R.P., S.K., K.S.K., L.S.G., and D.Z.M. drafted manuscript; J.D.Z., K.A.H., G.A., T.R.P., S.K., K.S.K., L.S.G., D.Z.M., G.W., K.L.T., C.C.S.C., amd E.N.C. edited and revised manuscript; J.D.Z., K.A.H., G.A., T.R.P., S.K., K.S.K., L.S.G., D.Z.M., G.W., K.L.T., C.C.S.C., and E.N.C. approved final version of manuscript.
REFERENCES
- 1.Ball EG. The development of our current concepts of biological oxidations. Mol Cell Biochem 5: 35–46, 1974. doi: 10.1007/BF01874170. [DOI] [PubMed] [Google Scholar]
- 2.Racker E. From Pasteur to Mitchell: a hundred years of bioenergetics. Fed Proc 39: 210–215, 1980. [PubMed] [Google Scholar]
- 3.Anderson KA, Madsen AS, Olsen CA, Hirschey MD. Metabolic control by sirtuins and other enzymes that sense NAD+, NADH, or their ratio. Biochim Biophys Acta Bioenerg 1858: 991–998, 2017. doi: 10.1016/j.bbabio.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H, Gerstenecker C, Menzel H, Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett 27: 171–175, 1972. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- 5.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem 86: 715–748, 2017. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 6.Strømland Ø, Niere M, Nikiforov AA, VanLinden MR, Heiland I, Ziegler M. Keeping the balance in NAD metabolism. Biochem Soc Trans 47: 119–130, 2019. doi: 10.1042/BST20180417. [DOI] [PubMed] [Google Scholar]
- 7.Hogan KA, Chini CCS, Chini EN. The multi-faceted ecto-enzyme CD38: roles in immunomodulation, cancer, aging, and metabolic diseases. Front Immunol 10: 1187, 2019. doi: 10.3389/fimmu.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi B, Wang W, Korman B, Kai L, Wang Q, Wei J, Bale S, Marangoni RG, Bhattacharyya S, Miller S, Xu D, Akbarpour M, Cheresh P, Proccissi D, Gursel D, Espindola-Netto JM, Chini CC, de Oliveira GC, Gudjonsson JE, Chini EN, Varga J. Targeting CD38-dependent NAD+ metabolism to mitigate multiple organ fibrosis. iScience 24: 101902, 2021. doi: 10.1016/j.isci.2020.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piedra-Quintero ZL, Wilson Z, Nava P, Guerau-de-Arellano M. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol 11: 597959, 2020. doi: 10.3389/fimmu.2020.597959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Su X, Quinn WJ 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 27: 1067–1080.e5, 2018. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chini CC, Zeidler JD, Kashyap S, Warner G, Chini EN. Evolving concepts in NAD+ metabolism. Cell Metab 33: 1076–1087, 2021. doi: 10.1016/j.cmet.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyes MP, Chen CY, Major EO, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J 326: 351–356, 1997. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 14.Burgos ES, Ho MC, Almo SC, Schramm VL. A phosphoenzyme mimic, overlapping catalytic sites and reaction coordinate motion for human NAMPT. Proc Natl Acad Sci USA 106: 13748–13753, 2009. doi: 10.1073/pnas.0903898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos ES, Schramm VL. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry 47: 11086–11096, 2008. doi: 10.1021/bi801198m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem 282: 24574–24582, 2007. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 17.de Figueiredo LF, Gossmann TI, Ziegler M, Schuster S. Pathway analysis of NAD+ metabolism. Biochem J 439: 341–348, 2011. doi: 10.1042/BJ20110320. [DOI] [PubMed] [Google Scholar]
- 18.Hara N, Yamada K, Terashima M, Osago H, Shimoyama M, Tsuchiya M. Molecular identification of human glutamine- and ammonia-dependent NAD synthetases. Carbon-nitrogen hydrolase domain confers glutamine dependency. J Biol Chem 278: 10914–10921, 2003. [Erratum in J Biol Chem 278:41572, 2003]. doi: 10.1074/jbc.M209203200. [DOI] [PubMed] [Google Scholar]
- 19.Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, Deterding LJ, Lim C, Xu X, Randall TA, Lee E, Li W, Fan W, Li JL, Sokolsky M, Kabanov AV, Li L, Migaud ME, Locasale JW, Li X. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab 31: 564–579.e7, 2020. doi: 10.1016/j.cmet.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasiak K, Saunders PP. Purification and properties of a human nicotinamide ribonucleoside kinase. Arch Biochem Biophys 333: 414–418, 1996. doi: 10.1006/abbi.1996.0409. [DOI] [PubMed] [Google Scholar]
- 21.Tempel W, Rabeh WM, Bogan KL, Belenky P, Wojcik M, Seidle HF, Nedyalkova L, Yang T, Sauve AA, Park HW, Brenner C. Nicotinamide riboside kinase structures reveal new pathways to NAD+. PLoS Biol 5: e263, 2007. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117: 495–502, 2004. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Mohammed FS, Zhang N, Sauve AA. Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem 294: 9295–9307, 2019. doi: 10.1074/jbc.RA118.005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Zhang N, Zhang G, Sauve AA. NRH salvage and conversion to NAD+ requires NRH kinase activity by adenosine kinase. Nat Metab 2: 364–379, 2020. doi: 10.1038/s42255-020-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giroud-Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, Zapata-Pérez R, Sánchez-García JL, Houtkooper RH, Migaud ME, Moco S, Canto C. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol Metab 30: 192–202, 2019. doi: 10.1016/j.molmet.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lüscher B, Bütepage M, Eckei L, Krieg S, Verheugd P, Shilton BH. ADP-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem Rev 118: 1092–1136, 2018. doi: 10.1021/acs.chemrev.7b00122. [DOI] [PubMed] [Google Scholar]
- 27.Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab 2: 9–31, 2020. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 28.Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312: 1798–1802, 2006. [Erratum in Science 332: 664, 2011]. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 29.Bindesbøll C, Tan S, Bott D, Cho T, Tamblyn L, MacPherson L, Grønning-Wang L, Nebb HI, Matthews J. TCDD-inducible poly-ADP-ribose polymerase (TIPARP/PARP7) mono-ADP-ribosylates and co-activates liver X receptors. Biochem J 473: 899–910, 2016. doi: 10.1042/BJ20151077. [DOI] [PubMed] [Google Scholar]
- 30.Léger K, Hopp AK, Fey M, Hottiger MO. ARTD1 regulates cyclin E expression and consequently cell-cycle re-entry and G1/S progression in T24 bladder carcinoma cells. Cell Cycle 15: 2042–2052, 2016. doi: 10.1080/15384101.2016.1195530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho-Park PF, Steller H. Proteasome regulation by ADP-ribosylation. Cell 153: 614–627, 2013. doi: 10.1016/j.cell.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopp AK, Gruter P, Hottiger MO. Regulation of glucose metabolism by NAD+ and ADP-ribosylation. Cells 8: 890, 2019. doi: 10.3390/cells8080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veith S, Mangerich A. RecQ helicases and PARP1 team up in maintaining genome integrity. Ageing Res Rev 23: 12–28, 2015. doi: 10.1016/j.arr.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468, 2011. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirinen E, Cantó C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, Li W, Timmers S, Imhof R, Verbeek J, Pujol A, van Loon B, Viscomi C, Zeviani M, Schrauwen P, Sauve AA, Schoonjans K, Auwerx J. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab 19: 1034–1041, 2014. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chini CC, Tarragó MG, Chini EN. NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol 455: 62–74, 2017. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuller M, Butler RE, Ariza A, Tromans-Coia C, Jankevicius G, Claridge TD, Kendall SL, Goh S, Stewart GR, Ahel I. Molecular basis for DarT ADP-ribosylation of a DNA base. Nature 596: 597–602, 2021. doi: 10.1038/s41586-021-03825-4. [DOI] [PubMed] [Google Scholar]
- 38.Hong JY, Lin H. Sirtuin modulators in cellular and animal models of human diseases. Front Pharmacol 12: 735044, 2021. doi: 10.3389/fphar.2021.735044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin H, Su X, He B. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol 7: 947–960, 2012. doi: 10.1021/cb3001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 280: 21313–21320, 2005. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 41.Schuetz A, Min J, Antoshenko T, Wang CL, Allali-Hassani A, Dong A, Loppnau P, Vedadi M, Bochkarev A, Sternglanz R, Plotnikov AN. Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure 15: 377–389, 2007. doi: 10.1016/j.str.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954, 2006. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496: 110–113, 2013. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang YL, Lin H. An improved fluorogenic assay for SIRT1, SIRT2, and SIRT3. Org Biomol Chem 14: 2186–2190, 2016. doi: 10.1039/C5OB02609A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jing H, Zhang X, Wisner SA, Chen X, Spiegelman NA, Linder ME, Lin H. SIRT2 and lysine fatty acylation regulate the transforming activity of K-Ras4a. Elife 6: e32436, 2017. doi: 10.7554/eLife.32436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madsen AS, Andersen C, Daoud M, Anderson KA, Laursen JS, Chakladar S, Huynh FK, Colaço AR, Backos DS, Fristrup P, Hirschey MD, Olsen CA. Investigating the Sensitivity of NAD+-dependent Sirtuin Deacylation Activities to NADH. J Biol Chem 291: 7128–7141, 2016. doi: 10.1074/jbc.M115.668699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, Douros JD, Wagner GR, Thompson JW, Madsen AS, Green MF, Sivley RM, Ilkayeva OR, Stevens RD, Backos DS, Capra JA, Olsen CA, Campbell JE, Muoio DM, Grimsrud PA, Hirschey MD. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab 25: 838–855.e15, 2017. doi: 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334: 806–809, 2011. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, Ro J, Wagner GR, Green MF, Madsen AS, Schmiesing J, Peterson BS, Xu G, Ilkayeva OR, Muehlbauer MJ, Braulke T, Mühlhausen C, Backos DS, Olsen CA, McGuire PJ, Pletcher SD, Lombard DB, Hirschey MD, Zhao Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 19: 605–617, 2014. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J, Cheng Z, Shi L, Shang Y, Yu W. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun 7: 12235, 2016. doi: 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarragó MG, Chini CC, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ, Chini EN. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab 27: 1081–1095.e10, 2018. doi: 10.1016/j.cmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camacho-Pereira J, Tarragó MG, Chini CC, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab 23: 1127–1139, 2016. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/Interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93: 1334–1343.e5, 2017. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348: 453–457, 2015. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao ZY, Xie XJ, Li WH, Liu J, Chen Z, Zhang B, Li T, Li SL, Lu JG, Zhang L, Zhang LH, Xu Z, Lee HC, Zhao YJ. A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15: 452–466, 2019. doi: 10.1016/j.isci.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhan AK, Reinherz EL, Poppema S, McCluskey RT, Schlossman SF. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med 152: 771–782, 1980. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chini EN, Chini CC, Barata da Silva H, Zielinska W. The cyclic-ADP-ribose signaling pathway in human myometrium. Arch Biochem Biophys 407: 152–159, 2002. doi: 10.1016/s0003-9861(02)00486-1. [DOI] [PubMed] [Google Scholar]
- 58.Chini EN, de Toledo FG, Thompson MA, Dousa TP. Effect of estrogen upon cyclic ADP ribose metabolism: beta-estradiol stimulates ADP ribosyl cyclase in rat uterus. Proc Natl Acad Sci USA 94: 5872–5876, 1997. doi: 10.1073/pnas.94.11.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dogan S, Deshpande DA, Kannan MS, Walseth TF. Changes in CD38 expression and ADP-ribosyl cyclase activity in rat myometrium during pregnancy: influence of sex steroid hormones. Biol Reprod 71: 97–103, 2004. doi: 10.1095/biolreprod.103.026245. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Guo Y, Huang W, Deng KY, Qian Y, Xin HB. 17beta-estradiol promotes apoptosis in airway smooth muscle cells through CD38/SIRT1/p53 pathway. Front Endocrinol (Lausanne) 9: 770, 2018. doi: 10.3389/fendo.2018.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boslett J, Hemann C, Christofi FL, Zweier JL. Characterization of CD38 in the major cell types of the heart: endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am J Physiol Cell Physiol 314: C297–C309, 2018. doi: 10.1152/ajpcell.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 84: 207–217, 2013. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 63.Matalonga J, Glaria E, Bresque M, Escande C, Carbó JM, Kiefer K, Vicente R, León TE, Beceiro S, Pascual-Garcia M, Serret J, Sanjurjo L, Moron-Ros S, Riera A, Paytubi S, Juarez A, Sotillo F, Lindbom L, Caelles C, Sarrias MR, Sancho J, Castrillo A, Chini EN, Valledor AF. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep 18: 1241–1255, 2017. doi: 10.1016/j.celrep.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Chini CC, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab 2: 1284–1304, 2020. doi: 10.1038/s42255-020-00298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat Metab 2: 1265–1283, 2020. [Erratum in Nat Metab 3: 120–121, 2021]. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chini C, Hogan KA, Warner GM, Tarragó MG, Peclat TR, Tchkonia T, Kirkland JL, Chini E. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem Biophys Res Commun 513: 486–493, 2019. doi: 10.1016/j.bbrc.2019.03.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glaria E, Valledor AF. Roles of CD38 in the Immune Response to Infection. Cells 9: 228, 2020. doi: 10.3390/cells9010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kishimoto H, Hoshino S, Ohori M, Kontani K, Nishina H, Suzawa M, Kato S, Katada T. Molecular mechanism of human CD38 gene expression by retinoic acid. Identification of retinoic acid response element in the first intron. J Biol Chem 273: 15429–15434, 1998. doi: 10.1074/jbc.273.25.15429. [DOI] [PubMed] [Google Scholar]
- 69.Kang BN, Jude JA, Panettieri RA Jr, Walseth TF, Kannan MS. Glucocorticoid regulation of CD38 expression in human airway smooth muscle cells: role of dual specificity phosphatase 1. Am J Physiol Lung Cell Mol Physiol 295: L186–L193, 2008. doi: 10.1152/ajplung.00352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amici SA, Young NA, Narvaez-Miranda J, Jablonski KA, Arcos J, Rosas L, Papenfuss TL, Torrelles JB, Jarjour WN, Guerau-de-Arellano M. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol 9: 1593, 2018. doi: 10.3389/fimmu.2018.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musso T, Deaglio S, Franco L, Calosso L, Badolato R, Garbarino G, Dianzani U, Malavasi F. CD38 expression and functional activities are up-regulated by IFN-gamma on human monocytes and monocytic cell lines. J Leukoc Biol 69: 605–612, 2001. [PubMed] [Google Scholar]
- 72.Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62: 1084–1093, 2013. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des 15: 57–63, 2009. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 345: 1386–1392, 2006. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 75.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem 287: 31633–31640, 2012. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chini EN, Chini CC, Kato I, Takasawa S, Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem J 362: 125–130, 2002. doi: 10.1042/0264-6021:3620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chini EN, Dousa TP. Enzymatic synthesis and degradation of nicotinate adenine dinucleotide phosphate (NAADP), a Ca2+-releasing agonist, in rat tissues. Biochem Biophys Res Commun 209: 167–174, 1995. doi: 10.1006/bbrc.1995.1485. [DOI] [PubMed] [Google Scholar]
- 78.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600, 2009. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J Biol Chem 270: 3216–3223, 1995. [Erratum in J Biol Chem 270: 10359, 1995]. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- 80.Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN. NAADP as a second messenger: neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol 292: C227–C239, 2007. doi: 10.1152/ajpcell.00638.2005. [DOI] [PubMed] [Google Scholar]
- 81.Chini EN, De Toledo FG. Nicotinic acid adenine dinucleotide phosphate: a new intracellular second messenger? Am J Physiol Cell Physiol 282: C1191–C1198, 2002. doi: 10.1152/ajpcell.00475.2001. [DOI] [PubMed] [Google Scholar]
- 82.Cheng J, Yusufi AN, Thompson MA, Chini EN, Grande JP. Nicotinic acid adenine dinucleotide phosphate: a new Ca2+ releasing agent in kidney. J Am Soc Nephrol 12: 54–60, 2001. doi: 10.1681/ASN.V12154. [DOI] [PubMed] [Google Scholar]
- 83.Fang C, Li T, Li Y, Xu GJ, Deng QW, Chen YJ, Hou YN, Lee HC, Zhao YJ. Correction: CD38 produces nicotinic acid adenine dinucleotide phosphate in the lysosome. J Biol Chem 294: 19447, 2019. doi: 10.1074/jbc.AAC119.011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gunaratne GS, Brailoiu E, He S, Unterwald EM, Patel S, Slama JT, Walseth TF, Marchant JS. Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling. Sci Signal 14: eabd5605, 2021. doi: 10.1126/scisignal.abd5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roggenkamp HG, Khansahib I, Hernandez CL, Zhang Y, Lodygin D, Krüger A, Gu F, Möckl F, Löhndorf A, Wolters V, Woike D, Rosche A, Bauche A, Schetelig D, Werner R, Schlüter H, Failla AV, Meier C, Fliegert R, Walseth TF, Flügel A, Diercks BP, Guse AH. HN1L/JPT2: a signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci Signal 14: eabd5647, 2021. doi: 10.1126/scisignal.abd5647. [DOI] [PubMed] [Google Scholar]
- 86.Diercks BP, Werner R, Weidemüller P, Czarniak F, Hernandez L, Lehmann C, Rosche A, Krüger A, Kaufmann U, Vaeth M, Failla AV, Zobiak B, Kandil FI, Schetelig D, Ruthenbeck A, Meier C, Lodygin D, Flügel A, Ren D, Wolf IM, Feske S, Guse AH. ORAI1, STIM1/2, and RYR1 shape subsecond Ca2+ microdomains upon T cell activation. Sci Signal 11: eaat0358, 2018. doi: 10.1126/scisignal.aat0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu F, Krüger A, Roggenkamp HG, Alpers R, Lodygin D, Jaquet V, Möckl F, Hernandez CL, Winterberg K, Bauche A, Rosche A, Grasberger H, Kao JY, Schetelig D, Werner R, Schröder K, Carty M, Bowie AG, Huber S, Meier C, Mittrücker HW, Heeren J, Krause KH, Flügel A, Diercks BP, Guse AH. Dual NADPH oxidases DUOX1 and DUOX2 synthesize NAADP and are necessary for Ca2+ signaling during T cell activation. Sci Signal 14: eabe3800, 2021. doi: 10.1126/scisignal.abe3800. [DOI] [PubMed] [Google Scholar]
- 88.Schmid F, Bruhn S, Weber K, Mittrücker HW, Guse AH. CD38: a NAADP degrading enzyme. FEBS Lett 585: 3544–3548, 2011. doi: 10.1016/j.febslet.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 89.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262: 1056–1059, 1993. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 90.Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 589: 1515–1525, 2011. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A., Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem 288: 25938–25949, 2013. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shrimp JH, Hu J, Dong M, Wang BS, MacDonald R, Jiang H, Hao Q, Yen A, Lin H. Revealing CD38 cellular localization using a cell permeable, mechanism-based fluorescent small-molecule probe. J Am Chem Soc 136: 5656–5663, 2014. doi: 10.1021/ja411046j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov 8: 1156–1175, 2018. doi: 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Flora A, Guida L, Franco L, Zocchi E, Pestarino M, Usai C, Marchetti C, Fedele E, Fontana G, Raiteri M. Ectocellular in vitro and in vivo metabolism of cADP-ribose in cerebellum. Biochem J 320: 665–671, 1996. doi: 10.1042/bj3200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pissios P. Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme. Trends Endocrinol Metab 28: 340–353, 2017. doi: 10.1016/j.tem.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 277: 45099–45107, 2002. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 97.Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 21: 3629–3639, 2007. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 98.Wang LF, Cao Q, Wen K, Xiao YF, Chen TT, Guan XH, Liu Y, Zuo L, Qian YS, Deng KY, Xin HB. CD38 deficiency alleviates D-galactose-induced myocardial cell senescence through NAD+/Sirt1 signaling pathway. Front Physiol 10: 1125, 2019. doi: 10.3389/fphys.2019.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cynamon MH, Sorg TB, Patapow A. Utilization and metabolism of NAD by Haemophilus parainfluenzae. J Gen Microbiol 134: 2789–2799, 1988. doi: 10.1099/00221287-134-10-2789. [DOI] [PubMed] [Google Scholar]
- 100.Herbert M, Sauer E, Smethurst G, Kraiss A, Hilpert AK, Reidl J. Nicotinamide ribosyl uptake mutants in Haemophilus influenzae. Infect Immun 71: 5398–5401, 2003. doi: 10.1128/IAI.71.9.5398-5401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Partida-Sánchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, Lund FE. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 7: 1209–1216, 2001. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 102.Lischke T, Heesch K, Schumacher V, Schneider M, Haag F, Koch-Nolte F, Mittrücker HW. CD38 controls the innate immune response against Listeria monocytogenes. Infect Immun 81: 4091–4099, 2013. doi: 10.1128/IAI.00340-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, Mousseau BJ, Sumoza-Toledo A, Bhagat H, Walseth TF, Guse AH, Lund FE. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by ADP-ribose, the major product generated by the CD38 enzyme reaction. J Immunol 179: 7827–7839, 2007. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- 104.Estrada-Figueroa LA, Ramírez-Jiménez Y, Osorio-Trujillo C, Shibayama M, Navarro GF, García-Tovar C, Talamás-Rohana P. Absence of CD38 delays arrival of neutrophils to the liver and innate immune response development during hepatic amoebiasis by Entamoeba histolytica. Parasite Immunol 33: 661–668, 2011. doi: 10.1111/j.1365-3024.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- 105.Zidovec Lepej S, Vince A, Dakovic Rode O, Remenar A, Jeren T. Increased numbers of CD38 molecules on bright CD8+ T lymphocytes in infectious mononucleosis caused by Epstein-Barr virus infection. Clin Exp Immunol 133: 384–390, 2003. doi: 10.1046/j.1365-2249.2003.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carbone J, Gil J, Benito JM, Navarro J, Muñóz-Fernández A, Bartolomé J, Zabay JM, López F, Fernández-Cruz E. Increased levels of activated subsets of CD4 T cells add to the prognostic value of low CD4 T cell counts in a cohort of HIV-infected drug users. AIDS 14: 2823–2829, 2000. doi: 10.1097/00002030-200012220-00003. [DOI] [PubMed] [Google Scholar]
- 107.Booiman T, Wit FW, Girigorie AF, Maurer I, De Francesco D, Sabin CA, Harskamp AM, Prins M, Franceschi C, Deeks SG, Winston A, Reiss P, Kootstra NA; Co-morBidity in Relation to Aids (COBRA) Collaboration. Terminal differentiation of T cells is strongly associated with CMV infection and increased in HIV-positive individuals on ART and lifestyle matched controls. PLoS One 12: e0183357, 2017. doi: 10.1371/journal.pone.0183357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schiavoni I, Scagnolari C, Horenstein AL, Leone P, Pierangeli A, Malavasi F, Ausiello CM, Fedele G. CD38 modulates respiratory syncytial virus-driven proinflammatory processes in human monocyte-derived dendritic cells. Immunology 154: 122–131, 2018. doi: 10.1111/imm.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heer CD, Sanderson DJ, Voth LS, Alhammad YM, Schmidt MS, Trammell SA, Perlman S, Cohen MS, Fehr AR, Brenner C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: an actionable component of innate immunity. J Biol Chem 295: 17986–17996, 2020. doi: 10.1074/jbc.RA120.015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Camell CD, Yousefzadeh MJ, Zhu Y, Prata LG, Huggins MA, Pierson M, et al. Senolytics reduce coronavirus-related mortality in old mice. Science 373: eabe4832, 2021. doi: 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeidler JD, Kashyap S, Hogan KA, Chini EN. Implications of the NADase CD38 in COVID pathophysiology. Physiol Rev 102: 339–341, 2022. doi: 10.1152/physrev.00007.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horenstein AL, Faini AC, Malavasi F. CD38 in the age of COVID-19: a medical perspective. Physiol Rev 101: 1457–1486, 2021. doi: 10.1152/physrev.00046.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pang H, Jiang Y, Li J, Wang Y, Nie M, Xiao N, Wang S, Song Z, Ji F, Chang Y, Zheng Y, Yao K, Yao L, Li S, Li P, Song L, Lan X, Xu Z, Hu Z. Aberrant NAD+ metabolism underlies Zika virus-induced microcephaly. Nat Metab 3: 1109–1124, 2021. doi: 10.1038/s42255-021-00437-0. [DOI] [PubMed] [Google Scholar]
- 114.Altay O, Arif M, Li X, Yang H, Aydın M, Alkurt G, Kim W, Akyol D, Zhang C, Dinler-Doganay G, Turkez H, Shoaie S, Nielsen J, Borén J, Olmuscelik O, Doganay L, Uhlén M, Mardinoglu A. Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19. Adv Sci (Weinh) 8: e2101222, 2021. doi: 10.1002/advs.202101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anaya JM, Restrepo-Jiménez P, Ramírez-Santana C. The autoimmune ecology: an update. Curr Opin Rheumatol 30: 350–360, 2018. doi: 10.1097/BOR.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 116.Rahman S, Archana A, Jan AT, Dutta D, Shankar A, Kim J, Minakshi R. Molecular insights into the relationship between autoimmune thyroid diseases and breast cancer: a critical perspective on autoimmunity and ER stress. Front Immunol 10: 344, 2019. doi: 10.3389/fimmu.2019.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pavón EJ, Zumaquero E, Rosal-Vela A, Khoo KM, Cerezo-Wallis D, García-Rodríguez S, Carrascal M, Abian J, Graeff R, Callejas-Rubio JL, Ortego-Centeno N, Malavasi F, Zubiaur M, Sancho J. Increased CD38 expression in T cells and circulating anti-CD38 IgG autoantibodies differentially correlate with distinct cytokine profiles and disease activity in systemic lupus erythematosus patients. Cytokine 62: 232–243, 2013. doi: 10.1016/j.cyto.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 118.Garcia-Rodríguez S, Rosal-Vela A, Botta D, Cumba Garcia LM, Zumaquero E, Prados-Maniviesa V, Cerezo-Wallis D, Lo Buono N, Robles-Guirado JA, Guerrero S, González-Paredes E, Andrés-León E, Corbi A, Mack M, Koch-Nolte F, Merino R, Zubiaur M, Lund FE, Sancho J. CD38 promotes pristane-induced chronic inflammation and increases susceptibility to experimental lupus by an apoptosis-driven and TRPM2-dependent mechanism. Sci Rep 8: 3357, 2018. doi: 10.1038/s41598-018-21337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schneider M, Schumacher V, Lischke T, Lücke K, Meyer-Schwesinger C, Velden J, Koch-Nolte F, Mittrücker HW. CD38 is expressed on inflammatory cells of the intestine and promotes intestinal inflammation. PLoS One 10: e0126007, 2015. doi: 10.1371/journal.pone.0126007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Viegas MS, Silva T, Monteiro MM, do Carmo A, Martins TC. Knocking out of CD38 accelerates development of a lupus-like disease in lpr mice. Rheumatology (Oxford) 50: 1569–1577, 2011. doi: 10.1093/rheumatology/ker178. [DOI] [PubMed] [Google Scholar]
- 121.Cole S, Walsh A, Yin X, Wechalekar MD, Smith MD, Proudman SM, Veale DJ, Fearon U, Pitzalis C, Humby F, Bombardieri M, Axel A, Adams H 3rd, Chiu C, Sharp M, Alvarez J, Anderson I, Madakamutil L, Nagpal S, Guo Y. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 20: 85, 2018. doi: 10.1186/s13075-018-1578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fortuna G, Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin North Am 57: 631–655, 2013. doi: 10.1016/j.cden.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Tsokos GC. Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 124.Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol Med 23: 615–635, 2017. doi: 10.1016/j.molmed.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sibley JT, Haug BL, Lee JS. Altered metabolism of poly(ADP-ribose) in the peripheral blood lymphocytes of patients with systemic lupus erythematosus. Arthritis Rheum 32: 1045–1049, 1989. doi: 10.1002/anr.1780320815. [DOI] [PubMed] [Google Scholar]
- 126.Pavon EJ, Muñoz P, Navarro MD, Raya-Alvarez E, Callejas-Rubio JL, Navarro-Pelayo F, Ortego-Centeno N, Sancho J, Zubiaur M. Increased association of CD38 with lipid rafts in T cells from patients with systemic lupus erythematosus and in activated normal T cells. Mol Immunol 43: 1029–1039, 2006. doi: 10.1016/j.molimm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus 6: 708–712, 1997. doi: 10.1177/096120339700600905. [DOI] [PubMed] [Google Scholar]
- 128.Katsuyama E, Suarez-Fueyo A, Bradley SJ, Mizui M, Marin AV, Mulki L, Krishfield S, Malavasi F, Yoon J, Sui SJ, Kyttaris VC, Tsokos GC. The CD38/NAD/SIRTUIN1/EZH2 axis mitigates cytotoxic CD8 T cell function and identifies patients with SLE prone to infections. Cell Rep 30: 112–123.e4, 2020. doi: 10.1016/j.celrep.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao L, He C. Polarization of macrophages and microglia in inflammatory demyelination. Neurosci Bull 29: 189–198, 2013. doi: 10.3389/fncel.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roboon J, Hattori T, Ishii H, Takarada-Iemata M, Le TM, Shiraishi Y, Ozaki N, Yamamoto Y, Sugawara A, Okamoto H, Higashida H, Kitao Y, Hori O. Deletion of CD38 suppresses glial activation and neuroinflammation in a mouse model of demyelination. Front Cell Neurosci 13: 258, 2019. doi: 10.3389/fncel.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herrmann MM, Barth S, Greve B, Schumann KM, Bartels A, Weissert R. Identification of gene expression patterns crucially involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Dis Model Mech 9: 1211–1220, 2016. doi: 10.1242/dmm.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Langley MR, Choi CI, Peclat TR, Guo Y, Simon WL, Yoon H, Kleppe L, Lucchinetti CF, Chini CC, Chini EN, Scarisbrick IA. Critical role of astrocyte NAD+ glycohydrolase in myelin injury and regeneration. J Neurosci 41: 8644–8667, 2021. doi: 10.1523/JNEUROSCI.2264-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 28: 573–621, 2010. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48: 526–535, 2001. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ning L, Shan G, Sun Z, Zhang F, Xu C, Lou X, Li S, Du H, Chen H, Xu G. Quantitative proteomic analysis reveals the deregulation of nicotinamide adenine dinucleotide metabolism and CD38 in inflammatory bowel disease. Biomed Res Int 2019: 3950628, 2019. doi: 10.1155/2019/3950628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Funderburg NT, Stubblefield Park SR, Sung HC, Hardy G, Clagett B, Ignatz-Hoover J, Harding CV, Fu P, Katz JA, Lederman MM, Levine AD. Circulating CD4+ and CD8+ T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 140: 87–97, 2013. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Joosse ME, Menckeberg CL, de Ruiter LF, Raatgeep HR, van Berkel LA, Simons-Oosterhuis Y, Tindemans I, Muskens AF, Hendriks RW, Hoogenboezem RM, Cupedo T, de Ridder L, Escher JC, Veenbergen S, Samsom JN. Frequencies of circulating regulatory TIGIT+CD38+ effector T cells correlate with the course of inflammatory bowel disease. Mucosal Immunol 12: 154–163, 2019. doi: 10.1038/s41385-018-0078-4. [DOI] [PubMed] [Google Scholar]
- 138.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14: 738–747, 2008. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chauhan K, Jandu JS, Goyal A, Bansal P, Al-Dhahir MA. Rheumatoid arthritis. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [Google Scholar]
- 140.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6: 15, 2018. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong W, Li X, Liu H, Zhu P. Infiltrations of plasma cells in synovium are highly associated with synovial fluid levels of APRIL in inflamed peripheral joints of rheumatoid arthritis. Rheumatol Int 29: 801–806, 2009. doi: 10.1007/s00296-008-0773-7. [DOI] [PubMed] [Google Scholar]
- 142.Volkov M, van Schie KA, van der Woude D. Autoantibodies and B cells: the ABC of rheumatoid arthritis pathophysiology. Immunol Rev 294: 148–163, 2020. doi: 10.1111/imr.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang X, Yue L, Liu W, Wang Y, Wang L, Xu B, Wang Y, Pan J, Yan X. CD38 and E2F transcription factor 2 have uniquely increased expression in rheumatoid arthritis synovial tissues. Clin Exp Immunol 176: 222–231, 2014. doi: 10.1111/cei.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H, Li S, Zhang G, Wu H, Chang X. Potential therapeutic effects of cyanidin-3-O-glucoside on rheumatoid arthritis by relieving inhibition of CD38+ NK cells on Treg cell differentiation. Arthritis Res Ther 21: 220, 2019. doi: 10.1186/s13075-019-2001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frerichs KA, Verkleij CP, Bosman PW, Zweegman S, Otten H, van de Donk N. CD38-targeted therapy with daratumumab reduces autoantibody levels in multiple myeloma patients. J Transl Autoimmun 2: 100022, 2019. doi: 10.1016/j.jtauto.2019.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Korver W, Carsillo M, Yuan J, Idamakanti N, Wagoner M, Shi P, Xia CQ, Smithson G, McLean L, Zalevsky J, Fedyk ER. A Reduction in B, T, and natural killer cells expressing CD38 by TAK-079 inhibits the induction and progression of collagen-induced arthritis in cynomolgus monkeys. J Pharmacol Exp Ther 370: 182–196, 2019. doi: 10.1124/jpet.119.256602. [DOI] [PubMed] [Google Scholar]
- 147.Peclat TR, Shi B, Varga J, Chini EN. The NADase enzyme CD38: an emerging pharmacological target for systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis. Curr Opin Rheumatol 32: 488–496, 2020. doi: 10.1097/BOR.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barsotti S, Orlandi M, Codullo V, Di Battista M, Lepri G, Della Rossa A, Guiducci S. One year in review 2019: systemic sclerosis. Clin Exp Rheumatol 37: 3–14, 2019. [PubMed] [Google Scholar]
- 149.Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol 46: 101333, 2019. doi: 10.1016/j.smim.2019.101333. [DOI] [PubMed] [Google Scholar]
- 150.Gally F, Hartney JM, Janssen WJ, Perraud AL. CD38 plays a dual role in allergen-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol 40: 433–442, 2009. doi: 10.1165/rcmb.2007-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in CD38-deficient mice. Am J Respir Cell Mol Biol 32: 149–156, 2005. doi: 10.1165/rcmb.2004-0243OC. [DOI] [PubMed] [Google Scholar]
- 152.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 291: L1286–L1293, 2006. doi: 10.1152/ajplung.00187.2006. [DOI] [PubMed] [Google Scholar]
- 153.Deshpande DA, Guedes AG, Lund FE, Subramanian S, Walseth TF, Kannan MS. CD38 in the pathogenesis of allergic airway disease: potential therapeutic targets. Pharmacol Ther 172: 116–126, 2017. doi: 10.1016/j.pharmthera.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 155.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP Jr, May RM, Wu HP, Nguyen DM, Arcos-Burgos M, MacDonald SD, Gochuico BR. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 176: 698–705, 2007. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.El-Chemaly S, Cheung F, Kotliarov Y, O’Brien KJ, Gahl WA, Chen J, Perl SY, Biancotto A, Gochuico BR. The immunome in two inherited forms of pulmonary fibrosis. Front Immunol 9: 76, 2018. doi: 10.3389/fimmu.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang W, Hu Y, Yang C, Zhu S, Wang X, Zhang Z, Deng H. Decreased NAD activates STAT3 and integrin pathways to drive epithelial-mesenchymal transition. Mol Cell Proteomics 17: 2005–2017, 2018. doi: 10.1074/mcp.RA118.000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365: 495–506, 2016. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goplen NP, Wu Y, Son YM, Li C, Wang Z, Cheon IS, Jiang L, Zhu B, Ayasoufi K, Chini EN, Johnson AJ, Vassallo R, Limper AH, Zhang N, Sun J. Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci Immunol 5: eabc4557, 2020. doi: 10.1126/sciimmunol.abc4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chun TW, Justement JS, Sanford C, Hallahan CW, Planta MA, Loutfy M, Kottilil S, Moir S, Kovacs C, Fauci AS. Relationship between the frequency of HIV-specific CD8+ T cells and the level of CD38+CD8+ T cells in untreated HIV-infected individuals. Proc Natl Acad Sci USA 101: 2464–2469, 2004. doi: 10.1073/pnas.0307328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Z, Zhu L, Nguyen TH, Wan Y, Sant S, Quiñones-Parra SM, Crawford JC, Eltahla AA, Rizzetto S, Bull RA, Qiu C, Koutsakos M, Clemens EB, Loh L, Chen T, Liu L, Cao P, Ren Y, Kedzierski L, Kotsimbos T, McCaw JM, La Gruta NL, Turner SJ, Cheng AC, Luciani F, Zhang X, Doherty PC, Thomas PG, Xu J, Kedzierska K. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat Commun 9: 824, 2018. doi: 10.1038/s41467-018-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thai TL, Arendshorst WJ. Mice lacking the ADP ribosyl cyclase CD38 exhibit attenuated renal vasoconstriction to angiotensin II, endothelin-1, and norepinephrine. Am J Physiol Renal Physiol 297: F169–F176, 2009. doi: 10.1152/ajprenal.00079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moss NG, Vogel PA, Kopple TE, Arendshorst WJ. Thromboxane-induced renal vasoconstriction is mediated by the ADP-ribosyl cyclase CD38 and superoxide anion. Am J Physiol Renal Physiol 305: F830–F838, 2013. doi: 10.1152/ajprenal.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 165.Endlich N, Siegerist F, Endlich K. Are podocytes motile? Pflugers Arch 469: 951–957, 2017. doi: 10.1007/s00424-017-2016-9. [DOI] [PubMed] [Google Scholar]
- 166.Boini KM, Xia M, Xiong J, Li C, Payne LP, Li PL. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med 16: 1674–1685, 2012. doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38-/- mouse. Biochem Biophys Res Commun 346: 188–192, 2006. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 168.Mori V, Amici A, Mazzola F, Di Stefano M, Conforti L, Magni G, Ruggieri S, Raffaelli N, Orsomando G. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS One 9: e113939, 2014. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogura Y, Kitada M, Monno I, Kanasaki K, Watanabe A, Koya D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep 23: 153–159, 2018. doi: 10.1080/13510002.2018.1487174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a Sirtuin 1-dependent manner. J Am Soc Nephrol 28: 2337–2352, 2017. [Erratum in J Am Soc Nephrol 28: 2553, 2017]. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, Parikh SM. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shu B, Feng Y, Gui Y, Lu Q, Wei W, Xue X, Sun X, He W, Yang J, Dai C. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-kappaB signaling suppression. Cell Signal 42: 249–258, 2018. doi: 10.1016/j.cellsig.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 173.Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem 27: 681–690, 2011. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]
- 174.Ogura Y, Kitada M, Xu J, Monno I, Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging (Albany NY) 12: 11325–11336, 2020. doi: 10.18632/aging.103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Faivre A, Katsyuba E, Verissimo T, Lindenmeyer M, Rajaram RD, Naesens M, Heckenmeyer C, Mottis A, Feraille E, Cippà P, Cohen C, Longchamp A, Allagnat F, Rutkowski JM, Legouis D, Auwerx J, de Seigneux S. Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol Dial Transplant 36: 60–68, 2021. doi: 10.1093/ndt/gfaa124. [DOI] [PubMed] [Google Scholar]
- 176.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol 31: 395–414, 1978. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology 145: 375–382.e1-2, 2013. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melato M, Mucli E. Something new in liver cirrhosis epidemiology. Lancet 2: 395–396, 1989. doi: 10.1016/s0140-6736(89)90578-3. [DOI] [PubMed] [Google Scholar]
- 179.Gan BH, Ng GL, Bay BH, Chang CF. Altered CD38 expression in thioacetamide-induced rat model of liver cirrhosis. Liver Int 25: 1233–1242, 2005. doi: 10.1111/j.1478-3231.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 180.Abdeen SM, Olusi SO, Askar HA, Thalib L, Al-Azemi A, George S. The predictive value of CD38 positive hepatic stellate cell count for assessing disease activity and fibrosis in patients with chronic hepatitis. Acta Histochem 111: 520–530, 2009. doi: 10.1016/j.acthis.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 181.Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 128: 33–42, 2005. doi: 10.1053/j.gastro.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 182.Sekoguchi S, Nakajima T, Moriguchi M, Jo M, Nishikawa T, Katagishi T, Kimura H, Minami M, Itoh Y, Kagawa K, Tani Y, Okanoue T. Role of cell-cycle turnover and oxidative stress in telomere shortening and cellular senescence in patients with chronic hepatitis C. J Gastroenterol Hepatol 22: 182–190, 2007. doi: 10.1111/j.1440-1746.2006.04454.x. [DOI] [PubMed] [Google Scholar]
- 183.Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol 32: 327–332, 2001. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 184.Aravinthan A, Pietrosi G, Hoare M, Jupp J, Marshall A, Verrill C, Davies S, Bateman A, Sheron N, Allison M, Alexander GJ. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One 8: e72904, 2013. doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn-Lowe S, Harvey R, Davies SE, Allison M, Coleman N, Alexander G. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol 58: 549–556, 2013. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 186.Wood MJ, Gadd VL, Powell LW, Ramm GA, Clouston AD. Ductular reaction in hereditary hemochromatosis: the link between hepatocyte senescence and fibrosis progression. Hepatology 59: 848–857, 2014. doi: 10.1002/hep.26706. [DOI] [PubMed] [Google Scholar]
- 187.WHO. Obesity and overweight (Online). https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. [2020 Apr 28].
- 188.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med 26: 364–373, 2016. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 189.Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun 349: 353–359, 2006. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 190.Cai G, Cole SA, Freeland-Graves JH, MacCluer JW, Blangero J, Comuzzie AG. Principal component for metabolic syndrome risk maps to chromosome 4p in Mexican Americans: the San Antonio Family Heart Study. Hum Biol 76: 651–665, 2004. doi: 10.1353/hub.2005.0001. [DOI] [PubMed] [Google Scholar]
- 191.Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab 29: 1028–1044, 2019. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Aslibekyan S, Demerath EW, Mendelson M, Zhi D, Guan W, Liang L, Sha J, Pankow JS, Liu C, Irvin MR, Fornage M, Hidalgo B, Lin LA, Thibeault KS, Bressler J, Tsai MY, Grove ML, Hopkins PN, Boerwinkle E, Borecki IB, Ordovas JM, Levy D, Tiwari HK, Absher DM, Arnett DK. Epigenome-wide study identifies novel methylation loci associated with body mass index and waist circumference. Obesity (Silver Spring) 23: 1493–1501, 2015. doi: 10.1002/oby.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sanjabi B, Dashty M, Özcan B, Akbarkhanzadeh V, Rahimi M, Vinciguerra M, van Rooij F, Al-Lahham S, Sheedfar F, van Kooten TG, Spek CA, Rowshani AT, van der Want J, Klaassen R, Sijbrands E, Peppelenbosch MP, Rezaee F. Lipid droplets hypertrophy: a crucial determining factor in insulin regulation by adipocytes. Sci Rep 5: 8816, 2015. doi: 10.1038/srep08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xie L, Wen K, Li Q, Huang CC, Zhao JL, Zhao QH, Xiao YF, Guan XH, Qian YS, Gan L, Wang LF, Deng KY, Xin HB. CD38 deficiency protects mice from high fat diet-induced nonalcoholic fatty liver disease through activating NAD+/sirtuins signaling pathways-mediated inhibition of lipid accumulation and oxidative stress in hepatocytes. Int J Biol Sci 17: 4305–4315, 2021. doi: 10.7150/ijbs.65588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiang SH, Harrington WW, Luo G, Milliken NO, Ulrich JC, Chen J, Rajpal DK, Qian Y, Carpenter T, Murray R, Geske RS, Stimpson SA, Kramer HF, Haffner CD, Becherer JD, Preugschat F, Billin AN. Genetic ablation of CD38 protects against western diet-induced exercise intolerance and metabolic inflexibility. PLoS One 10: e0134927, 2015. doi: 10.1371/journal.pone.0134927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 197.Wang LF, Miao LJ, Wang XN, Huang CC, Qian YS, Huang X, Wang XL, Jin WZ, Ji GJ, Fu M, Deng KY, Xin HB. CD38 deficiency suppresses adipogenesis and lipogenesis in adipose tissues through activating Sirt1/PPARgamma signaling pathway. J Cell Mol Med 22: 101–110, 2018. doi: 10.1111/jcmm.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Benzi A, Grozio A, Spinelli S, Sturla L, Guse AH, De Flora A, Zocchi E, Heeren J, Bruzzone S. Role of CD38 in adipose tissue: tuning coenzyme availability? Nutrients 13: 3734, 2021. doi: 10.3390/nu13113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kunz HE, Hart CR, Gries KJ, Parvizi M, Laurenti M, Dalla Man C, Moore N, Zhang X, Ryan Z, Polley EC, Jensen MD, Vella A, Lanza IR. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am J Physiol Endocrinol Metab 321: E105–E121, 2021. doi: 10.1152/ajpendo.00070.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond) 35: 1165–1172, 2011. doi: 10.1038/ijo.2010.272. [DOI] [PubMed] [Google Scholar]
- 203.Benzi A, Sturla L, Heine M, Fischer AW, Spinelli S, Magnone M, Sociali G, Parodi A, Fenoglio D, Emionite L, Koch-Nolte F, Mittrücker HW, Guse AH, De Flora A, Zocchi E, Heeren J, Bruzzone S. CD38 downregulation modulates NAD+ and NADP(H) levels in thermogenic adipose tissues. Biochim Biophys Acta Mol Cell Biol Lipids 1866: 158819, 2021. doi: 10.1016/j.bbalip.2020.158819. [DOI] [PubMed] [Google Scholar]
- 204.Yamaguchi S, Franczyk MP, Chondronikola M, Qi N, Gunawardana SC, Stromsdorfer KL, Porter LC, Wozniak DF, Sasaki Y, Rensing N, Wong M, Piston DW, Klein S, Yoshino J. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc Natl Acad Sci USA 116: 23822–23828, 2019. doi: 10.1073/pnas.1909917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu F, Zheng X, Lin B, Liang H, Cai M, Cao H, Ye J, Weng J. Diet-induced obesity and insulin resistance are associated with brown fat degeneration in SIRT1-deficient mice. Obesity (Silver Spring) 24: 634–642, 2016. doi: 10.1002/oby.21393. [DOI] [PubMed] [Google Scholar]
- 206.Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol (NY) 11: 167–175, 2015. [PMC free article] [PubMed] [Google Scholar]
- 207.El-Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol 14: 4120–4126, 2008. doi: 10.3748/wjg.14.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 65: 589–600, 2016. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 209.Wu T, Liu YH, Fu YC, Liu XM, Zhou XH. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann Clin Lab Sci 44: 410–418, 2014. [PubMed] [Google Scholar]
- 210.Sun L, Fan Z, Chen J, Tian W, Li M, Xu H, Wu X, Shao J, Bian Y, Fang M, Xu Y. Corrigendum: transcriptional repression of SIRT1 by protein inhibitor of activated STAT 4 (PIAS4) in hepatic stellate cells contributes to liver fibrosis. Sci Rep 6: 30513, 2016. doi: 10.1038/srep30513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Simão AL, Afonso MB, Rodrigues PM, Gama-Carvalho M, Machado MV, Cortez-Pinto H, Rodrigues CM, Castro RE. Skeletal muscle miR-34a/SIRT1:AMPK axis is activated in experimental and human non-alcoholic steatohepatitis. J Mol Med 97: 1113–1126, 2019. doi: 10.1007/s00109-019-01796-8. [DOI] [PubMed] [Google Scholar]
- 212.Ishizuka K, Kon K, Lee-Okada HC, Arai K, Uchiyama A, Yamashina S, Yokomizo T, Ikejima K. Aging exacerbates high-fat diet-induced steatohepatitis through alteration in hepatic lipid metabolism in mice. J Gastroenterol Hepatol 35: 1437–1448, 2020. doi: 10.1111/jgh.15006. [DOI] [PubMed] [Google Scholar]
- 213.Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D, Sack MN, Jing E, Kahn CR, Friedman JE, Jonscher KR. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J 433: 505–514, 2011. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nassir F, Arndt JJ, Johnson SA, Ibdah JA. Regulation of mitochondrial trifunctional protein modulates nonalcoholic fatty liver disease in mice. J Lipid Res 59: 967–973, 2018. doi: 10.1194/jlr.M080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, Sindelar M, Pietka T, Patterson BW, Imai SI, Klein S. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 372: 1224–1229, 2021. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 48: 397–408, 2013. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pirinen E, Auranen M, Khan NA, Brilhante V, Urho N, Pessia A, Hakkarainen A, Kuula J, Heinonen U, Schmidt MS, Haimilahti K, Piirilä P, Lundbom N, Taskinen MR, Brenner C, Velagapudi V, Pietiläinen KH, Suomalainen A. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab 31: 1078–1090.e5, 2020. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 221.Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep 9: 9772, 2019. doi: 10.1038/s41598-019-46120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dolopikou CF, Kourtzidis IA, Margaritelis NV, Vrabas IS, Koidou I, Kyparos A, Theodorou AA, Paschalis V, Nikolaidis MG. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur J Nutr 59: 505–515, 2020. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 223.Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde-Jorgensen H, Møller N, Brenner C, Treebak JT, Jessen N. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr 108: 343–353, 2018. doi: 10.1093/ajcn/nqy132. [DOI] [PubMed] [Google Scholar]
- 224.Zhang C, Bjornson E, Arif M, Tebani A, Lovric A, Benfeitas R, Ozcan M, Juszczak K, Kim W, Kim JT, Bidkhori G, Ståhlman M, Bergh PO, Adiels M, Turkez H, Taskinen MR, Bosley J, Marschall HU, Nielsen J, Uhlén M, Borén J, Mardinoglu A. The acute effect of metabolic cofactor supplementation: a potential therapeutic strategy against non-alcoholic fatty liver disease. Mol Syst Biol 16: e9495, 2020. doi: 10.15252/msb.209495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7: e42357, 2012. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 6: e19194, 2011. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA 112: 2876–2881, 2015. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McReynolds MR, Chellappa K, Chiles E, Jankowski C, Shen Y, Chen L, Descamps HC, Mukherjee S, Bhat YR, Lingala SR, Chu Q, Botolin P, Hayat F, Doke T, Susztak K, Thaiss CA, Lu W, Migaud ME, Su X, Rabinowitz JD, Baur JA. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst 12: 1160–1172.e4, 2021. doi: 10.1016/j.cels.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schultz MB, Sinclair DA. Why NAD+ declines during aging: it’s destroyed. Cell Metab 23: 965–966, 2016. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Williams AC, Hill LJ, Ramsden DB. Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: hypotheses and lessons from pellagra. Curr Gerontol Geriatr Res 2012: 302875, 2012. doi: 10.1155/2012/302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab 27: 529–547, 2018. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 27: 513–528, 2018. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11: 535–546, 2015. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 235.Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Wong M, Apte RS, Imai SI. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab 30: 329–342.e5, 2019. doi: 10.1016/j.cmet.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jadeja RN, Powell FL, Jones MA, Fuller J, Joseph E, Thounaojam MC, Bartoli M, Martin PM. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging (Albany NY) 10: 1306–1323, 2018. doi: 10.18632/aging.101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Poljsak B. NAMPT-mediated NAD biosynthesis as the internal timing mechanism: in NAD+ world, time is running in its own way. Rejuvenation Res 21: 210–224, 2018. doi: 10.1089/rej.2017.1975. [DOI] [PubMed] [Google Scholar]
- 238.Anderson KA, Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem 52: 23–35, 2012. doi: 10.1042/bse0520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, Nakagawa T, Yoshino J, Imai S. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab 21: 706–717, 2015. doi: 10.1016/j.cmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 131: 21–28, 2010. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I, Lastra RR, Curtis M, Yamada SD, Perets R, McGregor SM, Andrade J, Fiehn O, Moellering RE, Mann M, Lengyel E. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569: 723–728, 2019. doi: 10.1038/s41586-019-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Komatsu M, Kanda T, Urai H, Kurokochi A, Kitahama R, Shigaki S, Ono T, Yukioka H, Hasegawa K, Tokuyama H, Kawabe H, Wakino S, Itoh H. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD+ metabolism. Sci Rep 8: 8637, 2018. doi: 10.1038/s41598-018-26882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, Pirinen E, Pulinilkunnil TC, Gong F, Wang YC, Cen Y, Sauve AA, Asara JM, Peroni OD, Monia BP, Bhanot S, Alhonen L, Puigserver P, Kahn BB. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508: 258–262, 2014. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Neelakantan H, Vance V, Wetzel MD, Wang HL, McHardy SF, Finnerty CC, Hommel JD, Watowich SJ. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem Pharmacol 147: 141–152, 2018. doi: 10.1016/j.bcp.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Neelakantan H, Brightwell CR, Graber TG, Maroto R, Wang HL, McHardy SF, Papaconstantinou J, Fry CS, Watowich SJ. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem Pharmacol 163: 481–492, 2019. doi: 10.1016/j.bcp.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sun C, Wang K, Stock AJ, Gong Y, Demarest TG, Yang B, Giri N, Harrington L, Alter BP, Savage SA, Bohr VA, Liu Y. Re-equilibration of imbalanced NAD metabolism ameliorates the impact of telomere dysfunction. EMBO J 39: e103420, 2020. doi: 10.15252/embj.2019103420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Biol Sci Med Sci 69: S4–S9, 2014. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 248.Prata LG, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol 40: 101275, 2018. doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tannous C, Booz GW, Altara R, Muhieddine DH, Mericskay M, Refaat MM, Zouein FA. Nicotinamide adenine dinucleotide: Biosynthesis, consumption and therapeutic role in cardiac diseases. Acta Physiol (Oxf) 231: e13551, 2021. doi: 10.1111/apha.13551. [DOI] [PubMed] [Google Scholar]
- 250.Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol 18: 809–823, 2021. doi: 10.1038/s41569-021-00569-6. [DOI] [PubMed] [Google Scholar]
- 251.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 133: 698–705, 2016. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee CF, Tian R. Mitochondrion as a target for heart failure therapy—role of protein lysine acetylation. Circ J 79: 1863–1870, 2015. doi: 10.1253/circj.CJ-15-0742. [DOI] [PubMed] [Google Scholar]
- 253.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134: 883–894, 2016. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gul R, Park DR, Shawl AI, Im SY, Nam TS, Lee SH, Ko JK, Jang KY, Kim D, Kim UH. Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR) mediate Ca2+ signaling in cardiac hypertrophy induced by beta-adrenergic stimulation. PLoS One 11: e0149125, 2016. doi: 10.1371/journal.pone.0149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gul R, Park JH, Kim SY, Jang KY, Chae JK, Ko JK, Kim UH. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc Res 81: 582–591, 2009. doi: 10.1093/cvr/cvn232. [DOI] [PubMed] [Google Scholar]
- 256.Guan XH, Hong X, Zhao N, Liu XH, Xiao YF, Chen TT, Deng LB, Wang XL, Wang JB, Ji GJ, Fu M, Deng KY, Xin HB. CD38 promotes angiotensin II-induced cardiac hypertrophy. J Cell Mol Med 21: 1492–1502, 2017. doi: 10.1111/jcmm.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gorski PA, Jang SP, Jeong D, Lee A, Lee P, Oh JG, Chepurko V, Yang DK, Kwak TH, Eom SH, Park ZY, Yoo YJ, Kim DH, Kook H, Sunagawa Y, Morimoto T, Hasegawa K, Sadoshima J, Vangheluwe P, Hajjar RJ, Park WJ, Kho C. Role of SIRT1 in modulating acetylation of the sarco-endoplasmic reticulum Ca2+-ATPase in heart failure. Circ Res 124: e63–e80, 2019. doi: 10.1161/CIRCRESAHA.118.313865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Takahashi J, Kagaya Y, Kato I, Ohta J, Isoyama S, Miura M, Sugai Y, Hirose M, Wakayama Y, Ninomiya M, Watanabe J, Takasawa S, Okamoto H, Shirato K. Deficit of CD38/cyclic ADP-ribose is differentially compensated in hearts by gender. Biochem Biophys Res Commun 312: 434–440, 2003. doi: 10.1016/j.bbrc.2003.10.143. [DOI] [PubMed] [Google Scholar]
- 259.Gan L, Jiang W, Xiao YF, Deng L, Gu LD, Guo ZY, Zhou ZC, Wu D, Xin HB. Disruption of CD38 gene enhances cardiac functions by elevating serum testosterone in the male null mice. Life Sci 89: 491–497, 2011. doi: 10.1016/j.lfs.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 260.Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, Lee DI, Yoo H, Kass DA, Szweda LI, Lavandero S, Verdin E, Gillette TG, Hill JA. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ Res 128: 1629–1641, 2021. doi: 10.1161/CIRCRESAHA.120.317046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med 13: eabd7064, 2021. doi: 10.1126/scitranslmed.abd7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Boslett J, Helal M, Chini E, Zweier JL. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J Mol Cell Cardiol 118: 81–94, 2018. doi: 10.1016/j.yjmcc.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298: 229–317, 2012. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu L, Wang P, Liu X, He D, Liang C, Yu Y. Exogenous NAD+ supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam Clin Pharmacol 28: 180–189, 2014. doi: 10.1111/fcp.12016. [DOI] [PubMed] [Google Scholar]
- 265.Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol 40: 215–224, 2018. doi: 10.1007/s00281-017-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gan L, Liu D, Liu J, Chen E, Chen C, Liu L, Hu H, Guan X, Ma W, Zhang Y, He Y, Liu B, Tang S, Jiang W, Xue J, Xin H. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal Transduct Target Ther 6: 223, 2021. doi: 10.1038/s41392-021-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li JP, Wei W, Li XX, Xu M. Regulation of NLRP3 inflammasome by CD38 through cADPR-mediated Ca2+ release in vascular smooth muscle cells in diabetic mice. Life Sci 255: 117758, 2020. doi: 10.1016/j.lfs.2020.117758. [DOI] [PubMed] [Google Scholar]
- 268.Morabia A. Joseph Goldberger's research on the prevention of pellagra. J R Soc Med 101: 566–568, 2008. doi: 10.1258/jrsm.2008.08k010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci 20: 974, 2019. doi: 10.3390/ijms20040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J 33: 1321–1340, 2014. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mizuguchi M, Otsuka N, Sato M, Ishii Y, Kon S, Yamada M, Nishina H, Katada T, Ikeda K. Neuronal localization of CD38 antigen in the human brain. Brain Res 697: 235–240, 1995. doi: 10.1016/0006-8993(95)00885-T. [DOI] [PubMed] [Google Scholar]
- 272.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535: 551–555, 2016. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Guerreiro S, Privat AL, Bressac L, Toulorge D. CD38 in neurodegeneration and neuroinflammation. Cells 9: 471, 2020. doi: 10.3390/cells9020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34: 185–204, 2011. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (NY) 4: 575–590, 2018. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wang YM, Liu ZY, Ai YH, Zhang LN, Zou Y, Peng QY. Blocking the CD38/cADPR pathway plays a double-edged role in LPS stimulated microglia. Neuroscience 361: 34–42, 2017. doi: 10.1016/j.neuroscience.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 277.Franco L, Bodrato N, Moreschi I, Usai C, Bruzzone S, Scarf I. S, Zocchi E, De Flora A. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. J Neurochem 99: 165–176, 2006. doi: 10.1111/j.1471-4159.2006.04031.x. [DOI] [PubMed] [Google Scholar]
- 278.Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MO, Hartmann T, Lund FE, Stein R, Levy A. Alzheimer's disease pathology is attenuated in a CD38-deficient mouse model. Ann Neurol 78: 88–103, 2015. doi: 10.1002/ana.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gao L, Zhang Z, Lu J, Pei G. Mitochondria are dynamically transferring between human neural cells and Alexander disease-associated GFAP mutations impair the astrocytic transfer. Front Cell Neurosci 13: 316, 2019. doi: 10.3389/fncel.2019.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yao S, Zhang X, Zou SC, Zhu Y, Li B, Kuang WP, Guo Y, Li XS, Li L, Wang XY. A transcriptome-wide association study identifies susceptibility genes for Parkinson’s disease. NPJ Parkinsons Dis 7: 79, 2021. doi: 10.1038/s41531-021-00221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang X, Meng X, Chen Y, Leng SX, Zhang H. The biology of aging and cancer: frailty, inflammation, and immunity. Cancer J 23: 201–205, 2017. doi: 10.1097/PPO.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 282.Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome—a key determinant of cancer cell biology. Nat Rev Cancer 12: 741–752, 2012. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 283.Pramono AA, Rather GM, Herman H, Lestari K, Bertino JR. NAD- and NADPH-contributing enzymes as therapeutic targets in cancer: an overview. Biomolecules 10: 358, 2020. doi: 10.3390/biom10030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chini CC, Guerrico AM, Nin V, Camacho-Pereira J, Escande C, Barbosa MT, Chini EN. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res 20: 120–130, 2014. doi: 10.1158/1078-0432.CCR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Espindola-Netto JM, Chini CCS, Tarragó M, Wang E, Dutta S, Pal K, Mukhopadhyay D, Sola-Penna M, Chini EN. Preclinical efficacy of the novel competitive NAMPT inhibitor STF-118804 in pancreatic cancer. Oncotarget 8: 85054–85067, 2017. doi: 10.18632/oncotarget.18841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.van de Donk NW, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 131: 13–29, 2018. doi: 10.1182/blood-2017-06-740944. [DOI] [PubMed] [Google Scholar]
- 287.Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol 43: 676–681, 2016. doi: 10.1053/j.seminoncol.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, Tanaka H, Sakai A, Asaoku H, Kuramoto A. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 81: 2658–2663, 1993. doi: 10.1182/blood.V81.10.2658.2658. [DOI] [PubMed] [Google Scholar]
- 289.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 121: 482–488, 2004. doi: 10.1309/74R4TB90BUWH27JX. [DOI] [PubMed] [Google Scholar]
- 290.Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, Zhou Z, Auger MJ, Bowles KM, Rushworth SA. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res 79: 2285–2297, 2019. doi: 10.1158/0008-5472.CAN-18-0773. [DOI] [PubMed] [Google Scholar]
- 291.Martin TG, Corzo K, Chiron M, Velde HV, Abbadessa G, Campana F, Solanki M, Meng R, Lee H, Wiederschain D, Zhu C, Rak A, Anderson KC. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cells 8: 1522, 2019. doi: 10.3390/cells8121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Richardson PG, Harrison SJ, Bringhen S, Schjesvold F, Yong K, Campana F, Le-Guennec S, Macé S, Dimopoulos MA. Isatuximab for relapsed/refractory multiple myeloma: review of key subgroup analyses from the Phase III ICARIA-MM study. Future Oncol 17: 4797–4812, 2021. doi: 10.2217/fon-2021-0568. [DOI] [PubMed] [Google Scholar]
- 293.Zannetti BA, Faini AC, Massari E, Geuna M, Maffini E, Poletti G, Cerchione C, Martinelli G, Malavasi F, Lanza F. Novel insights in anti-CD38 therapy based on CD38-receptor expression and function: the multiple myeloma model. Cells 9: 2666, 2020. doi: 10.3390/cells9122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186: 1840–1848, 2011. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 295.Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, Parren PW. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7: 311–321, 2015. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Phipps C, Chen Y, Gopalakrishnan S, Tan D. Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development. Ther Adv Hematol 6: 120–127, 2015. doi: 10.1177/2040620715572295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Redondo-Muñoz J, García-Pardo A, Teixidó J. Molecular players in hematologic tumor cell trafficking. Front Immunol 10: 156, 2019. doi: 10.3389/fimmu.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood 118: 3470–3478, 2011. doi: 10.1182/blood-2011-06-275610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Boonstra JG, van Lom K, Langerak AW, Graveland WJ, Valk PJ, Kraan J, van ’t Veer MB, Gratama JW. CD38 as a prognostic factor in B cell chronic lymphocytic leukaemia (B-CLL): comparison of three approaches to analyze its expression. Cytometry B Clin Cytom 70: 136–141, 2006. doi: 10.1002/cyto.b.20106. [DOI] [PubMed] [Google Scholar]
- 300.Vaisitti T, Audrito V, Serra S, Buonincontri R, Sociali G, Mannino E, Pagnani A, Zucchetto A, Tissino E, Vitale C, Coscia M, Usai C, Pepper C, Gattei V, Bruzzone S, Deaglio S. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia 29: 356–368, 2015. doi: 10.1038/leu.2014.207. [DOI] [PubMed] [Google Scholar]
- 301.Deaglio S, Aydin S, Grand MM, Vaisitti T, Bergui L, D’Arena G, Chiorino G, Malavasi F. CD38/CD31 interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol Med 16: 87–91, 2010. doi: 10.2119/molmed.2009.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mele S, Devereux S, Pepper AG, Infante E, Ridley AJ. Calcium-RasGRP2-Rap1 signaling mediates CD38-induced migration of chronic lymphocytic leukemia cells. Blood Adv 2: 1551–1561, 2018. doi: 10.1182/bloodadvances.2017014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Manna A, Aulakh S, Jani P, Ahmed S, Akhtar S, Coignet M, Heckman M, Meghji Z, Bhatia K, Sharma A, Sher T, Alegria V, Malavasi F, Chini EN, Chanan-Khan A, Ailawadhi S, Paulus A. Targeting CD38 enhances the antileukemic activity of ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 25: 3974–3985, 2019. doi: 10.1158/1078-0432.CCR-18-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Manna A, Kellett T, Aulakh S, Lewis-Tuffin LJ, Dutta N, Knutson K, Chini E, Pinilla-Ibarz J, Lamanna N, Manochakian R, Malavasi F, Sher T, Chanan-Khan AA, Ailawadhi S, Paulus A. Targeting CD38 is lethal to Breg-like chronic lymphocytic leukemia cells and Tregs, but restores CD8+ T-cell responses. Blood Adv 4: 2143–2157, 2020. doi: 10.1182/bloodadvances.2019001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sek K, Molck C, Stewart GD, Kats L, Darcy PK, Beavis PA. Targeting adenosine receptor signaling in cancer immunotherapy. Int J Mol Sci 19: 3837, 2018. doi: 10.3390/ijms19123837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2: e26246, 2013. doi: 10.4161/onci.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Horenstein AL, Chillemi A, Quarona V, Zito A, Roato I, Morandi F, Marimpietri D, Bolzoni M, Toscani D, Oldham RJ, Cuccioloni M, Sasser AK, Pistoia V, Giuliani N, Malavasi F. NAD+-Metabolizing ectoenzymes in remodeling tumor-host interactions: the human myeloma model. Cells 4: 520–537, 2015. doi: 10.3390/cells4030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Morandi F, Morandi B, Horenstein AL, Chillemi A, Quarona V, Zaccarello G, Carrega P, Ferlazzo G, Mingari MC, Moretta L, Pistoia V, Malavasi F. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget 6: 25602–25618, 2015. doi: 10.18632/oncotarget.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 359: 1350–1355, 2018. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mittal D, Vijayan D, Smyth MJ. Overcoming acquired PD-1/PD-L1 resistance with CD38 blockade. Cancer Discov 8: 1066–1068, 2018. doi: 10.1158/2159-8290.CD-18-0798. [DOI] [PubMed] [Google Scholar]
- 311.Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol 17: 1–12, 2020. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O’Brien S, Hicks PD, Basu D, Singhal S, Malavasi F, Rustgi AK. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res 75: 4074–4085, 2015. doi: 10.1158/0008-5472.CAN-14-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ben Baruch B, Blacher E, Mantsur E, Schwartz H, Vaknine H, Erez N, Stein R. Stromal CD38 regulates outgrowth of primary melanoma and generation of spontaneous metastasis. Oncotarget 9: 31797–31811, 2018. doi: 10.18632/oncotarget.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mottahedeh J, Haffner MC, Grogan TR, Hashimoto T, Crowell PD, Beltran H, Sboner A, Bareja R, Esopi D, Isaacs WB, Yegnasubramanian S, Rettig MB, Elashoff DA, Platz EA, De Marzo AM, Teitell MA, Goldstein AS. CD38 is methylated in prostate cancer and regulates extracellular NAD. Cancer Metab 6: 13, 2018. doi: 10.1186/s40170-018-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liu X, Grogan TR, Hieronymus H, Hashimoto T, Mottahedeh J, Cheng D, Zhang L, Huang K, Stoyanova T, Park JW, Shkhyan RO, Nowroozizadeh B, Rettig MB, Sawyers CL, Elashoff D, Horvath S, Huang J, Witte ON, Goldstein AS. Low CD38 identifies progenitor-like inflammation-associated luminal cells that can initiate human prostate cancer and predict poor outcome. Cell Rep 17: 2596–2606, 2016. doi: 10.1016/j.celrep.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chmielewski JP, Bowlby SC, Wheeler FB, Shi L, Sui G, Davis AL, Howard TD, D’Agostino RB Jr, Miller LD, Sirintrapun SJ, Cramer SD, Kridel SJ. CD38 inhibits prostate cancer metabolism and proliferation by reducing cellular NAD+ pools. Mol Cancer Res 16: 1687–1700, 2018. doi: 10.1158/1541-7786.MCR-17-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 66: 342–351, 2017. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lam JH, Ng HH, Lim CJ, Sim XN, Malavasi F, Li H, Loh JJ, Sabai K, Kim JK, Ong CCH, Loh T, Leow WQ, Choo SP, Toh HC, Lee SY, Chan CY, Chew V, Lim TS, Yeong J, Lim TK. Expression of CD38 on macrophages predicts improved prognosis in hepatocellular carcinoma. Front Immunol 10: 2093, 2019. doi: 10.3389/fimmu.2019.02093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chini EN. Of mice and men: NAD+ boosting with niacin provides hope for mitochondrial myopathy patients. Cell Metab 31: 1041–1043, 2020. doi: 10.1016/j.cmet.2020.05.013. [DOI] [PubMed] [Google Scholar]