Abstract
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that intensive blood pressure (BP) lowering (target<120 mmHg) was more effective in preventing heart failure (HF) compared with standard BP goals (target<140 mm Hg). However, intensive BP lowering also led to an increase in serious adverse events. We aimed to identify a subset of the clinical trial population who might derive the greatest benefit from intensive BP lowering for prevention of HF using a previously validated HF risk prediction model. SPRINT participants without prevalent cardiovascular disease were stratified into HF risk tertiles based on predicted HF risk. We performed Kaplan-Meier Survival analysis and multivariable Cox proportional hazards models to test the effect of intensive vs. standard BP lowering on incident HF in each tertile of predicted HF risk. A total of 6,911 individuals were included and 77 incident HF events occurred over a median follow-up time of 3.3 [IQR 2.9–3.8] years. A reduction in risk of HF was observed among those randomized to intensive BP lowering in each risk tertile but was significant only in the highest HF risk category (risk tertile 1: HR 0.86 [95% CI 0.29, 2.56]; risk tertile 2: 0.54 [0.23, 1.30]; risk tertile 3: 0.46 [0.24, 0.88]). Serious adverse events were frequent in all groups. While the short follow-up may lead to an underestimation of benefit in the lower predicted risk groups, prioritizing intensive BP lowering in those at highest predicted HF risk may help to reduce the high burden of HF in the United States.
Keywords: SPRINT, Heart Failure, PCP-HF, Prevention
INTRODUCTION
Contemporary analyses estimate nearly one in two adults in the United States has hypertension, and blood pressure (BP) control for those with hypertension remains suboptimal.1 Hypertension, especially uncontrolled hypertension, is a key risk factor for the development of heart failure (HF).2–4 While the risk of HF in those with elevated BP levels can be reduced through BP lowering, the optimal threshold for intervention and the target BP level to achieve has been a long-standing question that has undergone intense investigation. The Systolic Blood Pressure Intervention Trial (SPRINT) provided robust evidence that intensive BP lowering to a target systolic BP (SBP) of <120 mmHg reduces incident HF compared with a target of <140 mmHg.4–7 However, SPRINT also demonstrated a high incidence of serious adverse events in the intensive treatment group. As the frequency of adverse effects and possibly need for more extensive clinical monitoring may limit the adoption of intensive BP lowering, prioritizing those individuals most at risk for HF for intensive BP lowering may favorably balance the costs and benefits of intensive BP lowering to prevent HF.
Multi-society guidelines for blood pressure and cardiovascular disease prevention, including from the American Heart Association (AHA)/American College of Cardiology (ACC), currently recommend a lower BP goal (≤130/80 mmHg compared with ≤140/90mmHg) in patients with a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥10% based on, in part, the findings from SPRINT.8, 9 While SPRINT was not powered to examine reduction in cardiovascular disease (CVD) subtypes, the greatest relative risk reduction among CVD events occurred for HF events (HR 0.62 [0.45, 0.84]).6 However, BP targets based on predicted HF risk, which could complement those based on ASCVD risk, are not currently available.10 In this post-hoc analysis of SPRINT, we sought to determine differences in relative and absolute benefits and risks of intensive compared with standard BP lowering after stratifying patients based upon predicted HF risk. We applied a validated HF risk prediction tool, the Pooled Cohort Equations to Prevent HF (PCP-HF), to address this knowledge gap and inform the potential for risk-based, prevention of HF with intensive blood pressure lowering.11
METHODS
Study Design
All data for this analysis were obtained from the publicly available National Heart Lung and Blood Institute (NHLBI) BioLINCC data repository and can be accessed at https://biolincc.nhlbi.nih.gov/home/. This was a post-hoc analysis using data from SPRINT. Details of the trial can be found elsewhere but briefly, SPRINT aimed to determine whether intensive (SBP target <120 mm Hg) compared with standard (SBP target 135–139 mm Hg) BP lowering reduced CVD outcomes, including incident HF, in individuals at elevated CVD risk.12 All participants provided written informed consent before trial randomization, and the current analysis was approved by the Northwestern University Institutional Review Board.
Study Population
Between November 2010 to March 2013, 9,361 participants were enrolled in SPRINT across 102 sites in the United States and Puerto Rico. The trial was stopped early in August 2015 after an interim analysis showed evidence of benefit for those randomized to intensive BP lowering. Inclusion criteria are detailed elsewhere but included age ≥50 years, SBP ≥130 mm Hg, and at least one risk factor for CVD. Risk for CVD was defined as the presence of at least one of the following 1) clinical or subclinical CVD, 2) chronic kidney disease (estimated glomerular filtration rate [eGFR] by the Modification in Diet in Renal Diseases [MDRD] equation 20–59 ml/min/1.73m2), 3) Framingham Risk Score ≥ 15%) or 4) age ≥75 years. Clinical CVD was defined in SPRINT as myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, abnormal stress testing, peripheral arterial disease, known abdominal aortic aneurysm, carotid stenting/endarterectomy or coronary, carotid or peripheral artery stenosis >50%). Exclusion criteria included a history of stroke, HF or diabetes mellitus.
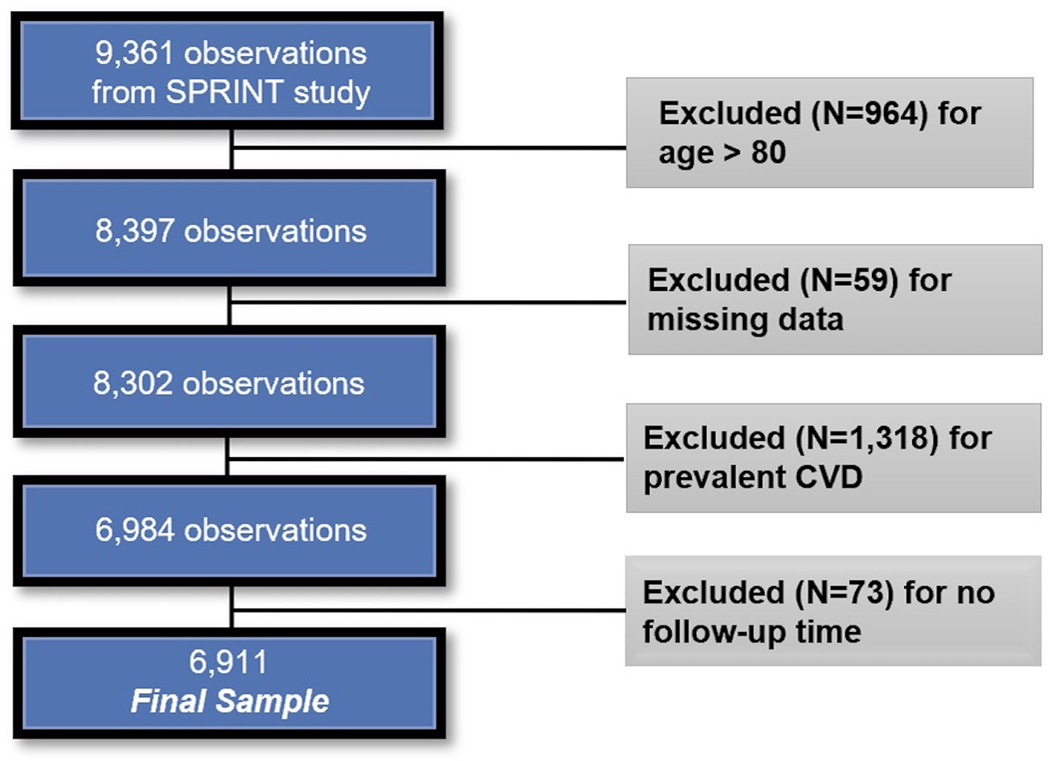
For this analysis, based on characteristics of the PCP-HF risk prediction tool which was derived from individuals aged 30–79 years free of CVD at baseline, we excluded the following trial participants: 1) those ≥ 80 years of age, 2) those with clinical CVD (as defined by SPRINT) and 3) those with incomplete data to calculate the PCP-HF score (described below) (N = 6911; Figure 1)
Figure 1.
Selection of participants from SPRINT included in the current analysis. CVD: cardiovascular disease.
PCP-HF Risk Prediction Model
The derivation and validation of the PCP-HF risk prediction model has been described previously11. Briefly, PCP-HF was derived and internally validated using data from 5 large US cohort studies (Atherosclerosis Risk in Communities [ARIC], Multiethnic Study of Atherosclerosis [MESA], Cardiovascular Health Study [CHS], Framingham Heart Study Offspring Cohort [FOF] and Coronary Artery Risk Development in Young Adults [CARDIA]) and externally validated in the Prevention of Renal and Vascular End-stage Diseases [PREVEND] and the Jackson Heart Study [JHS]). The resultant 10-year HF risk prediction tool includes twelve readily available demographic and clinical characteristics11. In this analysis, 10-year PCP-HF risk scores were calculated for each individual, after which participants were stratified into 1) low, 2) intermediate, or 3) high HF risk categories based upon tertiles of predicted HF risk.
Exposures and Outcome
The exposure of interest (independent variable) was assignment to intensive SBP lowering versus assignment to standard SBP lowering. The outcome (dependent variable) of interest was incident HF (a secondary outcome and component of the primary composite outcome in SPRINT). Incident HF was defined by an inpatient or emergency department encounter where the participant presented with multiple signs/symptoms of HF and required treatment with intravenous diuretics or inotropic agents. In SPRINT, adjudication of HF events was conducted by a blinded committee based on the HF adjudication model developed in the ARIC Study.6 The incidence of adverse events (AE) including serious adverse events (SAE) as defined by the SPRINT protocol was also examined by HF risk tertile and treatment status.6
Statistical Analysis
Univariate statistics (tabulations/frequencies, means/standard deviations [SD]), were used to describe the overall study population and tertiles of HF risk. Bivariate analysis of sample characteristics across tertiles of risk were conducted using ANOVA for normal continuous variables and Chi-squared comparison for categorical variables. Kaplan-Meier curves were utilized to show survival from incident HF across the tertiles of HF risk by intensive and standard BP treatment groups, and log rank tests assessed the null hypothesis of no difference in survival between treatment groups. Additionally, Cox proportional hazard models assessed the effect of intensive vs. standard BP lowering on incident HF in each baseline HF risk category. The proportional hazards assumption was determined to have been met via assessment of Schoenfeld residuals. Finally, the number needed to treat (NNT) and number needed to harm (NNH) was calculated as the inverse of the absolute risk reduction for intensive vs. standard BP lowering across the three HF risk levels. When treatment effects are not statistically significantly different, NNT and NNH confidence intervals include negative numbers and, thus, only point estimates are reported.13 All analyses were completed in SAS 9.4 (SAS Institute, Cary, North Carolina) and STATA 16.0 (STATA INC, College Station, TX). A two-tailed p-value <0.05 was considered statistically significant.
RESULTS
Table 1 summarizes the characteristics of the overall study population and by risk tertile. Individuals were stratified into tertiles of predicted HF risk using the PCP-HF 10-year risk prediction tool. Low, intermediate, and high HF risk categories were defined by ten-year HF risk scores of 0.6–5.4%, 5.4–9.0%, and 9.0–50.3%, with mean (SD) scores of 3.7% (1.1), 7.0% (1.0) and 14.0% (4.9) respectively, based on characteristics of this study population. Overall, the mean (SD) age of the sample at baseline was 65.7 (8.0) years, nearly two-thirds (63%) were male, and 35.4% identified as Black or African American. The mean BMI was 30.3 (5.8) kg/m2, and 89.6% reported treatment for hypertension at baseline. All examined characteristics differed by HR risk tertile; for example, individuals in the high risk tertile were older, more likely to be male, be on antihypertensive therapy and be current smokers (p<0.001 for all, Table 1).
Table 1.
Participant characteristics by heart failure risk tertiles
| Characteristic | Overall N=6,911 | Low Risk (Range 0.6–5.4%) N=2,303 | Intermediate Risk (Range 5.4–9.0%), N=2,304 | High Risk (Range 9.0–50.3%) N=2,304 | P-Value |
|---|---|---|---|---|---|
| Age, years* | 65.7 ±8.0 | 60.2 ±5.7 | 65.8 ±7.0 | 71.1 ±7.1 | <0.001 |
| Female gender, N (%) | 994 (14.4) | 1205 (52.3) | 901 (39.1) | 450 (19.5) | <0.001 |
| Black race, N(%) | 30.3 ±5.8 | 986 (42.8) | 904 (39.2) | 556 (24.1) | <0.001 |
| Current smoking, N (%) | 139.6 ±15.4 | 233 (10.1) | 325 (14.1) | 436 (18.9) | <0.001 |
| BMI, kg/m2 | 79.8 ±11.5 | 29.4 ±5.6 | 30.8 ±6.0 | 30.7 ±5.8 | <0.001 |
| Systolic blood pressure, mmHg | 6192 (89.6) | 136.1 ±14.9 | 139.9 ±15.1 | 143 ±15.5 | <0.001 |
| Diastolic blood pressure, mmHg | 195.2 ±39.9 | 81.4 ±10.8 | 80.2 ±11.3 | 77.9 ±12 | <0.001 |
| Hypertension treatment, N (%) | 53 ±14.6 | 1848 (80.2) | 2129 (92.4) | 2215 (96.1) | <0.001 |
| Total cholesterol, mg/dL | 98.8 ±13.6 | 203.2 ±40.4 | 194.9 ±40.1 | 187.4 ±37.7 | <0.001 |
| HDL cholesterol, mg/dL | 65.7 ±8.0 | 55.6 ±15.4 | 53.5 ±14.5 | 50.0 ±13.2 | <0.001 |
| Fasting glucose, mg/dL | 994 (14.4) | 94.9 ±10.8 | 98.2 ±11.0 | 103.5 ±16.8 | <0.001 |
Age limited to individuals 80 years or less
Values are N (%) or mean ±standard deviation. ANOVA comparison normal continuous variables and Chi-squared comparison for categorical variables. BMI: body mass index; HDL: high density lipoprotein.
In this analysis, 3,463 (50.1%) individuals were assigned to intensive BP treatment, and baseline characteristics did not significantly differ by treatment group (p>0.05 for all, Table S3). Baseline characteristics by race-sex specific groups are described in Table S1, and baseline characteristics by risk tertile and treatment group are described in Table S4.
The median follow-up time for this sample was 3.3 (IQR 2.9–3.8) years. Over the course of follow-up, 77 individuals developed HF. Over half (55%) of the incident HF cases were in the high risk tertile, with the intermediate and low risk tertiles accounting for 29% and 17% of cases of incident HF, respectively. Baseline characteristics of those with and without incident HF were similar apart from age and diastolic BP—individuals with incident HF were significantly older (p<0.001) and had lower DBP measurements (p=0.003) compared to individuals without incident HF (Table S2).
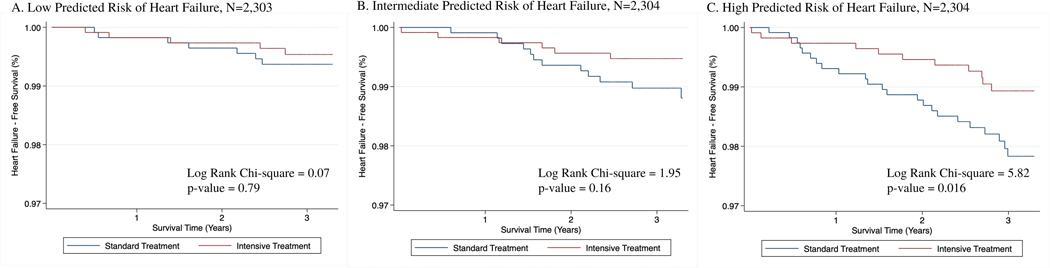
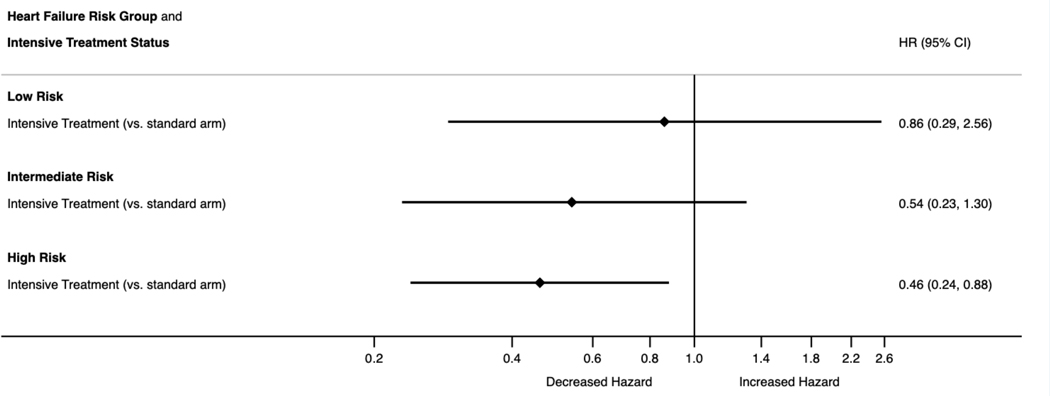
Figure 2 details the effects of intensive BP lowering compared with standard BP lowering across HF risk categories via Kaplan Meier curves. Across the three levels of risk, intensive BP lowering was favored but was only statistically significant for individuals in the high HF risk category (log-rank test Chi-sq=5.82, p-value=0.02). Figure 3 displays the hazard of heart failure by intensive treatment status for each tertile of HF risk (HR=0.86, 95% CI [0.29, 2.56]; 0.54, [0.23, 1.30]; 0.46, [0.24, 0.88], for low, intermediate and high HF risk categories respectively).
Figure 2.
Effects of intensive blood pressure lowering on incident heart failure events across the spectrum of 10-year predicted heart failure risk (low [0.6–5.4%], intermediate risk [5.4–9.0%], and high [9.0–50.3%]). Ten-year predicted heart failure risk was calculated using sex- and race-specific HF risk prediction equations, which include age, smoking status, body mass index, systolic blood pressure, total cholesterol, high density lipoprotein cholesterol, glucose, and treatment status for hypertension and diabetes. Intensive blood pressure lowering defined as a systolic blood pressure target <120 mmHg and standard blood pressure lowering defined as a systolic blood pressure target 135–139 mmHg.
Figure 3.
Hazard ratios with 95% confidence intervals for incident heart failure in those assigned to intensive compared with standard blood pressure lowering. Low, intermediate and high heart failure risk categories based on tertiles of the distribution of HF risk scores according to PCP-HF risk scores. Intensive blood pressure lowering defined as a systolic blood pressure target <120 mmHg and standard blood pressure lowering defined as a systolic blood pressure target 135–139 mmHg. PCP-HF: Pooled Cohort Equations to Prevent HF.
Table 2 describes SAEs by HF risk tertile and treatment arm. Across HF risk tertiles, SAEs affected 25.0% of low risk, 33.9% of intermediate risk, and 39.8% of high risk participants and were more common in the intensive treatment compared to standard treatment arms for both low HF risk and high HF risk categories (low: 27.2% vs. 22.8%, high: 40.9% vs. 38.7%). Related SAEs were more frequent among individuals in the intensive vs. standard treatment groups across all levels of risk. Additional AEs are available in Table S5.
Table 2.
Serious adverse events by heart failure risk tertile and SPRINT treatment arm
| Characteristic | Low Risk (Range 0.6–5.4%) | Intermediate Risk (Range 5.4–9.0%) | High Risk (Range 9.0–50.3%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Intensive N=1,149 | Standard N=1,154 | Intensive N=1,180 | Standard N=1,124 | Intensive N=1,134 | Standard N=1,170 | |
| Serious adverse events | 313 (27.2) | 263 (22.8) | 396 (33.6) | 386 (34.3) | 464 (40.9) | 453 (38.7) |
| Related serious adverse event | 29 (2.5) | 11 (1.0) | 40 (3.4) | 26 (2.3) | 69 (6.1) | 36 (3.1) |
| Hypotension | 16 (1.4) | 6 (0.5) | 18 (1.5) | 15 (1.3) | 36 (3.2) | 16 (1.4) |
| Syncope | 21 (1.8) | 9 (0.8) | 16 (1.4) | 17 (1.5) | 34 (3.0) | 20 (1.7) |
| Bradycardia | 8 (0.7) | 3 (0.3) | 15 (1.3) | 8 (0.7) | 21 (1.9) | 20 (1.7) |
| Electrolyte abnormality | 32 (2.8) | 17 (1.5) | 26 (2.2) | 29 (2.6) | 30 (2.6) | 25 (2.1) |
| Injurious fall | 12 (1.0) | 6 (0.5) | 20 (1.7) | 17 (1.5) | 21 (1.9) | 21 (1.8) |
| Acute kidney injury/acute renal failure | 25 (2.2) | 15 (1.3) | 32 (2.7) | 18 (1.6) | 55 (4.9) | 33 (2.8) |
| ER Visit or serious adverse event | ||||||
| Hypotension | 27 (2.3) | 10 (0.9) | 28 (2.4) | 20 (1.8) | 54 (4.8) | 23 (2.0) |
| Syncope | 33 (2.9) | 14 (1.2) | 26 (2.2) | 23 (2.0) | 46 (4.1) | 29 (2.5) |
| Bradycardia | 11 (1.0) | 4 (0.3) | 16 (1.4) | 11 (1.0) | 29 (2.6) | 21 (1.8) |
| Electrolyte abnormality | 41 (3.6) | 19 (1.6) | 34 (2.9) | 36 (3.2) | 42 (3.7) | 31 (2.6) |
| Injurious fall | 57 (5.0) | 35 (3.0) | 74 (6.3) | 64 (5.7) | 79 (7.0) | 84 (7.2) |
| Acute kidney injury or renal failure | 26 (2.3) | 15 (1.3) | 33 (2.8) | 19 (1.7) | 61 (5.4) | 33 (2.8) |
Table 3 presents the number needed to treat (NNT) to prevent one case of incident HF and the number needed to harm (NNH) to cause one incident SAE. For individuals in the high risk category, the NNT was estimated to be 76 (95% CI [42, 416]). The NNT for the intermediate and low risk categories were 177 and 1185, respectively, although these estimates were not statistically significant. For the low HF risk category, the NNH was estimated to be 23 (95% CI [13, 109]).
Table 3:
Number needed to treat with intensive blood pressure lowering to prevent one incident heart failure event and the number needed to harm with intensive blood pressure lowering to cause one incident serious adverse event
| Predicted HF Risk Tertile | NNT - Heart Failure | 95% Confidence Interval | NNH - SAE | 95% Confidence Interval |
|---|---|---|---|---|
| Low HF Risk | 1185 | ----- | 23 | 13, 109 |
| Intermediate HF Risk | 177 | ----- | 128 | ----- |
| High HF Risk | 76 | 42, 416 | 46 | ----- |
NNT=Number Needed to Treat; NNH=Number Needed to Harm; SAE=Serious Adverse Event; HF=Heart Failure.
Confidence intervals of non-significant NNT and NNH values not included.
DISCUSSION
In this post-hoc analysis of SPRINT, we found differences in HF prevention with intensive BP lowering based upon predicted HF risk grouping, with significant reductions in incident HF in the group at highest risk of HF at baseline based upon their PCP-HF score. There were also non-significant reductions in HF risk among those assigned to intensive BP lowering in the groups at low and intermediate baseline HF risk. We confirmed that SAE were common in all participants across HF risk groups in both control and treatment arms but were highest in those at highest risk of HF assigned to intensive BP lowering.
While the progression from hypertension to symptomatic HF can take decades, SPRINT demonstrated that intensive BP lowering over a relatively short time frame (approximately 3 years of follow-up) significantly reduced the risk of new onset HF in the overall trial population of older adults (aged 50 years and older) at-risk for CVD.6, 10 Our analysis adds to these findings by demonstrating that those at highest risk of HF derived the greatest benefit from intensive BP lowering, at least in the relatively short follow-up time provided by SPRINT. This is especially important given the prognostic implications of HF; incident HF was associated with a 27-fold increased risk of cardiovascular mortality after diagnosis in SPRINT.10
Our finding of a stepwise, dose-dependent, higher risk of HF based upon predicted risk groups is similar to the findings from Plante et al. which demonstrated a greater number of ASCVD events (not including HF) occurred in those with higher predicted ASCVD risk at baseline in SPRINT.14 Unlike their analysis which noted a consistent reduction in ASCVD events across risk groups, we did not find a significant reduction in HF events in those at low or intermediate HF risk. This difference is likely related to limited power with the small absolute numbers of HF events over the limited follow-up period, particularly among those with lower HF risk, or possibly due to pathophysiologic differences between ASCVD events and HF—e.g. a relatively slower progression from hypertension to symptomatic HF.
While current guidelines from the AHA/ACC recommend personalization of BP goals based on an individual’s 10-year ASCVD risk, our analysis highlights the substantial benefits of intensive BP lowering in individuals with high predicted risk of HF based on the PCP-HF model.8, 9 Consideration of the PCP-HF risk model in clinical decision-making tools and guidelines may help to identify additional individuals who might have greater benefit to emphasize intensive BP lowering. In addition, given the widespread problem of uncontrolled hypertension, regular utilization of a HF risk prediction tool in BP management may help facilitate conversations between providers and patients around HF prevention and would help to identify high-risk individuals who may benefit from additional HF risk stratification (e.g., measurement of brain natriuretic peptides, echocardiography) or further, targeted, risk factor modification. This is especially relevant given the higher morbidity burden (e.g., hospitalization) and poorer health-related quality of life observed in patients with HF, relative to ASCVD. As emerging therapies for HF prevention that also lower BP become available (e.g., sodium glucose co-transporter 2 inhibitors or finerenone, a nonsteroidal, selective mineralocorticoid receptor antagonist), further studies may be needed to identify which class of therapy to use in those with highest risk of HF relative to ASCVD as well.
Potential mechanism(s) responsible for the greater reduction in incident HF in those at high risk of HF may include higher prevalence of subclinical or Stage B HF at baseline. In the Flemish Study on Environment, Genes, and Health Outcomes, participants with a higher PCP-HF score had greater degree of subclinical heart maladaptation (e.g, abnormal LV longitudinal strain, diastolic dysfunction). However, previous analyses in SPRINT did not identify significant differences between indices of cardiac structure and CVD events, but may have been under-powered given that HF was not a very common event during follow-up.15, 16 In addition, the SPRINT-HEART sub-study (N=340) showed minimal differences in cardiac structure by cardiac magnetic resonance imaging between the two treatment arms in follow-up, indicating that favorable changes to cardiac structure are less likely to underlie the differences observed .15, 16 Intensive BP lowering also did not decrease the rates of HF precursors such as atrial fibrillation or new/progressive renal dysfunction.6, 17, 18 Potential pathways for HF prevention include class effects of particular antihypertensive agents or favorable changes in BNP, endothelial function, or systemic inflammation, which may be higher in those in higher predicted HF risk categories.19, 20
Although this analysis highlights the benefit associated with intensive BP lowering in those at high risk of HF, SAEs were common, particularly among those in the highest HF predicted risk tertile. Notably, the high rates of SAEs in this tertile were seen in both treatment arms and the relative increase in SAEs amongst those assigned to the intensive treatment arm were higher in those in the low risk compared with high HF risk category. Our finding of a stepwise increase in AEs by baseline HF predicted risk is consistent with prior analyses in SPRINT which have shown a significant association between baseline CVD risk—for example as defined by 10-year ASCVD risk—and SAEs.14, 21 In fact, one analysis showed that the number of medications (<5 medications vs. ≥5 medications; not limited to antihypertensive medications) a SPRINT participant was prescribed at baseline was associated with number of SAEs and that this association was not significantly modified by treatment arm assignment.22 Taken together, our findings, as well as those in prior analyses, point to baseline risk or comorbidity burden, defined in our analysis by PCP-HF, rather than treatment arm assignment, as the larger contributor to reported SAEs. Nonetheless, related SAEs were more common in those assigned to the intensive treatment arm and may limit the applicability of lower BP targets in clinical practice.
This analysis has important limitations to consider. First, as a non-prespecified, post-hoc analysis of SPRINT, characteristics of those included were limited by SPRINT’s enrollment criteria, preventing generalizability of our findings to a broader HF prevention population. This is particularly true given the categorical exclusion of those with baseline diabetes and those <50 years old. While the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial showed no significant difference in CVD outcomes (including HF) by intensive compared with standard BP lowering, its complex design may limit its application to contemporary HF prevention populations.23, 24 Future studies could consider investigating the benefits of intensive BP lowering in patients with diabetes at high HF risk. In addition, the exclusion of adults <50 limits these findings applicability to younger high-risk adults who may also benefit from intensive BP lowering. Second, we chose to categorize individuals by HF predicted risk tertiles in our sample. This limits our ability to draw conclusions about what might be the “optimal” risk-level for initiation of intensive BP lowering. Additional studies are needed to determine what level of HF risk maximizes the benefit of intensive BP lowering while minimizing the associated harms. The lowest risk tertile also included individuals that spanned 0.6–5.4% of predicted risk reflecting the relatively higher risk nature of the SPRINT population. Third, SPRINT was powered to observe differences in a composite CVD outcome but not for HF events. The low number of HF events in those with low and intermediate risk of HF limited our ability to detect significant differences between treatment arms and so our findings for these groups must be interpreted with caution. Further, our results do not detract from the importance of BP lowering in low or intermediate risk groups for long-term prevention of CVD but focus on prioritizing the relatively short-term benefits in those at highest risk of HF given the challenges of implementing intensive BP lowering in the overall population. Fourth, we were unable to determine whether there was a significant class effect related to specific antihypertensive agents. While this is a limitation of the SPRINT dataset itself it may be particularly relevant in HF prevention given the known benefit of specific antihypertensive classes (e.g., targeting renin-angiotensin-aldosterone system) for HF prevention.25
In conclusion, this post-hoc analysis of SPRINT demonstrates that participants assigned to intensive BP lowering with the highest predicted risk of HF based upon the PCP-HF score had a larger reduction in incident HF events and a smaller relative increase in total SAEs compared with those in the lowest HF predicted risk group. While risk prediction tools have been used to guide prevention strategies and balance benefits/risk of treatment in of atherosclerotic CVD, a similar paradigm has yet to have been adopted for HF prevention. HF prevention strategies, including BP lowering, may benefit from personalization to individual’s risk to appropriately target treatments and resources.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
While intensive blood pressure lowering in the Systolic Blood Pressure Intervention Trial (SPRINT) led to a reduction in heart failure events, challenges persist in optimizing blood pressure control with rates of blood pressure control declining in the general population in the United States
The present study demonstrates that identification of those individuals at greatest risk for heart failure can identify those who are most likely to benefit from aggressive blood pressure lowering interventions for heart failure prevention
What is Relevant?
Use of a simple and validated clinical risk score, the Pooled Cohort Equation to Prevent Heart Failure (PCP-HF), which integrates readily available clinical markers, can identify patients at greatest risk of heart failure, even among a population of individuals with hypertension
There was a 54% reduction in heart failure events in individuals in the highest predicted heart failure risk group with intensive blood pressure lowering
Summary?
Among patients with hypertension in SPRINT, targeting intensive blood pressure lowering led to greatest reduction in heart failure events among those at highest predicted heart failure risk at baseline and supports a risk-based paradigm to the primary prevention of heart failure
PERSPECTIVES.
Morbidity and mortality related to heart failure continues to increase in the United States, and hypertension, is a leading risk factor for heart failure. While it is well-accepted that blood pressure levels have a continuous, dose-dependent impact on heart failure risk and intensive blood pressure lowering can prevent heart failure, prevalence of controlled blood pressure has declined in the past decade. Once heart failure develops, prognosis is poor with a 5-year case fatality rate of 50%. Therefore, primary prevention of heart failure is of utmost importance. However, not all individuals with high blood pressure are at similar risk for heart failure. A population-level strategy identifying and targeting those at greatest risk for heart failure for intensive blood pressure lowering may have the greatest benefit. Use of a simple risk score that integrates readily available risk factor levels, the Pooled Cohort Equations to Prevent Heart Failure, is also appealing, in that it does not require addition of cardiac biomarkers or laboratory tests that are not routinely obtained in the general adult primary prevention population. This information on heart failure risk can then guide patient-clinician discussions and therapeutic considerations for intensive blood pressure lowering. However, primary prevention of heart failure also requires careful attention to blood pressure beginning early in life and long-term risk of heart failure should be considered in future studies to guide therapeutic decisions for younger adults who may have long-term benefit from intensive blood pressure lowering.
Acknowledgments:
The authors take responsibility for decision to submit the manuscript for publication. Dr. Khan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding: Research reported in this publication was supported, in part, by the National Institutes of Health, Grant Numbers KL2TR001424, P30AG059988; P30DK092939 (SSK) and the American Heart Association (#19TPA34890060) to SSK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Sanjiv J. Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, Tenax, and United Therapeutics. All remaining authors have nothing to disclose.
REFERENCES
- 1.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB and Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. Jama. 2020;324:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark D 3rd, Colantonio LD, Min YI, Hall ME, Zhao H, Mentz RJ, Shimbo D, Ogedegbe G, Howard G, Levitan EB, Jones DW, Correa A and Muntner P. Population-Attributable Risk for Cardiovascular Disease Associated With Hypertension in Black Adults. JAMA Cardiol. 2019;4:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenger NK, Arnold A, Bairey Merz CN, Cooper-DeHoff RM, Ferdinand KC, Fleg JL, Gulati M, Isiadinso I, Itchhaporia D, Light-McGroary K, Lindley KJ, Mieres JH, Rosser ML, Saade GR, Walsh MN and Pepine CJ. Hypertension Across a Woman’s Life Cycle. J Am Coll Cardiol. 2018;71:1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D, Larson MG, Vasan RS, Kannel WB and Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–62. [PubMed] [Google Scholar]
- 5.Upadhya B, Stacey RB and Kitzman DW. Preventing Heart Failure by Treating Systolic Hypertension: What Does the SPRINT Add? Current hypertension reports. 2019;21:9. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostis JB, Davis BR, Cutler J, Grimm RH Jr., Berge KG, Cohen JD, Lacy CR, Perry HM Jr., Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R and Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. Jama. 1997;278:212–6. [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD Sr. and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA, Yeboah J Sr. and Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, Johnson KC, Rodriguez CJ, Raj DS, Rastogi A, Tamariz L, Wiggers A and Kitzman DW. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, Mentz RJ, O’Brien E, Correa A, Suthahar N, de Boer RA, Wilkins JT and Lloyd-Jones DM. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. 2019;73:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG. Confidence intervals for the number needed to treat. Bmj. 1998;317:1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plante TB, Juraschek SP, Miller ER 3rd, Appel LJ, Cushman M and Littenberg B. Comparison of Frequency of Atherosclerotic Cardiovascular and Safety Events With Systolic Blood Pressure <120mm Hg Versus 135–139mm Hg in a Systolic Blood Pressure Intervention Trial Primary Prevention Subgroup. Am J Cardiol. 2018;122:1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upadhya B, Rocco MV, Pajewski NM, Morgan T, Blackshear J, Hundley WG, Oparil S, Soliman EZ, Cohen DL, Hamilton CA, Cho ME, Kostis WJ, Papademetriou V, Rodriguez CJ, Raj DS, Townsend R, Vasu S, Zamanian S and Kitzman DW. Effect of Intensive Blood Pressure Reduction on Left Ventricular Mass, Structure, Function, and Fibrosis in the SPRINT-HEART. Hypertension. 2019:Hypertensionaha11913073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman EZ, Ambrosius WT, Cushman WC, Zhang ZM, Bates JT, Neyra JA, Carson TY, Tamariz L, Ghazi L, Cho ME, Shapiro BP, He J, Fine LJ and Lewis CE. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation. 2017;136:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parcha V, Patel N, Kalra R, Kim J, Gutiérrez OM, Arora G and Arora P. Incidence and Implications of Atrial Fibrillation/Flutter in Hypertension: Insights From the SPRINT Trial. Hypertension. 2020;75:1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, Chonchol M, Sarnak M, Parikh CR, Shlipak MG and Ix JH. Effects of Intensive Blood Pressure Lowering on Kidney Tubule Injury in CKD: A Longitudinal Subgroup Analysis in SPRINT. Am J Kidney Dis. 2019;73:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messerli FH, Rimoldi SF and Bangalore S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017;5:543–551. [DOI] [PubMed] [Google Scholar]
- 20.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 21.Botchway A, Buhnerkempe MG, Prakash V, Al-Akchar M, Adekola B and Flack JM. Serious Adverse Events Cluster in Participants Experiencing the Primary Composite Cardiovascular Endpoint: A Post Hoc Analysis of the SPRINT Trial. Am J Hypertens. 2020;33:528–533. [DOI] [PubMed] [Google Scholar]
- 22.Derington CG, Gums TH, Bress AP, Herrick JS, Greene TH, Moran AE, Weintraub WS, Kronish IM, Morisky DE, Trinkley KE, Saseen JJ, Reynolds K, Bates JT, Berlowitz DR, Chang TI, Chonchol M, Cushman WC, Foy CG, Herring CT, Katz LA, Krousel-Wood M, Pajewski NM, Tamariz L and King JB. Association of Total Medication Burden With Intensive and Standard Blood Pressure Control and Clinical Outcomes: A Secondary Analysis of SPRINT. Hypertension. 2019:Hypertensionaha11912907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC and Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, Cutler J, Fine L, Boucher R, Wei G, Zhang C, Kramer H, Bress AP, Kimmel PL, Oparil S, Lewis CE, Rahman M and Cushman WC. Effects of Intensive Systolic Blood Pressure Lowering on Cardiovascular Events and Mortality in Patients With Type 2 Diabetes Mellitus on Standard Glycemic Control and in Those Without Diabetes Mellitus: Reconciling Results From ACCORD BP and SPRINT. J Am Heart Assoc. 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Jama. 2002;288:2981–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.