Abstract
Understanding immune responses toward viral infection will be useful for potential therapeutic intervention and offer insights into the design of prophylactic vaccines. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the COVID-19 pandemic. To understand the complex immune responses toward SARS-CoV-2 infection, here we developed a method to express and purify the recombinant and engineered viral receptor-binding domain (RBD) to more than 95% purity. We could encapsulate RNA molecules into the interior of a virion-sized liposome. We conjugated the purified RBD proteins onto the surface of the liposome in an orientation-specific manner with defined spatial densities. Both the encapsulation of RNAs and the chemical conjugation of the RBD protein on liposome surfaces were stable under physiologically relevant conditions. In contrast to soluble RBD proteins, a single injection of RBD-conjugated liposomes alone, in the absence of any other adjuvants, elicited RBD-specific B cell responses in BALB/c mice, and the resulting animal sera could potently neutralize HIV-1 pseudovirions that displayed the SARS-CoV-2 spike proteins. These results validate these supramolecular structures as a novel and effective tool to mimic the structure of enveloped viruses, the use of which will allow systematic dissection of the complex B cell responses to SARS-CoV-2 infection.
Graphical Abstract
INTRODUCTION
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unprecedented in its scale and impact on modern society. SARS-CoV-2 is an enveloped positive-strand RNA virus that encodes at least 20 different viral proteins.1 The immune responses triggered by SARS-CoV-2 infection are complex and multilayered,2,3 including components that are beneficial to the host, such as neutralizing antibodies and also components that are detrimental such as cytokine storm. Part of this complexity is caused by sophisticated constituents and assembly of viral particles that cause the infection. Although all viral-encoded components are foreign to the host and may elicit immune responses, only a few of them elicit immune responses that are useful for the host to fight against the viral infection. Understanding the spectrum of immune responses toward SARS-CoV-2 infection will be useful for both treatment and prophylactic intervention.
To unravel the molecular mechanisms of complex immune responses toward viral infection, we have taken a reductionist approach by focusing on the common qualitative and quantitative features of viruses.4 Through layer-by-layer construction of synthetic viral-like structures (sVLS) that resemble the key features of enveloped viruses and using them as immunogens, we hope to dissect and therefore understand the multilayered responses of the immune system toward viral infection.
There are two essential and common biochemical features among all enveloped viruses identified to date:5 (1) a closed lipid bilayer membrane with viral antigens on the surface and (2) nucleic acids that are encapsulated in the interior of the virus underneath the biological membrane. Within these two features, it is important to note that the viral antigens on the surface of individual virions are always orientation specific, which is a result of the complex protein biosynthesis, intracellular trafficking, and incorporation.6 This orientation specificity is a key feature for B cell antibody response because it allows a quantitative definition of the spatial density of surface epitopes.7 The variation of the epitope density is known to influence B cell antibody response in both qualitative and quantitative manners.8-10
To closely mimic enveloped viruses using synthetic materials, the method that we have recently developed11 involves the use of three key ingredients: (1) a highly purified recombinant viral surface protein, (2) a unilamellar liposome with a size that is typical of enveloped viruses, and (3) synthetic nucleic acid as a mimic of the viral genome. In our method, the nucleic acid was encapsulated inside the unilamellar liposome. The protein was conjugated in an orientation-specific manner onto the outer surface of the liposome, and the liposomes were purified away from both free nucleic acids and free proteins.
It is important to note that both nucleic acid encapsulation and protein conjugation are quantitative so that we can control both the amount of nucleic acids encapsulated inside the liposome and the spatial density of the viral protein conjugated on the liposome surface. These quantitative controls are necessary because naturally occurring viruses have varied epitope density4 and varied lengths of nucleic acid genome.12 Furthermore, the use of maleimide chemistry for site-specific conjugation of purified proteins onto the liposomal surface affords stable epitope density with time under physiologically relevant conditions, and the encapsulated nucleic acids were shielded from the degradation activity by nucleases in biologically relevant media.
To demonstrate the utility of these techniques, we have recently synthesized liposomes that display the hen egg lysozyme (HEL) on the liposomal surface in an orientation-specific manner.11 We showed that these liposomes can activate B cells in vitro in a highly antigen-specific manner.11 In this work, we show that the purified receptor-binding domain (RBD) from SARS-CoV-2 can be conjugated onto the surface of liposomes in an orientation-specific manner. A single injection of RBD-conjugated liposomes alone, in the absence of any other adjuvants, can elicit potent neutralizing antibodies against SARS-CoV-2 in immunized mice. Because these structures resemble authentic viruses in several critical aspects that include particle size, surface epitope density, and internal nucleic acids encapsulation,11 the use of these sVLS may offer tremendous insight on how protective immune responses were initiated during a viral infection, and the knowledge of which can better the design of next-generation vaccines.
RESULTS
Purification of Engineered RBD to Greater Than 95% Purity.
Among various proteins encoded by SARS-CoV-2, the virus uses the S protein for binding with host cell receptors to gain entry into host cells.13 In particular, the RBD within the S protein directly binds human ACE2 receptors (hACE2) to mediate viral entry.14 In BALB/c mice immunized with an inactivated SARS-CoV-2 vaccine and alum adjuvants,15 RBD-specific immunoglobulin G (IgG) accounts for half of S-induced antibody responses and potently neutralizes different strains of SARS-CoV-2. Moreover, RBD-specific antibodies that can cross-neutralize both SARS-CoV-1 and SARS-CoV-2 have been identified and characterized,16,17 suggesting that this immunogen has the potential to elicit cross-reactive antibodies. To understand the mechanisms behind the protective antibody responses, we have therefore chosen this protein to prepare sVLS.
The three-dimensional structures of SARS-CoV-2 RBD have been reported by several different groups in record time,18-21 as shown in Figure 1a for one of the high-resolution structures18 of SARS-CoV-2 RBD. Importantly, there are a total of four disulfide bonds in RBD, which are highlighted in space-filling models in this structure. Three of them are important to stabilize the folded protein structure, while one of them (C480–C488) constrains the three-dimensional conformation of a loop that is directly involved in binding with hACE2.18 Because of the important roles of these disulfide bonds in the RBD structure and interaction with the receptor, any perturbation of them likely compromises the function of this protein. To use maleimide chemistry for site-specific conjugation of the purified protein onto liposomes, it is therefore essential to purify this protein in native state, similar to what we did for the hen egg lysozyme in our recent work.11
Figure 1.
RBD protein and its purification. (a) Crystal structure of SARS-CoV-2 RBD showing four pairs of disulfide bonds, in which C480–C488 are important for binding to the hACE2 receptor (PDB 6m0j). The C-terminus of the RBD protein is also denoted. (b) Silver staining of a 15% (29:1 acrylamide/Bis) reducing sodium dodecyl sulfate (SDS)-polyacrylamide gel to assess the purity of the purified RBD protein. Three lanes were loaded at 2, 5, and 100% relative to the quantity of the purified protein.
Because of the similarity between HEL and RBD in their disulfide status, we started with the Escherichia coli system that we developed for HEL to overexpress and purify a recombinant RBD that carried a free engineered cysteine near the C-terminus of the protein followed by a hexahistidine tag for the purpose of purification. However, systematic variation of the expression conditions similar to that of HEL has not resulted in RBD that is more than 95% in purity. The RBD proteins were overexpressed but did not bind well to nickel-nitrilotriacetic acid (Ni-NTA) resin in either on-column or batch binding conditions, suggesting that the protein was not well folded and hexahistidine tags were not readily accessible. Coexpression of molecular chaperones to assist protein folding in this system resulted in soluble RBD proteins that are in a stable complex with the chaperone, further confirming this notion. These results are consistent with a recent report that RBD proteins produced in E. coli did not elicit specific detection by sera IgG from SARS-CoV-2 positive subjects, in contrast to the same protein produced from mammalian cells.22
We have thus decided to use the mammalian expression system 293F for the expression of RBD. We used the 293F system previously for the expression and purification of a monoclonal antibody Fab.23 Specifically, this system allowed us to express and purify the VRC01 Fab with an engineered cysteine near the C-terminus of the protein to more than 95% purity and achieve a site-specific conjugation at the engineered cysteine using maleimide chemistry with high efficiency in the context of multiple disulfide bonds without compromising the function of native proteins.23 Using the same expression system as we developed before, we could purify RBD that carried a specific cysteine near the C-terminus of the protein to greater than 95% purity, as shown in Figure 1b lanes 1–3.
Binding of RBD to hACE2 Monitored Using Biolayer Interferometry.
To assess the functionality of the purified RBD protein, we used biolayer interferometry to quantitate the binding between RBD and its target hACE2. In our experimental design, a biotinylated hACE2 was initially loaded onto a streptavidin sensor, and then the sensor was dipped into RBD solutions of varied concentrations, and the binding signals were monitored in real time. As shown in Figure 2a for the sensorgrams at various RBD concentrations, all of the association traces could be well described by the sum of two exponentials, which yielded two observed rate constants kobs,1 and kobs,2. The kobs,1 is linearly correlated with the concentration of RBD ([RBD]), indicated by the red-line fit in Figure 2b. The kobs,2 is more than 10-fold slower than kobs,1, but it also depends on [RBD] in a hyperbolic manner, as indicated by the red hyperbolic curve in Figure 2c.
Figure 2.
Kinetics of RBD binding to hACE2 in solution. (a) Sensorgrams from biolayer interferometry experiments showing RBD at five different concentrations binding to hACE2 immobilized on streptavidin sensors. The RBD concentrations are 202, 404, 605, 809, and 1010 nM, respectively. The experimental traces are shown in black, and the fits are shown in red. (b) Observed rate constant for phase 1 of RBD association with hACE2, kobs,1, follows a linear dependence on [RBD], which is shown by the red fitted line. (c) Observed rate constant for phase 2 of RBD association with hACE2, kobs,2, follows a hyperbolic dependence on [RBD], which is shown by the red fitted curve. (d) Observed rate constant for RBD dissociation from hACE2, kobs,dissociation, within error, is independent of [RBD]. The red straight line indicates the mean value of the dissociation rate. For (b–d), error bars represent the standard error from exponential fits of individual traces shown in (a). The quantitative data are representative of three independent repeats of the same experiments.
This biphasic dependence is quantitatively consistent with a two-step binding process,24 in which the bimolecular collision is followed by an isomerization step that can lead to a tighter binding between macromolecules. The bimolecular rate of collision determined from the slope in Figure 2b is (1.54 ± 0.03) × 105 M−1 s−1, which compares favorably to a previous measurement of 1.36 × 105 M−1 s−1 between SARS-CoV-2 RBD and hACE2 using the same technique14 as we deployed here. Both studies used the same original isolate of SARS-CoV-2 (GenBank: MN908947),1 although the RBD fragments used slightly differed. In the current study, the RBD encompasses residues 328–537 of the S protein, while the previous study used RBD with residues 319–591. In contrast to the biphasic association between RBD and hACE2, the dissociation of RBD from hACE2 as we monitored after transferring the sensor tip to phosphate-buffered saline (PBS) can be well described by single exponentials for all concentrations as shown in Figure 2a. The rate constant of dissociation is independent of [RBD] and averages (2.8 ± 0.1) × 10−3 s−1. This rate of dissociation is slower than the rate of dissociation of 4.7 × 10−3 s−1 measured previously for RBD from hACE2 but identical within error to the rate of dissociation measured for S protein from hACE2,14 which was 2.76 × 10−3 s−1. Based on these kinetic measurements of on and off rates, we estimate that the equilibrium dissociation constant between the RBD studied herein and hACE2 is at most 18.2 nM and likely to be even lower than this value due to the two-step binding process. This result thus compares very well with the KD of 14.7 nM measured for S protein and hACE2.14 In summary, the above results show that the RBD we so far purified retains its full function in binding to hACE2, and thus this protein is properly folded as we expect.
Site-Specific Conjugation of RBD to the Liposome Surface through Maleimide Chemistry.
We were able to conjugate the purified RBD in a site-specific manner onto the surface of liposomes through the maleimide-containing lipid and purify protein-conjugated liposomes away from free proteins by running a size exclusion column (SEC). This process is shown using a reducing SDS-polyacrylamide gel followed by silver staining.
As shown in Figure 3, this conjugate is indicated by the downward arrow in lane 2 and lane 4, respectively. The sample for lane 2 is before SEC, where the free protein present in the sample is also indicated by the upward arrow. Compared to the free protein, the amount of mobility shift on the gel by the conjugate is fully consistent with the molecular weight of one maleimide-containing lipid, which is 2941.605 Da. To confirm the site specificity of the maleimide reaction, we have overexpressed and purified another RBD protein with everything being identical to the RBD described above except that the cysteine near the C-terminus of RBD was now mutated to alanine (RBD-Ala). Under the same set of conditions as in lane 2, the purified RBD-Ala did not yield any conjugation between the protein and liposomes, as shown in lane 3. This result suggests that the conjugation that we observed in lane 2 is specific to the cysteine that we engineered near the C-terminus of the RBD. Under these conditions, none of the existing disulfide bonds in RBD were reduced and thus none of those cystines participated in the maleimide reaction. This result is important because it shows that we can achieve site-specific conjugation of RBD onto liposomes, which results in an orientation-specific display of RBD proteins on the surface of the liposome, similar to that in authentic viruses. By further using a gel filtration column, we can purify RBD-conjugated liposomes (pRBD) away from free proteins, as shown in lane 4. This result also suggests that RBDs were attached to liposomes through the covalent conjugation, and under these conditions, a nonspecific or noncovalent association between RBD and liposomes is negligible. It is worth noting that the epitope density for the pRBD shown in Figure 3 is 54 ± 5 (mean ± standard deviation herein) molecules of RBD per liposome, which is measured using the methods that we established previously.7,11 This value is also comparable to the RBD density of 72 ± 27 molecules per particle on intact SARS-CoV-2 virions.25 By systematically changing conjugate conditions, including the percentage of the maleimide and the concentration of either liposomes or proteins, we were able to prepare a set of pRBD with epitope density as low as eight molecules of RBD per liposome to as high as 200 molecules of RBD per liposome, similar to what we have done previously for liposomes site specifically conjugated with hen egg lysozyme.11 Moreover, we can encapsulate these liposomes with RNA oligos to mimic the presence of RNA genome in SARS-CoV-2 viral particles.
Figure 3.
Conjugation of RBD with maleimide-containing liposomes. The conjugation of purified RBD proteins with maleimide on the surface of liposomes was monitored using a 15% (29:1 acrylamide/Bis) reducing SDS-polyacrylamide gel followed by silver staining. Lane 1: molecular weight marker. Lane 2: the RBD–liposome conjugate before SEC. Lane 3: the admixture of RBD-Ala and liposome under the same conditions as RBD–liposome in lane 2. Lane 4: SEC-purified RBD–liposome.
RBD–Liposome Stability in 50% Serum.
We intended to use pRBD as prepared above for in vivo studies in animals; it is therefore critical that the structures should be stable under relevant physiological conditions. To examine the stability of pRBD under these conditions, we mixed pRBD with freshly thawed fetal bovine serum (FBS) in 1:1 volume ratio and incubated the mixture at 37 °C. At a designated time, aliquots of the mixture were taken for both the measurement of particle size and epitope density, following our previously established procedures.11 As shown in Figure 4a for pRBD with an initial epitope density of 196 ± 25 molecules per liposome, the size of liposomes did not change over a 2 week period, with an average diameter of 122 ± 2 nm, close to that of authentic SARS-CoV-2 virions13 and no sign of aggregation. These liposomes are also stable in their epitope density at 37 °C in 50% FBS. As shown in Figure 4b, the average number of RBD molecules per liposome was 196 ± 25 on Day 0 and slowly dropped to 168 ± 28 on Day 14 (Figure 4b). By the end of 2 weeks, more than 85% of the RBD remained covalently associated with liposomes. This trend recapitulates the stability of maleimide-based liposomes that we prepared recently for two different antigens11,26 and was much better than the stability of proteins conjugated onto the liposomal surface through Ni-NTA noncovalent chemistry as we reported previously.7 These results altogether establish a quantitative basis for the interpretation of in vivo immunization experiments in terms of epitope density. Finally, we also assayed the stability of RNA oligos encapsulated inside RBD-conjugated liposomes under the above conditions of 37 °C in 50% FBS. As shown in Figure 4c, more than 80% of the initial RNA content remained after 14 days in 50% FBS at 37 °C (solid circles). In sharp contrast, when the same initial amount of RNA was mixed with pRBD (without internal RNA) and then incubated in 50% FBS at 37 °C, the RNA molecules were quickly degraded within 24 h (hollow triangles). This result confirms that the RNA was in fact encapsulated inside the liposomes, which offers protection from degradation by nucleases in FBS.
Figure 4.

Stability of RBD–liposomes in biologically relevant conditions. (a) Size of RBD-conjugated liposomes as a function of time upon incubation in 50% FBS at 37 °C. (b) Average number of RBD molecules per liposome as a function of time upon incubation in 50% FBS at 37 °C. (c) Percentage of remaining RNA relative to Day 0 as a function of time upon incubation in 50% FBS at 37 °C, shown in black circles for the RNA encapsulated within liposomes and open triangles for the free RNA in an admixture with liposomes. Throughout, the error bars were standard deviations from three independent repeats of the same experiments.
pRBD Immunization in Mice.
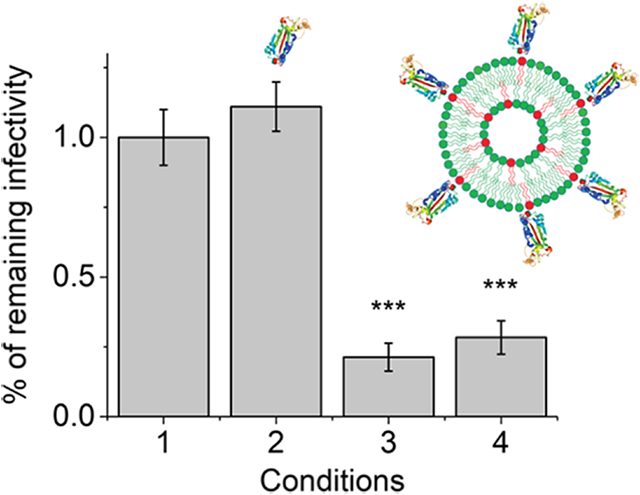
To examine immune responses toward pRBD in vivo, we conducted subcutaneous injection of pRBD in BALB/cJ mice and collected blood on day 5 and 12 post immunization to assay antibody responses. To study the effect of epitope density in isolation, liposomes were injected as prepared without alum or any other adjuvants. The pRBD used for injection did not contain internal RNA, either. As shown in Figure 5a, low as 0.48 μg (mass of protein on liposomes) of pRBD1 (94 ± 12 molecules of RBD per liposome) and pRBD2 (196 ± 25 molecules of RBD per liposome) elicited RBD-specific and class-switched IgG responses as early as Day 5 post a single injection of pRBD. The enzyme-linked immunosorbent assay (ELISA) signals for IgG were saturated for both pRBD1 and RBD2 on Day 12 post immunization. Serial dilution of the sera revealed that the IgG responses from both pRBD1 and pRBD2 reached a titer of 105 on day 12 post a single injection. These responses were in sharp contrast to either PBS-injected controls (condition 1) or soluble RBD protein (sRBD, condition 2) at the same antigen dose. Throughout, neither condition 1 nor condition 2 could elicit any measurable RBD-specific IgG in mice. These results suggest that RBD becomes highly immunogenic upon covalent and site-specific conjugation on the liposome surface and can activate antigen-specific B cells at a very low antigen dose. To assess the functionality of the IgG detected by ELISA, we prepared HIV-1 based pseudovirions that displayed the full-length S protein of the SARS-CoV-2 (see the Materials and Methods section). This pseudovirion-based reporter system has been recently rigorously compared with others and validated as an effective approach for quantitative assessment of serological immunity against SARS-CoV-2.27 As shown in Figure 5b, the sera from a mouse immunized with pRBD1 or pRBD2 can potently neutralize the infectivity of pseudovirions, suggesting that they would be functional in vivo that can offer host protection against the infection of SARS-CoV-2.
Figure 5.

Anti-RBD antibody response in wild-type BALB/cJ mice. (a) ELISA OD values for IgG antibody from 1:100 diluted mouse sera after a single inoculation using PBS (condition 1), sRBD (0.48 μg, condition 2), pRBD1 (condition 3), and pRBD2 (condition 4) containing 0.48 μg of RBD. Sera collected on both Day 5 and Day 12 after immunization were indicated. (b) Neutralization of HIV-1 virions pseudotyped with SARS-CoV-2 S protein by mouse sera collected on Day 12 post a single injection. Throughout the two panels, each data point in the figure represents the mean values obtained from four mice of each group. Error bars represent the standard errors. Statistical difference between sRBD and pRBD was determined by Student’s t-test (***p-value < 0.001).
DISCUSSION
The immune responses toward SARS-CoV-2 infection are very complex. To understand how protective humoral immunity arises as a result of the viral infection, we have taken a reductionist approach in this work to construct totally synthetic viral-like structures (sVLS) that resemble the key features of SARS-CoV-2 viruses and aim to use these structures for understanding of protective humoral immunity. To this end, we have developed a method to overexpress and purify the RBD of SARS-CoV-2 that carries an engineered cysteine and have conjugated the purified proteins onto the surface of unilamellar liposomes in a site-specific manner through the covalent maleimide chemistry. The epitope density on these particles is stable at 37 °C in 50% serum. We can also encapsulate RNA oligos into the interior of these particles. The encapsulated RNA molecules are shielded underneath the lipid bilayer membrane and protected from nuclease degradation. Thus, these structures are well suited for the investigation of mechanisms of humoral immunity in vivo.
We show that the oriented display of RBD proteins on the surface of a liposome, alone, in the absence of any other adjuvants, is able to trigger highly potent IgG secretion. These IgG can further neutralize HIV-1 pseudovirions that display the same isolate of SARS-CoV-2 envelope glycoproteins, suggesting that these IgG will be protective in vivo to counter SARS-CoV-2 infection. This result also suggests that the conjugation process by itself is unlikely to alter the conformation of RBD protein upon covalent attachment onto the surface of liposomes. The lipid components that we chose to prepare these sVLS are nonimmunogenic nor do they themselves possess any immune-stimulatory activity.28 These results are in sharp contrast to soluble RBD administered under the same dose, which did not elicit any measurable IgG response. The epitope density for RBD on intact SARS-CoV-2 virions is 72 ± 27 molecules of RBD per particle,25 which is very close to the epitope density on pRBD1 that we have tested for immunization. Our results thus suggest that the ordered display of RBDs on the virion surface, alone, is able to activate antigen-specific B cells for secretion of neutralizing IgG, aside from all other viral components including the viral RNA. Also, these results suggest that a distinct mechanism is likely to operate for activation of antigen-specific B cells by these structures as compared to their soluble counterparts. This result is consistent with our recent findings for a different peptide antigen, in which the liposomal display of the antigen above a threshold of epitope density is able to trigger T-independent B cell activation and class switch recombination,26 in contrast to the soluble peptides. Similar to this peptide antigen, it will be of future interest to examine how the RBD-specific IgG response may change with the conjugation density of RBD on the surface of liposomes and whether a threshold of epitope density may exist, below which the RBD-specific IgG is undetectable even though the RBD protein is conjugated on liposomal surface. Antigen organization is known to influence B cell responsiveness.8,10 However, it remains unclear what features in organized antigens such as viruses are essential for B cell responsiveness. Our previous work26 and the studies herein suggest that the ordered display of antigens on a spherical surface that is biophysically similar to a virion surface is likely the pattern that can be recognized in vivo for robust B cell activation. How a foreign antigen on the surface of a viral-like structure acts as a pattern for B cell activation, including the details of signal transduction, remains to be determined in the future.
At this point, it is worth mentioning other approaches reported in the literature to make particulate antigens for SARS-CoV-2 RBD. Using metal-chelation chemistry between cobalt and polyhistidine tag, Huang et al. reported the conjugation between RBD and cobalt-containing liposomes.29 This formulation could work as an admixture between RBD and liposomes. Together with the adjuvant monophosphoryl lipid A, they can elicit robust neutralizing antibodies in animals.29 A variety of other nanoparticle platforms have also been reported recently for use with SARS-CoV-2 RBD for potential prophylactic vaccines.30-33 In all of these studies, robust protective immune responses have been reported. Therefore, these platforms are well suited for the development of vaccine candidates. However, in all of these studies, the results are unclear in terms of understanding of SARS-CoV-2 immunogenicity because all of these nanoparticle platforms contained foreign proteins, and in all of these studies, various adjuvants have been included in the administration in addition to those particles. As a consequence, it is difficult to interpret the immunogenicity of the virus by itself based on these different nanoparticle platforms.
The particles we prepared herein elicited RBD-specific neutralizing IgG responses in vivo. B cell activation is a very complex process. It will be of great future interest to study both in vitro and in vivo how the epitope density on these liposomal particles influences the resulting IgG responses and how epitope density together with internal RNA contributes to the efficacy of IgG in pseudovirion neutralization, and whether they act independently or synergistically. The availability of these totally synthetic viral-like structures will allow us to unravel the mechanisms of B cell responses to viral antigens in detail, and in particular, how the characteristics of a viral particle influence each step in B cell activation, which will help understand the early immune responses toward a viral infection and also facilitate the design of next-generation vaccines.
MATERIALS AND METHODS
Purification of RBD Proteins.
The RBD proteins were expressed and purified following the protocols we developed previously for a recombinant antibody Fab using 293F system23 but with important modifications. The engineered RBD sequence is from the original isolate of SARS-CoV-2 (GenBank: MN908947)1 and codon optimized for expression in 293F cells. In the current study, the RBD encompasses residues 328–537 of the S protein. The C-terminal sequence of RBD is as follows: NLVKNKGGGCHHHHHH, where the cysteine residue underlined is the engineered cysteine designed for crosslinking with maleimide. The protein carries a hexahistidine tag at the C-terminus to facilitate its purification using nickel-nitrilotriacetic acid (Ni-NTA) technology as the first step of the purification. For the control protein RBD-Ala, the C-terminal sequence is as follows: NLVKNKGGGAHHHHHH, where the cysteine residue was mutated to alanine. RBD proteins were house expressed in 293F, and culture supernatants were harvested on Day 3 post transfection. We have developed a protocol to purify these recombinant RBD proteins to greater than 95% purity as judged by intensity comparisons on an acrylamide gel loaded with different amounts of final purified proteins (Figure 1b). All protein purification procedures were performed at 4 °C unless otherwise noted. Briefly, the 293F culture was harvested on Day 3 post transfection. The cell culture was spun down at 4 °C at 3000g for 10 min. The culture supernatant was filtered using a 0.45 μm filter unit and mixed with Ni-NTA agarose beads that were already equilibrated in buffer A (50 mM Na2HPO4, 0.5 M NaCl, and 10 mM imidazole, pH 8.0 at 4 °C). After overnight batch binding, the bead slurry was loaded onto an empty Bio-Rad Econo column. The column was first washed with buffer A at 1 mL/min to baseline and then sequentially washed with buffer A containing 19.8 mM imidazole for 120 min and buffer A containing 24.7 mM imidazole for 30 min, which was followed by a wash using an alkaline buffer containing 0.1 M Tris and 2 M KCl pH 10 at 4 °C. The bound RBD proteins were then eluted in a gradient from 24.7 to 500 mM increasing concentrations of imidazole in buffer A over 20 column volumes at a flow rate of 1 mL/min. The fractions containing RBD proteins were pooled, diluted with buffer C (10 mM Na2HPO4, pH 7.0 at 22 °C) to 50 mM NaCl, and loaded onto a HiTrap heparin column (GE) at a flow rate of 1.0 mL/min. The column was washed with buffer C to baseline and then sequentially washed with buffer C containing 100 and 150 mM NaCl, respectively, for 30 min. The bound RBD proteins were then eluted in a gradient from 150 to 1000 mM increasing concentrations of NaCl in buffer C over 25 column volumes at a flow rate of 1 mL/min. At this stage, the eluted RBD proteins were >95% pure. The protein was then filtered through a 0.1 μm sterile syringe filter, concentration determined by absorbance at 280 nm, flash-frozen in liquid N2, and stored in −80 °C freezer. RBD-Ala was purified using the same protocol as described above. For both RBD proteins, the introduction of mutations did not change the extinction coefficient of the protein at 280 nm under denaturing conditions, and an extinction coefficient of 3.11 × 104 M−1 cm−1 was used for all calculations of protein concentrations.
BioLayer Interferometry Experiments.
We used BioLayer interferometry to quantitate the binding between hACE2 and purified RBD proteins. Briefly, the streptavidin sensor (Sartorius Corp) was coated with 30 nM biotinylated hACE2 (CAT#10108-H08H, Sino Biological) in 1× PBS for 15 min at 20 °C, which was followed by a wash in PBS for 10 min. After this, the sensors were dipped into the RBD solutions of varied concentrations in PBS to measure the binding in real time using an OctetRed BioLayer Interferometer equipped with eight sensor positions that were read simultaneously. The measurement for binding was continued for 20 min, which was followed by dipping the sensors into PBS buffer for 20 min to monitor the dissociation between hACE2 and RBD in real time. Throughout, the measurements were done at 20 °C. PBS control was included and subtracted from all of the kinetic data before quantitative analysis.
Preparation of Maleimide-Containing Liposomes.
All liposomes used in this study were prepared using an oil-in-water emulsion precursor followed by membrane extrusion as originally described in literature.34-36 Three different lipids of designated molar ratios were used in the synthesis of liposomes as we described recently:11 1,2-distearoyl-sn-glycero-3-phos-phocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoetha-nolamine-N-[maleimide (polyethylene glycol)-2000] ammonium salt (DSPE-PEG (2000) maleimide), and cholesterol (Avanti lipids). The choice of a neutral lipid DSPC is meant to mimic that of cell membranes, from which enveloped viruses derive their viral membranes.5 Briefly, lipid mixture in chloroform was added to a round-bottom glass tube, blown dry with purified argon and desiccated by vacuum to form a thin film at the bottom of the tube. For the formation of empty liposomes, PBS buffer was added to hydrate the lipid film through sonication in a water bath. After hydration, the lipid film resuspension was extruded using a polycarbonate membrane with pore sizes of 100 nm for a minimum of 21 times at 70 °C. The resulting liposomes were then stored at 4 °C for all experiments. For encapsulation of nucleic acid molecules inside liposomes, an RNA oligo of the following sequence ACUGUUGAUUCAUCACAGGG (IDT, Coralville, Iowa) was dissolved in PBS buffer at a concentration of 1.02 mM, and 300 μL of this solution was used to hydrate the lipid film as described above, followed by extrusion using polycarbonate membrane with pore sizes of 100 nm for 39 times at 70 °C. The RNA sequence is part of the consensus genome of SARS-CoV-2 (GenBank: MN908947)1 and encodes motif V of an essential RNA helicase for the virus. The resulting liposomes were then applied to Sepharose CL-4B (GE Life Sciences) gel filtration column, as we described previously,7 to separate liposomes away from free excess nucleic acids. The encapsulation efficiency as we calculated from the percentage of nucleic acids molecules that were encapsulated inside the liposomes over the total initial input of nucleic acids molecules was 0.98 ± 0.18%. The average number of nucleic acid molecules per liposome ranged from 40 to 300, which was equivalent in length to 0.8–6 kb per liposome.
Conjugation of RBD to Liposomes, Purification, and Quantification.
Conjugation between RBD and liposomes was conducted at 22 °C for 1 h at a designated molar ratio between the proteins and the maleimide group. At the end of 1 h, 5 μL of 1 M cysteine solution was added to the reaction mixture and further incubated at 22 °C for 20 min. The mixture was then directly applied to the top of a 20 mL Sepharose CL-4B gel filtration column that was already equilibrated in PBS to remove excess free proteins not bound to liposomes. The sample was allowed to enter the column by gravity and then eluted with PBS at a flow rate of 0.35 mL/min at 4 °C. Absorbance at 280 nm was used to indicate fractions containing liposomes, which were further verified offline using Stewart assay as we described recently.11 The fractions with most of the liposomes were filtered through a 0.45 μm pore size membrane and stored at 4 °C. The molar concentrations of liposomes were determined based on Stewart assay for measurement of phospholipid content and geometric considerations of spherical lipid bilayers as we described recently without any modifications.11 The average diameters of the liposomes were measured using dynamic light scattering for each liposomal sample using Malvern Zetasizer Nano ZSP at 20 °C.
Quantitation of RBD Density on Liposomes.
Quantitation of RBD density follows the ensemble method that we described previously that was validated by single-molecule measurements.7 Briefly, the RBD density, or the average number of RBD molecules per liposomal particle, was determined by measuring both the protein concentration and the liposome concentration of the sample in molarity. To determine both concentrations, the liposome sample was first purified through SEC to remove free proteins. The purified liposome was then loaded onto a 15% (29:1 acrylamide/Bis) reducing SDS-polyacrylamide gel, together with a set of known quantities of RBD proteins, ranging from 5 to 200 ng, for construction of a standard curve. A minimum of eight reference points were included in each standard curve. The gel was stained with Sypro Red fluorescent dye, and the fluorescence was imaged using a multimode Typhoon scanner. The band intensities were quantitated using ImageQuant 5.0 (Molecular Dynamics). When FBS was included with liposomes, the gel was electrotransferred onto a supported nitrocellulose membrane and then further probed by western blotting. The primary antibody used for the detection of RBD was mouse anti-RBD monoclonal antibodies (CAT#-MAB10540, R&D Systems), which was diluted at 1:1000. The secondary antibody was anti-mouse alkaline phosphatase-conjugated secondary antibody (Santa Cruz Biotech), which was diluted at 1:2000. Protein bands were developed with the nitroblue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt substrates (NBT/BCIP, Roche) in a buffer containing 0.1 M Tris·HCl, 0.1 M NaCl, and 0.05 M MgCl2 at pH 9.5. The western blot was scanned using a Dell V305 all-in-one scanner, and the band intensities were quantitated using ImageJ (NIH, Bethesda, http://imagej.nih.gov/ij/). The concentration of proteins in the liposome sample was quantitated by comparison with the standard curve. All intensities to be measured were within the range of intensities shown by known reference samples. The average number of protein molecules per liposome was then taken as the ratio between the molar concentration of proteins and the molar concentration of liposomes.
Stability Assay for Liposomes.
The stability of liposomes under physiologically relevant conditions was measured as we described recently.11 Briefly, we mixed liposome samples with an equal volume of freshly thawed FBS on ice. An aliquot was taken immediately for both particle size and epitope density measurements as time zero. The mixture in a polypropylene test tube was then placed in an air-circulated incubator that was set at 37 °C. At a designated time, aliquots were taken out of the tube for both particle size and epitope density measurements. The original mixture in the test tube was immediately put back to the incubator for continued incubation and the rest of the time points. The RNA content within liposomes was determined using the same method as we established previously for DNA oligos in liposomes.11 Briefly, we ran each denatured sample in a 15% polyacrylamide gel (19:1 acrylamide/Bis) supplied with a TBE buffer. The gel was stained with SYBR Green II (ThermoFisher), and the fluorescence was imaged using a multimode Typhoon scanner. The band intensities were quantitated using ImageQuant 5.0 (Molecular Dynamics) and compared with a standard curve in the same gel constructed with the same RNA oligos of known quantities.
Mouse Immunization.
All animal procedures were approved by the University of Michigan Animal Care and Use Committee. Female BALB/c mice (8 weeks, Jackson Laboratory) were used for immunizations. All injection samples were filtered through a 0.45 μm pore size membrane to eliminate potential microbial contamination. One hundred microliters of samples were injected to each mouse subcutaneously, 50 μL on each flank. Mouse blood was collected submentally using a Microvette serum collection tube (Sarstedt) 3 days before immunization and 5 and 12 days after the immunization. The serum was harvested by centrifugation at 10 000g for 5 min, aliquoted, flash-frozen, and stored at −80 °C. Although in the current study, we did not report our results on mice immunization using pRBD that encapsulated single-stranded RNA oligos, the dose of RNA that can be offered by these sVLS would vary from 0.05 to 0.4 μg per animal based on our current dose for RBD proteins and the sVLS that we have characterized.
Enzyme-Linked Immunosorbent Assay (ELISA).
Blood serum was tested for ELISA to quantitate RBD-specific IgG responses to various immunizations. Ninety-six-well plates (Nunc MaxiSorp, Invitrogen) were coated overnight at 4 °C with 320 ng of sRBD per well in PBS. After blocking with 1% bovine serum albumin (BSA, Fisher) in PBS, mouse sera of specified dilution factors were added to each well for incubation at 22 °C for 2 h. After three washes using PBS with 0.05% Tween-20, secondary goat anti-mouse-IgG Fc-HRP antibody (# 1033-05, Southern Biotech) was added in the blocking buffer at 1:6000 dilution and incubated for 1 h at 22 °C. Following three washes, 100 μL of the substrate 3,3′,5,5′-tetramethylbenzidine (Thermal Scientific) was added to each well and incubated in the dark for 10 min. The reaction was stopped by the addition of 100 μL of 2 M sulfuric acid in each well. The optical density of each well at 450 nm was measured using a microplate reader (Bio-Tek Synergy HT). All of the OD values reported were background-subtracted by comparison between two wells that were coated with sRBD and PBS, respectively.
To determine the titers of anti-RBD IgG, we used a serial dilution of serum (from 1:100 dilution to 1:1 000 000 by a dilution factor of 10) for ELISA with the sRBD protein. Cutoff values were calculated using the following equation as reported:37 cutoff = + SD·f, where and SD are the mean and standard deviation of control well OD reading values and f is the standard deviation multiplier corresponding to different confidence levels. Specifically, in our assays, f = 2.631 when the number of control wells was 4 and confidence level was 95%. The titer value was determined as the highest dilution factor of the serum that still yielded an OD450 value higher than the above cutoff value in ELISA.
Preparation of HIV-1 Virions Pseudotyped with SARS-CoV-2 Envelope.
The HIV-1 pseudotyped with SARS-CoV-2 envelope was prepared following the published protocols38 but with modifications. Briefly, HEK 293T/17 cells (ATCC, Manassas, VA) were cultured at 37 °C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS (HyClone Laboratories, Logan, UT). Typically, 106 293T cells in a 2 mL of culture volume were seeded overnight in a 35 mm dish before transfection using the TransIT LT-1 transfection reagent (Mirus Bio, Madison, WI). For each dish, 2 μg of the provirus-containing plasmid pNL4-3 luc R−E−39 (ARP-3418, NIH AIDS Research and Reference Reagent Program) was used to make the transfection reagent mixture, together with 1 μg of envelope expression plasmid pcDNA3.1 SARS-CoV-2 S D614G.38 The plasmid pcDNA3.1 SARS-CoV-2 S D614G was a gift from Jeremy Luban (Addgene plasmid # 158075; http://n2t.net/addgene:158075; RRID:Addgene_158075). The RBD amino acid sequence 328–537 encoded in this plasmid is identical to the sequence of RBD that we studied here. The transfection reagent mixture was incubated at room temperature for 15 min before dropwise addition to the culture media, as we did previously.40 At 6 h post transfection, the culture media together with the transfection reagents was replaced with fresh complete media, and the incubation was continued at 37 °C with 5% CO2. At 48 h post transfection, the entire culture media containing single-cycle HIV-1 viruses was collected and filtered through a 0.45 μm syringe filter (Millex-HV poly- (vinylidene difluoride) (PVDF), Millipore). The filtrate was then aliquoted on ice, flash-frozen in liquid nitrogen, and stored in a −80 °C freezer. The concentration of virion particles was quantitated using an HIV-1 p24 ELISA kit (CAT#XB-1000, XpressBio) as we described previously.40
Virion Neutralization Assay.
Virion neutralization assay follows the protocols we established previously but with important modifications.40 HIV-1 virions pseudotyped with SARS-CoV-2 envelope containing 18 ng of HIV-1 p24 were incubated with various mouse sera at 20 °C for 1 h and then diluted with complete media by 25-fold in volume for the sera to initiate infection of Huh-7.5 cells at 37 °C for 2 h. At the end of 2 h, fresh media was added to each well in a 12-well plate, and the incubation was continued at 37 °C with 5% CO2. Luciferase activity was measured 48 h after infection. Briefly, culture media was removed and replaced with 100 μL of complete media. One hundred microliters of Bright-Glo reagent (CAT#E2610, Promega) that was just warmed up to room temperature was then added to each well. The cells were incubated for 3 min at room temperature to allow cell lysis. At the end of 3 min, 100 μL of lysate from each well was transferred to a single well in a 96-well black microtiter plate (Costar). Luminescence was measured using a Synergy HT multimode plate reader (BioTek Instruments Inc., Vermont), and background luminescence was subtracted using Huh-7.5 cells without virus infection. For comparison among different immunization groups, the luminescence readings for cells incubated with sera from PBS-injected mice were set as 100%. The luminescence readings from other groups were all normalized based on this and plotted as a percentage of the remaining infectivity.
ACKNOWLEDGMENTS
This work was supported by an NIH/NIAID grant (1R01AI155653-01A1) to W.C. The authors thank Dr. Charlie Rice at Rockefeller University for the kind gift of Huh-7.5 cells. The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): pNL4-3.Luc.R-E- from Dr. Nathaniel Landau. We thank Alex Meyer for a careful proofreading of the manuscript.
ABBREVIATIONS
- SARS-CoV-2
the severe acute respiratory syndrome coronavirus 2
- RBD
receptor-binding domain
- RBD-Ala
RBD that carries a cysteine to alanine mutation close to the C-terminus of the engineered RBD
- sRBD
free RBD proteins in solution
- pRBD
liposomes that display RBD at varied epitope densities
- Ni-NTA
nickel-nitrilotriacetic acid
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- DSPE-PEG maleimide
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] ammonium salt
Footnotes
The authors declare no competing financial interest.
Contributor Information
Wei-Yun Wholey, Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, Michigan 48109, United States.
Sekou-Tidiane Yoda, Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, Michigan 48109, United States.
Wei Cheng, Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, Michigan 48109, United States; Department of Biological Chemistry, University of Michigan Medical School, Ann Arbor, Michigan 48109, United States.
REFERENCES
- (1).Wu F; Zhao S; Yu B; Chen YM; Wang W; Song ZG; Hu Y; Tao ZW; Tian JH; Pei YY; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tay MZ; Poh CM; Renia L; MacAry PA; Ng LFP The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol 2020, 20, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Carvalho T; Krammer F; Iwasaki A The first 12 months of COVID-19: a timeline of immunological insights. Nat. Rev. Immunol 2021, 21, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cheng W The Density Code for the Development of a Vaccine? J. Pharm. Sci 2016, 105, 3223–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Knipe DM; Howley PM Fields Virology, 6th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, 2013. [Google Scholar]
- (6).Checkley MA; Luttge BG; Freed EO HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J. Mol. Biol 2011, 410, 582–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chen Z; Moon JJ; Cheng W Quantitation and Stability of Protein Conjugation on Liposomes for Controlled Density of Surface Epitopes. Bioconjugate Chem. 2018, 29, 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bachmann MF; Rohrer UH; Kundig TM; Burki K; Hengartner H; Zinkernagel RM The influence of antigen organization on B cell responsiveness. Science 1993, 262, 1448–1451. [DOI] [PubMed] [Google Scholar]
- (9).Chackerian B; Durfee MR; Schiller JT Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J. Immunol 2008, 180, 5816–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chackerian B; Lowy DR; Schiller JT Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J. Clin. Invest 2001, 108, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wholey WY; Mueller JL; Tan C; Brooks JF; Zikherman J; Cheng W Synthetic Liposomal Mimics of Biological Viruses for the Study of Immune Responses to Infection and Vaccination. Bioconjugate Chem. 2020, 31, 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cui J; Schlub TE; Holmes EC An allometric relationship between the genome length and virion volume of viruses. J. Virol 2014, 88, 6403–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhou P; Yang XL; Wang XG; Hu B; Zhang L; Zhang W; Si HR; Zhu Y; Li B; Huang CL; Chen HD; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wrapp D; Wang N; Corbett KS; Goldsmith JA; Hsieh CL; Abiona O; Graham BS; McLellan JS Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gao Q; Bao L; Mao H; Wang L; Xu K; Yang M; Li Y; Zhu L; Wang N; Lv Z; et al. Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yuan M; Wu NC; Zhu X; Lee CD; So RTY; Lv H; Mok CKP; Wilson IA A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wrapp D; De Vlieger D; Corbett KS; Torres GM; Wang N; Van Breedam W; Roose K; van Schie L; Team V-CC-R; Hoffmann M; et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell 2020, 1004.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lan J; Ge J; Yu J; Shan S; Zhou H; Fan S; Zhang Q; Shi X; Wang Q; Zhang L; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 215. [DOI] [PubMed] [Google Scholar]
- (19).Wang Q; Zhang Y; Wu L; Niu S; Song C; Zhang Z; Lu G; Qiao C; Hu Y; Yuen KY; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 894.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Shang J; Ye G; Shi K; Wan Y; Luo C; Aihara H; Geng Q; Auerbach A; Li F Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Yan R; Zhang Y; Li Y; Xia L; Guo Y; Zhou Q Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Shen H; Forgacs D; Chapla D; Moremen KW; Wells L; Hamer SA; Tompkins SM; Ross TM; Rouphael N; Edupuganti S; et al. A flexible, pan-species, multi-antigen platform for the detection and monitoring of SARS-CoV-2-specific antibody responses. medRxiv 2021, No. 2021.01.20.21249279. [Google Scholar]
- (23).DeSantis MC; Kim JH; Song H; Klasse PJ; Cheng W Quantitative Correlation between Infectivity and Gp120 Density on HIV-1 Virions Revealed by Optical Trapping Virometry. J. Biol. Chem 2016, 291, 13088–13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bjornson KP; Moore KJ; Lohman TM Kinetic mechanism of DNA binding and DNA-induced dimerization of the Escherichia coli Rep helicase. Biochemistry 1996, 35, 2268–2282. [DOI] [PubMed] [Google Scholar]
- (25).Ke Z; Oton J; Qu K; Cortese M; Zila V; McKeane L; Nakane T; Zivanov J; Neufeldt CJ; Cerikan B; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chen Z; Wholey WY; Hassani Najafabadi A; Moon JJ; Grigorova I; Chackerian B; Cheng W Self-Antigens Displayed on Liposomal Nanoparticles above a Threshold of Epitope Density Elicit Class-Switched Autoreactive Antibodies Independent of T Cell Help. J. Immunol 2020, 204, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Schmidt F; Weisblum Y; Muecksch F; Hoffmann HH; Michailidis E; Lorenzi JCC; Mendoza P; Rutkowska M; Bednarski E; Gaebler C; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med 2020, 217, No. e20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Watson DS; Endsley AN; Huang L Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Huang WC; Zhou S; He X; Chiem K; Mabrouk MT; Nissly RH; Bird IM; Strauss M; Sambhara S; Ortega J; et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv. Mater 2020, 32, No. 2005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kang YF; Sun C; Zhuang Z; Yuan RY; Zheng Q; Li JP; Zhou PP; Chen XC; Liu Z; Zhang X; et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates. ACS Nano 2021, 15, 2738–2752. [DOI] [PubMed] [Google Scholar]
- (31).Ma X; Zou F; Yu F; Li R; Yuan Y; Zhang Y; Zhang X; Deng J; Chen T; Song Z; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315.e9–1330.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Li H; Guo L; Zheng H; Li J; Zhao X; Li J; Liang Y; Yang F; Zhao Y; Yang J; et al. Self-Assembling Nanoparticle Vaccines Displaying the Receptor Binding Domain of SARS-CoV-2 Elicit Robust Protective Immune Responses in Rhesus Monkeys. Bioconjugate Chem. 2021, 32, 1034–1046. [DOI] [PubMed] [Google Scholar]
- (33).Walls AC; Fiala B; Schafer A; Wrenn S; Pham MN; Murphy M; Tse LV; Shehata L; O’Connor MA; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367.e17–1382.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hope MJ; Bally MB; Webb G; Cullis PR Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta, Biomembr 1985, 812, 55–65. [DOI] [PubMed] [Google Scholar]
- (35).Mayer LD; Hope MJ; Cullis PR Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta, Biomembr 1986, 858, 161–168. [DOI] [PubMed] [Google Scholar]
- (36).Olson F; Hunt CA; Szoka FC; Vail WJ; Papahadjopoulos D Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta, Biomembr 1979, 557, 9–23. [DOI] [PubMed] [Google Scholar]
- (37).Frey A; Di Canzio J; Zurakowski D A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [DOI] [PubMed] [Google Scholar]
- (38).Yurkovetskiy L; Wang X; Pascal KE; Tomkins-Tinch C; Nyalile TP; Wang Y; Baum A; Diehl WE; Dauphin A; Carbone C; et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 2020, 183, 739.e8–751.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Connor RI; Chen BK; Choe S; Landau NR Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995, 206, 935–944. [DOI] [PubMed] [Google Scholar]
- (40).Kim JH; Song H; Austin JL; Cheng W Optimized Infectivity of the Cell-Free Single-Cycle Human Immunodeficiency Viruses Type 1 (HIV-1) and Its Restriction by Host Cells. PLoS One 2013, 8, No. e67170. [DOI] [PMC free article] [PubMed] [Google Scholar]





