Abstract
Aim
We aimed to examine the gut permeability in patients with schizophrenia and its relevance to schizophrenia symptoms, medication, cognitive functions, and blood immune markers.
Methods
We selected 22 patients with schizophrenia (mean age: 37.9 ± 10.5 years) comprising 9 men and 13 women. Furthermore, we included 86 healthy controls (mean age: 43.5 ± 11.0 years) comprising 41 men and 45 women. All participants were biologically unrelated and of Japanese descent. We used the Positive and Negative Syndrome Scale (PANSS) and Brief Assessment of Cognition in Schizophrenia (BACS) to measure the severity of schizophrenia symptoms and cognitive functions, respectively. The lactulose‐mannitol loading test was used to measure the permeability of the small intestine. Furthermore, we used the lactulose to mannitol ratio (LMR) as an index of gut permeability. We measured the C‐reactive protein and natural killer (NK) cell activity in the blood as highly sensitive immune markers.
Results
The patients had a significantly higher rate of “leaky gut” (defined as LMR ≥ 0.1) compared to the control group (22.7% vs. 5.8%, odds ratio: 4.8 [95% confidence interval, 1.2‐18.3], Fisher's exact test, P = 0.03). There was no significant correlation between the LMR and PANSS scores or in the daily antipsychotic dose. In addition, the LMR was negatively correlated with the total Z‐score of the BACS and NK cell activity in the patients.
Conclusions
Our results suggest a higher rate of abnormally increased gut permeability in patients with schizophrenia than in controls. Moreover, gut permeability may be related to the cognitive and cellular immunity function of patients with schizophrenia.
Keywords: cognitive function, gut permeability, lactulose‐mannitol test, natural killer cell activity, schizophrenia
Frequency of “leaky gut” was increased in patients with schizophrenia. Gut permeability showed a negative correlation with cognitive function in patients. Gut permeability correlated negatively with natural killer cell activity in patients.

1. INTRODUCTION
There is accumulating evidence on the close relationship between the gut and the brain (brain‐gut correlation) involving the nervous, hormonal, and immune systems. Moreover, the gut bacteria strongly regulate the aforementioned association.1, 2, 3 Several studies have examined the relationship between the gut environment and various neuropsychiatric diseases, such as mood disorders,4, 5, 6, 7 autistic spectrum disorders,8, 9 and schizophrenia.10, 11, 12
The intestines are equipped with numerous defense mechanisms because they are considered foreign entities by living organisms. 13 However, dysbiosis of the intestinal bacteria can damage the tight junction of the intestinal epithelial cells, thus causing a “leaky gut.” Furthermore, few studies have examined the possible relationship between schizophrenia and the intestinal environment or gut microbiota, focusing on gut permeability.
An early study by Wood et al 14 reported on a significantly higher permeability of the small intestine in patients with schizophrenia (N = 17), compared to those with non‐schizophrenic psychiatric diseases (N = 15). The researchers used the cellobiose/mannitol test, thereby highlighting the possible role of increased gut permeability in the pathophysiology of the disease. In contrast, Lambert et al 15 found no significant difference between patients (N = 24) and healthy controls (N = 43), based on the 51Cr ethylenediaminetetraacetic acid (EDTA) test findings. Despite the difference in the methods used to assess gut permeability, the aforementioned results are inconsistent. No study has directly measured the permeability of the small intestine in patients with schizophrenia. However, there is indirect evidence based on an increase in inflammation markers in the intestinal mucosa.14, 15, 16, 17 Furthermore, the lactulose‐mannitol test (LMT) is considered a standard laboratory test to assess gut permeability.18, 19, 20 This can be attributed to the fact that both lactulose and mannitol are essentially harmless and are not metabolized in the human body.21, 22 In contrast, the presence of cellobiose degrading enzymes in the human body and the radioactivity in the 51Cr EDTA test have decreased the use of the above‐mentioned agents. However, no study has used the LMT to compare patients with schizophrenia and healthy controls.
The intestinal environment is associated with immune markers such as high sensitivity C‐reactive protein (hs‐CRP) and natural killer (NK) cell activity.23, 24 Alteration of these immune markers has been implicated in the pathophysiology of schizophrenia.25, 26, 27 To our knowledge, however, no study has been conducted to examine the relationship between gut permeability and such makers in schizophrenia.
We aimed to examine an alteration in the gut permeability of patients with schizophrenia. In addition, we investigated the relationship of gut permeability with clinical variables, such as the severity of schizophrenia symptoms, medication dose, and cognitive function. We also examined the relationship of gut permeability with hs‐CRP and NK cell activity.
2. METHODS
2.1. Subjects
Our study subjects included 22 patients with schizophrenia (mean age: 37.9 ± 10.5 years, 9 men and 13 women) and 86 healthy controls (mean age: 43.5 ± 11.0 years, 41 men and 45 women). All subjects were of Japanese descent. The participants were recruited at the National Center of Neurology and Psychiatry (NCNP) by means of advertisements at the NCNP Hospital, on our website, and in local free magazines. A board‐certificated research psychiatrist or a trained psychologist screened the participants for psychiatric disorders using the Japanese version of the Mini International Neuropsychiatric Interview (MINI).28, 29 They determined consensus diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, 30 MINI criteria, additional unstructured interviews, and medical records (if available). The healthy controls had no history of contact with any psychiatric service. Moreover, their total score for the Center for Epidemiologic Studies Depression Scale was <16. Participants with a history of central nervous system diseases, severe head injury, substance abuse, or mental retardation were excluded. The study was performed in accordance with the tenets of the Declaration of Helsinki. 31 We obtained written informed consent from every participant after providing them with a description of the study. Our study protocol was approved by the ethics committee of the NCNP (A2016‐019).
2.2. Clinical assessments
We used the Positive and Negative Syndrome Scale (PANSS) to evaluate the symptoms.32, 33 The Brief Assessment of Cognition in Schizophrenia (BACS) was administered to assess their cognitive function. Moreover, daily doses of antipsychotics were converted to chlorpromazine‐equivalent doses.
2.3. Lactulose‐mannitol test
Mannitol is a small molecule absorbed through the intestinal epithelial cells. In contrast, lactulose is absorbed through the tight junctions (ie, the gaps between the walls of adjacent epithelial cells). The lactulose/mannitol ratio of the absorption rates (LMR) is the standard for determining the gut permeability index. We used the LMT kit of Genova Diagnostics. Moreover, the measurement was performed at this company. Detailed information on the LMT procedures is provided in the supplementary file. According to Camilleri et al, 18 “leaky gut” was defined according to an LMR ≥ 0.1.
2.4. Blood sampling
The study participants were requested to fast for at least 12 hours, following which we collected the blood samples at 10:00 am. The samples were treated according to the instruction of SRL, Inc. We measured hs‐CRP using the latex immunity nephelometry method. Furthermore, the NK cell activity was measured with the 51Cr‐release assay. All measurements were performed at SRL, Inc.
2.5. Statistical analyses
We used the chi‐square test to compare the categorical variables between the groups. The Shapiro‐Wilk test was used for an assessment of the normal distribution. As the distribution of LMR deviated from the normal distribution, we used the Mann‐Whitney U‐test to compare the LMR of the two groups. In addition, Spearman's rank correlation was used to assess the correlation between the results of LMT and Z‐converted scores of BACS or the PANSS (only for patients). All statistical tests were two‐tailed, and a P‐value <0.05 was deemed statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (IBM Japan, Ltd.).
3. RESULTS
Table 1 summarizes the clinical characteristics of the participants. There was no significant difference in the distribution of sex and educational attainment. Nonetheless, age, body mass index (BMI), and the rate of smoking were significantly different between the groups.
TABLE 1.
Demographic and clinical characteristics of the participants
| Patients (n = 22) | Controls (n = 86) | P | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Age (y) | 37.9 ± 10.5 | 23‐57 | 43.5 ± 11.0 | 20‐62 | 0.03* |
| Sex, male (%) | 9 (40.9) | 41 (47.7) | 0.64 | ||
| Body mass index (kg/m2) | 24.0 ± 4.0 | 17.9‐37.0 | 21.8 ± 3.0 | 15.8‐30.2 | 0.009** |
| Education (y) | 14.3 ± 2.1 | 9‐16 | 15.3 ± 2.3 | 8‐22 | 0.11 |
| Smoking, yes (%) | 5 (22.7) | 5 (5.8) | 0.03* | ||
| Age at onset (y) | 21.1 ± 7.4 | 6‐39 | |||
| Chlorpromazine‐equivalent dose (mg/d) | |||||
| Total | 263 ± 284 | 0‐1050 | |||
| Typical | 42 ± 115 | 0‐450 | |||
| Atypical | 221 ± 228 | 0‐638 | |||
| Drug free, n (%) | 7 (31.8) | ||||
| Positive and Negative Syndrome Scale | |||||
| Total | 55.4 ± 15.7 | 31‐79 | |||
| Positive | 13.2 ± 5.2 | 7‐24 | |||
| Negative | 14.0 ± 5.2 | 7‐25 | |||
| General | 28.2 ± 7.3 | 17‐42 | |||
Drug‐free refers to lack of psychotropic medication use.
**P < 0.01.
*P < 0.05.
The results of LMT are shown in Figure 1. There was no significant difference in LMR between men and women in the patients or controls (data are not shown). Moreover, there was no significant correlation between the LMR and age or BMI in either group. There was no significant difference in the LMR between smokers and non‐smokers in the groups. However, the rate of “leaky gut” defined by an LMR ≥ 0.1 was significantly higher in the patients (n = 5, 22.7%) than in the controls (n = 5, 5.8%) (Fisher's exact test, P = 0.03, odds ratio 4.8 [95% CI 1.2‐18.3]).
FIGURE 1.
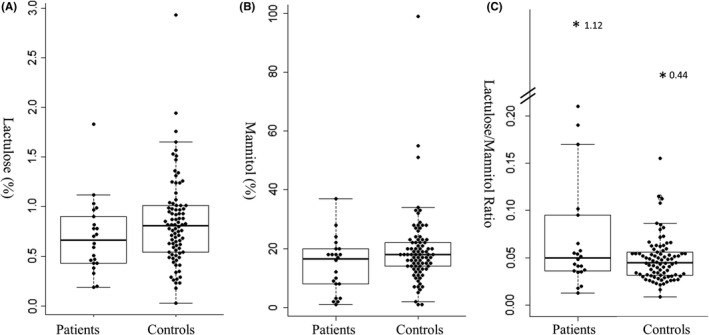
Comparing the results of the lactulose‐mannitol test between patients with schizophrenia and healthy controls. Rate of lactulose excretion (A), rate of mannitol excretion (B), and ratio of lactulose to mannitol (C). The bold horizontal bars represent the median. The boxes represent the interquartile range. Lactulose and mannitol values were calculated based on the percentage ratio of the amount excreted in urine to that of oral intake. An asterisk indicates an outlier, and the number next to that represents the value of lactulose/mannitol ratio
There was no significant correlation between the LMR and the daily dose of antipsychotic medication (ρ = 0.13, P = 0.56). However, we observed a negative correlation between the daily dose of antipsychotic medication and mannitol (ρ=−0.39, P = 0.07) (Table S1).
We investigated the possible relationship of LMR with schizophrenia severity, as assessed with the PANSS (total, positive, negative, and general scores). However, there was no significant correlation with any of the PANSS scores. Nonetheless, LMR was negatively correlated with cognitive function, as assessed by the total Z‐score (ρ = −0.52, P = 0.02. n = 21) (Table 2). Among the six subscales of the BACS, the LMR was negatively correlated with “Attention” (ρ = −0.37, P = 0.099, n = 21) and “Executive function” (ρ = −0.40, P = 0.07, n = 21) at trend level. There was no significant correlation between the total Z‐scores and daily dose of antipsychotics (ρ = −0.02, P = 0.93, n = 21), thus indicating our failure to ascribe the observed correlation between the LMR and cognitive function to antipsychotic medications.
TABLE 2.
Correlation between the lactulose/mannitol ratio and Z‐scores of the Brief Assessment of Cognition in Schizophrenia
|
Patients n = 21 |
||
|---|---|---|
| ρ | P | |
| Composite score | −0.52* | 0.02 |
| Verbal memory | −0.37 | 0.10 |
| Working memory | −0.13 | 0.58 |
| Motor speed | −0.17 | 0.47 |
| Verbal fluency | −0.25 | 0.27 |
| Attention | −0.37† | 0.099 |
| Executive function | −0.40† | 0.07 |
*P < 0.05.
† P < 0.10.
We calculated Spearman's rank correlation coefficients of the LMR with the immune markers (hs‐CRP and NK cell activity) (Table 3). This, in turn, facilitated determination of the relationship between gut permeability and inflammation and immune functions. LMR was negatively associated with the NK cell activity in patients (ρ = −0.43, P = 0.04). Nonetheless, such a correlation was absent in the control group. The Mann‐Whitney U‐test showed no significant difference between the groups for either hs‐CRP or NK cell activity (Table S2). Furthermore, Spearman's rank correlation was used to assess the relationship between the blood test values and the total PANSS scores and antipsychotic medications. However, there was no significant correlation for any of the aforementioned combinations (data are not shown).
TABLE 3.
Correlation between the lactulose/mannitol ratio and inflammatory and immune markers
| Group | ρ | P | n |
|---|---|---|---|
| Schizophrenia | |||
| C‐reactive protein | −0.03 | 0.90 | 22 |
| Natural killer cell activity | −0.43* | 0.04 | 22 |
| Control | |||
| C‐reactive protein | 0.06 | 0.61 | 75 |
| Natural killer cell activity | −0.05 | 0.66 | 75 |
Eleven subjects in the control group refused to undergo blood tests.
*P < 0.05.
4. DISCUSSION
This is the first study to compare gut permeability in patients with schizophrenia and healthy subjects using the LMT. We further examined the relationship of LMT with the severity of illness, medication dose, cognitive function, and plasma immune markers. The patients were more likely to have a “leaky gut” (LMR ≥ 0.1) than the healthy controls.
The mannitol values displayed a trend toward a negative correlation with the antipsychotic doses. This, in turn, possibly indicated a decrease in the absorption speed in patients who consumed higher doses of antipsychotics. However, the LMR was not significantly correlated with the antipsychotic doses. Therefore, the high frequency of “leaky gut” in the patients could not be entirely ascribed to the antipsychotic medications. Moreover, there was no association between the lactulose levels and the antipsychotics.
The LMR was negatively associated with the total Z‐scores of BACS. In addition, it showed a negative correlation with “Attention” and “Executive function” at a trend level. Hence, gut permeability might be related to cognitive decline in patients with schizophrenia. Moreover, a recent study reported on the recovery of cognitive function after the improvement of gut permeability in patients with cirrhosis. 34 Thus, interventions to enhance gut permeability might improve cognitive function in patients with schizophrenia. In addition, an animal study on old, obese mice provided evidence for the above‐mentioned possibility. 35
According to previous studies, the elevation of gut permeability increases the CRP.36, 37 However, there was no significant correlation between LMR and hs‐CRP in the present study. Thus, these inconsistent results necessitate further investigations.
The negative correlation between the LMR and NK cell activity was consistent with previous findings that highlighted the impact of chronic stress on the weakening of NK cell activity.38, 39, 40 This can be attributed to the stress posed by the penetration of materials because of reduced intestinal barrier function. Inflammatory bowel diseases (IBDs) and irritable bowel syndrome are closely related to a “leaky gut”. 41 IBDs, such as ulcerative colitis and Crohn's Disease show decreased NK cell activity. 42 Previous studies25, 26 have reported on reduced NK cell activity in patients with schizophrenia compared to controls. However, there are also contradictory negative reports. 43 Despite negative findings,44, 45 there is some evidence for changes in NK cell activity in a state‐dependent manner.46, 47 According to our results, altered gut permeability is related the reduced NK cell activity in schizophrenia. It is unclear why the significant inverse correlation between gut permeability and NK cell activity was observed in the patients with schizophrenia but not in the healthy controls. However, NK cell activity is known to decrease by chronic psychologic and/or physical stress, such as occupational stress, social conflicts, and smoking.48, 49 Patients with schizophrenia are vulnerable to various stressors50, 51 and the amount of chronic stress in patients with schizophrenia was likely to be greater than in healthy controls. For this reason, it is possible that the stress‐inducing effect of excessive gut permeability might be more exaggerated in schizophrenia patients, compared with the controls. It is also conceivable that gut environment, including the gut microbiota, might be different between schizophrenia and controls,52, 53 which may explain the observed differential effects of gut permeability on NK cell activity.
The cross‐sectional nature of our study precludes inference as to whether increased gut permeability is causal to schizophrenia. However, increased gut permeability leads to chronic inflammation in the body, and systemic inflammation, in turn, leads to neural inflammation, which is involved in the pathophysiology of schizophrenia.35, 54, 55, 56 Since NK cells play an important role in protection against infections and toxic substances, 57 decreased NK activity may further exaggerate the inflammation.
4.1. Limitations
Our study had several limitations. First, the number of patients with schizophrenia was relatively small (N = 22). This might have subjected our findings to type II errors. Second, there was a significant, albeit small, difference in the age distribution between the groups. However, there was no significant correlation between age and LMR. Therefore, the observed difference in LMR is an unlikely consequence of the age difference. Third, most patients were under medication, which might have exerted some effect on the LMR. Fourth, our patients represented a relatively milder form of the illness (mean PANSS score of 55.4). Therefore, patients with severe disease might possibly have extreme alterations in intestinal permeability. The aforementioned limitations necessitate further studies with a larger sample size including drug naïve patients.
5. CONCLUSION
In conclusion, patients with schizophrenia were shown to have a higher rate of abnormally increased gut permeability compared to their healthy counterparts. Moreover, there may be a correlation between gut permeability and the cognitive and cellular immunity function of the patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
HK designed and supervised the study, analyzed gut permeability data, and performed the statistical analyses, as well as wrote the manuscript approved by all authors. II recruited and performed cognitive tests, collected and analyzed gut permeability data, and performed the statistical analyses, as well as wrote the manuscript approved by all authors. SH, MO, SY, and YY made the diagnoses and evaluated the participants’ symptoms. JM recruited and performed cognitive tests. EA and JO collected gut permeability data.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The ethics committee of the NCNP approved this study protocol (A2016‐019).
INFORMED CONSENT
All of the participants provided written informed consent.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/a.
ANIMAL STUDIES
N/a.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
We thank all study participants.
Ishida I, Ogura J, Aizawa E, Ota M, Hidese S, Yomogida Y, et al. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol Rep. 2022;42:70–76. 10.1002/npr2.12227
Funding information
This work was supported by the Strategic Research Program for Brain Sciences from the Japan Agency for Medical Research and Development, AMED (Grant number 17dm0107100h0002 for H. K.). This funding source was involved only in the financial support
DATA AVAILABILITY STATEMENT
According to the study protocol approved by the institutional ethics committee, we did not obtain consent from the subjects as to publication of individual data. Therefore, we cannot disclose the individual data to the public. However, we will respond to data disclosure if there is a request from other researchers.
REFERENCES
- 1. Callaghan BL, Fields A, Gee DG, Gabard‐Durnam L, Caldera C, Humphreys KL, et al. Mind and gut: associations between mood and gastrointestinal distress in children exposed to adversity. Dev Psychopathol. 2020;32(1):309–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota‐gut‐brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 3. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. 2015;28(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5. Gondalia S, Parkinson L, Stough C, Scholey A. Gut microbiota and bipolar disorder: a review of mechanisms and potential targets for adjunctive therapy. Psychopharmacol. 2019;236(5):1433–43. [DOI] [PubMed] [Google Scholar]
- 6. Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–7. [DOI] [PubMed] [Google Scholar]
- 7. Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Bifidobacterium and Lactobacillus counts in the gut microbiota of patients with bipolar disorder and healthy controls. Front Psychiatry. 2018;9:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22(1):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. [DOI] [PubMed] [Google Scholar]
- 10. Karakuła‐Juchnowicz H, Dzikowski M, Pelczarska A, Dzikowska I, Juchnowicz D. The brain‐gut axis dysfunctions and hypersensitivity to food antigens in the etiopathogenesis of schizophrenia. Psychiatr Pol. 2016;50(4):747–60. [DOI] [PubMed] [Google Scholar]
- 11. Rodrigues‐Amorim D, Rivera‐Baltanás T, Regueiro B, Spuch C, de Las Heras ME, Vázquez‐Noguerol Méndez R, et al. The role of the gut microbiota in schizophrenia: current and future perspectives. World J Biol Psychiatry. 2018;19(8):571–85. [DOI] [PubMed] [Google Scholar]
- 12. Zhuo C, Yao Y, Xu Y, Liu C, Chen M, Ji F, et al. Schizophrenia and gut‐flora related epigenetic factors. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:49–54. [DOI] [PubMed] [Google Scholar]
- 13. Tanemoto S, Sujino T, Kanai T. Intestinal immune response is regulated by gut microbe. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(6):408–15. [DOI] [PubMed] [Google Scholar]
- 14. Wood NC, Hamilton I, Axon AT, Khan SA, Quirke P, Mindham RH, et al. Abnormal intestinal permeability. An aetiological factor in chronic psychiatric disorders? Br J Psychiatry. 1987;150:853–6. [DOI] [PubMed] [Google Scholar]
- 15. Lambert MT, Bjarnason I, Connelly J, Crow TJ, Johnstone EC, Peters TJ, et al. Small intestine permeability in schizophrenia. Br J Psychiatry. 1989;155:619–22. [DOI] [PubMed] [Google Scholar]
- 16. Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camilleri M, Nadeau A, Lamsam J, Nord SL, Ryks M, Burton D, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22(1):e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juby LD, Rothwell J, Axon AT. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989;96(1):79–85. [DOI] [PubMed] [Google Scholar]
- 20. Kushak RI, Buie TM, Murray KF, Newburg DS, Chen C, Nestoridi E, et al. Evaluation of intestinal function in children with autism and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2016;62(5):687–91. [DOI] [PubMed] [Google Scholar]
- 21. Clausen MR, Mortensen PB. Lactulose, disaccharides and colonic flora. Clinical consequences. Drugs. 1997;53(6):930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nissenson AR, Weston RE, Kleeman CR. Mannitol. West J Med. 1979;131(4):277–84. [PMC free article] [PubMed] [Google Scholar]
- 23. van den Munckhof ICL, Kurilshikov A, Ter Horst R, Riksen NP, Joosten LAB, Zhernakova A, et al. Role of gut microbiota in chronic low‐grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19(12):1719–34. [DOI] [PubMed] [Google Scholar]
- 24. Poggi A, Benelli R, Venè R, Costa D, Ferrari N, Tosetti F, et al. Human gut‐associated natural killer cells in health and disease. Front Immunol. 2019;10:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdeljaber MH, Nair MPN, Schork MA, Schwartz SA. Depressed natural killer cell activity in schizophrenic patients. Immunol Invest. 1994;23(4–5):259–68. [DOI] [PubMed] [Google Scholar]
- 26. DeLisi LE, Ortaldo JR, Maluish AE, Wyatt RJ. Deficient natural killer cell (NK) activity and macrophage functioning in schizophrenic patients. J Neural Transm. 1983;58(1–2):99–106. [DOI] [PubMed] [Google Scholar]
- 27. Miller BJ, Goldsmith DR. Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2017;42(1):299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, et al. Reliability and validity of Japanese version of the Mini‐International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59(5):517–26. [DOI] [PubMed] [Google Scholar]
- 29. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- 30. American Psychiatric Association . Diagnostic and statistical manual of mental misorders. American Psychiatric Association; 2013. [cited 2020 Oct 9]. Available from https://psychiatryonline.org/doi/book/ 10.1176/appi.books.9780890425596 [DOI]
- 31. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 32. Igarashi Y, Hayashi N, Yamashina M, Otsuka N, Kuroki N, Anzai N, et al. Interrater reliability of the Japanese version of the Positive and Negative Syndrome Scale and the appraisal of its training effect. Psychiatry Clin Neurosci. 1998;52(5):467–70. [DOI] [PubMed] [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. [DOI] [PubMed] [Google Scholar]
- 34. Román E, Nieto JC, Gely C, Vidal S, Pozuelo M, Poca M, et al. Effect of a multistrain probiotic on cognitive function and risk of falls in patients with cirrhosis: a randomized trial. Hepatol Commun. 2019;3(5):632–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmadi S, Razazan A, Nagpal R, Jain S, Wang B, Mishra SP, et al. Metformin reduces aging‐related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol A Biol Sci Med Sci. 2020;75(7):e9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiecolt‐Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. 2020;5(2):e134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt‐Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behav Neurosci. 1986;100(5):675–8. [DOI] [PubMed] [Google Scholar]
- 39. Halvorsen R, Vassend O. Effects of examination stress on some cellular immunity functions. J Psychosom Res. 1987;31(6):693–701. [DOI] [PubMed] [Google Scholar]
- 40. Locke SE, Kraus L, Leserman J, Hurst MW, Heisel JS, Williams RM. Life change stress, psychiatric symptoms, and natural killer cell activity. Psychosom Med. 1984;46(5):441–53. [DOI] [PubMed] [Google Scholar]
- 41. Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ginsburg CH, Dambrauskas JT, Ault KA, Falchuk ZM. Impaired natural killer cell activity in patients with inflammatory bowel disease: evidence for a qualitative defect. Gastroenterology. 1983;85(4):846–51. [PubMed] [Google Scholar]
- 43. Caldwell CL, Irwin M, Lohr J. Reduced natural killer cell cytotoxicity in depression but not in schizophrenia. Biol Psychiatry. 1991;30(11):1131–8. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez‐Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, Hatton A, et al. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment‐resistant schizophrenia. PLoS One. 2016;11(5):e0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):509–18. [DOI] [PubMed] [Google Scholar]
- 46. Sasaki T, Nanko S, Fukuda R, Kawate T, Kunugi H, Kazamatsuri H. Changes of immunological functions after acute exacerbation in schizophrenia. Biol Psychiatry. 1994;35(3):173–8. [DOI] [PubMed] [Google Scholar]
- 47. Sperner‐Unterweger B, Whitworth A, Kemmler G, Hilbe W, Thaler J, Weiss G, et al. T‐cell subsets in schizophrenia: a comparison between drug‐naive first episode patients and chronic schizophrenic patients. Schizophr Res. 1999;38(1):61–70. [DOI] [PubMed] [Google Scholar]
- 48. Capellino S, Claus M, Watzl C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol Immunol. 2020;17(7):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morimoto K, Takeshita T, Inoue‐Sakurai C, Maruyama S. Lifestyles and mental health status are associated with natural killer cell and lymphokine‐activated killer cell activities. Sci Total Environ. 2001;270(1–3):3–11. [DOI] [PubMed] [Google Scholar]
- 50. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Upthegrove R, Khandaker GM. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci. 2020;44:49–66. [DOI] [PubMed] [Google Scholar]
- 52. Butler MI, Mörkl S, Sandhu KV, Cryan JF, Dinan TG. The gut microbiome and mental health: what should we tell our patients?: Le microbiote Intestinal et la Santé Mentale : que Devrions‐Nous dire à nos Patients? Can J Psychiatry. 2019;64(11):747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Szeligowski T, Yun AL, Lennox BR, Burnet PWJ. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. 2020;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hidese S, Hattori K, Sasayama D, Tsumagari T, Miyakawa T, Matsumura R, et al. Cerebrospinal fluid inflammatory cytokine levels in patients with major psychiatric disorders: a multiplex immunoassay study. Front Pharmacol. 2020;11:594394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ishii T, Hattori K, Miyakawa T, Watanabe K, Hidese S, Sasayama D, et al. Increased cerebrospinal fluid complement C5 levels in major depressive disorder and schizophrenia. Biochem Biophys Res Commun. 2018;497(2):683–8. [DOI] [PubMed] [Google Scholar]
- 56. Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, et al. Increased cerebrospinal fluid interleukin‐6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47(3):401–6. [DOI] [PubMed] [Google Scholar]
- 57. O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43(4):634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
According to the study protocol approved by the institutional ethics committee, we did not obtain consent from the subjects as to publication of individual data. Therefore, we cannot disclose the individual data to the public. However, we will respond to data disclosure if there is a request from other researchers.


