Abstract
This study examined how short sleep impacts dietary consumption in adolescents by testing whether experimentally shortening sleep influences the amount, macronutrient content, food types, and timing of food consumed. Ninety-three adolescents completed a within-subjects crossover paradigm comparing five nights of short sleep (6.5-hour sleep opportunity) to five nights of Healthy Sleep (9.5-hour sleep opportunity). Within each condition, adolescents completed three multiple-pass dietary recalls that recorded the types, amount, and timing of food intake. The following outcomes were averaged across days of dietary recall within condition: kilocalories, grams of carbohydrates, fat, protein, and added sugars, glycemic load of foods, and servings of specific types of foods (low-calorie drinks, sweetened drinks, fruits/vegetables, meats/proteins, processed snacks, “fast food” entrees, grains, and sweets/desserts). Timing of consumption of kilocalorie and macronutrient outcomes were also examined across four noncumulative time bins: 06:00–10:59, 11:00–15:59, 16:00–20:59, and 21:00–01:00. Adolescents slept 2 h and 20 min longer in Healthy Sleep than in Short Sleep (p < .0001). While in Short Sleep, adolescents ate more grams of carbohydrates (p = .031) and added sugars (p = .047), foods higher in glycemic load (p = .013), and servings of sweet drinks (p = .023) and ate fewer servings of fruits/vegetables (p = .006) compared to Healthy Sleep. Differences in consumption of kilocalories, fat, and carbohydrates emerged after 9:00 pm (ps = .012, .043, .006, respectively). These experimental findings suggest that adolescents who have insufficient sleep exhibit dietary patterns that may increase the risk for negative weight and cardiometabolic outcomes. Future health promotion efforts should include promoting optimal sleep to increase healthy dietary habits.
Keywords: adolescent, sleep restriction, dietary outcomes, meal timing, obesity
Statement of Significance.
This mechanistic study (using the largest pediatric experimental sleep manipulation sample to date) demonstrated that short sleep increases adolescent consumption of carbohydrates and foods high in glycemic load, particularly in the late evening, compared to healthy sleep. This study uncovers a critical mechanism linking short sleep with increased obesity and cardiometabolic risk in adolescents and provides evidence for the implementation of pediatric sleep intervention in health promotion efforts. It is vital to continue to explore this relationship while examining additional outcomes of sleep beyond sleep duration (e.g. sleep timing, circadian alignment).
Introduction
Most adolescents are chronically sleep restricted on school nights, with over two-thirds of adolescents obtaining fewer than 8 h of sleep per night [1]. The resulting sleep debt appears to be related to multiple converging factors, including altered internal sleep mechanisms (i.e. delayed circadian phase, slowed sleep pressure accumulation), early school start times, and increased academic demands, screen exposure, evening social opportunities, and bedtime autonomy [2, 3]. Adolescents who obtain insufficient sleep are at risk for numerous negative mental and physical health outcomes [2, 4, 5], including heightened risk for the development of obesity [6] and cardiometabolic risk [7]. Recent review articles [8, 9] outline several potential mechanisms that may link shortened sleep with increased risk for developing obesity among adolescents, particularly highlighting the role of altered dietary mechanisms following shortened sleep.
Shortened sleep in adults appears to lead to increased caloric consumption, which over time could result in weight gain [10]. However, the adolescent literature is less conclusive [8]. Correlational findings on the relationship between adolescent sleep length and caloric intake have varied, with some demonstrating a positive correlation [11], no relationship [12], or a negative correlation [13]. Similarly, a limited amount of experimental research demonstrates that when adolescents have their sleep experimentally shortened, they demonstrate either no changes in caloric intake within artificial laboratory environments [14] or, as a pilot study from our group would suggest, increases in caloric consumption in naturalistic environments [15, 16]. Given the variability in these findings across research methodology and design, literature reviews have called for further examination of sleep and dietary outcomes using both internally valid and generalizable research methods [8, 9, 17].
More recently, there has been a shift from examining exclusively the amount of food consumed in relation to sleep restriction to the quality of the diet. Dietary quality has been conceptualized in a variety of ways, with some considering the macronutrients of the foods consumed (e.g. total fat, carbohydrate, and protein content), while others use categories such as food types (e.g. fast foods, fruits, vegetables, and sweets/desserts). Dietary quality may play a critical role in the development of cardiometabolic risk. For example, consuming foods higher in glycemic load, a measure of how quickly foods raise blood sugar levels, may decrease insulin sensitivity [18] and risk for obesity [19]. Currently, it is unclear as to whether shortened sleep impacts the macronutrient content of the diet in adolescents, with cross-sectional research demonstrating mixed results [8] and our previous experimental research observing no relationship between sleep duration and macronutrient content [16]. The literature is more consistent regarding sleep duration in relation to various food categories, particularly sweets and desserts. Our initial pilot work demonstrated that adolescents undergoing experimentally induced sleep restriction consumed more sweet/dessert foods than when they are well-rested [15, 16], though sleep restriction had no impact on consumption of other food types (e.g. grains, fast foods, eggs and meats, fruits and vegetables).
Research among adolescents (both cross-sectional and experimental) has largely ignored whether changes in sleep impact the time of day when food is consumed. In one study, sleep-restricted adults consumed fewer calories between 8:00 am and 2:59 pm but more calories between 10:00 pm and 3:59 am compared to when they were well-rested [20]. With observational studies suggesting links between obesity and energy intake concentrated later in the day [21, 22], such an effect could suggest increased risk for obesity even beyond total daily energy intake. Further, it could suggest a secondary point of intervention during periods of unavoidable sleep restriction. Finally, such findings might suggest that evening is the best time for future research to look for sleep-related dietary alterations.
Other factors must also be considered when exploring how sleep influences the adolescent diet. Very few studies that examine dietary outcomes following sleep restriction consider whether physical activity levels are also altered [8]. If increased intake is offset by increased calorie-burning moderate to vigorous physical activity, this could attenuate long-term health risk associated with energy balance. Furthermore, in noting the heterogeneity of findings, relevant review articles have called for exploration of whether the impact of sleep manipulations varies across individual factors (e.g. sex, race, and income) and elements of the experimental design (e.g. experimental order) [8, 9].
The first aim of this study was to replicate our prior work and reconcile inconsistencies noted in prior literature by examining the impact of experimental sleep restriction on the amount, macronutrient content, and types of food consumed by adolescents. Specifically, we compared the total caloric intake, macronutrient content of foods (i.e. grams of fat, proteins, carbohydrates, added sugars), glycemic load of foods, and servings of various food categories consumed (low-calorie drinks, sweet drinks, fruits/vegetables, meats/eggs, processed snacks, fast food, grains, and sweets/desserts) across multiple measurement occasions during five nights of short sleep (6.5 h sleep opportunity) and five nights of healthy sleep (9.5 h sleep opportunity). To mimic a schedule in which adolescents had to rise relatively early on school days, they maintained a consistent wake time but changed bedtimes to change sleep opportunities. We hypothesized that while in short sleep, adolescents would consume greater calories, more carbohydrates and added sugars (even after controlling for overall caloric intake), and more foods higher in glycemic load than while in the healthy sleep condition. We also hypothesized that adolescents undergoing short sleep would consume greater amounts of sweet/desserts compared to when they were well-rested.
For our second aim, we extended the research literature by examining how the timing of caloric and macronutrient consumption differed across the day by experimental condition. Based on adult findings, we hypothesized that adolescents would consume more calories and added sugars in the evening during short sleep, compared to healthy sleep.
Our third aim was to explore potential moderators to help determine under what circumstances dietary changes occur following sleep restriction relative to healthy sleep in adolescents. We were particularly interested in whether there were experimental or individual factors that increased the likelihood that adolescents would have negative changes in dietary outcomes following sleep restriction. We explored the moderating impact of sex, norm-adjusted body mass index (BMI), race, mid-point of sleep, income, experimental order, and the difference of how much adolescents slept across both experimental conditions for our first aim. Across all three aims, analyses were run before and after covarying for moderate-to-vigorous activity levels and, when appropriate, for energy intake within each condition.
Methods
The institutional review board at Cincinnati Children’s Hospital Medical Center approved all study procedures, which took place across four consecutive summers from 2015 to 2018 while participants were on break from school to minimize the likelihood of sleep restriction negatively impacting school performance. At the first study appointment, all parents provided permission for adolescents to complete the study procedures, and adolescents provided assent to participate. Participants and procedures have also been described in a prior article that reported on changes in the perceived reward value of foods but did not examine actual intake [23].
Participants
Healthy adolescents (ages 14–17) were recruited to participate in this research study through the use of social media advertisements, community flyers, and emails within a large pediatric hospital. Exclusion criteria included factors that may impact sleep or ability to adhere to sleep requirements as part of the study, including medications that may alter sleep outcomes (e.g. stimulants), self- or parent-report of organic sleep disorder symptoms assessed via a validated screener [24], highly atypical sleeping patterns (regularly sleeping <6 h or >10 h on school nights), or obligations that would prevent an adolescent from adhering to the prescribed bedtimes or wake times. Further exclusionary criteria included the presence of ongoing psychiatric disorders that could be negatively impacted by sleep restriction (e.g. bipolar disorder), history of neurological illness or injury (e.g. epilepsy, traumatic brain injury), intellectual disability or autism spectrum disorder, recurrent use of substances, BMI above 30, or daily caffeine consumption that exceeded more than a single cup of coffee/energy drink or more than two caffeinated sodas per day.
Study procedures
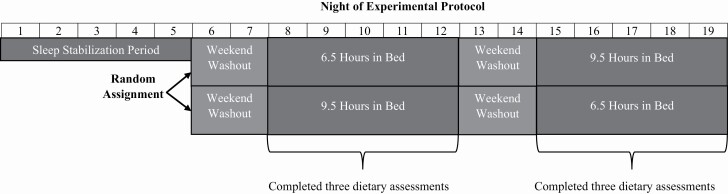
The 3-week study protocol is outlined in Figure 1 and has been summarized previously [23, 25]. Adolescents who were interested in participating in the study were screened for eligibility by study staff over the phone. After verbal consent, eligible adolescents were mailed study instructions, a sleep diary, and two accelerometers (one worn on the wrist for measuring sleep and the other on the hip for physical activity; see below). Each adolescent determined a wake-time that would allow for them to attend an 8:00 am study visit; this prescribed wake-time was held constant throughout the entire 3-week protocol. Bedtimes were manipulated across the study to either promote a Short Sleep (6.5-hour sleep opportunity) or Healthy Sleep (9.5-hour sleep opportunity) each day for five nights. This approach of keeping wake-time constant while manipulating bedtime was selected to mirror school-like waking patterns (i.e. consistently early wake-time due to school obligations, with bedtime more adjustable by the adolescent).
Figure 1.
Overview of the sleep manipulation protocol. Across the entire experimental paradigm, wake-time was held constant. Only bedtimes were manipulated to either extend or restrict time in bed. During the sleep stabilization period and weekend washout periods, adolescents were allowed to self-select their bedtime.
The first five nights of the experimental protocol were designed to stabilize sleep with the early rise time and to habituate to wearing the study devices. During this stabilization period, all adolescents woke at their prescribed wake time for the experiment, self-selected their bedtimes, wore their accelerometers, and completed a sleep diary. Following the stabilization period, adolescents attended their first in-person office visit, actigraphy was reviewed to determine adherence to wake-time instructions, basic demographic data were provided, and study measures were administered. The participants were then randomized to an experimental order in which they started the experimental protocol by undergoing either the Short Sleep condition (spending 6.5 h in bed for five nights) or the Healthy Sleep condition (spending 9.5 h in bed for five nights) first. The Healthy Sleep condition was designed to maximize chances that adolescents would meet consensus recommendations for nightly sleep duration [26], whereas the Short Sleep condition was designed to induce a level of sleep restriction that is common on school nights [1]. Following each experimental condition, adolescents returned for a Saturday morning office visit to review actigraphy, determine adherence, and complete study measures. For the two nights prior to each experimental condition (including the washout between conditions), adolescents returned to the sleep stabilization instructions; they were allowed to self-select their own bedtimes, while waking at their prescribed wake time. Within each Short Sleep and Healthy Sleep condition, adolescents completed three 24-hour dietary recalls (mean number of recalls = 5.88, SD = 0.44) over the phone, which were set to randomly occur on three of the five days across the first experimental condition. Recall days during the second experimental condition were yoked to the first; 97.6% of the recalls occurred on the same days of the week for both experimental conditions. During the entire study, adolescents were instructed to wear both accelerometers, fill out sleep diaries daily, avoid naps, and not to consume more than one cup of coffee or energy drinks or more than two caffeinated sodas.
Participants who completed the entire study participated in both the Short Sleep and Healthy Sleep condition, with the order randomly counterbalanced, and completed six dietary assessments. This general experimental approach was selected due to previous success in achieving adherence to the experimental protocol in a manner that mimics adolescents’ real-world sleep patterns [15, 16, 27].
Measures
Background information (descriptive information and exploratory moderators)
During the first Saturday morning assessment period, all adolescents and parents provided demographic information including family income, as well as participant age, sex, race, and ethnicity (using categories set by the US National Institutes of Health, which funded the project). Adolescent height and weight were measured in triplicate with research-quality calibrated equipment. These estimates were used to determine BMI (kg/m2), which was then converted to age- and sex-corrected BMI z-scores based on data from the US Centers for Disease Control and Prevention.
Sleep (adherence measure)
Sleep was monitored throughout each experimental condition using wrist-worn actigraphy (Motionlogger Micro Watch; Ambulatory Monitoring, Inc., Ardsley, NY), cross-checked against nightly diaries completed by the adolescent that included nightly bedtimes and daily waketimes. At each Saturday morning assessment, a member of the study staff met with each adolescent and parent to compare nightly diary and actigraphy estimates; in instances where the two sources conflicted, clarification was obtained. These meetings also served to identify artifacts (e.g. removal of watch) and to promote adherence to sleep instructions on subsequent nights. Actigraphy data for sleep were scored using the Sadah algorithm [28] to obtain estimates of sleep onset, sleep offset, sleep period duration (onset to offset, ignoring nocturnal arousals), and sleep efficiency (the percent of time spent asleep during the sleep period). As previously published, adherence to the sleep protocol was defined as nightly sleep that averaged at least 1 h longer in the Healthy Sleep Condition than the Short Sleep condition [15, 16, 23].
Physical activity (covariate)
Throughout the study, participants were asked to wear a waist-worn accelerometer (wGT3x-BT; ActiGraph, Pensacola, FL) during all waking hours. These accelerometers were fastened with an adjustable belt on the dominant hip. Participants were instructed to put on the accelerometer immediately upon waking, and to take off the accelerometer before getting into bed. They were also instructed to remove the accelerometer for activities that were high-impact or underwater. Data were downloaded in 30-second epochs and analyzed using Actilife software (ActiGraph, Pensacola, FL). A minimum of 10 h of recorded wear time were required for consideration as a valid day. For use as a covariate, the software extracted minutes/day spent in moderate-to-vigorous activity (≥ 2296 counts per minute) [29] averaged within each sleep condition for each participant.
Dietary assessments (primary outcomes)
Across each experimental condition, participants completed three 24-hour dietary recall interviews via telephone randomly out of the five days, with the interviews occurring across Tuesday through Saturday. These interviews were conducted by a trained member of the institution’s bionutrition research core who was blinded to randomization. These interviews used the USDA multiple-pass approach to assess for dietary consumption that occurred in the previous day, which consisted of asking which food items were consumed in each eating/drinking occasion across the day, portions of each food/drink item consumed, and timing and location of eating/drinking. This approach has been well validated in youth [30, 31], is relatively unobtrusive, reduces reporting bias [32], and allows staff to follow-up as needed to promote accuracy. This collected information produced nutritional data through the Nutrition Data Systems for Research (Nutrition Coordinating Center, University of Minnesota) software, and the following estimates for each eating occasion were obtained: kilocalories (Kcal), grams of protein, carbohydrates, fat, and added sugars, and glycemic load [33]. Each eating occasion was time-stamped, as to allow for mapping these primary outcomes across time each day. This allowed us to aggregate data within a day, across days within a given condition, and across time of day within a given condition, as needed.
Study staff also coded each food into the categories described by Beebe et al. [16]: low-calorie drinks (e.g. water, tea, coffee, and artificially sweetened drinks); sweetened drinks (e.g. soda, Frappuccino, and chocolate milk); fruits/vegetables (excluding potatoes); meats/proteins; processed snacks (e.g. crackers, French fries, and fruit snacks); “fast food” entrees; grains (e.g. bread, rice, and pasta); and sweets and desserts. Two research staff independently coded all food items to fall within one of these eight food categories. Initial interrater reliability was 0.93, with discrepancies reconciled via subsequent discussion. Number of servings for each food category consumed was summed across each day, and then averaged across all days for each experimental condition.
Analytic plan
Statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, NC). Preliminary analyses included descriptive analyses of sample demographics and sleep in each experimental condition, as well as paired-sample t-tests to examine within-participant sleep differences across those conditions. Preliminary analyses then examined whether there were systematic differences in day-to-day intake across each experimental condition via a linear mixed-effect model with condition-by-day interaction to examine whether the impact of sleep condition differed by experimental day on all primary dietary outcomes. As shown in the Supplemental Materials, condition-by-day effects were negligible, allowing us to simplify subsequent analyses by averaging dietary data across days within each experimental condition for each participant.
For our first aim, we conducted a series of mixed-effect models to determine the impact of sleep condition on Kcals consumed, macronutrient consumption (i.e. grams of fat, carbohydrates, protein, added sugars), glycemic load of food consumed, and daily servings of low-calorie drinks, sweetened drinks, fruits/vegetables, meats/eggs, processed snacks, fast food, grains, and sweets/desserts consumed. For our second aim, we created four noncumulative time bins (6:00 am–10:59 am, 11:00 am–3:59 pm, 4:00 pm–8:59 pm, and 9:00 pm–1:00 am) across a given day to model when caloric and macronutrient consumption was occurring. These time bins split at times when eating episodes were least frequent in this sample and roughly correspond to traditional periods for breakfast, lunch, dinner, and late-evening snacking. We then conducted condition (Short Sleep, Healthy Sleep) by time bin (6:00–10:59, 11:00–15:59, 16:00–20:59, and 21:00–1:00) linear mixed-modeling to determine the condition difference in Kcals, grams of fat, carbohydrates, and added sugars consumed. For analyses examining macronutrient consumption, we controlled averaged daily Kcal consumption and averaged daily physical activity. For analyses examining Kcal or food grouping servings, we covaried only for physical activity. We ran models with and without included covariates and reported significance values for each. Significance threshold for hypothesis-driven aims 1 and 2 was set at p = .05. For our third (exploratory) aim, we repeated all analyses to include the moderators of age, sex, race, income status, BMI z-score, experimental order, and the difference of sleep duration across both conditions, with a critical p-value of .005 to reduce the likelihood of obtaining a type 1 error given the large number of exploratory analyses. We evaluated model assumptions via residual plots. For all analyses, Cohen’s d estimates were established for estimates of effect size by using the mean and SD of within-subject differences between short sleep and healthy sleep.
Results
Participant characteristics
A total of 149 adolescents were recruited to participate in the study. Of these, 22 were excluded prior to randomization due to failure to adhere to the sleep parameters set during the stabilization week or because ineligibility criteria emerged (e.g. endorsed psychiatric symptoms during a structured interview conducted at the first in-person visit). Of the remaining 127 participants, 10 and 6 dropped out during the first and second experimental conditions, respectively. We also excluded 18 from analyses for nonadherence to sleep protocol during the experimental conditions. The final sample included 93 adolescents who completed all study procedures and were adherent to the study requirements. Demographic information and sleep characteristics during the stabilization week are presented in Table 1. Our final sample did not significantly differ from those who were excluded or dropped from the study on sex, weight status, or in their sleep characteristics during the stabilization week (ps > .05). However, those who were excluded or dropped from the study were more likely to be from households with lower income (p < .0001).
Table 1.
Sample characteristics at the sleep stabilization office visit (N = 93)
| Percent or mean ± SD | |
|---|---|
| Female (%) | 62.4 |
| Race/ethnicity (%) | |
| White | 63.4 |
| Black | 21.5 |
| Hispanic (White) | 2.5 |
| Asian | 3.0 |
| Multiracial | 9.6 |
| Age (years) | 15.78 ± 1.04 |
| Family yearly income (%) | |
| 50K or less | 19.4 |
| >50K and <=100K | 32.3 |
| >100K | 48.4 |
| BMI-z | 0.33 ± 0.91 |
| Sleep stabilization sleep onset (time) | 00:03 ± 0:59 |
| Sleep stabilization sleep offset (time) | 7:03 ± 0.03 |
| Sleep stabilization mid-point of sleep (time) | 3:35 ± 41.23 |
| Sleep stabilization sleep period (h) | 6.93 ± 1.05 |
| Sleep stabilization sleep efficiency | 90.91 ± 6.29 |
Sample features at time of randomization. Categorical variables are listed in percentages, while continuous variables are listed as mean ± SD.
Sleep characteristics
As expected, we observed significant differences in sleep duration across the two experimental sleep conditions (p < .0001; see Table 2); participants averaged 2 h and 20 min longer nightly sleep in the Healthy Sleep condition than in the Short Sleep condition. Consistent with the protocol, these differences were driven by changes in slee onset time (p = .002), not sleep offset times (p = .118). Sleep efficiency was significantly higher in the Short Sleep condition compared to the Healthy Sleep condition (p < .0001), likely driven by the increased homeostatic pressure that accompanies periods of shortened sleep; however, sleep efficiency remained in the normal range overall across both sleep conditions. Teens slept an average of 7 h and 20 min (SD = 51 min) and 7 h and 34 min (SD = 48 min) across the two nights immediately prior to the short and extended sleep conditions, respectively.
Table 2.
Actigraph-estimated sleep parameters across experimental conditions
| Short Sleep (M ± SD) | Healthy Sleep (M ± SD) | P* | Cohen’s d | |
|---|---|---|---|---|
| Sleep onset (time) | 00:44 ± 0:31 | 22:30 ± 0:43 | <.0001 | 3.57 |
| Sleep offset (time) | 07:01 ± 0:21 | 06:58 ± 0:27 | .188 | 0.12 |
| Sleep period (h) | 06.27 ± 0.52 | 08.47 ± 0.64 | <.0001 | 3.77 |
| Sleep efficiency (%) | 92.65 ± 4.40 | 88.84 ± 5.97 | <.0001 | 0.73 |
*P values were derived from paired-sample t-tests.
Dietary intake per day (aim 1)
As shown in Table 3, we did not observe a main effect of sleep condition on amount of energy consumed, with or without adjustment for covariates (unadjusted p = .401, adjusted p = .403). During Short Sleep, adolescents ate a small but statistically significant amount more carbohydrates (unadjusted p = .031, d = 0.16; adjusted p = .019) and had a small but statistically significant increase in the glycemic load of foods consumed, with or without adjusting for covariates (unadjusted p = .013, d = 0.19; adjusted p = .012). During Short Sleep, they also consumed a small amount more added sugars, an effect that straddled statistical significance across unadjusted and covariate-adjusted analyses (unadjusted p = .047, d = 0.16; adjusted p = .095). Additionally, during Healthy Sleep, they ate slightly more grams of fat, but this effect was very small and was statistically significant only following adjusting for covariates (unadjusted p = .698, d = 0.03; adjusted p = .046). Adolescents consumed moderately more daily servings of sweetened drinks (unadjusted p = .023, d = 0.29; adjusted p = .034) and moderately fewer daily servings of fruits and vegetables (unadjusted p = .006, d = 0.27; adjusted p = .001) during Short Sleep compared to Healthy Sleep. Differences in other dietary outcomes were nonsignificant (ps > .055).
Table 3.
Effects of experimental condition on kilocalories, macronutrients, and food groupings, averaged across all days
| Primary outcome | Mean (SE) Healthy | Mean (SE) Short | Cohen’s d | P, unadjusted | P, adjusted |
|---|---|---|---|---|---|
| Energy (kilocalories) | 1814.3 (59.8) | 1856.3 (59.3) | 0.06 | .401 | .403 |
| Macronutrient properties | |||||
| Fat (g) | 74.1 (2.7) | 72.8 (2.7) | 0.03 | .698 | .046 |
| Carbohydrates (g) | 223.0 (8.1) | 238.6 (8.1) | 0.16 | .031 | .019 |
| Protein (g) | 69.3 (2.7) | 68.0 (2.7) | 0.05 | .503 | .120 |
| Added sugar (g) | 65.4 (4.2) | 74.0 (4.2) | 0.16 | .047 | .095 |
| Glycemic load | 182.1 (6.9) | 197.2 (6.8) | 0.19 | .013 | .012 |
| Food groupings (servings) | |||||
| Low-calorie drinks | 4.2 (0.3) | 4.0 (0.3) | 0.06 | .549 | .619 |
| Sweet drinks | 1.3 (0.2) | 1.6 (0.2) | 0.29 | .023 | .034 |
| Fruits/veggies | 0.9 (0.1) | 0.6 (0.1) | 0.27 | .006 | .001 |
| Meats/eggs | 1.3 (0.1) | 1.1 (0.1) | 0.14 | .214 | .167 |
| Processed snacks | 1.3 (0.1) | 1.3 (0.1) | 0.07 | .529 | .676 |
| Fast food | 1.3 (0.1) | 1.3 (0.1) | 0.08 | .556 | .692 |
| Grains | 1.8 (0.2) | 1.9 (0.2) | 0.09 | .420 | .373 |
| Sweets/desserts | 1.3 (0.2) | 1.2 (0.2) | 0.03 | .816 | .731 |
For macronutrient outcomes, adjusted analyses covaried for averaged kilocalories consumed and averaged moderate/vigorous physical activity within each experimental condition. For the energy (kilocalorie) outcome and food grouping outcomes, adjusted analyses covaried for the averaged moderate/vigorous physical activity within each experimental condition.
Energy and macronutrient accumulation across time of day (aim 2)
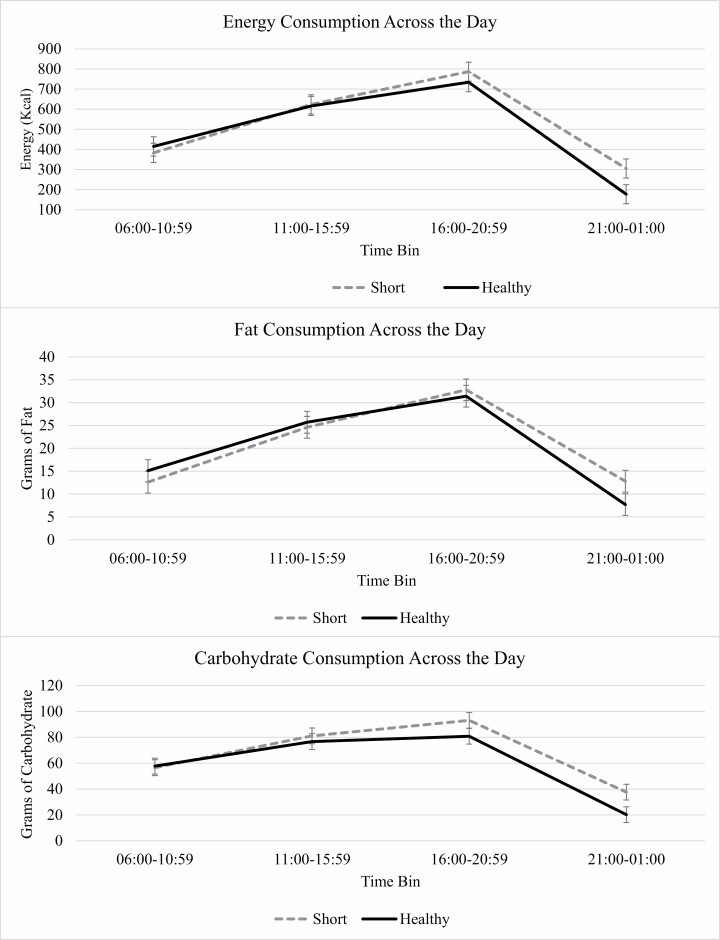
We observed nonsignificant interactions of time bins and sleep condition on all energy and macronutrient outcomes, though some interactions trended toward significance when controlling for covariates, including energy (unadjusted p = .118, adjusted p = .058), fat (unadjusted p = .113, adjusted p = .066), and carbohydrates (unadjusted p = .153, adjusted p = .083). As detailed in Table 4 and illustrated in Figure 2, planned contrasts showed differences in energy, fat, and carbohydrate consumption in the late evening. There were no significant differences in consumption of energy or any macronutrients in the first three-time bins (ps > .064). Within the final time bin (9:00 pm–1:00 am), adolescents in Short Sleep consumed significantly more energy (t(608) = −2.51, unadjusted p = .012, d = 0.38; adjusted p = .011), fat (t(608) = −2.03, unadjusted p = .043, d = 0.31; adjusted p = .043), and carbohydrates (t(608) = −2.77, unadjusted p = .006, d = 0.44; adjusted p = .006) than during Healthy Sleep. There were no significant interactions of cumulative time bins on grams of fat or protein or added sugar (ps > .13).
Table 4.
Primary energy and macronutrient outcomes across time of day and experimental condition
| Short Mean (SD) | Healthy Mean (SD) | t | d | Short Mean (SD) | Healthy Mean (SD) | t | d | |
|---|---|---|---|---|---|---|---|---|
| 06:00–10:59 | 11:00–15:59 | |||||||
| Energy (Kcal) | 382.6 (264.8) | 414.7 (272.3) | 0.78 | 0.13 | 624.0 (350.7) | 616.1 (316.4) | −0.04 | 0.05 |
| Fat (g) | 12.6 (14.1) | 15.1 (15.5) | 0.22 | 0.19 | 24.6 (15.1) | 25.7 (14.8) | 0.63 | 0.13 |
| Carbohydrate (g) | 56.5 (34.4) | 57.7 (33.8) | 0.29 | 0.08 | 81.1 (52.1) | 76.7 (41.7) | −0.60 | 0.03 |
| Protein (g) | 12.9 (12.5) | 14.0 (11.5) | 0.55 | 0.10 | 21.7 (13.2) | 21.5 (12.9) | −0.19 | 0.02 |
| Added sugar (g) | 18.6 (14.4) | 18.2 (14.5) | −0.00 | 0.02 | 27.0 (35.2) | 21.8 (21.8) | −1.38 | 0.20 |
| 16:00–20:59 | 21:00–01:00 | |||||||
| Energy (Kcal) | 787.0 (464.7) | 734.4 (363.2) | −0.58 | 0.05 | 305.0 (365.3) | 177.3 (299.3) | −2.51* | 0.38 |
| Fat (g) | 32.8 (22.1) | 31.4 (18.4) | −0.11 | 0.01 | 12.8 (16.8) | 7.7 (16.1) | −2.03* | 0.31 |
| Carbohydrate (g) | 93.1 (56.4) | 80.8 (42.5) | −1.69 | 0.21 | 37.6 (44.8) | 20.2 (33.9) | −2.77* | 0.44 |
| Protein (g) | 31.6 (22.0) | 33.1 (18.3) | 1.79 | 0.23 | 10.7 (14.3) | 7.1 (13.9) | −1.45 | 0.26 |
| Added sugar (g) | 27.6 (28.6) | 20.4 (20.5) | −1.85 | 0.30 | 13.5 (22.2) | 7.6 (21.9) | 1.73 | 0.27 |
*P < .05 across conditions both with and without accounting for covariates.
Figure 2.
Interaction of time bin with experimental condition on consumption of energy, fat, and carbohydrates.
Exploratory moderation analyses
We did not observe significant interactions of sleep condition and the mid-point of baseline sleep, differences in length of the sleep conditions, order of experimental condition, income, race, sex, or baseline BMI z-score on any dietary outcome (i.e. Kcals, grams of fat, carbohydrates, protein, and added sugars consumed, glycemic load, and servings of low-calorie drinks, sweetened drinks, fruits/vegetables, meats/eggs, processed snacks, fast food, grains, and sweets/desserts consumed). Thus, there was no clear evidence that any of these factors altered the impact of the sleep manipulation.
Discussion
Adolescents did not consume significantly more calories across a full day while experiencing shortened versus healthy sleep across the experimental condition, but they did consume more calories during the late evening hours. Adolescents particularly consumed a higher glycemic load and more carbohydrates when experiencing short sleep compared to healthy sleep, with this higher carbohydrate intake emerging in the evening hours. Adolescents undergoing shortened sleep also averaged more servings of sugar-sweetened beverages and less servings of fruits and vegetables, compared to when obtaining healthy sleep. These study findings did not significantly differ across several potential moderating factors, including adolescent sex and BMI, family income, and order in which participants experienced the two experimental conditions. Overall findings suggest that adolescents are more likely to engage in unhealthy eating practices during phases of shortened sleep, particularly seeking out fast-energy foods, with effects on consumption most evident in the late evening hours. This highlights a critical mechanism that may underlie the relationship between shortened sleep and increased obesity and cardiometabolic disorder risk in adolescents [6, 7, 19].
Contrary to our first hypothesis, we observed no significant difference in overall kilocalorie consumption among adolescents when undergoing short sleep compared to healthy sleep. While the cross-sectional correlations between adolescent sleep duration and caloric consumption have varied [8, 9], previous experimental studies in adolescents have demonstrated either modest increases in caloric consumption following sleep restriction in naturalistic environments [15, 16] or no changes in a highly controlled laboratory setting [14]. Our findings, paired with previous literature, would suggest that change in total energy intake consumed by adolescents following a short bout of experimentally induced sleep restriction is small to negligible.
However, current findings add to a growing literature showing an impact of short sleep on the quality and timing of foods consumed. During this study, adolescents undergoing shortened sleep consumed a higher glycemic load, more grams of carbohydrates and, in unadjusted analyses, more added sugars compared to healthy sleep. In each case, observed effect sizes were relatively small in a 24-hour period, but could have an outsized cumulative impact over time. If an adolescent obtains insufficient sleep during the 180 nights of a school year (which is commonplace) [1], an extra 12 g of added sugar each school day could result in over 4.5 pounds of extra sugar each year. Similarly, based on present findings, an adolescent who is chronically sleep-deprived on school nights could consume over 6 pounds of extra carbohydrates yearly.
Although prior studies have not shown a consistent relationship between adolescent sleep duration and most aspects of macronutrient intake [8], we previously found a trend toward greater consumption of carbohydrates following shortened sleep in adolescents [16]. The current study also suggested that adolescents undergoing shortened sleep consumed slightly less fat than when undergoing healthy sleep; however, it is hard to interpret that effect because it was very small, emerged only after controlling for covariates, and has not been evident in similar experimental studies [16]. Sleep-restricted adolescents may seek out foods that are specifically higher in carbohydrates/glycemic load, perhaps as a strategy to receive fast-acting energy that may counteract the sleepiness experienced following sleep restriction. Adolescent consumption of high carbohydrate foods increases the risk for both obesity [19] and cardiometabolic abnormalities [34]; as such, promotion of healthy sleep may be one mechanism to reduce such risk. Indeed, research is increasingly arguing for sleep to be directly addressed by the public health community, promoting sleep with the same intensity as promoting healthy nutrition and optimal physical activity [35].
When examining what types of foods are being consumed by adolescents undergoing shortened sleep, this study found them drinking more sugar-sweetened beverages when experiencing shortened sleep compared to healthy sleep. Our previous pilot experimental sleep research also showed that adolescents drank half of a serving more of sugar-sweetened beverages when experiencing shortened sleep compared to healthy sleep, though the effect did not reach statistical significance in that smaller sample [16]. In a large population of adults, shortened sleep also was significantly associated with increased sugar-sweetened beverages [36]. This finding is noteworthy, as the odds ratio of developing obesity among youth increases 1.6 times for each additional daily glass of sugar-sweetened beverage [37]. Interestingly, we observed negligible differences in consumption of sweets/desserts during this study, in contrast to the small but significant effects reported in our pilot work [15, 16]. Thus, while there is growing evidence that short sleep selectively impacts foods that are high in added sugars, the exact nature of such foods so far has differed across studies.
Additionally, adolescents in the current sample were less likely to consume fruits and vegetables when experiencing shortened sleep, compared to healthy sleep. The impact of sleep duration on fruit and vegetable consumption has been variable in past studies [8, 9]. In a large sample, longer adolescent self-reported habitual sleep was consistently associated with more fruit/vegetable consumption, even after adjusting for several potential confounding factors [38]. While one experimental sleep manipulation study did not observe changes in fruit/vegetable consumption in adolescents following sleep restriction [16], another observed that adolescents consumed more servings of fruits and vegetables following an intervention to improve their bedtimes [39]. Taken together, these findings suggest that sleep-restricted adolescents gravitate towards high-energy foods/drinks, rather than those associated with health benefits (e.g. fruits and vegetables), compared to when they are well-rested. It is notable that average servings for fruit and vegetable consumption during both conditions were markedly low.
To extend the current literature beyond asking how much and what types of foods are consumed following adolescent experimental sleep restriction, this study examined when these dietary alterations occurred throughout the day. We observed an impact of short sleep on consumption of total energy, carbohydrates, and fat consumed in the late evening hours, after 9:00 pm. Some caution is recommended in interpreting those findings, as omnibus analyses yielded significance levels just shy of statistical significance. However, current findings are similar to evidence in adults that suggests that sleep restriction is most likely to impact dietary patterns in the evening [20, 40, 41]. This could be important because late evening eating (particularly in foods higher in glycemic load) has negative health consequences on body weight and cardiometabolic health in adults [42, 43]. While the literature is emerging as to how evening eating impacts risk for developing obesity in adolescence [44, 45], late evening eating may also alter adolescent circadian rhythms in a way that contributes to future sleep problems [46]. Future research that aims to examine the impact of shortened sleep on dietary outcomes should particularly focus on evening eating patterns.
The current study has several salient strengths. To our knowledge, this is the largest pediatric experimental sleep manipulation study completed to date. Further, this study deliberately balanced internal and external validity. By allowing adolescents to sleep and consume foods within their natural environment while using realistic experimental sleep conditions that were objectively monitored to ensure adherence, we were able to uncover findings that are more generalizable to adolescents than if this study had been conducted in a laboratory [17]. We also had high completion of our rigorous dietary recalls and strong adherence to our experimental protocol among those included in the analyses. Finally, this study found that effects persisted even after controlling for an objective measure of physical activity, all of which increases our confidence in results.
However, the study also had limitations. Generalizability is limited by the brevity of the sleep manipulation. It could be that adolescents readjust their intakes over longer time spans, though consistencies with correlational findings are reassuring. Further, though well validated in free-living adolescents and adults, dietary recall interviews are subject to recall inaccuracies and biases. Additionally, although validated and objective, accelerometers yield indirect estimates of both sleep and physical activity. Further, while our experimental manipulation was intended to differentially alter sleep duration, it also impacted the timing of sleep. This is because an early rise time was maintained during both conditions to mimic prevailing constraints during the school year when altering bedtime is the only option available for most adolescents to change their sleep duration. This leaves open the possibility that effects found here might be as attributable to sleep timing as sleep duration; future work is needed to untangle these effects. Exploratory analyses found no moderating effect of the order of sleep conditions, so there was no evidence of carryover effects from one to the other; however, we recognize that two nights of a washout-period between experimental conditions may not provide adequate time for an adolescent to recover from accumulated sleep debt. Our sample also was predominantly middle- to upper-socioeconomic class, which limits our ability to extend these findings to lower-income adolescents and the study was not powered to examine racial/ethnic differences. This is of particular note, given that adolescents of lower socioeconomic class are at increased risk for sleep concerns [47, 48] and unhealthy eating patterns [49, 50]. Finally, despite the correction of alpha for all secondary analyses, testing multiple primary outcomes increased the risk for type 1 error, though the consistency of findings related to sugar-sweetened foods across studies provides reassurance that those effects were not spurious.
Conclusion
The mechanisms driving the relationship between sleep and cardiometabolic risk in adolescents are only just beginning to be clarified [9], but current findings contribute to our understanding of this link. It appears that short sleep impacts the types and quality of foods that adolescents are consuming. When sleep-restricted, adolescents consume more carbohydrates, high glycemic load foods, and sugar-sweetened beverages, and consume fewer fruits/vegetables, compared to when they are obtaining healthy levels of sleep. Furthermore, our study suggests that these dietary alterations are most evident in the late evening. These study findings suggest that future adolescent health promotion efforts should include the promotion of healthy sleep habits in efforts to improve dietary outcomes and subsequent cardiometabolic health.
Supplementary Material
Acknowledgments
The authors thank the families who participated in this research and would like to acknowledge the support of the many assistants who helped to run the study but are not authors on this article, especially Shealan McAlister, Nathan Lutz, Taylor Howarth, Megan Pfeiffer, Juliana Rizzo, Caitlin Brammer, Perry Catlin, Angela Moore, and Tori Van Dyk.
Funding
This project was supported by the United States National Institutes of Health (NIH; R01HL120879 and 5UL1TR001425). The primary author was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of the General Pediatrics Research Fellowship. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, the NIH, HRSA, HHS, or the US Government.
Disclosure Statement
Financial disclosure: None
Nonfinancial disclosure: None
References
- 1. Wheaton AG, et al. Short sleep duration among middle school and high school students—United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(3):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowley SJ, et al. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dewald JF, et al. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14(3):179–189. [DOI] [PubMed] [Google Scholar]
- 5. Baum KT, et al. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55(2):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller MA, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41(4). doi: 10.1093/sleep/zsy018. [DOI] [PubMed] [Google Scholar]
- 7. Quist JS, et al. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev. 2016;29:76–100. [DOI] [PubMed] [Google Scholar]
- 8. Krietsch KN, et al. Sleep and weight-related factors in youth: a systematic review of recent studies. Sleep Med Rev. 2019;46:87–96. [DOI] [PubMed] [Google Scholar]
- 9. Duraccio KM, et al. Poor sleep and adolescent obesity risk: a narrative review of potential mechanisms. Adolesc Health Med Ther. 2019;10:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Khatib HK, et al. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur J Clin Nutr. 2017;71(5):614–624. [DOI] [PubMed] [Google Scholar]
- 11. Ievers-Landis CE, et al. Dietary intake and eating-related cognitions related to sleep among adolescents who are overweight or obese. J Pediatr Psychol. 2016;41(6):670–679. [DOI] [PubMed] [Google Scholar]
- 12. Kelly NR, et al. Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eat Behav. 2016;22:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golley RK, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37(4):546–551. [DOI] [PubMed] [Google Scholar]
- 14. Klingenberg L, et al. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr. 2012;96(2):240–248. [DOI] [PubMed] [Google Scholar]
- 15. Simon SL, et al. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS One. 2015;10(2):e0115434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beebe DW, et al. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36(6):827–834. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beebe DW. Practical aspects of experimental sleep extension research with adolescents. Sleep Med Rev. 2021;58:101483. [DOI] [PubMed] [Google Scholar]
- 18. Kracht CL, et al. Association between meeting physical activity, sleep, and dietary guidelines and cardiometabolic risk factors and adiposity in adolescents. J Adolesc Health. 2020;66(6):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludwig DS, et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. 2021; 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spaeth AM, et al. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy Adults. Sleep. 2013;36(7):981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez-Minguez J, et al. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients. 2019;11(11):2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. St-Onge MP, et al. ; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Meal timing and frequency: implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duraccio KM, et al. The impact of short sleep on food reward processes in adolescents. J Sleep Res. 2021;30(2):e13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chervin RD, et al. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2(6):501–510. [DOI] [PubMed] [Google Scholar]
- 25. Beebe DW, et al. Short sleep and adolescents’ performance on a concussion assessment battery: an experimental sleep manipulation study. Clin J Sport Med. 2018;28(4):395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirshkowitz M, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 27. Van Dyk TR, et al. Inducing more sleep on school nights reduces sedentary behavior without affecting physical activity in short-sleeping adolescents. Sleep Med. 2018;47:7–10. [DOI] [PubMed] [Google Scholar]
- 28. Sadeh A, et al. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 29. Trost SG, et al. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc. 2011;43(7):1360–1368. [DOI] [PubMed] [Google Scholar]
- 30. Johnson RK, et al. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–1144. [DOI] [PubMed] [Google Scholar]
- 31. Klesges RC, et al. Validation of the 24-hour dietary recall in preschool children. J Am Diet Assoc. 1987;87(10):1383–1385. [PubMed] [Google Scholar]
- 32. Moshfegh AJ, et al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. [DOI] [PubMed] [Google Scholar]
- 33. Monro JA, et al. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. 2008;87(1):237S–243S. [DOI] [PubMed] [Google Scholar]
- 34. Welsh JA, et al. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaput J-P, et al. Lack of sleep as a contributor to obesity in adolescents: impacts on eating and activity behaviors. Int J Behav Nutr Phys Act. 2016;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prather AA, et al. Short and sweet: associations between self-reported sleep duration and sugar-sweetened beverage consumption among adults in the United States. Sleep Health. 2016;2(4):272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harrington S. The role of sugar-sweetened beverage consumption in adolescent obesity: a review of the literature. J Sch Nurs. 2008;24(1):3–12. [DOI] [PubMed] [Google Scholar]
- 38. Kruger AK, et al. Do sleep-deprived adolescents make less-healthy food choices? Br J Nutr. 2014;111(10):1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Asarnow LD, et al. The impact of sleep improvement on food choices in adolescents with late bedtimes. J Adolesc Health. 2017;60(5):570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baron KG, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19(7):1374–1381. [DOI] [PubMed] [Google Scholar]
- 41. Markwald RR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110(14):5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JB, et al. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27(Suppl 2):255–262. [DOI] [PubMed] [Google Scholar]
- 43. Bo S, et al. Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population-based prospective cohort study. PLoS One. 2014;9(9):e108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thompson OM, et al. Dietary pattern as a predictor of change in BMI z-score among girls. Int J Obes (Lond). 2006;30(1):176–182. [DOI] [PubMed] [Google Scholar]
- 45. Eng S, et al. Eating late in the evening is associated with childhood obesity in some age groups but not in all children: the relationship between time of consumption and body weight status in US children. Int J Behav Nutr Phys Act. 2009;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wehrens SMT, et al. Meal timing regulates the human circadian system. Curr Biol. 2017;27(12):1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marco CA, et al. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 2011;10(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013;79:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williamson VG, et al. The influence of socioeconomic status on snacking and weight among adolescents: a scoping review. Nutrients. 2020;12(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hanson MD, et al. Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med. 2007;30(3):263–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.