Abstract
Study Objectives
Post-traumatic stress disorder (PTSD) and rapid eye movement (REM) sleep behavior disorder (RBD) share some common features including prominent nightmares and sleep disturbances. We aimed to comparatively analyze REM sleep without atonia (RSWA) between patients with chronic PTSD with and without dream enactment behavior (DEB), isolated RBD (iRBD), and controls.
Methods
In this retrospective study, we comparatively analyzed 18 PTSD with DEB (PTSD+DEB), 18 PTSD without DEB, 15 iRBD, and 51 controls matched for age and sex. We reviewed medical records to determine PTSD clinical features and quantitatively analyzed RSWA. We used nonparametric analyses to compare clinical and polysomnographic features.
Results
PTSD patients, both with and without DEB, had significantly higher RSWA than controls (all p < .025, excepting submentalis phasic duration in PTSD+DEB). Most RSWA measures were also higher in PTSD+DEB than in PTSD without DEB patients (all p < .025).
Conclusions
PTSD patients have higher RSWA than controls, whether DEB is present or not, indicating that REM sleep atonia control is abnormal in chronic PTSD. Further prospective studies are needed to determine whether neurodegenerative risk and disease markers similar to RBD might occur in PTSD patients.
Keywords: post-traumatic stress disorder, REM sleep behavior disorder, dream enactment behavior, REM sleep without atonia
Statement of Significance.
Post-traumatic stress disorder (PTSD) and isolated rapid eye movement (REM) sleep behavior disorder (iRBD) both have prominent nightmares and sleep disturbances. In this retrospective study, we comparatively analyzed REM sleep without atonia (RSWA) between chronic PTSD with and without dream enactment behavior (DEB), and iRBD patients and controls. PTSD patients had higher RSWA than controls, whether DEB was present or not. RSWA is abnormal in chronic PTSD. Further prospective studies are needed to determine whether neurodegenerative risk and disease markers may occur in PTSD patients.
Chronic post-traumatic stress disorder (PTSD) develops in individuals after exceptionally threatening and distressing events [1]. PTSD symptoms are divided into the following four clusters: (1) re-experiencing of the traumatic event; (2) avoidance of trauma cues; (3) negative changes in thinking and mood; and (4) changes in physical and emotional reactions [2,3]. Exposure to traumatic experiences is unfortunately common, with an estimated 37%–92% lifetime frequency [4,5]. It is estimated that 6.8% of adult Americans have PTSD, and prevalence is significantly higher in military personnel, with 13.8% of veterans of the wars in Iraq and Afghanistan meeting DSM-IV criteria for PTSD [6,7].
Sleep difficulties are common among individuals with PTSD, and are likely a predictor for the development of PTSD [8–10]. Sleep disturbances are considered a “hallmark of PTSD,” as symptoms of insomnia and recurrent nightmares affect up to 87% of patients with PTSD [2,11,12]. Post-traumatic nightmares are frequent, ranging from 19% to 96% [13]. Some studies indicate that motor disturbances during both rapid eye movement (REM) and non-rapid eye movement (NREM) sleep are more frequent in PTSD than controls, although this finding is not entirely consistent across studies (see, e.g. [14]) [14–16]. REM sleep mechanisms have been postulated to be abnormal in PTSD [10,15]. For example, a meta-analysis of PSG studies showed that PTSD patients demonstrated greater REM density than controls [17]. Some studies also suggest greater REM sleep fragmentation than controls [17], and that REM sleep fragmentation predicts development of PTSD symptoms early after trauma [18], but may later correct, resulting in recovered or increased REM sleep [19]. Another meta-analysis indicates that REM sleep alteration in PTSD varies with age; older PTSD patients have more REM sleep than healthy controls, whereas younger PTSD have similar or decreased REM sleep amounts compared to healthy individuals [20,21]. Regions involved in emotion expression and emotion regulation, including the medial prefrontal cortex, amygdala, hippocampus, raphe nuclei, and locus coeruleus, may mediate sleep dysfunction in PTSD [22].
Rapid eye movement sleep behavior disorder (RBD) is characterized by REM sleep without atonia (RSWA) on polysomnography and dream enactment behaviors (DEB) [23]. RBD has been shown to be strongly associated with the future development of alpha-synucleinopathy neurodegenerative diseases including Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) [24]. When idiopathic (aka isolated) RBD has its onset in older adults (i.e. >50–55 years old), the symptoms of DEB together with RSWA are thought to reflect the pathophysiological manifestations of an underlying covert neurodegenerative process that has not yet progressed to cause clearly evident symptomatic motor, cognitive, and autonomic impairments. However, subtle signs of impairment indicating an emerging Lewy body disease may be found already at this stage in the majority of iRBD patients who undergo detailed longitudinal assessment [24].
Many younger adults with PTSD also frequently report sleep-related vocalizations and movements during sleep, similar to those seen in older adults with RBD. Recently, RBD was found to be significantly more frequent in a large cohort of Portland veterans who underwent polysomnography (9%) [25] than reported previously in the general population (1%–2%) [26–29] and RBD was even more frequent in those endorsing PTSD symptoms (15%), particularly among those with a prior history of traumatic brain injury (21%) [25].
The goal of this project was to characterize RSWA type (phasic and/or tonic), muscle distribution, and quantitative amount in patients with chronic PTSD with and without DEB in comparison to patients with iRBD and controls. We hypothesized that RSWA levels would be higher in PTSD patients than controls. We also sought to comparatively analyze RSWA levels in PTSD patients who did or did not have concomitant DEBs.
Methods
A total of 102 patients seen between 2012 and 2018 at the Mayo Clinic Center for Sleep Medicine were selected for retrospective analysis following a ICD-10 code search for PTSD from our electronic medical record system, with identification of polysomnogram (PSG) electronic signals retrieved from our PSG database. All medical records were reviewed to confirm the diagnosis of PTSD and to determine whether DEB was present or absent. The patient groups included 18 chronic PTSD patients with DEB, 18 chronic PTSD patients without DEB, and 15 antidepressant naive iRBD patients. All of the patients’ electronic medical records were reviewed to ensure diagnoses by a psychiatrist of chronic PTSD documented in a psychiatric clinic visit preceding polysomnography, and iRBD diagnosed by a sleep neurologist. All iRBD patients met the ICSD-2 criteria for RBD diagnosis and were without any clinical signs of parkinsonism or dementia, and all iRBD patients had an absence of a past history of PTSD [23]. All PTSD patients included in this study had PTSD diagnoses by a board-certified Mayo Clinic psychiatrist during at least one clinic visit prior to PSG. Medical record review was also performed to confirm that historical features consistent with PTSD diagnosis were present for all subjects, including previous traumatic exposure, trauma-related intrusion symptoms of nightmares and/or flashbacks, avoidance of trauma-related thoughts or feelings, post-traumatic negative cognitive and mood alterations, and post-traumatic alterations in arousal and reactivity, with symptom duration for at least 1 month and causing functional impairment and/or distress, as per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for PTSD, without specification for presence of dissociative symptoms or delayed expression [30]. Presence or absence of DEB was assessed in both RBD and PTSD patients and controls in all patients by a standard sleep clinic intake questionnaire which poses a slight alteration of the Mayo Sleep Questionnaire core dream enactment question, “Have you ever been told that you scream, shout, or make unusual movements such as swinging arms about, acting out dreams, etc…during sleep?” [31,32]. DEB was considered positive by endorsement of this question as positive, or alternatively by bedpartner or self-report of complex vocal and/or motor behaviors mirroring dream content, or when PSG recorded complex vocal and/or motor behaviors during REM sleep. None of the iRBD patients were receiving medications known to affect REM sleep muscle activity at the time of PSG. All of the iRBD patients were reported in a previous study [33]. The control group included 51 antidepressant-naive healthy controls matched for age and sex to the PTSD and iRBD cohorts, and were diagnosed with primary snoring (71%), mild obstructive sleep apnea (20%), or no specific sleep diagnosis (9%). Medical records were utilized to determine clinical and demographic factors for all patients.
Polysomnography Recordings
Video PSG recordings were conducted on a 16-channel Nicolet NicVue digital system with acquisition methods as previously described [33–35]. Electromyogram (EMG) recording included submentalis SM and bipolar linked anterior tibialis (AT) electromyography in both RBD patients and controls and extensor digitorum communis (EDC) for iRBD patients only as determined by discretion of the ordering sleep physician for evaluation of parasomnia behavior. Thirty-second PSG epochs were used for standard sleep stage scoring and scoring of arousals, breathing events, and periodic limb movements [36]. Following established rules for scoring REM sleep in the setting of RWSA for all subjects, the occurrence of the first REM in the EOG channel was used to determine the onset of REM, with end of REM sleep determined when either no REMs were detected in 3 consecutive minutes or when an awakening, K complexes, or spindles were observed [37] SM and AT EMG channels were viewed at 5 μV/mm with low- and high-frequency filters set at 10 and 70 Hz, respectively, with a sampling rate of 500 Hz, as per previously established methods [33–35].
Analysis of REM sleep muscle activity
Reference background EMG amplitude for each study was determined by measuring the average peak-to-peak amplitude in a 3-second mini-epoch within REM sleep that displayed the lowest amount of EMG activity throughout the study. The reference background EMG amplitude during REM sleep varied from 0.5 to 2.0 μV in all subjects. Quantitative analysis of EMG activity was performed utilizing HypnoLab sleep software, a scoring platform which enables both signal visualization and manual scoring, as well as automated analysis of the REM atonia index in the submentalis muscle as described further below (ATES Medica Labs, Verona, Italy). Overall tonic, phasic, and “any” (either tonic, phasic, or both forms of muscle activity occurring within the same mini-epoch) percent muscle activity were manually scored by previously published methods [33,34]. Phasic and “any” percent muscle activity were also calculated separately for SM and AT muscles. In addition, the duration of each phasic muscle burst during REM sleep was directly measured, and those bursts fulfilling scoring standards were individually recorded for each muscle, resulting in an overall average phasic muscle activity burst duration. Normative RSWA percentiles were calculated based on individual patients’ age decile and RSWA densities using algorithms developed and published previously [38]. Any 3-second mini-epoch containing a breathing-related, snoring-related, or spontaneous arousal was considered as artifact and excluded from final analysis.
Phasic muscle activity was defined as having duration between 0.1 and 14.9 seconds with a measured average peak-to-peak amplitude of greater than four times the lowest background muscle activity voltage. Return of muscle activity to background amplitude levels for at least 200 msec was considered to be the end of a phasic burst. Percent phasic muscle activity was calculated based on the number of 3-second mini-epochs containing phasic muscle activity divided by the total number of analyzable artifact-free 3-second mini-epochs during REM sleep.
Thirty-second epochs were used to score tonic muscle activity in both the SM and AT muscles. An epoch was considered positive for tonic activity if greater than 50% of the epoch (i.e. 15 or more seconds in duration), had muscle activity continuously greater than double the background EMG, or ≥10 μV. Tonic muscle activity percentage was calculated as the total number of positive 30-second epochs divided by the total number of analyzable 30-second REM sleep epochs. “Phasic-on-tonic” muscle activity (i.e. concurrent phasic and tonic muscle activity occurring within the same 3-second mini-epoch) was scored positively in addition to underlying tonic activity only if the overlying phasic burst was greater than twice the background tonic EMG activity within that same 3-second mini-epoch.
Percent “any” muscle activity was calculated as the number of 3-second mini-epochs containing phasic and/or tonic muscle activity divided by the total number of analyzable REM 3-second mini-epochs (and additionally, any 3-second mini-epoch containing both phasic and tonic muscle activity was only counted once to avoid artificially inflated muscle activity percentages).
The automated REM Atonia Index (RAI) for the SM muscle was also calculated using HypnoLab sleep scoring software (ATES Medica Labs, Verona, Italy). Prior to RAI analysis, 30-second epochs containing a breathing-related artifact, snoring, or arousal were excluded and the SM signal was notch filtered at 60 Hz and rectified. In distinction to visually determined RSWA scoring and calculations, the RAI computes RSWA on a scale from 1 (which indicates completely preserved atonia) down to 0 (indicating complete absence of REM atonia), so that lower RAI scores indicate greater RSWA amounts, while higher RAI conversely demonstrates lower amounts of RSWA [39,40]. RSWA was also analyzed using American Academy of Sleep Medicine (AASM) criteria for excessive phasic muscle activity, defined as a 30-second epoch containing five or more 3-second mini-epochs containing phasic muscle activity (with similar duration and amplitude criteria as described above for our visual methods) within them (while excluding any 30-second epoch not containing 5 or more positive phasic miniepochs, which is excluded from further analyses and AASM phasic RSWA metric calculation) [36]. This definition was used to generate AASM phasic percent muscle activity for the SM and AT muscles individually and combined.
Scorers of RSWA were blinded to patient group and had high inter-rater reliability with a ĸ coefficient of 0.886. ĸ coefficients were calculated according to previously published methods [33,34].
Statistical analysis
Clinical, demographic, and PSG data are presented as means, standard deviations, and frequencies. The primary outcome for RSWA analyses was considered to be the combined submentalis and anterior tibialis (SM+AT) any muscle activity percentage, which reflects the overall most parsimonious measure for RSWA across types (phasic, tonic) and recording sites. Other RSWA metric comparisons were considered as secondary outcomes. RSWA variables between groups were initially analyzed using principal component analysis. The principal components were assessed using scree plots to determine components with eigenvalues above 1. Cumulative variance plots of principal components were developed to assess the variance that each principal component accounted for within the data. The first two principal components were correlated against each other to display group separation. Principal components were correlated with raw RSWA values to determine the contribution of each RSWA metric within the component. Quantitative variables were analyzed using non-parametric Kruskal–Wallis tests while chi-square tests were used to analyze categorical variables with R statistical software [41]. Sensitivity analyses were performed to analyze the potential impact of outliers on results, with outliers included and excluded in the analyses. Relationships between clinical independent variables and dependent tonic, phasic, and “any” muscle activity and average phasic muscle burst duration RSWA measures were analyzed utilizing multivariable linear or logistic regression. For regression models with AT or SM+AT RSWA measures as the outcome variable, adjustment was made for periodic limb movement index (PLMI) and PLM arousal index (PLMAI), measures of periodic limb movements that are not considered RSWA and that could potentially artificially inflate AT RSWA measures (since we include all REM sleep phasic muscle activity measured in our AT RSWA calculations) [38], and age. When SM RSWA measures were the sole outcome variables in regression, only age adjustments were made. Following Bonferroni correction for multiple comparisons, p-value for key RSWA metric comparisons was set to .025, while p < .05 was used for other exploratory secondary outcomes and non-hypothesis-based comparisons.
Data availability
All data obtained are included within the manuscript and Supplementary Material.
Results
Clinical and demographic data
Of the 102 patients, 47 (46%) were men with an average age at PSG of 57.8 (range 26–88) years. RBD was diagnosed at an average age of 56.2 ± 11.8 (range 34–74, iRBD mean = 63.1, PTSD+DEB mean = 50.4, p < .05). PTSD was diagnosed at an average age of 45.4 ± 15.8 (range 21–88, PTSD mean = 47.0, PTSD+DEB mean = 43.7, p = .63). Within the PTSD+DEB group, the average time of DEB onset after PTSD diagnosis was 6.7 years (range −3 to 30 years, with negative values indicating DEB onset prior to PTSD diagnosis in 3 patients). Key demographic and clinical summary features, as well as sleep architecture metrics and antidepressant use with significant group differences are shown in Table 1. Most patients were referred to the Sleep Center for evaluation of obstructive sleep apnea (n = 21, n = 12 PTSD group, n = 9 PTSD+DEB group) while two were referred for snoring (both from PTSD group), two for insomnia (one from PTSD and one from PTSD+DEB), five for nonrestorative sleep (three from PTSD and two from PTSD+DEB), one for idiopathic hypersomnia (from PTSD+DEB group), and five for parasomnia behaviors (all from PTSD+DEB). None of the patients in the PTSD (without dream enactment) or controls had reported or PSG-recorded DEBs. iRBD patients were older at the time of PSG, and there were more women in the control than PTSD or iRBD cohorts, but there was no difference between the sexes for the PTSD and iRBD groups. Significant group differences in sleep architecture between PTSD+DEB, PTSD, and controls (all p < .05) included the following: longer TST in controls than PTSD patients without DEB, and in PTSD+DEB vs. iRBD patients; higher N1% in PTSD and iRBD than PTSD+DEB; greater N3% in controls than all PTSD patients; higher REM% in PTSD+DEB than controls; greater REM time in controls, PTSD+DEB, and iRBD than in PTSD; higher sleep efficiency in PTSD+DEB than other groups; and higher PLMI in PTSD+DEB and iRBD than controls, while controls had higher periodic limb movement arousal index (PLMAI) than PTSD+DEB. AI was significantly higher in iRBD than controls (p < .05), and the PTSD without DEB group had significantly higher AI than the PTSD+DEB group (p < .05). REM AHI was higher in PTSD patients and controls than in iRBD patients.
Table 1.
Comparative demographic, clinical, and polysomnography characteristics between PTSD patients with and without DEB, idiopathic/isolated RBD patients, and controls
| ControlA (n = 51) | PTSDBn = 18) | PTSD+DEBC (n = 18) | iRBDD (n = 15) | p < .05 | |
|---|---|---|---|---|---|
| Sex (F) | 25 | 14 | 14 | 2 | A > B,C,D |
| Age at PSG | 59.2 (14.3) | 53.6 (17.4) | 50.4 (12.6) | 66.8 (5.1) | D > B,C |
| Age at DEB/RBD Dx | - | - | 50.4 (12.5) | 63.1 (5.9) | D > C |
| Age at PTSD Dx | - | 47.0 (19.1) | 43.7 (11.9) | - | - |
| Antidepressant Use (Y) | 0 | 9 | 17 | 0 | - |
| BMI | 30.1 (7.2) | 32.3 (6.8) | 31.5 (7.3) | 29.3 (4.2) | - |
| ESS | 9.1 (5.4) | 8.7 (6.9) | 10.1 (6.6) | 9.7 (3.9) | - |
| REM AHI | 3.3 (5.6) | 6.1 (7.1) | 3.4 (2.9) | 1.5 (3.5) | A,B,C > D |
| TST (Min.) | 311.1 (109.0) | 207.6 (129.2) | 328.5 (106.0) | 290.8 (116.7) | A > B C > B |
| N1% | 11.2 (8.8) | 16.3 (11.0) | 8.5 (5.2) | 13.5 (6.2) | B,D > C |
| N2% | 52.7 (20.4) | 51.0 (17.6) | 50.6 (15.6) | 47.8 (14.6) | - |
| N3% | 21.0 (10.8) | 12.9 (15.5) | 13.7 (10.7) | 18.1 (15.5) | A > B,C |
| REM% | 18.3 (6.8) | 20.1 (10.2) | 27.2 (15.9) | 20.6 (7.2) | C > A |
| REM Time (Min.) | 59.3 (34.8) | 36.8 (22.8) | 79.9 (39.3) | 57.1 (24.4) | A,C,D > B C > D |
| SE | 73.4 (16.9) | 68.8 (19.4) | 85.1 (10.1) | 72.9 (17.8) | C > A,B,D |
| ISL | 18.2 (15.0) | 24.2 (29.4) | 23.0 (20.0) | 18.5 (21.6) | - |
| IRL | 98.2 (65.3) | 124.1 (119.0) | 148.0 (114.2) | 95.8 (77.4) | - |
| PLMI | 4.8 (6.7) | 21.5 (36.0) | 35.3 (29.7) | 32.3 (38.8) | C,D > A |
| PLMAI | 5.9 (14.7) | 7.2 (9.6) | 5.6 (4.9) | 6.6 (7.9) | A > C |
| AI (/hour) | 17.9 (8.8) | 28.0 (17.4) | 16.6 (7.1) | 22.0 (7.5) | B > C D > A |
AHI, apnea-hypopnea Index; AI, arousal index; BMI, body mass index; DEB, dream enactment behavior; ESS, Epworth Sleepiness Scale; ISL, initial sleep latency; IRL, initial REM latency; PLMI, periodic limb movement index; PLMAI, periodic limb movement arousal index; RBD, REM sleep behavior disorder; SE, sleep efficiency; TST, total sleep time.
The traumatic event associated with PTSD diagnosis is outlined in Supplementary Table 1. None of our PTSD without DEB patients reported or demonstrated DEBs during PSG, while all of the PTSD+DEB patients either reported (n = 15) DEB by history or demonstrated (n = 6) DEB during PSG.
Within the PTSD without DEB and PTSD+DEB groups, 28% of the subjects experienced symptoms of sleep behavioral disturbance within a few months of the inciting traumatic experience (Supplementary Table 2). Fifty percent of PTSD+DEB patients reported having dream mentation related to their trauma, compared with only 11% of PTSD patients without DEB (p < .05). No subjects had EEG epileptiform activity on PSG.
RSWA analysis
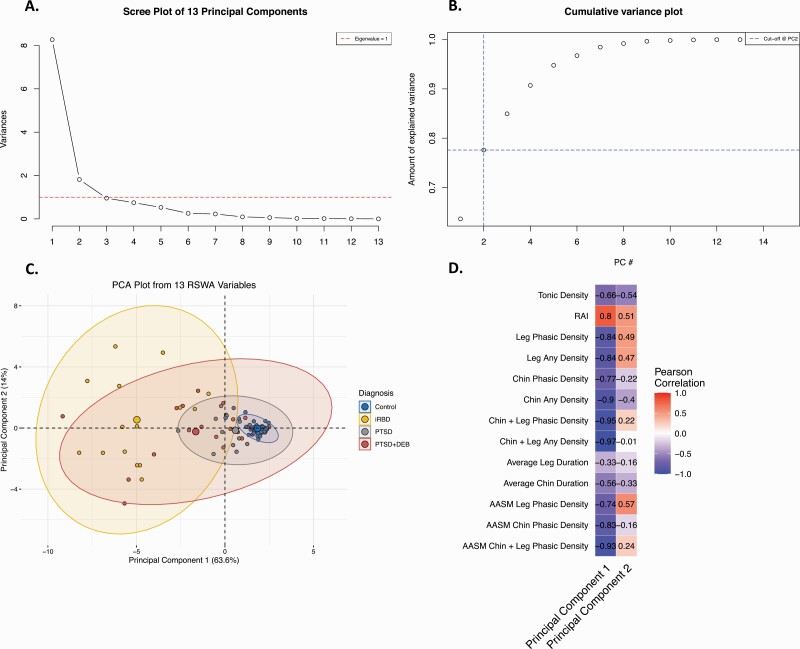
Principal component analysis determined the first two components to have eigen values above 1 (Figure 1A). These two components accounted for 78% of the variance seen within the RSWA values (Figure 1B). Separation between the groups (Control, iRBD, PTSD, PTSD + DEB) is apparent in Figure 1C. All RSWA metrics except Average Leg Duration were highly correlated with the first principal component (Figure 1D). Tonic density, RAI, and AASM Leg Phasic RSWA metrics were correlated with the second principal component (Figure 1D).
Figure 1.
Principal Component Analysis for REM sleep without atonia (RSWA) values in PTSD with and without dream enactment and control groups yielded 13 principal components. (A) Scree plot of the 13 principal components showed that principal components #1 and 2 exceeded eigenvalues of 1. (B) Cumulative variance plot demonstrating the eigenvalues and amount of RSWA variance explained for each of the 13 principal components, demonstrating that components 1 and 2 together explained 78% of the variance in RSWA values. (C) Principal Components Plot for the 13 RSWA principal components shows clear separation between the iRBD, PTSD, PTSD+DEB, and control groups. (D) Pearson correlation coefficients for the RSWA principal components 1 and 2 and manually and automatically (RAI) determined RSWA metrics demonstrate that all RSWA metrics (except average leg duration) were highly correlated with principal component 1, while tonic density, RAI, and AASM leg phasic RSWA metrics were correlated with component 2.
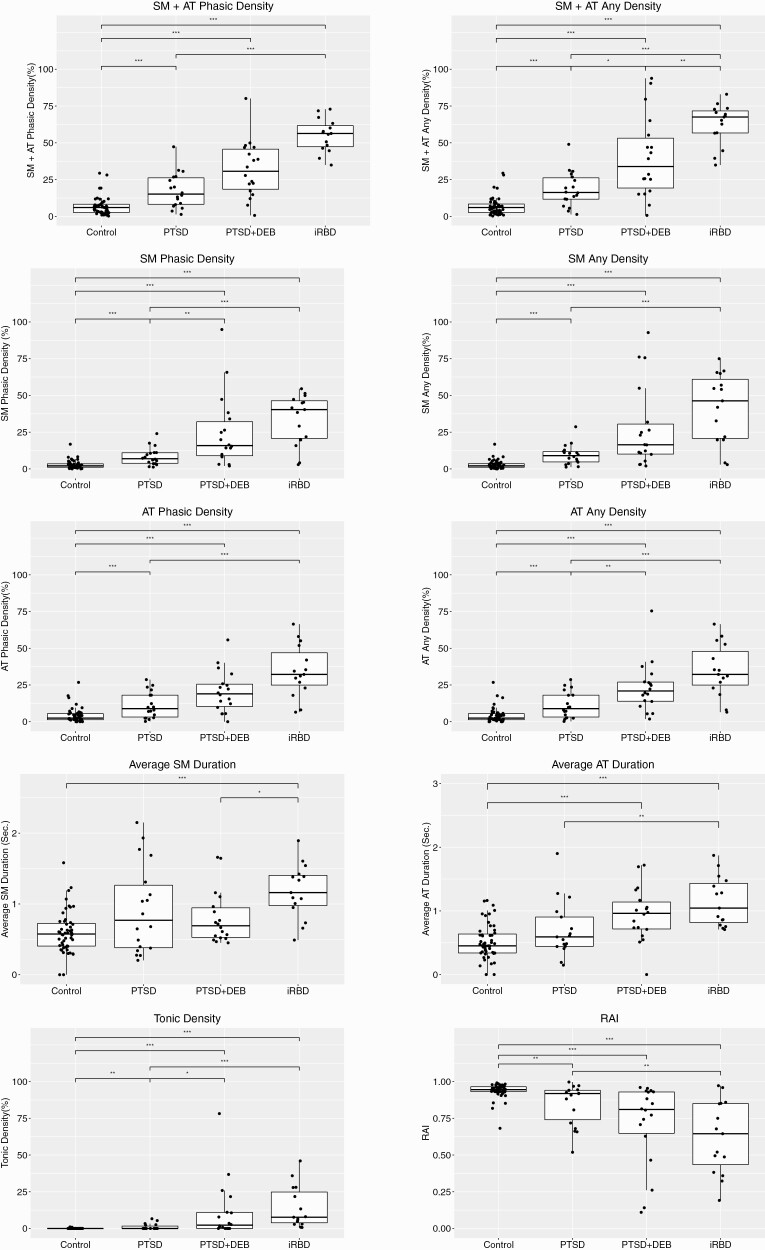
All measures of RSWA were significantly greater in iRBD patients compared with PTSD patients or controls. All RSWA measures were also significantly greater in PTSD+DEB and PTSD without DEB patients compared with controls, including the primary outcome of SM+AT “any” muscle activity, except for average SM duration (Table 2, Figure 2; for AASM metrics, see also Supplementary Figure 1). Sensitivity analyses with outliers included and removed in each of the subgroup comparisons did not change results. Most RSWA measures were higher in the PTSD+DEB subgroup than in the PTSD without DEB patients, including higher SM+AT Any, SM Phasic, AT Any, and Tonic muscle activity (p < 0.025 for all). Eighty-nine percent of all patients with PTSD met at least one diagnostic RSWA threshold for RBD, including all patients in the PTSD+DEB subgroup [33] (Supplementary Table 3). Additionally, 78% of all PTSD patients met at least one 95th normative RSWA percentile threshold, including all of the PTSD+DEB subgroup [38]. Normative percentiles within each group are shown in Supplementary Table 4, demonstrating similar trends across RSWA metric comparisons (Table 2).
Table 2.
Comparative quantitative RSWA metrics between PTSD patients with and without DEB, idiopathic/isolated RBD patients, and controls. The primary outcome of combined SM+AT “Any” RSWA Density was highest in iRBD patients, and intermediately elevated in PTSD+DEB and PTSD without RBD patient groups in comparison to controls. Most other RSWA metrics followed a similar pattern, with details provided below.
| Control A | PTSD B | PTSD+DEB C | iRBD D | p < .05 | p < .025 | |
|---|---|---|---|---|---|---|
| Average SM Duration (Sec.) | 0.6 (0.3) | 0.9 (0.6) | 0.8 (0.4) | 1.2 (0.4) | D > A,C C > A |
D > A |
| Average AT Duration (Sec.) | 0.5 (0.3) | 1.2 (2.1) | 0.9 (0.4) | 1.1 (0.4) | C,D > A D > B |
C,D > A D > B |
| SM Phasic Density (%) | 2.8 (2.9) | 8.4 (6.0) | 24.8 (24.3) | 33.8 (17.1) | B,C,D> A C,D > B |
B,C,D > A C,D > B |
| AT Phasic Density (%) | 4.3 (5.0) | 11.3 (9.0) | 20.4 (14.2) | 34.6 (17.6) | B,C,D > A C,D > B D > C |
B,C,D > A D > B D > C |
| SM Any Density (%) | 2.9 (2.9) | 9.5 (6.7) | 27.5 (28.1) | 41.8 (23.6) | B,C,D > A C,D > B |
B,C,D > A C,D > B |
| AT Any Density (%) | 4.2 (5.0) | 11.3 (9.0) | 23.4 (16.9) | 34.8 (17.7) | B,C,D > A C,D > B D > C |
B,C,D > A C,D > B |
| SM+AT Phasic Density (%) | 6.9 (6.1) | 17.6 (11.9) | 31.9 (19.2) | 55.1 (11.2) | B,C,D > A C,D > B D > C |
B,C,D > A C,D > B D > C |
| SM+AT Any Density (%) | 7.0 (6.2) | 18.5 (11.9) | 40.1 (27.7) | 62.8 (13.9) | B,C,D> A C,D > B D > C |
B,C,D > A C,D > B D > C |
| RAI | 0.94 (0.05) | 0.85 (0.14) | 0.75 (0.22) | 0.62 (0.25) | B,C,D < A D < B |
B,C,D < A D < B |
| Tonic Density (%) | 0.04 (0.18) | 1.1 (2.1) | 11.2 (19.8) | 14.2 (14.2) | B,C,D> A C,D > B |
B,C,D > A C,D > B |
| AASM SM Phasic (%) | 0.2 (0.5) | 0.6 (1.1) | 9.0 (15.8) | 33.3 (21.2) | C,D > A D > B C > B |
C,D > A D > B C > B |
| AASM AT Phasic (%) | 0.6 (1.8) | 2.6 (4.3) | 9.0 (9.3) | 33.1 (25.6) | C > B | C,D > A D > B |
| AASM Combined (%) | 1.1 (2.8) | 4.9 (7.8) | 20.4 (19.9) | 61.8 (18.4) | C,D > A C,D > B |
AT, anterior tibialis; DEB, dream enactment behavior; RSWA, REM sleep without atonia; SM, Submentalis.
Figure 2.
Quantitative REM sleep (REM sleep without atonia) analyses in PTSD patients with and without dream enactment behavior, IRBD patients, and controls. Shown are group differences between phasic, any, tonic, and automated RAI RSWA metrics in the chin (SM, submentalis) and leg (AT, anterior tibialis) between PTSD patients with and without dream enactment (PTSD+DEB, PTSD) patients, idiopathic/isolated RBD (iRBD) patients, and age- and sex-matched controls. Levels of significant group differences are designated by: one asterisk (*) = p < .025; two asterisks (**) = p < .01; and three asterisks (***) = p < .001. RAI, automated REM Atonia Index.
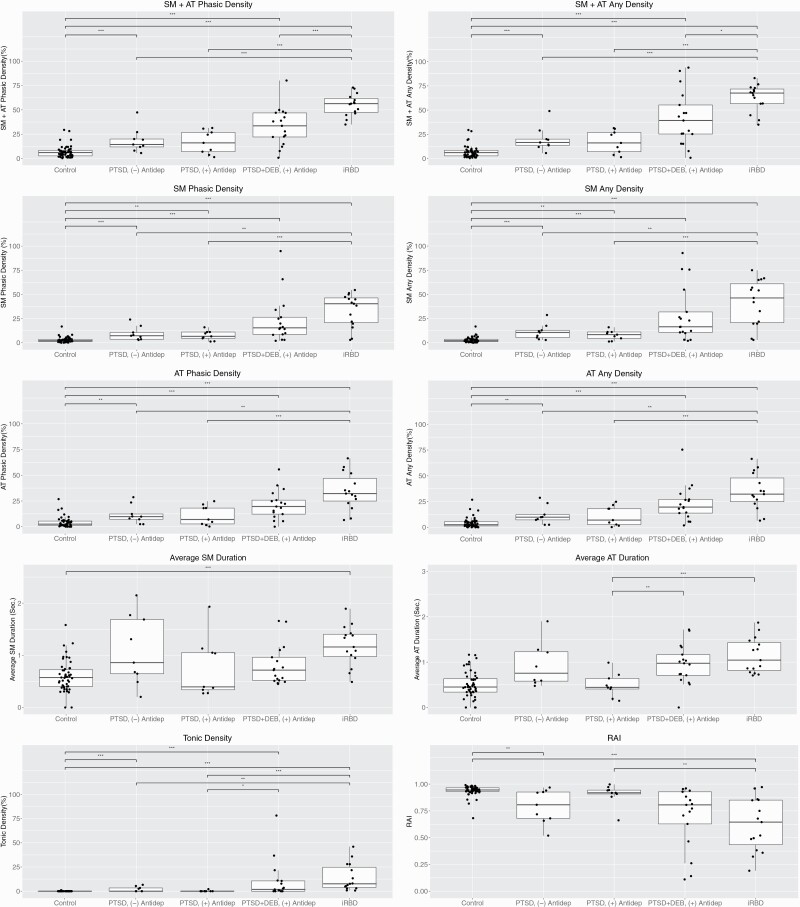
PTSD patients not receiving concurrent antidepressant treatment also had higher RSWA than controls including the primary outcome of SM+AT “any” muscle activity (p < .025 for all, Figure 3). Within the PTSD without DEB group, RSWA levels were similar regardless of concurrent antidepressant treatment (Figure 3). PTSD patients with concurrent antidepressant treatment only had significantly higher RSWA than controls for the SM phasic and “any” RSWA metrics (both p < .025).
Figure 3.
Quantitative REM sleep (REM sleep without atonia) analyses in PTSD patients with and without dream enactment behavior according to antidepressant use, IRBD patients, and controls. Shown are group differences between phasic, any, tonic, and automated RAI RSWA metrics in the chin (SM, submentalis) and leg (AT, anterior tibialis) between PTSD patients with and without dream enactment receiving ((+) Antidep) or not receiving ((-) Antidep) antidepressant medications, idiopathic/isolated RBD (iRBD) patients, and age- and sex-matched controls. Levels of significant group differences are designated by: one asterisk (*) = p < .025; two asterisks (**) = p < .01; and three asterisks (***) = p < .001. RAI, automated REM Atonia Index.
PTSD+DEB, PTSD without DEB patients, and iRBD patients each had higher RSWA than controls for all measures in regression modeling accounting for age and PLMI (Table 3). Additionally, all RSWA density measures were higher in PTSD+DEB and iRBD than PTSD patients without DEB while accounting for age and PLMI (Table 3).
Table 3.
Multivariate regression modeling of quantitative RSWA between PTSD patients with and without DEB, idiopathic/isolated RBD patients, and controls, accounting for age and PLMI
| Average SM duration accounting for age | p-value | ||
| PTSD > Controls | .007 | ||
| PTSD + RBD > Controls | .03 | ||
| iRBD > Controls | <.001 | ||
| Average AT duration accounting for age | |||
| PTSD > Controls | .04 | ||
| PTSD + RBD > Controls | <.001 | ||
| iRBD > Controls | <.001 | ||
| Tonic density accounting for age | |||
| PTSD > Controls | .001 | ||
| PTSD+RBD > Controls | <.001 | ||
| iRBD > PTSD | .003 | ||
| PTSD + RBD > PTSD | .02 | ||
| SM phasic density accounting for age | SM any density accounting for age | ||
| PTSD > Controls | <0.001 | PTSD > Controls | <0.001 |
| PTSD+RBD > Controls | <0.001 | PTSD+RBD > Controls | <0.001 |
| iRBD > Controls | <0.001 | iRBD > Controls | <0.001 |
| iRBD > PTSD | <0.001 | iRBD > PTSD | <0.001 |
| PTSD + RBD > PTSD | 0.01 | PTSD + RBD > PTSD | 0.007 |
| AT phasic density accounting for age and PLMI | AT any density accounting for age and PLMI | ||
| PTSD > Controls | <0.001 | PTSD > Controls | <0.001 |
| PTSD+RBD > Controls | <0.001 | PTSD+RBD > Controls | <0.001 |
| iRBD > Controls | <0.001 | iRBD > Controls | <0.001 |
| iRBD > PTSD | <0.001 | iRBD > PTSD | <0.001 |
| PTSD + RBD > PTSD | 0.02 | PTSD + RBD > PTSD | 0.01 |
| SM + AT phasic density accounting for age and PLMI | SM + AT any density accounting for age and PLMI | ||
| PTSD > Controls | <0.001 | PTSD > Controls | <0.001 |
| PTSD + RBD > Controls | <0.001 | PTSD + RBD > Controls | <0.001 |
| iRBD > Controls | <0.001 | iRBD > Controls | <0.001 |
| iRBD > PTSD | <0.001 | iRBD > PTSD | <0.001 |
| iRBD > PTSD + RBD | 0.03 | PTSD + RBD > RBD | 0.003 |
| PTSD + RBD > RBD | 0.01 |
AT, anterior tibialis; DEB, dream enactment behavior; RSWA, REM sleep without atonia; SM, Submentalis.
Discussion
We found that PTSD patients have higher RSWA than controls, regardless of the presence of clinical manifestations of DEB. Given the results of our principal component analysis, most RSWA metrics contributed toward components that explained the data variance. These data provide the first complete objective profile of abnormal REM sleep atonia control in patients with chronic PTSD including those with DEB. While RSWA in PTSD was lower than in “traditional” iRBD, RSWA associated with PTSD is clearly distinguishable from controls. REM atonia control is abnormal in iRBD and chronic PTSD (whether or not dream-enactment history is present in the latter) compared to controls. All patients with PTSD and DEB were on antidepressants, which could potentially indicate PTSD severity and contribute to medication-induced RSWA. Given this parallel in biology between these conditions, and given previous evidence that PTSD patients are at higher risk for developing defined synucleinopathies including dementia with Lewy bodies [42] and Parkinson disease [43], prospective cohort studies should determine whether a subset of PTSD patients could also be at risk for evolving dementia or parkinsonism (as seen in the at-risk population of iRBD) and whether any PTSD patients have evidence for possible covert synucleinopathy pathology suggested by presence of neurodegenerative markers such as objective hyposmia, orthostatic hypotension, or subtle motor abnormalities suggestive of bradykinesia, rigidity, or postural instability.
Previous studies have described associations between PTSD, RBD, and other REM sleep disturbances [16,44,45]. The presumptive neurobiology of PTSD is complex and multifold, but may involve increased norepinephrine neurotransmission and turnover, altered hypothalamic-pituitary-adrenal axis functioning and altered glucocorticoid signaling, and possible eventual norepinephrine depletion and apoptotic neuronal cell death in the locus coeruleus as suggested in a prolonged stress animal model of PTSD [46,47]. Recent evidence has suggested that norepinephrine and cortisol interact to increase negative intrusive memories in PTSD [48]. While REM sleep regulation involves pontine centers, including the glutamatergic subcoreuleus and cholinergic pedunculopontine and laterodorsal tegmental nuclei, the medullary magnocellular reticular formation is also involved, with additional modulation by the hypothalamus, substantia nigra, basal forebrain, limbic system, and frontal cortex. The locus coeruleus may also be involved in mediating REM sleep atonia control, in addition to the greater role served by the neighboring subcoeruleus [49–53]. The overlapping pathophysiology underlying PTSD and RBD could lead to a similar alteration in REM sleep atonia control and related clinical manifestations of dream enactment symptoms during sleep.
A recent cross-sectional study that compared intrusive memories following viewing of pictures with positive, negative, or neutral valence in PTSD patients with controls who were exposed or unexposed to previous trauma, found that probable RBD symptoms mediated greater negative waking intrusive memories in PTSD patients than controls, suggesting that REM sleep dream enactment (or, perhaps, reenactment of one’s own previous traumatic experiences) may consolidate waking intrusive memories and learned fear in PTSD.[54] It was recently postulated that altered network connectivity between the medial prefrontal cortico-striatal executive control pathways and the central amygdalar nucleus may underlie maladaptive emotional processing in PTSD [55,56]. Evidence for deficient emotional memory-consolidative processing during the REM sleep state in PTSD was provided by findings of relatively reduced right prefrontal theta frequencies in PTSD patients compared to resilient, adaptive trauma-exposed individuals [57,58]. Another similar experiment showed an alternative hyperarousal EEG signature in PTSD, with reduced NREM centro-parietal delta activity, accompanied by increased antero-frontal gamma during both NREM and REM sleep [58], which could correlate with the potential for post-traumatic nightmares to arise from either NREM or REM sleep as has been shown in another recent study [59]. Similar reductions in frontal slow-wave activity and increased fast activity in PTSD relative to controls were also recently independently demonstrated [60].
Our PTSD without RBD patients had elevated RSWA compared to controls, with 89% meeting RSWA thresholds for iRBD diagnosis, albeit not rising to the usually even higher levels seen in iRBD. Importantly, while there were imbalances in the frequency of antidepressant use across the PTSD groups, our PTSD patients without DEB had similar RSWA levels regardless of whether they were receiving antidepressants, demonstrating that abnormal REM sleep atonia control correlates with disease processes in PTSD specifically rather than simply being driven by antidepressant use. This finding stands in contrast to some previous studies showing that antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), are associated with higher levels of RSWA [35]. This difference could be due in part to our study-inclusion criteria that required a DSM-5 PTSD diagnosis, in comparison to previous studies measuring RSWA levels within broader and less specific psychiatric populations, where antidepressant therapies rather than the underlying psychiatric diagnosis may drive elevated RSWA. Additional future prospective studies comparing RSWA in antidepressant naive PTSD patients to those receiving antidepressants should be undertaken to confirm these findings.
Several hypotheses could be developed from these data. First, the symptoms of DEB in PTSD that mimic RBD could be due to neurochemical and functional neurophysiologic changes intrinsic to PTSD, unassociated with synucleinopathy. Second, given altered REM sleep atonia control distinct from controls (yet not at the levels seen in iRBD), synuclein neuropathology could underlie PTSD similar to (but even earlier than in) “traditional” iRBD, with PTSD later developing into usual iRBD or other Lewy body disease trajectories. If this were the case, then patients with PTSD should also harbor additional symptoms or covert signs of synucleinopathy neurodegeneration (e.g. objective hyposmia, color vision loss, “soft” cognitive, motor, or autonomic markers), intermediate in expression compared to iRBD patients and controls, as previously shown in a cross-sectional study of patients with iRBD who were receiving or not receiving antidepressant medications [61]. Third, antidepressants could differentially impact RSWA levels within the psychiatric population depending on the underlying psychiatric disorder/disease. Further larger-scale comparative cross-sectional and prospective cohort studies may elucidate whether synucleinopathy is present in a subset of PTSD survivors and clarify mechanisms of disease and outcomes for this patient population.
This study has several limitations. As a retrospective study at a tertiary center, selection and referral biases are likely. Also, in our RBD patients alone, we had only limited availability of arm-muscle recordings, which have been shown to further improve diagnostic accuracy for identifying RSWA in RBD [62]. Additionally, in this retrospective study, we had no information on other possible markers for neurodegeneration, such as hyposmia, or detailed cognitive, motor, or autonomic tests. Our PTSD+DEB and iRBD groups were based on a clinical history positive for PTSD in the PTSD+DEB group and absence of PTSD history in the iRBD group, but these groups could have had some overlapping clinical features. All PTSD diagnoses were confirmed by board certified psychiatrists, although consistency of diagnoses with DSM V criteria was verified retrospectively by medical record review without the use of standardized assessment tools, which could have limited diagnostic accuracy for PTSD. Our retrospective design may have also underestimated dream mentation related to previous trauma, which was reported in only 11% of our PTSD without DEB and 50% of PTSD with DEB patients in our cohort. However, in a recent study of veterans with PTSD using ambulatory polysomnography, only 7 (29.2%) of 24 reported nightmares involving traumatic replay, whereas in the remaining 17 (70.8%) nightmares, either purely non-replay, or mixed replay/non-replay content was reported [59]. Given the paucity of recorded DEB and nightmares captured during PSG, future prospective studies should delineate whether or not PTSD+DEB and iRBD patients may have clinically distinct nightmares and DEBs. Future studies should also seek to illuminate the complex relationship between disruptive nocturnal behaviors and dream mentation, since it remains unclear whether RBD and PTSD patients who exhibit complex nocturnal behaviors are actually “acting out dreams” (i.e. with “top-down” limbic and motor cortex generated dream content driving downstream DEBs given permissive altered REM sleep atonia control at the brainstem level), or instead, “dreaming out acts” caused by “bottom up” brain stem generation of complex motor behaviors, with sensory feedback from limb movements modulating dream imagery and content [63]. Information concerning the temporal relationship of initial traumatic events and onset of sleep disturbances was not available in our retrospective study, and further prospective cohort studies following traumatic life experiences will be necessary to better understand the complex temporal relationships between initial stress and later development of sleep disturbances. Most of our PTSD+DEB patients received antidepressants, although subgroup analyses of those patients on and off antidepressants did not suggest that medication differences alone explained findings of elevated RSWA in PTSD. Further limitations of our retrospective study design included that it was also difficult to determine the frequency of dream mentation related to trauma, and the temporal relationship between PTSD symptom and DEB onset for a small subset of our PTSD patients. Future prospective cohort studies utilizing polysomnography will be needed to accurately classify PTSD diagnoses, clarify the relationship between previous trauma and dream mentation and the temporal relationship between PTSD symptom and DEB onset, determine the influence of antidepressant medications on dream enactment, and understand the influences of PTSD severity and treatment on RSWA amounts. Sex imbalances within subgroups could contribute toward unexpected findings with respect to sleep stage differences, as REM fragmentation might be more pronounced in women with PTSD than men with PTSD [64]. While sex differences in RSWA levels, such as older men demonstrating higher amounts, have been reported, further research is necessary to understand the impact of sex on RSWA [38,65]. Future research is also needed to clarify whether our findings apply to community-based and military samples of PTSD patients and to determine the extent of overlap or clear distinctions between the PTSD and RBD patient populations.
Conclusion
PTSD appears to have elevated RSWA, albeit not to the levels seen within iRBD, suggesting abnormal REM atonia control is part of the spectrum of objective REM sleep abnormalities typical of PTSD, similar to the pathophysiology seen in RBD. Our data provide systematic evidence for abnormal REM sleep atonia control in chronic PTSD, which is a distinctive neurobiological characteristic, independent of antidepressant use. Future prospective studies of PTSD patients using polysomnography are necessary to confirm these retrospective findings, and to determine whether quantitative RSWA is a useful objective disease marker in PTSD and to define the overlap and distinctions between PTSD and iRBD.
Supplementary Material
Acknowledgments
The authors are also grateful for the assistance of Ms. Lea Dacy, Department of Neurology, for assistance with secretarial support for manuscript submission.
Funding
This publication was supported by NIH/NCRR/NCATS CCaTS Grant Number UL1 TR002377, and by NIH/NIA R34AG056639 (NAPS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Deposit of Material in a Data Repository
Not applicable.
Disclosure Statement
R.R.A. receives research support from the American Sleep Foundation. B.F.B. receives royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2017). He receives research support from the NIH, and the Mangurian Foundation and Little Family Foundation. E.K.S.L. reports that he receives research support from the Mayo Clinic Center for Translational Science Activities (CCaTS), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01; from the Mayo Clinic Alzheimer’s Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574); from NIH/NIA (R34AG056639); from Michael J. Fox Foundation; and from Sunovion, Inc. R.R.A. is a member of the DSM-5-TR Study Section, Sleep Wake Disorders Section: Circadian Rhythm Sleep-Wake Disorders Subsection and chairs the American Academy of Sleep Medicine, ICSD-3 Circadian Rhythm Sleep-Wake Disorders Work Group. B.F.B. reports that he is an investigator in clinical trials sponsored by Biogen, Alector, and EIP Pharma. He is on the Scientific Advisory Board of the Tau Consortium.
References
- 1. Bisson JI, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97–104. [DOI] [PubMed] [Google Scholar]
- 2. Pillar G, et al. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med Rev. 2000;4(2):183–200. [DOI] [PubMed] [Google Scholar]
- 3. Spoormaker VI, et al. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. [DOI] [PubMed] [Google Scholar]
- 4. Kearns MC, et al. Early interventions for PTSD: a review. Depress Anxiety. 2012;29(10):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breslau N, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626–632. [DOI] [PubMed] [Google Scholar]
- 6. Tanielian TL, et al.. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. MG-720-CCF. Santa Monica, CA:RAND Corporation; 2008. https://www.rand.org/pubs/monographs/MG720.1.htm [Google Scholar]
- 7. Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 8. Lamarche LJ, et al. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257–1270. [DOI] [PubMed] [Google Scholar]
- 9. Green BL, et al. Post-traumatic stress disorder in victims of disasters. Psychiatr Clin North Am. 1994;17(2):301–309. [PubMed] [Google Scholar]
- 10. Harvey AG, et al. The relationship between acute stress disorder and posttraumatic stress disorder: a prospective evaluation of motor vehicle accident survivors. J Consult Clin Psychol. 1998;66(3):507–512. [DOI] [PubMed] [Google Scholar]
- 11. Ross RJ, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. [DOI] [PubMed] [Google Scholar]
- 12. Khazaie H, et al. Sleep disturbances in veterans with chronic war-induced PTSD. J Inj Violence Res. 2016;8(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leskin GA, et al. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449–452. [DOI] [PubMed] [Google Scholar]
- 14. Woodward SH, et al. Movement during sleep: associations with posttraumatic stress disorder, nightmares, and comorbid panic disorder. Sleep. 2002;25(6):681–688. [PubMed] [Google Scholar]
- 15. Ross RJ, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. [DOI] [PubMed] [Google Scholar]
- 16. Husain AM, et al. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148–157. [DOI] [PubMed] [Google Scholar]
- 17. Richards A, et al. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology 2020;45:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mellman TA, et al. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. [DOI] [PubMed] [Google Scholar]
- 19. Mellman TA, et al. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;37(8):1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi I, et al. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, et al. Sleep in posttraumatic stress disorder: a systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev. 2019;48:101210. [DOI] [PubMed] [Google Scholar]
- 22. Germain A, et al. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res. 2013;211(2):176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Postuma RB, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elliott JE, et al.. Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in Veterans with and without comorbid traumatic brain injury. Sleep 2020;43(3). doi: 10.1093/sleep/zsz237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohayon MM, et al. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–478. [DOI] [PubMed] [Google Scholar]
- 27. Kang SH, et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haba-Rubio J, et al.. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 2018;41(2). doi: 10.1093/sleep/zsx197 [DOI] [PubMed] [Google Scholar]
- 29. Shprecher DR, et al. Prevalence of REM sleep behavior disorder in Sun City, Arizona. Heliyon. 2020;6(1):e03140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Publishing. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.. Washington, DC: American Psychiatric Publishing; 2013 [Google Scholar]
- 31. Boeve BF, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12(5):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boeve BF, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. 2013;9(5):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarter SJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017;33:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCarter SJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014;37(10):1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarter SJ, et al. Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep. 2015;38(6):907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications: Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 37. Lapierre O, et al. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42(7):1371–1374. [DOI] [PubMed] [Google Scholar]
- 38. Feemster JC, et al. Normative and isolated rapid eye movement sleep without atonia in adults without REM sleep behavior disorder. Sleep 2019;42(10). doi: 10.1093/sleep/zsz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferri R, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res. 2008;17(1):89–100. [DOI] [PubMed] [Google Scholar]
- 40. Ferri R, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010;11(9):947–949. [DOI] [PubMed] [Google Scholar]
- 41. R Core Team. R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/. [Google Scholar]
- 42. Yaffe K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White DL, et al. Post-traumatic stress disorder is associated with further increased Parkinson’s disease risk in veterans with traumatic brain injury. Ann Neurol. 2020;88(1):33–41. [DOI] [PubMed] [Google Scholar]
- 44. Elliott JE, et al. Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in Veterans with and without comorbid traumatic brain injury. Sleep. 2020;43(3). doi: 10.1093/sleep/zsz237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ross RJ, et al. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35(3):195–202. [DOI] [PubMed] [Google Scholar]
- 46. Yehuda R, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. [DOI] [PubMed] [Google Scholar]
- 47. Zhao W, et al. IRE1alpha pathway of endoplasmic reticulum stress induces neuronal apoptosis in the locus coeruleus of rats under single prolonged stress. Prog Neuropsychopharmacol Biol Psychiatry 2016;69:11–18. [DOI] [PubMed] [Google Scholar]
- 48. Nicholson EL, et al. Interaction of noradrenaline and cortisol predicts negative intrusive memories in posttraumatic stress disorder. Neurobiol Learn Mem. 2014;112:204–211. [DOI] [PubMed] [Google Scholar]
- 49. Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967;47(2):117–177. [DOI] [PubMed] [Google Scholar]
- 50. Sakai K, et al. Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res. 1979;176(2):233–254. [DOI] [PubMed] [Google Scholar]
- 51. St Louis EK, et al. REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin Proc. 2017;92(11):1723–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu J, et al. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. [DOI] [PubMed] [Google Scholar]
- 53. Luppi PH, et al. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med. 2013;14(8):714–718. [DOI] [PubMed] [Google Scholar]
- 54. Ney LJ, et al. The effect of self-reported REM behavior disorder symptomology on intrusive memories in post-traumatic stress disorder. Behav Sleep Med. 2021;19(2):178–191. [DOI] [PubMed] [Google Scholar]
- 55. Ney LJ, et al. The effect of self-reported REM behavior disorder symptomology on intrusive memories in post-traumatic stress disorder. Behav Sleep Med 2020:1–14. [DOI] [PubMed] [Google Scholar]
- 56. Murkar ALA, et al. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med Rev. 2018;41:173–184. [DOI] [PubMed] [Google Scholar]
- 57. Cowdin N, et al. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232(5):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang C, et al. An attempt to identify reproducible high-density EEG markers of PTSD during sleep. Sleep 2020;43(1). doi: 10.1093/sleep/zsz207 [DOI] [PubMed] [Google Scholar]
- 59. Phelps AJ, et al. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep 2018;41(1). doi: 10.1093/sleep/zsx188 [DOI] [PubMed] [Google Scholar]
- 60. de Boer M, et al. The spectral fingerprint of sleep problems in post-traumatic stress disorder. Sleep 2020;43(4). doi: 10.1093/sleep/zsz269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Postuma RB, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013;36(11):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Frauscher B, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blumberg MS, et al. A new view of “dream enactment” in REM sleep behavior disorder. Sleep Med Rev. 2016;30:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kobayashi I, et al. Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behav Sleep Med. 2012;10(3):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCarter SJ, et al. Greatest rapid eye movement sleep atonia loss in men and older age. Ann Clin Transl Neurol. 2014;1(9):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data obtained are included within the manuscript and Supplementary Material.