Abstract
Gonadal steroids and gender are risk factors for sleep disruptions and insomnia in women. However, the relationship between ovarian steroids and sleep is poorly understood. In rodent models, estradiol (E2) suppresses sleep in females suggesting that E2 may reduce homeostatic sleep need. The current study investigates whether E2 decreases sleep need and the potential mechanisms that govern E2 suppression of sleep. Our previous findings suggest that the median preoptic nucleus (MnPO) is a key nexus for E2 action on sleep. Using behavioral, neurochemical, and pharmacological approaches, we tested whether (1) E2 influenced the sleep homeostat and (2) E2 influenced adenosine signaling in the MnPO of adult female rats. In both unrestricted baseline sleep and recovery sleep from 6-h sleep deprivation, E2 significantly reduced nonrapid eye movement (NREM) sleep-delta power, NREM-slow wave activity (NREM-SWA, 0.5–4.0 Hz), and NREM-delta energy suggesting that E2 decreases homeostatic sleep need. However, coordinated with E2-induced changes in physiological markers of homeostatic sleep was a marked increase in MnPO extracellular adenosine (a molecular marker of homeostatic sleep need) during unrestricted and recovery sleep in E2-treated but not oil control animals. While these results seemed contradictory, systemically administered E2 blocked the ability of CGS-21680 (adenosine A2A receptor agonist) microinjected into the MnPO to increase NREM sleep suggesting that E2 may block adenosine signaling. Together, these findings provide evidence that E2 may attenuate the local effects of the A2A receptors in the MnPO, which in turn may underlie estrogenic suppression of sleep behavior as well as changes in homeostatic sleep need.
Keywords: estradiol, adenosine, median preoptic nucleus, sleep
Statement of Significance.
While gonadal steroids and gender are implicated as risk factors for sleep disruptions and insomnia, the relationship between ovarian steroids and sleep is poorly understood. Understanding the mechanisms through which estradiol (E2) is working to influence sleep–wake behavior is a critical first step toward a better understanding of the role of estrogens in sleep pathologies. Using a rodent model, the current study presents novel findings suggesting that estradiol (E2) is influencing adenosinergic actions in the MnPO. The ability of E2 to attenuate the local effects of the A2A receptors in the MnPO suggests that E2 modulation of A2A receptor signaling may underlie estrogenic suppression of sleep behavior as well as changes in homeostatic sleep need.
Introduction
Primary sleep disorders are among the most common medical conditions, as many as one in three individuals may have sleep problems [1–3] with an annual economic impact of over $100 billion [4]. Clinical data show women are 40% more likely than men to experience one or more symptoms of insomnia over their lifespan [5–7]. This increased risk emerges at puberty [8, 9] and has been associated with fluctuations in ovarian steroids, particularly estrogens, suggesting that gonadal steroids and biological sex are significant risk factors for sleep disruptions [10]. While chronic insufficient sleep is a risk factor for a variety of psychological [11–15], neurological, and neurodegenerative pathologies [16–23] as well as cardiovascular and metabolic dysfunctions [24–28], clinical studies reveal that women suffering from sleep disturbances and insufficient sleep are at greater risk compared with men for mood disorders such as depression [29] as well as metabolic [30] and cardiovascular dysfunction [28, 31–33]. Thus, given the increased risks to psychological and physiological well-being, sleep disorders among women are a significant public health concern.
Despite a deepening understanding of sleep regulatory mechanisms, how estrogens influence sleep circuitry is poorly understood. In women approaching menopause, the loss of estrogens is associated with insomnia, frequent nighttime awakenings, and poor sleep [10]. In contrast, recent data from young women of reproductive age suggest that the presence of estrogens are a contributing factor to sleep disturbances [34–38]. In order to better understand the biological basis for these apparent paradoxical effects of estrogens on sleep disturbances across a women’s lifespan, it is necessary to understand the mechanisms more fully through which estrogens are influencing the sleep circuitry.
Historically, male rodents have served as the cornerstone for elucidating the neural circuitries governing sleep. Unfortunately, this has resulted in a significant gap in our understanding of how estrogens modulate these circuits in females. Female rodents offer the opportunity to probe the sleep circuitry in order to elucidate the mechanisms by which ovarian steroids modulate sleep. Sleep patterns in the female rat are exquisitely sensitive to fluctuations in endogenous levels of ovarian steroids such as estradiol (E2) [39–41]. The highly reproducible effects of exogenously administered E2 on sleep in female rodents provide an informative bioassay to investigate how E2 may be affecting sleep mechanisms. Using adult female rats, studies consistently demonstrate that sleep time is significantly reduced when endogenous ovarian steroids or exogenous E2 are elevated in females [40, 42] but not males [42]. Our previous findings suggest this change in sleep may be mediated through E2 actions in the preoptic area (POA) nuclei associated with sleep induction and maintenance which includes the ventrolateral preoptic (VLPO) area [40, 43, 44] and the median preoptic nucleus (MnPO) [45]. Moreover, these both the VLPO and MnPO have been implicated in sensing homeostatic sleep need [46–49].
The sleep homeostat governs the amount of sleep needed after a given period of wake to maintain homeostasis. While the mechanism and circuitry of the sleep homeostat remains elusive, sleep intensity, as measured by electroencephalogram (EEG) slow wave activity (0.5–4 Hz) during nonrapid eye movement (NREM) sleep (referred to within as NREM-SWA) and NREM sleep duration are characteristic hallmarks of sleep need. Increases in the intensity of NREM-SWA are proportional to the amount of prior wake time, while time spent in NREM sleep will dissipate the amount of SWA [50–55].
The neuromodulator adenosine is highly recognized as both a sleep-promoting substance and a marker of homeostatic need [56, 57]. Adenosine levels increase proportionally with wake time and decrease during sleep in the basal forebrain and cortex [58–60]. Furthermore, activation of adenosine receptors, A2A and A1, in key sleep- and wake-regulating nuclei modulate sleep need and arousal [56, 57]. Although the exact mechanisms underlying sleep homeostasis have yet to be clearly elucidated, numerous pharmacological studies and transgenic models have indicated a role for the A1 receptor in SWA and sleep homeostasis [57]. Emerging evidence from the POA also suggest that A2A receptors residing in the MnPO and VLPO may also play a role in sleep homeostasis [49, 61].
While numerous independent studies in rodents clearly demonstrate that E2 markedly increases wake and suppresses NREM sleep particularly in the dark or active phase, little attention has been paid to E2 effects on sleep homeostasis in the light phase. Indeed, previous studies demonstrate that E2 does not significantly change the duration of light phase NREM sleep [41, 42, 62, 63]; however two studies in gonadally intact cycling females have reported a significant decrease in NREM-SWA toward the end of the light phase on proestrus when endogenous E2 levels are high [41, 62]. Nevertheless, it is not entirely clear if increases in E2 result in changes to homeostatic sleep pressure and/or adenosine during the light or quiescent phase. Here, we seek to investigate whether interactions between E2 and the adenosine system are involved in mediating E2 effects on sleep and homeostatic sleep pressure. Overall, the present findings suggest that (1) markers of homeostatic sleep need were decreased in the presence of E2; (2) despite an apparent reduction in sleep need, extracellular adenosine concentration in the MnPO was markedly increased in the presence of E2; and (3) E2 attenuated the effects of MnPO A2A receptor activation on NREM sleep. Taken together, these data suggest that E2 may be decreasing homeostatic sleep need by disrupting adenosine signaling in the MnPO.
Methods
Animals
Adult female Sprague–Dawley rats (250–350 g) were purchased from Charles River Laboratories (Kingston, NY) and housed in the Laboratory Animal Facilities at the University of Maryland, School of Medicine under a 12 h:12 h dark: light cycle. Upon arrival, animals were acclimated to the animal facility for at 7–10 days prior to the start of the experiments. Food and water were available ad libitum (AL).
In all experiments, zeitgeber time 0 (Zt 0) marks the start of the light phase. All sleep–wake recordings occurred in a designated animal room that was shielded from noise and with the room temperature ranging from 20 to 22°C. All experimental procedures were run in cohorts that consisted of at least one animal from each treatment groups and at most four animals per group. The cohorts for each experiment design were run over consecutive days until the a priori designated sample size as determined by power calculations was achieved of that experiment. Of note, due to the labor-intensive nature of the microdialysis procedure, only two animals (one E2 and one oil control) could be processed in a day. As a result, spontaneous sleep and sleep deprivation (SD) experiments were run as separate experiments and are statistically treated as such. All procedures were performed in accordance with the National Institutes of Health guide for care and use of laboratory animals and were approved by and in accordance with the guidelines of the University of Maryland Institutional Animal Care and Use Committee.
Surgical procedures
Surgical procedures were performed under isoflurane anesthesia with a maintenance flow of 1%–3% isoflurane with oxygen. All animals were allowed at least 7 days to recover from the surgical procedures before the start of any experiments.
Ovariectomy and transmitter implantation.
Animals were ovariectomized (OVX) and simultaneously implanted with TL11M2-F40-EET transmitters (Data Sciences International, St. Paul, MN) in a single surgical procedure. Briefly, a midline dorsal skin incision (2–2.5 cm) approximately halfway between the middle of the back and the base of the tail was made to allow access to the underlying abdominal muscles. Right and left abdominal incisions (0.5 cm) through the muscle were made allowing for the bilateral removal of the ovaries. Through one of the existing abdominal incisions, a bipotential-lead transmitter (DSI Inc., St. Paul, MN) was implanted intraperitoneally. Before closing the muscle wall, the transmitter leads were bundled together, placed in the subcutaneous space, and the muscle wall closed so that the base of the transmitter leads (ends arising from the transmitter) are anchored in place.
Next, a dorsal neck incision (~3 cm) was made through the skin along the dorsal midline from the posterior margin of the eyes to a point midway between the scapulae exposing the skull and neck muscle. The bundled electrode leads were threaded through this incision to their appropriate implant sites in the lateral cervical muscles and cranium. For cranial implantation, one burr hole was drilled on either side of the midline. The placement of the burr holes were asymmetrical where the left-side placement was 1.5 mm left lateral to the midline and 2 mm anterior from Bregma, and the ride-side placement was 1.5 mm right lateral to the midline and 7 mm posterior from the Bregma (Figure 1, A). Stainless steel screws (Plastics One, Roanoke, VA) were carefully implanted into each burr hole avoiding cortical contact. One set of leads (for the EEG) were wrapped around the screws and secured with a dental cement cap. The DSI telemeters are internally grounded as each set of biopotential leads contain one negative and one positive channel. The second set of leads (for the electromyogram; EMG) were implanted approximately 1.0 mm apart in the sternomastoid muscle (a lateral cervical muscle that runs diagonally from the behind the ear to the sternum). The leads were sutured into the muscle for stability.
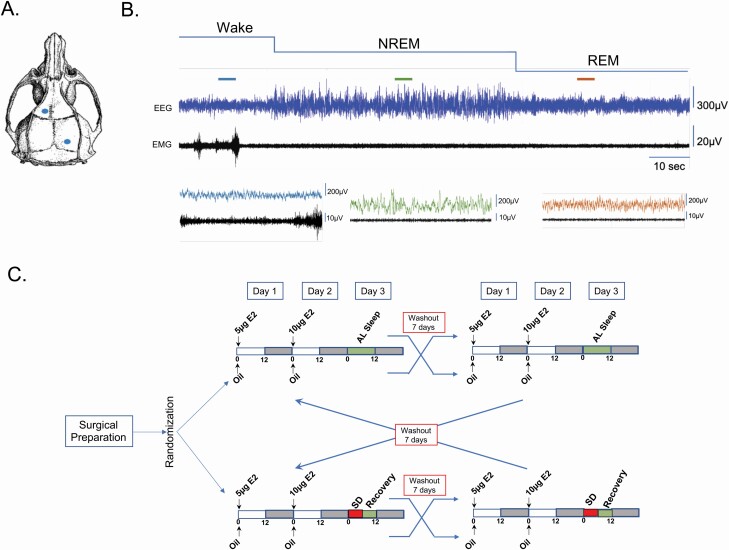
Figure 1.
Vigilant state scoring. (A) Configuration of recording electrode placement. Two burr holes were drilled asymmetrically at 2 mm anterior/1.5 mm lateral and 7 mm posterior/1.5 mm lateral to bregma. Stainless steel screws were placed in the holes and the EEG leads were wrapped around the screws. The screws and leads are secured with dental cement. Additionally, a second set of leads were implanted directly into the sternomastoid muscle approximately 1.0 mm apart and served to record nuchal EMG. (B) Top: Representative continuous recording of EEG and EMG. Bottom: Expanded view of a 5 s epoch from each state. The colored lines above the main trace indicate the specific epoch. From the acquired continuous EEG/EMG traces, vigilance states were first scored as wake, NREM sleep, or REM sleep using a custom program DJP. Next, experimenters blinded to the treatment groups confirmed the automated scores. (C) Double cross-over experimental design. OVX Sprague–Dawley rats (n = 11) were implanted with transmitters and randomized to one of two sleep conditions: AL sleep or SD. Once assigned to a sleep condition, animals were administered either oil vehicle or E2 according to our standard paradigm. At the end of the first recording period and 7 day washout period, the animals were crossed over to the other treatment but remained in the same sleep condition. At the end of the first cross over (and washout period), the animals were crossed to the other sleep conditions and treated as described above. Figures 2–5 represent the findings from this experimental design.
Guide cannula implantation.
Local drug infusions were made through a guide cannula targeted to the MnPO The cannulas were implanted at the time of the ovariectomy and transmitter implantation surgery. Briefly, the guide cannula (Plastics One #C315G, 0.46 mm outer diameter) were stereotaxically implanted after the EEG electrode placement and prior to the placement of the dental cement cap. A single burr hole was drilled in the skull bone 0.45 mm posterior and 1.0 mm lateral to bregma. The guide cannula was inserted at an angle of 9 degrees to a depth of 6.5 mm (~0.5 mm above the MnPO). The combination of a 9-degree angle insertion starting 1 mm from bregma facilitated placement of the guide cannula tip in the MnPO at midline without causing hemorrhaging that is typically seen if the cannula were inserted directly over bregma. The cannula and EEG leads were secured with a dental cement cap. Finally, the cannula opening was closed with a dummy cap provided by manufacturer.
For the microdialysis of MnPO extracellular content, animals were OVX as described earlier and implanted with microdialysis guide cannula (SciPro Inc., #MAB-6.14.G, 1 mm outer diameter, Sanborn, NY) in a single surgery. Briefly, the guide cannula were stereotaxically targeted to ~1 mm above the MnPO. A single burr hole was drilled in the skull bone 0.45 mm posterior and 1.0 mm lateral to Bregma. The guide cannula was inserted to a depth of 6 mm at an angle of 9 degrees, secured in place with a dental cement cap and the cannula opening closed with a dummy cap provided by manufacturer. In small cohort of animals (n = 4/group), transmitters and associated leads were implanted with microdialysis guide cannula in a single surgery as described earlier.
Verification of guide cannula placement.
Cannula placements were verified at the conclusions of the sample collections. Animals were euthanized via CO2 asphyxiation followed by decapitation. Brains were collected and submersion fixed in a solution of 9% formalin in potassium phosphate buffered saline (kPBS; 0.5 M, pH 7.4), at 4°C, followed by cryoprotection in 30% sucrose in kPBS. After cryoprotection, the brains were frozen on dry ice and stored at −80°C until processing. Brains were sectioned in 30 μm thick coronal sections in a cryostat. The sections were directly collected on gelatin-subbed slides, dried, and processed for neutral red staining. Cannula placement was determined by visual identification under a light microscope of the guide cannula bore and/or lesion created by cannula insertion. Such points falling within the POA boundary of Bregma −0.3 to Bregma +0.4 mm and within 1 mm above the MnPO (Figure 6, B; expected tip of the guide cannula) were counted as hits. Data from misses (Figures 6, B and 7, B) were not analyzed.
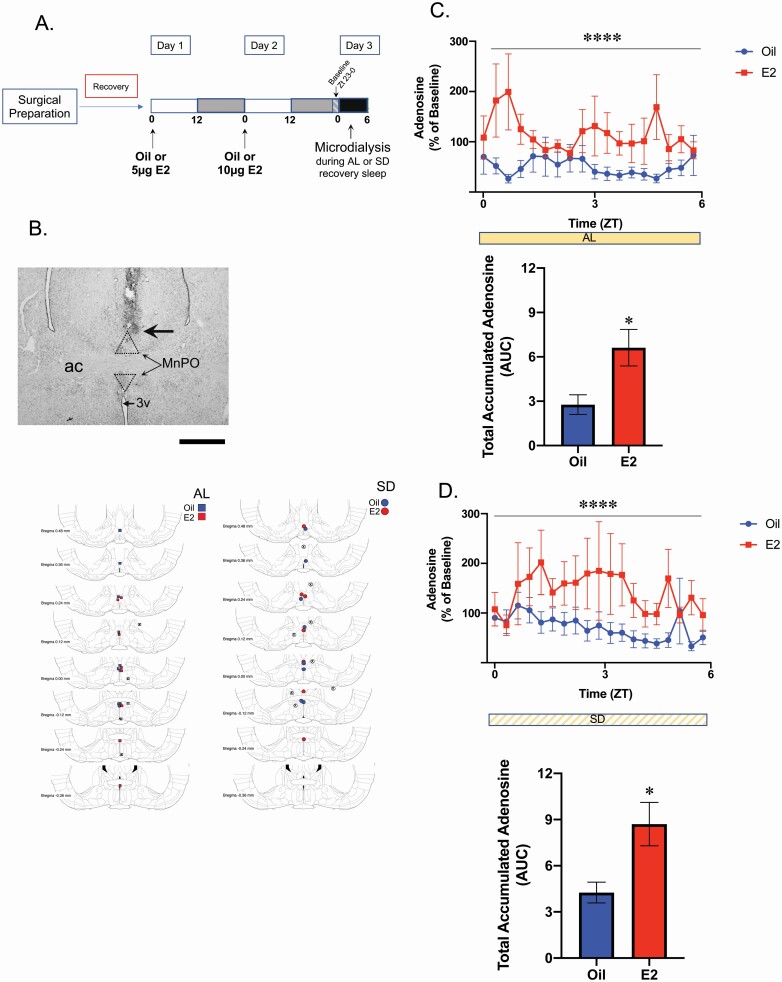
Figure 6.
Microdialysis of MnPO extracellular fluid and adenosine concentration analysis. (A) Experimental timeline. In two separate experiments (AL sleep and SD), Sprague–Dawley rats were OVX and implanted with microdialysis guide cannula targeted to the MnPO. Following recovery from surgery, animals in the AL sleep experiment were treated with E2 or oil vehicle on two consecutive days prior of microdialysis. At Zt 9 of the second treatment day, the microdialysis probe was inserted and allowed to acclimate until Zt 23. The collection of dialysates began in the last hour of the dark phase (Zt 23). These sampled served as the normalization baseline. For the remainder of the experiment, animals were allowed spontaneous sleep (AL sleep) during sample collection. Following the end of the collection period, the brains were collected and canula placement assessed (B). Only those animals with correctly targeted canula were included in the analysis (E2; n = 8 and oil vehicle; n = 10). The second experimental cohort was treated as described except during the sample collection the animals were subjected to the total SD protocol (E2; n = 7 and oil; n = 7). Of note, an a priori power analysis determined that to achieve greater than 80% power, samples sizes of n = 10 were required, however due to attrition is the final sample size was smaller in three of the four groups. Nevertheless, the resulting sample sizes achieved 77% power in the AL sleep cohort and 75% in the SD cohort. (B) Cannula Placement. Top. Representative photomicrograph of the cannula and microdialysis probe placement. Probe placement was determined visually using neutral red staining. The large arrow demarcates the end of the guide cannula from where the dialysis probe extended 1 mm. The stippled lines represent the area of the MnPO. ac, anterior commissure; 3V, 3rd ventricle. Scale bar = 100 µm. Bottom. Maps of the probe placements (including misses) for the AL sleep and SD sleep recovery cohorts. Samples were analyzed if the probe placement fell within the POA boundary defined by Bregma −0.3 mm to Bregma +0.4 mm and within with boundary of the MnPO (as marked by the box in the photomicrograph above). Blue symbols, oil; Red symbols, E2; X-mark, misses. Adenosine levels were collected by microdialysis (6 kd-cutoff probes) and analyzed by HPLC-Mass Spectrometry. Adenosine levels were normalized to each animal’s baseline adenosine collected from Zt 23 to 0. (C) AL Sleep Cohort. Top. Extracellular adenosine levels following E2 treatment were significantly elevated across the 6-h collection compared with oil (two-way ANOVA, main effect of E2; F1,302=35.44 ****p < 0.0001). Bottom. Area under the curve analysis demonstrated that the total levels of extracellular adenosine were markedly increased following E2 treatment compared with oil (two-tailed t-test; t(17)=2.832, p = 0.0115). (D) SD Cohort. Top. Similar to AL sleep, extracellular adenosine levels following E2 treatment were significantly elevated across the 6-h of SD compared with oil (two-way ANOVA, main effect of E2; F1,221=28.41, ****p < 0.0001). Bottom. Area under the curve analysis demonstrated that the total levels of extracellular adenosine during SD were markedly increased following E2 treatment (two-tailed t-test; t(12) = 2.842, p = 0.0148). Comparing the AUC calculations across the four treatment groups suggested that SD did increase total adenosine concentration in both the oil- and E2-treated groups compared with AL sleep; however, the increase did not reach statistical significance (Supplemental Figure S5). This could be due to the experimental variability between the AL sleep and SD sleep recovery cohorts which were run as separate experiments. Data are the mean + SEM.
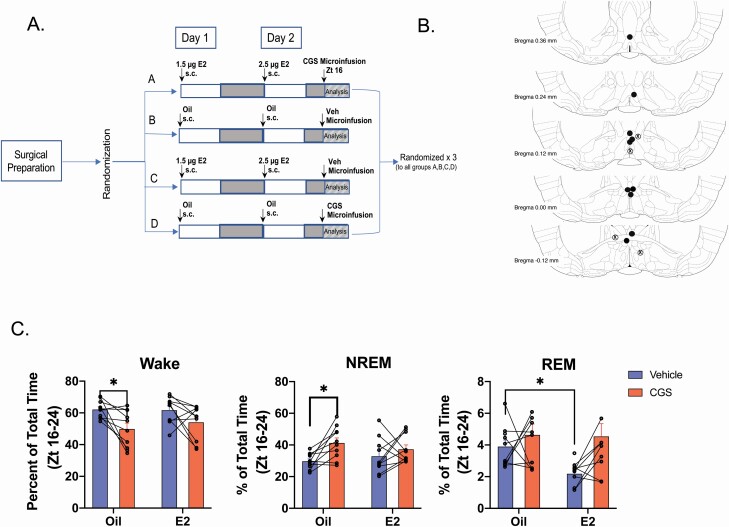
Figure 7.
A2A receptor agonist CGS-21680 decreased wake and increases NREM sleep following oil but not E2 treatment. Experimental timeline. Sprague–Dawley rats (n = 10) were OVX, fitted with telemeters and implanted with infusion guide cannula targeted to the MnPO. Following recovery from surgery, animals were randomly placed into one of four treatment arms where they received two subcutaneous injections of either oil or a subthreshold dose of E2 (that does not affect sleep–wake behavior) and at Zt16 on Day 2 infusion into the MnPO of either the A2A receptor agonist CGS-21680 (CGS; 24 nmol) or DMSO vehicle. The EEG/EMG recordings began immediately after the MnPO infusions. At the end of the recording period, the animals were allowed a 7-day washout period before being randomly assigned to another treatment arm. This protocol was repeated until all animals had been run through each treatment arm. The EEG/EMG traces from Zt 16 to 0 (8 h total) were scored and analyzed for changes in sleep–wake states. As CGS was predicted to increase NREM sleep at the expense of wake, the dark phase was chosen to maximize the probability of detecting changes in these two vigilant states. (B) Cannula Placement. Cannula placement was determined visually using neutral red staining. Representation of guide cannula placements (including misses). Placements were deemed a hit when they fell within the POA boundary defined by Bregma −0.3 mm to Bregma +0.4 mm and within with boundary of the MnPO (as marked by the box Figure 6B). Black circles = hits; Circled X = misses. (C) Effects of CGS on wake, NREM, and REM sleep in the presence and absence of E2. Local infusion of CGS into the MnPO significantly decreased wake following oil treatment, but not in the presence of E2 (repeated measures two-way ANOVA; main effect of CGS F1,9 = 15.16; p = 0.0037; no effect of E2; F1,9 = 0.2983, p = 0.5982; Šídák’s multiple comparison test, Adjusted p value *p = 0.0474). The decrease in wake was accompanied by an increase in NREM sleep following oil treatment only (repeated measures two-way ANOVA; main effect of CGS F1,9 = 8.822; p = 0.0157; no effect of E2; F1,9 = 0.0178, p = 0.8969; Šídák’s multiple comparison test, Adjusted p value *p = 0.0312). Curiously, REM sleep showed a significant effect of E2 but not CGS (repeated measures two-way ANOVA; main effect of E2 F1,9 = 5.576; p = 0.0425; no effect of CGS; F1,9 = 2.233, p = 0.1693; Šídák’s multiple comparison test, Adjusted p value *p = 0.0246). Individual lines represent paired measurements from the same animal. Data are the mean + SEM.
Steroid treatments
Estradiol replacement paradigm followed our established protocol that mimics the natural physiological rise of E2 that occurs on the day of proestrus [42, 64]. The replacement paradigm reliably reproduces (1) physiological levels of E2 equivalent to the day of proestrus [64] and (2) changes in sleep–wake behaviors observed by intact cycling female rats [40, 42]. Briefly, OVX females received 5 μg 17-β-estradiol benzoate in 5 µL sesame oil (E2, Sigma Aldrich, St. Louis, MO) followed by 10 μg E2 in 10 µL sesame oil 24 h later, or equivalent amounts (5 µL/10 µL) of sesame oil vehicle, through subcutaneous flank injections. The day following the last injection (Day 3 in the current set of experiments) is analogous to the day of proestrus in gonadally intact females in terms of E2 levels, and sleep–wake patterns were expected to be similar under these conditions [40, 42, 64]. 17-β-estradiol benzoate is a synthetic ester of estradiol, which nonspecific steroidal esterases deesterify to produce biologically active E2 in vivo [65, 66]. Of note, when testing the efficacy of the A2A receptor agonist, CGS-2168, to induce sleep in the absence or presence of E2, a sub-physiological dose of E2 was administered (1.25 and 2.5 µg) in order to avoid the E2-mediated effects on sleep–wake behavior.
Data acquisition, sleep scoring, and spectral analysis
EEG and EMG data were collected at a sampling rate of 500 Hz using the Ponemah Software (DSI Inc.). While the EEG/EMG recordings were autoscored with a custom MATLAB program described later, the digitized signal data were viewed and the autoscoring was confirmed offline using NeuroScore DSI v3.3.9 (DSI Inc.). The EEG/EMG signals were parsed into 10 s epochs. Wake was designated by low-amplitude, high-frequency EEG combined with high-amplitude EMG. NREM sleep was characterized as high-amplitude, low-frequency EEG with low-amplitude EMG. REM sleep consisted of low-amplitude, high-frequency EEG with very low EMG tone. Figure 1, B is a representative 2-min trace of the EGG/EMG signal with higher resolution examples of a 5 s epoch from each vigilance state.
For the autoscoring script, the DSI module for powerbands was used to generate a Fast Fourier transform (Hamming window, 4096 samples) of the EEG frequency bands. The frequency bands were designated as follows: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), beta (16–24 Hz), gamma (24–50 Hz), and total (0.5–50 Hz). For the EMG, a bandpass filter (20–200 Hz) was applied, and the mean of the absolute value for the EMG amplitude was calculated. Next, these data were exported to MATLAB (MATLAB R2015, Mathworks, Natick, MA) where vigilant states were automatically scored using a custom program developed in house (DJP). In the automated program, scoring decisions for each 10 s epoch were based on whether the 10 s epoch values were greater than or less than the threshold value for the following parameters: (1) threshold levels of EEG delta power, (2) threshold levels of theta power, (3) the ratio of theta to delta, and (4) the mean of the absolute value for the EMG amplitude (Supplementary Table S1A).
Briefly, the power of the frequency bands in each 10 s epoch were normalized to the 24-h mean power of the experimental day being scored. Next, a threshold level for each parameter was set based on the median of the normalized data. Data values from each 10 s epoch were then compared with the threshold value. Values were considered high if they were greater than the median normalized value, and low if they were less than the median normalized value. To further increase the accuracy of the REM sleep calls, the ratio of theta/delta was applied when the threshold levels of theta power were high. If the theta/delta ratio was >1 and the EMG value low the epoch was assigned to REM sleep. However, if the ratio was <1 with a low EMG values, the epoch was assigned to wake. Finally, REM transitions could only occur from NREM sleep. Additionally, potential artifacts were flagged based on the normalized frequencies. If the normalized frequencies were greater than five standard deviations from the frequency mean of the surrounding epochs (4 min before and after) the signal was considered an outlier and marked as an artifact. In an independent analysis of 24-h continuous recordings from 6 animals, this custom MATLAB script proved to be ~88%–92% in agreement with hand-scored traces from three independent scorers. The agreement between the independent hand-scored traces was ~91% suggesting that the autoscore script had similar accuracy to that of visually scored traces by different experimenters.
Finally, to ensure the highest level of scoring accuracy of the traces used in this study, the autoscored data was reimported into Neuroscore using a custom MATLAB program by the courtesy of Dr Michael Rempe (Whitworth University). Once in Neuroscore, visual inspection of the scored traces by an experimenter blinded to the treatment groups confirmed the automated scores and changes were made where necessary including the identification of artifacts. All confirmed artifacts were removed from the final analysis of the sleep–wake behavior and homeostatic parameters. The mean duration of artifacts as well as range for each treatment paradigm is listed in Supplementary Table S1B. Overall, the mean duration of artifacts was less than a minute for the entire 12 h of recordings for each treatment/sleep condition. Under the AL baseline sleep condition, a two-way ANOVA (steroid × hour) revealed no main effects of treatment (F1,9 = 0.7949; p = 0.3958) or time (F11,99 =0.7976; p = 0.7976) nor an interaction (F11,76 =1.042; p = 0.4192) between the groups. Similarly, in the SD recovery condition, a two-way ANOVA (steroid × hour) revealed no main effects of treatment (F1,9 = 0.1422; p = 0.7148) or time (F5,45 =0.7651; p = 0.5798) nor an interaction (F5,39 = 1.068; p = 0.3930) between the two.
To further test whether E2 influences sleep homeostasis, three measures of sleep homeostasis (1) the NREM-power spectral density (PSD); (2) NREM-SWA (representing the average delta activity); and (3) delta energy (the product of NREM-SWA and NREM sleep time representing total delta activity) were quantified from the corrected Neuroscore traces. The powerbands of the NREM sleep epochs per treatment condition were imported into MATLAB and used to calculate the EEG PSD for the following frequency bandwidths: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), and beta (16–24 Hz). The PSD was calculated into 0.25 Hz stepwise bins and reported as volts2/Hz. NREM-SWA was assessed via the EEG power spectra in the 0.5–4 Hz range. The frequencies of the SWA in NREM bouts were averaged into 1 h bins during the light phase and reported as µV2.
Experimental protocols
Effect of E2 on sleep and sleep homeostasis in the light phase.
To test whether E2 influences homeostatic sleep in the light phase, animals were randomly assigned to treatment arms in a double cross-over design. The use of a within-animal blocking design mitigates the variance in the data resulting from differences in individual animal’s sleep–wake behavior. Adult female Sprague–Dawley rats (n = 11) were OVX, implanted with EEG/EMG transmitters and randomly assigned to one of two sleep conditions: AL sleep or SD recovery. In the SD arm, animals were sleep deprived for the first 6 h of the light phase on Day 3 and then allowed recovery sleep in the second 6 h. The EEG/EMG recordings for all groups started at Zt 0 and ended at Zt 12 (light phase) on Day 3.
Once assigned to a sleep condition arm, animals were administered either oil vehicle or E2 according to our standard paradigm [40, 42]. After Day 3, light phase recordings were collected, animals received a 7-day washout/rest period before crossing over into the other steroid/oil treatment. Again, recordings were collected on Day 3 of treatment. Following a second 7-day washout/rest period, all animals were crossed over to the opposite sleep condition arm. The steroid treatments and EEG/EMG recordings proceeded as in the first round.
SD was induced in the animal’s home cage between Zt 0 and 6. The animals were never removed or handled but were presented with novel objects that included wooden blocks, paper towel, and cotton balls to induce exploration. When the novel objects were no longer sufficient to maintain arousal, animals were gently stroked on the nape of the neck or back with a long cotton tip applicator. Finally, in the late stages of SD, when the first two methods failed to prevent the initiation of sleep, it was necessary to gently rotate the home cage. Overall, the amount of accumulated wake, NREM sleep and REM sleep was similar for both oil and E2 treatment days where both groups were awake ~88% of the SD period (Supplementary Figure S1). The majority of accumulated NREM sleep occurred in bouts that were less than 1 min and most often less than 30 s (Supplementary Figure S1; inset). There was less than 0.1% of accumulated REM in either group (data not shown).
Sleep–wake behavior was scored from the recorded EEG/EMG traces from Day 3 (Zt 0–12) of each treatment arm of the experiment. The degree of sleep pressure was assessed by established measures described earlier (vigilant-state durations, bout length distributions, PSD, NREM-SWA, and delta energy). A priori comparisons between the oil and E2 treatments under the specific sleep condition were made to test whether E2 affected sleep homeostasis.
To further assess whether E2 differentially affects homeostatic responses from unrestricted sleep–wake (during the previous dark phase) or recovery sleep from light phase SD were compared. Since under spontaneous AL sleep conditions, NREM sleep pressure is highest during the early part of the light phase, we chose to use the homeostatic measures from first 6 h of the AL sleep light phase to compare with the homeostatic responses following SD. These two periods are referred to as “Hours from wake” and represent the highest NREM sleep pressure for the two sleep conditions.
Effect of E2 on MnPO extracellular adenosine levels.
Microdialysis collection during the light phase and HPLC analysis of extracellular content from the MnPO was used to investigate whether E2 altered levels of extracellular adenosine in both times of normal (AL sleep) and induced sleep need (SD). In one cohort, OVX animals were treated with either E2 (n = 9) or oil (n = 10) and were allowed AL sleep during the sample collection (AL sleep cohort). Within this group, a small cohort of animals (n = 4/group) were fitted with telemeters to confirm that the AL sleep cohort was accumulating sleep. In a separate cohort, OVX animals were treated with E2 (n = 7) or oil (n = 7) and were subjected to 6 h of SD during the collection period. To maximize the likelihood of measuring differences between E2- and oil-treated animals, the baseline measurements, which were used to normalize changes during the light phase collection period, were collected during the last hour of the dark phase when adenosine was expected to be at its peak.
Briefly, adult female rats were OVX and implanted with a microdialysis guide cannula targeted to the 1 mm above the MnPO (see earlier section). Following a 7-day recovery, the steroid treatment started with injections of either E2 or oil vehicle. On Day 2, approximately 6 h after treatment injections, the microdialysis probe (6 kD membrane cutoff; Scipro Inc., model #MAB-9.14.1) was inserted into the guide cannula. The 7 mm probe extended 1 mm beyond the end of the guide cannula to rest within the MnPO. The surrounding tissue was allowed approximately 12 h of probe acclimation without dialysis (~Zt 22.5).
Prior to the start of dialysis, the probe was attached via polyethylene tubing to a 1 mL Hamilton syringe (700 series, Hamilton, Reno, NV). The flowrate of the syringe was controlled by a BASi Bee pump attached to a Bee Hive controller (Bioanalytical Systems, Inc., West Lafayette, IN). The inserted dialysis probe was perfused at a rate of 1.167 µL/min with Ringer’s Solution (147 mM NaCl, 4 mM KCl, 1.4 mM CaCl2, in distilled water). Probes were primed for at least 30 min before baseline collections began at Zt 23 under red light conditions. During collection, animals could move freely about the cage and were provided food and water AL.
Dialysis samples were collected in fractions of 20 min (23.3 µL dialysate) for 7 h. Upon collection, the dialysates were immediately frozen and stored at −20°C until LC-MS analysis. The first three fractions were collected within the last hour of the dark phase (Zt 23–0) and constituted the baseline of extracellular adenosine levels that was used to calculate the percent change in the light phase from Zt 0 to 6 (see later section). Probe placement was verified as stated earlier.
To calculate the approximate in vitro probe recovery rate, free microdialysis probes (n = 3) were inserted into a solution of 100 nM adenosine (Tocris Biosciences, Bristol, UK) in Ringer’s Solution, and perfused with Ringer’s Solution at 1.167 µL/min for 2 h, with 20-min dialysate fractions collected.
Adenosine quantification was performed by the Proteomics Core Laboratory in the Center for Vascular and Inflammatory Diseases at the University of Maryland, School of Medicine. Adenosine concentration in the collected fractions was quantified by liquid chromatography tandem-mass spectrometry by monitoring the transition pair of m/z 268.1/136.1 and quantified by plotting the area under the curve versus the known concentrations of the standards from a calibration curve. Analysis was performed on a Perkin Elmer (Waltham, MA) Qsight LX50 HPLC system and a QSight 210 triple-quadrupole mass spectrometer. The chromatographic solvents used were 0.1% formic acid in water (Solvent A) and 0.1% formic acid in methanol (Solvent B). The column was a YMC Triart 3 µ C18, 2.1 mm × 150 mm operated at a flow rate of 400 µL/min at 45°C. An isocratic separation was used with a solvent composition of 94% Solvent A and 6% Solvent B. The effluent from the column was introduced into the mass spectrometer by electrospray ionization in positive polarity and the transition pair of m/z 268.1/136.1 at unit mass resolution was used for detection of adenosine. The run time was 3 min. A stock solution of adenosine (Sigma) was prepared from dry powder and finally diluted in Solvent A to obtain a 6-point calibration curve that ranged from 5 to 1500 pg injected on column. The area under the curve (AUC) for the adenosine standards was plotted against their known concentrations to quantify the amount of adenosine in the experimental samples.
Effect of E2 on adenosine A2A receptor activation.
To test whether E2 attenuates A2A receptor signaling in the MnPO, a highly selective A2A receptor agonist, CGS-21680 (CGS; Tocris Biosciences), was locally infused into the MnPO in the presence and absence of E2. Adult female Sprague–Dawley rats (n = 10) were OVX and implanted with EEG/EMG transmitters and guide cannula. Following recovery, animals were randomly assigned to one of four treatment arms that consisted of subcutaneous steroid/oil injections and local infusions of CGS or Vehicle. To reduce inter-animal variability, all animals served as their own controls and received all treatment (oil/DMSO vehicle, E2/DMSO, oil/CGS, and E2/CGS) in a random order. At the end of one treatment, animals were allowed a 7-day washout period before being randomly assigned to the next treatment.
To circumvent E2 suppression of sleep behavior, a sub-physiological dose of E2 that does not induce sleep suppression was administered (1.25 and 2.5 µg) according to the standard timing. Local MnPO microinfusions occurred at just prior to Zt 16 following the last E2 or oil injection. The dummy stylets were removed and replaced by 33-gauge microneedles (Plastics One) that project 1 mm past the end of the guide cannula. Infusion stylets were attached via polyethylene tubing to a 25 μL Hamilton syringe (700 series, Hamilton). The flowrate of the syringe was controlled by a BASi Bee pump attached to a Bee Hive controller (Bioanalytical Systems). Infusions of 24 nmol of CGS-21680 or equivalent volume of vehicle 5 µL in 4.0% DMSO/sterile saline occurred over 10 min. At the end of the infusion, the needle was left in place for another 5 min to prevent back flow of the injection. The dose of CGS was previously reported to markedly increase sleep in male rats when infused into the lateral ventricle [61]. Once the needle was removed and dummy stylets replaced, animals were returned to their home cages where EEG/EMG recordings were collected for the 8 h following the MnPO microinjection. Guide cannula placement was verified as stated earlier.
Statistics
Results are expressed as means ± SEM. All statistical tests were conducted using the Graph Pad Prism program (San Diego, CA) on a PC. Statistical test and results are described in detail in the figure legends.
Results
E2 increased wake and decreased NREM sleep durations in recovery sleep but not during AL sleep
Previous studies in female rats have demonstrated that E2 does not significantly influence unrestricted NREM sleep durations during the light phase unlike its marked effects in the dark [40, 42, 64, 67]. The current set of experiments sought to test whether E2 influenced homeostatic sleep pressure during (1) unrestricted light phase sleep (i.e., AL sleep) and (2) recovery sleep following a homeostatic challenge of 6 h of SD. As previously reported, there was no effect on NREM sleep or wake durations in AL sleep across the 12-h light phase (Figure 2, A). However, in SD recovery sleep (Zt 6–12), E2 significantly decreased the time spent in NREM sleep by ~10% and increased the time spent in wake during by ~20% compared with the oil control day (Figure 2, B). When Zt 6–12 in the AL sleep condition, which match the time period of SD recovery, was analyzed no significant differences in vigilance states were detected (Data not shown; two-way repeated measure, ANOVA F(1, 27) = 0.2784, p = 0.6021).
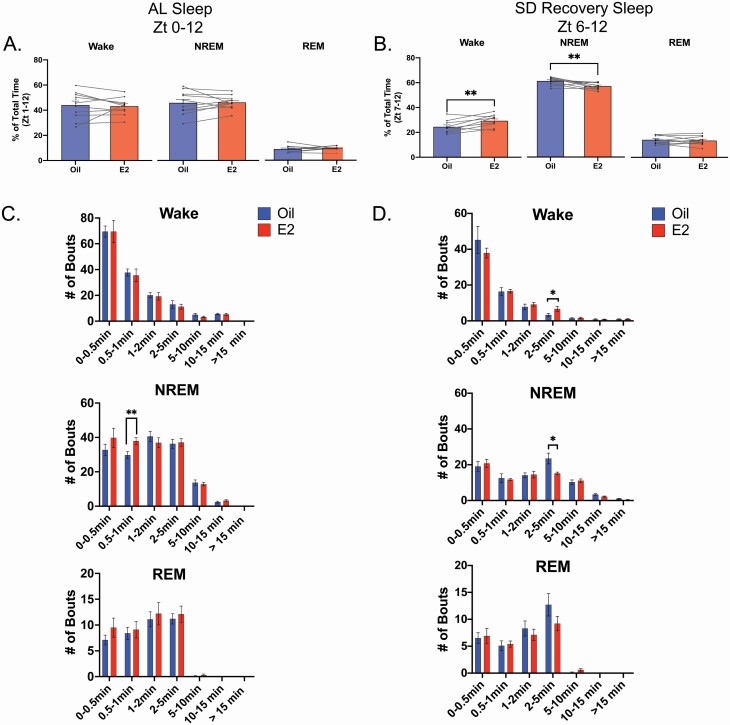
Figure 2.
E2 increased wake and decreased NREM sleep durations in recovery sleep but not during AL sleep. (A) E2 increased wake and decreased NREM sleep following SD. In the AL sleep across the 12 h light phase of Day 3, E2 did not significantly affect the percent of total time spent in any vigilance state (repeated measure two-way ANOVA; F(1,27) = 0.002, p = 0.96). Data are the mean + SEM. Individual lines represent paired measurements. (B) During SD recovery sleep, E2 increased wake at the expense of NREM sleep. When the period of SD recovery sleep (Zt 6–12) was analyzed for the time spent in each vigilant state, E2 treatment significantly increased the percent of total time spent in wake, while significantly decreasing the percent of NREM sleep (repeated measure two-way ANOVA; Interaction E2 × State, F(2,27) = 13.62, p < 0.0001; Šídák multiple comparisons, wake: **p = 0.0016 and NREM: **p = 0.0067). The amount of REM sleep was unchanged. Data are the mean + SEM. (C, D) In AL sleep and SD recovery sleep, E2-treatment significantly influenced bout length distribution. Wake, NREM sleep, and REM sleep bouts were binned into seven time intervals representing short (bouts lasting less than 1 min), medium (bouts between 1 and 5 min), and long (bout greater than 5 min) durations. A Wilcoxon matched-pairs signed rank test demonstrated that E2 increased the number of 0.5–1 min NREM bouts in AL sleep on Day 3 (Šídák correction for multiple comparisons, *p = 0.0232). In SD recovery sleep, the Wilcoxon matched-pairs signed rank test revealed that E2 increased the number of 2–5 min wake bouts (Holm–Šídák correction for multiple comparisons, *p = 0.0409), while decreasing the number of NREM sleep bouts in the same interval (Šídák correction for multiple comparisons, *p = 0.0403). Data are the mean + SEM.
Analysis of bout length distributions for the 12-h AL sleep period demonstrated that E2 significantly increased NREM bouts in the 0.5–1 min range only (Figure 2, C); but this effect was not present when only the Zt 6–12 period was analyzed (Data not shown). In the SD recovery sleep period, E2 specifically increased SD recovery wake and decreased NREM bouts that were between 2 and 5 min (Figure 2, D). Wake, NREM, and REM bout numbers in AL sleep and in SD recovery sleep were unchanged by E2 (Supplementary Table S2).
E2 decreased homeostatic sleep pressure in AL sleep and SD recovery sleep conditions
To explore E2 effects on sleep homeostasis, the PSD, SWA (0.5–4.0 Hz), and delta energy during light phase NREM sleep bouts were compared with oil controls (Figures 3–5) under both sleep conditions (AL sleep and SD recovery).
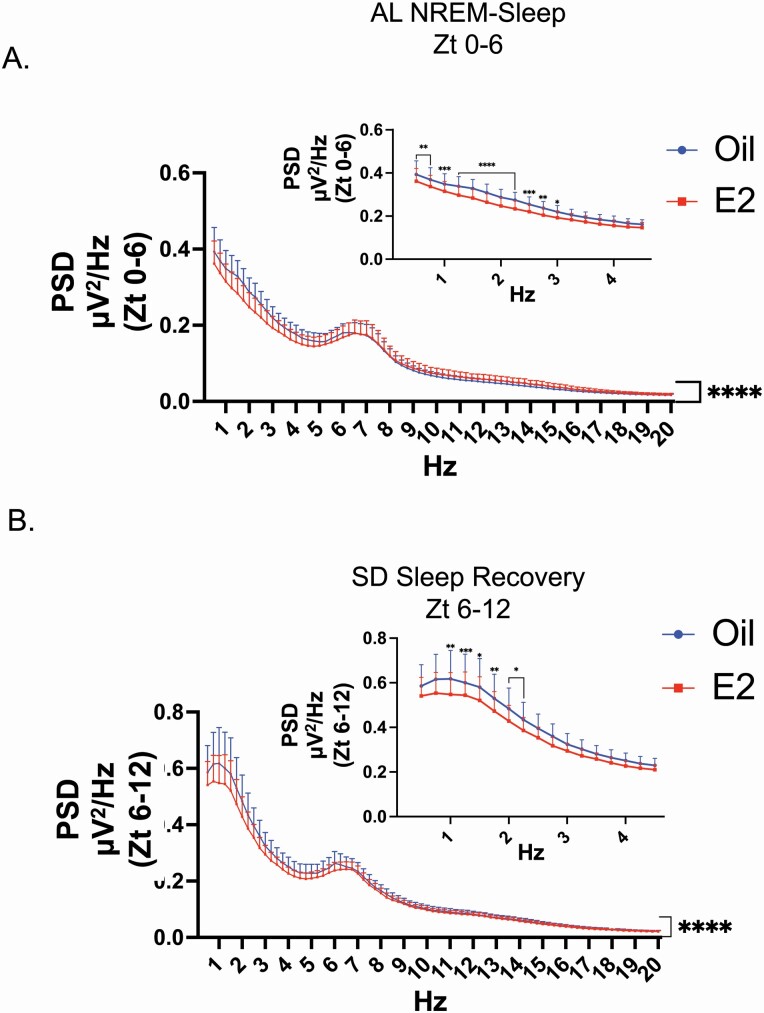
Figure 3.
E2 decreased PSD in AL sleep and SD recovery sleep. (A) PSD of the first 6 h of AL-NREM sleep. In AL sleep, analysis of the spectral power distribution from Zt 0 to 6 for NREM sleep revealed that E2 treatment significantly decreased the spectral distribution compared with oil treatment (repeated measure two-way ANOVA; main effect of E2: F(1,553) = 27.87, ****p < 0.0001; main effect of frequency: F(78,553) = 17.93, ****p < 0.0001 and interaction between E2 and Hz: F(78,553) = 4.263, ****p < 0.0001). (Inset) A multiple comparison post hoc test further revealed that the significant differences were limited to the lower delta frequency range of 0.5–3 Hz (Šídák correction for multiple comparisons; **p < 0.01, ***p < 0.005; see Supplemental Table S3 for adjusted p values and confidence intervals). Data are the mean + SEM. (B) PSD of the 6 h SD recovery sleep. In SD recovery sleep, analysis of the spectral power distribution from Zt 6 to 12 for NREM sleep revealed that E2 treatment significantly decreased the spectral distribution compared with oil treatment (repeated measure two-way ANOVA; main effect of E2: F(1,553) = 59.46, ****p < 0.0001; main effect of frequency: F(78,553) = 17.82, ****p < 0.0001 and interaction between E2 and frequency: F(78,553) = 1.370, ****p < 0.025). (Inset) A multiple comparison post hoc test further revealed that the significant differences were limited to the lower delta frequency range of 0.75–2 Hz (Šídák correction for multiple comparisons; *p < 0.05, **p < 0.01, ***p < 0.005; see Supplemental Table S3 for adjusted p values and confidence intervals).
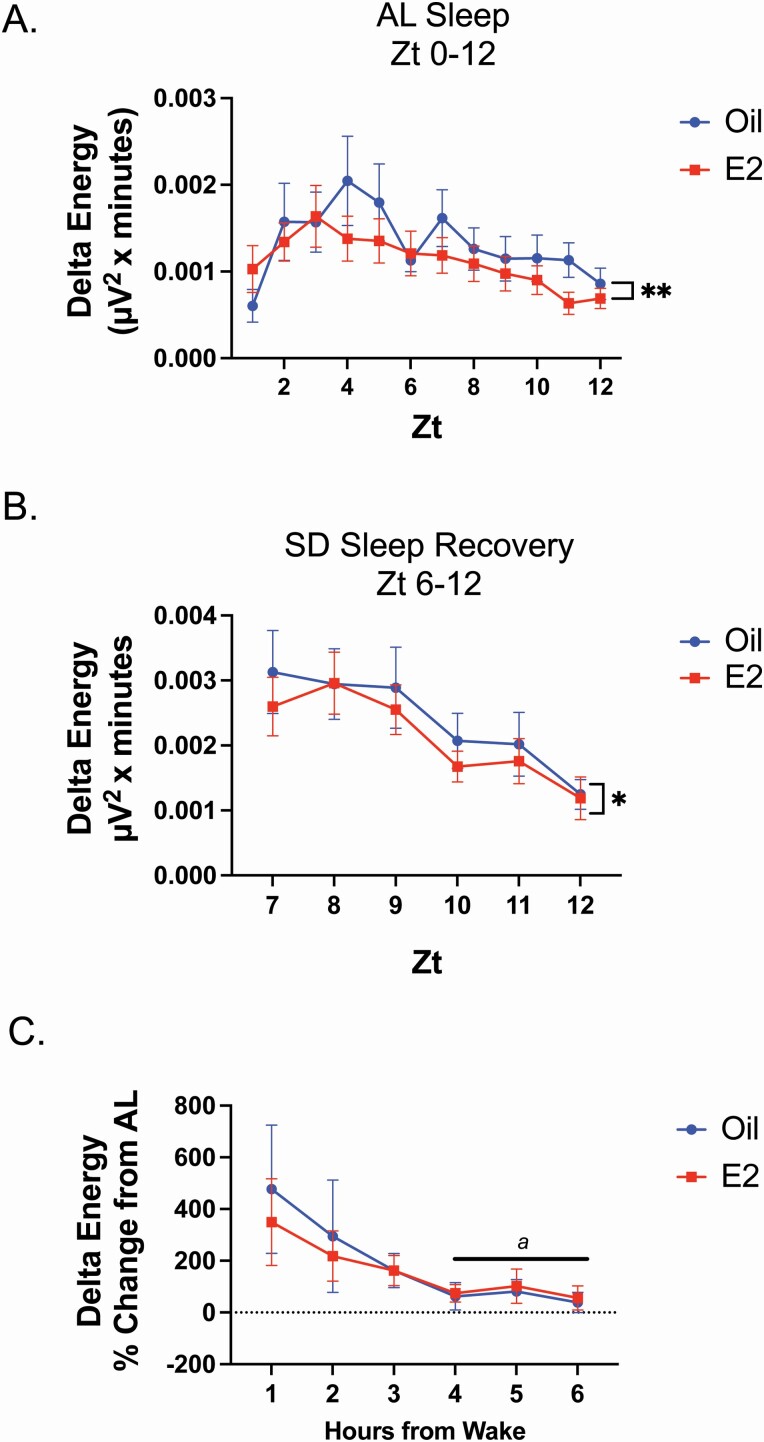
Figure 5.
E2 decreased delta energy in AL sleep and SD recovery sleep. (A) Hourly totals for delta energy across the 12-h AL sleep phase. For all NREM bouts on Day 3 (Zt 1–12), delta energy (NREM-SWA power × NREM duration) was calculated. In AL sleep, E2 treatment significantly decreased the overall delta energy compared with oil treatment across the 12-h period (repeated measure two-way ANOVA; main effect of E2, F(1, 94) = 9.174; **p = 0.0032). (B) Hourly totals for delta energy across the SD recovery phase. In SD recovery sleep, E2 treatment significantly decreased the overall delta energy compared with oil treatment across the recovery period (repeated measure two-way ANOVA; main effect of E2, F(1,48) = 5.264; *p = 0.0262). (C) Comparison of delta energy rebound between oil and E2 treatment. The delta rebound was derived for both oil and E2 treatments by calculating the percent change of SD recovery sleep percent change from AL sleep (Zt 0–6; period of greatest homeostatic pressure resulting from the preceding dark phase) from each respective treatment. The percent change of the delta energy rebound was not significantly different between oil and E2 treatment (repeated measure two-way ANOVA; main effect of E2, F(1,7)=0.0992; p = 0.7620). A comparison of the delta energy rebound decline over time revealed that only oil treatment induced a significant difference from the hour 1 (repeated measure two-way ANOVA; main effect of time, F(5,66)=3.826; **p = 0.0072). The a denotes hours that are significantly different from hour 1 (Dunnett’s multiple comparison test; Zt 1 vs. Zt 4, *p = 0.0304, Zt 1 vs. Zt 5, *p = 0.0433, and Zt 1 vs. Zt 6, *p = 0.0213).
Power spectral density.
Given that homeostatic sleep pressure is typically greatest after periods of prolonged wake, the analysis of the spectral distribution for the AL sleep condition was restricted to the first 6 h of the light phase (Zt 0–6) following dark phase unrestricted sleep–wake. Comparisons of the PSD revealed a main effect of E2 treatment for both AL sleep and SD recovery sleep conditions for the 6 h following wake (Figure 3, A and B). More specifically, in the first 6 h of AL sleep, E2 treatment significantly decreased delta power in the lower frequency range (0.5–3 Hz) compared with oil treatment (Figure 3, A inset). As expected, SD recovery sleep exhibited an increased power in the delta frequencies range for both E2 and oil treatments (Supplementary Figure S3, A). Similar to AL sleep, E2 treatment significantly decreased delta power in the lower frequencies, but only in the 1–2.5 Hz range (Figure 3, B inset). Of note, the PSD across the 12 h of AL sleep demonstrated a main effect of E2 and post hoc analysis demonstrated significant decreases in the 0.5–3.0 Hz frequencies compared with oil treatment (Supplementary Figure S2).
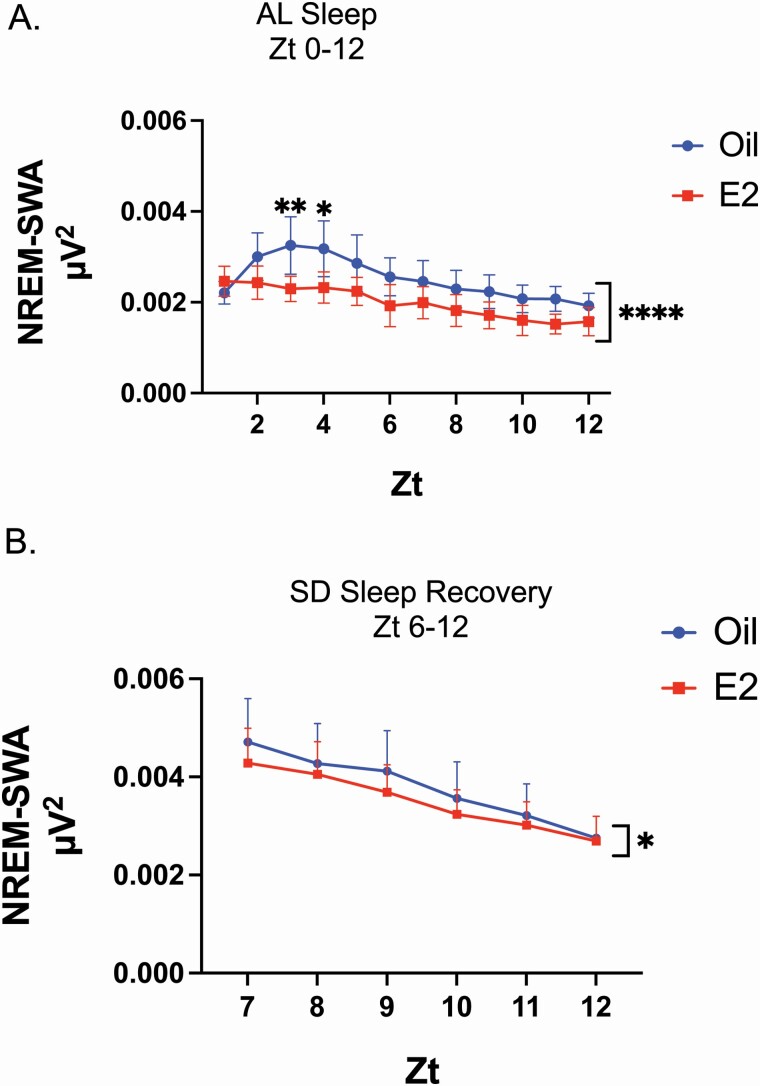
Nonrapid eye movement-slow wave activity.
Analysis of the NREM-SWA per hour revealed that E2-treatment significantly decreased the hourly amount of SWA during NREM bouts compared with oil treatment in AL and SD recovery sleep (Figure 4, A and B). Additionally, as expected SD significantly increased NREM-SWA during recovery sleep compared with the first 6 h of AL sleep (Supplementary Figure S3, B). For both E2 and oil treatment, significant declines across time in NREM-SWA were observed in SD recovery sleep, but not in the first 6 h of AL sleep (Supplementary Figure S3, B, a denotes hours that are significantly different from hour 1). Specifically, in the oil control condition, NREM-SWA was significantly decreased starting at hour 4 of SD recovery sleep. Following E2 treatment, NREM-SWA was significantly decreased starting at hour 3 of SD recovery sleep.
Figure 4.
E2 decreased NREM-SWA in AL sleep and SD recovery sleep. (A) Hourly totals of SWA in NREM sleep across the 12 h AL sleep phase. For all NREM bouts on Day 3 (Zt 1–12), the EEG power spectra in the 0.5–4 Hz range (also known as delta power) was calculated for hourly totals. In AL sleep, E2 significantly decreased the amount of NREM-SWA compared with oil treatment across the 12 h period (repeated measure two-way ANOVA; main effect of E2, F(1,84) = 57.00; ****p < 0.0001). A post hoc multiple comparison revealed significant differences Zt 3 and Zt 4 (Šídák’s multiple comparison test; Zt 3, **p < 0.01; Zt 4, *p < 0.05; see Supplemental Table S2 for adjusted p values and confidence intervals). (B) Hourly totals of SWA in NREM sleep across the SD recovery phase. In SD recovery sleep, E2 induced a main effect with an overall decrease in the amount of NREM-SWA compared with oil treatment (repeated measure two-way ANOVA; main effect of E2, F(1,48) = 5.158; *p = 0.0277).
Delta energy.
Analysis of delta energy per hour revealed that E2-treatment significantly decreased the hourly amount of delta energy compared with oil treatment in AL sleep and SD recovery sleep (Figure 5, A and B). Of note, the hourly duration of NREM sleep was not significant different between oil and E2 treatments for both the AL sleep and SD recovery sleep periods (Supplementary Figure S4, A and B). However, comparison of the cumulative duration for the last 3 h of SD recovery sleep did reveal E2 induced a significant decrease in NREM sleep (Supplementary Figure S4, B, inset) which likely contributed to the observed differences in Figure 2, B.
When the delta energy for SD recovery and AL sleep were compared between oil and E2 treatments, a main effect of the sleep condition was observed for both oil and E2 treatments (Supplementary Figure S3, C). Following oil treatment, SD recovery sleep had greater delta energy in the first 3 h of recovery sleep compared with the first 3 h of AL. Similarly, following E2 treatment, SD recovery sleep had greater delta energy in the first 2 h of recovery sleep compared with the first 2 h of AL sleep. Like NREM-SWA, significant declines in delta energy were observed in SD recovery sleep only for both E2 and oil treatments (Supplementary Figure S3, Ca denotes hours that are significantly different from hour 1). Following oil treatment delta energy was significantly decreased starting at hour 5 of SD recovery; while following E2 treatment, delta energy was significantly decreased starting at hour 4 of SD recovery sleep.
The findings from the PSD, NREM-SWA, and delta energy indicate that under normal physiological sleep pressure (AL sleep) and increased homeostatic need (SD recovery sleep), E2 reduced homeostatic sleep pressure. To test whether, E2 differentially influenced the degree of sleep pressure between AL sleep and SD recovery sleep, the delta energy rebound (% change of delta energy in SD recovery from AL sleep) was compared between E2 and oil treatment. The delta energy rebound was not significantly different between oil and E2 treatment (Figure 5, C). Interestingly, a comparison of the delta energy rebound decline over time revealed that only oil treatment induced a significant difference from the hour 1 (Figure 5, C; a denotes hours that are significantly different from hour 1).
E2 increased extracellular adenosine concentration in the MnPO of adult female rats
The analysis of homeostatic sleep measures strongly suggests that E2 decreased homeostatic sleep pressure in both AL sleep and SD recovery sleep. To test whether this decrease coincided with decreases in extracellular adenosine concentrations, we next measured extracellular concentrations of adenosine in the POA via microdialysis. Animals were treated according to timeline in Figure 6, A. The microdialysis probe placements within the MnPO are presented in Figure 6, B. Based on the mean in vitro probe recovery of 12%, the average extracellular baseline adenosine concentration in the MnPO of OVX females was calculated to be ~ 203 nM, which is near the range of reported values of 50–200 nM [68].
Extracellular adenosine concentration was significantly greater in E2-treated females compared with the oil controls during the first 6 h of light phase AL sleep (Figure 6, C). An AUC analysis over the 6 h revealed that the amount of adenosine over time was significantly increased by approximately twofold in E2-treated OVX females compared with oil controls (Figure 6, C). Similarly, extracellular adenosine concentration was significantly greater in E2-treated OVX females compared with the oil controls during SD that occurred over the first 6 h of the light phase. An AUC analysis for this SD period revealed that the amount of adenosine over time was significantly increased by approximately threefold in E2-treated OVX females compared with oil controls (Figure 6, D).
Comparing the AUC calculations across the four different treatment conditions demonstrated that SD did increase total adenosine concentration in both the oil and E2-treated groups compared with AL sleep; however, the increase did not reach statistical significance (Supplemental Figure S5). This could be due to the experimental variability between the AL sleep and SD recovery sleep cohorts which were run as separate experiments. Finally, when AL sleep–wake durations were analyzed in a subset of animals undergoing microdialysis and implanted with transmitters, E2- and oil-treated animals had equivalent amounts of light phase sleep (NREM and REM) and wake (data not shown but similar to the results in Figure 2, B), despite the higher adenosine levels in E2-treated animals.
E2 treatment attenuated the sleep-promoting actions of an adenosine A2A receptor agonist in the MnPO
Adenosine is widely accepted as both a molecular marker and mediator of sleep pressure [56, 59, 69]. The current finding that E2 increased MnPO adenosine concentration but reduced NREM-SWA presented an interesting paradox. Taken together, these finding suggested that E2 may be working to attenuate adenosine signaling and subsequently reduce the detection of homeostatic sleep pressure. To test this prediction, we investigated whether A2AR activation via local infusion of CGS, a highly specific A2AR agonist, induced sleep and decreased NREM-SWA in OVX females with and without E2 (Figure 7). Animals were treated according to timeline in Figure 7, A. The cannula placements for drug infusion into the MnPO are represented in Figure 7, B.
In OVX females treated with oil, local infusion of the CGS into the MnPO significantly reduced wake and increased NREM sleep in the dark phase by ~20% compared with vehicle infusion (Figure 7, C). This effect is similar to those reported in male rats [61]. However, when CGS was infused in the presence of E2-treated animals, the CGS-induced increase in NREM sleep and decrease in wake was significantly attenuated (Figure 7, C). The agonist did not have a significant effect on REM sleep with or without E2 (Figure 7, C). Of note, the reduced dose of E2 did not significantly change wake or NREM but did decrease REM sleep compared with oil/vehicle controls (Figure 7, C).
Discussion
The current set of findings provide evidence that E2 is involved in altering homeostatic sleep. Our previous work suggested that E2 might lower homeostatic need [42, 64, 67]. In the current study, we investigated estrogenic effects on specific behavioral, electrophysiological and neuromodulatory markers of sleep homeostasis in the light phase. In undisturbed light-phase sleep as well as in recovery sleep following 6 h of SD, E2 reduced delta power in the PSD, NREM-SWA, and delta energy, all markers of homeostatic sleep need. Interestingly, no significant difference in the magnitude of the delta energy rebound was detected between oil control and E2 treatments suggesting that E2 lowered the overall homeostatic set-point for sleep (i.e., less sleep need) compared with oil but did not affect the relative amount of delta energy rebound. Additionally, in the recovery period following total SD, E2 reduced the time spent in NREM further suggesting a decrease in homeostatic sleep pressure. Surprisingly, extracellular adenosine in the MnPO markedly increased during AL sleep and SD conditions following E2-treatment. Increases in extracellular adenosine are reliably linked to sleep induction [58]. Nevertheless, the current finding that E2 attenuated A2A receptor actions in the MnPO suggest a mechanism through which E2 blocked the actions of adenosine leading to decreased sleep need. Taken together, the current set of findings provide evidence that E2 may be working to increase the homeostatic set-point for sleep allowing for a decrease in sleep propensity despite periods of increased wake duration.
Estradiol and sleep homeostasis
In rodents, the absence of circulating gonadal steroids, eliminate sex differences in sleep behavior and architecture [40, 42, 70], suggesting that sex differences in sleep are primarily dependent on circulating sex steroids. In adult female rats, endogenous and exogenous E2 markedly suppresses dark-phase NREM sleep; however, findings as to whether E2 significantly affects light-phase sleep are mixed [40–42, 62, 63, 71]. One explanation for the mixed findings of E2 effects on light-phase sleep is the varied steroid-replacement paradigms and variability of endogenous steroidal milieus across animals. In our previous work, we found that Day 3 of the E2-replacement paradigm reliably mimics proestrus reproductive behaviors [72, 73], sleep [40, 42], and serum E2 levels [64]. Thus, using this replacement paradigm which allows for a more standardized physiological level of circulating E2, here, we investigated whether E2-influenced markers of homeostatic sleep in the light phase.
Although the mechanisms are not completely understood, the mammalian brain senses intrinsic sleep pressure or need. This is best exemplified by the increased sleep and SWA following periods of SD [51, 52, 74]. The current findings demonstrate that E2 replacement significantly reduced markers of homeostatic sleep need in baseline sleep (AL sleep) as well as in recovery sleep. In AL sleep, the amounts of NREM-SWA, delta energy, and power densities in the low-frequency delta bands were significantly decreased in E2-treated OVX females. Our current findings agree with studies from gonadally intact female rats demonstrating a significant reduction of NREM-SWA on the day of proestrus (when E2 levels are at their peak) [39, 41].
A similar pattern was seen in SD recovery sleep. Typically, SD leads to an increase in SWA during recovery sleep. Indeed, this was observed for both oil and E2 treatments. However, compared with oil controls, homeostatic sleep need was reduced following E2 treatment. Moreover, the E2-induced increase in wake and decrease in NREM durations further suggests that sleep drive is diminished compared with oil controls. The E2-induced increase in wake was not due to increased fragmentation of sleep, but rather an increase in wake bouts and a decrease in NREM bouts in the 2–5 min range. Importantly, the E2-induced decrease in SD recovery delta power suggests a true homeostatic response that was not a consequence of less NREM sleep. These findings taken together further supports the assertion that E2 is damping the homeostatic sleep drive and allowing for stable consolidated wake bouts.
Comparing the homeostatic responses between AL and SD recovery sleep demonstrated that the change in magnitude of the homeostatic markers was not significantly different between E2 and oil treatment. As expected, SD increased the amount of SWA (i.e., PSD, NREW-SWA, and delta energy) compared with AL sleep despite only achieving ~88% total wake during the deprivation. Moreover, recovery sleep facilitated the dissipation of NREM-SWA and delta energy to baseline levels for both treatments. Interestingly, the delta energy rebound (calculated as the magnitude of change of SD sleep from AL sleep), was not significantly different between oil and E2 treatments. Thus, when taken together with the findings that E2-reduced homeostatic measures in both sleep conditions, the findings suggest that E2 may lower the set-point for homeostatic sleep need, without affecting the recovery of sleep need, which is also lower in the presence of E2.
Estradiol and adenosine
The MnPO is a putative site for the sleep-inducing actions of adenosine as intracerebroventricular injections of an A2AR agonist increase NREM sleep duration, sleep propensity and NREM-SWA, and activation of sleep-active GABAergic neurons [61, 75]. Nevertheless, the current finding that E2 markedly increased MnPO extracellular adenosine during AL sleep and SD but markers of homeostatic sleep pressure where reduced compared with oil treatment, presents a curious paradox about adenosine action in the presence of E2. Interestingly, other groups have reported increased wake behavior following pharmacologically induced increases in POA adenosine [75, 76]. When levels of extracellular adenosine were experimentally increased by either (1) local microdialysis of nitrobenzyl–thioinosine (an adenosine transport inhibitor) or adenosine into the lateral POA [75] or (2) local microinjection of high concentrations of adenosine into the ventral lateral POA [76], NREM sleep is reduced, and wake increased in male rats. Taken together with our current results, these findings raise a critical question concerning adenosinergic receptor signaling in the presence of elevated adenosine levels; specifically whether changes in adenosinergic signaling in the MnPO underlie E2-induced sleep suppression. Of note, another recent study has reported sex and estrous cycle dependent differences in adenosine release in the hippocampus, basal lateral amygdala, and prefrontal cortex also suggesting that E2 may increase adenosine release [77]. However, to our knowledge, it is not known whether E2 affects adenosine concentrations in other sleep- or wake-associated nuclei.
Estradiol and adenosine A2A receptors
The current findings suggest that E2 blocked the sleep-inducing effects of the A2A receptor agonist, CGS, when the agonist was locally infused in the MnPO. The central somnogenic actions of adenosine occur via the A1 and A2A receptors located in brain nuclei associated with sleep–wake behaviors. Sleep induction via A1R occurs through the inhibition of several wake-promoting areas including the cholinergic arousal system in the brainstem [78] and basal forebrain [79], the orexinergic system in the lateral hypothalamus [80] and the histaminergic system in the tuberomammillary nucleus in the posterior hypothalamus [81]. In contrast, more recent evidence suggests that A2A receptors also play a significant role in sleep induction by exciting the GABAergic sleep-active neurons residing in the VLPO area [82] and the MnPO [49, 61]. Pharmacological experiments in male rats demonstrate that infusion of a highly selective A1R agonist, N6-cyclo-pentyladenosine (CPA) into the basal forebrain increases NREM and REM sleep [83, 84]. Subsequent work focusing on the POA sleep-active nuclei suggest that activation of A2A receptor with CGS stimulates sleep-active neurons in the VLPO [85] and MnPO [61] and increases GABA release in key arousal centers like the TMN [86].
In the current study, given that the ventral portion of the MnPO is located next to the third ventricle, the possibility exists that local CGS infusion may have had additional off-target (i.e., outside of the MnPO) effects via diffusion through the cerebral spinal fluid that contributed to the increase in NREM sleep. Nevertheless, given our findings that the MnPO is a key site of E2 action [45] and that the MnPO would have received a significantly higher dose of locally infused CGS compared with other sites as a result of diffusion via the 3rd ventricle, the current findings at least suggest a role for the MnPO in mediating E2 effects on sleep.
Interestingly, activation of POA A1 receptors by local infusion of CPA markedly increases wake at the expense of NREM sleep [75]. Indeed, the observation that activation of POA A1 receptors induce wake may offer a possible insight into how E2 is increasing wake in the presence of increased adenosine; E2 may be shifting the adenosinergic balance of the excitatory A2A tone that activates the GABAergic sleep-active neurons to an inhibitory A1 tone which inhibits the sleep-active neuronal population in the MnPO. The current findings suggest that E2 attenuated the signaling of the MnPO A2A receptors when activated by CGS. However, the E2 was administered at subthreshold levels to prevent behavioral changes in sleep–wake. Thus, it remains unclear whether E2-induced increases in adenosine activate A1 receptors leading to an increase in wake. These experiments are currently ongoing.
While the exact mechanism for how elevated levels of extracellular adenosine induce wake is not known, previous findings do suggest several possibilities including a shift in the excitatory/inhibitory balance [87]; direct post synaptic actions on neurons activated during sleep such as an uncoupling/downregulation of A2A signaling [75] or A1-mediated disinhibition of GABAergic inputs to sleep-active neurons [88]. Our current findings strongly suggest that the E2-induced increases in extracellular MnPO adenosine may be the upstream-trigger of these potential subsequent actions on the signaling inputs that result in an inhibition of MnPO sleep neurons and increases in wake. Future work will seek to determine whether E2 requires a marked increase in extracellular MnPO adenosine to mediate its effects on sleep.
Conclusions
While sex steroids and biological sex have been implicated as risk factors for sleep disruptions and insomnia, the relationship between ovarian steroids and normal sleep continues to be under investigated and poorly understood. Moreover, the mechanisms mediating ovarian steroid control of sleep are unknown. The current findings begin to lay the foundation for understanding potential cellular mechanisms underlying estrogenic effects on vigilance states and possibly sleep homeostasis by demonstrating a novel interaction between E2 and adenosinergic signaling in a major sleep-active nucleus, the MnPO. Future work will continue to elucidate the significance of this E2-adenosine nexus on regulation of sleep. Understanding the role of estrogenic regulation of sleep mechanisms is a critical first step toward a better understanding of roles ovarian steroids play in sleep pathologies and ultimately identification of targets for improved interventions for treating sleep disturbances in women.
Supplementary Material
Acknowledgments
Adenosine measurement was performed at University of Maryland, School of Medicine Center for Innovative Biomedical Resources, Protein Analysis Core, Baltimore, MD. LC/MS was performed at the Molecular Characterization and Analysis Complex at the University of Maryland, Baltimore County, Arbutus, MD. The scoring algorithm was developed with programming assistance from Dr Michael Rempe, Department of Mathematics and Computer Science, Whitworth University, Spokane, Wash.
Funding
This research was funded by the National Heart Lung and Blood Institute (NHLBI) #1F30HL145901 (granted to PCS) and grant # 5R01HL129138 (granted to JAM).
Disclosure Statement
There are no financial or conflicts of interest associated with this work. A preprint version of this manuscript was submitted to bioRxiv (2021 Pages 2021.05.27.445868).
References
- 1. Ford ES, et al. Trends in self-reported sleep duration among US Adults from 1985 to 2012. Sleep. 2015;38(5):829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ford ES, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watson NF, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wickwire EM, et al. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. [DOI] [PubMed] [Google Scholar]
- 5. Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31(3):333–346. [DOI] [PubMed] [Google Scholar]
- 6. Hu XZ, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kravitz HM, et al. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson EO, et al. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–e256. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, et al. Emergence of sex differences in Insomnia symptoms in Adolescents: a large-scale school-based study. Sleep. 2016;39(8):1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mong JA, et al. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parry BL, et al. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30(3):285–303. [DOI] [PubMed] [Google Scholar]
- 12. Dzaja A, et al. Women’s sleep in health and disease. J Psychiatr Res. 2005;39(1):55–76. [DOI] [PubMed] [Google Scholar]
- 13. Joffe H, et al. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28(5):404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shechter A, et al. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shechter A, et al. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Redline S, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–726. [DOI] [PubMed] [Google Scholar]
- 17. Méndez M, et al. Interactions between sleep and epilepsy. J Clin Neurophysiol. 2001;18(2):106–127. [DOI] [PubMed] [Google Scholar]
- 18. Vock J, et al. Evolution of sleep and sleep EEG after hemispheric stroke. J Sleep Res. 2002;11(4):331–338. [DOI] [PubMed] [Google Scholar]
- 19. Petit D, et al. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56(5):487–496. [DOI] [PubMed] [Google Scholar]
- 20. Somers VK, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080–1111. [DOI] [PubMed] [Google Scholar]
- 21. Pasic Z, et al. Incidence and types of sleep disorders in patients with stroke. Med Arh. 2011;65(4):225–227. [DOI] [PubMed] [Google Scholar]
- 22. Ju YE, et al. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Musiek ES, et al. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayas NT, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. [DOI] [PubMed] [Google Scholar]
- 25. Meier-Ewert HK, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. [DOI] [PubMed] [Google Scholar]
- 26. Patel SR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. [DOI] [PubMed] [Google Scholar]
- 27. Gangwisch JE, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. [DOI] [PubMed] [Google Scholar]
- 28. Cappuccio FP, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(Suppl 1B):S19–S28. [DOI] [PubMed] [Google Scholar]
- 30. Lyytikäinen P, et al. Association of sleep duration with weight and weight gain: a prospective follow-up study. J Sleep Res. 2011;20(2):298–302. [DOI] [PubMed] [Google Scholar]
- 31. Ferrie JE, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller MA, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32(7):857–864. [PMC free article] [PubMed] [Google Scholar]
- 33. Kronholm E, et al. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12(3):215–221. [DOI] [PubMed] [Google Scholar]
- 34. Merklinger-Gruchala A, et al. Low estradiol levels in women of reproductive age having low sleep variation. Eur J Cancer Prev. 2008;17(5):467–472. [DOI] [PubMed] [Google Scholar]
- 35. Baker FC, et al. Sex differences and menstrual-related changes in sleep and circadian rhythms. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier Saunders; 2010: 1562–1571. [Google Scholar]
- 36. Mong JA, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shechter A, et al. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. 2012;13(8):1071–1078. [DOI] [PubMed] [Google Scholar]
- 38. Sharkey KM, et al. Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med. 2014;15(6):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwierin B, et al. Prolonged effects of 24-h total sleep deprivation on sleep and sleep EEG in the rat. Neurosci Lett. 1999;261(1–2):61–64. [DOI] [PubMed] [Google Scholar]
- 40. Hadjimarkou MM, et al. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27(7):1780–1792. [DOI] [PubMed] [Google Scholar]
- 41. Swift KM, et al. Sex differences within sleep in gonadally intact rats. Sleep. 2020;43(5):1–4. doi: 10.1093/sleep/zsz289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cusmano DM, et al. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology. 2014;155(1):204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mong JA, et al. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: evidence from high-density oligonucleotide arrays and in situ hybridization. Proc Natl Acad Sci U S A. 2003;100(1):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mong JA, et al. Reduction of lipocalin-type prostaglandin D synthase in the preoptic area of female mice mimics estradiol effects on arousal and sex behavior. Proc Natl Acad Sci U S A. 2003;100(25):15206–15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith PC, et al. Estradiol action at the median preoptic nucleus is necessary and sufficient for sleep suppression in female rats. bioRxiv. 2020. doi: 10.1101/2020.07.29.223669 [DOI] [Google Scholar]
- 46. Lu J, et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20(10):3830–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gvilia I, et al. Different neuronal populations of the rat median preoptic nucleus express c-fos during sleep and in response to hypertonic saline or angiotensin-II. J Physiol. 2005;569(Pt 2):587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gvilia I, et al. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26(37):9426–9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alam MA, et al. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol. 2014;111(2):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borbély AA, et al. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–495. [DOI] [PubMed] [Google Scholar]
- 51. Franken P, et al. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261(1 Pt 2):R198–R208. [DOI] [PubMed] [Google Scholar]
- 52. Franken P, et al. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130(2):141–144. [DOI] [PubMed] [Google Scholar]
- 53. Borbely AA. Sleep mechanisms. Sleep and Biological Rhythms. 2004;2:S67–S68. [Google Scholar]
- 54. Borbély AA, et al. Manifestations and functional implications of sleep homeostasis. Handb Clin Neurol. 2011;98:205–213. [DOI] [PubMed] [Google Scholar]
- 55. Deboer T. Behavioral and electrophysiological correlates of sleep and sleep homeostasis. Curr Top Behav Neurosci. 2015;25:1–24. [DOI] [PubMed] [Google Scholar]
- 56. Lazarus M, et al. Adenosine and sleep. Handb Exp Pharmacol. 2019;253:359–381. [DOI] [PubMed] [Google Scholar]
- 57. Lazarus M, et al. Gating and the need for sleep: dissociable effects of adenosine A1 and A2A receptors. Front Neurosci. 2019;13:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Porkka-Heiskanen T, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porkka-Heiskanen T, et al. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99(3):507–517. [DOI] [PubMed] [Google Scholar]
- 60. Strecker RE, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115(2):183–204. [DOI] [PubMed] [Google Scholar]
- 61. Kumar S, et al. Adenosine A(2A) receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2013;305(1):R31–R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schwierin B, et al. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811(1–2):96–104. [DOI] [PubMed] [Google Scholar]
- 63. Deurveilher S, et al. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep. 2009;32(7):865–877. [PMC free article] [PubMed] [Google Scholar]
- 64. Schwartz MD, et al. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R271–R280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oriowo MA, et al. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception. 1980;21(4):415–424. [DOI] [PubMed] [Google Scholar]
- 66. Lund-Pero M, et al. Non-specific steroidal esterase activity and distribution in human and other mammalian tissues. Clin Chim Acta. 1994;224(1):9–20. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz MD, et al. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol Behav. 2011;104(5):962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Latini S, et al. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–484. [DOI] [PubMed] [Google Scholar]
- 69. Blanco-Centurion C, et al. Adenosine and sleep homeostasis in the Basal forebrain. J Neurosci. 2006;26(31):8092–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paul KN, et al. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463(3):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Deurveilher S, et al. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep. 2011;34(4):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Holder MK, et al. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology. 2010;35(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holder MK, et al. Methamphetamine enhances paced mating behaviors and neuroplasticity in the medial amygdala of female rats. Horm Behav. 2010;58(3):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Donlea JM, et al. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol. 2017;44:228–235. [DOI] [PubMed] [Google Scholar]
- 75. Methippara MM, et al. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1715–R1723. [DOI] [PubMed] [Google Scholar]
- 76. Zhang J, et al. Microinjection of adenosine into the hypothalamic ventrolateral preoptic area enhances wakefulness via the A1 receptor in rats. Neurochem Res. 2013;38(8):1616–1623. [DOI] [PubMed] [Google Scholar]
- 77. Borgus JR, et al. Complex sex and estrous cycle differences in spontaneous transient adenosine. J Neurochem. 2020;153(2):216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rainnie DG, et al. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263(5147):689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alam MN, et al. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521 Pt 3:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu ZW, et al. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97(1):837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oishi Y, et al. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105(50):19992–19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scammell TE, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107(4):653–663. [DOI] [PubMed] [Google Scholar]
- 83. Benington JH, et al. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692(1–2):79–85. [DOI] [PubMed] [Google Scholar]
- 84. Yang C, et al. Adenosine inhibits the excitatory synaptic inputs to Basal forebrain cholinergic, GABAergic, and parvalbumin neurons in mice. Front Neurol. 2013;4:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gallopin T, et al. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134(4):1377–1390. [DOI] [PubMed] [Google Scholar]
- 86. Hong CJ, et al. Association studies of the adenosine A2a receptor (1976T > C) genetic polymorphism in Parkinson’s disease and schizophrenia. J Neural Transm (Vienna). 2005;112(11):1503–1510. [DOI] [PubMed] [Google Scholar]
- 87. Qi G, et al. Adenosine differentially modulates synaptic transmission of excitatory and inhibitory microcircuits in layer 4 of Rat Barrel Cortex. Cereb Cortex. 2017;27(9):4411–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morairty S, et al. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123(2):451–457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.