Abstract
Introduction
Cerebral glucose and insulin metabolism is impaired in Alzheimer's disease (AD). Ketones provide alternative energy. Will medium chain triglyceride (MCT) oil, a nutritional source of ketones, impact cognition in AD?
Methods
This was a 6‐month randomized, double‐blind, placebo‐controlled, crossover study, with 6‐month open‐label extension in probable AD subjects, on stable medications. MCT dose was 42 g/day, or maximum tolerated. Cognition was assessed with Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Cognigram®.
Results
Twenty subjects, average age 72.6 years, 45% women, 70% university educated had baseline MMSE 22.6/30 (10–29); MoCA 15.6/30 (4–27); baseline Cognigram® Part 1: 65–106, Part 2: 48–107. Average MCT oil consumption was 1.8 tablespoons/day (25.2 g, 234 kcal). Eighty percent remained stable or improved. Longer MCT exposure and age > 73, resulted in higher final MMSE (P < .001) and Cognigram® 1 scores.
Discussion
This is the longest duration MCT AD study to date. Eighty percent had stabilization or improvement in cognition, and better response with 9‐month continual MCT oil.
Keywords: betahydroxybutyrate, coconut oil, cognigram, Cognitive Impairment, computer based cognitive testing, ketones, Montreal Cognitive Assessment
1. INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia affecting one in four people over the age of 80. Worldwide prevalence is estimated at 50 million cases, 1 with cases expected to double every 20 years. 2 Current pharmacotherapy provides symptomatic relief at best, and does not affect the disease trajectory. 3 No new pharmaceutical agents have reached the Canadian market since 1997.
The brain is an obligate glucose metabolizer using 120 to 130 g/day of glucose. 4 It uses 16% of the body's total O2 consumption, despite representing only 2.0% to 2.3% of adult body weight. In conditions of low carbohydrate intake or fasting, the body uses ketones (acetoacetate and beta hydroxybutyrate [BHB]) as an alternative energy source to glucose. Ketones are normally generated in fasting states from beta‐oxidation of adipose stores to maintain cerebral function. In long‐term fasting, ketones can supply > 60% of the brain's energy requirements, 4 , 5 and are preferentially taken up by the brain over glucose. 6 , 7 This occurs in cognitively normal younger and older adults, as well as in those with mild cognitive impairment (MCI) and AD. 8 , 9
Ketones can also be induced with a very low carbohydrate high fat (VLCHF) diet. 13 Medium chain triglyceride (MCT) oil has the potential to produce a nutritional source of ketones for an alternative brain fuel to glucose, 10 , 11 , 12 , 13 , 14 , 15 , 16 or by the consumption of MCT oil or esterases in freeze‐dried form. 15 This is independent of the fasting state or carbohydrate intake. Long‐term compliance with fasting or VLCHF and LCHF diet regimes is challenging and requires strict medical supervision. 17 Hence, the potential advantage of nutritional ketone sources (MCT) over these restrictive diets. Our recent study showed a clear dose‐dependent effect on ketone (BHB) generation with varying doses of MCT supplementation, and was found to be equivalent in young, elderly, and AD subjects 18 .
In AD, the brain is unable to use glucose normally, 19 , 20 causing hypofunction of 20% to 40% in key areas of the brain responsible for the symptoms in AD. This hypometabolism can be demonstrated with fluorodeoxyglucose positron emission tomography scanning 21 even in preclinical AD. It results from several factors: an abnormality of cerebral glucose receptors 22 , cerebral insulin resistance 23 , and abnormal cerebral glucose metabolism 24 . Hence, MCT oil may provide benefit as a source of readily available alternative energy: ketones. (Metabolism of MCT is discussed in the supporting information Appendix A). Because ketones generated in the liver cannot be used by the liver for energy, all therefore flow from the liver to extra‐hepatic tissues as fuel. 27 Given their origin from saturated fat sources (palm and coconut oil), there is potential concern for effects on the lipid profile or body composition in subjects consuming the oil regularly. 28
In humans, ketone infusions have been shown to reduce hormonal responses to acute hypoglycemia and improve cognitive functioning. 29 They also increase cerebral blood flow. 9 This has had a clinical benefit in conditions such as intractable epilepsy 30 . However, the data in cognitive impairment is mixed 12 , 14 , 15 , 16 , 31 , 32 , 33 , 34 , continuing the speculation about their potential role in aging and AD. 35 The few clinical studies that have been done are only in subjects with MCI or early AD, and are of short duration—a few weeks or months.
HIGHLIGHTS
Alzheimer's disease (AD) is a progressive neurodegenerative disease, currently with no effective therapy.
The brain is an obligate glucose user. AD is associated with impaired cerebral glucose metabolism.
Ketones are an alternative source of cerebral fuel.
Medium chain triglyceride oil (MCT) is a nutritional source of ketones.
This study suggests consistent MCT oil intake stabilizes cognition in AD subjects, especially in mild to moderate disease.
The purpose of this study was to use MCT oil supplementation to address some of the gaps in the current literature, including its effect on cognition in subjects with mild to moderate AD, the effect of longer duration (> 6 months), and the tolerance and safety (weight, lipid profile) with longer duration therapy.
We hypothesized that intermittent elevation of serum BHB, provided by regular ingestion of MCT oil, would result in stabilization or improvement of cognitive function in subjects with mild–moderate AD, without significant cardiovascular safety concerns.
RESEARCH IN CONTEXT
Systematic review: The authors searched relevant keywords in MEDLINE, including PubMed and Epub Ahead of Print with no restriction on publication dates. The relevant dementia clinical studies are summarized in the discussion. None were as long in duration as the current study. None included subjects with moderate–severe AD. None used the Cognigram® assessment tool. All reported a trend toward improvement, but this was not statistically significant in all cases.
Interpretation: This 15‐month, double‐blind, randomized, placebo‐controlled trial of community‐dwelling moderate–severe AD subjects suggests a stabilization of cognition with exposure to continuous daily nutritional ketones (in the form of medium chain triglyceride [MCT] oil) over a 9‐month period.
Future direction: Larger studies are needed to confirm this response, and look at other stages of AD, including mild cognitive impairment. Further research also needs to look at ways to improve compliance with different formulations of MCT.
2. METHODS
2.1. Design
This study is a randomized, double‐blind, placebo‐controlled, convenience sample, crossover study, with an open‐label MCT arm extension, in subjects with probable AD (see Figure 1; ClinicalTrials.gov Identifier NCT04396015). Each crossover arm includes a 1‐month dose titration followed by 3 months of therapy. The open‐label phase includes a 1‐month titration then 6 months of therapy, for a total study duration of 15 months. It was carried out at a single tertiary care hospital outpatient site in Edmonton, Alberta, Canada, with one principal investigator (PI) and one research nurse.
FIGURE 1.
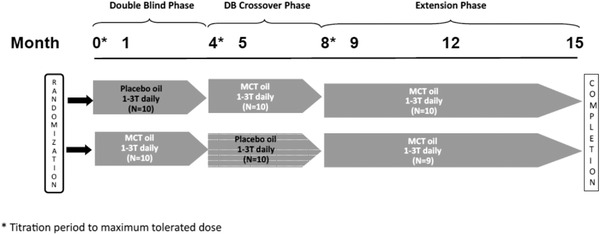
Study design
2.2. Ethics
This study was approved by the Health Products and Food Branch of Health Canada (HC6‐24‐C186660) and the local University of Alberta Health Research Ethics Board (Pro 000054165). Procedures followed were also in accordance with the Declaration of Helsinki Declaration as revised in 1983. All human subjects provided informed consent or assent.
2.3. Participants
Participants were community dwelling, with a diagnosis of dementia of the Alzheimer's type (mild to moderate), using standard criteria. AD diagnosis was based on the revised National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) 36 and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) 37 criteria, of at least 12 months duration.
Participants could continue AD medications (acetyl cholinesterase inhibitors [AChEI] and/or memantine), antidepressants, and antipsychotics as long as the doses were stable for at least 3 months prior to enrollment. All comorbidities needed to be stable. A Mini‐Mental Status Examination (MMSE) 38 score between 10 and 29 was required, as was a reliable caregiver. Given the unknown effect size, a convenience sample of 20 participants was enrolled.
Exclusion criteria included age < 50 years; non‐English speaking; allergy to coconut or MCT oil; swallowing issues; unstable medical conditions or malignancy; diabetes; history of alcohol abuse; known liver or renal disease; clinically significant abnormal lipid, hepatic, or renal function tests at screening; and high cardiovascular risk.
2.4. Protocol
Comprehensive Geriatric Assessment, medication documentation, and MMSE 38 score evaluated for eligibility Eligible subjects who chose to participate signed an ethically approved consent or assent form, depending on their cognitive ability.
Further baseline cognitive testing included Montreal Cognitive Assessment (MoCA) 39 and the computer based Cognigram®. 40 This is a validated computer‐based cognitive test that assesses processing speed, attention, visual learning, and working memory. It can be repeated frequently as there is no learning component. It is independent of education, which is very important, particularly in groups with low or high education (as in this study). See supporting information for more details on Cogstate's Cognigram® test.
2.4.1. Justification for cognitive tools
MMSE and MoCA were included as these tests are the ones most clinicians are familiar with, and therefore makes the understanding and clinical relevance of the study results that much greater. Although the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS‐Cog) is considered the gold standard cognitive test in AD pharmaceutical trials, it is not clinically practical. And although there may be statistically significant changes in the score (given it is a 70‐point scale), this often does not translate to any clinically perceivable/relevant difference.
The Cognigram® was included because of its validity for repeated frequent use, standardization of administration given it is a computer‐based test, acceptance of participants (it is a card game–based test), and its ability to detect small changes over time, unlike the MMSE and MoCA, which are primarily validated for screening and not ongoing evaluation (although this is how they are used in clinical practice).
The Euroqol EQ‐5D quality of life questionnaire 41 was administered at baseline and every study visit.
Apolipoprotein E (APOE) ε4 status was evaluated with saliva testing.
Randomization was done using a randomization sequence (Randomization.com) with 1:1 allocation and two blocks, by a study administrator (JK) who was not involved in any of the clinical assessments.
Caregivers completed a study oil intake diary, and returned all unused study oil at each visit.
Safety monitoring included monthly bloodwork including evaluation of lipid status. At every visit, weight, waist/hip circumference, body mass index (BMI) and ultrasound body composition status, blood pressure and heart rate, gastrointestinal (GI) and cardiac symptom screening, and current medication update were done. Baseline and end‐of study dual‐energy absorptiometry (DXA) body composition was completed.
2.5. Outcome measures
Primary outcome measures were: Cognigram® tests (1 and 2), MMSE, and MoCA. Secondary outcomes included impact of APOE ε 4 status, quality of life (QOL), and BHB level. Safety measures included lipid profile, fasting insulin, homocysteine, BMI, body fat composition (DXA), and clinical adverse events.
2.6. Intervention
Test MCT oil was Bulletproof Brain Octane ® (NPN 80057199) and placebo oil was olive oil (Hermes Olive Pomace oil ®).
Study oil was provided in a solid white plastic bottle. Both subjects and investigators were blinded to the contents. Standardized 15 mL measuring spoons were provided. Titration was to maximum tolerated dose (or 3 T [45 mL[ daily) for each stage (see Figure 1).
Bulletproof MCT oil is clear, colorless, and tasteless, and was chosen because of the Health Canada (HC) requirement for a Drug Identification Number (DIN). Pomace olive oil was chosen for its light taste and color. Study oil bottles were filled by the study administrators (JK, DS) and labeled according to HC guidelines identifying the subject, study, and test dose number only.
As both oils are over‐the‐counter products, for the validity of this study, the oil content was independently verified by an unrelated lipid research laboratory (see Acknowledgements).
The first month of the study was a week of test oil with dosing titration from 15 mL once daily to three times daily by week three, if clinically tolerated, or to the maximum tolerated dose. This dose was continued for each arm (4 months in total), and then the protocol was repeated when the crossover occurred. A similar titration schedule was followed for the open‐label extension (7 months in total).
2.7. Compliance
Returned test oil was weighed, and intake dairies reviewed.
2.8. Study visits
Study visits occurred monthly in the randomization/crossover stages, and three times a month in the open‐label phase. Different versions of the MoCA test (versions 1, 2, or 3) 39 were used sequentially at each assessment to minimize any learning effect. The Cognigram® was done monthly, as this has no learning component. The cognitive testing was done under the same conditions at each visit, either by the PI or the research nurse, to ensure reliability and consistency in administration of the cognitive tests.
2.9. Adverse events
Adverse events (AE), recorded as per standard HC protocols and reporting, were enquired for at each study visit, and at telephone contacts with the study nurse between visits, or spontaneously reported by subjects or caregivers. They were classified as mild, moderate, or severe based on pre‐specified criteria. 42 . Serious adverse events (SAE) were defined as death; life threatening event; hospitalization—initial or prolonged; disability; or medically important event. Any adverse events was also classified as: “Related” to study intervention = events identified in regulatory documents such as Investigator Brochure or product monograph and occurring within expected frequency estimates, or those identified in research ethics board submission and Letter of Information to participants; or “Unrelated” to study intervention = the result of the natural progression of the person's disease/illness and/or state of health; or “Unexpected (Unanticipated)” = events not identified in Investigator's Brochure, Product Monograph, or local protocol or occurring with more than expected frequency.
2.10. Statistical analysis
Data analysis was completed using the SAS 9.0 statistical software (version 9.4; SAS Institute Inc.). P‐values ≤.05 were indicative of statistical significance. Data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) unless otherwise specified. The Shapiro‐Wilk test was conducted to assess the normality of distribution. Repeated measures analysis of variance was performed to assess the effects of MCT supplementation on primary outcomes (Cognigram® 1/Cognigram® 2, MMSE, and MoCA tests) and secondary outcomes (serum BHB, safety) over the course of the study. Analysis of covariance was performed to adjust for any variables influencing these outcomes (baseline MMSE, MOCA, Cognigram® 1/Cognigram® 2, age, sex). Chi‐square tests were used to measure differences in categorical data. Univariate and multivariate analyses were conducted to assess potential relationships between MCT supplementation and primary outcomes.
3. RESULTS
3.1. Demographic and anthropometric data
From January to June 2016, 30 subjects were screened, and 10 did not meet inclusion criteria. Twenty subjects participated in the double‐blind phase, and 19 in the open‐label extension. One dropped out of the open‐label extension due to long‐term care admission. The remaining 19 subjects completed the total study duration (15 months).
Table 1 shows baseline demographics of the two arms. There were no statistically significant differences in demographic and anthropometric data, except for baseline Cognigram® Part 1, which was lower in the group randomized to the MCT oil start (P < .001). The MCT start group had a higher prevalence of APOE ε4 positivity, but this difference was not statistically significant.
TABLE 1.
Baseline demographics in groups assigned to Placebo oil or MCT oil start
| Variable | Placebo (n = 10) | MCT oil (n = 10) | P‐values |
|---|---|---|---|
| Age (years) | 75.7 ± 8.8 (53‐84) | 68.4 ± 9.9 (56‐84) | .67 |
| Sex (M:F) | 4:6 | 7:3 | .89 |
| Height (in) | 64.7 ± 3.9 (59‐70) | 68.3 ± 4.6 (63‐78) | .07 |
| Weight (lbs) | 171.9 ± 30.2 (133‐210) | 161.4 ± 31.8 (114‐220) | .82 |
| BMI (kg/m2) | 28.5 ± 3.0 (24‐33) | 24.1 ± 3.5 (19‐30) | .73 |
| Level of education (% college/university) | 50.0 | 90.0 | .49 |
| APOE ε4 status | N = 10 | N = 9 | .27 |
| Absent | 6 (60%) | 3 (33%) | .25 |
| Heterozygote | 2 (20%) | 5 (56%) | .11 |
| Homozygote | 2 (20%) | 1 (11%) | .60 |
| AChEI use | 6 (60%) | 8 (80%) | .35 |
| Memantine use | 2 (20%) | 2 (20%) | .82 |
| Baseline MMSE | 23 (12–28) | 22.3 (10–29) | .79 |
| Baseline MoCA | 15.9 (0–29) | 15.4 (4–27) | .65 |
| Baseline Cog 1 | 88.5 (65–110) | 67.1 (0–106) | <.001* |
| Baseline Cog 2 | 70.9 (0–102) | 61.7 (0–107) | .43 |
Note:
*Statistically significant difference.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; MCT, medium chain triglyceride; MMSE, Mini0‐Mental State Examination; MoCA, Montreal Cognitive Assessment.
3.2. Study oil analysis
All the MCT oil and olive oil came from the same lot number (1507075254, expiration 07/17; 16036E15, expiration 02/18, respectively) as that tested in the independent lipid research laboratory (see Acknowledgements). Bulletproof MCT oil was verified as 99.3% C8, 0.6% C10, and 0.1% C12:0. Placebo oil was 72.2% C18:1, 11.3% 18:2 (oleic acid), 12% C16:0 confirming it as a long chain, predominantly olive, oil.
3.3. MCT oil consumption
Average oil consumption was 30 mL (two tablespoons) daily. There was no difference between the two groups (P > .05). Intake was limited by compliance (lunchtime dose often forgotten by caregiver), or tolerance (predominantly GI related, such as abdominal pain or diarrhea). There was no change in morning fasting BHB levels over the course of the study.
3.4. Outcomes
A minimum dose of 15 mL (1 tablespoon, 14 g) of MCT oil over the entire study period resulted in stabilization in MMSE and MoCA, and improvement in Cognigram® 1 scores (see Table 2).
TABLE 2.
Cognitive Scores in each phase of the study
| Double‐blind placebo‐controlled Phase 1 Visits 0–4 | Double‐blind placebo‐ controlled Phase 2 (crossover) Visits 5–8 | Open label MCT extension Phase 3 Visits 9–12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Placebo start | MCT start | P | Placebo start | MCT start | P | Placebo start | MCT start | P | P model for group effects |
| MMSE | 23.8 ± 4.7 | 22.8 ± 6.4 | .75 | 23.7± 4.8 | 21±6.9 | .44 | 23.4±5.5 | 20.1±7.7 | .06 | .05 |
| MoCA | 18.6± 6.5 | 25.4±7.2 | .04 | 19.7±5.4 | 14.8±7.9 | .12 | 17.1±5.1 | 16±7.3 | .5 | .08 |
| Cog 1 | 90.2±15.9 | 76.7±21.6 | .12 | 91.3±13.7 | 79.2 ± 22.9 | .17 | 89.8±19.3 | 80.5±21.2 | .09 | .003* |
| Cog 2 | 84.9±13.1 | 80.8±24.3 | .62 | 84.4±12.9 | 73.1±27.7 | .24 | 88.7 ± 13.5 | 83.8±21.9 | .3 | .02 |
Cognitive scores in each phase of the study. (Placebo start refers to group starting study in placebo arm, MCT start refers to group starting in MCT arm).
Abbreviations: MCT, medium chain triglyceride; MMSE, Mini Mental Status Examination; MoCA, Montreal Cognitive Assessment; Cog 1, Cognigram® Part 1; Cog 2, Cognigram® Part 2.
*Statistically significant result.
A statistically significant difference in Cognigram® 1 (attention and psychomotor function) scores between the two groups at the study completion (after the additional 7 months open‐label MCT oil), P = .003, occurred in those who has started with the placebo oil (i.e., subjects who had 11 months of uninterrupted MCT oil consumption vs. those interrupted by placebo oil).
However, no statistically significant difference in Cognigram® 2 scores occurred between the two groups.
Of note, four subjects, three in the MCT start group and one in the placebo start group, were unable to complete the Cognigram® due to their level of cognitive impairment, and inability to follow the testing instructions.
To evaluate whether the intervention affected the usual downward trajectory of MMSE in AD, subjects were evaluated using data published by Doody et al. 43 reporting what the expected decline would be based on baseline MMSE as follows: Slow, with initial MMSE 24 = 0–1.9 pts/year; Interim, with initial MMSE = 18 2–4.9/year; or Rapid, with initial MMSE 14 > = 5/year. In 20% (four subjects), there was a decline in MMSE. These participants did not differ statistically from the other participants in baseline parameters. Three of the four decliners were on AChEI therapy, and all were homozygous or heterozygous for the APOE ε4 allele.
Those with a higher baseline MMSE were more likely to be a “responder”; P = .04. MCT starters had a trend toward greater declines in MMSE scores than placebo starters (P = .06), again suggesting more continuous exposure to MCT may be an advantage. Therefore, based on the Doody et al. 43 classification, 80% of subjects were “responders” based on their baseline and expected final MMSE scores (see Figure 2A).
FIGURE 2.
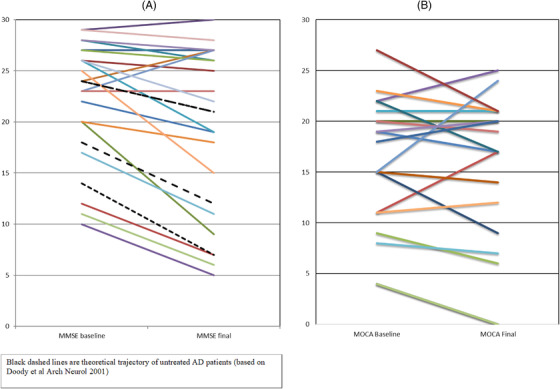
A, Graph showing change from baseline to final Mini‐Mental State Examination (MMSE; 15 months for 19 subjects, 8 months for one). Black lines represent theoretical decline anticipated, trajectory depending on starting MMSE level (Doody et al. 43 ). B, Graph showing change in Montreal Cognitive Assessment (MoCA) from baseline to final (15 months for 19 subjects, 8 months for one)
Overall, there was a noticeable stability or improvement in MMSE and MoCA scores in 80% of the participants, unrelated to their group assignment (Figure 2A,B). Age appeared to impact response to MCT oil supplementation based on stabilization and/or increases in MMSE scores. Fewer patients under the median age of 73 responded to MCT oil supplementation (by MMSE score changes): (3/10) versus 8/9 over the median age of 73, P = .009. Baseline MMSE also affected response, with those having an MMSE 25 to 29 at the start more likely to be responders.
Responses to MCT oil supplementation were independent of weight (P = .4), height (P = .22), and hip circumference (P = .33), but were related to BMI (P = .01) and waist circumference (WC; P = .02), with higher BMI/WC being indicative of a better response. This was primarily due to the differences in body composition related to age. No differences in baseline MMSE scores were noted between patients above and below the median age, but the older patients had higher BMI and WC than the younger patients.
Post hoc power analysis was done, given that this was a convenience sample. Statistical analysis revealed adequate power for change in MoCA (> 80%), but not for MMSE, Cognigram® 1, or Cognigram® 2 (< 40%).
Average fasting baseline BHB was 0.19 mmol/L (0–0.4), and at study completion was 0.22 mmol/L (0.1–0.9), showing, as expected, no permanent change in baseline BHB production with exposure to nutritionally induced ketones. This result also confirmed no significant dietary pattern changes (toward a ketogenic diet) during the study.
There was no measurable effect of APOE ε4 status on the response to MCT oil.
Perceived QOL reported by participants was high throughout the study. The EQ‐5D‐5L average visual analogue scale score was 84.4/100 with a range from 40 to 100. There was no difference between baseline (average 85.1) and study completion (average 85.95).
There was no statistically significant change in lipids (total cholesterol [TC], low density lipoprotein [LDL], triglyceride [TG]) in either group (on or off MCT oil; P ≥ .05). See Table 3. A weak association was noted for lower LDL and TC in those consuming higher doses of MCT oil. DXA body composition showed no significant change in percent body fat in both groups, suggesting no adverse effect on body composition related to prolonged MCT oil intake.
TABLE 3.
Lipid Profile safety evaluation
| Variable (mmol/L) | Baseline (n = 20) | Study completion (n = 19) | P‐values |
|---|---|---|---|
| Total cholesterol | 5.17 ± 1.09 (3.9–7.9) | 5.16 ± 1.48 (3.2–8.2) | .47 |
| Triglyceride | 1.14 ± 0.42 (0.49–2.18) | 1.35 ± 1.02 (0.34–4.74) | .10 |
| HDL | 1.79 ± 0.40 (1.2–2.8) | 1.73 ± 0.47 (1.0–2.7) | .52 |
| LDL | 2.90 ± 0.92 (1.08–5.62) | 2.81 ± 1.11 (1.17–5.43) | .72 |
| Ratio Cholesterol/HDL | 3.03 ± 0.65 (2.0–4.7) | 3.40 ± 1.11 (1.67–5.74) | .15 |
| Reference ranges (mmol/L) | |||
| Total cholesterol | <4.15 | ||
| Triglyceride | 0.0‐1.7 | ||
| HDL | >1.15 | ||
| LDL | 0.0‐3.4 | ||
| Ratio Cholesterol/HDL | <4.0 |
Note: Safety data: Lipid profile (average ± standard deviation).
Abbreviations: HDL, high density lipoprotein; LDL, low density lipoprotein.
In addition, there was no impact on glycemic control (HbA1c and fasting insulin), P = .06, during the study.
3.5. Adverse events
Thirty‐nine mild to moderate adverse events were reported in 17 (85%) subjects. Of these, 25 (64%) were GI and included vomiting, diarrhea, or abdominal cramping, felt to be related to the study oil. In all cases the subjects were taking MCT oil when they reported their GI symptoms. In no cases was this severe enough to require study discontinuation, and symptoms resolved with a dose decrease. Their average oil consumption did not change whether on the placebo or MCT oil.
There were two unrelated SAEs. Both serious adverse events were hospitalizations unrelated to the study intervention. The first was for a total abdominal hysterectomy for dysfunctional uterine bleeding (no malignancy identified), and the second was for orthopedic surgery after a fall and shoulder fracture dislocation.
3.6. Concomitant medications
None of the participants changed AD medications or started an antipsychotic agent during the double‐blind study period. One subject had AChEI treatment discontinued due to bradycardia, in the open‐label phase.
3.7. Dropouts
There was one dropout (in the open‐label stage) due to admission to long‐term care resulting in loss of direct caregiver supervision of oil consumption, and inability to attend study visits.
4. DISCUSSION
This study shows that participants taking MCT supplementation for 11 months continuously did better cognitively than their peers who had their 11 months of MCT interrupted by 4 months of placebo (olive) oil. Given that most patients should experience a drop in their cognitive scores over the 15 months, the fact that those on longer continuous MCT did not, could be a sample size error for the outcomes other than MoCA, but it could also be that the difference in scores (showing stability) is valid. Although previous studies have been shorter and in less cognitively impaired groups, they have all shown a statistically significant cognitive change 13 , 15 , 44 or a trend toward one. 12 , 14 , 16 , 32 , 34 None of the previous studies had a cross‐over design, or were as long in duration (15 months) as the present study, or included moderate AD subjects. All suggested a positive cognitive signal/result, with most using either ADAS‐Cog 45 or MMSE. This study chose to use cognitive tests that are more accessible and applicable to clinicians in daily practice. Although the ADAS‐Cog is considered the gold standard for clinical dementia drug trials, it is not used in clinical settings as it is time‐consuming to administer, and changes of up to 5 points may be statistically significant, but are not usually clinically relevant. Unlike the published data 15 , 16 , 44 (including some smaller than this study), we did not show any effect of APOE ε4 status on the response to MCT oil.
Baseline MMSE seemed to be the most important factor, suggesting the provision of alternative brain energy as ketones may be more beneficial earlier in the course of the disease. Other authors have also suspected this. Hence, of the few clinical studies done, 50% have been in MCI.
Published studies suggest other roles of MCT as an anti‐inflammatory agent 45 , 46 or a neuroprotective agent. 47 (See Supplement SD). Previous data, and the present study, raise the question as to whether there should be MCT dose adjustments or additive dietary changes in those with more advanced disease. Given the intolerance reported with higher MCT oil supplementation, however, it is unlikely that this can be done purely by increasing the MCT intake. The only option would be a combination of a ketogenic diet and MCT. However, clinicians caring for patients with AD know the challenges involved with food intake, given the change in taste and food preferences as the disease progresses. 49 It is practically unlikely that a moderate AD subject is going to comply with a ketogenic diet. This challenge was reported by Taylor et al. 13 in their study in moderate AD. There was a 33% dropout in their diet/MCT combination study because of caregiver burden, suggesting a lack of long‐term feasibility. Their data also showed a marked individual variability in BHB response to their intervention. We also previously demonstrated 18 a marked individual response to BHB levels affected not only by amount of MCT consumed, but also by concomitant carbohydrate intake, and phenotypic percentage of visceral fat. These variables may be an added reason why, with similar interventions, there may be an individual response to cognitive outcomes, but not necessarily a group effect.
In a population with MCI, Krikorian et al. 32 used a very low carbohydrate diet alone (rather than the high fat diet used by Taylor et al., or the MCT supplement alone used in this study) to induce ketosis. They showed a trend to improved cognitive function in those on the diet. Yet, despite their intervention group reporting perceived better health and function, only one subject (of 12) was planning to continue the lifestyle change long term.
Another unknown is whether energy provided by the ketones allows for cognitive function at that time only, or whether it has any lasting, clinically meaningful neuroprotective effects. There is evidence that ketone intake can reverse amyloid accumulation already present in cell cultures. 47 The results of the present study raise the intriguing possibility of a prolonged neuroprotective effect, given that those on the continuous 11 months of MCT supplementation appeared to have a better cognitive outcome than those whose MCT intake was interrupted by 4 months of placebo oil.
5. STRENGTHS
This study is currently the longest duration (15 months) prospective MCT AD study, and the only study including subjects with moderate‐stage AD. It shows that regular intake (≥ 28 g of MCT daily) is feasible long term for both patients and caregivers. It also suggests that longer continuous intake of MCT oil provides greater cognitive benefit. Using a validated computerized tool (Cognigram®) was useful for frequent evaluations, and acceptable in AD subjects who found it less stressful than typical cognitive testing. We also showed that long‐term supplemental MCT at a maximum daily dose of 42 g was safe, with no adverse effects on serum lipid profiles, body fat composition, or glucose metabolism.
6. LIMITATIONS
Limitations of the study include: small sample size; wide spectrum of ages and AD disease state; lack of continuous BHB monitoring so preventing assessment of maximum BHB level achieved; difficulty reaching maximum (42 g) dose due to side effects or missing the lunchtime dose, highlighting the long‐term practicality of a liquid, three times daily formulation. Additionally, Cognigram® requires preservation of baseline coordination and, at least in our study, was not useful in those with MMSE score < 10.
7. CONCLUSION
Overall, there was marked stability of the cognitive function over the 15 months of the trial.
For efficacy, this study showed (1) an effect on attention and psychomotor domains of Cognigram® proportional to amount of MCT oil consumed; (2) better response in those with a higher baseline MMSE, independent of age; and (3) no apparent effect of APOE ε4 status on response.
In terms of safety, there was (1) no effect on body weight, composition, or serum lipids with 11 months of MCT oil and 4 months of olive oil consumption; and (2) MCT at three tablespoons daily (42 g) was difficult for some to tolerate (due to GI side effects)
Given the paucity of new therapeutic options for AD treatment, it is increasingly urgent to explore other therapeutic options. The expanding basic science and clinical research highlighting the physiological and clinical basis for efficacy of ketone supplementation, raises an intriguing and hopeful new therapeutic addition for AD dementia treatment.
CONFLICTS OF INTEREST
AGJ received indpendent, peer‐reviewed study funding for this study from the Glenrose Rehabilitation Hospital Foundation, Edmonton, Canada. Unrelated to this work, AGJ has received consulting fees from Amgen Canada, and Paladin Labs Inc, and presentation fees from Amgen Canada. TEB has no disclosures. DRM has received an unrelated grant from the Kidney Foundation of Canada. Angela G. Juby, Diana R. Mager, Toni E. Blackburn, all have no conflicts of interest with respect to this study
Supporting information
Supplementary material
ACKNOWLEDGMENTS
The authors convey grateful thanks to the AD participants and their caregivers, and all our funders. Ms. Joan Kravic provided administrative assistance and carried out the subject randomization and oil distribution. Ms. Debbie Smith provided assistance with data entry, appointment scheduling, oil distribution, and compliance assessment. Dr. Vera Mazurak's Lipid Research laboratory assessed the MCT and placebo oil composition. Use of the Euroqol EQ‐5D‐5L QOL questionnaire permission was kindly approved. This work was supported by funding from a peer‐reviewed research grant from the Glenrose Rehabilitation Hospital Foundation, Edmonton, Canada. Bulletproof Brain Octane provided the MCT oil. Cognigram® test costs were covered by Cogstate Ltd Australia. Dual Energy X‐Ray Absorptiometry body composition was provided by Medical Imaging Consultants (MIC) Edmonton, Alberta, Canada.
Juby AG, Blackburn TE, Mager DR. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer's disease: A randomized, double‐blind, placebo‐controlled, crossover study, with an open label extension. Alzheimer's Dement. 2022;8:e12259. 10.1002/trc2.12259
ClinicalTrials.gov Identifier NCT04396015.
REFERENCES
- 1. Alzheimer's society: 2019 Alzheimer's statistics. https://www.alzheimer%27s.net/resources/alzheimers‐statistics
- 2. Prince M, Bryce E, Albanese A, Wimo F, Ribeiro Wagner P, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alz Dement. 2013;9:63–75. [DOI] [PubMed] [Google Scholar]
- 3. Eleti S. Drugs in Alzheimer's disease dementia: an overview of current pharmacological management and future directions. Psychiatria Danubina. 2016;28(suppl 1):136–140. [PubMed] [Google Scholar]
- 4. Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drenick EJ, Alvarez LC, Tamasi GC, Brickman AS. Resistance to symptomatic insulin reactions after fasting. J Clin Invest. 1972;51:2757–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasselbach SG, Madsen PL, Hageman LP, et al. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–51. [DOI] [PubMed] [Google Scholar]
- 7. Corchesne loyer A, Croteau E, Castellano CA, St Pierre V, Hennebelle M, Cunnane SC. Inverse relationship between brain ketone metabolism in adults during short‐term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. JCBFM. 2017;37:2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nugent S, Croteau E, Pifferi F, et al. Brain and systemic glucose metabolism in the healthy elderly following fish oil supplementation. PLEFA. 2011;85:287–291. [DOI] [PubMed] [Google Scholar]
- 9. Croteau E, Castellano CA, Fortier M, et al. A cross‐sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer's disease. Exp Gerontol. 2018;107:18–26. [DOI] [PubMed] [Google Scholar]
- 10. Fernando WMADB, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN. The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action. Brit J Nutr. 2015;114:1–14. [DOI] [PubMed] [Google Scholar]
- 11. Lei E, Vacy K, Boon WC. Fatty acids and their therapeutic potential in neurological disorders. Neurochem Int. 2016;95:75–84. [DOI] [PubMed] [Google Scholar]
- 12. Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone monoester in a case of Alzheimer's disease. Alz Demen. 2015;11:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alz Dem: transl Res clin Interven. 2018;4:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebello CJ, Keller JN, liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 2015;3:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendersen ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Constantini LC. Study of the ketogenic agent AC‐1202 in mild to moderate Alzheimer's disease: a randomized, double‐blind, placebo‐controlled, multicenter trial. Nutr Metab. 2009: 6–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reger MA, Henderson ST, Hale C, et al. Effect of B‐hydroxybutyrate on cognition in memory‐ impaired adults. Neurobiol Aging. 2004;25:311–314. [DOI] [PubMed] [Google Scholar]
- 17. Caprio M, Infante M, Moriconi E, et al. Very‐low‐calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of endocrinology (SIE). J Endocrinol Inv. 2019;42:1365–1386. [DOI] [PubMed] [Google Scholar]
- 18. Juby AG, Brocks DR, Jay DA, Davis CMJ, Mager DR. Assessing the impact of factors that influence the ketogenic response to varying doses of Medium Chain Triglyceride (MCT) oil. J Prev Alz Dis. 2020. press. [DOI] [PubMed] [Google Scholar]
- 19. Hoyer S. Oxidative energy metabolism in Alzheimer's brains. Studies in early‐ onset and late onset cases. Mol Chem Neuropathol. 1992;16:207–224. [DOI] [PubMed] [Google Scholar]
- 20. Cunnane S, Nugent S, Roy M, et al. Brain fuel metabolism, aging and Alzheimer's disease. Nutr. 2011: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosconi L, Brys M, Glodzik‐Sobanska L, De Santi S, Rusinek H, De Leon MJ. Early detection of Alzheimer's disease using neuroimaging. Exp Gerontol. 2007;42:129–138. [DOI] [PubMed] [Google Scholar]
- 22. Laybaert L, De Bock M, Van Moorhem M, Decrock E, De Vuyst E. Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res. 2007;85:3213–3220. [DOI] [PubMed] [Google Scholar]
- 23. Cholerton B, Baker LD, Craft S. Insulin, cognition and dementia. Eur J Pharmacol. 2013;719:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. An Y, Varma VR, Varma S, et al. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimers Dement. 2018;14:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramirez M, Amate L, Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Develop. 2001;65:S95‐S101. Suppl. [DOI] [PubMed] [Google Scholar]
- 26. Marten B, Pfeuffer M, Schrezenmeir J. Review: medium‐chain triglycerides. Int Dairy J. 2006;16:1374–1382. [Google Scholar]
- 27. Bach AC, Ingenbleek Y, Frey A. The usefulness of dietary medium chain triglycerides in body weight control: fact or fancy?. J Lip Res. 1996;37(4):708–726. [PubMed] [Google Scholar]
- 28. Cater NB, Heller HJ, Denke MA. Comparison of the effects of medium‐chain triacylglycerols, palm oil, and high ooleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr. 1997;65(1):41–45. [DOI] [PubMed] [Google Scholar]
- 29. Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal human. Diabetes. 1994;43:1311–1317. [DOI] [PubMed] [Google Scholar]
- 30. Vining EP, Freeman JM, Ballaban‐Gil K, Camfield CS, Camfield PR, Holmes GL. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55:1433–1437. [DOI] [PubMed] [Google Scholar]
- 31. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketpgenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2000;70:309–319. [DOI] [PubMed] [Google Scholar]
- 32. Krikorian R, Shidler MD, Dangelo K, Couch SC, Bemoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;425:E19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lange KW, Lange KM, Makulska‐Gertruda E, et al. Ketogenic diets and Alzheimer's Disease. Food Sci Hum Well. 2017;6:1–9. [Google Scholar]
- 34. Fortier M, Castellano CA, Croteau E, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alz Demen. 2019;15:625–634. [DOI] [PubMed] [Google Scholar]
- 35. Cunnane SC, Courchesne‐Loyer A, Vandenberghe C, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci. 2016;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 37. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- 38. Folstein MF, Folstein SE. Mini Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiat. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 39. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;52:695–699. [DOI] [PubMed] [Google Scholar]
- 40. Mielke MM, Machulda MM, Hagen CE, et al. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alz Dem. 2015;11:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brooks RG, Rabin R, De Charro F. The measurement and valuation of health status using EQ‐5D: a European perspective: evidence from the EuroQol BIOMED Research Programme. Dordrecht: Kluwer Academic; 2003. [Google Scholar]
- 42. Government of Canada, Clinical trials for Natural health products, Appendix 8:Adverse Reaction report form for Clinical trials. https://www.canada.ca/en/health‐canada/services/drugs‐health‐products/natural‐non‐prescription/legislation‐guidelines/guidance‐documents/clinical‐trials.html
- 43. Doody RS, Massman P, Dunn K. A method for estimating progression rates in Alzheimer's disease. Arch Neurol. 2001;58:449–454. [DOI] [PubMed] [Google Scholar]
- 44. Hendersen ST, Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer's disease: a randomized, double‐blind, placebo‐controlled study. BMC Med Genetics. 2011;12:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psych. 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 46. Pinto A, Bonucci A, magi E, Corsi M, Busraino R. Anti‐oxidant and anti‐ inflammatory activity of ketogenic diet: new perspective for neuroprotection in Alzheimer's disease. Antioxidants. 2018;7:63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res. 2017;95(4):943–972. [DOI] [PubMed] [Google Scholar]
- 48. Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Veech CRL. D‐Beta‐ hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci USA. 2000;97:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's Disease. Neurobiol Aging. 1990;11:465–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


