Abstract
Background:
Scarring has significant aesthetic and functional consequences for patients. A need exists for anti-scarring therapeutics. Light emitting diode-red light (LED-RL) has been shown to modulate skin fibrosis.
Objective:
Evaluate the safety and efficacy of LED-RL to reduce post-operative scarring.
Methods:
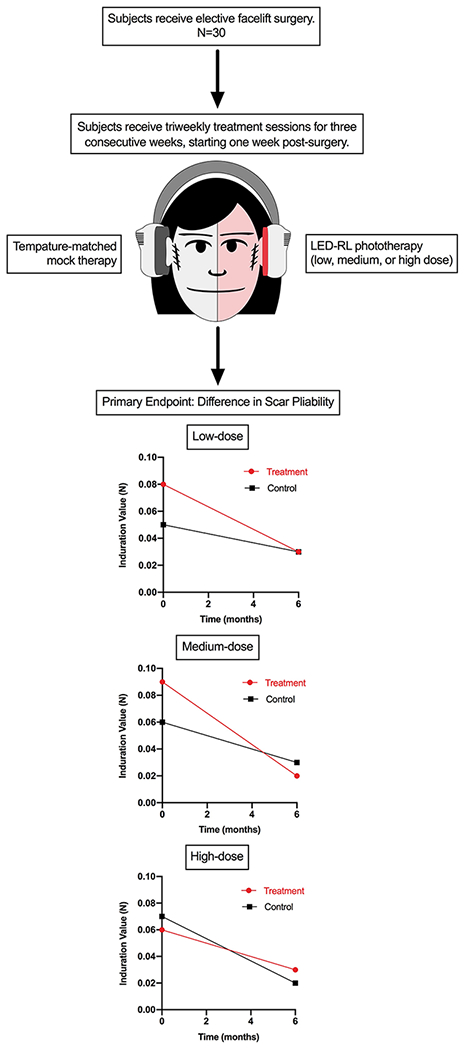
CURES (Cutaneous Understanding of Red-light Efficacy on Scarring) was a randomized, mock-controlled, single-blind, dose-ranging, split-face phase II clinical trial. Starting one week post-surgery, patients received LED-RL irradiation and temperature-controlled mock therapy to incision sites at fluences of 160 J/cm2, 320 J/cm2, or 480 J/cm2, triweekly for three weeks. Efficacy was assessed at 1, 3, and 6-12 months. The primary endpoint was difference in scar pliability between LED-RL-treated and control sites. Secondary outcomes included Patient and Observer Scar Assessment Scale, collagen and water concentration, and adverse events.
Results:
There were no significant differences in scar pliability between treated and control scars. At certain fluences, treated scars showed greater improvements in observer rating and scar pliability, reflected by greater reductions in induration, from baseline to 6 months compared to control scars. Treatment-site adverse events included blistering (n=2) and swelling (n=1), which were mild and resolved without sequelae.
Conclusions:
LED-RL phototherapy is safe in the early postoperative period and may reduce scarring.
Trial registration:
ClinicalTrials.gov, NCT03795116. Registered 20 December 2018, https://clinicaltrials.gov/ct2/show/NCT03795116
Keywords: low level light therapy, phototherapy, scarring, wound healing
Graphical Abstract
Scarring has significant functional and aesthetic consequences for patients, yet there are few effective and durable anti-scarring therapeutics available. Light emitting diode-red light (LED-RL) has been shown to modulate skin fibrosis, an abnormal wound healing response following tissue damage. This phase II trial demonstrates that LED-RL phototherapy can be safely used in the early postoperative period on facial skin and may reduce post-surgical scarring, as shown by improved scar pliability and cosmesis of treated sites at certain fluences.
INTRODUCTION
Scar tissue formation is a natural consequence of wound healing after injury to the skin, and outcomes can range from faint scarring to aberrant scarring such as hypertrophic scars and keloids.1, 2 Scar prevention is a key consideration in postoperative wound management, as scarring has significant aesthetic and functional consequences for patients.3, 4 The understanding of the molecular biology of wound healing is still evolving, and many strategies exist to prevent and treat scarring.5–8 Skin fibrosis is an abnormal wound healing response following tissue damage (e.g., burns, surgery, trauma), characterized by excessive fibroblast proliferation and collagen deposition in the dermis, which may manifest clinically as scar hypertrophy.9–12 Skin fibrosis is a significant global health problem with an estimated incidence of greater than 100 million persons affected per year in the developed world.13, 14 Cutaneous scars have a profoundly negative impact on patients’ quality of life due to associated pain and pruritus, functional impairment, cosmetic disfigurement, and psychosocial distress.13, 15, 16
There is great research interest and consumer demand for therapeutic modalities that prevent, reduce, or remove scars, as evidenced by an estimated $12 billion annual market for scar treatment in the United States.17 Despite the substantial socioeconomic burden associated with skin fibrosis, there are few effective and durable anti-scarring therapeutics available, making scar treatment a major unmet medical need.18–20 Furthermore, current scar management strategies may be invasive, cause undesirable side effects, or lack high-level evidence to support their use.18 Therefore, it is important to research and develop novel approaches to treat and prevent skin fibrosis.
Visible light (400-700 nm) is ubiquitous in the environment and comprises 44% of total solar energy, yet its cutaneous biologic effects have not been fully elucidated.21, 22 Visible light therapy delivered by light emitting diode (LED) devices is a therapeutic modality of increasing clinical importance in dermatology, as different wavelengths can alter skin physiology and provide benefits such as in wound healing and skin rejuvenation.23–25 Due to the significant advances in LED technology in recent years, LED phototherapy has become a valuable and effective treatment for a wide variety of medical and aesthetic conditions.26 In 2018, members of the American Society for Dermatologic Surgery performed 3.49 million procedures using lasers, lights, and energy-based devices.27 Furthermore, LED devices are commercially available and have U.S. Food and Drug Administration (FDA) clearance for various dermatologic conditions including acne and photoaging.25, 28 Red light (630-700 nm) has the deepest tissue penetration depth of the visible light colors, reaching the entirety of the dermis where skin fibrosis occurs.24, 29, 30 Recently published clinical observations indicate that red light in combination with other modalities, such as photosensitizers for photodynamic therapy, can decrease skin fibrosis.31–33
According to our in vitro data, light emitting diode-red light (LED-RL) at high fluences (defined as equal to or greater than 160 J/cm2) can exert anti-fibrotic cutaneous effects by decreasing the proliferation, collagen production, and migration speed of human skin fibroblasts.34–37 Prior to our studies on the anti-fibrotic properties of LED-RL, limited data existed regarding red light photobiomodulation of dermal fibroblasts. In two phase I, dose escalation, randomized controlled trials (Safety Trial Assessing Red-light on Skin [STARS 1 and STARS 2], n=115), we evaluated the safety and tolerability of LED-RL administered at fluences up to 640 J/cm2 on normal skin.38 Adverse events (AEs) included treatment-site erythema, hyperpigmentation, and blistering, all of which were mild and resolved without permanent sequelae.38 We concluded that LED-RL is safe up to 480 J/cm2 and may exert differential cutaneous effects depending on race and ethnicity, with darker skin being more photosensitive.38
This report describes findings from CURES (Cutaneous Understanding of Red-light Efficacy on Scarring), a phase II randomized controlled trial designed to evaluate the safety and efficacy of LED-RL treatment on fresh post-surgical scars (National Clinical Trials identifier NCT03795116, registered December 20, 2018).
METHODS
Study design
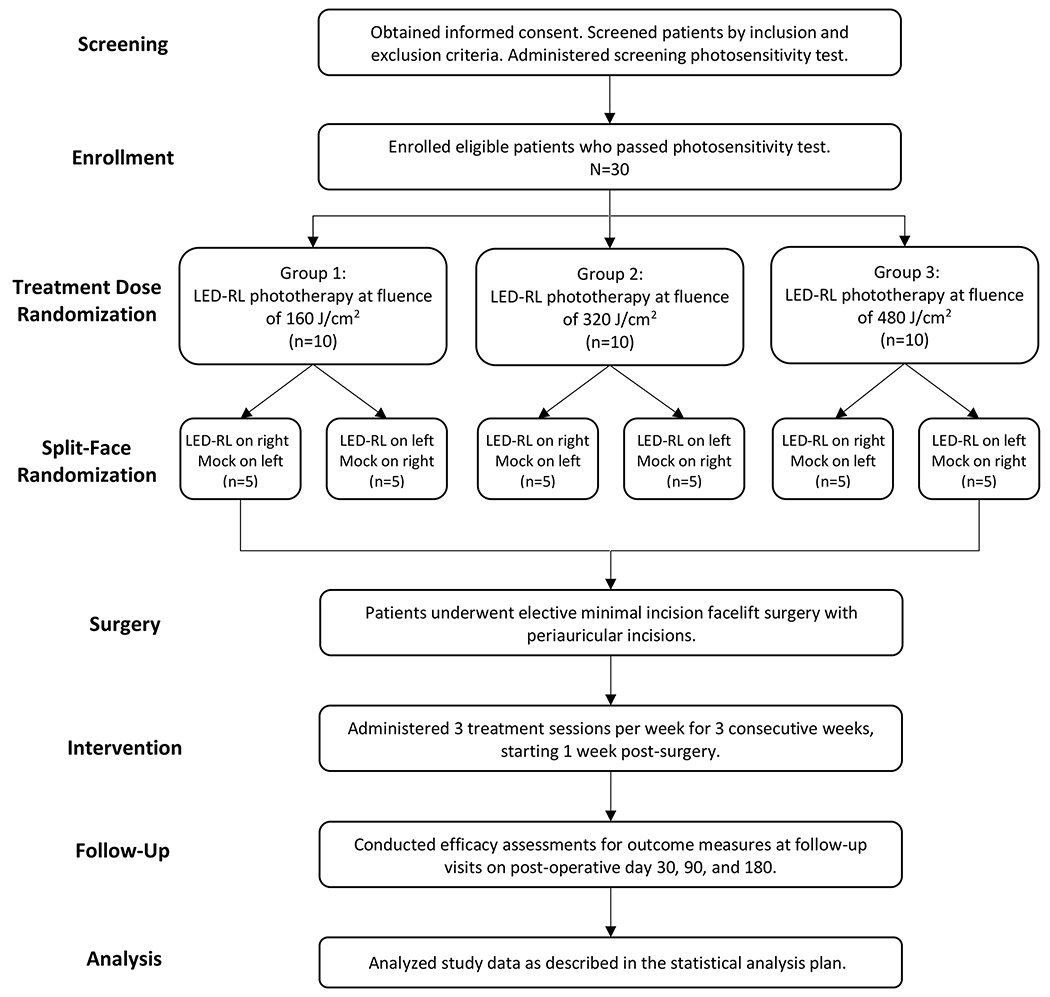
This randomized, temperature-matched mock therapy-controlled, single-blind, dose-ranging, split-face phase II clinical trial was conducted at SUNY Downstate between April 18, 2019 and October 26, 2020. The study protocol was approved by an institutional review board and previously published.39 The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent. Refer to Figure 1 for a schematic of the study design.
Figure 1.
Schematic of study design for the CURES trial.
Patients
Eligible patients were adults (age ≥18 years) who planned to undergo elective minimal incision facelift surgery with the same surgeon. Patients were screened according to the inclusion and exclusion criteria (Table 1). Prior to enrollment, a screening photosensitivity test was conducted; the patient was exposed to LED-RL for 20 minutes on the non-dominant upper forearm, and evaluated 24 hours later for evidence of photosensitivity (e.g., persistent erythema, rash, pain).40
Table 1.
Eligibility criteria for the CURES trial.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Provision of written informed consent for all study procedures • Stated willingness to comply with all study procedures and availability for the duration of the study • Suitable candidate for elective mini-facelift surgery • Pass a screening photosensitivity test |
• Current use of any photosensitizing medications • Light-sensitive conditions • Diabetes mellitus • Systemic lupus erythematosus • Current tobacco use • History of bleeding or coagulation disorder • Lax skin associated with genetic disorders • Open wounds on the face or neck • Fibrotic skin disease, pre-existing scar(s), or other skin conditions affecting the periauricular skin • History of surgery or procedure involving or affecting the periauricular skin within the past 6 months (e.g., prior facelift, fillers, laser therapy) • Tattoos that cover the proposed treatment sites on the periauricular skin • Any other medical condition(s) that could be compromised by exposure to the proposed treatment |
Treatment
Starting one week after surgery (postoperative days 7 to 10), patients received LED-RL irradiation and mock therapy to the periauricular skin (i.e., sites of the surgical incisions). The treatment side (right face versus left face) was randomized, with the untreated side receiving temperature-matched mock therapy. The fluence range was based on published reports of LED-RL maximum recommended starting dose and the maximum tolerated dose in our phase I studies.28, 31, 32 Treatment sessions were administered in-office triweekly for three weeks. Patients were randomly assigned to three treatment groups via block randomization:
Group 1 (Low dose): LED-RL 160 J/cm2 and mock phototherapy – 30 minutes
Group 2 (Medium dose): LED-RL 320 J/cm2 and mock phototherapy – 60 minutes
Group 3 (High dose): LED-RL 480 J/cm2 and mock phototherapy – 90 minutes
The treatment devices were positioned in close contact with the skin (within 10 mm), held in place via a custom-designed headset (Figure 2). Patients were blinded to the LED-RL treated side, as the treatment areas were outside of the range of view. For the entire duration of the study, patients were asked to avoid scar treatments (e.g., topical medications, intralesional corticosteroids, laser therapy), excluding topical agents recommended for routine postoperative wound care.
Figure 2.

Custom-designed headset containing an LED-RL treatment device and mock phototherapy device on opposing sides.
Treatment devices
The LED-RL source was the Omnilux handheld LED system (GlobalMed Technologies, Glen Ellen, CA, USA), FDA-cleared for the treatment of periorbital rhytides.40, 41 The LED-RL treatment device emitted visible red light (633 nm + 6 nm) at a power density of 360.2 W/m2 at room temperature and a distance of 10 mm from the skin surface.40, 42 The mock treatment device simulated the LED-RL treatment device (i.e., had the same physical components and thermal output) but did not emit red light.38 The use of mock phototherapy controlled for environmental factors that may affect wound healing, such as ambient light and temperature.35
Assessments and Outcomes
Efficacy
Assessments were conducted at baseline (i.e., at the first treatment session) and at follow-up visits at approximately 1 month, 3 months, and 6-12 months post-surgery. While patients were originally scheduled for a final 6-month follow-up visit, due to the COVID-19 pandemic, many patients were required to delay their final visit. The primary endpoint was the difference in quantitative scar pliability between the LED-RL-treated and control scars. Skin induration, which reflects scar pliability, was measured by an indentation instrument, the SkinFibroMeter (Delfin Technologies, Kuopio, Finland) at the midpoint of width and length of each scar.43–45
Secondary outcome measures included the Patient and Observer Scar Assessment Scale (POSAS) and quantitative measurements of collagen and moisture. The POSAS was performed by a blinded reviewer in conjunction with the patient. The two subscales of the POSAS each consist of six items rated from 1 to 10, where 1 is “normal skin” and 10 is the “worst imaginable scar.” The observer evaluated scar vascularity, pigmentation, thickness, relief, pliability, and surface area while the patient assessed pain, itching, color, stiffness, thickness, and irregularity. The Dermo spectroscopy probe (Connected Physics, Orsay, France) was used to measure collagen and water concentration in the dermis. Hydration of keratinocytes has been associated with reductions in collagen secretion and restoration of the barrier function of skin, helping reduce scar formation.46, 47
Safety
Treatment sessions were monitored for the occurrence of safety concerns and AEs, as reported by the patient or observed by the research team. Patients recorded AEs during the three-week treatment period in a home diary. Common expected post-treatment effects, including warmth, erythema, and edema, were not considered AEs unless they were prolonged (i.e., lasting more than 24 hours).38
Statistical analysis
This clinical trial was designed to be a preliminary study to obtain estimates of feasibility and outcome variability. We estimated that a difference of 15% in scar pliability would be clinically meaningful, based on the minimum decrease in fibroblast number in response to LED-RL irradiation in vitro.34 A sample size of 30 patients (with the split-face, intra-individual comparison design) allowed for an estimate of the variance in scar pliability change in this population.
SAS version 9.4 statistical package (SAS Institute, Cary, NC, USA) was used for intention-to-treat analysis and per-protocol analysis. Each primary outcome was used as a dependent variable (DV) in mixed linear models. Fixed factors in each model were treatment group, whether treated, side of face (left versus right), and time (three follow-up assessments). Baseline score was introduced as a scored covariate. Tests of interaction among fixed factors were conducted, and the utility of polynomial terms in the baseline DV investigated. DV scores were power-transformed to remove skew of model residuals.
RESULTS
Patient demographics
A total of 30 patients were enrolled and received at least one treatment session. All patients were female, mostly non-Hispanic Caucasian (63.3%), and the mean age was 54.1 years (Table 2). Most patients (n=20, [66.7%]) completed the study per-protocol (i.e., received all 9 treatment sessions). The most common reasons for study discontinuation were loss to follow-up (n=2, [6.7%]) and personal reasons (n=4, [13.3%]).
Table 2.
Baseline demographics of patients. Age is presented as mean (SD). Categorical variables are presented as n (%).
| Characteristic | Total (n = 30) | Group 1 LED-RL 160 J/cm2 (n = 10) | Group 2 LED-RL 320 J/cm2 (n = 10) | Group 3 LED-RL 480 J/cm2 (n = 10) |
|---|---|---|---|---|
| Age, years | 54.1 (7.5) | 53.7 (8.2) | 52.4 (7.2) | 56.3 (7.4) |
| Sex | ||||
| Female | 30 (100) | 30 (100) | 30 (100) | 30 (100) |
| Male | -- | -- | -- | -- |
| Race and ethnicity | ||||
| White non-Hispanic | 19 (63.3) | 5 (50) | 6 (60) | 8 (80) |
| White Hispanic | 6 (20) | 2 (20) | 3 (30) | 1 (10) |
| Black or African American | 4 (13.3) | 2 (20) | 1 (0) | 1 (10) |
| Two or more races | 1 (3.3) | 1 (10) | -- | -- |
Safety and tolerability
During the entire study period, no serious AEs were reported. All patients experienced warmth during treatment sessions and reported bilateral post-treatment erythema, which resolved within 24 hours. Treatment-site AEs occurred in 3 patients (10%): 2 incidences of localized bulla formation on the LED-RL-treated side and 1 incidence of localized facial swelling. There were no discontinuations due to AEs.
Efficacy
Skin Induration (SkinFibroMeter)
No significant differences were detected between treatment and control in the three groups at 6 months. However, the scars treated with a medium LED-RL dose (group 2), had lower induration values at 6 months compared to the control, reflecting greater scar pliability on the treatment side (0.02 vs 0.03). In addition, the scars treated with low and medium LED-RL doses (group 1 and 2) showed greater improvements in scar pliability from baseline to 6 months compared to the control scars. The low dose-treated scars (group 1) showed a 62.5% decrease in induration from baseline to 6 months compared to a 40.0% decrease for the control scars (Figure 3a). The medium dose-treated scars (group 2) showed a 77.8% decrease in induration from baseline to 6 months compared to 50.0% decrease for the control scars (Figure 3b). Detailed primary outcome results are displayed in Table 3.
Figure 3.
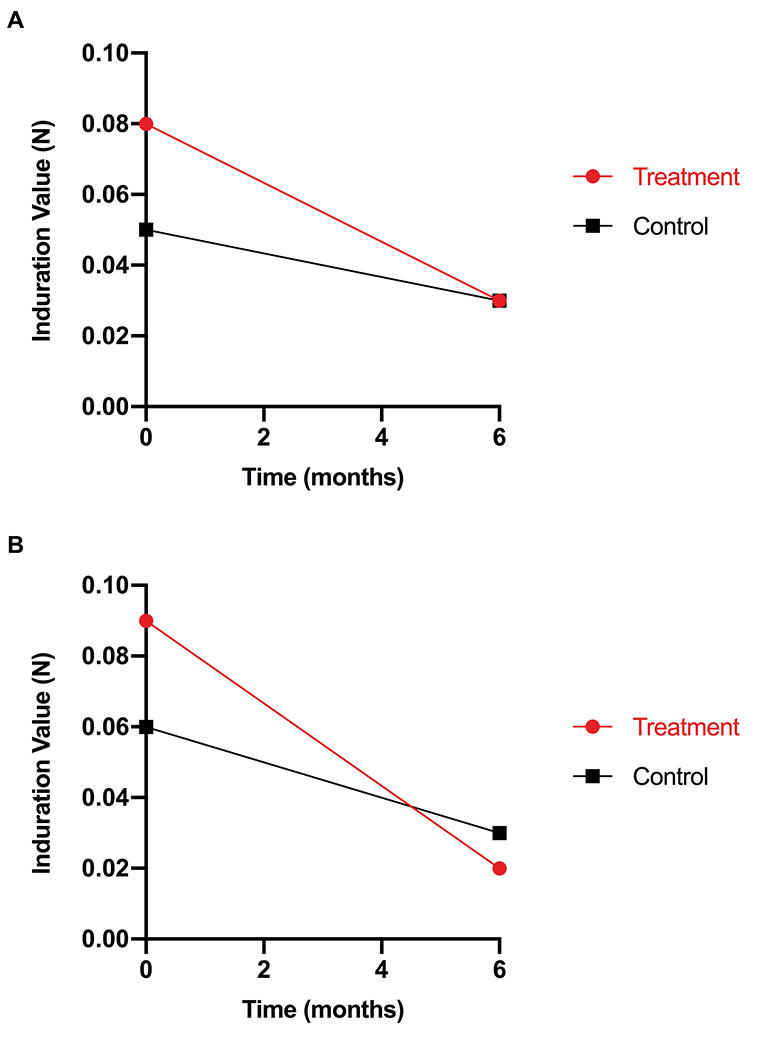
The low and medium dose-treated scars in (Groups 1 and 2) showed greater improvements in scar pliability compared to the control scars. (A) In group 1 (low dose), the treated scars showed a 62.5% decrease in induration from baseline to 6 months compared to a 40.0% decrease for the control scars. (B) In group 2 (medium dose), the treated scars showed a 77.8% decrease in induration from baseline to 6 months compared to a 50.0% decrease for the control scars.
Table 3.
Results of primary endpoint
| Treatment Group | Treatment or Control Side | Time Point | Median | Percent Change ([Baseline – 6 month]/Baseline) | |
|---|---|---|---|---|---|
| SkinFibroMeter (N) | Group 1 | Treatment | Baseline | 0.08 | ↓62.5 |
| 6 month | 0.03 | ||||
| Control | Baseline | 0.05 | ↓40.0 | ||
| 6 month | 0.03 | ||||
| Group 2 | Treatment | Baseline | 0.09 | ↓77.8 | |
| 6 month | 0.02 | ||||
| Control | Baseline | 0.06 | ↓50.0 | ||
| 6 month | 0.03 | ||||
| Group 3 | Treatment | Baseline | 0.06 | ↓50.0 | |
| 6 month | 0.03 | ||||
| Control | Baseline | 0.07 | ↓71.4 | ||
| 6 month | 0.02 |
POSAS - Patient Rating
At 6 months, the high dose-treated scars (group 3) had better (lower) total PSAS scores compared to the control scars (13.0 vs. 17.0), while the low and medium dose-treated scars (groups 1 and 2) had worse patient ratings compared to the control scars (12 vs. 10.5 and 23 vs. 18, respectively). Both the treated and control scars in all three groups showed improvement in patient ratings from baseline to 6 months. Detailed secondary outcome results are displayed in Table 4.
Table 4.
Results of secondary endpoints
| Treatment Group | Treatment or Control | Time Point | Median | Percent Change ([Baseline – 6 month]/Baseline) | |
|---|---|---|---|---|---|
| POSAS- Patient Rating | Group 1 | Treatment | Baseline | 36.0 | ↓66.7 |
| 6 month | 12.0 | ||||
| Control | Baseline | 37.0 | ↓71.6 | ||
| 6 month | 10.5 | ||||
| Group 2 | Treatment | Baseline | 32.0 | ↓28.1 | |
| 6 month | 23.0 | ||||
| Control | Baseline | 30.5 | ↓41.0 | ||
| 6 month | 18.0 | ||||
| Group 3 | Treatment | Baseline | 26.5 | ↓50.9 | |
| 6 month | 13.0 | ||||
| Control | Baseline | 28.5 | ↓40.4 | ||
| 6 month | 17.0 | ||||
| POSAS- Observer Rating | Group 1 | Treatment | Baseline | 16.5 | ↓45.5 |
| 6 month | 9.0 | ||||
| Control | Baseline | 16.5 | ↓24.2 | ||
| 6 month | 12.5 | ||||
| Group 2 | Treatment | Baseline | 19.0 | ↓57.9 | |
| 6 month | 8.0 | ||||
| Control | Baseline | 14.0 | 0 | ||
| 6 month | 14.0 | ||||
| Group 3 | Treatment | Baseline | 18.0 | ↓27.8 | |
| 6 month | 13.0 | ||||
| Control | Baseline | 16.5 | ↓27.3 | ||
| 6 month | 12.0 | ||||
| Collagen | Group 1 | Treatment | Baseline | 48.7 | ↑17.0 |
| 6 month | 57.0 | ||||
| Control | Baseline | 52.0 | ↑8.7 | ||
| 6 month | 56.5 | ||||
| Group 2 | Treatment | Baseline | 51.0 | ↑17.6 | |
| 6 month | 60.0 | ||||
| Control | Baseline | 50.5 | ↑20.8 | ||
| 6 month | 61.0 | ||||
| Group 3 | Treatment | Baseline | 47.7 | ↑18.9 | |
| 6 month | 56.7 | ||||
| Control | Baseline | 52.5 | ↑13.0 | ||
| 6 month | 59.3 | ||||
| Moisture (%) | Group 1 | Treatment | Baseline | 63.0 | ↓0.8 |
| 6 month | 62.5 | ||||
| Control | Baseline | 65.3 | ↓6.3 | ||
| 6 month | 61.2 | ||||
| Group 2 | Treatment | Baseline | 60.7 | ↑4.9 | |
| 6 month | 63.7 | ||||
| Control | Baseline | 62.7 | ↑0.5 | ||
| 6 month | 63.0 | ||||
| Group 3 | Treatment | Baseline | 63.2 | ↓1.9 | |
| 6 month | 62.0 | ||||
| Control | Baseline | 66.2 | ↓3.3 | ||
| 6 month | 64.0 |
POSAS - Observer Rating
At 6 months, the low and medium dose-treated scars (groups 1 and 2) had more favorable (lower) total OSAS scores on the treatment side compared to the control side (9.0 vs 12.5 and 8.0 vs 14.0, respectively), while the high dose-treated scars (group 3) had a slightly worse observer rating on the treatment side compared to the control side (13.0 vs 12.0). The low and medium dose-treated scars (groups 1 and 2) showed greater improvements in observer rating from baseline to 6 months compared to the control scars. The low dose-treated scars (group 1) showed a 45.5% improvement from baseline to 6 months compared to 24.2% for the control scars (Figure 4a). The medium dose-treated scars (group 2) showed a 57.9% improvement from baseline to 6 months compared to no improvement for the control scars (Figure 4b). Refer to figure 5 for comparative clinical photos.
Figure 4.
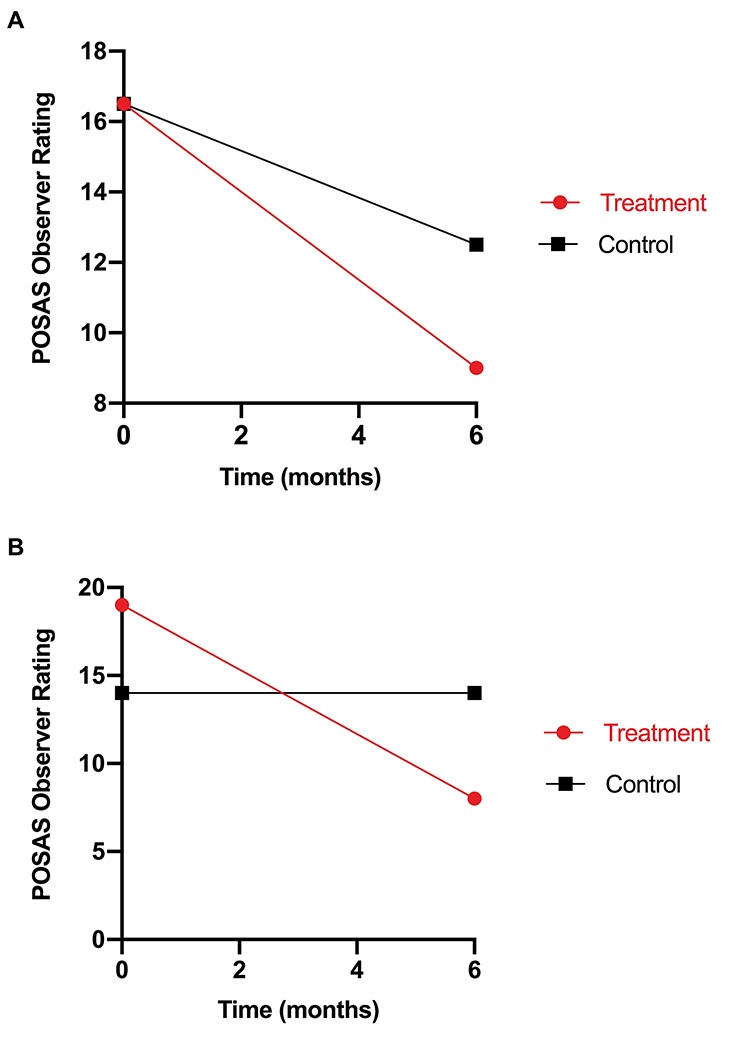
The low and medium dose-treated scars in (groups 1 and 2) showed greater improvements in observer rating from baseline to 6 months compared to the control scars. (A) In Group 1 (low dose), the treated scars showed a 45.5% improvement from baseline to 6 months compared to 24.2% for the control scar. (B) In Group 2 (medium dose), the treated scars showed a 57.9% improvement from baseline to 6 months compared to no improvement for the control scars.
Figure 5.
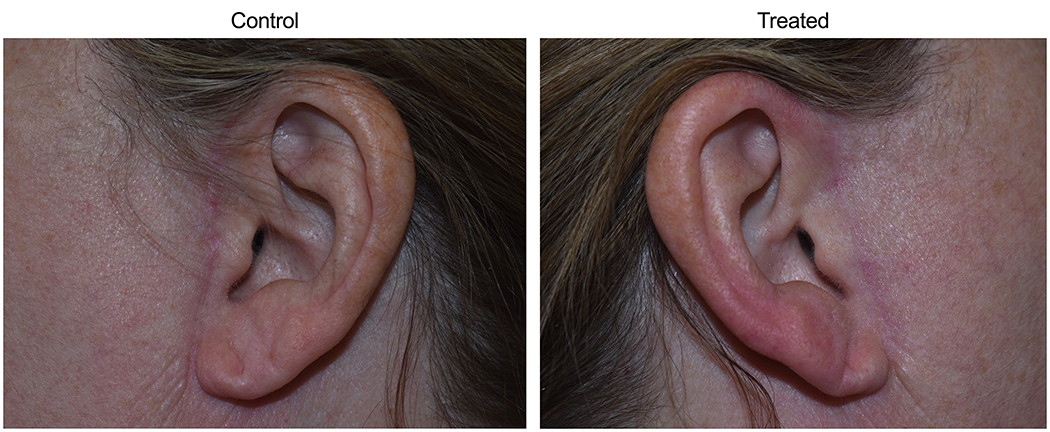
Control scar compared to low dose LED-RL-treated scar (group 1) at 6-month visit.
Collagen
Both the treatment and control sides of all three groups showed increases in collagen from baseline to 6 months, as expected in wound healing. The medium and high dose-treated scars (groups 2 and 3) had lower collagen compared to the control scars at 6 months (60.0 vs 61.0 and 56.7 and 59.3, respectively), a favorable outcome for the treatment side as excess collagen in scar tissue is associated with worse healing.
Moisture
At 6 months, there were negligible differences in water concentration between treatment and control in all three groups.
Patient Satisfaction
70.8% of patients reported they are likely or very likely to recommend the LED-RL treatment to a friend. 62.5% of patients reported they are likely or very likely to use this treatment again after a procedure that may produce a scar.
DISCUSSION
To our knowledge, no clinical trials have evaluated the safety and efficacy of red light for the treatment or prevention of cutaneous scarring. As in vitro data show that LED-RL can attenuate profibrotic cellular processes that contribute to skin fibrosis, LED-RL is a promising strategy to minimize scar formation after surgery.34, 35 In this study, LED-RL phototherapy was initiated within one week post-surgery, coinciding with the early proliferation phase of wound healing, to help answer important questions about the impact of intervention time on final scar outcomes.48–50 While no statistically significant difference in primary outcome between treatment and control were detected, LED-RL therapy demonstrated improvements in multiple endpoints. The low and medium dose-treated scars (groups 1 and 2) showed greater improvements in scar pliability compared to the control as demonstrated by the larger reductions in skin induration over the study period. In addition, lower collagen levels (group 2 and 3) were measured on the treated side at 6 months. Greater improvements in observer ratings on the low and medium dose-treated scars (groups 1 and 2) compared to the control were also noted. Interestingly, the 6-month observer and patient ratings favored opposite sides for each of the three treatment groups. This discordance highlights that scar outcomes are subject to interpretation and what one person perceives to be improved, may not be the same for others.
A dose-ranging study design was implemented as the safety of LED-RL phototherapy in a facelift scar model may differ from the safety in normal skin. For example, LED-RL fluences determined to be safe in normal forearm skin (the treatment site in STARS 1 and STARS 2) may have different effects on the face, as physiological properties of skin vary depending on anatomic location.51, 52 Thus, the MTD established in our Phase I studies served as the upper limit of treatment dose in this study. We now demonstrated that LED-RL therapy can be safely used on fresh surgical wounds on facial skin. No serious AEs were reported, and the few non-serious AEs reported were temporary and resolved without sequelae. Patients expressed high satisfaction with the treatment as the majority reported that they are likely or very likely to use the treatment again and recommend the treatment to a friend.
This study’s methodology offered several advantages compared to other clinical trials that evaluate scar management strategies. The split-face study design allowed each patient to serve as their own control, such that comparisons of clinical efficacy between treated and control scars are within-patient (i.e., intra-individual). Therefore, any measured changes in scar characteristics can be attributed to the treatment, eliminating the confounding factor of inter-individual differences in wound healing. It is important to note that in the prospective evaluation of scar reduction therapy, it is assumed that if left untreated, the bilateral facelift incisions would heal with identical scars. Furthermore, the treated side of the face was randomized to account for possible differences in skin quality (e.g., asymmetry of sun exposure in automobile drivers).53 Lastly, this study employed both objective and subjective outcome measures. The use of quantitative measurements allowed detection of mechanical skin properties changes not easily appreciated with subjective assessment of skin appearance.
This study had several limitations. There was a bias in age toward middle-aged and elderly individuals, as these are the typical facelift patients.54, 55 Increased age is associated with reduced collagen turnover due to a decrease in fibroblast collagen synthesis, which may affect the penetration and cutaneous effects of LED-RL.56, 57 Furthermore, since cell turnover is a major contributor to the development of scar tissue in a healing wound, elderly individuals tend to have better outcomes for scar cosmesis and are less susceptible to pathologic scarring.58–61 The majority of observer ratings reflected very subtle scarring, with over 70% of the scar characteristics (vascularity, pigmentation, etc.) rated between 1-3 out of 10. Because the majority of patients had minimal scarring, it was difficult to detect differences between the treated and control scars. A greater number of treatment sessions over additional weeks to months may have also correlated with greater differences between treated and control scars. Additionally, the thermal output of the mock device may have improved the appearance of the control scars. Lastly, while patients were blinded to the treatment side, they commonly reported greater sensation of warmth during treatment and greater degree of post-treatment erythema on the LED-RL-treated side.
Given the complexity of wound healing and the many potential confounding variables, it is challenging to devise and conduct robust clinical trials evaluating scar therapies. Studies are often limited by small sample sizes, subjective assessment tools, and well-healing scars that make it difficult to detect differences between treated and control scars. For instance, several systematic reviews have concluded that silicone gel significantly improves scar outcomes. However, in a randomized double-blind placebo-controlled clinical trial including 12 patients, no statistically significant difference between scars treated with silicone gel and scars treated with a placebo after direct brow lift surgery were detected.62 The researchers believed that because brow lift scars tend to heal well and not form hypertrophic scars, it was difficult to detect differences between the treated and control scars. Better scar outcomes, albeit non-significantly, were observed on the treated side and perhaps the silicone gel would have had greater effects on hypertrophy-prone scars. Our study faced similar challenges as the facelift scars were subtle and in an anatomic location not prone to pathologic scarring. Thus, the improvements observed on the LED-RL treated side, while not statistically significant compared to the control, should not be overlooked.
With LED-RL therapy showing great promise, the modality warrants further evaluation in a high-powered study with a larger sample size. Because all doses displayed excellent tolerability and conferred improvements in multiple endpoints, all three doses merit further assessment in a phase III study. To mimic real-world practice, home-use LED-RL devices should be evaluated. Lastly, the effects of LED-RL therapy on hypertrophy-prone scars should also be investigated.
CONCLUSION
There is a large unmet need for innovative therapeutic strategies to prevent cutaneous scarring after surgery. Despite the substantial healthcare burden of skin fibrosis, there is no “gold standard” or universally effective scar therapy, and current treatment options have limited clinical efficacy and durability.8, 18, 63 This study demonstrates that LED-RL phototherapy can be safely used in the early postoperative period on facial skin and may reduce post-surgical scarring, as shown by improved scar cosmesis of the treated sites. Future studies may extend beyond scar prevention and investigate the use of LED-RL to treat existing scars.
Funding
The study was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. K23GM117309.
Abbreviations:
- LED
light emitting diode
- LED-RL
light emitting diode-red light
- STARS
Safety Trial Assessing Red-light on Skin
- CURES
Cutaneous Understanding of Red-light Efficacy on Scarring
- AE
adverse event
- POSAS
Patient and Observer Scar Assessment Scale
- DV
dependent variable
Footnotes
Conflict of Interest
Dr. Jared Jagdeo and Dr. Daniel Siegel are on the Scientific Advisory Board for Global Med Tech. Dr. Jared Jagdeo also serves as a consultant for Global Med Tech. The other authors have no conflict of interests to declare.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Jagdeo J, Shumaker PR JAMA dermatology. 2017, 153, 364–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Profyris C, Tziotzios C, Do Vale I Journal of the American Academy of Dermatology. 2012, 66, 1–10. [DOI] [PubMed] [Google Scholar]
- [3].Son D, Harijan A Journal of Korean medical science. 2014, 29, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Commander SJ, Chamata E, Cox J, Dickey RM, Lee EI in Dermatology for Plastic Surgery: Update on Postsurgical Scar Management, Vol. 30 (Ed.Êds.: Editor), Thieme Medical Publishers, City, 2016, pp.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kerwin LY, El Tal AK, Stiff MA, Fakhouri TM International journal of dermatology. 2014, 53, 922–936. [DOI] [PubMed] [Google Scholar]
- [6].Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT Advances in wound care. 2018, 7, 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barnes LA, Marshall CD, Leavitt T, Hu MS, Moore AL, Gonzalez JG, Longaker MT, Gurtner GC Advances in wound care. 2018, 7, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Block L, Gosain A, King TW Advances in wound care. 2015, 4, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wynn TA The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2008, 214, 199–210. [Google Scholar]
- [10].Wynn TA, Ramalingam TR Nature medicine. 2012, 18, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Trace AP, Enos CW, Mantel A, Harvey VM American journal of clinical dermatology. 2016, 17, 201–223. [DOI] [PubMed] [Google Scholar]
- [12].Lee HJ, Jang YJ Int J Mol Sci. 2018, 19, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bayat A, McGrouther D, Ferguson M Bmj. 2003, 326, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bush JA, McGrouther DA, Young VL, Herndon DN, Longaker MT, Mustoe TA, Ferguson MW Wound Repair and Regeneration. 2011, 19, s32–s37. [DOI] [PubMed] [Google Scholar]
- [15].Bock O, Schmid-Ott G, Malewski P, Mrowietz U Arch Dermatol Res. 2006, 297, 433. [DOI] [PubMed] [Google Scholar]
- [16].Brown B, McKenna S, Siddhi K, McGrouther D, Bayat A Journal of Plastic, Reconstructive & Aesthetic Surgery. 2008, 61, 1049–1058. [DOI] [PubMed] [Google Scholar]
- [17].Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT Wound repair and regeneration. 2009, 17, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tziotzios C, Profyris C, Sterling J Journal of the American Academy of Dermatology. 2012, 66, 13–24. [DOI] [PubMed] [Google Scholar]
- [19].Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, C. Murcia Dermatologic Surgery. 2014, 40, 817–824. [DOI] [PubMed] [Google Scholar]
- [20].Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, Wearda T, Muhonen E, McArdle A, Tevlin R Plastic and reconstructive surgery. 2015, 135, 907–917. [DOI] [PubMed] [Google Scholar]
- [21].Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD Journal of Investigative Dermatology. 2012, 132, 1901–1907. [DOI] [PubMed] [Google Scholar]
- [22].Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW Photochemistry and photobiology. 2008, 84, 450–462. [DOI] [PubMed] [Google Scholar]
- [23].Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR Annals of biomedical engineering. 2012, 40, 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Opel DR, Hagstrom E, Pace AK, Sisto K, Hirano-ALi SA, Desai S, Swan J J Clin Aesthet Dermatol. 2015, 8, 36. [PMC free article] [PubMed] [Google Scholar]
- [25].Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM Lasers in surgery and medicine. 2018, 50, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ablon G J Clin Aesthet Dermatol. 2018, 11, 21. [PMC free article] [PubMed] [Google Scholar]
- [27].American Society for Dermatologic Surgery in American Society for Dermatologic Surgery (ASDS) Survey on Dermatologic Procedures, Vol. (Ed.Êds.: Editor), City, 2018. [Google Scholar]
- [28].Hession MT, Markova A, Graber EM Dermatologic Surgery. 2015, 41, 307–320. [DOI] [PubMed] [Google Scholar]
- [29].Sakamoto F, Avram M, Anderson R Dermatology. 3rd ed. London: Mosby. 2012. [Google Scholar]
- [30].Jagdeo JR, Adams LE, Brody NI, Siegel DM PloS one. 2012, 7, e47460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Campbell S, Tyrrell J, Marshall R, Curnow A Photodiagnosis and photodynamic therapy. 2010, 7, 183–188. [DOI] [PubMed] [Google Scholar]
- [32].Nie Z, Bayat A, Behzad F, Rhodes LE Photodermatology, photoimmunology & photomedicine. 2010, 26, 330–332. [DOI] [PubMed] [Google Scholar]
- [33].Sakamoto F, Izikson L, Tannous Z, Zurakowski D, Anderson RR British Journal of Dermatology. 2012, 166, 413–416. [DOI] [PubMed] [Google Scholar]
- [34].Mamalis A, Jagdeo J Dermatologic Surgery. 2015, 41, 35–39. [DOI] [PubMed] [Google Scholar]
- [35].Mamalis A, Koo E, Garcha M, Murphy WJ, Isseroff RR, Jagdeo J Journal of biophotonics. 2016, 9, 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lev-Tov H, Mamalis A, Brody N, Siegel D, Jagdeo J Dermatologic Surgery. 2013, 39, 1167–1170. [DOI] [PubMed] [Google Scholar]
- [37].Mamalis A, Jagdeo J Dermatologic Surgery. 2018, 44, 1317–1322. [DOI] [PubMed] [Google Scholar]
- [38].Jagdeo J, Nguyen JK, Ho D, Wang EB, Austin E, Mamalis A, Kaur R, Kraeva E, Schulman JM, Li CS Journal of biophotonics. 2020, 13, e201960014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nguyen JK, Weedon J, Jakus J, Heilman E, Isseroff RR, Siegel DM, Jagdeo JR Trials. 2019, 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Photo Therapeutics Inc. in Omnilux New-U User Guide, Vol. (Ed.^Eds.: Editor), City, 2008, pp.1–17. [Google Scholar]
- [41].Food D Administration in 510 (k) summary of safety and effectiveness for the photo therapeutics limited omnilux new-u. 2007, Vol. (Ed.Êds.: Editor), City. [Google Scholar]
- [42].Mamalis A, Koo E, Tepper C, Jagdeo J Journal of biophotonics. 2019, 12, e201800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sun D, Yu Z, Chen J, Wang L, Han L, Liu N Lymphatic research and biology. 2017, 15, 70–76. [DOI] [PubMed] [Google Scholar]
- [44].Kim M, Kim E, Lee H Skin Research and Technology. 2018, 24, 466–471. [DOI] [PubMed] [Google Scholar]
- [45].Virén T, Iivarinen J, Sarin J, Harvima I, Mayrovitz H International journal of cosmetic science. 2018, 40, 134–140. [DOI] [PubMed] [Google Scholar]
- [46].Tandara AA, Kloeters O, Mogford JE, Mustoe TA Wound repair and regeneration. 2007, 15, 497–504. [DOI] [PubMed] [Google Scholar]
- [47].Meaume S, Le Pillouer-Prost A, Richert B, Roseeuw D, Vadoud J European Journal of Dermatology. 2014, 24, 435–443. [DOI] [PubMed] [Google Scholar]
- [48].Karmisholt K, Haerskjold A, Karlsmark T, Waibel J, Paasch U, Haedersdal M Journal of the European Academy of Dermatology and Venereology. 2018, 32, 1099–1110. [DOI] [PubMed] [Google Scholar]
- [49].Karmisholt KE, Wenande E, Thaysen-Petersen D, Philipsen PA, Paasch U, Haedersdal M Lasers in surgery and medicine. 2018, 50, 28–36. [DOI] [PubMed] [Google Scholar]
- [50].Song H, Tan J, Fu Q, Huang L, Ao M J Cosmet Dermatol. 2019, 18, 874–878. [DOI] [PubMed] [Google Scholar]
- [51].Wa CV, Maibach HI Skin Research and Technology. 2010, 16, 38–54. [DOI] [PubMed] [Google Scholar]
- [52].Kleesz P, Darlenski R, Fluhr J Skin pharmacology and physiology. 2012, 25, 25–33. [DOI] [PubMed] [Google Scholar]
- [53].Butler ST, Fosko SW Journal of the American Academy of Dermatology. 2010, 63, 1006–1010. [DOI] [PubMed] [Google Scholar]
- [54].Gupta V, Winocour J, Shi H, Shack RB, Grotting JC, Higdon KK Aesthetic surgery journal. 2016, 36, 1–13. [DOI] [PubMed] [Google Scholar]
- [55].American Society of Plastic Surgeons in 2017 Plastic Surgery Statistics Report, Vol. (Ed.Êds.: Editor), City, 2017. [Google Scholar]
- [56].Farage MA, Miller KW, Elsner P, Maibach HI Aging clinical and experimental research. 2008, 20, 195–200. [DOI] [PubMed] [Google Scholar]
- [57].Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ The American journal of pathology. 2006, 168, 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thomas D, Hopkinson I, Harding K, Shepherd J International journal of oral and maxillofacial surgery. 1994, 23, 232–236. [DOI] [PubMed] [Google Scholar]
- [59].Sgonc R, Gruber J Gerontology. 2013, 59, 159–164. [DOI] [PubMed] [Google Scholar]
- [60].Bond JS, Duncan JA, Sattar A, Boanas A, Mason T, O’Kane S, Ferguson MW Plastic and Reconstructive Surgery. 2008, 121, 1650–1658. [DOI] [PubMed] [Google Scholar]
- [61].Marcus JR, Tyrone JW, Bonomo S, Xia Y, Mustoe TA Plastic and reconstructive surgery. 2000, 105, 1591–1599. [DOI] [PubMed] [Google Scholar]
- [62].Cadet N, Hardy I, Dudek D, Miszkiewicz K, Boulos P, Nguyen Q, Wong J Canadian Journal of Ophthalmology. 2018, 53, 29–33. [DOI] [PubMed] [Google Scholar]
- [63].Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, C. Murcia Dermatologic Surgery. 2014, 40, 825–831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


