Abstract
Background:
Pregnant women are ubiquitously exposed to per- and polyfluoroalkyl substances (PFAS). Prenatal exposure to PFAS has been associated with lower birth weight but also with excess adiposity and higher weight in childhood. These mixed findings warrant investigation of the relationship between PFAS and dynamic offspring growth.
Objectives:
To investigate the association between prenatal PFAS exposure and early-life growth trajectories during the first 2 y.
Methods:
Pregnant women () were recruited from 2013 to 2016 from the Shanghai Birth Cohort (SBC) Study, and their children were followed up from birth to 2 y of age. Seven PFAS congeners were quantified in pregnant women’s serum during the first trimester. Our study population was restricted to 1,350 children who had five repeated measurements for at least one anthropometric measure. Four anthropometric measures, including weight, length/height, weight-for-length, and head circumference, were evaluated at birth, 42 d, 6 months, 12 months, and 24 months, and standardized into z-scores using the World Health Organization reference. Trajectories of each measure were classified into five groups using group-based trajectory modeling. Multinomial logistic regression was used to estimate odds ratio (OR) and 95% confidence interval (CI) for trajectory groups according to -transformed PFAS concentrations, and the moderate-stable group was selected as the reference group for all measures.
Results:
Higher prenatal exposure to PFAS was associated with elevated odds for the low-rising weight-for-age z-score (WAZ) trajectory, and the high-rising length-for-age z-score (LAZ) trajectory. Meanwhile, PFAS levels were associated with decreased odds for the low-rising and high-rising weight-for-length z-score (WLZ) trajectories. In addition, the associations of PFAS with growth trajectory groups differed by sex, where males had greater odds for the low-rising and low-stable WAZ trajectories and for the high-stable and low-rising WLZ trajectories. In contrast, inverse associations were consistently observed with trajectories of the high-stable, low-stable, and low-rising head-circumference-for-age z-score (HCZ) in relation to most individual PFAS congeners. PFAS mixtures analysis further confirmed the above findings.
Discussion:
Trajectory analysis approach provided insight into the complex associations between PFAS exposure and offspring growth. Future studies are warranted to confirm the present findings with trajectory modeling strategies and understand the clinical significance of these trajectory groups. https://doi.org/10.1289/EHP9875
Introduction
Per- and polyfluoroalkyl substances (PFAS) have been produced since the 1940s and used for a wide variety of industrial and commercial applications (Prevedouros et al. 2006). Due to their global distribution, environmental persistence, long half-lives in humans, and toxicity, PFAS are recognized as environmental contaminants of high and emerging concern (Blum et al. 2015; Ritscher et al. 2018; Scheringer et al. 2014). Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), the most studied congeners, have been listed as persistent organic pollutants (POPs) in Annex B and Annex A of the Stockholm Convention in 2009 and 2019, respectively (UNEP 2009, 2019). More restrictive regulations have been introduced, limiting the production and use of PFAS in North America and Europe (EFSA CONTAM Panel 2020; U.S. EPA 2020). China has also restricted the usage of PFOA and PFOS since 2011(NDRC 2011). However, PFAS will present an intractable, potentially never - ending challenge to ecosystem and human health, given the continuing production and usage, the large number of existing substances, the manufacture of novel PFAS congeners and PFAS substitutes, and the lack of effective control measures (Wang et al. 2017).
The universal exposure to PFAS in pregnant women has been reported from different regions worldwide (Aimuzi et al. 2020; Han et al. 2018; Kim et al. 2020; Lebeaux et al. 2020). PFAS can transfer across the placenta during pregnancy (Eryasa et al. 2019; Wang et al. 2019), making them a potential threat to fetuses during the most sensitive early stages of life. Previous epidemiological studies have suggested that prenatal PFAS exposures, mainly PFOS and PFOA, may be associated with decreased birth weight (Chen et al. 2021; Gyllenhammar et al. 2018; Lee et al. 2021; Liew et al. 2018; Maisonet et al. 2012; Marks et al. 2019; Meng et al. 2018; Starling et al. 2017) and increased risk of childhood overweight or adiposity (Braun et al. 2016; Chen et al. 2017, 2019; Gyllenhammar et al. 2018; Lee et al. 2021; Maisonet et al. 2012; Starling et al. 2019). However, several inconsistent results have also been reported, such as inverse or null associations for childhood growth (Andersen et al. 2013; Barry et al. 2014; Shoaff et al. 2018). For other types of PFAS and other growth measures besides weight, the evidence was very limited and more inconclusive as reviewed by Lee et al. (Lee et al. 2021). Taken together, prenatal PFAS exposures may show mixed and conflicting associations with human growth at different developmental stages, raising concerns over their long-term health influence.
Although numerous studies have examined the impact of prenatal PFAS exposure on birth size or adiposity at birth and later in childhood (Alkhalawi et al. 2016; Gyllenhammar et al. 2018; Maisonet et al. 2012), the relationship between PFAS and early-life growth remains inconclusive (Andersen et al. 2010; Shoaff et al. 2018; Starling et al. 2019). Growth processes during the first 2 y of life, when the greatest variations in rates of weight gain usually occur, deserve more attention (Ong et al. 2000; Pryor et al. 2011). In addition, prior studies have almost exclusively used static anthropometric measures, which lack information on the dynamic growth processes (Braun et al. 2016; Gyllenhammar et al. 2018; Kashino et al. 2020; Lee et al. 2021). The heterogeneity in early-life growth calls for finer modeling of these variations into several typical trajectories instead of an average growth pattern (Herle et al. 2020). Previous studies have also demonstrated that life course changes in anthropometric measures, especially during critical developmental period, are more predictive for diseases in later life in comparison with measures at a single time point (Song et al. 2018; Zheng et al. 2017).
Many approaches have been proposed to identify distinctive early-life growth trajectories that may be related to high health risk in later life (Tu et al. 2013). Among them, the group-based trajectory modeling [GBTM; also called latent class growth analysis (LCGA)] provides a flexible approach for identifying distinct groups of trajectories within the same population (Nagin and Tremblay 2001). Psychology and criminology have already used GBTM to identify high-risk subgroups and risk factors based on individual-level behavior trajectories (Swartout et al. 2015). Recently, the GBTM approach has been increasingly used for biomedical research, such as obesity studies (Pryor et al. 2011; Song 2019; Zheng et al. 2017). However, to our knowledge, no study has looked at the association of prenatal exposure to PFAS with postnatal growth trajectories using GBTM.
To address the knowledge gap regarding prenatal PFAS exposure and offspring growth, we used GBTM to characterize early postnatal growth trajectories and to evaluate whether prenatal exposure to PFAS, individually and collectively, was associated with particular growth trajectories through the first 2 y of life in a prospective birth cohort study.
Methods
Study Population
We used data from the Shanghai Birth Cohort (SBC) Study, a prospective cohort study designed to examine the impacts of environmental chemicals and behavioral and genetic factors on fecundability, birth outcomes, postnatal growth and development, and risks of childhood diseases, which has been described in details elsewhere (Zhang et al. 2019). Briefly, pregnant women () from six participating hospitals in Shanghai, China, were enrolled from 2013 to 2016. After excluding those lost to follow-up or whose pregnancies ended in miscarriages or stillbirths, 3,331 pregnant women who had live births remained. Multiple pregnancy (more than one fetus) and maternal history of chronic diseases may seriously affect intrauterine or postnatal growth. Thus, women with a multiple pregnancy, diabetes, cancer, thyroid disorders, schizophrenia, and HIV infection before enrollment were excluded, leaving 3,078 pregnant women. We further excluded children who had severe congenital diseases, specifically congenital heart defects, neural tube defects, hydrocephalus, dwarfism, and intestinal atresia (), or who had no maternal PFAS exposure information (). We further restricted our study population to children who had five measurements for at least one anthropometric measure (weight, length/height, weight-for-length, or head circumference) from birth to 24 months (at birth, 42 d, 6 months, 12 months, and 24 months) ( in total; see flowchart in Figure S1). The study was approved by the ethics committees of all six hospitals. All participants signed informed consent prior to their enrollment.
PFAS Measurement
Maternal plasma for PFAS measurement was collected between 9 and 16 wk of gestation, and 10 PFAS congeners [PFOA, PFOS, perfluorononanoic acid (PFNA), perfluoroundecanoic acid (PFUA), perfluorodecanoic acid (PFDA), perfluorohexanesulfonate (PFHxS), perfluoroheptanoic acid (PFBS), perfluorododecanoic acid (PFDoA), perfluoroheptanoic acid (PFHpA), and perfluorooctane sulfonamide (PFOSA)] were measured using a liquid chromatography system coupled with tandem mass spectrometry (HPLC-MS/MS; Agilent Technologies Inc.). Intra- and inter-day variation coefficients were below 10%. Detailed methods for PFAS measurements have been reported previously (Wang et al. 2016). The limit of detection (LOD) for PFAS ranged from 0.009 to , and concentrations below the LOD were replaced by the LOD divided by the square root of 2. We excluded three PFAS (PFOSA, PFBS, and PFDoA) with detection rates lower than 80% in further analyses.
Anthropometric Measurements
Standardized measurements of weight (in grams), length/height (in centimeters), and head circumference (in centimeters) were taken when children were born, at 42 d (), 6 months [: ], 12 months (: ), and 24 months (: ) by trained research assistants who were blinded to the mothers’ PFAS concentrations. For analysis of infant/child growth, we generated weight-for-age z-score (WAZ), length-for-age z-score (LAZ), weight-for-length z-score (WLZ), and head-circumference-for-age z-score (HCZ), using the World Health Organization (WHO) reference data for children younger than 2 y old (version 3.2.2) (WHO Multicentre Growth Reference Study Group 2006). The American Academy of Pediatrics recommends using weight-for-length for children younger than 24 months rather than body mass index (BMI) (Daniels et al. 2015).
We systemically cleaned data to eliminate apparent data entry errors, such as incorrect units (e.g., years vs. months, grams vs. kilograms), and nonmonotonic growth in each consecutive visit.
Covariates
Directed acyclic graph (DAG) was used to select maternal sociodemographic, nutritional, prenatal, postnatal, and environmental factors as covariates in our analysis (Figure S2). Self-reported data on maternal/child characteristics and behaviors were obtained from the mothers during pregnancy or during follow-up visits, including: maternal age at delivery (continuous, years), maternal educational level (below Bachelor, Bachelor, above Bachelor), maternal prepregnancy BMI (continuous, kilograms per square meter), active or passive smoking during pregnancy (yes/no), fish or seafood intake during pregnancy (no, , 1–3 times/wk, 4–7 times/wk, ), nutrient supplementation during pregnancy (yes/no), breastfeeding duration (0, , ), infant nutrient supplementation (yes/no), and duration of outdoor activities per day of children (, ). Other important covariates were obtained from hospital medical records, including parity (nulliparous/multiparous), child sex (male, female), type of delivery (vaginal delivery, forceps delivery, caesarean), maternal weight at delivery (continuous, kilograms), and plasma creatinine (continuous, micromolar). Maternal weight gain during pregnancy (continuous, kilograms) was calculated as maternal weight at delivery minus prepregnancy weight. Maternal estimated glomerular filtration rate (eGFR) was calculated using levels of plasma creatinine by the Cockroft-Gault formula. Exact age (in days) for each individual child at anthropometry measurement was calculated as the date at examination minus the birth date of the child.
Statistical Analyses
First, we identified the growth trajectories of WAZ, LAZ, WLZ, and HCZ using GBTM to evaluate the evolution of these measures over time. GBTM assumes that all participants are from the same population but composed of distinct groups defined by their growth trajectories (Nagin and Tremblay 2001; Nagin and Odgers 2010; Nagin et al. 2018; Song 2019). The time and growth were linked using a polynomial function in GBTM. More technical details about the statistic basis of GBTM can be found elsewhere (Nagin and Tremblay 2001; Nagin 2014; Nagin et al. 2018).
Model selection is a key issue in GBTM, and we followed suggestions from early studies to complete the task (Song 2019). We first identified the optimal number of trajectory groups using the Bayesian information criteria (BIC). Specifically, we fit models with different numbers (from one to six) of trajectory groups using a quadratic form for all trajectories and then generated corresponding BICs. The quadratic function used in this step was to help us determine the optimal number of groups and did not represent the final shape of trajectories in this study. The higher BIC indicates better model fit, and a difference lower than 2 means not enough evidence against the null model and was only used to determine the optimal group number. Then, we determined the trajectory shapes that best describe the observed trajectories. Linear, quadratic, cubic, and quartic functions were all tried. Linear and quadratic functions were not enough to capture the variations in trajectories. Because we had only five measurements, the quartic function could always perfectly fit the observed data, no matter how unrealistic the predictive trajectory was, which led to concerns of overfitting. Therefore, as a compromise, the cubic function was finally selected to describe the trajectory shape. After obtaining the optimal number of trajectory groups and the proper trajectory shape, we visually presented the trajectories for each measure and selected the group that had a stable trend near the null z-score as the reference group.
Second, multinomial logistic regression was used to evaluate the association between prenatal PFAS exposure and the trajectory groups with adjustment for potential confounders. Maternal age, maternal education, prepregnancy BMI, fish and seafood intake during pregnancy, parity, and pregnancy eGFR were identified as the minimal set for necessary adjustment according the DAG and were adjusted in the primary analysis. Odds ratios (OR) and 95% confidence intervals (CI) were estimated. Meanwhile, PFAS concentrations were -transformed and treated as a continuous variable in the regression models. The effect estimate was thus interpreted as the OR for different growth trajectories for doubling in prenatal PFAS concentrations in comparison with the reference group. We further performed sex-stratified analyses to evaluate potential effect modification by child sex, because prior studies have reported potential sex difference in the effect of prenatal PFAS exposure (Ernst et al. 2019; Liew et al. 2014). Tests of heterogeneity were performed by assessing the -value of the interaction term for each PFAS and child sex in the regression models.
To account for the potential selection bias due to the inclusion criterion, we constructed inverse probability weights (IPW) for each anthropometric measure according to factors assessed for all women in the SBC at baseline that may affect participation, including maternal education, maternal age, and parity (Härkänen et al. 2014). All the regression models that evaluate the association between prenatal PFAS exposure and trajectory groups were weighted by corresponding IPW, and we presented the weighted estimates in this study.
Moreover, we used weighted quantile sum approach (WQS) to estimate the joint exposure effect of all PFAS congeners on each of the trajectories (Carrico et al. 2015). A WQS index was created using all PFAS congeners in this study, and each PFAS was assigned a weight that reflected the contribution of that PFAS to the association between PFAS mixture and outcomes. The WQS index was created using the PFAS quartiles, and one-unit increase in WQS index was interpreted as per quartile increase in PFAS mixture exposure. Because WQS performs unidirectional evaluation of mixture effects, we used the results from individual chemical analysis to guide the directionality of WQS analysis. For most trajectory groups, the direction was clear and consistent. For groups that had no clear direction, we estimated both positive and negative mixture associations and reported the estimates that were further away from null. We ran 500 bootstraps for the WQS regressions.
Fewer than 4% of participants had missing values in potential confounders. Missing confounders were assumed missing at random and replaced by multiple imputations that included all PFAS and the aforementioned variables. Ten complete data sets were generated and analyzed as recommended (Yuan 2010).
In the sensitivity analysis, we additionally adjusted for active or passive smoking during pregnancy, gestational weight gain, breastfeeding after birth, delivery methods, child sex, nutrient supplementation during pregnancy, infant nutrient supplementation, and infancy outdoor activity, aiming to control for all residual confounding in the back-door paths. We also used general additive models (GAM) to test nonlinearity using penalized smoothing regression splines with a degree of 3. A -value less than 0.10 for spline was considered as departure from linearity.
The GBTM was performed using PROC TRAJ in SAS (Jones et al. 2001). The WQS analysis was performed using R package gWQS (version 4.1.1, R Core Development Team). All other statistical analysis was performed using SAS (version 9.4; SAS Institute Inc.).
Results
The distributions of selected characteristics in the overall study population (), participants with five WAZ measurements (), participants with five LAZ measurements (), participants with five WLZ measurements (), and participants with five HCZ measurements () are presented in Table 1. The majority of pregnant women in this study were 25–29 y of age, received an education above high school, and had normal prepregnancy BMI. There was no substantial difference in demographic characteristics between the source population and the study population (Table S1).
Table 1.
Distribution of child anthropometric z-scores by maternal characteristics in Shanghai Birth Cohort, Shanghai, China, recruited from 2013 to 2016.
| Selected characteristics | Overall () |
WAZ () |
LAZ () |
WLZ () |
HCZ () |
|---|---|---|---|---|---|
| Maternal age (y) | |||||
| 99 (7.3) | 98 (7.3) | 94 (8.3) | 93 (8.2) | 89 (8.2) | |
| 25–29 | 717 (53.2) | 716 (53.3) | 615 (54.0) | 613 (54.1) | 580 (53.7) |
| 30–34 | 406 (30.1) | 405 (30.1) | 325 (28.5) | 323 (28.5) | 316 (29.2) |
| 126 (9.3) | 125 (9.3) | 105 (9.2) | 104 (9.2) | 96 (8.9) | |
| Missing | 2 | 2 | 1 | 1 | 1 |
| Maternal education | |||||
| High school or less | 147 (10.9) | 147 (10.9) | 137 (12.0) | 136 (12.0) | 135 (12.5) |
| Associate or bachelor | 1,074 (79.7) | 1,071 (79.7) | 908 (79.9) | 904 (79.9) | 857 (79.4) |
| Graduate | 126 (9.4) | 125 (9.3) | 92 (8.1) | 91 (8.0) | 88 (8.1) |
| Missing | 3 | 3 | 3 | 3 | 2 |
| Parity | |||||
| Nulliparous | 1,151 (85.3) | 1,148 (85.3) | 978 (85.8) | 974 (85.9) | 924 (85.4) |
| Multiparous | 199 (14.7) | 198 (14.7) | 162 (14.2) | 160 (14.1) | 158 (14.6) |
| Active or passive smoking during pregnancy | |||||
| No | 845 (63.1) | 842 (63.1) | 723 (63.9) | 720 (64.0) | 679 (63.2) |
| Yes | 494 (36.9) | 493 (36.9) | 408 (36.1) | 405 (36.0) | 395 (36.8) |
| Missing | 11 | 11 | 9 | 9 | 8 |
| Prepregnancy BMI () | |||||
| 208 (15.6) | 207 (15.5) | 189 (16.7) | 188 (16.7) | 181 (16.9) | |
| 18.5–24.9 | 981 (73.4) | 978 (73.4) | 821 (72.7) | 817 (72.7) | 773 (72.1) |
| 148 (11.1) | 148 (11.1) | 120 (10.6) | 119 (10.6) | 118 (11.0) | |
| Missing | 13 | 13 | 10 | 10 | 10 |
| Fish or seafood intake | |||||
| No | 53 (4.0) | 53 (4.0) | 30 (2.7) | 30 (2.7) | 28 (2.7) |
| /wk | 148 (11.3) | 148 (11.3) | 146 (13.1) | 146 (13.2) | 133 (12.6) |
| 1–3 /wk | 917 (69.7) | 914 (69.7) | 772 (69.4) | 767 (69.3) | 735 (69.7) |
| 4–7/wk | 186 (14.1) | 185 (14.1) | 159 (14.3) | 158 (14.3) | 153 (14.5) |
| /wk | 11 (0.8) | 11 (0.8) | 6 (0.5) | 6 (0.5) | 6 (0.6) |
| Missing | 35 | 35 | 27 | 27 | 27 |
| Gestational weight gain (kg) | |||||
| 327 (25.4) | 327 (25.5) | 278 (25.3) | 277 (25.4) | 264 (25.3) | |
| 12–14.9 | 319 (24.8) | 318 (24.8) | 266 (24.2) | 265 (24.3) | 252 (24.2) |
| 15–17.9 | 323 (25.1) | 320 (24.9) | 278 (25.3) | 275 (25.2) | 265 (25.4) |
| 319 (24.8) | 319 (24.8) | 275 (25.1) | 275 (25.2) | 261 (25.0) | |
| Missing | 62 | 62 | 43 | 42 | 40 |
| Breastfeeding after birth | |||||
| No | 40 (3.0) | 40 (3.0) | 40 (3.5) | 39 (3.4) | 36 (3.3) |
| Yes, less than 6 months | 350 (25.9) | 350 (26.0) | 281 (24.7) | 281 (24.8) | 268 (24.8) |
| Yes, more than 6 months | 960 (71.1) | 956 (71.0) | 819 (71.8) | 814 (71.8) | 778 (71.9) |
| Delivery method | |||||
| Vaginal delivery | 770 (57.0) | 768 (57.1) | 615 (53.9) | 611 (53.9) | 575 (53.1) |
| Cesarean delivery | 580 (43.0) | 578 (42.9) | 525 (46.1) | 523 (46.1) | 507 (46.9) |
| Child sex | |||||
| Female | 644 (47.7) | 643 (47.8) | 532 (46.7) | 530 (46.7) | 501 (46.3) |
| Male | 706 (52.3) | 703 (52.2) | 608 (53.3) | 604 (53.3) | 581 (53.7) |
| Pregnancy eGFR ()a | 157.8 (139.0–183.1) | 157.8 (139.0–183.0) | 158.9 (139.2–185.4) | 158.8 (139.2–185.3) | 158.7 (139.0–186.3) |
Note: BMI, body mass index; eGFR, estimated glomerular filtration rate; HCZ, head-circumference-for-age -score; LAZ, length-for-age -score; min, minute; WAZ, weight-for-age -score; WLZ, weight-for-length -score.
Median (interquartile range).
Infant anthropometric measures are presented in Table S2. For infants at birth, 42 d, 6 months, 12 months, and 24 months of age, average weights (SD) were 3.38 (0.44), 5.14 (0.62), 8.61 (1.02), 10.26 (1.14), and 12.87 (1.44) kg, respectively; average lengths (SD) were 49.92 (1.19), 56.74 (2.28), 68.65 (2.64), 76.18 (2.60), and 88.66 (3.25) cm, respectively; and average head circumferences (SD) were 34.36 (1.18), 37.91 (1.17), 43.50 (1.35), 45.90 (1.35), and 48.36 (1.33) cm, respectively. The corresponding WAZ, LAZ, WLZ, and HCZ are also presented in Table S2 as well.
GBTM identified five trajectory groups for each anthropometric measure (WAZ, LAZ, WLZ, and HCZ) in this study. The trajectories for each measure are presented in Figure 1. The trajectories were labeled according to their initial values (low, moderate, high) and subsequent trends (rising, stable, falling). For example, a low-rising trajectory means that the trajectory starts with a low value but is followed by an increase afterward. The five trajectory groups for WAZ were: high-rising, high-stable, moderate-stable, low-rising, and low-stable. The five trajectory groups for LAZ were: high-rising, moderate-rising, moderate-stable, moderate-falling, and low-rising. The five trajectory groups for WLZ were: high-rising, high-stable, moderate-stable, low-rising, and low-stable. The five trajectory groups for HCZ were: high-stable, moderate-rising, moderate-stable, low-stable, and low-rising. In each measure, there was one moderate-stable group. This group demonstrated a steady trend near and had a considerable portion of the study populations. Therefore, the moderate-stable group was selected as the reference group for all measures. Detailed definitions for five trajectory groups for each anthropometrical measure are presented in Table S3. The BIC used for model selection and posterior probability for model goodness of fit can be found in Table S4.
Figure 1.
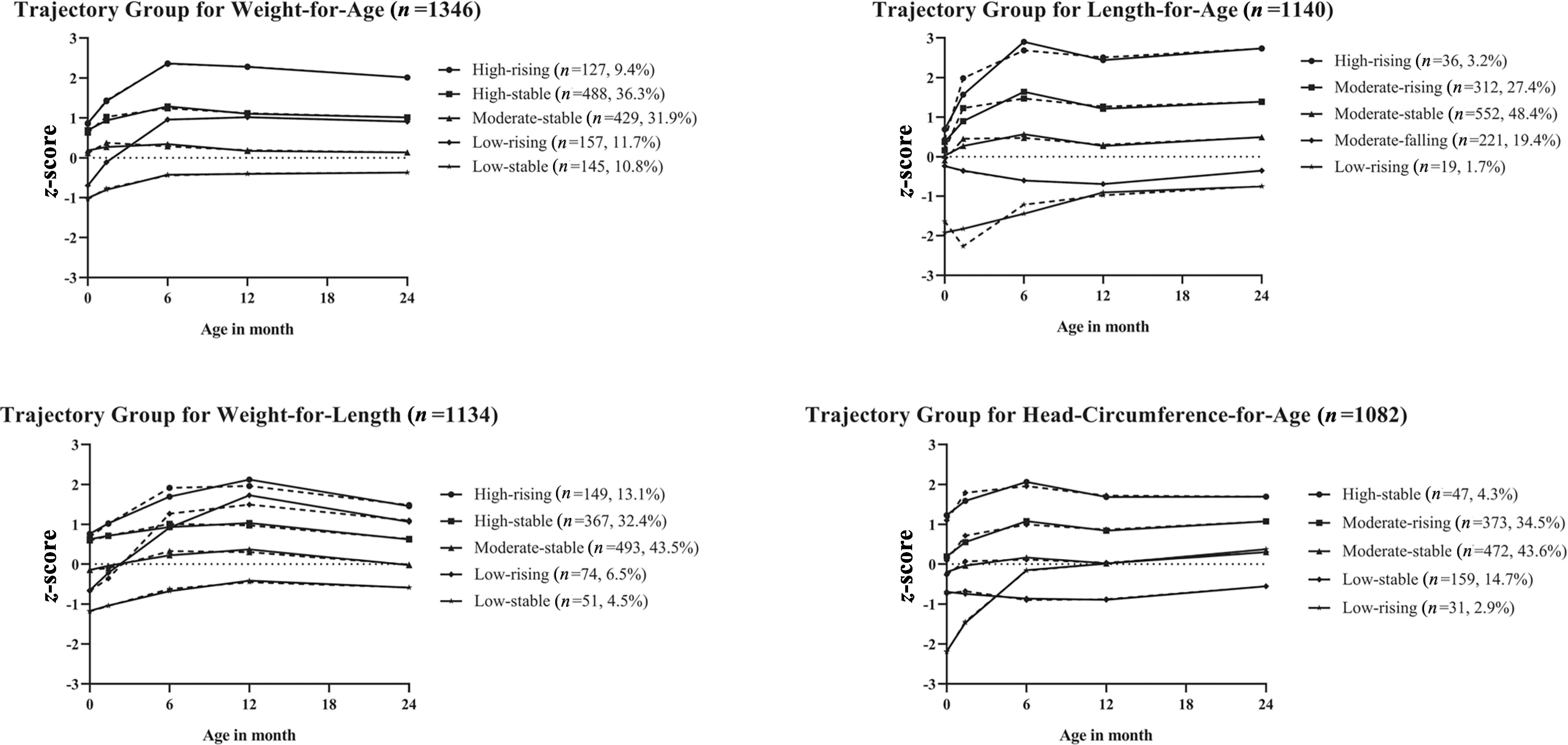
Developmental trajectories for weight-for-age z-score (WAZ), length-for-age z-score (LAZ), weight-for-length z-score (WLZ), and head-circumference-for-age z-score (HCZ) from birth to 24 months of age in Shanghai Birth Cohort, Shanghai, China, recruited from 2013 to 2016. The groups are labeled according to the initial value and following trend. The solid line indicates predicted trajectory, whereas the dashed line indicates observed trajectory.
First-trimester maternal plasma concentrations of 10 PFAS congeners are shown in Table S5. The detection frequencies were greater than 80% for seven PFAS congeners that were included in the analysis. Median concentrations [interquartile ranges (IQRs)] were 11.66 (9.24–14.80), 9.68 (6.75–13.60), 1.82 (1.23–2.67), 1.77 (1.27–2.41), 1.48 (1.02–2.10), 0.54 (0.43–0.68), and 0.06 for PFOA, PFOS, PFDA, PFNA, PFUA, PFHxS, and PFHpA, respectively.
The associations between prenatal PFAS exposures and child growth trajectories by each anthropometric measure are presented in Figure 2 (numeric values are presented in Tables S6 and S7). With regard to WAZ, it was noted that positive associations were consistently observed for most PFAS exposures and the low-rising group (except PFOS and PFHpA). When using WQS analysis, PFAS mixture was associated with higher odds (; 95% CI: 1.09, 1.65) for the low-rising group, whereas PFHxS had the highest weights (0.74).
Figure 2.
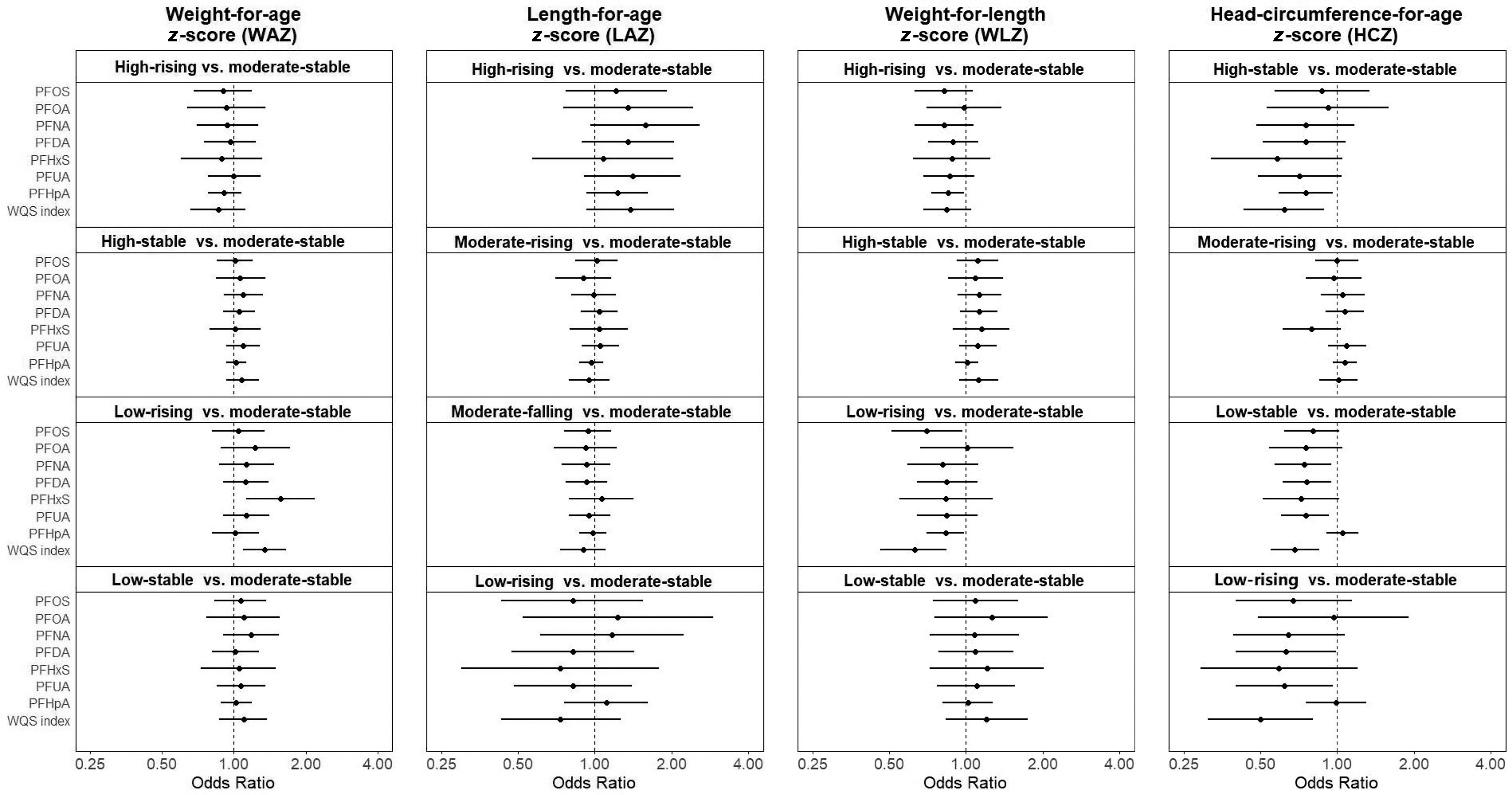
OR and 95% CI for trajectory groups in each anthropometrical measure according to per doubling increase in prenatal PFAS level (nanograms per milliliter) in logistic regression models in Shanghai Birth Cohort, Shanghai, China, recruited from 2013 to 2016. A WQS index was created using all seven types of PFAS to reflect the mixture exposure level. All models were adjusted for maternal age, maternal education, prepregnancy BMI, fish and seafood intake during pregnancy, parity, and pregnancy eGFR. Numeric values are presented in Table S6. Note: BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HCZ, head-circumference-for-age z-score; for LAZ, length-for-age z-score; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUA, perfluoroundecanoic acid; OR, odds ratio; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score; WQS, weighted quantile sum.
For LAZ (Figure 2; numeric values are presented in Tables S6 and S7), most PFAS exposures were consistently associated with higher OR for the high-rising group. When using WQS analysis, PFAS mixture was marginally significantly associated with higher odds (; 95% CI: 0.93, 2.05) for the high-rising group, whereas PFDA had the highest weights (0.53).
For WLZ (Figure 2; numeric values are presented in Tables S6 and S7), inverse associations were observed between PFAS congeners and the high-rising and the low-rising groups. PFAS mixture was associated with lower odds for the high-rising group (; 95% CI: 0.68, 1.05) and for the low-rising group (; 95% CI: 0.46, 0.74), whereas PFHxS had the highest weight (0.33) for the high-rising group, and PFHpA had the highest weight (0.58) for the low-rising group.
For HCZ (Figure 2; numeric values are presented in Tables S6 and S7), inverse associations were consistently observed between PFAS congeners and the high-stable, low-stable, and low-rising groups. When PFAS mixture was considered, associations were strengthened. WQS index was negatively associated with the high-stable group (; 95% CI: 0.43, 0.89), the low-stable group (; 95% CI: 0.55, 0.85), and the low-rising group (; 95% CI: 0.31, 0.80), whereas PFHxS, PFUA, and PFDA had the highest weights (0.34, 0.51, and 0.33), respectively.
In stratified analysis by sex (Figure 3: numeric values are presented in Tables S8–S10), PFAS were associated with greater ORs for the low-rising and low-stable WAZ trajectories and the high-stable and low-rising WLZ trajectories among males but not females. Most pronounced differences between males and females were found for the low-rising WLZ trajectory. For example, PFNA (; 95% CI: 0.33, 0.85), PFDA (; 95% CI: 0.40, 0.89), and PFUA (; 95% CI: 0.37, 0.83) were related with decreased OR in females, whereas PFNA (; 95% CI: 0.77, 2.01), PFDA (; 95% CI: 0.79, 1.82), and PFUA (; 95% CI: 0.84, 1.97) were related with increased OR in males, and for interaction were 0.03, 0.04, and 0.01, respectively.
Figure 3.
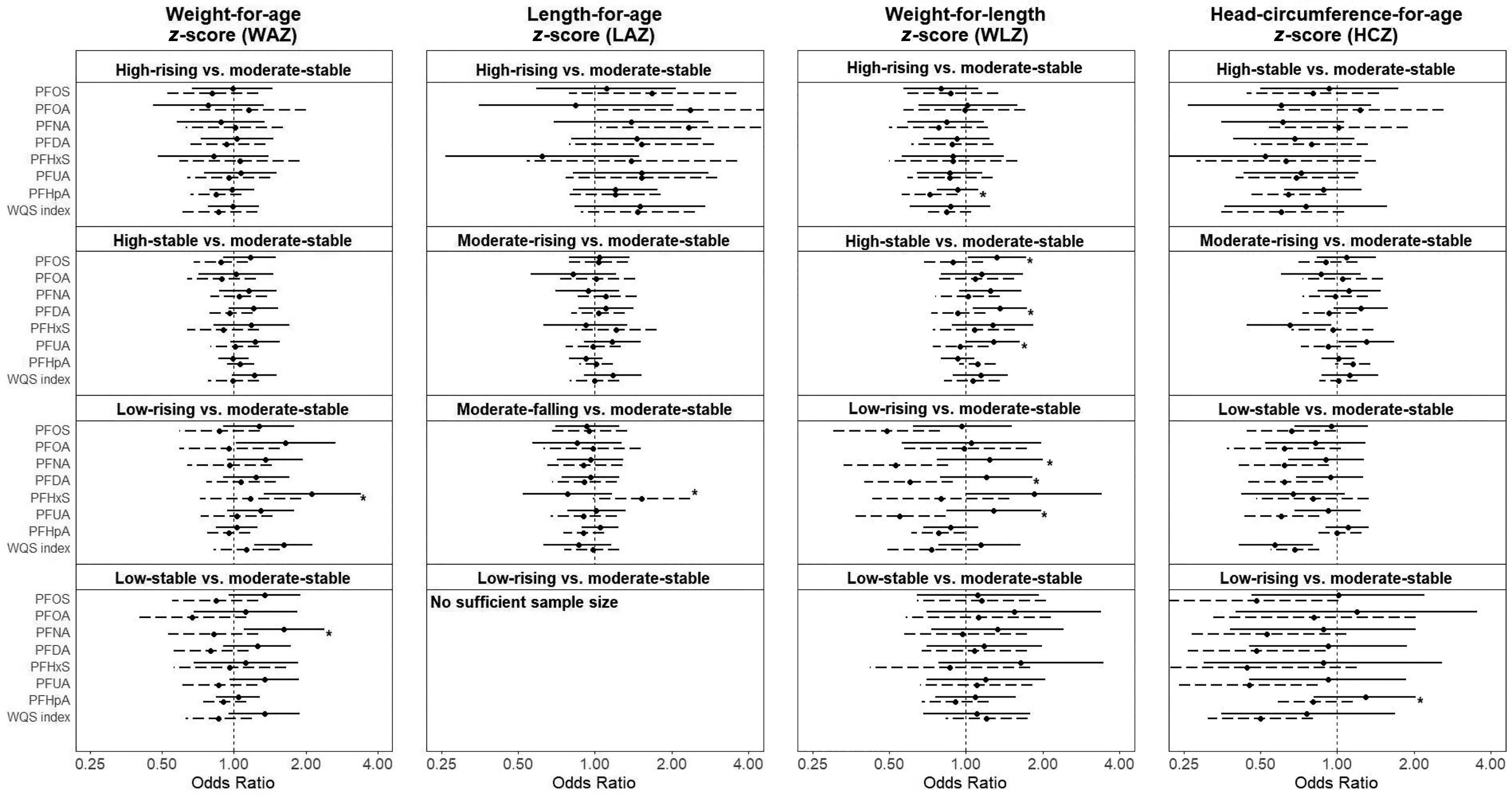
Stratified analysis by sex using logistic regression models in Shanghai Birth Cohort, Shanghai, China, recruited from 2013 to 2016. The solid line represents males, and the dashed line represents females. Lines with asterisk mean a for the comparison between males and females. A WQS index was created using all seven types of PFAS to reflect the mixture exposure level. All models were adjusted for maternal age, maternal education, prepregnancy BMI, fish and seafood intake during pregnancy, parity, and pregnancy eGFR. Numeric values are presented in Tables S8 and S9. Note: BMI, body mass index; eGFR, estimated glomerular filtration rate; HCZ, head-circumference-for-age z-score; for LAZ, length-for-age z-score; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUA, perfluoroundecanoic acid; OR, odds ratio; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score; WQS, weighted quantile sum.
In the sensitivity analysis, when additionally adjusted for active or passive smoking during pregnancy, gestational weight gain, breastfeeding after birth, delivery method, child sex, nutrient supplementation during pregnancy, infant nutrient supplementation, and infancy outdoor activity, although some changes were observed, the overall results were consistent with our main analysis (Table S11). In GAM (Table S12), we found no evidence for nonlinear relationship between prenatal PFAS exposures and trajectories of WAZ, LAZ, and WLZ. However, GAM suggested nonlinear relationship between PFAS and the high-stable, low-rising, and low-stable HCZ trajectories. The splines demonstrated a U-shaped curve only for the high-stable group. As for the low-rising and low-stable HCZ trajectories, though nonlinear, the splines exhibited a monotonic trend (Figure S3).
Discussion
In a—to our knowledge—novel application of GBTM to environmental epidemiology, we characterized postnatal growth trajectories during the first 2 y of life as outcome variables in relation to prenatal PFAS exposure levels during the first trimester. We found that higher PFAS levels were associated with elevated odds for the low-rising WAZ trajectory and for the high-rising LAZ trajectory in comparison with the moderate-stable trajectory. The associations of PFAS with weight and length/height trajectories might differ by sex. In contrast, high PFAS concentrations were associated with decreased odds of being in the high-stable, low-stable, or low-rising HCZ trajectories in comparison with the moderate-stable trajectory. PFAS mixtures analysis further confirmed the above findings.
Our findings are generally in line with the growing evidence regarding the association of prenatal PFAS exposure both with fetal growth restriction and overweight or obesity during infancy and early childhood, including low birth weight (Fei et al. 2007; Meng et al. 2018; Starling et al. 2019), greater weight and adiposity at 5 months of age (Starling et al. 2019), heavier at 20 months (Maisonet et al. 2012), higher BMI at 3–5 y of age (Gyllenhammar et al. 2018), and a more rapid increase in BMI between 2–8 y (Braun et al. 2016). More previous studies investigated growth in mid-childhood and afterward; however, several inconsistencies were also noted. For example, in a Danish study, maternal PFOS and PFOA levels during pregnancy were inversely related to child weight up to 7 y of age, although not statistically significant (Andersen et al. 2013). The Avon Longitudinal Study found a nonsignificant association between PFAS exposure and childhood adiposity in girls at 9 y of age (Hartman et al. 2017). The cohort study around mid-Ohio valley showed that elevated levels of PFOA exposure in early life were not associated with overweight and obesity risk in adulthood (Barry et al. 2014). Great heterogeneity in the study populations and residual confounding may partly explain the inconsistent results. Our study focuses on the first 2 y after birth. In comparison with later life periods, prenatal PFAS exposure may play a more important role in growth variance during infancy and early childhood, which may explain the difference between results in this study and the null findings during later childhood in other studies. It is commonly observed that growth-restricted newborns are more likely to experience rapid postnatal weight gain during the first 2 y of life to compensate for intrauterine restraint (Ong et al. 2000), which may be a precursor for metabolic diseases in later life (Ibáñez et al. 2006; Kerkhof et al. 2012; Teller et al. 2018). Thus, the potential impact of prenatal exposure to PFAS at early postnatal growth is of concern for public health.
Most prior literature almost exclusively used static growth measures. Limited longitudinal studies investigated postnatal growth at multiple time points using conventional analysis methods, such as linear mixed-effects and marginal models to estimate cumulative growth metrics instead of trajectory patterns (Braun et al. 2016; Shoaff et al. 2018; Starling et al. 2019). These conventional methods are cross-sectional in nature and fail to capture the dynamic features of growth. In contrast, growth trajectory modeling can provide more complete characterization of dynamic growth processes (Pryor et al. 2011), which may allow more accurate identification and quantification of modifiable risk factors and prediction of health outcomes as well as identification of critical windows for intervention (Regnault and Gillman 2014). To our knowledge, only a recent study of Swedish children () explored how prenatal PFAS exposure may influence child weight trajectory. The Swedish study investigated the weight trajectory from birth to age 5.5 y with a double logistic function modeling and found that higher prenatal PFOA exposure was related to lower birth weight as well as higher infant weight plateau at later stages (Tanner et al. 2020). Using a different trajectory model, we confirmed their findings by showing an association between prenatal PFAS exposure and the low-rising WAZ trajectory that has a higher WAZ from 6 months. The Swedish study focused only on PFOA and weight trajectory, whereas our study included seven PFAS congeners and multiple anthropometric measures, providing more evidence regarding the influence of prenatal PFAS exposure on postnatal growth.
In this study, using age- and sex-standardized measures, we observed an association between prenatal PFAS exposure and low-rising WAZ trajectory. These findings support the hypothesis that reduced fetal growth due to in utero exposure to PFAS may result in early-life catch-up growth gain (Gyllenhammar et al. 2018; Starling et al. 2019). Moreover, this catch-up weight gain appears to be excessive because the late-stage z-score in the low-rising WAZ trajectory was larger than that in the average trajectory. Because excessive catch-up growth is an established risk factor for obesity and metabolic disease in later life (Ibáñez et al. 2006; Kerkhof et al. 2012; Lobstein et al. 2004; Teller et al. 2018), our findings warrant the concern over the long-term health influence of prenatal PFAS exposure. In addition to WAZ, we found that high PFAS levels were also associated with the risk of high-rising LAZ and low-stable WAZ, suggesting a complex and heterogeneous relationship of PFAS with postnatal growth. Our results further support that growth trajectory modeling is particularly useful for allowing a close scrutiny of heterogeneity in growth patterns, whereas most previous studies focused on average growth pattern as a whole, which may have overlooked individual heterogeneity of growth patterns.
Unexpectedly, we found that in contrast to WAZ and LAZ, inverse associations were consistently observed with HCZ for high-stable, low-stable, and low-rising trajectories in relation to most individual PFAS congeners as well as PFAS mixture. It suggests that PFAS exposure might be related to increased odds for average development of HCZ, neither too high nor too low. A U-shaped relationship between PFAS exposure and HCZ high-stable trajectory was observed in our study, which implies divergent associations of different PFAS exposure levels. Head circumference is far less studied than weight in the context of PFAS exposures, and results were inconsistent among available studies. For example, studies from the Danish National Birth Cohort and Avon Longitudinal Study showed an inverse association between prenatal exposure to PFOA or PFOS and head circumference (Marks et al. 2019; Xiao et al. 2020), whereas studies from the Aarhus Birth Cohort, Shanghai Birth Cohort, and Hokkaido, Japan, showed no association with head circumference (Bach et al. 2016; Chen et al. 2021; Kashino et al. 2020). Another study in Zhoukou City, China, reported that PFHxS was positively associated with postnatal (mean 19 months) head circumference (Cao et al. 2018). Head circumference is believed to be related to cognitive and neurological development in children. As shown in a recent study from six sites worldwide, fetal cranial growth trajectories within a 20- to 25-wk gestational age window were associated with brain development at 2 y of age (Villar et al. 2021). In addition, macrocephaly and brain overgrowth have already been reported to be associated with autism spectrum disorder (ASD) (Sacco et al. 2015). Epidemiological evidence remains inconsistent regarding association between prenatal PFAS exposures and child ASD. For example, positive associations between prenatal PFAS and increased risk of child ASD were found in a high-risk ASD cohort (Oh et al. 2021) as well as in a population-based case–control study (Shin et al. 2020). However, several studies have also reported an inverse association (Braun et al. 2014; Long et al. 2019; Lyall et al. 2018), which might be related to the weak estrogenic activities and antiandrogenic activities of PFAS (Long et al. 2019) but also could be due to some unmeasured confounders (Lyall et al. 2018). Because prior studies suggest that high-stable head circumference trajectory may be an indicator for autism (Fukumoto et al. 2011; Sacco et al. 2015), our observations might partly explain the mixed findings in earlier studies evaluating the association between prenatal PFAS exposure and autism: PFAS might be inversely associated with autism at low levels but positively associated with autism at high levels. However, in view of the inconsistencies in the limited amount of available literature, we should not overinterpret the results. More research on this issue is needed.
In addition, we found potential differential effects between sex. It seems that boys might be more sensitive to PFAS exposures in our study, with more pronounced OR for the associations of PFAS with WAZ and WLZ trajectories than those reported for girls. Sex-specific effect estimates in fetal or childhood growth related to PFAS have also been reported in other studies, but the results were mixed. Some studies showed that prenatal PFAS concentrations were associated with lower birth weight in boys (Li et al. 2017; Manzano-Salgado et al. 2017), whereas others suggested that girls could be more vulnerable (Cao et al. 2018; Chen et al. 2019; Kashino et al. 2020; Kishi et al. 2015), and some reported no obvious sex differences (Bach et al. 2016; Gyllenhammar et al. 2018). A recent study also showed PFAS exposures were related to cholesterol metabolism with significant differences in sex (Blomberg et al. 2021). The modification effects by child sex should be considered in future studies.
To our knowledge, ours is one of the largest and most comprehensive studies to date on the potential growth effects of prenatal exposure to PFAS. We employed GBTM to characterize postnatal growth trajectories during the first 2 y of life, which provided a more complete characterization of growth trajectory in comparison with cumulative weight measures. Repeated measurement of weight, length/height and head circumference from birth to 2 y old using WHO standardized protocols is an important strength of our study, which is superior to most existing studies that relied on medical records. This study also benefited from the prospective study design and comprehensive collected covariates, including maternal age, maternal education, prepregnancy BMI, fish and seafood intake, parity, and pregnancy eGFR. In addition, with reliable biological measures of seven PFAS congeners, we estimated effect estimates of individual PFAS as well as joint estimates of PFAS mixtures, which may provide additional insight of overall findings to further confirm the single PFAS analyses and reduce the possibility of chance findings.
Several limitations of the present study should also be noted. First, potential selection bias might have been induced by the inclusion criterion that all participants should have all five measurements of one anthropometric measure. Nevertheless, we constructed inverse probability weights according to factors measured for all women in the SBC at baseline that may affect inclusion in each measurement, and there was no substantial difference in demographic characteristics between participants for different anthropometric measures, indicating selection bias was minimal. Second, despite including multiple confounders, residual confounding cannot be ruled out from other unmeasured or unknown confounders, such as environmental pollutants and lifestyle factors. Studies have reported that PFAS are only weakly correlated with other persistent chemicals (Fisher et al. 2016). Thus, potential confounding from other persistent environmental pollutants may be small. Third, it is difficult to assess the clinical relevance for the growth trajectories used as an outcome in this study. It is a complex and challenging endeavor to demonstrate the influence of growth trajectories on later diseases at this moment. Life-course studies combined with multiple approaches are required to unravel the complexity (Tu et al. 2013). Because we plan to follow these children for a long time, we will try to understand the clinical significance of these trajectories with health outcomes in the future. Finally, GBTM, as well as other trajectory analysis approaches, cannot perfectly delineate the trajectory for each individual, and thus misclassification cannot be ruled out. Other alternative trajectory modeling strategies are also encouraged to verify our results.
In conclusion, using trajectory analysis of repeated measurements of anthropometric metric during the first 2 y of life, we found that higher PFAS levels were associated with elevated odds for low-rising WAZ trajectory and high-rising LAZ trajectory. However, inverse associations were observed with HCZ for average development of HCZ trajectories, and a sex-specific effect might exist. GBTM, a trajectory analysis approach, provided insight into the complex effects of PFAS exposure on growth at different developmental stages. Follow-up of the SBC participants will help further characterize how prenatal exposures may impact late childhood growth trajectories. Future studies are warranted to confirm the present findings with trajectory modeling strategies.
Supplementary Material
Acknowledgments
The authors express their thanks to all the participants in the Shanghai Birth Cohort and the workers in the Ministry of Education – Shanghai Key Laboratory of Children’s Environmental Health in Xin Hua Hospital affiliated with Shanghai Jiao Tong University School of Medicine.
This work is supported by the National Natural Science Foundation of China (41991314, 81773387, 81872629), the Shanghai Municipal Health Commission (GWIII-26, GWIV-26, 2020CXJQ01), and the Shanghai Jiao Tong University School of Medicine, Xinhua Hospital, and the National Human Genetic Resources Sharing Service Platform (2005DKA21300).
References
- Aimuzi R, Luo K, Huang R, Huo X, Nian M, Ouyang F, et al. 2020. Perfluoroalkyl and polyfluroalkyl substances and maternal thyroid hormones in early pregnancy. Environ Pollut 264:114557, PMID: , 10.1016/j.envpol.2020.114557. [DOI] [PubMed] [Google Scholar]
- Alkhalawi E, Kasper-Sonnenberg M, Wilhelm M, Völkel W, Wittsiepe J. 2016. Perfluoroalkyl acids (PFAAs) and anthropometric measures in the first year of life: results from the Duisburg Birth Cohort. J Toxicol Environ Health A 79(22–23):1041–1049, PMID: , 10.1080/15287394.2016.1219552. [DOI] [PubMed] [Google Scholar]
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TI, Olsen J. 2010. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol 172(11):1230–1237, PMID: , 10.1093/aje/kwq289. [DOI] [PubMed] [Google Scholar]
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TI, Olsen J. 2013. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol 178(6):921–927, PMID: , 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bonefeld-Jørgensen EC, et al. 2016. Perfluoroalkyl acids in maternal serum and indices of fetal growth: the Aarhus Birth Cohort. Environ Health Perspect 124(6):848–854, PMID: , 10.1289/ehp.1510046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Darrow LA, Klein M, Winquist A, Steenland K. 2014. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res 132:62–69, PMID: , 10.1016/j.envres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Blomberg AJ, Shih YH, Messerlian C, Jørgensen LH, Weihe P, Grandjean P. 2021. Early-life associations between per- and polyfluoroalkyl substances and serum lipids in a longitudinal birth cohort. Environ Res 200:111400, PMID: , 10.1016/j.envres.2021.111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, et al. 2015. The Madrid statement on poly- and perfluoroalkyl substances (PFASs). Environ Health Perspect 123(5):A107–A111, PMID: , 10.1289/ehp.1509934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity (Silver Spring) 24(1):231–237, PMID: , 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 122(5):513–520, PMID: , 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Liu X, Liu X, Zhou Y, Zhang X, Tian H, et al. 2018. Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort. Environ Int 116:197–205, PMID: , 10.1016/j.envint.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120, PMID: , 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Ng S, Hsieh CJ, Lin CC, Hsieh WS, Chen PC. 2017. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci Total Environ 607–608:669–675, PMID: , 10.1016/j.scitotenv.2017.06.273. [DOI] [PubMed] [Google Scholar]
- Chen L, Tong C, Huo X, Zhang J, Tian Y. 2021. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: a longitudinal cohort with repeated measurements. Chemosphere 267:128899, PMID: , 10.1016/j.chemosphere.2020.128899. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang X, Zhao Y, Lu W, Wu J, Zhao S, et al. 2019. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: a prospective birth cohort study in Shanghai, China. Chemosphere 226:17–23, PMID: , 10.1016/j.chemosphere.2019.03.095. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Hassink SG, Committee on Nutrition. 2015. The role of the pediatrician in primary prevention of obesity. Pediatrics 136(1):e275-292–e292, PMID: , 10.1542/peds.2015-1558. [DOI] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (European Food Safety Authority Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, et al. 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):e06223, PMID: , 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect 127(1):17004, PMID: , 10.1289/EHP3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130:104874, PMID: , 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: , 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, et al. 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ Health 15(1):59, PMID: , 10.1186/s12940-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto A, Hashimoto T, Mori K, Tsuda Y, Arisawa K, Kagami S. 2011. Head circumference and body growth in autism spectrum disorders. Brain Dev 33(7):569–575, PMID: , 10.1016/j.braindev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Diderholm B, Gustafsson J, Berger U, Ridefelt P, Benskin JP, et al. 2018. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ Int 111:191–199, PMID: , 10.1016/j.envint.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Han W, Gao Y, Yao Q, Yuan T, Wang Y, Zhao S, et al. 2018. Perfluoroalkyl and polyfluoroalkyl substances in matched parental and cord serum in Shandong, China. Environ Int 116:206–213, PMID: , 10.1016/j.envint.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Härkänen T, Kaikkonen R, Virtala E, Koskinen S. 2014. Inverse probability weighting and doubly robust methods in correcting the effects of non-response in the reimbursed medication and self-reported turnout estimates in the ATH survey. BMC Public Health 14:1150, PMID: , 10.1186/1471-2458-14-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, et al. 2017. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obes 13(3):222–230, PMID: , 10.1089/chi.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herle M, Micali N, Abdulkadir M, Loos R, Bryant-Waugh R, Hübel C, et al. 2020. Identifying typical trajectories in longitudinal data: modeling strategies and interpretations. Eur J Epidemiol 35(3):205–222, PMID: , 10.1007/s10654-020-00615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez L, Ong K, Dunger DB, de Zegher F. 2006. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab 91(6):2153–2158, PMID: , 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- Jones BL, , Nagin DS, Roeder K. 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 29(3):374–393, 10.1177/0049124101029003005. [DOI] [Google Scholar]
- Kashino I, Sasaki S, Okada E, Matsuura H, Goudarzi H, Miyashita C, et al. 2020. Prenatal exposure to 11 perfluoroalkyl substances and fetal growth: a large-scale, prospective birth cohort study. Environ Int 136:105355, PMID: , 10.1016/j.envint.2019.105355. [DOI] [PubMed] [Google Scholar]
- Kerkhof GF, Willemsen RH, Leunissen RW, Breukhoven PE, Hokken-Koelega AC. 2012. Health profile of young adults born preterm: negative effects of rapid weight gain in early life. J Clin Endocrinol Metab 97(12):4498–4506, PMID: , 10.1210/jc.2012-1716. [DOI] [PubMed] [Google Scholar]
- Kim K, Bennett DH, Calafat AM, Hertz-Picciotto I, Shin HM. 2020. Temporal trends and determinants of serum concentrations of per- and polyfluoroalkyl substances among Northern California mothers with a young child, 2009–2016. Environ Res 186:109491, PMID: , 10.1016/j.envres.2020.109491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, Nakajima T, Goudarzi H, Kobayashi S, Sasaki S, Okada E, et al. 2015. The association of prenatal exposure to perfluorinated chemicals with maternal essential and long-chain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: the Hokkaido Study. Environ Health Perspect 123(10):1038–1045, PMID: , 10.1289/ehp.1408834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaux RM, Doherty BT, Gallagher LG, Zoeller RT, Hoofnagle AN, Calafat AM, et al. 2020. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Environ Res 185:109395, PMID: , 10.1016/j.envres.2020.109395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jung HW, Kim HY, Choi YJ, Lee YA. 2021. Early-life exposure to per- and poly-fluorinated alkyl substances and growth, adiposity, and puberty in children: a systematic review. Front Endocrinol (Lausanne) 12:683297, PMID: , 10.3389/fendo.2021.683297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zeng XW, Qian ZM, Vaughn MG, Sauvé S, Paul G, et al. 2017. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ Int 102:1–8, PMID: , 10.1016/j.envint.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: , 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bonefeld-Jørgensen EC, Henriksen TB, Nohr EA, Bech BH, et al. 2014. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am J Epidemiol 180(6):574–581, PMID: , 10.1093/aje/kwu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobstein T, Baur L, Uauy R. 2004. Obesity in children and young people: a crisis in public health. Obes Rev 5(suppl 1):4–104, PMID: , 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, et al. 2019. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case–control study. Mol Autism 10:1, PMID: , 10.1186/s13229-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. 2018. Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environ Health Perspect 126(1):017001, PMID: , 10.1289/EHP1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. 2012. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect 120(10):1432–1437, PMID: , 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, et al. 2017. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int 108:278–284, PMID: , 10.1016/j.envint.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K, Hartman TJ. 2019. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int J Hyg Environ Health 222(5):889–895, PMID: , 10.1016/j.ijheh.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. 2018. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health 15(9):1832, PMID: , 10.3390/ijerph15091832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS. 2014. Group-based trajectory modeling: an overview. Ann Nutr Metab 65(2–3):205–210, PMID: , 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Jones BL, Passos VL, Tremblay RE. 2018. Group-based multi-trajectory modeling. Stat Methods Med Res 27(7):2015–2023, PMID: , 10.1177/0962280216673085. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Odgers CL. 2010. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109–138, PMID: , 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. 2001. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods 6(1):18–34, PMID: , 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- NDRC (National Development and Reform Commission) People’s Republic of China. 2011. Industrial Reconstructing Guide Dictionary, 2011. http://www.gov.cn/flfg/2011-04/26/content_1852729.htm.
- Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, et al. 2021. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ Int 147:106328, PMID: , 10.1016/j.envint.2020.106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. 2000. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320(7240):967–971, PMID: , 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. 2006. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44, PMID: , 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Pryor LE, Tremblay RE, Boivin M, Touchette E, Dubois L, Genolini C, et al. 2011. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med 165(10):906–912, PMID: , 10.1001/archpediatrics.2011.153. [DOI] [PubMed] [Google Scholar]
- Regnault N, Gillman MW. 2014. Importance of characterizing growth trajectories. Ann Nutr Metab 65(2–3):110–113, PMID: , 10.1159/000365893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritscher A, Wang Z, Scheringer M, Boucher JM, Ahrens L, Berger U, et al. 2018. Zurich statement on future actions on per- and polyfluoroalkyl substances (PFASs). Environ Health Perspect 126(8):84502, PMID: , 10.1289/EHP4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, Persico AM. 2015. Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis. Psychiatry Res 234(2):239–251, PMID: , 10.1016/j.pscychresns.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Scheringer M, Trier X, Cousins IT, de Voogt P, Fletcher T, Wang Z, et al. 2014. Helsingør statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114:337–339, PMID: , 10.1016/j.chemosphere.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Calafat AM, Tancredi D, Hertz-Picciotto I. 2020. Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: a case-control study. Environ Res 186:109514, PMID: , 10.1016/j.envres.2020.109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. 2018. Prenatal exposure to perfluoroalkyl substances: infant birth weight and early life growth. Environ Epidemiol 2:, PMID: , 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. 2019. Trajectory analysis in obesity epidemiology: a promising life course approach. Curr Opin Endocr Metab Res 4:37–41, PMID: , 10.1016/j.coemr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MY, Zheng Y, Qi L, Hu FB, Chan AT, Giovannucci EL. 2018. Associations between genetic variants associated with body mass index and trajectories of body fatness across the life course: a longitudinal analysis. Int J Epidemiol 47(2):506–515, PMID: , 10.1093/ije/dyx255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Dabelea D. 2019. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: the healthy start study. Environ Int 131:104983, PMID: , 10.1016/j.envint.2019.104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Ye X, et al. 2017. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the Healthy Start study. Environ Health Perspect 125(6):067016, PMID: , 10.1289/EHP641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartout KM, Koss MP, White JW, Thompson MP, Abbey A, Bellis AL. 2015. Trajectory analysis of the campus serial rapist assumption. JAMA Pediatr 169(12):1148–1154, PMID: , 10.1001/jamapediatrics.2015.0707. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Bornehag CG, Gennings C. 2020. Dynamic growth metrics for examining prenatal exposure impacts on child growth trajectories: application to perfluorooctanoic acid (PFOA) and postnatal weight gain. Environ Res 182:109044, PMID: , 10.1016/j.envres.2019.109044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller IC, Hoyer-Kuhn H, Brönneke H, Nosthoff-Horstmann P, Oosting A, Lippach G, et al. 2018. Complex lipid globules in early-life nutrition improve long-term metabolic phenotype in intra-uterine growth-restricted rats. Br J Nutr 120(7):763–776, PMID: , 10.1017/S0007114518001988. [DOI] [PubMed] [Google Scholar]
- Tu YK, Tilling K, Sterne JAC, Gilthorpe MS. 2013. A critical evaluation of statistical approaches to examining the role of growth trajectories in the developmental origins of health and disease. Int J Epidemiol 42(5):1327–1339, PMID: , 10.1093/ije/dyt157. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2020. EPA PFAS Action Plan: Program Update. https://www.epa.gov/sites/production/files/2020-01/documents/pfas_action_plan_feb2020.pdf.
- UNEP (United Nations Environment Programme). 2009. Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the Work of its Fourth Meeting. http://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.4-38.English.pdf [accessed 2 March 2022]. [Google Scholar]
- UNEP. 2019. Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the work of its Ninth Meeting. http://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.9-30.English.pdf [accessed 2 March 2022]. [Google Scholar]
- Villar J, Gunier RB, Tshivuila-Matala COO, Rauch SA, Nosten F, Ochieng R, et al. 2021. Fetal cranial growth trajectories are associated with growth and neurodevelopment at 2 years of age: INTERBIO-21st Fetal Study. Nat Med 27(4):647–652, PMID: , 10.1038/s41591-021-01280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Chen Q, Shen L, Zhao S, Pang W, Zhang J. 2016. Perfluoroalkyl and polyfluoroalkyl substances in cord blood of newborns in Shanghai, China: implications for risk assessment. Environ Int 97:7–14, PMID: , 10.1016/j.envint.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Wang ZY, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: , 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Wang Y, Han W, Wang C, Zhou Y, Shi R, Bonefeld-Jørgensen EC, et al. 2019. Efficiency of maternal–fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environ Sci Pollut Res Int 26(3):2691–2698, PMID: , 10.1007/s11356-018-3686-3. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (World Health Organization Multicentre Growth Reference Study Group). 2006. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 450:76–85, PMID: , 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Xiao C, Grandjean P, Valvi D, Nielsen F, Jensen TK, Weihe P, et al. 2020. Associations of exposure to perfluoroalkyl substances with thyroid hormone concentrations and birth size. J Clin Endocrinol Metab 105:, PMID: , 10.1210/clinem/dgz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YC. 2010. Multiple Imputation for Missing Data: Concepts and New Development (version 9.0), vol. 40. Rockville, MD: SAS Institute Inc., 1–11. [Google Scholar]
- Zhang J, Tian Y, Wang W, Ouyang F, Xu J, Yu X, et al. 2019. Cohort profile: the Shanghai Birth Cohort. Int J Epidemiol 48(1):21–21g, PMID: , 10.1093/ije/dyy277. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Song M, Manson JE, Giovannucci EL, Hu FB. 2017. Group-based trajectory of body shape from ages 5 to 55 years and cardiometabolic disease risk in 2 US cohorts. Am J Epidemiol 186(11):1246–1255, PMID: , 10.1093/aje/kwx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


