Abstract
Calorie restriction (CR) (25–40%) is the most commonly studied strategy for curtailing age-related disease and has also been found to extend reproductive lifespan in female mice. However, the effects of mild CR (10%), which is sustainable, on ovarian aging has not yet been addressed. 17α-estradiol (17α-E2) is another intervention shown to positively modulate healthspan and lifespan in mice but its effects on female reproduction remain unclear. We evaluated the effects of mild CR (10%) and 17α-E2 treatment on ovarian reserve and female fertility over a 24-week period, and compared these effects with the more commonly employed 30% CR regimen. Both 10% and 30% CR elicited positive effects on the preservation of ovarian reserve, whereas 17α-E2 did not alter parameters associated with ovarian function. Following refeeding, both 10% and 30% increased fertility as evidenced by greater pregnancy rates. In aligned with the ovarian reserve data, 17α-E2 also failed to improve fertility. Collectively, these data indicate that 10% CR is effective in preserving ovarian function and fertility, while 17α-E2 does not appear to have therapeutic potential for delaying ovarian aging.
Keywords: Follicular progression, Menopause, Ovarian aging, Pro-longevity interventions
1. Introduction
Human life expectancy has increased significantly over the past several decades, primarily due to public health initiatives and improvements in western medicine capabilities (Christensen et al., 2009). An unanticipated consequence of extending human lifespan was the concomitant increase in chronic disease burden which has more recently been linked to multimorbidity and the onset of frailty syndrome (Lopez-Otin et al., 2013). In particular, postmenopausal females display an accelerated aging phenotype (Levine et al., 2016) and greater disease burden as compared to premenopausal females. Furthermore, an early onset of menopause has been linked to a reduction in life expectancy (Ossewaarde et al., 2005). These observations suggest a connection between gonadal and systemic aging processes, which has led to the search for interventional strategies that not only extend lifespan and compress the period of morbidity in mid-to-late life, but also curtail ovarian aging.
The most commonly studied strategy for extending lifespan and curtailing age-related disease is calorie restriction (CR). Preclinical studies employing CR have been found to extend lifespan and delay disease onset in a variety of species ranging from invertebrates to mammals (Fontana and Partridge, 2015; Masoro, 2005). The mechanisms underlying these observations are multifactorial, but have been linked to declines in growth signaling & pro-inflammatory stress and improvements in insulin sensitivity & nutrient-sensing plasticity. In addition to extending lifespan and delaying disease onset, CR has also been found to slow the ovarian aging process, thereby extending reproductive longevity (Schneider et al., 2021).
Female reproductive longevity is dictated by the ovarian reserve, which is determined by the number of quiescent primordial follicles in the ovary (te Velde et al., 1998). Ovarian reserve is finite, and once activated, primordial follicles enter an irreversible growth phase that results in either ovulation or atresia (Richardson et al., 2014). Therefore, a greater activation rate of primordial follicles will more rapidly result in the exhaustion of ovarian reserve which results in the onset of menopause (Broekmans et al., 2009). Commonly employed CR regimens (30–40%) have been shown to reduce primordial follicle activation and extend ovarian reserve in mice (Garcia et al., 2019; Xiang et al., 2012). The primary mechanism underlying these effects is believed to be the suppression of mammalian target of rapamycin (mTOR) activity (Li et al., 2015). CR has also been shown to decrease ROS production, increase SIRT3 activity, improve oocyte-specific meiotic spindle assembly & maintenance, while enhancing chromosome stability (Selesniemi et al., 2011).
Despite the fact that chronic CR improves ovarian reserve, ovulation and fertility are actually reduced during an active bout of CR due to declines in GnRH activity, which adversely affects cyclicity (Sun et al., 2021). However, the aforementioned improvements in primordial follicle number and oocyte quality promote greater fertility, fecundity, and offspring survival when refeeding periods follow bouts of chronic CR (Selesniemi et al., 2008). Although the benefits of CR on ovarian reserve and fertility outcomes while refeeding are well-established, human compliance when employing a robust CR regimen (25–30%) is poor (Flanagan et al., 2020), and tends to decrease over time. For example, in the first 6 months of the CALERIE 2 trial, in which the goal was 25% CR, the percentage of CR achieved dropped to only 9% after the first six months (Ravussin et al., 2015). Intriguingly, even this level of CR was capable of eliciting health benefits including weight loss and declines in metabolic risk factors. In rats, mild CR (10–20%) has also been shown to elicit benefits that are comparable to more stringent levels of CR (Richardson et al., 2016), despite there being a scarcity of data addressing the idea. However, no studies to date have evaluated the effects of mild CR (10%) on ovarian aging, which could represent a more viable intervention option for individuals attempting to delay ovarian failure and extend reproductive lifespan.
In addition to exploring the effects of mild CR on reproductive outcomes in these studies, we also evaluated the effects of 17α-estradiol (17α-E2); a natural-occurring diastereomer of 17β-estradiol (17β-E2) that has been shown to extend lifespan (Harrison et al., 2014; Strong et al., 2016) and elicit beneficial effects on metabolic and aging-related parameters in a manner similar to CR (Miller et al., 2020; Stout et al., 2017). We have also established that 17α-E2 suppresses calorie intake by modulating hypothalamic anorexigenic pathways in male mice (Steyn et al., 2018), which at least partially underlies the improvements in metabolic outcomes following 17α-E2 administration. We more recently reported that 17α-E2 reverses ovariectomy-mediated changes in adiposity and bone density in female mice (Mann et al., 2020b), indicating that specific populations of females may also benefit from 17α-E2 treatment. What remains unclear is if 17α-E2 can beneficially alter ovarian reserve or fertility in a manner similar to CR. We have previously shown that 17α-E2 treatment appears to have negligible effects on ovarian reserve in WT mice, although we did not evaluate fertility in that study (Isola et al., 2020). Based on the aforementioned results, the aim of this study was to evaluate the effects of a mild CR (10%) and 17α-E2 treatment on ovarian reserve and female fertility, while also comparing these interventions to a more standard CR regimen (30%).
2. Methodology
2.1. Animal diets
TestDiet, a division of Purina Mills (Richmond, IN), prepared the diets for these studies. We used TestDiet 58YP (66.4% CHO, 20.5% PRO, 13.1% FAT) ± 17α-E2 (14.4 ppm; Steraloids, Newport, RI).
2.2. Animals and experimental design
Ten- to eleven-week old female C57BL/6 mice (N = 72) were obtained from UFPel Central Vivarium and housed three per cage at 24 ± 0.5°C on a 12:12-hour light-dark cycle with ad libitum access to water throughout the study. Following a two-week facility acclimation, mice were randomly assigned into one of four groups: Control (CTL; n = 18) receiving ad libitum access to TestDiet 58YP; 17α-E2 (n = 18) receiving ad libitum access to TestDiet 58YP + 17α-E2; 10% calorie restriction (CR10; n = 18) receiving TestDiet 58YP; or 30% calorie restriction (CR30; n = 18) receiving TestDiet 58YP. The number of calories that the 10% and 30% CR groups received was determined by assessing calorie consumption in the CTL group, with 10% or 30% fewer calories being provided per day during the following week. Daily food rations were provided at noon each day for the calorie restricted groups. The experimental period was 24 weeks. Body mass was evaluated at baseline, week four, and every two weeks thereafter for the first 18 weeks of treatment. After 18 weeks of treatment, half of the females were prepared for mating. These females began ad libitum feeding at this point in preparation for mating, therefore body mass assessments were halted. All procedures were approved by the Animal Experimentation Ethics Committee from the Universidade Federal de Pelotas.
2.3. Embryo and ovary collections
Following 24 weeks of treatment, half of the mice in each group (n = 9) were IP injected with equine chorionic gonadotrophin (eCG; 5 IU) five days prior to euthanasia and human chorionic gonadotrophin (hCG; 5 IU) three days prior to euthanasia for synchronized superovulation. Immediately following the hCG injections, all females were paired with three-month old males and allowed to mate. Mice were fasted for 12 h and euthanized. After euthanasia, embryos were then collected by washing the uterine tubes with saline solution. Any female found to have at least one embryonic structure were considered responsive to the synchronization protocol. From those females that were fertilized the total number of embryos collected was recorded. Ovaries were also collected from these mice for the evaluation of ovarian reserve and gene expression.
2.4. Histological assessment of ovarian reserve
Ovaries were collected and processed as previously described (Isola et al., 2020). Nine sections (three from the distal end, three from the middle, three from the proximal end) from each ovary (n = 5/group) were evaluated for follicular density. Only follicles with clearly visible oocyte nuclei were counted. Follicles were classified as done previously (Isola et al., 2020) The total number of follicles from each classification were then divided by the total section area (mm2), thereby providing follicular density (follicles/mm2) (Ansere et al., 2021).
2.5. DNA damage by immunofluorescence
Immunofluorescence was performed to assess DNA damage in ovarian follicles as previously described (Saccon et al., 2020).
2.6. Ovarian gene expression
Total ovarian RNA was extracted and processed as previously described (Stout et al., 2011). B2m (Beta-2 microglobulin), Actb (Actin beta) and Ppia (Peptidylprolyl isomerase A) were evaluated as candidate housekeeper genes using the geNorm software (Vandesonpele et al., 2002). B2m was found to be the most stably expressed gene and used as the internal control. The target genes evaluated were Mtor (Mechanistic target of rapamycin kinase), Kit (Proto-oncogene receptor tyrosine kinase), Kitl (Kit ligand), Foxo3 (Forkhead box O3), Amh (Anti-Mullerian hormone) Igf1 (Insulin-like growth factor 1), Esr1 (Estrogen receptor alpha) and Esr2 (Estrogen receptor beta). Relative gene expression was calculated using the comparative CT method (Livak and Schmittgen, 2001). Primer sequences used in these analyses are shown in Table 1.
Table 1.
Primers used in the study.
| Gene | NCBI | Forward Primer Sequence | Reverse Primer Sequence | Amplicon Size (bp) |
|---|---|---|---|---|
|
| ||||
| Actb | NM_007393.5 | GAGACCTTCAACACCCCAGC | ATGTCACGCACGATTTCCC | 263 |
| B2m | NM_009735.3 | AAGTATACTCACGCCACCCA | CAGGCGTATGTATCAGTCTC | 217 |
| Ppia | NM_008907 | GAGCTGTTTGCAGACAAAGTTC | CCCTGGCACATGAATCCTGG | 125 |
| Mtor | NM_020009.2 | CGGCAACTTGACCATCCTCT | TGCTGGAAGGCGTCAATCTT | 101 |
| Kitl | NM_013598. | TTCGCACAGTGGCTGGTAAC | TTCACAGCGAAGCACTCTGC | 163 |
| Kit | NM_001122733.1 | CTCCCCCAACAGTGTATTCAC | TAGCCCGAAATCGCAAATCTT | 90 |
| Foxo3a | NM_001376967.1 | CGGCTCACTTTGTCCCAGAT | GCCGGATGGAGTTCTTCCA | 106 |
| Amh | NM_007445.3 | TCCTACATCTGGCTGAAGTGATATG | CAGGTGGAGGCTCTTGGAACT | 66 |
| Igf1 | NM_010512.5 | CTGAGCTGGTGGATGCTCTT | CACTCATCCACAATGCCT | 118 |
| Erα | NM_001302531.1 | CCTCCCGCCTTCTACAGGT | CACACGGCACAGTAGCGAG | 128 |
| Erβ | NM_010157.3 | CTGTGATGAACTACAGTGTTCCC | CACATTTGGGCTTGCAGTCTG | 80 |
2.7. Fertility assessment
Following 18 weeks of treatment, the other half of the females, those females not randomly selected to be subjected to superovulation (n = 9/group), were provided ad libitum access to their respective diets for 10 days in order to restore normal ovulatory patterns (Selesniemi et al., 2008). The group receiving 17α-E2 was kept under treatment for the whole period, including pregnancy. After the refeeding period, the females were paired with three-month old males and allowed to mate for a period of 14 days. One breeding cage in the 30% CR group had to be excluded due to death of the male. Visibly pregnant females were separated into individual cages. After birth, all pups were counted and weighed. The number of females that delivered pups were used to calculate the pregnancy rate.
2.8. Statistical analysis
Results are presented as mean ± SEM with p values less than 0.05 considered to be significant unless otherwise specified. Normal distribution of data was evaluated using the Shapiro-Wilk test. Differences between groups in body mass were evaluated using the mixed procedure repeated measures test in SAS (Cary, NC, USA). Follicular density was also compared using the mixed procedure test in SAS. Of note, different ovarian histological sections were accounted for in the model as within subject replicates. The remaining statistical analyses were performed using GraphPad Prism 8 software (La Jolla, CA, USA). Variables expressed as a percentage, including pregnancy rate and percent of females responsive to ovarian synchronization, were compared using the Chi square test. The other continuous variables were compared using one-way ANOVA followed by the Tukey post-hoc test.
3. Results
3.1. CR prevents gains in body mass more robustly than 17α-E2 in lean female mice
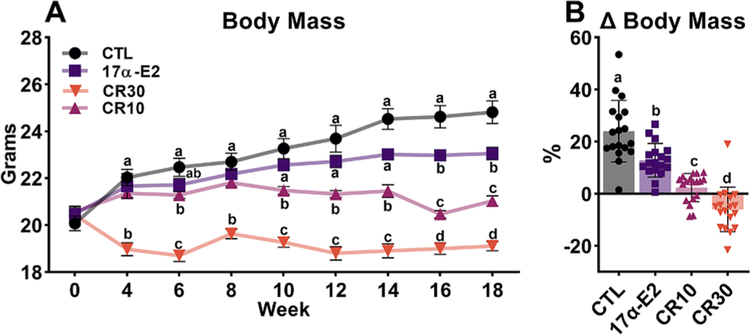
As expected, both regimens of CR (10% & 30%) reduced gains in body mass over the treatment period as compared to ad libitum fed CTL mice (Fig. 1A). 30% CR immediately affected body mass, whereas 10% CR only elicited effects after 6 weeks, and 17α-E2, interestingly did not cause body mass differences from the CTL until 16 weeks of treatment (Fig. 1A). As shown in Fig. 1B, the CTL mice increased their body mass by over 20% during the course of the study, whereas 17α-E2 and 10% CR groups gained approximately 13% and 2% respectively, and 30% CR females lost 6% of body mass during this period.
Fig. 1.
Calorie restriction (CR) and 17α-E2 differentially modulate body mass in female mice. (A) Body mass and (B) % change in body mass in female control (CTL) mice and those treated with 17α-E2 or CR regimens (10% or 30%) for 18 weeks starting at 12 weeks of age. All data are presented as mean ± SEM and were analyzed by repeated measures ANOVA or one-way ANOVA where appropriate. Different letters indicate statistical differences (p < 0.05) between groups (n = 18/group).
3.2. Mild CR positively affects ovarian reserve
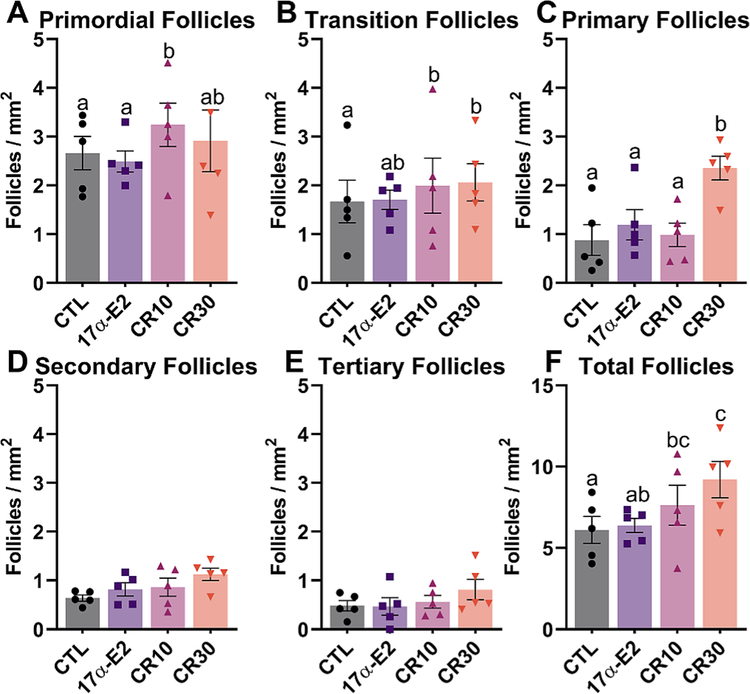
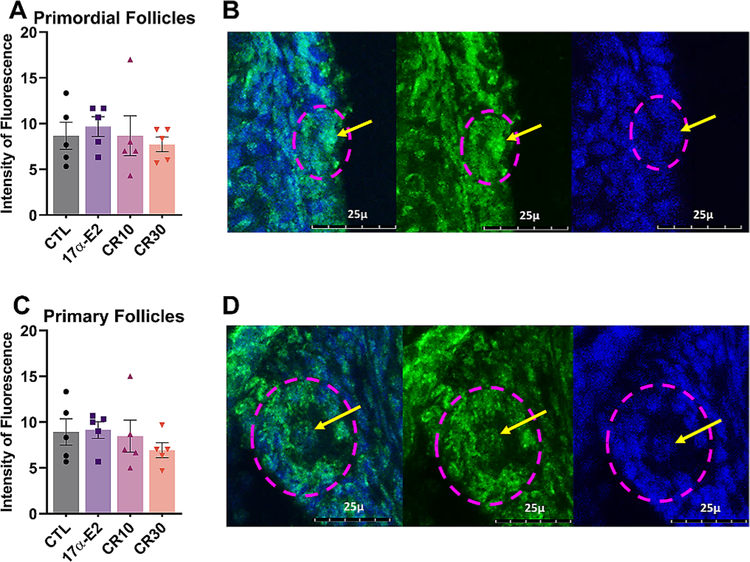
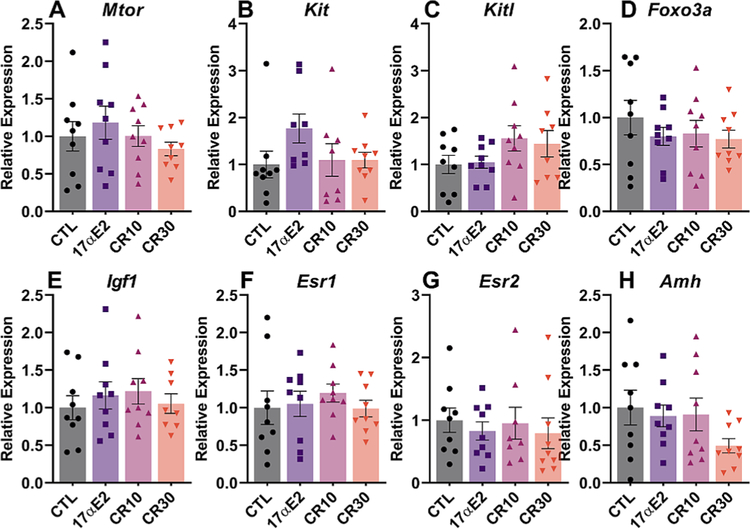
30% CR positively affected the ovarian reserve, as seen by the overall greater follicle density in this group. However, no statistical difference was observed for primordial follicles, as would be expected. This result can be due to the methodology used, which compares the number of follicles/mm2 and do not estimate the number of follicles in the ovary, which makes the discrepancy less obvious. Moreover, 10% CR had an effect on the number of both primordial and total follicles (Fig. 2). The overall number of follicles in the 17α-E2 group, however, was similar to the control group, suggesting that 17α-E2 does not affect the ovarian reserve. We also performed anti-γH2AX immunostaining, which is a marker of DNA damage and observed no difference between groups for both primordial and primary follicles (Fig. 3). We also assessed ovarian gene expression, and found no differences for genes involved on the pathway of follicle activation (Igf1, Mtor, Kit, Kitl, Foxo3), a fertility marker (Amh) or the estrogen receptors (Esr1, Esr2) (Fig. 4).
Fig. 2.
Both mild (10%) and moderate (30%) calorie restriction (CR) regimens positively affect ovarian reserve. Number of primordial (A), transition (B), primary (C), secondary (D), tertiary (E) and total follicles (F) per section area (mm2) from female control (CTL) mice and those treated with 17α-E2 or CR regimens (10% or 30%) for 24 weeks starting at 12 weeks of age (n = 9 sections/mouse; 5 mice/group). All data are presented as mean ± SEM and were analyzed using the mixed procedure test in SAS. Different letters indicate statistical differences (p < 0.05) between groups.
Fig. 3.
Calorie restriction (CR) and 17α-E2 fail to reduce DNA damage in the oocyte of primordial and primary follicles. Average fluorescence intensity of γH2AX immunostaining in oocytes from (A) primordial and (C) primary follicles from female control (CTL) mice and those treated with 17α-E2 or CR regimens (10% or 30%) for 24 weeks starting at 12 weeks of age. Representative images demonstrate γH2AX protein (green) and blue represent DAPI staining of genetic material in (B) primordial and (D) primary follicles. The dashed pink line represents the follicles being evaluated, whereas the line arrows point out the nuclei being evaluated. The images are stacked slices in the maximum projection mode, at a 400× magnification in a TCS SP8 CLSM Leica microscope. Values presented are averages of 3 follicles nuclei for each mouse (n = 5 mice/group). All data are presented as mean ± SEM and were analyzed by one-way ANOVA.
Fig. 4.
Calorie restriction (CR) and 17α-E2 did not alter the expression of genes related to fertility and follicle activation. Relative expression of (A) Mtor (Mechanistic target of rapamycin kinase), (B) Kit (Proto-oncogene receptor tyrosine kinase), Kitl (Kit ligand), Foxo3 (Forkhead box O3), Amh (Anti-Mullerian hormone), Igf1 (Insulin-like growth factor 1), Esr1 (Estrogen receptor alpha) and Esr2 (Estrogen receptor beta) in ovaries of female control (CTL) mice and those treated with 17α-E2 or CR regimens (10% or 30%) for 24 weeks starting at 12 weeks of age (n = 9/group). All data are presented as mean ± SEM and were analyzed by one-way ANOVA.
3.3. In the absence of refeeding, CR decreases responsiveness to ovulatory synchronization
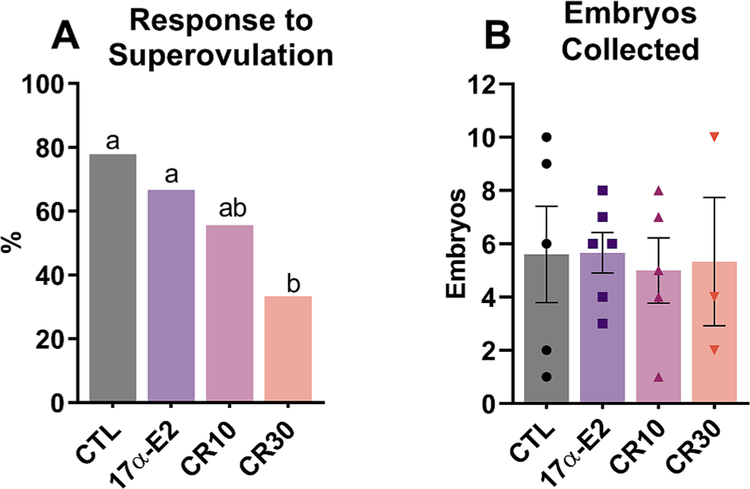
The 30% CR group had a poorer response to the superovulation protocol than other groups (33.33%), whereas the 10% CR group had an intermediate response (55.55%) and the CTL and 17α-E2 groups had a higher and similar response (77.77% and 66.66%, respectively) (Fig. 5A). Regarding the number of embryos recovered, no difference was observed among groups (p = 0.985) (Fig. 5).
Fig. 5.
Calorie restriction (CR) decreases % of females responsive to superovulation treatment, but does not affect the number of structures (oocytes/embryos) produced by those that respond. (A) Percentage of females that had at least one embryo or oocyte recovered by uterine flushing after ovulation induction and mating (n = 9/group) and (B) number of embryos produced by those that responded to the protocol and were fertilized (n = 5 CTL; 6 17α-E2; 5 CR10; 3 CR30) among female control (CTL) mice and those treated with 17α-E2 or CR regimens (10% or 30%) for 24 weeks, starting at 12 weeks of age. (A) data are presented in % and were analyzed by Chi-square (7/9 CTL; 7/9 17α-E2; 5/3 CR10; 3/9 CR30), (B) data are presented as mean ± SEM and were analyzed by one-way ANOVA. Different letters indicate statistical differences (p < 0.05) between groups.
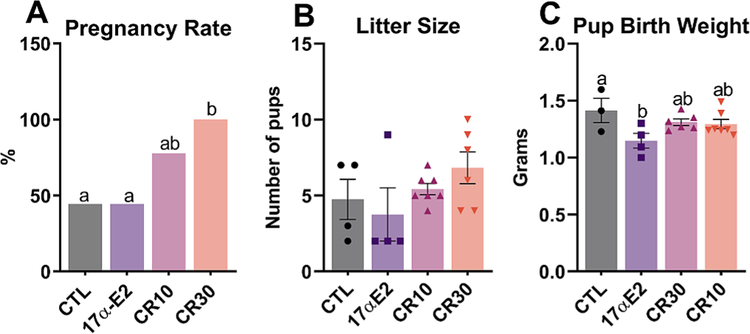
3.4. Both mild and moderate CR regimens increase pregnancy rates after refeeding
After 10 days of refeeding for the CR groups, we observed a pregnancy rate of 100% in females submitted to 30% CR, while groups CTL and 17α-E2, presented a lower rate (44.44%) and the group 10% CR presented an intermediate rate (77.77%) (Fig. 6). Litter size was also similar among groups. The weight of neonates in the 17α-E2 group, interestingly, was lower than that of the CTL group, although it was not different from the CR groups (Fig. 6).
Fig. 6.
After refeeding, calorie restriction (CR) increases pregnancy rate in female mice. Pregnancy rate (A), litter size (B), pup birth weight (C) from female control (CTL) mice and those treated with 17α-E2 for 24 weeks or CR regimens (10% or 30%) for 18 weeks starting at 12 weeks of age and then refed for 10 days before mating. A) data are presented in % and were analyzed by Chi-square (4/9 CTL; 4/9 17α-E2; 7/9 CR10; 6/6 CR30), (B & C) data are presented as mean ± SEM and were analyzed by one-way ANOVA. Different letters indicate statistical differences (p < 0.05) between groups.
4. Discussion
In the present study we evaluated the effects of CR (10% & 30%) and 17α-E2 treatment on ovarian reserve and female mice fertility. As a means of demonstrating tangible treatment effects, we assessed body mass for comparison purposes. As expected, 30% CR immediately prevented gains in body mass, whereas 10% CR required a longer duration of treatment before effects on body mass were observed. 17α-E2 treatment also mildly prevented gains in body mass but these effects were less pronounced when compared to the two CR regimens. Our findings support previous reports demonstrating minimal, if any, effects on body mass in wild-type females (Garratt et al., 2019; Mann et al., 2020a; Mann et al., 2020b; Sidhom et al., 2021). In fact, 17α-E2 seems to fail eliciting beneficial effects in intact females. Conversely, 17α-E2 has been found to prevent gains in body mass and adiposity, prevent bone loss, and alter the metabolome in ovariectomized mice (Garratt et al., 2018; Mann et al., 2020b), which suggests that the presence of functional ovaries and/or their endocrine factors may attenuate 17α-E2 actions in females. However, previous studies from our group have shown that life-extending strategies such as GH deficiency (Saccon et al., 2017), 30% CR (Garcia et al., 2019) and rapamycin (Garcia et al., 2019) also result in improved ovarian reserve. Therefore, we hypothesized that 17α-E2 could have some beneficial effect in the reproductive axis or even a negative effect that would counterbalance its positive endocrine effects in females.
With regard to ovarian reserve, we observed an overall trend for a greater number of follicles in both CR treatment groups as evidenced by more primordial and transition follicles in the 10% CR group and more transition and primary follicles in the 30% CR group. In addition, the 30% CR group had slightly higher total follicle density than the 10% CR group, suggesting an incremental effect. Several previous studies have found that between 3 and 6 months of 30% CR increases the ovarian reserve (Garcia et al., 2019; Li et al., 2015; Xiang et al., 2012; Liu et al., 2015). In the current study we did not find statistical differences in the number of primordial follicles between 30% CR and the CTL group, although we did observe more primary follicles in this group. This discrepancy could be due to differences in methodological approaches for quantifying primordial follicles. Most previous reports provide an estimation of the total number of follicles in the ovaries, whereas we report the number of follicles in a given area of histological sections. Potential differences are less obvious using our methodology. However, overall, both 10 and 30% CR improved primordial/transition/primary follicle density compared to the control group, reflecting in increased total follicle density, in line with previous evidence. Of note, 17α-E2 did not alter ovarian reserve in this study, indicating its overall endocrine effect is not sufficient to affect folliculogenesis.
Interestingly, 10% CR appears to have elicited protective effects on ovarian reserve similarly to those seen in the 30% CR group, despite much milder effects on body mass. This observation is aligned with previous reports demonstrating that mild CR in preclinical studies can positively affect age-related parameters associated with disease burden (Richardson et al., 2016; Duffy et al., 2001). We perceive this to be an important observation because human compliance to more stringent CR regimens is poor (Ravussin et al., 2015). In fact, most humans only achieve a small percentage of their prescribed energy deficit (Flanagan et al., 2020), indicating that milder CR regimens may serve as an effective alternative. In addition to evaluating ovarian reserve, we also assessed fertility in this study. CR has previously been reported to modulate female fertility (Sun et al., 2021). During the period when CR in employed, cyclicity is impaired due to CR-mediated effects on the hypothalamus-pituitary-ovarian axis due to changes in gonadotrophin pulsatility (Sun et al., 2021). However, female fertility is significantly enhanced during refeeding periods that follow bouts of CR (Selesniemi et al., 2008; Sluczanowska-Glabowska et al., 2015). In the current study, females that were used for embryo collection were not subjected to refeeding prior to initiating the superovulation protocol because it provided additional confirmation that our CR regimens were indeed modulating cyclicity. As expected, the 30% CR group displayed a significant reduction in ovulation, in alignment with other reports in the literature (Liu et al., 2015). Interestingly, 10% CR also reduced ovulation to a lesser extent, although this finding did not rise to the level of statistical significance. Nevertheless, despite fewer females responding to superovulation treatment in the CR groups, the mice that did respond produced similar numbers of structures (oocytes/embryos) as those mice in the CTL and 17α-E2 treatment groups. Response to synchronization and embryo numbers were also not affected by 17α-E2, further indicating its lack of effect in the reproductive axis.
In addition to the experiments outlined above, we also evaluated the effects of CR regimens on fertility in mice that were subjected to a tenday ad libitum refeeding period. In alignment with previous reports (Selesniemi et al., 2008, Sluczanowska-Glabowska et al., 2015) we found that both regimens of CR increased pregnancy rates, although the changes in the 10% CR group did not reach statistical significance. Litter sizes and pup birth weights were not affected by either CR regimen following the refeeding period, suggesting that lower body masses during pregnancy did not adversely affect the ability of females to carry and deliver multiple pups. Females provided 17α-E2 did not display any changes in pregnancy rates, although pup birth weights were found to be lower, which has been associated with poor health outcomes (Beauchamp et al., 2015). Future studies will be needed to determine if 17α-E2 treatment during pregnancy adversely affects pup morbidity during maturation and adulthood. Despite the fact that CR clearly improved ovarian reserve and fertility in our studies, the underlying mechanism(s) promoting these benefits remain unclear. Previous studies have linked the protective effects of CR to declines in ovarian DNA damage and oxidative stress (Luo et al., 2017), which certainly contribute to improvements in follicle number and quality (Prasad et al., 2016; Selesniemi et al., 2011). However, we did not observe difference in primordial or primary oocyte DNA damage, or any transcriptional changes in genes associated with follicle activation or fertility. Future studies will need to explore alternative mechanisms by which mild CR may be modulating ovarian reserve, including its effects on mTOR activity. The suppression of mTOR activity in the ovary decreases primordial follicle activation, thereby extending ovarian reserve (Li et al., 2015). This has been linked to declines in FOXO3a phosphorylation, which is the primary signal for the activation of dormant primordial follicles (Saccon et al., 2017; Castrillon et al., 2003). To date, it remains unclear if mild CR regimens also modulate mTOR and FOXO3a activity in the ovary.
There are a few notable limitations to our studies. First, the methodology used to assess follicle reserve consists of counting of follicles/mm2 on histological sections, which often times leads to less obvious differences between groups as compared to estimating the overall number of follicles in the ovary. Despite not being the best methodology for comparing different treatments in age matched mice, our approach provides an exact quantification of different follicle types within the sections being evaluated. Secondly, the females being evaluated in these studies for changes in DNA damage responses were relatively young (9 months old), which may explain why we did not observe a beneficial effect of CR. Also, transcriptional analyses were done in whole ovarian extracts, therefore it prevents us from making any conclusions related to cell-type-specific mechanisms of ovarian aging. Lastly, the fertility assessments were not similar for all groups, since CR was withdrawn and 17α-E2 was maintained prior to mating and during pregnancy, explaining the higher pregnancy in CR groups. Also, we did not measure endocrine markers during estrous cycle and pregnancy. However, CR is known to impair the hypothalamus-pituitary-ovarian axis and the ovulation rate and estradiol/progesterone levels (Sun et al., 2021). However, the results suggest that 17α-E2 does not have deleterious effects on the reproductive axis, as the fertility was similar to the control group. Despite these limitations, this is the first report to our knowledge that demonstrates that mild CR can prolong ovarian reserve and increase fertility in female mice.
Acknowledgements and funding
This work was supported by CAPES (J.V.V.I.), CNPq and FAPERGS (A.S.) and the National Institutes of Health [R01 AG069742 to M.B.S.].
Footnotes
Declaration of competing interest
The authors declare no conflicts or competing interests.
References
- Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, Henseb JD, Saccon TD, Ocanas SR, Tooley KB, Stout MB, Schneider A, Freeman WM, 2021. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech. Ageing Dev. 194, 111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp B, Ghosh S, Dysart MW, Kanaan GN, Chu A, Blais A, Rajamanickam K, Tsai EC, Patti ME, Harper ME, 2015. Low birth weight is associated with adiposity, impaired skeletal muscle energetics and weight loss resistance in mice. Int. J. Obes. 39, 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC, 2009. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 30, 465–493. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, Depinho RA, 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301, 215–218. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW, 2009. Ageing populations: the challenges ahead. Lancet 374, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PH, Seng JE, Lewis SM, Mayhugh MA, Aidoo A, Hattan DG, Casciano DA, Feuers RJ, 2001. The effects of different levels of dietary restriction on aging and survival in the Sprague-Dawley rat: implications for chronic studies. Aging (Milano) 13, 263–272. [DOI] [PubMed] [Google Scholar]
- Flanagan EW, Most J, Mey JT, Redman LM, 2020. Calorie restriction and aging in humans. Annu. Rev. Nutr. 40, 105–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DN, Saccon TD, Pradiee J, Rincon JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB, Schneider A, 2019. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience 41, 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA, 2018. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell 17, e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M, Leander D, Pifer K, Bower B, Herrera JJ, Day SM, Fiehn O, Brooks SV, Miller RA, 2019. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell 18, e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA, 2014. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola JVV, Zanini BM, Sidhom S, Kopchick JJ, Bartke A, Masternak MM, Stout MB, Schneider A, 2020. 17alpha-estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp. Gerontol. 129, 110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, Bandinelli S, Salfati E, Manson JE, Quach A, Kusters CD, Kuh D, Wong A, Teschendorff AE, Widschwendter M, Ritz BR, Absher D, Assimes TL, Horvath S, 2016. Menopause accelerates biological aging. Proc. Natl. Acad. Sci. U. S. A. 113, 9327–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL, 2015. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod. Sci. 22, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Zhang XM, Wang N, Zhou XL, Fu YC, Luo LL, 2015. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. Eur. J. Med. Res. 20, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Chiang HH, Louw M, Susanto A, Chen D, 2017. Nutrient sensing and the oxidative stress response. Trends Endocrinol. Metab. 28, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann SN, Hadad N, Nelson Holte M, Rothman AR, Sathiaseelan R, Ali Mondal S, Agbaga MP, Unnikrishnan A, Subramaniam M, Hawse J, Huffman DM, Freeman WM, Stout MB, 2020. Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann SN, Pitel KS, Nelson-Holte MH, Iwaniec UT, Turner RT, Sathiaseelan R, Kirkland JL, Schneider A, Morris KT, Malayannan S, Hawse JR, Stout MB, 2020b. 17alpha-estradiol prevents ovariectomy-mediated obesity and bone loss. Exp. Gerontol. 142, 111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ, 2005. Overview of caloric restriction and ageing. Mech. Ageing Dev. 126, 913–922. [DOI] [PubMed] [Google Scholar]
- Miller BF, Pharaoh GA, Hamilton KL, Peelor FF, Kirkland JL, Freeman WM, Mann SN, Kinter M, Price JC, Stout MB, 2020. Short-term calorie restriction and 17alpha-estradiol administration elicit divergent effects on proteostatic processes and protein content in metabolically active tissues. J. Gerontol. A Biol. Sci. Med. Sci. 75, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT, 2005. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 16, 556–562. [DOI] [PubMed] [Google Scholar]
- Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK, 2016. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 23, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB, Group, C. S., 2015. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MC, Guo M, Fauser BC, Macklon NS, 2014. Environmental and developmental origins of ovarian reserve. Hum. Reprod. Update 20, 353–369. [DOI] [PubMed] [Google Scholar]
- Richardson A, Austad SN, Ikeno Y, Unnikrishnan A, McCarter RJ, 2016. Significant life extension by ten percent dietary restriction. Ann. N. Y. Acad. Sci. 1363, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccon TD, Moreira F, Cruz LA, Mondadori RG, Fang Y, Barros CC, Spinel L, Bartke A, Masternak MM, Schneider A, 2017. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol. Cell. Endocrinol. 455, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccon TD, Rovani MT, Garcia DN, Mondadori RG, Cruz LAX, Barros CC, Bartke A, Masternak MM, Schneider A, 2020. Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice. Exp. Gerontol. 132, 110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Saccon TD, Garcia DN, Zanini BM, Isola JVV, Hense JD, Alvarado-Rincon JA, Cavalcante MB, Mason JB, Stout MB, Bartke A, Masternak MM, 2021. The interconnections between somatic and ovarian aging in murine models. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL, 2008. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 7, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL, 2011. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. U. S. A. 108, 12319–12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhom S, Schneider A, Fang Y, McFadden S, Darcy J, Sathiaseelan R, Palmer AK, Steyn FJ, Grillari J, Kopchick JJ, Bartke A, Siddiqi S, Masternak MM, Stout MB, 2021. 17alpha-estradiol modulates IGF1 and hepatic gene expression in a sex-specific manner. J. Gerontol. A Biol. Sci. Med. Sci. 76, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Grabowska M, Grymula K, Ratajczak MZ, 2015. Caloric restriction increases ratio of estrogen to androgen receptors expression in murine ovaries–potential therapeutic implications. J. Ovarian Res. 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn FJ, Ngo ST, Chen VP, Bailey-Downs LC, Xie TY, Ghadami M, Brimijoin S, Freeman WM, Rubinstein M, Low MJ, Stout MB, 2018. 17alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Liu LF, Belury MA, 2011. Hepatic steatosis by dietary-conjugated linoleic acid is accompanied by accumulation of diacylglycerol and increased membrane-associated protein kinase C epsilon in mice. Mol. Nutr. Food Res. 55, 1010–1017. [DOI] [PubMed] [Google Scholar]
- Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, De Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, Lebrasseur NK, Von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL, 2017. 17alpha-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J. Gerontol. A Biol. Sci. Med. Sci. 72, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhaes JP, Martinez PA, McCord JM, Miller BF, Muller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE, 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Shen X, Liu H, Lu S, Peng J, Kuang H, 2021. Caloric restriction in female reproduction: is it beneficial or detrimental? Reprod. Biol. Endocrinol. 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC, 1998. Developmental and endocrine aspects of normal ovarian aging. Mol. Cell. Endocrinol. 145, 67–73. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 18 3 (7). RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Xu J, Li L, Lin X, Chen X, Zhang X, Fu Y, Luo L, 2012. Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene 493, 77–82. [DOI] [PubMed] [Google Scholar]