ABSTRACT
Active immunization in pregnancy is recommended for the influenza and the tetanus, diphtheria, and acellular pertussis (Tdap) vaccines. Evidence indicates vaccine effectiveness in preventing influenza-related hospitalizations and pertussis in early infancy. We investigate vaccine uptake in pregnant and non-pregnant women through a sample of young women and consultant gynecologists, along with the potential predisposing and/or enabling factors affecting attitudes to vaccination (knowledge, beliefs, barriers). A cross-sectional study was conducted between June and September 2019, with a sample of 251 women and 14 consultant gynecologists at the Local Health Authority (ASL01) of the Abruzzo Region (Italy), using an anonymous, self-report questionnaire survey. Among the participants, 5.6% of women had received influenza vaccination, 16.4% had received Tdap during pregnancy and only 1.2% had received both vaccines. The assessment of the psychometric attitudinal variables has suggested a more positive willingness to receive Tdap than influenza vaccine among women, as the former is considered more important for the maternal and neonatal health. Health care workers have reported vaccine safety concerns, lack of information, and misconceptions about the need for vaccination as barriers to immunization in pregnant women. The results of this study will contribute to defining the goals and strategies to increase vaccine uptake under the current recommendations, through promoting effective training programs for all health care workers involved (gynecologists, obstetricians, public health physicians).
KEYWORDS: Vaccinations, pregnancy, attitudes, influenza vaccine, Tdap
Introduction
Pregnancy is a remarkable moment for the female body that has to adjust to the presence of a new organism. Latent changes occur, such as a maternal immune system modulation that is necessary to tolerate the coexistence of two semi-allogeneic individuals, mediated by female hormones. High levels of estradiol induce Th2 responses and humoral (antibody-mediated) immunity, whereas progesterone is able to shift the Th1/Th2 balance toward the Th2 response.1,2 Such shift allows the maternal-fetal tolerance during pregnancy but, at the same time, the suppression of cell-mediated immunity may contribute to higher susceptibility to infections, with increased severity of certain infectious diseases during pregnancy.3,4 Thus, pregnant women, infants, older people, and subjects with chronic health conditions are at increased risk for severe illness from infection. Active immunization in pregnancy for some vaccines is not currently recommended (MMR, IPV) or should be used with caution (HBV, HPV); conversely, it is recommended for the seasonal influenza and Tdap (tetanus-diphtheria-acellular pertussis) vaccines because, due to their composition, they are safe and effective in preventing specific infectious diseases and complications in the maternal and fetal health. Influenza in pregnancy may cause acute cardiopulmonary hospitalizations,5,6 and it increases the risk of stillbirth, neonatal death, preterm birth, low birth weight,5,7–9 and congenital cardiopathy.10
During the 2018–2019 influenza season, 812 serious cases were reported in Italy, of which 8 (1%) pregnant women were admitted to intensive care units.11 Studies demonstrated the effectiveness of maternal influenza vaccination in preventing influenza-associated hospitalizations of infants by 81%, and the risk of infant influenza by 61% in their first 6 months12 with a reduction of pneumonia cases.13
Efficacy of maternal influenza immunization is confirmed by antibodies titers in pregnant women, which demonstrate no diminution of immunogenicity and the maintenance of immune memory response.14–16 Furthermore, several studies in the literature found no increased risk for congenital abnormalities nor other adverse events for the fetus.17,18
Pertussis, infectious and contagious diseases caused by Bordetella pertussis, may affect any age group. However, the disease is most dangerous in infants below 1 year of age, and in particular those under 3 months. Pneumonia, bronchiolitis, otitis, respiratory failure, apnea may occur in the infant.19 In 2017, 42,242 cases of pertussis were reported in Europe. The most affected age group was children aged less than 1 year, of which 70% were below 6 months of age. In the same year, 964 cases were reported in Italy.20 The Italian epidemiological data on pertussis are recognized to be under-diagnosed and under-reported, indeed the surveys conducted by the Italian National Health Institute (Istituto Superiore di Sanità) show higher incidence rates of the B. pertussis among adults (20–29 y and >60 y) than in the pediatric population. Conversely, an analysis of the hospitalizations over the period 2002–2016 showed that 63.39% of the pertussis cases were registered among children aged less than 1 year.21 Vaccination against pertussis in pediatric patients starts at 3 months, according to the National Immunization Prevention Plan (PNPV).22 Thus, infants in the first weeks of life are most vulnerable to B.pertussis infection and complications. Vaccination in the third trimester of pregnancy, along with the natural postnatal transmission of passive immunity, is an effective strategy to reduce any risk. Moreover, fathers and other household contacts are frequently identified as the main source of infection among infants,23 therefore Tdap vaccination is recommended also to all those who will be in close contact with the infant (‘cocoon strategy’). Safety of Tdap vaccination, both for the mother and the infant, is well documented24–26 as well as the absence of any interference between passive immunization (maternal antibodies) and efficacy of active immunoprophylaxis in infants.25
In Italy, influenza vaccination is recommended at the beginning of the winter season for all women in the second and third trimesters of pregnancy, under the Circular of 21/11/2018 issued by the Italian Ministry of Health concerning the “Immunizations recommended for women of reproductive age and in pregnancy” (ITALIAN MINISTRY OF HEALTH).27 On the other hand, the Tdap vaccine is recommended between 27 and 36 weeks of gestation, preferably at week 28, when the transfer of maternal protective immunoglobulin antibodies to the fetus is more likely to occur. Despite the law provisions and the public awareness campaigns by the local health authorities, vaccination coverages in pregnant women remain below the recommended levels for influenza in Europe,28,29 as well as for Tdap in Europe (20) and USA.30
The study aim is to describe the active immunization in pregnancy and the potential predisposing and/or enabling factors. In particular: 1) to assess the percentage of vaccinated women or willing to receive the influenza and Tdap vaccines during pregnancy; 2) to identify the level of knowledge about vaccination and the attitudinal variables influencing compliance with institutional recommendations (i.e., the National Immunization Prevention Plan) both among the target women and the consultant physicians (gynecologists). The findings of this study will allow the definition of the goals and strategies to promote and improve vaccine uptake under the current recommendations, starting from extensive training programs for all the health care workers involved (gynecologists, obstetricians, public health doctors).
Materials and methods
Between June–September 2019, a cross-sectional survey was conducted on a sample of 300 women attending the immunization department at the Local Health Authority ASL01 in the Abruzzo Region (Italy). Eligibility criteria included: mothers of children with age lower than 1 year and/or no more than 27 weeks pregnant women, able to read and write Italian. A questionnaire, already used in related studies,31 was firstly adapted into the Italian language, divided into three sections (eliciting general data, knowledge, and attitudes about seasonal influenza vaccination, knowledge and attitudes about Tdap vaccination). It was administered in writing and self-filled in by interview. Women were approached and screened for eligibility by trained health care workers. Signed informed consent was obtained from all eligible women interested in participating. Questionnaires were self-reported and anonymous. From literature data, the prevalence of women who underwent influenza vaccination during pregnancy was variable from none to a value next to 10%.10,32–34 On the basis of these values, it was estimated that already with a sample size of 85 women interviewed, a study power greater than 80.0% would be reached (with an alpha error of 0.05), and with a sample of 100 women a power of more than 90.0%. Therefore, the minimum sample size is estimated to be between 85 and 100 women.
In the same period, 30 consultant gynecologists working in the same Local Health Authority (ASL 01) were recruited and administered an adapted questionnaire with the same format, items, and method of administration. The questionnaire included multiple-choice closed-ended questions, open-ended questions with short responses, and 1- to 4-point Likert-type scales (1 = “strongly disagree”, 4 = “strongly agree”) for the psychometric assessment of the attitudinal variables.
The software STATA/SE 14.2 was used to perform all data analyses. The frequency for the categorical variable was measured and tests were conducted to analyze the statistical significance of the difference between groups (difference of proportions test) and to determine the association with correlates, for example, occupational status (Chi-squared test, Fisher’s Exact Test) and between the vaccination status against the two preventable infectious diseases in the same woman (McNemar’s test for paired data). Measures of central tendency and of variability were used for the Likert scale points treated as ordinals (arithmetic mean, standard deviation, min–max interval variability). The statistical significance of the differences was assessed using the Wilcoxon non-parametric test for paired data, by comparing different psychometric variables assessed in the form of interval scale on the same woman.
Results
Overall, 83.7% (251 out of 300) of the questionnaires administered to the women and 46.7% (14 out of 30) of those administered to the gynecologists were returned and considered usable for the study.
The sample consisted of 87.7% women of Italian nationality (220 out of 251), 72.5% (182) were aged older than 31, and 93.6% (235) had a medium-high level of education. The percentage of women employed was 61.4% (154), in particular, 9.2% (23) worked in the health care With regard to the family status, 56.6% (142) were married, 47.8% (120) had no prior births, 38.2% (96) had only one child, and 12.74% (32) had more than one child (Table 1).
Table 1.
Socio-demographic characteristics of the women surveyed
| Characteristics | No. (total 251) |
% |
|---|---|---|
| Age (years) <30 31–35 >35 Unknown |
67 85 97 2 |
26.7% 33.9% 38.6% 0.8% |
| Nationality Italian Foreign |
220 31 |
87.7% 12.3% |
| Level of education Low Medium High |
16 116 119 |
6.4% 46.2% 47.4% |
| Occupation Employed Unemployed Health care professional Unknown |
131 73 23 24 |
52.2% 29.1% 9.2% 9.5% |
| Marital status Married Other Unknown |
142 106 3 |
56.6% 42.23% 1.2% |
| No. of children None One More than one Unknown |
120 96 32 3 |
47.9% 38.2% 12.7% 1.2% |
Separately for influenza and Tdap, we calculated the proportions of women that never received vaccination in their life (tagged “Never vaccinated”) and of women that received the vaccination but not during the current or previous pregnancy (tagged “Vaccinated Not in the pregnancy”). Moreover, in women that received the two vaccinations during pregnancy, we distinguished these three conditions: “Vaccinated ‘only’ against Influenza”, “Vaccinated ‘only’ Tdap” and “Vaccinated against Influenza ‘and’ Tdap”.
Among the women surveyed, 81.3% (204 out of 251) reported never being vaccinated against influenza, and 5.6% (14) reported being vaccinated during current or previous pregnancy (Figure 1). In the sub-sample of the women working in health care only 8.7% received the annual influenza vaccine and none reported being vaccinated during pregnancy.
Figure 1.
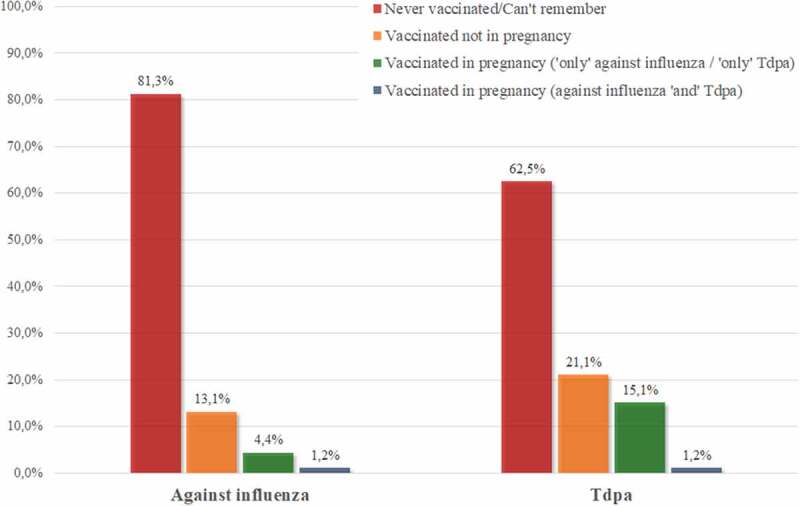
Vaccination status of pregnant women for the seasonal influenza and Tdap vaccines (frequency related to the denominator equal to 251 surveyed women).
Women who reported having influenza during their pregnancy accounted for 10.4% (26) (2 cases had influenza despite vaccination in pregnancy). This percentage is higher among health care workers, at 17.9% (4 out of 23), though the result is not statistically significant (p = .2749).
Among the whole sample, 62.5% (157) never received the Tdap vaccine (34.8% of health care workers, 8 out of 23), whereas 16.4% (41) were vaccinated during pregnancy (13.0% of health care workers, 3 out of 23) (Figure 1), and 32.3% (81) reported lack of knowledge and awareness of vaccination (8.7% of health care workers, 2 out of 23).
Only 1.2% of the sample (3 out of 251) reported receiving both vaccines during pregnancy (Figure 1). The McNemar’s test showed that there is no association between being vaccinated against seasonal influenza and the Tdap because the statistical significance excludes the homogeneity of the distribution of paired data (McNemar’s test = 14.88, p < .001).
Table 2 reports the frequency of the possible reasons for not being vaccinated and the statistical significance of the differences between the responses regarding influenza and Tdap vaccines. The most commonly reported reason for not receiving vaccination was “lack of knowledge regarding the possibility of receiving vaccination” for both vaccines, with higher values for the Tdap (48.9%) vs the influenza vaccine (69.5%, p < .001). Other most cited reasons, with increased frequency for influenza vaccine vs Tdap, were the belief that vaccination “is not necessary” (28.3% vs 10.5%, p < .001); “no time” (8.0% vs 2.9%, p < .05); and “concerns about vaccination safety” (5.1% vs 2.4%, p = .1378). ‘Cost’ was never reported as a barrier to receiving both vaccines.
Table 2.
Reasons reported for not receiving influenza and Tdap vaccinations: frequency of adherence to responses and significance level in the test for the difference in the proportions
| Influenza (total 237)a |
Tdap (total 210)a |
Statistical significance | |
|---|---|---|---|
| I did not know about the possibility of receiving vaccination | 48.9% (116) | 69.5% (146) | p < .001 |
| I do not think vaccination is necessary | 28.3% (67) | 10.5% (22) | p < .001 |
| No time | 8.0% (19) | 2.9% (6) | p < .05 |
| I doubt vaccination safety | 5.1% (12) | 2.4% (5) | n.s. |
| I am concerned about vaccination costs | 0.0% | 0.0% | - |
aThe column totals are different between the two vaccination questions due to missing data.
A stratified analysis to assess barriers to vaccination by the occupation of young women interviewed (Table 3) highlighted significant differences between health care workers and women working in other fields. The ‘no time’ reason for not receiving influenza vaccine is more frequently reported by health care workers (21.7% vs 6.1%, p < .01) vs the Tdap (10.0% vs 2.1%, p < .05) vaccine. The ‘I did not know about the possibility of receiving vaccination’ reason for the Tdap vaccine is less frequently cited by health care workers, although it is nearly close to half of this stratum (45.0% vs 71.6%, p < .05). There are no significant differences in the barriers to accepting vaccination, in particular the opinion that ‘vaccination is not necessary’ is reported both for seasonal influenza (34.8% of health care workers vs 27.6% of other professions, p = .4648) and Tdap (15.0% vs 10.0%, p = .4874).
Table 3.
Reasons reported for not receiving vaccination in pregnant women: frequency of adherence to closed-ended responses stratified by vaccination and profession and significance level in the test for the difference in the proportions
| Influenza vaccine | Health care workera (total 23) | Other professiona (total 228) | Statistical significance |
|---|---|---|---|
| I doubt vaccination safety | 4.3% (1) | 5.1% (12) | n.s. |
| I do not think vaccination is necessary | 34.8% (8) | 27.6% (63) | n.s. |
| No time | 21.7% (5) | 6.1% (14) | p < .01 |
| I am concerned about vaccination costs | 0.0% (0) | 0.0% (0) | - |
| I did not know about the possibility of receiving vaccination during pregnancy |
34.8% (8) |
49.5% (113) |
ns |
| Tdap |
Health care workera (total 20) |
Other professiona (total 190) |
Statistical significance |
| I doubt vaccination safety | 0.0% (0) | 2.6% (5) | n.s. |
| I do not think vaccination is necessary | 15.0% (3) | 10.0% (19) | n.s. |
| No time | 10.0% (2) | 2.1% (4) | p < .05 |
| I am concerned about vaccination costs | 0.0% (0) | 0.0% (0) | - |
| I did not know about the possibility of receiving vaccination during pregnancy | 45.0% (9) | 71.6% (136) | p < .05 |
n.s. = not statistically significant, the detailed ‘p’ values in the text.
aThe column totals are different between the two vaccination questions due to missing data.
The coverage of vaccines stratified by other potential socio-demographic variables (age, level of education, citizenship, marital status, and prior childbirth) does not show significant associations. The percentage of women that reported receiving information and educational materials from their gynecologists is 14.7% (37) for influenza and 19.5% (49) for Tdap. A comparison of the vaccination history with the provision of educational materials about vaccination shows that the rates of seasonal influenza (24.3% vs 2.3%, p < .001 in the Chi-square test) and of Tdap (59.2% vs 5.9%, p < .001) vaccines are statistically higher among women who had obtained information. Prevalence ratio for influenza immunization is PR = 10.41 (IC 95%: 3.69–29.3, p < .001), and prevalence ratio for Tdap is PR = 9.96 (I.C. 95%: 5.49–18.1, p < 001) suggesting that vaccination rates among women who received information are tenfold higher than the percentage of uninformed women, in a statistically significant way (Figure 2).
Figure 2.

Percentage of women that received influenza and Tdap vaccines, stratified based on the information received from health care workers (counseling and educational material).
The comparison between the psychometric measurements of the beliefs on the two recommended vaccines highlights a more positive attitude toward the Tdap than the influenza vaccine. The mean values of the scores are always higher in the items with ‘positive’ statements (i.e., 3.18 vs 2.72, for the item ‘I think that people should be vaccinated’, p < .001) and lower in those items with a ‘negative’ statement (i.e., 2.02 vs 2.25, for the item “I am more concerned about the side effects of vaccination for my child than about the risks associated with the disease”, p < .001) as outlined in Table 4.
Table 4.
Beliefs of pregnant women on influenza and Tdap vaccines. Arithmetic mean of the scores collected by 1- to 4-point Likert-type scales and statistical significance of the difference by the Wilcoxon non-parametric test
| Influenza (total 250) |
Tdap(total 250) | Statistical significance | |
|---|---|---|---|
| I think that people should be vaccinated | 2.72 | 3.18 | p < .001 |
| It is important to be vaccinated during pregnancy for my safety and security | 2.65 | 2.95 | p < .001 |
| It is important to be vaccinated during pregnancy for the safety and security of my child | 2.74 | 3.09 | p < .001 |
| I am more concerned about the side effects of vaccination for my child than about the risks associated with the disease | 2.25 | 2.02 | p < .001 |
More than half of the 14 consultant gynecologists recruited for the study were males (57.1%), and two-thirds with an age higher than 60 (64.3%). With regard to the influenza vaccine in pregnancy, 28.6% reported safety concerns if the vaccine is given in the first trimester; however, this percentage is equal to zero when the influenza vaccine is given in the following semesters. Among the gynecologists, 64.3% recommends vaccination only to the women that, at the beginning of the influenza season, are in their second or third trimester of pregnancy. Overall, 71.5% reported being “concerned o very concerned” about the risk that women may get influenza during pregnancy. Gynecologists report that the major reasons for not receiving the influenza vaccine among pregnant women are concerns about vaccination safety and the lack of information on vaccination. With regard to the Tdap vaccination in pregnancy, 87.5% of the gynecologists recommend vaccination during the third trimester and 85.7% report being “little not at all concerned” about vaccination safety. On the other hand, 93% of the gynecologists report being “concerned or very concerned” about the risk that the new-born infant might contract pertussis during the first 2 months of life. The reasons cited for not accepting the Tdap vaccination during pregnancy are concerns about vaccination safety, lack of information about the possibility of vaccination, and the general misperceptions about the necessity of vaccines.
Educational handouts, as an integral part of counseling practice, are used only by 28% of the gynecologists for influenza and by 42.9% for Tdap.
The immunization history of the surveyed gynecologists highlights that 42.9% never received the influenza vaccine, and 78.6% did not receive it during the last influenza season. About 14.3% of the surveyed gynecologists reported having influenza during the last influenza season, whereas 85% of the gynecologists have not received a booster dose of Tdap in the past 10 y.
Discussion
Influenza is an infectious disease that causes annual epidemics associated with high morbidity and mortality rates among the principal high-risk groups. Thus, vaccination has a double function since it protects both women in pregnancy and the infants in their first months of life. Every year, the Italian Ministry of Health offers influenza vaccination free of charge to all subjects at risk and therefore to pregnant women.
Nonetheless, the present study has found significantly low coverage rates for seasonal influenza (5.6%) and Tdap (16.4%) vaccines. Only 1.2% of women have received both vaccines. The prevalence of influenza vaccination uptake reported in the present study is higher than the literature data,17,32,33 lower than other data,31,34,35 consistent with the findings reported by Descamps.36 Our data (16.4%) about adherence to Tdap vaccination is higher than the rates reported by Agricola37 and lower than other findings.35,38 The discrepancy between influenza and Tdap uptake rates led us to assess whether influenza immunization might be a predictor of accepting Tdap vaccination and vice versa; however, no association between the two vaccinations was identified (McNemar’s test). This finding does not align with a previous study conducted by Wales38 where Tdap vaccine adherence was predictive of influenza vaccine acceptance. The immunization history of the women surveyed highlights low willingness to receive active immunization because more than three-quarters of the women (81.3%) reported never receiving influenza vaccination and almost two-thirds (62.6%) did not remember receiving a Tdap dose. The “lack of knowledge” of the recommendations from the Ministry of Health and the perceived “lack of necessity” of immunization are the two main reasons for low adherence to the two vaccines. Concerns about vaccine safety are relatively lower (5.1% for influenza and 2.4% for Tdap) than the rates reported in the literature.31,32,39 Among the mothers not vaccinated with Tdap during their pregnancy, 8.7% believed to be protected by previous vaccination, a finding also reported by Murthy.35 This highlights a lack of knowledge about the decrease in vaccine effectiveness over time, also supported by the data collected regarding access and exposure to educational messages and type of vaccine information sources. Among the women surveyed, a small percentage reported receiving information from their gynecologist also through the distribution of handouts (14.7% for influenza, 19.5% for Tdap), a finding that is consistent with another study32 In the literature, gynecologists and health care workers are reported as the most trusted source of information on vaccination for pregnant women.31,32,38,40 This is confirmed in our study since the prevalence ratio of women vaccinated during pregnancy is tenfold higher than the percentage of women who had received neither advice nor information material about vaccinations from their gynecologists (p < .001). Thus, the information given by gynecologists and health care workers plays a critical role in improving vaccination coverage. Several studies highlight that the recommendation is essential to increase awareness of maternal immunization32,38 also through informative material.31 Women reported that, in addition to counseling, educational materials on the influenza and Tdap vaccines were provided only in 2.8% and 11.1% of cases, respectively. These findings do not align with the data collected through the questionnaires completed by the gynecologists, who reported using information material along with counseling in 28% of cases for the influenza vaccine and in 42% of cases for Tdap.
The assessment of the psychometric attitudinal variables suggested a more favorable attitude toward the Tdap than the influenza vaccine among pregnant women because the former is considered more important for the maternal and neonatal health protection. This finding is confirmed by the analysis of the determinants of vaccine hesitancy though the result is not statistically significant. In fact, the concerns about the influenza vaccine safety are higher than about Tdap (5.1 vs 2.4 p = .1378), along with the perception of the influenza vaccine as unnecessary (28.3% vs 10.5% p < .001), without significant differences between mothers employed in health care sector vs other professions.
The different attitudes toward the two recommended vaccinations could be explained as misconceptions about the severity of the illnesses, in the health care personnel too, as found in our study and in the literature.39,41 Moreover, their immunization history highlights poor adherence to the vaccinations with a not negligible finding about the risk of contracting influence, higher in health care workers vs other professions though not statistically significant (17.4% vs 9.6%, p = .2411).
By analyzing the responses given by the gynecologists, the first relevant data is their high median age as two-thirds of them are 61 y or older. The assessment of the responses of the gynecologists highlights high awareness of the risks related to influenza during pregnancy, although they recommend being vaccinated only from the second trimester. The Italian Ministry of Health in fact recommended, solely on precautionary grounds, to be vaccinated against seasonal influenza in the second and third trimesters; however, published studies have found no indication of adverse events from vaccination in the first trimester.18,42,43 For the Tdap vaccination, the majority of gynecologists (93%) report being concerned about the risk for infants to contract the disease in their first weeks of life; in fact, they advise (87.5%) to be vaccinated in the recommended periods, thus showing also trust in vaccine safety. Gynecologists reported vaccination safety concerns as the main barrier to immunization among pregnant women, followed by lack of information and by the misconception of the vaccine as not necessary. However, such statements are not consistent with the responses given by the women who, for the influenza and Tdap vaccines, reported “concerns about vaccine safety” at 5.1% and 2.4%, respectively. These data suggest that the recent health communication campaigns promoted by the Ministry of Health may have had a positive impact in contrasting the spread of misinformation in online social media and the internet.
Gynecologists report a lack of accurate information as one of the main barriers to immunization. It is paradoxical that such a statement is reported by those who should play a key role in providing women with the correct recommendations to make informed decisions. This need for information is highlighted also by other studies where health care workers report a lack of support by communication experts and opportunities in their practice.41,44 The distribution of handouts contributes to increasing awareness and vaccination adherence, as highlighted in our study (5.3 times higher the chance of being informed among vaccinated women). However, few gynecologists use information material during their counseling. Thus, training for gynecologists on communication strategies and the efficacy of information material is fundamental.
The data on the immunization history of the gynecologists in this study are not very encouraging (42.9% never vaccinated against influenza and 85% never against Tdap). The data are in line with the literature41,45,46 and highlight a low immunization compliance of subjects that should play a pivotal role to influence vaccination compliance among patients.47 Extensive information and training programs on new scientific evidence in vaccinations and on communication strategies should be provided to maternal healthcare workers, gynecologists, obstetricians, primary care physicians, and nurses. Information alone may not necessarily be an enabler to vaccination among pregnant women. Vaccination visits of parous women might be a moment to access educational information and their immunization records. The active involvement of physicians, nurses, pharmacists, and all health care professionals in promoting and supporting the best practices on vaccine-preventable diseases is the main strategy for the success of any vaccination awareness campaign.
Our study should be interpreted in light of some limitations. First, the cross-sectional design imposes restraints in assessing the efficacy of educational information in influencing vaccination decisions. A second limitation is that our sample came from a specific geographic area and this limits the generalizability of the results to the Italian population. Moreover, one exclusion criterion was having a good level of the Italian language, which was necessary for reading and understanding the questionnaire, thus leading to the exclusion of several women coming from foreign countries. The third limitation was that questionnaires were self-reported and this could have led to under- or overestimation of some data about the immunization history. However, the data collected regarding the prevalence of maternal influenza and Tdap vaccines are valuable because to date their availability is still limited. The novelty of our study is that it conducts a comparative analysis between the opinions of gynecologists, the main source of information for pregnant women, and the knowledge and practices about maternal vaccinations, thus contributing to the debate on vaccination in pregnancy.
Funding Statement
The authors have no funding to report.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical issues
Approval of the Local Ethics Committee was obtained.
References
- 1.Crescini C, Lopalco P, Fantini D, Giornelli R, Greggi S, Morgera R, Ragusa A, Stigliano CM, Viora E.. Vaccini in gravidanza: Proteggere madre e bambino. Superare diffidenze e pregiudizi. Cogliere l’opportunità. Riv Ostet Ginecol Prat Med Perinat. 2018. [accessed 2020 Jun];XXXII(1). https://www.aogoi.it/media/5580/rivista-di-ostetricia-ginecologia-pratica-e-medicina-perinatale-vol-xxxii-n-1-2018.pdf [Google Scholar]
- 2.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012. Aug;62(3):263–71. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtis AP, Read J, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–18. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vojtek I, Dieussaert I, Doherty TM, Franck V, Hanssens L, Miller J, Bekkat-Berkani R, Kandeil W, Prado-Cohrs D, Vyse A, et al. Maternal immunization: where are we now and how to move forward? Ann Med. 2018;50(3):193–208. doi: 10.1080/07853890.2017.1421320. [DOI] [PubMed] [Google Scholar]
- 5.Fell DB, Bhutta ZA, Hutcheon JA, Karron RA, Knight M, Kramer MS, Monto AS, Swamy GK, Ortiz JR, Savitz DA, et al. Report of the WHO technical consultation on the effect of maternal influenza and influenza vaccination on the developing fetus: Montreal, Canada, September 30-October 1, 2015. Vaccine. 2017;35(18):2279–87. Epub 2017 Mar 24. doi: 10.1016/j.vaccine.2017.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 7.Lindley MC, Kahn KE, Bardenheier BH, D’Angelo DV, Dawood FS, Fink RV, Havers F, Skoff TH. Vital signs: burden and prevention of influenza and pertussis among pregnant women and infants — United States. MMWR Morb Mortal Wkly Rep. 2019;68(40):885–92. doi: 10.15585/mmwr.mm6840e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijer WJ, van Noortwijk AG, Bruinse HW, Wensing AM. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94(8):797–819. Epub 2015 Jun 13. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 9.Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M; UKOSS . Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia YQ, Zhao KN, Zhao AD, Zhu JZ, Hong HF, Wang YL, Li HS. Associations of maternal upper respiratory tract infection/influenza during early pregnancy with congenital heart disease in offspring: evidence from a case-control study and meta-analysis. BMC Cardiovasc Disord. 2019;19(1):277. doi: 10.1186/s12872-019-1206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bella A, Castrucci MR. La sorveglianza integrata dell’influenza in Italia: i risultati della stagione 2018-19 Not Ist Super Sanità. Inserto BEN. 2019; 32(7–8): 17. http://old.iss.it/binary/publ/cont/BEN_LUGLIO_AGOSTO.pdf. [Google Scholar]
- 12.Shakib JH, Korgenski K, Presson AP, Sheng X, Varner MW, Pavia AT, Byington CL. Influenza in infants born to women vaccinated during pregnancy. Pediatrics. 2016;137:e20152360. doi: 10.1542/peds.2015-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi SA, Nunes MC. Experience and challenges on influenza and pertussis vaccination in pregnant women. Hum Vaccin Immunother. 2018;14(9):2183–88. Epub 2018 Jul 24. doi: 10.1080/21645515.2018.1483810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling RS, Engel SM, Wallenstein S, Kraus TA, Garrido J, Singh T, Kellerman L, Moran TM. Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum. Obstet Gynecol. 2012. Mar;119(3):631–39. doi: 10.1097/AOG.0b013e318244ed20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohfuji S, Fukushima W, Deguchi M, Kawabata K, Yoshida H, Hatayama H, Maeda A, Hirota Y. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203:1301–08. doi: 10.1093/infdis/jir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsatsaris V, Capitant C, Schmitz T, Chazallon C, Bulifon S, Riethmuller D, Picone O, Poulain P, Lewin F, Lainé F, et al. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine: a single-group trial. Ann Intern Med. 2011;155:733–41. doi: 10.7326/0003-4819-155-11-201112060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Fabiani M, Bella A, Rota MC, Clagnan E, Gallo T, D’Amato M, Pezzotti P, Ferrara L, Demicheli V, Martinelli D, et al. A/H1N1 pandemic influenza vaccination: a retrospective evaluation of adverse maternal, fetal and neonatal outcomes in a cohort of pregnant women in Italy. Vaccine. 2015;; 33(19):2240–224. doi: 10.1016/j.vaccine.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Baum U, Leino T, Gissler M, Kilpi T, Jokinen J. Perinatal survival and health after maternal influenza A (H1N1) pdm09 vaccination: a cohort study of pregnancy stratified by trimester of vaccination. Vaccine. 2015;33(38):4850–57. doi: 10.1016/j.vaccine.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek MC, Ware RS, McEniery JA, Coulthard MG, Lambert SB. Epidemiology of pertussis-related paediatric intensive care unit (ICU) admissions in Australia, 1997–2013: an observational study. BMJ Open. 2016;6:e010386. doi: 10.1136/bmjopen-2015-010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ECDC Pertussis . Annual epidemiological report for 2017. [accessed 2020 Jun]. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-pertussis.pdf.
- 21.Fiasca F, Gabutti G, Mattei A. Trends in hospital admissions for pertussis infections: a nationwide retrospective observational study in Italy, 2002–2016. Int J Environ Res Public Health. 2019. Nov 15;16(22):4531. doi: 10.3390/ijerph16224531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministero della Salute (Italian Minister of Health). Piano Nazionale Prevenzione Vaccinale PNPV 2017–2019. [accessed 2020 Jun]. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- 23.Fedele G, Carollo M, Palazzo R, Stefanelli P, Pandolfi E, Gesualdo F, Tozzi AE, Carsetti R, Villani A, Nicolai A, et al. Parents as source of pertussis transmission in hospitalized young infants. Infection. 2017. Apr;45(2):171–78. Epub 2016 Sep 10. doi: 10.1007/s15010-016-0943-6. [DOI] [PubMed] [Google Scholar]
- 24.Kerr SM, Van Bennekom CM, Mitchell AA. Tetanus, diphtheria, and pertussis vaccine (Tdap) in pregnancy and risk of major birth defects in offspring. Birth Defects Res. 2020;112(5):393–403. doi: 10.1002/bdr2.1642. [DOI] [PubMed] [Google Scholar]
- 25.Campbell H, Gupta S, Dolan GP, Kapadia SJ, Singh A, Andrews N, Amirthalingam G. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018. Oct;67(10):1426–56. doi: 10.1099/jmm.0.000829. [DOI] [PubMed] [Google Scholar]
- 26.Vygen-Bonnet S, Hellenbrand W, Garbe E, von Kries R, Bogdan C, Heininger U, Röbl-Mathieu M, Harder T. Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review. BMC Infect Dis. 2020. 13;20(1):136. doi: 10.1186/s12879-020-4824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministero della Salute . Vaccinazioni raccomandate per le donne in età fertile e in gravidanza. Aggiornamento. 2019. Nov [accessed 2020 Jun]. http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=71540&parte=1%20&serie=null.
- 28.ECDC . Technical report: seasonal influenza vaccination and antiviral use in EU/EEA member states. 2018. [accessed 2020 Jun]. https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-antiviral-use-2018.pdf.
- 29.Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine. 2015;33:2125–31. doi: 10.1016/j.vaccine.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, Lee GM, Hambidge S, Jackson ML, Omer SB, et al. Maternal Tdap vaccination: coverage and acute safety outcomes in the vaccine safety datalink, 2007–2013. Vaccine. 2016;34(7):968–73. doi: 10.1016/j.vaccine.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strassberg ER, Power M, Schulkin J, Stark LM, Mackeen AD, Murtough KL, Paglia MJ. Patient attitudes toward influenza and tetanus, diphtheria and acellular pertussis vaccination in pregnancy. Vaccine. 2018;36:4548–54. doi: 10.1016/j.vaccine.2018.05.121. [DOI] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Napolitano F, D’Ambrosio A, Angelillo IF. Vaccination knowledge and acceptability among pregnant women in Italy. Hum Vaccin Immunother. 2018. Jul 3;14(7):1573–79. doi: 10.1080/21645515.2018.1483809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurici M, Dugo V, Zaratti L, Paulon L, Pellegrini MG, Baiocco E, Rizzo G, Franco E. Knowledge and attitude of pregnant women toward flu vaccination: a cross-sectional survey. J Maternal Fetal Neonatal Med. 2016;29:19,3147–3150. doi: 10.3109/14767058.2015.1118033. [DOI] [PubMed] [Google Scholar]
- 34.Napolitano F, Napolitano P, Angelillo IF. Seasonal influenza vaccination in pregnant women: knowledge, attitudes, and behaviors in Italy. BMC Infect Dis. 2017;17(1):48. doi: 10.1186/s12879-016-2138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy NC, Black C, Kahn KE, Ding H, Ball S, Fink RV, Devlin R, D’Angelo D, Fiebelkorn AP. Tetanus, diphtheria, and acellular pertussis and influenza vaccinations among women with a live birth, internet panel survey, 2017–2018. Infect Dis (Auckl). 2020. Feb 10;13:1178633720904099. doi: 10.1177/1178633720904099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Descamps A, Launay O, Bonnet C, Blondel B. Seasonal influenza vaccine uptake and vaccine refusal among pregnant women in France: results from a national survey. Hum Vaccin Immunother. 2019. Nov 14;1–8. doi: 10.1080/21645515.2019.1688035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agricola E, Gesualdo F, Alimenti L, Pandolfi E, Carloni E, D’Ambrosio A, Russo L, Campagna I, Ferretti B, Tozzi AE, et al. Knowledge attitude and practice toward pertussis vaccination during pregnancy among pregnant and postpartum Italian women. Hum Vaccin Immunother. 2016;12(8):1982–88. doi: 10.1080/21645515.2016.1188242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wales DP, Khan S, Suresh D, Ata A, Morris B. Factors associated with Tdap vaccination receipt during pregnancy: a cross-sectional study. Public Health. 2020. Feb;179:38–44. doi: 10.1016/j.puhe.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Riccò M, Vezzosi L, Gualerzi G, Balzarini F, Capozzi VA, Volpi L. Knowledge, attitudes, beliefs and practices of obstetrics-gynecologists on seasonal influenza and pertussis immunizations in pregnant women: preliminary results from north-western Italy. Minerva Ginecol. 2019. Aug;71(4):288–97. doi: 10.23736/S0026-4784.19.04294. [DOI] [PubMed] [Google Scholar]
- 40.Marchetti F, Vilca LM, Cetin I. Insights and expectations for Tdap vaccination of pregnant women in Italy. J Maternal Fetal Neonatal Med. 2019:1–8. doi: 10.1080/14767058.2019.165924. [DOI] [PubMed] [Google Scholar]
- 41.Pinto L, Falsaperla R, Villan A, Corsello G, Del Gado R, Mazzeo A, Lubrano R. Influenza vaccination: opinions of health care professionals working in pediatric emergency departments. Ital J Pediatr. 2019;45(1):45–47. doi: 10.1186/s13052-019-0643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheffild JS, Greer LG, Rogers VL, Roberts SW, Lytle H, McIntire DD, Wendel GD Jr. Effect of influenza in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–37. doi: 10.1097/AOG.0b013e318263a278. [DOI] [PubMed] [Google Scholar]
- 43.Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, Klein NP, Lee G, Jackson ML, Hambidge SJ, et al. First trimester influenza vaccination and risks for major structural birth defects in offspring. J Pediatr. 2017;187:234–39. doi: 10.1016/j.jpeds.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzilli S, Tavoschi L, Lopalco PL. Factors affecting the implementation process of pertussis (Tdpa) immunization in pregnant women in an Italian regione: a qualitative study. FrontPublic Health. 2020;8. doi: 10.3389/fpubh.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scatigna M, Fabiani L, Micolucci G, Santilli F, Mormile P, Giuliani AR. Attitudinal variables and a possible mediating mechanism for vaccination practice in health care workers of a local hospital in L’Aquila (Italy). Hum Vaccin Immunother. 2017;13(1):198–205. doi: 10.1080/21645515.2016.1225638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese C, Picerno IAM, Trimarchi G, Cannavò G, Egitto G, Cosenza B, Merlina V, Icardi G, Panatto D, Amicizia D, et al. Vaccination coverage in healthcare workers: a multicenter cross-sectional study in Italy. J Prev Med Hyg. 2019. Mar 29;60(1):E12–E17. doi: 10.15167/2421-4248/jpmh2019.60.1.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohom S, Robl-Mathieu M, Scheele B, Wojcinski M, Wichmann O, Hellenbrand W. Influenza and pertussis vaccination during pregnancy- attitudes, practices and barriers in gynaecological practice in Germany. BMC Health Serv Res. 2019;19(1):616. doi: 10.1186/s12913-019-4437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


