Abstract
Context
Amauroderma rugosum (Blume & T. Nees) Torrend (Ganodermataceae) is an edible mushroom with medicinal properties. However, the effects of A. rugosum on gastric ulcer remain unclear.
Objective
To investigate the gastroprotective efficacy of water extract of A. rugosum (WEA) on gastric ulcer.
Materials and methods
Sprague-Dawley rats were randomly grouped as control, model, lansoprazole and 200, 100 and 50 mg/kg of WEA. After pre-treatment for seven days, ethanol- and indomethacin-induced gastric ulcer models were established. The gastric ulcer and histopathology were investigated. Enzyme-linked immunosorbent assay (ELISA), quantitative polymerase chain reaction (Q-PCR) and Western blot assays were conducted to explore the potential anti-inflammatory effect and mechanism of WEA. Additionally, the pyloric ligation model was used to explore the influence of WEA on gastric acid and mucus.
Results
Pre-treatment with WEA (200, 100 and 50 mg/kg) effectively reduced ulcerous area in both ethanol-induced (71%, 88% and 71%) and indomethacin-induced (77%, 65% and 86%) gastric ulcer model. The gastric levels of tumour necrosis factor-alpha (TNF-α) (34% and 50 mg/kg), interleukin-6 (IL-6) (32% and 100 mg/kg) and interleukin-1β (IL-1β) (36%, 45% and 41%) were reduced significantly (p < 0.05) by WEA. Serum nitric oxide was decreased significantly (p < 0.05) at 200 and 50 mg/kg and PGE2 concentration was increased remarkably (p < 0.05) at 100 mg/kg. Gene expression of inflammasome Nlrp3, and the nuclear translocation of nuclear factor-κB (NF-κB) P65 were significantly decreased by WEA pre-treatment. However, the pH of gastric acid and secretion of mucus did not show any significant change.
Conclusions
The gastroprotective effect of WEA on gastric damage is attributed to anti-inflammation through the inhibition on NF-κB P65 nuclear migration and Nlrp3 gene expression.
Keywords: Stomach, Traditional Chinese Medicine, anti-inflammatory, NF-κB/NLRP3
Introduction
Gastric ulcer is a common and recurrent gastrointestinal disease affecting millions of people yearly (Greenwood-Van Meerveld et al. 2017). Gastric ulcer occurs due to various noxious factors, such as nonsteroidal anti-inflammatory drugs (NSAIDs), Helicobacter pylori infection, alcohol consumption, smoking, poor dietary habits and stress (Hunt et al. 2015). The imbalance between these noxious and protective factors (mucin, bicarbonate, prostaglandins, and so on) would trigger an interruption in the mucosal integrity, resulting in gastric mucosal lesions.
Numerous studies have indicated that inflammatory responses play an important role in the pathogenesis of acute gastric ulcer (Chien et al. 2015; Fu et al. 2018; Luo et al. 2018). The up-regulation of pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α) and interleukins factors (IL‐1β and IL‐6), was observed in the acute phase of gastric ulceration (Hernández et al. 2014; Li Q et al. 2018). The nuclear translocation of nuclear factor-κB (NF-κB) P65 plays an essential role in the increment of TNF-α and interleukin-6 (IL-6), as well as NACHT, LRR and PYD domains containing protein 3 (NLRP3) inflammasome, which regulates the synthesis of interleukin-1β (IL-1β) (Doyle and O'Neill 2006; Zhen and Zhang 2019). Inhibition of gastric inflammatory responses is an effective therapy except for the control of gastric acid secretion by targeting type II histamine receptors and proton pumps for gastric ulcer treatment.
Amauroderma rugosum (Blume & T. Nees) Torrend (Ganodermataceae) is a traditional medicinal and edible mushroom. A notable characteristic is that the mushroom’s lower surface becomes red when it is touched (Chan et al. 2013), so it is also known as ‘blood Lingzhi’ in Chinese. A. rugosum lives in the tropics and subtropics, grows in humid and humus soil, attaches to the buried roots in hardwood forest (Seng et al. 2017). In recent years, the mushroom has been widely consumed because of both its nutritional value and wide range of medicinal properties. We have cultivated A. rugosum since 2015 in order to meet the ever-increasing market demand. A. rugosum has been used as a remedy to reduce inflammation, treat anuresis and upset stomach in China since ancient times (Lin et al. 2021). Modern pharmacological studies have shown that A. rugosum exhibited anti-inflammatory and antioxidant effects in vitro (Chan et al. 2015). However, pharmacological research of A. rugosum on gastrointestinal disease in vivo is rarely reported.
The present study investigated the gastroprotective efficacy of domesticated A. rugosum water extract (WEA) on rat gastric ulcer by using ethanol, indomethacin and pylorus ligation models. The gastroprotective and anti-inflammatory effects of WEA in vivo were demonstrated. Results provided a pharmacological basis for the use of A. rugosum in treating gastrointestinal diseases.
Materials and methods
Drugs and animals
Absolute ethanol was purchased from Tianjin Zhiyuan Reagent Co., Ltd. (Tianjin, China). Indomethacin was purchased from Meilunbio (Dalian, China). Lansoprazole was purchased from Renhe Pharmaceutical Co., Ltd. (Zhangshu, China). Male Sprague-Dawley rats (6–7 weeks old, 200–250 g) were obtained from Guangdong Medical Laboratory Animal Center (Foshan, China, SYXK (yue) 2018-0085). The rats were housed under specific pathogen-free conditions in a temperature-controlled room illuminated for 12 h every day and received humane care. This study was approved by the Animal Ethics Committee of Guangzhou University of Chinese Medicine (no. 20200826004). Prior to experimentation, all rats were fasted for 24 h and housed in cages with raised floors made of wide mesh to prevent coprophagy.
Preparation of water extract of domesticated Amauroderma rugosum
Dried domesticated A. rugosum was purchased from Longmen Maling Ganoderma lucidum planting base (Huizhou, China) and identified by morphological, microscopic and gene sequencing (the internal transcribed spacer (ITS). Sequence analysis showed that the similarity between the sample (GenBank accession number: MK660145) and A. rugosum (GenBank accession number: MG021113.1) was 99%. The authenticated voucher specimen (voucher 20-07-02) was kept in Huizhou Health Sciences Polytechnic. Dried domesticated A. rugosum mycelia powder as above was extracted for 1 h in hot water (98 °C) in proportion (1:17, w/v) by heating reflux, thrice. The extracting solution was mixed, filtered, concentrated and lyophilized to obtain WEA. The powder of WEA was dissolved in appropriate distilled water to prepare the extract solution (1 mL equivalent to 1 g of A. rugosum mycelia powder) for test. The polysaccharide content was determined according to Chinese Pharmacopoeia (version 2020) based on the method of Ganoderma lucidum (Curtis: Fr.) P. Karst. (Ganodermataceae) polysaccharide quantification. The nucleosides were determined by high-performance liquid chromatography (HPLC). The total polysaccharides of WEA were analysed by anthrone-sulphuric acid colorimetry at 625 nm. The contents of four nucleosides, namely, including cytidine, uridine, guanosine and adenosine, were determined by HPLC.
InertSustain AQ-C18 (1.9 μm, 2.1 × 100 mm) column was used at 40 °C, and the flow rate was 0.3 mL/min. The injection volume was 1 μL, and the detection wavelength was set at 259 nm. The eluent was methanol (A)–water (B), and the gradient elution under HPLC conditions was as follows: 0–2 min, 0% A; 2–2.5 min, 0–2% A; 2.5–4 min, 2%A; 4–4.5 min, 2–10% A; 4.5–8.5 min, 10%A; 8.5–9.0 min, 10–30% A; 9.0–10.5 min, 30–90% A.
Groups and drug administration
Rats were randomly divided into six groups, namely, the blank control (n = 6), the gastric ulcer model (n = 8), lansoprazole (n = 6), H-WEA (n = 6), M-WEA (n = 6) and L-WEA (n = 6). The number of rats were adjusted to six in pyloric ligation assay. The details of drug administration were as follows. The blank control group and gastric ulcer model rats were treated with distilled water (1 mL/100 g, p.o.) for seven consecutive days. The rats in the lansoprazole were treated with 30 mg/kg lansoprazole. An acute toxicity assay of A. rugosum indicated that oral administration of a single dose of the powder (2000 mg/kg) had no adverse effect (Fung et al. 2017). According to the results of our pre-experiment, the H-WEA, M-WEA and L-WEA groups were treated with 200, 100 and 50 mg/kg WEA (p.o.) for seven consecutive days, respectively. All rats were fasted for 24 h with free access to drinking water in the last day.
Ethanol-induced gastric ulcer model assay
One hour after the last treatment, rats (except for those in the blank control group) received an oral dose of 1 mL of absolute ethanol. After 1 h, all rats were anaesthetized with isoflurane, blood was collected from the abdominal aorta and serum was separated after centrifugation. Stomachs were harvested. The stomachs were then opened from the greater curvature and rinsed with cold saline to remove the gastric contents and blood clots. The flattened stomach samples were photographed, and the ulcerated area (mm2) was measured using Image J software (developed by the National Institutes of Health, Bethesda, MD). The stomach was cut into three parts, as follows. One was fixed in 10% paraformaldehyde for pathological section observation. One was frozen for the detection of inflammatory response. One was dipped in RNAwait (Meilunbio, Dalian, China) solution to measure gene expression.
Indomethacin-induced gastric ulcer model assay
Thirty minutes after the last treatment, rats (except for the rats in the blank control group) were given an oral dose of 100 mg/kg of indomethacin. After 5 h, all rats were euthanized with carbon dioxide, and the subsequent procedures were performed in accordance with the ethanol-induced gastric ulcer protocol.
Pyloric ligation model assay
After the oral administration of the last drug or distilled water for 1 h, the rats (except for the rats in the control) were subjected to longitudinal incisions slightly below the xiphoid apophysis to place a pyloric ligature (Moawad et al. 2019). After 4 h, the rats were sacrificed, the abdomen was opened, and another ligature was placed around the oesophagus close to the diaphragm. The stomach was opened along the greater curvature, and the gastric contents were collected and centrifuged for 10 min at 5000×g. The pH of gastric juice was measured by pH meter. The glandular portion of the stomach was weighed and immersed for 2 h in alcian blue solution (Sigma-Aldrich, St. Louis, MO) with vortexing every 10 min for the mucus quantification procedure. The absorbance was measured in a spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Waltham, MA) at a wavelength of 585 nm, and the results were expressed as μg Alcian Blue/g tissue (Rafatullah et al. 1990).
Inflammatory cytokines and nitrogen monoxide (NO) evaluation
Tissues were homogenized in normal saline. Supernatant was harvested after centrifuging at 10,000×g for 15 min at 4 °C. Tumour necrosis factor-α, IL-1β and IL-6 were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits from 4 A Biotech (Beijing, China). Prostaglandin E2 (PGE2) was determined by commercial kit from ANRC (Tianjin, China). NO was measured by commercial kits from Beyotime Biotechnology (Shanghai, China).
Haematoxylin and eosin staining assay
Stomach tissues were fixed in formalin, paraffin-embedded, sectioned and stained with haematoxylin and eosin. The histological examination of gastric tissue was performed according to the methods presented previously (Luo et al. 2018).
Real-time quantitative polymerase chain reaction (q-PCR)
Total RNA was isolated by homogenizing the tissues in RNApure Tissue & Cell Kit (Beijing, China), and single standard cDNA was synthesized by using Tiangen cDNA kit (Beijing, China). Quantitative real-time PCR was performed with Thermo Scientific QuanStudio™ 5 Real-Time PCR instrument (Waltham, MA). Primer sequences are listed in Table 1.
Table 1.
Primer sequences for real-time PCR assays.
| Gene | Primer (5′–3′) |
|---|---|
| Gapdh | f GGCACAGTCAAGGCTGAGAATG |
| r ATGGTGGTGAAGACGCCAGTA | |
| Cox2 | f TGAACACGGACTTGCTCACTTTG |
| r AGGCCTTTGCCACTGCTTGTA | |
| Nlrp3 | f CTGAAGCATCTGCTCTGCAACC |
| r AACCAATGCGAGATCCTGACAAC | |
| Caspase-1 | f ACTCGTACACGTCTTGCCCTCA |
| r CTGGGCAGGCAGCAAATTC | |
| Asc | f TTGCTGGATGCTCTGTATGG |
| r CAGGTCTGTCACCAAGTAGG |
Sequences: 5′–3′. Forward primers are designated by f and reverse primers by r.
Western blot
Nuclear and cytoplasmic proteins were extracted using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents from Thermo Fisher (Waltham, MA). Protein extracts were fractionated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were then blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 for 2 h at room temperature and incubated with anti-β-actin (SAB, MD, USA, 1:5000), anti-Histone H3 (SAB, 1:5000) and anti-NF-κB P65 (SAB, 1:1000) at 4 °C overnight. They were rinsed thrice with TBST and incubated with respective secondary antibodies for 2 h at room temperature. Protein bands were visualized with Millipore Immobilon Western Chemiluminescent HRP Substrate (Boston, MA) and captured using the Bio-Rad ChemiDoc™ Imaging System imaging system (Shanghai, China).
Data analysis
All results were expressed as means ± standard error. Data from more than two groups were analysed by one-way ANOVA followed by LSD or Dunnett’s test for multiple comparisons. Student’s t-test was performed to identify differences between two groups. p < 0.05 was considered significant.
Results
Quantification of four nucleosides and total polysaccharides in WEA
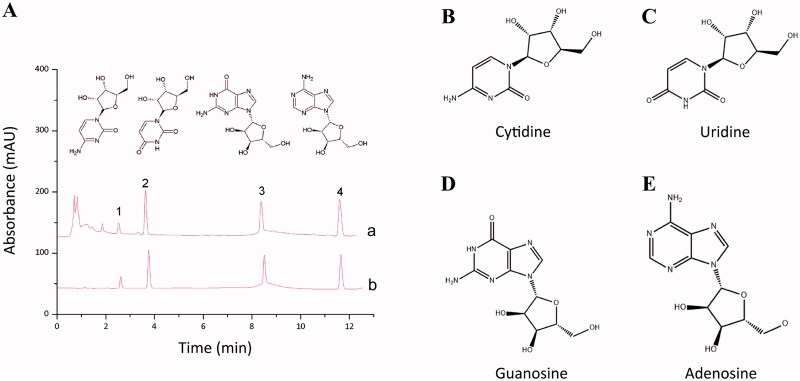
The yield of WEA was 6.13%, RSD was 7.52%. HPLC and visible spectrophotometry analysis were conducted to quantify the four nucleosides and total polysaccharides in the WEA. The cytidine, uridine, vernine and adenosine were 0.2465, 0.6301, 0.4064 and 0.4989 mg/mL, respectively. The total polysaccharides were 7.2 mg/mL (Table 2, Figure 1).
Table 2.
Composition of WEA analysed by HPLC and visible spectrophotometry (n = 3).
| Nucleosides and polysaccharides | Concentration (mg/mL) | RSD (%) |
|---|---|---|
| Cytidine | 0.2465 | 3.87 |
| Uridine | 0.6301 | 3.00 |
| Vernine | 0.4064 | 4.40 |
| Adenosine | 0.4989 | 3.99 |
| Total nucleosides | 1.7819 | 1.53 |
| Total polysaccharides | 7.20 | 2.92 |
Figure 1.
Specific components of WEA determined by HPLC. (A) High-performance liquid chromatography result of WEA (a: test solution; b: reference solution); (B) cytidine; (C) uridine; (D) guanosine; (E) adenosine.
WEA exhibited gastroprotective effect in ethanol-induced gastric ulcer
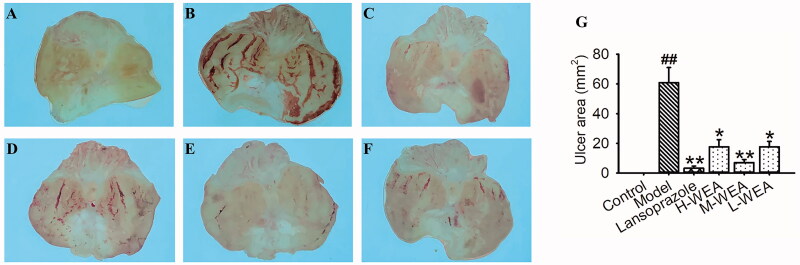
After receiving absolute ethanol 1 h, ethanol-induced gastric ulcer model rats exhibited severe damage in the ulcerated area (60.84 ± 10.16 mm2) (Figure 2(B,G)). This severe ulcerative injury was reversed by lansoprazole pre-treatment, and the ulcerated area was reduced significantly with lansoprazole pre-treatment (3.22 ± 1.43 mm2, reduced 95%) (Figure 2(C,G)). Meanwhile, all three dosages of WEA pre-treatment attenuated the ulcerative damage (17.68 ± 4.74, 7.07 ± 2.01 and 17.70 ± 3.57 mm2; reduced 71%, 88% and 71%) (Figure 2(D–G)). Reduction of ulcerated area was observed in WEA pre-treatment group (Figure 2(G)).
Figure 2.
Effects of WEA on the appearance of the gastric mucosa in ethanol-induced gastric lesions in rats. (A) Intact gastric mucosa of the normal control; (B) gastric mucosa of ethanol-induced gastric lesions; (C) gastric mucosa after lansoprazole administration; (D–F) gastric mucosa after the H-WEA (200 mg/kg), M-WEA (100 mg/kg) and L-WEA (50 mg/kg) administration; (G) gastric ulcer area (mm2) of rat stomach induced by ethanol after pre-treatment with lansoprazole and WEA at different dosages. Values are means ± SEMs (n = 8 for model group, n = 6 for other groups). ##p< 0.01 vs. the control group; *p< 0.05, **p< 0.01 vs. the model group.
WEA could protect rat from indomethacin-induced gastric ulcer
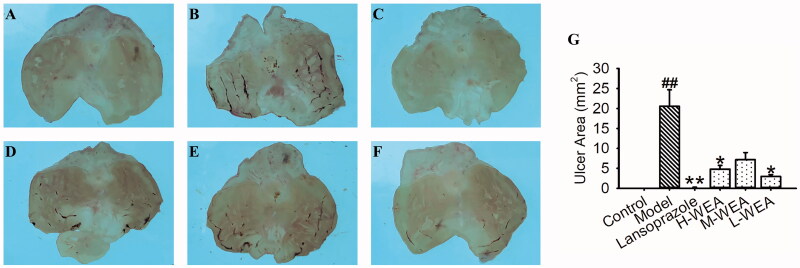
After intervention with indomethacin at 100 mg/kg, rats showed mild mucosal injury in the ulcerated area (20.58 ± 4.12 mm2) (Figure 3(B,G)). Consistent with ethanol-induced gastric ulcer, mucosal injury and ulcerated area were also alleviated with lansoprazole pre-treatment (0.22 ± 0.05 mm2, reduced 99%) (Figure 3(C,G)). H-WEA and L-WEA pre-treatments ameliorated the indomethacin-induced mucosal injury, and the ulcerated area decreased after WEA pre-treatment (4.80 ± 0.95, 7.17 ± 1.80 and 2.97 ± 0.45 mm2; reduced 77%, 65% and 86%) (Figure 3(D–G)).
Figure 3.
Effects of WEA on the appearance of the gastric mucosa in indomethacin-induced gastric lesions in rats. (A) Intact gastric mucosa of normal control; (B) gastric mucosa of indomethacin-induced gastric lesions; (C) gastric mucosa after lansoprazole administration; (D–F) gastric mucosa after H-WEA (200 mg/kg), M-WEA (100 mg/kg) and L-WEA (50 mg/kg) administration; (G) gastric ulcer area (mm2) of rat stomach induced by indomethacin after pre-treatment with lansoprazole and WEA at different dosages. Values are means ± SEMs (n = 8 for model group, n = 6 for other groups). ##p< 0.01 vs. control group; *p< 0.05, **p< 0.01 vs. model group.
Histological evaluations of ethanol-induced gastric ulcer
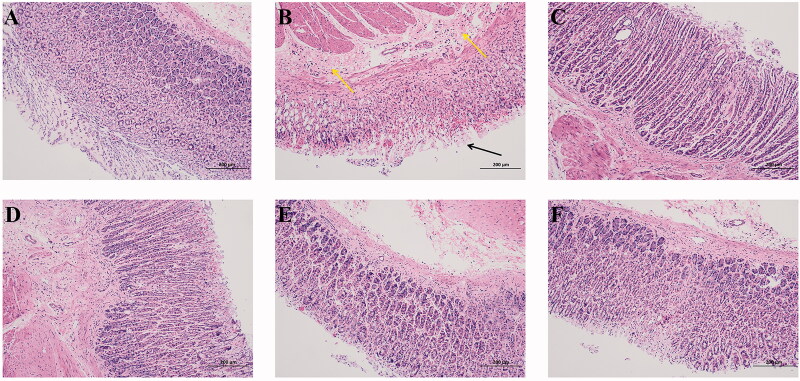
In the histological analysis, rats exhibited extensive erosion in the mucosal layer (Figure 4(B)). Necrocytosis of gastric epithelium and oedema of submucosa with inflammatory infiltration were observed (Figure 4(B)). Lansoprazole pre-treatment could prevent the rat from ethanol-induced erosion in the mucosal layer; necrocytosis of gastric epithelium and oedema of submucosa were also diminished (Figure 4(C)). The necrocytosis of gastric epithelium and oedema of submucosa, as well as the erosion of the mucosal layer, were reversed by 200 mg/kg WEA (H-WEA) pre-treatment. (Figure 4(D)). Rats pre-treated with 100 mg/kg WEA (M-WEA) could also improve the histological exhibition in the gastric epithelium and submucosa (Figure 4(E)). In the 50 mg/kg WEA (L-WEA) pre-treatment group, slight exfoliation of epithelial cells and oedema of submucosa were observed (Figure 4(E)). These results showed that WEA pre-treatment can prevent ethanol-induced damage on the gastric area.
Figure 4.
Effects of WEA on histological evaluation in ethanol-induced gastric lesions in rats (HE staining; magnification ×100). (A) Intact gastric epithelium with organized glandular structure and normal submucosa; (B) ethanol-induced ulcer (black arrow: necrocytosis of gastric epithelium; yellow arrow: oedema of submucosa); (C) lansoprazole administration; (D–F) H-WEA (200 mg/kg), M-WEA (100 mg/kg) and L-WEA (50 mg/kg) administration.
Effects of WEA on inflammatory cytokines, NO and PGE2 secretion in ethanol-induced gastric ulcer
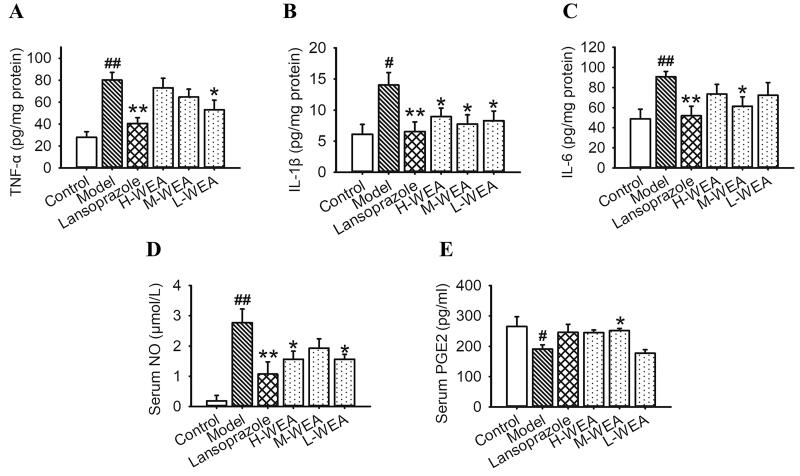
Based on the above findings that WEA attenuated the ethanol-induced gastric ulcer and improved the histological morphology, we assumed that the secretion of inflammatory cytokines was suppressed by WEA pre-treatment. A significant increase in TNF-α, IL-1β and IL-6 secretions in stomach tissues was observed after ethanol intervention, which indicated that severe inflammatory infiltration occurred in the stomach (80.12 ± 7.10, 14.03 ± 2.01 and 90.73 ± 5.20 pg/mg) (Figure 5(A–C)). Lansoprazole pre-treatment significantly reduced the levels of these inflammatory cytokine (40.68 ± 5.19, 6.52 ± 1.59 and 51.92 ± 9.48 pg/mg; reduced 49%, 54% and 43%) (Figure 5(A–C)). L-WEA pre-treatment significantly decreased the gastric level of TNF-α (53.03 ± 8.78 pg/mg, reduced 34%) (Figure 5(A)). Moreover, all three dosages of WEA exhibited significant effects on the lowering of IL-1β level (8.95 ± 1.37, 7.77 ± 1.45 and 8.29 ± 1.56 pg/mg; reduced 36%, 45% and 41%) (Figure 5(B)). M-WEA could significantly reduce IL-6 level (61.40 ± 9.38 pg/mg, reduced 32%) (Figure 5(C)). Consistent with the results on inflammatory cytokines, serum NO also increased after ethanol administration (2.77 ± 0.45 µmol/L) (Figure 5(D)). However, this effect was neutralized by both H-WEA and L-WEA pre-treatments (1.56 ± 0.27, 1.57 ± 0.16 µmol/L; reduced 44%, 43%) (Figure 5(D)). Serum PGE2 decreased with ethanol administration (190.83 ± 14.15 pg/mL) (Figure 5(E)), but this result was reversed by M-WEA pre-treatment (251.55 ± 7.18 pg/mL, increased 31.82%) (Figure 5(E)).
Figure 5.
Effects of WEA on inflammatory cytokines, NO and PGE2 secretion in ethanol-induced gastric lesions in rats. (A) Gastric TNF-α; (B) gastric IL-1β; (C) gastric IL-6; (D) serum NO; (E) serum PGE2. Values are means ± SEMs (n = 8 for model group, n = 6 for other groups). #p< 0.05, ##p< 0.01 vs. the control group; *p< 0.05, **p< 0.01 vs. the model group.
Effects of WEA on gene expression in gastric ulcer
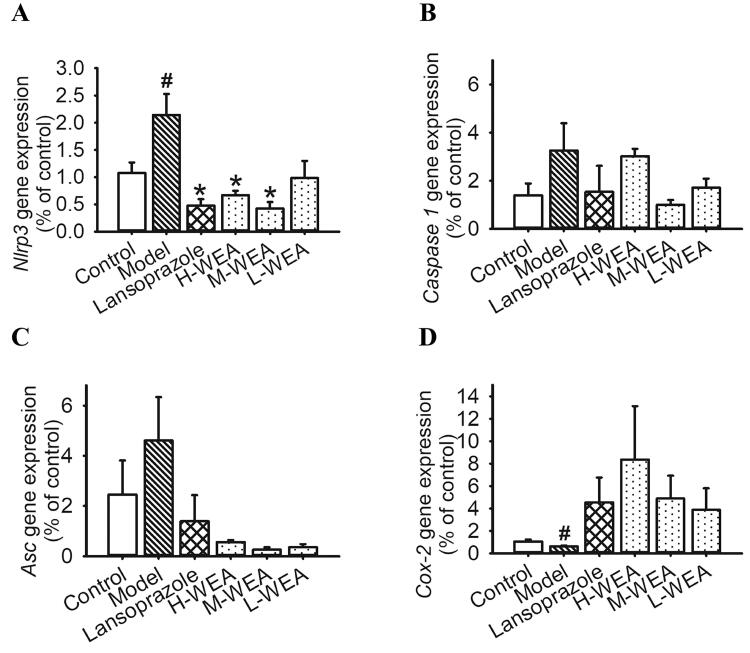
After investigating the anti-inflammatory effects of WEA on rats, we determined the inflammatory relative gene expression in ethanol- and indomethacin-induced gastric ulcer rats after WEA intervention. In rats with ethanol-induced ulcer, a critical pro-inflammatory mediator Nlrp3 was over-expressed in the stomach after ethanol administration (Figure 6(A)). Lansoprazole, H-WEA and M-WEA significantly suppressed gene expression of Nlrp3 in the gastric tissue (Figure 6(A)). In the indomethacin-induced ulcer rats, the gene expression of Cox-2 was inhibited (Figure 6(A)). Although WEA pre-treatment enhanced Cox-2 expression, no significant difference was found (Figure 6(A)).
Figure 6.
Effects of WEA on gastric Nlrp3, Caspase-1, Asc and Cox-2 mRNA expression in ethanol- and indomethacin-induced gastric lesions in rats. (A–C) Nlrp3, Caspase-1 and Asc relative gene expression in rat stomach induced by ethanol after pre-treatment with lansoprazole and WEA at different dosages (n = 8 for the model group, n = 6 for the other groups); (D) Cox-2 gene expression in the rat stomach induced by indomethacin after pre-treatment with lansoprazole and WEA at different dosages (n = 5 per group). Values are means ± SEMs. #p< 0.05 vs. the control group; *p< 0.05 vs. the model group.
WEA inhibited the nuclear migration of NF-κB P65 in ethanol-induced gastric ulcer
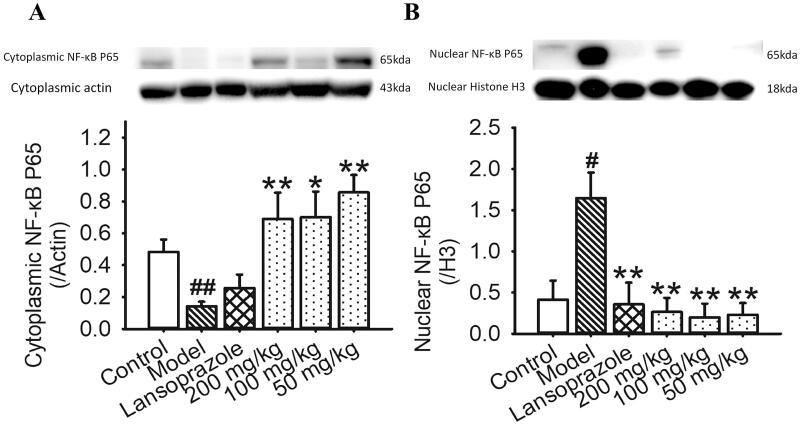
We estimated the levels of cytoplasmic/nuclear NF-κB P65 to further explore the anti-inflammatory mechanism of WEA in gastric ulcer treatment. The level of gastric nuclear NF-κB P65 was significantly increased after ethanol intervention, and the level of cytoplasmic NF-κB P65 decreased (Figure 7). These data suggested that the cytoplasm–nuclear transfection was activated by ethanol stimulation, which was a critical target for acute inflammation activation. Lansoprazole pre-treatment could suppress the nuclear migration of NF-κB P65 significantly (Figure 7). Compared with lansoprazole treatment, WEA pre-treatment inhibited the increase of nuclear NF-κB P65 and recovered the cytoplasmic level of NF-κB P65 (Figure 7). Thus, WEA pre-treatment can prevent the ethanol-induced cytoplasm-nuclear NF-κB P65 transfection.
Figure 7.
Effects of WEA on the expression of NF-κB P65 in ethanol-induced gastric lesions in rats. (A) Visualization and quantification of cytoplasmic NF-κB P65; (B) visualization and quantification of the expression of nuclear NF-κB P65. Values are means ± SEMs (n = 4 per group). #p< 0.05, ##p< 0.01 vs. the control group; *p< 0.05, **p< 0.01 vs. the model group.
Effects of WEA on pyloric ligation rats
After rats were subjected to pyloric ligation for 4 h, the retention of gastric juice in the stomach was observed (Table 3). We compared the gastric mucus and pH of gastric juice after pyloric ligation with WEA administration. However, the three dosages of WEA pre-treatment failed to normalize the pH of gastric juice (Table 3). Lansoprazole pre-treatment even up-regulated the pH of gastric juice (Table 3). On the contrary, gastric mucus was diminished after pyloric ligation (Table 3). However, both lansoprazole and WEA administration had no significant effects on this parameter (Table 3).
Table 3.
Effects of WEA on the pH and mucus in rats with pyloric ligation.
| Groups | pH | Mucus (μg Alcian blue/g tissue) |
|---|---|---|
| Control | – | 1809.93 ± 135.32 |
| Model | 2.55 ± 0.10 | 1065.59 ± 64.47## |
| Lansoprazole | 6.53 ± 0.25** | 896.34 ± 79.01 |
| 200 mg/kg | 2.30 ± 0.05 | 1238.84 ± 164.63 |
| 100 mg/kg | 2.52 ± 0.06 | 1344.66 ± 112.10 |
| 50 mg/kg | 2.43 ± 0.08 | 1211.00 ± 144.85 |
Values are means ± SEMs, n = 6 per group.
##p< 0.01 vs. control group.
**p< 0.01 vs. model group.
Discussion
Gastric ulcer is among the most prevalent gastrointestinal diseases in the world. Many studies revealed the protective effects of edible mushrooms and nature products against gastric mucosa diseases (Wang M et al. 2015; Khémiri and Bitri 2019; Wang L et al. 2020). A. rugosum, an edible and medicinal mushroom, was demonstrated to have anti-inflammation and antioxidant effects in recent years (Chan et al. 2013, 2015; Li J et al. 2021). However, the effects of A. rugosum on gastric mucosa damage is still unclear. In this study, several gastric ulcerative animal models were established to investigate the gastroprotective efficacy of WEA, and results indicated that WEA exhibited anti-inflammatory effects on various aspects.
Oral administration of absolute ethanol is a well-recognized method for inducing gastric injury in animal models (Kwon et al. 2019). In this model, rats subjected to ethanol showed severe gastric lesions, most of which involved extensive erosion in the mucosal layer and necrocytosis of the gastric epithelium. Meanwhile, ethanol administration led to elevated serum NO. NO, a free radical molecule, plays different roles under physiological and pathophysiological conditions. At physiological concentration, NO plays a role against gastric mucosal damage (Elliott and Wallace 1998). Recent research indicated that ethanol administration could enhance inducible nitric oxide synthase (iNOS) expression and inhibit the expression of endothelial nitric oxide synthase (eNOS), leading to NO overload and subsequent cytotoxic effects and free radical generation (Bagyánszki et al. 2011; Amirshahrokhi and Khalili 2015). WEA pre-treatment significantly ameliorated the ethanol-induced gastric mucosal lesion and reversed the elevated serum NO, which suggested that WEA has potential effects on the restoration of antioxidants in ethanol-induced gastric ulceration. In addition, ethanol administration decreased the serum PGE2, which is an endogenous gastroprotective mediator that promotes gastric ulcer healing by improving the secretion of bicarbonate and mucus. PGE2 can also increase gastric mucosal blood flow and protect against reactive oxygen species (ROS) in the stomach (Amirshahrokhi and Khalili 2017; Cheng et al. 2017). WEA pre-treatment significantly increased the concentration of serum PGE2.
Inflammatory cytokines play pivotal roles in gastric mucosal damage (Chien et al. 2015; Fu et al. 2018; Luo et al. 2018). In this research, ethanol administration stimulated inflammatory infiltration. The increase of several inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, in gastric tissue was observed. These cytokines could deteriorate oxidative stress by accelerating mitochondrial ROS generation and cytotoxicity (Rozza et al. 2014; Rajamanickam et al. 2020). Moreover, TNF-α can induce the immune response to generate cytotoxic metabolites, suppress gastric microcirculation and delay the recovery (Verma and Kumar 2016). Many studies have indicated that the levels of IL-6 and IL-1β in gastric tissue are correlated with the severity of gastric ulceration (Li WF et al. 2014). In the present study, WEA pre-treatment significantly decreased the levels of gastric TNF-α, IL-6 and IL-1β, which indicated that WEA possessed anti-inflammatory effects on ethanol-induced gastric ulceration. We aimed to investigate the mechanism underlying the gastroprotective effects of WEA. Interestingly, another critical pro-inflammatory mediator involved in gastric ulceration, NLRP3, was over-expressed in gastric tissue. NLRP3 inflammasome, assembled by NLRP3, ASC and pro-CASPASE-1, would be activated under gastric ulcer conditions, resulting in the proteolysis of pro-CASPASE-1 into cle-CASPASE-1 and leading to the release of functional IL-1β (Swanson et al. 2019). The activation of NLRP3 inflammasome can be triggered by NF-κB pathway and result in the increase of IL-1β and TNF-α secretion (Chi et al. 2015; Ma et al. 2018), as well as the increase in nuclear migration of NF-κB P65. In agreement with the effects of inflammatory cytokines, WEA pre-treatment also suppressed the nuclear migration of NF-κB P65 and inactivated the NLRP3 inflammasome gene expression. We suggested that WEA could inhibit gastric inflammation by suppressing the NF-κB/NLRP3 pathway and attenuating the inflammatory cytokine-associated oxidative stress.
Indomethacin, an NSAID, was used to induce a gastric ulcer model, which is used to confirm the gastroprotective effects of WEA in different ulcerous animal models. Indomethacin is a non-selective inhibitor of both COX-1 and COX-2 and can cause damage in the stomach (Zheng et al. 2014; Stachowicz 2020). WEA pre-treatment could also attenuate indomethacin-induced gastric ulceration. Although WEA had no significant effect on the gastric Cox-2 gene expression, the gastroprotective effects and the reliable anti-inflammation effect of WEA were still robust. A pylorus ligation gastric ulcer model was performed to investigate the different gastroprotective effects of WEA on gastric ulceration and the underlying mechanism. In this model, the ligation of the pyloric end leads to the accumulation of gastric acid, which is another pathological feature of gastric ulceration. The decrement of mucus secretion results in the erosion by gastric acid, which is the pivotal factor for the start of ulceration (Adeniyi et al. 2018). Therefore, gastric acid accumulated in the stomach, and the mucus secretion was reduced after pylorus ligation, thereby resulting in gastric mucosa damage. WEA pre-treatment had no significant effects on the pH of gastric acid and the secretion of mucus. Based on the above evidence, we suggested that the therapeutic effects of WEA on gastric ulcer are independent of hydrochloric acid production.
WEA can alleviate gastric ulcer through the inhibition of nuclear migration of the critical inflammatory factor NF-κB P65 and the inactivation of the NF-κB/NLRP3 pathway. Although the reliable anti-inflammation effect of WEA may contribute to the improvement of indomethacin-induced gastric ulcer, the specific mechanism of WEA in non-steroid anti-inflammatory drug-induced ulcer may need further investigation. A. rugosum has potential to be further developed into a promising candidate for the treatment of gastric ulcer.
Acknowledgements
We thank Prof. Shaoju Guo and Prof. Bin Huang for the technical and funding support.
Funding Statement
This research was supported by Youth Innovative Talents Project of Colleges and Universities of Guangdong Province (Project number: 2018GkQNCX068); Shenzhen Science and Technology Innovation Commission (Project number: JCYJ20210324111602007); Scientific Research Project of Guangdong Bureau of Traditional Chinese Medicine (Project number: 20221349); Featured innovation Project of Colleges and Universities of Guangdong Province (Project number: 2018GKTSCX097).
Authors contributions
Conceived and designed the experiments: Y.F. Xu and Y.Z. Mai; performed the experiments and analysed the data: Y.Z. Mai, S.Y. Xu, R. S. and B.R. Feng; Wrote and revised the manuscript: Y.Z. Mai, H. H and Y.F. Xu. All authors read and approved the final manuscript.
Disclosure statement
The authors have no conflict of interest to declare.
References
- Adeniyi OS, Emikpe BO, Olaleye SB.. 2018. Accelerated gastric ulcer healing in thyroxine treated rats: roles of gastric acid, mucus, and inflammatory response. Can J Physiol Pharmacol. 96(6):597–602. [DOI] [PubMed] [Google Scholar]
- Amirshahrokhi K, Khalili AR.. 2015. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation. 38(2):476–484. [DOI] [PubMed] [Google Scholar]
- Amirshahrokhi K, Khalili AR.. 2017. Methylsulfonylmethane is effective against gastric mucosal injury. Eur J Pharmacol. 811:240–248. [DOI] [PubMed] [Google Scholar]
- Bagyánszki M, Torfs P, Krecsmarik M, Fekete E, Adriaensen D, Van Nassauw L, Timmermans JP, Kroese AB.. 2011. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol Motil. 23(6):e237–e248. [DOI] [PubMed] [Google Scholar]
- Chan PM, Kanagasabapathy G, Tan YS, Sabaratnam V, Kuppusamy UR.. 2013. Amauroderma rugosum (Blume & T. Nees) Torrend: nutritional composition and antioxidant and potential anti-inflammatory properties. Evid Based Complement Alternat Med. 2013:304713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PM, Tan YS, Chua KH, Sabaratnam V, Kuppusamy UR.. 2015. Attenuation of inflammatory mediators (TNF-α and nitric oxide) and up-regulation of IL-10 by wild and domesticated basidiocarps of Amauroderma rugosum (Blume & T. Nees) torrend in LPS-stimulated RAW264.7 cells. PLOS One. 10(10):e0139593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YT, Lu CC, Yen GC.. 2017. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol Nutr Food Res. 61. [DOI] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y.. 2015. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J Neuroinflammation. 12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien MY, Lin YT, Peng FC, Lee HJ, Chang JM, Yang CM, Chen CH.. 2015. Gastroprotective potential against indomethacin and safety assessment of the homology of medicine and food formula cuttlebone complex. Food Funct. 6(8):2803–2812. [DOI] [PubMed] [Google Scholar]
- Doyle SL, O'Neill LA.. 2006. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 72(9):1102–1113. [DOI] [PubMed] [Google Scholar]
- Elliott SN, Wallace JL.. 1998. Nitric oxide: a regulator of mucosal defense and injury. J Gastroenterol. 33(6):792–803. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wu HQ, Cui HL, Li YY, Li CZ.. 2018. Gastroprotective and anti-ulcer effects of oxymatrine against several gastric ulcer models in rats: possible roles of antioxidant, antiinflammatory, and prosurvival mechanisms. Phytother Res. 32(10):2047–2058. [DOI] [PubMed] [Google Scholar]
- Fung S, Tan N, Kong B, Lee S, Tan Y, Sabaratnam V.. 2017. Acute toxicity study and the in vitro cytotoxicity of a black Lingzhi medicinal mushroom, Amauroderma rugosum (Agaricomycetes), from Malaysia. Int J Med Mushrooms. 19(12):1093–1099. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Johnson AC, Grundy D.. 2017. Gastrointestinal physiology and function. Handb Exp Pharmacol. 239:1–16. [DOI] [PubMed] [Google Scholar]
- Hernández C, Serrano C, Einisman H, Villagrán A, Peña A, Duarte I, Torres J, Riera F, Harris PR.. 2014. Peptic ulcer disease in Helicobacter pylori-infected children: clinical findings and mucosal immune response. J Pediatr Gastroenterol Nutr. 59(6):773–778. [DOI] [PubMed] [Google Scholar]
- Hunt RH, Camilleri M, Crowe SE, El-Omar EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM, Rugge M, et al. 2015. The stomach in health and disease. Gut. 64(10):1650–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khémiri I, Bitri L.. 2019. Effectiveness of Opuntia ficus indica L. inermis seed oil in the protection and the healing of experimentally induced gastric mucosa ulcer. Oxid Med Cell Longev. 2019:1568720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DA, Kim YS, Baek SH, Kim SK, Kim HK, Jo SK, Jung U, Park HR, Lee HS.. 2019. Protective effects of a standardized extract (HemoHIM) using indomethacin- and ethanol/HCl-induced gastric mucosal injury models. Pharm Biol. 57(1):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li R, Wu X, Hoo RL, Lee SM, Cheung TM, Ho BS, Leung GP.. 2021. Amauroderma rugosum protects PC12 cells against 6-OHDA-induced neurotoxicity through antioxidant and antiapoptotic effects. Oxid Med Cell Longev. 2021:6683270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Hu X, Xuan Y, Ying J, Fei Y, Rong J, Zhang Y, Zhang J, Liu C, Liu Z.. 2018. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochim Biophys Sin. 50(3):246–253. [DOI] [PubMed] [Google Scholar]
- Li WF, Hao DJ, Fan T, Huang HM, Yao H, Niu XF.. 2014. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem Biol Interact. 208:18–27. [DOI] [PubMed] [Google Scholar]
- Lin W, Shi Y, Jia G, Sun H, Sun T, Hou D.. 2021. Genome sequencing and annotation and phylogenomic analysis of the medicinal mushroom Amauroderma rugosum, a traditional medicinal species in the family Ganodermataceae. Mycologia. 113(2):268–277. [DOI] [PubMed] [Google Scholar]
- Luo C, Chen H, Wang Y, Lin G, Li C, Tan L, Su Z, Lai X, Xie J, Zeng H.. 2018. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: characterization of potential molecular mechanisms. Life Sci. 193:47–56. [DOI] [PubMed] [Google Scholar]
- Ma C, Liu S, Zhang S, Xu T, Yu X, Gao Y, Zhai C, Li C, Lei C, Fan S, et al. 2018. Evidence and perspective for the role of the NLRP3 inflammasome signaling pathway in ischemic stroke and its therapeutic potential (review). Int J Mol Med. 42(6):2979–2990. [DOI] [PubMed] [Google Scholar]
- Moawad H, El Awdan SA, Sallam NA, El-Eraky WI, Alkhawlani MA.. 2019. Gastroprotective effect of cilostazol against ethanol- and pylorus ligation-induced gastric lesions in rats. Naunyn Schmiedebergs Arch Pharmacol. 392(12):1605–1616. [DOI] [PubMed] [Google Scholar]
- Rafatullah S, Tariq M, Al-yahya MA, Mossa JS, Ageel AM.. 1990. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J. Ethnopharmacol. 29(1):25–34. [DOI] [PubMed] [Google Scholar]
- Rajamanickam V, Yan T, Wu L, Zhao Y, Xu X, Zhu H, Chen X, Wang M, Liu Z, Liu Z, et al. 2020. Allylated curcumin analog CA6 inhibits TrxR1 and leads to ROS-dependent apoptotic cell death in gastric cancer through Akt-FoxO3a. Cancer Manag Res. 12:247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozza AL, Meira de Faria F, Souza Brito AR, Pellizzon CH.. 2014. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLOS One. 9(1):e86686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng CK, Abdullah N, Aminudin N.. 2017. Antioxidative and inhibitory effects of the fruiting body of black lingzhi mushroom, Amauroderma rugosum (Agaricomycetes), on LDL oxidation and HMG-CoA reductase activity. Int J Med Mushrooms. 19(9):797–807. [DOI] [PubMed] [Google Scholar]
- Stachowicz K. 2020. Indomethacin, a nonselective cyclooxygenase inhibitor, does not interact with MTEP in antidepressant-like activity, as opposed to imipramine in CD-1 mice. Eur J Pharmacol. 888:173585. [DOI] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP.. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 19(8):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Kumar VL.. 2016. Attenuation of gastric mucosal damage by artesunate in rat: modulation of oxidative stress and NF-κB mediated signaling. Chem Biol Interact. 257:46–53. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao H, Brennan M, Guan W, Liu J, Wang M, Wen X, He J, Brennan C.. 2020. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chem. 330:127214. [DOI] [PubMed] [Google Scholar]
- Wang M, Konishi T, Gao Y, Xu D, Gao Q.. 2015. Anti-gastric ulcer activity of polysaccharide fraction isolated from mycelium culture of lion's mane medicinal mushroom, Hericium erinaceus (higher basidiomycetes). Int J Med Mushrooms. 17(11):1055–1060. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Zhang H.. 2019. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol. 10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YF, Xie JH, Xu YF, Liang YZ, Mo ZZ, Jiang WW, Chen XY, Liu YH, Yu XD, Huang P, et al. 2014. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem Biol Interact. 222:27–36. [DOI] [PubMed] [Google Scholar]