Abstract
Background:
Relative to White adults, Black adults have a substantially higher prevalence of hypertension and diabetes, both key risk factors for stroke, cardiovascular disease, cognitive impairment, and dementia. Blood biomarkers have shown promise in identifying contributors to racial disparities in many chronic diseases.
Methods:
We outline the study design and related statistical considerations for a nested cohort study, the Biomarker Mediators of Racial Disparities in Risk Factors (BioMedioR) study, within the 30,239-person biracial REasons for Geographic And Racial Differences in Stroke (REGARDS) study (2003-present). Selected biomarkers will be assessed for contributions to racial disparities in risk factor development over median 9.4 years of follow-up, with initial focus on hypertension and diabetes. Here we outline study design decisions and statistical considerations for the sampling of 4,400 BioMedioR participants.
Results:
The population for biomarker assessment was selected using a random sample study design balanced across race and sex to provide the optimal opportunity to describe association of biomarkers with the development of hypertension and diabetes. Descriptive characteristics of the BioMedioR sample and analytic plans are provided for this nested cohort study.
Conclusions:
This nested biomarker study will examine pathways with the target to help explain racial differences in hypertension and diabetes incidence.
Keywords: study design, racial disparity, biomarkers, hypertension, diabetes
Stroke, cardiovascular disease, cognitive impairment, and dementia share many risk factors including hypertension and diabetes which have high population attributable risks [1]. These same risk factors disproportionately affect Black compared to White adults in the US [2, 3], and substantially contribute to the excess burden of both adverse health outcomes in Black adults, including stroke, heart disease, kidney disease, [2, 4–6] and cognitive impairment and dementia [7–9]. Racial differences in hypertension and diabetes are also important contributors to higher mortality in Black than White people [10–12]. Genetic differences between races explains little of the observed differences in cardiovascular disease and associated risk factors [13, 14]. Examining molecular biomarkers may reveal potential pathways for pharmacological, behavioral, or societal interventions to address racial differences in risk factors to reduce disease burden [15].
Inflammatory biomarkers, such as C-reactive protein (CRP), are associated with increased risk of incident hypertension [16–19] and diabetes [20–22], and elevated CRP is more common in Black than White adults in the US [23–25]. This higher prevalence of a risk factor in Black people contributed to our observation that C-reactive protein mediated the association of race with hypertension via an inflammation pathway of hypertension risk factors in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study [26]. Other biomarkers, such as N-terminal-proB-type natriuretic peptide (NT-proBNP), interleukin-6, lipoprotein(a), and D-dimer, have differential distributions between Black and White people [27] and are associated with increased risk of stroke [28–30], mortality [31–33], myocardial infarction [34], kidney disease [35] and incident cognitive impairment [36]. This raises the possibility that the higher level of these factors in Black people may contribute to disparities in cardiovascular and cognitive outcomes, a possibility that can be formally investigated with mediation analysis [37]. Lipoprotein (a) was a race-specific stroke risk factor, important in Black but not White REGARDS participants [38], suggesting effect modification that could also contribute to the disparity in stroke. These compelling findings suggest future work on biomarkers might improve understanding of racial disparities in the incidence of hypertension and diabetes.
Identifying contributors to the higher incidence of hypertension and diabetes in the Black population is a primary aim of the REGARDS study. Effect modification (interaction) and mediation analyses of biomarker measurements can provide complimentary information on potential effect heterogeneity and pathways, jointly informing both potential interventions and targeted populations [39]. Nevertheless, given the cost of biomarker assaying and finite resources, measuring such biomarkers in all participants within large cohort studies is not feasible, and can be considered unnecessary. Therefore, an efficiently designed sub-study within a larger cohort, taking advantage of various sampling approaches available, may be the optimal approach to studying biomarker relationships with incident hypertension and diabetes. We report herein the study design and statistical considerations of the Biomarkers as Mediators of racial disparities in Risk factors (BioMedioR) study, a nested sub-study within the REGARDS cohort, with aims to determine the role of biomarkers in racial disparities in the development of hypertension and diabetes. Our goals were to preserve the ability to assess multiple disease outcomes, minimize assay costs, and maximize the number of biomarkers we could assess with finite resources.
METHODS
Original study population
REGARDS is a national cohort study of 30,239 participants from the contiguous US who self-identified as non-Hispanic Black or White, with oversampling from the southeastern United States and Black adults; the study is described in more detail elsewhere [40]. The study recruited participants aged 45 years or older between 2003 and 2007, selected from commercially available contact information. Upon enrollment, participants completed a computer-assisted telephone interview and an in-home visit to collect demographic, clinical measures and blood and urine samples [40]. The REGARDS team continues to contact participants (or designated proxies) every six months via telephone to collect information about stroke events, stroke symptoms, hospitalizations, and death, and to assess cognitive functioning [41–43]. Approximately 10 years after enrollment (2013–2016), 16,150 (53.4%) of enrolled REGARDS participants underwent a second visit. This second visit contained both a telephone interview and in-person examination, providing researchers the opportunity to examine the development of hypertension and diabetes between the study visits [15, 44]. Institutional review boards of all participating institutions approved the study, and all participants provided written informed consent.
Biospecimens
Fasting morning blood samples were drawn with venipuncture at both study visits, as previously described [41–43, 45]. Samples were centrifuged and shipped overnight to a central laboratory at the University of Vermont, recentrifuged at 30,000g, and either analyzed or aliquoted and stored in −80C freezers. Blood glucose was measured using colorimetric reflectance spectrophotometry on the Ortho Vitros 950 IRC Clinical Analyzer (Johnson & Johnson Clinical Diagnostics).
Hypertension and Diabetes Definitions
Hypertension was defined as either self-reported use of medication to control blood pressure or measured systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg (blood pressure thresholds included in guidelines at the time of the assessments). Diabetes was defined as self-reported use of glucose lowering medication or fasting glucose ≥ 126 mg/dL (or random glucose ≥ 200 mg/dL among the participants failing to fast). Blood pressure measurements utilized were the mean of two measures taken by research technicians following a standardized protocol after the participant had been seated for five minutes. Hypertension and diabetes definitions were applied to both baseline and second visit assessments. Given that participants with hypertension or diabetes at baseline cannot develop these conditions by the second visit, we categorized hypertension and diabetes outcomes herein as prevalent (present at baseline visit), incident (absent at baseline visit and present at follow-up visit), and neither (absent at both baseline and follow-up visits).
BioMedioR Design Considerations
We initially considered a case-cohort study design [46] as an efficient means to identify participants for laboratory analyses of a range of biomarkers as potential factors involved in racial disparities in hypertension and/or diabetes. In a case-cohort design, we would identify cases of incident hypertension and diabetes, and compare baseline concentrations of biomarkers with a subcohort sample. Case-cohort studies have been successful in several REGARDS projects, examining biomarker differences in stroke and coronary disease risk, cognitive impairment, and venous thromboembolism [29, 30, 34, 38, 47–52]. However, case-cohort studies are most efficient for rare diseases [46, 53, 54]. As incident hypertension and diabetes are not rare among those at-risk in the REGARDS population (36.5% of those at risk developed hypertension, 12.5% of those at risk developed diabetes; Supplemental Table S1), sampling all eligible REGARDS participants with incident hypertension and diabetes is not financially efficient.
To help inform the choice of the BioMedioR subcohort sampling design, a statistical simulation study designed to detect effect modification (statistical interaction) of any biomarker with race was implemented to guide the sampling framework (Supplemental Material). The simulation study was intended to verify the expected statistical properties of different sampling approaches when resampling from the REGARDS population. Both race and sex are important factors on which the scientific team preferred balance, particularly given the potential role of sex in these complex race-biomarker relationships. Therefore race/sex stratified sampling should provide more flexible and powerful analyses due to its balance characteristic. Specifically, we compared simulated sub-cohorts selected through (1) simple random sampling, (2) stratified random sampling with equal allocation across race, and (3) stratified random sampling with equal allocation across race and sex. For comparison, the simulation study also considered the hypothetical ideal scenario where biomarkers could be measured in all eligible REGARDS participants. Across all simulated sampling scenarios, bias, power and Type I error rates were similar (Supplemental Figure 2), suggesting our preference for race-sex stratification was justifiable compared to other similar sampling strategies.
BioMedioR Sample
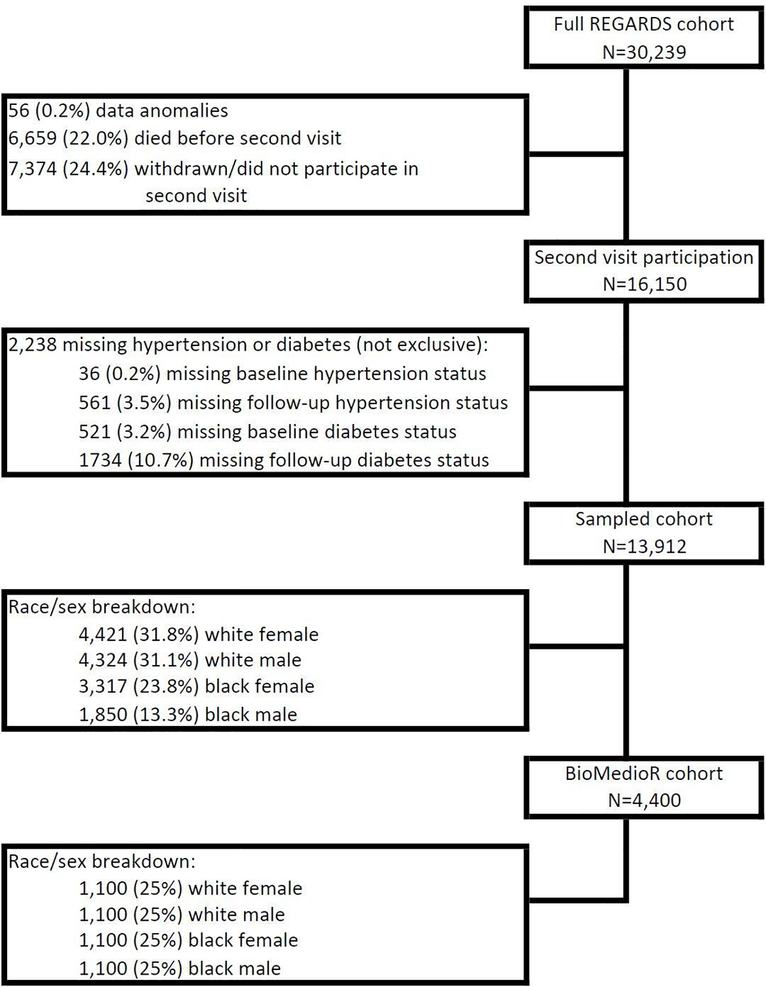
The BioMedioR subcohort was sampled from the REGARDS participants with second in-home assessment of both hypertension and diabetes (N=13,912) using stratified random sampling by race and sex. The target sample size for BioMedioR subcohort was 4,000. To account for the possibility that up to 10% of participants may not have sufficient blood samples for biomarker assessments, the REGARDS statistical team provided a list of 4,400 unique participant identifiers. The stratified selection employed the sequential algorithm [55] which incorporates the flexibility to sequentially add more participants in the future. For each race/sex combination, 1,100 participants were randomly selected to establish the sample of 4,400 BioMedioR participants. These 4,400 provide the foundation for sampling weight creation, allowing future BioMedioR analyses to account for the stratified sampling design. Figure 1 presents the flow diagram of participants selected for BioMedioR. It should be noted that the number of Black participants eligible for inclusion in BioMedioR is lower than the number of White participants.
Figure 1:
BioMedioR cohort flow diagram
Table 1 presents the baseline characteristics of the BioMedioR 4,400 participants stratified by race and sex, weighted to reflect the sampling. The sample includes 391 White and 319 Black participants with incident hypertension, and 184 White and 291 Black participants with incident diabetes (Table 1). Of those BioMedioR participants at risk for incident hypertension, White women and Black women experienced the lowest and highest proportions developing disease, respectively (29.9%, 51.5%). Among those without diabetes at baseline, Black men had the largest fraction developing diabetes (19.2%) and White women had the lowest (8.1%). The baseline characteristics of the BioMedioR sample stratified by hypertension and diabetes status at the second visit are presented in Table 2. Using logistic regression accounting for the sampling design, similar estimated associations between individual risk factors and incident hypertension and diabetes were found between BioMedioR and the entire REGARDS cohort, with the latter having increased efficiency due to larger sample size (Supplemental Table 1).
Table 1.
BioMedioR hypertension and diabetes status and sample characteristics by race and sex (N=4,400), weighted
| Male | Female | ||||
|---|---|---|---|---|---|
| Overall | White | Black | White | Black | |
| Sample size | 4400 | 1100 | 1100 | 1100 | 1100 |
| Weighted N | 13912 | 4324 | 1850 | 4421 | 3317 |
| Hypertension status, unweighted n (weighted %) | |||||
| Prevalent hypertension | 2477 (53.3) | 520 (47.3) | 739 (67.2) | 458 (41.6) | 760 (69.1) |
| Incident hypertension | 710 (16.7) | 199 (18.1) | 144 (13.1) | 192 (17.5) | 175 (15.9) |
| No hypertension | 1213 (30.0) | 381 (34.6) | 217 (19.7) | 450 (40.9) | 165 (15.0) |
| Diabetes status, unweighted n (weighted %) | |||||
| Prevalent diabetes | 930 (18.8) | 151 (13.7) | 338 (30.7) | 125 (11.4) | 316 (28.7) |
| Incident diabetes | 475 (10.2) | 105 (9.5) | 146 (13.3) | 79 (7.2) | 145 (13.2) |
| No diabetes | 2995 (71.0) | 844 (76.7) | 616 (56.0) | 896 (81.4) | 639 (58.1) |
| Characteristics a , mean (SE) or % | |||||
| Demographics | |||||
| Age, years | 63.1 (0.13) | 64.2 (0.24) | 62.3 (0.24) | 62.9 (0.26) | 62.4 (0.25) |
| Follow-up time, years, median | 9.5 | 9.8 | 9.7 | 9.3 | 9.4 |
| Stroke belt residenceb | 55.1 | 53.4 | 52.5 | 58.8 | 53.9 |
| Household income ≤ $35,000 | 35.0 | 22.5 | 36.4 | 34.7 | 51.0 |
| Has no health insurance | 5.5 | 2.6 | 8.0 | 4.4 | 9.4 |
| Self-reported health of fair/poor | 20.2 | 12.4 | 25.0 | 14.8 | 32.0 |
| High school education or less | 31.0 | 22.5 | 39.1 | 27.7 | 41.9 |
| Anthropometrics | |||||
| BMI, kg/m2 | 29.3 (0.1) | 28.4 (0.1) | 29.5 (0.2) | 28.3 (0.2) | 31.9 (0.2) |
| Waist circumference, cm | 95.5 (0.3) | 100.0 (0.4) | 100.0 (0.4) | 88.4 (0.5) | 96.4 (0.5) |
| Lifestyle factors | |||||
| Current smoker | 10.7 | 8.2 | 16.3 | 9.6 | 12.4 |
| Heavy alcohol use | 6.9 | 2.8 | 5.3 | 2.2 | 4.7 |
| Physical activity frequency <1 time per week (none) | 29.1 | 19.9 | 25.6 | 32.8 | 38.0 |
| Clinical factors | |||||
| Estimated GFR (CKD-EPI), mL/min per 1.73m2 | 88 (0.3) | 85 (0.4) | 90 (0.6) | 86 (0.5) | 93 (0.6) |
| Albumin-creatinine ratio, mg/mmol | 29 (2.5) | 27 (5.4) | 44 (7.2) | 16 (1.7) | 42 (6.2) |
| Systolic blood pressure, mm Hg | 125 (0.2) | 125 (0.4) | 130 (0.5) | 122 (0.4) | 129 (0.5) |
| Prediabetesc | 22.1 | 23.8 | 25.8 | 16.8 | 24.8 |
| LDL cholesterol, mg/dL | 115 (0.5) | 111 (1.0) | 115 (1.1) | 115 (1.0) | 119 (1.1) |
| HDL cholesterol, mg/dL | 52 (0.3) | 44 (0.4) | 48 (0.4) | 58 (0.5) | 57 (0.5) |
| Triglycerides, mg/dL | 129 (1.3) | 141 (2.9) | 120 (3.2) | 137 (2.4) | 106 (1.9) |
Table values account for sampling weights.
Stroke belt: Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee
Prediabetes is defined as fasting serum glucose between 100 – 125 mg/dL or a random glucose between 140 – 199 mg/dL.
Table 2:
BioMedioR sample baseline characteristics by hypertension and diabetes status at follow-up (N=4,400), weighted
| Hypertension | Diabetes mellitus | |||||
|---|---|---|---|---|---|---|
| Prevalent disease at baseline | Incident disease at follow-up | No disease | Prevalent disease at baseline | Incident disease at follow-up | No disease | |
| Sample size | 2477 | 710 | 1213 | 930 | 475 | 2995 |
| Weighted N | 7419 | 2324 | 4169 | 2617 | 1413 | 9882 |
| Characteristics a , mean (SE) or % | ||||||
| Demographics | ||||||
| Black | 47.6 | 33.1 | 20.7 | 58.1 | 48.3 | 30.0 |
| Female | 55.7 | 55.9 | 55.3 | 55.6 | 53.4 | 55.9 |
| Age, years | 64.3 (0.17) | 62.2 (0.31) | 61.6 (0.25) | 63.9 (0.28) | 62.5 (0.37) | 63.0 (0.16) |
| Stroke belt residenceb | 56.2 | 56.9 | 52.3 | 56.5 | 60.0 | 54.1 |
| Household income ≤ $35,000 | 40.9 | 30.9 | 27.0 | 46.4 | 42.8 | 30.9 |
| No health insurance | 5.4 | 6.3 | 5.3 | 6.5 | 10.5 | 4.5 |
| Self-reported health of fair/poor | 27.5 | 14.5 | 8.33 | 39.1 | 22.0 | 13.8 |
| High school education or less | 36.2 | 31.1 | 21.7 | 38.1 | 39.7 | 27.8 |
| Anthropometrics | ||||||
| BMI, kg/m2 | 30.8 (0.1) | 29.1 (0.2) | 26.9 (0.1) | 32.8 (0.2) | 31.5 (0.3) | 28.1 (0.1) |
| Waist circumference, cm | 99.1 (0.3) | 94.5 (0.7) | 89.7 (0.4) | 104 (0.5) | 101 (0.7) | 92.4 (0.3) |
| Lifestyle factors | ||||||
| Current smoker | 11.3 | 11.8 | 9.2 | 9.8 | 14.7 | 10.4 |
| Heavy alcohol use | 4.6 | 5.7 | 4.5 | 1.4 | 3.2 | 5.8 |
| Physical activity frequency < 1 time per week (none) | 32.1 | 29.0 | 23.8 | 35.9 | 31.5 | 26.9 |
| Clinical factors | ||||||
| Estimated GFR (CKD-EPI), mL/min per 1.73m2 | 86 (0.4) | 90 (0.6) | 90 (0.4) | 88 (0.7) | 90 (0.8) | 87 (0.3) |
| Albumin-creatinine ratio, mg/mmol | 44 (4.6) | 14 (2.0) | 11 (0.9) | 68 (8.3) | 31 (6.2) | 19 (2.6) |
| Systolic blood pressure, mmHg | 131 (0.3) | 123 (0.4) | 116 (0.3) | 130 (0.5) | 128 (0.7) | 124 (0.2) |
| Prediabetesc | 26.4 | 20.7 | 15.1 | 27.9 | 56.2 | 15.7 |
| LDL cholesterol, mg/dL | 111 (0.7) | 119 (1.3) | 119 (1.0) | 104 (1.2) | 117 (1.6) | 117 (0.6) |
| HDL cholesterol, mg/dL | 51 (0.3) | 53 (0.7) | 54 (0.5) | 48 (0.5) | 47 (0.7) | 54 (0.3) |
| Triglycerides, mg/dL | 134 (1.7) | 132 (4.5) | 118 (2.0) | 145 (3.6) | 141 (3.4) | 123 (1.5) |
Table values account for sampling weights.
Stroke belt: Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee
Prediabetes is defined as fasting serum glucose between 100 – 125 mg/dL or a random glucose between 140 – 199 mg/dL.
Biomarker assessment
Blood samples from the BioMedioR participants will be retrieved from the baseline visit biorepository to measure biomarkers of interest. Biomarker selection was based upon the literature or preliminary data of cross-sectional associations of biomarkers measured previously in other REGARDS samples and required that each biomarker have some evidence of a racial difference in distribution and/or potential importance in identifying risk of incident hypertension or diabetes. Biomarkers also had to be robust to REGARDS sample procurement, shipping and storage methods [45].
Upon biomarker assay completion, laboratory data will be transmitted to the REGARDS Coordinating Center at the University of Alabama at Birmingham for data cleaning and integration into the REGARDS dataset. A BioMedioR data set will be maintained with unique participant identifiers, sampling weights and biomarker assay results.
BioMedioR Statistical Analysis Plan
Because hypertension and diabetes status were only evaluated at two time-points (baseline and second in-person visits), the analysis of incident hypertension and diabetes will be performed using Poisson regression with robust standard error estimation [56]. Each biomarker will be examined in analytic models for incident hypertension and for incident diabetes at the second visit. Risk factors for hypertension or diabetes will be adjusted for in the models as appropriate. For some analyses we may consider studying the joint influence of a set of biomarkers considered together, using analytical approaches tailored to the goal of that analysis. To address the role of biomarkers in explaining racial differences in hypertension and diabetes incidence, we will assess effect modification between biomarker and race in the analytic models, as well as perform mediation analyses. If effect modification is present, mediation methods that allow for biomarker-race interactions will be utilized [57]. Specifically mediation methods, such as inverse odds ratio weighting methods [58, 59], will be used to examine biomarker contributions to race differences in incident hypertension and diabetes. One advantage of inverse odds ratio weighting methodology is the accommodation of multiple mediators when interest lies in a set of biomarkers.
DISCUSSION
We have outlined the goals, study design and analytic plan for a nested biomarker study, BioMedioR, which will efficiently identify pathways that may help explain racial differences in hypertension and diabetes incidence in a large US cohort study. While the primary focus of BioMedioR is incident hypertension and diabetes, the proposed design also allows flexibility to analyze biomarkers and racial disparities in the incidence of other risk factors (e.g., incident kidney disease, dyslipidemia). Associations of risk factors with incident hypertension and diabetes were similar in the BioMedioR sample, suggesting it reflects the larger sample.
During the approximately ten years between the baseline and second visit, 22% of participants died and 24% withdrew or chose not to participate in the second visit, invoking selective attrition concerns. Our previous investigation of this possibility of selection bias in the study of the Black-White disparity in incident hypertension did not find evidence of measurable selection bias due to differential attrition [60]. Future BioMedioR analyses may choose to incorporate methods to account for differential attrition, such as inverse probability weighting or survival average causal effects, when examining other outcomes such as diabetes [60]. However, since no baseline covariates, including race, were notably predictive of study withdrawal (that is, no second visit among participants alive), we expect results to be similar between complete case and methods accounting for study withdrawal in analyses of other exposure-outcome relationships.
With the goal of advancing scientific understanding of contributors to the higher cardiovascular disease burden in Black adults, a first and pivotal step in designing interventions is to reduce or eliminate disparities in the contributors (a.k.a., risk factors) for these cardiovascular diseases. With these advances, we hope that interventions can be mounted to reduce disparities in the risk factors, and that this will subsequently reduce the disparities in cardiovascular health that have proven persistent over decades in the US.
Supplementary Material
Acknowledgements
We thank the other investigators, the staff, and the participants of the Reasons for Geographic and Racial Differences in Stroke study for their valuable contributions. A full list of participating Reasons for Geographic and Racial Differences in Stroke investigators and institutions can be found at www.uab.edu/soph/regardsstudy/.
Funding
This research project is supported and co-funded by the National Institute of Neurological Disorders and Stroke and the National Institute on Aging (cooperative agreement U01 NS041588). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institute on Aging. Representatives of the National Institute of Neurological Disorders and Stroke were involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D. Leann Long, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama.
Boyi Guo, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama.
Leslie A. McClure, Department of Epidemiology and Biostatistics, Drexel University, Philadelphia, Pennsylvania
Byron C. Jaeger, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama
Stephanie Tison, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama.
George Howard, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama.
Suzanne E. Judd, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama
Virginia J. Howard, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama
Timothy B. Plante, Department of Medicine, Larner College of Medicine at the University of Vermont
Neil A. Zakai, Department of Medicine, Larner College of Medicine at the University of Vermont Department of Pathology & Laboratory Medicine, Larner College of Medicine at the University of Vermont.
Insu Koh, Department of Pathology & Laboratory Medicine, Larner College of Medicine at the University of Vermont.
Katharine L. Cheung, Department of Medicine, Larner College of Medicine at the University of Vermont
Mary Cushman, Department of Medicine, Larner College of Medicine at the University of Vermont; Department of Pathology & Laboratory Medicine, Larner College of Medicine at the University of Vermont.
References
- 1.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard G, Moy CS, Howard VJ, McClure LA, Kleindorfer DO, Kissela BM, et al. Where to Focus Efforts to Reduce the Black-White Disparity in Stroke Mortality: Incidence Versus Case Fatality? Stroke. 2016;47(7):1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136(21):e393–e423. [DOI] [PubMed] [Google Scholar]
- 4.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, et al. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64(5):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17(6):1710–5. [DOI] [PubMed] [Google Scholar]
- 7.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Hayward MD, Yu YL. Life Course Pathways to Racial Disparities in Cognitive Impairment among Older Americans. J Health Soc Behav. 2016;57(2):184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–92. [DOI] [PubMed] [Google Scholar]
- 11.Howard G, Peace F, Howard VJ. The contributions of selected diseases to disparities in death rates and years of life lost for racial/ethnic minorities in the United States, 1999–2010. Prev Chronic Dis. 2014;11:E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajeu GS, Safford MM, Howard G, Howard VJ, Chen L, Long DL, et al. Black-White Differences in Cardiovascular Disease Mortality: A Prospective US Study, 2003–2017. Am J Public Health. 2020;110(5):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amos W, Manica A. Global genetic positioning: evidence for early human population centers in coastal habitats. Proc Natl Acad Sci U S A. 2006;103(3):820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster MW, Sharp RR. Race, ethnicity, and genomics: social classifications as proxies of biological heterogeneity. Genome Res. 2002;12(6):844–50. [DOI] [PubMed] [Google Scholar]
- 15.Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, et al. Association of Clinical and Social Factors With Excess Hypertension Risk in Black Compared With White US Adults. JAMA. 2018;320(13):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108(24):2993–9. [DOI] [PubMed] [Google Scholar]
- 17.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44(6):859–65. [DOI] [PubMed] [Google Scholar]
- 18.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–51. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49(3):432–8. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. [DOI] [PubMed] [Google Scholar]
- 21.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Valkonen VP, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47(8):1403–10. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem. 2009;55(9):1627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42(12):3369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–9. [DOI] [PubMed] [Google Scholar]
- 26.Plante TB, Long DL, Guo B, Howard G, Carson AP, Howard VJ, et al. C-reactive protein and hypertension incidence in black and white Americans: REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Am J Hypertens. 2021;34(7):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, et al. Racial Differences in Plasma Levels of N-Terminal Pro-B-Type Natriuretic Peptide and Outcomes: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol. 2018;3(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landry K, Alexander K, Zakai N, Judd S, Kleindorfer D, Howard V, et al. Association of stroke risk biomarkers with stroke symptoms: the Reasons for Geographic and Racial Differences in Stroke cohort. Journal of Thrombosis and Haemostasis. 2017;15(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke; a journal of cerebral circulation. 2014;45(6):1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenny NS, Callas PW, Judd SE, McClure LA, Kissela B, Zakai NA, et al. Inflammatory cytokines and ischemic stroke risk. The REGARDS cohort. 2019;92(20):e2375–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, et al. Racial differences in plasma levels of N-terminal pro–B-type natriuretic peptide and outcomes: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. JAMA cardiology. 2018;3(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu WJ, Ma GZ, Ni Y, Hu XS, Luo DZ, Zeng XW, et al. Copeptin and NT-proBNP for prediction of all-cause and cardiovascular death in ischemic stroke. Neurology. 2017;88(20):1899–905. [DOI] [PubMed] [Google Scholar]
- 33.Wolsk E, Claggett B, Pfeffer MA, Diaz R, Dickstein K, Gerstein HC, et al. Role of B-Type Natriuretic Peptide and N-Terminal Prohormone BNP as Predictors of Cardiovascular Morbidity and Mortality in Patients With a Recent Coronary Event and Type 2 Diabetes Mellitus. J Am Heart Assoc. 2017;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterling MR, Durant RW, Bryan J, Levitan EB, Brown TM, Khodneva Y, et al. N-terminal pro-B-type natriuretic peptide and microsize myocardial infarction risk in the reasons for geographic and racial differences in stroke study. BMC cardiovascular disorders. 2018;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spanaus KS, Kronenberg F, Ritz E, Schlapbach R, Fliser D, Hersberger M, et al. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem. 2007;53(7):1264–72. [DOI] [PubMed] [Google Scholar]
- 36.Cushman M, Callas PW, McClure LA, Unverzagt FW, Howard VJ, Gillett SR, et al. N-terminal pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. Journal of Alzheimer’s Disease. 2016;54(2):497–503. [DOI] [PubMed] [Google Scholar]
- 37.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arora P, Kalra R, Callas PW, Alexander KS, Zakai NA, Wadley V, et al. Lipoprotein(a) and Risk of Ischemic Stroke in the REGARDS Study. Arterioscler Thromb Vasc Biol. 2019;39(4):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWeele TJ. Explanation in causal inference : methods for mediation and interaction. New York: Oxford University Press; 2015. [Google Scholar]
- 40.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. [DOI] [PubMed] [Google Scholar]
- 41.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of neurology. 2011;69(4):619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard VJ, Safford MM, Allen S, Judd SE, Rhodes JD, Kleindorfer DO, et al. Stroke Symptoms as a Predictor of Future Hospitalization. J Stroke Cerebrovasc Dis. 2016;25(3):702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph JJ, Bennett A, Echouffo Tcheugui JB, Effoe VS, Odei JB, Hidalgo B, et al. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia. 2019;62(3):426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 47.Alexander KS, Zakai NA, Gillett S, McClure LA, Wadley V, Unverzagt F, et al. ABO blood type, factor VIII, and incident cognitive impairment in the REGARDS cohort. Neurology. 2014;83(14):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One. 2018;13(3):e0194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung KL, Zakai NA, Callas PW, Howard G, Mahmoodi BK, Peralta CA, et al. Mechanisms and mitigating factors for venous thromboembolism in chronic kidney disease: the REGARDS study. J Thromb Haemost. 2018;16(9):1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cushman M, Callas PW, McClure LA, Unverzagt FW, Howard VJ, Gillett SR, et al. N-Terminal Pro-B-Type Natriuretic Peptide and Risk of Future Cognitive Impairment in the REGARDS Cohort. J Alzheimers Dis. 2016;54(2):497–503. [DOI] [PubMed] [Google Scholar]
- 51.Venkatraman A, Callas P, McClure LA, Unverzagt F, Arora G, Howard V, et al. Galectin-3 and incident cognitive impairment in REGARDS, a cohort of blacks and whites. Alzheimers Dement (N Y). 2018;4:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panwar B, Judd SE, Howard VJ, Jenny NS, Wadley VG, Gutierrez OM. Vitamin D, Fibroblast Growth Factor 23 and Incident Cognitive Impairment: Findings from the REGARDS Study. PLoS One. 2016;11(11):e0165671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–72. [DOI] [PubMed] [Google Scholar]
- 54.Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics. 2004;60(4):1015–24. [DOI] [PubMed] [Google Scholar]
- 55.Fan CT, Muller ME, Rezucha I. Development of Sampling Plans by Using Sequential (Item by Item) Selection Techniques and Digital Computers. Journal of the American Statistical Association. 1962;57(298):387–402. [Google Scholar]
- 56.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 57.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181(5):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tchetgen Tchetgen EJ. Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med. 2013;32(26):4567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long DL, Howard G, Long DM, Judd S, Manly JJ, McClure LA, et al. An Investigation of Selection Bias in Estimating Racial Disparity in Stroke Risk Factors. Am J Epidemiol. 2019;188(3):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.