Abstract
Estrogen mainly binds to estrogen receptors (ERs) to regulate menstrual cycles and reproduction. The expression of ERalpha (ERα), ERbeta (ERβ), and G-protein-coupled estrogen receptor (GPER) mRNA could be detected in ovary, suggesting that they play an important role in estrogen signal transduction in ovary. And many studies have revealed that abnormal expression of estrogen and its receptors is closely related to ovarian disease or malignant tumors. With the continuous development and research of animal models, tissue-specific roles of both ERα and ERβ have been demonstrated in animals, which enable people to have a deeper understanding of the potential role of ER in regulating female reproductive diseases. Nevertheless, our current understanding of ERs expression and function in ovarian disease is, however, incomplete. To elucidate the biological mechanism behind ERs in the ovary, this review will focus on the role of ERα and ERβ in polycystic ovary syndrome (PCOS), ovarian cancer and premature ovarian failure (POF) and discuss the major challenges of existing therapies to provide a reference for the treatment of estrogen target tissue ovarian diseases.
Keywords: estrogen, estrogen receptor α, estrogen receptor β, ovary, biosynthesis
Introduction
In the female reproductive system, there are two major components, the uterus, which is used for pregnancy, and the ovary, which generates ova. Ovaries are the central organ of female reproduction, providing hormones such as testosterone, inhibin, progesterone, and estrogen, which play crucial roles in menstrual cycle regulation and maintenance of fertility (1). Hormones produced by the ovaries also cause periodic changes in the endometrium, the period of endometrium proliferation corresponding to the follicular phase of the ovary and forms the basis for a successful pregnancy. In particular, estrogen is an important class of steroid hormones that include estrone (E1), estradiol (E2 or 17β-estradiol), and estriol (E3) (2), of which E2 is the most abundant and active estrogen. Estrogen exerts a critical influence on female reproduction via the two principal estrogen receptors (ERs), ERα and ERβ.
Deficient endogenous estrogen production can result in functional reproductive disorders in women, which profoundly influence infertility. Common diseases in women (e.g., polycystic ovary syndrome, endometriosis, breast cancer, and ovarian cancer) are associated with aberrant estrogen-mediated functions and the expression of corresponding ERα and ERβ. A better understanding of the mechanism has been achieved through extensive studies of ERs. Nevertheless, further analyses to reach convincing conclusions and determining efficient precautions remain necessary. This review will focus on the physiological roles of the estrogen and ERs in regulating ovary and current major medications used to stimulate ovarian function.
Estrogen and ERs Expression Profile in the Ovary
Estrogen Synthesis
Estrogen plays a fundamental role in the development and normal physiological function of the human female reproductive tract, including the ovaries and fallopian tubes. Before puberty, only low concentrations of endogenous estrogen is present in girls; the onset of puberty occurs along with an increased level of hypothalamic gonadotropin-releasing hormone (3). Gonadotropin-releasing hormone triggers the secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the anterior pituitary gland; subsequently, LH and FSH cause the ovarian follicle to generate large quantities of estrogen. In females, estrogen is produced by locally expressed p450 aromatase from follicular androgens in the ovary and other estrogen-responsive tissues ( Figure 1 ). Specifically, three major forms of endogenous estrogen with estrogenic hormonal activity are present in women: E1, E2, and E3. In pre-menopausal women, E1 and E2 are primarily synthesized in and secreted from the ovarian follicle. E2 is the most potent and prevalent estrone steroid (70-500μg daily, depending on the phase of the menstrual cycle); accordingly, E2 is the major product of the overall estrogen biosynthesis process. In post-menopausal women, E1 becomes the most prevalent endogenous estrogen; it is produced by conversion of androstenedione (secreted by the adrenal cortex) to E1 by peripheral tissues (4). E3 is produced in large quantities by the placenta during pregnancy; it is formed from E1 through 16α-hydroxylation (5). The process of differentiation during the embryonic and prenatal period, as well as the maturation of these tissues during puberty, determines fertility in females.
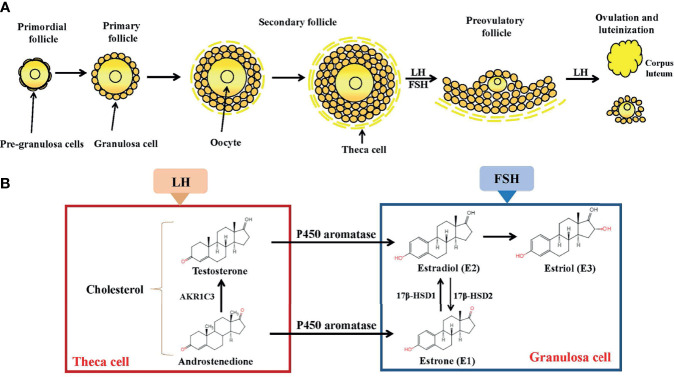
Figure 1.
Folliculogenesis and follicular estrogen synthesis. (A) Estrogen biosynthesis in ovarian follicles is known as the ‘two-cell-two-gonadotropin system’ because both cells (granulosa and theca cells) and gonadotropins (FSH and LH) are indispensable for the generation of estrogen in ovarian follicles. Follicles are mainly composed of oocytes, granulosa cells and theca cells. Folliculogenesis develops from primordial, primary, secondary follicles and preovulatory follicles before ovulation occurs. After ovulation, the corpus luteum activity was restored. (B) Synthesis of estrogen. In theca cells, LH stimulation produces androstenedione and testosterone. In granulosa cells, testosterone is converted by P450aromatase into estradiol. LH, Luteinizing Hormone; FSH, follicle stimulating hormone; AKR1C3, aldo-keto reductase 1C3; 17β-HSD, 17β-hydroxysteroid dehydrogenase.
Although circulating estrogen exists in a dynamic equilibrium with metabolic interconversions, E2 is the principal intracellular human estrogen because its potency is substantially greater than that of its metabolites, E1 and E3, at the receptor level (6). It is well known that in the late follicular phase of the menstrual cycle when concentrations of E2 reach a persistently high critical concentration (≥ 200pg/mL), a dramatic shift occurs in E2 action, such that feedback changes from negative to positive at both pituitary and hypothalamic levels; after the shift, E2 becomes a positive inducer of the anterior pituitary and triggers it to release more FSH and LH. These increased levels of FSH and LH stimulate ovarian follicles to produce more E2 (7–9). Following ovulation, E2 concentrations temporarily decrease, but are restored by corpus luteum activity ( Figure 1 ).
ERs Gene, Protein, and Expression in the Ovary
The levels of ERα and ERβ expression in different tissues varied greatly. In rats, ERα mRNA is mainly expressed in predominant in the uterus, mammary gland, testis, pituitary, adipose tissue, kidney and skeletal muscle, while ERβ mRNA is dominant in the ovary, prostate, lung, cardiovascular and central nervous systems (10–12). For example, the most obvious is that ERβ/ERα in the ovary is much higher than in uterus (11). In human tissues, ERs have two isoforms, ERα and ERβ. ERα is encoded by ESR1 gene on chromosome 6q25.1 while ERβ is encoded by ESR2 on chromosome 14q23.2 (13, 14). Northern blot analysis revealed that ERβ transcripts were detected in human testis, thymus, spleen and ovary (14). ERα mainly participates E2 signaling in the uterus, mammary glands, pituitary, skeletal muscle, adipose tissue and bone (15, 16).
Furthermore, with the development of research, several discrepancies in ERα and ERβ are gradually prominent, even in the same tissue, the expressions of ERα and ERβ positions are also different. In the rat ovary, ERβ is detectable in granulosa cells of maturing follicles; conversely, ERα is more abundant in the interstitium and theca cells (17, 18). ERα and ERβ are differentially expressed in human bone, prostate and breast tissue (15, 19, 20). Both normal and neoplastic human tissues and cell lines express detectable ERβ mRNA (21–23).
The data of ER expression location in the ovary is obtained by in situ hybridization of premenopausal female reproductive tissue ( Table 1 ). In the human ovary, immunohistochemical studies have shown that ERα and ERβ are differentially expressed in granulosa cells that develop follicles. ERβ immunoreactivity is found in granulosa cells of growing follicles at all stages from primary to mature follicles, and also in some interstitial gland cells (15, 29). ERα predominantly occurs in the thecal, interstitial gland and germinal epithelium cells. Before the discovery of ERβ, ER was described in 1993 in the ampullary and fimbrial sections of the fallopian tube (30, 31). The present observations suggest that estrogen stimulates the proliferation of granulosa cells in small follicles, enhance the sensitivity of granulosa cells to FSH and LH, and modulate the biosynthesis of progestins and cAMP (32). Several studies have also reported GPER mRNA and protein is localized on the ovary (27, 28). However, the exact role and mechanism of estrogen in human ovaries remain to be elucidated.
Table 1.
Expression of ERs in normal ovary tissue.
| Tissue | Specific location | Method | Results | References |
|---|---|---|---|---|
| Ovary | the granulosa cells of the developing follicles | Immunocytochemistry | ERα+; ERβ+ (ERα and β are differentially expressed) | (15, 24) |
| Ovary | pre-antral follicles | Immunohistochemistry | ERβ+ | (24) |
| Ovary | mature antral follicles | Immunohistochemistry | ERα+; ERβ+ | (24) |
| Ovary | in the surface epithelium | RT-PCR | ERα+; ERβ+ | (25) |
| Ovary | granulosa cells, endothelial cells | Immunocytochemistry | ERβ2+; ERβ4+; ERβ5+ | (26) |
| Ovary | granulosa cells | RT-PCR; Western blotting | GPER+ | (27, 28) |
+, expressed; ER, estrogen receptor; RT-PCR, reverse transcription-polymerase chain reaction.
Mechanism of ERs Action in the Ovary
Estrogen is produced in the ovaries and is present in highest concentrations in this organ. In theory, it continuously saturates and activates homologous receptors and could lead to continuous transcriptional activation. Estrogen signaling pathways are mainly mediated by ERα and ERβ which located at different chromosomal locations. At the cellular level, they function synergistically or antagonistically, therefore the final cellular outcome will be determined by their cumulative interaction of activation and/or inhibition pathways (33). Numerous mRNA splice variants exist for both receptors in both normal and diseased tissues (34). ER consists of six components: an amino-terminal domain (A/B domain), a DNA-binding domain (DBD; C domain), a hinge region (D domain), a ligand-binding domain (LBD; E domain), and a carboxy-terminal domain (F domain) (35). ERα and ERβ share similar structures and they form homodimers (ERαα/ERββ) or heterodimers (ERαβ) upon estrogen binding (36). The amino-terminal A/B domains of ERα have ligand-independent AF-1, while the LBD includes the ligand-dependent AF-2 which is significant in ligand-dependent transcriptional regulation (37). In contrast, ERβ displays a lower affinity for ERE binding and considerably lower transcriptional activity in the E2-induced ERE-dependent genomic signaling pathway.
ER-mediated genomic pathway can be subdivided into ERE-dependent and ERE-independent subtypes, and both processes typically occur within hours (34). In the “classical” mechanism of estrogen action, the nuclear ER-estrogen complex directly binds to a specific DNA sequence called the estrogen responsive element (ERE) in the promoter regions of estrogen-responsive genes, then regulates the transcription of these genes (38). Alternatively, the nuclear ER-estrogen complex can bind to ERE sequences indirectly through protein-protein interactions with transcription factors, like activator protein 1 (AP-1) or specificity protein 1 (SP-1) that occur in the promoter regions of ERE sequences. These interactions result in the recruitment of co-regulatory proteins (co-activators or co-repressors) to the promoter, changes in mRNA levels, and the production of proteins that are associated with physiological responses (39–42).
Furthermore, ERα was found to be association with the cell membrane, allowing for rapid ‘non-genomic’ estrogen signaling. The non-genomic pathway typically involves cytoplasmic proteins, growth factors, and other membrane-initiated signaling pathways (43–45). Alternatively, in addition to hormone-mediated activation, it is now well accepted that ER function can be modulated by extracellular signals in the absence of E2. Polypeptide growth factors, such as epidermal growth factor (EGF) and insulin-like growth factor-I (IGF-I), activate ER and increase the expression of ER target genes in an estrogen-independent manner (46). Although the molecular mechanisms involved in ligand independent ER activation have been characterized, the biological role of these processes requires further understanding.
GPER is a novel membrane estrogen receptor responsible for rapid non-genomic estrogen response and transcriptional regulation that activates numerous signal transduction pathways. A study demonstrated that ERα is one of the transcription factors downstream of GPER, suggesting the interaction between non-genomic and genomic estrogen receptors (47).
The Role of ERs: Evidence From Animal Models
The ERKO mice are an optimal biological model to the research work for understanding the physiological action and mechanisms of estrogen action ( Table 2 ). Estrogen via ERs is essential for the establishment and maintenance of pregnancy. As evidenced by the αERKO mouse model, adult αERKO female mice are infertile due to hypoplastic uteri and hyperemic ovaries without corpora (48, 49). In wild-type rodents, the primary site of estrogen feedback is hypothalamus, where αERKO females cause increased hypothalamic gonadotrophin releasing hormone (GnRH) secretion, which then manifests as hypergonadotropism, resulting in ovarian cyst formation. Female mice with αERKO have elevated plasma estradiol and androstendione levels, as well as elevated testosterone levels. In addition, an obvious endocrine sex reversal observed in αERKO/αβERKO ovaries, characterized by ectopic expression of HSD17β3, as this enzyme is unique to the testes (48, 50).
Table 2.
Animal models of estrogen receptors in the hypothalamic-pituitary-ovarian axis.
| Models | Estrous cycle | Ovarian cyst | Fertility | Androgen levels | LH levels | Notes | References |
|---|---|---|---|---|---|---|---|
| αERKO mouse | Irregular | YES | Infertile | Androstendione levels↑; testosterone levels↑ | Circulating LH↑; plasma LH levels were significantly lower than ovariectomized wild-type females | Hypoplastic uteri; hyperemic ovaries; E2 levels↑ | (48, 49) |
| αβERKO mice | NA | NA | Infertile | Plasma testosterone levels↑ | Serum LH levels was higher than αERKO females | Normal reproductive tract; no corpora lutea were observed; apparent sex reversal | (48, 50) |
| ENERKI mice | NA | YES | Infertile | Testosterone levels↑ | Serum LH levels↑ | Have hypoplastic uterine tissues; rudimentary mammary gland ductal trees; E2 levels↑ | (51) |
| βERKO | NA | No | Exhibit variable degrees of Subfertility | Plasma testosterone levels↑ | Exhibited wild type-like levels | Aromatase activity↓; estradiol synthesis↓; have normal mammary histology | (53, 55) |
| A neuron-specific ERα mutant mouse | Failed to exhibit estrous cycles | NA | Infertile | NA | Basal LH levels are not elevated | Lack estrogen positive feedback; have dilated, fluid-filled uteri; increased numbers of antral follicles; ack of corpora lutea | (60, 61) |
| neuron-specific ERβ null mice | Normal estrous cycles | NA | Impairment of fertility | NA | Increase in LH secretion after ovariectomy | Normal negative feedback | (61, 70) |
| ERαflox/flox αGSUcre mice | Irregular | NA | Infertile | NA | Basal serum LH levels were not elevated | Maintain a basal level of serum FSH; their ovulatory capacity is comparable to controls; | (64) |
| PitESR1KO | Irregular | YES | Subfertile or infertile | NA | Serum LH levels↑ | A decrease in the number of litters and size of the litters; | (65) |
| PitERtgKO | Irregular | YES | NA | Testosterone levels↑ | Serum LH levels were normal | Pituitary ERα is involved in negative feedback regulation of estrogen; an anomalous sporadic LH secretion profile | (66) |
| GPER KO mouse | Regular | NO | Fertility | NA | Serum LH levels were normal | Normal function of the HPG axis; ovaries developed normally | (69) |
↑ = upregulated; ↓ = downregulated; NA: not available or not assessed. ER, estrogen receptor; GPER, G-protein-coupled estrogen receptor; FSH, follicle stimulating hormone; LH, luteinizing hormone; HPG, hypothalamic-pituitary-gonadal.
ENERKI mice are a mutation converts glycine to leucine at residues 525 (G525L) in LBD of ERα, lacking ERα interaction and response with endogenous estrogen. The phenotypes of ENERKI mice and αERKO mice are similar. The mammary gland ductal trees of ENERKI mice are rudimentary, with no terminal end bud formation, ductal elongation, branching, or alveolar differentiation (51). ENERKI mice were infertile due to being unable to ovulate normally and had hypoplastic uterine tissues. Phenotypical evaluation of the ENERKI mice has also established that ERα ligand-induced, but not ligand-independent, signaling is critical in female mammary gland and reproductive tract development. Furthermore, ENERKI mice have elevated LH levels, cystic ovaries and high androgen secretion, reminiscent of human polycystic ovary syndrome (PCOS) (52, 53). And ENERKI females’ granulosa cells are disordered and not encapsulated in follicular structures, similar to certain types of ovarian granulosa cell tumors.
Female mice lacking ERβ are normally fertile and show normal sexual behavior, but in a continuous mating study, they have reduced litter numbers and sizes compared with wild-type mice (54). This decrease in fertility is the result of impaired follicular maturation and a decrease in the number of follicles responsive to LH. There are fewer corpora lutea in the ovaries of βERKO mice than WT ovary after superovulation, indicating that some follicles failed to rupture and discharge oocytes during the ovulatory hCG surge. ERβ mediates the stimulating effect of estrogen on granulosa cell proliferation and βERKO mice have an inefficient and rare ovulation response (55). Granulosa cells of βERKO ovaries exhibited an attenuated response to FSH-induced differentiation after PMSG treatment, which is reflected in the insufficient expression of LH receptors, decreased aromatase activity and reduced estradiol synthesis, accompanied by incomplete expansion of the cumulus-oocyte complex (56). The female mice with ERβ mutations have normal mammary histology and lactate effectively. These studies might help to determine that ERβ is essential for expelling healthy oocytes but not essential for female sexual differentiation, fertility, or lactation.
Briefly, αERKO and βERKO female mice have an appropriate development female reproductive tract (57). However, lacking sensitivity to estrogen severely affects sexual maturation of the entire reproductive tract in the αERKO female and ovarian function in βERKO female. Mice lacking ERα and ERβ exhibit a phenotype similar to αERKO mice, as female mice are infertile (58). Further investigation is required to determine whether there are potential compensatory and synergistic effects between ERα and ERβ.
There are two known forms of nuclear ER, ERα and ERβ, in the tissues of hypothalamic-pituitary-gonadal (HPG) axis. Serum sex hormone levels are strictly regulated by the HPG axis and are associated with follicular growth in the ovaries. The symptoms of female reproductive disease PCOS may result from a disturbance of feedback regulation system of the HPG axis. The regulation of the GnRH neuronal network by E2 involves both negative and positive feedback effects, which together are responsible for producing the ovarian cycle in adult women (59). Estrogen regulates GnRH neurons to control ovulation in mammals. As GnRH neurons do not express ERα, and the positive feedback of estrogen to GnRH neurons is indirect. Mice harboring a neuron-specific ERα deletion was infertile and lacked estrogen positive feedback, and the ERα-expressing neurons have been found to modulate GnRH neurons located within the periventricular nuclei of the rostral hypothalamus (60). The study has provided evidence to show that the ovulation is mediated by estrogen actions on ERα-expressing neuronal afferents to GnRH neurons. Furthermore, ablation of ERα from neurons expressing calmodulin kinase IIα using a tamoxifen-based inducible Cre-LoxP approach displayed that ERα is a key receptor responsible for acute negative estrogen feedback in the mouse brain (61). However, ERβ is expressed in GnRH neurons. Global ERβ mutant mice showed normal patterns of estrogen-induced GnRH activation and LH surge secretion. This indicated that ERβ-regulated signaling in GnRH neurons is not important for estrogen positive feedback (60). Mutant mice with selective deletion of ERβ and its functional splice variants in GnRH neurons also suggested that ERβ has no critical role in acute negative feedback in GnRH neurons (61, 62).
Both ERα and ERβ are expressed in the rat pituitary gland, yet diverse lines of evidence indicate that estrogen-induced pituitary changes are presumably mediated by ERα (12, 63). Gieske et al. produced a ERαflox/flox αGSUcre mice line by deleting ERα in pituitary gonadotroph (64). The basal LH and FSH levels of female pituitary-specific ERα knockout mice were not elevated and their ovulation ability was comparable to that in controls, their estrous cycles were irregular and infertile, suggesting that the LH surge is mistimed, attenuated, or absent. Taken together, ERα in the pituitary gonadotroph is required for estrous cyclicity and fertility in females. In addition, these findings support that the pituitary is not the primary target of negative estrogen feedback in mice, but rather the hypothalamus, compared to the ERα -/- phenotype.
In another study, however, estrogen positive and negative feedback was disrupted in female mice lacking ERα in the pituitary gonadotroph (PitESR1KO) (65). Female PitESR1KO mice were subfertile or infertile, had higher serum LH levels than wild-type, along with a lack of surges in LH values and regular estrous cycles. Some hemorrhagic cystic follicles were also noted in these mice.
Due to the varied phenotypes reported in pituitary-specific ERα KO mouse models, Arao et al. develop a unique model that reintroduces ERα expression exclusively in the pituitary on the background of a global ERα-null (PitERtgKO) mouse (66). The findings suggested that serum E2 and LH levels were normalized in PitERtgKO females. While PitERtgKO female mouse caused a more severe cystic and hemorrhagic phenotype than observed in ERαKO females, implying that anomalous sporadic LH secretion is an important factor to induce severe ovarian phenotype. The results also further suggested that pituitary ERα is involved in negative feedback regulation of estrogen and hypothalamus ERα is required for precise control of LH secretion (66).
Wang et al. reported that GPER expression in hamster ovaries depends on the estrous cycle, with GPER mRNA and protein abundance in the granulosa and theca cell layers peaking on day 3 of the estrus cycle and decreasing on day 4 (67). GPER has been shown to regulate the E2-mediated stimulation of primordial follicle formation in the hamster ovary (68). However, the GPER KO mouse model exhibits normal fertility and reproduction, which may explain the lack of particularly strong effects of GPER on normal reproductive physiology (69). Due to species differences, reproductive data from rodents may not apply to humans.
ER knock-out models suggest that ERα plays an active role in the reproductive system, and ERβ mediates estrogen stimulation of granulocyte proliferation, which is important during ovulation, and the role of GPER appears to be less significant than that of ERα and ERβ. In general, studies using animal models have highlighted the importance of ERα and ERβ signaling in the ovary and the effect of ERs on the gonadal axis on the ovary.
ERs and the Main Pathological Conditions of Ovary
ER is a marker of ovarian disease in the numerous conditions affecting the ovarian functions. Therefore, the mechanism of ER involved in inducing ovarian disease and cancer cell growth should be further studied ( Figure 2 ).
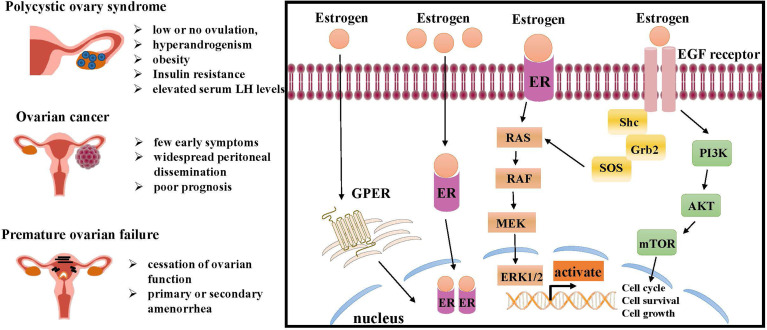
Figure 2.
Molecular pathways of ER regulation in ovarian lesions, like PCOS, ovarian cancer and premature ovarian failure (POF). The interaction of estrogen and ER can initiate intracellular signaling cascades leading to downstream activation of MAPK signaling and PI3K/AKT signaling, both of which are critical for cell growth and proliferation. Estrogen also promotes ovarian disease by regulating GPER. ER, estrogen receptor; RAS, renin-angiotensin system; RAF, residual adversarial fusion; MEK, mitogen-activated protein kina; ERK, extracellular regulated kinase; Grb, cytotoxic protease granzyme B; EGF, epidermal growth factor; PI3K, phosphoinositide 3-kinase; AKT, mTOR, mammalian target of rapamycin; GPER, G-protein-coupled estrogen receptor.
PCOS
PCOS is a state of unbalanced steroid hormone production and activity that affects approximately 4%-18% of women of reproductive age worldwide (71). The most common phenomena are anovulation (or oligo-ovulation) and endometrial changes, which typically result in female infertility. According to the conclusion of the 2003 Rotterdam consensus workshop, the clinical manifestations of PCOS include low or no ovulation, hyperandrogenism, and obesity (72). Insulin resistance and elevated serum LH levels are also common characteristics of PCOS. The criteria included a wider range of symptoms of ovarian dysfunction than the previous NIH classification of 1990. And it has been recognized that not all females with the disease have the biochemical and clinical features defined by PCOS, and that some women with the syndrome have regular cycles, or do not have excess androgens, but will show evidence of ovarian dysfunction (73).
Unlike higher testosterone and lower progesterone levels, estrogen levels in women with PCOS are typically similar to those of healthy women (74). ERβ stimulates follicular growth, induces the expression of specific genes, and increases the number of ovulated oocytes. Disruption of the ERβ gene, therefore, would be expected to have effects on ovarian function and subsequent fertility in the female. In contrast, ERα inhibited ovulation, presumably through effects on the HPG axis and uterine growth (75) ( Figure 2 ). The mechanism of estrogen deficiency or disrupted estrogen synthesis by PCOS remains a longstanding challenge. Upregulation and activation of the WNT2/β-catenin pathway was reportedly tightly associated with estrogen deficiency in PCOS cumulus cells (76). Moreover, the mRNA expression levels of ERα and ERβ in cumulus cells from patients with PCOS are significantly lower than those from healthy controls (75), and considerable alterations in the expression levels of ERα and ERβ are also found in theca and granulosa cells (77); taken together, these findings suggest abnormal folliculogenesis in patients with PCOS.
Interestingly, women with PCOS possess various endometrial abnormalities. Women with PCOS present with alterations in the hypothalamic-pituitary-ovarian axis, which results in persistent circulating levels of estrogen, and complex effects on the endometrium, leading to implantation failure and proliferative aberrations (78). Several endometrial markers associated with the PCOS phenotype have been carried out, explaining some of the adverse endometrium-related clinical manifestations. ER may be the most prominent marker in women with PCOS, as the expression of ER is reportedly increased in the stroma and glandular epithelium of women with PCOS (79–83). Moreover, endometrial ERα immunostaining is reportedly higher in the stroma and epithelium of women with PCOS during the proliferative phase of the menstrual cycle (83, 84), but some studies have shown no alterations in ER expression among patients with PCOS (81). Recent studies have demonstrated that, compared with body mass index-matched controls, proliferative endometrium in women with obesity who have PCOS exhibits lower mRNA levels of ERα and ERα36, as well as a lower ERα/ERβ mRNA ratio (85). It has been reported that the endometrium from PCOS-like rodents and patients with PCOS is morphologically normal, but exhibits structurally and biochemically abnormal responses to hormone stimulation; uterine E2-regulated glycolysis via ERα activation may contribute to successful implantation and the establishment of pregnancy, as E2 is widely regarded as a master regulator of endometrial cell proliferation (86, 87). ERβ-/- mice have defective ovulation reminiscent of PCOS in humans (88). Studies focused on polymorphisms in ER genes have shown no associations between the polymorphisms PvuII and XbaI in ERα and the presence of PCOS (89). In contrast, another study reported that ERα rs9340799 was associated with susceptibility to PCOS in Pakistani women in a sequencing analysis of DNA samples from 96 patients with genetically unrelated PCOS and 96 controls (90). Jiao et al. found that the GA genotype of rs1999805 in ERα increased the risk of PCOS in the Chinese population (91). Furthermore, a study evaluating polymorphisms in ERβ gene demonstrated that the ERβ gene +1730 G/A polymorphism may be associated with pathophysiologic aberrancies observed in patients with PCOS (92).
A normal endocrine feedback loop between organs of the HPG axis exerts a critical influence on reproductive potential. ERs are necessary for the normal function of the hypothalamic-pituitary-ovarian axis. Attenuated E2 negative feedback action by neuronal ERα of the mediobasal hypothalamus may lead to GnRH hypersecretion, and thus LH excess, contributing to ovarian hyperandrogenism in PCOS.
There are a number of reports that the hypothalamic-pituitary-adrenal axis plays a role in the development of PCOS. For example, patients with congenital adrenal hyperplasia possess features of PCOS, are characterized by elevated LH levels and ovarian hyperandrogenism (93). Notably, studies have shown that ERα and ERβ are expressed in the hypothalamus, where they induce GnRH release and upregulate anterior pituitary GnRH receptor expression, thereby promoting the positive regulation of the hypothalamic-pituitary axis by estrogen and maintaining normal ovulation (94, 95). Considering the direct and indirect effects of estrogen on follicle development, maturation and ovulation mediated by ERα and ERβ, it is expected that polymorphisms in ERα and/or ERβ play a role in the persistent anovulation in PCOS. However, studies on SNPs in estrogen signaling and susceptibility to PCOS have been inconsistent. Zhou et al. concluded that no significant association between ERα rs2234693, RS9340799, and ERβ RS4936938 variants and PCOS (96). But we should note that although no statistical significance was found, the rate of RS2234693 polymorphism in ERα in patients with PCOS tended to be higher than in control women.
Studies provide evidence that estrogen may induce its effect on the adrenal gland through receptor-mediated mechanism (97). Although ovaries are the major source of increased androgens in PCOS, studies have found that the adrenal glands also contribute to hyperandrogenism in the syndrome. Approximately 20-33% of PCOS patients present with an excess of adrenal androgens, which is primarily detected by elevated levels of dehydroepiandrosterone sulfate (98). Studies have illustrated that circulating adrenal androgens serve as precursors of ovarian androgens via intraovarian conversion, and that if serum adrenal androgen levels are elevated, it is possible to induce polycystic ovaries functionally and morphologically (99). In contrast, data indicate that in PCOS, the ovaries promoted adrenal androgen overproduction (100, 101). Thus, in PCOS, the ovaries and adrenal glands may be able to interact in a bidirectional manner in addition to the traditional regulation exerted by the hypothalamus and pituitary on either peripheral gland. Nevertheless, the relationship between ERs expression and human PCOS and adrenal gland remains to be further investigated.
Several researches have examined a possible association between GPER and oocyte maturation. For instance, Pang and colleagues treated Atlantic croaker oocytes with E2 and G-1 (GPR30 ligand), indicating GPER maintained oocyte meiotic arrest by activating adenylate cyclase (102). The high expression GPER mRNA in carp oocytes showed the inhibitory effect of E2 on oocyte maturation (103). Peyton et al. also confirmed this idea in zebrafish experiments (104). Subsequently, the relationship between GPER and follicular dysplasia was further revealed in recent studies. Zang et al. analyzed the differences in GPER expression between patients with PCOS and women without PCOS (28). The data indicated the GPER mRNA and protein levels of granulosa cells in PCOS patients were higher than those in the control group at the germinal vesicle stage. Therefore, E2 may be involved in inhibiting oocyte meiosis through GPER. This results in abnormalities oocyte maturation, increased follicular density and augmented androgen synthesis, which is a confluence of factors that induce PCOS.
Ovarian Cancer
Among gynecological malignancies, epithelial ovarian cancer is the most common cause of gynecological cancer death and it is the seventh most common female cancer in the world (105). Every year around the world, 230000 women will be diagnosed and 150000 will die (106). Although many efforts have been made to elucidate the etiology of ovarian cancer and the molecular mechanisms involved in ovarian cell proliferation, the disease remains poorly understood.
The expression levels of ERα are implicated in estrogen-dependent proliferation, invasion and response to endocrine therapy in ovarian cancer (107). ERα is a direct target of the tumor suppressor microRNA (miR)-206, and the introduction of miR-206 mimics inhibits cell proliferation and invasion of ERα-positive ovarian cancer cell lines, CAOV-3 and BG-1 (108). The effects of E2 on cancer progression can be mediated by coding RNAs and non-coding RNAs (ncRNAs). Studies have indicated that three long non-coding RNAs (TC0100223, TC0101686 and TC0101441) are abnormally expressed in ERα-positive epithelial ovarian cancer (EOC) tissues, suggesting that they may have potential for cancer progression (109). And TC0101441 is considered to be an independent prognostic factor for overall survival (109). In vitro studies have used cDNA microarrays to confirm changes in gene expression in E2-treated PEO1 cells (an ER-positive, estrogen-responsive ovarian cancer cell line); the activation of ERα-mediated transcription was shown to be responsible for the observed changes in gene expression, as well as the estrogen-driven growth of epithelial ovarian carcinoma, whereas ERβ played no meaningful role (110).
The level of ERα mRNA in malignant tumors is higher than that in benign tumors, while ERβ mRNA in benign is higher than in malignant tumors. Therefore, we hypothesize that ERβ may have the opposing effects on ovarian cancer. ERβ levels and/or the ERβ/ERα ratio decrease along with the development of ovarian cancer, indicating that loss of ERβ expression affects carcinogenesis. Treatment with the ERβ agonist DPN or re-introduction of ERβ results in notably slower cell growth in both ovarian cell lines SKOV3 and OV2008, and xenografts (111, 112). ERβ decreased the frequency of S phase cells and increased the cells in G2/M phase cells. At the molecular level, the inhibitory effects of ERβ are mediated via the low expression levels of total retinoblastoma (Rb), phosphorylated Rb and phospho-AKT cellular content as well as cyclins D1 and A2, accompanied by a more than 10-fold increase of cyclin-dependent kinase inhibitor p21 (WAF1) transcript levels and upregulation fibulin 1c (112, 113). In addition, part of the anti-proliferative action of ERβ can be explained the direct effect of ERβ on ERα by strongly inhibiting its expression and activity.
In the normal ovary, the levels of ERβ are high, being ERβ1, ERβ2, and ERβ5 the most represented isoforms (114). Different ERβ isoforms may have different roles. Patients with cytoplasmic ERβ2 expression have markedly worse, the 5-year survival rate of patients with serous ovarian cancer expressing cytoplasmic ERβ2 was 32% lower than that of negative patients (115). Cytoplasmic ERβ2 expression was also reported to be associated with chemoresistance. These findings suggested that the sudden diminish in ovarian estrogen might, confer a more aggressive phenotype by the modulation of ERβ2 status, and ultimately contribute to ovarian cancer development.
GPER mRNA is expressed in both benign and malignant ovarian tumors, but GPER mRNA is overexpressed in one-third of malignant tumors (116). GPER is localized in the nucleus and cytoplasm of the biopsy tissues of serous and mucinous ovarian adenocarcinoma. GPER is also expressed in certain cell lines, such as SKOV-3, OVCAR-3, and OVCAR5, and is abundantly co-expressed with ERα and ERβ in the SKOV3 cell line (117, 118). GPER regulates the expression of c-fos and cyclin D1 proteins as well as cancer cell invasion and metastasis factors. In ERα-positive BG-1 ovarian cancer cells, GPER mediated growth response to estrogen and G-1 through activation of the EGFR pathway (119). Atrazine is one of the most common pesticide contaminants in groundwater and surface water (120, 121). In ovarian cancer, atrazine stimulates the expression of multiple estrogen target genes and the proliferation of ovarian cancer cells through the participation of GPER and ERα (122). On cursory consideration, the role of GPER in ovarian cancer may require the co-expression of ERα. On the opposite hand, it has been reported that GPER stimulation in the opposite way, inhibiting the development of cancer. G-1 is a selective GPER agonist that can inhibit the G2/M cell cycle progression and promote apoptosis to control the proliferation of ovarian cancer cells (117).
POF
POF is a heterogeneous condition clinically defined as cessation of ovarian function, with elevated gonadotropins and low estrogen levels under the age of 40 years, presenting approximately 1% of women of reproductive age (123, 124). Recently, the term primary ovarian insufficiency (POI) has been proposed to scientifically describe the state of ovarian function (125). It is characterized by the occurrence of primary or secondary amenorrhea (126). Although it has an iatrogenic or spontaneous etiology, to date, there is no effective treatment to restore ovulation function. However, underlying genetic abnormalities may still be associated with POF, and sequence variations in genes involved in estrogen metabolism may lead to ovarian dysfunction. Given that initial follicular pool size and follicular recruitment rates are associated with age at menopause, genetic variants in sex hormone receptor genes can be considered important risk factors to POF development.
The results related to the distribution of ERα PvuII and XbaI genotypes in patients with POF are contradictory. Bretherick et al. have uncovered in fifty-five POF patients, compared with 107 women from the general population, and 27 women who had proven fertility after age 37, an association between the polymorphic C allele of PvuII polymorphism in ERα gene and secondary POF risk (127). In keeping with this result, recently, M’Rabet et al. found a positive association of the CC-allele of the PvuII polymorphic variant in ERα gene to women suffering from infertility (128). Further, Cordts et al. associated the presence of the C allele of ERα PvuII polymorphism (rs2234693) with POF in Brazilian population, and none of the ERβ polymorphisms evaluated were associated with POF (129). These findings could be due to the decreased of ERα transcription and receptors number in the presence of C allele in PvuII site, which impacts the estrogenic response of tissues, leading to low levels of nuclear transcription factors, allowing apoptotic events with multiple estrogen-responsive target tissues to occur.
Other investigators also have reported the relation of ERα genetic variant and POF. The X allele of XbaI and specific haplo- and diplotype of PvuII and XbaI polymorphisms were associated with a marginally reduced risk of POF occurrence (130). On the other hand, A study detected ERα gene polymorphisms by MGB primer/probe taqman assay from 126 idiopathic POF patients and 221 post-menopausal controls (131). As is shown in the results, a significantly higher frequency of TT genotypes was observed in the POF patients among the ERα PvuII polymorphisms assessed. When the POF patients were further divided into the primary and the secondary POF groups, secondary POF had a higher prevalence of TT genotype than the control. Indeed, the ERα gene polymorphisms may be associated with the risk of idiopathic POF. Overall, ERs could be involved in the pathogenesis of ovarian failure but, to strengthen this hypothesis, more studies are needed to confirm these results.
Drugs Potential for Treating Ovarian Disorders
Estrogen mediate cell proliferation in both normal and malignant cells; therefore, ER antagonists that can inhibit transcription by promoting the binding of co-repressors to the ERE are useful for the prevention of proliferation in malignant tissues. Mixed agonist-antagonists, such as tamoxifen and raloxifene, have distinct pharmacological effects on estrogen target tissues (132). Transcriptional activation of ERα is mediated by AF-1 in the N terminal and AF-2 in LBD, which is needed by the binding of ERα to cofactors (133). AF-1 activity is regulated by the phosphorylation of Ser118 through the growth factor signaling pathway, whilst the activity of AF-2 responds to ligand binding (134, 135). Agonist binding triggers AF-2 activity, but the binding antagonist does not (136). Tamoxifen has a weak agonistic activity. This mild agonistic behavior is caused by phosphorylation of serine 305 of ERα by protein kinase A (PKA) (137). By contrast, human ERβ lacks AF-1 domain activity and is therefore completely dependent on ligand-dependent AF-2 (138). The major determinant of the weak agonist response to tamoxifen is the AF-1 domain of ERα rather than the AF-1 domain of ERβ, which explains the different responses to anti-estrogen tamoxifen (139). In addition, anti-estrogen raloxifene also can block the action of ER by blocking the interaction of AF-2 with p160 coactivator. These drugs are regarded as selective estrogen receptor modulators (SERMs). The use of SERM-targeted ER may be an appropriate approach for the treatment of associated endocrine diseases.
Clomiphene citrate (CC) is another SERM which has ER agonist and antagonist properties, acting as an estrogen agonist at low endogenous estrogen concentrations, otherwise primarily as a competitive antagonist of ER (140, 141). CC is metabolized in the liver and excreted in the stool with a relatively long half-life of 5–7 days (142). CC is highly effective in promoting ovulation in women with PCOS and is recommended as the most used medication for most anovulatory or oligo-ovulatory infertility. And CC induced ovulation in women with PCOS is accompanied by increased secretion of LH and FSH by the pituitary gland and enhanced estrogen secretion (143). It is believed that CC affects hypothalamic ERα which implies enhanced secretion of hypothalamic GnRH pulses, thereby increasing the pulse frequency of LH to promote ovulation (144). Although there is some data regarding its potential harmful effects like affecting endometrial thickness (145, 146). A randomized multicenter trial comparing CC, metformin, and a combination of two drugs in patients with PCOS found that the conception rate was significantly higher in the clomiphene group than in the metformin group (147).
Aromatase, also known as CYP19A1, is responsible for the aromatization of androgen to estrogen. Given that aromatase inhibitors significantly reduce systemic estrogen concentrations, researchers have used aromatase inhibitors to induce ovulation by preventing negative feedback of estrogen on FSH (148). Studies have shown that the aromatase inhibitor letrozole is effective in inducing ovulation and increasing follicle recruitment in patients with polycystic ovary syndrome with few side effects (149). Compared with patients who exhibited progression of ovarian cancer, tumors from stable patients (using CA125 marker criteria of which response is associated with ER expression in a phase II trial of letrozole in ovarian cancer) showed significantly lower expression levels of several estrogen-responsive genes, which were demonstrated to be ERα-dependent and upregulated by E2 in the PEO4 ovarian cancer cell line; moreover, higher aromatase expression has been observed in stable patients (150, 151). Legro et al. conducted a trial in which 750 women with PCOS were randomly assigned to receive letrozole or clomiphene according to modified Rotterdam criteria and demonstrated that letrozole was superior to CC in patients with PCOS, resulting in recommendation to switch from CC to letrozole as the first line agent for ovulation induction in this setting (152).
Oral contraceptives (OCs) are used to treat hirsutism, acne, menstrual irregularities, and irregular menses and of male-pattern alopecia. We should note that adolescents with PCOS may experience distress due to clinical manifestations of hyperandrogenemia. Thus, OC pills could be used as a first line therapy for young women with PCOS who have no reproductive requirements (153). OCs have been proven to contain low doses of estrogen and progestin which regulate ovulation by inhibiting the hypothalamic-pituitary-ovarian axis (154). The positive effect of OCs in the treatment of PCOS is predominantly attributed to the reduction of LH secretion, inhibition of ovarian and adrenal androgen secretion, which decreases free testosterone levels and increases sex hormone-binding globulins production in the liver (155).
The effectiveness of PCOS treatment depends on the dose of estrogen in the contraceptive drug. In general, long-term follow-up and treatment with combined OC is required and at least 6 months of OC therapy is needed to detect improvement in hirsutism or acne in women with PCOS (156). Metformin, an insulin sensitizer, is used to treat PCOS in women with modulated hyperinsulinemia, androgen levels, and menstrual irregularities in women with PCOS (157). However, OCs and metformin, as well as oral contraceptives alone, were reported to be superior to metformin alone in reducing free testosterone levels (158). Contraceptive pills can reduce the risk of endometrial cancer in a long lasting period due to the protective effect of progestin, which is present in OCs (159, 160). However, some studies have provided evidence that OCs may impair tissue insulin sensitivity and increase the potential risk of thrombosis and metabolic disease (154, 155).
Conclusion
Although it has long been suspected that ERs play an important role in in the ovaries, and there is strong evidence that this is the case in many models, the role of estrogen and its receptors in human disease is extraordinarily complex. The co-dependent, redundant, and independent aspects of E2 signaling through both nuclear and membrane-associated ERs are complex and specific to particular cell types, tissues, ligands, and diseases (161). The relative levels of synthesis of ERs and ER variants can also have profound effects on the dynamic and integrated network of cellular events, both in terms of physiology and pathophysiology, in a tissue-specific manner (162). Finally, ER signaling depends on coregulators. Thus, distinct post-translational modifications modulate unliganded or liganded ER functions at each level, and can alter ligand pharmacology (163, 164). GPER inhibits oocyte meiosis to induce polycystic ovary syndrome, and some studies have also shown that GPER is involved in the occurrence of ovarian cancer, but the effect of GPER on ovaries is not mainstream. We mainly review the dominant role of ERα and ERβ in ovarian diseases. The expression levels of ERα and ERβ are significantly altered in PCOS and ovarian cancer, and the polymorphism of ERα gene is associated with POF.
There is mounting evidence suggesting that changing the ratio of ERα/ERβ expression may play a key role in tumor development and progression. In fact, pharmacological activated or inhibited of ERα and/or ERβ has provided the basis for treating many diseases. Studies targeting ERβ and ERα provide a method to search for novel specific SERMs. But Long-term estrogen treatment-related adverse events induced by SERM treatment should be closely monitored as well; identifying ideal SERMs that can exert favorable tissue-selective estrogenic agonist activities on target tissues, while remaining neutral with respect to ER and anti-estrogenic activities in the reproductive system, is the future direction of research in this field (165, 166). Moreover, in order to better understand the complex regulatory mechanisms of ERs actions in women, it is necessary to build a database of female reproductive diseases. Because most knowledge about the role of ERs in disease has been obtained through animal studies.
In this paper, we discuss animal disease models and the causes of different ovarian diseases that provide insight into the roles of estrogen and its receptor in the female ovary. Also, an overview of available evidence indicating a potential role of ERs as a diagnostic or therapeutic target in reproductive endocrine diseases. In view of these considerations, a better understanding of the complex regulatory mechanisms that underlie ER actions is necessary to identify therapeutic approaches that could protect female ovarian health from estrogen, estrogen target tissue malignancies, and diseases associated with estrogen deficiency.
Author Contributions
KY, X-LX, and Z-YH reviewed the literature, wrote the manuscript and designed the figures and tables. JL revised the draft. X-WF and S-LD made substantial contributions to the conception and design of the work, and provided input into manuscript content and composition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Nature Science Foundation Project of China (No. 32072722, 31101714 & 31372307).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Wei Yan, Ph.D. and Ryan Chastain-Gross, Ph.D.(from Liwen Bianji, Edanz Group China www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
- 1. Colvin CW, Abdullatif H. Anatomy of Female Puberty: The Clinical Relevance of Developmental Changes in the Reproductive System. Clin Anat (2013) 26:115–29. doi: 10.1002/ca.22164 [DOI] [PubMed] [Google Scholar]
- 2. Boon WC, Chow JD, Simpson ER. The Multiple Roles of Estrogens and the Enzyme Aromatase. Prog Brain Res (2010) 181:209–32. doi: 10.1016/S0079-6123(08)81012-6 [DOI] [PubMed] [Google Scholar]
- 3. Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson A-M. The Sensitivity of the Child to Sex Steroids: Possible Impact of Exogenous Estrogens. Hum Reprod Update (2006) 12:341–9. doi: 10.1093/humupd/dml018 [DOI] [PubMed] [Google Scholar]
- 4. Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, et al. Estrogen–the Good, the Bad, and the Unexpected. Endocr Rev (2005) 26:322–30. doi: 10.1210/er.2004-0020 [DOI] [PubMed] [Google Scholar]
- 5. Cui J, Shen Y, Li R. Estrogen Synthesis and Signaling Pathways During Aging: From Periphery to Brain. Trends Mol Med (2013) 19:197–209. doi: 10.1016/j.molmed.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible Formation of Breast Cancer Stem Cells and Their Dynamic Equilibrium With non-Stem Cancer Cells via IL6 Secretion. Proc Natl Acad Sci (2011) 108:1397–402. doi: 10.1073/pnas.1018898108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vollman RF. The Menstrual Cycle. Major Probl Obstet Gynecol (1977) 7:1–193. [PubMed] [Google Scholar]
- 8. Knobil E. The Neuroendocrine Control of the Menstrual Cycle. Recent Prog Horm Res (1980) 36:53–88. doi: 10.1016/B978-0-12-571136-4.50008-5 [DOI] [PubMed] [Google Scholar]
- 9. Treloar AE, Boynton RE, Behn BG, Brown BWJI. Variation of the Human Menstrual Cycle Through Reproductive Life. Int J Fertil (1967) 12:77–126. [PubMed] [Google Scholar]
- 10. Byers M, Kuiper GGJM, Gustafsson JA, ParkSarge OK. Estrogen Receptor-Beta mRNA Expression in Rat Ovary: Down-Regulation by Gonadotropins. Mol Endocrinol (1997) 11:172–82. doi: 10.1210/mend.11.2.9887 [DOI] [PubMed] [Google Scholar]
- 11. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue Distribution and Quantitative Analysis of Estrogen Receptor-Alpha (ER Alpha) and Estrogen Receptor-Beta (ER Beta) Messenger Ribonucleic Acid in the Wild-Type and ER Alpha-Knockout Mouse. Endocrinology (1997) 138:4613–21. doi: 10.1210/endo.138.11.5496 [DOI] [PubMed] [Google Scholar]
- 12. Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors Alpha and Beta. Endocrinology (1997) 138:863–70. doi: 10.1210/endo.138.3.4979 [DOI] [PubMed] [Google Scholar]
- 13. Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, et al. Cloning of the Human Estrogen-Receptor Cdna. Proc Natl Acad Sci USA (1985) 82:7889–93. doi: 10.1073/pnas.82.23.7889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mosselman S, Polman J, Dijkema R. ER Beta: Identification and Characterization of a Novel Human Estrogen Receptor. FEBS Lett (1996) 392:49–53. doi: 10.1016/0014-5793(96)00782-X [DOI] [PubMed] [Google Scholar]
- 15. Pelletier G, El-Alfy M. Immunocytochemical Localization of Estrogen Receptors Alpha and Beta in the Human Reproductive Organs. J Clin Endocr Metab (2000) 85:4835–40. doi: 10.1210/jcem.85.12.7029 [DOI] [PubMed] [Google Scholar]
- 16. Yeh CR, Da J, Song W, Fazili A, Yeh S. Estrogen Receptors in Prostate Development and Cancer. Am J Clin Exp Urol (2014) 2:161–8. [PMC free article] [PubMed] [Google Scholar]
- 17. Sar M, Welsch F. Differential Expression of Estrogen Receptor-Beta and Estrogen Receptor-Alpha in the Rat Ovary. Endocrinology (1999) 140:963–71. doi: 10.1210/endo.140.2.6533 [DOI] [PubMed] [Google Scholar]
- 18. Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, et al. Differential Immunolocalization of Estrogen Receptor Alpha and Beta in Rat Ovary and Uterus. J Mol Endocrinol (1999) 22:37–44. doi: 10.1677/jme.0.0220037 [DOI] [PubMed] [Google Scholar]
- 19. Braidman IP, Hainey L, Batra G, Selby PL, Saunders PTK, Hoyland JA. Localization of Estrogen Receptor Beta Protein Expression in Adult Human Bone. J Bone Miner Res (2001) 16:214–20. doi: 10.1359/jbmr.2001.16.2.214 [DOI] [PubMed] [Google Scholar]
- 20. Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, et al. Estrogen Receptors Alpha and Beta in the Normal, Hyperplastic and Carcinomatous Human Prostate. J Endocrinol (2001) 168:447–54. doi: 10.1677/joe.0.1680447 [DOI] [PubMed] [Google Scholar]
- 21. Dotzlaw H, Leygue E, Watson PH, Murphy LC. Expression of Estrogen Receptor-Beta in Human Breast Tumors. J Clin Endocr Metab (1997) 82:2371–4. doi: 10.1210/jcem.82.7.4212 [DOI] [PubMed] [Google Scholar]
- 22. Vladusic EA, Hornby AE, Guerra-Vladusic FK, Lupu R. Expression of Estrogen Receptor Beta Messenger RNA Variant in Breast Cancer. Cancer Res (1998) 58:210–4. [PubMed] [Google Scholar]
- 23. Dotzlaw H, Leygue E, Watson PH, Murphy LC. Estrogen Receptor-Beta Messenger RNA Expression in Human Breast Tumor Biopsies: Relationship to Steroid Receptor Status and Regulation by Progestins. Cancer Res (1999) 59:529–32. [PubMed] [Google Scholar]
- 24. Saunders PTK, Millar MR, Williams K, Macpherson S, Harkiss D, Anderson RA, et al. Differential Expression of Estrogen Receptor-Alpha and -Beta and Androgen Receptor in the Ovaries of Marmosets and Humans. Biol Reprod (2000) 63:1098–105. doi: 10.1095/biolreprod63.4.1098 [DOI] [PubMed] [Google Scholar]
- 25. Hillier SG, Anderson RA, Williams ARW, Tetsuka M. Expression of Oestrogen Receptor Alpha and Beta in Cultured Human Ovarian Surface Epithelial Cells. Mol Hum Reprod (1998) 4:811–5. doi: 10.1093/molehr/4.8.811 [DOI] [PubMed] [Google Scholar]
- 26. Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PTK. Human Oestrogen Receptors: Differential Expression of ERalpha and Beta and the Identification of ERbeta Variants. Steroids (2002) 67:985–92. doi: 10.1016/S0039-128X(02)00047-8 [DOI] [PubMed] [Google Scholar]
- 27. Maiti K, Paul JW, Read M, Chan EC, Riley SC, Nahar P. Smith, R. G-1-Activated Membrane Estrogen Receptors Mediate Increased Contractility of the Human Myometrium. Endocrinology (2011) 152:2448–55. doi: 10.1210/en.2010-0979 [DOI] [PubMed] [Google Scholar]
- 28. Zang LL, Zhang Q, Zhou Y, Zhao Y, Lu LL, Jiang Z, et al. Expression Pattern of G Protein-Coupled Estrogen Receptor 1 (GPER) in Human Cumulus Granulosa Cells (CGCs) of Patients With PCOS. Syst Biol Reprod Med (2016) 62:184–91. doi: 10.3109/19396368.2016.1164260 [DOI] [PubMed] [Google Scholar]
- 29. Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human Estrogen Receptor Beta-Gene Structure, Chromosomal Localization, and Expression Pattern. J Clin Endocr Metab (1997) 82:4258–65. doi: 10.1210/jc.82.12.4258 [DOI] [PubMed] [Google Scholar]
- 30. Coppens MT, Deboever JG, Dhont MA, Serreyn RF, Vandekerckhove DA, Roels HJ. Topographical Distribution of Estrogen and Progesterone Receptors in the Human Endometrium and Fallopian-Tube - an Immunocytochemical Study. Histochemistry (1993) 99:127–31. doi: 10.1007/BF00571873 [DOI] [PubMed] [Google Scholar]
- 31. Amso NN, Crow J, Shaw RW. Comparative Immunohistochemical Study of Estrogen and Progesterone Receptors in the Fallopian-Tube and Uterus at Different Stages of the Menstrual-Cycle and the Menopause. Hum Reprod (1994) 9:1027–37. doi: 10.1093/oxfordjournals.humrep.a138628 [DOI] [PubMed] [Google Scholar]
- 32. Welsh TH, Zhuang LZ, Hsueh AJW. Estrogen Augmentation of Gonadotropin-Stimulated Progestin Biosynthesis in Cultured Rat Granulosa-Cells. Endocrinology (1983) 112:1916–24. doi: 10.1210/endo-112-6-1916 [DOI] [PubMed] [Google Scholar]
- 33. Tang ZR, Zhang R, Lian ZX, Deng SL, Yu K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells (2019) 8:1123. doi: 10.3390/cells8101123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deroo BJ, Korach KS. Estrogen Receptors and Human Disease. J Clin Invest (2006) 116:561–70. doi: 10.1172/JCI27987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The Nuclear Receptor Superfamily - The 2nd Decade. Cell (1995) 83:835–9. doi: 10.1016/0092-8674(95)90199-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chakraborty S, Willett H, Biswas PK. Insight Into Estrogen Receptor Beta-Beta and Alpha-Beta Homo- and Heterodimerization: A Combined Molecular Dynamics and Sequence Analysis Study. Biophys Chem (2012) 170:42–50. doi: 10.1016/j.bpc.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bourguet W, Germain P, Gronemeyer H. Nuclear Receptor Ligand-Binding Domains Three-Dimensional Structures, Molecular Interactions and Pharmacological Implications. Trends Pharmacol Sci (2000) 21:381–8. doi: 10.1016/S0165-6147(00)01548-0 [DOI] [PubMed] [Google Scholar]
- 38. Klinge CM. Estrogen Receptor Interaction With Estrogen Response Elements. Nucleic Acids Res (2001) 29:2905–19. doi: 10.1093/nar/29.14.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung E, Acevedo ML, Cole PA, Kraus WL. Altered Pharmacology and Distinct Coactivator Usage for Estrogen Receptor-Dependent Transcription Through Activating Protein-1. Proc Natl Acad Sci USA (2005) 102:559–64. doi: 10.1073/pnas.0407113102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bjornstrom L, Sjoberg M. Mutations in the Estrogen Receptor DNA-Binding Domain Discriminate Between the Classical Mechanism of Action and Cross-Talk With Stat5b and Activating Protein 1 (AP-1). J Biol Chem (2002) 277:48479–83. doi: 10.1074/jbc.C200570200 [DOI] [PubMed] [Google Scholar]
- 41. Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, et al. Estrogen Receptor Pathways to AP-1. J Steroid Biochem Mol Biol (2000) 74:311–7. doi: 10.1016/S0960-0760(00)00108-4 [DOI] [PubMed] [Google Scholar]
- 42. Marino M, Ascenzi P, Acconcia F. S-Palmitoylation Modulates Estrogen Receptor Alpha Localization and Functions. Steroids (2006) 71:298–303. doi: 10.1016/j.steroids.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 43. Kelly MJ, Levin ER. Rapid Actions of Plasma Membrane Estrogen Receptors. Trends Endocrinol Metab (2001) 12:152–6. doi: 10.1016/S1043-2760(01)00377-0 [DOI] [PubMed] [Google Scholar]
- 44. Segars JH, Driggers PH. Estrogen Action and Cytoplasmic Signaling Cascades. Part I: Membrane-Associated Signaling Complexes. Trends Endocrinol Metab (2002) 13:349–54. doi: 10.1016/S1043-2760(02)00633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prossnitz ER, Barton M. Estrogen Biology: New Insights Into GPER Function and Clinical Opportunities. Mol Cell Endocrinol (2014) 389:71–83. doi: 10.1016/j.mce.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith CL. Cross-Talk Between Peptide Growth Factor and Estrogen Receptor Signaling Pathways. Biol Reprod (1998) 58:627–32. doi: 10.1095/biolreprod58.3.627 [DOI] [PubMed] [Google Scholar]
- 47. Clark S, Rainville J, Zhao X, Katzenellenbogen BS, Pfaff D, Vasudevan N. Estrogen Receptor-Mediated Transcription Involves the Activation of Multiple Kinase Pathways in Neuroblastoma Cells. J Steroid Biochem Mol Biol (2014) 139:45–53. doi: 10.1016/j.jsbmb.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 48. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the Hypothalamic-Pituitary-Gonadal Axis in Estrogen Receptor (ER) Null Mice Reveals Hypergonadism and Endocrine Sex Reversal in Females Lacking ER Alpha But Not ER Beta. Mol Endocrinol (2003) 17:1039–53. doi: 10.1210/me.2002-0398 [DOI] [PubMed] [Google Scholar]
- 49. Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, et al. Theca-Specific Estrogen Receptor-Alpha Knockout Mice Lose Fertility Prematurely. Endocrinology (2009) 150:3855–62. doi: 10.1210/en.2008-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, et al. Postnatal Sex Reversal of the Ovaries in Mice Lacking Estrogen Receptors Alpha and Beta. Science (1999) 286:2328–31. doi: 10.1126/science.286.5448.2328 [DOI] [PubMed] [Google Scholar]
- 51. Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, et al. An Estrogen Receptor-Alpha Knock-in Mutation Provides Evidence of Ligand-Independent Signaling and Allows Modulation of Ligand-Induced Pathways In Vivo . Endocrinology (2008) 149:2970–9. doi: 10.1210/en.2007-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rulli SB, Huhtaniemi I. What Have Gonadotrophin Overexpressing Transgenic Mice Taught Us About Gonadal Function? Reproduction (2005) 130:283–91. doi: 10.1530/rep.1.00661 [DOI] [PubMed] [Google Scholar]
- 53. Couse JF, Yates MM, Sanford R, Nyska A, Nilson JH, Korach KS. Formation of Cystic Ovarian Follicles Associated With Elevated Luteinizing Hormone Requires Estrogen Receptor-Beta. Endocrinology (2004) 145:4693–702. doi: 10.1210/en.2004-0548 [DOI] [PubMed] [Google Scholar]
- 54. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and Reproductive Phenotypes of Mice Lacking Estrogen Receptor Beta. Proc Natl Acad Sci USA (1998) 95:15677–82. doi: 10.1073/pnas.95.26.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of Single and Compound Knockouts of Estrogen Receptors Alpha (ER Alpha) and Beta (ER Beta) on Mouse Reproductive Phenotypes. Development (2000) 127:4277–91. doi: 10.1242/dev.127.19.4277 [DOI] [PubMed] [Google Scholar]
- 56. Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen Receptor-Beta Is Critical to Granulosa Cell Differentiation and the Ovulatory Response to Gonadotropins. Endocrinology (2005) 146:3247–62. doi: 10.1210/en.2005-0213 [DOI] [PubMed] [Google Scholar]
- 57. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of Reproductive Function But Not Prenatal Sexual Development After Insertional Disruption of the Mouse Estrogen-Receptor Gene. P Natl Acad Sci USA (1993) 90:11162–6. doi: 10.1073/pnas.90.23.11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Couse JF, Korach KS. Estrogen Receptor Null Mice: What Have We Learned and Where Will They Lead Us? (Vol 20, Pg 358, 1999). Endocrine Rev (1999) 20:459–9. doi: 10.1210/edrv.20.3.0370 [DOI] [PubMed] [Google Scholar]
- 59. Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and Control of Estrous Cycle in Rats. Rev Endocr Metab Disord (2007) 8:21–9. doi: 10.1007/s11154-007-9032-6 [DOI] [PubMed] [Google Scholar]
- 60. Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, et al. Definition of Estrogen Receptor Pathway Critical for Estrogen Positive Feedback to Gonadotropin-Releasing Hormone Neurons and Fertility. Neuron (2006) 52:271–80. doi: 10.1016/j.neuron.2006.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheong RY, Porteous R, Chambon P, Abrahám I, Herbison AE. Effects of Neuron-Specific Estrogen Receptor (ER) α and Erβ Deletion on the Acute Estrogen Negative Feedback Mechanism in Adult Female Mice. Endocrinology (2014) 155:1418–27. doi: 10.1210/en.2013-1943 [DOI] [PubMed] [Google Scholar]
- 62. Antal MC, Krust A, Chambon P, Mark M. Sterility and Absence of Histopathological Defects in Nonreproductive Organs of a Mouse ERbeta-Null Mutant. Proc Natl Acad Sci USA (2008) 105:2433–8. doi: 10.1073/pnas.0712029105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mitchner NA, Garlick C, Ben-Jonathan N. Cellular Distribution and Gene Regulation of Estrogen Receptors Alpha and Beta in the Rat Pituitary Gland. Endocrinology (1998) 139:3976–83. doi: 10.1210/endo.139.9.6181 [DOI] [PubMed] [Google Scholar]
- 64. Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, et al. Pituitary Gonadotroph Estrogen Receptor-Alpha is Necessary for Fertility in Females. Endocrinology (2008) 149:20–7. doi: 10.1210/en.2007-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, et al. Impaired Estrogen Feedback and Infertility in Female Mice With Pituitary-Specific Deletion of Estrogen Receptor Alpha (Esr1). Biol Reprod (2009) 81:488–96. doi: 10.1095/biolreprod.108.075259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arao Y, Hamilton KJ, Wu SP, Tsai MJ, DeMayo FJ, Korach KS. Dysregulation of Hypothalamic-Pituitary Estrogen Receptor Alpha-Mediated Signaling Causes Episodic LH Secretion and Cystic Ovary. FASEB J (2019) 33:7375–86. doi: 10.1096/fj.201802653RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang C, Prossnitz ER, Roy SK. Expression of G Protein-Coupled Receptor 30 in the Hamster Ovary: Differential Regulation by Gonadotropins and Steroid Hormones. Endocrinology (2007) 148:4853–64. doi: 10.1210/en.2007-0727 [DOI] [PubMed] [Google Scholar]
- 68. Wang C, Prossnitz ER, Roy SKJE. G Protein-Coupled Receptor 30 Expression is Required for Estrogen Stimulation of Primordial Follicle Formation in the Hamster Ovary. Endocrinology (2008) 149:4452–61. doi: 10.1210/en.2008-0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, et al. GPR30 Does Not Mediate Estrogenic Responses in Reproductive Organs in Mice. Biol Reprod (2009) 80:34–41. doi: 10.1095/biolreprod.108.071175 [DOI] [PubMed] [Google Scholar]
- 70. Novaira HJ, Negron AL, Graceli JB, Capellino S, Schoeffield A, Hoffman GE, et al. Impairments in the Reproductive Axis of Female Mice Lacking Estrogen Receptor β in GnRH Neurons. Am J Physiol Endocrinol Metab (2018) 315:E1019–e33. doi: 10.1152/ajpendo.00173.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hu M, Li J, Zhang Y, Li X, Brannstrom M, Shao LR, et al. Endometrial Progesterone Receptor Isoforms in Women With Polycystic Ovary Syndrome. Am J Transl Res (2018) 10:2696–705. [PMC free article] [PubMed] [Google Scholar]
- 72. Chang J, Azziz R, Legro R, Dewailly D, Franks S, Tarlatzis BC, et al. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil Steril (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 73. Carmina E, Lobo RA. Polycystic Ovaries in Hirsute Women With Normal Menses. Am J Med (2001) 111:602–6. doi: 10.1016/S0002-9343(01)00979-2 [DOI] [PubMed] [Google Scholar]
- 74. Kowalczyk K, Franik G, Kowalczyk D, Pluta D, Blukacz L, Madej P. Thyroid Disorders in Polycystic Ovary Syndrome. Eur Rev Med Pharmacol Sci (2017) 21:346–60. [PubMed] [Google Scholar]
- 75. Artimani T, Saidijam M, Aflatoonian R, Amiri I, Ashrafi M, Shabab N, et al. Estrogen and Progesterone Receptor Subtype Expression in Granulosa Cells From Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2015) 31:379–83. doi: 10.3109/09513590.2014.1001733 [DOI] [PubMed] [Google Scholar]
- 76. Qiao GY, Dong BW, Zhu CJ, Yan CY, Chen BL. Deregulation of WNT2/FZD3/beta-Catenin Pathway Compromises the Estrogen Synthesis in Cumulus Cells From Patients With Polycystic Ovary Syndrome. Biochem Biophys Res Commun (2017) 493:847–54. doi: 10.1016/j.bbrc.2017.07.057 [DOI] [PubMed] [Google Scholar]
- 77. Jakimiuk AJ, Weitsman SR, Yen H-W, Bogusiewicz M, Magoffin DA. Estrogen Receptor α and β Expression in Theca and Granulosa Cells From Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2002) 87:5532–8. doi: 10.1210/jc.2002-020323 [DOI] [PubMed] [Google Scholar]
- 78. Ferreira SR, Motta AB. Uterine Function: From Normal to Polycystic Ovarian Syndrome Alterations. Curr Med Chem (2018) 25:1792–804. doi: 10.2174/0929867325666171205144119 [DOI] [PubMed] [Google Scholar]
- 79. Piltonen TT. Polycystic Ovary Syndrome: Endometrial Markers. Best Pract Res Clin Obstet Gynaecol (2016) 37:66–79. doi: 10.1016/j.bpobgyn.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 80. Gregory CW, Wilson EM, Apparao K, Lininger RA, Meyer WR, Kowalik A, et al. Steroid Receptor Coactivator Expression Throughout the Menstrual Cycle in Normal and Abnormal Endometrium. J Clin Endocrinol Metab (2002) 87:2960–6. doi: 10.1210/jcem.87.6.8572 [DOI] [PubMed] [Google Scholar]
- 81. Kim JY, Song H, Kim H, Kang HJ, Jun JH, Hong SR, et al. Transcriptional Profiling With a Pathway-Oriented Analysis Identifies Dysregulated Molecular Phenotypes in the Endometrium of Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2009) 94:1416–26. doi: 10.1210/jc.2008-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quezada S, Avellaira C, Johnson MC, Gabler F, Fuentes A, Vega M. Evaluation of Steroid Receptors, Coregulators, and Molecules Associated With Uterine Receptivity in Secretory Endometria From Untreated Women With Polycystic Ovary Syndrome. Fertil Steril (2006) 85:1017–26. doi: 10.1016/j.fertnstert.2005.09.053 [DOI] [PubMed] [Google Scholar]
- 83. Villavicencio A, Bacallao K, Avellaira C, Gabler F, Fuentes A, Vega M. Androgen and Estrogen Receptors and Co-Regulators Levels in Endometria From Patients With Polycystic Ovarian Syndrome With and Without Endometrial Hyperplasia. Gynecol Oncol (2006) 103:307–14. doi: 10.1016/j.ygyno.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 84. Maliqueo M, Clementi M, Gabler F, Johnson MC, Palomino A, Sir-Petermann T, et al. Expression of Steroid Receptors and Proteins Related to Apoptosis in Endometria of Women With Polycystic Ovary Syndrome. Fertil Steril (2003) 80:812–9. doi: 10.1016/S0015-0282(03)00987-7 [DOI] [PubMed] [Google Scholar]
- 85. Hulchiy M, Nybacka Å, Sahlin L, Hirschberg AL. Endometrial Expression of Estrogen Receptors and the Androgen Receptor in Women With Polycystic Ovary Syndrome: A Lifestyle Intervention Study. J Clin Endocrinol Metab (2016) 101:561–71. doi: 10.1210/jc.2015-3803 [DOI] [PubMed] [Google Scholar]
- 86. Gellersen B, Brosens JJ. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocrine Rev (2014) 35:851–905. doi: 10.1210/er.2014-1045 [DOI] [PubMed] [Google Scholar]
- 87. Hu M, Zhang Y, Egecioglu E, Li X, Shao LR, Billig H. Uterine Glycolytic Enzyme Expression is Affected by Knockout of Different Estrogen Receptor Subtypes. Biomed Rep (2019) 11:135–44. doi: 10.3892/br.2019.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen Receptor Beta in Health and Disease. Biol Reprod (2005) 73:866–71. doi: 10.1095/biolreprod.105.043497 [DOI] [PubMed] [Google Scholar]
- 89. Silva F, Sóter M, Sales M, Candido A, Reis F, Silva I, et al. Estrogen Receptor αlpha Gene (ESR1) PvuII and XbaI Polymorphisms Are Associated to Metabolic and Proinflammatory Factors in Polycystic Ovary Syndrome. Gene (2015) 560:44–9. doi: 10.1016/j.gene.2015.01.037 [DOI] [PubMed] [Google Scholar]
- 90. Liaqat I, Jahan N, Krikun G, Taylor HS. Genetic Polymorphisms in Pakistani Women With Polycystic Ovary Syndrome. Reprod Sci (Thousand Oaks Calif) (2015) 22:347–57. doi: 10.1177/1933719114542015 [DOI] [PubMed] [Google Scholar]
- 91. Jiao X, Chen W, Zhang J, Wang W, Song J, Chen D, et al. Variant Alleles of the ESR1, PPARG, HMGA2, and MTHFR Genes Are Associated With Polycystic Ovary Syndrome Risk in a Chinese Population: A Case-Control Study. Front Endocrinol (Lausanne) (2018) 9:504. doi: 10.3389/fendo.2018.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim JJ, Choi YM, Choung SH, Yoon SH, Lee GH, Moon SY. Estrogen Receptor Beta Gene+ 1730 G/A Polymorphism in Women With Polycystic Ovary Syndrome. Fertil Steril (2010) 93:1942–7. doi: 10.1016/j.fertnstert.2008.12.040 [DOI] [PubMed] [Google Scholar]
- 93. Levin JH, Carmina E, Lobo RA. Is the Inappropriate Gonadotropin-Secretion of Patients With Polycystic-Ovary-Syndrome Similar to That of Patients With Adult-Onset Congenital Adrenal-Hyperplasia. Fertil Steril (1991) 56:635–40. doi: 10.1016/S0015-0282(16)54592-0 [DOI] [PubMed] [Google Scholar]
- 94. Mahesh VB, Brann DW. Regulation of the Preovulatory Gonadotropin Surge by Endogenous Steroids. Steroids (1998) 63:616–29. doi: 10.1016/S0039-128X(98)00075-0 [DOI] [PubMed] [Google Scholar]
- 95. Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of Estrogen Receptor-Alpha in the Anterior Pituitary Gland. Mol Endocrinol (1997) 11:674–81. doi: 10.1210/mend.11.6.0019 [DOI] [PubMed] [Google Scholar]
- 96. Zhou S, Wen S, Sheng Y, Yang M, Shen X, Chen Y, et al. Association of Estrogen Receptor Genes Polymorphisms With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis Based on Observational Studies. Front Endocrinol (Lausanne) (2021) 12:726184. doi: 10.3389/fendo.2021.726184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gonzalez F. Adrenal Involvement in Polycystic Ovary Syndrome. Semin Reprod Endocrinol (1997) 15:137–57. doi: 10.1055/s-2007-1016296 [DOI] [PubMed] [Google Scholar]
- 98. Yildiz BO, Azziz R. The Adrenal and Polycystic Ovary Syndrome. Rev Endocr Metab Dis (2007) 8:331–42. doi: 10.1007/s11154-007-9054-0 [DOI] [PubMed] [Google Scholar]
- 99. Greenblatt RB, Mahesh VB. The Androgenic Polycystic Ovary. Am J Obstet Gynecol (1976) 125:712–26. doi: 10.1016/0002-9378(76)90801-2 [DOI] [PubMed] [Google Scholar]
- 100. Fruzzetti F, De Lorenzo D, Ricci C, Teti G. Ovarian Influence on Adrenal Androgen Secretion in Polycystic Ovary Syndrome. Fertil Steril (1995) 63:734–41. doi: 10.1016/S0015-0282(16)57474-3 [DOI] [PubMed] [Google Scholar]
- 101. Carmina E, Gonzalez F, Chang L, Lobo RA. Reassessment of Adrenal Androgen Secretion in Women With Polycystic Ovary Syndrome. Obstet Gynecol (1995) 85:971–6. doi: 10.1016/0029-7844(95)00065-Y [DOI] [PubMed] [Google Scholar]
- 102. Pang Y, Dong J, Thomas P. Estrogen Signaling Characteristics of Atlantic Croaker G Protein-Coupled Receptor 30 (GPR30) and Evidence It Is Involved in Maintenance of Oocyte Meiotic Arrest. Endocrinology (2008) 149:3410–26. doi: 10.1210/en.2007-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Majumder S, Das S, Moulik SR, Mallick B, Pal P, Mukherjee D. G-Protein Coupled Estrogen Receptor (GPER) Inhibits Final Oocyte Maturation in Common Carp, Cyprinus Carpio. Gen Comp Endocr (2015) 211:28–38. doi: 10.1016/j.ygcen.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 104. Peyton C, Thomas P. Involvement of Epidermal Growth Factor Receptor Signaling in Estrogen Inhibition of Oocyte Maturation Mediated Through the G Protein-Coupled Estrogen Receptor (Gper) in Zebrafish (Danio Rerio). Biol Reprod (2011) 85:42–50. doi: 10.1095/biolreprod.110.088765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int J Cancer (2015) 136:E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 106. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial Ovarian Cancer. Lancet (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2 [DOI] [PubMed] [Google Scholar]
- 107. Englert-Golon M, Andrusiewicz M, Zbikowska A, Chmielewska M, Sajdak S, Kotwicka M. Altered Expression of ESR1, ESR2, PELP1 and C-SRC Genes Is Associated With Ovarian Cancer Manifestation. Int J Mol Sci (2021) 22:6216. doi: 10.3390/ijms22126216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li SR, Li Y, Wen ZF, Kong FJ, Guan XL, Liu WH. microRNA-206 Overexpression Inhibits Cellular Proliferation and Invasion of Estrogen Receptor Alpha-Positive Ovarian Cancer Cells. Mol Med Rep (2014) 9:1703–8. doi: 10.3892/mmr.2014.2021 [DOI] [PubMed] [Google Scholar]
- 109. Qiu JJ, Ye LC, Ding JX, Feng WW, Jin HY, Zhang Y, et al. Expression and Clinical Significance of Estrogen-Regulated Long Non-Coding RNAs in Estrogen Receptor Alpha-Positive Ovarian Cancer Progression. Oncol Rep (2014) 31:1613–22. doi: 10.3892/or.2014.3000 [DOI] [PubMed] [Google Scholar]
- 110. O’Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen Receptor-Alpha Mediates Gene Expression Changes and Growth Response in Ovarian Cancer Cells Exposed to Estrogen. Endocr Relat Cancer (2005) 12:851–66. doi: 10.1677/erc.1.01039 [DOI] [PubMed] [Google Scholar]
- 111. Chan KKL, Leung THY, Chan DW, Wei N, Lau GTY, Liu SS, et al. Targeting Estrogen Receptor Subtypes (ER Alpha and ER Beta) With Selective ER Modulators in Ovarian Cancer. J Endocrinol (2014) 221:325–36. doi: 10.1530/JOE-13-0500 [DOI] [PubMed] [Google Scholar]
- 112. Treeck O, Pfeiler G, Mitter D, Lattrich C, Piendl G, Ortmann O. Estrogen Receptor Beta 1 Exerts Antitumoral Effects on SK-OV-3 Ovarian Cancer Cells. J Endocrinol (2007) 193:421–33. doi: 10.1677/JOE-07-0087 [DOI] [PubMed] [Google Scholar]
- 113. Bossard C, Busson M, Vindrieux D, Gaudin F, Machelon R, Brigitte M, et al. Potential Role of Estrogen Receptor Beta as a Tumor Suppressor of Epithelial Ovarian Cancer. PloS One (2012) 7:e44787. doi: 10.1371/journal.pone.0044787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, et al. Cloning and Characterization of Human Estrogen Receptor Beta Isoforms. Biochem Bioph Res Co (1998) 247:75–8. doi: 10.1006/bbrc.1998.8738 [DOI] [PubMed] [Google Scholar]
- 115. Ciucci A, Zannoni GF, Travaglia D, Petrillo M, Scambia G, Gallo D. Prognostic Significance of the Estrogen Receptor Beta (ER Beta) Isoforms ER Beta 1, ER Beta 2, and ER Beta 5 in Advanced Serous Ovarian Cancer. Gynecol Oncol (2014) 132:351–9. doi: 10.1016/j.ygyno.2013.12.027 [DOI] [PubMed] [Google Scholar]
- 116. Kolkova Z, Casslen V, Henic E, Ahmadi S, Ehinger A, Jirstrom K, et al. The G Protein-Coupled Estrogen Receptor 1 (GPER/GPR30) Does Not Predict Survival in Patients With Ovarian Cancer. J Ovarian Res (2012) 5:9. doi: 10.1186/1757-2215-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ignatov T, Modl S, Thulig M, Weissenborn C, Treeck O, Ortmann O, et al. GPER-1 Acts as a Tumor Suppressor in Ovarian Cancer. J Ovarian Res (2013) 6:51. doi: 10.1186/1757-2215-6-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yan Y, Jiang XL, Zhao Y, Wen HX, Liu GY. Role of GPER on Proliferation, Migration and Invasion in Ligand-Independent Manner in Human Ovarian Cancer Cell Line SKOV3. Cell Biochem Funct (2015) 33:552–9. doi: 10.1002/cbf.3154 [DOI] [PubMed] [Google Scholar]
- 119. Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17 Beta-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res (2007) 67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909 [DOI] [PubMed] [Google Scholar]
- 120. Miller SM, Green ML, Depinto JV, Hornbuckle KC. Results From the Lake Michigan Mass Balance Study: Concentrations and Fluxes of Atmospheric Polychlorinated Biphenyls and Trans-Nonachlor. Environ Sci Technol (2001) 35:278–85. doi: 10.1021/es991463b [DOI] [PubMed] [Google Scholar]
- 121. Solomon KR, Giesy JP, LaPoint TW, Giddings JM, Richards RP. Ecological Risk Assessment of Atrazine in North American Surface Waters. Environ Toxicol Chem (2013) 32:10–1. doi: 10.1002/etc.2050 [DOI] [PubMed] [Google Scholar]
- 122. Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, et al. G-Protein-Coupled Receptor 30 and Estrogen Receptor-Alpha Are Involved in the Proliferative Effects Induced by Atrazine in Ovarian Cancer Cells (Retracted Article. See Vol. 122, Pg. A42, 2014). Environ Health Perspect (2008) 116:1648–55. doi: 10.1289/ehp.11297 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123. Coulam CB, Adamson SC, Annegers JF. Incidence of Premature Ovarian Failure. Obstet Gynecol (1986) 67:604–6. [PubMed] [Google Scholar]
- 124. Meskhi A, Seif MW. Premature Ovarian Failure. Curr Opin Obstet Gyn (2006) 18:418–26. doi: 10.1097/01.gco.0000233937.36554.d3 [DOI] [PubMed] [Google Scholar]
- 125. Welt CK. Primary Ovarian Insufficiency: A More Accurate Term for Premature Ovarian Failure. Clin Endocrinol (2008) 68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x [DOI] [PubMed] [Google Scholar]
- 126. Goswami D, Conway GS. Premature Ovarian Failure. Hum Reprod Update (2005) 11:391–410. doi: 10.1093/humupd/dmi012 [DOI] [PubMed] [Google Scholar]
- 127. Bretherick KL, Hanna CW, Currie LM, Fluker MR, Hammond GL, Robinson WP. Estrogen Receptor Alpha Gene Polymorphisms Are Associated With Idiopathic Premature Ovarian Failure. Fertil Steril (2008) 89:318–24. doi: 10.1016/j.fertnstert.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 128. M’Rabet N, Moffat R, Helbling S, Kaech A, Zhang H, de Geyter C. The CC-Allele of the PvuII Polymorphic Variant in Intron 1 of the Alpha-Estrogen Receptor Gene is Significantly More Prevalent Among Infertile Women at Risk of Premature Ovarian Aging. Fertil Steril (2012) 98:965–72.e965. doi: 10.1016/j.fertnstert.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 129. Cordts EB, Santos AA, Peluso C, Bianco B, Barbosa CP, Christofolini DM. Risk of Premature Ovarian Failure Is Associated to the PvuII Polymorphism at Estrogen Receptor Gene ESR1. J Assisted Reprod Genet (2012) 29:1421–5. doi: 10.1007/s10815-012-9884-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yang JJ, Cho LY, Lim YJ, Ko KP, Lee KS, Kim H, et al. Estrogen Receptor-1 Genetic Polymorphisms for the Risk of Premature Ovarian Failure and Early Menopause. J Womens Health (2010) 19:297–304. doi: 10.1089/jwh.2008.1317 [DOI] [PubMed] [Google Scholar]
- 131. Yoon SH, Choi YM, Hong MA, Lee GH, Kim JJ, Im HJ, et al. Estrogen Receptor Alpha Gene Polymorphisms in Patients With Idiopathic Premature Ovarian Failure. Hum Reprod (2010) 25:283–7. doi: 10.1093/humrep/dep375 [DOI] [PubMed] [Google Scholar]
- 132. Martinkovich S, Shah D, Planey SL, Arnott J. Selective Estrogen Receptor Modulators: Tissue Specificity and Clinical Utility. Clin Interv Aging (2014) 9:1437. doi: 10.2147/CIA.S66690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Metivier R, Penot G, Flouriot G, Pakdel F. Synergism Between ER Alpha Transactivation Function 1 (AF-1) and AF-2 Mediated by Steroid Receptor Coactivator Protein-1: Requirement for the AF-1 Alpha-Helical Core and for a Direct Interaction Between the N- and C-Terminal Domains. Mol Endocrinol (2001) 15:1953–70. doi: 10.1210/mend.15.11.0727 [DOI] [PubMed] [Google Scholar]
- 134. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the Estrogen-Receptor Through Phosphorylation by Mitogen-Activated Protein-Kinase. Science (1995) 270:1491–4. doi: 10.1126/science.270.5241.1491 [DOI] [PubMed] [Google Scholar]
- 135. Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional Domains of the Human Estrogen-Receptor. Cell (1987) 51:941–51. doi: 10.1016/0092-8674(87)90581-2 [DOI] [PubMed] [Google Scholar]
- 136. Berry M, Metzger D, Chambon P. Role of the 2 Activating Domains of the Estrogen-Receptor in the Cell-Type and Promoter-Context Dependent Agonistic Activity of the Antiestrogen 4-Hydroxytamoxifen. EMBO J (1990) 9:2811–8. doi: 10.1002/j.1460-2075.1990.tb07469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, et al. Tamoxifen Resistance by a Conformational Arrest of the Estrogen Receptor Alpha After PKA Activation in Breast Cancer. Cancer Cell (2004) 5:597–605. doi: 10.1016/j.ccr.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 138. Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, et al. The Estrogen Receptor Enhances AP-1 Activity by Two Distinct Mechanisms With Different Requirements for Receptor Transactivation Functions. Mol Endocrinol (1999) 13:1672–85. doi: 10.1210/mend.13.10.0357 [DOI] [PubMed] [Google Scholar]
- 139. Zwart W, de Leeuw R, Rondaij M, Neefjes J, Mancini MA, Michalides R. The Hinge Region of the Human Estrogen Receptor Determines Functional Synergy Between AF-1 and AF-2 in the Quantitative Response to Estradiol and Tamoxifen. J Cell Sci (2010) 123:1253–61. doi: 10.1242/jcs.061135 [DOI] [PubMed] [Google Scholar]
- 140. Clark JH, Markaverich BM. The Agonistic-Antagonistic Properties of Clomiphene - A Review. Pharmacol Therapeut (1981) 15:467–519. doi: 10.1016/0163-7258(81)90055-3 [DOI] [PubMed] [Google Scholar]
- 141. Medicine, P.C.o.t.A.S.f.R . Use of Clomiphene Citrate in Women. Fertil Steril (2003) 80:1302–8. doi: 10.1016/S0015-0282(03)01184-1 [DOI] [PubMed] [Google Scholar]
- 142. Mikkelson TJ, Kroboth PD, Cameron WJ, Dittert LW, Chungi V, Manberg PJ. Single-Dose Pharmacokinetics of Clomiphene Citrate in Normal Volunteers. Fertil Steril (1986) 46:392–6. doi: 10.1016/S0015-0282(16)49574-9 [DOI] [PubMed] [Google Scholar]
- 143. Kettel LM, Roseff SJ, Berga SL, Mortola JF, Yen SSC. Hypothalamic-Pituitary-Ovarian Response to Clomiphene Citrate in Women With Polycystic-Ovary-Syndrome. Fertil Steril (1993) 59:532–8. doi: 10.1016/S0015-0282(16)55795-1 [DOI] [PubMed] [Google Scholar]
- 144. Sir T, Alba F, Devoto L, Rossmanith W. Clomiphene Citrate and Lh Pulsatility in Pco Syndrome. Horm Metab Res (1989) 21:583–3. doi: 10.1055/s-2007-1009293 [DOI] [PubMed] [Google Scholar]
- 145. Bhagavath B, Carson SA. Ovulation Induction in Women With Polycystic Ovary Syndrome: An Update. Am J Obstet Gynecol (2012) 206:195–8. doi: 10.1016/j.ajog.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 146. Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, et al. Effect of Clomiphene Citrate on Endometrial Thickness, Ovulation, Pregnancy and Live Birth in Anovulatory Women: Systematic Review and Meta-Analysis. Ultrasound Obst Gyn (2018) 51:64–76. doi: 10.1002/uog.18933 [DOI] [PubMed] [Google Scholar]
- 147. Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, Metformin, or Both for Infertility in the Polycystic Ovary Syndrome. N Engl J Med (2007) 356:551–66. doi: 10.1056/NEJMoa063971 [DOI] [PubMed] [Google Scholar]
- 148. Casper RF, Mitwally MFM. A Historical Perspective of Aromatase Inhibitors for Ovulation Induction. Fertil Steril (2012) 98:1352–5. doi: 10.1016/j.fertnstert.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 149. Mitwally MFM, Casper RF. Use of an Aromatase Inhibitor for Induction of Ovulation in Patients With an Inadequate Response to Clomiphene Citrate. Fertil Steril (2001) 75:305–9. doi: 10.1016/S0015-0282(00)01705-2 [DOI] [PubMed] [Google Scholar]
- 150. Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Estrogen-Regulated Gene Expression Predicts Response to Endocrine Therapy in Patients With Ovarian Cancer. Gynecol Oncol (2007) 106:461–8. doi: 10.1016/j.ygyno.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 151. Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, et al. CA125 Response Is Associated With Estrogen Receptor Expression in a Phase II Trial of Letrozole in Ovarian Cancer: Identification of an Endocrine-Sensitive Subgroup. Clin Cancer Res (2002) 8:2233–9. [PubMed] [Google Scholar]
- 152. Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole Versus Clomiphene for Infertility in the Polycystic Ovary Syndrome. N Engl J Med (2014) 371:119–29. doi: 10.1056/NEJMoa1313517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bruni V, Dei M, Pontello V, Vangelisti P. The Management of Polycystic Ovary Syndrome. Ann N Y Acad Sci (2003) 997:307–21. doi: 10.1196/annals.1290.034 [DOI] [PubMed] [Google Scholar]
- 154. Yildiz BO. Approach to the Patient: Contraception in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2015) 100:794–802. doi: 10.1210/jc.2014-3196 [DOI] [PubMed] [Google Scholar]
- 155. Kahn JA, Gordon CM. Polycystic Ovary Syndrome. Adolesc Med (Philadelphia Pa) (1999) 10:321–36. [PubMed] [Google Scholar]
- 156. Yildiz BO. Assessment, Diagnosis and Treatment of a Patient With Hirsutism. Nat Clin Practice Endocrinol Metab (2008) 4:294–300. doi: 10.1038/ncpendmet0789 [DOI] [PubMed] [Google Scholar]
- 157. Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS. Metformin Versus Ethinyl Estradiol-Cyproterone Acetate in the Treatment of Nonobese Women With Polycystic Ovary Syndrome: A Randomized Study. J Clin Endocrinol Metab (2003) 88:148–56. doi: 10.1210/jc.2002-020997 [DOI] [PubMed] [Google Scholar]
- 158. Yang YM, Choi EJ. Efficacy and Safety of Metformin or Oral Contraceptives, or Both in Polycystic Ovary Syndrome. Ther Clin Risk Manage (2015) 11:1345–53. doi: 10.2147/TCRM.S89737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wiegratz I, Kuhl H. Long-Cycle Treatment With Oral Contraceptives. Drugs (2004) 64:2447–62. doi: 10.2165/00003495-200464210-00006 [DOI] [PubMed] [Google Scholar]
- 160. Group ECW. Noncontraceptive Health Benefits of Combined Oral Contraception. Hum Reprod Update (2005) 11:513–25. doi: 10.1093/humupd/dmi019 [DOI] [PubMed] [Google Scholar]
- 161. Prossnitz ER, Barton M. The G-Protein-Coupled Estrogen Receptor GPER in Health and Disease. Nat Rev Endocrinol (2011) 7:715–26. doi: 10.1038/nrendo.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yasar P, Ayaz G, User SD, Gupur G, Muyan M. Molecular Mechanism of Estrogen-Estrogen Receptor Signaling. Reprod Med Biol (2017) 16:4–20. doi: 10.1002/rmb2.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir J-M, Corbo L. Cracking the Estrogen Receptor’s Posttranslational Code in Breast Tumors. Endocrine Rev (2011) 32:597–622. doi: 10.1210/er.2010-0016 [DOI] [PubMed] [Google Scholar]
- 164. Maggi A. Liganded and Unliganded Activation of Estrogen Receptor and Hormone Replacement Therapies. Biochim Biophys Acta (BBA)-Mol Basis Dis (2011) 1812:1054–60. doi: 10.1016/j.bbadis.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Taylor HS. Designing the Ideal Selective Estrogen Receptor Modulator—an Achievable Goal? Menopause (New York NY) (2009) 16:609. doi: 10.1097/gme.0b013e3181906fa3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Maximov Y, Lee M, Craig Jordan V. The Discovery and Development of Selective Estrogen Receptor Modulators (SERMs) for Clinical Practice. Curr Clin Pharmacol (2013) 8:135–55. doi: 10.2174/1574884711308020006 [DOI] [PMC free article] [PubMed] [Google Scholar]