Key Points
Question
What is the association between statin-induced reductions in low-density lipoprotein cholesterol (LDL-C) levels and the absolute and relative reductions in individual clinical outcomes, such as all-cause mortality, myocardial infarction, or stroke?
Findings
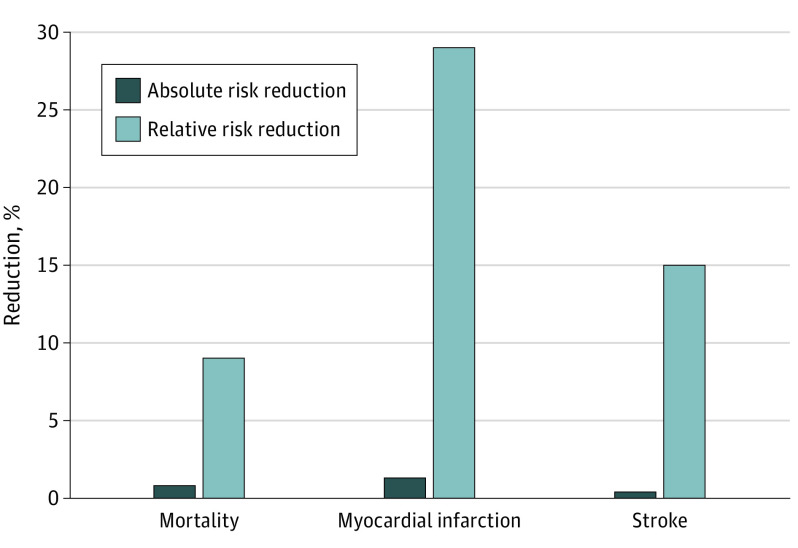
In this meta-analysis of 21 randomized clinical trials in primary and secondary prevention that examined the efficacy of statins in reducing total mortality and cardiovascular outcomes, there was significant heterogeneity but also reductions in the absolute risk of 0.8% for all-cause mortality, 1.3% for myocardial infarction, and 0.4% for stroke in those randomized to treatment with statins compared with control, with relative risk reductions of 9%, 29%, and 14%, respectively. A meta-regression was inconclusive regarding the association between the magnitude of statin-induced LDL-C reduction and all-cause mortality, myocardial infarction, or stroke.
Meaning
The study results suggest that the absolute benefits of statins are modest, may not be strongly mediated through the degree of LDL-C reduction, and should be communicated to patients as part of informed clinical decision-making as well as to inform clinical guidelines and policy.
Abstract
Importance
The association between statin-induced reduction in low-density lipoprotein cholesterol (LDL-C) levels and the absolute risk reduction of individual, rather than composite, outcomes, such as all-cause mortality, myocardial infarction, or stroke, is unclear.
Objective
To assess the association between absolute reductions in LDL-C levels with treatment with statin therapy and all-cause mortality, myocardial infarction, and stroke to facilitate shared decision-making between clinicians and patients and inform clinical guidelines and policy.
Data Sources
PubMed and Embase were searched to identify eligible trials from January 1987 to June 2021.
Study Selection
Large randomized clinical trials that examined the effectiveness of statins in reducing total mortality and cardiovascular outcomes with a planned duration of 2 or more years and that reported absolute changes in LDL-C levels. Interventions were treatment with statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) vs placebo or usual care. Participants were men and women older than 18 years.
Data Extraction and Synthesis
Three independent reviewers extracted data and/or assessed the methodological quality and certainty of the evidence using the risk of bias 2 tool and Grading of Recommendations, Assessment, Development and Evaluation. Any differences in opinion were resolved by consensus. Meta-analyses and a meta-regression were undertaken.
Main Outcomes and Measures
Primary outcome: all-cause mortality. Secondary outcomes: myocardial infarction, stroke.
Findings
Twenty-one trials were included in the analysis. Meta-analyses showed reductions in the absolute risk of 0.8% (95% CI, 0.4%-1.2%) for all-cause mortality, 1.3% (95% CI, 0.9%-1.7%) for myocardial infarction, and 0.4% (95% CI, 0.2%-0.6%) for stroke in those randomized to treatment with statins, with associated relative risk reductions of 9% (95% CI, 5%-14%), 29% (95% CI, 22%-34%), and 14% (95% CI, 5%-22%) respectively. A meta-regression exploring the potential mediating association of the magnitude of statin-induced LDL-C reduction with outcomes was inconclusive.
Conclusions and Relevance
The results of this meta-analysis suggest that the absolute risk reductions of treatment with statins in terms of all-cause mortality, myocardial infarction, and stroke are modest compared with the relative risk reductions, and the presence of significant heterogeneity reduces the certainty of the evidence. A conclusive association between absolute reductions in LDL-C levels and individual clinical outcomes was not established, and these findings underscore the importance of discussing absolute risk reductions when making informed clinical decisions with individual patients.
This systematic review and meta-analysis examines the association between absolute reductions in low-density lipoprotein cholesterol levels with treatment with statin therapy and all-cause mortality, myocardial infarction, and stroke.
Introduction
The accumulation of low-density lipoprotein cholesterol (LDL-C) in vessel walls is purported to be the causative factor in the development of atherosclerosis.1,2 Hence, the reduction of LDL-C has become an important target for preventing atherosclerotic cardiovascular disease. The aggressive lowering of LDL-C levels with treatment with statins (3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitors) is reflected in the various iterations of expert guidelines for preventing cardiovascular disease (CVD), giving rise to the popular theory that the lower the LDL-C level, the better.1,2,3,4
A log-linear association between LDL-C and cardiovascular events has been reported by the Cholesterol Treatment Trialists’ (CTT) collaboration, which published a series of meta-analyses that suggested that a reduction of 38.7 mg/dL (to convert to mmol/L, multiply by 0.0259) in LDL-C levels with statins yields about a 21% relative risk reduction (RRR) of major vascular events and a 10% RRR in all-cause mortality.5 More recently, Silverman and colleagues6 reported a log-linear association between LDL-C levels and major vascular events.
However, CTT analyses were based on individual patient data (IPD),5,7,8 which are inaccessible to independent researchers and not replicable. In addition, the use of composite outcomes in such analyses5,6,7,8 are a point of concern.9 For example, the outcomes reported in Silverman et al6 comprised various composites as defined by the included trials rather than a universally defined composite. Reported RRRs in composite outcomes may be associated with reductions in potentially subjective outcomes, such as revascularization or hospitalization, the frequency of which may depend on opinions or preferences of the attending physician, rather than more objective outcomes (eg, all-cause mortality, myocardial infarction [MI], or stroke), leading to misleading impressions of the effect of treatment.10 Hence, an analysis focusing on hard, singular end points (total mortality, MI, and stroke) is less susceptible to bias.
Reporting the reduction in cardiovascular outcomes as RRR without reporting the corresponding absolute risk reduction (ARR) has the potential to inflate the clinical importance of an intervention and may exaggerate trivial associations.11 Therefore, to enable better decision-making between clinicians and patients, we assessed ARRs and RRRs from treatment with statins in hard outcomes, such as all-cause mortality, MI, and stroke, as well as exploring the association between LDL-C reduction and statin treatment effects.
Methods
This review was conducted according to the methods of the Cochrane Handbook for Systematic Reviews of Interventions and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline statement.12,13 The protocol for our review was published on PROSPERO.14
Details of the search strategy are described in eFigures 1 (PRISMA Flowchart) and 2 (PICOTTS format) in the Supplement. In summary, we searched PubMed and EMBASE in September 2020 and updated the search in June 2021. Potential studies were identified using the combined search terms clinical outcomes, LDL lowering, ischemic heart disease, and hydroxymethylglutaryl COA reductase inhibitors; were published between January 1966 and June 2021, limited to randomized clinical trials (RCTs); were written in English; and included human participants. Potential studies were also identified through the reference files of relevant studies. The search results were independently screened by P.B. and R.D. by title and abstract, and they obtained full-text versions of potentially relevant studies. Relevant studies were selected by P.B. and R.D. by reading the full texts and applying the inclusion and exclusion criteria. Covidence systematic review software15 was used to manage the searches and extraction of data. Any differences of opinion were resolved by discussion or by consulting another review author (S.S.).
We included any RCT that examined the efficacy of statins on total mortality and cardiovascular outcomes and stroke in adults, had a planned duration of 2 years or longer, had an enrollment of more than 1000 participants (to ensure reasonable statistical power), whose comparator was placebo or usual care, and reported absolute changes in LDL-C levels. Data extraction was performed by P.B. and R.D. and any differences resolved by consensus.
The methodological quality of included studies was assessed using the revised Cochrane Risk-of-bias tool for RCTs (version 2)16 by P.B., R.D., and K.O.B. Discrepancies were resolved by consensus. The overall certainty of the evidence for each outcome individually depending on the risk of bias (ROB), indirectness of evidence, inconsistency, imprecision of effect estimates, and potential publication bias was analyzed by P.B. and K.O.B. using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.17
We calculated the absolute difference in LDL-C levels between treatment arms based on the mean difference averaged over the course of follow-up. If unavailable, we used the mean absolute difference in LDL-C levels at the point closest to the median follow-up period (usually 1-2 years). We conducted a random-effects meta-analysis for each of the prespecified outcomes that were reported as log relative risks and absolute risk differences using the method of DerSimonian and Laird and reported the results by primary prevention, secondary prevention, and overall. We conducted a subgroup meta-analysis for each of the outcomes by study population (primary, secondary, mixed). We used this LDL-C absolute difference as an explanatory variable in a random-effects meta-regression analysis of the treatment effect estimated for each study (absolute risk difference and log relative risk) using the metareg command in Stata, version 16 (StataCorp; https://journals.sagepub.com/doi/pdf/10.1177/1536867X0800800403). Statistical heterogeneity was assessed using I2 and the Cochrane Q test. We conducted exploratory meta-regressions of absolute risk differences with adjustment for control event rates to control for differences in baseline risk and length of follow-up between trials.
Results
An initial database search identified 275 studies, with a further 31 studies identified from other sources (eFigure 1 in the Supplement). Following the removal of duplicates, 275 studies were eligible for title and abstract screening. Of these, 36 studies (13.1%) were selected for full-text review, and 15 (41.7%) of these were excluded. Details of the included and excluded studies are described in eTables 1 and 2 in the Supplement. Following full-text review, 21 studies were included in this review.
The 21 included trials were approximately equally distributed between primary prevention trials (7 [33%]), secondary prevention trials (6 [29%]), and trials that included participants from primary and secondary prevention populations (8 [38%]). Achieved LDL-C differences ranged from 16.99 mg/dL to 67.57 mg/dL for each individual trial. The average trial follow-up period was 4.4 years, ranging from 1.9 to 6.1 years, and the number of trial participants ranged from 1255 to 20 536 (eTable 1 in the Supplement).
As stated in our protocol,14 we initially intended to extract data on the composite outcomes of major vascular events and major coronary events. However, these outcomes were inconsistently defined across the trials. Thus, we concluded that a meta-analysis of these outcomes would be inappropriate. We found similar difficulties in the definitions of cardiovascular death and cardiovascular events. Hence, we restricted our analysis to all-cause mortality, spontaneous MI, and spontaneous stroke (excluding procedure-related MI and stroke when specified).
Overall, the ROB of the included trials was low (eFigure 3 in the Supplement). Of the 5 ROB domains, the randomization process was generally well conducted, most studies were placebo-controlled and blinded so that participants, health care professionals, and those conducting the intervention were unaware of the participants’ assigned intervention. For the outcomes of interest, most studies had little missing data despite there being a number of withdrawals in some of the studies. The outcomes were generally measured in an appropriate manner and analyzed as indicated in the individual study protocols.
There were a few aspects of the trials that caused concern. In the 4D trial,18 it was unclear from the protocol how the authors planned to measure or analyze fatal stroke, while in the ASPEN trial,19 there were changes to the inclusion criteria for participants after 2 years. However, it was unclear if this would have introduced bias. Four trials (JUPITER, CARDS, AFCAPS/TexCAPS, and ASCOT-LLA20,21,22,23) were terminated early, and this may have been a source of bias. In addition, we noted that all of the included trials were funded, in part or wholly, by the pharmaceutical industry.
The certainty of the evidence from these trials was appraised using the GRADE method, in which an assessment is made for each reported outcome. The certainty of the evidence was rated high for 1 outcome (MI secondary prevention) and low for 1 outcome (all-cause mortality secondary prevention). The outcomes of all-cause mortality all trials, stroke primary prevention, stroke secondary prevention, and stroke all trials were considered to be of moderate to low certainty. The outcomes of all-cause mortality primary prevention and MI all trials were rated moderate certainty, and the outcome of MI primary prevention was rated moderate to high certainty (eTable 3 in the Supplement).
A meta-analysis was conducted on 19 of the 21 trials that reported data on all-cause mortality and 18 trials reporting data on MI and stroke (Table 1). The ARR was 0.8% for all-cause mortality, 1.3% for MI, and 0.4% for stroke. The RRR for all-cause mortality was 9%, 29% for MI, and 14% for stroke for the groups randomized to receive statin therapy compared with placebo or usual care (Figure 1; eFigures 4, 5, 6, 7, 8, and 9 in the Supplement). Meta-analyses of relative associations of treatment with all-cause mortality in primary and secondary prevention trials were also conducted (Table 1). The ARR was 0.6% for all-cause mortality, 0.7% for MI, and 0.3% for stroke in primary prevention and 0.9%, 2.2%, and 0.7%, respectively, in secondary prevention. The RRR was 13% for all-cause mortality, 38% for MI, and 24% for stroke in primary prevention and 14%, 27%, and 13%, respectively, in secondary prevention (eFigures 10, 11, 12, 13, 14, and 15 in the Supplement). Results for statistical heterogeneity were mixed, with a range of low to high heterogeneity depending on the outcome and how it was assessed and the study population (primary prevention, secondary prevention, overall) (Table 1).
Table 1. Summary Results of Meta-analysis of the Relative and Absolute Association of Statin Therapy With All-Cause Mortality, MI, and Stroke.
| All studies | No. of trials | No. in statin group | No. in control group | Outcome | Effect size % (95% CI) | I2 % | P value (Q test) |
|---|---|---|---|---|---|---|---|
| RRR | 19 | 66 366 | 66 397 | All death | 9 (5 to 14) | 52 | .01 |
| 18 | 60 569 | 60 621 | MI | 29 (22 to 34) | 36 | .07 | |
| 18 | 65 522 | 65 564 | Stroke | 14 (5 to 22) | 49 | .01 | |
| ARR | 19 | 66 366 | 66 397 | All death | 0.8 (0.4 to 1.2) | 29 | .11 |
| 18 | 60 569 | 60 621 | MI | 1.3 (0.9 to 1.7) | 82 | <.001 | |
| 18 | 65 522 | 65 564 | Stroke | 0.4 (0.2 to 0.6) | 27 | .14 | |
| 1° Prevention | |||||||
| RRR | 6 | 29 028 | 29 028 | All death | 13 (3 to 22) | 44 | .11 |
| 6 | 27 162 | 27 215 | MI | 38 (29 to 46) | 0 | .56 | |
| 6 | 29 028 | 29 099 | Stroke | 24 (9 to 37) | 41 | .13 | |
| ARR | 6 | 29 028 | 29 028 | All death | 0.6 (0.2 to 1) | 7 | .37 |
| 6 | 27 162 | 27 215 | MI | 0.7 (0.4 to 1) | 73 | .003 | |
| 6 | 29 028 | 29 099 | Stroke | 0.3 (0.2 to 0.5) | 0 | .46 | |
| 2° Prevention | |||||||
| RRR | 5 | 12 227 | 12 213 | All death | 14 (–2 to 27) | 82 | <.001 |
| 5 | 13 464 | 13 458 | MI | 27 (18 to 35) | 4 | .39 | |
| 4 | 11 383 | 11 380 | Stroke | 13 (–6 to 28) | 57 | .07 | |
| ARR | 5 | 12 227 | 12 213 | All death | 0.9 (0.5 to 2.4) | 66 | .02 |
| 5 | 13 464 | 13 458 | MI | 2.2 (0.4 to 3.9) | 89 | <.001 | |
| 4 | 11 383 | 11 380 | Stroke | 0.7 (0.2 to 1.6) | 65 | .04 | |
Abbreviations: ARR, absolute risk reduction; I2, Proportion of total variation because of heterogeneity; Q test, test for heterogeneity in which P < .10 provides evidence of heterogeneity; MI, myocardial infarction; RRR, relative risk reduction; 1° prevention, primary prevention; 2° prevention, secondary prevention.
Figure 1. Comparison of Absolute and Relative Risk Reductions of Statins, All Trials.
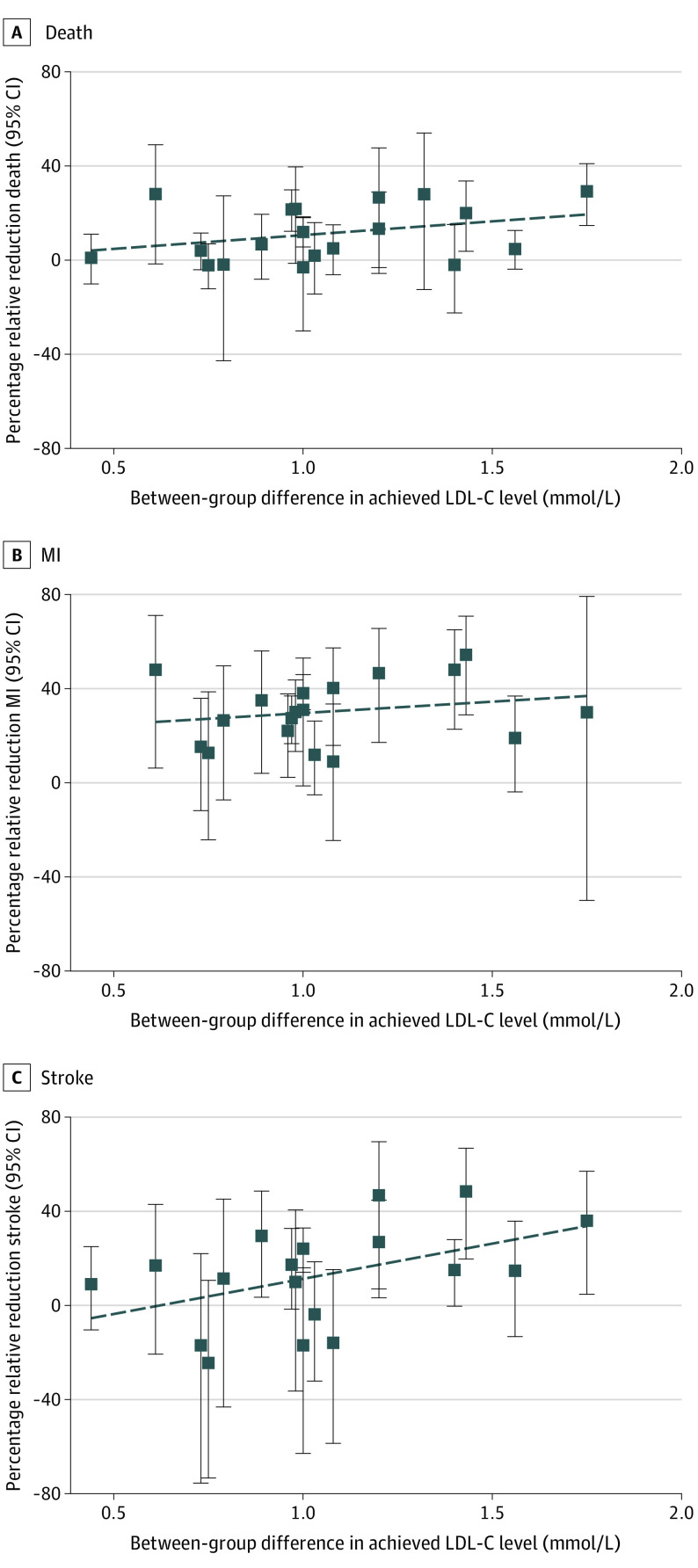
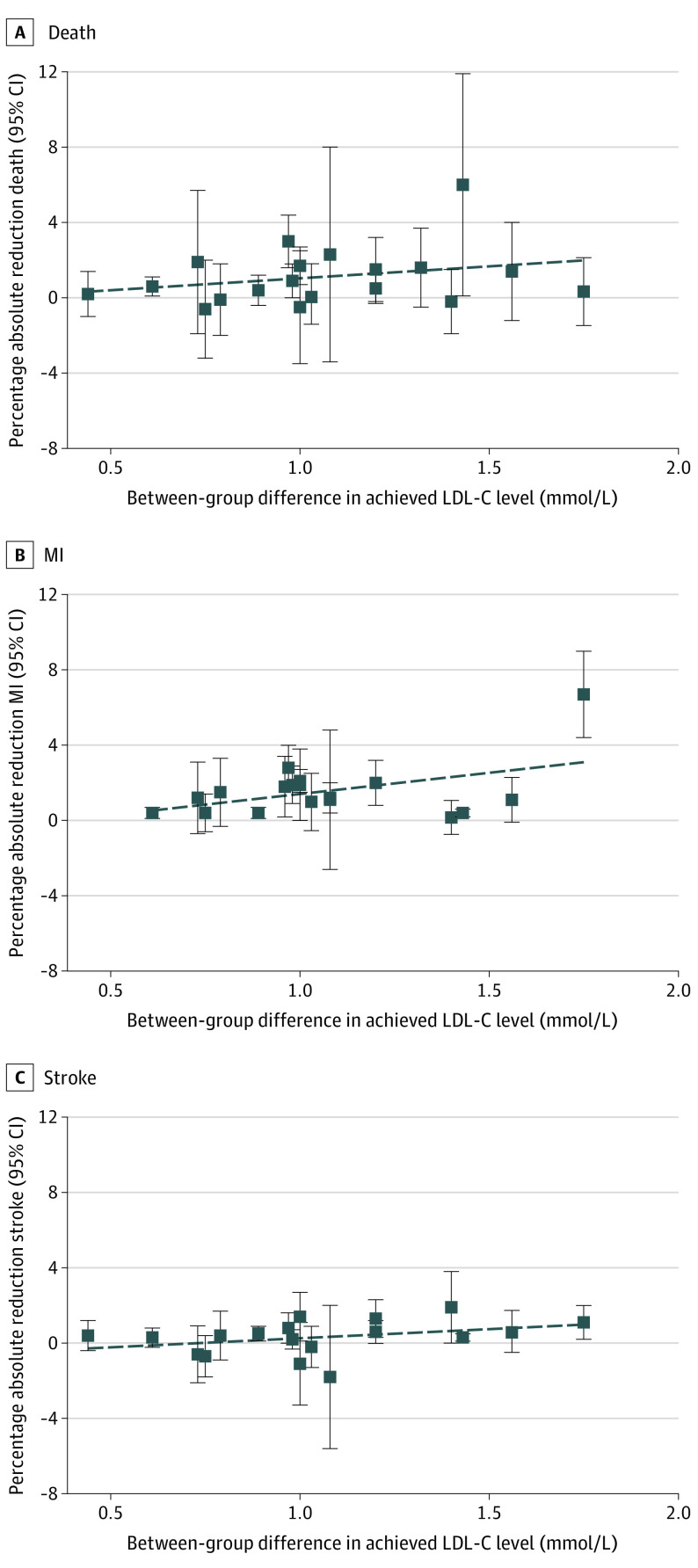
Meta-regression was undertaken as planned to explore the potential mediating association of LDL-C reduction with relative and absolute treatment effects. Findings were inconclusive, with regression slope confidence intervals all including the null value of 0. The proportion of between-study variance explained by LDL-C ranged from 0% to 14%, indicating very little, if any, association between the magnitude of LDL-C reduction and size of the treatment effect (eTable 4 in the Supplement). However, exploratory meta-regressions that adjusted for control event rates (or study population) and length of follow-up provided some differences from the unadjusted regressions (Table 224). These meta-regressions revealed inconsistent associations between the magnitude of LDL-C reduction and the size of treatment effects on clinical outcomes. Some association was found for the relative effects on all-cause mortality and stroke, but not for MI. Similarly, some association was found between the magnitude of LDL-C reduction and the size of the absolute treatment effect on stroke, but not for all-cause mortality or MI. Plots of the association between LDL-C reduction and the relative and absolute treatment effects on individual outcomes are shown in Figure 2 and Figure 3.
Table 2. Adjusted Meta-Regression Results of the Association of Mean Difference in LDL-C With All-Cause Mortality, MI, and Stroke.
| Outcome | No. of trials | Coefficient (95% CI) | P value | R2 % | |
|---|---|---|---|---|---|
| Baseline model | Full model | ||||
| All death | |||||
| logRR | 19 | –0.19 (–0.33 to –0.05) | .01 | 0 | 45 |
| Death ARD | 19 | –0.006 (–0.02 to 0.008) | .41 | 0 | 0 |
| MI | |||||
| logRR | 18 | –0.31 (–0.69 to 0.07) | .11 | 38 | 69 |
| ARD | 17a | –0.008 (–0.025 to 0.01) | .38 | 11 | 0 |
| Stroke | |||||
| logRR | 18 | –0.50 (–0.82 to –0.19) | .002 | 0 | 52 |
| ARD | 18 | –0.009 (–0.016 to –0.002) | .01 | 0 | 62 |
Abbreviations: ARD, absolute risk difference; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; RR, relative risk.
The 4S study24 was excluded from the meta-regression because of an outlying ARD that made the results uninterpretable (coefficient = estimate of slope in meta-regression model; R2 = proportion of between-study variance explained by the model). Baseline model only includes the adjusting variables; full model includes adjusting variables and explanatory variable: mean difference in LDL-C. Adjusting variables were study population (primary prevention, secondary prevention, or mixed) and length of follow up for logRR, and control event rate and length of follow up for risk difference.
Figure 2. Association Between Relative Risk Reductions in All-Cause Mortality, Myocardial Infarction (MI), and Stroke and Between-Group Difference in Achieved Low-Density Lipoprotein Cholesterol (LDL-C) Level.
Figure 3. Association Between Absolute Risk Reductions in All-Cause Mortality, Myocardial Infarction (MI), and Stroke and Between-Group Difference in Achieved Low-Density Lipoprotein Cholesterol (LDL-C) Level.
Discussion
The primary focus of this systematic review and meta-analysis was to estimate and compare the ARRs and RRRs in total mortality and cardiovascular events achieved with statin treatment and explore whether treatment effect was mediated by the magnitude of LDL-C lowering. The results of the meta-analysis suggested a benefit in all clinical outcomes. However, the presence of significant clinical and statistical heterogeneity suggests that pooling studies for a meta-analysis may be ill-advised; therefore, these results may be unreliable.25 Additional meta-analyses that were conducted on subgroups using trials that included only primary prevention or secondary prevention participants suggested that differences in study populations in terms of previous CVD explains little of the observed heterogeneity. Our unadjusted meta-regression analyses showed only a weak or inconsistent association between absolute LDL-C reductions and clinical outcomes (Table 2; Figure 2 and Figure 3). However, after adjusting for control event rates (or study population) and length of follow-up, evidence of an association was found for the relative effect on all-cause mortality and relative and absolute effect on stroke (Table 2). Because the meta-regression analysis yielded inconsistent results, we concluded that our meta-regression was inconclusive in proving or disproving an association between the magnitude of LDL-C reduction and the size of treatment effect. We noted that the R2 values ranged from 0% to 69%, which made little clinical sense; if the magnitude of LDL-C is associated with the relative reduction in all-cause mortality, it should also be associated with the absolute reduction in all-cause mortality, as well as with MI.
The cardiovascular benefits of treatment with statins are sometimes reported as RRR.7 However, reporting RRR without the corresponding ARR or number-needed-to-treat of a treatment can be misleading.25,26 For example, in our analysis, the RRR for MI was 29%, whereas the ARR was 1.3%. In other words, 77 participants would need to be treated with a statin for roughly 4.4 years on average to prevent 1 MI.
Moreover, the benefits of a treatment also depend on baseline risk (eTable 1 in the Supplement). Reporting RRR without baseline risk has been described as “the first sin against transparent reporting,”27 as it increases people’s willingness to receive a treatment, advise treatment, and pay to prevent the risk compared with ARR or other methods for communicating risk.28,29 Some patients who experience harms might choose to discontinue their use of the drug if they were counseled about the absolute degree of risk and benefit.30
Thus, we believe that ARR is essential for clinical decision-making and provides the clinician with a more accurate means of discussing the true benefits and harms of a specific therapy with their patients.31,32 Framed this way, our analysis found that when considering the ARR of statins, the benefits are quite modest, and most trial participants who took statins derived no clinical benefit.
While it is an advantage to patients that clinicians report the benefits of interventions in terms of ARR, this practice may not be as widespread as it should. Some studies report that physicians have been “found to recommend treatment options more on basis of relative than of absolute risk reduction.”33 Physicians may have difficulty in interpreting health risk statistics,33,34,35,36 are more likely to prescribe a treatment if the risk reduction is presented as relative rather than absolute,33 and some consider the association between cholesterol levels and CVD to be a “generally accepted truth.”37,38
In addition, our analyses considered the association between LDL-C reduction and the individual outcomes of all-cause mortality, MI, and stroke rather than focusing on the composite outcomes reported in other meta-analyses.5,6,39,40,41 There are inherent problems with composite outcomes as they can be inconsistently defined and inadequately reported.32 Mora et al10 analyzed the components of the composite outcome total CVD events in 1 large trial and found that women had a significant reduction in revascularizations and unstable angina, but not in other components of the composite outcome, including stroke. Patients or prescribers may alter their decision-making regarding the potential benefits of treatment with statins even though larger treatment effects may be associated with the less clinically important components of the composite.32
Current cholesterol clinical guidelines are based on an assumption of a log linear association between LDL-C and cardiovascular outcomes. For example, the 2019 European Guidelines for the Management of Dylipidaemias2 note that “RCTs have consistently demonstrated a log linear relationship between the absolute changes in plasma LDL-C and the risk of ASCVD.” Similarly, the 2018 American cholesterol guidelines state, “the more LDL-C is reduced on statin therapy, the greater will be subsequent risk reduction.”1 In contrast with these clinical guidelines, our findings are inconclusive in proving or disproving an association between the magnitude of LDL-C reduction and the size of treatment effect.
A strength of our review was the identification and acknowledgment of significant clinical and statistical heterogeneity, well-described limitations of meta-analysis that to our knowledge have received little or no attention in prior meta-analyses of statins. Our analysis identified marked clinical heterogeneity in the individual trials, the sources of which can be classified into those that are likely associated with LDL-C reduction and those that are not. For example, the type of statin, dosage of statin, compliance with treatment, and baseline level of LDL-C are all likely to be associated with LDL-C reduction, whereas population characteristics (for example, those taking statins for primary prevention compared with those taking them for secondary prevention), study duration, control treatment, and outcome definitions may not be. Therefore, if LDL-C reduction is strongly associated with treatment effect, then we should see this explain a sizeable portion of heterogeneity, but not all of it. However, our findings were inconsistent, and the meta-regression was inconclusive in proving or disproving an association between the magnitude of LDL-C reduction and the size of treatment effect. While pooling of studies may be inadvisable when this degree of clinical heterogeneity exists,42 we completed the meta-analysis as per protocol.
We also identified evidence of statistical heterogeneity in the I2 and Q statistic for all outcomes. In the context of significant heterogeneity, the calculations of ARR and RRR in all-cause mortality and cardiovascular events must be interpreted with caution. However, these limitations similarly apply to previous meta-analyses of statins.5,6,39,40,41
The calculated RRRs were similar to those reported in other meta-analyses,5,6,39,40,41 although each review used somewhat different trial selection criteria and methods. However, by avoiding composite end points, our review may have been able to eliminate a potential source of bias. Unlike other reviews, we formally compared ARR and RRR and acknowledged the importance of heterogeneity on the validity of our results.
Compared with the CTT meta-analysis, our trial list was similar, but CTT weighted their meta-analysis based on the degree of LDL-C reduction reported in individual patient data. Independent researchers do not have access to that individual patient data, so our analysis was based on aggregate published data, and we used traditional weighting based on individual trial variances.
In addition to considering the absolute benefit, patients and clinicians need to examine the potential for harm from treatment with statin therapy. This has been an area of considerable controversy, and the exact incidence and definition of statin harms have been debated. Collins et al43 reported that treating 10 000 patients with statins for 5 years could result in 5 cases of myopathy, 50 to 100 new cases of diabetes, and 5 to 10 cases of hemorrhagic stroke. The authors asserted that these harms are outweighed by the benefits of statins. This argument has merit if a patient’s baseline risk of serious CVD events is greater than their risk of harm from taking the medicine. However, the definition of myopathy used by Collins et al43 may be a high bar for diagnosing muscle symptoms among patients who may simply define myopathy as any muscle symptom.32 Observational data suggest that the frequency of statin myopathy may be higher. Buettner et al44 reported that 22.0% (95% CI, 18.0%-26.7%) of those taking statins in their study reported musculoskeletal pain in at least 1 anatomical region during the previous 30 days compared with 16.7% (95% CI, 15.1%-18.4%) of those who did not use a statin. Fernandez et al45 reported that the observational studies included in their review suggest that the frequency of statin myopathy is 9% to 20%.
Limitations
By restricting the analysis to only hard end points (total mortality, spontaneous MIs, and strokes), we may have excluded important clinical events, such as revascularization procedures or hospital admissions for angina. However, while we acknowledge that these are important clinical events, they lack uniform indications and are left to the discretion of the attending physician. Physicians in this context may be influenced by the lower LDL-C levels in patients who are receiving statin therapy. Similarly, composite end points could have been included in the analysis because they better reflect all cardiovascular events. Nevertheless, we felt it inappropriate to analyze composite clinical end points because they lack a universal definition and composite outcomes, such as major vascular events, are defined differently in different trials. The analysis also relied on aggregate data with potential biases associated with ecological fallacy. Although we intended to undertake subgroup analyses by age and sex as described in our protocol, we did not because of outcomes not reported within these subgroups of interest in the included trials. Meta-regressions were based on fewer than 20 trials, which may explain the inconsistent findings obtained across the different outcomes.
There are several possible explanations for why some of our conclusions differ from previous meta-analyses. Our trial list was similar to CTT meta-analyses, but CTT weighted their meta-analysis based on IPD, whereas our analysis was based on aggregate published data, using traditional weighting based on individual trial variances. Unlike other meta-analysis, we did not include trials of high-dose vs low-dose statins or trials that enrolled fewer than 1000 participants. Finally, we included an intercept term in our meta-regression models because of concerns it may be inappropriate to force the regression line through the origin.
Conclusions
This systematic review and meta-analysis found that the ARR of statins appears to be modest compared with the RRR, but these calculated benefits must be interpreted with caution because of the presence of significant heterogeneity. Our findings were inconsistent and inconclusive regarding the association between the magnitude of LDL-C reduction because of treatment with statins and all-cause mortality, MI, or stroke. The transparent communication of RRR and ARR by clinicians, as well as the potential for harm, to their patients may lead to more informed decision-making about the true benefits and risks of statins.31 In addition, our findings have implications for future clinical guideline development and for policy makers and payers considering the opportunity cost of statin therapy.
eFigure 1. PRISMA Flow Diagram
eFigure 2. PICOTTS format
eTable 1. Included trials & references
eTable 2. Excluded trials & reasons for exclusion
eFigure 3. ROB of included trials for all outcomes
eTable 3. GRADE Table
eFigure 4. Meta-analysis of relative effects of treatment on all-cause mortality, all trials
eFigure 5. Meta-analysis of absolute effects of treatment on all-cause mortality, all trials
eFigure 6. Meta-analysis of relative effects of treatment on myocardial infarction, all trials
eFigure 7. Meta-analysis of absolute effects of treatment on myocardial infarction, all trials
eFigure 8. Meta-analysis of relative effects of treatment on stroke, all-trials
eFigure 9. Meta-analysis of absolute effects of treatment on stroke, all trials
eFigure 10. Meta-analysis of relative effects of treatment on all-cause mortality in primary and secondary prevention trials
eFigure 11. Meta-analysis of absolute effects of treatment on all-cause mortality in primary and secondary prevention trials
eFigure 12. Meta-analysis of relative effects of treatment on myocardial infarction in primary and secondary prevention trials
eFigure 13. Meta-analysis of absolute effects of treatment on myocardial infarction in primary and secondary prevention trials
eFigure 14. Meta-analysis of relative effects of treatment on stroke from primary and secondary prevention trials
eFigure 15. Meta-analysis of absolute effects of treatment on stroke from primary and secondary prevention trials
eTable 4. Meta-regression results of outcomes by mean difference in LDL-C (unadjusted)
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/ AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486-2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Keech A, Kearney PM, et al. ; Cholesterol Treatment Trialists’ Collaborators . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267-1278. doi: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 8.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121(9):1069-1077. doi: 10.1161/CIRCULATIONAHA.109.906479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vine DL, Hastings GE. Ischaemic heart disease and cholesterol: absolute risk more informative than relative risk. BMJ. 1994;308:1040-1041. [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne P, Demasi M, Smith SM, Diamond D, Jones M, Dubroff R. Relation of LDL-cholesterol reduction with statin treatment on relative risk reduction compared to absolute risk reduction of cardiovascular events and mortality: protocol for a systematic review. Accessed September 1, 2020. https://www.crd.york.ac.uk/prospero/#recordDetails
- 15.Covidence. Better systematic review management. Accessed October 27, 2020. http://www.covidence.org
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380-382. doi: 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 18.Wanner C, Krane V, März W, et al. ; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248. doi: 10.1056/NEJMoa043545 [DOI] [PubMed] [Google Scholar]
- 19.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29(7):1478-1485. doi: 10.2337/dc05-2415 [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 21.Colhoun HM, Betteridge DJ, Durrington PN, et al. ; CARDS investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696. doi: 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 22.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279(20):1615-1622. doi: 10.1001/jama.279.20.1615 [DOI] [PubMed] [Google Scholar]
- 23.Sever PS, Dahlöf B, Poulter NR, et al. ; ASCOT investigators . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi: 10.1016/S0140-6736(03)12948-0 [DOI] [PubMed] [Google Scholar]
- 24.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389. [PubMed] [Google Scholar]
- 25.Diamond DM, Ravnskov U. How statistical deception created the appearance that statins are safe and effective in primary and secondary prevention of cardiovascular disease. Expert Rev Clin Pharmacol. 2015;8(2):201. doi: 10.1586/17512433.2015.1012494 [DOI] [PubMed] [Google Scholar]
- 26.Demasi M. Statin wars: have we been misled about the evidence? a narrative review. Br J Sports Med. 2018;52(14):905-909. doi: 10.1136/bjsports-2017-098497 [DOI] [PubMed] [Google Scholar]
- 27.Gigerenzer G, Wegwarth O, Feufel M. Misleading communication of risk. BMJ. 2010;341:c4830. doi: 10.1136/bmj.c4830 [DOI] [PubMed] [Google Scholar]
- 28.Visschers VH, Meertens RM, Passchier WW, de Vries NN. Probability information in risk communication: a review of the research literature. Risk Anal. 2009;29(2):267-287. doi: 10.1111/j.1539-6924.2008.01137.x [DOI] [PubMed] [Google Scholar]
- 29.Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8(10):543-548. doi: 10.1007/BF02599636 [DOI] [PubMed] [Google Scholar]
- 30.Edwards A, Elwyn G, Covey J, Matthews E, Pill R. Presenting risk information—a review of the effects of “framing” and other manipulations on patient outcomes. J Health Commun. 2001;6(1):61-82. doi: 10.1080/10810730150501413 [DOI] [PubMed] [Google Scholar]
- 31.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452-454. doi: 10.1136/bmj.310.6977.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne P, Cullinan J, Smith A, Smith SM. Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open. 2019;9(4):e023085. doi: 10.1136/bmjopen-2018-023085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcatto F, Rolison JJ, Ferrante D. Communicating clinical trial outcomes: effects of presentation method on physicians' evaluations of new treatments. Judgment and Decision Making. 2013;8(1):29. http://journal.sjdm.org/12/12913/jdm12913.pdf [Google Scholar]
- 34.Gaissmaier W, Gigerenzer G. Statistical illiteracy undermines informed shared decision making. Z Evid Fortbild Qual Gesundhwes. 2008;102(7):411-413. doi: 10.1016/j.zefq.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 35.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz LM, Woloshin S. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest. 2007;8(2):53-96. doi: 10.1111/j.1539-6053.2008.00033.x [DOI] [PubMed] [Google Scholar]
- 36.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135(6):943-973. doi: 10.1037/a0017327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrne P, O’Donovan Ó, Smith SM, Cullinan J. Medicalisation, risk and the use of statins for primary prevention of cardiovascular disease: a scoping review of the literature. Health, Risk & Society. 2019;21(7-8):390-406. doi: 10.1080/13698575.2019.1667964 [DOI] [Google Scholar]
- 38.Hann A, Peckham S. Cholesterol screening and the Gold Effect. Health, risk & society. 2010;12(1):33-50. doi: 10.1080/13698570903499608 [DOI] [Google Scholar]
- 39.Chou R, Dana T, Blazina I, et al. Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 40.Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med. 2010;170(12):1024-1031. doi: 10.1001/archinternmed.2010.182 [DOI] [PubMed] [Google Scholar]
- 41.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1001/jama.2013.281348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao G, Lopez-Jimenez F, Boyd J, et al. ; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. 2017;136(10):e172-e194. doi: 10.1161/CIR.0000000000000523 [DOI] [PubMed] [Google Scholar]
- 43.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532-2561. doi: 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 44.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23(8):1182-1186. doi: 10.1007/s11606-008-0636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78(6):393-403. doi: 10.3949/ccjm.78a.10073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. PICOTTS format
eTable 1. Included trials & references
eTable 2. Excluded trials & reasons for exclusion
eFigure 3. ROB of included trials for all outcomes
eTable 3. GRADE Table
eFigure 4. Meta-analysis of relative effects of treatment on all-cause mortality, all trials
eFigure 5. Meta-analysis of absolute effects of treatment on all-cause mortality, all trials
eFigure 6. Meta-analysis of relative effects of treatment on myocardial infarction, all trials
eFigure 7. Meta-analysis of absolute effects of treatment on myocardial infarction, all trials
eFigure 8. Meta-analysis of relative effects of treatment on stroke, all-trials
eFigure 9. Meta-analysis of absolute effects of treatment on stroke, all trials
eFigure 10. Meta-analysis of relative effects of treatment on all-cause mortality in primary and secondary prevention trials
eFigure 11. Meta-analysis of absolute effects of treatment on all-cause mortality in primary and secondary prevention trials
eFigure 12. Meta-analysis of relative effects of treatment on myocardial infarction in primary and secondary prevention trials
eFigure 13. Meta-analysis of absolute effects of treatment on myocardial infarction in primary and secondary prevention trials
eFigure 14. Meta-analysis of relative effects of treatment on stroke from primary and secondary prevention trials
eFigure 15. Meta-analysis of absolute effects of treatment on stroke from primary and secondary prevention trials
eTable 4. Meta-regression results of outcomes by mean difference in LDL-C (unadjusted)