Abstract
Background:
Youth with anxiety disorders struggle with managing emotions relative to peers, but the neural basis of this difference has not been examined.
Methods:
Youth (Mage = 13.6; range = 8–17) with (n = 37) and without (n = 24) anxiety disorders completed a cognitive reappraisal task while undergoing functional magnetic resonance imaging. Emotional reactivity and regulation, functional activation, and beta-series connectivity were compared across groups.
Results:
Groups did not differ on emotional reactivity or regulation. However, fronto-limbic activation after viewing aversive imagery with and without regulation, as well as affect ratings without regulation, were higher for anxious youth. Neither group demonstrated age-related changes in regulation, though anxious youth became less reactive with age. Stronger amygdala-ventromedial prefrontal cortex connectivity related to greater anxiety in control youth, but less anxiety in anxious youth.
Conclusion:
Anxious youth regulated when instructed, but regulation ability did not relate to age. Viewing aversive imagery related to heightened fronto-limbic activation even after reappraisal. Emotion dysregulation in youth anxiety disorders may stem from heightened emotionality and potent bottom-up neurobiological responses to aversive stimuli. Findings suggest the importance of treatments focused on both reducing initial emotional reactivity and bolstering regulatory capacity.
Keywords: anxiety, children & adolescents, emotion regulation, fMRI
1 |. INTRODUCTION
Youth anxiety disorders are pervasive and impairing (Merikangas et al., 2010) and can persist through adulthood (Beesdo-Baum & Knappe, 2012). Affected youth exhibit impairments in managing emotional experiences adaptively (Suveg & Zeman, 2004) and may face particular difficulty when distress intensifies (Legerstee et al., 2010). Evidence-based treatments, such as cognitive behavioral therapy (CBT) teach youth to regulate distress via cognitive reappraisal of anxiogenic thoughts. Although this technique is effective (Peris et al., 2015), and CBT more generally is efficacious for treating childhood and adolescent anxiety disorders (Higa-McMillan et al., 2016), anxiety symptoms often persist following treatment (Ginsburg et al., 2018); further, traditional anxiety-focused CBT does not improve emotion-related impairments other than worry (Suveg et al., 2009). Treatments may be improved by better understanding the neurobiological mechanisms driving the effects of cognitive reappraisal in anxious youth.
Neural correlates of cognitive reappraisal are commonly studied using emotion regulation paradigms in which participants view negative emotional stimuli and are instructed to either reduce their evoked negative affect or simply experience it. These paradigms isolate emotional reactivity, or the intensity of an emotional response, from emotion regulation, or the capacity to modify the intensity of an emotional response. Distinguishing between these constructs can elucidate whether emotion dysregulation in anxious youth stems from heightened reactivity, insufficient regulation of reactivity, or both (Lewis et al., 2010). Emotional reactivity decreases with age across both anxious and non-anxious youth (Carthy et al., 2010), though others have found no age-related changes in control youth (McRae et al., 2012; Silvers et al., 2012). In contrast, emotion regulation capacity develops across age in control youth, demonstrating both linear and quadratic trajectories (McRae et al., 2012; Silvers et al., 2016). While anxious youth regulate as successfully as non-anxious peers when cued to regulate (Carthy et al., 2010; Carthy et al., 2010), they do not increase their tendency to use adaptive emotion regulation with age (Schäfer et al., 2017). As such, low regulation use may become a stable characteristic by adulthood (Aldao et al., 2010); however, the developmental trajectory of regulation ability has not been examined in anxious youth.
Development of affective control brain regions relates to improved emotion regulation abilities in typically developing youth (Ahmed et al., 2015). Cognitive reappraisal studies find age-related decreases in amygdala activation during downregulation of affect (see Buhle et al., 2014 for meta-analysis) though not always (McRae et al., 2012). Higher adolescent trait anxiety attenuates age-related trajectories of decreased amygdala activation (Hare et al., 2008). Healthy adults demonstrate greater left ventrolateral prefrontal (vlPFC) activation during reappraisal (Kohn et al., 2014); similarly, better emotion regulation in youth relates to age-related increases in vlPFC activation (Silvers et al., 2012). Ventromedial prefrontal cortex (vmPFC) activation relates to downregulation of negative affect during reappraisal (Diekhof et al., 2011) and valuation of stimulus valence (Ochsner et al., 2012). Youth at risk for anxiety (e.g., have experienced early life trauma) demonstrate heightened prefrontal activation during reappraisal (McLaughlin et al., 2015), possibly reflecting enhanced effort to employ reappraisal, as these youth also exhibit greater emotional reactivity and greater activation in brain regions encoding emotional salience when viewing negative stimuli (Hein & Monk, 2017).
Refinement of subcortical-cortical connectivity (Casey, Heller, Gee, & Cohen, 2019) also contributes to improvements in emotion regulation abilities in youth. Age predicts more negative amygdala–vmPFC connectivity during passive viewing of fearful faces and relates to less anxiety (Gee et al., 2013), as well as more negative amygdala–vlPFC connectivity during reappraisal (Silvers et al., 2015). Youth with stronger negative amygdala–vmPFC connectivity also evince left vlPFC activation that mediates the relationship between age and decreased amygdala activation (Silvers et al., 2016). In contrast, healthy adults with less negative affect demonstrate positive fronto-limbic coupling during reappraisal (Banks et al., 2007).
Here, we used an functional magnetic resonance imaging (fMRI) paradigm to investigate behavioral and neurobiological differences in emotional reactivity and regulation between youth with and without anxiety disorders. Although emotion regulation requires coordination across several brain regions, the amygdala, vlPFC, and vmPFC can serve as targets for which prior literature consistently finds effects related to cognitive reappraisal in both healthy adults and children. As such, and given this is the first study examining neurobiological differences between youth with and without anxiety in emotion regulation (Young et al., 2019), we focused on this circumscribed portion of the emotion regulation circuit as a priori seed regions in activation and connectivity analyses, while also exploring whole-brain activation. We hypothesized that anxious youth would display heightened emotional reactivity but comparable emotion regulation relative to control youth. Age-specific hypotheses for emotional reactivity were not formulated given limited and mixed prior findings; however, emotion regulation was expected to develop linearly and quadratically in control youth, but not expected to demonstrate age-related changes in anxious youth. Amygdala activation during reappraisal was expected to decline across age in control but not in anxious youth, and to correlate with greater anxiety severity across both groups. We did not formulate directional hypotheses relating amygdala-frontal connectivity to reappraisal, given mixed prior findings. However, age was hypothesized to relate to amygdala–vmPFC and amygdala–vlPFC connectivity; anxiety severity was hypothesized to moderate this association. Exploratory whole-brain analyses were also conducted for activation. Results can offer insights into mechanisms at play during CBT and suggest techniques to bolster treatment efficacy.
2 |. METHODS
2.1 |. Participants
The sample consists of 61 children and adolescents 8–17 years old: 37 participants with anxiety (28 females, Mage = 13.8, SDage = 3.0) and 24 control youth (17 female, Mage = 13.3, SDage = 3.3). Nine participants (n = 5 anxious, n = 4 controls) were included in behavioral analyses but excluded from imaging analyses for excessive motion (Supplementary Materials). The greater proportion of females in this sample is consistent with disorder prevalence (Hantsoo & Epperson, 2017). All participants were evaluated by a trained clinical psychologist using the anxiety disorder interview schedule IV to assess for an anxiety disorder (Albano & Silverman, 1996). Youth were excluded if they had any contraindications to MRI, present or current history of neurodevelopmental or neurological disorder, or any psychiatric medication use. Additionally, control youth were excluded if they had any previous or current history of psychiatric disorder. Full scale intelligence quotient (IQ) was estimated using the vocabulary and matrix reasoning subtests from the Weschler abbreviated scale of intelligence (Wechsler, 1999); youth with IQ below 70 were excluded. Maternal education was used as a proxy for socioeconomic status.
2.2 |. Anxiety severity
All participants completed the 39-item multidimensional anxiety scale for children (March et al., 1997) to assess anxiety symptom severity. Participants reported on a scale from 0 to 3 to describe how often they experience symptoms, where 0 indicates never, 1 rarely, 2 sometimes, and 3 often. Scores were averaged to examine composite anxiety severity.
2.3 |. Data collection
Task fMRI data were obtained using a Siemens 3T Prisma (20-channel head coil) or Siemens Trio at UCLA. Each participant received a matched-bandwidth echo-planar image for registration (TR = 5000 ms, TE = 35 ms, FOV = 192 mm, 34 slices, slice thickness 4 mm, in-plane voxel size 1.5 × 1.5 mm). The T2*-weighted task fMRI sequence (TR = 2000 ms, TE = 30 ms, FOV = 192 mm, 34 slices, slice thickness 4 mm, in-plane voxel size 3×3mm) was acquired while participants completed the emotion regulation task (Figure 1).
FIGURE 1.
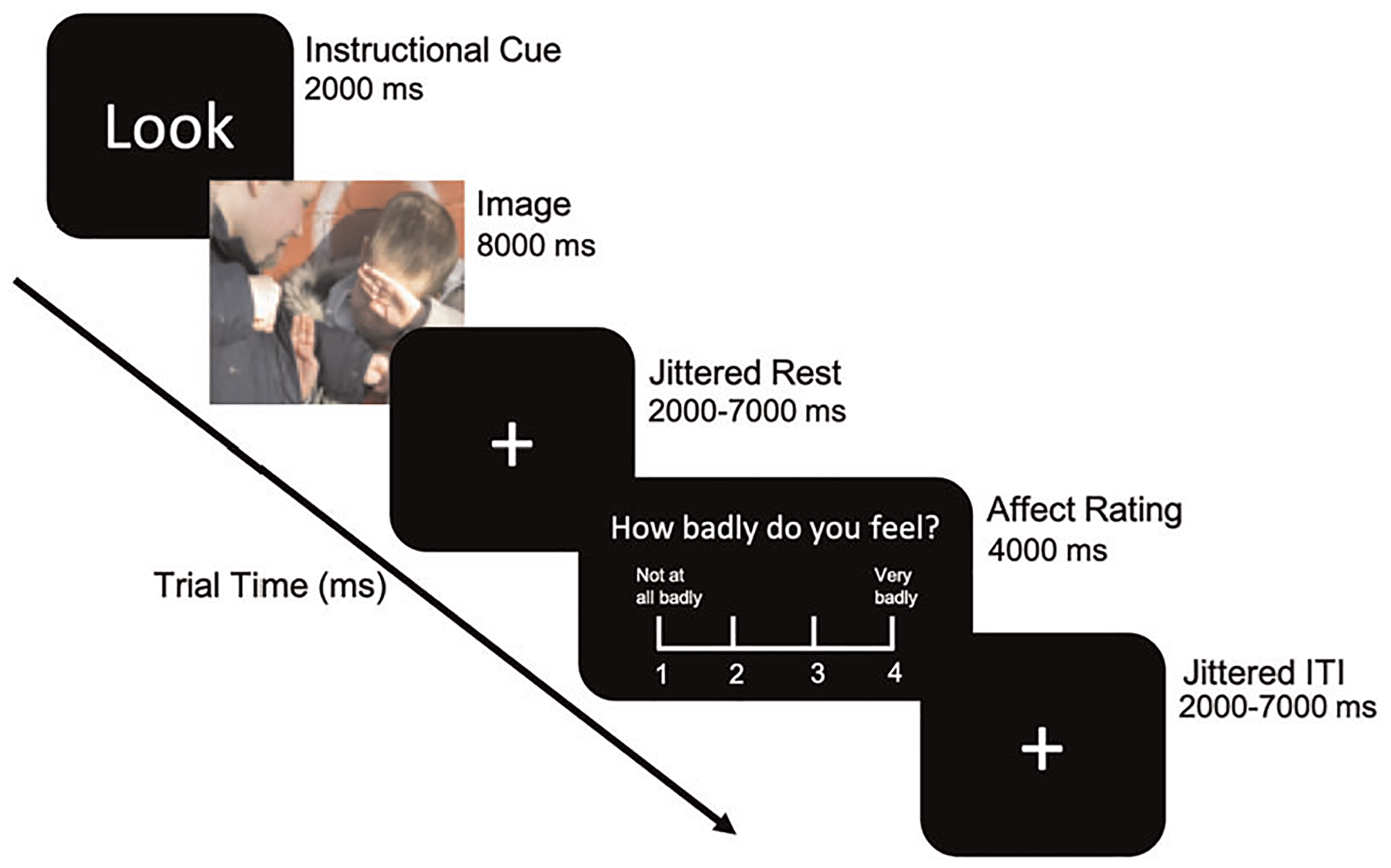
Emotion regulation paradigm. Each trial begins with an instructional cue, followed by either an aversive or neutral image and a jittered rest period. Participants are then presented with an affect rating scale and a jittered intertrial interval before the next trial
Each trial started with a 2 s instructional cue (“Look,” the instruction to react naturally or “Decrease,” the instruction to regulate emotions). Either an aversive or a neutral picture followed for 8 s, after which participants were presented with a rating scale for 4 s to report the strength of their negative affect. The scale ranged from 1 to 4 where 1 indicates feeling “not at all badly” and 4 indicates feeling “very badly.” Trials were presented in an event-related design, with “Look” and “Decrease” trials interspersed. A total of 24 Look and 24 Decrease trials were presented across 2 runs, separated into 12 negative and 12 neutral trials. Conditions of interest include look aversive (emotional reactivity), look neutral (nonemotional responding), and decrease aversive (emotion regulation).
Emotion regulation was calculated as the difference in aversiveness ratings between look aversive and decrease aversive trials. Emotional reactivity was calculated as the difference in aversiveness ratings between look aversive and look neutral trials. One anxious youth was an outlier in emotional reactivity (+3 standard deviations above group average). Results are only reported if they remained significant with and without this participant.
2.4 |. Neuroimaging data analysis
Analyses were performed using FMRIB’s Software Library (FSL) 5.0.9 (http://www.fmrib.ox.ac.uk/fsl) (Supplementary Methods). Following pre-processing, participants’ individual run-level data were analyzed using a fixed-effects general linear model. Regressors for each trial type were created by convolving a delta function representing the onset time with a canonical double-gamma hemodynamic response function. Six total regressors were created: four for stimuli (decrease aversive, decrease neutral, look aversive, look neutral), one representing all cues, and one representing all rating events.
Group level analyses were conducted using a mixed effects model in FSL (FLAME1), thresholded at z > 3.1 (p < .001) and corrected for multiple comparisons at p < .05. As five participants were scanned before institutional scanner upgrade, scanner type was included as a covariate of no interest in all group level analyses. Conditions of interest included decrease aversive, look aversive, and look neutral. Contrasts of interest investigated emotion regulation (decrease aversive >look aversive) and emotional reactivity (look aversive >look neutral). Given a priori interest in activation of the amygdala, vmPFC, and vlPFC, parameter estimates were extracted for these regions of interest (ROI). Exploratory whole-brain analyses were conducted as well given the novelty of the sample.
Trial-by-trial functional connectivity was generated for each subject using a least-squares separate beta-series regression analysis (Mumford et al., 2012; Rissman et al., 2004) in FSL. Time series were extracted from generated ROI masks.
3 |. RESULTS
3.1 |. Behavioral results
3.1.1 |. Participant characteristics
Anxious youth displayed greater anxiety severity than control youth (t(55.3) = 5.41, p < .001; Table 1). There was a trending difference in maternal education between groups (χ2(4) = 8.44, p = .08). IQ was included as a covariate of no interest in all analyses given a significant group difference (t(46.7) = −2.48, p = .02). There were no significant differences between groups in age (t(45.6) = 0.66, p = .51), sex (χ2(1) = 0.18, p = .67), race/ethnicity (χ2(16) = 6.07, p = .19), or mean relative motion (t(34.1) = −0.34, p = .73). Youth who did and did not (n = 9) provide usable imaging data did not differ in anxiety severity (t(14.6) = 0.89, p = .39), race/ethnicity (χ2(16) = 2.09, p = .72), maternal education (t(12.2) = 0.98, p = .35), or IQ (t(12.2) = −1.21, p = .25); however, youth with usable data were older (t(35.1) = 9.91, p < .001) and female (χ2(1) = 4.69, p = .03), consistent with prior findings (Dosenbach et al., 2017).
Table 1.
Participant characteristics
| Anxious, n = 37 | Controls, n = 24 | p | |
|---|---|---|---|
| Age | 13.8 (3.0) | 13.3 (3.3) | .51 |
| Sex | 28 F, 9 M | 17 F, 7 M | .67 |
| Mean relative motion (mm) | 0.11 (0.09) | 0.12 (0.12) | .73 |
| MASC | 2.47 (0.44) | 1.91 (0.37) | <.001 |
| Aversiveness ratings | .05 | ||
| Emotional reactivity | 1.33 (0.64) | 1.05 (0.58) | .11 |
| Emotion regulation | 0.20 (0.44) | 0.09 (0.55) | .49 |
| Look aversive | 2.58 (0.58) | 2.18 (0.65) | .04 |
| Look neutral | 1.25 (0.32) | 1.14 (0.21) | .37 |
| Decrease aversive | 2.38 (0.58) | 2.10 (0.65) | .14 |
| Self-reported race/ethnicity | .19 | ||
| Asian | 4 | 3 | - |
| Black | 5 | 1 | - |
| Hispanic | 2 | 5 | - |
| More than one race | 8 | 7 | - |
| White | 18 | 7 | - |
| Missing | - | 1 | - |
| Maternal education | .08 | ||
| Has not completed high school | 1 | 4 | - |
| Completed high Sshool | 1 | 1 | - |
| Completed Associate’s degree | 6 | 0 | - |
| Completed Bachelor’s degree | 11 | 10 | - |
| Completed Master’s degree or above | 11 | 5 | - |
| Full scale IQ | 105.7 (14.6) | 115.6 (15.7) | .02 |
Abbreviation: MASC, multidimensional anxiety scale for children.
3.1.2 |. Aversiveness ratings
Aversiveness ratings averaged across all trials demonstrated that anxious youth displayed greater negative affect compared to control youth for all image types (β = −0.22, p = .05; Table 1). Specifically, anxious youth reported significantly greater negative affect during look aversive trials (β = −0.35, p = .04). Ratings were not significantly different during decrease aversive (β = −0.26, p = .14) or look neutral trials (β = −0.07, p = .37).
3.1.3 |. Emotion reactivity and regulation
Across both groups, youth reported significantly greater negative affect during look aversive relative to look neutral trials (t(60) = −15.3, p < .001), demonstrating that aversive images elicited a negative affective response. Groups did not differ in the extent of emotional reactivity (β = −0.28, p = .11; Table 1, Figure 2a).
FIGURE 2.
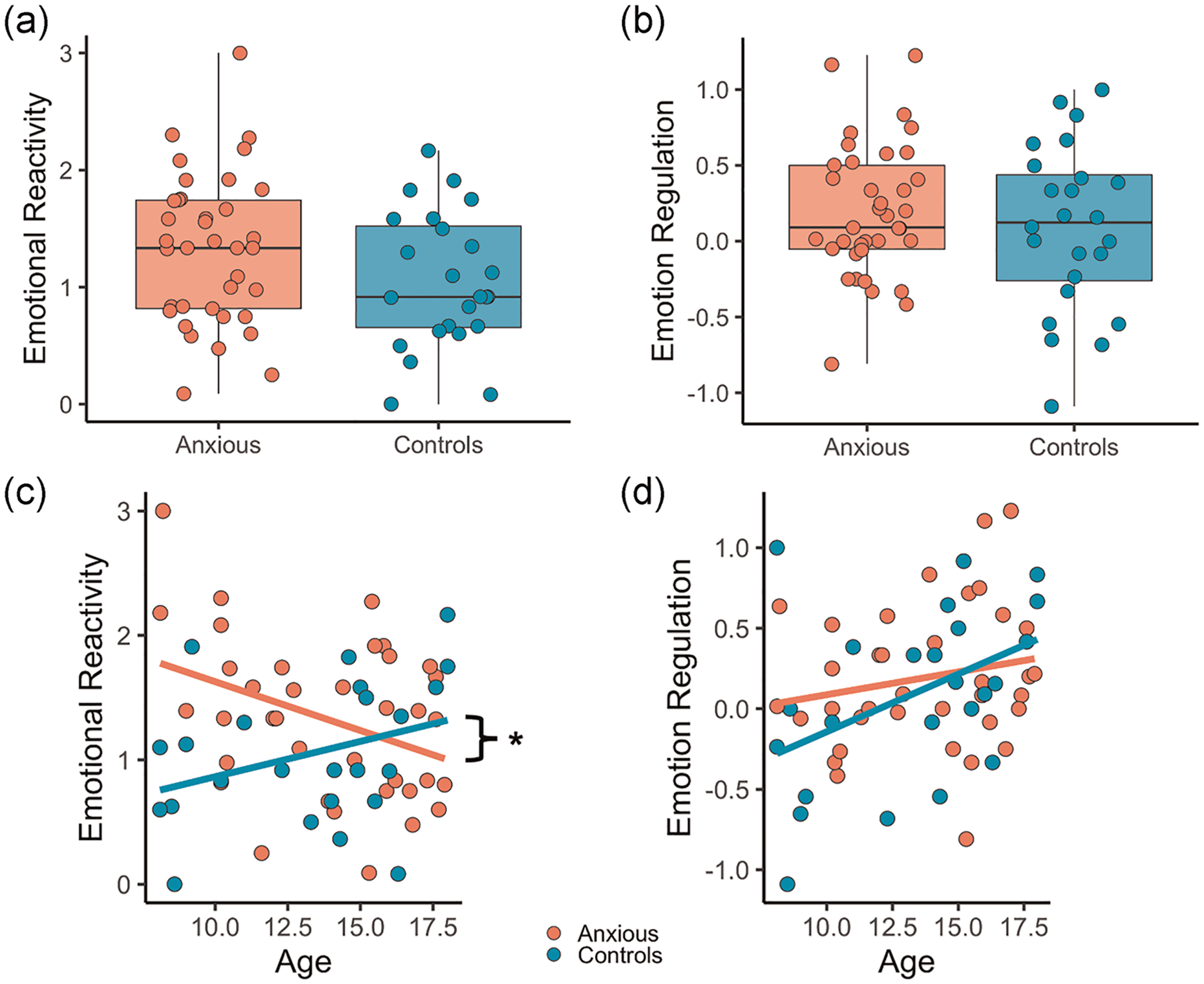
Emotional reactivity and regulation. (a) Emotional reactivity (look aversive–look neutral affect ratings). (b) Emotion regulation (decrease aversive–look aversive affect ratings). (c) Emotional reactivity across age. (d) Emotion regulation across age. *p < .05
Youth reported significantly lower negative affect during decrease aversive relative to look aversive trials (t(60) = −2.51, p = .01), demonstrating that negative affect was being regulated. Groups did not differ in the extent of emotion regulation (β = −0.09, p = .49; Table 1, Figure 2b).
3.1.4 |. Effects of anxiety severity and age on emotional reactivity and regulation
There was no significant main effect of anxiety severity (β = −0.07, p = .76), group (β = .56, p = .53), or anxiety severity by group interaction (β = −0.47, p = .27) on emotional reactivity. Age (β = −0.08, p = .02) and group (β = −2.10, p = .003) both predicted emotional reactivity. However, there was a significant group by age interaction (β = .13, p = .008); post hoc simple slopes analyses revealed that emotional reactivity decreased with age in anxious youth, but age and emotional reactivity were unrelated in control youth (Figure 2c).
There was no significant main effect of anxiety severity (β = −0.01, p = .95), group (β = .30, p = .68), or anxiety severity by group interaction (β = −0.21, p = .54) on emotion regulation. There was also no significant main effect of age (β = .04, p = .19), group (β = −0.74, p = .18), or age by group interaction (β = .05, p = .23) on emotion regulation (Figure 2d).
3.2 |. Functional activation results
3.2.1 |. Functional activation differences within look aversive, decrease aversive, and look neutral
Whole-brain analyses revealed activation in anxious youth in the thalamus, lateral occipital cortex (LOC), and fronto-parietal regions across all three conditions (Supplementary Figures and Tables 1–3). In controls, activation during all three conditions occurred mainly in temporal and occipital regions. Between-group analyses revealed greater activation in anxious youth in the middle frontal and precentral gyri during look aversive and decrease aversive trials; anxious youth also showed greater activation in right LOC, supramarginal gyrus, and caudate during decrease aversive trials. In look neutral trials, anxious youth showed greater activation in left occipital pole and right LOC.
TABLE 3.
Peak coordinates of brain activity during emotion regulation (decrease aversive >look aversive)
| Peak MNI coordinates | Voxels (mm3) | ||||||
|---|---|---|---|---|---|---|---|
| Hemisphere | Region label | x | y | z | z-Max | ||
| Anxious | Right | Lateral occipital cortex, superior division | 56 | −60 | 32 | 4.58 | 199 |
| 54 | −64 | 36 | 4.39 | ||||
| 52 | −60 | 44 | 4.25 | ||||
| 54 | −62 | 40 | 4.23 | ||||
| Angular gyrus | 56 | −54 | 32 | 4.55 | |||
Anxious youth displayed significantly higher activation of the bilateral amygdala relative to control youth during look aversive (β = 4.89, p = .003; Figure 3a), decrease aversive (β = 3.99, p = .009; Figure 3b), and look neutral trials (β = 6.74, p = .0003; Figure 3c). vlPFC activation was higher in anxious youth during look aversive (β = 4.31, p = .04), decrease aversive (β = 4.11, p = .05), and look neutral trials (β = 5.94, p = .01). vmPFC activation did not differ between groups during look aversive (β = 1.23, p = .71), decrease aversive (β = 2.17, p = .71), or look neutral trials (β = 3.91, p = .48).
FIGURE 3.
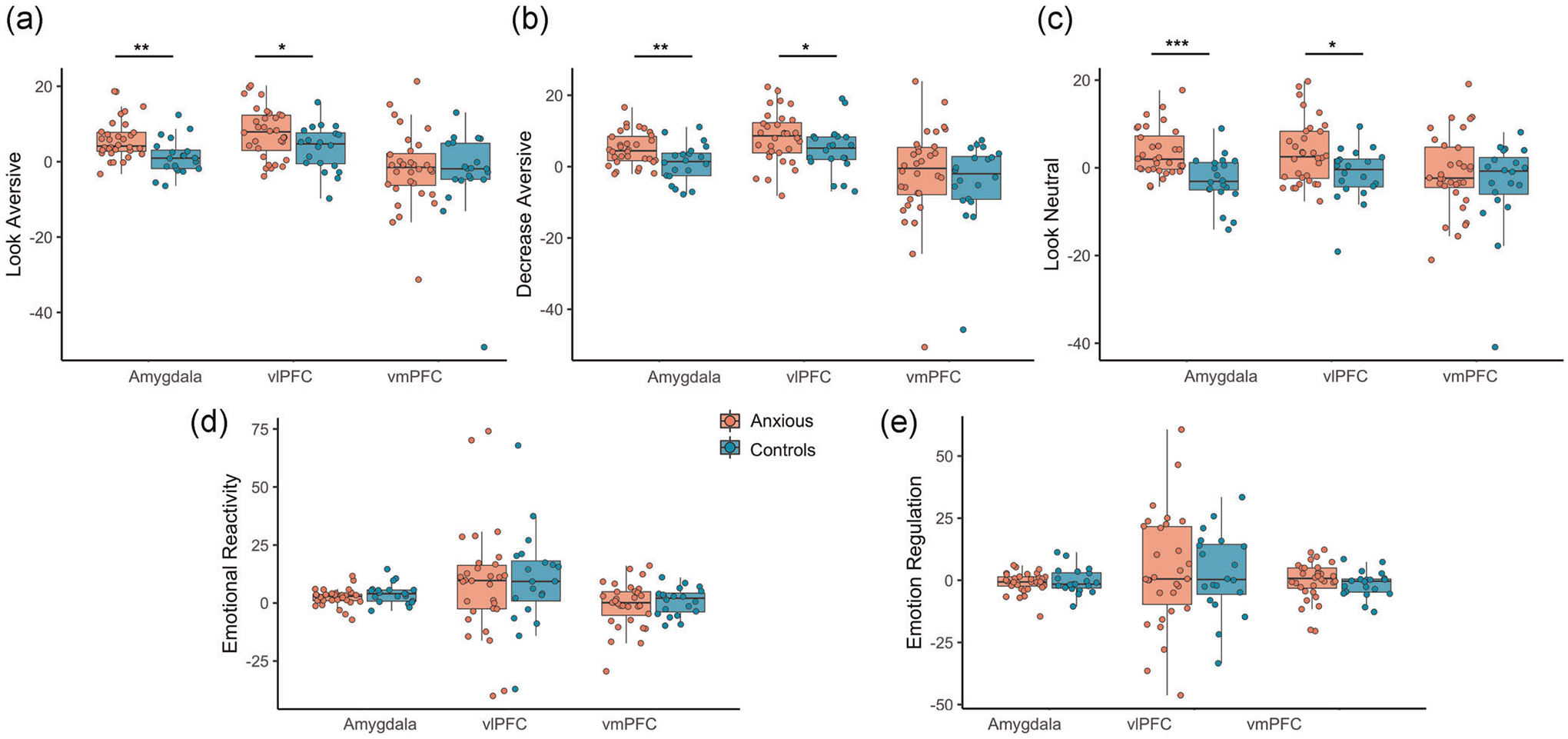
Amygdala, vlPFC, and vmPFC parameter estimates. Parameter estimates extracted from a priori regions of interest during (a) look aversive trials, (b) decrease aversive trials, (c) look neutral trials, (d) emotional reactivity, and (e) emotion regulation. vlPFC, ventrolateral prefrontal; vmPFC, ventromedial prefrontal cortex
3.2.2 |. Functional activation during emotional reactivity
Whole-brain analyses revealed activation in both groups during emotional reactivity (look aversive >look neutral; Figure 4, Table 2) in temporo-occipital regions, superior and inferior frontal gyri, and several subcortical regions including the bilateral thalamus, striatum, and amygdala. No significant activation was observed for control youth, nor any observed between-group differences.
FIGURE 4.
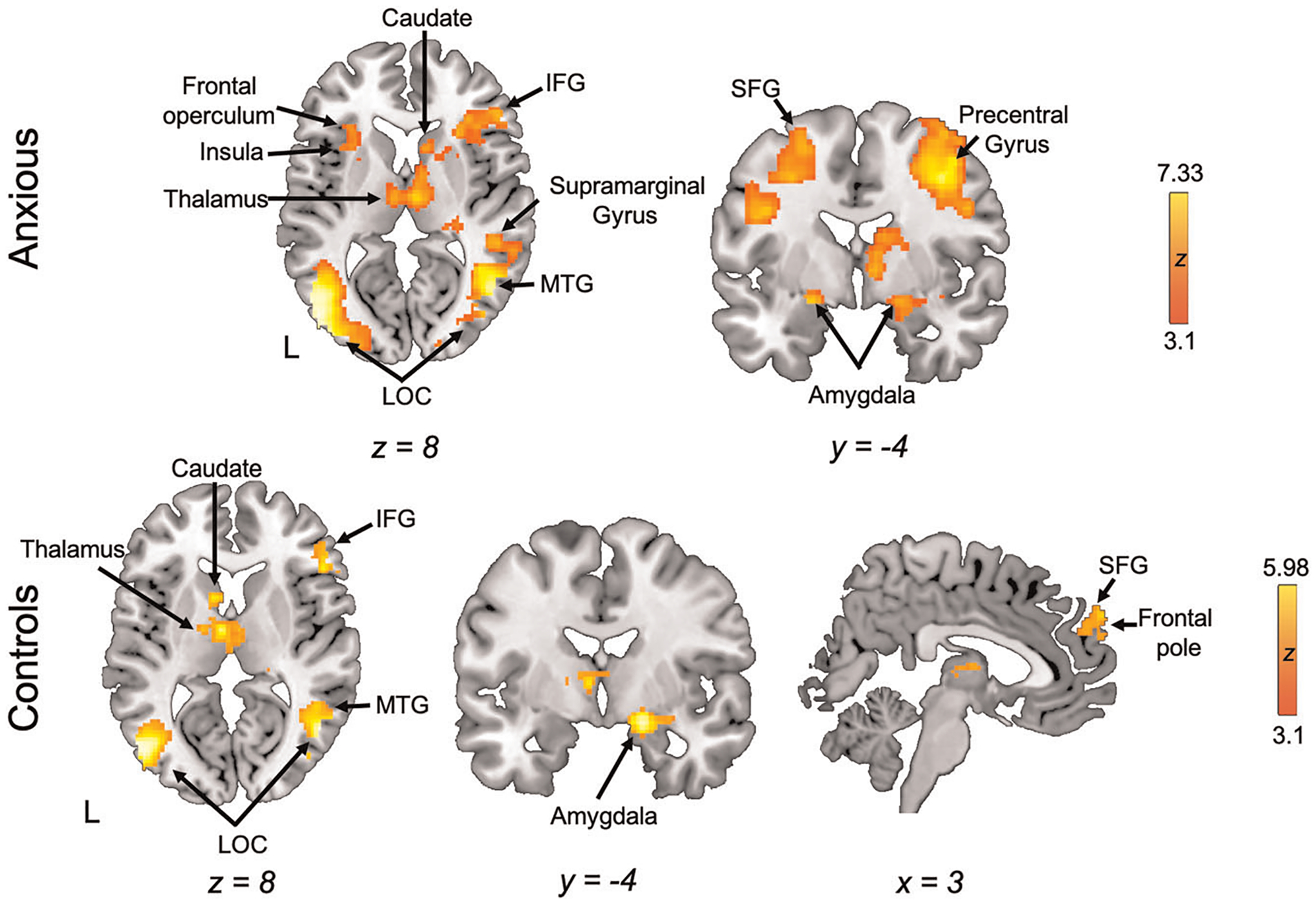
Neural activation during emotional reactivity. Whole-brain activation for the emotional reactivity contrast (look aversive >look neutral). IFG, inferior frontal gyrus; LOC; lateral occipital cortex; MTG, middle temporal gyrus; SFG, sperior frontal gyrus
TABLE 2.
Peak coordinates of brain activity during emotional reactivity (look aversive >look neutral)
| Peak MNI coordinates | Voxels (mm3) | ||||||
|---|---|---|---|---|---|---|---|
| Hemisphere | Region label | x | y | z | z-Max | ||
| Anxious | Right | Precentral gyrus | 42 | 0 | 46 | 6.31 | 4692 |
| 42 | 4 | 32 | 5.69 | ||||
| 38 | 4 | 30 | 5.67 | ||||
| 48 | 4 | 28 | 5.35 | ||||
| 52 | 10 | 30 | 5.02 | ||||
| Medial | Superior frontal gyrus | 4 | 10 | 56 | 5.03 | ||
| Left | Lateral occipital cortex, inferior division | −48 | −78 | 6 | 7.33 | 4445 | |
| −48 | −66 | 4 | 7 | ||||
| −48 | −70 | 10 | 6.84 | ||||
| −44 | −84 | 10 | 6.83 | ||||
| −42 | −60 | 0 | 6.51 | ||||
| Temporal occipital fusiform cortex | −40 | −46 | −16 | 6.57 | |||
| Right | Lateral occipital cortex, inferior division | 44 | −64 | −2 | 6.74 | 3503 | |
| 46 | −68 | 2 | 6.67 | ||||
| 46 | −64 | 4 | 6.58 | ||||
| 48 | −62 | 12 | 6.21 | ||||
| Middle temporal gyrus, temporooccipital part | 40 | −54 | 4 | 6.59 | |||
| Lateral occipital cortex, superior division | 42 | −60 | 16 | 6.23 | |||
| Right | Thalamus | 18 | −30 | 0 | 5.46 | 1988 | |
| 4 | −12 | 10 | 5.04 | ||||
| 14 | −28 | −4 | 4.99 | ||||
| 10 | −26 | −2 | 4.93 | ||||
| Left | Thalamus | −6 | −28 | −2 | 5.1 | ||
| Amygdala | −18 | −6 | −12 | 4.88 | |||
| Left | Precentral gyrus | −32 | −6 | 46 | 5.53 | 1169 | |
| −34 | −6 | 50 | 5.52 | ||||
| −46 | 2 | 34 | 5.33 | ||||
| −42 | 0 | 30 | 4.99 | ||||
| Superior frontal gyrus | −24 | −2 | 58 | 4.42 | |||
| Middle frontal gyrus | −42 | 10 | 30 | 4.01 | |||
| Left | Frontal operculum cortex | −32 | 26 | 6 | 4.45 | 221 | |
| Insular cortex | −34 | 16 | 6 | 4.27 | |||
| −28 | 16 | 10 | 4.1 | ||||
| −34 | 14 | −4 | 3.51 | ||||
| −34 | 14 | −8 | 3.47 | ||||
| Putamen | −24 | 10 | 10 | 3.29 | |||
| Right | Supramarginal gyrus, anterior division | 48 | −28 | 40 | 4.01 | 179 | |
| 60 | −24 | 38 | 3.76 | ||||
| 62 | −20 | 40 | 3.75 | ||||
| 64 | −28 | 30 | 3.58 | ||||
| Controls | Right | Middle temporal gyrus, temporooccipital part | 50 | −60 | 2 | 5.82 | 873 |
| Lateral occipital cortex, inferior division | 48 | −62 | 8 | 5.75 | |||
| 46 | −68 | 2 | 5.52 | ||||
| 44 | −72 | 12 | 4.8 | ||||
| Inferior temporal gyrus, temporooccipital part | 44 | −58 | −6 | 5.25 | |||
| Angular gyrus | 42 | −56 | 16 | 4.79 | |||
| Left | Lateral occipital cortex, inferior division | −46 | −74 | 4 | 5.98 | 670 | |
| −52 | −70 | 8 | 5.7 | ||||
| −40 | −62 | 0 | 4.29 | ||||
| Temporal occipital fusiform cortex | −38 | −52 | −14 | 4.8 | |||
| −38 | −46 | −16 | 4.25 | ||||
| −38 | −58 | −4 | 3.99 | ||||
| Right | Inferior frontal gyrus, pars triangularis | 50 | 26 | 8 | 4.44 | 487 | |
| 44 | 32 | 14 | 4.24 | ||||
| 58 | 28 | 14 | 4.06 | ||||
| 52 | 28 | 12 | 3.86 | ||||
| Middle frontal gyrus | 44 | 26 | 24 | 4.44 | |||
| Frontal pole | 54 | 40 | 12 | 4.43 | |||
| Right | Precentral gyrus | 42 | 6 | 28 | 4.53 | 410 | |
| 46 | 6 | 28 | 4.44 | ||||
| 50 | 10 | 32 | 4.42 | ||||
| Middle frontal gyrus | 54 | 16 | 40 | 3.39 | |||
| Left | Thalamus | −6 | −6 | 6 | 5.03 | 347 | |
| Caudate | −8 | 10 | 10 | 4.58 | |||
| −10 | 8 | 2 | 3.69 | ||||
| Pallidum | −16 | −6 | 6 | 3.87 | |||
| Right | Thalamus | 4 | −8 | 8 | 3.65 | ||
| 4 | −14 | 8 | 3.51 | ||||
| Left | Frontal pole | −10 | 64 | 26 | 4.65 | 225 | |
| −8 | 62 | 34 | 4 | ||||
| Right | 4 | 62 | 36 | 4.06 | |||
| 8 | 64 | 28 | 3.79 | ||||
| 8 | 58 | 40 | 3.67 | ||||
| Superior frontal gyrus | 0 | 54 | 28 | 3.53 | |||
| Left | Thalamus | −16 | −32 | 2 | 4.61 | 186 | |
| Hippocampus | −16 | −24 | −8 | 4.07 | |||
| −20 | −24 | −8 | 4.04 | ||||
| −22 | −28 | −6 | 4.01 | ||||
| Putamen | −28 | −22 | −6 | 3.71 | |||
| Right | Amygdala | 18 | −6 | −14 | 5.51 | 161 | |
| 30 | −6 | −14 | 3.66 | ||||
| Right | Thalamus | 18 | −32 | 0 | 4.79 | 147 | |
| 22 | −26 | −4 | 4.28 | ||||
| Left | Precentral gyrus | −46 | 4 | 32 | 4.39 | 127 | |
| Middle frontal gyrus | −56 | 10 | 40 | 3.39 | |||
ROI analyses revealed no between-group differences during emotional reactivity in activation of the amygdala (β = 1.66, p = .31), vlPFC (β = 2.65, p = .73), or vmPFC (β = 2.78, p = .49); Figure 3d). Extracted parameter estimates from bilateral amygdala, bilateral vlPFC, and vmPFC did not correlate with age, anxiety severity, or extent of emotional reactivity.
3.2.3 |. Functional activation during emotion regulation
In whole-brain emotion regulation analyses (decrease aversive >look aversive; Figure 5, Table 3), anxious youth demonstrated significant activation of LOC and left angular gyrus. No significant activation was observed for control youth, nor any significant between-group differences.
FIGURE 5.
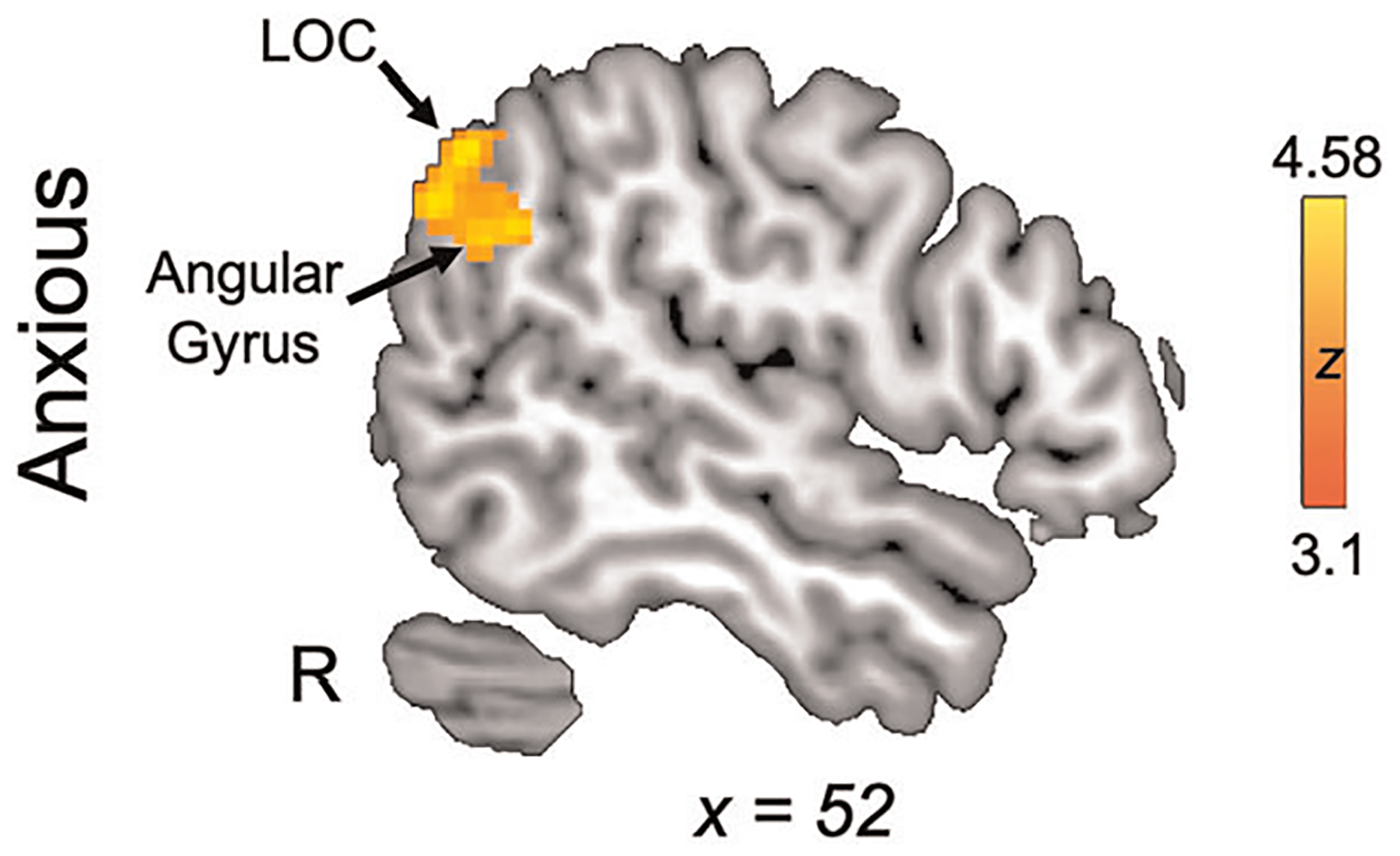
Neural activation during emotion regulation. Whole-brain activation for the emotion regulation contrast (decrease aversive >look aversive). LOC, lateral occipital cortex
ROI analyses revealed no between-group differences during emotion regulation in activation of the amygdala (β = .91, p = .49), vlPFC (β = −2.14, p = .73), or vmPFC (β = −1.16, p = .57); Figure 3e). Extracted parameter estimates from bilateral amygdala, bilateral vlPFC, and vmPFC did not correlate with age, anxiety severity, or extent of emotion regulation.
3.3 |. Functional connectivity results
Amygdala–vlPFC connectivity did not differ between groups during look aversive (β = −0.01, p = .91), decrease aversive (β = .12, p = .65), or look neutral trials (β = −0.04, p = .91). Similarly, amygdala–vmPFC connectivity did not differ between groups during look aversive (β = −0.02, p = .79), decrease aversive (β = .09, p = .79), or look neutral trials (β = .03, p = .79). Connectivity did not relate to age in either group in any condition.
Amygdala–vmPFC connectivity during decrease aversive trials showed a significant group by anxiety severity interaction (β = .43, p = .05; Figure 6). Whereas greater anxiety severity was associated with greater amygdala–vmPFC connectivity in control youth, greater anxiety was associated with less amygdala–vmPFC connectivity in anxious youth. Amygdala–vlPFC connectivity did not relate to anxiety severity.
FIGURE 6.
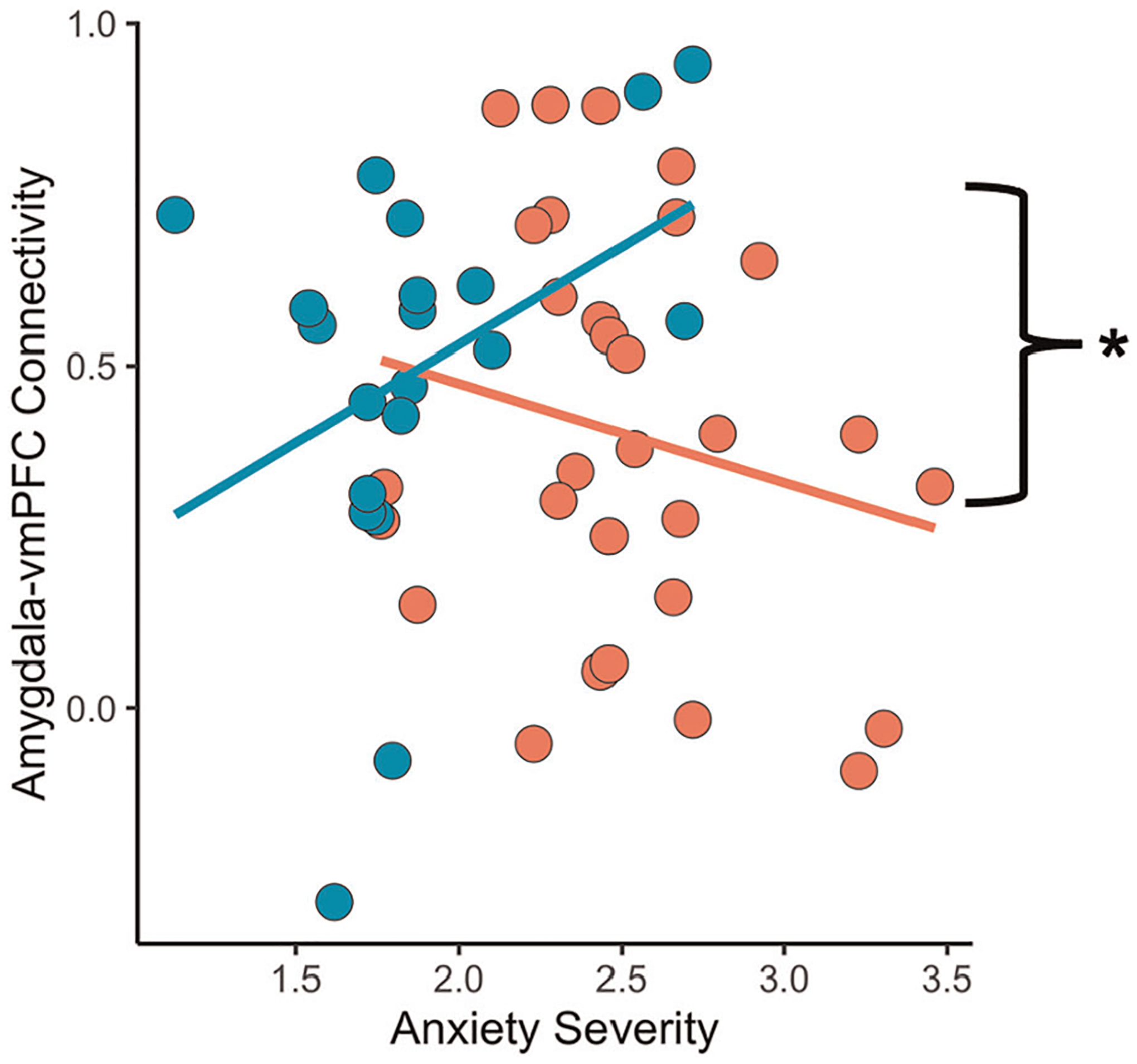
Beta-series amygdala-frontal connectivity. Amygdala–vmPFC functional connectivity as a function of anxiety severity. vmPFC, ventromedial prefrontal cortex. *p < .05
4 |. DISCUSSION
To our knowledge, this is the first study to examine neurobiological differences during emotional reactivity and regulation among youth with and without clinical anxiety, with an eye toward understanding processes engaged during a key component of current CBT treatments: cognitive reappraisal. We found similar emotion regulation in both groups which did not correlate with anxiety severity. There was no observed developmental relationship with emotion regulation capacity in either group. Targeted ROI analyses revealed heightened amygdala and vlPFC activation in anxious relative to control youth during all conditions. Amygdala connectivity with vmPFC during emotion regulation differentially related to anxiety severity in youth with and without anxiety.
Contrary to expectations, anxious youth were not significantly more emotionally reactive than control youth. Anxious youth did however self-report more distress following look aversive trials which index youth’s dispositional reactions to negatively valanced images. Higher reactivity suggests that anxious youth may generally experience greater negative emotions in response to aversive imagery. Anxious youth also displayed significantly greater amygdala and vlPFC activation during look aversive trials, consistent with work in anxious adults suggesting an overall heightened behavioral and neurobiological response to negative images (Fitzgerald et al., 2017). Altogether, findings suggest that the look aversive condition was more distressing for anxious compared to control youth. Coupled with prior findings that anxious youth rely on maladaptive coping strategies (Schäfer et al., 2017), negative situations may present a source of heightened and difficult-to-control emotions.
In line with our hypothesis, instructing anxious youth to regulate resulted in regulation capacity at the level of their non-anxious peers. Examining decrease aversive trials specifically revealed similar ratings across all youth, further demonstrating efficacious reappraisal following instruction. Despite similar behavioral performance, anxious youth displayed greater amygdala and vlPFC activation during decrease aversive trials. Heightened amygdala activation during reappraisal could reflect attentional bias toward threatening (Bar-Haim et al., 2007) and emotionally salient (McRae et al., 2012) stimuli, which persists in parallel with reduced negative affect. The general tendency of anxious youth to not use reappraisal could result in heightened amygdala responsivity that is difficult to downregulate. Indeed, anxious youth also exhibited greater vlPFC activation which could reflect greater effort (Etkin et al., 2015) required to employ effective reappraisal, especially if they are less experienced with this emotion regulation strategy (Silvers et al., 2016). The lack of subjective perception of negative affect despite heightened neurobiological response suggests that therapies should specifically address strategies for incentivizing adolescents to employ reappraisal when faced with heightened emotions, in addition to skill development. Emotion regulation tendency and capacity may codevelop in adolescence (Silvers & Moreira, 2017), further suggesting the need to encourage reappraisal as this may boost its efficacy. Examining both the tendency to use and efficacy of reappraisal in anxious youth can elucidate whether youth who reappraise more often also exhibit less amygdala activation during reappraisal.
Neither emotional reactivity nor emotion regulation related to age in control youth in contrast with prior work demonstrating linear increases in emotion regulation across age (McRae et al., 2012; Silvers et al., 2012). Stable emotional reactivity combined with lack of emotion regulation development may contribute to vulnerability for developing affective disorders during the period in which regulatory capabilities may not yet be developed enough to manage reactivity (Meyer & Lee, 2019). In contrast, emotional reactivity decreased over age in anxious youth, but emotion regulation did not relate to age. This lack of development may contribute to the continuation or exacerbation of anxious symptomatology. Continued dysregulation across development may serve as a risk factor for comorbid psychopathology (Sloan et al., 2017); emotion regulation deficits predict risk for both future anxiety and depressive disorder diagnoses (Schäfer et al., 2017). Reduced emotion regulation development, coupled with infrequent use of adaptive emotion regulation strategies, may contribute to deficits in emotion regulation use in adulthood (Hofmann et al., 2012). Longitudinal studies are necessary for investigating developmental trajectories of emotion regulation in anxious youth as they transition into adulthood.
We did not observe developmental relationships involving amygdala activation or connectivity unlike prior work (Gee et al., 2013; Silvers et al., 2016). Stronger amygdala–vmPFC connectivity related to significantly fewer anxiety symptoms in anxious youth, but more anxiety symptoms in control youth, in line with work in adults (Young et al., 2017). While beta series connectivity analyses cannot address directionality within circuits, anxious youth may have recruited prefrontal regions to downregulate the heightened emotionality and amygdala activation observed during decrease aversive trials relative to control youth. Perhaps positive coupling between the amygdala and regulatory regions is necessary for attenuating heightened amygdala responsivity but is not necessary in the absence.
These findings should be interpreted with limitations in mind. The modest sample size may have limited our power to detect significant differences. Future studies should aim for larger sample sizes, which may also allow for investigating sex differences. As such, this study should be interpreted as pilot work in need of replication in larger studies of pediatric anxiety. Further, as emotion regulation develops with age, longitudinal studies are crucial for identifying differential trajectories of development across psychiatric conditions. Capitalizing on dimensional anxiety rather than dichotomizing participants may also help isolate the relationship between anxiety symptoms and emotional development.
Despite these limitations, this study provides novel context for emotion dysregulation in anxiety disorders. Anxious youth exhibit intact regulatory abilities relative to non-anxious peers, though still demonstrate greater fronto-limbic activation following aversive imagery. Regulation may require additional effort in anxious youth, as potentially indexed both by greater recruitment of lateral prefrontal cortex and greater amygdala-frontal connectivity. Therapeutics may confer greater clinical benefit by addressing strategies for attenuating heightened emotional response following negative experiences in addition to encouraging reappraisal use in daily life to support healthy emotional development into adulthood.
Supplementary Material
ACKNOWLEDGMENTS
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (1650604 to NTP), the National Institute of Child Health and Human Development (1T32HD091059 to NTP), and the Friends of Semel (to TSP). We are grateful for the support from the Staglin Institute for Neuroscience and Human Behavior Center for Cognitive Neuroscience. We would like to thank Kathy T Do, Jocelyn Perez, Leoneh Ohanian, Kayla Oakley, Anna Sedykin, and Katherine Soto for assistance in data collection and study coordination. Thank you to the participants and their families for sharing their time.
Funding information
National Institute of Child Health and Human Development, Grant/Award Number: 1T32HD091059; Friends of Semel; National Science Foundation, Grant/Award Number: 1650604
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA AVAILABILITY STATEMENT
Data can be made available upon request.
REFERENCES
- Ahmed SP, Bittencourt-Hewitt A, & Sebastian CL (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. 10.1016/J.DCN.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano AM, & Silverman WK (1996). The anxiety disorders interview schedule for DSM-IV: Clinician manual (child and parent versions). Psychological Corporation. [Google Scholar]
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Luan Phan K (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Beesdo-Baum K, & Knappe S (2012). Developmental epidemiology of anxiety disorders. Child and Adolescent Psychiatric Clinics of North America, 21(3), 457–478. 10.1016/j.chc.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, & Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Edge MD, & Gross JJ (2010). Emotional reactivity and cognitive regulation in anxious children. Behaviour Research and Therapy, 48, 384–393. 10.1016/j.brat.2009.12.013 [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, & Gross JJ (2010). Patterns of emotional reactivity and regulation in children with anxiety disorders. Journal of Psychopathology and Behavioral Assessment, 32, 23–36. 10.1007/s10862-009-9167-8 [DOI] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, & Cohen AO (2019). Development of the emotional brain. Neuroscience letters, 693, 29–34. 10.1016/j.neulet.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, & Gruber O (2011). Fear is only as deep as the mind allows. A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage, 58(1), 275–285. 10.1016/j.neuroimage.2011.05.073 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Koller JM, Earl EA, Miranda-Dominguez O, Klein RL, Van AN, & Fair DA (2017). Real-time motion analytics during brain MRI improve data quality and reduce costs. NeuroImage, 161, 80–93. 10.1016/j.neuroimage.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, & Klumpp H (2017). Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. Journal of Affective Disorders, 218, 398–406. 10.1016/j.jad.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, & Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Becker-Haimes EM, Keeton C, Kendall PC, Iyengar S, Sakolsky D, & Piacentini J (2018). Results from the child/adolescent anxiety multimodal extended long-term study (CAMELS): Primary anxiety outcomes. Journal of the American Academy of Child and Adolescent Psychiatry, 57(7), 471–480. 10.1016/j.jaac.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Hantsoo L, & Epperson CN (2017). Anxiety disorders among women: A female lifespan approach. Focus, 15(2), 162–172. 10.1176/appi.focus.20160042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. 10.1016/J.BIOPSYCH.2008.03.015015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research review: Neural response to threat in children, adolescents, and adults after child maltreatment—A quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58(3), 222–230. 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- Higa-McMillan CK, Francis SE, Rith-Najarian L, & Chorpita BF (2016). Evidence base update: 50 Years of research on treatment for child and adolescent anxiety. Journal of Clinical Child & Adolescent Psychology, 45(2), 91–113. 10.1080/15374416.2015.1046177 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, & Asnaani A (2012). Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety, 29(5), 409–416. 10.1002/da.21888 [DOI] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U (2014). Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/J.NEUROIMAGE.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerstee JS, Garnefski N, Jellesma FC, Verhulst FC, & Utens EMWJ (2010). Cognitive coping and childhood anxiety disorders. European Child and Adolescent Psychiatry, 19(2), 143–150. 10.1007/s00787-009-0051-6 [DOI] [PubMed] [Google Scholar]
- Lewis AR, Zinbarg RE, & Durbin CE (2010). Advances, problems, and challenges in the study of emotion regulation: A commentary. Journal of Psychopathology and Behavioral Assessment, 32(1), 83–91. 10.1007/s10862-009-9170-0 [DOI] [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, & Conners CK (1997). The multidimensional anxiety scale for children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry, 36(4), 554–565. 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, & Ochsner KN (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social cognitive and affective neuroscience, 7, 11–22. 10.1093/scan/nsr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, & Gross JJ (2012). Bottom-up and top-down emotion generation: implications for emotion regulation. Social Cognitive and Affective Neuroscience, 7(3), 253–262. 10.1093/scan/nsq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, & Swendsen J (2010). Lifetime prevalence of mental disorders in U. S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. 10.1016/J.JAAC.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, & Lee FS (2019). Translating developmental neuroscience to understand risk for psychiatric disorders. American Journal of Psychiatry, 176(3), 179–185. 10.1176/appi.ajp.2019.19010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, & Poldrack RA (2012). Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage, 59(3), 2636–2643. 10.1016/J.NEUROIMAGE.2011.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris TS, Compton SN, Kendall PC, Birmaher B, Sherrill J, March J, & Piacentini J (2015). Trajectories of change in youth anxiety during cognitive-behavior therapy. Journal of Consulting and Clinical Psychology, 83(2), 239–252. 10.1037/a0038402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, & D’Esposito M (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage, 23(2), 752–763. 10.1016/J.NEUROIMAGE.2004.06.035 [DOI] [PubMed] [Google Scholar]
- Schäfer JÖ, Naumann E.va, Emily Holmes A, Tuschen-Caffier B, & Samson AC (2017). Emotion Regulation Strategies in Depressive and Anxiety Symptoms in Youth: A Meta-Analytic Review. Journal of Youth and Adolescence, 46, 261–276. 10.1007/s10964-016-0585-0 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, & Ochsner KN (2016). vlPFC–vmPFC–Amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7). 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, & Ochsner KN (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion (Washington, D.C.), 12(6), 1235–1247. 10.1037/a0028297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, & Moreira JFG (2017). Capacity and tendency: A neuroscientific framework for the study of emotion regulation. Neuroscience Letters, 693, 35–39. 10.1016/j.neulet.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, & Ochsner KN (2015). Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental science, 18(5), 771–784. 10.1111/desc.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E, Hall K, Moulding R, Bryce S, Mildred H, & Staiger PK (2017). Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clinical Psychology Review, 57, 141–163. 10.1016/j.cpr.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Suveg C, Sood E, Comer JS, & Kendall PC (2009). Changes in emotion regulation following cognitive-behavioral therapy for anxious youth. Journal of Clinical Child & Adolescent Psychology, 38(3), 390–401. 10.1080/15374410902851721 [DOI] [PubMed] [Google Scholar]
- Suveg C, & Zeman J (2004). Emotion regulation in children with anxiety disorders. Journal of Clinical Child and Adolescent Psychology, 33(4), 750–759. 10.1207/s15374424jccp3304_10 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence (WASI). Psychological Corporation. [Google Scholar]
- Young KS, Burklund LJ, Torre JB, Saxbe D, Lieberman MD, & Craske MG (2017). Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Research: Neuroimaging, 261, 44–51. 10.1016/j.pscychresns.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, Sandman CF, & Craske MG (2019). Positive and negative emotion regulation in adolescence: Links to anxiety and depression. Brain Sciences. 10.3390/brainsci9040076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available upon request.


