Abstract
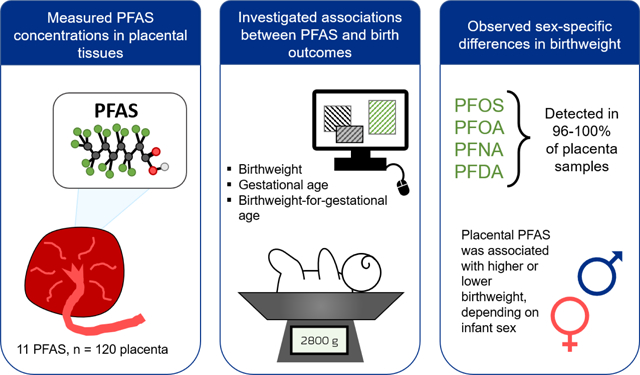
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous environmental contaminants commonly detected in human serum. Previous studies have observed associations between maternal serum PFAS and adverse pregnancy and birth outcomes such as lower birth weight or pre-eclampsia; however, few studies have explored these associations with birth outcomes and placental tissue PFAS concentration. The placenta is a vital contributor to a healthy pregnancy and may be involved in the mechanism of PFAS reproductive toxicity. Our goal was to measure placental PFAS concentrations and examine associations with birth outcomes (e.g., birth weight, gestational duration). Placenta samples (n=120) were collected during delivery from women enrolled in the Healthy Pregnancy, Healthy Baby cohort (HPHB) in Durham, North Carolina. All placenta samples contained detectable PFAS, with perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) being the most abundant and most frequently detected (all >96% detection frequency). While placental PFAS concentrations did not differ by infant sex, higher PFAS levels were observed in placenta from nulliparous women, suggesting that parity influences the accumulation of PFAS in the placenta. We used linear regression models to examine associations between placental PFAS and birth outcomes. After adjustment for parity, tobacco use, maternal age, and maternal race, we found that placental PFOS was associated with lower birth weight for gestational age in male infants and higher birth weight for gestational age in female infants. Similar findings were seen for PFNA for birth weight for gestational age. These differences in birth outcomes based on infant sex highlight a need to explore mechanistic differences in PFAS toxicity during gestation for male and female infants.
Keywords: placenta, PFAS, perfluoroalkyl, polyfluoroalkyl, birth weight, birth outcomes
Graphical Abstract
1. Introduction
PFAS, or per- and polyfluoroalkyl substances, are a large group of chemical contaminants that can be found ubiquitously in the environment and in human bodies. Their occurrence is so frequent in part because PFAS are widely used for their water- and grease-repellant properties in a range of products such as non-stick cookware, textiles, carpeting, food packaging, fire-fighting foam, and consumer products (Wang et al., 2017). In addition to being used in many products, many PFAS chemicals do not break down easily due to the strength of their multiple carbon-fluorine bonds (Lau et al., 2007).
PFAS are frequently detected in human serum and urine, and over 95% of the U.S. population has PFAS in their blood according to data collected by the National Health and Nutrition Examination Survey (NHANES) within the Centers for Disease Control and Prevention (CDC) (Calafat et al., 2007a; Calafat et al., 2007b). PFAS can be persistent in the human body due to the long elimination half-lives of some PFAS, particularly longer-chain PFAS (Olsen et al., 2007; Bartell et al., 2010; Zhang et al., 2013a; Li et al., 2018). PFAS have been found in human serum (Calafat et al., 2006; Calafat et al., 2019), breast milk (Macheka-Tendenguwo et al., 2018; Jin et al., 2020), and umbilical cord blood (Chen et al., 2017; Mamsen et al., 2017; Wang et al., 2020). These findings are particularly concerning as they suggest the potential for placental and lactational transfer of PFAS to developing fetuses and infants; some PFAS are known to have developmental and reproductive toxicity (Washino et al., 2009; Bach et al., 2015; Chang et al., 2018; Blake and Fenton, 2020).
These contaminants are multi-system toxicants with effects on the liver, kidney, immune system, thyroid, cholesterol, and infant birthweight (ATSDR, 2021). Epidemiological studies on PFAS have observed significant associations with adverse health outcomes, including cancer, immune impairment, and thyroid disease, among others (Lau et al., 2007; Grandjean et al., 2012; Barry et al., 2013; NTP, 2016; Rappazzo et al., 2017; Pelch et al., 2019; Sunderland et al., 2019; Fenton et al., 2021). These observations in humans have been further reinforced by data from animal studies (Lau et al., 2007; Lilienthal et al., 2017; ATSDR, 2021; Fenton et al., 2021).
While the mechanisms of PFAS toxicity are still being elucidated, the placenta has come forward as a potential target organ of PFAS toxicity (Blake and Fenton, 2020). Placental effects have been observed after exposure to PFOA and GenX in mice (Blake et al., 2020), and transplacental transfer of PFAS has been found experimentally in mice (Fenton et al., 2009) and observationally in humans (Yang et al., 2016a; Yang et al., 2016b; Chen et al., 2017; Mamsen et al., 2017; Mamsen et al., 2019). The placenta is a vital contributor to a healthy pregnancy as it mediates the exchange of nutrients, hormones, and other factors between mother and fetus. PFAS effects on the placenta may play a role in the mechanism of PFAS reproductive toxicity (Gao et al., 2019; Bangma et al., 2020a; Blake et al., 2020; Szilagyi et al., 2020a; Szilagyi et al., 2020b; Lu et al., 2021). For example, the placenta is a very important source of reproductive hormones, and prenatal exposure to short-chain PFAS such as PFBA and PFHpA has been associated with perturbations in fetal reproductive hormones (Nian et al., 2020). A few prior studies have reported PFAS concentrations in placenta (Zhang et al., 2013b; Martin et al., 2016; Chen et al., 2017; Mamsen et al., 2017; Mamsen et al., 2019; Bangma et al., 2020b; Lu et al., 2021; Vela-Soria et al., 2021). However, the potential health implications of PFAS accumulation in the placenta remain largely unknown. To our knowledge, only one study (Bangma et al., 2020b) has examined associations between placental PFAS with health or birth outcomes in a high-risk cohort. In this study, researchers investigated whether PFAS placental levels were associated with gestational age at delivery, fetal growth, or hypertensive disorders of pregnancy; they did not find evidence for any associations.
In addition to being a potential target of toxicity, the placenta may also be a better measure of fetal PFAS exposure than maternal measures. While maternal serum PFAS is frequently used in studies focusing on birth outcomes, maternal serum PFAS concentrations during pregnancy can be impacted by other changes that occur during pregnancy. For example as pregnancy progresses, increases in blood volume, plasma volume, and glomerular filtration rate (GFR) can occur (Peck and Arias, 1979; Odutayo and Hladunewich, 2012). These changes may impact serum PFAS concentrations. The extent of change in blood volume can be highly variable, and normal pregnancies can show increases in blood volume of 20–100% of nonpregnant blood volume (Pritchard, 1965). It is unclear how these changes in blood volume and GFR affect the partitioning of contaminants between maternal serum and placental tissues.
The goals of the present study were to evaluate the concentration of several PFAS in placenta collected from women living in central North Carolina in 2010–2011 and to further explore the associations between placental PFAS and birth outcomes.
2. Materials and Methods
2.1. Study population and sample collection
Placental tissues were obtained from participants in the Healthy Pregnancy, Healthy Baby (HPHB) Study. This prospective cohort study aimed to explore racial disparities in pregnancy outcomes due to social, environmental, and maternal factors (Miranda et al., 2010; Miranda et al., 2011; Stapleton et al., 2011; Swamy et al., 2011; Maxson et al., 2012; Buttke et al., 2013; Johnston et al., 2014; Sanders et al., 2014; Edwards et al., 2015; Gona et al., 2015; Miranda et al., 2015; Leonetti et al., 2016a; Maxson et al., 2016; Grotegut et al., 2017). This study was carried out in accordance with a human subjects research protocol approved by the Duke University Medical Center Institutional Review Board (IRB), and all women provided informed consent prior to participation. In brief, pregnant women were enrolled from the Duke University Medical Center (DUMC) Obstetrics Clinic and the Durham County Health Department Prenatal Clinic. Women were excluded from participation if they were younger than 18 years old, were not English-literate, were less than 18 or greater than 28 weeks gestation at time of enrollment, lived outside of Durham County, North Carolina, had a multi-fetal gestation, had a known fetal genetic or congenital abnormality, or were not planning to deliver at DUMC (Miranda et al., 2010). This population has previously been studied while investigating associations between brominated flame retardants and thyroid hormone levels in the placenta (Leonetti et al., 2016a; Leonetti et al., 2016b).
Our analyses include a subset of women from the HPHB study who delivered between March 2010 and December 2011 and from whom sufficient placental tissue was available (n = 120). Placenta tissue subsamples (approximately 5–20 g) were taken at time of delivery at the Duke University Medical Center, and tissues were stored in Nalgene™ polypropylene screw-top cryo-vials at −80 °C until analysis.
2.2. Sample extraction and purification
Extraction of PFAS from placenta was adapted from several methods previously described in the literature (Taniyasu et al., 2005; Liu et al., 2015; Martin et al., 2016). Whole-thickness placenta tissue aliquots (~1.0 g wet weight) were thawed, lightly rinsed with ultra-pure water to remove blood, and minced with dissecting scissors in 50-mL polypropylene centrifuge tubes. Tissue was spiked with 13C-mass-labeled internal standards (Wellington Laboratories, Guelph, Ontario, Canada), acidified with formic acid, and extracted with acetonitrile. Extracts (30 mL acetonitrile) were concentrated under nitrogen in an N-EVAP on low heat (Organomation) to ~1.0 mL. Extracts were then purified using solid-phase extraction with weak-anion-exchange cartridges (Waters Oasis WAX) and eluted in 10 mL of methanol with 0.1% ammonium hydroxide in 15-mL polypropylene centrifuge tubes. Extracts were concentrated under nitrogen again to ~300 μL, spiked with 60 μL of 1 mM sodium hydroxide, and stored at −20 °C. Prior to LC-MS/MS analysis, 500 μL of 2 mM ammonium acetate in water was added to each extract, and samples were transferred to 1-mL polypropylene push-top LC vials (Agilent).
2.3. HPLC-MS/MS analysis
Eleven PFAS (Table S1) were quantified in each extract using an Agilent 1260 Infinity II high-performance liquid chromatograph (HPLC) instrument coupled to an Agilent 6460A triple quadrupole mass spectrometer. The mass spectrometer was operated in negative electrospray ionization mode (HPLC-ESI-MS/MS). Separation of analytes by LC was performed using a 4.6 mm (I.D.) × 50 mm Agilent ZORBAX Eclipse XDB-C18 reversed-phase HPLC column (1.8 μm particle size) preceded by a 4.6 mm × 5 mm XDB-C18 guard cartridge.
Mobile phases were 2 mM ammonium acetate in water (mobile phase A) and 2 mM ammonium acetate in methanol (mobile phase B) using a flow rate of 0.4 mL/min. Gradient conditions for chromatographic separation were as follows: initial condition (30% B) was increased to 60% B over 1.5 minutes; then increased to 95% B over 2 minutes and held for 5.5 minutes. The gradient was then increased to 100% B over 3 minutes, before finally returning to initial conditions (30% B) over 0.5 minutes, and holding for 5.5 minutes. The column temperature was set at 45°C and the injection volume was 20 μL. Data were acquired under multiple reaction monitoring (MRM) transitions using optimized parameters. Additional methods information, including transitions, is included in Tables S2, S3, and S4.
2.4. Quality control and quality assurance (QA/QC)
Laboratory processing blanks were included in each batch and extracted alongside placenta tissue samples. A Lake Michigan fish tissue Standard Reference Material (SRM 1947) (NIST, 2017) of approximately 2.0 g was analyzed for PFAS alongside samples to examine accuracy. In total we included 7 lab processing blanks, 7 SRM extracts, and 12 duplicate samples (to assess precision) for our QA/QC assessment. Isotopically-labeled internal standards (13C-mass-labeled, listed in Table S2) were used in all samples. Recovery of 13C-labeled PFAS was calculated to assess the recovery efficiency of the extraction and clean-up methods; recovery averaged 133% for M3-GenX and ranged between 56–82% for all other mass-labeled standards as listed in Table S5. Our SRM values were extremely similar to the reference values for SRM 1947 as reported in Table S6.
2.5. Statistical analysis
Method detection limits (MDLs) for each analyte were calculated as three times the standard deviation of the blank values. Concentrations of each PFAS in samples were blank-corrected using the average laboratory blank levels. Only PFAS that were detected in more than 50% of placenta samples (PFOS, PFOA, PFDA, PFNA) were included in subsequent statistical analyses. Values less than the MDL were imputed with MDL divided by 2. Statistical analyses were performed using JMP Pro (version 14.0.0) and GraphPad Prism (version 9.0.2). The following data were abstracted from medical records: gestational age at delivery, birth weight, infant sex, maternal age, parity, maternal tobacco use during pregnancy, and maternal race. Birth weight was normalized for gestational age using the INTERGROWTH-21st standards and is reported as a percentile (Stirnemann et al., 2017). Preliminary analyses using the Shapiro-Wilk test indicated the PFAS concentration data were not normally distributed, so non-parametric tests were used for analyses. Spearman rank correlation coefficients were calculated to evaluate the associations between individual placental PFAS concentrations. To evaluate whether placental PFAS differed by demographic characteristics, the two-tailed Mann-Whitney test was performed.
Linear regression models were conducted to determine if placental PFAS and other factors were associated with continuous measures of birth outcomes, including birth weight, gestational age, and birth weight for gestational age. For these models, placental PFAS concentrations were categorized into tertiles. Each tertile included n=40 pregnancies. Pregnancies were grouped into tertile solely based on placental PFAS concentration, without regard for infant sex; previous analyses in Figure S1 showed no difference in placental PFAS by infant sex and the distribution of placental PFAS concentration data by infant sex was fairly even. Tertiles were approximately 55% female and 45% male, mirroring the sex distribution in the total study population. Gestational age in days, birth weight in grams, and birth weight for gestational age in percentile were treated as continuous variables for regression analyses.
The potential for demographic and behavioral factors to confound associations between PFAS and birth outcomes was also considered. We identified possible confounding variables based on a literature review and selected variables for inclusion in adjusted analysis if they were associated with both placental PFAS and birth outcomes.
The final adjustment set included maternal race (non-Hispanic black vs. other); parity: (nulliparous (first birth) vs. multiparous (second or later birth)); maternal smoking status (any tobacco use during pregnancy vs. no use during pregnancy) and maternal age at gestation (dichotomized at the median of 27 years for analysis). Maternal education was considered for inclusion in fully adjusted models, but it was not associated with any of the outcomes in this population and did not appreciably change models when included.
Previous work suggests sex differences in associations with PFAS and birth outcomes. Thus, analyses were stratified by infant sex to explore potential sex differences in birth outcomes. The threshold for statistical significance was p<0.05 for all analyses. Main analyses were performed on our full study population (n=120), including preterm births. However, to evaluate whether results were driven by the inclusion of premature babies, analyses were repeated excluding babies born before 37 complete weeks gestation (n=12); results were very similar in direction and magnitude (Supplemental Excel File); our conclusions and interpretations are unchanged by the inclusion or exclusion of preterm births.
3. Results and Discussion
3.1. Demographic information
Placenta samples from 120 participants were included in this study. Table 1 summarizes the demographic characteristics of the women and children in this study cohort.
Table 1:
Demographic characteristics of cohort (n = 120)
| Characteristic | N | % |
|---|---|---|
|
| ||
| Total | 120 | 100.0 |
| Maternal age at gestation | ||
| 18–19 | 11 | 9.2 |
| 20–26 | 44 | 36.7 |
| 27–34 | 46 | 38.3 |
| 35–39 | 14 | 11.7 |
| 40–46 | 5 | 4.2 |
| Maternal race | ||
| Non-Hispanic black | 72 | 60.0 |
| Non-Hispanic white | 26 | 21.7 |
| Hispanic | 12 | 10.0 |
| Other | 10 | 8.3 |
| Male infant | 54 | 45.0 |
| Gestational age <37 weeks | 12 | 10.0 |
| Tobacco use during pregnancy | 26 | 21.7 |
| Parity | ||
| Nulliparous | 38 | 31.7 |
| Multiparous | 82 | 68.3 |
Of the 120 participants, 60% were non-Hispanic black, 22% were non-Hispanic white, 10% were Hispanic, and 8% were either non-Hispanic Asian, other, or multi-racial. Approximately 54% of the women were 27 years of age or older, and approximately 78% of women reported no tobacco use during pregnancy.
3.2. PFAS in placenta
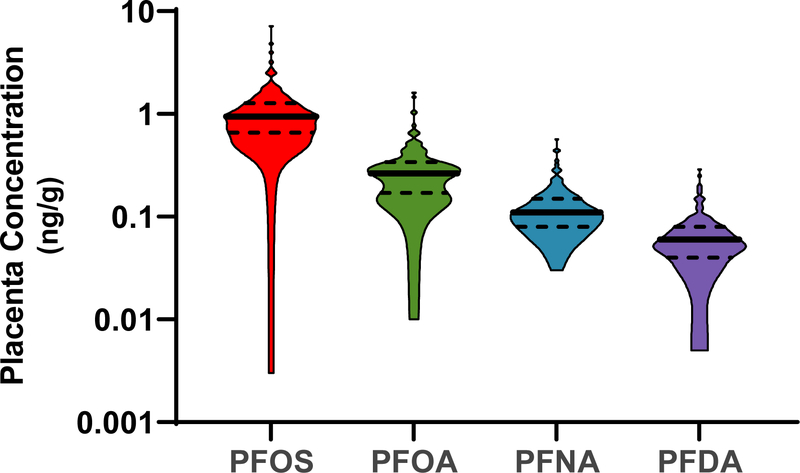
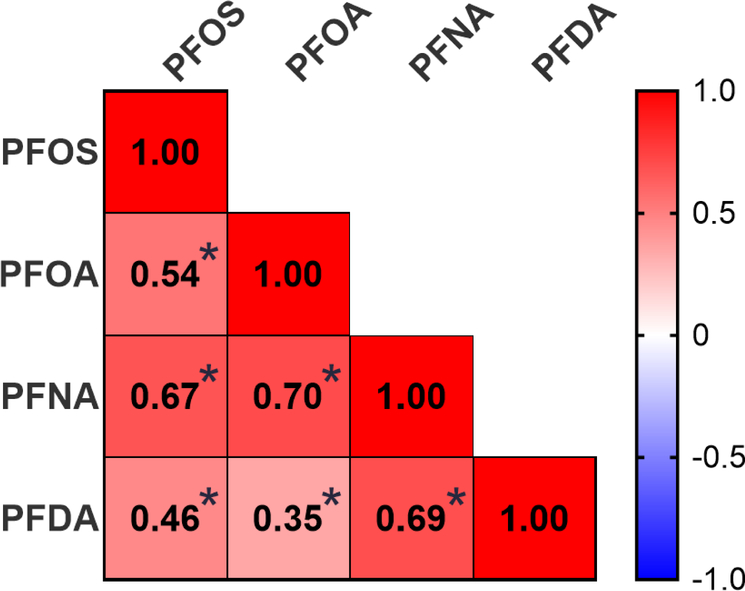
At least one PFAS analyte was detected in all placental samples (n=120) as reported in Table 2. The most frequently detected PFAS were PFOS, PFOA, PFNA, and PFDA, which were detected in more than 95% of placental samples. As displayed in Figure 1, PFOS and PFOA were the most abundant analytes with median concentrations of 0.95 and 0.27 ng/g, respectively. The concentrations of individual PFAS in placenta were correlated with the concentrations of other frequently-detected PFAS, with Spearman correlations ranging from 0.35 to 0.70, p<0.05 for all (Figure 2).
Table 2:
Detection frequency, method detection limit (MDL), minimum, median, 95th percentile, and maximum concentrations of PFAS in ng/g wet weight in placental tissues (n = 120).
| Analyte | Detection Frequency | MDL (ng/g) | Minimum (ng/g) | Median (ng/g) | 95th percentile (ng/g) | Maximum (ng/g) |
|---|---|---|---|---|---|---|
|
| ||||||
| PFOS | 99% | 0.01 | <MDL | 0.95 | 2.5 | 7.2 |
| PFOA | 98% | 0.02 | <MDL | 0.27 | 0.7 | 1.6 |
| PFNA | 100% | 0.01 | 0.03 | 0.11 | 0.3 | 0.6 |
| PFDA | 96% | 0.01 | <MDL | 0.06 | 0.2 | 0.3 |
|
| ||||||
| PFBA | 12% | 2.36 | <MDL | <MDL | 19.2 | 29.4 |
| PFPeA | 0% | 1.06 | <MDL | <MDL | <MDL | <MDL |
| PFHxA | 7% | 0.03 | <MDL | <MDL | 0.1 | 0.1 |
| PFHpA | 0% | 1.50 | <MDL | <MDL | <MDL | <MDL |
| PFBS | 4% | 8.10 | <MDL | <MDL | <MDL | 10.3 |
| PFHxS | 19% | 0.17 | <MDL | <MDL | 0.5 | 0.5 |
| GenX | 0% | 0.06 | <MDL | <MDL | <MDL | <MDL |
Figure 1:
PFAS concentrations (ng/g) in placenta (n = 120) for analytes detected in more than 95% of samples. Violin plots show the distribution of concentration data with solid lines and dotted lines demarcating median and quartiles, respectively. Concentrations are plotted on a logarithmic scale.
Figure 2:
Spearman correlation coefficients for analytes detected in more than 95% of placental samples (n = 120). Color indicates strength and directionality of correlation. *indicates p<0.05
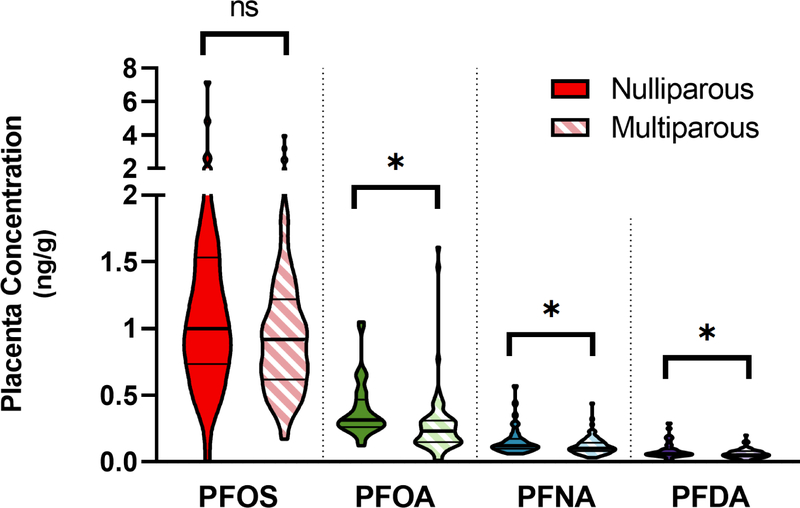
Placental PFAS concentrations for PFOS, PFOA, PFNA, and PFDA were analyzed to determine if concentrations differed based on infant sex, but no statistically significant differences were found. Figure S1 displays the concentrations of these four PFAS stratified by infant sex. However, placental PFOA, PFNA, and PFDA were significantly higher in placenta from nulliparous women compared to multiparous women; nulliparous women had 20–40% higher median concentrations (Figure 3). This is not a surprising result given the potential for transplacental and lactational transfer of these chemicals to reduce the PFAS body burden in the mother. Previous studies have found that PFAS concentration in the placenta may decrease with parity as lactational and placental transfer offloads the body burden of PFAS from the mother to the infant (Fei et al., 2007; Kim et al., 2011; Lee et al., 2013). For example, higher PFOA concentrations have been reported in nulliparous women compared to multiparous women (Lee et al., 2013), and higher parity was associated with lower serum levels for PFOA and seven other PFAS (Shu et al., 2018).
Figure 3:
Parity and placental PFAS concentrations. Placenta samples from nulliparous (n = 38) and multiparous (n = 82) women were compared. PFOA, PFNA, and PFDA placental concentrations were significantly higher in nulliparous women. Violin plots show the distribution of concentration data with thick solid lines and thin solid lines demarcating median and quartiles, respectively. *indicates p<0.05, and “ns” indicates not significant.
We also examined differences in placental PFAS concentrations by maternal race. Previous studies have observed differences in PFAS serum concentrations based on race, with non-Hispanic whites having higher serum concentrations of PFOS, PFOA, and PFHxS than non-Hispanic blacks and Mexican Americans (Calafat et al., 2006). In our study, we found no significant differences in placental PFAS by maternal race (Figure S2). However, it is important to note that the racial and ethnic breakdown of our cohort necessitated combining non-Hispanic white, Hispanic, and other races or ethnicities into a single category for comparison with non-Hispanic black women. It is likely this limited our ability to detect differences between groups.
We also analyzed a small group of anonymous placenta samples (n=10) collected more recently in 2018 for PFAS. Methods for the collection, storage, and processing of these tissues from 2018 are included in Supporting Information. Previous research in our lab has observed higher concentrations of brominated flame retardants in the fetal side of the placenta compared to the maternal side (Ruis et al., 2019). To investigate whether PFAS would also partition preferentially to one side of the placenta, we sectioned these ten placenta samples into the maternal and fetal sides, using the same method reported in Ruis et al. (2019), and analyzed them for PFAS. No significant differences were observed between the maternal and fetal placenta concentrations of PFAS. These ten placenta samples were observed to have slightly lower PFAS concentrations than the 120 placenta samples collected in 2010–2011, though this comparison is limited due to the small sample size (Table S7).
3.3. Placental PFAS concentrations and birth outcomes
Placental PFAS concentrations were analyzed for associations with birth outcomes using linear regression models and adjusted for maternal race, maternal age at gestation, parity, and tobacco use. For infant males, the highest exposure to PFOS was associated with lower birth weight for gestational age; the highest exposure exhibited a 13% decrease (95% CI: −23, −1.6) in birth weight percentile in comparison to the reference (lowest exposure) tertile (Figure 4). To put that difference into context, for a baby born at 40 weeks gestation, a 13% decrease in percentile is equivalent to approximately a 130-gram decrease in birth weight. Conversely, for infant females, the highest exposure of PFOS was associated with higher birth weight for gestational age; the highest exposure group exhibited an 11% increase (95% CI: 2.8, 19) in birth weight percentile in comparison to the reference tertile. Placental PFNA had a complex association with birth weight for gestational age; while modeling results for female infants were null and showed no difference, male infants with the highest exposure had lower weight percentiles (14% percentile decrease; 95% CI: −24, −3.9) and male infants within the middle tertile of exposure had higher weight percentiles (11% percentile increase; 95% CI: 0.2, 21) in comparison to the reference tertile.
Figure 4:
Results for regression models of birth weight for gestational age stratified by male and female infants. Analyses were performed using tertiles of placenta PFAS exposure with the lowest tertile as the reference group and were adjusted for maternal tobacco use, race, age, and parity. Horizontal bars reflect the 95% confidence interval (CI), *indicates p<0.05.
For models of gestational age, a significant association was observed in male infants exposed to the highest placental PFDA (Figure S3). Placental PFDA in males was associated with a shorter gestational age; male babies with the highest levels of exposure were born approximately ten days earlier on average. All other models of gestational age were null, suggesting no association between placental PFAS and the timing of parturition. Analyses of birth weight in grams, not normalized to gestational age, are included in (Figure S4), and a significant association was observed only in female infants exposed to the highest placental PFOS.
A sensitivity analysis was performed to examine the influence of preterm births on the model outcomes. This restricted model excluded the samples from preterm births (gestational age less than 37 complete weeks, n=108). Analyses from the restricted model were very similar in direction and magnitude as results from the full model and conclusions were not altered; additional details are included in Supplemental Information.
Many studies have explored associations between certain PFAS in maternal blood and birth weight or gestational age. Low birth weight has been associated with maternal serum PFOA and PFOS in previous studies (Fei et al., 2007; Darrow et al., 2013; Johnson et al., 2014), particularly for male infants (Marks et al., 2019). Manzano-Salgado et al. (2017) reported weak associations between maternal plasma PFOA, PFHxS, and PFNA and reduced birth weight in a Spanish birth cohort with samples collected from 2003–2008. Of note, they also report that higher PFOS in first-trimester maternal plasma (n=1,202) was associated with low birth weight in boys, similar to the results reported here (Manzano-Salgado et al., 2017).
In a study comparing placental PFAS with maternal serum and fetal tissue PFAS, Mamsen et al. (2019) noted that the placenta:maternal serum ratio was higher in pregnancies with male fetuses compared to those with female fetuses. While we did not see differences in placental concentrations between male and female pregnancies, we also did not have maternal serum available to compare with this study or to determine the placenta:maternal serum ratio. Additionally, there are several reported sex differences in placental epigenetics that may also affect infant birth weight (Martin et al., 2017; Clark et al., 2021). This sexually-dimorphic epigenetic placental signature may explain the differential response to gestational PFAS exposure.
Impacts on birth weight and gestational age are not the only potential deleterious effects of PFAS exposure. Maternal PFAS exposure has been associated with adverse reproductive outcomes such as miscarriage (Wikström et al., 2021) and earlier menopause (Ding et al., 2020), though there is evidence of reverse causation with regards to PFAS and menopause (Dhingra et al., 2017). Maternal PFAS exposure could also lead to disruptions in inflammatory pathways that impact pregnancy outcomes and reproductive health (Liu et al., 2020).
3.4. Comparisons with other placenta studies
Results from this study were compared with placenta PFAS concentrations reported in previous studies and are summarized in Table S8 and Figure S5. Overall, our PFAS concentration data are very similar to previously reported data for placental concentrations. Mamsen et al. (2017) reports PFAS concentrations in placental and fetal tissues from fetuses terminated in the first trimester of pregnancy. A second study by this group, reported in Mamsen et al. (2019), expands on their research by including tissues from the second and third trimester of pregnancy from fetuses that died in utero. Placental PFAS concentrations in their study were similar to fetal organ concentrations, and the placenta:maternal serum ratio for PFOS, PFOA, and PFNA increased in later trimesters, suggesting placental bioaccumulation or hemodilution due to plasma volume increase (Mamsen et al., 2019). Given the similarity in PFAS concentrations between placenta and fetal organs seen in Mamsen et al. (2019), it is possible that our placenta data would be similar to the tissue concentration in the infants. However, Chen et al. (2017) measured PFAS in maternal serum, placenta, and cord serum from maternal-fetal pairs, and they found that PFAS concentrations in maternal serum and cord serum were higher than in placenta.
Zhang et al. (2013b) measured PFAS in maternal blood, placenta, cord blood, and amniotic fluid. They noted that maternal transfer efficiency (moving from maternal blood to cord blood) decreased with increasing PFCA chain length. Zhang et al. (2013b) found that shorter-carbon chain PFAS (i.e., shorter than PFDA) partitioned more in the cord blood and maternal blood than in the placenta, possibly due to greater water solubility. In our study, longer-chain PFAS were abundantly detected in placenta, but shorter-chain PFAS were detected much less frequently. However, this absence of shorter-chain PFAS may also be reflective of a lack of exposure to shorter-chain PFAS in our study. The placenta may also not be the best matrix for measuring certain PFAS. While the longer-chain PFAS were frequently detected in our placenta, shorter-chain PFAS were not detected or detected very rarely. As these placenta samples were collected in 2010–2011, this may be due to a lack of exposure to the shorter-chain PFAS. It may also be an indication that these other PFAS do not accumulate in the placenta as efficiently. We were unable to compare our placenta PFAS to matched maternal serum PFAS. Having both the placenta and maternal serum, or fetal or cord serum, would have been valuable in determining whether the absence of shorter-chain PFAS was due to a lack of exposure or due to a limitation of the placenta matrix.
Bangma et al. (2020b) measured PFAS in placenta from a geographically similar cohort as our study, with placental tissues collected more recently (2015–2018 compared to 2010–2011). While their study did not find any significant associations between placental PFAS concentrations and fetal growth, gestational age, or hypertensive disorders of pregnancy, this may be explained by the fact that their study cohort was comprised of women who were at increased risk for spontaneous preterm birth and included predominantly white women.
As seen in Table S8 and Figure S5, PFOS and PFOA are the most abundant PFAS measured in placental samples across the world. Over time, it appears that placental concentrations are slowly declining for PFOS, PFOA, PFNA, and PFDA. However, geographic location plays an important role in placenta PFAS concentration in addition to year of sample collection. Although both the present study and Zhang et al. (2013b) analyzed placenta samples collected in 2010–2011, our median and maximum concentrations were considerably lower. As Zhang et al. (2013b) collected placenta from healthy women at a hospital in Tianjin, China, the differences in placental PFAS exposure may be a result of differential use of consumer products and furnishings in each region (e.g., furniture, carpeting, stain-repellent).
3.5. Limitations
Limitations to our study include the fact that our study cohort consisted exclusively of women living in central North Carolina and may not be representative of other regions. Demographic differences in our cohort may also be a limitation; our cohort is unique as it consists primarily of non-Hispanic black women, an under-represented population in contaminant exposure studies. However, while this may limit the generalizability of our findings to other populations, we do not expect the demographic characteristics of the study population to limit the internal validity of our findings; the homogeneity of our cohort may have helped to reduce unmeasured confounding. Additionally, exposures to PFAS have been changing over time as legacy PFAS such as PFOA and PFOS are phased out of use and replaced with newer, emerging PFAS. Our study examined associations with 120 placenta samples collected in 2010–2011. The placenta concentrations observed in these samples may not be consistent with current PFAS exposure as can be evidenced by the changing placental concentrations observed in more recent studies (Table S8) and in our limited observation of ten placenta samples collected in 2018 (Table S7). In addition, our results indicate that evaluating sex-specific associations between PFAS in placenta and birth outcomes is critical. However, our relatively small sample size (n=120) may have limited our power to detect more subtle associations.
4. Conclusions
Our present study shows that several PFAS are frequently detected in placenta, and our observed associations with birth outcomes indicate the potential concern for adverse health effects on infants exposed to the highest tertiles of PFAS exposure. Our concentrations are similar in magnitude to the few other studies on placenta PFAS conducted across the world, highlighting the widespread reach of PFAS exposure. In our study PFAS placental exposure was associated with sex-specific birth outcomes, similar to other studies that analyzed maternal serum PFAS. We found that the highest exposure of placental PFOS was associated with lower birth weight for gestational age in infant males and higher birth weight in female infants.
Supplementary Material
Highlights.
Several PFAS were detected in human placental tissues collected from 2010–2011 in the U.S.
PFOS, PFOA, PFNA, and PFDA were most abundant in placenta
Sex-specific differences were observed in birth outcome associations with PFAS
Placental PFAS was higher in nulliparous pregnancies
Acknowledgments
The authors would like to thank all the participants in this research study. The authors would also like to thank Claire Osgood for database management. We thank Matthew Ruis, Brian Antczak, and Liping Feng for help with collection of placenta samples in 2018 for data included in supporting information.
Funding:
This work was supported by grants from the National Institute of Environmental Health Sciences of the National Institutes of Health [R01 ES031419 to HMS/MLM; T32-ES021432 to SMH (the Duke University Program in Environmental Health)], and the US Environmental Protection Agency (RD-83329301-0 to MLM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US EPA.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary information
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Wang Z, DeWitt JC, Higgins CP, Cousins IT, 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51, 2508–2518. DOI: 10.1021/acs.est.6b04806 [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J, 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99, 366–394. DOI: 10.1093/toxsci/kfm128 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL, 2007a. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES). Environ Sci Technol 41, 2237–2242. DOI: 10.1021/es062686m [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL, 2007b. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115, 1596–1602. DOI: 10.1289/ehp.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR, 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115, 1298–1305. DOI: 10.1289/ehp.10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K, 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118, 222–228. DOI: 10.1289/ehp.0901252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW, 2013a. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47, 10619–10627. DOI: 10.1021/es401905e [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K, 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75, 46–51. DOI: 10.1136/oemed-2017-104651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL, 2006. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ Sci Technol 40, 2128–2134. DOI: 10.1021/es0517973 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY, 2019. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 131, 105048. DOI: 10.1016/j.envint.2019.105048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheka-Tendenguwo LR, Olowoyo JO, Mugivhisa LL, Abafe OA, 2018. Per- and polyfluoroalkyl substances in human breast milk and current analytical methods. Environ Sci Pollut Res Int 25, 36064–36086. DOI: 10.1007/s11356-018-3483-z [DOI] [PubMed] [Google Scholar]
- Jin H, Mao L, Xie J, Zhao M, Bai X, Wen J, Shen T, Wu P, 2020. Poly- and perfluoroalkyl substance concentrations in human breast milk and their associations with postnatal infant growth. Sci Total Environ 713, 136417. DOI: 10.1016/j.scitotenv.2019.136417 [DOI] [PubMed] [Google Scholar]
- Chen F, Yin S, Kelly BC, Liu W, 2017. Isomer-specific transplacental transfer of perfluoroalkyl acids: results from a survey of paired maternal, cord sera, and placentas. Environ Sci Technol 51, 5756–5763. DOI: 10.1021/acs.est.7b00268 [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Jonsson BAG, Lindh CH, Olesen RH, Larsen A, Ernst E, Kelsey TW, Andersen CY, 2017. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ 596–597, 97–105. DOI: 10.1016/j.scitotenv.2017.04.058 [DOI] [PubMed] [Google Scholar]
- Wang J, Pan Y, Wei X, Dai J, 2020. Temporal trends in prenatal exposure (1998–2018) to emerging and legacy per- and polyfluoroalkyl substances (PFASs) in cord plasma from the Beijing Cord Blood Bank, China. Environ Sci Technol 54, 12850–12859. DOI: 10.1021/acs.est.0c01877 [DOI] [PubMed] [Google Scholar]
- Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R, 2009. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect 117, 660–667. DOI: 10.1289/ehp.11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JPE, Henriksen TB, 2015. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol 45, 53–67. DOI: 10.3109/10408444.2014.952400 [DOI] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB, Seed JG, 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. Reprod Toxicol 78, 150–168. DOI: 10.1016/j.reprotox.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Blake BE, Fenton SE, 2020. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443, 152565. DOI: 10.1016/j.tox.2020.152565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2021. Toxicological Profile for Perfluoroalkyls. pp. 1–852. DOI: 10.15620/cdc:59198 [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C, 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307, 391–397. DOI: 10.1001/jama.2011.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K, 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121, 1313–1318. DOI: 10.1289/ehp.1306615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP, 2016. NTP Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) or Perfluorooctane Sulfonate (PFOS). in: Services, U.S.D.o.H.a.H. (Ed.), Research Triangle Park, NC: National Toxicology Program, pp. 1–147 [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP, 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature. Int J Environ Res Public Health 14, 691. DOI: 10.3390/ijerph14070691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelch KE, Reade A, Wolffe TAM, Kwiatkowski CF, 2019. PFAS health effects database: Protocol for a systematic evidence map. Environ Int 130, 104851. DOI: 10.1016/j.envint.2019.05.045 [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29, 131–147. DOI: 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, Roberts SM, 2021. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40, 606–630. DOI: 10.1002/etc.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Dieter HH, Holzer J, Wilhelm M, 2017. Recent experimental results of effects of perfluoroalkyl substances in laboratory animals - Relation to current regulations and guidance values. Int J Hyg Environ Health 220, 766–775. DOI: 10.1016/j.ijheh.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, Scott B, Stapleton HM, Strynar MJ, Elmore SA, Fenton SE, 2020. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect 128, 27006. DOI: 10.1289/EHP6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou SE, 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27, 365–372. DOI: 10.1016/j.reprotox.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li J, Lai J, Luan H, Cai Z, Wang Y, Zhao Y, Wu Y, 2016a. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing Prenatal Exposure Study. Sci Rep 6, 21699. DOI: 10.1038/srep21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Shi Y, Li J, Wang Y, Zhao Y, Wu Y, Cai Z, 2016b. Human placental transfer of perfluoroalkyl acid precursors: Levels and profiles in paired maternal and cord serum. Chemosphere 144, 1631–1638. DOI: 10.1016/j.chemosphere.2015.10.063 [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Bjorvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY, Damdimopoulou P, 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124, 482–492. DOI: 10.1016/j.envint.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Gao K, Zhuang T, Liu X, Fu J, Zhang J, Fu J, Wang L, Zhang A, Liang Y, Song M, Jiang G, 2019. Prenatal exposure to per- and polyfluoroalkyl substances (PFASs) and association between the placental transfer efficiencies and dissociation constant of serum proteins-PFAS complexes. Environ Sci Technol 53, 6529–6538. DOI: 10.1021/acs.est.9b00715 [DOI] [PubMed] [Google Scholar]
- Bangma J, Szilagyi J, Blake BE, Plazas C, Kepper S, Fenton SE, Fry RC, 2020a. An assessment of serum-dependent impacts on intracellular accumulation and genomic response of per- and polyfluoroalkyl substances in a placental trophoblast model. Environ Toxicol 35, 1395–1405. DOI: 10.1002/tox.23004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Avula V, Fry RC, 2020a. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator-activated receptors (PPARs). Curr Environ Health Rep 7, 222–230. DOI: 10.1007/s40572-020-00279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Freedman AN, Kepper SL, Keshava AM, Bangma JT, Fry RC, 2020b. Per- and polyfluoroalkyl substances differentially inhibit placental trophoblast migration and invasion in vitro. Toxicol Sci 175, 210–219. DOI: 10.1093/toxsci/kfaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Meng L, Ma D, Cao H, Liang Y, Liu H, Wang Y, Jiang G, 2021. The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ Pollut 273, 116460. DOI: 10.1016/j.envpol.2021.116460 [DOI] [PubMed] [Google Scholar]
- Nian M, Luo K, Luo F, Aimuzi R, Huo X, Chen Q, Tian Y, Zhang J, 2020. Association between prenatal exposure to PFAS and fetal sex hormones: Are the short-chain PFAS safer? Environ Sci Technol 54, 8291–8299. DOI: 10.1021/acs.est.0c02444 [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun H, Lin Y, Qin X, Zhang Y, Geng X, Kannan K, 2013b. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47, 7974–7981. DOI: 10.1021/es400937y [DOI] [PubMed] [Google Scholar]
- Martin J, Rodriguez-Gomez R, Zafra-Gomez A, Alonso E, Vilchez JL, Navalon A, 2016. Validated method for the determination of perfluorinated compounds in placental tissue samples based on a simple extraction procedure followed by ultra-high performance liquid chromatography-tandem mass spectrometry analysis. Talanta 150, 169–176. DOI: 10.1016/j.talanta.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Bangma J, Eaves LA, Oldenburg K, Reiner JL, Manuck T, Fry RC, 2020b. Identifying risk factors for levels of per- and polyfluoroalkyl substances (PFAS) in the placenta in a high-risk pregnancy cohort in North Carolina. Environ Sci Technol 54, 8158–8166. DOI: 10.1021/acs.est.9b07102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela-Soria F, Garcia-Villanova J, Mustieles V, de Haro T, Antignac JP, Fernandez MF, 2021. Assessment of perfluoroalkyl substances in placenta by coupling salt assisted liquid-liquid extraction with dispersive liquid-liquid microextraction prior to liquid chromatography-tandem mass spectrometry. Talanta 221, 121577. DOI: 10.1016/j.talanta.2020.121577 [DOI] [PubMed] [Google Scholar]
- Peck TM, Arias F, 1979. Hematologic changes associated with pregnancy. Clin Obstet Gynecol 22, 785–798. DOI: 10.1097/00003081-197912000-00002 [DOI] [PubMed] [Google Scholar]
- Odutayo A, Hladunewich M, 2012. Obstetric nephrology: Renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol 7, 2073–2080. DOI: 10.2215/cjn.00470112 [DOI] [PubMed] [Google Scholar]
- Pritchard JA, 1965. Changes in the blood volume during pregnancy and delivery. Anesthesiology 26, 393–399. DOI: 10.1097/00000542-196507000-00004 [DOI] [PubMed] [Google Scholar]
- Miranda ML, Edwards SE, Swamy GK, Paul CJ, Neelon B, 2010. Blood lead levels among pregnant women: historical versus contemporaneous exposures. Int J Environ Res Public Health 7, 1508–1519. DOI: 10.3390/ijerph7041508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Edwards S, Maxson PJ, 2011. Mercury levels in an urban pregnant population in Durham County, North Carolina. Int J Environ Res Public Health 8, 698–712. DOI: 10.3390/ijerph8030698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML, 2011. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect 119, 1454–1459. DOI: 10.1289/ehp.1003235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy GK, Garrett ME, Miranda ML, Ashley-Koch AE, 2011. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am J Med Genet A 155A, 1264–1271. DOI: 10.1002/ajmg.a.33583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson PJ, Edwards SE, Ingram A, Miranda ML, 2012. Psychosocial differences between smokers and non-smokers during pregnancy. Addict Behav 37, 153–159. DOI: 10.1016/j.addbeh.2011.08.011 [DOI] [PubMed] [Google Scholar]
- Buttke DE, Wolkin A, Stapleton HM, Miranda ML, 2013. Associations between serum levels of polybrominated diphenyl ether (PBDE) flame retardants and environmental and behavioral factors in pregnant women. J Expo Sci Environ Epidemiol 23, 176–182. DOI: 10.1038/jes.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JE, Valentiner E, Maxson P, Miranda ML, Fry RC, 2014. Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a North Carolina cohort. PLOS ONE 9, e109661. DOI: 10.1371/journal.pone.0109661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, Smeester L, Rojas D, DeBussycher T, Wu MC, Wright FA, Zhou YH, Laine JE, Rager JE, Swamy GK, Ashley-Koch A, Lynn Miranda M, Fry RC, 2014. Cadmium exposure and the epigenome: Exposure-associated patterns of DNA methylation in leukocytes from mother-baby pairs. Epigenetics 9, 212–221. DOI: 10.4161/epi.26798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SE, Maxson P, Miranda ML, Fry RC, 2015. Cadmium levels in a North Carolina cohort: Identifying risk factors for elevated levels during pregnancy. J Expo Sci Environ Epidemiol 25, 427–432. DOI: 10.1038/jes.2014.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gona S, Sanders AP, Miranda ML, Fry RC, 2015. Prenatal exposure to cadmium and cotinine and CpG island DNA methylation in mother-infant pairs. Genom Data 5, 378–380. DOI: 10.1016/j.gdata.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Anthopolos R, Wolkin A, Stapleton HM, 2015. Associations of birth outcomes with maternal polybrominated diphenyl ethers and thyroid hormones during pregnancy. Environ Int 85, 244–253. DOI: 10.1016/j.envint.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM, 2016a. Brominated flame retardants in placental tissues: Associations with infant sex and thyroid hormone endpoints. Environ Health 15, 113. DOI: 10.1186/s12940-016-0199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson PJ, Edwards SE, Valentiner EM, Miranda ML, 2016. A multidimensional approach to characterizing psychosocial health during pregnancy. Matern Child Health J 20, 1103–1113. DOI: 10.1007/s10995-015-1872-1 [DOI] [PubMed] [Google Scholar]
- Grotegut CA, Ngan E, Garrett ME, Miranda ML, Ashley-Koch AE, Swamy GK, 2017. The association of single-nucleotide polymorphisms in the oxytocin receptor and G protein-coupled receptor kinase 6 (GRK6) genes with oxytocin dosing requirements and labor outcomes. Am J Obstet Gynecol 217, 367 e361–367 e369. DOI: 10.1016/j.ajog.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM, 2016b. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int 88, 23–29. DOI: 10.1016/j.envint.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyasu S, Kannan K, So MK, Gulkowska A, Sinclair E, Okazawa T, Yamashita N, 2005. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A 1093, 89–97. DOI: 10.1016/j.chroma.2005.07.053 [DOI] [PubMed] [Google Scholar]
- Liu Y, Pereira Ados S, Martin JW, 2015. Discovery of C5-C17 poly- and perfluoroalkyl substances in water by in-line SPE-HPLC-Orbitrap with in-source fragmentation flagging. Anal Chem 87, 4260–4268. DOI: 10.1021/acs.analchem.5b00039 [DOI] [PubMed] [Google Scholar]
- NIST, 2017. Standard Reference Material® 1947 Certificate of Analysis: Lake Michigan Fish Tissue. National Institute of Standards & Technology., pp. 1–17 [Google Scholar]
- Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG, Nosten F, Craik R, Munim S, Cheikh Ismail L., Barros FC, Lambert A, Norris S, Carvalho M, Jaffer YA, Noble JA, Bertino E, Gravett MG, Purwar M, Victora CG, Uauy R, Bhutta Z, Kennedy S, Papageorghiou AT, 2017. International estimated fetal weight standards of the INTERGROWTH-21(st) Project. Ultrasound Obstet Gynecol 49, 478–486. DOI: 10.1002/uog.17347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J, 2007. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ Health Perspect 115, 1677–1682. DOI: 10.1289/ehp.10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, Kim CK, Lee JS, Park PS, Yoo YW, Ha JY, Shin YS, Lee JH, 2011. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut 159, 169–174. DOI: 10.1016/j.envpol.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim MK, Bae J, Yang JH, 2013. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 90, 1603–1609. DOI: 10.1016/j.chemosphere.2012.08.035 [DOI] [PubMed] [Google Scholar]
- Shu H, Lindh CH, Wikstrom S, Bornehag CG, 2018. Temporal trends and predictors of perfluoroalkyl substances serum levels in Swedish pregnant women in the SELMA study. PLOS ONE 13, e0209255. DOI: 10.1371/journal.pone.0209255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis MT, Rock KD, Hall SM, Horman B, Patisaul HB, Stapleton HM, 2019. PBDEs concentrate in the fetal portion of the placenta: Implications for thyroid hormone dysregulation. Endocrinology 160, 2748–2758. DOI: 10.1210/en.2019-00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Stein CR, Steenland K, 2013. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect 121, 1207–1213. DOI: 10.1289/ehp.1206372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, Woodruff TJ, 2014. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122, 1028–1039. DOI: 10.1289/ehp.1307893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K, Hartman TJ, 2019. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int J Hyg Environ Health 222, 889–895. DOI: 10.1016/j.ijheh.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iniguez C, Martinez D, Costa O, Santa-Marina L, Pereda-Pereda E, Schettgen T, Sunyer J, Vrijheid M, 2017. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int 108, 278–284. DOI: 10.1016/j.envint.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Martin E, Smeester L, Bommarito PA, Grace MR, Boggess K, Kuban K, Karagas MR, Marsit CJ, O’Shea TM, Fry RC, 2017. Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period. Epigenomics 9, 267–278. DOI: 10.2217/epi-2016-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Eaves LA, Gaona AR, Santos HP Jr., Smeester L, Bangma JT, Rager JE, O’Shea TM, Fry RC, 2021. Pre-pregnancy BMI-associated miRNA and mRNA expression signatures in the placenta highlight a sexually-dimorphic response to maternal underweight status. Sci Rep 11, 15743. DOI: 10.1038/s41598-021-95051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström S, Hussein G, Lingroth Karlsson A., Lindh CH, Bornehag C-G, 2021. Exposure to perfluoroalkyl substances in early pregnancy and risk of sporadic first trimester miscarriage. Sci Rep 11, 3568. DOI: 10.1038/s41598-021-82748-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Randolph JF, Calafat AM, Mukherjee B, Batterman S, Gold EB, Park SK, 2020. Associations of perfluoroalkyl substances with incident natural menopause: The study of women’s health across the nation. J Clin Endocrinol Metab 105, e3169–3182. DOI: 10.1210/clinem/dgaa303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K, 2017. A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125, 416–421. DOI: 10.1289/ehp273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen D, Wang B, Xu F, Pang Y, Zhang L, Zhang Y, Jin L, Li Z, Ren A, 2020. Does low maternal exposure to per- and polyfluoroalkyl substances elevate the risk of spontaneous preterm birth? A nested case-control study in China. Environ Sci Technol 54, 8259–8268. DOI: 10.1021/acs.est.0c01930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.