Abstract
Modern nosologies (e.g., ICD-11, DSM-5) for alcohol use disorder (AUD) and dependence prioritize reliability and clinical presentation over etiology, resulting in a diagnosis that is not always strongly grounded in basic theory and research. Within these nosologies, DSM-5 AUD is treated as a discrete, largely categorical, but graded, phenomenon, which results in additional challenges (e.g., significant phenotypic heterogeneity). Efforts to increase the compatibility between AUD diagnosis and modern conceptualizations of alcohol dependence, which describe it as dimensional and partially overlapping with other psychopathology (e.g., other substance use disorders) will inspire a stronger scientific framework and strengthen AUD’s validity. We conducted a systematic review of 144 reviews to integrate addiction constructs and theories into a comprehensive framework with the aim of identifying fundamental mechanisms implicated in AUD. The product of this effort was the Etiologic, Theory-Based, Ontogenetic Hierarchical Framework (ETOH Framework) of AUD mechanisms, which outlines superdomains of cognitive control, reward, as well as negative valence and emotionality, each of which subsume narrower, hierarchically-organized components. We also outline opponent processes and self-awareness as key moderators of AUD mechanisms. In contrast with other frameworks, we recommend an increased conceptual role for negative valence and compulsion in AUD. The ETOH framework serves as a critical step towards conceptualizations of AUD as dimensional and heterogeneous. It has the potential to improve AUD assessment and aid in the development of evidence-based diagnostic measures that focus on key mechanisms in AUD, consequently facilitating treatment matching.
Keywords: alcohol use disorder, addiction, classification, diagnosis, multidimensionality, heterogeneity, transdiagnostic, translational, systematic review of reviews
Introduction
Diagnosis is central to research on etiology, course, nosology, treatment, and prevention, but available frameworks for diagnosing alcohol use disorder (AUD) are characterized by unknown construct validity. Over time, AUD diagnoses have moved from imprecise, ill-defined concepts reflecting hypothetical etiological constructs (e.g., Diagnostic and Statistical Manual of Mental Disorders, First [DSM-I; APA, 1952] and Second [DSM-II; APA, 1968] Editions) to a “theory-free” criteria set based on clinical consensus of presenting symptoms (from the DSM-III [APA, 1980] thru the current DSM-5 [APA, 2013]). Arguably, reliability has been prioritized over validity, resulting in a diagnostic approach that is not as strongly grounded in basic theory and research (Brown & Barlow, 2009; Kozak & Cuthbert, 2016; Strain, 2021). Moreover, there are known issues with our current diagnostic systems, including that our diagnostic constructs are of poorly defined construct validity (Charney et al., 2002), exhibit significant phenotypic heterogeneity (Litten et al., 2015), and are substantially overlapping with other putatively discrete conditions (Krueger & Markon, 2006).
In an effort to improve the validity of AUD diagnoses, we conducted a systematic review of reviews to describe and integrate the literature on translational AUD mechanisms. The overall goal of this review was to address the shortcomings of existing diagnostic systems (e.g., DSM, ICD) and mechanism-based frameworks of AUD (e.g., Alcohol Addiction Research Domain Criteria [Litten et al., 2015], Addictions Neuroclinical Assessment [Kwako et al., 2017]) by: (a) integrating the existing literature on AUD etiology into a dimensional, hierarchically-organized framework, (b) differentiating premorbid, dispositional, and acquired features of AUD,1 and (c) distinguishing substance-use-general and alcohol-specific mechanisms. We termed the resulting framework the Etiologic, Theory-Based, Ontogenetic Hierarchical Framework (ETOH Framework). Ultimately, we are optimistic that the ETOH Framework can refine AUD research, assessment, diagnosis, prevention, and treatment by organizing AUD-relevant etiologic mechanisms into higher-order domains, which clarifies sources of within-disorder heterogeneity and points to sources responsible for AUD’s comorbidity with other forms of psychopathology.
Problems with the Classification of DSM-5 Alcohol Use Disorder
DSM-5 intended to resolve many of the issues with prior versions of the DSM, both broadly and specifically related to AUD. With respect to AUD diagnosis, DSM-5 made three major changes: (1) the shift from abuse and dependence categories to a unidimensional structure, (2) the removal of the legal problems criterion, and (3) the addition of a craving criterion. Regarding the shift to a unidimensional structure, the DSM no longer subdivides AUD (and substance use disorders [SUDs] more generally) into abuse or dependence diagnoses, where dependence was thought to reflect a more severe manifestation of AUD (e.g., APA, 1994). The distinction between abuse and dependence was determined empirically arbitrary given that some abuse criteria appear more severe than dependence criteria (e.g., Compton et al., 2009; Saha et al., 2007), and because abuse and dependence form a single factor (or two highly correlated factors) rather than two distinct factors (see Hasin et al., 2013 for a review of AUD dimensionality). DSM-5 AUD is now classified as a unitary construct that grades along a severity dimension based on the number of criteria endorsed.
Regarding the legal problems criterion, it was removed from AUD due to its: (a) low prevalence in the general population and high severity, which was inconsistent with its classification as a milder abuse criterion (Compton et al., 2009; Gelhorn et al., 2008; Harford et al., 2009; Hartman et al., 2008); (b) poor ability to discriminate between people with high and low AUD severity (Hasin et al., 2012; Martin et al., 2006; Piontek et al., 2011; Saha et al., 2006); (c) poor associations with other SUD criteria (including AUD), which increases construct multidimensionality (Langenbucher et al., 2004; Martin et al., 2006); (d) failure to measure the same construct across different genders (Martin et al., 2006) and racial/ethnic groups (Gizer et al., 2013; Harford et al., 2009); (e) and failure to increment other SUD criteria in terms of the information it provided to the latent trait (Lynskey & Agrawal, 2007; Martin et al., 2006; Saha et al., 2012; Schmulewitz et al., 2010). Finally, DSM-5 also added craving as a criterion given that it increases consistency of AUD diagnosis between diagnostic systems (i.e., International Classification of Diseases, Tenth Edition [ICD-10] and Eleventh Edition [ICD-11]) and may have utility as a pharmacological treatment target (Hasin et al., 2013).
Although these changes may reflect some progress towards improving the validity of the AUD construct, DSM-5 AUD remains plagued with several other problems. These include but are not limited to: (1) inadequate construct validity of DSM-5 AUD symptoms, (2) high degrees of within-disorder heterogeneity, (3) a failure to explicitly consider etiologic mechanisms, (4) substantial comorbidity with other forms of psychopathology, and (5) unknown construct validity of the diagnostic criteria. The current review addresses each of these issues.
Inadequate Construct Validity
DSM-5 AUD criteria are organized to fit in the overall groupings of impaired control, social impairment, risky use, and pharmacological criteria (APA, 2013). These groupings rely mostly on expert consensus rather than empirical classification whereby signs and symptoms are determined by experts who undoubtedly carry with them different backgrounds, traditions, and biases (Krueger et al., 2018). Indeed, these groupings appear more conceptual than empirical (Watts et al., in press).
Additionally, an AUD diagnosis contains a complex mix of fundamental (or primary; e.g., loss of control, craving) and secondary (or accessory; e.g., consequences such as failure to fulfill role obligations) features. Fundamental features are those that are specific to and present throughout the course of AUD, whereas secondary features are those that represent epiphenomena or sequelae. Thus, secondary features may be non-specific to AUD and can be prominent or absent throughout the course of AUD, or they may moderate AUD’s expression. Many criteria pertain to potential consequences of AUD (e.g., social/interpersonal impairment), that are defined, in part, on the basis of contextual factors (Martin et al., 2014; Sher & Vergés, 2018). Additionally, defining a diagnosis based on criteria that reflect impairment may guarantee that a diagnosis reflects a secondary outcome (e.g., negative affect) that does not cause AUD. Thus, it is often difficult to determine whether a given symptom arises due to chronic and excessive alcohol use (i.e., fundamental/primary), or some other factor. The inclusion of secondary outcomes, including consequences, in a diagnosis also may contribute to comorbidity among AUD and other psychopathology. For instance, social/interpersonal problems in some form are included as diagnostic criteria in a number of disorders’ criteria (e.g., depression, social anxiety) and are requisite for others (i.e., personality disorders), so it may be a non-specific marker of psychopathology given that it is common to many disorders.
Ultimately, the “mixing” of fundamental, accessory, and secondary features within an AUD diagnosis suggests that some of the symptoms outlined by our current diagnostic systems may only be distally related to fundamental AUD features, which likely compromises the construct validity of a diagnosis and increases the likelihood of diagnostic comorbidity by including features that are multiply determined. “Mixing” types of features also creates a difficult scenario in terms of determining the most effective treatment targets. It also potentially obscures investigations into the causes of alcohol use and addiction.
Significant Heterogeneity
There are more than 2,000 possible combinations of the 11 DSM-5 criteria that are sufficient for an AUD diagnosis (i.e., two or more criteria). When considering two criteria alone, there are still 55 different combinations of criteria. As such, two individuals could both receive an AUD diagnosis despite having no or few overlapping symptoms. Therefore, those diagnosed with AUD exhibit considerable heterogeneity in terms of clinical presentation as well as patterns of consumption, profiles of risk (e.g., family history of alcohol-related problems, age of first drink), alcohol-related consequences, and patterns of comorbid psychopathology (Litten et al., 2015; Martin et al., 2011). Within-disorder heterogeneity is further complicated by the fact that DSM criteria are considered interchangeable, meaning that they contribute equally towards a diagnosis. There is clear evidence that AUD criteria vary, sometimes considerably, in their severities (Boness et al., 2019; Lane & Sher, 2014), so different criteria cannot be assumed to be equivalent indicators of AUD. Arguably, an individual who meets criteria solely on the basis of tolerance and withdrawal (indicating physiological dependence) is quite different from an individual who meets criteria solely on the basis of social or interpersonal problems and giving up activities to use. Ultimately, a unitary, heterogeneous diagnosis of AUD may preclude a thorough understanding of the development and nature of the disorder and impede prevention and treatment efforts as a result.
Failure to Consider Etiology
The DSM-5 prioritizes clinical description and presentation and fails to systematically consider etiology in the construction of AUD diagnostic criteria (e.g., Charney et al., 2002). Certain AUD criteria (i.e., tolerance, withdrawal) are caused by distinct genetic and environmental risk pathways in rodents and humans (e.g., Crabbe et al., 2011; Kendler et al., 2012), and several well-established etiologic models of AUD and addiction (which we describe later) posit numerous explanatory AUD mechanisms (e.g., reward sensitivity, negative emotionality). It remains unclear how multiple etiologic mechanisms are accommodated in DSM-5 AUD. That is, does a given symptom represent one or multiple etiologic mechanisms? Does a single etiologic mechanism give rise to multiple symptoms? If AUD comprises etiologically distinct symptoms, why is it considered unitary? These and other questions have yet to be satisfactorily addressed by the DSM. Moreover, current diagnostic criteria fail to explicitly probe such mechanisms. Improved attention to etiology may result in an AUD diagnosis that is more informative of development, treatment targets, and prognosis.
Substantial Comorbidity
There is extensive comorbidity, or diagnostic co-occurrence, of AUD with other virtually all other disorders including, other SUDs (e.g., Glass et al., 2014; Sher & Trull, 2002), externalizing disorders (Krueger et al., 2002; Slutske et al., 2002), personality disorders (e.g., antisocial, borderline; Helle et al., 2020), and internalizing disorders (Kessler et al., 2005; Tully & Iacono, 2016). Potential explanations for comorbidity include artefactual reasons (e.g., drawing arbitrary categorical boundaries between disorders where they do not exist, suboptimal diagnostic decision rules, definitional overlap), shared underlying mechanisms (e.g., common etiologies; Krueger et al., 2002), causal associations between disorders (Brown & Barlow, 2009; Krueger & Markon, 2006; Sher & Trull, 1996), and, as we noted earlier, the inclusion of non-specific consequences of psychopathology as diagnostic criteria. Given our limited knowledge of etiology, it is difficult to distinguish true comorbidity from a poor diagnostic framework (Aragona, 2009; Lilienfeld et al., 1994).
Others have suggested that comorbidity may reflect dysregulation in some higher-order dimension (e.g., disinhibition, negative emotionality, reward; Kotov et al., 2017). For instance, much of the variance in AUD is shared with other forms of externalizing psychopathology (e.g., antisocial personality disorder), which likely arises from an underlying tendency toward disinhibition (Krueger et al., 2002). Regardless of the nature of comorbidity observed between AUD and other disorders, it suggests that our current diagnostic approaches, such as the DSM-5, might not be accurately capturing AUD’s distinctive features.
Potential Solution: A Mechanistic Focus
A potential solution to the aforementioned concerns involves shifting conceptualizations of psychopathology away from clinical description and towards mechanisms. The shift towards mechanisms would prioritize focusing on the etiology of mental disorders over factors like clinical presentation, allowing an evaluation of how well proposed mechanisms converge upon a disorder. Mechanism-focused approaches also emphasize the importance of integrating translational research, which refers to the application of findings from basic science (e.g., neural circuits) to the etiology, pathophysiology, and trajectory of mental disorders (NIH, 2017). Translational research is important for integrating research across different units of measurement (e.g., cells, circuits, genes, behavior), which has been a challenge in psychopathology research for many decades. In our view, the shift towards focusing on mechanisms will improve the understanding, diagnosis, and treatment of mental disorders. In the sections that follow, we elaborate on the mechanism-based alternatives to DSM and ICD. Notably, these systems are complementary in many respects. Table 2 provides an overview of each system or framework.
Table 2.
Comparison of Dominant Psychopathology Systems and Frameworks
| System or Framework |
||||||
|---|---|---|---|---|---|---|
| Characteristic | ETOH Framework | ANA/AARDoC | PhAB | RDoC | HiTOP | DSM-5/ICD-10/11 |
| Target | Addiction | Addiction | Addiction | Psychopathology | Psychopathology | Psychopathology |
| Goal | Explanation, description, assessment, diagnosis | Explanation (AARDoC), assessment (ANA) | Assessment | Explanation | Description, assessment, diagnosis | Diagnosis |
| Focus | Mechanisms | Mechanisms | Mechanisms | Mechanisms | Empirical description of covariance among signs and symptoms | Clinical description |
| Units of analysis | Genotypes, endophenotypes, phenotypes | Endophenotypes, phenotypes | Endophenotypes, phenotypes | Genes, molecules, cells, circuits, physiology, behavior, self-report, paradigms | Genotypes, endophenotypes, phenotypes | Phenotypes |
| Classification type | Dimensional, Hierarchical | Dimensional | Dimensional | Dimensional | Dimensional, Hierarchical | Categorical,a Independent Taxa |
| Identification of disorder/dysfunction | Dysfunction in functional domains | Dysfunction in functional domains | Dysfunction in functional domains | Dysfunction in functional domains | Dysfunction in functional domains, with potentially clinically meaningful thresholds along dimensions | Signs and symptoms based on consequences |
| Intended use | Research, Clinical | Research, Clinical | Research | Research | Research, Clinical | Clinical |
| Classification of AUD within system/framework | Broad functional domains of cognitive control, reward, and negative valence & emotionality | Broad functional domains of executive function, reward, negative emotionality | Broad functional domains of metacognition, interoception, cognition/executive function, reward/incentive salience, emotion/negative emotionality, and sleep/circadian rhythm | (see AARDoC) | Disinhibited externalizing | Substance use disorders |
Note. ETOH-Framework = Etiologic, Theory-Based, Ontogenetic Hierarchical Framework; AARDoC = Alcohol Addiction Research Domain Criteria; ANA = Addictions Neuroclinical Assessment; PhAB = Phenotyping Assessment Battery; RDoC = Research Domain Criteria; HiTOP = Hierarchical Taxonomy of Psychopathology; DSM-5 = Diagnostic and Statistical Manual of Mental Diseases, Fifth Edition; ICD-10/11 = International Classification of Diseases, Tenth and Eleventh Editions.
We acknowledge that the DSM-5 has attempted to move away from a categorical approach through the inclusion of a symptom count.
Research Domain Criteria (RDoC)
Research Domain Criteria (RDoC; e.g., Sainslow et al., 2010) adopts a mechanistic framework of psychopathology that characterizes it in terms of basic dimensions of functioning (constructs, subconstructs) that span multiple units of analysis (from genes to paradigms). There is a wealth of research on mechanisms of psychopathology, but those mechanisms are not well-mapped onto diagnoses, perhaps especially in the case of SUDs and other behavioral addictions (Belin-Rauscent et al., 2016; Kozak & Cuthbert, 2016). RDoC assumes that coherence between diagnosis and mechanism can increase with the development of data-driven diagnostic groupings or categories (versus the traditional diagnostic constructs). RDoC also aims to develop, test, and validate biological and behavioral markers across multiple units of analysis, ranging from genes to behavior. These efforts may assist in matching treatment, which is consistent with precision medicine and evaluating treatment response (Litten et al., 2016), and may also help illuminate mechanisms of treatment response and sustained behavior change (Feldstein & Chung, 2013), which may inform the development of improved interventions (NIH, 2017).
Other Alternatives to DSM and ICD
The Hierarchical Taxonomy of Psychopathology (HiTOP; Kotov et al., 2017) describes psychopathology in terms of hierarchically-organized, empirically-based dimensions. The National Institute on Drug Abuse’s (2018) emerging Phenotyping Assessment Battery (PhAB) aims to develop an addiction assessment battery that contains self-reported questionnaires and fMRI. RDoC, HiTOP, and NIDA PhAB are compatible and complementary in many respects (see Table 2 for a comparison and Michelini et al., 2020 for a review of HiTOP and RDoC). Because they focus on empirically-derived, transdiagnostic dimensions thought to map onto etiologic mechanisms, each of these approaches have the potential to address some of the aforementioned limitations with existing classification systems (e.g., rampant comorbidity).
Alcohol Addiction Research Domain Criteria (AARDoC) and Addictions Neuroclinical Assessment (ANA)
To extend the RDoC framework to alcohol addiction more specifically, Litten and colleagues (2015) introduced the Alcohol Addiction RDoC (AARDoC; Sher, 2015) as the first AUD-specific mechanistic framework that synthesizes research on its behavioral, neurobiological, and genetic features. Based off of AARDoC, Kwako and colleagues (2015) proposed a clinical framework and addiction assessment battery termed the Addictions Neuroclinical Assessment (ANA; Kwako et al., 2017; Voon et al., 2020). ANA focuses on three domains – reward/incentive salience, negative emotionality, and cognitive control/executive function – described in Koob and Le Moal’s (1997) theory of addiction.
Koob and LeMoal (1997) propose that addiction results from a cycle of dysregulation within brain reward systems that progressively escalates and spirals into addiction (i.e., compulsive use and a loss of control over substance use). The first stage, binge-intoxication, is characterized by heavy consumption and experiences of pleasurable effects following from substance use. As use increases, reward valuation and hedonic set points shift. The second stage, withdrawal-negative affect, is characterized by increases in substance use to alleviate the stress and anxiety-like responses resulting from acute and protracted abstinence (i.e., negative reinforcement). The third stage, preoccupation-anticipation, is characterized by an intense need, or craving, for the substance after a period of abstinence. Preoccupation-anticipation is thought to entail compromised executive functioning, leading to loss of control over consumption, which reverts back to the binge-intoxication phase. The Koob and LeMoal model posits initial failures in behavioral self-regulation within a given stage results in distress, leading to a cycle of repeated self-regulation failures. Each failure is thought to result in additional distress, which escalates the “spiral” into addiction (i.e., progression through the addiction cycle). Therefore, addiction is thought to arise as a result of attempts to regulate the emotional distress that follows from failed self-regulation at each stage of the model.
Accordingly, Kwako and colleagues’ (2017) ANA domains relate directly to the three stages in Koob and Le Moal’s cycle of addiction. Incentive salience is thought to correspond to binge-intoxication, negative emotionality with withdrawal-negative affect, and cognitive control with preoccupation-anticipation. According to the ANA model, AUD is an acquired, atypical form of learning, and these three functional domains relate to each other in a causal, staged process whereby prolonged alcohol exposure leads to alterations in the neurocircuitry underlying the stress response, reward, and executive functioning, resulting in compulsive use (e.g., Koob, 2003). Thus, each domain is hypothesized to be causally implicated in the initiation and progression of addictive behaviors. Ultimately, the ANA aims to clarify sources of heterogeneity within AUD through the characterization of these three domains, which, ideally, will serve as useful targets in precision medicine efforts.
Reward or incentive salience describes the processes that transform otherwise neutral stimuli or events (e.g., cues) into attractive and wanted stimuli (Berridge, 1996; Kwako et al., 2015). Through continued use of alcohol, cues or stimuli associated with alcohol may become salient, or attractive. From this theoretical perspective, excessive attribution of incentive salience to cues contributes to the development of compulsive behavioral disorders, including but not limited to AUD (Robinson & Berridge, 1993). Incentive salience is well-documented in the laboratory, where it is described by the shift from goal-tracking behavior (e.g., pursuit of alcohol consumption) to sign-tracking behavior (e.g., pursuit of cues associated with alcohol; Berridge & Robinson, 2003; Flagel et al., 2009; Srey et al., 2015), and at the neural level, where changes in connectivity and neuronal activity in the basal ganglia occur with the incentivization of alcohol and its cues. Incentive salience is conceptually equivalent to the reward learning construct within the RDoC positive valence systems domain, although RDoC outlines psychopathology-general as opposed to alcohol-specific reward processing.
Negative emotionality refers to increased negative emotional responses to alcohol-related stimuli with chronic consumption as well as higher overall levels of low mood observed in individuals diagnosed with AUD (Kwako et al., 2015, 2017). ANA focuses on acquired negative affective states (e.g., dysphoria, anhedonia, alexithymia, and anxiety) as a result of excessive alcohol consumption and withdrawal, which leads to craving. This form of negative affect from chronic alcohol use can be traced to neuroadaptations with molecular (e.g., corticotropin-releasing hormone; Zorrilla et al., 2014) and neural substrates (e.g., decreased GABAergic and increased NMDA glutamatergic transmission in the nucleus accumbens; Dahchour et al., 1998; Davidson et al., 1995). ANA’s negative emotionality domain is intended to be conceptually equivalent to the “negative valence systems” domain in RDoC, although, again, RDoC outlines mechanisms that are more general to other psychopathology and ANA emphasizes acquired as opposed to premorbid negative affect. As we argue later, we believe ANA’s negative emotionality domain is a narrower conceptualization of negative valence compared with RDoC’s.
Executive function describes the ability to regulate one’s cognitions or responses in relation to goals and to temporally organize behavior (Lyon & Kradsnegor, 1996), and cognitive control describes a subset of executive functions that guide behavior toward or away from a particular task by goal setting and inhibiting habitual and impulsive acts (Wilcox et al., 2014). ANA focuses on acquired dysregulation of cognitive control mechanisms relevant to addiction rather than preexisting vulnerabilities, including but not limited to the subdomains of attention, response inhibition, planning, working memory, decision-making, cognitive flexibility, set shifting, and valuation of future events (Kwako et al., 2015; Kwako et al., 2017). A number of recent reviews have demonstrated a significant association between AUD and impaired executive function (Bickel et al., 2012; Montgomery et al., 2012; Stephan et al., 2017; Wilcox et al., 2014). For example, individuals diagnosed with AUD are more likely to have impairment in planning (Joyce & Robbins, 1991), set-shifting, problem-solving (Stephan et al., 2017), and response inhibition (Noël et al., 2007; Stephan et al., 2017). Further, alcohol use appears to result in specific neuroadaptations that manifest as deficits in cognitive control, including excessive glucocorticoid receptor activity in the prefrontal cortex (e.g., Pahng et al., 2017). Non-human animal research is beginning to illuminate explicit mechanisms for alcohol-induced frontal cortex dysfunction, such as volume and myelin density loss as well as metabolite abnormalities (Kwako et al., 2017; Wilcox et al., 2014). ANA’s cognitive control domain is conceptually equivalent to the cognitive control construct within the cognitive systems RDoC domain.
Together, the ANA domains capture narrower constructs within three of the six RDoC domains – positive valence, negative valence, cognitive systems – and exclude three others – sensorimotor systems, arousal/regulatory systems, and systems for social processes (see NIDA PhAB [Keyser-Marcus et al., 2021], for a more comprehensive integration of RDoC domains into addiction).
Limitations of AARDoC and ANA
Although the AARDoC and ANA offer an excellent starting place for a mechanistic-based approach that aims to identify and assess the constructs/domains of most relevance to AUD, the ANA framework has several limitations. Briefly, these include: (a) questionable empirical support for early operationalizations of the model and measurement approaches, (b) the exclusion of some addiction-relevant domains, (c) the failure to consider the distinction between premorbid risk versus acquired features, (d) the lack of resolution regarding the overlap between domains and constructs, (e) the lack of consideration of the distinction between general substance use and alcohol-specific mechanisms, and (f) the clinical feasibility of ANA. Although we choose to focus on the AARDoC and ANA frameworks given their relevance to AUD, other mechanistic-based frameworks (e.g., RDoC, HiTOP) also suffer from the same limitations, such as a lack of resolution regarding overlapping domains and feasibility in a clinical setting (e.g., Lilienfeld, 2014; Lilienfeld & Treadway, 2016). These criticisms may also apply to NIDA PhAB, but it is in its relative nascence.
Interpretability of Empirical Examinations of the ANA Model
It is unclear how well the ANA conceptual model is supported by the recent operationalizations of the model. Kwako and colleagues (2019) reported that both confirmatory and exploratory factor analyses supported their proposed three-factor ANA framework, but their empirical models are somewhat difficult to interpret. First, Kwako and colleagues (2019) include mostly trait/dispositional measures (e.g., personality) in their factor models, which is inconsistent with ANA’s major conceptual focus is on acquired features of AUD. For instance, dispositional negative emotionality measures are used to assess acquired negative emotionality associated with alcohol withdrawal. Second, extraversion, which is made up of positive emotionality among other things, loads substantially negatively onto ANA negative emotionality. This loading is puzzling given that negative and positive emotionality (and negative and positive affectivity) are largely empirically distinct dimensions (e.g., Tellegen & Waller, 2008; Watson & Clark, 1997; Watson et al., 1988, 1999). Third, the negative emotionality factor is essentially defined by aggression (and reverse agreeableness), whose factor loading well exceeds that of negative emotionality. The ANA negative emotionality factor might be better described as tendencies towards disinhibition or antisociality (or what Eysenck referred to as Psychoticism; Eysenck & Eysenck, 1975).2 Together, the inconsistencies between the conceptual and operationalized ANA models, along with questionable empirical support for the operationalized model, suggest the need for further development and validation of this framework and associated assessment.
Exclusion of Other AUD-Relevant Domains
A consequence of ANA’s exclusive focus on Koob and Le Moal’s (1997) model of addiction is that it neglects other important functional domains and mechanisms. For instance, an initial low level of response to alcohol, or subjective response, which describes individual differences in how one experiences the effects of alcohol, is not explicitly considered or assessed as part of the ANA despite the fact that it has been repeatedly implicated in the etiology of AUD (e.g., Morean & Corbin 2010; Quinn & Fromme, 2011; Ray et al., 2016). Subjective response, along with other etiologically relevant mechanisms – such as an inability to abstain, habit, and punishment sensitivity – have not been incorporated into AARDoC and ANA.
Emphasis on Acquired as Opposed to Premorbid Features
Another consequence of the exclusive focus on Koob and Le Moal’s (1997) model is that it is concentrated on features that are acquired as a result of chronic alcohol consumption as opposed to premorbid. Although Kwako and colleagues (2019) acknowledge the existence of premorbid AUD features, they are neither incorporated systematically into the ANA framework, nor, more critically, adequately distinguished from acquired features. We view the exclusion of premorbid features as an important oversight should AARDoC and ANA aim to reflect a comprehensive explanatory model of the alcohol addiction process. The relevance of premorbid features is especially necessary to address given that acquired and premorbid factors are each implicated in the development of addiction. Complicating matters further, ANA domains may reflect a combination of acquired and premorbid influences, so their assessment and interpretation of mechanisms within the context of a staged AUD development process becomes challenging. As we noted earlier, the inclusion of mechanisms that likely reflect a blend of acquired and premorbid factors has other implications, including that it makes it more difficult to identify etiologic mechanisms and that premorbid and acquired features may require different treatment and prevention efforts and goals. The chronicity of premorbid factors alone suggests that they may require more intensive treatments than acquired ones.
One example of a relevant premorbid factor to AUD is negative emotionality. Within the ANA, negative emotionality is largely described as an adaptation to chronic consumption, although it is also a premorbid risk factor. Indeed, much research has demonstrated that negative emotionality, and depressive symptomology in particular, is a risk factor for the development of AUD. In fact, negative emotionality is referred to as the “internalizing pathway” to substance use risk (e.g., Hussong et al., 2011). Negative emotionality assessed in childhood is prospectively associated with precocious alcohol use, heavy drinking in adolescence, and subsequent development of AUD and other substance use problems (Elkins, et al., 2006; King et al., 2004). Additionally, individuals with early onset and persistent AUDs fail to exhibit the normative declines in negative emotionality across the lifespan, suggesting that negative emotionality may be involved in the maintenance of AUD over time (Hicks et al., 2012).
Poorly Delineated Boundaries Among Domains
The ANA domains are heterogeneous and there may be overlap between constructs they subsume. For example, although ANA designates impulsivity as related to executive function, research suggests that negative urgency – which is one component of impulsivity that describes the tendency to act rashly when experiencing extreme negative affect and – is highly overlapping with negative emotionality (Cyders & Smith, 2008). By defining impulsivity broadly under executive function, the ANA overlooks the potential for impulsivity subdomains to better relate to other ANA domains, and certain aspects of the ANA assessment battery, at least as operationalized by Kwako and colleagues (2019), may probe relatively nonspecific features of the addiction process. Also, the relationships of components or mechanisms with a given domain may also vary as a function of the stage of addiction. For example, negative emotionality (ANA’s negative emotionality domain) might be implicated as a preexisting vulnerability in the early stages of addiction but become more associated with craving (ANA’s reward domain) in the later stages (Koob & Volkow, 2010). As such, the stage of addiction may change the nature of the association between constructs and domains.
The potential for overlap between domains is acknowledged by ANA advocates (e.g., Kwako et al., 2019) but it is neither explicitly incorporated in nor resolved by their framework. Explicit consideration of the overlap of constructs between domains, and how overlap varies as a function of stage, could assist in the identification of mechanisms that cut across functional domains. Such cross-cutting mechanisms (e.g., negative emotionality) might serve as potential treatment targets for reducing dysfunction in multiple domains concurrently.
Failure to Demarcate Alcohol-Specific and Substance-General Features
The ANA fails to demarcate mechanisms that are general to SUDs (substance-general) versus specific to alcohol (alcohol-specific), which is important given that SUDs share genetic influences, but also contain substance-specific genetic influences (Kendler et al., 2007; Krueger et al., 2002; Palmer et al., 2012; Tsuang et al., 1998; Walters et al., 2018). One example of an alcohol-specific mechanism is a variant of the aldehyde dehydrogenase gene, ALDH2 (ALDH2**2). The ALDH2 gene regulates the activity of acetaldehyde dehydrogenase, which is critical in the catabolism of acetaldehyde into acetate. Carriers of the ALDH2**2 variant experience a build up of acetaldehyde after consuming alcohol, resulting in an alcohol-flush reaction that is aversive and protective against AUD among carriers of the variant (primarily East Asians; e.g., Luczak et al., 2006). Importantly, the ALDH2 gene does not influence metabolism of other substances (Vanyukov et al., 2003).3 Although ANA is based on AARDoC, which is alcohol specific, ANA articulates a model for addiction more broadly (Kwako et al., 2015). This is problematic because, as demonstrated with ALDH2, there is a need to differentiate between substance-general and substance-specific mechanisms. An explicit consideration of how substance-specific mechanisms (e.g., acetaldehyde accumulation after alcohol consumption) differ from substance-general mechanisms may be important in understanding an individual’s profile and developing tailored treatments.
Feasibility of the ANA Assessment Battery
The ANA proposes numerous measures (spanning self-report, behavioral laboratory tasks, and neuroimaging assessments) to be included in their comprehensive, 10-hour long assessment battery (Kwako et al., 2015). Given the length of time it takes to complete the battery, one would expect significant burden on the participant, particularly individuals diagnosed with a SUD, which may not be feasible for most research or clinical settings (DeVito et al., 2016). NIDA PhAB aims to develop an abbreviated battery in light of concerns with ANA’s length.
Additionally, laboratory tasks have been widely criticized due to poor reliability (i.e., measurement error; Dang et al., 2020; Hedge et al., 2018) and an inability to assess between-subjects (or individual difference) effects given that many are designed to detect within-subjects effects (Dang et al., 2020). It may therefore be necessary to prioritize more efficient and reliable assessment approaches and further refine those proposed to improve their reliability and suitability for studying individual differences. Refining these assessments could address concerns related to the significant length of the proposed battery as well as address issues related to method variance which arise when several different methods are used to assess a construct (or domain). It is worth noting, though, that relying on a single method (e.g., self-report) could also introduce concerns, such as single-reporter method variance.
The Development of an Evidence-Based Alcohol Use Disorder Framework
AARDoC and ANA offer excellent foundations for mapping the etiologic mechanisms implicated in AUD, but there are important shortcomings of those efforts that require further refinement and elaboration. Although not unlike ANA and AARDoC, the overall goal of the current project was to systematically integrate theoretical and empirical addiction constructs using a translational, mechanism-focused framework that explicitly addresses shortcomings of current diagnostic frameworks and other mechanism-based approaches. This effort resulted in a mechanism-based, hierarchical framework of AUD that we call the ETOH Framework.
We focused on published literature on AUD etiology, core theories, and important endophenotypes (i.e., measurable phenotypes ostensibly associated with genotypes; Gottesman, & Gould, 2003) that spanned various units of analysis, from basic biology to clinical research. The specific emphasis of the current review was on theoretical and meta-analytic reviews that systematically address and integrate relevant constructs, rather than on primary studies. This methodology, known as a review of reviews (Cooper & Koenka, 2012), is an efficient and robust way to examine the current state of evidence for a topic (Akram et al., 2014). Unlike a narrative review, in which eligible reviews may be determined solely based on author’s expertise, a review of reviews takes a more systematic and less biased approach to considering reviews for inclusion. Such a systematic effort is important given that existing diagnostic systems and frameworks have largely been a product of authoritative classification.
By clearly specifying the mechanisms implicated in AUD, the ETOH Framework has the potential to more explicitly address components of AUD that are overlooked by current diagnostic systems. Arguably, limitations associated with current and past diagnostic conceptualizations of AUD have impeded progress in its prevention, diagnosis, and treatment matching which makes addressing them a top priority in addiction research. Thus, the ETOH Framework also aims to provide a tool to comprehensively characterize individuals diagnosed with AUD, which will allow for the identification of subpopulations based on profiles of risk and can improve treatment matching.
Method
All methods were specified in advance and were documented by the first author in a protocol. The present review of reviews was conducted according to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher et al., 2015) and the reporting standards of APA’s Publications and Communications Board Task Force Report (Levitt et al., 2018).
Search Strategy and Data Sources
The search aimed to identify all systematic reviews, including meta-analyses, examining AUD etiology (e.g., factors related to family history and environmental factors), core theories (e.g., allostasis and incentive sensitization), and important endophenotypes (e.g., impulsivity).
Reviews were identified by searching electronic databases, forward and backward searching manually, and consulting with coauthors and experts to ensure comprehensive searches. Twenty-nine ProQuest databases were searched through the ProQuest multidatabase search option, and 37 EBSCO databases were searched via the university’s discovery layer. Notable databases included PsycINFO with PsycARTICLES, CINAHL, MEDLINE, GenderWatch, ProQuest Dissertations & Theses A&I, ScienceDirect, Social Services Abstracts, Sociological Abstracts, ERIC, Academic Search Complete, Education Full Text, and the Directory of Open Access Journals. The University Libraries catalog was additionally searched to identify relevant manuscripts, with book chapters isolated as individual records.
A Boolean strategy was applied to include all combinations of relevant terminology: (“alcohol use disorder” OR “alcohol abuse” OR “alcohol dependence” OR alcoholi* OR addiction) AND (translation* OR etiolog* OR endophenotype OR genetic* OR neurobiologic* OR environment* OR experience* OR incentive OR “subjective response” OR “biological markers” OR “gene-environment” OR family OR families OR familial) AND (“systematic review” OR “meta-analysis” OR “literature review” OR “review of the literature” OR commentar*). Inclusion criteria required search terms to appear in either the “Subject” or “Title” fields of results to narrow the scope and ensure results were reviews focused on etiology. In addition, inclusion criteria required results to originate from peer-reviewed sources. No limits were applied for the start date of searches across databases. The database search was initially conducted in March of 2018, with all searches performed by the third author.
Eligibility Criteria
The citation management tool Mendeley (2016) was used to organize records and detect duplicates. The identified abstracts and titles were examined for inclusion by the first author, and records were retained if they provided information on AUD or addiction etiology, theory, or endophenotypes. Inclusion criteria were broad to ensure most relevant reviews were included at least initially. Further exclusion criteria at this stage included the mention of other co-occurring physical/mental health problems (e.g., liver disease, depression) or a sole focus on non-pathological alcohol consumption rather than heavy use. Of note, reviews focused on methodology were considered eligible for inclusion as long as they were related to etiology, theory, or endophenotypes. When the review focused on addiction or SUDs more generally, these reviews were considered eligible as long as alcohol was explicitly included under those umbrella terms. Similarly, when reviews were focused on “drug addiction,” it needed to be clear that alcohol was considered a drug, otherwise the review was considered not eligible. Once a review was included as eligible, we considered relevant etiologic factors regardless of whether they were described for heavy consumption or AUD more specifically. There were no exclusions based on review type (e.g., quantitative synthesis, narrative review).
Study Selection
Searching initially retrieved 2,331 records containing search terms in title and subject fields. Removal of duplicates and examination of abstracts based on inclusion and exclusion criteria resulted in a total of 141 unique records.4 Full-text versions of each record were acquired and read by the first author to determine eligibility. A further 17 articles were removed at this stage based on exclusion criteria, leaving 124 articles (see Figure 1 for a full description of reasons of exclusion).
Figure 1.
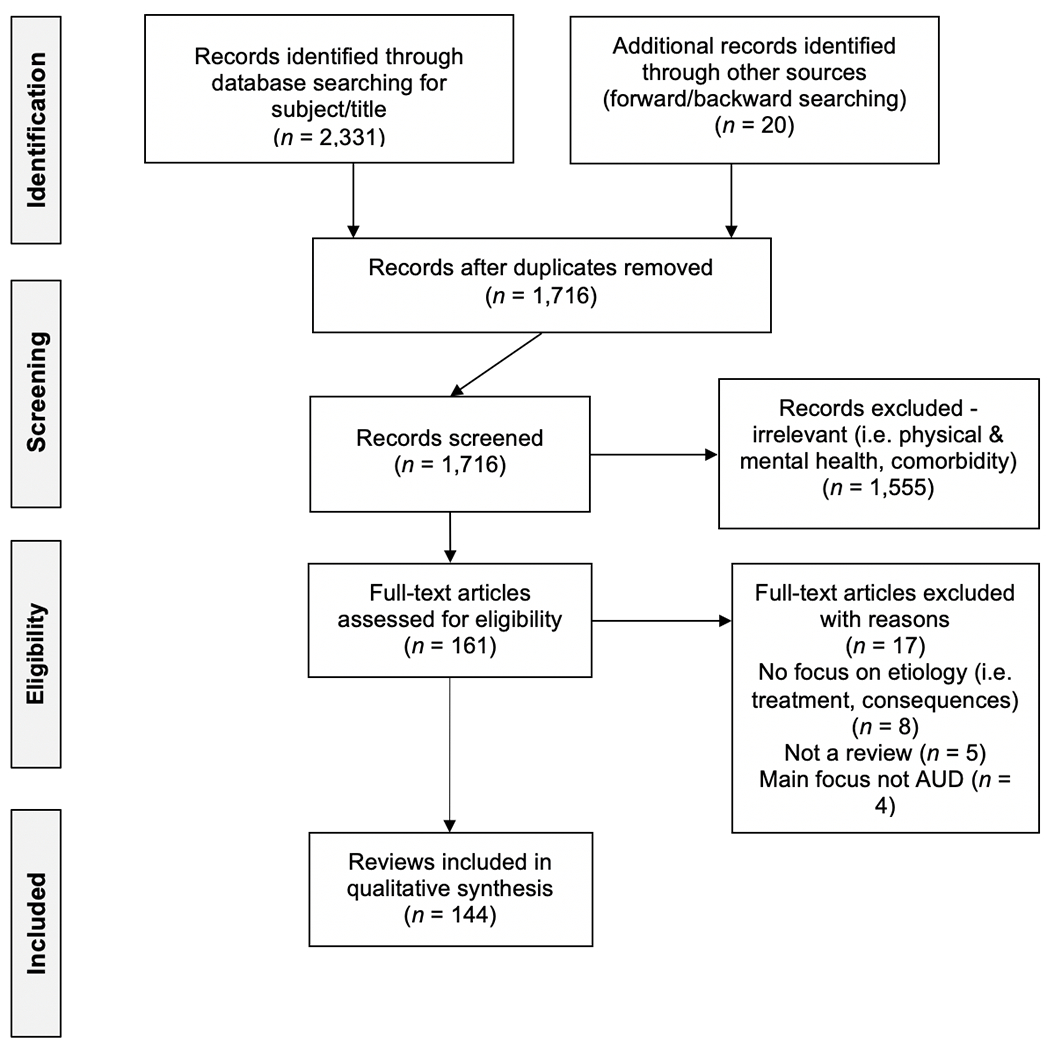
Flow Diagram of Systematic Review of Reviews Search Strategy and Results
Supplemental forward and backward searches were conducted on the remaining 122 eligible reviews5, resulting in 1,825 possibly eligible records, 945 of which were determined eligible records for inclusion following review of the full text. The authors speculate that the “Subject” and “Title” field limitations in the initial search strategy resulted in the high number of records identified through forward and backward searching. Additionally, subject and title limitations were not applied during forward and backwards searching which further explains the large number of records identified through this supplemental search. Indeed, the large majority of additional reviews located through the supplemental search were merely a recapitulation of the findings of the 124 reviews located in the original search. To minimize redundancy and keep the scope of the current review manageable, a randomly selected sample of 20 records from these 945 eligible reviews were included, resulting in 144 total reviews for inclusion in the current systematic review of reviews (see Figure 1). Characteristics of included reviews are described in Table 1.
Table 1.
Summary of Reviews Included in Review
| Target Demographics | AARDoC Domain | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||||
| Source | Type of Publication | Type of Review | Main Topic(s) | Age | Gender | Race/Ethnicity | Sexual Orientation | Species | Search Strategy? | Start Year | End Year | Cognitive Control | Reward | Negative Emotionality | None | Other | Quality |
|
|
|
||||||||||||||||
| Adler & Raphael, 1983 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Agarwal & Goedde, 1989 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | X | High | |||
| Agrawal & Lynskey, 2014 | Book Chapter | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | 3 | X | High | |||
| Akerlind & Hornquist, 1992 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Allan & Cooke, 1985 | Journal article | Lit. Review | Etiology | NR | Women | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Alterman & Tarter, 1983 | Journal article | Lit. Review | Genetics, Etiology | NR | Men | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Amlung et al., 2017 | Journal article | Meta-Analysis | Intermediate phenotype/endophenotype, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2015 | 3 | X2 | Moderate | |||
| Anthenelli & Schuckit, 1990 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Arias et al., 2006 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1997 | 2005 | X | Moderate | ||||
| Benyamina et al., 2010 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1990 | 2009 | X | Moderate | ||||
| Berridge & Robinson, 2006 | Book Chapter | Commentary | Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | X | X | High | ||
| Bhaskar & Kumar, 2014 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X |
High | ||||
| Borsari et al., 2009 1 | Journal article | Lit. Review | Etiology | Young Adults or College Students | NR | NR | NR | Humans | Yes | 1999 | 2009 | X | High | ||||
| Brady & Back, 2012 | Journal article | Lit. Review | Epidemiology, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Brady & Sonne, 1999 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | 3 | X | X | High | ||
| Buisman-Pijlman et al., 2014 1 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | X | High | |||
| Cablov et al., 2014 | Journal article | Lit. Review | Theory, Etiology | Children, Adolescents | NR | NR | NR | Humans | Yes | 1995 | 2012 | X | High | ||||
| Cao et al., 2013a | Journal article | Meta-Analysis | Genetics | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| Cao et al., 2013b | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2012 | X | High | ||||
| Chen et al., 2012 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | Asian or Asian American, European American or Caucasian | NR | Humans | Yes | M | 2011 | X | High | ||||
| Clarke et al., 2012 | Journal article | Lit. Review | Genetics, Etiology | Adolescents | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | 3 | X | High | |||
| Collins, 1990 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Colrain & Baker, 2012 | Journal article | Commentary | Intermediate phenotype/endophenotype, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Cooper, 1983 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Davis & Tunks, 1991 | Journal article | Lit. Review | Etiology, Course | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Dew et al., 2007 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Dick et al., 2017 | Journal article | Lit. Review | Epidemiology, Genetics | NR | NR | African American | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Doria, 1995 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Du et al., 2011 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2009 | X | High | ||||
| El-Guebaly, 1995 | Journal article | Lit. Review | Epidemiology, Etiology, Course | NR | Women | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Elliott et al., 2012 | Journal article | Meta-Analysis | Genetics, Etiology | Young Adults or College Students | NR | NR | NR | Humans | Yes | M | 2010 | X | High | ||||
| Ellis et al., 1997 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X2 | X | High | |||
| Ezard, 2012 | Journal article | Lit. Review | Epidemiology, Etiology | NR | NR | NR | NR | Humans | Yes | 1950 | 2010 | X | High | ||||
| Feinn et al., 2005 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| Fillmore et al., 1993 | Journal article | Meta-Analysis | Course | NR | NR | NR | NR | Humans | No | NA | NA | X | High | ||||
| Freed, 2010 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Galizio & Maisto, 1985 1 | Book Chapter | Lit. Review | Theory, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Goodwin, 1976 1 | Book Chapter | Lit. Review | Course, Clinical presentation or diagnosis | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Guerrini et al., 2014 | Journal article | Lit. Review | Genetics, Etiology | Adolescents | NR | NR | NR | Humans | No (NA) | NA | NA | X | X | X | High | ||
| Hall, 1990 | Journal article | Lit. Review | Theory, Etiology | NR | Women | NR | Lesbian | Humans | No | NA | NA | X | X | High | |||
| Heath et al., 2011 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Heath, 1995 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Herold & Conlon, 1981 1 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | X | High | |||
| Hesselbrock et al., 2013 | Journal article | Lit. Review | Genetics, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Hill, 1985 | Journal article | Lit. Review | Theory, Clinical presentation or diagnosis | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Hill, 1995 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics, Etiology | NA | Women | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Ho et al., 2018 1 | Journal article | Lit. Review | Etiology, Course | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | X | High | |||
| Israelstam & Lambert, 1983 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | Not heterosexual | Humans | No (NA) | NA | NA | X | High | ||||
| Jackson et al., 2014 | Journal article | Lit. Review | Etiology | Adolescents | NR | NR | NR | Humans | Yes | 1806 | 2014 | X | High | ||||
| Jacob & Johnson, 1997 | Journal article | Lit. Review | Etiology | Children, Adolescents | NR | NR | NR | Humans | No (NA) | NA | NA | X2 | 3 | High | |||
| Jacob, 1992 | Journal article | Lit. Review | Etiology, Course | NR | NR | NR | NR | Humans | No | NA | NA | X | High | ||||
| Johnson et al., 2006 1 | Journal article | Symposium Proceedings | Etiology, Clinical presentation or diagnosis | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| King et al., 2011 | Journal article | Commentary | Intermediate phenotype/endophenotype, Etiology | NR | NR | NR | NR | Humans | No | NA | NA | X |
High | ||||
| Koob & Volkow, 2016 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Rodents, Humans | Yes, NSTD | M | M | X | X | X | X2 | High | |
| Kuhn, 2011 1 | Journal article | Lit. Review | Etiology | NR | Women | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Kuntsche et al., 2015 | Journal article | Lit. Review | Etiology, Course | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| Lettieri, 1985 | Book Chapter | Lit. Review | Theory, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | X | X | X2 | High | |
| Luczak et al., 2006 | Journal article | Meta-Analysis | Intermediate phenotype/endophenotype, Genetics | NR | NR | Asian or Asian American | NR | Humans | Yes | 1966 | 2005 | X | High | ||||
| Ma et al., 2017 a | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1995 | 2015 | X | High | ||||
| Magid & Moreland, 2014 | Journal article | Lit. Review | Etiology | Adolescents | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Maimaris & McCambridge,2014 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | Yes | M | 2013 | X | High | ||||
| Malouff et al., 2008 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | Yes | M | 2007 | X | High | ||||
| Marlatt et al., 1988 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | NR | Humans | No (NA) | 1980 | 1987 | X | High | ||||
| McGue, 1997 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Monteiro & Schuckit, 1988 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics, Etiology | Young Adults or College Students | Men | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Morean & Corbin, 2010 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Etiology | NR | NR | NR | NR | Humans | No (NA) | 1990 | M | X | High | ||||
| Munafo et al., 2007 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1990 | 2006 | X | High | ||||
| Murphy et al., 2012 | Journal article | Lit. Review | Etiology | Adults | NR | NR | NR | Humans | Yes | 1991 | 2011 | X | High | ||||
| Noble, 1998 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X3 | 2 | High | |||
| Onuoha et al., 2016 | Journal article | Meta-analysis | Etiology | Adults | NR | NR | NR | Humans | Yes | 1990 | 2015 | X | High | ||||
| Oo et al., 2016 | Journal article | Meta-Analysis | Genetics, Etiology | Adults, young adults or college students, middle-aged adults, older adults | NR | NR | NR | Humans | Yes | 1996 | 2009 | X | High | ||||
| Ouzir & Errami, 2016 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X2 | X | X | High | ||
| Panitz et al., 1983 | Journal article | Lit. Review | Etiology | NR | Men | Latino or Hispanic | NR | Humans | No | NA | NA | X | Moderate | ||||
| Pohorecky, 1991 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Polcin, 1997 | Journal article | Lit. Review | Etiology, Clinical presentation or diagnosis | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X2 | 3 | High | |||
| Preuss et al., 2012 1 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | X | High | |||
| Prom-Wormley et al., 2017 | Journal article | Lit. Review | Epidemiology, Genetics | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Quinn & Fromme, 2011 | Journal article | Meta-Analysis | Intermediate phenotype/endophenotype, Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| Reilly et al., 2017 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | X | X | X2 | High | |
| Rew, 1989 | Book Chapter | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | 3 | X2 | High | |||
| Robinson & Berridge, 1993 | Journal article | Lit. Review | Theory, Etiology | NR | NR | NR | NR | Rodents, Primates, Humans | No (NA) | NA | NA | X | High | ||||
| Roche et al., 2015 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | Yes, NSTD | 1990 | 2012 | X | High | ||||
| Rossow et al., 2016 | Journal article | Lit. Review | Etiology | Children, Adolescents | NR | NR | NR | Humans | Yes | 1980 | 2013 | X | High | ||||
| Sadava, 1980 | Journal article | Lit. Review | Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Salvatore et al., 2015 1 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics | NR | NR | NR | NR | Rodents, Fruit Flies, Humans | No (NA) | NA | NA | X | X | High | |||
| Schacht et al., 2013 | Journal article | Meta-Analysis | Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| Schneeberger et al., 2014 | Journal article | Lit. Review | Etiology, Course | Adults | NR | NR | Sexual minorities | Humans | Yes | 1990 | 2013 | 3 | X2 | High | |||
| Schulsinger, 1980 | Journal article | Lit. Review | Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Schulte et al., 2009 1 | Journal article | Lit. Review | Etiology | Adolescents, young adults or college students | NR | NR | NR | Humans | No (NA) | NA | NA | X2 | 3 | High | |||
| Scott, 2017 | Journal article | Commentary | Genetics, Etiology | NA | NA | Diverse racial/ethnic groups | NA | Humans | No (NA) | NA | NA | X | High | ||||
| Serrano & Parsons, 2011 1 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Rodents, Primates, Humans | No (NA) | NA | NA | X | X | High | |||
| Serre et al., 2015 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Genetics, Course, Clinical presentation or diagnosis | NR | NR | NR | NR | Humans | Yes | 1996 | 2013 | 3 | X | X | High | ||
| Sher et al., 2010 1 | Journal article | Lit. Review | Genetics, Etiology, Course | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Sillaber & Henniger, 20041 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Sloan et al., 2008 | Journal article | Lit. Review | Genetics, Etiology | NA | NA | NA | NA | Rodents, Humans | NA | NA | NA | X | High | ||||
| Smith et al., 2008 | Journal article | Meta-analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2006 | X | High | ||||
| Stautz & Cooper, 2013 | Journal article | Meta-Analysis | Etiology | Adolescents | NR | NR | NR | Humans | Yes | M | 2013 | X | X | X | High | ||
| Steinhausen, 1995 | Journal article | Lit. Review | Etiology | Children | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Stewart, 1996 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Tarter & Edwards, 1988 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Tarter et al., 1985 | Journal article | Lit. Review | Etiology | NR | Men | NR | NR | Humans | No (NA) | NA | NA | X2 | X |
X2 | High | ||
| Tawa et al., 2016 1 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 2005 | 2016 | X | High | ||||
| Thacker et al., 1984 | Journal article | Lit. Review | Epidemiology, Genetics | NR | NR | NR | NR | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Thatcher & Clark, 2008 1 | Journal article | Lit. Review | Intermediate phenotype/endophenotype, Etiology | Adolescents | NR | NR | NR | Humans | No (NA) | NA | NA | X | X | High | |||
| Thombs & Osborn, 2013 1 | Book Chapter | Lit. Review | Theory, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | X2 | X2 | High | ||
| Topel, 1988 | Journal article | Lit. Review | Genetics, Etiology | NA | NA | NA | NA | Rodents, Humans | No (NA) | NA | NA | X | High | ||||
| Trice & Sonnenstuhl, 1988 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Velleman, 1992 | Journal article | Lit. Review | Etiology | NR | NR | NA | NA | Humans | No | NA | NA | X | High | ||||
| Villalba et al., 2015 | Journal article | Meta-analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | High | ||||
| West, 2005 1 | Book Chapter | Lit. Review | Theory, Etiology | NA | NA | NA | NA | Humans | No (NA) | NA | NA | X | X | X | X2 | High | |
| Wiles et al., 2007 | Journal article | Lit. Review | Etiology | Children, Adolescents | NR | NR | NR | Humans | Yes | 1806 | 2005 | X | High | ||||
| Wilson & Elston, 1995 1 | Book Chapter | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | High | ||||
| Wilson et al., 2017 | Journal article | Meta-Analysis | Etiology | NR | NR | NR | NR | Humans | Yes | M | 2015 | X | High | ||||
| Wimer & Wimer, 1985 | Journal article | Lit. Review | Animal models, Etiology | NA | NA | NA | NA | Rodents, Primates, Fruit Flies, Canines | No (NA) | NA | NA | X | High | ||||
| Worby & Organista, 2007 | Journal article | Lit. Review | Epidemiology, Etiology | NR | Men | Latino or Hispanic | NR | Humans | Yes | 1985 | 2007 | X | High | ||||
| Xiao et al., 2015 | Journal article | Meta-Analysis | Etiology | NR | NR | NR | NR | Humans | Yes | 2000 | 2014 | X | X | X2 | High | ||
| Yap et al., 2017 | Journal article | Meta-Analysis | Etiology | Adolescents | NR | NR | NR | Humans | Yes | 1986 | 2016 | X | High | ||||
| Young-Wolff et al., 2011 | Journal article | Lit. Review | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2010 | X | X | High | |||
| Zintzaras, 2012 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 2004 | 2010 | X | High | ||||
|
| |||||||||||||||||
| Reviews Excluded Due to Low Quality | |||||||||||||||||
|
| |||||||||||||||||
| Barry, 2007 | Journal article | Lit. Review | Epidemiology, Theory, Etiology | Young Adults or College Students | NR | NR | NR | Humans | Yes | 1984 | 2003 | X | X | X | Critically low | ||
| Brennan et al., 1986 | Journal article | Lit. Review | Etiology, Course | Young Adults or College Students | NR | NR | NR | Humans | Yes, NSTD | 1953 | 1984 | X | X | X | Critically low | ||
| Bühler & Mann, 2011 | Journal article | Lit. Review | Etiology | NR | NR | NR | NR | Humans | Yes | 1975 | 2009 | X |
Low | ||||
| Field et al., 2009 | Journal article | Meta-Analysis | Theory | NR | NR | NR | NR | Humans | Yes, NSTD | 1993 | 2008 | X | Low | ||||
| Forero et al., 2015 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | Low | ||||
| Hong et al., 2011 | Journal article | Lit. Review | Etiology | Adolescents | NR | South Korean | NR | Humans | Yes | 1980 | 2010 | X | Critically low | ||||
| Hummel et al., 2012 | Journal article | Lit. Review | Etiology | Adolescents | NR | NR | NR | Humans | Yes | M | 2012 | X | Low | ||||
| Kebir et al., 2011 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | European American or Caucasian | Humans | Yes | 1990 | 2010 | X | Low | ||||
| Kuhn & Gallinat, 2011 | Journal article | Meta-Analysis | Etiology | NR | NR | NR | NR | Humans | Yes | M | 2010 | X | Critically low | ||||
| Li et al., 2011 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | M | X | Critically low | ||||
| McHugh et al., 2010 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | M | 2009 | X | Low | ||||
| Mulligan et al., 2006 | Journal article | Meta-Analysis | Genetics, Animal models | NA | NA | NA | NA | Rodents | No | NA | NA | X | Low | ||||
| Noori et al., 2014 | Journal article | Meta-Analysis | Animal models | NA | NA | NA | NA | Rodents | Yes | M | M | X | Critically low | ||||
| Pan et al., 2013 | Journal article | Meta-Analysis | Intermediate phenotype/endophenotype, Genetics | NR | NR | NR | NR | Humans | No (NA) | NA | NA | X | Critically low | ||||
| Polich et al., 1994 | Journal article | Meta-analysis | Etiology | NR | Men | NR | NR | Humans | Yes | M | 1993 | X | Low | ||||
| Pollock et al., 1987 | Journal article | Meta-Analysis | Etiology | NR | NR | NR | NR | Humans | Yes, NSTD | 1930 | 1985 | X | Critically low | ||||
| Rozin & Zagonel, 2012 | Journal article | Lit. Review | Etiology | Adolescents | NR | NR | NR | Humans | Yes | 2000 | 2009 | X | Low | ||||
| Schepis et al., 2011 | Journal article | Lit. Review | Etiology | Children, Adolescents | NR | NR | NR | Humans | Yes | M | M | X | Low | ||||
| Verhulst et al., 2015 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes, NSTD | M | M | X | Critically low | ||||
| Walters, 2002 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1970 | 2000 | X | Low | ||||
| Wang et al., 2011 | Journal article | Meta-analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1990 | 2012 | X | Low | ||||
| Wang et al., 2013 | Journal article | Meta-analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | No | NA | NA | X | Low | ||||
| Whitfield, 1997 | Journal article | Meta-analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | Yes | 1990 | 1996 | X | Critically low | ||||
| Zuo et al., 2014 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Rodents, Humans | Yes | M | M | X | Low | ||||
| Zuo et al., 2015 | Journal article | Meta-Analysis | Genetics, Etiology | NR | NR | NR | NR | Humans | No | NA | NA | X | Low | ||||
Note. AARDoC = Alcohol Addiction Research Domain Criteria. Lit. Review = Lit. Review. NR = no restrictions; NA = not applicable; M = missing; NSTD = no search terms described.
= Ma et al., 2016 has an erratum (Ma et al., 2017) which was incorporated into the conclusions reported in this systematic review of reviews.
= review was added as a result of forward/backward searching;
= review was not initially coded into this domain, but upon further inspection by the first (CB) and third (KS) authors was added;
= review was initially coded into this domain, but upon further inspection by the first (CB) and third (KS) authors was removed.
Data Extraction and Coding
Eligible studies were coded using a data extraction sheet developed by the first author. The sheet included items on review characteristics (e.g., year, type of review, main theory/concept); methods (e.g., inclusion criteria, search strategy, number of primary studies included); outcome data (e.g., RDoC matrix units of analysis to consider, AARDoC/ANA construct, summary of results); assessment of quality (e.g., assessment of primary study quality by authors and assessment of review quality by coders [described below]); and coder characteristics (i.e., coder name, date, and duration of coding). The full codebook is available here: https://osf.io/xz83s/.
The coding sheet was pilot tested on 10 randomly selected eligible reviews and refined accordingly through consultation with coauthors. Trained research assistants (RAs) were responsible for extracting data from included reviews and each review was coded by two RAs. Discrepancies were resolved through weekly meetings with the first author until consensus was reached. Missing information was expected in this context and was accounted for using codes such as “not reported” (NR) and “not applicable” (NA; see Table 1).
Quality of Reviews
All reviews were subjected to Cooper’s (2015) quality assurance checklist to evaluate the methodological quality of each eligible review. Although there exist a range of possible systems for coding the methodology of research syntheses (e.g., the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA; Moher et al., 2009]), Cooper’s checklist is the only system developed with behavioral science research synthesis in mind (Cooper & Koenka, 2012). The Cooper (2015) system presents 20 questions evaluating several relevant domains of systematic reviews (e.g., formulating the problem, searching the literature, evaluating the quality of studies, and interpreting the evidence) which are answered “yes” or “no,” with “yes” indicating more rigorous methods were used (Cooper & Koenka, 2012).
This system is not intended to produce an overall score based on a count of items for which a response of “yes” is indicated. In fact, researchers generally agree that the practice of summing across the dimensions is inappropriate as it may disguise critical weaknesses while still resulting in an adequate sum score. Cooper and Koenka (2012) point out that comparing single scores derived from different quality rating systems can result in conflicting scores simply because the systems have different foci. Therefore, we did not compare overall sum scores for agreement. Instead, each rater indicated their overall confidence in the results of the review based on Cooper’s checklist. Overall confidence ratings were adapted from AMSTAR2 (Shea et al., 2017) and included: critically low, low, moderate, and high. The use of this system required identification of critical domains (based on the goals of the current systematic review), which was detailed in the study protocol. Identification of weaknesses in these areas, therefore, undermined confidence in the results of the review. All eligible reviews were double coded by trained RAs and discrepancies in overall ratings were resolved through consensus.
Any review coded as critically low or low was reviewed by the first author (regardless of whether or not there were coding discrepancies) to ensure that excluded reviews suffered from flaws in critical domains. In some cases, we contacted authors in an attempt to acquire or clarify information with the goal of trying to improve the accuracy of review’s overall quality rating. Three research teams were contacted. Two responded promptly with the information requested and this additional information was considered in their quality ratings, whereas the third reported they did not have the information requested. Of the 144 eligible reviews, 15 (10.42%) were rated as having “critically low” quality and 10 (6.90%) were rated as having “low” quality. This resulted in 25 total reviews being excluded at this stage (see Table 1).
Integration
Final reviews were integrated and used to define and outline an AARDoC/ANA-informed conceptual framework of AUD (see Table 1 for a final list of reviews considered at this stage). Based on the reviews included, superdomains, domains, subdomains, and specific components or mechanisms for each of the subdomains were identified. Specific superdomains, domains, subdomains, and components or mechanisms were only retained for inclusion if there was robust evidence of such components as etiologic mechanisms. In instances where it was unclear from the current review how robust the evidence was for a given mechanism, additional literature was consulted, and areas of further research were noted. Cases in which it was unclear where a particular finding fit into the domains were discussed among colleagues and expert consultants.
Additionally, although all studies of adequate quality listed in Table 1 were considered eligible, not all were retained for final integration because: a) the findings were supplanted by more recent work or critical revisions (e.g., Hill, 1985 was supplanted by Koob & Volkow, 2016 [described in detail below]; in these cases the later findings and concepts were relied on and/or the discrepancy was explicitly highlighted), b) the results were not robust enough (e.g., due to methodological concerns not fully captured by Cooper’s checklist or failure to replicate) to further consider the construct as a core mechanism (e.g., Onuoha et al., 2016), or c) upon further inspection, the review’s focal construct tended to be more of a broad risk factor (e.g., early life stress; Schneeberger et al., 2014) rather than an etiologically-relevant mechanism with demonstrated biological substrates (e.g., altered hypothalamic-pituitary adrenal axis responses; Sinha et al., 2009).
Results and Discussion
The results of the current systematic review of reviews suggested that the ANA in its current form is a useful starting point for the articulation of a dimensional, translational, research-based AUD framework. Specifically, it provides concrete suggestions regarding the neuroscience-based domains most relevant to AUD, which are largely supported by work from other experts in addiction (i.e., Yücel et al., 2019). In the sections that follow, we integrate our findings across the ANA domains of cognitive control, reward, and negative emotionality.
At the same time, in our systematic review of reviews, it became clear that several components and mechanisms identified through the current systematic review of reviews did not correspond with any of the three ANA domains. Thus, we included one additional domain, compulsivity, which is organized hierarchically. We partition each domain into subdomains that contain components and mechanisms. Notably, the ETOH Framework extends ANA’s negative emotionality to also include negative valence and bifurcates cognitive control into impulsivity and compulsivity. We also introduce two critical constructs that are thought to moderate the expression of the ETOH domains but are not fundamental AUD constructs: opponent process and self-awareness. The resulting framework is a fine-grained, hierarchical conceptualization of etiologic mechanisms implicated in AUD and other substances. A comparison of the ETOH Framework’s major components and those of other models of addiction and psychopathology is offered in Table 3.
Table 3.
Overview of Associations between the ETOH Framework and Other Models of Addiction and Psychopathology
| ETOH Framework | ANA/AARDoC | PhAB | RDoC | HiTOP | |||
|---|---|---|---|---|---|---|---|
| Superdomain | Domain | Subdomain | Domain | Construct (Subconstruct) | Spectrum (Subfactor) | ||
| Cognitive Control | Impulsivity | Conscientiousness | Cognitive Control/Executive Function | Cognition/Executive Function | Cognitive Systems | Cognitive Control | Disinhibited Externalizing |
| Response Inhibition | |||||||
| Compulsivity | Compulsive Use | -- | -- | ||||
| Reward | -- | Habit | -- | Reward/Incentive Salience | Positive Valence | Reward Learning | -- |
| -- | Positive Expectancies | -- | Reward Responsiveness (Reward Anticipation) | Disinhibited Externalizing | |||
| -- | Reward Sensitivity | -- | Reward Responsiveness | Disinhibited Externalizing | |||
| -- | Positive Emotionality | -- | Reward Responsiveness (Initial Response to Reward) | Detachment (−), Internalizing (Distress) | |||
| -- | Incentive Salience | Inventive Salience | Reward Satiation | -- | |||
| -- | Reward Discounting | Cognitive Control/Executive Function | Reward Valuation | -- | |||
| Negative Valence & Emotionality | -- | Negative Emotionality | Negative Emotionality | Negative Emotionality | Negative Valence | Acute Threat, Potential Treat, Sustained Threat, Loss, Frustrative Nonreward | Internalizing |
| Coping | Negative Emotionality | ||||||
| -- | Punishment Sensitivity | -- | |||||
| Negative Expectancies | -- | ||||||
Note. ETOH Framework = Etiologic, Theory-Based, Ontogenetic Hierarchical Framework; AARDoC = Alcohol Addiction Research Domain Criteria; ANA = Addictions Neuroclinical Assessment; PhAB = Phenotyping Assessment Battery; RDoC = Research Domain Criteria; HiTOP = Hierarchical Taxonomy of Psychopathology.
As we noted, consistent with a hierarchical conceptualization, we also outline domains and subdomains subordinate to each superdomain. Subdomains are included for the sake of comprehensiveness and to provide a crosswalk among terms that are used in different literatures, but subdomains are likely overlapping in many cases. We organized ETOH components hierarchically, because it is increasingly clear that psychopathology more generally is organized in this manner (e.g., Krueger et al., 2018; Krueger & DeYoung, 2016). It is important to note that we view this framework as evolving along with the state of the literature and that it will likely be refined with time. To facilitate the ETOH Framework’s refinement, an open commentary space where readers can provide feedback has been created: https://osf.io/t5jqm/.
Figure 2 displays the ETOH Framework. Of note, it includes several dashed lines that indicate possible cross-loadings between domains (each are described in more detail later). For example, the habit subdomain is encompassed by the reward domain, but there is also evidence for the relationship between habit and the compulsivity domain. In some cases, there exists empirical evidence for the overlap and, in other cases, the dashed lines represent hypothesized cross-loadings that we advanced because we think they warrant further attention.
Figure 2.
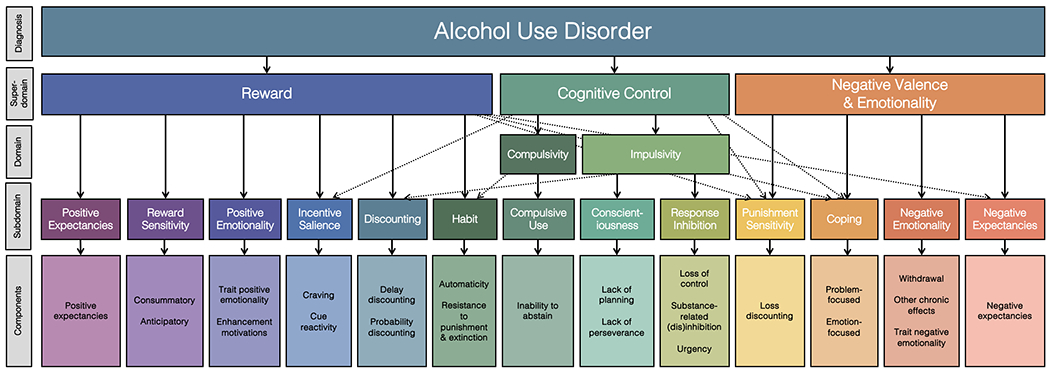
The Etiologic, Theory-Based, Ontogenetic Hierarchical Framework for Alcohol Use Disorder Diagnosis
Note. This figure depicts a visual of the ETOH Framework derived as a result of a systematic review of reviews on alcohol use disorder etiology. It is intended to be iterative in nature and is, therefore, subject to updates as the literature develops further. The dashed lines indicate possible cross-loadings between domains. In some cases, components incorporate fine-grained facets that are described in text.
Reward Superdomain
As previously described, ANA defines the reward domain broadly as encompassing the processes that transform otherwise neutral stimuli or events (e.g., cues) into attractive and wanted stimuli. Importantly, reward valuation was considered the most relevant construct for vulnerability to addictions by a group of addiction experts in a recent consensus study (Yücel et al., 2019) and AUD has been considered a “reward deficiency syndrome” by others (e.g., Bowirrat & Oscar-Berman, 2005). The reviews in the current synthesis that constitute the “reward” superdomain describe at least one of six subdomains: habit, positive expectancies, reward sensitivity, positive emotionality, incentive salience, and reward discounting. Notably, this reflects an extension of the ANA’s conceptualization of reward, which mostly encompasses incentive salience, and broadly corresponds with RDoC’s positive valence systems domain. In this way, our “reward” domain is broader than that of ANA, inasmuch as ANA’s coverage of RDoC’s positive valence systems is limited to the construct of reward valuation. The ETOH Framework encompasses reward valuation as well as most other RDoC positive valence systems constructs (see Table 3).
Reward: Habit
Habit describes a consequence of reward learning whereby alcohol or drug use becomes more ingrained and automatic (Corbit & Janak, 2016) and less susceptible to voluntary control and decision making (Hogarth, 2020). Although habit is a function of stimulus-response reward learning, once habits are established, they can become functionally autonomous from reward value and therefore not goal directed (Belin et al., 2013). In individuals diagnosed with AUD, habit indicates that continued use can occur after alcohol is no longer rewarding or desirable, such that individuals continue to drink sometimes even without conscious awareness (Ray, 2012). Several reviews point to the importance of habit as a relevant reward-related mechanism (Berridge & Robinson, 2006; Lettieri, 1985; Reilly et al., 2017; Xiao et al., 2015). Habit has identifiable neurobiological underpinnings in the dorsal striatum, which is implicated in reward processing (Everitt et al., 2008).
Habit includes more specific processes, such as automaticity (Johnson et al., 2006; West, 2005) and resistance to punishment and extinction (Robinson & Berridge, 1993), which are relevant to several substances (e.g., cocaine) in addition to alcohol (Everitt et al., 2008; Everitt & Robbins, 2016). As such, these are described as substance-general within the current framework. The categorization of habit under the reward domain (which corresponds to RDoC’s Positive Valence System) is also consistent with the findings of previous work that used expert consensus to delineate the “primary” RDoC constructs most relevant to substance and behavioral addictions (Yücel et al., 2019).
Although we consider habit primarily a reward-related mechanism, habit is likely also a precursor to compulsion. Research suggests that it is the pairing of habit and drug-induced aberrant motivational processes (e.g., incentive salience to drug cues), resulting in an “incentive habit process,” that facilitates the transition from habit to compulsion (Belin et al., 2013). Indeed, Everitt and Robbins (2016) refer to habits as “the building blocks of compulsive drug seeking” (p. 24). Consequently, we believe habit, although primarily falling under the reward domain, may also cross-load on compulsivity (see Figure 2).
Importantly, the acquisition of habit requires consumption. That is, without stimulus-response learning, habit is unlikely to develop. Therefore, repeated consumption, or drug-taking more generally, is a necessary condition for habit as an etiologic mechanism in AUD. Additionally, habit may serve as a maintenance mechanism whereby habit maintains consumption and AUD.
We recognize that there is significant debate about the role of habit and habitual responding in addiction (e.g., some literature has suggested that evidence for habit can instead be explained by general task disengagement driven by cognitive impairment [Hogarth, 2020]), and we recognize that not all drug use is habit-based (e.g., Robinson & Berridge, 2008). However, we choose to retain habit in the ETOH framework for several reasons. First, there is considerable evidence for the role of habit in drug seeking within animal models of addiction (e.g., Lüscher et al., 2020). Similar findings in humans are lacking to date (see Hogarth, 2020), for which there may be several explanations. For example, the shift to habitual responding may be more complicated among humans compared with animals (e.g., due to environmental contingencies), making it difficult to model experimentally in the laboratory. Some research suggests that human paradigms test drug taking, rather than drug seeking, which is problematic because most animal research has focused on drug seeking and these two behaviors likely have different underlying neurobiological processes (see Lüscher et al., 2020). Also, there may be important between-individual differences in the vulnerability towards habitual responding which may not be fully accounted for by current behavioral paradigms. For example, humans may need longer exposure to the stimulus-outcome associations or more training than what is common among current human studies of habit. Second, there is strong evidence in humans for the role of habit in the cases of binge eating disorder (Voon et al., 2015) and obsessive-compulsive disorder (Gillan et al., 2016; Voon et al., 2015), pointing to its relevance as a transdiagnostic process. Other research suggests that habitual responding is relevant to other SUDs, including stimulant use disorder (Voon et al., 2015; Lüscher et al., 2020) and nicotine use disorder (Luijten et al., 2020; Piasecki et al., 2011). However, given habitual and goal-directed processes likely operate in parallel (Wood & Rünger, 2016), it remains unclear whether it is the development of habitual processes or the failure of goal-directed processes that tips the balance to habitual responding (Gillian et al., 2015; Luijten et al., 2020). Because of the relevance of habit in other SUDs and psychopathology more generally, we retain habit as a relevant mechanism in the ETOH framework but also emphasize that more research is needed at this point in time to further clarify the role of habit in the development and maintenance of AUD among humans.
Reward: Positive Expectancies
The general construct of expectancies describes cognitive schema regarding the anticipated outcome(s) of a given behavior or action (e.g., West, 2005). Alcohol- or substance-related outcome expectancies refer to what one expects to happen as a result of their substance use (Lettieri, 1985; Schulte et al., 2009; Thombs & Osborn, 2013). That is, what affective, cognitive, physiological, and behavioral effects does one expect as a result of their use? Expectancies can be learned through direct pharmacological experience, social learning including interpersonal modeling (e.g., parents and peers), and mass media, among other factors (Ellis et al., 1997; Goldman et al., 1991; Jacob & Johnson, 1997; Thombs & Osborn, 2013). There is additional evidence that expectancies are heavily influenced by personality (e.g., McCarthy et al., 2001), suggesting they might not result solely from learning but that personality traits might exert influence upon relevant psychosocial learning mechanisms (see Smith and Anderson’s [2001] Acquired Preparedness model of drinking risk).
Goldman and colleagues (1999) categorize expectancies along three basic dimensions: (a) positive versus negative expected outcomes (e.g., increased sociability versus increased aggressiveness); (b) positive versus negative reinforcement (e.g., social facilitation versus tension reduction); and (c) arousal versus sedation (e.g., stimulant versus depressant effects). In this section, we concentrate solely on positive outcome expectancies, which are focused on the expectation regarding how positive a given outcome will be. Examples of positive expectancies include pleasant feelings and enhancement (e.g., Alcohol will make me feel more sociable, and I will enjoy drinking) as well as the alleviation or avoidance of negative states such as emotional distress, pain, and withdrawal (e.g., Alcohol will alleviate my pain and alcohol will decrease my anxiety; Thombs & Osborn, 2013). Therefore, a positive outcome expectancy can be related to either positive or negative reinforcement.
Research has shown consistently that positive expectancies related to positive reinforcement are associated with moderate and heavy drinking (e.g., Kuntsche et al., 2005) as well as frequency of consumption (Cho et al., 2019). In comparison, positive expectancies related to negative reinforcement are more strongly associated with risk of alcohol-related problems and dependence (Cho et al., 2019). Findings are consistent with research suggesting that addiction proceeds through several stages, beginning with positive reinforcement and transitioning to negative reinforcement with repeated use whereby individuals are drinking to alleviate distress associated with abstinence and withdrawal (e.g., Koob & Volkow, 2010). As such, positive outcome expectancies related to positive reinforcement (e.g., Alcohol will help me feel more sociable) are likely more relevant during the earlier stages of drinking, and as an individual transitions to the later stages of addiction, such positive expectancies may become more related to negative reinforcement (e.g., Alcohol will decrease my anxiety). It is worth considering whether positive expectancies related to negative reinforcement may be overlapping with other mechanisms (e.g., negative emotionality, coping), given that an individual may drink to avoid or alleviate negative mood states (e.g., Tension-Reduction Theory) which in itself may be rewarding.
Positive expectancies in early adulthood predict alcohol-relevant outcomes, such as initiation of alcohol use and quantity of consumption in adulthood (Anderson et al., 2013). Indeed, children of “alcoholics,” who are known to be at higher risk of AUD themselves, tend to have more positive expectancies about the reinforcing effects of alcohol (i.e., are more likely to expect that alcohol will make them feel good; Ellis et al., 1997) compared to individuals without a family history of AUD. Thus, there is consistent evidence for positive expectancies as an etiologic factor in AUD (especially those related to negative reinforcement) within the current review (Schulte et al., 2009) and the larger literature, although this relationship may be complex and influenced by other more distal factors (e.g., personality; Settles et al., 2010).
Positive expectancies are also implicated in other types of substance use and dependence. For example, heavy smokers tend to report more positive expectancies about the effects of smoking compared to less heavy smokers (e.g., Copeland et al., 1995; Wetter et al., 1994), and positive expectancies, especially those related to negative reinforcement, are associated with withdrawal severity and decreased likelihood of cessation (Wetter et al., 1994). Over the course of smoking cessation treatment, expectancies become less positive, particularly among abstainers (Copeland et al., 1995). Positive expectancies appear to reflect a more substance-general mechanism in the development and maintenance of SUDs, although research with other substances beyond alcohol and nicotine are needed (see Kouimtsidis et al., 2014 for preliminary work in this area). Taken together, the previously noted research points to expectancies as a relevant mechanism within in the current framework, although the exact influence of expectancies on consumption and SUDs may be complex.
Reward: Reward Sensitivity
Reward sensitivity describes the tendency to detect, pursue, learn from, and experience pleasure from positive stimuli (Koob & Volkow, 2016). It is conceptualized as a component of temperament and personality (Gray, 1970; Gray, 1982), with individual differences in reward sensitivity ostensibly arising from neurobiological mechanisms (e.g., dopamine D2 receptor levels) that give rise to physiologic and affective or emotional experiences (Stephens et al., 2010). Reward sensitivity can be further broken down into anticipatory and consummatory mechanisms. These are typically described as components of pleasure (Gard et al., 2006) but have also been described as motivational states (Joseph et al., 2015). Anticipatory pleasure is defined as the forecasting of, and likely pursuit of, pleasure for a given a positive stimulus. It is related to motivation and goal-directed behavior and leads to an individual wanting more of something. As such, anticipatory pleasure may also be related to positive outcome expectancies. In comparison, consummatory pleasure is defined as the experience of pleasure from a desired positive stimulus and is linked to satiation or a reduction in desire (Klein, 1984). Research has found that anticipatory and consummatory pleasure have distinguishable neurocircuitry and neurotransmitter systems which, in both cases, are found in regions typically related to reward. Anticipatory pleasure is linked to dopamine and the mesolimbic pathway, whereas consummatory pleasure is linked to serotonergic and opioid systems (Berridge & Robinson, 1998; Schultz, 2002; Wise, 2002).
We view anticipatory and consummatory mechanisms as relevant to reward sensitivity given that one or both are likely dysregulated in addiction. For example, individuals diagnosed with a SUD may want more alcohol (anticipatory) and have difficulty feeling satiated (consummatory). Robinson and Berridge (1993) distinguished anticipatory and consummatory experiences in terms of “wanting” and “liking,” respectively, by drawing on nonhuman animal models. “Wanting” a drug is akin to anticipatory pleasure and “liking” a drug is akin to consummatory pleasure. As one transitions into problematic substance use, they shift from “liking” to “wanting,” or experience a compulsive desire to use (“wanting”) even when the substance stops being pleasurable (“liking”). Individuals diagnosed with a SUD experience a shift from “liking” to “wanting” for both specific substance(s) and other, more general natural reinforcers (e.g., food, sex, money).6 This change demonstrates a shift (neuroadaptation) in natural reward processes among those diagnosed with a SUD whereby there is a narrowing of one’s response towards the substance and away from other natural reinforcers (Koob & Volkow, 2016; Ouzir & Errami, 2016).
Given the evidence for shifting reward processes in the development of addiction, it is unsurprising that reward sensitivity is a key component to many other addiction theories and models. Several behavioral neuroscience theories posit that changes in reward mechanisms as a consequence of substance use are what underlie the development of addiction (Bhaskar & Kumar, 2014; Koob & Le Moal, 2001; Koob & Volkow, 2016; Nestler, 2005; Robinson & Berridge, 2003). Robbins and Everitt’s (1999) theory of addiction supposes that neural circuits underlying the encoding of natural rewards are “hijacked” as a result of chronic use of substances, resulting in lasting neuroadaptations associated with addiction that results in decreased sensitivity to rewards. Also, the reward deficiency hypothesis proposes that addiction results from a predisposition towards blunted neural response to reward (Topel, 1988) and the addiction-to-pleasure theory proposes that addiction results from a conditioned need for pleasure, which causes future pleasure-seeking (Lettieri, 1985). Reward sensitivity is therefore widely recognized as central to AUD.
Although a large majority of prominent addiction theories tend to focus on reward sensitivity in terms of a chronic adaptation, there is a literature suggesting that reward sensitivity is also a premorbid risk factor for AUD. According to Reinforcement Sensitivity Theory, response sensitivity is an individual difference construct whereby individuals with high reward sensitivity are especially sensitive to rewarding stimuli (Gray, 1970, 1982), such as alcohol. Research on adolescents and young adults supports the association between high reward sensitivity and increased use (O’Connor & Colder, 2005; van Hemel-Ruiter et al., 2015). Further, high reward sensitivity predicts reactivity to alcohol cues (Kambouropoulos & Staiger, 2001), consistent with the mechanism of incentive salience as described later. As consumption becomes chronic, sensitivity to natural reinforcers decreases and alcohol and other substances are used to compensate for this deficit, thus leading to the neurobiological adaptations (e.g., decreases in the levels of the dopamine D2 receptors and in the amount of dopamine released by dopamine cells; Volkow et al., 2010).
Within reward sensitivity, we also include subjective response to alcohol and sensation seeking. Subjective response to alcohol is a consummatory mechanism that refers to the individual experience of the pharmacological effects of alcohol that reflects a premorbid risk factor for AUD. There are two dominant theories regarding the relationship between subjective response and risk for AUD. The Low Level of Response Model (Schuckit, 1984) posits that individuals with a family history of AUD are more likely to exhibit low sensitivity to the stimulating and sedating effects of alcohol. According to the Low Level of Response Model, individuals with low sensitivity need to consume more alcohol to experience both the rewarding and punishing effects of alcohol to the same extent as someone high in sensitivity, which results in high levels of consumption that increases risk for AUD. In comparison, the Differentiator Model (Newlin & Thomson, 1990) posits that increased stimulation and decreased sedation from alcohol are what confer risk for AUD. Both low stimulation/low sedation (LRRM) and high stimulation/low sedation (Differentiator Model) have been associated with risk factors for AUD (Quinn & Fromme, 2011). Further, subjective response to alcohol has been shown to prospectively predict the development and progression of AUD, above and beyond other predictors, such as expectancies (King et al., 2016; Morean & Corbin, 2010; Schuckit, 1984; Quinn & Fromme, 2011). In light of this research, we believe there is merit to reward sensitivity as a premorbid risk factor for AUD.
We also include sensation seeking as a consummatory reward-related mechanism. Sensation seeking is the tendency to seek out novel and thrilling stimulation (e.g., Cyders & Smith, 2007; Peterson & Smith, 2019; Zuckerman et al., 1980), with emphasis on the experience of pleasure in the moment (i.e., consummatory pleasure). Sensation seeking may manifest as the pursuit of pleasure (e.g., substance use) at the expense of potential risks (e.g., health problems). The majority of research suggests that sensation seeking acts as a premorbid risk factor rather than an adaptation resulting from chronic alcohol use. Within the ETOH Framework, sensation seeking also encompasses novelty and fun seeking, which all tend to be highly correlated and are conceptually similar (see Statuz & Cooper, 2013 for a review). Sensation seeking predicts alcohol use quantity and frequency, binge drinking, alcohol-related problems, initiation of alcohol use, early-onset AUD, and dependence (Coskunpinar et al., 2013; Stautz & Cooper, 2013; Tarter & Edwards, 1988; Watts et al., 2020). Sensation seeking has been successfully targeted as a mechanism for reducing alcohol consumption in various interventions for adolescents (e.g., Conrod et al., 2006; Sargent et al., 2010) and has been shown to be predictive of relapse in abstinent individuals (Marra et al., 1998), further demonstrating the importance of this construct for alcohol use.
Anhedonia, also included in reward sensitivity, is the loss of pleasure (both consummatory and anticipatory) or decreased reactivity to pleasurable stimuli, which also encompasses loss of motivation for natural rewards (Koob & Volkow, 2010). Anhedonia is thought to be a consequence of AUD and other SUDs: as one develops alcohol dependence, they may exhibit increased levels of anhedonia. Interestingly, anhedonia can be reduced with sustained abstinence (Martinotti et al., 2008, 2011), which suggests it is amendable and lends further merit to the conceptualization of anhedonia as a chronic adaptation.
We deviate from ANA by categorizing anhedonia under reward sensitivity as opposed to negative emotionality (Kwako & Koob, 2017). We argue that anhedonia is more a reward-related mechanism than an affect-related mechanism. Preclinical animal models have demonstrated that anhedonia can also occur as a result of acute and protracted withdrawal due to decreases in D2 receptor expression and dopamine release (Garfield et al., 2014; Koob & Le Moal, 2001) which likely explains why anhedonia was categorized as negative emotionality (corresponding to the withdrawal/negative affect stage of the addiction cycle) within AARDoC/ANA (Koob & Volkow, 2010; Kwako & Koob, 2017). However, since anhedonia is only overcome by powerful rewards, such as drugs, it is implied that drugs work to reduce anhedonia through reward pathways as well, thus suggesting anhedonia is a reward-related mechanism. Relatedly, anhedonia tends to be positively correlated with craving, which is also thought to result as a consequence of reward dysregulation (as described later) in abstinent individuals with alcohol dependence (see Garfield et al., 2014). Also, others have argued that the distinction between the reward-related processes of anticipatory and consummatory pleasure is central to understanding anhedonia (e.g., Gard et al., 2006). Thus, for the purpose of the current framework, we argue anhedonia is most relevant to reward sensitivity, albeit, scaled in the opposite direction of reward, and particularly anticipatory and consummatory mechanisms.
Importantly, reward sensitivity is also implicated in other forms of psychopathology such as major depression, bipolar disorder, and psychosis suggesting it is likely also a transdiagnostic mechanism underlying numerous forms of psychopathology (e.g., Baskin-Sommers & Foti, 2015; Dichter et al., 2012; Garfield et al., 2014; Kapur, 2003).
Reward: Positive Emotionality
Positive emotionality is defined as a tendency towards experiencing positive emotions (e.g., Lopez-Vergara et al., 2016). Others have described positive emotionality as the extent to which a person feels pleasantly alert, with high positive emotionality resulting in a state of positive engagement (e.g., Watson et al., 1988). Positive emotionality is a broad construct. Here, we distinguish among trait positive emotionality and drinking to increase state positive emotionality (enhancement motivations) within the ETOH Framework.
Trait positive emotionality reflects individual differences in the predisposition towards emotions such as cheerfulness, enthusiasm, and energy. The associations between alcohol use, “misuse”, and AUD, on the one hand, and trait positive emotionality, on the other, are mixed. Some research has found that individuals with lower trait positive emotionality are at increased risk of alcohol misuse (Lopez-Vergara et al., 2016) as well as higher levels of alcohol involvement and number of AUD symptoms (Colder et al., 2010; Simons et al., 2014; Wray et al., 2012), but other research found that trait positive emotionality is not associated with increased drinking in young adults (Hussong et al., 2001). These mixed findings may suggest that potential moderators, such as impulsivity and environmental contexts (e.g., Colder & Chassin, 1997; Wray et al., 2012), may goad or inhibit consumption.
Drinking to increase state positive emotionality, referred to as an enhancement-related drinking motive (Cooper, 1994), is associated with a stronger desire to consume alcohol (Ralston et al., 2013). Enhancement-related drinking motives are associated with heavier consumption and increased intoxication (Simons et al., 2014), frequency of consumption (Cooper, 1994), and binge drinking (Palfai et al., 2011), particularly among college students (see Kuntsche et al., 2005 for a review). There may be overlap between enhancement-related drinking motives and positive expectancies, as well, given that drinking to increase positive emotionality is likely a strong correlate of positive expectancies (and provides positive reinforcement; Stautz & Cooper, 2013). Importantly, drinking to increase positive emotions has been shown to increase craving (Berridge & Robinson, 2006; Serre et al., 2015). Positive emotionality is undergirded, at least in part, by dopaminergic functioning, which promotes wanting and approach. Regarding positive emotionality’s relationship with AUD, it may result in craving and chronic consumption, thereby contributing to the development of AUD.
Reward: Incentive Salience
Incentive salience describes a motivational property whereby alcohol- and drug-related cues (i.e., conditioned stimuli), such as being in a bar in the case of AUD, become increasingly rewarding with repeated exposure. Berridge and Robinson (2003) refer to this motivational property as drug “wanting,” meaning the drug cues, rather than the drugs themselves, becomes the target of appeal as a result of classic conditioning processes that occur with chronic substance use. Incentive salience has been consistently described as an AUD-relevant etiologic mechanism (Berridge & Robinson, 2003, 2016; Robinson & Berridge, 1993).
The shift away from neutrality of alcohol- and drug-related cues towards incentive salience can be assessed by enhanced physiological reactivity to substance-related cues among those with and without SUDs, including AUD. In individuals diagnosed with AUD, incentive salience is apparent in physiologic responses such as increased salivation and heart rate in the presence of alcohol-related cues. Indeed, cue reactivity has been repeatedly characterized in behavioral paradigms (Field & Cox, 2008), neuroimaging studies (Schacht et al., 2013; Wrase et al., 2007), and electrophysiological studies (Namkoong et al., 2004) and has been demonstrated to be predictive of future drinking behavior (Rohsenow et al., 1994). This consistent translational characterization provides support for incentive salience as a neuroadaptation associated with the development of AUD and suggests a robust relationship between the two.
Importantly, there are also meaningful premorbid individual differences in the tendency toward attributing incentive salience to reward cues that make some individuals more likely to develop an AUD than others. In the rodent literature, rodents can be categorized as sign- versus goal-trackers based on their behavior following a Pavlovian conditioning paradigm. Following the conditioning task, sign-trackers become attracted to the reward cue whereas goal-trackers treat the cue as predictive of the reward. A recent translational review by Colaizzi and colleagues (2020) demonstrated that humans can be categorized into similar phenotypes and, as with rodents, humans with sign-tracking traits may have greater self-reported impulsivity, more compulsive behaviors, and attentional bias to reward cues compared to those with goal-tracking traits (Colaizzi et al., 2020). Compared with human goal-trackers, human sign-trackers (or those with a greater propensity towards developing incentive salience) are more likely to be vulnerable to the development and maintenance of AUD as well as externalizing psychopathology more generally (e.g., Flagel et al., 2010). Nevertheless, preclinical data suggest that the development of sign-tracking is associated with the development of self-regulation deficits, but conceptually they are distinct processes (Flagel et al., 2010). Although conceptually distinct, incentive salience and self-regulation may share etiologic influences. For example, the development of incentive sensitization is associated with both craving and impulsivity (Flagel et al., 2010; Lovic et al., 2011). Consequently, it is important to be mindful of correlated processes when seeking to identify the unique role of a mechanism of interest. Taken together, numerous reviews in the current synthesis supported incentive salience, and particularly the components of craving and cue reactivity, as relevant mechanisms in AUD (Berridge & Robinson, 2006; Guerrini et al., 2014; Koob & Volkow, 2016; Kuhn & Gallinat, 2011; Serre et al., 2015). Figure 2 displays a cross-loading from cognitive control to incentive salience given evidence suggesting that inhibitory control and cognitive flexibility may be predictive of sign-versus goal-tracking behaviors (Colaizzi et al., 2020; for a review see Diamond, 2013).
Craving, and “cue-elicited craving” in particular, is thought to be an expression of reactivity (i.e., wanting) towards substance-related cues. Thus, craving is itself considered to be a result of these shifting reward valuations (Robinson & Berridge, 1993), although there also exists between-individual variation in craving responses to substances (Everitt & Robbins, 2016). Cue-reactivity (typically assessed behaviorally [e.g., Field & Cox, 2008] or via autonomic or central nervous system activity [e.g., Schacht et al., 2013]) and craving may be partially overlapping but distinct mechanisms given that they are only modestly correlated and tend to be differentially predictive of future drinking with cue-reactivity but not craving being a significant predictor (Rohsenow et al., 1994). Craving may also be influenced by additional factors, such as the interpretation of the physiological response (Tiffany & Conklin, 2000; Witteman et al., 2015). Further, cue reactivity may simply be a result of reward learning rather than indicative of incentive salience. That is, individuals diagnosed with AUD have had more alcohol cue and outcome pairings than those not diagnosed AUD. It is perhaps unsurprising, then, that people diagnosed with AUD would show a greater response to alcohol-related cues, but it doesn’t necessarily mean they have developed incentive salience. Indeed, cue-reactivity is unreliably associated with dependence severity (see Hogarth, 2020 for a discussion). Although the differences between cue reactivity and craving are not fully understood, there is a strong evidence base for incentive salience in the maintenance of AUD and craving and cue-reactivity may be associated with this shift in reward valuation.
Reward: Reward Discounting
Reward discounting is a broad concept that has sometimes been described as how much value a given reinforcer loses as a function of a manipulated variable, such as size or probability of the reward and time (Bickel et al., 2014). Here, it encompasses delay discounting and probability discounting.
Delay discounting describes an impulsive choice whereby an individual prefers immediately available smaller rewards over larger but delayed rewards. It allows one to answer the question: How much does the value of a reinforcer decrease as a function of temporal distance? Individuals diagnosed with AUD, and SUDs in general, tend to exhibit more delay discounting in that they tend to prefer immediately available, smaller rewards over delayed rewards. Stronger delay discounting is associated with heavier alcohol use and greater AUD severity, and prospectively predicts treatment outcomes (Amlung et al., 2017). Delay discounting also plays an etiologic role in smoking acquisition (Audrain-McGovern et al., 2009). This body of research suggests that delay discounting plays a role in both the etiology and maintenance of addictive behavior (Amlung et al., 2017), as well as psychopathology more broadly (i.e., bipolar disorder, borderline personality disorder; Amlung et al., 2019).
Probability discounting describes how much the uncertainty of a reinforcer decreases the value of that reward. It allows one to answer the question: How much does the value of a reinforcer decrease as a function of decreased probability? and provides information about an individual’s preference between larger, but less certain rewards, and comparatively smaller but more definite rewards. The relationship between AUD and probability discounting is less clear than is the relationship between AUD and delay discounting. Some research has found that individuals who use substances exhibit greater probability discounting than nonusers in both human and nonhuman animal research, but other research failed to find such effects (see Bickel et al., 2014 for a review). Additional research with nonhuman animals suggests that rats exposed to alcohol in adolescence display a preference for larger, more uncertain rewards when compared to those not exposed (Nasrallah et al., 2009). Although this work provides tentative support for the association between alcohol use and probability discounting, it is unclear whether probability discounting is a premorbid feature or an acquired feature resulting from heavy consumption.
Accordingly, alcohol and other drugs may provide an immediate and reasonably certain source of reinforcement (e.g., euphoria, stress reduction) compared to other, more delayed or uncertain alternatives, such as natural reinforcers (e.g., sex), health behaviors (e.g., exercise), and academic/vocational outcomes (e.g., going to college to obtain a fulfilling career). Thus, individuals who devalue delayed or uncertain outcomes may therefore display a preference for substance use relative to many other alternatives (Bidwell et al., 2013). This preference typically results in more discounting among those with SUDs (Dalley et al., 2011; Voon et al., 2020), although delay discounting may be a more robust predictor of substance use and SUDs than probability discounting (Bidwell et al., 2013).
The subdomain of reward discounting (primary superdomain: reward) may overlap with the superdomain of cognitive control (Amlung et al., 2017). Reward discounting is primarily a product of reward dysregulation (i.e., shifting reward processes as a result of substance use), but it is also related to impulsivity and response inhibition (Dalley et al., 2011; Lee et al., 2019; Salvatore et al., 2015), which are subsumed under the cognitive control superdomain. There is some evidence that executive functioning plays a role in both cognitive control (e.g., impulsivity) and reward discounting among individuals with SUDs (Bickel et al., 2011; West, 2005). Thus, reward discounting may also be related to the cognitive control superdomain (as indicated in Figure 2 by a dashed line between discounting and impulsivity).
Reward: Summary
Taken together, the reward superdomain encompasses the subdomains of habit, positive reinforcement expectancies, reward sensitivity, positive emotionality, incentive salience, and reward discounting, which are proposed to be relevant mechanisms in the development, and at times the maintenance, of AUD. Notably, this domain extends the ANA’s conceptualization of reward given that ANA tends to focus mostly on incentive salience.
Negative Valence and Emotionality Superdomain
AARDoC and ANA propose “negative emotionality” as a domain that is intended to be equivalent to RDoC’s negative valence system. RDoC’s negative valence system encompasses systems “…primarily responsible for responses to aversive situations or context, such as fear, anxiety, and loss” (NIH, 2019). However, AARDoC and ANA conceptualize negative emotionality within the context of Koob and LeMoal’s (2001) model, which occurs as a result of chronic adaptation to alcohol, and thus negative emotionality within ANA is acquired as opposed to premorbid. AARDoC and ANA cover a narrower set of negative valence mechanisms that focus on increases in negative emotional response to alcohol (Kwako et al., 2015). This characterization overlooks other relevant mechanisms related to AUD such as dispositional negative emotionality, reduced punishment sensitivity, and negative expectancies.
To address AARDoC and ANA’s potentially overly narrow focus on negative emotional response to alcohol, the ETOH Framework puts forth Negative Valence and Emotionality as a superdomain. We separate emotionality (i.e., mechanisms related more to negative emotion specifically) and valence (i.e., mechanisms related more to other negative effects such as physical pain or those resulting from substance use) in this label in order to include AARDoC’s narrower negative emotionality domain but also to broaden the scope of negative valence to include other addiction-relevant other constructs captured in RDoC but not ANA/AARRDC. Additional research is likely needed to determine if valence and emotionality mechanisms are distinct from one another. Our review suggested four subdomains for the domain of negative valence and emotionality: negative emotionality, coping, punishment sensitivity, and negative expectancies.
Negative Valence and Emotionality: Negative Emotionality
Negative emotionality is a negative mood state that includes alexithymia, anxiety, irritability, and depression. It has been implicated as one, if not the most, important mechanism across addiction (Berridge & Robinson, 2006) and is considered both a premorbid risk factor and acquired feature resulting from chronic substance use.
Regarding negative emotionality as a premorbid risk factor, research has demonstrated associations between high levels of negative emotionality and heavy alcohol use (Gomberg 1997; Chassin et al., 2013; Hussong et al., 2011; King et al., 2004; Simons et al., 2014; see van Lier et al., 2018, for a review). For example, individuals with higher negative emotionality (e.g., nervousness, depression) tend to drink more frequently (Flynn, 2000; Simons et al., 2014; Swendsen et al., 2000) and individuals are more likely to drink on days characterized by more sadness (Armeli et al., 2000; c.f., Hussong et al., 2005). Accordingly, negative emotionality is also associated with increased odds of AUD and AUD severity (King et al., 2004; Simons et al., 2014). This research provides some evidence for negative emotionality as a relevant risk factor for AUD. Nevertheless, research on the association between negative emotionality and alcohol-related outcomes has been somewhat inconsistent, particularly among adolescents, and the association may vary based on factors, such as the specific type of negative emotionality (e.g., anxiety versus depression) and the specific alcohol-related outcomes assessed (see Hussong et al., 2017, for a review). Additional research should continue to examine to what extent negative emotionality explains variance in alcohol-related outcomes, including AUD.
According to Koob and Volkow (2016), heightened negative emotionality resulting from chronic alcohol consumption is the result of neuroadaptation, including the downregulation of mesolimbic reward circuits and increases in stress responding within motivational circuits. As a result of these changes, relief from negative emotions (which is also thought to be related to craving; Reilly et al., 2017) replaces hedonic pleasure as the driving force behind substance use (known as “hedonic allostasis;” Koob et al., 2004). Subsequent relief from negative emotions after drinking is negatively reinforcing, which maintains use. Indeed, negative emotionality is particularly associated with both withdrawal and protracted abstinence and may be one mechanism through which consumption escalates and relapse becomes more likely (Koob & Volkow, 2016). These findings are consistent with research demonstrating that alcohol craving is positively associated with negative emotionality and that craving can be evoked by inducing negative emotion (Serre et al., 2015). Negative emotionality as a chronic adaptation has been well characterized within several neurotransmitter systems and molecular neurocircuits, particularly within the withdrawal/negative affect stage of addiction, pointing to the biological bases of increased negative emotionality observed in those with addiction (see Koob & Volkow, 2016 for a review). In light of the research on hedonic allostasis and trait negative emotionality, it is likely that negative emotionality is both an acquired feature of and a premorbid risk factor for AUD.
Taken together, negative emotionality is likely implicated in AUD multiple stages in the addiction cycle, serving as premorbid risk factor and arising from chronic adaptation to use. This construal broadens the ANA’s negative emotionality domain as a neuroadaptation resulting from chronic use by explicitly considering its role as a premorbid risk factor for AUD.
Negative Valence and Emotionality: Coping
Coping describes the ways in which individuals manage negative emotions and involves complex cognitive processes (e.g., appraisal) and behaviors in response to stress or problems (Lazarus & Folkman, 1984). Coping is likely uniquely associated with AUD above and beyond negative emotionality more generally. Thus, coping is closely related to negative emotionality. Several models of addiction theorize that individuals drink in an attempt to cope with or ameliorate negative emotions (Cooper, 1994; Cox & Klinger, 1988; Shiffman & Wills, 1985), including the self-medication model of addiction (Khantzian, 1997; West, 2005), the Tension-Reduction Theory (Cappell & Herman, 1972), and the Coping Theory (Milkman & Frosch, 1980; Lettieri, 1985).
Self-reported coping motives are particularly implicated in alcohol-related problems and AUD, especially among those who use alcohol to avoid or deal with negative emotions (i.e., emotion-focused coping; Berridge & Robinson, 2006; Hogarth, 2020; Martens et al., 2008). The specific mechanisms noted under the coping subdomain include problem-focused and emotion-focused coping. Problem-focused coping aims to remove the stressor from the environment whereas emotion-focused coping strategies (which tend to be more consistent with “coping motives” described in the literature) aim to alleviate the emotional responses to the stressor (Lazarus & Folkman, 1984). The distinction between problem- and emotion-focus coping is important because problem-focused coping is associated with less alcohol consumption than emotion-focused coping (Brady & Sonne, 1999; West, 2005). Emotion-focused copers, those who try alleviating emotional responses to stressors (e.g., alleviation of negative emotion resulting from a stressor), tend to drink more, potentially as a result of the negative reinforcement that occurs when alcohol provides alleviation from negative emotion (Reilly et al., 2017).
Drinking to reduce negative emotion is longitudinally associated with alcohol dependence (Carpenter & Hasin, 1998). This relationship is further supported by research by Marten and colleagues (2008) which showed a three-way interaction among alcohol use, negative emotion, and coping motives in predicting alcohol-related problems in college students. For those low in coping motives, the association between alcohol use and related problems was the same regardless of level of negative emotion. For those high in coping motives, the association between use and related problems was significantly stronger for individuals high as opposed to low in negative emotionality. Thus, negative emotionality and coping may work together to increase risk for heavy consumption and AUD. For example, negative emotionality may increase the relative reinforcing value of alcohol compared to other alternatives, particularly among those with drinking to cope motives (Rousseau et al., 2011), which may confer risk for dependence above and beyond consumption by increasing impairment or promoting symptoms of dependence such as craving. The reasons for why coping motives predict alcohol-related problems beyond what is explainable by consumption has yet to be resolved.
Negative Valence and Emotionality: Punishment Sensitivity
Within the context of alcohol use, punishment sensitivity is intended to describe a reduced sensitivity to the aversive effects of alcohol (e.g., flushing or hangover; Agarwal & Goedde, 1989), which is closely related to the mechanism of “loss discounting” (i.e., a decrease in loss aversion when a loss is delayed versus immediate). When coupled with increased delayed discounting of rewarding effects of alcohol, such differences in punishment sensitivity and loss discounting are likely potent contributors to vulnerability to AUD. Reduced sensitivity to punishment is associated with familial history of AUD (Finn et al., 1994) and has been implicated as a significant factor in early onset AUD (Finn et al., 2002). Indeed, reduced sensitivity to punishment is also related to heavier alcohol use (Jonker et al., 2014; Tapper et al., 2015), but its statistical associations with alcohol use are attenuated after taking into account reward sensitivity (Jonker et al., 2014). This research suggests that reduced sensitivity to punishment and reward sensitivity both account for unique variance in alcohol consumption and AUD, but effects are stronger for reward sensitivity. Note, also, that as substance use progresses to addiction and becomes more compulsive, punishing outcomes become less effective in suppressing substance seeking behavior (Lüscher et al., 2020). Consequently, it is important to consider stage of addiction in evaluating the role of punishment sensitivity.
In light of Gray’s revised Reinforcement Sensitivity Theory (rRST; Gray & McNaughton, 2000), sensitivity to punishment is viewed as a premorbid risk factor for AUD. Unlike reward sensitivity, punishment sensitivity remains relatively stable from childhood to adulthood (Fox et al., 2005). Preliminary evidence also suggests that low punishment sensitivity is especially related to alcohol and cannabis use in individuals with low levels of inhibitory control (e.g., Kahn et al., 2018), pointing to the need for more research examining the role of effortful control in the link between punishment sensitivity and substance use behaviors.
Within the ETOH Framework, the mechanism of loss discounting is included to represent sensitivity to punishment. Loss discounting describes a decrease in loss aversion (e.g., punishment) when the loss is delayed as opposed to immediate. Compared with controls, individuals diagnosed with AUD exhibit more discounting of future losses (Bailey et al., 2018). This finding offers preliminary support for loss discounting as a mechanism in AUD, but its inclusion in the ETOH Framework is more tentative and requires further research.
Negative Valence and Emotionality: Negative Expectancies
Compared with positive expectancies which were covered in the reward superdomain, here we include negative expectancies, and specifically negative outcome expectancies. Negative outcome expectancies describe beliefs people hold about the likelihood of experiencing negative effects from alcohol (West, 2005). Examples of negative expectancies include: Alcohol will make me feel sick and alcohol will make me more aggressive.
Compared with positive expectancies, negative expectancies have been less consistently examined in the alcohol literature and the research that does exist is fairly mixed. Regarding the relationship between negative expectancies and consumption, some authors have found that they are inversely related whereas others have found no association between the two and suggest, instead, that such associations are better accounted for by variables such as sex or personality (see Jones et al., 2001 and Patrick et al., 2009 for overviews of this literature). It has also been hypothesized that negative expectancies might provide motivation to reduce or stop drinking (Jones et al., 2001). This hypothesis is supported by work demonstrating that pre-treatment negative expectancies predict treatment outcomes whereas positive expectancies do not (Jones & McMahon, 1996). Additional work has suggested that adolescents with more negative expectancies drink less alcohol one to two years later, yet there also exists other work to the contrary demonstrating that negative expectancies at age 16 do not predict alcohol use or problems in adulthood (see Patrick et al., 2009 for an overview). Indeed, the research on negative expectancies in the role of facilitating AUD has been highly mixed.
It is conceivable, based on these findings, that early on in one’s drinking career, consumption is more easily influenced by the expectancy of punishment (i.e., negative expectancies) such that it is associated with inhibition of consumption. However, as an individual’s drinking becomes heavier, and perhaps more compulsive, negative expectancies tend to influence consumption less. Thus, the role of negative expectancies may be stage dependent. Thus, we have chosen to retain negative outcome expectancies in the ETOH Framework. We advocate for additional research into the role of negative expectancies in the etiology and maintenance of AUD.
Several reviews categorized as “negative emotionality” focused on the stress system and stress response specifically (Buisman-Pijlman et al., 2014; Guerrini et al., 2014; Ouzir & Errami, 2016; Rew, 1989; Schneeberger et al., 2014; Thatcher & Clark, 2008). Some of these reviews describe states of stress as a component of the withdrawal/negative affect stage of addiction (e.g., Koob & Volkow, 2016), whereas others describe stress and trauma exposure as an early-life risk factor (e.g., Clarke et al., 2012). In general, though, the literature seems to lack clear directionality for the relationship between stress and AUD or consumption (i.e., is dysregulation in the stress system a cause or consequence of AUD?). Numerous studies have tried to characterize stress-induced drinking (i.e., stressor exposure as an independent variable and alcohol-related behavior as an outcome) and stress-reduction or stress-response dampening (i.e., where alcohol consumption is the independent variable and stress response is the dependent variable).
Overall, there is some support for both stress-induced drinking effects and stress-response dampening effects, but both are highly conditional on both dispositional and contextual variables (Sher & Grekin, 2007). For example, laboratory studies suggest that stressful situations are more likely to result in increased consumption when the risk of punishment for intoxication is low and when more effective methods for coping with the stressor are not available (Sher, 1987). Field, diary, and EMA studies lead to similar conclusions (Sher & Grekin, 2007). Laboratory studies also indicate that whether a moderate dose of alcohol is likely to lead to reduced response to a discrete stressor is highly dependent upon both personological and situational variables (Hull, 1987; Sayette, 1999; Steele & Josephs, 1990), although more unconditional effects can be observed at higher doses (Donohue et al., 2007). Thus, whether one chooses to drink when stressed is highly contextual. Given the highly conditional nature of the stress-alcohol relation, we have not designated stress as its own subdomain in the current mechanistic framework, although it is clearly closely related to both trait and state negative emotionality, especially state negative emotionality associated with acute or prolonged stress. Future research should further explore stress as a mechanism in AUD, determine if it is distinct from negative emotionality in general, and disentangle the extent to which it is acquired, premorbid, or both.
Negative Valence and Emotionality: Summary
The superdomain of negative valence and emotionality extends the ANA’s narrower conceptualization of negative emotionality to also include mechanisms such as negative expectancies and punishment sensitivity.
Cognitive Control Superdomain
The superdomain of cognitive control broadly represents mechanisms traditionally considered a part of executive functioning. As previously described, AARDoC articulates this domain as including mechanisms related to the organization of behavior towards future goals and argues it encompasses things such as set shifting, response inhibition, and planning. Like ANA’s conceptualization of cognitive control, the cognitive control superdomain in the ETOH framework includes mechanisms related to deficits in conscientiousness but is expanded to include a compulsivity domain. These superdomains encompass the subdomains of conscientiousness, response inhibition, and compulsive use.
Cognitive control bifurcates into impulsivity and compulsivity given research suggesting that impaired cognitive control mechanisms are linked to both impulsive and compulsive drug seeking. For example, preclinical research suggests that impulsivity is a precursor to compulsive use (Belin et al., 2008). Indeed, compulsivity is likely underpinned by dysregulation in response inhibition (i.e., impulsivity) and contingency-related cognitive inflexibility (e.g., Dalley et al., 2011; Lee, et al., 2019). Importantly, though, it seems that impulsivity and compulsivity are overlapping in that they share elements of disinhibition but that they are distinct constructs (Lee et al., 2019). This research supports the decision to construe impulsivity and compulsivity as separate domains while acknowledging their association.
Finally, in the case of both domains being placed under cognitive control, they appear to serve as both premorbid risk factors and downstream consequences of chronic alcohol consumption. Indeed, impairments in cognitive control likely have bidirectional, causal associations with AUD.
Cognitive Control: Impulsivity
Impulsivity: Conscientiousness.
Conscientiousness is a broad, hierarchically-organized personality trait that reflects tendencies towards cautiousness, dutifulness, and planfulness, and contains narrower components such as self-control, deliberation, and order, among others (e.g., Costa & McCrae, 1998). Within the subdomain of Conscientiousness, we include the narrower traits of lack of planning and lack of perseverance.
Within the UPPS-P model of impulsivity, lack of planning and lack of perseverance cohere into a higher-order “deficits in conscientiousness” factor (e.g., Cyders & Smith, 2007; Dick et al., 2010). Lack of planning (also called lack of premeditation) is the tendency to act without forethought, while lack of perseverance (also called lack of persistence) is conceptualized as the inability to sustain the attention or motivation necessary to complete a task (Smith et al., 2007). There is a significant literature regarding the relationships between both conscientiousness subdomains and AUD.
In addition to deficits in conscientiousness, there are two other higher-order factors in the UPPS-P model, urgency (which contains lower-order negative and positive urgency factors) and sensation seeking, which indicates that deficits in conscientiousness are subsumed under, but are not isomorphic with, the broader trait of impulsivity. As we mentioned earlier, we deviate from ANA in that we place the UPPS-P deficits in conscientiousness (lack of planning, lack of perseverance) dimensions and positive/negative urgency under the Cognitive Control superdomain and sensation seeking factor under the Reward superdomain. We have intentionally disaggregated UPPS-P impulsivity dimensions within the ETOH Framework because UPPS-P dimensions relate differentially with alcohol use and alcohol-related outcomes (Coskunpinar et al., 2013; Smith et al., 2007), and may be associated with different cognitive systems (e.g., Jentsch et al., 2014). For example, response inhibition appears dependent upon the lateral prefrontal and dorsal striatal systems, whereas other facets of impulsivity are dependent upon medial prefrontal and ventral striatal functioning (Stevens et al., 2014).
In a meta-analysis of adolescents, Stautz and Cooper (2013) demonstrated that lack of planning and lack of perseverance were significantly associated with alcohol consumption and problematic use. Other meta-analyses of a wider age range of people similarly found that lack of perseverance was associated with drinking quantity (r = 0.32), and lack of planning was associated with drinking problems (r = 0.27; Coskunpinar et al., 2013). Thus, individuals who act without forethought and have difficulty sustaining attention or motivation (i.e., low conscientiousness) may be more at risk for alcohol-related problems. Further, individuals lower in conscientiousness are at increased risk of relapse following treatment (Bottlender & Soyka, 2005; Ouzir & Errami, 2016; Preuss et al., 2012). Deficits in conscientiousness are implicated in various points of addiction, from premorbid vulnerability to chronic adaptation and relapse, and thus are likely a core mechanism by which AUD is initiated and maintained. Further, deficits in conscientiousness are also implicated in other forms of psychopathology that commonly co-occur with AUD (e.g., antisocial personality disorder, attention deficit/hyperactivity disorder, particularly inattention), it may be an important transdiagnostic mechanism that accounts for their co-occurrence (Roberts et al., 2009; Stanton & Watson, 2016).
Impulsivity: Response Inhibition.
Response inhibition describes “the ability to suppress dominant, automatic, or prepotent responses” (Friedman & Miyake, 2004, p. 104). Response inhibition has also been described as rapid-response impulsivity, which is “a tendency toward immediate action that occurs with diminished forethought and is out of context with the present demands of the environment” (Hamilton et al., 2015, p. 1).7 Specific components and mechanisms within the response inhibition subdomain include loss of control, substance-related disinhibition, and positive and negative urgency.
Loss of control broadly describes the failure to stop an ongoing or prepotent action, such as substance use, once use of the substance has begun. The concept is attributed to E.M. Jellinek (1952), who describes loss of control as an uncontrollable urge to consume more alcohol, leading to heavy drinking or bingeing (Hill, 1985). Within the alcohol literature, loss of control is often described more specifically as impaired control over drinking. Within the ETOH framework, we choose the more general term “loss of control” rather than “impaired control over drinking” for two reasons. First, “impaired control over drinking” is a broader label that includes several mechanisms such as the inability to abstain (designated under the “compulsive use” subdomain) and loss of control over consumption (which we designate here within the “response inhibition” subdomain). We treat the inability to abstain and loss of control as separate given research suggesting that the two mechanisms are conceptually and neurobiologically distinct (e.g., Hamilton et al., 2015). This approach is also consistent with the DSM-5, which classifies loss of control (i.e., drinking longer or large amounts than intended; APA, 2013) as distinct from the inability to abstain (i.e., failed attempts to quit or cut down) in that they are separate diagnostic criteria. In comparison, ICD-10 and ICD-11 include a single impaired control criterion, which describes “Difficulties in controlling substance-taking behaviour in terms of its onset, termination, or levels of use…” (WHO, 1992, p.75; Saunders et al., 2019).8 Second, “loss of control” is a term that is more substance-general when compared to “impaired control over drinking,” allowing for more a direct comparison of this mechanism across substances.
Many researchers argue that loss of control is a cardinal feature of AUD (Edwards & Gross, 1976; Koob & Volkow, 2016; c.f., Hill, 1985) but several reviews failed to report specific empirical evidence in support of this position (Polcin, 1997; West, 2005). In general, the larger literature fails to distinguish between the inability to abstain and loss of control, making it difficult to characterize the literature in regard to how certain outcomes are related uniquely to loss of control. Despite this failure, there is a significant literature to suggest that impaired control over drinking, broadly defined, is associated with several alcohol-related outcomes, both as a premorbid risk factor and a result of chronic adaptation to substance use. For example, impaired control over drinking is related to the onset of alcohol-related problems (prospectively and retrospectively), frequency of consumption, and heavy drinking (Leeman et al., 2014; Vogel-Sprott et al., 2001; Weafer & Fillmore, 2012). Moreover, there is evidence for the relationship between impaired control over drinking and neurobiological dysfunction (e.g., in the insula), suggesting that impaired control over drinking may be closely linked with identifiable biological mechanisms among those diagnosed with AUD (Leeman et al., 2014), as well as among humans more generally (Field et al., 2010). Impaired control may be at least partially attributable to one’s alcohol-related expectancies (Marlatt et al., 1973).
Substance-related disinhibition describes difficulty in suppressing motivated behaviors (e.g., eating, sexual activity, aggression) to engage in socially sanctioned behavior while under the influence of substances such as alcohol (Polivy, 1998; Weafer & Fillmore, 2012). Research has consistently demonstrated that alcohol impairs inhibitory control (Day et al., 2015; Weafer & Fillmore, 2012). Consequently, intoxication can result in difficulty suppressing behaviors that result in negative consequences (e.g., aggression, provoking fights, driving while intoxicated). Some theories suggest there is relationship between alcohol-related disinhibition and AUD and related problems (West, 2005), although few studies have elaborated on the specific empirical evidence for such associations. In terms of aggression, the effects of alcohol appear to be conditional on baseline executive function and explicit provocation (Giancola, 2004). Taken together, research suggests that alcohol-related disinhibition may be a relevant alcohol-specific mechanism in AUD, but more research is needed to clarify the exact nature of the relationship.
Positive and negative urgency describe the tendency to engage in rash behavior when in an extreme positive or negative mood, respectively (Cyders & Smith, 2007; Stuatz & Cooper, 2013). Urgency more generally reflects difficulties with response inhibition while in an extreme positive or negative mood state, which is why it is included here rather than under the subdomains of positive or negative emotionality.
A cross-sectional meta-analysis of adolescent samples demonstrated that positive urgency is associated with alcohol consumption (r = .27) and problematic alcohol use (r = .31; Stautz & Cooper, 2013). Positive urgency is also associated with alcohol use initiation (e.g., Gunn & Smith, 2010; Watts et al., 2020), quantity of consumption, and alcohol-related problems (Coskunpinar et al., 2013; Peterson & Smith, 2019). Thus, positive urgency is likely a premorbid (prospectively-assessed) risk factor for AUD. Moreover, positive urgency is also associated with other risky and potentially harmful behaviors (e.g., risky sexual behavior, non-suicidal self-injury), so it is regarded as an important transdiagnostic mechanism (Um & Cyders, 2019). Although the association between positive urgency and alcohol related problems seems clear, the relationship between positive emotionality and alcohol involvement remains to be fully resolved. Some research suggests that extraversion, which contains positive emotionality, is not associated with drinking among college students, but it is associated with selecting (i.e., niche picking) into high-risk environments such as the Greek system where drinking takes place. So, positive emotionality may influence the selection of high-risk environments but not selection of high-risk behaviors per se (see Littlefield & Sher, 2010 for a review).
There is similarly consistent evidence for negative urgency’s association with alcohol-related outcomes including, alcohol use initiation (Gunn & Smith, 2010; Peterson et al., 2018; Stautz & Cooper, 2013), alcohol-related problems (e.g., Coskunpinar et al., 2013), and alcohol dependence symptoms (Stautz & Cooper, 2013). This research suggests that individuals who tend to engage in risky behaviors under negative emotions are at risk for increased alcohol involvement. Further, Smith and Anderson’s (2001) Acquired Preparedness model implicates the role of negative urgency in alcohol-related risk and posits that negative urgency may serve to predispose individuals towards viewing alcohol use as an adaptive response to distress (Settles et al., 2010). This finding lends merit to negative urgency as a premorbid risk factor for AUD.
Overall, the response inhibition domain appears to be clearly relevant to AUD and was among the constructs identified by addiction experts as fundamental to AUD (Yücel et al., 2019). Further, response inhibition has also been noted as a heritable endophenotype for addiction (Jentsch et al., 2014) and others (i.e., Reilly et al., 2017) have demonstrated how dopamine receptors (i.e., DRD2 and DRD4) are implicated in the modulation of neural mechanisms that underlie response inhibition in the binge-intoxication stage of addiction. Interestingly, some research demonstrates that response inhibition is genetically related to behavioral disinhibition (e.g., Young et al., 2009). It is therefore plausible that response inhibition, if indeed closely associated with behavioral disinhibition, may also be general to all externalizing psychopathology (e.g., Krueger et al., 2002) rather than specific to addiction. Therefore, we consider response inhibition a relevant mechanism in the etiology of AUD, but it may be general to other psychopathology.
Cognitive Control: Compulsivity
Compulsivity: Compulsion.
Within the addiction literature, compulsion is described as a loss of voluntary control over behavior such that punishment no longer suppresses ostensible reward seeking. Compulsion can manifest in difficulty resisting urges to use, as well as repetitive behaviors performed in a habitual or stereotyped manner, typically in situations where the actions are inappropriate (e.g., Robbins et al., 2012). In individuals diagnosed with AUD, compulsion can result in an inability to control one’s drinking despite a desire to do so and/or negative consequences due to drinking. Some models suggest that there is a shift from positive to negative reinforcement as individuals transition from impulsive to compulsive use. This shift is thought to reflect a change in neurocircuitry whereby there are (a) decreases in reward function and (b) increases in stress function in the motivational circuits of the ventral striatum, extended amygdala, and habenula. The shift from impulsive to compulsive use is thought to maintain consumption through habitual drug-seeking and use (Koob & Volkow, 2016). Interestingly, despite its relevance, compulsion is not consistently included in modern conceptualizations of addiction (e.g., AARDoC/ANA). Compulsion has also been criticized by some authors (e.g., Pickard, 2020) as problematic in that it largely ignores evidence in support of the impact of environmental and context-specific contingencies on choice and research suggesting that the choice to use is actually voluntary and value-based and, therefore, not compulsive. However, given compulsion-related shifts in neurocircuitry are well-established in the literature, we advocate for its inclusion within the ETOH Framework at this point in time.
Compulsion is distinct from habit (i.e., the automation of behavior based on initial reinforcement learning); the former is associated with the experience of being forced or compelled to act despite negative consequences or punishment, whereas the latter is not necessarily associated with the expected reward value of the substance once established, is more automatic, and can be modified by the direct experience of the substance (e.g., loss of positive reinforcement; Everitt & Robbins, 2005, 2016; Hogarth, 2020). Given that they are likely closely associated and potentially sequential in nature, with habit thought to serve as the “building blocks” for compulsive drug seeking (Everitt & Robbins, 2016), we have included a cross-loading between compulsion and habit (reward superdomain).
In an expert consensus study (Yücel et al., 2019), compulsion was identified as a critical construct that had been omitted from the RDoC model. The results of our review support this conclusion. As such, we believe that compulsion is necessary to include in the current framework as well as any other mechanism-based conceptualization of AUD. Of note, RDoC’s positive valence domain was revised in the summer of 2018 and it now includes aspects of compulsivity (e.g., reward valuation). However, we believe compulsivity is a core feature of AUD that warrants its own domain. Included under the domain of compulsivity is the subdomain of compulsive use, which we believe is general to SUDs and reflects the inability to abstain from substance use (Prom-Wormley et al., 2017).
Cognitive Control: Summary
Dysregulated cognitive control serves as a major mechanism in the development and maintenance of AUD and is described within the ETOH Framework as encompassing the lower order subdomains of conscientiousness, response inhibition, and compulsive use.
Additional AUD-relevant Constructs
Our review discovered four constructs that were not covered by existing models (e.g., ANA/AARDoC, NIDA PhAB): negative valence, compulsivity, opponent process, and self-awareness. As we noted earlier, we deemed negative valence and compulsion as worthy of inclusion in the ETOH Framework because they appear causally implicated in AUD, either as premorbid risk factors or consequences of use, or both. We determined that the two other constructs, opponent process and self-awareness, appear to act as critical moderators of the ETOH Framework domains. As such, we do not include them in the overall framework (Figure 2) but have elected to emphasize their apparent roles in AUD by describing them here in a “Critical Moderators” section. Additionally, we end by describing relevant social and environmental mechanisms that are worth further consideration.
Critical Moderating Processes
We designated opponent processes and self-awareness as critical moderating mechanisms rather than superdomains, domains, or subdomains given they are thought to act upon the other mechanisms articulated within the ETOH Framework. That is, they are thought to moderate the expression of ETOH subdomains that are more directly implicated in the etiology, onset, or maintenance of AUD. At the same time, we elected to describe them in our review because they were discussed by a number of reviews and because we believe they are necessary to consider in any comprehensive, mechanistic framework of AUD etiology.
Opponent Process.
Opponent process theories describe hedonic processes that are altered as a consequence of substance use. The more one uses, the more the pleasurable effects of the substance (or other reinforcers) will decrease with time as a result of the recruitment of countervailing opponent processes that offset the pleasurable effects. Opponent process theories also posit that this process occurs in the opposite direction whereby negative effects decrease with time as a result of the positive effects intensifying (e.g., pain relief; see Leknes et al., 2008). Thus, opponent processes can dampen both pleasurable and punishing effects of substances and are viewed as a primary mechanism of tolerance development.
Opponent process theories rest on the idea that our central nervous system works to maintain a hedonic balance through the use of opponent processes whereby an “a-process” is evoked from the use of a substance and is followed by a “b-process” that has the opposite effect to the substance (Solomon, 1980; West, 2005). As use continues, the a-state remains the same as the b-state intensifies, becoming stronger and longer in duration, thus decreasing or counteracting, the strength of the a-state (e.g., Solomon, 1980) resulting in acquired tolerance to substances. Critically, these countervailing b-process can come to be elicited by cues surrounding substance administration through a process of classical conditioning, in effect, moving the post-consumptive b-state into an anticipatory b-state (Siegel et al., 1987).
Koob and Volkow (2016) hypothesize that as the binge-intoxication stage triggers opponent-process responses, pleasure is diminished (a-state) via dopamine and brain stress system activity (b-state) increases (see also Reilly et al., 2017). Over time, the repeated elicitation of opponent processes results in a change in hedonic set point such that there is a chronic effect of b-states resulting in tonic changes in affective tone (Koob & Le Moal, 2001), which results in apparent withdrawal-like phenomena and the need for increasing doses of the drug to maintain normal reward or hedonic impact (Koob & Le Moal, 2001). When the drug is not present, this allostatic state results in the negative reinforcement stage of addiction which is associated with acute and protracted withdrawal, and, thus, contributes to compulsive drug-seeking and addiction (Koob & Volkow, 2016). Because opponent processes act on rewarding and punishing effects, we designated them as critical moderators separate from, rather than overlapping with or encompassed by, the superdomains of reward and negative valence.
Self-Awareness.
Self-awareness encompasses three more specific components: awareness of disorder, awareness of internal states, and awareness of self in social contexts. Generally, these describe insight-related constructs whereby attention is self-directed (Fenigstein et al., 1975) and information is organized according to its self-relevance (Hull & Leavy, 1979). Awareness of disorder describes an awareness of one’s substance-related behavioral impairment or need for treatment, whereas awareness of internal states has been described as a cognitive problem of self-regulation whereby an individual is unable to regulate their own behavior due to impairment in self-evaluative assessments (e.g., related to one’s mood, which is known more specifically as alexithymia; Lettieri, 1985; Stewart, 1996; Thombs & Osborn, 2013). Traditionally, awareness of internal states has been described as private self-consciousness, the aspect of self-consciousness whereby one engages in a private “mulling over self” (Fenigstein et al., 1975, p. 525) or looking inwards to one’s feelings and motives. In comparison, awareness of self in social contexts describes the mechanism of public self-consciousness, awareness of how one relates to others in social situations (Fenigstein et al., 1975). Public self-consciousness can include, for example, the awareness of other’s perspectives of self and is often focused on the reactions of others.
Research has demonstrated a relationship between AUD and impaired insight, broadly defined (e.g., Rafferty et al., 2020; Reilly et al., 2017; Tarter et al., 1985).9 Based on our review, we concluded that self-awareness likely moderates the expression of ETOH domains because lack of self-awareness in isolation need not indicate AUD per se, but it likely impacts the manifestation of AUD. For instance, lack of awareness of disorder may prevent efforts to cut down, whereas lack of awareness of internal states and lack of awareness of self in social contexts may prohibit insights into levels of impairment, thereby increasing the likelihood that someone may drive under the influence of alcohol because they think they are less drunk than they are (e.g., Rafferty et al., 2020). Lack of self-awareness may also moderate AUD’s expression such that it increases the extent to which AUD is associated with impairment (e.g., Hull, 1987). Alternatively, high self-awareness can be viewed as a predisposing factor for experiencing self-conscious emotions such as guilt and embarrassment. Because alcohol acutely interferes with the cognitive processes maintaining self-awareness (Hull, 1981) high self-aware individuals are likely to experience greater stress reduction when confronting self-relevant stressors (e.g., failure experiences). Within RDoC, self-awareness, described as the perception and understanding of self or “self-knowledge,” is categorized under the domain of “social mechanisms.” NIDA PhAB highlights a more central role of social mechanisms than does ANA by outlining a Metacognition domain (Keyser-Marcus et al., 2021).
Some empirical research suggests that lack of self-awareness is thought to reduce the chances of initiating and maintaining reductions in drinking (Le Berre & Sullivan, 2016). Similarly, lack of awareness of internal states is associated with increased probability of relapse among individuals following detoxification, particularly in the face of relatively negative self-relevant life events (Hull et al., 1986). Further supporting the role of self-awareness as a critical moderator of AUD domains, high self-consciousness is associated with increased attitude-behavior consistency (Kernis & Grannemann, 1988), meaning that it could serve to increase or decrease risk for AUD depending on individual expectancies and attitudes.
Self-awareness may also serve as a premorbid risk factor or consequence of AUD, but research supporting this possibility is fairly meager. Regarding self-awareness’ role as a premorbid risk factor in AUD, some research has shown that high private self-consciousness is related to increased alcohol use whereby individuals drink to avoid self-awareness, especially in the face of personal failure (e.g., Hull & Leavy, 1979; see also Sher & Epler, 2004). Public self-consciousness, in comparison has been demonstrated as a predictor of alcohol use (e.g., LaBrie, Hummer, et al. 2008; LaBrie, Pedersen, et al., 2008) and is relevant to several explanations and models of substance use (Lettieri, 1985), although the direction of this relationship has been mixed (e.g., Foster & Neighbors, 2013).
Regarding self-awareness as a consequence of AUD, others have argued that acute and chronic alcohol use impairs one’s ability to encode self-relevant information, thus decreasing self-awareness and failed problem recognition (Hull, 1987; Sher & Epler, 2004). Failures in self-awareness and problem recognition are thought to occur both (a) acutely, or in the moment, as a result of alcohol use which results in a narrowing of one’s attention away from the self, and (b) more chronically, resulting in so called “alcoholic denial,” for example. The specific role of self-awareness in AUD, and psychopathology more generally, requires more attention.
Social and Environmental Mechanisms
Several of the reviews focused on social and environmental factors. As such, it may be worthwhile to consider these factors within the current framework in future work. Although the interplay of environmental and contextual factors in AUD and treatment outcomes has been acknowledged by AARDoC/ANA, we do not explicitly consider environmental and contextual factors as their own ETOH domains. We have made this decision because it is difficult to determine which of these environmental factors serve truly as mechanisms rather than general risk factors, and many environmental factors may be associated with specific ETOH domains. Although not explicitly included in our framework, we do not dismiss the critical importance of social and environmental factors on outcomes such as rates of consumption and excessive consumption and, therefore, still support environmental and policy approaches to the prevention of excessive consumption and AUDs (e.g., Borsari et al., 2007).
Summary of the ETOH Framework
Taken together, the ETOH Framework organizes mechanisms of AUD by arranging them in a hierarchical framework consisting of superdomains, domains, subdomains, and specific components and mechanisms. Relevant mechanisms are grouped into the higher-order superdomains of cognitive control, reward, and negative valence and emotionality. Each superdomain subsumes narrower, hierarchically organized components (i.e., domains, subdomains, and components and mechanisms). In contrast with frameworks such as AARDoC/ANA and NIDA PhAB, the ETOH Framework recommends an increased conceptual role for negative valence and compulsion given their importance in the development and progression of AUD. Further, the current framework designates each mechanism as substance-general or alcohol-specific and premorbid or acquired, features that are not well resolved by other dimensional models of AUD but would appear to be critical for theory, assessment, and treatment. Last, the ETOH Framework describes two key moderating mechanisms, opponent process and self-awareness, which are proposed to exert influence upon the mechanisms included within the framework.
Implications for Research, Diagnosis, and Treatment
Our proposed framework is intended to inform research, diagnosis, and treatment of AUD as well as other forms of psychopathology. We believe that our framework will contribute to the further refinement and adaptation of the AARDoC, ANA, and NIDA PhAB frameworks by providing an alternative approach that considers etiology in combination with dimensional phenotypes, similar to systems such as HiTOP, RDoC, and PhAB.
The ETOH Framework provides a starting point for the consideration of dimensional, transdiagnostic factors related to SUDs and psychopathology more broadly. Take, for example, abnormal reward functioning and negative emotionality. Abnormal reward functioning is implicated in AUD and other disorders (e.g., Major Depressive Disorder; e.g., Forbes, 2009), so it likely serves as a transdiagnostic mechanism that underlies much of psychopathology. Similarly, negative emotionality is implicated in most all forms of psychopathology (Widiger & Oltmanns, 2017; cf., Stanton, 2020). Such transdiagnostic mechanisms may be related to the significant comorbidity observed among AUD and other diagnoses (Krueger & Eaton, 2015). As such, the ETOH Framework provides a useful starting point for examining these factors within the context of SUDs and addiction more broadly. Further, it incorporates etiology and dimensional phenotypes and extends RDoC’s heightened focus on basic units of analysis to self-report and laboratory tasks (see Table 2).
The ETOH Framework has the ability inform the shortcomings of existing diagnostic approaches. By offering an evidence-based and theory-informed mechanistic conceptualization, the ETOH Framework directly addresses issues within traditional nosologies, including that they are characterized by unknown construct validity and fail to systematically consider etiology (e.g., DSM-5, ICD-10/11). Practically, the ETOH framework can be used as a guide for developing AUD criteria that more closely map on to key mechanisms implicated in AUD and facilitate translation between human and nonhuman models.
Further, the ETOH Framework has the ability to resolve some of the within-disorder heterogeneity observed under traditional nosologic approaches by identifying higher order domains that reflect associations among lower-order components. “Lumping” into hierarchical domains based on shared mechanisms can help to reduce the number of AUD symptoms needed to assess AUD and, thus, the possible combinations of AUD symptoms. For example, Table 4 demonstrates how (a) prevailing AUD diagnostic criteria (e.g., DSM-5, ICD-11, ICD-10) map on to multiple mechanisms and (b) some mechanisms implicated in AUD are almost entirely overlooked by current criteria (e.g., those related to punishment sensitivity). This table suggests that current diagnostic criteria may assess more than one relevant etiologic mechanism while neglecting others. Additionally, some current criteria (e.g., hazardous use) are likely etiologically heterogeneous reflecting conceptually and clinically distinct mechanisms (e.g., low conscientiousness vs. compulsion). Thus, the ETOH Framework can help to increase comprehensiveness while decreasing repetitiveness and avoid conflating distinct mechanisms associated with etiologically heterogeneous symptoms.
Table 4.
Overview of Associations between the ETOH Framework and Other Diagnostic Frameworks
| Diagnostic Criteria | ETOH Framework | ||||
|---|---|---|---|---|---|
|
| |||||
| DSM-5 Alcohol Use Disordera | ICD-11 Alcohol Dependenceb | ICD-10 Alcohol Dependencec | Superdomain (Domain) | Domain | Subdomain(s) (or Moderating Variables) |
| Craving | Impaired control over alcohol use | Craving | Reward | Incentive Salience | |
| Drank more or longer than intended | Difficulties controlling use | Cognitive Control | Impulsivity | Response Inhibition, Conscientiousness | |
| Cognitive Control | Compulsivity | Compulsive Use | |||
| Persistent desire or unsuccessful efforts to quit or cut down | Cognitive Control | Impulsivity | Response Inhibition, Conscientiousness | ||
| Cognitive Control | Compulsivity | Compulsive Use | |||
| Important activities given up or reduced to drink | Alcohol use an increasing priority | Higher priority given to use than other activities | Reward | Reward Discounting, Incentive Salience | |
| Failure to fulfill major role obligations due to drinking | Cognitive Control | Compulsivity | Compulsive Use | ||
| Much time spent obtaining, using, or recovering from drinking | Reward | Incentive Salience, Reward Sensitivity, Positive Expectancies | |||
| Negative Valence & Emotionality | Reward Sensitivity, Negative Expectancies | ||||
| Continued use despite physical or psychological harm | Continued use despite harmful consequences | Cognitive Control | Compulsivity | Compulsive Use | |
| Reward | Reward Discounting | ||||
| Negative Valence & Emotionality | Punishment Sensitivity | ||||
| Self-Awareness d | |||||
| Continued use despite social or interpersonal harm | Cognitive Control | Compulsivity | Compulsive Use | ||
| Reward | Reward Discounting | ||||
| Self-Awareness d | |||||
| Tolerance | Physiological features indicative of neuroadaptation to alcohol | Tolerance | Opponent Processes d | ||
| Withdrawal | Withdrawal | Negative Valence & Emotionality | Negative Emotionality | ||
| Recurrent use in hazardous situations | Cognitive Control | Impulsivity | Response Inhibition, Conscientiousness | ||
| Cognitive Control | Compulsivity | Compulsive Use | |||
| Reward | Reward Discounting | ||||
Note.
= Severity gradient for a diagnosis corresponds to ≥ 2 criteria = Mild, ≥ 4 criteria = Moderate, and ≥ 6 criteria = Severe.
= A diagnosis corresponds to ≥ 2 criteria.
= A diagnosis corresponds to ≥ 3 criteria.
= this is treated as a moderating mechanism within the ETOH Framework, meaning it likely moderates the expression of ETOH subdomains.
In addition, developing criteria that align with key mechanisms and organizing them into hierarchical domains may also inform and help to standardize the algorithm used to combine symptoms into a diagnosis. Currently, scoring algorithms vary widely based on the diagnostic framework (e.g., 2/11 symptoms in DSM-5 AUD; 3/6 for ICD-10 alcohol dependence; see Table 4). When this framework is extended to other substances or psychopathologies, it has the potential to clarify sources of comorbidity, which may be due to shared etiologies, by identifying common components of disorders at the domain or subdomain level. For example, consider a person diagnosed with AUD whose risk profile includes a high degree of impulsivity deficits. They also meet diagnostic criteria for antisocial personality disorder by virtue of a chronic, lifelong pattern of criminal behavior. This person’s problems in life may be most parsimoniously explained by broad, transdiagnostic externalizing psychopathology (or disinhibition) rather than alcohol problems per se. Characterizing AUD and other conditions in terms of their components allows us to clarify critical sources of comorbidity among putatively distinct conditions. These more specific sources may be the best treatment targets moving forward.
Clinically, psychopathology could be assessed broadly at the domain-level. Those domains with dysregulation would warrant more specific adaptive assessment to identify the specific subdomains and components of dysregulation within that domain. Thus, a diagnosis would consist of an individual’s dimensional profile of dysregulation which allows for the consideration of multiple domains or mechanisms at once. In this way, the ETOH framework allows for the consideration of unique risk profiles while at the same time aiming to reduce the heterogeneity at the diagnosis level by considering a smaller number of domains. Thus, diagnostic heterogeneity is reduced by operating at the domain-level but not eliminated in that individual profiles of risk can be clarified by finer-grained assessments of specific subdomains or components.
Future research should consider where to draw the necessary cut-offs for clinical decision making. For example, where is the diagnostic threshold for whether someone receives a diagnosis of AUD or not? More specifically, how much dysregulation is required within a given domain for it to be considered problematic? Such questions are especially important given that the ETOH Framework emphasizes a dimensional conceptualization of constructs whereby we consider the full range of the construct from normal to abnormal. Such decisions could be made empirically through the use of quantitative approaches, including factor analysis, item response theory models, and statistical optimization, to name a few.
Another relevant diagnostic issue is whether an individual’s drinking pattern needs to be considered. Although the shift from consequences to mechanisms in defining AUD is in many cases superior, there is the possibility that dysfunction in these domains exists even in the absence of alcohol consumption. For example, one can experience dysfunction in cognitive control and reward mechanisms but not be a current drinker. Thus, it might be necessary to incorporate information on consumption into an AUD diagnosis based on this framework.
The identification of a specific diagnostic profile would have clear indications for treatment. For example, the identification of higher levels of dysregulation (e.g., response inhibition at the subdomain level) could be addressed with interventions focused on treating general, transdiagnostic dysregulation (e.g., through mindfulness-based interventions) or alcohol-specific dysregulation (e.g., with drink refusal skills). Further, based on the superdomains, domains, and subdomains of dysregulation identified, subpopulations most likely to respond to a given treatment or medication could be identified, which may increase the treatment’s effectiveness (Kwako et al., 2017). Relatedly, certain mechanistic domains may serve as useful experimental targets in medication development (Ray et al., 2020). As such, the ETOH Framework is consistent with precision medicine approaches as it would allow certain identification of dysregulation at various levels of the hierarchy to be specifically targeted. Table 5 reflects a preliminary identification of treatments that may effectively target the subdomains within this framework. Although our framework does not focus significantly on psychopathology-general (i.e., transdiagnostic) mechanisms, we acknowledge that several of the components described throughout are indeed transdiagnostic (e.g., reward sensitivity, negative emotionality) and are therefore potentially useful targets for addressing psychopathology broadly construed. Future research could focus on more explicitly classifying the superdomains within the given framework based on their shared patterns of impairment with other forms of psychopathology and identifying or developing treatments that directly target each of the identified superdomains, domains, subdomains, or components with the most dysfunction.
Table 5.
Relevant Interventions for Targeting Alcohol Use Disorder Mechanisms at the Sub-Domain Level
| Interventions |
|||
|---|---|---|---|
| Superdomain | Subdomain | Transdiagnostic | Alcohol-Specific |
|
| |||
| Cognitive Control | Conscientiousness | Episodic future thinking | Episodic future thinking |
| Functional analysis (DBT) | Behavioral self-control training | ||
| Cognitive Remediation Therapy | Self-management planning (CBT for AUD) | ||
|
| |||
| Response Inhibition | Mindfulness-based intervention | Mindfulness-based addiction treatment | |
| Functional analysis (DBT) | Drink refusal skills | ||
|
| |||
| Compulsive Use | Mindfulness-based intervention | Acamprosate | |
| Motivational Enhancement Training | Exposure and response prevention | ||
|
| |||
| Reward | Habit | Mindfulness-based intervention | Mindfulness-based addiction treatment |
| Exposure-based interventions | -- | ||
|
| |||
| Positive Expectancies | Cognitive restructuring (CBT) | Cognitive restructuring (CBT for AUD) | |
| Cognitive bias modification training | Cognitive bias modification training | ||
| -- | Contingency management | ||
|
| |||
| Reward Sensitivity | Mindfulness-based intervention | Naltrexone | |
|
| |||
| Positive Emotionality | Cognitive restructuring (CBT) | Substance Free Activity Session | |
| -- | Alternatives to drinking (CBT for AUD) | ||
|
| |||
| Incentive Salience | Cue exposure | Naltrexone | |
| Attentional bias modification training | Attentional bias modification training | ||
| Mindfulness-based intervention | Mindfulness-based addiction treatment | ||
|
| |||
| Reward Discounting | Contingency management | Community Reinforcement Approach | |
|
| |||
| Negative Valence & Emotionality | Negative Emotionality | Cognitive restructuring (CBT) | Cognitive restructuring (CBT for AUD) |
| Unified Protocol | Mindfulness-based relapse prevention | ||
| Emotion regulation skills (DBT) | -- | ||
|
| |||
| Coping | Coping skills training | Relapse prevention | |
| Distress tolerance skills (DBT) | Acamprosate | ||
| Emotion regulation skills (DBT) | -- | ||
|
| |||
| Punishment Sensitivity | -- | Disulfiram | |
| -- | Aversive conditioning (emetic and covert sensitization) | ||
|
| |||
| Negative Expectancies | Cognitive restructuring (CBT) | Cognitive restructuring (CBT for AUD) | |
| Cognitive bias modification training | Cognitive bias modification training | ||
| -- | Contingency management | ||
Note. This table is only intended to serve as an example and is not an exhaustive list of interventions. Interventions within a given domain are also likely to be effective across the sub-domains as well. This table is intended to elucidate mechanistic targets and are not necessarily representative of the latest evidence of efficacy for any given treatment. Mindfulness-based addiction treatment can include mindfulness-based relapse prevention. AUD = alcohol use disorder; CBT = cognitive behavioral therapy; DBT = dialectical behavior therapy
Limitations and Future Directions
Although the systematic review of reviews approach provides a more methodical way of considering literature on a given topic when compared to techniques such as the narrative review, there still remain several limitations. Most notably, the synthesis of findings in a systematic review of reviews is still largely subjective in nature. This methodology can introduce a level of imprecision when compared to an approach such as meta-analysis which, arguably, may be viewed as more objective. Further, due to this subjectivity, such syntheses may therefore be vulnerable to various biases held by the authors. The use of different decision rules throughout the systematic review of reviews process may also result in dissimilar conclusions. To combat this potential for bias, we have attempted to be fully transparent in our search strategies, decision making, and “researcher degrees of freedom” within the main text as well as by providing our codebook online (https://osf.io/xz83s/). Despite these limitations, it is our opinion that the systematic review of reviews method still provides a useful methodology for characterizing the literature on a broad topic area and synthesizing information in a way that offers a valuable starting point for answering additional questions in future research. This is not to say that the framework would not benefit from more targeted meta-analytic work that aims to clarify some of the specific relationships between a given mechanism and AUD moving forward.
One limitation of the ETOH framework, as well as other classification frameworks, is that it fails to fully account for overlap between domains. Although the RDoC website (NIMH, 2020) describes RDoC-informed research as being guided by the principle of assuming “interactions among constructs,” the research on this topic is essentially nonexistent at this point in time, making it unclear how to best account for such interactions. Relatedly, the superdomains, domains, and subdomains delineated here may be neither conceptually nor empirically distinct in practice, bringing up the historical problem of how to best “carve nature at its joints” and suggests there may be value in the combined consideration of multiple domains/components and their overlap. A related issue to consider is that certain superdomains (e.g., negative emotionality, cognitive control/executive function) may interact to predict alcohol consumption and disorder (Witkiewitz et al., 2019). Indeed, some research (e.g., Jentsch & Taylor, 1999) has demonstrated that as incentive habit increases, executive function decreases, suggesting an important interplay among at least some of the mechanisms within the current framework.
The issue around carving nature at its joints requires consideration of another common problem in psychopathology research: “jingle” and “jangle” fallacies (Block, 1995). The jingle fallacy refers to the phenomenon whereby two different constructs are given the same label, whereas the jangle fallacy refers to the same construct being given different names. These fallacies are common in psychopathology research and, unfortunately, thwart progress towards cumulative knowledge. Any useful framework, especially one that is translational in nature, must be careful to consider such fallacies. We must continue to cautiously consider what qualifies as a relevant construct within the current framework (e.g., whether negative emotionality and negative valence are indeed distinct), as well as the minimum qualifications for including a given construct, both of which remains to be fully resolved. Additionally, we must aim to refine language for basic and clinical phenotypes such that translational research can be more easily facilitated (see Ray et al., 2020 for a review). Despite these limitations and remaining questions, we are confident that this framework provides an evidence-based etiologic framework for AUD that is informed by the extant literature and serves as a necessary starting point for further clarifying these remaining issues, especially empirically.
A major challenge for this framework is related to the measurement and assessment of the domains described, particularly given the wide range of constructs and mechanisms included. A multimodal assessment approach (i.e., the use of multiple assessments that comprise different modalities such as self-report or behavioral tasks) – such as that commonly recommended in attention-deficit/hyperactivity disorder evaluations (Ferguson, 2000) – may prove useful. Multimodal assessment would allow the flexibility required for measuring a wide range of constructs, which is vital considering some constructs (e.g., discounting, extinction, self-awareness) that are not easily or most accurately measured solely by self-report assessments. However, this approach leads to several practical issues such as how to integrate information from multiple measures, how to deal with method variance (Lilienfeld & Treadway, 2016), and how to design an assessment battery that is reliable, valid, efficient, and appropriate for its intended use (e.g., research versus clinical use). In contrast to this approach, emerging work suggests that the AARDoC domains – specifically the negative emotionality domain – can be validated solely on the basis of self-report (among individuals seeking treatment for AUD; e.g., Votaw et al., 2020). Such issues are imperative to consider and resolve before this framework can be used appropriately.
Future work should more explicitly and systematically consider how these AUD mechanisms are related to the stages of addiction. That is, are some mechanisms more relevant as premorbid risk factors (i.e., vulnerability) or as consequences of use (i.e., chronic adaptation)? For example, some superdomains (e.g., negative emotionality) may become increasingly relevant at later stages of addiction (e.g., withdrawal) than others. In fact, experts have explicitly acknowledged the relevance of each mechanism at different stages as an important issue to consider among the conceptualization of addiction (Yücel et al., 2019). Further consideration of staging would result in a more comprehensive framework of AUD as a chronic, progressive disorder. Better understanding staging would also, in theory, allow identification of dysregulation in these mechanisms by their stage which could inform the level of prevention that is indicated (i.e., primary, secondary, or tertiary; Leavell & Clark, 1958). Staging is an approach that is becoming increasingly recommended in psychiatry (Maj, 2020) and is consistent with general medicine’s use of staging models for physical disorders such as cancer and diabetes (e.g., Scott et al., 2013). It relies on the assumption that stage-appropriate treatment can modify disease progression, an assumption that is tenable as AUD mechanisms and phenotypes become better understood. Interestingly, this consideration has been largely neglected within mechanistic frameworks of psychopathology to date. Clearly, though, there is utility in continuing to consider the stages of addiction within this developing framework.
A particular challenge is the interconnections across domains. For example, despite being conceptually distinct, incentive salience appears to be related to impulsive action more generally, at least in non-human animals (e.g., Lovic et al., 2011). Thus, there can be correlations between distinct mechanisms that make identification of critical mechanisms more complex. Such correlations highlight the importance of studying these different domains together so as not to misidentify a critical mechanism. It also highlights the preceding issue of staging in that we need to think of cross-domain progression that reflects the possibility of shared underlying or transactional processes.
Another major challenge related to staging is the fact that the domains outlined here can, in many cases, be premorbid and acquired as a result of chronic adaptation. This challenge raises important issues related to diagnostic thresholds and treatment goals. First, regarding diagnostic thresholds, it is quite possible one’s standing on the dimension (e.g., level of the trait) could be just below the diagnostic threshold prior to drinking initiation (i.e., premorbid). Thus, as consumption continues, they are quickly “pushed over” the diagnostic threshold within that superdomain (e.g., cognitive control). In contrast, one could have very little impairment prior to initiation, and then quickly exceed the diagnostic threshold with chronic consumption (i.e., acquired). Thus, these individuals’ “baseline” levels of impairment are very different, but both would exceed the diagnostic threshold. Such a difference may or may not be clinically meaningful, but it is worth further considering how some mechanisms may vary in the degree to which they are premorbid or acquired across individuals. We must then consider additional questions related to treatment goals and targets. For example, should the goal be to resolve the impairment to baseline levels, or to just below the diagnostic threshold? It will be necessary to consider these issues as the ETOH Framework (and other related frameworks) continue to develop.
We look forward to additional research focused on extending the ETOH Framework to other substances and addiction. This additional work is especially important given the high rate of polysubstance use in the general population (e.g., Connor et al., 2014) and the increasing need to account for polysubstance use in SUD research (Rounsaville et al., 2003). Although we would not expect the framework’s main superdomains to change significantly, additional domains, subdomains, or components may be elucidated by incorporating the consideration of other substances and polysubstance use.
Future research should also aim to resolve known limitations of translational work within the field of addiction. For example, translational approaches may be inherently limited in that some aspects of AUD, and SUDs more generally, may be uniquely human (e.g., craving, failed attempts to quit or control use) and, therefore, difficult to translate from, and model in, nonhuman animals (e.g., Bickel et al., 2019). This limitation highlights the known problem that human phenotypes have yet to reach consilience with non-human animal models and suggests a clear area in need of more research.
In addition, further work must prioritize consideration of how each ETOH mechanism contributes to AUD, and if they do so equally, across individuals of diverse backgrounds and environments. That is, are all mechanisms equally implicated in AUD across people? Although literature in this area is lacking, we anticipate the answer to this question is “no.”
It is likely that the strength of the relationship between a given ETOH mechanism and AUD varies based on attributes of individuals such as sociodemographic factors (e.g., sex, gender, race, ethnicity, socioeconomic status). For instance, subjective response to alcohol and craving, well-known predictors of AUD, may be influenced by endogenous factors such as ovarian hormones (see McHugh et al., 2018 for a review). Further, there is evidence for sex and gender differences in stress and negative affect responses such that women tend to have higher temperamental negative emotionality. Thus, the link between negative affect and AUD may be heightened among females compared to males, especially since they may be more likely to experience stressful life events (Allan & Cooke, 1985; Brady & Sonne, 1999; see Guinle & Sinah, 2020 for a review). Ultimately, the relationship between specific ETOH mechanisms and AUD may be moderated by sex and gender. Indeed, some work supports the notion that there may be stronger associations between the risk factors that women experience and problematic alcohol outcomes when compared to men (see Foster et al., 2015 for a discussion of mean versus structural gender effects in the etiology of AUD). In addition, associations between stressful events and SUDs vary for both men and women as a function of sexual orientation, such that gay and bisexual men and all sexual minority subgroups of women report higher rates of SUD than do heterosexual individuals. Consistent with the potential that sociodemographic factors may moderate the relations between ETOH mechanisms and AUD, the relations between sexual minority status and SUDs appear further mediated by stressful life events and perceived discrimination (e.g., Krueger et al., 2020).
Ultimately, the role of sociodemographics in the associations between ETOH mechanisms and AUD is complex but critical to consider given that holding a marginalized identity or identities is likely associated with disparities in SUD diagnosis and treatment. Future research must systematically recognize and explore how mechanisms may be differentially implicated in AUD on the basis of sociodemographic factors. A shift towards mechanistic conceptualizations of AUD will appears promising for reducing bias compared with dominant consequence- or problem-based conceptualizations of AUD (e.g., Zapolski et al., 2014).
The next logical step in the development of this framework is to begin empirically testing the conceptual model (consistent with the movement towards quantitative nosology; see Hopwood et al., 2019). This type of testing is likely to be best achieved through a combination of laboratory-based tasks and self-report, amenable to a multimodal assessment approach and multitrait-multimethod validation approaches (Campbell & Fiske, 1959). Such an evaluation will help elucidate the areas in need of further development and refinement and aid in decision making regarding important features such as statistically derived cutoff scores. This work may also serve as an important starting point for the creation of a flexible diagnostic measure informed by fundamental mechanisms which can be used to aid in making subsequent treatment decisions. Such a diagnostic measure could start by assessing higher levels of the framework (i.e., superdomain-level dysfunction) and progress to more focused assessments based on this information (see Hopwood et al., 2019 for an example using the HiTOP hierarchy and suggestions on integrating such a system into psychotherapy).
Summary
This is the first systematic research synthesis to integrate the AUD literature into an etiologic, theory-based, ontogenetic hierarchical framework. The resulting ETOH Framework addresses challenges with traditional nosologic systems and more recent dimensional models of AUD by integrating etiologic mechanisms into a comprehensive framework that prioritizes construct validity and directly considers (a) how mechanisms may be relevant at different stages of the addiction cycle (e.g., premorbid versus acquired) and (b) which mechanisms may be alcohol-specific or substance-general. Importantly, it also extends the AARDoC/ANA model to include negative valence and compulsivity, as well as the moderators of opponent process and self-awareness. The ETOH Framework serves as an important starting place for reducing phenotypic heterogeneity and comorbidity with other forms of psychopathology by lumping related mechanisms into higher order domains which can be used to inform diagnosis (e.g., criteria and algorithms for combining them, differential diagnosis), assessment (e.g., adaptive testing based domains of dysregulation), and treatment of AUD (e.g., treatment matching based on profiles of dysfunction and stage). It is our hope that this framework will continue to be modified on an ongoing basis as the literature progresses rather than serve as a static model.
Public Significance Statement:
Alcohol use disorder is a significant public health problem and there is a need for improving the identification and treatment of individuals with the disorder. The current review aims to identify the casual factors implicated in alcohol use disorder by synthesizing key findings into a comprehensive etiologic framework. This framework serves as a starting point for additional research and offers several implications for the diagnosis and treatment of alcohol use disorder.
Acknowledgments
The present study was supported by NIH grants F31AA026177 (PI: Boness) and K99AA028306 (PI: Watts). The funders played no role in the current review. We would like to thank John Crabbe, Uma Vaidyanathan, and Tori Votaw for feedback on previous versions of this manuscript and Harris Cooper for consulting on quality evaluations. We would also like to acknowledge the contributions of Adam Arand, Natalie Gatten, Jennifer Gray, Stella Peters, Sydney Rapp, and McKenna Treece who served as coders. We’d also like to thank the anonymous reviewers and editor for their useful comments on earlier versions of this manuscript.
Footnotes
Supporting materials for this manuscript, including a full codebook, can be found at the following link: https://osf.io/xz83s/
Much of our review focuses on the distinction between premorbid and acquired features of AUD, although it is worth noting that the term “premorbid” can take on two different meanings. Here, we distinguish premorbid and dispositional. Not all premorbid characteristics are dispositional, but all dispositional characteristics are premorbid. What distinguishes them is chronicity. Dispositional characteristics are definitionally more chronic than are premorbid characteristics that are not dispositional. Consider trait reward sensitivity, a relatively stable individual difference characteristic, as an example of a dispositional feature implicated in AUD. When reward sensitivity is assessed early in life, even late childhood, it predicts AUD onset. In this case, trait reward sensitivity is premorbid because it precedes AUD onset. Consider a depressive episode as an example of a premorbid characteristic that is not necessarily dispositional. A single depressive episode may elicit AUD, but if it is the first and only episode, it is not considered dispositional. In contrast, in the case of a person who experiences chronic depressivity, such as dysthymia with depressive episodes (“double depression”), a single depressive episode may be better characterized as dispositional. Ultimately, we highlight the potential difference between premorbid and dispositional because the stability or chronicity, in addition to severity, of features that predict onset or elicit AUD is likely important to consider in treatment. We suspect AUD features may be differentially malleable based on their chronicity.
This conclusion is supported by considerable research in the general personality literature, where the convergence of negative emotionality and aggression has long been termed a trait called Psychoticism (Eysenck & Eysenck, 1975); it is important to note that Psychoticism is almost certainly misnamed, and most argue that it reflects antisocial personality features, as opposed to psychotic-like experiences.
In addition, this polymorphism cannot serve as a mechanism in White and Black populations because it is absent, making it not only alcohol-specific but also population-specific (Vanyukov et al., 2003).
Of note, Ma, Fan, & Li (2016) published an erratum. For the present review, we maintained the original article given it was more comprehensive, but used the erratum (Ma et al., 2017) for the purposes of coding their findings and conclusions.
Forward and backward searching was conducted on 122 reviews. An additional 2 reviews (Jackson et al., 2014; Murphy et al., 2012) were included based on feedback during the revise and resubmit process. Although originally excluded due to a sole focus on consumption rather than AUD, these two additions focus on excessive or heavy consumption, therefore fitting the criteria of this review.
Although, a more dated review included in this synthesis suggested that individuals with addiction may actually overrespond to reinforcers (Berridge & Robinson, 2006).
We have retained conscientiousness and response inhibition as separate subdomains because the broader literature describes them as empirically distinct (Cyders & Coskunpinar, 2011, 2012) despite the fact that they are conceptually overlapping. We suspect part of the reason they are described as distinct is because the two are studied in different silos. Conscientiousness is typically probed with questionnaires and response inhibition is typically probed with laboratory tasks, and so method variance precludes considerable overlap between measures of these constructs.
Notably, ICD-11 also includes a single impaired control criterion but extends the ICD-10 definition to include “…a subjective sensation of urge or craving to use alcohol” (Saunders et al., 2019, p.5).
We use the phrase “broadly defined” because lack of awareness is a multifaceted construct that has been described by a number of terms. For example, in the neuropsychiatry addiction literature, this process has been referred to as “anosognosia,” or an ignorance of the presence of disease (e.g., Le Berre & Sullivan, 2016), and in the psychodynamic and psychosocial addiction literature as “alcoholic denial” which is loosely defined, but sometimes used to refer to a lack of insight (Sher & Epler, 2004). Given research that suggests these constructs are likely encompassed by a more general lack of awareness (David et al., 2012), meaning the distinctions between these terms are likely arbitrary, we chose the label “lack of awareness” to describe this subdomain.
Contributor Information
Cassandra L. Boness, Department of Psychological Science, University of Missouri
Ashley L. Watts, Department of Psychological Science, University of Missouri
Kimberly N. Moeller, Instructional Services, University of Missouri
Kenneth J. Sher, Department of Psychological Science, University of Missouri
References
- *Adler R, & Raphael B (1983). Children of alcoholics. Australian and New Zealand Journal of Psychiatry, 17(1), 3–8. 10.3109/00048678309159980 [DOI] [PubMed] [Google Scholar]
- *Agarwal DP, & Goedde HW (1989). Human aldehyde dehydrogenases: Their role in alcoholism. Alcohol, 6(6), 517–523. 10.1016/0741-8329(89)90061-X [DOI] [PubMed] [Google Scholar]
- *Agrawal A, & Lynskey MT (2014). Genetics of Substance Use Disorders. In Rhee S & Ronald A (Eds.), Behavior Genetics of Psychopathology (pp. 185–230). Springer, New York, NY. 10.1007/978-1-4614-9509-3_7 [DOI] [Google Scholar]
- *Åkerlind I, & Hörnquist JO (1992). Loneliness and alcohol abuse: A review of evidences of an interplay. Social Science & Medicine, 34(4), 405–414. 10.1016/0277-9536(92)90300-F [DOI] [PubMed] [Google Scholar]
- Akram Y, Copello A, & Moore D (2014). Family-based interventions for substance misuse: a systematic review of systematic reviews---protocol. Systematic Reviews, 3(1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Allan CA, & Cooke DJ (1985). Stressful life events and alcohol misuse in women: A critical review. Journal of Studies on Alcohol, 46(2), 147–152. 10.15288/jsa.1985.46.147 [DOI] [PubMed] [Google Scholar]
- *Alterman AI, & Tarter RE (1983). The transmission of psychological vulnerability: Implications for alcoholism etiology. Journal of Nervous and Mental Disease, 171(3), 147–154. 10.1097/00005053-198303000-00003 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th Ed.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd Ed.). Arlington, VA: Author. [Google Scholar]
- American Psychiatric Association. (1968). Diagnostic and statistical manual of mental disorders (2nd Ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (1952). Diagnostic and statistical manual of mental disorders (1st Ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, … & McCabe RE (2019). Delay Discounting as a transdiagnostic Process in psychiatric disorders. JAMA Psychiatry, 76(11), 1176–1186. 10.1001/jamapsychiatry.2019.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Amlung M, Vedelago L, Acker J, Balodis I, & MacKillop J (2017). Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction, 112(1), 51. 10.1111/add.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Briggs KE, & White HR (2013). Motives to drink or not to drink: Longitudinal relations among personality, motives, and alcohol use across adolescence and early adulthood. Alcoholism: Clinical and Experimental Research, 37, 860–867. 10.1111/acer.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Anthenelli RM, & Schuckit MA (1990). Genetic studies of alcoholism. International Journal of the Addictions, 25(1-A), 81–94. 10.3109/10826089009067006 [DOI] [PubMed] [Google Scholar]
- Aragona M (2009). The role of comorbidity in the crisis of the current psychiatric classification system. Philosophy, Psychiatry, & Psychology, 16(1), 1–11. [Google Scholar]
- *Arias A, Feinn R, & Kranzler HR (2006). Association of an Asn40Asp (A118G) polymorphism in the µ-opioid receptor gene with substance dependence: A meta-analysis. Drug and Alcohol Dependence, 83(3), 262–268. 10.1016/j.drugalcdep.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Armeli S, Tennen H, Affleck G, & Kranzler HR (2000). Does affect mediate the association between daily events and alcohol use?. Journal of Studies on Alcohol, 61(6), 862–871. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, & Wileyto EP (2009). Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence, 103(3), 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ, Gerst K, & Finn PR (2018). Delay discounting of losses and rewards in alcohol use disorder: The effect of working memory load. Psychology of Addictive Behaviors, 32(2), 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Barry AE (2007). Using theory-based constructs to explore the impact of Greek membership on alcohol-related beliefs and behaviors: A systematic Lit. Review. Journal of American College Health, 56(3), 307–315. 10.3200/JACH.56.3.307-316 [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2), 227–239. [DOI] [PubMed] [Google Scholar]
- *Benyamina A, Kebir O, Blecha L, Reynaud M, & Krebs M-O (2011). CNR1 gene polymorphisms in addictive disorders: A systematic review and a meta-analysis. Addiction Biology, 16(1), 1–6. Retrieved from 10.1111/j.1369-1600.2009.00198.x [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, & Everitt BJ (2013). Addiction: Failure of control over maladaptive incentive habits. Current Opinion in Neurobiology, 23(4), 564–572. 10.1016/J.CONB.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, & Everitt BJ (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science, 320(5881), 1352–1355. 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, & Belin D (2016). How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biological Psychiatry, 79(1), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (1996). Food reward: Brain substrates of wanting and liking. Neuroscience & Biobehavioral Reviews, 20(1), 1–25. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?. Brain Research Reviews, 28(3), 309–369. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. [DOI] [PubMed] [Google Scholar]
- *Berridge KC, & Robinson TE (2006). Automatic processes in addiction: A commentary. Handbook of Implicit Cognition and Addiction, 477–481. Department of Psychology, University of Michigan: SAGE Publications Inc. 10.4135/9781412976237.n31 [DOI] [Google Scholar]
- Berridge KC, & Robinson TE (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71(8), 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bhaskar LVKS, & Kumar SA (2014). Polymorphisms in genes encoding dopamine signaling pathway and risk of alcohol dependence: A systematic review. Acta Neuropsychiatrica, 26(2), 69–80. 10.1017/neu.2013.27 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Crabbe JC, & Sher KJ (2019). What is addiction? How can animal and human research be used to advance research, diagnosis, and treatment of alcohol and other substance use disorders? Alcoholism: Clinical and Experimental Research, 43(1), 6–21. 10.1111/acer.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, & McClure SM (2012). Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology, 221(3), 361–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, & Murphy JG (2014). The behavioral economics of substance use disorders: Reinforcement pathologies and their repair. Annual Review of Clinical Psychology, 10, 641–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, & Baxter C (2011). Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry, 69(3), 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell L, MacKillop J, Murphy JG, Grenga A, Swift RM, & McGeary JE (2013). Biphasic effects of alcohol on delay and probability discounting. Experimental and Clinical Psychopharmacology, 21(3), 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J (1995). A contrarian view of the five-factor approach to personality description. Psychological Bulletin, 117, 187–215. [DOI] [PubMed] [Google Scholar]
- Boness CL, Lane SP, & Sher KJ (2019). Not all alcohol use disorder criteria are equally severe: Toward severity grading of individual criteria in college drinkers. Psychology of Addictive Behaviors, 33(1), 35–49. 10.1037/adb0000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Borsari B, Hustad JTP, & Capone C (2009). Alcohol use in the Greek system, 1999-2009: A decade of progress. Current Drug Abuse Reviews, 2(3), 216–255. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20443768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsari B, Murphy JG, & Barnett NP (2007). Predictors of alcohol use during the first year of college: Implications for prevention. Addictive Behaviors, 32(10), 2062–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottlender M, & Soyka M (2005). Impact of different personality dimensions (NEO Five-Factor Inventory) on the outcome of alcohol-dependent patients 6 and 12 months after treatment. Psychiatry Research, 136(1), 61–67. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, & Oscar-Berman M (2005). Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 132(1), 29–37. [DOI] [PubMed] [Google Scholar]
- *Brady KT, & Back SE (2012). Childhood trauma, posttraumatic stress disorder, and alcohol dependence. Alcohol Research: Current Reviews 34(4), 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Brady KT, & Sonne SC (1999). The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Research and Health, 23(4), 263–271. [PMC free article] [PubMed] [Google Scholar]
- *Brennan AF, Walfish S, & AuBuchon P (1986). Alcohol use and abuse in college students: II. Social/environmental correlates, methodological issues, and implications for intervention. International Journal of the Addictions, 21(4-5), 475–493. 10.3109/10826088609083537 [DOI] [PubMed] [Google Scholar]
- Brown TA, & Barlow DH (2009). A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: Implications for assessment and treatment. Psychological Assessment, 21(3), 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bühler M, & Mann K (2011). Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcoholism: Clinical & Experimental Research, 35(10), 1771–1793. 10.1111/j.1530-0277.2011.01540.x [DOI] [PubMed] [Google Scholar]
- *Buisman-Pijlman FTA, Sumracki NM, Gordon JJ, Hull PR, Carter CS, & Tops M (2014). Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacology Biochemistry and Behavior, 119, 22–38. 10.1016/j.pbb.2013.09.005 [DOI] [PubMed] [Google Scholar]
- *Cablov L, Pazderkov K, & Miovsk M (2014). Parenting styles and alcohol use among children and adolescents: A systematic review. Drugs: Education, Prevention & Policy, 21(1), 1–13. 10.3109/09687637.2013.817536 [DOI] [Google Scholar]
- Campbell DT, & Fiske DW (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin, 56(2), 81–105. [PubMed] [Google Scholar]
- *Cao J, Hudziak JJ, & Li D (2013a). Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology, 38(9), 1737–1747. 10.1038/npp.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cao J, LaRocque E, & Li D (2013b). Associations of the 5-hydroxytryptamine (serotonin) receptor 1B gene (HTR1B) with alcohol, cocaine, and heroin abuse. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(2), 169–176. 10.1002/ajmg.b.32128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, & Herman CP (1972). Alcohol and tension reduction; A review. Quarterly Journal of Studies on Alcohol, 33(1), 33–64. [PubMed] [Google Scholar]
- Carpenter KM, & Hasin DS (1998). Reasons for drinking alcohol: Relationships with DSM-IV alcohol diagnoses and alcohol consumption in a community sample. Psychology of Addictive Behaviors, 12(3), 168. [Google Scholar]
- Charney DS, Barlow DH, Botteron K, Cohen JD, Goldman D, Gur RE, et al. (2002). Neuroscience research agenda to guide development of a pathophysiologically based classification system. In Kupfer DJ, First MB, & Regier DA (Eds.), A research agenda for DSM–IV (pp. 31–83). Washington, DC: American Psychiatric Association. [Google Scholar]
- Chassin L, Sher KJ, Hussong A, & Curran P (2013). The developmental psychopathology of alcohol use and alcohol disorders: Research achievements and future directions. Development and Psychopathology, 25(4 0 2), 1567–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Chen D, Liu L, Xiao Y, Peng Y, Yang C, & Wang Z (2012). Ethnic-specific meta-analyses of association between the OPRM1 A118G polymorphism and alcohol dependence among Asians and Caucasians. Drug & Alcohol Dependence, 123(1-3), 1–6. 10.1016/j.drugalcdep.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Cho S. Bin, Su J Kuo SIC, Bucholz KK, Chan G, Edenberg HJ, … & Dick DM (2019). Positive and negative reinforcement are differentially associated with alcohol consumption as a function of alcohol dependence. Psychology of Addictive Behaviors, 33(1), 58–68. 10.1037/adb0000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Clarke T-K, Nymberg C, & Schumann G (2012). Genetic and environmental determinants of stress responding. Alcohol Research: Current Reviews, 34(4), 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaizzi JM, Flagel SB, Joyner MA, Gearhardt AN, Stewart JL, & Paulus MP (2020). Mapping sign-tracking and goal-tracking onto human behaviors. Neuroscience and Biobehavioral Reviews, 111(January), 84–94. 10.1016/j.neubiorev.2020.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, & Chassin L (1997). Affectivity and impulsivity: Temperament risk for adolescent alcohol involvement. Psychology of Addictive Behaviors, 11(2), 83–97. [Google Scholar]
- Colder CR, Chassin L, Lee MR, & Villalta IK (2010). Developmental perspectives: Affect and adolescent substance use. In Kassel JD (Ed.), Substance abuse and emotion (pp. 109–135). American Psychological Association. [Google Scholar]
- *Collins AC (1990). Genetic influences on tobacco use: A review of human and animal studies. International Journal of the Addictions, 25(1-A), 35–55. 10.3109/10826089009067004 [DOI] [PubMed] [Google Scholar]
- Colrain IM, & Baker FC (2012). Sleep EEG as a potential marker of alcoholism predisposition-Commentary on “Adolescence and parental history of alcoholism: Insights from the sleep EEG.” Alcoholism: Clinical & Experimental Research, 36(9), 1477–1478. 10.1111/j.1530-0277.2012.01892.x [DOI] [PubMed] [Google Scholar]
- Compton WM, Saha TD, Conway KP, Grant BF (2009). The role of cannabis use within a dimensional approach to cannabis use disorders. Drug and Alcohol Dependence, 100, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, & Quinn EP (1995). The Smoking Consequences Questionnaire-Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment, 7(4), 484. [Google Scholar]
- Connor JP, Gullo MJ, White A, & Kelly AB (2014). Polysubstance use: Diagnostic challenges, patterns of use and health. Current Opinion in Psychiatry, 27(4), 269–275. 10.1097/YCO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Stewart SH, Comeau N, & Maclean AM (2006). Efficacy of cognitive–behavioral interventions targeting personality risk factors for youth alcohol misuse. Journal of Clinical Child and Adolescent Psychology, 35(4), 550–563. [DOI] [PubMed] [Google Scholar]
- Cooper ML (1994). Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment, 6, 117–128. [Google Scholar]
- Cooper H (2015). Research synthesis and meta-analysis: A step-by-step approach (Vol. 2). Sage Publications. [Google Scholar]
- Cooper H, & Koenka AC (2012). The overview of reviews: Unique challenges and opportunities when research syntheses are the principal elements of new integrative scholarship. American Psychologist, 67(6), 446–462. [DOI] [PubMed] [Google Scholar]
- *Cooper SE (1983). Survey of studies on alcoholism. International Journal of the Addictions, 18(7), 971–985. 10.3109/10826088309033064 [DOI] [PubMed] [Google Scholar]
- Corbit LH, & Janak PH (2016). Habitual alcohol seeking: neural bases and possible relations to alcohol use disorders. Alcoholism: Clinical and Experimental Research, 40(7), 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, & Cyders MA (2013). Multidimensionality in impulsivity and alcohol use: A meta-analysis using the UPPS model of impulsivity. Alcoholism: Clinical and Experimental Research, 37(9), 1441–1450. 10.1111/acer.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT Jr, & McCrae RR (1998). Six approaches to the explication of facet-level traits: Examples from conscientiousness. European Journal of Personality, 12(2), 117–134. [Google Scholar]
- Cox WM, & Klinger E (1988). A motivational model of alcohol use. Journal of Abnormal Psychology, 97(2), 168–180. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kendler KS, & Hitzemann RJ (2011). Modeling the diagnostic criteria for alcohol dependence with genetic animal models. Behavioral Neurobiology of Alcohol Addiction, 13, 187–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, & Coskunpinar A (2011). Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review, 31(6), 965–982. [DOI] [PubMed] [Google Scholar]
- Cyders MA, & Coskunpinar A (2012). The relationship between self-report and lab task conceptualizations of impulsivity. Journal of Research in Personality, 46(1), 121–124. [Google Scholar]
- Cyders MA, & Smith GT (2007). Mood-based rash action and its components: Positive and negative urgency. Personality and Individual Differences, 43, 839–850. 10.1016/j.paid.2007.02.008 [DOI] [Google Scholar]
- Cyders MA, & Smith GT (2008). Emotion-based dispositions to rash action: Positive and negative urgency. Psychological Bulletin, 134(6), 807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P, Bolo N, Nédélec JF, Muzet M, Durbin P, & Macher JP (1998). Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Research: Neuroimaging, 82(2), 107–114. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, & Robbins TW (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69(4), 680–694. [DOI] [PubMed] [Google Scholar]
- Dang J, King KM, & Inzlicht M (2020). Why Are self-report and behavioral measures weakly correlated?. Trends in Cognitive Sciences, 24(4), 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, & Gilleen J (2012). Failures of metacognition and lack of insight in neuropsychiatric disorders. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1594), 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Shanley B, & Wilce P (1995). Increased NMDA-induced excitability during ethanol withdrawal: a behavioural and histological study. Brain Research, 674(1), 91–96. [DOI] [PubMed] [Google Scholar]
- *Davis JR, & Tunks E (1990). Environments and addiction: A proposed taxonomy. International Journal of the Addictions, 25(sup7), 805–826. 10.3109/10826089109071025 [DOI] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Ahern DC, & Clark US (2015). Executive functioning in alcohol use studies: A brief review of findings and challenges in assessment. Current drug abuse reviews, 8(1), 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Carroll KM, & Sofuoglu M (2016). Toward refinement of our understanding of the fundamental nature of addiction. Biological Psychiatry, 80(3), 172–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dew B, Elifson K, & Dozier M (2007). Social and environmental factors and their influence on drug use vulnerability and resiliency in rural populations. Journal of Rural Health Official Journal of the American Rural Health Association and the National Rural Health Care Association, 23(s1), 16–21. 10.1111/j.1748-0361.2007.00119.x [DOI] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, & Allen JA (2012). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. Journal of Neurodevelopmental Disorders, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dick DM, Barr P, Guy M, Nasim A, & Scott D (2017). Review: Genetic research on alcohol use outcomes in African American populations: A review of the literature, associated challenges, and implications. American Journal on Addictions, 26(5), 486. 10.1111/ajad.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, & Sher K (2010). Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology, 15(2), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, & Lang AR (2007). Intoxication level and emotional response. Emotion, 7(1), 103–112. [DOI] [PubMed] [Google Scholar]
- *Doria JJ (1995). Gene variability and vulnerability to alcoholism. Alcohol Health & Research World, 19(3), 245–248. [PMC free article] [PubMed] [Google Scholar]
- *Du Y, Nie Y, Li Y, & Wan Y-JY (2011). The association between the SLC6A3 VNTR 9-repeat allele and alcoholism-A Meta-Analysis. Alcoholism: Clinical & Experimental Research, 35(9), 1625–1634. 10.1111/j.1530-0277.2011.01509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, & Gross MM (1976). Alcohol dependence: Provisional description of a clinical syndrome. British Medical Journal, 1, 1058–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *El-Guebaly N (1995). Alcohol and polysubstance abuse among women. The Canadian Journal of Psychiatry/La Revue Canadienne de Psychiatrie, 40(2), 73–79. 10.1177/070674379504000204 [DOI] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, & Iacono WG (2006). Personality traits and the development of nicotine, alcohol, and illicit drug disorders: Prospective links from adolescence to young adulthood. Journal of Abnormal Psychology, 115(1), 26. [DOI] [PubMed] [Google Scholar]
- *Elliott JC, Carey KB, & Bonafide KE (2012). Does family history of alcohol problems influence college and university drinking or substance use? A meta-analytical review. Addiction, 107(10), 1774–1785. 10.1111/j.1360-0443.2012.03903.x [DOI] [PubMed] [Google Scholar]
- *Ellis DA, Zucker RA, & Fitzgerald HE (1997). The role of family influences in development and risk. Alcohol Health & Research World, 21(3), 218–226. [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annual Review of Psychology, 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, & Eysenck SBG (1975). Manual of the Eysenck personality questionnaire. Hodder and Stoughton. [Google Scholar]
- *Ezard N (2012). Substance use among populations displaced by conflict: A Lit. Review. Disasters, 36(3), 533–557. 10.1111/j.1467-7717.2011.01261.x [DOI] [PubMed] [Google Scholar]
- Fenigstein A, Scheier MF, & Buss AH (1975). Public and private self-consciousness: Assessment and theory. Journal of Consulting and Clinical Psychology, 43(4), 522–527. [Google Scholar]
- *Feinn R, Nellissery M, & Kranzler HR (2005). Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 133B(1), 79–84. 10.1002/ajmg.b.30132 [DOI] [PubMed] [Google Scholar]
- Feldstein S, & Chung T (2013). Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: Emerging translational approaches that bridge biology and behavior: Psychology of Addictive Behaviors, 18(9), 1199–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JH (2000). National Institutes of Health Consensus Development Conference Statement: Diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child & Adolescent Psychiatry, 39, 182–193. 10.1097/00004583-200002000-00018 [DOI] [PubMed] [Google Scholar]
- Field M, & Cox WM (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence, 97(1-2), 1–20. [DOI] [PubMed] [Google Scholar]
- *Field M, Munafò MR, & Franken IHA (2009). A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin, 135(4), 589–607. 10.1037/a0015843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, & Verster JC (2010). Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research, 34(8), 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fillmore KM, Johnstone BM, Leino EV, & Ager CR (1993). A cross-study contextual analysis of effects from individual-level drinking and group-level drinking factors: A meta-analysis of multiple longitudinal studies from the Collaborative Alcohol-Related Longitudinal Project. Journal of Studies on Alcohol, 54(1), 37–47. 10.15288/jsa.1993.54.37 [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, & Hussong AM (1994). Risk for alcoholism and classical conditioning to signals for punishment: Evidence for a weak behavioral inhibition system. Journal of Abnormal Psychology, 103(2), 292–301. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, & Steinmetz J (2002). Early-onset alcoholism with conduct disorder: Go/no go learning deficits, working memory capacity, and personality. Alcoholism: Clinical and Experimental Research, 26(2), 186–206 [PubMed] [Google Scholar]
- Flagel SB, Akil H, & Robinson TE (2009). Salience to reward-related cues: Implications for addiction, Neuropharmacology, 56, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, … & Akil H (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology, 35(2), 388–400. https://www.nature.com/articles/npp2009142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE (2009). Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry, 66(3), 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Forero DA, López-León S, Shin HD, Park BL, & Kim D-J (2015). Meta-analysis of six genes (BDNF, DRD1, DRD3, DRD4, GRIN2B and MAOA) involved in neuroplasticity and the risk for alcohol dependence. Drug and Alcohol Dependence, 149, 259–263. 10.1016/j.drugalcdep.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, & McGue M (2015). Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychological Medicine, 45, 3047–3058. 10.1017/S0033291715001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DW, & Neighbors C (2013). Self-consciousness as a moderator of the effect of social drinking motives on alcohol use. Addictive Behaviors, 38(4), 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, & Ghera MM (2005). Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu. Rev. Psychol, 56, 235–262. [DOI] [PubMed] [Google Scholar]
- *Freed CR (2010). “In the Spirit of Selden Bacon: The Sociology of Drinking and Drug Problems.” Sociology Compass, 4(10), 856–868. 10.1111/j.1751-9020.2010.00325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 133, 101–135. doi: 10.1037/0096-3445.133.1.101 [DOI] [PubMed] [Google Scholar]
- Flynn HA (2000). Comparison of cross-sectional and daily reports in studying the relationship between depression and use of alcohol in response to stress in college students. Alcoholism: Clinical and Experimental Research, 24(1), 48–52. [PubMed] [Google Scholar]
- *Galizio M, & Maisto SA (1985). Toward a biopsychosocial theory of substance abuse. In Galizio M & Maistro SA (Eds.), Determinants of substance abuse (pp. 425–429). Springer, Boston, MA. 10.1007/978-1-4757-9990-3_13 [DOI] [Google Scholar]
- Gard DE, Gard MG, Kring AM, & John OP (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. [Google Scholar]
- Garfield JBB, Lubman DI, & Yücel M (2014). Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Australian and New Zealand Journal of Psychiatry, 48(1), 36–51. 10.1177/0004867413508455 [DOI] [PubMed] [Google Scholar]
- Gelhorn H, Hartman C, Sakai J, Stallings M, Young S, Rhee SH, Corley R, Hewitt J, Hopfer C, & Crowley T (2008). Toward DSM-V: An item response theory analysis of the diagnostic process of DSM-IV alcohol abuse and dependence in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR (2004). Executive functioning and alcohol-related aggression. Journal of Abnormal Psychology, 113(4), 541–555. [DOI] [PubMed] [Google Scholar]
- Gillan CM, Otto AR, Phelps EA, & Daw ND (2015). Model-based learning protects against forming habits. Cognitive, Affective and Behavioral Neuroscience, 15(3), 523–536. 10.3758/s13415-015-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Robbins TW, Sahakian BJ, van den Heuvel OA, & van Wingen G (2016). The role of habit in compulsivity. European Neuropsychopharmacology, 26(5), 828–840. 10.1016/j.euroneuro.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Gilder DA, Lau P, Wang T, Wilhelmsen KC, & Ehlers CL (2013). Contributions of ethnicity to differential item functioning of cannabis abuse and dependence symptoms. Journal of Studies on Alcohol and Drugs, 74(2), 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JE, Williams EC, & Bucholz KK (2014). Psychiatric comorbidity and perceived alcohol stigma in a nationally representative sample of individuals with DSM-5 alcohol use disorder. Alcoholism: Clinical and Experimental Research, 38(6), 1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Brown SA, Christiansen BA, & Smith GT (1991). Alcoholism and memory: Broadening the scope of alcohol-expectancy research. Psychological Bulletin, 110, 137−146 [DOI] [PubMed] [Google Scholar]
- Goldman MS, Del Boca FK, & Darkes J (1999). Alcohol expectancy theory: The application of cognitive neuroscience. In Blane HT & Leonard KE (Eds.), Psychological theories of drinking and alcoholism (pp. 203–246). Guilford Press. [Google Scholar]
- Gomberg ESL (1997) Alcohol abuse: Age and gender differences. In: Wilsnack RW & Wilsnack SC, (Eds.) Gender and Alcohol: Individual Social Perspectives, pp. 225–244. New Brunswick, NJ: Rutgers Center of Alcohol Studies. [Google Scholar]
- *Goodwin DW (1976). Psychiatric description and evaluation of the alcoholic. Alcoholism: Interdisciplinary Approaches to an Enduring Problem, 203–223. [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160, 636–645. 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Gray JA (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8(3), 249–266. [DOI] [PubMed] [Google Scholar]
- Gray JA (1982). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Behavioral and Brain Sciences, 5(3), 469–484. [Google Scholar]
- Gray JA, & McNaughton N (2000). The Neuropsychology of Anxiety. Oxford University Press. [Google Scholar]
- *Guerrini I, Quadri G, & Thomson AD (2014). Genetic and environmental interplay in risky drinking in adolescents: A Lit. Review. Alcohol and Alcoholism, 49(2), 138–142. 10.1093/alcalc/agu003 [DOI] [PubMed] [Google Scholar]
- Guinle MIB, & Sinha R (2020). The role of stress, trauma, and negative affect in alcohol misuse and alcohol use disorder in women. Alcohol Research: Current Reviews, 40(2), 05. 10.35946/arcr.v40.2.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RL, & Smith GT (2010). Risk factors for elementary school drinking: Pubertal status, personality, and alcohol expectancies concurrently predict fifth grade alcohol consumption. Psychology of Addictive Behaviors, 24, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hall JM (1990). Alcoholism in lesbians: Developmental, symbolic interactionist, and critical perspectives. Health Care for Women International, 11(1), 89–107. 10.1080/0739933900951587 [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Littlefield AK, Anastasio NC, Cunningham KA, Fink LHL, Wing VC, … Potenza MN (2015). Rapid-response impulsivity: Definitions, measurement issues, and clinical implications. Personality Disorders: Theory, Research, and Treatment, 6(2), 168–181. 10.1037/per0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford TC, Yi HY, Faden VB, & Chen CM (2009). The dimensionality of DSM-IV alcohol use disorders among adolescent and adult drinkers and symptom patterns by age, gender, and race/ethnicity. Alcoholism: Clinical and Experimental Research, 33, 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings M, Young SE, Rhee SH, Corley R, Hewitt JK, & Hopfer CJ (2008). Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Fenton MC, Beseler C, Park JY, & Wall MM (2012). Analyses related to the development of DSM-5 criteria for substance use related disorders: 2. Proposed DSM-5 criteria for alcohol, cannabis, cocaine and heroin disorders in 663 substance abuse patients. Drug and Alcohol Dependence, 122, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, … Grant BF (2013). DSM-5 criteria for substance use disorders: Recommendations and rationale. American Journal of Psychiatry, 170(8), 834–851. 10.1176/appi.ajp.2013.12060782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Heath AC (1995). Genetic influences on alcoholism risk: A review of adoption and twin studies. Alcohol Health & Research World, 19(3), 166–171. [PMC free article] [PubMed] [Google Scholar]
- *Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, … Montgomery GW (2011). A quantitative-trait genome-wide association study of alcoholism risk in the community: Findings and implications. Biological Psychiatry, 70(6), 513–518. 10.1016/j.biopsych.2011.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, & Sumner P (2018). The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behavior Research Methods, 50(3), 1166–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle AC, Trull TJ, Watts AL, McDowell Y, & Sher KJ (2020). Psychiatric comorbidity as a function of severity: DSM-5 alcohol use disorder and HiTOP classification of mental disorders. Alcoholism: Clinical and Experimental Research, 44(3), 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Herold DM, & Conlon EJ (1981). Work factors as potential causal agents of alcohol abuse. Journal of Drug Issues, 11(3), 337–356. 10.1177/002204268101100305 [DOI] [Google Scholar]
- *Hesselbrock MN, Hesselbrock VM, & Chartier KG (2013). Genetics of alcohol dependence and social work research: Do they mix? Social Work in Public Health, 28(3-4), 178–193. 10.1080/19371918.2013.758999 [DOI] [PubMed] [Google Scholar]
- Hicks BM, Durbin CE, Blonigen DM, Iacono WG, & McGue M (2012). Relationship between personality change and the onset and course of alcohol dependence in young adulthood. Addiction, 107(3), 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hill SY (1985). The disease concept of alcoholism: A review. Drug and Alcohol Dependence, 16(3), 193–214. 10.1016/0376-8716(85)90045-6 [DOI] [PubMed] [Google Scholar]
- *Hill SY (1995). Neurobiological and clinical markers for a severe form of alcoholism in women. Alcohol Health & Research World, 19(3), 249–256. [PMC free article] [PubMed] [Google Scholar]
- *Ho AL, Salib A-MN, Pendharkar AV, & Sussman E (2018). The nucleus accumbens and alcoholism: A target for deep brain stimulation. Journal of Neurosurgery, 45(2), 1–10. 10.3171/2018.5.FOCUS18157. [DOI] [PubMed] [Google Scholar]
- Hogarth L (2020). Addiction is driven by excessive goal-directed drug choice under negative affect: Translational critique of habit and compulsion theory. Neuropsychopharmacology, (December 2019), 1–16. 10.1038/s41386-020-0600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hong JS, Lee NY, Grogan-Kaylor A, & Huang H (2011). Alcohol and tobacco use among South Korean adolescents: An ecological review of the literature. Children and Youth Services Review, 33(7), 1120–1126. 10.1016/j.childyouth.2011.02.004 [DOI] [Google Scholar]
- Hopwood CJ, Bagby RM, Gralnick T, Ro E, Ruggero C, Mullins-Sweatt S, … Zimmermann J (2019). Integrating psychotherapy with the Hierarchical Taxonomy of Psychopathology (HiTOP). Journal of Psychotherapy Integration, 87(12), 1069–1084. 10.1037/int0000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JG, Young RD, & Jouriles E (1986). Applications of the self-awareness model of alcohol consumption: Predicting patterns of use and abuse. Journal of Personality and Social Psychology, 51(4), 790–796. [DOI] [PubMed] [Google Scholar]
- Hull JG (1987). Self-awareness model. In Blane HT & Leonard KE (Eds.), Psychological Theories of Drinking and Alcoholism (pp. 272–304). [Google Scholar]
- Hull JG, & Levy AS (1979). The organizational functions of the self: An alternative to the Duval and Wicklund Model of self-awareness. Journal of Personality and Social Psychology, 37(5), 756–768. [Google Scholar]
- *Hummel A, Shelton KH, Heron J, Moore L, & Bree MBM (2013). A systematic review of the relationships between family functioning, pubertal timing and adolescent substance use. Addiction, 108(3), 487–496. 10.1111/add.12055 [DOI] [PubMed] [Google Scholar]
- Hussong AM, Ennett ST, Cox MJ, & Haroon M (2017). A systematic review of the unique prospective association of negative affect symptoms and adolescent substance use controlling for externalizing symptoms. Psychology of Addictive Behaviors, 31(2), 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Galloway CA, & Feagans LA (2005). Coping motives as a moderator of daily mood-drinking covariation. Journal of Studies on Alcohol, 66(3), 344–353. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Hicks RE, Levy SA, & Curran PJ (2001). Specifying the relations between affect and heavy alcohol use among young adults. Journal of Abnormal Psychology, 110(3), 449–461. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, & Boeding S (2011). An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors, 25(3), 390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Israelstam S, & Lambert S (1983). Homosexuality as a cause of alcoholism: A historical review. International Journal of the Addictions, 18(8), 1085–1107. 10.3109/10826088309027372 [DOI] [PubMed] [Google Scholar]
- *Jackson N, Denny S, & Ameratunga S (2014). Social and socio-demographic neighborhood effects on adolescent alcohol use: A systematic review of multi-level studies. Social Science & Medicine, 115, 10–20. 10.1016/j.socscimed.2014.06.004 [DOI] [PubMed] [Google Scholar]
- *Jacob T (1992). Family studies of alcoholism. Journal of Family Psychology, 5(3-4), 319–338. 10.1037/0893-3200.5.3-4.319 [DOI] [Google Scholar]
- *Jacob T, & Johnson S (1997). Parenting influences on the development of alcohol abuse and dependence. Alcohol Health & Research World, 21(1), 204–209. [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM (1952). Phases of alcohol addiction. Quarterly Journal of Studies on Alcohol, 13(4), 673–684. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, James AS, Groman SM, & Pennington ZT (2014). Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences, 1327, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, & Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology, 146(4), 373–390. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Koob GF, Schuckit MA, Mason BJ, & Ait-Daoud N (2006). Understanding and treating alcohol dependence. Alcoholism: Clinical & Experimental Research, 20(3), 567–584. 10.1111/j.1530-0277.2005.00066.x [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, & Fromme K (2001). A review of expectancy theory and alcohol consumption. Addiction, 96, 57–72. 10.1080/09652140020016969 [DOI] [PubMed] [Google Scholar]
- Jones BT & McMahon J (1996). Changes in alcohol expectancies during treatment relate to sub-sequent abstinence survivorship, British Journal of Clinical Psychology, 35, 221–234. [DOI] [PubMed] [Google Scholar]
- Jonker NC, Ostafin BD, Glashouwer KA, van Hemel-Ruiter ME, & de Jong PJ (2014). Reward and punishment sensitivity and alcohol use: The moderating role of executive control. Addictive Behaviors, 39(5), 945–948. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Zhu X, Corbly CR, DeSantis S, Lee DC, Baik G, … & Kelly TH (2015). Influence of neurobehavioral incentive valence and magnitude on alcohol drinking behavior. NeuroImage, 104, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, & Robbins TW (1991). Frontal lobe function in Korsakoff and non-Korsakoff alcoholics: planning and spatial working memory. Neuropsychologia, 29(8), 709–723. [DOI] [PubMed] [Google Scholar]
- Kahn RE, Chiu PH, Deater-Deckard K, Hochgraf AK, King-Casas B, & Kim-Spoon J (2018). The interaction between punishment sensitivity and effortful control for emerging adults’ substance use behaviors. Substance Use & Misuse, 53(8), 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouropoulos N, & Staiger PK (2001). The influence of sensitivity to reward on reactivity to alcohol-related cues. Addiction, 96(8), 1175–1185. [DOI] [PubMed] [Google Scholar]
- Kapur S (2003). Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry, 160, 13–23. [DOI] [PubMed] [Google Scholar]
- *Kebir O, Gorsane M-A, Blecha L, Krebs M-O, Reynaud M, & Benyamina A (2011). Association of inflammation genes with alcohol dependence/abuse: A systematic review and a Meta-Analysis. European Addiction Research, 17(3), 146–153. 10.1159/000324849 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Prescott C. a, Crabbe J, & Neale MC. (2012). Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence. Molecular Psychiatry, 17(12), 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, & Prescott CA (2007). Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry, 64(11), 1313–1320. [DOI] [PubMed] [Google Scholar]
- Kernis MH, & Grannemann BD (1988). Private self-consciousness and perceptions of self-consistency. Personality and Individual Differences, 9(5), 897–902. [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser-Marcus LA, Ramey T, Bjork J, Adams A, & Moeller FG (2021). Development and feasibility study of an addiction - focused Phenotyping Assessment Battery. The American Journal on Addictions, 1–8. 10.1111/ajad.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harv Rev Psychiatry, 4, 231–244. [DOI] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, & Cao D (2016). A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biological Psychiatry, 79(6), 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, & McGue M (2004). Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction, 99, 1548–1559. [DOI] [PubMed] [Google Scholar]
- *King AC, Roche DJ, & Rueger SY (2011). Subjective Responses to Alcohol: A Paradigm Shift May Be Brewing. Alcoholism: Clinical and Experimental Research 35(10) 1726–1728. 10.1111/j.1530-0277.2011.01629.x [DOI] [PubMed] [Google Scholar]
- Klein D (1984). Depression and anhedonia. In Clark DC & Fawcett J (Eds.), Anhedonia and affect deficit states (pp. 1–14). New York: PMA Publishing. [Google Scholar]
- Koob GF (2003). Alcoholism: Allostasis and beyond. Alcoholism: Clinical and Experimental Research, 27(2), 232–243. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, … & Sanna PP. (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews, 27(8), 739–749. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: Hedonic homeostatic dysregulation. Science, 278(5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Koob GF, & Volkow ND (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, … & Eaton NR. (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454–477. [DOI] [PubMed] [Google Scholar]
- Kouimtsidis C, Stahl D, West R & Drummond C (2014), Can outcome expectancies be measured across substances? Development and validation of a questionnaire for populations in treatment. Drugs and Alcohol Today, 14(4), 172–186. 10.1108/DAT-02-2014-0007 [DOI] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH Research Domain Criteria Initiative: Background, issues, and pragmatics. Psychophysiology, 53(3), 286–297. [DOI] [PubMed] [Google Scholar]
- Krueger RF, & DeYoung CG (2016). The RDoC initiative and the structure of psychopathology. Psychophysiology, 53(3), 351–354. 10.1111/psyp.12551 [DOI] [PubMed] [Google Scholar]
- Krueger RF, & Eaton NR (2015). Transdiagnostic factors of mental disorders. World Psychiatry, 14(1), 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger EA, Fish JN, & Upchurch DM (2020). Sexual orientation disparities in substance use: Investigating social stress mechanisms in a national sample. American Journal of Preventive Medicine, 58(1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, & McGue M (2002). Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology, 111(3), 411–424. [PubMed] [Google Scholar]
- Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, … Zimmermann J (2018). Progress in achieving quantitative classification of psychopathology. World Psychiatry, 17(3), 282–293. 10.1002/wps.20566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, & Markon KE (2006). Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology, 2, 111–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kuhn CM (2011). Alcohol and women: What is the role of biologic factors? Alcoholism Treatment Quarterly, 29(4), 479–504. 10.1080/07347324.2011.608340 [DOI] [Google Scholar]
- *Kühn S, & Gallinat J (2011). Common biology of craving across legal and illegal drugs- a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience, 33(7), 1318. 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2005). Why do young people drink? A review of drinking motives. Clinical Psychology Review, 25(7), 841–861. [DOI] [PubMed] [Google Scholar]
- *Kuntsche E, Rossow I, Engels R, & Kuntsche S (2015). Is “age at first drink” a useful concept in alcohol research and prevention? We doubt that. Addiction, 111(6), 957–965. 10.1111/add.12980 [DOI] [PubMed] [Google Scholar]
- Kwako LE, & Koob GF (2017). Neuroclinical framework for the role of stress in addiction. Chronic Stress, 1, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Grodin EN, Litten RZ, Koob GF, & Goldman D (2017). Addictions Neuroclinical Assessment: A reverse translational approach. Neuropharmacology, 122, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, & Goldman D (2015). Addictions Neuroclinical Assessment: A neuroscience-based framework for addictive disorders. Biological Psychiatry, 80(3), 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, … Goldman D (2019). Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. American Journal of Psychiatry, 176, 744–753. 10.1176/appi.ajp.2018.18030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBrie JW, Hummer JF, & Neighbors C (2008). Self-consciousness moderates the relationship between perceived norms and drinking in college students. Addictive Behaviors, 33(12), 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBrie J, Pedersen ER, Neighbors C, & Hummer JF (2008). The role of self-consciousness in the experience of alcohol-related consequences among college students. Addictive Behaviors, 33(6), 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SP, & Sher KJ (2014). Limits of current approaches to diagnosing severity based on criterion counts: An example with DSM-5 alcohol use disorder. Clinical Psychological Science, 3(6), 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, & Chung T (2004). An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. Journal of Abnormal Psychology, 113, 72–80. [DOI] [PubMed] [Google Scholar]
- Lazarus R, & Folkman S (1984). Stress, appraisal, and coping. New York: Springer. [Google Scholar]
- Leavell HR, & Clark EG (1958). Preventive Medicine for the Doctor in his Community. An Epidemiologic Approach. Preventive Medicine for the Doctor in his Community. An Epidemiologic Approach, 2nd Edition. New York: McGraw-Hill Book Co. [Google Scholar]
- Le Berre AP, & Sullivan EV (2016). Anosognosia for memory impairment in addiction: insights from neuroimaging and neuropsychological assessment of metamemory. Neuropsychology Review, 26(4), 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Hoppenbrouwers S, & Franken I (2019). A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychology Review, 14–26. 10.1007/s11065-019-09402-x [DOI] [PubMed] [Google Scholar]
- Leeman RF, Beseler CL, Helms CM, Patock-Peckham JA, Wakeling VA, & Kahler CW (2014). A brief, critical review of research on impaired control over alcohol use and suggestions for future studies. Alcoholism: Clinical and Experimental Research, 38(2), 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Brooks JC, Wiech K, & Tracey I (2008). Pain relief as an opponent process: A psychophysical investigation. European Journal of Neuroscience, 28(4), 794–801. [DOI] [PubMed] [Google Scholar]
- *Lettieri DJ (1985). Drug abuse: A review of explanations and models of explanation. Advances in Alcohol & Substance Abuse, 4(3-4), 9–40. 10.1300/J251v04n03_02 [DOI] [PubMed] [Google Scholar]
- Levitt HM, Bamberg M, Creswell JW, Frost DM, Josselson R, & Suárez-Orozco C (2018). Journal article reporting standards for qualitative primary, qualitative meta-analytic, and mixed methods research in psychology: The APA Publications and Communications Board task force report. American Psychologist, 73(1), 26–46. [DOI] [PubMed] [Google Scholar]
- *Li C-Y, Zhou W-Z, Wei L, Zhang P-W, Johnson C, & Uhl GR (2011). Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics, 12(1), 508. 10.1186/1471-2164-12-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO (2014). The Research Domain Criteria (RDoC): An analysis of methodological and conceptual challenges. Behaviour Research and Therapy, 62, 129–139. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, & Treadway MT (2016). Clashing Diagnostic Approaches: DSM-ICD Versus RDoC. Annual Review of Clinical Psychology, 12(1), 435–463. 10.1146/annurev-clinpsy-021815-093122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Waldman ID, & Israel AC (1994). A critical examination of the use of the term and concept of comorbidity in psychopathology research. Clinical Psychology: Science and Practice, 1(1), 71–83. [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, & Fertig JB (2016). Discovery, development, and adoption of medications to treat alcohol use disorder: Goals for the phases of medications development. Alcoholism: Clinical and Experimental Research, 40(7), 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, & Koob GF (2015). Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment. Alcoholism: Clinical and Experimental Research, 39(4), 579–584. [DOI] [PubMed] [Google Scholar]
- Littlefield AK, & Sher KJ (2010). The multiple, distinct ways that personality contributes to alcohol use disorders. Social and Personality Psychology Compass, 4(9), 767–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergara HI, Spillane NS, Merrill JE, & Jackson KM (2016). Developmental trends in alcohol use initiation and escalation from early to middle adolescence: Prediction by urgency and trait affect. Psychology of Addictive Behaviors, 30(5), 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, & Robinson TE (2011). Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural Brain Research, 223(2), 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Luczak SE, Glatt SJ, & Wall TJ (2006). Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychological Bulletin, 132(4), 607–621. [DOI] [PubMed] [Google Scholar]
- Luijten M, Gillan CM, De Wit S, Franken IHA, Robbins TW, & Ersche KD (2020). Goal-directed and habitual control in smokers. Nicotine and Tobacco Research, 22(2), 188–195. 10.1093/ntr/ntz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Robbins TW, & Everitt BJ (2020). The transition to compulsion in addiction. Nature Reviews Neuroscience, 21, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, & Agrawal A (2007). Psychometric properties of DSM assessments of illicit drug abuse and dependence: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychological Medicine, 37, 1345–1355. [DOI] [PubMed] [Google Scholar]
- Lyon G, & Krasnegor NA (1996). Attention, memory, and executive function. Paul H Brookes Publishing. [Google Scholar]
- *Ma Y, Fan R, & Li MD (2016). Meta-analysis reveals significant association of the 3’-UTR VNTR in SLC6A3 with alcohol dependence. Alcoholism: Clinical & Experimental Research, 40(7), 1443–1453. 10.1111/acer.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ma Y, Fan R, & Li MD (2017). “Erratum: Meta-analysis reveals significant association of the 3’-UTR VNTR in SLC6A3 with alcohol dependence.” Alcoholism: Clinical & Experimental Research, 41(9), 1656–1659. 10.1111/acer.13465 [DOI] [PubMed] [Google Scholar]
- *Magid V, & Moreland AD (2014). The role of substance use initiation in adolescent development of subsequent substance-related problems. Journal of Child & Adolescent Substance Abuse, 23(2), 78–86. 10.1080/1067828X.2012.748595 [DOI] [Google Scholar]
- *Maimaris W, & McCambridge J (2014). Age of first drinking and adult alcohol problems: Systematic review of prospective cohort studies. Journal of Epidemiology & Community Health, 68(3), 268–274. 10.1136/jech-2013-203402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M (2020). Beyond diagnosis in psychiatric practice. Annals of General Psychiatry, 1–6. 10.1186/s12991-020-00279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Malouff JM, Rooke SE, & Schutte NS (2008). The heritability of human behavior: Results of aggregating meta-analyses. Current Psychology, 27(3), 153–161. 10.1007/s12144-008-9032-z [DOI] [Google Scholar]
- *Marlatt GA, Baer JS, Donovan DM, & Kivlahan DR (1988). Addictive behaviors: Etiology and treatment. Annual Review of Psychology, 39, 223–252. 10.1146/annurev.ps.39.020188.001255 [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Demming B, & Reid JB (1973). Loss of control drinking in alcoholics: An experimental analogue. Journal of Abnormal Psychology, 81(3), 233–241. [DOI] [PubMed] [Google Scholar]
- Marra D, Warot D, Payan C, Hispard E, Dally S, & Puech AJ (1998). Anhedonia and relapse in alcoholism. Psychiatry Research, 80(2), 187–196. [DOI] [PubMed] [Google Scholar]
- Martens MP, Neighbors C, Lewis MA, Lee CM, Oster-Aaland L, & Larimer ME (2008). The roles of negative affect and coping motives in the relationship between alcohol use and alcohol-related problems among college students. Journal of Studies on Alcohol and Drugs, 69(3), 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Chung T, Kirisci L, & Langenbucher JW (2006). Item Response Theory analysis of diagnostic criteria for alcohol and cannabis use disorders in adolescents: Implications for DSM-V. Journal of Abnormal Psychology, 115(4), 807–814. [DOI] [PubMed] [Google Scholar]
- Martin CS, Langenbucher JW, Chung T, Kenneth J, & Louis S (2014). Truth or consequences in the diagnosis of substance use disorders. Addiction, 109(11), 1773–1778. 10.1111/add.12615.Truth [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Steinley DL, Vergés A, & Sher KJ (2011). Letter to the Editor: The proposed 2/11 symptom algorithm for DSM-5 substance-use disorders is too lenient. Psychological Medicine, 41(9), 2008–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Andreoli S, Reina D, Di Nicola M, Ortolani I, Tedeschi D, … & D’Iddio S (2011). Acetyl-l-Carnitine in the treatment of anhedonia, melancholic and negative symptoms in alcohol dependent subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 953–958. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P & Janiri L (2008). Alcohol protracted withdrawal syndrome: The role of anhedonia. Substance Use & Misuse, 43(3-4), 271–284. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Kroll LS, & Smith GT (2001). Integrating disinhibition and learning risk for alcohol use. Experimental and Clinical Psychopharmacology, 9(4), 389–398. 10.1037/1064-1297.9.4.389 [DOI] [PubMed] [Google Scholar]
- *McGue M (1997). A behavioral-genetic perspective on children of alcoholics. Alcohol Health & Research World. 21(3), 210–217. [PMC free article] [PubMed] [Google Scholar]
- *McHugh RK, Hofmann SG, Asnaani A, Sawyer AT, & Otto MW (2010). The serotonin transporter gene and risk for alcohol dependence: A meta-analytic review. Drug & Alcohol Dependence, 108(1-2), 1–6. 10.1016/j.drugalcdep.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Sugarman DE, & Greenfield SF (2018). Sex and gender differences in substance use disorders. Clinical Psychology Review, 66(November 2017), 12–23. 10.1016/j.cpr.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini G, Palumbo IM, DeYoung CG, Latzman R, & Kotov R (2020). Linking RDoC and HiTOP: A new interface for advancing psychiatric nosology and neuroscience. 10.31234/osf.io/ps7tc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman H, & Frosch WA (1980). Theory of drug use. In Lettieri DJ, Sayers M, and Pearson HW (eds.), Theories of Drug Abuse: Selected Contemporary Perspectives. Research Monograph 30. Rockville, D: National Institute on Drug Abuse. [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, … & Stewart LA (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Monteiro MG, & Schuckit MA (1988). Populations at high alcoholism risk: Recent findings. The Journal of Clinical Psychiatry, 49(9, Suppl), 3–7. [PubMed] [Google Scholar]
- Montgomery C, Fisk JE, Murphy PN, Ryland I, & Hilton J (2012). The effects of heavy social drinking on executive function: A systematic review and meta-analytic study of existing literature and new empirical findings. Human Psychopharmacology: Clinical and Experimental, 27(2), 187–199. [DOI] [PubMed] [Google Scholar]
- *Morean ME, & Corbin WR (2010). Subjective response to alcohol: A critical review of the literature. Alcoholism: Clinical & Experimental Research, 34(3), 385–395. 10.1111/j.1530.0277.2009.01103.x [DOI] [PubMed] [Google Scholar]
- *Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, … & Bloom FE. (2006). Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proceedings of the National Academy of Sciences of the United States of America, 103(16), 6368–6373. 10.1073/pnas.0510188103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Munafò MR, Matheson IJ, & Flint J (2007). Association of the DRD2 gene Taq1A polymorphism and alcoholism: A meta-analysis of case-control studies and evidence of publication bias. Molecular Psychiatry, 12(5), 454–461. 10.1038/sj.mp.4001938 [DOI] [PubMed] [Google Scholar]
- *Murphy A, Roberts B, Stickley A, & McKee M (2012). Social factors associated with alcohol consumption in the former Soviet Union: A systematic review. Alcohol and Alcoholism, 47(6), 711–718. [DOI] [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, & An SK (2004). Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research, 28(9), 1317–1323. [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, & Bernstein IL (2009). Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proceedings of the National Academy of Sciences, 106(41), 17600–17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2018). Feasibility and validation of a standard Phenotyping Assessment Battery (PhAB). Retrieved from https://clinicaltrials.gov/ct2/show/NCT03495869
- National Institute of Health (NIH). (2017). Division of translational research: Overview. Retrieved from https://www.nimh.nih.gov/about/organization/dtr/index.shtml
- National Institute of Health (NIH). (2019). Research Domain Criteria: RDoC Constructs. Retrieved from https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/negative-valence-systems.shtml
- National Institute of Mental Health (NIMH). (2020). RDoC Frequently Asked Questions (FAQ). Retrieved from https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/resources/rdoc-frequently-asked-questions-faq.shtml
- Nestler EJ (2005). Is there a common molecular pathway for addiction?. Nature Neuroscience, 8(11), 1445–1449. [DOI] [PubMed] [Google Scholar]
- Newlin DB, & Thomson JB (1990). Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin, 108(3), 383–402. [DOI] [PubMed] [Google Scholar]
- *Noble EP (1998). The D2 dopamine receptor gene: A review of association studies in alcoholism and phenotypes. Alcohol, 16(1), 33–45. 10.1016/S0741-8329(97)00175-4 [DOI] [PubMed] [Google Scholar]
- Noël X, Bechara A, Dan B, Hanak C, & Verbanck P (2007). Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology, 21(6), 778. [DOI] [PubMed] [Google Scholar]
- *Noori HR, Helinski S, & Spanagel R (2014). Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addiction Biology, 19(2), 225. 10.1111/adb.12125 [DOI] [PubMed] [Google Scholar]
- O’Connor RM, & Colder CR (2005). predicting alcohol patterns in first-year college students through motivational systems and reasons for drinking. Psychology of Addictive Behaviors, 19(1), 10–20. 10.1037/0893-164X.19.1.10 [DOI] [PubMed] [Google Scholar]
- *Onuoha RC, Quintana DS, Lyvers M, & Guastella AJ (2016). A meta-analysis of theory of mind in alcohol use disorders. Alcohol & Alcoholism, 51(4), 410. 10.1093/alcalc/agv137 [DOI] [PubMed] [Google Scholar]
- *Oo KZ, Aung YK, Jenkins MA, & Win AK (2016). Associations of 5HTTLPR polymorphism with major depressive disorder and alcohol dependence: A systematic review and meta-analysis. The Australian and New Zealand Journal of Psychiatry, 50(9), 842–857. 10.1177/0004867416637920 [DOI] [PubMed] [Google Scholar]
- *Ouzir M, & Errami M (2016). Etiological theories of addiction: A comprehensive update on neurobiological, genetic and behavioural vulnerability. Pharmacology Biochemistry and Behavior, 148, 59–68. 10.1016/j.pbb.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Pahng AR, McGinn MA, Paulsen RI, & Edwards S (2017). The prefrontal cortex as a critical gate of negative affect and motivation in alcohol use disorder. Current Opinion in Behavioral Sciences, 13, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfai TP, Ralston TE, & Wright LL (2011). Understanding university student drinking in the context of life goal pursuits: The mediational role of enhancement motives. Personality and Individual Differences, 50(2), 169–174. [Google Scholar]
- Palmer RH, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, … & Hewitt JK. (2012). Genetic etiology of the common liability to drug dependence: evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug and Alcohol Dependence, 123, S24–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Pan Y, Lou X, Liu X, Wu L-Y, Zhang Q, Wang L … & Wang K-S. (2013). Genome-wide association studies of maximum number of drinks. Journal of Psychiatric Research. 47(11), 1717–1724. 10.1016/j.jpsychires.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Panitz DR, McConchie RD, Sauber SR, & Fonseca JA (1983). The role of machismo and the Hispanic family in the etiology and treatment of alcoholism in Hispanic American males. American Journal of Family Therapy, 11(1), 31–44. 10.1080/01926188308250109 [DOI] [Google Scholar]
- Patrick ME, Wray-Lake L, Finlay AK, & Maggs JL (2009). The long arm of expectancies: Adolescent alcohol expectancies predict adult alcohol use. Alcohol and Alcoholism, 45(1), 17–24. 10.1093/alcalc/agp066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SJ, Davis HA, & Smith GT (2018). Personality and learning predictors of adolescent alcohol consumption trajectories. Journal of Abnormal Psychology, 127, 482–495. 10.1037/abn0000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SJ, & Smith GT (2019). Impulsigenic personality: Is urgency an example of the jangle fallacy? Psychological Assessment, 31(9), 1135–1144. 10.1037/pas0000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, McGinn MA, & Paulsen RI (2017). The prefrontal cortex as a critical gate of negative affect and motivation in alcohol use disorder. Current Opinion in Behavioral Sciences, 13, 139–143. 10.1016/J.COBEHA.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB, & Hunt-Carter EE (2011). WISDM primary and secondary dependence motives: Associations with self-monitored motives for smoking in two college samples. Drug and Alcohol Dependence, 114(2-3), 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard H (2020). What we’re not talking about when we talk about addiction. Hastings Center Report, 50(4), 37–46. 10.1002/hast.1172 [DOI] [PubMed] [Google Scholar]
- Piontek D, Kraus L, Legleye S, Buhringer G (2011). The validity of DSM-IV cannabis abuse and dependence criteria in adolescents and the value of additional cannabis use indicators. Addiction, 106, 1137–1145. [DOI] [PubMed] [Google Scholar]
- *Pohorecky LA (1991). Stress and alcohol interaction: An update of human research. Alcoholism: Clinical and Experimental Research, 15(3), 438–459. 10.1111/j.1530-0277.1991.tb00543.x [DOI] [PubMed] [Google Scholar]
- *Polcin DL (1997). The etiology and diagnosis of alcohol dependence: Differences in the professional literature. Psychotherapy: Theory, Research, Practice, Training, 34(3), 297–306. 10.1037/h0087704 [DOI] [Google Scholar]
- *Polich J, Pollock VE, & Bloom FE (1994). Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin, 115(1), 55–73. 10.1037/0033-2909.115.1.55 [DOI] [PubMed] [Google Scholar]
- Polivy J (1998). The effects of behavioral inhibition: Integrating internal cues, cognition, behavior, and affect. Psychological Inquiry, 9(3), 181–204. [Google Scholar]
- *Pollock VE, Schneider LS, Gabrielli WF, & Goodwin DW (1987). Sex of parent and offspring in the transmission of alcoholism: A meta-analysis. Journal of Nervous and Mental Disease, 175(11), 668–673. 10.1097/00005053-198711000-00004 [DOI] [PubMed] [Google Scholar]
- *Preuss UW, Walther C, & Wong JWM (2012). Genetics of alcohol dependence in humans: Recent findings and perspectives. SUCHT, 58(1), 9–21. 10.1024/0939-5911.a000157 [DOI] [Google Scholar]
- *Prom-Wormley EC, Ebejer J, Dick DM, & Bowers MS (2017). The genetic epidemiology of substance use disorder: A review. Drug and Alcohol Dependence, 180, 241–259. 10.1016/j.drugalcdep.2017.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Quinn PD, & Fromme K (2011). Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical & Experimental Research, 35(10), 1759–1770. 10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery D, Kelly PJ, Deane FP, Baker AL, Goh MCW, Lubman DI, … Mcketin R (2020). Insight in substance use disorder: A systematic review of the literature. Addictive Behaviors, 106549. 10.1016/j.addbeh.2020.106549 [DOI] [PubMed] [Google Scholar]
- Ralston TE, Palfai TP, & Rinck M (2013). The influence of depressed mood on action tendencies toward alcohol: The moderational role of drinking motives. Addictive Behaviors, 38(12), 2810–2816. [DOI] [PubMed] [Google Scholar]
- Ray LA (2012). Clinical neuroscience of addiction: Applications to psychological science and practice. Clinical Psychology: Science and Practice, 19(2), 154–166. 10.1111/j.1468-2850.2012.01280.x [DOI] [Google Scholar]
- Ray LA, Bujarski S, & Roche DJO (2016). Subjective response to alcohol as a Research Domain Criterion. Alcoholism: Clinical and Experimental Research, 40(1), 6–17. [DOI] [PubMed] [Google Scholar]
- Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Feldstein Ewing SW, … Koob GF (2020). The future of translational research on alcohol use disorder. Addiction Biology, (March), 1–11. 10.1111/adb.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Reilly MT, Noronha A, Goldman D, & Koob GF (2017). Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology, 122, 3–21. 10.1016/j.neuropharm.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Rew L (1989). Long-term effects of childhood sexual exploitation. Issues in Mental Health Nursing, 10(3-4), 229–244. 10.3109/01612848909140847 [DOI] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, & Ersche KD (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences, 16(1), 81–91. [DOI] [PubMed] [Google Scholar]
- Robbins TW, & Everitt BJ (1999). Drug addiction: Bad habits add up. Nature, 398(6728), 567–570. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Berger J, & Trautwein U (2009). Conscientiousness and extemalizing psychopathology: Overlap, developmental patterns, and etiology of two related constructs. Development and Psychopathology, 21, 871–888. [DOI] [PubMed] [Google Scholar]
- *Robinson TE, & Berridge KC (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews, 18(3), 247–291. 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2003). Addiction. Annual Review of Psychology, 54(1), 25–53. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3137–3146. 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Roche AM, Lee NK, Battams S, Fischer JA, Cameron J, & McEntee A (2015). Alcohol use among workers in male-dominated industries: A systematic review of risk factors. Safety Science, 78, 124–141. 10.1016/j.ssci.2015.04.007 [DOI] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, … & Abrams DB. (1994). Cue reactivity as a predictor of drinking among male alcoholics. Journal of Consulting and Clinical Psychology, 62(3), 620–626. [DOI] [PubMed] [Google Scholar]
- *Rossow I, Felix L, Keating P, & McCambridge JIM (2016). Parental drinking and adverse outcomes in children: A scoping review of cohort studies. Drug & Alcohol Review, 35(4), 397–405. 10.1111/dar.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Petry NM, & Carroll KM (2003). Single versus multiple drug focus in substance abuse clinical trials research. Drug and Alcohol Dependence, 70(2), 117–125. 10.1016/S0376-8716(03)00033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau GS, Irons JG, & Correia CJ (2011). The reinforcing value of alcohol in a drinking to cope paradigm. Drug and Alcohol Dependence, 118(1), 1–4. [DOI] [PubMed] [Google Scholar]
- *Rozin L, & Zagonel IPS (2012). Risk factors for alcohol dependence in adolescents. Acta Paulista de Enfermagem, 25(2), 314–318. 10.1590/S0103-21002012000200025 [DOI] [Google Scholar]
- *Sadava SW (1980). Towards a molar interactional psychology. Canadian Journal of Behavioural Science/Revue Canadienne Des Sciences Du Comportement, 12(1), 33–51. 10.1037/h0081047 [DOI] [Google Scholar]
- Saha TD, Chou SP, Grant BF (2006). Toward an alcohol use disorder continuum using item response theory: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine, 36, 931–941. [DOI] [PubMed] [Google Scholar]
- Saha TD, Stinson FS, & Grant BF (2007). The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Dependence, 89, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Compton WM, Chou SP, Smith S Ruan WJ. Huang B, Pickering RP, Grant BF. (2012). Analyses related to the development of DSM-5 criteria for substance use related disorders: 1. Toward amphetamine, cocaine and prescription drug use disorder continua using item response theory. Drug and Alcohol Dependence, 122, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainslow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, & Cuthbert BN (2010). Developing constructs for psychopathology research: Research Domain Criteria. Journal of Abnormal Psychology, 119, 631–639. [DOI] [PubMed] [Google Scholar]
- *Salvatore JE, Gottesman II, & Dick DM (2015). Endophenotypes for alcohol use disorder: An update on the field. Current Addiction Reports, 2(1), 76–90. 10.1007/s40429-015-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent JD, Tanski S, Stoolmiller M, & Hanewinkel R (2010). Using sensation seeking to target adolescents for substance use interventions. Addiction, 105(3), 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Degenhardt L, Reed GM, & Poznyak V (2019). Alcohol Use Disorders in ICD-11: Past, Present, and Future. Alcoholism: Clinical and Experimental Research, 43(8), 1617–1631. [DOI] [PubMed] [Google Scholar]
- Sayette MA (1999). Does drinking reduce stress?. Alcohol Research & Health, 23(4), 250–255. [PMC free article] [PubMed] [Google Scholar]
- *Schacht JP, Anton RF, & Myrick H (2013). Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology, 18(1) 121–133. 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schepis TS, Rao U, Yadav H, & Adinoff B (2011). The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcoholism: Clinical & Experimental Research, 35(4), 595–605. 10.1111/j.1530-0277.2010.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmulewitz D, Keyes K, Beseler C, Aharonovich E, Aivadyan C, Spivak B, & Hasin D (2010). The dimensionality of alcohol use disorders: Results from Israel. Drug and Alcohol Dependence, 111(1-2), 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schneeberger A, Dietl M, Muenzenmaier K, Huber C, & Lang U (2014). Stressful childhood experiences and health outcomes in sexual minority populations: a systematic review. Social Psychiatry & Psychiatric Epidemiology, 49(9), 1427–1445. 10.1007/s00127-014-0854-8 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry, 41(9), 879–884. [DOI] [PubMed] [Google Scholar]
- *Schulsinger F (1980). Biological psychopathology. Annual Review of Psychology, 31, 583–606. 10.1146/annurev.ps.31.020180.003055 [DOI] [PubMed] [Google Scholar]
- *Schulte MT, Ramo D, & Brown SA (2009). Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clinical Psychology Review, 29(6), 535–547. 10.1016/J.CPR.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2002). Getting formal with dopamine and reward. Neuron, 36(2), 241–263. [DOI] [PubMed] [Google Scholar]
- *Scott MS (2017). Commentary: Perspectives on alcohol-related gene and environment interplay in diverse populations. The American Journal on Addictions, 26(5), 526–531. 10.1111/ajad.12584 [DOI] [PubMed] [Google Scholar]
- Scott J, Leboyer M, Hickie I, Berk M, Kapczinski F, Frank E, … & McGorry P. (2013). Clinical staging in psychiatry: A cross-cutting model of diagnosis with heuristic and practical value. British Journal of Psychiatry, 202(4), 243–245. 10.1192/bjp.bp.112.110858 [DOI] [PubMed] [Google Scholar]
- *Serrano A, & Parsons LH (2011). Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacology & Therapeutics, 132(3), 215–41. 10.1016/j.pharmthera.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20. 10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Settles RF, Cyders M, & Smith GT (2010). Longitudinal validation of the acquired preparedness model of drinking risk. Psychology of Addictive Behaviors, 24(2), 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, … & Henry DA. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ, 358, j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ (1987). Stress response dampening. In Blane HT & Leonard KE (Eds.), Psychological theories of drinking and alcoholism (pp. 227–271). Guilford Press. [Google Scholar]
- Sher KJ (2015). Moving the Alcohol Addiction RDoC forward. Alcoholism: Clinical and Experimental Research, 39(4), 579–584. 10.1111/acer.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, & Epler AJ (2004). Alcoholic denial: Self-Awareness and beyond. In Beitman BD & Nair J (Eds.), Self-awareness deficits in psychiatric patients: Neurobiology, assessment, and treatment (p. 184–212). W W Norton & Co. [Google Scholar]
- *Sher KJ, Dick DM, Crabbe JC, Hutchison KE, O’Malley SS, & Heath AC (2010). Consilient research approaches in studying gene x environment interactions in alcohol research. Addiction Biology, 15(2), 200–216. 10.1111/j.1369-1600.2009.00189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, & Grekin ER (2007). Alcohol and affect regulation. In Gross J (Ed.), Handbook of Emotion Regulation (pp. 560–580). New York: Guilford. [Google Scholar]
- Sher KJ, & Trull TJ (1996). Methodological issues in psychopathology research. Annual Review of Psychology, 16(4), 371–400. [DOI] [PubMed] [Google Scholar]
- Sher KJ, & Trull TJ (2002). Substance use disorder and personality disorder. Current Psychiatry Reports, 4(1), 25–29. [DOI] [PubMed] [Google Scholar]
- Sher KJ, & Vergés A (2018). Anticipating the future of research on substance use and substance use disorders. In Sher KJ (Ed.), The Oxford Handbook of Substance Use and Substance Use Disorders. Oxford Press. [Google Scholar]
- Shiffman S, & Wills TA (1985). Coping and substance use: A conceptual framework. In Shiffman S & Willis T (Eds.). Coping and substance use (pp. 3–24). Academic Press. [Google Scholar]
- Siegel S, Krank MD, & Hinson RE (1987). Anticipation of pharmacological and nonpharmacological events: Classical conditioning and addictive behavior. Journal of Drug Issues, 17(1), 83–110. [Google Scholar]
- *Sillaber I, & Henniger MSH (2009). Stress and alcohol drinking. Annals of Medicine, 36(8), 596–605. 10.1080/07853890410018862 [DOI] [PubMed] [Google Scholar]
- Simons JS, Wills TA, & Neal DJ (2014). The many faces of affect: A multilevel model of drinking frequency/quantity and alcohol dependence symptoms among young adults. Journal of Abnormal Psychology, 123(3), 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, & Siedlarz KM (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 34(5), 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Sloan CD, Sayarath V, & Moore JH (2008). Systems genetics of alcoholism. Alcohol Research & Health, 31(1), 14–25. [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, & Martin NG (2002). Personality and the genetic risk for alcohol dependence. Journal of Abnormal Psychology, 111(1), 124–133. [PubMed] [Google Scholar]
- Smith GT, & Anderson KG (2001). Adolescent risk for alcohol problems as acquired preparedness: A model and suggestions for intervention. In Monti PM, Colby SM, & O’Leary TA (Eds.), Adolescents, alcohol, and substance abuse: Reaching teens through brief interventions (pp. 109–141). New York: Guilford Press. [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, & McCarthy DM (2007). On the validity and utility of discriminating among impulsivity-like traits. Assessment, 14(2), 155–170. [DOI] [PubMed] [Google Scholar]
- *Smith L, Watson M, Gates S, Ball D, & Foxcroft D (2008). Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: A huge gene-disease association review. American Journal of Epidemiology, 167(2), 125–138. 10.1093/aje/kwm281 [DOI] [PubMed] [Google Scholar]
- Solomon RL (1980). The opponent-process theory of acquired motivation: The costs of pleasure and the benefits of pain. American Psychologist, 35(8), 691–712. [DOI] [PubMed] [Google Scholar]
- Srey CS, Maddux JMN, & Chaudhri N (2015). The attribution of incentive salience to Pavlovian alcohol cues: A shift from goal-tracking to sign-tracking. Frontiers in Behavioral Neuroscience, 9, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton K (2020). Increasing diagnostic emphasis on negative affective dysfunction: potentially negative consequences for psychiatric classification and diagnosis. Clinical Psychological Science. 10.31219/osf.io/7ysfa [DOI] [Google Scholar]
- Stanton K, & Watson D (2016). Adult ADHD: Associations with personality and other psychopathology. Journal of Psychopathology and Behavioral Assessment, 38(2), 195–208. [Google Scholar]
- *Stautz K, & Cooper A (2013). Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clinical Psychology Review, 33(4), 574–592. 10.1016/j.cpr.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Steele CM, & Josephs RA (1990). Alcohol myopia: Its prized and dangerous effects. American Psychologist, 45(8), 921–933. [DOI] [PubMed] [Google Scholar]
- *Steinhausen H-C (1995). Children of alcoholic parents: A review. European Child & Adolescent Psychiatry, 4(3), 143–152. 10.1007/BF01980453 [DOI] [PubMed] [Google Scholar]
- Stephan RA, Alhassoon OM, Allen KE, Wollman SC, Hall M, Thomas WJ, … & Dalenberg CJ. (2017). Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. The American Journal of Drug and Alcohol Abuse, 43(1), 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, & Crabbe JC (2010). Reward sensitivity: Issues of measurement, and achieving consilience between human and animal phenotypes. Addiction Biology, 15(2), 146–168. 10.1111/j.1369-1600.2009.00193.x [DOI] [PubMed] [Google Scholar]
- Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, & Vanderplasschen W (2014). Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. Journal of Substance Abuse Treatment, 47(1), 58–72. [DOI] [PubMed] [Google Scholar]
- *Stewart SH (1996). Alcohol abuse in individuals exposed to trauma: A critical review. Psychological Bulletin, 120(1), 83–112. 10.1037/0033-2909.120.1.83 [DOI] [PubMed] [Google Scholar]
- Strain EC (2021). Hegemony, homogeneity, and DSM-5 SUD. Drug and Alcohol Dependence, 221, 108660–108660. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Tennen H, Carney MA, Affleck G, Willard A, & Hromi A (2000). Mood and alcohol consumption: an experience sampling test of the self-medication hypothesis. Journal of Abnormal Psychology, 109(2), 198–204. [PubMed] [Google Scholar]
- Tapper K, Baker L, Jiga-Boy G, Haddock G, & Maio GR (2015). Sensitivity to reward and punishment: Associations with diet, alcohol consumption, and smoking. Personality and Individual Differences, 72, 79–84. [Google Scholar]
- *Tarter RE, & Edwards K (1988). Psychological factors associated with the risk for alcoholism. Alcoholism: Clinical and Experimental Research, 12(4), 471–480. 10.1111/j.1530-0277.1988.tb00229.x [DOI] [PubMed] [Google Scholar]
- *Tarter RE, Alterman AI, & Edwards KL (1985). Vulnerability to alcoholism in men: A behavior-genetic perspective. Journal of Studies on Alcohol, 46(4), 329–356. 10.15288/jsa.1985.46.329 [DOI] [PubMed] [Google Scholar]
- *Tawa EA, Hall SD, & Lohoff FW. (2016). Overview of the genetics of alcohol use disorder. Alcohol and Alcoholism, 51(5), 507–514. 10.1093/alcalc/agw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A, & Waller NG (2008). Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. The SAGE Handbook of Personality Theory and Assessment, 2, 261–292. [Google Scholar]
- *Thacker SB, Veech RL, Vernon AA, & Rutstein DD (1984). Genetic and biochemical factors relevant to alcoholism. Alcoholism: Clinical and Experimental Research, 8(4), 375–383. 10.1111/j.1530-0277.1984.tb05683.x [DOI] [PubMed] [Google Scholar]
- *Thatcher DL, & Clark DB (2008). Adolescents at risk for substance use disorders: Role of psychological dysregulation, endophenotypes, and environmental influences. Alcohol Research & Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism, 31(2), 168–176. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23584818 [PMC free article] [PubMed] [Google Scholar]
- *Thombs DL, & Osborn CJ (2013). Cognitive models. In Introduction to Addictive Behaviors (pp. 160–184). Guilford Press. [Google Scholar]
- Tiffany ST, & Conklin CA (2000). A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction, 95(8), S145–S153. 10.1080/09652140050111717 [DOI] [PubMed] [Google Scholar]
- *Topel H (1988). Beta-endorphin genetics in the etiology of alcoholism. Alcohol, 5(2), 159–165. 10.1016/0741-8329(88)90014-6 [DOI] [PubMed] [Google Scholar]
- *Trice HM, & Sonnenstuhl WJ (1988). Drinking behavior and risk factors related to the work place: Implications for research and prevention. The Journal of Applied Behavioral Science, 24(4), 327–346. 10.1177/002188638802400403 [DOI] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, … & Eaves L (1998). Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Archives of General Psychiatry, 55(11), 967–972. [DOI] [PubMed] [Google Scholar]
- Tully EC, & Iacono WG (2016). An integrative common liabilities model for the comorbidity of substance use disorders with externalizing and internalizing disorders. In Sher KJ (Eds.), The Oxford Handbook of Substance Use and Substance Use Disorders: Volume 2 (pp. 187–214). New York, New York: Oxford University Press. [Google Scholar]
- Um M & Cyders MA (2019). Positive emotion-based impulsivity as a transdiagnostic endophenotype. Gruber J (Ed.), The Oxford handbook of positive emotion and psychopathology (pp. 197–222). Oxford University Press. [Google Scholar]
- van Hemel-Ruiter ME, de Jong PJ, Ostafin BD, & Wiers RW (2015). Reward sensitivity, attentional bias, and executive control in early adolescent alcohol use. Addictive Behaviors, 40, 84–90. [DOI] [PubMed] [Google Scholar]
- van Lier HG, Pieterse ME, Schraagen JMC, Postel MG, Vollenbroek-Hutten MMR, de Haan HA, & Noordzij ML (2018). Identifying viable theoretical frameworks with essential parameters for real-time and real world alcohol craving research: A systematic review of craving models. Addiction Research & Theory, 26(1), 35–51. 10.1080/16066359.2017.1309525 [DOI] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, & Clark DB (2003). Liability to substance use disorders: 1. Common mechanisms and manifestations. Neuroscience and Biobehavioral Reviews, 27, 507–515. 10.1016/j.neubiorev.2003.08.002 [DOI] [PubMed] [Google Scholar]
- *Velleman R (1992). Intergenerational effects—A review of environmentally oriented studies concerning the relationship between parental alcohol problems and family disharmony in the genesis of alcohol and other problems. I: The intergenerational effects of alcohol problems. International Journal of the Addictions, 27(3), 253–280. 10.3109/10826089209068741 [DOI] [PubMed] [Google Scholar]
- *Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Villalba K, Attonito J, Mendy A, Devieux JG, Gasana J, & Dorak TM (2015). A meta-analysis of the associations between the SLC6A4 promoter polymorphism (5HTTLPR) and the risk for alcohol dependence. Psychiatric Genetics, 25(2), 47–58. 10.1097/YPG.0000000000000078 [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, Easdon C, Fillmore M, Finn P, & Justus A (2001). Alcohol and behavioral control: Cognitive and neural mechanisms. Alcoholism: Clinical and Experimental Research, 25(1), 117–121. [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Tomasi D, & Baler R (2010). Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioeassays, 32(9), 748–755. 10.1002/bies.201000042.Addiction [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, … Bullmore ET (2015). Disorders of compulsivity: A common bias towards learning habits. Molecular Psychiatry, 20(3), 345–352. 10.1038/mp.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Grodin E, Mandali A, Morris L, Kwako L, Goldman D, & Koob GF (2020). Addictions NeuroImaging Assessment (ANIA): Towards an integrative framework for alcohol use disorder. Neuroscience and Biobehavioral Reviews 10.1016/j.neubiorev.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Votaw VR, Pearson MR, Stein E, & Witkiewitz K (2020). The Addictions Neuroclinical Assessment negative emotionality domain among treatment-seekers with alcohol use disorder: construct validity and measurement invariance. Alcoholism: Clinical and Experimental Research. 10.1111/acer.14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Walters GD (2002). The heritability of alcohol abuse and dependence: A meta-analysis of behavior genetic research. The American Journal of Drug and Alcohol Abuse, 28(3), 557–584. 10.1081/ADA-120006742 [DOI] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, … & Biernacka JM. (2018). Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience, 21(12), 1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wang K-S, Liu X, Zhang Q, Pan Y, Aragam N, & Zeng M (2011). A meta-analysis of two genome-wide association studies identifies 3 new loci for alcohol dependence. Journal of Psychiatric Research, 45(11), 1419–1425. 10.1016/j.jpsychires.2011.06.005 [DOI] [PubMed] [Google Scholar]
- *Wang F, Simen A, Arias A, Lu Q-W, & Zhang H (2013). A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Human Genetics, 132(3), 347–358. 10.1007/s00439-012-1251-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1997). Extraversion and its positive emotional core. In: Hogan R, Johnson J, & Briggs S, Eds. Handbook of Personality Psychology (pp. 767–793). Academic Press. [Google Scholar]
- Watson D, Wiese D, Vaidya J, & Tellegen A (1999). The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology, 76, 820–838. [Google Scholar]
- Watts AL, Boness CL, Loeffelman JE, Steinley D, & Sher KJ (in press). Does crude measurement contribute to observed unidimensionality of psychological constructs? An example with DSM-5 alcohol use disorder. Journal of Abnormal Psychology. https://psyarxiv.com/paxd4/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AL, Wood P, Jackson KM, Lisdahl K, Heitzeg M, Gonzalez R, … Sher KJ (2020). Incipient alcohol use in childhood: Early alcohol sipping and its relations with psychopathology and personality. Development and Psychopathology 10.31234/osf.io/bpu4r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2012). Comparison of alcohol impairment of behavioral and attentional inhibition. Drug and Alcohol Dependence, 126(1–2), 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *West R (2005). Beginning the journey: Addiction as choice. In West R (Ed.), Theory of addiction. (pp. 41–94). [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, … & Baker TB (1994). Smoking outcome expectancies: Factor structure, predictive validity, and discriminant validity. Journal of Abnormal Psychology, 103(4), 801–811. [DOI] [PubMed] [Google Scholar]
- *Whitfield JB (1997). Meta-analysis of the effects of alcohol dehydrogenase genotype on alcohol dependence and alcoholic liver disease. Alcohol & Alcoholism, 32(5), 613–619. 10.1093/oxfordjournals.alcalc.a008303 [DOI] [PubMed] [Google Scholar]
- Widiger TA, & Oltmanns JR (2017). Neuroticism is a fundamental domain of personality with enormous public health implications. World Psychiatry, 16(2), 144–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, & Turner JA (2014). Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences, 25(1), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wiles NJ, Lingford-Hughes A, Daniel J, Hickman M, Farrell M, Macleod J, … & Lewis G (2007). Socio-economic status in childhood and later alcohol use: A systematic review. Addiction, 102(10), 1546–1563. 10.1111/j.1360-0443.2007.01930.x [DOI] [PubMed] [Google Scholar]
- *Wilson AF, & Elston RC (1995). Linkage analysis in the study of the genetics of alcoholism. In Begleiter H & Kissin B (Eds.), The genetics of alcoholism (pp. 353–376). [Google Scholar]
- *Wilson S, Bair JL, Thomas KM, & Iacono WG (2017). Problematic alcohol use and reduced hippocampal volume: A meta-analytic review. Psychological Medicine, 47(13), 2288–2301. 10.1017/S0033291717000721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wimer RE, & Wimer CC (1985). Animal behavior genetics: A search for the biological foundations of behavior. Annual Review of Psychology, 36, 171–218. 10.1146/annurev.ps.36.020185.001131 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, & Leggio L (2019). Advances in the science and treatment of alcohol use disorder. Science Advances, 5(9), eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2002). Brain reward circuitry: Insights from unsensed incentives. Neuron, 36(2), 229–240. [DOI] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna EDSF, Ramaekers JG, & Wiers RW (2015). Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology, 232(20), 3685–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, & Rünger D (2016). Psychology of habit. Annual Review of Psychology, 67, 289–314. 10.1146/annurev-psych-122414-033417 [DOI] [PubMed] [Google Scholar]
- *Worby PA, & Organista KC (2007). Alcohol use and problem drinking among male Mexican and Central American im/migrant laborers: A review of the literature. Hispanic Journal of Behavioral Sciences, 29(4), 413–455. 10.1177/0739986307308765 [DOI] [Google Scholar]
- World Health Organization (WHO) Staff. (1992). ICD-10 classifications of mental and behavioural disorder: Clinical descriptions and diagnostic guidelines. World Health Organization [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, … & Heinz A (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage, 35(2), 787–794. [DOI] [PubMed] [Google Scholar]
- Wray TB, Simons JS, Dvorak RD, & Gaher RM (2012). Trait-based affective processes in alcohol-involved “risk behaviors”. Addictive behaviors, 37(11), 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Xiao P, Zhong J, Zhu Y, Shi H, Pan P, & Dai Z (2015). Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. 10.1016/j.drugalcdep.2015.05.030 [DOI] [PubMed] [Google Scholar]
- *Yap MBH, Cheong TWK, Zaravinos-Tsakos F, Lubman DI, & Jorm AF (2017). Modifiable parenting factors associated with adolescent alcohol misuse: A systematic review and meta-analysis of longitudinal studies. Addiction, 112(7), 1142–1162. 10.1111/add.13785 [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118(1), 117–130. 10.1037/a0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Young-Wolff KC, Enoch M-A, & Prescott CA (2011). The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review, 31(5), 800–816. 10.1016/j.cpr.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Carter A, … Fernandez MJ (2019). A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction, 114(6), 1095–1109. 10.1111/add.14424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TCB, Pedersen SL, McCarthy DM, & Smith GT (2014). Less drinking, yet more problems: Understanding African American drinking and related problems. Psychological Bulletin, 140(1), 188–223. 10.1037/a0032113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Zintzaras E (2012). Gamma-aminobutyric acid A receptor, alpha-2 (GABRA2) variants as individual markers for alcoholism: A meta-analysis. Psychiatric Genetics, 22(4), 189–196. 10.1097/YPG.0b013e328353ae53 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, & Koob GF (2014). Corticotropin Releasing Factor: A key role in the neurobiology of addiction. Frontiers in Neuroendocrinology, 35(2), 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Buchsbaum MS, & Murphy DL (1980). Sensation seeking and its biological correlates. Psychological Bulletin, 88(1), 187–214. 10.1037/0033-2909.88.1.187 [DOI] [Google Scholar]
- *Zuo L, Lu L, Tan Y, Pan X, Cai Y, Wang X, … & Vanerlinden LA (2014). Genome-wide association discoveries of alcohol dependence. American Journal on Addictions, 23(6), 526–539. 10.1111/j.1521-0391.2014.12147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Zuo L, Tan Y, Zhang X, Wang X, Krystal J, Tabakoff B, … & Luo X (2015). A new genomewide association meta-analysis of alcohol dependence. Alcoholism: Clinical & Experimental Research, 39(8), 1388–1395. 10.1111/acer.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]


