Highlights
-
•
RRMDD patients show deviant uninstructed neural emotion regulation.
-
•
This is reflected in residual problems in daily strategy use.
-
•
RRMDD patients are capable to engage frontolimbic areas upon instructed reappraisal.
-
•
Neural regulation capacity is related to inadequate rumination.
-
•
Positive, next to negative, affect is highly relevant for understanding vulnerability.
Keywords: Depression, Remission, Emotion regulation, Rumination, fMRI
Abstract
The recurrent nature of Major Depressive Disorder (MDD) necessitates a better understanding of mechanisms facilitating relapse. MDD has often been associated with abnormal emotion regulation, underpinned by aberrant interactions between the prefrontal cortex and subcortical areas. We assessed whether neural regulation abnormalities remain after remission and relate to emotion regulation problems in daily life.
At the baseline measurement of a randomized controlled trial, an emotion regulation task was performed during fMRI scanning by 46 remitted recurrent (rrMDD) patients and 24 healthy controls. We assessed both fMRI peak activity and the temporal dynamics of the neural response during passive attendance and explicit regulation of positive and negative emotions. Furthermore, we assessed regulation strategy use in daily life using questionnaires, and attentional biases using a modified attentional dot-probe task.
RrMDD patients showed lower activation and different temporal dynamics in occipital, parietal, and prefrontal brain regions during passive attendance of emotional material compared to healthy controls. During explicit downregulation of negative emotions, no group differences were found. However, during explicit upregulation of positive emotions, rrMDD patients showed a different neural response over time in the insula. Behaviourally, rrMDD patients were characterized by dysfunctional regulation strategies in daily life. Within rrMDD patients, rumination was associated with activation within a limbic- prefrontal network.
After remission, immediate emotional processing seems unaffected, but regulatory abnormalities remain, especially uninstructed and in daily life. Abnormal insula activation during positive upregulation suggests decreased monitoring of positive emotions. The relation between inadequate rumination and brain activity during emotion regulation suggests that regulation of both positive and negative affect is important in understanding neurocognitive underpinnings of resilience.
1. Introduction
Major Depressive Disorder (MDD) is the leading cause of disease burden worldwide, partly because of its highly recurrent character (Kessing et al., 2004, Richards, 2011, Ferrari et al., 2013). Some models of depression propose that abnormalities in the processing and regulation of emotional information are important in understanding how even minor negative events may lead to subsequent MDD episodes. Negative stressors may trigger latent dysfunctional schemas, which modify the processing of internal and external information, (dis)favouring information with a certain emotional valence, and facilitating the occurrence of negative mood states (Beck, 2008, Disner et al., 2011, Gotlib and Joormann, 2010, De Raedt and Koster, 2010). Dysfunctional regulatory control may then lead to the prolongation of negative mood, spiralling down into a sustained negative mood, and eventually a (new) depressive episode (Disner et al., 2011, Berking and Wupperman, 2012, Ehring et al., 2008, Berking et al., 2014). Insight into neurocognitive abnormalities after remission may contribute to understanding relapse vulnerability and improving preventive treatment.
Dysfunctional emotion regulation has been proposed as a transdiagnostic therapeutic target for overcoming affective problems occurring in psychiatric disorders such as MDD (Gotlib and Joormann, 2010, Holtzheimer and Mayberg, 2011, Ochsner and Gross, 2005, Phillips et al., 2008, Phillips et al., 2003, Sloan et al., 2017, Cludius et al., 2020, Aldao and Nolen-Hoeksema, 2010). Behaviourally, emotion regulation abnormalities may manifest as a tendency to use inadequate emotion regulation strategies typically related to MDD, including greater use of rumination and expressive suppression but lower use of cognitive reappraisal (Aldao et al., 2010, Joormann et al., 2007, Joormann and Michael, 2014, Visted et al., 2018). These strategies are all aimed to improve affect, although rumination and suppression appear less effective and less beneficial for mental wellbeing than reappraisal (Nolen-Hoeksema et al., 2008, Gross and John, 2003, Gross, 1998, Garnefski and Kraaij, 2006). To what extent residual abnormalities in daily use of emotion regulation strategies reflect abnormalities in neural regulation capacity is not yet fully understood.
Emotion regulation takes place on a continuum from implicit and automatic (for example automatic selective attention or habitual responses), to more explicit and controlled (for example cognitive reappraisal) (Braunstein et al., 2017, Gyurak et al., 2011). During implicit forms of emotion regulation, MDD has been associated with increased recruitment of parietal and lateral prefrontal cortices, suggestive of compensatory yet inadequate regulatory control (Rive et al., 2013, Hamilton et al., 2012). During explicit forms of emotion regulation, abnormally decreased lateral prefrontal cortex activity has been most consistently observed (Rive et al., 2013, Picó-Pérez et al., 2017, Zilverstand et al., 2017), suggesting inadequate recruitment of regulatory resources resulting in an unregulated depressive mood (as reflected in increased amygdala activity (Zilverstand et al., 2017). Whether this reflects deficient performance of control mechanisms and/or inadequate signalling for the need for control is currently unresolved (Hamilton et al., 2012, Teasdale and Dent, 1987, Moll et al., 2005, Miller and Cohen, 2001, Disner et al., 2011). Problems in sustaining positive mood, reflected in diminished frontostriatal activation, seem related to anhedonic symptoms (Heller et al., 2009) and recurrence (de Jonge et al., 2017). Most studies so far, however, have focused on negative emotion regulation (Zilverstand et al., 2017).
Whether neural abnormalities in implicit or explicit emotion regulation persist after remission is not yet clear. Some studies suggest normalization of automatic emotion processing abnormalities following treatment (Fu et al., 2004, Fu et al., 2008, Victor et al., 2010, Godlewska et al., 2012), while other studies suggest that abnormalities remain in the remitted phase (Bocharov et al., 2017, Leppänen, 2006, Ruhe et al., 2019, Goulden et al., 2012). Regarding explicit emotion regulation, both impaired (Kanske et al., 2012, Smoski et al., 2015) and normal (Rive et al., 2015a) frontal regulation of limbic activity have been reported in remitted recurrent MDD (rrMDD), leaving uncertainty as to the specifics of frontal regulatory abnormalities after remission. Furthermore, in fMRI studies in this population, only averaged peak height of the haemodynamic response has been studied, while for positive emotion regulation in acute MDD it has proven fruitful to additionally assess the temporal dynamics of sustained regulation capacity (Heller et al., 2009). Studying the temporal dynamics of frontal response profiles during emotion regulation may comprehensively add to our understanding of neurocognitive mechanisms underlying recurrence (Goldin et al., 2008), specifically whether rrMDD is characterized by a failure to engage the prefrontal cortex (PFC) or by abnormalities in sustaining PFC activation (Zhang et al., 2019).
Explicit emotion regulation may be affected by abnormalities in early emotional processing, by dysfunctional gating of emotional information (Beck, 2008, Zvielli et al., 2016, Keller et al., 2019, Disner et al., 2017). MDD is typically characterized by attentional biases favouring negative information or disfavouring positive information (Peckham et al., 2010, Armstrong and Olatunji, 2012, Winer and Salem, 2016). It is not yet clear to what extent attentional biases are related to MDD state, and whether they play a role in the neurocognitive mechanisms facilitating relapse (Joormann and Gotlib, 2007, Elgersma et al., 2018, Elgersma et al., 2019). Attentional biases have been related to rumination (during acute MDD) (Joormann et al., 2006, Sanchez-Lopez et al., 2019, Grafton et al., 2016, Owens and Gibb, 2017), and it has been suggested that attentional biases may not directly lead to depressive symptoms, but in interaction with ineffective emotion regulation strategies (Demeyer et al., 2012, Everaert et al., 2017) (though not consistently (Figueroa et al., 2019). Further examining emotion regulation in relation to attentional biases in rrMDD might enhance our understanding of residual vulnerability factors.
In the present study we examined for the first time whether medication-free rrMDD patients at high risk for recurrence are characterized by residual abnormalities in emotional processing and explicit emotion regulation on both the neural and behavioural level, for both positive and negative emotionality. To this end, we investigated both average peak activity and the temporal response profile of the hemodynamic response function (HRF) during an emotion regulation task, and assessed self-reported use of emotion regulation strategies, in rrMDD patients and healthy controls. Furthermore, within rrMDD patients, we examined whether neural activity during explicit emotion regulation is reflected in regulation strategy use in daily life. Finally, we evaluated the role of early gating of emotional information in emotion regulation capacity.
2. Materials and methods
2.1. Participants
In the present study, baseline data was used from participants included in the context of a randomized controlled trial, the Neurocognitive Working Mechanisms of the Prevention of Relapse in Depression (NEWPRIDE) study (Van Kleef et al., 2019). The NEWPRIDE study was approved by the Medical Ethical Committee of the University Medical Centre Groningen, and all participants provided written informed consent.
Fifty participants with rrMDD were included in the present study, together with 25 healthy control (HC) participants. Inclusion criteria for rrMDD patients were: at least two Major Depressive Episodes in the past five years, being currently in remission from their last MDE for more than two months, but no longer than two years, and no current other DSM-IV diagnosis (as assessed with the Structural Clinical Interview for DSM-IV Axis I disorders (SCID-I; (Spitzer et al., 1992), no psychotropic medication use, and scoring 13 or lower on the Self-Rated version of the Inventory of Depressive Symptomatology (IDS-SR; (Rush et al., 1996) at the time of screening. Controls were selected based on the (lifetime) absence of any DSM-IV diagnosis (as assessed with the SCID-I). All participants showed normal intelligence, did not report neurological problems or lifetime alcohol or substance dependency, and met standard MRI compatibility criteria.
2.2. Measures
2.2.1. Clinical measures
In order to assess current depressive symptomatology, the Inventory of Depressive Symptoms-Self Report (IDS-SR; (Rush et al., 1996) was administered. The IDS-SR is a 30-item scale assessing a full range of depressive symptoms, with good psychometric properties (Trivedi et al., 2004). Furthermore, the Positive and Negative Affect Schedule (PANAS, showing adequate reliability and validity (Watson et al., 1988, Crawford and Henry, 2004), a self-report questionnaire with two 10-item scales on positive and negative affect, was used to assess affect at the time of testing (using a visual analogue scale).
2.2.2. Emotion regulation strategies
The Leuven adaptation of the Rumination on Sadness Scale (RSS) is a 17-item self-report questionnaire with good reliability and validity, designed to measure ruminative thinking on sadness, and consists of three sub-scales (Analysis, Understanding, Uncontrollability) (Raes et al., 2008).
The Dutch version of the Responses to Positive Affect (RPA; showing adequate psychometric properties (Feldman et al., 2008, Raes et al., 2009) measures rumination in response to positive affect, and consists of three scales: Self-focused rumination, Emotion-focused rumination and Dampening (based on factor analysis; (Feldman et al., 2008).
The Emotion Regulation Questionnaire (ERQ; (Gross and John, 2003), a 10-item self-report questionnaire with good psychometric properties (Ioannidis and Siegling, 2015), assesses to which extent participants tend to use expressive suppression and cognitive reappraisal (confirmed with factor analysis (Melka et al., 2011).
2.2.3. Attentional bias task
An adjusted version of the Attentional Response to Distal vs Proximal Emotional Information task (ARDPEI; (Grafton and MacLeod, 2014) was administered outside of the scanner, to assess attentional biases towards negative information and away from positive information. The ARDPEI is a modified dot-probe task that allows for the calculation of both an engagement bias index and a disengagement bias index. The task consists of two types of conditions: attentional selectivity (engagement or disengagement) and valence (positive or negative visual stimuli). Details on the task design and calculation of the bias indices can be found in Supplementary materials 1.
2.2.4. Emotion regulation task
During fMRI scanning, participants performed the Emotion Regulation Task. The task was designed to measure regional brain activity during passive attending and active regulating emotions, elicited by neutral, negative emotional or positive emotional pictures (each presented for eight seconds). During the attend condition, participants were solely asked to watch the picture. During the downregulation condition, participants were instructed to apply cognitive reappraisal techniques to reduce the intensity of negative emotions, and during the upregulation condition, to increase the intensity of positive emotions. The regulation techniques were trained prior to scanning. Details on the task design can be found in Supplementary materials 1.
2.3. Image acquisition
Scanning was performed on a 3 Tesla Philips Intera scanner (Philips, Best, the Netherlands). Functional images were acquired using a T2*-gradient echo-planar sequence with the following parameters: TR = 2000 ms, TE = 30 ms, FOV = 224 × 129.5 mm, image matrix = 64 × 62, and voxel size = 3.5 × 3.5 × 3.5 mm. Whole-brain coverage was achieved by acquisition of 37 descending slices. Furthermore, a T1-weighted structural scan (170 slices, TR = 9 ms, TE = 3.53 ms, FOV = 256 × 256 mm, image matrix = 256 × 256 mm, voxel size = 2 × 2 × 2 mm) was acquired to be used in spatial preprocessing of the functional imaging data.
2.4. Data analyses
2.4.1. Demographic and clinical characteristics
Questionnaire and behavioural data were analysed with IBM SPSS 25 (SPSS Inc., Chicago, IL, USA). Because normality of questionnaire and behavioural scores could not be assumed, non-parametric tests were performed. First, groups were compared on demographic characteristics, using nonparametric Mann-Whitney U tests for age and educational level, and chi square tests for sex. To compare clinical characteristics, IDS total scores and PANAS subscale (Negative and Positive) scores were entered in non-parametric Mann-Whitney U tests.
2.4.2. Group differences in behavioural measures
Group differences in emotion regulation strategies were examined with Mann-Whitney nonparametric U tests of the RSS, RPA and ERQ subscales. Effects were considered significant at p < 0.05 (one-tailed). We corrected the alpha level for possible non-independency of effects by using the Simple Interactive Statistical Analysis Bonferroni tool (SISA Bonferroni; https://www.quantitativeskills.com/sisa/calculations/bonfer.htm). We corrected for eight tests, with an average correlation of 0.27, leading to an adjusted alpha of 0.011.
Finally, to assess group differences in attentional biases, we performed non-parametric Mann-Whitney U tests on the ARDPEI negative and positive engagement- and disengagement bias indices. Effects were considered significant at p < 0.05 (one-tailed), SISA-Bonferroni corrected (based on 4 tests, with an average correlation of 0.2, leading to an adjusted alpha of 0.017). For all tests, standardized effect sizes (Hedges’ g) were calculated.
2.4.3. FMRI data preprocessing
Functional MRI data was preprocessed and analysed on the subject-level with statistical parametric mapping software (SPM12 v7487; Welcome Trust Centre for Neuroimaging, London, UK), implemented in Matlab 8.5.0 (R2015a; Mathworks, Natick, MA, USA). Scans were manually reoriented to the anterior-posterior commissure plane, realigned, co-registered (anatomical to functional plane), normalised to MNI space, and spatially smoothed (full-width half-maximum Gaussian kernel of 8 mm). Furthermore, framewise displacement was calculated using a threshold of 0.9 mm (Siegel et al., 2014), and volumes with slices containing high intensity values (related to motion and scanner artefacts) were identified to assess data quality and to be censored in first level models.
2.4.4. FMRI group differences in processing and regulating emotions
On the subject level, data was analysed within the framework of the general linear model (using SPM12). The task data was modelled in an event-related fashion at the subject level including regressors for the five task conditions, instructions, rating periods, volumes marked as motion or artefact, and time and dispersion derivatives, and convolved with a canonical HRF. Due to long between-block intervals within conditions, the high pass filter was set to 0.0052 Hz (192 s).
Non-parametric permutation-based between-group comparisons (HC vs rrMDD patients) were performed using FSL Randomise (5000 permutations) (Winkler et al., 2014), with contrast images from the subject level (negative-attend-vs-baseline, positive-attend-vs-baseline, negative-downregulate-vs-attend and positive-upregulate-vs-attend) as input. Tests were performed both on whole-brain level and within a composite ROI mask. With ‘whole-brain’ we mean a voxel-based analysis of the brain regions included in the brain mask containing adequate signal for fMRI analysis. The ventromedial frontal and anterior temporal brain regions were not included in this brain mask (see Supplement 2). The composite ROI mask was created using the WFU Pickatlas, including the bilateral middle frontal gyrus, frontal operculum, inferior frontal gyrus, superior medial frontal gyrus, anterior cingulate gyrus, amygdala and insula (AAL atlas based). The selection of regions of interest was based on areas commonly involved in emotional processing and regulation (Buhle et al., 2014, Frank et al., 2014, Kohn et al., 2014). Significance was determined at p < 0.05, family-wise error rate corrected for multiple comparisons, based on threshold free cluster-enhancement (TFCE). TFCE has been shown to be the non-parametric correction method of choice in terms of reliability (Han et al., 2019).
2.4.5. FMRI group differences in temporal dynamics of BOLD response during processing and regulating emotions
Furthermore, to assess the hemodynamic response during emotion regulation over time, without a priori assumptions of its shape, we built a general linear model with a Finite Impulse Response (FIR) basis set (method adapted from (Zhang et al., 2019). The model was set up using log-transformed scans, to allow interpretation of meaningful effect values (i.e. percent signal change) on group level (Langers et al., 2014). The time windows were set to the start of the event, and were divided into four bins of 2 s (matching the TR; see Supplementary materials 1 for an overview of the time bins). FIR estimates of the hemodynamic response were calculated per time bin, per condition, for each participant. Equal to the canonical HRF model, other regressors included task instructions, rating periods, and volumes marked as motion or artefact.
To account for the intercorrelation among the within-subject time bins and task conditions, we set up a group-level multivariate model using 3dMVM (AFNI-based software; (Chen et al., 2014, Chen et al., 2015). Two repeated measures-ANOVAs were built, with: (Kessing et al., 2004) negative-attend-vs-baseline and positive-attend-vs-baseline contrast images or (Richards, 2011) negative-downregulate-vs-attend and positive-upregulate-vs-attend contrast images as dependent variables, time (bin 1, bin 2, bin 3 and bin 4) as within-subjects factor and group (HC vs rrMDD) as between-subjects factor. We were particularly interested in the GroupxTime interactions as these would represent differential temporal characteristics of the BOLD signal.
Clusters were considered significant at a height of p < 0.001 and a cluster extent threshold (k > 59 for whole-brain analyses and k > 13 for within-ROI analyses), which was based on Monte Carlo simulated cluster-size distributions (AFNI 3DClustSim, 1000 simulations), retaining two-sided p < 0.05 significance after FWE-correction at the cluster level (NN1). Smoothness of the residuals was calculated using the autocorrelation function for each subject (AFNI’s 3DFWHMx).
2.4.6. Within-rrMDD relations between neural emotion regulation capacity, daily emotion regulation strategy use and attentional bias
We performed multiple regression analyses within the rrMDD group, to assess the relationship between neural responses during emotion regulation and daily use of regulation strategies. Multiple regression models were built using FSL Randomise, to assess the effects of the ERQ subscales, RSS total scale and RPA subscales on the canonical HRF-modelled negative downregulation-vs-negative-attend and positive-upregulate-vs-positive-attend contrast images.
In order to examine the relationship between the neural responses during emotion regulation and attentional biases, multiple regression models were built using FSL Randomise, including the ARDPEI indices as regressors and the canonical HRF-modelled regulation contrast images as dependent variables. For all multiple regression analyses, 5000 permutations were performed, results were considered significant at p < 0.05, family-wise error rate corrected for multiple comparisons, based on TFCE.
Finally, we calculated Spearman correlations between the ERQ, RSS and RPA subscales with the ARDPEI bias indices (considered significant at p < 0.05 (one-tailed), SISA-Bonferroni corrected (based on 32 correlational tests, with an average correlation of 0.16, leading to an adjusted alpha level of 0.003)).
3. Results
3.1. Demographic and clinical characteristics
Initially, we included data from the baseline measurement of 50 rrMDD patients and 25 HC participating in the RCT. Five participants were excluded from these current analyses due to excessive motion or insufficient data quality, leading to a sample of 46 rrMDD patients and 24 HC. Sample characteristics, questionnaire scores and behavioural results are presented in Table 1. Sex, age and education level did not differ between rrMDD and HC. Clinically, rrMDD patients showed more depressive symptoms (IDS, p < 0.001) and experienced more negative and less positive affect (PANAS; Negative p = 0.001, Positive p = 0.001) than HC.
Table 1.
Demographic and clinical characteristics. Significance is indicated with an asterisk.
| rrMDD M (SD) | HC M (SD) | χ2 | U | p | Hedges’ g | |
|---|---|---|---|---|---|---|
| N | 46 | 24 | ||||
| Sex ratio (men/women) | 10/36 | 6/18 | 0.095 | 0.771 | ||
| Age | 35.11 (11.32) | 36.67 (12.88) | 535.00 | 0.832 | 0.13 | |
| Educational level | 5.85 (1.36) | 6.29 (0.69) | 460.00 | 0.223 | 0.37 | |
| Number of MDD episodes | 7.42 (10.07) | – | ||||
| Months in remission | 9.46 (6.29) | – | ||||
| Cognitive (behavioural) therapy use in the past (yes/no) | 33/13 | – | ||||
| Antidepressant use in the past (yes/no) | 25/21 | |||||
| IDS score | 8.33 (5.36) | 2.88 (3.09) | 201.50 | <0.001* | 1.16 | |
| PANAS score | ||||||
| Negative scale | 30.13 (17.97) | 17.51 (13.72) | 322.00 | 0.001* | 0.76 | |
| Positive scale | 61.36 (17.02) | 73.41 (11.60) | 318.00 | 0.001* | 0.78 | |
Abbreviations: RrMDD = remitted recurrent Major Depressive Disorder; HC = healthy controls; M = mean; SD = standard deviation; χ2 = chi square statistic; U = Mann-Whitney U statistic; p = significance level; MDD = Major Depressive Disorder; IDS = Inventory of Depressive Symptoms; PANAS = Positive and Negative Affect Schedule.
3.2. Group differences in behavioural measures
3.2.1. Group differences in emotion regulation strategies
All questionnaire and ARDPEI results are presented in Table 2. RrMDD patients showed a significantly higher tendency to ruminate on negative content (RSS Causal Analysis, Understanding and Uncontrollability all p’s < 0.001, high effect sizes (all g’s > 1.21)). Furthermore, in the face of positive affect, they reported a higher tendency to dampen positive feelings (RPA Dampening p < 0.001, medium/high effect size: g = 0.73) and a lower tendency to react with experiencing/savouring the positive emotion (RPA Self-focused rumination p = 0.001, large effect size, g = 0.81). No difference in emotion-focused rumination in response to positive affect was found (RPA Emotion-focused rumination p = 0.148). Regarding emotion regulation strategies, rrMDD patients reported to use cognitive reappraisal less (ERQ Cognitive Reappraisal p = 0.002, medium/large effect size: g = 0.67), and expressive suppression more (ERQ Expressive Suppression p = 0.005, medium/large effect size: g = 0.71) than HC.
Table 2.
Overview of results of group differences in attentional biases and emotion regulation strategies. Significance is indicated with an asterisk.
| rrMDD M (SD) | HC M (SD) | U | p | Hedges’ g | |
|---|---|---|---|---|---|
| Rumination on Sadness Scale | |||||
| Causal analysis | 17.00 (3.98) | 12.25 (3.83) | 228.00 | <0.001* | 1.21 |
| Understanding | 16.24 (5.16) | 10.29 (3.93) | 197.50 | 0.001* | 1.24 |
| Uncontrollability | 18.43 (5.66) | 10.25 (3.58) | 119.00 | 0.001* | 1.62 |
| Responses to Positive Affect | |||||
| Dampening | 14.78 (6.21) | 10.88 (2.95) | 263.50 | 0.001* | 0.73 |
| Self-focused rumination | 7.70 (2.85) | 9.96 (2.65) | 300.00 | 0.001* | 0.81 |
| Emotion-focused rumination | 12.93 (3.58) | 13.79 (2.92) | 468.50 | 0.148 | 0.26 |
| Emotion Regulation Questionnaire | |||||
| Cognitive reappraisal | 24.82 (8.09) | 30.02 (7.04) | 324.50 | 0.002* | 0.67 |
| Expressive suppression | 14.89 (5.82) | 11.25 (3.30) | 350.00 | 0.005* | 0.71 |
| ARDPEI biases | |||||
| Engagement negative | −36.27 (153.50) | 8.18 (97.95) | 347.00 | 0.210 | 0.32 |
| Engagement positive | −106.50 (129.25) | −98.16 (108.80) | 360.50 | 0.278 | 0.07 |
| Disengagement negative | −0.86 (108.58) | 15.32 (104.81) | 348.00 | 0.214 | 0.15 |
| Disengagement positive | 55.35 (154.24) | −20.08 (121.01) | 306.50 | 0.076 | 0.52 |
Abbreviations: RrMDD = remitted recurrent Major Depressive Disorder, HC = healthy controls, M = mean, SD = standard deviation, U = Mann-Whitney U value, p = significance level, ARDPEI = Attentional Responses to Distal vs Proximal Emotional Information.
3.2.2. Group differences in attention biases
The mean percentage of correct trials during the ARDPEI was 95.24, in line with previous studies using this task (Grafton and MacLeod, 2014, Southworth et al., 2017, Jonker et al., 2019). RrMDD patients and HC did not differ with regard to the attentional engagement and disengagement biases for both negative stimuli and positive stimuli (all p’s < 0.076), although for disengagement from positive material, effect sizes indicated a medium effect size (g = 0.52).
3.3. FMRI group differences in attending to emotional images
3.3.1. Peak activation in canonical HRF modelled BOLD responses during attending
RrMDD patients showed less peak activity during negative-attend-vs-baseline in the bilateral occipital lobe and precuneus, and the right visual association area and ventral part of the posterior cingulate cortex (PCC), dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG) and frontal pole than HC (see Fig. 1A). During positive-attend-vs-baseline, rrMDD patients showed less peak activity in the bilateral occipital lobe, extending to the precuneus, and the left superior temporal gyrus, extending to the supramarginal gyrus (see Fig. 3B).
Fig. 1.
Clusters in which rrMDD show significantly different activation than HC patients during 1) negative-attend-vs-baseline and (1B) positive-attend-vs-baseline, with yellow/orange representing clusters with lower peak activity in rrMDD patients compared to HC in the HRF model, and green/blue representing clusters in which a significant GroupxTime interaction was found within the FIR model, plus plots of the percent signal change of the beta images per condition over the four time bins for each group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Significant clusters in multiple regressions with RSS scores (3A) and RPA Self-focused rumination scores (3B) during explicit negative regulation (versus attending), and RPA Dampening scores (3C) during explicit positive regulation (versus attending) within rrMDD patients.
3.3.2. Responses over time in FIR modelled BOLD responses during attending
RrMDD patients and HC showed a different temporal pattern of BOLD response height in the superior parietal gyrus during negative-attend-vs-baseline, and in the bilateral precuneus and postcentral gyrus during positive-attend-vs-baseline. Plotting activity over the time bins within these clusters (see Fig. 1B) showed that during attending emotional information, HC had an initial dip in activation around 2–4 s in these areas, after which activity increased, and rrMDD patients show a more blunted response.
3.4. FMRI group differences in explicit emotion regulation
3.4.1. Peak activation in canonical HRF modelled BOLD responses during explicit regulation
There were no significant group differences in peak BOLD activity during explicit emotion regulation versus attending emotional images for both negative and positive conditions.
3.4.2. Responses over time in FIR modelled BOLD responses during explicit regulation
There were no significant differences in the temporal dynamics of the BOLD response during negative-downregulate-vs-attend.
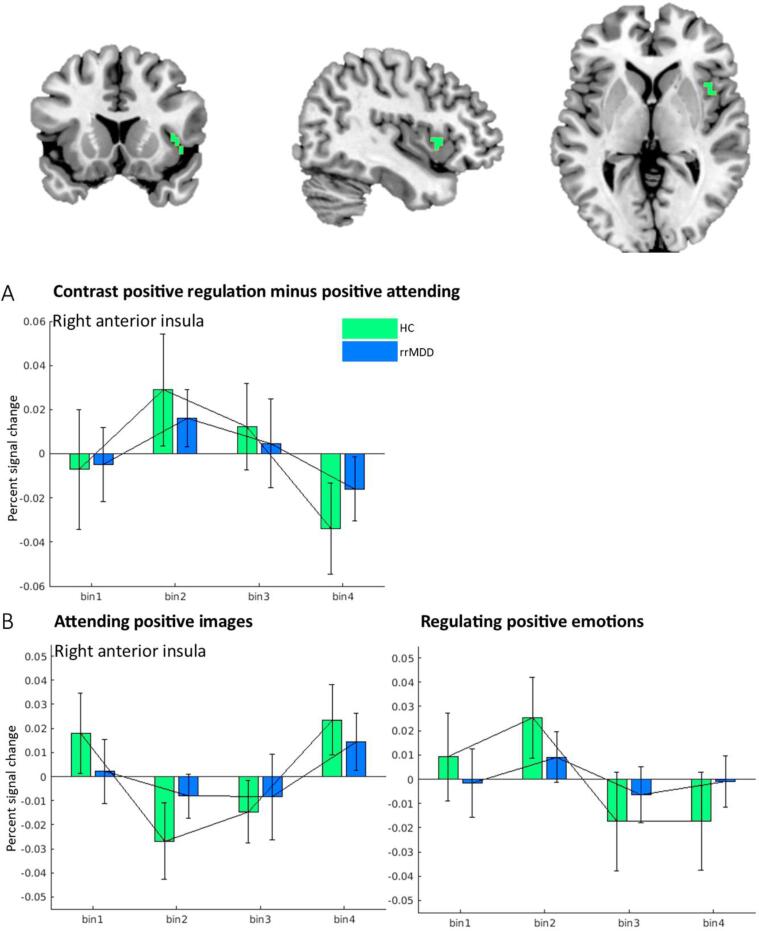
During positive-upregulate-vs-attend, rrMDD patients showed both lower overall activation and a differential temporal pattern of the BOLD response in the right dorsal anterior insula, compared to HC. Plotting the mean activity during these conditions over time within the right insula (see Fig. 2) showed that regulation of positive emotions is characterized by an initial increase and a later decrease in activity in HCs, and only a slight initial increase in rrMDD patients.
Fig. 2.
Percent signal change over time in rrMDD patients and HC showing (2A) contrast images for positive-upregulation-vs-attend and (2B) positive-attend-vs-baseline and positive-upregulate-vs-attend images separately, within the clusters in which a significant GroupxTime interaction was found.
3.5. Within-rrMDD relations between neural emotion regulation capacity and daily emotion regulation strategy use
RrMDD patients who tend to ruminate on negative content (RSS total score), showed lower PCC activation during negative-downregulation-vs-attend (see Fig. 3A).
RrMDD patients who tend to focus more on themselves in response to positive affect (RPA Self-focused rumination) showed lower activation in the left amygdala, hippocampus, ACC, orbitofrontal cortex (OFC), inferior temporal lobe, and bilateral medial PFC and occipital lobe during negative-downregulation-vs-attend (see Fig. 3B). Furthermore, rrMDD patients who tend to dampen their positive emotions (RPA Dampening) showed higher activity in the right OFC and right ventral anterior insula during positive-upregulation-vs-attend (see Fig. 3C).
Use of expressive suppression or cognitive reappraisal was not significantly related to fMRI activity during explicit emotion regulation.
3.6. Within-rrMDD relations between emotion regulation and attentional bias
Within rrMDD patients, there was no significant relationship between explicit negative or positive regulation and the attentional bias scores. Within rrMDD patients, the attentional bias scores did not correlate significantly with emotion regulation strategy use (all p’s > 0.016). Correlations can be found in Supplementary materials 6.
4. Discussion
The recurrent character of MDD calls for a better understanding of neurocognitive mechanisms underlying relapse vulnerability. Dysfunctional emotion regulation is often mentioned in the context of affective disorders. However, the concept of emotion regulation in (especially recurrent) MDD has to date not been fully explored. This study is the first to investigate whether remitted patients suffering from recurrent MDD (rrMDD) are characterized by residual emotion regulation abnormalities on both a neural and behavioural level, for both negative and positive emotionality. We demonstrated that rrMDD patients are characterized by several residual neural regulation abnormalities. More specifically, rrMDD patients showed lower activation in areas within a visual-parietal-prefrontal network, and different temporal dynamics in parietal areas during attending emotional images, but no clear abnormalities during explicit negative emotion regulation. During explicit regulation of positive emotions, rrMDD patients showed differential temporal dynamics in dorsal anterior insula activation. Behaviourally, rrMDD patients reported more dysfunctional emotion regulation strategies, which were related to engagement of posterior midline areas and a frontal-limbic network during regulation of negative and positive emotions within rrMDD patients. Finally, early processing biases do not seem to characterize remitted depression or underlie emotion regulation abnormalities.
4.1. Residual emotion regulation abnormalities in rrMDD patients
4.1.1. Abnormal neural activation during emotion regulation
RrMDD patients showed different activity in several brain regions during attending to emotional images, which could be indicative of abnormalities in the unintentional use of strategies to regulate emotional responses (also considered implicit regulation (Gyurak et al., 2011, Webb et al., 2015, Reinecke et al., 2015). First, lower (and different temporal) activation in the occipital lobe, precuneus, superior parietal gyrus, postcentral gyrus, and PCC during attending emotional images in rrMDD was observed. As these regions have been primarily implicated in visual, self-related and attentional processing, these abnormalities could indicate that abnormal resource deployment during these important aspects of processing of emotional information underpins the vulnerability to recurrent depression (Leech and Sharp, 2014, Frodl et al., 2009, Ochsner et al., 2004), for example in the form of distancing (Picó-Pérez et al., 2017, Moodie et al., 2020). Abnormal activation in these areas might not be limited to the process of emotion regulation, but might for example also reflect deficient mental imagery, as evidenced by wider neuroscience literature covering the neural correlates of mental imagery (Holmes et al., 2016, Pearson et al., 2015, Skottnik and Linden, 2019). Second, lower peak activation in frontal regions (IFG and DLPFC) relative to healthy individuals suggests that these brain regions, previously associated with signalling the need for control and exerting control, are sub-optimally involved in the regulation process when no explicit efforts are made (Urry et al., 2009, Buhle et al., 2014, Frank et al., 2014, Kohn et al., 2014). No differences in brain activation were found during negative or positive emotion processing (versus neutral processing), suggesting neural differences during implicit processing are not necessarily valence-specific but could reflect a more general abnormality in complex information processing.
In line with Rive and colleagues (Rive et al., 2015b) we did not find significant differences in neural activity between rrMDD patients and HC during explicit negative emotion regulation, which suggests at least a certain degree of normalization upon remission. As a side note, it is possible that participants used a broader set of regulation strategies during the task than merely cognitive reappraisal. Given that emotion regulation-related neural activity has been shown to be dependent on specific regulatory strategy use (Moodie et al., 2020), this might in part account for the different results in rrMDD patients reported by (Kanske et al., 2012) and (Smoski et al., 2015).
During regulation of positive emotions, rrMDD patients showed a lower overall height of the neural response and different temporal dynamics in the right dorsal insula during positive emotion regulation, relative to the control group. The dorsal part of the anterior insula has been implicated in cognitive control processes (Uddin et al., 2017). In healthy individuals, an early increase and later decrease in positive regulation-induced activation of the insula was found, somewhat similar to the temporal pattern as described in (Goldin et al., 2008) during negative emotion regulation. Right anterior insula activity has been related to processing/regulating both negative and positive emotions before (Frank et al., 2014, Damasio et al., 2000), and may contribute to emotional state monitoring and salience detection. In rrMDD patients, this initial increase and later decrease during regulation was less pronounced, suggesting lower affective monitoring during active engagement with positive emotions (Sliz and Hayley, 2012).
4.1.2. Dysfunctional daily emotion regulation strategy use
RrMDD patients reported to use less cognitive reappraisal and savouring of positive affect, in comparison with never-depressed individuals, but more expressive suppression and negative rumination. Dysfunctional rumination tendencies after remission of MDD play a key role in models of recurrence (Disner et al., 2011, Nolen-Hoeksema et al., 2008, Marchetti et al., 2012, Figueroa et al., 2017). RrMDD patients in our study showed a tendency to dampen and not savour positive affect, which was related to negative affect (see Supplementary materials 6), and has previously been linked to anhedonia (Nelis et al., 2015). Expressive suppression has been related to lower affect and depressogenic cognitions and depressive symptoms, and has repeatedly been reported in remitted depression (Visted et al., 2018, Liu and Thompson, 2017).
Less use of cognitive reappraisal, on the other hand, has previously been found in current, but not remitted depression (Visted et al., 2018, Liu and Thompson, 2017). However, most reported studies included a broader group of remitted patients, with much longer periods since remission and lower numbers of previous episodes than our sample, suggesting that cognitive reappraisal abnormalities may be dependent on level of recurrence. All in all, the regulation of both positive and negative affect seems disturbed on a behavioural level in highly recurrent remitted MDD patients, probably reflecting a vulnerability for relapse.
4.2. Relevance of neural regulation abnormalities for daily regulation problems
Within rrMDD patients, lower PCC activation during downregulation of negative emotions (versus attending) was related to higher rumination on negative content in rrMDD patients. This suggests lower involvement of regions involved in autobiographical self-related processing during effortful regulation of negative emotion, indicative of problems in self-relevant information processing during regulation of emotions in ruminators.
Cross-valence, lower activity in the left ACC, OFC, hippocampus, amygdala and the inferior temporal lobe during downregulating negative emotions was related to a higher tendency to savour positive affect in relation to oneself. Lower activation in these areas, belonging to an affective network, has been related to successful emotion regulation (Buhle et al., 2014), suggesting that more adaptive levels of positive affect savouring may add to cognitive-affective resilience.
Regarding the regulation of positive emotions, different topological parts of the insula seem involved in relapse vulnerability. While different dorsal anterior insula activation was found to be characteristic of rrMDD patients, higher ventral anterior insula activation during upregulating positive emotions was related to dampening of positive affect within rrMDD patients. The ventral anterior insula has been related to affective (Uddin et al., 2017) and interoceptive (Barrett and Simmons, 2015) processes. These results suggest lower affective monitoring of positive emotions in rrMDD when effortfully engaging with positive emotions, but higher experienced arousal and interoceptive awareness in rrMDD patients with a tendency to dampen, possibly reflecting discomfort with experiencing positive emotions.
Furthermore, there was no relationship between brain activity during explicitly regulation emotions (vs attend) and reported use of expressive suppression and cognitive reappraisal, in contrast to earlier studies in non-clinical or clinical (PTSD) populations (Fitzgerald et al., 2018, Drabant et al., 2009). It has previously been reported that even though remitted MDD patients did report less use of cognitive reappraisal, they did not show a different ability to use this strategy when instructed to do so (Liu and Thompson, 2017, Ehring et al., 2010), suggesting they are capable of cognitive reappraisal similarly to never-depressed individuals, but tend to choose less functional strategies (Reinecke et al., 2015).
Finally, of all studied emotion regulation strategies, especially the tendency to dampen positive emotions, the experienced uncontrollability of negative thinking, and the decreased tendencies to savour positive affect and to use cognitive reappraisal seem to have clinical relevance in rrMDD, given their relation with clinical state characteristics (Supplementary materials 8). Furthermore, increased activity in limbic emotion processing areas during upregulation of positive emotions was associated with higher daily negative affect, suggesting higher emotional reactivity when in a negative mood (Supplementary materials 8). Given the relation of negative affect with symptomatology, these findings seem clinically relevant. Taken together, the results of this study suggest that especially focusing on positive regulation skills in preventive treatment (either in the form of specific regulatory skills training (Radkovsky et al., 2014, Berking et al., 2013) or cognitive preventive treatment (Bockting et al., 2005) could be of clinical benefit.
4.3. Early processing biases not relevant for understanding emotion regulation and relapse risk
The present results suggest that there are no residual attentional biases in MDD patients after remission, neither for negative or positive stimuli. These findings should be interpreted with caution, given the difficulty of reliably measuring these kinds of attentional phenomena using reaction time tasks, especially giving the use of long stimulus durations and difference scores (Chapman et al., 2019). Our null findings are in line with earlier studies, questioning the relevance of attentional biases for understanding current (Marchetti et al., 2018) or remitted MDD (Elgersma et al., 2019). However, residual attentional abnormalities have been reported before (Zvielli et al., 2016, Elgersma et al., 2018, Gupta and Kar, 2012), which could be explained by the use of different task paradigms and/or longer stimulus durations. Even though 500 ms is considered long in attention bias research, it has been suggested that attentional biases in (rr)MDD are only present after longer stimulus durations (Koster et al., 2005, Oehlberg et al., 2012). Therefore, we also included a 1000 ms condition (see Supplementary materials 7), however similar null results were found. Finally, no significant correlations with clinical characteristics were found within the rrMDD group (see Supplementary materials 6), suggesting attentional biases have no significant clinical implications for MDD patients in remission. Future research has to specify whether attentional bias is not at all present after remission, or whether biases do remain present but are only observable under certain conditions, for example using self-relevant stimuli (Ji et al., 2017).
Furthermore, early processing biases do not seem relevant for understanding elaborate emotion processing and regulation abnormalities, nor clinical outcomes. Even though it has been suggested that attentional biases in MDD are primarily influential in relation to other cognitive abnormalities (Marchetti et al., 2018, Everaert et al., 2012) such as rumination (Demeyer et al., 2012, Kaiser et al., 2018), in our remitted sample attentional biases did not correlate with emotion regulation strategies, nor with brain activation during explicit emotion regulation. In line with these results, and in reference to the emotion regulation model of (Gross, 2015, Bebko et al., 2014) suggested that early emotion regulation processes, as attentional control, may be less important for understanding emotion regulation success than late elaborate emotion regulation processes as appraisal or response modulation. Since mechanisms for MDD onset can be different than for relapse, early processing biases may be relevant for understanding onset, but based on these results seem of limited importance for understanding relapse vulnerability.
4.4. Strengths and limitations
This study is the first to investigate several related emotion processing and regulation biases, all considered key concepts in understanding depression vulnerability according to the neurocognitive model of MDD (Disner et al., 2011), in rrMDD patients. Investigating the relevance of these abnormalities not just separately but also in relation to each other, may add to the neurocognitive understanding of recurrence in MDD (Everaert et al., 2012). Furthermore, it is important to address not just negative but also positive emotionality when studying rrMDD, since this has been suggested to reflect additional mechanisms underlying vulnerability (Heller et al., 2009, Carl et al., 2013, Tugade and Fredrickson, 2007), which is underscored by our findings. One of the main strengths of the present study is the patient sample, consisting of highly recurrent (with an average of at least seven previous episodes) remitted patients, without confounding effects of comorbidity or medication use. Furthermore, we believe that additionally modelling the fMRI data with a FIR basis set, allowing for investigation of the temporal dynamics of brain activation during emotion regulation, is a valuable addition to the emotion regulation literature (Goldin et al., 2008, Zhang et al., 2019).
Unfortunately, the emotion regulation task setup made it difficult to investigate longer temporal differences in emotion regulation capacity, given the delay of the BOLD response and the close proximity of the events in time. Also, a common technical challenge in fMRI research involves signal coverage in the frontal cortex (Stenger et al., 2006). The current study did not include ventromedial and anterior temporal brain regions (see Supplement 2). Our null results in these regions should therefore be interpreted with caution. Furthermore, given the context of the trial in which this data was collected, our number of subjects is relatively small (although considered sufficient for fMRI studies into moderate effect sizes (Thirion et al., 2007). Finally, the cross-sectional data presented here is useful for understanding neurocognitive residual abnormalities after remission, but more prospective longitudinal designs are necessary for assessing whether emotion regulation abnormalities are representing premorbid vulnerability factors or are the result of earlier episodes, and for identifying neurocognitive relapse predictors (Brouwer et al., 2019).
4.5. Conclusions
We found several neural emotion regulation abnormalities in remitted depression. Even though recurrent MDD patients after remission seem not so much characterized by problems with explicit, instructed regulation of negative emotions, they may engage less in spontaneous adequate regulation when not instructed, in the MR scanner and in daily life. RrMDD patients tend to apply less implicit regulation during negative emotions and engage less with positive emotions, which manifests in dysfunctional use of emotion regulation strategies in daily life and in lower affect.
Furthermore, the present findings highlight the importance of studying the regulation of positive emotions in addition to negative emotions. The insula, mostly studied in the context of negative emotion processing, may play a key role in abnormal regulation of positive emotions in rrMDD patients. Furthermore, the ability to savour positive affect seems highly relevant for emotion regulation success, a finding with important clinical implications. Finally, early processing biases do not seem relevant for either characterizing rrMDD, nor for understanding emotion regulation abnormalities underlying relapse vulnerability.
Clinically, these results demonstrate the importance of addressing residual abnormalities in affect and the regulation of negative, but especially positive emotions after remission, and may guide further preventive treatment improvement. Future research into neurocognitive changes in emotion regulation after effective preventive intervention and into the predictive value of emotional and self-referential processing for relapse risk will further contribute to understanding neurocognitive relapse vulnerability.
Funding
The NEWPRIDE study is supported by a Dutch Research Council (NWO/ZonMW) grant (VENI grant number 016.156.077) and a Dutch Brain Foundation (Hersenstichting) grant (Fellowship grant number F2014(1)-21) in name of Marie-José van Tol. The funding sources had no involvement in conducting the research.
CRediT authorship contribution statement
Rozemarijn S. van Kleef: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization, Project administration. Jan-Bernard C. Marsman: Methodology, Software, Writing – review & editing, Supervision. Evelien van Valen: Resources, Writing – review & editing. Claudi L.H. Bockting: Resources, Writing – review & editing. André Aleman: Writing – review & editing, Supervision. Marie-José van Tol: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our gratitude towards the participants who took the time and effort to participate in the NEWPRIDE study. Furthermore, we would like to thank Remco Renken for his help with improving data quality and methodological advice, Anita Sibeijn-Kuiper and Judith Streurman for their assistance with fMRI scanning, Peter de Jong and Nienke Jonker for sharing their attentional bias task, and the research assistants and students for their contribution to the data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102988.
Contributor Information
Rozemarijn S. van Kleef, Email: r.s.van.kleef@umcg.nl.
Jan-Bernard C. Marsman, Email: j.b.c.marsman@umcg.nl.
Evelien van Valen, Email: e.vanvalen@umcutrecht.nl.
Claudi L.H. Bockting, Email: c.l.bockting@amsterdamumc.nl.
André Aleman, Email: a.aleman@umcg.nl.
Marie-José van Tol, Email: m.j.van.tol@umcg.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Aldao A., Nolen-Hoeksema S. Specificity of cognitive emotion regulation strategies: a transdiagnostic examination. Behav. Res. Ther. 2010;48:974–983. doi: 10.1016/j.brat.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Armstrong T., Olatunji B.O. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin. Psychol. Rev. 2012;32:704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Simmons W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko G.M., Franconeri S.L., Ochsner K.N., Chiao J.Y. Attentional deployment is not necessary for successful emotion regulation via cognitive reappraisal or expressive suppression. Emotion. 2014;14:504–512. doi: 10.1037/a0035459. [DOI] [PubMed] [Google Scholar]
- Beck A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Berking M., Ebert D., Cuijpers P., Hofmann S.G. Emotion regulation skills training enhances the efficacy of inpatient cognitive behavioral therapy for Major Depressive Disorder: a randomized controlled trial. Psychother. Psychosom. 2013;82:234–245. doi: 10.1159/000348448. [DOI] [PubMed] [Google Scholar]
- Berking M., Wupperman P. Emotion regulation and mental health: recent findings, current challenges, and future directions. Curr. Opin. Psychiatry. 2012;25:128–134. doi: 10.1097/YCO.0b013e3283503669. [DOI] [PubMed] [Google Scholar]
- Berking M., Wirtz C.M., Svaldi J., Hofmann S.G. Emotion regulation predicts symptoms of depression over five years. Behav. Res. Ther. 2014;57:13–20. doi: 10.1016/j.brat.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Bocharov A.V., Knyazev G.G., Savostyanov A.N. Depression and implicit emotion processing: an EEG study. Neurophysiol. Clin. 2017;47:225–230. doi: 10.1016/j.neucli.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Bockting C.L.H., Schene A.H., Spinhoven P., Koeter M.W.J., Wouters L.F., Huyser J., et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. J. Consult. Clin. Psychol. 2005;73:647–657. doi: 10.1037/0022-006X.73.4.647. [DOI] [PubMed] [Google Scholar]
- Braunstein L.M., Gross J.J., Ochsner K.N. Explicit and implicit emotion regulation: a multi-level framework. Soc. Cogn. Affect. Neurosci. 2017;12:1545–1557. doi: 10.1093/scan/nsx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M.E., Williams A.D., Kennis M., Fu Z., Klein N.S., Cuijpers P., et al. Psychological theories of depressive relapse and recurrence: A systematic review and meta-analysis of prospective studies. Clin. Psychol. Rev. 2019;74 doi: 10.1016/j.cpr.2019.101773. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wage T.D., Lopez R., Onyemekwu C., Kober H., et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl J.R., Soskin D.P., Kerns C., Barlow D.H. Positive emotion regulation in emotional disorders: a theoretical review. Clin. Psychol. Rev. 2013;33:343–360. doi: 10.1016/j.cpr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Chapman A., Devue C., Grimshaw G.M. Fleeting reliability in the dot-probe task. Psychol. Res. 2019;83:308–320. doi: 10.1007/s00426-017-0947-6. [DOI] [PubMed] [Google Scholar]
- Chen G., Adleman N.E., Saad Z.S., Leibenluft E., Cox R.W. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Adleman N.E., Leibenluft E., Cox R.W. Detecting the subtle shape differences in hemodynamic responses at the group level. Front. Neurosci. 2015;9:1–18. doi: 10.3389/fnins.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cludius B., Mennin D., Ehring T. Emotion regulation as a transdiagnostic process. Emotion. 2020;20:37–42. doi: 10.1037/emo0000646. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Henry J.D. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L.B., Parvizi J., et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- de Jonge M., Dekker J.J.M., Kikkert M.J., Peen J., van Rijsbergen G.D., Bockting C.L.H. The role of affect in predicting depressive symptomatology in remitted recurrently depressed patients. J. Affect. Disord. 2017;210:66–71. doi: 10.1016/j.jad.2016.12.015. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Koster E.H.W. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cogn. Affect. Behav. Neurosci. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Demeyer I., De Lissnyder E., Koster E.H.W., De Raedt R. Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: a prospective study in remitted depressed adults. Behav. Res. Ther. 2012;50:292–297. doi: 10.1016/j.brat.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A.P., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Shumake J.D., Beevers C.G. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cogn. Emot. 2017;31:632–644. doi: 10.1080/02699931.2016.1146123. [DOI] [PubMed] [Google Scholar]
- Drabant E.M., McRae K., Manuck S.B., Hariri A.R., Gross J.J. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol. Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T., Fischer S., Schnülle J., Bösterling A., Tuschen-Caffier B. Characteristics of emotion regulation in recovered depressed versus never depressed individuals. Pers. Individ. Dif. 2008;44:1574–1584. [Google Scholar]
- Ehring T., Tuschen-Caffier B., Schnülle J., Fischer S., Gross J.J. Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Elgersma H.J., Koster E.H.W., Van Tuijl L.A., Hoekzema A., Penninx B.W.J.H., Bockting C.L.H., et al. Attentional bias for negative, positive, and threat words in current and remitted depression. PLoS ONE. 2018;13:1–23. doi: 10.1371/journal.pone.0205154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma H.J., Koster E.H.W., Vugteveen J., Hoekzema A., Penninx B.W.J.H., Bockting C.L.H., et al. Predictive value of attentional bias for the recurrence of depression: A 4-year prospective study in remitted depressed individuals. Behav. Res. Ther. 2019;114:25–34. doi: 10.1016/j.brat.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Everaert J., Koster E.H.W., Derakshan N. The combined cognitive bias hypothesis in depression. Clin. Psychol. Rev. 2012;32:413–424. doi: 10.1016/j.cpr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Everaert J., Grahek I., Duyck W., Buelens J., Van den Bergh N., Koster E.H.W. Mapping the interplay among cognitive biases, emotion regulation, and depressive symptoms. Cogn. Emot. 2017;31:726–735. doi: 10.1080/02699931.2016.1144561. [DOI] [PubMed] [Google Scholar]
- Feldman G.C., Joormann J., Johnson S.L. Responses to positive affect: A self-report measure of rumination and dampening. Cognit. Ther. Res. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J.L., et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa C.A., Mocking R.J.T., van Wingen G., Martens S., Ruhé H.G., Schene A.H. Aberrant default-mode network-hippocampus connectivity after sad memory-recall in remitted-depression. Soc. Cogn. Affect. Neurosci. 2017;12:1803–1813. doi: 10.1093/scan/nsx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa C.A., DeJong H., Mocking R.J.T., Fox E., Rive M.M., Schene A.H., et al. Attentional control, rumination and recurrence of depression. J. Affect. Disord. 2019;256:364–372. doi: 10.1016/j.jad.2019.05.072. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J.M., Macnamara A., Kennedy A.E., Rabinak C.A., Rauch S.A.M., Liberzon I., et al. Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD. Depress Anxiety. 2018;34:79–88. doi: 10.1002/da.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., Schaeffer D.J., Ball B.H., Schwarz N.F., et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Frodl T., Scheuerecker J., Albrecht J., Kleemann A.M., Müller-Schunk S., Koutsouleris N., et al. Neuronal correlates of emotional processing in patients with major depression. World J. Biol. Psychiatry. 2009;10:202–208. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Williams S.C.R., Cleare A.J., Brammer M.J., Walsh N.D., Kim J., et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Williams S.C.R., Cleare A.J., Scott J., Mitterschiffthaler M.T., Walsh N.D., et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Pers. Individ. Dif. 2006;40:1659–1669. [Google Scholar]
- Godlewska B.R., Norbury R., Selvaraj S., Cowen P.J., Harmer C.J. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol. Med. 2012;42:2609–2617. doi: 10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N., McKie S., Thomas E.J., Downey D., Juhasz G., Williams S.R., et al. Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol. Psychiatry. 2012;72:604–611. doi: 10.1016/j.biopsych.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton B., MacLeod C. Enhanced probing of attentional bias: The independence of anxiety-linked selectivity in attentional engagement with and disengagement from negative information. Cogn. Emot. 2014;28:1287–1302. doi: 10.1080/02699931.2014.881326. [DOI] [PubMed] [Google Scholar]
- Grafton B., Southworth F., Watkins E., MacLeod C. Stuck in a sad place: Biased attentional disengagement in rumination. Emotion. 2016;16:63–72. doi: 10.1037/emo0000103. [DOI] [PubMed] [Google Scholar]
- Gross J.J. The emerging field of emotion regulation: An integrative review. Rev. Gen. Psychol. 1998;2:271–299. [Google Scholar]
- Gross J.J. Emotion regulation: current status and future prospects. Psychol. Inq. 2015;26:1–26. [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gupta R., Kar B.R. Attention and memory biases as stable abnormalities among currently depressed and currently remitted individuals with unipolar depression. Front. Psychiatry. 2012;3:1–7. doi: 10.3389/fpsyt.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Han H., Glenn A.L., Dawson K.J. Evaluating alternative correction methods for multiple comparison in functional neuroimaging research. Brain Sci. 2019;9:198. doi: 10.3390/brainsci9080198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Shackman A.J., Light S.N., Peterson M.J., Kolden G.G., et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.A., Blackwell S.E., Burnett Heyes S., Renner F., Raes F. Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu. Rev. Clin. Psychol. 2016;12:249–280. doi: 10.1146/annurev-clinpsy-021815-092925. [DOI] [PubMed] [Google Scholar]
- Holtzheimer P.E., Mayberg H.S. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis C.A., Siegling A.B. Criterion and incremental validity of the emotion regulation questionnaire. Front. Psychol. 2015;6:247. doi: 10.3389/fpsyg.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J.L., Grafton B., MacLeod C. Referential focus moderates depression-linked attentional avoidance of positive information. Behav. Res. Ther. 2017;93:47–54. doi: 10.1016/j.brat.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker N.C., Glashouwer K.A., Ostafin B.D., de Jong P.J. Attentional engagement with and disengagement from food cues in Anorexia Nervosa. Behaviour Research and Therapy. 2019;114:15–24. doi: 10.1016/j.brat.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Joormann J., Gotlib I.H. Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J., Michael V.W. Emotion regulation in depression: The role of biased cognition and reduced cognitive control. Clin. Psychol. Sci. 2014;2:402–421. [Google Scholar]
- Joormann J., Dkane M., Gotlib I.H. Adaptive and maladaptive components of rumination? diagnostic specificity and relation to depressive biases. Behav. Ther. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Joormann J., Siemer M., Gotlib I.H. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J. Abnorm. Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Snyder H.R., Goer F., Clegg R., Ironside M., Pizzagalli D.A. Attention bias in rumination and depression: cognitive mechanisms and brain networks. Clin. Psychol. Sci. 2018;6:765–782. doi: 10.1177/2167702618797935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Keller A.S., Leikauf J.E., Holt-Gosselin B., Staveland B.R., Williams L.M. Paying attention to attention in depression. Transl. Psychiatry. 2019;9:1–12. doi: 10.1038/s41398-019-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing L.V., Hansen M.G., Andersen P.K., Angst J. The predictive effect of episodes on the risk of recurrence in depressive and bipolar disorders – a life-long perspective. Acta Psychiatr. Scand. 2004;109:339–344. doi: 10.1046/j.1600-0447.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation – An ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster E.H.W., De Raedt R., Goeleven E., Franck E., Crombez G. Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion. 2005;5:446–455. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Langers D.R.M., Krumbholz K., Bowtell R.W., Hall D.A. Neuroimaging paradigms for tonotopic mapping (I): The influence of sound stimulus type. Neuroimage. 2014;100:650–662. doi: 10.1016/j.neuroimage.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M. Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Liu D.Y., Thompson R.J. Selection and implementation of emotion regulation strategies in major depressive disorder: an integrative review. Clin. Psychol. Rev. 2017;57:183–194. doi: 10.1016/j.cpr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H.W., Sonuga-Barke E.J., De Raedt R. The Default Mode Network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol. Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Everaert J., Dainer-Best J., Loeys T., Beevers C.G., Koster E.H.W. Specificity and overlap of attention and memory biases in depression. J. Affect. Disord. 2018;225:404–412. doi: 10.1016/j.jad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Melka S.E., Lancaster S.L., Bryant A.R., Rodriquez B.F. Confirmatory factor and measurement invariance analyses of the emotion regulation questionnaire. J. Clin. Psychol. 2011;67:1283–1293. doi: 10.1002/jclp.20836. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moll J., Zahn R., De O.-S., Krueger F. The neural basis of human moral cognition. Nat. Rev. Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Moodie C.A., Suri G., Goerlitz D.S., Mateen M.A., Sheppes G., McRae K., et al. The neural bases of cognitive emotion regulation: The roles of strategy and intensity. Cogn. Affect. Behav. Neurosci. 2020;387–407 doi: 10.3758/s13415-020-00775-8. [DOI] [PubMed] [Google Scholar]
- Nelis S., Holmes E.A., Raes F. Response styles to positive affect and depression: concurrent and prospective associations in a community sample. Cognit. Ther. Res. 2015;39:480–491. doi: 10.1007/s10608-015-9671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. Rethinking rumination. Perspect. Psychol. Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005 doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D.E., et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Oehlberg K.A., Revelle W., Mineka S. Time-course of attention to negative stimuli: negative affectivity, anxiety, or dysphoria? Emotion. 2012;12:943–959. doi: 10.1037/a0027227. [DOI] [PubMed] [Google Scholar]
- Owens M., Gibb B.E. Brooding rumination and attentional biases in currently non-depressed individuals: an eye-tracking study. Cogn. Emot. 2017;31:1062–1069. doi: 10.1080/02699931.2016.1187116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Naselaris T., Holmes E.A., Kosslyn S.M. Mental imagery: functional mechanisms and clinical applications. Trends Cogn. Sci. 2015;19:590–602. doi: 10.1016/j.tics.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham A.D., McHugh R.K., Otto M.W. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó-Pérez M., Radua J., Steward T., Menchón J.M., Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:96–104. doi: 10.1016/j.pnpbp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Radkovsky A., McArdle J.J., Bockting C.L.H., Berking M. Successful emotion regulation skills application predicts subsequent reduction of symptom severity during treatment of major depressive disorder. J. Consult. Clin. Psychol. 2014;82:248–262. doi: 10.1037/a0035828. [DOI] [PubMed] [Google Scholar]
- Raes F., Hermans D., Williams J.M.G., Bijttebier P., Eelen P. A ‘triple W’-model of rumination on sadness: why am I feeling sad, what’s the meaning of my sadness, and wish I could stop thinking about my sadness (but I can’t!) Cognit. Ther. Res. 2008;32:526–541. [Google Scholar]
- Raes F., Daems K., Feldman G., Johnson S., Van Gucht D. A psychometric evaluation of the Dutch version of the Responses to Positive Affect Questionnaire. Psychol. Belg. 2009;49:293–310. [Google Scholar]
- Reinecke A., Filippini N., Berna C., Western D.G., Hanson B., Cooper M.J., et al. Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: implications for psychological treatment action. Transl. Psychiatry. 2015;5:e673–10. doi: 10.1038/tp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. Prevalence and clinical course of depression: a review. Clin. Psychol. Rev. 2011;31:1117–1125. doi: 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Rive M.M., Van Rooijen G., Veltman D.J., Mary M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rive M.M., Mocking R.J.T., Koeter M.W.J., van Wingen G., de Wit S.J., van den Heuvel O.A., et al. State-dependent differences in emotion regulation between unmedicated Bipolar Disorder and Major Depressive Disorder. JAMA Psychiatry. 2015;72:687–696. doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- Rive M.M., Mocking R.J.T., Koeter M.W.J., van Wingen G., de Wit S.J., van den Heuvel O.A., et al. State-dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- Ruhe H.G., Mocking R.J.T., Figueroa C.A., Seeverens P.W.J., Ikani N., Tyborowska A., et al. Emotional biases and recurrence in major depressive disorder. Results of 2.5 years follow-up of drug-free cohort vulnerable for recurrence. Front. Psychiatry. 2019;10:1–18. doi: 10.3389/fpsyt.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.J., Gullion C.M., Basco M.R., Jarrett R.B., Trivedi M.H. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol. Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez A., Koster E.H.W., Van Put J., De Raedt R. Attentional disengagement from emotional information predicts future depression via changes in ruminative brooding: A five-month longitudinal eye-tracking study. Behav. Res. Ther. 2019;118:30–42. doi: 10.1016/j.brat.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottnik L., Linden D.E.J. Mental imagery and brain regulation—new links between psychotherapy and neuroscience. Front. Psychiatry. 2019;10:1–14. doi: 10.3389/fpsyt.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliz D., Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging (fMRI) research. Front. Hum. Neurosci. 2012;6:1–14. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E., Hall K., Moulding R., Bryce S., Mildred H., Staiger P.K. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin. Psychol. Rev. 2017;57:141–163. doi: 10.1016/j.cpr.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Keng S.-L., Ji J.L., Moore T., Minkel J., Dichter G.S. Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Soc. Cogn. Affect. Neurosci. 2015;10:1187–1194. doi: 10.1093/scan/nsv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski M.J., Keng S.L., Ji J.L., Moore T., Minkel J., Dichter G.S. Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Soc. Cogn. Affect. Neurosci. 2015;10:1187–1194. doi: 10.1093/scan/nsv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southworth F., Grafton B., MacLeod C., Watkins E. Heightened ruminative disposition is associated with impaired attentional disengagement from negative relative to positive information: support for the “impaired disengagement” hypothesis. Cognition and Emotion. 2017;31(3):422–434. doi: 10.1080/02699931.2015.1124843. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stenger V.A. In: The orbitofrontal cortex. Zald D.H., Rauch S.L., editors. Oxford University Press; New York, NY, US: 2006. Technical considerations for BOLD fMRI of the orbitofrontal cortex; pp. 423–446. [Google Scholar]
- Teasdale J.D., Dent J. Cognitive vulnerability to depression: An investigation of two hypotheses. Br. J. Clin. Psychol. 1987;26:113–126. doi: 10.1111/j.2044-8260.1987.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thirion B., Pinel P., Mériaux S., Roche A., Dehaene S., Poline J.B. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Trivedi M.H., Rush A.J., Ibrahim H.M., Carmody T.J., Biggs M.M., Suppes T., et al. The Inventory of Depressive Symptomatology, Clinical Rating (IDS-C) and Self-Report (IDS_SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psycho. Psychol. Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Tugade M.M., Fredrickson B.L. Regulation of positive emotions: emotion regulation strategies that promote resilience. J. Happiness Stud. 2007;8:311–333. [Google Scholar]
- Uddin L.Q., Nomi J.S., Hebert-Seropian B., Ghaziri J., Boucher O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017;34:300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Davidson R.J. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kleef R.S., Bockting C.L.H., Van Valen E., Aleman A., Marsman J.B.C., Van Tol M.J. Neurocognitive working mechanisms of the prevention of relapse in remitted recurrent depression (NEWPRIDE): Protocol of a randomized controlled neuroimaging trial of preventive cognitive therapy. BMC Psychiatry. 2019;19:409. doi: 10.1186/s12888-019-2384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T., Furey M., Fromm S. Relationship of emotional processing to masked faces in the amygdala to mood state and treatment in major depressive disorder. Arch. Gen. Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visted E., Vøllestad J., Nielsen M.B., Schanche E. Emotion regulation in current and remitted depression: A systematic review and meta-analysis. Front. Psychol. 2018;9:1–20. doi: 10.3389/fpsyg.2018.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Webb T.L., Totterdell P., Ibar D.N.H. Foundations and extensions for the extended model: more on implicit and explicit forms of emotion regulation. Psychol. Inq. 2015;26:123–129. [Google Scholar]
- Winer E.S., Salem T. Reward devaluation: dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychol. Bull. 2016;142:1–61. doi: 10.1037/bul0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ai H., Opmeer E.M., Marsman J.B.C., Van Der Meer L., Ruhé H.G., et al. Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychol. Med. 2019;50:413–421. doi: 10.1017/S0033291719000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A., Parvaz M.A., Goldstein R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvielli A., Vrijsen J.N., Koster E.H.W., Bernstein A. Attentional bias temporal dynamics in remitted depression. J. Abnorm. Psychol. 2016;125:768–776. doi: 10.1037/abn0000190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.