Abstract
Formation of specialized pro-resolving lipid mediators (SPMs) such as lipoxins or resolvins usually involves arachidonic acid 5-lipoxygenase (5-LO, ALOX5) and different types of arachidonic acid 12- and 15-lipoxygenating paralogues (15-LO1, ALOX15; 15-LO2, ALOX15B; 12-LO, ALOX12). Typically, SPMs are thought to be formed via consecutive steps of oxidation of polyenoic fatty acids such as arachidonic acid, eicosapentaenoic acid or docosahexaenoic acid. One hallmark of SPM formation is that reported levels of these lipid mediators are much lower than typical pro-inflammatory mediators including the monohydroxylated fatty acid derivatives (e.g., 5-HETE), leukotrienes or certain cyclooxygenase-derived prostaglandins. Thus, reliable detection and quantification of these metabolites is challenging. This paper is aimed at critically evaluating i) the proposed biosynthetic pathways of SPM formation, ii) the current knowledge on SPM receptors and their signaling cascades and iii) the analytical methods used to quantify these pro-resolving mediators in the context of their instability and their low concentrations. Based on current literature it can be concluded that i) there is at most, a low biosynthetic capacity for SPMs in human leukocytes. ii) The identity and the signaling of the proposed G-protein-coupled SPM receptors have not been supported by studies in knock-out mice and remain to be validated. iii) In humans, SPM levels were neither related to dietary supplementation with their ω-3 polyunsaturated fatty acid precursors nor were they formed during the resolution phase of an evoked inflammatory response. iv) The reported low SPM levels cannot be reliably quantified by means of the most commonly reported methodology. Overall, these questions regarding formation, signaling and occurrence of SPMs challenge their role as endogenous mediators of the resolution of inflammation.
Keywords: lipoxygenase, SPM, lipoxin, resolvin, resolution of inflammation, leukotriene, FPR, LC-MS-based lipid mediator analysis
In Vitro Formation of Lipoxins and Resolvins
In contrast to originally identified oxylipins such as leukotrienes that are conventionally implicated in inflammatory responses, specialized pro-resolving mediators (SPM) represent an alternative group that have been suggested to be important for inhibition of inflammation and promotion of its resolution.
SPMs such as lipoxins and resolvins were reported to foster resolution of inflammation. For example, resolvin D2 (RvD2) was reported to be a potent regulator of leukocyte function and to control microbial sepsis at very low concentrations in mice (Spite et al., 2009). Resolvin E2 (RvE2) was shown to inhibit zymosan-induced leukocyte infiltration and peritoneal inflammation (Tjonahen et al., 2006). Later on, a plethora of biological and pharmacological activities of SPMs were reported such as the limitation of PMN tissue infiltration, enhancement of macrophage phagocytosis and efferocytosis, increased microbial killing and enhanced tissue regeneration [for review see (Serhan, 2017b; Serhan and Levy, 2018)]. The relevance of these findings to the physiological and pathophysiological roles of endogenous SPMs depends on their in vivo concentrations and the accuracy with which they can be measured in body fluids.
The biosynthetic pathways for lipoxins and resolvins have been suggested to involve at least two consecutive oxygenase reactions. Formation of lipoxins can occur via the oxygenation of arachidonic acid (AA) by 5-lipoxygenase (5-LO, ALOX5) to LTA4 followed by further conversion of this intermediate by murine 12/15-LO (Alox15), human 15-LOs (ALOX15, ALOX15B) or 12-LO (ALOX12) to LXA4 and LXB4, further referred to as the 5-LO:12/15-LO pathway or via the consecutive action of 12/15-LO and 5-LO which is referred to as the 12/15-LO:5-LO pathway (Figure 1). Both SPM biosynthesis pathways can involve transcellular cooperation in which the first oxygenation product is transferred to a neighboring cell type for the second oxygenation. This will be discussed below in more detail. It should also be mentioned here that the mouse orthologue (12/15-LO, Alox15) of the human 15-LO (ALOX15) generates a different product pattern (see The 12/15-LO:5-LO Pathway of Lipoxin, D-Series Resolvin and RvE4 Formation and 5-LO-Independent Lipoxin Formation).
FIGURE 1.
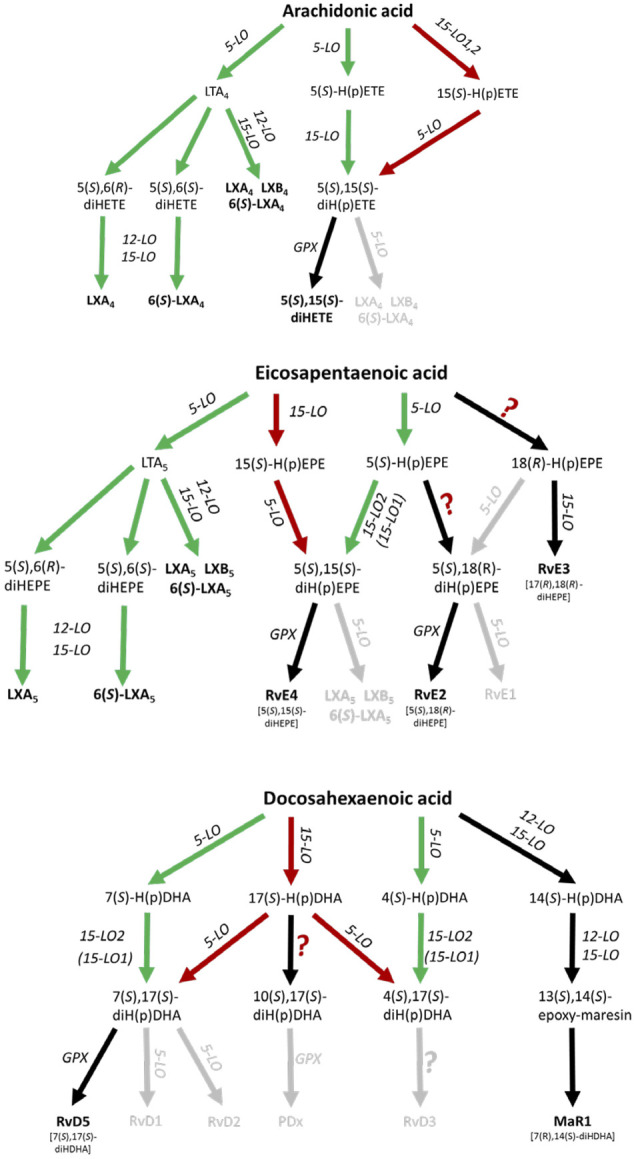
Scheme of human leukocyte-dependent SPM formation from AA, EPA and DHA where SPMs with significant formation are highlighted. The 5-LO:12/15-LO pathway is shown in green and the 12/15-LO:5-LO pathway is depicted in red. Inefficient SPM biosynthesis routes are grey colored. GPX, glutathione peroxidase.
In view of the findings reviewed here, there are significant differences in the biosynthetic capacities for SPM formation. Recent data are compatible with the hypothesis that trihydroxylated SPMs are formed at much lower rates than dihydroxylated SPMs such as resolvin D5, D6, E2 and E4.
The 5-LO:12/15-LO Pathway of SPM Formation
Here, 5-LO first generates 5-HpETE, 5-HpEPE and 7-HpDHA from AA, EPA and DHA, respectively, and these metabolites are further converted by AA 15-lipoxygenating enzymes (Figure 1). Indeed, it has been shown that isolated 15-LO2 (ALOX15B) equally accepts 5-HETE and 5-HpETE for the formation of 5,15-diHETE (Green et al., 2016). Furthermore, the enzyme also accepts 7-HDHA and 7-HpDHA for conversion to RvD5 (7,17-diHDHA). Human 15-LO1 (ALOX15) also accepts 7-H(p)DHA as substrate but 7,14-diH(p)DHA was identified as major (90%) reaction product (Perry et al., 2020a). These data are compatible with RvD5 formation via the 5-LO:15-LO pathway being 5-LO:15-LO2 restricted.
Efficient transcellular lipoxin formation was detected in platelet/leukocyte co-incubations stimulated with Ca2+ ionophore or a combination of fMLF and thrombin. 5-LO-derived LTA4 is released from granulocytes and then further converted by platelet 12-LO (ALOX12) to lipoxins (Serhan and Sheppard, 1990; Edenius et al., 1991; Lehmann et al., 2015) (Figure 1). Furthermore, it was shown that LTA4 can serve as a substrate for various ALOX15 orthologues (Tornhamre et al., 2000). Thus, in principle, 12-LO and 15-LO are functionally redundant in the biosynthetic cascade.
In contrast to granulocyte (PMNL)/platelet co-incubations, co-expression of 5-LO together with 15-LO1 or 15-LO2 in the same cell type did not result in efficient lipoxin formation via the 5-LO:12/15-LO pathway. Human macrophages differentiated in the presence of IL-4 co-express ALOX5 and ALOX15 (Wuest et al., 2012; Ebert et al., 2020; Von Hegedus et al., 2020) and should be able to form lipoxins on their own. However, release of lipoxins upon stimulation with Ca2+ ionophore in presence of exogenous AA was barely detectable in these cells (Ebert et al., 2020). At present, the reason for the lack of SPM formation under these experimental conditions is unknown.
Freshly isolated PMNL suspensions contain eosinophils which are known to co-express ALOX5 and ALOX15 (Nadel et al., 1991; Archambault et al., 2018) yet produce only minute amounts of lipoxins, compared to other lipoxygenase metabolites, even when stimulated with Ca2+ ionophore (Serhan et al., 1987; Edenius et al., 1991; Mainka et al., 2021). It is not known whether this is due to the fact that PMNL are mainly composed of neutrophils which mainly express ALOX5, while ALOX15 expression is more or less restricted to eosinophils which represent only a minor fraction of the PMNLs. Furthermore, ALOX15B expression was recently demonstrated in neutrophils (Archambault et al., 2018) whereas another study failed to detect this enzyme in granulocytes (Mainka et al., 2021).
Of note, leukocyte preparations are usually contaminated with platelets since these cells tend to adhere to leukocytes during isolation. Thus, contamination with platelet-derived 12-LO cannot be avoided. Therefore, the small amounts of lipoxins occasionally observed in PMNL and monocyte/macrophage preparations might in part be related to platelet contamination.
The 12/15-LO:5-LO Pathway of Lipoxin, D-Series Resolvin and RvE4 Formation
As an alternative to the 5-LO:12/15-LO pathway, 5-LO together with 15-LO1 (ALOX15) or 15-LO2 (ALOX15B) may produce lipoxins, D-series resolvins and RvE4 from their corresponding PUFAs (Figure 1). In a first step AA, eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) can be oxidized to 15-H(p)ETE, 15-H(p)EPE and 17-H(p)DHA, respectively by an AA 15-lipoxygenating enzyme. 15-H(p)ETE on one hand and 15-H(p)EPE on the other are then further converted by 5-LO to 5,15-diHETE, LXA4, LXB4 and RvE4 (5,15-diHEPE), LXA5 and LXB5. The formation of SPMs from DHA is more complex and 17-H(p)DHA is reported to be converted to RvD1-6 (Chiang and Serhan, 2017; Serhan and Levy, 2018). With DHA, human 15-LO1 exhibits a pronounced dual specificity since DHA is oxygenated to a 1:1 mixture of 17-HDHA and 14-HDHA (Kutzner et al., 2017) (Figure 1). In contrast, mouse Alox15 forms 100% 14-HDHA (Kutzner et al., 2017). These differences in the reaction specificity between mouse and human ALOX15 orthologues may impact the SPM synthase activities of the two ALOX15 orthologues from different substrates, although this is not known.
A hallmark of the proposed 12/15-LO:5-LO SPM formation route is that the substrate fatty acids (AA, EPA, DHA) are first oxygenated by 15-LO1/15-LO2 or other oxygenases to the corresponding monohydro(pero)xy-fatty acids (15-H(p)ETE, 15-H(p)EPE, 17-H(p)DHA) and subsequently by 5-LO (Figure 1). It should be stressed at this point that the substrates for the second (5-LO-catalyzed) oxygenation reaction (15-H(p)ETE, 15-H(p)EPE, 17-H(p)DHA) may also originate from fatty acid auto-oxidation. If one follows the proposed mechanistic scenario of the 12/15-LO:5-LO pathway there are two major prerequisites for SPM formation:
i) Involvement of leukocytes. ALOX5 expression is largely restricted to these immune cells, as red blood cells and platelets are 5-LO deficient (Rådmark et al., 2007).
ii) 5-LO must accept 15-H(p)ETE, 15-H(p)EPE and/or 17-H(p)DHA as substrates for SPM formation. Interestingly, as will be discussed in Lipoxin and Resolvin Formation in Human PMNL and Lipoxin and Resolvin Formation in Human Macrophages, the conversion rate of most of these oxylipins is dependent on FLAP, which is normally present in 5-LO-containing cells. In the absence of FLAP, the conversion rate of these oxylipins by purified human 5-LO is very low compared to AA or EPA. In fact, the conversion rate of 15-HpETE to 5,15-diHpETE by human 5-LO was found to be 10-fold lower compared to the conversion of AA to 5-HpETE. Moreover, no conversion of 5,15-diHpETE to LXA4 was observed (Green et al., 2018). In line with this finding, 15-HETE is hardly converted to LXA4 isomers by purified human 5-LO (Lehmann et al., 2015; Mainka et al., 2021). In fact, compared with AA, conversion of 15-HETE and 15-HpETE by 5-LO was found to be 130- and 85-fold slower, respectively (Perry et al., 2020a). Similarly, the oxygenation rates of 17-HDHA and 17-HpDHA by human 5-LO are more than 100-fold lower when compared with the parent fatty acid DHA (Perry et al., 2020b). In accordance, 17-HDHA was not accepted as substrate by recombinant human 5-LO and 17-HDHA was not converted to resolvins such as RvD5 (Mainka et al., 2021). These data clearly show that in the tested in vitro systems in the absence of FLAP, oxylipins are poor substrates for purified human 5-LO. Furthermore, there seem to be no significant differences between the turnover of the hydroxy fatty acids compared to their corresponding hydroperoxy derivatives (Mainka et al., 2021).
Formation of EPA-Derived 18-HEPE and of the Resolvin E-Series
In a recent publication, the patterns of oxygenation products of EPA and 18-HEPE were analyzed with particular emphasis on the formation of double and triple oxygenation products (Kutzner et al., 2020) (Table 1). It was found that recombinant 5-LO as well as a combination of 5-LO with 15-LO1 oxygenated these substrates to a complex mixture of mono-, double- and triple oxygenation products. Recombinant 5-LO and ALOX15 paralogues formed different double oxygenation products (Table 1) including 5,15-diHEPE and various 8,15-diHEPE isomers (Figure 1). Similar product patterns were identified when the two enzymes were simultaneously added to the assay system. Of note, formation of triple oxygenation products, i.e., RvE1 (Table 1), which has an 18R hydroxyl group was below the detection limits of the employed LC-MS method (see Quantitative Analysis and SPM Levels in Humans). When 18(R,S)-HEPE was used as substrate, a number of dihydroxy derivatives including 5,18-diHEPE (RvE2) and 17,18-diHEPE (RvE3) but also small amounts of trihydroxy compounds such as 5,17,18-triHEPE were detected (Kutzner et al., 2020). Thus, it has to be considered whether 18-HEPE is likely to be present as a potential substrate for this in vivo. While 18R-HEPE is a precursor in the biosynthesis of different resolvins (RvE1, RvE2, RvE3) (Figure 1), the origin of 18R-HEPE in biological systems remains speculative. LO isoforms have been suggested as a metabolic source but considering the reaction specificity of mammalian LO-isoforms, effective formation of 18R-HEPE from EPA is not very likely. When EPA was incubated with recombinant human 5-LO, 12-LO, 15-LO2 and 15-LO1, very small amounts of 18-HEPE were formed in the 15-LO1 incubations and its S/R-ratio was less than 3:1. However, this compound which was hardly detected in the incubation mixtures of the other LO-isoforms, only contributed 1% to the mixture of all oxygenation products (Kutzner et al., 2020). These data indicate that 18-HEPE formation from EPA via the 15-LO pathway is very inefficient and that the stereochemistry of 18-HEPE oxygenation is not tightly controlled by the enzyme. The molecular basis for the low degree of enantio-control has not been explored in detail but it might be speculated that hydrogen abstraction from C(16) is catalyzed by the enzymes whereas oxygen insertion at C(18) proceeds in part non-enzymatically.
TABLE 1.
Di- and trihydroxylated oxylipins derived from AA, EPA and DHA.
| Hydroxylation | Trivial name | Hydroxylation pattern | Proposed pathway of formation |
|---|---|---|---|
| dihydroxy derivatives | 5,15-diHETE | 5,15-diHETE a | 12/15-LO:5-LO, 5-LO:12/15-LO |
| 8,15-diHETE | 8,15-diHETE | 12/15-LO | |
| resolvin D5 | 7,17-diHDHAb | 12/15-LO:5-LO | |
| resolvin D6 | 4,17-diHDHA | 12/15-LO:5-LO | |
| resolvin E2 | 5,18-diHEPE c | CYP or (12/15LO):5-LO | |
| resolvin E3 | 17,18-diHEPE | CYP, (12/15-LO) | |
| resolvin E4 | 5,15-diHEPE | 12/15-LO:5-LO, 5-LO:12/15-LO | |
| trihydroxy derivatives | lipoxin A4 | 5,6,15-triHETE | 5-LO:12/15-LO |
| lipoxin B4 | 5,14,15-triHETE | 5-LO:12/15-LO | |
| resolvin D1 | 7,8,17-triHDHA | 12/15-LO:5-LO | |
| resolvin D2 | 7,16,17-triHDHA | 12/15-LO:5-LO | |
| resolvin D3 | 4,11,17-triHDHA | 12/15-LO:5-LO | |
| resolvin D4 | 4,5,17-triHDHA | 12/15-LO:5-LO | |
| resolvin E1 | 5,12,18-triHEPE | CYP or (12/15-LO):5-LO |
Hydroxyeicosatetraenoic acid.
Hydroxydocosahexaenoic acid.
Hydroxyeicosapentaenoic acid.
Alternatively, 18R-HEPE might arise from oxygenation of EPA by CYP450 enzymes (Isobe et al., 2018), but there is no evidence for the physiological relevance of this biosynthetic pathway so far. In this respect, it is of interest that CYP4F18 oxidizes LTB4 to 18-OH-LTB4 (Christmas et al., 2006) which is a structural analogue to the EPA-derived RvE1 (chemically a 18-OH-LTB5). However, it is not known whether LTB5 is also a CYP4F18 substrate.
Formation of 15R-HETE from AA by COX2 in stimulated endothelial cells has been reported after COX2 acetylation by aspirin, leading to the so called aspirin-triggered (AT) lipoxins which represent C(15) epimers (Claria and Serhan, 1995). Later it was shown that aspirin treatment of endothelial cells can generate 18R-HEPE from EPA (Serhan et al., 2000). However, in a subsequent publication, it was reported that aspirin treatment of human recombinant COX2 fosters the formation of 18S-HEPE but not the 18R configuration (Oh et al., 2011). In agreement with this finding, slightly enhanced 18S-HEPE formation was found in serum from aspirin-treated patients (Oh et al., 2011). At present, it is unclear whether 18R-HETE is formed in vivo to serve as precursor for SPM formation. Thus, we doubt that aspirin-triggered 18-HEPE formation significantly contributes to the resolvin E formation. All of these observations depend on aspirin treatment in vitro. Their relevance to the pharmacological actions of aspirin in vivo remain entirely speculative.
5-LO-Independent Lipoxin Formation
It has been reported 40 years ago that soybean LOX1 is capable of oxygenating its own AA oxygenation product (15-HpETE) to a mixture of several dihydroxy eicosanoids and the major products of this AA double oxygenation were identified as 8S,15S-diHpETE and 5S,15S-diHpETE (Van Os et al., 1981). The reaction rate of the second oxygenation step (conversion of 15S-HpETE to the mixture of 8S,15S-diHpETE and 5S,15S-diHpETE) was about 5-fold lower than the rate of AA oxygenation (Van Os et al., 1981). As a mechanistic reason for C(5) oxygenation of arachidonic acid derivatives, an inverse head-to-tail orientation of the fatty acid substrate at the active site of the enzyme has been suggested. Free polyenoic fatty acids penetrate the active site of AA 15-lipoxygenating ALOX-isoforms leading with their methyl end and this allows optimal alignment of the substrate for C(15) lipoxygenation. In 15-H(p)ETE, the presence of the hydro(pero)xy group in close proximity to the methyl end of the fatty acid substrate reduces the hydrophobicity of this part of the substrate molecule and thus, the substrate might be inversely aligned at the active site. This inverse substrate alignment favors oxygenation at C(5) and C(8). Similar experiments were carried out with pure rabbit 15-LO. When this enzyme was incubated with AA at 37°C significant amounts of 8,15-diHpETE, 5,15-diHpETE as well as 14,15-LTA4 were detected as reaction products (Bryant et al., 1985). These data are compatible with AA 15-lipoxygenating LO isoforms capable of catalyzing the oxygenation of other carbon atoms of polyenoic fatty acids when oxygenation of the preferred carbon atom is blocked. 5S,15S-diH(p)ETE may be considered as an intermediate in the formation of lipoxin isomers (5,6,15-triHETE is LXA4 and 5,14,15-triHETE is LXB4). In fact, when pure rabbit ALOX15 was incubated with 15-HETE as substrate, significant amounts of LXB4 (5,14,15-triHETE) were formed (Kühn et al., 1984). The structure of this compound was characterized comprehensively (Kühn et al., 1987) and experiments with heavy oxygen isotopes indicated that the oxygen introduced at carbons 5 and 14 originated from atmospheric 18O2. These data indicate that LXB4 can be formed via 5-LO-independent pathways and that the substrate (15-HETE derivatives) is metabolized via two consecutive steps of 15-LO catalyzed oxygenation. It should, however, be emphasized that in principle, the formation of 5,14,15-triHETE (LXB4) may also proceed via an alternative mechanism. For instance, the 14,15-LTA4 synthase activity of rabbit 15-LO (Bryant et al., 1985) may lead to the conversion of 15-HpETE to 14,15-LTA4. This metabolite quickly undergoes non-enzymatic hydrolysis yielding various 14,15-diHETE isomers. When these isomers undergo 5-lipoxygenation either by 5-LO or by the 5-lipoxygenating activity of 15-LO, 5,14,15-triHETE is formed.
The biosynthetic capacity of human and mouse ALOX-isoforms for lipoxin isomer formation depends on the reaction specificity of these enzymes with arachidonic acid. Human 15-LO1 is an AA 15-lipoxygenating enzyme (Sigal et al., 1990) while its mouse orthologue is dominantly AA 12-lipoxygenating (Sun and Funk, 1996). It may therefore not be possible to conclude the efficiency of lipoxin synthase activity of mouse 12/15-LO (Alox15) from the activity of the human orthologue. In other words, a biosynthetic scheme worked out for humans might not adequately mirror the situation in mice. A similar situation exists for mouse and human ALOX15B. Here, the human enzyme is AA 15-lipoxygenating (Jisaka et al., 2000) whereas mouse Alox15B converts AA mainly to 8-HpETE (Krieg et al., 1998).
Taken together, most of these studies on lipoxin biosynthesis were performed with purified enzymes and under incubation conditions which do not necessarily reflect the cellular environment. Considering the data from intact cell incubations and the FLAP dependency of lipoxin formation, it is rather unlikely that 5-LO-independent pathways are a plausible route to SPM formation in-vivo.
Lipoxin and Resolvin Formation in Human PMNL
Formation of lipoxins by 5-LO was first reported in human leukocytes (Serhan et al., 1984b; a). Although the oxygenation of 15-HETE to 5,15-diHETE by purified human 5-LO is two orders of magnitude less effective than AA oxygenation (Perry et al., 2020a), the enzyme accepts 15-HETE as substrate in a cellular setup where 5-LO interacts with FLAP which transfers the fatty acid substrates to 5-LO (Dixon et al., 1990). In fact, in intact PMNL, 5-LO oxygenates 12- and 15-HETE in a FLAP-dependent manner (Hill et al., 1992; Mancini et al., 1998) (Figure 1). Subsequently, it was found that FLAP is also required for the biosynthesis of LXA4 and RvD1 from 15-HETE and 17-HDHA, respectively (Lehmann et al., 2015). Similarly, formation of 5,15-diHETE, RvD1, RvD5 and RvE1 is FLAP-dependent since their formation can be efficiently inhibited by the FLAP inhibitor MK-886 (Mainka et al., 2021). This infers that the investigation of SPM formation with purified enzymes might not adequately mirror the biosynthetic capacity of cellular systems where additional protein-protein and protein-lipid interactions occur. Further investigation of SPM formation by 5-LO was performed in ionophore-stimulated PMNL from AA, DHA as well as the SPM precursors 15-HETE, 17-HDHA and 18-HEPE (Figure 1). Due to the low cellular concentrations of 15-LO1, 15-LO2 and 12-LO in PMNL, no substantial amounts of AA- and DHA-derived SPMs were detected (Mainka et al., 2021). In contrast, human and mouse eosinophils which express ALOX15 biosynthesized docosanoids (14-HDHA, 17-HDHA) in the presence of DHA, but the resolvin levels were very low: RvD5 was the most prominent of these (Archambault et al., 2022). Noteworthy, the stimulation of eosinophils with platelet-activating factor (which increases intracellular Ca2+ concentrations and thus 5-lipoxygenase activity) did not increase docosanoids despite increasing leukotrienes.
When 15-HETE was used as a substrate for human PMNL, LXA4 and 6S-LXA4 formation was about 75- and 110-times lower compared to 5,15-diHETE, suggesting that formation of the 5,6-epoxy-15-hydroxy intermediate is unfavorable (Mainka et al., 2021). Similarly, only the dihydroxylated SPM RvD5 was formed from 17-DHDA in easily detectable amounts. 18-HEPE was barely converted by 5-LO in the PMNL incubations and the formed amounts of RvE1 and RvE2 were more than 30- and 90-fold lower as compared to 17-HDHA-derived RvD5 (Mainka et al., 2021). In contrast to RvE1, detectable RvE2 formation was observed in ionophore-stimulated human macrophages in the presence of EPA as substrate (Ebert et al., 2020). In line with this, it was found that recombinant human 5-LO can catalyze the conversion of 18-HEPE to RvE2 (Kutzner et al., 2020) (Figure 1).
Taken together, all these data suggest that the 12/15-LO:5-LO pathway where the granulocyte 5-LO employs SPM precursors released e.g. from 15-LO1, 12-LO or 15-LO2 expressing cells is an inefficient source of trihydroxy SPMs (lipoxins, resolvins D1-4, resolvin E1) (Figure 1; Table 1). In contrast, the formation of dihydroxy SPMs, such as RvD5 (7,17-diHDHA), RvE2 (5,18-diHEPE), and RvE4 (5,15-diHEPE) appears to be more efficient (Lam et al., 1987), at least if DHA, EPA or the respective oxylipin precursors are added and the cells are stimulated with ionophore. The low formation of trihydroxylated SPMs, even in these in-vitro systems, could be related to the fact that formation of the 5,6-epoxide from hydroperoxy precursors by 5-LO is rather slow and that the 5-hydroperoxy intermediates generated by this enzyme are rapidly reduced by glutathione peroxidases to the corresponding alcohols. This peroxide reduction prevents epoxide formation via the leukotriene A4 synthase activity of 5-LO.
A major prerequisite for the formation of 5-LO-derived products is the Ca2+-dependent translocation of the enzyme to nuclear membranes where it interacts with FLAP. Indeed, a number of stimuli capable of elevating the cytosolic Ca2+ concentration are frequently used to obtain detectable SPM formation in human leukocytes. Ca2+ ionophore triggers lipoxin (Serhan et al., 1986a; Serhan et al., 1986b; Serhan et al., 1987; Serhan, 1989; Edenius et al., 1991; Sheppard et al., 1992; Chavis et al., 1996; Lehmann et al., 2015; Ebert et al., 2020; Mainka et al., 2021) as well as D-series (Spite et al., 2009; Lehmann et al., 2015; Ebert et al., 2020) and E-series (Tjonahen et al., 2006; Isobe et al., 2012; Ebert et al., 2020) resolvin formation by neutrophils and macrophages. Furthermore, stimuli that activate Gq- and Gi-coupled receptors such as thrombin and fMLF were reported to trigger SPM biosynthesis (Serhan and Sheppard, 1990; Sheppard et al., 1992). Resolvin formation was proposed to be induced by yeast-derived zymosan (Tjonahen et al., 2006; Sun et al., 2007; Spite et al., 2009) and under cell stress conditions such as hypoxia (Norris et al., 2019) and apoptosis (Dalli and Serhan, 2012) or upon co-incubation of macrophages with apoptotic PMNL (Dalli and Serhan, 2012). All these stimuli are also known to mobilize Ca2+ from intracellular stores. Furthermore, live/growing bacteria (E. coli, Staphylococcus aureus) or bacterial toxins were shown to stimulate SPM biosynthesis in human macrophages (Werz et al., 2018; Rao et al., 2019; Jordan et al., 2020). In these studies, prolonged incubation times of 90–180 min were used.
In recent years, short term incubations (up to 15 min) employing stimuli such as Ca2+ ionophore have been shown to produce low levels of SPMs. The SPM formation capacity of different 5-LO stimuli (LPS/fMLF, S1P, phenol soluble modulin-α, Ca2+ ionophore and osmotic stress) was recently compared in human PMNL supplemented with 15-HETE and 17-HDHA in short term incubations. These experiments showed that the SPM synthesizing capacity differed markedly depending on the stimulus. Nevertheless, Ca2+ ionophore was the most potent stimulus, probably due to the strong intracellular Ca2+ mobilization triggered by this compound. The pattern of SPM formation (high levels of dihydroxylated and minute amounts of trihydroxylated lipids) was comparable for all stimuli tested (Mainka et al., 2021) and did not substantially differ from other studies. Longer incubation times were not tested since 5-LO is rapidly auto-inactivated after stimulation (Aharony et al., 1987; Rouzer and Kargman, 1988; Wong et al., 1988; Kargman and Rouzer, 1989). Moreover, during long-term incubations, the probability of nonspecific autoxidation is strongly increased. This clearly argues for the use of Ca2+ ionophore in investigations relating to SPM biosynthetic capacity since this non-physiological stimulus is potent enough to trigger SPM biosynthesis in amounts sufficient for LC-MS detection (Quantitative Analysis and SPM Levels in Humans) with SPM patterns comparable to other stimuli.
However, it should be emphasized that these conditions (stimulation of leukocytes with Ca2+ ionophore, addition of AA, EPA and DHA) do not reflect the in vivo situation where weaker cell stimulation and lower substrate availability occurs. Furthermore, in leukocytes, AA is present and released at much higher levels than EPA or DHA from cell membranes upon cell stimulation. This favors formation of AA-derived oxylipins rather than EPA or DHA derived oxylipins under physiological conditions (Lee et al., 1985; Pratt et al., 2002). However, a ω-3 PUFA rich diet or ω-3 PUFA supplementation strongly increases their content in the membrane and several enzymes of the AA cascade including LOs (Kutzner et al., 2017) and CYPs (Fischer et al., 2014) preferably convert ω-3 PUFA compared to AA. Despite this, such dietary manipulation does not result in augmented SPM formation (see Quantitative Analysis and SPM Levels in Humans).
Taken together, it becomes obvious that detectable formation of SPMs in leukocytes is mostly restricted to non-physiological conditions such as ionophore-mediated cell stimulation and exogenous supply with fatty acid substrates.
Lipoxin and Resolvin Formation in Human Macrophages
In contrast to neutrophils, human monocytes/macrophages express AA 15-lipoxygenating paralogues (15-LO1, 15-LO2) depending on their differentiation status. Different 15-LO1 expression levels can be found in M1 and M2 macrophages (Wuest et al., 2012; Ebert et al., 2020). Expression of 15-LO2 is induced after stimulation of M2-differentiated macrophages with the TLR ligands LPS and zymosan as well as hypoxia (Rydberg et al., 2004; Ebert et al., 2020; Von Hegedus et al., 2020). 15-LO1 expression is IL4-dependent and is strongly upregulated during differentiation of monocytes to M2-like macrophages by IL4 (Conrad et al., 1992; Ebert et al., 2020). Since macrophage phenotype switching has been proposed as an important step in the transition of a pro-inflammatory reaction into the resolution phase (Gordon, 2003), the alterations of 15-LO1 and 15-LO2 expression during macrophage phenotype switching have been proposed to be part of a lipid mediator switch from pro-inflammatory lipid mediators such as leukotrienes and prostaglandins to SPMs (Chiang and Serhan, 2017). In addition to 15-LO1 and 15-LO2, macrophages also express 5-LO and FLAP (Ebert et al., 2020). Thus, in principle, these cells should be capable of producing SPMs. However, their SPM forming capacity is very limited. TLR-2 and -4 mediated stimulation of M1 macrophages primarily triggered the release of pro-inflammatory cytokines. Persistent stimulation of human M2 macrophages with LPS induced a coordinated upregulation of 5-LO and 15-LO2 expression (Ebert et al., 2020). 15-HETE but no SPMs were found in the conditioned media of the M2 macrophages and also after fMLF-stimulation of these cells. After stimulation with Ca2+ ionophore combined with supplementation of AA, EPA and DHA, M2 macrophages released small amounts of trihydroxy SPMs (RvD1, RvD2, RvE1, LXA4, LXB4). However, the concentrations of these metabolites were 100–1,000-fold lower than those of LTB4 or different monohydroxylated PUFAs. On the other hand, the dihydroxy SPMs RvE2 and RvD5 as well as 5,15-diHETE were formed in stimulated M2 macrophages in detectable amounts (Ebert et al., 2020). Similar results were obtained in another study where the lipid mediator profile in M1 and M2 polarized macrophages in the presence of a pathogenic E. coli strain was investigated: Here, consistently detectable RvD5 and MaR1 (7,14-diHDHA) levels were found in stimulated M2 macrophages but the formation of the other SPMs of the D- and E-series of resolvins was low, i.e. >400-fold and 40-fold lower than prostaglandin E2 and leukotriene B4 (LTB4) formation in the corresponding M1 macrophages, respectively (Werz et al., 2018). Similar lipid mediator profiles were reported in two subsequent reports by the same group (Rao et al., 2019; Jordan et al., 2020). An interesting finding of these studies on macrophages was that stimulation of M1 and M2 macrophages leads to a different lipid mediator profile released from the cells which seems to be due to the upregulation of 15-LO1 during M2 polarization and reduction of classical 5-LO metabolites such as 5-HETE and LTB4, probably via inhibition of 5-LO activity by the 15-LO reaction products such as 15-HETE (Vanderhoek et al., 1980; Camp and Fincham, 1985). In fact, stimulation of M2 macrophages led to the very prominent release of 15-HETE (Rao et al., 2019; Ebert et al., 2020; Jordan et al., 2020).
From these data, it becomes evident that macrophage polarization to M2 leads to a lipid mediator switch from the 5-LO to the 15-LO pathway. However, leukocytes have a very low capability of synthesizing trihydroxylated SPMs even in the presence of both, 5-LO and 15-LO, so that only 5,15-diHETE, RvD5, RvE2 and MaR1 (a 12/15-LO product) could be consistently detected in macrophages (Figure 1). Whether these levels are biologically relevant remains to be determined. Of note, 5S,15S-diHETE formation by dual lipoxygenase action was already reported 40 years ago (Borgeat et al., 1982), but up to now, this compound has never been classified as an SPM. The corresponding EPA-derived 5S,15S-diHEPE was recently assigned as resolvin E4 (Reinertsen et al., 2021). The relevance of the formed concentrations of either of these compounds to the resolution of inflammation remains to be determined.
The spectrum of the SPMs formed by M2 macrophages is more or less in agreement with the in vitro data obtained with purified LO paralogues (see above). Furthermore, it should be kept in mind that even the levels of the more prominent dihydroxylated SPMs formed in leukocytes tend to be significantly lower compared to pro-inflammatory lipid mediators, even if the cells are provided with exogenous substrates and different stimuli.
Based on the currently available experimental data, it appears unlikely that many of the proposed SPMs, especially trihydroxylated SPMs, are formed in leukocytes in sufficient amounts to counteract the functionality of the pro-inflammatory lipid mediators unless AA release is inhibited. However, this would be difficult to achieve, given the numerous cytosolic and secreted phospholipases A2 that can contribute to PUFA release. From the SPMs that involve 5-LO in their biosynthesis, only 5,15-diHETE, RvE2, RvE4 (5,15-diHEPE) and RvD5 seem to be formed in detectable quantities in leukocytes where 5-LO acts as first or second oxygenase. Alternatively, based on studies from platelet-leukocyte interactions, the 5-LO:12/15-LO pathway where LTA4 and LTA5 are released from leukocytes and further converted to lipoxins via a transcellular mechanism might have the capacity to be a potential source for lipoxins. Studies with 12-LO (Alox12) knockout mice do not support a role of this pathway in the resolution of inflammation (see SPMs and Lipoxygenase Knockout Data).
Autoxidation of PUFAs
Polyunsaturated fatty acids (PUFAs) such as EPA and DHA are susceptible to autoxidation (Frankel, 2005). Compared to enzymatic lipid peroxidation catalyzed by ALOX-isoforms, this process is slow but can be dramatically accelerated many fold in the presence of transition metals or other non-enzymatic catalysts (Leung et al., 2015). When the content of hydroxylated EPA and DHA derivatives was analyzed in a number of commercial fatty acid preparations, monohydroxylated and dihydroxylated derivatives were always detected. 15-HETE, 18-HEPE and 20-HDHA were dominantly found but 5-HETE, 5-HEPE and 4-HDHA were also detected. Since the latter compounds rapidly undergo lactonization, their relative abundance as primary auto-oxidation products is usually underestimated. By contrast, trihydroxy PUFAs such as LXA4 and LXB4 isomers were never observed in other than trace quantities. However, the presence of mono- and dihydroxy PUFAs in commercial PUFA preparations is a serious problem since these compounds may be used as substrates for trihydroxy-SPM formation. In fact, when commercial AA preparations were incubated with leukocytes or with different recombinant LO isoforms, the formation of lipoxin isomers was sometimes detected. In contrast, with purified AA these products were never observed. Thus, when using exogenous PUFAs to stimulate SPM formation in any cellular and subcellular system, it is highly recommended to purify the fatty acids prior their use.
A feature of non-enzymatic oxidation of PUFAs is the formation of multiple isomeric products (combination of different epimers as well as positional and cis/trans isomers) confounding instrumental analysis if not specifically considered and assessed. It should be noted that SPMs present in a biological sample could arise from autoxidation of either the parent PUFA or from autoxidation of monohydroperoxy PUFAs which were generated nonenzymatically or by the above-mentioned lipoxygenases. In other words, the different oxygenation reactions involved in the (bio)synthesis of double and triple oxygenated SPMs may be catalyzed by enzymes and/or non-enzymatic catalysts. The big difference between enzymatic and non-enzymatic oxygenation reactions is the degree of product specificity. If a given SPM is present as single isomer, its enzymatic origin is most likely. However, if a complex mixture of isomers is found, non-enzymatic oxidation reactions were probably involved.
The complexity of SPM structures with a multitude of isomers is a challenge for the instrumental analysis of these oxylipins (Gladine et al., 2019). For instance, LXA4 (5,6,15-triHETE) is often formed together with several isomers (enantiomer, diastereomers, cis/trans isomers and positional isomers). Up to now, only selected isomers have been chemically synthesized and for many of them no authentic standards are currently available. Thus, some of these SPM isomers may overlap during elution in commonly used reversed phase (RP) chromatography. This problem has been addressed for a few SPMs by employing chiral phase chromatography (Ferreiros et al., 2014) or ion mobility spectrometry (Jonasdottir et al., 2015) demonstrating the presence of interfering isomers in biological samples. Unfortunately, these analytical techniques require higher amounts of analytes or have not been implemented in routine analyses. As summarized in Quantitative Analysis and SPM Levels in Humans only RP-LC-MS/MS analysis is routinely used for quantification. With respect to SPM isomers formed by autoxidation, one should keep in mind that the chromatographic peaks may contain several isomers, even if the method has been developed and calibrated using a specific isomer available as pure standard.
Summary on SPM Biosynthesis Pathways
The proposed biosynthetic pathways for most SPMs involve 5-LO (ALOX5) as well as 12/15-LO activities (15-LO1, 15-LO2, 12-LO). Since 5-LO expression is mainly restricted to leukocytes, these cells are supposed to be key players in SPM formation. Biochemical studies with purified lipoxygenases revealed that their capacity for formation of trihydroxylated SPM is extremely low and dihydroxylated SPMs are formed at much lower rates than the classical reaction products (e.g., HETEs, HEPEs, HDHAs and leukotrienes). In accordance with these in vitro studies, formation of most SPMs is only detected after stimulation of leukocytes with non-physiological stimuli such as ionophore after addition of exogenous fatty acid substrates. The capacity of leukocytes for the formation of trihydroxylated SPMs is very low. Dihydroxylated SPMs such as RvE2, RvE4 (5,15-diHEPE) and RvD5 can be detected but at levels considerably below those of classical pro-inflammatory oxylipins. Based on the published biochemical and cellular data, the plausibility of at least some of the proposed SPM biosynthetic pathways is questionable. Whether any of them are operable in in vivo systems and whether they generate SPMs of functional relevance can only be verified using highly reliable analytical methodology for their detection in complex biological matrices (see Quantitative Analysis and SPM Levels in Humans).
SPMs and Lipoxygenase Knockout Data
The role of lipoxygenases in inflammatory processes has also been investigated by genetic knockout of different mouse Alox paralogues. Knockout of Alox5 has been shown to ameliorate certain inflammatory insults (Chen et al., 1994), protect from pulmonary fibrosis (Peters-Golden et al., 2002), attenuate renal ischemia-reperfusion injury (Patel et al., 2004), lower LPS-induced endotoxemia (Collin et al., 2004), impair the adaptive immune responses during fungal infections (Secatto et al., 2012) and attenuate antibacterial activity and phagocytosis (Bailie et al., 1996). Furthermore, it has been shown that 5-LO plays a pivotal role in lung injury after experimental sepsis (Monteiro et al., 2014). Using FLAP and LTC4 synthase knockout mice, it was found that cysteinyl leukotrienes contribute to renal fibrosis and to the progression of chronic kidney disease (Montford et al., 2019). Alox5 knockout mice also showed improved wound healing (Brogliato et al., 2014). Other data suggest that the 5-LO pathway may play a role in the development of neurodegenerative diseases (Joshi and Pratico, 2014). However, in the experimental acute encephalomyelitis (EAE) system, which is considered a suitable mouse model for human multiple sclerosis, Alox5−/− mice developed more severe neurological symptoms than corresponding controls (Emerson and Levine, 2004). The mechanisms behind this observation are still unclear, one possible explanation would be that Alox5 knockout impairs SPM formation which enhances inflammation. However, it should be emphasized that most knockout studies point to a pro-inflammatory role for the 5-LO pathway.
Interestingly, Alox5-knock-in mice which express an AA 15-lipoxygenating 5-LO enzyme mutant (Marbach-Breitruck et al., 2021) are leukotriene deficient, but these animals were not protected from the development of inflammatory symptoms in different mouse inflammation models. Studies on the role of 5-LO in atherosclerosis have delivered controversial results. Initial studies identified 5-LO as a contributor to atherosclerosis development in mice (Mehrabian et al., 2002) which could not be confirmed in later studies [for review, see (Poeckel and Funk, 2010)].
Taken together, it has become evident that the 5-LO pathway is part of the innate and adaptive immune system. It is involved in host defense reactions and promotes inflammatory processes (Peters-Golden et al., 2005; Flamand et al., 2007), but there are leukotriene independent elements in the immune response. This physiological profile is in agreement with many reported functions of the leukotriene receptors BLT1, CysLT1 and CysLT2 (Sasaki and Yokomizo, 2019).
Since 15-LO has been implicated in the lipid mediator switch during human macrophage polarization towards M2, the effects of Alox15 knockout on inflammatory reactions and on the resolution of inflammation are of special interest. In humans, a loss-of-function variant in the ALOX15 gene was shown to protect against the development of nasal polyps and chronic rhinosinusitis (Kristjansson et al., 2019). Furthermore, functional inactivation of the Alox15 gene resulted in reduced expression of pro-inflammatory genes and less severe colitis in the DSS mouse model (Kroschwald et al., 2018), diminished atherosclerosis in ApoE or LDL receptor deficient mice (Cyrus et al., 1999; Rong et al., 2012), reduced airway allergic inflammation in response to ovalbumin (Andersson et al., 2008), improved survival in mouse models of acute lung injury (Rossaint et al., 2012), reduced insulin resistance induced by high fat diet (Sears et al., 2009) and protected hyperlipidemic mice from nonalcoholic fatty acid liver disease (Martinez-Clemente et al., 2010). However, besides these pro-inflammatory effects of 12/15-LO (Alox15), several reports demonstrated an anti-inflammatory role of this enzyme. It was found that 12/15-LO promotes wound healing and host defense as well as counteracts fibroblast activation and fibrosis (Gronert et al., 2005; Kronke et al., 2012; Ogawa et al., 2020). A protective role for 12/15-LO has also been described in arthritis (Kronke et al., 2009) and in IL-33-induced eosinophilic airway inflammation which seems to be at least partially due to the 12/15-LO product 14-HDHA (Miyata et al., 2021). In a context of high EPA and DHA tissue levels, 12/15-LO led to protection from DSS-induced colitis, which was abolished by functional inactivation of the enzyme (Rohwer et al., 2021), further underlining a context-specific pro- or anti-inflammatory role of the enzyme. When human 15-LO1 is overexpressed in transgenic rabbits and in mice, an anti-atherogenic effect was observed (Serhan et al., 2003; Merched et al., 2008). However, the experimental strategy of overexpressing the human 15-LO1 in rabbits and mice deserves more detailed discussion. Like humans, rabbits express an AA 15-lipoxygenating Alox15 orthologue, whereas mice express an AA 12-lipoxygenating enzyme. This means that the transgenic rabbits express their endogenous AA 15-lipoxygenating enzyme and in addition the human transgenic enzyme, which is also AA 15-lipoxygenating. In contrast, the ALOX15 transgenic mice express their endogenous AA 12-lipoxygenating enzyme and in addition the AA 15-lipoxygenating transgenic gene product. These functional differences in the reaction specificity of the endogenous Alox15 orthologues and the transgenic enzymes make the system very complex and hamper straightforward interpretations.
Taken together, it is becoming clear that 12/15-LO can induce both pro- and anti-inflammatory effects which may depend on the expression level of the enzyme(s), on the availability of different substrates and on the regulatory peculiarities of the enzyme in different mammals. The multiplicity and availability of substrates and enzymatic products which are generated and decomposed in a tissue as well as species-specific manner may play an important role for the outcome of these experiments. When it comes to the functionality of Alox15 and Alox15B orthologues, species-specificity is particularly critical since for these enzymes, remarkable functional differences have been described. Moreover, it has never been explored in detail, which role the AA 8-lipoxygenating mouse Alox15B might play in the biosynthetic cascade of SPMs.
Even in those experimental systems in which the formation of SPMs has been reported (Merched et al., 2008; Fredman et al., 2016; Chiang and Serhan, 2017) a causal relation between the formation of these compounds and the observed anti-inflammatory or pro-resolving effects is unclear. There may be statistical correlations, but in most cases, it remains unclear whether the formation of SPMs is the basis for the observed biological effects. Additional knockout of the proposed receptor(s) (see Proposed SPM Receptors) would be helpful to dissect the role of individual lipid mediators in the animal models.
The 5-LO:12/15-LO pathway (Figure 1), in which LTA4 and LTA5 are released from leukocytes and further converted to lipoxins via transcellular mechanisms has been suggested as an effective source for lipoxins (Edenius et al., 1991). Platelet 12-LO has been implicated in the regulation of platelet activation (Tourdot and Holinstat, 2017) and cancer metastasis (Pidgeon et al., 2002). Platelets derived from Alox12 knockout mice showed modified ADP-induced platelet aggregation (Johnson et al., 1998) and the animals experience an increased transepidermal loss of water (Johnson et al., 1999). Moreover, Alox12 knockout inhibited skin carcinogenesis (Virmani et al., 2001) and reduced atherosclerosis and abdominal aneurysm (Allen-Redpath et al., 2019). However, to the best of our knowledge, impairment of inflammatory resolution has never been reported in these mice although the animals have been available for more than 30 years. On the contrary, knockout of Alox12 protected mice from injury-induced neuroinflammation suggesting a pro-inflammatory activity of 12-LO (Li et al., 2019). Thus, at present there is no experimental evidence from Alox12 knockout studies that the 5-LO+12-LO mediated formation of lipoxins may play a significant role in resolution of inflammation in vivo.
Summary on SPM Biosynthesis in Genetically Modified Mice
Since 5-LO has been suggested as key enzyme in the biosynthesis of many SPMs, Alox5−/− mice should not be capable of synthesizing these mediators and should show a defect in the resolution of inflammation. By contrast, most phenotypes of these knockout mice are anti-inflammatory. However, it has to be considered that a possible pro-resolution role of 5-LO might not be observed in the absence of pro-inflammatory 5-LO products in knockout mice. 15-LO can induce both pro- and anti-inflammatory effects which may depend on its expression level and on the availability of different substrates, but it is unclear which 15-LO enzymatic products are responsible for these observations. There is no clear evidence that any of the described lipoxygenase knockouts result in phenotypes which are due to disrupted formation of SPMs.
Proposed SPM Receptors
FPR2/ALX
After initial binding studies (Fiore et al., 1992), it was proposed that a close homologue of the formyl peptide receptor 1 (FPR1), FPR2, also named ALX (Brink et al., 2003; Chiang et al., 2006; Ye et al., 2009), is the receptor through which LXA4 exerts its effects (Fiore et al., 1994). FPR2 was later shown to serve as a receptor for a large number of ligands belonging to different chemical classes, including both formylated peptides and non-formylated peptides/proteins as well as small molecules (He and Ye, 2017). The initial ligand-receptor pairing of LXA4 and FPR2/ALX was based on the demonstration that [3H]-LXA4 binds to the surface of intact human neutrophils, a binding that was reduced not only by non-labeled LXA4 but also by the non-hydrolysable GTP analogue GppNHp (Fiore et al., 1992). [3H]-LXA4 also specifically bound to isolated neutrophil plasma membranes and to intact lysosomal granules as well as to FPR2/ALX expressing CHO cells that were first freeze thawed to facilitate binding (Fiore et al., 1994). It was also shown that GTPγS affected binding of LXA4 to the receptors present on the granule vesicle membrane, and that LXA4 increased the GTPase rate in permeabilized CHO cells expressing FPR2/ALX. In this, as well as in subsequent reports, a negative control in which binding and GTPase experiments are performed in mock-transfected cells was missing (Fiore et al., 1994; Chiang et al., 2000). However, it was demonstrated that cells transfected with FPR2/ALX showed a pertussis toxin-sensitive release of arachidonic acid in response to LXA4, while mock-transfected CHO cells did not (Fiore et al., 1994), even if in these experiments, concentration-dependency and EC50 values of the LXA4 effect were not determined.
Several other reports demonstrated that cells responded to LXA4 in a FPR2/ALX-dependent manner. For instance, in RBL-2H3 cells as well as in human monocytes, LXA4 induced a transient increase in [Ca2+]i depending on FPR2/ALX (Maddox et al., 1997; Bae et al., 2003). The rise in [Ca2+]i was rather weak as compared to the FPR2/ALX-selective peptide agonist WKYMVM (Bae et al., 2003), and LXA4 did not induce ERK phosphorylation whereas WKYMVM did so (Bae et al., 2003). In addition, siRNA-mediated knock-down of FPR2/ALX blocked LXA4-induced Ca2+ transients in rat goblet cells (Hodges et al., 2017). Barnig et al. showed that LXA4 can act on NK cells to induce eosinophil apoptosis as well as on type 2 innate lymphoid cells (ILCs) to decrease IL-13 release, which in both cases was inhibited by the FPR2 receptor antagonist WRW4 (Barnig et al., 2013). While these data suggest that LXA4 acts as an FPR2/ALX agonist, several other reports were not able to confirm a role of FPR2/ALX as an LXA4 receptor. Two groups reported independently that LXA4 was unable to induce effects in HL60 cells transfected with FPR2/ALX or in human neutrophils known to express FPR2/ALX endogenously, whereas WKYMVM was active (Christophe et al., 2002; Planaguma et al., 2013). Subsequent studies showed that LXA4 from at least two different commercial sources was not able to induce FPR2/ALX-dependent effects in neutrophils (Forsman and Dahlgren, 2009; Forsman et al., 2011). Failure of LXA4 to induce an increase in [Ca2+]i in the presence of FPR2/ALX was also reported by other groups (Hanson et al., 2013; Bang et al., 2018).
A large number of experiments using receptor antagonists have been published in order to test a direct link between effects of SPMs and FPR2/ALX. However, in many of these studies antagonistic peptides that preferentially inhibit FPR1 rather than FPR2, such as Boc-1, Boc-MLF, or Boc-2, BocFLFLF have been used (Krishnamoorthy et al., 2010; Wu et al., 2014; Zhang et al., 2017; Mai et al., 2018; Yang et al., 2018; Lyngstadaas et al., 2021).
Regarding alternative receptor down-stream signaling events, a publication reported that LXA4 increased the luminescence signal using a complementation β-arrestin assay in FPR2/ALX-expressing cells (Krishnamoorthy et al., 2010). In the same study, the authors also described RvD1 as an agonistic ligand of FPR2/ALX, and knock-down of FPR2/ALX led to strongly reduced effects of RvD1 on macrophages (Krishnamoorthy et al., 2010; Lee and Surh, 2013). While these data suggest that LXA4 may act as a biased FPR2/ALX agonist, several other reports were not able to confirm such a role of FPR2/ALX as an LXA4 receptor. Two subsequent studies showed that recruitment of β-arrestin was not induced by LXA4 from at least two different commercial sources whereas peptide agonists specific for FPR2/ALX induced translocation of β-arrestin (Forsman and Dahlgren, 2009; Forsman et al., 2011). Similarly, in HEK293 and CHO cells expressing FPR2/ALX, no effect of LXA4 from different sources could be observed on β-arrestin 2 membrane translocation, whereas again the FPR2/ALX agonist WKYMVM was active (Hanson et al., 2013). This study also ruled out that LXA4 was degraded before or during the experiment by performing mass spectrometry determination of LXA4 concentrations in the assay buffer after completion of the experiment.
More recently, it was shown that so called aspirin-triggered 15-epi-lipoxin A4 (ATL), an isomer of LXA4 with unclear in vivo relevance, interacted in a complex manner with FPR2/ALX, using a modified version of FPR2/ALX, which allows determination of conformational changes of FPR2/ALX by fusing FPR2/ALX with CFP and by inserting a CCPGCC motif into the first or third intracellular loop (Ge et al., 2020). ATL, which has not yet been shown to be formed under in vivo conditions, induced only a small increase in [Ca2+]i and of β-arrestin 2 membrane translocation at concentrations higher than 1 µM. However, it had a partial agonistic effect on the inhibition of cAMP accumulation already at a concentration of 1 nM. In addition, the authors found opposing effects on the FPR2/ALX conformation at concentrations between 1 and 100 pM as well as 100 pM and 1 µM. At the same time LXA4 was unable to displace WKYMVM from the receptor at concentrations up to 1 μM, whereas ATL was able to affect binding of WKYMVM and WKYMVM downstream signaling at much lower concentrations. The authors concluded that ATL at subnanomolar concentrations functions as an inverse agonist, most likely in an allosteric fashion, whereas it functions as an orthosteric partial agonist at much higher concentrations.
In terms of biology of the receptor, various ligands of different chemical natures can have similar or opposite cellular effects. For example, while LXA4, RvD1 and Cmp43 have anti-inflammatory properties, formyl peptides produce a pro-inflammatory response. Since the known signaling events that take place upon receptor activation such as [Ca2+]i mobilization are shared for example by Cmp43 and formylated peptides, simple signaling differences have not yet been identified that are responsible for the functional differences at the cellular level. To explain this inconsistency, Cooray et al. have proposed that each ligand could stabilize distinct conformations of FPR2/ALX with different outcomes in terms of dimerization of the receptor and subsequent activation of signaling pathways (Cooray et al., 2013). However, there is no experimental evidence in support of this conjecture.
Several studies explored the potential role of FPR2/ALX as a mediator of LXA4 effects in vivo using Fpr2/Alx-deficient mice. The establishment of a relevant mouse model is, however, problematic since initial characterizations identified both FPR2/ALX and FPR3 as the mouse receptor for LXA4 (Takano et al., 1997; Vaughn et al., 2002). The first study using knock-out mice later turned out to have employed an (Fpr2/Alx)/Fpr3 double knock-out model (Dufton et al., 2010). In addition, subsequent studies showed that mice lacking FPR2/ALX have already a phenotype under basal conditions, which may be due to loss of basal (FPR2/ALX)/FPR3 activity or loss of the effects of other (FPR2/ALX)/FPR3 receptor agonists such as Cmp43 and the endogenous ligands annexin I and several non-amyloidogenic peptides (He and Ye, 2017). In another study it was shown that deletion of FPR2/ALX has an anti-inflammatory effect in an airway inflammation model (Chen et al., 2010). This indicates that even if endogenous LXA4 can activate this receptor and exert an anti-inflammatory effect, it cannot overcome the pro-inflammatory effects of other endogenous FPR2/ALX ligands, suggesting that the interaction of LXA4 with FPR2/ALX is of limited in vivo relevance, at least in the context of airway inflammation. It should also be noted that, in addition to the prominent differences that exist in gene expansion of formyl peptide receptors (FPRs) between humans and mice, there is also increasing evidence indicating that human FPRs and their murine counterparts differ substantially with respect to their ligand-binding profiles, as illustrated by the fact that some of the potent and selective antagonists for the FPRs lack effects on the mouse receptors, and an earlier described FPR2/ALX inhibitor/antagonist actually activates FPR2/ALX (Winther et al., 2018). No such comparative studies of mice and men involving SPMs have been performed, which highlights the importance in future research studies of choosing appropriate ligands when designing animal experiments. Together, this makes the interpretation of differential effects of LXA4 in animal knock-out models difficult (Dufton et al., 2010; Maderna et al., 2010; Norling et al., 2012; Brancaleone et al., 2013; Vital et al., 2016; Petri et al., 2017). There are, thus, still conflicting data regarding the specific role and signaling properties of FPR2/ALX in the action of LXA4 and the related lipid ATL, and this also goes for the action of RvD1 (see the GPR32 dealing with GPR32 for more details).
GPR18
A β-arrestin recruitment assay was used to screen a large number of RvD2 receptor candidates, leading to the nomination of GPR18 (Chiang et al., 2015). Specific binding of [3H]-RvD2 to CHO cells expressing human GPR18 with a KD value in the lower nanomolar range was shown, but negative controls using non-transfected cells were not reported. However, knock-down of GPR18 in macrophages resulted in loss of RvD2-induced cAMP formation and other cellular effects (Chiang et al., 2015), although previous data from the same group suggested that RvD2 acts through a Gi-coupled receptor based on pertussis toxin sensitivity of RvD2 effects on endothelial cells (Spite et al., 2009). Also, the ability of RvD2 to exert anti-inflammatory and protective activity in vivo was lost or strongly reduced in GPR18-deficient mice (Chiang et al., 2015). The same group later provided additional evidence for an in vivo role of GPR18 as a receptor for RvD2 in various models of infectious inflammation (Chiang et al., 2017). So far, the activity of RvD2 as an agonist of GPR18 has not been reported by an independent laboratory, but one independent study was unable to reproduce agonism for RvD2 in a β-arrestin recruitment assay (Schoeder et al., 2020).
Previously, GPR18 has also been described as a receptor for N-arachidonoyl glycine (NAGly) as well as for Δ9-tetrahydrocannabinol (Kohno et al., 2006; Mchugh et al., 2010; Mchugh et al., 2012). However, agonism of these two ligands for GPR18 was not reproduced in three reports, which used β-arrestin assays (Yin et al., 2009; Southern et al., 2013; Schoeder et al., 2020).
GPR32
RvD1 has been shown to interact with neutrophils and to have anti-inflammatory and pro-resolving effects fairly similar to those of LXA4. Based on a screen for candidate GPCRs able to mediate inhibition of TNFα-stimulated NFκB activity by RvD1, not only FPR2/ALX but also GPR32 was found to function as a receptor for RvD1 (Krishnamoorthy et al., 2010). GPR32 was shown to have an amino acid sequence partly shared with the FPRs (i.e., 39 and 35% amino acid identity with FPR1 and FPR2/ALX, respectively). Screening data were confirmed by showing that RvD1 increased β-arrestin recruitment to the plasma membrane in cells expressing either GPR32 or FPR2/ALX. In the same study, the authors also showed that RvD1 increased phagocytosis by macrophages, and that this effect was not obtained after knock-down of GPR32 (Krishnamoorthy et al., 2010; Krishnamoorthy et al., 2012). The same group later reported that RvD3 and RvD5 are also able to increase β-arrestin activity in CHO cells overexpressing human GPR32. However, control experiments using untransfected cells were not reported (Chiang et al., 2012; Dalli et al., 2013). In addition, both reports demonstrated that RvD3 and RvD5 show increased activity to stimulate macrophage phagocytosis after transfection of cells with GPR32, but no knock-down control data were reported (Chiang et al., 2012; Dalli et al., 2013). GPR32 has also been found to recruit β-arrestin in response to LXA4 and the dual FPR1/(FPR2/ALX) agonist Cmp43, and these ligands were as potent as RvD1 (Krishnamoorthy et al., 2010). Studies in mice lacking GPR32 have not been possible since GPR32 is a pseudo-gene in mice and rats (Haitina et al., 2009). An ability of RvD1, other D-series resolvins, LXA4 or Cmp43 to activate GPR32 has not been reported by an independent laboratory so far, and agonism of RvD1 for GPR32 could not be replicated in a study using a different assay system (Foster et al., 2019), or in studies using the same β-arrestin recruitment assay (Southern et al., 2013).
Chemerin Receptor 1
In a small candidate screen for GPCRs mediating inhibition of NFκB-dependent luciferase activity by RvE1, ChemR23 was reported to function as a receptor for RvE1 (Arita et al., 2005). In two publications by the same group, it was demonstrated that RvE1 bound to CHO cells transfected with ChemR23 but not to untransfected cells, and that RvE1-induced inhibition of IL-12 production by dendritic cells was significantly reduced after knock-down of ChemR23 (Arita et al., 2005; Arita et al., 2007). Specific binding of RvE1 to ChemR23 and a role of ChemR23 in typical GPCR downstream signaling events in response to RvE1 has not yet been reported by an independent group. It has been shown by an independent publication that RvE1 inhibited oxLDL uptake by peritoneal macrophages and that this effect was lost in peritoneal macrophages from ChemR23-deficient mice (Laguna-Fernandez et al., 2018).
BLT1
In addition to ChemR23, RvE1 has also been reported to be a partial agonist of the leukotriene B4 (LTB4) receptor (BLT1) having relatively low potency as compared to LTB4 (Arita et al., 2007). RvE1 showed specific binding to human neutrophils and HEK cells overexpressing BLT1 and decreased forskolin-induced cAMP formation in a BLT1-dependent manner. The same group reported later that also RvE2 binds to HEK cells overexpressing BLT1 and to reduce LTB4-induced β-arrestin signaling (Oh et al., 2012). These findings suggest that RvE1 and RvE2 induce their effects through a partial agonism at the BLT1 receptor thereby dampening the pro-inflammatory effects of LTB4. Evidence for an interaction between BLT1 and RvE1 has not yet been reported by other independent groups.
Other Receptors
Several other receptors for SPMs have been proposed by single publications. LXA4 was reported to function as a positive allosteric modulator of cannabinoid receptor 1 (CB1) (Pamplona et al., 2012). This was based on the observation that LXA4 only partially displaced a specific ligand from the CB1 receptor while enhancing the affinity of anandamide and other synthetic CB1 agonists for CB1. Consistent with this, LXA4 potentiated effects of CB1 agonists without showing any effect on its own. In addition to several GPCRs, LXA4 has also been proposed to interact with nuclear receptors. One report suggests that LXA4 functions as an agonist of the aryl-hydrocarbon receptor (AhR) (Schaldach et al., 1999). In a competition binding assay, LXA4 displaced a radio-labeled AhR ligand. However, direct binding of LXA4 to AhR was not demonstrated. Another publication reported that LXA4 activates the estrogen receptor (ER) (Russell et al., 2011). The report showed that LXA4 displaced estrogen from its receptor and induced an increase in ER transcriptional activity independent of AhR or FPR2/ALX. However, no negative control experiments were performed.
Neuroprotectin D1 (NPD1) was reported to interact with the orphan receptor GPR37 (Bang et al., 2018). NPD1 was shown to induce calcium mobilization in a GPR37-dependent manner with an EC50 in the low nanomolar range. Binding of NPD1 to GPR37 was analyzed using a dot blot assay. Since the ligand is not labeled and solubilized cellular proteins were tested, caution is warranted when interpreting the data. However, NPD1 was shown to increase the phagocytosis in macrophages in a GPR37-dependent manner.
After conducting a screen of orphan GPCRs using the β-arrestin recruitment assay, maresin 1 (MaR1) was reported to interact specifically with the G-protein-coupled receptor LGR6 (Chiang et al., 2019). MaR1 induced an increase in cAMP and β-arrestin recruitment in LGR6 overexpressing cells but not in mock transfected cells. A radiolabeled methylester of MaR1 was shown to bind to CHO cells expressing LGR6. Binding of the radiolabeled ligand in mock transfected cells was not reported. However, MaR1-induced phagocytosis in human macrophages depended on LGR6.
After conducting another screen of orphan GPCRs using the β-arrestin recruitment assay, ω-3 docosapentaenoic acid-derived resolvin D5 (RvD5n-3 DPA) was reported to be able to function through various GPCRs, of which GPR101 mediated the strongest effect (Flak et al., 2020). Radiolabeled RvD5n-3 DPA bound to HEK cells overexpressing Gpr101. However, a negative control by testing the binding of labeled RvD5n-3 DPA to untransfected cells was not shown. Several effects of RvD5n-3 DPA on macrophages were affected by knock-down of GPR101.
Summary on SPM Receptors
Generally, it has been difficult to describe the interaction of lipids with putative receptors due to their physicochemical properties, including nonspecific binding to proteins, formation of micelles, detergent or surfactant effects as well as indirect effects due to unspecific interactions with membranes. Particular caution has therefore to be taken before it can be concluded that a certain lipid ligand receptor pair exists. A good starting point for a rigorous analysis of such interactions are the published recommendations for new pairings of ligands and GPCRs (Davenport et al., 2013; Laschet et al., 2018). These include that two or more refereed papers from independent research groups should demonstrate activity of the ligand at the receptor with a potency consistent with a physiological function. The experiments need to be properly controlled, e.g., exclusion of unspecific effects in the absence of the receptor. In addition, it is highly recommended that both radio-ligand binding and functional assays should be employed, both in vitro and in native tissues/cells. If available, well validated selective agonists should mimic and selective antagonist as well as allosteric modulators should block/modulate the action of the endogenous ligand. Naturally, the putative endogenous ligand should be present in tissues in appropriate concentrations, and a plausible mechanism for the proposed ligand to reach physiologically significant concentration in tissues expressing its cognate receptor should be provided. Finally, deleting the gene encoding the receptor in mice, use of naturally occurring deletion mutations in human tissues or RNA silencing or other techniques to knock down receptor expression should be used to provide further evidence for the proposed ligand-receptor pairing. By these criteria, endogenous receptors for SPMs remain to be validated.
Quantitative Analysis and SPM Levels in Humans
There are two major requirements for the assignment of the biological function of a given lipid mediator in inflammatory resolution which partly depend on each other: i) The cells involved in this process must exhibit a sufficiently high biosynthetic capacity for the formation of these SPMs. ii) These lipid mediators must reach local concentrations which are high enough to activate their receptor(s) and to counteract the bioactivity of the pro-inflammatory mediators that are formed in parallel. The quantitative analysis of SPMs is indispensable for assessing both requirements.
The capacity for SPM generation under optimal circumstances in leukocytes even after activation with powerful stimuli is low (In Vitro Formation of Lipoxins and Resolvins). Consistently, very low levels have been reported or SPMs cannot be detected at all in human plasma (Figure 2) (Calder, 2020). Since eicosanoids and other oxylipins are believed to predominantly act as autocrine or paracrine mediators, their local concentration in tissues is of greater biological relevance than that in plasma. Unlike prostanoids which are found in tissues such as colon at high levels (ng/g) (Gottschall et al., 2018; Rohwer et al., 2020), the reported tissue concentrations of SPMs are also low, i.e. in the range of pg/g (Serhan, 2017a). Thus, very sensitive analytical methods are required for the reliable quantification of SPMs in biological samples.
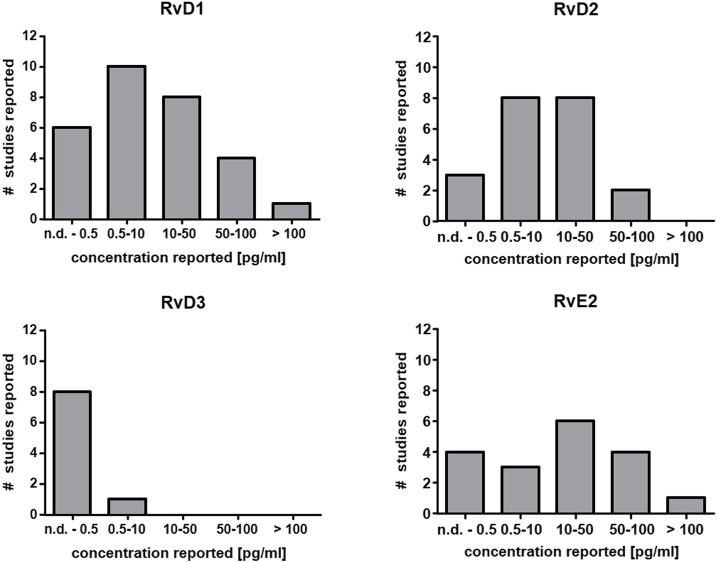
FIGURE 2.
Reported concentration ranges of selected SPMs in human plasma. Based on the comprehensive review from Calder (Calder, 2020) the number of studies and the detected concentration range are summarized for RvD1, RvD2, RvD3 and RvE2 in plasma from human subjects. In the majority of the studies the concentration is low (<50 pg/ml) which is close to the detection limit of several methods or below (Table 2).
Enzyme-Linked Immunosorbent Assays
Several enzyme-linked immunosorbent assays (ELISAs) have been developed for different types of SPMs. They are commercially available and are frequently used. However, taking into account the large number of structurally similar SPMs and other oxylipins (Willenberg et al., 2015), it is unlikely that these kits reach the required selectivity. They have been tested for selected SPM isomers but it remains unclear to which extent they pick-up alternative isomers. For instance, an ELISA developed for 15-epi-LXA4 cross reacts with LXA4 (Fedirko et al., 2017). However, it remains unclear to which extent geometric double bond isomers are also quantified. Moreover, various oxylipins such as 15-HETE, 5,15-diHETE and LXA4 on the one hand and 5-HETE, 5,15-diHETE and LXB4 on the other share at least single epitopes. Since the epitopes used for the different antibodies have not been carefully characterized the degree of cross-reactivity with other SPMs, never mind other lipids, cannot be predicted. Thus, detailed cross-reactivity studies are required for reliable analytical data. For the interpretation of ELISA-based SPM data another point must be considered: It has been reported that 5,6-diHETE cross reacts by 5% in the Neogen LXA4 kit. Since LXA4 is found in biological samples at much lower abundance than 5,6-diHETE, the documented LXA4 levels may at least in part be due to 5,6-diHETE. Another example is RvD1, its plasma concentration was ∼30 pg/ml determined by LC-MS. In contrast, by using ELISA assays, levels above 2000 pg/ml were found, as summarized recently (Calder, 2020). In summary, ELISAs for SPMs are unreliable for complex biological matrices.
LC-MS Analysis of SPMs
The required sensitivity and selectivity for oxylipin analysis including SPMs can only be reached using chromatography coupled to mass spectrometry (MS) (Willenberg et al., 2015; Gladine et al., 2019). To date, only liquid chromatography coupled via electrospray ionization (ESI) to tandem MS is frequently used for quantitative SPM analysis. In fact, the techniques employed are almost identical using commercially available “ultra high” performance (sub-2 µm particle filled) chromatographic RP-columns with a diameter of 2.1 mm and flow rates of about ∼300 μl/min (Table 2; Supplementary Table S1), “standard” ion sources and triple quadrupoles as analyzers operating in selected/multiple reaction monitoring mode (MRM, Table 2) (Willenberg et al., 2015; Gladine et al., 2019). Thus, with nearly identical instrumentation and method parameters one would assume that the sensitivity for the detection is comparable between different labs.
TABLE 2.
Sensitivity of state-of-the-art analysis of SPMs using LC-MS/MS.
| Laboratory | References | Instrument LLOQ | LLOQ plasma/serum/fluid | Instrument | LLOQ definition | |
|---|---|---|---|---|---|---|
| pg on column | pg/ml vial | pg/ml | ||||
| Dalli | Colas et al. (2014) | 0.05–0.22 a , c , d5-LXA4 0.05, d5-RvD2, 0.09 | 1.3–5.5, LXA4 1.3, RvD2 2.3 | 0.05–0.22, d5-LXA4 0.05, d5-RvD2, 0.09 | Sciex 6500 | |
| Dalli | Gomez et al. (2020), Koenis et al. (2021), 10.21203/rs.3.pex-1147/v1 | 0.05–5.00, LXA4 0.1, RvD2 0.1 b , c | 1.4–142, LXA4 2.9, RvD2 2.9 | 0.05–5.00, LXA4 0.1, RvD2 0.1 | Sciex 5500 | |
| Dennis | Dumlao et al. (2011), Deems et al. (2007), Quehenberger et al. (2011) | 1 a , LXA4 1 | 25 | 2.8 | Sciex 4000 | S/N ≥ 3 (n = 3) (LOD) |
| Geisslinger | Toewe et al. (2018) | 1–2, LXA4 2, RvD2 2 | 100–200, LXA4 200, RvD2 200 | 25–50, LXA4 50, RvD2 50 | Sciex 5500 | S/N ≥ 10, ± 20% accuracy and precision |
| Giera | Jónasdóttir et al. (2015) | 0.5, LXA4 0.5, RvD2 0.5 | 25, LXA4 25, RvD2 25 | 200 | Sciex 6500 | S/N > 10 |
| Giera | Jonasdottir et al. (2017), Giera et al. (2012) | 0.4, LXA4 0.4 | 10 d , LXA4 10, RvD2 no calibration | Synovial fluid 12 | Sciex 6500 | S/N > 10 |
| Hammock | Yang et al. (2009) | 0.21 | 21 | 8 | Sciex 4000 | S/N ≥ 10 |
| Hersberger | Hartling et al. (2021) | 0.002–0.063, LXA4 0.008, RvD2 0.002 | 0.2–6.3, LXA4 0.8, RvD2 0.2 | 0.4–12.5, d5-LXA4 0.4, d5-RvD2 3.2 c | Sciex 6500+ | S/N > 10 |
| Mori | Mas et al. (2012) | 6 | 250 | 25 | Thermo TSQ Quantum | S/N ≥ 10 |
| Newmann | Pedersen et al. (2021) | 0.2–1, LXA4 0.4, RvD2 0.8 | 40–199, LXA4 80, RvD2 159 | 201–995, LXA4 398, RvD2 794 | Sciex 6500 | 3 × t n-1,0.95 × STD e |
| Nicholson | Wolfer et al. (2015) | 0.05–5, LXA4 1.3, RvD2 0.5 | 10–1000, LXA4 260, RvD2 100 | 12–1200, LXA4 312, RvD2 120 | Waters Xeno TQS | S/N > 5, intraday RSD <20% (n = 4), accuracy ±20% |
| Ramsden | Yuan et al. (2018) | 1–5, LXA4 2, RvD2 5 | 100–500, LXA4 200, RvD2 500 | 20–100, LXA4 40 RvD2 100 |
Sciex5500 | S/N > 5, intraday RSD <20% (n = 4), accuracy ±30% |
| Schebb | Kutzner et al. (2019) | 0.6–3.6, LXA4 0.6, RvD2 1.4 | 61–360, LXA4 61, RvD2 141 | 6–36, LXA4 6, RvD2 14 | Sciex 6500 | S/N ≥ 5, ±20% accuracy |
| Werz | Werner et al. (2019), Werner et al. (2020) | 0.195–1.56, LXA4 0.195, RvD2 1.56 a | 19.5–156, LXA4 19.5, RvD2 156 a | 1–8, LXA4 1, RvD2 8 a | Sciex 5500 | S/N > 3, >5 data points (LOD) |
| Zhu | Wang et al. (2020) | 0.18–4.5, LXA4 0.18, RvD2 0.9 | 1.8–45, LXA4 1.8, RvD2 9 | 5.4–135, LXA4 5.4, RvD2 27 | Agilent 6470 | S/N > 7 |
The reported sensitivity for all covered SPMs and exemplary LXA4 and RvD2 is summarized. Shown is the lower limit of quantification of the instrument (LLOQ on column and the corresponding concentration), the effective LLOQ for liquid biological samples in pg/ml, as well the instrument and method used for definition of the LLOQ during method validation. Please note, that this is a simplified table. An extended version of the table including all required information and more method parameters is provided in the supplemental information (Supplementary Table S1).
Only LLOD is given in the publication.
Not specified between LLOD/LLOQ.
Determined in plasma matrix.
Lowest calibration level injected.
LLOD/LLOQ was determined based on a significant change (one-tailed t-test) in the sensitivity between successive calibration standards using the standard deviation (STD) of the concentration level significantly different than the preceding concentration level and the t-distribution (t-value: one-tailed, 95% confidence).
However, as shown in Table 2, the lower limit of quantification (LLOQ) - which is the lowest concentration that can be reliably quantified in a sample - varies between 0.05 and 6 pg on column. In case of LXA4 and RvD2 this means, with an LLOQ of 0.1 - 2 or 0.1–6 pg on column, that the very same instrumentation and equipment appears to be 20–60 fold more sensitive in one lab compared to another.
In our hands, the state-of-the-art instruments operated as described above reach a sensitivity of 0.5–5 pg on column for SPMs, in case of LXA4 0.6 pg and RvD2 1.4 pg on column (Kutzner et al., 2019). In fact, this is the same range as for other oxylipins (Gladine et al., 2019) which is consistent with their structural similarity.
If methods are dramatically more sensitive (which means at least factor 5, not 2-3 which could easily depend on the skills of the person who optimized separation and detection, or on the performance of the specific instrument) there must be another methodologic explanation. This could reflect for example the use of nano-LC, i.e., chromatographic separation at low flow rates enhancing ionization efficacy (Wilson et al., 2015). However, probably due to the lack of robustness this has not been frequently applied in SPM analysis. Recently, Hartling et al. described a method with an LOQ of 8 fg for LXA4 and 2 fg for RvD2 on column. Unlike other methods they used a base modified LC solvent, thus enhancing the ionization process in negative ESI mode. This alternative analytical technique is most probably the reason why their method is 100-fold more sensitive (Hartling et al., 2021).
However, Colas et al. (2014) and Koenis et al. (2021) report a sensitivity which is 5–10 fold better (0.05–5 pg on column, 0.1 pg for LXA4 and RvD1) than all the other methods (Table 2) using almost the same conditions. This can only be explained by a different definition of the sensitivity, i.e., the LLOQ. Commonly for LC-MS, the LLOQ (LLOQ-definition I) is defined by the height of the peak compared to the noise as well as the accuracy of the calibration at this concentration (European medicines Agency, 2011; US Food and Drug Administration, 2018). This assures that not only a signal of the compound is detected beyond doubt (which would be the limit of detection), but also allows calculation of a meaningful concentration with acceptable variation and precision. However, in the field of SPMs several groups use a different definition of the LLOQ (LLOQ-definition II), in which the ratio between peak and noise is irrelevant (Colas et al., 2020; Koenis et al., 2021): If the peak exceeds i) a threshold intensity (2,000 counts), ii) a minimum of 4 data points [12–20 points are commonly used for peak definition in LC-MS/MS (European medicines Agency, 2011; US Food and Drug Administration, 2018)] and iii) if the MS/MS spectrum contains ions matching the m/z of the SPM fragments, then the peak is integrated and used for quantification (Colas et al., 2020). Using this technique, peaks are considered which would be regarded as too low to determine a reliable concentration by common LLOQ-definition I. Particularly in extracts from biological samples, noise can easily exceed the threshold set (e.g., 2,000 cps over four data points) because of the sensitivity of the instruments. The fragment spectra of these background ions contain a variety of nonspecific ions, some of which may have similar m/z values as those derived from SPMs (Kutzner et al., 2020). This is illustrated by the chromatograms shown by Gomez et al., e.g., for RvD2, RvD5, RvD6 and LXA4, where the integrated peaks are hardly distinguishable from noise (Gomez et al., 2020) and the method used here, which is widely applied to SPM analysis, has been criticized (O'Donnell et al., 2021). Thinking about the quantification of pharmaceuticals (which are not so different from SPMs with respect to molecular weight, functional groups and polarity), one should be aware that the LLOQ-definition II is unacceptable for method validation by either the US Federal Drug Administration or the European Medicines Agency (European medicines Agency, 2011; US Food and Drug Administration, 2018).
Regarding the power to detect SPMs in blood and tissue, the sensitivity of the method also depends on an appropriate sample preparation. This is critical both to reduce interfering matrix compounds such as phospholipids (Ostermann et al., 2015), and for pre-concentration. Commonly applied solid phase extraction (SPE) allows reconstitution in a lower volume compared to the initial sample volume facilitating effective LLOQs which are 10- to 25-fold better than for the injected solution (Table 2). If this step is omitted and samples are injected following protein precipitation, low LLOQs are sacrificed making the detection of SPMs generally impossible (Jonasdottir et al., 2017; Pedersen et al., 2021). However, online-SPE methods may allow injecting enough sample volume to gain sensitivity (Wang et al., 2020). Of course, the volume injected, i.e., the amount/percentage of the extracted biological material/volume of plasma (Table 2), impacts the effective sensitivity of SPM detection in biological materials. Pre-concentration is again limited by the available sample volume and even more so by interfering co-extracted matrix components disturbing the detection of SPMs (Ostermann et al., 2015). However, data on extraction efficacy and ion suppression as well as method details and validation data are missing in most reports describing the analysis of SPMs, making the evaluation of the adequacy of the methods difficult.
Consistent with the sensitivity of the analysis (Table 2; Supplementary Table S1) and the described levels (Figure 2), several groups failed to detect SPMs in plasma of healthy individuals (Jónasdóttir et al., 2015; Skarke et al., 2015; Kutzner et al., 2019). Even under conditions anticipated to maximize their formation such as supplementation with their putative ω-3 PUFA precursors by fish oil, SPMs could not be detected (Skarke et al., 2015; Ostermann et al., 2019). Thus, the supplementation of increasing doses of the fish oil to healthy volunteers lead to a dose dependent shift from ω-6 to ω-3 oxylipins, while SPMs were largely undetectable (Skarke et al., 2015; Ostermann et al., 2019). Even after administration of an acute inflammatory stimulus, i.e. bacterial lipopolysaccharide, to such volunteers, SPMs were unaltered in the inflammatory or resolution phase (Skarke et al., 2015). Similarly, Mazaleuskaya et al. largely failed to detect SPMs in stimulated whole blood from healthy volunteers (Mazaleuskaya et al., 2016). Elevated SPM concentrations have been detected in samples from patients suffering from severe inflammation such as COVID-19 or septic shock. However, this also coincided with a strong increase in all oxylipins including pro-inflammatory eicosanoids (Kutzner et al., 2019; Archambault et al., 2021).
Summary on Quantitative Analysis and SPM Levels
The capacity of tissues to form SPMs is very low and tissue or cellular capacity to generate traditional eicosanoids and other oxylipins greatly exceeds actual rates of SPM biosynthesis. As such it is not surprising that SPM levels in biological fluids would be at or below the limits of detection of the most sensitive MS instrumentation. The specificity of the most commonly applied approach on SPM detection (Dalli and Serhan, 2012; Colas et al., 2014; Dalli et al., 2017; Gomez et al., 2020; Koenis et al., 2021) is not in accordance with internationally agreed standards and has recently been fundamentally questioned (O'Donnell et al., 2021). It remains to be established that SPMs are formed in vivo at concentrations of relevance to the resolution of inflammation.
Conclusion
The resolution of inflammation is now considered as an active process, which coincides with a switch in the profile of cytokines, lipid mediators and other signaling molecules. Some prostaglandins and leukotrienes are typically pro-inflammatory lipid mediators, others have an anti-inflammatory effect. SPMs such as lipoxins and resolvins which are biosynthesized via the 5-LO plus 12/15-LO pathway, have been suggested to play important roles in the resolution of inflammation. However, in vitro studies with purified enzymes and studies with leukocytes as a source of 5-LO revealed that many of the lipoxins and resolvins of the D- and E-series are only formed in minute amounts, if at all, since their biosynthetic precursors are hardly accepted by human 5-LO as a substrate for SPM formation, calling into question whether the proposed schemes on their enzymatic formation are biologically plausible. Deletion of their putative biosynthetic enzymes have failed to provide evidence consistent with a role for SPMs in the resolution of inflammation. Evidence that resolvins and lipoxins exert their effects through specific receptors remains controversial and incomplete. Finally, evidence that SPMs are formed in biologically active concentrations in humans that promote the resolution of inflammation remains to be provided.
Author Contributions
NS, HK, SO, and DS structured the paper, AK, HK, KR, NS, MA, SO, and DS wrote the paper, VO’D, NF, MP-G, P-JJ, KW, NR, RM, GG, GF, JH, and CD commented and amended the paper.
Funding
NHS was supported by the Deutsche Forschungsgemeinschaft (DFG) (Sche 1801). DS, AK, and GG were supported by the DFG (SFB 1039, TP A02, Z01; GRK 2336, TP4), the Fraunhofer CIMD and the Fraunhofer Leistungszentrum TheraNova. SO was supported by the DFG (SFB 1039, TP A04). HK was supported by DFG (KU 961/13-1 and KU 961/14-1), KW was supported by DFG (WE 2908), NF by the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2021-03777), MP-G by an Outstanding Investigator Award from NIH (R35 HL144979). P-JJ received support from the Swedish Research Council (2020-01817). Funding from the Wellcome Trust is gratefully acknowledged for VO’D, for LIPID MAPS (203014/Z/16/Z). JH is a F.R.S-FNRS Senior Research Associate and was supported by a F.R.S-FNRS Research Project Grant (PDRT.0111.19). GF has a Merit Award from the AHA and is the McNeil Professor of Translational Medicine and Therapeutics. Supported by 2U54TR001878 from the NIH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.838782/full#supplementary-material
References
- Aharony D., Redkar-Brown D. G., Hubbs S. J., Stein R. L. (1987). Kinetic Studies on the Inactivation of 5-lipoxygenase by 5(S)-hydroperoxyeicosatetraenoic Acid. Prostaglandins 33, 85–100. 10.1016/0090-6980(87)90307-8 [DOI] [PubMed] [Google Scholar]
- Allen-Redpath K., Aldrovandi M., Lauder S. N., Gketsopoulou A., Tyrrell V. J., Slatter D. A., et al. (2019). Phospholipid Membranes Drive Abdominal Aortic Aneurysm Development through Stimulating Coagulation Factor Activity. Proc. Natl. Acad. Sci. U S A. 116, 8038–8047. 10.1073/pnas.1814409116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C. K., Claesson H. E., Rydell-Törmänen K., Swedmark S., Hällgren A., Erjefält J. S. (2008). Mice Lacking 12/15-lipoxygenase Have Attenuated Airway Allergic Inflammation and Remodeling. Am. J. Respir. Cel. Mol. Biol. 39, 648–656. 10.1165/rcmb.2007-0443OC [DOI] [PubMed] [Google Scholar]
- Archambault A. S., Brassard J., Bernatchez É., Martin C., Di Marzo V., Laviolette M., et al. (2022). Human and Mouse Eosinophils Differ in Their Ability to Biosynthesize Eicosanoids, Docosanoids, the Endocannabinoid 2-Arachidonoyl-Glycerol and its Congeners. Cells 11, 141. 10.3390/cells11010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault A. S., Turcotte C., Martin C., Provost V., Larose M. C., Laprise C., et al. (2018). Comparison of Eight 15-lipoxygenase (LO) Inhibitors on the Biosynthesis of 15-LO Metabolites by Human Neutrophils and Eosinophils. PLoS One 13, e0202424. 10.1371/journal.pone.0202424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault A. S., Zaid Y., Rakotoarivelo V., Turcotte C., Dore E., Dubuc I., et al. (2021). High Levels of Eicosanoids and Docosanoids in the Lungs of Intubated COVID-19 Patients. FASEB J. 35, e21666. 10.1096/fj.202100540R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., et al. (2005). Stereochemical Assignment, Antiinflammatory Properties, and Receptor for the omega-3 Lipid Mediator Resolvin E1. J. Exp. Med. 201, 713–722. 10.1084/jem.20042031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. (2007). Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation. J. Immunol. 178, 3912–3917. 10.4049/jimmunol.178.6.3912 [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Park J. C., He R., Ye R. D., Kwak J. Y., Suh P. G., et al. (2003). Differential Signaling of Formyl Peptide Receptor-like 1 by Trp-Lys-Tyr-Met-Val-Met-CONH2 or Lipoxin A4 in Human Neutrophils. Mol. Pharmacol. 64, 721–730. 10.1124/mol.64.3.721 [DOI] [PubMed] [Google Scholar]
- Bailie M. B., Standiford T. J., Laichalk L. L., Coffey M. J., Strieter R., Peters-Golden M. (1996). Leukotriene-deficient Mice Manifest Enhanced Lethality from Klebsiella Pneumonia in Association with Decreased Alveolar Macrophage Phagocytic and Bactericidal Activities. J. Immunol. 157, 5221–5224. [PubMed] [Google Scholar]
- Bang S., Xie Y. K., Zhang Z. J., Wang Z., Xu Z. Z., Ji R. R. (2018). GPR37 Regulates Macrophage Phagocytosis and Resolution of Inflammatory Pain. J. Clin. Invest. 128, 3568–3582. 10.1172/JCI99888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnig C., Cernadas M., Dutile S., Liu X., Perrella M. A., Kazani S., et al. (2013). Lipoxin A4 Regulates Natural Killer Cell and Type 2 Innate Lymphoid Cell Activation in Asthma. Sci. Transl Med. 5, 174ra126. 10.1126/scitranslmed.3004812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Picard S., Drapeau J., Vallerand P. (1982). Metabolism of Arachidonic Acid in Leukocytes: Isolation of a 5,15-Dihydroxy-Eicosatetraenoic Acid. Lipids 17, 676–681. 10.1007/BF02534650 [DOI] [PubMed] [Google Scholar]
- Brancaleone V., Gobbetti T., Cenac N., Le Faouder P., Colom B., Flower R. J., et al. (2013). A Vasculo-Protective Circuit Centered on Lipoxin A4 and Aspirin-Triggered 15-Epi-Lipoxin A4 Operative in Murine Microcirculation. Blood 122, 608–617. 10.1182/blood-2013-04-496661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink C., Dahlen S. E., Drazen J., Evans J. F., Hay D. W., Nicosia S., et al. (2003). International Union of Pharmacology XXXVII. Nomenclature for Leukotriene and Lipoxin Receptors. Pharmacol. Rev. 55, 195–227. 10.1124/pr.55.1.8 [DOI] [PubMed] [Google Scholar]
- Brogliato A. R., Moor A. N., Kesl S. L., Guilherme R. F., Georgii J. L., Peters-Golden M., et al. (2014). Critical Role of 5-lipoxygenase and Heme Oxygenase-1 in Wound Healing. J. Invest. Dermatol. 134, 1436–1445. 10.1038/jid.2013.493 [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Schewe T., Rapoport S. M., Bailey J. M. (1985). Leukotriene Formation by a Purified Reticulocyte Lipoxygenase Enzyme. Conversion of Arachidonic Acid and 15-hydroperoxyeicosatetraenoic Acid to 14,15-leukotriene A4. J. Biol. Chem. 260, 3548–3555. 10.1016/s0021-9258(19)83657-8 [DOI] [PubMed] [Google Scholar]
- Calder P. C. (2020). Eicosapentaenoic and Docosahexaenoic Acid Derived Specialised Pro-resolving Mediators: Concentrations in Humans and the Effects of Age, Sex, Disease and Increased omega-3 Fatty Acid Intake. Biochimie 178, 105–123. 10.1016/j.biochi.2020.08.015 [DOI] [PubMed] [Google Scholar]
- Camp R. D., Fincham N. J. (1985). Inhibition of Ionophore-Stimulated Leukotriene B4 Production in Human Leucocytes by Monohydroxy Fatty Acids. Br. J. Pharmacol. 85, 837–841. 10.1111/j.1476-5381.1985.tb11082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis C., Vachier I., Chanez P., Bousquet J., Godard P. (1996). 5(S),15(S)-dihydroxyeicosatetraenoic Acid and Lipoxin Generation in Human Polymorphonuclear Cells: Dual Specificity of 5-lipoxygenase towards Endogenous and Exogenous Precursors. J. Exp. Med. 183, 1633–1643. 10.1084/jem.183.4.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Le Y., Liu Y., Gong W., Ying G., Huang J., et al. (2010). A Critical Role for the G Protein-Coupled Receptor mFPR2 in Airway Inflammation and Immune Responses. J. Immunol. 184, 3331–3335. 10.4049/jimmunol.0903022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. S., Sheller J. R., Johnson E. N., Funk C. D. (1994). Role of Leukotrienes Revealed by Targeted Disruption of the 5-lipoxygenase Gene. Nature 372, 179–182. 10.1038/372179a0 [DOI] [PubMed] [Google Scholar]
- Chiang N., Dalli J., Colas R. A., Serhan C. N. (2015). Identification of Resolvin D2 Receptor Mediating Resolution of Infections and Organ protection. J. Exp. Med. 212, 1203–1217. 10.1084/jem.20150225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., De La Rosa X., Libreros S., Serhan C. N. (2017). Novel Resolvin D2 Receptor axis in Infectious Inflammation. J. Immunol. 198, 842–851. 10.4049/jimmunol.1601650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Fierro I. M., Gronert K., Serhan C. N. (2000). Activation of Lipoxin A(4) Receptors by Aspirin-Triggered Lipoxins and Select Peptides Evokes Ligand-specific Responses in Inflammation. J. Exp. Med. 191, 1197–1208. 10.1084/jem.191.7.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Fredman G., Backhed F., Oh S. F., Vickery T., Schmidt B. A., et al. (2012). Infection Regulates Pro-resolving Mediators that Lower Antibiotic Requirements. Nature 484, 524–528. 10.1038/nature11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Libreros S., Norris P. C., De La Rosa X., Serhan C. N. (2019). Maresin 1 Activates LGR6 Receptor Promoting Phagocyte Immunoresolvent Functions. J. Clin. Invest. 129, 5294–5311. 10.1172/JCI129448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W., Rovati G. E., et al. (2006). The Lipoxin Receptor ALX: Potent Ligand-specific and Stereoselective Actions In Vivo . Pharmacol. Rev. 58, 463–487. 10.1124/pr.58.3.4 [DOI] [PubMed] [Google Scholar]
- Chiang N., Serhan C. N. (2017). Structural Elucidation and Physiologic Functions of Specialized Pro-resolving Mediators and Their Receptors. Mol. Aspects Med. 58, 114–129. 10.1016/j.mam.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas P., Tolentino K., Primo V., Berry K. Z., Murphy R. C., Chen M., et al. (2006). Cytochrome P-450 4F18 Is the Leukotriene B4 omega-1/omega-2 Hydroxylase in Mouse Polymorphonuclear Leukocytes: Identification as the Functional Orthologue of Human Polymorphonuclear Leukocyte CYP4F3A in the Down-Regulation of Responses to LTB4. J. Biol. Chem. 281, 7189–7196. 10.1074/jbc.M513101200 [DOI] [PubMed] [Google Scholar]
- Christophe T., Karlsson A., Rabiet M. J., Boulay F., Dahlgren C. (2002). Phagocyte Activation by Trp-Lys-Tyr-Met-Val-Met, Acting Through FPRL1/LXA4R, is Not Affected by Lipoxin A4. Scand. J. Immunol. 56, 470–476. 10.1046/j.1365-3083.2002.01149.x [DOI] [PubMed] [Google Scholar]
- Claria J., Serhan C. N. (1995). Aspirin Triggers Previously Undescribed Bioactive Eicosanoids by Human Endothelial Cell-Leukocyte Interactions. Proc. Natl. Acad. Sci. U S A. 92, 9475–9479. 10.1073/pnas.92.21.9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas R. A., Gomez E. A., Dalli J. (2020). Methodologies and Procedures Employed in the Identification and Quantitation of Lipid Mediators via LC-MS/MS. Res. Square 1, 1. 10.21203/rs.3.pex-1147/v1 [DOI] [Google Scholar]
- Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. (2014). Identification and Signature Profiles for Pro-resolving and Inflammatory Lipid Mediators in Human Tissue. Am. J. Physiol. Cel .Physiol. 307, C39–C54. 10.1152/ajpcell.00024.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M., Rossi A., Cuzzocrea S., Patel N. S., Di Paola R., Hadley J., et al. (2004). Reduction of the Multiple Organ Injury and Dysfunction Caused by Endotoxemia in 5-lipoxygenase Knockout Mice and by the 5-lipoxygenase Inhibitor Zileuton. J. Leukoc. Biol. 76, 961–970. 10.1189/jlb.0604338 [DOI] [PubMed] [Google Scholar]
- Conrad D. J., Kühn H., Mulkins M., Highland E., Sigal E. (1992). Specific Inflammatory Cytokines Regulate the Expression of Human Monocyte 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 89, 217–221. 10.1073/pnas.89.1.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray S. N., Gobbetti T., Montero-Melendez T., Mcarthur S., Thompson D., Clark A. J., et al. (2013). Ligand-specific Conformational Change of the G-Protein-Coupled Receptor ALX/FPR2 Determines Proresolving Functional Responses. Proc. Natl. Acad. Sci. U S A. 110, 18232–18237. 10.1073/pnas.1308253110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus T., Witztum J. L., Rader D. J., Tangirala R., Fazio S., Linton M. F., et al. (1999). Disruption of the 12/15-lipoxygenase Gene Diminishes Atherosclerosis in Apo E-Deficient Mice. J. Clin. Invest. 103, 1597–1604. 10.1172/JCI5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J., Colas R. A., Quintana C., Barragan-Bradford D., Hurwitz S., Levy B. D., et al. (2017). Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations with Survival and Clinical Outcomes. Crit. Care Med. 45, 58–68. 10.1097/CCM.0000000000002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J., Serhan C. N. (2012). Specific Lipid Mediator Signatures of Human Phagocytes: Microparticles Stimulate Macrophage Efferocytosis and Pro-resolving Mediators. Blood 120, e60–72. 10.1182/blood-2012-04-423525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J., Winkler J. W., Colas R. A., Arnardottir H., Cheng C. Y., Chiang N., et al. (2013). Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Potent Immunoresolvents. Chem. Biol. 20, 188–201. 10.1016/j.chembiol.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A. P., Alexander S. P., Sharman J. L., Pawson A. J., Benson H. E., Monaghan A. E., et al. (2013). International Union of Basic and Clinical Pharmacology. LXXXVIII. G Protein-Coupled Receptor List: Recommendations for New Pairings with Cognate Ligands. Pharmacol. Rev. 65, 967–986. 10.1124/pr.112.007179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deems R., Buczynski M. W., Bowers-Gentry R., Harkewicz R., Dennis E. A. (2007). Detection and Quantitation of Eicosanoids via High Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. Methods Enzymol. 432, 59–82. 10.1016/S0076-6879(07)32003-X [DOI] [PubMed] [Google Scholar]
- Dixon R. A. F., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., et al. (1990). Requirement of a 5-Lipoxygenase-Activating Protein for Leukotriene Synthesis. Nature 343, 282–284. 10.1038/343282a0 [DOI] [PubMed] [Google Scholar]
- Dufton N., Hannon R., Brancaleone V., Dalli J., Patel H. B., Gray M., et al. (2010). Anti-inflammatory Role of the Murine Formyl-Peptide Receptor 2: Ligand-specific Effects on Leukocyte Responses and Experimental Inflammation. J. Immunol. 184, 2611–2619. 10.4049/jimmunol.0903526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumlao D. S., Buczynski M. W., Norris P. C., Harkewicz R., Dennis E. A. (2011). High-throughput Lipidomic Analysis of Fatty Acid Derived Eicosanoids and N-Acylethanolamines. Biochim. Biophys. Acta 1811, 724–736. 10.1016/j.bbalip.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert R., Cumbana R., Lehmann C., Kutzner L., Toewe A., Ferreiros N., et al. (2020). Long-term Stimulation of Toll-like Receptor-2 and -4 Upregulates 5-LO and 15-LO-2 Expression Thereby Inducing a Lipid Mediator Shift in Human Monocyte-Derived Macrophages. Biochim. Biophys. Acta 1865, 158702. 10.1016/j.bbalip.2020.158702 [DOI] [PubMed] [Google Scholar]
- Edenius C., Stenke L., Lindgren J. A. (1991). On the Mechanism of Transcellular Lipoxin Formation in Human Platelets and Granulocytes. Eur. J. Biochem. 199, 401–409. 10.1111/j.1432-1033.1991.tb16137.x [DOI] [PubMed] [Google Scholar]
- Emerson M. R., Levine S. M. (2004). Experimental Allergic Encephalomyelitis Is Exacerbated in Mice Deficient for 12/15-lipoxygenase or 5-lipoxygenase. Brain Res. 1021, 140–145. 10.1016/j.brainres.2004.06.045 [DOI] [PubMed] [Google Scholar]
- European medicines Agency (2011). “Guideline on Bioanalytical Method Validation,” in EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** 1. (Commitee for Medicinal Products for Human Use CHMP; ). [Google Scholar]
- Fedirko V., Mckeown-Eyssen G., Serhan C. N., Barry E. L., Sandler R. S., Figueiredo J. C., et al. (2017). Plasma Lipoxin A4 and Resolvin D1 Are Not Associated with Reduced Adenoma Risk in a Randomized Trial of Aspirin to Prevent colon Adenomas. Mol. Carcinog. 56, 1977–1983. 10.1002/mc.22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiros N., Homann J., Labocha S., Grossmann N., Hahn J. S., Brüne B., et al. (2014). Lipoxin A(4): Problems with its Determination Using Reversed Phase Chromatography-Tandem Mass Spectrometry and Confirmation with Chiral Chromatography. Talanta 127, 82–87. 10.1016/j.talanta.2014.03.051 [DOI] [PubMed] [Google Scholar]
- Fiore S., Maddox J. F., Perez H. D., Serhan C. N. (1994). Identification of a Human cDNA Encoding a Functional High Affinity Lipoxin A4 Receptor. J. Exp. Med. 180, 253–260. 10.1084/jem.180.1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S., Ryeom S. W., Weller P. F., Serhan C. N. (1992). Lipoxin Recognition Sites. Specific Binding of Labeled Lipoxin A4 with Human Neutrophils. J. Biol. Chem. 267, 16168–16176. 10.1016/s0021-9258(18)41982-5 [DOI] [PubMed] [Google Scholar]
- Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., et al. (2014). Dietary omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-Epoxygenase Pathway. J. Lipid Res. 55, 1150–1164. 10.1194/jlr.M047357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak M. B., Koenis D. S., Sobrino A., Smith J., Pistorius K., Palmas F., et al. (2020). GPR101 Mediates the Pro-resolving Actions of RvD5n-3 DPA in Arthritis and Infections. J. Clin. Invest. 130, 359–373. 10.1172/JCI131609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand N., Mancuso P., Serezani C. H., Brock T. G. (2007). Leukotrienes: Mediators that Have Been Typecast as Villains. Cell. Mol. Life Sci. 64, 2657–2670. 10.1007/s00018-007-7228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman H., Dahlgren C. (2009). Lipoxin A(4) Metabolites/analogues from Two Commercial Sources Have No Effects on TNF-Alpha-Mediated Priming or Activation through the Neutrophil Formyl Peptide Receptors. Scand. J. Immunol. 70, 396–402. 10.1111/j.1365-3083.2009.02311.x [DOI] [PubMed] [Google Scholar]
- Forsman H., Onnheim K., Andreasson E., Dahlgren C. (2011). What Formyl Peptide Receptors, if Any, Are Triggered by Compound 43 and Lipoxin A4? Scand. J. Immunol. 74, 227–234. 10.1111/j.1365-3083.2011.02570.x [DOI] [PubMed] [Google Scholar]
- Foster S. R., Hauser A. S., Vedel L., Strachan R. T., Huang X. P., Gavin A. C., et al. (2019). Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 179, 895–908 e821. 10.1016/j.cell.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel R. N. (2005). Lipid Oxidation. 2nd edition. Elsevier, 1–455. [Google Scholar]
- Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., et al. (2016). An Imbalance between Specialized Pro-resolving Lipid Mediators and Pro-inflammatory Leukotrienes Promotes Instability of Atherosclerotic Plaques. Nat. Commun. 7, 12859. 10.1038/ncomms12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y., Zhang S., Wang J., Xia F., Wan J. B., Lu J., et al. (2020). Dual Modulation of Formyl Peptide Receptor 2 by Aspirin-Triggered Lipoxin Contributes to its Anti-inflammatory Activity. FASEB J. 34, 6920–6933. 10.1096/fj.201903206R [DOI] [PubMed] [Google Scholar]
- Giera M., Ioan-Facsinay A., Toes R., Gao F., Dalli J., Deelder A. M., et al. (2012). Lipid and Lipid Mediator Profiling of Human Synovial Fluid in Rheumatoid Arthritis Patients by Means of LC-MS/MS. Biochim. Biophys. Acta 1821, 1415–1424. 10.1016/j.bbalip.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladine C., Ostermann A. I., Newman J. W., Schebb N. H. (2019). MS-based Targeted Metabolomics of Eicosanoids and Other Oxylipins: Analytical and Inter-individual Variabilities. Free Radic. Biol. Med. 144, 72–89. 10.1016/j.freeradbiomed.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Gomez E. A., Colas R. A., Souza P. R., Hands R., Lewis M. J., Bessant C., et al. (2020). Blood Pro-resolving Mediators Are Linked with Synovial Pathology and Are Predictive of DMARD Responsiveness in Rheumatoid Arthritis. Nat. Commun. 11, 5420. 10.1038/s41467-020-19176-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. (2003). Alternative Activation of Macrophages. Nat. Rev. Immunol. 3, 23–35. 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- Gottschall H., Schmöcker C., Hartmann D., Rohwer N., Rund K., Kutzner L., et al. (2018). Aspirin Alone and Combined with a Statin Suppresses Eicosanoid Formation in Human colon Tissue. J. Lipid Res. 59, 864–871. 10.1194/jlr.M078725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Barbour S., Horn T., Carlos J., Raskatov J. A., Holman T. R. (2016). Strict Regiospecificity of Human Epithelial 15-lipoxygenase-2 Delineates its Transcellular Synthesis Potential. Biochemistry 55, 2832–2840. 10.1021/acs.biochem.5b01339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Freedman C., Tena J., Tourdot B. E., Liu B., Holinstat M., et al. (2018). 5S,15S-Dihydroperoxyeicosatetraenoic Acid (5,15-diHpETE) as a Lipoxin Intermediate: Reactivity and Kinetics with Human Leukocyte 5-lipoxygenase, Platelet 12-lipoxygenase, and Reticulocyte 15-lipoxygenase-1. Biochemistry 57, 6726–6734. 10.1021/acs.biochem.8b00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Laniado Schwartzman M. (2005). A Role for the Mouse 12/15-lipoxygenase Pathway in Promoting Epithelial Wound Healing and Host Defense. J. Biol. Chem. 280, 15267–15278. 10.1074/jbc.M410638200 [DOI] [PubMed] [Google Scholar]
- Haitina T., Fredriksson R., Foord S. M., Schioth H. B., Gloriam D. E. (2009). The G Protein-Coupled Receptor Subset of the Dog Genome Is More Similar to that in Humans Than Rodents. BMC Genomics 10, 24. 10.1186/1471-2164-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J., Ferreiros N., Pirotte B., Geisslinger G., Offermanns S. (2013). Heterologously Expressed Formyl Peptide Receptor 2 (FPR2/ALX) Does Not Respond to Lipoxin A(4). Biochem. Pharmacol. 85, 1795–1802. 10.1016/j.bcp.2013.04.019 [DOI] [PubMed] [Google Scholar]
- Hartling I., Cremonesi A., Osuna E., Lou P. H., Lucchinetti E., Zaugg M., et al. (2021). Quantitative Profiling of Inflammatory and Pro-resolving Lipid Mediators in Human Adolescents and Mouse Plasma Using UHPLC-MS/MS. Clin. Chem. Lab. Med. 59, 1811–1823. 10.1515/cclm-2021-0644 [DOI] [PubMed] [Google Scholar]
- He H. Q., Ye R. D. (2017). The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 22, 455. 10.3390/molecules22030455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Maclouf J., Murphy R. C., Henson P. M. (1992). Reversible Membrane Association of Neutrophil 5-lipoxygenase Is Accompanied by Retention of Activity and a Change in Substrate Specificity. J. Biol. Chem. 267, 22048–22053. 10.1016/s0021-9258(18)41633-x [DOI] [PubMed] [Google Scholar]
- Hodges R. R., Li D., Shatos M. A., Bair J. A., Lippestad M., Serhan C. N., et al. (2017). Lipoxin A4 Activates ALX/FPR2 Receptor to Regulate Conjunctival Goblet Cell Secretion. Mucosal Immunol. 10, 46–57. 10.1038/mi.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., et al. (2012). Identification and Structure Determination of Novel Anti-inflammatory Mediator Resolvin E3, 17,18-dihydroxyeicosapentaenoic Acid. J. Biol. Chem. 287, 10525–10534. 10.1074/jbc.M112.340612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y., Itagaki M., Ito Y., Naoe S., Kojima K., Ikeguchi M., et al. (2018). Comprehensive Analysis of the Mouse Cytochrome P450 Family Responsible for omega-3 Epoxidation of Eicosapentaenoic Acid. Sci. Rep. 8, 7954. 10.1038/s41598-018-26325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jisaka M., Kim R. B., Boeglin W. E., Brash A. R. (2000). Identification of Amino Acid Determinants of the Positional Specificity of Mouse 8S-Lipoxygenase and Human 15S-Lipoxygenase-2. J. Biol. Chem. 275, 1287–1293. 10.1074/jbc.275.2.1287 [DOI] [PubMed] [Google Scholar]
- Johnson E. N., Brass L. F., Funk C. D. (1998). Increased Platelet Sensitivity to ADP in Mice Lacking Platelet-type 12-lipoxygenase. Proc. Natl. Acad. Sci. U S A. 95, 3100–3105. 10.1073/pnas.95.6.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. N., Nanney L. B., Virmani J., Lawson J. A., Funk C. D. (1999). Basal Transepidermal Water Loss Is Increased in Platelet-type 12-lipoxygenase Deficient Mice. J. Invest. Dermatol. 112, 861–865. 10.1046/j.1523-1747.1999.00595.x [DOI] [PubMed] [Google Scholar]
- Jonasdottir H. S., Brouwers H., Kwekkeboom J. C., Van Der Linden H. M. J., Huizinga T., Kloppenburg M., et al. (2017). Targeted Lipidomics Reveals Activation of Resolution Pathways in Knee Osteoarthritis in Humans. Osteoarthritis Cartilage 25, 1150–1160. 10.1016/j.joca.2017.01.018 [DOI] [PubMed] [Google Scholar]
- Jónasdóttir H. S., Ioan-Facsinay A., Kwekkeboom J., Brouwers H., Zuurmond A. M., Toes R., (2015). An Advanced LC–MS/MS Platform for the Analysis of Specialized Pro-resolving Lipid Mediators. Chromatographia 78, 391–401. 10.1007/s10337-014-2779-5 [DOI] [Google Scholar]
- Jonasdottir H. S., Papan C., Fabritz S., Balas L., Durand T., Hardardottir I., et al. (2015). Differential Mobility Separation of Leukotrienes and Protectins. Anal. Chem. 87, 5036–5040. 10.1021/acs.analchem.5b00786 [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Gerstmeier J., Pace S., Bilancia R., Rao Z., Borner F., et al. (2020). Staphylococcus Aureus-Derived Alpha-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution. Cell Rep 33, 108247. 10.1016/j.celrep.2020.108247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Y. B., Pratico D. (2014). The 5-lipoxygenase Pathway: Oxidative and Inflammatory Contributions to the Alzheimer's Disease Phenotype. Front. Cel. Neurosci. 8, 436. 10.3389/fncel.2014.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargman S., Rouzer C. A. (1989). Studies on the Regulation, Biosynthesis, and Activation of 5-lipoxygenase in Differentiated HL60 Cells. J. Biol. Chem. 264, 13313–13320. 10.1016/s0021-9258(18)51630-6 [DOI] [PubMed] [Google Scholar]
- Koenis D. S., Beegun I., Jouvene C. C., Aguirre G. A., Souza P. R., Gonzalez-Nunez M., et al. (2021). Disrupted Resolution Mechanisms Favor Altered Phagocyte Responses in COVID-19. Circ. Res. 129, e54–e71. 10.1161/CIRCRESAHA.121.319142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., et al. (2006). Identification of N-Arachidonylglycine as the Endogenous Ligand for Orphan G-Protein-Coupled Receptor GPR18. Biochem. Biophys. Res. Commun. 347, 827–832. 10.1016/j.bbrc.2006.06.175 [DOI] [PubMed] [Google Scholar]
- Krieg P., Kinzig A., Heidt M., Marks F., Fürstenberger G. (1998). cDNA Cloning of a 8-lipoxygenase and a Novel Epidermis-type Lipoxygenase from Phorbol Ester-Treated Mouse Skin. Biochim. Biophys. Acta 1391, 7–12. 10.1016/s0005-2760(97)00214-2 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C. N. (2012). Resolvin D1 Receptor Stereoselectivity and Regulation of Inflammation and Proresolving microRNAs. Am. J. Pathol. 180, 2018–2027. 10.1016/j.ajpath.2012.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C. H., Yang R., et al. (2010). Resolvin D1 Binds Human Phagocytes with Evidence for Proresolving Receptors. Proc. Natl. Acad. Sci. U S A. 107, 1660–1665. 10.1073/pnas.0907342107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson R. P., Benonisdottir S., Davidsson O. B., Oddsson A., Tragante V., Sigurdsson J. K., et al. (2019). A Loss-Of-Function Variant in ALOX15 Protects against Nasal Polyps and Chronic Rhinosinusitis. Nat. Genet. 51, 267–276. 10.1038/s41588-018-0314-6 [DOI] [PubMed] [Google Scholar]
- Kronke G., Katzenbeisser J., Uderhardt S., Zaiss M. M., Scholtysek C., Schabbauer G., et al. (2009). 12/15-lipoxygenase Counteracts Inflammation and Tissue Damage in Arthritis. J. Immunol. 183, 3383–3389. 10.4049/jimmunol.0900327 [DOI] [PubMed] [Google Scholar]
- Kronke G., Reich N., Scholtysek C., Akhmetshina A., Uderhardt S., Zerr P., et al. (2012). The 12/15-lipoxygenase Pathway Counteracts Fibroblast Activation and Experimental Fibrosis. Ann. Rheum. Dis. 71, 1081–1087. 10.1136/annrheumdis-2011-200745 [DOI] [PubMed] [Google Scholar]
- Kroschwald S., Chiu C. Y., Heydeck D., Rohwer N., Gehring T., Seifert U., et al. (2018). Female Mice Carrying a Defective Alox15 Gene Are Protected from Experimental Colitis via Sustained Maintenance of the Intestinal Epithelial Barrier Function. Biochim. Biophys. Acta 1863, 866–880. 10.1016/j.bbalip.2018.04.019 [DOI] [PubMed] [Google Scholar]
- Kühn H., Wiesner R., Alder L., Fitzsimmons B. J., Rokach J., Brash A. R. (1987). Formation of Lipoxin B by the Pure Reticulocyte Lipoxygenase via Sequential Oxygenation of the Substrate. Eur. J. Biochem. 169, 593–601. 10.1111/j.1432-1033.1987.tb13650.x [DOI] [PubMed] [Google Scholar]
- Kühn H., Wiesner R., Stender H. (1984). The Formation of Products Containing a Conjugated Tetraenoic System by Pure Reticulocyte Lipoxygenase. FEBS Lett. 177, 255–259. 10.1016/0014-5793(84)81294-6 [DOI] [PubMed] [Google Scholar]
- Kutzner L., Goloshchapova K., Heydeck D., Stehling S., Kuhn H., Schebb N. H. (2017). Mammalian ALOX15 Orthologs Exhibit Pronounced Dual Positional Specificity with Docosahexaenoic Acid. Biochim. Biophys. Acta 1862, 666–675. 10.1016/j.bbalip.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Kutzner L., Goloshchapova K., Rund K. M., Jubermann M., Blum M., Rothe M., et al. (2020). Human Lipoxygenase Isoforms Form Complex Patterns of Double and Triple Oxygenated Compounds from Eicosapentaenoic Acid. Biochim. Biophys. Acta 1865, 158806. 10.1016/j.bbalip.2020.158806 [DOI] [PubMed] [Google Scholar]
- Kutzner L., Rund K. M., Ostermann A. I., Hartung N. M., Galano J. M., Balas L., et al. (2019). Development of an Optimized LC-MS Method for the Detection of Specialized Pro-resolving Mediators in Biological Samples. Front. Pharmacol. 10, 169. 10.3389/fphar.2019.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna-Fernandez A., Checa A., Carracedo M., Artiach G., Petri M. H., Baumgartner R., et al. (2018). ERV1/ChemR23 Signaling Protects against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages. Circulation 138, 1693–1705. 10.1161/CIRCULATIONAHA.117.032801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam B. K., Hirai A., Yoshida S., Tamura Y., Wong P. Y. (1987). Transformation of 15-hydroperoxyeicosapentaenoic Acid to Lipoxin A5 and B5, Mono- and Dihydroxyeicosapentaenoic Acids by Porcine Leukocytes. Biochim. Biophys. Acta 917, 398–405. 10.1016/0005-2760(87)90118-4 [DOI] [PubMed] [Google Scholar]
- Laschet C., Dupuis N., Hanson J. (2018). The G Protein-Coupled Receptors Deorphanization Landscape. Biochem. Pharmacol. 153, 62–74. 10.1016/j.bcp.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Lee H. N., Surh Y. J. (2013). Resolvin D1-Mediated NOX2 Inactivation Rescues Macrophages Undertaking Efferocytosis from Oxidative Stress-Induced Apoptosis. Biochem. Pharmacol. 86, 759–769. 10.1016/j.bcp.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., et al. (1985). Effect of Dietary Enrichment with Eicosapentaenoic and Docosahexaenoic Acids on In Vitro Neutrophil and Monocyte Leukotriene Generation and Neutrophil Function. N. Engl. J. Med. 312, 1217–1224. 10.1056/NEJM198505093121903 [DOI] [PubMed] [Google Scholar]
- Lehmann C., Homann J., Ball A. K., Blöcher R., Kleinschmidt T. K., Basavarajappa D., et al. (2015). Lipoxin and Resolvin Biosynthesis Is Dependent on 5-lipoxygenase Activating Protein. FASEB J. 29, 5029–5043. 10.1096/fj.15-275487 [DOI] [PubMed] [Google Scholar]
- Leung K. S., Galano J. M., Durand T., Lee J. C. (2015). Current Development in Non-enzymatic Lipid Peroxidation Products, Isoprostanoids and Isofuranoids, in Novel Biological Samples. Free Radic. Res. 49, 816–826. 10.3109/10715762.2014.960867 [DOI] [PubMed] [Google Scholar]
- Li J. L., Liang Y. L., Wang Y. J. (2019). Knockout of ALOX12 Protects against Spinal Cord Injury-Mediated Nerve Injury by Inhibition of Inflammation and Apoptosis. Biochem. Biophys. Res. Commun. 516, 991–998. 10.1016/j.bbrc.2019.06.118 [DOI] [PubMed] [Google Scholar]
- Lyngstadaas A. V., Olsen M. V., Bair J. A., Hodges R. R., Utheim T. P., Serhan C. N., et al. (2021). Pro-resolving Mediator Annexin A1 Regulates Intracellular Ca(2+) and Mucin Secretion in Cultured Goblet Cells Suggesting a New Use in Inflammatory Conjunctival Diseases. Front. Immunol. 12, 618653. 10.3389/fimmu.2021.618653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Hachicha M., Takano T., Petasis N. A., Fokin V. V., Serhan C. N. (1997). Lipoxin A4 Stable Analogs Are Potent Mimetics that Stimulate Human Monocytes and THP-1 Cells via a G-Protein-Linked Lipoxin A4 Receptor. J. Biol. Chem. 272, 6972–6978. 10.1074/jbc.272.11.6972 [DOI] [PubMed] [Google Scholar]
- Maderna P., Cottell D. C., Toivonen T., Dufton N., Dalli J., Perretti M., et al. (2010). FPR2/ALX Receptor Expression and Internalization Are Critical for Lipoxin A4 and Annexin-Derived Peptide-Stimulated Phagocytosis. FASEB J. 24, 4240–4249. 10.1096/fj.10-159913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J., Liu W., Fang Y., Zhang S., Qiu Q., Yang Y., et al. (2018). The Atheroprotective Role of Lipoxin A4 Prevents oxLDL-Induced Apoptotic Signaling in Macrophages via JNK Pathway. Atherosclerosis 278, 259–268. 10.1016/j.atherosclerosis.2018.09.025 [DOI] [PubMed] [Google Scholar]
- Mainka M., George S., Angioni C., Ebert R., Goebel T., Kampschulte N., et al. (2021). On the Biosynthesis of Specialized Pro-resolving Mediators in Human Neutrophils and the Influence of Cell Integrity. Biochim. Biophys. Acta Mol. Cel Biol. Lipids 1867 (3), 159093. 10.1016/j.bbalip.2021.159093 [DOI] [PubMed] [Google Scholar]
- Mancini J. A., Waterman H., Riendeau D. (1998). Cellular Oxygenation of 12-hydroxyeicosatetraenoic Acid and 15-hydroxyeicosatetraenoic Acid by 5-lipoxygenase Is Stimulated by 5-Lipoxygenase-Activating Protein. J. Biol. Chem. 273, 32842–32847. 10.1074/jbc.273.49.32842 [DOI] [PubMed] [Google Scholar]
- Marbach-Breitruck E., Rohwer N., Infante-Duarte C., Romero-Suarez S., Labuz D., Machelska H., et al. (2021). Knock-in Mice Expressing a 15-lipoxygenating Alox5 Mutant Respond Differently to Experimental Inflammation Than Reported Alox5(-/-) Mice. Metabolites 11, 698. 10.3390/metabo11100698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente M., Ferre N., Titos E., Horrillo R., Gonzalez-Periz A., Moran-Salvador E., et al. (2010). Disruption of the 12/15-lipoxygenase Gene (Alox15) Protects Hyperlipidemic Mice from Nonalcoholic Fatty Liver Disease. Hepatology 52, 1980–1991. 10.1002/hep.23928 [DOI] [PubMed] [Google Scholar]
- Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. (2012). Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood Following N-3 Fatty Acid Supplementation. Clin. Chem. 58, 1476–1484. 10.1373/clinchem.2012.190199 [DOI] [PubMed] [Google Scholar]
- Mazaleuskaya L. L., Lawson J. A., Li X., Grant G., Mesaros C., Grosser T., et al. (2016). A Broad-Spectrum Lipidomics Screen of Antiinflammatory Drug Combinations in Human Blood. JCI Insight 1, e87031. 10.1172/jci.insight.87031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchugh D., Hu S. S., Rimmerman N., Juknat A., Vogel Z., Walker J. M., et al. (2010). N-arachidonoyl glycine, an Abundant Endogenous Lipid, Potently Drives Directed Cellular Migration through GPR18, the Putative Abnormal Cannabidiol Receptor. BMC Neurosci. 11, 44. 10.1186/1471-2202-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchugh D., Page J., Dunn E., Bradshaw H. B. (2012). Delta(9) -Tetrahydrocannabinol and N-Arachidonyl glycine Are Full Agonists at GPR18 Receptors and Induce Migration in Human Endometrial HEC-1B Cells. Br. J. Pharmacol. 165, 2414–2424. 10.1111/j.1476-5381.2011.01497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian M., Allayee H., Wong J., Shi W., Wang X. P., Shaposhnik Z., et al. (2002). Identification of 5-lipoxygenase as a Major Gene Contributing to Atherosclerosis Susceptibility in Mice. Circ. Res. 91, 120–126. 10.1161/01.res.0000028008.99774.7f [DOI] [PubMed] [Google Scholar]
- Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008). Atherosclerosis: Evidence for Impairment of Resolution of Vascular Inflammation Governed by Specific Lipid Mediators. FASEB J. 22, 3595–3606. 10.1096/fj.08-112201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J., Yokokura Y., Moro K., Arai H., Fukunaga K., Arita M. (2021). 12/15-Lipoxygenase Regulates IL-33-Induced Eosinophilic Airway Inflammation in Mice. Front. Immunol. 12, 687192. 10.3389/fimmu.2021.687192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A. P., Soledade E., Pinheiro C. S., Dellatorre-Teixeira L., Oliveira G. P., Oliveira M. G., et al. (2014). Pivotal Role of the 5-lipoxygenase Pathway in Lung Injury after Experimental Sepsis. Am. J. Respir. Cel. Mol. Biol. 50, 87–95. 10.1165/rcmb.2012-0525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montford J. R., Bauer C., Dobrinskikh E., Hopp K., Levi M., Weiser-Evans M., et al. (2019). Inhibition of 5-lipoxygenase Decreases Renal Fibrosis and Progression of Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 316, F732–F742. 10.1152/ajprenal.00262.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel J. A., Conrad D. J., Ueki I. F., Schuster A., Sigal E. (1991). Immunocytochemical Localization of Arachidonate 15-lipoxygenase in Erythrocytes, Leukocytes and Airway Cells. J. Clin. Invest. 87, 1139–1145. 10.1172/JCI115110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling L. V., Dalli J., Flower R. J., Serhan C. N., Perretti M. (2012). Resolvin D1 Limits Polymorphonuclear Leukocyte Recruitment to Inflammatory Loci: Receptor-dependent Actions. Arterioscler. Thromb. Vasc. Biol. 32, 1970–1978. 10.1161/ATVBAHA.112.249508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P. C., Libreros S., Serhan C. N. (2019). Resolution Metabolomes Activated by Hypoxic Environment. Sci. Adv. 5, eaax4895. 10.1126/sciadv.aax4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell V. B., Schebb N. H., Milne G. L., Murphy M. H., Thomas C. P., Steinhilber D., et al. (2021). Failure to Apply Standard Limit-Of-Detection or Limit-Of-Quantitation Criteria to Specialized Pro-resolving Mediator Analysis Incorrectly Characterizes Their Presence in Biological Samples. Zenodo 1, 1. 10.5281/zenodo.5766267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Ishihara T., Isobe Y., Kato T., Kuba K., Imai Y., et al. (2020). Eosinophils Promote Corneal Wound Healing via the 12/15-lipoxygenase Pathway. FASEB J. 34, 12492–12501. 10.1096/fj.202000483R [DOI] [PubMed] [Google Scholar]
- Oh S. F., Dona M., Fredman G., Krishnamoorthy S., Irimia D., Serhan C. N. (2012). Resolvin E2 Formation and Impact in Inflammation Resolution. J. Immunol. 188, 4527–4534. 10.4049/jimmunol.1103652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. (2011). Pro-resolving Actions and Stereoselective Biosynthesis of 18S E-Series Resolvins in Human Leukocytes and Murine Inflammation. J. Clin. Invest. 121, 569–581. 10.1172/JCI42545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann A. I., West A. L., Schoenfeld K., Browning L. M., Walker C. G., Jebb S. A., et al. (2019). Plasma Oxylipins Respond in a Linear Dose-Response Manner with Increased Intake of EPA and DHA: Results from a Randomized Controlled Trial in Healthy Humans. Am. J. Clin. Nutr. 109, 1251–1263. 10.1093/ajcn/nqz016 [DOI] [PubMed] [Google Scholar]
- Ostermann A. I., Willenberg I., Schebb N. H. (2015). Comparison of Sample Preparation Methods for the Quantitative Analysis of Eicosanoids and Other Oxylipins in Plasma by Means of LC-MS/MS. Anal. Bioanal. Chem. 407, 1403–1414. 10.1007/s00216-014-8377-4 [DOI] [PubMed] [Google Scholar]
- Pamplona F. A., Ferreira J., Menezes De Lima O., Jr., Duarte F. S., Bento A. F., Forner S., et al. (2012). Anti-inflammatory Lipoxin A4 Is an Endogenous Allosteric Enhancer of CB1 Cannabinoid Receptor. Proc. Natl. Acad. Sci. U S A. 109, 21134–21139. 10.1073/pnas.1202906109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. S., Cuzzocrea S., Chatterjee P. K., Di Paola R., Sautebin L., Britti D., et al. (2004). Reduction of Renal Ischemia-Reperfusion Injury in 5-lipoxygenase Knockout Mice and by the 5-lipoxygenase Inhibitor Zileuton. Mol. Pharmacol. 66, 220–227. 10.1124/mol.66.2.220 [DOI] [PubMed] [Google Scholar]
- Pedersen T. L., Gray I. J., Newman J. W. (2021). Plasma and Serum Oxylipin, Endocannabinoid, Bile Acid, Steroid, Fatty Acid and Nonsteroidal Anti-inflammatory Drug Quantification in a 96-well Plate Format. Anal. Chim. Acta 1143, 189–200. 10.1016/j.aca.2020.11.019 [DOI] [PubMed] [Google Scholar]
- Perry S. C., Horn T., Tourdot B. E., Yamaguchi A., Kalyanaraman C., Conrad W. S., et al. (2020a). Role of Human 15-Lipoxygenase-2 in the Biosynthesis of the Lipoxin Intermediate, 5S,15S-diHpETE, Implicated with the Altered Positional Specificity of Human 15-Lipoxygenase-1. Biochemistry 59, 4118–4130. 10.1021/acs.biochem.0c00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. C., Kalyanaraman C., Tourdot B. E., Conrad W. S., Akinkugbe O., Freedman J. C., et al. (2020b). 15-Lipoxygenase-1 Biosynthesis of 7S,14S-diHDHA Implicates 15-lipoxygenase-2 in Biosynthesis of Resolvin D5. J. Lipid Res. 61, 1087–1103. 10.1194/jlr.RA120000777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M., Bailie M., Marshall T., Wilke C., Phan S. H., Toews G. B., et al. (2002). Protection from Pulmonary Fibrosis in Leukotriene-Deficient Mice. Am. J. Respir. Crit. Care Med. 165, 229–235. 10.1164/ajrccm.165.2.2104050 [DOI] [PubMed] [Google Scholar]
- Peters-Golden M., Canetti C., Mancuso P., Coffey M. J. (2005). Leukotrienes: Underappreciated Mediators of Innate Immune Responses. J. Immunol. 174, 589–594. 10.4049/jimmunol.174.2.589 [DOI] [PubMed] [Google Scholar]
- Petri M. H., Laguna-Fernandez A., Arnardottir H., Wheelock C. E., Perretti M., Hansson G. K., et al. (2017). Aspirin-triggered Lipoxin A4 Inhibits Atherosclerosis Progression in Apolipoprotein E(-/-) Mice. Br. J. Pharmacol. 174, 4043–4054. 10.1111/bph.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon G. P., Kandouz M., Meram A., Honn K. V. (2002). Mechanisms Controlling Cell Cycle Arrest and Induction of Apoptosis after 12-lipoxygenase Inhibition in Prostate Cancer Cells. Cancer Res. 62, 2721–2727. [PubMed] [Google Scholar]
- Planaguma A., Domenech T., Jover I., Ramos I., Sentellas S., Malhotra R., et al. (2013). Lack of Activity of 15-Epi-Lipoxin A(4) on FPR2/ALX and CysLT1 Receptors in Interleukin-8-Driven Human Neutrophil Function. Clin. Exp. Immunol. 173, 298–309. 10.1111/cei.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeckel D., Funk C. D. (2010). The 5-lipoxygenase/leukotriene Pathway in Preclinical Models of Cardiovascular Disease. Cardiovasc. Res. 86, 243–253. 10.1093/cvr/cvq016 [DOI] [PubMed] [Google Scholar]
- Pratt V. C., Watanabe S., Bruera E., Mackey J., Clandinin M. T., Baracos V. E., et al. (2002). Plasma and Neutrophil Fatty Acid Composition in Advanced Cancer Patients and Response to Fish Oil Supplementation. Br. J. Cancer 87, 1370–1378. 10.1038/sj.bjc.6600659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O., Yamashita T., Armando A. M., Dennis E. A., Palinski W. (2011). Effect of Gestational Hypercholesterolemia and Maternal Immunization on Offspring Plasma Eicosanoids. Am. J. Obstet. Gynecol. 205, 156 e115–125. 10.1016/j.ajog.2011.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2007). 5-Lipoxygenase: Regulation of Expression and Enzyme Activity. Trends Biochem. Sci. 32, 332–341. 10.1016/j.tibs.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Rao Z., Pace S., Jordan P. M., Bilancia R., Troisi F., Borner F., et al. (2019). Vacuolar (H(+))-ATPase Critically Regulates Specialized Proresolving Mediator Pathways in Human M2-like Monocyte-Derived Macrophages and Has a Crucial Role in Resolution of Inflammation. J. Immunol. 203, 1031–1043. 10.4049/jimmunol.1900236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen A. F., Primdahl K. G., Shay A. E., Serhan C. N., Hansen T. V., Aursnes M. (2021). Stereoselective Synthesis and Structural Confirmation of the Specialized Pro-resolving Mediator Resolvin E4. J. Org. Chem. 86, 3535–3545. 10.1021/acs.joc.0c02913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N., Chiu C. Y., Huang D., Smyl C., Rothe M., Rund K. M., et al. (2021). Omega-3 Fatty Acids Protect from Colitis via an Alox15-Derived Eicosanoid. FASEB J. 35, e21491. 10.1096/fj.202002340RR [DOI] [PubMed] [Google Scholar]
- Rohwer N., Kühl A. A., Ostermann A. I., Hartung N. M., Schebb N. H., Zopf D., et al. (2020). Effects of Chronic Low-Dose Aspirin Treatment on Tumor Prevention in Three Mouse Models of Intestinal Tumorigenesis. Cancer Med. 9, 2535–2550. 10.1002/cam4.2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Cao Q., Liu M., Seo J., Jia L., Boudyguina E., et al. (2012). Macrophage 12/15 Lipoxygenase Expression Increases Plasma and Hepatic Lipid Levels and Exacerbates Atherosclerosis. J. Lipid Res. 53, 686–695. 10.1194/jlr.M022723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint J., Nadler J. L., Ley K., Zarbock A. (2012). Eliminating or Blocking 12/15-lipoxygenase Reduces Neutrophil Recruitment in Mouse Models of Acute Lung Injury. Crit. Care 16, R166. 10.1186/cc11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Kargman S. (1988). Translocation of 5-lipoxygenase to the Membrane in Human Leukocytes Challenged with Ionophore A23187. J. Biol. Chem. 263, 10980–10988. 10.1016/s0021-9258(18)38066-9 [DOI] [PubMed] [Google Scholar]
- Russell R., Gori I., Pellegrini C., Kumar R., Achtari C., Canny G. O. (2011). Lipoxin A4 Is a Novel Estrogen Receptor Modulator. FASEB J. 25, 4326–4337. 10.1096/fj.11-187658 [DOI] [PubMed] [Google Scholar]
- Rydberg E. K., Krettek A., Ullstrom C., Ekstrom K., Svensson P. A., Carlsson L. M., et al. (2004). Hypoxia Increases LDL Oxidation and Expression of 15-lipoxygenase-2 in Human Macrophages. Arterioscler. Thromb. Vasc. Biol. 24, 2040–2045. 10.1161/01.ATV.0000144951.08072.0b [DOI] [PubMed] [Google Scholar]
- Sasaki F., Yokomizo T. (2019). The Leukotriene Receptors as Therapeutic Targets of Inflammatory Diseases. Int. Immunol. 31, 607–615. 10.1093/intimm/dxz044 [DOI] [PubMed] [Google Scholar]
- Schaldach C. M., Riby J., Bjeldanes L. F. (1999). Lipoxin A4: a New Class of Ligand for the Ah Receptor. Biochemistry 38, 7594–7600. 10.1021/bi982861e [DOI] [PubMed] [Google Scholar]
- Schoeder C. T., Mahardhika A. B., Drabczynska A., Kiec-Kononowicz K., Muller C. E. (2020). Discovery of Tricyclic Xanthines as Agonists of the Cannabinoid-Activated Orphan G-Protein-Coupled Receptor GPR18. ACS Med. Chem. Lett. 11, 2024–2031. 10.1021/acsmedchemlett.0c00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears D. D., Miles P. D., Chapman J., Ofrecio J. M., Almazan F., Thapar D., et al. (2009). 12/15-lipoxygenase Is Required for the Early Onset of High Fat Diet-Induced Adipose Tissue Inflammation and Insulin Resistance in Mice. PLoS One 4, e7250. 10.1371/journal.pone.0007250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secatto A., Rodrigues L. C., Serezani C. H., Ramos S. G., Dias-Baruffi M., Faccioli L. H., et al. (2012). 5-Lipoxygenase Deficiency Impairs Innate and Adaptive Immune Responses during Fungal Infection. PLoS One 7, e31701. 10.1371/journal.pone.0031701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. (2000). Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from omega-3 Fatty Acids via Cyclooxygenase 2-nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 192, 1197–1204. 10.1084/jem.192.8.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. (2017a). Discovery of Specialized Pro-resolving Mediators marks the Dawn of Resolution Physiology and Pharmacology. Mol. Aspects Med. 58, 1–11. 10.1016/j.mam.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B. (1984a). Lipoxins: Novel Series of Biologically Active Compounds Formed from Arachidonic Acid in Human Leukocytes. Proc. Natl. Acad. Sci. U S A. 81, 5335–5339. 10.1073/pnas.81.17.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B., Morris J., Wishka D. G. (1986a). On the Stereochemistry and Biosynthesis of Lipoxin B. Proc. Natl. Acad. Sci. U S A. 83, 1983–1987. 10.1073/pnas.83.7.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B. (1984b). Trihydroxytetraenes: a Novel Series of Compounds Formed from Arachidonic Acid in Human Leukocytes. Biochem. Biophys. Res. Commun. 118, 943–949. 10.1016/0006-291x(84)91486-4 [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Hirsch U., Palmblad J., Samuelsson B. (1987). Formation of Lipoxin A by Granulocytes from Eosinophilic Donors. FEBS Lett. 217, 242–246. 10.1016/0014-5793(87)80671-3 [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Jain A., Marleau S., Clish C., Kantarci A., Behbehani B., et al. (2003). Reduced Inflammation and Tissue Damage in Transgenic Rabbits Overexpressing 15-lipoxygenase and Endogenous Anti-inflammatory Lipid Mediators. J. Immunol. 171, 6856–6865. 10.4049/jimmunol.171.12.6856 [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Levy B. D. (2018). Resolvins in Inflammation: Emergence of the Pro-resolving Superfamily of Mediators. J. Clin. Invest. 128, 2657–2669. 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Nicolaou K. C., Webber S. E., Veale C. A., Dahlen S. E., Puustinen T. J., et al. (1986b). Lipoxin A. Stereochemistry and Biosynthesis. J. Biol. Chem. 261, 16340–16345. 10.1016/s0021-9258(18)66571-8 [DOI] [PubMed] [Google Scholar]
- Serhan C. N. (1989). On the Relationship between Leukotriene and Lipoxin Production by Human Neutrophils: Evidence for Differential Metabolism of 15-HETE and 5-HETE. Biochim. Biophys. Acta 1004, 158–168. 10.1016/0005-2760(89)90264-6 [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Sheppard K. A. (1990). Lipoxin Formation during Human Neutrophil-Platelet Interactions. Evidence for the Transformation of Leukotriene A4 by Platelet 12-lipoxygenase In Vitro . J. Clin. Invest. 85, 772–780. 10.1172/JCI114503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. (2017b). Treating Inflammation and Infection in the 21st century: New Hints from Decoding Resolution Mediators and Mechanisms. FASEB J. 31, 1273–1288. 10.1096/fj.201601222R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard K. A., Greenberg S. M., Funk C. D., Romano M., Serhan C. N. (1992). Lipoxin Generation by Human Megakaryocyte-Induced 12-lipoxygenase. Biochim. Biophys. Acta 1133, 223–234. 10.1016/0167-4889(92)90073-k [DOI] [PubMed] [Google Scholar]
- Sigal E., Grunberger D., Highland E., Gross C., Dixon R. A., Craik C. S. (1990). Expression of Cloned Human Reticulocyte 15-lipoxygenase and Immunological Evidence that 15-lipoxygenases of Different Cell Types Are Related. J. Biol. Chem. 265, 5113–5120. 10.1016/s0021-9258(19)34092-x [DOI] [PubMed] [Google Scholar]
- Skarke C., Alamuddin N., Lawson J. A., Li X., Ferguson J. F., Reilly M. P., et al. (2015). Bioactive Products Formed in Humans from Fish Oils. J. Lipid Res. 56, 1808–1820. 10.1194/jlr.M060392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern C., Cook J. M., Neetoo-Isseljee Z., Taylor D. L., Kettleborough C. A., Merritt A., et al. (2013). Screening Beta-Arrestin Recruitment for the Identification of Natural Ligands for Orphan G-Protein-Coupled Receptors. J. Biomol. Screen. 18, 599–609. 10.1177/1087057113475480 [DOI] [PubMed] [Google Scholar]
- Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., et al. (2009). Resolvin D2 Is a Potent Regulator of Leukocytes and Controls Microbial Sepsis. Nature 461, 1287–1291. 10.1038/nature08541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Funk C. D. (1996). Disruption of 12/15-lipoxygenase Expression in Peritoneal Macrophages. Enhanced Utilization of the 5-lipoxygenase Pathway and Diminished Oxidation of Low Density Lipoprotein. J. Biol. Chem. 271, 24055–24062. 10.1074/jbc.271.39.24055 [DOI] [PubMed] [Google Scholar]
- Sun Y. P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., et al. (2007). Resolvin D1 and its Aspirin-Triggered 17R Epimer. Stereochemical Assignments, Anti-inflammatory Properties, and Enzymatic Inactivation. J. Biol. Chem. 282, 9323–9334. 10.1074/jbc.M609212200 [DOI] [PubMed] [Google Scholar]
- Takano T., Fiore S., Maddox J. F., Brady H. R., Petasis N. A ., Serhan C. N. (1997). Aspirin-triggered 15-Epi-Lipoxin A4 (LXA4) and LXA4 Stable Analogues Are Potent Inhibitors of Acute Inflammation: Evidence for Anti-inflammatory Receptors. J. Exp. Med. 185, 1693–1704. 10.1084/jem.185.9.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E., Oh S. F., Siegelman J., Elangovan S., Percarpio K. B., Hong S., et al. (2006). Resolvin E2: Identification and Anti-inflammatory Actions: Pivotal Role of Human 5-lipoxygenase in Resolvin E Series Biosynthesis. Chem. Biol. 13, 1193–1202. 10.1016/j.chembiol.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Toewe A., Balas L., Durand T., Geisslinger G., Ferreiros N. (2018). Simultaneous Determination of PUFA-Derived Pro-resolving Metabolites and Pathway Markers Using Chiral Chromatography and Tandem Mass Spectrometry. Anal. Chim. Acta 1031, 185–194. 10.1016/j.aca.2018.05.020 [DOI] [PubMed] [Google Scholar]
- Tornhamre S., Elmqvist A., Lindgren J. A. (2000). 15-Lipoxygenation of Leukotriene A(4). Studies of 12- and 15-lipoxygenase Efficiency to Catalyze Lipoxin Formation. Biochim. Biophys. Acta 1484, 298–306. 10.1016/s1388-1981(00)00017-2 [DOI] [PubMed] [Google Scholar]
- Tourdot B. E., Holinstat M. (2017). Targeting 12-lipoxygenase as a Potential Novel Antiplatelet Therapy. Trends Pharmacol. Sci. 38, 1006–1015. 10.1016/j.tips.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Us Food and Drug Administration (2018). Bioanalytical Method Validation - Guidance for Industry. Silver Spring: U.S. Department of Health and Human Services. [Google Scholar]
- Van Os C. P., Rijke-Schilder G. P., Van Halbeek H., Verhagen J., Vliegenthart J. F. (1981). Double Dioxygenation of Arachidonic Acid by Soybean Lipoxygenase-1. Kinetics and Regio-Stereo Specificities of the Reaction Steps. Biochim. Biophys. Acta 663, 177–193. 10.1016/0005-2760(81)90204-6 [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Bryant R. W., Bailey J. M. (1980). Inhibition of Leukotriene Biosynthesis by the Leukocyte Product 15-Hydroxy-5,8,11,13-Eicosatetraenoic Acid. J. Biol. Chem. 255, 10064–10066. 10.1016/s0021-9258(19)70428-1 [DOI] [PubMed] [Google Scholar]
- Vaughn M. W., Proske R. J., Haviland D. L. (2002). Identification, Cloning, and Functional Characterization of a Murine Lipoxin A4 Receptor Homologue Gene. J. Immunol. 169, 3363–3369. 10.4049/jimmunol.169.6.3363 [DOI] [PubMed] [Google Scholar]
- Virmani J., Johnson E. N., Klein-Szanto A. J., Funk C. D. (2001). Role of 'platelet-type' 12-lipoxygenase in Skin Carcinogenesis. Cancer Lett. 162, 161–165. 10.1016/s0304-3835(00)00634-0 [DOI] [PubMed] [Google Scholar]
- Vital S. A., Becker F., Holloway P. M., Russell J., Perretti M., Granger D. N., et al. (2016). Formyl-peptide Receptor 2/3/lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation 133, 2169–2179. 10.1161/CIRCULATIONAHA.115.020633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hegedus J. H., Kahnt A. S., Ebert R., Heijink M., Toes R. E. M., Giera M., et al. (2020). Toll-like Receptor Signaling Induces a Temporal Switch towards a Resolving Lipid Profile in Monocyte-Derived Macrophages. Biochim. Biophys. Acta 1865, 158740. 10.1016/j.bbalip.2020.158740 [DOI] [PubMed] [Google Scholar]
- Wang T., Li H., Han Y., Wang Y., Gong J., Gao K., et al. (2020). A Rapid and High-Throughput Approach to Quantify Non-esterified Oxylipins for Epidemiological Studies Using Online SPE-LC-MS/MS. Anal. Bioanal. Chem. 412, 7989–8001. 10.1007/s00216-020-02931-y [DOI] [PubMed] [Google Scholar]
- Werner M., Jordan P. M., Romp E., Czapka A., Rao Z., Kretzer C., et al. (2019). Targeting Biosynthetic Networks of the Proinflammatory and Proresolving Lipid Metabolome. FASEB J. 33, 6140–6153. 10.1096/fj.201802509R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Pace S., Czapka A., Jordan P. M., Gerstmeier J., Koeberle A., et al. (2020). Communication between Human Macrophages and Epithelial Cancer Cell Lines Dictates Lipid Mediator Biosynthesis. Cel. Mol. Life Sci. 77, 4365–4378. 10.1007/s00018-019-03413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz O., Gerstmeier J., Libreros S., De La Rosa X., Werner M., Norris P. C., et al. (2018). Human Macrophages Differentially Produce Specific Resolvin or Leukotriene Signals that Depend on Bacterial Pathogenicity. Nat. Commun. 9, 59. 10.1038/s41467-017-02538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenberg I., Ostermann A. I., Schebb N. H. (2015). Targeted Metabolomics of the Arachidonic Acid cascade: Current State and Challenges of LC-MS Analysis of Oxylipins. Anal. Bioanal. Chem. 407, 2675–2683. 10.1007/s00216-014-8369-4 [DOI] [PubMed] [Google Scholar]
- Wilson S. R., Vehus T., Berg H. S., Lundanes E. (2015). Nano-LC in Proteomics: Recent Advances and Approaches. Bioanalysis 7, 1799–1815. 10.4155/bio.15.92 [DOI] [PubMed] [Google Scholar]
- Winther M., Dahlgren C., Forsman H. (2018). Formyl Peptide Receptors in Mice and Men: Similarities and Differences in Recognition of Conventional Ligands and Modulating Lipopeptides. Basic Clin. Pharmacol. Toxicol. 122, 191–198. 10.1111/bcpt.12903 [DOI] [PubMed] [Google Scholar]
- Wolfer A. M., Gaudin M., Taylor-Robinson S. D., Holmes E., Nicholson J. K. (2015). Development and Validation of a High-Throughput Ultrahigh-Performance Liquid Chromatography-Mass Spectrometry Approach for Screening of Oxylipins and Their Precursors. Anal. Chem. 87, 11721–11731. 10.1021/acs.analchem.5b02794 [DOI] [PubMed] [Google Scholar]
- Wong A., Hwang S. M., Cook M. N., Hogaboom G. K., Crooke S. T. (1988). Interactions of 5-lipoxygenase with Membranes: Studies on the Association of Soluble Enzyme with Membranes and Alterations in Enzyme Activity. Biochemistry 27, 6763–6769. 10.1021/bi00418a018 [DOI] [PubMed] [Google Scholar]
- Wu R., Zhou W., Chen S., Shi Y., Su L., Zhu M., et al. (2014). Lipoxin A4 Suppresses the Development of Endometriosis in an ALX Receptor-dependent Manner via the P38 MAPK Pathway. Br. J. Pharmacol. 171, 4927–4940. 10.1111/bph.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest S. J., Crucet M., Gemperle C., Loretz C., Hersberger M. (2012). Expression and Regulation of 12/15-lipoxygenases in Human Primary Macrophages. Atherosclerosis 225, 121–127. 10.1016/j.atherosclerosis.2012.07.022 [DOI] [PubMed] [Google Scholar]
- Yang J., Schmelzer K., Georgi K., Hammock B. D. (2009). Quantitative Profiling Method for Oxylipin Metabolome by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 81, 8085–8093. 10.1021/ac901282n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Kong Y., Zhang X., Zhang H., Gang Y., et al. (2018). The Therapeutic Effects of Lipoxin A4 during Treadmill Exercise on Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Mol. Immunol. 103, 35–45. 10.1016/j.molimm.2018.08.027 [DOI] [PubMed] [Google Scholar]
- Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., et al. (2009). International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 61, 119–161. 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., et al. (2009). Lipid G Protein-Coupled Receptor Ligand Identification Using Beta-Arrestin PathHunter Assay. J. Biol. Chem. 284, 12328–12338. 10.1074/jbc.M806516200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. X., Majchrzak-Hong S., Keyes G. S., Iadarola M. J., Mannes A. J., Ramsden C. E. (2018). Lipidomic Profiling of Targeted Oxylipins with Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 410, 6009–6029. 10.1007/s00216-018-1222-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. L., Zhuo X. J., Lin J., Luo L. C., Ying W. Y., Xie X., et al. (2017). Maresin1 Stimulates Alveolar Fluid Clearance through the Alveolar Epithelial Sodium Channel Na,K-ATPase via the ALX/PI3K/Nedd4-2 Pathway. Lab. Invest. 97, 543–554. 10.1038/labinvest.2016.150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.