Abstract
Objective
To study the association between covid-19 vaccines, SARS-CoV-2 infection, and risk of immune mediated neurological events.
Design
Population based historical rate comparison study and self-controlled case series analysis.
Setting
Primary care records from the United Kingdom, and primary care records from Spain linked to hospital data.
Participants
8 330 497 people who received at least one dose of covid-19 vaccines ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, or Ad.26.COV2.S between the rollout of the vaccination campaigns and end of data availability (UK: 9 May 2021; Spain: 30 June 2021). The study sample also comprised a cohort of 735 870 unvaccinated individuals with a first positive reverse transcription polymerase chain reaction test result for SARS-CoV-2 from 1 September 2020, and 14 330 080 participants from the general population.
Main outcome measures
Outcomes were incidence of Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. Incidence rates were estimated in the 21 days after the first vaccine dose, 90 days after a positive test result for SARS-CoV-2, and between 2017 and 2019 for background rates in the general population cohort. Indirectly standardised incidence ratios were estimated. Adjusted incidence rate ratios were estimated from the self-controlled case series.
Results
The study included 4 376 535 people who received ChAdOx1 nCoV-19, 3 588 318 who received BNT162b2, 244 913 who received mRNA-1273, and 120 731 who received Ad26.CoV.2; 735 870 people with SARS-CoV-2 infection; and 14 330 080 people from the general population. Overall, post-vaccine rates were consistent with expected (background) rates for Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome. Self-controlled case series was conducted only for Bell’s palsy, given limited statistical power, but with no safety signal seen for those vaccinated. Rates were, however, higher than expected after SARS-CoV-2 infection. For example, in the data from the UK, the standardised incidence ratio for Bell’s palsy was 1.33 (1.02 to 1.74), for encephalomyelitis was 6.89 (3.82 to 12.44), and for Guillain-Barré syndrome was 3.53 (1.83 to 6.77). Transverse myelitis was rare (<5 events in all vaccinated cohorts) and could not be analysed.
Conclusions
No safety signal was observed between covid-19 vaccines and the immune mediated neurological events of Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. An increased risk of Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome was, however, observed for people with SARS-CoV-2 infection.
Introduction
As of 13 January 2022, the covid-19 pandemic has resulted in more than 5.5 million deaths worldwide. After the rapid development of anti-SARS-CoV-2 vaccines, 9.2 billion doses have been administered through national vaccination programmes.1 To date, five vaccines against SARS-CoV-2 have received a conditional marketing authorisation by the European Medicines Agency. These include two mRNA vaccines: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna); two viral vector vaccines: ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and Ad.26.COV2.S (Janssen/Johnson & Johnson); and one adjuvanted, recombinant spike protein nanoparticle vaccine: NVX-CoV2373 (Novavax).2 All of these vaccines have shown high efficacy in preventing severe covid-19 and acceptable safety profiles in clinical trials.3 4 5 6 7 However, potential adverse events related to these new vaccines have been reported, and continuous vaccine safety surveillance is needed as mass immunisation against covid-19 continues.
More recently, concerns have been raised about immune mediated neurological disorders post-covid-19 vaccination. Owing to reports of people developing Guillain-Barré syndrome after vaccination with Ad.26.COV2.S (108 of 21 million people vaccinated as of late June 2021),8 and ChAdOx1 nCoV-19 (833 people of 592 million doses administered as of late July 2021),9 10 the EMA listed Guillain-Barré syndrome as a rare side effect related to these vaccines. Guillain-Barré syndrome has also been associated with mRNA vaccines in a few people.11 12 In addition, Bell’s palsy, encephalomyelitis, and transverse myelitis events have been described in case series studies after covid-19 vaccination with both viral vector and mRNA vaccines.13 14 15 16 17 Although these events are not necessarily due to covid-19 vaccines, the temporal association between the events and vaccination warrants robust post-vaccination surveillance. Large scale epidemiological studies are required to determine whether covid-19 vaccination increases the risks of these events above background rates in the general population.
We leveraged large routinely collected datasets including millions of vaccinated people in the UK and Spain to study the potential association between covid-19 vaccines and the short term risk of developing Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. To place these risks in context, we also studied the association between SARS-CoV-2 infection and risk of these immune mediated neurological events.
Methods
Data sources
For this study we used data from primary care records in both the UK and Spain. The Clinical Practice Research Datalink (CPRD) AURUM contains routinely collected data from primary care practices in the UK,18 19 representing 20% of the current UK population.20 Data from Spain came from the Information System for Research in Primary Care (SIDIAP; www.sidiap.org), a primary care database that covers 80% of the population in Catalonia, Spain, and is linked at an individual level to hospital data. These hospital data included information from all public and private hospitals in Catalonia (Conjunt Mínim Bàsic de Dades d’Alta Hospitalària, CMBD-AH).21 Both databases have been mapped to the Observational Medical Outcomes Partnership (OMOP) common data model,22 which allowed the same analytical code to be run against both datasets without the need to share patient level data.
Study participants
The populations of interest were individuals who had received at least one dose of a covid-19 vaccine and people with SARS-CoV-2 infection. Vaccination cohorts were constructed of people vaccinated according to the product (ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, or Ad.26.COV2.S) and dose administered (first or second dose, with only a single dose cohort for Ad.26.COV2.S as this vaccine is comprises a one dose regimen). Ad26.COV2.S and mRNA-1273 cohorts were only available in SIDIAP. Vaccinated individuals were required to have received their first dose between the start of the vaccination campaign in each country (8 December 2020 in the UK, 27 December 2020 in Spain) and one week before the end of data availability of each database (9 May 2021 for CPRD AURUM, 30 June 2021 for SIDIAP), with the vaccination date used as index date. We excluded those who received more than one brand of a covid-19 vaccine. For the second dose cohorts, participants were required to have received their second dose in prespecified intervals after the first dose. For both databases, the interval allowed between doses of two dose regimens (except Ad26.COV2.S) was 14 to 180 days. The SARS-CoV-2 cohort included people with a first positive reverse transcription polymerase chain reaction (RT-PCR) test result or antigen test result between 1 September 2020 and one week before the end of data availability of each database, with the test date used as index date. Data from both RT-PCR and antigen tests were available in SIDIAP, whereas only RT-PCR test results were available in CPRD AURUM. From the SARS-CoV-2 cohort we excluded individuals vaccinated against covid-19 before infection. We also identified a background population cohort that included all individuals registered in CPRD AURUM and SIDIAP as of 1 January 2017 (index date).
All study participants were required to be 18 years or older and to have at least 365 days of data availability before their index date. For each specific outcome, we excluded those who had experienced the outcome in the year before the index date. For each cohort, we followed participants from the index date until the earliest of end of follow-up (21 days for vaccinated people, 90 days for those with a diagnosis of covid-19, and 31 December 2019 for the general population cohort), first occurrence of the adverse event, end of data availability, or until transference out of the database or death. For cohorts that had received a first vaccine dose, we also censored follow-up if a second dose was observed before 21 days.
Events of interest
The events of interest were four immune mediated neurological disorders prespecified as potential adverse events of special interest for covid-19 vaccine safety: Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis.2 We identified these events using previously published clinical codes from electronic health records.23 Supplementary appendix table 1 provides details of the Systematized Nomenclature of Medicine (SNOMED) codes used to define the outcomes.
Study design
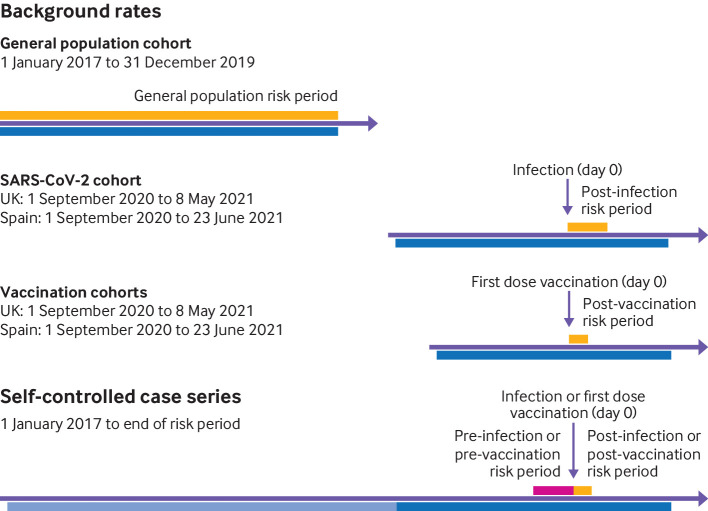
Firstly, we used the historical rate comparison method (see fig 1). Incidence rates of each outcome in the vaccinated and SARS-CoV-2 cohorts were used as observed rates and compared with the expected background incidence rates estimated from the general population cohort. For the vaccinated cohorts, we estimated the rates during 1-21 days after a first vaccine dose (day 0). For the SARS-CoV-2 cohort we used a post-test period of 90 days.
Fig 1.
Study design. Potential risk period (dark blue) for vaccination cohorts was defined as time between the start of the vaccination campaign and one week before the end of data availability for each database (CPRD AURUM: 8 December 2020 to 2 May 2021; SIDIAP: 27 December 2020 to 23 June 2021). For the SARS-CoV-2 infected cohort, the potential risk period started on 1 September 2020. The baseline period for the self-controlled case series analysis (light blue) was defined from 1 January 2017 to 21 days before the day of vaccination or SARS-CoV-2 positive test result. The pre-risk period (pink) was defined as −21 to −1 days before vaccination or SARS-CoV-2 positive test result, and the risk period (orange) was defined as 1 to 21 days after vaccination and 1-90 days after a SARS-CoV-2 positive test result
Secondly, we used a self-controlled case series method. In this approach, only individuals who experience the outcome are included, with participants acting as their own controls and thereby eliminating time fixed confounding.2 Within person comparisons of event rates were made between the baseline period before vaccination and the period at risk of an outcome. We defined the at risk period as the 0-21 days after a first vaccine dose or after a SARS-CoV-2 positive test result. The 0-21 day period was subdivided into several prespecified time periods: 0, 1-7, 8-14, 15-21 days, as well as for the 1-21 day period overall. We considered events at day 0 separately, as these events might precipitate hospital admissions and subsequent covid-19 testing, which could result in a positive association between SARS-CoV-2 infection and the studied events.25 The risk period for the SARS-CoV-2 cohort was also extended to 90 days. The study period of the self-controlled case series analysis was defined from 1 January 2017 to 21 days after the first vaccine dose or 90 days after a positive test result.
Statistical analysis
We characterised the participants in each cohort by personal characteristics, such as age and sex; comorbidities any time before vaccination; and recent drug use during the six months before the index date. Supplementary appendix table 2 shows the codes used for definitions of comorbidities and drug use. We estimated the observed rates during the 21 days after immunisation for the vaccinated cohorts and 90 days after testing for the SARS-CoV-2 cohort. Similarly, background rates were estimated for the general population from 1 January 2017 to 31 December 2019. We calculated crude incidence rates as the total number of events divided by the person time at risk per 100 000 person years. Indirect standardisation is used to account for differences between the age structure of the vaccinated or SARS-CoV-2 cohorts and the general population.26 Observed and expected rates were compared using standardised incidence ratios with corresponding 95% confidence intervals.
For the self-controlled case series analysis, only the first event was considered for each participant. Because events could potentially decrease the probability of being vaccinated, we removed a 21 day pre-risk period from the baseline period and reported separately. To assess potential for event dependent observation periods, we plotted a histogram of the time between the occurrence of the event and the end of observation for individuals censored and uncensored, and we calculated the number of deaths that occurred after each event (day 0 to day 7). Conditional Poisson regression models were fitted to estimate incidence rate ratios and 95% confidence intervals for each outcome,27 comparing the risk period with the baseline period. These models were estimated separately for each cohort of interest and were adjusted for age (in five year bands) and seasonality (four seasons). Self-controlled case series analyses were only conducted for comparisons with a ≤2 minimum detectable relative risk.28
We applied two sensitivity analyses to assess the impact of study design choices. Firstly, to exclude events potentially related to covid-19, in the vaccinated cohorts we excluded individuals infected with SARS-CoV-2 before the index date. For this, as RT-PCR tests were not routinely performed during the first wave of the pandemic, the covid-19 definition was broadened to include positive SARS-CoV-2 results (RT-PCR or antigen test) or a compatible covid-19 clinical code (see supplementary appendix table 3). Secondly, to include those with little previous use of healthcare, we also replicated the analyses after removing the one year before observation requirement for participants.
Any subgroups with fewer than five people were blinded and reported as less than five, following the requirements of information governance.
Patient and public involvement
No patients or members of the public were directly involved in the design or analysis of the reported data. The independent scientific advisory committee responsible for the approval of our protocol involved patients in the evaluation of our data access application.
Results
Figure 1 shows the study design and table 1 and table 2 show the distribution of personal characteristics, comorbidities, and recent drug use for each database and cohort. The study sample comprised 8 330 497 people who received at least one dose of a covid-19 vaccine (CPRD AURUM: 5 477 859; SIDIAP: 2 852 638), including 4 376 535 with ChAdOx1 nCoV-19, 3 588 318 with BNT162b2, 244 913 with mRNA-1273, and 120 731 with Ad26.COV2.S. Among those who received two vaccine doses (possible for all except Ad26.COV2.S), 3 745 017 completed the vaccination course (CPRD AURUM: 2 260 880; SIDIAP: 1 644 365), including 1 324 666 with ChAdOx1 nCoV-19, 2 420 351 with BNT162b2, and 160 228 with mRNA-1273. Among those vaccinated, 594 407 had covid-19 before the first dose. In addition, the study sample comprised 735 870 unvaccinated people with SARS-CoV-2 infection (CPRD AURUM: 447 233; SIDIAP: 288 637) and 14 330 080 people from the general population (CPRD AURUM: 9 651 568; SIDIAP: 4 678 512). Participants who received the first dose of any of the covid-19 vaccines were older (median age 56-64 years in CPRD AURUM, 51-62 years in SIDIAP) than the general population (median age 48 years in CPRD AURUM, 47 years in SIDIAP). In CPRD AURUM, participants with SARS-CoV-2 infection were younger than those vaccinated against covid-19 (median age 41 years). Vaccinated individuals also had more comorbidities than the general population, including autoimmune diseases, cancer, diabetes, obesity, heart disease, and renal impairment (table 1 and table 2).
Table 1.
Baseline characteristics of study participants in UK’s Clinical Practice Research Datalink AURUM database
| Characteristics | General population (n=9 651 568) | ChAdOx1 nCoV-19 | BNT162b2 | SARS-CoV-2 infection (n=447 233) | |||
|---|---|---|---|---|---|---|---|
| First dose (n=3 782 401) | Second dose (n=1 093 812) | First dose (n=1 695 458) | Second dose (n=1 167 068) | ||||
| Median (IQR) age (years) | 48 (33-63) | 56 (47-66) | 71 (59-76) | 64 (49-76) | 69 (52-78) | 41 (30-54) | |
| Men | 4 825 624 (50.0) | 1 829 719 (48.4) | 482 007 (44.1) | 711 217 (41.9) | 462 061 (39.6) | 204 798 (45.8) | |
| Median (IQR) past observation time (years) | 12.8 (4.9-23.7) | 16.1 (6.7-27.6) | 20.4 (8.2-32.4) | 18.0 (7.1-30.1) | 19.1 (7.5-31.3) | 11.6 (4.5-22.2) | |
| Comorbidities | |||||||
| Autoimmune disease | 198 170 (2.1) | 105 128 (2.8) | 47 582 (4.4) | 62 384 (3.7) | 45 003 (3.9) | 8988 (2.0) | |
| Malignant neoplastic disease | 606 442 (6.3) | 319 601 (8.4) | 185 702 (17.0) | 243 369 (14.4) | 191 533 (16.4) | 17 519 (3.9) | |
| Diabetes mellitus | 685 313 (7.1) | 383 250 (10.1) | 181 246 (16.6) | 263 365 (15.5) | 167 689 (14.4) | 32 705 (7.3) | |
| Obesity | 349 425 (3.6) | 190 790 (5.0) | 67 163 (6.1) | 98 771 (5.8) | 61 431 (5.3) | 20 436 (4.6) | |
| Heart disease | 827 039 (8.6) | 413 054 (10.9) | 232 267 (21.2) | 319 053 (18.8) | 236 329 (20.2) | 28 603 (6.4) | |
| Hypertensive disorder | 1 761 421 (18.3) | 915 008 (24.2) | 449 684 (41.1) | 592 676 (35.0) | 445 346 (38.2) | 59 638 (13.3) | |
| Renal impairment | 512 050 (5.3) | 242 170 (6.4) | 157 400 (14.4) | 208 112 (12.3) | 168 226 (14.4) | 15 982 (3.6) | |
| Drug use (183 days before to four days before index date) | |||||||
| Systemic corticosteroids | 545 205 (5.6) | 187 454 (5.0) | 77 668 (7.1) | 103 444 (6.1) | 73 838 (6.3) | 18 932 (4.2) | |
| Antithrombotics and anticoagulants | 190 145 (2.0) | 70 593 (1.9) | 43 189 (3.9) | 58 379 (3.4) | 44 663 (3.8) | 4388 (1.0) | |
| Lipid modifying agents | 290 710 (3.0) | 137 925 (3.6) | 71 958 (6.6) | 95 123 (5.6) | 69 719 (6.0) | 9110 (2.0) | |
| Antineoplastic and immunomodulating agents | 170 815 (1.8) | 40 343 (1.1) | 14 601 (1.3) | 25 199 (1.5) | 18 078 (1.5) | 9041 (2.0) | |
IQR=interquartile range.
Table 2.
Baseline characteristics of study participants in Spain’s Information System for Research in Primary Care (SIDIAP) database
| Characteristics | General population (n=4 678 512) | ChAdOx1 nCoV-19 | BNT162b2 | mRNA-1273 | Ad26.COV2.S (n=120 731) | SARS-CoV-2 infection (n=288 637) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First dose (n=594 134) | Second dose (n=230 854) | First dose (n=1 892 860) | Second dose (n=1 253 283) | First dose (n=244 913) | Second dose (n=160 228) | ||||||
| Median (IQR) age (years) | 47 (36-63) | 62 (59-65) | 60 (42-63) | 56 (47-75) | 72 (55-79) | 53 (47-57) | 54 (49-60) | 51 (43-67) | 46 (34-59) | ||
| Men | 2 287 403 (48.9) | 270 830 (45.6) | 103 686 (44.9) | 859 915 (45.4) | 527 395 (42.1) | 117 409 (47.9) | 73 139 (45.6) | 64 435 (53.4) | 135 813 (47.1) | ||
| Median (IQR) past observation time (years) | 11.0 (11.0-11.0) | 15.3 (15.2-15.3) | 15.5 (15.4-15.5) | 15.3 (15.2-15.4) | 15.3 (15.2-15.4) | 15.4 (15.3-15.4) | 15.4 (15.3-15.4) | 15.4 (15.3-15.5) | 14.9 (14.8-15.1) | ||
| Comorbidities | |||||||||||
| Autoimmune disease | 79 044 (1.7) | 14 501 (2.4) | 5075 (2.2) | 49 029 (2.6) | 40 899 (3.3) | 13 012 (5.3) | 10 776 (6.7) | 2242 (1.9) | 5857 (2.0) | ||
| Malignant neoplastic disease | 339 004 (7.2) | 60 074 (10.1) | 18 504 (8.0) | 241 140 (12.7) | 220 040 (17.6) | 38 401 (15.7) | 32 964 (20.6) | 9397 (7.8) | 22 242 (7.7) | ||
| Diabetes mellitus | 464 603 (9.9) | 80 492 (13.5) | 23 582 (10.2) | 280 876 (14.8) | 243 412 (19.4) | 25 669 (10.5) | 19 699 (12.3) | 15 049 (12.5) | 30 542 (10.6) | ||
| Obesity | 863 167 (18.4) | 161 047 (27.1) | 50 787 (22.0) | 502 689 (26.6) | 396 021 (31.6) | 55 920 (22.8) | 38 727 (24.2) | 30 154 (25.0) | 66 933 (23.2) | ||
| Heart disease | 568 547 (12.2) | 87 455 (14.7) | 26 640 (11.5) | 403 280 (21.3) | 367 372 (29.3) | 30 494 (12.5) | 24 061 (15.0) | 15 827 (13.1) | 37 800 (13.1) | ||
| Hypertensive disorder | 1 139 337 (24.4) | 200 957 (33.8) | 56 782 (24.6) | 701 276 (37.0) | 628 916 (50.2) | 60 731 (24.8) | 46 745 (29.2) | 33 102 (27.4) | 65 030 (22.5) | ||
| Renal impairment | 229 136 (4.9) | 21 359 (3.6) | 5650 (2.4) | 194 837 (10.3) | 187 662 (15.0) | 14 622 (6.0) | 12 998 (8.1) | 4923 (4.1) | 16 807 (5.8) | ||
| Drug use (183 days before to four days before index date) | |||||||||||
| Systemic corticosteroids | 258 775 (5.5) | 32 229 (5.4) | 11 213 (4.9) | 123 581 (6.5) | 98 008 (7.8) | 20 231 (8.3) | 15 854 (9.9) | 7296 (6.0) | 15 654 (5.4) | ||
| Antithrombotics and anticoagulants | 110 809 (2.4) | 16 968 (2.9) | 4851 (2.1) | 68 891 (3.6) | 59 083 (4.7) | 10 122 (4.1) | 7838 (4.9) | 3548 (2.9) | 7618 (2.6) | ||
| Lipid modifying agents | 80 561 (1.7) | 18 339 (3.1) | 5310 (2.3) | 45 250 (2.4) | 37 985 (3.0) | 6292 (2.6) | 4804 (3.0) | 2934 (2.4) | 4268 (1.5) | ||
| Antineoplastic and immunomodulating agents | 59 360 (1.3) | 7097 (1.2) | 3277 (1.4) | 24 460 (1.3) | 18 331 (1.5) | 7861 (3.2) | 6455 (4.0) | 1310 (1.1) | 5046 (1.7) | ||
IQR=interquartile range.
Bell’s palsy
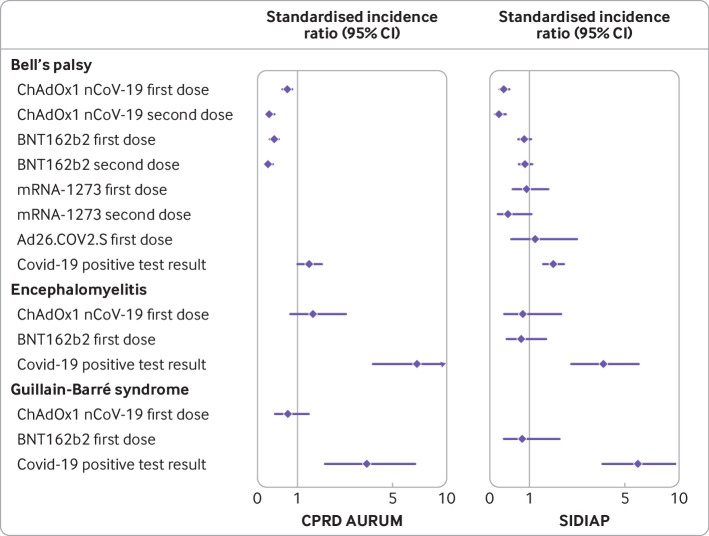
Within 21 days after a first dose of ChAdOx1 nCoV-19, Bell’s palsy was observed in 144 people: 117 observed in CPRD AURUM compared with 164.5 expected given background rates in the general population (standardised incidence ratio 0.71, 95% confidence interval 0.59 to 0.85; table 3). In SIDIAP, the corresponding values were 27 observed compared with 82.6 expected (0.33, 0.22 to 0.48; table 4). Thirty five patients (25 in CPRD AURUM; 10 in SIDIAP) were observed after the second dose of ChAdOx1 nCoV-19, resulting in a standardised incidence ratio of 0.26 (0.18 to 0.39) in CPRD AURUM and 0.21 (0.11 to 0.39) in SIDIAP (table 3, table 4, and fig 2). Bell’s palsy was observed in 46 and 24 patients after a first and second dose of BNT162b2 in CPRD AURUM, compared with 116.4 and 99.5 expected (0.40, 0.30 to 0.53, and 0.24, 0.16 to 0.36, respectively). In SIDIAP, rates of Bell’s palsy after BNT162b2 (first and second doses) and mRNA-1273 and Ad26.COV2.S first doses were similar to those expected, with equivalent standardised incidence ratios of 0.86 (0.70 to 1.04), 0.88 (0.71 to 1.08), 0.92 (0.54 to 1.55) and 1.15 (0.52 to 2.56). Incidence rate ratios could only be stratified by age and sex for Bell’s palsy, as it was the only event in which some of the strata had more than five occurrences. None of the strata showed significantly higher rates after vaccination in either CPRD AURUM or SIDIAP (see appendix figure 2). Rates of Bell’s palsy were higher than expected among those infected with SARS-CoV-2, with 53 and 93 events in the 90 days after an infection registered in CPRD AURUM and in SIDIAP, compared with 39.8 and 54.7 expected events, respectively. Equivalent standardised incidence ratios were 1.33 (1.02 to 1.74) in CPRD AURUM and 1.70 (1.39 to 2.08) in SIDIAP.
Table 3.
Association between covid-19 vaccination or SARS-CoV-2 infection and occurrence of immune mediated neurological disorders of special interest in UK’s Clinical Practice Research Datalink AURUM database
| Event by vaccine and dose | No of participants | Person years | Observed events | Expected events | Standardised incidence ratio (95% CI) |
|---|---|---|---|---|---|
| Bell’s palsy | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 3 776 803 | 384 250 | 117 | 164.5 | 0.71 (0.59 to 0.85) |
| Second dose | 1 093 258 | 218 629 | 25 | 94.7 | 0.26 (0.18 to 0.39) |
| BNT162b2: | |||||
| First dose | 1 693 453 | 275 333 | 46 | 116.4 | 0.40 (0.30 to 0.53) |
| Second dose | 1 166 571 | 235 351 | 24 | 99.5 | 0.24 (0.16 to 0.36) |
| SARS-CoV-2 positive test result | 446 851 | 106 342 | 53 | 39.8 | 1.33 (1.02 to 1.74) |
| Encephalomyelitis | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 3 778 358 | 384 420 | 11 | 7.6 | 1.45 (0.80 to 2.62) |
| BNT162b2: | |||||
| First dose | 1 694 161 | 275 451 | <5 | 5.4 | |
| Second dose | 1 167 028 | 235 447 | <5 | 4.6 | |
| SARS-CoV-2 positive test result | 447 037 | 106 391 | 11 | 1.6 | 6.89 (3.82 to 12.44) |
| Guillain-Barré syndrome | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 3 778 430 | 384 427 | 11 | 14.9 | 0.74 (0.41 to 1.33) |
| Second dose | 1 093 778 | 218 737 | <5 | 9.5 | |
| BNT162b2: | |||||
| First dose | 1 694 167 | 275 452 | <5 | 10.4 | |
| Second dose | 1 167 031 | 235 448 | <5 | 9.0 | |
| SARS-CoV-2 positive test result | 447 029 | 106 390 | 9 | 2.6 | 3.53 (1.83 to 6.77) |
| Transverse myelitis | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 3 778 434 | 384 430 | <5 | 7.6 | |
| BNT162b2: | |||||
| First dose | 1 694 190 | 275 456 | <5 | 4.7 | |
| SARS-CoV-2 positive test result | 447 041 | 106 393 | <5 | 1.9 | |
Events with fewer than five occurrences have been omitted for privacy reasons. No events were observed for omitted cohorts.
Table 4.
Association between covid-19 vaccination or SARS-CoV-2 infection and occurrence of immune mediated neurological disorders of special interest in Spain’s Information System for Research in Primary Care (SIDIAP) database
| Event by vaccine and dose | No of participants | Person years | Observed events | Expected events | Standardised incidence ratio (95% CI) |
|---|---|---|---|---|---|
| Bell’s palsy | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 592 365 | 89 055 | 27 | 82.6 | 0.33 (0.22 to 0.48) |
| Second dose | 230 692 | 55 659 | 10 | 47.3 | 0.21 (0.11 to 0.39) |
| BNT162b2: | |||||
| First dose | 1 890 434 | 102 623 | 100 | 116.7 | 0.86 (0.70 to 1.04) |
| Second dose | 1 251 680 | 74 638 | 85 | 97.1 | 0.88 (0.71 to 1.08) |
| mRNA-1723: | |||||
| First dose | 244 467 | 17 648 | 14 | 15.2 | 0.92 (0.54 to 1.55) |
| Second dose | 159 995 | 12 563 | 5 | 11.3 | 0.44 (0.18 to 1.06) |
| Ad26.COV2.S: | |||||
| First dose | 120 470 | 5,228 | 6 | 5.2 | 1.15 (0.52 to 2.56) |
| SARS-CoV-2 positive test result | 288 030 | 66 603 | 93 | 54.7 | 1.70 (1.39 to 2.08) |
| Encephalomyelitis | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 592 843 | 89 121 | 5 | 6.1 | 0.82 (0.34 to 1.97) |
| Second dose | 230 844 | 55 696 | <5 | 3.4 | |
| BNT162b2: | |||||
| First dose | 1 892 409 | 102 738 | 9 | 11.7 | 0.77 (0.40 to 1.48) |
| Second dose | 1 253 202 | 74 733 | <5 | 10.3 | |
| mRNA-1273: | |||||
| First dose | 244 744 | 17 669 | <5 | 1.2 | |
| Ad26.COV2.S: | |||||
| First dose | 120 588 | 5,233 | <5 | 0.4 | |
| SARS-CoV-2 positive test result | 288 374 | 66 689 | 17 | 4.5 | 3.75 (2.33 to 6.02) |
| Guillain-Barré syndrome | |||||
| ChAdOx1 nCoV-19: | |||||
| First dose | 592 860 | 89 123 | <5 | 5 | |
| BNT162b2: | |||||
| First dose | 1 892 423 | 102 739 | 5 | 6.3 | 0.79 (0.33 to 1.91) |
| Second dose | 1 253 201 | 74 733 | <5 | 5.3 | |
| mRNA-1273: | |||||
| Second dose | 160 213 | 12,58 | <5 | 0.7 | |
| SARS-CoV-2 positive test | 288 392 | 66 693 | 17 | 2.9 | 5.92 (3.68 to 9.53) |
| Transverse myelitis | |||||
| BNT162b2: | |||||
| First dose | 1 892 510 | 102 744 | <5 | 0.9 | |
| mRNA-1723: | |||||
| Second dose | 160 222 | 12 581 | <5 | 0.1 | |
Events with fewer than five occurrences have been omitted for privacy reasons. No events were observed for omitted cohorts.
Fig 2.
Standardised incidence ratios of immune mediated neurological disorders of special interest. CPRD AURUM=Clinical Practice Research Datalink AURUM (UK); SIDIAP=Information System for Research in Primary Care (Spain)
The self-controlled case series analysis was only sufficiently powered to study those with a first dose of BNT162b2, ChAdOx1 nCoV-19, and mRNA-1273 (in SIDIAP only), and those with SARS-CoV-2 infection (see supplementary appendix tables 4 and 5). There was some indication of event dependent observation periods (see supplementary appendix figure 1). In the self-controlled case series analysis, an adjusted incidence rate ratio for Bell’s palsy of 0.81 (0.66 to 0.98) was observed for ChAdOx1 nCoV-19 and 0.83 (0.61 to 1.10) for BNT162b2 between one and 21 days in CPRD AURUM (table 5). The values in SIDIAP were 0.92 (0.60 to 1.33) and 0.83 (0.66 to 1.02) between eight and 14 days (table 6). The adjusted incidence rate ratio for mRNA-1273 was 0.99 (0.54 to 1.64), and for SARS-CoV-2 infection was 1.82 (1.21 to 2.61). An increased risk of Bell’s palsy was observed on the day of a SARS-CoV-2 positive test result, and 1-21 days before and 1-7 days after the test result (table 5 and table 6).
Table 5.
Incidence rate ratios (95% confidence intervals) for covid-19 vaccination or SARS-CoV-2 infection and occurrence of Bell’s palsy using self-controlled case series analysis in UK’s Clinical Practice Research Datalink AURUM database
| ChAdOx1 nCoV-19 first dose | BNT162b2 first dose | SARS-CoV-2 infection | ||||||
|---|---|---|---|---|---|---|---|---|
| No of events | Incidence rate ratio (95% CI) | No of events | Incidence rate ratio (95% CI) | No of events | Incidence rate ratio (95% CI) | |||
| Baseline | 7403 | Ref | 4564 | Ref | 2335 | Ref | ||
| Days pre-vaccination/pre-infection: | ||||||||
| −21 to −1 | 83 | 0.68 (0.54 to 0.84) | 41 | 0.74 (0.54 to 1.00) | 14 | 1.03 (0.58 to 1.68) | ||
| Day 0 | 5 | 0.83 (0.30 to 1.79) | <5 | <5 | ||||
| Days post-vaccination/post-infection: | ||||||||
| 1-7 | 25 | 0.59 (0.38 to 0.85) | 14 | 0.75 (0.42 to 1.22) | 6 | 1.30 (0.52 to 2.65) | ||
| 8-14 | 34 | 0.81 (0.56 to 1.11) | 17 | 0.91 (0.54 to 1.41) | <5 | |||
| 15-21 | 43 | 1.03 (0.75 to 1.37) | 16 | 0.84 (0.49 to 1.33) | 7 | 1.51 (0.64 to 2.93) | ||
| 1-21 | 102 | 0.81 (0.66 to 0.98) | 47 | 0.83 (0.61 to 1.10) | * | 0.94 (0.51 to 1.55) | ||
| 1-90 | 48 | 0.76 (0.56 to 1.01) | ||||||
Events with fewer than five occurrences have been omitted for privacy reasons.
Since event number <5 was blinded, the total number of events during the period was also blinded.
Table 6.
Incidence rate ratios (95% confidence intervals) for covid-19 vaccination or SARS-CoV-2 infection and the occurrence of Bell’s palsy estimated using self-controlled case series analysis in in SIDIAP
| ChAdOx1 nCoV-19 | BNT162b2 | mRNA-1273 | SARS-CoV-2 infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of events | Incidence rate ratio (95% CI) | No of events | Incidence rate ratio (95% CI) | No of events | Incidence rate ratio (95% CI) | No of events | Incidence rate ratio (95% CI) | ||||
| Baseline | 4588 | Ref | 9150 | Ref | 3791 | Ref | 3892 | Ref | |||
| Days pre-vaccination/pre-infection: | |||||||||||
| −21 to −1 | 12 | 0.44 (0.23 to 0.73) | 74 | 0.69 (0.54 to 0.86) | 11 | 0.80 (0.41 to 1.38) | 44 | 2.93 (2.13 to 3.92) | |||
| Day 0 | <5 | <5 | <5 | 8 | 11.13 (5.07 to 20.80) | ||||||
| Days post-vaccination/post-infection: | |||||||||||
| 1-7 | 7 | 0.77 (0.33 to 1.49) | 22 | 0.62 (0.39 to 0.91) | <5 | 17 | 3.38 (2.01 to 5.28) | ||||
| 8-14 | 7 | 0.77 (0.33 to 1.50) | 35 | 1.02 (0.72 to 1.40) | 6 | 1.37 (0.54 to 2.79) | 6 | 1.21 (0.48 to 2.47) | |||
| 15-21 | 11 | 1.22 (0.63 to 2.10) | 27 | 0.85 (0.57 to 1.22) | <5 | <5 | |||||
| 1-21 | 25 | 0.92 (0.60 to 1.33) | 84 | 0.83 (0.66 to 1.02) | * | 0.99 (0.54 to 1.64) | * | 1.82 (1.21 to 2.61) | |||
| 1-90 | 77 | 1.31 (1.03 to 1.64) | |||||||||
Events with fewer than five occurrences have been omitted for privacy reasons.
Since event number <5 was blinded, the total number of events during the period was also blinded.
Encephalomyelitis
Overall, 16 encephalomyelitis events were observed after a first dose of ChAdOx1 nCoV-19: standardised incidence ratio 1.45 (0.80 to 2.62) in CPRD AURUM and 0.82 (0.34 to 1.97) in SIDIAP (table 3 and table 4). Nine events were observed after a first dose of BNT162b2 in SIDIAP (0.77, 0.40 to 1.48). No events were reported after a second dose of mRNA-1273, and fewer than five occurred among the remaining vaccination cohorts. Conversely, 28 encephalomyelitis events occurred after SARS-CoV-2 infection: 11 observed to 1.6 expected (6.89, 3.82 to 12.44) in CPRD AURUM and 17 observed to 4.5 expected (3.75, 2.33 to 6.02) in SIDIAP.
Guillain-Barré syndrome
In CPRD AURUM, 11 Guillain-Barré syndrome events were observed after a first dose of ChAdOx1 nCoV-19 and fewer than five after a second dose. The corresponding values in SIDIAP were fewer than five and zero events. Five or fewer events were observed after the first and second dose of BNT162b2 and the second dose of mRNA-1723, with no events after the first dose of mRNA or Ad26.COV2.S. However, 26 events occurred after SARS-CoV-2 infection: nine observed to 2.6 expected (3.53, 1.83 to 6.77) events in CPRD AURUM and 17 observed to 2.9 expected (5.92, 3.68 to 9.53) events in SIDIAP.
Transverse myelitis
Transverse myelitis events were only observed after the first dose of ChAdOx1 nCoV-19 (in CPRD AURUM), first dose of BNT162b2 (in CPRD AURUM and SIDIAP) and second dose of mRNA-1723 (in SIDIAP), with fewer than five events. Fewer than five events were also observed after SARS-CoV-2 infection in CPRD AURUM.
Sensitivity analyses
We found similar results in sensitivity analysis after excluding individuals with previous covid-19 among those vaccinated, removing the requirement for at least one year of history in all cohorts (see supplementary appendix table 6). We obtained similar results after reducing the follow-up period from 90 to 21 days in the SARS-CoV-2-infected cohorts, although standardised incidence ratios were higher and confidence intervals wider than those obtained in the main analysis. The first sensitivity analysis was also performed in the self-controlled case series, with similar results obtained to those of the main analysis (see supplementary appendix table 7). The results from primary and secondary analyses are available at https://livedataoxford.shinyapps.io/VaxAEsNeuroimmune.
Hospital admissions and deaths after outcome events
In SIDIAP, the percentage of outcome events associated with hospital admission ranged from 35.9% for Bell’s palsy to 86.3% for encephalomyelitis (see supplementary appendix table 8). Deaths in the first week after an event occurred in less than 0.5% participants across both databases (see supplementary appendix table 9).
Discussion
In this study, which included more than eight million vaccine recipients from two countries, we observed no safety signal between BNT162b2, ChAdOx1 nCoV-19, mRNA-1273, or Ad26.COV2.S and risk of developing the immune mediated neurological events of Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. An increased risk of Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome was, however, observed in two cohorts of 447 233 and 288 637 people with SARS-CoV-2 infection.
Findings in context
Immune mediated neurological disorders have been identified as adverse events of special interest by regulators, such as the Food and Drug Administration in the US and the EMA in Europe, and organisations such as the Brighton Collaboration. These adverse events of special interest have been closely monitored during immunisation campaigns,23 29 30 and several severe neurological disorders were reported as rare adverse events during the first clinical trials of covid-19 vaccines.4 5 31 32 33 34 35 Although a causal relationship was not established, the recommendation for further monitoring has led to the publication of many case reports worldwide.36
However, research findings from observational studies on the association between covid-19 vaccines and immune mediated neurological events have been mixed. For mRNA vaccines, for example, although two studies underpinned by safety surveillance databases found no association between mRNA vaccines and Bell’s palsy,37 38 two others observed an increased risk,39 40 with the case series and nested case-control study from Hong Kong also reporting an increased risk for recipients of an inactivated vaccine (CoronaVac; Sinovac Biotech). Another study from Hong Kong, however, found no such increased risk when comparing 1.1 million recipients of BNT162b2 or CoronaVac with 2.7 million unvaccinated individuals.41 Our findings are in line with this study, with no safety signal seen for either BNT162b2 or mRNA-1273.
Although a study of more than 20 million people vaccinated with ChAdOx1 nCoV-19 and 12 million vaccinated with BNT162b2 in the UK similarly did not observe any increased risk of hospital admissions for neurological events with BNT162b2, it did find an association for ChAdOx1 nCoV-19.42 For example, the incidence rate ratios in the self-controlled case series analysis for Bell’s palsy and Guillain-Barré syndrome were 1.3 (1.1 to 1.6) and 2.9 (2.2 to 3.9), respectively, 15-21 days after vaccination. Moreover, this association between ChAdOx1 nCoV-19 and Guillain-Barré syndrome was subsequently confirmed using analogous methods with Scottish data.42 In our study we found no such safety signals for ChAdOx1 nCoV-19. Meanwhile, in another study comparing events reported in a vaccine surveillance system with background rates, Ad26.COV2.S was associated with increased risks of Guillain-Barré syndrome (observed to expected rate ratio of 4.2 (3.5 to 5.0) in the 42 days after vaccination).43 In our study, we observed no safety signal for Ad26.COV2.S, but this vaccine was only available for the data from Spain, and was the smallest vaccine cohort.
In line with our findings, several other studies have also reported increased risks of immune mediated neurological events after SARS-CoV-2 infection.42 44 45 One study, for example, found increased risks of Bell’s palsy; encephalitis, meningitis, and myelitis; and Guillain-Barré syndrome after SARS-CoV-2 infection.42 The increase in risks after SARS-CoV-2 infection much exceed any of the previously mentioned associations reported after vaccination.
Limitations of this study
Our study has limitations. Firstly, CPRD AURUM only included primary care data from the UK. Therefore, diagnoses from inpatient settings might not be captured and the absolute risk could be underestimated. A previous study has, however, shown that CPRD AURUM captures immune mediated neurological disorders such as Guillain-Barré syndrome relatively accurately, even in the absence of linked hospital data.46 Meanwhile, the SIDIAP database did have patient level hospital linkage, and results were consistent between databases. Secondly, confounding by indication might affect the historical comparator method. Although we account for differences in age, individuals vaccinated also had more comorbidities than the comparator cohort and may well differ in other, unobserved characteristics. Thirdly, comparisons between the vaccinated and historical cohorts might be limited because of changes over time, including seasonal variations47 in outcomes and changes in exposure to other viral infections during the pandemic, which might have reduced the incidence of immune mediated neurological events.48 In addition, given public concerns over vaccine safety and complications from SARS-CoV-2 infection, individuals vaccinated against or infected with SARS-CoV-2 might have been more prone to seek care when showing symptoms. Although seasonality was not included in the historical comparison method, in our self-controlled case series analysis we adjusted for age and seasonality to control for time varying confounding. Fourthly, because we excluded individuals with a recent history of the same immune mediated neurological events, our results are not generalisable to people with chronic relapsing conditions such as multiple sclerosis or Devic’s disease. This exclusion criteria would also have led to a depletion-of-susceptible bias in the cohorts that received a second vaccine dose (because those who had an event after the first dose were excluded from the second dose cohort). Fifthly, we assessed outcomes using routinely recorded diagnoses without a formal adjudication, and outcome misclassification might therefore have been possible. However, we used code lists and algorithms for the identification of immune mediated neurological disorders previously published as part of a study on the background rates of covid-19 related adverse events of special interest.23
Our study focused on short term adverse events after vaccination, using similar time periods to other studies.41 42 The time at risk after vaccination was defined according to the shortest recommended interval between first and second vaccine doses. Although the second dose is not a consideration for Ad26.COV2.S, we used the same time at risk to ensure comparability between the results. Therefore, further evidence is required to understand the long term adverse events of vaccination and SARS-CoV-2 infection. Larger cohorts are also needed to study the effect of vaccination on different age groups, particularly among younger populations. Moreover, although it is reassuring that so few outcomes of transverse myelitis were seen in this study, additional data might allow a more detailed study of this event.
Conclusion
We found no safety signal for any of the studied immune mediated neurological events after vaccination against covid-19. Infection with SARS-CoV-2 was, however, associated with an increased risk of Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome.
What is already known on this topic
Spontaneous reporting indicated a potential association between immune mediated neurological disorders after covid-19 vaccination
The European Medicines Agency has listed Guillain-Barré syndrome as a rare side effect of exposure to viral vector vaccines
To date, however, the results from research into the risk of immune mediated neurological disorders after covid-19 vaccination have been mixed
What this study adds
Using routinely collected data from the UK and Spain, this study found no safety signal after covid-19 vaccination for the immune mediated neurological events of Bell’s palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis
An increased risk of Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome was, however, seen after SARS-CoV-2 infection
Acknowledgments
We thank healthcare professionals of the UK and Catalonia who daily register information in the populations’ electronic health records; the Institut Català de la Salut and the Programa d'analítica de dades per a la recerca i la innovació en salut for providing access to the different data sources accessible through SIDIAP; and Jennifer A de Beyer (Centre for Statistics in Medicine, University of Oxford) for help with English language editing.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-9 and figures 1 and 2
Contributors: XL and BR are joint first authors. DP-A, EB, and TD-S are joint senior authors. XL, BR, DP-A, TD-S, EB, and VS conceived the study and contributed to the study design. XL, BR, ER, and AP conducted the statistical analyses. XL, BR, ER, VS, DP-A, EB, and TD-S interpreted the results and wrote the manuscript. All authors contributed to writing the manuscript, approved the final version, and had final responsibility for the decision to submit for publication. TD-S, EB, and DP-A are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was partially funded by the UK National Institute for Health Research (NIHR) and European Health Data and Evidence Network (EHDEN). EHDEN has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 806968. The Innovative Medicines Initiative 2 Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and The European Federation of Pharmaceutical Industries and Associations (EFPIA). The study funders had no role in the conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript. ER and EMH were supported by Instituto de Salud Carlos III, Spain (grant No CM20/00174, and JR17/00012).
Competing interests: All authors have completed the ICMJE disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare the following interests: DP-A receives funding from the UK National Institute for Health Research (NIHR) in the form of a senior research fellowship and from the Oxford NIHR Biomedical Research Centre. XL receives the Clarendon Fund and Brasenose College scholarship (University of Oxford) to support her DPhil study. DP-A’s research group has received research grants from the European Medicines Agency; the Innovative Medicines Initiative; and Amgen, Chiesi, and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca, and UCB Biopharma.
The lead authors (XL and BR) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will disseminate a lay summary of our findings through our Twitter and other social media accounts.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The protocol for this research was approved by the independent scientific advisory committee for Medicine and Healthcare Products Regulatory Agency database research (protocol No 20_000211) and by the clinical research ethics committee of Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (project code: 21/052-PCV). Informed consent of individual patients was not required as anonymised information was obtained from medical records.
Data availability statement
Patient level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. Analytical code and detailed definitions of algorithms for identifying the events are available in a GitHub repository (https://github.com/SIDIAP/VaxAEsNeuroimmune).
References
- 1.WHO. COVID-19 Dashboard. Geneva: World Health Organization, 2020. https://covid19.who.int (accessed 14 January 2022).
- 2.European Medicines Agency. COVID-19 vaccines: authorised. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section (accessed 14 January 2022).
- 3. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadoff J, Gray G, Vandebosch A, et al. ENSEMBLE Study Group . Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021;384:2187-201. 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heath PT, Galiza EP, Baxter DN, et al. 2019nCoV-302 Study Group . Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med 2021;385:1172-83. 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency. COVID-19 Vaccine Janssen: Guillain-Barré syndrome listed as a very rare side effect. www.ema.europa.eu/en/news/covid-19-vaccine-janssen-guillain-barre-syndrome-listed-very-rare-side-effect (accessed 19 October 2021).
- 9. Matarneh AS, Al-Battah AH, Farooqui K, Ghamoodi M, Alhatou M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep 2021;9:e04756. 10.1002/ccr3.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann Med Surg (Lond) 2021;67:102540. 10.1016/j.amsu.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021;13:e13426. 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transpl 2022;28:134-7. 10.1002/lt.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goss AL, Samudralwar RD, Das RR, Nath A, ANA Investigates . ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann Neurol 2021;89:856-7. 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front Immunol 2021;12:653786. 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. QJM 2021;114:657-8. 10.1093/qjmed/hcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann Neurol 2021;90:506-11. 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogrig A, Janes F, Gigli GL, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg 2021;208:106839. 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol 2019;48:1740-1740g, g. 10.1093/ije/dyz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827-36. 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical Practice Research Datalink. CPRD Aurum June 2021 (Version 2021.06.001). Clinical Practice Research Datalink. June 7,2021. 10.48329/PYC2-WE97 [DOI]
- 21.Burn E, Fernández-Bertolín S, Voss EA, et al. Establishing and characterising large COVID-19 cohorts after mapping the Information System for Research in Primary Care in Catalonia to the OMOP Common Data Model. MedRxiv 2021:21266734. [cited 2021 Jan 5] 10.1101/2021.11.23.21266734. [DOI]
- 22. Hripcsak G, Schuemie MJ, Madigan D, Ryan PB, Suchard MA. Drawing reproducible conclusions from observational clinical data with OHDSI. Yearb Med Inform 2021;30:283-9. 10.1055/s-0041-1726481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ 2021;373;n1435. 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:i4515. 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 25. Fonseca-Rodríguez O, Fors Connolly AM, Katsoularis I, Lindmark K, Farrington P. Avoiding bias in self-controlled case series studies of coronavirus disease 2019. Stat Med 2021;40:6197-208. 10.1002/sim.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharples LD, Kirkwood BR. Essentials of medical statistics. J R Stat Soc Ser A Stat Soc 1989;152:263 10.2307/2982934. [DOI] [Google Scholar]
- 27. Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul 2013;23. 10.1145/2414416.2414791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med 2006;25:2618-31. 10.1002/sim.2477. [DOI] [PubMed] [Google Scholar]
- 29.bc-coordinator. Priority list of adverse events of special interest: COVID-19. 2020. https://brightoncollaboration.us/priority-list-aesi-covid/ (accessed 11 Mar 2021).
- 30.Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology. CBER Surveillance Program Background Rates of Adverse Events of Special Interest for COVID-19 Vaccine Safety Monitoring Protocol. www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf (accessed 11 Mar 2021).
- 31.Food and Drug Administration. Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. In: Vaccines and Related Biological Products Advisory Committee Meeting. www.fda.gov/media/144245/download (accessed 15 September 2021)
- 32. Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021;397:72-4. 10.1016/S0140-6736(20)32623-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med 2021;384:1824-35. 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré Syndrome in the Placebo and Active Arms of a COVID-19 Vaccine Clinical Trial: Temporal Associations Do Not Imply Causality. Neurology 2021;10.1212/WNL.0000000000011881. 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 35.Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults. NCT04516746. National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT04516746 [cited 2021 Apr 1]
- 36.Food and Drug Administration. Briefing Document. Moderna COVID-19 Vaccine. In: Vaccines and Related Biological Products Advisory Committee Meeting. www.fda.gov/media/144434/download
- 37. Klein NP, Lewis N, Goddard K, et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021;326:1390-9. 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Renoud L, Khouri C, Revol B, et al. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern Med 2021;181:1243-5. 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis 2021;111:310-2. 10.1016/j.ijid.2021.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis 2022;22:64-72. 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Gao L, Tong X, et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated people in Hong Kong. MedRxiv 2021:21265314. 10.1101/2021.10.21.21265314 [DOI] [PMC free article] [PubMed]
- 42. Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med 2021;27:2144-53. 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. JAMA 2021;326:1606-13. 10.1001/jama.2021.16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020;143:3104-20. 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varatharaj A, Thomas N, Ellul MA, et al. CoroNerve Study Group . Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020;7:875-82. 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol 2009;169:382-8. 10.1093/aje/kwn310. [DOI] [PubMed] [Google Scholar]
- 47. Webb AJ, Brain SA, Wood R, Rinaldi S, Turner MR. Seasonal variation in Guillain-Barré syndrome: a systematic review, meta-analysis and Oxfordshire cohort study. J Neurol Neurosurg Psychiatry 2015;86:1196-201. 10.1136/jnnp-2014-309056. [DOI] [PubMed] [Google Scholar]
- 48. Wouk J, Rechenchoski DZ, Rodrigues BCD, Ribelato EV, Faccin-Galhardi LC. Viral infections and their relationship to neurological disorders. Arch Virol 2021;166:733-53. 10.1007/s00705-021-04959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-9 and figures 1 and 2
Data Availability Statement
Patient level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. Analytical code and detailed definitions of algorithms for identifying the events are available in a GitHub repository (https://github.com/SIDIAP/VaxAEsNeuroimmune).