Abstract
Current global changes are reshaping ecological communities and modifying environmental conditions. We need to recognize the combined impact of these biotic and abiotic factors on species interactions, community dynamics and ecosystem functioning. Specifically, the strength of predator–prey interactions often depends on the presence of other natural enemies: it weakens with competition and interference or strengthens with facilitation. Such effects of multiple predators on prey are likely to be affected by changes in the abiotic environment, altering top-down control, a key structuring force in natural and agricultural ecosystems. Here, we investigated how warming alters the effects of multiple predators on prey suppression using a dynamic model coupled with empirical laboratory experiments with Drosophila–parasitoid communities. While multiple parasitoids enhanced top-down control under warming, parasitoid performance generally declined when another parasitoid was present owing to competitive interactions. This could reduce top-down control over multiple generations. Our study highlights the importance of accounting for interactive effects between abiotic and biotic factors to better predict community dynamics in a rapidly changing world and thus better preserve ecosystem functioning and services such as biological control.
Keywords: biodiversity-ecosystem functioning, global change, temperature, functional response, host–parasitoid networks, multiple predator effects
1. Introduction
Ongoing global anthropogenic changes alter the abiotic context, changing the outcome of species interactions [1,2]. Global warming can modify the strength of trophic interactions owing to changes in metabolic rates [3], shifts in spatial distributions and seasonal phenology [4], lethal effects on predators, or altered attack rates [5–7]. However, warming also alters the strength of non-trophic interactions among predators [8,9]. Altered non-trophic interactions among predators would change the effects of multiple predators on top-down control [10,11], yet to what extent is unclear. Effects of warming on non-trophic interactions among predators are often overlooked but essential to accurately forecast ecological consequences of warming for biological control and ecosystem integrity.
The effects of multiple predators on prey suppression are often not additive. Additivity would occur if predators had independent effects on prey, in which case increased predator density should enhance top-down control because of higher predatory pressure on the prey. However, direct and indirect interactions among predators may cause effects to deviate from additivity [12–14]. The effects of multiple predators on prey can be synergistic (i.e. the effects are greater than what would be expected if they were additive) owing to niche complementarity or facilitation (i.e. risk enhancement for the prey) [15]. By contrast, the effects of multiple predators on prey can be antagonistic owing to intraguild predation, competition or interference when the degree of overlap between predator's foraging areas or phenologies is too high (i.e. risk reduction) [16]. All such potential effects are called multiple predator effects (MPEs [17]). Emergent MPEs are particularly important in biological control where introducing one or several predator species might result in risk reduction for the prey because of competition among predators instead of planned risk enhancement [18].
Warming can alter both trophic and non-trophic interactions. Changes in the strength of these interactions could modify emergent MPEs, either enhancing or decreasing top-down control. Climate change also disrupts species composition of communities [4,19], which would change the outcome of pairwise interactions that are influenced by other species in the community [20–22]. Changes in species composition of communities are thus also likely to alter MPEs, affecting biological control. However, interactive effects between warming and community composition on top-down control remain poorly studied, and little is known about how warming alters the effects of multiple predators on top-down control.
Here, we used mathematical models in combination with a series of three laboratory experiments on Drosophila simulans and three of its co-occurring larval parasitoids to investigate the effects of warming on MPEs on top-down control. Host–parasitoid interactions are a particular type of predator–prey interaction in which parasitoid larvae feed and develop inside or on an arthropod host while adults are free-living [23]. When host is parasitized, three outcomes are possible: the parasitoid successfully develops and kills the host, the host survives and successfully eliminates its parasitoid through immune response (i.e. encapsulation and melanization) [24], or both parties die. The presence of multiple parasitoids can result in extrinsic competition between adults for space and oviposition (i.e. interference) and intrinsic competition within a host [25]. Intrinsic competition results from super- or multi-parasitism events when two parasitoids (conspecific or heterospecific) parasitize the same host individual. A single parasitoid can also lay multiple eggs in a single host as part of a strategy to overwhelm the host immune system. In solitary parasitoids, such as the species used in the present study, only one individual completes development in each host, suppressing the other(s) physically or physiologically. Parasitoids represent an excellent system to study how warming directly changes the effects of multiple predators on top-down control because the outcome of the interactions is easily observed by rearing the host, and intrinsic competitive interactions between parasitoids can be observed by dissecting the host larva. In this study, we empirically measured trophic interaction strength across temperatures and parasitoid assemblages. We recorded emergent effects of multiple parasitoids on host suppression by comparing empirical data with estimates in which multiple parasitoids would not interact (i.e. would have an additive effect) using a mathematical model for multiple co-occurring parasitoids with a functional response approach [26,27]. With this framework, we addressed three specific questions: (i) do multiple parasitoids have additive, synergistic, or antagonistic effects on host suppression? (ii) to what extent does temperature modify the outcomes of MPEs? and (iii) are changes in host immune response or competitive interaction strength causing emergent MPEs? Our results demonstrate the principal role of temperature for non-trophic interactions among parasitoids, with cascading effects on host suppression.
2. Material and methods
(a) . Biological system
Cultures of D. simulans and their associated parasitoids collected from two tropical rainforest locations in North Queensland Australia: Paluma (18°59.031′ S, 146°14.096′ E) and Kirrama Range (18°12.134′ S, 145°53.102′ E; both less than 100 m above sea level; [28]) were used for the experiments. Tropical species already live close to their upper thermal limits [29]. Drosophila species are limited in their evolutionary potential for thermal adaptation [30,31], making our tropical Drosophila–parasitoid community a relevant system to study effects of future warming conditions on communities. Drosophila simulans and parasitoid cultures were established between 2017 and 2018, identified using both morphology and DNA barcoding, and shipped to the Czech Republic under permit no. PWS2016-AU-002018 from Australian Government, Department of the Environment. All cultures were maintained at 23°C and 12 L : 12 D cycle at the Biology Centre, Czech Academy of Sciences. The three larval parasitoid species Asobara sp. (Braconidae: Alysiinae; strain KHB, reference voucher no. USNMENT01557097, reference sequence BOLD process ID: DROP043–21), Leptopilina sp. (Figitidae: Eucolinae; strain 111F, reference voucher no. USNMENT01557117, reference sequence BOLD process ID: DROP053-21) and Ganaspis sp. (Figitidae: Eucolinae; strain 84BC, reference voucher no. USNMENT01557102 and USNMENT01557297, reference sequence BOLD process ID: DROP164-21) were used (for more details on the parasitoid strains see [32]). Drosophila simulans isofemale lines were kept on standard Drosophila medium (corn flour, yeast, sugar, agar and methyl-4-hydroxybenzoate) for approximately 45–70 non-overlapping generations before the experiments. To revive genetic variation, five host lines were combined to establish two population cages of mass-bred lines before the start of the experiments. Single-parasitoid isofemale lines were used and maintained for approximately 25 to 40 non-overlapping generations before the start of the experiment by providing them every week with 2-day-old larvae of a different Drosophila species—Drosophila melanogaster.
(b) . Experiments
We used a functional response approach following McCoy's framework to investigate the effects of warming on the strength of trophic and non-trophic interactions [26]. We first obtained each parasitoid functional response parameter at ambient and warmed temperatures with single-parasitoid treatments (experiment 1). Then, we used these functional response parameter estimates to predict trophic interaction strength for each temperature and parasitoid combination with the null hypothesis that parasitoids were not interacting and thus had additive effects on host suppression. In experiment 2, we empirically measured the effects of temperature and parasitoid combinations on trophic interaction strength and compared the predicted and observed values to identify emergent effects of multiple parasitoids on host suppression and their dependence on the temperature regime. We performed the two first blocks of experiment 1 and the entire experiment 2 in parallel, and controls and single-parasitoid treatments were common to both experiments. In experiment 3, we investigated the mechanisms of multiple parasitoid effects by dissecting hosts rather than rearing them. This allowed us to measure super- and multi-parasitism rates and encapsulation depending on the temperature regime and parasitoid combinations.
A total of 22 920 D. simulans eggs were collected; 13 120 for experiment 1, 4800 for experiment 2 and 5000 for experiment 3 (from which 1000 larvae were dissected). In experiments 1 and 2, 12 990 eggs (73%) successfully developed into adults (8409 hosts and 4581 parasitoids). The remaining 23% either died naturally or the host was suppressed without successful development of the parasitoid.
(i) . Experiment 1: single-parasitoid experiment
Eggs of D. simulans were placed in a single 90 mm high and 28 mm diameter glass vial with 10 ml of Drosophila media at six different densities replicated eight times each (5, 10, 15, 25, 50 or 100 eggs per 10 ml of food media in a vial, total number of eggs = 13 120, n = 384 vials; figure 1b). To collect D. simulans eggs, an egg-washing protocol was adapted from [33]. The day before the egg-washing protocol was conducted, two batches of egg-laying medium (Petri dishes with agar gel topped with yeast paste) were introduced in each population cage for flies to lay eggs overnight. Eggs were transferred to the experimental vials. We placed half of the vials at ambient temperature (22.7°C ± 0.4 s.d.—the current mean yearly temperature at the two study sites [28]), and the other half under elevated temperature (27.4°C ± 0.5 s.d.—projected change in global mean surface temperature for the late twenty-first century is 3.7°C for the IPCC RCP8.5 baseline scenario [34]). Like in other Drosophila species, the thermal performance curve of D. simulans demonstrates a decrease in performance from temperatures above 25°C [35].
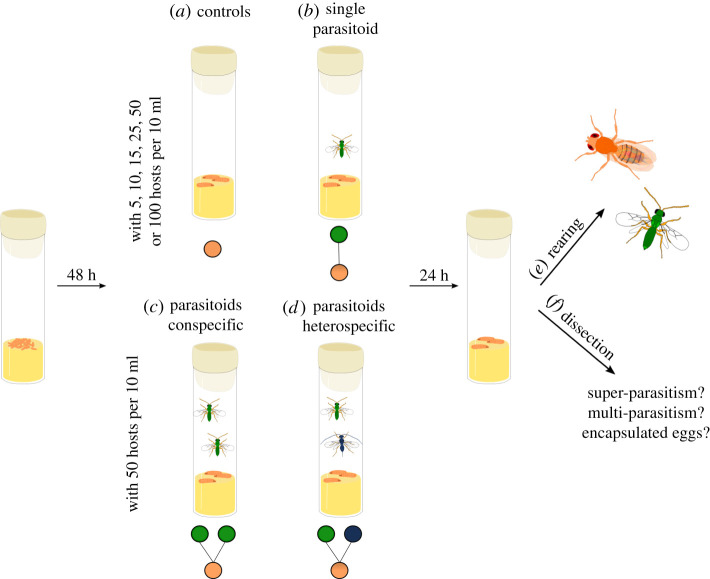
Figure 1.
Schematic representation of the experimental design. (a) Controls or (b) one single-parasitoid female with either 5, 10, 25, 50 or 100 D. simulans per 10 ml of media (n = 384 vials), (c) two parasitoids conspecific (n = 88 vials), or (d) two parasitoids heterospecific (n = 68 vials) with 50 D. simulans per 10 ml of media. (e) Rearing until adults emerge for experiments 1 and 2 (up to 41 days), or (f) dissection of 10 third instar larvae or pupae per vial 2, 3 or 4 days after infection for experiment 3. (Online version in colour.)
After 48 h, we placed one single naïve mated three to 5-day-old female parasitoid in each vial with D. simulans larvae. We removed the parasitoids 24 h later. We repeated this for all three parasitoid species, temperatures and host densities. We simultaneously performed controls without parasitoids to obtain the baseline for host survival without parasitism and potential variation during egg collection (figure 1a). We checked vials daily for adult emergences until the last emergence (up to 41 days for the species with the longest developmental time). We waited five consecutive days without any emergence to stop collecting and thus avoided confounding counts with a second generation. All emerged insects were collected, identified, sexed and stored in 95% ethanol. Each treatment was replicated eight times across eight experimental blocks.
(ii) . Experiment 2: multiple parasitoids experiment
To investigate the effect of warming on MPEs, we manipulated parasitoid assemblages and temperature in a fully factorial design (figure 1c,d). We followed the same protocol described above for experiment 1, using 50 D. simulans eggs per vial with two female parasitoids either from the same (figure 1c) or different species (figure 1d). Each treatment was replicated eight times across two blocks (n = 96). Controls and single-parasitoid treatments were standardized to experiment 1 with the 50 eggs per vial density. Fifty eggs per standard Drosophila vial corresponds to low competition between hosts, a suitable host/parasitoid ratio when using one or two parasitoids, and enough host per vial for meaningful statistics.
(iii) . Experiment 3: mechanisms of multiple predator effects
In a follow-up experiment, we conducted a subset of the treatments described for experiments 1 and 2 with Asobara sp. and Ganaspis sp. We put 50 D. simulans eggs per vial with 10 ml of food media under ambient and warming temperatures and introduced one parasitoid, two conspecific parasitoids or the two heterospecific parasitoids, resulting in five different parasitoid assemblages. Each treatment was replicated 10 times across two blocks (n = 100). Instead of rearing the insects to adults, we dissected 10 third instar larvae or pupae per vial (figure 1f). We individually transferred each host larva into a glass Petri dish containing phosphate buffered saline and dissected it under a stereomicroscope. We recorded the number of parasitoid larvae and eggs of each species to assess super- and multi-parasitism events. When possible, we also identified the number and species of encapsulated parasitoids. Pictures of the eggs, larvae and encapsulated parasitoids for each species observed during the experiment are presented in the electronic supplementary material, S1 and figure S1. At the elevated temperature, six replicates were dissected 2 days after infection (early dissection time) and four 3 days after infection (late dissection time). At the ambient temperature, four replicates were dissected 3 days after infection (early dissection time) and six 4 days after infection (late dissection time). We selected different times for dissection at each temperature to standardize parasitoid developmental stage while still being able to identify all the parasitoids that have parasitized the host. At the early dissection time, Asobara sp. were already at the larval stage, whereas Ganaspis sp. were still eggs. Ganaspis larvae were also observed at the late dissection time, sometimes simultaneously with a larva of Asobara sp. within the same host.
(c) . Data analysis and modelling
(i) . Experiment 1: single-parasitoid experiment
We combined numerical simulations of host density dynamics, accounting for host depletion [36]:
with Bayesian parameter estimation using the rstan package (e.g. [37]). p = 1 is the parasitoid density, and F(H) denotes the host density-dependent functional response. In the model fitting, we used Markov chain Monte Carlo to sample from the functional response's model parameters’ posterior probability distribution p(θ|Hsup) given the observations Hsup, based on the likelihood function p(Hsup|θ) and prior distributions p(θ), with θ the free parameters. Hsup is the number of D. simulans suppressed (the difference between adult hosts emerging from the controls without parasitoids and from the experiment). In each iteration, we computed numerical solutions of the equation with the built-in Runge–Kutta ordinary differential equation solver to predict densities after 1 day for each given initial host density, H0. The initial host densities were taken from the average number of hosts that emerged from the controls for each density and temperature to account for potential deviations between the aimed and actual densities (electronic supplementary material, table S1). The likelihood was evaluated assuming a binomial distribution for observed numbers of suppressed hosts Hsup with n = H0 trials and success probability. We used vague priors for all model parameters.
We fitted three different functional response models (Type II, Type III and generalized Type III) and retained the Type II functional response [38] after model comparison (see the electronic supplementary material, S2). The equation for the instantaneous attack rate of a parasitoid is as follows:
where a is the attack rate and h is the handling time. Type II functional responses are thought to characterize the attack rate of many types of predators and parasitoids [39]. Parameter estimates and the functional responses for each species at each temperature are presented in the electronic supplementary material, S2, table S2 and figure S2).
(ii) . Experiment 2: multiple parasitoids experiment
Host–parasitoid interaction strength was described with the combination of degree of infestation (DI; i.e. host suppression) and successful parasitism rate (SP; i.e. parasitoid performance). The observed degree of infestation (DIobs) and successful parasitism rate (SP) were measured as
where H is the number of adult hosts emerging from the experiment vial, HC is the mean number of adult hosts emerging from the controls without parasitoids and P is the number of parasitoid adults emerging from the experimental vial [40,41]. DIobs was set to zero if the number of hosts emerging from the treatment was greater than the controls. If no parasitoid emerged or the number of hosts suppressed was estimated to be zero, SP was set to zero. If the number of parasitoids that emerged was greater than the estimated number of hosts suppressed, SP was assigned to one. For treatments with parasitoid conspecifics, we assumed that each of the two parasitoid individuals was attacking the hosts equally; therefore, the number of parasitoid adults emerging was divided by two to calculate individual successful parasitism rate.
We analysed these data with generalized linear models (GLMs) and verified model assumptions with the DHARMa package [42]. To correct for overdispersion of the residuals and zero inflation, data were modelled using zero-inflation models with a beta-binomial error distribution and a logit function using the glmmTMB function from the TMB package [43]. Two categories of predictor variables were used in separate models with temperature treatment (two levels: ambient and warming): (i) parasitoid treatment (three levels; single parasitoid, two parasitoids conspecific, and two parasitoids heterospecific), and (ii) parasitoid species assemblage (nine levels). The two-way interactions between temperature and either parasitoid treatment or parasitoid assemblage were tested and kept in our models if judged to be significant based on backward model selection using likelihood-ratio tests. The significance of the effects was tested using Wald type III analysis of deviance with likelihood-ratio tests. Factor levels were compared using Tukey's HSD post hoc comparisons of all means and the emmeans package [44]. Results for developmental rate are presented in the electronic supplementary material, S3 and figure S3.
(d) . Estimation of multiple parasitoid effects
To predict the degree of infestation if parasitoids have independent effects on host suppression, we used the method developed by McCoy et al. [26], which considers host depletion. This method uses the functional responses obtained from experiment 1 in a population-dynamic model to predict how host density changes in time as a function of initial density and parasitoid combination for each temperature. We thus calculated the estimated degree of infestation (DI0) by integrating the aggregate attack rates over the duration of the experiment as host density declines. We first solved the equation
similar to the equation described for experiment 1 but adapted to n parasitoids. Then, we calculated the estimated degree of infestation as
where H0 is the initial host density and HT is the estimated host population at the end of the experiment (time T = 1 day). This method allows a reasonable estimate of DI0 for the null hypothesis that predators do not interact [27]. The lower and upper confidence intervals (CI) around the predicted values were estimated with a global sensitivity analysis based on the functional response parameters estimates to generate 100 random parameter sets using a Latin hypercube sampling algorithm [45]. The expected degree of infestation was calculated for each parameter set using the sensRange function in the R package FME. The 2.5% and the 97.5% quantiles of the values obtained from these simulations were used as 95% CIs around the predictions.
Predictions from the population-dynamic model were then compared with the observed values (DIobs). Estimated DI values greater than observed DI translate to risk reduction, while lower estimates reflect risk enhancement for the host with multiple parasitoids. We calculated the difference between DIobs and mean DI0 for each treatment and investigated the effects of temperature (ambient versus warmed), parasitoid diversity (one or two species), and their interaction if significant, using an analysis of variance with the aov function. We statistically compared the observed and estimated DI for each temperature regime using GLMs with a beta-binomial error distribution and a logit function with DI0 as an offset (i.e. predictor variable) following Sentis et al. [10]. A positive or negative significant intercept indicates that DI0 values underestimate or overestimate DIobs, respectively.
(i) . Experiment 3: multiple predator effects mechanisms
The frequency of super- and multi-parasitism events was calculated out of the larvae parasitized per vial (total of 1000 larvae dissected across 100 vials, out of which 868 were parasitized: the presence of either one or both parasitoid species and/or trace of melanization). The frequency of encapsulated parasitoids was calculated out of the total number of parasitoids per larva. Effects of temperature and parasitoid assemblages on these frequencies were analysed with generalized linear mixed models with the method described for experiment 2 and the time of dissection (early or late) as a random effect. All analyses were performed using R v. 4.0.2 [46].
3. Results
(a) . Effects of multiple parasitoids on host suppression under warming
The degree of infestation observed in the experiment varied from the model estimations (figure 2). Temperature significantly affected these differences (F1,93 = 13.9, p < 0.0001), but parasitoid diversity did not (F1,93 = 0.09, p = 0.766) (electronic supplementary material, tables S3 and S4), implying that parasitoid density rather than their diversity is important for host suppression. The comparison of the estimated and observed DI revealed that, in most cases, there was no significant difference between predicted and observed DI at ambient temperature, implying neutral effects with multiple parasitoids (when looking at the intercept of the GLM with DI0 as an offset; value ± s.e.: 0.12 ± 0.32, z value = 0.381, d.f. = 40, p = 0.703), whereas under warming, the predicted DI0 significantly underestimated the observed DIobs, implying risk enhancement for the host (value ± s.e.: 0.44 ± 0.18, z value = 2.431, d.f. = 40, p = 0.015; figure 2).
Figure 2.
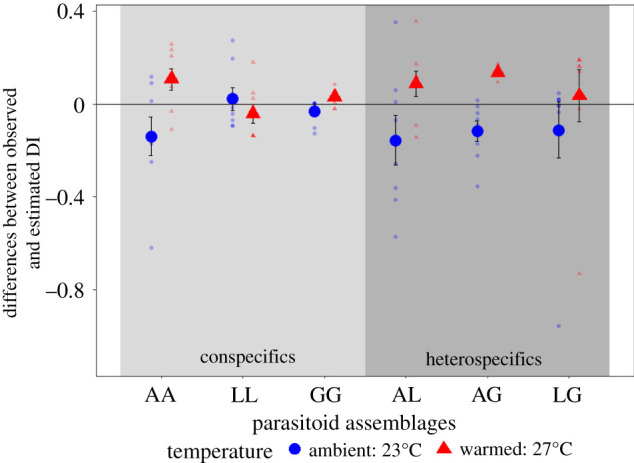
Differences between observed and estimated DI for each parasitoid assemblage and temperature (i.e. DIobs - DI0). Negative values translate to risk reduction, while positive values reflect risk enhancement for the host with multiple parasitoids. Light grey panel, two parasitoids conspecific; darker grey panel, two parasitoids heterospecific. Parasitoid abbreviations: A, Asobara sp.; L, Leptopilina sp.; and G, Ganaspis sp. Big dots represent the means (±s.e.) and small dots represent raw data. (Online version in colour.)
(b) . Effects of warming and parasitoid assemblages on the observed degree of infestation
Contrary to the effects of multiple parasitoids on host suppression, the observed degree of infestation DIobs was not significantly affected by temperature (, p = 0.306), or parasitoid treatment (single, two conspecific or two heterospecific parasitoid assemblages: , p = 0.119; electronic supplementary material, table S5) owing to species-specific effects. DI only varied with parasitoid species assemblages (, p < 0.0001, electronic supplementary material, table S6). DI was the highest in assemblages with Ganaspis sp., either alone, with a conspecific, or another parasitoid species (electronic supplementary material, figure S4).
(c) . Effect of warming and parasitoid assemblages on parasitoid performance
Despite having no effect on DI, parasitoid treatment (single, two conspecific or two heterospecific parasitoid assemblages) significantly affected successful parasitism rate, and the effect varied among parasitoid species (two-way interaction: , p = 0.002; table 1; electronic supplementary material, table S7). SP of Ganaspis sp. decreased by 95.7% (95% CI: 93.6–97.8%) with the presence of a parasitoid conspecific (post hoc odds ratio (OR) = 0.043, p < 0.0001 for contrast 2Pc/1P; electronic supplementary material, table S8), and by 83.4% (CI: 75.4–91.3%) with the presence of a parasitoid heterospecific compared to when alone (OR = 0.166, p < 0.001 for contrast 2Ph/1P; electronic supplementary material, table S8). However, it increased by 287.6% (CI: 178.8–396.4%) when the parasitoid competitor was from another species compared to a conspecific (OR = 3.876, p < 0.0001, for contrast 2Pc/2Ph; electronic supplementary material, table S8). SP of Asobara sp. decreased by 55.2% (CI: 41.5–69.7%) when a parasitoid conspecific was present compared to when alone (OR = 0.448, p = 0.036), but was not significantly affected by the presence of a parasitoid heterospecific (OR = 0.712, p = 0.484). There were no significant effects of parasitoid treatments for SP of Leptopilina sp. Effects of parasitoid assemblages on SP also varied between parasitoid species and are presented in the electronic supplementary material, S6 (electronic supplementary material, tables S9 and S10, and figure S5).
Table 1.
Odds ratios of a successful parasitism event between parasitoid treatments (single parasitoid, two parasitoids conspecific and two parasitoids heterospecific) for each parasitoid species. (Results are averaged over both temperatures because there was no significant interaction between temperature and parasitoid treatments. Values less than or greater than one denote a decrease or an increase in the odds of successful parasitism, respectively. Significant differences are highlighted in italics.)
| parasitoid species | contrast | odds ratio | p-value |
|---|---|---|---|
| Ganaspis sp. | two conspecifics/single | 0.043 | <0.0001 |
| two heterospecifics/single | 0.166 | 0.0007 | |
| heterospecifics/conspecifics | 3.876 | <0.0001 | |
| Asobara sp. | two conspecifics/single | 0.448 | 0.036 |
| two heterospecifics/single | 0.711 | 0.484 | |
| heterospecifics/conspecifics | 1.589 | 0.251 | |
| Leptopilina sp. | two conspecifics/single | 0.182 | 0.494 |
| two heterospecifics/single | 0.871 | 0.994 | |
| heterospecifics/conspecifics | 4.764 | 0.295 |
Effects of temperature on SP also depended on the species (two-way interaction: , p = 0.026; electronic supplementary material, table S7). Only Ganaspis sp. was significantly affected by temperature, and its SP decreased by 58.8% (CI: 69.8–47.8%) with warming (OR = 0.412, , p = 0.001). However, all species developed faster under warming (electronic supplementary material, figure S3).
(d) . Mechanisms of multiple predator effects
The frequency of either super- or multi-parasitism events, reflecting strength of intrinsic competition among parasitoids, was significantly affected by parasitoid assemblages (, p < 0.0001), temperature (, p = 0.034) and the interaction between parasitoid assemblages and temperature , p < 0.0001; figure 3, electronic supplementary material, table S11). Super-parasitism rate increased by 239% (CI: 230–308%) when Ganaspis sp. was with a conspecific (OR = 3.69, p < 0.0001), and by 581% (CI: 411–751%) when Asobara sp. was with a conspecific (OR = 6.81, p < 0.0001) compared to when they were alone, but without significant differences between temperature treatments. In the parasitoids heterospecific treatments, warming significantly increased the frequency of super- and multi-parasitism events by 173% (CI: 130–216%; OR = 2.73, p < 0.0001), indicating an increase in intrinsic competition among parasitoids with warming.
Figure 3.
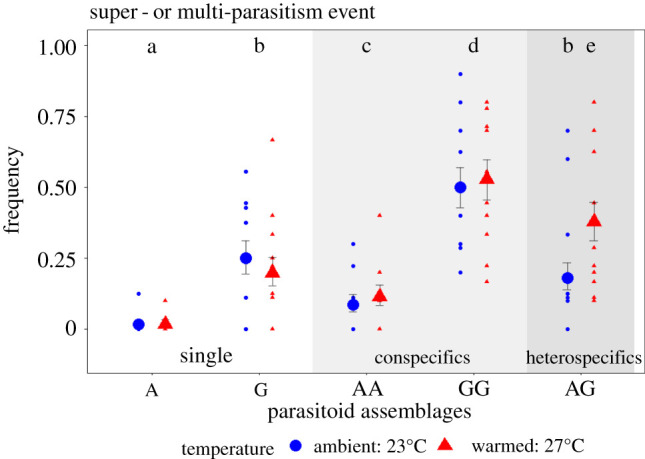
Frequency of super- or multi-parasitism events out of the total of parasitized hosts per vial (n = 100 vials) significantly changed depending on parasitoid assemblage and temperature regime, indicating changes in intrinsic competitive interaction strength among parasitoids. Within each plot, different small letters denote significant differences between parasitoid assemblages (and temperature regime if significant). White panel, single parasitoid; light grey panel, two parasitoids conspecific; darker grey panel, two parasitoids heterospecific. Parasitoid abbreviations: A, Asobara sp.; and G, Ganaspis sp. Big dots represent the estimated means (±95% CIs) and small dots represent raw data. (Online version in colour.)
The frequency of encapsulated parasitoids differed between parasitoid species, but not between treatments (results presented in the electronic supplementary material, S8, table S12 and figure S6), indicating that host immune response did not change depending on the treatments.
4. Discussion
The key result from our study is the synergistic effects of multiple predators for top-down control at elevated temperature across predator assemblages. However, parasitoid performance often decreased when multiple parasitoids were present owing to intrinsic competition among parasitoids, potentially limiting the long-term benefits for ecosystem functioning.
(a) . Warming increases the effects of multiple predators on the risk of predation
Our results showed that warming led to a higher top-down control than expected with multiple predators. Indeed, our mathematical model underestimated trophic interaction strength measured in multiple predators treatments at elevated temperatures. Our results are in concordance with previous studies on diverse systems on the importance of considering non-trophic interactions to predict the effect of multiple predators on top-down control under global changes. Drieu et al. [47] found that predator diversity enhanced the biological control of insect pests in vineyards under warming owing to functional complementarity among predator species, while effects were substitutive at ambient temperature. Cuthbert et al. [11] also found an impact of temperature on intraspecific MPEs on an invasive Gammaridae species (Amphipoda). Yet, the direction of the effects contrasted ours as they observed risk enhancement at low temperature and risk reduction with warming. Sentis et al. [10] found a general trend of predation risk reduction for the prey with multiple predators in an aquatic food web but without any effect of temperature on those emergent MPEs. Our study goes further by showing the important impact of warming on the impacts of multiple predators on prey suppression across multiple assemblages of conspecifics and heterospecifics. In addition to increasing prey suppression with multiple predators under warming in terrestrial ecosystems, a diverse predator community also increases the chances of complementarity in the face of environmental variation and disturbance [48]. Indeed, the presence of multiple predator species could mitigate the adverse effects of warming on top-down control owing to resource partitioning and/or functional redundancy [47,49,50]. Therefore, preserving predator biodiversity should be generally beneficial for top-down control under climate change.
(b) . Mechanisms behind emergent multiple predator effects on the prey
Because of the synergistic effects of multiple parasitoids on host suppression under warming found in our study, we could have hypothesized that warming weakens interference between parasitoids, similarly to predator–prey systems [51]. However, our host–parasitoid system allowed us to investigate further the potential mechanisms behind our results, especially the strength of intrinsic competitive interactions between parasitoids (i.e. frequency of super- and multi-parasitism events). We found generally higher intrinsic competition in multiple parasitoid treatments than single-parasitoid treatments and higher intrinsic competition under warming when the two species were present compared to ambient temperature. When superparasitized or multiparasitized, the host was less likely to survive, possibly because its immune response was less likely to overcome multiple parasitoids. Therefore, the higher top-down control observed under warming with multiple parasitoids was because of a higher parasitism pressure and not because of weaker interactions between parasitoids.
We conducted the experiments in simplified laboratory conditions and forced parasitoids to share the same habitat (a vial) and overlapped in time (24 h), which does not allow for resource partitioning [52]. This might have enhanced the rate of super- and multi-parasitism events and thus top-down control. In nature, warming could also change predator habitat use [8,9] and phenology [53,54], leading to changes in MPEs. However, the impact of temperature on MPEs was consistent across parasitoid assemblages, suggesting a general pattern for synergistic effects with multiple natural enemies under warming in our system.
(c) . Parasitoid performance was mostly affected by parasitoid assemblage
Despite multiple parasitoids enhancing host suppression under warming, the successful parasitism rate was often lower at both temperatures when another parasitoid individual was present, probably owing to the strong intrinsic competitive interactions observed through dissections. A decrease in parasitoid performance would potentially limit the synergistic effects of multiple parasitoids for host suppression in the long term. Similarly, another study on Drosophila–parasitoid interactions observed a significant impact of thermal regime on parasitoid success, but still without changes in the observed degree of infestation [55]. The long-term effects of warming on parasitoid populations are thus uncertain, and hosts from the next generation might benefit from lower parasitoid abundances owing to a lower rate of successful parasitism.
(d) . Similar effects of intra- versus interspecific multiple parasitoids on top-down control
Similar to other studies, we did not find significant differences between treatments with multiple conspecifics or heterospecific predators for prey suppression [56–58]. Therefore, it is essential to look at the effects of both predator diversity and density on prey suppression, rather than only using a substitutive approach (i.e. keeping predator density constant [52]), which might confound the results. When niche differentiation is allowed, for example, with habitat heterogeneity or a more prolonged timeframe that includes potential differences in phenology, an increase in predator diversity should intensify prey suppression because of functional diversity rather than because of diversity per se [58–60]. Here, two predators of the same species rather than a single predator intensified prey suppression at warmer temperatures despite the small scale of the experiment. Allowing for differentiation in habitat domain between predator species might have yielded higher prey suppression in treatments with heterospecifics and a lower rate of multi-parasitism. Given the likely ubiquity of resource partitioning in nature [61], preserving predator biodiversity would be the best strategy to maintain top-down control.
(e) . No effects of treatments on the observed degree of infestation
Warming had a significant effect on the differences between observed and estimated degree of infestation. However, temperature treatment had no significant effect on the observed degree of infestation. Moreover, prey suppression was generally higher when predator assemblages included the best-performing species, Ganaspis sp., no matter the predator treatment or temperature. A meta-analysis on the effects of predator diversity on prey suppression found a similar trend across the 46 studies taken into account [62], but also found a general positive effect of multiple predators on top-down control. Contrastingly, a meta-analysis of 108 biological control projects found no relationship between the number of agents released and biological control success for insect pests [63]. However, increasing predator diversity should be generally beneficial for top-down control by increasing the chances to have a more effective natural enemy species in the community, as was the case in our study (i.e. sampling effect model [64]). Moreover, the presence of multiple species in the community could buffer any mismatch between predator and prey species induced by warming [65]. Ganaspis sp. was the best-performing species in suppressing D. simulans across treatments. Still, its performance decreased with warming, suggesting that parasitism rate, and therefore host suppression, could also be reduced in the longer term owing to a decrease in parasitoid population.
5. Conclusion
Overall, pairwise interaction strength generally failed to accurately estimate the trophic interaction strength observed, indicating that non-trophic interactions must be considered to predict the effects of multiple predators on prey suppression and in food web studies in general [66]. While previous studies show altered MPEs with warming owing to changes in resource partitioning [8,11], our study is the first, to our knowledge, to show signs of direct effects of warming on predator interactions across predator assemblages, resulting in a higher top-down control with multiple predators at elevated temperature.
Acknowledgements
We thank Anna Mácová, Andrea Weberova, Grégoire Proudhom and Joel Brown for their help during the set-up of the experiments, and Tereza Holicová and Vincent Montbel for their help dissecting the larvae. Tereza Holicová made the drawings used for figure 1.
Data accessibility
All data used for this study are available from the Zenodo database: https://doi.org/10.5281/zenodo.5865217.
Authors' contributions
M.T.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft and writing—review and editing; N.A.P.: investigation and writing—review and editing; M.G.X.-E.: investigation and writing—review and editing; B.R.: formal analysis and writing—review and editing; J.H.: funding acquisition and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge funding support from the Czech Science Foundation grant no. 20-30690S. B.R. acknowledges the support of iDiv funded by the German Research Foundation (DFG-FZT 118, 202548816).
References
- 1.Song C, Von Ahn S, Rohr RP, Saavedra S. 2020. Towards a probabilistic understanding about the context-dependency of species interactions. Trends Ecol. Evol. 35, 384-396. ( 10.1016/j.tree.2019.12.011) [DOI] [PubMed] [Google Scholar]
- 2.Davis AJ, Lawton JH, Shorrocks B, Jenkinson LS. 1998. Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J. Anim. Ecol. 67, 600-612. ( 10.1046/j.1365-2656.1998.00223.x) [DOI] [Google Scholar]
- 3.Rall BÖC, Vucic-Pestic O, Ehnes RB, Emmerson M, Brose U. 2010. Temperature, predator-prey interaction strength and population stability. Glob. Chang. Biol. 16, 2145-2157. ( 10.1111/j.1365-2486.2009.02124.x) [DOI] [Google Scholar]
- 4.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637-669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 5.Thierry M, Hrček J, Lewis OT. 2019. Mechanisms structuring host–parasitoid networks in a global warming context: a review. Ecol. Entomol. 44, 581-592. ( 10.1111/een.12750) [DOI] [Google Scholar]
- 6.Hance T, van Baaren J, Vernon P, Boivin G. 2007. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 52, 107-126. ( 10.1146/annurev.ento.52.110405.091333) [DOI] [PubMed] [Google Scholar]
- 7.Uszko W, Diehl S, Englund G, Amarasekare P. 2017. Effects of warming on predator–prey interactions: a resource-based approach and a theoretical synthesis. Ecol. Lett. 20, 513-523. ( 10.1111/ele.12755) [DOI] [PubMed] [Google Scholar]
- 8.Barton BT, Schmitz OJ. 2009. Experimental warming transforms multiple predator effects in a grassland food web. Ecol. Lett. 12, 1317-1325. ( 10.1111/j.1461-0248.2009.01386.x) [DOI] [PubMed] [Google Scholar]
- 9.Schmitz OJ, Barton BT. 2014. Climate change effects on behavioral and physiological ecology of predator-prey interactions: implications for conservation biological control. Biol. Control 75, 87-96. ( 10.1016/j.biocontrol.2013.10.001) [DOI] [Google Scholar]
- 10.Sentis A, Gémard C, Jaugeon B, Boukal DS. 2017. Predator diversity and environmental change modify the strengths of trophic and nontrophic interactions. Glob. Chang. Biol. 23, 2629-2640. ( 10.1111/gcb.13560) [DOI] [PubMed] [Google Scholar]
- 11.Cuthbert RN, Wasserman RJ, Dalu T, Briski E. 2021. Warming mediates intraspecific multiple predator effects from an invasive crustacean. Mar. Biol. 168, 35. ( 10.1007/s00227-021-03840-z) [DOI] [Google Scholar]
- 12.Schmitz OJ. 2007. Predator diversity and trophic interactions. Ecology 88, 2415-2426. ( 10.1890/06-0937.1) [DOI] [PubMed] [Google Scholar]
- 13.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350-355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 14.Resetarits WJ, Bohenek JR, Pintar MR. 2021. Predator-specific responses and emergent multi-predator effects on oviposition site choice in grey treefrogs, Hyla chrysoscelis. Proc. R. Soc. B 288, 20210558. ( 10.1098/rspb.2021.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz OJ. 2009. Effects of predator functional diversity on grassland ecosystem function. Ecology 90, 2339-2345. ( 10.1890/08-1919.1) [DOI] [PubMed] [Google Scholar]
- 16.Laubmeier AN, Rebarber R, Tenhumberg B. 2020. Towards understanding factors influencing the benefit of diversity in predator communities for prey suppression. Ecosphere 11, e03271. ( 10.1002/ecs2.3271) [DOI] [Google Scholar]
- 17.Soluk DA. 1993. Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74, 219-225. ( 10.2307/1939516) [DOI] [Google Scholar]
- 18.Tylianakis JM, Romo CM. 2010. Natural enemy diversity and biological control: making sense of the context-dependency. Basic Appl. Ecol. 11, 657-668. ( 10.1016/j.baae.2010.08.005) [DOI] [Google Scholar]
- 19.Thierry M, Pardikes NA, Lue CH, Lewis OT, Hrček J. 2021. Experimental warming influences species abundances in a Drosophila host community through direct effects on species performance rather than altered competition and parasitism. PLoS ONE 16, e0245029. ( 10.1371/journal.pone.0245029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kéfi S, Berlow EL, Wieters EA, Joppa LN, Wood SA, Brose U, Navarrete SA. 2015. Network structure beyond food webs: mapping non-trophic and trophic interactions on Chilean rocky shores. Ecology 96, 291-303. ( 10.1890/13-1424.1) [DOI] [PubMed] [Google Scholar]
- 21.Wootton JT. 1997. Estimates and tests of per capita interaction strength: diet, abundance, and impact of intertidally foraging birds. Ecol. Monogr. 67, 45-64. ( 10.1890/0012-9615(1997)067[0045:EATOPC]2.0.CO;2) [DOI] [Google Scholar]
- 22.Thierry M, Pardikes NA, Ximénez-Embún MG, Proudhom G, Hrček J. 2021. Multiple parasitoid species enhance top-down control, but parasitoid performance is context-dependent. bioRxiv, 2021.07.16.452484. ( 10.1101/2021.07.16.452484) [DOI] [PubMed]
- 23.Godfray HCJ. 2013. Parasitoids. In Encyclopedia of biodiversity (ed. Levin SA), pp. 674-682. San Diego, CA: Academic Press. [Google Scholar]
- 24.Carton Y, Poirié M, Nappi AJ. 2008. Insect immune resistance to parasitoids. Insect Sci. 15, 67-87. ( 10.1111/j.1744-7917.2008.00188.x) [DOI] [Google Scholar]
- 25.Harvey JA, Poelman EH, Tanaka T. 2013. Intrinsic inter- and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 58, 333-351. ( 10.1146/annurev-ento-120811-153622) [DOI] [PubMed] [Google Scholar]
- 26.McCoy MW, Stier AC, Osenberg CW. 2012. Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecol. Lett. 15, 1449-1456. ( 10.1111/ele.12005) [DOI] [PubMed] [Google Scholar]
- 27.Sentis A, Boukal DS. 2018. On the use of functional responses to quantify emergent multiple predator effects. Sci. Rep. 8, 1-12. ( 10.1038/s41598-018-30244-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffs CT, et al. 2021. Molecular analyses reveal consistent food web structure with elevation in rainforest Drosophila - parasitoid communities. Ecography (Cop) 44, 403-413. ( 10.1111/ecog.05390) [DOI] [Google Scholar]
- 29.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellermann V, Overgaard J, Hoffmann AA, Fløjgaard C, Svenning J-C, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228-16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overgaard J, Kearney MR, Hoffmann AA. 2014. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob. Chang. Biol. 20, 1738-1750. ( 10.1111/gcb.12521) [DOI] [PubMed] [Google Scholar]
- 32.Lue C-H, et al. 2021. DROP: molecular voucher database for identification of Drosophila parasitoids. Mol. Ecol. Resour. 21, 2437-2454. ( 10.1111/1755-0998.13435) [DOI] [PubMed] [Google Scholar]
- 33.Nouhaud P, Mallard F, Poupardin R, Barghi N, Schlötterer C. 2018. High-throughput fecundity measurements in Drosophila. Sci. Rep. 8, 4469. ( 10.1038/s41598-018-22777-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IPCC . 2014. Climate change 2014: synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Meyer LA), p. 151. Geneva, Switzerland: IPCC. See http://www.ipcc.ch. [Google Scholar]
- 35.MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Phil. Trans. R. Soc. B 374, 20180548. ( 10.1098/rstb.2018.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum B, Rall BC. 2018. Fitting functional responses: direct parameter estimation by simulating differential equations. Methods Ecol. Evol. 9, 2076-2090. ( 10.1111/2041-210X.13039) [DOI] [Google Scholar]
- 37.Sohlström EH, Archer LC, Gallo B, Jochum M, Kordas RL, Rall BC, Rosenbaum B, O'Gorman EJ. 2021. Thermal acclimation increases the stability of a predator-prey interaction in warmer environments. Glob. Chang. Biol. 15715, 3765-3778. ( 10.1111/gcb.15715) [DOI] [PubMed] [Google Scholar]
- 38.Holling CS. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385-398. ( 10.4039/Ent91385-7) [DOI] [Google Scholar]
- 39.Fernández-Arhex V, Corley JC. 2003. The functional response of parasitoids and its implications for biological control. Biocontrol Sci. Technol. 13, 403-413. ( 10.1080/0958315031000104523) [DOI] [Google Scholar]
- 40.Carton Y, Kitano H. 1981. Evolutionary relationships to parasitism by seven species of the Drosophila melanogaster subgroup. Biol. J. Linn. Soc. 16, 227-241. ( 10.1111/j.1095-8312.1981.tb01849.x) [DOI] [Google Scholar]
- 41.Boulétreau M, Wajnberg E. 1986. Comparative responses of two sympatric parasitoid cynipids to the genetic and epigenetic variations of the larvae of their host, Drosophila melanogaster. Entomol. Exp. Appl. 41, 107-114. ( 10.1111/j.1570-7458.1986.tb00516.x) [DOI] [Google Scholar]
- 42.Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3, 3. See https://cran.r-project.org/web/packages/DHARMa.
- 43.Lüdecke D, Makowski D, Waggoner P. 2019. Performance: assessment of regression models performance. See https://cran.r-project.org/package=performance.
- 44.Lenth RV, Singman H, Love J, Buerkner P, Herve M. 2018. Emmeans: estimated marginal means, aka least-squares means. R package version 1(1),3. See https://cran.r-project.org/web/packages/emmeans.
- 45.Soetaert K, Petzoldt T. 2010. Inverse modelling, sensitivity and monte carlo analysis in R using package FME. J. Stat. Softw. 33, 1-28. ( 10.18637/jss.v033.i03)20808728 [DOI] [Google Scholar]
- 46.Team RC. 2017. R: the R project for statistical computing. See https://www.r-project.org.
- 47.Drieu R, Rusch A. 2017. Conserving species-rich predator assemblages strengthens natural pest control in a climate warming context. Agric. For. Entomol. 19, 52-59. ( 10.1111/afe.12180) [DOI] [Google Scholar]
- 48.Macfadyen S, Craze PG, Polaszek A, van Achterberg K, Memmott J. 2011. Parasitoid diversity reduces the variability in pest control services across time on farms. Proc. R. Soc. B 278, 3387-3394. ( 10.1098/rspb.2010.2673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cebolla R, Urbaneja A, van Baaren J, Tena A. 2018. Negative effect of global warming on biological control is mitigated by direct competition between sympatric parasitoids. Biol. Control 122, 60-66. ( 10.1016/j.biocontrol.2018.04.006) [DOI] [Google Scholar]
- 50.Pepi A, McMunn M. 2021. Predator diversity and thermal niche complementarity attenuate indirect effects of warming on prey survival. Am. Nat. 198, 33-43. ( 10.1086/714590) [DOI] [PubMed] [Google Scholar]
- 51.Lang B, Rall BC, Brose U. 2012. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 81, 516-523. ( 10.1111/j.1365-2656.2011.01931.x) [DOI] [PubMed] [Google Scholar]
- 52.Ives AR, Cardinale BJ, Snyder WE. 2005. A synthesis of subdisciplines: predator-prey interactions, and biodiversity and ecosystem functioning. Ecol. Lett. 8, 102-116. ( 10.1111/j.1461-0248.2004.00698.x) [DOI] [Google Scholar]
- 53.Abarca M, Spahn R. 2021. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 47, 67-74. ( 10.1016/j.cois.2021.04.008) [DOI] [PubMed] [Google Scholar]
- 54.Renner SS, Zohner CM. 2018. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165-182. ( 10.1146/annurev-ecolsys-110617-062535) [DOI] [Google Scholar]
- 55.Delava E, Fleury F, Gibert P. 2016. Effects of daily fluctuating temperatures on the Drosophila–Leptopilina boulardi parasitoid association. J. Therm. Biol. 60, 95-102. ( 10.1016/j.jtherbio.2016.06.012) [DOI] [PubMed] [Google Scholar]
- 56.Lampropoulos PD, Perdikis DC, Fantinou AA. 2013. Are multiple predator effects directed by prey availability? Basic Appl. Ecol. 14, 605-613. ( 10.1016/j.baae.2013.08.004) [DOI] [Google Scholar]
- 57.Griffin JN, Toscano BJ, Griffen BD, Silliman BR. 2015. Does relative abundance modify multiple predator effects? Basic Appl. Ecol. 16, 641-651. ( 10.1016/j.baae.2015.05.003) [DOI] [Google Scholar]
- 58.Finke DL, Snyder WE. 2008. Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488-1490. ( 10.1126/science.1160854) [DOI] [PubMed] [Google Scholar]
- 59.Krey KL, et al. 2021. Prey and predator biodiversity mediate aphid consumption by generalists. Biol. Control 160, 104650. ( 10.1016/j.biocontrol.2021.104650) [DOI] [Google Scholar]
- 60.Greenop A, Woodcock BA, Wilby A, Cook SM, Pywell RF. 2018. Functional diversity positively affects prey suppression by invertebrate predators: a meta-analysis. Ecology 99, 1771-1782. ( 10.1002/ecy.2378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chesson P. 1991. A need for niches? Trends Ecol. Evol. 6, 26-28. ( 10.1016/0169-5347(91)90144-M) [DOI] [PubMed] [Google Scholar]
- 62.Griffin JN, Byrnes JEK, Cardinale BJ. 2013. Effects of predator richness on prey suppression: a meta-analysis. Ecology 94, 2180-2187. ( 10.1890/13-0179.1) [DOI] [PubMed] [Google Scholar]
- 63.Denoth M, Frid L, Myers JH. 2002. Multiple agents in biological control: improving the odds? Biol. Control 24, 20-30. ( 10.1016/S1049-9644(02)00002-6) [DOI] [Google Scholar]
- 64.Myers JH, Higgins C, Kovacs E. 1989. How many insect species are necessary for the biological control of insects? Environ. Entomol. 18, 541-547. ( 10.1093/ee/18.4.541) [DOI] [Google Scholar]
- 65.Pardikes N, Revilla T, Lue CH, Mélanie T, Soutos-Villaros D, Hrcek J. 2021. Community context modifies response of host-parasitoid interactions to phenological mismatch under warming. Authorea ( 10.22541/au.162454818.82806593/v1) [DOI] [Google Scholar]
- 66.Kéfi S, et al. 2012. More than a meal… integrating non-feeding interactions into food webs. Ecol. Lett. 15, 291-300. ( 10.1111/j.1461-0248.2011.01732.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this study are available from the Zenodo database: https://doi.org/10.5281/zenodo.5865217.