Abstract
Objectives:
To examine whether attachment style moderates the relationship between polygenic risk scores (PRS) for posttraumatic stress disorder (PTSD) re-experiencing (PTSDREX) symptoms and the severity of and positive screen for traumatic loss-related PTSD.
Methods:
Data were analyzed from 631 U.S. veterans who endorsed “unexpected death of a loved one” as their ‘worst’ traumatic event. Multivariable models evaluated the association between PRS for PTSDREX, attachment style, and their interaction in predicting severity and positive screen for PTSD. A gene enrichment analysis was conducted to identify possible molecular mechanisms underlying the association between PTSDREX PRS and PTSD.
Results:
PTSDREX PRS (β=0.17; odds ratio [OR]=1.85), attachment style (β= −0.33; OR=0.14), and PTSDREX PRS x attachment style interaction (β= −0.12; OR=0.53) were significant predictors of the severity and positive screen for PTSD. The most significant gene set detected was the gene ontology (GO) cellular component podosome set (GO:0002102, p<3.95×10−5).
Conclusions:
Having a secure attachment style may help mitigate polygenic risk for developing traumatic loss-related PTSD in U.S. veterans. Podosomes, which are implicated in inflammatory and neuroplasticity processes, may contribute to the genetic liability to developing loss-related PTSD. Psychological treatments targeting attachment security may help mitigate increased polygenic risk for loss-related PTSD in this population.
Keywords: Veterans, posttraumatic stress disorder (PTSD), bereavement, Gene-environment interaction
1. INTRODUCTION
Posttraumatic stress disorder (PTSD) is one of the most prevalent psychiatric disorders among U.S. military veterans, with an estimated lifetime prevalence of 7.7 to 13.4%, versus 3.4 to 8.0% in the general U.S. adult population (Lehavot et al., 2018). Furthermore, veterans with PTSD have higher rates of other psychiatric disorders and suicidal behaviors relative to veterans without PTSD (Smith et al., 2016; Wisco et al., 2014). While the majority of military service members will be exposed to potentially traumatic events (PTEs), only an estimated 6.9% will develop PTSD (Smith et al., 2016). Individual differences in vulnerability for PTSD are mediated by a combination of genetic and environmental factors (Pape & Binder, 2016). Research efforts such as the National Health and Resilience in Veterans Study (NHRVS) (Pietrzak & Cook, 2013; Tamman et al., 2019; Wisco et al., 2014) and the Million Veteran Program (MVP; (Gaziano et al., 2016; Gelernter et al., 2019)), have therefore aimed to identify and characterize genetic, demographic, and psychosocial factors that may confer vulnerability or resilience to PTEs among veterans.
The most prevalent PTE globally is the unexpected death of a close family member or friend, which is experienced by 31.4% of adults (Benjet et al., 2016). Within the NHRVS sample of U.S. veterans, which is older, on average, than the general population, 61.3% experienced unexpected death of a close family member or friend, making it the most common PTE in this population (Wisco et al., 2014). Among this subset of individuals, 45.0% endorsed this traumatic loss as their ‘worst’ PTE, and we previously found that having a secure attachment style was associated with a significant reduction in PTSD symptom severity and risk for traumatic loss-related PTSD (Asch, et al. under review). In the NHRVS cohort, having a secure attachment style was also found to counteract the negative impact of both environmental (childhood trauma) and possible genetic (FKBP5 polymorphisms) risk factors for PTSD (Tamman et al., 2019).
In the current study, we tested the hypothesis that having a secure attachment style may protect against polygenic risk for the severity and probable diagnosis of traumatic loss-related PTSD. Polygenic risk, which was calculated per individual using summed weights of number of risk alleles for PTSD, was estimated using genome-wide association study (GWAS)-derived polygenic risk scores (PRS) for re-experiencing symptoms (PTSDREX) in the MVP, which sampled a large military veteran cohort (Gelernter et al., 2019). Re-experiencing or ‘intrusion’ symptoms, such as nightmares and flashbacks related to the traumatic event, are largely unique to PTSD, in contrast to negative mood symptoms, for example, which are characteristic of other disorders such as major depressive disorder or persistent complex bereavement disorder (Association, 2013; Malgaroli et al., 2018). Further, re-experiencing symptoms present a very high genetic correlation (rg>0.9) with other PTSD symptom clusters (Stein et al., 2019). To date, the MVP PTSDREX GWAS is the most powerful publicly available dataset we can use to investigate the genetic liability for PTSD (Gelernter et al., 2019). To examine whether this association was differentially related to the phenotypic presentation of this disorder, we additionally examined the relation between PTSDREX, attachment style, and the interaction of PTSDREX and attachment style in relation to severity of the four PTSD symptom clusters (DSM5): intrusions; avoidance, negative alterations in cognitions and mood; and alterations in arousal and reactivity. Finally, to provide insight into possible biological mechanisms driving any observed associations, we conducted a pathway enrichment analysis on the PTSDREX PRS gene set.
2. METHODS
2.1. Participants.
The sample consisted of 631 unrelated American veterans of European descent who reported “unexpected death of a loved one” as their ‘worst’ traumatic event. As previously reported (Tamman et al., 2020), ancestry and relatedness were verified using genetic information. These veterans completed a web-based survey and provided a saliva sample for genotyping as part of the NHRVS; 482 participated in the 2011 wave and 151 in the 2013 wave of the NHRVS. As described in detail elsewhere (Pietrzak & Cook, 2013; Wisco et al., 2014), veterans were recruited from KnowledgePanel, a survey research panel of over 50,000 U.S. households that is maintained by the survey research company Ipsos. Panel members are recruited through national random samples, which provide coverage of approximately 98% of US households. All participants provided informed consent. The Human Subjects Subcommittee of the Veterans Affairs (VA) Connecticut Healthcare System approved all study procedures. None of the authors have any conflicts of interest to disclose.
2.2. Data availability.
Data from the National Health and Resilience in Veterans Study are not publicly available. Individuals interested in collaborating on projects using data from this study may contact the corresponding author, Robert Pietrzak, PhD, MPH: robert.pietrzak@yale.edu.
2.3. Genotyping.
Saliva was collected using Oragene DNA (OG-250) kits and DNA was extracted using prepIT-L2 P reagent (DNA Genotek, Ontario, Canada). Samples were genotyped at the Gelernter Laboratory (VA Connecticut Healthcare System, West Haven, CT) using the PsychChip GWAS array and genotypes were called using GenomeStudio software V2011.1 with genotyping module V1.8.4 (Illumina, San Diego, CA, USA).
2.4. Polygenic risk scores.
PRS for NHRVS samples were computed using PRSice software (Choi & O’Reilly, 2019), using summary statistics generated from the PTSDREX GWAS conducted on the MVP cohort (N=146,660) (Gelernter et al., 2019). We verified that no overlap is present between NHRVS and MVP cohorts. The PRS were calculated after using p-value-informed clumping with a LD cut-off of R2 = 0.3 within a 500-kb window, and excluding the Major Histocompatibility Complex region of the genome because of its complex LD structure. In the line with the clumping/thresholding method (Choi et al., 2020), a range of genome-wide association p-value thresholds (PT=5×10−8, 10−7, 10−6, 10−5, 10−4, 0.001, 0.05, 0.3, 0.5, 1) were considered for SNP inclusion. Ultimately, the PRS defined at PT=0.3 was selected, as it had the largest magnitude association with both severity (r=0.16 vs. r= −0.01 to 0.15) and positive screen (r=0.14 vs. 0.01 to 0.13) for PTSD symptoms in this veteran population expressing sudden loss an their most significant trauma. PRS were standardized prior to analysis for ease of interpretation.
2.5. Trauma and PTSD.
Trauma history was assessed using the Trauma History Screen (Carlson et al., 2011) and PTSD symptoms using the PTSD Checklist (PCL) (Weathers et al., 1993). The DSM-IV version of the PCL was administered in the 2011 NHRVS cohort and the DSM-5 version in the 2013 cohort; scores were standardized separately in each sample and then harmonized for analyses; a score ≥50 on the DSM-IV version and ≥36 on the DSM-5 version (Moshier et al., 2019) were indicative of a positive screen for PTSD. Four PTSD symptom clusters were analyzed in secondary analyses: re-experiencing/intrusions; avoidance; emotional numbing/negative alterations in cognitions and mood; and hyperarousal/alterations in arousal and reactivity.
2.6. Attachment style.
Attachment style was assessed using the 3-Item Adult Attachment Style Questionnaire (ASQ) (Hazan & Shaver, 1987). Veterans were asked to select which of three statements best described their feelings and attitudes in relationships: a] “feeling that it is easy to get close to others and feeling comfortable with them”, b] “feeling uncomfortable being close to others”, or c] “feeling that others are reluctant to get close”, corresponding to secure, avoidant, and anxious/ambivalent attachments styles, respectively. As only 3% of veterans responded “c,” avoidant and anxious/ambivalent styles were collapsed into a single insecure attachment category.
2.7. Data analysis.
Multivariable linear and binary logistic regression analysis were conducted to examine the relation between PTSDREX PRS, attachment style, and their interaction in predicting severity of PTSD symptoms and positive screen for PTSD, respectively, among veterans endorsing “unexpected death of a loved one” as their most significant, or ‘worst’ traumatic experience. Age, sex, combat status, number of lifetime traumas other than traumatic loss, NHRVS cohort (2013 vs. 2011), and the top 10 ancestry principal components were entered as independent variables in these analyses (Table 1). Secondary linear regression analyses were then conducted to examine the relation between PTSDREX PRS, attachment style, and their interaction in predicting severity of the four PTSD symptom clusters.
Table 1.
Multivariable linear and binary logistic regression models examining the relation between PTSDREX PRS, attachment style, and their interaction in predicting severity of PTSD symptoms and positive screen for PTSD.
| Multivariable linear regression model predicting PTSD symptom severity Adjusted R2=0.47 |
Multivariable logistic regression model predicting positive screen for PTSD Nagelkerke R2=0.22 |
|||||
|---|---|---|---|---|---|---|
| β | t | p | Wald X2 | p | OR (95%CI) | |
| PTSDREX PRS | 0.17 | 2.87 | 0.004 | 7.97 | 0.005 | 1.85 (1.21–2.83) |
| Secure Attachment Style | −0.33 | 10.26 | <.001 | 35.39 | <.001 | 0.14 (0.07–0.27) |
| PTSDREX PRS x Attachment | −0.12 | 1.99 | 0.048 | 4.64 | 0.031 | 0.53 (0.30–0.94) |
Note. Results are adjusted for age, sex, combat veteran status, number of lifetime traumas other than traumatic loss, time since traumatic loss, NHRVS cohort, and the top 10 ancestry principal components.
2.8. Polygenic risk gene set enrichment.
The PRSice 2 software (Choi & O’Reilly, 2019) as used to perform gene set enrichment for three models (PT=0.3): (Model 1) PTSDREX predicting traumatic loss-related PTSD covaried for age, sex, and 10 principal components of ancestry, (Model 2) PTSDREX predicting traumatic loss-related PTSD additionally covaried for attachment style, and (Model 3) PTSDREX predicting traumatic loss-related PTSD additionally covaried for an attachment style by PTSDREX PRS interaction term. The PRSset analysis will calculate subsets of the PTSDREX PRS stratified by specific gene sets and test them with respect to the three models described. Differently from the standard PRS analysis, the clumping procedure is performed separately for each gene sets. PRSet gene sets were obtained from the Molecular Signatures Database (MSigDB) (Liberzon et al., 2015) for biological process, cellular component, and molecular function gene ontologies (GO). GO enrichments with p<0.05 were further analyzed using REVIGO (Reduce and Visualize Gene Ontology) (Supek et al., 2011), applying 0.4 similarity score and the Homo sapiens GO term database.
3. RESULTS
3.1. Sample characteristics.
On average, veterans were 63.9 years of age (SD=14.2, range=22–90), predominantly male (n=579, 93.1%) and non-combat veterans (n=456; 74.6%). Three-quarters (n=484; 75.2%) reported having a secure attachment style, while a fourth (n=147; 24.8%) reported having an insecure attachment style. The mean number of years since traumatic loss was 19.7 (SD=17.6, range=0–76 years), and the mean number of potentially traumatic events other than traumatic loss was 2.2 (SD=2.1, range=0–10). Seventy-four (12.7%) veterans screened positive for traumatic loss-related PTSD.
3.2. Traumatic loss-related PTSD.
As shown in Table 1, results of a linear regression analysis revealed significant main effects of PRS PTSDREX, secure attachment style, and a significant PRS x secure attachment style interaction (Figure 1a) in predicting severity of overall traumatic loss-related PTSD symptoms. Results of a logistic regression analysis revealed a significant main effect of PRS, secure attachment style, as well as a significant interaction of PRS x attachment style (Figure 2) in predicting a positive screen for loss-related PTSD.
Figure 1.
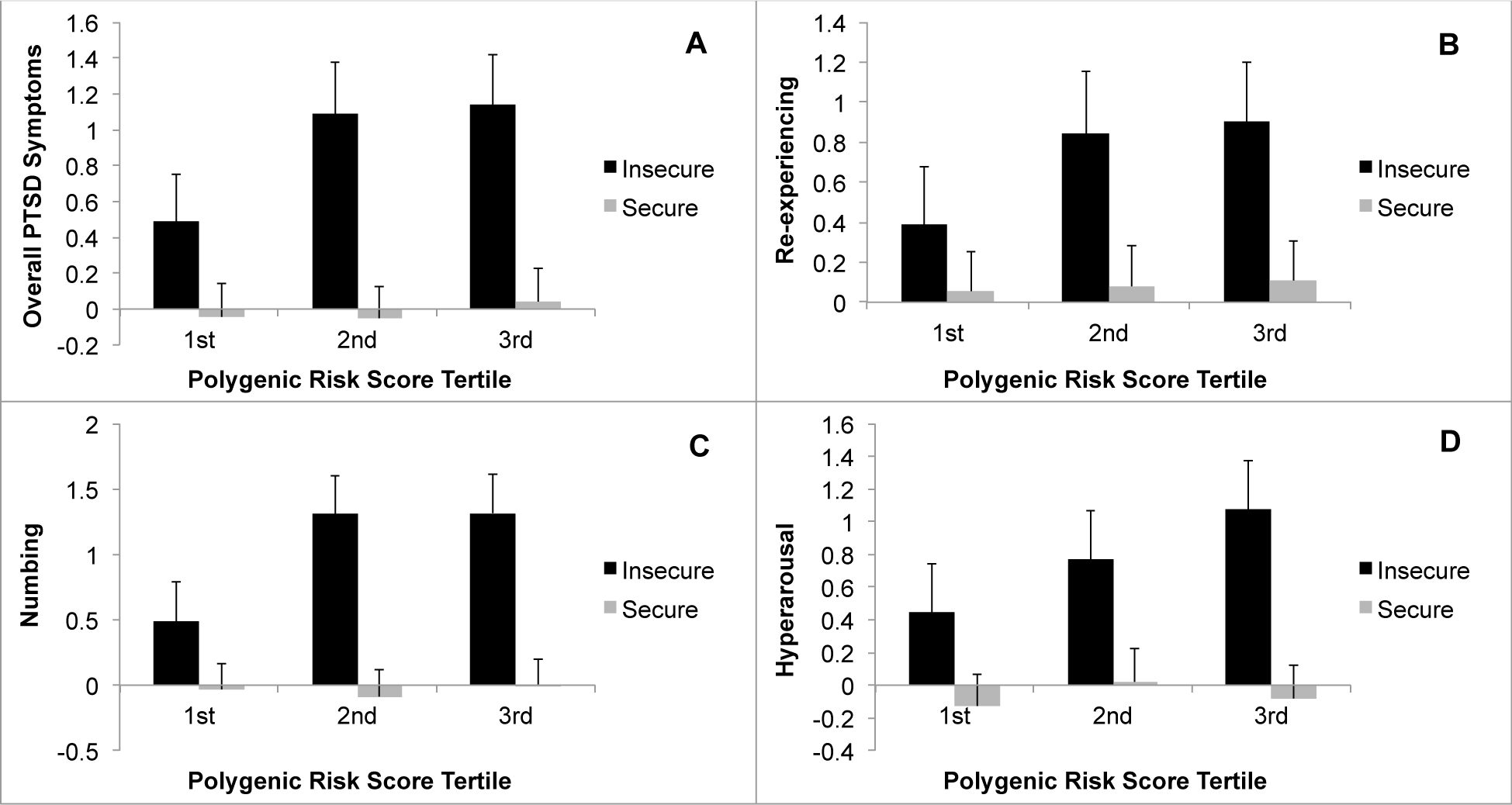
Interaction between polygenic risk score (PRS) of posttraumatic stress disorder re-experiencing (PTSDREX) and attachment style (insecure vs. secure) in predicting severity of overall traumatic loss-related PTSD symptoms (A), and re-experiencing (B), numbing (C), and hyperarousal (D) symptom clusters. Data presented as mean ±95% confidence intervals for PTSD symptom severity for veterans in the 1st, 2nd and 3rd PRS tertiles.
Figure 2.
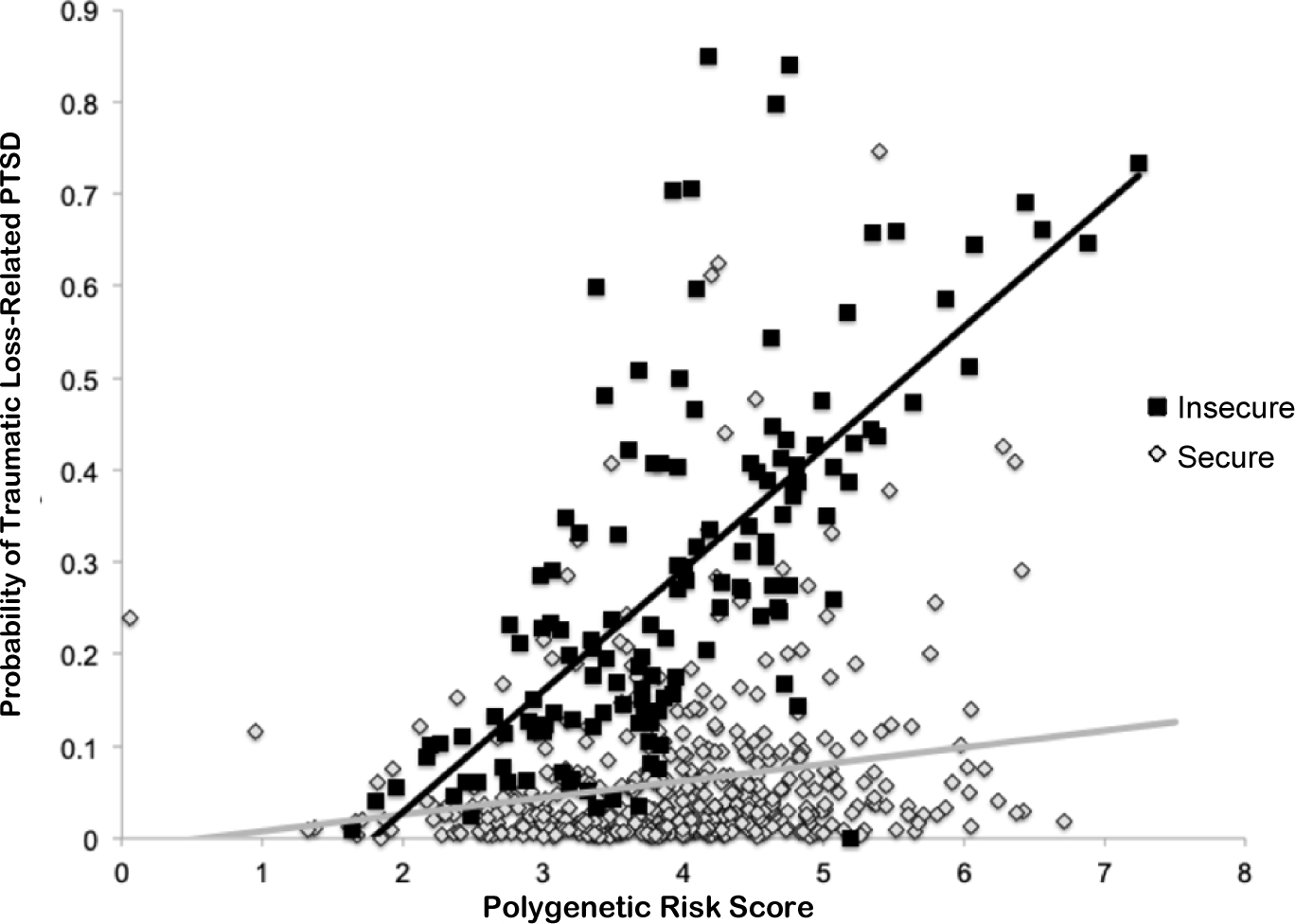
Results of multivariable logistic regression model illustrating the association between PTSDREX PRS and the probability of traumatic loss-related PTSD in veterans with insecure and secure attachment style.
3.3. Traumatic loss-related PTSD symptom clusters.
There were significant main effects of PTSD-REX PRS and attachment style, as well as significant PRS x attachment style interactions in predicting severity of intrusions (β=0.21, t=2.67, p=0.008; β= −0.24, t=5.22, p=2.8×10−7; and β= −0.19, t=2.40, p=0.017, respectively; Figure 1b), numbing (β=0.21, t=2.66, p=0.008; β= −0.39, t=8.73, p=6.9×10−17; and β= −0.17, t=2.23, p=0.026, respectively; Figure 1c), and hyperarousal (β=0.30, t=4.31, p=1.9×10−5; β= −0.35, t=8.95, p=5.8×10−18; and β= −0.22, t=3.24, p=0.001, respectively; Figure 1d). The main effect of PTSD-REX PRS and the interaction of PTSD-REX PRS x attachment style were not significant for avoidance symptoms (both β’s<0.02, p’s>0.84).
3.4. Gene set enrichment.
Although none of the gene sets survived multiple testing correction, the most significant gene set detected by all three PRS models was the GO cellular component podosome gene set (GO:0002102, p<3.95×10−5; Table 2 and Figure S1). Using nominally significant GO terms from each model, four biological processes, four cellular components, and nine molecular functions were deemed indispensable (REVIGO dispensability=0.0) across models (Table 2). All biological process, cellular component, and molecular function networks are shown in Figures S2–S4.
Table 2.
Gene ontology (GO) network summary of indispensable (i.e., REVIGO dispensability =0.0) gene sets from polygenic risk score (PRS) models of posttraumatic stress disorder re-experiencing (PTSDREX) predicting traumatic loss-related PTSD. Model 1 was covaried for age, sex, and 10 principal components of ancestry. Model 2 was additionally covaried for attachment style. Model 3 was additionally covaried for attachment style and the interactive term of attachment style x PTSDREX PRS. The proportion of genes contributing to each gene set hit relative to the total number of genes belonging to that gene set (PTSDREX PRS/ GO gene set) is provided. The full list of altered genes for each hit can be found in Table S1.
| Type | GO | Description | Model 1 P value | Model 2 P value | Model 3 P value | REVIGO uniqueness score mean (SD) | Genes PTSDREX PRS/ GO gene set |
|---|---|---|---|---|---|---|---|
| Biological Process | 0007029 | Endoplasmic reticulum organization | 0.001 | 0.001 | 9.63x10−4 | 0.947 (0.006) | 44/57 |
| 0010887 | Negative regulation of cholesterol storage | 2.09x10−4 | 2.10x10−4 | 2.22x10−4 | 0.880 (0) | 9/9 | |
| 0019230 | Proprioception | 5.92x10−4 | 5.83x10−4 | 5.61x10−4 | 0.960 (0) | 4/5 | |
| 0035640 | Exploration behavior | 0.013 | 0.013 | 0.012 | 0.980 (0) | 18/25 | |
| Cellular Component | 0002102 | Podosome | 3.54x10−5 | 3.56x10−5 | 3.95x10−5 | 0.683 (0.011) | 25/29 |
| 0099080 | Supramolecular complex | 0.017 | 0.017 | 0.016 | 0.950 (1.36x10−16) | 958/1285 | |
| 0099081 | Supramolecular polymer | 0.011 | 0.011 | 0.010 | 0.767 (0.025) | 756/985 | |
| 0071437 | Invadopodium | 4.27x10−4 | 4.30x10−4 | 4.47x10−4 | 0.903 (0.006) | 13/16 | |
| Molecular Function | 0004672 | Protein kinase activity | 9.61x10−4 | 0.61x10−4 | 9.04x10−4 | 0.823 (0.006) | 487/591 |
| 0008307 | Structural constituent of muscle | 0.018 | 0.018 | 0.012 | 0.950 (1.36x10−16) | 38/43 | |
| 0022842 | Narrow pore channel activity | 8.23x10−4 | 0.32x10−4 | 9.76x10−4 | 0.857 (0.011) | 18/19 | |
| 0030228 | Lipoprotein particle receptor activity | 0.049 | 0.049 | 0.049 | 0.970 (1.36x10−16) | 14/15 | |
| 0050544 | Arachidonic acid binding | 4.35x10−4 | 4.40x10−4 | 4.27x10−4 | 0.860 (0) | 4/5 | |
| 0099602 | Neurotransmitter receptor regulator activity | 0.005 | 0.005 | 0.006 | 0.900 (0) | 9/14 | |
| 0031013 | Troponin I binding | 0.004 | 0.004 | 0.004 | 0.903 (0.006) | 4/5 | |
| 0048037 | Cofactor binding | 5.40x10−4 | 5.43x10−4 | 4.85x10−4 | 0.960 (0) | 390/501 | |
| 1901567 | Fatty acid derivative binding | 0.023 | 0.023 | 0.025 | 0.960 (0) | 21/27 |
4. DISCUSSION
Using data from a nationally representative sample of U.S. veterans who reported unexpected traumatic loss of a loved one as their worst event, we found that both PRS for re-experiencing symptoms of PTSD (PTSDREX PRS) and attachment style were significantly associated with severity of traumatic loss-related PTSD symptoms. Further, a significant PTSDREX PRS x attachment style interaction emerged, suggesting that having a secure attachment style may help confer resilience to traumatic loss-related PTSD in individuals with high polygenic risk, such that the association between PTSDREX PRS and symptom severity was markedly reduced in securely attached veterans relative to those with insecure attachment style. A similar pattern was observed for re-experiencing, numbing, and hyperarousal symptom clusters. No significant effects of PTSDREX PRS or PTSDREX PRS by attachment style interactions were observed for avoidance symptoms. This finding may relate to the PRS itself, which was developed to be specific to re-experiencing symptoms (Gelernter et al., 2019), and could suggest different underlying polygenic and PRS x environmental mechanisms underlying avoidance symptoms. It is also possible, since all veterans included in these analyses experienced traumatic loss and, to promote generalizability, we purposely did not exclude for any comorbidities, that avoidance symptoms may be less specific to traumatic loss related-PTSD and more broadly associated with the expression of grief or bereavement (Baker et al., 2016; Schnider et al., 2007). It is certainly possible that the PRS for PTSDREX phenotype may – directly and interactively – be linked to other psychiatric phenotypes associated with traumatic loss, such as persistent complex bereavement, major depressive, or substance use disorders, and may not be unique to PTSD, per se. Further research is needed to evaluate this possibility.
A multivariable logistic regression model revealed that PTSDREX PRS was a significant predictor of a positive screen for traumatic loss-related PTSD. The significant PTSDREX PRS x attachment style interaction provides further evidence that secure attachment may mitigate the influence of polygenic risk on screening positive for traumatic loss-related PTSD. The present findings are consistent with our previous report that secure attachment counteracted the negative impact of candidate risk polymorphisms on PTSD symptom severity (Tamman et al., 2019). They extend these results to suggest that, by assessing polygenic risk, attachment style may additionally moderate polygenic risk for PTSD (i.e. not limited to candidate gene markers) in veterans who reported unexpected loss as their worst event. Our enrichment analysis provides further insight into possible biological mechanisms (i.e., contribution of multiple variants located in different genes) that may underlie this association.
In our exploratory PTSDREX PRS gene set enrichment analysis, the strongest result was observed for genes involved in podosome cellular structure. Genes involved in invadopodium structure and function (GO: 0071437) also emerged. Podosomes and invadopodia are microglial structures that mediate inflammatory responses and matrix remodeling following disease or injury (Vincent et al., 2012). Podosomes and invadopodia are further implicated neuron motility and neurite outgrowth, thereby influencing neurodevelopment and plasticity (Tanna et al., 2019). Although no known studies have investigated the role of these cellular structures in PTSD pathogenesis, a recent multivariate gene-by-environment genome-wide interaction study in >120,000 UK Biobank participants identified extracellular matrix biology and synaptic plasticity as biological mediators of the effects of PTSD and trauma on genetic risk for suicidal behavior (Wendt et al., 2020). Taken together, these findings suggest a need for further study into the possible role of extracellular matrix and glial structural elements in the pathophysiology of PTSD (Bach et al., 2019).
Another molecular function gene set, neurotransmitter receptor regulator activity (GO:0099602), stood out as particularly meaningful for the genetic relationships investigated in this study. Dysregulation of neurotransmitter signaling, especially dopamine, has been implicated in PTSD (Lee et al., 2016) and similar psychiatric disorders, while a secure attachment style has been found to ameliorate effects on dopamine dysregulation associated with these disorders (McCormick et al., 2019; Verbeke et al., 2014; Wazana et al., 2015). Adult attachment, which shapes internal working models of the self and others in close relationships, may influence how individuals perceive, cope, and process emotional memories related to objective and subjective threats and may thus influence responses to trauma in general, and traumatic loss in particular (Bowlby, 1977; Edelstein, 2006). While secure attachment may promote healthy adaptation to traumatic loss, it is also possible that living with traumatic loss-related PTSD may lead to an erosion of attachment security and social support (Kaniasty & Norris, 2008).
The cross-sectional design of this study is a recognized limitation. Longitudinal and prospective studies are needed to provide further insight as to the dynamics of the relationship between polygenic risk and attachment style and its relation to traumatic loss-related PTSD. Of note, recent work from our group revealed that higher PTSDREX PRS were associated with greater severity of PTSD symptoms, with evidence of bidirectional associations between attachment style and PTSD diagnosis and symptom severity (Tamman et al., 2020). This evidence, coupled with results of the current study, supports the notion of a possible shared biology (i.e., pathways, mechanisms, gene-sets) between attachment style and PTSD.
It is also noteworthy that the present study sample was comprised predominantly of older, European-American male, non-combat veterans. Replicating these findings in a non-veteran/civilian population is an important next-step in establishing whether the present findings may generalize to other populations. It was necessary to exclude our analysis to European-ancestry study subjects because we lack a sufficiently powerful reference GWAS for PTSDREX in any other ancestry group; this is a common limitation of GWAS and post-GWAS studies, and it underscores the need to replicate the results reported herein in more diverse samples of veterans, as well as other populations affected by traumatic loss.
We also recognize that the brief ASQ, while being a widely used and validated measure of adult attachment style (Crowell & Treboux, 1995; Hazan & Shaver, 1987), is limited and categorical in nature; nevertheless, responses on the ASQ have been found to correlate strongly with other measures of adult attachment (Sperling et al., 1996). Furthermore, as administered here, the ASQ captures only current feelings and attitudes toward relationships, which are likely influenced by PTSD in general and traumatic loss of a loved one in particular. As already mentioned, prospective studies would help in this regard, as well as incorporating more thorough assessments of adult attachment that would enable a more extensive analysis of factors influencing adult attachment. Future studies aimed at understanding how different insecure attachment subtypes and aspects of attachment may be more closely associated with emotional and behavioral responses to stress and predispose an individual to developing PTSD following trauma are warranted; how these relationships may change over the course of illness and respond to targeted treatment strategies is of particular interest and clinical relevance.
Notwithstanding these limitations, results of this study suggest that having a secure attachment style may help mitigate polygenic risk for traumatic loss-related PTSD in U.S. veterans who report the unexpected loss of a loved one as their worst traumatic life event. Further, they provide a basis for investigating the potential of new treatment strategies. For example, psychotherapeutic modalities targeted at promoting secure interpersonal support systems, such as interpersonal therapy (IPT), while initially developed within the context of mood disorders, could be tested for efficacy among individuals suffering with traumatic loss-related PTSD (Althobaiti et al., 2020; Markowitz & Weissman, 2004). The results of the exploratory genetic analysis encourage further investigations of specific plasma membrane signaling cascades, including both immune and neurotransmitter signaling, that may contribute to the pathophysiology of traumatic loss-related PTSD. Further research is needed to replicate these findings and examine how polygenic risk and attachment style relate to other aspects of loss-related psychopathology (e.g., prolonged grief disorder); identify biopsychosocial mechanisms that mediate the relationship between secure attachment style and loss-related PTSD; and evaluate the efficacy of interventions designed to promote secure attachment in mitigating risk for loss-related PTSD in other samples of veterans, as well as non-veteran populations affected by traumatic loss.
Supplementary Material
Figure S1. Summary of associations for three polygenic risk score (PRS) models of posttraumatic stress disorder re-experiencing (PTSDREX) predicting traumatic loss-related PTSD: (Model 1) was covaried for age, sex, and 10 principal components of ancestry, (Model 2) was additionally covaried for attachment style, and (Model 3) was additionally covaried for attachment style and the interactive term of attachment style by PTSDREX PRS. The most significant gene set is labeled.
Figure S2. Representative Gene Ontology network for biological processes detected by models for PTSDREX PRS predicting traumatic loss-related PTSD. Due to lack of distinct observational network sub-clusters, network node positions were adjusted to improve visual clarity.
Figure S3. Representative Gene Ontology network for cellular components detected by models for PTSDREX PRS predicting traumatic loss-related PTSD.
Figure S4. Representative Gene Ontology network for molecular functions detected by models for PTSDREX PRS predicting traumatic loss-related PTSD.
Acknowledgements:
The National Health and Resilience in Veterans Study is supported by the U.S. Department of Veterans Affairs National Center for Posttraumatic Stress Disorder. Preparation of this report was supported in part by T32 MH014276 (RHA) and F32 MH122058 (FRW). The authors thank the veterans who participated in this study and the Ipsos staff who helped coordinate it.
Footnotes
Statement of Interest: None to declare
REFERENCES
- Althobaiti S, Kazantzis N, Ofori-Asenso R, Romero L, Fisher J, Mills KE, & Liew D (2020). Efficacy of interpersonal psychotherapy for post-traumatic stress disorder: A systematic review and meta-analysis. Journal of Affective Disorders, 264, 286–294. 10.1016/j.jad.2019.12.021 [DOI] [PubMed] [Google Scholar]
- Association AP (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Bach DR, Brown SA, Kleim B, & Tyagarajan SK (2019). Extracellular matrix: a new player in memory maintenance and psychiatric disorders. Swiss medical weekly, 149, w20060. [DOI] [PubMed] [Google Scholar]
- Baker AW, Keshaviah A, Horenstein A, Goetter EM, Mauro C, Reynolds C 3rd, Zisook S, Shear MK, & Simon NM (2016). The role of avoidance in complicated grief: A detailed examination of the Grief-Related Avoidance Questionnaire (GRAQ) in a large sample of individuals with complicated grief. J Loss Trauma, 21(6), 533–547. 10.1080/15325024.2016.1157412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Bromet E, Karam E, Kessler R, McLaughlin K, Ruscio A, Shahly V, Stein DJ, Petukhova M, & Hill E (2016). The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychological medicine, 46(2), 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J (1977). The making and breaking of affectional bonds: I. Aetiology and psychopathology in the light of attachment theory. The British journal of psychiatry, 130(3), 201–210. [DOI] [PubMed] [Google Scholar]
- Carlson EB, Smith SR, Palmieri PA, Dalenberg C, Ruzek JI, Kimerling R, Burling TA, & Spain DA (2011). Development and validation of a brief self-report measure of trauma exposure: the Trauma History Screen. Psychol Assess, 23(2), 463–477. 10.1037/a0022294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Mak TS, & O’Reilly PF (2020). Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc, 15(9), 2759–2772. 10.1038/s41596-020-0353-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, & O’Reilly PF (2019). PRSice-2: Polygenic Risk Score software for biobank-scale data. GigaScience, 8(7), giz082. 10.1093/gigascience/giz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JA, & Treboux D (1995). A Review of Adult Attachment Measures: Implications for Theory and Research. Social Development, 4(3), 294–327. 10.1111/j.1467-9507.1995.tb00067.x [DOI] [Google Scholar]
- Edelstein RS (2006). Attachment and emotional memory: Investigating the source and extent of avoidant memory impairments. Emotion, 6(2), 340. [DOI] [PubMed] [Google Scholar]
- Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, Guarino P, Aslan M, Anderson D, LaFleur R, Hammond T, Schaa K, Moser J, Huang G, Muralidhar S, Przygodzki R, & O’Leary TJ (2016). Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol, 70, 214–223. 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, Lu Q, Hu Y, Li B, Radhakrishnan K, Aslan M, Cheung KH, Li Y, Rajeevan N, Sayward F, Harrington K, Chen Q, Cho K, Pyarajan S, Sullivan PF, Quaden R, Shi Y, Hunter-Zinck H, Gaziano JM, Concato J, Zhao H, & Stein MB (2019). Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci, 22(9), 1394–1401. 10.1038/s41593-019-0447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan C, & Shaver P (1987). Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology, 52(3), 511–524. 10.1037/0022-3514.52.3.511 [DOI] [PubMed] [Google Scholar]
- Kaniasty K, & Norris FH (2008). Longitudinal linkages between perceived social support and posttraumatic stress symptoms: sequential roles of social causation and social selection. J Trauma Stress, 21(3), 274–281. 10.1002/jts.20334 [DOI] [PubMed] [Google Scholar]
- Lee JC, Wang LP, & Tsien JZ (2016). Dopamine Rebound-Excitation Theory: Putting Brakes on PTSD. Front Psychiatry, 7, 163. 10.3389/fpsyt.2016.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K, Katon JG, Chen JA, Fortney JC, & Simpson TL (2018). Post-traumatic Stress Disorder by Gender and Veteran Status. American Journal of Preventive Medicine, 54(1), e1–e9. 10.1016/j.amepre.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, & Tamayo P (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell systems, 1(6), 417–425. 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli M, Maccallum F, & Bonanno GA (2018). Symptoms of persistent complex bereavement disorder, depression, and PTSD in a conjugally bereaved sample: a network analysis. Psychological medicine, 48(14), 2439–2448. [DOI] [PubMed] [Google Scholar]
- Markowitz JC, & Weissman MM (2004). Interpersonal psychotherapy: principles and applications. World psychiatry : official journal of the World Psychiatric Association (WPA), 3(3), 136–139. [PMC free article] [PubMed] [Google Scholar]
- McCormick EM, McElwain NL, & Telzer EH (2019). Alterations in adolescent dopaminergic systems as a function of early mother-toddler attachment: A prospective longitudinal examination. International Journal of Developmental Neuroscience, 78(1), 122–129. 10.1016/j.ijdevneu.2019.06.010 [DOI] [PubMed] [Google Scholar]
- Moshier SJ, Lee DJ, Bovin MJ, Gauthier G, Zax A, Rosen RC, Keane TM, & Marx BP (2019). An Empirical Crosswalk for the PTSD Checklist: Translating DSM-IV to DSM-5 Using a Veteran Sample. Journal of Traumatic Stress, 32(5), 799–805. 10.1002/jts.22438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape JC, & Binder EB (2016). The role of genetics and epigenetics in the pathogenesis of posttraumatic stress disorder. Psychiatric Annals, 46(9), 510–518. [Google Scholar]
- Pietrzak RH, & Cook JM (2013). Psychological resilience in older US veterans: results from the national health and resilience in veterans study. Depression and anxiety, 30(5), 432–443. [DOI] [PubMed] [Google Scholar]
- Schnider KR, Elhai JD, & Gray MJ (2007). Coping style use predicts posttraumatic stress and complicated grief symptom severity among college students reporting a traumatic loss. Journal of Counseling Psychology, 54(3), 344–350. 10.1037/0022-0167.54.3.344 [DOI] [Google Scholar]
- Smith SM, Goldstein RB, & Grant BF (2016). The association between post-traumatic stress disorder and lifetime DSM-5 psychiatric disorders among veterans: Data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). J Psychiatr Res, 82, 16–22. 10.1016/j.jpsychires.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MB, Foelsch P, & Grace C (1996). Measuring Adult Attachment: Are Self-Report Instruments Congruent? Journal of Personality Assessment, 67(1), 37–51. 10.1207/s15327752jpa6701_3 [DOI] [PubMed] [Google Scholar]
- Stein MB, Levey DF, Cheng Z, Wendt FR, Harrington K, Cho K, Quaden R, Radhakrishnan K, Girgenti MJ, Anne Ho Y-L, Posner D, Aslan M, Duman RS, Zhao H, Polimanti R, Concato J, & Gelernter J (2019). Genomic Characterization of Posttraumatic Stress Disorder in a Large US Military Veteran Sample. bioRxiv, 764001. 10.1101/764001 [DOI] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, & Šmuc T (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One, 6(7), e21800. 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamman AJF, Sippel LM, Han S, Neria Y, Krystal JH, Southwick SM, Gelernter J, & Pietrzak RH (2019). Attachment style moderates effects of FKBP5 polymorphisms and childhood abuse on post-traumatic stress symptoms: Results from the National Health and Resilience in Veterans Study. World J Biol Psychiatry, 20(4), 289–300. 10.1080/15622975.2017.1376114 [DOI] [PubMed] [Google Scholar]
- Tamman AJF, Wendt FR, Pathak GA, Krystal JH, Montalvo-Ortiz JL, Southwick SM, Sippel LM, Gelernter J, Polimanti R, & Pietrzak RH Attachment style moderates polygenic risk for posttraumatic stress in United States military veterans: Results from the National Health and Resilience in Veterans Study. Biological Psychiatry. 10.1016/j.biopsych.2020.09.018 [DOI] [PubMed] [Google Scholar]
- Tamman AJF, Wendt FR, Pathak GA, Krystal JH, Montalvo-Ortiz JL, Southwick SM, Sippel LM, Gelernter J, Polimanti R, & Pietrzak RH (2020). Attachment Style Moderates Polygenic Risk for Posttraumatic Stress in United States Military Veterans: Results From the National Health and Resilience in Veterans Study. Biological Psychiatry. 10.1016/j.biopsych.2020.09.018 [DOI] [PubMed] [Google Scholar]
- Tanna CE, Goss LB, Ludwig CG, & Chen PW (2019). Arf GAPs as Regulators of the Actin Cytoskeleton-An Update. Int J Mol Sci, 20(2). 10.3390/ijms20020442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke W, Bagozzi RP, & van den Berg WE (2014). The role of attachment styles in regulating the effects of dopamine on the behavior of salespersons. Front Hum Neurosci, 8, 32. 10.3389/fnhum.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C, Siddiqui TA, & Schlichter LC (2012). Podosomes in migrating microglia: components and matrix degradation. J Neuroinflammation, 9, 190. 10.1186/1742-2094-9-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazana A, Moss E, Jolicoeur-Martineau A, Graffi J, Tsabari G, Lecompte V, Pascuzzo K, Babineau V, Gordon-Green C, Mileva V, Atkinson L, Minde K, Bouvette-Turcot AA, Sassi R, St-André M, Carrey N, Matthews S, Sokolowski M, Lydon J, Gaudreau H, Steiner M, Kennedy JL, Fleming A, Levitan R, & Meaney MJ (2015). The interplay of birth weight, dopamine receptor D4 gene (DRD4), and early maternal care in the prediction of disorganized attachment at 36 months of age. Development and psychopathology, 27(4 Pt 1), 1145–1161. 10.1017/S0954579415000735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. annual convention of the international society for traumatic stress studies, San Antonio, TX, [Google Scholar]
- Wendt FR, Pathak GA, Levey DF, Nunez YZ, Overstreet C, Tyrrell C, Adhikari K, De Angelis F, Tylee DS, Goswami A, Krystal JH, Abdallah C, Stein MB, Kranzler HR, Gelernter J, & Polimanti R (2020). Trauma and posttraumatic stress interact with sex-specific risk loci for suicidality and converge on brain extracellular matrix biology and synaptic plasticity. medRxiv, 2020.2005.2018.20105734. 10.1101/2020.05.18.20105734 [DOI] [Google Scholar]
- Wisco BE, Marx BP, Wolf EJ, Miller MW, Southwick SM, & Pietrzak RH (2014). Posttraumatic stress disorder in the US veteran population: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry, 75(12), 1338–1346. 10.4088/JCP.14m09328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of associations for three polygenic risk score (PRS) models of posttraumatic stress disorder re-experiencing (PTSDREX) predicting traumatic loss-related PTSD: (Model 1) was covaried for age, sex, and 10 principal components of ancestry, (Model 2) was additionally covaried for attachment style, and (Model 3) was additionally covaried for attachment style and the interactive term of attachment style by PTSDREX PRS. The most significant gene set is labeled.
Figure S2. Representative Gene Ontology network for biological processes detected by models for PTSDREX PRS predicting traumatic loss-related PTSD. Due to lack of distinct observational network sub-clusters, network node positions were adjusted to improve visual clarity.
Figure S3. Representative Gene Ontology network for cellular components detected by models for PTSDREX PRS predicting traumatic loss-related PTSD.
Figure S4. Representative Gene Ontology network for molecular functions detected by models for PTSDREX PRS predicting traumatic loss-related PTSD.
Data Availability Statement
Data from the National Health and Resilience in Veterans Study are not publicly available. Individuals interested in collaborating on projects using data from this study may contact the corresponding author, Robert Pietrzak, PhD, MPH: robert.pietrzak@yale.edu.


