Abstract
BACKGROUND:
Intravesical Bacillus Calmette-Guerin (BCG), a live attenuated tuberculosis vaccine that acts as a non-specific immune system stimulant, is the most effective adjuvant treatment for patients with intermediate or high-risk non-muscle-invasive bladder cancer (NMIBC). However, to date, there are no reliable tests that are predictive of BCG treatment response. In this study, we evaluated the performance of Oncuria, a bladder cancer detection test, to predict response to intravesical BCG.
METHODS:
Oncuria data was evaluated in voided urine samples obtained from a prospectively collected cohort of 64 subjects with intermediate or high risk NMIBC prior to treatment with intravesical BCG. The Oncuria test, which measures 10 cancer-associated biomarkers was performed in an independent clinical laboratory. The ability of the test to identify those patients in whom BCG is ineffective against tumor recurrence was tested. Predictive models were derived using supervised learning and cross-validation analyses. Model performance was assessed using ROC curves.
RESULTS:
Pre-treatment urinary concentrations of MMP9, VEGFA, CA9, SDC1, PAI1, APOE, A1AT, ANG and MMP10 were increased in patients who developed disease recurrence. A combinatorial predictive model of treatment outcome achieved an AUROC 0.89 [95% CI: 0.80–0.99], outperforming any single biomarker, with a test sensitivity of 81.8% and a specificity of 84.9%. Hazard ratio analysis revealed that patients with higher urinary levels of ANG, CA9 and MMP10 had a significantly higher risk of disease recurrence.
CONCLUSIONS:
Monitoring the urinary levels of a cancer-associated biomarker panel enabled the discrimination of patients who did not respond to intravesical BCG therapy. With further study, the multiplex Oncuria test may be applicable for the clinical evaluation of bladder cancer patients considering intravesical BCG treatment.
Keywords: Biomarkers, bladder cancer, multiplex, protein, BCG
1. Introduction
Up to 60% of non-muscle invasive bladder cancer (NMIBC) cases that are treated by transurethral resection (TUR) will experience disease recurrence. Guidelines for NMIBC management include the recommendation for post-TUR intravesical instillation therapy [1, 2]. Intravesical Bacillus Calmette-Guerin (BCG), a live attenuated tuberculosis vaccine that acts as a non-specific immune system stimulant, has proven to be the most successful adjuvant treatment to date, assisting in the eradication of residual disease, reducing recurrence rates, and decreasing disease progression to muscle-invasive bladder cancer (MIBC) [1, 2]. However, despite considerable success, as many as 30% of patients will develop tumor recurrence and up to 15% can progress despite BCG therapy [3, 4]. Failure to intervene with definitive radical cystectomy prior to progression to MIBC is associated with a significant reduction in long-term survival probability [5, 6]. Thus, early identification of patients suited for bladder preservation with BCG treatment or for radical cystectomy is essential. At this time, the decision to preserve the bladder or to perform a cystectomy depends on models based on clinicopathological parameters [7, 8], but these tools have limited accuracy for predicting disease recurrence or progression [9, 10]. Furthermore, there is currently no established evaluation test available for the prediction of patient response to intravesical BCG.
Previously, we have identified a panel of protein biomarkers that are associated with bladder cancer [11, 12, 13, 14], and we have developed a multiplex immunoassay for the automated detection of the analyte panel in voided urine [15, 16, 17]. The test has been validated for non-invasive diagnosis, but in this study, we tested the potential clinical utility of the multiplex test for the prediction of BCG treatment response in a small prospective cohort.
2. Patients and methods
2.1. Patients, specimens and data collection
Patients with intermediate and high risk NMIBC (Tis, Ta or T1) [18] were previously recruited and reported in a clinical trial in which intravesical BCG was administered [19]. Briefly, spontaneous voided urine samples were collected prior to BCG treatment. All urine samples were centrifuged at 1,500 g for 10 min, and cell-free urine samples were stored at 80C prior to analysis. After the initiation of intravesical BCG treatment, all participants were followed up with periodic cystoscopic medical examination. The endpoint of interest in each patient was disease recurrence, defined as a newly identified bladder tumor after a previous negative follow-up cystoscopy. Patients with noted abnormality on cystoscopy, underwent cystoscopy with bladder biopsy or transurethral resection of a bladder tumor (TURBT) followed by pathological interpretation.
2.2. Multiplex immunoassay
The concentrations of the 10 proteins (A1AT, APOE, ANG, CA9, IL8, MMP9, MMP10, PAI1, SDC1 and VEGFA) were monitored using an analytically validated multiplex bead-based immunoassay (Oncuria) from R&D Systems Inc. (Minneapolis, MN) [15, 16, 17]. Urine samples were passively thawed on ice, centrifuged for 10 minutes 1,000 g and handled on ice prior to diluting 2-fold with R&D Assay Diluent. Samples, standards and controls (50 l) were added to the 96 well plate in duplicate. The multiplex immunoassay was conducted according to the manufacturer’s instructions. A seven-point standard curve across the dynamic range of the assays was included in the current assay design. Plates were read on the Luminex 200 plate reader (Luminex Corp, Austin, TX). Calibration curves were generated along with optimal fit in conjunction with Akaike’s information criteria (AIC) values [20].
2.3. Data analysis
Wilcoxon rank sum tests were used to determine the association between each biomarker and bladder cancer recurrence. Nonparametric receiver operating characteristic (ROC) curves were generated to plot assay sensitivity against the false-positive rate (1-specificity). The relative ability of each biomarker to predict bladder cancer recurrence was evaluated by calculating the area under the curve (AUC), and AUCs were compared by chi-square test. The sensitivity and specificity of each biomarker individually and in combination were estimated at the optimal cutoff value defined by the Youden index [21]. To assess the independent association between biomarkers and bladder cancer recurrence, we used logistic regression analysis with recurrence status (yes vs. no) as the response variable and biomarker concentrations as explanatory variables. Multivariate analysis using Cox proportional hazards models for recurrence was performed to evaluate the influences of each biomarker on disease-specific survival. The all-subset method was used to evaluate the predictive value of each possible combination of biomarkers, and the Bayesian information criterion (BIC) was used to compare models. The BIC, a widely used criterion in model selection, balances the model likelihood and the number of biomarkers included in the model [22]. The Bootstrap method (using 1000 Bootstrap samples) was used [23] to select the most efficient and stable predictive model. Statistical significance in this study was set at 0.05 and all reported values were 2-sided. All analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
3. Results
The study population was comprised of 64 subjects with NMIBC who were scheduled to be treated with intravesical BCG. Among the 64 study subjects in this application, all 64 had high-grade disease with 23 with intermediate risk disease (i.e., ALL Ta high-grade 3 cm) and the remaining 41 high risk disease (i.e., 29 with high-grade T1, 7 with high-grade Ta 3 cm and 5 with CIS). The mean age of subjects was 65.8 11.3 years, 78.1% of the subjects were men, 85.9% were Caucasian, and 54.7% of the subjects presented with Tis/Ta disease while 45.3% of the subjects presented with T1 disease (Table 1). Of the total 64 subjects, 11 (15.6%) were found to have post-treatment bladder cancer recurrence on follow-up. Median time to recurrence was 6 months (range 1–17 months). The recurrences were noted to be non-muscle invasive bladder cancer (NMIBC; stages Ta, Tis, T1) high-grade in 81.8% ( 9), and muscle invasive bladder cancer (MIBC; stage T2) high-grade in 18.2% ( 2). Of these 11 subjects, two were noted to have a second recurrence.
Table 1.
Demographic and clinical-pathologic characteristics of study cohorts
| No recurrence ( 53) | Recurrence ( 11) | |||||
|---|---|---|---|---|---|---|
| Variable | Value | % | % | |||
| Age | 18–54 | 9 | 17.0 | 2 | 18.2 | 0.37 |
| 55–64 | 16 | 30.2 | 4 | 36.4 | ||
| 65–74 | 17 | 32.1 | 1 | 9.1 | ||
| 75 | 11 | 20.8 | 4 | 36.4 | ||
| Sex | Female | 10 | 18.9 | 4 | 36.4 | 0.22 |
| Male | 43 | 81.1 | 7 | 63.6 | ||
| Race | White | 46 | 86.8 | 9 | 81.8 | 0.67 |
| Other | 7 | 13.2 | 2 | 18.2 | ||
| Stage | Ta | 26 | 49.1 | 4 | 36.4 | 0.21 |
| Tis | 5 | 9.4 | 0 | 0.0 | ||
| T1 | 22 | 41.5 | 7 | 63.6 | ||
| Cytology | Negative | 24 | 45.3 | 2 | 18.2 | 0.10 |
| Positive | 25 | 47.2 | 6 | 54.5 | ||
| Missing | 4 | 7.5 | 3 | 27.3 | ||
For each of the 64 clinical samples, we reported the mean SD and range of each biomarker, along with the percentage of samples in which the biomarker was detectable (Table 2). Each individual biomarker was detectable in 90% of the samples, except for A1AT (detected in 58% of samples). Increased urinary concentrations of MMP9 ( 0.53), VEFGA ( 0.20), CA9 ( 0.19), SDC1 ( 0.23), PAI1 ( 0.19), ApoE ( 0.21), A1AT ( 0.04), ANG ( 0.02) and MMP10 ( 0.15) were observed in subjects with disease recurrence. The urinary concentration of IL8 was unchanged.
Table 2.
Mean urinary ( SD) concentrations of 10 biomarkers assessed by Oncuria
| Biomarker pg/mL | Detectable % | No recurrence ( 53) | Recurrence ( 11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Cutoff | |||
| MMP-9 | 92 | 10,873 | 12,604 | 292 | 60,355 | 13,986 | 14,885 | 653 | 39,466 | 0.53 | 6,051 |
| CXCL8/IL-8 | 100 | 425.8 | 373.6 | 6.4 | 1,508.1 | 417.2 | 395.4 | 39.4 | 1,322.3 | 0.95 | 254.4 |
| VEGF-A | 100 | 209.9 | 114.6 | 40.6 | 496.7 | 327.6 | 279.7 | 86.0 | 900.5 | 0.20 | 209.3 |
| IX/CA9 | 91 | 2.07 | 3.46 | 0.07 | 20.81 | 6.87 | 11.31 | 0.32 | 32.32 | 0.19 | 1.55 |
| Syndecan-1 | 100 | 6,580 | 2,860 | 1,256 | 14,433 | 7,911 | 3,294 | 3,341 | 12,176 | 0.23 | 6,562 |
| Serpin E1/PAI-1 | 100 | 448.7 | 439.5 | 35.7 | 1,990.0 | 955.7 | 1,196.0 | 78.4 | 3,827.0 | 0.19 | 378.0 |
| ApoE | 100 | 8,714 | 9,513 | 676 | 53,748 | 12,925 | 9,763 | 2,555 | 34,151 | 0.21 | 7,664 |
| Serpin A1 | 58 | 771,434 | 716,725 | 38,750 | 1,940,791 | 1,257,290 | 645,741 | 66,035 | 1,631,774 | 0.04 | 629,949 |
| Angiogenin | 100 | 2,284 | 1,323 | 24 | 6,718 | 4,179 | 2,339 | 1,185 | 8,603 | 0.02 | 2,683 |
| MMP-10 | 95 | 236.6 | 286.5 | 7.5 | 1,341.3 | 405.9 | 346.5 | 84.7 | 1,059.6 | 0.15 | 192.1 |
Table 3 provides AUC data for each individual biomarker and for the combination of all 10 biomarkers in the Oncuria test. Using optimal cutoff values defined by the Youden index from this cohort, the 10-biomarker model resulted in an AUC of 0.8971 (95% confidence interval, 0.8000–0.9942), with a sensitivity value of 81.8% and a specificity value of 84.9% for the prediction of disease recurrence (Fig. 1). Patients with higher urinary levels of CA9 (HR: 3.44, 95% CI: 1.21–9.76; 0.02), ANG (HR: 42.89, 95% CI: 3.06–602.10; 0.005) and MMP10 (HR: 3.86, 95% CI: 1.06–14.05; 0.04) had a significantly higher risk of disease recurrence. Increased levels of PAI1 (HR: 3.54, 95% CI: 0.87–14.43; 0.08), APOE (HR: 4.64, 95% CI: 0.91–23.59; 0.06) and A1AT (HR: 3.72, 95% CI: 0.94–14.83; 0.06) approached significance (Table 4).
Table 3.
Performance of the Oncuria test for the prediction of BCG treatment response
| Biomarker | Area under the curve | 95% confidence interval | No. of correctly predicted events | No. of correctly predicted nonevents | No. of nonevents predicted as events | No. of events predicted as nonevents | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| A1AT | 0.6364 | (0.4606, 0.8121) | 9 | 30 | 23 | 2 | 0.818 | 0.566 | 0.281 | 0.938 |
| ANG | 0.7444 | (0.5700, 0.9189) | 7 | 43 | 10 | 4 | 0.636 | 0.811 | 0.412 | 0.915 |
| APOE | 0.6724 | (0.4985, 0.8463) | 9 | 27 | 26 | 2 | 0.818 | 0.509 | 0.257 | 0.931 |
| CA9 | 0.6878 | (0.5203, 0.8553) | 7 | 37 | 16 | 4 | 0.636 | 0.698 | 0.304 | 0.902 |
| IL8 | 0.5146 | (0.3146, 0.7146) | 4 | 41 | 12 | 7 | 0.364 | 0.774 | 0.250 | 0.854 |
| MMP9 | 0.542 | (0.3469, 0.7371) | 10 | 14 | 39 | 1 | 0.909 | 0.264 | 0.204 | 0.933 |
| MMP10 | 0.7238 | (0.5787, 0.8690) | 8 | 36 | 17 | 3 | 0.727 | 0.679 | 0.320 | 0.923 |
| PAI1 | 0.6261 | (0.4306, 0.8215) | 7 | 35 | 18 | 4 | 0.636 | 0.660 | 0.280 | 0.897 |
| SDC1 | 0.6106 | (0.3927, 0.8286) | 6 | 47 | 6 | 5 | 0.545 | 0.887 | 0.500 | 0.904 |
| VEGFA | 0.5935 | (0.3904, 0.7965) | 3 | 52 | 1 | 8 | 0.273 | 0.981 | 0.750 | 0.867 |
| 10-biomarker | 0.8971 | (0.8000, 0.9942) | 9 | 45 | 8 | 2 | 0.818 | 0.849 | 0.529 | 0.957 |
| combination |
Figure 1.
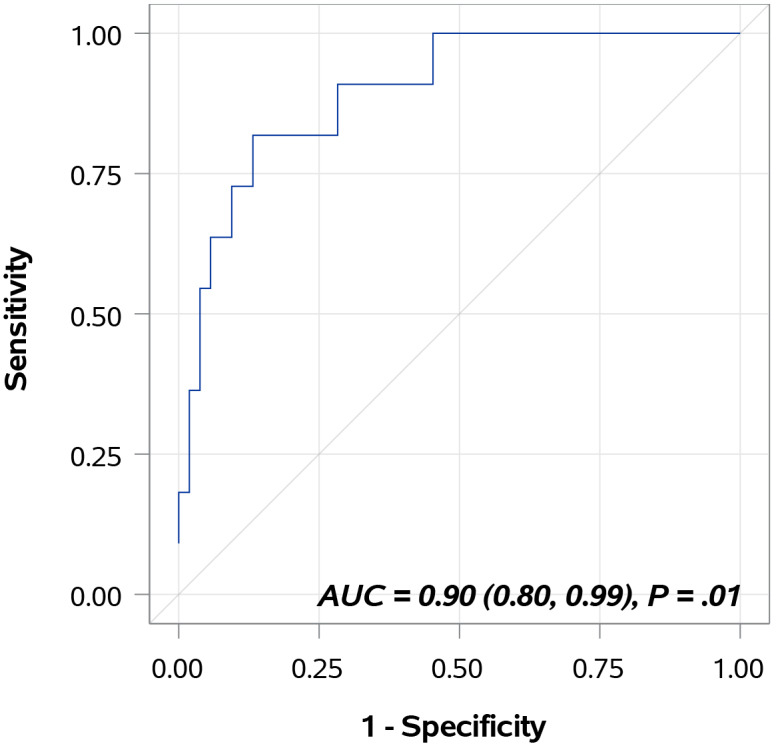
Diagnostic performance of bladder cancer-associated molecular signature. The areas under the curves was 0.8971 (95% confidence interval, 0.8000–0.9942), with a sensitivity value of 81.8% and a specificity value of 84.9% for the prediction of disease recurrence.
Table 4.
Hazard ratio of tumor recurrence based on molecular signature
| Biomarker (log10 pg/ml) | HR | LCL | UCL | -value |
|---|---|---|---|---|
| MMP-9 | 1.36 | 0.48 | 3.84 | 0.56 |
| CXCL8/IL-8 | 0.97 | 0.31 | 3.03 | 0.96 |
| VEGF-A | 7.38 | 0.68 | 80.43 | 0.10 |
| IX/CA9 | 3.44 | 1.21 | 9.76 | 0.02 |
| Syndecan-1 | 6.82 | 0.29 | 159.23 | 0.23 |
| Serpin E1/PAI-1 | 3.54 | 0.87 | 14.43 | 0.08 |
| ApoE | 4.64 | 0.91 | 23.59 | 0.06 |
| Serpin A1 | 3.72 | 0.94 | 14.83 | 0.06 |
| Angiogenin | 42.89 | 3.06 | 602.10 | 0.005 |
| MMP-10 | 3.86 | 1.06 | 14.05 | 0.04 |
4. Discussion
A comprehensive review of the literature regarding potential predictive biomarkers of BCG response reveals limited results. In 2003, investigators reported that the failure to detect urinary IL-2 during a BCG induction course correlated with the time to disease recurrence and progression [24]. Similarly, Kamat and others developed the CyPRIT assay and noted that an increase in nine cytokines, including IL-2, induced after a BCG treatment correlated with disease recurrence, and that combinatorial changes in the cytokine panel could best predict disease recurrence [19]. Monitoring cytokine profile changes is promising as a test of immune response once BCG treatment is initiated and may guide BCG treatment frequency or continuance in the individual.
Previous studies have assessed the ability of the diagnostic FISH test (UroVysionTM, Abbott Molecular Inc.) to predict whether patients with NMIBC would incur disease recurrence or progression after BCG treatment. UroVysion testing was applied at the beginning and the end of the BCG induction cycle (typically involving 6 weekly instillations), and urine cytology and cystoscopy were performed six weeks after cycle completion. In a multivariate analysis, the presence of high-grade disease and a positive UroVysion test after BCG initiation were significant predictors of disease recurrence [25, 26]. Again, in these studies, the urine-based results were only informative after the initiation of BCG treatment, and so are not predictive of BCG outcome prior to the first cycle of instillation. Therefore, the information would not be available to guide the clinical management of the individual patient regarding the decision to embark on an intravesical BCG treatment regimen. In a small prospective study, Lotan and others noted a positive FISH test prior to BCG to be associated with recurrence and progression, hazard ratio HR 2.59 (95% CI 1.42–4.73) [27].
Here, we evaluated the performance of the diagnostic Oncuria test to predict the response to BCG therapy, prior to treatment initiation. In this pilot study, we modified the established diagnostic algorithm, which weights biomarker values to produce a risk score, to the fit the predictive scenario. The 10 biomarkers that comprise the Oncuria test were reliably detected in almost all of the 64 urine samples, and the levels of 9 of the biomarkers were elevated in pre-treatment urine samples from subjects who had a subsequent bladder cancer recurrence after the completion of BCG treatment. As shown in diagnostic applications, it is the combination of the biomarker values through a weighted analytical algorithm that provides a model with sufficient predictive power for potential clinical application. In this case, the test shows promise for the evaluation of patients with respect to the decision to undergo BCG treatment.
The biomarkers that compose the bladder cancer diagnostic signature have a varied range of reported functions including angiogenesis, breakdown of extracellular matrix, serine protein inhibitor, catalyze the reversible hydration of carbon dioxide, lipoprotein metabolism and cell binding/signaling (Table 5) with the two primary functional groups being extracellular matrix remodeling (MMP9 and MMP10) and angiogenesis (IL8, VEGFA and ANG). Angiogenesis, the development of new blood vessels from existing blood vessels, is essential for normal growth and development of tissues and organs. A balance of pro-angiogenic factors and anti-angiogenic factors tightly controls this process [28, 29, 30]. However in solid tumors, the balance may favor pro-angiogenic factors, ensuring nutrients are provided to the rapidly dividing cancer cells, thus allowing the support of the abnormal growth seen in tumors [31]. Recent studies also suggest ANG and PAI1 can breakdown the extracellular matrix [32, 33], allowing cancer cells to invade and metastasize [34]. The extent of tumor vascularization differs between malignancies, and has been shown to correlate directly with metastatic potential [35].
Table 5.
Annotated urine-based bladder cancer associated diagnostic
| Full name | Abbreviation | Ascribed function | Location | Interacts with other members of signature |
|---|---|---|---|---|
| Interleukin 8 | IL8 | Chemoattractant & angiogenesis | Extracellular | MMP9, SDC1 |
| Angiogenin | ANG | Angiogenesis | Extracellular, nucleus | None |
| Vascular endothelial growth | VEGFA | Angiogenesis | Extracellular, cytoplasm | None |
| factor A | ||||
| Matrix metallopeptidase 9 | MMP9 | Breakdown of extracellular matrix | Extracellular | IL8, MMP10 |
| Matrix metallopeptidase 10 | MMP10 | Breakdown of extracellular matrix | Extracellular | MMP9 |
| Serpin peptidase inhibitor | SERPINA1 | Serine protease inhibitor | Extracellular | None |
| Serpin peptidase inhibitor | SERPINE1 | Serine endopeptidase inhibitor | Extracellular, plasma membrane | None |
| Carbonic anhydrase IX | CA9 | Catalyze the reversible hydration of carbon | Plasma membrane | None |
| dioxide | ||||
| Apolipoprotein E | APOE | Lipoprotein catabolism and metabolism | Extracellular, plasma membrane, | None |
| cytoplasm | ||||
| Syndecan 1 | SDC1 | Cell binding, cell signaling, cytoskeletal | Plasma membrane, cytoplasm | IL8 |
| organization |
We recognize that the study has several limitations. First, as a pilot study, with only 64 total subjects the study is small and underpowered. Second, defining truly independent disease recurrence and distinguishing new lesions from regrowth from a previous tumor resection site can be challenging. In future studies, we would propose noting the location of the primary tumor, as well as the location and timing of the recurrence, realizing that a second tumor within the previous tumor bed at 3 months follow-up may not necessarily be due to a lack of BCG response, while recurrences outside of the tumor bed may be more indicative of BCG failure. The evaluation of the test in a larger, prospective cohort with more information on lesion characteristics before and after BCG treatment may determine whether the urinary biomarker profile indicates residual disease or a field change in the urothelial or immune landscape which may be predictive of the response to intravesical BCG.
The development of an accurate and robust test that can predict BCG treatment response would benefit both patients and healthcare systems. The identification of predictive molecular signatures has the potential to better tailor treatment regimens for individual patients, avoiding potentially significant delays in clinical management. In this pilot study, the multiplex Oncuria test achieved encouraging predictive performance. The test uses established technology facilitating uptake in clinical laboratories once validated for defined applications. Additional studies are underway to evaluate the potential added value of the test in clinical decision making.
Ethics approval and consent to participate
MD Anderson Cancer Center local ethics review board approved.
Consent for publication
Not applicable.
Availability of data and materials
Reasonable requests for data will be made available for review.
Competing interests
Dr. Charles Rosser is an officer of Nonagen Bioscience. No financial or commercial conflicts of interest were declared by other co-authors.
Funding
This work was supported by research grants R01 CA1988887 (CJR) and R01 CA206584 (SG).
Author contributions
Conception, interpretation or analysis of data: KM.
Interpretation or analysis of data, revision for important intellectual content: AK.
Interpretation or analysis of data: IP, RC, YS.
Revision for important intellectual content: AG and SG.
Conception, supervision, preparation of the manuscript: CJR and HF.
Conflict of interest
CJR is an officer of Nonagen Bioscience Corporation.
References
- [1]. Hall M.C., Chang S.S., Dalbagni G., Pruthi R.S., Seigne J.D., Skinner E.C., Wolf J.S., Jr. and Schellhammer P.F., Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update, J Urol 178 (2007), 2314–2330. [DOI] [PubMed] [Google Scholar]
- [2]. Babjuk M., Burger M., Zigeuner R., Shariat S.F., van Rhijn B.W., Comperat E., Sylvester R.J., Kaasinen E., Bohle A., Palou Redorta J., Roupret M. and U. European Association of, EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013, Eur Urol 64 (2013), 639–653. [DOI] [PubMed] [Google Scholar]
- [3]. Witjes J.A., Management of BCG failures in superficial bladder cancer: A review, Eur Urol 49 (2006), 790–797. [DOI] [PubMed] [Google Scholar]
- [4]. Matulay J.T., Li R., Hensley P.J., Brooks N.A., Narayan V.M., Grossman H.B., Navai N., Dinney C.P.N. and Kamat A.M., Contemporary outcomes of patients with nonmuscle-invasive bladder cancer treated with bacillus Calmette-Guerin: Implications for clinical trial design, J Urol 205 (2021), 1612–1621. [DOI] [PubMed] [Google Scholar]
- [5]. von Rundstedt F.C. and Lerner S.P., Bacille-Calmette-Guerin non-responders: How to manage, Transl Androl Urol 4 (2015), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Gupta A., Lotan Y., Bastian P.J., Palapattu G.S., Karakiewicz P.I., Raj G.V., Schoenberg M.P., Lerner S.P., Sagalowsky A.I., Shariat S.F. and C. Bladder Cancer Research, Outcomes of patients with clinical T1 grade 3 urothelial cell bladder carcinoma treated with radical cystectomy, Urology 71 (2008), 302–307. [DOI] [PubMed] [Google Scholar]
- [7]. Yamada T., Tsuchiya K., Kato S., Kamei S., Taniguchi M., Takeuchi T., Yamamoto N., Ehara H. and Deguchi T., A pretreatment nomogram predicting recurrence- and progression-free survival for nonmuscle invasive bladder cancer in Japanese patients, Int J Clin Oncol 15 (2010), 271–279. [DOI] [PubMed] [Google Scholar]
- [8]. Kamat A.M., Li R., O’Donnell M.A., Black P.C., Roupret M., Catto J.W., Comperat E., Ingersoll M.A., Witjes W.P., McConkey D.J. and Witjes J.A., Predicting response to intravesical bacillus Calmette-Guerin immunotherapy: Are we there yet? A systematic review, Eur Urol 73 (2018), 738–748. [DOI] [PubMed] [Google Scholar]
- [9]. Lammers R.J., Hendriks J.C., Rodriguez Faba O.R., Witjes W.P., Palou J. and Witjes J.A., Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation, World J Urol 34 (2016), 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Zuiverloon T.C., Nieuweboer A.J., Vekony H., Kirkels W.J., Bangma C.H. and Zwarthoff E.C., Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: A systematic review, Eur Urol 61 (2012), 128–145. [DOI] [PubMed] [Google Scholar]
- [11]. Yang N., Feng S., Shedden K., Xie X., Liu Y., Rosser C.J., Lubman D.M. and Goodison S., Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification, Clin Cancer Res 17 (2011), 3349–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Kreunin P., Zhao J., Rosser C., Urquidi V., Lubman D.M. and Goodison S., Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling, J Proteome Res 6 (2007), 2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rosser C.J., Liu L., Sun Y., Villicana P., McCullers M., Porvasnik S., Young P.R., Parker A.S. and Goodison S., Bladder cancer-associated gene expression signatures identified by profiling of exfoliated urothelia, Cancer Epidemiol Biomarkers Prev 18 (2009), 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Urquidi V., Goodison S., Cai Y., Sun Y. and Rosser C.J., A candidate molecular biomarker panel for the detection of bladder cancer, Cancer Epidemiol Biomarkers Prev 21 (2012), 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Furuya H., Pagano I., Chee K., Kobayashi T., Wong R.S., Lee R. and Rosser C.J., Comparison of commercial ELISA kits, a prototype multiplex electrochemoluminescent assay, and a multiplex bead-based immunoassay for detecting a urine-based bladder-cancer-associated diagnostic signature, Diagnostics (Basel) 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Furuya H., Tabula L., Lee R., Kralovec P., Ramsden M., Wong R. and Rosser C.J., Analytical validation of ONCURIA a multiplex bead-based immunoassay for the non-invasive bladder cancer detection, Pract Lab Med 22 (2020), e00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Hirasawa Y., Pagano I., Chen R., Sun Y., Dai Y., Gupta A., Tikhonenkov S., Goodison S., Rosser C.J. and Furuya H., Diagnostic performance of Oncuria, a urinalysis test for bladder cancer, J Transl Med 19 (2021), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Chang S.S., Boorjian S.A., Chou R., Clark P.E., Daneshmand S., Konety B.R., Pruthi R., Quale D.Z., Ritch C.R., Seigne J.D., Skinner E.C., Smith N.D. and McKiernan J.M., Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline, J Urol 196 (2016), 1021–1029. [DOI] [PubMed] [Google Scholar]
- [19]. Kamat A.M., Briggman J., Urbauer D.L., Svatek R., Nogueras Gonzalez G.M., Anderson R., Grossman H.B., Prat F. and Dinney C.P., Cytokine panel for response to intravesical therapy (CyPRIT): Nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guerin, Eur Urol 69 (2016), 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Motulsky H. and Christopoulos A., Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting, Oxford University Press, 2004.
- [21]. Fluss R., Faraggi D. and Reiser B., Estimation of the Youden Index and its associated cutoff point, Biom J 47 (2005), 458–472. [DOI] [PubMed] [Google Scholar]
- [22]. Leon L.F. and Tsai C.L., Assessment of model adequacy for Markov regression time series models, Biometrics 54 (1998), 1165–1175. [PubMed] [Google Scholar]
- [23]. Austin P.C. and Tu J.V., Bootstrap methods for developing predictive models, The American Statistician 58 (2004), 131–137. [Google Scholar]
- [24]. Saint F., Kurth N., Maille P., Vordos D., Hoznek A., Soyeux P., Patard J.J., Abbou C.C. and Chopin D.K., Urinary IL-2 assay for monitoring intravesical bacillus Calmette-Guerin response of superficial bladder cancer during induction course and maintenance therapy, Int J Cancer 107 (2003), 434–440. [DOI] [PubMed] [Google Scholar]
- [25]. Whitson J.M., Berry A.B., Carroll P.R. and Konety B.R., UroVysion testing can lead to early identification of intravesical therapy failure in patients with high risk non-muscle invasive bladder cancer, Int Braz J Urol 35 (2009), 664–670; discussion 671-2. [DOI] [PubMed] [Google Scholar]
- [26]. Savic S., Zlobec I., Thalmann G.N., Engeler D., Schmauss M., Lehmann K., Mattarelli G., Eichenberger T., Dalquen P., Spieler P., Schoenegg R., Gasser T.C., Sulser T., Forster T., Zellweger T., Casella R. and Bubendorf L., The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin therapy, Int J Cancer 124 (2009), 2899–2904. [DOI] [PubMed] [Google Scholar]
- [27]. Lotan Y., Inman B.A., Davis L.G., Kassouf W., Messing E., Daneshmand S., Canter D., Marble H.T., Joseph A.M., Jewell S. and Boorjian S.A., Evaluation of the fluorescence in situ hybridization test to predict recurrence and/or progression of disease after bacillus Calmette-Guerin for primary high grade nonmuscle invasive bladder cancer: Results from a prospective multicenter trial, J Urol 202 (2019), 920–926. [DOI] [PubMed] [Google Scholar]
- [28]. Folkman J., Angiogenesis in cancer, vascular, rheumatoid and other disease, Nat Med 1 (1995), 27–31. [DOI] [PubMed] [Google Scholar]
- [29]. Folkman J. and Klagsbrun M., Angiogenic factors, Science 235 (1987), 442–447. [DOI] [PubMed] [Google Scholar]
- [30]. Hanahan D. and Folkman J., Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis, Cell 86 (1996), 353–364. [DOI] [PubMed] [Google Scholar]
- [31]. Blood C.H. and Zetter B.R., Tumor interactions with the vasculature: Angiogenesis and tumor metastasis, Biochim Biophys Acta 1032 (1990), 89–118. [DOI] [PubMed] [Google Scholar]
- [32]. Giacoia E.G., Miyake M., Lawton A., Goodison S. and Rosser C.J., PAI-1 leads to G1-phase cell-cycle progression through cyclin D3/cdk4/6 upregulation, Mol Cancer Res 12 (2014), 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Miyake M., Goodison S., Lawton A., Gomes-Giacoia E. and Rosser C.J., Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway, Oncogene 34 (2015), 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Liotta L.A., Kleinerman J. and Saidel G.M., Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation, Cancer Res 34 (1974), 997–1004. [PubMed] [Google Scholar]
- [35]. Weidner N., Folkman J., Pozza F., Bevilacqua P., Allred E.N., Moore D.H., Meli S. and Gasparini G., Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma, J Natl Cancer Inst 84 (1992), 1875–1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Reasonable requests for data will be made available for review.


