Abstract
COVID-19 is deemed as the most critical world health calamity of the 21st century, leading to dramatic life loss. There is a pressing need to understand the multi-stage dynamics, including transmission routes of the virus and environmental conditions due to the possibility of multiple waves of COVID-19 in the future. In this paper, a systematic examination of the literature is conducted associating the virus-laden-aerosol and transmission of these microparticles into the multimedia environment, including built environments. Particularly, this paper provides a critical review of state-of-the-art modelling tools apt for COVID-19 spread and transmission pathways. GIS-based, risk-based, and artificial intelligence-based tools are discussed for their application in the surveillance and forecasting of COVID-19. Primary environmental factors that act as simulators for the spread of the virus include meteorological variation, low air quality, pollen abundance, and spatial-temporal variation. However, the influence of these environmental factors on COVID-19 spread is still equivocal because of other non-pharmaceutical factors. The limitations of different modelling methods suggest the need for a multidisciplinary approach, including the ‘One-Health’ concept. Extended One-Health-based decision tools would assist policymakers in making informed decisions such as social gatherings, indoor environment improvement, and COVID-19 risk mitigation by adapting the control measurements.
Keywords: COVID-19, Multimedia environment, Risk assessment, One-health, Virus transmission, Environmental models
1. Introduction
Coronavirus disease 2019 (COVID-19) has changed the dynamic and perception of the whole world's human living style because of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). On March 11, 2020, the WHO announced COVID-19 disease as a pandemic (IPAC, 2020). The disease is continuously spreading, presumably because of virus mutation. This pandemic has caused 446 Million cases, including 6 Million deaths as of March2022 worldwide (WHO, 2021). More recently, the new variant of SARS-CoV-2, Omicron (B.1.1.529) was first found in South Africa made its way to other countries, including the Netherlands, Australia, North America, and many parts of Europe (Burki, 2022; WHO, 2021). Other variants such as Alpha (B.1.1.7) was found in the United Kingdom, Beta (B.1.351) was found in South Africa, Gamma (P.1) was found amongst travelers in Brazil, and another variant, i.e., Delta (B.1.617.2) was first identified in India (CDC, 2021; Rubin, 2021). Zoonotic diseases are considered infectious diseases caused by an agent, such as a virus, and jump from other animal species to humans. The possibility of reverse zoonotic transfer also exists (Munir et al., 2020). Kissler et al. (2020) studied the future of SARS-CoV-2 transmission based on immunity and seasonality using the USA database. This study also emphasized continuous surveillance because of possible resurgence in contagion as late as 2024, indicating that persistent social distancing may be required into 2022.
SARS-CoV-2 is mainly transmitted human-to-human through aerosols and droplets generated through cough, sneeze, talk, shouting, singing, or airborne particles (Jayaweera et al., 2020). The possible exposure pathways could be inhalation of virus carried in respiratory droplets, aerosol particles, and contact with the virus-contaminated surfaces (Jayaweera et al., 2020). A recent study shows that fine aerosol particles (≤ 5 µm) contain more copies of SARS-CoV-2 in contrast to coarse aerosols and may play a crucial role in virus transmission (Coleman et al., 2021). Thus, these fine particles persist for hours in the air as compared to larger droplets that are quickly deposited onto the ground or fomite surfaces (Saey, 2021). However, it is crucial to understand how ambient environmental conditions play a vital role in spreading and extending the virus in outdoor and indoor settings (Srivastava, 2020). The clinical impacts of the SARS-CoV-2 virus on the human host depend on age, sex, immunity system, and other demographic factors (Jin et al., 2020).
Simulation models are one of the effective ways to understand the dynamics of infectious disease. Models can be qualitative or quantitative and provide a prediction of a virus spread, risk assessment, and decision-making support (Scoones et al., 2017). Process-based mathematical models, usually called compartmental models, have been used to predict the disease spreads, reproduction number, the total number of infected persons, number of recovered patients, death rate, and other such epidemiological parameters (S Chang et al., 2020; Tolles & Luong, 2020). The outbreak of infectious diseases experiences complex interactions of multiple parameters, including environmental drivers and climate change. Weather conditions may affect the survival and reproduction rates of the vector populations (e.g., West Nile Virus (Paz, 2015)), habitat suitability, distribution, and abundance. That is why it is crucial to understand the virus spread through the lens of environmental/ weather conditions. Pattern-based simulation methods have been used to statistically associate the virus traits with the environmental variables (Bhattacharjee, 2020), to understand the spatiotemporal pattern of infection (Chen et al., 2020) for the projecting purpose (Qi et al., 2020). From the governmental perspective, non-pharmaceutical policies have been intact such as personal prevention practices (wearing masks, maintaining 6 feet distance, staying home when sick, and using sanitizers) since the first wave of the pandemic (O'Dowd et al., 2020). Most of the mathematical models are good to simulate the “what if” type of scenario under different interventions; it paves the path to open essential works, public places, schools, and offices in the low-risk area considering the socio-economic effects as well as preventive measurements (Parajuli et al., 2020). Besides process-based compartmental models, artificial intelligence (AI) has recently established an interest in developing an emerging infectious disease prediction model (Rustam et al., 2020). AI, such as the neural network model, has been used in modelling past pandemics, e.g., HINI influenza, dengue virus, and norovirus (Ardabili et al., 2020). These predictive models are usually based on assumptions and parameters used under simplified system conditions (Liu et al., 2021). Such assumptions may result in unrealistic prediction outcomes, which could hinder local stakeholders from policymaking (Taghizadeh et al., 2020) and require model validation using real-time monitoring data before making an informed decision (Eker, 2020). Hence, there is a dire need to re-examine existing models. An integrated decision support approach based on state-of-the-art modelling tools is desired by incorporating real-time database under the changing environmental conditions.
‘One-Health’ (OH) is a holistic approach that provides an integrated strategy to combat the outbreak, considering the health risk and human-animal-environment interface (Hinchliffe, 2015). OH's primary principle is to ensure interdisciplinary collaborations amongst policymakers, stakeholders/ relevant sectors considering the close dependency of the human-animal-environment (CDC, 2020). The OH approach is extensively relevant to strengthening the system and preparing, preventing, detecting, responding, and recovering from infectious diseases while resisting threats against humans, animals, and environmental health (World Bank, 2012). In 2004 wildlife conservation society organized a symposium titled as "One World, One-Health," which led to the term OH (Evans & Leighton, 2014). As a result of other subsequent conferences and OH programs on the same theme, OH became the recommended strategic approach to deal with avian influenza (Gibbs, 2014). These activities paved the way for the “world health organization” (WHO), “Food and Agriculture Organization of the United Nations” (FAO), and “World Organization for Animal Health” (OIE) tripartite partnership, which promoted the integration of zoonotic and foodborne diseases within the OH movement (Gibbs, 2014). The OH concept is a promising pathway to shape the current integrated studies on the control of coronaviruses (e.g., Scoones et al., 2017).
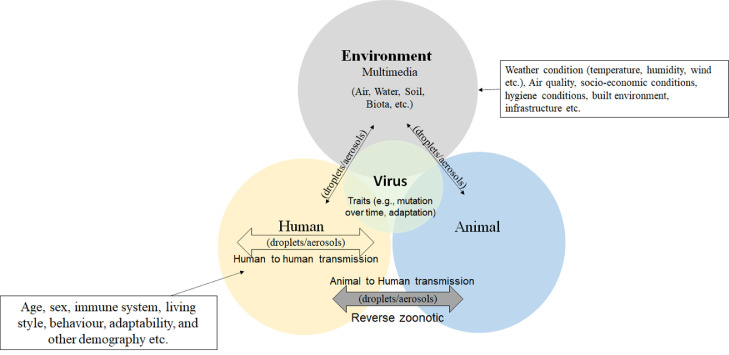
Referring to OH and environmental multimedia (i.e., atmosphere, water, soil, biota) concepts, Fig. 1 shows the dynamic exposure routes of virus-laden-aerosol transmissions between a host (human/animal) and environmental components. Although the multidisciplinary steps are the way forward to control this pandemic, there is still a need to better understand the factors related to each integrated approach actor, as shown in Fig. 1. For instance, environmental conditions can affect the virus growth, transmission, infection rate, and host susceptibility. Moreover, built infrastructure, air quality, and meteorological factors should be considered while finding strategies to deal with the disease. As animals are the primary host for such zoonotic viruses, their habitat, and other traits are important. Moreover, virus traits (e.g., mutation over time in the viral spike protein and viral genome) play a crucial role in human to human and animal to human transmissions. Like other RNA viruses, SARS-CoV-2 is prone to genetic evolution resulting in mutant variants that may have different characteristics than its ancestral strains while adapting to their new human hosts (Cascella et al., 2020).
Fig. 1.
Relevance of human, animal, and environment interaction to viral disease based on One-Health concept.
This paper aims first to explore the essential mechanisms of the virus spread under various environmental conditions, including the level of suspended fine particles and possible transmissions pathways in the multimedia environment, including indoor confined spaces. The focus will be on a systematic review of the modelling tools to understand the dynamics of COVID-19 under both short and long-term decision scenarios. This critical review helps identify the research gaps of existing decision analysis techniques and the need for an OH-based approach to encourage a multidisciplinary strategic solution to resilience against the pandemic. The integrated decision support system will help avoid multiple future waves by providing an equitable solution to optimizing mitigation measurements.
2. Methods
The peer-reviewed literature published in the years 2020, 2021 until now and relevant studies of past pandemics were searched. Articles were primarily found from database searches in PubMed Central, arXiv, and Web of Science using PRISMA guidelines. Also, the reference lists of relevant records were searched in google scholar to obtain articles that might have been missed in the database searches. The following search terminologies were used: “COVID-19 OR SARS-CoV-2 AND environment/climate”; “SARS-CoV-2 AND transmission”; “SARS-CoV-2 AND aerosol OR airborne particles”; “COVID-19 AND air quality”; “COVID-19 AND modelling”; COVID-19 AND risk assessment”; “COVID-19 AND surveillance/dashboard/tools”; “COVID-19 AND Artificial intelligence”; “COVID-19 AND One-Health”. All terms were searched using free text in title, abstract and controlled vocabulary. We collected information from 50 and 40 articles through arXiv, and PubMed Central, respectively. In addition, 82 articles were retrieved from google scholar and 10 articles from the Web of Science database. We excluded the duplicate papers and research papers that did not have data. We gathered the following data for each study: modelling method, transmission pathways, virus load or concentration value, study outcome, environmental factors, uncertainty factors, and other critical comments. The following questions are addressed in this systematic review:
-
1
To what extent do environmental factors potentially impact virus-laden-aerosol transmission into the multimedia and built environments?
-
2
What are the effective and novel approaches to combat the COVID-19 by incorporating environmental factors while considering the critical review and research gaps of state-of-the-art modelling techniques?
-
3
Does the multidisciplinary approach provide an equitable solution to resilience against the pandemic?
We addressed these questions using the literature database, summarized important factors responsible for the COVID-19 spread, and discussed integral components based on the OH concept.
3. Results
Due to the mutation of SARS-CoV-2, efforts continue to investigate the effect and role of environmental factors, transmission pathways, and modelling techniques to understand the difference in dynamics of COVID-19 across various regions and find suitable solutions to control its spreading. In Section 3.1, literature data for six different environmental factors (temperature, UV index, humidity, precipitation, wind speed, and humidity) and correlation of air pollution with COVID-19 cases in hot spot areas of different regions worldwide are evaluated. In Section 3.2, the possibility of transmitting SARS-CoV-2 through different pathways is examined, including its presence in the water environment, biota, and reverse zoonosis via airborne particles. In Section 3.3, various systematic analysis tools for COVID-19 are reviewed that would help to understand the virus survival, transmission pathways, risk of virus spread while considering the environmental factors. In Section 3.4, advanced strategies are examined that help decision-makers to implement suitable control measurements.
3.1. Environmental factors influence on the spread of SARS-CoV-2
Environmental conditions may influence the aerosol transmission of the SARS-CoV-2 virus that causes disease, its transmission, and host vulnerability, as in other past pandemics. Significant parameters that can impact the virus-laden-aerosol transmission and virus's temporal activity are temperature, humidity, precipitation, and wind speed (Bhattacharjee, 2020). Sajadi et al. (2020) found that the virus spread more in colder regions with an average of 5−11°C temperature and located within 30–50°N (30−50 ° north from the equator), such as Wuhan, Tokyo, Daegu, Qom, Milan, Paris, Seattle, and Madrid. Sil and Kumar (2020) observed that a range of temperature between -6.28 to +14.51°C was auspicious for COVID-19 spread during the first wave. Also, it was found that 13–17 COVID-19 cases were reduced per day with the one-degree rise in temperature based on data collection from positively affected countries (Europe, China, USA) and least affected countries (Siberia, Africa, and Canada). Hence, other such studies also provided considerable evidence that temperature was a crucial factor that plays a vital role in spreading the virus (Y Han et al., 2021; Islam, 2021; Rume & Islam, 2020). Eslami and Jalili, (2020) observed that rising 1°C ambient minimum temperature decreased COVID-19 cases by 0.86%.
Meanwhile, some other reported studies found a positive or no association between SARS-CoV-2 virus and temperature (Wang et al., 2020d), which came to opposite thoughts. For instance, Bashir et al. (2020) reported that there was no clear evidence that warm temperature would suppress the clinical COVID-19 cases. A similar observation was reported by To et al. (2021) and Lim et al. (2021). Jüni et al. (2020) raised another point that reduced COVID-19 patients were due to seasonal variation or public health interventions, including school closure and social distance. A cohort study of 144 geopolitical areas was performed to support this statement, including cities and provinces of the USA, Canada, and Australia (Jüni et al., 2020). It was observed that there was no considerable correlation of COVID-19 cases with latitude, temperature and a weak association with humidity (Jüni et al., 2020). In contrast, a negative correlation was found with school closure, restricted mass gathering, and social distancing (Jüni et al., 2020).
Overall, 66.6% of the included literature in this article shows that COVID 19 cases may be reduced by increasing temperature (Al-Rousan & Al-Najjar, 2020; Bhattacharjee, 2020; Wang et al., 2020a). These reported studies are mostly conducted during the first wave and the beginning of the second wave. Most of the confirmed cases and high mortalities were observed in New York (USA), Madrid (Spain), Lombardy (Italy), and London (UK) in the time from January to April 2020 (WHO, 2021). Where average temperature observed during this period was 9 °C, 11.9 °C, 11 °C, and 8.6 °C, respectively. Hence, Sharif et al. (2021) concluded that the optimal temperature range in which most of the COVID-19 cases were detected in these regions was from 7 to 14°C. In contrast to the USA and colder regions of European countries, some regions such as Western Cape (South Africa), Sao Paulo (Brazil), Dhaka (Bangladesh), a different sensitive temperature range was observed, i.e., 16.5 to 30°C (Sharif et al., 2021). The reduction in cases was observed when the temperature exceeded this range. During the second wave, significant cases were observed in the USA, followed by Brazil, India, the UK, France (WHO, 2021). There might be other reasons for maximum cases in various climate zone regions, including inconsistent implementation of lockdown, social distancing, and health facilities (Huang et al., 2020). The effect of temperature on COVID-19 based on various studies is summarized in Table 1 .
Table 1.
Examples of environmental conditions associated with the spread of SARS-CoV-2.
| Parameters studied | Study area | Time span | Major Findings | Correlation with COVID-19 | Refs. | |
|---|---|---|---|---|---|---|
| Meteorological/atmospheric factors | ||||||
| Temperature, absolute humidity (AH) | China (30 provincial capital cities) | 5 January to 22 March 2020 | Each 1°C rise in ambient and diurnal temperature, there is a decline in daily cases, and low humidity increase transmission | Negative correlation with temperature and AH | Liu et al. (2020a) | |
| Temperature, relative humidity (RH) and high windspeed | Four selected cities in China and five cities in Italy | 1 January to 13 March 2020 | RH and wind speed have no significant impact. Whereas maximum temperature decreased the cases | Negative correlation with temperature | Bhattacharjeet al. (2020) | |
| Temperature, humidity, windspeed, pressure | Chinese provinces | 22 January to 1 March 2020 | High temperature, windspeed, and pressure with low humidity increase confirmed cases and deaths in many of the provinces in China | Positive correlation with temperature, windspeed; negative correlation with humidity | Al-Rousan & Al-Najjar (2020) | |
| Temperature | China | 20 January to 4 February 2020 | Every 1°C increase in temperatures decrease the cumulative number of cases by 0.86 | Negative correlation with Temperature | Wang et al. (2020a) | |
| Temperature, humidity | China | 22 January to 16 February 2020 | 1°C rise in temperature above 5°C decreases the transmission by 10%; and no relation with humidity | Negative correlation with Temperature | Gupta (2020) | |
| Temperature, rainfall, average humidity, wind speed, and air quality | New York, USA | 1 March to 12 April 2020 | Temperature and air quality are significantly associated with the COVID-19 pandemic | Positive correlation with temperature | Bashir et al. (2020) | |
| Temperature, windspeed, precipitation | Four Canadian provinces (Quebec, Ontario, British Columbia, and Alberta) | January to May 2020 | Per unit rise in temperature,14.3 COVID-19 cases increase per 100,000 people | Positive correlation but statistically non-significant after windspeed and precipitation adjustment | To et al. (2021) | |
| Temperature, relative humidity, and UV radiation | More than 200 cities in China | Early January to early March 2020 | No association of temperature with cumulative daily cases | No correlation with temperature or humidity | Yao et al. (2020) | |
| Humidity | 50 states in the USA | 22 January to 26 March 2020 | Direct and significant humidity association with COVID-19 cases in all the states | Positive correlation between humidity and COVID-19 patient fatality | Li (2020) | |
| Windspeed, temperature | Delhi, India | Not specified | Possibility of a second wave of COVID-19 in autumn and winter where low temperatures and high wind speeds increase virus transmission and survival | Positive correlation with windspeed and negative correlation with temperature | Dbouk and Drikakis (2020) | |
| Temperature, precipitation, humidity, wind speed, and average solar radiation | Iran | 19 February to 22 March 2020 | Areas with low wind speed, humidity, and solar radiation exposure to a high rate of infection; Precipitation is not significantly related | Negative correlation with windspeed, humidity, and solar radiation | Ahmadi et al. (2020) | |
| Precipitation, temperature | International samples | 1 December 2019 to 30 March 2020 | Average daily temperature by 1°F reduced the COVID-19 cases by 6.4 cases/day; Average inch/day precipitation increased; 56.01 cases/day rise. | Negative correlation with temperature. Positive correlation with precipitation | Sobral et al. (2020) | |
| Pollen concentration, temperature, humidity, lockdown effect | 130 sites in 31 countries | 10 to 14 March 2020 | An increase of pollen abundance by 100 pollen/m3 resulted in a 4% average increase of infection rates. | Without lockdown, pollens have a positive correlation with infection rate | Damialis et al. (2021) | |
| Temperature and Absolute temperature | Several provinces in USA and China | 21 January to 6 May 2020 | 60.0% of the confirmed cases of COVID-19 occurred in places where the air temperature ranged from 5°C to 15°C. Approximately 73.8% of the confirmed cases were observed with absolute humidity of 3 g/m3 to 10 g/m3. | Optimal temperature and humidity range is found with increasing COVID-19 cases | Huang et al. (2020) | |
| Ambient air pollutants | ||||||
| PM2.5, SO2, NO2 | 355 municipalities in the Netherlands | February to June 2020 | A municipality with 1 μg/m3 more PM2.5 concentrations will have 9.4 more COVID-19 cases, 3.0 more hospital admissions, and 2.3 more deaths | Positive correlation with PM2.5 and NOx; SO2 is not statistically significant | Cole et al. (2020) | |
| PM2.5, NO2 | Italy | March-October 2020 | An increase of 1 (μg/m3) in PM2.5 and NO2 concentrations corresponded to an increase in incidence rates of 1.56 and 1.24 × 104 people, respectively, | Positive correlation with PM2.5 and NO2 | Fiasca et al. (2020) | |
| PM2.5 and other meteorological factors | USA (County-level) | January to 18 June 2020 | An increase of only 1 µg/m3 in PM2.5 is associated with an 8% increase in the COVID-19 death | Positive correlation | Wu et al. (2020a) | |
| PM2.5, PM10, CO, NO2, SO2 and O3 | 120 cities of China | 23 January to February 2020 | Positive associations of all pollutants with COVID-19 confirmed cases. SO2 levels are negatively associated with the number of daily confirmed cases |
Positive correlation with PM2.5, PM10, CO, NO2, and O3 Negative correlation with SO2 |
Zhu et al. (2020) | |
| PM2.5,CO2, NO2 | 25 major cities in India | 29 January to 18 May 2020 | Direct association with PM2.5 and COVID-19 death rate in India | Positive correlation | Mele and Magazzino (2021) | |
| NO2 | 6 administrative regions in Italy, Spain, France, and Germany | January to February 2020 | Contribution of long-term exposure to NO2 on coronavirus fatality | Positive correlation | Ogen (2020) | |
| SO2 and O3 | USA and China | 12 December to 22 April 2020 | Positive association of ambient air pollutant of SO2 and Ozone concentration with a high risk of COVID-19 spread | Positive correlation | Xu et al. (2020) | |
Wang et al. (2020a) investigated whether high temperature and relative humidity (RH) had a strong influence on the adequate reproduction number (R) of COVID-19 in the USA and China before lockdown based on first-quarter data. For instance, assuming a 25 % increase in RH at 30 °C temperature in the northern hemisphere, there would be a reduction of about 0.89 in the R-value from winter to summer (Wang et al., 2020b). Whereas, after lockdown, the correlation of RH and temperature with R-value was still negative (Wang et al., 2020c), it was not statistically significant because of non-pharmaceutical interventions in the USA. The same concept was discussed by Lowen and Steel (2014) that cold temperature and dry conditions with 20−35% RH increased the intensity of the influenza viruses then intermediate (50% RH) or high humidity (>80% RH) conditions. Hence, many other studies confirmed RH's negative association with COVID-19 cases (Qi et al., 2020; Xu et al., 2020). In some of the southern hemisphere and humid tropical regions such as Bogota and Antioquia in Colombia, it showed a steady record of COVID-19 even at 72 to 82 % humidity with a temperature range 13−27°C during October – December 2020 (Islam, 2021). Similarly, in some regions of Brazil, such as Rio de Janeiro, the weather changed to hot humid at the end of October, but SARS-CoV-2 kept spreading, indicating a weak correlation between humidity and COVID-19 cases (Islam, 2021). Humidity is also measured as absolute humidity (AH) (a water vapour measurement in the air without considering temperature). Bukhari and Jameel (2020) studied that COVID-19 was mostly spreading in regions with AH < 10 g/m3. The number of cases reduced in summer because many hot spot areas of Canada and the USA had high AH than 10 g/m3. Similar results were reported by Huang et al. (2020) indicated that 73.5% of confirmed cases were reported at AH of 3 g/m3 to 10 g/m3. In comparison to temperature, it is not clear in the literature whether AH or RH has a more potent influence on spreading the virus (Bashir et al., 2020; Biktasheva, 2020; Rume & Islam, 2020). However, in most studies, an insignificant correlation was observed between COVID-19 mortality rates and humidity (Eslami & Jalili, 2020; Harmooshi et al., 2020). Sharif et al. (2021) found that regardless of temperate climate zone, the majority of cases and fatalities at 65%–80% average RH per day was near optimum for droplet nuclei to survive longer in the environment.
Menebo (2020) indicated that precipitation was negatively correlated with the spread of the virus in a densely populated area. The reason could be that rainfall better supported social distancing and staying-at-home rules than dry weather. In comparison, Xu et al. (2020) reported a positive correlation between precipitation, wind speed, and virus transmission. Another study found that for each one-inch increment in rainfall per day, 56.01 cases per day increased globally (Sobral et al, 2020). Although the rainy season does not directly influence virus transmission, extreme precipitation events indirectly drive people to stay indoors (Bashir et al., 2020; Menebo, 2020). The indoor built environment, including the efficiency of heating, ventilation, and air conditioning (HVAC) systems, can play a significant role in fostering the transmission of infectious diseases (Dietz et al., 2020).
Wind speed may contribute to the transmission of the virus from the source to distant places. Past studies confirmed a positive correlation of dust particles with virus transportation, especially during dust storms. For instance, the influenza A virus concentration was found high in downwind South Korea and Japan (Chen et al., 2010). Further studies indicated that infectious diseases viruses theoretically might be transported by dust particles across oceans (Wu et al., 2016). Wei et al. (2020) investigated warm places with increasing wind speed had a higher exposure risk for COVID-19, but a negative correlation was observed if the windspeed range lies between 1.5–2.5 m/s. However, the negative correlation between wind speed and the COVID-19 infection rate was observed by Ahmadi et al. (2020) in those areas in Iran, where there were low humidity and low solar radiation. Isaia et al. (2021) examined that solar radiation explained up to 83.2% of the spatial variance of the COVID-19 in the Italian region. Sharif et al. (2021) found that average 11−22 (km/h) wind speed and 3−6 UV index were associated with the virus spread during the first wave of COVID-19 in the hot spot areas [e.g., New York (22 km/h), London (14 km/h), Lombardy (14 km/h), Western Cape (17 km/h), Madrid (14 km/h), and Dhaka (11 km/h)]. In addition, Ahmadi et al. (2020) suggested that the virus's spread depends on geographical location; and high population density with high intra-provincial transportation directly relates to COVID-19 infection rate. Damialis et al. (2021) investigated the correlation of airborne pollens and the temperature and humidity effect with and without the lockdown effect. Pollen abundance was found to be synergistic with temperature and humidity with a significant positive correlation with the infection rate; however, lockdown and weekends halved infection rates under similar pollen concentrations. However, pollen particles could not act as infection carriers or transmit the virus (Dunker et al., 2021). The reason might be that pollen suppressed the innate antiviral immunity of the population and made them more susceptible to viruses such as SARS-CoV-2. It suggested wearing a masque during outdoor activities in spring (pollen season) (Gilles et al., 2020). In addition to the environmental conditions, very few studies focused on spatial-temporal variation and seasonal variation to understand the geographical evolution of COVID-19 (Sartorius et al., 2021). For example, Matthew et al. (2021) examined the influence of spatial-temporal variations on the COVID-19 cases concerning environmental factors fluctuations in 61 countries worldwide for an approximately five-month period (December, 2019−May, 2020). It was found that the spread of COVID-19 was highest in the high latitudes regions with a temperate climate, and the primary route of the transmission was mainly from the epicentre to North America and Europe, imitating the travel patterns from China (Matthew et al., 2021). Furthermore, the influence of meteorological changes on the spread of COVID-19 varied across different climatic regions. i.e., warm and cold (Wang et al., 2021). Thus, positive and negative correlations have been observed for all environmental factors (temperature, humidity, and precipitation) (Matthew et al., 2021; Sartorius et al., 2021; Wang et al., 2021). It implies other crucial factors should also be considered, such as variations in socioeconomic, demographic, healthcare facilities, and other protocols (e.g., lockdown, wearing masks, social distancing, frequent washing of hands) to fight the pandemic (Matthew et al., 2021).
The research studies have highlighted that coronavirus's air-borne transmission is one of the most potent ways of infection (e.g., Morawska & Cao, 2020). Table 1 shows that exposure to criteria air pollutants [e.g., particulate matter (PM2.5, PM10), ozone (O3), oxide of nitrogen (NOx), and carbon monoxide (CO)]. Most studies found confirmed associations of pollutants’ concentration with COVID-19 spread or fatalities for various regions such as China, USA, Italy, Netherland, India, and Germany, with higher levels of air pollutants resulting in increased cases and mortality rates during the first wave (Cole et al., 2020; Ogen, 2020; Zhu et al., 2020). It is interesting to observe that in Sao Paulo, Brazil (tropical zone with 8006 persons per square kilometre population density), a 10 µg/m3 increase of PM2.5 results in a risk of 1.140 for COVID-19 cases (Risk value >1 means increased risk for the exposed group) (Ibarra-Espinosa et al., 2022). Such risk is 1.06 times higher than that caused by O3 (Ali & Islam, 2020; Ibarra-Espinosa et al., 2022). Another study indicated that for short-term exposure and a 10 μg/m3 increase in PM2.5, PM10, NO2, and O3 were associated with a 2.24, 1.76, 6.94, and 4.76% increase in the daily COVID-19 cases, respectively (Ali & Islam, 2020). For SO2, the association was not clear with the risk of COVID-19 infection (Zhu et al., 2020). Ali and Islam, (2020) found that about 78% of mortality occurred in five regions of northern Italy and central Spain for long-term exposure, where a high level of NO2 was present because of traffic and power plant emissions. After the first lockdown, air quality improved in many regions of India and China, where reduced mortality rates were observed in the second wave (Ali & Islam, 2020).
3.2. Transmission of virus-laden-aerosol within environmental media
The virus attached to aerosols can persist, survive and use the environment as a secondary reservoir until finding a living host (Li et al., 2020). There has been proof that SARS-CoV-2 remains alive and can stay for hours in the air (Coleman et al., 2021) and survive on the fomite surfaces from a few hours to many days (Chin & Poon, 2020). SARS-CoV-2 may transmit to the multimedia environment via airborne particles through multiple routes, which is not addressed in epidemiological studies.
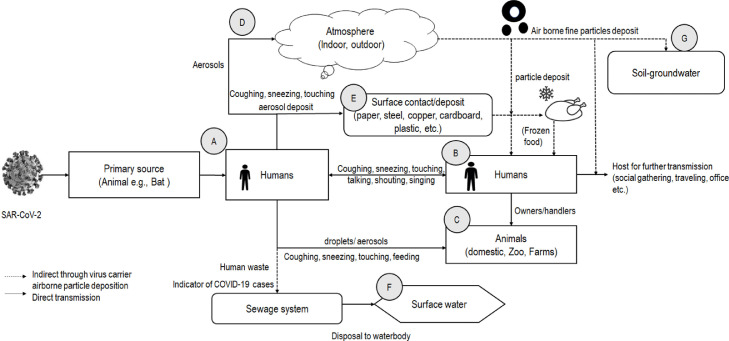
Considering the above discussion, Fig. 2 shows possible SARS-CoV-2 transmission pathways through primary reservoirs (e.g., atmosphere) to humans and then from human to airborne particles that could be deposited to various environmental media. Multiple pathways could transmit the virus mainly via human-to-human interaction directly or transmit between humans and other animal species. These animals (pets or other farm animals) could be sources of the secondary reservoir (i.e., host) of the virus (e.g., Chan et al., 2020). Simultaneously, the environmental transmission could be through a respiratory droplet and aerosol particles to the atmosphere (i.e., both indoor and outdoor environments). The transmission could be from the aerosolization through flushing, infected person's waste, and the virus could be found in the local sewage system (Wang et al., 2005). If sewage disposes into the surface water, it could contaminate the water bodies (e.g., rivers) (Guerrero-Latorre et al., 2020). Virus-laden-aerosol or airborne particulate matter could be deposited on the soil and other fomite surfaces through particle dispersion. Even an infected person could transmit the virus on the fomite surface via touching, sneezing, and coughing (Wijaya et al., 2020). The virus in soil could be further transported to groundwater (Kimura et al., 2008), possibly further to drinking well. Moreover, the deposition of virus-laden particles on the food item is suspected by scientists (Han et al., 2020; Wijaya et al., 2020). Thus, it shows that multiple aerosol transmission routes might result in dreadful loops for virus spread while considering the virus, host, and environment interactions based on Fig. 2.
Fig. 2.
SARS-CoV-2 transmission via airborne particles and multiple pathways to multimedia and the built environment. Note: Agent to host transmission and then host to host transmission: animal to human transmission (A), human to human transmission (B), human to animal transmission (C); virus-laden-aerosol deposit and transmission into various environments multimedia (D–G).
The respiratory droplet is responsible for the human-to-human and human-to-animal transmission mechanism within six feet distance. In contrast, aerosols are the virus carrier in the air that disperse to other environmental media (water, soil, and biota). Differences between respiratory droplets and aerosol particle dynamics and how virus-laden-aerosol transmission into the multimedia environment are further discussed.
3.2.1. Droplets and aerosols carry virus through the air
SARS-CoV-2 can transport in the air through respiratory droplets and aerosols (airborne particles) (WHO, 2021). The respiratory droplet generates through the cough, sneeze, and talk of the (infected) person and is larger in size (diameter > 5 μm) that abruptly drops to the ground under gravity, typically within 6 feet of the infected ones (Jayaweera et al., 2020). The aerosols are relatively smaller droplet nuclei (diameter ≤ 5 μm) that linger in the air over a long time and may disperse to some extent greater than 6 feet (Thompson, 2020).
Most of the published studies related to SARS-CoV-2 air sampling did not report the exact measurement of particle size. Fears et al. (2020) suggested that SARS-CoV-2 generally remained for a longer duration in 2−5 µm size particles. The residence time of ultrafine particles in the air is in the order of days to weeks, allowing transport up to thousands of kilometers in the atmosphere compared to the coarser particles (Wei et al., 2016). Gravity is the dominant mechanism for large droplets over Brownian motion (Zhou et al., 2020). Whereas smaller particles usually evaporate before reaching the surface, the evaporated residues persist in the atmosphere for a longer time (Jayaweera et al., 2020). These small droplets are also known as bioaerosols when they contain infectious viruses such as SARS-CoV-2 (Thompson, 2020). Even the liquid portion of the particle evaporates, the bioaerosol residue lingers for an extended time and can disperse easily in the air (Jayaweera et al., 2020). Table 2 shows that SARS-CoV-2 concentration is primarily found in respirable particles than inhalable size. Interestingly, it is observed based on Table 2 that a higher concentration of virus is found in personal protective equipment (PPE) disposal room, 12−40 (× 103) SARS-CoV-2 RNA copies/m3 for particles less than 1 μm, and 2.0−8.0 (× 103) SARS-CoV-2 RNA copies/m3 for 1 to 4 μm particle size. In medical staff offices, higher value, i.e., 7 (× 103) SARS-CoV-2 RNA copies/m3 for less than 1 μm and 13 (× 103) SARS-CoV-2 RNA copies/m3 is observed. Whereas 1−4 μm size aerosols are found in the public area toilets contaminated with 1545 SARS-CoV-2 copies/m3. The reason is toilet flushing might generate aerosol droplets that rise into the air to one metre if the lid is not closed (Laura Howes, 2020).
Table 2.
Aerosol size distribution and virus concentration in the built environment.
| SARS-CoV-2 RNA genome copies/ m3 0.103 | ||||||
|---|---|---|---|---|---|---|
| Aerosol size distribution | Patient room | Outside patient room | PPE removal room | Clinical sampling area | Medical staff offices | Toilet |
| Refs. | Chia et al. (2020) | Chia et al. (2020) | Liu et al. (2020) | Lee (2020) | Lui et al. (2020) | Zhou et al. (2020) |
| <1 µm | – | – | 12–40 | – | 7 | – |
| 1−4 µm | 1.3 | 0.92 | 2–8 | – | 13 | 1.55 |
| 4−10 µm | 2 | 0.93 | – | 2.35 | – | – |
| >10 µm | – | – | – | 0.7 | – | – |
Note. – means measured but found undetectable.
As presented earlier in Table 1, environmental conditions, including air quality, may influence the transmission through aerosol or airborne particles. Another person inhaled these particles or these particles deposited onto the ground/soil/surface. Twohy, Coakley, and Tahnk (2009) suggested that besides temperature, humidity in the atmosphere could cause a considerable growth of hygroscopic aerosol particles, which might offer a large surface area for adsorption in the atmosphere. Thus, it is crucial to understand the optimal humidity range in which the virus could be stable in aerosol particles. van Doremalen et al. (2020) studied that the survival rate of SARS-CoV-2 in aerosols was 3 hrs with a half-life of 1.09 h at 65% RH and 21°C–23°C temperature and compared it with SARS-CoV having 1.18 hrs half-life at same environmental. Smither et al. (2020) reported, based on the experimental study, that SARS-CoV-2 was more stable at 40–60% RH (decay rate, 2.27 percentage per min) as compared to 68–88% RH (decay rate, 0.40 percentage per min) in artificial saliva. Conversely, the opposite analysis was observed in tissue culture media, with a 0.90 percentage per min decay rate at medium RH and a 1.59 percentage per min decay rate at high RH.
3.2.2. Stability of virus-laden-aerosol in the built environment
Several past investigations have shown that the virus has higher transmission in confined indoor spaces (e.g., restaurants, shopping centres, cruise ships), workplaces (offices, factories), and indoor events, religious gatherings, parties, and transport vehicles (Qian et al., 2020). Especially in crowded places, the risk of COVID-19 disease transmission is higher, where it is challenging for individuals to remain spaced at least 6 feet apart (Thompson, 2020). For example, in 2020, 1.9 million people got infected in carnival celebrations in Germany and football matches in Italy (Bergamo city), highlighting the risk of crowding events outdoor (Sassano et al., 2020). At a call centre in South Korea, more than 43.5% of employees (total number of employees 216) got infected, indicating widespread transmission in a crowded indoor workplace environment (Park et al., 2020). Two people died, and approximately 103 people had tested positive amongst 2460 passengers and about 1111 crew members in Grand Princess cruise ship, USA (Shen et al., 2020). In another case, 24 of 68 people were tested positive on a bus in Ningbo City, Zhejiang Province, China (Shen et al., 2020). Forty people were found with COVID-19 at a shopping mall in Tianjin, China (Tang et al., 2020a).
Efficient heating, ventilation, and air conditioning (HVAC) systems help reduce the spread of the virus (Dietz et al., 2020). Higher ventilation is suggested to reduce the risk of airborne infection, also known as dilution ventilation (Qian et al., 2020). However, high ventilation also increases energy consumption and operating cost. Sha et al. (2021) suggested ventilative cooling and dilution ventilation that could reduce energy consumption in high-rise buildings and, thus, reduce the risk of virus transmission. Henriques et al. (2021) reported that natural ventilation strategies might be used in enclosed spaces such as offices regardless of the season since it is twice as effective during winter compared to summer. Viruses are found to be associated with particles in a range of sizes. Some of these particles could potentially penetrate high-efficiency filters, and a proper filtration system could reduce aerosol transmission risk. Operational factors in facilities such as air conditioning and ventilation play a vital role in indoor virus transmission regardless of outside environmental conditions (Dietz et al., 2020). It was reported that the virus present on the hospital bed could spread within 10–18 hrs to other fomites, including door handles, chairs in a waiting room, and books in different rooms (Lanese, 2020). SARS-CoV-2 can survive on fomite surfaces such as tissue papers and printing papers in offices for up to 3 h; treated-wood and furniture for up to 2 days; can stay longer on smoother surfaces, such as banknotes and glass surfaces up to 4 days; and can survive up to 7 days over the stainless steel and plastic surfaces (Chin & Poon, 2020). Another study reported that SARS-CoV-2 remained active for 8 h to 1 day on copper and 1–2 days on cardboard (Aboubakr et al., 2020). Table 3 shows that that concentration of virus in aerosols and fomite surfaces at various areas under hospital environment setting.
Table 3.
Concentration of SARS-CoV-2 in aerosols in hospital indoor environment.
| Hospitals | Huoshenshan Hospital Wuhan, China Guo et al. (2020) | Hospital of Guangzhou Medical University (FAHGMU), China Lei et al. (2020) | Renmin Hospital, Wuhan, China Liu et al. (2020a) | Fangcang Field Hospital Wuhan, China Liu et al., 2020a) | North West London teaching hospital, London, UK Zhou et al. (2020) | Institut Universitaire de Cardiologie et Pneumologie de Quebec (IUCPQ), Canada Dumont-Leblond et al. (2020) | National Centre for Infectious Diseases, Singapore Chia et al. (2020) | University of Nebraska Medical centre, USA Santarpia et al. (2020) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of sampling | Air | Fomite | Air | Fomite | Air | Air | Air | Fomite | Air | Air | Air | Fomite | Air | Fomite |
| Devices/method used during the study | SASS 2300a | Swabb | NIOSHc | Swab | Air sampler with gelatine filtersd | Coriolis μ air samplere | Swab | Air sampler with gelatine filters | 37 mm cassettef | NIOSH | Swab | Airport MD8 g | Swab | |
| Sampler flow rate (L/min) | 300 | NA | 3.5 | NA | 5 | NA | 100 | NA | 10 | NA | 3.5 | NA | 4–50 | NA |
| Sampling time | 30 min | 4 hr | 30 min | – | 300 min | – | 10 min | – | 4,6, 18 hrs | – | 4 hr | – | 15 min | – |
| Units | Copies/L | Copies per sample (0.103) | Copies/mL (0.105) | Copies per sample (0.105) | Copies/m3 | Copies/m3 | Copies/m3 | % | Copies/m3 | Copies/m3 | Copies/m3 | % | Copies/L | % |
| Isolation wards |
23.25–208.33 (overall values in patient's room and hospital) |
9.86–514.87 (overall values in patient's room and hospital) |
916–2000 (overall values in wards) |
|||||||||||
| Patient masque | NA | 3.3 | – | 0.98–700 | – | – | – | – | – | – | – | |||
| Trash can | NA | 34 | – | – | – | – | – | – | – | – | – | |||
| Computer mouse | NA | 28 | – | – | – | – | NA | 62–82 | – | – | – | |||
| Bed handrail or near bed | NA | 43 | – | .0022 | – | – | NA | 39–57 | 59 | 40 | – | |||
| Indoor air near patient | 1.40 | NA | .0045- 8.3 | – | – | – | – | – | – | 2.42- 45 | – | |||
| Indoor air near the doctor | 0.52 | NA | – | – | – | – | – | – | – | – | – | |||
| Doorknob | NA | ND | – | – | – | – | – | – | 48 | – | – | |||
| Air in ward | ND | NA | 700 | – | ND | ND | 7048 | NA | 60 i | – | – | |||
| Patient's room floor | NA | 66 | – | .0022 | – | – | – | – | 65 | – | 7–30 | |||
| Patient mobile | – | – | – | ND | – | – | – | – | – | – | 15 | |||
| Intensive care unit | 3.80 | NA | ND | ND | 113–31h | ND | 720 | ND | – | – | – | |||
| Pharmacy or departmental stores | ||||||||||||||
| Pharmacy floor | NA | 74.5 | – | – | ND | 3 | – | – | – | – | ||||
| PPE and changing room | ||||||||||||||
| PPE | – | – | ND | 16–42 | – | – | – | – | – | |||||
| Sleeve cuffs | NA | 7.10 | – | – | – | – | – | – | – | – | ||||
| Gloves | NA | 2.90 | – | – | – | – | – | – | – | – | ||||
| Shoe sole | NA | 32 | – | – | – | – | – | – | – | – | ||||
| Bathrooms | – | – | 170- 700 | – | ND | 19 | 464 | 69–81 | 32 | 28 | – | |||
| Staff office, and other stations | ||||||||||||||
| Telephones | – | – | – | – | – | – | NA | 35–43 | – | – | – | |||
| Workstation | ND | ND | – | – | ND | 1–9 | ND | ND | – | – | – | |||
| Staff office | ND | ND | ND | ND | ND | 6–20 | 404 | ND | – | 20.03 | – | |||
| Public area and halls | ||||||||||||||
| Public area | – | – | – | – | 7 | 3 | 1545 | NA | – | – | – | |||
| hallways | – | – | – | – | 6 | ND | 1574 | NA | – | 0.979–8.688 | – | |||
Notes. ND, Not determined. NA, Not applicable. –, means not included in the study.
SASS 2300 is a Wetted Wall Cyclone Sampler.
Swabs are wetted with viral transport medium (VTM) prior to sample collection and then placed in 15‐mL tubes for further lab analysis.
NIOSH (National Institute for Occupational Safety and Health) cyclone bioaerosol sampler.
IOM sampler with 3 µm gelatine filters (Sartorius Biotech, Gottingen, Germany).
air samples collected into a conical vial containing 5 mL Dulbeccos's minimal essential medium (DMEM) using a Coriolis μ air sampler (Bertin Technologies).
37 mm cassette sampler with 0.8 µm polycarbonate filters (PC) (SKC, Eighty-Four, PA, USA).
Sartorius Airport MD8 air sampler.
The reported values are virus aerosol deposition rates in copies m−2 h−1.
Sample collected from exhaust surface.
Based on Table 3, It was found that the Intensive care unit (ICU) indoor air had higher virus concentration followed by the air around the patients and air near the doctor compared to other areas. The virus was found usually on some patients’ masks, trash cans, computer mouse, bedrail, patient's bed floor, pharmacy floor, hospital's telephone, air exhaust surface, personal protective equipment (PPE) changing room, including shoe soles, sleeves, and gloves. In some studies, virus-laden aerosols were found in bathrooms. However, room ventilation, open spaces, and sanitization could limit the virus concentration in the air (Liu et al., 2020b). Table 3 also shows that values of the virus might depend on the sampler and air sampler flow rate. Dumont-Leblond et al. (2020) suggested that the air sampled volume might be increased using a high-flow sampling rate to capture maximum virus. However, the SASS® 2300 detecting the virus was unsuccessful using 300 L/min with a sampling time of 30 min in Guo et al. (2020) study. It indicates that the virus may be degraded or desiccated by increasing the flow rate without optimizing the sampling time. The instrument's short sampling time could also have reduced the chances of catching aerosols that the infected patient randomly produces.
3.2.3. Evidence of virus in wastewaters with aerosolization
Despite the COVID-19 pandemic spread across the globe, wide-ranging access to testing at the community level has thus far been constrained. While it is challenging and time-consuming to test every occupant for SARS-CoV-2, the virus has been found in the positive patient stool (Wu et al., 2020b). Thus, wastewater-based epidemiology could be one way to detect pathogens across the communities and estimate population incidence without individual testing (Zahedi et al., 2021). The chemical composition of faecal matter is mostly organic and can encourage the extended survival of the virus (Wu et al., 2020b). Thus, poor sanitation conditions, coupled with insufficient hygienic practices, can potentially spread the virus via fomites. Wang and Liu, (2021) study shows that wastewater plays a vital role in the transmission of COVID-19 within a city. SARS-CoV-2 can spread via oral, fomite, or flushing-aerosol routes that eventually reach the sewage systems (Wang & Liu, 2021). Sherchan et al. (2020) found SARS-CoV-2 first time in wastewater in Louisiana, USA. Medema et al. (2020) reported the SARS-CoV-2 viral RNA detection in wastewater from water resource recovery facilities (WRRFs) in seven different cities in the Netherlands. In another example, La Rosa et al. (2020) observed SARS-Cov-2 first time in the wastewater treatment plant influent in Milan and Rome, Italy.
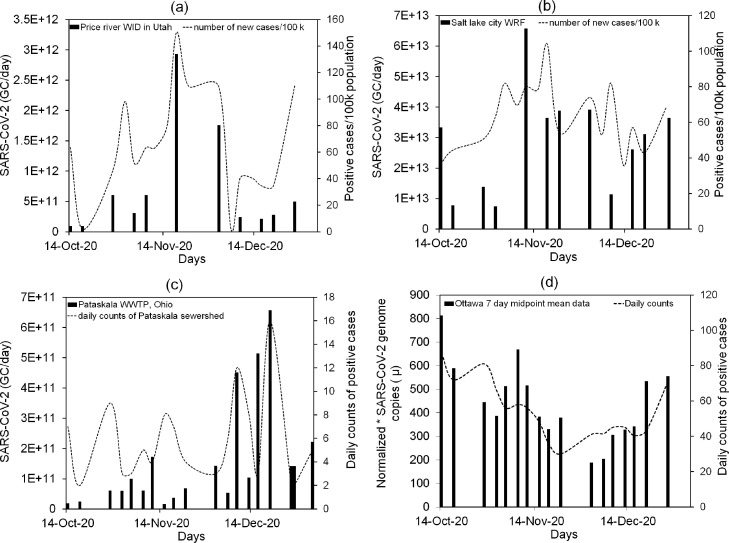
Fig. 3 shows the concentration of SARS-CoV-2 (genome copies/day) in wastewater from Coronavirus Wastewater Monitoring Network- Ohio (Pataskala-WWTP), Ottawa covid-19 wastewater surveillance, and two of the Utah SARS-CoV-2 sewage monitoring surveillance dashboards, i.e., Price River-Water Improvement District (WID) and Salt Lake City-Water Reclamation Facility (WRF). The maximum concentration observed in Price River-WID wastewater is 2.93 × 1012 genome copies (GC) per day in November 2020, having the highest number of positive cases (150) on the same day. In Salt Lake City-WRF, the maximum concentration of virus in wastewater is 6.57 × 1013 GC per day Fig. 3. (c) shows the 6.52 × 1011 SARS-CoV-2 GC per day in the influent of Pataskala-WWTP as the highest concentration. The examples show that surveillance helps to identify an increase in the number of cases without testing. Ottawa, a wastewater surveillance program, represented the virus concentration data in normalized GC as shown in Fig. 3 (d).
Fig. 3.
SARS-CoV-2 genome copies (GC)/day in wastewater compared to COVID-19 cases per 100,000 population from October to December 2020 with the time interval of 7 days: (a) Price river water improvement district (WID) in Utah, (b) Salt Lake City water reclamation facility (WRF) (Utah department of environmental quality, 2021); (c) SARS-CoV-2 genome copies (GC)/day in wastewater compared to COVID-19 daily count cases for Pataskala wastewater treatment plant (WWTP), Licking County, Ohio (Ohio Department of Health, 2021); (d) Normalized SARS-CoV-2 GC compared to daily counts of positive cases for Ottawa wastewater (Ottawa COVID-19, 2021). Notes. *Normalized copies: In wastewater, the proportion is from human waste, and the other proportion is from rainwater, snowmelt, etc. Viral copy data is normalized to subtract the runoff data using a seasonally stable faecal biomarker.
3.2.4. Presence of virus in freshwaters
Past outbreaks have an evident history that a virus might exist in surface water, groundwater, drinking water, and recreational water (Li et al., 2020). Whether the SARS-CoV-2 virus can spread through water is not clear. Although there is no evidence showing the link of transmission of coronavirus or its survival in water bodies. A few studies reported that the high concentration of SARS-CoV-2 virus was detected in natural waters from a low sanitation region, such as the sewage discharge points in urban rivers of Quito, Ecuador (Guerrero-Latorre et al., 2020). In April 2020, minuscule traces of the coronavirus were found in Paris's non-potable water used for parks, gardens, and cleaning streets drawn from rivers and canals (Al-Arabiya News, 2020). In Italy, the viral RNA was found in the Lambro River and Lambro Meridionale River samples (Giacobbo et al., 2021). The possible source of SARS-CoV-2 in freshwater is the discharge of raw sewage into surface water (Guerrero-Latorre et al., 2020). As in some developing countries (e.g., Brazil, Ecuador), there is a lack of separation of urban runoff water from sewage, leading to combined sewage overflows and disposal of sewage without proper treatment into surface waters is a common practice (Giacobbo et al., 2021). Another way that virus may enter the water bodies are improper disposal of single-use personal protective equipment (PPE) waste (e.g., face masks, face shields, gloves) (Tran et al., 2021). Unlike professional health care partitioners, most residents disposed of their masks and gloves with the mixed waste stream, which did not enter the treated biomedical waste stream (Benson et al., 2021). It has been reported that approximately 3.4 billion single-use PPE waste, including facemasks, are discarded daily globally during the COVID-19 pandemic (Benson et al., 2021). A considerable amount of PPE waste has escaped these waste streams, thus, ultimately washing up along coastlines and freshwater; hence residual viruses from this waste may enter the freshwater (Tran et al., 2021). As discussed earlier, evidence of the SARS-CoV-2 is found in the aqueous environment; however, no study on its persistence and survival in freshwater is available (Giacobbo et al., 2021). Past literature investigated that the SARS-CoV-1 could survive at 4−20 °C in the aqueous environment (Tran et al., 2021). Some studies also discussed that SARS-CoV-1 could persist in dechlorinated tap water for two days (Guerrero-Latorre et al., 2020). Such investigations should be made for the existence of SARS-CoV-2 under various environmental factors such as pH, temperature, UV index, and the presence of organic matter (Giacobbo et al., 2021). Recent studies started to examine if the SARS-CoV-2 virus could survive in streams and rivers under flow conditions and temperature (Dennehy, 2020).
3.2.5. Presence of virus in soils with aerosol deposition
As discussed in previous sections, research evidence has shown that the SARS-CoV-2 virus is found in air and wastewater, but few attempts are made to assess virus transport to soils (Núñez-Delgado, 2020). In general practice, wastewater is applied to irrigation or directly onto the soil surface in the absence of a nearby water stream or canal (Zhang & Shen, 2019). If sewage is applied without treatment, there are equal chances of soil and plant contamination, and the virus may become part of the food chain (Núñez-Delgado, 2020). Survival and transmission of viruses to groundwater depend on certain factors such as soil texture, adsorption property, organic matter, pH, moisture content, permeability, ionic strength, iron oxide level, and electric conductivity (Kimura et al., 2008). Also, environmental conditions, including rainfall, sunlight, and temperature, might associate with the transmission of the virus (Kimura et al., 2008). As far as viruses responsible for a pandemic are concerned, very few studies are available. For example, Buchy and Gutiérrez (2012) examined what type of soil was responsible for avian influenza virus transmission to animals under experimental conditions. It was found that soil collected from buildings and local nurseries in Cambodia could transmit the virus with a 100 % mortality rate in chickens than sandy soil from the rice field. However, natural conditions under different environmental settings might have a different conclusion. Recently, Zhang et al. (2020) found SARS-CoV-2 in 15 different soil samples (205–550 copies/g) close to the hospital in Wuhan. It could be that viruses in soil samples exhibited transmission potential through the aerosol deposition. These aerosols might be virus-laden droplets of COVID-19 patients in the hospital (Tang et al., 2020b). SARS-CoV-2 likely arose from raw wastewater before treatment and eventually deposited on soils. Future study can explore further how SARS-CoV-2 finds its way to host via soil transmission and under what environmental settings, virus transported to groundwater through the soil column.
3.2.6. Presence of virus in biota and reverse-zoonosis
Public health authorities, including the WHO, Food and Drug Administration (FDA) in the United States, and other regional food authorities, advised that there is currently no association that SARS-CoV-2 virus can transmit through food. However, some incidents have been reported, such as the viral particle was found on a cutting board used to process imported salmon at the Xinfadi agricultural market in Beijing (Han et al., 2020). In July 2020, approximately 8−9 cases of food contamination were reported, where viral particles of SARS-CoV-2 were detected on the packaging material of imported frozen shrimps imported from Ecuador (Han et al., 2020). In a later incident in Shenzhen, China, in August 2020, the virus was found in the packaging of frozen chicken wings (originated from Brazil), which became the first reported case where the coronavirus was found on food samples (Schwiegershausen, 2020). Recent studies reported that the virus remained stable on fish, and meat, for the 14–21 days at refrigerated and freezing temperatures (−20 and −80°C). Thus, food items are indirect at risk from farm to processing to the table, and further studies are needed to get more strong evidence (Fisher et al., 2020).
The above discussion shows that transmission could be from humans to food supplies or even to animals, indicating reverse zoonosis with the pathogen being transmitted from a person to another animal species (Munir et al., 2020). If this is possible for the SARS-CoV-2 virus, it could become problematic for other animal species to become potential reservoirs for secondary zoonotic infections (Munir et al., 2020). Many studies report transmission from infected owners and handlers to animals. For example, Mallapaty (2020) reported that a cat was tested positive because of the infected pet owner. Several tigers and lions tested positive in zoos due to close contact with infected employees in New York City (USDA, 2020). Sit et al. (2020) reported 2 out of a 15-dog sample tested positive in Hong Kong. In farms, sixteen minks were tested positive in various states (Utah, Oregon, Michigan, Michigan) (USDA, 2020). Forty-nine cats and thirty-five dogs reported testing positive in different US states (Munir et al., 2020). Oreshkova et al. (2020) reported interstitial pneumonia and SARS-CoV-2 RNA in organ and swab samples of minks in two farms in the Netherlands. Hence, till now virus has been detected in pets (dog and cat), Zoo animals (tiger, lion), and farm animals (Mink) through pet owners, animal caretakers, and farmworkers, respectively. There are scarce chances that the virus could transmit via contaminated feed or from an animal to animals in a cage or Zoo. However, there are some experimental cases where studies showed mild to moderate symptoms (Munir et al., 2020). SARS-CoV-2 infections caused none to mild respiratory signs. Food safety and reverse zoonosis require an in-depth investigation (Zhang et al., 2020b), including the transmission of the virus from the host or environmental surfaces to susceptible animals.
3.3. Opportunities to integrate environmental factors into modelling techniques to understand dynamics of COVID-19
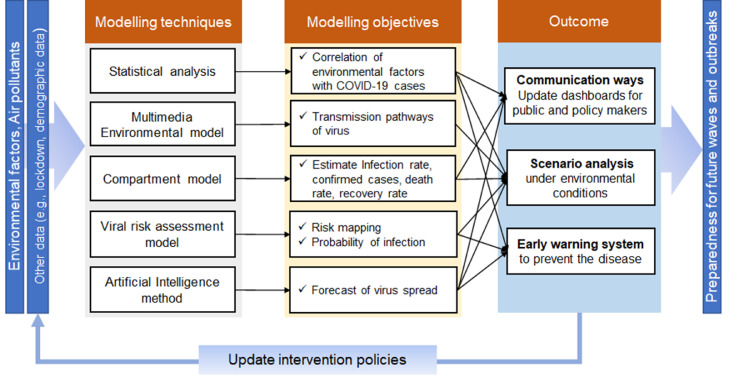
The mathematical modelling of pandemic diseases is advantageous in understanding epidemiological prototypes of viruses such as estimation of confirmed cases, recovery rate, and death by region; that information help decision-makers to implement social distancing measurement and other interventions (Naik et al., 2021; Sharif et al., 2021). However, these models are silent in investigating the evolution of the virus in response to climatic variation; and assume that the infection rate is constant with a homogenous environment (Merow & Urban, 2020). Environmental factors can influence the virus infection rate in two distinct ways: (1) From an epidemiological viewpoint, the spread of a virus depends on certain environmental conditions (Matthew et al., 2021) (as discussed in Section 3.1). (2) From a social behaviour perspective, weather influences mobility patterns and social distancing, affecting the virus spread across various regions (Sharif et al., 2021). Therefore, environmental granularity enables scientists to predict the situation closest to reality. In this section, various approaches are examined to understand the dynamics of COVID-19 by incorporating environmental factors. The statistical correlation of COVID-19 cases with the seasonal variations, transmission pathways, risk assessment to identify hot spots, and forecasting of virus spread under various environmental scenarios; all these opportunities are a way forward to combat the pandemic. As shown in Fig. 4 , the outcome of these tools would help to update the impact and forecast risk of future outbreaks and onset pandemic waves under various environmental conditions and assist policymakers in implementing non-pharmaceutical policies (e.g., lockdown) and planning, and proactive community actions.
Fig. 4.
Thematic aspects of various modelling techniques, their objectives, and outcomes to combat COVID-19 by incorporating environmental factors.
3.3.1. Statistical models to implicate environmental factors with COVID-19
Emerging pandemics such as the “severe acute respiratory syndrome” (SARS) of 2002–2003 and the “H1N1 swine flu” of 2009 show the importance of environmental conditions in the spread of infection and early outbreak detection (Hodges & Jackson, 2020). Toward this aim, the statistical modelling approach has boosted the potential in combating such pandemics, allowing for assessing emerging situations from an environmental perspective. The uncertainty in the data and the choice of the most suitable methodology are critical for constructing statistical models to examine the impact of climatic factors and air quality on the spread of the SARS-CoV-2 virus. For example, Tosepu et al. (2020) applied Spearman rank correlation analysis to associate weather conditions with COVID-19. Wu et al. (2020a) applied binomial regression and found that 1 µg/m3 increased in PM2.5 concentration was associated with the COVID-19 death rate by 8 %, after evaluating death counts for more than 3000 counties in the United States (representing 98% of the population of entire USA) up to April 22, 2020. Gupta (2020) used descriptive analysis to study the effects of weather conditions (e.g., temperature, wind, humidity) on the virus spread. Liu et al. (2021) used principal component analysis and the Pearson correlation coefficient to build a multiple linear regression model to investigate the impact of the built environment and urban density on the incident rate of COVID-19. Other statistical methods used to correlate climate change, meteorological factors, and seasonal variation with COVID-19 include multivariate linear regression (Pirouz et al., 2020), a time-series additive model (Qi et al., 2020), Bayesian approach (Merow & Urban, 2020). It is concluded that the statistical approach assists in correlating the environmental conditions with the spread of the SARS-CoV-2. However, these studies do not discuss transport mechanisms such as aerosol dispersion, multiple transmission pathways, or transmission to other environmental mediums.
3.3.2. Environmental models to estimate virus-laden-aerosol transport
The physical process-based environmental models determine the aerosol route while considering other environmental factors, including wind speed, temperature, humidity, to help identify dynamic exposure risk. For instance, Peng et al. (2020) demonstrated the effect of airflow on virus transmission in different environments (e.g., hospitals, school buildings) using the computational fluid dynamics (CFD) modelling approach and emphasized the significance of ventilation Bhattacharyya et al., (2020). also used CFD simulation to understand the spread of COVID-19 in hospitals' isolation rooms. In recent studies, Zhang et al. (2021) measured the SARS-CoV-2 transmission within the bus using CFD and experimental HVAC conditions. It was found that well fitted surgical masque and the effect of opening windows reduced the concentration to half. Desai et al. (2020) used CFD to study the optimum seat in the first business and economy section of the commercial plane, considering the temperature, air velocity, and CO2 mass fraction, which would help the public to decide which seat to occupy for their next flight during COVID-19 travelling. Moreover, air dispersion models such as AERMOD (Rezaali & Fouladi-Fard, 2021), and the three-dimensional Lagrangian dispersion models (Zhang et al., 2020b) were used to determine the virus-laden-aerosol dispersion under the ambient meteorological conditions to understand the exposure COVID-19 risk in the outdoor environment.
The ecological niche models (ENM) are mathematical-based and use process-based or biophysical approaches and have been essential to assessing diversity and disease distributions, indicating its application for spatial epidemiology. ENM predicts the viral particle distribution based on at least two organisms (i.e., host-virus; infected-susceptible), and biotic interactions lie at the core of the virus's ecological niche (Escobar, 2020). Recent studies to predict COVID-19 infection rate indicates that the ENM approach could study the probability distribution of virus in the environment (Coro, 2020).
3.3.3. Modelling epidemics with compartmental models
Compartment models are the deterministic type of epidemic model with ordinary differential equations, which is widely applied for infectious diseases (Thompson, 2020), in which each compartment is assigned with a category, e.g., “Susceptible” (S), “Infectious” (I), “Exposed” (E) or “Recovered” (R), and a susceptible person after getting infected is considered in “Infectious” category. Compartmental models are often based on Markov chains, which include SIR (Susceptible-Infected-Recovery), SEIAR (Susceptible-Exposed-Infected-Asymptomatic-Recovery), and SEIR (Susceptible-Exposed-Infected-Recovery) methods (Tolles & Luong, 2020). Thompson (2020) applied SIR model simulations to predict the effects of different non-pharmaceutical mitigation measures on several COVID-19 cases in the UK. The reported studies related to compartment models are mostly based on virus transmission mechanisms from individual to individual. They often assume a homogenous environment and epidemiological parameter considering known viruses, which is hardly suitable for heterogeneous conditions and evolving viruses (Tang et al., 2020a). Recently, Mahrouf et al. (2021) extended the conventional SIR compartmental model to a stochastic time-delayed model to predict the epidemiological trend of COVID-19. Hence, a stochastic (random) modelling framework has been used for influenza pandemics (Chang et al., 2014) and could extend the compartment models while incorporating heterogeneities in the population. Tuite et al. (2020) developed an SEIR-compartment model to estimate the number of COVID-19 cases in Ontario, Canada, different physical distancing scenarios, case detection, and isolation to support risk assessment and policy planning. During the COVID-19 waves, many countries enacted lockdown orders (Thompson, 2020). However, the policy makers must decide when to reopen the places, including schools, offices, gyms, and restaurants, or implement additional restriction policies. Chang et al. (2020) developed the SEIR model to capture the effects of mobility patterns on the spread of the virus and predict the people at risk across various census block groups (CBG) in the USA (The US census bureau uses CBGs; they are geographical units contain 600 to 3000 people). The number (Nci) of COVID-19 cases shows how many populations in CBG (ci) are in each disease state; where Nci is equal to the sum of Suspected (Sci), Exposed (Eci), Infectious (Ici) and Recovered (Rci) states. The following expression shows the distribution of the overall number of new exposures in CBG (ci) at the time (t) considering the mobility pattern to the point of interests (POIs) (e.g., restaurants, gyms, grocery stores, and other non-residential places) (Chang et al., 2020).
| (1) |
NSci→Eci is the number of new exposures, i.e., transition from the suspected population to the exposed state at the hour (t). λpj is the rate of infection at the point of interest (POIs) pj and represented as Poisson distribution. ωij is the number of people travelling from CBG (ci) to POI pj at t. λci is the base infection rate independent of visiting POIs and is distributed as binomial. Ψ is the transmission constant shared amongst all POIs and usually has a value of one. apj is a physical size in square feet. dpj is the median dwell time fraction d ∈ [0, 1] of an average visitor to pj. It is assumed that the proportion of infectious population amongst the ωkj visitors to pj from a CBG (ck) mirrors the overall density of infections [Ick/ Nck] in that CBG. In addition to visiting POIs, it is assumed that each susceptible in CBG (ci) has a probability of getting infected considering the base transmission rate (βbase). Overall, such compartment models help to understand the situation in metropolitan regions and restrict the maximum occupancy capacity at each point of interest.
3.3.4. Risk assessment methods to evaluate the COVID-19 risk
Risk assessments are generally based on four significant factors: health, behaviour, exposure, and social policy. Risk assessment modelling help to estimate the probability of undesired consequences because of exposure to the virus. Such a modelling approach can successfully quantify risks due to COVID-19 to measure the probability of infections/deaths and support decision-makers in understanding the likelihood of an action's outcomes and making informed decisions (Oliveira et al., 2020). Andersen et al. (2021) developed a population risk calculator for COVID-19 mortality using the Poisson regression model based on pre-existing conditions for the US and various sociodemographic factors. Zhang et al. (2020a) used a dose-response model to evaluate the infection risk of SARS-CoV-2 via aerosol transmission in China; the study was based on a quantitative microbial risk assessment (QMR) approach assuming an infected shopkeeper and assessing risk outside the market while considering the ambient wind conditions. A quantitative risk assessment of the viral disease and its spread via different transmission routes is difficult to evaluate their relative importance and strategize the control measures (Ashinyo et al., 2020). However, to avoid uncertainty in the analysis, it is suggested to incorporate real-time data of SARS-CoV-2. Risk assessment tools could be implemented as an early warning system at workplaces and help to establish whether an employee should return to the workplace or continue working remotely from home (Cook & El-Boghdadly, 2020).
3.3.5. Artificial intelligence method for forecasting of virus spread
Methods based on Artificial intelligence (AI) could be useful for finding the most suitable predictive model to forecast the maximum number of infected persons or recovered persons (Car et al., 2020). The AI-based method simulates the non-linear virus behaviours with or without human intervention. AI techniques have been used in medicine and health, including the neural network model to deep learning techniques. Unlike the if-then rules used in conventional computer programming, AI methods follow the decision-making process using two primary approaches. The first significant approach is supervised machine learning aiming to build up a predictive algorithm based on data analysis like regression and classification methods. Classification methods used in the prediction of infectious diseases in the past are “mixed linear regression (MLR)”, “artificial neural network (ANN)”, “decision tree (DT)”, “support vector machine (SVM)”, “Random forest (RF)”, “gradient boosting decision tree (GBDT)”, and “Bayesian Network (BN)” (e.g., Agany et al., 2020). The other AI approach is based on unsupervised machine learning, which permits computers to discover large amounts of unclassified data and learn treatment patterns. For example, Car et al. (2020) used an artificial neural network (ANN) model to study the spread of the Covid-19 and predicted the number of people who may contract the disease, recovered cases, and mortality rate per area each time unit. Pan et al. (2021) used the random forest (RF) machine learning method to understand the pandemic dynamics and make time-series predictions for Asian countries.
Table 4 shows the advantages and limitations of some of the essential modelling techniques discussed above, which could be used to predict the virus, associate socio-environmental factors with the spread of the virus and assess risk during the pandemic.
Table 4.
A state-of-the-art review of modelling approaches for COVID-19.
| Type of Models | Examples | Advantages | Limitation | Refs. |
|---|---|---|---|---|
| Statistical models | Spearman's rank correlation analysis | Study the correlation between COVID-19 and the environment (e.g., temperature, humidity, rainfall) and air pollutants | Metrological factors may have possible nonlinearity issues. Not consider virus transmissions |
Shakil et al. (2020) |
| Regression analysis using binomial | Help to identify the association of environmental factors with the spread of the virus | Need background data of environmental conditions and confirmed cases | Wu et al. (2020a) | |
| Descriptive analysis | Association of environmental factors and quantitative summary of COVID-19 daily cases | Findings are limited to theoretical/empirical explanation | Shakil et al. (2020) | |
| Environmental models | Lagrangian particle model | Fate and transport of aerosol transmission route under environmental conditions | Neglect the particle-particle interactions. | Wang et al. (2020a) |
| Gaussian plume dispersion models | Predict how much distance bio-aerosol or airborne particles may travel away from the infection source and spread the virus. Can estimate the spread at the field site, livestock area |
Cannot quantify the risk at the receptor level, could be combined with the risk assessment model. Not suitable for indoor air quality |
Van Leuken et al. (2016) | |
| CFD | Predict the spread of virus bearing droplets inside selected indoor environments, e.g., metro, hospitals, buildings | Not suitable for an open-air environment | Bhattacharyya et al. (2020) | |
| Ecological Niche model | Use a computer algorithm to predict the distribution of viruses across space and time in a particular region using environmental data. Could be used to estimate viruses amongst farm animals and livestock areas. |
Extensive details for geographical and climatic data are required for accurate results. | Coro (2020) | |
| Compartment model | SEIR | Estimate number of infection cases, reproductive number (Ro) of the virus, Evaluate the effect of non-pharmaceutical strategies |
Assume transmission rate is constant. Do not deal with uncertainties. Assume no external environmental conditions |
Tuite et al. (2020) |
| Risk Assessment models | Poisson regression model | Aid decision-maker to identify mortality rate due to COVID-19. Statistical distribution of virus spread across the region. |
Not incorporate other external factors such as environmental conditions. | Andersen et al. (2021) |
| AI | Artificial neural network (ANN) | Long term forecasting Can be used as a surveillance system. |
Complex Required large database for training. |
Car et al. (2020) |
| Gradient boosting machine (GBM) approach | GBM has high accuracy in the prediction of active and recovered cases of COVID-19. | The role of atmospheric factors, like temperature and humidity, in the transmission rate of COVID-19, is still uncertain and may vary according to location. | Shrivastav and Jha (2020) |
3.4. Strategies to tackle COVID-19 based on the One-Health approach
3.4.1. Environmental-assisted modelling system
There is no model or a framework that could address all the aspects of the outbreak and intrinsic factors such as environmental conditions, aerosol transmission to the multimedia environment, socio-economic factors, and the spatiotemporal disease pattern. Instead of building a complex model that may be subject to uncertainties challenging to parameterize and validate, Scoones et al. (2017) suggested an integrated modelling approach based on the OH concept. However, OH does not guide which model is suitable for predicting and controlling the disease but encourages combining models within the domain of virus disease dynamic modelling. For instance, spatial approach integration with conventional mechanistic epidemiological models might help to consider movement networks and geographical parameters influencing the disease spread (Allen et al., 2012). Scoones et al. (2017) recommended integrated process-based compartmental models using the OH approach, which can offer a spatial perspective on potential control of diseases (e.g., Lassa fever and Ebola virus). However, without insights from the transmission pathways to the multimedia environment and environmental drivers, it is crucial to better comprehend the dynamics of the disease first before proposing the OH-based integrated mode.
Previously, various OH-based developments were reported. For example, a strategic framework based on the OH approach was developed at the government level to prevent and control emerging infectious Nipah virus diseases in Bangladesh with WHO and FAO department (Ambat et al., 2019). In response to MERS-CoV in Qatar from 2012 to 2017, the OH approach was used (Farag et al., 2019). The road map of OH includes surveillance, investigation, epidemiological studies, and increased local diagnostic capacity (Farag et al., 2019).
Another future aspect of the OH approach is strengthening the management system's capacity by integrating with effective monitoring, risk assessment tools, and decision-making tools. Establishing the OH platform plays a crucial role in early warning systems, emergency preparedness, and control of infectious diseases. The quantitative viral risk assessment model would help provide the probability of infection for human-human transmission or reverse zoonotic and identify risk mapping of hot spot areas. The decision-making models help to generate various scenarios under socio-economic and environmental conditions. Examples of advanced surveillance, risk assessment tools, dashboards, and optimization methods are further discussed in the next section.
3.4.2. Advanced surveillance and risk assessment tools
There is a need for advanced and comprehensive risk assessment tools, surveillance, and continuous monitoring to estimate the health status, existing medical facilities, and people's behaviour. Moreover, public health surveillance could assist decision-makers in planning, managing on time, and becoming an important component of the OH-based system. For example, the “WHO Mass Gathering Religious Addendum Risk Assessment Tool” in the context of COVID-19 is an offline tool for planners and contains a control checklist for organizing events and mass gatherings (Chatterjee et al., 2020). “COVID Safe” is the Australian app that helps regional officials to contact the susceptible group who are vulnerable to COVID-19 quickly. Other such tools include “TraceTogether”, “COVIDWatch” (Chaturvedi, 2020). The assessment tool allows assessing sufficient measures concerning low to high-risk outcomes. The assessment tool also enables the measurement of risk assessment through the user's anxiety level in the current scenario.
GIS has become an important tool in visualizing the spread of COVID-19. Boulos (2004) discussed that GIS-driven virus spread-distribution maps such as Map Asia, SARS-GIS, through which visual information was provided for the public to locate infected buildings and zones during the 2003 SARS outbreak in Hong Kong. GIS was also used as an essential tool to help raise the community's spatial awareness for combating the virus, e.g., through an online GIS dashboard to provide real-time data of the spatial distribution of COVID-19 spread, including the confirmed cases, death rates, and recovery rate (Dangermond et al., 2020). Other examples of online GIS dashboards include “WHO-Covid19” (WHO-dashboard, 2021), “WorldPop” (Worldpop, 2021), and HealthMap (Healthmap, 2021). These dashboards are readily accessible to the public to track the virus (Kamel Boulos & Geraghty, 2020). Although GIS dashboards are handy but maximum benefits could be obtained by integrating them with other predictive models.
Internet of things (IoT) linked devices and applications could help lower the possible spread of COVID-19 to others by early monitoring. Nasajpour et al. (2020) surveyed the role of IoT tools, including robots, sensors, drones, wearables, smartphones, and IoT buttons applications, in helping the monitoring, warning and control of COVID-19. It is seen that IoT applications can be extremely efficient for this pandemic, but it is also difficult to consider data privacy. Big data and machine learning are promising ways to track infected people and timely preparedness against the pandemic. Big data provide a platform to integrate with effective modelling techniques such as statistics, environmental models, and risk assessment, making it possible to examine the pandemic dynamically and increase database capacity. For instance, the efficient use of big data methods helping to reduce the number of cases and deaths was reported in Taiwan (Wang et al., 2020a). Therefore, AI and monitoring data using the internet of things (IoT), could be an equitable and digital solution to the decision-makers.
3.4.3. Decision support system to optimize mitigation solutions
Decision support tools help build the community's resilience and capacity to control, anticipate, prepare, and manage future risks involving pandemic dynamics. During the COVID-19 pandemic, strategic decisions are crucial for the stakeholders, and worldwide resources are often allocated inefficiently. However, the attention is focused on financial survival and keeping health and safety as a priority. Moreover, the evolving nature of the COVID-19 provides additional aspects related to the urban areas' demographic and environmental characteristics, including population density, social gathering habits, seasonal variation, and economic factors to identify the risk of the virus spread (Sohrabi et al., 2020). The multi-criteria methods were reported useful to consider multiple key aspects for the virus risk assessment (De Almeida et al., 2017). Various methods such as optimization techniques, calibration methods, and AI neural networks can be formulated as multi-criteria approaches to assess different scenarios to find effective mitigation solutions (Sangiorgio & Parisi, 2020). However, limited studies were found on effective decision support tools, specifically considering environmental perspectives. Sangiorgio and Parisi (2020) integrated optimization and artificial neural network to predict the risk and optimize the strategies to restart or limit the local activities during lockdown under socio-economic conditions. It is expected that the decision-making system helps establish the policy and find equitable and optimize the solution, which would help eradicate the disease and act as control and adaptation stage during the pandemic. For example, the development of decision methods within the OH framework can provide technical support to the decision-makers by predicting the new infection cases under various scenarios, seasonal variations, and control policies. It should reflect the heterogeneous risks of different locations and interpret the disproportionate impact of the COVID-19 on public, animal, and ecosystem health.
4. Discussion
SARS-CoV-2 is transmitted by aerosol droplets and fomite surfaces in different environmental compartments. Different environmental factors can affect the viability of SARS-CoV-2 and its distribution to the multimedia environment. Temperature is one of the most significant environmental factors related to seasonal variations in various regions. A few studies discussed spatial variation of distinct locations such as China, USA, UK, Brazil, Spain, and India, along with optimal temperature range, humidity, and wind speed (Qian Wang et al., 2021). However, most of the reported studies covered the first and second COVID-19 wave, and datasets did not capture the full seasonal cycle for different continents, including northern and southern hemisphere regions (Ali & Islam, 2020; Sharif et al., 2021). The non-climatic factors such as lockdown duration and population density for the same time span should be considered. For instance, the influence of seasonal variation and population density on the COVID-19 cases in the USA shows that increased temperature cannot be considered a substitute for mitigation policies, and winter and lower temperature may increase the transmission intensity in the absence of mobility restriction policy measurements (Smith et al., 2021). In addition to the airborne transmission, the evidence of the existence of SARS-CoV-2 in untreated wastewater and river water has been found with potential transmission source of infected person stool and disposal of sewage into surface water, respectively. Continuous wastewater monitoring helps in indicating and tracking COVID-19 cases. However, there is seldom any study on the survival and persistency of SARS-CoV-2 in wastewater and water environment is available in the public domain (Tran et al., 2021).
In addition to the environmental factors, long-term transmission monitoring, inventory analysis of existing resources, trade, and economy of the region should be considered (Ali and Islam, 2020). An in-depth analysis of system uncertainty and randomness in the data should be considered, particularly for the heterogeneous environment, while calculating the reproduction number of viruses (Sharif et al., 2021). Future studies may combine multidisciplinary models (e.g., process models with data-driven techniques and real-time surveillance) to overcome this issue (Dangermond et al., 2020). For instance, GIS-based tools could also be used for real-time data sharing to support critical decision-making.
5. Conclusion
COVID-19 is a deadly pandemic globally, which is currently in its fourth wave and possibly to enter the fifth wave because of a new variant. The assembling of data from various regions shows that COVID-19 peak outbreak is observed in winter in most significant hot spots (New York, Madrid, Lombardy, and London), having an ambient temperature less than 15°C and 11−22 (km/h) range of wind speed. In contrast, severity in COVID-19 cases is observed in hot-humid seasons with a mean temperature above 16 °C (e.g., Brazil, Colombia, India, and South Africa). Thus, evidence of environmental sensitivity towards the COVID-19 infection cases in these humid areas is not strong, providing insights into other factors such as population density, mobility pattern, and exposure to air pollutants. Respiratory droplets and fine airborne particles are the dominant transmission routes of virus transport. Also, SARS-CoV-2 can linger longer in the air as the part of respirable size particles (1–4 µm) compared to inhalable size. These particles could deposit anywhere on the fomite surfaces. The investigation of the indoor built environment of hospitals shows that frequent cleaning and ventilation is necessary as SARS-CoV-2 is found even on doorknobs, PPE removal rooms, pharmacy floors, in addition to the patient room. This article examines various opportunities and modelling techniques to estimate the modulation effect of environmental influences on the SARS-CoV-2 spread and transmission into a multimedia environment. The limitations of these techniques indicate a signal to establish an environmental-assisted modelling system based on the OH concept to take precautionary measurements and lessons learned from the earlier COVID-19 waves. In conclusion, the advanced OH-based integrated approach can be used as an effective decision tool for policy and decision-makers, agencies, and the public to combat the highly transmissible virus at various stages and scenarios.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank all the participants in the study.
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases. 2020 doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agany D.D.M., Pietri J.E., Gnimpieba E.Z. Assessment of vector-host-pathogen relationships using data mining and machine learning. Computational and Structural Biotechnology Journal. 2020;18 doi: 10.1016/j.csbj.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M., Sharifi A., Dorosti S., Jafarzadeh Ghoushchi S., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Science of the Total Environment. 2020;729 doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Arabiya-News. (2020). Paris finds ‘minuscule traces’ of coronavirus in its non-potable water. https://english.alarabiya.net/en/coronavirus/2020/04/20/Paris-finds-minuscule-traces-of-coronavirus-in-its-non-potable-water.html.
- Ali N., Islam F. The effects of air pollution on COVID-19 infection and mortality-a review on recent evidence. Frontiers in Public Health. 2020;8 doi: 10.3389/fpubh.2020.580057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L.J.S., Brown V.L., Jonsson C.B., Klein S.L., Laverty S.M., Magwedere K., Owen J.C., van den Driessche P. Mathematical modeling of viral zoonoses in wildlife. Natural Resource Modeling. 2012;25(1):5–51. doi: 10.1111/j.1939-7445.2011.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rousan N., Al-Najjar H. The correlation between the spread of COVID-19 infections and weather variables in 30 Chinese provinces and the impact of Chinese government mitigation plans. European Review for Medical and Pharmacological Sciences. 2020;24(8):4565–4571. doi: 10.26355/eurrev_202004_21042. [DOI] [PubMed] [Google Scholar]
- Ambat A.S., Zubair S.M., Prasad N., Pundir P., Rajwar E., Patil D.S., Mangad P. Nipah virus: A review on epidemiological characteristics and outbreaks to inform public health decision making. Journal of Infection and Public Health. 2019;12(5):634–639. doi: 10.1016/j.jiph.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Andersen L.M., Harden S.R., Sugg M.M., Runkle J.D., Lundquist T.E. Analyzing the spatial determinants of local Covid-19 transmission in the United States. Science of the Total Environment. 2021;754 doi: 10.1016/j.scitotenv.2020.142396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardabili S.F., Mosavi A., Ghamisi P., Ferdinand F., Varkonyi-Koczy A.R., Reuter U., Rabczuk T., Atkinson P.M. COVID-19 outbreak prediction with machine learning. Algorithms. 2020;13(10) doi: 10.3390/a13100249. [DOI] [Google Scholar]
- Ashinyo M.E., Dubik S.D., Duti V., Amegah K.E., Ashinyo A., Larsen-Reindorf R., Kaba Akoriyea S., Kuma-Aboagye P. Healthcare workers exposure risk assessment: A survey among frontline workers in designated COVID-19 treatment centers in Ghana. Journal of Primary Care and Community Health. 2020;11 doi: 10.1177/2150132720969483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., Ma B., Bilal, Komal B., Bashir M., Tan D., Bashir M. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Science of the Total Environment. 2020;728 doi: 10.1016/j.scitotenv.2020.138835. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N.U., Bassey D.E., Palanisami T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee, S. (2020). Statistical investigation of relationship between spread of coronavirus disease (COVID-19) and environmental factors based on study of four mostly afected places of China and fve mostly afected places of Italy.
- Bhattacharyya S., Dey K., Paul A.R., Biswas R. A novel CFD analysis to minimize the spread of COVID-19 virus in hospital isolation room. Chaos, Solitons and Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biktasheva I.V. Role of a habitat's air humidity in Covid-19 mortality. Science of the Total Environment. 2020;736 doi: 10.1016/j.scitotenv.2020.138763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos M.N.K. Descriptive review of geographic mapping of severe acute respiratory syndrome (SARS) on the internet. International Journal of Health Geographics. 2004;3 doi: 10.1186/1476-072X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchy P., Gutiérrez R.A. Contaminated soil and transmission of influenza virus (H5N1) Emerging Infectious Diseases. 2012;18(9):1530–1532. doi: 10.3201/eid1809.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari Q., Jameel Y. Will coronavirus pandemic diminish by summer? SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3556998. [DOI] [Google Scholar]
- Burki T.K. Omicron variant and booster COVID-19 vaccines. The Lancet. Respiratory Medicine. 2022;10(2):e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Car Z., Baressi Šegota S., Anđelić N., Lorencin I., Mrzljak V. Modeling the spread of COVID-19 infection using a multilayer perceptron. Computational and Mathematical Methods in Medicine. 2020;2020 doi: 10.1155/2020/5714714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S.C., & Di Napoli, R. (2020). Features, evaluation and treatment coronavirus (COVID-19). In StatPearls. http://www.ncbi.nlm.nih.gov/pubmed/32150360. [PubMed]
- CDC (Centers for Disease Control and Prevention). (2020). One health. https://www.cdc.gov/onehealth/index.html.
- CDC . National Center for Immunization and Respiratory Diseases; 2021. About variants of the virus that causes COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html [Google Scholar]
- Chan, J.F.W., Zhang, A.J., Yuan, S., Poon, V.K.M., Chan, C.C.S., Lee, A.C.Y., Chan, W.M., Fan, Z., Tsoi, H.W., Wen, L., Liang, R., Cao, J., Chen, Y., Tang, K., Luo, C., Cai, J.P., Kok, K.H., Chu, H., Chan, K.H., … Yuen, K.Y. (2020). Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clinical infectious diseases : an official publication of the infectious diseases society of America. 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed]
- Chang, H.J., Chuang, J.-H., Chern, T.C., Stein, M., Coker, R., Wang, D.-W., & Hsu, T. (2014). A comparison between a deterministic, compartmental model and an individual based-stochastic model for simulating the transmission dynamics of pandemic influenza. 586–594. 10.5220/0005040905860594.
- Chang S., Pierson E., Koh P.W., Gerardin J., Redbird B., Grusky D., Leskovec J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2020 doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Bajwa S., Dwivedi D., Kanji R., Ahammed M., Shaw R. COVID-19 Risk Assessment Tool: Dual application of risk communication and risk governance. Progress in Disaster Science. 2020;7 doi: 10.1016/j.pdisas.2020.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi, A. (2020). Top 10 popular smartphone apps to track Covid-19. Geo Spatial World. https://www.geospatialworld.net/blogs/popular-apps-COVID-19/.
- Chen P.S., Tsai F.T., Lin C.K., Yang C.Y., Chan C.C., Young C.Y., Lee C.H. Ambient influenza and avian influenza virus during dust storm days and background days. Environmental Health Perspectives. 2010;118(9):1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.L., Zhang Q., Lu Y., Guo Z.M., Zhang X., Zhang W.J., Guo C., Liao C.H., Li Q.L., Han X.H., Lu J.H. Distribution of the COVID-19 epidemic and correlation with population emigration from Wuhan, China. Chinese Medical Journal. 2020;133(9):1044–1050. doi: 10.1097/CM9.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M.…Moses D. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications. 2020;11(1) doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions – Authors’ reply. The Lancet Microbe. 2020;1(4) doi: 10.1016/s2666-5247(20)30095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.A., Ozgen C., Strobl E. Air pollution exposure and COVID-19 in dutch municipalities. Environmental and Resource Economics. 2020;76(4):581–610. doi: 10.1007/s10640-020-00491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, K.K., Tay, D.J.W., Tan, K.S, Ong, S.W.X., Son, T.T., Koh, M.H., Chin, Y.Q., Nasir, H., Mak, T.M., Chu, J.J.H., Milton, D.K., Chow, V.T.K., Tambyah, P.A., Chen, M., & Wai, T.K. (2021). Viral load of SARS-CoV-2 in respiratory aerosols emitted by COVID-19 patients while breathing, talking, and singing. MedRxiv, 2021.07.15.21260561. https://www.medrxiv.org/content/10.1101/2021.07.15.21260561v2%0Ahttps://www.medrxiv.org/content/10.1101/2021.07.15.21260561v2.abstract. [DOI] [PMC free article] [PubMed]
- Cook T.M., El-Boghdadly K. COVID-19 risk tools should incorporate assessment of working environment risk and its mitigation. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coro G. A global-scale ecological niche model to predict SARS-CoV-2 coronavirus infection rate. Ecological Modelling. 2020;431 doi: 10.1016/j.ecolmodel.2020.109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damialis A., Gilles S., Sofiev M., Sofieva V., Kolek F., Bayr D., Plaza M.P., Leier-Wirtz V., Kaschuba S., Ziska L.H., Bielory L., Makra L., del Mar Trigo M., Traidl-Hoffmann C. Proceedings of the National Academy of Sciences of the United States of America. Vol. 118. 2021. Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangermond, J., De Vito, C., & Pesaresi, C. (2020). Using GIS in the Time of the COVID-19 Crisis, casting a glance at the future. A joint discussion. Copyright© Nuova Cultura Italian Association of Geography Teachers J-READING JOURNAL OF RESEARCH AND DIDACTICS IN GEOGRAPHY, 1(December 2019), 195–205. https://penn-chime.phl.io/.
- Dbouk T., Drikakis D. Weather impact on airborne coronavirus survival. Physics of Fluids. 2020;32(9) doi: 10.1063/5.0024272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida A.T., Alencar M.H., Garcez T.V., Ferreira R.J.P. A systematic literature review of multicriteria and multi-objective models applied in risk management. IMA Journal of Management Mathematics. 2017;28(2) doi: 10.1093/imaman/dpw021. [DOI] [Google Scholar]
- Dennehy, K. (2020). New study examines whether COVID-19 virus has entered rivers and streams. https://environment.yale.edu/news/article/has-the-coronavirus-entered-rivers-and-streams.
- Desai, P.S., Sawant, N., & Keene, A. (2020). On COVID-19-safety ranking of seats in intercontinental commercial aircrafts: A preliminary multiphysics computational perspective. In medRxiv. 10.1101/2020.08.17.20176909. [DOI] [PMC free article] [PubMed]
- Dietz L., Horve P.F., Coil D.A., Fretz M., Eisen J.A., Van Den Wymelenberg K. 2019 Novel coronavirus (COVID-19) pandemic: Built environment considerations to reduce transmission. MSystems. 2020;5(2) doi: 10.1128/msystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont-Leblond N., Veillette M., Mubareka S., Yip L., Longtin Y., Jouvet P., Paquet Bolduc B., Godbout S., Kobinger G., McGeer A., Mikszewski A., Duchaine C. Low incidence of airborne SARS-CoV-2 in acute care hospital rooms with optimized ventilation. Emerging Microbes & Infections. 2020;9(1):2597–2605. doi: 10.1080/22221751.2020.1850184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker S., Hornick T., Szczepankiewicz G., Maier M., Bastl M., Bumberger J., Treudler R., Liebert U.G., Simon J.C. No SARS-CoV-2 detected in air samples (pollen and particulate matter) in Leipzig during the first spread. Science of the Total Environment. 2021;755 doi: 10.1016/j.scitotenv.2020.142881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eker S. Validity and usefulness of COVID-19 models. Humanities and Social Sciences Communications. 2020;7(1) doi: 10.1057/s41599-020-00553-4. [DOI] [Google Scholar]
- Escobar L.E. Ecological niche modeling: An introduction for veterinarians and epidemiologists. Frontiers in Veterinary Science. 2020;7 doi: 10.3389/fvets.2020.519059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami H., Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10(1) doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B.R., Leighton F.A. A history of one health. OIE Revue Scientifique et Technique. 2014;33(2):413–420. doi: 10.20506/rst.33.2.2298. [DOI] [PubMed] [Google Scholar]
- Farag E., Nour M., Islam M.M., Mustafa A., Khalid M., Sikkema R.S., Alhajri F., Bu-Sayaa A., Haroun M., Van Kerkhove M.D., Elkholy A., Malik S.M.R., Reusken C., Koopmans M., AlHajri M.M. Qatar experience on one health approach for middle-east respiratory syndrome coronavirus, 2012–2017: A viewpoint. One Health. 2019;7 doi: 10.1016/j.onehlt.2019.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerging Infectious Diseases. 2020;26(9) doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiasca F., Minelli M., Maio D., Minelli M., Vergallo I., Necozione S., Mattei A. Associations between covid-19 incidence rates and the exposure to pm2.5 and no2: A nationwide observational study in Italy. International Journal of Environmental Research and Public Health. 2020;17(24):1–10. doi: 10.3390/ijerph17249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D., Reilly, A., Eng Zheng, A.K., Cook, A.R., & Anderson, D.E. (2020). Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. In bioRxiv. 10.1101/2020.08.17.255166.
- Giacobbo A., Rodrigues M.A.S., Zoppas Ferreira J., Bernardes A.M., de Pinho M.N. A critical review on SARS-CoV-2 infectivity in water and wastewater. What do we know? Science of the Total Environment. 2021;774 doi: 10.1016/j.scitotenv.2021.145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E.P.J. The evolution of one health: A decade of progress and challenges for the future. Veterinary Record. 2014;174(4):85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- Gilles S., Blume C., Wimmer M., Damialis A., Meulenbroek L., Gökkaya M., Bergougnan C., Eisenbart S., Sundell N., Lindh M., Andersson L.M., Dahl Å., Chaker A., Kolek F., Wagner S., Neumann A.U., Akdis C.A., Garssen J., Westin J.…Traidl-Hoffmann C. Pollen exposure weakens innate defense against respiratory viruses. Allergy: European Journal of Allergy and Clinical Immunology. 2020;75(3):576–587. doi: 10.1111/all.14047. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: Implications in low sanitation countries. Science of the Total Environment. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R.B, Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Liu K., Cao C., Liu B., Zhang K., Gao Y.W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infectious Diseases. 2020;26(7) doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. Effect of ambient temperature on COVID-19 infection rate. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3558470. [DOI] [Google Scholar]
- Han J., Zhang X., He S., Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environmental Chemistry Letters. 2020 doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Yang L., Jia K., Li J., Feng S., Chen W., Zhao W., Pereira P. Spatial distribution characteristics of the COVID-19 pandemic in Beijing and its relationship with environmental factors. Science of the Total Environment. 2021;761 doi: 10.1016/j.scitotenv.2020.144257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmooshi N.N., Shirbandi K., Rahim F. Environmental concern regarding the effect of humidity and temperature on 2019-nCoV survival: Fact or fiction. Environmental Science and Pollution Research. 2020;27(29):36027–36036. doi: 10.1007/s11356-020-09733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthmap. (2021). Healthmap-COVID-19. https://www.healthmap.org/covid-19/.
- Henriques, A., Rognlien, M.K., Devine, J., Azzopardi, G., Mounet, N., Elson, P.J., Andreini, M., & Tarocco, N. (2021). Modelling airborne transmission of SARS-CoV-2: Risk assessment for enclosed spaces. 10.17181/CERN.1GDQ.5Y75. [DOI] [PMC free article] [PubMed]
- Hinchliffe S. More than one world, more than one health: Re-configuring interspecies health. Social Science and Medicine. 2015;129:28–35. doi: 10.1016/j.socscimed.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Hodges K., Jackson J. Pandemics and the global environment. Science Advances. 2020;6(28) doi: 10.1126/sciadv.abd1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes L. Can you get COVID-19 in the bathroom? Chemical & Engineering News. 2020:21–23. doi: 10.47287/cen-09838-feature2. [DOI] [Google Scholar]
- Huang Z., Huang J., Gu Q., Du P., Liang H., Dong Q. Optimal temperature zone for the dispersal of COVID-19. Science of the Total Environment. 2020;736 doi: 10.1016/j.scitotenv.2020.139487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Espinosa S., Dias de Freitas E., Ropkins K., Dominici F., Rehbein A. Negative-Binomial and quasi-poisson regressions between COVID-19, mobility and environment in São Paulo, Brazil. Environmental Research. 2022;204 doi: 10.1016/j.envres.2021.112369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPAC. (2020). Infection prevention and contol Canda: SARS-CoV (Severe Acute Respiratory Syndrome). https://ipac-canada.org/sars.php.
- Isaia G., Diémoz H., Maluta F., Fountoulakis I., Ceccon D., di Sarra A., Facta S., Fedele F., Lorenzetto G., Siani A.M., Isaia G. Does solar ultraviolet radiation play a role in COVID-19 infection and deaths? An environmental ecological study in Italy. Science of the Total Environment. 2021;757 doi: 10.1016/j.scitotenv.2020.143757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. (2021). The Effect of Weather Pattern on the Second Wave of Coronavirus: A cross study between cold and tropical climates of France, Italy, Colombia, and Brazil. MedRxiv, 2021.12.28.21268496. 10.1101/2021.12.28.21268496.
- Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environmental Research. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender differences in patients with COVID-19: Focus on severity and mortality. Frontiers in Public Health. 2020;8 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüni P., Rothenbühler M., Bobos P., Thorpe K.E., Da Costa B.R., Fisman D.N., Slutsky A.S., Gesink D. Impact of climate and public health interventions on the COVID-19 pandemic: A prospective cohort study. CMAJ. 2020;192(21):E566–E573. doi: 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel Boulos M.N., Geraghty E.M. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: How 21st century GIS technologies are supporting the global fight against outbr. International Journal of Health Geographics. 2020;19(1) doi: 10.1186/s12942-020-00202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Jia Z.J., Nakayama N., Asakawa S. Ecology of viruses in soils: Past, present and future perspectives. Soil Science and Plant Nutrition. 2008;54(1):1–32. doi: 10.1111/j.1747-0765.2007.00197.x. [DOI] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493) doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Science of the Total Environment. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanese, N. (2020). New Coronavirus may spread as an airborne, aerosol like SARS. The Live Science, 2.
- Lee B.U. Minimum sizes of respiratory particles carrying SARS-CoV-2 and the possibility of aerosol generation. International Journal of Environmental Research and Public Health. 2020;17(19):1–8. doi: 10.3390/ijerph17196960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Ye F., Liu X., Huang Z., Ling S., Jiang Z., Cheng J., Huang X., Wu Q., Wu S., Xie Y., Xiao C., Ye D., Yang Z., Li Y., Leung N.H.L., Cowling B.J., He J., Wong S.S., Zanin M. SARS-CoV-2 environmental contamination associated with persistently infected COVID-19 patients. Influenza and Other Respiratory Viruses. 2020;14(6) doi: 10.1111/irv.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. The Link between Humidity and COVID-19 Caused Death. Journal of Biosciences and Medicines. 2020;08(06):50–55. doi: 10.4236/jbm.2020.86005. [DOI] [Google Scholar]
- Li M., Yang Y., Lu Y., Zhang D., Liu Y., Cui X., Yang L., Liu R., Liu J., Li G., Qu J. Natural host–environmental media–human: A new potential pathway of COVID-19 outbreak. Engineering. 2020 doi: 10.1016/j.eng.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.K., Kweon O.J., Kim H.R., Kim T.H., Lee M.K. The impact of environmental variables on the spread of COVID-19 in the Republic of Korea. Scientific Reports. 2021;11(1) doi: 10.1038/s41598-021-85493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Liu Z., Guan C.H. The impacts of the built environment on the incidence rate of COVID-19: A case study of King County, Washington. Sustainable Cities and Society. 2021;74 doi: 10.1016/j.scs.2021.103144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhou J., Yao J., Zhang X., Li L., Xu X., He X., Wang B., Fu S., Niu T., Yan J., Shi Y., Ren X., Niu J., Zhu W., Li S., Luo B., Zhang K. Impact of meteorological factors on the COVID-19 transmission: A multi-city study in China. Science of the Total Environment. 2020;726 doi: 10.1016/j.scitotenv.2020.138513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. fai. [DOI] [PubMed] [Google Scholar]
- Lowen A.C., Steel J. Roles of Humidity and Temperature in Shaping Influenza Seasonality. Journal of Virology. 2014;88(14):7692–7695. doi: 10.1128/jvi.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui R.N., Wong S.H., Sánchez-Luna S.A., Pellino G., Bollipo S., Wong M.Y., Chiu P.W.Y., Sung J.J.Y. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. Journal of Gastroenterology and Hepatology (Australia) 2020;35(5):749–759. doi: 10.1111/jgh.15053. [DOI] [PubMed] [Google Scholar]
- Mahrouf M., Boukhouima A., Zine H., Lotfi E.M., Torres D.F.M., Yousfi N. Modeling and Forecasting of COVID-19 Spreading by Delayed Stochastic Differential Equations. Axioms. 2021;10(1) doi: 10.3390/axioms10010018. [DOI] [Google Scholar]
- Mallapaty S. Coronavirus can infect cats-dogs, not so much. Nature. 2020 doi: 10.1038/d41586-020-00984-8. [DOI] [PubMed] [Google Scholar]
- Matthew O.J., Eludoyin A.O., Oluwadiya K.S. Spatio-temporal variations in COVID-19 in relation to the global climate distribution and fluctuations. Spatial and Spatio-Temporal Epidemiology. 2021;37 doi: 10.1016/j.sste.2021.100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environmental Science and Technology Letters. 2020;7(7) doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mele M., Magazzino C. Pollution, economic growth, and COVID-19 deaths in India: A machine learning evidence. Environmental Science and Pollution Research. 2021;28(3):2669–2677. doi: 10.1007/s11356-020-10689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menebo M.M. Temperature and precipitation associate with Covid-19 new daily cases: A correlation study between weather and Covid-19 pandemic in Oslo, Norway. Science of the Total Environment. 2020;737 doi: 10.1016/j.scitotenv.2020.139659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merow C., Urban M.C. Seasonality and uncertainty in global COVID-19 growth rates. Proceedings of the National Academy of Sciences. 2020;117(44) doi: 10.1073/pnas.2008590117. 27456 LP –27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir K., Ashraf S., Munir I., Khalid H., Muneer M.A., Mukhtar N., Amin S., Ashraf S., Imran M.A., Chaudhry U., Zaheer M.U., Arshad M., Munir R., Ahmad A., Zhao X. Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health. Emerging Microbes and Infections. 2020;9(1) doi: 10.1080/22221751.2020.1827984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P.A., Zu J., Ghori M.B., Naik M. Modeling the effects of the contaminated environments on COVID-19 transmission in India. Results in Physics. 2021;29 doi: 10.1016/j.rinp.2021.104774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasajpour M., Pouriyeh S., Parizi R.M., Dorodchi M., Valero M., Arabnia H.R. Internet of things for current COVID-19 and future pandemics: An exploratory study. Journal of Healthcare Informatics Research. 2020;4(4):325–364. doi: 10.1007/s41666-020-00080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. SARS-CoV-2 in soils. Environmental Research. 2020;190 doi: 10.1016/j.envres.2020.110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd K., Nair K.M., Forouzandeh P., Mathew S., Grant J., Moran R., Bartlett J., Bird J., Pillai S.C. Face masks and respirators in the fight against the COVID-19 pandemic: A review of current materials, advances and future perspectives. Materials. 2020;13(15) doi: 10.3390/ma13153363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Science of the Total Environment. 2020;726 doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohio Department of Health. (2021). Ohio coronavirus wastewater monitoring network. https://coronavirus.ohio.gov/wps/portal/gov/covid-19/dashboards/other-resources/wastewater.
- Oliveira, A., Duarte, H.O., Siqueira, P.G., & Moura, M., & das, C. (2020). Probabilistic model for quantitative risk assessment of COVID-19 : the case of a patchy environment with potential for migration between continents. 10.17632/3gyxtcbnj7.1.
- Oreshkova N., Molenaar R.J., Vreman S., Harders F., Oude Munnink B.B., Van Der Honing R.W.H., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S., Tacken M.G.J., De Rooij M.M.T., Weesendorp E., Engelsma M.Y., Bruschke C.J.M., Smit L.A.M., Koopmans M., Van Der Poel W.H.M., Stegeman A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020;25(23) doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottawa COVID-19 . 2021. Ottawa covid-19 wastewater surveillance.https://613covid.ca/wastewater/ [Google Scholar]
- Pan Y., Zhang L., Yan Z., Lwin M.O., Skibniewski M.J. Discovering optimal strategies for mitigating COVID-19 spread using machine learning: Experience from Asia. Sustainable Cities and Society. 2021;75 doi: 10.1016/j.scs.2021.103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli R.R., Mishra B., Banstola A., Ghimire B.R., Poudel S., Sharma K., Dixit S.M., Sah S.K., Simkhada P., van Teijlingen E. Multidisciplinary approach to COVID-19 risk communication: A framework and tool for individual and regional risk assessment. Scientific Reports. 2020;10(1):21650. doi: 10.1038/s41598-020-78779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Kim Y.M., Yi S., Lee S., Na B.J., Kim C.B., Kim J.Il, Kim H.S., Kim Y.B., Park Y., Huh I.S., Kim H.K., Yoon H.J., Jang H., Kim K., Chang Y., Kim I., Lee H., Gwack J.…Jeong E.K. Coronavirus disease outbreak in call center, South Korea. Emerging Infectious Diseases. 2020;26(8):1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz S. Climate change impacts on West Nile virus transmission in a global context. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1665):1–11. doi: 10.1098/rstb.2013.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Chen Q., Liu E. The role of computational fluid dynamics tools on investigation of pathogen transmission: Prevention and control. Science of the Total Environment. 2020;746 doi: 10.1016/j.scitotenv.2020.142090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirouz B., Haghshenas S.S., Pirouz B., Haghshenas S.S., Piro P. Development of an assessment method for investigating the impact of climate and urban parameters in confirmed cases of COVID-19: A new challenge in sustainable development. International Journal of Environmental Research and Public Health. 2020;17(8) doi: 10.3390/ijerph17082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W., Su Q., Wang W., Wang X., Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Science of the Total Environment. 2020;728 doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, H., Miao, T., Liu, L., Zheng, X., Luo, D., & Li, Y. (2020). Indoor transmission of SARS-CoV-2. Indoor Air. 10.1111/ina.12766. [DOI] [PubMed]
- Rezaali M., Fouladi-Fard R. Aerosolized SARS-CoV-2 exposure assessment: Dispersion modeling with AERMOD. Journal of Environmental Health Science and Engineering. 2021 doi: 10.1007/s40201-020-00602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. COVID-19 vaccines vs variants - determining how much immunity is enough. JAMA - Journal of the American Medical Association. 2021;325(13):1241–1243. doi: 10.1001/jama.2021.3370. [DOI] [PubMed] [Google Scholar]
- Rume T., Islam S.M.D.U. Environmental effects of COVID-19 pandemic and potential strategies of sustainability. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e04965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustam F., Reshi A.A., Mehmood A., Ullah S., On B.W., Aslam W., Choi G.S. COVID-19 future forecasting using supervised machine learning models. IEEE Access. 2020;8 doi: 10.1109/ACCESS.2020.2997311. [DOI] [Google Scholar]
- Saey, T.H. (2021). New studies hint that the coronavirus may be evolving to become more airborne. Science News. https://www.sciencenews.org/article/covid-coronavirus-aerosol-droplets-airborne-evolution.
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19) JAMA Network Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgio V., Parisi F. A multicriteria approach for risk assessment of Covid-19 in urban district lockdown. Safety Science. 2020;130 doi: 10.1016/j.ssci.2020.104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia, J.L., Rivera, D.N., Herrera, V., Morwitzer, M.J., Creager, H., Santarpia, G.W., Crown, K.K., Brett-Major, D.M., Schnaubelt, E., Broadhurst, M.J., Lawler, J.V., Reid, S.P., & Lowe, J.J. (2020). Transmission Potential of SARS-CoV-2 in viral shedding observed at the university of Nebraska medical center. MedRxiv, 2020.03.23.20039446. 10.1101/2020.03.23.20039446.
- Sartorius B., Lawson A.B., Pullan R.L. Modelling and predicting the spatio-temporal spread of COVID-19, associated deaths and impact of key risk factors in England. Scientific Reports. 2021;11(1):5378. doi: 10.1038/s41598-021-83780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano M., McKee M., Ricciardi W., Boccia S. Transmission of SARS-CoV-2 and other infections at large sports gatherings: A surprising gap in our knowledge. Frontiers in Medicine. 2020;7 doi: 10.3389/fmed.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiegershausen, E. (2020). The coronavirus was found on Frozen Chicken Wings. New York Magazine: Intelligencer. https://nymag.com/intelligencer/2020/08/covid-19-frozen-chicken-wings.html.
- Scoones I., Jones K., Lo Iacono G., Redding D.W., Wilkinson A., Wood J.L.N. Integrative modelling for one health: Pattern, process and participation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1725) doi: 10.1098/rstb.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha H., Zhang X., Qi D. Optimal control of high-rise building mechanical ventilation system for achieving low risk of COVID-19 transmission and ventilative cooling. Sustainable Cities and Society. 2021;74 doi: 10.1016/j.scs.2021.103256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakil M.H., Munim Z.H., Tasnia M., Sarowar S. COVID-19 and the environment: A critical review and research agenda. Science of the Total Environment. 2020;745 doi: 10.1016/j.scitotenv.2020.141022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N., Sarkar M.K., Ahmed S.N., Ferdous R.N., Nobel N.U., Parvez A.K., Talukder A.A., Dey S.K. Environmental correlation and epidemiologic analysis of COVID-19 pandemic in ten regions in five continents. Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Li C., Dong H., Wang Z., Martinez L., Sun Z., Handel A., Chen Z., Chen E., Ebell M., Wang F., Yi B., Wang H., Wang X., Wang A., Chen B., Qi Y., Liang L., Li Y.…Xu G. Airborne Transmission of COVID-19: Epidemiologic Evidence from Two Outbreak Investigations. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3567505. [DOI] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Science of the Total Environment. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav L.K., Jha S.K. A gradient boosting machine learning approach in modeling the impact of temperature and humidity on the transmission rate of COVID-19 in India. Applied Intelligence. 2020 doi: 10.1007/s10489-020-01997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil A., Kumar V.N. Does weather affect the growth rate of COVID-19, a study to comprehend transmission dynamics on human health. Journal of Safety Science and Resilience. 2020;1(1):3–11. doi: 10.1016/j.jnlssr.2020.06.004. [DOI] [Google Scholar]
- Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R.A.P.M., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586(7831) doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.P., Flaxman S., Gallinat A.S., Kinosian S.P., Stemkovski M., Juliette H., Watson O.J., Whittaker C., Cattarino L., Dorigatti I., Tristem M., Pearse W.D. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(25) doi: 10.1073/pnas.2019284118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither S.J., Eastaugh L.S., Findlay J.S., Lever M.S. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerging Microbes and Infections. 2020;9(1):1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral M.F.F., Duarte G.B., da Penha Sobral A.I.G., Marinho M.L.M., de Souza Melo A. Association between climate variables and global transmission oF SARS-CoV-2. Science of the Total Environment. 2020;729 doi: 10.1016/j.scitotenv.2020.138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020;76 doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. COVID-19 and air pollution and meteorology-an intricate relationship: A review. Journal of Microbiology and Immunology. 2020 doi: 10.1016/j.chemosphere.2020.128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh L., Karimi A., Heitzinger C. Uncertainty quantification in epidemiological models for the COVID-19 pandemic. Computers in Biology and Medicine. 2020;125 doi: 10.1016/j.compbiomed.2020.104011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Zhou Y., Wang L., Purkayastha S., Zhang L., He J., Wang F., Song P.X.K. A review of multi-compartment infectious disease models. International Statistical Review. 2020;88(2):462–513. doi: 10.1111/insr.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., Shen J., Lv Y., Pan L., Ding P., Wang X., Wang Y., MacIntyre C.R., Shi X. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environmental International. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.N. Epidemiological models are important tools for guiding COVID-19 interventions. BMC Medicine. 2020;18(1) doi: 10.1186/s12916-020-01628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T., Zhang K., Maguire B., Terebessy E., Fong I., Parikh S., Zhu J. Correlation of ambient temperature and COVID-19 incidence in Canada. Science of the Total Environment. 2021;750 doi: 10.1016/j.scitotenv.2020.141484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolles J., Luong T. Modeling Epidemics with compartmental models. JAMA - Journal of the American Medical Association. 2020;323(24):2515–2516. doi: 10.1001/jama.2020.8420. [DOI] [PubMed] [Google Scholar]
- Tosepu R., Gunawan J., Effendy D.S., Ahmad L.O.A.I., Lestari H., Bahar H., Asfian P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Science of the Total Environment. 2020;725 doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H.N., Le G.T., Nguyen D.T., Juang R.S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.P. SARS-CoV-2 coronavirus in water and wastewater: A critical review about presence and concern. Environmental Research. 2021;193 doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite A.R., Fisman D.N., Greer A.L. Mathematical modeling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. Canadian Medical Association Journal. 2020 doi: 10.1503/cmaj.200476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twohy C.H., Coakley J.A., Tahnk W.R. Effect of changes in relative humidity on aerosol scattering near clouds. Journal of Geophysical Research Atmospheres. 2009;114(5) doi: 10.1029/2008JD010991. [DOI] [Google Scholar]
- USDA. (2020). Cases of SARS-CoV-2 in Animals in the United States. https://www.aphis.usda.gov/animal_health/one_health/downloads/sars-cov2-in-animals.pdf.
- Utah department of environmental quality. (2021). SARS-CoV-2 Sewage Monitoring. https://deq.utah.gov/water-quality/sars-cov-2-sewage-monitoring.
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuken J.P.G., Swart A.N., Havelaar A.H., Van Pul A., Van der Hoek W., Heederik D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock - a review to inform risk assessment studies. Microbial Risk Analysis. 2016;1 doi: 10.1016/j.mran.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Wu H., Wan X.F. Transport and fate of human expiratory droplets - a modeling approach. Physics of Fluids. 2020;32(8) doi: 10.1063/5.0021280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: Big data analytics, new technology, and proactive testing. JAMA - Journal of the American Medical Association. 2020;323(14):1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. High Temperature and High Humidity Reduce the Transmission of COVID-19. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3551767. [DOI] [Google Scholar]
- Wang, M., Jiang, A., Gong, L., Lu, L., Guo, W., Li, C., Zheng, J., Li, C., Yang, B., Zeng, J., Chen, Y., Zheng, K., & Li, H. (2020). Temperature significantly change COVID-19 transmission in 429 cities. 10.1101/2020.02.22.20025791.
- Wang Q., Dong W., Yang K., Ren Z., Huang D., Zhang P., Wang J. Temporal and spatial analysis of COVID-19 transmission in China and its influencing factors. International Journal of Infectious Diseases. 2021;105:675–685. doi: 10.1016/j.ijid.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Liu L. On the critical role of human feces and public toilets in the transmission of COVID-19: Evidence from China. Sustainable Cities and Society. 2021;75 doi: 10.1016/j.scs.2021.103350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., Song N., Jin M., Xiao W., Zhu X., Gu C., Yin J., Wei W., Yao W., Liu C., Li J., Ou G., Wang M.…Li J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th Hospital of the Chinese People's liberation army. Water Science and Technology. 2005;52(8):213–221. doi: 10.2166/wst.2005.0266. [DOI] [PubMed] [Google Scholar]
- Wei J.Te, Liu Y.X., Zhu Y.C., Qian J., Ye R.Z., Li C.Y., Ji X.K., Li H.K., Qi C., Wang Y., Yang F., Zhou Y.H., Yan R., Cui X.M., Liu Y.L., Jia N., Li S.X., Li X.J., Xue F.Z.…Cao W.C. Impacts of transportation and meteorological factors on the transmission of COVID-19. International Journal of Hygiene and Environmental Health. 2020;230 doi: 10.1016/j.ijheh.2020.113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K., Zou Z., Zheng Y., Li J., Shen F., Wu C., Wu Y., Hu M., Yao M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Science of the Total Environment. 2016;550:751–759. doi: 10.1016/j.scitotenv.2016.01.137. [DOI] [PubMed] [Google Scholar]
- WHO. (2021). Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- WHO-dashboard. (2021). WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int/.
- Wijaya, K.P., Ganegoda, N., Jayathunga, Y., Götz, T., Bock, W., Schäfer, M., & Heidrich, P. (2020). A COVID-19 epidemic model integrating direct and fomite transmission as well as household structure. In medRxiv. 10.1101/2020.04.25.20079178. [DOI] [PMC free article] [PubMed]
- World Bank . Vol. 2. The World Bank; 2012. pp. 1–65. (People, pathogens and our planet volume 2 - the economics of one health). [Google Scholar]
- Worldpop. (2021). Worldpop COVID-19 research and data. https://www.worldpop.org/covid19.
- Wu, X., Nethery, R., Sabath, B., Braun, D., & Dominici, F. (2020). Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. MedRxiv : The Preprint Server for Health Sciences. 10.1101/2020.04.05.20054502.
- Wu X., Lu Y., Zhou S., Chen L., Xu B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environmental International. 2016;86:14–23. doi: 10.1016/j.envint.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in Faecal samples. The Lancet Gastroenterology and Hepatology. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Rahmandad H., Gupta M., DiGennaro C., Ghaffarzadegan N., Amini H., Jalali M. Weather conditions and COVID-19 transmission: Estimates and projections. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3593879. [DOI] [Google Scholar]
- Yao Y., Pan J., Liu Z., Meng X., Wang W., Kan H., Wang W. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. European Respiratory Journal. 2020;55(5) doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Monis P., Deere D., Ryan U. Wastewater-based epidemiology-surveillance and early detection of waterborne pathogens with a focus on SARS-CoV-2, Cryptosporidium and Giardia. Parasitology Research. 2021 doi: 10.1007/s00436-020-07023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., Yang, Y., Huang, X., Jiang, J., Li, M., Zhang, X., Ling, H., Li, J., Liu, Y., Li, G., Li, W., Yi, C., Zhang, T., Jiang, Y., Xiong, Y., He, Z., Wang, X., Deng, S., Zhao, P., & Qu, J. (2020). SARS-CoV-2 spillover into hospital outdoor environments. MedRxiv, 2020.05.12.20097105. 10.1101/2020.05.12.20097105. [DOI] [PMC free article] [PubMed]
- Zhang X., Ji Z., Yue Y., Liu H., Wang J. Infection risk assessment of COVID-19 through aerosol transmission: A case study of South China Seafood market. Environmental Science and Technology. 2020 doi: 10.1021/acs.est.0c02895. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shen Y. Wastewater irrigation: Past, present, and future. Wiley Interdisciplinary Reviews: Water. 2019;6(3) doi: 10.1002/wat2.1234. [DOI] [Google Scholar]
- Zhang Z., Han T., Yoo K.H., Capecelatro J., Boehman A.L., Maki K. Disease transmission through expiratory aerosols on an urban bus. Physics of Fluids. 2021;33(1) doi: 10.1063/5.0037452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Otter J.A., Price J.R., Cimpeanu C., Garcia D.M., Kinross J., Boshier P.R., Mason S., Bolt F., Holmes A.H., Barclay W.S. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Science of the Total Environment. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]