Take home messages
Bone marrow microenvironment in multiple myeloma provides a growth advantage for the malignant clones.
T cells expressing the inhibitory molecule T cell immunoglobulin and ITIM domain and myeloid-derived suppressor cells facilitate myeloma progression.
In Smoldering myeloma, early Tregs activation and defective antigen presentation are observed in the bone marrow microenvironment.
Introduction
Multiple myeloma (MM) is an incurable plasma cell malignancy with significant interpatient heterogeneity, accounting for 10% of all hematological malignancies.1 Despite the introduction of immunomodulatory drugs and proteasome inhibitors, many patients with high-risk features still have modest progression-free survival rates and poor overall survival.
MM usually progresses from asymptomatic precursor stages, namely monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM).2 The risk of progression from MGUS and SMM to overt myeloma constitutes a continuum, whereby certain patients progress rather quickly, while others never do. Several prognostic models have been developed to capture that variability and identify patients with high-risk MGUS and SMM who are likely to progress rapidly to overt MM. However, the majority of the biomarkers currently in use only measure tumor burden and disease activity or describe cytogenetics, which cannot fully account for the variability observed in risk of progression. Instead, the tumor microenvironment in the bone marrow niche should be interrogated in parallel, to better understand disease progression and resistance, to develop more tailored therapies, and to improve patient outcome.
Current state of the art
Bone marrow microenvironment in MM
Out of the microenvironment's cellular compartment, T cells are perhaps the most well studied in relation to cancer evolution and progression. Myeloma cells can harbor a number of genetic alterations that distinguish them from normal cells at the protein level, thereby inducing tumor-reactive T cell responses. However, those responses are quickly muted by tumor cells, which curtail T cell effector functions via a spectrum of immunosuppressive mechanisms. These mechanisms include the high expression of inhibitory molecules, such as programmed cell death 1 (PD-1), lymphocyte-activation gene 3 (Lag-3), cytotoxic T lymphocyte antigen-4 (CTLA-4), and T cell immunoglobulin and ITIM domain (TIGIT). Previous studies demonstrated that PD-L1 is expressed on MM plasma cells and especially in relapsed disease,3▪ while PD-1 expression was reported to be upregulated in NK and T cells of MM patients,4,5 whose T cells reportedly exhibit a senescent phenotype.6 Nonetheless, clinical results of single-agent PD-1 inhibitor in relapsed myeloma were underwhelming,7 and combination therapy with other antimyeloma drugs has been halted due to serious toxicities in a group of patients.8▪ However, recent studies demonstrated that myeloma burden in Vκ∗MYC mice correlates with the percentage of CD8 + TIGIT+ cells and that TIGIT is the most expressed checkpoint molecule in diseased mice. More importantly though, myeloma grew more slowly in TIGIT-null mice, and tumor burden was reduced with anti-TIGIT antibody treatment, raising the possibility of new immunotherapy options for MM patients.9▪ What is more, regulatory T cells (Tregs), that are normally involved in immune self-tolerance, can be coopted by cancer cells to aid in immune suppression and evasion. Indeed, there has been growing evidence supporting the role of Tregs in MM progression.10▪ Most importantly though, CD38-expressing Tregs were described as suppressive modulators of antitumor immune responses in MM, and CD38 targeting was shown to inhibit immunosuppression in ex vivo cocultures.11 These results suggest a potential for anti-CD38 monoclonal antibody treatment to regulate the immune compartment of the bone marrow niche, as well as the tumor.
Furthermore, in recent years, another microenvironmental cell type, myeloid-derived suppressor cells (MDSCs), has been implicated in cancer-related immunosuppression. In MM, CD14+HLA-DR−/low monocytic MDSCs (M-MDSCs) are significantly increased in patients with newly diagnosed and relapsed disease. A recent study showed that the IL-18 induces MDSC activity, which accelerates MM progression by suppressing T cell response, while IL-18-deficient Vk∗MYC mice were significantly protected from MM progression in a CD8 + T cell-dependent manner.12▪
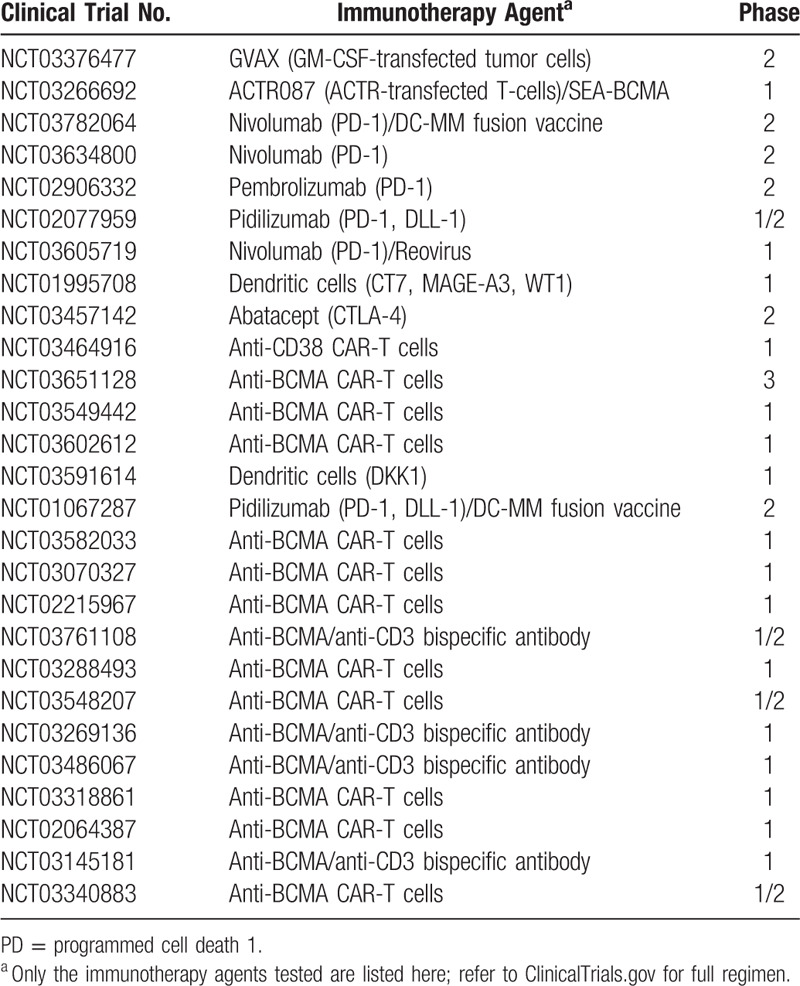
Collectively, these results underline the importance of a permissive bone marrow niche even in the presence of overt disease and suggest that bone marrow niche normalization might be necessary for disease eradication. A list of current clinical trials of immunotherapy in MM can be found in (Table 1).
Table 1.
Current Immunotherapy Trials Targeting the Immune Microenvironment in Multiple Myeloma
Bone marrow niche in MGUS and SMM
Using single-cell RNA sequencing, our group recently showed that the tumor immune microenvironment can already be altered at as early a stage as MGUS.13 Specifically, an enrichment in NK cells and nonclassical monocytes was observed, together with a decrease in plasmacytoid dendritic cells. On top of that the alterations extended beyond the mere composition of the immune bone marrow niche into functional dysregulation. Exemplifying that quality, HLA expression in CD14+ monocytes was found to be upregulated, although the corresponding surface protein levels were shown to be reduced, suggesting an antigen-presentation defect is established early in disease progression. Moreover, coculture experiments showed that MM cells are indeed responsible for this dysregulation, favorably shaping the immune microenvironment at the precursor stage. These results suggest that parallel characterization of the immune microenvironment could improve risk stratification and thus patient management.
In another study by our group, Tregs expansion was observed in bone marrow samples of patients with SMM, as well as Vk∗Myc mice with early-stage disease, suggesting early establishment of tumor-related immunosuppression in MM disease progression. More importantly, though, it was shown that in vivo Treg expansion negatively impacted survival, whereas mice survived longer in the absence of bone marrow Tregs, which designates Treg expansion as a potential therapeutic target for prevention of progression in MM. Indeed, it was shown that Treg expansion is driven by MM cells through secretion of type 1 IFN, which is reversible by anti-IFNAR1 antibodies.
Notwithstanding, in the era of single-cell RNA sequencing and immunotherapy, it is important to note that the tumor microenvironment comprises more than just immune cells. In fact, it consists of multiple other cell types that could be coopted by MM cells to promote progression, as well as the extracellular matrix (ECM), a noncellular component comprising a collection of proteins with an established role in various malignancies.14 In a recent study of the bone marrow ECM by our group, performed on bone marrow samples from patients with MGUS, newly diagnosed and relapsed MM, 2 ECM proteins, ANXA2 and LGALS1, were shown to only appear in the niche at progression to myeloma.15 These results were in concordance with tumor RNA expression data that showed an upregulation of the corresponding transcripts, indicating once again that the tumor itself is actively shaping its microenvironment. Intriguingly, though, we also observed proteins that were only altered at the MGUS stage of disease, suggesting that ECM alterations can take place early in disease progression, just like alterations of the immune microenvironment.
Future perspectives
An important implication of these discoveries on the precursor-stage microenvironment is that MM disease progression cannot be fully understood or predicted outside the context of the bone marrow niche. But more importantly, MM disease progression might be amenable to interception by early therapeutic targeting of bone marrow niche components.
Footnotes
Citation: Sklavenitis-Pistofidis R, Bustoros M, Ghobrial IM. Bone Marrow Niche in Multiple Myeloma and Its Precursor States. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000246.
Funding/support: The authors are supported by National Institutes of Health (NIH) grant R01CA181683, Multiple Myeloma Research Foundation (MMRF), Leukemia and Lymphoma Society (LLS) and Stand Up To Cancer (SU2C).
Disclosure: Mark Bustoros has received Honororia from Takeda and Dava Oncology. Irene Ghobrial has received Honoraria and research funding, served on advisory boards for Takeda, Celgene, Bristol Meyers Squib (BMS), Janssen, Amgen, and Sanofi.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood 2008; 111:2962–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss BM, Abadie J, Verma P, et al. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef S, Marvin J, Steinbach M, et al. Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J 2015; 5:e285.This paper reported that PD-L1 was overexpressed in myeloma patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paiva B, Azpilikueta A, Puig N, et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia 2015; 29:2110–2113. [DOI] [PubMed] [Google Scholar]
- 5.Ray A, Das DS, Song Y, et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015; 29:1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suen H, Brown R, Yang S, et al. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia 2016; 30:1716–1724. [DOI] [PubMed] [Google Scholar]
- 7.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016; 34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley NJ, Pazdur R. Immunotherapy combinations in multiple myeloma—known unknowns. N Engl J Med 2018; 379:1791–1795.This is a perespective on the use of checkpoint inhibitors in multiple myeloma. [DOI] [PubMed] [Google Scholar]
- Guillerey C, Harjunpaa H, Carrie N, et al. TIGIT immune checkpoint blockade restores CD8(+) T-cell immunity against multiple myeloma. Blood 2018; 132:1689–1694.This study demonstrated the overexpression of TGIT on CD8+ cells and its role in MM progression. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Zavidij O, Park J, et al. Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. J Clin Invest 2018; 128:2487–2499.This study reports the presence of Tregs in early stages of MM and the interaction between Tregs and MM cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, Zhang L, Acharya C, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res 2017; 23:4290–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kassem S, Cleynen A, et al. Dysregulated IL-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer cell 2018; 33:634–648.This Paper highlights the role of MDSCs in MM progression and the effect of IL-18 in inducing its activity. [DOI] [PubMed] [Google Scholar]
- 13.Zavidij O, Haradhvala N, Mouhieddine TH, et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Blood 2018; 132:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naba A, Clauser KR, Whittaker CA, et al. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer 2014; 14:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavey SV, Naba A, Manier S, et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia 2017; 31:2426–2434. [DOI] [PubMed] [Google Scholar]


