Abstract
Context
Adrenocortical carcinoma (ACC) is a rare malignancy with high rates of recurrence and poor prognosis. The role of radiotherapy (RT) in localized ACC has been controversial, and RT is not routinely offered.
Objective
To evaluate the benefit of adjuvant RT on outcomes in ACC.
Design
This is a retrospective propensity-matched analysis.
Setting
All patients were seen through the University of Michigan’s Endocrine Oncology program, and all those who underwent RT were treated at the University of Michigan.
Participants
Of 424 patients with ACC, 78 were selected; 39 patients underwent adjuvant radiation.
Intervention
Adjuvant RT to the tumor bed and adjacent lymph nodes.
Main Outcomes Measures
Time to local failure, distant failure, or death.
Results
Median follow-up time was 4.21 years (95% CI, 2.79 to 4.94). The median radiation dose was 55 Gy (range, 45 to 60). The 3-year overall survival estimate for patients improved from 48.6% for patients without RT (95% CI, 29.7 to 65.2) to 77.7% (95% CI, 56.3 to 89.5) with RT, with a hazard ratio (HR) of 3.59 (95% CI, 1.60 to 8.09; P = 0.002). RT improved local recurrence-free survival (RFS) from 34.2% (95% CI, 18.8 to 50.3) to 59.5% (95% CI, 39.0 to 75.0), with an HR of 2.67 (95% CI, 1.38 to 5.19; P = 0.0035). RT improved all RFS from 18.3% (95% CI, 6.7 to 34.3) to 46.7% (95% CI, 26.9 to 64.3), with an HR 2.59 (95% CI, 1.40 to 4.79; P = 0.0024).
Conclusions
In the largest single institution study to date, adjuvant RT after gross resection of ACC improved local RFS, all RFS, and overall survival in this propensity-matched analysis. Adjuvant RT should be considered a part of multidisciplinary management for patients with ACC.
It is unknown if adjuvant radiotherapy in patients with ACC improves outcomes. In this retrospective study, adjuvant radiotherapy improved outcomes for patients with ACC.
The standard of care for localized adrenocortical carcinoma (ACC) is surgical resection (1). Prognoses are poor after gross total resection, and there is a high failure rate with this treatment. Locoregional failures are a common component of this treatment, with estimated rates of local recurrence as high as 65% (1) with associated poor long-term survivorship (2). Distant failures are also quite high, with 2-year stage-by-stage rates of 27%, 46%, and 63% for stage I, II, and III, respectively (3). Therefore, there is a need for adjuvant therapy intensification to improve patient outcomes.
Adjuvant therapy, including mitotane, systemic chemotherapy, and radiotherapy (RT), have been explored, each being used in various settings (4). Due to the rarity of this disease (5), there is a paucity of prospective randomized studies to guide clinical practice in the use of these adjuvant therapies (6). The utility of adjuvant RT after gross resection in ACC is controversial due to a number of underpowered retrospective analyses with conflicting conclusions (7–9), and therefore it is infrequently used as a treatment option in the United States, with only 10% to 14% of patients with ACC receiving radiation (10, 11).
The Endocrine Oncology Program at the University of Michigan is a major tertiary referral center for ACC. We had previously shown that adjuvant RT improved local control in a smaller group of patients (9). In this analysis, we updated our ACC database with patients who received adjuvant radiation with modern radiation techniques compared with those who were not treated adjuvantly with radiation after gross total resection.
Materials and Methods
Patients
Patients who received adjuvant radiation from 2003 to 2017 were retrospectively identified from an internal database within the radiation oncology department at the University of Michigan. We selected patients with stage I to III or oligometastatic stage IV who underwent definitive surgical resection (including oligo-metastectomy for those with stage IV); all patients were staged according to the European Network for the Study of Adrenal Tumors staging system (12) followed by adjuvant RT. A minority of patients presented with local recurrence and underwent resection followed by adjuvant RT; for such patients, treatment characteristics and follow-up were calculated from the time of the second surgery. Patients who have common contraindications for radiation were excluded, including but not limited to pregnancy, p53 mutations, and inflammatory bowel disease. Locoregional recurrence was defined as abdominal recurrence. Tumor grade was based on mitotic counts.
Our institutional practice is to offer adjuvant radiation after gross total resection for adverse risk factors, including high-grade disease, nodal involvement, positive surgical margins, large tumors where pathologic sampling may not be complete, and after laparoscopic resections [which is associated with higher rates of recurrence (13)]. All patients are reviewed in a multidisciplinary Endocrine Oncology Tumor Board, and recommendations are made with input from endocrinology, endocrine surgery, radiation oncology, and medical oncology.
All radiation was delivered at the University of Michigan. Treatment was preceded by CT simulation. Beginning in 2008, four-dimensional motion assessment of the tumor bed (consisting of CT performed throughout the breathing cycle) was used in all patients. Patients with tumor bed motion of >5 to 10 mm were treated with a breath-hold technique if tolerated. The primary clinical target volume was defined as the tumor bed, which was constructed using a combination of presurgical imaging, operative reports, and surgical clips placed at the time of resection. These volumes are reviewed with the surgeon to ensure adequate coverage of the tumor bed. The adjacent para-aortic lymph node basin was contoured as a secondary clinical target volume because up to 62% of patients have positive lymph nodes at time of diagnosis (12). Doses to the primary target ranged from 45 to 60 Gy (median, 55 Gy).
Patients who received adjuvant RT were then matched to control subjects who received surgery with curative intent but without adjuvant RT. Control subjects were selected from an internal database of 424 patients with ACC seen by the Endocrine Oncology Program at the University of Michigan and were selected by propensity matching methods, matching the patient’s sex, age at diagnosis, stage at diagnosis, tumor grade, and surgical margin status. Scores were calculated using logistic regression models to estimate the probability of receiving adjuvant RT. Control subjects were limited to those who underwent resection after 2003 up to 2017 to better match the time frame in which adjuvant RT was administered. Cases were matched iteratively using nearest neighbors. All study subjects were matched.
Statistical analysis
Overall, recurrence-free, and local recurrence-free survival probabilities were estimated using the Kaplan-Meier product limit method. Distributions were compared between study subjects and control subjects using the log-rank test statistic. Cox proportional hazards regression models were used to estimate the hazard ratio (HR) for time-to-event endpoints. The median follow-up time was estimated by the product-limit method of Kaplan-Meier using the reverse-censoring method. For all statistical tests, P values <5% were considered statistically significant.
Three sensitivity analyses were performed. First, a second matching step was performed in which two control patients were selected for each case, using a nearest neighbor and caliper metric where possible. Control patients needed to have propensity scores within 0.1 of the study subjects to be selected. Thirty-eight of the 39 study subjects had at least one control subjects using this method, and two control subjects could be selected for 36 study subjects. The average difference between case and control propensity adjuvant RT was 0.008 (range, 0.00003 to 0.095).
A second sensitivity analysis was performed to guard against immortal time bias. To mitigate the possibility of this effect, study subjects known not to have undergone adjuvant RT have been screened for suitable follow-up without a recurrence (local or regional recurrence, metastatic failure, and/or death) to ensure that if adjuvant RT had been prescribed as part of the multi-modality treatment regimen, it would have been initiated. A period of 3 months was selected as the mandatory follow-up time. One-to-one matching was carried out, and all 39 study subjects were matched to control subjects. A third sensitivity analysis was performed to account for stage migration seen in control subjects who presented to the University of Michigan with more advanced disease. Patients who underwent adjuvant radiation were matched one to one with control subjects who did not receive adjuvant radiation and who had the same stage at diagnosis as compared with stage at University of Michigan presentation.
Results
A total of 424 patients were available to assess from the institutional database. Of these, 39 patients underwent postoperative adjuvant RT at the University of Michigan. These were matched one to one to patients who did not undergo adjuvant RT using propensity matching. Patients underwent resection between 2003 and 2017. All patients had localized or oligometastatic disease and underwent surgery with curative intent. Three patients in the RT cohort had adjuvant radiation after resection with curative intent of an isolated local recurrence that followed a prior surgery. Median follow-up time was 4.21 years (95% CI, 2.79 to 4.95).
Table 1 summarizes the baseline characteristics of patients by treatment group. There was no significant difference between the groups with respect to sex, age, stage, receipt of mitotane, tumor grade, tumor size, hormone production, or surgical margin status. The control subjects tended to present to the University of Michigan at higher stage than initial presentation to an outside facility, with 59% of patients at a higher stage than initially diagnosed (time from initial diagnosis calculated for analysis), whereas all patients treated with adjuvant RT were staged based on the surgical procedure that preceded radiation. The two study subjects with ENSAT stage I disease had a local recurrence treated with curative resection. The majority of patients treated with adjuvant RT were treated after 2012 (72.4%). There was an average of 64 days from the date of surgery to initiation of RT (range, 25 to 147 days). Five patients had >100 days between surgery and initiation of RT. Nearly all patients were treated with intensity-modulated RT (92.3%); only three patients were treated with three-dimensional conformal RT.
Table 1.
Baseline Characteristics of Patients
| No Radiation Therapy (n = 39) | Radiation Therapy (n = 39) | P Value | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 18 (46.2) | 18 (46.2) | 1 a , c |
| Female | 21 (53.9) | 21 (53.9) | |
| Mean age, y (range) | 44.9 (18–69) | 47.1 (13–74) | 0.260 b , c |
| Disease stage | |||
| I | 3 (7.7) | 2 (5.7) | 0.970 b , c |
| II | 16 (41.0) | 16 (41.0) | |
| III | 17 (43.6) | 18 (44.9) | |
| IV | 3 (7.7) | 3 (7.7) | |
| Mitotane use | |||
| Yes | 30 (76.9) | 30 (76.9) | 0.622 b |
| No | 9 (23.1) | 9 (23.1) | |
| Tumor grade | |||
| Low | 11 (28.2) | 10 (25.6) | 0.564 b , c |
| High | 28 (71.8) | 29 (74.4) | |
| Mean tumor size, cm (range) | 11.7 (4.1–23.0) | 10.4 (0.6–22.5) | 0.176 a |
| Hormone production | |||
| No | 18 (46.2) | 15 (38.5) | 0.172 b |
| Yes | 21 (53.8) | 19 (48.7) | |
| Not reported | 0 | 5 (12.8) | |
| Cortisol production | |||
| No | 22 (56.4) | 21 (53.9) | 0.149 b |
| Yes | 17 (43.6) | 13 (33.3) | |
| Not reported | 0 | 5 (12.8) | |
| Surgical margins | |||
| Negative | 32 (82.1) | 30 (76.9) | 0.801 b , c |
| Positive | 3 (7.7) | 5 (12.8) | |
| Not reported | 4 (10.3) | 4 (10.3) |
Paired t test.
McNemar test of dependent proportion or Bowker test of symmetry.
Accounted for in the model calculating the propensity weights for adjuvant RT.
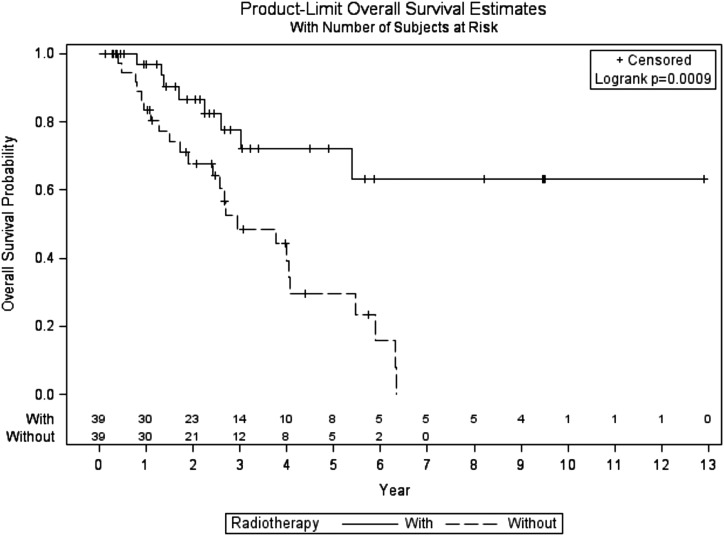
The overall survival (OS) distributions for case subjects and control subjects were significantly different (log-rank P = 0.0009) (Fig. 1). A total of 32 (41%) patients [eight (20.5%) treated with adjuvant RT and 24 (61.5%) treated without adjuvant RT] are known to have died. Overall survival estimates at 3 and 5 years were 77.7% (95% CI, 56.3 to 89.5) and 72.1% (95% CI, 49.2 to 86.0) in the adjuvant RT group vs 48.6% (95% CI, 29.7 to 65.2) and 29.5% (95% CI, 13.1 to 48.0), respectively, with an adjusted HR of 3.59 (95% CI, 1.60 to 8.09; P = 0.002).
Figure 1.
Overall survival of patients with ACC treated with or without adjuvant RT.
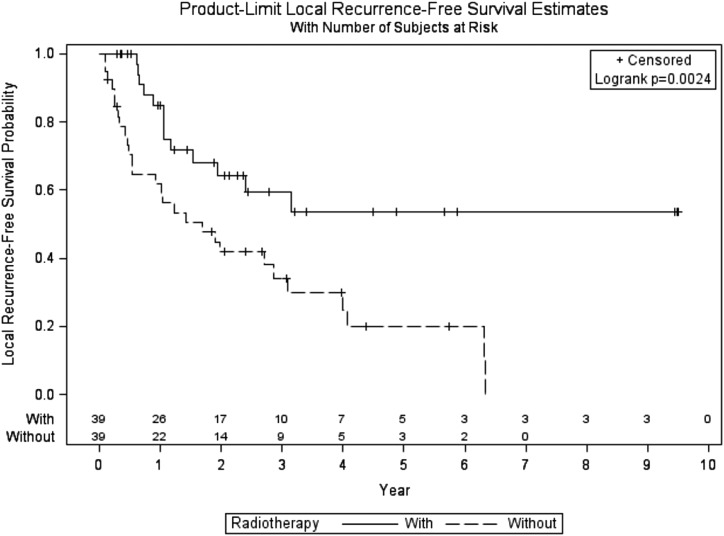
Locoregional recurrences occurred in 41 (52.7%) patients [13 (33.3%) patients treated with RT and 28 (71.8%) treated with resection alone]. Locoregional recurrence-free survival (RFS) was significantly higher for patients who received adjuvant RT (log-rank P = 0.0024) (Fig. 2). Locoregional RFS estimates at 3 and 5 years were 59.5% (95% CI, 39.0 to 75.0) and 53.5% (95% CI, 32.2 to 70.8) vs 34.2% (95% CI, 18.8 to 50.3) and 20.0% (95% CI, 7.4 to 37.2), respectively, with an adjusted HR of 2.67 (95% CI, 1.38 to 5.19; P = 0.0035).
Figure 2.
Local recurrence-free survival of patients with ACC treated with or without adjuvant RT.
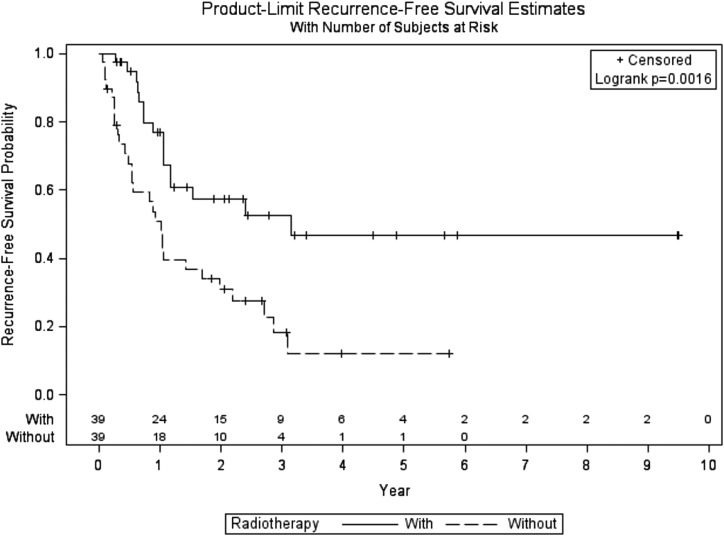
Any recurrence, including distant failures or death, occurred in 45 (57.7%) patients [16 (41.0%) treated with RT and 29 (74.4%) treated with resection alone]. The RFS was significantly higher for patients who received adjuvant RT than for those who underwent resection only (log-rank P value 0.0016) (Fig. 3). RFS estimates at 3 and 5 years were 46.7% (95% CI, 26.9 to 64.3) and 46.7% (95% CI, 26.9 to 64.3) vs 18.3% (95% CI, 6.7 to 34.3) and 12.2% (95% CI, 2.8 to 29.1), respectively, with an adjusted HR of 2.59 (95% CI, 1.40 to 4.79; P = 0.0024).
Figure 3.
All recurrence-free survival of patients with ACC treated with or without adjuvant RT.
Sensitivity analyses were performed to assess the robustness of the results. Matching with multiple control subjects produced results similar to those found in single-matched analyses (14). Analyses to adjust for immortal time bias were performed, excluding failures within 3 months of surgery. Patients were well matched, and again a benefit of adjuvant RT was seen in all outcomes (14). Finally, stage migration sensitivity analyses were performed (14). All three sensitivity analyses confirmed the results and maintained the advantage noted with adjuvant RT.
Discussion
This retrospective study represents the largest single-institution study to date analyzing the benefit of adjuvant RT for ACC. We have found that adjuvant RT significantly improves locoregional control and OS.
Assessing the role of adjuvant therapy in this rare disease site has been challenging. The use of mitotane, an adrenolytic agent, as systemic therapy in ACC is largely based on retrospective data (5). Varying chemotherapy regimens have been examined as well. The poor prognosis of ACC has driven the search for effective therapies. Analysis of the German ACC registry in 2006 suggested an improvement in local control with adjuvant RT (7). A subsequent retrospective analysis in 2013 did not show any benefit in local control (8), but there was no central evaluation of RT plan quality in this study, and patients were treated at low-volume sites. Prior work by our group (9) showed a local control benefit with adjuvant RT. This has prompted investigations into the role of adjuvant RT in ACC.
Registry analyses using National Cancer Database and the National Cancer Institute’s Surveillance, Epidemiology, and End Results database have resulted in conflicting conclusions to the benefit of adjuvant radiation (10, 11, 15). However, these studies are limited by the lack of granular data; heterogeneous treatment quality; and a mixture of adjuvant, salvage, and palliative treatment intent. More detailed examinations of the role of adjuvant radiation have been hampered by small numbers of patients who received this therapy [e.g., 10 (16), 14 (7), 16 (8), and 20 (9) patients].
A recent population-based publication on the role of adjuvant RT for ACC used the National Cancer Database and included 171 patients who received adjuvant RT (11). Only 14.4% of patients received adjuvant radiation from a cohort of 1184 patients; patients who received adjuvant RT were more likely to be higher-risk patients who had positive surgical margins and who had received chemotherapy as part of the treatment regimen. Adjuvant radiation improved overall survival with a 40% decreased risk of death but only in patients with positive margins. A lack of stage, grade, and recurrence information limited the scope of this finding.
Single-institution analyses provide a source of homogenous patient populations treated uniformly with rigorous treatment evaluations. All patients at the University of Michigan are treated with guidance from a tumor board in a large tertiary referral center specializing in endocrine malignancies, allowing for homogenous recommendations and improved treatment quality. The use of modern RT techniques (including breath-hold, daily image guidance, and intensity modulation) allows for delivery of high-quality radiation plans that show a distinct improvement as compared with older radiation techniques in terms of toxicity (17) and accuracy of delivery (18, 19) in other disease sites. Additionally, direct surgeon guidance in outlining high-risk areas to aid in volume delineation allows precise and consistent coverage of the areas most likely to fail. Previous studies assessing the benefit of adjuvant RT in ACC have limited quality assurance.
In our experience, patients tend to tolerate RT well. Side effects of RT include fatigue, nausea, diarrhea, potential kidney/liver damage, and obstruction. The side effects of mitotane include nausea, fatigue, anorexia, and diarrhea. Given the overlap of these side effect profiles, patients may experience nausea and diarrhea while undergoing RT. This review precluded comparison of adverse events due to the lack of prospective gathering of data.
Our study had weaknesses typical of retrospective designs for rare disease types. There are a limited number of study subjects. This cohort consisted mostly of adult patients, and results are not generalizable to the unique pediatric population of patients with ACC, ∼80% of whom harbor pathogenic germline variants. Sensitivity analyses have suggested the results are robust. Prospective confirmation of the data presented here would be ideal; given the rarity of this disease, completion of a prospective trial with sufficient accrual would be challenging, and retrospective reviews currently offer the best guidance available for treatment decisions.
In summary, the evolution of literature on adjuvant radiation in ACC has shown a clear arc in the efficacy of treatment with RT as radiation techniques have improved. There have been nearly a dozen retrospective single-institution studies examining local control since the 1970s with at least 130 patients. Studies prior to the 2000s did not show a local control benefit to RT, with local control ranging from 0% to 60%; however, since 2006, there have been several publications reporting local control rates from 56% to 100% (Table 2). Improvements in local control with RT have translated to improvements in OS in other cancer types [breast cancer (20), rectal cancer (21), head and neck cancers (22)], although studies typically required large patient populations to show this advantage. The current study highlights that a survival benefit with improved locoregional control may also be seen in ACC in the modern treatment era.
Table 2.
Prior Single-Institution Studies of Adjuvant RT for ACC
| Study | Year | No. of Cases | Radiation Dose (Gy) | Chemotherapy or Mitotane, n (%) | Local Control, n (%) |
|---|---|---|---|---|---|
| Percarpio (26) | 1976 | 4 | 28–40 | NR | 1/4 (25) |
| Henley (27) | 1983 | 10 | NR | NR | 1/10 (10) |
| Markoe (28) | 1991 | 5 | 42–60 | 2/5 (40) | 3/5 (60) |
| Pommier (29) | 1992 | 3 | 39–45 | NR | 0/3 (0) |
| Fassnacht (7) | 2006 | 14 | 40-54 | 5/14 (36) | 12/14 (86) |
| Hermsen (30) | 2010 | 3 | NR | NR | 3/3 (100) |
| Habra (8) | 2013 | 16 | 36–59.4 | 4/16 (25) | 9/16 (56) |
| Sabolch (9) | 2015 | 20 | 45–60 | 15/20 (75) | 19/20 (95) |
| Atallah (31) | 2017 | 6 | NR | 2/6 (33) | 5/6 (83) |
| Srougi (16) | 2017 | 10 | 45–54 | NR | 6/10 (60) |
| Gharzai | Present study | 39 | 45–60 | 30/39 (77) | 26/39 (67) |
Abbreviation: NR, not reported.
Our findings demonstrate that adjuvant RT is important for local tumor control for patients with ACC. Current recommendations for adjuvant RT in ACC suggest consideration of RT in high-risk patients, including R0 with large size (8, 23), incomplete/R1 resection (24), or stage III disease (25). Our data suggest that adjuvant RT provides substantial improvements in locoregional control regardless of margin status. Therefore, in the absence of randomized trials, resection followed by adjuvant RT should be considered for all patients with ACC.
Acknowledgments
Author Contributions: L.A.G. and M.D.G. collected, analyzed, and interpreted the data; prepared the initial draft of manuscript; and critically reviewed manuscript. K.A.G. analyzed and interpreted the data, analyzed the statistics, and critically reviewed the manuscript. T.E. designed the study, collected and interpreted the data, and critically reviewed the manuscript. C.S.M. collected the data and critically reviewed the manuscript. E.S., D.E.S., E.B.J., A.S., B.S.M., F.W., T.G., and G.D.H. interpreted the data and critically reviewed the manuscript. S.J. designed the study, interpreted the data, critically reviewed the manuscript, and supervised the study. S.J. has full access to all the data in the study and final responsibility for the decision to submit for publication.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- HR
hazard ratio
- OS
overall survival
- RFS
recurrence-free survival
- RT
radiotherapy
References and Notes
- 1. Kendrick ML, Lloyd R, Erickson L, Farley DR, Grant CS, Thompson GB, Rowland C, Young WF Jr, van Heerden JA. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136(5):543–549. [DOI] [PubMed] [Google Scholar]
- 2. Tran TB, Postlewait LM, Maithel SK, Prescott JD, Wang TS, Glenn J, Phay JE, Keplinger K, Fields RC, Jin LX, Weber SM, Salem A, Sicklick JK, Gad S, Yopp AC, Mansour JC, Duh QY, Seiser N, Solorzano CC, Kiernan CM, Votanopoulos KI, Levine EA, Hatzaras I, Shenoy R, Pawlik TM, Norton JA, Poultsides GA. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol. 2016;114(8):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, Dousset B, Bertagna X, Bertherat J. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650–2655. [DOI] [PubMed] [Google Scholar]
- 4. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–3136. [DOI] [PubMed] [Google Scholar]
- 6. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B; FIRM-ACT Study Group. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 7. Fassnacht M, Hahner S, Polat B, Koschker AC, Kenn W, Flentje M, Allolio B. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91(11):4501–4504. [DOI] [PubMed] [Google Scholar]
- 8. Habra MA, Ejaz S, Feng L, Das P, Deniz F, Grubbs EG, Phan A, Waguespack SG, Ayala-Ramirez M, Jimenez C, Perrier ND, Lee JE, Vassilopoulou-Sellin R. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabolch A, Else T, Griffith KA, Ben-Josef E, Williams A, Miller BS, Worden F, Hammer GD, Jolly S. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2015;92(2):252–259. [DOI] [PubMed] [Google Scholar]
- 10. Luo Y, Chen SS, Zheng XG, Luo L, Wang S. The efficacy of radiation therapy in adrenocortical carcinoma: a propensity score analysis of a population-based study. Medicine (Baltimore). 2017;96(17):e6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson DW, Chang SC, Bandera BC, Fischer TD, Wollman R, Goldfarb M. Adjuvant radiation is associated with improved survival for select patients with non-metastatic adrenocortical carcinoma. Ann Surg Oncol. 2018;25(7):2060–2066. [DOI] [PubMed] [Google Scholar]
- 12. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio BGerman Adrenocortical Carcinoma Registry GroupEuropean Network for the Study of Adrenal Tumors . Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. [DOI] [PubMed] [Google Scholar]
- 13. Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152(6):1150–1157. [DOI] [PubMed] [Google Scholar]
- 14. Gharzai LA, Green MD, Griffith KA, Else T, Mayo CS, Hesseltine E, Spratt DE, Ben-Josef E, Sabolch A, Miller BS, Worden F, Giordano TJ, Hammer GD, Jolly S. Data from: Adjuvant radiation improves recurrence-free survival and overall survival in adrenocortical carcinoma. Deep Blue Data Repository at University of Michigan. Deposited 2 January 2019. https://deepblue.lib.umich.edu/data/concern/data_sets/gx41mj832?locale=en. [DOI] [PMC free article] [PubMed]
- 15. Wang S, Chen SS, Gao WC, Bai L, Luo L, Zheng XG, Luo Y. Prognostic factors of adrenocortical carcinoma: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Asian Pac J Cancer Prev. 2017;18(10):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srougi V, de Bessa J Jr., Tanno FY, Ferreira AM, Hoff AO, Bezerra JE, Almeida CM, Almeida MQ, Mendonça BB, Nahas WC, Chambô JL, Srougi M, Fragoso MCBV. Adjuvant radiotherapy for the primary treatment of adrenocortical carcinoma: are we offering the best? Int Braz J Urol. 2017;43:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, Gifford K, Gaffney DK, Small W Jr, Thompson S, Doncals DE, Cantuaria GHC, Yaremko BP, Chang A, Kundapur V, Mohan DS, Haas ML, Kim YB, Ferguson CL, Pugh SL, Kachnic LA, Bruner DW. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol. 2018;36(24):2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta T, Narayan CA. Image-guided radiation therapy: physician’s perspectives. J Med Phys. 2012;37(4):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kilburn JM, Soike MH, Lucas JT, Ayala-Peacock D, Blackstock W, Isom S, Kearns WT, Hinson WH, Miller AA, Petty WJ, Munley MT, Urbanic JJ. Image guided radiation therapy may result in improved local control in locally advanced lung cancer patients. Pract Radiat Oncol. 2016;6(3):e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23(24):5644–5650. [DOI] [PubMed] [Google Scholar]
- 22. Wadsley JC, Bentzen SM. Investigation of relationship between change in locoregional control and change in overall survival in randomized controlled trials of modified radiotherapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1405–1409. [DOI] [PubMed] [Google Scholar]
- 23. Polat B, Fassnacht M, Pfreundner L, Guckenberger M, Bratengeier K, Johanssen S, Kenn W, Hahner S, Allolio B, Flentje M. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115(13):2816–2823. [DOI] [PubMed] [Google Scholar]
- 24. Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, Pentheroudakis G; ESMO Guidelines Working Group. Adrenal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii131–vii138. [DOI] [PubMed] [Google Scholar]
- 25. Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46. [DOI] [PubMed] [Google Scholar]
- 26. Percarpio B, Knowlton AH. Radiation therapy of adrenal cortical carcinoma. Acta Radiol Ther Phys Biol. 1976;15(4):288–292. [DOI] [PubMed] [Google Scholar]
- 27. Henley DJ, van Heerden JA, Grant CS, Carney JA, Carpenter PC. Adrenal cortical carcinoma: a continuing challenge. Surgery. 1983;94(6):926–931. [PubMed] [Google Scholar]
- 28. Markoe AM, Serber W, Micaily B, Brady LW. Radiation therapy for adjunctive treatment of adrenal cortical carcinoma. Am J Clin Oncol. 1991;14(2):170–174. [DOI] [PubMed] [Google Scholar]
- 29. Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112(6):963–970, discussion 970–971. [PubMed] [Google Scholar]
- 30. Hermsen IG, Groenen YE, Dercksen MW, Theuws J, Haak HR. Response to radiation therapy in adrenocortical carcinoma. J Endocrinol Invest. 2010;33(10):712–714. [DOI] [PubMed] [Google Scholar]
- 31. Atallah S, Al-Assaf H, Xu Y, El-Sayed S. Adrenocortical carcinoma: patterns of care and role of adjuvant radiation therapy: a population-based study and review of the literature. Curr Oncol. 2017;24(4):e316–e322. [DOI] [PMC free article] [PubMed] [Google Scholar]