Solution Set Oversight Committee
Ty J. Gluckman, MD, MHA, FACC, Chair
Niti R. Aggarwal, MD, FACC
Nicole M. Bhave, MD, FACC
Biykem Bozkurt, MD, PhD, FACC
Gregory J. Dehmer, MD, MACC
Chayakrit Krittanawong, MD
Dharam J. Kumbhani, MD, SM, FACC
Javier A. Sala-Mercado, MD, PhD
David E. Winchester, MD, MS, FACC
Megan Coylewright, MD, MPH, FACC, Ex Officio
Table of Contents
Preface 1719
-
1.
Introduction 1719
-
2.Methods 1719
-
2.1.Background 1719
-
2.2.Process 1720
-
2.1.
-
3.Rationale 1720
-
3.1.Scope of the Document 1720
- Figure 1. Scope of the Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults 1720
-
3.1.1.Myocarditis and Other Myocardial Involvement 1721
-
3.1.2.Post-acute Sequelae of SARS-CoV-2 Infection 1721
-
3.1.3.Return to Play 1721
-
3.1.
-
4.
Assumptions and Definitions 1721
-
5.Pathway Summary Graphic 1721
- Figure 2. Framework for Evaluating and Managing Cardiovascular Sequelae of COVID-19 in Adults 1722
-
6.Description of Pathway 1721
-
6.1.Section 1: Myocarditis and Other Myocardial Involvement 1721
-
6.1.1.Overview 1721
-
6.1.2.Definitions and Clinical Presentation 1723
-
6.1.3.Epidemiology 1724
-
6.1.4.Mechanisms 1724
-
6.1.5.Evaluation 1725
- Figure 3. Evaluation and Management of Patients With Suspected Myocarditis or Myocardial Involvement 1726
-
6.1.6.Management 1726
-
6.1.7.Postvaccination Myocarditis 1727
- Figure 4. Favorable Benefit-to-Risk Ratio for COVID-19 mRNA Vaccination Among Those at Highest Risk for Postvaccination Myocarditis 1729
-
6.1.8.Future Directions 1730
-
6.1.1.
-
6.2.Section 2: Post-acute Sequelae of SARS-CoV-2 Infection 1730
-
6.2.1.Overview 1730
- Figure 5. Symptoms of PASC and Potential Mechanisms 1730
-
6.2.2.Definition 1731
-
6.2.3.Framework 1731
-
6.2.4.Epidemiology 1732
-
6.2.5.Mechanisms 1733
- Figure 6. Downward Spiral of Deconditioning: A Potential Mechanism of Exercise Intolerance and Excessive Tachycardia in COVID-19 1734
-
6.2.6.Evaluation 1735
- Figure 7. Evaluation of Cardiovascular Symptoms Suggestive of PASC 1736
-
6.2.7.Management 1737
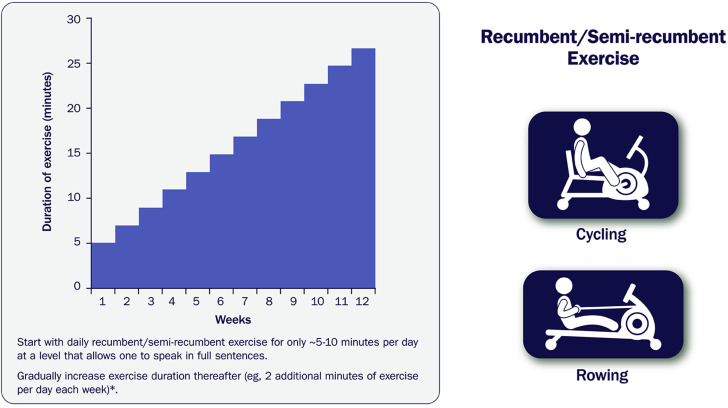
- Figure 8. Sample Recumbent Exercise Therapy Prescription 1737
-
6.2.8.Future Directions 1738
-
6.2.1.
-
6.3.Section 3: Return to Play 1739
-
6.3.1.Overview 1739
-
6.3.2.Epidemiology 1739
-
6.3.3.Case Series 1740
-
6.3.4.Registries 1740
-
6.3.5.Limitations of Screening With CMR 1741
-
6.3.6.Evaluation and Management 1741
-
6.3.7.Return to Exercise and Athletic Training 1741
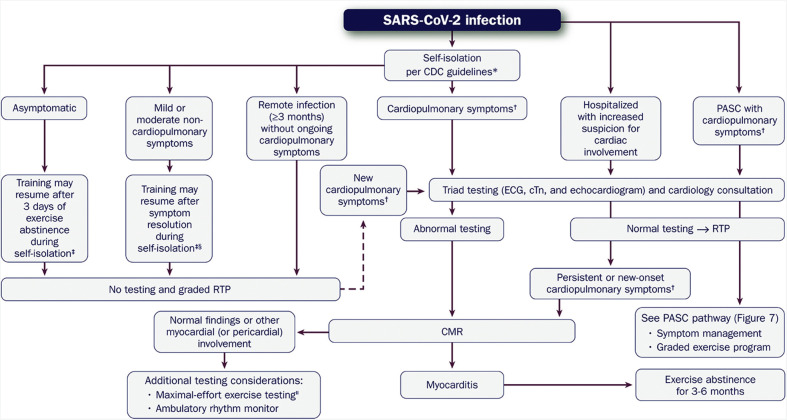
- Figure 9. Evaluation of the Athletic Patient Convalesced From COVID-19 and Guidance on RTP and/or Intense Training 1742
-
6.3.8.Remote History of COVID-19 1743
-
6.3.9.Nonspecific and Isolated Screening Abnormalities 1743
-
6.3.10.Myocarditis and Exercise Training Restrictions 1743
-
6.3.11.PASC and Return to Exercise 1745
-
6.3.12.Future Directions 1745
-
6.3.1.
-
6.1.
-
7.
Implication of Pathway 1745
References 1746
- Appendix 1
- Author Relationships With Industry and Other Entities (Relevant) 1752
- Appendix 2
- Peer Reviewer Relationships With Industry and Other Entities (Comprehensive) 1754
- Appendix 3
- Abbreviations 1756
Preface
The American College of Cardiology (ACC) has a long history of developing documents (eg, decision pathways, health policy statements, appropriate use criteria) to provide members with guidance on both clinical and nonclinical topics relevant to cardiovascular care. In most circumstances, these documents have been created to complement clinical practice guidelines and to inform clinicians about areas where evidence may be new and evolving or where sufficient data may be more limited. Despite this, numerous care gaps continue to exist, highlighting the need for more streamlined and efficient processes to implement best practices in service to improved patient care.
Central to the ACC’s strategic plan is the generation of “actionable knowledge”—a concept that places emphasis on making clinical information easier to consume, share, integrate, and update. To this end, the ACC has evolved from developing isolated documents to developing integrated “solution sets.” Solution sets are groups of closely related activities, policy, mobile applications, decision support, and other tools necessary to transform care and/or improve heart health. Solution sets address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care. They use both established and emerging methods to disseminate information for cardiovascular conditions and their related management. The success of the solution sets rests firmly on their ability to have a measurable impact on the delivery of care. Because solution sets reflect current evidence and ongoing gaps in care, the associated content will be refined over time to best match changing evidence and member needs.
Expert Consensus Decision Pathways (ECDPs) represent a key component of solution sets. The methodology for ECDPs is grounded in assembling a group of clinical experts to develop content that addresses key questions facing our members across a range of high-value clinical topics.1 This content is used to inform the development of various tools that accelerate real-time use of clinical policy at the point of care. They are not intended to provide a single correct answer; rather, they encourage clinicians to ask questions and consider important factors as they define treatment plans for their patients. Whenever appropriate, ECDPs seek to provide unified articulation of clinical practice guidelines, appropriate use criteria, and other related ACC clinical policy. In some cases, covered topics will be addressed in subsequent clinical practice guidelines as the evidence base evolves. In other cases, these will serve as stand-alone policy.
Ty J. Gluckman, MD, MHA, FACC
Chair, ACC Solution Set Oversight Committee
1. Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has had an unprecedented impact worldwide. In the United States alone, more than 78 million cases have been reported, with greater than 948,000 deaths attributed directly to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as of the writing of this ECDP.2 Among patients hospitalized for COVID-19, various abnormalities in cardiac testing (eg, electrocardiographic abnormalities, elevated cardiac biomarkers)3, 4, 5 and a wide range of cardiovascular complications (eg, myocardial injury, thrombosis, arrhythmia) have been reported.4, 5, 6, 7
For some patients infected with SARS-CoV-2, cardiac symptoms (eg, chest pain, shortness of breath, fatigue, and palpitations) persist, lasting months after the initial illness.8 , 9 Laboratory and imaging evidence of myocardial injury and involvement has also been observed in both symptomatic and asymptomatic individuals,4 , 10 , 11 as well as after receipt of the COVID-19 mRNA vaccine.12 For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity.
To help gather further insights, the ACC held a virtual Heart House Roundtable in May 2021, bringing together experts in the following areas: 1) myocarditis; 2) post-acute sequelae of SARS-CoV-2 infection (PASC); and 3) sports cardiology. This ECDP represents a key output from that meeting and is intended to serve as a resource for clinicians caring for these patients, largely in the outpatient setting. More specifically, this document attempts to address commonly asked questions regarding the care of adults (aged ≥18 years) with cardiac symptoms (eg, chest pain, dyspnea, palpitations, syncope) after SARS-CoV-2 infection. In the absence of robust clinical trial data, it is intended to provide practical guidance related to evaluation and management.
2. Methods
2.1. Background
On May 26, 2021, the ACC’s Cardiovascular Conundrums in the COVID-19 Era Roundtable was convened to bring together expert clinicians along with a broad set of stakeholders, including representatives from patient advocacy groups, professional societies, and content experts, to discuss ongoing questions faced by cardiovascular clinicians caring for individuals with COVID-19. Participants in this ACC Heart House Roundtable identified the need for expert consensus guidance regarding the evaluation and management of patients with ongoing cardiac symptoms, along with resumption of physical activity after SARS-CoV-2 infection.
2.2. Process
Guidance issued in this document was informed by scientific evidence presented and expert opinions considered during the Heart House Roundtable, with additional review and deliberation by the writing committee. Although practical approaches and ongoing gaps in knowledge were discussed during the Roundtable meeting, this document is a separate and independent endeavor that specifically aims to address some of the most common questions that were raised. The work of the writing committee was supported exclusively by the ACC without commercial support. Writing committee members volunteered their time to this effort. Conference calls of the writing committee were confidential and attended only by committee members and society staff.
The ACC and the Solution Set Oversight Committee recognize the importance of avoiding real or perceived relationships with industry (RWI) or other entities that may affect clinical policy. The ACC maintains a database that tracks all relevant relationships for ACC members and persons who participate in ACC activities, including those involved in the development of ECDPs. ECDPs follow ACC RWI policy in determining what constitutes a relevant relationship, with additional vetting by the Solution Set Oversight Committee.
ECDP writing groups must be chaired or co-chaired by an individual with no relevant RWI. Although vice chairs and writing group members may have relevant RWI, they must constitute less than 50% of the writing group. Relevant disclosures for the writing group and comprehensive disclosures for external peer reviewers can be found in Appendixes 1 and 2. To ensure complete transparency, a comprehensive list of disclosure information for the writing group, including relationships not pertinent to this document, is available in a Supplemental Appendix. Writing group members are discouraged from acquiring relevant RWI during the writing process.
3. Rationale
3.1. Scope of the Document
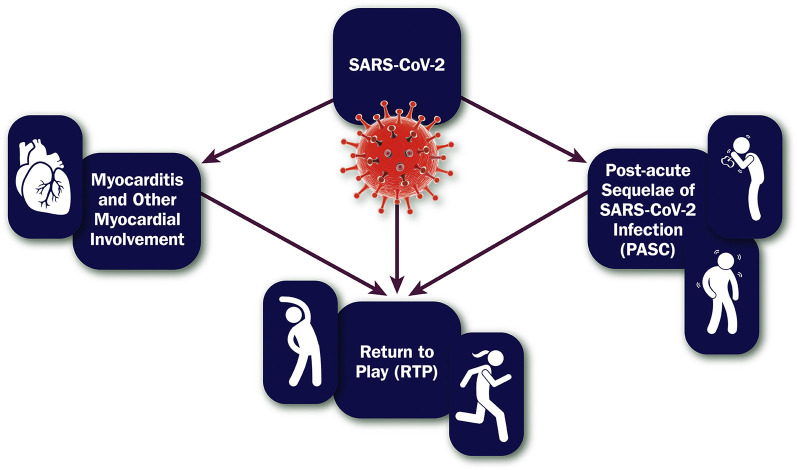
Recognizing that COVID-19 may have short- and long-term impacts on multiple organ systems, recommendations made in this ECDP are restricted to some of the more common cardiovascular sequelae in adults. Evaluation of cardiovascular symptoms after SARS-CoV-2 infection is imperative, and this document focuses on conditions that clinicians may struggle to diagnose and manage—myocarditis and PASC—while providing guidance on return to play (RTP) (see Figure 1 ). Recommendations made in this ECDP are intended to be applied to a broad, unselected population; individualized approaches may be needed for specific populations (eg, those who are pregnant, those with adult congenital heart disease, those with pre-existing heart failure).13 Finally, although other cardiovascular sequelae exist with COVID-19 (eg, thrombosis), they are not covered in this ECDP.
Figure 1.
Scope of the Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults
PASC = post-acute sequelae of SARS-CoV-2 infection; RTP = return to play; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.1.1. Myocarditis and Other Myocardial Involvement
Myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination. Other myocardial abnormalities have also been noted on cardiac magnetic resonance imaging (CMR), even in the absence of cardiac symptoms. In this section, we review potential underlying mechanisms for these conditions and preferred approaches for their evaluation and management.
3.1.2. Post-acute Sequelae of SARS-CoV-2 Infection
PASC encompasses a constellation of symptoms that emerge or persist weeks to months after recovery from COVID-19.14 , 15 Although evidence guiding the care of these patients continues to evolve, there is a need to develop common taxonomies and approaches to care that can be updated iteratively as new data become available. In this section, we segment PASC patients into 2 groups—those with discernable cardiovascular disease (PASC-cardiovascular disease [CVD]) and those with test results that are either normal or unable to fully explain reported symptoms (PASC-cardiovascular syndrome [CVS])—and present patient-centered approaches to their evaluation and management.
3.1.3. Return to Play
Early in the pandemic, reports of significant myocardial injury among patients hospitalized with COVID-19 raised concern about risks borne by athletes as part of RTP after SARS-CoV-2 infection. Since then, the prevalence of clinical myocarditis, myocardial involvement, and myocardial injury has been further defined among athletes. Several large observational studies have also helped outline the utility and drawbacks of different diagnostic strategies. In this section, we review the compilation of data, update RTP recommendations, and highlight areas of future investigation.
4. Assumptions and Definitions
To limit inconsistencies in interpretation and to develop guidance that is complementary to current terminology, specific definitions and assumptions were considered by the writing committee in the development of consensus recommendations:
-
1.Clinical spectrum of SARS-CoV-2 infection:16
-
1.1.Asymptomatic (or presymptomatic): Individual who tests positive for SARS-CoV-2 infection but has no symptoms consistent with COVID-19;
-
1.2.Mild illness: Individual who has symptoms or signs of COVID-19 (eg, fever, cough, sore throat, and malaise), without dyspnea or abnormal chest imaging;
-
1.3.Moderate illness: Individual who has lower respiratory disease (eg, pneumonia) with oxygen saturation ≥94% on room air at sea level;
-
1.4.Severe illness: Individual who has lower respiratory disease with oxygen saturation <94% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, a respiratory rate >30 breaths per minute, or infiltrates involving >50% of the lung fields on chest imaging;
-
1.5.Critical illness: Individual who has lower respiratory disease with respiratory failure, septic shock, and/or multiple organ dysfunction.
-
1.1.
-
2.
Myocarditis: Condition defined by the presence of cardiac symptoms (eg, chest pain, dyspnea, palpitations, syncope), an elevated cardiac troponin (cTn), and abnormal electrocardiographic, echocardiographic, CMR, and/or histopathologic findings on biopsy or postmortem evaluation in the absence of flow-limiting coronary artery disease.
-
3.
Myocardial involvement: Condition defined by abnormal myocardium manifest by electrocardiographic, echocardiographic, CMR and/or histopathologic findings, with or without symptoms and with or without an elevated cTn.
-
4.
Myocardial injury: Condition defined by a cTn level above the 99th percentile upper reference limit.17
-
5.
PASC: Condition defined by a constellation of symptoms that emerge or persist after recovery from COVID-19, usually lasting for 4-12 weeks and beyond.
-
6.
Athlete: Individual who places a high premium on exercise training, competition, and sports achievement, extending across the age spectrum from youth (aged <18 years) to masters-level (aged >35 years) participation.
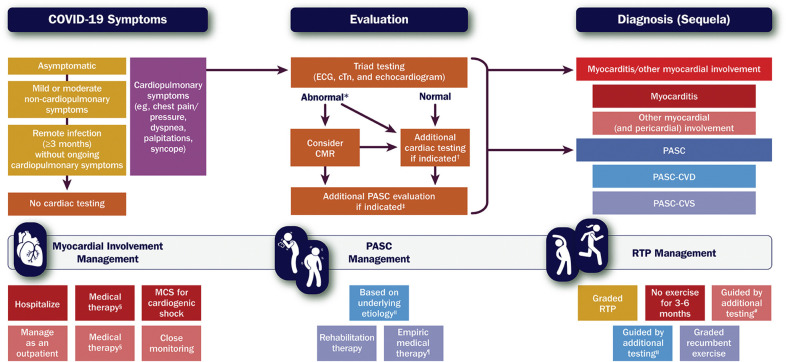
5. Pathway Summary Graphic
Figure 2 provides a framework for the evaluation of cardiovascular symptoms after SARS-CoV-2 infection and management of myocarditis/other myocardial involvement, PASC, and RTP.
Figure 2.
Framework for Evaluating and Managing Cardiovascular Sequelae of COVID-19 in Adults
Gold and purple boxes = COVID-19 symptoms. Orange boxes = cardiac testing. Red and blue boxes = diagnoses (sequelae). ∗Includes elevated cTn; ECG with diffuse T-wave inversion, ST-segment elevation without reciprocal ST-segment depression, and/or prolongation of the QRS complex duration; and echocardiogram with ventricular wall motion abnormalities, often in a noncoronary distribution, and/or abnormal ventricular strain. †Informed by the presentation and may include coronary angiography for suspected acute coronary syndrome or CT pulmonary angiography for suspected pulmonary embolism (see Figure 3). ‡Includes other laboratory testing (eg, complete blood count, basic metabolic panel, C-reactive protein), an ambulatory rhythm monitor, a chest X-ray or CT imaging, and pulmonary function tests, along with additional testing for suspected PASC-CVD or PASC-CVS (see Figure 7). §For patients with myocarditis, medical therapy may include immunosuppressive drugs (eg, corticosteroids); for patients with pericardial involvement, medical therapy may include nonsteroidal anti-inflammatory drugs, colchicine, and corticosteroids (see Figure 3). ‖See Figure 7. ¶For patients with PASC-CVS, empiric medical therapy may include a beta-blocker, a non-dihydropyridine calcium-channel blocker, ivabradine, fludrocortisone, and/or midodrine. #Includes maximal-effort exercise testing and/or an ambulatory rhythm monitor (see Figure 9). CMR = cardiac magnetic resonance imaging; COVID-19 = novel coronavirus disease 2019; CT = computed tomography; cTn = cardiac troponin; ECG = electrocardiogram, MCS = mechanical circulatory support; PASC = post-acute sequelae of SARS-CoV-2 infection; PASC-CVD = PASC-Cardiovascular Disease; PASC-CVS = PASC-Cardiovascular Syndrome; RTP = return to play; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
6. Description of Pathway
6.1. Section 1: Myocarditis and Other Myocardial Involvement
6.1.1. Overview
Infection with SARS-CoV-2 and complications of COVID-19 mRNA vaccination have drawn increased attention to myocarditis (and pericarditis) during the COVID-19 pandemic. Early case reports of fulminant myocarditis, combined with frequently observed increases in cTn among patients hospitalized with COVID-19, led to initial concern that myocardial involvement was common.3 Although cases of severe myocarditis with both viral infection and vaccination remain relatively rare, they do exist, and have complicated immunization efforts.18 At the same time, various abnormalities on CMR (and positron emission tomography [PET]) have been reported in those with COVID-19, even in the absence of cardiac symptoms.11 , 19 Collectively, these findings challenge our understanding of viral-mediated myocardial (and pericardial) involvement20, 21, 22, 23, 24, 25 and raise questions about the pandemic’s long-term cardiovascular consequences.
The definition and approach to diagnosis of myocarditis following SARS-CoV-2 infection continues to evolve, reflecting advances in diagnostic testing (particularly CMR) and improved understanding of its immunopathogenesis. Older definitions based on pathology (eg, Dallas and immunohistologic criteria)26 historically relied upon abnormal histopathologic findings on endomyocardial biopsy, with myocyte necrosis and inflammatory myocardial infiltrates that were nonischemic in origin.27 Subsequent definitions have incorporated use of an elevated cTn and abnormalities on CMR and fluorodeoxyglucose (FDG)-PET to characterize and diagnose myocarditis in the absence of endomyocardial biopsy (eg, Lake Louise criteria).28, 29, 30 Importantly, however, numerous ambiguities exist with this approach, driven, in part, by discrepant findings and diverse practices in CMR and the lack of a standardized clinical definition for myocarditis.31 The absence of uniform methods for noninvasively evaluating these patients further limits comparison of diagnostic methods and has posed a barrier to the development of normative ranges and prognostic cutoffs that can be used to inform subsequent management steps.31 Furthermore, existing definitions of myocarditis were developed in models of viral-mediated inflammation with focal areas of necrosis (infarct-like myocarditis); in contrast, the underlying myocardial processes with COVID-19 appear to be more heterogeneous and diffuse.
Myocardial injury with COVID-19 has been extensively reported, with rates widely varying depending upon the population studied.4 The underlying causes are numerous and include but are not limited to myocarditis,22 acute coronary syndrome (myocardial infarction type 1),17 demand ischemia (myocardial infarction type 2),25 , 32, 33, 34, 35 multisystem inflammatory syndrome in children (MIS-C) and multisystem inflammatory syndrome in adults (MIS-A),20 , 21 , 24 takotsubo/stress cardiomyopathy,36 , 37 cytokine storm,34 acute cor pulmonale resulting from macropulmonary or micropulmonary emboli,23 , 25 myocardial injury from chronic conditions like pre-existing heart failure,38, 39, 40, 41 and acute viral infection unmasking subclinical heart disease.38 Because 1 or more of these etiologies may coexist, it can sometimes be challenging to identify a specific underlying cause.
Cognizant of the wide spectrum of potential myocardial involvement that may be seen with COVID-19, we have chosen to focus on myocarditis and other forms of cardiac involvement. For those with myocardial injury unrelated to these conditions (most often observed in those hospitalized for COVID-19), a targeted evaluation and management plan should be undertaken based on the suspected underlying cause.4 , 42
6.1.2. Definitions and Clinical Presentation
A variety of terms and associated definitions are offered in the following text, highlighting the spectrum of myocardial abnormalities that may be seen with COVID-19.
Myocarditis: This condition is defined by the presence of 1) cardiac symptoms (eg, chest pain, dyspnea, palpitations, syncope); 2) an elevated cTn; and 3) abnormal electrocardiographic (eg, diffuse T-wave inversion, ST-segment elevation without reciprocal ST-segment depression, prolongation of the QRS complex duration), echocardiographic (eg, left ventricular [LV] wall motion abnormalities, often noted in a noncoronary distribution), CMR (eg, nonischemic late gadolinium enhancement [LGE] pattern with prolonged native T1 and T2 relaxation times), and/or histopathologic findings on biopsy or postmortem evaluation (eg, inflammatory myocardial infiltrates associated with myocyte degeneration and necrosis) in the absence of flow-limiting epicardial coronary artery disease. It is further categorized into levels of certainty (possible, probable, and definite) based on the number of features present.
-
▪
Possible myocarditis: This includes individuals with: 1) cardiac symptoms; 2) an elevated cTn; and 3) abnormal electrocardiographic and/or echocardiographic findings but a) absence of acute myocarditis by endomyocardial biopsy or CMR or b) circumstances where neither biopsy nor CMR were performed. Ideally, flow-limiting coronary artery disease would have been excluded in men older than 50 years and women older than 55 years.
-
▪
Probable myocarditis: This condition is defined by all features of possible myocarditis, but follow-up CMR and/or biopsy within 6 months of SARS-CoV-2 infection demonstrates abnormalities consistent with previous myocarditis.
-
▪
Definite myocarditis: This condition is defined by all features of possible myocarditis, but CMR and/or biopsy performed at the time of SARS-CoV-2 infection demonstrates findings consistent with active myocarditis.
Myocardial involvement: This broader term is intended to include myocardial abnormalities noted to occur in the setting of SARS-CoV-2 infection that do not meet the criteria for possible, probable, or definite myocarditis. Importantly, affected individuals may or may not have cardiac symptoms and may have wide variation in presentation, ranging from asymptomatic individuals (eg, athletes who have undergone screening with CMR)43 to those evaluated at autopsy.44 , 45
Myocardial injury: This condition is defined by a cTn level (preferably using a high-sensitivity assay) above the 99th percentile upper reference limit.17 This may be related to widely varying mechanisms, ranging from demand ischemia to cytokine storm. Regardless of the etiology, myocardial injury with COVID-19 carries a worse prognosis.
Symptoms
Fever, dyspnea, cough, and chest pain are among the most common symptoms with COVID-19 myocarditis.46 Additional symptoms include other types of chest discomfort, postexertional fatigue, palpitations, and syncope.47 Although symptoms may resolve within 3 months of initial diagnosis, persistence of symptoms for greater than 12 months has been reported.48
Severity
Symptomatology and hemodynamics should be used to help define myocarditis severity. Although historically categorized as asymptomatic, mild, moderate, and fulminant,49 assessment may alternatively be made with a 4-grade symptom scale for chest pain,50 dyspnea,51 and functional status.52 Importantly, symptom intensity does not correlate particularly well with left ventricular ejection fraction (LVEF), nor with levels of cTn, natriuretic peptides, and C-reactive protein. Classic findings on CMR include increased native T1 (fibrosis or inflammation) and T2 (inflammation or edema) signals along with nonischemic LGE.
Although fulminant myocarditis with cardiogenic shock can occur with COVID-19,53, 54, 55 distributive shock resulting from sepsis and/or a hyperinflammatory state can occur as well. In a systematic review of 41 cases of myocarditis associated with COVID-19, cardiogenic shock and distributive shock were noted in 27% and 12% of individuals, respectively.46 Of note, these rates exceed that seen with viral myocarditis unrelated to COVID-19.56
6.1.3. Epidemiology
Incidence and Prevalence
Historically, viral infection has been regarded as the most common cause of myocarditis. Its reported prevalence is variable and has been estimated at 10 to 106 cases per 100,000 people worldwide, with an annual incidence of 1.8 million.57 With COVID-19, the true incidence of myocarditis has proven challenging to assess because of ambiguous definitions, evaluation of nonrepresentative populations, and lack of systematic data collection. Although a recent population-based study of young adults (aged <20 years) from 48 U.S. health care organizations estimated the incidence of myocarditis with COVID-19 at about 450 per million,22 prospective and retrospective studies of hospitalized patients, autopsy data, and CMR suggest that the overall incidence is higher. Fortunately, fulminant myocarditis appears to be quite rare.53, 54, 55
Echocardiographic data from prospective, cross-sectional studies of patients hospitalized for COVID-19 suggest that myocardial dysfunction may be present in up to 40%.58 , 59 This can range from abnormal ventricular strain to overt left and right ventricular systolic dysfunction.60 Although the latter may be global, wall motion abnormalities with myocarditis are classically segmental in a noncoronary distribution. Pericardial effusion and thickening may also be seen (especially in cases of myopericarditis); however, data on their frequency and degree of overlap with myocarditis are limited.61 In selected studies, these echocardiographic findings correlate with cTn levels10 and risk of mortality.59 , 62 The underlying mechanisms for this (eg, myocarditis, demand ischemia, stress cardiomyopathy, right ventricular pressure overload), however, are not always clearly defined.
CMR is the most sensitive imaging modality for identifying myocardial (and pericardial) involvement. It has been used in several studies to evaluate symptomatic and asymptomatic individuals with COVID-19, in both hospital and ambulatory settings. In a study of 100 patients (33% hospitalized) imaged a median of 71 days after testing positive for COVID-19, nonischemic LGE was found in 20% and prolonged native T1 and T2 relaxation times in 73% and 60%, respectively.11 Similar findings have been observed in other CMR studies, with variable degrees of LGE and mapping abnormalities in those convalescing from COVID-19.19 , 63, 64, 65, 66, 67 When performed in athletes as part of a RTP screening protocol, various abnormalities have been noted, with 0.6%-3% of subjects meeting modified Lake Louise criteria for clinical myocarditis.43 , 68 , 69 Variability in observed findings with CMR likely reflect differences in the populations studied, timing relative to infection onset, and the specific imaging protocols and interpretations used. It is likely, however, that MIS-C represents an important subset, with limited case series demonstrating widely varying rates of imaging abnormalities suggestive of myocarditis (0%-51%).20 , 21 , 70, 71, 72
Autopsy findings among those with COVID-19 have also varied. In a study of 277 cardiac autopsies from 22 studies, classic myocarditis was identified in 7.2%, nonmyocarditis inflammatory infiltrate in 12.6%, single-cell ischemia in 13.7%, and acute myocardial infarction in 4.7%.25 At least one cardiovascular histopathologic abnormality (eg, macrovascular or microvascular thrombus, inflammation, or intraluminal megakaryocytes) was noted in 47.8%. Collectively, these data suggest that although fulminant myocarditis as a cause of death is rare (using historical histopathologic criteria), nonspecific cardiac inflammation and/or injury is not.
Risk Factors
Identification of risk factors for myocarditis with COVID-19 is still an area of active investigation. In general, viral myocarditis unrelated to SARS-CoV-2 is more common in men. Although a similar pattern has been observed in those with COVID-19, one cannot exclude the possibility of selection bias. In a large review of 38 patients with myocarditis associated with SARS-CoV-2 infection, however, 26 (68%) were men.54 Male predominance has also been observed in studies of myocarditis in athletes,43 , 68 , 73 , 74 MIS-C,20 , 21 , 70, 71, 72 and myocarditis following vaccination for COVID-19.12 By comparison, a greater number of risk factors exist for myocardial injury with COVID-19, such as advanced age, male sex, underlying cardiovascular disease, obesity, diabetes mellitus, hypertension, immunosuppression, and severe systemic disease.33, 34, 35
6.1.4. Mechanisms
Viral-induced myocarditis has historically been defined by three phases: acute viral exposure with an innate immune response (<1 week); activation of an acquired immune response with cytokine and chemokine release (1-4 weeks); and disease progression with clearance of the virus and development of fibrosis, remodeling, and cardiomyopathy (>4 weeks).75 , 76 Although cases of myocarditis following SARS-CoV-2 infection are rare, they largely follow this pattern, with a delay in onset after viral infection of days to weeks. For some individuals with myocarditis, elevation of cTn and abnormalities on CMR may be observed early after infection; much more often, however, there is delayed onset of cardiac symptoms, elevation of cardiac biomarkers, and abnormalities on cardiac imaging. Because of variation in how these patients are evaluated and varying sensitivity thresholds of the different imaging methods used, the exact incidence of myocarditis with COVID-19 remains unclear.
Investigators have proposed several mechanisms by which SARS-CoV-2 may contribute to myocarditis and other forms of myocardial involvement, including direct virus invasion, host inflammatory or immune responses, and microvascular angiopathy. Emerging data indicate that a maladaptive host immune response occurs, with excessive activation of innate immune pathways, a surge of proinflammatory cytokines, deregulated thrombo-inflammation, thrombotic microangiopathy, endothelial dysfunction, and even molecular mimicry.77, 78, 79, 80 Other hypothesized contributing mechanisms include demand ischemia, stress cardiomyopathy, and hypoxia-induced myocardial injury.81 Although baseline comorbidities, including metabolic syndrome, hypertension, and cardiovascular disease, may potentiate these effects along with subsequent adverse outcomes, they are not required for cardiovascular involvement, as seen in MIS-C.
Even though SARS-CoV-2 mRNA has been detected in the myocardium of 25%-50% of COVID-19 patients undergoing autopsy, the virus is largely found in pericytes and within the subendothelium, not within cardiac myocytes. Autopsy findings have also largely demonstrated the absence of diffuse lymphocytic myocarditis or confluent myocyte necrosis—findings typically observed with other forms of viral myocarditis.25 , 82, 83, 84, 85 Although lymphocytic infiltration has been noted on endomyocardial biopsy of some patients with myocardial involvement by CMR,11 it is more often characterized by increased CD68+ macrophage or monocytic infiltration, endothelialitis, microvascular dysfunction, and individual cell necrosis.44 , 45 , 79 , 83 , 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102 Importantly, CMR has also provided additional insights with the identification of nonischemic myocardial and pericardial LGE, along with prolonged native T1 and T2 relaxation times among some in this population.11 , 63, 64, 65, 66, 67
6.1.5. Evaluation
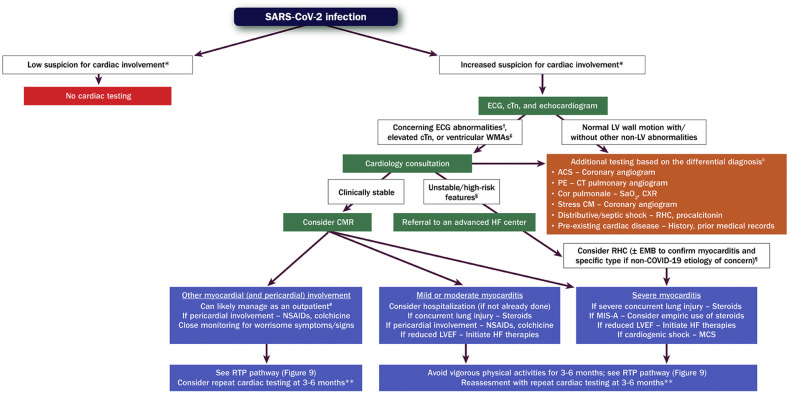
Cognizant of 1) the spectrum of myocardial involvement that may be seen with COVID-19; 2) the timing of evaluation relative to SARS-CoV-2 infection; and 3) the range of diagnostic tests available, the writing committee proposes a multiparametric approach to help guide decision-making (see Figure 3 ). This is limited, however, by a paucity of evidence about how best to diagnose and manage myocarditis and other forms of myocardial involvement with COVID-19.31
Figure 3.
Evaluation and Management of Patients With Suspected Myocarditis or Myocardial Involvement
Green boxes = cardiac testing for evaluation of myocarditis/myocardial involvement. Orange box = other cardiac/noncardiac testing. Purple boxes = management. ∗Informed by symptoms suggestive of cardiac involvement, including chest pain/pressure, dyspnea, palpitations, and syncope. †Includes diffuse T-wave inversion, ST-segment elevation without reciprocal ST-segment depression, and prolongation of the QRS complex duration. ‡Often in a noncoronary distribution; may also include abnormal ventricular strain. §Includes hypotension, cardiogenic shock, sustained ventricular arrhythmias, and/or advanced atrioventricular block. ‖This is an incomplete list of potential etiologies. ¶Testing for viral genomes should be performed on frozen heart tissue to exclude other causes of myocarditis, if possible. #Assumes chest pain is the only symptom, LV systolic function is preserved, and there are no ventricular arrhythmias. ∗∗Includes an ECG, echocardiogram, ambulatory rhythm monitor, and CMR. ACS = acute coronary syndrome; CM = cardiomyopathy; CMR = cardiac magnetic resonance imaging; COVID-19 = novel coronavirus disease 2019; CT = computed tomography; cTn = cardiac troponin; CXR = chest X-ray; ECG = electrocardiogram; EMB = endomyocardial biopsy; HF = heart failure; LV = left ventricular; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; MIS-A = multisystem inflammatory syndrome in adults; NSAIDs = nonsteroidal anti-inflammatory drugs; PE = pulmonary embolism; RHC = right heart catheterization; SaO2 = arterial oxygen saturation; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; WMAs = wall motion abnormalities.
Among patients with SARS-CoV-2 infection, pre-existing cardiovascular disease and other cardiovascular and noncardiovascular risk factors (eg, advanced age, male sex, immunosuppression) represent important determinants of clinical presentation.53 , 65 , 81 , 103 , 104 When suspicion for cardiac involvement is low, no further cardiac testing is generally recommended. In contrast, when suspicion for cardiac involvement is moderate or high, initial testing should generally consist of an electrocardiogram (ECG), measurement of cTn (preferably using a high-sensitivity assay), and an echocardiogram (transthoracic or point-of-care ultrasound). Because patients hospitalized with an elevated cTn face higher risk of adverse outcomes,34 closer monitoring for potential deterioration is usually warranted.
In those with a rising cTn, ECG abnormalities concerning for myocarditis (eg, diffuse T-wave inversion, ST-segment elevation without reciprocal ST-segment depression, prolongation of the QRS complex duration), and/or echocardiographic abnormalities concerning for myocarditis (eg, ventricular wall motion abnormalities often in a noncoronary distribution, abnormal ventricular strain),105 cardiology consultation is recommended. Importantly, the presence of normal LV wall motion and/or other non-LV echocardiographic abnormalities should invite testing for other conditions, informed by the presentation (eg, coronary angiography for suspected acute coronary syndrome, computerized tomography (CT) pulmonary angiography for suspected pulmonary embolism).
In those with suspected myocardial involvement, CMR is recommended if the patient is hemodynamically stable. In symptomatic patients, CMR is the most sensitive method to exclude ischemia and preexisting cardiomyopathies106 while confirming cardiac changes due to SARS-CoV-2 infection, including myocardial inflammation, nonischemic epicardial scar, and pericardial effusion/enhancement, all of which are demonstrable after 4 weeks.106 , 107 For patients who are unstable or exhibit high-risk features (eg, hypotension, ventricular arrhythmias), evaluation at an advanced heart failure center is recommended. Endomyocardial biopsy should be considered in those with clinical deterioration, particularly if heart block or ventricular arrhythmias are present and obstructive coronary artery disease has been excluded.83 , 108, 109, 110
6.1.6. Management
Management of patients with myocardial involvement in the setting of COVID-19 is primarily dictated by the clinical presentation and course. Because myocarditis is associated with a heightened risk of cardiac complications early in the disease course,34 , 111 a proactive management plan should be in place.
Asymptomatic (Subclinical) Myocardial Involvement
Asymptomatic myocardial involvement has been reported following SARS-CoV-2 infection (eg, following CMR screening of athletes as part of a RTP protocol). Because most asymptomatic individuals do not undergo cardiac testing, however, this group is likely to be quite small. Recognizing that the long-term consequences of this condition are not known, it is still reasonable to manage these individuals expectantly, with instruction to share any worrisome symptoms or signs (eg, chest pain, shortness of breath, syncope, edema) should they occur. For those interested in resuming physical activity, further recommendations are provided in the RTP section.
Symptomatic Myocardial Involvement and Myocarditis
The treatment approach for symptomatic myocardial involvement in the setting of COVID-19 greatly depends upon the patient’s clinical presentation, as well as the severity of abnormalities noted on testing. Acknowledging the potential for overlap between some forms of myocardial involvement and possible myocarditis, patients who have chest pain as their only symptom, preserved LV systolic function, and no ventricular arrhythmias can likely be managed in the ambulatory setting with close monitoring for worrisome symptoms or signs (eg, shortness of breath, syncope, edema). Consideration should be given to follow-up testing (eg, ECG, echocardiogram, ambulatory rhythm monitor, CMR) 3-6 months after presentation, particularly in those with ongoing cardiac symptoms and/or findings suggestive of significant/worsening myocardial involvement.
In those with definite myocarditis that is either mild or moderate in severity, hospitalization is recommended (if not already done), ideally at an advanced heart failure center. Those with a fulminant course (eg, those with cardiogenic shock, sustained ventricular arrhythmias, and/or advanced atrioventricular block) should be managed similar to other forms of cardiogenic shock at centers with expertise in advanced heart failure, mechanical circulatory support (including use of V-A extracorporeal membrane oxygenation), and other advanced therapies.112 , 113
Further decision-making about treatment depends upon whether concurrent pneumonia is present. In general, patients with myocarditis and COVID-19 pneumonia with ongoing need for supplemental oxygen should be treated with corticosteroids.114 For those with associated pericardial involvement, nonsteroidal anti-inflammatory drugs may be used to help alleviate chest pain and inflammation.115 Low-dose colchicine or prednisone may be added for persistent chest pain, with a plan to taper the dose based on symptoms and clinical findings.
Treatment of myocarditis in stable patients should be based on standard pathways unrelated to COVID-19. A low-dose beta-blocker along with a renin-angiotensin-aldosterone system inhibitor may be used empirically in patients with mildly reduced LV systolic function and stable hemodynamics. A beta-blocker may also be helpful in patients with supraventricular arrhythmia, if hemodynamically stable. In fact, treatment with intravenous metoprolol tartrate was observed to improve respiratory status in a small, randomized trial of patients with COVID-19 and acute respiratory distress syndrome requiring mechanical ventilation.116 Beta-blockade can, however, precipitate cardiogenic shock in patients with greater compromise of cardiac function.
Use of intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A, a hyperinflammatory state with acute heart failure and/or cardiogenic shock in the absence of sepsis, as this approach was associated with a favorable prognosis in a small series.77 , 117 MIS-A is often associated with delayed-onset myocarditis, and affected individuals may have high levels of inflammatory biomarkers and ferritin.80 Although similar presentations have been observed in children (MIS-C),118 a review of treatment approaches for this condition is beyond the scope of this ECDP.
Empiric use of immunosuppressive therapy (eg, corticosteroids) may also be considered in those with biopsy evidence of severe myocardial inflammatory infiltrates or fulminant myocarditis, balanced against infection risk.113 It is important to note, however, that these biopsy findings are very rare in COVID-19, and the magnitude of benefit from such immune-modifying therapy is not fully known.45
Once a patient with cardiogenic shock or hemodynamic instability from suspected myocarditis has stabilized, CMR should be performed (before hospital discharge) to confirm the diagnosis and assess the extent of ventricular dysfunction and inflammation. Guideline-directed medical therapy for heart failure should be initiated before discharge and titrated as appropriate in the outpatient setting. Strenuous physical activity should be avoided for 3-6 months, as outlined in the RTP section. Importantly, follow-up surveillance testing (eg, ECG, echocardiogram, ambulatory rhythm monitor, CMR) can be helpful in all patients with myocarditis to: 1) gauge recovery of cardiac function and inflammation; 2) guide heart failure management; and 3) assess prognosis.
6.1.7. Postvaccination Myocarditis
Clinical Presentation
Myocarditis following COVID-19 mRNA vaccination is an entity separate from but related to myocarditis following SARS-CoV-2 infection.119 In case reports and case series of vaccine-associated myocarditis, chest pain has been noted in the vast majority,12 occurring most commonly 2-3 days after the second mRNA vaccine dose.12 Much less often, it has been observed after the first dose.12 ST-segment elevation has been the most common ECG abnormality, and most cases have reported an elevated cTn level, peaking approximately 3 days after vaccination. Other laboratory findings include elevated natriuretic peptide and C-reactive protein levels. Although a small percentage have had an LVEF <50% by echocardiography, CMR findings suggestive of myocarditis have been present in most who were tested,120 with evidence of LGE and myocardial edema. In these cases, testing failed to identify evidence of SARS-CoV-2, other viral infection, or autoimmune disorders.12 Fortunately, most patients have had a nonfulminant course, with symptom resolution along with improved laboratory tests and imaging findings, with or without treatment.12 , 120 , 121
Epidemiology and Risk Factors
Available data from the U.S. Vaccine Adverse Event Reporting System suggest that myocarditis following COVID-19 mRNA vaccination is rare.12 , 122 As of June 11, 2021, the reported rate of myocarditis following administration of the second dose of the COVID-19 mRNA vaccine was 40.6 cases per million in male individuals aged 12-29 years and 2.4 cases per million in male individuals aged ≥30 years.123 Corresponding rates in female individuals for these age groups were 4.2 and 1.0 cases per million after the second vaccine dose, respectively. Male individuals aged 12−17 years and 18−24 years had the highest reported rates of postvaccination myocarditis, with 62.8 and 50.5 cases per million following the second vaccine dose, respectively. Among 323 individuals <30 years of age with adjudicated postvaccination myocarditis, 90% were male, with a median age of 19 years. Importantly, even though 96% of these individuals were hospitalized, most had a mild clinical course, with no reported deaths.
Similar findings have been observed in other retrospective analyses using coded clinical data to screen for cases of postvaccination myocarditis. In a study that evaluated 5.1 million Israelis treated with 2 doses of a COVID-19 mRNA vaccine between December 2020 and May 2021, 136 cases of definite or probable postvaccination myocarditis were identified.124 Although most affected individuals (95%) had a mild clinical course, one individual died. Myocarditis risk in the overall population was higher after the second dose (risk difference of 18 per million) and was highest among male individuals between the ages of 16 and 19 years (risk difference of 137 per million). Consistent findings were observed in a separate study of 2.5 million Israelis who were part of a large health care organization, where the incidence of postvaccination myocarditis after at least one dose of a COVID-19 mRNA vaccine was 21 cases per million for the all-comer population and 107 cases per million for male individuals aged 16-29 years.125 Finally, a higher rate of vaccine-associated myocarditis was observed in a study of 153,438 individuals in the Kaiser Permanente health system between December 2020 and October 2021.126 After receiving a second mRNA vaccine dose, rates of myopericarditis among male individuals aged 12-17 years and 18-24 years were 377 and 537 cases per million, respectively. This was driven, in part, by identification of affected individuals through combined use of coded clinical data with specific search terms within the electronic medical record.
Accrued observational data also suggest that rates of postvaccination myocarditis may differ between the 2 mRNA vaccines (BNT162b2 [Pfizer-BioNTech] and mRNA-1273 [Moderna]), particularly after the second dose. In a population-based cohort study using individual-level data for nearly 5 million individuals in the Danish Vaccination Register, rates of postvaccination myocarditis were noted to be higher with the mRNA-1273 (Moderna) vaccine.127 Similar observations were noted in an analysis of patient-level data for more than 38 million vaccinated adults in the English National Immunisation database, where rates of postvaccination myocarditis were higher with the mRNA-1273 (Moderna) vaccine.128 Of note, a small but significantly increased risk of postvaccination myocarditis was also observed after the first dose of the adenoviral vector (non-mRNA) vaccine, ChAdOx1 (University of Oxford); however, this effect was not observed with the second dose.128
Mechanism
Although the exact mechanism of vaccine-associated myocarditis is not currently known, it has been hypothesized that molecular mimicry (between the spike protein of SARS-CoV-2 and self-antigens), autoantibody formation, triggered immune dysregulation with activation of natural killer cells, and a dysregulated cytokine and immune response to mRNA may all play a role.12 , 129 , 130 The observed male predominance may also be related to sex hormone differences in immune response.131
Endomyocardial biopsy has been reported in only a small number of cases of postvaccination myocarditis. In 2 cases, specimens showed inflammatory infiltrates largely consisting of T cells and macrophages, admixed with eosinophils, B cells, and plasma cells.18 In other cases, biopsy has failed to demonstrate myocardial infiltrates132 and evidence of myocarditis.133 Cases reported to date do not suggest a delayed hypersensitivity reaction, such as a serum sickness–like reaction or eosinophilic infiltrate.134 Furthermore, no cases have had associated thrombotic events, thrombocytopenia, or disseminated intravascular coagulation.12
Benefit-to-Risk Ratio
Currently approved COVID-19 mRNA vaccines are highly effective and have been shown to be safe in large-scale trials.135 , 136 Most reported systemic reactions have been mild and transient, albeit more common among younger individuals and after the second vaccine dose. Adverse cardiovascular effects in these trials have been largely isolated, with an incidence of <0.05%, and there have been no reported cases of myocarditis. Rates of hypertension, bradycardia, atrial fibrillation, acute coronary syndrome, cerebrovascular events, and heart failure have been similar between vaccine and placebo arms.
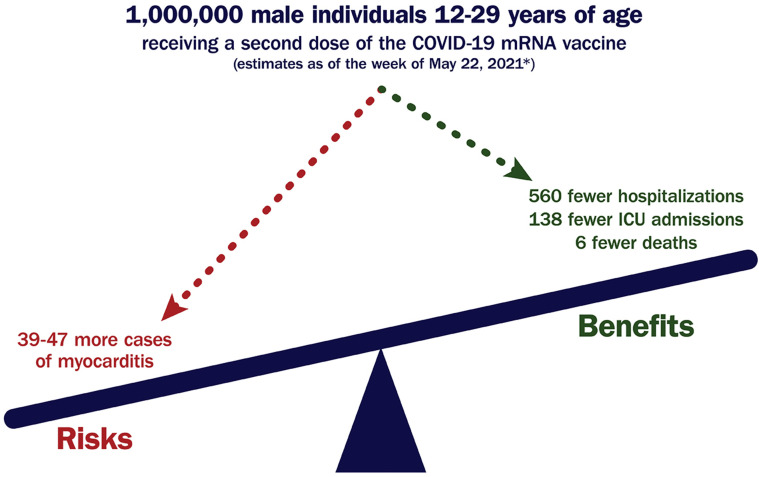
Importantly, a very favorable benefit-to-risk ratio exists with the COVID-19 vaccine for all age and sex groups evaluated thus far.12 , 121 , 123 , 128 , 137 , 138 This is particularly true when inventorying the severe risks of SARS-CoV-2 infection, including a mortality rate of 1-10 per million individuals aged 12-29 years. As of May 22, 2021, it was estimated that for every 1 million male individuals aged 12-29 years administered a second dose of the COVID-19 mRNA vaccine, approximately 39-47 cases of myocarditis would be expected, but 560 hospitalizations, 138 intensive care unit admissions, and 6 deaths would be prevented (see Figure 4 ).123 In addition, beyond helping to mitigate the risk of hospitalization and death related to SARS-CoV-2 infection, vaccination can help reduce the risk of related complications, such as myocarditis, MIS-A,139 and PASC.12 , 140
Figure 4.
Favorable Benefit-to-Risk Ratio for COVID-19 mRNA Vaccination Among Those at Highest Risk for Postvaccination Myocarditis
∗Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET).12,141 COVID-19 = novel coronavirus disease 2019; ICU = intensive care unit, mRNA = messenger RNA.
Evaluation and Management
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection (see Figure 3). Individuals presenting with chest pain early after receiving the COVID-19 mRNA vaccine should be evaluated for possible myocarditis. Initial testing should include an ECG, measurement of cTn, and an echocardiogram. If myocarditis is suspected, cardiology consultation and further testing with CMR should be performed. Evaluation for current (and potentially prior) SARS-CoV-2 infection is also recommended. Coronary angiography and endomyocardial biopsy are rarely indicated unless a significant level of concern exists for flow-limiting coronary artery disease or specific forms of myocarditis (eg, giant cell).
Patients with 1) chest pain; 2) an elevated cTn; 3) abnormal electrocardiographic, echocardiographic, and/or CMR findings; 4) concerning arrhythmias; and/or 5) hemodynamic instability should be hospitalized for close monitoring. For those with rapidly improving symptoms, a normal or improving cTn level, and a normal LVEF, anti-inflammatory medications may not be needed. Use of nonsteroidal anti-inflammatory drugs, colchicine, and corticosteroids should be considered, however, in those with ongoing symptoms. Analogous to fulminant myocarditis related to SARS-CoV-2 infection, intravenous corticosteroids, along with other forms of circulatory support, may be considered in those with LV systolic dysfunction, worsening heart failure, ventricular arrhythmias, and/or hemodynamic instability. Once clinically stable, guideline-directed medical therapy for heart failure should be initiated, with continuation at discharge. In addition, strenuous physical activity should be avoided for 3-6 months, as outlined in the RTP section.
Limited data currently exist regarding the safety of COVID-19 vaccination for individuals with a history of myocarditis or pericarditis unrelated to COVID-19 mRNA vaccines. Centers for Disease Control and Prevention (CDC) guidelines currently recommend that such individuals be vaccinated against COVID-19 after their myocarditis/pericarditis episode has completely resolved.142 In contrast, the guidelines recommend that no further doses of any COVID-19 vaccine be given to those who developed myocarditis/pericarditis following COVID-19 mRNA vaccination. The guidelines acknowledge that this decision should be informed, however, by an individual’s risk for severe COVID-19 illness.
6.1.8. Future Directions
The incidence, risk factors, mechanisms, preferred diagnostic and therapeutic approaches, and prognosis for viral-mediated and mRNA vaccine-associated myocarditis remain incompletely understood. Because of this, additional systematic, cross-sectional data are needed to describe the frequency, type, and severity of myocarditis and myocardial involvement observed. Randomized trials are also needed to better understand the preferred means to test and treat patients with myocarditis related both to SARS-CoV-2 infection and mRNA vaccination. Although randomized trials in myocarditis have been challenging historically, the current pandemic has created unique opportunities for further investigation.
6.2. Section 2: Post-acute Sequelae of SARS-CoV-2 Infection
6.2.1. Overview
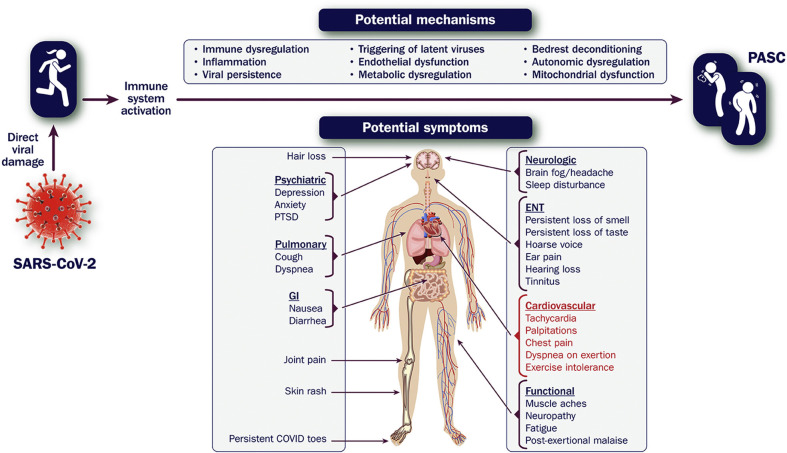
PASC is a term used to describe a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after SARS-CoV-2 infection.15 Patients with this condition commonly experience wide-ranging symptoms, including fatigue, cognitive dysfunction, sleep disturbance, and exercise intolerance. Reported symptoms encompass nearly every organ system, with varying impacts on quality of life (see Figure 5 ).
Figure 5.
Symptoms of PASC and Potential Mechanisms
COVID-19 = novel coronavirus disease 2019; ENT = ear, nose, and throat; GI = gastrointestinal; PASC = post-acute sequelae of SARS-CoV-2 infection; PTSD = posttraumatic stress disorder; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Chest pain, dyspnea, and palpitations represent some of the key symptoms that draw attention to the cardiovascular system. Affected individuals may also experience elevated blood pressure, tachycardia out of proportion to that expected for effort, and/or drops in oxygen saturation. Given that the pathophysiology of PASC is poorly understood and the underlying drivers may be heterogeneous, a unifying explanation is often lacking. Added to this is a paucity of data to help guide diagnosis, treatment, and prognosis.
Cognizant of this, we nonetheless propose a systematic approach to assist in the evaluation and management of PASC, recognizing that the evidence base will evolve over time. Importantly, patient-centered care models are needed to optimally address this condition, with coordination by multidisciplinary teams that include primary care clinicians, specialists (eg, pulmonologists, cardiologists, neurologists, rheumatologists, psychiatrists, infectious disease experts), social workers, psychologists, and physical therapists.
6.2.2. Definition
PASC is a heterogeneous disorder without a universally accepted definition for its widely varying presentations. Patients with PASC have symptoms that present after SARS-CoV-2 infection, usually persisting for 4-12 weeks and beyond.143 , 144 Although some groups have differentiated an earlier “postacute period” (4-12 weeks after acute infection) from a later “chronic period” (>12 weeks after acute infection), it is not clear whether this distinction is important as it relates to the underlying mechanisms, evaluation, or management and whether symptoms that extend even beyond 12 months should be considered separately.111 , 145 , 146 The United Kingdom’s National Institute for Health Care and Excellence has defined PASC as a condition in which symptoms and signs developing during or after an infection consistent with COVID-19 continue for more than 12 weeks and are not explained by an alternative diagnosis.147 This definition mirrors that of the World Health Organization, which defines PASC as a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection (usually 3 months from the onset of COVID-19), with symptoms that last for at least 2 months that are not explained by an alternative diagnosis.14 The CDC uses an earlier timepoint and defines PASC as a wide range of new, returning, or ongoing health problems that are present 4 or more weeks after first being infected with SARS-CoV-2.15 Irrespective of the definition used, it is important to realize that individuals without symptoms in the days or weeks after infection can still develop PASC.15
Details related to a patient’s acute illness can help place persistent symptoms into context and identify alternative diagnoses. For example, those with extensive lung injury, requirement for mechanical ventilation, and/or serious cardiovascular complications may experience protracted sequelae related to the initial insult. Some patients may experience postintensive care syndrome, which includes physical, cognitive, and mental health problems that can either be directly or indirectly related to critical illness from SARS-CoV-2 infection.148 Similarly, persistence and/or marked worsening of symptoms (eg, fever, lightheadedness, chest pain, shortness of breath, gastrointestinal symptoms) throughout the early post-acute period may suggest a hyperinflammatory state, such as MIS-A.149 , 150
PASC may occur in individuals with widely varying initial presentations, ranging from asymptomatic infection to critical illness.111 Debilitating PASC symptoms have been reported in individuals with mild SARS-CoV-2 infection without underlying cardiopulmonary disease, resulting in declines in health status and quality of life that have affected their ability to return to work, participate in daily activities, or exercise.111 , 151, 152, 153 Cardiac evaluation in these patients may also fail to identify a specific etiology for their reported symptoms.154, 155, 156, 157, 158 Collectively, this can be a source of tremendous frustration for patients and clinicians alike and may lead to significant variation in care.
6.2.3. Framework
To help provide clarity as to the potential etiology of cardiovascular symptoms with PASC, the writing committee proposes a taxonomy that differentiates known cardiovascular disease entities that can present during the early post-acute or chronic periods of COVID-19 (PASC-CVD) from cardiovascular symptoms that extend beyond the acute infection and are not fully explained by initial testing (PASC-CVS). Differentiating PASC-CVD from PASC-CVS can help direct appropriate referrals and establish pathways for efficient evaluation and management. Moreover, focused attention on these conditions can accelerate research and educational efforts aimed at improving outcomes.
Distinguishing between PASC-CVD and PASC-CVS may depend, in part, on the testing approach taken and the findings that follow. Of note, identified abnormalities may or may not relate to reported symptoms. Several echocardiographic studies have shown limited structural and functional abnormalities in those who have recovered from their acute illness 159, 160, 161 and in those with persistent cardiopulmonary symptoms suggestive of PASC. In a study of 145 patients recovering from COVID-19 (41% of whom had persistent symptoms) 60 and 100 days after infection, diastolic dysfunction was detected in 60% and 55%, respectively; LV systolic dysfunction, however, was detected in only 4 patients.160 In a separate study of 47 patients convalescing from COVID-19 (an average of 67 days after diagnosis), CMR and FDG-PET were performed.19 Nineteen patients (40%) had ongoing cardiac symptoms, and 8 (17%) were noted to have myocardial inflammation by FDG-PET that correlated with CMR abnormalities and laboratory markers of inflammation. Although cardiac symptoms were approximately twice as common in individuals with abnormalities on FDG-PET, this was not statistically significant. Recognizing that different imaging studies may identify less overt forms of underlying myocardial involvement,11 , 63 , 157 it is not always clear whether identified abnormalities relate to prior SARS-CoV-2 infection, a different disease process, or an incidental finding unrelated to the current presentation.
PASC-CVD
PASC-CVD refers to a broad group of cardiovascular conditions that manifest ≥4 weeks after SARS-CoV-2 infection. Timing, however, may vary depending upon initial illness severity. PASC-CVD includes, but is not limited to, myocarditis and other forms of myocardial involvement, pericarditis, new or worsening myocardial ischemia due to obstructive coronary artery disease, microvascular dysfunction, nonischemic cardiomyopathy with involvement of the left and/or right ventricles, thromboembolism, cardiovascular sequelae of pulmonary disease (eg, pulmonary hypertension, right ventricular failure), and arrhythmia (eg, atrial fibrillation, premature ventricular contractions, nonsustained ventricular tachycardia).
Discerning whether PASC-CVD began with acute infection, during illness resolution, or as a new condition postrecovery can be challenging. This is, in part, related to variability in the timing of clinical presentation and the type of diagnostic testing performed. Self-isolation may further limit early detection; moreover, some patients may choose to defer care, with the expectation that any lingering symptoms will improve over time. Where possible, evaluation and management strategies for the most common PASC-CVD entities (eg, cardiomyopathy, ischemic heart disease, arrhythmia) should follow existing guideline recommendations.
PASC-CVS
PASC-CVS is a heterogeneous disorder that includes widely ranging cardiovascular symptoms, without objective evidence of cardiovascular disease using standard diagnostic tests. Exercise intolerance and tachycardia are two of the most common reported symptoms; others include chest pain and dyspnea, with or without exercise intolerance. Additional accompanying symptoms include fatigue; cognitive complaints, including memory impairment, attention deficit, and poor executive function (frequently described as brain fog);162 sleep disturbance or nonrestorative sleep; and postexertional malaise.
There are no established timelines for when a diagnosis of PASC-CVS should be considered. Importantly, its diagnosis should balance the potential benefits of early detection, diagnosis, and intervention against the pitfalls of unnecessary testing and medicalization of people who would otherwise recover without intervention. PASC-CVS should be considered when cardiovascular symptoms persist beyond a timeframe typical for the severity of acute infection and projected recovery based on a person’s age and underlying health status. For example, it may be reasonable to consider PASC when symptoms persist beyond 4 weeks after mild acute infection, as opposed to waiting more than 12 weeks as suggested in the National Institute for Health and Care Excellence and World Health Organization definitions.
Associated Conditions
Patients with PASC-CVS may meet criteria for other established syndromes. Debate currently exists, however, as to whether these other conditions fully characterize the experience of patients with PASC-CVS phenotypes.
Postural Orthostatic Tachycardia Syndrome
Orthostatic intolerance with excessive tachycardia upon standing may reflect postural orthostatic tachycardia syndrome (POTS).163 Patients with POTS generally have a heart rate >30 beats per minute above the supine heart rate after 5-10 minutes of quiet standing (and frequently >120 beats per minute) in the absence of orthostatic hypotension. Other postural symptoms are common, including palpitations, lightheadedness, weakness, fatigue, blurry vision, and exercise intolerance.164 In a study of 152 patients with POTS predating COVID-19, 42% of cases were preceded by a viral infection.165 Although much remains to be learned about the relationship between POTS and PASC,166 it is important to recognize that other forms of tachycardia (eg, sinus, inappropriate sinus) may be commonly found.167
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
The clustering of exercise intolerance with symptoms of fatigue, postexertional malaise, and brain fog has prompted comparison of PASC to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).168 ME/CFS is defined by a triad of symptoms: 1) substantial impairment in the ability to function at home or at work, lasting for more than 6 months, accompanied by profound fatigue of new or definite onset (but not lifelong) that is not appreciably alleviated by rest; 2) postexertional malaise; and 3) unrefreshing sleep.169 Patients must also have either orthostatic intolerance or cognitive impairment.169 Although ME/CFS has been reported after infection (eg, Epstein-Barr virus, cytomegalovirus, human herpesvirus 6 and 8, Borrelia burgdorferi), a direct link between infection and prolonged symptoms is usually lacking. Even though some have suggested that ME/CFS and PASC share a common pathophysiology (including immune and metabolic system dysregulation), ME/CFS still remains poorly understood, with relatively few practitioners experienced in diagnosing and treating it.
6.2.4. Epidemiology
Data suggest that as many as 10%-30% of individuals may experience prolonged symptoms following SARS-CoV-2 infection, a subset of which may relate to the cardiovascular system.9 In a community sample of >500,000 U.K. residents participating in the REACT-2 (Real-Time Assessment of Community Transmission-2) study, one-third of those self-reporting a history of COVID-19 noted at least one symptom (such as shortness of breath, chest pain, or tiredness) and nearly 15% experienced 3 or more symptoms lasting 12 weeks or longer.153 Similarly, in a study of Swedish health care workers, 10% reported persistent symptoms more than 8 months following mild infection, with dyspnea and palpitations among the top 10 symptoms reported.151 Finally, in a study of patients hospitalized for COVID-19 in Hong Kong, 10% reported tachycardia at 6 months, with no significant change at 12 months.146
Further insight comes from an online survey conducted by a self-organized group of citizen scientists attempting to better understand the long-term sequalae of COVID-19.152 More than 3,700 self-described COVID-19 long haulers from 56 countries participated in the survey, most of whom were White women between the ages of 30 and 60 years. Only 8% were hospitalized for COVID-19, but >90% reported symptoms persisting beyond 35 weeks and one-half were unable to return to work at 6 months. Although fatigue, breathing difficulties, and cognitive impairment were the most common symptoms, 85% self-reported cardiovascular symptoms (eg, chest pain/burning, palpitations, tachycardia). Among those with tachycardia (n = 2,308), 73% were able to measure their heart rate and 52% noted a >30 beats per minute increase in heart rate with standing. Similar observations come from a separate survey of more than 5,000 individuals with prior COVID-19, where an average of 21 symptoms were reported >3 weeks after infection, with palpitations or persistent chest pain/pressure noted by more than one-third.170
Importantly, these studies have major design limitations, including response bias and lack of a control group. Moreover, few studies have assessed important nuances in symptom presentation that may suggest specific cardiovascular diagnoses or syndromes. For example, in a large observational study of 3,597 U.S. collegiate athletes, cardiovascular diagnoses (consistent with PASC-CVD) were found in a minority of those with persistent exertional chest pain or shortness of breath and in none with exercise intolerance.171 Similarly, in a study of 126 consecutive patients with PASC evaluated in a cardiology clinic for cardiovascular symptoms, only 23% were found to have a corresponding cardiovascular diagnosis.158 Because most had symptoms that could not be attributed to cardiovascular disease, there is a pressing need to more fully research this condition. It is anticipated that the ongoing multicenter, prospective CDC study that is comparing long-term outcomes of symptomatic adults who test positive for SARS-CoV-2 to those who test negative will help shed light on persistent symptom prevalence, duration, and trajectory.172
6.2.5. Mechanisms
Multiple mechanistic underpinnings for PASC-CVS have been proposed, including inflammation,173 immune activation,173 , 174 viral persistence,175 triggering of latent viruses,176 endothelial dysfunction,177 , 178 impaired exercise metabolism,179 and profound cardiac deconditioning following viral infection.180 , 181 Importantly, some or all of these may be at play within a given patient. Rather than focusing on a specific mechanism (which may be a challenge to identify and address), we present some of the most common cardiovascular symptoms and mechanisms that may underlie them.
Tachycardia and exercise intolerance
Under normal conditions, heart rate is determined by 3 factors: 1) intrinsic heart rate, 2) parasympathetic (vagal) neural tone, and 3) sympathetic (adrenergic) neural tone. Multiple central regulatory pathway inputs influence neural control of heart rate, both at rest and during exercise. This includes arterial and cardiac baroreceptors, feed-forward influences (central command), and feedback from group III mechanoreceptors and group IV metaboreceptors within skeletal muscle. Collectively, these inputs precisely regulate oxygen delivery, response of cardiac output to exercise, and changes in metabolic demand. Because heart rate and cardiac output are so tightly regulated, sinus tachycardia is usually an appropriate compensatory response and rarely reflects dysautonomia.166 , 182, 183, 184
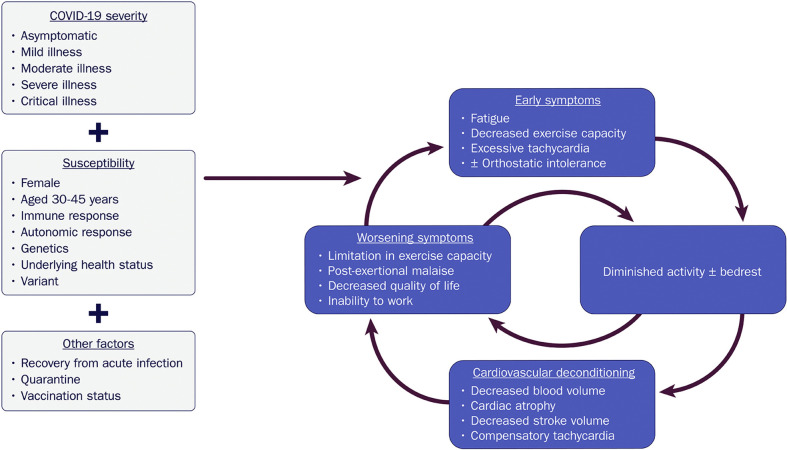
Fatigue and exercise intolerance may have several underlying causes, including alterations in immune activity and metabolism. Identification of these changes in clinical settings, however, can be challenging. Deconditioning represents a final common pathway that can either incite or exacerbate these symptoms, triggered by as little as 20 hours of bedrest and a sudden decline in physical activity from baseline.181 , 185, 186, 187 Reduced plasma volume and secondary cardiac atrophy may ensue,183 with a shift in the LV pressure-volume curve (because of hypovolemia). This results in a reduced stroke volume at any degree of orthostatic stress and ultimately, compensatory tachycardia.182 , 183 , 188
Although some degree of deconditioning with moderate and severe illness may be unavoidable, it is unclear why limited durations of bedrest and modest physical inactivity in those who are asymptomatic or with mild COVID-19 illness result in similar degrees of deconditioning.181 In a study of 247 patients with COVID-19 who isolated at home, fatigue and dyspnea were reported by 30% and 15%, respectively, at 6 months.189 Underlying drivers for this include an enhanced hyperadrenergic state, volume dysregulation, mast cell activation, peripheral autonomic neuropathy, and/or autonomic dysfunction.166 Regardless of the inciting cause, once symptoms develop, there is the potential for rapid decompensation in a downward spiral, where short periods of bedrest produce orthostatic/exercise intolerance and tachycardia, leading to further inactivity, worsening of cardiovascular deconditioning, and even more debilitating symptoms (see Figure 6 ).164 In fact, this is considered to be one of the key mechanisms underlying POTS.184
Figure 6.
Downward Spiral of Deconditioning: A Potential Mechanism of Exercise Intolerance and Excessive Tachycardia in COVID-19
COVID-19 = novel coronavirus disease 2019.
Importantly, although exercise therapy is essential to recovery, standard exercise involving upright activity (eg, walking, jogging) may worsen symptoms of PASC-CVS, further prolonging recovery. This is because upright activity perpetuates the physiology of bedrest deconditioning,181 including decreased blood volume, decreased venous return, and cardiac atrophy, with accompanying vagal withdrawal and sympathetic activation. Acknowledging that other mechanisms for fatigue, exercise intolerance, and excessive tachycardia likely exist, the construct of bedrest deconditioning provides a unifying explanation for these symptoms, as well as a paradigm to focus recovery.
Chest pain
Multiple factors may contribute to chest pain (or other types of chest discomfort) with PASC-CVS in the absence of known forms of PASC-CVD. Vascular endothelial injury may result directly from SARS-CoV-2 infection or indirectly from an exaggerated immune response. Endothelial dysfunction resulting from acute infection or ongoing inflammation may also increase the potential for coronary vasospasm.177 , 190 , 191 In a study of 11 PASC patients with angina and suspected ischemia, invasive coronary vasomotion testing revealed abnormal endothelial-dependent responses to acetylcholine in 82%; this was driven by a mix of endothelial-dependent and endothelial-independent (smooth muscle) effects.192 Other potential contributors include myocardial and/or pericardial disease that may have gone undetected with initial testing. Finally, chest pain is a common feature of POTS and ME/CFS; the underlying mechanisms for this, however, remain poorly understood.
Dyspnea
Shortness of breath, cough, and pleuritic chest pain represent commonly reported symptoms with PASC. Some may also note inability to take a full breath or air hunger with exertion, prompting interruption of ambulation on flat ground and/or basic activities of daily living.
Although dyspnea is common in patients with myocardial ischemia, heart failure, and arrhythmia, it may also be seen in those with pulmonary complications following SARS-CoV-2 infection. These include: 1) pulmonary embolism, especially if dyspnea is accompanied by exercise-induced oxygen desaturation, tachycardia, and/or presyncope/syncope; 2) pneumonia; 3) impaired diffusion capacity for carbon monoxide; 4) pulmonary fibrosis; 5) neuromuscular weakness; 6) new or worsening asthma; and 7) bronchial hyperreactivity due to pulmonary vascular inflammation. Among patients hospitalized for COVID-19, lung function impairment is common 6 months after discharge.33 , 193 , 194
Unexplained dyspnea (in the absence of cardiopulmonary abnormalities) may also be seen with PASC and may relate to deconditioning with poor cardiovascular fitness. In a study of 70 patients with COVID-19, 41 (59%) had persistent dyspnea at 3 months.195 Although women were more likely to experience dyspnea, there was no association with age, baseline comorbidities, hospitalization for COVID-19, echocardiographic findings, or cardiac biomarkers. On cardiopulmonary exercise testing (CPET), however, those with dyspnea had a lower peak VO2, lower VO2 at anaerobic threshold, and greater ventilatory inefficiency. Similarly, in a case-control study that compared 10 patients with unexplained dyspnea and exercise intolerance 11 months after COVID-19 to 10 age- and sex-matched controls, those with PASC had an appreciably lower peak aerobic exercise capacity.188 Patients with PASC also had lower stroke volumes, lower left-sided filling pressures, higher heart rates, lower systemic oxygen extraction, and greater ventilatory inefficiency. Finally, in a study of 41 patients with PASC and unexplained dyspnea, ventilatory abnormalities were found in 88% by CPET.196 Further studies are still needed to better understand drivers of dyspnea in this population, including assessment of pulmonary flow-volume loops during exercise to look for dynamic hyperinflation, expiratory flow limitation, and exercise-induced hypoxemia.
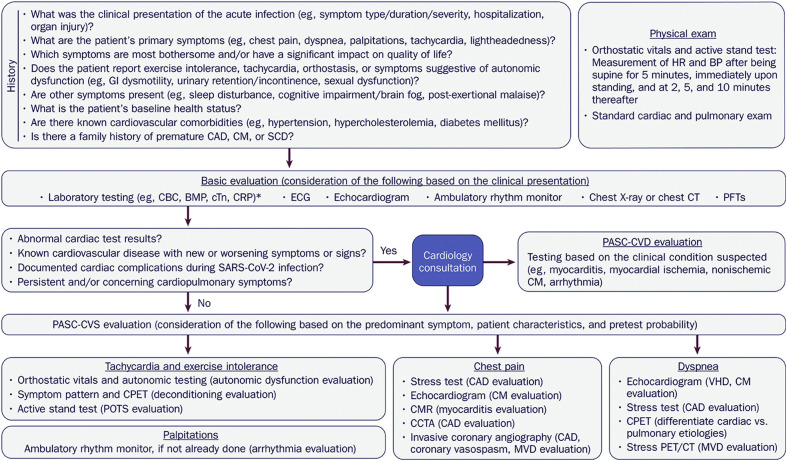
6.2.6. Evaluation
A multidisciplinary approach should be used in the evaluation of most patients with PASC. Primary care clinicians are usually the first point of contact for these patients, helping to oversee and coordinate care with other specialists, including pulmonologists, cardiologists, neurologists, rheumatologists, psychiatrists, and infectious disease experts. Within this framework, a basic cardiopulmonary evaluation can usually be performed up front to determine the need for specialty care.
Although symptom type and severity should inform diagnostic testing, a reasonable initial approach for those with cardiovascular symptoms includes: 1) basic laboratory testing (eg, complete blood count, basic metabolic panel, cTn, C-reactive protein); 2) an ECG; 3) an echocardiogram; 4) an ambulatory rhythm monitor; 5) chest imaging (X-ray and/or CT); and/or 6) pulmonary function tests. Cardiology consultation is recommended for those with: 1) abnormal cardiac test results; 2) known cardiovascular disease with new or worsening symptoms or signs (eg, worsening dyspnea in a patient with known heart failure); 3) documented cardiac complications during SARS-CoV-2 infection; and/or 4) persistent cardiopulmonary symptoms that are not otherwise explained.
As part of a PASC evaluation, cardiologists should perform a thorough history and physical examination, reviewing all relevant tests to help determine the probability of PASC-CVD. Importantly, previously unrecognized cardiac disease may only become clinically evident in the setting of acute illness. If myocarditis, pericarditis, or other myocardial involvement is suspected, further evaluation should be performed as recommended in the myocarditis section. Additional testing may also be required based on the clinical presentation (eg, CT pulmonary angiography for suspected pulmonary embolism). Ultimately, for those with persistent symptoms but without PASC-CVD, additional evaluation for PASC-CVS should be performed, dictated by: 1) the most prominent symptom(s); 2) the patient’s baseline characteristics; and 3) the pretest probability of cardiovascular disease (see Figure 7 ).
Figure 7.
Evaluation of Cardiovascular Symptoms Suggestive of PASC
∗Consideration should be given to additional laboratory testing (eg, D-dimer, B-type natriuretic peptide/N-terminal pro-B-type natriuretic peptide, thyroid function tests) based on the clinical presentation. BMP = basic metabolic panel; BP = blood pressure; CAD = coronary artery disease; CBC = complete blood count; CCTA = coronary computed tomography angiography; CM = cardiomyopathy; CMR = cardiac magnetic resonance imaging; CPET = cardiopulmonary exercise test; CRP = C-reactive protein; CT = computed tomography; cTn = cardiac troponin; CV = cardiovascular; ECG = electrocardiogram; GI = gastrointestinal; HR = heart rate; MVD = microvascular dysfunction; PASC = post-acute sequelae of SARS-CoV-2 infection; PASC-CVD = PASC-Cardiovascular Disease; PASC-CVS = PASC-Cardiovascular Syndrome; PET = positron emission tomography; PFTs = pulmonary function tests; POTS = postural orthostatic tachycardia syndrome; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SCD = sudden cardiac death; VHD = valvular heart disease.
Tachycardia and exercise intolerance
While other potential causes of tachycardia (eg, arrhythmia, heart failure, pulmonary embolism) are considered, initial outpatient testing should include a 10-minute active stand test to assess for dynamic changes in blood pressure and heart rate. This involves measurement of blood pressure and heart rate 5 minutes after being supine, immediately upon standing, and at 2, 5, and 10 minutes thereafter. Orthostatic hypotension is defined by a drop in systolic blood pressure of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing. In the absence of orthostatic hypotension, POTS is defined by an increase in heart rate of >30 beats per minute in those aged ≥19 years or >40 beats per minute in those aged <19 years and/or a heart rate of >120 beats per minute during the 10-minute active stand test. Of note, tachycardia should last for more than 30 seconds and be accompanied by symptoms. It is also important that the patient stand quietly for the full 10 minutes, as an increase in heart rate may take time. Tachycardia unrelated to position is likely due to inappropriate sinus tachycardia, especially if the heart rate does not slow at night. If feasible, a 6-minute walk test can help to assess functional capacity, while monitoring heart rate and oxygen saturation.
Ambulatory rhythm monitoring should also be considered to exclude arrhythmia and define the pattern of heart rate elevation. Whereas the latter can likely be done with a 24- to 48-hour Holter monitor, longer-duration monitoring (eg, extended Holter monitor, event monitor) should be considered in those with episodic palpitations, depending upon their reported frequency. Mobile health devices capable of heart rate and ECG monitoring can also help in evaluation and surveillance monitoring during recovery.
Even though affected individuals may have a resting heart rate that is higher than that expected for one's age and training state, and higher than that reported before COVID-19, such increases may be physiologically appropriate and should not automatically invite assumption of autonomic dysregulation. Increases in heart rate may be observed at unexpected times throughout the day or night, including during sleep and sedentary periods. Some may also note excessive tachycardia in response to minimal exercise, with greater heart rate recovery times following activity. Ultimately, although potential underlying mechanisms for persistent tachycardia (eg, increased adrenergic tone, deconditioning) should be considered, they may prove challenging to discern in most clinical settings.
Chest pain
If not previously performed, an ischemia evaluation should be conducted based on guideline recommendations.197 For low-risk patients, either coronary artery calcium testing or exercise testing without imaging is a reasonable first-line approach. For higher-risk patients, either CT coronary angiography or stress imaging (stress echocardiography, PET/single-photon emission computed tomography myocardial perfusion imaging, or CMR) should be performed, especially if there is an inability to exercise and/or the ECG is uninterpretable. If microvascular dysfunction is suspected, PET myocardial perfusion imaging can be particularly useful; when unavailable, stress imaging using echocardiography, single-photon emission computed tomography myocardial perfusion imaging, or CMR should be considered. Invasive coronary angiography should be reserved for those with abnormal noninvasive test results or those in whom there is a very strong suspicion for obstructive coronary artery disease or vasospasm.158 , 192 Invasive coronary vasomotor testing can help with evaluation of the latter; however, referral to a specialized center may be needed.
Dyspnea
Given the proclivity of SARS-CoV-2 infection to involve the lungs, initial diagnostic testing should focus on exclusion of pulmonary processes. Most commonly, this involves a chest X-ray and/or a noncontrast CT scan of the lungs, along with pulmonary function testing. If unrevealing, consideration should be given to CPET with flow-volume loops (if available) to characterize cardiac and pulmonary reserve. A CT pulmonary angiogram or ventilation-perfusion (V/Q) scan should also be considered if pulmonary embolism is suspected. Finally, an ischemia evaluation should be pursued if obstructive coronary artery disease or microvascular dysfunction is suspected.
6.2.7. Management
Most treatment recommendations related to PASC-CVD should be based on current guidelines; those related to PASC-CVS largely depend on the underlying symptom(s).
Tachycardia and exercise intolerance
For those with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, interventions normally used for POTS should be considered. The benefits of exercise training following bedrest deconditioning187 , 198 and that resulting from POTS184 , 185 , 199 are well-described. Exercise training increases cardiac mass and blood volume, improves ventricular compliance, and shifts the Frank-Starling curve upward, increasing stroke volume, improving maximal oxygen uptake, and ultimately increasing functional capacity.
To achieve these effects, however, specific types of exercise training are recommended. Importantly, such patients are usually unable to tolerate upright exercise (eg, power walking, jogging); moreover, upright exercise can worsen fatigue, resulting in postexertional malaise. This has led the U.K.’s NICE guidelines to caution against graded exercise therapy for patients recovering from COVID-19, specifically those diagnosed with ME/CFS.200 However, not all exercise is problematic.201 Instead of upright exercise, recumbent or semi-recumbent exercise (eg, rowing, swimming, or cycling) is recommended initially (see Figure 8 ), with transition to upright exercise over time as orthostatic intolerance resolves.185 , 198 , 202
Figure 8.
Sample Recumbent Exercise Therapy Prescription
∗For a more precise prescription for exercise therapy with heart rate targets and patient exercise logs, see appendix to Bryarly et al.164
In addition, exercise duration should be short initially, increasing gradually as functional capacity increases. Exercise intensity should also be at a submaximal level, sustained for the duration of exercise, with natural increases as tolerance improves. For example, patients should generally start with daily recumbent exercise for only ∼5-10 minutes at a level that allows them to speak in full sentences; thereafter, gradual increases in exercise (eg, 2 additional minutes of exercise per day each week) are recommended (see Figure 8). These principles help to avoid worsening fatigue or setbacks and usually can be applied to all patients with deconditioning, regardless of age and whether POTS is present.203 This type of program has also been shown to support long-term cardiovascular health.204 Ultimately, a formalized exercise program should be initiated. For some patients, this may be best done in a supervised setting with a physical therapist; alternatively, specific instructions may be provided on how to implement such a program at home or in a gym.164 , 199
Given the reduction in plasma volume that follows deconditioning,183, 184, 185 nonpharmacological interventions should be considered that address this pathophysiology. This includes salt and fluid loading (provided blood pressure is controlled), elevation of the head of one’s bed with 4–6-inch (10–15-cm) blocks during sleep, and use of support stockings (waist-high to ensure sufficient support of central blood volume).164 Salt loading may be achieved through liberalized sodium intake (5-10 g or 1-2 teaspoons of table salt per day); salt tablets, however, should be avoided to minimize nausea and vomiting. Patients should also be encouraged to drink 3 liters of water or an electrolyte-balanced fluid per day. Attention should be paid to avoid factors that contribute to dehydration, such as consumption of alcohol and/or caffeine, ingestion of large heavy meals, and excessive heat exposure.164
Although no pharmacological therapies are currently approved for PASC-CVS, various treatments may be used empirically. For example, if palpitations predominate, a low-dose beta-blocker (eg, bisoprolol, metoprolol, nebivolol, propranolol) or a nondihydropyridine calcium-channel blocker (eg, diltiazem, verapamil) may be added and gradually titrated to slow the heart rate. This may help to modestly improve exercise tolerance and alleviate symptoms, and patients can be weaned from these therapies as their fitness and activity improve. Even though they have not been shown to consistently improve quality of life beyond exercise training in those with POTS,205 nonselective beta-blockers that inhibit beta-2 adrenergic receptor–mediated vasodilation (eg, propranolol) may help to control debilitating symptoms in those with orthostatic intolerance with a hyperadrenergic state.206 , 207 Propranolol may also prove useful in those with coexisting anxiety or migraine.
Ivabradine has also been used in those with severe fatigue exacerbated by beta-blockers and calcium-channel blockers. Support for this approach comes from a trial of 22 patients with POTS, where improvement in heart rate and quality of life was observed following treatment with ivabradine for one month.208 Fludrocortisone (up to 0.2 mg taken at night) may also be used in conjunction with salt loading to increase blood volume and help with orthostatic intolerance; careful monitoring to guard against hypokalemia, however, is critical. Finally, midodrine (2.5-10 mg) may help with orthostatic intolerance, with the first dose taken in the morning before getting out of bed and the last dose taken no later than 4 pm.
Chest pain
For patients with chest pain without evidence of ischemia, a variety of empiric treatments may be considered. If the pain is pleuritic in nature or there is an underlying inflammatory component (eg, costochondritis), a 1- to 2-week trial of nonsteroidal anti-inflammatory drugs is reasonable, with addition of low-dose colchicine as needed. If the symptoms worsen with nonsteroidal anti-inflammatory drugs, esophagitis and esophageal spasm should be considered. For those with suspected endothelial dysfunction, a calcium-channel blocker, a long-acting nitrate, and/or ranolazine may be tried. Aspirin and a high-intensity statin represent key components in those with underlying atherosclerotic cardiovascular disease. Finally, some nutritional supplements (eg, beetroot extract, l-arginine) can increase nitric oxide and may be considered in those with persistent chest pain refractory to other therapies or in those with suspected microvascular dysfunction. Beetroot extract can be taken 1 hour before exercise to achieve maximal vasodilation, and l-arginine may be used at a dose of 4 mg twice a day.209, 210, 211
Dyspnea
For patients with dyspnea unrelated to cardiovascular disease, consideration should be given to pulmonary consultation to guide further evaluation and management. Importantly, low preload states can result in dyspnea, underscoring the importance of volume loading and recumbent exercise, as described in the tachycardia and exercise intolerance section.212 Physical therapy that incorporates aerobic training and diaphragmatic breathing exercises can be a helpful adjunct to address underlying ventilatory abnormalities and muscle dysfunction,196 , 213 as noted with other pulmonary diseases.
6.2.8. Future Directions
Both PASC-CVD and PASC-CVS may be encountered after SARS-CoV-2 infection, with varying effects on quality of life. Because much remains to be learned about these conditions,214 substantial opportunities exist to better understand their epidemiology, underlying mechanisms, and best approaches for evaluation and management.214 In addition, little is also currently known about the incidence of PASC in children, those with vaccine breakthrough infection, and those affected by newer SARS-CoV-2 variants (eg, Delta, Omicron). All of this underscores the need to conduct studies that leverage rigorous data collection and precise enrollment to define best practices for distinct phenotypes.172 , 215 , 216 Given that the total cost of PASC to patients and their families, the health care system, payers, and society at large is likely to be substantial, there is a critical need to identify ways to better meet the needs of those with this condition.
6.3. Section 3: Return to Play
6.3.1. Overview
Observation of myocardial injury among patients hospitalized with COVID-19,33 , 217 , 218 coupled with uncertainty around cardiovascular sequalae after mild illness,11 fueled early apprehension regarding the safety of competitive sports for athletes recovering from SARS-CoV-2 infection. Several consensus screening recommendations have followed,219 , 220 including 2 by the ACC’s Sports and Exercise Cardiology Council,221 , 222 endorsing a conservative RTP screening approach consisting of so-called triad testing43 with a 12-lead ECG, cTn (preferably using a high-sensitivity assay), and an echocardiogram. Subsequent protocols incorporating universal CMR in U.S. collegiate athletes identified myocardial and pericardial involvement in some recovering from COVID-19.73 , 74 , 223 , 224 Data from large registries have demonstrated, however, an overall low prevalence of clinical myocarditis,43 , 68 , 69 without a rise in reported acute adverse cardiac events.43 , 69 Cognizant of additional data that has accrued over the past year, we provide updated guidance related to resumption of athletics and intense exercise training after SARS-CoV-2 infection. Included below is a summary of literature related to athletes with COVID-19 and a practical, evidence-based framework to guide RTP evaluation.
6.3.2. Epidemiology
Multiple studies have affirmed that myocarditis is a common cause of sudden cardiac death in athletes.225 Unfortunately, early RTP recommendations related to COVID-19 were limited by: 1) an uncertain prevalence of myocarditis with SARS-CoV-2 infection; 2) unknown rates of adverse outcomes with exercise resumption; and 3) reports of significant myocardial injury and poor outcomes among some hospitalized patients.10 , 11
6.3.3. Case Series
Initial reports detailing the prevalence of myocardial involvement in athletes with COVID-19 were largely single-center studies using CMR as an additional screening tool. In the first published case series of largely asymptomatic U.S. collegiate athletes who underwent triad testing along with screening CMR,223 4 of 26 (15%) athletes met modified CMR Lake Louise criteria29 for myocarditis, 8 (31%) had evidence of LGE without edema, and none had pericarditis.223 This was followed soon thereafter by a study of 54 asymptomatic or minimally symptomatic student-athletes, of whom 19 (40%) demonstrated evidence of pericarditis with pericardial enhancement and/or pericardial effusion; no athlete, however, fulfilled CMR criteria for myocarditis.74 It is noteworthy that no other COVID-19 athlete study has reported similar degrees of pericardial involvement.
Additional small, single-center case series have followed73 , 224 , 226, 227, 228, 229 (see Table 1 ), all limited by: 1) lack of appropriate controls as well as independent and blinded review of imaging studies; 2) heterogeneity in CMR abnormalities; and 3) lack of a standardized approach to timing of CMR acquisition (ranging from 10-40+ days after testing positive for COVID-19). Data from these studies, along with well-publicized cases in the media,230, 231, 232 have nonetheless led some organizations to mandate the use of CMR to screen all competitive athletes following SARS-CoV-2 infection.
Table 1.
Case Series Evaluating the Prevalence of Myocarditis, Other Myocardial Involvement, and Pericardial Involvement in Athletes Following SARS-CoV-2 Infection∗
| Case Series | Competition Level | Sample Size | CMR Strategy | Age, Mean or Median Years | Sex, % Female | Symptomatic, n (%) | Myocarditis or Pericarditis, n (%) | Additional Observations |
|---|---|---|---|---|---|---|---|---|
| Rajpal et al223 (Online September 11, 2020) | Collegiate | 26 | All athletes | 20 | 42% | 12 (46%) | Myocarditis: 4 (15%) Pericarditis: 0 (0%) |
LGE: 12 (46%) Pericardial effusion: 2 (8%) |
| Brito et al74 (Online November 4, 2020) | Collegiate | 54 | Symptomatic athletes and those with an abnormal ECG and/or echocardiogram (N = 48, 89%) | 19 | 15% | 38 (70%) | Myocarditis: 0 (0%) Pericarditis: 19 (40%) |
Any CMR abnormality: 27 (56%) |
| Vago et al226 (Online December 16, 2020) | Professional | 12 | All athletes | 23 | 83% | 10 (83%) | Myocarditis: 0 (0%) Pericarditis: 0 (0%) |
NA |
| Clark et al224 (Online December 17, 2020) | Collegiate | 59 with COVID-19 60 athlete controls 27 healthy controls |
All subjects | 20 25 30 |
63% | 46 (78%) | Myocarditis: 2 (3%) Pericarditis: 1 (2%) |
RV insertion point LGE: 13 (22%) with COVID-19, 10 (24%) of 41 athlete controls evaluated with gadolinium, 0 (0%) healthy controls |
| Starekova et al73 (Online January 14, 2021) | Collegiate | 145 | All athletes | 20 | 26% | 111 (77%) | Myocarditis: 2 (1%) Pericarditis: 0 (0%) |
RV insertion point LGE: 38 (26%) Mid-myocardial LGE: 2 (1%) |
| Malek et al227 (Online January 20, 2021) |
Professional | 26 | All athletes | 24 | 81% | 20 (77%) | Myocarditis: 0 (0%) Pericarditis: 1 (4%) |
Any CMR abnormality: 5 (19%), includes 1 mid-myocardial LGE (4%) |
| Hendrickson et al228 (Online May 11, 2021) | Collegiate | 137 | Selective (based on abnormal triad testing, N = 5, 4%) | 20 | 32% | 112 (82%) | Myocarditis: 0 (0%) Pericarditis: 0 (0%) |
Abnormal ECG: 0 (0%) Abnormal cTn: 4 (3%) Abnormal echocardiogram: 4 (3%) Any CMR abnormality (of those tested): 0 (0%) |
| Hwang et al229 (Online June 24, 2021) | Collegiate | 55 | Selective (based on abnormal triad testing, N = 8, 15%) | NA | NA | 38 (69%) | Myocarditis: 0 (0%) Pericarditis: 1 (2%) |
Abnormal ECG: 3 (5%) Abnormal cTn: 1 (2%) Abnormal echocardiogram: 6 (11%) Any CMR abnormality (of 8 tested): 2 (25%) |
CMR = cardiac magnetic resonance imaging; cTn = cardiac troponin; ECG = electrocardiogram; LGE = late gadolinium enhancement; NA = not available; RV = right ventricle.
Studies included standard triad testing (ECG, cTn, and echocardiogram) or standard triad testing along with mandated CMR.
6.3.4. Registries
Throughout 2020, multiple large registries were created to track U.S. professional and collegiate athletes who had recovered from COVID-19. Both the U.S. professional cohort (N = 789)69 and multicenter collegiate Outcomes Registry for Cardiac Conditions in Athletes (N = 3,018)43 documented a significantly lower prevalence of myocarditis (0.6%-0.7%) among previously infected athletes, most of whom underwent cardiac triad testing regardless of symptomatology (see Table 2 ). Within these registries, CMR was generally obtained if any component of triad testing was abnormal or if symptoms were suggestive of myocarditis (excluding 198 athletes in the Outcomes Registry for Cardiac Conditions in Athletes with mandated CMR).43 , 69 No adverse cardiac outcomes were reported as a direct consequence of COVID-19.43 , 69
Table 2.
Registries Evaluating the Prevalence of Myocarditis, Other Myocardial Involvement, and Pericardial Involvement in Athletes Following SARS-CoV-2 Infection
| Registry | Competition Level | Sample Size | CMR Strategy | Age, Mean Years | Sex, % Female) | Symptomatic, n (%) | Myocarditis or Pericarditis, n (%) | Additional Observations |
|---|---|---|---|---|---|---|---|---|
| Martinez et al69 (Online March 4, 2021) | Professional | 789 | Selective, based on abnormal triad testing, (N = 30, 4%) | 25 | 2% | 460 (58%) | Myocarditis: 3 (0.4%) Pericarditis: 2 (0.3%) |
Abnormal ECG: 9 (1%) Abnormal cTn: 6 (0.8%) Abnormal echocardiogram: 19 (2%) Any CMR abnormality: 5/30 (17%) |
| Moulson et al43 (Online April 17, 2021) | Collegiate (Total N = 3,018) | 2,820 | Selective, based on symptoms or abnormal triad testing, (N = 119, 4.2%) | 20 | 32% | 2,022 (67%) | Myocarditis: 12 (0.4%) Pericarditis: 3 (0.1%) |
Abnormal ECG: 12 (0.4%) Abnormal cTn: 9 (0.3%) Abnormal echocardiogram: 15 (0.5%) Any CMR abnormality: 15/119 (13%) |
| 198 | Nonselective, all athletes | NA | NA | 62 (31%)∗ | Myocardial involvement:† 6 (3%) | Definite or probable myocardial involvement:† 3 (2%) | ||
| Daniels et al68 (Online May 27, 2021) | Collegiate | 1,597 | All athletes | NA | 40% | NA | Myocarditis:‡ 37 (2%) Pericarditis: 0 (0%) |
Clinical myocarditis: 9 (0.6%) |
CMR = cardiac magnetic resonance imaging; cTn = cardiac troponin; ECG = electrocardiogram; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; NA = not available.
N = 62 with moderate COVID-19 symptoms or abnormal baseline cardiac testing.
Myocardial involvement defined as: 1) definite: T1 abnormality + LGE and T2 abnormality, or T2 abnormality + additional supportive criteria (LVEF ≤45%, pericardial effusion or enhancement, or cTn level above the 99th percentile upper reference limit); 2) probable: T1 abnormality + LGE and 1 or more supportive criterion; or 3) possible: isolated T1 abnormality or LGE.
Myocarditis defined as clinical or subclinical (CMR-defined myocardial inflammation in the absence of symptoms suggestive of clinical myocarditis and elevated cTn).
Most recently, the Big Ten Conference published data on 1,597 athletes who underwent universal screening with CMR in addition to triad testing, irrespective of symptom status68 (see Table 2). Abnormalities on CMR consistent with myocarditis29 were reported in 2.3% of athletes. Similar to data from single-center studies, however, there was marked heterogeneity in the prevalence (0%-7.6%) and type of CMR abnormalities among the 13 programs that participated. Results were also remarkably comparable to those of the Outcomes Registry for Cardiac Conditions in Athletes and the professional athlete registries, as only 9 athletes (0.6%) had findings consistent with clinical myocarditis.
6.3.5. Limitations of Screening With CMR
Although CMR is a powerful diagnostic tool capable of identifying tissue necrosis, inflammation, and edema, significant limitations exist when used as a screening tool for those infected with SARS-CoV-2. First, the modified Lake Louise criteria are predicated on a clinical suspicion of myocarditis and have not been validated as a screening tool for low-to-intermediate risk patients.29 Moreover, diagnostic algorithms for myocarditis from the European Society of Cardiology and American Heart Association rely on both a clinical presentation suggestive of myocarditis and abnormal diagnostic testing.108 , 233 Thus, the clinical relevance of abnormal tissue characterization on CMR in individuals without a clinical suspicion for myocarditis is unknown. Second, a paucity of athlete-specific normative data complicates use of CMR as a generalized screening tool in this population. For example, numerous studies have demonstrated nonspecific LGE in masters-level athletes.234 A recent study also noted comparable rates of focal LGE at RV insertion points among athletes with SARS-CoV-2 (22%) and noninfected athlete controls (24%).224 Third, parametric mapping requires a high level of expertise. Accordingly, marked heterogeneity in reported CMR results from different centers may relate to regional variations in CMR performance and interpreter bias, given the lack of blinded review by an independent core laboratory. Fourth, the absence of an appropriate control group limits the interpretation of clinical significance and application of these CMR findings in a testing algorithm. Finally, cost, limited availability, and equitable resource allocation all represent real-world challenges when considering use of CMR for RTP screening.
Importantly, to date, there have been no confirmed cases of cardiac demise in the registries of athletes with COVID-19.43 , 68 , 69 In addition, most athletes at all levels of competition resume participation without undergoing CMR.
6.3.6. Evaluation and Management
The first consensus statement endorsed by the ACC Sports and Exercise Cardiology Section recommended that triad testing be part of RTP screening for all competitive athletes with symptoms.221 Subsequent recommendations by this group were tempered, recommending cardiac testing only in those with moderate, severe, and/or worsening symptoms related to COVID-19.222 Changes in consensus recommendations (see Supplemental Appendix Tables 1 and 2) reflected an effort to balance the uncertainty of cardiac sequalae with mild illness,11 , 74 , 223 the absence of robust prevalence data related to myocarditis or myocardial involvement,222 and the time-sensitive need for guidance within the sports medicine community. Despite these changes, most collegiate and professional athletes continued with triad testing before RTP, even for those who were asymptomatic.43 , 68 , 69 Recent data from multi-institutional registries, however, have now established a low prevalence of clinical myocarditis among competitive athletes with COVID-19.43 , 68 , 69 In addition, symptoms suggestive of myocarditis can help to predict cardiac involvement.43
Historically, an athlete has been defined as an individual who places a high premium on exercise training, competition, and sports achievement.235 Individuals who self-identify as athletes extend across the age spectrum, from youth sports (aged <18 years) to masters-level (aged >35 years) exercise enthusiasts.235 Previous recommendations were given to individual athletic populations recovering from COVID-19, with encouragement to observe younger athletes (aged <21 years) for several weeks to monitor for the development of MIS-C.236 , 237 Masters-level athletes may also have traditional risk factors for cardiovascular disease,238 , 239 increasing the possibility of more severe manifestations with COVID-19.
Current data suggest that competitive athletes recovering from COVID-19 with mild, noncardiopulmonary symptoms are unlikely to have myocarditis and clinically significant myocardial involvement.43 , 69 Consistent observations have been noted in an unselected, nonathletic cohort of infected health care workers (N = 149, aged 18-63 years), where mild symptoms were not associated with discernable cardiovascular pathology.66 Instead, cardiopulmonary symptoms that are persistent are likely to be of greater relevance than age or competition level when assessing the probability of clinically-important myocardial disease.43
6.3.7. Return to Exercise and Athletic Training
For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms concerning for myocarditis or myocardial involvement (chest pain or tightness, dyspnea, palpitations, lightheadedness, or syncope) and/or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation should be performed before resuming exercise (see Figure 9 ). For all others who are asymptomatic or with symptoms less suggestive of a cardiopulmonary etiology (eg, fever [temperature ≥100.4 °F], chills, lethargy, myalgias), additional cardiac testing is not recommended.
Figure 9.
Evaluation of the Athletic Patient Convalesced From COVID-19 and Guidance on RTP and/or Intense Training
∗CDC Guidelines: COVID-19 Quarantine and Isolation.240 †Cardiopulmonary symptoms include chest pain/tightness, dyspnea, palpitations, and lightheadedness/syncope; this also includes symptoms occurring ≤1 week following COVID-19 mRNA vaccination. ‡Strategies to minimize transmission of SARS-CoV-2 to other athletes 3-10 days following a positive COVID-19 test include 1) training in isolation, 2) participating in socially-distanced outdoor training, 3) training with a face mask in a well-ventilated facility with appropriate social distancing, and 4) participating in group training after a single negative NAAT (eg, RT-PCR test) or 2 negative rapid antigen tests 24-48 hours apart. §Excludes prolonged, isolated anosmia/ageusia, which should not delay return to training. ‖Maximal-effort exercise testing should be deferred until myocarditis has been excluded. CDC = Centers for Disease Control and Prevention; CMR = cardiac magnetic resonance imaging; COVID-19 = novel coronavirus disease 2019, cTn = cardiac troponin; ECG = electrocardiogram; NAAT = nucleic acid amplification test, PASC = post-acute sequelae of SARS-CoV-2 infection; RTP = return to play; RT-PCR = reverse transcription polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Current CDC guidelines stipulate at least 5 days of self-isolation from symptom onset or upon testing positive for COVID-19.240 Past consensus RTP guidance recommended that individuals abstain from exercise throughout a 10-day self-isolation period, in part, because of the potential for clinical deterioration.221 , 222 Studies demonstrating a lack of clinically significant myocardial involvement among athletes with mild COVID-1943 , 69 suggest that such activity restriction is no longer necessary (see Figure 9). For asymptomatic athletes with COVID-19, 3 days of abstinence from training is recommended to ensure that symptoms do not develop.
For individuals with mild non-cardiopulmonary symptoms, exercise training should generally be withheld until symptom resolution. One exception is isolated anosmia or ageusia, which may have a more prolonged course. For athletes with cardiopulmonary symptoms, intense exercise training should be limited until symptoms resolve, self-isolation is complete, and further cardiac testing can be obtained. In addition, a graded RTP regimen should be emphasized in all individuals with prior COVID-19 to ensure close monitoring for new cardiopulmonary symptoms. For those participating in organized competitive sports, graded exercise programs should be individualized and implemented, with support by athletic trainers and primary care sports medicine clinicians. For most individuals participating in high-level recreational athletics, a graded return-to-exercise program equates to more qualitative gradual increases in effort. This remains a point of key emphasis, as many high-level recreational exercise enthusiasts do not have immediate access to cardiac testing and sports cardiology referrals, regardless of symptom severity.
For athletes with cardiopulmonary symptoms, initial evaluation should ideally be with an ECG, cTn (high-sensitivity assay preferred), and an echocardiogram (see Figure 9). The presence of abnormal findings with triad testing or persistence of cardiopulmonary symptoms (specifically chest pain or tightness, palpitations, or syncope) after initial testing suggest that additional evaluation with CMR should be performed. Maximal-effort exercise testing may be a useful adjunct in cases of persistent cardiopulmonary symptoms, only after myocarditis has been excluded with CMR. Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, it is also reasonable to apply these recommendations to high-school athletes (aged ≥14-15 years) along with masters-level recreational exercise enthusiasts.
Use of CMR to screen athletes who are asymptomatic or with non-cardiopulmonary symptoms is likely to be low yield. Such testing may, however, be considered in those with abnormal triad testing along with increased concern of cardiac involvement or in those with either persistent or new cardiopulmonary symptoms. Importantly, further research is needed to better understand the significance of other forms of myocardial involvement detected by CMR,68 , 223 particularly in the absence of symptoms.
6.3.8. Remote History of COVID-19
It is likely that clinicians will increasingly see asymptomatic athletes who are returning for sports physicals after having had COVID-19 without prior RTP evaluation. Regardless of the amount of time that has passed since infection, athletes who have remained asymptomatic or who have had non-cardiopulmonary symptoms and are exercising without limitation require no further cardiac testing. In contrast, for athletes with prior COVID-19 and a history of cardiopulmonary symptoms, the duration of time since symptom resolution and the athlete’s current clinical status should dictate the approach. If <1 month has elapsed since resolution of cardiopulmonary symptoms, triad testing should be performed. If >3 months have elapsed since resolution of cardiopulmonary symptoms and no exercise limitations are present, no further cardiac testing is likely required. The rationale for this stems from prior guidance recommending avoidance of exercise for at least 3 months in cases of confirmed myocarditis.241 Finally, if 1-3 months have elapsed since cardiopulmonary symptom resolution and athletes have returned on their own to training without exercise limitation, it is reasonable to allow continued exercise training without further cardiac evaluation. This decision should be individualized and based on clinical judgement, informed by the type and severity of prior symptoms. Factors warranting further cardiac evaluation include prior worrisome cardiopulmonary symptoms (eg, syncope, sustained exertional palpitations, and/or exertional chest tightness or dyspnea).
Regardless of vaccination status, a growing number of professional and collegiate athletes have been diagnosed with COVID-19, likely because of more transmissible variants (eg, Delta, Omicron). For those with recurrent COVID-19, repeat cardiac testing is not warranted in the absence of cardiopulmonary symptoms. RTP guidance should follow the same pathway as those with initial infection (see Figure 9).
6.3.9. Nonspecific and Isolated Screening Abnormalities
In general, routine surveillance monitoring and/or additional testing of athletes who have successfully returned to play after COVID-19 is not recommended. Incidental findings of unclear significance may be encountered with such testing, particularly in those with lower pretest probability of concerning cardiac abnormalities. For example, detection of isolated LGE without concomitant edema has been reported with CMR.68 , 223 , 224 Recognition of isolated LGE as a manifestation of resolving myocarditis should be more fully considered in those with: 1) a high clinical suspicion of myocarditis; 2) supportive abnormal findings on other testing (ECG, cTn); and 3) a pattern of LGE suggestive of myocarditis.242 , 243 Additional abnormalities warranting evaluation include: 1) mildly reduced LV systolic function that may be representative of either exercise-induced cardiac remodeling or a pathologic phenotype;244 2) isolated cTn elevation; and/or 3) ECG abnormalities. With these findings, if it is determined that acute myocarditis is less likely, consideration can be given to maximal-effort exercise testing (with stress echocardiography if there is reduced LV systolic function to assess for appropriate augmentation with exercise)245 and ambulatory rhythm monitoring.
6.3.10. Myocarditis and Exercise Training Restrictions
In a 2015 guidance document related to sports eligibility, individuals with clinical myocarditis were recommended to have 3-6 months of complete exercise abstinence.241 Thereafter, decision-making regarding RTP would be based on: 1) absence of cardiopulmonary symptoms; 2) resolution of laboratory evidence of myocardial injury; 3) normalization of LV systolic function; and 4) absence of spontaneous/inducible cardiac arrhythmias on ECG monitoring and exercise stress testing. Whether this 3-month minimum of exercise abstinence independently reduces the risk of acute cardiac events is uncertain. In the Big Ten COVID-19 cardiac registry, 27 athletes (27 of 37, 73%) with clinical or subclinical myocarditis completed repeat CMR within 4-14 weeks after initially testing positive for COVID-19.68 In 11 of these athletes (41%), LGE and T2 mapping abnormalities had completely resolved after a median of 8 weeks. Whether this relatively rapid resolution reflects disproportionately subclinical findings, however, is unknown. Indeed, just 1 of 6 athletes with clinical myocarditis had resolution of inflammatory CMR findings after 10 weeks.68 Collectively, these data suggest that it is reasonable to reassess for resolution of myocardial inflammation in those with clinical myocarditis <3 months since testing positive for COVID-19, especially if they have had rapid resolution of symptoms and prior demonstration of normal LV systolic function or in those with subclinical myocarditis identified with CMR screening. The best time for this reassessment should likely be individualized, but no sooner than 1 month from the time of initial diagnosis of clinical or subclinical myocarditis. For asymptomatic athletes with complete resolution of myocardial inflammation and no spontaneous arrhythmias, a RTP approach using conservative, graded exercise should be pursued. Finally, screening for myocarditis in the absence of suggestive symptoms following receipt of the COVID-19 mRNA vaccine is not recommended. In addition, although self-limited side effects following vaccination (eg, fatigue, headache, fever, muscle pain) may lead some individuals to stop exercising until symptoms resolve, vaccination per se does not require a set time away from exercise training.
6.3.11. PASC and Return to Exercise
Decision-making regarding RTP in PASC should be informed by whether cardiopulmonary symptoms are present. If they are, a similar approach to that recommended above should be pursued, using: 1) triad testing; 2) CMR (if appropriate); and 3) limitation of strenuous physical activity (see Figure 9). If PASC-CVD is excluded, further evaluation with CPET and pulmonary function testing may be considered as part of the PASC-CVS evaluation. Although individuals with PASC may be unable to readily resume their prior activity levels, structured and individualized graded exercise, as recommended in the PASC section, should be performed throughout the recovery process.
6.3.12. Future Directions
As our understanding of the cardiovascular effects of COVID-19 evolves, it is anticipated that RTP guidance for athletes will change as well. Several areas of future investigation are noteworthy. First, although it is extremely reassuring that adverse events among infected athletes have been rare, further study is needed to determine how long recovery takes after documented abnormalities and the role that imaging can play in guiding this. Second, the risks and benefits of exercise resumption among patients with PASC remains to be rigorously defined. Given the therapeutic role of training in this population, further investigation into associated risks will be particularly important. Third, our current knowledge stems largely from study of those infected with early variants of SARS-CoV-2. Further research is needed to understand whether the extent and severity of cardiac involvement is different with Delta, Omicron, and other variants. It will also be important to define the temporal pattern of myocardial involvement, along with predictors of myocarditis progression and resolution with SARS-CoV-2 and other common respiratory viral pathogens. Finally, widespread resumption of competitive sports will come with rare but devastating adverse events, including sudden cardiac death, that well predate COVID-19. Accordingly, it will be important to limit automatic attribution of such events to COVID-19 without further detailed evaluation.
7. Implication of Pathway
This ECDP provides a framework for evaluation and management of adults with cardiovascular sequelae following SARS-CoV-2 infection. Guidance provided is based on expert consensus, with multiple key takeaways (see Table 3 ). Importantly, it is intended to help clinicians understand not only when testing may be warranted, but also when it is not. Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves.
Table 3.
Summary of Diagnostic Criteria and Key Recommendations Related to Cardiovascular Sequelae of COVID-19 in Adults
| Myocarditis and other myocardial involvement |
|
| PASC |
|
| RTP |
|
CMR = cardiac magnetic resonance imaging; COVID-19 = novel coronavirus disease 2019; CT = computed tomography; cTn = cardiac troponin; ECG = electrocardiogram; MIS-A = multisystem inflammatory syndrome in adults; mRNA = messenger RNA; NSAID = nonsteroidal anti-inflammatory drug; PASC = post-acute sequelae of SARS-CoV-2 infection; PASC-CVD = post-acute sequelae of SARS-CoV-2 infection–cardiovascular disease; PASC-CVS = post-acute sequelae of SARS-CoV-2 infection–cardiovascular syndrome; RTP = return to play; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
For both myocarditis and PASC, there exist significant opportunities to better understand their epidemiology, underlying mechanisms, predisposing risk factors, and preferred approaches for evaluation and management. For RTP, there is a need to more rigorously define how long cardiovascular abnormalities persist and the relative benefits of exercise training in those with PASC.
Beyond the indirect effects and undesirable consequences that COVID-19 has had on care delivery, it also remains to be seen how new variants and vaccine breakthrough infection will affect cardiovascular health. Improved understanding of the associated near- and long-term consequences will be key in helping to improve clinical outcomes.
President and Staff
Dipti Itchhaporia, MD, FACC, President
Cathleen C. Gates, Chief Executive Officer
Richard J. Kovacs, MD, MACC, Chief Medical Adviser/Chief Medical Officer
Joseph M. Allen, MA, Team Lead, Clinical Standards and Solution Sets
Amy Dearborn, Team Lead, Clinical Policy Content Development
Ashleigh M. Covington, MA, Team Lead, Clinical Pathways and Heart House Roundtables
Severa Chavez, Project Manager, Clinical Pathways and Heart House Roundtables
Grace Ronan, Team Lead, Clinical Policy Publication
Footnotes
This document was approved by the American College of Cardiology Clinical Policy Approval Committee in February 2022.
The American College of Cardiology requests that this document be cited as follows: Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, Baggish AL, Bozkurt B, Cornwell WK III, Harmon KG, Kim JH, Lala A, Levine BD, Martinez MW, Onuma O, Phelan D, Puntmann VO, Rajpal S, Taub PR, Verma AK. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2022;79:1717–1756.
Copies: This document is available on the website of the American College of Cardiology (www.acc.org). For copies of this document, please contact Elsevier Inc. Reprint Department via fax (212) 633-3820 or e-mail (reprints@elsevier.com).
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American College of Cardiology. Requests may be completed online via the Elsevier site (https://www.elsevier.com/about/policies/copyright/permissions).
Appendix 1. Author Relationships With Industry and Other Entities (Relevant)— 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-acute Sequelae of SARS-CoV-2 Infection, and Return to Play
Relationships with Industry and Other Entities
To avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the writing committee, all members of the writing committee, as well as peer reviewers of the document, are asked to disclose all current healthcare-related relationships, including those existing 12 months before initiation of the writing effort. The ACC Solution Set Oversight Committee reviews these disclosures to determine what companies make products (on market or in development) that pertain to the document under development. Based on this information, a writing committee is formed to include a majority of members with no relevant relationships with industry (RWI), led by a chair with no relevant RWI. RWI is reviewed on all conference calls and updated as changes occur. Author RWI pertinent to this document is disclosed in the table below and peer reviewer RWI is disclosed in Appendix 2. Additionally, to ensure complete transparency, authors’ comprehensive disclosure information—including RWI not pertinent to this document—is available in a Supplemental Appendix. Disclosure information for the ACC Solution Set Oversight Committee is also available online at http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/guidelines-and-documents-task-forces, as well as the ACC disclosure policy for document development at http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy.
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/ Partnership/ Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Ty J. Gluckman (Co-Chair) | Providence Heart Institute Providence St. Joseph Health—Medical Director, Center for Cardiovascular Analytics, Research, and Data Science (CARDS) |
None | None | None | None | None | None |
| Nicole M. Bhave (Co-Chair) | University of Michigan Medical School—Associate Professor of Medicine Cardiovascular Medicine |
None | None | None | None | None | None |
| Larry A. Allen (Vice Chair) | University of Colorado School of Medicine—Professor of Medicine; Kenneth Poirier Chair; Associate Head for Clinical Affairs, Cardiology Medical Director, Advanced Heart Failure |
|
None | None | None | None | None |
| Eugene H. Chung (Vice Chair) | University of Michigan Medical School—Professor of Medicine; Director, Sports Cardiology Clinic | None | None | None | None | None | None |
| Erica S. Spatz (Vice Chair) | Yale University School of Medicine—Associate Professor, Section of Cardiovascular Medicine; Director, Preventive Cardiovascular Health Program | None | None | None | None | None | None |
| Enrico Ammirati | Niguarda Hospital, Milan, Italy—Cardiologist, Advanced Heart Failure, LVAD and Cardiac Transplant | None | None | None | None |
|
None |
| Aaron L. Baggish | Massachusetts General Hospital—Cardiovascular Performance Program Director | None | None | None | None | None | None |
| Biykem Bozkurt | Baylor College of Medicine—The Mary and Gordon Cain Chair and Professor of Medicine; Associate Provost of Faculty Affairs; Senior Associate Dean of Faculty Development; Director, Winters Center for Heart Failure Research; Associate Director, Cardiovascular Research Institute; Vice-Chair of Medicine |
|
None | None | None |
|
None |
| William K. Cornwell, III | University of Colorado Anschutz Medical Campus—Cardiologist, Advanced Heart Failure, LVAD and Cardiac Transplant | None | None | None | None | None | None |
| Kimberly G. Harmon | University of Washington—Team Physician | None | None | None | None | None | None |
| Jonathan H. Kim | Emory Clinical Cardiovascular Research Institute, Emory School of Medicine—Associate Professor of Medicine, Director of Sports Cardiology | None | None | None | None | None | None |
| Anuradha Lala | Icahn School of Medicine at Mount Sinai—Associate Professor, Cardiology & Population Health Science; Program Director, Advanced Heart Failure & Transplant Fellowship | None | None | None | None | None | None |
| Benjamin D. Levine | Institute for Exercise & Environmental Medicine—Director; Texas Health Presbyterian Dallas—Chair for Wellness and Chair for Cardiovascular Research; The University of Texas Southwestern Medical Center—Professor of Medicine and Cardiology | None | None |
|
None | None | None |
| Matthew M. Martinez | Morristown Medical Center—Director Sports Cardiology and Hypertrophic Cardiomyopathy Center |
|
None | None | None | None | None |
| Oyere Onuma | Yale University School of Medicine— Assistant Professor of Cardiology | None | None | None | None | None | None |
| Dermot Phelan | Sanger Heart and Vascular Institute, Atrium Health—Director of Sports Cardiology |
|
None | None | None | None | None |
| Valentina O. Puntmann | University Hospital Frankfurt—Associate Professor, Consultant Cardiologist (Cardiovascular Magnetic Resonance Imaging) |
|
None | None | None | None | None |
| Saurabh Rajpal | The Ohio State University Wexner Medical Center—Assistant Professor | None | None | None | None | None | None |
| Pam R. Taub | University of California San Diego Medical Center—Professor of Medicine; Director of Step Family Foundation Cardiovascular Rehabilitation and Wellness Center |
|
None | None | None | None | None |
| Amanda K. Verma | Washington University School of Medicine—Assistant Professor of Medicine, Advanced Heart Failure and Transplant Cardiology; Director of COVID Cardiology Clinics | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted.
According to the ACC, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document or makes a competing drug or device addressed in the document; or c) the person or a member of the person’s household, has a reasonable potential for financial, professional or other personal gain or loss as a result of the issues/content addressed in the document.
ACC = American College of Cardiology; LVAD = left ventricular assist device.
Significant relationship.
Appendix 2. Peer Reviewer Relationships With Industry and Other Entities (Comprehensive)—2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-acute Sequelae of SARS-CoV-2 Infection, and Return to Play.
| Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|---|
| Geoffrey D. Barnes | Content Reviewer—ACC Expert | University of Michigan—Assistant Professor of Internal Medicine; Co-director, Michigan Anticoagulation Quality Improvement Initiative |
|
None | None |
|
None | None |
| Diana Berrent | Content Reviewer—Patient Advocate | Survivor Corps—Founder | None | None | None | None | None | None |
| Devyani Chowdhury | Official Reviewer—ACC Adult Congenital & Pediatric Cardiology Council | Cardiology Care for Children and Ai Dupont Children's Hospital—Director of Cardiology Care for Children; Medical Director of ACHD at Nemour's |
|
None | None | None | None | |
| Leslie T. Cooper | Content Reviewer—ACC Expert | Mayo Clinic—Professor of Medicine | None | None |
|
None | ||
| Peter N Dean | Content Reviewer—ACC Expert | University of Virginia— Associate Professor | None | None | None | None | None | None |
| James L. Januzzi Jr. | Content Reviewer—ACC Expert | Harvard Medical School—Hutter Family Professor of Medicine; Director, Dennis and Marilyn Barry Fellowship in Cardiology Research; Senior Cardiometabolic Faculty, Baim Institute for Clinical Research | None | None | None | None | ||
| Thomas M. Maddox | Content Reviewer—ACC Expert | BJC HealthCare and Washington University School of Medicine—Executive Director, Healthcare Innovation Lab; Professor of Medicine | None | None | None |
|
|
None |
| Matthew Oster | Content Reviewer—ACC Expert | Children's Healthcare of Atlanta—Pediatric Cardiologist | None | None | None | None | None | None |
| Satish R. Raj | Content Reviewer—ACC Expert | University of Calgary & Alberta Health Services— Professor of Cardiac Sciences |
|
None | None |
|
||
| Ashwin Kumar Ravichandran | Official Reviewer—ACC Heart Failure & Transplant Council | St. Vincent Heart Center—Cardiologist |
|
None | None | None | ||
| Ashley Schenk | Official Reviewer—American Society of Health System Pharmacists | UK College of Pharmacy; University of Kentucky HealthCare—Acute Care Pharmacy Services; Cardiology Assistant Adjunct Professor |
None | None | None | None | None | None |
| Ravi V. Shah | Content Reviewer—ACC Expert | Massachusetts General Hospital—Cardiologist |
|
None | None | None | None | None |
| Cynthia C. Taub | Official Reviewer—American Society of Echocardiography | Dartmouth Hitchcock Medical Center—Chief, Section of Cardiovascular Medicine | None | None | None | None | None | None |
| David E. Winchester | Official Reviewer—Solution Set Oversight Committee | University of Florida—Associate Professor of Medicine | None | None | None | None |
|
None |
This table represents all relationships of reviewers with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to https://www.acc.org/Guidelines/About-Guidelines-and-Clinical-Documents/Relationships-with-Industry-Policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
ACC = American College of Cardiology; AHA = American Heart Association
Significant relationship.
No financial benefit.
Relationship with this company is limited to enrolling patients in clinical trials. This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system. To appear in this category, the author acknowledges that there is no direct or institutional relationship with the trial sponsor as defined in the ACC/AHA Disclosure Policy for Writing Committees.
Appendix 3. Abbreviations.
ACC = American College of Cardiology
CDC = Centers for Disease Control and Prevention
CMR = cardiac magnetic resonance imaging
CT = computed tomography
cTn = cardiac troponin
COVID-19 = novel coronavirus disease 2019
ECDP = Expert Consensus Decision Pathway
ECG = electrocardiogram
LGE = late gadolinium enhancement
LV = left ventricular
ME/CFS = myalgic encephalomyelitis/chronic fatigue syndrome
MIS-A = multisystem inflammatory syndrome in adults
MIS-C = multisystem inflammatory syndrome in children
mRNA = messenger RNA
PASC = post-acute sequelae of SARS-CoV-2 infection
PASC-CVD = post-acute sequelae of SARS-CoV-2 infection–cardiovascular disease
PASC-CVS = post-acute sequelae of SARS-CoV-2 infection–cardiovascular syndrome
POTS = postural orthostatic tachycardia syndrome
RWI = relationships with industry
RTP = return to play
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2
Appendix
References
- 1.Januzzi J.L., Jr., Ahmad T., Binder L.G., et al. 2019 methodology for creating expert consensus decision pathways: a report of the American College of Cardiology. J Am Coll Cardiol. 2019;74:1138–1150. doi: 10.1016/j.jacc.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Johns Hopkins University & Medicine Coronarvirus Resource Center https://coronavirus.jhu.edu/map.html
- 3.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough S.A., Goyal P., Krishnan U., et al. Electrocardiographic findings in coronavirus disease-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26:626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochi A.N., Tagliari A.P., Forleo G.B., et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfi A., Bernabei R., Landi F., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt A.S., Adler E.D., Albert N.M., et al. Coronavirus disease-2019 and heart failure: a scientific statement from the Heart Failure Society of America. J Card Fail. 2022;28:93–112. doi: 10.1016/j.cardfail.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- 15.Centers for Disease Control and Prevention Post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 16.COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 17.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 18.Verma A.K., Lavine K.J., Lin C.Y. Myocarditis after COVID-19 mRNA vaccination. N Engl J Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanneman K., Houbois C., Schoffel A., et al. Combined cardiac fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging assessment of myocardial injury in patients who recently recovered from COVID-19. JAMA Cardiol. Published online January 12, 2022 doi: 10.1001/jamacardio.2021.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster G., Patel A.B., Carr M.R., et al. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. 2021;23:86. doi: 10.1186/s12968-021-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theocharis P., Wong J., Pushparajah K., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22:896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer M.E., Taub I.B., Kaelber D.C. Risk of myocarditis from COVID-19 infection in people under age 20: a population-based analysis. medRxiv. Published online July 27, 2021 doi: 10.1101/2021.07.23.21260998. [DOI] [Google Scholar]
- 23.Pellegrini J.A.S., Rech T.H., Schwarz P., et al. Incidence of venous thromboembolism among patients with severe COVID-19 requiring mechanical ventilation compared to other causes of respiratory failure: a prospective cohort study. J Thromb Thrombolysis. 2021;52:482–492. doi: 10.1007/s11239-021-02395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nune A., Iyengar K.P., Goddard C., et al. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V) BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-243888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aretz H.T., Billingham M.E., Edwards W.D., et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 27.Baughman K.L. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich M.G., Sechtem U., Schulz-Menger J., et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 30.Francone M., Chimenti C., Galea N., et al. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. J Am Coll Cardiol Img. 2014;7:254–263. doi: 10.1016/j.jcmg.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Nagel E., Kwong R.Y., Chandrashekhar Y.S. CMR in nonischemic myocardial inflammation: solving the problem of diagnosing myocarditis or still diagnostic ambiguity? J Am Coll Cardiol Img. 2020;13:163–166. doi: 10.1016/j.jcmg.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Cornelissen A., Kutyna M., Cheng Q., et al. Effects of simulated COVID-19 cytokine storm on stent thrombogenicity. Cardiovasc Revasc Med. Published online April 8, 2021 doi: 10.1016/j.carrev.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer P., Degrauwe S., Van Delden C., et al. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen D., Nguyen T., De Bels D., et al. A case of Takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging. 2020;21:1052. doi: 10.1093/ehjci/jeaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Garcia J., Jaladanki S., Rivas-Lasarte M., et al. New heart failure diagnoses among patients hospitalized for COVID-19. J Am Coll Cardiol. 2021;77:2260–2262. doi: 10.1016/j.jacc.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyal P., Reshetnyak E., Khan S., et al. Clinical characteristics and outcomes of adults with a history of heart failure hospitalized for COVID-19. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.121.008354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkegaard C., Falco-Roget A., Sanchez-Montalva A., et al. Incidence and risk factors for early readmission after hospitalization for SARS-CoV-2 infection: results from a retrospective cohort study. Infection. Published online July 30, 2021 doi: 10.1007/s15010-021-01662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo J., Grewal P., Hotelling J., et al. Admission NT-proBNP and outcomes in patients without history of heart failure hospitalized with COVID-19. ESC Heart Fail. 2021;8:4278–4287. doi: 10.1002/ehf2.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Z.Y., Geng Y.J., Huang J., et al. Pathogenesis and management of myocardial injury in coronavirus disease 2019. Eur J Heart Fail. 2020;22:1994–2006. doi: 10.1002/ejhf.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 45.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathore S.S., Rojas G.A., Sondhi M., et al. Myocarditis associated with COVID-19 disease: a systematic review of published case reports and case series. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14470. [DOI] [PubMed] [Google Scholar]
- 47.Grant M.C., Geoghegan L., Arbyn M., et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abelmann W.H. Virus and the heart. Circulation. 1971;44:950–956. doi: 10.1161/01.cir.44.5.950. [DOI] [PubMed] [Google Scholar]
- 50.Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- 51.UK Research and Innovation MRC dyspnoea scale / MRC breathlessness scale. https://mrc.ukri.org/research/facilities-and-resources-for-researchers/mrc-scales/mrc-dyspnoea-scale-mrc-breathlessness-scale/
- 52.Klok F.A., Boon G., Barco S., et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1):2001494. doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27:251–261. doi: 10.1007/s10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaine S., Devitt P., Coughlan J.J., et al. COVID-19-associated myocarditis presenting as new-onset heart failure and atrial fibrillation. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-244027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ammirati E., Cipriani M., Moro C., et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 57.Dai H., Lotan D., Much A.A., et al. Global, regional, and national burden of myocarditis and cardiomyopathy, 1990-2017. Front Cardiovasc Med. 2021;8:610989. doi: 10.3389/fcvm.2021.610989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun W., Zhang Y., Wu C., et al. Incremental prognostic value of biventricular longitudinal strain and high-sensitivity troponin I in COVID-19 patients. Echocardiography. 2021;38:1272–1281. doi: 10.1111/echo.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrizales-Sepulveda E.F., Vera-Pineda R., Flores-Ramirez R., et al. Echocardiographic manifestations in COVID-19: a review. Heart Lung Circ. 2021;30:1117–1129. doi: 10.1016/j.hlc.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furqan M.M., Verma B.R., Cremer P.C., et al. Pericardial diseases in COVID19: a contemporary review. Curr Cardiol Rep. 2021;23:90. doi: 10.1007/s11886-021-01519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rath D., Petersen-Uribe A., Avdiu A., et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109:1491–1499. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knight D.S., Kotecha T., Razvi Y., et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joy G., Artico J., Kurdi H., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. J Am Coll Cardiol Img. 2021;14:2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galea N., Marchitelli L., Pambianchi G., et al. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a Cardiovascular Magnetic Resonance study. J Cardiovasc Magn Reson. 2021;23:68. doi: 10.1186/s12968-021-00764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 71.Sirico D., Basso A., Reffo E., et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10(15):3360. doi: 10.3390/jcm10153360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palabiyik F., Akcay N., Sevketoglu E., et al. Imaging of multisystem inflammatory disease in children (MIS-C) associated With COVID-19. Acad Radiol. 2021;28:1200–1208. doi: 10.1016/j.acra.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starekova J., Bluemke D.A., Bradham W.S., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brito D., Meester S., Yanamala N., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. J Am Coll Cardiol Img. 2021;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ammirati E., Varrenti M., Veronese G., et al. Prevalence and outcome of patients with acute myocarditis and positive viral search on nasopharyngeal swab. Eur J Heart Fail. 2021;23:1242–1245. doi: 10.1002/ejhf.2247. [DOI] [PubMed] [Google Scholar]
- 76.Tschope C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chau V.Q., Giustino G., Mahmood K., et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
- 78.Skendros P., Mitsios A., Chrysanthopoulou A., et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox S.E., Falgout L., Vander Heide R.S. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021;54:107361. doi: 10.1016/j.carpath.2021.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bajaj R., Sinclair H.C., Patel K., et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med. 2021;9:e32–e34. doi: 10.1016/S2213-2600(21)00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendren N.S., Drazner M.H., Bozkurt B., et al. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawakami R., Sakamoto A., Kawai K., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fox S.E., Li G., Akmatbekov A., et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 86.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barton L.M., Duval E.J., Stroberg E., et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youd E., Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840–844. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 89.Edler C., Schroder A.S., Aepfelbacher M., et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buja L.M., Wolf D.A., Zhao B., et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Michele S., Sun Y., Yilmaz M.M., et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanley B., Naresh K.N., Roufosse C., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bradley B.T., Maioli H., Johnston R., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao X.H., Li T.Y., He Z.C., et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 97.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox S.E., Akmatbekov A., Harbert J.L., et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tombolini A., Scendoni R. SARS-CoV-2-related deaths in routine forensic autopsy practice: histopathological patterns. Int J Legal Med. 2020;134:2205–2208. doi: 10.1007/s00414-020-02354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Remmelink M., De Mendonca R., D'Haene N., et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dweck M.R., Bularga A., Hahn R.T., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fayol A., Livrozet M., Boutouyrie P., et al. Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Heart Fail. 2021;8:2232–2239. doi: 10.1002/ehf2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kelle S., Bucciarelli-Ducci C., Judd R.M., et al. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson. 2020;22:61. doi: 10.1186/s12968-020-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen S.E., Friedrich M.G., Leiner T., et al. Cardiovascular magnetic resonance for patients with COVID-19. J Am Coll Cardiol Img. Published online October 7, 2021 doi: 10.1016/j.jcmg.2021.08.021. [DOI] [Google Scholar]
- 108.Caforio A.L., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 109.Seferovic P.M., Tsutsui H., McNamara D.M., et al. Heart Failure Association, Heart Failure Society of America, and Japanese Heart Failure Society position statement on endomyocardial biopsy. J Card Fail. 2021;27:727–743. doi: 10.1016/j.cardfail.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Cooper L.T., Baughman K.L., Feldman A.M., et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 112.Ammirati E., Frigerio M., Adler E.D., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 114.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adler Y., Charron P., Imazio M., et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clemente-Moragon A., Martinez-Milla J., Oliver E., et al. Metoprolol in critically ill patients with COVID-19. J Am Coll Cardiol. 2021;78:1001–1011. doi: 10.1016/j.jacc.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hekimian G., Kerneis M., Zeitouni M., et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belhadjer Z., Meot M., Bajolle F., et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 119.Truong D.T., Dionne A., Muniz J.C., et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults. Circulation. 2022;145(5):345–356. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 120.Montgomery J., Ryan M., Engler R., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klein N.P., Lewis N., Goddard K., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jain S.S., Steele J.M., Fonseca B., et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5) doi: 10.1542/peds.2021-053427. [DOI] [PubMed] [Google Scholar]
- 123.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: ppdate from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Witberg G., Barda N., Hoss S., et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharff K.A., Dancoes D.M., Longueil J.L., et al. Risk of myopericarditis following COVID-19 mRNA vaccination in a large integrated health system: a comparison of completeness and timeliness of two methods. medRxiv. Published online December 27, 2021 doi: 10.1101/2021.12.21.21268209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Husby A., Hansen J.V., Fosbol E., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. Published online on December 14, 2021 doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caforio A.L., Mahon N.J., Tona F., et al. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 130.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heymans S., Eriksson U., Lehtonen J., et al. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 132.Larson K.F., Ammirati E., Adler E.D., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rosner C.M., Genovese L., Tehrani B.N., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.D'Angelo T., Cattafi A., Carerj M.L., et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boehmer T.K., Kompaniyets L., Lavery A.M., et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Most Z.M., Hendren N., Drazner M.H., et al. Striking similarities of multisystem inflammatory syndrome in children and a myocarditis-like syndrome in adults: overlapping manifestations of COVID-19. Circulation. 2021;143:4–6. doi: 10.1161/CIRCULATIONAHA.120.050166. [DOI] [PubMed] [Google Scholar]
- 140.Hageman J.R. Long COVID-19 or post-acute sequelae of SARS-CoV-2 infection in children, adolescents, and young adults. Pediatr Ann. 2021;50:e232–e233. doi: 10.3928/19382359-20210519-02. [DOI] [PubMed] [Google Scholar]
- 141.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
- 142.Centers for Disease Control and Prevention Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the United States. https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
- 143.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amenta E.M., Spallone A., Rodriguez-Barradas M.C., et al. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa509. ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.National Institute for Health and Care Excellence Royal College of General Practitioners, and Scottish Intercolleciate Guidelines Network COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://www.nice.org.uk/guidance/NG188 [PubMed]
- 148.Biehl M., Sese D. Post-intensive care syndrome and COVID-19 - implications post pandemic. Cleve Clin J Med. Published online August 5, 2020 doi: 10.3949/ccjm.87a.ccc055. [DOI] [PubMed] [Google Scholar]
- 149.Patel P., DeCuir J., Abrams J., et al. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Havervall S., Rosell A., Phillipson M., et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325:2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whitaker M., Elliott J., Chadeau-Hyam M., et al. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. medRxiv. 2021;18(9) doi: 10.1101/2021.06.28.21259452. [DOI] [Google Scholar]
- 154.Cassar M.P., Tunnicliffe E.M., Petousi N., et al. Symptom persistence despite improvement in cardiopulmonary health— insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021;41:101159. doi: 10.1016/j.eclinm.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Greenhalgh T., Knight M., A'Court C., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 156.Richter D., Guasti L., Koehler F., et al. Late phase of COVID-19 pandemic in general cardiology. a position paper of the ESC Council for Cardiology Practice. ESC Heart Fail. 2021;8:3483–3494. doi: 10.1002/ehf2.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saricam E., Dursun A.D., Turkmen Sariyildiz G., et al. Laboratory and imaging evaluation of cardiac involvement in patients with post-acute COVID-19. Int J Gen Med. 2021;14:4977–4985. doi: 10.2147/IJGM.S321156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang S.Y., Adejumo P., See C., et al. Characteristics of patients referred to a cardiovascular disease clinic for post-acute sequelae of SARS-CoV-2 infection. medRxiv. Published online December 5, 2021 doi: 10.1101/2021.12.04.21267294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Catena C., Colussi G., Bulfone L., et al. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J Am Soc Echocardiogr. 2021;34:193–195. doi: 10.1016/j.echo.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Daher A., Balfanz P., Cornelissen C., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Becker J.H., Lin J.J., Doernberg M., et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johansson M., Stahlberg M., Runold M., et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. J Am Coll Cardiol Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bryarly M., Phillips L.T., Fu Q., et al. Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol. 2019;73:1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 165.Thieben M.J., Sandroni P., Sletten D.M., et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 166.Raj S.R., Arnold A.C., Barboi A., et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res. 2021;31:365–368. doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stahlberg M., Reistam U., Fedorowski A., et al. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med. 2021;134:1451–1456. doi: 10.1016/j.amjmed.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Komaroff A.L., Lipkin W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. 2021;27:895–906. doi: 10.1016/j.molmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clayton E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313:1101–1102. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 170.Lambert N., Corps S., El-Azab S.A., et al. COVID-19 survivors’ reports of the timing, duration, and health impacts of post-acute sequelae of SARS-CoV-2 (PASC) Infection. medRxiv. Published online March 22, 2021 doi: 10.1101/2021.03.22.21254026. [DOI] [Google Scholar]
- 171.Petek B.J., Moulson N., Baggish A.L., et al. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) Br J Sports Med. Published online November 1, 2021 doi: 10.1136/bjsports-2021-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O’Laughlin K.N., Thompson M., Hota B., et al. Study protocol for the Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE): a longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection. medRxiv. Published online August 5, 2021 doi: 10.1101/2021.08.01.21261397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peluso M.J., Lu S., Tang A.F., et al. Markers of immune activation and inflammation in individuals with post-acute sequelae of SARS-CoV-2 infection. medRxiv. Published online July 11, 2021 doi: 10.1101/2021.07.09.21260287. [DOI] [Google Scholar]
- 174.Wang E.Y., Mao T., Klein J., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 175.Visvabharathy L., Hanson B., Orban Z., et al. Neuro-COVID long-haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv. Published online October 29, 2021 doi: 10.1101/2021.08.08.21261763. [DOI] [Google Scholar]
- 176.Gold J.E., Okyay R.A., Licht W.E., et al. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 2021;10(6):763. doi: 10.3390/pathogens10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Evans P.C., Rainger G.E., Mason J.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Charfeddine S., Ibn Hadj, Amor H., Jdidi J., et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV Study. Front Cardiovasc Med. 2021;8:745758. doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baratto C., Caravita S., Faini A., et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985) 2021;130:1470–1478. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Clavario P., De Marzo V., Lotti R., et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. medRxiv. Published online November 16, 2020 doi: 10.1101/2020.11.15.20231985. [DOI] [Google Scholar]
- 181.Rinaldo R.F., Mondoni M., Parazzini E.M., et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58(2):2100870. doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Benarroch E.E. “Dysautonomia”: a plea for precision. Clin Auton Res. 2021;31:27–29. doi: 10.1007/s10286-020-00749-3. [DOI] [PubMed] [Google Scholar]
- 183.Levine B.D., Zuckerman J.H., Pawelczyk J.A. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 184.Fu Q., Vangundy T.B., Galbreath M.M., et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shibata S., Fu Q., Bivens T.B., et al. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590:3495–3505. doi: 10.1113/jphysiol.2012.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gaffney F.A., Nixon J.V., Karlsson E.S., et al. Cardiovascular deconditioning produced by 20 hours of bedrest with head-down tilt (-5 degrees) in middle-aged healthy men. Am J Cardiol. 1985;56:634–638. doi: 10.1016/0002-9149(85)91025-2. [DOI] [PubMed] [Google Scholar]
- 187.Saltin B., Blomqvist G., Mitchell J.H., et al. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–VII78. [PubMed] [Google Scholar]
- 188.Singh I., Joseph P., Heerdt P.M., et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161:54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Blomberg B., Mohn K.G., Brokstad K.A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Quyyumi A.A., Cannon R.O., 3rd, Panza J.A., et al. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–1871. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 191.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Espejo C., Mejia-Renteria H., Travieso A., et al. Myocardial ischaemia of non-obstructive origin as a cause of new onset anginal chest pain in the long COVID syndrome. Eur Heart J. 2021;42 [Google Scholar]
- 193.Bellan M., Soddu D., Balbo P.E., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Finney L.J., Doughty R., Lovage S., et al. Lung function deficits and symptom burden in survivors of COVID-19 requiring mechanical ventilation. Ann Am Thorac Soc. 2021;18:1740–1743. doi: 10.1513/AnnalsATS.202102-099RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aparisi A., Ybarra-Falcon C., Garcia-Gomez M., et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10(12):2591. doi: 10.3390/jcm10122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mancini D.M., Brunjes D.L., Lala A., et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. J Am Coll Cardiol HF. 2021;9:927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78:e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 198.Hastings J.L., Krainski F., Snell P.G., et al. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol (1985) 2012;112:1735–1743. doi: 10.1152/japplphysiol.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.George S.A., Bivens T.B., Howden E.J., et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13:943–950. doi: 10.1016/j.hrthm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 200.Torjesen I. NICE advises against using graded exercise therapy for patients recovering from COVID-19. BMJ. 2020;370:m2912. doi: 10.1136/bmj.m2912. [DOI] [PubMed] [Google Scholar]
- 201.Lindsay R.K., Wilson J.J., Trott M., et al. What are the recommendations for returning athletes who have experienced long term COVID-19 symptoms? Ann Med. 2021;53:1935–1944. doi: 10.1080/07853890.2021.1992496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fu Q., Levine B.D. Exercise and non-pharmacological treatment of POTS. Auton Neurosci. 2018;215:20–27. doi: 10.1016/j.autneu.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Howden E.J., Sarma S., Lawley J.S., et al. Reversing the cardiac effects of sedentary aging in middle age-a randomized controlled trial: implications for heart failure prevention. Circulation. 2018;137:1549–1560. doi: 10.1161/CIRCULATIONAHA.117.030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bhella P.S., Hastings J.L., Fujimoto N., et al. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–1266. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fu Q., Vangundy T.B., Shibata S., et al. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58:167–175. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arnold A.C., Okamoto L.E., Diedrich A., et al. Low-dose propranolol and exercise capacity in postural tachycardia syndrome: a randomized study. Neurology. 2013;80:1927–1933. doi: 10.1212/WNL.0b013e318293e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raj S.R., Black B.K., Biaggioni I., et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Taub P.R., Zadourian A., Lo H.C., et al. Randomized trial of ivabradine in patients with hyperadrenergic postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2021;77:861–871. doi: 10.1016/j.jacc.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 209.Rector T.S., Bank A.J., Mullen K.A., et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 210.Lundberg J.O., Carlstrom M., Larsen F.J., et al. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 211.Lerman A., Burnett J.C., Jr., Higano S.T., et al. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 212.Tooba R., Mayuga K.A., Wilson R., et al. Dyspnea in chronic low ventricular preload states. Ann Am Thorac Soc. 2021;18:573–581. doi: 10.1513/AnnalsATS.202005-581CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gea J., Pascual S., Casadevall C., et al. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis. 2015;7:e418–e438. doi: 10.3978/j.issn.2072-1439.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Adeloye D., Elneima O., Daines L., et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467–1478. doi: 10.1016/S2213-2600(21)00286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.American Heart Association $10 million invested to study long-term impact of COVID-19 on heart and brain health. https://newsroom.heart.org/news/10-million-invested-to-study-long-term-impact-of-covid-19-on-heart-and-brain-health
- 216.National Institutes of Health NIH builds large nationwide study population of tens of thousands to support research on long-term effects of COVID-19. https://www.nih.gov/news-events/news-releases/nih-builds-large-nationwide-study-population-tens-thousands-support-research-long-term-effects-covid-19
- 217.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 218.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baggish A., Drezner J.A., Kim J., et al. Resurgence of sport in the wake of COVID-19: cardiac considerations in competitive athletes. Br J Sports Med. 2020;54:1130–1131. doi: 10.1136/bjsports-2020-102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bhatia R.T., Marwaha S., Malhotra A., et al. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of Sports Cardiology & Exercise of the European Association of Preventive Cardiology (EAPC) Eur J Prev Cardiol. 2020;27:1242–1251. doi: 10.1177/2047487320930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Phelan D., Kim J.H., Chung E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020;5:1085–1086. doi: 10.1001/jamacardio.2020.2136. [DOI] [PubMed] [Google Scholar]
- 222.Kim J.H., Levine B.D., Phelan D., et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6:219–227. doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 223.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Clark D.E., Parikh A., Dendy J.M., et al. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Eichhorn C., Biere L., Schnell F., et al. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. J Am Coll Cardiol Img. 2020;13:494–507. doi: 10.1016/j.jcmg.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 226.Vago H., Szabo L., Dohy Z., et al. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. J Am Coll Cardiol Img. 2021;14:1279–1281. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Malek L.A., Marczak M., Milosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hendrickson B.S., Stephens R.E., Chang J.V., et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143:1926–1928. doi: 10.1161/CIRCULATIONAHA.121.053982. [DOI] [PubMed] [Google Scholar]
- 229.Hwang C.E., Kussman A., Christle J.W., et al. Findings from cardiovascular evaluation of National Collegiate Athletic Association Division I collegiate student-athletes after asymptomatic or mildly symptomatic SARS-CoV-2 infection. Clin J Sport Med. Published online June 24, 2021 doi: 10.1097/JSM.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 230.Kilgore A. Health experts worry coronavirus could cause lasting heart complications for athletes. https://www.washingtonpost.com/sports/2020/08/08/athletes-coronavirus-heart-complications/
- 231.The Big Ten conference adopts stringent medical protocols; football season to resume October 23-24, 2020. https://bigten.org/news/2020/9/16/the-big-ten-conference-adopts-stringent-medical-protocols-football-season-to-resume-october-23-24-2020.aspx
- 232.Anderson R. Red Sox pitcher Eduardo Rodriguez confirms he's dealing with heart issue stemming from COVID-19 infection. https://www.cbssports.com/mlb/news/red-sox-pitcher-eduardo-rodriguez-confirms-hes-dealing-with-heart-issue-stemming-from-covid-19-infection/
- 233.Bozkurt B., Colvin M., Cook J., et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 234.Malek L.A., Bucciarelli-Ducci C. Myocardial fibrosis in athletes-Current perspective. Clin Cardiol. 2020;43:882–888. doi: 10.1002/clc.23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Baggish A.L., Battle R.W., Beckerman J.G., et al. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70:1902–1918. doi: 10.1016/j.jacc.2017.08.055. [DOI] [PubMed] [Google Scholar]
- 236.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yonker L.M., Neilan A.M., Bartsch Y., et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52 e45. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Merghani A., Maestrini V., Rosmini S., et al. Prevalence of subclinical coronary artery disease in Masters Endurance Athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126–137. doi: 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- 239.Svedberg N., Sundstrom J., James S., et al. Long-term incidence of atrial fibrillation and stroke among cross-country skiers. Circulation. 2019;140:910–920. doi: 10.1161/CIRCULATIONAHA.118.039461. [DOI] [PubMed] [Google Scholar]
- 240.Centers for Disease Control and Prevention Quarantine and Isolation https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html
- 241.Maron B.J., Udelson J.E., Bonow R.O., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 242.La Gerche A., Burns A.T., Mooney D.J., et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 243.Abdullah S.M., Barkley K.W., Bhella P.S., et al. Lifelong physical activity regardless of dose is not associated with myocardial fibrosis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim J.H., Baggish A.L. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the “grey zone” of clinical uncertainty. Can J Cardiol. 2016;32:429–437. doi: 10.1016/j.cjca.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 245.Millar L.M., Fanton Z., Finocchiaro G., et al. Differentiation between athlete's heart and dilated cardiomyopathy in athletic individuals. Heart. 2020;106:1059–1065. doi: 10.1136/heartjnl-2019-316147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.