Abstract
Wallerian degeneration is a widespread mechanism of programmed axon degeneration. In the three decades since the discovery of the Wallerian degeneration slow (WldS) mouse, research has generated extensive knowledge of the molecular mechanisms underlying Wallerian degeneration, demonstrated its involvement in non-injury disorders and found multiple ways to block it. Recent developments have included: the detection of NMNAT2 mutations that implicate Wallerian degeneration in rare human diseases; the capacity for lifelong rescue of a lethal condition related to Wallerian degeneration in mice; the discovery of ‘druggable’ enzymes, including SARM1 and MYCBP2 (also known as PHR1), in Wallerian pathways; and the elucidation of protein structures to drive further understanding of the underlying mechanisms and drug development. Additionally, new data have indicated the potential of these advances to alleviate a number of common disorders, including chemotherapy-induced and diabetic peripheral neuropathies, traumatic brain injury, and amyotrophic lateral sclerosis.
Wallerian degeneration was originally defined as the degeneration of an axon that takes place distal to an injury, characterized by granular disintegration of the cytoskeleton, mitochondrial swelling and axon fragmentation1; however, the mechanism is now known to also occur in many non-injury disorders (TABLE 1, FIG. 1). Wallerian degeneration has gained importance in the fields of neurodegenerative and axonal disorders because of its widespread occurrence, its well-characterized and ‘druggable’ mechanism and the ability to alleviate — and sometimes completely prevent — axon loss by blocking it2. Our deep mechanistic understanding of Wallerian degeneration stems largely from axon injury studies, but the regulatory genes that have been identified in these studies are now known to also influence axon survival in many other circumstances3. Recent advances (highlighted below) include the identification of a genetic association between Wallerian degeneration and human disease, new rescue effects in animal models, and the discovery of new drug targets with unexpected enzyme activities associated with Wallerian degeneration and progress towards the elucidation of their protein structures.
Table 1 |.
Human diseases associated with the Wallerian degeneration pathway
| Human disease | Findings | Comment | Refs |
|---|---|---|---|
| Alzheimer disease | NMNAT2 protein and mRNA are reduced in brains of individuals with Alzheimer disease | This finding suggests that reduced NMNAT2 is associated with, and may be a risk factor for, the development of Alzheimer disease | 95 |
| Erythromelalgia and peripheral neuropathy | A homozygous NMNAT2 partial loss-of-function mutation in two individuals with erythromelalgia and peripheral neuropathy | Loss-of-function mutation in NMNAT2 results in development of peripheral neuropathy | 89 |
| Fetal akinesia deformation syndrome | Compound heterozygous NMNAT2 severe loss-of-function mutations in two fetuses with fetal akinesia deformation syndrome | Severe loss-of-function mutation in NMNAT2 results in stillbirth, mimicking observations in NMNAT2 null mice | 90 |
| ALS | GWAS show association of the SARM1 locus with ALS | A pathogenic role of SARM1 in ALS must involve gain-of-function variants that increase the risk of axonal degeneration | 101,102 |
| ALS | An ALS-causing mutation in TARDBP reduces levels of stathmin 2 in iPSC-derived motor neurons; a similar decrease is common in sporadic ALS | Low levels of stathmin 2 increase the likelihood of Wallerian degeneration and could therefore be an important modifier or risk factor for ALS | 96,97 |
ALS, amyotrophic lateral sclerosis; GWAS, genome-wide association studies; iPSC, induced pluripotent stem cell; NMNAT2, nicotinamide mononucleotide adenylyltransferase 2; SARM1, sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1; TDP43, transactive response DNA binding protein 43 kDa.
Fig. 1 |. Activation of Wallerian degeneration in injury and disease.
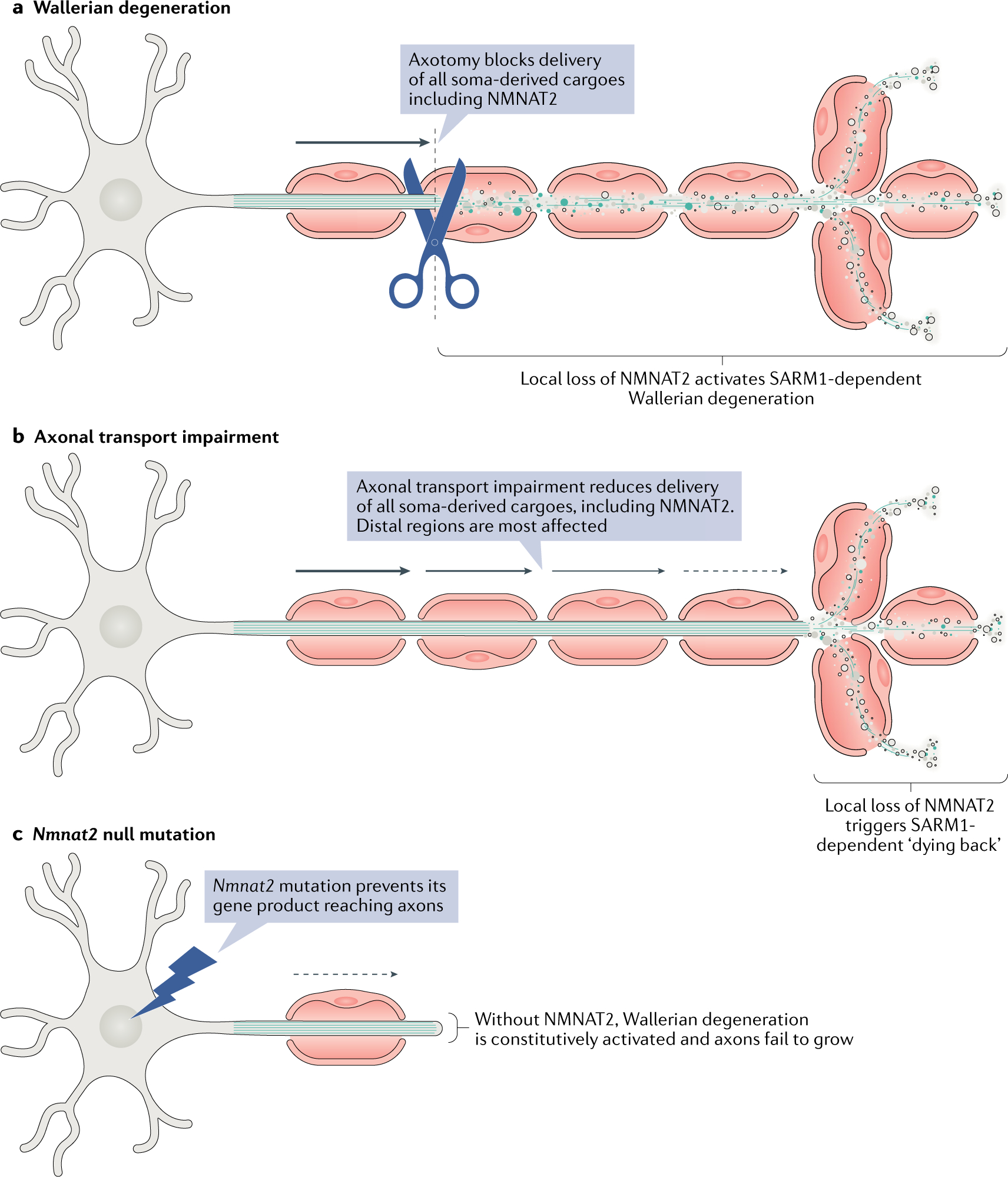
The figure shows three ways in which the Wallerian degeneration mechanism can be activated. a | In classical Wallerian degeneration paradigms, axon injury prevents the delivery of nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) from the soma along with all other cargoes12. Because of its short half-life, NMNAT2 is quickly lost in the distal stump, which triggers sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1-dependent degeneration27. b | Axonal transport impairment (for example, due to a toxin, a protein defect or ageing) limits the supply of all cargoes to distal axons. Delivery of NMNAT2 to distal ends of the axon is particularly affected because of its short half-life. When NMNAT2 falls below the threshold for survival, the distal terminal dies back. c | Mutation of the Nmnat2 gene leads to loss or dysfunction of the protein13,89,90. Without it, the axon cannot grow, causing a developmental disorder (unless rescued through a Sarm1 deletion, for example). If the mutation causes only a partial loss of function, the axon grows but is more susceptible to degeneration.
The discovery, three decades ago, of a mouse strain with an unusual phenotype began the research that has led us to today’s detailed molecular understanding of Wallerian degeneration (FIG. 2). In these Wallerian degeneration slow (WldS) mice, transected axons survive tenfold longer than normal4. Until this finding, Wallerian degeneration was generally considered to result from a lack of ‘nourishment’ of the distal axon by the soma in transected axons1. Alternatively, Lubinska5 had proposed that one specific, essential substance, delivered by axonal transport, inhibits Wallerian degeneration. Lubinska had no means of identifying this substance; the existence of a mutant mouse has now made this possible.
Fig. 2 |. Wallerian degeneration timeline.
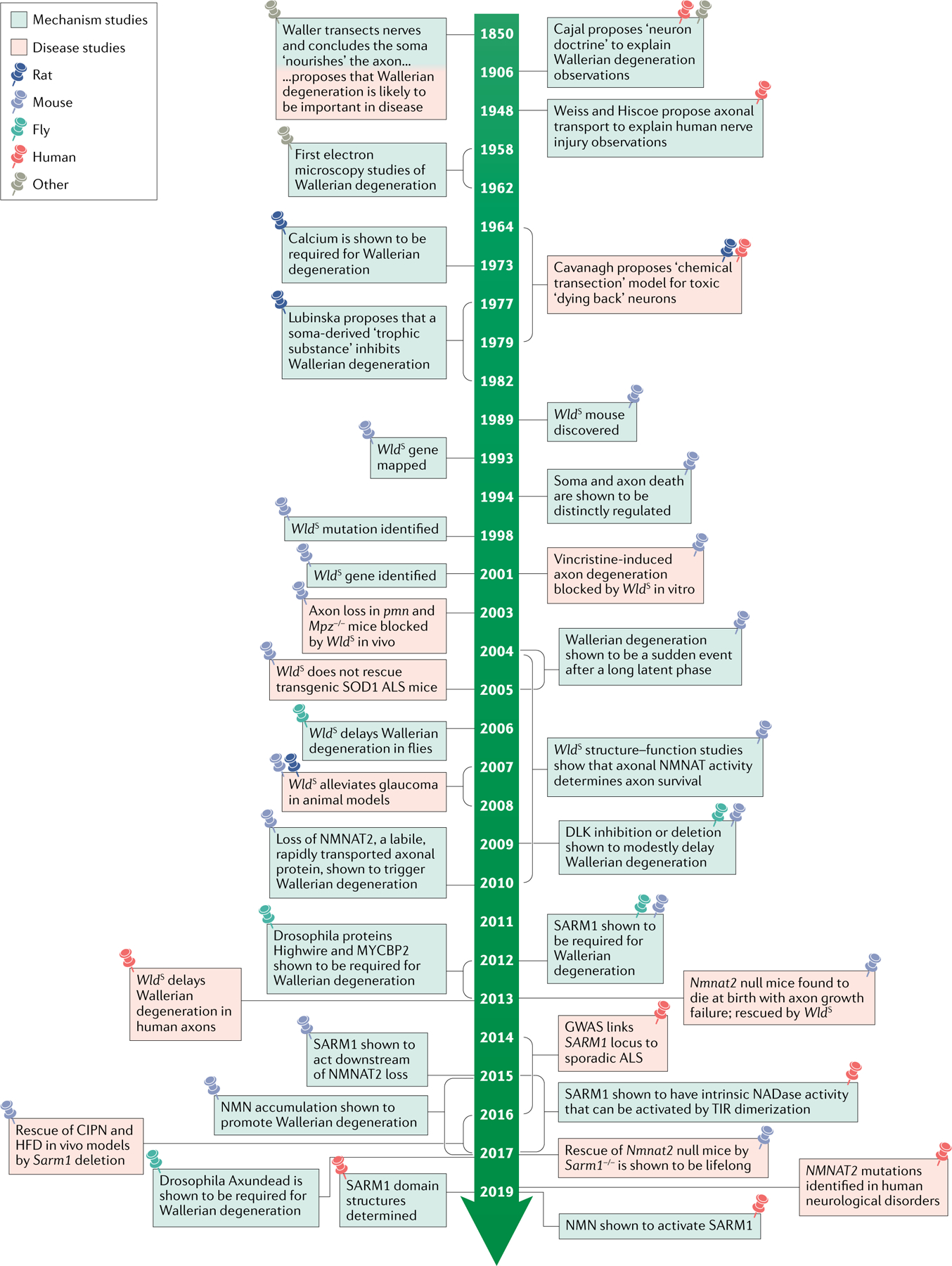
Prior to discovery of the Wallerian degeneration slow (WldS) mouse, there was wide acceptance that axonal transport was required for axon survival and that axon injury could serve as a model for disease, albeit with no molecular understanding of the process5. The identification of the axon-protective gene encoded by WldS made it possible to address this question and led to the identification of nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) as the best match for the previously proposed ‘Wallerian degeneration inhibitor’12. Conservation of the Wallerian mechanism in Drosophila melanogaster has enabled the discovery of other major players in the pathway, including sterile-α and TIR motif containing protein 1 (SARM1)18. More recently, human genetic studies have started to identify the clinical disorders involving this preventable degeneration pathway, with promising leads in the quest to develop drugs to block it89,90. ALS, amyotrophic lateral sclerosis; CIPN, chemotherapy-induced peripheral neuropathy; DLK, dual leucine zipper kinase; GWAS, genome-wide association studies; HFD, high-fat diet; NMN, nicotinamide mononucleotide; SOD1, superoxide dismutase 1; TIR, Toll/interleukin 1 receptor.
Structure–function analysis of the protective WldS gene product, a unique fusion of nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1; an enzyme involved in the synthesis of the redox and signalling cofactor nicotinamide adenine dinucleotide (NAD)), with part of the ubiquitin ligase UBE4B6, suggested a key role for axonal NAD metabolism in axon maintenance7. Indeed, most subsequent studies have suggested that the NMNAT enzyme activity of the WldS gene product, localized to axons by the fused protein, is required for the axon protection phenotype8–10. NMNAT2, a related protein that is normally found in the axon and for which WLDS can substitute, was subsequently found to be essential for axon growth and survival11–13 and remains the best fit for Lubinska’s natural ‘inhibitor’ of Wallerian degeneration. A key property of NMNAT2 is its short half-life12,14, which necessitates constant resupply by new protein synthesis and axonal transport. When NMNAT2 is lost from axons, whether owing to injury, mutation, silencing, axonal transport disruption, or other causes, the resulting degeneration can be blocked by WLDS, indicating that it involves a Wallerian-like mechanism3 (FIG. 1).
Wallerian degeneration slow (WldS) mice
A mutant strain of mouse showing a tenfold delay in the onset of Wallerian degeneration after axotomy as well as axon protection in many disease models.
Axonal transport
The ATP-dependent, bidirectional trafficking of axonal proteins, organelles, mRNAs and other cargoes delivering axonal constituents to where they are required, often over large distances.
The discovery of the mouse WldS gene also led to the finding that the pathway is conserved in Drosophila melanogaster15,16 (FIG. 2). Powerful D. melanogaster genetic studies subsequently revealed a set of additional positive and negative regulators of Wallerian degeneration17–19 that are largely conserved in mammals and have recently provided a strong basis for drug development efforts (FIG. 3). The key roles of D. melanogaster in elucidating this pathway, and the similarities and differences between the pathway in mammals and flies, have recently been reviewed elsewhere20; therefore, they are not the main focus of this article.
Fig. 3 |. A working model of the Wallerian degeneration pathway.
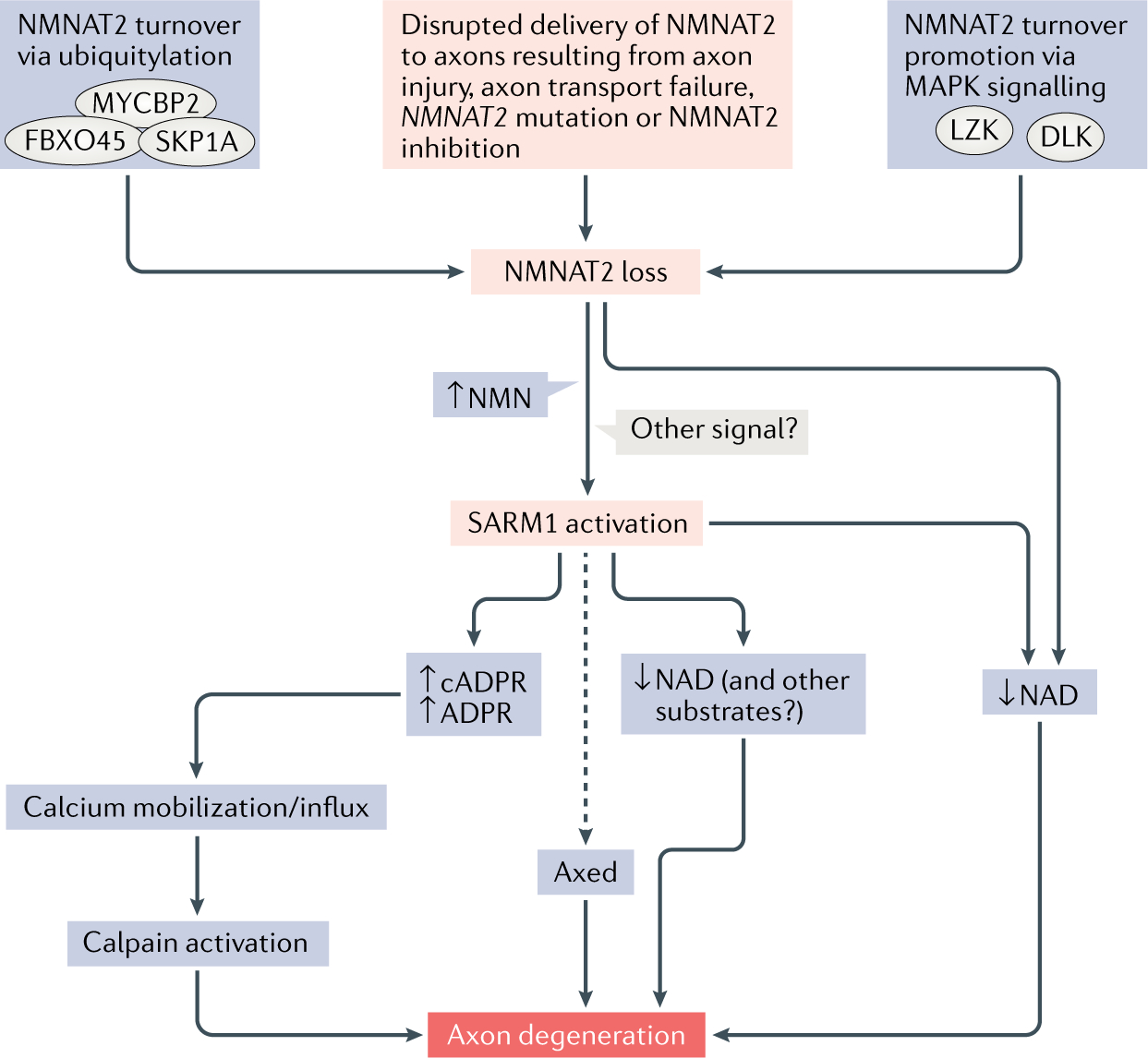
The figure shows current knowledge of the factors that determine nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) activity in axons, how NMNAT2 loss activates sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1) and events downstream of SARM1 leading to axon degeneration. Various factors disrupt the delivery of functional NMNAT2 to axons. NMNAT2 turnover is mediated by the mitogen-activated protein kinases (MAPK) dual leucine zipper kinase (DLK) and leucine zipper-bearing kinase (LZK), as well as a ubiquitin ligase complex consisting of MYC-binding protein 2 (MYCBP2), s-phase kinase-associated protein 1A (SKP1A) and F-box protein 45 (FBXO45)38. In combination, this reduces NMNAT2 levels and causes downstream activation of SARM1, probably as a result of increased levels of nicotinamide mononucleotide (NMN) but potentially also in other ways35. The combined effect of decreased NMNAT2 and increased activation of SARM1 leads to a great decrease in axonal nicotinamide adenine dinucleotide (NAD), which may itself cause axon degeneration through ATP synthesis failure25. Alternatively, other SARM1 substrates or its calcium mobilizing products could be important for the later stages of Wallerian degeneration. In Drosophila melanogaster, there is also an as-yet undefined role for Axundead (Axed) in Wallerian axon degeneration19. ADPR, ADP-ribose; cADPR, cyclic ADP-ribose.
This Review describes the major recent advances in our understanding of the Wallerian degeneration mechanism and distinguishes areas of agreement and ongoing debate. It reviews what we have learned from animal models about the roles of Wallerian degeneration in disease and explains why complementary human studies are vital. Finally, it discusses which proteins in the pathway might be targeted for therapeutic intervention and considers outstanding questions that remain to be addressed in order to move the field towards clinical application.
Wallerian mechanisms: recent advances
Mechanisms driving NAD loss.
NAD levels are known to decline in injured axons and nerves21,22. Part of the explanation for this decrease is the rapid loss of its normal synthetic axonal enzyme, NMNAT2, which has a short half-life and cannot be replenished by axon transport from the soma after injury. Indeed, the presence of a more stable axonally targeted NMNAT, such as WLDS, stabilizes NAD levels after injury22. However, an important recent discovery was the identification of the NAD-degrading activity of another axonal protein, sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1), as well as its activation as part of the molecular pathway that induces Wallerian degeneration23 (FIG. 3).
SARM1 is a Toll-like receptor adapter protein that is required for the normal, rapid rate of Wallerian degeneration18. It has an ‘execution’ role in Wallerian degeneration, analogous to the roles of caspases as executors of apoptosis, although the molecular mechanism is distinct. This function was discovered in D. melanogaster, replicated using Sarm1−/− mice18 and later confirmed in a separate mammalian RNA interference study24. However, the mechanism by which it kills axons was not immediately apparent. In 2015, experiments employing forced dimerization of the SARM1 TIR signalling domain demonstrated an unknown NADase activity that generates ADP-ribose (ADPR) and cyclic ADPR (cADPR) from NAD25. It was subsequently shown that murine SARM1 is required for extensive NAD loss after injury25 and that a mutated human SARM1 that is unable to degrade NAD cannot, unlike wild-type human SARM1, support fast Wallerian degeneration when introduced to Sarm1−/− mouse neurons23. Isotopic labelling of NAD in mouse dorsal root ganglion (DRG) neuron cultures showed that there is both NAD synthesis failure at around 2 h after axon transection (likely owing to NMNAT2 loss) and a SARM1-dependent, fourfold acceleration of NAD degradation26. The intrinsic NADase activity of SARM1’s own TIR domain was then demonstrated by experiments with cell-free transcribed and translated TIR protein23.
SARM1 is known to act downstream of NMNAT2 loss in the Wallerian degeneration pathway as its removal in mice protects NMNAT2 null axons without fully preserving NAD levels27. SARM1 activation exacerbates the effects of slowing NAD generation caused by NMNAT2 loss through an increase in NAD degradation, causing a precipitous decline in NAD levels (FIG. 3). The discovery of this enzyme activity has also changed the perception of the suitability of SARM1 as a drug target (discussed below).
Toll-like receptor
A family of receptors on plasma and endosomal membranes that detect molecular patterns associated with infection or cell damage, activating signalling pathways leading to inflammation or cell death.
The contribution of NMN to Wallerian degeneration.
Given the well-known roles of NAD in ATP synthesis and many other cellular activities28, its loss is an obvious and attractive candidate mechanism to explain the axon degeneration that occurs after injury. However, it remains unclear whether the loss of NAD offers a full explanation. Manipulating NAD levels through methods that do not involve altering NMNATs (or their substrates and precursors) has been repeatedly found not to alter Wallerian degeneration22,29,30. Moreover, inhibitors of nicotinamide phosphoribosyl transferase (NAMPT), the synthetic enzyme that generates NMN, such as FK866, lower NAD by up to 90%; however, rather than killing axons as expected, they partially phenocopy the effects of WLDS, protecting injured axons22,29–31. To explain this paradox, a harmful role for the NAD precursor nicotinamide mononucleotide (NMN) was proposed22 NMN accumulates in the axon distal to the injury, at least transiently, during Wallerian degeneration22,26 and both WLDS and FK866 prevent this accumulation — WLDS maintains NMN removal from axons by metabolizing it to produce NAD, whereas FK866 stops NMN synthesis22 (FIG. 4). Supporting the idea of NMN toxicity, ectopically expressed bacterial NMN deamidase, which removes NMN without altering NAD (at least after axon injury), strongly protects cut axons in mice22,26,32 and exogenous NMN or ectopically expressed NMN synthase both restore degeneration to cut murine neurites that have been protected by FK866 (REFS30,33,34). However, in conditions in which NAD levels are also high, NMN does not activate axon degeneration26.
Fig. 4 |. Activation of SARM1 by NMN.
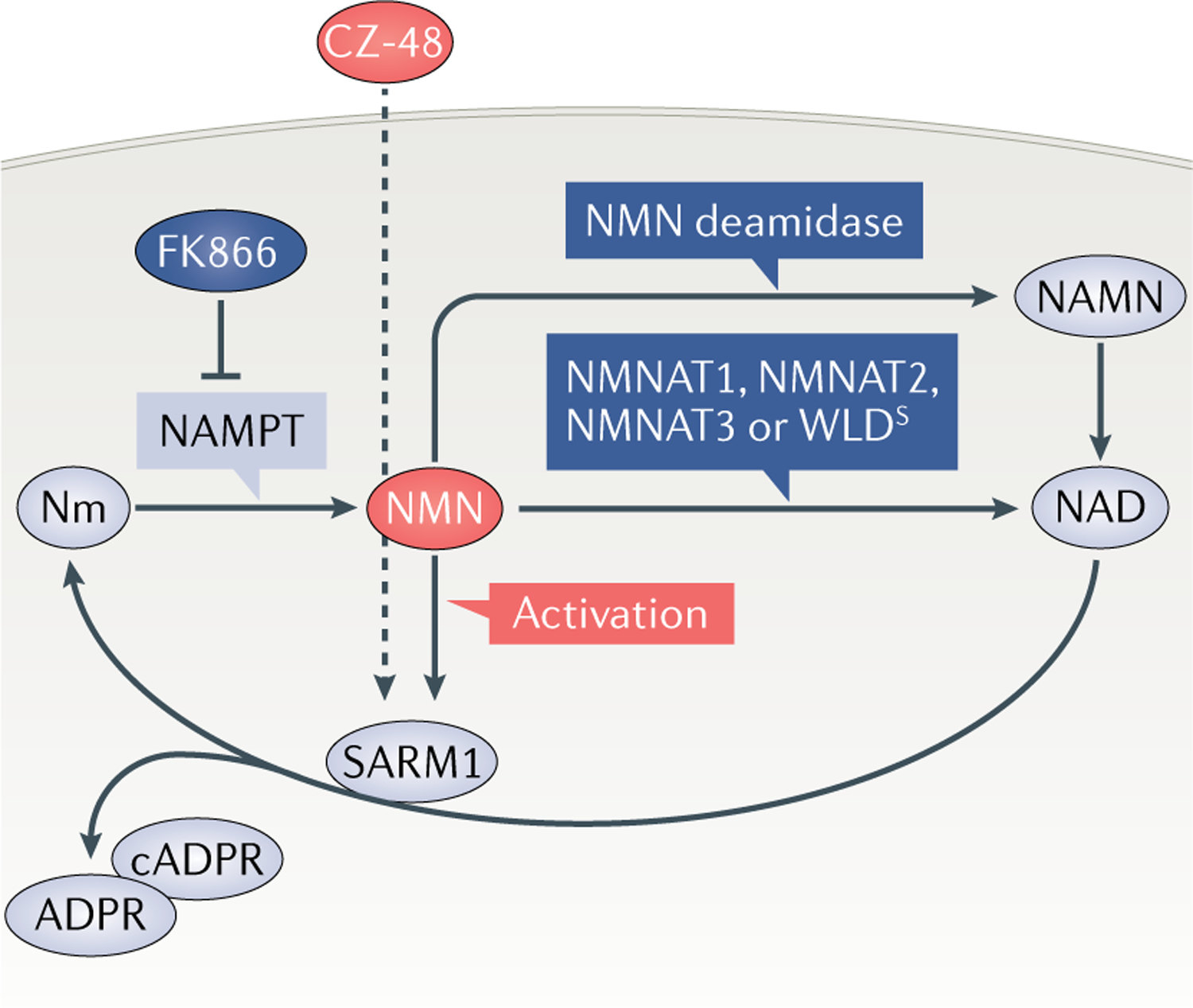
The figure summarizes evidence supporting activation of sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1) by nicotinamide mononucleotide (NMN). NMN accumulates after axon injury22 because the enzyme that normally removes it, nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2), is quickly degraded12. If left unchecked, this activates SARM1, leading to axon degeneration27. However, NMN accumulation can be prevented in three ways (dark blue): through inhibition of its synthetic enzyme, nicotinamide phosphoribosyl transferase (NAMPT)22,29–31; by overexpression, or increased axonal targeting, of any isoform of NMNAT3,10, its normal processing enzyme in mammals; or by ectopic expression of bacterial NMN deamidase22,32. Conversely, its membrane-permeable analogue CZ-48 activates SARM1 directly35. Thus, NMN, or a close analogue, appears to be necessary and sufficient for SARM1 activation. ADPR, ADP-ribose; cADPR, cyclic ADP-ribose; NAD, nicotinamide adenine dinucleotide; NAMN, nicotinic acid mononucleotide; Nm, nicotinamide; WLDS, Wallerian degeneration slow.
Since neither NAD loss nor NMN accumulation by themselves explain all available data, it can be hypothesized that both may be part of the mechanism. For example, the ratio between NAD and NMN levels may be important. Alternatively, the recent finding that NMN, or a synthetic membrane-permeable analogue (CZ-48), can activate SARM1 suggests that NMN could drive further NAD loss35 (FIG. 3). However, such NMN-dependent SARM1 activation has not yet been demonstrated in axons.
Downstream executors of degeneration.
Even once SARM1 is activated, it is still unclear whether the cause of the axon degeneration is NAD loss, an increase in the level of a SARM1 enzyme product or the increased metabolism of an alternative substrate35. If it is NAD loss, it is clear that levels must become very low to have an effect: NAD declines by 50% in transected sciatic nerves in Sarm1−/− mice within 5 days of injury27 and by 80% in severed DRG neurites in Sarm1−/− mice within 36 h of injury36; however, in both cases, the neurons survive for a considerably longer period. Changes in calcium mobilization and/or influx provide one alternative mechanism. The SARM1 products ADPR and cADPR are powerful calcium mobilizers28 and, in mice, NMN activates a SARM1-dependent rise in intra-axonal calcium that immediately precedes the demise of the axon34. Interestingly, in D. melanogaster, the protein Axundead (Axed), which (like SARM1 and its D. melanogaster orthologue dSarm) is required for rapid Wallerian degeneration, appears to act downstream of dSarm19. Axed is likely to act either as an executioner of degeneration or as a negative regulator of a powerful compensatory mechanism. Axed is a BTB/BACK-containing protein with no obvious role in calcium or NAD metabolism, although its ability to protect either in the presence of activated dSarm or in the absence of dNmnat19 (the only known NAD synthetic enzyme in D. melanogaster) strongly suggests that it influences NAD in some way. Elucidation of its function, as well as the identification and characterization of any mammalian orthologue will be crucial steps in understanding and preventing Wallerian degeneration.
Regulating NMNAT2 stability.
Understanding the regulation of NMNAT2 stability is another rapidly developing area. NMNAT2 had a half-life of less than 40 min in human embryonic kidney cell experiments14 and was greatly depleted in mouse neurites 4 h after transection12. How such a labile protein can reach distal axons before being degraded, a journey that takes 2 days in the longest human axons, remains unclear37. The existence of two distinct NMNAT2 pools — vesicular and non-vesicular — is probably part of the explanation14. It has been shown that, in mice, the distribution between vesicular and non-vesicular Nmnat2 is regulated in part by palmitoylation14,38. However, studies that hypothesized that the moving, vesicular form of the enzyme may be more stable than non-vesicular NMNAT2 found that the opposite was true: disrupting vesicle targeting lengthened NMNAT2’s half-life and greatly strengthened its capacity to protect axons14,39. Although candidate palmitoylation and depalmitoylation enzymes were identified40, these are probably not good drug targets as they have many substrates. It has also been shown that mitogen-activated protein kinase (MAPK) signalling promotes NMNAT2 turnover as depletion of MKK4 (also known as MAP2K4) and MKK7 or blockage of their upstream regulators dual leucine zipper kinase (DLK) and leucine zipper-bearing kinase (LZK) extends the half-life of the palmitoylated form of NMNAT2 and increases the survival of transected murine neurites38,41. MAPK signalling is also influenced by SARM1, but whether this influences axon survival remains unclear38,42.
Turnover of the soluble, more stable NMNAT2 pool14 is influenced by the MYC-binding protein 2 (MYCBP2)–S-phase kinase-associated protein 1 (SKP1)-F-box protein 45 (FBXO45) ubiquitin ligase complex38, each member of which has been separately implicated in Wallerian degeneration17,43–46. Ubiquitylation determines NMNAT2 turnover rate14, but the newly discovered serine/threonine monoubiquitylation activity of MYCBP2 (REF.47) raises important questions about the role of classical lysine polyubiquitylation45 in this process. Like SARM1 and MAPK signalling, this is another promising area for drug development, especially as the structural basis of serine/threonine ubiquitylation is known47.
Calcium mobilization
The release of calcium from intracellular stores such as endoplasmic reticulum and mitochondria, potentially activating calcium-activated proteases, under the control of second messenger molecules (many of which are NAD metabolites).
Next steps.
The recent rapid progress in our understanding of Wallerian degeneration mechanisms has raised many new questions. Prominent among them is ‘how is SARM1 activated?’ The recently discovered activation of SARM1 by NMN appears to provide one possible answer to this question35 and, if confirmed in axons, could explain why NMN promotes Wallerian degeneration22,32,34 (FIG. 4); however, whether NMN binds directly to SARM1 (and if so where), whether other proteins are involved and whether NAD can block this activation (and if so, what level of NAD is required to do so)26 remain unclear. Emerging information about the structure of SARM1 (REFS48,49) will be important in answering these questions.
Both in axons and in non-neuronal cells there are indications of other SARM1 activation mechanisms that need to be brought together for a full understanding of how this protein is regulated. On the proximal side of an axon injury, SARM1 is required for proinflammatory responses50; however, in this location, there is no NMNAT2 loss12 nor a rise in NMN, so there must be another activation mechanism at work. SARM1 expression level appears to be another point of regulation as this is altered by some viruses and Toll-like receptor ligands in neuronal and/or non-neuronal cells51,52. A full understanding of SARM1 regulation also requires a better understanding of the roles of post-translational modifications, such as the reported JNK-dependent phosphorylation53 and of mitochondrial targeting, reported by some to be important for the prodegenerative function of SARM1 (REFS53,54) (but not by others24). The normal subcellular location of SARM1 also remains to be resolved. While overexpressed and fluorescent protein-tagged SARM1 colocalizes with mitochondria under basal conditions55,56, the endogenous protein does not colocalize with mitochondria18, or at least not completely24,57.
Other questions arise about the evolution of SARM1 and its relationship to NAD hydrolysis by other, evolutionary-divergent TIR domains49,58,59. The wider involvement of this newly discovered enzyme activity in innate immunity suggests that there may be an ancient mechanism of pathogen defence involving NAD modulation49,59. It could be hypothesized that axon injury may have co-opted this mechanism later in evolution to remove damaged axons. However, the ultimate effector of this mechanism remains unknown.
Questions also remain about other regulators of NMNAT2. Could local translation of NMNAT2 (REF.60) as well as the existence of different NMNAT2 pools14,38 help explain how this unstable protein reaches distal axons without being degraded? It is not known precisely how MYCBP2, DLK and LZK influence the steady state level of each NMNAT2 pool; specifically, it is unclear which NMNAT2 residues they may phosphorylate or ubiquitylate and whether they are responsible for its polyubiquitylation or monoubiquitylation. Stabilizing, boosting the synthesis of61 and activating NMNAT2 have all been proposed as important therapeutic strategies for axonopathies, making these key questions for advancement in this area. Finally, identifying proteins that control NMNAT2 expression level, enzyme activity and axonal transport is also important. Given the absolute requirement for NMNAT2 for axon survival and the enhanced level of protection provided by the soluble form of this enzyme39, such modulators are likely to influence axon survival; however, very few of these factors have yet been identified62,63.
Linking degeneration to disease
Animal models of disease.
The notion that Wallerian degeneration mechanisms are important in disease dates back to the work of Waller1 (FIG. 2) but could only be tested after mutant WldS mice were discovered. Axon degeneration in many animal models of disease can be alleviated by the presence of WLDS (as previously summarized3), which has prompted a number of studies investigating the effects of manipulating the Wallerian degeneration pathway in other ways in animal models of disease.
Local translation
The synthesis of proteins directly within axons using mRNAs delivered by axonal transport.
Several studies have attempted to prevent distal axonal degeneration in mouse models of peripheral neuropathy by Sarm1 deletion. Peripheral neuropathy caused by the commonly used chemotherapy drugs vincristine or bortezomib was prevented in Sarm1−/− mice64,65. Similarly, peripheral neuropathy was prevented in Sarm1−/− mice given either paclitaxel, another common chemotherapy drug that causes neuropathy, or a high-fat diet (HFD) (a model of metabolic syndrome that results in neuropathy)66. In each study, the primary end point was the loss of intraepidermal nerve fibres, the distal endings of unmyelinated sensory axons that tend to degenerate first in most axonal peripheral neuropathies. Thus, it is possible that the distal axonal degeneration seen in these toxic and metabolic syndromes involves molecular mechanisms that are shared with those of Wallerian degeneration.
What about the other types of axonal injury seen in human diseases? For example, traumatic brain injury (TBI) causes stretching and shearing of axons. One would expect that axons in WldS and Sarm1−/− mice would be protected from axon degeneration following TBI, as the mechanism may be similar to traumatic axotomy. Indeed, in two separate studies, Sarm1−/− mice showed decreased axonal damage in TBI models67,68, with functional preservation also observed in the milder model67. Furthermore, blocking NAMPT with FK866 partially phenocopied Sarm1−/−, mirroring findings in peripheral nerves68. Obviously, traumatic injuries pose a therapeutic challenge as any clinical intervention has to happen after injury. It is unclear how effective a delayed blockade of SARM1 will be in these models; nevertheless, interventions such as the application of FK866, cleavage of SARM1, or transduction of NMNAT1 protein have been found to be effective several hours after injury in animal models22,25,69. Combined with the observation that delayed overexpression of WLDS in axons up to 4–5 h after axotomy can prevent Wallerian degeneration70, these results suggest that intervention early after TBI could be feasible.
Secondary or primary progressive multiple sclerosis, in which gradual axonal degeneration is important in the progressive loss of neurological function, may be another potential therapeutic target for Wallerian degeneration-blocking drugs. Axonal injury has been shown to be present in several rodent models of multiple sclerosis, with studies showing that behavioural deficits and axonal loss are attenuated in an experimental autoimmune encephalitis (EAE) model of multiple sclerosis in WldS mice71,72. No studies have been published to date investigating the effects of EAE in Sarm1−/− mice.
Manipulation of the Wallerian degeneration pathway through treatment with nicotinamide, a precursor of NAD, has been shown to be effective in a genetic mouse model of glaucoma in which there is an age-associated decline in retinal NAD levels and axonal degeneration of retinal ganglion cells73,74. Furthermore, the same approach has been used successfully in murine models of diabetic peripheral neuropathy75. Dietary supplementation of nicotinamide riboside (NR) prevented neuropathy both in a mouse model of HFD-induced metabolic syndrome and in mice that received both HFD and low-dose streptozotocin to model type 2 diabetes75. However, in these studies, it is unclear whether NR truly acts by blocking the Wallerian degeneration pathway as no mechanistic studies were carried out. Furthermore, the dose required for effect in these models is much higher than equivalent doses feasible in humans: the maximum tested dose of nicotinamide in humans is 1,000 mg twice a day, which showed no efficacy against several metabolomic parameters in obese men despite raising blood NAD levels76. Perhaps combining NR or nicotinic acid riboside with the manipulation of enzymes in the NAD synthesis pathway (using NAMPT inhibitors, for example) may be more effective, as observed in a recent vincristine-induced neuropathy model in murine primary cultures30 (FIG. 4). Similarly, in models of increased intraocular pressure glaucoma, both axons77,78 and NAD levels73 are preserved in WldS mice and, when supplemented with additional nicotinamide, axon protection is further increased. A similar treatment also further abrogated the disease phenotype and axonal loss in EAE in mice71.
The molecular mechanism by which Sarm1 deletion prevents distal axonal degeneration is likely to be cell-autonomous within axons, but the role of non-neuronal cells in peripheral neuropathies needs to be considered. Examples of sites of non-neuronal actions of SARM1 include macrophages79 and in D. melanogaster, glia80. Overexpressed SARM1 was found to be present in both the cytosol and the mitochondrial matrix of human embryonic kidney cells and endogenous SARM1 was associated with the matrix proapoptotic Nod-like receptor, NLRX1 (REF.57). Although apoptosis in non-neuronal cells was dependent on both SARM1 and NLRX1, axonal degeneration in murine primary neurons induced by vinblastine was dependent only on SARM1, indicating perhaps a different role for SARM1 in non-neuronal cells.
Despite these promising results, the findings of animal studies suggest that manipulating SARM1 or other components of the Wallerian degeneration pathway may not prevent all axonal degeneration. Although one of the earliest events in a mouse model of amyotrophic lateral sclerosis (ALS) driven by mutations in superoxide dismutase (SOD1) is distal axonal degeneration and denervation at the neuromuscular junctions81, when the mutant Sod1 mice were crossed to WldS mice, there was minimal impact on disease onset or survival82. Similarly, when Sod1G93A mutant mice were bred with Sarm1−/− mice, there was no rescue83. Thus, neurodegeneration in this particular model of ALS is unlikely to depend on the molecular players involved in Wallerian degeneration82, although there is evidence that Wallerian degeneration influences axon survival in a TDP-43 model of ALS84.
The role that SARM1 plays in axon degeneration versus neuronal cell death has also been shown to vary significantly across cell populations and disease models. For example, in an optic nerve crush-induced retinal ganglion cell (RGC) death model of glaucoma, Sarm1−/− mice showed preservation of distal optic nerve axons but no prevention of DLK–JNK pathway activation in the somanor of RGC death85. Similarly, in a prion disease model, Sarm1−/− mice had an exacerbated neuronal death upon infection, independent of any role of SARM1 in immune cells86. This observation mirrors the findings of the initial study that described the development of a Sarm1−/− mouse line, in which infection with West Nile virus led to increased neuronal death in Sarm1−/− mice due to impairment of microglial activation87. Finally, the expression of mutant proteins that cause ALS in Caenorhabditis elegans motor neurons, induces an innate immune response that propagates neuronal death88; this process requires the SARM1 orthologue. Therefore, these studies point to a potentially complex role for SARM1 in neuronal and non-neuronal cells and their interactions in disease states.
Mechanistic basis of disease-modifying effects.
As outlined above, axotomy studies have revealed Wallerian degeneration to be a pathway that can be activated in multiple ways, the most specific of which is the removal of just one protein, NMNAT2. Under these circumstances, SARM1 is absolutely required for degeneration as axons are rescued permanently in Nmnat2::Sarm1 double null mutant mice2. Less specific lesions, such as axotomy or general axonal transport impairment, prevent the delivery of all cargoes from the soma. Eventually, the levels of other proteins must fall below the threshold for survival, which we believe may explain why rescue through Sarm1 knockout is only temporary in these models.
With this in mind, there are several scenarios in which blocking Wallerian degeneration in the clinic is likely to be particularly effective. One is in disorders caused or exacerbated by specific disruption of NMNAT2, for example, those driven by its genetic mutation89,90 or inhibition91. Another is in disorders in which there is an ongoing modest impairment of the delivery of many cargoes to distal axons but in which only NMNAT2 falls below the threshold for survival; this could include age-related disorders of long or highly branched axons, where the age-related decline in axonal transport62 and disease-specific mechanisms together impair the delivery of cargoes to axon terminals. A third scenario is in disorders in which there is a temporary impairment of transport or synthesis that is resolved before the loss of other more stable proteins becomes limiting. For example, the treatment of DRG primary cultures with vincristine for 24 h, followed by washout, causes the degeneration of wild-type neurites from which WLDS provides permanent protection92. A similar outcome was seen when protein synthesis was transiently blocked and then restored in superior cervical ganglion neurons12. The treatment of chemotherapy-induced peripheral neuropathy (CIPN) is an obvious application of this knowledge, as chemotherapy is temporary, the timing predictable, and rescue has been very widely replicated and extended to in vivo studies9,42,64–66,93.
There are many other ways in which axons may be depleted of functional NMNAT2 in disease, which we suggest may explain why blocking Wallerian degeneration alleviates symptoms in some disease models that are not obviously caused by axonal transport impairment. Axonal NMNAT2 activity is likely to be lowered if there is impaired synthesis at the transcriptional, post-transcriptional or translational level, or by reduced protein stability, enzyme inhibition or posttranslational modifications that influence any of the above. NMNAT2 transcription is regulated in part by cAMP response element-binding protein (CREB)63 and Ras-responsive element-binding protein 1 (RREB1; the mammalian orthologue of D. melanogaster zinc transcription factor Pebbled) is another candidate regulator of NMNAT2 transcription given its influence on Wallerian degeneration94. There is huge variation between individuals in NMNAT2 mRNA expression in human post mortem brains, suggesting that there are multiple transcriptional and epigenetic regulators of this protein95. Acute protein synthesis blockade causes axon degeneration through the Wallerian pathway12, suggesting that disorders of protein synthesis may activate it. Normal ageing lowers NMNAT2 axonal transport by 40–50%, potentially contributing to axon loss in age-related diseases62. Depletion of stathmin 2 (SCG10), a protein that co-migrates with NMNAT2 in axons and shares several properties with it14, potentiates Wallerian degeneration and is frequently seen in sporadic ALS96,97, raising the prospect of a role in that disease. Finally, environmental inhibitors of NMNAT2 enzyme activity91 are likely to activate a Wallerian mechanism and have been associated with peripheral neuropathy98. These many influences on axonal NMNAT2 help to explain how diverse primary causes could activate a common Wallerian degeneration response99.
Stathmin 2
A protein that, like NMNAT2, is palmitoylated, has a short half-life and negatively regulates Wallerian degeneration. Its loss is insufficient to activate Wallerian degeneration but accelerates it after injury and its overexpression delays degeneration. The same protein is depleted in many induced pluripotent stem cell-derived motor neurons from sporadic amyotrophic lateral sclerosis patients.
Wallerian degeneration in human disease.
Identifying the pathogenic or disease-modifying effects of Wallerian-regulating proteins directly in human disorders promises to be a powerful indicator of where best to apply Wallerian-blocking drugs (TABLE 1). Animal studies are extremely useful but also have unavoidable caveats, making it essential to complement them with clinical data. For example, EAE is a very limited model of human MS and extrapolating directly from effectiveness in this model to the human disease is risky. Conversely, the failure of WLDS, or SARM1 ablation, to rescue SOD1 ALS mouse models by no means rules out the potential for therapy in other inherited forms of human ALS or in the much more prevalent sporadic forms.
Although no major neurological disease has so far been associated with mutations in genes involved in Wallerian degeneration, levels of NMNAT2 mRNA and protein are reduced in brains of individuals with Alzheimer disease95. Whether this is a cause or consequence of axonal and neuronal degeneration in Alzheimer disease remains unknown. Nevertheless, two recent studies have identified potentially pathogenic mutations in NMNAT2 in two rare neurological conditions89,90. The first of these describes two sisters who developed childhood onset peripheral neuropathy and erythromelalgia and were found to be homozygous for a T94M mutation in the NMNAT2 gene, likely indicating an autosomal recessive pattern of inheritance89. The T94M mutation confers partial loss of function for NMNAT2 (full loss of function of NMNAT2 is likely to be lethal). A companion report describes two fetuses with severe fetal akinesia deformation sequence and multiple developmental neurological abnormalities leading to stillbirth. These two siblings carried biallelic loss-of-function mutations in the NMNAT2 gene, with one (NMNAT2R232Q) disrupting both NAD+ synthase and chaperone activities and the other (NMNAT2Q135Pfs*44) greatly lowering protein expression. Each variant NMNAT2 was unable to prevent Wallerian degeneration when overexpressed in neurons90.
In another study, a 72-year-old patient with a 2-year history of progressive brainstem atrophy was shown to have a heterozygous mutation in NMNAT2 that had the potential to result in haploinsufficiency, although no functional analysis was presented100. Given the normalcy of the heterozygous parents and siblings of the individuals carrying biallelic loss-of-function mutations of NMNAT2 (REFS89,90), it is unclear whether NMNAT2 haploinsufficiency necessarily leads to axonopathies and which other environmental and genetic factors influence this. Furthermore, in the report on the patient with progressive brainstem atrophy, whole exome sequencing identified other potential candidate genes100.
Loss-of-function mutations of SARM1 would be expected to be protective, meaning that exome sequencing of disease cases will not find these mutations. However, evidence that SARM1 loss of function reduces disease risk or severity in humans would indicate involvement of the Wallerian degeneration mechanism in the disease. An ideal cohort in which to evaluate this possibility would be patients undergoing chemotherapy, assessing whether those who do not develop peripheral neuropathy despite high doses of chemotherapy carry more loss-of-function mutations in SARM1.
A genome-wide association study (GWAS) showed linkage to the SARM1 locus in sporadic ALS101, an observation that was later replicated in a separate GWAS study102. Although the authors in the second GWAS suggested that a cis-expression quantitative trait loci (eQTL) effect on Polymerase delta-interacting protein 2 (POLDIP2) might be the driver of linkage at this locus, a role for SARM1 has not been ruled out. For example, SARM1 expression might respond in a genetically determined way to environmental ALS risk factors that are not represented in the expression quantitative trait loci data. Two recent studies provide a further link between Wallerian degeneration and ALS. ALS-causing mutations in TDP43 reduce levels of the JNK substrate SCG10 in patient fibroblast-derived motor neurons and this is associated with reduced axonal growth96,97. This phenotype could be rescued by overexpression of SCG10 in human induced pluripotent stem cell-derived motor neurons. Previously, it had been shown that axotomy in mouse DRG neurons leads to a rapid reduction in SCG10 levels and that axonal degeneration could be delayed by its overexpression, indicating that SCG10 is a moderately strong modifier of Wallerian degeneration103. These observations do not imply that SCG10 depletion by itself is sufficient to cause axonal degeneration but that, when SCG10 is lowered as part of another pathogenic mechanism, Wallerian-like axon degeneration becomes more likely. Moreover, upregulation of SCG10 levels may be sufficient to protect axons from degeneration.
Following the discovery of NMNAT2 loss-of-function mutations in rare disorders89,90, it will be important to see what other pathogenic mutations emerge in these, or related, disorders. The human population exome and genome sequence database, the Genome Aggregation Database (gnomAD)104, compares the observed and expected frequencies of nonsense, frameshift and splicing mutations to estimate a ‘loss-of-function intolerance’ score, likely related to pathogenicity. Within this database, NMNAT2 has a 0.99 probability of loss-of-function intolerance, one of the highest scores, suggesting that many heterozygous alleles can also cause lethality or disease, at least in combination with other genes and/or environmental factors. SARM1 gain of function (for example, resulting in a mutant that is overexpressed, more stable or more easily activated) would also be expected to cause or increase disease risk.
Wallerian gene variants are likely to have wider clinical significance through disease-modifying effects: that is, by influencing risk and severity in both common and rare disorders. Evidence is emerging of a spectrum of axon vulnerability in the human population (FIG. 5) caused by both coding and gene expression variation of Wallerian pathway genes89,90,95. Data from mouse experiments already indicate that gene expression alterations have disease-modifying effects105. With reports that NMNAT2 expression level varies widely in post mortem brains95, it will be important to determine the consequences of this variation for disease and which genetic and environmental factors bring this about, especially as low Nmnat2 expression is associated with sensory and motor deficiencies in mice105. Similarly, increased SARM1 expression (for example, in viral infections51,52,106 or following prolonged low doses of rotenone107) is likely to increase disease risk. Interestingly, rotenone also causes SARM1-dependent axon degeneration in vitro108. The effect of other pesticides and environmental toxins on the pathway91 is another important area for study.
Fig. 5 |. An axon vulnerability spectrum in humans and mice.
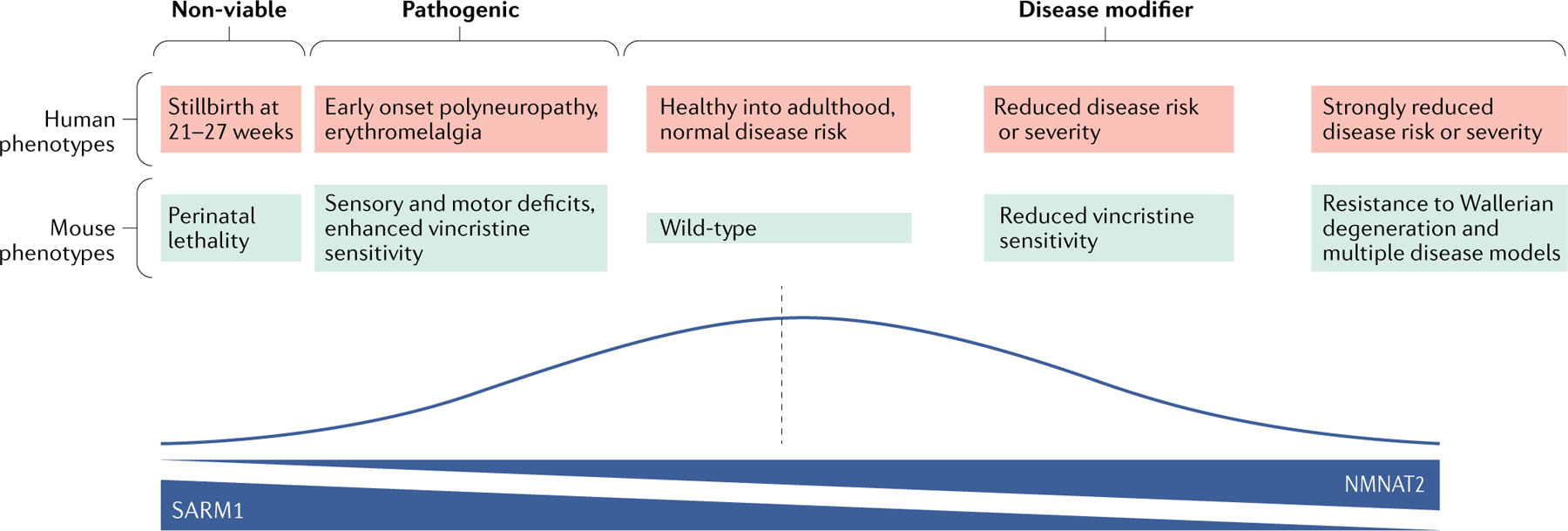
The figure shows the range of known axon survival phenotypes in mice and proposed analogous phenotypes in the human population for which evidence is growing. The line graph depicts the likely prevalence in the human population and the gradients underneath depict protein and enzyme activity levels of nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) and sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1) in axons. The pink and blue boxes show five distinct phenotypes in humans and mice, respectively, all of which have been extensively characterized in mice6,13,105. In humans, the first two phenotypes (non-viable and pathogenic) have been linked to NMNAT2 mutation89,90. Causes of the proposed disease-modifying effects indicated towards the right side, corresponding to lower axon vulnerability, may include (1) SARM1 splicing alleles that are present in the Genome Aggregation Database (gnomAD), which are likely to disrupt the function of this pro-degeneration protein and (2) higher than average expression levels of NMNAT295, which are likely to result in gain of function of this pro-survival protein.
Factors increasing axonal demand for NMNAT2 or reducing the soma’s ability to supply it are another risk; for example, ageing lowers NMNAT2 axonal transport62 and increases motor unit size (the number of muscle fibres that each motor neuron innervates) by up to 2.4-fold109, as surviving axons sprout to innervate neuromuscular junctions vacated by degenerating axons. Combined with reduced axonal transport, this is likely to create a ‘double hit’ on the ability to deliver sufficient NMNAT2 to keep distal axons alive. Thus, even minor impairment of the motor neuron soma could underlie the onset of a dying-back disorder such as ALS or ageing associated neuromuscular junction denervation110. Nigrostriatal neurons have even larger axonal arbours than motor neurons, with a combined length for a single neuron estimated at up to 4.5 m (REFS111,112). Age-related compensatory sprouting in this pathway, similar to that seen following a partial lesion113, would put a huge strain on the ability of surviving neurons to deliver critical cargoes such as NMNAT2. Thus, it will be important to determine whether such a mechanism could contribute to the dying-back observed in the striatum in Parkinson disease114.
In future studies, it will be important to directly test for Wallerian degeneration mechanisms in human disorders. In axonal transport disorders such as some forms of CIPN, it will be important to ask whether genetic variation in NMNAT2, SARM1 or other Wallerian-regulating proteins influences neuropathy risk. Carefully phenotyped patient populations are vital to firmly link neuropathy to a specific risk factor such as chemotherapy or diabetes; such cohorts exist115 but await careful genotyping. Genetic disruption of axonal transport is another important area for future research. For example, the mutation of tubulin folding cofactor E (TBCE) in early-onset encephalopathy with distal spinal muscular atrophy116 suggests the involvement of Wallerian degeneration as its mouse orthologue is disrupted in a model that was particularly strongly protected by WldS, progressive motor neuronopathy (pmn)117.
Criteria for involvement of Wallerian degeneration in disease.
As the field moves forward to clinical translation, it is important to establish strong criteria for the involvement of Wallerian degeneration in a particular disease (TABLE 2). For a long time, in animal models, the gold standard was evidence for significant alleviation of axon loss (and, ideally, symptoms) by WldS. To this we can now add alleviation of axon loss and symptoms through the deletion of Sarm1, which in some models is more effective than WldS2. Biochemical changes specifically associated with loss of NMNAT2 (such as changes in the NMN-to-NAD ratio22) or activation of SARM1 (such as a large increase in cADPR levels35) may play an increasing role in defining the presence of Wallerian degeneration, both in animal tissue and in human biopsy material. Meanwhile, significant disease associations with human genetic variants of Wallerian-regulating genes will become another strong indicator of its presence in human disorders. In these cases, it is expected that increased disease risk should correlate with NMNAT2 loss of function or SARM1 gain of function and that protective effects would be associated with alleles having the opposite effects. Disease modelling in human induced pluripotent stem cell lines will also help establish roles for Wallerian degeneration as this allows molecular pathways to be manipulated genetically or pharmacologically to test for rescue effects.
Table 2 |.
Criteria for defining the contribution of Wallerian degeneration to disease
| Criterion | Established in (disease) | Limitations | Refs |
|---|---|---|---|
| Protection by WLDS, NMNATs or SARM1 ablation in animal or cell culture models | Many (listed in REF.3) as well as metabolic syndrome and/or type II diabetes, CIPN, TBI, nerve growth failure and ALS | Animal and cell culture models only partially represent corresponding human diseases | 2,3,27,64–68,84 |
| Causation or exacerbation by removal and/or depletion of NMNAT2 in animal or cell culture models | Nerve growth failure, small fibre neuropathy (especially thermosensitivity and late onset motor impairment) and CIPN | Animal and cell culture models only partially represent corresponding human diseases | 11,13,105 |
| Genetic or protein expression association in humans with NMNAT2, SARM1 or other Wallerian degeneration regulator | Polyneuropathy with erythromelalgia, FADS, brainstem degeneration, ALS and Alzheimer disease | Correlation, not causation, although some supported by functional evidence; GWAS link may reflect another nearby gene | 89,90,95–97,100–102 |
| Chemical markers of Wallerian degeneration (raised levels of NMN or cADPR or lowered levels of NAD) in animal or cell culture models | Glaucoma, CIPN, nerve outgrowth failure and type II diabetes | Correlation not causation; animal and cell culture models only partially represent the corresponding human diseases | 21,27,73–75 |
ALS, amyotrophic lateral sclerosis; cADPR, cyclic ADP-ribose; CIPN, chemotherapy-induced peripheral neuropathy; FADS, fetal akinesia deformation sequence; GWAS, genome-wide association study; NMN, nicotinamide mononucleotide; NMNATs, nicotinamide mononucleotide adenylyltransferases; SARM1, sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1; TBI, traumatic brain injury; WLDs, Wallerian degeneration slow.
Drug development.
As the Wallerian degeneration mechanism and its involvement in disease, including human disease, become clearer, it is timely to consider what will be needed to drive the application of Wallerian degeneration-blocking therapies in the clinic. Safe and effective drugs will need to be trialled in appropriate disorders. Where different mechanisms underlie the same or closely related disorders (for example, peripheral neuropathies), the focus needs to be on the most relevant subtype. Finally, biomarkers are needed to measure efficacy.
One attractive clinical target is CIPN, which allows intervention before significant axonal injury occurs. The possibility of pre-medicating patients with a potentially neuroprotective drug (such as a SARM1 inhibitor or an FK866-like drug) before they receive chemotherapy would also simplify clinical trial design. These studies would be relatively short, as most chemotherapy drugs cause peripheral neuropathy within 6 months of starting treatment. The presence of validated outcome measures for CIPN would also simplify trial design and the recent development of neurofilament light chain levels as a potential biomarker of axonal injury make it even simpler118,119.
In terms of CNS diseases, TBI and primary and/or secondary progressive multiple sclerosis may be good initial targets. In TBI, one could potentially intervene immediately after an injury and contain the consequences of axonal injury. However, variability in the severity of injury and timely access to patients may make clinical trial design challenging. In multiple sclerosis, despite the development of effective immune-modulating therapies120, there are no treatments that prevent secondary axonal degeneration. Given the observations in EAE in WldS mice71, it is reasonable to consider axon-protective strategies that modulate the Wallerian degeneration pathway as a therapeutic target for multiple sclerosis. Clinical trials in this area will, however, be challenging as they will require long-term treatment and monitoring. Nevertheless, the use of biomarkers, such as neurofilament light chain levels, may help.
Given that the preclinical data show the efficacy of targeting several different components of the Wallerian degeneration pathway, which one is likely to be the most suitable for drug development? One of the most attractive targets is SARM1 as this molecule plays a relatively downstream role in Wallerian degeneration, meaning that one drug may be useful in multiple diseases. However, it will be challenging to develop a SARM1 inhibitor that does not affect other NAD-utilizing enzymes in neurons and/or non-neuronal cells, in order to avoid unwanted side effects. An inhibitor that targets the activation of SARM1 by preventing TIR domain dimerization may be a more suitable target. Recent observations that SARM1 forms an octomeric structure and that inhibition of oligomerization is able to prevent the cell death-promoting activity of SARM1, favour this approach48,49. Another attractive approach is to develop a dominant negative SARM1 gene therapy121 as this avoids the side effects of inhibiting other NADases; however, the delivery of such a therapy to appropriate neuronal populations remains a big challenge. Finally, anti-sense oligonucleotides have blossomed into FDA-approved therapies for rare neurodegenerative diseases such as amyloid neuropathy and spinal muscular atrophy122 and could therefore offer a more suitable delivery path.
Other therapeutic interventions may target other components of the Wallerian pathway. For example, drugs may target NMNAT2 expression level61 by preventing its degradation or by using a combined approach to prevent NMN synthesis (using FK866, for example) and providing an alternative NAD+ precursor such as nicotinic acid riboside30. Alternatively, drugs targeting pathway components that play a more prominent role in specific neuronal populations and disease states (such as DLK and/or LZK in RGCs in glaucoma) may lead to the development of disease-specific therapies.
Concluding remarks
The recent pace of discoveries in Wallerian degeneration research, the choice of suitable drug targets and the attractiveness of CIPN as a primary application, leaves us in little doubt that the next 5–10 years will see significant advances in translation in this field, as well as further developments in the mechanistic understanding. Genetics — whether in mice, flies or humans — will continue to provide the major advances that it has done for three decades. The biggest question is perhaps whether Wallerian-blocking drugs will be limited to rare, inherited disorders, where they are likely to be highly effective, or whether they will find extremely wide application across the neurodegenerative disease treatment spectrum. The prospects for the next three decades appear even more exciting than the last three.
Acknowledgements
The authors thank members of the Coleman group for constructive feedback.
Footnotes
Competing interests
M.P.C. has an academic collaboration with AstraZeneca and is a consultant for Proneurotech. A.H. serves on the scientific advisory board of Disarm Therapeutics.
References
- 1.Waller A Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 140, 423–429 (1850). [Google Scholar]
- 2. Gilley J, Ribchester RR & Coleman M R Sarm 1 deletion, but not Wlds, confers lifelong rescue in a mouse model of severe axonopathy. Cell Rep. 21, 10–16(2017). This study shows that blocking Wallerian degeneration can permanently rescue axons in some circumstances.
- 3.Conforti L, Gilley J & Coleman M R Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 15, 394–409 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Lunn ER, Perry VH, Brown MC, Rosen H & Gordon S Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur. J. Neurosci. 1,27–33 (1989). The discovery of the Wlds (formerly ‘Ola’) mouse, which initiated a molecular understanding of Wallerian degeneration.
- 5.Lubińska L Patterns of Wallerian degeneration of myelinated fibres in short and long peripheral stumps and in isolated segments of rat phrenic nerve. Interpretation of the role of axoplasmic flow of the trophic factor. Brain Res. 233, 227–240 (1982). [DOI] [PubMed] [Google Scholar]
- 6. Mack TGA et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4, 1199–1206 (2001). The identification of the protective gene and protein in Wlds mice implicated NAD biology.
- 7.Coleman MP & Freeman MR Wallerian degeneration, Wlds, and Nmnat. Annu. Rev. Neurosci. 33, 245–267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araki T Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Conforti L et al. Wlds protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 184, 491–500 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babetto E et al. Targeting NMNAT 1 to axons and synapses transforms its neuroprotective potency in vivo. J. Neurosci. 30, 13291–13304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks AN et al. Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLOS ONE 7, e47869 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilley J & Coleman MP Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLOS Biol. 8, e1000300 (2010). The identification of NMNAT2 as a pro-survival, endogenous regulator of Wallerian degeneration.
- 13.Gilley J, Adalbert R, Yu G & Coleman MP Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J. Neurosci. 33, 13410–13424 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milde S, Gilley J & Coleman MP Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLOS Biol. 11, e1001539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald JM et al. The Drosophila cell corpse engulfment receptor draper mediates glial clearance of severed axons. Neuron 50, 869–881 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Hoopfer ED et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50, 883–895 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Xiong X et al. The highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLOS Biol. 10, e1001440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osterloh JM et al. dSarm/Sarm 1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484 (2012). The identification of dSarm/SARMl as a prodegenerative, endogenous regulator of Wallerian degeneration.
- 19. Neukomm LJ et al. Axon death pathways converge on axundead to promote functional and structural axon disassembly. Neuron 95, 78–91.e5 (2017). The identification of a Wallerian degeneration execution step downstream of dSarm.
- 20.Llobet Rosell A & Neukomm LJ Axon death signalling in Wallerian degeneration among species and in disease. Open Biol. 9, 190118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 170, 349–355 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Stefano M et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 22, 731–742 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Essuman K et al. The SARM1 toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343.e5 (2017). This study provides evidence that an NADase activity in full-length SARM 1 is linked to axon degeneration.
- 24.Gerdts J, Summers DW, Sasaki Y, DiAntonio A & Milbrandt J Sarml-mediated axon degeneration requires both SAM and TIR interactions. J. Neurosci. 33, 13569–13580(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A & Milbrandt J SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki Y, Nakagawa T, Mao X, DiAntonio A & Milbrandt J NMNAT 1 inhibits axon degeneration via blockade of SARM 1-mediated NAD+ depletion. eLife 5, e1749 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilley J, Orsomando G, Nascimento-Ferreira I & Coleman M R Absence of SARM 1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 10, 1974–1981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikiforov A, Kulikova V & Ziegler M The human NAD metabolome: functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 50, 284–297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki Y, Vohra BPS, Lund FE & Milbrandt J Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci. 29, 5525–5535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H et al. Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc. Natl Acad. Sci. USA 115, 10654–10659 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark DE et al. Application of virtual screening to the discovery of novel nicotinamide phosphoribosyltransferase (NAMPT) inhibitors with potential for the treatment of cancer and axonopathies. Bioorg. Med. Chem. Lett. 26, 2920–2926(2016). [DOI] [PubMed] [Google Scholar]
- 32.Di Stefano M et al. NMN deamidase delays wallerian degeneration and rescues axonal defects caused by NMNAT2 deficiency in vivo. Curr. Biol. 27, 784–794 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Feinberg K et al. A neuroprotective agent that inactivates prodegenerative TrkA and preserves mitochondria. J. Cell Biol. 216, 3655–3675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loreto A, Di Stefano M, Gering M & Conforti L Wallerian degeneration is executed by an NMN-SARM1-dependent late Ca2+ influx but only modestly influenced by mitochondria. Cell Rep. 13, 2539–2552 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Zhao ZY et al. A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15, 452–466 (2019). This study demonstrates that NMN can activate SARM1.
- 36.Summers DW, Gibson DA, DiAntonio A & Milbrandt J SARM1-specific motifs in the TIR domain enable NAD + loss and regulate injury-induced SARM1 activation. Proc. Natl Acad. Sci. USA 113, E6271–E6280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maday S, Twelvetrees AE, Moughamian AJ & Holzbaur ELF Axonal transport: cargo-pecific mechanisms of motility and regulation. Neuron 84, 292–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers DW, Milbrandt J & DiAntonio A Palmitoylation enables MAPK-dependent proteostasis of axon survival factors. Proc. Natl Acad. Sci. USA 115, E8746–E8754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milde S, Fox AN, Freeman MR & Coleman MP Deletions within its subcellular targeting domain enhance the axon protective capacity of Nmnat2 in vivo. Sci. Rep. 3, 2567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milde S & Coleman MP Identification of palmitoyltransferase and thioesterase enzymes that control the subcellular localization of axon survival factor nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2). J. Biol. Chem. 289, 32858–32870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker LJ et al. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. eLife 6, e22540 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J et al. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 160, 161–176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babetto E, Beirowski B, Russler EV, Milbrandt J & DiAntonio A The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 3, 1422–1429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamagishi Y & Tessier-Lavigne M An atypical SCF-like ubiquitin ligase complex promotes wallerian degeneration through regulation of axonal Nmnat2. Cell Rep. 17, 774–782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desbois M et al. PAM forms an atypical SCF ubiquitin ligase complex that ubiquitinates and degrades NMNAT2. J. Biol. Chem. 293, 13897–13909 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brace EJ, Wu C, Valakh V & DiAntonio A SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury. J. Neurosci. 34, 8398–8410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pao K-C et al. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity. Nature 556, 381–385 (2018). A novel ubiquitin ligase mechanism in MYCBP2 indicates new opportunities for drug development.
- 48.Sporny M et al. Structural evidence for an octameric ring arrangement of SARM1. J. Mol. Biol. 431, 3591–3605 (2019). [DOI] [PubMed] [Google Scholar]
- 49. Horsefield S et al. NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365, 793–799 (2019). The first report of the structure of SARM1 complexed with a low MW substrate.
- 50. Wang Q et al. Sarm1/Myd88–5 regulates neuronal intrinsic immune response to traumatic axonal injuries. Cell Rep. 23, 716–724 (2018). This study provides evidence of a retrograde injury signalling function of SARM1 that is likely to be distinct from its role in Wallerian degeneration.
- 51.Morale MG, da Silva Abjaude W, Silva AM, Villa LL & Boccardo E HPV-transformed cells exhibit altered HMGB1-TLR4/MyD88-SARM1 signaling axis. Sci. Rep. 8, 3476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee P, Woods TA, Moore RA & Peterson KE Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity 38, 705–716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murata H et al. c-Jun N-terminal kinase (JN)-mediated phosphorylation of SARM1 regulates NAD+ cleavage activity to inhibit mitochondrial respiration. J. Biol. Chem. 293, 18933–18943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panneerselvam P et al. T-cell death following immune activation is mediated by mitochondria-localized SARM. Cell Death Differ. 20, 478–489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y et al. MyD88–5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J. Exp. Med. 204, 2063–2074 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murata H, Sakaguchi M, Kataoka K & Huh NH SARM1 and TRAF6 bind to and stabilize PINK1 on depolarized mitochondria. Mol. Biol. Cell 24, 2772–2784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Killackey SA et al. The mitochondrial Nod-like receptor NLRX1 modifies apoptosis through SARM1. Mol. Cell. Biochem. 453, 187–196 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Essuman K et al. TIR domain proteins are an ancient family of NAD+-consuming enzymes. Curr. Biol. 28, 421–430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan L et al. TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365, 799–803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shigeoka T et al. Dynamic axonal translation in developing and mature visual circuits. Cell 166, 181–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ali YO, Bradley G & Lu HC Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Science Rep. 7, 43846 (2017). This study shows a substantial variation of NMNAT2 expression level in humans and a correlation with dementia.
- 62.Milde S, Adalbert R, Elaman MH & Coleman MP Axonal transport declines with age in two distinct phases separated by a period of relative stability. Neurobiol. Aging 36, 971–981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ljungberg MC et al. CREB-activity and Nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum. Mol. Genet. 21, 251–267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geisler S et al. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 139, 3092–3108 (2016). This study provides in vivo evidence of protection from CIPN by removal of SARM1.
- 65.Geisler S et al. Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program. JCI Insight 4, e129920 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Turkiew E, Falconer D, Reed N & Höke A Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J. Peripher. Nerv. Syst. 22, 162–171 (2017). This study reports the alleviation of in vivo models of CIPN and metabolic disorder.
- 67.Henninger N et al. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 139, 1094–1105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziogas NK & Koliatsos VE Primary traumatic axonopathy in mice subjected to impact acceleration: a reappraisal of pathology and mechanisms with high-resolution anatomical methods. J. Neurosci. 38, 4031–4047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki Y & Milbrandt J Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J. Biol. Chem. 285, 41211–41215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang JT, Medress ZA, Vargas ME & Barres BA Local axonal protection by WldS as revealed by conditional regulation of protein stability. Proc. Natl Acad. Sci. USA 112, 10093–10100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaneko S Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chitnis T et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am. J. Pathol. 170, 1695–1712 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams PA et al. Nicotinamide and WLDS act together to prevent neurodegeneration in glaucoma. Front. Neurosci. 11, 232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams PA et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760 (2017). This study indicates the therapeutic potential of NMNAT and NAD in glaucoma.
- 75.Trammell SA et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 6, 26933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dollerup OL et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 108, 343–353 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Howell GR et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 179, 1523–1537 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beirowski B, Babetto E, Coleman MP & Martin KR The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 28, 1166–1179 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Carty M et al. Cell survival and cytokine release after inflammasome activation is regulated by the Toll-IL-1R protein SARM. Immunity 50, 1412–1424 (2019). [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin CN, Perry-Richardson JJ, Coutinho-Budd JC & Broihier HT Dying neurons utilize innate immune signaling to prime glia for phagocytosis during development. Dev. Cell 48, 506–522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer LR et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 185, 232–240 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Fischer LR et al. The WldS gene modestly prolongs survival in the SOD1G93A fALS mouse. Neurobiol. Dis. 19, 293–300 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Peters OM et al. Loss of Sarm1 does not suppress motor neuron degeneration in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Hum. Mol. Genet. 27, 3761–3771 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White MA et al. Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss. Acta Neuropathol. Commun. 7, 166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandes KA et al. Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury. Exp. Eye Res. 171, 54–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu C, Li B, Frontzek K, Liu Y & Aguzzi A SARM1 deficiency up-regulates XAF1, promotes neuronal apoptosis, and accelerates prion disease. J. Exp. Med. 216, 743–756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szretter KJ et al. The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts west Nile virus pathogenesis. J. Virol. 83, 9329–9338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vérièpe J, Fossouo L & Parker JA Neurodegeneration in C. elegans models of ALS requires TIR-1/Sarm1 immune pathway activation in neurons. Nat. Commun. 6, 7319 (2015). [DOI] [PubMed] [Google Scholar]
- 89. Huppke P et al. Homozygous NMNAT2 mutation in sisters with polyneuropathy and erythromelalgia. Exp. Neurol. 320, 112958 (2019). This study reports a human NMNAT2 mutation that is associated with paediatric neurological disease.
- 90. Lukacs M et al. Severe biallelic loss-of-function mutations in nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) in two fetuses with fetal akinesia deformation sequence. Exp. Neurol. 320, 112961 (2019). This study reports a human NMNAT2 mutation that is associated with a stillbirth phenotype similar to Nmnat2 null mice.
- 91.Buonvicino D et al. Identification of the nicotinamide salvage pathway as a new toxification route for antimetabolites. Cell Chem. Biol. 25, 471–482 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Wang MS et al. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann. Neurol. 50, 773–779 (2001). [DOI] [PubMed] [Google Scholar]
- 93.Sasaki Y, Vohra BPS, Baloh RH & Milbrandt J Transgenic mice expressing the nmnat1 protein manifest robust delay in axonal degeneration in vivo. J. Neurosci. 29, 6526–6534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farley JE et al. Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc. Natl Acad. Sci. USA 115, 1358–1363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ali YO et al. NMNAT2:HSP90 complex mediates proteostasis in proteinopathies. PLOS Biol. 14, e1002472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klim JR et al. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci. 22, 167–179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melamed Z et al. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat. Neurosci. 22, 180–190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.LeWitt PA Neurotoxicity of the rat poison vacor — a clinical study of 12 cases. N. Engl. J. Med. 302, 73–77 (1980). [DOI] [PubMed] [Google Scholar]
- 99.Coleman M Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Schulz A, Wagner F, Ungelenk M, Kurth I & Redecker C Stroke-like onset of brain stem degeneration presents with unique MRI sign and heterozygous NMNAT2 variant: a case report. Transl. Neurodegener. 5, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fogh I et al. A genome-wide association metaanalysis identifies a novel locus at 17q11.2 associated with sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 23, 2220–2231 (2014). In REFs 101 and 102 GWAS report a linkage between ALS and the SARM1 locuse.
- 102.Consortium SLAGEN et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shin JE et al. SCG10 is a JNK target in the axonal degeneration pathway. Proc. Natl Acad. Sci. USA 109, E3696–E3705 (2012). This study reports that SCG10 modifies the rate of Wallerian degeneration.
- 104.Karczewski KJ et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. preprint at bioRxiv 10.1101/531210 (2019). [DOI]
- 105.Gilley J, Mayer PR, Yu G & Coleman MP Low levels of NMNAT2 compromise axon development and survival. Hum. Mol. Genet. 28, 448–458 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Hou Y-J et al. SARM is required for neuronal injury and cytokine production in response to central nervous system viral infection. J. Immunol. 191, 875–883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sur M et al. Sarm1 induction and accompanying inflammatory response mediates age-dependent susceptibility to rotenone-induced neurotoxicity. Cell Death Discov. 4, 114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Summers DW, DiAntonio A & Milbrandt J Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J. Neurosci. 34, 9338–9350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Valdez G, Tapia JC, Lichtman JW, Fox MA & Sanes JR Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLOS ONE 7, e34640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chung T et al. Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline: dying-back axonal degeneration. Muscle Nerve 55, 894–901 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsuda W et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 29, 444–453 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolam JP & Pissadaki EK Living on the edge with too many mouths to feed: why dopamine neurons die. Mov. Disord. 27, 1478–1483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song DD & Haber SN Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J. Neurosci. 20, 5102–5114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng H-C, Ulane CM & Burke RE Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 67, 715–725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas S et al. Peripheral neuropathy research registry: a prospective cohort. J. Peripher. Nerv. Syst. 24, 39–47 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Sferra A et al. TBCE mutations cause early-onset progressive encephalopathy with distal spinal muscular atrophy. Am. J. Hum. Genet.99974–983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferri A, Sanes JR, Coleman MP, Cunningham JM & Kato AC Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr. Biol. 13, 669–673 (2003). [DOI] [PubMed] [Google Scholar]
- 118.Shahim P et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 36791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varhaug KN et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 5, e422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coles AJ et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380, 1829–1839 (2012). [DOI] [PubMed] [Google Scholar]
- 121.Geisler S et al. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J. Exp. Med. 216, 294–303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corey DR Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat. Neurosci. 20, 497–499 (2017). [DOI] [PubMed] [Google Scholar]


